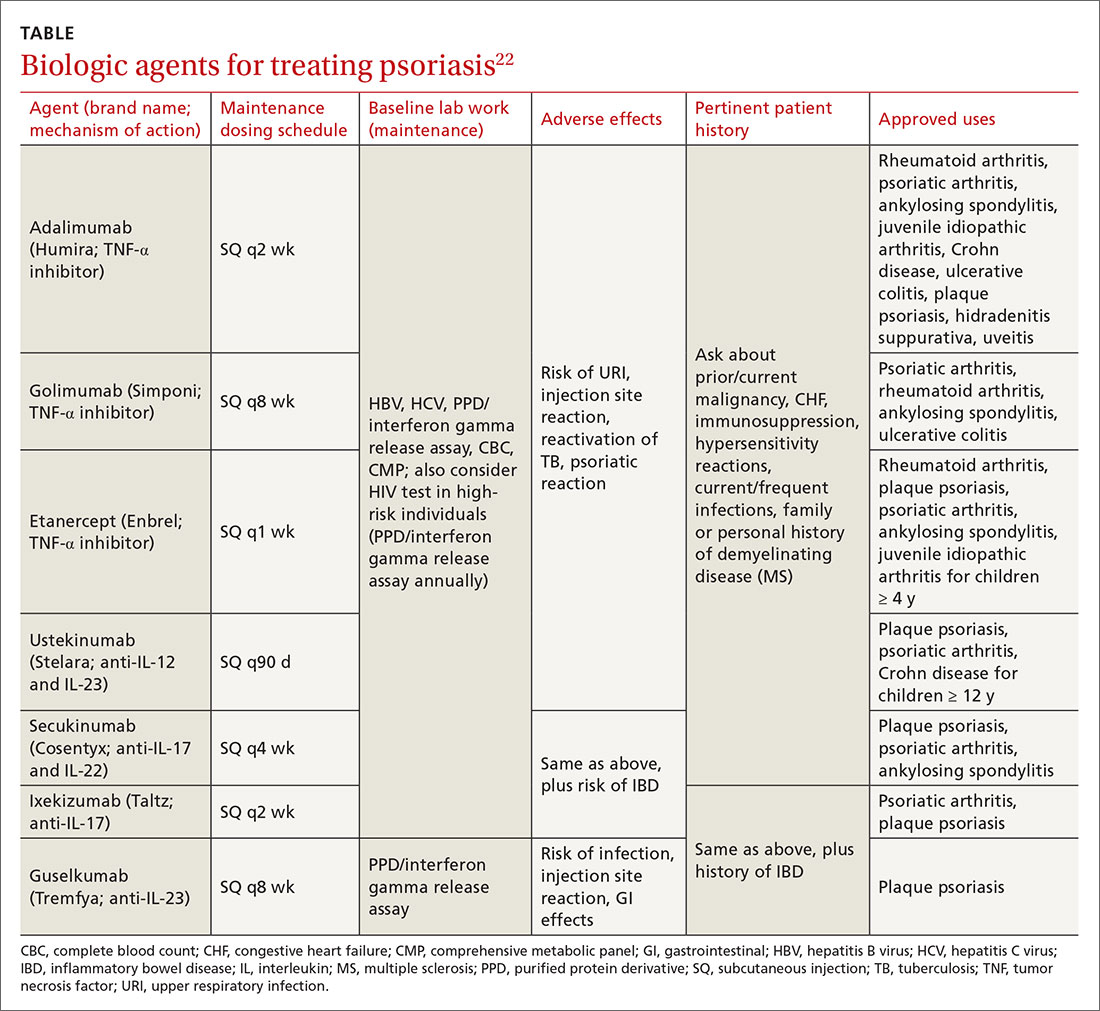
User login
Tips and tools for safe opioid prescribing
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
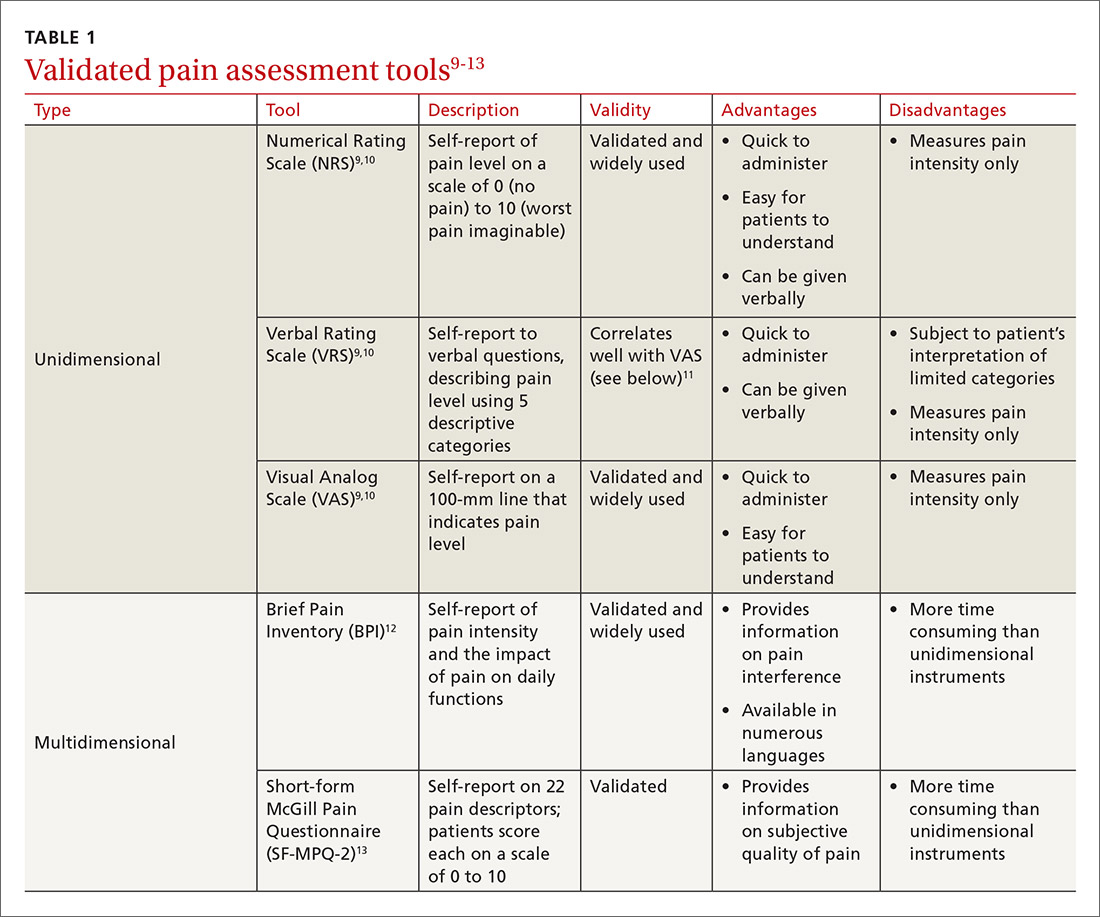
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
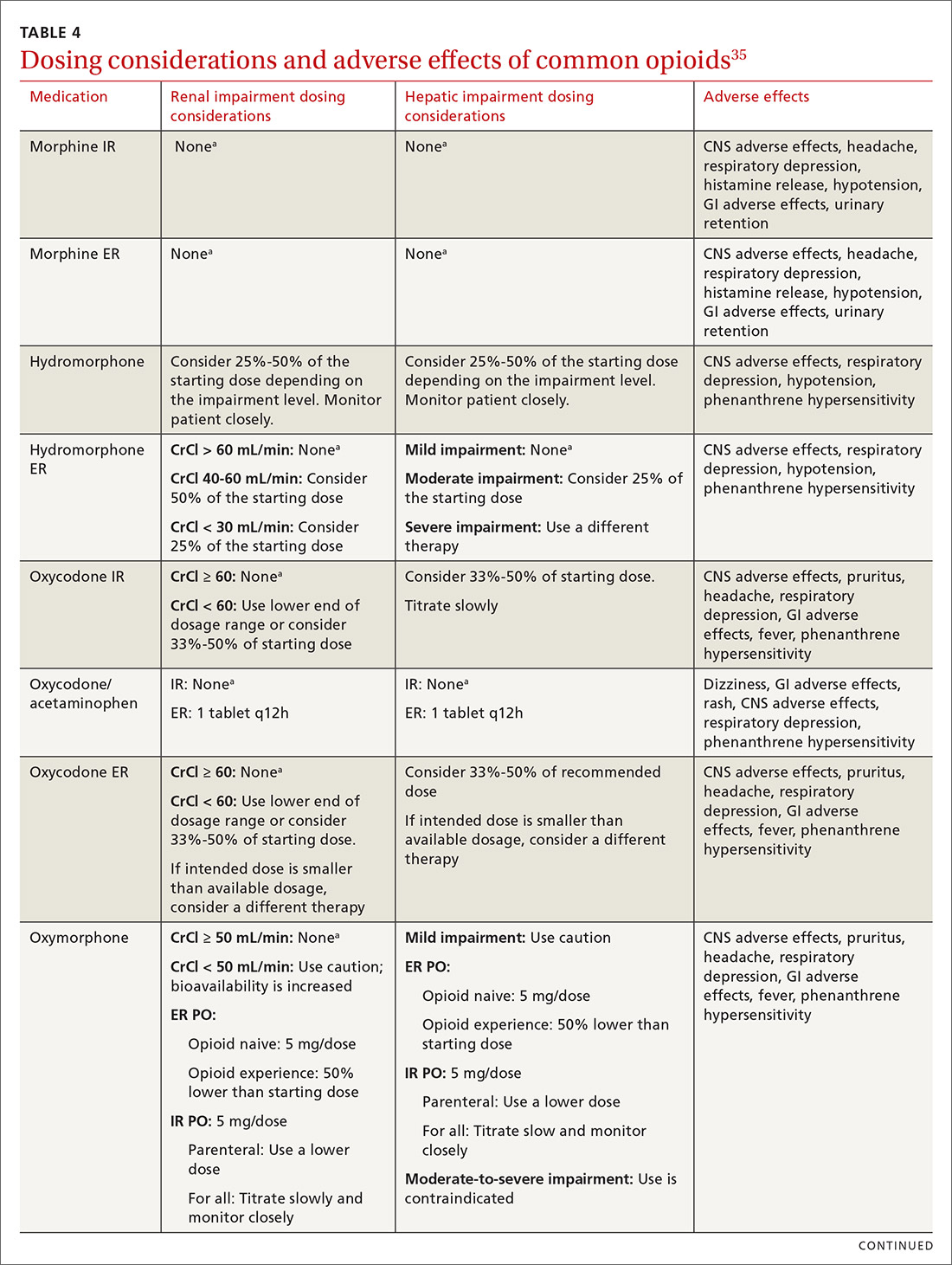
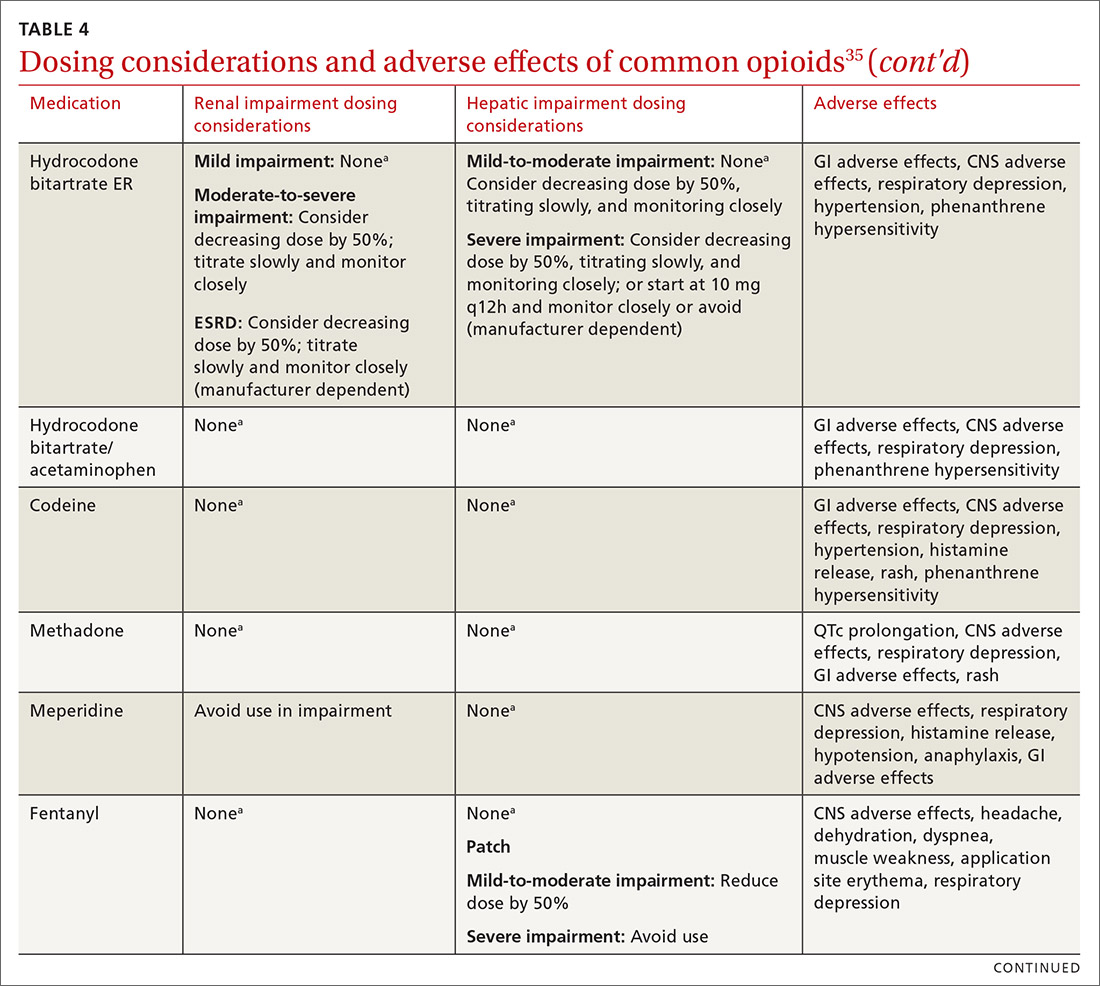
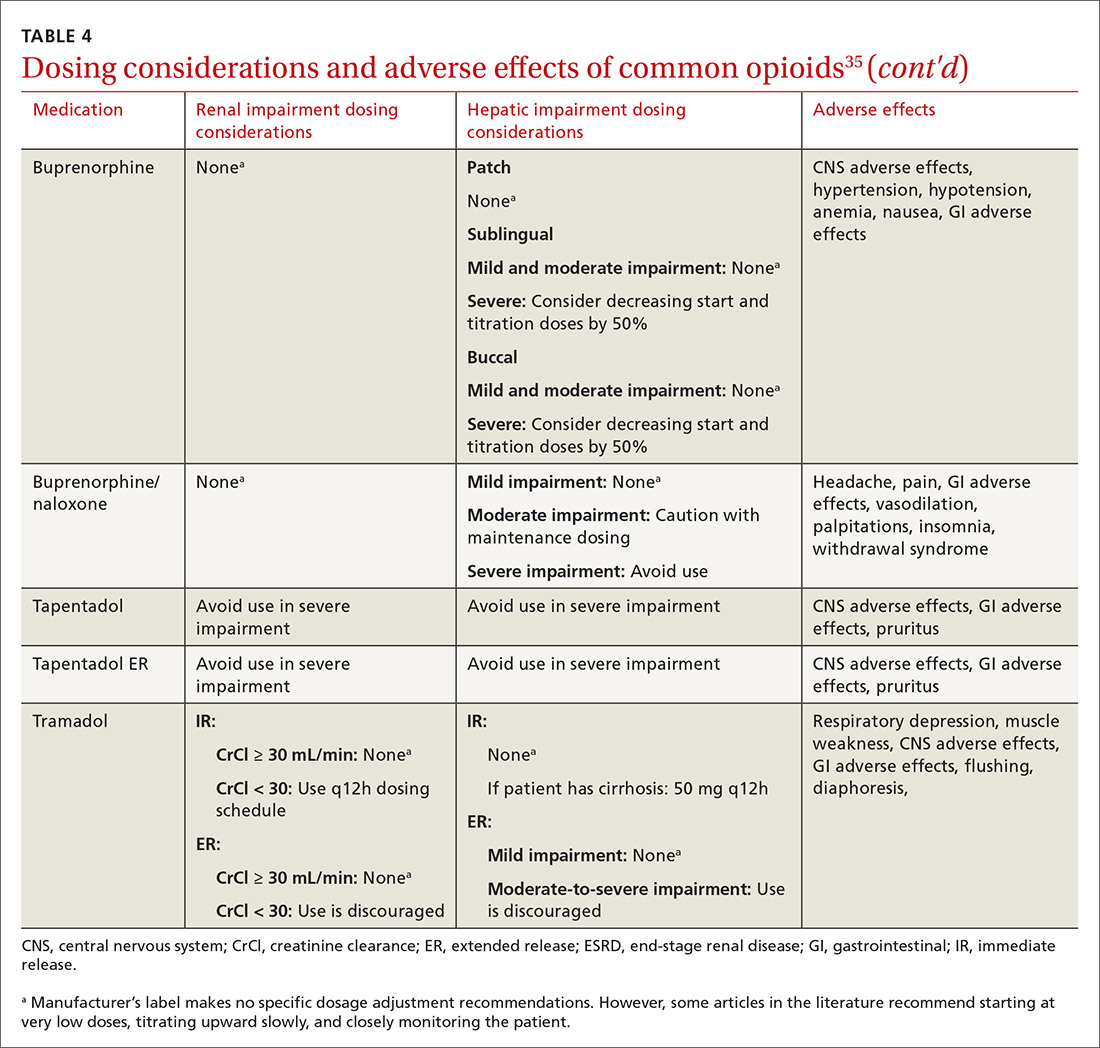
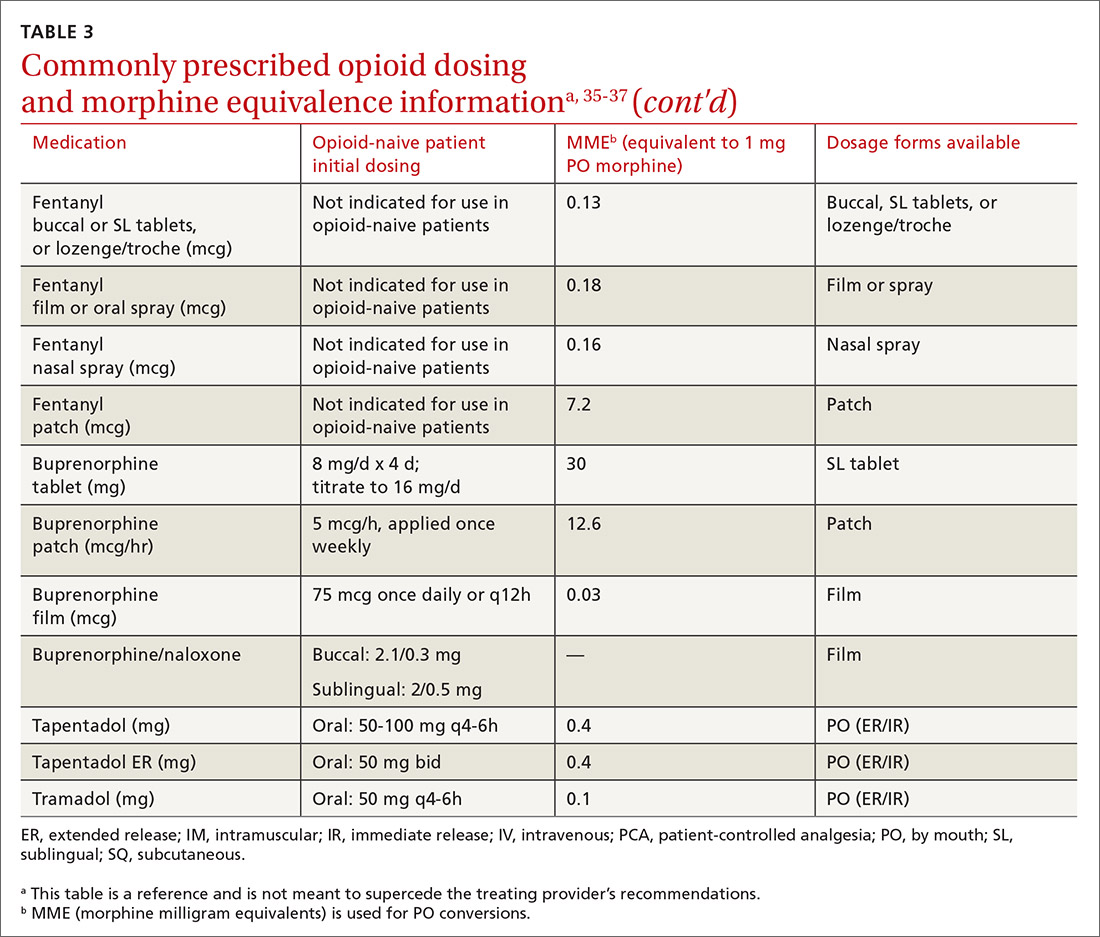
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).
Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
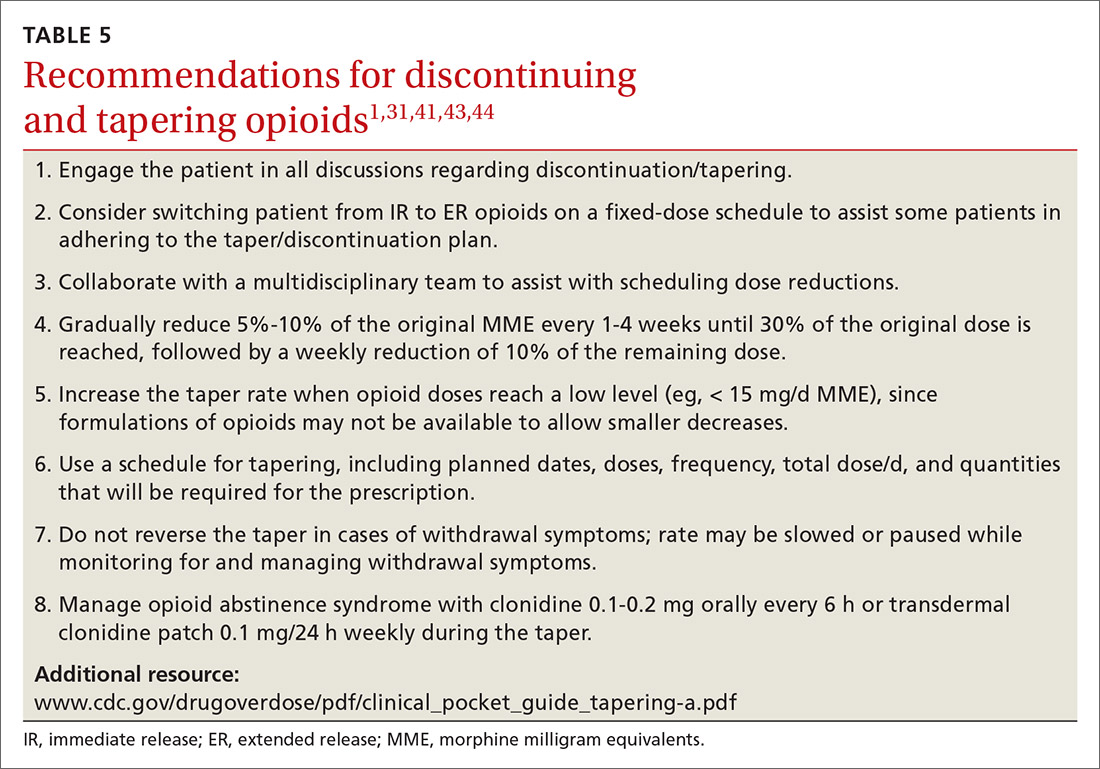
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
PRACTICE RECOMMENDATIONS
› Use a screening instrument such as the Opioid Risk Tool or the DIRE assessment to gauge a patient’s risk of opioid misuse and determine the frequency of monitoring. C
› Give as much priority to improving functional activity and minimizing adverse opioid effects as you do to relieving pain. C
› Prescribe an immediate-release, short-acting agent at first instead of a long-acting formulation; start with the lowest effective dosage and calculate total daily dose in terms of morphine milligram equivalents (MME). C
› Reduce the original MME dose by 5% to 10% every week when discontinuing an opioid. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Acute rhinosinusitis: When to prescribe an antibiotic
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9
Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; [email protected].
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9
Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; [email protected].
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9
Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; [email protected].
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
PRACTICE RECOMMENDATIONS
› Reserve antibiotics for patients who meet diagnostic criteria for acute bacterial rhinosinusitis (ABRS). Patients must have purulent nasal drainage that is accompanied by either nasal obstruction or facial pain/pressure/fullness and EITHER symptoms that persist without improvement for at least 10 days OR symptoms that worsen within 10 days of initial improvement (“double sickening”). A
› Offer watchful waiting and delay antibiotics for up to 7 days after diagnosing ABRS in a patient if adequate access to follow-up is available; otherwise, treat with amoxicillin (with or without clavulanate) for 5 to 10 days. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing a woman with BRCA mutations? Shared decision-making is key
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; [email protected].
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; [email protected].
CASE
Sara T* recently moved back to the area to be closer to her family. The 34-year-old patient visited our office to discuss the benefits and potential risks of genetic counseling. She explained that her aunt had just died at age 64 of ovarian cancer. Also, her maternal cousin had been diagnosed at age 42 with breast cancer, and her maternal grandmother had died at age 45 of an unknown “female cancer.” She was scared to find out if she had high-risk genes because she felt it would change her life forever. However, if she ignored the issue, she thought she might worry too much.
We discussed the implications of a positive result, such as having to live with the knowledge and to make decisions about potential screening and risk-reducing surgery. On the other hand, not knowing could allow for the undetected growth of cancer that might otherwise be mitigated to some degree if she knew her risk status and pursued an aggressive screening program.
We worked with Ms. T to map out her next steps.
*The patient’s name has been changed to protect her identity.
Breast cancer is the most commonly diagnosed cancer in women worldwide, representing nearly one-quarter of all female cancer diagnoses in 2018.1 It is the second-leading cause of cancer death in women in developed nations and the leading cause of cancer death in women in developing nations.1 In the United States, 1 in 8 women will develop breast cancer in her lifetime.2 By comparison, the rate of ovarian cancer is much lower, with a lifetime prevalence of 1 in 70 to 80 women.3,4 Although ovarian cancer is less common than breast cancer, its associated mortality is high, and most cases are discovered at advanced stages.
The outsized threat of BRCA mutations. It is estimated that 5% to 10% of all breast cancers are hereditary, with 80% of these attributable to BRCA1 (45%) and BRCA2 (35%).5 These autosomal dominant mutations occur at the germline level, within the egg or sperm, and are therefore incorporated into the DNA of every cell and passed from one generation to the next. Families with BRCA mutations have much higher lifetime rates of cancer. The lifetime risk of breast cancer due to BRCA mutations is estimated at > 80% (BRCA1) and 45% (BRCA2).5BRCA mutations account for between
Male BRCA carriers have a lifetime breast cancer risk of 1% to 5% with BRCA1 and 5% to 10% with BRCA2,7,8 compared with about 1:1000 lifetime incidence in the unselected male population. Male carriers are also at risk for more aggressive prostate cancers.7,8
Continue to: Certain populatiosn carry undue burden of BRCA-related disease
Certain populations carry undue burden of BRCA-related disease due to specific founder mutations. While the estimated global prevalence of BRCA mutations is 0.2% to 1%, for those of Ashkenazi Jewish descent the range is 2% to 3%, representing a relative risk up to 15 times that of the general population.9 Hispanic Americans also appear to have higher rates of BRCA-related cancers.10 Ongoing genetics research continues to identify founder mutations worldwide,10 which may inform future screening guidelines.
In addition to BRCA mutations, there are other, less common mutations (TABLE 15) known to cause hereditary breast and ovarian cancer.
Identifying BRCA genes enables treatment planning. Compared with sporadic cancers, BRCA-related breast cancers
Similarly, BRCA-related ovarian cancer is more likely to be high-grade and endometrioid or serous subtype.14
Shared decision-making helps give clarity to the way forward
Shared decision-making is a process of communication whereby the clinician and the patient identify a decision to be made, review data relevant to clinical options, discuss patient perspectives and preferences regarding each option, and arrive at the decision together.17 Shared decision-making is important when treating women with BRCA mutations because there is no single correct plan. Individual values and competing medical issues may strongly guide each woman’s decisions about screening and cancer prevention treatment decisions.
Continue to: Shared decision-making in this situation...
Shared decision-making in this situation is a strategy to use the evidence of risk along with patient preferences around fertility issues to help come to a decision that is the right one for the patient. Primary care clinicians aware of the general risks and benefits of each available option can refer women at high risk for breast or ovarian cancer to a specialist multidisciplinary clinic that can provide tailored risk assessment and risk reduction counseling as needed.18-20
Genetic screening recommendations
Screening is recommended for women who have any 1 of several family risk factors (TABLE 221). A number of risk assessment tools are available for primary care clinicians to determine which patients are at high enough risk for a hereditary breast or ovarian cancer to warrant referral to a genetic counselor.22-25 If screening suggests high risk, the US Preventive Services Task Force (USPSTF) recommends (Grade B) referral for genetic counseling.21
Explain to patients who are candidates for further investigation that a genetic counselor will review their family history and recommend testing for the specific mutations that increase cancer risk. Discuss potential benefits and harms of genetic testing. A benefit of genetic testing is that aggressive screening may suggest preventive procedures to reduce the risk of future cancer. Most tests come back definitively positive or negative, but an indeterminate result may cause harm. A small minority of results may indicate a genetic variant of unknown significance. The ramifications of this variant may not be known. Some women will experience anxiety about nonspecific test results and will be afraid to share them with family members. There is also some concern about privacy issues, potential insurance bias, and coverage of any preventive strategies.26
CASE
Based on Ms. T’s family history and her desire to know more, we referred her to a genetic counselor and she decided to undergo genetic testing. She screened positive for BRCA1. Ms. T was in a serious relationship and thought she would like to have children at some point. She returned to our office after receiving the positive genetic test results, wondering about screening for breast and ovarian cancer.
Breast cancer screening and risk-reduction strategies
Screening. Because the risk of breast cancer is high in women with BRCA mutations, and because cancer in these women is more likely to be advanced at diagnosis, starting a screening program at an early age is prudent. Observational studies suggest that breastfeeding reduces the risk of breast and ovarian cancer in women with BRCA mutations, as it does for women in the general population.27 Women should return for a clinical breast exam every 6 to 12 months starting at age 25; they should start radiologic screening with magnetic resonance imaging at age 25 and mammography at age 30 (TABLE 327,28).
Continue to: Risk-reduction strategies
Risk-reduction strategies. There is weak evidence to support the use of tamoxifen or other synthetic estrogen reuptake modulators (SERMs) to reduce breast cancer risk in women with BRCA mutations. Many of these cancers do not express estrogen receptors, which may explain the lack of efficacy in certain cases. Several observational studies have shown that tamoxifen can reduce the risk of contralateral breast cancer in women with BRCA mutations who have already been diagnosed with cancer in the other breast.29-31 However, tamoxifen does not reduce a patient’s risk of ovarian cancer, and it may increase her risk of uterine cancer.
Prophylactic bilateral mastectomy is the mainstay of breast cancer prevention in this population. Data from a systematic review suggest that this surgery may prevent the incidence of breast cancer in women with BRCA mutations by 90% to 95%.32 However, this review did not demonstrate a reduction in mortality from breast cancer, likely due to poor data quality.32 The National Comprehensive Cancer Network (NCCN) recommends discussing prophylactic mastectomy with all women who have BRCA mutations.28 Further conversations are important to review the risk of tissue left behind and quality-of-life issues, including the inability to breastfeed if the woman wants more children and the cosmetic changes with reconstruction.
Ovarian cancer screening and risk-reduction strategies
Screening. No effective screening strategy has been endorsed for ovarian cancer, as most previous studies have shown screening to be ineffective.26,33 Recently, studies both in the United Kingdom and the United States have investigated a screening strategy using the risk-of-ovarian-cancer algorithm (ROCA), which calculates an individual’s risk based on serum levels of cancer antigen 125 (CA-125).34,35 These studies measured CA-125 levels every 3 to 4 months followed by transvaginal ultrasound if CA-125 increased substantially (as determined by ROCA). Absent an abnormal increase in CA-125, transvaginal ultrasound was performed annually. These screening strategies showed improved specificity over annual screening programs, and the cancers detected were more likely to be diagnosed at an early stage (stage II vs stage III) and had higher rates of zero residual disease after surgery compared with those detected 1 year after screening ended.34,35 However, survival data are not yet available. More research is needed to determine if more frequent screening approaches could improve survival in high-risk women.
NCCN and the American College of Obstetricians and Gynecologists (ACOG) do not endorse routine screening with transvaginal ultrasound and serum CA-125 for high-risk women, as the benefits are uncertain. However, they do advise that these screens may be considered as a short-term strategy for women ages 30 to 35 who defer risk-reducing surgery.26,36 The USPSTF does not make a recommendation regarding ovarian cancer screening in high-risk women.37
Risk-reduction strategies. Risk-reducing bilateral salpingo-oophorectomy (RRSO) is the only recommended technique for reducing the risk of ovarian cancer in women at high risk.26,33,36 Meta-analyses have shown an 80% reduction in ovarian cancer risk16 and 68% reduction in all-cause mortality with this approach.38 The NCCN recommends RRSO for women with a known BRCA1 mutation between the ages of 35 and 40 who have completed childbearing.36 Since the onset of ovarian cancer tends to be later in women with BRCA2 mutations, it is reasonable to delay RRSO until age 40 to 45 in this population if they have taken other steps to maximize breast cancer prevention (ie, bilateral mastectomy).36
Continue to: Adverse effects of RRSO...
