User login
How to integrate shared decision-making into your practice
Shared decision-making (SDM), a methodology for improving patient communication, education, and outcomes in preference-sensitive health care decisions, debuted in 1989 with the Ottawa Decision Support Framework1 and the creation of the Foundation for Informed Medical Decision Making (now the Informed Medical Decisions Foundation).2 SDM enhances care by actively involving patients as partners in their health care choices. This approach can not only increase patient knowledge and satisfaction with care but also has a beneficial effect on adherence and outcomes.3-5
Despite the significant benefits of SDM, overall uptake of SDM practices remains low—even in situations in which SDM is a requirement for reimbursement, such as in lung cancer screening.6-8 The ever-shifting list of conditions that warrant the implementation of SDM in a family practice can be daunting. Our review seeks to highlight current best practices, review common situations in which SDM would be beneficial, and describe tools and frameworks that can facilitate effective SDM conversations in the typical primary care practice.
Preference-sensitive care
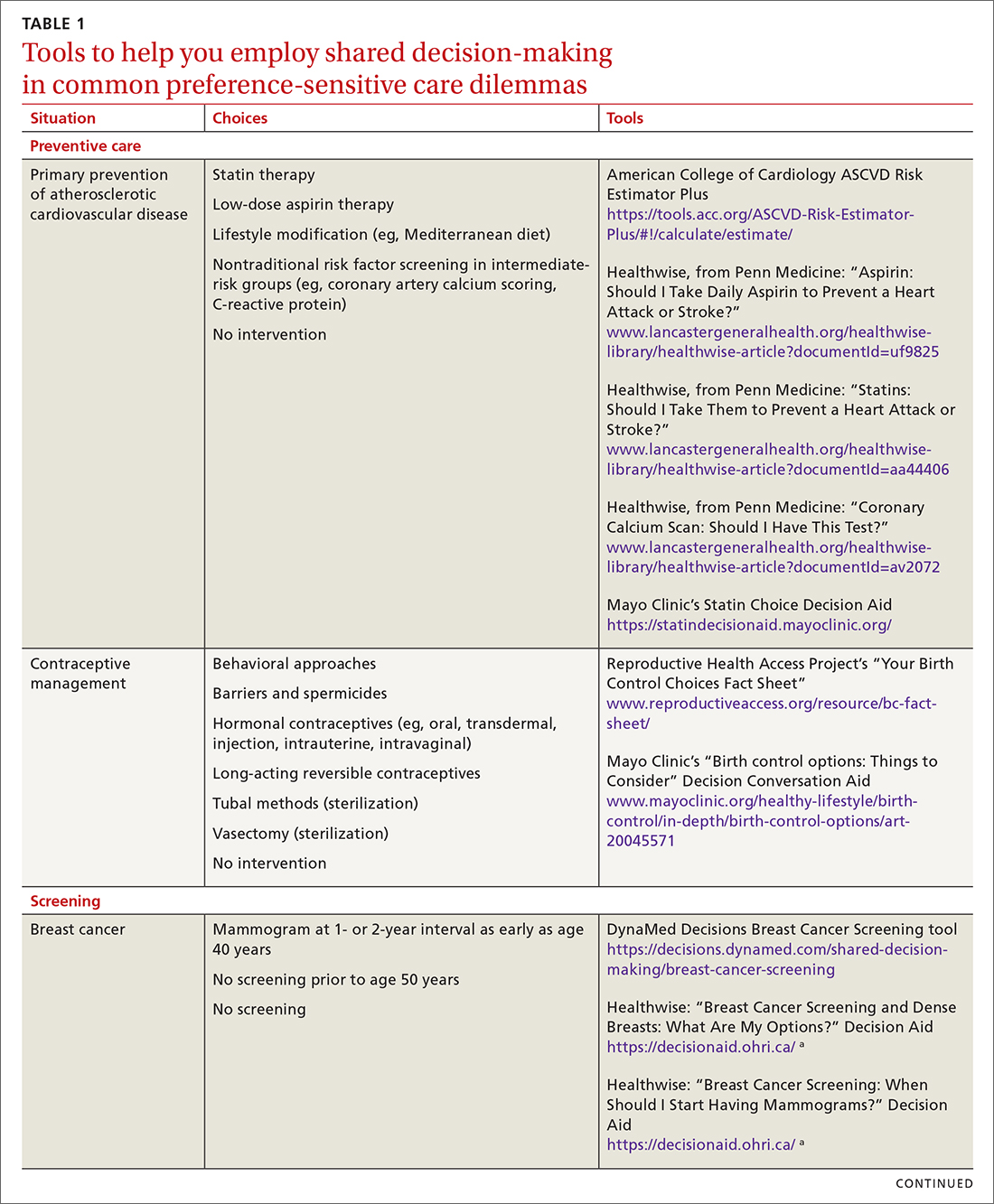
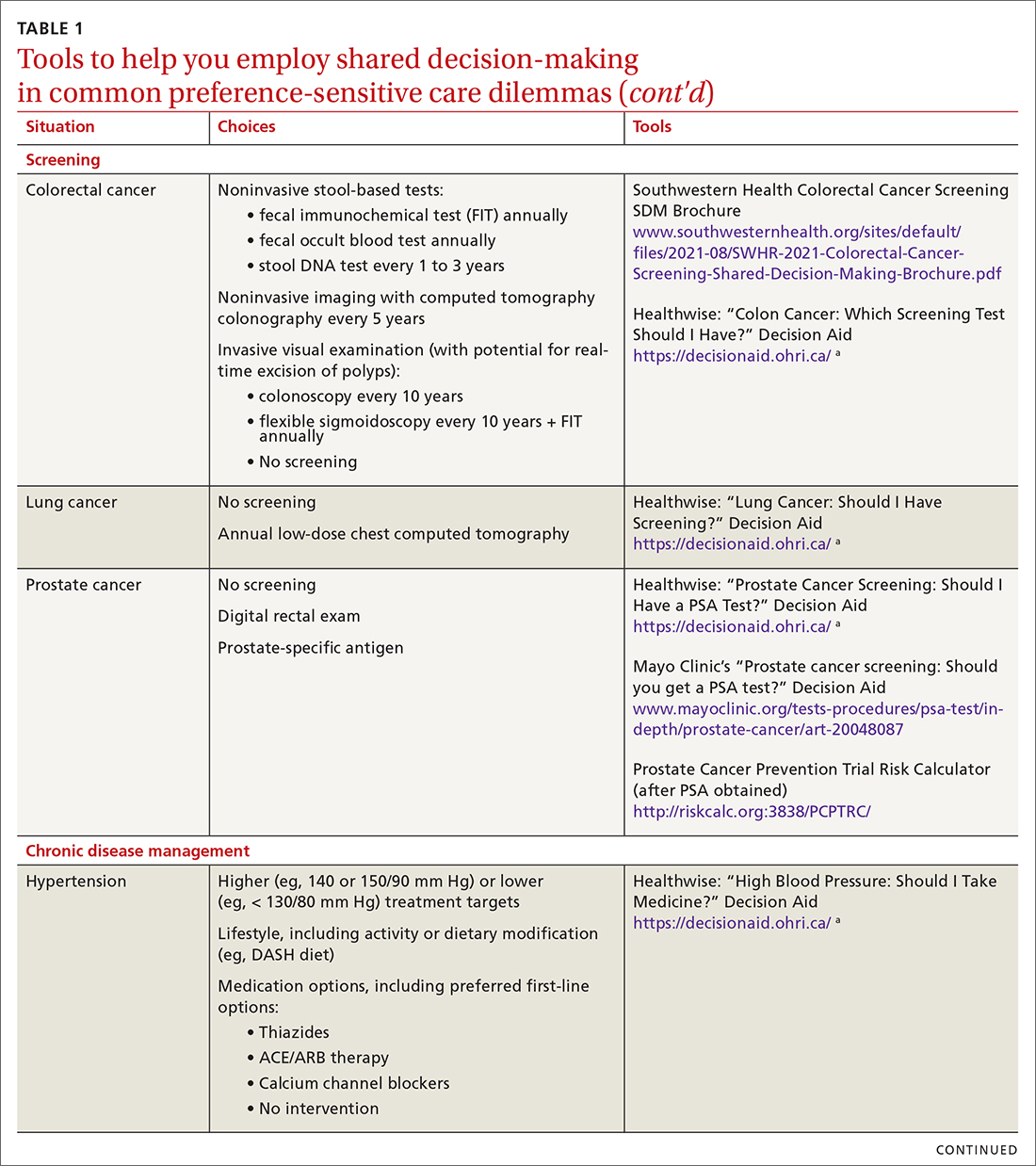
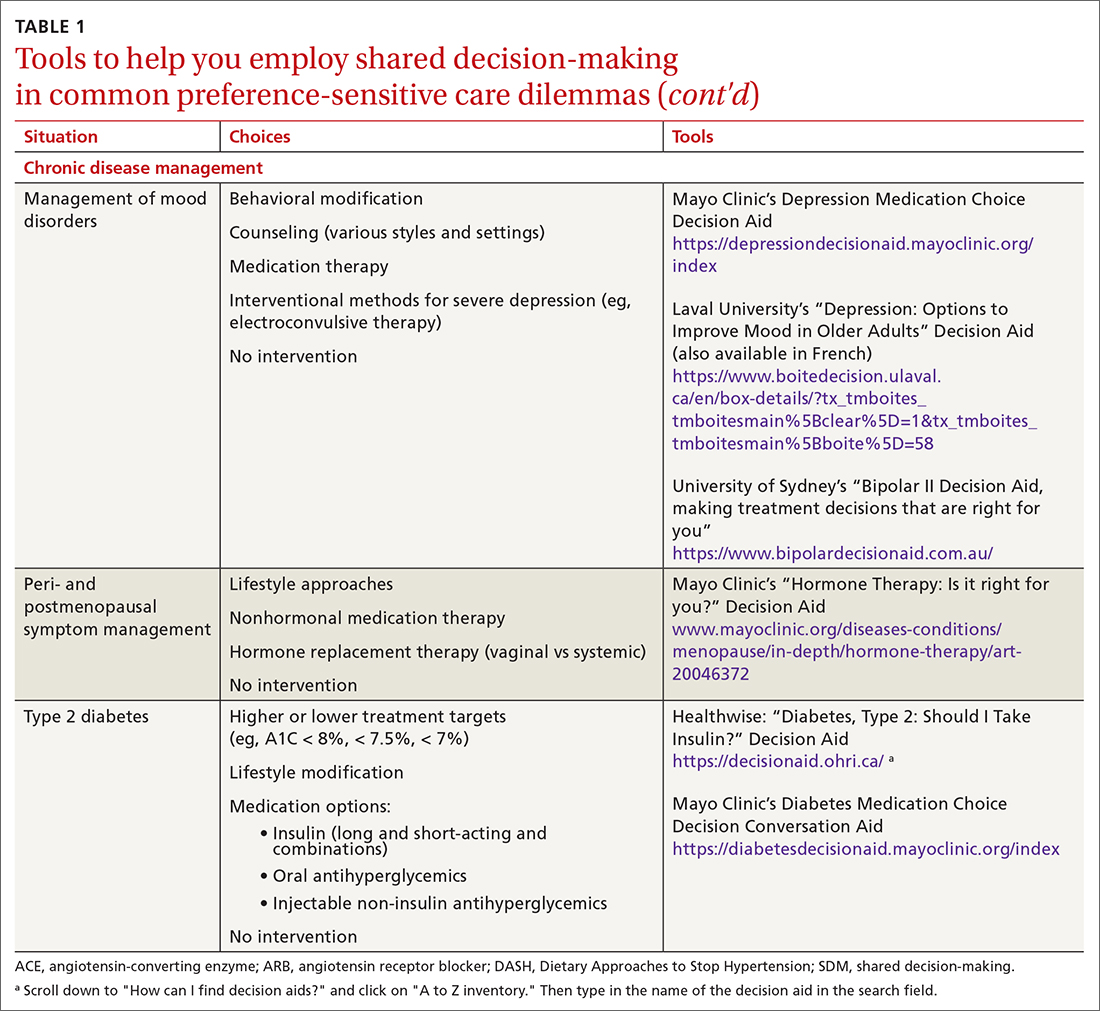
SDM is designed to enhance the role of patient preference, considering a patient’s own personal values for managing clinical conditions when more than one reasonable strategy exists. Such situations are often referred to as preference-sensitive conditions—ie, since evidence is limited on a single “best” treatment approach, patients’ values should impact decision-making.9 Examples of common preference-sensitive situations that include preventive care, screening, and chronic disease management are outlined in TABLE 1.
How to engage patients
In preference-sensitive care situations, SDM endeavors to address uncertainty by laying out what the options are, as well as providing risk and benefit data. This helps inform patients and guides providers about individual patient preference on whether to screen (eg, for average-risk female patients, breast cancer screening between ages 40-50 years). SDM can assist with determining whether to screen and if so, at what interval (eg, at 1- or 2-year intervals), while acknowledging that no single decision would be “best” for every patient.
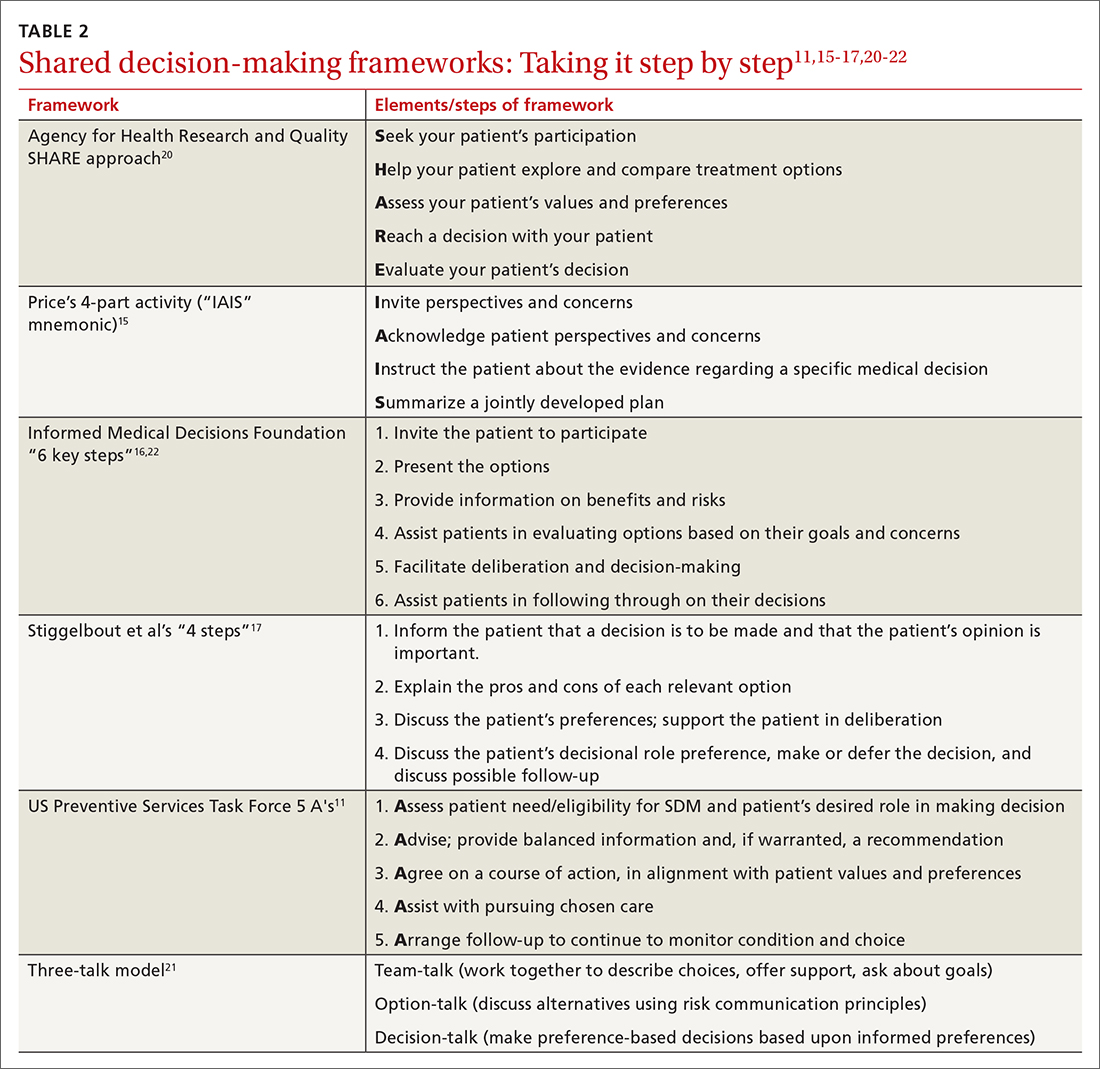
While there are formalized tools to provide information to patients and help them consider their values and choices,3,10 SDM does not hinge on the use of an explicit tool.11-18 There are many approaches to and interpretations of SDM; the Ottawa Decision Support Framework reviews and details these many considerations at length in its 2020 revision.19 TABLE 211,15-17,20-22 highlights various SDM frameworks and the steps involved.
These 3 elements are commonamong SDM frameworks
In a 2019 systematic review, the following 3 elements were highlighted as the most prevalent over time across SDM frameworks and could be considered core to any meaningful SDM process23:
Explicit effort by 2 or more experts. The patient is an expert in their own values. The clinician, as an expert in relevant medical knowledge, clarifies that the current medical situation will benefit from incorporating the patient’s preferences to arrive at an appropriate shared decision.
Continue to: Effort to provide relevant...
Effort to provide relevant, evidence-based information. The clinician provides treatment options applicable to the patient, including the risks and benefits of each (potentially using one of the decision aids in the following section), to facilitate a values-based discussion and decision.
Patient support and assistance. The clinician assists the patient in navigating next steps based on the treatment decision and arranges necessary follow-up.
Various case studies and examples of SDM conversations have been published.15-17,24 Video examples of optimal25 and less than optimal26 SDM conversations are available on the Massachusetts General Hospital Health Decision Sciences Center website (https://mghdecisionsciences.org/) under the section “Tools & Training >> Videos about Shared Decision-Making.”27
SDM and motivational interviewing: Both can serve you well
SDM and motivational interviewing share many common elements,28 and it’s useful to take advantage of both techniques. Preference-sensitive care situations may require a combination of approaches.
For example, motivational interviewing may be a beneficial tool when dealing with a patient who is initially against colon cancer screening (evidence clearly favors screening in some form over no screening) and has a history of avoiding medical care. Through an SDM approach, motivational interviewing may identify an opportunity to prioritize the patient’s preference to minimize medical intervention by ensuring that the patient is familiar with noninvasive colon cancer screening options. After sufficiently eliciting a patient value aligned with screening and engaging the patient’s own motivations for follow-through, a more thorough SDM conversation can then help clarify the best options.
Continue to: A proposed framework...
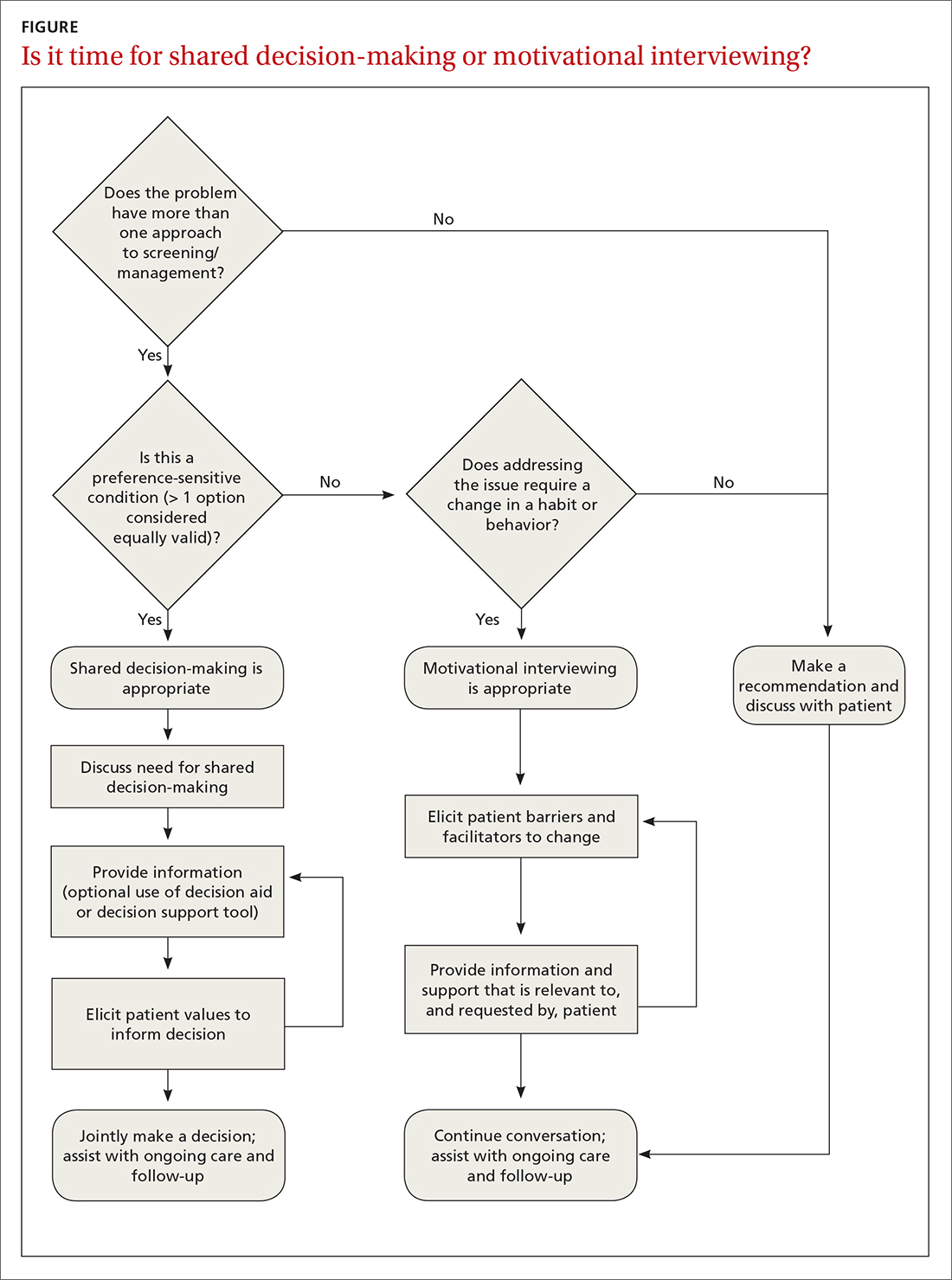
A proposed framework for identifying whether SDM or motivational interviewing is appropriate is featured in the FIGURE. In their paper, Elwyn et al29 further define and discuss the distinguishing features and roles of SDM and behavioral support interventions, such as motivational interviewing.
Tools to facilitate SDM conversations
Decision aids
SDM has historically been operationalized for study through the use of decision aids: formally structured materials describing, in detail, the available treatment options under consideration, including the relative risks and benefits. Frequently, such tools are framed from a patient perspective, with digestible information presented in a multimedia format (eg, visual risk representations of “1 out of 10” in an icon array vs “10%”), leveraging effective risk communication strategies (eg, absolute risk rates vs relative risks and “balanced framing”). For instance, the physician would note that 1 out of 10 patients have an outcome and 9 out of 10 do not.
Additional information on risk communication skills is available at the Agency for Healthcare Research and Quality’s webpage on the SHARE approach (www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html).30 Decision aids have been shown to enhance health literacy, increase patient knowledge and understanding, and promote the frequency of “values-concordant” choices.3
Point-of-care decision support
A more recent trend in SDM is increased development and use of point-of-care decision support tools that emphasize information reflecting individual patient circumstances (eg, leveraging heart risk calculators to individualize risk conversations when considering statins for primary prevention of heart disease based on lipids and other demographic factors). An advantage to using such tools is that they provide “just-in-time” detailed and personalized evidence-based information, guiding the discussion and minimizing the need for an extensive advance review of each topic by emphasizing the “key facts.” To ensure effective use of SDM tools, avoid oversaturating patients with data, maintain a focus on patient values, and engage in a 2-way discussion that considers the unique mix of preferences and circumstances.
Proprietorship of tools and decision aids
Until recently, SDM materials were compiled primarily within not-for-profit entities such as the Informed Medical Decisions Foundation, which became a division of Healthwise in 2014.2 In recent years, there has been an increasing trend of for-profit companies acquiring or developing their own decision aids and decision-support tools, eg, EBSCO Health (Option Grid31 and Health Decision32) and Wolters Kluwer (EMMI33). The extensive work of curating SDM and educational tools to keep up with best medical evidence is costly, and the effort to defray costs can give rise to potential conflicts of interest. Therefore, the interests of the creators of such tools—whether commercial or academic—should always be considered when evaluating the use of a given decision-support tool.
Contunue to: An online listing...
An online listing of publicly available decision aids is maintained by the Ottawa Hospital Research Institute,34 which reviews decision-aid quality by objective criteria in addition to providing direct links to resources.35 EBSCO health’s DynaMed Decisions also maintains a list of shared decision-making tools (https://decisions.dynamed.com/).
Effectiveness of decision aids
There is a robust body of research focused on decision aids for SDM. An example is a 2017 Cochrane review that concluded SDM facilitated by decision aids significantly improved patient engagement and satisfaction and increased patient knowledge, accuracy in risk perception, and congruency in making value-aligned care choices. Beyond decision aids, studies show SDM practices increase patient knowledge, engagement, and satisfaction, particularly among low-literacy or disadvantaged groups.4,36,37
Barriers to implementation
Clinicians frequently cite time constraints as a barrier to successfully implementing SDM in practice, although studies that explicitly compare the time/cost of SDM to “usual care” are limited.38 A Cochrane review of 105 studies evaluating the use of decision aids vs usual care found that only 10 studies examined the effects of decision aids on the length of the office visit.3 Two of these studies (one evaluating decision aids for prenatal diagnostic screening and the other for atrial fibrillation) found a median increase in visit length of 2.6 minutes (24 vs 21; 7.5% increase); the other 8 studies reported no increase in visit length.3
Studies focusing on the time impact of using SDM in an office visit, rather than decision aids as a proxy for SDM, are few. A study by Braddock et al39 assessed the elements of SDM, measuring the quality and the time-efficiency of 141 surgical decision-making interactions between patients and 89 orthopedic surgeons. Researchers found 57% of the discussions had elements of SDM sufficient to meet a “reasonable minimum” standard (eg, nature of the decision, patient’s role, patient’s preference). These conversations took 20 minutes compared to a median of 16 minutes for a more typical conversation.39 The study used audiotaped interviews, which were coded and scored based on the presence of SDM elements; treatment choice, outcomes of the choices, and satisfaction were not reported. A separate study by Loh et al5 looking at SDM in primary care for patients with depression sought to determine whether patient participation in the decision-making process improved treatment adherence, outcomes, and patient satisfaction without increasing consultation time. This study, which included 23 physicians and 405 patients, found improved participation and satisfaction outcomes in the intervention group and no difference in consultation time between the intervention and control groups.5
Care costs appear similar
The impact of SDM on cost and patient-centered clinical outcomes is not well defined. One study by Arterburn et al40 found decision aids and SDM lowered the rates of elective surgery for hip and knee arthritis, as well as associated health system costs. However, other studies suggest this phenomenon likely varies by demographic, demonstrating that certain populations with a generally lower baseline preference for surgery on average chose surgery more often after SDM interventions.41,42 Evidence does support patient acceptability and efficacy for SDM in longitudinal care when the approach is incorporated into decisions over multiple visits or long-term decisions for chronic conditions.4 Studies comparing patient groups receiving decision aids to usual care have shown similar or lower overall care costs for the decision-aid group.3
Continue to: Limitations to the evidence
Limitations to the evidence
Systematic reviews routinely note substantial heterogeneity in the literature on SDM use, owing to variable definitions of what steps are essential to constitute an SDM intervention and a wide variety of outcome measures used, as well as the broad range of conditions to which SDM is potentially applicable.3,4,10,36,37,43-45 While efforts in SDM education, uptake, and study frequently adapt frameworks such as those outlined in TABLE 2,11,15-17,20-22 there is as yet no one consensus on the “best” approach to SDM, and explicit study of any given approach is limited.18,23,36,44-46 There remains a clear need to improve the uptake of existing reporting standards to ensure the future evidence base will be of high quality.44 In the meantime, a large portion of the impetus for expanding the use of SDM remains based on principles of effective communication and championing a patient-centered philosophy of care.
Cultivating an effective approach
An oft-cited objection to the use of SDM in day-to-day clinical care is that it “takes too much time.”47 Like all excellent communication skills, SDM is best incorporated into a clinician’s approach to patient care. With practice, we have found this can be accomplished during routine patient encounters—eg, when providing general counsel, giving advice, providing education, answering questions. Given the interdependent relationship between evidence-based medicine and SDM, particularly in preference-sensitive conditions, SDM skills can facilitate efficient decision-making and patient satisfaction.48 To that end, clinician training on SDM techniques, especially those that emphasize the 3 core elements, can be particularly beneficial. These broadly applicable skills can be leveraged in an “SDM mindset,” even outside traditional preference-sensitive care situations, to enhance clinician–patient rapport, relationship, and satisfaction.
The future of SDM
More than 2 decades after SDM was introduced to clinical care, there remains much to do to improve uptake in primary care settings. An important strategy to increase the successful uptake of SDM for the typical clinician and patient is to emphasize the approach to framing the topic and discussion rather than to overemphasize decision aids.23 Continuing the trend of well-designed and accessible tools for clinical decision support at the point of care for clinicians, in addition to the sustained evolution of decision aids for patients, should help minimize the need for extensive background knowledge on a topic, increase accessibility, and enable an effective partnership with patients in their health care decisions.46 Ongoing, well-structured study and the use of common proposed standards in developing these tools and studying SDM implementation will provide long-term quality assurance.44
SDM has a role to play in health equity
SDM has a clear role to play in addressing health inequities. Values vary from person to person, and individuals exist along a variety of cultural, community, and other spectra that strongly influence their perception of what is most important to them. Moreover, clinicians’ assumptions typically do not correspond to a patient’s actual desire to engage in SDM nor to their overall likelihood of choosing any given treatment option.46 While many clinicians believe patients do not participate in SDM because they simply do not wish to, a systematic review and thematic synthesis by Joseph-Williams et al46 suggested a great number of patients are instead unable to take part in SDM due to barriers such as a lack of time availability, challenges in the structure of the health care system itself, and factors specific to the clinician–patient interaction such as patients feeling as though they don’t have “permission” to participate in SDM.
SDM may improve health equity, adherence, and outcomes in certain groups. For example, SDM has been suggested as a potential means to address disparities in outcomes for populations disproportionately affected by hypertension.24 The increased implementation of SDM practices, coupled with a genuine partnership between patients and care teams, may improve patient–clinician communication, enhance understanding of patient concerns and goals, and perhaps ultimately increase patient engagement and adherence.
Continue to: Being the change
Being the change
Effective framing of medical decisions in the context of best medical evidence and eliciting patient values supports continued evolution in health care delivery. The traditional, physician-directed patriarchal “one-size-fits-all” approach has evolved. Through the continued development and implementation of SDM techniques, the clinician’s approach to care will continue to advance.
Ultimately, patients and clinicians both benefit from the use of SDM—the patient benefits from explicit framing of the medical facts most relevant to their decision, and the physician benefits from enhanced knowledge of the patient’s values and considerations. When done well, SDM increases the likelihood that patients will receive the best care possible, concordant with their values and preferences and within the context of their unique circumstances, leading to improved knowledge, adherence, outcomes, and satisfaction.
CORRESPONDENCE
Matthew Mackwood, MD, One Medical Center Drive, Lebanon, NH 03756; [email protected]
1. Ottawa Hospital Research Institute. Mission and history—patient decision aids. Accessed October 20, 2022. https://decisionaid.ohri.ca/mission.html
2. Healthwise. Informed Medical Decision Foundation. Accessed October 20, 2022. www.healthwise.org/specialpages/imdf.aspx
3. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5
4. Joosten EAG, DeFuentes-Merillas L, De Weert G, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219-226. doi: 10.1159/000126073
5. Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324-332. doi: 10.1016/j.pec.2007.03.023
6. Goodwin JS, Nishi S, Zhou J, et al. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179:716-718. doi: 10.1001/jamain ternmed.2018.6405
7. Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision-making for lung cancer screening. JAMA Intern Med. 2018;178:1311-1316. doi: 10.1001/jamainternmed.2018.3054
8. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi: 10.1016/j.chest.2021.01.041
9. Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69-79. doi: 10.1353/pbm.2003.000
10. Hoefel L, O’Connor AM, Lewis KB, et al. 20th Anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555-581. doi: 10.1177/0272989X20936209
11. Sheridan SL, Harris RP, Woolf SH. Shared decision-making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56-66. doi: 10.1016/j.amepre.2003.09.011
12. Elwyn G, Frosch D, Thomson R, et al. Shared decision-making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367. doi: 10.1007/s11606-012-2077-6
13. Fowler FJ Jr, Barry MJ, Sepucha KR, et al. Let’s require patients to review a high-quality decision aid before receiving important tests and treatments. Med Care. 2021;59:1-5. doi: 10.1097/MLR.0000000000001440
14. Hargraves IG, Fournier AK, Montori VM, et al. Generalized shared decision-making approaches and patient problems. Adapting AHRQ’s SHARE approach for purposeful SDM. Patient Educ Couns. 2020;103:2192-2199. doi: 10.1016/j.pec.2020.06.022
15. Price D. Sharing clinical decisions by discussing evidence with patients. Perm J. 2005;9:70-73. doi: 10.7812/TPP/05-006
16. Schrager S, Phillips G, Burnside E. Shared decision-making in cancer screening. Fam Pract Manag. 2017;24:5-10.
17. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision-making: concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. doi: 10.1016/j.pec.2015.06.022
18. Hargraves I, LeBlanc A, Shah ND, et al. Shared decision-making: the need for patient-clinician conversation, not just information. Health Aff (Milford). 2016;35:627-629. doi: 10.1377/hlthaff.2015.1354
19. Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa Decision Support Framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. 2020;40:379-398. doi: 10.1177/0272989X20911870
20. Agency for Health Research and Quality. The SHARE Approach. Accessed November 24, 2021, www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
21. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision-making: multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891
22. Healthwise – Informed Medical Decisions Foundation. The six steps of shared decision making. Accessed December 21, 2022. http://cdn-www.informedmedicaldecisions.org/imdfdocs/SixStepsSDM_CARD.pdf
23. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, et al. Key components of shared decision-making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-03176
24. Langford AT, Williams SK, Applegate M, et al. Partnerships to improve shared decision making for patients with hypertension - health equity implications. Ethn Dis. 2019;29(suppl 1):97-102. doi: 10.18865/ed.29.S1.97
25. MGH Health Decision Sciences Center. High cholesterol visit version 2. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=o2mZ9duJW0A
26. MGH Health Decision Sciences Center. High cholesterol visit version 1. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=0NdDMKS8DwU
27. MGH Health Decision Sciences Center. Videos about shared decision-making. Accessed October 20, 2022. https://mghdecision sciences.org/tools-training/sdmvideos/
28. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision-making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12:270-275. doi: 10.1370/afm.1615. Published correction in Ann Fam Med. 2014;12:301. doi: 10.1370/afm.1674
29. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision-making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75
30. Agency for Health Research and Quality. The SHARE approach—communicating numbers to your patients: a reference guide for health care providers. Workshop curriculum: tool 5. Accessed October 21, 2022. www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html
31. EBSCO. Accessed October 21, 2022. https://optiongrid.ebsco.com/about
32. HealthDecision. HealthDecision - Decision Support & Shared decision-making for Clinicians & Patients at the Point of Care. Accessed November 24, 2021. www.healthdecision.com/ [Now DynaMed Decisions, https://decisions.dynamed.com/]
33. Wolters Kluwer. EmmiEngage: guide patients in their care journeys. Accessed October 21, 2022. www.wolterskluwer.com/en/solutions/emmi/emmi-engage
34. The Ottawa Hospital Research Institute. Patient decision aids. Accessed October 21, 2022. https://decisionaid.ohri.ca/Azinvent.php
35. The Ottawa Hospital Research Institute. Alphabetical list of decision aids by health topic. Accessed October 21, 2022. https://decisionaid.ohri.ca/AZlist.html
36. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision-making and patient outcomes. Med Decis Making. 2015;35:114-131. doi: 10.1177/0272989X14551638
37. Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670
38. Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision-making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32:268-275. doi: 10.1377/hlthaff.2012.1084
39. Braddock C 3rd, Hudak PL, Feldman JJ, et al. “Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgery. J Bone Joint Surg Am. 2008;90:1830-1838. doi: 10.2106/JBJS.G.00840
40. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31:2094-2104. doi: 10.1377/hlthaff.2011.0686.
41. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res. 2016;474:1755-1764. doi: 10.1007/s11999-016-4834-z
42. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for Black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152:e164225. doi: 10.1001/jamasurg.2016.4225
43. Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9-18. doi: 10.1016/j.pec.2011.02.004
44. Trenaman L, Jansen J, Blumenthal-Barby J, et al. Are we improving? Update and critical appraisal of the reporting of decision process and quality measures in trials evaluating patient decision aids. Med Decis Making. 2021;41:954-959. doi: 10.1177/0272989x211011120
45. Hoefel L, Lewis KB, O’Connor A, et al. 20th anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. 2020;40:522-539. doi: 10.1177/0272989X20924645
46. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision-making. Patient Educ Couns. 2014;94:291-309. doi: 10.1016/j.pec.2013.10.031
47. Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535. doi: 10.1016/ j.pec.2008.07.018
48. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision-making. JAMA. 2014;312:1295-1296. doi:10.1001/jama.2014.10186
Shared decision-making (SDM), a methodology for improving patient communication, education, and outcomes in preference-sensitive health care decisions, debuted in 1989 with the Ottawa Decision Support Framework1 and the creation of the Foundation for Informed Medical Decision Making (now the Informed Medical Decisions Foundation).2 SDM enhances care by actively involving patients as partners in their health care choices. This approach can not only increase patient knowledge and satisfaction with care but also has a beneficial effect on adherence and outcomes.3-5
Despite the significant benefits of SDM, overall uptake of SDM practices remains low—even in situations in which SDM is a requirement for reimbursement, such as in lung cancer screening.6-8 The ever-shifting list of conditions that warrant the implementation of SDM in a family practice can be daunting. Our review seeks to highlight current best practices, review common situations in which SDM would be beneficial, and describe tools and frameworks that can facilitate effective SDM conversations in the typical primary care practice.
Preference-sensitive care
SDM is designed to enhance the role of patient preference, considering a patient’s own personal values for managing clinical conditions when more than one reasonable strategy exists. Such situations are often referred to as preference-sensitive conditions—ie, since evidence is limited on a single “best” treatment approach, patients’ values should impact decision-making.9 Examples of common preference-sensitive situations that include preventive care, screening, and chronic disease management are outlined in TABLE 1.



