User login
Juvenile idiopathic arthritis: Old disease, new tactics
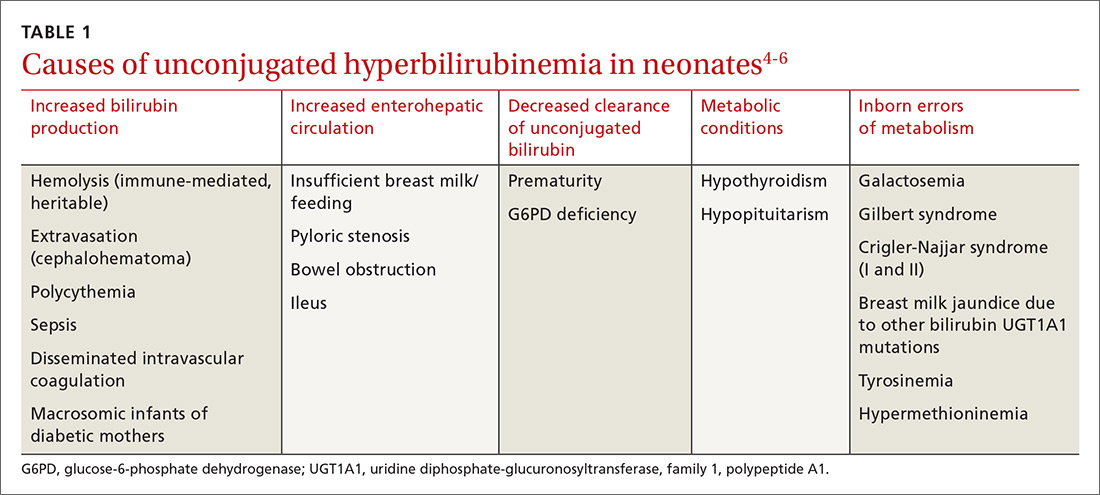
Juvenile idiopathic arthritis (JIA) is a clinically heterogeneous group of arthritides that are characterized by onset before 16 years of age and defined in part as lasting ≥6 weeks.1 Significantly, the etiology of JIA is unknown, making it a diagnosis of exclusion.2
The most common autoimmune condition of childhood, JIA has a prevalence of 3.8 to 400 affected children for every 100,000 people.3,4 As the leading cause of musculoskeletal disability in children,5 and comprising 7 categories of disease, JIA must be managed with appropriate initial and ongoing intervention.
The amalgam of care that a JIA patient requires—medical, social, physical, psychological—calls for a primary care physician’s expert ability to collaborate and coordinate with medical specialists and subspecialists, including rheumatology, ophthalmology, social work, physical and occupational therapy, and psychology. The goal? As this article describes, the goal is to provide prompt diagnosis, suitable and effective intervention, and continuity of care. (JIA is a lifelong disease, in many cases.)
How JIA is classifiedfor diagnosis and treatment
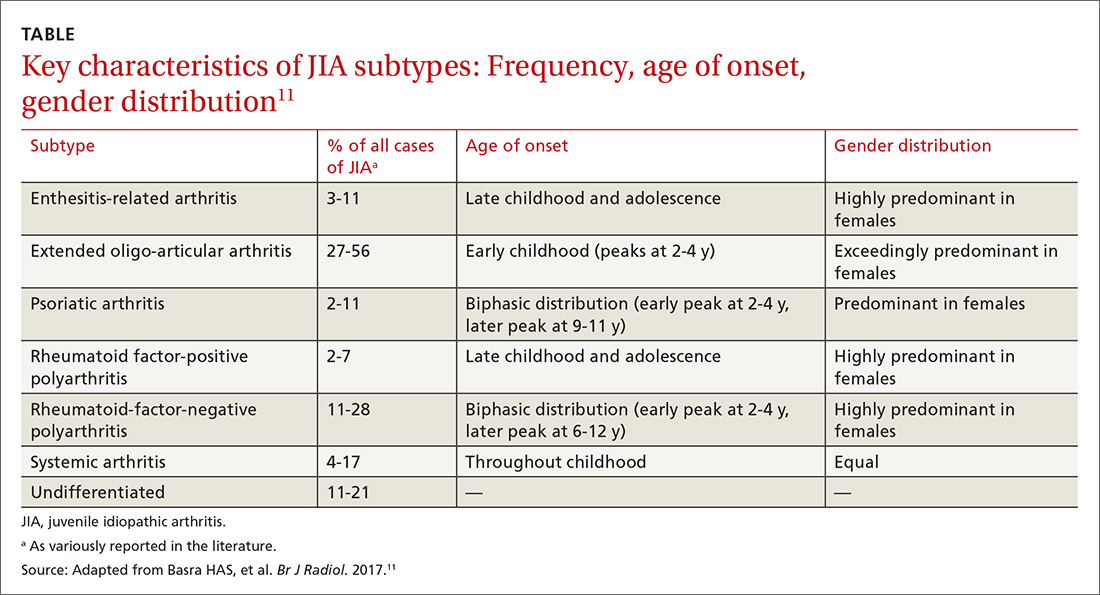
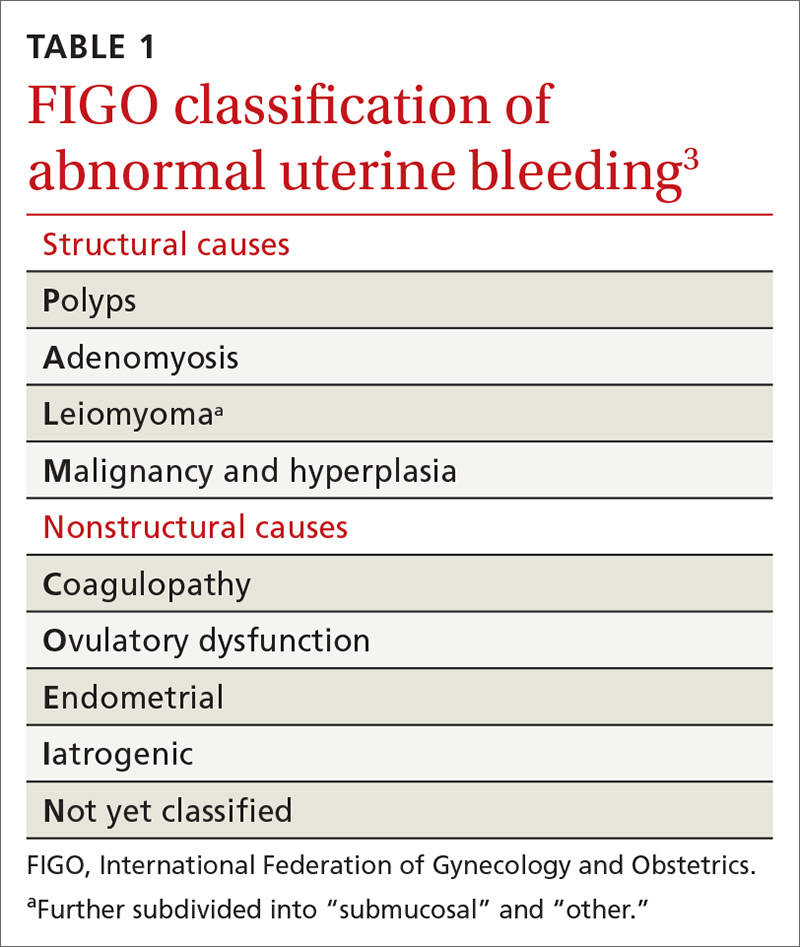
JIA comprises 7 categories, or classes.6 The scheme devised by the International League of Associations for Rheumatology (ILAR), now widely accepted, classifies JIA on the basis of clinical and biochemical markers that aid detection and treatment of the disorder, as well as research. (See “How efforts to classify JIA have caused confusion.”7-10) The ILAR classes (TABLE11) are:
- enthesitis-related arthritis (ERA)
- extended oligo-articular JIA (eoJIA), which involves ≤4 joints
- juvenile psoriatic arthritis (jPsA)
- rheumatoid factor (RF)-positive polyarticular JIA (RF+ pJIA)
- RF-negative polyarticular JIA (RF– pJIA)
- systemic-onset JIA (sJIA)
- undifferentiated JIA, which, generally, involves ≥4 joints.
SIDEBAR
How efforts to classiy JIA have caused confusion7-10
Various classifications of juvenile arthritis have been proposed and used over the past 3 decades. First was the American College of Rheumatology’s 1972 criteria for juvenile rheumatoid arthritis7; next came the European League against Rheumatism (EULAR) criteria for juvenile chronic arthritis, developed in 1977.8 Being contemporaneous, the 2 classifications led to a complicated, dichotomous definition of JIA among clinicians and researchers.
As a result of this disarray, the 1997 Durban, South Africa, meeting of the Pediatric Standing Committee of the International League of Associations for Rheumatology (ILAR)9 proposed that juvenile idiopathic arthritis be adopted as the umbrella term for the misunderstood terms juvenile rheumatoid arthritis and juvenile chronic arthritis. The intent of including “idiopathic” in the term was to acknowledge that the cause of these diseases was (and is still) unknown.
The novel classification proposed by the Pediatric Standing Committee was followed, in 2001, by an ILAR task force meeting in Edmonton, Alberta, Canada, on the classification of childhood arthritis. The outcome was a recommendation to add exclusion and inclusion criteria, to make all classes of JIA mutually exclusive.10 Most recently, as discussed in the body of this article, updated ILAR guidelines on JIA classification emphasize 1) heterogeneity among the 7 disease subtypes and 2) the fact that overlapping and exclusive features exist from class to class.
Updated guidelines regarding the 7 ILAR classes of JIA emphasize heterogeneity among disease subtypes, with overlapping and exclusive features noted from class to class.11
Extended oligo-articular JIA (27%-56%), pJIA (13%-35%), sJIA (4%-17%), and ERA,(3%-11%) are the most common JIA subtypes,12 with age of onset and sex predilection differing according to JIA class.11 The disease occurs more often in girls than in boys,11 and the predisposition is higher among Whites and Asians. The incidence of JIA (all classes taken together, for every 100,000 people) is: in Japan, 10 to 15 cases13; in Turkey, 64 cases14; in Norway, 65 cases15; and in the United States and Canada, taken together, 10 to 15 cases.16
What causes JIA?
The etiology of JIA remains unclear. It is known that the disease involves inflammation of the synovium and destruction of hard and soft tissues in joints.17 It has been postulated, therefore, that a combination of genetic, environmental, and immunogenic mechanisms might be responsible for JIA.
Continue to: For example, there is an increased...
For example, there is an increased frequency of autoimmune diseases among JIA patients.18 There are also reports documenting an increased rate of infection, including with enteric pathogens, parvovirus B,19 rubella, mumps, hepatitis B, Epstein-Barr virus, mycoplasma, and chlamydia.19 Stress and trauma have also been implicated.12
The T-lymphocyte percentage is increased in the synovial fluid of JIA patients, although that percentage varies from subtype to subtype.20 This elevation results in an increase in the number of macrophages, which are induced by secreted cytokines to produce interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-a). This activity of cellular immunity leads to joint destruction.21
Clinical features
The most common signs and symptoms of JIA are arthralgias (39%), arthritis (25%), fever (18%), limping (9%), rash (8%), abdominal pain (1.3%), and uveitis (1.3%).15 Forty percent of JIA patients are reported to have temporomandibular joint involvement at some point in their life; mandibular asymmetry secondary to condylar resorption and remodeling17 is the most common presenting complaint—not arthralgia or pain, as would be expected.
Most JIA patients (52%) first present to the emergency department; another 42% present to the office of a general medical practitioner.15 On average, 3 visits to a physician, over the course of approximately 3 months, are made before a definitive diagnosis (usually by a pediatric rheumatologist) is made.15
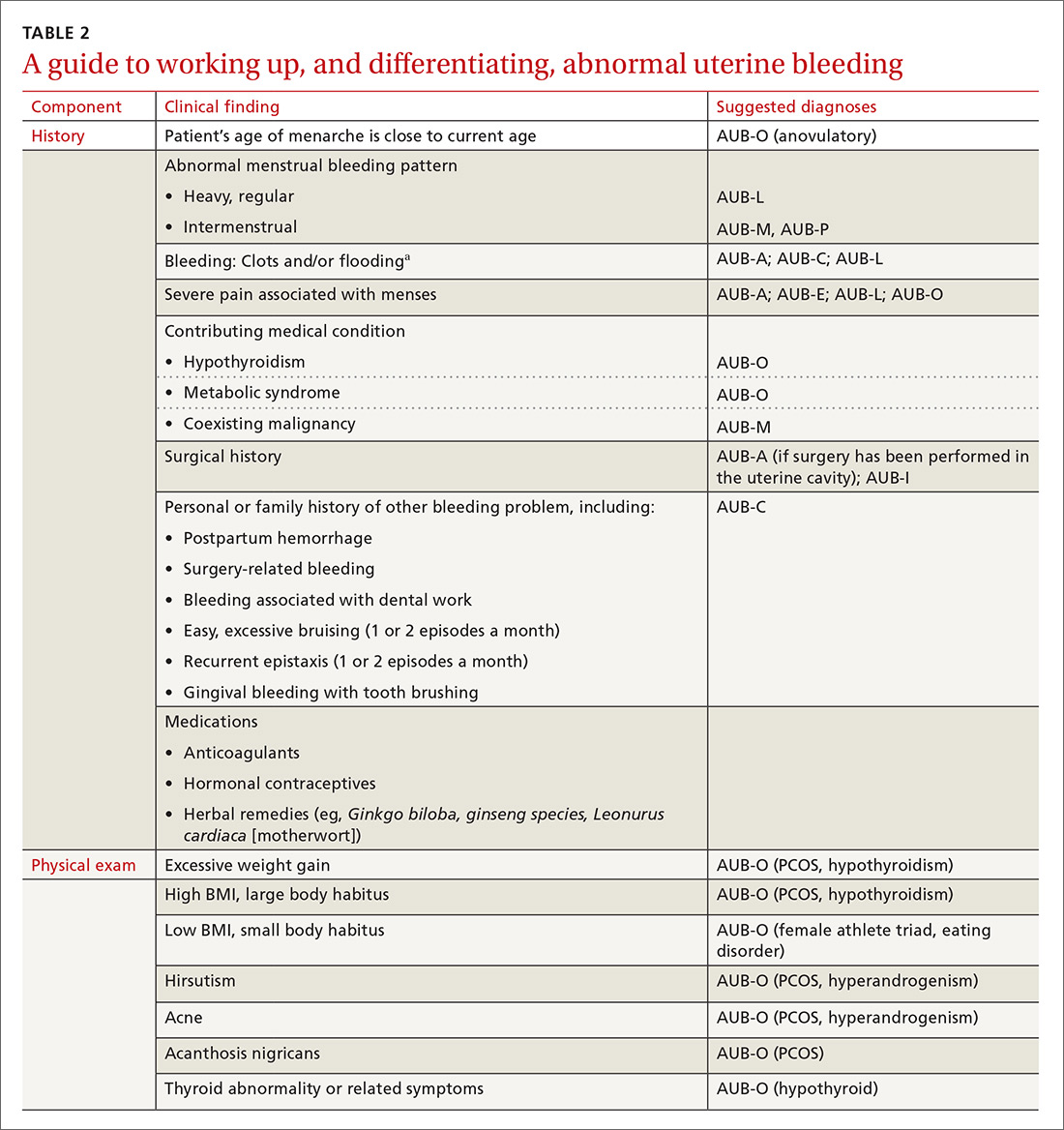
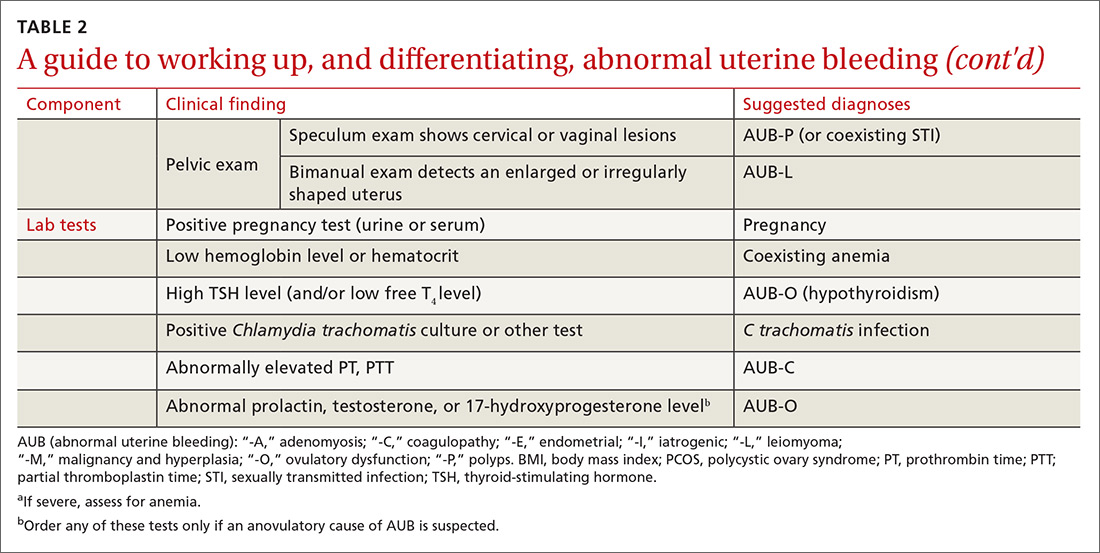
Pertinent questions to ask a patient who has a confirmed diagnosis of JIA include the nature, severity, and duration of morning stiffness and pain, as well as any encumbering factors to regular functioning at home or school.22 Different scoring charts can be used to determine the extent of pain and disability, including the Juvenile Arthritis Disease Activity Score (JADAS)23 and the clinical JADAS (cJADAS),24 which measure minimal disease activity25 and clinically inactive disease26 cutoffs.
Continue to: Macrophage-activating syndrome increases risk of morbidity, mortality
Macrophage-activating syndrome increases risk of morbidity, mortality
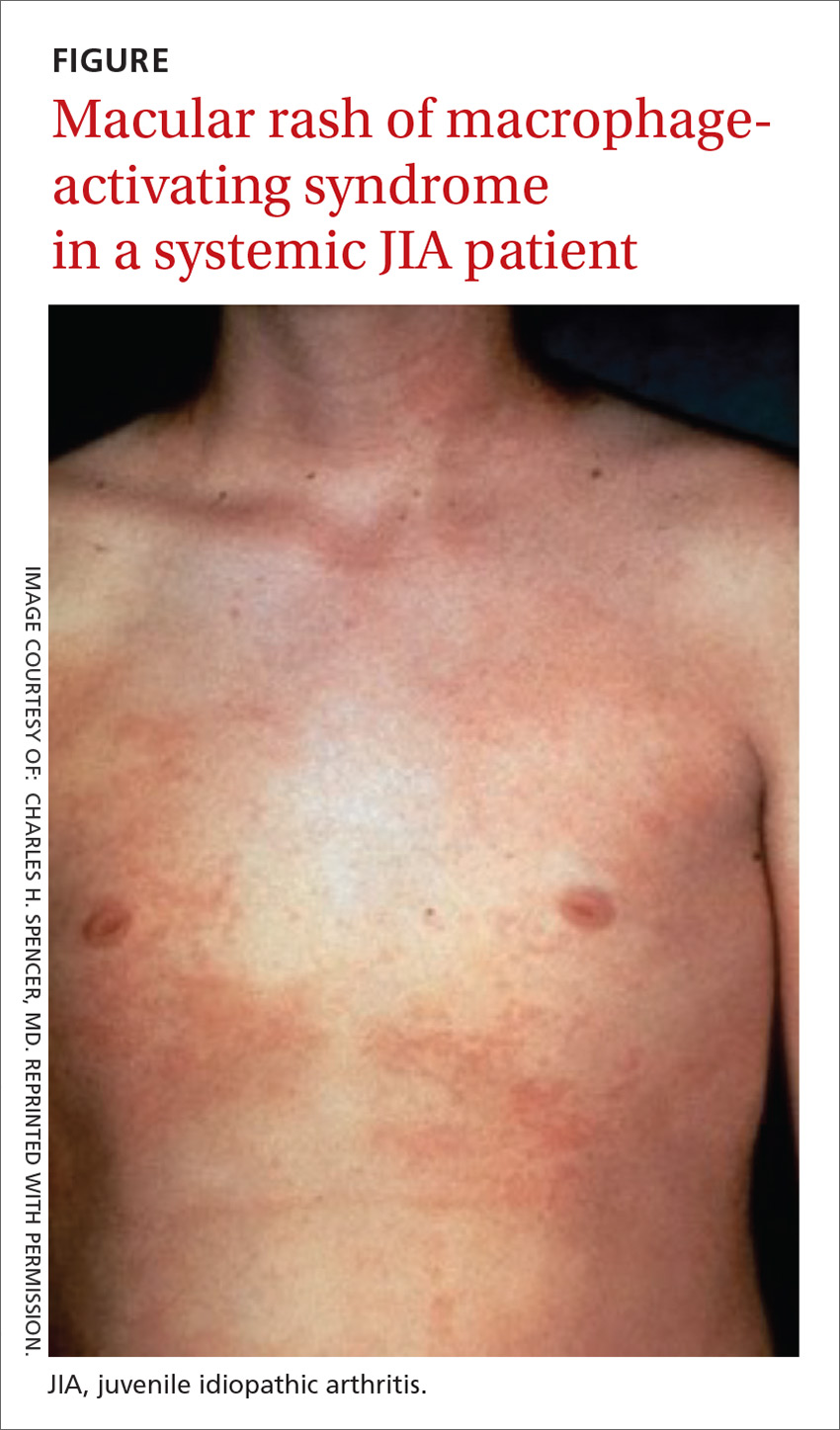
An overactivation and expansion of T lymphocytes and macrophagic histiocytes with hemophagocytic activity, macrophage-activating syndrome (MAS) occurs in approximately 10% of JIA patients,27 increasing their risk of morbidity and mortality. The syndrome, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly, and occasionally, multisystem organ failure, is seen in 30% to 40% of sJIA patients; approximately 11% of them experience sudden death as a consequence.28
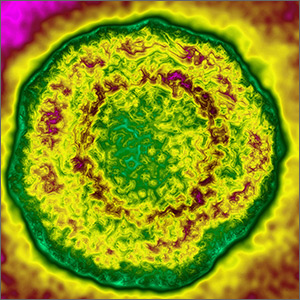
The clinical setting of MAS includes presenting symptoms of fever and a salmon-pink macular rash (FIGURE). For many sJIA patients with MAS, the diagnosis is made when laboratory results show hyperferritinemia, thrombocytopenia, anemia, leukopenia, coagulopathy, and elevated levels of C-reactive protein and D-dimer.27
Different classes, different features
The following clinical profiles have been documented in different classes of JIA:
Systemic JIA presents with intermittent fever of at least 2 weeks’ duration, arthritis, and occasionally, a rash.
