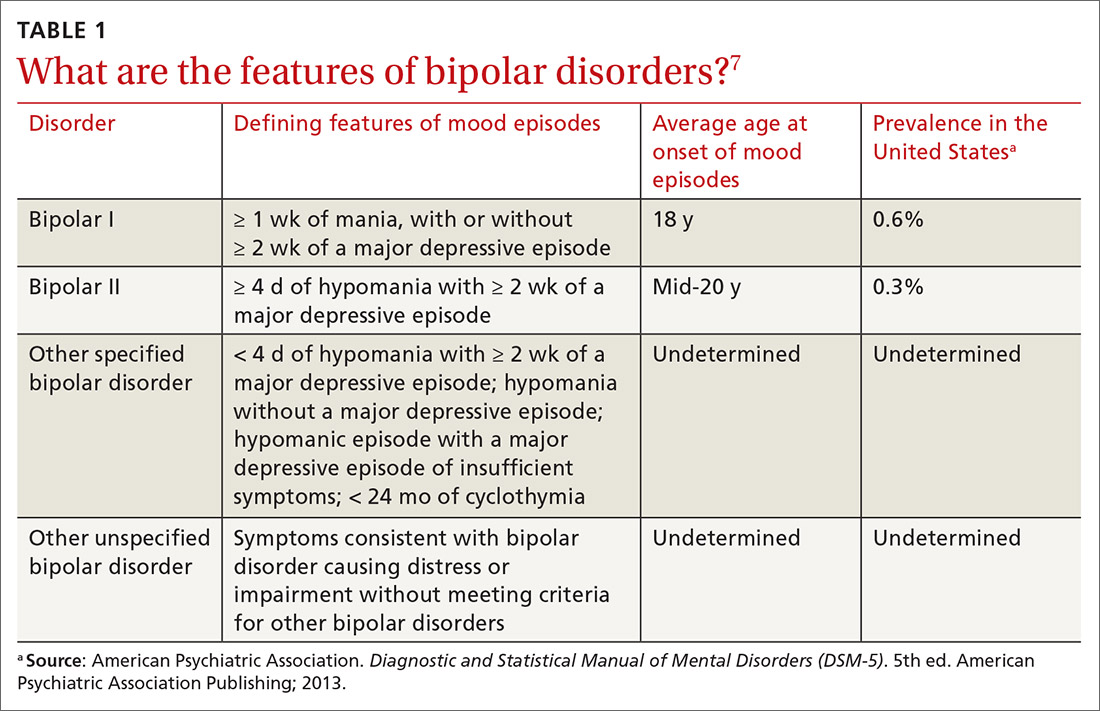
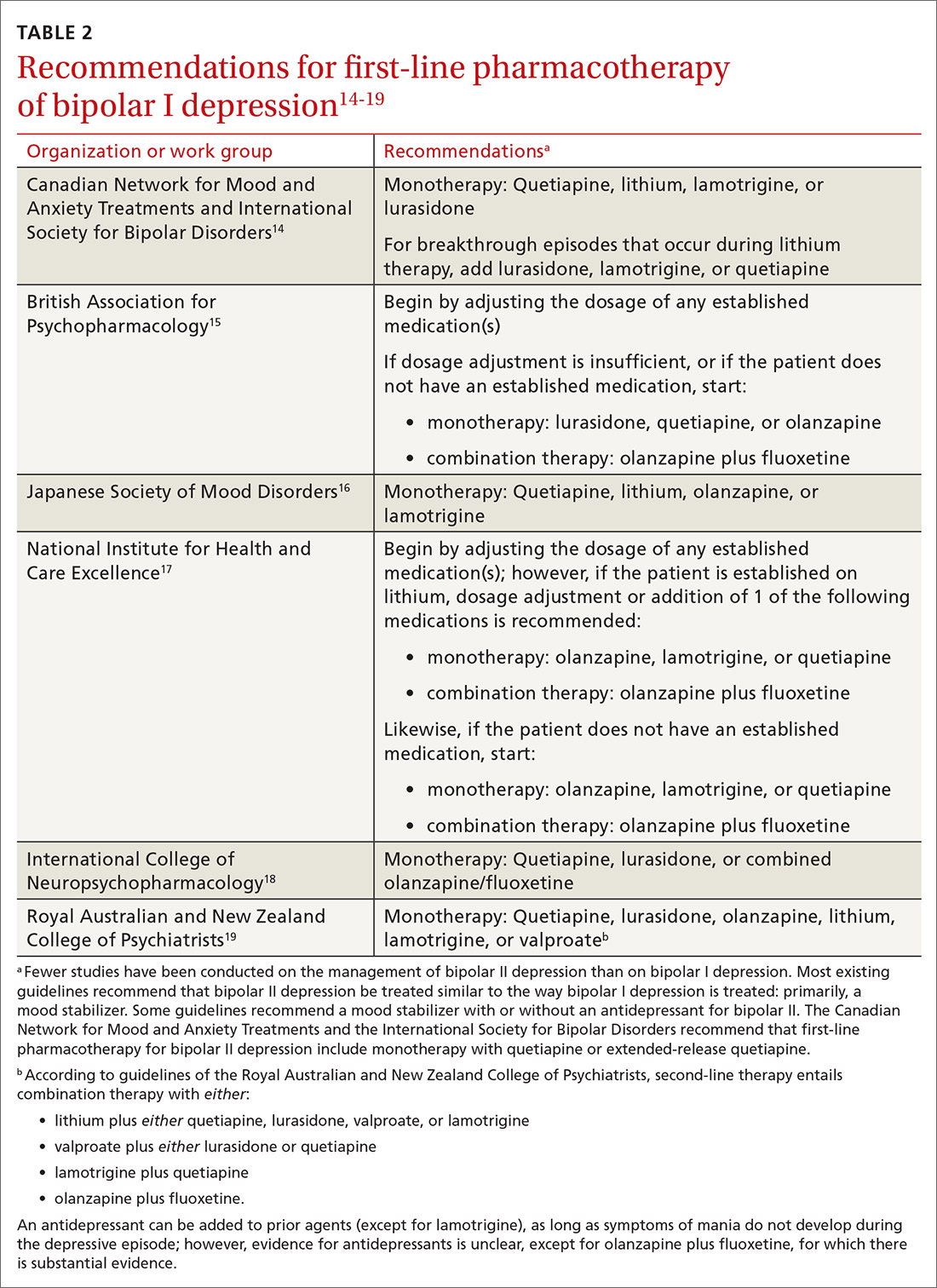
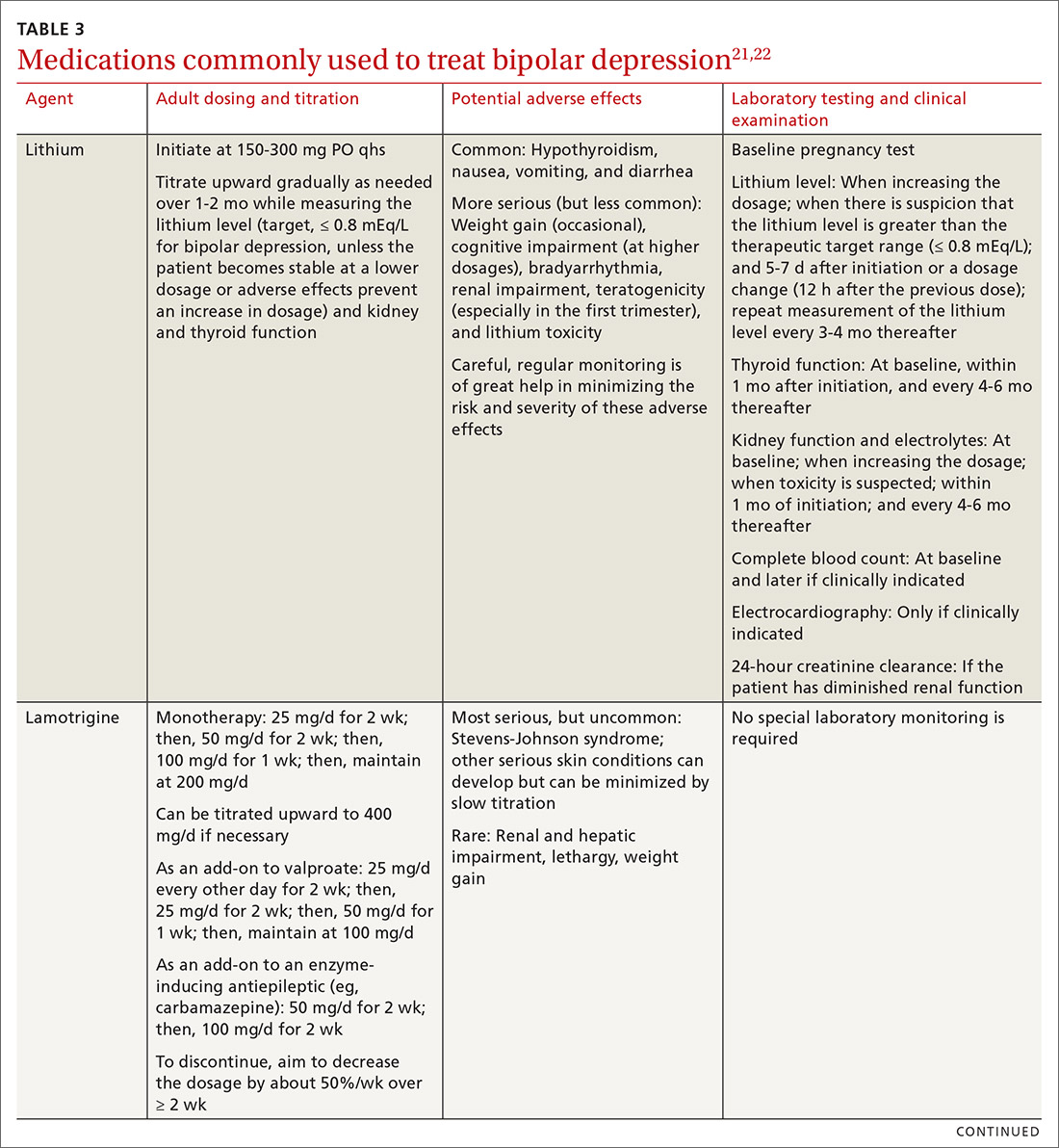
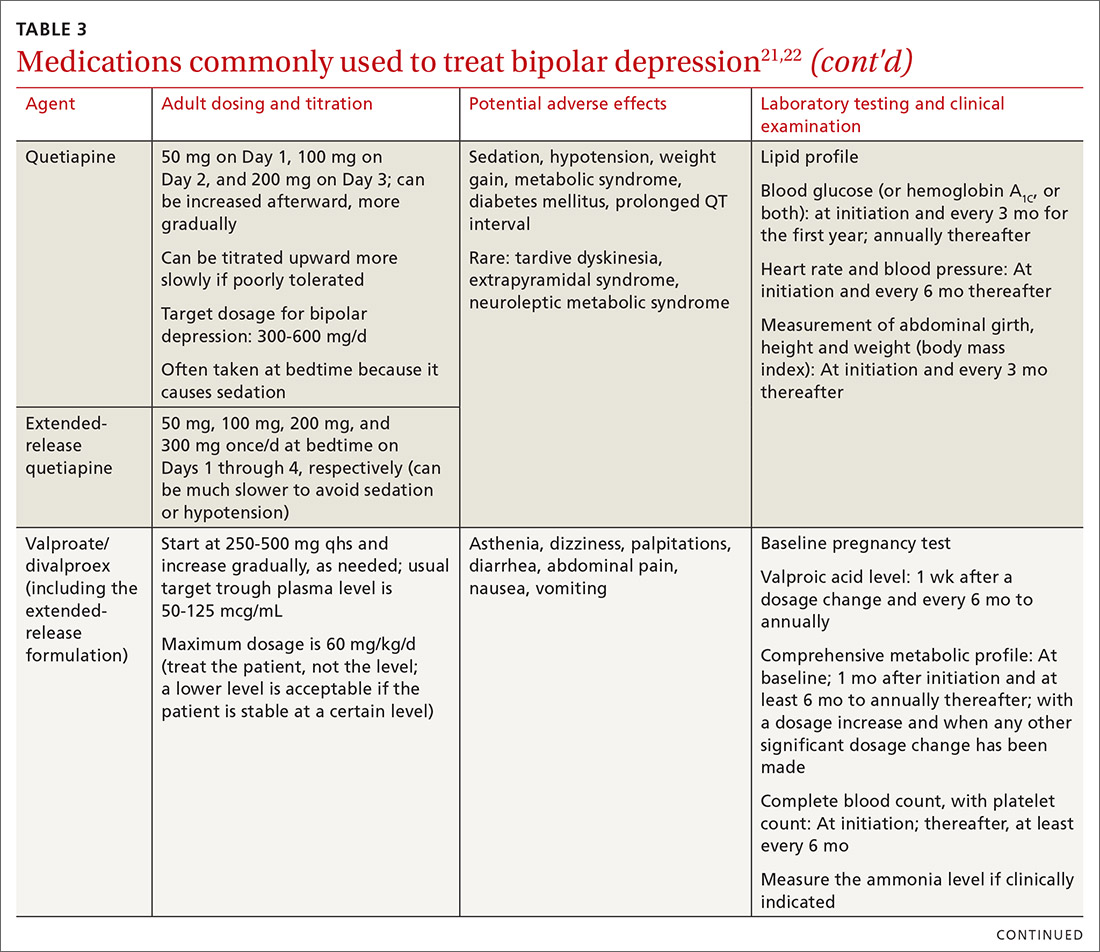
User login
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
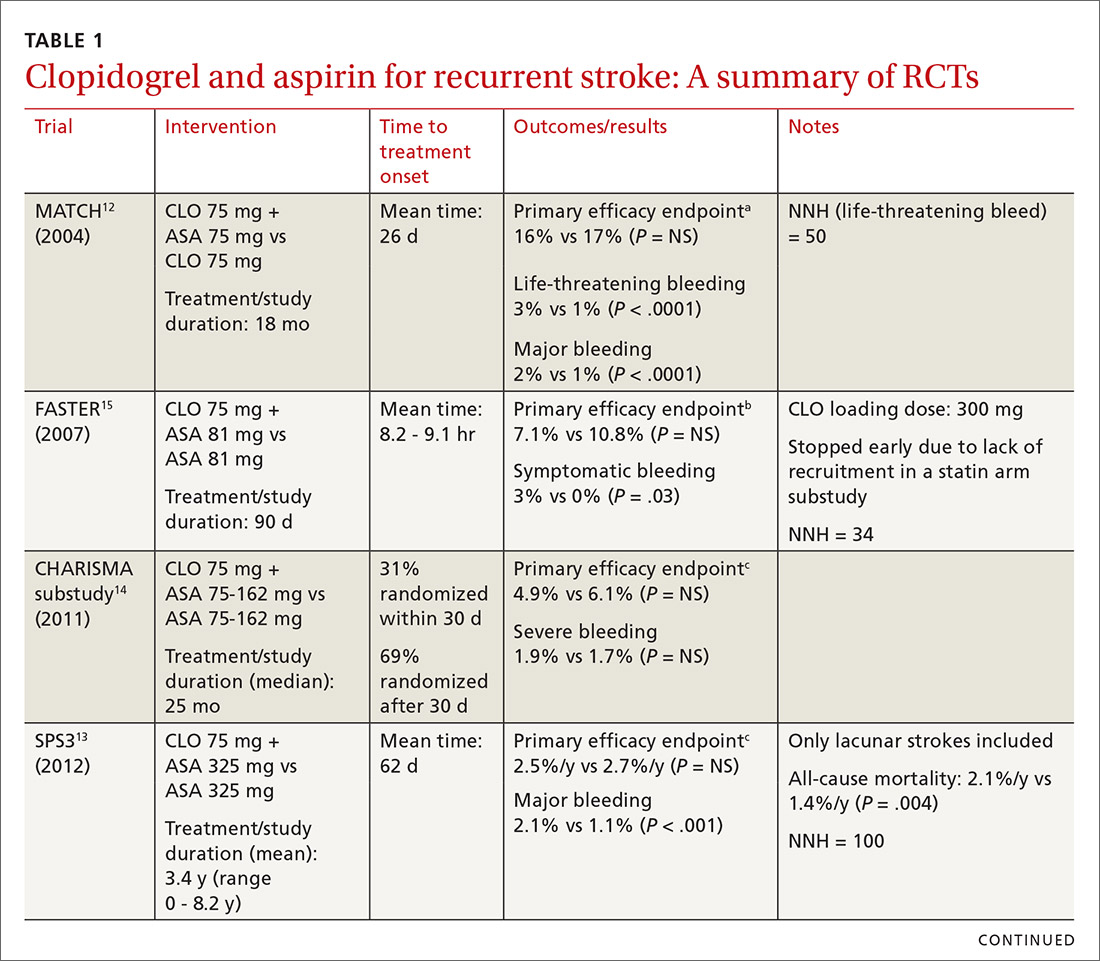
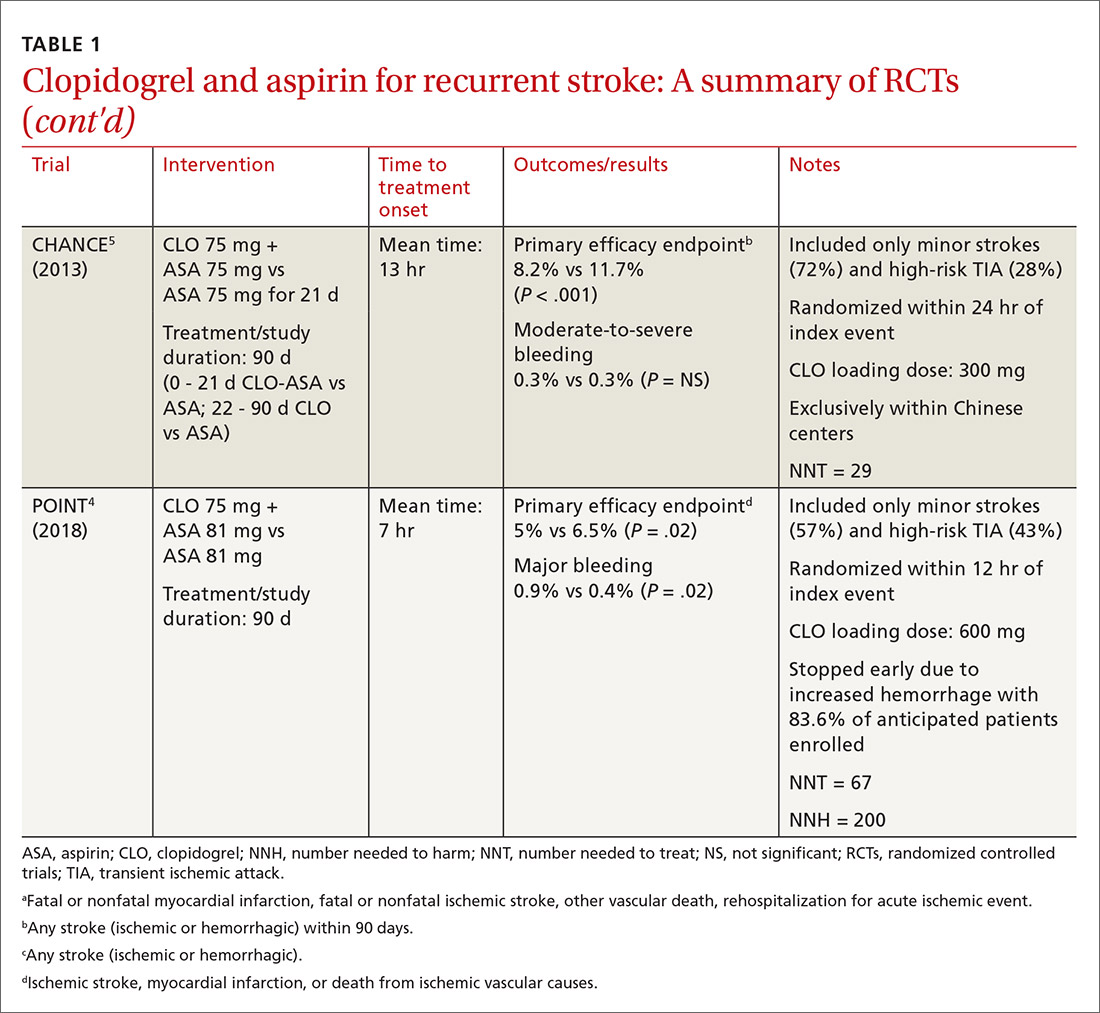
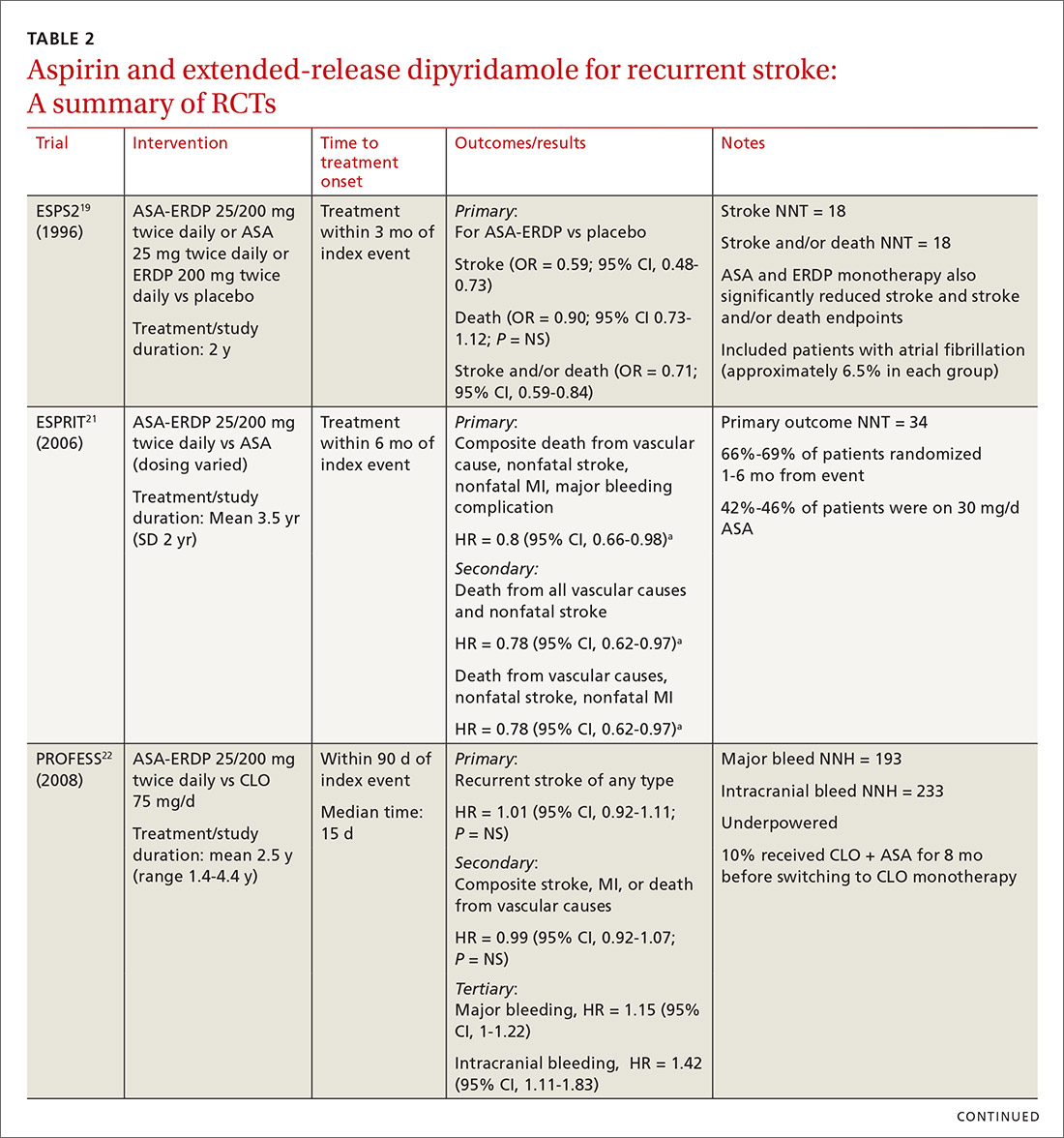
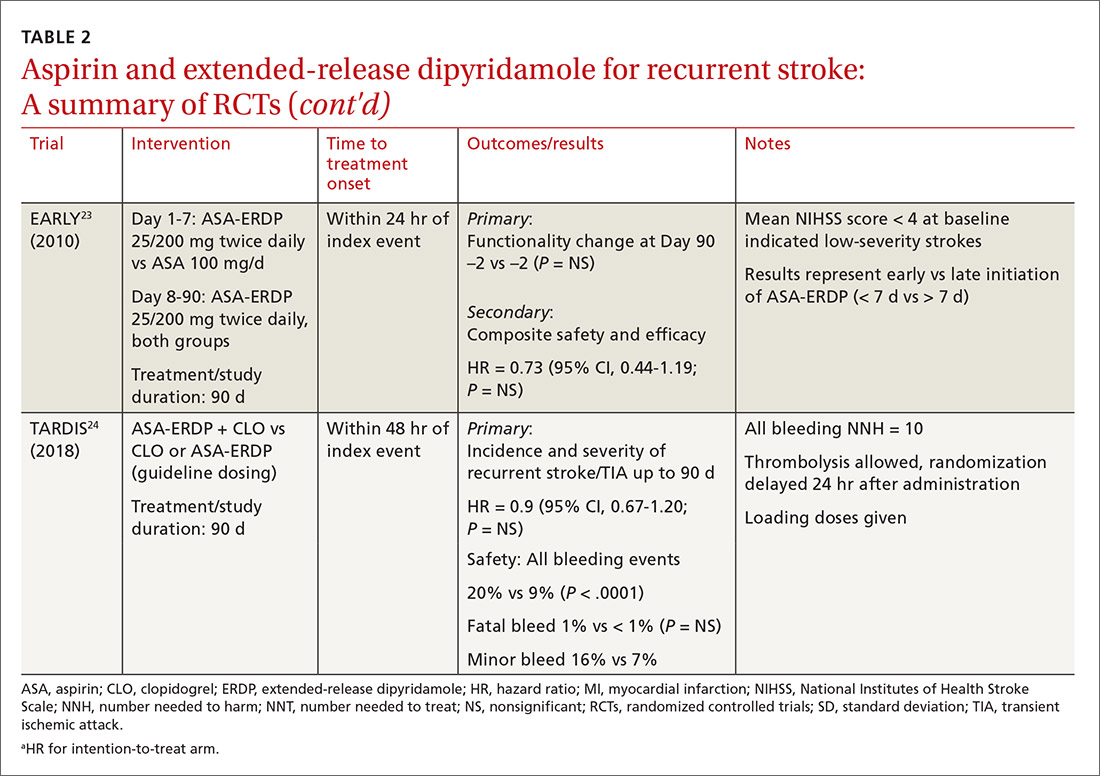
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
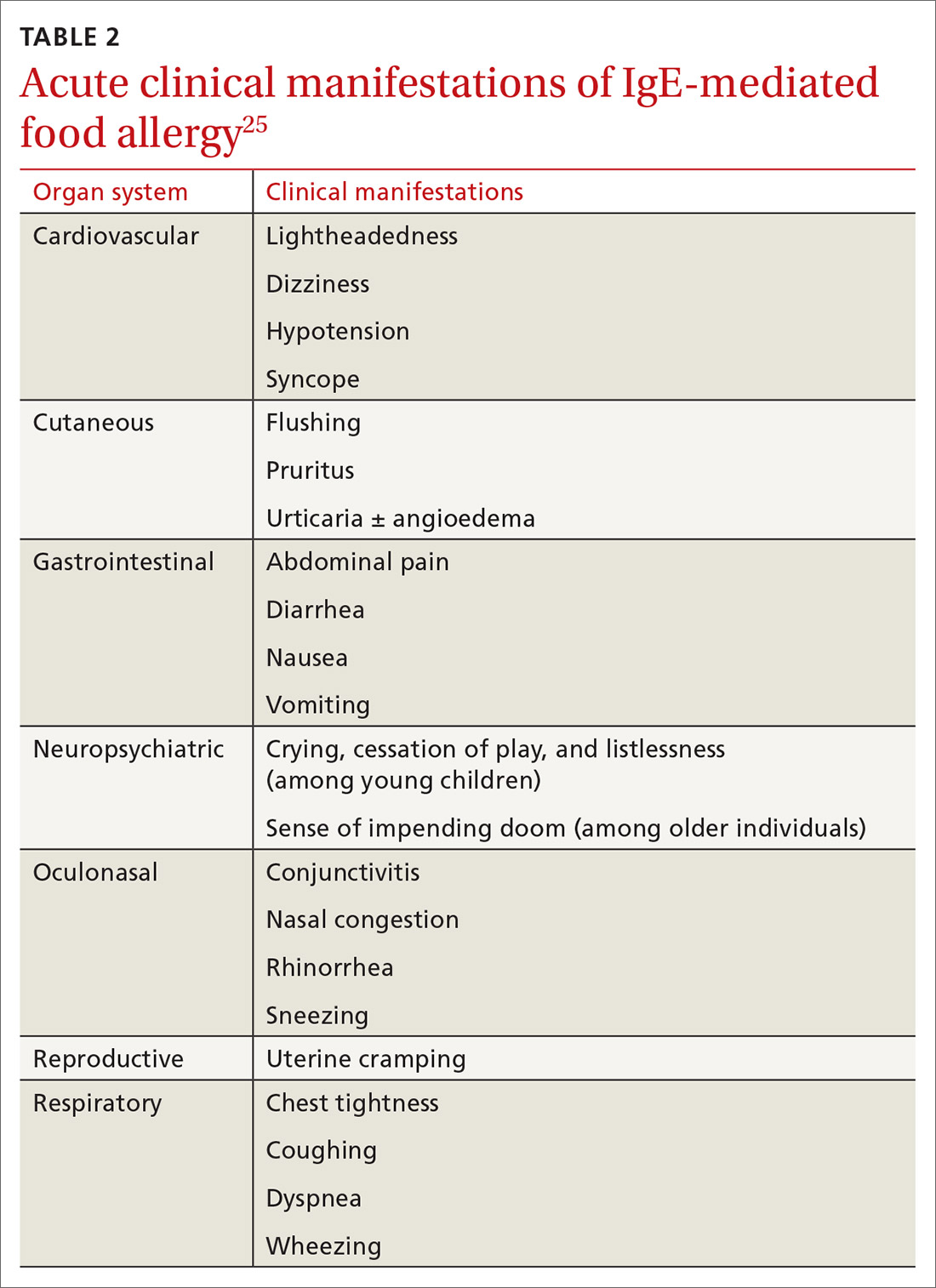
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
Your role in early diagnosis & Tx of metastatic bone disease
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
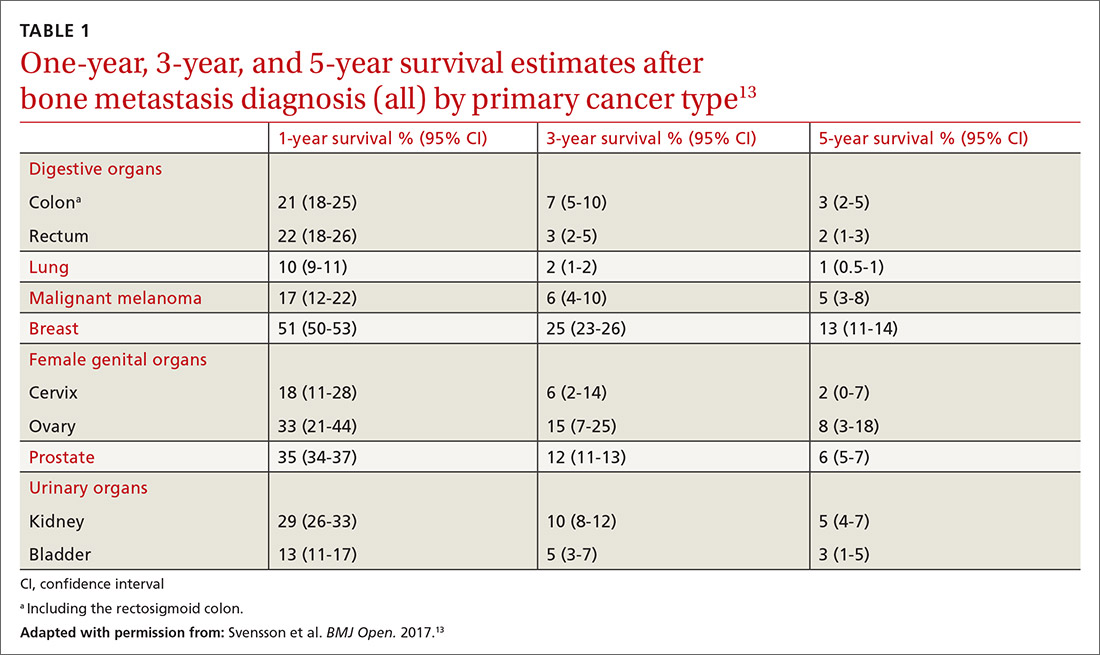
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
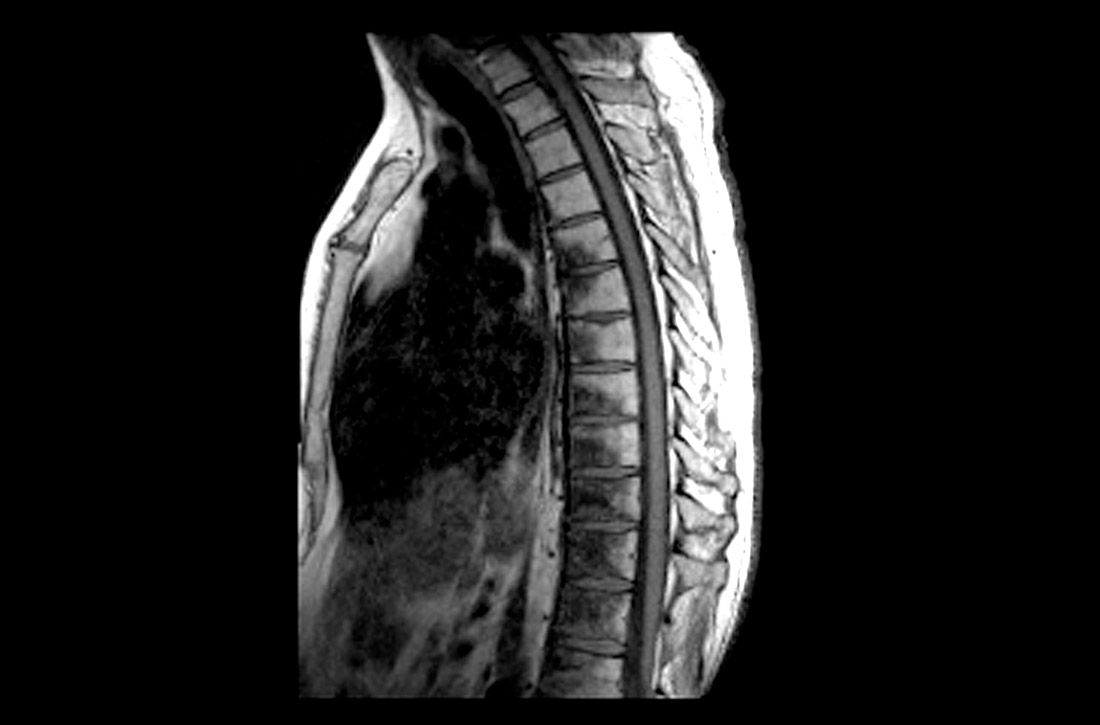
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
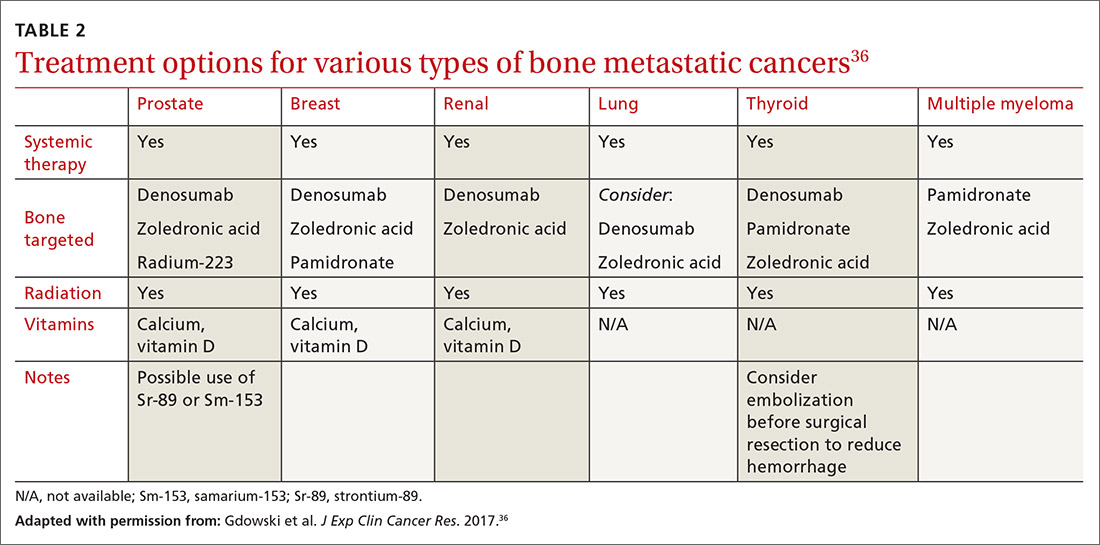
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; [email protected].
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
PRACTICE RECOMMENDATIONS
› Initiate appropriate lab and imaging work-ups for any patient without known malignancy who has a suspicious bone lesion. C
› Prescribe protected weight-bearing for the patient who has a painful bone lesion, and refer promptly to an orthopedic surgeon to prevent pathologic fracture. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A practical approach to knee OA
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8
Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)
Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.
Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8
Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)
Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.
Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8
Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)
Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.
Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
PRACTICE RECOMMENDATIONS
› Treat pain from knee osteoarthritis (OA) with weight management and low-impact exercise to decrease the risk of disease progression. A
› Prescribe oral or topical nonsteroidal anti-inflammatory drugs to relieve pain from knee OA, as both forms are equally effective. B
› Recommend a medial unloading (valgus) knee brace for short-term relief of medial knee OA. B
› Consider a trial of intra-articular corticosteroids or intra-articular hyaluronic acid derivatives for short-term relief of knee OA pain. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A primary care guide to bipolar depression treatment
Bipolar disorder is a prevalent disorder in the primary care setting.1,2 Primary care providers therefore commonly encounter bipolar depression (BD; a major depressive episode in the context of bipolar disorder), which might be (1) an emerging depressive episode in previously undiagnosed bipolar disorder or (2) a recurrent episode during the course of chronic bipolar illness.3,4
A primary care–based collaborative model has been identified as a potential strategy for effective management of chronic mental health conditions such as bipolar disorder.5,6 However, this collaborative treatment model isn’t widely available; many patients with bipolar disorder are, in fact, treated solely by their primary care provider.
Two years ago in this journal,7 we addressed how to precisely identify an episode of BD and differentiate it from major depressive disorder (MDD; also known as unipolar depression). In this review, in addition to advancing clinical knowledge of BD, we provide:
- an overview of treatment options for BD (in contrast to the treatment of unipolar depression)
- the pharmacotherapeutic know-how to initiate and maintain treatment for uncomplicated episodes of BD.
We do not discuss management of manic, hypomanic, and mixed episodes of bipolar disorder.
How to identify bipolar depression
Understanding the (sometimes) unclear distinction between bipolar I and bipolar II disorders in an individual patient is key to formulating a therapeutic regimen for BD.
Bipolar I disorder consists of manic episodes, alternating (more often than not) with depressive episodes. Bipolar I usually manifests first with a depressive episode.
Bipolar II disorder manifests with depressive episodes and hypomanic episodes (but never manic episodes).