How to engage patients
In preference-sensitive care situations, SDM endeavors to address uncertainty by laying out what the options are, as well as providing risk and benefit data. This helps inform patients and guides providers about individual patient preference on whether to screen (eg, for average-risk female patients, breast cancer screening between ages 40-50 years). SDM can assist with determining whether to screen and if so, at what interval (eg, at 1- or 2-year intervals), while acknowledging that no single decision would be “best” for every patient.
While there are formalized tools to provide information to patients and help them consider their values and choices,3,10 SDM does not hinge on the use of an explicit tool.11-18 There are many approaches to and interpretations of SDM; the Ottawa Decision Support Framework reviews and details these many considerations at length in its 2020 revision.19 TABLE 211,15-17,20-22 highlights various SDM frameworks and the steps involved.

These 3 elements are commonamong SDM frameworks
In a 2019 systematic review, the following 3 elements were highlighted as the most prevalent over time across SDM frameworks and could be considered core to any meaningful SDM process23:
Explicit effort by 2 or more experts. The patient is an expert in their own values. The clinician, as an expert in relevant medical knowledge, clarifies that the current medical situation will benefit from incorporating the patient’s preferences to arrive at an appropriate shared decision.
Continue to: Effort to provide relevant...
Effort to provide relevant, evidence-based information. The clinician provides treatment options applicable to the patient, including the risks and benefits of each (potentially using one of the decision aids in the following section), to facilitate a values-based discussion and decision.
Patient support and assistance. The clinician assists the patient in navigating next steps based on the treatment decision and arranges necessary follow-up.
Various case studies and examples of SDM conversations have been published.15-17,24 Video examples of optimal25 and less than optimal26 SDM conversations are available on the Massachusetts General Hospital Health Decision Sciences Center website (https://mghdecisionsciences.org/) under the section “Tools & Training >> Videos about Shared Decision-Making.”27
SDM and motivational interviewing: Both can serve you well
SDM and motivational interviewing share many common elements,28 and it’s useful to take advantage of both techniques. Preference-sensitive care situations may require a combination of approaches.
For example, motivational interviewing may be a beneficial tool when dealing with a patient who is initially against colon cancer screening (evidence clearly favors screening in some form over no screening) and has a history of avoiding medical care. Through an SDM approach, motivational interviewing may identify an opportunity to prioritize the patient’s preference to minimize medical intervention by ensuring that the patient is familiar with noninvasive colon cancer screening options. After sufficiently eliciting a patient value aligned with screening and engaging the patient’s own motivations for follow-through, a more thorough SDM conversation can then help clarify the best options.
Continue to: A proposed framework...
A proposed framework for identifying whether SDM or motivational interviewing is appropriate is featured in the FIGURE. In their paper, Elwyn et al29 further define and discuss the distinguishing features and roles of SDM and behavioral support interventions, such as motivational interviewing.