Adverse effects of RRSO include surgery complications (wound infection, small bowel obstruction, bladder perforation) and effects of early menopause (vasomotor symptoms, decreased sexual functioning, and increased risk of osteoporosis, cardiovascular disease, and all-cause mortality).39-41 In the absence of contraindications, ACOG recommends using hormone therapy in women undergoing RRSO until the natural age of menopause,42 particularly if their breast tissue has been removed.
Salpingectomy as an alternative. In an attempt to reduce these adverse effects of early menopause, and because a large proportion of high-grade serous tumors originate in the fallopian tube,43 interest has increased in the use of risk-reducing salpingectomy (removal of fallopian tubes) and delayed oophorectomy in women at high risk of ovarian cancer.42 Studies have shown this may be a cost-effective approach and an acceptable alternative in BRCA mutation carriers who are unwilling to undergo RRSO.44,45 A clinical trial investigating this approach in women with BRCA mutations is currently underway in the United States.46 Many centers offer salpingectomy to high-risk patients < 40 years old, understanding that ovary removal is an eventuality for these patients.
When oral contraceptive pills might be beneficial. In younger women with BRCA mutations, there may also be a role for oral contraceptive pills (OCPs) as a risk-reducing strategy. Meta-analyses have shown an approximately 50% reduction in the risk of ovarian cancer among women with BRCA mutations who use OCPs.47-49
ACOG advises that it is appropriate for women with BRCA mutations to use oral contraceptives if indicated (for pregnancy prevention or menstrual cycle regulation), and that it is reasonable to use them for cancer prevention.26 NCCN does not make a formal recommendation, although it does state OCPs may reduce the risk of ovarian cancer in women with a BRCA mutation.36 Case-control studies have produced conflicting data on the association between OCP use and breast cancer risk in BRCA mutation carriers,50-53 although 2 meta-analyses found no significant association in this population.47,48
Decision aids for women with BRCA mutations
Decision aids are visual displays of risk that help patients work through complex decisions. Most decision aids are in print or digital format and include information about the decision to be made as well as pictorial examples of possible outcomes. Pictographs are especially helpful in communicating information. Some decision aids for women with BRCA mutations can be complicated with multiple outcomes (ie, breast cancer and ovarian cancer) and multiple potential interventions (risk-reducing surgery, enhanced screening options).54
Continue to: A Cochrane review...
A Cochrane review found that decision aids increased patients’ knowledge, helped patients clarify their values, and may improve value-concordant decisions.55 Two papers describing the use of decision aids for women with BRCA mutations56,57 documented decreased decisional conflict and increased satisfaction.
CASE
Ms. T underwent the recommended mammogram and MRI screening for breast cancer, as well as testing with serum CA-125 and ultrasound examinations for ovarian cancer. Her initial mammogram and MRI revealed early stage, triple-negative right breast cancer. She chose to undergo bilateral mastectomy and reconstruction. She has now completed treatment and continues to work closely with her oncology team for appropriate breast follow-up.
One year after her initial diagnosis, at the age of 35, she returned to discuss fertility. She was recently married, and she and her husband wanted to start having children. She was concerned about a safe timeline for her to pursue pregnancy, saying she felt “like a ticking time-bomb” given her prior cancer and carrier status. She wanted to discuss the risks and benefits of pregnancy and when she should consider prophylactic oophorectomy. She had a few options. She could have a baby and then undergo an RRSO, or she could talk to her gynecologist about having a salpingectomy to reduce her risk now and use assisted reproductive technology to get pregnant. She could also freeze eggs or embryos, have an RRSO, and then use a surrogate to get pregnant. We informed her that pregnancy would not affect her risk of ovarian cancer and discussed the options for pre-implantation genetic testing to assure that her children would not carry the genetic mutation.58
We provided Ms. T and her husband with a decision aid to help them navigate the decision. They are currently evaluating the options and said they would let us know when they made a decision.
CORRESPONDENCE
Sarina Schrager, MD, MS, Northeast Family Medicine Center, 3209 Dryden Drive, Madison, WI, 53704; [email protected].
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953.
2. SEER Cancer Statistics Review, 1975-2016. Cancer of the female breast. [Table 4.1] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_04_breast.pdf. Accessed May 27, 2020.
3. SEER Cancer Statistics Review, 1975-2016. Cancer of the ovary. [Table 21.10] National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_21_ovary.pdf. Accessed May 22, 2020.
4. Torre LA, Trabert B, DeSantis C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284-296.
5. Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665-676.
6. Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807-2816.
7. Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814.
8. Evans DG, Susnerwala I, Dawson J, et al. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47:710-711.
9. CDC. Jewish women and BRCA gene mutations. www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/jewish_women_brca.htm. Accessed May 22, 2020.
10. Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39:593-620.
11. Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330.
12. Wang YA, Jian JW, Hung CF, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315.
13. Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine. 2016;95:e4975.
14. Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004;10:2473-2481.
15. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72-78.
16. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80-87.
17. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Int Med. 2012;27:1361-1367.
18. Ardern-Jones A, Eeles R. Developments in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic.” Hered Cancer Clin Pract. 2004;2:77-79.
19. Yerushalmi R, Rizel S, Zoref D, et al. A dedicated follow-up clinic for BRCA mutation carriers. Isr Med Assoc J. 2016;18:549-552.
20. Pichert G, Jacobs C, Jacobs I, et al. Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer. 2010;9:313-319.
21. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652-665.
22. Evans D, Eccles D, Rahman N, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41:474-480.
23. Bellcross CA, Lemke AA, Pape LS, et al. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783-789.
24. Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population based screening. Cancer. 2006;107:1769-1776.
25. Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299-308.
26. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017;130:e110-e126.
27. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110.
28. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2019. NCCN Clinical Practice Guidelines in Oncology. www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed May 22, 2020.
29. Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091-3099.
30. Foulkes WD, Goffin J, Brunet JS, et al. Tamoxifen may be an effective adjuvant treatment for BRCA1-related breast cancer irrespective of estrogen receptor status. J Natl Cancer Inst. 2002;94:1504-1506.
31. Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer. 2006;118:2281-2284.
32. Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surgery. 2016;212:660-669.
33. Bougie O, Weberpals JI. Clinical considerations of BRCA1- and BRCA2-mutation carriers: a review. Int J Surg Oncol. 2011;2011:374012.
34. Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411-1420.
35. Skates SJ, Greene MH, Buys SS, et al. Early detection of ovarian cancer using the Risk of Ovarian Cancer Algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin Cancer Res. 2017;23:3628-3637.
36. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9-20.
37. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:588-594.
38. Marchetti C, De Felice F, Palaia I, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health. 2014;14:150.
39. Nelson HD, Pappas M, Zakher B, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2014;160:255-266.
40. Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709-716.
41. Faubion SS, Kuhle CL, Shuster LT, et al. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483-491.
42. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909-927.
43. Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
44. Kwon JS, Tinker A, Pansegrau G, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121:14-24.
45. Holman LL, Friedman S, Daniels MS, et al. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133:283-286.
46. MD Anderson Cancer Center. Prophylactic salpingectomy with delayed oophorectomy, risk-reducing salpingo-oophorectomy, and ovarian cancer screening among BRCA mutation carriers: a proof-of-concept study. www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2013-0340.html. Accessed May 22, 2020.
47. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
48. Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188-4198.
49. Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju091.
50. Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863-1870.
51. Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170-3178.
52. Narod SA, Dubé MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773-1779.
53. Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350-356.
54. Culver JO, MacDonald DJ, Thornton AA, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. 2011;20:294-307.
55. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2017;4:CD001431.
56. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28:11-19.
57. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Gen Med. 2017;19:330-336.
58. Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;34:83-86.
PRACTICE RECOMMENDATIONS
› Recommend genetic screening for the BRCA mutation if a patient’s family history includes a breast cancer diagnosis before age 50, occurrences of both breast and ovarian cancers, or other suggestive features. C
› Advise women with the BRCA gene to return for a clinical breast exam every 6 to 12 months starting at age 25, and to start radiologic screening at age 30. C
› Consider recommending bilateral salpingo-oophorectomy to prevent ovarian cancer in women 35 to 40 years of age with a BRCA1 mutation who have completed childbearing. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Leveraging CAM to treat depression and anxiety
Almost 8% of Americans ages ≥ 12 years have depression and 19.1% of Americans ages ≥ 18 years have experienced an anxiety disorder in the past year.1,2 Furthermore, suicide, which can result from depression and anxiety, is the 10th leading cause of death in the United States, claiming about 40,000 to 49,000 lives per year since 2012, with increasing yearly rates.3 While multiple conventional medication and therapy treatments are available, patients remain interested in complementary and alternative medicine (CAM) options. According to the National Center for Complementary and Integrative Health, more than 30% of American adults use CAM treatments.4
This article provides an overview of the evidence for commonly used CAM treatments for unipolar depression and anxiety in adults. It is designed to serve as a useful resource when patients are interested in looking beyond conventional medications.
St. John’s wort: ‘Yes’ for depression; ‘no’ for anxiety
Hypericum perforatum, more commonly known as St. John’s wort, is a widely used antidepressant, especially in Europe where it is prescribed, rather than offered over the counter as it is here. Its mechanism of action is not completely understood because its various constituents have different neuropharmacologic activities.5,6
A 2008 Cochrane review evaluated 29 randomized, double-blind studies (N = 5489) that compared St. John’s wort with placebo or standard antidepressants in the treatment of depression.7 St. John’s wort was found to be superior to placebo and comparable to standard antidepressants. More recently, a 2017 meta-analysis of 27 studies (N = 3808) had similar findings.8 In patients with mild-to-moderate depression, St. John’s wart produced rates of remission that were comparable to those produced by selective serotonin reuptake inhibitors (SSRIs) but with a lower discontinuation rate.
Mood disorders other than depression. Studies do not support a role for St. John’s wort in the treatment of anxiety disorders. There are no trials that assess the efficacy of St. John’s wort for the reduction of symptoms of general anxiety disorder as a primary outcome of treatment. Some small clinical trials have investigated the efficacy of St. John’s wort in obsessive-compulsive disorder and social anxiety disorder. In those studies, St. John’s wort performed no better than placebo.9,10
A few words of caution. Preparations of St. John’s wort in the United States are not standardized, so St. John’s wort should be used in America with caution. Furthermore, long-term use of St. John’s wort for depression is questionable given that most studies have evaluated only up to 12 weeks of use.8
If used, studies indicate that the St. John’s wort extract that should be used is 0.3% hypericin or 5% hyperforin administered in a dosage of 300 to 400 mg tid.7,8,11 Physicians and patients can use www.consumerlabs.com to find St. John’s wort brands that have met specified quality criteria based on independent laboratory studies. This Web site can also be used to investigate the quality of the brands available for the other supplements discussed in this article.
Continue to: Adverse effects
Adverse effects. St. John’s wort and an SSRI can lead to serotonin syndrome, which is a constellation of symptoms involving mental status changes and autonomic and neuromuscular hyperactivity caused by serotonin overactivity.12 Furthermore, treatment failures with anticoagulants, digoxin, hormonal contraceptives, immunosuppressants, and narcotics due to concomitant use with St. John’s wort have been reported.13
The most common adverse reactions to St. John’s wort include gastrointestinal symptoms, dizziness, sedation, photosensitivity, dry mouth, urinary frequency, anorgasmia, and swelling.14 However, multiple studies have supported St. John’s wort to be equally or better tolerated than conventional antidepressants.7,8
Certain forms of folate can be adjunctive treatment for depression
Methylfolate is the form of folate that crosses the blood–brain barrier. A prospective observational study evaluated the cerebral spinal fluid of 33 patients with refractory depression. The authors found metabolic abnormalities in the cerebrospinal fluid of most of those patients, the most common of which was folate deficiency in 12 patients despite normal serum folate levels.15
Additionally, current understanding of the role of the Methylenetetrahydrofolate reductase (MTHFR) gene and the folate cycle in depression supports a potential role of methylfolate in depression treatment.16 The MTHFR gene encodes for an enzyme called MTHFR. The MTHFR enzyme converts 5,10-MTHF to 5-MTHF, which then crosses the blood–brain barrier and donates a methyl group for the conversion of homocysteine to methionine. Methionine is a precursor to monoamine neurotransmitters. Thus, decreased expression of the MTHFR gene leads to decreased methylfolate levels, which, in turn, potentially leads to insufficient neurotransmitter synthesis and homocysteine excess.
MTHFR gene polymorphisms and increased homocysteine levels have been found to be associated with the occurrence of depression. One thought is that methylfolate supplementation compensates for an underlying MTHFR enzyme deficiency in patients with depression. Further studies are needed to determine if screening depressed patients for MTHFR gene polymorphisms is of benefit.16
Continue to: A 2012 randomized controlled trial...
A 2012 randomized controlled trial (RCT) (N = 75) compared L-methylfolate 15 mg/d plus an SSRI with placebo plus an SSRI in patients with SSRI-resistant major depression.17 The trial found that a reduction of baseline symptoms by ≥ 50% occurred in more patients who received adjunctive L-methylfolate than placebo (32% vs 15%) and tolerability was comparable. These findings were again supported in 2016 with a 12-month study showing L-methylfolate to have long-term tolerability comparable to placebo,18 and in 2017 with a randomized trial (N = 260) that found escitalopram 10 mg/d plus L-methylfolate 15 mg/d to be significantly more effective at treating depression than escitalopram 10 mg/d alone.19 Thus, methylfolate may be an effective adjunctive treatment for depression at a dosage of 15 mg/d.
S-adenosyl methionine (SAMe) is a metabolite of folate derived from methionine that facilitates the synthesis of neurotransmitters including dopamine, norepinephrine, and serotonin. In Europe, as is the case with St. John’s wort, it is a prescription medication.
A randomized trial (N = 73) compared adjunctive SAMe 800 mg bid with placebo in the treatment of patients with unipolar major depression who did not experience improvement with SSRI treatment alone.20 The investigators found that more patients who received SAMe than who received placebo had improvement in their depression (36.1% vs 17.6%), and more patients who received SAMe compared to placebo went into depression remission (25.8% vs 11.7%).
Adverse effects were comparable in both groups. Thus, SAMe at a dosage of 400 to 1600 mg/d may be effective in the treatment of depression.20,21 The findings of 1 study (N = 65) suggest that patients could experience further improvement in their depression symptoms with SAMe at doses of as much as 3200 mg/d; however, 3200 mg/d increased the occurrence of gastrointestinal adverse effects (31.3% in the SAMe arm vs 3.8% in the placebo group).21
Folate. With regard to folate itself, randomized trials have not supported its efficacy in the treatment of depression in the general population.22
Continue to: VItamin D may improve anxiety/depression in those with low levels
Vitamin D may improve anxiety/depression in those with low levels
Vitamin D supplementation is also being used more frequently in the treatment of depression. Case-control, cross-sectional, and cohort studies have linked low vitamin D levels to the occurrence of depression. A 2013 systematic review of 14 such studies (N = 31,424) found lower vitamin D levels in people with depression compared with controls.23 Further studies are needed to determine if this relationship is causal, and quality RCTs investigating the effect of vitamin D supplementation on depression are lacking.
A 2019 study (N = 30) evaluated the impact of vitamin D supplementation on generalized anxiety disorder in patients with co-occurring vitamin D deficiency. Half received standard-of-care general anxiety disorder treatment plus 50,000 IU of vitamin D weekly for 3 months, while the other half received standard of care alone. Significant improvements in anxiety scores, increases in serum serotonin, and decreases in serum neopterin (an inflammatory marker) were observed in the vitamin D–treated group compared to the group that did not receive vitamin D.24
It is not currently standard of care to check vitamin D levels in all patients presenting with mood disorders. However, if screening is indicated for another reason and low levels are confirmed, vitamin D replacement may improve anxiety and/or depressive symptoms. Despite this, no evidence exists to support vitamin D supplementation for depression or anxiety in patients with normal vitamin D levels.24,25
The effects of omega-3 fatty acids are largely unclear
Research has shown that omega-3 polyunsaturated fatty acids (n-3 PUFA), which are found in fish oil, protect glutamatergic neurotransmission from glucocorticoids, which are released in the body during a stress response.26 Small clinical trials have found n-3 PUFAs to reduce the symptoms of anxiety compared with placebo, but the acids have not been studied directly for anxiety disorders.27,28
With regard to depression, evidence is conflicting as to whether n-3 PUFAs are of any benefit in treatment.29 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are hypothesized to be the components in omega-3 fatty acid preparations that could lead to a reduction in depressive symptoms. However, randomized trials have shown that EPA-predominant omega-3 fatty acid formulations have only a moderate or no clinically significant effect on depression over placebo, and that DHA-predominant omega-3 fatty acid formulations are only comparable or inferior to placebo.30
Continue to: Response to EPA...