Extended oligo-articular JIA involves pain, in a mono-articular lower-extremity joint, that can develop suddenly or insidiously, and is characterized by early-morning stiffness and uveitis (especially in early-onset, antinuclear antibody-positive JIA patients).
Continue to: Poly-articular JIA
Poly-articular JIA patients present with mild fever, weight loss, and anemia.
Enthesis-related arthritis patients have findings of enthesopathy; asymmetric arthritis of the lower extremities, particularly the Achilles tendon29; and recurrent acute, symptomatic iridocyclitis.30
Juvenile psoriatic arthritis can involve any joint but is readily differentiated from pJIA by involvement of distal interphalangeal joints and psoriatic skin and nail changes.29
Investigations
Imaging
Radiography is still the most widely used imaging tool for making the diagnosis of JIA. Plain films demonstrate structural joint damage and disturbances of growth and maturation in bones. Radiography has poor sensitivity for detecting acute synovitis and limited utility in visualizing erosion changes early in the course of disease, however, which has led to increased use of ultrasonography (US) and contrast-enhanced magnetic resonance imaging (MRI) to diagnose JIA.30
Contrast-enhanced MRI is superior to US for detecting early inflammation and monitoring subsequent joint disease. Of course, MRI is more expensive than US, and less widely available. Other imaging options are computed tomography and positron emission tomography, but these scans are not as sensitive as contrast-enhanced MRI and have the disadvantage of radiation exposure (in the former) and cost (in the latter).
Continue to: Laboratory testing
Laboratory testing
No diagnostic tests for JIA exist. Assays of acute-phase reactants, including C-reactive protein, the erythrocyte sedimentation rate, and serum amyloid-A proteins, can be utilized to demonstrate inflammation but not to confirm the diagnosis. For some classes of JIA, various tests, including rheumatoid factor, antinuclear antibody, human leukocyte antigen B-27, and cyclic citrullated peptide antibodies, can be used to confirm a specific class but, again, are not recommended for confirming JIA.6
The complete blood count, blood cultures, and tests of uric acid and lactate dehydrogenase can be ordered during treatment to monitor for complications, such as malignancy, infection, MAS, and sepsis.
Treatment is based on disease class
Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular steroids are used in all JIA classes, as an adjunct to class-specific treatment, or as induction agents.31 These therapies, although they alleviate acute signs and symptoms, such as pain, inflammation, swelling and joint contractures, are not useful for long-term treatment of JIA because they do not halt disease progression.
Systemic steroids can be utilized in exceptional cases, including chronic uveitis with arthritis or in patients with destructive arthritis and poor prognostic features, including cyclic citrullated peptide antibodies, positive RF, erosions, and joint-space narrowing.32
Other drugs. Options include traditional disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate and leflunomide; biologic agents, such as TNF-a inhibitors (eg, etanercept, adalimumab, and infliximab); and anti-IL monoclonal antibody drugs (eg, the IL-6 inhibitor tocilizumab and IL-1 inhibitors anakinra, and canakinumab).31 Indications by class include:
- csDMARDs as first-line therapy in persistent eoJIA and pJIA;
- TNF-Symbolα inhibitors for refractory eoJIA and for pJIA episodes31;
- tocilizumab, recommended for sJIA patients who have persistent systemic signs; and
- anakinra and canakinumab for refractory SJIA patients.32
Continue to: Failure
Failure
When treatment of JIA fails with a given drug, options include increasing the dosage; switching to another agent in the same drug class; switching to a different class; and combining an NSAID with a csDMARD or a biologic agent.32 In class-specific JIA cases, a change in a drug regimen is warranted on the basis of the evidence-based historical clinical response rate.32
What is the prognosis?
Treatment of JIA with novel agents, such as biologics, has opened up the possibility that JIA patients can live not just with suppressed symptoms but immunologically inactive disease. This is the result of better understanding of the pathogenesis of JIA and the mechanism of action of targeted drugs, and identification of biomarkers that are helpful in predicting prognosis, adverse effects, and response to treatment.
JIA is often a lifelong disease; one-third of patients continue to exhibit symptoms into adulthood.4 If their disease is properly managed, however, these patients do not develop typical features of rheumatoid arthritis, including hand, limb, and spine deformities. Last, patients with JIA who have only intermittent disease tend to do better over the long term than those whose disease is continual.32
The mortality rate of JIA has dropped: from 1% to 4% in the mid-1970s to 0.3% to 1% today4—an improvement in life expectancy that is echoed in enhanced quality of life for patients. According to the 4-level Steinbrocker functional classification scale33 (used to rate the extent of physical disability), 15% of JIA patients were Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care) in the period from 1976 to 1994—a percentage that had declined to 5% by 2002.34
The family physician plays pivotal role in JIA care
For the family physician, appropriate initial intervention in the management of JIA is imperative. This includes ordering imaging (whether plain films or MRI), laboratory tests as described earlier (although not to make the diagnosis), and the use of NSAIDs, intra-articular steroids, and other induction agents. Once the diagnosis is made, and a drug regimen is put in place, you will need to monitor for adverse effects. This monitoring will need to occur when a patient is escalated to csDMARDs, biological agents, or systemic steroids; is maintained on an NSAID; or is placed on a combination regimen.
Continue to: Before beginning therapy with a biologic agent...
Before beginning therapy with a biologic agent, it’s important to screen for hepatitis B, hepatitis C, human immunodeficiency virus infection, tuberculosis, and fungal infection (eg, Histoplasma capsulatum, Coccidioides immitis32). Be sure to make a timely referral to the ophthalmology service for a bi-annual eye exam and, in the event that surgery is necessary, conduct a preoperative evaluation, with the knowledge of how long before surgery a biologic agent must be withheld (duration varies by drug).32
CORRESPONDENCE
Tobe Momah, MD, Department of Family Medicine, Clinical Science Building, 4th Floor, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Adriano LS, de França Fonteles MM, de Fátima Menezes Azevedo M, et al. Medication adherence in patients with juvenile idiopathic arthritis. Rev Bras Reumatol Engl Ed. 2017;57:23-29.
2. Akioka S. A better understanding of juvenile idiopathic arthritis with classification criteria. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:513-521.
3. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112-117.
4. Petty RE, Laxer RM, Lindsley CB, et al. Pediatric Rheumatology. Philadelphia, PA: Elsevier; 2016:188-201.e6.
5. Scott C, Brice N. Juvenile idiopathic arthritis–an update on its diagnosis and management. S Afr Med J. 2015;105:1077.
6. Giancane G, Consolaro A, Lanni S, et al. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3:187-207.
7. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1972;23:712-719.
8. Wood PHN: Special meeting on nomenclature and classification of arthritis in children. In: Munthe E, ed. The Care of Rheumatic Children. Basel, Switzerland: EULAR Publishers; 1978:47-50.
9. Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-1994.
10. Petty RE, Southwood TR, Manners P, et al; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
11. Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920.
12. Weiss J, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442, vi.
13. Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Pediatric Jpn. 1997:39:242-244.
14. Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25:2445-2449.
15. Aoust L, Rossi-Semerano L, Koné-PauL I, et al. Time to diagnosis in juvenile idiopathic arthritis: a French perspective. Orphanet J Rare Dis. 2017;12:43.
16. Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway; a ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99-101.
17. Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg. 2016;45:801-812.
18. Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851-1856.
19. Gonzalez B, Larrañaga C, León O, et al. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol. 2007;34:1336-1340.
20. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149.
21. Zhou J, Ding Y, Zhang Y, et al. CD3+CD56+ natural killer T cell activity in children with different forms of juvenile idiopathic arthritis and the influence of etanercept treatment on polyarticular subgroup. Clin Immunol. 2016;176:1-11.
22. Shoop-Worrall SJW, Verstappen SMM, Baildam E, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017;0:1-8.
23. Nordal EB, Zak M, Berntson L, et al. Juvenile Arthritis Disease Activity Score (JADAS) based on CRP; validity and predictive ability in a Nordic population-based setting. Pediatr Rheumatol Online J. 2011;9(suppl 1):155.
24. Swart JF, Dijkhuizen EHP, Wulffraat NM, et al. Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77:336-342.
25. Horneff G, Klein A, Ganser G, et al. Protocols on classification, monitoring and therapy in children’s rheumatology (PRO-KIND): results of the working group polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78.
26. Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70:1519-1529.
27. Ahn SS, Yoo BW, Jung SM, et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Sem Arthritis Rheum. 2017;.47:216-221.
28. Yokota S, Mori M, Imagawa T, et al. Proposal for juvenile idiopathic arthritis guidance on diagnosis and treatment for primary care pediatricians and nonpediatric rheumatologists (2007). Mod Rheumatol. 2007;17:353-363.
29. Barut K, Adrovic A, Şahin S, et al. Juvenile idiopathic arthritis. Balkan Med J. 2017;34:90-101.
30. Colebatch-Bourn AN, Edwards CJ, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74:1946-1957.
31. Blazina Š, Markelj G, AvramoviČ MZ, et al. Management of juvenile idiopathic arthritis: a clinical guide. Pediatr Drugs. 2016;18:397-412.
32. Santos MJ, Conde M, Mourão AF, et al. 2016 update of the Portuguese recommendations for the use of biologic therapies in children and adolescents with juvenile idiopathic arthritis. Acta Rheumatol Port. 2016;41:194-212.
33. Steinbrocker 0, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
34. Oen K, Malleson PN, Cabral D, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989-1999.
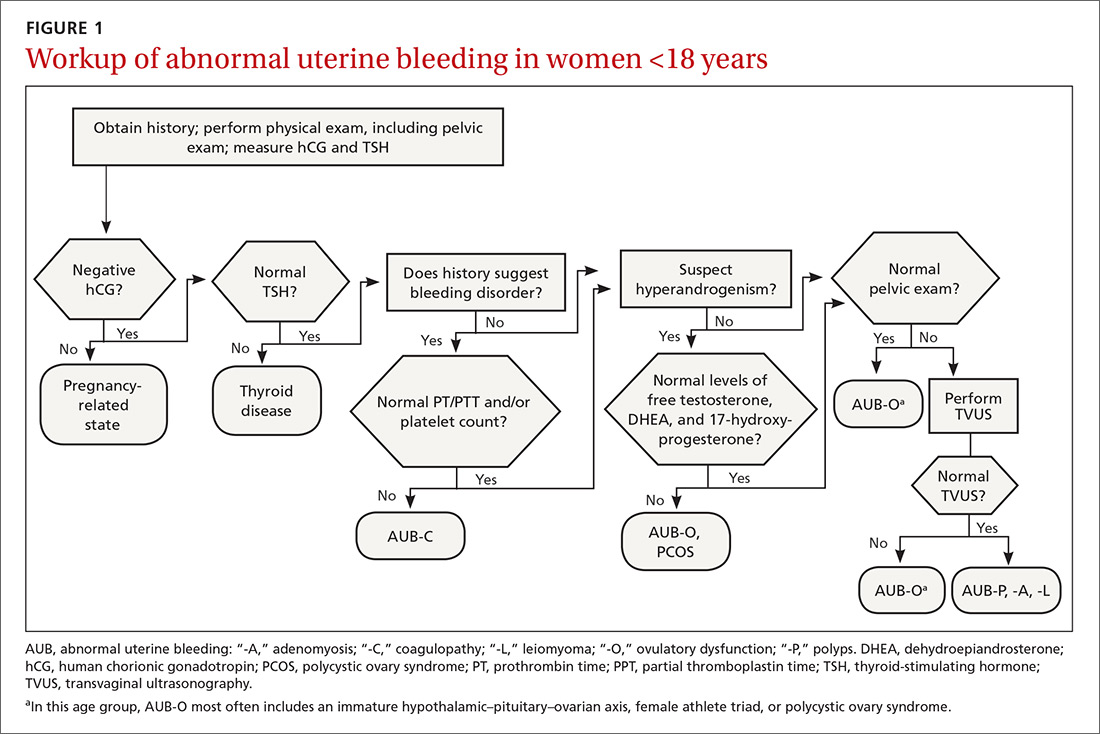
Juvenile idiopathic arthritis (JIA) is a clinically heterogeneous group of arthritides that are characterized by onset before 16 years of age and defined in part as lasting ≥6 weeks.1 Significantly, the etiology of JIA is unknown, making it a diagnosis of exclusion.2
The most common autoimmune condition of childhood, JIA has a prevalence of 3.8 to 400 affected children for every 100,000 people.3,4 As the leading cause of musculoskeletal disability in children,5 and comprising 7 categories of disease, JIA must be managed with appropriate initial and ongoing intervention.
The amalgam of care that a JIA patient requires—medical, social, physical, psychological—calls for a primary care physician’s expert ability to collaborate and coordinate with medical specialists and subspecialists, including rheumatology, ophthalmology, social work, physical and occupational therapy, and psychology. The goal? As this article describes, the goal is to provide prompt diagnosis, suitable and effective intervention, and continuity of care. (JIA is a lifelong disease, in many cases.)
How JIA is classifiedfor diagnosis and treatment
JIA comprises 7 categories, or classes.6 The scheme devised by the International League of Associations for Rheumatology (ILAR), now widely accepted, classifies JIA on the basis of clinical and biochemical markers that aid detection and treatment of the disorder, as well as research. (See “How efforts to classify JIA have caused confusion.”7-10) The ILAR classes (TABLE11) are:
- enthesitis-related arthritis (ERA)
- extended oligo-articular JIA (eoJIA), which involves ≤4 joints
- juvenile psoriatic arthritis (jPsA)
- rheumatoid factor (RF)-positive polyarticular JIA (RF+ pJIA)
- RF-negative polyarticular JIA (RF– pJIA)
- systemic-onset JIA (sJIA)
- undifferentiated JIA, which, generally, involves ≥4 joints.
SIDEBAR
How efforts to classiy JIA have caused confusion7-10
Various classifications of juvenile arthritis have been proposed and used over the past 3 decades. First was the American College of Rheumatology’s 1972 criteria for juvenile rheumatoid arthritis7; next came the European League against Rheumatism (EULAR) criteria for juvenile chronic arthritis, developed in 1977.8 Being contemporaneous, the 2 classifications led to a complicated, dichotomous definition of JIA among clinicians and researchers.
As a result of this disarray, the 1997 Durban, South Africa, meeting of the Pediatric Standing Committee of the International League of Associations for Rheumatology (ILAR)9 proposed that juvenile idiopathic arthritis be adopted as the umbrella term for the misunderstood terms juvenile rheumatoid arthritis and juvenile chronic arthritis. The intent of including “idiopathic” in the term was to acknowledge that the cause of these diseases was (and is still) unknown.
The novel classification proposed by the Pediatric Standing Committee was followed, in 2001, by an ILAR task force meeting in Edmonton, Alberta, Canada, on the classification of childhood arthritis. The outcome was a recommendation to add exclusion and inclusion criteria, to make all classes of JIA mutually exclusive.10 Most recently, as discussed in the body of this article, updated ILAR guidelines on JIA classification emphasize 1) heterogeneity among the 7 disease subtypes and 2) the fact that overlapping and exclusive features exist from class to class.
Updated guidelines regarding the 7 ILAR classes of JIA emphasize heterogeneity among disease subtypes, with overlapping and exclusive features noted from class to class.11
Extended oligo-articular JIA (27%-56%), pJIA (13%-35%), sJIA (4%-17%), and ERA,(3%-11%) are the most common JIA subtypes,12 with age of onset and sex predilection differing according to JIA class.11 The disease occurs more often in girls than in boys,11 and the predisposition is higher among Whites and Asians. The incidence of JIA (all classes taken together, for every 100,000 people) is: in Japan, 10 to 15 cases13; in Turkey, 64 cases14; in Norway, 65 cases15; and in the United States and Canada, taken together, 10 to 15 cases.16
What causes JIA?
The etiology of JIA remains unclear. It is known that the disease involves inflammation of the synovium and destruction of hard and soft tissues in joints.17 It has been postulated, therefore, that a combination of genetic, environmental, and immunogenic mechanisms might be responsible for JIA.
Continue to: For example, there is an increased...
For example, there is an increased frequency of autoimmune diseases among JIA patients.18 There are also reports documenting an increased rate of infection, including with enteric pathogens, parvovirus B,19 rubella, mumps, hepatitis B, Epstein-Barr virus, mycoplasma, and chlamydia.19 Stress and trauma have also been implicated.12
The T-lymphocyte percentage is increased in the synovial fluid of JIA patients, although that percentage varies from subtype to subtype.20 This elevation results in an increase in the number of macrophages, which are induced by secreted cytokines to produce interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-a). This activity of cellular immunity leads to joint destruction.21
Clinical features
The most common signs and symptoms of JIA are arthralgias (39%), arthritis (25%), fever (18%), limping (9%), rash (8%), abdominal pain (1.3%), and uveitis (1.3%).15 Forty percent of JIA patients are reported to have temporomandibular joint involvement at some point in their life; mandibular asymmetry secondary to condylar resorption and remodeling17 is the most common presenting complaint—not arthralgia or pain, as would be expected.
Most JIA patients (52%) first present to the emergency department; another 42% present to the office of a general medical practitioner.15 On average, 3 visits to a physician, over the course of approximately 3 months, are made before a definitive diagnosis (usually by a pediatric rheumatologist) is made.15
Pertinent questions to ask a patient who has a confirmed diagnosis of JIA include the nature, severity, and duration of morning stiffness and pain, as well as any encumbering factors to regular functioning at home or school.22 Different scoring charts can be used to determine the extent of pain and disability, including the Juvenile Arthritis Disease Activity Score (JADAS)23 and the clinical JADAS (cJADAS),24 which measure minimal disease activity25 and clinically inactive disease26 cutoffs.
Continue to: Macrophage-activating syndrome increases risk of morbidity, mortality
Macrophage-activating syndrome increases risk of morbidity, mortality
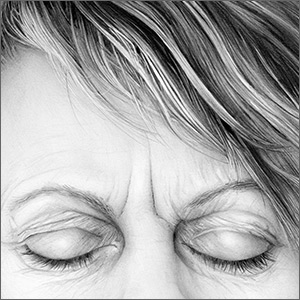
An overactivation and expansion of T lymphocytes and macrophagic histiocytes with hemophagocytic activity, macrophage-activating syndrome (MAS) occurs in approximately 10% of JIA patients,27 increasing their risk of morbidity and mortality. The syndrome, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly, and occasionally, multisystem organ failure, is seen in 30% to 40% of sJIA patients; approximately 11% of them experience sudden death as a consequence.28
The clinical setting of MAS includes presenting symptoms of fever and a salmon-pink macular rash (FIGURE). For many sJIA patients with MAS, the diagnosis is made when laboratory results show hyperferritinemia, thrombocytopenia, anemia, leukopenia, coagulopathy, and elevated levels of C-reactive protein and D-dimer.27
Different classes, different features
The following clinical profiles have been documented in different classes of JIA:
Systemic JIA presents with intermittent fever of at least 2 weeks’ duration, arthritis, and occasionally, a rash.
Extended oligo-articular JIA involves pain, in a mono-articular lower-extremity joint, that can develop suddenly or insidiously, and is characterized by early-morning stiffness and uveitis (especially in early-onset, antinuclear antibody-positive JIA patients).
Continue to: Poly-articular JIA
Poly-articular JIA patients present with mild fever, weight loss, and anemia.
Enthesis-related arthritis patients have findings of enthesopathy; asymmetric arthritis of the lower extremities, particularly the Achilles tendon29; and recurrent acute, symptomatic iridocyclitis.30
Juvenile psoriatic arthritis can involve any joint but is readily differentiated from pJIA by involvement of distal interphalangeal joints and psoriatic skin and nail changes.29
Investigations
Imaging
Radiography is still the most widely used imaging tool for making the diagnosis of JIA. Plain films demonstrate structural joint damage and disturbances of growth and maturation in bones. Radiography has poor sensitivity for detecting acute synovitis and limited utility in visualizing erosion changes early in the course of disease, however, which has led to increased use of ultrasonography (US) and contrast-enhanced magnetic resonance imaging (MRI) to diagnose JIA.30
Contrast-enhanced MRI is superior to US for detecting early inflammation and monitoring subsequent joint disease. Of course, MRI is more expensive than US, and less widely available. Other imaging options are computed tomography and positron emission tomography, but these scans are not as sensitive as contrast-enhanced MRI and have the disadvantage of radiation exposure (in the former) and cost (in the latter).