Tools to facilitate SDM conversations
Decision aids
SDM has historically been operationalized for study through the use of decision aids: formally structured materials describing, in detail, the available treatment options under consideration, including the relative risks and benefits. Frequently, such tools are framed from a patient perspective, with digestible information presented in a multimedia format (eg, visual risk representations of “1 out of 10” in an icon array vs “10%”), leveraging effective risk communication strategies (eg, absolute risk rates vs relative risks and “balanced framing”). For instance, the physician would note that 1 out of 10 patients have an outcome and 9 out of 10 do not.
Additional information on risk communication skills is available at the Agency for Healthcare Research and Quality’s webpage on the SHARE approach (www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html).30 Decision aids have been shown to enhance health literacy, increase patient knowledge and understanding, and promote the frequency of “values-concordant” choices.3
Point-of-care decision support
A more recent trend in SDM is increased development and use of point-of-care decision support tools that emphasize information reflecting individual patient circumstances (eg, leveraging heart risk calculators to individualize risk conversations when considering statins for primary prevention of heart disease based on lipids and other demographic factors). An advantage to using such tools is that they provide “just-in-time” detailed and personalized evidence-based information, guiding the discussion and minimizing the need for an extensive advance review of each topic by emphasizing the “key facts.” To ensure effective use of SDM tools, avoid oversaturating patients with data, maintain a focus on patient values, and engage in a 2-way discussion that considers the unique mix of preferences and circumstances.
Proprietorship of tools and decision aids
Until recently, SDM materials were compiled primarily within not-for-profit entities such as the Informed Medical Decisions Foundation, which became a division of Healthwise in 2014.2 In recent years, there has been an increasing trend of for-profit companies acquiring or developing their own decision aids and decision-support tools, eg, EBSCO Health (Option Grid31 and Health Decision32) and Wolters Kluwer (EMMI33). The extensive work of curating SDM and educational tools to keep up with best medical evidence is costly, and the effort to defray costs can give rise to potential conflicts of interest. Therefore, the interests of the creators of such tools—whether commercial or academic—should always be considered when evaluating the use of a given decision-support tool.
Contunue to: An online listing...
An online listing of publicly available decision aids is maintained by the Ottawa Hospital Research Institute,34 which reviews decision-aid quality by objective criteria in addition to providing direct links to resources.35 EBSCO health’s DynaMed Decisions also maintains a list of shared decision-making tools (https://decisions.dynamed.com/).
Effectiveness of decision aids
There is a robust body of research focused on decision aids for SDM. An example is a 2017 Cochrane review that concluded SDM facilitated by decision aids significantly improved patient engagement and satisfaction and increased patient knowledge, accuracy in risk perception, and congruency in making value-aligned care choices. Beyond decision aids, studies show SDM practices increase patient knowledge, engagement, and satisfaction, particularly among low-literacy or disadvantaged groups.4,36,37
Barriers to implementation
Clinicians frequently cite time constraints as a barrier to successfully implementing SDM in practice, although studies that explicitly compare the time/cost of SDM to “usual care” are limited.38 A Cochrane review of 105 studies evaluating the use of decision aids vs usual care found that only 10 studies examined the effects of decision aids on the length of the office visit.3 Two of these studies (one evaluating decision aids for prenatal diagnostic screening and the other for atrial fibrillation) found a median increase in visit length of 2.6 minutes (24 vs 21; 7.5% increase); the other 8 studies reported no increase in visit length.3
Studies focusing on the time impact of using SDM in an office visit, rather than decision aids as a proxy for SDM, are few. A study by Braddock et al39 assessed the elements of SDM, measuring the quality and the time-efficiency of 141 surgical decision-making interactions between patients and 89 orthopedic surgeons. Researchers found 57% of the discussions had elements of SDM sufficient to meet a “reasonable minimum” standard (eg, nature of the decision, patient’s role, patient’s preference). These conversations took 20 minutes compared to a median of 16 minutes for a more typical conversation.39 The study used audiotaped interviews, which were coded and scored based on the presence of SDM elements; treatment choice, outcomes of the choices, and satisfaction were not reported. A separate study by Loh et al5 looking at SDM in primary care for patients with depression sought to determine whether patient participation in the decision-making process improved treatment adherence, outcomes, and patient satisfaction without increasing consultation time. This study, which included 23 physicians and 405 patients, found improved participation and satisfaction outcomes in the intervention group and no difference in consultation time between the intervention and control groups.5
Care costs appear similar
The impact of SDM on cost and patient-centered clinical outcomes is not well defined. One study by Arterburn et al40 found decision aids and SDM lowered the rates of elective surgery for hip and knee arthritis, as well as associated health system costs. However, other studies suggest this phenomenon likely varies by demographic, demonstrating that certain populations with a generally lower baseline preference for surgery on average chose surgery more often after SDM interventions.41,42 Evidence does support patient acceptability and efficacy for SDM in longitudinal care when the approach is incorporated into decisions over multiple visits or long-term decisions for chronic conditions.4 Studies comparing patient groups receiving decision aids to usual care have shown similar or lower overall care costs for the decision-aid group.3
Continue to: Limitations to the evidence
Limitations to the evidence
Systematic reviews routinely note substantial heterogeneity in the literature on SDM use, owing to variable definitions of what steps are essential to constitute an SDM intervention and a wide variety of outcome measures used, as well as the broad range of conditions to which SDM is potentially applicable.3,4,10,36,37,43-45 While efforts in SDM education, uptake, and study frequently adapt frameworks such as those outlined in TABLE 2,11,15-17,20-22 there is as yet no one consensus on the “best” approach to SDM, and explicit study of any given approach is limited.18,23,36,44-46 There remains a clear need to improve the uptake of existing reporting standards to ensure the future evidence base will be of high quality.44 In the meantime, a large portion of the impetus for expanding the use of SDM remains based on principles of effective communication and championing a patient-centered philosophy of care.
Cultivating an effective approach
An oft-cited objection to the use of SDM in day-to-day clinical care is that it “takes too much time.”47 Like all excellent communication skills, SDM is best incorporated into a clinician’s approach to patient care. With practice, we have found this can be accomplished during routine patient encounters—eg, when providing general counsel, giving advice, providing education, answering questions. Given the interdependent relationship between evidence-based medicine and SDM, particularly in preference-sensitive conditions, SDM skills can facilitate efficient decision-making and patient satisfaction.48 To that end, clinician training on SDM techniques, especially those that emphasize the 3 core elements, can be particularly beneficial. These broadly applicable skills can be leveraged in an “SDM mindset,” even outside traditional preference-sensitive care situations, to enhance clinician–patient rapport, relationship, and satisfaction.
The future of SDM
More than 2 decades after SDM was introduced to clinical care, there remains much to do to improve uptake in primary care settings. An important strategy to increase the successful uptake of SDM for the typical clinician and patient is to emphasize the approach to framing the topic and discussion rather than to overemphasize decision aids.23 Continuing the trend of well-designed and accessible tools for clinical decision support at the point of care for clinicians, in addition to the sustained evolution of decision aids for patients, should help minimize the need for extensive background knowledge on a topic, increase accessibility, and enable an effective partnership with patients in their health care decisions.46 Ongoing, well-structured study and the use of common proposed standards in developing these tools and studying SDM implementation will provide long-term quality assurance.44
SDM has a role to play in health equity
SDM has a clear role to play in addressing health inequities. Values vary from person to person, and individuals exist along a variety of cultural, community, and other spectra that strongly influence their perception of what is most important to them. Moreover, clinicians’ assumptions typically do not correspond to a patient’s actual desire to engage in SDM nor to their overall likelihood of choosing any given treatment option.46 While many clinicians believe patients do not participate in SDM because they simply do not wish to, a systematic review and thematic synthesis by Joseph-Williams et al46 suggested a great number of patients are instead unable to take part in SDM due to barriers such as a lack of time availability, challenges in the structure of the health care system itself, and factors specific to the clinician–patient interaction such as patients feeling as though they don’t have “permission” to participate in SDM.
SDM may improve health equity, adherence, and outcomes in certain groups. For example, SDM has been suggested as a potential means to address disparities in outcomes for populations disproportionately affected by hypertension.24 The increased implementation of SDM practices, coupled with a genuine partnership between patients and care teams, may improve patient–clinician communication, enhance understanding of patient concerns and goals, and perhaps ultimately increase patient engagement and adherence.
Continue to: Being the change
Being the change
Effective framing of medical decisions in the context of best medical evidence and eliciting patient values supports continued evolution in health care delivery. The traditional, physician-directed patriarchal “one-size-fits-all” approach has evolved. Through the continued development and implementation of SDM techniques, the clinician’s approach to care will continue to advance.
Ultimately, patients and clinicians both benefit from the use of SDM—the patient benefits from explicit framing of the medical facts most relevant to their decision, and the physician benefits from enhanced knowledge of the patient’s values and considerations. When done well, SDM increases the likelihood that patients will receive the best care possible, concordant with their values and preferences and within the context of their unique circumstances, leading to improved knowledge, adherence, outcomes, and satisfaction.
CORRESPONDENCE
Matthew Mackwood, MD, One Medical Center Drive, Lebanon, NH 03756; [email protected]
Shared decision-making (SDM), a methodology for improving patient communication, education, and outcomes in preference-sensitive health care decisions, debuted in 1989 with the Ottawa Decision Support Framework1 and the creation of the Foundation for Informed Medical Decision Making (now the Informed Medical Decisions Foundation).2 SDM enhances care by actively involving patients as partners in their health care choices. This approach can not only increase patient knowledge and satisfaction with care but also has a beneficial effect on adherence and outcomes.3-5
Despite the significant benefits of SDM, overall uptake of SDM practices remains low—even in situations in which SDM is a requirement for reimbursement, such as in lung cancer screening.6-8 The ever-shifting list of conditions that warrant the implementation of SDM in a family practice can be daunting. Our review seeks to highlight current best practices, review common situations in which SDM would be beneficial, and describe tools and frameworks that can facilitate effective SDM conversations in the typical primary care practice.
Preference-sensitive care
SDM is designed to enhance the role of patient preference, considering a patient’s own personal values for managing clinical conditions when more than one reasonable strategy exists. Such situations are often referred to as preference-sensitive conditions—ie, since evidence is limited on a single “best” treatment approach, patients’ values should impact decision-making.9 Examples of common preference-sensitive situations that include preventive care, screening, and chronic disease management are outlined in TABLE 1.



How to engage patients
In preference-sensitive care situations, SDM endeavors to address uncertainty by laying out what the options are, as well as providing risk and benefit data. This helps inform patients and guides providers about individual patient preference on whether to screen (eg, for average-risk female patients, breast cancer screening between ages 40-50 years). SDM can assist with determining whether to screen and if so, at what interval (eg, at 1- or 2-year intervals), while acknowledging that no single decision would be “best” for every patient.
While there are formalized tools to provide information to patients and help them consider their values and choices,3,10 SDM does not hinge on the use of an explicit tool.11-18 There are many approaches to and interpretations of SDM; the Ottawa Decision Support Framework reviews and details these many considerations at length in its 2020 revision.19 TABLE 211,15-17,20-22 highlights various SDM frameworks and the steps involved.

These 3 elements are commonamong SDM frameworks
In a 2019 systematic review, the following 3 elements were highlighted as the most prevalent over time across SDM frameworks and could be considered core to any meaningful SDM process23:
Explicit effort by 2 or more experts. The patient is an expert in their own values. The clinician, as an expert in relevant medical knowledge, clarifies that the current medical situation will benefit from incorporating the patient’s preferences to arrive at an appropriate shared decision.
Continue to: Effort to provide relevant...
Effort to provide relevant, evidence-based information. The clinician provides treatment options applicable to the patient, including the risks and benefits of each (potentially using one of the decision aids in the following section), to facilitate a values-based discussion and decision.
Patient support and assistance. The clinician assists the patient in navigating next steps based on the treatment decision and arranges necessary follow-up.
Various case studies and examples of SDM conversations have been published.15-17,24 Video examples of optimal25 and less than optimal26 SDM conversations are available on the Massachusetts General Hospital Health Decision Sciences Center website (https://mghdecisionsciences.org/) under the section “Tools & Training >> Videos about Shared Decision-Making.”27
SDM and motivational interviewing: Both can serve you well
SDM and motivational interviewing share many common elements,28 and it’s useful to take advantage of both techniques. Preference-sensitive care situations may require a combination of approaches.
For example, motivational interviewing may be a beneficial tool when dealing with a patient who is initially against colon cancer screening (evidence clearly favors screening in some form over no screening) and has a history of avoiding medical care. Through an SDM approach, motivational interviewing may identify an opportunity to prioritize the patient’s preference to minimize medical intervention by ensuring that the patient is familiar with noninvasive colon cancer screening options. After sufficiently eliciting a patient value aligned with screening and engaging the patient’s own motivations for follow-through, a more thorough SDM conversation can then help clarify the best options.
Continue to: A proposed framework...
A proposed framework for identifying whether SDM or motivational interviewing is appropriate is featured in the FIGURE. In their paper, Elwyn et al29 further define and discuss the distinguishing features and roles of SDM and behavioral support interventions, such as motivational interviewing.