Response to EPA may be greater in patients with depression who have high levels of inflammatory biomarkers, such as interleuken (IL)-1 receptor antagonist (1Ra), IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin, and adiponectin, than in patients with low levels. An 8-week trial randomly assigned patients with unipolar major depression (N = 155) to receive either EPA, DHA, or placebo and found that improvement for the 3 groups was comparable; however, in the subgroup of patients with high levels of inflammatory markers, improvement in depressive symptoms was significantly greater with EPA than with either DHA or placebo.31
It is unclear whether different sources of n-3 PUFA, such as whole fish vs fish oil vs prescription omega-3 acid ethyl esters (Lovaza), are more or less efficacious in the treatment of anxiety or depression. Furthermore, there is no standard dosing for n-3 PUFA in the treatment of mood disorders. Given that the US Food and Drug Administration recommends no more than 2 g/d of combined EPA and DHA supplementation, we recommend using 2 g/d if one decides to treat depression/anxiety with n-3 PUFA.32
N-3 PUFA supplementation is fairly benign. There have been previous concerns about n-3 PUFA supplementation increasing patients’ risk for gastrointestinal bleeding, but a 2006 systematic review that included 9 trials (N = 2612) that looked at clinically significant bleeding episodes found that even patients at high risk for bleeding (ie, those taking aspirin or warfarin) had no increased bleeding risk from taking n-3 PUFA supplementation at up to 4 g/d.33
Don’t underestimate exercise and meditation; consider acupuncture
Exercise. Multiple practice guidelines, including the American Psychiatric Association’s “Practice Guideline for the Treatment of Patients with Major Depressive Disorder,” and meta-analyses have supported the use of exercise to treat unipolar major depression and anxiety.34-37 However, only about 26% of American men and 19% of American women met the US Department of Health and Human Services’ “Federal Physical Activity Guidelines for Americans” in 2016.38
Exercise alone is a reasonable monotherapy, as long as patients are monitored closely for worsening symptoms. Additionally, exercise as an add-on treatment can be helpful for more severe depression or anxiety.39 The best type, duration, and frequency of exercise specifically for the treatment of depression or anxiety has yet to be determined, but physicians may base their exercise recommendations on the “Federal Physical Activity Guidelines for Americans” for general good health (TABLE).38
Continue to: Meditation
Meditation, especially mindfulness meditation, is another strategy that has gained popularity in the treatment of anxiety and depression. Mindfulness has been defined as “the practice of maintaining a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis.”40 A 2014 systematic review and meta-analysis of 47 trials with 3515 participants found that mindfulness meditation programs led to clinically significant moderate reductions in anxiety.41 Smaller effects were found for depression.
Nevertheless, meditation may be beneficial as an adjunctive treatment for depression. A small randomized trial (N = 25) compared an adjunctive breathing-based meditation intervention with a waitlist control (delayed yoga) in patients with unipolar major depression who failed to respond to at least 8 weeks of antidepressant treatment.42 The meditation intervention consisted of a group program with sitting meditation, breathing exercises, and yoga postures. Participants engaged in the meditation intervention for 2 to 3.5 hours per day for 8 weeks and demonstrated significant improvement in depression symptoms compared with the control group.42
Acupuncture. A 2018 meta-analysis of 64 studies (N = 7104) suggests that acupuncture results in a small-to-moderate reduction in depressive symptoms when compared to no treatment, control/sham acupuncture, or medication.43 Furthermore, acupuncture plus medication compared to medication alone results in a higher reduction in depressive symptoms without an increase in adverse events.43
Additionally, a 2019 analysis of 10 systematic reviews found acupuncture to be more effective than control/sham acupuncture in the treatment of general anxiety.44 It should be noted, however, that a lot of heterogeneity and potential for bias existed across all of the studies. The studies analyzed were very low to low in quality. Thus, the evidence is insufficient to strongly recommend the use of acupuncture for depression or anxiety, although acupuncture is a safe intervention with low rates of adverse events.
Emotional support animals: Beneficial, but evidence is weak
Emotional support animals are gaining in popularity with Americans who have mood disorders. An important distinction must be made, however, between service animals and emotional support animals. A service animal is one “that is individually trained to do work or perform tasks for the benefit of an individual with a disability, including a physical, sensory, psychiatric, intellectual, or other mental disability.”45 Under the Americans with Disabilities Act (ADA), service animals are limited to dogs, and, in some cases, specially trained miniature horses. Psychiatric service dogs can be trained to do anything from reminding their owner to take medicine to stopping self-mutilation activities.
Continue to: Emotional support animals...
Emotional support animals are not specially trained to perform tasks to help with disabilities. It’s their companionship that helps relieve symptoms of depression and/or anxiety.45 Thus, emotional support animals are not covered under federal laws that apply to service animals. However, the Air Carrier Access Act does require airlines to allow emotional support animals to fly in the cabin for free. Furthermore, the Fair Housing Act allows emotional support animals to circumvent no-pet rules in housing and dorms. Airplanes and housing are the only places legally required to allow the unrestricted presence of emotional support animals.46
Also, there are important distinctions between emotional support animals and pets. While anyone can own a pet, an emotional support animal is prescribed by a licensed mental health professional as a treatment for a mood disorder. Housing facilities and airlines will usually require an emotional support animal “prescription” or letter from a physician to recognize animals as such.
A 2018 systematic review evaluated the evidence behind emotional support animals, which included 17 peer-reviewed journal articles, conference papers, and research dissertations (N = 1727) mostly containing qualitative evidence.47 Unfortunately, there are no RCTs, and there are limited case-control and cohort studies evaluating the effect of an emotional support animal on mood disorders. Based on the available evidence, there does seem to be a psychological benefit to owning an animal for both those with a diagnosable mental health disorder and the general population. This benefit seems to stem from a perceived reduction in social isolation and an increase in emotional support. Factors that determine the psychological benefit of emotional support animals include the type of pet, the number of pets, the attachment to the pet, and the perceived friendliness of the pet.47
Animal-assisted therapy. A 2014 systematic review evaluated higher-level evidence behind animal-assisted therapy (AAT).48 Although participating in therapy that involves interaction with animals is not the same as owning an emotional support animal, the concept—using an animal to improve mental health—is the same. The systematic review looked at 11 RCTs (N = 411) that studied the effect of AAT on mental health. Animals studied included dogs, cats, dolphins, birds, cows, rabbits, ferrets, and guinea pigs. Mental health disorders studied included schizophrenia, depression, anxiety, alcohol/drug abuse, and other addictive behaviors.
Therapeutic animal exposure led to reported improvements in mood, quality of life, and social behavior. These improvements were attributed to the animals buffering people’s reactions to mental stressors. The animals provided a sense of comfort and safety and diverted attention away from immediate stressors. Furthermore, the memory of the animals brought participants a sense of comfort/happiness when they were later without the animal. However, the majority of participants were people who liked animals at baseline.48
CORRESPONDENCE
Amanda E. Olagunju, DO, Operational Medicine Clinic, Langley AFB Hospital, 77 Nealy Avenue, Langley AFB, VA 23665; [email protected].
1. National Center for Health Statistics, Centers for Disease Control and Prevention. FastStats: Depression. Last reviewed October 7, 2015. www.cdc.gov/nchs/fastats/depression.htm. Accessed May 26, 2020.
2. National Institute of Mental Health. Mental health information—statistics: any anxiety disorder. Last updated November 2017. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml. Accessed May 26, 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief, no 362. Hyattsville, MD: National Center for Health Statistics; 2020.
4. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? Last updated July 2018. www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed May 26, 2020.
5. Bennett DA Jr, Phun L, Polk JF, et al. Neuropharmacology of St. John’s wort (Hypericum). Ann Pharmacother. 1998;32:1201-1208.
6. Müller WE, Singer A, Wonnemann M, et al. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(suppl 1):16-21.
7. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448.
8.
9. Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20:299-304.
10. Kobak KA, Taylor LV, Warner G, et al. St. John’s wort versus placebo in social phobia: results from a placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25:51-58.
11. Product reviews: St. John’s wort supplements review. ConsumerLab.com. September 23, 2016. www.consumerlab.com/reviews/St_Johns_Wort/stjohnswort/. Accessed May 26, 2020.
12. Simhan S. Serotonin syndrome. In: Abd-Elsayed A. (ed) Pain: A Review Guide. New York, NY: Springer; 2019.
13. Chrubasik-Hausmann S, Vlachojannis J, McLachlan A. Understanding drug interactions with St. John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71:129-138.
14. Knüppel L, Linde K. Adverse effects of St. John’s wort: a systematic review. J Clin Psychiatry. 2004;65:1470-1479.
15. Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174:42-50.
16. Kandler C, Lam S. Methylenetetrahydrofolate reductase screening in treatment-resistant depression. Fed Pract. 2019;36:207-208.
17. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267-1274.
18. Zajecka J, Fava M, Shelton R, et al. Long-term efficacy, safety, and tolerability of L-methylfolate calcium 15 mg as adjunctive therapy with selective serotonin reuptake inhibitors: a 12-month, open-label study following a placebo-controlled acute study. J Clin Psychiatry. 2016;77:654-660.
19. Kakar MS, Jehangir S, Mustafa M, et al. Therapeutic efficacy of combination therapy of L-methylfolate and escitalopram in depression. Pakistan Armed Forces Med J. 2017;67:976-981.
20. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942-948.
21.
22. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
23. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
24. Eid A, Khoja S, AlGhamdi S, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. 2019;34:1781-1786.
25. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767.
26. Hennebelle M, Champeil-Potokar G, Lavialle M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99-112.
27. Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725-1734.
28. Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568-575.
29. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012;17:1161-1163.
30. Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192-201.
31. Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71-79.
32. National Institutes of Health Office of Dietary Supplements. Omega-3 fatty acids. Updated October 17, 2019. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional. Accessed May 26, 2020.
33. Wang C, Harris WS, Chung M, et al. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linoleic acid, benefit cardiovascular disease outcomes in primary- and secondary- prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5-17.
34. American Psychiatric Association Practice. Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed May 26, 2020.
35. Gordon B, McDowell C, Lyons M, et al. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521-2532.
36. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102-108.
37. Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19:204-212.
38. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
39. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;CD004366.
40. Merriam-Webster Dictionary. "Mindfulness." www.merriam-webster.com/dictionary/mindfulness. Accessed May 26, 2020.
41. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368.
42. Sharma A, Barrett MS, Cucchiara AJ, et al. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63.
43. Smith CA, Armour M, Soo Lee M, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;CD004046.
44. Li M
45. Brennan J. Service animals and emotional support animals. ADA National Network Information Guidance and Training on the Americans with Disabilities Act. Last updated April 2020. adata.org/publication/service-animals-booklet. Accessed May 26, 2020.
46. Clay RA. Is that a pet or therapeutic aid? Monitor on Psychology. 2016;47:38.
47. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18:31.
48. Kamioka H, Okada S, Tsutani K, et al. Effectiveness of animal-assisted therapy: a systematic review of randomized controlled trials. Complement Ther Med. 2014;22:371-390.
Almost 8% of Americans ages ≥ 12 years have depression and 19.1% of Americans ages ≥ 18 years have experienced an anxiety disorder in the past year.1,2 Furthermore, suicide, which can result from depression and anxiety, is the 10th leading cause of death in the United States, claiming about 40,000 to 49,000 lives per year since 2012, with increasing yearly rates.3 While multiple conventional medication and therapy treatments are available, patients remain interested in complementary and alternative medicine (CAM) options. According to the National Center for Complementary and Integrative Health, more than 30% of American adults use CAM treatments.4
This article provides an overview of the evidence for commonly used CAM treatments for unipolar depression and anxiety in adults. It is designed to serve as a useful resource when patients are interested in looking beyond conventional medications.
St. John’s wort: ‘Yes’ for depression; ‘no’ for anxiety
Hypericum perforatum, more commonly known as St. John’s wort, is a widely used antidepressant, especially in Europe where it is prescribed, rather than offered over the counter as it is here. Its mechanism of action is not completely understood because its various constituents have different neuropharmacologic activities.5,6
A 2008 Cochrane review evaluated 29 randomized, double-blind studies (N = 5489) that compared St. John’s wort with placebo or standard antidepressants in the treatment of depression.7 St. John’s wort was found to be superior to placebo and comparable to standard antidepressants. More recently, a 2017 meta-analysis of 27 studies (N = 3808) had similar findings.8 In patients with mild-to-moderate depression, St. John’s wart produced rates of remission that were comparable to those produced by selective serotonin reuptake inhibitors (SSRIs) but with a lower discontinuation rate.
Mood disorders other than depression. Studies do not support a role for St. John’s wort in the treatment of anxiety disorders. There are no trials that assess the efficacy of St. John’s wort for the reduction of symptoms of general anxiety disorder as a primary outcome of treatment. Some small clinical trials have investigated the efficacy of St. John’s wort in obsessive-compulsive disorder and social anxiety disorder. In those studies, St. John’s wort performed no better than placebo.9,10
A few words of caution. Preparations of St. John’s wort in the United States are not standardized, so St. John’s wort should be used in America with caution. Furthermore, long-term use of St. John’s wort for depression is questionable given that most studies have evaluated only up to 12 weeks of use.8
If used, studies indicate that the St. John’s wort extract that should be used is 0.3% hypericin or 5% hyperforin administered in a dosage of 300 to 400 mg tid.7,8,11 Physicians and patients can use www.consumerlabs.com to find St. John’s wort brands that have met specified quality criteria based on independent laboratory studies. This Web site can also be used to investigate the quality of the brands available for the other supplements discussed in this article.
Continue to: Adverse effects
Adverse effects. St. John’s wort and an SSRI can lead to serotonin syndrome, which is a constellation of symptoms involving mental status changes and autonomic and neuromuscular hyperactivity caused by serotonin overactivity.12 Furthermore, treatment failures with anticoagulants, digoxin, hormonal contraceptives, immunosuppressants, and narcotics due to concomitant use with St. John’s wort have been reported.13
The most common adverse reactions to St. John’s wort include gastrointestinal symptoms, dizziness, sedation, photosensitivity, dry mouth, urinary frequency, anorgasmia, and swelling.14 However, multiple studies have supported St. John’s wort to be equally or better tolerated than conventional antidepressants.7,8
Certain forms of folate can be adjunctive treatment for depression
Methylfolate is the form of folate that crosses the blood–brain barrier. A prospective observational study evaluated the cerebral spinal fluid of 33 patients with refractory depression. The authors found metabolic abnormalities in the cerebrospinal fluid of most of those patients, the most common of which was folate deficiency in 12 patients despite normal serum folate levels.15
Additionally, current understanding of the role of the Methylenetetrahydrofolate reductase (MTHFR) gene and the folate cycle in depression supports a potential role of methylfolate in depression treatment.16 The MTHFR gene encodes for an enzyme called MTHFR. The MTHFR enzyme converts 5,10-MTHF to 5-MTHF, which then crosses the blood–brain barrier and donates a methyl group for the conversion of homocysteine to methionine. Methionine is a precursor to monoamine neurotransmitters. Thus, decreased expression of the MTHFR gene leads to decreased methylfolate levels, which, in turn, potentially leads to insufficient neurotransmitter synthesis and homocysteine excess.
MTHFR gene polymorphisms and increased homocysteine levels have been found to be associated with the occurrence of depression. One thought is that methylfolate supplementation compensates for an underlying MTHFR enzyme deficiency in patients with depression. Further studies are needed to determine if screening depressed patients for MTHFR gene polymorphisms is of benefit.16
Continue to: A 2012 randomized controlled trial...
A 2012 randomized controlled trial (RCT) (N = 75) compared L-methylfolate 15 mg/d plus an SSRI with placebo plus an SSRI in patients with SSRI-resistant major depression.17 The trial found that a reduction of baseline symptoms by ≥ 50% occurred in more patients who received adjunctive L-methylfolate than placebo (32% vs 15%) and tolerability was comparable. These findings were again supported in 2016 with a 12-month study showing L-methylfolate to have long-term tolerability comparable to placebo,18 and in 2017 with a randomized trial (N = 260) that found escitalopram 10 mg/d plus L-methylfolate 15 mg/d to be significantly more effective at treating depression than escitalopram 10 mg/d alone.19 Thus, methylfolate may be an effective adjunctive treatment for depression at a dosage of 15 mg/d.
S-adenosyl methionine (SAMe) is a metabolite of folate derived from methionine that facilitates the synthesis of neurotransmitters including dopamine, norepinephrine, and serotonin. In Europe, as is the case with St. John’s wort, it is a prescription medication.
A randomized trial (N = 73) compared adjunctive SAMe 800 mg bid with placebo in the treatment of patients with unipolar major depression who did not experience improvement with SSRI treatment alone.20 The investigators found that more patients who received SAMe than who received placebo had improvement in their depression (36.1% vs 17.6%), and more patients who received SAMe compared to placebo went into depression remission (25.8% vs 11.7%).
Adverse effects were comparable in both groups. Thus, SAMe at a dosage of 400 to 1600 mg/d may be effective in the treatment of depression.20,21 The findings of 1 study (N = 65) suggest that patients could experience further improvement in their depression symptoms with SAMe at doses of as much as 3200 mg/d; however, 3200 mg/d increased the occurrence of gastrointestinal adverse effects (31.3% in the SAMe arm vs 3.8% in the placebo group).21
Folate. With regard to folate itself, randomized trials have not supported its efficacy in the treatment of depression in the general population.22
Continue to: VItamin D may improve anxiety/depression in those with low levels
Vitamin D may improve anxiety/depression in those with low levels
Vitamin D supplementation is also being used more frequently in the treatment of depression. Case-control, cross-sectional, and cohort studies have linked low vitamin D levels to the occurrence of depression. A 2013 systematic review of 14 such studies (N = 31,424) found lower vitamin D levels in people with depression compared with controls.23 Further studies are needed to determine if this relationship is causal, and quality RCTs investigating the effect of vitamin D supplementation on depression are lacking.
