User login
Epistaxis: A guide to assessment and management
Epistaxis is a common presenting complaint in family medicine. Successful treatment requires knowledge of nasal anatomy, possible causes, and a step-wise approach.
Epistaxis predominantly affects children between the ages of 2 and 10 years and older adults between the ages of 45 and 65.1-4 Many presentations are spontaneous and self-limiting; often all that is required is proper first aid. It is important, however, to recognize the signs and symptoms that are suggestive of more worrisome conditions.
Management of epistaxis requires good preparation, appropriate equipment, and adequate assistance. If any of these are lacking, prompt nasal packing followed by referral to an emergency department or ear, nose, and throat (ENT) service is recommended.
Anatomy of the nasal cavity
The nasal cavity has a rich and highly varied blood supply arising from the internal and external carotid arteries with multiple anastomoses and a crossover between the left and right arterial systems.2,4,5 The internal maxillary artery (IMAX) supplies 80% of the nasal vault.2 The sphenopalatine artery (SPA) supplies most of the nasal septum and the turbinates, while the greater palatine artery (GPA) supplies the floor of the nasal septum.3,5 The ethmoidal arteries course through the cribriform plate to supply the roof of the nasal cavity. The ethmoidal arteries communicates with branches of the SPA posteriorly and several branches anteriorly (FIGURE 1).
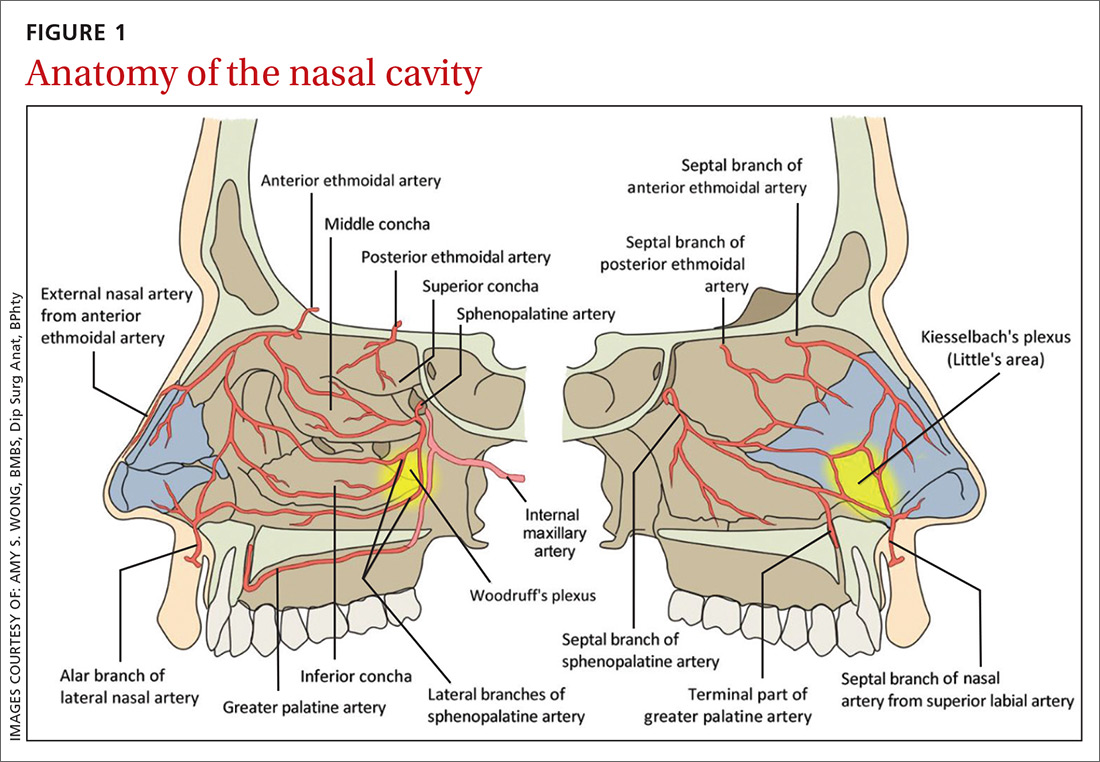
Kiesselbach’s plexus is a highly vascularized region of cartilaginous nasal septum anteroinferiorly that is also known as Little’s area. It is supplied by the SPA, GPA, superior labial artery, and ethmoidal arteries.5 Woodruff’s plexus is the richly vascularized posterior aspect of the nasal cavity primarily supplied by the SPA.3,5
Is the bleed anterior or posterior; primary or secondary?
Epistaxis is classified as anterior or posterior based on the arterial supply and the location of the bleed in relation to the piriform aperture.2,3 Anterior epistaxis occurs in >90% of patients and arises in Little’s area.6 Posterior epistaxis arises from Woodruff’s plexus in the posterior nasal septum or lateral nasal wall. It occurs in 5% to 10% of patients, is usually arterial in origin, and leads to a greater risk of airway compromise, aspiration, and difficulty in controlling the hemorrhage.2,6
Epistaxis can be classified further as primary or secondary hemorrhage. Primary epistaxis is idiopathic, spontaneous bleeds without any precipitants.2 Blood vessels within the nasal mucosa run superficially and are relatively unprotected. Damage to this mucosa and to vessel walls can result in bleeding.4 Spontaneous rupture of vessels may occur occasionally, during, say the Valsalva maneuver or when straining to lift heavy objects.4 Secondary epistaxis occurs when there is a clear and definite cause (eg trauma, anticoagulant use, or surgery).
Continue to: Numerous causes...
Numerous causes: From trauma to medications
Epistaxis can be caused by local, systemic, or environmental factors; medications; or be idiopathic in nature (TABLE 12). It commonly arises due to self-inflicted trauma from nose picking, particularly in children; trauma to nasal bones or septum; and mucosal irritation from topical nasal drugs, such as corticosteroids and antihistamines. Other local factors include septal abnormalities, such as septal perforation, inflammatory diseases, rhinosinusitis, illicit drug use (eg cocaine), iatrogenic causes, and neoplasia.
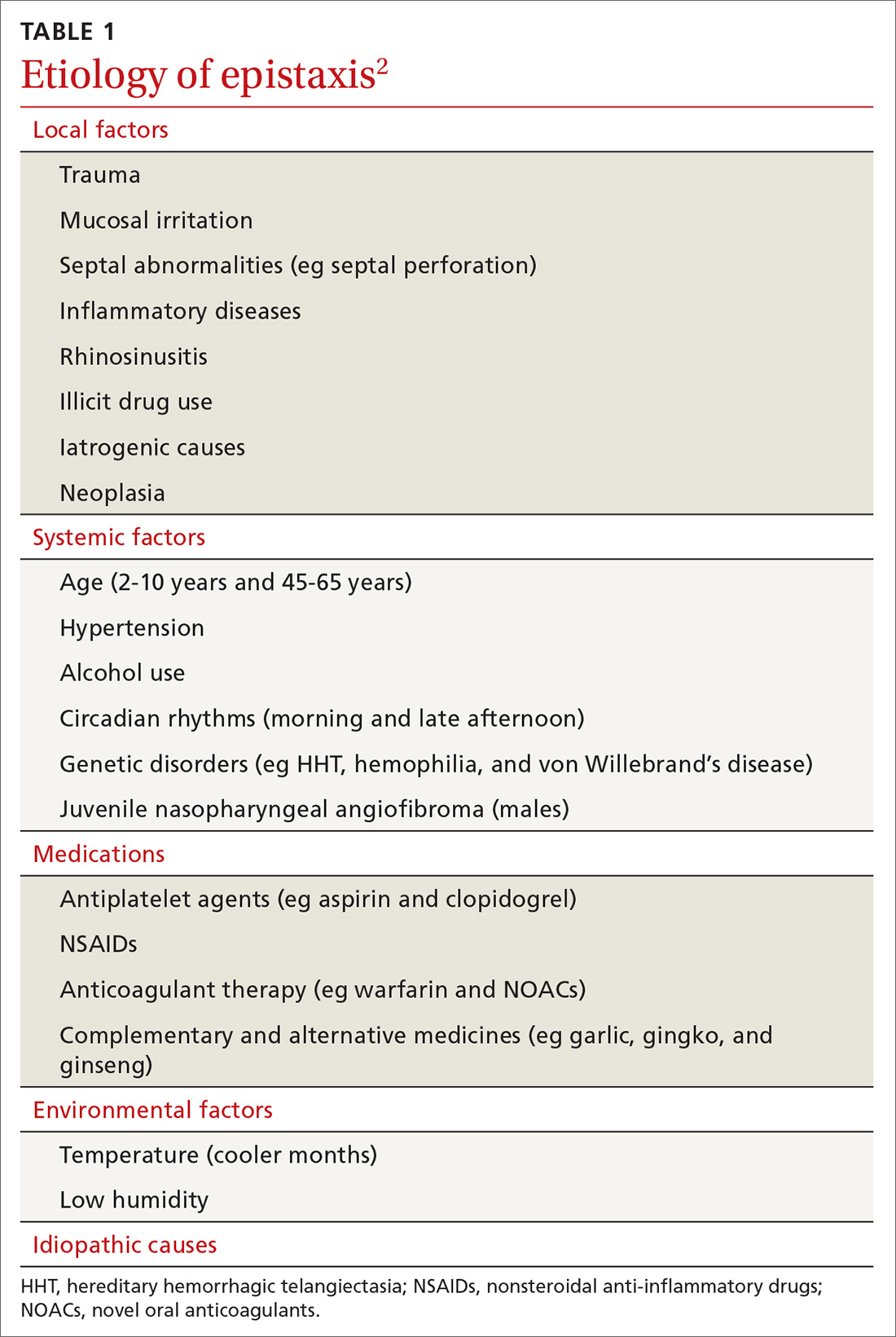
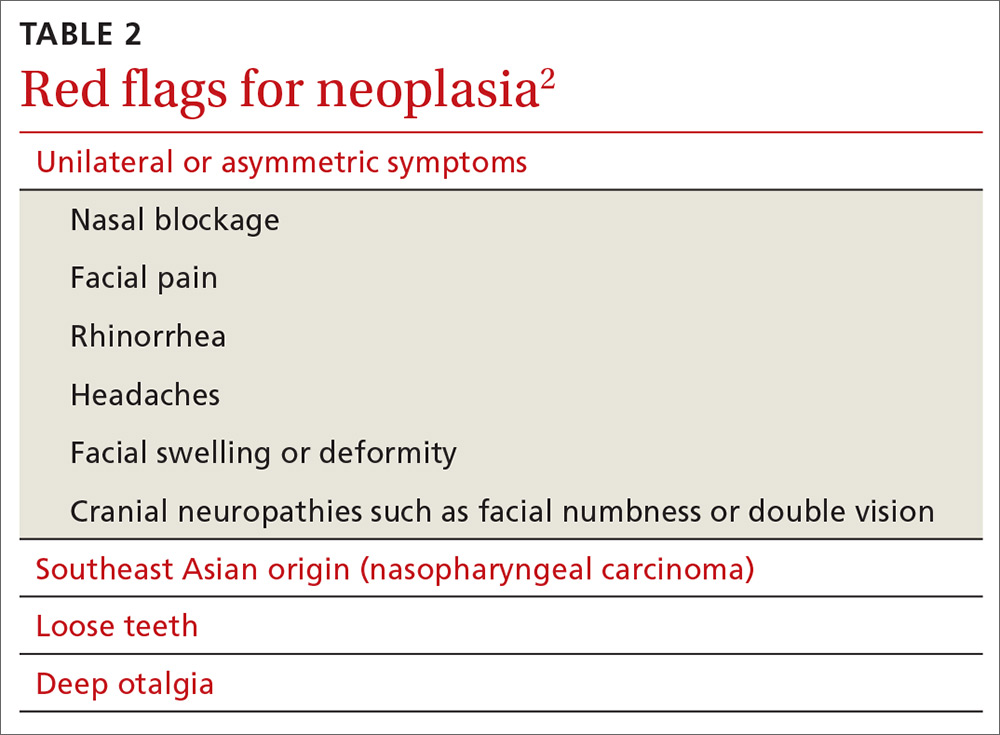
Red flags for neoplasia include unilateral or asymmetric symptoms, such as nasal blockage, facial pain, rhinorrhea, headaches, facial swelling or deformity, and cranial neuropathies (ie, facial numbness or double vision). Other red flags include Southeast Asian origin (nasopharyngeal carcinoma), loose maxillary teeth, and deep otalgia (TABLE 22). In adolescent males, it is important to consider juvenile nasopharyngeal angiofibroma, a benign tumor that can bleed extensively.
Systemic factors include age, hypertension, alcohol use, acquired coagulopathies due to liver or renal disease, hematologic abnormalities, circadian rhythms, and genetic disorders such as hereditary hemorrhagic telangiectasia (HHT), hemophilia, and von Willebrand’s disease.2
Medications that contribute to epistaxis include antiplatelet agents, such as aspirin and clopidogrel; nonsteroidal anti-inflammatory drugs (NSAIDs); warfarin and novel oral anticoagulants (NOACs); and complementary and alternative medicines, such garlic, gingko, and ginseng. Environmental factors include temperature and humidity.2
Ask about trauma, but also about upper GI hemorrhage
Resuscitation and control of bleeding (which we’ll discuss in a moment) should always take priority. A thorough history and examination are also essential. It’s important to elicit details of the acute episode and any previous episodes, including the duration, severity, frequency, laterality of bleed, and contributing or inciting factors.1,2 Posterior epistaxis often occurs from both nostrils and feels as though blood is dripping down the throat rather than the nose.
Continue to: Hematemesis and melena from upper gastrointestinal hemorrhage...
Hematemesis and melena from upper gastrointestinal hemorrhage can often be overlooked. Elicit history of local trauma, including nose picking, possible foreign body (particularly batteries in children), and recurrent upper respiratory tract infections.
Treatments, including methods previously used to control episodes, can be instructive. Pinching over the nasal bones—rather than the soft cartilaginous part of the nose—unfortunately remains relatively common. Ask about any past medical history that can give clues to the cause of bleeding, such as hypertension, hepatic impairment, easy bruising, family history of coagulation disorders, and social history including alcohol intake, smoking, and recreational drug use—particularly cocaine use. A detailed medication history, as discussed earlier, is vital.
Initial management: Digital pressure
Epistaxis is potentially a life-threatening event. All patients who are actively bleeding require full assessment, resuscitation, and control of the bleeding.4 To protect the airway sit the patient upright and lean them forward to prevent aspiration of blood posteriorly into the pharynx. To control bleeding, get the patient to apply digital pressure at the cartilaginous part of the nose for a minimum of 10 minutes. This provides tamponade of the anterior septal vessels. Applying ice packs around the neck and having the patient suck on ice significantly reduces nasal mucosa blood flow and can slow down the bleeding.7
If there is significant bleeding
Monitor the patient’s vital signs, in particular, the pulse and respiratory rate. Assess the patient’s hemodynamic stability and look for signs of shock, such as sweating and pallor. Insert 2 large-bore (16 G) intravenous cannula and draw blood for type and crossmatch for potential transfusion if significant bleeding has occurred, in high risk patients (eg patients who are elderly or anticoagulated or have a suspected bleeding diathesis), or if further bleeding is likely to occur.2
Consider fluid resuscitation with intravenous saline initially and blood transfusions based on hemoglobin level, symptoms, and history of ischemic heart disease.3,6 Routine clotting studies need to be performed if there is a suspected bleeding diathesis or the patient is anticoagulated. Test for hepatic or renal dysfunction in patients with systemic conditions that could lead to coagulopathy. The clinical state of an elderly patient may deteriorate rapidly, so aggressive resuscitation is vital.4
Continue to: Getting a better look requires the proper equipment
Getting a better look requires the proper equipment
Universal precautions including facemask, eye protection, and gloves should be worn. Have equipment easily accessible, including sufficient lighting and suction. A headlight enables the use of both hands to assess and treat the patient. The nasal cavity often is obscured by clots, so ask the patient to blow and clear their nose. Although this may lead to a recurrence of bleeding, it could assist in identifying the bleeding point.2
Local anesthetic with a vasoconstrictor should be applied to the nasal mucosa over Little’s area either via a solution applied on cotton-tipped applicator or as a nasal spray. Once adequate local anesthesia is achieved, the nasal cavity can be examined and treatment instigated to stem the hemorrhage. Perform anterior rhinoscopy with a Thudicum’s speculum with one hand (FIGURE 2) while suctioning simultaneously with the other. Assess the nasal cavity systematically, paying particular attention to the septum and Little’s area for an anterior bleed. Look for scabbed and excoriated areas.