Tools to facilitate SDM conversations
Decision aids
SDM has historically been operationalized for study through the use of decision aids: formally structured materials describing, in detail, the available treatment options under consideration, including the relative risks and benefits. Frequently, such tools are framed from a patient perspective, with digestible information presented in a multimedia format (eg, visual risk representations of “1 out of 10” in an icon array vs “10%”), leveraging effective risk communication strategies (eg, absolute risk rates vs relative risks and “balanced framing”). For instance, the physician would note that 1 out of 10 patients have an outcome and 9 out of 10 do not.
Additional information on risk communication skills is available at the Agency for Healthcare Research and Quality’s webpage on the SHARE approach (www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html).30 Decision aids have been shown to enhance health literacy, increase patient knowledge and understanding, and promote the frequency of “values-concordant” choices.3
Point-of-care decision support
A more recent trend in SDM is increased development and use of point-of-care decision support tools that emphasize information reflecting individual patient circumstances (eg, leveraging heart risk calculators to individualize risk conversations when considering statins for primary prevention of heart disease based on lipids and other demographic factors). An advantage to using such tools is that they provide “just-in-time” detailed and personalized evidence-based information, guiding the discussion and minimizing the need for an extensive advance review of each topic by emphasizing the “key facts.” To ensure effective use of SDM tools, avoid oversaturating patients with data, maintain a focus on patient values, and engage in a 2-way discussion that considers the unique mix of preferences and circumstances.
Proprietorship of tools and decision aids
Until recently, SDM materials were compiled primarily within not-for-profit entities such as the Informed Medical Decisions Foundation, which became a division of Healthwise in 2014.2 In recent years, there has been an increasing trend of for-profit companies acquiring or developing their own decision aids and decision-support tools, eg, EBSCO Health (Option Grid31 and Health Decision32) and Wolters Kluwer (EMMI33). The extensive work of curating SDM and educational tools to keep up with best medical evidence is costly, and the effort to defray costs can give rise to potential conflicts of interest. Therefore, the interests of the creators of such tools—whether commercial or academic—should always be considered when evaluating the use of a given decision-support tool.
Contunue to: An online listing...
An online listing of publicly available decision aids is maintained by the Ottawa Hospital Research Institute,34 which reviews decision-aid quality by objective criteria in addition to providing direct links to resources.35 EBSCO health’s DynaMed Decisions also maintains a list of shared decision-making tools (https://decisions.dynamed.com/).
Effectiveness of decision aids
There is a robust body of research focused on decision aids for SDM. An example is a 2017 Cochrane review that concluded SDM facilitated by decision aids significantly improved patient engagement and satisfaction and increased patient knowledge, accuracy in risk perception, and congruency in making value-aligned care choices. Beyond decision aids, studies show SDM practices increase patient knowledge, engagement, and satisfaction, particularly among low-literacy or disadvantaged groups.4,36,37
Barriers to implementation
Clinicians frequently cite time constraints as a barrier to successfully implementing SDM in practice, although studies that explicitly compare the time/cost of SDM to “usual care” are limited.38 A Cochrane review of 105 studies evaluating the use of decision aids vs usual care found that only 10 studies examined the effects of decision aids on the length of the office visit.3 Two of these studies (one evaluating decision aids for prenatal diagnostic screening and the other for atrial fibrillation) found a median increase in visit length of 2.6 minutes (24 vs 21; 7.5% increase); the other 8 studies reported no increase in visit length.3
Studies focusing on the time impact of using SDM in an office visit, rather than decision aids as a proxy for SDM, are few. A study by Braddock et al39 assessed the elements of SDM, measuring the quality and the time-efficiency of 141 surgical decision-making interactions between patients and 89 orthopedic surgeons. Researchers found 57% of the discussions had elements of SDM sufficient to meet a “reasonable minimum” standard (eg, nature of the decision, patient’s role, patient’s preference). These conversations took 20 minutes compared to a median of 16 minutes for a more typical conversation.39 The study used audiotaped interviews, which were coded and scored based on the presence of SDM elements; treatment choice, outcomes of the choices, and satisfaction were not reported. A separate study by Loh et al5 looking at SDM in primary care for patients with depression sought to determine whether patient participation in the decision-making process improved treatment adherence, outcomes, and patient satisfaction without increasing consultation time. This study, which included 23 physicians and 405 patients, found improved participation and satisfaction outcomes in the intervention group and no difference in consultation time between the intervention and control groups.5
Care costs appear similar
The impact of SDM on cost and patient-centered clinical outcomes is not well defined. One study by Arterburn et al40 found decision aids and SDM lowered the rates of elective surgery for hip and knee arthritis, as well as associated health system costs. However, other studies suggest this phenomenon likely varies by demographic, demonstrating that certain populations with a generally lower baseline preference for surgery on average chose surgery more often after SDM interventions.41,42 Evidence does support patient acceptability and efficacy for SDM in longitudinal care when the approach is incorporated into decisions over multiple visits or long-term decisions for chronic conditions.4 Studies comparing patient groups receiving decision aids to usual care have shown similar or lower overall care costs for the decision-aid group.3
Continue to: Limitations to the evidence
Limitations to the evidence
Systematic reviews routinely note substantial heterogeneity in the literature on SDM use, owing to variable definitions of what steps are essential to constitute an SDM intervention and a wide variety of outcome measures used, as well as the broad range of conditions to which SDM is potentially applicable.3,4,10,36,37,43-45 While efforts in SDM education, uptake, and study frequently adapt frameworks such as those outlined in TABLE 2,11,15-17,20-22 there is as yet no one consensus on the “best” approach to SDM, and explicit study of any given approach is limited.18,23,36,44-46 There remains a clear need to improve the uptake of existing reporting standards to ensure the future evidence base will be of high quality.44 In the meantime, a large portion of the impetus for expanding the use of SDM remains based on principles of effective communication and championing a patient-centered philosophy of care.
Cultivating an effective approach
An oft-cited objection to the use of SDM in day-to-day clinical care is that it “takes too much time.”47 Like all excellent communication skills, SDM is best incorporated into a clinician’s approach to patient care. With practice, we have found this can be accomplished during routine patient encounters—eg, when providing general counsel, giving advice, providing education, answering questions. Given the interdependent relationship between evidence-based medicine and SDM, particularly in preference-sensitive conditions, SDM skills can facilitate efficient decision-making and patient satisfaction.48 To that end, clinician training on SDM techniques, especially those that emphasize the 3 core elements, can be particularly beneficial. These broadly applicable skills can be leveraged in an “SDM mindset,” even outside traditional preference-sensitive care situations, to enhance clinician–patient rapport, relationship, and satisfaction.
The future of SDM
More than 2 decades after SDM was introduced to clinical care, there remains much to do to improve uptake in primary care settings. An important strategy to increase the successful uptake of SDM for the typical clinician and patient is to emphasize the approach to framing the topic and discussion rather than to overemphasize decision aids.23 Continuing the trend of well-designed and accessible tools for clinical decision support at the point of care for clinicians, in addition to the sustained evolution of decision aids for patients, should help minimize the need for extensive background knowledge on a topic, increase accessibility, and enable an effective partnership with patients in their health care decisions.46 Ongoing, well-structured study and the use of common proposed standards in developing these tools and studying SDM implementation will provide long-term quality assurance.44
SDM has a role to play in health equity
SDM has a clear role to play in addressing health inequities. Values vary from person to person, and individuals exist along a variety of cultural, community, and other spectra that strongly influence their perception of what is most important to them. Moreover, clinicians’ assumptions typically do not correspond to a patient’s actual desire to engage in SDM nor to their overall likelihood of choosing any given treatment option.46 While many clinicians believe patients do not participate in SDM because they simply do not wish to, a systematic review and thematic synthesis by Joseph-Williams et al46 suggested a great number of patients are instead unable to take part in SDM due to barriers such as a lack of time availability, challenges in the structure of the health care system itself, and factors specific to the clinician–patient interaction such as patients feeling as though they don’t have “permission” to participate in SDM.
SDM may improve health equity, adherence, and outcomes in certain groups. For example, SDM has been suggested as a potential means to address disparities in outcomes for populations disproportionately affected by hypertension.24 The increased implementation of SDM practices, coupled with a genuine partnership between patients and care teams, may improve patient–clinician communication, enhance understanding of patient concerns and goals, and perhaps ultimately increase patient engagement and adherence.
Continue to: Being the change
Being the change
Effective framing of medical decisions in the context of best medical evidence and eliciting patient values supports continued evolution in health care delivery. The traditional, physician-directed patriarchal “one-size-fits-all” approach has evolved. Through the continued development and implementation of SDM techniques, the clinician’s approach to care will continue to advance.
Ultimately, patients and clinicians both benefit from the use of SDM—the patient benefits from explicit framing of the medical facts most relevant to their decision, and the physician benefits from enhanced knowledge of the patient’s values and considerations. When done well, SDM increases the likelihood that patients will receive the best care possible, concordant with their values and preferences and within the context of their unique circumstances, leading to improved knowledge, adherence, outcomes, and satisfaction.
CORRESPONDENCE
Matthew Mackwood, MD, One Medical Center Drive, Lebanon, NH 03756; [email protected]
1. Ottawa Hospital Research Institute. Mission and history—patient decision aids. Accessed October 20, 2022. https://decisionaid.ohri.ca/mission.html
2. Healthwise. Informed Medical Decision Foundation. Accessed October 20, 2022. www.healthwise.org/specialpages/imdf.aspx
3. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5
4. Joosten EAG, DeFuentes-Merillas L, De Weert G, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219-226. doi: 10.1159/000126073
5. Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324-332. doi: 10.1016/j.pec.2007.03.023
6. Goodwin JS, Nishi S, Zhou J, et al. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179:716-718. doi: 10.1001/jamain ternmed.2018.6405
7. Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision-making for lung cancer screening. JAMA Intern Med. 2018;178:1311-1316. doi: 10.1001/jamainternmed.2018.3054
8. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi: 10.1016/j.chest.2021.01.041
9. Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69-79. doi: 10.1353/pbm.2003.000
10. Hoefel L, O’Connor AM, Lewis KB, et al. 20th Anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555-581. doi: 10.1177/0272989X20936209
11. Sheridan SL, Harris RP, Woolf SH. Shared decision-making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56-66. doi: 10.1016/j.amepre.2003.09.011
12. Elwyn G, Frosch D, Thomson R, et al. Shared decision-making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367. doi: 10.1007/s11606-012-2077-6
13. Fowler FJ Jr, Barry MJ, Sepucha KR, et al. Let’s require patients to review a high-quality decision aid before receiving important tests and treatments. Med Care. 2021;59:1-5. doi: 10.1097/MLR.0000000000001440
14. Hargraves IG, Fournier AK, Montori VM, et al. Generalized shared decision-making approaches and patient problems. Adapting AHRQ’s SHARE approach for purposeful SDM. Patient Educ Couns. 2020;103:2192-2199. doi: 10.1016/j.pec.2020.06.022
15. Price D. Sharing clinical decisions by discussing evidence with patients. Perm J. 2005;9:70-73. doi: 10.7812/TPP/05-006
16. Schrager S, Phillips G, Burnside E. Shared decision-making in cancer screening. Fam Pract Manag. 2017;24:5-10.
17. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision-making: concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. doi: 10.1016/j.pec.2015.06.022
18. Hargraves I, LeBlanc A, Shah ND, et al. Shared decision-making: the need for patient-clinician conversation, not just information. Health Aff (Milford). 2016;35:627-629. doi: 10.1377/hlthaff.2015.1354
19. Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa Decision Support Framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. 2020;40:379-398. doi: 10.1177/0272989X20911870
20. Agency for Health Research and Quality. The SHARE Approach. Accessed November 24, 2021, www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
21. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision-making: multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891
22. Healthwise – Informed Medical Decisions Foundation. The six steps of shared decision making. Accessed December 21, 2022. http://cdn-www.informedmedicaldecisions.org/imdfdocs/SixStepsSDM_CARD.pdf
23. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, et al. Key components of shared decision-making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-03176
24. Langford AT, Williams SK, Applegate M, et al. Partnerships to improve shared decision making for patients with hypertension - health equity implications. Ethn Dis. 2019;29(suppl 1):97-102. doi: 10.18865/ed.29.S1.97
25. MGH Health Decision Sciences Center. High cholesterol visit version 2. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=o2mZ9duJW0A
26. MGH Health Decision Sciences Center. High cholesterol visit version 1. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=0NdDMKS8DwU
27. MGH Health Decision Sciences Center. Videos about shared decision-making. Accessed October 20, 2022. https://mghdecision sciences.org/tools-training/sdmvideos/
28. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision-making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12:270-275. doi: 10.1370/afm.1615. Published correction in Ann Fam Med. 2014;12:301. doi: 10.1370/afm.1674
29. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision-making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75
30. Agency for Health Research and Quality. The SHARE approach—communicating numbers to your patients: a reference guide for health care providers. Workshop curriculum: tool 5. Accessed October 21, 2022. www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html
31. EBSCO. Accessed October 21, 2022. https://optiongrid.ebsco.com/about
32. HealthDecision. HealthDecision - Decision Support & Shared decision-making for Clinicians & Patients at the Point of Care. Accessed November 24, 2021. www.healthdecision.com/ [Now DynaMed Decisions, https://decisions.dynamed.com/]
33. Wolters Kluwer. EmmiEngage: guide patients in their care journeys. Accessed October 21, 2022. www.wolterskluwer.com/en/solutions/emmi/emmi-engage
34. The Ottawa Hospital Research Institute. Patient decision aids. Accessed October 21, 2022. https://decisionaid.ohri.ca/Azinvent.php
35. The Ottawa Hospital Research Institute. Alphabetical list of decision aids by health topic. Accessed October 21, 2022. https://decisionaid.ohri.ca/AZlist.html
36. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision-making and patient outcomes. Med Decis Making. 2015;35:114-131. doi: 10.1177/0272989X14551638
37. Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670
38. Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision-making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32:268-275. doi: 10.1377/hlthaff.2012.1084
39. Braddock C 3rd, Hudak PL, Feldman JJ, et al. “Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgery. J Bone Joint Surg Am. 2008;90:1830-1838. doi: 10.2106/JBJS.G.00840
40. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31:2094-2104. doi: 10.1377/hlthaff.2011.0686.
41. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res. 2016;474:1755-1764. doi: 10.1007/s11999-016-4834-z
42. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for Black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152:e164225. doi: 10.1001/jamasurg.2016.4225
43. Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9-18. doi: 10.1016/j.pec.2011.02.004
44. Trenaman L, Jansen J, Blumenthal-Barby J, et al. Are we improving? Update and critical appraisal of the reporting of decision process and quality measures in trials evaluating patient decision aids. Med Decis Making. 2021;41:954-959. doi: 10.1177/0272989x211011120
45. Hoefel L, Lewis KB, O’Connor A, et al. 20th anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. 2020;40:522-539. doi: 10.1177/0272989X20924645
46. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision-making. Patient Educ Couns. 2014;94:291-309. doi: 10.1016/j.pec.2013.10.031
47. Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535. doi: 10.1016/ j.pec.2008.07.018
48. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision-making. JAMA. 2014;312:1295-1296. doi:10.1001/jama.2014.10186
1. Ottawa Hospital Research Institute. Mission and history—patient decision aids. Accessed October 20, 2022. https://decisionaid.ohri.ca/mission.html
2. Healthwise. Informed Medical Decision Foundation. Accessed October 20, 2022. www.healthwise.org/specialpages/imdf.aspx
3. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5
4. Joosten EAG, DeFuentes-Merillas L, De Weert G, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219-226. doi: 10.1159/000126073
5. Loh A, Simon D, Wills CE, et al. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67:324-332. doi: 10.1016/j.pec.2007.03.023
6. Goodwin JS, Nishi S, Zhou J, et al. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179:716-718. doi: 10.1001/jamain ternmed.2018.6405
7. Brenner AT, Malo TL, Margolis M, et al. Evaluating shared decision-making for lung cancer screening. JAMA Intern Med. 2018;178:1311-1316. doi: 10.1001/jamainternmed.2018.3054
8. Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi: 10.1016/j.chest.2021.01.041
9. Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69-79. doi: 10.1353/pbm.2003.000
10. Hoefel L, O’Connor AM, Lewis KB, et al. 20th Anniversary update of the Ottawa decision support framework part 1: a systematic review of the decisional needs of people making health or social decisions. Med Decis Making. 2020;40:555-581. doi: 10.1177/0272989X20936209
11. Sheridan SL, Harris RP, Woolf SH. Shared decision-making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56-66. doi: 10.1016/j.amepre.2003.09.011
12. Elwyn G, Frosch D, Thomson R, et al. Shared decision-making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367. doi: 10.1007/s11606-012-2077-6
13. Fowler FJ Jr, Barry MJ, Sepucha KR, et al. Let’s require patients to review a high-quality decision aid before receiving important tests and treatments. Med Care. 2021;59:1-5. doi: 10.1097/MLR.0000000000001440
14. Hargraves IG, Fournier AK, Montori VM, et al. Generalized shared decision-making approaches and patient problems. Adapting AHRQ’s SHARE approach for purposeful SDM. Patient Educ Couns. 2020;103:2192-2199. doi: 10.1016/j.pec.2020.06.022
15. Price D. Sharing clinical decisions by discussing evidence with patients. Perm J. 2005;9:70-73. doi: 10.7812/TPP/05-006
16. Schrager S, Phillips G, Burnside E. Shared decision-making in cancer screening. Fam Pract Manag. 2017;24:5-10.
17. Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision-making: concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172-1179. doi: 10.1016/j.pec.2015.06.022
18. Hargraves I, LeBlanc A, Shah ND, et al. Shared decision-making: the need for patient-clinician conversation, not just information. Health Aff (Milford). 2016;35:627-629. doi: 10.1377/hlthaff.2015.1354
19. Stacey D, Légaré F, Boland L, et al. 20th anniversary Ottawa Decision Support Framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. 2020;40:379-398. doi: 10.1177/0272989X20911870
20. Agency for Health Research and Quality. The SHARE Approach. Accessed November 24, 2021, www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
21. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision-making: multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891
22. Healthwise – Informed Medical Decisions Foundation. The six steps of shared decision making. Accessed December 21, 2022. http://cdn-www.informedmedicaldecisions.org/imdfdocs/SixStepsSDM_CARD.pdf
23. Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, et al. Key components of shared decision-making models: a systematic review. BMJ Open. 2019;9:e031763. doi: 10.1136/bmjopen-2019-03176
24. Langford AT, Williams SK, Applegate M, et al. Partnerships to improve shared decision making for patients with hypertension - health equity implications. Ethn Dis. 2019;29(suppl 1):97-102. doi: 10.18865/ed.29.S1.97
25. MGH Health Decision Sciences Center. High cholesterol visit version 2. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=o2mZ9duJW0A
26. MGH Health Decision Sciences Center. High cholesterol visit version 1. YouTube. February 28, 2020. Accessed October 20, 2022. www.youtube.com/watch?v=0NdDMKS8DwU
27. MGH Health Decision Sciences Center. Videos about shared decision-making. Accessed October 20, 2022. https://mghdecision sciences.org/tools-training/sdmvideos/
28. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision-making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12:270-275. doi: 10.1370/afm.1615. Published correction in Ann Fam Med. 2014;12:301. doi: 10.1370/afm.1674
29. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision-making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi: 10.1186/1748-5908-4-75
30. Agency for Health Research and Quality. The SHARE approach—communicating numbers to your patients: a reference guide for health care providers. Workshop curriculum: tool 5. Accessed October 21, 2022. www.ahrq.gov/health-literacy/professional-training/shared-decision/tool/resource-5.html
31. EBSCO. Accessed October 21, 2022. https://optiongrid.ebsco.com/about
32. HealthDecision. HealthDecision - Decision Support & Shared decision-making for Clinicians & Patients at the Point of Care. Accessed November 24, 2021. www.healthdecision.com/ [Now DynaMed Decisions, https://decisions.dynamed.com/]
33. Wolters Kluwer. EmmiEngage: guide patients in their care journeys. Accessed October 21, 2022. www.wolterskluwer.com/en/solutions/emmi/emmi-engage
34. The Ottawa Hospital Research Institute. Patient decision aids. Accessed October 21, 2022. https://decisionaid.ohri.ca/Azinvent.php
35. The Ottawa Hospital Research Institute. Alphabetical list of decision aids by health topic. Accessed October 21, 2022. https://decisionaid.ohri.ca/AZlist.html
36. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision-making and patient outcomes. Med Decis Making. 2015;35:114-131. doi: 10.1177/0272989X14551638
37. Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PloS One. 2014;9:e94670. doi: 10.1371/journal.pone.0094670
38. Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision-making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32:268-275. doi: 10.1377/hlthaff.2012.1084
39. Braddock C 3rd, Hudak PL, Feldman JJ, et al. “Surgery is certainly one good option”: quality and time-efficiency of informed decision-making in surgery. J Bone Joint Surg Am. 2008;90:1830-1838. doi: 10.2106/JBJS.G.00840
40. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood). 2012;31:2094-2104. doi: 10.1377/hlthaff.2011.0686.
41. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res. 2016;474:1755-1764. doi: 10.1007/s11999-016-4834-z
42. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for Black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 2017;152:e164225. doi: 10.1001/jamasurg.2016.4225
43. Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9-18. doi: 10.1016/j.pec.2011.02.004
44. Trenaman L, Jansen J, Blumenthal-Barby J, et al. Are we improving? Update and critical appraisal of the reporting of decision process and quality measures in trials evaluating patient decision aids. Med Decis Making. 2021;41:954-959. doi: 10.1177/0272989x211011120
45. Hoefel L, Lewis KB, O’Connor A, et al. 20th anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. 2020;40:522-539. doi: 10.1177/0272989X20924645
46. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision-making. Patient Educ Couns. 2014;94:291-309. doi: 10.1016/j.pec.2013.10.031
47. Légaré F, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535. doi: 10.1016/ j.pec.2008.07.018
48. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision-making. JAMA. 2014;312:1295-1296. doi:10.1001/jama.2014.10186
A practical guide to hidradenitis suppurativa
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic, recurrent, inflammatory occlusive disease affecting the terminal follicular epithelium in apocrine gland–bearing skin areas.1 HS manifests as painful nodules, abscesses, fistulas, and scarring and often has a severe psychological impact on the affected patient.2
When HS was first identified in the 1800s, it was believed to result from a dysfunction of the sweat glands.3 In 1939, scientists identified the true cause: follicular occlusion.3
Due to its chronic nature, heterogeneity in presentation, and apparent low prevalence,4 HS is considered an orphan disease.5 Over the past 10 years, there has been a surge in HS research—particularly in medical management—which has provided a better understanding of this condition.6,7
In this review, we discuss the most updated evidence regarding the diagnosis and treatment of HS to guide the family physician (FP)’s approach to managing this debilitating disease. But first, we offer a word about the etiology and pathophysiology of the condition.
3 events set the stage for hidradenitis suppurativa
Although the exact cause of HS is still unknown, some researchers have hypothesized that HS results from a combination of genetic predisposition and environmental and lifestyle factors.8-12 The primary mechanism of HS is the obstruction of the terminal follicular epithelium by a keratin plug.1,13,14 A systematic review of molecular inflammatory pathways involved in HS divides the pathogenesis of HS into 3 events: follicular occlusion followed by dilation, follicular rupture and inflammatory response, and chronic inflammatory state with sinus tracts.8
An underreported condition
HS is often underreported and misdiagnosed.4,15 Globally, the prevalence of HS varies from < 1% to 4%.15,16 A systematic review with meta-analysis showed a higher prevalence of HS in females compared to males in American and European populations.17 In the United States, the overall frequency of HS is 0.1%, or 98 per 100,000 persons.16 The prevalence of HS is highest among patients ages 30 to 39 years; there is decreased prevalence in patients ages 55 years and older.16,18
Who is at heightened risk?
Recent research has shown a relationship between ethnicity and HS.16,19,20 African American and biracial groups (defined as African American and White) have a 3-fold and 2-fold greater prevalence of HS, respectively, compared to White patients.16 However, the prevalence of HS in non-White ethnic groups may be underestimated in clinical trials due to a lack of representation and subgroup analyses based on ethnicity, which may affect generalizability in HS recommendations.21
Continue to: Genetic predisposition
Genetic predisposition. As many as 40% of patients with HS report having at least 1 affected family member. A positive family history of HS is associated with earlier onset, longer disease duration, and severe disease.22 HS is genetically heterogeneous, and several mutations (eg, gamma secretase, PSTPIP1, PSEN1 genes) have been identified in patients and in vitro as the cause of dysregulation of epidermal proliferation and differentiation, immune dysregulation, and promotion of amyloid formation.8,23-25
Obesity and metabolic risk factors. There is a strong relationship between HS and obesity. As many as 70% of patients with HS are obese, and 9% to 40% have metabolic syndrome.12,18,26-28 Obesity is associated with maceration and mechanical stress, increased fragility of the dermo-epidermal junction, changes in cutaneous blood flow, and subdermal fat inflammation—all of which favor the pathophysiology of HS.29,30
Smoking. Tobacco smoking is associated with severe HS and a lower chance of remission.12 Population-based studies have shown that as many as 90% of patients with HS have a history of smoking ≥ 20 packs of cigarettes per year.1,12,18,31,32 The nicotine and thousands of other chemicals present in cigarettes trigger keratinocytes and fibroblasts, resulting in epidermal hyperplasia, infundibular hyperkeratosis, excessive cornification, and dysbiosis.8,23,24
Hormones. The exact role sex hormones play in the pathogenesis of HS remains unclear.8,32 Most information is based primarily on small studies looking at antiandrogen treatments, HS activity during the menstrual cycle and pregnancy, HS exacerbation related to androgenic effects of hormonal contraception, and the association of HS with metabolic-endocrine disorders (eg, polycystic ovary syndrome [PCOS]).8,33
Androgens induce hyperkeratosis that may lead to follicular occlusion—the hallmark of HS pathology.34 A systematic review looking at the role of androgen and estrogen in HS found that while some patients with HS have elevated androgen levels, most have androgen and estrogen levels within normal range.35 Therefore, increased peripheral androgen receptor sensitivity has been hypothesized as the mechanism of action contributing to HS manifestation.34
Continue to: Host-defense defects
Host-defense defects. HS shares a similar cytokine profile with other well-established immune-mediated inflammatory diseases, including pyoderma gangrenosum (PG)36,37 and Crohn disease.38-40 HS is characterized by the expression of several immune mediators, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-8, IL-17, and the IL-23/T helper 17 pathway, all of which are upregulated in other inflammatory diseases and also result in an abnormal innate immune response.8,24 The recently described clinical triad of PG, acne, and HS (PASH) and the tetrad of pyogenic arthritis, PG, acne, and HS (PAPASH) further support the role of immune dysregulation in the pathogenesis of HS.40 Nonetheless, further studies are needed to determine the exact pathways of cytokine effect in HS.41