Continue to: Depressive episodes in the bipolar disorders
Depressive episodes in the bipolar disorders. Bipolar depression can be seen in the settings of both bipolar I and II disorders. When a patient presents with a manic episode, a history of depressive episodes is common (although not essential) to diagnose bipolar I; alternatively, a history of hypomania (but no prior mania) and depression is needed to make the diagnosis of bipolar II. The natural history of the bipolar disorders is therefore alternating manic and almost always depressive episodes (bipolar I) and alternating hypomanic and always depressive episodes (bipolar II).8
Symptoms of hypomanic episodes are similar to what are seen in manic episodes, but are of shorter duration (≥ 4 days [episodes of mania are at least of 1 week’s duration]), lower intensity (no psychotic symptoms), and not associated with significant functional impairment or hospitalization. Table 17 further describes the differentiating features of bipolar I and bipolar II. A history of an unequivocal manic or hypomanic episode makes the diagnosis of BD relatively easy. However, an unclear history of manic or hypomanic symptoms or episodes frequently leads to misdiagnosis or underdiagnosis of BD.
In both bipolar I and II, it is depressive symptoms and episodes that place the greatest burden on patients across the lifespan: They are the most commonly experienced features of the bipolar disorders9,10 and lead to significant distress and functional impairment11; in fact, patients with bipolar disorder spend 3 (or more) times as long in depressive episodes as in manic or hypomanic episodes.12,13 In addition, subthreshold depressive symptoms occur commonly between major mood episodes.
Failure to identify and adequately treat depressive episodes of the bipolar disorders can have serious consequences: Patients are at risk of a worsening course of illness, alcohol use disorder, substance use disorder, chronic disability, mixed states, rapid cycling of mood episodes, and suicide.
Guidelines for treating bipolar depression
Despite the similarity in presenting symptoms and signs of depressive episodes in bipolar disorders and MDD, treating episodes of BD is significantly different than treating MDD. Antidepressant monotherapy, a mainstay of treatment for MDD, has limited utility in BD (especially depressive episodes of bipolar I) because of its limited efficacy and potential to destabilize mood, lead to rapid cycling, and induce mania or hypomania. Treatment options for BD include pharmacotherapy (the primary modality), psychological intervention (a useful adjunct, described later), and electroconvulsive therapy (ECT; highly worth considering in severe or treatment-resistant cases).
Continue to: For this article...
For this article, we searched PubMed and Google Scholar for guidelines for the management of bipolar disorders in adults that were published between July 2013 (when the US Food and Drug Administration [FDA] approved lurasidone for the treatment of BD) and March 2019. Related guideline-referenced articles and clinical trials were also reviewed.
Our search identified 6 guidelines issued during the search period, developed by the:
- Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD),14
- British Association for Psychopharmacology (BAP),15
- Japanese Society of Mood Disorders (JSMD),16
- National Institute for Health and Care Excellence (NICE),17
- International College of Neuropsychopharmacology (CINP),18 and
- Royal Australian and New Zealand College of Psychiatrists.19
How to manage an episode of bipolar depression
First-line pharmacotherapeutic agents for the management of BD in acute bipolar I are listed and described in Table 2.4-19 Compared to the number of studies and reports on the management of BD in bipolar I, few studies have been conducted that specifically examine the treatment of BD in acute bipolar II. In practice, evidence from the treatment of BD in bipolar I has been extrapolated to the treatment of bipolar II depression. CANMAT–ISBD guidelines recommend quetiapine as the only first-line therapy for BD in bipolar II; JSMD, CINP, and NICE guidelines do not make distinct recommendations for treating BD in bipolar II.