Anterior bleeds can be managed safely in primary care, provided that appropriate equipment is available. Consider transfer to an emergency department or referral to an ENT specialist if bleeding continues or if a posterior bleed is suspected.2 Examination of the entire nasal cavity via nasendoscopy may be required to identify the source of bleeding—especially with posterior bleeds.
Nonsurgical management
Topical agents
Topical vasoconstrictor and local anaesthetic agents are widely available, and their limited adverse effect profiles make them a convenient first-line therapy.6,8 These agents reduce hemorrhage to allow for better visualization and analgesia for possible cautery or nasal packing.2 Common preparations include cophenylcaine (topical 5% lidocaine solution with 0.5% phenylephrine) and lidocaine injection (0.5%, 1%, or 2%) with epinephrine 1 in 200,000 and cocaine topical solutions (2% or 5%). Topical tranexamic acid has shown significant benefits in acute epistaxis in a systematic review.9
Cautery
If direct pressure and medical therapy fail to stop the bleeding, cautery or nasal packing can be performed.2,8 Chemical cautery entails application of 75% silver nitrate sticks to the bleeding point with firm pressure for 5 to 10 seconds to produce a local chemical burn.4 Only one side of the septum should be cauterized, as there is a small risk of septal perforation resulting from decreased vascularization to the septal cartilage.2,4,8 This can be performed at 4 to 6 week intervals. Electric bipolar cautery with a metal loop is performed by otolaryngologists under local anesthesia.4 Compared with electric cautery, silver nitrate cautery is cheap, readily available, easy to perform, equal in effectiveness, and has fewer complications.10
Continue to: Nasal packing...
Nasal packing
Nasal packing can be performed if cautery is unsuccessful in controlling the bleed or if no bleeding point is seen on examination.2 It provides direct mechanical compression and acts as a platelet aggregator, thereby facilitating coagulation.
Anterior packing. Packs should be directed posteriorly along the floor of the nasal cavity, rather than superiorly.2 After packing, examine the patient for ongoing bleeding from the contralateral nares or posteriorly in the oropharynx using a tongue depressor. If bleeding is seen, consider packing the other side before removal of the already inserted pack to increase the tamponade pressure over the septum.4
Anterior packs are effective, easy to use, widely available, and inexpensive.8 Types of packs include traditional packing, nasal tampons, and absorbable packing materials.
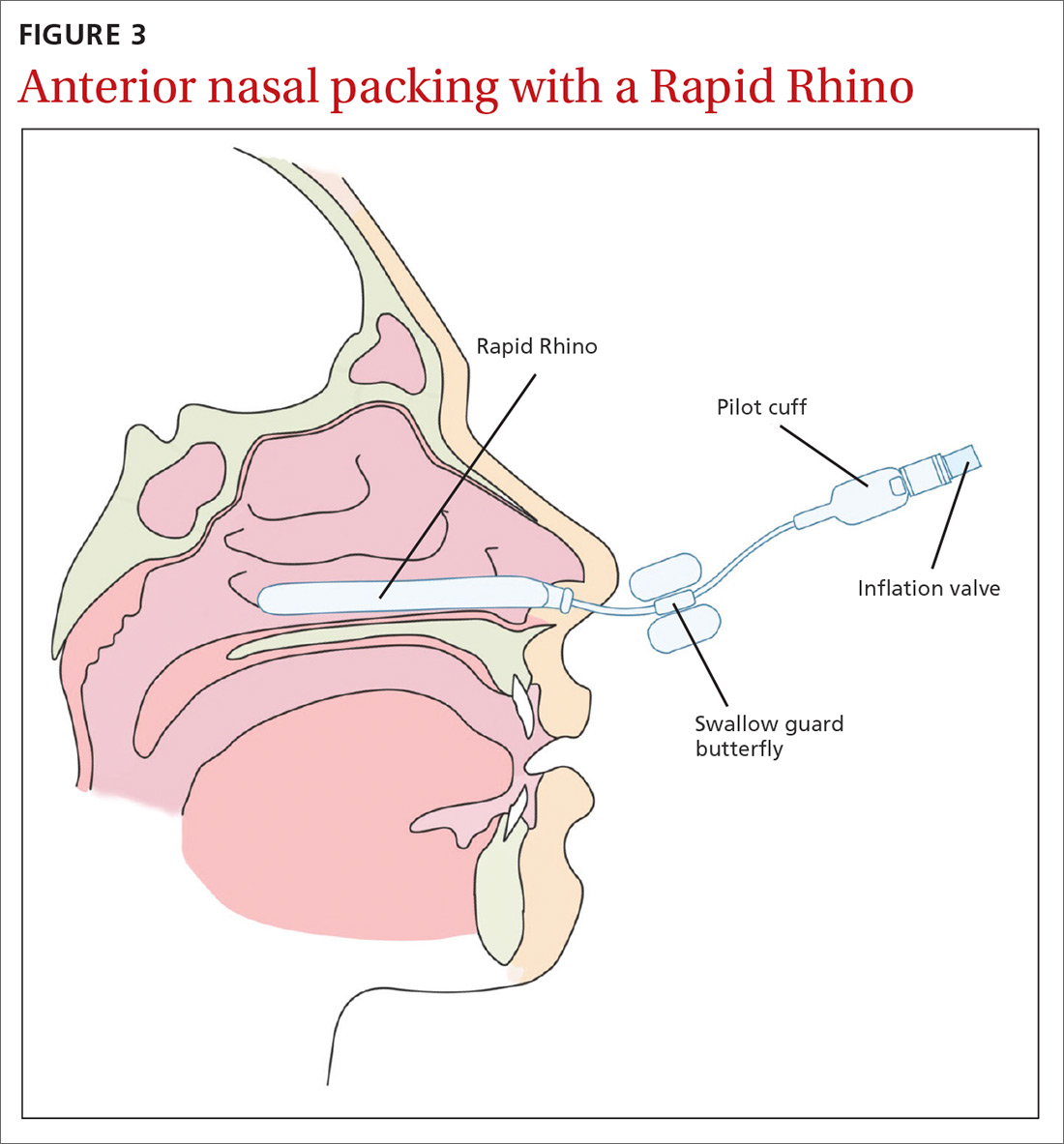
The Rapid Rhino is also an option. It’s an inflatable balloon pack coated with a lubricating compound. It remains in contact with the mucosa when deflated and can be left in situ for up to 4 days (FIGURE 3). It has the same rate of control of epistaxis when compared with polyvinyl alcohol. Both patients and physicians found insertion and removal of the Rapid Rhino easier with less patient discomfort.11-13
Absorbable packs do not require formal removal and are useful for patients with or without coagulopathies. They can be applied topically with a syringe that conforms to the 3-dimensional structure of the nasal cavity.1 The decision regarding which product to use is based on availability, cost, and physician preference.
Continue to: Posterior packing...
Posterior packing may be required if epistaxis continues despite anterior packing and may take the form of a balloon or a formal pack. A Foley catheter inflated with 3 to 4 mL of water or air is inserted through the anterior nares, along the floor of the nasal cavity into the posterior pharynx and pulled forward until the balloon engages the posterior choana (FIGURE 4). This provides local tamponade and tamponade at the sphenopalatine foramen.2,4 The balloon is held firmly in place with an umbilical clamp at the anterior nares. To prevent pressure necrosis, the columella can be protected with a soft dressing that is regularly checked by the nursing staff. The nasal cavity is then packed anteriorly with ribbon gauze or a nasal sponge to stem any potential anterior bleeds.
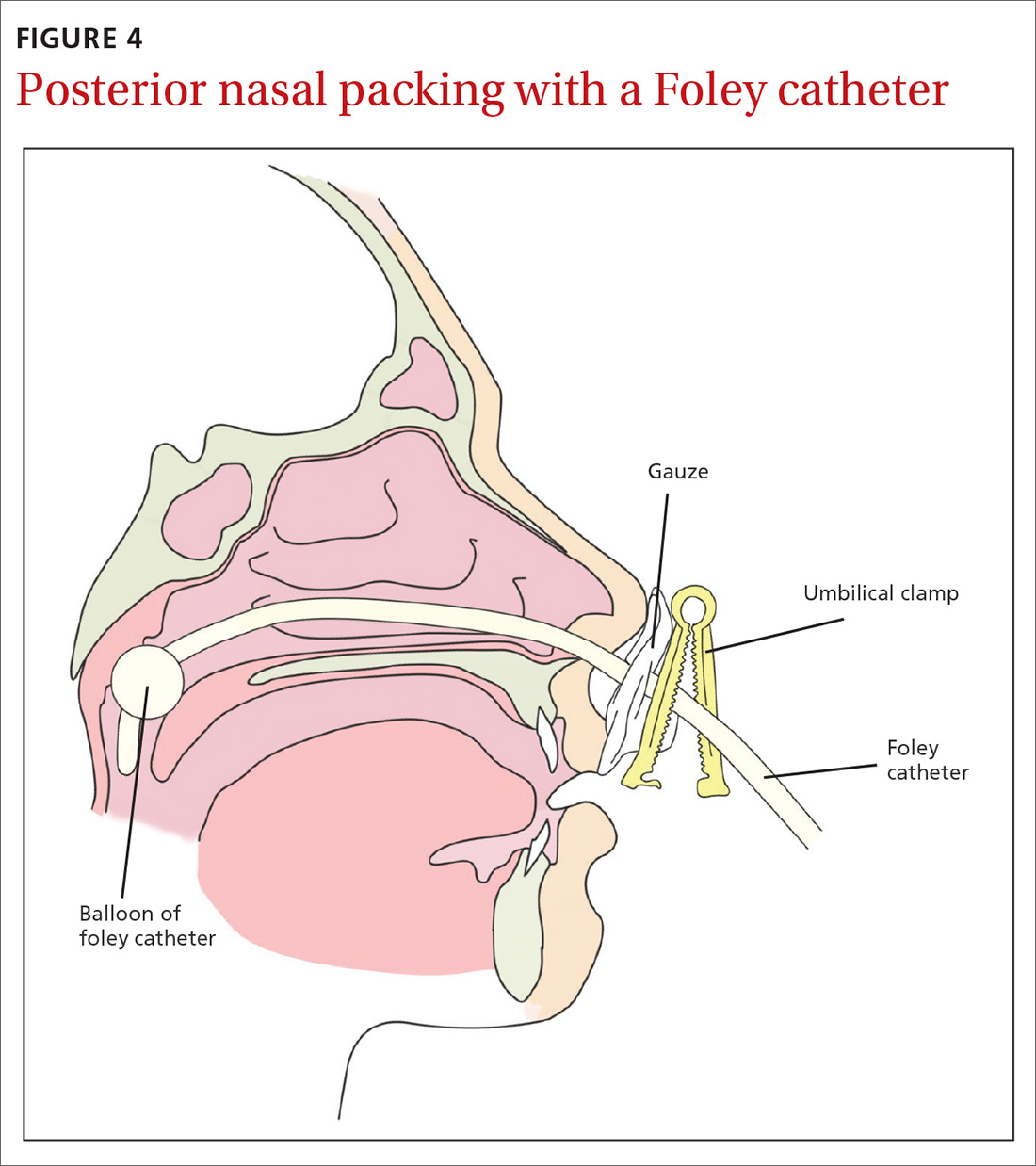
Potential complications include posterior displacement of the balloon with potential airway compromise, deflation in situ, and rupture of the balloon—which could result in aspiration.4 It is important to note the Foley catheter is, in fact, not licensed for nasal use.4 Insertion only should be performed by a clinician who has been trained in this skill.
Traditional nasopharyngeal packs are rolled gauze attached to tapes or sutured to a catheter. Compared with balloons, they were found to be more effective in controlling epistaxis and produce less short- and long-term complications.2 However, they are rather uncomfortable and hence normally performed under general anesthesia.4 Posterior packing has many disadvantages. They have a 50% failure rate, which increases to 70% in patients with bleeding disorders.8
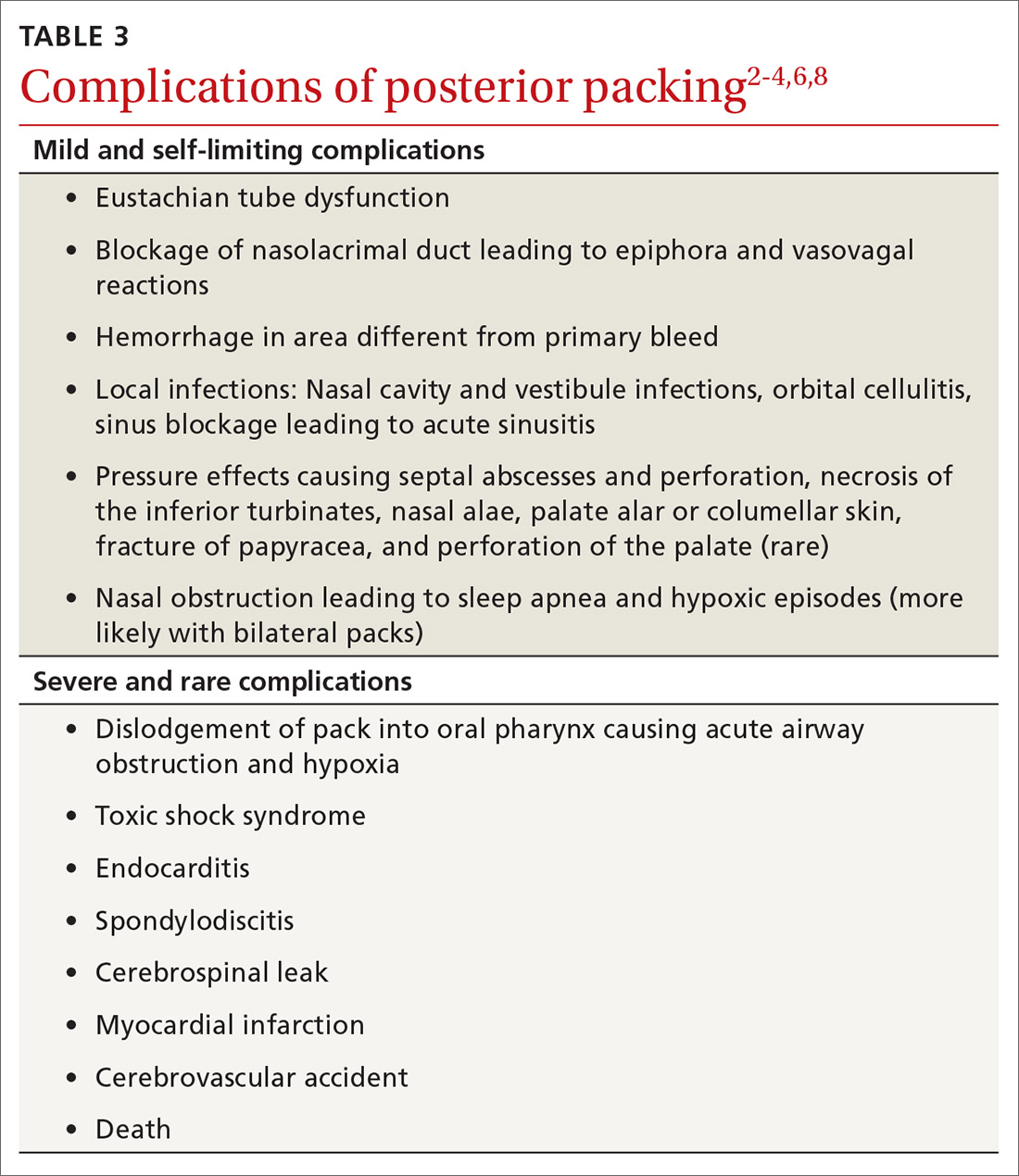
Complications vary from mild and self-limiting such as infection, hemorrhage, and pressure effects to severe such as toxic shock syndrome, myocardial infarction, and death (TABLE 32-4,6,8). There is little evidence supporting the use of prophylactic oral antibiotics after packing. Prophylactic antibiotics are reserved for those with posterior packs or if packs remain in situ for more than 24 hours.6
Warm water irrigation
Warm water irrigation via Foley catheter has a reported 82% success rate.8 It results in earlier discharge, less pain, less trauma to the nose, and reduced hospital length of stay.13 The balloon catheter is used to close off the posterior choana and water irrigation is applied at 45° C to 50° C for about 3 minutes with the help of a caloric stimulator.4,8 It helps clear blood clots from the nose and reduces local blood flow by causing mucosal edema, which compresses the bleeding vessels.
Continue to: Surgical management
Surgical management
Any bleeding that fails to stop, despite an escalation of management, requires surgical intervention. This includes cases in which the bleeding continues after pack removal.4 Options include4:
- Diathermy, with bipolar or radiofrequency laser, can be used to localize the bleeding site.
- Septoplasty allows for better access to the nasal cavity, reduction of blood flow to the nasal mucosa by raising a mucoperichondrial flap, correction of a deviated septum, and removal of a septal spur that may be responsible for the epistaxis.
- Arterial ligation involves identification of the bleeding vessel that is clipped or coagulated with bipolar diathermy.
- Endoscopic SPA ligation is an excellent, well-tolerated, and cost-effective method of treating recurrent epistaxis.6,14 It controls 98% of posterior epistaxis, and is superior to posterior nasal packing and embolization.2,3,10 It results in a shorter hospital stay, reduction in repeated hemorrhage and painful packing procedures, and a cost saving of >$7,000 per patient if performed early.7 Concomitant ligation of the anterior ethmoidal artery may be performed in traumatic epistaxis or when severe bleeding is from the ethmoidal region.4,6
- Ligation of the IMAX and external carotid arteries is performed rarely due to potential complications and high failure rates.
Arterial embolization
When arterial ligation fails, or is not possible due to anesthetic concerns, selective embolization of the maxillary or facial arteries by specialist radiologists can be considered.6 Access to the vascular system through a femoral punch leads to identification of the bleeding point. A catheter is then placed in the artery and the bleeding vessel is embolized. Possible candidates include patients with HHT, bleeding tumors, poor surgical candidates, or patient preference.3
Other management considerations
Once bleeding is controlled, factors that contributed to the epistaxis should be addressed.3 Hypertension needs to be managed. Antiplatelet or anticoagulant therapy may need to be temporarily halted in consultation with specialist physicians. Local treatments such as cautery are unlikely to be effective in patients who are anticoagulated. Nasal packing with a ‘procoagulant’ dressing, such as Kaltostat or Rapid Rhino, is often required.
Patient education and follow-up
Patients should be started on saline sprays or irrigation to maintain nasal hygiene after acute epistaxis. It’s a good idea to teach patients about proper first aid for recurrence (eg, sitting upright with digital pressure applied to the cartilaginous part of the nose, ice packs around the neck and ice to suck) and to encourage them to refrain from activities that may stimulate bleeding (blowing or picking the nose, heavy lifting, strenuous exercise). Also advise patients to abstain from alcohol and hot drinks that cause vasodilatation of nasal vessels as much as possible.4 Advise patients that topical gels, lotions, and ointments such as kenacomb, nasalate, or paraffin can be used to moisturise the mucosa and promote healing.1
All patients with a history of severe or recurrent epistaxis require formal examination of the nasal cavity to rule out a neoplastic lesion.
CORRESPONDENCE
Amy Wong, BMBS, Department ENT/Head and Neck Surgery, Monash ENT Building, PO Box 72, Rear 867 Centre Road, Bentleigh East 3165 Australia, [email protected].
1. Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784-789.
2. Yau S. An update on epistaxis. Aust Fam Physician. 2015;44:653-656.
3. McClurg SW, Carrau R. Endoscopic management of posterior epistaxis: a review. Acta Otorhinolaryngol Ital. 2014;34:1-8.
4. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
5. Dubel GJ, Ahn SH, Soares GM. Transcatheter embolization in the management of epistaxis. Semin Intervent Radiol. 2013;30:249-262.
6. Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol. 2012;37:382-389.
7. Porter M, Marais J, Tolley N. The effect of ice packs upon nasal mucosal blood flow. Acta Otolaryngol. 1991;111:1122-1125.
8. Traboulsi H, Alam E, Hadi U. Changing Trends in the Management of Epistaxis. Int J Otolaryngol. 2015;2015:263987.
9. Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771-776.
10. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot-water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
11. Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
12. Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol. 2005;30:333-337.
13. Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol. 2006;263:719-722.
14. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: randomized prospective trial. J Otolaryngol. 2004;33:185-188.
Epistaxis is a common presenting complaint in family medicine. Successful treatment requires knowledge of nasal anatomy, possible causes, and a step-wise approach.
Epistaxis predominantly affects children between the ages of 2 and 10 years and older adults between the ages of 45 and 65.1-4 Many presentations are spontaneous and self-limiting; often all that is required is proper first aid. It is important, however, to recognize the signs and symptoms that are suggestive of more worrisome conditions.
Management of epistaxis requires good preparation, appropriate equipment, and adequate assistance. If any of these are lacking, prompt nasal packing followed by referral to an emergency department or ear, nose, and throat (ENT) service is recommended.
Anatomy of the nasal cavity
The nasal cavity has a rich and highly varied blood supply arising from the internal and external carotid arteries with multiple anastomoses and a crossover between the left and right arterial systems.2,4,5 The internal maxillary artery (IMAX) supplies 80% of the nasal vault.2 The sphenopalatine artery (SPA) supplies most of the nasal septum and the turbinates, while the greater palatine artery (GPA) supplies the floor of the nasal septum.3,5 The ethmoidal arteries course through the cribriform plate to supply the roof of the nasal cavity. The ethmoidal arteries communicates with branches of the SPA posteriorly and several branches anteriorly (FIGURE 1).

Kiesselbach’s plexus is a highly vascularized region of cartilaginous nasal septum anteroinferiorly that is also known as Little’s area. It is supplied by the SPA, GPA, superior labial artery, and ethmoidal arteries.5 Woodruff’s plexus is the richly vascularized posterior aspect of the nasal cavity primarily supplied by the SPA.3,5
Is the bleed anterior or posterior; primary or secondary?
Epistaxis is classified as anterior or posterior based on the arterial supply and the location of the bleed in relation to the piriform aperture.2,3 Anterior epistaxis occurs in >90% of patients and arises in Little’s area.6 Posterior epistaxis arises from Woodruff’s plexus in the posterior nasal septum or lateral nasal wall. It occurs in 5% to 10% of patients, is usually arterial in origin, and leads to a greater risk of airway compromise, aspiration, and difficulty in controlling the hemorrhage.2,6
Epistaxis can be classified further as primary or secondary hemorrhage. Primary epistaxis is idiopathic, spontaneous bleeds without any precipitants.2 Blood vessels within the nasal mucosa run superficially and are relatively unprotected. Damage to this mucosa and to vessel walls can result in bleeding.4 Spontaneous rupture of vessels may occur occasionally, during, say the Valsalva maneuver or when straining to lift heavy objects.4 Secondary epistaxis occurs when there is a clear and definite cause (eg trauma, anticoagulant use, or surgery).
Continue to: Numerous causes...
Numerous causes: From trauma to medications
Epistaxis can be caused by local, systemic, or environmental factors; medications; or be idiopathic in nature (TABLE 12). It commonly arises due to self-inflicted trauma from nose picking, particularly in children; trauma to nasal bones or septum; and mucosal irritation from topical nasal drugs, such as corticosteroids and antihistamines. Other local factors include septal abnormalities, such as septal perforation, inflammatory diseases, rhinosinusitis, illicit drug use (eg cocaine), iatrogenic causes, and neoplasia.

Red flags for neoplasia include unilateral or asymmetric symptoms, such as nasal blockage, facial pain, rhinorrhea, headaches, facial swelling or deformity, and cranial neuropathies (ie, facial numbness or double vision). Other red flags include Southeast Asian origin (nasopharyngeal carcinoma), loose maxillary teeth, and deep otalgia (TABLE 22). In adolescent males, it is important to consider juvenile nasopharyngeal angiofibroma, a benign tumor that can bleed extensively.

Systemic factors include age, hypertension, alcohol use, acquired coagulopathies due to liver or renal disease, hematologic abnormalities, circadian rhythms, and genetic disorders such as hereditary hemorrhagic telangiectasia (HHT), hemophilia, and von Willebrand’s disease.2
Medications that contribute to epistaxis include antiplatelet agents, such as aspirin and clopidogrel; nonsteroidal anti-inflammatory drugs (NSAIDs); warfarin and novel oral anticoagulants (NOACs); and complementary and alternative medicines, such garlic, gingko, and ginseng. Environmental factors include temperature and humidity.2
Ask about trauma, but also about upper GI hemorrhage
Resuscitation and control of bleeding (which we’ll discuss in a moment) should always take priority. A thorough history and examination are also essential. It’s important to elicit details of the acute episode and any previous episodes, including the duration, severity, frequency, laterality of bleed, and contributing or inciting factors.1,2 Posterior epistaxis often occurs from both nostrils and feels as though blood is dripping down the throat rather than the nose.
Continue to: Hematemesis and melena from upper gastrointestinal hemorrhage...
Hematemesis and melena from upper gastrointestinal hemorrhage can often be overlooked. Elicit history of local trauma, including nose picking, possible foreign body (particularly batteries in children), and recurrent upper respiratory tract infections.
Treatments, including methods previously used to control episodes, can be instructive. Pinching over the nasal bones—rather than the soft cartilaginous part of the nose—unfortunately remains relatively common. Ask about any past medical history that can give clues to the cause of bleeding, such as hypertension, hepatic impairment, easy bruising, family history of coagulation disorders, and social history including alcohol intake, smoking, and recreational drug use—particularly cocaine use. A detailed medication history, as discussed earlier, is vital.
Initial management: Digital pressure
Epistaxis is potentially a life-threatening event. All patients who are actively bleeding require full assessment, resuscitation, and control of the bleeding.4 To protect the airway sit the patient upright and lean them forward to prevent aspiration of blood posteriorly into the pharynx. To control bleeding, get the patient to apply digital pressure at the cartilaginous part of the nose for a minimum of 10 minutes. This provides tamponade of the anterior septal vessels. Applying ice packs around the neck and having the patient suck on ice significantly reduces nasal mucosa blood flow and can slow down the bleeding.7
If there is significant bleeding
Monitor the patient’s vital signs, in particular, the pulse and respiratory rate. Assess the patient’s hemodynamic stability and look for signs of shock, such as sweating and pallor. Insert 2 large-bore (16 G) intravenous cannula and draw blood for type and crossmatch for potential transfusion if significant bleeding has occurred, in high risk patients (eg patients who are elderly or anticoagulated or have a suspected bleeding diathesis), or if further bleeding is likely to occur.2
Consider fluid resuscitation with intravenous saline initially and blood transfusions based on hemoglobin level, symptoms, and history of ischemic heart disease.3,6 Routine clotting studies need to be performed if there is a suspected bleeding diathesis or the patient is anticoagulated. Test for hepatic or renal dysfunction in patients with systemic conditions that could lead to coagulopathy. The clinical state of an elderly patient may deteriorate rapidly, so aggressive resuscitation is vital.4
Continue to: Getting a better look requires the proper equipment
Getting a better look requires the proper equipment
Universal precautions including facemask, eye protection, and gloves should be worn. Have equipment easily accessible, including sufficient lighting and suction. A headlight enables the use of both hands to assess and treat the patient. The nasal cavity often is obscured by clots, so ask the patient to blow and clear their nose. Although this may lead to a recurrence of bleeding, it could assist in identifying the bleeding point.2
Local anesthetic with a vasoconstrictor should be applied to the nasal mucosa over Little’s area either via a solution applied on cotton-tipped applicator or as a nasal spray. Once adequate local anesthesia is achieved, the nasal cavity can be examined and treatment instigated to stem the hemorrhage. Perform anterior rhinoscopy with a Thudicum’s speculum with one hand (FIGURE 2) while suctioning simultaneously with the other. Assess the nasal cavity systematically, paying particular attention to the septum and Little’s area for an anterior bleed. Look for scabbed and excoriated areas.

Anterior bleeds can be managed safely in primary care, provided that appropriate equipment is available. Consider transfer to an emergency department or referral to an ENT specialist if bleeding continues or if a posterior bleed is suspected.2 Examination of the entire nasal cavity via nasendoscopy may be required to identify the source of bleeding—especially with posterior bleeds.
Nonsurgical management
Topical agents
Topical vasoconstrictor and local anaesthetic agents are widely available, and their limited adverse effect profiles make them a convenient first-line therapy.6,8 These agents reduce hemorrhage to allow for better visualization and analgesia for possible cautery or nasal packing.2 Common preparations include cophenylcaine (topical 5% lidocaine solution with 0.5% phenylephrine) and lidocaine injection (0.5%, 1%, or 2%) with epinephrine 1 in 200,000 and cocaine topical solutions (2% or 5%). Topical tranexamic acid has shown significant benefits in acute epistaxis in a systematic review.9
Cautery
If direct pressure and medical therapy fail to stop the bleeding, cautery or nasal packing can be performed.2,8 Chemical cautery entails application of 75% silver nitrate sticks to the bleeding point with firm pressure for 5 to 10 seconds to produce a local chemical burn.4 Only one side of the septum should be cauterized, as there is a small risk of septal perforation resulting from decreased vascularization to the septal cartilage.2,4,8 This can be performed at 4 to 6 week intervals. Electric bipolar cautery with a metal loop is performed by otolaryngologists under local anesthesia.4 Compared with electric cautery, silver nitrate cautery is cheap, readily available, easy to perform, equal in effectiveness, and has fewer complications.10
Continue to: Nasal packing...
Nasal packing
Nasal packing can be performed if cautery is unsuccessful in controlling the bleed or if no bleeding point is seen on examination.2 It provides direct mechanical compression and acts as a platelet aggregator, thereby facilitating coagulation.
Anterior packing. Packs should be directed posteriorly along the floor of the nasal cavity, rather than superiorly.2 After packing, examine the patient for ongoing bleeding from the contralateral nares or posteriorly in the oropharynx using a tongue depressor. If bleeding is seen, consider packing the other side before removal of the already inserted pack to increase the tamponade pressure over the septum.4
Anterior packs are effective, easy to use, widely available, and inexpensive.8 Types of packs include traditional packing, nasal tampons, and absorbable packing materials.
The Rapid Rhino is also an option. It’s an inflatable balloon pack coated with a lubricating compound. It remains in contact with the mucosa when deflated and can be left in situ for up to 4 days (FIGURE 3). It has the same rate of control of epistaxis when compared with polyvinyl alcohol. Both patients and physicians found insertion and removal of the Rapid Rhino easier with less patient discomfort.11-13

Absorbable packs do not require formal removal and are useful for patients with or without coagulopathies. They can be applied topically with a syringe that conforms to the 3-dimensional structure of the nasal cavity.1 The decision regarding which product to use is based on availability, cost, and physician preference.
Continue to: Posterior packing...
Posterior packing may be required if epistaxis continues despite anterior packing and may take the form of a balloon or a formal pack. A Foley catheter inflated with 3 to 4 mL of water or air is inserted through the anterior nares, along the floor of the nasal cavity into the posterior pharynx and pulled forward until the balloon engages the posterior choana (FIGURE 4). This provides local tamponade and tamponade at the sphenopalatine foramen.2,4 The balloon is held firmly in place with an umbilical clamp at the anterior nares. To prevent pressure necrosis, the columella can be protected with a soft dressing that is regularly checked by the nursing staff. The nasal cavity is then packed anteriorly with ribbon gauze or a nasal sponge to stem any potential anterior bleeds.