Continue to: Laboratory testing
Laboratory testing
No diagnostic tests for JIA exist. Assays of acute-phase reactants, including C-reactive protein, the erythrocyte sedimentation rate, and serum amyloid-A proteins, can be utilized to demonstrate inflammation but not to confirm the diagnosis. For some classes of JIA, various tests, including rheumatoid factor, antinuclear antibody, human leukocyte antigen B-27, and cyclic citrullated peptide antibodies, can be used to confirm a specific class but, again, are not recommended for confirming JIA.6
The complete blood count, blood cultures, and tests of uric acid and lactate dehydrogenase can be ordered during treatment to monitor for complications, such as malignancy, infection, MAS, and sepsis.
Treatment is based on disease class
Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular steroids are used in all JIA classes, as an adjunct to class-specific treatment, or as induction agents.31 These therapies, although they alleviate acute signs and symptoms, such as pain, inflammation, swelling and joint contractures, are not useful for long-term treatment of JIA because they do not halt disease progression.
Systemic steroids can be utilized in exceptional cases, including chronic uveitis with arthritis or in patients with destructive arthritis and poor prognostic features, including cyclic citrullated peptide antibodies, positive RF, erosions, and joint-space narrowing.32
Other drugs. Options include traditional disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate and leflunomide; biologic agents, such as TNF-a inhibitors (eg, etanercept, adalimumab, and infliximab); and anti-IL monoclonal antibody drugs (eg, the IL-6 inhibitor tocilizumab and IL-1 inhibitors anakinra, and canakinumab).31 Indications by class include:
- csDMARDs as first-line therapy in persistent eoJIA and pJIA;
- TNF-Symbolα inhibitors for refractory eoJIA and for pJIA episodes31;
- tocilizumab, recommended for sJIA patients who have persistent systemic signs; and
- anakinra and canakinumab for refractory SJIA patients.32
Continue to: Failure
Failure
When treatment of JIA fails with a given drug, options include increasing the dosage; switching to another agent in the same drug class; switching to a different class; and combining an NSAID with a csDMARD or a biologic agent.32 In class-specific JIA cases, a change in a drug regimen is warranted on the basis of the evidence-based historical clinical response rate.32
What is the prognosis?
Treatment of JIA with novel agents, such as biologics, has opened up the possibility that JIA patients can live not just with suppressed symptoms but immunologically inactive disease. This is the result of better understanding of the pathogenesis of JIA and the mechanism of action of targeted drugs, and identification of biomarkers that are helpful in predicting prognosis, adverse effects, and response to treatment.
JIA is often a lifelong disease; one-third of patients continue to exhibit symptoms into adulthood.4 If their disease is properly managed, however, these patients do not develop typical features of rheumatoid arthritis, including hand, limb, and spine deformities. Last, patients with JIA who have only intermittent disease tend to do better over the long term than those whose disease is continual.32
The mortality rate of JIA has dropped: from 1% to 4% in the mid-1970s to 0.3% to 1% today4—an improvement in life expectancy that is echoed in enhanced quality of life for patients. According to the 4-level Steinbrocker functional classification scale33 (used to rate the extent of physical disability), 15% of JIA patients were Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care) in the period from 1976 to 1994—a percentage that had declined to 5% by 2002.34
The family physician plays pivotal role in JIA care
For the family physician, appropriate initial intervention in the management of JIA is imperative. This includes ordering imaging (whether plain films or MRI), laboratory tests as described earlier (although not to make the diagnosis), and the use of NSAIDs, intra-articular steroids, and other induction agents. Once the diagnosis is made, and a drug regimen is put in place, you will need to monitor for adverse effects. This monitoring will need to occur when a patient is escalated to csDMARDs, biological agents, or systemic steroids; is maintained on an NSAID; or is placed on a combination regimen.
Continue to: Before beginning therapy with a biologic agent...
Before beginning therapy with a biologic agent, it’s important to screen for hepatitis B, hepatitis C, human immunodeficiency virus infection, tuberculosis, and fungal infection (eg, Histoplasma capsulatum, Coccidioides immitis32). Be sure to make a timely referral to the ophthalmology service for a bi-annual eye exam and, in the event that surgery is necessary, conduct a preoperative evaluation, with the knowledge of how long before surgery a biologic agent must be withheld (duration varies by drug).32
CORRESPONDENCE
Tobe Momah, MD, Department of Family Medicine, Clinical Science Building, 4th Floor, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
Juvenile idiopathic arthritis (JIA) is a clinically heterogeneous group of arthritides that are characterized by onset before 16 years of age and defined in part as lasting ≥6 weeks.1 Significantly, the etiology of JIA is unknown, making it a diagnosis of exclusion.2
The most common autoimmune condition of childhood, JIA has a prevalence of 3.8 to 400 affected children for every 100,000 people.3,4 As the leading cause of musculoskeletal disability in children,5 and comprising 7 categories of disease, JIA must be managed with appropriate initial and ongoing intervention.
The amalgam of care that a JIA patient requires—medical, social, physical, psychological—calls for a primary care physician’s expert ability to collaborate and coordinate with medical specialists and subspecialists, including rheumatology, ophthalmology, social work, physical and occupational therapy, and psychology. The goal? As this article describes, the goal is to provide prompt diagnosis, suitable and effective intervention, and continuity of care. (JIA is a lifelong disease, in many cases.)
How JIA is classifiedfor diagnosis and treatment
JIA comprises 7 categories, or classes.6 The scheme devised by the International League of Associations for Rheumatology (ILAR), now widely accepted, classifies JIA on the basis of clinical and biochemical markers that aid detection and treatment of the disorder, as well as research. (See “How efforts to classify JIA have caused confusion.”7-10) The ILAR classes (TABLE11) are:
- enthesitis-related arthritis (ERA)
- extended oligo-articular JIA (eoJIA), which involves ≤4 joints
- juvenile psoriatic arthritis (jPsA)
- rheumatoid factor (RF)-positive polyarticular JIA (RF+ pJIA)
- RF-negative polyarticular JIA (RF– pJIA)
- systemic-onset JIA (sJIA)
- undifferentiated JIA, which, generally, involves ≥4 joints.
SIDEBAR
How efforts to classiy JIA have caused confusion7-10
Various classifications of juvenile arthritis have been proposed and used over the past 3 decades. First was the American College of Rheumatology’s 1972 criteria for juvenile rheumatoid arthritis7; next came the European League against Rheumatism (EULAR) criteria for juvenile chronic arthritis, developed in 1977.8 Being contemporaneous, the 2 classifications led to a complicated, dichotomous definition of JIA among clinicians and researchers.
As a result of this disarray, the 1997 Durban, South Africa, meeting of the Pediatric Standing Committee of the International League of Associations for Rheumatology (ILAR)9 proposed that juvenile idiopathic arthritis be adopted as the umbrella term for the misunderstood terms juvenile rheumatoid arthritis and juvenile chronic arthritis. The intent of including “idiopathic” in the term was to acknowledge that the cause of these diseases was (and is still) unknown.
The novel classification proposed by the Pediatric Standing Committee was followed, in 2001, by an ILAR task force meeting in Edmonton, Alberta, Canada, on the classification of childhood arthritis. The outcome was a recommendation to add exclusion and inclusion criteria, to make all classes of JIA mutually exclusive.10 Most recently, as discussed in the body of this article, updated ILAR guidelines on JIA classification emphasize 1) heterogeneity among the 7 disease subtypes and 2) the fact that overlapping and exclusive features exist from class to class.
Updated guidelines regarding the 7 ILAR classes of JIA emphasize heterogeneity among disease subtypes, with overlapping and exclusive features noted from class to class.11
Extended oligo-articular JIA (27%-56%), pJIA (13%-35%), sJIA (4%-17%), and ERA,(3%-11%) are the most common JIA subtypes,12 with age of onset and sex predilection differing according to JIA class.11 The disease occurs more often in girls than in boys,11 and the predisposition is higher among Whites and Asians. The incidence of JIA (all classes taken together, for every 100,000 people) is: in Japan, 10 to 15 cases13; in Turkey, 64 cases14; in Norway, 65 cases15; and in the United States and Canada, taken together, 10 to 15 cases.16
What causes JIA?
The etiology of JIA remains unclear. It is known that the disease involves inflammation of the synovium and destruction of hard and soft tissues in joints.17 It has been postulated, therefore, that a combination of genetic, environmental, and immunogenic mechanisms might be responsible for JIA.
Continue to: For example, there is an increased...
For example, there is an increased frequency of autoimmune diseases among JIA patients.18 There are also reports documenting an increased rate of infection, including with enteric pathogens, parvovirus B,19 rubella, mumps, hepatitis B, Epstein-Barr virus, mycoplasma, and chlamydia.19 Stress and trauma have also been implicated.12
The T-lymphocyte percentage is increased in the synovial fluid of JIA patients, although that percentage varies from subtype to subtype.20 This elevation results in an increase in the number of macrophages, which are induced by secreted cytokines to produce interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-a). This activity of cellular immunity leads to joint destruction.21
Clinical features
The most common signs and symptoms of JIA are arthralgias (39%), arthritis (25%), fever (18%), limping (9%), rash (8%), abdominal pain (1.3%), and uveitis (1.3%).15 Forty percent of JIA patients are reported to have temporomandibular joint involvement at some point in their life; mandibular asymmetry secondary to condylar resorption and remodeling17 is the most common presenting complaint—not arthralgia or pain, as would be expected.
Most JIA patients (52%) first present to the emergency department; another 42% present to the office of a general medical practitioner.15 On average, 3 visits to a physician, over the course of approximately 3 months, are made before a definitive diagnosis (usually by a pediatric rheumatologist) is made.15
Pertinent questions to ask a patient who has a confirmed diagnosis of JIA include the nature, severity, and duration of morning stiffness and pain, as well as any encumbering factors to regular functioning at home or school.22 Different scoring charts can be used to determine the extent of pain and disability, including the Juvenile Arthritis Disease Activity Score (JADAS)23 and the clinical JADAS (cJADAS),24 which measure minimal disease activity25 and clinically inactive disease26 cutoffs.
Continue to: Macrophage-activating syndrome increases risk of morbidity, mortality
Macrophage-activating syndrome increases risk of morbidity, mortality
An overactivation and expansion of T lymphocytes and macrophagic histiocytes with hemophagocytic activity, macrophage-activating syndrome (MAS) occurs in approximately 10% of JIA patients,27 increasing their risk of morbidity and mortality. The syndrome, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly, and occasionally, multisystem organ failure, is seen in 30% to 40% of sJIA patients; approximately 11% of them experience sudden death as a consequence.28
The clinical setting of MAS includes presenting symptoms of fever and a salmon-pink macular rash (FIGURE). For many sJIA patients with MAS, the diagnosis is made when laboratory results show hyperferritinemia, thrombocytopenia, anemia, leukopenia, coagulopathy, and elevated levels of C-reactive protein and D-dimer.27
Different classes, different features
The following clinical profiles have been documented in different classes of JIA:
Systemic JIA presents with intermittent fever of at least 2 weeks’ duration, arthritis, and occasionally, a rash.
Extended oligo-articular JIA involves pain, in a mono-articular lower-extremity joint, that can develop suddenly or insidiously, and is characterized by early-morning stiffness and uveitis (especially in early-onset, antinuclear antibody-positive JIA patients).
Continue to: Poly-articular JIA
Poly-articular JIA patients present with mild fever, weight loss, and anemia.
Enthesis-related arthritis patients have findings of enthesopathy; asymmetric arthritis of the lower extremities, particularly the Achilles tendon29; and recurrent acute, symptomatic iridocyclitis.30
Juvenile psoriatic arthritis can involve any joint but is readily differentiated from pJIA by involvement of distal interphalangeal joints and psoriatic skin and nail changes.29
Investigations
Imaging
Radiography is still the most widely used imaging tool for making the diagnosis of JIA. Plain films demonstrate structural joint damage and disturbances of growth and maturation in bones. Radiography has poor sensitivity for detecting acute synovitis and limited utility in visualizing erosion changes early in the course of disease, however, which has led to increased use of ultrasonography (US) and contrast-enhanced magnetic resonance imaging (MRI) to diagnose JIA.30
Contrast-enhanced MRI is superior to US for detecting early inflammation and monitoring subsequent joint disease. Of course, MRI is more expensive than US, and less widely available. Other imaging options are computed tomography and positron emission tomography, but these scans are not as sensitive as contrast-enhanced MRI and have the disadvantage of radiation exposure (in the former) and cost (in the latter).
Continue to: Laboratory testing
Laboratory testing
No diagnostic tests for JIA exist. Assays of acute-phase reactants, including C-reactive protein, the erythrocyte sedimentation rate, and serum amyloid-A proteins, can be utilized to demonstrate inflammation but not to confirm the diagnosis. For some classes of JIA, various tests, including rheumatoid factor, antinuclear antibody, human leukocyte antigen B-27, and cyclic citrullated peptide antibodies, can be used to confirm a specific class but, again, are not recommended for confirming JIA.6
The complete blood count, blood cultures, and tests of uric acid and lactate dehydrogenase can be ordered during treatment to monitor for complications, such as malignancy, infection, MAS, and sepsis.
Treatment is based on disease class
Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular steroids are used in all JIA classes, as an adjunct to class-specific treatment, or as induction agents.31 These therapies, although they alleviate acute signs and symptoms, such as pain, inflammation, swelling and joint contractures, are not useful for long-term treatment of JIA because they do not halt disease progression.
Systemic steroids can be utilized in exceptional cases, including chronic uveitis with arthritis or in patients with destructive arthritis and poor prognostic features, including cyclic citrullated peptide antibodies, positive RF, erosions, and joint-space narrowing.32
Other drugs. Options include traditional disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate and leflunomide; biologic agents, such as TNF-a inhibitors (eg, etanercept, adalimumab, and infliximab); and anti-IL monoclonal antibody drugs (eg, the IL-6 inhibitor tocilizumab and IL-1 inhibitors anakinra, and canakinumab).31 Indications by class include:
- csDMARDs as first-line therapy in persistent eoJIA and pJIA;
- TNF-Symbolα inhibitors for refractory eoJIA and for pJIA episodes31;
- tocilizumab, recommended for sJIA patients who have persistent systemic signs; and
- anakinra and canakinumab for refractory SJIA patients.32
Continue to: Failure
Failure
When treatment of JIA fails with a given drug, options include increasing the dosage; switching to another agent in the same drug class; switching to a different class; and combining an NSAID with a csDMARD or a biologic agent.32 In class-specific JIA cases, a change in a drug regimen is warranted on the basis of the evidence-based historical clinical response rate.32
What is the prognosis?
Treatment of JIA with novel agents, such as biologics, has opened up the possibility that JIA patients can live not just with suppressed symptoms but immunologically inactive disease. This is the result of better understanding of the pathogenesis of JIA and the mechanism of action of targeted drugs, and identification of biomarkers that are helpful in predicting prognosis, adverse effects, and response to treatment.
JIA is often a lifelong disease; one-third of patients continue to exhibit symptoms into adulthood.4 If their disease is properly managed, however, these patients do not develop typical features of rheumatoid arthritis, including hand, limb, and spine deformities. Last, patients with JIA who have only intermittent disease tend to do better over the long term than those whose disease is continual.32
The mortality rate of JIA has dropped: from 1% to 4% in the mid-1970s to 0.3% to 1% today4—an improvement in life expectancy that is echoed in enhanced quality of life for patients. According to the 4-level Steinbrocker functional classification scale33 (used to rate the extent of physical disability), 15% of JIA patients were Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care) in the period from 1976 to 1994—a percentage that had declined to 5% by 2002.34
The family physician plays pivotal role in JIA care
For the family physician, appropriate initial intervention in the management of JIA is imperative. This includes ordering imaging (whether plain films or MRI), laboratory tests as described earlier (although not to make the diagnosis), and the use of NSAIDs, intra-articular steroids, and other induction agents. Once the diagnosis is made, and a drug regimen is put in place, you will need to monitor for adverse effects. This monitoring will need to occur when a patient is escalated to csDMARDs, biological agents, or systemic steroids; is maintained on an NSAID; or is placed on a combination regimen.
Continue to: Before beginning therapy with a biologic agent...
Before beginning therapy with a biologic agent, it’s important to screen for hepatitis B, hepatitis C, human immunodeficiency virus infection, tuberculosis, and fungal infection (eg, Histoplasma capsulatum, Coccidioides immitis32). Be sure to make a timely referral to the ophthalmology service for a bi-annual eye exam and, in the event that surgery is necessary, conduct a preoperative evaluation, with the knowledge of how long before surgery a biologic agent must be withheld (duration varies by drug).32
CORRESPONDENCE
Tobe Momah, MD, Department of Family Medicine, Clinical Science Building, 4th Floor, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Adriano LS, de França Fonteles MM, de Fátima Menezes Azevedo M, et al. Medication adherence in patients with juvenile idiopathic arthritis. Rev Bras Reumatol Engl Ed. 2017;57:23-29.
2. Akioka S. A better understanding of juvenile idiopathic arthritis with classification criteria. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:513-521.
3. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112-117.
4. Petty RE, Laxer RM, Lindsley CB, et al. Pediatric Rheumatology. Philadelphia, PA: Elsevier; 2016:188-201.e6.
5. Scott C, Brice N. Juvenile idiopathic arthritis–an update on its diagnosis and management. S Afr Med J. 2015;105:1077.
6. Giancane G, Consolaro A, Lanni S, et al. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3:187-207.
7. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1972;23:712-719.
8. Wood PHN: Special meeting on nomenclature and classification of arthritis in children. In: Munthe E, ed. The Care of Rheumatic Children. Basel, Switzerland: EULAR Publishers; 1978:47-50.
9. Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-1994.
10. Petty RE, Southwood TR, Manners P, et al; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
11. Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920.
12. Weiss J, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442, vi.
13. Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Pediatric Jpn. 1997:39:242-244.
14. Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25:2445-2449.
15. Aoust L, Rossi-Semerano L, Koné-PauL I, et al. Time to diagnosis in juvenile idiopathic arthritis: a French perspective. Orphanet J Rare Dis. 2017;12:43.
16. Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway; a ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99-101.
17. Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg. 2016;45:801-812.
18. Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851-1856.
19. Gonzalez B, Larrañaga C, León O, et al. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol. 2007;34:1336-1340.
20. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149.
21. Zhou J, Ding Y, Zhang Y, et al. CD3+CD56+ natural killer T cell activity in children with different forms of juvenile idiopathic arthritis and the influence of etanercept treatment on polyarticular subgroup. Clin Immunol. 2016;176:1-11.
22. Shoop-Worrall SJW, Verstappen SMM, Baildam E, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017;0:1-8.
23. Nordal EB, Zak M, Berntson L, et al. Juvenile Arthritis Disease Activity Score (JADAS) based on CRP; validity and predictive ability in a Nordic population-based setting. Pediatr Rheumatol Online J. 2011;9(suppl 1):155.
24. Swart JF, Dijkhuizen EHP, Wulffraat NM, et al. Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77:336-342.
25. Horneff G, Klein A, Ganser G, et al. Protocols on classification, monitoring and therapy in children’s rheumatology (PRO-KIND): results of the working group polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78.
26. Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70:1519-1529.
27. Ahn SS, Yoo BW, Jung SM, et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Sem Arthritis Rheum. 2017;.47:216-221.
28. Yokota S, Mori M, Imagawa T, et al. Proposal for juvenile idiopathic arthritis guidance on diagnosis and treatment for primary care pediatricians and nonpediatric rheumatologists (2007). Mod Rheumatol. 2007;17:353-363.
29. Barut K, Adrovic A, Şahin S, et al. Juvenile idiopathic arthritis. Balkan Med J. 2017;34:90-101.
30. Colebatch-Bourn AN, Edwards CJ, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74:1946-1957.
31. Blazina Š, Markelj G, AvramoviČ MZ, et al. Management of juvenile idiopathic arthritis: a clinical guide. Pediatr Drugs. 2016;18:397-412.
32. Santos MJ, Conde M, Mourão AF, et al. 2016 update of the Portuguese recommendations for the use of biologic therapies in children and adolescents with juvenile idiopathic arthritis. Acta Rheumatol Port. 2016;41:194-212.
33. Steinbrocker 0, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
34. Oen K, Malleson PN, Cabral D, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989-1999.
1. Adriano LS, de França Fonteles MM, de Fátima Menezes Azevedo M, et al. Medication adherence in patients with juvenile idiopathic arthritis. Rev Bras Reumatol Engl Ed. 2017;57:23-29.
2. Akioka S. A better understanding of juvenile idiopathic arthritis with classification criteria. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:513-521.
3. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112-117.
4. Petty RE, Laxer RM, Lindsley CB, et al. Pediatric Rheumatology. Philadelphia, PA: Elsevier; 2016:188-201.e6.
5. Scott C, Brice N. Juvenile idiopathic arthritis–an update on its diagnosis and management. S Afr Med J. 2015;105:1077.
6. Giancane G, Consolaro A, Lanni S, et al. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3:187-207.
7. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1972;23:712-719.
8. Wood PHN: Special meeting on nomenclature and classification of arthritis in children. In: Munthe E, ed. The Care of Rheumatic Children. Basel, Switzerland: EULAR Publishers; 1978:47-50.
9. Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-1994.
10. Petty RE, Southwood TR, Manners P, et al; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
11. Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920.