Patients who have BD can present de novo (ie, not taking any medication for bipolar disorder) or with a breakthrough episode while on maintenance medication(s). In either case, monotherapy for BD is preferred, although combinations of medications (Table 214-19) can be more effective in some cases. Treatment guidelines overlap to a high degree, especially in regard to first-line treatments, but there is variation, especially beyond first-line therapeutics.20
The top recommended medications for BD are lithium, quetiapine, olanzapine, lamotrigine, and combined olanzapine/fluoxetine. FDA-approved agents for treating acute BD specifically include quetiapine, lurasidone, and combined olanzapine/fluoxetine. Guidelines generally recommend a first step of adjusting the dosage of medications in any established regimen before changing or adding other agents. If clinical improvement is not seen using any recommended medications, psychiatric referral is recommended. See Table 321,22 for dosing and titration guidance and highlights of both common and rare but serious adverse effects.
Continue to: Recommendations, best options for acute bipolar depression
Recommendations, best options for acute bipolar depression
Start with lithium, lamotrigine, quetiapine, or lurasidone as the first-line medication at the dosages given in Table 3.21,22 Olanzapine alone, or in combination with fluoxetine, can be used when it has been determined that the medications listed above are ineffective.
Note that lithium requires regular blood monitoring (Table 321,22). However, lithium has the advantage of strong supporting evidence of benefit in all mood episodes of bipolar disorders (depressive, manic, hypomanic), as well as maintenance, prevention of recurrence, and anti-suicidal properties.
Also of note: Lurasidone is much more costly than other recommended medications because it is available only by brand name in the United States; the other agents are available as generics. Consider generic equivalents of the recommended agents when cost is an important factor, in part because of the impact that cost has on medication adherence for some patients.
Last, olanzapine should be used later in the treatment algorithm, unless rapid control of symptoms is needed or other first-line medications are ineffective or not tolerated—given the higher propensity of the drug to produce weight gain and cause metabolic problems, including obesity and hyperglycemia.
The importance of maintenance therapy
Almost all patients with BD require maintenance treatment to prevent subsequent episodes, reduce residual symptoms, and restore functioning and quality of life. Maintenance therapy is formulated on the basis of efficacy and tolerability in the individual patient.
Continue to: As a general rule...
As a general rule, the strongest evidence for preventing recurrent BD episodes favors lithium—and most guidelines therefore support lithium as first-line maintenance therapy. It is important to note, however, that if a medication (or medications) successfully aborted an acute BD episode in a given patient, that agent (or agents) should be continued for maintenance purposes to prevent or minimize future episodes—generally, at the same dosage. First-line pharmacotherapeutic agents for the maintenance of bipolar disorder, and thus to prevent subsequent episodes of BD, are listed in Table 4.14-19
℞ antidepressantsin bipolar depression?
The use of antidepressants to treat BD remains a topic of ongoing deliberation. Antidepressant treatment of BD has historically raised concern for depressive relapse due to ineffectiveness and the ability of antidepressants to increase (1) the frequency of manic and hypomanic episodes23 and (2) mood instability in the form of induction of mixed states or rapid cycling. Among most authorities, the recommendation against using antidepressants for BD in both bipolar I and II is the same; however, limited evidence allows the use of antidepressant monotherapy in select cases of BD episodes in bipolar II,24,25 although not bipolar I.
The consensus in the field is that medications with mood-stabilizing effects should be considered as monotherapy before adding an antidepressant (if an antidepressant is to be added) to treat BD in bipolar II.26 In other words, if an antidepressant is to be used at all, it should be combined with a mood stabilizer or atypical antipsychotic15,27 and should probably not be used long term. The efficacy of antidepressants in treating BD in bipolar II should be assessed periodically at follow-up.
Nonpharmaceutical treatment options
Although pharmacotherapy is the mainstay of treatment of BD, adjunctive psychotherapy can be useful for treating acute BD episodes that occur during the maintenance phase of the disorder. Psychoeducation (ie, education on psychiatric illness and the importance of medication adherence), alone or in combination with interpersonal and social rhythm therapy (IPSRT), family-focused therapy (FFT), and cognitive behavioral therapy (CBT) can add to the overall efficacy of pharmacotherapy by lowering the risk of relapse and enhancing psychosocial functioning.28
IPSRT is supported by what is known as the instability model, which specifies that 3 interconnected pathways trigger recurrences of a bipolar episode: stressful life events, medication nonadherence, and social-rhythm disruption. IPSRT also uses principles of interpersonal psychotherapy that are applied in treating MDD, “arguing that improvement in interpersonal relationships can ameliorate affective symptoms and prevent their return.”29,30
Continue to: FFT
FFT focuses on communication styles between patients and their spouses and families. The goal is to improve relationship functioning. FFT is delivered to the patient and the family.
Attention to social factors. For psychotherapy to provide adequate results as an adjunct to pharmacotherapy, social stressors (eg, homelessness and financial concerns) might also need to be considered and addressed through social services or a social work consult.
NICE guidelines recommend psychological intervention (in particular, with CBT and FFT) for acute BD. CANMAT–ISBD guidelines recommend either adjunctive psychoeducation, CBT, or FFT during the maintenance phase. Again, medication is the mainstay of treatment for BD in bipolar disorders; psychotherapy has an adjunctive role—unlike the approach to treatment of MDD, in which psychotherapy can be used alone in cases of mild, or even moderate, severity.
Referral for specialty care
In the primary care setting, providers might choose to manage BD by initiating first-line pharmacotherapeutic agents or continuing established treatment regimens with necessary dosage adjustments. These patients should be monitored closely until symptoms remit.
However, it is important for the primary care provider to identify patients who need psychiatric referral. Complex presentations, severe symptoms, and poor treatment response might warrant evaluation and management by a psychiatrist. Furthermore, patients with comorbid psychotic features, catatonia, or severely debilitating depression (with or without suicidality) need referral to the emergency department.
Continue to: Electroconvulsive therapy (ECT)
Electroconvulsive therapy (ECT). Patients might also need referral to Psychiatry for ECT, which is recommended by CANMAT–ISBD and JSMD guidelines as a second-line option; by the Royal Australian and New Zealand College of Psychiatrists as a third-line option; and by BAP for cases that are resistant to conventional treatment, with or without a high risk of suicide; in pregnancy; and in life-threatening situations.15,31,32
Telemedicine. There is a considerable shortage of mental health care professionals.33,34 The fact that nearly all (96%) counties in the United States have an unmet need for prescribers of mental health services (mainly psychiatrists) makes it crucial that primary care physicians be knowledgeable and prepared to manage BD—often with infrequent psychiatry consultation or, even, without psychiatry consultation. For primary care facilities that lack access to psychiatric services, telemedicine can be used as a consultative resource.
Psychiatric consultation using telemedicine technologies has provided significant cost savings for medical centers and decreased the likelihood of hospital admission,35 thereby alleviating health care costs and improving care, as shown in a rural Kansas county study.36 Furthermore, the burden on emergency departments in several states has been significantly reduced with psychiatric consultations via interactive telemedicine technologies.37
ACKNOWLEDGEMENT
Mark Yassa, BS, provided editing assistance.
CORRESPONDENCE
Nagy Youssef, MD, PhD, Medical College of Georgia at Augusta University, Department of Psychiatry and Health Behavior, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
1. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
2. Stubbs B, Vancampfort D, Solmi M, et al. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Aust N Z J Psychiatry. 2016;50:631-639.
3. Carta MG, Norcini-Pala A, Moro MF, et al. Does mood disorder questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord. 2015;183:173-178.
4. Fonseca-Pedrero E, Ortuno-Sierra J, Paino M, et al. Screening the risk of bipolar spectrum disorders: validity evidence of the Mood Disorder Questionnaire in adolescents and young adults. Rev Psiquiatr Salud Ment. 2016;9:4-12.
5. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
6. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790-804.
7. Aquadro E, Youssef NA. Combine these screening tools to detect bipolar depression. J Fam Pract. 2018;67:500-503.
8. Bipolar and related disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association Publishing; 2013:123.
9. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2013;73:19-32.
10. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
11. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68:1237-1245.
12. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-394.
13. Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49-58.
14. Yatham LN, Kennedy SH, Parikh S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170.
15. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495-553.
16. Kanba S, Kato T, Terao T, et al; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders, 2012. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285-300.
17. National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. Clinical Guideline CG185. September 24, 2014. www.nice.org.uk/guidance/cg185. Accessed August 17, 2020.
18. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-195.
19. Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225.
20. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. 2017;29:266-282.
21. Gabbard GO, ed. Gabbard’s Treatments of Psychiatric Disorders. 5th ed. American Psychiatric Association Publishing; 2014: chap 12-15.
22. National Health Service. Guidelines for the monitoring of antimanic and prophylactic medication in bipolar disorder. NHT policy number MM-G-023. 2009. http://fac.ksu.edu.sa/sites/default/files/bipolar_disorder_guidelines.pdf. Accessed August 17, 2020.
23. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
24. Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord. 2015;185:31-37.
25. Parker G, Tully L, Olley A, et al. SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord. 2006;92:205-214.
26. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249-1262.
27. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust N Z J Psychiatry. 2004;38:280-305.
28. Chiang K-J, Tsai J-C, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176849.
29. de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
30. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581-601.
31. Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3:95-104.
32. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118:55-59.
33. Ellis AR, Konrad TR, Thomas KC, et al. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315-1322.
34. Thomas KC, Ellis AR, Konrad TR, et al. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60:1323-1328.
35. Narasimhan M, Druss BG, Hockenberry JM, et al. Impact of a telepsychiatry program at emergency departments statewide on the quality, utilization, and costs of mental health services. Psychiatr Serv. 2015;66:1167-1172.
36. Spaulding R, Belz N, DeLurgio S, et al. Cost savings of telemedicine utilization for child psychiatry in a rural Kansas community. Telemed J E Health. 2010;16:867-871.
37. States leverage telepsychiatry solutions to ease ED crowding, accelerate care. ED Manag. 2015;27:13-17.
Bipolar disorder is a prevalent disorder in the primary care setting.1,2 Primary care providers therefore commonly encounter bipolar depression (BD; a major depressive episode in the context of bipolar disorder), which might be (1) an emerging depressive episode in previously undiagnosed bipolar disorder or (2) a recurrent episode during the course of chronic bipolar illness.3,4
A primary care–based collaborative model has been identified as a potential strategy for effective management of chronic mental health conditions such as bipolar disorder.5,6 However, this collaborative treatment model isn’t widely available; many patients with bipolar disorder are, in fact, treated solely by their primary care provider.
Two years ago in this journal,7 we addressed how to precisely identify an episode of BD and differentiate it from major depressive disorder (MDD; also known as unipolar depression). In this review, in addition to advancing clinical knowledge of BD, we provide:
- an overview of treatment options for BD (in contrast to the treatment of unipolar depression)
- the pharmacotherapeutic know-how to initiate and maintain treatment for uncomplicated episodes of BD.
We do not discuss management of manic, hypomanic, and mixed episodes of bipolar disorder.
How to identify bipolar depression
Understanding the (sometimes) unclear distinction between bipolar I and bipolar II disorders in an individual patient is key to formulating a therapeutic regimen for BD.
Bipolar I disorder consists of manic episodes, alternating (more often than not) with depressive episodes. Bipolar I usually manifests first with a depressive episode.
Bipolar II disorder manifests with depressive episodes and hypomanic episodes (but never manic episodes).
Continue to: Depressive episodes in the bipolar disorders
Depressive episodes in the bipolar disorders. Bipolar depression can be seen in the settings of both bipolar I and II disorders. When a patient presents with a manic episode, a history of depressive episodes is common (although not essential) to diagnose bipolar I; alternatively, a history of hypomania (but no prior mania) and depression is needed to make the diagnosis of bipolar II. The natural history of the bipolar disorders is therefore alternating manic and almost always depressive episodes (bipolar I) and alternating hypomanic and always depressive episodes (bipolar II).8
Symptoms of hypomanic episodes are similar to what are seen in manic episodes, but are of shorter duration (≥ 4 days [episodes of mania are at least of 1 week’s duration]), lower intensity (no psychotic symptoms), and not associated with significant functional impairment or hospitalization. Table 17 further describes the differentiating features of bipolar I and bipolar II. A history of an unequivocal manic or hypomanic episode makes the diagnosis of BD relatively easy. However, an unclear history of manic or hypomanic symptoms or episodes frequently leads to misdiagnosis or underdiagnosis of BD.
In both bipolar I and II, it is depressive symptoms and episodes that place the greatest burden on patients across the lifespan: They are the most commonly experienced features of the bipolar disorders9,10 and lead to significant distress and functional impairment11; in fact, patients with bipolar disorder spend 3 (or more) times as long in depressive episodes as in manic or hypomanic episodes.12,13 In addition, subthreshold depressive symptoms occur commonly between major mood episodes.
Failure to identify and adequately treat depressive episodes of the bipolar disorders can have serious consequences: Patients are at risk of a worsening course of illness, alcohol use disorder, substance use disorder, chronic disability, mixed states, rapid cycling of mood episodes, and suicide.
Guidelines for treating bipolar depression
Despite the similarity in presenting symptoms and signs of depressive episodes in bipolar disorders and MDD, treating episodes of BD is significantly different than treating MDD. Antidepressant monotherapy, a mainstay of treatment for MDD, has limited utility in BD (especially depressive episodes of bipolar I) because of its limited efficacy and potential to destabilize mood, lead to rapid cycling, and induce mania or hypomania. Treatment options for BD include pharmacotherapy (the primary modality), psychological intervention (a useful adjunct, described later), and electroconvulsive therapy (ECT; highly worth considering in severe or treatment-resistant cases).
Continue to: For this article...
For this article, we searched PubMed and Google Scholar for guidelines for the management of bipolar disorders in adults that were published between July 2013 (when the US Food and Drug Administration [FDA] approved lurasidone for the treatment of BD) and March 2019. Related guideline-referenced articles and clinical trials were also reviewed.
Our search identified 6 guidelines issued during the search period, developed by the:
- Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD),14
- British Association for Psychopharmacology (BAP),15
- Japanese Society of Mood Disorders (JSMD),16
- National Institute for Health and Care Excellence (NICE),17
- International College of Neuropsychopharmacology (CINP),18 and
- Royal Australian and New Zealand College of Psychiatrists.19
How to manage an episode of bipolar depression
First-line pharmacotherapeutic agents for the management of BD in acute bipolar I are listed and described in Table 2.4-19 Compared to the number of studies and reports on the management of BD in bipolar I, few studies have been conducted that specifically examine the treatment of BD in acute bipolar II. In practice, evidence from the treatment of BD in bipolar I has been extrapolated to the treatment of bipolar II depression. CANMAT–ISBD guidelines recommend quetiapine as the only first-line therapy for BD in bipolar II; JSMD, CINP, and NICE guidelines do not make distinct recommendations for treating BD in bipolar II.
Patients who have BD can present de novo (ie, not taking any medication for bipolar disorder) or with a breakthrough episode while on maintenance medication(s). In either case, monotherapy for BD is preferred, although combinations of medications (Table 214-19) can be more effective in some cases. Treatment guidelines overlap to a high degree, especially in regard to first-line treatments, but there is variation, especially beyond first-line therapeutics.20
The top recommended medications for BD are lithium, quetiapine, olanzapine, lamotrigine, and combined olanzapine/fluoxetine. FDA-approved agents for treating acute BD specifically include quetiapine, lurasidone, and combined olanzapine/fluoxetine. Guidelines generally recommend a first step of adjusting the dosage of medications in any established regimen before changing or adding other agents. If clinical improvement is not seen using any recommended medications, psychiatric referral is recommended. See Table 321,22 for dosing and titration guidance and highlights of both common and rare but serious adverse effects.
Continue to: Recommendations, best options for acute bipolar depression
Recommendations, best options for acute bipolar depression
Start with lithium, lamotrigine, quetiapine, or lurasidone as the first-line medication at the dosages given in Table 3.21,22 Olanzapine alone, or in combination with fluoxetine, can be used when it has been determined that the medications listed above are ineffective.
Note that lithium requires regular blood monitoring (Table 321,22). However, lithium has the advantage of strong supporting evidence of benefit in all mood episodes of bipolar disorders (depressive, manic, hypomanic), as well as maintenance, prevention of recurrence, and anti-suicidal properties.
Also of note: Lurasidone is much more costly than other recommended medications because it is available only by brand name in the United States; the other agents are available as generics. Consider generic equivalents of the recommended agents when cost is an important factor, in part because of the impact that cost has on medication adherence for some patients.
Last, olanzapine should be used later in the treatment algorithm, unless rapid control of symptoms is needed or other first-line medications are ineffective or not tolerated—given the higher propensity of the drug to produce weight gain and cause metabolic problems, including obesity and hyperglycemia.
The importance of maintenance therapy
Almost all patients with BD require maintenance treatment to prevent subsequent episodes, reduce residual symptoms, and restore functioning and quality of life. Maintenance therapy is formulated on the basis of efficacy and tolerability in the individual patient.
Continue to: As a general rule...
As a general rule, the strongest evidence for preventing recurrent BD episodes favors lithium—and most guidelines therefore support lithium as first-line maintenance therapy. It is important to note, however, that if a medication (or medications) successfully aborted an acute BD episode in a given patient, that agent (or agents) should be continued for maintenance purposes to prevent or minimize future episodes—generally, at the same dosage. First-line pharmacotherapeutic agents for the maintenance of bipolar disorder, and thus to prevent subsequent episodes of BD, are listed in Table 4.14-19
℞ antidepressantsin bipolar depression?
The use of antidepressants to treat BD remains a topic of ongoing deliberation. Antidepressant treatment of BD has historically raised concern for depressive relapse due to ineffectiveness and the ability of antidepressants to increase (1) the frequency of manic and hypomanic episodes23 and (2) mood instability in the form of induction of mixed states or rapid cycling. Among most authorities, the recommendation against using antidepressants for BD in both bipolar I and II is the same; however, limited evidence allows the use of antidepressant monotherapy in select cases of BD episodes in bipolar II,24,25 although not bipolar I.
The consensus in the field is that medications with mood-stabilizing effects should be considered as monotherapy before adding an antidepressant (if an antidepressant is to be added) to treat BD in bipolar II.26 In other words, if an antidepressant is to be used at all, it should be combined with a mood stabilizer or atypical antipsychotic15,27 and should probably not be used long term. The efficacy of antidepressants in treating BD in bipolar II should be assessed periodically at follow-up.
Nonpharmaceutical treatment options
Although pharmacotherapy is the mainstay of treatment of BD, adjunctive psychotherapy can be useful for treating acute BD episodes that occur during the maintenance phase of the disorder. Psychoeducation (ie, education on psychiatric illness and the importance of medication adherence), alone or in combination with interpersonal and social rhythm therapy (IPSRT), family-focused therapy (FFT), and cognitive behavioral therapy (CBT) can add to the overall efficacy of pharmacotherapy by lowering the risk of relapse and enhancing psychosocial functioning.28
IPSRT is supported by what is known as the instability model, which specifies that 3 interconnected pathways trigger recurrences of a bipolar episode: stressful life events, medication nonadherence, and social-rhythm disruption. IPSRT also uses principles of interpersonal psychotherapy that are applied in treating MDD, “arguing that improvement in interpersonal relationships can ameliorate affective symptoms and prevent their return.”29,30
Continue to: FFT
FFT focuses on communication styles between patients and their spouses and families. The goal is to improve relationship functioning. FFT is delivered to the patient and the family.
Attention to social factors. For psychotherapy to provide adequate results as an adjunct to pharmacotherapy, social stressors (eg, homelessness and financial concerns) might also need to be considered and addressed through social services or a social work consult.
NICE guidelines recommend psychological intervention (in particular, with CBT and FFT) for acute BD. CANMAT–ISBD guidelines recommend either adjunctive psychoeducation, CBT, or FFT during the maintenance phase. Again, medication is the mainstay of treatment for BD in bipolar disorders; psychotherapy has an adjunctive role—unlike the approach to treatment of MDD, in which psychotherapy can be used alone in cases of mild, or even moderate, severity.
Referral for specialty care
In the primary care setting, providers might choose to manage BD by initiating first-line pharmacotherapeutic agents or continuing established treatment regimens with necessary dosage adjustments. These patients should be monitored closely until symptoms remit.
However, it is important for the primary care provider to identify patients who need psychiatric referral. Complex presentations, severe symptoms, and poor treatment response might warrant evaluation and management by a psychiatrist. Furthermore, patients with comorbid psychotic features, catatonia, or severely debilitating depression (with or without suicidality) need referral to the emergency department.
Continue to: Electroconvulsive therapy (ECT)
Electroconvulsive therapy (ECT). Patients might also need referral to Psychiatry for ECT, which is recommended by CANMAT–ISBD and JSMD guidelines as a second-line option; by the Royal Australian and New Zealand College of Psychiatrists as a third-line option; and by BAP for cases that are resistant to conventional treatment, with or without a high risk of suicide; in pregnancy; and in life-threatening situations.15,31,32
Telemedicine. There is a considerable shortage of mental health care professionals.33,34 The fact that nearly all (96%) counties in the United States have an unmet need for prescribers of mental health services (mainly psychiatrists) makes it crucial that primary care physicians be knowledgeable and prepared to manage BD—often with infrequent psychiatry consultation or, even, without psychiatry consultation. For primary care facilities that lack access to psychiatric services, telemedicine can be used as a consultative resource.