A 2019 study (N = 30) evaluated the impact of vitamin D supplementation on generalized anxiety disorder in patients with co-occurring vitamin D deficiency. Half received standard-of-care general anxiety disorder treatment plus 50,000 IU of vitamin D weekly for 3 months, while the other half received standard of care alone. Significant improvements in anxiety scores, increases in serum serotonin, and decreases in serum neopterin (an inflammatory marker) were observed in the vitamin D–treated group compared to the group that did not receive vitamin D.24
It is not currently standard of care to check vitamin D levels in all patients presenting with mood disorders. However, if screening is indicated for another reason and low levels are confirmed, vitamin D replacement may improve anxiety and/or depressive symptoms. Despite this, no evidence exists to support vitamin D supplementation for depression or anxiety in patients with normal vitamin D levels.24,25
The effects of omega-3 fatty acids are largely unclear
Research has shown that omega-3 polyunsaturated fatty acids (n-3 PUFA), which are found in fish oil, protect glutamatergic neurotransmission from glucocorticoids, which are released in the body during a stress response.26 Small clinical trials have found n-3 PUFAs to reduce the symptoms of anxiety compared with placebo, but the acids have not been studied directly for anxiety disorders.27,28
With regard to depression, evidence is conflicting as to whether n-3 PUFAs are of any benefit in treatment.29 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are hypothesized to be the components in omega-3 fatty acid preparations that could lead to a reduction in depressive symptoms. However, randomized trials have shown that EPA-predominant omega-3 fatty acid formulations have only a moderate or no clinically significant effect on depression over placebo, and that DHA-predominant omega-3 fatty acid formulations are only comparable or inferior to placebo.30
Continue to: Response to EPA...
Response to EPA may be greater in patients with depression who have high levels of inflammatory biomarkers, such as interleuken (IL)-1 receptor antagonist (1Ra), IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin, and adiponectin, than in patients with low levels. An 8-week trial randomly assigned patients with unipolar major depression (N = 155) to receive either EPA, DHA, or placebo and found that improvement for the 3 groups was comparable; however, in the subgroup of patients with high levels of inflammatory markers, improvement in depressive symptoms was significantly greater with EPA than with either DHA or placebo.31
It is unclear whether different sources of n-3 PUFA, such as whole fish vs fish oil vs prescription omega-3 acid ethyl esters (Lovaza), are more or less efficacious in the treatment of anxiety or depression. Furthermore, there is no standard dosing for n-3 PUFA in the treatment of mood disorders. Given that the US Food and Drug Administration recommends no more than 2 g/d of combined EPA and DHA supplementation, we recommend using 2 g/d if one decides to treat depression/anxiety with n-3 PUFA.32
N-3 PUFA supplementation is fairly benign. There have been previous concerns about n-3 PUFA supplementation increasing patients’ risk for gastrointestinal bleeding, but a 2006 systematic review that included 9 trials (N = 2612) that looked at clinically significant bleeding episodes found that even patients at high risk for bleeding (ie, those taking aspirin or warfarin) had no increased bleeding risk from taking n-3 PUFA supplementation at up to 4 g/d.33
Don’t underestimate exercise and meditation; consider acupuncture
Exercise. Multiple practice guidelines, including the American Psychiatric Association’s “Practice Guideline for the Treatment of Patients with Major Depressive Disorder,” and meta-analyses have supported the use of exercise to treat unipolar major depression and anxiety.34-37 However, only about 26% of American men and 19% of American women met the US Department of Health and Human Services’ “Federal Physical Activity Guidelines for Americans” in 2016.38
Exercise alone is a reasonable monotherapy, as long as patients are monitored closely for worsening symptoms. Additionally, exercise as an add-on treatment can be helpful for more severe depression or anxiety.39 The best type, duration, and frequency of exercise specifically for the treatment of depression or anxiety has yet to be determined, but physicians may base their exercise recommendations on the “Federal Physical Activity Guidelines for Americans” for general good health (TABLE).38
Continue to: Meditation
Meditation, especially mindfulness meditation, is another strategy that has gained popularity in the treatment of anxiety and depression. Mindfulness has been defined as “the practice of maintaining a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis.”40 A 2014 systematic review and meta-analysis of 47 trials with 3515 participants found that mindfulness meditation programs led to clinically significant moderate reductions in anxiety.41 Smaller effects were found for depression.
Nevertheless, meditation may be beneficial as an adjunctive treatment for depression. A small randomized trial (N = 25) compared an adjunctive breathing-based meditation intervention with a waitlist control (delayed yoga) in patients with unipolar major depression who failed to respond to at least 8 weeks of antidepressant treatment.42 The meditation intervention consisted of a group program with sitting meditation, breathing exercises, and yoga postures. Participants engaged in the meditation intervention for 2 to 3.5 hours per day for 8 weeks and demonstrated significant improvement in depression symptoms compared with the control group.42
Acupuncture. A 2018 meta-analysis of 64 studies (N = 7104) suggests that acupuncture results in a small-to-moderate reduction in depressive symptoms when compared to no treatment, control/sham acupuncture, or medication.43 Furthermore, acupuncture plus medication compared to medication alone results in a higher reduction in depressive symptoms without an increase in adverse events.43
Additionally, a 2019 analysis of 10 systematic reviews found acupuncture to be more effective than control/sham acupuncture in the treatment of general anxiety.44 It should be noted, however, that a lot of heterogeneity and potential for bias existed across all of the studies. The studies analyzed were very low to low in quality. Thus, the evidence is insufficient to strongly recommend the use of acupuncture for depression or anxiety, although acupuncture is a safe intervention with low rates of adverse events.
Emotional support animals: Beneficial, but evidence is weak
Emotional support animals are gaining in popularity with Americans who have mood disorders. An important distinction must be made, however, between service animals and emotional support animals. A service animal is one “that is individually trained to do work or perform tasks for the benefit of an individual with a disability, including a physical, sensory, psychiatric, intellectual, or other mental disability.”45 Under the Americans with Disabilities Act (ADA), service animals are limited to dogs, and, in some cases, specially trained miniature horses. Psychiatric service dogs can be trained to do anything from reminding their owner to take medicine to stopping self-mutilation activities.
Continue to: Emotional support animals...
Emotional support animals are not specially trained to perform tasks to help with disabilities. It’s their companionship that helps relieve symptoms of depression and/or anxiety.45 Thus, emotional support animals are not covered under federal laws that apply to service animals. However, the Air Carrier Access Act does require airlines to allow emotional support animals to fly in the cabin for free. Furthermore, the Fair Housing Act allows emotional support animals to circumvent no-pet rules in housing and dorms. Airplanes and housing are the only places legally required to allow the unrestricted presence of emotional support animals.46
Also, there are important distinctions between emotional support animals and pets. While anyone can own a pet, an emotional support animal is prescribed by a licensed mental health professional as a treatment for a mood disorder. Housing facilities and airlines will usually require an emotional support animal “prescription” or letter from a physician to recognize animals as such.
A 2018 systematic review evaluated the evidence behind emotional support animals, which included 17 peer-reviewed journal articles, conference papers, and research dissertations (N = 1727) mostly containing qualitative evidence.47 Unfortunately, there are no RCTs, and there are limited case-control and cohort studies evaluating the effect of an emotional support animal on mood disorders. Based on the available evidence, there does seem to be a psychological benefit to owning an animal for both those with a diagnosable mental health disorder and the general population. This benefit seems to stem from a perceived reduction in social isolation and an increase in emotional support. Factors that determine the psychological benefit of emotional support animals include the type of pet, the number of pets, the attachment to the pet, and the perceived friendliness of the pet.47
Animal-assisted therapy. A 2014 systematic review evaluated higher-level evidence behind animal-assisted therapy (AAT).48 Although participating in therapy that involves interaction with animals is not the same as owning an emotional support animal, the concept—using an animal to improve mental health—is the same. The systematic review looked at 11 RCTs (N = 411) that studied the effect of AAT on mental health. Animals studied included dogs, cats, dolphins, birds, cows, rabbits, ferrets, and guinea pigs. Mental health disorders studied included schizophrenia, depression, anxiety, alcohol/drug abuse, and other addictive behaviors.
Therapeutic animal exposure led to reported improvements in mood, quality of life, and social behavior. These improvements were attributed to the animals buffering people’s reactions to mental stressors. The animals provided a sense of comfort and safety and diverted attention away from immediate stressors. Furthermore, the memory of the animals brought participants a sense of comfort/happiness when they were later without the animal. However, the majority of participants were people who liked animals at baseline.48
CORRESPONDENCE
Amanda E. Olagunju, DO, Operational Medicine Clinic, Langley AFB Hospital, 77 Nealy Avenue, Langley AFB, VA 23665; [email protected].
Almost 8% of Americans ages ≥ 12 years have depression and 19.1% of Americans ages ≥ 18 years have experienced an anxiety disorder in the past year.1,2 Furthermore, suicide, which can result from depression and anxiety, is the 10th leading cause of death in the United States, claiming about 40,000 to 49,000 lives per year since 2012, with increasing yearly rates.3 While multiple conventional medication and therapy treatments are available, patients remain interested in complementary and alternative medicine (CAM) options. According to the National Center for Complementary and Integrative Health, more than 30% of American adults use CAM treatments.4
This article provides an overview of the evidence for commonly used CAM treatments for unipolar depression and anxiety in adults. It is designed to serve as a useful resource when patients are interested in looking beyond conventional medications.
St. John’s wort: ‘Yes’ for depression; ‘no’ for anxiety
Hypericum perforatum, more commonly known as St. John’s wort, is a widely used antidepressant, especially in Europe where it is prescribed, rather than offered over the counter as it is here. Its mechanism of action is not completely understood because its various constituents have different neuropharmacologic activities.5,6
A 2008 Cochrane review evaluated 29 randomized, double-blind studies (N = 5489) that compared St. John’s wort with placebo or standard antidepressants in the treatment of depression.7 St. John’s wort was found to be superior to placebo and comparable to standard antidepressants. More recently, a 2017 meta-analysis of 27 studies (N = 3808) had similar findings.8 In patients with mild-to-moderate depression, St. John’s wart produced rates of remission that were comparable to those produced by selective serotonin reuptake inhibitors (SSRIs) but with a lower discontinuation rate.
Mood disorders other than depression. Studies do not support a role for St. John’s wort in the treatment of anxiety disorders. There are no trials that assess the efficacy of St. John’s wort for the reduction of symptoms of general anxiety disorder as a primary outcome of treatment. Some small clinical trials have investigated the efficacy of St. John’s wort in obsessive-compulsive disorder and social anxiety disorder. In those studies, St. John’s wort performed no better than placebo.9,10
A few words of caution. Preparations of St. John’s wort in the United States are not standardized, so St. John’s wort should be used in America with caution. Furthermore, long-term use of St. John’s wort for depression is questionable given that most studies have evaluated only up to 12 weeks of use.8
If used, studies indicate that the St. John’s wort extract that should be used is 0.3% hypericin or 5% hyperforin administered in a dosage of 300 to 400 mg tid.7,8,11 Physicians and patients can use www.consumerlabs.com to find St. John’s wort brands that have met specified quality criteria based on independent laboratory studies. This Web site can also be used to investigate the quality of the brands available for the other supplements discussed in this article.
Continue to: Adverse effects
Adverse effects. St. John’s wort and an SSRI can lead to serotonin syndrome, which is a constellation of symptoms involving mental status changes and autonomic and neuromuscular hyperactivity caused by serotonin overactivity.12 Furthermore, treatment failures with anticoagulants, digoxin, hormonal contraceptives, immunosuppressants, and narcotics due to concomitant use with St. John’s wort have been reported.13
The most common adverse reactions to St. John’s wort include gastrointestinal symptoms, dizziness, sedation, photosensitivity, dry mouth, urinary frequency, anorgasmia, and swelling.14 However, multiple studies have supported St. John’s wort to be equally or better tolerated than conventional antidepressants.7,8
Certain forms of folate can be adjunctive treatment for depression
Methylfolate is the form of folate that crosses the blood–brain barrier. A prospective observational study evaluated the cerebral spinal fluid of 33 patients with refractory depression. The authors found metabolic abnormalities in the cerebrospinal fluid of most of those patients, the most common of which was folate deficiency in 12 patients despite normal serum folate levels.15
Additionally, current understanding of the role of the Methylenetetrahydrofolate reductase (MTHFR) gene and the folate cycle in depression supports a potential role of methylfolate in depression treatment.16 The MTHFR gene encodes for an enzyme called MTHFR. The MTHFR enzyme converts 5,10-MTHF to 5-MTHF, which then crosses the blood–brain barrier and donates a methyl group for the conversion of homocysteine to methionine. Methionine is a precursor to monoamine neurotransmitters. Thus, decreased expression of the MTHFR gene leads to decreased methylfolate levels, which, in turn, potentially leads to insufficient neurotransmitter synthesis and homocysteine excess.
MTHFR gene polymorphisms and increased homocysteine levels have been found to be associated with the occurrence of depression. One thought is that methylfolate supplementation compensates for an underlying MTHFR enzyme deficiency in patients with depression. Further studies are needed to determine if screening depressed patients for MTHFR gene polymorphisms is of benefit.16
Continue to: A 2012 randomized controlled trial...
A 2012 randomized controlled trial (RCT) (N = 75) compared L-methylfolate 15 mg/d plus an SSRI with placebo plus an SSRI in patients with SSRI-resistant major depression.17 The trial found that a reduction of baseline symptoms by ≥ 50% occurred in more patients who received adjunctive L-methylfolate than placebo (32% vs 15%) and tolerability was comparable. These findings were again supported in 2016 with a 12-month study showing L-methylfolate to have long-term tolerability comparable to placebo,18 and in 2017 with a randomized trial (N = 260) that found escitalopram 10 mg/d plus L-methylfolate 15 mg/d to be significantly more effective at treating depression than escitalopram 10 mg/d alone.19 Thus, methylfolate may be an effective adjunctive treatment for depression at a dosage of 15 mg/d.
S-adenosyl methionine (SAMe) is a metabolite of folate derived from methionine that facilitates the synthesis of neurotransmitters including dopamine, norepinephrine, and serotonin. In Europe, as is the case with St. John’s wort, it is a prescription medication.
A randomized trial (N = 73) compared adjunctive SAMe 800 mg bid with placebo in the treatment of patients with unipolar major depression who did not experience improvement with SSRI treatment alone.20 The investigators found that more patients who received SAMe than who received placebo had improvement in their depression (36.1% vs 17.6%), and more patients who received SAMe compared to placebo went into depression remission (25.8% vs 11.7%).
Adverse effects were comparable in both groups. Thus, SAMe at a dosage of 400 to 1600 mg/d may be effective in the treatment of depression.20,21 The findings of 1 study (N = 65) suggest that patients could experience further improvement in their depression symptoms with SAMe at doses of as much as 3200 mg/d; however, 3200 mg/d increased the occurrence of gastrointestinal adverse effects (31.3% in the SAMe arm vs 3.8% in the placebo group).21
Folate. With regard to folate itself, randomized trials have not supported its efficacy in the treatment of depression in the general population.22
Continue to: VItamin D may improve anxiety/depression in those with low levels
Vitamin D may improve anxiety/depression in those with low levels
Vitamin D supplementation is also being used more frequently in the treatment of depression. Case-control, cross-sectional, and cohort studies have linked low vitamin D levels to the occurrence of depression. A 2013 systematic review of 14 such studies (N = 31,424) found lower vitamin D levels in people with depression compared with controls.23 Further studies are needed to determine if this relationship is causal, and quality RCTs investigating the effect of vitamin D supplementation on depression are lacking.
A 2019 study (N = 30) evaluated the impact of vitamin D supplementation on generalized anxiety disorder in patients with co-occurring vitamin D deficiency. Half received standard-of-care general anxiety disorder treatment plus 50,000 IU of vitamin D weekly for 3 months, while the other half received standard of care alone. Significant improvements in anxiety scores, increases in serum serotonin, and decreases in serum neopterin (an inflammatory marker) were observed in the vitamin D–treated group compared to the group that did not receive vitamin D.24
It is not currently standard of care to check vitamin D levels in all patients presenting with mood disorders. However, if screening is indicated for another reason and low levels are confirmed, vitamin D replacement may improve anxiety and/or depressive symptoms. Despite this, no evidence exists to support vitamin D supplementation for depression or anxiety in patients with normal vitamin D levels.24,25
The effects of omega-3 fatty acids are largely unclear
Research has shown that omega-3 polyunsaturated fatty acids (n-3 PUFA), which are found in fish oil, protect glutamatergic neurotransmission from glucocorticoids, which are released in the body during a stress response.26 Small clinical trials have found n-3 PUFAs to reduce the symptoms of anxiety compared with placebo, but the acids have not been studied directly for anxiety disorders.27,28
With regard to depression, evidence is conflicting as to whether n-3 PUFAs are of any benefit in treatment.29 Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are hypothesized to be the components in omega-3 fatty acid preparations that could lead to a reduction in depressive symptoms. However, randomized trials have shown that EPA-predominant omega-3 fatty acid formulations have only a moderate or no clinically significant effect on depression over placebo, and that DHA-predominant omega-3 fatty acid formulations are only comparable or inferior to placebo.30
Continue to: Response to EPA...