12. Weiss J, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442, vi.
13. Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Pediatric Jpn. 1997:39:242-244.
14. Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25:2445-2449.
15. Aoust L, Rossi-Semerano L, Koné-PauL I, et al. Time to diagnosis in juvenile idiopathic arthritis: a French perspective. Orphanet J Rare Dis. 2017;12:43.
16. Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway; a ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99-101.
17. Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg. 2016;45:801-812.
18. Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851-1856.
19. Gonzalez B, Larrañaga C, León O, et al. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol. 2007;34:1336-1340.
20. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149.
21. Zhou J, Ding Y, Zhang Y, et al. CD3+CD56+ natural killer T cell activity in children with different forms of juvenile idiopathic arthritis and the influence of etanercept treatment on polyarticular subgroup. Clin Immunol. 2016;176:1-11.
22. Shoop-Worrall SJW, Verstappen SMM, Baildam E, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017;0:1-8.
23. Nordal EB, Zak M, Berntson L, et al. Juvenile Arthritis Disease Activity Score (JADAS) based on CRP; validity and predictive ability in a Nordic population-based setting. Pediatr Rheumatol Online J. 2011;9(suppl 1):155.
24. Swart JF, Dijkhuizen EHP, Wulffraat NM, et al. Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77:336-342.
25. Horneff G, Klein A, Ganser G, et al. Protocols on classification, monitoring and therapy in children’s rheumatology (PRO-KIND): results of the working group polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78.
26. Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70:1519-1529.
27. Ahn SS, Yoo BW, Jung SM, et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Sem Arthritis Rheum. 2017;.47:216-221.
28. Yokota S, Mori M, Imagawa T, et al. Proposal for juvenile idiopathic arthritis guidance on diagnosis and treatment for primary care pediatricians and nonpediatric rheumatologists (2007). Mod Rheumatol. 2007;17:353-363.
29. Barut K, Adrovic A, Şahin S, et al. Juvenile idiopathic arthritis. Balkan Med J. 2017;34:90-101.
30. Colebatch-Bourn AN, Edwards CJ, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74:1946-1957.
31. Blazina Š, Markelj G, AvramoviČ MZ, et al. Management of juvenile idiopathic arthritis: a clinical guide. Pediatr Drugs. 2016;18:397-412.
32. Santos MJ, Conde M, Mourão AF, et al. 2016 update of the Portuguese recommendations for the use of biologic therapies in children and adolescents with juvenile idiopathic arthritis. Acta Rheumatol Port. 2016;41:194-212.
33. Steinbrocker 0, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
34. Oen K, Malleson PN, Cabral D, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989-1999.
PRACTICE RECOMMENDATIONS
› Pair the findings of your clinical exam with the results of imaging and laboratory testing to make the diagnosis of juvenile idiopathic arthritis (JIA), as it is a diagnosis of exclusion. B
› Individualize treatment based on where the patient falls in the JIA disease spectrum to increase the likelihood that medical therapy will be effective. A
› Consider treating diagnosed JIA with an available biologic agent, which can provide a long asymptomatic period. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To avoid Hep B reactivation, screen before immunosuppression
CASE A 53-year-old woman you are seeing for the first time has been taking 10 mg of prednisone daily for a month, prescribed by another practitioner for polymyalgia rheumatica. Testing is negative for hepatitis B surface antigen but is positive for hepatitis B core antibody total, indicating a resolved hepatitis B infection. The absence of hepatitis B DNA is confirmed.
How would you proceed with this patient?
Patients with resolved hepatitis B virus (HBV) or chronic hepatitis B (CHB) infections are at risk for HBV reactivation (HBVr) if they undergo immunosuppressive therapy for a condition such as cancer. HBVr can in turn lead to delays in treatment and increased morbidity and mortality.
HBVr is a well-documented adverse outcome in patients treated with rituximab and in those undergoing stem cell transplantation. Current oncology guidelines recommend screening for HBV prior to initiating these treatments.1,2 More recent evidence shows that many other immunosuppressive therapies can also lead to HBVr.3 Such treatments are now used across a multitude of specialties and conditions. For many of these conditions, there are no consistent guidelines regarding HBV screening.
In 2013, the US Food and Drug Administration (FDA) announced the requirement of a Boxed Warning for the immunosuppressive drugs ofatumumab and rituximab. In 2016, the FDA announced the same requirement for certain direct-acting antiviral medicines for hepatitis C virus.
Among patients who are positive for hepatitis-B surface antigen (HBsAg) and who are treated with immunosuppression, the frequency of HBVr has ranged from 0% to 39%.4,5
As the list of immunosuppressive therapies that can cause HBVr grows, specialty guidelines are evolving to address the risk that HBVr poses.
Continue to: An underrecognized problem
An underrecognized problem. CHB affects an estimated 350 million people worldwide6 but remains underrecognized and underdiagnosed. An estimated 1.4 million Americans6 have CHB, but only a minority of them are aware of their positive status and are followed by a hepatologist or receive medical care for their disease.7 Compared with the natural-born US population, a higher prevalence of CHB exists among immigrants to this country from the Asian Pacific and Eastern Mediterranean regions, sub-Saharan Africa, and certain parts of South America.8-10 In 2008, the Centers for Disease Control and Prevention (CDC) updated its recommendations on screening for HBV to include immigrants to the United States from intermediate and high endemic areas.6 Unfortunately, data published on physicians’ adherence to the CDC guidelines for screening show that only 60% correctly screened at-risk patients.11
Individuals with CHB are at risk and rely on a robust immune system to keep their disease from becoming active. During infection, the virus gains entry into the hepatocytes and the double-stranded viral genome is imported into the nucleus of the cell, where it is repaired into covalently closed circular DNA (cccDNA). Research has demonstrated the stability of cccDNA and its persistence as a latent reservoir for HBV reactivation, even decades after recovery from infection.12
Also at risk are individuals who have unrecovered from HBV infection and are HBsAg negative and anti-HBc positive. To avert reverse seroconversion, they also rely on a robust immune system.13 Reverse seroconversion is defined as a reappearance of HBV DNA and HBsAg positivity in individuals who were previously negative.13 In these individuals, HBV DNA may not be quantifiable in circulation, but trace amounts of viral DNA found in the liver are enough to pose a reactivation risk in the setting of immune suppression.14
Moreover, often overlooked is the fact that reactivation or reverse seroconversion can necessitate disruptions and delays in immunosuppressive treatment for other life-threatening disease processes.14,15
Universal screening reduces risk for HBVr. Patients with CHB are at risk for reactivation, as are patients with resolved HBV infection. Many patients, however, do not know their status. By screening all patients before beginning immunosuppressive therapy, physicians can provide effective prophylaxis, which has been shown to significantly reduce the risk for HBVr.8.15
Continue to: Recognizing the onset of HBVr
Recognizing the onset of HBVr
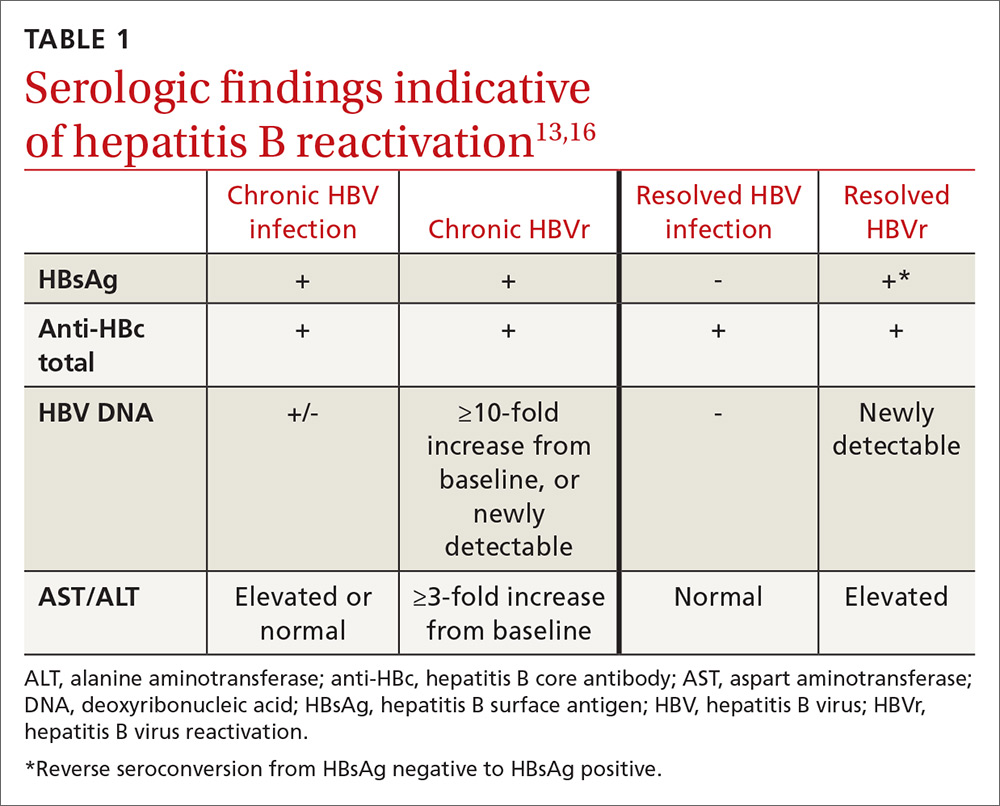
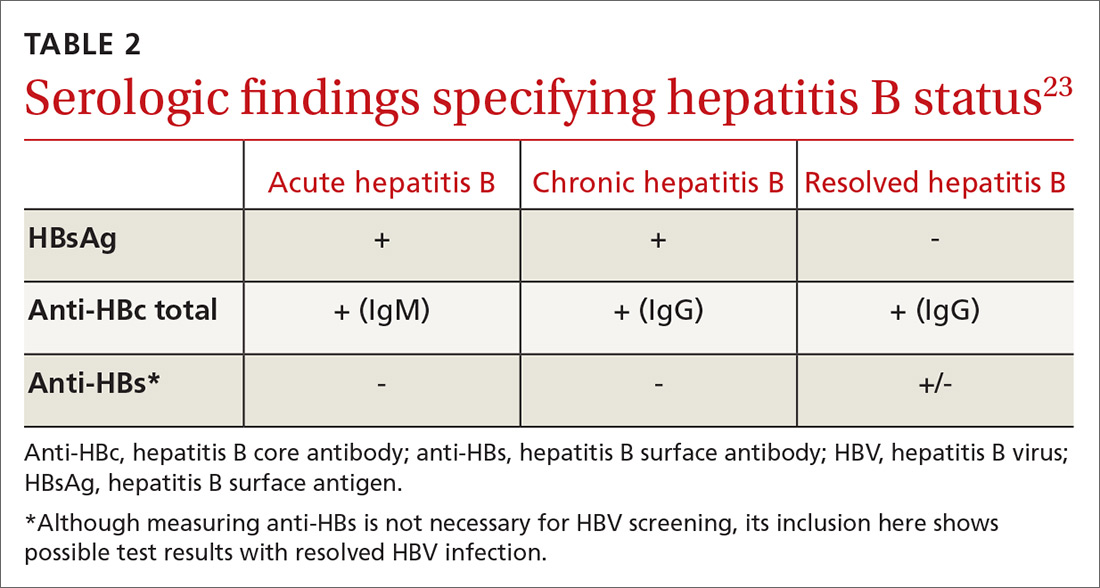
In patients with CHB, HBVr is defined as at least a 3-fold increase in aspart aminotransferase (AST) and alanine aminotransferase (ALT) and at least a 10-fold increase from baseline in HBV DNA. In patients with resolved HBV infection, there may be reverse seroconversion from HbsAg-negative to HBsAg-positive status (TABLE 113,16).
Not all elevations in AST/ALT in patients undergoing chemotherapy or immunosuppressive therapy indicate HBVr. Very often, derangements in AST/ALT may be related to the toxic effects of therapy or to the underlying disease process. However, as immunosuppressive therapy is now used for a wide array of medical conditions, consider HBVr as a potential cause of abnormal liver function in all patients receiving such therapy
A patient is at risk for HBVr when starting immunosuppression and up to a year following the completion of therapy. With suppression of the immune system, HBV replication increases and serum AST/ALT concentrations may rise. HBVr may also present with the appearance of HBV DNA in patients with previously undetectable levels.12,17
Most patients remain asymptomatic, and abnormal AST/ALT levels eventually resolve after completion of immunosuppression. However, some patients' liver enzymes may rise, indicating a more severe hepatic flare. These patients may present with right upper-quadrant tenderness, jaundice, or fatigue. In these cases, recognizing HBVr and starting antivirals may reduce hepatitis flare.
Unfortunately, despite early recognition of HBVr and initiation of appropriate therapy, some patients can progress to hepatic decompensation and even fulminant hepatic failure that may have been prevented with prophylaxis.
Continue to: The justification for universal screening
The justification for universal screening
Although nongastroenterology societies differ in their recommendations on screening for HBV, universal screening before implementing prolonged immunosuppressive treatment is recommended by the CDC,6 the American Association for the Study of Liver Diseases,18 the Asian Pacific Association for the Study of the Liver,19 the European Association for the Study of the Liver,20 and the American Gastroenterological Association (AGA).21
Older guidelines recommended screening only high-risk populations. But such screening has downfalls. It requires that patients or their physicians recognize that they are at high risk. In one study, nearly 65% of an infected Asian-American population was unaware of their positive HBV status.22 Risk-based screening also requires that physicians ask the appropriate questions and that patients admit to high-risk behavior. Screening patients based only on risk factors may easily overlook patients who need prophylaxis against HBVr.
Common arguments against universal screening include the cost of testing, the possibility of false-positive results, and the implications of a new diagnosis of hepatitis B. However, the potential benefits of screening are significant, and HBV screening in the general population has been shown to be cost effective when the prevalence of HBV is 0.3%.21 In the United States, conservative estimates are a prevalence of HBsAg positivity of 0.4% and past infection of 3%, making screening a cost-effective recommendation.16 It is therefore prudent to screen all patients before starting immunosuppressive therapy.
How to screen
All guidelines agree on how to test for HBV. Measuring levels of HBsAg and hepatitis B core antibody (anti-HBc total) allows the clinician to ascertain whether the patient’s HBV infection status is acute, chronic, or resolved (TABLE 223) and to perform HBVr risk stratification (discussed later).
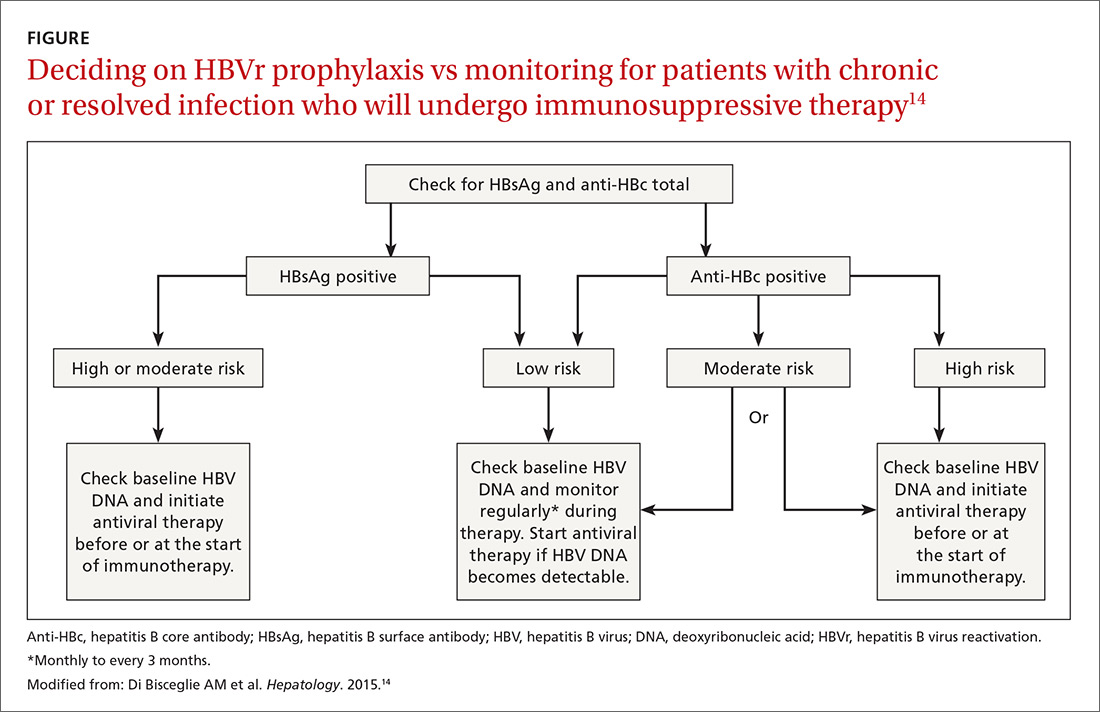
Patients with acute infections should be referred to a hepatologist. With chronic or resolved HBV, stratify patients into a prophylaxis group or monitoring group (FIGURE14). Stratification involves identifying HBV status (chronic or resolved) and selecting a type of immunosuppressive therapy. Whether the patient falls into prophylaxis or monitoring, obtain a baseline level of viral DNA, as this has proven to be the best predictor of HBV reactivation.16
Continue to: In screening, be sure the appropriate...
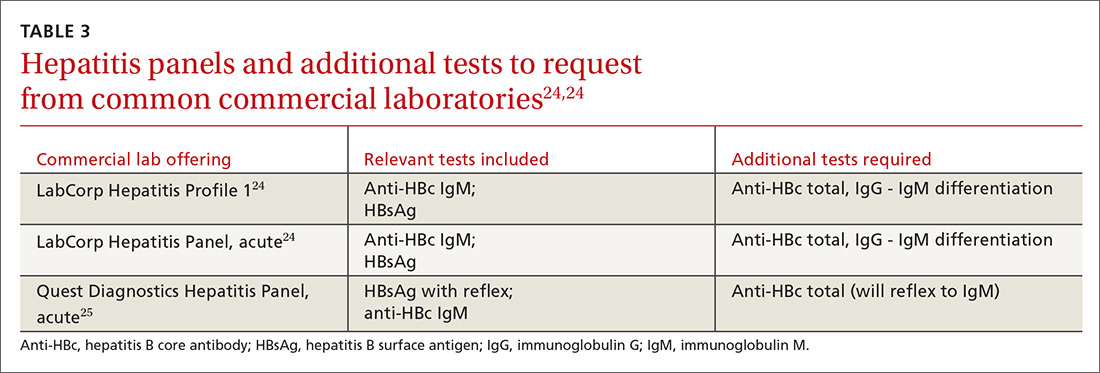
In screening, be sure the appropriate anti-HBc testing is covered. Common usage of the term anti-HBc may refer to immunoglobulin G (IgG) or immunoglobulin M (IgM)or total core antibody, containing both IgG and IgM. But in this context, accurate screening requires either total core antibody or anti-HBc IgG. Anti-IgM alone is inadequate. Many commercial laboratories offer acute hepatitis panels or hepatitis profiles (TABLE 324,25), and it is important to confirm that such order sets contain the tests necessary to allow for risk stratification.
Testing for hepatitis B surface antibody (anti-HBs) is not useful in screening. Although it was hypothesized that the presence of this antibody lowered risk, recent studies have proven no change in risk based on this value.21
How to assess HBVr risk
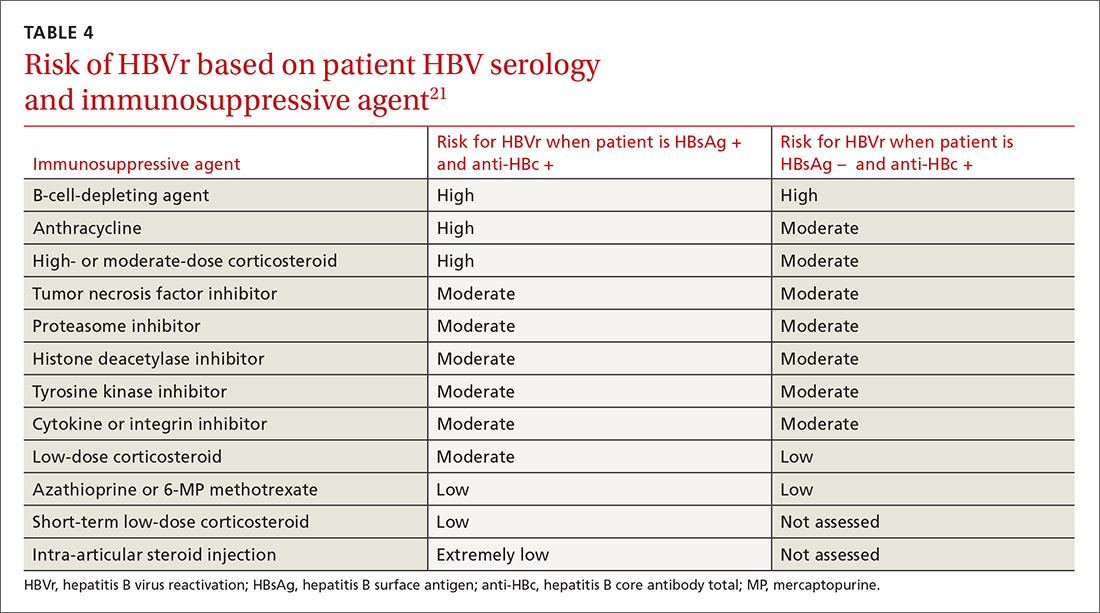
Assessing risk for HBVr takes into account both the patient’s serology and intended treatment. Reddy et al delineated patient groups into high, moderate, and low risk (TABLES 4 and 5).21 The high-risk group was defined by anticipated incidence of HBVr in > 10% of cases; the moderate-risk group had an anticipated incidence of 1% to 10%; and the low-risk group had an anticipated incidence of <1%.21 Evidence was strongest in the high-risk group.