Psychiatric consultation using telemedicine technologies has provided significant cost savings for medical centers and decreased the likelihood of hospital admission,35 thereby alleviating health care costs and improving care, as shown in a rural Kansas county study.36 Furthermore, the burden on emergency departments in several states has been significantly reduced with psychiatric consultations via interactive telemedicine technologies.37
ACKNOWLEDGEMENT
Mark Yassa, BS, provided editing assistance.
CORRESPONDENCE
Nagy Youssef, MD, PhD, Medical College of Georgia at Augusta University, Department of Psychiatry and Health Behavior, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
Bipolar disorder is a prevalent disorder in the primary care setting.1,2 Primary care providers therefore commonly encounter bipolar depression (BD; a major depressive episode in the context of bipolar disorder), which might be (1) an emerging depressive episode in previously undiagnosed bipolar disorder or (2) a recurrent episode during the course of chronic bipolar illness.3,4
A primary care–based collaborative model has been identified as a potential strategy for effective management of chronic mental health conditions such as bipolar disorder.5,6 However, this collaborative treatment model isn’t widely available; many patients with bipolar disorder are, in fact, treated solely by their primary care provider.
Two years ago in this journal,7 we addressed how to precisely identify an episode of BD and differentiate it from major depressive disorder (MDD; also known as unipolar depression). In this review, in addition to advancing clinical knowledge of BD, we provide:
- an overview of treatment options for BD (in contrast to the treatment of unipolar depression)
- the pharmacotherapeutic know-how to initiate and maintain treatment for uncomplicated episodes of BD.
We do not discuss management of manic, hypomanic, and mixed episodes of bipolar disorder.
How to identify bipolar depression
Understanding the (sometimes) unclear distinction between bipolar I and bipolar II disorders in an individual patient is key to formulating a therapeutic regimen for BD.
Bipolar I disorder consists of manic episodes, alternating (more often than not) with depressive episodes. Bipolar I usually manifests first with a depressive episode.
Bipolar II disorder manifests with depressive episodes and hypomanic episodes (but never manic episodes).
Continue to: Depressive episodes in the bipolar disorders
Depressive episodes in the bipolar disorders. Bipolar depression can be seen in the settings of both bipolar I and II disorders. When a patient presents with a manic episode, a history of depressive episodes is common (although not essential) to diagnose bipolar I; alternatively, a history of hypomania (but no prior mania) and depression is needed to make the diagnosis of bipolar II. The natural history of the bipolar disorders is therefore alternating manic and almost always depressive episodes (bipolar I) and alternating hypomanic and always depressive episodes (bipolar II).8
Symptoms of hypomanic episodes are similar to what are seen in manic episodes, but are of shorter duration (≥ 4 days [episodes of mania are at least of 1 week’s duration]), lower intensity (no psychotic symptoms), and not associated with significant functional impairment or hospitalization. Table 17 further describes the differentiating features of bipolar I and bipolar II. A history of an unequivocal manic or hypomanic episode makes the diagnosis of BD relatively easy. However, an unclear history of manic or hypomanic symptoms or episodes frequently leads to misdiagnosis or underdiagnosis of BD.
In both bipolar I and II, it is depressive symptoms and episodes that place the greatest burden on patients across the lifespan: They are the most commonly experienced features of the bipolar disorders9,10 and lead to significant distress and functional impairment11; in fact, patients with bipolar disorder spend 3 (or more) times as long in depressive episodes as in manic or hypomanic episodes.12,13 In addition, subthreshold depressive symptoms occur commonly between major mood episodes.
Failure to identify and adequately treat depressive episodes of the bipolar disorders can have serious consequences: Patients are at risk of a worsening course of illness, alcohol use disorder, substance use disorder, chronic disability, mixed states, rapid cycling of mood episodes, and suicide.
Guidelines for treating bipolar depression
Despite the similarity in presenting symptoms and signs of depressive episodes in bipolar disorders and MDD, treating episodes of BD is significantly different than treating MDD. Antidepressant monotherapy, a mainstay of treatment for MDD, has limited utility in BD (especially depressive episodes of bipolar I) because of its limited efficacy and potential to destabilize mood, lead to rapid cycling, and induce mania or hypomania. Treatment options for BD include pharmacotherapy (the primary modality), psychological intervention (a useful adjunct, described later), and electroconvulsive therapy (ECT; highly worth considering in severe or treatment-resistant cases).
Continue to: For this article...
For this article, we searched PubMed and Google Scholar for guidelines for the management of bipolar disorders in adults that were published between July 2013 (when the US Food and Drug Administration [FDA] approved lurasidone for the treatment of BD) and March 2019. Related guideline-referenced articles and clinical trials were also reviewed.
Our search identified 6 guidelines issued during the search period, developed by the:
- Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD),14
- British Association for Psychopharmacology (BAP),15
- Japanese Society of Mood Disorders (JSMD),16
- National Institute for Health and Care Excellence (NICE),17
- International College of Neuropsychopharmacology (CINP),18 and
- Royal Australian and New Zealand College of Psychiatrists.19
How to manage an episode of bipolar depression
First-line pharmacotherapeutic agents for the management of BD in acute bipolar I are listed and described in Table 2.4-19 Compared to the number of studies and reports on the management of BD in bipolar I, few studies have been conducted that specifically examine the treatment of BD in acute bipolar II. In practice, evidence from the treatment of BD in bipolar I has been extrapolated to the treatment of bipolar II depression. CANMAT–ISBD guidelines recommend quetiapine as the only first-line therapy for BD in bipolar II; JSMD, CINP, and NICE guidelines do not make distinct recommendations for treating BD in bipolar II.
Patients who have BD can present de novo (ie, not taking any medication for bipolar disorder) or with a breakthrough episode while on maintenance medication(s). In either case, monotherapy for BD is preferred, although combinations of medications (Table 214-19) can be more effective in some cases. Treatment guidelines overlap to a high degree, especially in regard to first-line treatments, but there is variation, especially beyond first-line therapeutics.20
The top recommended medications for BD are lithium, quetiapine, olanzapine, lamotrigine, and combined olanzapine/fluoxetine. FDA-approved agents for treating acute BD specifically include quetiapine, lurasidone, and combined olanzapine/fluoxetine. Guidelines generally recommend a first step of adjusting the dosage of medications in any established regimen before changing or adding other agents. If clinical improvement is not seen using any recommended medications, psychiatric referral is recommended. See Table 321,22 for dosing and titration guidance and highlights of both common and rare but serious adverse effects.
Continue to: Recommendations, best options for acute bipolar depression
Recommendations, best options for acute bipolar depression
Start with lithium, lamotrigine, quetiapine, or lurasidone as the first-line medication at the dosages given in Table 3.21,22 Olanzapine alone, or in combination with fluoxetine, can be used when it has been determined that the medications listed above are ineffective.
Note that lithium requires regular blood monitoring (Table 321,22). However, lithium has the advantage of strong supporting evidence of benefit in all mood episodes of bipolar disorders (depressive, manic, hypomanic), as well as maintenance, prevention of recurrence, and anti-suicidal properties.
Also of note: Lurasidone is much more costly than other recommended medications because it is available only by brand name in the United States; the other agents are available as generics. Consider generic equivalents of the recommended agents when cost is an important factor, in part because of the impact that cost has on medication adherence for some patients.
Last, olanzapine should be used later in the treatment algorithm, unless rapid control of symptoms is needed or other first-line medications are ineffective or not tolerated—given the higher propensity of the drug to produce weight gain and cause metabolic problems, including obesity and hyperglycemia.
The importance of maintenance therapy
Almost all patients with BD require maintenance treatment to prevent subsequent episodes, reduce residual symptoms, and restore functioning and quality of life. Maintenance therapy is formulated on the basis of efficacy and tolerability in the individual patient.
Continue to: As a general rule...
As a general rule, the strongest evidence for preventing recurrent BD episodes favors lithium—and most guidelines therefore support lithium as first-line maintenance therapy. It is important to note, however, that if a medication (or medications) successfully aborted an acute BD episode in a given patient, that agent (or agents) should be continued for maintenance purposes to prevent or minimize future episodes—generally, at the same dosage. First-line pharmacotherapeutic agents for the maintenance of bipolar disorder, and thus to prevent subsequent episodes of BD, are listed in Table 4.14-19
℞ antidepressantsin bipolar depression?
The use of antidepressants to treat BD remains a topic of ongoing deliberation. Antidepressant treatment of BD has historically raised concern for depressive relapse due to ineffectiveness and the ability of antidepressants to increase (1) the frequency of manic and hypomanic episodes23 and (2) mood instability in the form of induction of mixed states or rapid cycling. Among most authorities, the recommendation against using antidepressants for BD in both bipolar I and II is the same; however, limited evidence allows the use of antidepressant monotherapy in select cases of BD episodes in bipolar II,24,25 although not bipolar I.
The consensus in the field is that medications with mood-stabilizing effects should be considered as monotherapy before adding an antidepressant (if an antidepressant is to be added) to treat BD in bipolar II.26 In other words, if an antidepressant is to be used at all, it should be combined with a mood stabilizer or atypical antipsychotic15,27 and should probably not be used long term. The efficacy of antidepressants in treating BD in bipolar II should be assessed periodically at follow-up.
Nonpharmaceutical treatment options
Although pharmacotherapy is the mainstay of treatment of BD, adjunctive psychotherapy can be useful for treating acute BD episodes that occur during the maintenance phase of the disorder. Psychoeducation (ie, education on psychiatric illness and the importance of medication adherence), alone or in combination with interpersonal and social rhythm therapy (IPSRT), family-focused therapy (FFT), and cognitive behavioral therapy (CBT) can add to the overall efficacy of pharmacotherapy by lowering the risk of relapse and enhancing psychosocial functioning.28
IPSRT is supported by what is known as the instability model, which specifies that 3 interconnected pathways trigger recurrences of a bipolar episode: stressful life events, medication nonadherence, and social-rhythm disruption. IPSRT also uses principles of interpersonal psychotherapy that are applied in treating MDD, “arguing that improvement in interpersonal relationships can ameliorate affective symptoms and prevent their return.”29,30
Continue to: FFT
FFT focuses on communication styles between patients and their spouses and families. The goal is to improve relationship functioning. FFT is delivered to the patient and the family.
Attention to social factors. For psychotherapy to provide adequate results as an adjunct to pharmacotherapy, social stressors (eg, homelessness and financial concerns) might also need to be considered and addressed through social services or a social work consult.
NICE guidelines recommend psychological intervention (in particular, with CBT and FFT) for acute BD. CANMAT–ISBD guidelines recommend either adjunctive psychoeducation, CBT, or FFT during the maintenance phase. Again, medication is the mainstay of treatment for BD in bipolar disorders; psychotherapy has an adjunctive role—unlike the approach to treatment of MDD, in which psychotherapy can be used alone in cases of mild, or even moderate, severity.
Referral for specialty care
In the primary care setting, providers might choose to manage BD by initiating first-line pharmacotherapeutic agents or continuing established treatment regimens with necessary dosage adjustments. These patients should be monitored closely until symptoms remit.
However, it is important for the primary care provider to identify patients who need psychiatric referral. Complex presentations, severe symptoms, and poor treatment response might warrant evaluation and management by a psychiatrist. Furthermore, patients with comorbid psychotic features, catatonia, or severely debilitating depression (with or without suicidality) need referral to the emergency department.
Continue to: Electroconvulsive therapy (ECT)
Electroconvulsive therapy (ECT). Patients might also need referral to Psychiatry for ECT, which is recommended by CANMAT–ISBD and JSMD guidelines as a second-line option; by the Royal Australian and New Zealand College of Psychiatrists as a third-line option; and by BAP for cases that are resistant to conventional treatment, with or without a high risk of suicide; in pregnancy; and in life-threatening situations.15,31,32
Telemedicine. There is a considerable shortage of mental health care professionals.33,34 The fact that nearly all (96%) counties in the United States have an unmet need for prescribers of mental health services (mainly psychiatrists) makes it crucial that primary care physicians be knowledgeable and prepared to manage BD—often with infrequent psychiatry consultation or, even, without psychiatry consultation. For primary care facilities that lack access to psychiatric services, telemedicine can be used as a consultative resource.
Psychiatric consultation using telemedicine technologies has provided significant cost savings for medical centers and decreased the likelihood of hospital admission,35 thereby alleviating health care costs and improving care, as shown in a rural Kansas county study.36 Furthermore, the burden on emergency departments in several states has been significantly reduced with psychiatric consultations via interactive telemedicine technologies.37
ACKNOWLEDGEMENT
Mark Yassa, BS, provided editing assistance.
CORRESPONDENCE
Nagy Youssef, MD, PhD, Medical College of Georgia at Augusta University, Department of Psychiatry and Health Behavior, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
1. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
2. Stubbs B, Vancampfort D, Solmi M, et al. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Aust N Z J Psychiatry. 2016;50:631-639.
3. Carta MG, Norcini-Pala A, Moro MF, et al. Does mood disorder questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord. 2015;183:173-178.
4. Fonseca-Pedrero E, Ortuno-Sierra J, Paino M, et al. Screening the risk of bipolar spectrum disorders: validity evidence of the Mood Disorder Questionnaire in adolescents and young adults. Rev Psiquiatr Salud Ment. 2016;9:4-12.
5. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
6. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790-804.
7. Aquadro E, Youssef NA. Combine these screening tools to detect bipolar depression. J Fam Pract. 2018;67:500-503.
8. Bipolar and related disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association Publishing; 2013:123.
9. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2013;73:19-32.
10. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
11. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68:1237-1245.
12. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-394.
13. Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49-58.
14. Yatham LN, Kennedy SH, Parikh S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170.
15. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495-553.
16. Kanba S, Kato T, Terao T, et al; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders, 2012. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285-300.
17. National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. Clinical Guideline CG185. September 24, 2014. www.nice.org.uk/guidance/cg185. Accessed August 17, 2020.
18. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-195.
19. Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225.
20. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. 2017;29:266-282.
21. Gabbard GO, ed. Gabbard’s Treatments of Psychiatric Disorders. 5th ed. American Psychiatric Association Publishing; 2014: chap 12-15.
22. National Health Service. Guidelines for the monitoring of antimanic and prophylactic medication in bipolar disorder. NHT policy number MM-G-023. 2009. http://fac.ksu.edu.sa/sites/default/files/bipolar_disorder_guidelines.pdf. Accessed August 17, 2020.
23. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
24. Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord. 2015;185:31-37.
25. Parker G, Tully L, Olley A, et al. SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord. 2006;92:205-214.
26. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249-1262.
27. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust N Z J Psychiatry. 2004;38:280-305.
28. Chiang K-J, Tsai J-C, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176849.
29. de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
30. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581-601.
31. Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3:95-104.
32. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118:55-59.
33. Ellis AR, Konrad TR, Thomas KC, et al. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315-1322.
34. Thomas KC, Ellis AR, Konrad TR, et al. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60:1323-1328.
35. Narasimhan M, Druss BG, Hockenberry JM, et al. Impact of a telepsychiatry program at emergency departments statewide on the quality, utilization, and costs of mental health services. Psychiatr Serv. 2015;66:1167-1172.
36. Spaulding R, Belz N, DeLurgio S, et al. Cost savings of telemedicine utilization for child psychiatry in a rural Kansas community. Telemed J E Health. 2010;16:867-871.
37. States leverage telepsychiatry solutions to ease ED crowding, accelerate care. ED Manag. 2015;27:13-17.
1. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in general primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
2. Stubbs B, Vancampfort D, Solmi M, et al. How common is bipolar disorder in general primary care attendees? A systematic review and meta-analysis investigating prevalence determined according to structured clinical assessments. Aust N Z J Psychiatry. 2016;50:631-639.
3. Carta MG, Norcini-Pala A, Moro MF, et al. Does mood disorder questionnaire identify sub-threshold bipolarity? Evidence studying worsening of quality of life. J Affect Disord. 2015;183:173-178.
4. Fonseca-Pedrero E, Ortuno-Sierra J, Paino M, et al. Screening the risk of bipolar spectrum disorders: validity evidence of the Mood Disorder Questionnaire in adolescents and young adults. Rev Psiquiatr Salud Ment. 2016;9:4-12.
5. Reilly S, Planner C, Gask L, et al. Collaborative care approaches for people with severe mental illness. Cochrane Database Syst Rev. 2013;(11):CD009531.
6. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790-804.
7. Aquadro E, Youssef NA. Combine these screening tools to detect bipolar depression. J Fam Pract. 2018;67:500-503.
8. Bipolar and related disorders. In: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association Publishing; 2013:123.
9. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2013;73:19-32.
10. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
11. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68:1237-1245.
12. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-394.
13. Judd LL, Schettler PJ, Solomon DA, et al. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Affect Disord. 2008;108:49-58.
14. Yatham LN, Kennedy SH, Parikh S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97-170.
15. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495-553.
16. Kanba S, Kato T, Terao T, et al; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders, 2012. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285-300.
17. National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. Clinical Guideline CG185. September 24, 2014. www.nice.org.uk/guidance/cg185. Accessed August 17, 2020.
18. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-195.
19. Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust. 2018;208:219-225.
20. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. 2017;29:266-282.
21. Gabbard GO, ed. Gabbard’s Treatments of Psychiatric Disorders. 5th ed. American Psychiatric Association Publishing; 2014: chap 12-15.
22. National Health Service. Guidelines for the monitoring of antimanic and prophylactic medication in bipolar disorder. NHT policy number MM-G-023. 2009. http://fac.ksu.edu.sa/sites/default/files/bipolar_disorder_guidelines.pdf. Accessed August 17, 2020.
23. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653-661.
24. Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord. 2015;185:31-37.
25. Parker G, Tully L, Olley A, et al. SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord. 2006;92:205-214.
26. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249-1262.
27. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust N Z J Psychiatry. 2004;38:280-305.
28. Chiang K-J, Tsai J-C, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0176849.
29. de Mello MF, de Jesus Mari J, Bacaltchuk J, et al. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:75-82.
30. Zhou X, Teng T, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:581-601.
31. Daly JJ, Prudic J, Devanand DP, et al. ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disord. 2001;3:95-104.
32. Medda P, Perugi G, Zanello S, et al. Response to ECT in bipolar I, bipolar II and unipolar depression. J Affect Disord. 2009;118:55-59.
33. Ellis AR, Konrad TR, Thomas KC, et al. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315-1322.
34. Thomas KC, Ellis AR, Konrad TR, et al. County-level estimates of mental health professional shortage in the United States. Psychiatr Serv. 2009;60:1323-1328.
35. Narasimhan M, Druss BG, Hockenberry JM, et al. Impact of a telepsychiatry program at emergency departments statewide on the quality, utilization, and costs of mental health services. Psychiatr Serv. 2015;66:1167-1172.
36. Spaulding R, Belz N, DeLurgio S, et al. Cost savings of telemedicine utilization for child psychiatry in a rural Kansas community. Telemed J E Health. 2010;16:867-871.
37. States leverage telepsychiatry solutions to ease ED crowding, accelerate care. ED Manag. 2015;27:13-17.
PRACTICE RECOMMENDATIONS
› Become knowledgeable in identifying an episode of bipolar depression and differentiating it from major depressive disorder, so as to provide effective treatment for bipolar depression. A
› Begin treatment of bipolar depression with one of the recommended first-line medications, especially lithium, lamotrigine, quetiapine, or lurasidone. A
› Treat bipolar II depression similar to the way bipolar I depression is treated: primarily, using a mood stabilizer alone or, occasionally, using a mood stabilizer plus an antidepressant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing food allergy in children: An evidence-based update
Food allergy is a complex condition that has become a growing concern for parents and an increasing public health problem in the United States. Food allergy affects social interactions, school attendance, and quality of life, especially when associated with comorbid atopic conditions such as asthma, atopic dermatitis, and allergic rhinitis.1,2 It is the major cause of anaphylaxis in children, accounting for as many as 81% of cases.3 Societal costs of food allergy are great and are spread broadly across the health care system and the family. (See “What is the cost of food allergy?”2.)
SIDEBAR
What is the cost of food allergy?
Direct costs of food allergy to the health care system include medications, laboratory tests, office visits to primary care physicians and specialists, emergency department visits, and hospitalizations. Indirect costs include family medical and nonmedical expenses, lost work productivity, and job opportunity costs. Overall, the cost of food allergy in the United States is $24.8 billion annually—averaging $4184 for each affected child. Parents bear much of this expense.2
What a food allergy is—and isn’t
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”4 An adverse reaction to food or a food component that lacks an identified immunologic pathophysiology is not considered food allergy but is classified as food intolerance.4
Food allergy is caused by either immunoglobulin E (IgE)-mediated or non-IgE-mediated immunologic dysfunction. IgE antibodies can trigger an intense inflammatory response to certain allergens. Non-IgE-mediated food allergies are less common and not well understood.
This article focuses only on the diagnosis and management of IgE-mediated food allergy.
The culprits
More than 170 foods have been reported to cause an IgE-mediated reaction. Table 15-8 lists the 8 foods that most commonly cause allergic reactions in the United States and that account for > 50% of allergies to food.9 Studies vary in their methodology for estimating the prevalence of allergy to individual foods, but cow’s milk and peanuts appear to be the most common, each affecting as many as 2% to 2.5% of children.7,8 In general, allergies to cow’s milk and to eggs are more prevalent in very young and preschool children, whereas allergies to peanuts, tree nuts, fish, and shellfish are more prevalent in older children.10 Labels on all packaged foods regulated by the US Food and Drug Administration must declare if the product contains even a trace of these 8 allergens.
How common is food allergy?