Response to EPA may be greater in patients with depression who have high levels of inflammatory biomarkers, such as interleuken (IL)-1 receptor antagonist (1Ra), IL-6, high-sensitivity C-reactive protein (hs-CRP), leptin, and adiponectin, than in patients with low levels. An 8-week trial randomly assigned patients with unipolar major depression (N = 155) to receive either EPA, DHA, or placebo and found that improvement for the 3 groups was comparable; however, in the subgroup of patients with high levels of inflammatory markers, improvement in depressive symptoms was significantly greater with EPA than with either DHA or placebo.31
It is unclear whether different sources of n-3 PUFA, such as whole fish vs fish oil vs prescription omega-3 acid ethyl esters (Lovaza), are more or less efficacious in the treatment of anxiety or depression. Furthermore, there is no standard dosing for n-3 PUFA in the treatment of mood disorders. Given that the US Food and Drug Administration recommends no more than 2 g/d of combined EPA and DHA supplementation, we recommend using 2 g/d if one decides to treat depression/anxiety with n-3 PUFA.32
N-3 PUFA supplementation is fairly benign. There have been previous concerns about n-3 PUFA supplementation increasing patients’ risk for gastrointestinal bleeding, but a 2006 systematic review that included 9 trials (N = 2612) that looked at clinically significant bleeding episodes found that even patients at high risk for bleeding (ie, those taking aspirin or warfarin) had no increased bleeding risk from taking n-3 PUFA supplementation at up to 4 g/d.33
Don’t underestimate exercise and meditation; consider acupuncture
Exercise. Multiple practice guidelines, including the American Psychiatric Association’s “Practice Guideline for the Treatment of Patients with Major Depressive Disorder,” and meta-analyses have supported the use of exercise to treat unipolar major depression and anxiety.34-37 However, only about 26% of American men and 19% of American women met the US Department of Health and Human Services’ “Federal Physical Activity Guidelines for Americans” in 2016.38
Exercise alone is a reasonable monotherapy, as long as patients are monitored closely for worsening symptoms. Additionally, exercise as an add-on treatment can be helpful for more severe depression or anxiety.39 The best type, duration, and frequency of exercise specifically for the treatment of depression or anxiety has yet to be determined, but physicians may base their exercise recommendations on the “Federal Physical Activity Guidelines for Americans” for general good health (TABLE).38
Continue to: Meditation
Meditation, especially mindfulness meditation, is another strategy that has gained popularity in the treatment of anxiety and depression. Mindfulness has been defined as “the practice of maintaining a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis.”40 A 2014 systematic review and meta-analysis of 47 trials with 3515 participants found that mindfulness meditation programs led to clinically significant moderate reductions in anxiety.41 Smaller effects were found for depression.
Nevertheless, meditation may be beneficial as an adjunctive treatment for depression. A small randomized trial (N = 25) compared an adjunctive breathing-based meditation intervention with a waitlist control (delayed yoga) in patients with unipolar major depression who failed to respond to at least 8 weeks of antidepressant treatment.42 The meditation intervention consisted of a group program with sitting meditation, breathing exercises, and yoga postures. Participants engaged in the meditation intervention for 2 to 3.5 hours per day for 8 weeks and demonstrated significant improvement in depression symptoms compared with the control group.42
Acupuncture. A 2018 meta-analysis of 64 studies (N = 7104) suggests that acupuncture results in a small-to-moderate reduction in depressive symptoms when compared to no treatment, control/sham acupuncture, or medication.43 Furthermore, acupuncture plus medication compared to medication alone results in a higher reduction in depressive symptoms without an increase in adverse events.43
Additionally, a 2019 analysis of 10 systematic reviews found acupuncture to be more effective than control/sham acupuncture in the treatment of general anxiety.44 It should be noted, however, that a lot of heterogeneity and potential for bias existed across all of the studies. The studies analyzed were very low to low in quality. Thus, the evidence is insufficient to strongly recommend the use of acupuncture for depression or anxiety, although acupuncture is a safe intervention with low rates of adverse events.
Emotional support animals: Beneficial, but evidence is weak
Emotional support animals are gaining in popularity with Americans who have mood disorders. An important distinction must be made, however, between service animals and emotional support animals. A service animal is one “that is individually trained to do work or perform tasks for the benefit of an individual with a disability, including a physical, sensory, psychiatric, intellectual, or other mental disability.”45 Under the Americans with Disabilities Act (ADA), service animals are limited to dogs, and, in some cases, specially trained miniature horses. Psychiatric service dogs can be trained to do anything from reminding their owner to take medicine to stopping self-mutilation activities.
Continue to: Emotional support animals...
Emotional support animals are not specially trained to perform tasks to help with disabilities. It’s their companionship that helps relieve symptoms of depression and/or anxiety.45 Thus, emotional support animals are not covered under federal laws that apply to service animals. However, the Air Carrier Access Act does require airlines to allow emotional support animals to fly in the cabin for free. Furthermore, the Fair Housing Act allows emotional support animals to circumvent no-pet rules in housing and dorms. Airplanes and housing are the only places legally required to allow the unrestricted presence of emotional support animals.46
Also, there are important distinctions between emotional support animals and pets. While anyone can own a pet, an emotional support animal is prescribed by a licensed mental health professional as a treatment for a mood disorder. Housing facilities and airlines will usually require an emotional support animal “prescription” or letter from a physician to recognize animals as such.
A 2018 systematic review evaluated the evidence behind emotional support animals, which included 17 peer-reviewed journal articles, conference papers, and research dissertations (N = 1727) mostly containing qualitative evidence.47 Unfortunately, there are no RCTs, and there are limited case-control and cohort studies evaluating the effect of an emotional support animal on mood disorders. Based on the available evidence, there does seem to be a psychological benefit to owning an animal for both those with a diagnosable mental health disorder and the general population. This benefit seems to stem from a perceived reduction in social isolation and an increase in emotional support. Factors that determine the psychological benefit of emotional support animals include the type of pet, the number of pets, the attachment to the pet, and the perceived friendliness of the pet.47
Animal-assisted therapy. A 2014 systematic review evaluated higher-level evidence behind animal-assisted therapy (AAT).48 Although participating in therapy that involves interaction with animals is not the same as owning an emotional support animal, the concept—using an animal to improve mental health—is the same. The systematic review looked at 11 RCTs (N = 411) that studied the effect of AAT on mental health. Animals studied included dogs, cats, dolphins, birds, cows, rabbits, ferrets, and guinea pigs. Mental health disorders studied included schizophrenia, depression, anxiety, alcohol/drug abuse, and other addictive behaviors.
Therapeutic animal exposure led to reported improvements in mood, quality of life, and social behavior. These improvements were attributed to the animals buffering people’s reactions to mental stressors. The animals provided a sense of comfort and safety and diverted attention away from immediate stressors. Furthermore, the memory of the animals brought participants a sense of comfort/happiness when they were later without the animal. However, the majority of participants were people who liked animals at baseline.48
CORRESPONDENCE
Amanda E. Olagunju, DO, Operational Medicine Clinic, Langley AFB Hospital, 77 Nealy Avenue, Langley AFB, VA 23665; [email protected].
1. National Center for Health Statistics, Centers for Disease Control and Prevention. FastStats: Depression. Last reviewed October 7, 2015. www.cdc.gov/nchs/fastats/depression.htm. Accessed May 26, 2020.
2. National Institute of Mental Health. Mental health information—statistics: any anxiety disorder. Last updated November 2017. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml. Accessed May 26, 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief, no 362. Hyattsville, MD: National Center for Health Statistics; 2020.
4. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? Last updated July 2018. www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed May 26, 2020.
5. Bennett DA Jr, Phun L, Polk JF, et al. Neuropharmacology of St. John’s wort (Hypericum). Ann Pharmacother. 1998;32:1201-1208.
6. Müller WE, Singer A, Wonnemann M, et al. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(suppl 1):16-21.
7. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448.
8.
9. Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20:299-304.
10. Kobak KA, Taylor LV, Warner G, et al. St. John’s wort versus placebo in social phobia: results from a placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25:51-58.
11. Product reviews: St. John’s wort supplements review. ConsumerLab.com. September 23, 2016. www.consumerlab.com/reviews/St_Johns_Wort/stjohnswort/. Accessed May 26, 2020.
12. Simhan S. Serotonin syndrome. In: Abd-Elsayed A. (ed) Pain: A Review Guide. New York, NY: Springer; 2019.
13. Chrubasik-Hausmann S, Vlachojannis J, McLachlan A. Understanding drug interactions with St. John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71:129-138.
14. Knüppel L, Linde K. Adverse effects of St. John’s wort: a systematic review. J Clin Psychiatry. 2004;65:1470-1479.
15. Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174:42-50.
16. Kandler C, Lam S. Methylenetetrahydrofolate reductase screening in treatment-resistant depression. Fed Pract. 2019;36:207-208.
17. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267-1274.
18. Zajecka J, Fava M, Shelton R, et al. Long-term efficacy, safety, and tolerability of L-methylfolate calcium 15 mg as adjunctive therapy with selective serotonin reuptake inhibitors: a 12-month, open-label study following a placebo-controlled acute study. J Clin Psychiatry. 2016;77:654-660.
19. Kakar MS, Jehangir S, Mustafa M, et al. Therapeutic efficacy of combination therapy of L-methylfolate and escitalopram in depression. Pakistan Armed Forces Med J. 2017;67:976-981.
20. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942-948.
21.
22. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
23. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
24. Eid A, Khoja S, AlGhamdi S, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. 2019;34:1781-1786.
25. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767.
26. Hennebelle M, Champeil-Potokar G, Lavialle M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99-112.
27. Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725-1734.
28. Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568-575.
29. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012;17:1161-1163.
30. Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192-201.
31. Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71-79.
32. National Institutes of Health Office of Dietary Supplements. Omega-3 fatty acids. Updated October 17, 2019. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional. Accessed May 26, 2020.
33. Wang C, Harris WS, Chung M, et al. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linoleic acid, benefit cardiovascular disease outcomes in primary- and secondary- prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5-17.
34. American Psychiatric Association Practice. Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed May 26, 2020.
35. Gordon B, McDowell C, Lyons M, et al. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521-2532.
36. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102-108.
37. Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19:204-212.
38. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
39. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;CD004366.
40. Merriam-Webster Dictionary. "Mindfulness." www.merriam-webster.com/dictionary/mindfulness. Accessed May 26, 2020.
41. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368.
42. Sharma A, Barrett MS, Cucchiara AJ, et al. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63.
43. Smith CA, Armour M, Soo Lee M, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;CD004046.
44. Li M
45. Brennan J. Service animals and emotional support animals. ADA National Network Information Guidance and Training on the Americans with Disabilities Act. Last updated April 2020. adata.org/publication/service-animals-booklet. Accessed May 26, 2020.
46. Clay RA. Is that a pet or therapeutic aid? Monitor on Psychology. 2016;47:38.
47. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18:31.
48. Kamioka H, Okada S, Tsutani K, et al. Effectiveness of animal-assisted therapy: a systematic review of randomized controlled trials. Complement Ther Med. 2014;22:371-390.
1. National Center for Health Statistics, Centers for Disease Control and Prevention. FastStats: Depression. Last reviewed October 7, 2015. www.cdc.gov/nchs/fastats/depression.htm. Accessed May 26, 2020.
2. National Institute of Mental Health. Mental health information—statistics: any anxiety disorder. Last updated November 2017. www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml. Accessed May 26, 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief, no 362. Hyattsville, MD: National Center for Health Statistics; 2020.
4. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? Last updated July 2018. www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. Accessed May 26, 2020.
5. Bennett DA Jr, Phun L, Polk JF, et al. Neuropharmacology of St. John’s wort (Hypericum). Ann Pharmacother. 1998;32:1201-1208.
6. Müller WE, Singer A, Wonnemann M, et al. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(suppl 1):16-21.
7. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448.
8.
9. Kobak KA, Taylor LV, Bystritsky A, et al. St John’s wort versus placebo in obsessive-compulsive disorder: results from a double-blind study. Int Clin Psychopharmacol. 2005;20:299-304.
10. Kobak KA, Taylor LV, Warner G, et al. St. John’s wort versus placebo in social phobia: results from a placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25:51-58.
11. Product reviews: St. John’s wort supplements review. ConsumerLab.com. September 23, 2016. www.consumerlab.com/reviews/St_Johns_Wort/stjohnswort/. Accessed May 26, 2020.
12. Simhan S. Serotonin syndrome. In: Abd-Elsayed A. (ed) Pain: A Review Guide. New York, NY: Springer; 2019.
13. Chrubasik-Hausmann S, Vlachojannis J, McLachlan A. Understanding drug interactions with St. John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71:129-138.
14. Knüppel L, Linde K. Adverse effects of St. John’s wort: a systematic review. J Clin Psychiatry. 2004;65:1470-1479.
15. Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174:42-50.
16. Kandler C, Lam S. Methylenetetrahydrofolate reductase screening in treatment-resistant depression. Fed Pract. 2019;36:207-208.
17. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267-1274.
18. Zajecka J, Fava M, Shelton R, et al. Long-term efficacy, safety, and tolerability of L-methylfolate calcium 15 mg as adjunctive therapy with selective serotonin reuptake inhibitors: a 12-month, open-label study following a placebo-controlled acute study. J Clin Psychiatry. 2016;77:654-660.
19. Kakar MS, Jehangir S, Mustafa M, et al. Therapeutic efficacy of combination therapy of L-methylfolate and escitalopram in depression. Pakistan Armed Forces Med J. 2017;67:976-981.
20. Papakostas GI, Mischoulon D, Shyu I, et al. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942-948.
21.
22. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575-587.
23. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
24. Eid A, Khoja S, AlGhamdi S, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. 2019;34:1781-1786.
25. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767.
26. Hennebelle M, Champeil-Potokar G, Lavialle M, et al. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr Rev. 2014;72:99-112.
27. Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725-1734.
28. Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:568-575.
29. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry 2012;17:1161-1163.
30. Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192-201.
31. Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71-79.
32. National Institutes of Health Office of Dietary Supplements. Omega-3 fatty acids. Updated October 17, 2019. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional. Accessed May 26, 2020.
33. Wang C, Harris WS, Chung M, et al. n-3 fatty acids from fish or fish-oil supplements, but not alpha-linoleic acid, benefit cardiovascular disease outcomes in primary- and secondary- prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5-17.
34. American Psychiatric Association Practice. Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed May 26, 2020.
35. Gordon B, McDowell C, Lyons M, et al. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47:2521-2532.
36. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102-108.
37. Rethorst CD, Trivedi MH. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J Psychiatr Pract. 2013;19:204-212.
38. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd edition. Washington, DC: US Department of Health and Human Services; 2018.
39. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;CD004366.
40. Merriam-Webster Dictionary. "Mindfulness." www.merriam-webster.com/dictionary/mindfulness. Accessed May 26, 2020.
41. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368.
42. Sharma A, Barrett MS, Cucchiara AJ, et al. A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: a randomized pilot study. J Clin Psychiatry. 2017;78:e59-e63.
43. Smith CA, Armour M, Soo Lee M, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;CD004046.
44. Li M
45. Brennan J. Service animals and emotional support animals. ADA National Network Information Guidance and Training on the Americans with Disabilities Act. Last updated April 2020. adata.org/publication/service-animals-booklet. Accessed May 26, 2020.
46. Clay RA. Is that a pet or therapeutic aid? Monitor on Psychology. 2016;47:38.
47. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18:31.
48. Kamioka H, Okada S, Tsutani K, et al. Effectiveness of animal-assisted therapy: a systematic review of randomized controlled trials. Complement Ther Med. 2014;22:371-390.
PRACTICE RECOMMENDATIONS
› Consider standardized preparations of St. John’s wort for the treatment of mild to moderate depression in certain patients. A
› Encourage patients with depression or anxiety to engage in exercise and meditation to help with symptom management. A
› Consider methylfolate and S-adenosyl methionine as adjunctive treatments to improve depression. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Painful foot or ankle? Don't overlook these 5 injuries
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29
Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; [email protected]
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29
Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; [email protected]
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29
Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; [email protected]
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
PRACTICE RECOMMENDATIONS
› Suspect higher-grade syndesmotic disruption (which typically requires surgical intervention) in patients whose ankle pain persists after 3 weeks of immobilization or who have a tibial or fibular diastasis on a plain film. C
› Order weight-bearing x-rays to make an accurate diagnosis of Lisfranc injury. Refer for potential surgical intervention if diastasis is evident at the base between the first and second metatarsals. C
› Distinguish between proximal diaphysial (Jones) fracture of the fifth metatarsal, diaphysial stress fracture, and avulsion fracture—essential because avulsion fracture can be treated nonoperatively but the other 2 require surgical intervention. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Whom should you screen for abdominal aortic aneurysm?
Too few patients are being screened for abdominal aortic aneurysm (AAA), resulting in severe morbidity and mortality. Many patients with AAA aren’t identified until they present with rupture, leading to mortality as high as 90%.1 Early detection is critical.
Medicare offers one-time free screening to eligible individuals > 65 years of age, and several professional organizations promote screening with published guidelines, which we discuss later in this article.