Patients with CHB (HBsAg positive and anti-HBc positive) are considered high risk for reactivation with a wide variety of immunosuppressive therapies. Such patients are 5 to 8 times more likely to develop HBVr than patients with an HBsAg-negative status signifying a resolved infection.16
Immunosuppressive agents and associated risks. The AGA guidelines consider treatment with B-cell-depleting agents, such as rituximab and ofatumumab, to be high risk, regardless of a patient’s surface antigen status. Additionally, for patients who are HBsAg positive, high-risk treatments include anthracycline derivatives, such as doxorubicin and epirubicin, or high- or moderate-dose steroids. These treatments are considered moderate risk when used in patients who have resolved HBV infection (HBsAg negative/anti-HBc positive). Moderate-risk modalities also include tumor necrosis factor inhibitors and tyrosine kinase inhibitors, regardless of surface antigen status; and low-dose steroids or cytokine or integrin inhibitors in HbsAg-positive individuals.21
Continue to: Other immunosuppression modalities...
Other immunosuppression modalities considered to be moderate risk independent of HBV serology include proteasome inhibitors, such as bortezomib, used for multiple myeloma treatment, and histone deacetylase inhibitors, such as romidepsin, used to treat T-cell lymphoma.13 Low-dose steroids or cytokine or integrin inhibitors are considered to be low risk in surface antigen-negative individuals; azathioprine, mercaptopurine, or methotrexate are low risk regardless of HBsAg status.21 Intra-articular steroid injections are considered extremely low risk in HbsAg-positive individuals, and are unclassified for HbsAg-negative individuals.13
More recent evidence has implicated other medication classes in triggering HBVr — (eg, direct-acting antivirals.)26
Prophylaxis options: High to moderate risk vs low risk
The consensus of major guideline issuers is to offer prophylaxis to high-risk patients and to monitor low-risk patients. The AGA additionally recommends prophylaxis for patients at moderate risk.
Controversy surrounding the moderate-risk group. Some authors argue that monitoring HBV DNA in the moderate-risk group is preferable to committing patients to long periods of prophylaxis, and that rescue treatment could be initiated as needed. However, the ideal monitoring period has not been determined, and the effectiveness of prophylaxis over monitoring is so significant that monitoring is losing favor.
Perrillo et al performed a meta-analysis of 5 randomized controlled trials evaluating antiviral agents vs no prophylaxis.16 The analysis included 139 patients receiving prophylaxis and 137 controls. The pooled results demonstrated an 87% relative risk reduction with prophylaxis, supporting the trend toward treating patients with moderate risk.16
Continue to: Prophylactic treatment options are safe...
Prophylactic treatment options are safe and well tolerated. For this reason, committing a high- or moderate-risk patient to a course of treatment should be less of a concern than the risk for HBVr.
In the early randomized controlled trials for HBVr prophylaxis, lamivudine, although effective, unfortunately led to a high incidence of viral resistance after prolonged use, thus diminishing its desirability.18 Newer agents, such as entecavir and tenofovir, have proven just as effective as lamivudine and are largely unaffected by viral resistance.27
In retrospective and prospective studies on HBVr prophylaxis, patients treated with entecavir had less HBV-related hepatitis, less delay in chemotherapy, and a lower rate of HBVr when compared with lamivudine.28,29 Tenofovir is recommended, however, if patients were previously treated with lamivudine.30
A recent meta-analysis demonstrated that tenofovir and entecavir are preferable to lamivudine in preventing HBVr.31
Looking ahead
Screening for HBsAg and anti-HBc total before starting immunosuppressive therapy can reduce morbidity and mortality in patients undergoing such treatment. The AGA recommends screening all patients about to begin high- or moderate-risk therapy or patients in populations with a prevalence of CHB ≥2%, per the CDC.6,21
Continue to: Classes of medications...
Classes of medications other than immunosuppressants may also trigger HBVr. The FDA has issued a warning regarding direct-acting antivirals, but optimal management of these patients is still evolving.
Once HBV status is established, a patient’s risk for HBVr can be specified as high, moderate, or low using their HBV status and the type of therapy being initiated. The AGA recommends prophylactic treatment with well-tolerated and effective agents for patients classified as high or moderate risk. If a patient’s risk is low, regular monitoring of HBV DNA and AST and ALT levels is sufficient. Recommendations of monitoring intervals span from monthly to every 3 months.13,14
CASE Given the patient’s status of resolved HBV infection and her current moderate-dose regimen of prednisone, her risk for HBV reactivation is moderate. She could either receive antiviral prophylaxis or undergo regular monitoring. Following a discussion of the options, she opts for referral to a hepatologist to discuss possible prophylactic treatment.
Increased awareness of HBVr risk associated with immunosuppressive therapy, coupled with a planned approach to appropriate screening and risk stratification, can help health care providers prevent the reactivation of HBV or initiate early intervention for CHB.
CORRESPONDENCE
Ronan Farrell, MD, Rhode Island Hospital, 593 Eddy Street, Providence, RI 02903; [email protected].
1. Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199-3202.
2. Day FL, Link E, Thursky K, et al. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract. 2011;7:141-147.
3. Paul S, Saxena A, Terrin N, et al. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Internal Med. 2016;164:30-40.
4. Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med. 2007;22:237-243.
5. Esteve M, Saro C, González-Huix F, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363-1365.
6. Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20.
7. Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62:1893-1908.
8, , , A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329-1339.
9. WHO. Hepatitis B. www.who.int/en/news-room/fact-sheets/detail/hepatitis-b. Accessed February 28, 2019.
10. Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422-433.
11. Foster T, Hon H, Kanwal F, et al. Screening high risk individuals for hepatitis B: physician knowledge, attitudes, and beliefs. Dig Dis Sci. 2011;56:3471-3487.
12. Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104-1108.
13. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297-1309.
14. Di Bisceglie AM, Lok AS, Martin P, et al. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703-711.
15. Lok AS, Ward JW, Perrillo RP, et al. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743-745.
16. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.
17. Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209-219.
18. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662.
19. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561.
20. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185.
21. Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-219.
, , . Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034-1040.
23. Hwang JP, Artz AS, Somerfield MR. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Oncol Pract. 2015;11:e487-489.
24. LabCorp. Hepatitis B core antibody, IgG, IgM, differentiation. www.labcorp.com/test-menu/27196/hepatitis-b-core-antibody-igg-igm-differentiation. Accessed February 28, 2019.
25. Quest diagnostics. Hepatitis B Core Antibody, Total. www.questdiagnostics.com/testcenter/TestDetail.action?ntc=501.Accessed November 5, 2018.
26. The Food and Drug Administration Adverse Event Reporting System (FAERS). www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed February 28, 2019.
27. Lim YS. Management of antiviral resistance in chronic hepatitis B. Gut Liver. 2017;11:189-195.
28. Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521-2530.
29. Chen WC, Cheng JS, Chiang PH, et al. A comparison of entecavir and lamivudine for the prophylaxis of hepatitis B virus reactivation in solid tumor patients undergoing systemic cytotoxic chemotherapy. PLoS One. 2015;10:e0131545.
30. Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514.
31. Zhang MY, Zhu GQ, Shi KQ, et al. Systematic review with network meta-analysis: comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642-30658.
CASE A 53-year-old woman you are seeing for the first time has been taking 10 mg of prednisone daily for a month, prescribed by another practitioner for polymyalgia rheumatica. Testing is negative for hepatitis B surface antigen but is positive for hepatitis B core antibody total, indicating a resolved hepatitis B infection. The absence of hepatitis B DNA is confirmed.
How would you proceed with this patient?
Patients with resolved hepatitis B virus (HBV) or chronic hepatitis B (CHB) infections are at risk for HBV reactivation (HBVr) if they undergo immunosuppressive therapy for a condition such as cancer. HBVr can in turn lead to delays in treatment and increased morbidity and mortality.
HBVr is a well-documented adverse outcome in patients treated with rituximab and in those undergoing stem cell transplantation. Current oncology guidelines recommend screening for HBV prior to initiating these treatments.1,2 More recent evidence shows that many other immunosuppressive therapies can also lead to HBVr.3 Such treatments are now used across a multitude of specialties and conditions. For many of these conditions, there are no consistent guidelines regarding HBV screening.
In 2013, the US Food and Drug Administration (FDA) announced the requirement of a Boxed Warning for the immunosuppressive drugs ofatumumab and rituximab. In 2016, the FDA announced the same requirement for certain direct-acting antiviral medicines for hepatitis C virus.
Among patients who are positive for hepatitis-B surface antigen (HBsAg) and who are treated with immunosuppression, the frequency of HBVr has ranged from 0% to 39%.4,5
As the list of immunosuppressive therapies that can cause HBVr grows, specialty guidelines are evolving to address the risk that HBVr poses.
Continue to: An underrecognized problem
An underrecognized problem. CHB affects an estimated 350 million people worldwide6 but remains underrecognized and underdiagnosed. An estimated 1.4 million Americans6 have CHB, but only a minority of them are aware of their positive status and are followed by a hepatologist or receive medical care for their disease.7 Compared with the natural-born US population, a higher prevalence of CHB exists among immigrants to this country from the Asian Pacific and Eastern Mediterranean regions, sub-Saharan Africa, and certain parts of South America.8-10 In 2008, the Centers for Disease Control and Prevention (CDC) updated its recommendations on screening for HBV to include immigrants to the United States from intermediate and high endemic areas.6 Unfortunately, data published on physicians’ adherence to the CDC guidelines for screening show that only 60% correctly screened at-risk patients.11
Individuals with CHB are at risk and rely on a robust immune system to keep their disease from becoming active. During infection, the virus gains entry into the hepatocytes and the double-stranded viral genome is imported into the nucleus of the cell, where it is repaired into covalently closed circular DNA (cccDNA). Research has demonstrated the stability of cccDNA and its persistence as a latent reservoir for HBV reactivation, even decades after recovery from infection.12
Also at risk are individuals who have unrecovered from HBV infection and are HBsAg negative and anti-HBc positive. To avert reverse seroconversion, they also rely on a robust immune system.13 Reverse seroconversion is defined as a reappearance of HBV DNA and HBsAg positivity in individuals who were previously negative.13 In these individuals, HBV DNA may not be quantifiable in circulation, but trace amounts of viral DNA found in the liver are enough to pose a reactivation risk in the setting of immune suppression.14
Moreover, often overlooked is the fact that reactivation or reverse seroconversion can necessitate disruptions and delays in immunosuppressive treatment for other life-threatening disease processes.14,15
Universal screening reduces risk for HBVr. Patients with CHB are at risk for reactivation, as are patients with resolved HBV infection. Many patients, however, do not know their status. By screening all patients before beginning immunosuppressive therapy, physicians can provide effective prophylaxis, which has been shown to significantly reduce the risk for HBVr.8.15
Continue to: Recognizing the onset of HBVr
Recognizing the onset of HBVr
In patients with CHB, HBVr is defined as at least a 3-fold increase in aspart aminotransferase (AST) and alanine aminotransferase (ALT) and at least a 10-fold increase from baseline in HBV DNA. In patients with resolved HBV infection, there may be reverse seroconversion from HbsAg-negative to HBsAg-positive status (TABLE 113,16).
Not all elevations in AST/ALT in patients undergoing chemotherapy or immunosuppressive therapy indicate HBVr. Very often, derangements in AST/ALT may be related to the toxic effects of therapy or to the underlying disease process. However, as immunosuppressive therapy is now used for a wide array of medical conditions, consider HBVr as a potential cause of abnormal liver function in all patients receiving such therapy
A patient is at risk for HBVr when starting immunosuppression and up to a year following the completion of therapy. With suppression of the immune system, HBV replication increases and serum AST/ALT concentrations may rise. HBVr may also present with the appearance of HBV DNA in patients with previously undetectable levels.12,17
Most patients remain asymptomatic, and abnormal AST/ALT levels eventually resolve after completion of immunosuppression. However, some patients' liver enzymes may rise, indicating a more severe hepatic flare. These patients may present with right upper-quadrant tenderness, jaundice, or fatigue. In these cases, recognizing HBVr and starting antivirals may reduce hepatitis flare.
Unfortunately, despite early recognition of HBVr and initiation of appropriate therapy, some patients can progress to hepatic decompensation and even fulminant hepatic failure that may have been prevented with prophylaxis.
Continue to: The justification for universal screening
The justification for universal screening
Although nongastroenterology societies differ in their recommendations on screening for HBV, universal screening before implementing prolonged immunosuppressive treatment is recommended by the CDC,6 the American Association for the Study of Liver Diseases,18 the Asian Pacific Association for the Study of the Liver,19 the European Association for the Study of the Liver,20 and the American Gastroenterological Association (AGA).21
Older guidelines recommended screening only high-risk populations. But such screening has downfalls. It requires that patients or their physicians recognize that they are at high risk. In one study, nearly 65% of an infected Asian-American population was unaware of their positive HBV status.22 Risk-based screening also requires that physicians ask the appropriate questions and that patients admit to high-risk behavior. Screening patients based only on risk factors may easily overlook patients who need prophylaxis against HBVr.
Common arguments against universal screening include the cost of testing, the possibility of false-positive results, and the implications of a new diagnosis of hepatitis B. However, the potential benefits of screening are significant, and HBV screening in the general population has been shown to be cost effective when the prevalence of HBV is 0.3%.21 In the United States, conservative estimates are a prevalence of HBsAg positivity of 0.4% and past infection of 3%, making screening a cost-effective recommendation.16 It is therefore prudent to screen all patients before starting immunosuppressive therapy.
How to screen
All guidelines agree on how to test for HBV. Measuring levels of HBsAg and hepatitis B core antibody (anti-HBc total) allows the clinician to ascertain whether the patient’s HBV infection status is acute, chronic, or resolved (TABLE 223) and to perform HBVr risk stratification (discussed later).
Patients with acute infections should be referred to a hepatologist. With chronic or resolved HBV, stratify patients into a prophylaxis group or monitoring group (FIGURE14). Stratification involves identifying HBV status (chronic or resolved) and selecting a type of immunosuppressive therapy. Whether the patient falls into prophylaxis or monitoring, obtain a baseline level of viral DNA, as this has proven to be the best predictor of HBV reactivation.16
Continue to: In screening, be sure the appropriate...
In screening, be sure the appropriate anti-HBc testing is covered. Common usage of the term anti-HBc may refer to immunoglobulin G (IgG) or immunoglobulin M (IgM)or total core antibody, containing both IgG and IgM. But in this context, accurate screening requires either total core antibody or anti-HBc IgG. Anti-IgM alone is inadequate. Many commercial laboratories offer acute hepatitis panels or hepatitis profiles (TABLE 324,25), and it is important to confirm that such order sets contain the tests necessary to allow for risk stratification.
Testing for hepatitis B surface antibody (anti-HBs) is not useful in screening. Although it was hypothesized that the presence of this antibody lowered risk, recent studies have proven no change in risk based on this value.21
How to assess HBVr risk
Assessing risk for HBVr takes into account both the patient’s serology and intended treatment. Reddy et al delineated patient groups into high, moderate, and low risk (TABLES 4 and 5).21 The high-risk group was defined by anticipated incidence of HBVr in > 10% of cases; the moderate-risk group had an anticipated incidence of 1% to 10%; and the low-risk group had an anticipated incidence of <1%.21 Evidence was strongest in the high-risk group.
Patients with CHB (HBsAg positive and anti-HBc positive) are considered high risk for reactivation with a wide variety of immunosuppressive therapies. Such patients are 5 to 8 times more likely to develop HBVr than patients with an HBsAg-negative status signifying a resolved infection.16
Immunosuppressive agents and associated risks. The AGA guidelines consider treatment with B-cell-depleting agents, such as rituximab and ofatumumab, to be high risk, regardless of a patient’s surface antigen status. Additionally, for patients who are HBsAg positive, high-risk treatments include anthracycline derivatives, such as doxorubicin and epirubicin, or high- or moderate-dose steroids. These treatments are considered moderate risk when used in patients who have resolved HBV infection (HBsAg negative/anti-HBc positive). Moderate-risk modalities also include tumor necrosis factor inhibitors and tyrosine kinase inhibitors, regardless of surface antigen status; and low-dose steroids or cytokine or integrin inhibitors in HbsAg-positive individuals.21
Continue to: Other immunosuppression modalities...
Other immunosuppression modalities considered to be moderate risk independent of HBV serology include proteasome inhibitors, such as bortezomib, used for multiple myeloma treatment, and histone deacetylase inhibitors, such as romidepsin, used to treat T-cell lymphoma.13 Low-dose steroids or cytokine or integrin inhibitors are considered to be low risk in surface antigen-negative individuals; azathioprine, mercaptopurine, or methotrexate are low risk regardless of HBsAg status.21 Intra-articular steroid injections are considered extremely low risk in HbsAg-positive individuals, and are unclassified for HbsAg-negative individuals.13
More recent evidence has implicated other medication classes in triggering HBVr — (eg, direct-acting antivirals.)26
Prophylaxis options: High to moderate risk vs low risk
The consensus of major guideline issuers is to offer prophylaxis to high-risk patients and to monitor low-risk patients. The AGA additionally recommends prophylaxis for patients at moderate risk.
Controversy surrounding the moderate-risk group. Some authors argue that monitoring HBV DNA in the moderate-risk group is preferable to committing patients to long periods of prophylaxis, and that rescue treatment could be initiated as needed. However, the ideal monitoring period has not been determined, and the effectiveness of prophylaxis over monitoring is so significant that monitoring is losing favor.
Perrillo et al performed a meta-analysis of 5 randomized controlled trials evaluating antiviral agents vs no prophylaxis.16 The analysis included 139 patients receiving prophylaxis and 137 controls. The pooled results demonstrated an 87% relative risk reduction with prophylaxis, supporting the trend toward treating patients with moderate risk.16
Continue to: Prophylactic treatment options are safe...
Prophylactic treatment options are safe and well tolerated. For this reason, committing a high- or moderate-risk patient to a course of treatment should be less of a concern than the risk for HBVr.
In the early randomized controlled trials for HBVr prophylaxis, lamivudine, although effective, unfortunately led to a high incidence of viral resistance after prolonged use, thus diminishing its desirability.18 Newer agents, such as entecavir and tenofovir, have proven just as effective as lamivudine and are largely unaffected by viral resistance.27
In retrospective and prospective studies on HBVr prophylaxis, patients treated with entecavir had less HBV-related hepatitis, less delay in chemotherapy, and a lower rate of HBVr when compared with lamivudine.28,29 Tenofovir is recommended, however, if patients were previously treated with lamivudine.30
A recent meta-analysis demonstrated that tenofovir and entecavir are preferable to lamivudine in preventing HBVr.31
Looking ahead
Screening for HBsAg and anti-HBc total before starting immunosuppressive therapy can reduce morbidity and mortality in patients undergoing such treatment. The AGA recommends screening all patients about to begin high- or moderate-risk therapy or patients in populations with a prevalence of CHB ≥2%, per the CDC.6,21
Continue to: Classes of medications...
Classes of medications other than immunosuppressants may also trigger HBVr. The FDA has issued a warning regarding direct-acting antivirals, but optimal management of these patients is still evolving.
Once HBV status is established, a patient’s risk for HBVr can be specified as high, moderate, or low using their HBV status and the type of therapy being initiated. The AGA recommends prophylactic treatment with well-tolerated and effective agents for patients classified as high or moderate risk. If a patient’s risk is low, regular monitoring of HBV DNA and AST and ALT levels is sufficient. Recommendations of monitoring intervals span from monthly to every 3 months.13,14
CASE Given the patient’s status of resolved HBV infection and her current moderate-dose regimen of prednisone, her risk for HBV reactivation is moderate. She could either receive antiviral prophylaxis or undergo regular monitoring. Following a discussion of the options, she opts for referral to a hepatologist to discuss possible prophylactic treatment.
Increased awareness of HBVr risk associated with immunosuppressive therapy, coupled with a planned approach to appropriate screening and risk stratification, can help health care providers prevent the reactivation of HBV or initiate early intervention for CHB.
CORRESPONDENCE
Ronan Farrell, MD, Rhode Island Hospital, 593 Eddy Street, Providence, RI 02903; [email protected].
CASE A 53-year-old woman you are seeing for the first time has been taking 10 mg of prednisone daily for a month, prescribed by another practitioner for polymyalgia rheumatica. Testing is negative for hepatitis B surface antigen but is positive for hepatitis B core antibody total, indicating a resolved hepatitis B infection. The absence of hepatitis B DNA is confirmed.
How would you proceed with this patient?
Patients with resolved hepatitis B virus (HBV) or chronic hepatitis B (CHB) infections are at risk for HBV reactivation (HBVr) if they undergo immunosuppressive therapy for a condition such as cancer. HBVr can in turn lead to delays in treatment and increased morbidity and mortality.
HBVr is a well-documented adverse outcome in patients treated with rituximab and in those undergoing stem cell transplantation. Current oncology guidelines recommend screening for HBV prior to initiating these treatments.1,2 More recent evidence shows that many other immunosuppressive therapies can also lead to HBVr.3 Such treatments are now used across a multitude of specialties and conditions. For many of these conditions, there are no consistent guidelines regarding HBV screening.
In 2013, the US Food and Drug Administration (FDA) announced the requirement of a Boxed Warning for the immunosuppressive drugs ofatumumab and rituximab. In 2016, the FDA announced the same requirement for certain direct-acting antiviral medicines for hepatitis C virus.