The Centers for Disease Control and Prevention (CDC) estimates that 4% to 6% of children in the United States have a food allergy.11,12 Almost 40% of food-allergic children have a history of severe food-induced reactions.13 Other developed countries cite similar estimates of overall prevalence.14,15
However, many estimates of the prevalence of food allergy are derived from self-reports, without objective data.9 Accurate evaluation of the prevalence of food allergy is challenging because of many factors, including differences in study methodology and the definition of allergy, geographic variation, racial and ethnic variations, and dietary exposure. Parents and children often confuse nonallergic food reactions, such as food intolerance, with food allergy. Precise determination of the prevalence and natural history of food allergy at the population level requires confirmatory oral food challenges of a representative sample of infants and young children with presumed food allergy.16
Continue to: The CDC concludes that the prevalence...
The CDC concludes that the prevalence of food allergy in children younger than 18 years increased by 18% from 1997 through 2007.17,18 The cause of this increase is unclear but likely multifactorial; hypotheses include an increase in associated atopic conditions, delayed introduction of allergenic foods, and living in an overly sterile environment with reduced exposure to microbes.19 A recent population-based study of food allergy among children in Olmsted County, Minnesota, found that the incidence of food allergy increased between 2002 and 2007, stabilized subsequently, and appears to be declining among children 1 to 4 years of age, following a peak in 2006-2007.19
What are the risk factors?
Proposed risk factors for food allergy include demographics, genetics, a history of atopic disease, and environmental factors. Food allergy might be more common in boys than in girls, and in African Americans and Asians than in Whites.12,16 A child is 7 times more likely to be allergic to peanuts if a parent or sibling has peanut allergy.20 Infants and children with eczema or asthma are more likely to develop food allergy; the severity of eczema correlates with risk.12,20 Improvements in hygiene in Western societies have decreased the spread of infection, but this has been accompanied by a rise in atopic disease. In countries where health standards are poor and exposure to pathogens is greater, the prevalence of allergy is low.21
Conversely, increased microbial exposure might help protect against atopy via a pathway in which T-helper cells prevent pro-allergic immune development and keep harmless environmental exposures from becoming allergens.22 Attendance at daycare and exposure to farm animals early in life reduces the likelihood of atopic disease.16,21 The presence of a dog in the home lessens the probability of egg allergy in infants.23 Food allergy is less common in younger siblings than in first-born children, possibly due to younger siblings’ increased exposure to infection and alterations in the gut microbiome.23,24
Diagnosis: Established by presentation, positive testing
Onset of symptoms after exposure to a suspected food allergen almost always occurs within 2 hours and, typically, resolves within several hours. Symptoms should occur consistently after ingestion of the food allergen. Subsequent exposures can trigger more severe symptoms, depending on the amount, route, and duration of exposure to the allergen.25 Reactions typically follow ingestion or cutaneous exposures; inhalation rarely triggers a response.26 IgE-mediated release of histamine and other mediators from mast cells and basophils triggers reactions that typically involve one or more organ systems (Table 2).25
Cutaneous symptoms are the most common manifestations of food allergy, occurring in 70% to 80% of childhood reactions. Gastrointestinal and oral or respiratory symptoms occur in, respectively, 40% to 50% and 25% of allergic reactions to food. Cardiovascular symptoms develop in fewer than 10% of allergic reactions.26
Continue to: Anaphylaxis
Anaphylaxis is a serious allergic reaction that develops rapidly and can cause death; diagnosis is based on specific criteria (Table 3).27 Data for rates of anaphylaxis due to food allergy are limited. The incidence of fatal reaction due to food allergy is estimated to be 1 in every 800,000 children annually.3
Clinical suspicion. Food allergy should be suspected in infants and children who present with anaphylaxis or other symptoms (Table 225) that occur within minutes to hours of ingesting food.4 Parental and self-reports alone are insufficient to diagnose food allergy. NIAID guidelines recommend that patient reports of food allergy be confirmed, because multiple studies demonstrate that 50% to 90% of presumed food allergies are not true allergy.4 Health care providers must obtain a detailed medical history and pertinent family history, plus perform a physical exam and allergy sensitivity testing. Methods to help diagnose food allergies include skin-prick tests, allergen-specific serum IgE tests, and oral food challenges.4
General principles and utility of testing
Before ordering tests, it’s important to distinguish between food sensitization and food allergy and to inform the families of children with suspected food allergy about the limitations of skin-prick tests and serum IgE tests. A child with IgE antibodies specific to a food or with a positive skin-prick test, but without symptoms upon ingestion of the food, is merely sensitized; food allergy indicates the appearance of symptoms following exposure to a specific food, in addition to the detection of specific IgE antibodies or a positive skin-prick test to that same food.28
Skin-prick testing. Skin-prick tests can be performed at any age. The procedure involves pricking or scratching the surface of the skin, usually the volar aspect of the forearm or the back, with a commercial extract. Testing should be performed by a physician or other provider who is properly trained in the technique and in interpreting results. The extract contains specific allergenic proteins that activate mast cells, resulting in a characteristic wheal-and-flare response that is typically measured 15 to 20 minutes after application. Some medications, such as H1- and H2-receptor blockers and tricyclic antidepressants, can interfere with results and need to be held for 3 to 5 days before testing.
A positive skin-prick test result is defined as a wheal ≥ 3 mm larger in diameter than the negative control. The larger the size of the wheal, the higher the likelihood of a reaction to the tested food.29 Patients who exhibit dermatographism might experience a wheal-and-flare response from the action of the skin-prick test, rather than from food-specific IgE antibodies. A negative skin-prick test has > 90% negative predictive value, so the test can rule out suspected food allergy.30 However, the skin-prick test alone cannot be used to diagnose food allergy because it has a high false-positive rate.
Continue to: Allergen-specific serum IgE testing
Allergen-specific serum IgE testing. Measurement of food-specific serum IgE levels is routinely available and requires only a blood specimen. The test can be used in patients with skin disease, and results are not affected by concurrent medications. The presence of food-specific IgE indicates that the patient is sensitized to that allergen and might react upon exposure; children with a higher level of antibody are more likely to react.29
Food-specific serum IgE tests are sensitive but nonspecific for food allergy.31 Broad food-allergy test panels often yield false-positive results that can lead to unnecessary dietary elimination, resulting in years of inconvenience, nutrition problems, and needless health care expense.32
It is appropriate to order tests of specific serum IgE to foods ingested within the 2 to 3–hour window before onset of symptoms to avoid broad food allergy test panels. Like skin-prick testing, positive allergen-specific serum IgE tests alone cannot diagnose food allergy.
Oral food challenge. The double-blind, placebo-controlled oral food challenge is the gold standard for the diagnosis of food allergy. Because this test is time-consuming and technically difficult, single-blind or open food challenges are more common. Oral food challenges should be performed only by a physician or other provider who can identify and treat anaphylaxis.
The oral challenge starts with a very low dose of suspected food allergen, which is gradually increased every 15 to 30 minutes as vital signs are monitored carefully. Patients are observed for an allergic reaction for 1 hour after the final dose.
Continue to: A retrospective study...
A retrospective study showed that, whereas 19% of patients reacted during an open food challenge, only 2% required epinephrine.33 Another study showed that 89% of children whose serum IgE testing was positive for specific foods were able to reintroduce those foods into the diet after a reassuring oral food challenge.34
Other diagnostic tests. The basophil activation assay, measurement of total serum IgE, atopy patch tests, and intradermal tests have been used, but are not recommended, for making the diagnosis of food allergy.4
How can food allergy be managed?
Medical options are few. No approved treatment exists for food allergy. However, it’s important to appropriately manage acute reactions and reduce the risk of subsequent reactions.1 Parents or other caregivers can give an H1 antihistamine, such as diphenhydramine, to infants and children with acute non-life-threatening symptoms. More severe symptoms require rapid administration of epinephrine.1 Auto-injectable epinephrine should be prescribed for parents and caregivers to use as needed for emergency treatment of anaphylaxis.
Team approach. A multidisciplinary approach to managing food allergy—involving physicians, school nurses, dietitians, and teachers, and using educational materials—is ideal. This strategy expands knowledge about food allergies, enhances correct administration of epinephrine, and reduces allergic reactions.1
Avoidance of food allergens can be challenging. Parents and caregivers should be taught to interpret the list of ingredients on food packages. Self-recognition of allergic reactions reduces the likelihood of a subsequent severe allergic reaction.35
Continue to: Importance of individualized care
Importance of individualized care. Health care providers should develop personalized management plans for their patients.1 (A good place to start is with the “Food Allergy & Anaphylaxis Emergency Care Plan”a developed by Food Allergy Research & Education [FARE]). Keep in mind that children with multiple food allergies consume less calcium and protein, and tend to be shorter4; therefore, it’s wise to closely monitor growth in these children and consider referral to a dietitian who is familiar with food allergy.
Potential of immunotherapy. Current research focuses on immunotherapy to induce tolerance to food allergens and protect against life-threatening allergic reactions. The goal of immunotherapy is to lessen adverse reactions to allergenic food proteins; the strategy is to have patients repeatedly ingest small but gradually increasing doses of the food allergen over many months.36 Although immunotherapy has successfully allowed some patients to consume larger quantities of a food without having an allergic reaction, it is unknown whether immunotherapy provides permanent resolution of food allergy. In addition, immunotherapy often causes serious systemic and local reactions.1,36,37
Is prevention possible?
Maternal diet during pregnancy and lactation does not affect development of food allergy in infants.38,39 Breastfeeding might prevent development of atopic disease, but evidence is insufficient to determine whether breastfeeding reduces the likelihood of food allergy.39 In nonbreastfed infants at high risk of food allergy, extensively or partially hydrolyzed formula might help protect against food allergy, compared to standard cow’s milk formula.9,39 Feeding with soy formula rather than cow’s milk formula does not help prevent food allergy.39,40 Pregnant and breastfeeding women should not restrict their diet as a means of preventing food allergy.39
Diet in infancy. Over the years, physicians have debated the proper timing of the introduction of solid foods into the diet of infants. Traditional teaching advocated delaying introduction of potentially allergenic foods to reduce the risk of food allergy; however, this guideline was based on inconsistent evidence,41 and the strategy did not reduce the incidence of food allergy. The prevalence of food allergy is lower in developing countries where caregivers introduce foods to infants at an earlier age.20
A recent large clinical trial indicates that early introduction of peanut-containing foods can help prevent peanut allergy. The study randomized 4- to 11-month-old infants with severe eczema, egg allergy, or both, to eat or avoid peanut products until 5 years of age. Infants assigned to eat peanuts were 81% less likely to develop peanut allergy than infants in the avoidance group. Absolute risk reduction was 14% (number need to treat = 7).42 Another study showed a nonsignificant (20%) lower relative risk of food allergy in breastfed infants who were fed potentially allergenic foods starting at 3 months of age, compared to being exclusively breastfed.43
Continue to: Based on these data...
Based on these data,42,43 NIAID instituted recommendations in 2017 aimed at preventing peanut allergy44:
- In healthy infants without known food allergy and those with mild or moderate eczema, caregivers can introduce peanut-containing foods at home with other solid foods.Parents who are anxious about a possible allergic reaction can introduce peanut products in a physician’s office.
- Infants at high risk of peanut allergy (those with severe eczema or egg allergy, or both) should undergo peanut-specific IgE or skin-prick testing:
- Negative test: indicates low risk of a reaction to peanuts; the infant should start consuming peanut-containing foods at 4 to 6 months of age, at home or in a physician’s office, depending on the parents’ preference
- Positive test: Referral to an allergist is recommended.
Do children outgrow food allergy?
Approximately 85% of children who have an allergy to milk, egg, soy, or wheat outgrow their allergy; however, only 15% to 20% who have an allergy to peanuts, tree nuts, fish, or shellfish eventually tolerate these foods. The time to resolution of food allergy varies with the food, and might not occur until adolescence.4 No test reliably predicts which children develop tolerance to any given food. A decrease in the food-specific serum IgE level or a decrease in the size of the wheal on skin-prick testing might portend the onset of tolerance to the food.4
CORRESPONDENCE
Catherine M. Bettcher, MD, FAAFP, Briarwood Family Medicine, 1801 Briarwood Circle, Building #10, Ann Arbor, MI 48108; [email protected].
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al; . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008-1025.
2. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
3. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32:165-195.
4., Boyce JA, Assa’ad A, Burks WA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
5. Vierk KA, Koehler KM, Fein SB, et al. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504-1510.
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:35-50.
7. Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep. 2018;20:17.
8. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235.
9. Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856.
10. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992-1007.
11. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief No. 10. National Center for Health Statistics. October 2008. www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed August 19, 2020.
12. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798-806.e13.
13. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17.
14. Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986-988.
15. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354-359.
16. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45-59.
17. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549-1555.
18. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief No. 121. National Center for Health Statistics. May 2013. www.cdc.gov/nchs/products/databriefs/db121.htm. Accessed August 19, 2020.
19. Willits EK, Park MA, Hartz MF, et al. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93:1423-1430.
20. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331-1336.
21. Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
22. Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21 Suppl 3:2-7.
23. Prince BT, Mandel MJ, Nadeau K, et al. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479-1492.
24. Kusunoki T, Mukaida K, Morimoto T, et al. Birth order effect on childhood food allergy. Pediatr Allergy Immunol. 2012;23:250-254.
25. Abrams EM, Sicherer SH. Diagnosis and management of food allergy. CMAJ. 2016;188:1087-1093.
26. Perry TT, Matsui EC, Conover-Walker MK, et al. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164-1168.
27. Sampson HA, A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397.
28. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981-989.
29. Lieberman JA, Sicherer SH. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58-64.
30. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116-S125.
31. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76-86.
32. Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97-100.
33. Lieberman JA, Cox AL, Vitale M, et al. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128:1120-1122.
34. Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578-583.e1.
35. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111-115.
36. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
37. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016-1025.e43.
38. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133.
39. de Silva D, Geromi M, Halken S, et al; . Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581-589.
40. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004;(3):CD003741.
41. Filipiak B, Zutavern A, Koletzko S, et al; GINI-Group. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151:352-358.
42. Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
43. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
44. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
Food allergy is a complex condition that has become a growing concern for parents and an increasing public health problem in the United States. Food allergy affects social interactions, school attendance, and quality of life, especially when associated with comorbid atopic conditions such as asthma, atopic dermatitis, and allergic rhinitis.1,2 It is the major cause of anaphylaxis in children, accounting for as many as 81% of cases.3 Societal costs of food allergy are great and are spread broadly across the health care system and the family. (See “What is the cost of food allergy?”2.)
SIDEBAR
What is the cost of food allergy?
Direct costs of food allergy to the health care system include medications, laboratory tests, office visits to primary care physicians and specialists, emergency department visits, and hospitalizations. Indirect costs include family medical and nonmedical expenses, lost work productivity, and job opportunity costs. Overall, the cost of food allergy in the United States is $24.8 billion annually—averaging $4184 for each affected child. Parents bear much of this expense.2
What a food allergy is—and isn’t
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”4 An adverse reaction to food or a food component that lacks an identified immunologic pathophysiology is not considered food allergy but is classified as food intolerance.4
Food allergy is caused by either immunoglobulin E (IgE)-mediated or non-IgE-mediated immunologic dysfunction. IgE antibodies can trigger an intense inflammatory response to certain allergens. Non-IgE-mediated food allergies are less common and not well understood.
This article focuses only on the diagnosis and management of IgE-mediated food allergy.
The culprits
More than 170 foods have been reported to cause an IgE-mediated reaction. Table 15-8 lists the 8 foods that most commonly cause allergic reactions in the United States and that account for > 50% of allergies to food.9 Studies vary in their methodology for estimating the prevalence of allergy to individual foods, but cow’s milk and peanuts appear to be the most common, each affecting as many as 2% to 2.5% of children.7,8 In general, allergies to cow’s milk and to eggs are more prevalent in very young and preschool children, whereas allergies to peanuts, tree nuts, fish, and shellfish are more prevalent in older children.10 Labels on all packaged foods regulated by the US Food and Drug Administration must declare if the product contains even a trace of these 8 allergens.
How common is food allergy?
The Centers for Disease Control and Prevention (CDC) estimates that 4% to 6% of children in the United States have a food allergy.11,12 Almost 40% of food-allergic children have a history of severe food-induced reactions.13 Other developed countries cite similar estimates of overall prevalence.14,15
However, many estimates of the prevalence of food allergy are derived from self-reports, without objective data.9 Accurate evaluation of the prevalence of food allergy is challenging because of many factors, including differences in study methodology and the definition of allergy, geographic variation, racial and ethnic variations, and dietary exposure. Parents and children often confuse nonallergic food reactions, such as food intolerance, with food allergy. Precise determination of the prevalence and natural history of food allergy at the population level requires confirmatory oral food challenges of a representative sample of infants and young children with presumed food allergy.16
Continue to: The CDC concludes that the prevalence...
The CDC concludes that the prevalence of food allergy in children younger than 18 years increased by 18% from 1997 through 2007.17,18 The cause of this increase is unclear but likely multifactorial; hypotheses include an increase in associated atopic conditions, delayed introduction of allergenic foods, and living in an overly sterile environment with reduced exposure to microbes.19 A recent population-based study of food allergy among children in Olmsted County, Minnesota, found that the incidence of food allergy increased between 2002 and 2007, stabilized subsequently, and appears to be declining among children 1 to 4 years of age, following a peak in 2006-2007.19
What are the risk factors?
Proposed risk factors for food allergy include demographics, genetics, a history of atopic disease, and environmental factors. Food allergy might be more common in boys than in girls, and in African Americans and Asians than in Whites.12,16 A child is 7 times more likely to be allergic to peanuts if a parent or sibling has peanut allergy.20 Infants and children with eczema or asthma are more likely to develop food allergy; the severity of eczema correlates with risk.12,20 Improvements in hygiene in Western societies have decreased the spread of infection, but this has been accompanied by a rise in atopic disease. In countries where health standards are poor and exposure to pathogens is greater, the prevalence of allergy is low.21
Conversely, increased microbial exposure might help protect against atopy via a pathway in which T-helper cells prevent pro-allergic immune development and keep harmless environmental exposures from becoming allergens.22 Attendance at daycare and exposure to farm animals early in life reduces the likelihood of atopic disease.16,21 The presence of a dog in the home lessens the probability of egg allergy in infants.23 Food allergy is less common in younger siblings than in first-born children, possibly due to younger siblings’ increased exposure to infection and alterations in the gut microbiome.23,24
Diagnosis: Established by presentation, positive testing
Onset of symptoms after exposure to a suspected food allergen almost always occurs within 2 hours and, typically, resolves within several hours. Symptoms should occur consistently after ingestion of the food allergen. Subsequent exposures can trigger more severe symptoms, depending on the amount, route, and duration of exposure to the allergen.25 Reactions typically follow ingestion or cutaneous exposures; inhalation rarely triggers a response.26 IgE-mediated release of histamine and other mediators from mast cells and basophils triggers reactions that typically involve one or more organ systems (Table 2).25
Cutaneous symptoms are the most common manifestations of food allergy, occurring in 70% to 80% of childhood reactions. Gastrointestinal and oral or respiratory symptoms occur in, respectively, 40% to 50% and 25% of allergic reactions to food. Cardiovascular symptoms develop in fewer than 10% of allergic reactions.26
Continue to: Anaphylaxis
Anaphylaxis is a serious allergic reaction that develops rapidly and can cause death; diagnosis is based on specific criteria (Table 3).27 Data for rates of anaphylaxis due to food allergy are limited. The incidence of fatal reaction due to food allergy is estimated to be 1 in every 800,000 children annually.3
Clinical suspicion. Food allergy should be suspected in infants and children who present with anaphylaxis or other symptoms (Table 225) that occur within minutes to hours of ingesting food.4 Parental and self-reports alone are insufficient to diagnose food allergy. NIAID guidelines recommend that patient reports of food allergy be confirmed, because multiple studies demonstrate that 50% to 90% of presumed food allergies are not true allergy.4 Health care providers must obtain a detailed medical history and pertinent family history, plus perform a physical exam and allergy sensitivity testing. Methods to help diagnose food allergies include skin-prick tests, allergen-specific serum IgE tests, and oral food challenges.4
General principles and utility of testing
Before ordering tests, it’s important to distinguish between food sensitization and food allergy and to inform the families of children with suspected food allergy about the limitations of skin-prick tests and serum IgE tests. A child with IgE antibodies specific to a food or with a positive skin-prick test, but without symptoms upon ingestion of the food, is merely sensitized; food allergy indicates the appearance of symptoms following exposure to a specific food, in addition to the detection of specific IgE antibodies or a positive skin-prick test to that same food.28
Skin-prick testing. Skin-prick tests can be performed at any age. The procedure involves pricking or scratching the surface of the skin, usually the volar aspect of the forearm or the back, with a commercial extract. Testing should be performed by a physician or other provider who is properly trained in the technique and in interpreting results. The extract contains specific allergenic proteins that activate mast cells, resulting in a characteristic wheal-and-flare response that is typically measured 15 to 20 minutes after application. Some medications, such as H1- and H2-receptor blockers and tricyclic antidepressants, can interfere with results and need to be held for 3 to 5 days before testing.
A positive skin-prick test result is defined as a wheal ≥ 3 mm larger in diameter than the negative control. The larger the size of the wheal, the higher the likelihood of a reaction to the tested food.29 Patients who exhibit dermatographism might experience a wheal-and-flare response from the action of the skin-prick test, rather than from food-specific IgE antibodies. A negative skin-prick test has > 90% negative predictive value, so the test can rule out suspected food allergy.30 However, the skin-prick test alone cannot be used to diagnose food allergy because it has a high false-positive rate.