Use these criteria to make the diagnosis
The US and Canadian Hidradenitis Suppurativa Foundations (HSF) guidelines base the clinical diagnosis of HS on the following criteria2:
- Typical HS lesions: Erythematous skin lesions; inflamed, deep-seated painful nodules; “tombstone” double-ended comedones; sinus tracts; scarring; deformity. FIGURES 1A-1E show typical lesions seen in patients with HS.
- Typical locations: Intertriginous regions—apocrine gland–containing areas in axilla, groin, perineal region, buttocks, gluteal cleft, and mammary folds; beltline and waistband areas; areas of skin compression and friction.
- Recurrence and chronicity: Recurrent painful or suppurating lesions that appear more than twice in a 6-month period.2,41-43
Patients with HS usually present with painful recurrent abscesses and scarring and often report multiple visits to the emergency department for drainage or failed antibiotic treatment for abscesses.15,44
Ask patients these 2 questions. Vinding et al45 developed a survey for the diagnosis of HS using 2 simple questions based on the 3 criteria established by the HSF:
- “Have you had an outbreak of boils during the last 6 months?” and
- “Where and how many boils have you had?” (This question includes a list of the typical HS locations—eg, axilla, groin, genitals, area under breast.)
In their questionnaire, Vinding et al45 found that an affirmative answer to Question 1 and reports of > 2 boils in response to Question 2 correlated to a sensitivity of 90%, specificity of 97%, positive predictive value of 96%, and negative predictive value of 92% for the diagnosis of HS. The differential diagnosis of HS is summarized in TABLE 1.42,45-52
Continue to: These tools can help you to stage hidradenitis suppurativa
These tools can help you to stage hidradenitis suppurativa
Multiple tools are available to assess the severity of HS.53 We will describe the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System (IHS4). Other diagnostic tools, such as the Sartorius score and the Hidradenitis Suppurativa Physician’s Global Assessment Scale (HS-PGA), can be time-consuming and challenging to interpret, limiting their use in the clinical setting.2,54
Hurley staging system (available at www.hsdiseasesource.com/hs-disease-staging) considers the presence of nodules, abscesses, sinus tracts, and scarring affecting an entire anatomical area.13,55 This system is most useful as a rapid classification tool for patients with HS in the clinical setting but should not be used to assess clinical response.2,13,56
The IHS4 (available at https://online library.wiley.com/doi/10.1111/bjd.15748) is a validated and easy-to-use tool for assessing HS and guiding the therapeutic strategy in clinical practice.54 With IHS4, the clinician must calculate the following:
- total number of nodules > 10 mm in diameter
- total number of abscesses multiplied by 2, and
- total number of draining tunnels (fistulae/sinuses) multiplied by 4.
Mild HS is defined as a score ≤ 3 points; moderate HS, 4 to 10 points; and severe HS, ≥ 11 points.54
No diagnostic tests, but ultrasound may be helpful
There are currently no established biological markers or specific tests for diagnosing HS.15 Ultrasound is emerging as a tool to assess dermal thickness, hair follicle morphology, and number and extent of fluid collections. Two recent studies showed that pairing clinical assessment with ultrasound findings improves accuracy of scoring in 84% of cases.57,58 For patients with severe HS, skin biopsy can be considered to rule out squamous cell carcinoma. Cultures, however, have limited utility except for suspected superimposed bacterial infection.2
Continue to: Screening for comorbidities
Screening for comorbidities
HSF recommends clinicians screen patients for comorbidities associated with HS (TABLE 2).2 Overall, screening patients for active and past history of smoking is strongly recommended, as is screening for metabolic syndrome, hyperlipidemia, type 2 diabetes (1.5- to 3-fold greater risk of type 2 diabetes in HS patients), and PCOS (3-fold greater risk).2,26,27,59 Screening patients for depression and anxiety is also routinely recommended.2 However, the authors of this article strongly recommend screening all patients with HS for psychiatric comorbidities, as research has shown a 2-fold greater risk of depression and anxiety, social isolation, and low self-esteem that severely limits quality of life (QOL) in this patient population.60,61
Management
Treat existing lesions, reduce formation of new ones
The main goals of treatment for patients with HS are to treat existing lesions and reduce associated symptoms, reduce the formation of new lesions, and minimize associated psychological morbidity.15 FPs play an important role in the early diagnosis, treatment, and comprehensive care of patients with HS. This includes monitoring patients, managing comorbidities, making appropriate referrals to dermatologists, and coordinating the multidisciplinary care that patients with HS require.
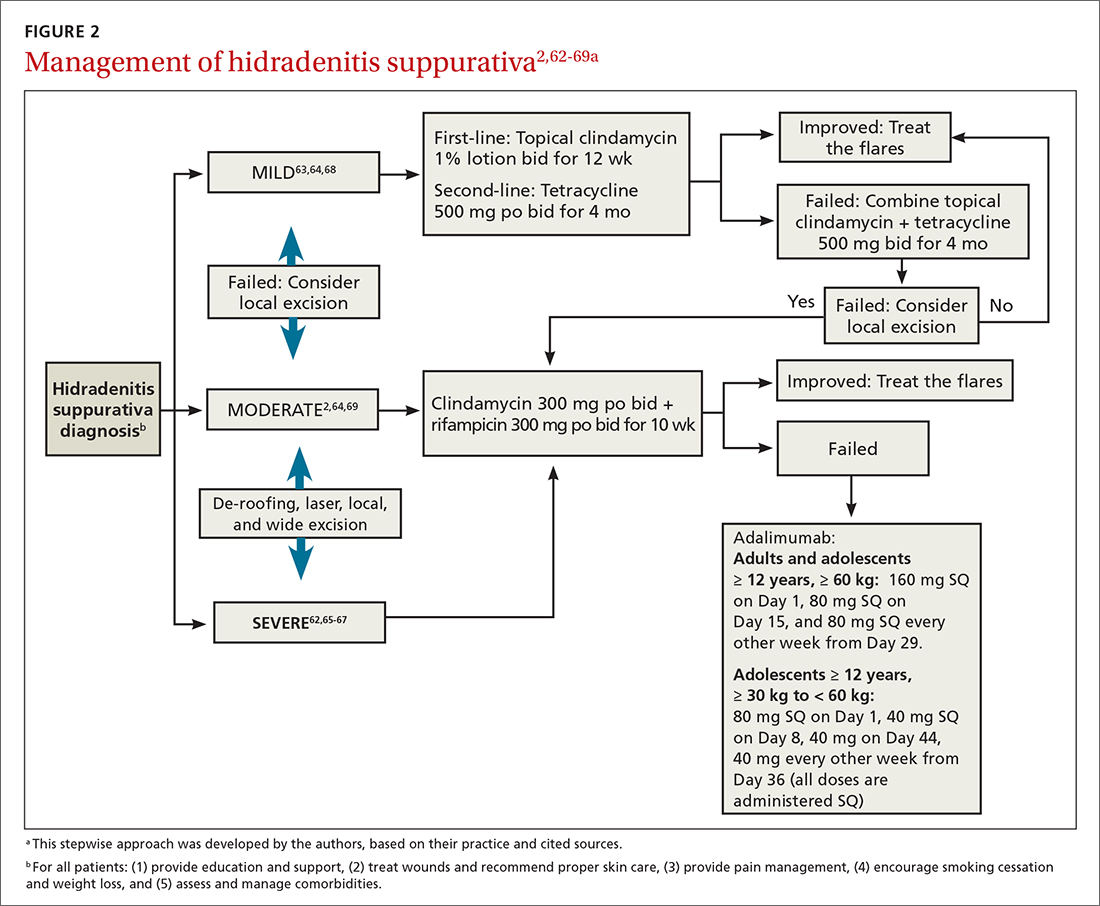
A systematic review identified more than 50 interventions used to treat HS, most based on small observational studies and randomized controlled trials (RCTs) with a high risk of bias.62 FIGURE 22,62-69 provides an evidence-based treatment algorithm for HS, and TABLE 32,63,64,70-75 summarizes the most commonly used treatments.
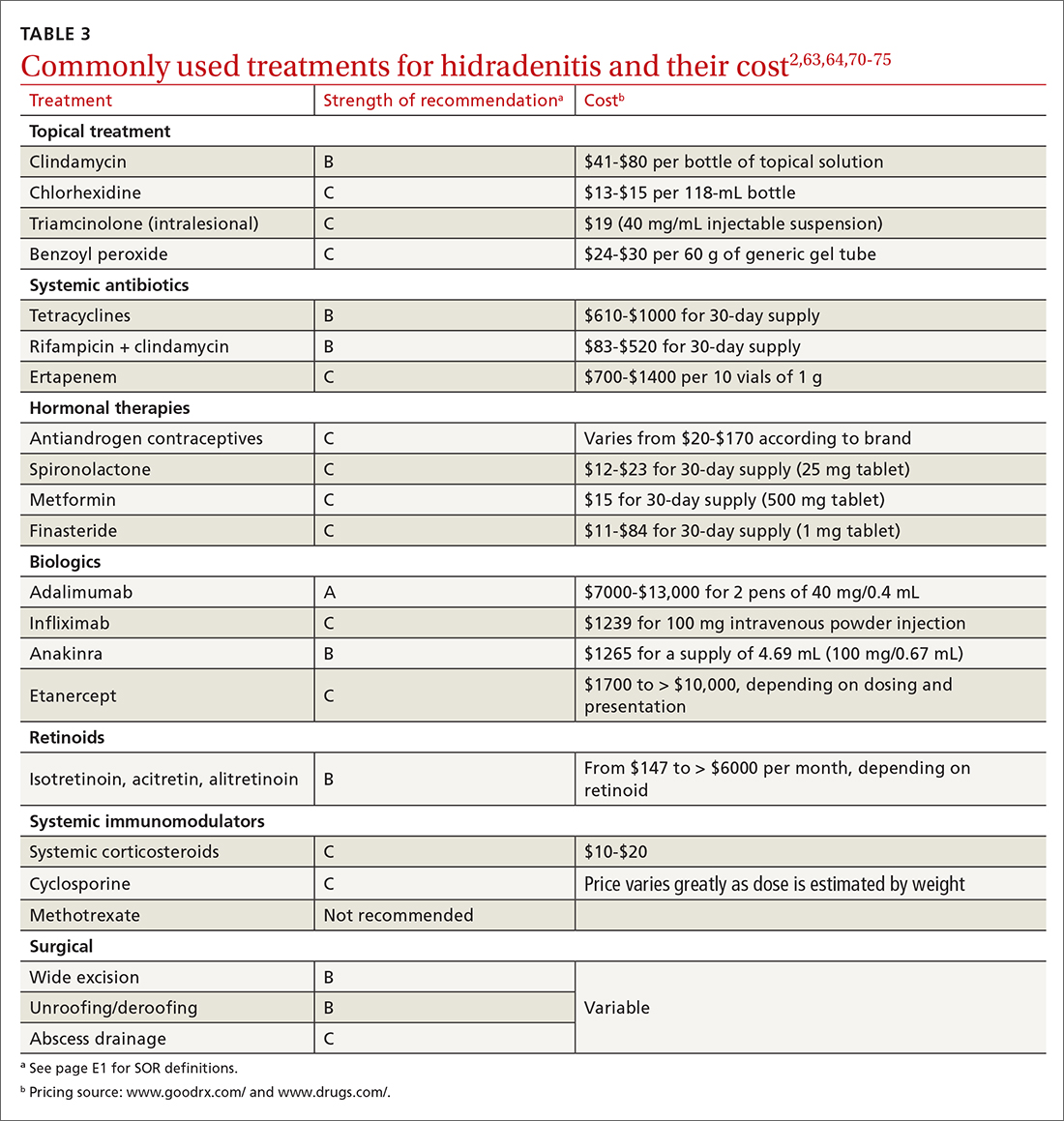
Biologic agents
Adalimumab (ADA) is a fully human immunoglobulin G1 monoclonal antibody that binds to TNF-alpha, neutralizes its bioactivity, and induces apoptosis of TNF-expressing mononuclear cells. It is the only medication approved by the US Food and Drug Administration for active refractory moderate and severe HS.62,65 Several double-blinded RCTs, including PIONEER I and PIONEER II, studied the effectiveness of ADA for HS and found significant clinical responses at Week 12, 50% reduction in abscess and nodule counts, no increase in abscesses or draining fistulas at Week 12, and sustained improvement in lesion counts, pain, and QOL.66,67,76
IL-1 and IL-23 inhibitors. The efficacy of etanercept and golimumab (anti-TNF), as well as anakinra (IL-1 inhibitor) and ustekinumab (IL-1/IL-23 inhibitor), continue to be investigated with variable results; they are considered second-line treatment for active refractory moderate and severe HS after ADA.65,77-80 Infliximab (IL-1 beta inhibitor) has shown no effect on reducing disease severity.70Compared to other treatments, biologic therapy is associated with higher costs (TABLE 3),2,63,64,70-75 an increased risk for reactivation of latent infections (eg, tuberculosis, herpes simplex, and hepatitis C virus [HCV], and B [HBV]), and an attenuated response to vaccines.81 Prior to starting biologic therapy, FPs should screen patients with HS for tuberculosis and HBV, consider HIV and HCV screening in at-risk patients, and optimize the immunization status of the patient.82,83 While inactivated vaccines can be administered without discontinuing biologic treatment, patients should avoid live-attenuated vaccines while taking biologics.83
Continue to: Antibiotic therapy
Antibiotic therapy
Topical antibiotics are considered first-line treatment for mild and moderate uncomplicated HS.63,64 Clindamycin 1%, the only topical antibiotic studied in a small double-blind RCT of patients with Hurley stage I and stage II HS, demonstrated significant clinical improvement after 12 weeks of treatment (twice- daily application), compared to placebo.84 Topical clindamycin is also recommended to treat flares in patients with mild disease.2,64
Oral antibiotics. Tetracycline (500 mg twice daily for 4 months) is considered a second-line treatment for patients with mild HS.64,68 Doxycycline (200 mg/d for 3 months) may also be considered as a second-line treatment in patients with mild disease.85
Combination oral clindamycin (300 mg) and rifampicin (300 mg) twice daily for 10 weeks is recommended as first-line treatment for patients with moderate HS.2,64,69 Combination rifampin (300 mg twice daily), moxifloxacin (400 mg/d), and metronidazole (500 mg three times a day) is not routinely recommended due to increased risk of toxicity.2
Ertapenem (1 g intravenously daily for 6 weeks) is supported by lower-level evidence as a third-line rescue therapy option and as a bridge to surgery; however, limitations for home infusions, costs, and concerns for antibiotic resistance limit its use.2,86
Corticosteroids and systemic immunomodulators
Intralesional triamcinolone (2-20 mg) may be beneficial in the early stages of HS, although its use is based on a small prospective open study of 33 patients.87 A recent double-blind placebo-controlled RCT comparing varying concentrations of intralesional triamcinolone (10 mg/mL and 40 mg/mL) vs normal saline showed no statistically significant difference in inflammatory clearance, pain reduction, or patient satisfaction.88
Continue to: Short-term systemic corticosteroid tapers...
Short-term systemic corticosteroid tapers (eg, prednisone, starting at 0.5-1 mg/kg) are recommended to treat flares. Long-term corticosteroids and cyclosporine are reserved for patients with severe refractory disease; however, due to safety concerns, their regular use is strongly discouraged.63,64,85 There is limited evidence to support the use of methotrexate for severe refractory disease, and its use is not recommended.63
Hormonal therapy
The use of hormonal therapy for HS is limited by the low-quality evidence (eg, anecdotal evidence, small retrospective analyses, uncontrolled trials).33,63 The only exception is a small double-blind controlled crossover trial from 1986 showing that the antiandrogen effects of combination oral contraceptives (ethinyloestradiol 50 mcg/cyproterone acetate in a reverse sequential regimen and ethinyloestradiol 50 mcg/norgestrel 500 mcg) improved HS lesions.89
Spironolactone, an antiandrogen diuretic, has been studied in small case report series with a high risk for bias. It is used mainly in female patients with mild or moderate disease, or in combination with other agents in patients with severe HS. Further research is needed to determine its utility in the treatment of HS.63,90,91
Metformin, alone or in combination with other therapies (dapsone, finasteride, liraglutide), has been analyzed in small prospective studies of primarily female patients with different severities of HS, obesity, and PCOS. These studies have shown improvement in lesions, QOL, and reduction of workdays lost.92,93
Finasteride. Studies have shown finasteride (1.25-5 mg/d) alone or in combination with other treatments (metformin, liraglutide, levonorgestrel-ethinyl estradiol, and dapsone) provided varying degrees of resolution or improvement in patients with severe and advanced HS. Finasteride has been used for 4 to 16 weeks with a good safety profile.92,94-96
Continue to: Retinoids
Retinoids
Acitretin, alitretinoin, and isotretinoin have been studied in small retrospective studies to manage HS, with variable results.97-99 Robust prospective studies are needed. Retinoids, in general, should be considered as a second- or third-line treatment for moderate to severe HS.63
Surgical intervention
Surgical interventions, which should be considered in patients with widespread mild, moderate, or severe disease, are associated with improved daily activity and work productivity.100 Incision and drainage should be avoided in patients with HS, as this technique does not remove the affected follicles and is associated with 100% recurrence.101
Wide excision is the preferred surgical technique for patients with Hurley stage II and stage III HS; it is associated with lower recurrence rates (13%) compared to local excision (22%) and deroofing (27%).102 Secondary intention healing is the most commonly chosen method, based on lower recurrence rates than primary closure.102
STEEP and laser techniques. The skin-tissue-sparing excision with electrosurgical peeling (STEEP) procedure involves successive tangential excision of affected tissue until the epithelized bottom of the sinus tracts has been reached. This allows for the removal of fibrotic tissue and the sparing of the deep subcutaneous fat. STEEP is associated with 30% of relapses after 43 months.71
Laser surgery has also been studied in patients with Hurley stage II and stage III HS. The most commonly used lasers for HS are the 1064-nm neodymium-doped yttrium aluminum garnet (Nd: YAG) and the carbon dioxide laser; they have been shown to reduce disease severity in inguinal, axillary, and inflammatory sites.72-74
Pain management: Start with lidocaine, NSAIDs
There are few studies about HS-associated pain management.103 For acute episodes, short-acting nonopioid local treatment with lidocaine, topical or oral nonsteroidal anti-inflammatory drugs, and acetaminophen are preferred. Opioids should be reserved for moderate-to-severe pain that has not responded to other analgesics. Adjuvant therapy with pregabalin, gabapentin, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors can also be considered for the comanagement of pain and depression.62,104
Consider this tool to measure treatment response
The HS clinical response (HiSCR) tool is an outcome measure used to evaluate treatment outcomes. The tool uses an HS-specific binary score with the following criteria:
- ≥ 50% reduction in the number of inflammatory nodules;
- no increase in the number of abscesses; and
- no increase in the number of draining fistulas.105
The HiSCR was developed for the PIONEER studies105,106 to assess the response to ADA treatment. It is the only HS scoring system to undergo an extensive validation process with a meaningful clinical endpoint for HS treatment evaluation that is easy to use. Compared to the HS-PGA score (clear, minimal, mild), HiSCR was more responsive to change in patients with HS.105,106
CORRESPONDENCE
Cristina Marti-Amarista, MD, 101 Nicolls Road, Stony Brook, NY, 11794-8228; [email protected]
1. Bergler-Czop B, Hadasik K, Brzezińska-Wcisło L. Acne inversa: difficulties in diagnostics and therapy. Postepy Dermatol Alergol. 2015;32:296-301. doi: 10.5114/pdia.2014.44012
2. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
3. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2:9-16. doi: 10.4161/derm.2.1.12490
4. Kokolakis G, Wolk K, Schneider-Burrus S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236:421-430. doi: 10.1159/000508787
5. Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22:71-77. doi: 10.1177/1203475417736290
6. Savage KT, Gonzalez Brant E, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi: 10.1111/jdv.16213.
7. Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.26083.1
8. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965
9. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. doi: 10.12688/f1000 research.17267.1
10. Sabat R, Jemec GBE, Matusiak Ł. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1
11. Shlyankevich J, Chen AJ, Kim GE, et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144-1150. doi: 10.1016/j.jaad.2014.09.012
12. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. doi: 10.1111/j.1365-2133.2009.09198.x
13. von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537. doi: 10.1111/j.1600-0625.2009.00915.x
14. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999. doi: 10.1016/s0190-9622(96)90277-7
15. Ballard K, Shuman VL. Hidradenitis suppurativa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Updated July 15, 2022. Accessed November 28, 2022. www.ncbi.nlm.nih.gov/books/NBK534867/
16. Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi: 10.1001/jamadermatol.2017.0201
17. Phan K, Charlton O, Smith SD. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. BioMed Dermatol. 2020;4. doi: 10.1186/s41702-019-0052-0
18. Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi: 10.1038/jid.2012.255
19. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi: 10.1177/1203475420972348
20. Vaidya T, Vangipuram R, Alikhan A. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J. 2017;23:13030/qt9xc0n0z1. doi: 10.5070/D3236035391
21. Price KN, Hsiao JL, Shi VY. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatology. 2021;237:97-102. doi: 10.1159/000504911
22. Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460-467. doi: 10.1016/j.jaad.2014.04.001
23. Frew JW, Vekic DA, Wood J, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177:987-998. doi: 10.1111/bjd.15441
24. Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183:999-1010. doi: 10.1111/bjd.19556
25. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi: 10.1016/j.jaad.2019.08.090
26. Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PloS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810
27. Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2021;237:119-124. doi: 10.1159/000501611
28. Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Association between hidradenitis suppurativa and metabolic syndrome: a systematic review and meta-analysis. Actas Dermosifiliogr (Engl Ed). 2019;110:279-288. doi: 10.1016/j.ad.2018.10.020
29. Walker JM, Garcet S, Aleman JO, et al. Obesity and ethnicity alter gene expression in skin. Sci Rep. 2020;10:14079. doi: 10.1038/s41598-020-70244-2.
30. Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43. doi: 10.1016/j.det.2015.08.011
31. Vossen ARJV, van Straalen KR, Swolfs EFH, et al. Nicotine dependency and readiness to quit smoking among patients with hidradenitis suppurativa. Dermatology. 2021;237:383-385. doi: 10.1159/000514028
32. Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi: 10.1111/bjd.13090
33. Clark AK, Quinonez RL, Saric S, et al. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23:13030/qt6383k0n4. doi: 10.5070/D32310036990
34. Saric-Bosanac S, Clark AK, Sivamani RK, et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26:13030/qt8949296f. doi: 10.5070/D3262047430
35. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
36. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270. doi: 10.1001/archdermatol.2010.328
37. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671. doi: 10.1111/j.1365-2230.2005.01897.x
38. Kirthi S, Hellen R, O’Connor R, et al. Hidradenitis suppurativa and Crohn’s disease: a case series. Ir Med J. 2017;110:618.
39. Dumont LM, Landman C, Sokol H, et al; CD-HS Study Group. Increased risk of permanent stoma in Crohn’s disease associated with hidradenitis suppurativa: a case-control study. Aliment Pharmacol Ther. 2020;52:303-310. doi: 10.1111/apt.15863
40. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. doi: 10.1097/MD.0000000000000187.
41. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
42. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
43. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781. doi: 10.1111/bjd.16998.
44. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221; doi: 10.1136/postgradmedj-2013-131994
45. Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170:884-889. doi: 10.1111/bjd.12787
46. Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016;107(suppl 2):27-31. doi: 10.1016/S0001-7310(17) 30006-6
47. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. doi: 10.2147/IDR.S39601
48. Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Dtsch Dermatol Ges. 2014;12:451-463. doi: 10.1111/ddg.12310
49. Hap W, Frejlich E, Rudno-Rudzińska J, et al. Pilonidal sinus: finding the righttrack for treatment. Pol Przegl Chir. 2017;89:68-75. doi: 10.5604/01.3001.0009.6009
50. Al-Hamdi KI, Saadoon AQ. Acne onglobate of the scalp. Int J Trichology. 2020;12:35-37. doi: 10.4103/ijt.ijt_117_19
51. Balestra A, Bytyci H, Guillod C, et al. A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int Med Case Rep J. 2018;11:313-318. doi: 10.2147/IMCRJ.S178561
52. Ibler KS, Kromann CB. Recurrent furunculosis – challenges and management: a review. Clin Cosmet Investig Dermatol. 2014;7:59-64. doi: 10.2147/CCID.S35302
53. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175:263-272. doi: 10.1111/bjd.14475
54. Zouboulis CC, Tzellos T, Kyrgidis A, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (I4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401-1409. doi: 10.1111/bjd.15748
55. Hidradenitis Suppurativa Clinical Resource. Hidradenitis suppurativa stages: Hurley Staging System. www.hsdiseasesource.com/hs-disease-staging. Accessed October 11, 2022.
56. Ovadja ZN, Schuit MM, van der Horst CMAM, et al. Inter- and interrater reliability of Hurley staging for hidradenitis suppurativa. Br J Dermatol. 2019;181:344-349. doi: 10.1111/bjd.17588
57. Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340-1342. doi: 10.1111/j.1524-4725.2007.33286.x
58. Napolitano M, Calzavara-Pinton PG, Zanca A, et al. Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e87. doi: 10.1111/jdv.15235
59. Bukvić Mokos Z, Miše J, Balić A, et al. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat. 2020;28:9-13.
60. Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29:371-376. doi: 10.1111/jdv.12567
61. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology. 2016;232:687-691. doi: 10.1159/000453355
62 Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978. doi: 10.1111/bjd.14418
63. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi: 10.1016/j.jaad.2019.02.068
64. Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343-351. doi: 10.1007/s11154-016-9328-5
65. Vena GA, Cassano N. Drug focus: adalimumab in the treatment of moderate to severe psoriasis. Biologics. 2007;1:93-103.
66. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-55. doi: 10.7326/0003-4819-157-12-201212180-00004
67. Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi: 10.1016/j.jaad.2018.05.040
68. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi: 10.1016/s0190-9622(98)70272-5
69. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi: 10.1159/000228334
70. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. doi: 10.1016/j.jaad.2009.06.050
71. Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi: 10.1111/jdv.12376
72. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. doi: 10.1016/j.jaad.2009.07.048
73. Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. doi: 10.1111/j.1524-4725.2009.01214.x
74. Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. doi: 10.1111/j.1524-4725.2009.01427.x
75. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi: 10.1016/j.jaad.2009.12.018
76. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi: 10.1056/NEJMoa1504370. PMID: 27518661.
77. Adams DR, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501-504. doi: 10.1001/archdermatol.2010.72
78. Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48:1511-1512. doi: 10.1016/j.dld.2016.09.010
79. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi: 10.1001/jamadermatol.2015.3903.
80. Romaní J, Vilarrasa E, Martorell A, et al. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236:21-24. doi: 10.1159/000501075
81. Kane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterol Hepatol (N Y). 2011;7:544-546.
82. Davis W, Vavilin I, Malhotra N. Biologic therapy in HIV: to screen or not to screen. Cureus. 2021;13:e15941. doi: 10.7759/cureus.15941
83. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50-74. doi: 10.1177/1203475418811335
84. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328. doi: 10.1111/j.1365-4362.1983.tb02150.x
85. Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233:113-119. doi: 10.1159/000477459
86. Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19-31. doi: 10.1111/jdv.15233
87. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi: 10.1016/j.jaad.2016.06.049
88. Fajgenbaum K, Crouse L, Dong L, et al. Intralesional triamcinolone may not be beneficial for treating acute hidradenitis suppurativa lesions: a double-blind, randomized, placebo-controlled trial. Dermatol Surg. 2020;46:685-689. doi: 10.1097/DSS.0000000000002112
89. Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268. doi: 10.1111/j.1365-2133.1986.tb05740.x
90. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131. doi: 10.2310/7750.2007.00019
91. Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196. doi: 10.1111/ajd.12362
92. Khandalavala BN. A disease-modifying approach for advanced hidradenitis suppurativa (regimen with metformin, liraglutide, dapsone, and finasteride): a case report. Case Rep Dermatol. 2017;9:70-78. doi: 10.1159/000473873
93. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi: 10.1111/j.1468-3083.2012.04668.x
94. Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
95. Mota F, Machado S, Selores M. Hidradenitis suppurativa in children treated with finasteride-a case series. Pediatr Dermatol. 2017;34:578-583. doi: 10.1111/pde.13216
96. Doménech C, Matarredona J, Escribano-Stablé JC, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology. 2012;224:307-308. doi: 10.1159/000339477
97. Patel N, McKenzie SA, Harview CL, et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J Dermatolog Treat. 2021;32:473-475. doi: 10.1080/09546634.2019.1670779
98. Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-76. doi: 10.1016/s0190-9622(99) 70530-x
99. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125. doi: 10.1159/000477207
100. Prens LM, Huizinga J, Janse IC. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941-1946. doi: 10.1111/jdv.15706
101. Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168. doi: 10.1007/s003840050159
102. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70-S77. doi: 10.1016/j.jaad.2015.07.044.
103. Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444. doi: 10.1097/AJP.0b013e3181ceb80c
104. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi: 10.1016/j.jaad.2015.07.046
105. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994. doi: 10.1111/jdv.13216
106. Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434-1442. doi: 10.1111/bjd.13270
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic, recurrent, inflammatory occlusive disease affecting the terminal follicular epithelium in apocrine gland–bearing skin areas.1 HS manifests as painful nodules, abscesses, fistulas, and scarring and often has a severe psychological impact on the affected patient.2
When HS was first identified in the 1800s, it was believed to result from a dysfunction of the sweat glands.3 In 1939, scientists identified the true cause: follicular occlusion.3
Due to its chronic nature, heterogeneity in presentation, and apparent low prevalence,4 HS is considered an orphan disease.5 Over the past 10 years, there has been a surge in HS research—particularly in medical management—which has provided a better understanding of this condition.6,7
In this review, we discuss the most updated evidence regarding the diagnosis and treatment of HS to guide the family physician (FP)’s approach to managing this debilitating disease. But first, we offer a word about the etiology and pathophysiology of the condition.
3 events set the stage for hidradenitis suppurativa
Although the exact cause of HS is still unknown, some researchers have hypothesized that HS results from a combination of genetic predisposition and environmental and lifestyle factors.8-12 The primary mechanism of HS is the obstruction of the terminal follicular epithelium by a keratin plug.1,13,14 A systematic review of molecular inflammatory pathways involved in HS divides the pathogenesis of HS into 3 events: follicular occlusion followed by dilation, follicular rupture and inflammatory response, and chronic inflammatory state with sinus tracts.8
An underreported condition
HS is often underreported and misdiagnosed.4,15 Globally, the prevalence of HS varies from < 1% to 4%.15,16 A systematic review with meta-analysis showed a higher prevalence of HS in females compared to males in American and European populations.17 In the United States, the overall frequency of HS is 0.1%, or 98 per 100,000 persons.16 The prevalence of HS is highest among patients ages 30 to 39 years; there is decreased prevalence in patients ages 55 years and older.16,18
Who is at heightened risk?
Recent research has shown a relationship between ethnicity and HS.16,19,20 African American and biracial groups (defined as African American and White) have a 3-fold and 2-fold greater prevalence of HS, respectively, compared to White patients.16 However, the prevalence of HS in non-White ethnic groups may be underestimated in clinical trials due to a lack of representation and subgroup analyses based on ethnicity, which may affect generalizability in HS recommendations.21
Continue to: Genetic predisposition
Genetic predisposition. As many as 40% of patients with HS report having at least 1 affected family member. A positive family history of HS is associated with earlier onset, longer disease duration, and severe disease.22 HS is genetically heterogeneous, and several mutations (eg, gamma secretase, PSTPIP1, PSEN1 genes) have been identified in patients and in vitro as the cause of dysregulation of epidermal proliferation and differentiation, immune dysregulation, and promotion of amyloid formation.8,23-25
Obesity and metabolic risk factors. There is a strong relationship between HS and obesity. As many as 70% of patients with HS are obese, and 9% to 40% have metabolic syndrome.12,18,26-28 Obesity is associated with maceration and mechanical stress, increased fragility of the dermo-epidermal junction, changes in cutaneous blood flow, and subdermal fat inflammation—all of which favor the pathophysiology of HS.29,30
Smoking. Tobacco smoking is associated with severe HS and a lower chance of remission.12 Population-based studies have shown that as many as 90% of patients with HS have a history of smoking ≥ 20 packs of cigarettes per year.1,12,18,31,32 The nicotine and thousands of other chemicals present in cigarettes trigger keratinocytes and fibroblasts, resulting in epidermal hyperplasia, infundibular hyperkeratosis, excessive cornification, and dysbiosis.8,23,24
Hormones. The exact role sex hormones play in the pathogenesis of HS remains unclear.8,32 Most information is based primarily on small studies looking at antiandrogen treatments, HS activity during the menstrual cycle and pregnancy, HS exacerbation related to androgenic effects of hormonal contraception, and the association of HS with metabolic-endocrine disorders (eg, polycystic ovary syndrome [PCOS]).8,33
Androgens induce hyperkeratosis that may lead to follicular occlusion—the hallmark of HS pathology.34 A systematic review looking at the role of androgen and estrogen in HS found that while some patients with HS have elevated androgen levels, most have androgen and estrogen levels within normal range.35 Therefore, increased peripheral androgen receptor sensitivity has been hypothesized as the mechanism of action contributing to HS manifestation.34
Continue to: Host-defense defects
Host-defense defects. HS shares a similar cytokine profile with other well-established immune-mediated inflammatory diseases, including pyoderma gangrenosum (PG)36,37 and Crohn disease.38-40 HS is characterized by the expression of several immune mediators, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-8, IL-17, and the IL-23/T helper 17 pathway, all of which are upregulated in other inflammatory diseases and also result in an abnormal innate immune response.8,24 The recently described clinical triad of PG, acne, and HS (PASH) and the tetrad of pyogenic arthritis, PG, acne, and HS (PAPASH) further support the role of immune dysregulation in the pathogenesis of HS.40 Nonetheless, further studies are needed to determine the exact pathways of cytokine effect in HS.41
Use these criteria to make the diagnosis
The US and Canadian Hidradenitis Suppurativa Foundations (HSF) guidelines base the clinical diagnosis of HS on the following criteria2:
- Typical HS lesions: Erythematous skin lesions; inflamed, deep-seated painful nodules; “tombstone” double-ended comedones; sinus tracts; scarring; deformity. FIGURES 1A-1E show typical lesions seen in patients with HS.
- Typical locations: Intertriginous regions—apocrine gland–containing areas in axilla, groin, perineal region, buttocks, gluteal cleft, and mammary folds; beltline and waistband areas; areas of skin compression and friction.
- Recurrence and chronicity: Recurrent painful or suppurating lesions that appear more than twice in a 6-month period.2,41-43

