User login
Medication-assisted recovery for opioid use disorder: A guide
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.
Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
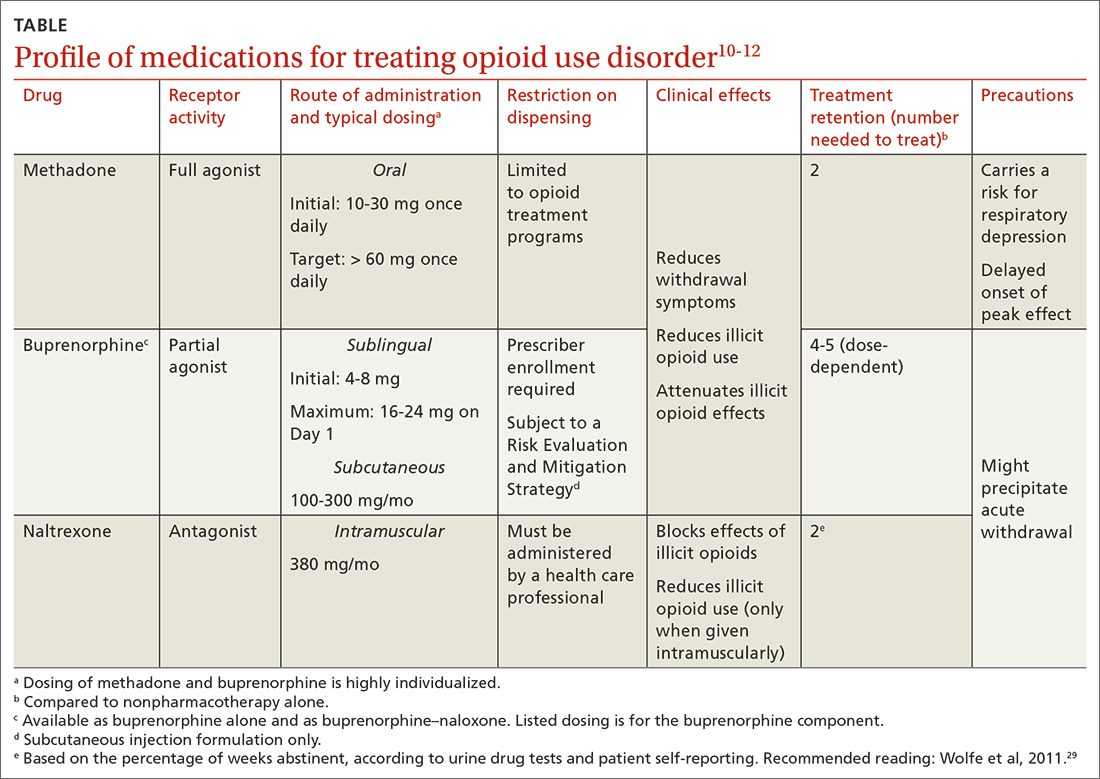
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13
The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.

Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13

The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.

Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13

The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; [email protected]
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
PRACTICE RECOMMENDATIONS
› Consider resource availability (eg, treatment programs and regulatory barriers), in addition to patient- and medicationspecific factors, when designing the most individualized, advantageous medication-assisted recovery plan, to reduce the risk for mortality. B
› Schedule early (< 2 weeks) and frequent follow-up with patients who are starting medications for opioid use disorder (particularly methadone), to manage risk when mortality is highest and to support recovery. C
› Set and manage patient expectations for control of withdrawal symptoms when initiating medications for opioid use disorder (particularly buprenorphine). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sports: An underutilized tool for patients with disabilities
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6
However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; [email protected]
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; [email protected]
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; [email protected]
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
PRACTICE RECOMMENDATIONS
› Recommend physical activity as an adjunct to traditional medical management to maximize physical and psychosocial benefits in patients with intellectual/developmental disabilities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hyperlipidemia management: A calibrated approach
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12
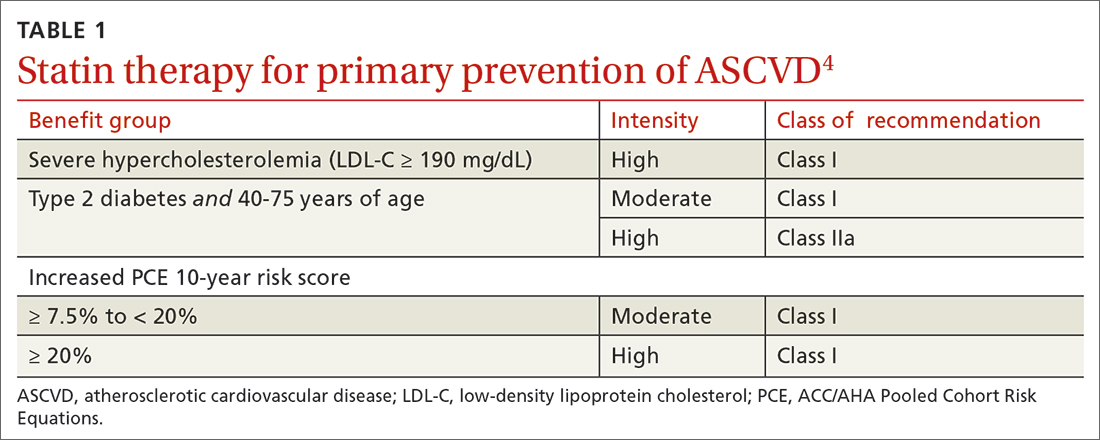
Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).
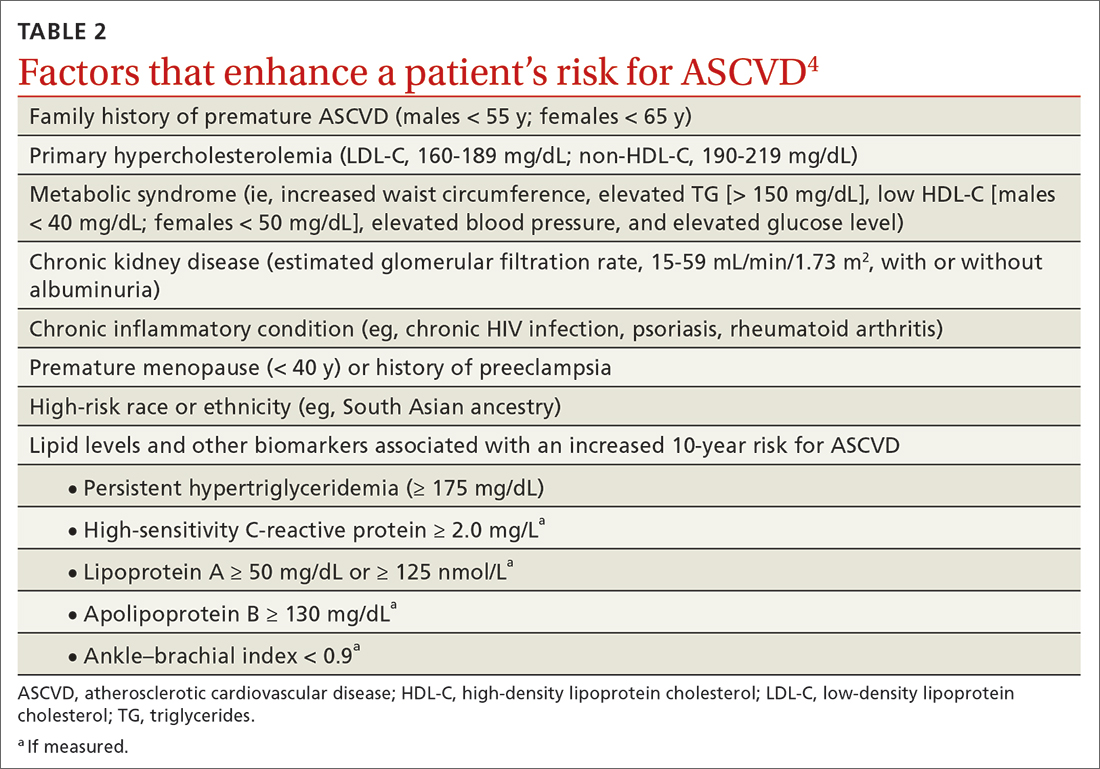
If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4
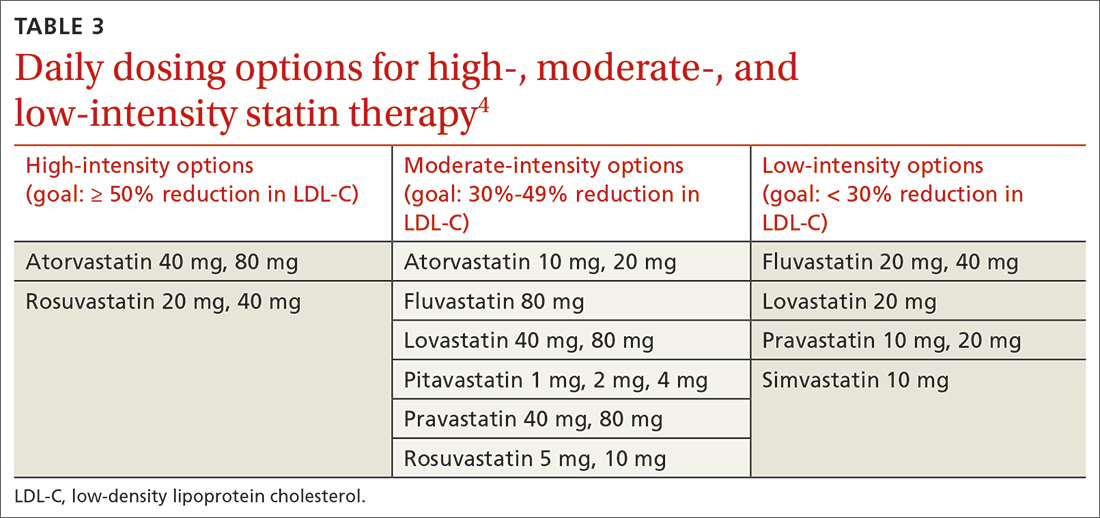
Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2

The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2

The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
Estimating risk for ASCVDby ascertaining LDL-C
The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL), estimation of LDL-C is invalid.
The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very-low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC – HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
Continue to: Apolipoprotein B
Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
Continue to: How much does lifestyle modification actually matter?
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention:Stratification by age
40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