Continue to: Allergen-specific serum IgE testing
Allergen-specific serum IgE testing. Measurement of food-specific serum IgE levels is routinely available and requires only a blood specimen. The test can be used in patients with skin disease, and results are not affected by concurrent medications. The presence of food-specific IgE indicates that the patient is sensitized to that allergen and might react upon exposure; children with a higher level of antibody are more likely to react.29
Food-specific serum IgE tests are sensitive but nonspecific for food allergy.31 Broad food-allergy test panels often yield false-positive results that can lead to unnecessary dietary elimination, resulting in years of inconvenience, nutrition problems, and needless health care expense.32
It is appropriate to order tests of specific serum IgE to foods ingested within the 2 to 3–hour window before onset of symptoms to avoid broad food allergy test panels. Like skin-prick testing, positive allergen-specific serum IgE tests alone cannot diagnose food allergy.
Oral food challenge. The double-blind, placebo-controlled oral food challenge is the gold standard for the diagnosis of food allergy. Because this test is time-consuming and technically difficult, single-blind or open food challenges are more common. Oral food challenges should be performed only by a physician or other provider who can identify and treat anaphylaxis.
The oral challenge starts with a very low dose of suspected food allergen, which is gradually increased every 15 to 30 minutes as vital signs are monitored carefully. Patients are observed for an allergic reaction for 1 hour after the final dose.
Continue to: A retrospective study...
A retrospective study showed that, whereas 19% of patients reacted during an open food challenge, only 2% required epinephrine.33 Another study showed that 89% of children whose serum IgE testing was positive for specific foods were able to reintroduce those foods into the diet after a reassuring oral food challenge.34
Other diagnostic tests. The basophil activation assay, measurement of total serum IgE, atopy patch tests, and intradermal tests have been used, but are not recommended, for making the diagnosis of food allergy.4
How can food allergy be managed?
Medical options are few. No approved treatment exists for food allergy. However, it’s important to appropriately manage acute reactions and reduce the risk of subsequent reactions.1 Parents or other caregivers can give an H1 antihistamine, such as diphenhydramine, to infants and children with acute non-life-threatening symptoms. More severe symptoms require rapid administration of epinephrine.1 Auto-injectable epinephrine should be prescribed for parents and caregivers to use as needed for emergency treatment of anaphylaxis.
Team approach. A multidisciplinary approach to managing food allergy—involving physicians, school nurses, dietitians, and teachers, and using educational materials—is ideal. This strategy expands knowledge about food allergies, enhances correct administration of epinephrine, and reduces allergic reactions.1
Avoidance of food allergens can be challenging. Parents and caregivers should be taught to interpret the list of ingredients on food packages. Self-recognition of allergic reactions reduces the likelihood of a subsequent severe allergic reaction.35
Continue to: Importance of individualized care
Importance of individualized care. Health care providers should develop personalized management plans for their patients.1 (A good place to start is with the “Food Allergy & Anaphylaxis Emergency Care Plan”a developed by Food Allergy Research & Education [FARE]). Keep in mind that children with multiple food allergies consume less calcium and protein, and tend to be shorter4; therefore, it’s wise to closely monitor growth in these children and consider referral to a dietitian who is familiar with food allergy.
Potential of immunotherapy. Current research focuses on immunotherapy to induce tolerance to food allergens and protect against life-threatening allergic reactions. The goal of immunotherapy is to lessen adverse reactions to allergenic food proteins; the strategy is to have patients repeatedly ingest small but gradually increasing doses of the food allergen over many months.36 Although immunotherapy has successfully allowed some patients to consume larger quantities of a food without having an allergic reaction, it is unknown whether immunotherapy provides permanent resolution of food allergy. In addition, immunotherapy often causes serious systemic and local reactions.1,36,37
Is prevention possible?
Maternal diet during pregnancy and lactation does not affect development of food allergy in infants.38,39 Breastfeeding might prevent development of atopic disease, but evidence is insufficient to determine whether breastfeeding reduces the likelihood of food allergy.39 In nonbreastfed infants at high risk of food allergy, extensively or partially hydrolyzed formula might help protect against food allergy, compared to standard cow’s milk formula.9,39 Feeding with soy formula rather than cow’s milk formula does not help prevent food allergy.39,40 Pregnant and breastfeeding women should not restrict their diet as a means of preventing food allergy.39
Diet in infancy. Over the years, physicians have debated the proper timing of the introduction of solid foods into the diet of infants. Traditional teaching advocated delaying introduction of potentially allergenic foods to reduce the risk of food allergy; however, this guideline was based on inconsistent evidence,41 and the strategy did not reduce the incidence of food allergy. The prevalence of food allergy is lower in developing countries where caregivers introduce foods to infants at an earlier age.20
A recent large clinical trial indicates that early introduction of peanut-containing foods can help prevent peanut allergy. The study randomized 4- to 11-month-old infants with severe eczema, egg allergy, or both, to eat or avoid peanut products until 5 years of age. Infants assigned to eat peanuts were 81% less likely to develop peanut allergy than infants in the avoidance group. Absolute risk reduction was 14% (number need to treat = 7).42 Another study showed a nonsignificant (20%) lower relative risk of food allergy in breastfed infants who were fed potentially allergenic foods starting at 3 months of age, compared to being exclusively breastfed.43
Continue to: Based on these data...
Based on these data,42,43 NIAID instituted recommendations in 2017 aimed at preventing peanut allergy44:
- In healthy infants without known food allergy and those with mild or moderate eczema, caregivers can introduce peanut-containing foods at home with other solid foods.Parents who are anxious about a possible allergic reaction can introduce peanut products in a physician’s office.
- Infants at high risk of peanut allergy (those with severe eczema or egg allergy, or both) should undergo peanut-specific IgE or skin-prick testing:
- Negative test: indicates low risk of a reaction to peanuts; the infant should start consuming peanut-containing foods at 4 to 6 months of age, at home or in a physician’s office, depending on the parents’ preference
- Positive test: Referral to an allergist is recommended.
Do children outgrow food allergy?
Approximately 85% of children who have an allergy to milk, egg, soy, or wheat outgrow their allergy; however, only 15% to 20% who have an allergy to peanuts, tree nuts, fish, or shellfish eventually tolerate these foods. The time to resolution of food allergy varies with the food, and might not occur until adolescence.4 No test reliably predicts which children develop tolerance to any given food. A decrease in the food-specific serum IgE level or a decrease in the size of the wheal on skin-prick testing might portend the onset of tolerance to the food.4
CORRESPONDENCE
Catherine M. Bettcher, MD, FAAFP, Briarwood Family Medicine, 1801 Briarwood Circle, Building #10, Ann Arbor, MI 48108; [email protected].
Food allergy is a complex condition that has become a growing concern for parents and an increasing public health problem in the United States. Food allergy affects social interactions, school attendance, and quality of life, especially when associated with comorbid atopic conditions such as asthma, atopic dermatitis, and allergic rhinitis.1,2 It is the major cause of anaphylaxis in children, accounting for as many as 81% of cases.3 Societal costs of food allergy are great and are spread broadly across the health care system and the family. (See “What is the cost of food allergy?”2.)
SIDEBAR
What is the cost of food allergy?
Direct costs of food allergy to the health care system include medications, laboratory tests, office visits to primary care physicians and specialists, emergency department visits, and hospitalizations. Indirect costs include family medical and nonmedical expenses, lost work productivity, and job opportunity costs. Overall, the cost of food allergy in the United States is $24.8 billion annually—averaging $4184 for each affected child. Parents bear much of this expense.2
What a food allergy is—and isn’t
The National Institute of Allergy and Infectious Diseases (NIAID) defines food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.”4 An adverse reaction to food or a food component that lacks an identified immunologic pathophysiology is not considered food allergy but is classified as food intolerance.4
Food allergy is caused by either immunoglobulin E (IgE)-mediated or non-IgE-mediated immunologic dysfunction. IgE antibodies can trigger an intense inflammatory response to certain allergens. Non-IgE-mediated food allergies are less common and not well understood.
This article focuses only on the diagnosis and management of IgE-mediated food allergy.
The culprits
More than 170 foods have been reported to cause an IgE-mediated reaction. Table 15-8 lists the 8 foods that most commonly cause allergic reactions in the United States and that account for > 50% of allergies to food.9 Studies vary in their methodology for estimating the prevalence of allergy to individual foods, but cow’s milk and peanuts appear to be the most common, each affecting as many as 2% to 2.5% of children.7,8 In general, allergies to cow’s milk and to eggs are more prevalent in very young and preschool children, whereas allergies to peanuts, tree nuts, fish, and shellfish are more prevalent in older children.10 Labels on all packaged foods regulated by the US Food and Drug Administration must declare if the product contains even a trace of these 8 allergens.
How common is food allergy?
The Centers for Disease Control and Prevention (CDC) estimates that 4% to 6% of children in the United States have a food allergy.11,12 Almost 40% of food-allergic children have a history of severe food-induced reactions.13 Other developed countries cite similar estimates of overall prevalence.14,15
However, many estimates of the prevalence of food allergy are derived from self-reports, without objective data.9 Accurate evaluation of the prevalence of food allergy is challenging because of many factors, including differences in study methodology and the definition of allergy, geographic variation, racial and ethnic variations, and dietary exposure. Parents and children often confuse nonallergic food reactions, such as food intolerance, with food allergy. Precise determination of the prevalence and natural history of food allergy at the population level requires confirmatory oral food challenges of a representative sample of infants and young children with presumed food allergy.16
Continue to: The CDC concludes that the prevalence...
The CDC concludes that the prevalence of food allergy in children younger than 18 years increased by 18% from 1997 through 2007.17,18 The cause of this increase is unclear but likely multifactorial; hypotheses include an increase in associated atopic conditions, delayed introduction of allergenic foods, and living in an overly sterile environment with reduced exposure to microbes.19 A recent population-based study of food allergy among children in Olmsted County, Minnesota, found that the incidence of food allergy increased between 2002 and 2007, stabilized subsequently, and appears to be declining among children 1 to 4 years of age, following a peak in 2006-2007.19
What are the risk factors?
Proposed risk factors for food allergy include demographics, genetics, a history of atopic disease, and environmental factors. Food allergy might be more common in boys than in girls, and in African Americans and Asians than in Whites.12,16 A child is 7 times more likely to be allergic to peanuts if a parent or sibling has peanut allergy.20 Infants and children with eczema or asthma are more likely to develop food allergy; the severity of eczema correlates with risk.12,20 Improvements in hygiene in Western societies have decreased the spread of infection, but this has been accompanied by a rise in atopic disease. In countries where health standards are poor and exposure to pathogens is greater, the prevalence of allergy is low.21
Conversely, increased microbial exposure might help protect against atopy via a pathway in which T-helper cells prevent pro-allergic immune development and keep harmless environmental exposures from becoming allergens.22 Attendance at daycare and exposure to farm animals early in life reduces the likelihood of atopic disease.16,21 The presence of a dog in the home lessens the probability of egg allergy in infants.23 Food allergy is less common in younger siblings than in first-born children, possibly due to younger siblings’ increased exposure to infection and alterations in the gut microbiome.23,24
Diagnosis: Established by presentation, positive testing
Onset of symptoms after exposure to a suspected food allergen almost always occurs within 2 hours and, typically, resolves within several hours. Symptoms should occur consistently after ingestion of the food allergen. Subsequent exposures can trigger more severe symptoms, depending on the amount, route, and duration of exposure to the allergen.25 Reactions typically follow ingestion or cutaneous exposures; inhalation rarely triggers a response.26 IgE-mediated release of histamine and other mediators from mast cells and basophils triggers reactions that typically involve one or more organ systems (Table 2).25
Cutaneous symptoms are the most common manifestations of food allergy, occurring in 70% to 80% of childhood reactions. Gastrointestinal and oral or respiratory symptoms occur in, respectively, 40% to 50% and 25% of allergic reactions to food. Cardiovascular symptoms develop in fewer than 10% of allergic reactions.26
Continue to: Anaphylaxis
Anaphylaxis is a serious allergic reaction that develops rapidly and can cause death; diagnosis is based on specific criteria (Table 3).27 Data for rates of anaphylaxis due to food allergy are limited. The incidence of fatal reaction due to food allergy is estimated to be 1 in every 800,000 children annually.3
Clinical suspicion. Food allergy should be suspected in infants and children who present with anaphylaxis or other symptoms (Table 225) that occur within minutes to hours of ingesting food.4 Parental and self-reports alone are insufficient to diagnose food allergy. NIAID guidelines recommend that patient reports of food allergy be confirmed, because multiple studies demonstrate that 50% to 90% of presumed food allergies are not true allergy.4 Health care providers must obtain a detailed medical history and pertinent family history, plus perform a physical exam and allergy sensitivity testing. Methods to help diagnose food allergies include skin-prick tests, allergen-specific serum IgE tests, and oral food challenges.4
General principles and utility of testing
Before ordering tests, it’s important to distinguish between food sensitization and food allergy and to inform the families of children with suspected food allergy about the limitations of skin-prick tests and serum IgE tests. A child with IgE antibodies specific to a food or with a positive skin-prick test, but without symptoms upon ingestion of the food, is merely sensitized; food allergy indicates the appearance of symptoms following exposure to a specific food, in addition to the detection of specific IgE antibodies or a positive skin-prick test to that same food.28
Skin-prick testing. Skin-prick tests can be performed at any age. The procedure involves pricking or scratching the surface of the skin, usually the volar aspect of the forearm or the back, with a commercial extract. Testing should be performed by a physician or other provider who is properly trained in the technique and in interpreting results. The extract contains specific allergenic proteins that activate mast cells, resulting in a characteristic wheal-and-flare response that is typically measured 15 to 20 minutes after application. Some medications, such as H1- and H2-receptor blockers and tricyclic antidepressants, can interfere with results and need to be held for 3 to 5 days before testing.
A positive skin-prick test result is defined as a wheal ≥ 3 mm larger in diameter than the negative control. The larger the size of the wheal, the higher the likelihood of a reaction to the tested food.29 Patients who exhibit dermatographism might experience a wheal-and-flare response from the action of the skin-prick test, rather than from food-specific IgE antibodies. A negative skin-prick test has > 90% negative predictive value, so the test can rule out suspected food allergy.30 However, the skin-prick test alone cannot be used to diagnose food allergy because it has a high false-positive rate.
Continue to: Allergen-specific serum IgE testing
Allergen-specific serum IgE testing. Measurement of food-specific serum IgE levels is routinely available and requires only a blood specimen. The test can be used in patients with skin disease, and results are not affected by concurrent medications. The presence of food-specific IgE indicates that the patient is sensitized to that allergen and might react upon exposure; children with a higher level of antibody are more likely to react.29
Food-specific serum IgE tests are sensitive but nonspecific for food allergy.31 Broad food-allergy test panels often yield false-positive results that can lead to unnecessary dietary elimination, resulting in years of inconvenience, nutrition problems, and needless health care expense.32
It is appropriate to order tests of specific serum IgE to foods ingested within the 2 to 3–hour window before onset of symptoms to avoid broad food allergy test panels. Like skin-prick testing, positive allergen-specific serum IgE tests alone cannot diagnose food allergy.
Oral food challenge. The double-blind, placebo-controlled oral food challenge is the gold standard for the diagnosis of food allergy. Because this test is time-consuming and technically difficult, single-blind or open food challenges are more common. Oral food challenges should be performed only by a physician or other provider who can identify and treat anaphylaxis.
The oral challenge starts with a very low dose of suspected food allergen, which is gradually increased every 15 to 30 minutes as vital signs are monitored carefully. Patients are observed for an allergic reaction for 1 hour after the final dose.
Continue to: A retrospective study...
A retrospective study showed that, whereas 19% of patients reacted during an open food challenge, only 2% required epinephrine.33 Another study showed that 89% of children whose serum IgE testing was positive for specific foods were able to reintroduce those foods into the diet after a reassuring oral food challenge.34
Other diagnostic tests. The basophil activation assay, measurement of total serum IgE, atopy patch tests, and intradermal tests have been used, but are not recommended, for making the diagnosis of food allergy.4
How can food allergy be managed?
Medical options are few. No approved treatment exists for food allergy. However, it’s important to appropriately manage acute reactions and reduce the risk of subsequent reactions.1 Parents or other caregivers can give an H1 antihistamine, such as diphenhydramine, to infants and children with acute non-life-threatening symptoms. More severe symptoms require rapid administration of epinephrine.1 Auto-injectable epinephrine should be prescribed for parents and caregivers to use as needed for emergency treatment of anaphylaxis.
Team approach. A multidisciplinary approach to managing food allergy—involving physicians, school nurses, dietitians, and teachers, and using educational materials—is ideal. This strategy expands knowledge about food allergies, enhances correct administration of epinephrine, and reduces allergic reactions.1
Avoidance of food allergens can be challenging. Parents and caregivers should be taught to interpret the list of ingredients on food packages. Self-recognition of allergic reactions reduces the likelihood of a subsequent severe allergic reaction.35
Continue to: Importance of individualized care
Importance of individualized care. Health care providers should develop personalized management plans for their patients.1 (A good place to start is with the “Food Allergy & Anaphylaxis Emergency Care Plan”a developed by Food Allergy Research & Education [FARE]). Keep in mind that children with multiple food allergies consume less calcium and protein, and tend to be shorter4; therefore, it’s wise to closely monitor growth in these children and consider referral to a dietitian who is familiar with food allergy.
Potential of immunotherapy. Current research focuses on immunotherapy to induce tolerance to food allergens and protect against life-threatening allergic reactions. The goal of immunotherapy is to lessen adverse reactions to allergenic food proteins; the strategy is to have patients repeatedly ingest small but gradually increasing doses of the food allergen over many months.36 Although immunotherapy has successfully allowed some patients to consume larger quantities of a food without having an allergic reaction, it is unknown whether immunotherapy provides permanent resolution of food allergy. In addition, immunotherapy often causes serious systemic and local reactions.1,36,37
Is prevention possible?
Maternal diet during pregnancy and lactation does not affect development of food allergy in infants.38,39 Breastfeeding might prevent development of atopic disease, but evidence is insufficient to determine whether breastfeeding reduces the likelihood of food allergy.39 In nonbreastfed infants at high risk of food allergy, extensively or partially hydrolyzed formula might help protect against food allergy, compared to standard cow’s milk formula.9,39 Feeding with soy formula rather than cow’s milk formula does not help prevent food allergy.39,40 Pregnant and breastfeeding women should not restrict their diet as a means of preventing food allergy.39
Diet in infancy. Over the years, physicians have debated the proper timing of the introduction of solid foods into the diet of infants. Traditional teaching advocated delaying introduction of potentially allergenic foods to reduce the risk of food allergy; however, this guideline was based on inconsistent evidence,41 and the strategy did not reduce the incidence of food allergy. The prevalence of food allergy is lower in developing countries where caregivers introduce foods to infants at an earlier age.20
A recent large clinical trial indicates that early introduction of peanut-containing foods can help prevent peanut allergy. The study randomized 4- to 11-month-old infants with severe eczema, egg allergy, or both, to eat or avoid peanut products until 5 years of age. Infants assigned to eat peanuts were 81% less likely to develop peanut allergy than infants in the avoidance group. Absolute risk reduction was 14% (number need to treat = 7).42 Another study showed a nonsignificant (20%) lower relative risk of food allergy in breastfed infants who were fed potentially allergenic foods starting at 3 months of age, compared to being exclusively breastfed.43
Continue to: Based on these data...
Based on these data,42,43 NIAID instituted recommendations in 2017 aimed at preventing peanut allergy44:
- In healthy infants without known food allergy and those with mild or moderate eczema, caregivers can introduce peanut-containing foods at home with other solid foods.Parents who are anxious about a possible allergic reaction can introduce peanut products in a physician’s office.
- Infants at high risk of peanut allergy (those with severe eczema or egg allergy, or both) should undergo peanut-specific IgE or skin-prick testing:
- Negative test: indicates low risk of a reaction to peanuts; the infant should start consuming peanut-containing foods at 4 to 6 months of age, at home or in a physician’s office, depending on the parents’ preference
- Positive test: Referral to an allergist is recommended.
Do children outgrow food allergy?
Approximately 85% of children who have an allergy to milk, egg, soy, or wheat outgrow their allergy; however, only 15% to 20% who have an allergy to peanuts, tree nuts, fish, or shellfish eventually tolerate these foods. The time to resolution of food allergy varies with the food, and might not occur until adolescence.4 No test reliably predicts which children develop tolerance to any given food. A decrease in the food-specific serum IgE level or a decrease in the size of the wheal on skin-prick testing might portend the onset of tolerance to the food.4
CORRESPONDENCE
Catherine M. Bettcher, MD, FAAFP, Briarwood Family Medicine, 1801 Briarwood Circle, Building #10, Ann Arbor, MI 48108; [email protected].
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al; . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008-1025.
2. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
3. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32:165-195.
4., Boyce JA, Assa’ad A, Burks WA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
5. Vierk KA, Koehler KM, Fein SB, et al. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504-1510.
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:35-50.
7. Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep. 2018;20:17.
8. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235.
9. Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856.
10. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992-1007.
11. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief No. 10. National Center for Health Statistics. October 2008. www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed August 19, 2020.
12. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798-806.e13.
13. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17.
14. Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986-988.
15. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354-359.
16. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45-59.
17. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549-1555.
18. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief No. 121. National Center for Health Statistics. May 2013. www.cdc.gov/nchs/products/databriefs/db121.htm. Accessed August 19, 2020.
19. Willits EK, Park MA, Hartz MF, et al. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93:1423-1430.
20. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331-1336.
21. Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
22. Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21 Suppl 3:2-7.
23. Prince BT, Mandel MJ, Nadeau K, et al. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479-1492.
24. Kusunoki T, Mukaida K, Morimoto T, et al. Birth order effect on childhood food allergy. Pediatr Allergy Immunol. 2012;23:250-254.