Patients with HS usually present with painful recurrent abscesses and scarring and often report multiple visits to the emergency department for drainage or failed antibiotic treatment for abscesses.15,44
Ask patients these 2 questions. Vinding et al45 developed a survey for the diagnosis of HS using 2 simple questions based on the 3 criteria established by the HSF:
- “Have you had an outbreak of boils during the last 6 months?” and
- “Where and how many boils have you had?” (This question includes a list of the typical HS locations—eg, axilla, groin, genitals, area under breast.)
In their questionnaire, Vinding et al45 found that an affirmative answer to Question 1 and reports of > 2 boils in response to Question 2 correlated to a sensitivity of 90%, specificity of 97%, positive predictive value of 96%, and negative predictive value of 92% for the diagnosis of HS. The differential diagnosis of HS is summarized in TABLE 1.42,45-52

Continue to: These tools can help you to stage hidradenitis suppurativa
These tools can help you to stage hidradenitis suppurativa
Multiple tools are available to assess the severity of HS.53 We will describe the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System (IHS4). Other diagnostic tools, such as the Sartorius score and the Hidradenitis Suppurativa Physician’s Global Assessment Scale (HS-PGA), can be time-consuming and challenging to interpret, limiting their use in the clinical setting.2,54
Hurley staging system (available at www.hsdiseasesource.com/hs-disease-staging) considers the presence of nodules, abscesses, sinus tracts, and scarring affecting an entire anatomical area.13,55 This system is most useful as a rapid classification tool for patients with HS in the clinical setting but should not be used to assess clinical response.2,13,56
The IHS4 (available at https://online library.wiley.com/doi/10.1111/bjd.15748) is a validated and easy-to-use tool for assessing HS and guiding the therapeutic strategy in clinical practice.54 With IHS4, the clinician must calculate the following:
- total number of nodules > 10 mm in diameter
- total number of abscesses multiplied by 2, and
- total number of draining tunnels (fistulae/sinuses) multiplied by 4.
Mild HS is defined as a score ≤ 3 points; moderate HS, 4 to 10 points; and severe HS, ≥ 11 points.54
No diagnostic tests, but ultrasound may be helpful
There are currently no established biological markers or specific tests for diagnosing HS.15 Ultrasound is emerging as a tool to assess dermal thickness, hair follicle morphology, and number and extent of fluid collections. Two recent studies showed that pairing clinical assessment with ultrasound findings improves accuracy of scoring in 84% of cases.57,58 For patients with severe HS, skin biopsy can be considered to rule out squamous cell carcinoma. Cultures, however, have limited utility except for suspected superimposed bacterial infection.2
Continue to: Screening for comorbidities
Screening for comorbidities
HSF recommends clinicians screen patients for comorbidities associated with HS (TABLE 2).2 Overall, screening patients for active and past history of smoking is strongly recommended, as is screening for metabolic syndrome, hyperlipidemia, type 2 diabetes (1.5- to 3-fold greater risk of type 2 diabetes in HS patients), and PCOS (3-fold greater risk).2,26,27,59 Screening patients for depression and anxiety is also routinely recommended.2 However, the authors of this article strongly recommend screening all patients with HS for psychiatric comorbidities, as research has shown a 2-fold greater risk of depression and anxiety, social isolation, and low self-esteem that severely limits quality of life (QOL) in this patient population.60,61

Management
Treat existing lesions, reduce formation of new ones
The main goals of treatment for patients with HS are to treat existing lesions and reduce associated symptoms, reduce the formation of new lesions, and minimize associated psychological morbidity.15 FPs play an important role in the early diagnosis, treatment, and comprehensive care of patients with HS. This includes monitoring patients, managing comorbidities, making appropriate referrals to dermatologists, and coordinating the multidisciplinary care that patients with HS require.