Potential complications include posterior displacement of the balloon with potential airway compromise, deflation in situ, and rupture of the balloon—which could result in aspiration.4 It is important to note the Foley catheter is, in fact, not licensed for nasal use.4 Insertion only should be performed by a clinician who has been trained in this skill.
Traditional nasopharyngeal packs are rolled gauze attached to tapes or sutured to a catheter. Compared with balloons, they were found to be more effective in controlling epistaxis and produce less short- and long-term complications.2 However, they are rather uncomfortable and hence normally performed under general anesthesia.4 Posterior packing has many disadvantages. They have a 50% failure rate, which increases to 70% in patients with bleeding disorders.8
Complications vary from mild and self-limiting such as infection, hemorrhage, and pressure effects to severe such as toxic shock syndrome, myocardial infarction, and death (TABLE 32-4,6,8). There is little evidence supporting the use of prophylactic oral antibiotics after packing. Prophylactic antibiotics are reserved for those with posterior packs or if packs remain in situ for more than 24 hours.6

Warm water irrigation
Warm water irrigation via Foley catheter has a reported 82% success rate.8 It results in earlier discharge, less pain, less trauma to the nose, and reduced hospital length of stay.13 The balloon catheter is used to close off the posterior choana and water irrigation is applied at 45° C to 50° C for about 3 minutes with the help of a caloric stimulator.4,8 It helps clear blood clots from the nose and reduces local blood flow by causing mucosal edema, which compresses the bleeding vessels.
Continue to: Surgical management
Surgical management
Any bleeding that fails to stop, despite an escalation of management, requires surgical intervention. This includes cases in which the bleeding continues after pack removal.4 Options include4:
- Diathermy, with bipolar or radiofrequency laser, can be used to localize the bleeding site.
- Septoplasty allows for better access to the nasal cavity, reduction of blood flow to the nasal mucosa by raising a mucoperichondrial flap, correction of a deviated septum, and removal of a septal spur that may be responsible for the epistaxis.
- Arterial ligation involves identification of the bleeding vessel that is clipped or coagulated with bipolar diathermy.
- Endoscopic SPA ligation is an excellent, well-tolerated, and cost-effective method of treating recurrent epistaxis.6,14 It controls 98% of posterior epistaxis, and is superior to posterior nasal packing and embolization.2,3,10 It results in a shorter hospital stay, reduction in repeated hemorrhage and painful packing procedures, and a cost saving of >$7,000 per patient if performed early.7 Concomitant ligation of the anterior ethmoidal artery may be performed in traumatic epistaxis or when severe bleeding is from the ethmoidal region.4,6
- Ligation of the IMAX and external carotid arteries is performed rarely due to potential complications and high failure rates.
Arterial embolization
When arterial ligation fails, or is not possible due to anesthetic concerns, selective embolization of the maxillary or facial arteries by specialist radiologists can be considered.6 Access to the vascular system through a femoral punch leads to identification of the bleeding point. A catheter is then placed in the artery and the bleeding vessel is embolized. Possible candidates include patients with HHT, bleeding tumors, poor surgical candidates, or patient preference.3
Other management considerations
Once bleeding is controlled, factors that contributed to the epistaxis should be addressed.3 Hypertension needs to be managed. Antiplatelet or anticoagulant therapy may need to be temporarily halted in consultation with specialist physicians. Local treatments such as cautery are unlikely to be effective in patients who are anticoagulated. Nasal packing with a ‘procoagulant’ dressing, such as Kaltostat or Rapid Rhino, is often required.
Patient education and follow-up
Patients should be started on saline sprays or irrigation to maintain nasal hygiene after acute epistaxis. It’s a good idea to teach patients about proper first aid for recurrence (eg, sitting upright with digital pressure applied to the cartilaginous part of the nose, ice packs around the neck and ice to suck) and to encourage them to refrain from activities that may stimulate bleeding (blowing or picking the nose, heavy lifting, strenuous exercise). Also advise patients to abstain from alcohol and hot drinks that cause vasodilatation of nasal vessels as much as possible.4 Advise patients that topical gels, lotions, and ointments such as kenacomb, nasalate, or paraffin can be used to moisturise the mucosa and promote healing.1
All patients with a history of severe or recurrent epistaxis require formal examination of the nasal cavity to rule out a neoplastic lesion.
CORRESPONDENCE
Amy Wong, BMBS, Department ENT/Head and Neck Surgery, Monash ENT Building, PO Box 72, Rear 867 Centre Road, Bentleigh East 3165 Australia, [email protected].
Epistaxis is a common presenting complaint in family medicine. Successful treatment requires knowledge of nasal anatomy, possible causes, and a step-wise approach.
Epistaxis predominantly affects children between the ages of 2 and 10 years and older adults between the ages of 45 and 65.1-4 Many presentations are spontaneous and self-limiting; often all that is required is proper first aid. It is important, however, to recognize the signs and symptoms that are suggestive of more worrisome conditions.
Management of epistaxis requires good preparation, appropriate equipment, and adequate assistance. If any of these are lacking, prompt nasal packing followed by referral to an emergency department or ear, nose, and throat (ENT) service is recommended.
Anatomy of the nasal cavity
The nasal cavity has a rich and highly varied blood supply arising from the internal and external carotid arteries with multiple anastomoses and a crossover between the left and right arterial systems.2,4,5 The internal maxillary artery (IMAX) supplies 80% of the nasal vault.2 The sphenopalatine artery (SPA) supplies most of the nasal septum and the turbinates, while the greater palatine artery (GPA) supplies the floor of the nasal septum.3,5 The ethmoidal arteries course through the cribriform plate to supply the roof of the nasal cavity. The ethmoidal arteries communicates with branches of the SPA posteriorly and several branches anteriorly (FIGURE 1).

Kiesselbach’s plexus is a highly vascularized region of cartilaginous nasal septum anteroinferiorly that is also known as Little’s area. It is supplied by the SPA, GPA, superior labial artery, and ethmoidal arteries.5 Woodruff’s plexus is the richly vascularized posterior aspect of the nasal cavity primarily supplied by the SPA.3,5
Is the bleed anterior or posterior; primary or secondary?
Epistaxis is classified as anterior or posterior based on the arterial supply and the location of the bleed in relation to the piriform aperture.2,3 Anterior epistaxis occurs in >90% of patients and arises in Little’s area.6 Posterior epistaxis arises from Woodruff’s plexus in the posterior nasal septum or lateral nasal wall. It occurs in 5% to 10% of patients, is usually arterial in origin, and leads to a greater risk of airway compromise, aspiration, and difficulty in controlling the hemorrhage.2,6
Epistaxis can be classified further as primary or secondary hemorrhage. Primary epistaxis is idiopathic, spontaneous bleeds without any precipitants.2 Blood vessels within the nasal mucosa run superficially and are relatively unprotected. Damage to this mucosa and to vessel walls can result in bleeding.4 Spontaneous rupture of vessels may occur occasionally, during, say the Valsalva maneuver or when straining to lift heavy objects.4 Secondary epistaxis occurs when there is a clear and definite cause (eg trauma, anticoagulant use, or surgery).
Continue to: Numerous causes...
Numerous causes: From trauma to medications
Epistaxis can be caused by local, systemic, or environmental factors; medications; or be idiopathic in nature (TABLE 12). It commonly arises due to self-inflicted trauma from nose picking, particularly in children; trauma to nasal bones or septum; and mucosal irritation from topical nasal drugs, such as corticosteroids and antihistamines. Other local factors include septal abnormalities, such as septal perforation, inflammatory diseases, rhinosinusitis, illicit drug use (eg cocaine), iatrogenic causes, and neoplasia.

Red flags for neoplasia include unilateral or asymmetric symptoms, such as nasal blockage, facial pain, rhinorrhea, headaches, facial swelling or deformity, and cranial neuropathies (ie, facial numbness or double vision). Other red flags include Southeast Asian origin (nasopharyngeal carcinoma), loose maxillary teeth, and deep otalgia (TABLE 22). In adolescent males, it is important to consider juvenile nasopharyngeal angiofibroma, a benign tumor that can bleed extensively.

Systemic factors include age, hypertension, alcohol use, acquired coagulopathies due to liver or renal disease, hematologic abnormalities, circadian rhythms, and genetic disorders such as hereditary hemorrhagic telangiectasia (HHT), hemophilia, and von Willebrand’s disease.2
Medications that contribute to epistaxis include antiplatelet agents, such as aspirin and clopidogrel; nonsteroidal anti-inflammatory drugs (NSAIDs); warfarin and novel oral anticoagulants (NOACs); and complementary and alternative medicines, such garlic, gingko, and ginseng. Environmental factors include temperature and humidity.2
Ask about trauma, but also about upper GI hemorrhage
Resuscitation and control of bleeding (which we’ll discuss in a moment) should always take priority. A thorough history and examination are also essential. It’s important to elicit details of the acute episode and any previous episodes, including the duration, severity, frequency, laterality of bleed, and contributing or inciting factors.1,2 Posterior epistaxis often occurs from both nostrils and feels as though blood is dripping down the throat rather than the nose.
Continue to: Hematemesis and melena from upper gastrointestinal hemorrhage...
Hematemesis and melena from upper gastrointestinal hemorrhage can often be overlooked. Elicit history of local trauma, including nose picking, possible foreign body (particularly batteries in children), and recurrent upper respiratory tract infections.
Treatments, including methods previously used to control episodes, can be instructive. Pinching over the nasal bones—rather than the soft cartilaginous part of the nose—unfortunately remains relatively common. Ask about any past medical history that can give clues to the cause of bleeding, such as hypertension, hepatic impairment, easy bruising, family history of coagulation disorders, and social history including alcohol intake, smoking, and recreational drug use—particularly cocaine use. A detailed medication history, as discussed earlier, is vital.
Initial management: Digital pressure
Epistaxis is potentially a life-threatening event. All patients who are actively bleeding require full assessment, resuscitation, and control of the bleeding.4 To protect the airway sit the patient upright and lean them forward to prevent aspiration of blood posteriorly into the pharynx. To control bleeding, get the patient to apply digital pressure at the cartilaginous part of the nose for a minimum of 10 minutes. This provides tamponade of the anterior septal vessels. Applying ice packs around the neck and having the patient suck on ice significantly reduces nasal mucosa blood flow and can slow down the bleeding.7
If there is significant bleeding
Monitor the patient’s vital signs, in particular, the pulse and respiratory rate. Assess the patient’s hemodynamic stability and look for signs of shock, such as sweating and pallor. Insert 2 large-bore (16 G) intravenous cannula and draw blood for type and crossmatch for potential transfusion if significant bleeding has occurred, in high risk patients (eg patients who are elderly or anticoagulated or have a suspected bleeding diathesis), or if further bleeding is likely to occur.2
Consider fluid resuscitation with intravenous saline initially and blood transfusions based on hemoglobin level, symptoms, and history of ischemic heart disease.3,6 Routine clotting studies need to be performed if there is a suspected bleeding diathesis or the patient is anticoagulated. Test for hepatic or renal dysfunction in patients with systemic conditions that could lead to coagulopathy. The clinical state of an elderly patient may deteriorate rapidly, so aggressive resuscitation is vital.4
Continue to: Getting a better look requires the proper equipment
Getting a better look requires the proper equipment
Universal precautions including facemask, eye protection, and gloves should be worn. Have equipment easily accessible, including sufficient lighting and suction. A headlight enables the use of both hands to assess and treat the patient. The nasal cavity often is obscured by clots, so ask the patient to blow and clear their nose. Although this may lead to a recurrence of bleeding, it could assist in identifying the bleeding point.2
Local anesthetic with a vasoconstrictor should be applied to the nasal mucosa over Little’s area either via a solution applied on cotton-tipped applicator or as a nasal spray. Once adequate local anesthesia is achieved, the nasal cavity can be examined and treatment instigated to stem the hemorrhage. Perform anterior rhinoscopy with a Thudicum’s speculum with one hand (FIGURE 2) while suctioning simultaneously with the other. Assess the nasal cavity systematically, paying particular attention to the septum and Little’s area for an anterior bleed. Look for scabbed and excoriated areas.

Anterior bleeds can be managed safely in primary care, provided that appropriate equipment is available. Consider transfer to an emergency department or referral to an ENT specialist if bleeding continues or if a posterior bleed is suspected.2 Examination of the entire nasal cavity via nasendoscopy may be required to identify the source of bleeding—especially with posterior bleeds.
Nonsurgical management
Topical agents
Topical vasoconstrictor and local anaesthetic agents are widely available, and their limited adverse effect profiles make them a convenient first-line therapy.6,8 These agents reduce hemorrhage to allow for better visualization and analgesia for possible cautery or nasal packing.2 Common preparations include cophenylcaine (topical 5% lidocaine solution with 0.5% phenylephrine) and lidocaine injection (0.5%, 1%, or 2%) with epinephrine 1 in 200,000 and cocaine topical solutions (2% or 5%). Topical tranexamic acid has shown significant benefits in acute epistaxis in a systematic review.9
Cautery
If direct pressure and medical therapy fail to stop the bleeding, cautery or nasal packing can be performed.2,8 Chemical cautery entails application of 75% silver nitrate sticks to the bleeding point with firm pressure for 5 to 10 seconds to produce a local chemical burn.4 Only one side of the septum should be cauterized, as there is a small risk of septal perforation resulting from decreased vascularization to the septal cartilage.2,4,8 This can be performed at 4 to 6 week intervals. Electric bipolar cautery with a metal loop is performed by otolaryngologists under local anesthesia.4 Compared with electric cautery, silver nitrate cautery is cheap, readily available, easy to perform, equal in effectiveness, and has fewer complications.10
Continue to: Nasal packing...
Nasal packing
Nasal packing can be performed if cautery is unsuccessful in controlling the bleed or if no bleeding point is seen on examination.2 It provides direct mechanical compression and acts as a platelet aggregator, thereby facilitating coagulation.
Anterior packing. Packs should be directed posteriorly along the floor of the nasal cavity, rather than superiorly.2 After packing, examine the patient for ongoing bleeding from the contralateral nares or posteriorly in the oropharynx using a tongue depressor. If bleeding is seen, consider packing the other side before removal of the already inserted pack to increase the tamponade pressure over the septum.4
Anterior packs are effective, easy to use, widely available, and inexpensive.8 Types of packs include traditional packing, nasal tampons, and absorbable packing materials.
The Rapid Rhino is also an option. It’s an inflatable balloon pack coated with a lubricating compound. It remains in contact with the mucosa when deflated and can be left in situ for up to 4 days (FIGURE 3). It has the same rate of control of epistaxis when compared with polyvinyl alcohol. Both patients and physicians found insertion and removal of the Rapid Rhino easier with less patient discomfort.11-13

Absorbable packs do not require formal removal and are useful for patients with or without coagulopathies. They can be applied topically with a syringe that conforms to the 3-dimensional structure of the nasal cavity.1 The decision regarding which product to use is based on availability, cost, and physician preference.
Continue to: Posterior packing...
Posterior packing may be required if epistaxis continues despite anterior packing and may take the form of a balloon or a formal pack. A Foley catheter inflated with 3 to 4 mL of water or air is inserted through the anterior nares, along the floor of the nasal cavity into the posterior pharynx and pulled forward until the balloon engages the posterior choana (FIGURE 4). This provides local tamponade and tamponade at the sphenopalatine foramen.2,4 The balloon is held firmly in place with an umbilical clamp at the anterior nares. To prevent pressure necrosis, the columella can be protected with a soft dressing that is regularly checked by the nursing staff. The nasal cavity is then packed anteriorly with ribbon gauze or a nasal sponge to stem any potential anterior bleeds.

Potential complications include posterior displacement of the balloon with potential airway compromise, deflation in situ, and rupture of the balloon—which could result in aspiration.4 It is important to note the Foley catheter is, in fact, not licensed for nasal use.4 Insertion only should be performed by a clinician who has been trained in this skill.
Traditional nasopharyngeal packs are rolled gauze attached to tapes or sutured to a catheter. Compared with balloons, they were found to be more effective in controlling epistaxis and produce less short- and long-term complications.2 However, they are rather uncomfortable and hence normally performed under general anesthesia.4 Posterior packing has many disadvantages. They have a 50% failure rate, which increases to 70% in patients with bleeding disorders.8
Complications vary from mild and self-limiting such as infection, hemorrhage, and pressure effects to severe such as toxic shock syndrome, myocardial infarction, and death (TABLE 32-4,6,8). There is little evidence supporting the use of prophylactic oral antibiotics after packing. Prophylactic antibiotics are reserved for those with posterior packs or if packs remain in situ for more than 24 hours.6