25. Abrams EM, Sicherer SH. Diagnosis and management of food allergy. CMAJ. 2016;188:1087-1093.
26. Perry TT, Matsui EC, Conover-Walker MK, et al. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164-1168.
27. Sampson HA, A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397.
28. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981-989.
29. Lieberman JA, Sicherer SH. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58-64.
30. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116-S125.
31. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76-86.
32. Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97-100.
33. Lieberman JA, Cox AL, Vitale M, et al. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128:1120-1122.
34. Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578-583.e1.
35. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111-115.
36. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
37. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016-1025.e43.
38. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133.
39. de Silva D, Geromi M, Halken S, et al; . Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581-589.
40. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004;(3):CD003741.
41. Filipiak B, Zutavern A, Koletzko S, et al; GINI-Group. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151:352-358.
42. Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
43. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
44. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
1. Muraro A, Werfel T, Hoffmann-Sommergruber K, et al; . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008-1025.
2. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
3. Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32:165-195.
4., Boyce JA, Assa’ad A, Burks WA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
5. Vierk KA, Koehler KM, Fein SB, et al. Prevalence of self-reported food allergy in American adults and use of food labels. J Allergy Clin Immunol. 2007;119:1504-1510.
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. 2012;32:35-50.
7. Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep. 2018;20:17.
8. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235.
9. Chafen JJS, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848-1856.
10. Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992-1007.
11. Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief No. 10. National Center for Health Statistics. October 2008. www.cdc.gov/nchs/products/databriefs/db10.htm. Accessed August 19, 2020.
12. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798-806.e13.
13. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9-e17.
14. Soller L, Ben-Shoshan M, Harrington DW, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986-988.
15. Venter C, Pereira B, Voigt K, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354-359.
16. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35:45-59.
17. Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549-1555.
18. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief No. 121. National Center for Health Statistics. May 2013. www.cdc.gov/nchs/products/databriefs/db121.htm. Accessed August 19, 2020.
19. Willits EK, Park MA, Hartz MF, et al. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93:1423-1430.
20. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331-1336.
21. Okada H, Kuhn C, Feillet H, et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1-9.
22. Liu AH. Hygiene theory and allergy and asthma prevention. Paediatr Perinat Epidemiol. 2007;21 Suppl 3:2-7.
23. Prince BT, Mandel MJ, Nadeau K, et al. Gut microbiome and the development of food allergy and allergic disease. Pediatr Clin North Am. 2015;62:1479-1492.
24. Kusunoki T, Mukaida K, Morimoto T, et al. Birth order effect on childhood food allergy. Pediatr Allergy Immunol. 2012;23:250-254.
25. Abrams EM, Sicherer SH. Diagnosis and management of food allergy. CMAJ. 2016;188:1087-1093.
26. Perry TT, Matsui EC, Conover-Walker MK, et al. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164-1168.
27. Sampson HA, A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391-397.
28. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981-989.
29. Lieberman JA, Sicherer SH. Diagnosis of food allergy: epicutaneous skin tests, in vitro tests, and oral food challenge. Curr Allergy Asthma Rep. 2011;11:58-64.
30. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 suppl 2):S116-S125.
31. Soares-Weiser K, Takwoingi Y, Panesar SS, et al. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76-86.
32. Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr. 2015;166:97-100.
33. Lieberman JA, Cox AL, Vitale M, et al. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128:1120-1122.
34. Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578-583.e1.
35. Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet. 2001;357:111-115.
36. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
37. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update—2014. J Allergy Clin Immunol. 2014;134:1016-1025.e43.
38. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133.
39. de Silva D, Geromi M, Halken S, et al; . Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69:581-589.
40. Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004;(3):CD003741.
41. Filipiak B, Zutavern A, Koletzko S, et al; GINI-Group. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr. 2007;151:352-358.
42. Du Toit G, Roberts G, Sayre PH, et al; LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
43. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
44. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
PRACTICE RECOMMENDATIONS
› Diagnose food allergy based on a convincing clinical history paired with positive diagnostic testing. A
› Use a multidisciplinary approach to improve caregiver and patient understanding of food allergy and to reduce allergic reactions. B
› Recommend early introduction of peanut products to infants to reduce the likelihood of peanut allergy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A guide to managing disorders of the ear pinna and canal
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
PRACTICE RECOMMENDATIONS
› Prescribe topical antibiotics for uncomplicated otitis externa, reserving systemic agents for infection extending outside the ear canal, necrotizing otitis externa, or patients who are immunodeficient. C
› Avoid clearing cerumen if a patient is asymptomatic and advise patients/parents on Do’s and Don’ts for ear wax accumulation. C
› Consider flooding the ear canal with xylocaine, alcohol, or mineral oil before attempting insect removal. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s time to rethink your approach to C diff infection
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; [email protected]
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; [email protected]
CASE 1
Beth O, a 63-year-old woman, presents to the emergency department (ED) with a 2-week history of diarrhea (6 very loose, watery stools per day) and lower abdominal pain. The patient denies any vomiting, sick contacts, or recent travel. Past medical history includes varicose veins. Her only active medication is loperamide, as needed, for the past 2 weeks. Ms. O also recently completed a 10-day course of clindamycin for an infected laceration on her finger.
Ms. O’s laboratory values are unremarkable, with a normal white blood cell (WBC) count and serum creatinine (SCr) level. Abdominal computed tomography (CT) reveals some abnormal bowel dilatation and a slight increase in colon wall thickness. There is a high suspicion for Clostridioides difficile (formerly Clostridium difficile) infection (CDI), and stool sent for polymerase chain reaction (PCR) testing comes back positive for C difficile toxin B. It is revealed to be a strain other than the BI/NAP1/027 epidemic strain (which has a higher mortality rate).
How should this patient be treated?
CASE 2
Sixty-eight-year-old Barbara Z presents to the ED from her skilled nursing facility with persistent diarrhea and abdominal cramping. She was diagnosed with CDI about 2 months ago and reports that her symptoms resolved within 4 to 5 days after starting a 14-day course of oral metronidazole.
Her past medical history is notable for multiple myeloma with bone metastasis, for which she is actively undergoing chemotherapy treatment. She also has chronic kidney disease (baseline SCr, 2.2 mg/dL), hypertension, and anemia of chronic disease. The patient’s medications include amlodipine and cholecalciferol. Her chemotherapy regimen consists of bortezomib, lenalidomide, and dexamethasone. CT of the abdomen shows diffuse colon wall thickening with surrounding inflammatory stranding—concerning for pancolitis. There is no evidence of toxic megacolon or ileus.
Ms. Z’s laboratory values are notable for a WBC count of 15,900 cells/mL and an SCr of 4.1 mg/dL. She is started on oral levofloxacin and metronidazole due to concern for an intra-abdominal infection. PCR testing is positive for C difficile, and an enzyme immunoassay (EIA) for C difficile toxin is positive.
What factors put Ms. Z at risk for C difficile, and how should she be treated?
Continue to: C difficile is one of the most...
C difficile is one of the most commonly reported pathogens in health care–associated infections and affects almost 1% of all hospitalized patients in the United States each year.1 From 2001 to 2010, the incidence of CDI doubled in patients discharged from hospitals,2 with an estimated cost of more than $5 billion annually.3 Furthermore, rates of community-associated CDI continue to increase and account for about 40% of cases.4
After colonization in the intestine, C difficile releases 2 toxins (TcdA and TcdB) that cause colitis.5 Patients may present with mild diarrhea that can progress to abdominal pain, cramping, fever, and leukocytosis. Fulminant CDI can lead to the formation of pseudomembranes in the colon, toxic megacolon, bowel perforation, shock, and death.2
Beginning in the early 2000s, hospitals reported increases in severe cases of CDI.6 A specific strain known as BI/NAP1/027 was identified and characterized by fluoroquinolone resistance, increased spore formation, and a higher mortality rate.6
Further complicating matters … Recurrent CDI occurs in up to 10% to 30% of patients,7 typically within 14 to 45 days of completion of antibiotic pharmacotherapy for CDI.8 Recurrence is characterized by new-onset diarrhea or abdominal symptoms after completion of treatment for CDI.5
It typically begins with an antibiotic
Risk factors for CDI are listed in TABLE 1.9 The most important modifiable risk factor for initial and recurrent CDI is recent use of antibiotics.10 Most antibiotics can disrupt normal intestinal flora, causing colonization of C difficile, but the strongest association seems to be with third- and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and clindamycin.11 The risk for CDI occurs during antibiotic treatment, as well as up to 3 months after completion of antibiotic therapy.7 Exposure to multiple antibiotics and extended duration of antibacterial therapy can greatly increase the risk for CDI, so antimicrobial stewardship is key.11
Continue to: Continuing antibiotics while attempting...
Continuing antibiotics while attempting to treat CDI reduces the patient’s clinical response to CDI treatment, which can lead to recurrence.12 The Infectious Diseases Society of America (IDSA) guidelines include a strong recommendation to discontinue concurrent antibiotics as soon as possible in these scenarios.11
Acid-suppression therapy has also been associated with CDI. The mechanism is thought to be an interruption in the protection provided by stomach acid, and use over time may reduce the diversity of flora within the gut microbiome.13 The data demonstrating an association between acid-suppression therapy and CDI is conflicting, which may be a result of confounding factors such as the severity of CDI illness and diarrhea induced by use of proton pump inhibitors (PPIs).4 IDSA guidelines do not provide a recommendation regarding discontinuation of PPI therapy for the prevention of CDI, although inappropriate PPI therapy should always be discontinued.11
Advanced age is an important nonmodifiable risk factor for CDI. Older adults who live in long-term care facilities are at a higher risk for CDI, and these facilities have colonization rates as high as 50%.12
Community-associated risk. In an analysis of community-associated cases of CDI, 82% of patients reported some sort of health care exposure (ranging from physician office visit to surgery admission), 64% reported the receipt of antimicrobial therapy, and 31% reported the use of PPIs.14 Inflammatory bowel disease (IBD) may also put community dwellers at higher risk for CDI and its complications.15
CASES 1 & 2
Both CASE patients have risk factors for CDI. Ms. O (CASE 1) is likely at risk for CDI after completion of her recent course of clindamycin. Ms. Z (CASE 2) has several risk factors for recurrent CDI, including advanced age (≥ 65 years), residence in a long-term care facility, prior antibiotic exposure, and immunodeficiency because of chemotherapy/steroid use.
Continue to: Diagnosis
Diagnosis: Who and how to test
CDI should be both a clinical and laboratory-confirmed diagnosis. Patients should be tested for CDI if they have 3 or more episodes of unexplainable, new-onset unformed stools in 24 hours.11 Asymptomatic patients should not be tested to avoid unnecessary testing and treatment of those who are colonized but not infected.11 It is not recommended to routinely test patients who have taken laxatives within the previous 48 hours.11
There are several stool-based laboratory test options for the diagnosis of CDI (TABLE 211,12,16) but no definitive recommendation for all institutions.11 Many institutions have now implemented PCR testing for the diagnosis of CDI. However, while the benefits of this test include reduced need for repeat testing and possible identification of carriers, it’s estimated that reports of CDI increase more than 50% when an institution switches to PCR testing.1 Nonetheless, a one-step, highly sensitive test such as PCR may be used if strict criteria are implemented and followed.
The increase in positive PCR tests has prompted evaluation of using another test in addition to or in place of PCR. Multistep testing options include a glutamate dehydrogenase assay (GDH) with a toxin EIA, GDH with a toxin EIA and final decision via PCR, or PCR with toxin EIA.11 Use of a multistep diagnostic algorithm may increase overall specificity up to 100%, which may improve determination of asymptomatic colonization vs active infection.16 (Patients who have negative toxin results with positive PCR likely have colonization but not infection and often do not require treatment.) IDSA guidelines recommend that the stool toxin test should be part of a multistep algorithm for diagnosis, rather than PCR alone, if strict criteria are not implemented for stool test submission.11
There is no need to perform a test of cure after a patient has been treated for CDI, and no repeat testing should be performed within 7 days of the previous test.11 After successful treatment, patients will continue to shed spores and test positively via PCR for weeks to months.11 When patients have a positive PCR test, there are several important infection control efforts that institutions should consider; see “IDSA weighs in on measures to combat C difficile.”
SIDEBAR
IDSA weighs in on measures to combat C difficile
The spores produced by Clostridioides difficile can survive for 5 months or longer on dry surfaces because of resistance to heat, acid, antibiotics, and many cleaning products.38 Unfortunately, spores transmitted from health care workers and the environment are the most likely cause of infection spreading in health care institutions. To prevent transmission of C difficile infection (CDI) throughout institutions, appropriate infection control measures are necessary.
Clinical practice guidelines from the Infectious Diseases Society of America (IDSA) recommend that patients with CDI be isolated to a private room with a dedicated toilet. Health care staff should wear gloves and gowns when entering the room of, or taking care of, a patient with CDI. For patients who are suspected of having CDI, contact precautions should be implemented while awaiting test results. When the diagnosis is confirmed, contact precautions should remain in place for at least 48 hours after resolution of diarrhea but may be continued until discharge.11
Practicing good hand hygiene is essential, especially in institutions with high rates of CDI or if fecal contamination is likely.11 Hand hygiene with soap and water is preferred, due to evidence of a higher spore removal rate, but alcohol-based alternatives may be used if necessary.11 In institutions with high rates of CDI, terminal (post-discharge) cleaning of rooms with a sporicidal agent should be considered.11
Asymptomatic carriers are also a concern for transmission of CDI in institutional settings. Screening and isolating patients who are carriers may prevent transmission, and some institutions have implemented this process to reduce the risk for CDI that originates in a health care facility.39 The IDSA guidelines do not make a recommendation regarding screening or isolation of asymptomatic carriers, so the decision is institution specific.11 These guidelines also recommend that patients presenting with similar infectious organisms be housed in the same room, if needed, to avoid cross-contamination to others or additional surfaces.11
For pediatric patients, testing recommendations vary by age. Testing is not generally recommended for neonates or infants ≤ 2 years of age with diarrhea because of the prevalence of colonization with C difficile.11 For children older than 2 years, testing for CDI is only recommended in the setting of prolonged or worsening diarrhea and if the patient has risk factors such as IBD, immunocompromised state, health care exposure, or recent antibiotic use.11 In addition, testing in this population should only be considered once other infectious and noninfectious causes of diarrhea have been excluded.11
Continue to: First-line treatment? Drug of choice has changed
First-line treatment? Drug of choice has changed
In 2018, the IDSA published new treatment guidelines that provide important updates from the 2010 guidelines.11 Chief among these was the elimination of metronidazole as a first-line therapy. Vancomycin or fidaxomicin are now recommended as first-line treatment options because of superior eradication of C difficile when compared with metronidazole.11 In the opinion of the authors, vancomycin should be considered the drug of choice because of cost. (See “The case for vancomycin.”)
SIDEBAR
The case for vancomycin
The majority of studies conducted prior to publication of the 2010 Infectious Diseases Society of America guidelines described numerically worse eradication rates of Clostridioides difficile infection (CDI) with metronidazole compared with vancomycin for all severities of infection, but statistical significance was not achieved. These studies also showed a nonsignificant increase in CDI recurrence with metronidazole.17,40,41
A 2005 systematic review demonstrated increased treatment failure rates with metronidazole.42 The rates of metronidazole discontinuation and transition to alternative options more than doubled in 2003-2004, to 25.7% of patients compared with 9.6% in earlier years.42 Metronidazole efficacy was further questioned in a prospective observational study conducted in 2005, in which only 50% of patients were cured after an initial course of treatment, while 28% had recurrence within 90 days.43
Vancomycin was found to be the superior treatment option to metronidazole and tolevamer in a 2014 randomized controlled trial.18 This study also demonstrated that vancomycin was the superior therapy when comparing treatment-naïve vs experienced patients and severity of CDI.18 A 2017 retrospective cohort study demonstrated decreased 30-day all-cause mortality for patients taking vancomycin vs metronidazole (adjusted relative risk = 0.86; 95% confidence interval, 0.74-0.98), although it should be noted that this difference was driven by those with severe CDI, and there was no statistically significant difference in mortality for patients with mild-to-moderate CDI.44
The results of these studies led to the recommendation of vancomycin over metronidazole as first-line pharmacotherapy for CDI in practice, despite the historical perspective that overutilization of oral vancomycin could potentially increase rates of vancomycinresistant Enterococcus.11
Metronidazole should only be used in the treatment of CDI as a lastresort medication because of cost or insurance coverage. Although the price of oral vancomycin is higher, favorable patient outcomes are substantially greater, and recent analyses have shown that vancomycin is actually more cost-effective than metronidazole as a result.24 Adverse effects for metronidazole include neurotoxicity, gastrointestinal discomfort, and disulfiram-like reaction.
Vancomycin does not harbor as many adverse effects because of extremely low systemic absorption when taken orally, but patients may experience gastrointestinal discomfort.45 While systemic exposure with oral administration of vancomycin is very low (< 1%), there have been case reports of nephrotoxicity and “red man syndrome” that are more typically seen with intravenous vancomycin.44
Given the low rate of systemic exposure, routine monitoring of renal function and serum drug levels is not usually necessary during oral vancomycin therapy. However, it may be appropriate to monitor renal function and serum levels of vancomycin in patients who have renal failure, have altered intestinal integrity, are age ≥ 65 years, or are receiving high doses of vancomycin.46
10-day vs 14-day treatment of CDI. Most studies for the treatment of CDI have used a 10-day regimen rather than increasing the duration to a 14-day regimen, and nearly all studies conducted have displayed high rates of symptom resolution at the end of 10 days of treatment.17,18 Thus, treatment duration beyond 10 days should only be considered for patients who continue to have symptoms or complications with CDI on Day 10 of treatment.
First recurrence. Metronidazole is no longer the recommended treatment for first recurrence of CDI treated initially with metronidazole; instead, a 10-day course of vancomycin should be used.11 For recurrent cases in patients initially treated with vancomycin, a tapered and pulsed regimen of vancomycin is recommended11:
- vancomycin PO 125 mg four times daily for 10 to 14 days followed by
- vancomycin PO 125 mg twice daily for 7 days, then
- vancomycin PO 125 mg once daily for 7 days, then
- vancomycin PO 125 mg every 2 to 3 days for 2 to 8 weeks.
Pediatric patients. The IDSA guidelines recommend use of metronidazole or vancomycin to treat an initial case or first recurrence of mild-to-moderate CDI in this population.11 Due to a lack of quality evidence, the drug of choice for initial treatment is inconclusive, so patient-specific factors and cost should be considered when choosing an agent.11 If not cost prohibitive, vancomycin should be the drug of choice for most cases of pediatric CDI, and for severe cases or multiple recurrences of CDI, vancomycin is clearly the drug of choice.
Recommended agents: A closer look
Oral vancomycin products. Vancocin, a capsule, and Firvanq, an oral solution, are 2 vancomycin products currently on the market for CDI. Although the capsules are a readily available treatment option, the cost of the full course of treatment can be a barrier for patients without insurance, or with high copays or deductibles (brand name, $4000; generic, $1252).19
Continue to: Historically, in an effort to keep costs down...
Historically, in an effort to keep costs down, an oral solution was often inexpensively compounded at hospitals or pharmacies.20
Fidaxomicin, an oral macrocyclic antibiotic with minimal systemic absorption, was first approved by the US Food and Drug Administration (FDA) for CDI in 2011.21 The IDSA guidelines recommend fidaxomicin for initial, and recurrent, cases of CDI as an alternative to vancomycin.11 This recommendation is based on 2 randomized double-blind trials comparing fidaxomicin to standard-dose oral vancomycin for initial or recurrent CDI.21,22
Pooled data from these 2 similar studies found that fidaxomicin was noninferior (10% noninferiority margin) to vancomycin for the primary outcome of clinical cure.23 Fidaxomicin was shown to be superior to vancomycin regarding rate of CDI recurrence (relative risk [RR] = 0.61; 95% confidence interval [CI], 0.43-0.87). These results were similar regardless of whether the CDI was an initial or recurrent case.23
Given the lack of systemic absorption, fidaxomicin is generally very well tolerated. The largest downside to fidaxomicin is its cost, which can be nearly $5000 for a standard 10-day course (vs as little as $165 for oral vancomycin).19 As a result, oral vancomycin solution is likely the most cost-effective therapy for initial cases of CDI.24 In patients with poor medication adherence, fidaxomicin offers the advantage of less-frequent dosing (twice daily vs 4 times daily with vancomycin).
For cases of recurrent CDI, when treatment failure occurred with vancomycin, fidaxomicin should be considered as an efficacious alternative. If fidaxomicin is used, it is advisable to verify coverage with the patient’s insurance plan, since prior authorization is frequently required.
Continue to: When meds fail, consider a fecal microbiota transplant
When meds fail, consider a fecal microbiota transplant
Another important change in the IDSA guidelines for CDI management is the strong recommendation for fecal microbiota transplantation (FMT) in patients with multiple recurrences of CDI for whom appropriate antibiotic treatment courses have failed.11,25 The goal of FMT is to “normalize” an abnormal gut microbiome by transplanting donor stool into a recipient.26
FMT has been shown to be highly effective in 5 randomized clinical trials conducted since 2013, with CDI cure rates between 85% and 94%.11 This rate of cure is particularly impressive given that the studies only included patients with refractory CDI.
Patients with recurrent CDI who may be candidates for FMT should be referred to a center or specialist with experience in FMT. These transplants can be expensive because of the screening process involved in obtaining donor samples. (Historically, a single FMT has cost $3000-$5000, and it is seldom covered by insurance.27) The emergence of universal stool banks offers a streamlined solution to this process.26
Fresh or frozen stool is considered equally effective in treating refractory CDI.26 Oral capsule and freeze-dried stool formulations have been studied, but their use is considered investigational at this time.26
Delivery via colonoscopy to the right colon is the preferred route of infusion; however, delivery via enema or nasogastric, nasojejunal, or nasoduodenal infusion can be considered as well.26
Continue to: In preparing for stool transplantation...