A systematic review identified more than 50 interventions used to treat HS, most based on small observational studies and randomized controlled trials (RCTs) with a high risk of bias.62 FIGURE 22,62-69 provides an evidence-based treatment algorithm for HS, and TABLE 32,63,64,70-75 summarizes the most commonly used treatments.

Biologic agents
Adalimumab (ADA) is a fully human immunoglobulin G1 monoclonal antibody that binds to TNF-alpha, neutralizes its bioactivity, and induces apoptosis of TNF-expressing mononuclear cells. It is the only medication approved by the US Food and Drug Administration for active refractory moderate and severe HS.62,65 Several double-blinded RCTs, including PIONEER I and PIONEER II, studied the effectiveness of ADA for HS and found significant clinical responses at Week 12, 50% reduction in abscess and nodule counts, no increase in abscesses or draining fistulas at Week 12, and sustained improvement in lesion counts, pain, and QOL.66,67,76
IL-1 and IL-23 inhibitors. The efficacy of etanercept and golimumab (anti-TNF), as well as anakinra (IL-1 inhibitor) and ustekinumab (IL-1/IL-23 inhibitor), continue to be investigated with variable results; they are considered second-line treatment for active refractory moderate and severe HS after ADA.65,77-80 Infliximab (IL-1 beta inhibitor) has shown no effect on reducing disease severity.70Compared to other treatments, biologic therapy is associated with higher costs (TABLE 3),2,63,64,70-75 an increased risk for reactivation of latent infections (eg, tuberculosis, herpes simplex, and hepatitis C virus [HCV], and B [HBV]), and an attenuated response to vaccines.81 Prior to starting biologic therapy, FPs should screen patients with HS for tuberculosis and HBV, consider HIV and HCV screening in at-risk patients, and optimize the immunization status of the patient.82,83 While inactivated vaccines can be administered without discontinuing biologic treatment, patients should avoid live-attenuated vaccines while taking biologics.83
Continue to: Antibiotic therapy
Antibiotic therapy
Topical antibiotics are considered first-line treatment for mild and moderate uncomplicated HS.63,64 Clindamycin 1%, the only topical antibiotic studied in a small double-blind RCT of patients with Hurley stage I and stage II HS, demonstrated significant clinical improvement after 12 weeks of treatment (twice- daily application), compared to placebo.84 Topical clindamycin is also recommended to treat flares in patients with mild disease.2,64
Oral antibiotics. Tetracycline (500 mg twice daily for 4 months) is considered a second-line treatment for patients with mild HS.64,68 Doxycycline (200 mg/d for 3 months) may also be considered as a second-line treatment in patients with mild disease.85
Combination oral clindamycin (300 mg) and rifampicin (300 mg) twice daily for 10 weeks is recommended as first-line treatment for patients with moderate HS.2,64,69 Combination rifampin (300 mg twice daily), moxifloxacin (400 mg/d), and metronidazole (500 mg three times a day) is not routinely recommended due to increased risk of toxicity.2
Ertapenem (1 g intravenously daily for 6 weeks) is supported by lower-level evidence as a third-line rescue therapy option and as a bridge to surgery; however, limitations for home infusions, costs, and concerns for antibiotic resistance limit its use.2,86
Corticosteroids and systemic immunomodulators
Intralesional triamcinolone (2-20 mg) may be beneficial in the early stages of HS, although its use is based on a small prospective open study of 33 patients.87 A recent double-blind placebo-controlled RCT comparing varying concentrations of intralesional triamcinolone (10 mg/mL and 40 mg/mL) vs normal saline showed no statistically significant difference in inflammatory clearance, pain reduction, or patient satisfaction.88
Continue to: Short-term systemic corticosteroid tapers...
Short-term systemic corticosteroid tapers (eg, prednisone, starting at 0.5-1 mg/kg) are recommended to treat flares. Long-term corticosteroids and cyclosporine are reserved for patients with severe refractory disease; however, due to safety concerns, their regular use is strongly discouraged.63,64,85 There is limited evidence to support the use of methotrexate for severe refractory disease, and its use is not recommended.63
Hormonal therapy
The use of hormonal therapy for HS is limited by the low-quality evidence (eg, anecdotal evidence, small retrospective analyses, uncontrolled trials).33,63 The only exception is a small double-blind controlled crossover trial from 1986 showing that the antiandrogen effects of combination oral contraceptives (ethinyloestradiol 50 mcg/cyproterone acetate in a reverse sequential regimen and ethinyloestradiol 50 mcg/norgestrel 500 mcg) improved HS lesions.89
Spironolactone, an antiandrogen diuretic, has been studied in small case report series with a high risk for bias. It is used mainly in female patients with mild or moderate disease, or in combination with other agents in patients with severe HS. Further research is needed to determine its utility in the treatment of HS.63,90,91
Metformin, alone or in combination with other therapies (dapsone, finasteride, liraglutide), has been analyzed in small prospective studies of primarily female patients with different severities of HS, obesity, and PCOS. These studies have shown improvement in lesions, QOL, and reduction of workdays lost.92,93
Finasteride. Studies have shown finasteride (1.25-5 mg/d) alone or in combination with other treatments (metformin, liraglutide, levonorgestrel-ethinyl estradiol, and dapsone) provided varying degrees of resolution or improvement in patients with severe and advanced HS. Finasteride has been used for 4 to 16 weeks with a good safety profile.92,94-96
Continue to: Retinoids
Retinoids
Acitretin, alitretinoin, and isotretinoin have been studied in small retrospective studies to manage HS, with variable results.97-99 Robust prospective studies are needed. Retinoids, in general, should be considered as a second- or third-line treatment for moderate to severe HS.63
Surgical intervention
Surgical interventions, which should be considered in patients with widespread mild, moderate, or severe disease, are associated with improved daily activity and work productivity.100 Incision and drainage should be avoided in patients with HS, as this technique does not remove the affected follicles and is associated with 100% recurrence.101
Wide excision is the preferred surgical technique for patients with Hurley stage II and stage III HS; it is associated with lower recurrence rates (13%) compared to local excision (22%) and deroofing (27%).102 Secondary intention healing is the most commonly chosen method, based on lower recurrence rates than primary closure.102
STEEP and laser techniques. The skin-tissue-sparing excision with electrosurgical peeling (STEEP) procedure involves successive tangential excision of affected tissue until the epithelized bottom of the sinus tracts has been reached. This allows for the removal of fibrotic tissue and the sparing of the deep subcutaneous fat. STEEP is associated with 30% of relapses after 43 months.71
Laser surgery has also been studied in patients with Hurley stage II and stage III HS. The most commonly used lasers for HS are the 1064-nm neodymium-doped yttrium aluminum garnet (Nd: YAG) and the carbon dioxide laser; they have been shown to reduce disease severity in inguinal, axillary, and inflammatory sites.72-74
Pain management: Start with lidocaine, NSAIDs
There are few studies about HS-associated pain management.103 For acute episodes, short-acting nonopioid local treatment with lidocaine, topical or oral nonsteroidal anti-inflammatory drugs, and acetaminophen are preferred. Opioids should be reserved for moderate-to-severe pain that has not responded to other analgesics. Adjuvant therapy with pregabalin, gabapentin, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors can also be considered for the comanagement of pain and depression.62,104
Consider this tool to measure treatment response
The HS clinical response (HiSCR) tool is an outcome measure used to evaluate treatment outcomes. The tool uses an HS-specific binary score with the following criteria:
- ≥ 50% reduction in the number of inflammatory nodules;
- no increase in the number of abscesses; and
- no increase in the number of draining fistulas.105
The HiSCR was developed for the PIONEER studies105,106 to assess the response to ADA treatment. It is the only HS scoring system to undergo an extensive validation process with a meaningful clinical endpoint for HS treatment evaluation that is easy to use. Compared to the HS-PGA score (clear, minimal, mild), HiSCR was more responsive to change in patients with HS.105,106
CORRESPONDENCE
Cristina Marti-Amarista, MD, 101 Nicolls Road, Stony Brook, NY, 11794-8228; [email protected]
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic, recurrent, inflammatory occlusive disease affecting the terminal follicular epithelium in apocrine gland–bearing skin areas.1 HS manifests as painful nodules, abscesses, fistulas, and scarring and often has a severe psychological impact on the affected patient.2
When HS was first identified in the 1800s, it was believed to result from a dysfunction of the sweat glands.3 In 1939, scientists identified the true cause: follicular occlusion.3
Due to its chronic nature, heterogeneity in presentation, and apparent low prevalence,4 HS is considered an orphan disease.5 Over the past 10 years, there has been a surge in HS research—particularly in medical management—which has provided a better understanding of this condition.6,7
In this review, we discuss the most updated evidence regarding the diagnosis and treatment of HS to guide the family physician (FP)’s approach to managing this debilitating disease. But first, we offer a word about the etiology and pathophysiology of the condition.
3 events set the stage for hidradenitis suppurativa
Although the exact cause of HS is still unknown, some researchers have hypothesized that HS results from a combination of genetic predisposition and environmental and lifestyle factors.8-12 The primary mechanism of HS is the obstruction of the terminal follicular epithelium by a keratin plug.1,13,14 A systematic review of molecular inflammatory pathways involved in HS divides the pathogenesis of HS into 3 events: follicular occlusion followed by dilation, follicular rupture and inflammatory response, and chronic inflammatory state with sinus tracts.8
An underreported condition
HS is often underreported and misdiagnosed.4,15 Globally, the prevalence of HS varies from < 1% to 4%.15,16 A systematic review with meta-analysis showed a higher prevalence of HS in females compared to males in American and European populations.17 In the United States, the overall frequency of HS is 0.1%, or 98 per 100,000 persons.16 The prevalence of HS is highest among patients ages 30 to 39 years; there is decreased prevalence in patients ages 55 years and older.16,18
Who is at heightened risk?
Recent research has shown a relationship between ethnicity and HS.16,19,20 African American and biracial groups (defined as African American and White) have a 3-fold and 2-fold greater prevalence of HS, respectively, compared to White patients.16 However, the prevalence of HS in non-White ethnic groups may be underestimated in clinical trials due to a lack of representation and subgroup analyses based on ethnicity, which may affect generalizability in HS recommendations.21
Continue to: Genetic predisposition
Genetic predisposition. As many as 40% of patients with HS report having at least 1 affected family member. A positive family history of HS is associated with earlier onset, longer disease duration, and severe disease.22 HS is genetically heterogeneous, and several mutations (eg, gamma secretase, PSTPIP1, PSEN1 genes) have been identified in patients and in vitro as the cause of dysregulation of epidermal proliferation and differentiation, immune dysregulation, and promotion of amyloid formation.8,23-25
Obesity and metabolic risk factors. There is a strong relationship between HS and obesity. As many as 70% of patients with HS are obese, and 9% to 40% have metabolic syndrome.12,18,26-28 Obesity is associated with maceration and mechanical stress, increased fragility of the dermo-epidermal junction, changes in cutaneous blood flow, and subdermal fat inflammation—all of which favor the pathophysiology of HS.29,30
Smoking. Tobacco smoking is associated with severe HS and a lower chance of remission.12 Population-based studies have shown that as many as 90% of patients with HS have a history of smoking ≥ 20 packs of cigarettes per year.1,12,18,31,32 The nicotine and thousands of other chemicals present in cigarettes trigger keratinocytes and fibroblasts, resulting in epidermal hyperplasia, infundibular hyperkeratosis, excessive cornification, and dysbiosis.8,23,24
Hormones. The exact role sex hormones play in the pathogenesis of HS remains unclear.8,32 Most information is based primarily on small studies looking at antiandrogen treatments, HS activity during the menstrual cycle and pregnancy, HS exacerbation related to androgenic effects of hormonal contraception, and the association of HS with metabolic-endocrine disorders (eg, polycystic ovary syndrome [PCOS]).8,33
Androgens induce hyperkeratosis that may lead to follicular occlusion—the hallmark of HS pathology.34 A systematic review looking at the role of androgen and estrogen in HS found that while some patients with HS have elevated androgen levels, most have androgen and estrogen levels within normal range.35 Therefore, increased peripheral androgen receptor sensitivity has been hypothesized as the mechanism of action contributing to HS manifestation.34
Continue to: Host-defense defects
Host-defense defects. HS shares a similar cytokine profile with other well-established immune-mediated inflammatory diseases, including pyoderma gangrenosum (PG)36,37 and Crohn disease.38-40 HS is characterized by the expression of several immune mediators, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-8, IL-17, and the IL-23/T helper 17 pathway, all of which are upregulated in other inflammatory diseases and also result in an abnormal innate immune response.8,24 The recently described clinical triad of PG, acne, and HS (PASH) and the tetrad of pyogenic arthritis, PG, acne, and HS (PAPASH) further support the role of immune dysregulation in the pathogenesis of HS.40 Nonetheless, further studies are needed to determine the exact pathways of cytokine effect in HS.41
Use these criteria to make the diagnosis
The US and Canadian Hidradenitis Suppurativa Foundations (HSF) guidelines base the clinical diagnosis of HS on the following criteria2:
- Typical HS lesions: Erythematous skin lesions; inflamed, deep-seated painful nodules; “tombstone” double-ended comedones; sinus tracts; scarring; deformity. FIGURES 1A-1E show typical lesions seen in patients with HS.
- Typical locations: Intertriginous regions—apocrine gland–containing areas in axilla, groin, perineal region, buttocks, gluteal cleft, and mammary folds; beltline and waistband areas; areas of skin compression and friction.
- Recurrence and chronicity: Recurrent painful or suppurating lesions that appear more than twice in a 6-month period.2,41-43

Patients with HS usually present with painful recurrent abscesses and scarring and often report multiple visits to the emergency department for drainage or failed antibiotic treatment for abscesses.15,44
Ask patients these 2 questions. Vinding et al45 developed a survey for the diagnosis of HS using 2 simple questions based on the 3 criteria established by the HSF:
- “Have you had an outbreak of boils during the last 6 months?” and
- “Where and how many boils have you had?” (This question includes a list of the typical HS locations—eg, axilla, groin, genitals, area under breast.)
In their questionnaire, Vinding et al45 found that an affirmative answer to Question 1 and reports of > 2 boils in response to Question 2 correlated to a sensitivity of 90%, specificity of 97%, positive predictive value of 96%, and negative predictive value of 92% for the diagnosis of HS. The differential diagnosis of HS is summarized in TABLE 1.42,45-52

Continue to: These tools can help you to stage hidradenitis suppurativa
These tools can help you to stage hidradenitis suppurativa
Multiple tools are available to assess the severity of HS.53 We will describe the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System (IHS4). Other diagnostic tools, such as the Sartorius score and the Hidradenitis Suppurativa Physician’s Global Assessment Scale (HS-PGA), can be time-consuming and challenging to interpret, limiting their use in the clinical setting.2,54
Hurley staging system (available at www.hsdiseasesource.com/hs-disease-staging) considers the presence of nodules, abscesses, sinus tracts, and scarring affecting an entire anatomical area.13,55 This system is most useful as a rapid classification tool for patients with HS in the clinical setting but should not be used to assess clinical response.2,13,56
The IHS4 (available at https://online library.wiley.com/doi/10.1111/bjd.15748) is a validated and easy-to-use tool for assessing HS and guiding the therapeutic strategy in clinical practice.54 With IHS4, the clinician must calculate the following:
- total number of nodules > 10 mm in diameter
- total number of abscesses multiplied by 2, and
- total number of draining tunnels (fistulae/sinuses) multiplied by 4.
Mild HS is defined as a score ≤ 3 points; moderate HS, 4 to 10 points; and severe HS, ≥ 11 points.54
No diagnostic tests, but ultrasound may be helpful
There are currently no established biological markers or specific tests for diagnosing HS.15 Ultrasound is emerging as a tool to assess dermal thickness, hair follicle morphology, and number and extent of fluid collections. Two recent studies showed that pairing clinical assessment with ultrasound findings improves accuracy of scoring in 84% of cases.57,58 For patients with severe HS, skin biopsy can be considered to rule out squamous cell carcinoma. Cultures, however, have limited utility except for suspected superimposed bacterial infection.2
Continue to: Screening for comorbidities
Screening for comorbidities
HSF recommends clinicians screen patients for comorbidities associated with HS (TABLE 2).2 Overall, screening patients for active and past history of smoking is strongly recommended, as is screening for metabolic syndrome, hyperlipidemia, type 2 diabetes (1.5- to 3-fold greater risk of type 2 diabetes in HS patients), and PCOS (3-fold greater risk).2,26,27,59 Screening patients for depression and anxiety is also routinely recommended.2 However, the authors of this article strongly recommend screening all patients with HS for psychiatric comorbidities, as research has shown a 2-fold greater risk of depression and anxiety, social isolation, and low self-esteem that severely limits quality of life (QOL) in this patient population.60,61

Management
Treat existing lesions, reduce formation of new ones
The main goals of treatment for patients with HS are to treat existing lesions and reduce associated symptoms, reduce the formation of new lesions, and minimize associated psychological morbidity.15 FPs play an important role in the early diagnosis, treatment, and comprehensive care of patients with HS. This includes monitoring patients, managing comorbidities, making appropriate referrals to dermatologists, and coordinating the multidisciplinary care that patients with HS require.

A systematic review identified more than 50 interventions used to treat HS, most based on small observational studies and randomized controlled trials (RCTs) with a high risk of bias.62 FIGURE 22,62-69 provides an evidence-based treatment algorithm for HS, and TABLE 32,63,64,70-75 summarizes the most commonly used treatments.