Warm water irrigation
Warm water irrigation via Foley catheter has a reported 82% success rate.8 It results in earlier discharge, less pain, less trauma to the nose, and reduced hospital length of stay.13 The balloon catheter is used to close off the posterior choana and water irrigation is applied at 45° C to 50° C for about 3 minutes with the help of a caloric stimulator.4,8 It helps clear blood clots from the nose and reduces local blood flow by causing mucosal edema, which compresses the bleeding vessels.
Continue to: Surgical management
Surgical management
Any bleeding that fails to stop, despite an escalation of management, requires surgical intervention. This includes cases in which the bleeding continues after pack removal.4 Options include4:
- Diathermy, with bipolar or radiofrequency laser, can be used to localize the bleeding site.
- Septoplasty allows for better access to the nasal cavity, reduction of blood flow to the nasal mucosa by raising a mucoperichondrial flap, correction of a deviated septum, and removal of a septal spur that may be responsible for the epistaxis.
- Arterial ligation involves identification of the bleeding vessel that is clipped or coagulated with bipolar diathermy.
- Endoscopic SPA ligation is an excellent, well-tolerated, and cost-effective method of treating recurrent epistaxis.6,14 It controls 98% of posterior epistaxis, and is superior to posterior nasal packing and embolization.2,3,10 It results in a shorter hospital stay, reduction in repeated hemorrhage and painful packing procedures, and a cost saving of >$7,000 per patient if performed early.7 Concomitant ligation of the anterior ethmoidal artery may be performed in traumatic epistaxis or when severe bleeding is from the ethmoidal region.4,6
- Ligation of the IMAX and external carotid arteries is performed rarely due to potential complications and high failure rates.
Arterial embolization
When arterial ligation fails, or is not possible due to anesthetic concerns, selective embolization of the maxillary or facial arteries by specialist radiologists can be considered.6 Access to the vascular system through a femoral punch leads to identification of the bleeding point. A catheter is then placed in the artery and the bleeding vessel is embolized. Possible candidates include patients with HHT, bleeding tumors, poor surgical candidates, or patient preference.3
Other management considerations
Once bleeding is controlled, factors that contributed to the epistaxis should be addressed.3 Hypertension needs to be managed. Antiplatelet or anticoagulant therapy may need to be temporarily halted in consultation with specialist physicians. Local treatments such as cautery are unlikely to be effective in patients who are anticoagulated. Nasal packing with a ‘procoagulant’ dressing, such as Kaltostat or Rapid Rhino, is often required.
Patient education and follow-up
Patients should be started on saline sprays or irrigation to maintain nasal hygiene after acute epistaxis. It’s a good idea to teach patients about proper first aid for recurrence (eg, sitting upright with digital pressure applied to the cartilaginous part of the nose, ice packs around the neck and ice to suck) and to encourage them to refrain from activities that may stimulate bleeding (blowing or picking the nose, heavy lifting, strenuous exercise). Also advise patients to abstain from alcohol and hot drinks that cause vasodilatation of nasal vessels as much as possible.4 Advise patients that topical gels, lotions, and ointments such as kenacomb, nasalate, or paraffin can be used to moisturise the mucosa and promote healing.1
All patients with a history of severe or recurrent epistaxis require formal examination of the nasal cavity to rule out a neoplastic lesion.
CORRESPONDENCE
Amy Wong, BMBS, Department ENT/Head and Neck Surgery, Monash ENT Building, PO Box 72, Rear 867 Centre Road, Bentleigh East 3165 Australia, [email protected].
1. Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784-789.
2. Yau S. An update on epistaxis. Aust Fam Physician. 2015;44:653-656.
3. McClurg SW, Carrau R. Endoscopic management of posterior epistaxis: a review. Acta Otorhinolaryngol Ital. 2014;34:1-8.
4. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
5. Dubel GJ, Ahn SH, Soares GM. Transcatheter embolization in the management of epistaxis. Semin Intervent Radiol. 2013;30:249-262.
6. Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol. 2012;37:382-389.
7. Porter M, Marais J, Tolley N. The effect of ice packs upon nasal mucosal blood flow. Acta Otolaryngol. 1991;111:1122-1125.
8. Traboulsi H, Alam E, Hadi U. Changing Trends in the Management of Epistaxis. Int J Otolaryngol. 2015;2015:263987.
9. Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771-776.
10. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot-water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
11. Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
12. Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol. 2005;30:333-337.
13. Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol. 2006;263:719-722.
14. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: randomized prospective trial. J Otolaryngol. 2004;33:185-188.
1. Schlosser RJ. Clinical practice. Epistaxis. N Engl J Med. 2009;360:784-789.
2. Yau S. An update on epistaxis. Aust Fam Physician. 2015;44:653-656.
3. McClurg SW, Carrau R. Endoscopic management of posterior epistaxis: a review. Acta Otorhinolaryngol Ital. 2014;34:1-8.
4. Pope LE, Hobbs CG. Epistaxis: an update on current management. Postgrad Med J. 2005;81:309-314.
5. Dubel GJ, Ahn SH, Soares GM. Transcatheter embolization in the management of epistaxis. Semin Intervent Radiol. 2013;30:249-262.
6. Spielmann PM, Barnes ML, White PS. Controversies in the specialist management of adult epistaxis: an evidence-based review. Clin Otolaryngol. 2012;37:382-389.
7. Porter M, Marais J, Tolley N. The effect of ice packs upon nasal mucosal blood flow. Acta Otolaryngol. 1991;111:1122-1125.
8. Traboulsi H, Alam E, Hadi U. Changing Trends in the Management of Epistaxis. Int J Otolaryngol. 2015;2015:263987.
9. Kamhieh Y, Fox H. Tranexamic acid in epistaxis: a systematic review. Clin Otolaryngol. 2016;41:771-776.
10. Stangerup SE, Dommerby H, Siim C, et al. New modification of hot-water irrigation in the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1999;125:686-690.
11. Douglas R, Wormald PJ. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180-183.
12. Badran K, Malik TH, Belloso A, et al. Randomized controlled trial comparing Merocel and RapidRhino packing in the management of anterior epistaxis. Clin Otolaryngol. 2005;30:333-337.
13. Moumoulidis I, Draper MR, Patel H, et al. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol. 2006;263:719-722.
14. Moshaver A, Harris JR, Liu R, et al. Early operative intervention versus conventional treatment in epistaxis: randomized prospective trial. J Otolaryngol. 2004;33:185-188.
From The Journal of Family Practice | 2018;67(12):E13-E20.
PRACTICE RECOMMENDATIONS
› Use topical vasoconstrictor and local anesthetic agents as a first line therapy for epistaxis. Consider the additional use of topical tranexamic acid. A
› Perform chemical cautery with silver nitrate in cases of anterior epistaxis. This approach is cheap, easy to perform, and silver nitrate is readily available. A
› Consider endoscopic sphenopalatine artery ligation in the acute management of posterior epistaxis. It is superior to posterior nasal packing and embolization when it comes to pain, cost-effectiveness, risk, and overall control of bleeding. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dermoscopy in family medicine: A primer
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.
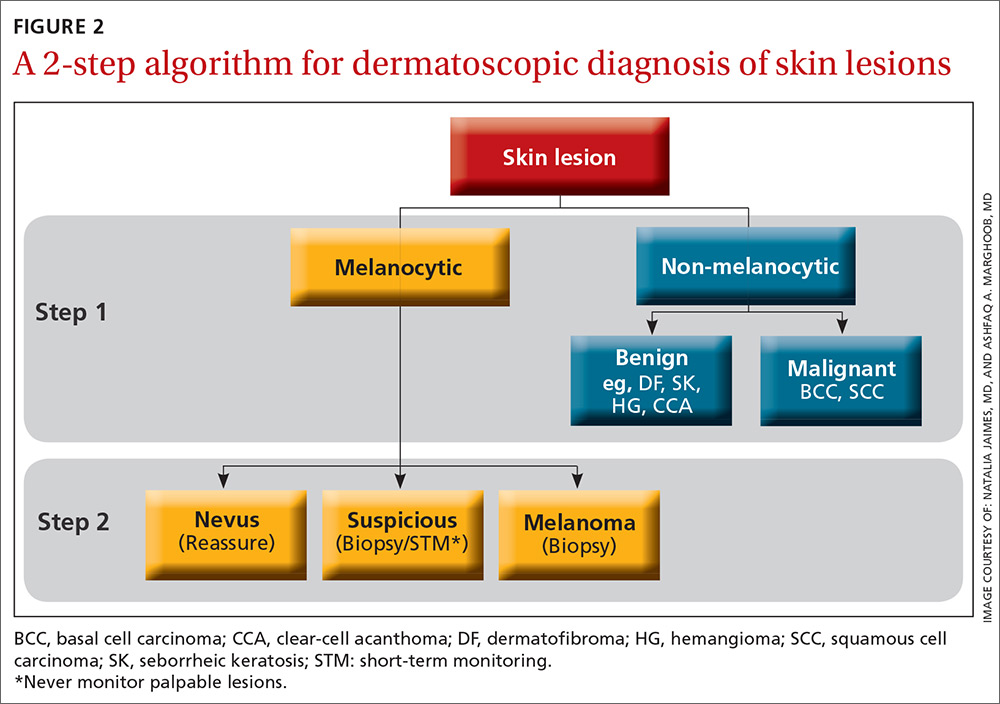
Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
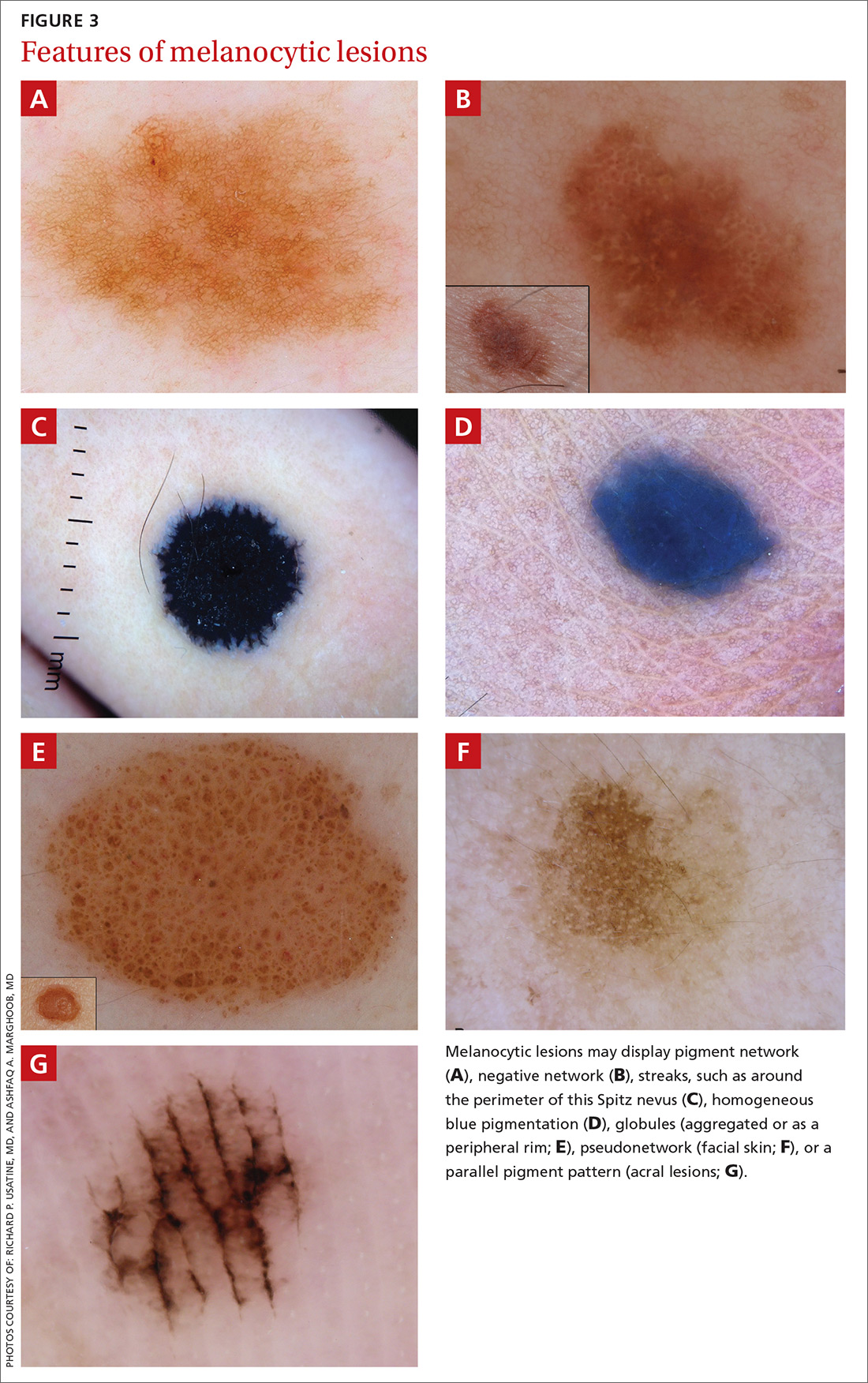
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).
Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
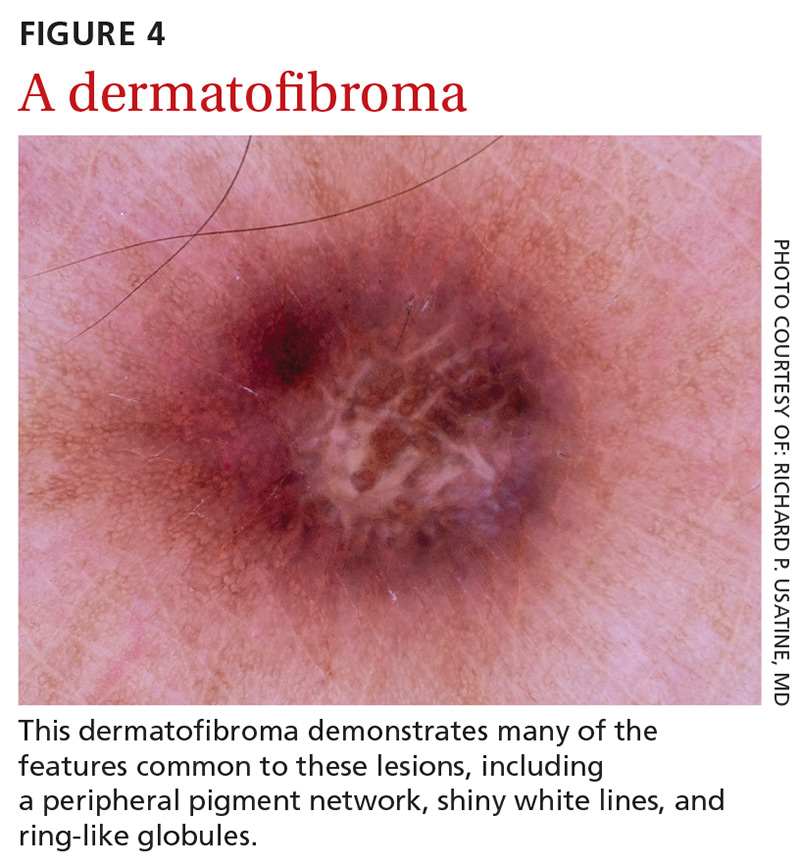
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.
Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
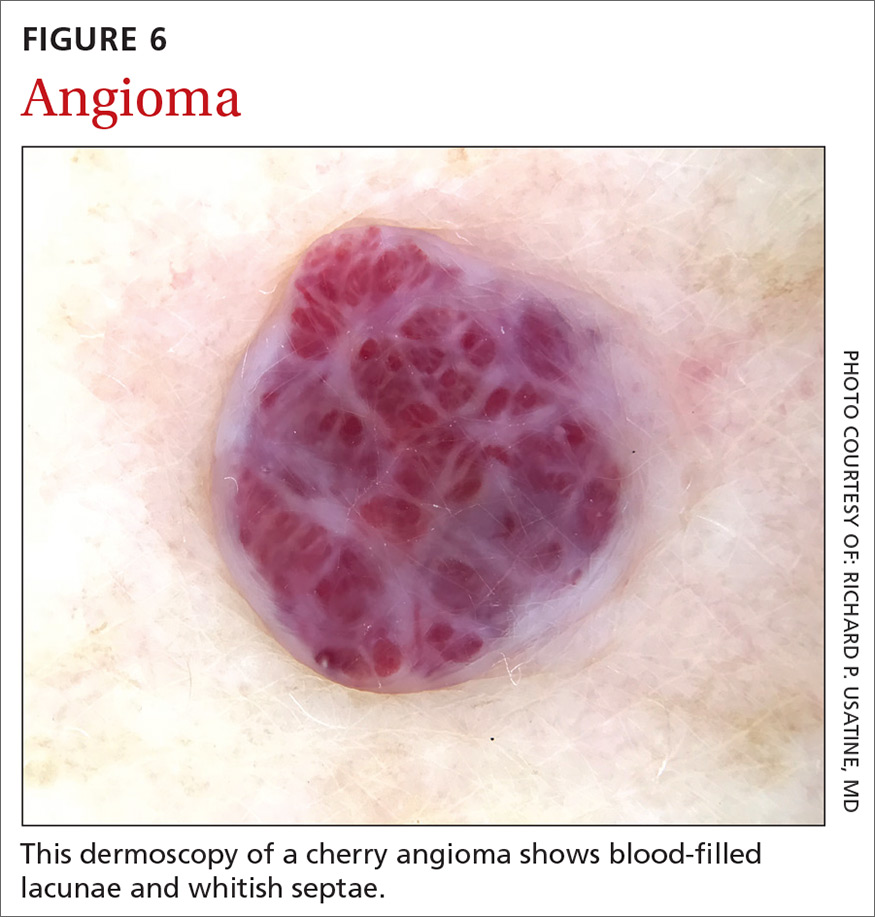
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).
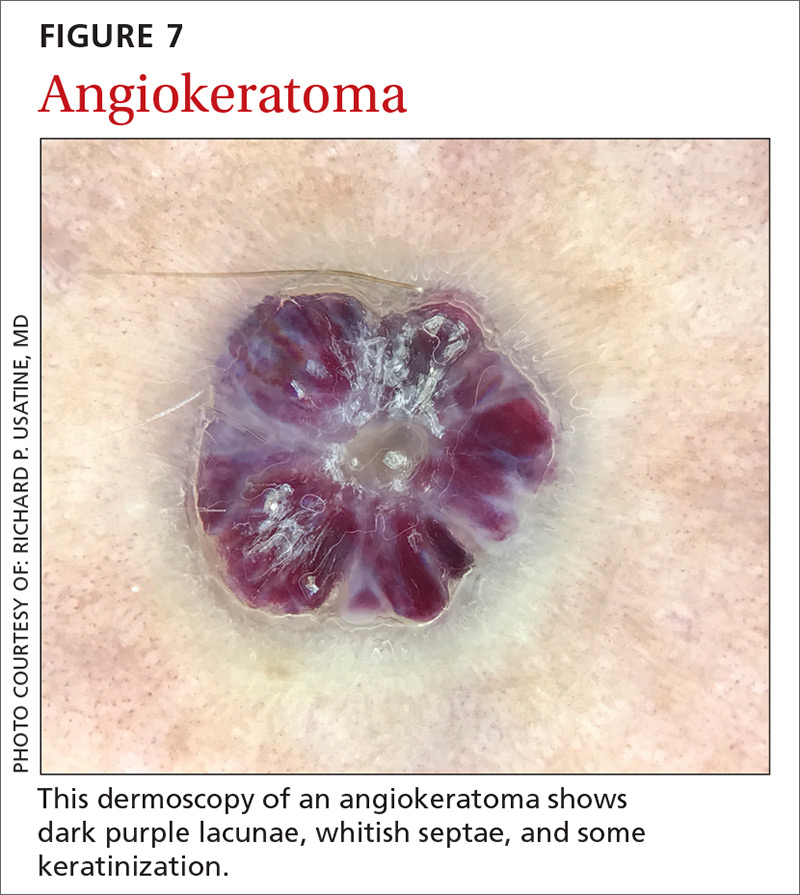
Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.
Continue to: Sebaceous hyperplasia...
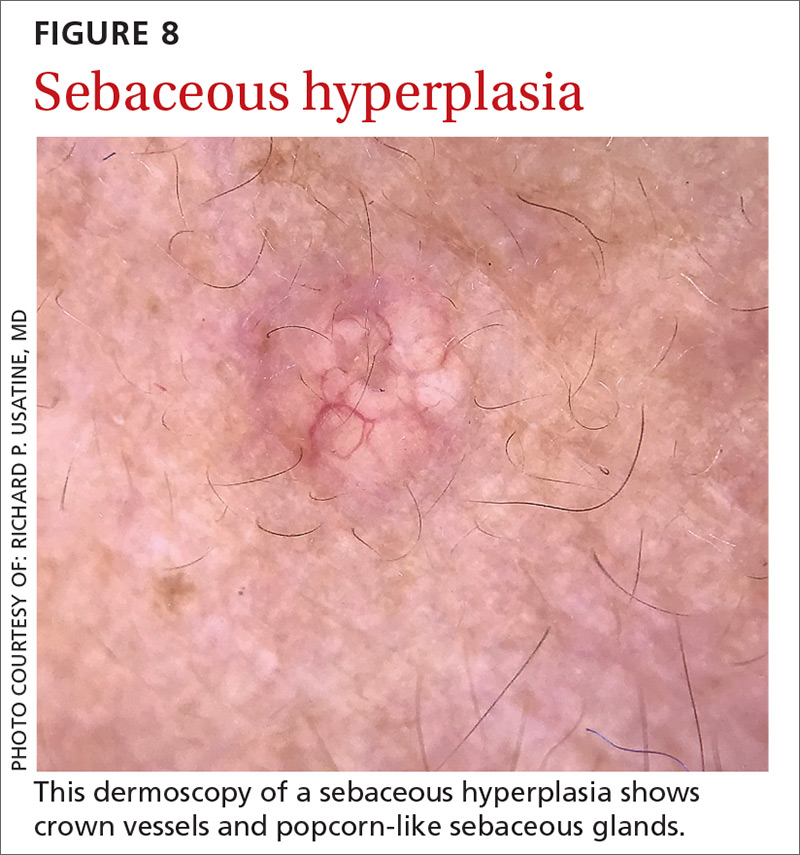
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).
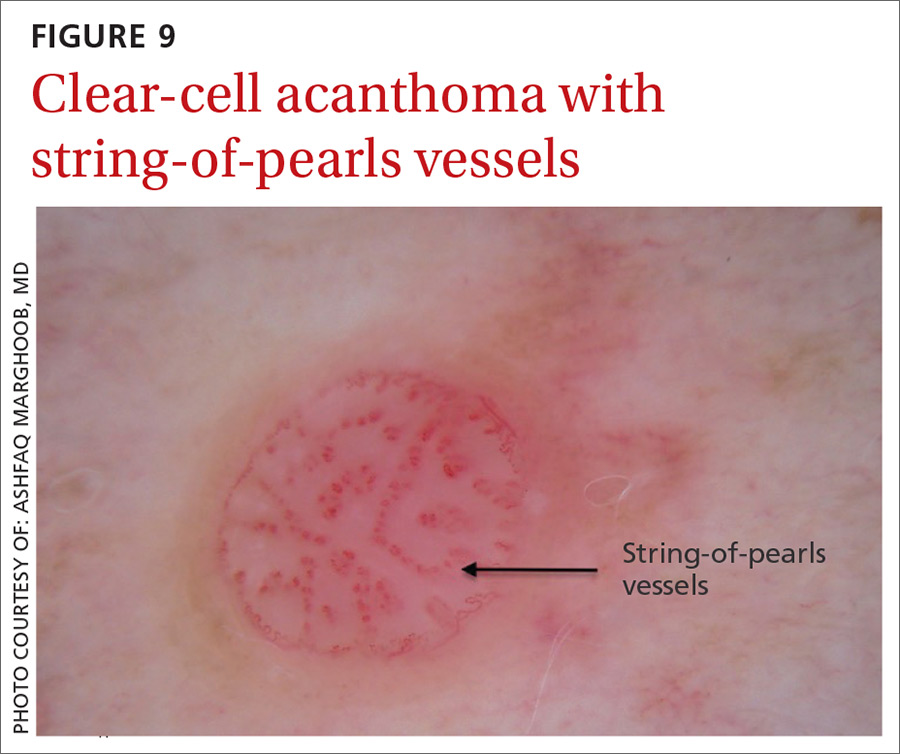
Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).
Malignant nonmelanocytic lesions
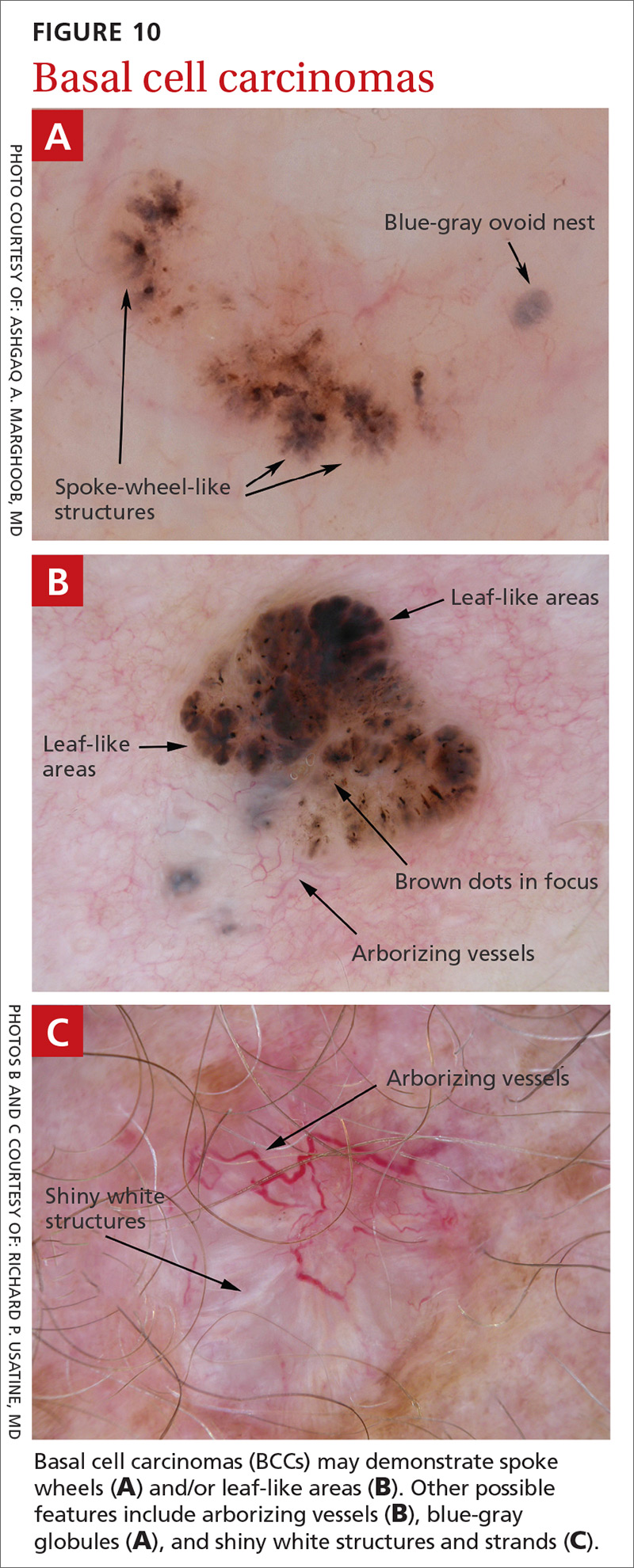
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).
Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
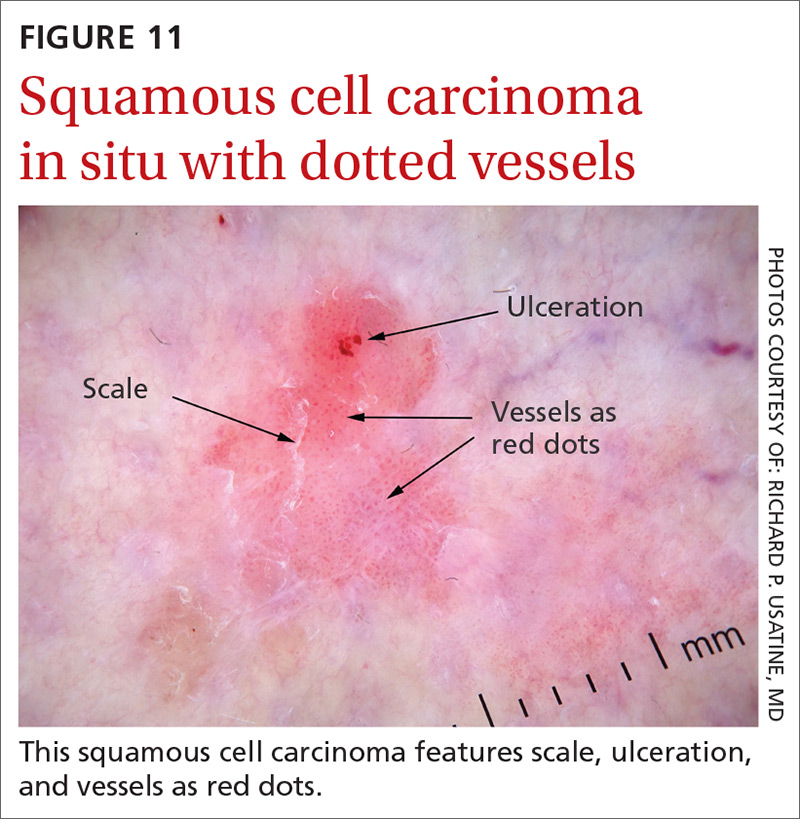
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.
Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17
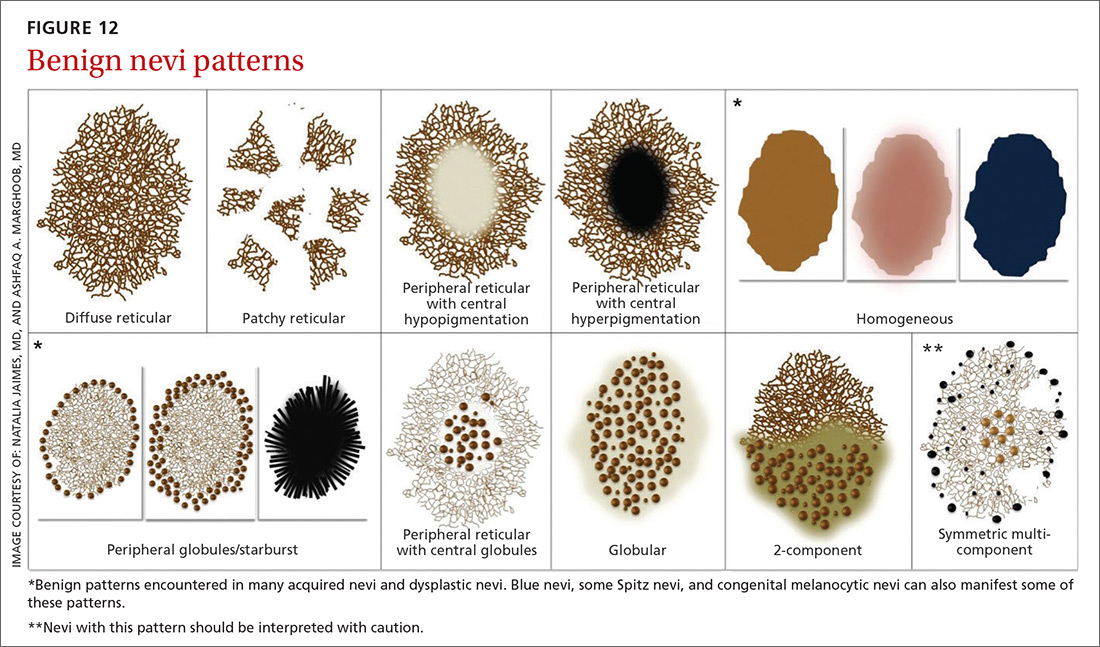
Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).
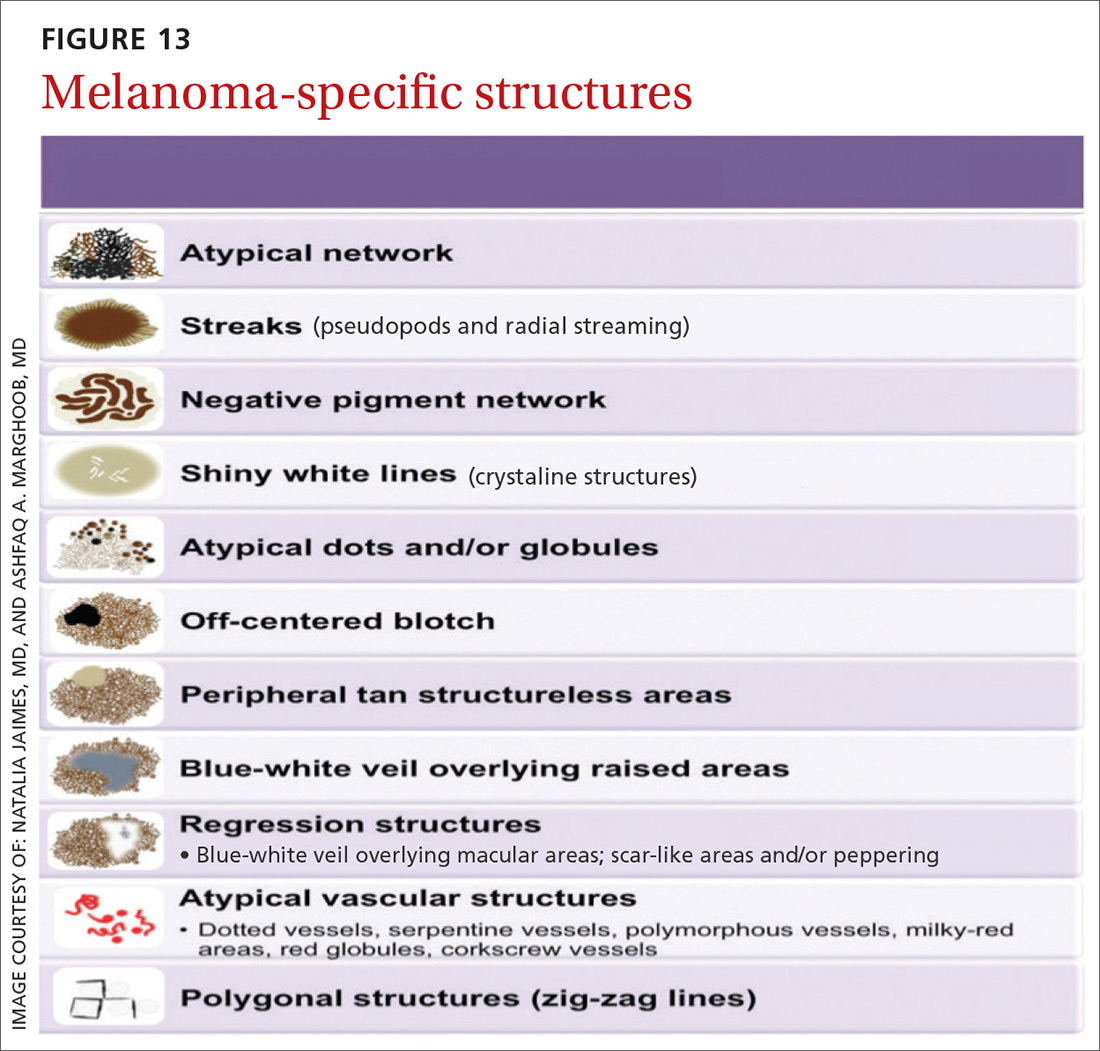
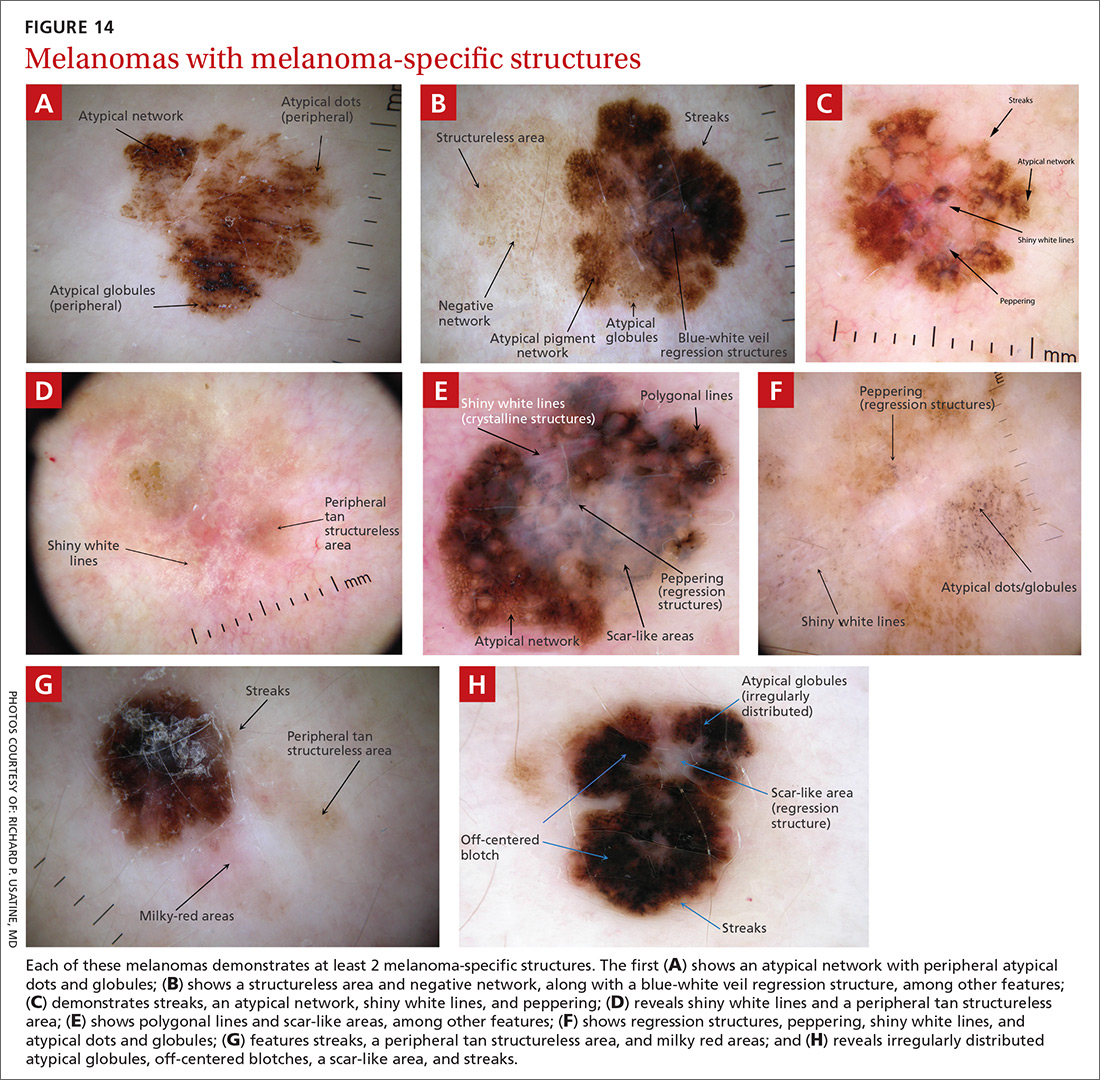
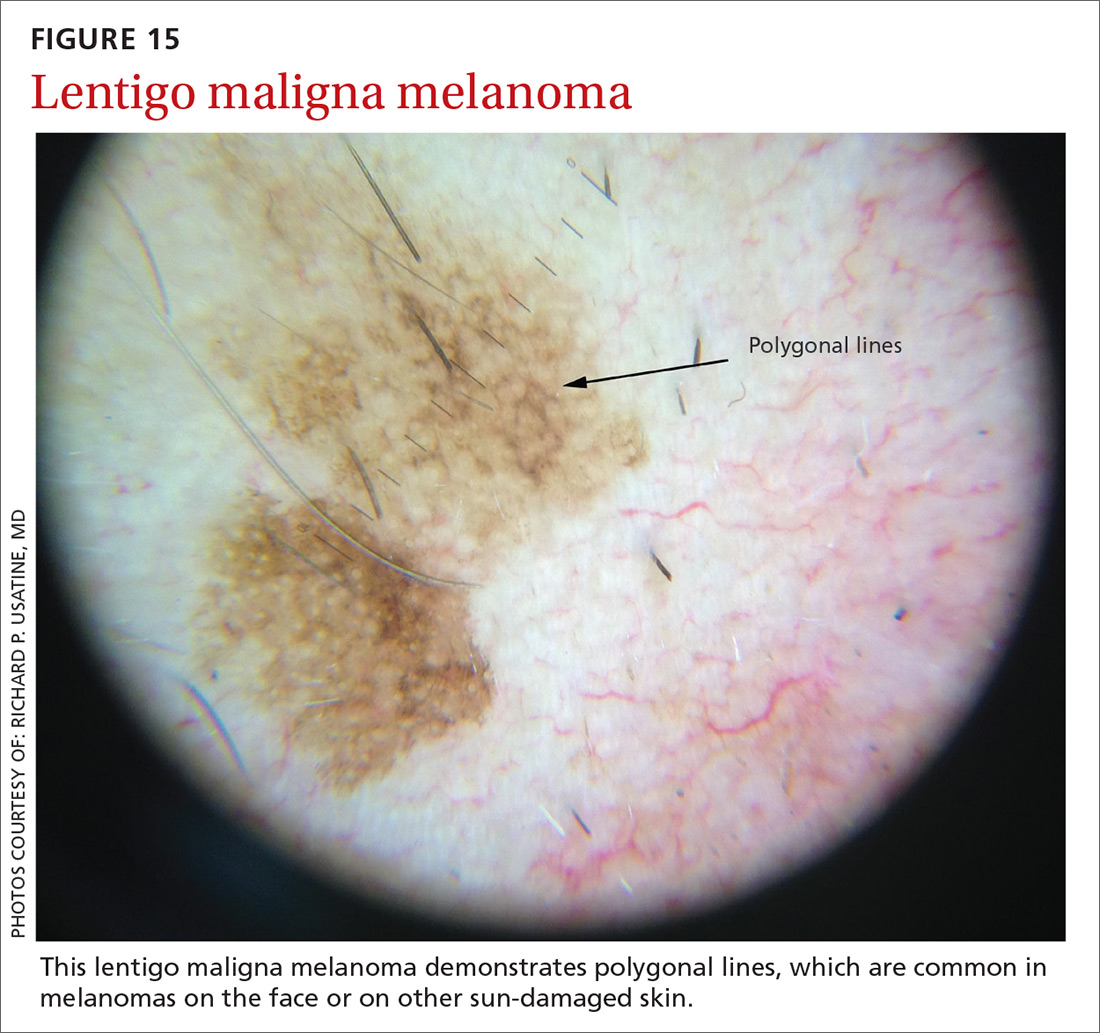
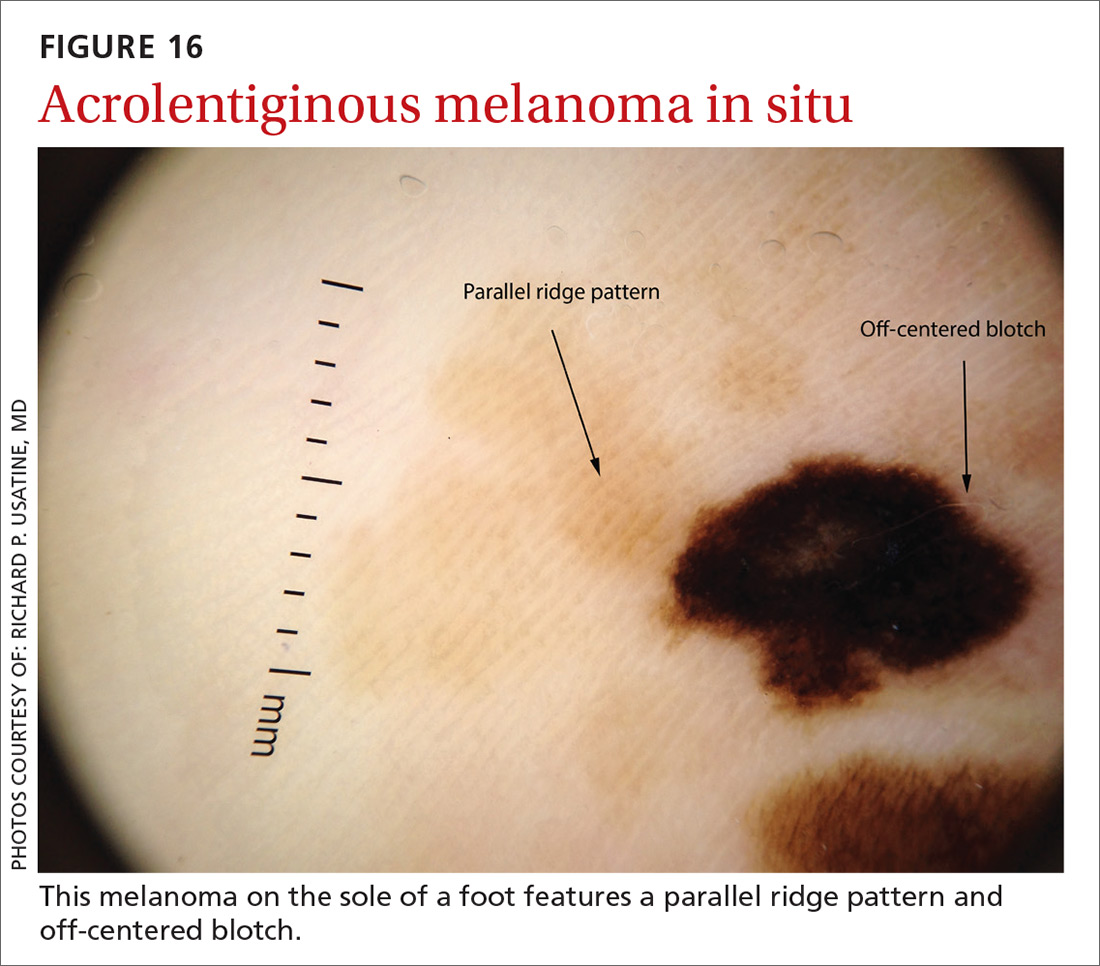
Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).
How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.
This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.
Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.

Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).

Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.

Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).

Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.

Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).

Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).

Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).

Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.

Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17

Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).

Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).

How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.

This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.

Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.

Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).

Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.

Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).

Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.

Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).

Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).

Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).

Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.

Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17

Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).

Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).

How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.

This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.

Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
The art of delivering evidence-based dual antiplatelet therapy
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
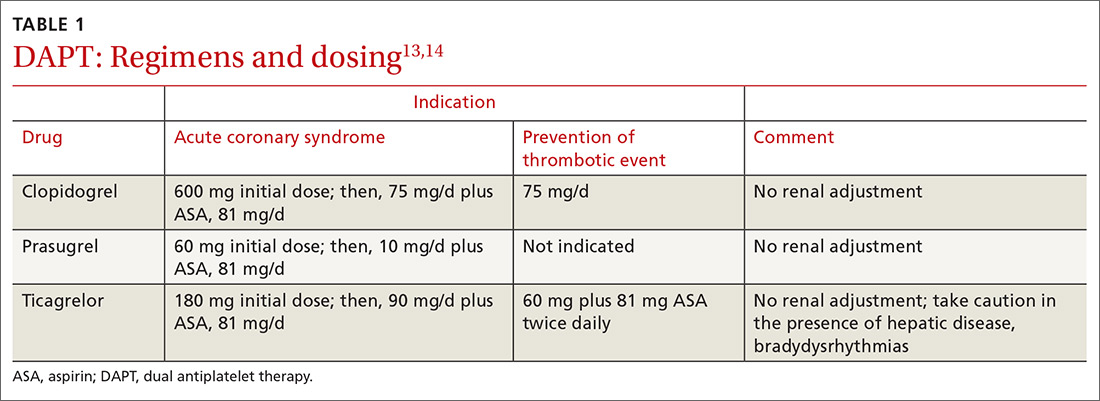
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
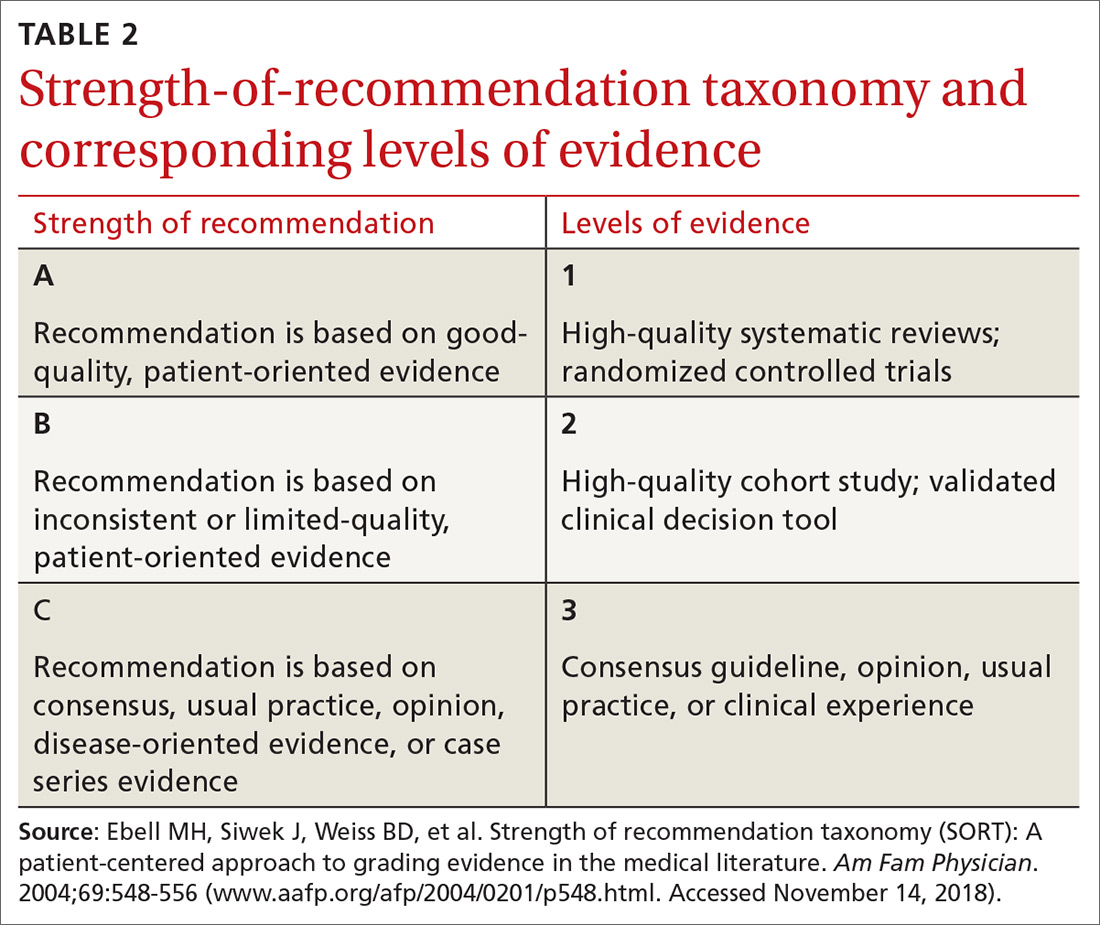
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
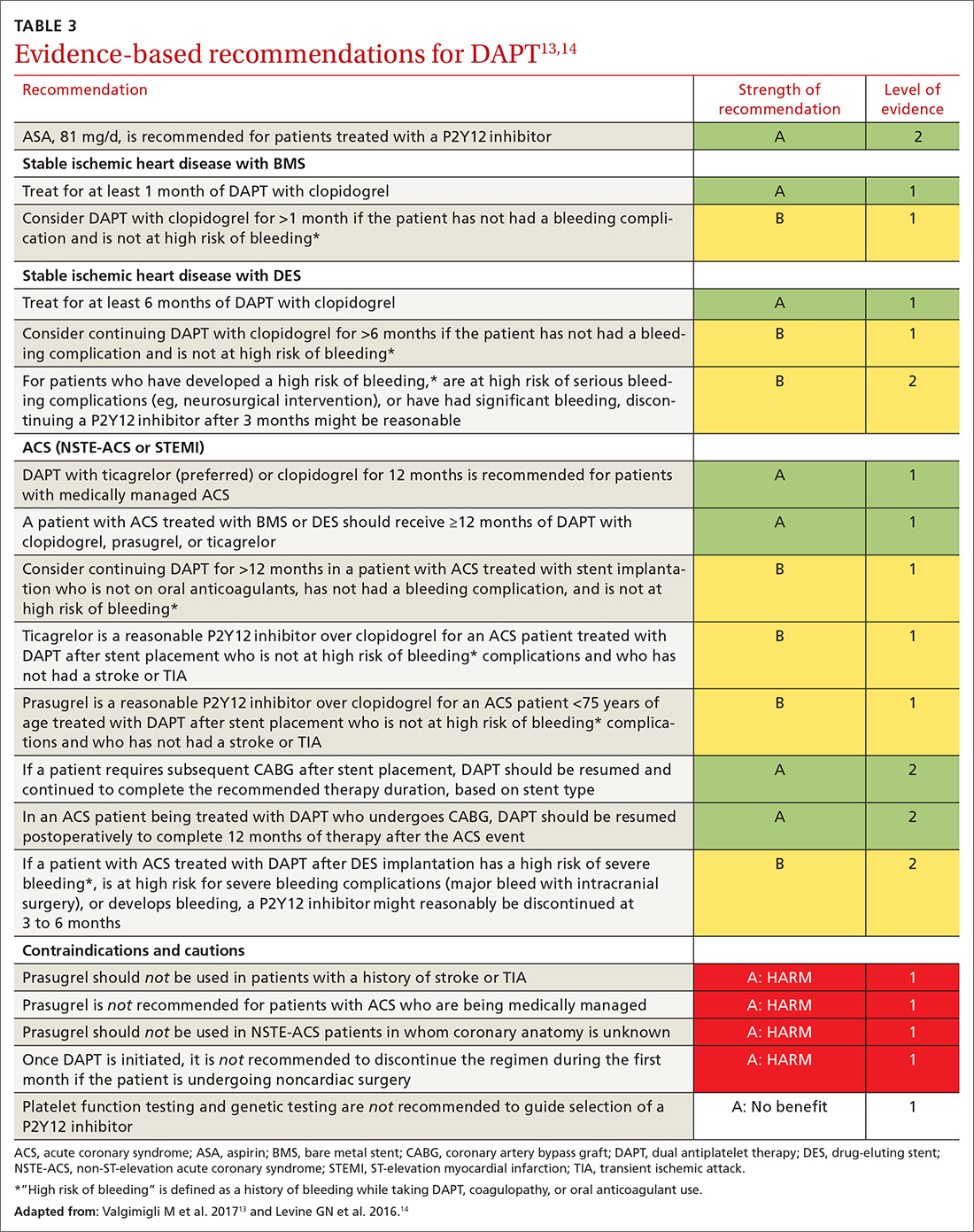
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; [email protected].
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.

DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14

When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)

For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.

How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; [email protected].
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.

DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14

When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)

For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.