So who is at risk, who should be screened, and what is the best way to screen your patients?
Risk factors and sex differences
AAA has a prevalence of between 1% and 5% in men > 65 years old,2,3 and it is 4 to 6 times more common in men than women.4 Major risk factors include smoking, older age, family history, and genetic factors, while hypertension, history of coronary artery disease, hyperlipidemia, and peripheral arterial disease have weaker associations.3,4 Exercise and diabetes seem to have protective effects.5
The incidence and mortality of AAA increased between the 1950s and the mid-1990s; however, both indicators have decreased in numerous countries in the 21st century.6 Although the prevalence is much lower in women, they have a higher risk of rupture than men at equivalent lesion diameters.3 The prevalence of AAA in women who smoke and are > 70 years of age is > 1%.3
Silent but deadly
Most patients with AAA are asymptomatic. Their lesions are often detected incidentally on magnetic resonance imaging of the spine obtained for back pain, on an abdominal ultrasound (US) for gallstones, or on a routine computed tomography (CT) scan for the evaluation of abdominal pain. Some patients will experience vague abdominal discomfort from rapid expansion of an aneurysm prior to rupture, necessitating urgent repair. Also, some large aneurysms can erode into the spine and cause chronic back pain prior to rupture. An infrarenal abdominal aortic diameter > 30 mm defines an aneurysm,7 and once the diameter reaches 55 mm, the threat of rupture often justifies operative repair. (See “The preferred approach to repair.”)
SIDEBAR
The preferred approach to repair
Since the introduction of endovascular aneurysm repair (EVAR) in the latter part of the 20th century, it has become the standard of care for the surgical management of aneurysmal disease. Currently, > 80% of patients with an abdominal aortic aneurysm (AAA) who undergo repair are treated with EVAR.23
Typically, the AAA diameter is assessed via ultrasound. If repair is indicated, a computed tomography arteriogram is obtained to define the anatomy and help determine if the AAA is amenable to endografting. The most common contraindications to EVAR are either a short proximal neck (not enough distance below the renal arteries to safely anchor the stent graft) or an iliac artery diameter that is too small to allow delivery of the device. The operation can be performed under local, regional, or general anesthesia, and patients are usually discharged on the first postoperative day. These patients require lifelong surveillance due to the risk of delayed endoleak and reperfusion of the aneurysm sac.24
Ruptured aneurysms will classically manifest with severe abdominal and/or back pain. Often a ruptured aneurysm will be contained in the retroperitoneum, allowing the patient to remain hemodynamically stable for a period of time and thus providing a window of opportunity for emergent repair.
Continue to: What is the evidence that screening is effective?
What is the evidence that screening is effective?
In 1988, researchers in Chichester, England, randomized > 6000 men ages 65 to 80 years to either a control group or a group that was offered a one-time US screen for AAA. After 15 years of follow-up, no significant difference in AAA mortality was seen between the groups, although 26% of those invited for screening declined to participate and accounted for more than half of the AAA-related deaths in the group receiving an invitation for screening.8
The MASS Trial,9 another British study, began in 1997 and screened men ages 65 to 74. More than 67,000 men were randomized, with 1 group invited for AAA screening and the other serving as a control. The final report on this trial was published in 2012. After 13 years of follow-up, there was a 42% reduction in AAA-related deaths in the group invited for screening, a small reduction in all-cause mortality, and a significant reduction in risk of AAA rupture (hazard ratio = 0.57). The researchers noted that 216 patients would have to be invited for screening to prevent 1 death over 13 years. They also reported that 21% of the invited patients that had an AAA-related death had an initial scan that was negative for AAA (aortic diameter < 3 cm). However, despite this finding, screening still appeared to be beneficial.
Lindholdt et al10 randomized > 12,000 Danish men ages 64 to 73 to serve as controls or to be invited for US screening for AAA. After 13 years of follow-up, those invited for screening had a 66% relative risk reduction in AAA-related mortality, with screening considered cost effective. There were no differences between the groups in all-cause mortality. Conversely, the Western Australia Trial studied an older group of men, ages 64 to 83, but was unable to show a benefit of screening in lowering AAA-related mortality.11
Tikagi et al6 performed a meta-analysis on the data from the 4 trials above and reported up to 15 years of follow-up. Patients who attended screening sessions had a reduction in all-cause mortality with an odds ratio (OR) of 0.6, and a marked reduction in AAA-related mortality with an OR of 0.4. The favorable data on screening have prompted the United Kingdom and Sweden to offer screening to all men ≥ 65 years, based on the current estimate of a 1% prevalence of AAA, although screening is felt to remain cost effective down to a prevalence of 0.35%.12
Massachusetts General Hospital also reported13 that the detection rate of AAA increased, with the diagnosis made at smaller aneurysm dimensions, following publication of the US Preventive Services Task Force recommendations (reviewed below).
Continue to: When to screen
When to screen
Several professional organizations, as well as Medicare, have published AAA screening recommendations. While there are notable differences among them, their shared message is crucial: screen. Consider adding applicable reminders to your practice’s electronic medical record system to increase screening rates for eligible patients.
US Preventive Services Task Force 14
- Recommend one-time AAA screening with ultrasonography for men ages 65 to 75 years who have ever smoked (B recommendation).
- Selectively offer AAA screening to men ages 65 to 75 years who have never smoked, rather than routinely screening all men in this group (C recommendation). Individual attributes that could favor screening include a family history of AAA, the presence of other arterial aneurysms, and the number of risk factors for cardiovascular disease.
- Do not routinely screen women for AAA if they have never smoked and have no family history of AAA (D recommendation). For women ages 65 to 75 years who have ever smoked or have a family history of AAA, current evidence is insufficient to assess the balance of benefits and harms of screening for AAA (I statement). (See the related Practice Alert.)
Canadian Task Force on Preventive Health Care4
- Recommend one-time screening for AAA with US for men 65 to 80 years of age (weak level of recommendation; moderate-quality evidence).
- Do not recommend screening for men older than 80 years of age (weak recommendation; low quality of evidence).
- Do not recommend screening for women (strong recommendation; very low quality of evidence).
Society for Vascular Surgery15
- Recommend one-time US screening for AAA for men or women 65 to 75 years of age with a history of tobacco use (strong recommendation with high-quality evidence).
- Recommend one-time screening if there is a history of smoking for men or women > 75 years of age who are in good health and have not previously been screened. (Weak recommendation with low-quality evidence).
- Recommend one-time screening of men or women 65 to 75 years of age who are first-degree relatives of someone with AAA, or in those older than age 75 and in good health (weak recommendation with low-quality evidence).
How to screen
Physical exam. The abdominal aorta is often palpable in the epigastric region, and a thorough abdominal exam should include an attempt to detect it. It is critical that the patient be supine during palpation, to allow compression of the aorta against the lumbar spine. Even with a well-performed exam, however, its sensitivity is just 76% in the detection of AAA ≥ 5 cm.16
Continue to: Imaging
Imaging. US is the preferred imaging procedure when screening for AAA, given its high sensitivity and specificity.17 If US yields poor image quality, noncontrast CT is suggested, with magnetic resonance angiography being another alternative.18 Handheld US has the potential to supplement the physical exam, and has been shown to be a viable method to detect AAA in an outpatient primary care setting at a reasonable cost.19 Typically, a formal aortic US requires a patient to go without food or liquids for 8 hours before the procedure to obtain the best image; however, a good estimate of aortic diameter can be obtained without this restriction.
Despite Medicare coverage and recs, few people are screened
In 2007, Medicare started the SAAVE Program (Screening Aortic Aneurysm Very Effectively), offering a one-time US screening for AAA for eligible patients, as part of the Welcome to Medicare Program. Eligible individuals are men between 65 and 75 years of age with a history of smoking at least 100 cigarettes in their lifetime, and men or women in the same age group with a family history of AAA.2
Despite these recommendations, few patients receive screening. Centers for Medicare & Medicaid Services data show that < 10% of eligible men were screened between 2004 and 2008,20 and Olchanski et al21 report that < 1% of eligible patients were screened from 2005-2009. A simulation model estimates that 131 additional life years could be gained per 1000 patients screened if the utilization rate could be increased to 80%, a seemingly achievable goal.21 Moreover, expanding the screening program to include female smokers could increase 10-year life expectancy by 13%.21 Reasons for underutilization of this Medicare screening benefit may include lack of awareness by physicians and patients, costs of co-pays, and underutilization of the basic Welcome to Medicare exam.21
An additional consequence of low utilization of AAA screening is a high percentage of patients who are identified only late in the course of the disease. Mell et al22 report that 39% of patients undergoing AAA repair were identified < 6 months prior to surgery, a higher percentage than would be expected in a well-screened population. They also determined that slightly more than one-third of patients undergoing surgery for ruptured AAA had diagnostic imaging performed > 6 months prior to surgery, suggesting the possibility that these patients may not have been properly surveilled for aneurysm expansion, although the authors note that other potential explanations include delays in treatment due to comorbidities, and patient-related factors such as refusal of surgery or noncompliance with follow-up.
CORRESPONDENCE
Jeffrey S. Todd, MD, 127 McClanahan Street, Suite 300, Roanoke, VA 24014; [email protected].
1. Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85:268-273.
2. SBU—Swedish agency for Health Technology Assessment and Assessment of Social Services. Screening for abdominal aortic aneurysm. 2018. www.sbu.se/en/publications/sbu-assesses/screening-for-abdominal-aortic-aneurysm/. Accessed April 24, 2020.
3. Spanos K, Labropoulos N, Giannoukas A. Abdominal aortic aneurysm screening: do we need to shift toward a targeted strategy? Angiology. 2018;69:192-194.
4. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-1145.
5. Stackelberg O, Wolk, A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725.
6. Tikagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) group. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2017;69:205-211.
7. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
8. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701.
9. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
10. Lindholdt JS, Sørenson J, Søgaard R et al. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826-834.
11. McCaul KA, Lawrence-Brown M, Dickinson JA, et al. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761-1766.
12. Earnshaw JJ, Lees T. Update on screening for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:1-2.
13. Zucker EJ, Misono AS, Prabhakar AM. Abdominal aortic aneurysm screening practices: impact of the 2014 US Preventive Services Task Force recommendations. J Am Coll Radiol. 2017;14:868-874.
14. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement.
15. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.
16. Venkatasubramaniam AK, Mehta T, Chetter IC, et al. The value of abdominal examination in the diagnosis of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;24:56-60.
17. Lindholdt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
18. Desjardins B, Dill KE, Flamm SD, et al. ACR Appropriateness Criteria®, pulsatile abdominal mass, suspected abdominal aortic aneurysm. Int J Cardiovasc Imaging. 2013;29:177-183.
19. Sisó-Almirall A, Kostov B, Navarro-González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
20. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462.
21. Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155-1161.
22. Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg. 2013;57:1519-1523.
23. England A, McWilliams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J. 2013;82:3-10.
24. Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446-451.
Too few patients are being screened for abdominal aortic aneurysm (AAA), resulting in severe morbidity and mortality. Many patients with AAA aren’t identified until they present with rupture, leading to mortality as high as 90%.1 Early detection is critical.
Medicare offers one-time free screening to eligible individuals > 65 years of age, and several professional organizations promote screening with published guidelines, which we discuss later in this article.
So who is at risk, who should be screened, and what is the best way to screen your patients?
Risk factors and sex differences
AAA has a prevalence of between 1% and 5% in men > 65 years old,2,3 and it is 4 to 6 times more common in men than women.4 Major risk factors include smoking, older age, family history, and genetic factors, while hypertension, history of coronary artery disease, hyperlipidemia, and peripheral arterial disease have weaker associations.3,4 Exercise and diabetes seem to have protective effects.5
The incidence and mortality of AAA increased between the 1950s and the mid-1990s; however, both indicators have decreased in numerous countries in the 21st century.6 Although the prevalence is much lower in women, they have a higher risk of rupture than men at equivalent lesion diameters.3 The prevalence of AAA in women who smoke and are > 70 years of age is > 1%.3
Silent but deadly
Most patients with AAA are asymptomatic. Their lesions are often detected incidentally on magnetic resonance imaging of the spine obtained for back pain, on an abdominal ultrasound (US) for gallstones, or on a routine computed tomography (CT) scan for the evaluation of abdominal pain. Some patients will experience vague abdominal discomfort from rapid expansion of an aneurysm prior to rupture, necessitating urgent repair. Also, some large aneurysms can erode into the spine and cause chronic back pain prior to rupture. An infrarenal abdominal aortic diameter > 30 mm defines an aneurysm,7 and once the diameter reaches 55 mm, the threat of rupture often justifies operative repair. (See “The preferred approach to repair.”)
SIDEBAR
The preferred approach to repair
Since the introduction of endovascular aneurysm repair (EVAR) in the latter part of the 20th century, it has become the standard of care for the surgical management of aneurysmal disease. Currently, > 80% of patients with an abdominal aortic aneurysm (AAA) who undergo repair are treated with EVAR.23
Typically, the AAA diameter is assessed via ultrasound. If repair is indicated, a computed tomography arteriogram is obtained to define the anatomy and help determine if the AAA is amenable to endografting. The most common contraindications to EVAR are either a short proximal neck (not enough distance below the renal arteries to safely anchor the stent graft) or an iliac artery diameter that is too small to allow delivery of the device. The operation can be performed under local, regional, or general anesthesia, and patients are usually discharged on the first postoperative day. These patients require lifelong surveillance due to the risk of delayed endoleak and reperfusion of the aneurysm sac.24
Ruptured aneurysms will classically manifest with severe abdominal and/or back pain. Often a ruptured aneurysm will be contained in the retroperitoneum, allowing the patient to remain hemodynamically stable for a period of time and thus providing a window of opportunity for emergent repair.
Continue to: What is the evidence that screening is effective?
What is the evidence that screening is effective?
In 1988, researchers in Chichester, England, randomized > 6000 men ages 65 to 80 years to either a control group or a group that was offered a one-time US screen for AAA. After 15 years of follow-up, no significant difference in AAA mortality was seen between the groups, although 26% of those invited for screening declined to participate and accounted for more than half of the AAA-related deaths in the group receiving an invitation for screening.8
The MASS Trial,9 another British study, began in 1997 and screened men ages 65 to 74. More than 67,000 men were randomized, with 1 group invited for AAA screening and the other serving as a control. The final report on this trial was published in 2012. After 13 years of follow-up, there was a 42% reduction in AAA-related deaths in the group invited for screening, a small reduction in all-cause mortality, and a significant reduction in risk of AAA rupture (hazard ratio = 0.57). The researchers noted that 216 patients would have to be invited for screening to prevent 1 death over 13 years. They also reported that 21% of the invited patients that had an AAA-related death had an initial scan that was negative for AAA (aortic diameter < 3 cm). However, despite this finding, screening still appeared to be beneficial.
Lindholdt et al10 randomized > 12,000 Danish men ages 64 to 73 to serve as controls or to be invited for US screening for AAA. After 13 years of follow-up, those invited for screening had a 66% relative risk reduction in AAA-related mortality, with screening considered cost effective. There were no differences between the groups in all-cause mortality. Conversely, the Western Australia Trial studied an older group of men, ages 64 to 83, but was unable to show a benefit of screening in lowering AAA-related mortality.11
Tikagi et al6 performed a meta-analysis on the data from the 4 trials above and reported up to 15 years of follow-up. Patients who attended screening sessions had a reduction in all-cause mortality with an odds ratio (OR) of 0.6, and a marked reduction in AAA-related mortality with an OR of 0.4. The favorable data on screening have prompted the United Kingdom and Sweden to offer screening to all men ≥ 65 years, based on the current estimate of a 1% prevalence of AAA, although screening is felt to remain cost effective down to a prevalence of 0.35%.12
Massachusetts General Hospital also reported13 that the detection rate of AAA increased, with the diagnosis made at smaller aneurysm dimensions, following publication of the US Preventive Services Task Force recommendations (reviewed below).
Continue to: When to screen
When to screen
Several professional organizations, as well as Medicare, have published AAA screening recommendations. While there are notable differences among them, their shared message is crucial: screen. Consider adding applicable reminders to your practice’s electronic medical record system to increase screening rates for eligible patients.
US Preventive Services Task Force 14
- Recommend one-time AAA screening with ultrasonography for men ages 65 to 75 years who have ever smoked (B recommendation).
- Selectively offer AAA screening to men ages 65 to 75 years who have never smoked, rather than routinely screening all men in this group (C recommendation). Individual attributes that could favor screening include a family history of AAA, the presence of other arterial aneurysms, and the number of risk factors for cardiovascular disease.
- Do not routinely screen women for AAA if they have never smoked and have no family history of AAA (D recommendation). For women ages 65 to 75 years who have ever smoked or have a family history of AAA, current evidence is insufficient to assess the balance of benefits and harms of screening for AAA (I statement). (See the related Practice Alert.)
Canadian Task Force on Preventive Health Care4
- Recommend one-time screening for AAA with US for men 65 to 80 years of age (weak level of recommendation; moderate-quality evidence).
- Do not recommend screening for men older than 80 years of age (weak recommendation; low quality of evidence).
- Do not recommend screening for women (strong recommendation; very low quality of evidence).