Continue to: In adults at borderline risk...
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
Continue to: > 75 years, without ASCVD
> 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy
Options for lipid-lowering pharmacotherapy
Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
Continue to: Inclisiran
Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoic acid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL) and TG (42 mg/dL).31,32
Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C < 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
1. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
2. Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
3. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001/jama.2013.280532
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161/CIR.0000000000000625
5. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
6. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi: 10.1161/CIRCOUTCOMES.110.959247
7. Framingham Heart Study. Cardiovascular disease (10-year risk). Accessed February 14, 2023. www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/
8. Stone NJ, Robinson JG, Lichtenstein AH, et al; . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
10. Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146
11. Eckel RH, Jakicic JM, Ard JD, et al; . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
12. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi: 10.1001/jama.2016.15450
13. Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j.jcmg.2018.04.015
14. Valenti V, Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016/j.jcmg.2015.01.025
15. Armitage J, Baigent C, Barnes E, et al; . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407-415. doi: 10.1016/S0140-6736(18)31942-1
16. Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161/CIRCULATIONAHA.117.028271
17. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
18. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
19. Cannon CP, Blazing MA, Giugliano RP, et al; . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
20. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384. doi: 10.1001/jama.2016.16951
21. Sabatine MS, Giugliano RP, Wiviott SD, et al; . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
22. Robinson JG, Farnier M, Krempf M, et al; . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
23. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
24. Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
25. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
26. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
27. Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001/jamacardio.2021.1157
28. US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
29. Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
30. Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
31. Landray MJ, Haynes R, Hopewell JC, et al; . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212. doi: 10.1056/NEJMoa1300955
32. Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
33. Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001/jamacardio.2016.4828
34. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056/NEJMoa1001282
PRACTICE RECOMMENDATIONS
› Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
› Consider the impact of ASCVD risk-enhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
› Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Isolated third nerve palsy: Lessons from the literature and 4 case studies
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.
Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.
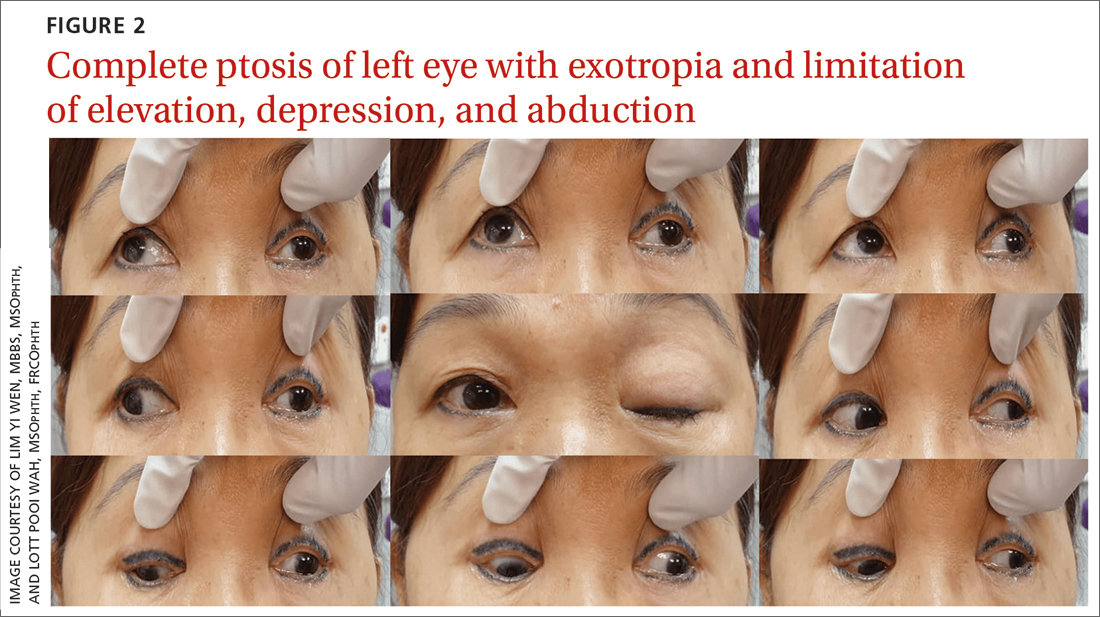
Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.
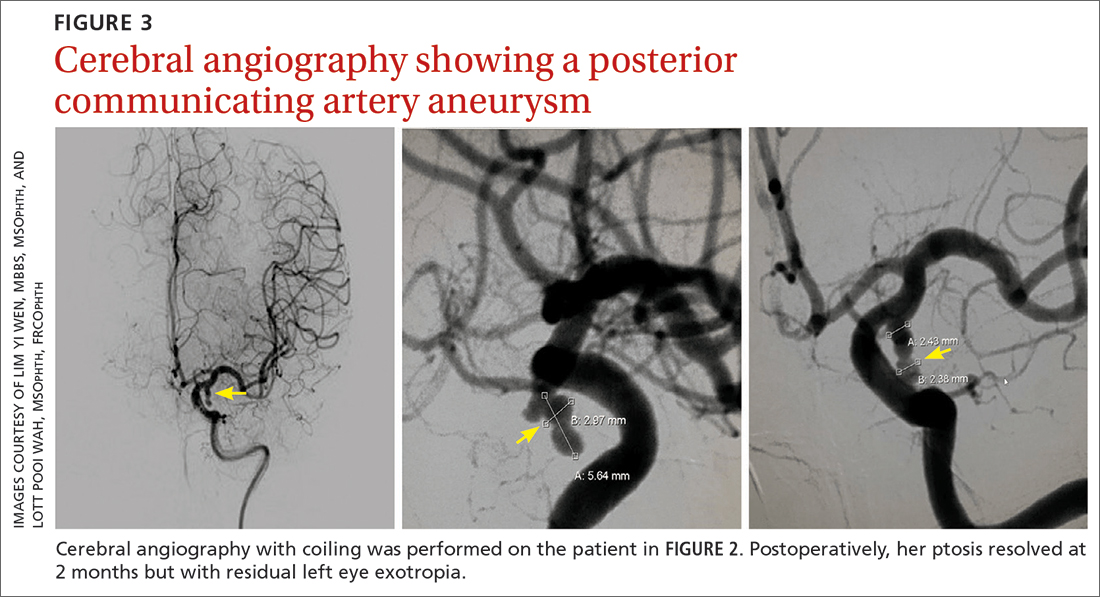
CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.
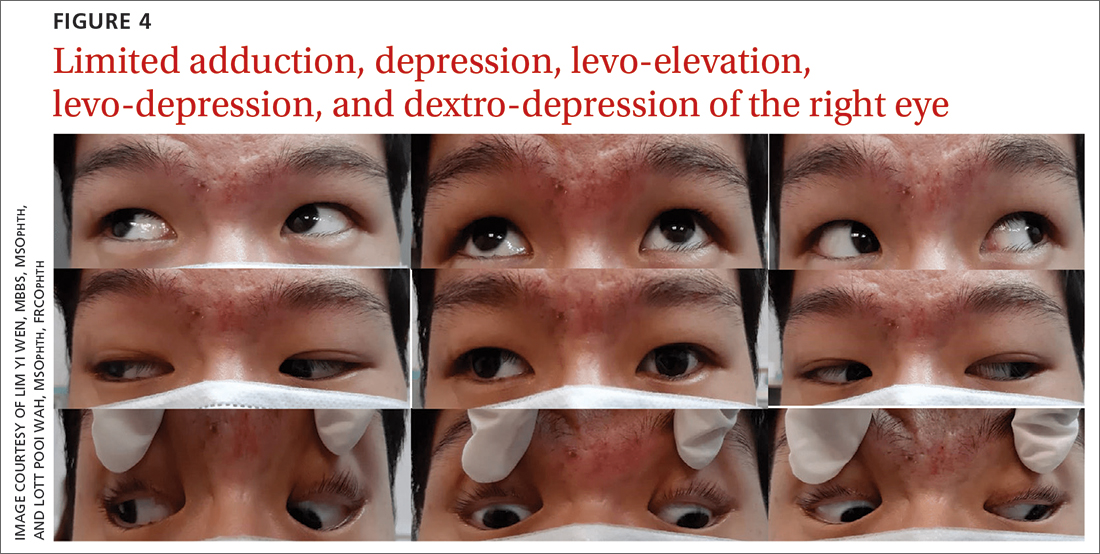
CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.
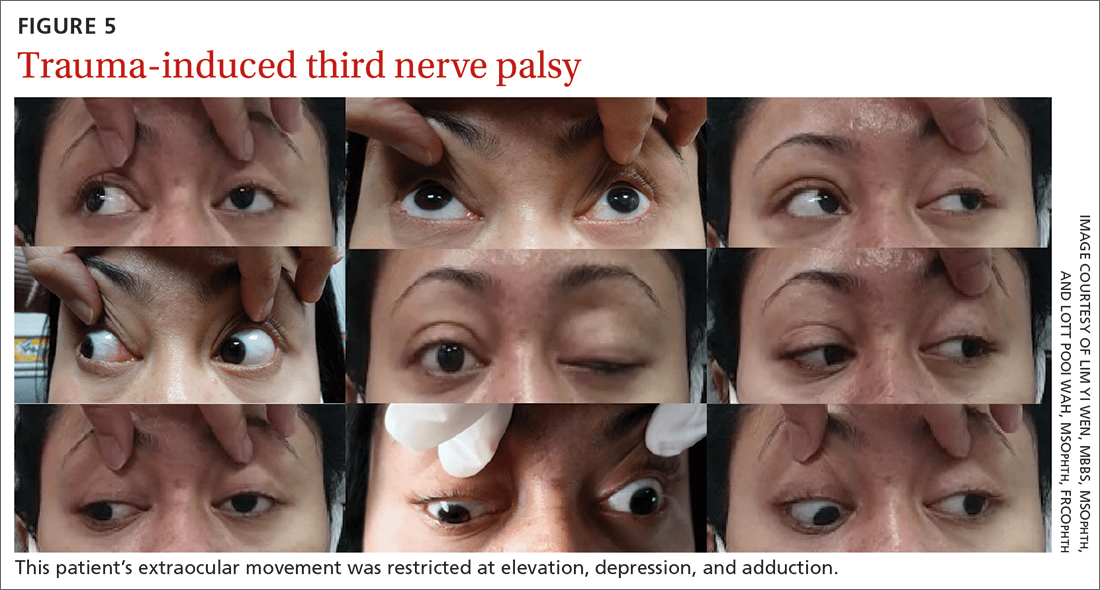
CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of
Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.

Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.

Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.

CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.

CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.

CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of

Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.

Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.

Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.

CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.

CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.

CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of

Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
PRACTICE RECOMMENDATIONS
› Consider microvascular ischemia if third nerve palsy is pupil sparing. C
› Consider computerized tomography (CT) angiography as an alternative to plain CT for first-line study of suspected aneurysm. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tips for treating patients with late-life depression
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients withinsomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients withinsomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients withinsomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
PRACTICE RECOMMENDATIONS
› Begin treatment with a selective serotonin reuptake inhibitor (SSRI) unless another antidepressant has worked well in the past. A
› Consider augmenting therapy with bupropion XL, mirtazapine, aripiprazole, or quetiapine for any patient who responds only partially to an SSRI. C
› Add psychotherapy to antidepressant pharmacotherapy, particularly for patients who have difficulties with executive functions such as planning and organization. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Pulmonary hypertension: An update of Dx and Tx guidelines
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.
Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.
Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7
Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6
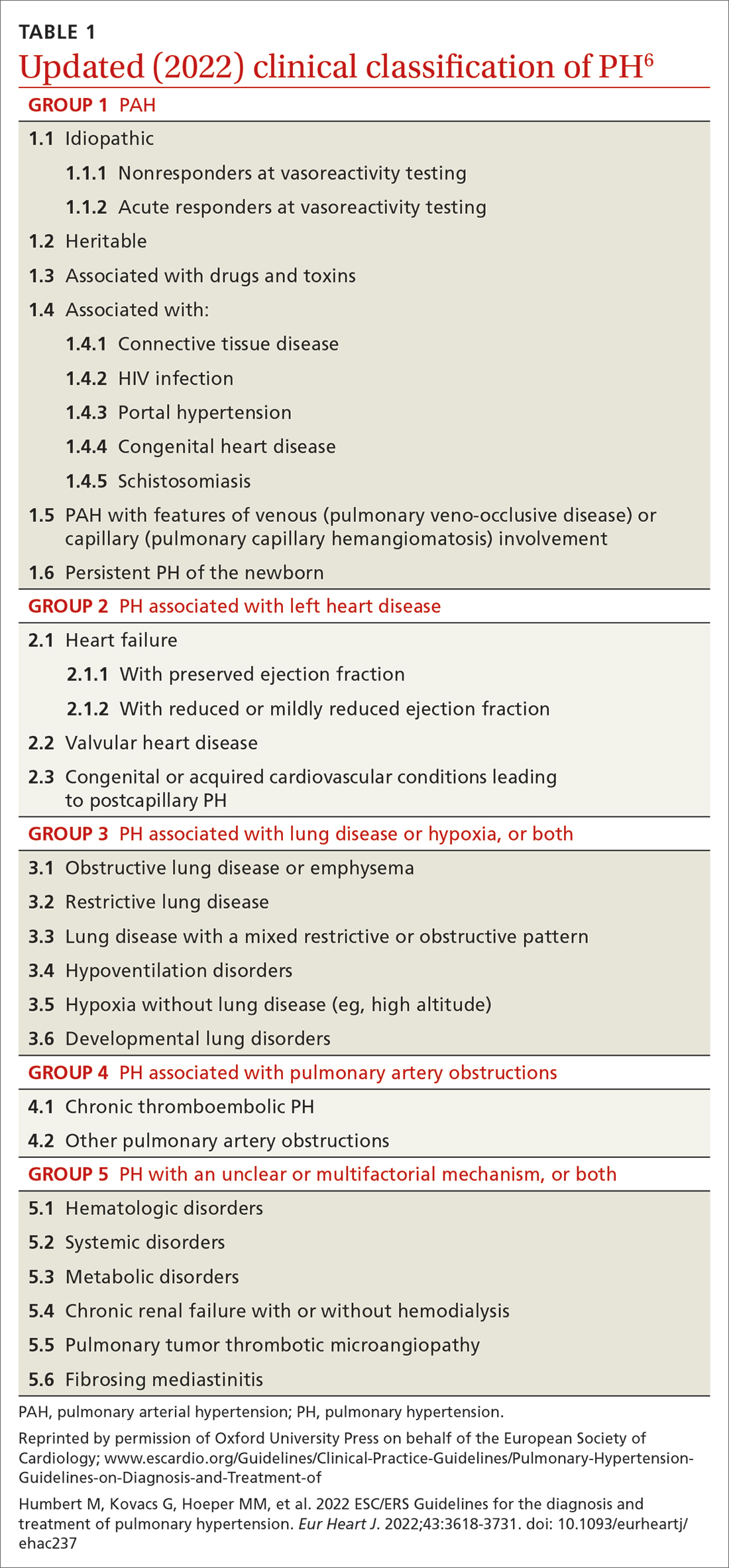
Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).
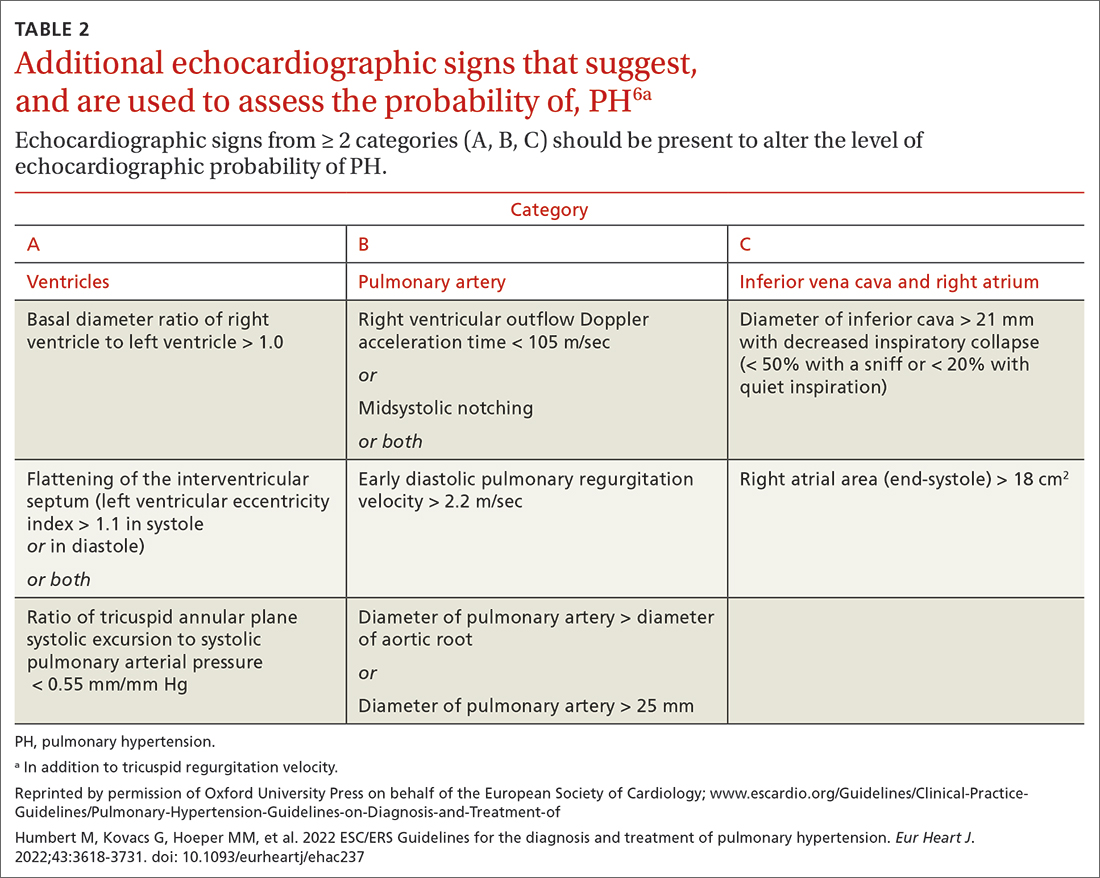
Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18