How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; [email protected].
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
PRACTICE RECOMMENDATIONS
› Use a dual antiplatelet therapy (DAPT) risk calculator to encourage patient-centric decisions when presenting information to the health care team and the patient. B
› Consider the potential benefit of a shorter duration of DAPT for patients who 1) have prior bleeding complications or 2) are taking an oral anticoagulant, chronic corticosteroid, or nonsteroidal anti-inflammatory drug. B
› Continue aspirin use upon completion of DAPT or if a P2Y12 inhibitor is being held for surgery. A
› Reduce the risk of recurrent stroke in patients who have had a mild ischemic stroke or transient ischemic attack by providing DAPT for 21 to 28 days, followed by aspirin indefinitely—so long as treatment can begin within 24 hours of the event. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Primary hyperparathyroidism: Labs to order, Tx to consider
Since the advent of multichannel serum chemistry screening in the 1970s, large numbers of asymptomatic cases of primary hyperparathyroidism (PHPT) have been discovered. The clinical spectrum of the disease has changed from the classic “moans, groans, bones, and stones” to an asymptomatic and subtle presentation of hypercalcemia.1,2 PHPT and malignancy are the most common causes for hypercalcemia, accounting for 90% of cases.3 In the United States, the estimated incidence of PHPT between 1998 and 2010 was about 50 per 100,000 person-years. Most patients with PHPT are older women (ages >50 years) who are asymptomatic at the time of diagnosis.1
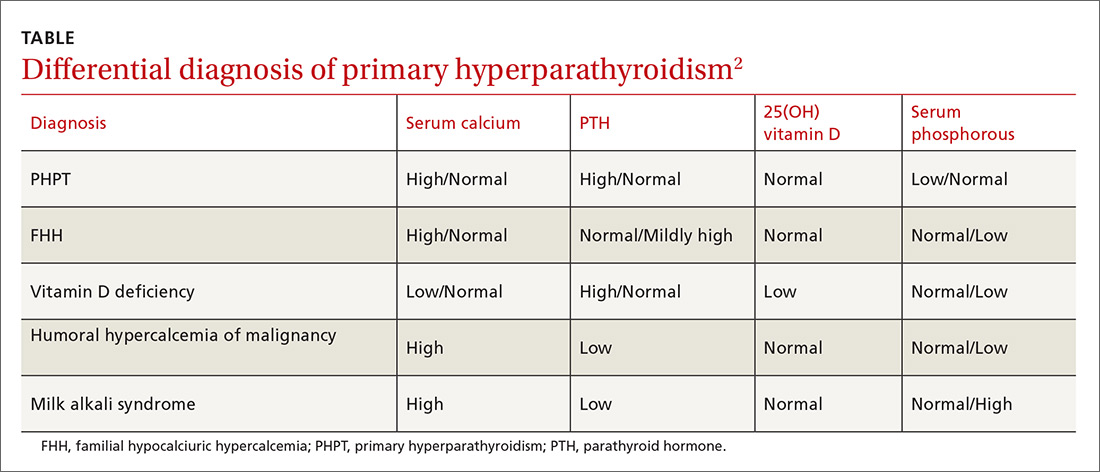
Vigilance needed in primary care. PHPT is slowly progressive, and the patient might accept symptoms as a process of aging. Therefore, it is essential that primary care physicians (PCPs) be aware of the diagnostic and management options. A systematic approach to the diagnosis of PHPT helps differentiate the causes of hypercalcemia (TABLE2; FIGURE2). But before we discuss PHPT diagnostic clues, it’s helpful to quickly review the workings of the parathyroid glands.
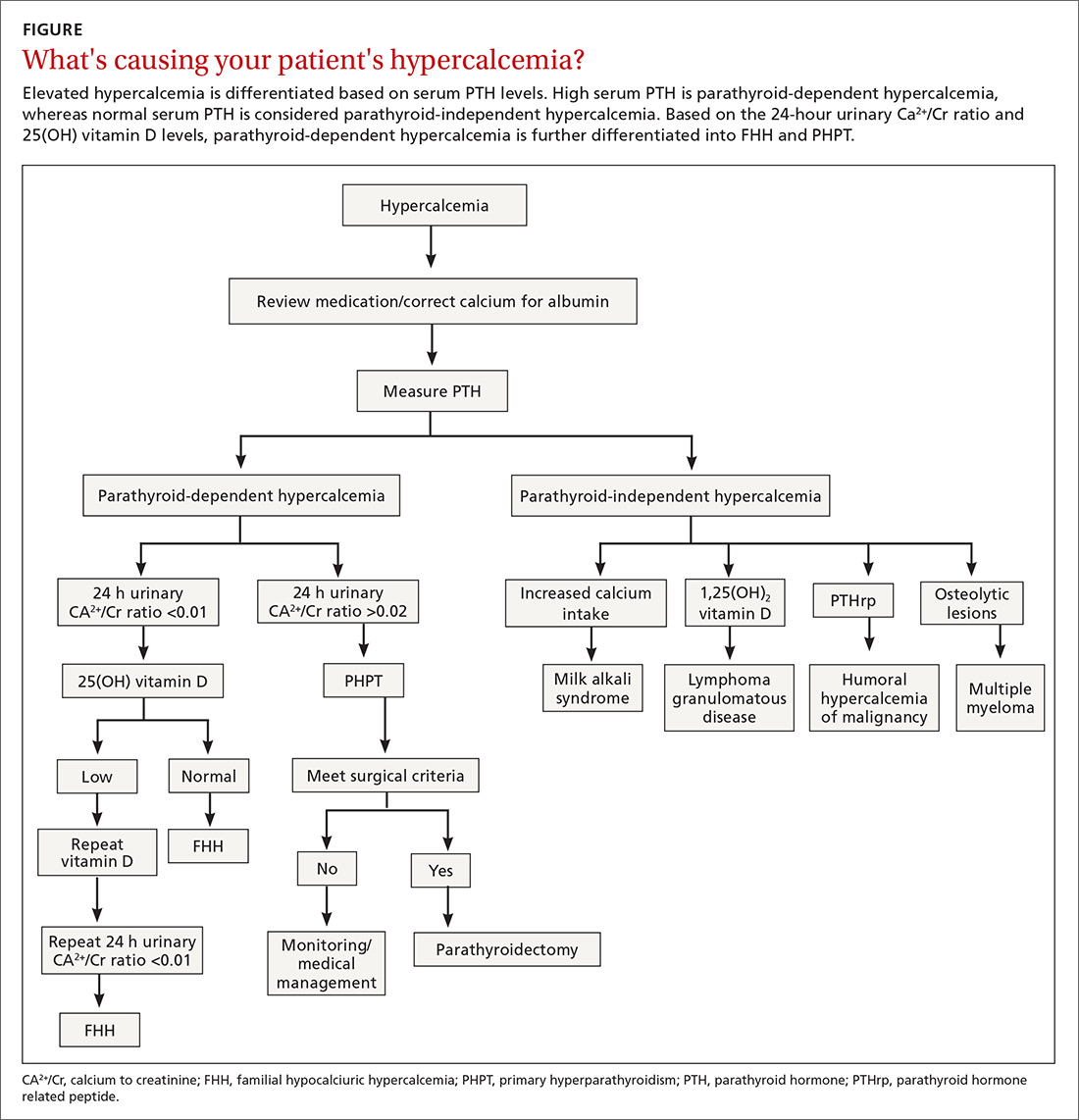
How the glands work, what can go wrong
Parathyroid hormone (PTH) is secreted by 4 pea-sized parathyroid glands located posterior to the thyroid. PTH regulates the levels of calcium (Ca2+) and phosphorous and controls the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme 1 alpha-hydroxylase.
PHPT is regarded as an abnormal secretion of PTH that does not correlate with the levels of Ca2+ in the blood.1 Eighty percent of PHPT is due to a solitary adenoma in one of the parathyroid glands, 2% to 4% is secondary to multiple parathyroid adenomas, 15% is due to parathyroid hyperplasia, and 0.5% due to parathyroid carcinoma.4
Nonspecific symptomsare subtle clues of PHPT
Patients with PHPT can present with nonspecific symptoms, such as weakness, fatigue, anorexia, polyuria, polydipsia, bone and joint pain, mild depression, and mild cognitive or neuromuscular dysfunction.5 A careful history is essential to elicit these symptoms, as the patient may attribute these to aging or other causes. PHPT should also be considered when patients present with kidney stones, unexplained osteoporosis, or fragility fractures. A physical examination is seldom helpful, as parathyroid adenomas are hardly ever palpable. A slit-lamp examination may reveal corneal diseases in rare cases of hypercalcemia.6
Which lab tests, imaging should you order?
Serum Ca2+
Repeat measurements of serum Ca2+ to confirm hypercalcemia. Volume depletion, underlying malignancy, medications such as hydrochlorothiazide, lithium, and excess intake of Ca2+ carbonate can cause hypercalcemia.7 Therefore, a review of the patients’ home medications and dietary preferences in the evaluation of hypercalcemia is essential. The 2 most common causes of hypercalcemia are hyperparathyroidism and hypercalcemia of malignancy.
For hypercalcemia, establish a differential diagnosis by measuring intact PTH. An increased serum Ca2+ level along with an elevated PTH concentration should suggest PTH-dependent hypercalcemia, whereas hypercalcemia with suppressed or low-normal PTH values should suggest PTH-independent hypercalcemia (granulomatous disorders, hypercalcemia of malignancy).
Continue to: Hypercalcemia of malignancy is due to...
Hypercalcemia of malignancy is due to increased production of parathyroid hormone-related peptide from various tumor cells that initiate bone resorption and increased renal Ca2+ absorption. It can also be due to osteolysis from bone metastasis.7 It is generally severe and is a common cause of hypercalcemia in the inpatient setting.
Meticulous evaluation is vital to diagnose PHPT. Measurement of serum ionized Ca2+ reflects the biologically active Ca2+. Studies by Ong and colleagues suggest that about 24% of patients with the histologically proven parathyroid disease had isolated ionized hypercalcemia.8 It is also an important adjunct to diagnose the presumed normocalcemic PHPT in which both the ionized Ca2+ levels and serum total Ca2+ levels should be normal.9
In patients with hypoalbuminemia, a corrected serum Ca2+ is calculated using the equation: corrected Ca2+ = [0.8 × (normal albumin-patient’s albumin)] + serum Ca2+ level.
Serum PTH
Second-generation PTH assays (intact PTH) and third-generation PTH assays (bioactive PTH) are equally reliable in diagnosing PHPT.10 The results obtained with intact and bioactive PTH assays are highly correlated. Several studies have found no improvement in diagnostic accuracy when using the bioactive PTH assay.11,12
Serum PTH can be low, normal, or elevated in hypercalcemia. Hypercalcemia with a high PTH level is parathyroid-dependent hypercalcemia, whereas hypercalcemia with a suppressed PTH level is considered parathyroid-independent.
Continue to: Serum 25(OH) vitamin D
Serum 25(OH) vitamin D
Vitamin D levels are normal in PHPT and normocalcemic PHPT. However, measuring 25(OH) vitamin D in all patients with suspected PHPT is recommended to evaluate for secondary hyperparathyroidism that is due to hypocalcemia or renal failure, which can occur concomitantly with PHPT.
Normocalcemic PHPT can be differentiated from secondary hyperparathyroidism of chronic kidney disease by measuring the 1,25(OH)2 vitamin D level; it will be low in secondary hyperparathyroidism.4
Serum 1,25(OH)2 vitamin D
1,25(OH)2 vitaminD levels are elevated in about one-third of patients with PHPT, as PTH stimulates the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D.13 Although this is not a routine test, it is useful in the evaluation of parathyroid-independent hypercalcemia caused by granulomatous disease, such as sarcoidosis where there is an autonomous production of 1,25(OH)2 vitamin D leading to hypercalcemia.14
Serum creatinine and estimated glomerular filtration rate
Serum creatinine (Cr) helps assess renal function. Reduction in serum Cr clearance to <60 mL/min with no other underlying cause is an indication for parathyroidectomy.10
Serum phosphorous
PTH increases the excretion of phosphorous by inhibiting reabsorption from the proximal tubule. Therefore, serum phosphorus tends to be in the lower range of normal in PHPT, but hypophosphatemia is present in less than a quarter of patients.4
Continue to: 24-hour urinary Ca2+
24-hour urinary Ca2+
A 24-hour urinary Ca2+ excretion is used to assess the risk of renal stones and to differentiate PHPT from familial hypocalciuric hypercalcemia (FHH). Patients with FHH have an abnormality in Ca2+ receptor gene expression in parathyroid cells and renal tubular cells that could lead to parathyroid-mediated hypercalcemia and hypocalciuria. FHH is differentiated from PHPT by calculating a 24-hour urinary Ca2+/Cr ratio. A value of <0.01 is diagnostic of FHH; whereas values >0.02 indicate PHPT. The test can be more accurate when the patient is on a normal Ca2+ and salt diet, when the estimated glomerular filtration rate is >60 mL/min/1.73 m2, and when the serum 25(OH) vitamin D level is >30 ng/dL.15 Adequate urine volume is necessary for the 24-hour Ca2+/Cr ratio to be valid.
Renal imaging
Kidney stones and high Ca2+ deposits in the kidneys are the common manifestations of PHPT. Renal X-ray, computed tomography (CT), or ultrasonography are recommended in the evaluation of patients with PHPT. An incidental finding of either kidney stones or high Ca2+ deposits in the kidneys is an indication for surgery.10
Bone density/DEXA (dual energy X-ray absorptiometry) scan with a vertebral fracture assessment (VFA)
Asymptomatic PHPT individuals with osteoporosis (T-score < 2.5) or vertebral compression fracture benefit from surgical management.10 It is essential to obtain densitometry at 3 sites: the lumbar spine, the hip, and the distal third of the radius. Due to differing amounts of cortical and cancellous bone at the 3 sites and the differential effects of PTH on the cortical and cancellous bone, measurement at all 3 sites allows a clear estimation of the severity of the hyperparathyroid process on the skeleton.16 Therefore, consider measuring serum PTH if the patient has severe osteoporosis or fragility fractures that cannot be explained or that are unresponsive to treatment.
Management
The primary modality of treatment in PHPT is parathyroidectomy. The benefits are many, including an increase in bone mineral density (BMD) and reduction in fractures and kidney stones.10 With modern imaging and intra-operative PTH measurement, the success of minimally invasive parathyroidectomy is high in experienced hands. Patients with PHPT should be referred to an endocrinologist before surgery.
Surgery
Consider surgery if the patient meets any one of the following criteria:
1) overt clinical manifestations (stones, fractures)
2) serum Ca2+ >1 mg/dL above the upper limit of normal
3) Cr clearance <60 mL/min
4) low BMD with a T score ≤2.5 at any site
5) age <50 years
6) uncertain prospect for follow-up.
Continue to: Perform imaging before surgery to identify...
Perform imaging before surgery to identify the overactive parathyroid glands. Ultrasound can detect enlargement of the parathyroid glands. A sestamibi scan, which measures the uptake of Tc99-sestamibi by the parathyroid glands, reflects the activity of the parathyroid glands. In cases of nonlocalization by these 2 modalities, other imaging techniques like 4D CT scan and contrast-enhanced ultrasound can be used. Of note: Imaging is used for localization, but not for diagnosis.
Intra-operative PTH measurement has added to the efficacy of minimally invasive parathyroidectomy. A drop in PTH of >50% after 10 to 15 minutes of excising the gland is considered to be positive.10
Medication management
Monitor patients who refuse surgery or those who do not meet the criteria after surgery. Serum Ca2+ and PTH are monitored annually. DEXA scan needs to be repeated every 1 to 2 years based on the clinical picture. Also assess patients for any fragility fractures and renal endpoints. Recommend taking vitamin D to keep the level above 20 ng/dL.10 Ca2+ intake should follow normally recommended guidelines.
Bisphosphonates are primarily used for the treatment of osteoporosis accompanying PHPT. They decrease bone resorption and, to a lesser extent, bone formation. Alendronate increases BMD at the lumbar spine, but does not have much effect on Ca2+ and PTH levels.
Calcimimetics act by mimicking the effects of Ca2+ on the Ca2+ receptors present on the surface of the parathyroid cells. Therefore, calcimimetics reduce the level of parathyroid hormone and Ca2+ levels. (Long-term benefits have not been established.) Bisphosphonates are prescribed for osteoporosis and calcimimetics for hypercalcemia.10
Continue to: Conclusion
Conclusion
Although largely asymptomatic, consider PHPT when patients present with unexplained kidney stones, osteoporosis, or any nonspecific symptoms described earlier. PHPT is diagnosed by detecting an inappropriately high or normal PTH in relation to the Ca2+ level. Medications need to be reviewed, and conditions such as FHH that produce similar symptoms need to be ruled out. Measurement of 25(OH) vitamin D levels is recommended in all patients with PHPT.
Parathyroidectomy is the definitive form of treatment and should be offered to patients who meet any one of the surgical criteria, as described earlier. It can also be offered to patients who do not meet the criteria if they prefer. It is known to decrease the risk of kidney stones and osteoporosis. Medical therapy is primarily for patients who do not meet the criteria as mentioned earlier and for those who cannot and/or are unwilling to undergo surgery.
CORRESPONDENCE
Padmaja Sanapureddy, MD, Department of Primary Care and Medicine, G.V. (Sonny) Montgomery VA Medical Center, 1500 E Woodrow Wilson Ave, Jackson, MS 39216; [email protected].
1. Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7.
2. Melmed S, Polonsky, KS, Larsen PR, et al. Williams Textbook of Endocrinology: Hormones and Disorders of Mineral Metabolism, 12th ed. Philadelphia, PA: Elsevier Inc, 2011:1262-1263.
3. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3(2):71-79.
4. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;19;2:16033.
5. Roman S, Sosa JA. Psychiatric and cognitive aspects of primary hyperparathyroidism. Curr Opin Oncol. 2007;19:1-5.
6. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66:812-824.
7. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
8. Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:3138-3145.
9. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348-5352.
10. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570-3579.
11. Boudou P, Ibrahim F, Cormier C, et al. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:6370-6372.
12. Carnevale V, Dionisi S, Nofroni I, et al. Potential clinical utility of a new IRMA for parathyroid hormone in postmenopausal patients with primary hyperparathyroidism. Clin Chem. 2004;50:626-631.
13. Jameson JL, De Groot L. Endocrinology: Adult and Pediatric. 7thed. Philadelphia, PA: Elsevier Inc, 2016:1109.
14. Tebben PJ, Singh RJ, Kumar R. Vitamin d-mediated hypercalcemia: mechanisms, diagnosis and treatment. Endocr Rev. 2016;37:521-547.
15. Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19:697-702.
16. Castellano E, Attanasio R, Gianotti L, et al. Forearm DXA increases the rate of patients with asymptomatic primary hyperparathyroidism meeting surgical criteria. J Clin Endocrinol Metab. 2016;101:2728-2732.
Since the advent of multichannel serum chemistry screening in the 1970s, large numbers of asymptomatic cases of primary hyperparathyroidism (PHPT) have been discovered. The clinical spectrum of the disease has changed from the classic “moans, groans, bones, and stones” to an asymptomatic and subtle presentation of hypercalcemia.1,2 PHPT and malignancy are the most common causes for hypercalcemia, accounting for 90% of cases.3 In the United States, the estimated incidence of PHPT between 1998 and 2010 was about 50 per 100,000 person-years. Most patients with PHPT are older women (ages >50 years) who are asymptomatic at the time of diagnosis.1

Vigilance needed in primary care. PHPT is slowly progressive, and the patient might accept symptoms as a process of aging. Therefore, it is essential that primary care physicians (PCPs) be aware of the diagnostic and management options. A systematic approach to the diagnosis of PHPT helps differentiate the causes of hypercalcemia (TABLE2; FIGURE2). But before we discuss PHPT diagnostic clues, it’s helpful to quickly review the workings of the parathyroid glands.