Among patients who are positive for hepatitis-B surface antigen (HBsAg) and who are treated with immunosuppression, the frequency of HBVr has ranged from 0% to 39%.4,5
As the list of immunosuppressive therapies that can cause HBVr grows, specialty guidelines are evolving to address the risk that HBVr poses.
Continue to: An underrecognized problem
An underrecognized problem. CHB affects an estimated 350 million people worldwide6 but remains underrecognized and underdiagnosed. An estimated 1.4 million Americans6 have CHB, but only a minority of them are aware of their positive status and are followed by a hepatologist or receive medical care for their disease.7 Compared with the natural-born US population, a higher prevalence of CHB exists among immigrants to this country from the Asian Pacific and Eastern Mediterranean regions, sub-Saharan Africa, and certain parts of South America.8-10 In 2008, the Centers for Disease Control and Prevention (CDC) updated its recommendations on screening for HBV to include immigrants to the United States from intermediate and high endemic areas.6 Unfortunately, data published on physicians’ adherence to the CDC guidelines for screening show that only 60% correctly screened at-risk patients.11
Individuals with CHB are at risk and rely on a robust immune system to keep their disease from becoming active. During infection, the virus gains entry into the hepatocytes and the double-stranded viral genome is imported into the nucleus of the cell, where it is repaired into covalently closed circular DNA (cccDNA). Research has demonstrated the stability of cccDNA and its persistence as a latent reservoir for HBV reactivation, even decades after recovery from infection.12
Also at risk are individuals who have unrecovered from HBV infection and are HBsAg negative and anti-HBc positive. To avert reverse seroconversion, they also rely on a robust immune system.13 Reverse seroconversion is defined as a reappearance of HBV DNA and HBsAg positivity in individuals who were previously negative.13 In these individuals, HBV DNA may not be quantifiable in circulation, but trace amounts of viral DNA found in the liver are enough to pose a reactivation risk in the setting of immune suppression.14
Moreover, often overlooked is the fact that reactivation or reverse seroconversion can necessitate disruptions and delays in immunosuppressive treatment for other life-threatening disease processes.14,15
Universal screening reduces risk for HBVr. Patients with CHB are at risk for reactivation, as are patients with resolved HBV infection. Many patients, however, do not know their status. By screening all patients before beginning immunosuppressive therapy, physicians can provide effective prophylaxis, which has been shown to significantly reduce the risk for HBVr.8.15
Continue to: Recognizing the onset of HBVr
Recognizing the onset of HBVr
In patients with CHB, HBVr is defined as at least a 3-fold increase in aspart aminotransferase (AST) and alanine aminotransferase (ALT) and at least a 10-fold increase from baseline in HBV DNA. In patients with resolved HBV infection, there may be reverse seroconversion from HbsAg-negative to HBsAg-positive status (TABLE 113,16).
Not all elevations in AST/ALT in patients undergoing chemotherapy or immunosuppressive therapy indicate HBVr. Very often, derangements in AST/ALT may be related to the toxic effects of therapy or to the underlying disease process. However, as immunosuppressive therapy is now used for a wide array of medical conditions, consider HBVr as a potential cause of abnormal liver function in all patients receiving such therapy
A patient is at risk for HBVr when starting immunosuppression and up to a year following the completion of therapy. With suppression of the immune system, HBV replication increases and serum AST/ALT concentrations may rise. HBVr may also present with the appearance of HBV DNA in patients with previously undetectable levels.12,17
Most patients remain asymptomatic, and abnormal AST/ALT levels eventually resolve after completion of immunosuppression. However, some patients' liver enzymes may rise, indicating a more severe hepatic flare. These patients may present with right upper-quadrant tenderness, jaundice, or fatigue. In these cases, recognizing HBVr and starting antivirals may reduce hepatitis flare.
Unfortunately, despite early recognition of HBVr and initiation of appropriate therapy, some patients can progress to hepatic decompensation and even fulminant hepatic failure that may have been prevented with prophylaxis.
Continue to: The justification for universal screening
The justification for universal screening
Although nongastroenterology societies differ in their recommendations on screening for HBV, universal screening before implementing prolonged immunosuppressive treatment is recommended by the CDC,6 the American Association for the Study of Liver Diseases,18 the Asian Pacific Association for the Study of the Liver,19 the European Association for the Study of the Liver,20 and the American Gastroenterological Association (AGA).21
Older guidelines recommended screening only high-risk populations. But such screening has downfalls. It requires that patients or their physicians recognize that they are at high risk. In one study, nearly 65% of an infected Asian-American population was unaware of their positive HBV status.22 Risk-based screening also requires that physicians ask the appropriate questions and that patients admit to high-risk behavior. Screening patients based only on risk factors may easily overlook patients who need prophylaxis against HBVr.
Common arguments against universal screening include the cost of testing, the possibility of false-positive results, and the implications of a new diagnosis of hepatitis B. However, the potential benefits of screening are significant, and HBV screening in the general population has been shown to be cost effective when the prevalence of HBV is 0.3%.21 In the United States, conservative estimates are a prevalence of HBsAg positivity of 0.4% and past infection of 3%, making screening a cost-effective recommendation.16 It is therefore prudent to screen all patients before starting immunosuppressive therapy.
How to screen
All guidelines agree on how to test for HBV. Measuring levels of HBsAg and hepatitis B core antibody (anti-HBc total) allows the clinician to ascertain whether the patient’s HBV infection status is acute, chronic, or resolved (TABLE 223) and to perform HBVr risk stratification (discussed later).
Patients with acute infections should be referred to a hepatologist. With chronic or resolved HBV, stratify patients into a prophylaxis group or monitoring group (FIGURE14). Stratification involves identifying HBV status (chronic or resolved) and selecting a type of immunosuppressive therapy. Whether the patient falls into prophylaxis or monitoring, obtain a baseline level of viral DNA, as this has proven to be the best predictor of HBV reactivation.16
Continue to: In screening, be sure the appropriate...
In screening, be sure the appropriate anti-HBc testing is covered. Common usage of the term anti-HBc may refer to immunoglobulin G (IgG) or immunoglobulin M (IgM)or total core antibody, containing both IgG and IgM. But in this context, accurate screening requires either total core antibody or anti-HBc IgG. Anti-IgM alone is inadequate. Many commercial laboratories offer acute hepatitis panels or hepatitis profiles (TABLE 324,25), and it is important to confirm that such order sets contain the tests necessary to allow for risk stratification.
Testing for hepatitis B surface antibody (anti-HBs) is not useful in screening. Although it was hypothesized that the presence of this antibody lowered risk, recent studies have proven no change in risk based on this value.21
How to assess HBVr risk
Assessing risk for HBVr takes into account both the patient’s serology and intended treatment. Reddy et al delineated patient groups into high, moderate, and low risk (TABLES 4 and 5).21 The high-risk group was defined by anticipated incidence of HBVr in > 10% of cases; the moderate-risk group had an anticipated incidence of 1% to 10%; and the low-risk group had an anticipated incidence of <1%.21 Evidence was strongest in the high-risk group.
Patients with CHB (HBsAg positive and anti-HBc positive) are considered high risk for reactivation with a wide variety of immunosuppressive therapies. Such patients are 5 to 8 times more likely to develop HBVr than patients with an HBsAg-negative status signifying a resolved infection.16
Immunosuppressive agents and associated risks. The AGA guidelines consider treatment with B-cell-depleting agents, such as rituximab and ofatumumab, to be high risk, regardless of a patient’s surface antigen status. Additionally, for patients who are HBsAg positive, high-risk treatments include anthracycline derivatives, such as doxorubicin and epirubicin, or high- or moderate-dose steroids. These treatments are considered moderate risk when used in patients who have resolved HBV infection (HBsAg negative/anti-HBc positive). Moderate-risk modalities also include tumor necrosis factor inhibitors and tyrosine kinase inhibitors, regardless of surface antigen status; and low-dose steroids or cytokine or integrin inhibitors in HbsAg-positive individuals.21
Continue to: Other immunosuppression modalities...
Other immunosuppression modalities considered to be moderate risk independent of HBV serology include proteasome inhibitors, such as bortezomib, used for multiple myeloma treatment, and histone deacetylase inhibitors, such as romidepsin, used to treat T-cell lymphoma.13 Low-dose steroids or cytokine or integrin inhibitors are considered to be low risk in surface antigen-negative individuals; azathioprine, mercaptopurine, or methotrexate are low risk regardless of HBsAg status.21 Intra-articular steroid injections are considered extremely low risk in HbsAg-positive individuals, and are unclassified for HbsAg-negative individuals.13
More recent evidence has implicated other medication classes in triggering HBVr — (eg, direct-acting antivirals.)26
Prophylaxis options: High to moderate risk vs low risk
The consensus of major guideline issuers is to offer prophylaxis to high-risk patients and to monitor low-risk patients. The AGA additionally recommends prophylaxis for patients at moderate risk.
Controversy surrounding the moderate-risk group. Some authors argue that monitoring HBV DNA in the moderate-risk group is preferable to committing patients to long periods of prophylaxis, and that rescue treatment could be initiated as needed. However, the ideal monitoring period has not been determined, and the effectiveness of prophylaxis over monitoring is so significant that monitoring is losing favor.
Perrillo et al performed a meta-analysis of 5 randomized controlled trials evaluating antiviral agents vs no prophylaxis.16 The analysis included 139 patients receiving prophylaxis and 137 controls. The pooled results demonstrated an 87% relative risk reduction with prophylaxis, supporting the trend toward treating patients with moderate risk.16
Continue to: Prophylactic treatment options are safe...
Prophylactic treatment options are safe and well tolerated. For this reason, committing a high- or moderate-risk patient to a course of treatment should be less of a concern than the risk for HBVr.
In the early randomized controlled trials for HBVr prophylaxis, lamivudine, although effective, unfortunately led to a high incidence of viral resistance after prolonged use, thus diminishing its desirability.18 Newer agents, such as entecavir and tenofovir, have proven just as effective as lamivudine and are largely unaffected by viral resistance.27
In retrospective and prospective studies on HBVr prophylaxis, patients treated with entecavir had less HBV-related hepatitis, less delay in chemotherapy, and a lower rate of HBVr when compared with lamivudine.28,29 Tenofovir is recommended, however, if patients were previously treated with lamivudine.30
A recent meta-analysis demonstrated that tenofovir and entecavir are preferable to lamivudine in preventing HBVr.31
Looking ahead
Screening for HBsAg and anti-HBc total before starting immunosuppressive therapy can reduce morbidity and mortality in patients undergoing such treatment. The AGA recommends screening all patients about to begin high- or moderate-risk therapy or patients in populations with a prevalence of CHB ≥2%, per the CDC.6,21
Continue to: Classes of medications...
Classes of medications other than immunosuppressants may also trigger HBVr. The FDA has issued a warning regarding direct-acting antivirals, but optimal management of these patients is still evolving.
Once HBV status is established, a patient’s risk for HBVr can be specified as high, moderate, or low using their HBV status and the type of therapy being initiated. The AGA recommends prophylactic treatment with well-tolerated and effective agents for patients classified as high or moderate risk. If a patient’s risk is low, regular monitoring of HBV DNA and AST and ALT levels is sufficient. Recommendations of monitoring intervals span from monthly to every 3 months.13,14
CASE Given the patient’s status of resolved HBV infection and her current moderate-dose regimen of prednisone, her risk for HBV reactivation is moderate. She could either receive antiviral prophylaxis or undergo regular monitoring. Following a discussion of the options, she opts for referral to a hepatologist to discuss possible prophylactic treatment.
Increased awareness of HBVr risk associated with immunosuppressive therapy, coupled with a planned approach to appropriate screening and risk stratification, can help health care providers prevent the reactivation of HBV or initiate early intervention for CHB.
CORRESPONDENCE
Ronan Farrell, MD, Rhode Island Hospital, 593 Eddy Street, Providence, RI 02903; [email protected].
1. Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199-3202.
2. Day FL, Link E, Thursky K, et al. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract. 2011;7:141-147.
3. Paul S, Saxena A, Terrin N, et al. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Internal Med. 2016;164:30-40.
4. Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med. 2007;22:237-243.
5. Esteve M, Saro C, González-Huix F, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363-1365.
6. Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20.
7. Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62:1893-1908.
8, , , A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329-1339.
9. WHO. Hepatitis B. www.who.int/en/news-room/fact-sheets/detail/hepatitis-b. Accessed February 28, 2019.
10. Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422-433.
11. Foster T, Hon H, Kanwal F, et al. Screening high risk individuals for hepatitis B: physician knowledge, attitudes, and beliefs. Dig Dis Sci. 2011;56:3471-3487.
12. Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104-1108.
13. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297-1309.
14. Di Bisceglie AM, Lok AS, Martin P, et al. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703-711.
15. Lok AS, Ward JW, Perrillo RP, et al. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743-745.
16. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.
17. Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209-219.
18. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662.
19. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561.
20. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185.
21. Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-219.
, , . Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034-1040.
23. Hwang JP, Artz AS, Somerfield MR. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Oncol Pract. 2015;11:e487-489.
24. LabCorp. Hepatitis B core antibody, IgG, IgM, differentiation. www.labcorp.com/test-menu/27196/hepatitis-b-core-antibody-igg-igm-differentiation. Accessed February 28, 2019.
25. Quest diagnostics. Hepatitis B Core Antibody, Total. www.questdiagnostics.com/testcenter/TestDetail.action?ntc=501.Accessed November 5, 2018.
26. The Food and Drug Administration Adverse Event Reporting System (FAERS). www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed February 28, 2019.
27. Lim YS. Management of antiviral resistance in chronic hepatitis B. Gut Liver. 2017;11:189-195.
28. Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521-2530.
29. Chen WC, Cheng JS, Chiang PH, et al. A comparison of entecavir and lamivudine for the prophylaxis of hepatitis B virus reactivation in solid tumor patients undergoing systemic cytotoxic chemotherapy. PLoS One. 2015;10:e0131545.
30. Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514.
31. Zhang MY, Zhu GQ, Shi KQ, et al. Systematic review with network meta-analysis: comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642-30658.
1. Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199-3202.
2. Day FL, Link E, Thursky K, et al. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract. 2011;7:141-147.
3. Paul S, Saxena A, Terrin N, et al. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Internal Med. 2016;164:30-40.
4. Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med. 2007;22:237-243.
5. Esteve M, Saro C, González-Huix F, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363-1365.
6. Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20.
7. Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62:1893-1908.
8, , , A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329-1339.
9. WHO. Hepatitis B. www.who.int/en/news-room/fact-sheets/detail/hepatitis-b. Accessed February 28, 2019.
10. Kowdley KV, Wang CC, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422-433.
11. Foster T, Hon H, Kanwal F, et al. Screening high risk individuals for hepatitis B: physician knowledge, attitudes, and beliefs. Dig Dis Sci. 2011;56:3471-3487.
12. Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104-1108.
13. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297-1309.
14. Di Bisceglie AM, Lok AS, Martin P, et al. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703-711.
15. Lok AS, Ward JW, Perrillo RP, et al. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743-745.
16. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.
17. Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209-219.
18. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662.
19. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561.
20. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185.
21. Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-219.
, , . Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034-1040.
23. Hwang JP, Artz AS, Somerfield MR. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Oncol Pract. 2015;11:e487-489.
24. LabCorp. Hepatitis B core antibody, IgG, IgM, differentiation. www.labcorp.com/test-menu/27196/hepatitis-b-core-antibody-igg-igm-differentiation. Accessed February 28, 2019.
25. Quest diagnostics. Hepatitis B Core Antibody, Total. www.questdiagnostics.com/testcenter/TestDetail.action?ntc=501.Accessed November 5, 2018.
26. The Food and Drug Administration Adverse Event Reporting System (FAERS). www.fda.gov/Drugs/DrugSafety/ucm522932.htm. Accessed February 28, 2019.
27. Lim YS. Management of antiviral resistance in chronic hepatitis B. Gut Liver. 2017;11:189-195.
28. Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521-2530.
29. Chen WC, Cheng JS, Chiang PH, et al. A comparison of entecavir and lamivudine for the prophylaxis of hepatitis B virus reactivation in solid tumor patients undergoing systemic cytotoxic chemotherapy. PLoS One. 2015;10:e0131545.
30. Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514.
31. Zhang MY, Zhu GQ, Shi KQ, et al. Systematic review with network meta-analysis: comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642-30658.
PRACTICE RECOMMENDATIONS
› Measure levels of hepatitis B surface antigen and core antibody total. Although testing for IgG alone can be acceptable, testing for IgM alone is unacceptable. C
› Use both a patient’s serologic findings and the recognized risk associated with intended therapy to determine the threat of hepatitis B virus (HBV) reactivation. C
› Offer antiviral prophylaxis when risk for HBV reactivation is high. Consider prophylaxis or monitoring for those at moderate risk. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A systematic approach to chronic abnormal uterine bleeding
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).
CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).
CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).
CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
PRACTICE RECOMMENDATIONS
› Perform endometrial biopsy on all women who have abnormal uterine bleeding and risk factors for endometrial cancer and on all women ≥45 years, regardless of risk. C
› Initiate a workup for a coagulation disorder in women who are close to the onset of menarche and have a history of heavy menstrual bleeding. C
› Promote lifestyle changes and weight loss as primary treatments for polycystic ovary syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
COPD and asthma: Diagnostic accuracy requires spirometry
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; [email protected].
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; [email protected].
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; [email protected].
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
PRACTICE RECOMMENDATIONS
› Perform spirometry in all patients with symptoms and risk factors suggestive of chronic obstructive pulmonary disease (COPD) or asthma. B
› Consider having a patient use a peak flow meter to support a diagnosis of asthma if spirometry is unavailable. B
› Consider the possibility of a diagnostic error if COPD or asthma is unresponsive to treatment and the initial diagnosis was made without spirometry. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Attitudes of Women Toward the Gynecologic Examination
Neonatal hyperbilirubinemia: An evidence-based approach
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
PRACTICE RECOMMENDATIONS
› Diagnose hyperbilirubinemia in infants with bilirubin measured at >95th percentile for age in hours. Do not use visual assessment of jaundice for diagnosis as it may lead to errors. C
› Determine the threshold for initiation of phototherapy by applying serum bilirubin and age in hours to the American Academy of Pediatrics phototherapy nomogram along a risk curve assigned based on gestational age and neurotoxicity risk factors (not major and minor risk factors for severe hyperbilirubinemia). C
› Make arrangements to ensure that all infants are seen by a health care provider within 2 days of discharge (within 1 day if significant risk factors for development of severe hyperbilirubinemia are present). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The role of home BP monitoring: Answers to 10 common questions
National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014 revealed that 29% of adults in the United States have hypertension.1 Prevalence increases with age, so that 7% of adults ages 18 to 39 years, 32% of adults ages 40 to 59, and 65% of adults ages ≥60 years have the disease.1 This national survey data also showed that 53% of those given the diagnosis had uncontrolled hypertension, and that control of hypertension did not change significantly from 2009 to 2014.1
Elevated blood pressure (BP) has been the leading risk factor for death related to cardiovascular disease globally for the last 3 decades.2 Yet in 2 nationally representative samples, only 1 in 6 patients with documented BP ≥140/90 mm Hg received treatment intensification with new medication during primary care visits.3 Uncertainty about the representativeness of any single clinic BP measurement is a prominent reason for health care providers not to intensify therapy.4
Confirming the Dx outside the office. The 2015 US Preventive Services Task Force (USPSTF) guidelines on screening for hypertension state that, for most patients, a diagnosis of hypertension should be confirmed with out-of-office BP monitoring before initiating treatment.5 The USPSTF states that ambulatory BP monitoring (ABPM) is accurate for hypertension diagnosis and monitoring, and that home BP monitoring (HBPM) is an acceptable alternative, based on good quality evidence.
Access to ABPM, however, is often limited. In a 2015 survey of primary care clinics, only 25% of the 123 clinics that completed the questionnaire reported having access to it.6 Conversely, HBPM is widely available and acceptable to most patients. A recent NHANES survey showed that 43.5% of patients who were aware of their hypertension diagnosis engaged in HBPM.7
So what, exactly, should the role of HBPM be in the management of patients with hypertension? The evidence-based answers to the 10 questions that follow provide useful insights.
[polldaddy:10224678]
1. Can HBPM be used to confirm a Dx of hypertension?
Yes (Strength of recommendation [SOR] C).
In reviewing the diagnostic accuracy of various methods to confirm the diagnosis of hypertension, the USPSTF identified ABPM as the most accurate, followed by HBPM, with clinic BP measurements bringing up the rear.5 In adults ≥18 years of age, the USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment unless the patient’s BP is ≥180/110 mm Hg, there is evidence of end-organ damage, or the patient has a diagnosis of secondary hypertension.5 The USPSTF recommends HBPM as an acceptable alternative to ABPM based on 6 studies including a total of 1253 participants.8 The percentage of patients with elevated office BP confirmed by HBPM to have hypertension was 45% to 84% across these 6 studies.
Sixteen studies from another systematic review evaluated the diagnostic accuracy of HBPM while using ABPM as a reference.9 This review found that HBPM had high specificity and negative predictive value, but low sensitivity and positive predictive value. There was moderate diagnostic agreement between HBPM and ABPM, with kappa statistic values of 0.37 to 0.73 across all studies.9
Continue to: In yet another study...
In yet another study, home BP and ambulatory BP measurements were identical when the same dual-mode device was used to measure both ambulatory and home BP.10
2. What are the diagnostic and treatment targets for home BP monitoring?
Treat patients if home BP is ≥130/80 mm Hg and categorize patients as normotensive if home BP is <125/76 mm Hg (SOR C). Monitor patients who are in between.