Society for Vascular Surgery15
- Recommend one-time US screening for AAA for men or women 65 to 75 years of age with a history of tobacco use (strong recommendation with high-quality evidence).
- Recommend one-time screening if there is a history of smoking for men or women > 75 years of age who are in good health and have not previously been screened. (Weak recommendation with low-quality evidence).
- Recommend one-time screening of men or women 65 to 75 years of age who are first-degree relatives of someone with AAA, or in those older than age 75 and in good health (weak recommendation with low-quality evidence).
How to screen
Physical exam. The abdominal aorta is often palpable in the epigastric region, and a thorough abdominal exam should include an attempt to detect it. It is critical that the patient be supine during palpation, to allow compression of the aorta against the lumbar spine. Even with a well-performed exam, however, its sensitivity is just 76% in the detection of AAA ≥ 5 cm.16
Continue to: Imaging
Imaging. US is the preferred imaging procedure when screening for AAA, given its high sensitivity and specificity.17 If US yields poor image quality, noncontrast CT is suggested, with magnetic resonance angiography being another alternative.18 Handheld US has the potential to supplement the physical exam, and has been shown to be a viable method to detect AAA in an outpatient primary care setting at a reasonable cost.19 Typically, a formal aortic US requires a patient to go without food or liquids for 8 hours before the procedure to obtain the best image; however, a good estimate of aortic diameter can be obtained without this restriction.
Despite Medicare coverage and recs, few people are screened
In 2007, Medicare started the SAAVE Program (Screening Aortic Aneurysm Very Effectively), offering a one-time US screening for AAA for eligible patients, as part of the Welcome to Medicare Program. Eligible individuals are men between 65 and 75 years of age with a history of smoking at least 100 cigarettes in their lifetime, and men or women in the same age group with a family history of AAA.2
Despite these recommendations, few patients receive screening. Centers for Medicare & Medicaid Services data show that < 10% of eligible men were screened between 2004 and 2008,20 and Olchanski et al21 report that < 1% of eligible patients were screened from 2005-2009. A simulation model estimates that 131 additional life years could be gained per 1000 patients screened if the utilization rate could be increased to 80%, a seemingly achievable goal.21 Moreover, expanding the screening program to include female smokers could increase 10-year life expectancy by 13%.21 Reasons for underutilization of this Medicare screening benefit may include lack of awareness by physicians and patients, costs of co-pays, and underutilization of the basic Welcome to Medicare exam.21
An additional consequence of low utilization of AAA screening is a high percentage of patients who are identified only late in the course of the disease. Mell et al22 report that 39% of patients undergoing AAA repair were identified < 6 months prior to surgery, a higher percentage than would be expected in a well-screened population. They also determined that slightly more than one-third of patients undergoing surgery for ruptured AAA had diagnostic imaging performed > 6 months prior to surgery, suggesting the possibility that these patients may not have been properly surveilled for aneurysm expansion, although the authors note that other potential explanations include delays in treatment due to comorbidities, and patient-related factors such as refusal of surgery or noncompliance with follow-up.
CORRESPONDENCE
Jeffrey S. Todd, MD, 127 McClanahan Street, Suite 300, Roanoke, VA 24014; [email protected].
Too few patients are being screened for abdominal aortic aneurysm (AAA), resulting in severe morbidity and mortality. Many patients with AAA aren’t identified until they present with rupture, leading to mortality as high as 90%.1 Early detection is critical.
Medicare offers one-time free screening to eligible individuals > 65 years of age, and several professional organizations promote screening with published guidelines, which we discuss later in this article.
So who is at risk, who should be screened, and what is the best way to screen your patients?
Risk factors and sex differences
AAA has a prevalence of between 1% and 5% in men > 65 years old,2,3 and it is 4 to 6 times more common in men than women.4 Major risk factors include smoking, older age, family history, and genetic factors, while hypertension, history of coronary artery disease, hyperlipidemia, and peripheral arterial disease have weaker associations.3,4 Exercise and diabetes seem to have protective effects.5
The incidence and mortality of AAA increased between the 1950s and the mid-1990s; however, both indicators have decreased in numerous countries in the 21st century.6 Although the prevalence is much lower in women, they have a higher risk of rupture than men at equivalent lesion diameters.3 The prevalence of AAA in women who smoke and are > 70 years of age is > 1%.3
Silent but deadly
Most patients with AAA are asymptomatic. Their lesions are often detected incidentally on magnetic resonance imaging of the spine obtained for back pain, on an abdominal ultrasound (US) for gallstones, or on a routine computed tomography (CT) scan for the evaluation of abdominal pain. Some patients will experience vague abdominal discomfort from rapid expansion of an aneurysm prior to rupture, necessitating urgent repair. Also, some large aneurysms can erode into the spine and cause chronic back pain prior to rupture. An infrarenal abdominal aortic diameter > 30 mm defines an aneurysm,7 and once the diameter reaches 55 mm, the threat of rupture often justifies operative repair. (See “The preferred approach to repair.”)
SIDEBAR
The preferred approach to repair
Since the introduction of endovascular aneurysm repair (EVAR) in the latter part of the 20th century, it has become the standard of care for the surgical management of aneurysmal disease. Currently, > 80% of patients with an abdominal aortic aneurysm (AAA) who undergo repair are treated with EVAR.23
Typically, the AAA diameter is assessed via ultrasound. If repair is indicated, a computed tomography arteriogram is obtained to define the anatomy and help determine if the AAA is amenable to endografting. The most common contraindications to EVAR are either a short proximal neck (not enough distance below the renal arteries to safely anchor the stent graft) or an iliac artery diameter that is too small to allow delivery of the device. The operation can be performed under local, regional, or general anesthesia, and patients are usually discharged on the first postoperative day. These patients require lifelong surveillance due to the risk of delayed endoleak and reperfusion of the aneurysm sac.24
Ruptured aneurysms will classically manifest with severe abdominal and/or back pain. Often a ruptured aneurysm will be contained in the retroperitoneum, allowing the patient to remain hemodynamically stable for a period of time and thus providing a window of opportunity for emergent repair.
Continue to: What is the evidence that screening is effective?
What is the evidence that screening is effective?
In 1988, researchers in Chichester, England, randomized > 6000 men ages 65 to 80 years to either a control group or a group that was offered a one-time US screen for AAA. After 15 years of follow-up, no significant difference in AAA mortality was seen between the groups, although 26% of those invited for screening declined to participate and accounted for more than half of the AAA-related deaths in the group receiving an invitation for screening.8
The MASS Trial,9 another British study, began in 1997 and screened men ages 65 to 74. More than 67,000 men were randomized, with 1 group invited for AAA screening and the other serving as a control. The final report on this trial was published in 2012. After 13 years of follow-up, there was a 42% reduction in AAA-related deaths in the group invited for screening, a small reduction in all-cause mortality, and a significant reduction in risk of AAA rupture (hazard ratio = 0.57). The researchers noted that 216 patients would have to be invited for screening to prevent 1 death over 13 years. They also reported that 21% of the invited patients that had an AAA-related death had an initial scan that was negative for AAA (aortic diameter < 3 cm). However, despite this finding, screening still appeared to be beneficial.
Lindholdt et al10 randomized > 12,000 Danish men ages 64 to 73 to serve as controls or to be invited for US screening for AAA. After 13 years of follow-up, those invited for screening had a 66% relative risk reduction in AAA-related mortality, with screening considered cost effective. There were no differences between the groups in all-cause mortality. Conversely, the Western Australia Trial studied an older group of men, ages 64 to 83, but was unable to show a benefit of screening in lowering AAA-related mortality.11
Tikagi et al6 performed a meta-analysis on the data from the 4 trials above and reported up to 15 years of follow-up. Patients who attended screening sessions had a reduction in all-cause mortality with an odds ratio (OR) of 0.6, and a marked reduction in AAA-related mortality with an OR of 0.4. The favorable data on screening have prompted the United Kingdom and Sweden to offer screening to all men ≥ 65 years, based on the current estimate of a 1% prevalence of AAA, although screening is felt to remain cost effective down to a prevalence of 0.35%.12
Massachusetts General Hospital also reported13 that the detection rate of AAA increased, with the diagnosis made at smaller aneurysm dimensions, following publication of the US Preventive Services Task Force recommendations (reviewed below).
Continue to: When to screen
When to screen
Several professional organizations, as well as Medicare, have published AAA screening recommendations. While there are notable differences among them, their shared message is crucial: screen. Consider adding applicable reminders to your practice’s electronic medical record system to increase screening rates for eligible patients.
US Preventive Services Task Force 14
- Recommend one-time AAA screening with ultrasonography for men ages 65 to 75 years who have ever smoked (B recommendation).
- Selectively offer AAA screening to men ages 65 to 75 years who have never smoked, rather than routinely screening all men in this group (C recommendation). Individual attributes that could favor screening include a family history of AAA, the presence of other arterial aneurysms, and the number of risk factors for cardiovascular disease.
- Do not routinely screen women for AAA if they have never smoked and have no family history of AAA (D recommendation). For women ages 65 to 75 years who have ever smoked or have a family history of AAA, current evidence is insufficient to assess the balance of benefits and harms of screening for AAA (I statement). (See the related Practice Alert.)
Canadian Task Force on Preventive Health Care4
- Recommend one-time screening for AAA with US for men 65 to 80 years of age (weak level of recommendation; moderate-quality evidence).
- Do not recommend screening for men older than 80 years of age (weak recommendation; low quality of evidence).
- Do not recommend screening for women (strong recommendation; very low quality of evidence).
Society for Vascular Surgery15
- Recommend one-time US screening for AAA for men or women 65 to 75 years of age with a history of tobacco use (strong recommendation with high-quality evidence).
- Recommend one-time screening if there is a history of smoking for men or women > 75 years of age who are in good health and have not previously been screened. (Weak recommendation with low-quality evidence).
- Recommend one-time screening of men or women 65 to 75 years of age who are first-degree relatives of someone with AAA, or in those older than age 75 and in good health (weak recommendation with low-quality evidence).
How to screen
Physical exam. The abdominal aorta is often palpable in the epigastric region, and a thorough abdominal exam should include an attempt to detect it. It is critical that the patient be supine during palpation, to allow compression of the aorta against the lumbar spine. Even with a well-performed exam, however, its sensitivity is just 76% in the detection of AAA ≥ 5 cm.16
Continue to: Imaging
Imaging. US is the preferred imaging procedure when screening for AAA, given its high sensitivity and specificity.17 If US yields poor image quality, noncontrast CT is suggested, with magnetic resonance angiography being another alternative.18 Handheld US has the potential to supplement the physical exam, and has been shown to be a viable method to detect AAA in an outpatient primary care setting at a reasonable cost.19 Typically, a formal aortic US requires a patient to go without food or liquids for 8 hours before the procedure to obtain the best image; however, a good estimate of aortic diameter can be obtained without this restriction.
Despite Medicare coverage and recs, few people are screened
In 2007, Medicare started the SAAVE Program (Screening Aortic Aneurysm Very Effectively), offering a one-time US screening for AAA for eligible patients, as part of the Welcome to Medicare Program. Eligible individuals are men between 65 and 75 years of age with a history of smoking at least 100 cigarettes in their lifetime, and men or women in the same age group with a family history of AAA.2
Despite these recommendations, few patients receive screening. Centers for Medicare & Medicaid Services data show that < 10% of eligible men were screened between 2004 and 2008,20 and Olchanski et al21 report that < 1% of eligible patients were screened from 2005-2009. A simulation model estimates that 131 additional life years could be gained per 1000 patients screened if the utilization rate could be increased to 80%, a seemingly achievable goal.21 Moreover, expanding the screening program to include female smokers could increase 10-year life expectancy by 13%.21 Reasons for underutilization of this Medicare screening benefit may include lack of awareness by physicians and patients, costs of co-pays, and underutilization of the basic Welcome to Medicare exam.21
An additional consequence of low utilization of AAA screening is a high percentage of patients who are identified only late in the course of the disease. Mell et al22 report that 39% of patients undergoing AAA repair were identified < 6 months prior to surgery, a higher percentage than would be expected in a well-screened population. They also determined that slightly more than one-third of patients undergoing surgery for ruptured AAA had diagnostic imaging performed > 6 months prior to surgery, suggesting the possibility that these patients may not have been properly surveilled for aneurysm expansion, although the authors note that other potential explanations include delays in treatment due to comorbidities, and patient-related factors such as refusal of surgery or noncompliance with follow-up.
CORRESPONDENCE
Jeffrey S. Todd, MD, 127 McClanahan Street, Suite 300, Roanoke, VA 24014; [email protected].
1. Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85:268-273.
2. SBU—Swedish agency for Health Technology Assessment and Assessment of Social Services. Screening for abdominal aortic aneurysm. 2018. www.sbu.se/en/publications/sbu-assesses/screening-for-abdominal-aortic-aneurysm/. Accessed April 24, 2020.
3. Spanos K, Labropoulos N, Giannoukas A. Abdominal aortic aneurysm screening: do we need to shift toward a targeted strategy? Angiology. 2018;69:192-194.
4. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-1145.
5. Stackelberg O, Wolk, A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725.
6. Tikagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) group. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2017;69:205-211.
7. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
8. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701.
9. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
10. Lindholdt JS, Sørenson J, Søgaard R et al. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826-834.
11. McCaul KA, Lawrence-Brown M, Dickinson JA, et al. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761-1766.
12. Earnshaw JJ, Lees T. Update on screening for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:1-2.
13. Zucker EJ, Misono AS, Prabhakar AM. Abdominal aortic aneurysm screening practices: impact of the 2014 US Preventive Services Task Force recommendations. J Am Coll Radiol. 2017;14:868-874.
14. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement.
15. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.
16. Venkatasubramaniam AK, Mehta T, Chetter IC, et al. The value of abdominal examination in the diagnosis of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;24:56-60.
17. Lindholdt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
18. Desjardins B, Dill KE, Flamm SD, et al. ACR Appropriateness Criteria®, pulsatile abdominal mass, suspected abdominal aortic aneurysm. Int J Cardiovasc Imaging. 2013;29:177-183.
19. Sisó-Almirall A, Kostov B, Navarro-González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
20. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462.
21. Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155-1161.
22. Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg. 2013;57:1519-1523.
23. England A, McWilliams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J. 2013;82:3-10.
24. Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446-451.
1. Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgrad Med J. 2009;85:268-273.
2. SBU—Swedish agency for Health Technology Assessment and Assessment of Social Services. Screening for abdominal aortic aneurysm. 2018. www.sbu.se/en/publications/sbu-assesses/screening-for-abdominal-aortic-aneurysm/. Accessed April 24, 2020.
3. Spanos K, Labropoulos N, Giannoukas A. Abdominal aortic aneurysm screening: do we need to shift toward a targeted strategy? Angiology. 2018;69:192-194.
4. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-1145.
5. Stackelberg O, Wolk, A, Eliasson K, et al. Lifestyle and risk of screening-detected abdominal aortic aneurysm in men. J Am Heart Assoc. 2017;6:e004725.
6. Tikagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) group. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2017;69:205-211.
7. Fleming C, Whitlock EP, Beil TL, et al. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142:203-211.
8. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701.
9. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656.
10. Lindholdt JS, Sørenson J, Søgaard R et al. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg. 2010;97:826-834.
11. McCaul KA, Lawrence-Brown M, Dickinson JA, et al. Long-term outcomes of the Western Australian trial of screening for abdominal aortic aneurysms: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761-1766.
12. Earnshaw JJ, Lees T. Update on screening for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2017;54:1-2.
13. Zucker EJ, Misono AS, Prabhakar AM. Abdominal aortic aneurysm screening practices: impact of the 2014 US Preventive Services Task Force recommendations. J Am Coll Radiol. 2017;14:868-874.
14. USPSTF. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement.
15. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.
16. Venkatasubramaniam AK, Mehta T, Chetter IC, et al. The value of abdominal examination in the diagnosis of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;24:56-60.
17. Lindholdt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
18. Desjardins B, Dill KE, Flamm SD, et al. ACR Appropriateness Criteria®, pulsatile abdominal mass, suspected abdominal aortic aneurysm. Int J Cardiovasc Imaging. 2013;29:177-183.
19. Sisó-Almirall A, Kostov B, Navarro-González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
20. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462.
21. Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155-1161.
22. Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg. 2013;57:1519-1523.
23. England A, McWilliams R. Endovascular aortic aneurysm repair (EVAR). Ulster Med J. 2013;82:3-10.
24. Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446-451.
How to minimize the pain of local anesthetic administration
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
PRACTICE RECOMMENDATIONS
› Add epinephrine and sodium bicarbonate buffer to local anesthetic solution to reduce pain and procedural blood loss. A
› Use such techniques as counter-stimulation, a perpendicular angle of injection, a subcutaneous depth of injection, and a slow rate of injection to minimize patient discomfort. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The many variants of psoriasis
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.
Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.
Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.
Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
PRACTICE RECOMMENDATIONS
› Consider guttate psoriasis if small (often < 1 cm) pink scaly papules appear suddenly, particularly in a child who has an upper respiratory tract infection. C
› Document extent of disease using a tool such as the Psoriasis Area and Severity Index, which calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions. C
› Consider prescribing UV light treatment or a combination of alcitretin and topical corticosteroid if > 10% of the body surface area is involved but joints are not affected. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series