How the glands work, what can go wrong
Parathyroid hormone (PTH) is secreted by 4 pea-sized parathyroid glands located posterior to the thyroid. PTH regulates the levels of calcium (Ca2+) and phosphorous and controls the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme 1 alpha-hydroxylase.
PHPT is regarded as an abnormal secretion of PTH that does not correlate with the levels of Ca2+ in the blood.1 Eighty percent of PHPT is due to a solitary adenoma in one of the parathyroid glands, 2% to 4% is secondary to multiple parathyroid adenomas, 15% is due to parathyroid hyperplasia, and 0.5% due to parathyroid carcinoma.4
Nonspecific symptomsare subtle clues of PHPT
Patients with PHPT can present with nonspecific symptoms, such as weakness, fatigue, anorexia, polyuria, polydipsia, bone and joint pain, mild depression, and mild cognitive or neuromuscular dysfunction.5 A careful history is essential to elicit these symptoms, as the patient may attribute these to aging or other causes. PHPT should also be considered when patients present with kidney stones, unexplained osteoporosis, or fragility fractures. A physical examination is seldom helpful, as parathyroid adenomas are hardly ever palpable. A slit-lamp examination may reveal corneal diseases in rare cases of hypercalcemia.6
Which lab tests, imaging should you order?
Serum Ca2+
Repeat measurements of serum Ca2+ to confirm hypercalcemia. Volume depletion, underlying malignancy, medications such as hydrochlorothiazide, lithium, and excess intake of Ca2+ carbonate can cause hypercalcemia.7 Therefore, a review of the patients’ home medications and dietary preferences in the evaluation of hypercalcemia is essential. The 2 most common causes of hypercalcemia are hyperparathyroidism and hypercalcemia of malignancy.
For hypercalcemia, establish a differential diagnosis by measuring intact PTH. An increased serum Ca2+ level along with an elevated PTH concentration should suggest PTH-dependent hypercalcemia, whereas hypercalcemia with suppressed or low-normal PTH values should suggest PTH-independent hypercalcemia (granulomatous disorders, hypercalcemia of malignancy).
Continue to: Hypercalcemia of malignancy is due to...
Hypercalcemia of malignancy is due to increased production of parathyroid hormone-related peptide from various tumor cells that initiate bone resorption and increased renal Ca2+ absorption. It can also be due to osteolysis from bone metastasis.7 It is generally severe and is a common cause of hypercalcemia in the inpatient setting.
Meticulous evaluation is vital to diagnose PHPT. Measurement of serum ionized Ca2+ reflects the biologically active Ca2+. Studies by Ong and colleagues suggest that about 24% of patients with the histologically proven parathyroid disease had isolated ionized hypercalcemia.8 It is also an important adjunct to diagnose the presumed normocalcemic PHPT in which both the ionized Ca2+ levels and serum total Ca2+ levels should be normal.9
In patients with hypoalbuminemia, a corrected serum Ca2+ is calculated using the equation: corrected Ca2+ = [0.8 × (normal albumin-patient’s albumin)] + serum Ca2+ level.
Serum PTH
Second-generation PTH assays (intact PTH) and third-generation PTH assays (bioactive PTH) are equally reliable in diagnosing PHPT.10 The results obtained with intact and bioactive PTH assays are highly correlated. Several studies have found no improvement in diagnostic accuracy when using the bioactive PTH assay.11,12
Serum PTH can be low, normal, or elevated in hypercalcemia. Hypercalcemia with a high PTH level is parathyroid-dependent hypercalcemia, whereas hypercalcemia with a suppressed PTH level is considered parathyroid-independent.
Continue to: Serum 25(OH) vitamin D
Serum 25(OH) vitamin D
Vitamin D levels are normal in PHPT and normocalcemic PHPT. However, measuring 25(OH) vitamin D in all patients with suspected PHPT is recommended to evaluate for secondary hyperparathyroidism that is due to hypocalcemia or renal failure, which can occur concomitantly with PHPT.
Normocalcemic PHPT can be differentiated from secondary hyperparathyroidism of chronic kidney disease by measuring the 1,25(OH)2 vitamin D level; it will be low in secondary hyperparathyroidism.4
Serum 1,25(OH)2 vitamin D
1,25(OH)2 vitaminD levels are elevated in about one-third of patients with PHPT, as PTH stimulates the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D.13 Although this is not a routine test, it is useful in the evaluation of parathyroid-independent hypercalcemia caused by granulomatous disease, such as sarcoidosis where there is an autonomous production of 1,25(OH)2 vitamin D leading to hypercalcemia.14
Serum creatinine and estimated glomerular filtration rate
Serum creatinine (Cr) helps assess renal function. Reduction in serum Cr clearance to <60 mL/min with no other underlying cause is an indication for parathyroidectomy.10
Serum phosphorous
PTH increases the excretion of phosphorous by inhibiting reabsorption from the proximal tubule. Therefore, serum phosphorus tends to be in the lower range of normal in PHPT, but hypophosphatemia is present in less than a quarter of patients.4
Continue to: 24-hour urinary Ca2+
24-hour urinary Ca2+
A 24-hour urinary Ca2+ excretion is used to assess the risk of renal stones and to differentiate PHPT from familial hypocalciuric hypercalcemia (FHH). Patients with FHH have an abnormality in Ca2+ receptor gene expression in parathyroid cells and renal tubular cells that could lead to parathyroid-mediated hypercalcemia and hypocalciuria. FHH is differentiated from PHPT by calculating a 24-hour urinary Ca2+/Cr ratio. A value of <0.01 is diagnostic of FHH; whereas values >0.02 indicate PHPT. The test can be more accurate when the patient is on a normal Ca2+ and salt diet, when the estimated glomerular filtration rate is >60 mL/min/1.73 m2, and when the serum 25(OH) vitamin D level is >30 ng/dL.15 Adequate urine volume is necessary for the 24-hour Ca2+/Cr ratio to be valid.
Renal imaging
Kidney stones and high Ca2+ deposits in the kidneys are the common manifestations of PHPT. Renal X-ray, computed tomography (CT), or ultrasonography are recommended in the evaluation of patients with PHPT. An incidental finding of either kidney stones or high Ca2+ deposits in the kidneys is an indication for surgery.10
Bone density/DEXA (dual energy X-ray absorptiometry) scan with a vertebral fracture assessment (VFA)
Asymptomatic PHPT individuals with osteoporosis (T-score < 2.5) or vertebral compression fracture benefit from surgical management.10 It is essential to obtain densitometry at 3 sites: the lumbar spine, the hip, and the distal third of the radius. Due to differing amounts of cortical and cancellous bone at the 3 sites and the differential effects of PTH on the cortical and cancellous bone, measurement at all 3 sites allows a clear estimation of the severity of the hyperparathyroid process on the skeleton.16 Therefore, consider measuring serum PTH if the patient has severe osteoporosis or fragility fractures that cannot be explained or that are unresponsive to treatment.
Management
The primary modality of treatment in PHPT is parathyroidectomy. The benefits are many, including an increase in bone mineral density (BMD) and reduction in fractures and kidney stones.10 With modern imaging and intra-operative PTH measurement, the success of minimally invasive parathyroidectomy is high in experienced hands. Patients with PHPT should be referred to an endocrinologist before surgery.
Surgery
Consider surgery if the patient meets any one of the following criteria:
1) overt clinical manifestations (stones, fractures)
2) serum Ca2+ >1 mg/dL above the upper limit of normal
3) Cr clearance <60 mL/min
4) low BMD with a T score ≤2.5 at any site
5) age <50 years
6) uncertain prospect for follow-up.
Continue to: Perform imaging before surgery to identify...
Perform imaging before surgery to identify the overactive parathyroid glands. Ultrasound can detect enlargement of the parathyroid glands. A sestamibi scan, which measures the uptake of Tc99-sestamibi by the parathyroid glands, reflects the activity of the parathyroid glands. In cases of nonlocalization by these 2 modalities, other imaging techniques like 4D CT scan and contrast-enhanced ultrasound can be used. Of note: Imaging is used for localization, but not for diagnosis.
Intra-operative PTH measurement has added to the efficacy of minimally invasive parathyroidectomy. A drop in PTH of >50% after 10 to 15 minutes of excising the gland is considered to be positive.10
Medication management
Monitor patients who refuse surgery or those who do not meet the criteria after surgery. Serum Ca2+ and PTH are monitored annually. DEXA scan needs to be repeated every 1 to 2 years based on the clinical picture. Also assess patients for any fragility fractures and renal endpoints. Recommend taking vitamin D to keep the level above 20 ng/dL.10 Ca2+ intake should follow normally recommended guidelines.
Bisphosphonates are primarily used for the treatment of osteoporosis accompanying PHPT. They decrease bone resorption and, to a lesser extent, bone formation. Alendronate increases BMD at the lumbar spine, but does not have much effect on Ca2+ and PTH levels.
Calcimimetics act by mimicking the effects of Ca2+ on the Ca2+ receptors present on the surface of the parathyroid cells. Therefore, calcimimetics reduce the level of parathyroid hormone and Ca2+ levels. (Long-term benefits have not been established.) Bisphosphonates are prescribed for osteoporosis and calcimimetics for hypercalcemia.10
Continue to: Conclusion
Conclusion
Although largely asymptomatic, consider PHPT when patients present with unexplained kidney stones, osteoporosis, or any nonspecific symptoms described earlier. PHPT is diagnosed by detecting an inappropriately high or normal PTH in relation to the Ca2+ level. Medications need to be reviewed, and conditions such as FHH that produce similar symptoms need to be ruled out. Measurement of 25(OH) vitamin D levels is recommended in all patients with PHPT.
Parathyroidectomy is the definitive form of treatment and should be offered to patients who meet any one of the surgical criteria, as described earlier. It can also be offered to patients who do not meet the criteria if they prefer. It is known to decrease the risk of kidney stones and osteoporosis. Medical therapy is primarily for patients who do not meet the criteria as mentioned earlier and for those who cannot and/or are unwilling to undergo surgery.
CORRESPONDENCE
Padmaja Sanapureddy, MD, Department of Primary Care and Medicine, G.V. (Sonny) Montgomery VA Medical Center, 1500 E Woodrow Wilson Ave, Jackson, MS 39216; [email protected].
Since the advent of multichannel serum chemistry screening in the 1970s, large numbers of asymptomatic cases of primary hyperparathyroidism (PHPT) have been discovered. The clinical spectrum of the disease has changed from the classic “moans, groans, bones, and stones” to an asymptomatic and subtle presentation of hypercalcemia.1,2 PHPT and malignancy are the most common causes for hypercalcemia, accounting for 90% of cases.3 In the United States, the estimated incidence of PHPT between 1998 and 2010 was about 50 per 100,000 person-years. Most patients with PHPT are older women (ages >50 years) who are asymptomatic at the time of diagnosis.1

Vigilance needed in primary care. PHPT is slowly progressive, and the patient might accept symptoms as a process of aging. Therefore, it is essential that primary care physicians (PCPs) be aware of the diagnostic and management options. A systematic approach to the diagnosis of PHPT helps differentiate the causes of hypercalcemia (TABLE2; FIGURE2). But before we discuss PHPT diagnostic clues, it’s helpful to quickly review the workings of the parathyroid glands.

How the glands work, what can go wrong
Parathyroid hormone (PTH) is secreted by 4 pea-sized parathyroid glands located posterior to the thyroid. PTH regulates the levels of calcium (Ca2+) and phosphorous and controls the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme 1 alpha-hydroxylase.
PHPT is regarded as an abnormal secretion of PTH that does not correlate with the levels of Ca2+ in the blood.1 Eighty percent of PHPT is due to a solitary adenoma in one of the parathyroid glands, 2% to 4% is secondary to multiple parathyroid adenomas, 15% is due to parathyroid hyperplasia, and 0.5% due to parathyroid carcinoma.4
Nonspecific symptomsare subtle clues of PHPT
Patients with PHPT can present with nonspecific symptoms, such as weakness, fatigue, anorexia, polyuria, polydipsia, bone and joint pain, mild depression, and mild cognitive or neuromuscular dysfunction.5 A careful history is essential to elicit these symptoms, as the patient may attribute these to aging or other causes. PHPT should also be considered when patients present with kidney stones, unexplained osteoporosis, or fragility fractures. A physical examination is seldom helpful, as parathyroid adenomas are hardly ever palpable. A slit-lamp examination may reveal corneal diseases in rare cases of hypercalcemia.6
Which lab tests, imaging should you order?
Serum Ca2+
Repeat measurements of serum Ca2+ to confirm hypercalcemia. Volume depletion, underlying malignancy, medications such as hydrochlorothiazide, lithium, and excess intake of Ca2+ carbonate can cause hypercalcemia.7 Therefore, a review of the patients’ home medications and dietary preferences in the evaluation of hypercalcemia is essential. The 2 most common causes of hypercalcemia are hyperparathyroidism and hypercalcemia of malignancy.
For hypercalcemia, establish a differential diagnosis by measuring intact PTH. An increased serum Ca2+ level along with an elevated PTH concentration should suggest PTH-dependent hypercalcemia, whereas hypercalcemia with suppressed or low-normal PTH values should suggest PTH-independent hypercalcemia (granulomatous disorders, hypercalcemia of malignancy).
Continue to: Hypercalcemia of malignancy is due to...
Hypercalcemia of malignancy is due to increased production of parathyroid hormone-related peptide from various tumor cells that initiate bone resorption and increased renal Ca2+ absorption. It can also be due to osteolysis from bone metastasis.7 It is generally severe and is a common cause of hypercalcemia in the inpatient setting.
Meticulous evaluation is vital to diagnose PHPT. Measurement of serum ionized Ca2+ reflects the biologically active Ca2+. Studies by Ong and colleagues suggest that about 24% of patients with the histologically proven parathyroid disease had isolated ionized hypercalcemia.8 It is also an important adjunct to diagnose the presumed normocalcemic PHPT in which both the ionized Ca2+ levels and serum total Ca2+ levels should be normal.9
In patients with hypoalbuminemia, a corrected serum Ca2+ is calculated using the equation: corrected Ca2+ = [0.8 × (normal albumin-patient’s albumin)] + serum Ca2+ level.
Serum PTH
Second-generation PTH assays (intact PTH) and third-generation PTH assays (bioactive PTH) are equally reliable in diagnosing PHPT.10 The results obtained with intact and bioactive PTH assays are highly correlated. Several studies have found no improvement in diagnostic accuracy when using the bioactive PTH assay.11,12
Serum PTH can be low, normal, or elevated in hypercalcemia. Hypercalcemia with a high PTH level is parathyroid-dependent hypercalcemia, whereas hypercalcemia with a suppressed PTH level is considered parathyroid-independent.
Continue to: Serum 25(OH) vitamin D
Serum 25(OH) vitamin D
Vitamin D levels are normal in PHPT and normocalcemic PHPT. However, measuring 25(OH) vitamin D in all patients with suspected PHPT is recommended to evaluate for secondary hyperparathyroidism that is due to hypocalcemia or renal failure, which can occur concomitantly with PHPT.
Normocalcemic PHPT can be differentiated from secondary hyperparathyroidism of chronic kidney disease by measuring the 1,25(OH)2 vitamin D level; it will be low in secondary hyperparathyroidism.4
Serum 1,25(OH)2 vitamin D
1,25(OH)2 vitaminD levels are elevated in about one-third of patients with PHPT, as PTH stimulates the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D.13 Although this is not a routine test, it is useful in the evaluation of parathyroid-independent hypercalcemia caused by granulomatous disease, such as sarcoidosis where there is an autonomous production of 1,25(OH)2 vitamin D leading to hypercalcemia.14
Serum creatinine and estimated glomerular filtration rate
Serum creatinine (Cr) helps assess renal function. Reduction in serum Cr clearance to <60 mL/min with no other underlying cause is an indication for parathyroidectomy.10
Serum phosphorous
PTH increases the excretion of phosphorous by inhibiting reabsorption from the proximal tubule. Therefore, serum phosphorus tends to be in the lower range of normal in PHPT, but hypophosphatemia is present in less than a quarter of patients.4
Continue to: 24-hour urinary Ca2+
24-hour urinary Ca2+
A 24-hour urinary Ca2+ excretion is used to assess the risk of renal stones and to differentiate PHPT from familial hypocalciuric hypercalcemia (FHH). Patients with FHH have an abnormality in Ca2+ receptor gene expression in parathyroid cells and renal tubular cells that could lead to parathyroid-mediated hypercalcemia and hypocalciuria. FHH is differentiated from PHPT by calculating a 24-hour urinary Ca2+/Cr ratio. A value of <0.01 is diagnostic of FHH; whereas values >0.02 indicate PHPT. The test can be more accurate when the patient is on a normal Ca2+ and salt diet, when the estimated glomerular filtration rate is >60 mL/min/1.73 m2, and when the serum 25(OH) vitamin D level is >30 ng/dL.15 Adequate urine volume is necessary for the 24-hour Ca2+/Cr ratio to be valid.
Renal imaging
Kidney stones and high Ca2+ deposits in the kidneys are the common manifestations of PHPT. Renal X-ray, computed tomography (CT), or ultrasonography are recommended in the evaluation of patients with PHPT. An incidental finding of either kidney stones or high Ca2+ deposits in the kidneys is an indication for surgery.10
Bone density/DEXA (dual energy X-ray absorptiometry) scan with a vertebral fracture assessment (VFA)
Asymptomatic PHPT individuals with osteoporosis (T-score < 2.5) or vertebral compression fracture benefit from surgical management.10 It is essential to obtain densitometry at 3 sites: the lumbar spine, the hip, and the distal third of the radius. Due to differing amounts of cortical and cancellous bone at the 3 sites and the differential effects of PTH on the cortical and cancellous bone, measurement at all 3 sites allows a clear estimation of the severity of the hyperparathyroid process on the skeleton.16 Therefore, consider measuring serum PTH if the patient has severe osteoporosis or fragility fractures that cannot be explained or that are unresponsive to treatment.
Management
The primary modality of treatment in PHPT is parathyroidectomy. The benefits are many, including an increase in bone mineral density (BMD) and reduction in fractures and kidney stones.10 With modern imaging and intra-operative PTH measurement, the success of minimally invasive parathyroidectomy is high in experienced hands. Patients with PHPT should be referred to an endocrinologist before surgery.
Surgery
Consider surgery if the patient meets any one of the following criteria:
1) overt clinical manifestations (stones, fractures)
2) serum Ca2+ >1 mg/dL above the upper limit of normal
3) Cr clearance <60 mL/min
4) low BMD with a T score ≤2.5 at any site
5) age <50 years
6) uncertain prospect for follow-up.
Continue to: Perform imaging before surgery to identify...
Perform imaging before surgery to identify the overactive parathyroid glands. Ultrasound can detect enlargement of the parathyroid glands. A sestamibi scan, which measures the uptake of Tc99-sestamibi by the parathyroid glands, reflects the activity of the parathyroid glands. In cases of nonlocalization by these 2 modalities, other imaging techniques like 4D CT scan and contrast-enhanced ultrasound can be used. Of note: Imaging is used for localization, but not for diagnosis.
Intra-operative PTH measurement has added to the efficacy of minimally invasive parathyroidectomy. A drop in PTH of >50% after 10 to 15 minutes of excising the gland is considered to be positive.10
Medication management
Monitor patients who refuse surgery or those who do not meet the criteria after surgery. Serum Ca2+ and PTH are monitored annually. DEXA scan needs to be repeated every 1 to 2 years based on the clinical picture. Also assess patients for any fragility fractures and renal endpoints. Recommend taking vitamin D to keep the level above 20 ng/dL.10 Ca2+ intake should follow normally recommended guidelines.
Bisphosphonates are primarily used for the treatment of osteoporosis accompanying PHPT. They decrease bone resorption and, to a lesser extent, bone formation. Alendronate increases BMD at the lumbar spine, but does not have much effect on Ca2+ and PTH levels.
Calcimimetics act by mimicking the effects of Ca2+ on the Ca2+ receptors present on the surface of the parathyroid cells. Therefore, calcimimetics reduce the level of parathyroid hormone and Ca2+ levels. (Long-term benefits have not been established.) Bisphosphonates are prescribed for osteoporosis and calcimimetics for hypercalcemia.10
Continue to: Conclusion
Conclusion
Although largely asymptomatic, consider PHPT when patients present with unexplained kidney stones, osteoporosis, or any nonspecific symptoms described earlier. PHPT is diagnosed by detecting an inappropriately high or normal PTH in relation to the Ca2+ level. Medications need to be reviewed, and conditions such as FHH that produce similar symptoms need to be ruled out. Measurement of 25(OH) vitamin D levels is recommended in all patients with PHPT.
Parathyroidectomy is the definitive form of treatment and should be offered to patients who meet any one of the surgical criteria, as described earlier. It can also be offered to patients who do not meet the criteria if they prefer. It is known to decrease the risk of kidney stones and osteoporosis. Medical therapy is primarily for patients who do not meet the criteria as mentioned earlier and for those who cannot and/or are unwilling to undergo surgery.
CORRESPONDENCE
Padmaja Sanapureddy, MD, Department of Primary Care and Medicine, G.V. (Sonny) Montgomery VA Medical Center, 1500 E Woodrow Wilson Ave, Jackson, MS 39216; [email protected].
1. Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7.
2. Melmed S, Polonsky, KS, Larsen PR, et al. Williams Textbook of Endocrinology: Hormones and Disorders of Mineral Metabolism, 12th ed. Philadelphia, PA: Elsevier Inc, 2011:1262-1263.
3. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3(2):71-79.
4. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;19;2:16033.
5. Roman S, Sosa JA. Psychiatric and cognitive aspects of primary hyperparathyroidism. Curr Opin Oncol. 2007;19:1-5.
6. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66:812-824.
7. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
8. Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:3138-3145.
9. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348-5352.
10. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570-3579.
11. Boudou P, Ibrahim F, Cormier C, et al. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:6370-6372.
12. Carnevale V, Dionisi S, Nofroni I, et al. Potential clinical utility of a new IRMA for parathyroid hormone in postmenopausal patients with primary hyperparathyroidism. Clin Chem. 2004;50:626-631.
13. Jameson JL, De Groot L. Endocrinology: Adult and Pediatric. 7thed. Philadelphia, PA: Elsevier Inc, 2016:1109.
14. Tebben PJ, Singh RJ, Kumar R. Vitamin d-mediated hypercalcemia: mechanisms, diagnosis and treatment. Endocr Rev. 2016;37:521-547.
15. Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19:697-702.
16. Castellano E, Attanasio R, Gianotti L, et al. Forearm DXA increases the rate of patients with asymptomatic primary hyperparathyroidism meeting surgical criteria. J Clin Endocrinol Metab. 2016;101:2728-2732.
1. Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7.
2. Melmed S, Polonsky, KS, Larsen PR, et al. Williams Textbook of Endocrinology: Hormones and Disorders of Mineral Metabolism, 12th ed. Philadelphia, PA: Elsevier Inc, 2011:1262-1263.
3. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3(2):71-79.
4. Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;19;2:16033.
5. Roman S, Sosa JA. Psychiatric and cognitive aspects of primary hyperparathyroidism. Curr Opin Oncol. 2007;19:1-5.
6. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66:812-824.
7. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
8. Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:3138-3145.
9. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348-5352.
10. Eastell R, Brandi ML, Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570-3579.
11. Boudou P, Ibrahim F, Cormier C, et al. Third- or second-generation parathyroid hormone assays: a remaining debate in the diagnosis of primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:6370-6372.
12. Carnevale V, Dionisi S, Nofroni I, et al. Potential clinical utility of a new IRMA for parathyroid hormone in postmenopausal patients with primary hyperparathyroidism. Clin Chem. 2004;50:626-631.
13. Jameson JL, De Groot L. Endocrinology: Adult and Pediatric. 7thed. Philadelphia, PA: Elsevier Inc, 2016:1109.
14. Tebben PJ, Singh RJ, Kumar R. Vitamin d-mediated hypercalcemia: mechanisms, diagnosis and treatment. Endocr Rev. 2016;37:521-547.
15. Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19:697-702.
16. Castellano E, Attanasio R, Gianotti L, et al. Forearm DXA increases the rate of patients with asymptomatic primary hyperparathyroidism meeting surgical criteria. J Clin Endocrinol Metab. 2016;101:2728-2732.
PRACTICE RECOMMENDATIONS
› Evaluate suspected cases of primary hyperparathyroidism (PHPT) with serum total calcium, parathyroid hormone (PTH), creatinine, and 25-hydroxy vitamin D levels. A
› Consider 24-hour urine measurement of calcium and creatinine in patients undergoing evaluation for possible PHPT. A
› Obtain bone densitometry at the spine, hip, and distal radius in patients with PHPT. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Adult foot fractures: A guide
The evaluation and management of acute musculoskeletal conditions are frequently handled by primary care providers.1 It’s estimated that up to 14% of orthopedic complaints encountered by family physicians involve fractures,2 and approximately 15% of these are foot fractures.2 Diagnosis requires radiographic evaluation, but ultrasound is proving useful, too. This article reviews the diagnosis and management of adult foot fractures, with an emphasis on when advanced imaging and referral are indicated (TABLE1,3-10).
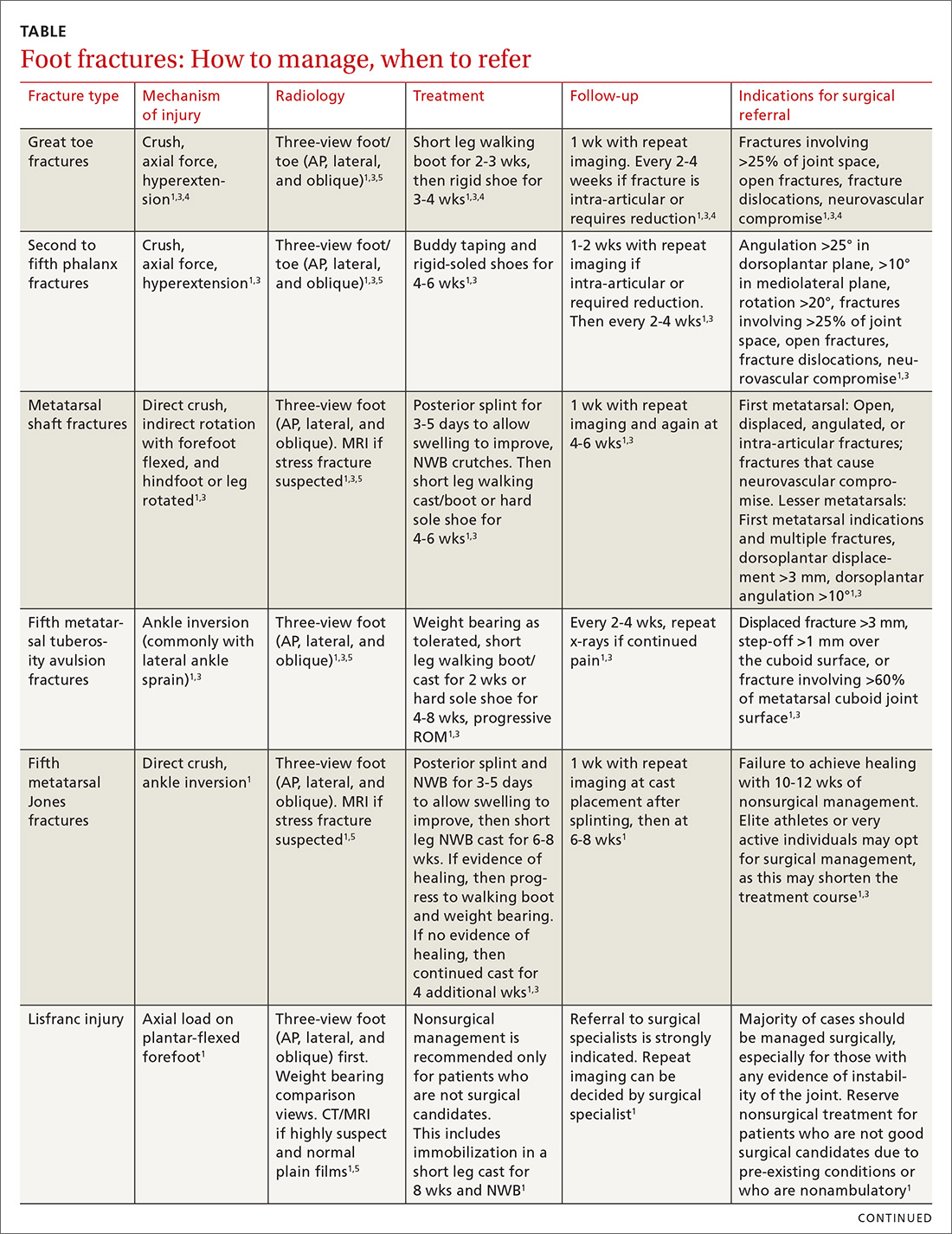
Phalanx fractures: The most common foot fractures
Phalanx fractures typically occur by crush injury, hyperextension, or direct axial force (eg, stubbing the toe).3 Patients with phalanx fractures typically present with pain at or near the site of injury, edema, ecchymosis, and erythema. Throbbing pain is characteristic, and dependent position may worsen the pain.1 Emergently evaluate any fracture causing tenting of the skin, protrusion from the skin, or neurovascular compromise, and attempt realignment to regain neurovascular function.