A 2017 joint statement from the American College of Cardiology/American Heart Association (ACC/AHA) Task Force states that the target BP for HBPM should be <130/80 mm Hg.11 The Joint National Commission (JNC) 8 issued BP goals of <140/90 mm Hg for adults <60 years of age and those with diabetes and/or chronic kidney disease, and a goal of <150/90 mm Hg for adults ≥60 years of age with no diabetes or chronic kidney disease,12 but much debate has recently surrounded these guidelines. JNC 8 does not provide a separate BP goal for HBPM.
Although based solely on evidence (and not patient-oriented outcomes), a home BP threshold of ≥135/85 mm Hg for the diagnosis and treatment of hypertension has been supported by the European Society of Hypertension consensus guidelines,13 results of a longitudinal study,14 meta-analyses of published studies, and a meta-analysis using individual subject data.15
Support for a home BP measurement of <125/76 mm Hg as normal is limited to a single cross-sectional study of 48 patients with 2 elevated office BP readings where the threshold of 125/76 mm Hg on home BP was shown to exclude 80% of patients diagnosed with hypertension by ambulatory readings.16 If home BP measurements are >125/76 mm Hgbut <135/85 mm Hg, 24-hour ambulatory BP monitoring is recommended to assess hypertension control.17
3. Does home BP monitoring improve hypertension control?
Yes, in the short term, but not in the long term (SOR C).
A meta-analysis of 13 comparative studies looking at HBPM alone vs usual care showed a small, but statistically significant, benefit of achieving target BP at 6 months with a relative risk ratio (RRR) of 1.3 (95% confidence interval [CI], 1.00-1.68; I2=77%).18 However, the pooled effects from 3 studies that measured the benefit of achieving a predefined BP target at the 12-month follow-up mark were not significant in this review (RRR=1.18; 95% CI, 0.95-1.46, I2=86%).18 The pooled effect from 19 studies from the same review showed that there was a statistically significant weighted mean difference of -3.9 mm Hg in systolic BP and a weighted mean difference of -2.4 mm Hg in diastolic BP at 6 months; however, the changes were no longer significant at the 12-month follow-up mark.18
Continue to: More than half of the studies included...
More than half of the studies included in the meta-analysis were of low quality, and none of the studies recruited patients based on differences in clinic BP and home BP patterns, but rather on controlled or uncontrolled hypertension status. The studies included in this meta-analysis measured final BP outcomes by measuring ambulatory BP or clinic BP.
Another systematic review of 19 studies and 7100 participants looking at how HBPM compared with ABPM as a measurement standard for BP control and patient outcomes found insufficient data to determine the benefit of using HBPM as a measurement standard for BP control.19
HBPM + added support. There was high-quality evidence from the meta-analysis that HBPM plus additional support vs usual care led to a reduction in BP and a higher proportion of patients achieving target BP.18 However, the additional support interventions in the studies were heterogeneous.
4. Should HBPM be used to detect a change in BP associated with medication alterations?
Yes (SOR B).
A 2008 meta-analysis20 and several other studies21,22 showed that HBPM has greater accuracy than office BP for identifying drug-induced BP changes. The 2008 meta-analysis looked at changes in office and home BP measurements produced by various antihypertensive drugs. In 7 studies that compared office BP measurements with home and ambulatory BP measurements, the 24-hour ambulatory BP measurements and home BP measurements showed less dramatic BP reductions with medications than clinic BP measurements.20 This meta-analysis included 30 studies with 6794 participants and showed that home BP readings fell 20% less than office BP readings; the difference was statistically significant. These findings suggest that treatment-attributable changes in home BP and clinic BP measurements are linearly related, with the treatment effect on home BP measurements being around 80% of the effect on clinic BP measurements.
5. Do home BP measurements correlate with clinical outcomes?
Yes, and better than office BP measurements do; however, most studies comparing home BP measurements with usual care while looking at clinical outcomes are observational or quasi-experimental (SOR B).
For example, a 2015 systematic review looking at associations between BP measurement type (office, home, and ambulatory) and patient mortality found 5 observational studies that showed that adding home or ambulatory BP information improved cardiovascular risk prediction models. Moreover, all-cause mortality was associated with home BP and ambulatory BP levels only and not with office BP levels.19 The number of participants in these 5 studies varied between 210 and 2051 with study duration between 2.4 and 12.3 years. Of note, every study had a distinct population, affecting the generalizability of the results.
Continue to: One quasi-experimental study...
One quasi-experimental study with 450 participants showed that home BP measurements were at least as good as ambulatory BP measurements at predicting end organ damage related to hypertension when organ damage was measured by cardiac echocardiography, detection of microalbuminuria, and carotid echocardiography.23 Similarly, a systematic review of 14 studies and 2485 participants comparing home, ambulatory, and office BP readings showed that home BP measurements’ association with left ventricular mass index is as good as that of ambulatory BP measurements, and superior to clinic BP readings.24
6. Does HBPM help improve medication adherence?
The jury is still out on this one (SOR B).
A 2006 systematic review of randomized controlled trials (RCTs) incorporating HBPM and evaluating medication adherence outcomes found that in 6 of the 11 studies identified, there was some improvement in medication adherence with HBPM.25 However, only 1 of the 6 studies in this review involved HBPM as the sole intervention; the remaining 5 studies employed additional adherence-enhancing strategies.
Another systematic review looking at HBPM vs usual care included 8 studies (3 of moderate quality and 5 of low quality) that measured medication adherence (using varying measures of adherence) of which only 3 studies showed some improvement in medication adherence with HBPM.18
7. Does HBPM reduce therapeutic inertia?
Yes (SOR B).
A meta-analysis of 15 studies showed that therapeutic inertia was less common with HBPM than with office BP monitoring alone; the relative risk for unchanged medication was 0.82 (95% CI, 0.68 to 0.99) with HBPM.26 However, 10 of the 15 studies were of low quality with a Jadad score ≤3.
8. Does HBPM, along with titration of treatment, improve BP outcomes?
Yes (SOR B).
Two RCTs that looked at self-monitoring of BP and self-titration of hypertensive medications showed significant reductions in BP levels.27,28 In a cluster RCT of home BP telemonitoring, in which the pharmacist adjusted antihypertensives based on transmitted BP measurements, hypertension control was significantly better in the intervention group than in the usual care group (57.2% vs 30%).29
Continue to: What are the recommended techniques for HBPM?
9. What are the recommended techniques for HBP
Patients should use a device that is validated, fully automated, and has an upper arm cuff (not a wrist monitor), according to a joint statement from the AHA, the American Society of Hypertension, and the Preventive Cardiovascular Nurses Association.17 (SOR C). (See validated BP monitor list at http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.)
Patients should measure their BP in their nondominant arm after 5 minutes of rest with the arm at heart level, back supported, and feet flat on the ground. Patient technique and the accuracy of the home BP monitor should be checked annually. It is also recommended that patients check their BP 2 to 3 times every morning and evening. An average of 12 morning and evening measurements should be used for monitoring and treatment changes. An AHA informational sheet that shows how to measure BP properly can be found on their Web si
Several studies examining the accuracy of measuring BP over clothing did not find significant differences in BP measurements performed on a bare arm vs over a sleeve.30-33
10. What are the predictors of differences between home and office BP measurements?
Gender is one of the biggest predictors (SOR B).
A 2016 meta-analysis reported a total of 60 different hypothesized predictors of differences between home and clinic BP measurements (eg, gender, age, body mass index, systolic BP, diastolic BP). Masked hypertension was defined as a normal clinic BP reading and an elevated home BP reading. White coat hypertension was defined as an elevated clinic BP measurement with an acceptable home BP measurement. The researchers extracted odds ratios (ORs) for each study describing the association between patient characteristics and white coat or masked hypertension.34 Studies of masked hypertension diagnosed from HBPM showed male gender as the most significant predictor of home-clinic BP differences (OR=1.47, 95% CI, 1.18-1.75). In contrast, female gender was the only significant predictor of white coat hypertension (OR=3.38; 95% CI, 1.64-6.96) when comparing home BP with clinic BP measurements
Literature limitations and barriers to greater implementation
Most studies looking at HBPM outcomes have measured outcomes using ABPM or office BP measurements. The authors of studies using office BP as the outcome measure usually performed multiple BP measurements at often multiple office or clinic visits to calculate the true BP—a procedure that primary care practices rarely follow.35 Additionally, there are significant methodologic differences in HBPM and ABPM; home BP is measured at rest, while ambulatory BP is measured while the patient is mobile and functioning. There are insufficient prospective studies looking at HBPM effects on clinical and patient-oriented outcomes.
The evidence clearly supports using HBPM in the diagnosis of hypertension and suggests its benefit in hypertension management. However, there are significant barriers to incorporating HBPM into practice—barriers that are largely unaddressed in the literature.
For HBPM to be successful, patients need affordable validated home BP monitors covered by insurance that can translate home BP readings into usable information. Additional administrative and/or nursing assistance is required for patient education and support. Uploaded data need to be summarized in a way that is actionable and linked to the electronic health record.
Continue to: As the volume of patient-generated home date increases...
As the volume of patient-generated home data increases, there is a risk of information overload. Thus, meaningful summarization of the data is required to enable the physician, patient, and/or pharmacist to take prompt and effective action.
CORRESPONDENCE
Sonal J. Patil, MD, MSPH, Curtis W. and Ann H. Long Department of Family and Community Medicine, University of Missouri, MA306 Medical Sciences Building, DC032.00 Columbia, MO 65212; [email protected].
ACKNOWLEDGEMENTS
The authors are grateful to Dr. David R. Mehr for his valuable expertise in editing and proofreading this manuscript.
1. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief. 2015;(220):1-8.
2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634-647.
3. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188.
4. Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717-727.
5. U.S. Preventive Services Task Force. Final recommendation statement: High blood pressure in adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed July 19, 2017.
6. Woolsey S, Brown B, Ralls B, et al. Diagnosing hypertension in primary care clinics according to current guidelines. J Am Board Fam Med. 2017;30:170-177.
7. Ostchega Y, Zhang G, Kit BK, et al. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011-2014. Am J Hypertens. 2017;30:1126-1132.
8. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
9. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123-134.
10. Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2009;24:207-212.
11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13-e115.
12. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. Erratum in JAMA. 2014;311:1809.
13. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848.
14. Tsunoda S, Kawano Y, Horio T, et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167-173.
15. Staessen JA, Thijs L. Development of diagnostic thresholds for automated self-measurement of blood pressure in adults. First International Consensus Conference on Blood Pressure Self-Measurement. Blood Press Monit. 2000;5:101-109.
16. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017-1022.
17. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008;23:299-323.
18. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185-194.
19. Breaux-Shropshire TL, Judd E, Vucovich LA, et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43-49.
20. Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856-864.
21. Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179-185.
22. Vaur L, Dubroca I, Dutrey-Dupagne C, et al. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3:107-114.
23. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
24. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299.
25. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8:174-180.
26. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38.
27. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799-808.
28. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163-172.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: The HyperLink Cluster Randomized Trial. JAMA. 2013;310:46-56.
30. Ma G, Sabin N, Dawes M. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ. 2008;178:585-589.
31. Kahan E, Yaphe J, Knaani-Levinz H, et al. Comparison of blood pressure measurements on the bare arm, below a rolled-up sleeve, or over a sleeve. Fam Pract. 2003;20:730-732.
32. Liebl M, Holzgreve H, Schulz M, et al. The effect of clothes on sphygmomanometric and oscillometric blood pressure measurement. Blood Press. 2004;13:279-282.
33. Holleman DR Jr., Westman EC, McCrory DC, et al. The effect of sleeved arms on oscillometric blood pressure measurement. J Gen Intern Med. 1993;8:325-326.
34. Sheppard JP, Fletcher B, Gill P, et al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens. 2016;29:614-625.
35. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745-1751.
National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014 revealed that 29% of adults in the United States have hypertension.1 Prevalence increases with age, so that 7% of adults ages 18 to 39 years, 32% of adults ages 40 to 59, and 65% of adults ages ≥60 years have the disease.1 This national survey data also showed that 53% of those given the diagnosis had uncontrolled hypertension, and that control of hypertension did not change significantly from 2009 to 2014.1
Elevated blood pressure (BP) has been the leading risk factor for death related to cardiovascular disease globally for the last 3 decades.2 Yet in 2 nationally representative samples, only 1 in 6 patients with documented BP ≥140/90 mm Hg received treatment intensification with new medication during primary care visits.3 Uncertainty about the representativeness of any single clinic BP measurement is a prominent reason for health care providers not to intensify therapy.4
Confirming the Dx outside the office. The 2015 US Preventive Services Task Force (USPSTF) guidelines on screening for hypertension state that, for most patients, a diagnosis of hypertension should be confirmed with out-of-office BP monitoring before initiating treatment.5 The USPSTF states that ambulatory BP monitoring (ABPM) is accurate for hypertension diagnosis and monitoring, and that home BP monitoring (HBPM) is an acceptable alternative, based on good quality evidence.
Access to ABPM, however, is often limited. In a 2015 survey of primary care clinics, only 25% of the 123 clinics that completed the questionnaire reported having access to it.6 Conversely, HBPM is widely available and acceptable to most patients. A recent NHANES survey showed that 43.5% of patients who were aware of their hypertension diagnosis engaged in HBPM.7
So what, exactly, should the role of HBPM be in the management of patients with hypertension? The evidence-based answers to the 10 questions that follow provide useful insights.
[polldaddy:10224678]
1. Can HBPM be used to confirm a Dx of hypertension?
Yes (Strength of recommendation [SOR] C).
In reviewing the diagnostic accuracy of various methods to confirm the diagnosis of hypertension, the USPSTF identified ABPM as the most accurate, followed by HBPM, with clinic BP measurements bringing up the rear.5 In adults ≥18 years of age, the USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment unless the patient’s BP is ≥180/110 mm Hg, there is evidence of end-organ damage, or the patient has a diagnosis of secondary hypertension.5 The USPSTF recommends HBPM as an acceptable alternative to ABPM based on 6 studies including a total of 1253 participants.8 The percentage of patients with elevated office BP confirmed by HBPM to have hypertension was 45% to 84% across these 6 studies.
Sixteen studies from another systematic review evaluated the diagnostic accuracy of HBPM while using ABPM as a reference.9 This review found that HBPM had high specificity and negative predictive value, but low sensitivity and positive predictive value. There was moderate diagnostic agreement between HBPM and ABPM, with kappa statistic values of 0.37 to 0.73 across all studies.9
Continue to: In yet another study...
In yet another study, home BP and ambulatory BP measurements were identical when the same dual-mode device was used to measure both ambulatory and home BP.10
2. What are the diagnostic and treatment targets for home BP monitoring?
Treat patients if home BP is ≥130/80 mm Hg and categorize patients as normotensive if home BP is <125/76 mm Hg (SOR C). Monitor patients who are in between.
A 2017 joint statement from the American College of Cardiology/American Heart Association (ACC/AHA) Task Force states that the target BP for HBPM should be <130/80 mm Hg.11 The Joint National Commission (JNC) 8 issued BP goals of <140/90 mm Hg for adults <60 years of age and those with diabetes and/or chronic kidney disease, and a goal of <150/90 mm Hg for adults ≥60 years of age with no diabetes or chronic kidney disease,12 but much debate has recently surrounded these guidelines. JNC 8 does not provide a separate BP goal for HBPM.
Although based solely on evidence (and not patient-oriented outcomes), a home BP threshold of ≥135/85 mm Hg for the diagnosis and treatment of hypertension has been supported by the European Society of Hypertension consensus guidelines,13 results of a longitudinal study,14 meta-analyses of published studies, and a meta-analysis using individual subject data.15
Support for a home BP measurement of <125/76 mm Hg as normal is limited to a single cross-sectional study of 48 patients with 2 elevated office BP readings where the threshold of 125/76 mm Hg on home BP was shown to exclude 80% of patients diagnosed with hypertension by ambulatory readings.16 If home BP measurements are >125/76 mm Hgbut <135/85 mm Hg, 24-hour ambulatory BP monitoring is recommended to assess hypertension control.17
3. Does home BP monitoring improve hypertension control?
Yes, in the short term, but not in the long term (SOR C).
A meta-analysis of 13 comparative studies looking at HBPM alone vs usual care showed a small, but statistically significant, benefit of achieving target BP at 6 months with a relative risk ratio (RRR) of 1.3 (95% confidence interval [CI], 1.00-1.68; I2=77%).18 However, the pooled effects from 3 studies that measured the benefit of achieving a predefined BP target at the 12-month follow-up mark were not significant in this review (RRR=1.18; 95% CI, 0.95-1.46, I2=86%).18 The pooled effect from 19 studies from the same review showed that there was a statistically significant weighted mean difference of -3.9 mm Hg in systolic BP and a weighted mean difference of -2.4 mm Hg in diastolic BP at 6 months; however, the changes were no longer significant at the 12-month follow-up mark.18
Continue to: More than half of the studies included...
More than half of the studies included in the meta-analysis were of low quality, and none of the studies recruited patients based on differences in clinic BP and home BP patterns, but rather on controlled or uncontrolled hypertension status. The studies included in this meta-analysis measured final BP outcomes by measuring ambulatory BP or clinic BP.
Another systematic review of 19 studies and 7100 participants looking at how HBPM compared with ABPM as a measurement standard for BP control and patient outcomes found insufficient data to determine the benefit of using HBPM as a measurement standard for BP control.19
HBPM + added support. There was high-quality evidence from the meta-analysis that HBPM plus additional support vs usual care led to a reduction in BP and a higher proportion of patients achieving target BP.18 However, the additional support interventions in the studies were heterogeneous.
4. Should HBPM be used to detect a change in BP associated with medication alterations?
Yes (SOR B).
A 2008 meta-analysis20 and several other studies21,22 showed that HBPM has greater accuracy than office BP for identifying drug-induced BP changes. The 2008 meta-analysis looked at changes in office and home BP measurements produced by various antihypertensive drugs. In 7 studies that compared office BP measurements with home and ambulatory BP measurements, the 24-hour ambulatory BP measurements and home BP measurements showed less dramatic BP reductions with medications than clinic BP measurements.20 This meta-analysis included 30 studies with 6794 participants and showed that home BP readings fell 20% less than office BP readings; the difference was statistically significant. These findings suggest that treatment-attributable changes in home BP and clinic BP measurements are linearly related, with the treatment effect on home BP measurements being around 80% of the effect on clinic BP measurements.
5. Do home BP measurements correlate with clinical outcomes?
Yes, and better than office BP measurements do; however, most studies comparing home BP measurements with usual care while looking at clinical outcomes are observational or quasi-experimental (SOR B).
For example, a 2015 systematic review looking at associations between BP measurement type (office, home, and ambulatory) and patient mortality found 5 observational studies that showed that adding home or ambulatory BP information improved cardiovascular risk prediction models. Moreover, all-cause mortality was associated with home BP and ambulatory BP levels only and not with office BP levels.19 The number of participants in these 5 studies varied between 210 and 2051 with study duration between 2.4 and 12.3 years. Of note, every study had a distinct population, affecting the generalizability of the results.
Continue to: One quasi-experimental study...
One quasi-experimental study with 450 participants showed that home BP measurements were at least as good as ambulatory BP measurements at predicting end organ damage related to hypertension when organ damage was measured by cardiac echocardiography, detection of microalbuminuria, and carotid echocardiography.23 Similarly, a systematic review of 14 studies and 2485 participants comparing home, ambulatory, and office BP readings showed that home BP measurements’ association with left ventricular mass index is as good as that of ambulatory BP measurements, and superior to clinic BP readings.24
6. Does HBPM help improve medication adherence?
The jury is still out on this one (SOR B).
A 2006 systematic review of randomized controlled trials (RCTs) incorporating HBPM and evaluating medication adherence outcomes found that in 6 of the 11 studies identified, there was some improvement in medication adherence with HBPM.25 However, only 1 of the 6 studies in this review involved HBPM as the sole intervention; the remaining 5 studies employed additional adherence-enhancing strategies.
Another systematic review looking at HBPM vs usual care included 8 studies (3 of moderate quality and 5 of low quality) that measured medication adherence (using varying measures of adherence) of which only 3 studies showed some improvement in medication adherence with HBPM.18
7. Does HBPM reduce therapeutic inertia?
Yes (SOR B).
A meta-analysis of 15 studies showed that therapeutic inertia was less common with HBPM than with office BP monitoring alone; the relative risk for unchanged medication was 0.82 (95% CI, 0.68 to 0.99) with HBPM.26 However, 10 of the 15 studies were of low quality with a Jadad score ≤3.
8. Does HBPM, along with titration of treatment, improve BP outcomes?
Yes (SOR B).
Two RCTs that looked at self-monitoring of BP and self-titration of hypertensive medications showed significant reductions in BP levels.27,28 In a cluster RCT of home BP telemonitoring, in which the pharmacist adjusted antihypertensives based on transmitted BP measurements, hypertension control was significantly better in the intervention group than in the usual care group (57.2% vs 30%).29
Continue to: What are the recommended techniques for HBPM?
9. What are the recommended techniques for HBP
Patients should use a device that is validated, fully automated, and has an upper arm cuff (not a wrist monitor), according to a joint statement from the AHA, the American Society of Hypertension, and the Preventive Cardiovascular Nurses Association.17 (SOR C). (See validated BP monitor list at http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.)