Biologic agents
Adalimumab (ADA) is a fully human immunoglobulin G1 monoclonal antibody that binds to TNF-alpha, neutralizes its bioactivity, and induces apoptosis of TNF-expressing mononuclear cells. It is the only medication approved by the US Food and Drug Administration for active refractory moderate and severe HS.62,65 Several double-blinded RCTs, including PIONEER I and PIONEER II, studied the effectiveness of ADA for HS and found significant clinical responses at Week 12, 50% reduction in abscess and nodule counts, no increase in abscesses or draining fistulas at Week 12, and sustained improvement in lesion counts, pain, and QOL.66,67,76
IL-1 and IL-23 inhibitors. The efficacy of etanercept and golimumab (anti-TNF), as well as anakinra (IL-1 inhibitor) and ustekinumab (IL-1/IL-23 inhibitor), continue to be investigated with variable results; they are considered second-line treatment for active refractory moderate and severe HS after ADA.65,77-80 Infliximab (IL-1 beta inhibitor) has shown no effect on reducing disease severity.70Compared to other treatments, biologic therapy is associated with higher costs (TABLE 3),2,63,64,70-75 an increased risk for reactivation of latent infections (eg, tuberculosis, herpes simplex, and hepatitis C virus [HCV], and B [HBV]), and an attenuated response to vaccines.81 Prior to starting biologic therapy, FPs should screen patients with HS for tuberculosis and HBV, consider HIV and HCV screening in at-risk patients, and optimize the immunization status of the patient.82,83 While inactivated vaccines can be administered without discontinuing biologic treatment, patients should avoid live-attenuated vaccines while taking biologics.83
Continue to: Antibiotic therapy
Antibiotic therapy
Topical antibiotics are considered first-line treatment for mild and moderate uncomplicated HS.63,64 Clindamycin 1%, the only topical antibiotic studied in a small double-blind RCT of patients with Hurley stage I and stage II HS, demonstrated significant clinical improvement after 12 weeks of treatment (twice- daily application), compared to placebo.84 Topical clindamycin is also recommended to treat flares in patients with mild disease.2,64
Oral antibiotics. Tetracycline (500 mg twice daily for 4 months) is considered a second-line treatment for patients with mild HS.64,68 Doxycycline (200 mg/d for 3 months) may also be considered as a second-line treatment in patients with mild disease.85
Combination oral clindamycin (300 mg) and rifampicin (300 mg) twice daily for 10 weeks is recommended as first-line treatment for patients with moderate HS.2,64,69 Combination rifampin (300 mg twice daily), moxifloxacin (400 mg/d), and metronidazole (500 mg three times a day) is not routinely recommended due to increased risk of toxicity.2
Ertapenem (1 g intravenously daily for 6 weeks) is supported by lower-level evidence as a third-line rescue therapy option and as a bridge to surgery; however, limitations for home infusions, costs, and concerns for antibiotic resistance limit its use.2,86
Corticosteroids and systemic immunomodulators
Intralesional triamcinolone (2-20 mg) may be beneficial in the early stages of HS, although its use is based on a small prospective open study of 33 patients.87 A recent double-blind placebo-controlled RCT comparing varying concentrations of intralesional triamcinolone (10 mg/mL and 40 mg/mL) vs normal saline showed no statistically significant difference in inflammatory clearance, pain reduction, or patient satisfaction.88
Continue to: Short-term systemic corticosteroid tapers...
Short-term systemic corticosteroid tapers (eg, prednisone, starting at 0.5-1 mg/kg) are recommended to treat flares. Long-term corticosteroids and cyclosporine are reserved for patients with severe refractory disease; however, due to safety concerns, their regular use is strongly discouraged.63,64,85 There is limited evidence to support the use of methotrexate for severe refractory disease, and its use is not recommended.63
Hormonal therapy
The use of hormonal therapy for HS is limited by the low-quality evidence (eg, anecdotal evidence, small retrospective analyses, uncontrolled trials).33,63 The only exception is a small double-blind controlled crossover trial from 1986 showing that the antiandrogen effects of combination oral contraceptives (ethinyloestradiol 50 mcg/cyproterone acetate in a reverse sequential regimen and ethinyloestradiol 50 mcg/norgestrel 500 mcg) improved HS lesions.89
Spironolactone, an antiandrogen diuretic, has been studied in small case report series with a high risk for bias. It is used mainly in female patients with mild or moderate disease, or in combination with other agents in patients with severe HS. Further research is needed to determine its utility in the treatment of HS.63,90,91
Metformin, alone or in combination with other therapies (dapsone, finasteride, liraglutide), has been analyzed in small prospective studies of primarily female patients with different severities of HS, obesity, and PCOS. These studies have shown improvement in lesions, QOL, and reduction of workdays lost.92,93
Finasteride. Studies have shown finasteride (1.25-5 mg/d) alone or in combination with other treatments (metformin, liraglutide, levonorgestrel-ethinyl estradiol, and dapsone) provided varying degrees of resolution or improvement in patients with severe and advanced HS. Finasteride has been used for 4 to 16 weeks with a good safety profile.92,94-96
Continue to: Retinoids
Retinoids
Acitretin, alitretinoin, and isotretinoin have been studied in small retrospective studies to manage HS, with variable results.97-99 Robust prospective studies are needed. Retinoids, in general, should be considered as a second- or third-line treatment for moderate to severe HS.63
Surgical intervention
Surgical interventions, which should be considered in patients with widespread mild, moderate, or severe disease, are associated with improved daily activity and work productivity.100 Incision and drainage should be avoided in patients with HS, as this technique does not remove the affected follicles and is associated with 100% recurrence.101
Wide excision is the preferred surgical technique for patients with Hurley stage II and stage III HS; it is associated with lower recurrence rates (13%) compared to local excision (22%) and deroofing (27%).102 Secondary intention healing is the most commonly chosen method, based on lower recurrence rates than primary closure.102
STEEP and laser techniques. The skin-tissue-sparing excision with electrosurgical peeling (STEEP) procedure involves successive tangential excision of affected tissue until the epithelized bottom of the sinus tracts has been reached. This allows for the removal of fibrotic tissue and the sparing of the deep subcutaneous fat. STEEP is associated with 30% of relapses after 43 months.71
Laser surgery has also been studied in patients with Hurley stage II and stage III HS. The most commonly used lasers for HS are the 1064-nm neodymium-doped yttrium aluminum garnet (Nd: YAG) and the carbon dioxide laser; they have been shown to reduce disease severity in inguinal, axillary, and inflammatory sites.72-74
Pain management: Start with lidocaine, NSAIDs
There are few studies about HS-associated pain management.103 For acute episodes, short-acting nonopioid local treatment with lidocaine, topical or oral nonsteroidal anti-inflammatory drugs, and acetaminophen are preferred. Opioids should be reserved for moderate-to-severe pain that has not responded to other analgesics. Adjuvant therapy with pregabalin, gabapentin, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors can also be considered for the comanagement of pain and depression.62,104
Consider this tool to measure treatment response
The HS clinical response (HiSCR) tool is an outcome measure used to evaluate treatment outcomes. The tool uses an HS-specific binary score with the following criteria:
- ≥ 50% reduction in the number of inflammatory nodules;
- no increase in the number of abscesses; and
- no increase in the number of draining fistulas.105
The HiSCR was developed for the PIONEER studies105,106 to assess the response to ADA treatment. It is the only HS scoring system to undergo an extensive validation process with a meaningful clinical endpoint for HS treatment evaluation that is easy to use. Compared to the HS-PGA score (clear, minimal, mild), HiSCR was more responsive to change in patients with HS.105,106
CORRESPONDENCE
Cristina Marti-Amarista, MD, 101 Nicolls Road, Stony Brook, NY, 11794-8228; [email protected]
1. Bergler-Czop B, Hadasik K, Brzezińska-Wcisło L. Acne inversa: difficulties in diagnostics and therapy. Postepy Dermatol Alergol. 2015;32:296-301. doi: 10.5114/pdia.2014.44012
2. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
3. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2:9-16. doi: 10.4161/derm.2.1.12490
4. Kokolakis G, Wolk K, Schneider-Burrus S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236:421-430. doi: 10.1159/000508787
5. Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22:71-77. doi: 10.1177/1203475417736290
6. Savage KT, Gonzalez Brant E, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi: 10.1111/jdv.16213.
7. Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.26083.1
8. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965
9. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. doi: 10.12688/f1000 research.17267.1
10. Sabat R, Jemec GBE, Matusiak Ł. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1
11. Shlyankevich J, Chen AJ, Kim GE, et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144-1150. doi: 10.1016/j.jaad.2014.09.012
12. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. doi: 10.1111/j.1365-2133.2009.09198.x
13. von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537. doi: 10.1111/j.1600-0625.2009.00915.x
14. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999. doi: 10.1016/s0190-9622(96)90277-7
15. Ballard K, Shuman VL. Hidradenitis suppurativa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Updated July 15, 2022. Accessed November 28, 2022. www.ncbi.nlm.nih.gov/books/NBK534867/
16. Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi: 10.1001/jamadermatol.2017.0201
17. Phan K, Charlton O, Smith SD. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. BioMed Dermatol. 2020;4. doi: 10.1186/s41702-019-0052-0
18. Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi: 10.1038/jid.2012.255
19. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi: 10.1177/1203475420972348
20. Vaidya T, Vangipuram R, Alikhan A. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J. 2017;23:13030/qt9xc0n0z1. doi: 10.5070/D3236035391
21. Price KN, Hsiao JL, Shi VY. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatology. 2021;237:97-102. doi: 10.1159/000504911
22. Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460-467. doi: 10.1016/j.jaad.2014.04.001
23. Frew JW, Vekic DA, Wood J, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177:987-998. doi: 10.1111/bjd.15441
24. Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183:999-1010. doi: 10.1111/bjd.19556
25. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi: 10.1016/j.jaad.2019.08.090
26. Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PloS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810
27. Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2021;237:119-124. doi: 10.1159/000501611
28. Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Association between hidradenitis suppurativa and metabolic syndrome: a systematic review and meta-analysis. Actas Dermosifiliogr (Engl Ed). 2019;110:279-288. doi: 10.1016/j.ad.2018.10.020
29. Walker JM, Garcet S, Aleman JO, et al. Obesity and ethnicity alter gene expression in skin. Sci Rep. 2020;10:14079. doi: 10.1038/s41598-020-70244-2.
30. Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43. doi: 10.1016/j.det.2015.08.011
31. Vossen ARJV, van Straalen KR, Swolfs EFH, et al. Nicotine dependency and readiness to quit smoking among patients with hidradenitis suppurativa. Dermatology. 2021;237:383-385. doi: 10.1159/000514028
32. Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi: 10.1111/bjd.13090
33. Clark AK, Quinonez RL, Saric S, et al. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23:13030/qt6383k0n4. doi: 10.5070/D32310036990
34. Saric-Bosanac S, Clark AK, Sivamani RK, et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26:13030/qt8949296f. doi: 10.5070/D3262047430
35. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
36. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270. doi: 10.1001/archdermatol.2010.328
37. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671. doi: 10.1111/j.1365-2230.2005.01897.x
38. Kirthi S, Hellen R, O’Connor R, et al. Hidradenitis suppurativa and Crohn’s disease: a case series. Ir Med J. 2017;110:618.
39. Dumont LM, Landman C, Sokol H, et al; CD-HS Study Group. Increased risk of permanent stoma in Crohn’s disease associated with hidradenitis suppurativa: a case-control study. Aliment Pharmacol Ther. 2020;52:303-310. doi: 10.1111/apt.15863
40. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. doi: 10.1097/MD.0000000000000187.
41. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
42. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
43. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781. doi: 10.1111/bjd.16998.
44. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221; doi: 10.1136/postgradmedj-2013-131994
45. Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170:884-889. doi: 10.1111/bjd.12787
46. Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016;107(suppl 2):27-31. doi: 10.1016/S0001-7310(17) 30006-6
47. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. doi: 10.2147/IDR.S39601
48. Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Dtsch Dermatol Ges. 2014;12:451-463. doi: 10.1111/ddg.12310
49. Hap W, Frejlich E, Rudno-Rudzińska J, et al. Pilonidal sinus: finding the righttrack for treatment. Pol Przegl Chir. 2017;89:68-75. doi: 10.5604/01.3001.0009.6009
50. Al-Hamdi KI, Saadoon AQ. Acne onglobate of the scalp. Int J Trichology. 2020;12:35-37. doi: 10.4103/ijt.ijt_117_19
51. Balestra A, Bytyci H, Guillod C, et al. A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int Med Case Rep J. 2018;11:313-318. doi: 10.2147/IMCRJ.S178561
52. Ibler KS, Kromann CB. Recurrent furunculosis – challenges and management: a review. Clin Cosmet Investig Dermatol. 2014;7:59-64. doi: 10.2147/CCID.S35302
53. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175:263-272. doi: 10.1111/bjd.14475
54. Zouboulis CC, Tzellos T, Kyrgidis A, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (I4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401-1409. doi: 10.1111/bjd.15748
55. Hidradenitis Suppurativa Clinical Resource. Hidradenitis suppurativa stages: Hurley Staging System. www.hsdiseasesource.com/hs-disease-staging. Accessed October 11, 2022.
56. Ovadja ZN, Schuit MM, van der Horst CMAM, et al. Inter- and interrater reliability of Hurley staging for hidradenitis suppurativa. Br J Dermatol. 2019;181:344-349. doi: 10.1111/bjd.17588
57. Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340-1342. doi: 10.1111/j.1524-4725.2007.33286.x
58. Napolitano M, Calzavara-Pinton PG, Zanca A, et al. Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e87. doi: 10.1111/jdv.15235
59. Bukvić Mokos Z, Miše J, Balić A, et al. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat. 2020;28:9-13.
60. Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29:371-376. doi: 10.1111/jdv.12567
61. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology. 2016;232:687-691. doi: 10.1159/000453355
62 Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978. doi: 10.1111/bjd.14418
63. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi: 10.1016/j.jaad.2019.02.068
64. Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343-351. doi: 10.1007/s11154-016-9328-5
65. Vena GA, Cassano N. Drug focus: adalimumab in the treatment of moderate to severe psoriasis. Biologics. 2007;1:93-103.
66. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-55. doi: 10.7326/0003-4819-157-12-201212180-00004
67. Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi: 10.1016/j.jaad.2018.05.040
68. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi: 10.1016/s0190-9622(98)70272-5
69. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi: 10.1159/000228334
70. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. doi: 10.1016/j.jaad.2009.06.050
71. Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi: 10.1111/jdv.12376
72. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. doi: 10.1016/j.jaad.2009.07.048
73. Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. doi: 10.1111/j.1524-4725.2009.01214.x
74. Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. doi: 10.1111/j.1524-4725.2009.01427.x
75. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi: 10.1016/j.jaad.2009.12.018
76. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi: 10.1056/NEJMoa1504370. PMID: 27518661.
77. Adams DR, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501-504. doi: 10.1001/archdermatol.2010.72
78. Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48:1511-1512. doi: 10.1016/j.dld.2016.09.010
79. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi: 10.1001/jamadermatol.2015.3903.
80. Romaní J, Vilarrasa E, Martorell A, et al. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236:21-24. doi: 10.1159/000501075
81. Kane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterol Hepatol (N Y). 2011;7:544-546.
82. Davis W, Vavilin I, Malhotra N. Biologic therapy in HIV: to screen or not to screen. Cureus. 2021;13:e15941. doi: 10.7759/cureus.15941
83. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50-74. doi: 10.1177/1203475418811335
84. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328. doi: 10.1111/j.1365-4362.1983.tb02150.x
85. Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233:113-119. doi: 10.1159/000477459
86. Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19-31. doi: 10.1111/jdv.15233
87. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi: 10.1016/j.jaad.2016.06.049
88. Fajgenbaum K, Crouse L, Dong L, et al. Intralesional triamcinolone may not be beneficial for treating acute hidradenitis suppurativa lesions: a double-blind, randomized, placebo-controlled trial. Dermatol Surg. 2020;46:685-689. doi: 10.1097/DSS.0000000000002112
89. Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268. doi: 10.1111/j.1365-2133.1986.tb05740.x
90. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131. doi: 10.2310/7750.2007.00019
91. Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196. doi: 10.1111/ajd.12362
92. Khandalavala BN. A disease-modifying approach for advanced hidradenitis suppurativa (regimen with metformin, liraglutide, dapsone, and finasteride): a case report. Case Rep Dermatol. 2017;9:70-78. doi: 10.1159/000473873
93. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi: 10.1111/j.1468-3083.2012.04668.x
94. Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
95. Mota F, Machado S, Selores M. Hidradenitis suppurativa in children treated with finasteride-a case series. Pediatr Dermatol. 2017;34:578-583. doi: 10.1111/pde.13216
96. Doménech C, Matarredona J, Escribano-Stablé JC, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology. 2012;224:307-308. doi: 10.1159/000339477
97. Patel N, McKenzie SA, Harview CL, et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J Dermatolog Treat. 2021;32:473-475. doi: 10.1080/09546634.2019.1670779
98. Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-76. doi: 10.1016/s0190-9622(99) 70530-x
99. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125. doi: 10.1159/000477207
100. Prens LM, Huizinga J, Janse IC. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941-1946. doi: 10.1111/jdv.15706
101. Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168. doi: 10.1007/s003840050159
102. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70-S77. doi: 10.1016/j.jaad.2015.07.044.
103. Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444. doi: 10.1097/AJP.0b013e3181ceb80c
104. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi: 10.1016/j.jaad.2015.07.046
105. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994. doi: 10.1111/jdv.13216
106. Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434-1442. doi: 10.1111/bjd.13270
1. Bergler-Czop B, Hadasik K, Brzezińska-Wcisło L. Acne inversa: difficulties in diagnostics and therapy. Postepy Dermatol Alergol. 2015;32:296-301. doi: 10.5114/pdia.2014.44012
2. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
3. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2:9-16. doi: 10.4161/derm.2.1.12490
4. Kokolakis G, Wolk K, Schneider-Burrus S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236:421-430. doi: 10.1159/000508787
5. Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22:71-77. doi: 10.1177/1203475417736290
6. Savage KT, Gonzalez Brant E, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi: 10.1111/jdv.16213.
7. Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.26083.1
8. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965
9. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. doi: 10.12688/f1000 research.17267.1
10. Sabat R, Jemec GBE, Matusiak Ł. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1
11. Shlyankevich J, Chen AJ, Kim GE, et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144-1150. doi: 10.1016/j.jaad.2014.09.012
12. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. doi: 10.1111/j.1365-2133.2009.09198.x
13. von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537. doi: 10.1111/j.1600-0625.2009.00915.x
14. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999. doi: 10.1016/s0190-9622(96)90277-7
15. Ballard K, Shuman VL. Hidradenitis suppurativa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Updated July 15, 2022. Accessed November 28, 2022. www.ncbi.nlm.nih.gov/books/NBK534867/
16. Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi: 10.1001/jamadermatol.2017.0201
17. Phan K, Charlton O, Smith SD. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. BioMed Dermatol. 2020;4. doi: 10.1186/s41702-019-0052-0
18. Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi: 10.1038/jid.2012.255
19. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi: 10.1177/1203475420972348
20. Vaidya T, Vangipuram R, Alikhan A. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J. 2017;23:13030/qt9xc0n0z1. doi: 10.5070/D3236035391
21. Price KN, Hsiao JL, Shi VY. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatology. 2021;237:97-102. doi: 10.1159/000504911
22. Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460-467. doi: 10.1016/j.jaad.2014.04.001
23. Frew JW, Vekic DA, Wood J, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177:987-998. doi: 10.1111/bjd.15441
24. Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183:999-1010. doi: 10.1111/bjd.19556
25. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi: 10.1016/j.jaad.2019.08.090
26. Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PloS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810
27. Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2021;237:119-124. doi: 10.1159/000501611
28. Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Association between hidradenitis suppurativa and metabolic syndrome: a systematic review and meta-analysis. Actas Dermosifiliogr (Engl Ed). 2019;110:279-288. doi: 10.1016/j.ad.2018.10.020
29. Walker JM, Garcet S, Aleman JO, et al. Obesity and ethnicity alter gene expression in skin. Sci Rep. 2020;10:14079. doi: 10.1038/s41598-020-70244-2.
30. Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43. doi: 10.1016/j.det.2015.08.011
31. Vossen ARJV, van Straalen KR, Swolfs EFH, et al. Nicotine dependency and readiness to quit smoking among patients with hidradenitis suppurativa. Dermatology. 2021;237:383-385. doi: 10.1159/000514028
32. Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi: 10.1111/bjd.13090
33. Clark AK, Quinonez RL, Saric S, et al. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23:13030/qt6383k0n4. doi: 10.5070/D32310036990
34. Saric-Bosanac S, Clark AK, Sivamani RK, et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26:13030/qt8949296f. doi: 10.5070/D3262047430
35. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
36. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270. doi: 10.1001/archdermatol.2010.328
37. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671. doi: 10.1111/j.1365-2230.2005.01897.x
38. Kirthi S, Hellen R, O’Connor R, et al. Hidradenitis suppurativa and Crohn’s disease: a case series. Ir Med J. 2017;110:618.
39. Dumont LM, Landman C, Sokol H, et al; CD-HS Study Group. Increased risk of permanent stoma in Crohn’s disease associated with hidradenitis suppurativa: a case-control study. Aliment Pharmacol Ther. 2020;52:303-310. doi: 10.1111/apt.15863
40. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. doi: 10.1097/MD.0000000000000187.
41. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
42. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
43. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781. doi: 10.1111/bjd.16998.
44. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221; doi: 10.1136/postgradmedj-2013-131994
45. Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170:884-889. doi: 10.1111/bjd.12787
46. Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016;107(suppl 2):27-31. doi: 10.1016/S0001-7310(17) 30006-6
47. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. doi: 10.2147/IDR.S39601
48. Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Dtsch Dermatol Ges. 2014;12:451-463. doi: 10.1111/ddg.12310
49. Hap W, Frejlich E, Rudno-Rudzińska J, et al. Pilonidal sinus: finding the righttrack for treatment. Pol Przegl Chir. 2017;89:68-75. doi: 10.5604/01.3001.0009.6009
50. Al-Hamdi KI, Saadoon AQ. Acne onglobate of the scalp. Int J Trichology. 2020;12:35-37. doi: 10.4103/ijt.ijt_117_19
51. Balestra A, Bytyci H, Guillod C, et al. A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int Med Case Rep J. 2018;11:313-318. doi: 10.2147/IMCRJ.S178561
52. Ibler KS, Kromann CB. Recurrent furunculosis – challenges and management: a review. Clin Cosmet Investig Dermatol. 2014;7:59-64. doi: 10.2147/CCID.S35302
53. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175:263-272. doi: 10.1111/bjd.14475
54. Zouboulis CC, Tzellos T, Kyrgidis A, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (I4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401-1409. doi: 10.1111/bjd.15748
55. Hidradenitis Suppurativa Clinical Resource. Hidradenitis suppurativa stages: Hurley Staging System. www.hsdiseasesource.com/hs-disease-staging. Accessed October 11, 2022.
56. Ovadja ZN, Schuit MM, van der Horst CMAM, et al. Inter- and interrater reliability of Hurley staging for hidradenitis suppurativa. Br J Dermatol. 2019;181:344-349. doi: 10.1111/bjd.17588
57. Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340-1342. doi: 10.1111/j.1524-4725.2007.33286.x
58. Napolitano M, Calzavara-Pinton PG, Zanca A, et al. Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e87. doi: 10.1111/jdv.15235
59. Bukvić Mokos Z, Miše J, Balić A, et al. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat. 2020;28:9-13.
60. Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29:371-376. doi: 10.1111/jdv.12567
61. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology. 2016;232:687-691. doi: 10.1159/000453355
62 Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978. doi: 10.1111/bjd.14418
63. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi: 10.1016/j.jaad.2019.02.068
64. Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343-351. doi: 10.1007/s11154-016-9328-5
65. Vena GA, Cassano N. Drug focus: adalimumab in the treatment of moderate to severe psoriasis. Biologics. 2007;1:93-103.
66. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-55. doi: 10.7326/0003-4819-157-12-201212180-00004
67. Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi: 10.1016/j.jaad.2018.05.040
68. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi: 10.1016/s0190-9622(98)70272-5
69. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi: 10.1159/000228334
70. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. doi: 10.1016/j.jaad.2009.06.050
71. Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi: 10.1111/jdv.12376
72. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. doi: 10.1016/j.jaad.2009.07.048
73. Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. doi: 10.1111/j.1524-4725.2009.01214.x
74. Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. doi: 10.1111/j.1524-4725.2009.01427.x
75. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi: 10.1016/j.jaad.2009.12.018
76. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi: 10.1056/NEJMoa1504370. PMID: 27518661.
77. Adams DR, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501-504. doi: 10.1001/archdermatol.2010.72
78. Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48:1511-1512. doi: 10.1016/j.dld.2016.09.010
79. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi: 10.1001/jamadermatol.2015.3903.
80. Romaní J, Vilarrasa E, Martorell A, et al. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236:21-24. doi: 10.1159/000501075
81. Kane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterol Hepatol (N Y). 2011;7:544-546.
82. Davis W, Vavilin I, Malhotra N. Biologic therapy in HIV: to screen or not to screen. Cureus. 2021;13:e15941. doi: 10.7759/cureus.15941
83. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50-74. doi: 10.1177/1203475418811335
84. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328. doi: 10.1111/j.1365-4362.1983.tb02150.x
85. Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233:113-119. doi: 10.1159/000477459
86. Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19-31. doi: 10.1111/jdv.15233
87. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi: 10.1016/j.jaad.2016.06.049
88. Fajgenbaum K, Crouse L, Dong L, et al. Intralesional triamcinolone may not be beneficial for treating acute hidradenitis suppurativa lesions: a double-blind, randomized, placebo-controlled trial. Dermatol Surg. 2020;46:685-689. doi: 10.1097/DSS.0000000000002112
89. Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268. doi: 10.1111/j.1365-2133.1986.tb05740.x
90. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131. doi: 10.2310/7750.2007.00019
91. Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196. doi: 10.1111/ajd.12362
92. Khandalavala BN. A disease-modifying approach for advanced hidradenitis suppurativa (regimen with metformin, liraglutide, dapsone, and finasteride): a case report. Case Rep Dermatol. 2017;9:70-78. doi: 10.1159/000473873
93. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi: 10.1111/j.1468-3083.2012.04668.x
94. Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
95. Mota F, Machado S, Selores M. Hidradenitis suppurativa in children treated with finasteride-a case series. Pediatr Dermatol. 2017;34:578-583. doi: 10.1111/pde.13216
96. Doménech C, Matarredona J, Escribano-Stablé JC, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology. 2012;224:307-308. doi: 10.1159/000339477
97. Patel N, McKenzie SA, Harview CL, et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J Dermatolog Treat. 2021;32:473-475. doi: 10.1080/09546634.2019.1670779
98. Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-76. doi: 10.1016/s0190-9622(99) 70530-x
99. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125. doi: 10.1159/000477207
100. Prens LM, Huizinga J, Janse IC. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941-1946. doi: 10.1111/jdv.15706
101. Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168. doi: 10.1007/s003840050159
102. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70-S77. doi: 10.1016/j.jaad.2015.07.044.
103. Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444. doi: 10.1097/AJP.0b013e3181ceb80c
104. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi: 10.1016/j.jaad.2015.07.046
105. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994. doi: 10.1111/jdv.13216
106. Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434-1442. doi: 10.1111/bjd.13270
PRACTICE RECOMMENDATIONS
› Screen patients with hidradenitis suppurativa (HS) for depression, anxiety, history of smoking, metabolic syndrome, and type 2 diabetes. A
› Look into early surgical and dermatology referrals for patients with mild diffused, moderate, and severe disease. B
› Consider biopsy to rule out skin cancer in patients with severe and longstanding HS refractory to treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The family physician’s role in long COVID management
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
PRACTICE RECOMMENDATIONS
› Acknowledge and address the persistence of COVID-19 symptoms when meeting with patients. C
› Continue to monitor persistent, fluctuating symptoms of COVID-19 well after hospital discharge or apparent resolution of initial symptoms. C
› Provide psychological support and resources for mental health care to patients regarding their ongoing fears and frustrations with persistent COVID-19 symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Asthma management: How the guidelines compare
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.
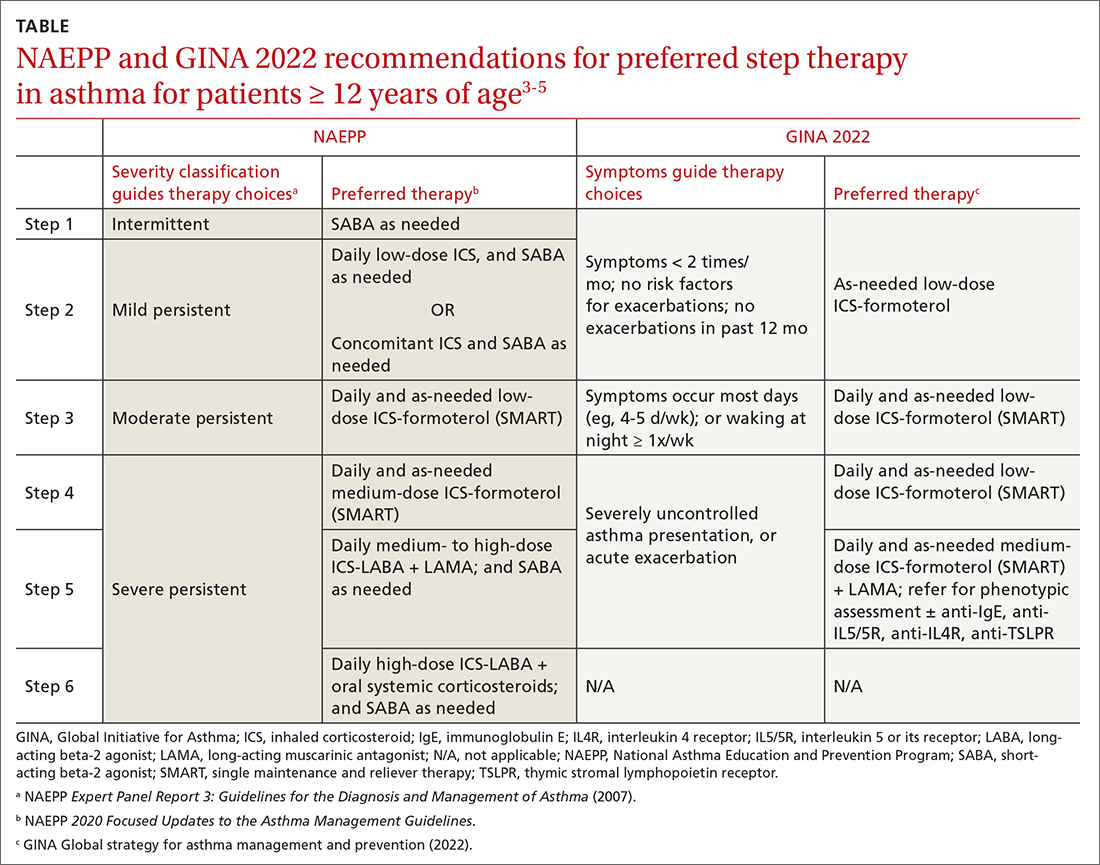
A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; [email protected]
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; [email protected]
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; [email protected]
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
PRACTICE RECOMMENDATIONS
› Consider early initiation of intermittent inhaled corticosteroid (ICS)- formoterol over a short-acting beta-2 agonist for reliever therapy. A
› Start prescribing single maintenance and reliever therapy (SMART) with ICS-formoterol to reduce exacerbation rates and simplify application. A
› Consider FeNO assessment when the diagnosis of asthma remains unclear despite history and spirometry findings. B
› Consider adding a longacting antimuscarinic agent to a medium- or high-dose ICS-LABA (long-acting beta-2 agonist) combination in uncontrolled asthma. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Make room for continuous glucose monitoring in type 2 diabetes management
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.
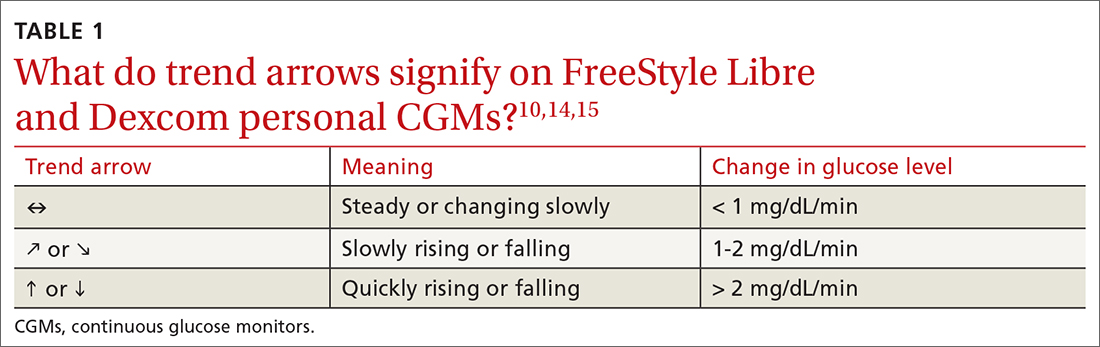
For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17
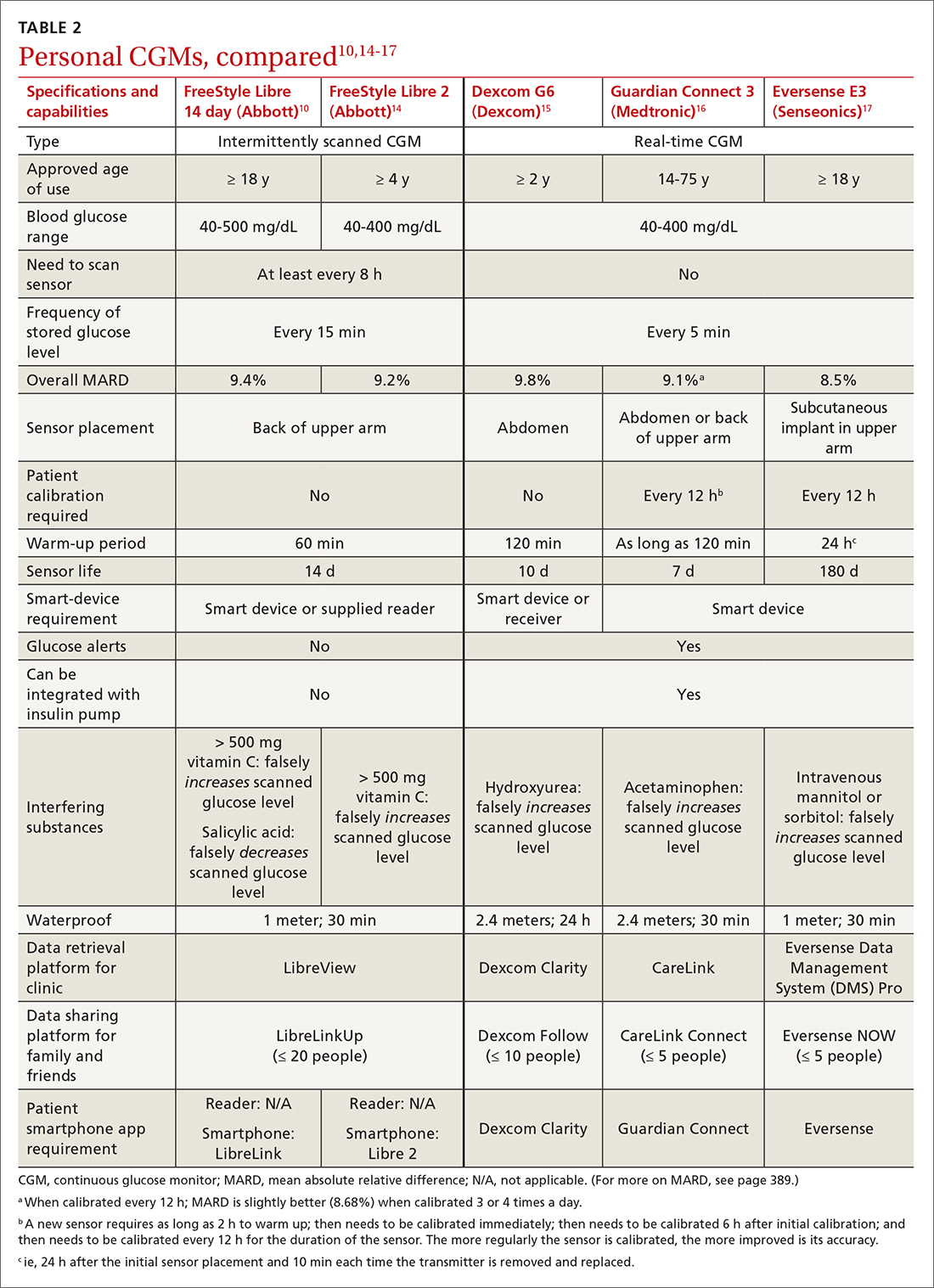
The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32
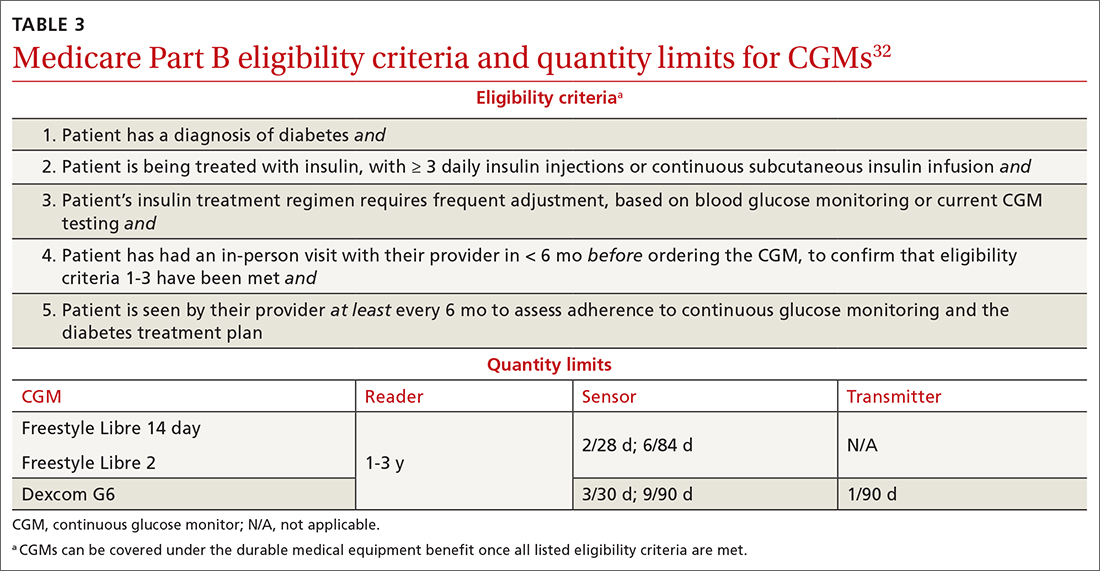
Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
PRACTICE RECOMMENDATIONS
› Initiate continuous glucose monitoring early in the disease process, based on a patient’s needs or preferences. C
› Interpret a continuous glucose monitor (CGM) report with the understanding that time within target range is the most important factor to evaluate. Minimizing or eliminating time below range is of paramount importance. B
› Advise patients who use a CGM to continue to have access to a glucometer and instruct them on appropriate times when such confirmation might be necessary. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Lung cancer screening: New evidence, updated guidance
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.
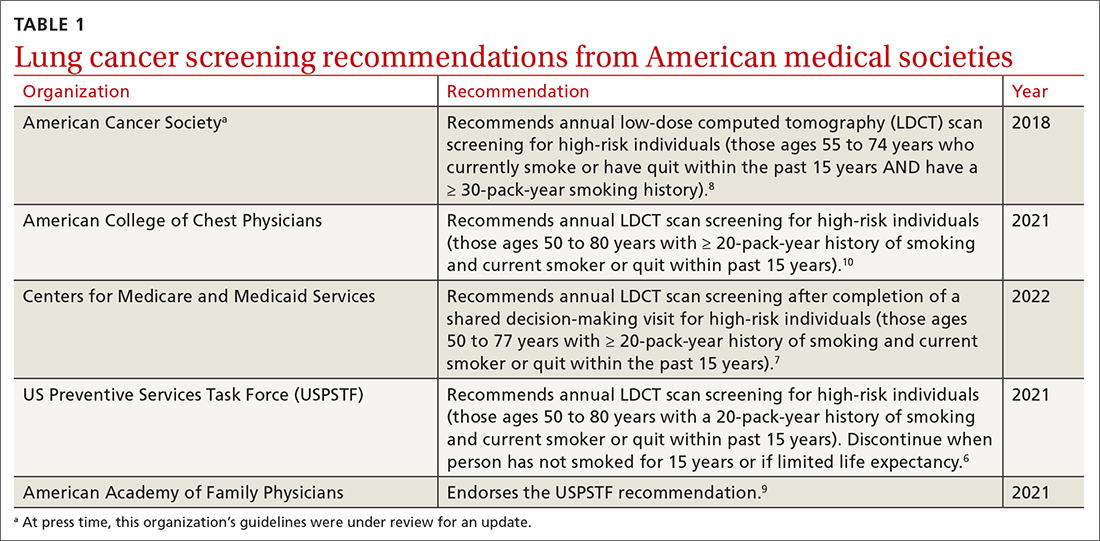
The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSONtrials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.
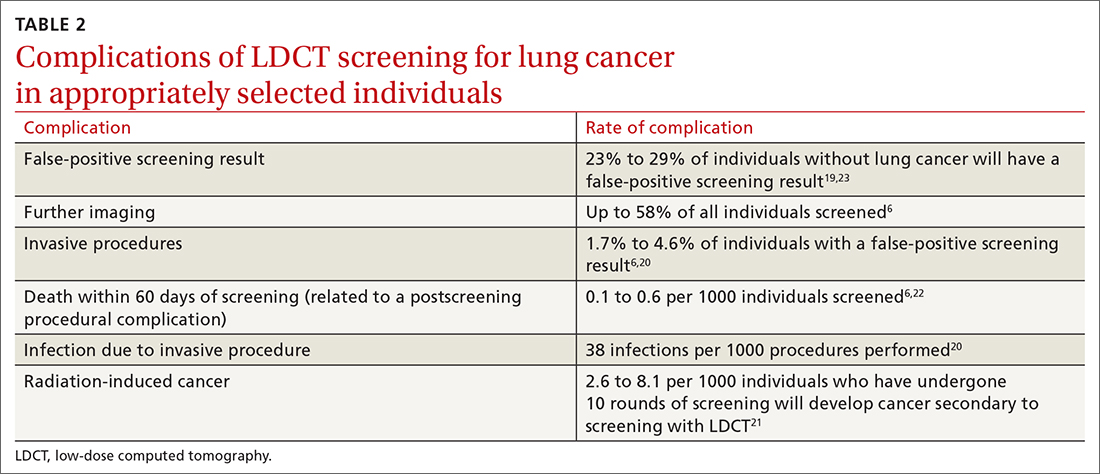
The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSONtrials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSONtrials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
PRACTICE RECOMMENDATIONS
› Recommend annual lung cancer screening for all highrisk adults ages 50 to 80 years using low-dose computed tomography. A
› Do not pursue lung cancer screening in patients who quit smoking ≥ 15 years ago, have a health problem that limits their life expectancy, or are unwilling to undergo lung surgery. A
› Recommend varenicline as first-line pharmacotherapy for smokers who would like to quit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
COVID-19 vaccine insights: The news beyond the headlines
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24
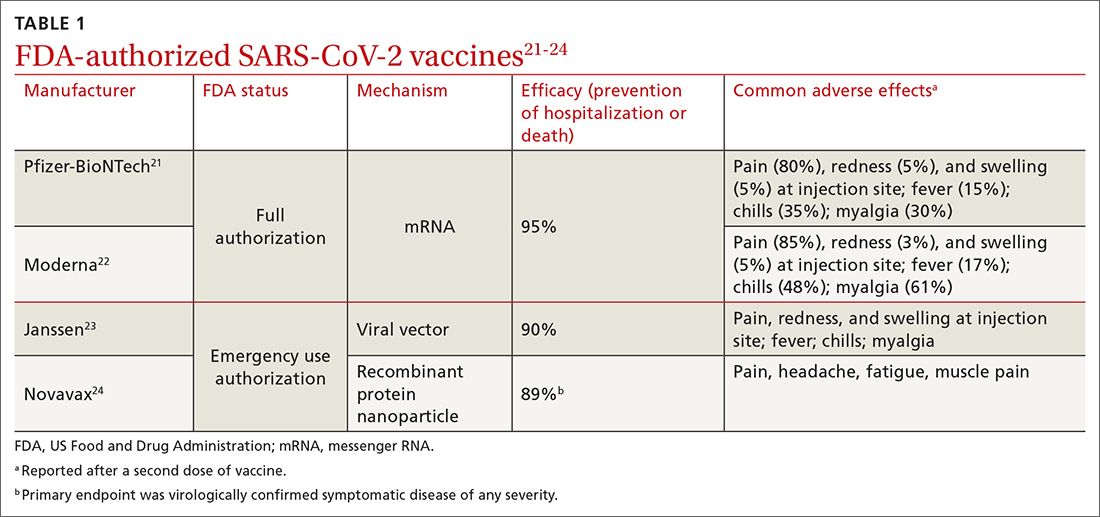
Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).
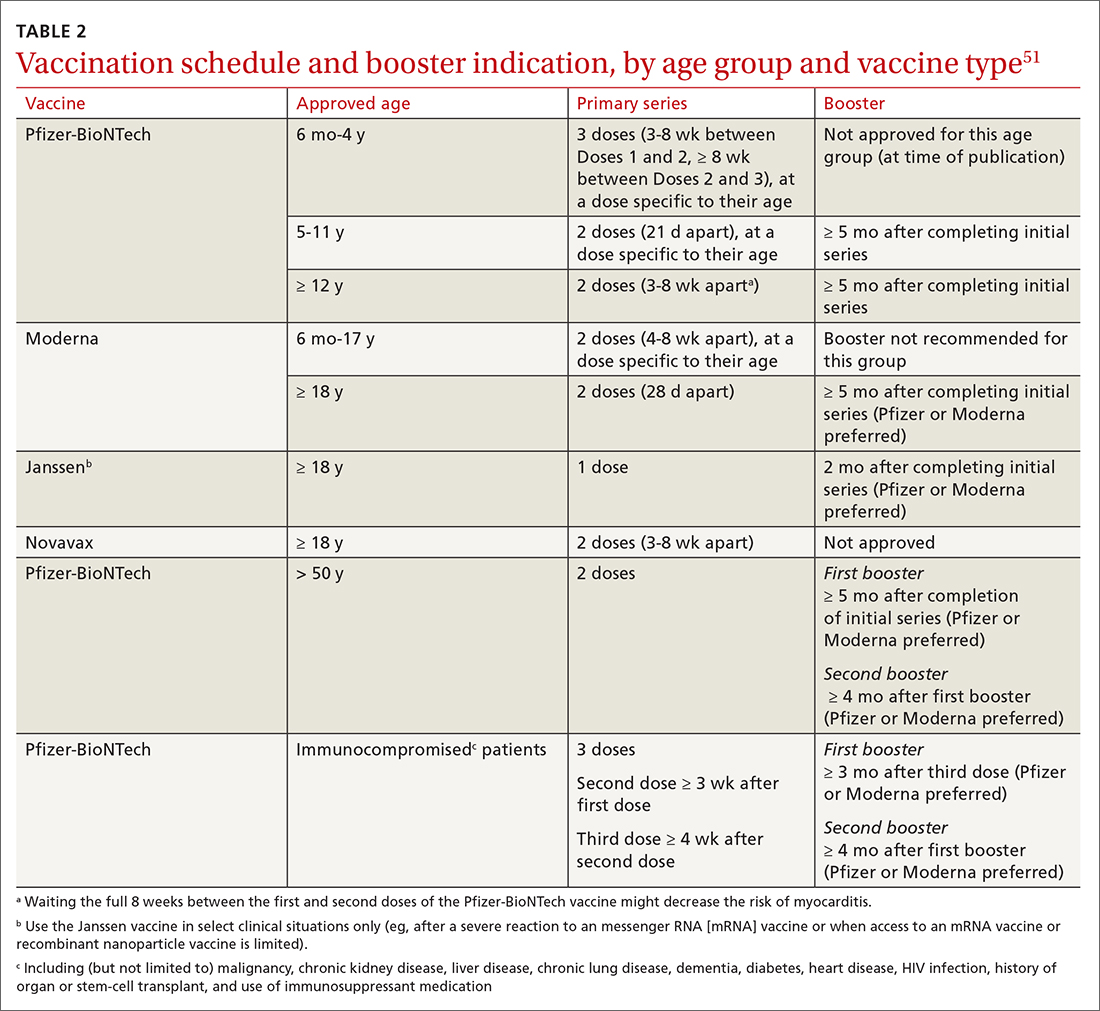
Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
awww.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
awww.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
awww.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
PRACTICE RECOMMENDATIONS
› Vaccinate all adults (≥ 18 years) against COVID-19, based on recommendations for the initial series and boosters. A
› Vaccinate patients against COVID-19 with evidence-based assurance that doing so reduces disease-related risk of hospitalization, myocardial infarction, stroke, need for mechanical ventilation, and death. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series