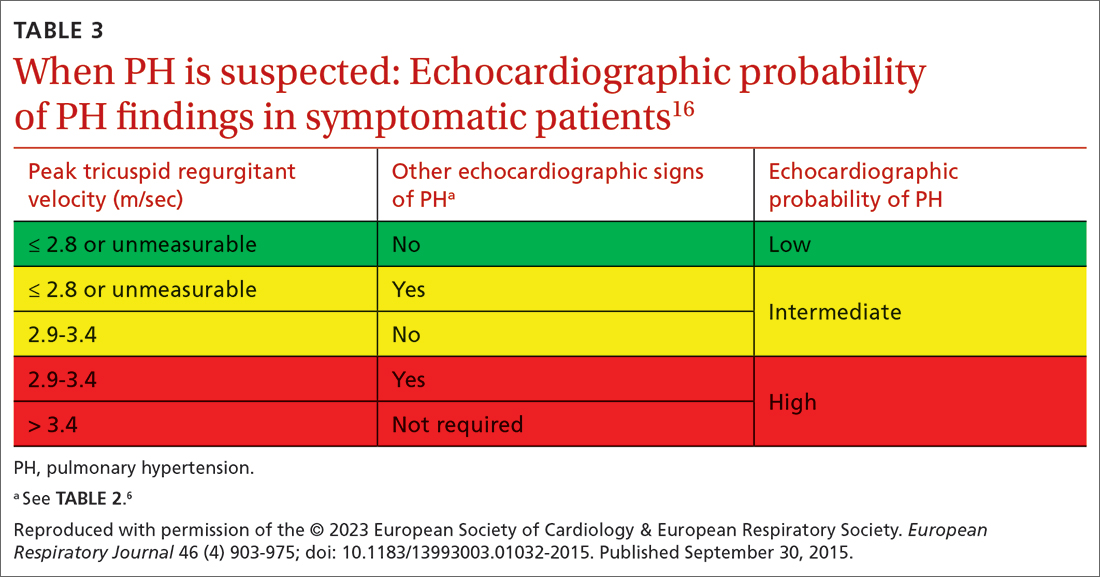
TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
PRACTICE RECOMMENDATIONS
› Employ echocardiography as the first-line diagnostic test when pulmonary hypertension (PH) is suspected. C
› Order a ventilation– perfusion scan in patients with unexplained PH to exclude chronic thromboembolic PH. C
› Order lung function testing with diffusion capacity for carbon monoxide as part of the initial evaluation of PH. C
› Use right heart catheterization to confirm the diagnosis of pulmonary arterial hypertension. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Insomnia diagnosis and treatment across the lifespan
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings,and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59
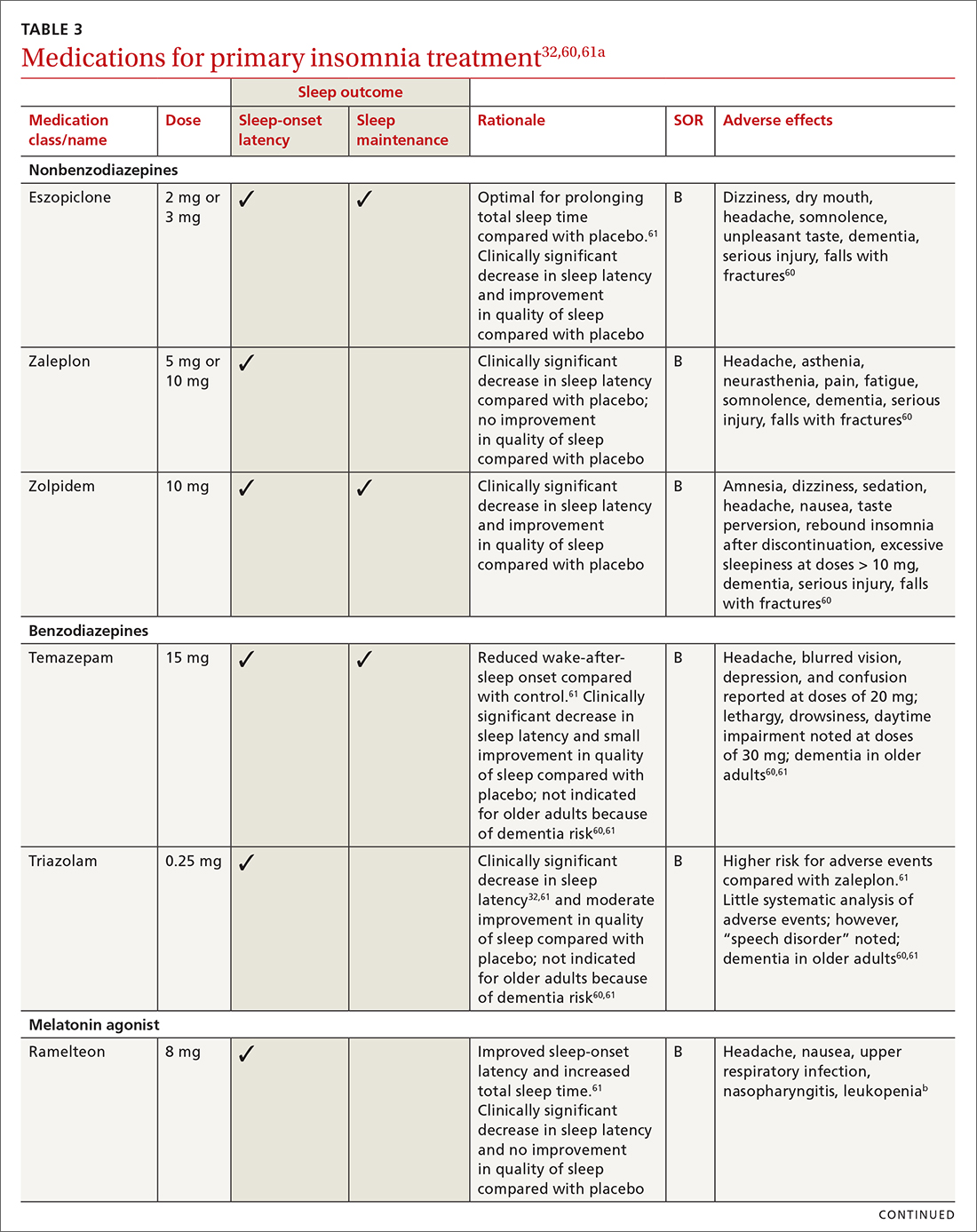
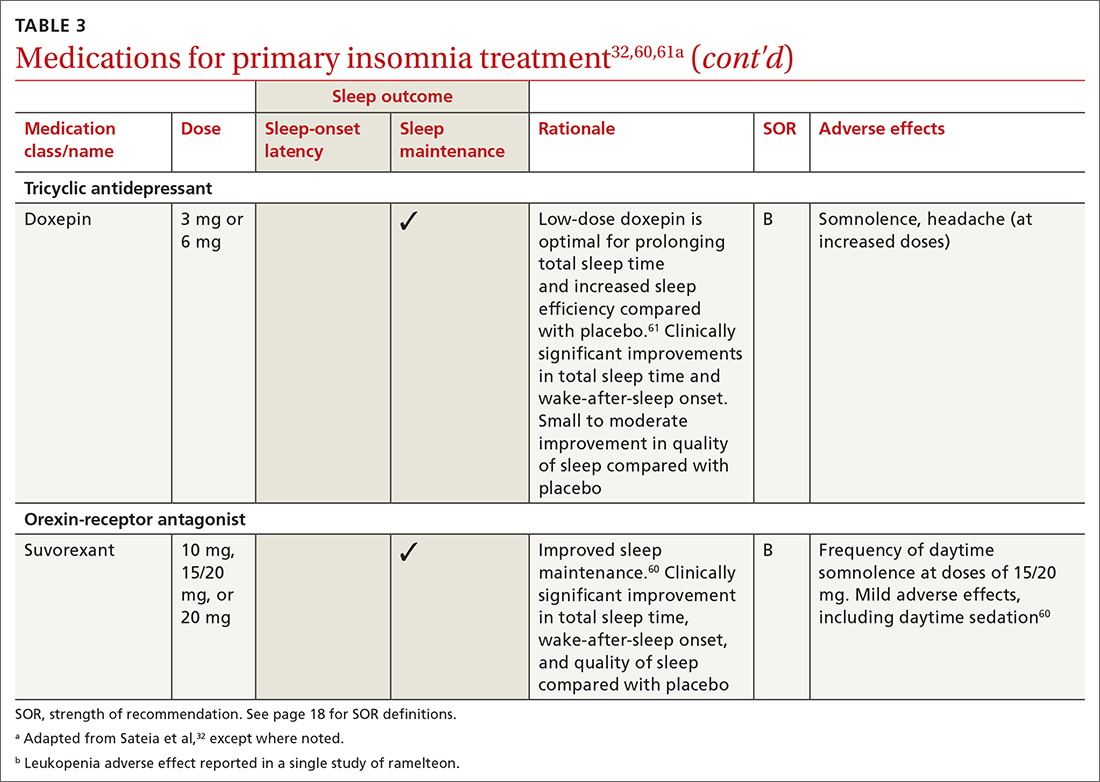
Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61)are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32
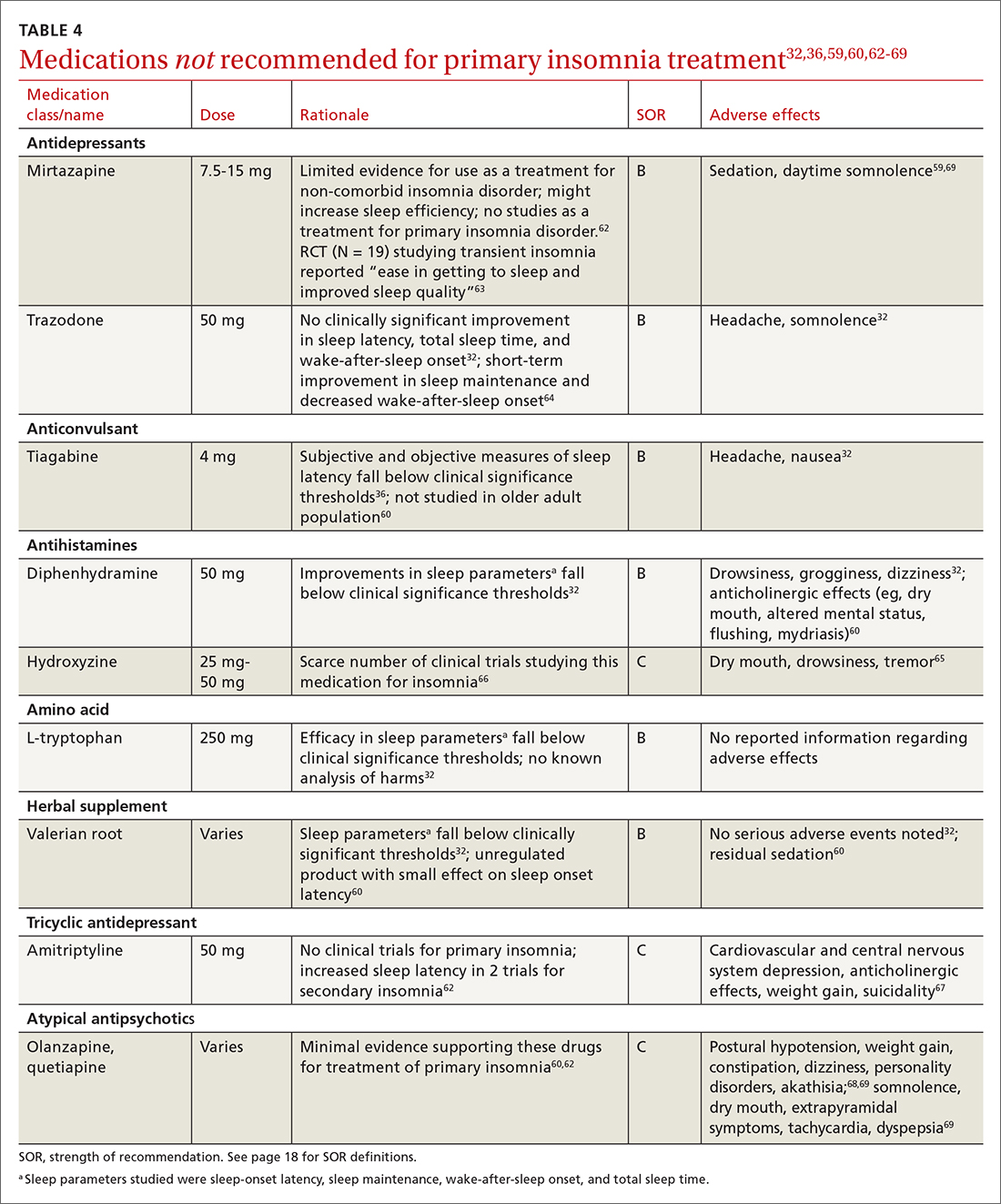
Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; [email protected]
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings,and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59


Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61)are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32

Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; [email protected]
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings,and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59


Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61)are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32

Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; [email protected]
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
PRACTICE RECOMMENDATIONS
› Use a standard validated screening tool for the diagnosis of insomnia in all age groups. A
› Employ nonpharmacologic interventions as first-line treatment for insomnia in all populations. A
› Utilize sleep hygiene or cognitive behavioral therapy for insomnia in adolescents and all adults. A
› Initiate independent cognitive or behavioral therapies with younger children. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series