Patients should measure their BP in their nondominant arm after 5 minutes of rest with the arm at heart level, back supported, and feet flat on the ground. Patient technique and the accuracy of the home BP monitor should be checked annually. It is also recommended that patients check their BP 2 to 3 times every morning and evening. An average of 12 morning and evening measurements should be used for monitoring and treatment changes. An AHA informational sheet that shows how to measure BP properly can be found on their Web si
Several studies examining the accuracy of measuring BP over clothing did not find significant differences in BP measurements performed on a bare arm vs over a sleeve.30-33
10. What are the predictors of differences between home and office BP measurements?
Gender is one of the biggest predictors (SOR B).
A 2016 meta-analysis reported a total of 60 different hypothesized predictors of differences between home and clinic BP measurements (eg, gender, age, body mass index, systolic BP, diastolic BP). Masked hypertension was defined as a normal clinic BP reading and an elevated home BP reading. White coat hypertension was defined as an elevated clinic BP measurement with an acceptable home BP measurement. The researchers extracted odds ratios (ORs) for each study describing the association between patient characteristics and white coat or masked hypertension.34 Studies of masked hypertension diagnosed from HBPM showed male gender as the most significant predictor of home-clinic BP differences (OR=1.47, 95% CI, 1.18-1.75). In contrast, female gender was the only significant predictor of white coat hypertension (OR=3.38; 95% CI, 1.64-6.96) when comparing home BP with clinic BP measurements
Literature limitations and barriers to greater implementation
Most studies looking at HBPM outcomes have measured outcomes using ABPM or office BP measurements. The authors of studies using office BP as the outcome measure usually performed multiple BP measurements at often multiple office or clinic visits to calculate the true BP—a procedure that primary care practices rarely follow.35 Additionally, there are significant methodologic differences in HBPM and ABPM; home BP is measured at rest, while ambulatory BP is measured while the patient is mobile and functioning. There are insufficient prospective studies looking at HBPM effects on clinical and patient-oriented outcomes.
The evidence clearly supports using HBPM in the diagnosis of hypertension and suggests its benefit in hypertension management. However, there are significant barriers to incorporating HBPM into practice—barriers that are largely unaddressed in the literature.
For HBPM to be successful, patients need affordable validated home BP monitors covered by insurance that can translate home BP readings into usable information. Additional administrative and/or nursing assistance is required for patient education and support. Uploaded data need to be summarized in a way that is actionable and linked to the electronic health record.
Continue to: As the volume of patient-generated home date increases...
As the volume of patient-generated home data increases, there is a risk of information overload. Thus, meaningful summarization of the data is required to enable the physician, patient, and/or pharmacist to take prompt and effective action.
CORRESPONDENCE
Sonal J. Patil, MD, MSPH, Curtis W. and Ann H. Long Department of Family and Community Medicine, University of Missouri, MA306 Medical Sciences Building, DC032.00 Columbia, MO 65212; [email protected].
ACKNOWLEDGEMENTS
The authors are grateful to Dr. David R. Mehr for his valuable expertise in editing and proofreading this manuscript.
National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014 revealed that 29% of adults in the United States have hypertension.1 Prevalence increases with age, so that 7% of adults ages 18 to 39 years, 32% of adults ages 40 to 59, and 65% of adults ages ≥60 years have the disease.1 This national survey data also showed that 53% of those given the diagnosis had uncontrolled hypertension, and that control of hypertension did not change significantly from 2009 to 2014.1
Elevated blood pressure (BP) has been the leading risk factor for death related to cardiovascular disease globally for the last 3 decades.2 Yet in 2 nationally representative samples, only 1 in 6 patients with documented BP ≥140/90 mm Hg received treatment intensification with new medication during primary care visits.3 Uncertainty about the representativeness of any single clinic BP measurement is a prominent reason for health care providers not to intensify therapy.4
Confirming the Dx outside the office. The 2015 US Preventive Services Task Force (USPSTF) guidelines on screening for hypertension state that, for most patients, a diagnosis of hypertension should be confirmed with out-of-office BP monitoring before initiating treatment.5 The USPSTF states that ambulatory BP monitoring (ABPM) is accurate for hypertension diagnosis and monitoring, and that home BP monitoring (HBPM) is an acceptable alternative, based on good quality evidence.
Access to ABPM, however, is often limited. In a 2015 survey of primary care clinics, only 25% of the 123 clinics that completed the questionnaire reported having access to it.6 Conversely, HBPM is widely available and acceptable to most patients. A recent NHANES survey showed that 43.5% of patients who were aware of their hypertension diagnosis engaged in HBPM.7
So what, exactly, should the role of HBPM be in the management of patients with hypertension? The evidence-based answers to the 10 questions that follow provide useful insights.
[polldaddy:10224678]
1. Can HBPM be used to confirm a Dx of hypertension?
Yes (Strength of recommendation [SOR] C).
In reviewing the diagnostic accuracy of various methods to confirm the diagnosis of hypertension, the USPSTF identified ABPM as the most accurate, followed by HBPM, with clinic BP measurements bringing up the rear.5 In adults ≥18 years of age, the USPSTF recommends obtaining BP measurements outside of the clinical setting for diagnostic confirmation before starting treatment unless the patient’s BP is ≥180/110 mm Hg, there is evidence of end-organ damage, or the patient has a diagnosis of secondary hypertension.5 The USPSTF recommends HBPM as an acceptable alternative to ABPM based on 6 studies including a total of 1253 participants.8 The percentage of patients with elevated office BP confirmed by HBPM to have hypertension was 45% to 84% across these 6 studies.
Sixteen studies from another systematic review evaluated the diagnostic accuracy of HBPM while using ABPM as a reference.9 This review found that HBPM had high specificity and negative predictive value, but low sensitivity and positive predictive value. There was moderate diagnostic agreement between HBPM and ABPM, with kappa statistic values of 0.37 to 0.73 across all studies.9
Continue to: In yet another study...
In yet another study, home BP and ambulatory BP measurements were identical when the same dual-mode device was used to measure both ambulatory and home BP.10
2. What are the diagnostic and treatment targets for home BP monitoring?
Treat patients if home BP is ≥130/80 mm Hg and categorize patients as normotensive if home BP is <125/76 mm Hg (SOR C). Monitor patients who are in between.
A 2017 joint statement from the American College of Cardiology/American Heart Association (ACC/AHA) Task Force states that the target BP for HBPM should be <130/80 mm Hg.11 The Joint National Commission (JNC) 8 issued BP goals of <140/90 mm Hg for adults <60 years of age and those with diabetes and/or chronic kidney disease, and a goal of <150/90 mm Hg for adults ≥60 years of age with no diabetes or chronic kidney disease,12 but much debate has recently surrounded these guidelines. JNC 8 does not provide a separate BP goal for HBPM.
Although based solely on evidence (and not patient-oriented outcomes), a home BP threshold of ≥135/85 mm Hg for the diagnosis and treatment of hypertension has been supported by the European Society of Hypertension consensus guidelines,13 results of a longitudinal study,14 meta-analyses of published studies, and a meta-analysis using individual subject data.15
Support for a home BP measurement of <125/76 mm Hg as normal is limited to a single cross-sectional study of 48 patients with 2 elevated office BP readings where the threshold of 125/76 mm Hg on home BP was shown to exclude 80% of patients diagnosed with hypertension by ambulatory readings.16 If home BP measurements are >125/76 mm Hgbut <135/85 mm Hg, 24-hour ambulatory BP monitoring is recommended to assess hypertension control.17
3. Does home BP monitoring improve hypertension control?
Yes, in the short term, but not in the long term (SOR C).
A meta-analysis of 13 comparative studies looking at HBPM alone vs usual care showed a small, but statistically significant, benefit of achieving target BP at 6 months with a relative risk ratio (RRR) of 1.3 (95% confidence interval [CI], 1.00-1.68; I2=77%).18 However, the pooled effects from 3 studies that measured the benefit of achieving a predefined BP target at the 12-month follow-up mark were not significant in this review (RRR=1.18; 95% CI, 0.95-1.46, I2=86%).18 The pooled effect from 19 studies from the same review showed that there was a statistically significant weighted mean difference of -3.9 mm Hg in systolic BP and a weighted mean difference of -2.4 mm Hg in diastolic BP at 6 months; however, the changes were no longer significant at the 12-month follow-up mark.18
Continue to: More than half of the studies included...
More than half of the studies included in the meta-analysis were of low quality, and none of the studies recruited patients based on differences in clinic BP and home BP patterns, but rather on controlled or uncontrolled hypertension status. The studies included in this meta-analysis measured final BP outcomes by measuring ambulatory BP or clinic BP.
Another systematic review of 19 studies and 7100 participants looking at how HBPM compared with ABPM as a measurement standard for BP control and patient outcomes found insufficient data to determine the benefit of using HBPM as a measurement standard for BP control.19
HBPM + added support. There was high-quality evidence from the meta-analysis that HBPM plus additional support vs usual care led to a reduction in BP and a higher proportion of patients achieving target BP.18 However, the additional support interventions in the studies were heterogeneous.
4. Should HBPM be used to detect a change in BP associated with medication alterations?
Yes (SOR B).
A 2008 meta-analysis20 and several other studies21,22 showed that HBPM has greater accuracy than office BP for identifying drug-induced BP changes. The 2008 meta-analysis looked at changes in office and home BP measurements produced by various antihypertensive drugs. In 7 studies that compared office BP measurements with home and ambulatory BP measurements, the 24-hour ambulatory BP measurements and home BP measurements showed less dramatic BP reductions with medications than clinic BP measurements.20 This meta-analysis included 30 studies with 6794 participants and showed that home BP readings fell 20% less than office BP readings; the difference was statistically significant. These findings suggest that treatment-attributable changes in home BP and clinic BP measurements are linearly related, with the treatment effect on home BP measurements being around 80% of the effect on clinic BP measurements.
5. Do home BP measurements correlate with clinical outcomes?
Yes, and better than office BP measurements do; however, most studies comparing home BP measurements with usual care while looking at clinical outcomes are observational or quasi-experimental (SOR B).
For example, a 2015 systematic review looking at associations between BP measurement type (office, home, and ambulatory) and patient mortality found 5 observational studies that showed that adding home or ambulatory BP information improved cardiovascular risk prediction models. Moreover, all-cause mortality was associated with home BP and ambulatory BP levels only and not with office BP levels.19 The number of participants in these 5 studies varied between 210 and 2051 with study duration between 2.4 and 12.3 years. Of note, every study had a distinct population, affecting the generalizability of the results.
Continue to: One quasi-experimental study...
One quasi-experimental study with 450 participants showed that home BP measurements were at least as good as ambulatory BP measurements at predicting end organ damage related to hypertension when organ damage was measured by cardiac echocardiography, detection of microalbuminuria, and carotid echocardiography.23 Similarly, a systematic review of 14 studies and 2485 participants comparing home, ambulatory, and office BP readings showed that home BP measurements’ association with left ventricular mass index is as good as that of ambulatory BP measurements, and superior to clinic BP readings.24
6. Does HBPM help improve medication adherence?
The jury is still out on this one (SOR B).
A 2006 systematic review of randomized controlled trials (RCTs) incorporating HBPM and evaluating medication adherence outcomes found that in 6 of the 11 studies identified, there was some improvement in medication adherence with HBPM.25 However, only 1 of the 6 studies in this review involved HBPM as the sole intervention; the remaining 5 studies employed additional adherence-enhancing strategies.
Another systematic review looking at HBPM vs usual care included 8 studies (3 of moderate quality and 5 of low quality) that measured medication adherence (using varying measures of adherence) of which only 3 studies showed some improvement in medication adherence with HBPM.18
7. Does HBPM reduce therapeutic inertia?
Yes (SOR B).
A meta-analysis of 15 studies showed that therapeutic inertia was less common with HBPM than with office BP monitoring alone; the relative risk for unchanged medication was 0.82 (95% CI, 0.68 to 0.99) with HBPM.26 However, 10 of the 15 studies were of low quality with a Jadad score ≤3.
8. Does HBPM, along with titration of treatment, improve BP outcomes?
Yes (SOR B).
Two RCTs that looked at self-monitoring of BP and self-titration of hypertensive medications showed significant reductions in BP levels.27,28 In a cluster RCT of home BP telemonitoring, in which the pharmacist adjusted antihypertensives based on transmitted BP measurements, hypertension control was significantly better in the intervention group than in the usual care group (57.2% vs 30%).29
Continue to: What are the recommended techniques for HBPM?
9. What are the recommended techniques for HBP
Patients should use a device that is validated, fully automated, and has an upper arm cuff (not a wrist monitor), according to a joint statement from the AHA, the American Society of Hypertension, and the Preventive Cardiovascular Nurses Association.17 (SOR C). (See validated BP monitor list at http://www.dableducational.org/sphygmomanometers/devices_2_sbpm.html.)
Patients should measure their BP in their nondominant arm after 5 minutes of rest with the arm at heart level, back supported, and feet flat on the ground. Patient technique and the accuracy of the home BP monitor should be checked annually. It is also recommended that patients check their BP 2 to 3 times every morning and evening. An average of 12 morning and evening measurements should be used for monitoring and treatment changes. An AHA informational sheet that shows how to measure BP properly can be found on their Web si
Several studies examining the accuracy of measuring BP over clothing did not find significant differences in BP measurements performed on a bare arm vs over a sleeve.30-33
10. What are the predictors of differences between home and office BP measurements?
Gender is one of the biggest predictors (SOR B).
A 2016 meta-analysis reported a total of 60 different hypothesized predictors of differences between home and clinic BP measurements (eg, gender, age, body mass index, systolic BP, diastolic BP). Masked hypertension was defined as a normal clinic BP reading and an elevated home BP reading. White coat hypertension was defined as an elevated clinic BP measurement with an acceptable home BP measurement. The researchers extracted odds ratios (ORs) for each study describing the association between patient characteristics and white coat or masked hypertension.34 Studies of masked hypertension diagnosed from HBPM showed male gender as the most significant predictor of home-clinic BP differences (OR=1.47, 95% CI, 1.18-1.75). In contrast, female gender was the only significant predictor of white coat hypertension (OR=3.38; 95% CI, 1.64-6.96) when comparing home BP with clinic BP measurements
Literature limitations and barriers to greater implementation
Most studies looking at HBPM outcomes have measured outcomes using ABPM or office BP measurements. The authors of studies using office BP as the outcome measure usually performed multiple BP measurements at often multiple office or clinic visits to calculate the true BP—a procedure that primary care practices rarely follow.35 Additionally, there are significant methodologic differences in HBPM and ABPM; home BP is measured at rest, while ambulatory BP is measured while the patient is mobile and functioning. There are insufficient prospective studies looking at HBPM effects on clinical and patient-oriented outcomes.
The evidence clearly supports using HBPM in the diagnosis of hypertension and suggests its benefit in hypertension management. However, there are significant barriers to incorporating HBPM into practice—barriers that are largely unaddressed in the literature.
For HBPM to be successful, patients need affordable validated home BP monitors covered by insurance that can translate home BP readings into usable information. Additional administrative and/or nursing assistance is required for patient education and support. Uploaded data need to be summarized in a way that is actionable and linked to the electronic health record.
Continue to: As the volume of patient-generated home date increases...
As the volume of patient-generated home data increases, there is a risk of information overload. Thus, meaningful summarization of the data is required to enable the physician, patient, and/or pharmacist to take prompt and effective action.
CORRESPONDENCE
Sonal J. Patil, MD, MSPH, Curtis W. and Ann H. Long Department of Family and Community Medicine, University of Missouri, MA306 Medical Sciences Building, DC032.00 Columbia, MO 65212; [email protected].
ACKNOWLEDGEMENTS
The authors are grateful to Dr. David R. Mehr for his valuable expertise in editing and proofreading this manuscript.
1. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief. 2015;(220):1-8.
2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634-647.
3. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188.
4. Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717-727.
5. U.S. Preventive Services Task Force. Final recommendation statement: High blood pressure in adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed July 19, 2017.
6. Woolsey S, Brown B, Ralls B, et al. Diagnosing hypertension in primary care clinics according to current guidelines. J Am Board Fam Med. 2017;30:170-177.
7. Ostchega Y, Zhang G, Kit BK, et al. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011-2014. Am J Hypertens. 2017;30:1126-1132.
8. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
9. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123-134.
10. Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2009;24:207-212.
11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13-e115.
12. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. Erratum in JAMA. 2014;311:1809.
13. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848.
14. Tsunoda S, Kawano Y, Horio T, et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167-173.
15. Staessen JA, Thijs L. Development of diagnostic thresholds for automated self-measurement of blood pressure in adults. First International Consensus Conference on Blood Pressure Self-Measurement. Blood Press Monit. 2000;5:101-109.
16. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017-1022.
17. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008;23:299-323.
18. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185-194.
19. Breaux-Shropshire TL, Judd E, Vucovich LA, et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43-49.
20. Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856-864.
21. Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179-185.
22. Vaur L, Dubroca I, Dutrey-Dupagne C, et al. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3:107-114.
23. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
24. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299.
25. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8:174-180.
26. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38.
27. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799-808.
28. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163-172.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: The HyperLink Cluster Randomized Trial. JAMA. 2013;310:46-56.
30. Ma G, Sabin N, Dawes M. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ. 2008;178:585-589.
31. Kahan E, Yaphe J, Knaani-Levinz H, et al. Comparison of blood pressure measurements on the bare arm, below a rolled-up sleeve, or over a sleeve. Fam Pract. 2003;20:730-732.
32. Liebl M, Holzgreve H, Schulz M, et al. The effect of clothes on sphygmomanometric and oscillometric blood pressure measurement. Blood Press. 2004;13:279-282.
33. Holleman DR Jr., Westman EC, McCrory DC, et al. The effect of sleeved arms on oscillometric blood pressure measurement. J Gen Intern Med. 1993;8:325-326.
34. Sheppard JP, Fletcher B, Gill P, et al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens. 2016;29:614-625.
35. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745-1751.
1. Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011-2014. NCHS Data Brief. 2015;(220):1-8.
2. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634-647.
3. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188.
4. Kerr EA, Zikmund-Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717-727.
5. U.S. Preventive Services Task Force. Final recommendation statement: High blood pressure in adults: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed July 19, 2017.
6. Woolsey S, Brown B, Ralls B, et al. Diagnosing hypertension in primary care clinics according to current guidelines. J Am Board Fam Med. 2017;30:170-177.
7. Ostchega Y, Zhang G, Kit BK, et al. Factors associated with home blood pressure monitoring among US adults: National Health and Nutrition Examination Survey, 2011-2014. Am J Hypertens. 2017;30:1126-1132.
8. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
9. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011;24:123-134.
10. Stergiou GS, Tzamouranis D, Nasothimiou EG, et al. Are there really differences between home and daytime ambulatory blood pressure? Comparison using a novel dual-mode ambulatory and home monitor. J Hum Hypertens. 2009;24:207-212.
11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:e13-e115.
12. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. Erratum in JAMA. 2014;311:1809.
13. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-848.
14. Tsunoda S, Kawano Y, Horio T, et al. Relationship between home blood pressure and longitudinal changes in target organ damage in treated hypertensive patients. Hypertens Res. 2002;25:167-173.
15. Staessen JA, Thijs L. Development of diagnostic thresholds for automated self-measurement of blood pressure in adults. First International Consensus Conference on Blood Pressure Self-Measurement. Blood Press Monit. 2000;5:101-109.
16. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017-1022.
17. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008;23:299-323.
18. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185-194.
19. Breaux-Shropshire TL, Judd E, Vucovich LA, et al. Does home blood pressure monitoring improve patient outcomes? A systematic review comparing home and ambulatory blood pressure monitoring on blood pressure control and patient outcomes. Integr Blood Press Control. 2015;8:43-49.
20. Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52:856-864.
21. Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179-185.
22. Vaur L, Dubroca I, Dutrey-Dupagne C, et al. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3:107-114.
23. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
24. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299.
25. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006;8:174-180.
26. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38.
27. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799-808.
28. McManus RJ, Mant J, Bray EP, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163-172.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: The HyperLink Cluster Randomized Trial. JAMA. 2013;310:46-56.
30. Ma G, Sabin N, Dawes M. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ. 2008;178:585-589.
31. Kahan E, Yaphe J, Knaani-Levinz H, et al. Comparison of blood pressure measurements on the bare arm, below a rolled-up sleeve, or over a sleeve. Fam Pract. 2003;20:730-732.
32. Liebl M, Holzgreve H, Schulz M, et al. The effect of clothes on sphygmomanometric and oscillometric blood pressure measurement. Blood Press. 2004;13:279-282.
33. Holleman DR Jr., Westman EC, McCrory DC, et al. The effect of sleeved arms on oscillometric blood pressure measurement. J Gen Intern Med. 1993;8:325-326.
34. Sheppard JP, Fletcher B, Gill P, et al. Predictors of the home-clinic blood pressure difference: a systematic review and meta-analysis. Am J Hypertens. 2016;29:614-625.
35. Stergiou GS, Skeva II, Baibas NM, et al. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18:1745-1751.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Migraine: Expanding our Tx arsenal
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
PRACTICE RECOMMENDATIONS
› Offer treatment with a triptan to adult patients with moderate-to-severe episodic migraine. A
› Consider prescribing topiramate, divalproex sodium, metoprolol, propranolol, or the herbal, Petasites hybridum, for the prevention of recurrent episodic migraine that has not responded to a reduction in headache triggers. A
› Add onabotulinumtoxinA injection to your therapeutic toolbox as an effective preventive treatment for chronic migraine (≥15 headache days a month for 3 months). B
› Recommend magnesium and feverfew as adjunctive preventive treatments for migraine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series