In preparing for stool transplantation, patients should be treated with standard doses of oral vancomycin or fidaxomicin for 3 days before the procedure to suppress intestinal C difficile, and the last dose of antibiotics should be given 12 to 48 hours before the procedure.26 Bowel lavage with polyethylene glycol is recommended, regardless of whether stool is delivered via colonoscopy or upper GI route.
Short-term adverse events associated with FMT appear to be minimal; data is lacking for long-term safety outcomes.28 While only recommended currently for cases of recurrent CDI, there is promising data emerging for use of FMT for severe cases, even without recurrence.29
The role of probiotics remains unclear
Probiotics have been explored in numerous trials to determine if they are effective in preventing CDI in patients who have been prescribed antibiotics.11 While no randomized trials have conclusively shown benefit, several meta-analyses have shown that the use of probiotics may result in a 60% to 65% relative risk reduction in CDI incidence.30,31
One proviso to these meta-analyses is that the incorporated studies have typically included patients at very high risk for CDI, and subanalyses have only found a reduction in CDI incidence when patients are at a very high baseline risk. In addition, there are many differences in probiotic types, formulations, treatment durations, and follow-up. As a result, the IDSA guidelines state that there is “insufficient data at this time” to recommend routine administration of probiotics for either primary or secondary CDI prophylaxis.11
Due to insufficient high-quality data, the IDSA guidelines do not provide a recommendation regarding use as an adjunct treatment option for acute CDI.11 Probiotics should not be routinely used to prevent CDI; however, they may provide benefit if reserved for patients at the highest risk for CDI (eg, history of CDI, prolonged use of broad-spectrum antibiotics, high local incidence).
Continue to: What about surgical intervention?
What about surgical intervention?
In severe cases of CDI, surgery may be necessary and can reduce mortality.32 The surgical procedure with the strongest recommendation in the IDSA guidelines is the subtotal colectomy, though the diverting loop ileostomy is an alternative option.11 Patients who may benefit from surgery include those with a WBC count ≥ 25,000; lactate > 5 mmol/L11; altered mental status; megacolon; perforation of the colon; acute abdomen on physical examination; or septic shock due to CDI.33 Although surgery can be beneficial, the mortality rate remains high for those with CDI who undergo colectomy.33
Reserve bezlotoxumab for prevention of recurrence
Bezlotoxumab, a human monoclonal immunoglobulin GI/kappa antibody, was approved by the FDA in 2016 for the prevention of recurrent CDI. Its mechanism of action is to bind and neutralize C difficile toxin B. It was approved as a single infusion for adults who are receiving active antibiotic therapy for CDI and are considered to be at high risk for recurrence.34
This approval was based on 2 trials of more than 2500 patients, in which participants received bezlotoxumab or placebo while receiving treatment for primary or recurrent CDI. The primary outcome of these studies was recurrent infection within 12 weeks after infusion, which was significantly lower for bezlotoxumab in both studies: 17% vs 28% (P < 0.001) in one trial and 16% vs 26% (P < 0.001) in the other trial.35
Bezlotoxumab should only be used as an adjunct to prevent recurrence.32 There is no recommendation for or against bezlotoxumab in the IDSA guidelines because of the recent date of the drug’s approval. Its frequency of use will likely depend on the number of patients who meet criteria as high risk for recurrence and its estimated cost of $4560 per dose.34,36
CASES
CASE 1: In light of Ms. O’s recent completion of a course of clindamycin and unremarkable lab work, she should be treated for mild-to-moderate CDI. She has no comorbid conditions to warrant fidaxomicin, and thus vancomycin (capsules or oral solution) would be the best treatment option. Ms. O is started on vancomycin PO 125 mg qid for 10 days. She is also advised to discontinue loperamide as soon as possible, based on poor outcomes data seen with the use of antimotility agents in CDI.37
Continue to: CASE 2
CASE 2: Ms. Z has several risk factors for recurrent CDI and has an elevated WBC count and SCr level (WBC ≥ 15,000 and SCr > 1.5 mg/dL). Thus, she is classified as having severe, recurrent CDI. Oral levofloxacin and metronidazole should be discontinued, because they increase the risk for treatment failure and development of more virulent CDI strains, such as BI/NAP1/027. Since Ms. Z used metronidazole for treatment of her initial CDI, vancomycin or fidaxomicin should be used at this time. Either vancomycin PO 125 mg qid for 10 days or fidaxomicin 200 mg bid for 10 days would be an appropriate regimen; however, because of cost and unknown insurance coverage, vancomycin is the most appropriate regimen.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, University of Wyoming School of Pharmacy, Saint Joseph Family Medicine Residency, 1000 E. University Avenue, Dept 3375, Laramie, WY 82071; [email protected]
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
1. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175:1792-1801.
2. Reveles KR, Lee GC, Boyd NK, et al. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42:1028-1032.
3. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88-S92.
4. Tariq R, Singh S, Gupta A, et al. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:784-791.
5. Kachrimanidou M, Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit Rev Microbiol. 2011;37:178-187.
6. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913-1924.
7. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C difficile infection. JAMA. 2009;301:954-962.
8. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154-S161.
9. Napolitano LM, Edmiston CE Jr. Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325-348.
10. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460.
11. McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1-e48.
12. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498; quiz 499.
13. Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
14. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173:1359-1367.
15. Negrón ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691-704.
16. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408.
17. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
18. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345-354.
19. Vancomycin: product details. Redbook Online. www.micromedexsolutions.com. Published 2018. Accessed June 13, 2020.
20. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics. 2014;32:639-650.
21. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
22. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281-289.
23. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55 suppl 2:S93-103.
24. Ford DC, Schroeder MC, Ince D, et al. Cost-effectiveness analysis of initial treatment strategies for mild-to-moderate Clostridium difficile infection in hospitalized patients. Am J Health Syst Pharm. 2018;75:1110-1121.
25. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
26. Panchal P, Budree S, Scheeler A, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. 2018;20:14.
27. Arbel LT, Hsu E, McNally K. Cost-effectiveness of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: a literature review. Cureus. 2017;9:e1599.
28. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580.
29. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis. 2018;66:645-650.
30. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
31. Johnston BC, Lytvyn L, Lo CK, et al. Microbial preparations (probiotics) for the prevention of Clostridium difficile infection in adults and children: an individual patient data meta-analysis of 6,851 participants. Infect Control Hosp Epidemiol. 2018:1-11.
32. Stewart DB, Hollenbeak CS, Wilson MZ. Is colectomy for fulminant Clostridium difficile colitis life saving? A systematic review. Colorectal Dis. 2013;15:798-804.
33. Julien M, Wild JL, Blansfield J, et al. Severe complicated Clostridium difficile infection: can the UPMC proposed scoring system predict the need for surgery? J Trauma Acute Care Surg. 2016;81:221-228.
34. Merck & Co, Inc. Sharp M. ZinplavaTM (bezlotoxumab [package insert] US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761046s000lbl.pdf. Revised October 2016. Accessed May 29, 2020.
35. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305-317.
36. Chahine EB, Cho JC, Worley MV. Bezlotoxumab for the Prevention of Clostridium difficile recurrence. Consult Pharm. 2018;33:89-97.
37. Koo HL, Koo DC, Musher DM, et al. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605.
38. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526-536.
39. Longtin Y, Paquet-Bolduc B, Gilca R, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796-804.
40. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-1046.
41. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813-818.
42. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
43. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
44. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med. 2017;177:546-553.
45. CutisPharma. FirvanqTM (vancomycin hydrochloride) for oral solution [package insert]. US Food and Drug Administration Web site. www.accessdata.fda.gov/drugsatfda_docs/label/2018/208910s000lbl.pdf. Revised January 2018. Accessed May 29, 2020.
46.
PRACTICE RECOMMENDATIONS
› Keep in mind that previous exposure to antibiotics is the most important risk factor for initial and recurrent Clostridioides difficile infection (CDI). Thus, appropriate antimicrobial stewardship is key to prevention. C
› Begin with vancomycin or fidaxomicin (over metronidazole) for first-line treatment of CDI in adults. A
› Consider fecal microbiota transplantation in high-risk patients with recurrent CDI for whom antimicrobial therapy has failed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dual antiplatelet Tx for stroke prevention: Worth the risk?
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; [email protected].
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; [email protected].
The incidence of ischemic stroke in the United States is estimated to be more than 795,000 events each year.1 After an initial stroke, the rate of recurrence is 5% to 20% within the first year, with the greatest prevalence in the first 90 days following an event.2-5 Although dual antiplatelet therapy, often with aspirin and a P2Y12 inhibitor such as clopidogrel, reduces the risk for recurrent cardiovascular events, cerebrovascular events, and death following acute coronary syndromes and percutaneous intervention, the role of combination antiplatelet therapy for secondary prevention of ischemic stroke continues to be debated.6 Reconciling currently available data can be challenging, as many studies vary considerably in both the time to antiplatelet initiation and duration of therapy.
For many years, aspirin alone was the drug of choice for secondary prevention of noncardioembolic ischemic stroke.7 Efficacy is similar at dosages anywhere between 50 and 1500 mg/d; higher doses incur a greater risk for gastrointestinal hemorrhage.7 Current secondary prevention guidelines recommend a dosage of aspirin somewhere between 50 and 325 mg/d.7
Alternative agents have also been evaluated for secondary stroke prevention, but only clopidogrel is currently considered an acceptable alternative for monotherapy based on a subgroup analysis of the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.7,8 Other alternatives, including cilostazol, ticlopidine, and ticagrelor, are limited by a lack of data, adverse drug reactions, or unproven efficacy and are not recommended in current guidelines.7,9 The ongoing THALES (Acute Stroke or Transient Ischaemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death) trial, assessing combination ticagrelor and aspirin, may identify an additional option for antiplatelet therapy following acute stroke.10
The current guidelines from the American Heart Association/American Stroke Association (AHA/ASA) support the combination of aspirin and extended-release dipyridamole (ASA-ERDP) as a long-term alternative to aspirin monotherapy.7,11 Additionally, the combination of clopidogrel and aspirin (CLO-ASA) is now recommended for limited duration in the early management of ischemic stroke.11
This review will explore the role of dual antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke or transient ischemic attack (TIA), with particular focus on acute use of CLO-ASA.
Clopidogrel and aspirin: When to initiate, when to stop
The combined use of clopidogrel and aspirin has been well-studied for secondary prevention of ischemic stroke and TIA. However, interpreting and applying the results of these trials can be challenging given key differences in both time to treatment initiation and the duration of combination therapy. Highlights of the major randomized controlled trials (RCTs) evaluating the safety and efficacy of CLO-ASA are detailed in TABLE 1.4,5,12-15
Initial trials evaluating CLO-ASA for secondary stroke prevention, including the MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients),12 SPS3 (Secondary Prevention of Small Subcortical Strokes),13 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization, Management and Avoidance)14 trials assessed the long-term benefits of combination therapy, with most patients initiating treatment a month or more following an initial stroke and continuing therapy for at least 18 months.12-14 Results from these trials indicate that long-term use (> 18 months) of CLO-ASA does not reduce recurrent events but increases rates of clinically significant bleeding.12-14
Continue to: A look at Tx timing
A look at Tx timing. Since these initial attempts failed to show a long-term benefit with CLO-ASA, subsequent trials attempted to establish an appropriate balance between the optimal time to initiate CLO-ASA and the optimal duration of therapy. The FASTER (Fast Assessment of Stroke and Transient ischaemic attack to prevent Early Recurrence) trial was a small pilot study of 392 patients randomized to CLO-ASA or aspirin within 24 hours of stroke or TIA onset and continued for only 3 months.15 While this trial did not find a significant reduction in ischemic or hemorrhagic stroke with combination therapy, there was a large numerical difference in event rates between the 2 groups (7.1% CLO-ASA vs 10.8% aspirin).15 An underpowered sample size (due to difficulty recruiting participants) is likely responsible for the lack of statistical significance.15 Despite the trial’s failure to show a benefit with acute use of CLO-ASA, it suggested a possible benefit that led to further investigation in the CHANCE (Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events)5 and POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) 4 trials.
The CHANCE trial conducted in China included more than 5000 patients with acute minor ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 3) or high-risk TIA (ABCD2 [a scale that assesses the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes] score ≥ 4).5 Similar to FASTER, patients were randomized within 24 hours of symptom onset to CLO-ASA or aspirin. However, CHANCE utilized combination therapy for only 21 days, after which the patients were continued on clopidogrel monotherapy for up to 90 days; the aspirin monotherapy group continued aspirin for 90 days.
After 90 days, patients initially using combination therapy had significantly lower rates of ischemic or hemorrhagic stroke vs those assigned to aspirin monotherapy. This result was driven heavily by the reduction in ischemic stroke (7.9% CLO-ASA vs 11.4% aspirin; P < .001). Additionally, there was no significant difference in moderate or severe bleeding events between the 2 groups.5 Efficacy and safety results were similar among a subgroup of patients who were randomized to treatment within 12 hours rather than 24 hours from symptom onset.16 The CHANCE trial was the first major study to demonstrate a clinical benefit of CLO-ASA to prevent recurrent stroke. Accordingly, the 2018 AHA/ASA guidelines included a new recommendation regarding secondary prevention for the use of CLO-ASA initiated within 24 hours and continued for 21 days following a minor stroke or TIA.11
A drawback of the CHANCE trial was its narrow patient population of only Chinese patients, which may limit applicability in clinical practice. There are known genetic variations in cytochrome P450 2C19 (CYP2C19) that may affect clopidogrel metabolism. CYP2C19 is responsible for the conversion of clopidogrel into its activated form in vivo. Carriers of a CYP2C19 loss-of-function allele may have reduced clopidogrel activation and subsequent reduced antiplatelet activity. Such loss-of-function alleles are more common in Asian populations vs non-Asian populations.17
A substudy of CHANCE found that CLO-ASA’s efficacy benefit was preserved in noncarrier patients; however, patients with the CYP2C19 loss-of-function allele did not benefit from combination therapy.18 Interestingly, these genetic differences did not affect bleeding outcomes. Given that approximately 60% of patients in the CHANCE substudy were loss-of-function allele carriers and that the overall study results still showed benefit with combination therapy, application of CHANCE’s findings to broader populations may not be a concern after all.18
Continue to: In efforts to gain insight...
In efforts to gain insight on CLO-ASA’s use in a more diverse patient population, the POINT trial included almost 5000 patients, with 82% from the United States, who were randomized within 12 hours of symptom onset to CLO-ASA or aspirin monotherapy for 90 days.4 Similar to the CHANCE study, the POINT study included patients with mild ischemic strokes (NIHSS ≤ 3) or high-risk TIA (ABCD2 ≥ 4). Combination therapy significantly reduced the primary endpoint of ischemic stroke, myocardial infarction (MI), or death from an ischemic event. Contrary to CHANCE, there was a significant increase in major bleeding in those assigned to combination therapy, which resulted in the trial being stopped early.4
A closer look at safety differences. CHANCE and POINT were the first major trials to show a benefit of CLO-ASA for secondary prevention of stroke, yet their differences in safety outcomes, specifically major hemorrhage, argued for a deeper reconciliation of their results.4,5 While both trials initiated secondary prevention within 24 hours of symptom onset, the difference in duration of combination therapy (21 days in CHANCE vs 90 days in POINT) likely impacted the rates of hemorrhage. When results from POINT were stratified by time period, particularly within the first 30 days of therapy (similar to the 21-day treatment duration of CHANCE), combination therapy significantly reduced the primary endpoint of ischemic stroke, MI, or death from an ischemic event (3.9% CLO-ASA vs 5.8% aspirin; P = .02) without an increased risk for major hemorrhage. Between 30 and 90 days, this efficacy benefit disappeared. However, bleeding rates between groups continued to separate throughout the 90-day course. In this light, the 30-day outcomes of POINT are largely similar to CHANCE and support the short-term use of CLO-ASA for secondary prevention without an associated increase in major bleeding.4,5
Antiplatelet dosing in POINT and CHANCE may also play a role in the contrasting safety results between the trials.4,5 While both studies utilized clopidogrel loading doses, POINT used 600 mg while CHANCE used 300 mg. Clopidogrel maintenance dosing was the same at 75 mg/d. In CHANCE, aspirin dosing was protocolized to 75 mg/d; however, in POINT, 31% of patients used > 100 mg/d aspirin.4,5 It is possible that the higher doses of both aspirin and clopidogrel in the POINT trial contributed to the difference in the occurrence of major hemorrhage between the treatment groups in these trials.
The takeaway. Based on currently available data, patients who are best suited to benefit from CLO-ASA are those who have had minor noncardioembolic ischemic strokes or high-risk TIAs.4,5,11 Clopidogrel should be given as a 300-mg loading dose followed by 75 mg/d given concomitantly with aspirin at a dose no higher than 100 mg/d. CLO-ASA therapy should be initiated within 24 hours of symptom onset and be continued for no longer than 1 month, after which chronic preventive therapy with either aspirin or clopidogrel monotherapy should be started.4,5,11
Dipyridamole and aspirin: A controversial option
Since the approval of the combination product ASA-ERDP, there has been considerable controversy about using this combination over other therapies, such as aspirin or clopidogrel, for recurrent ischemic stroke prevention. Much of this controversy arises from limitations in the trial designs.
Continue to: The first trial to show benefit...
The first trial to show benefit with ASA-ERDP was ESPS2 (European Stroke Prevention Study 2), which demonstrated superiority of the combination over placebo in reducing recurrent stroke when treatment was added within 3 months of an index stroke.19 A few studies have evaluated ASA-ERDP compared to aspirin monotherapy; however, most of these studies were small and did not show any difference in outcomes.20 Only ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)21 carried significant weight in a 2013 meta-analysis, which showed a significant reduction in recurrent events with the combination product compared to aspirin monotherapy.20
Both the ESPS2 and ESPRIT trials had significant limitations.19,21 Patients in both studies had vascular comorbidities including atherosclerotic cardiovascular disease (ASCVD); however, pharmacotherapies designated to treat these diseases were not mentioned in the demographic data, nor were these medications taken into consideration to limit potential bias.19,21 Retrospectively, a significant proportion of aspirin doses utilized as a control in ESPRIT were inferior to the guideline-recommended dosing with 42% to 46% of patients receiving 30 mg/d.21 Despite these controversies, ASA-ERDP is still considered an alternative to aspirin monotherapy in the guidelines.7
The timing of ASA-ERDP initiation appears to be inversely related to the efficacy of the combination over therapeutic alternatives. Studies in which the therapy was initiated 3 to 6 months from the index stroke indicated favorable outcomes for the combination when compared to ASA or ERDP monotherapy.19,21 Studies utilizing early initiation (ie, within 24 or 48 hours of the index event) or even within 3 weeks showed no difference in outcomes; however, this may be due in part to the use of clopidogrel or other combination antiplatelet therapy as active comparators.22-24
Early initiation of ASA-ERDP also demonstrated a higher risk of major and intracranial bleeding compared to clopidogrel.22 Additionally, use of triple therapy with ASA-ERDP plus clopidogrel increased bleeding events without improving efficacy.24 More recent studies of ASA-ERDP are focusing on earlier initiation of therapy; it is unknown whether the benefits of late initiation will be confirmed in future studies. Highlights of the major RCTs evaluating the safety and efficacy of ASA-ERDP are detailed in TABLE 219,21-24.
The takeaway. Methodological issues and potential confounding factors in many of the key trials for ASA-ERDP make it challenging to fully discern the role that ASA-ERDP may play in the secondary prevention of stroke. Further evidence utilizing appropriate controls, timing, and assessment of confounders is needed. Additionally, ASA-ERDP is plagued by tolerability issues such as headache, nausea, and vomiting, leading to higher rates of discontinuation than its comparators in clinical trials. Accordingly, the maintenance use of ASA-ERDP for secondary stroke prevention may be considered less preferred than other recommended alternatives such as aspirin or clopidogrel monotherapies.
CORRESPONDENCE
Robert S. Helmer, PharmD, BCPS, Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy, 650 Clinic Drive, Suite 2100, Mobile, AL 36688; [email protected].
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
1. CDC. Stroke Facts. Last updated January 31, 2020. www.cdc.gov/stroke/facts.htm. Accessed June 29, 2020.
2. Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
3. Amarenco P, Lavallee PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182-2190.
4. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
5. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
6. Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol. 2008;101:960-966.
7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
8. Gent M, Beaumont D, Blanchard J, et al. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329-1339.
9. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
10. Johnston SC, Amarenco P, Denison H, et al. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745‐751.
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110.
12. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
13. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
14. Hankey GJ, Johnston SC, Easton JD, et al. Effect of clopidogrel plus ASA vs. ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trial. Int J Stroke. 2011;6:3-9.
15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961-969.
16. Li Z, Wang Y, Zhao X, et al. Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. J Am Heart Assoc. 2016;5:e003038.
17. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317-323.
18. Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70-78.
19. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
20. Li X, Zhou G, Zhou X, et al. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following TIA or stroke: a meta-analysis of randomized controlled trials. J Neurol Sci. 2013;332:92-96.
21. Halkes PH, van Gijn J, Kapelle IJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
22. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
23. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166.
24. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391:850-859.
PRACTICE RECOMMENDATIONS
› Initiate combined clopidogrel plus aspirin within 24 hours of a minor stroke or TIA and continue for no longer than 1 month; then switch patients to aspirin or clopidogrel monotherapy. A
› Do not use combined clopidogrel plus aspirin for long-term secondary stroke prevention. A
› Limit use of aspirin plus extended-release dipyridamole as a first choice for secondary stroke prevention because of limitations in efficacy and poor tolerability. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series