Most patients with phalanx fractures have point tenderness over the site of the fracture; however, this may also occur with contusions. Placing a gentle loading force along the long axis of the bone distal to the injury may help you differentiate between a contusion and a fracture.4 Pain observed with axial loading of the bone during examination points to a fracture rather than a contusion.
Differential diagnosis
Obtain imaging, including anterior-posterior (AP), lateral, and oblique views at a minimum, for all patients in whom you suspect fractures.5 Multiple fractures of the phalanges are common; therefore, always thoroughly examine the phalanges adjacent to the injured one.
Sesamoid bone fractures are uncommon but do occur and are usually due to direct injury from jumping or landing. The most common sesamoid to be injured is the medial sesamoid of the great toe, although the lateral sesamoid can also be injured. Bipartite sesamoids can occur and may confuse the examiner due to their similar appearance on x-rays to a sesamoid fracture.1 These normal variants often appear smooth and are commonly bilateral as opposed to the jagged or abrupt edges of a fracture. Stress fractures occur as well and are typically due to overuse-type injuries.
Other causes of pain similar to that experienced with phalanx fractures include soft tissue injuries to adjacent ligaments, tendons, and muscles. To help discern the cause of pain, evaluate nail beds for subungual hematomas, indicating injury to the nail bed causing bleeding and pressure under the nail. Obvious deformities of the toes or metatarsal-phalangeal joints signal the possibility of a fracture-dislocation. First metatarsophalangeal (MTP) sprain (“turf toe,” a condition common in athletes who hyperextend the toe, such as when pushing off from hard surfaces like turf) and gout can also present with acute pain in the first phalanx.
Treatment
Due to the role of the great toe in weight bearing and balance, great toe fractures are sometimes managed differently than fractures in Toes 2 through 5. Proper alignment and healing from a fracture in the first toe are critical to prevent future pain and other sequelae. Refer for orthopedic evaluation great toe fractures with displacement, angulation, rotational deformity, neurovascular compromise, >25% involvement of the joint space, or obvious dislocation.1 If referral is not indicated, treat great toe fractures with a short leg walking cast/boot for 2 to 3 weeks followed by buddy taping and use of a hard-soled shoe for 3 to 4 weeks.1
Continue to: With regard to the lesser toes...
With regard to the lesser toes, refer patients with fracture-dislocations, displaced intra-articular fractures, and fractures that do not reduce easily. Nondisplaced fractures of the lesser toes do not require surgical referral.1 These can be treated with splinting (buddy taping) and use of rigid-sole shoes for 4 to 6 weeks. Treatment duration depends largely on patient compliance; generally, continue treatment until point tenderness resolves.1
Treatment of sesamoid fractures consists of resting the affected foot with a walking boot, hard-soled shoe, or “donut” pad under the sesamoid bone to help distribute weight on the foot when standing. Length of treatment is approximately 6 to 8 weeks for most fractures.1 Consider surgical referral if nonoperative management is unsuccessful.
Metatarsal fractures: Look for malalignment
Metatarsal fractures account for 5% of all foot fractures encountered in primary care.2 These fractures typically occur as a result of falls, direct trauma, or rotational injuries (eg, ankle and foot sprains).1 In athletes, the most common cause of these fractures is high rotational force. Patients typically present with pain over the injury site, swelling, bruising, and pain with weight bearing.
As part of your exam, look for malalignment, rotational deformities, and evidence of open fracture. Palpation at the site of the fracture may increase the pain; however, as is true with phalanx fractures, contusions may also cause significant tenderness upon palpation. Also assess range of motion—with special attention to signs of malrotation—and evaluate the adjacent metatarsals, as multiple bones, ligaments, or both are often involved.1
Fifth metatarsal fractures are the most common in adults, likely because of decreased cortical thickness as compared with the other metatarsals.3,11 In addition, multiple soft tissue attachments connect at the proximal aspect of the fifth metatarsal. Classification of these types of metatarsal fractures is based on anatomic location.3 Jones fractures are one type of fracture at the proximal aspect of the fifth metatarsal that occur at the metaphyseal-diaphyseal junction specifically (FIGURE 1). Because this area receives its blood supply from small terminal vessels, fractures here have a high risk of non-union and, thus, should be top of mind in any patient with tenderness at the base of the fifth metatarsal.
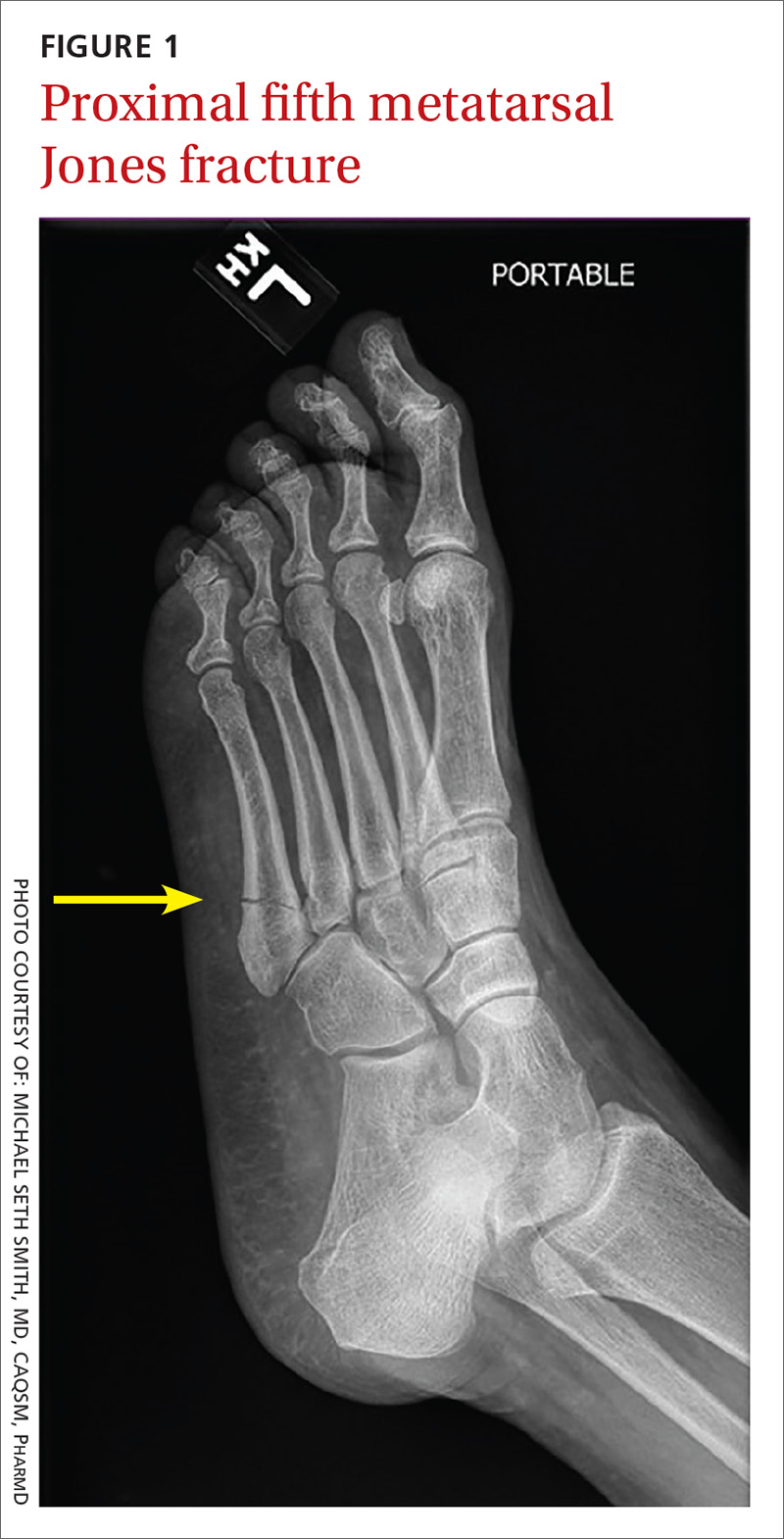
Continue to: MRI/ultrasound in addition to plain films?
MRI/ultrasound in addition to plain films?
Use the Ottawa Ankle and Foot Rules to determine the need for foot radiographs in the acute setting.12 If indicated, imaging should include AP, lateral, and oblique views of the foot.5 Consider magnetic resonance imaging (MRI) if you suspect a stress fracture, which typically presents as an overuse injury in athletes.
Ultrasound may be effective for identifying metatarsal fractures, as well.13 Ultrasound can be used to visualize fractures of the long metatarsal bones and identify displacement, angulation, and step-offs. Although its use in the United States has been limited for this purpose, studies in other countries are showing that it yields results comparable to plain films in the emergency department setting for diagnosis and initial management of these fractures.13
Differential diagnosis
Multiple other diagnoses present similarly to metatarsal fractures including: ligamentous/tendon/soft tissue injuries, interdigital neuroma, sesamoid fractures, and Lisfranc ligament injury (which we'll discuss in a bit). Stress fractures of the metatarsal, most commonly seen in the second metatarsal, are insidious in nature and are common with repetitive movement such as that made by gymnasts, dancers, and track athletes.14 Metatarsalgia, which causes pain at the ball of the foot, is a condition that can stem from a myriad of causes including a low or high arch, biomechanics, and previous injury.
Treatment
Nondisplaced, minimally displaced (<3 mm), and minimally angulated (<10° dorsal/plantar angulation) fractures of any metatarsal shaft may be managed conservatively with a hard-soled shoe or walking boot with weight bearing as tolerated for 4 to 6 weeks.1 Most stress fractures (excluding fifth metatarsal stress fractures) may be treated similarly. Surgical referral is indicated for patients with any open fracture, first metatarsal fractures with displacement, central metatarsal fractures with >10° deformity, >3 mm of translation, multiple fractures, or fifth metatarsal stress fracture.1
Base of fifth metatarsal tuberosity avulsion fractures can be managed nonoperatively with protected weight bearing in a boot or cast for 2 weeks or hard sole shoe for 4 to 8 weeks if the fracture is at the proximal tubercle (see Zone I, FIGURE 2). Jones fractures (Zone II, FIGURE 2) or fractures of the proximal diaphysis (Zone III, FIGURE 2) of the fifth metatarsal may also be managed nonoperatively in patients who are not competitive athletes. This can be done with a non-weight bearing (NWB) short leg cast for 6 to 8 weeks at a minimum.4 If the patient is an athlete who wishes to resume competition, surgical referral is indicated, as the risk of nonunion is high with these fractures.15
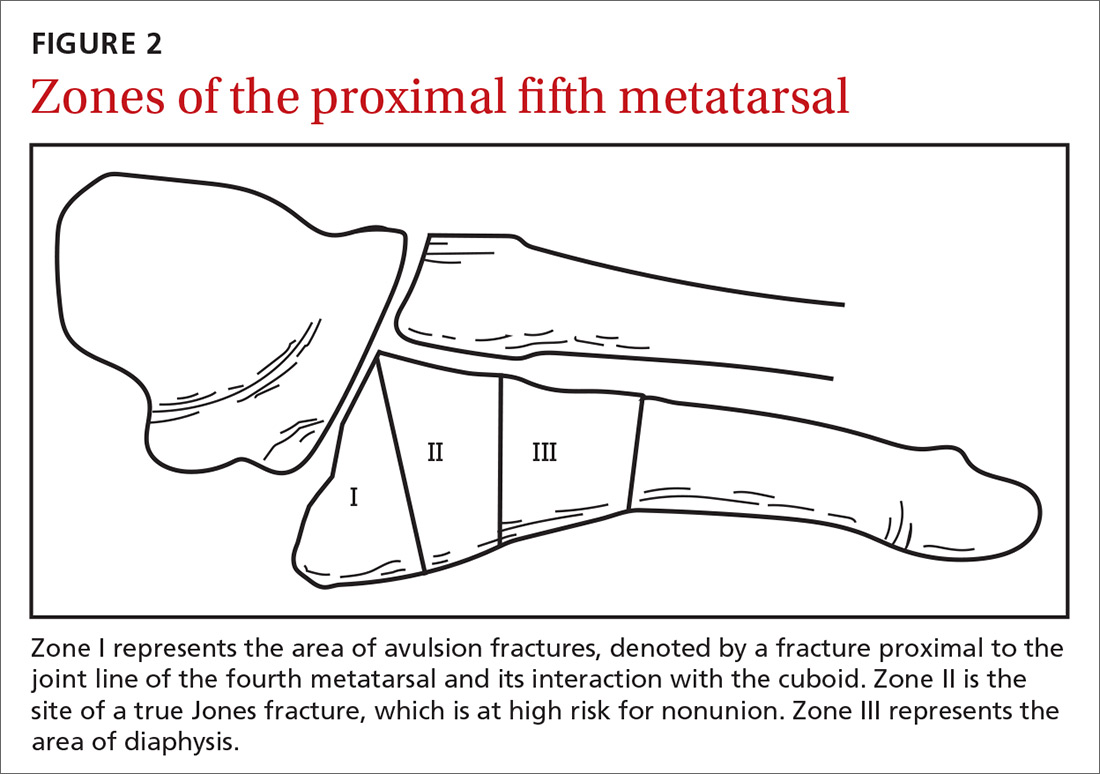
Continue to: Stress fractures and stress reactions...
Stress fractures and stress reactions (early evidence of bone edema seen on MRI, indicating progression to stress fracture) of the proximal fifth metatarsal should be managed with strict activity modifications and a NWB short leg cast for 6 to 8 weeks, given the high risk of nonunion.
Midfoot fractures: The cuboid, cuneiforms, and navicular bone
Although fractures are less common in the midfoot, the midfoot serves an important role in weight bearing and stabilization. In addition, along with the metatarsal bones, the midfoot is critical to proper alignment and articulation.
Cuboid and cuneiform fractures
Cuboid fractures may occur with high-velocity trauma, foot inversion with external rotation of the tibia, a direct blow, or axial load on a plantar-flexed heel. Pain with weight bearing or with walking on toes is usually present. Cuneiform fractures are less common and rarely occur in isolation.6 Mechanisms of injury for cuneiform fractures include direct impact, axial loading on a dorsiflexed or plantar-flexed foot with rotational force, and severe rotation of the midfoot section in a fixed foot. Pain is usually localized to the dorsal or dorsomedial aspect of the foot.
With these 2 midfoot injuries, the exam should include palpation of the cuboid, navicular, and cuneiform bones and careful inspection of the Lisfranc joint, as this can be injured in midfoot fractures.7 Obtain AP, lateral, and oblique views of the foot,5 and strongly consider bilateral weight-bearing films to evaluate for tarsometatarsal (TMT) joint complex injuries (typically Lisfranc joint complex injuries), given their association with midfoot fractures1 (FIGURE 3).
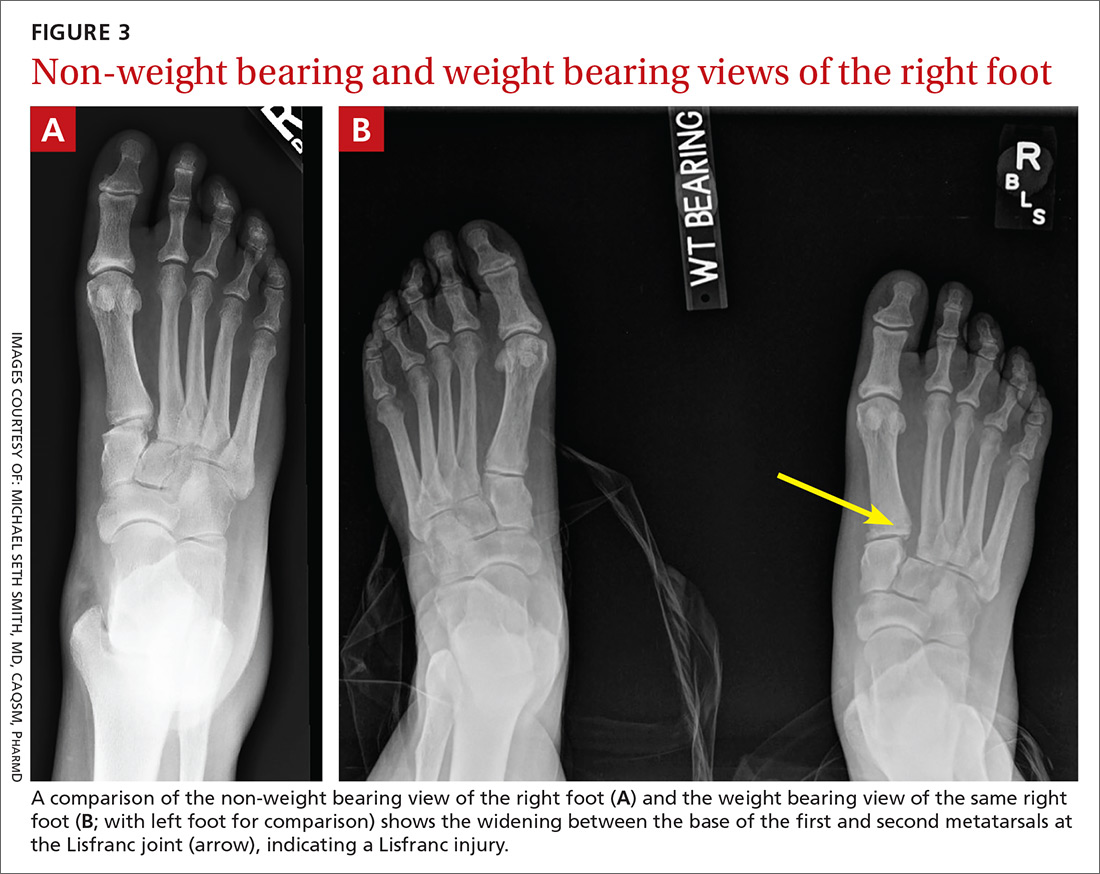
If the fracture is at or near the TMT joint complex, obtain a CT scan or MRI regardless of plain film findings to evaluate the Lisfranc joint complex. Because the Lisfranc joint is particularly important in midfoot stability, untreated injuries can lead to impaired gait or chronic foot pain and deformity. Early identification and surgical referral for these injuries is crucial.
Continue to: Navicular fractures
Navicular fractures
Navicular fractures are typically caused by a twisting mechanism with forced plantar flexion or forced dorsiflexion of the midfoot. They present with severe pain over the dorsal or dorsomedial foot, particularly while bearing weight. Tenderness to palpation over the navicular bone generally warrants imaging studies to rule out fracture, as undiagnosed fractures can lead to severe long-term disability. Use Ottawa Ankle and Foot Rules12 in the acute setting to determine the need for radiographs. If imaging is indicated, obtain AP, lateral, and oblique views.
Tuberosity fractures may be seen on the AP view, while dorsal avulsions, talonavicular joint disruptions, and naviculo-cuneiform joint injuries are better seen on lateral views.16 Patients, particularly cross-country and track athletes, presenting with insidious onset of pain over the navicular bone should be evaluated for stress fracture using MRI, even in the presence of normal radiographs.
Differential diagnosis. Suspect Lisfranc joint complex injuries in any patient with mid-foot pain or fracture. The transverse arch of the foot is reliant upon the articulation of the second metatarsal with all 5 neighboring bones. The Lisfranc ligament is the strongest of 3 supporting ligaments to anchor the TMT joint complex. Other causes of mid-foot pain include soft tissue injury, contusion, and tendinopathy. In addition, other conditions that may cause pain in this area include cuboid syndrome, peroneal tendinopathy, Jones fracture, stress fracture, anterior calcaneal fracture, and sinus tarsi syndrome.
Treatment. Nondisplaced fractures of the cuboid or cuneiform may be treated with a short leg walking cast/boot for 6 weeks followed by the use of a shoe with a thin, rigid, longitudinal arch support for an additional 6 weeks.1 Fractures requiring referral for surgical evaluation include fractures that are open and fractures with vascular or neurological compromise. Also refer comminuted fractures and those that present with >2 mm step-off. Lastly, midfoot fractures that involve the Lisfranc joint should be immobilized and referred for orthopedic evaluation with instructions to the patient to avoid weight bearing until orthopedic evaluation.8
Avulsion fractures of the navicular bone may be managed nonoperatively with a short leg walking cast/boot if there is <20% involvement of the talonavicular surface. Simple nondisplaced body fractures may also be managed conservatively with immobilization and protected weight bearing for 6 to 8 weeks.15 Refer for surgical evaluation avulsion fractures that are intra-articular or dorsal involving 20% or more of the talonavicular surface and tuberosity fractures, given their risk of nonunion.9 All navicular body fractures that are not longitudinal in nature should also be referred for surgical evaluation. Navicular stress fractures that do not extend into the plantar cortex may be managed conservatively with a minimum of 6 weeks of a short leg cast and strict NWB with close follow-up.17
Continue to: Calcaneal fractures
Calcaneal fractures
Calcaneal fractures typically occur from severe axial load or fall from a height. Weight bearing is usually limited and secondary to significant pain. Tenderness to palpation over the calcaneus or with squeezing of the heel will produce pain on exam. Initial x-rays should include a lateral and axial view of the calcaneus. Additional imaging, including a CT scan, may be indicated for further evaluation to determine the extent of the fracture or to determine if a fracture is present despite normal x-rays.10
Acute compartment syndrome occurs in 10% of calcaneal fractures and must be considered in patients with calcaneal fractures from severe trauma.18 Tendon injuries of the ankle, hindfoot, and midfoot may present similarly but can be ruled out with clinical exam and appropriate imaging.
Treatment
Avulsion fractures that do not involve more than 25% of the calcaneocuboid joint and nondisplaced calcaneal fractures may be managed conservatively by instructing patients to wear a NWB short leg cast/boot for 4 to 6 weeks.1 Refer for surgical evaluation patients with calcaneal fracture fragments >1 cm, displacement >3 mm, open fractures, joint involvement >25%, and those whose symptoms fail to resolve with conservative management. Stress fractures can be managed conservatively with cessation of aggravating activities and immobilization in a walking boot until symptoms resolve, which typically takes 4 to 6 weeks.1
CORRESPONDENCE
Michael Seth Smith, MD, CAQSM, PharmD, 3450 Hull Road, Gainesville, FL 32611; [email protected]
1. Eiff MP, Hatch RL, et al. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
2. Hatch RL, Rosenbaum Cl. Fracture care by family physicians. A review of 295 cases. J Fam Pract. 1994;38:238-244.
3. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
4. Hatch RL, Hacking S. Evaluation and management of toe fractures. Am Fam Physician. 2003;68:2413-2418.
5. Snider RK. Essentials of Musculoskeletal Care. 2nd ed. Rosemont, IL: American Orthopedic Surgeons; 2001.
6. Guler F, Baz AB, Turan A, et al. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Spec. 2011;4:306-309.
7. Borrelli J, De S, Van Pelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20:472.
8. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001:32:21-33.
9. Rosenbaum AJ, Uhl R, DiPreta JA. Acute fractures of the tarsal navicular. Orthopedics. 2014;37:541-546.
10. Sanders RW, Clare MP. Calcaneous fractures. In: Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:2064.
11. Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772-776.
12. Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384-390.
13. Ekinci S, Polat O, Günalp M, et al. The accuracy of ultrasound evaluation in foot and ankle trauma. Am J Emerg Med. 2013;31:1551-1555.
14. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
15. Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817-826.
16. Schildhauer TA, Coulibaly MO, Hoffman MF. Fractures and dislocations of the midfoot and forefoot. In: Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:2690.
17. Mayer SW, Joyner PW, Almekinders LC, et al. Stress fractures of the foot and ankle in athletes. Sports Health. 2014;6:481-491.
18. Kalsi R, Dempsey A, Bunney EB. Compartment syndrome of the foot after calcaneal fracture. J Emerg Med. 2012;43:e101-106.
The evaluation and management of acute musculoskeletal conditions are frequently handled by primary care providers.1 It’s estimated that up to 14% of orthopedic complaints encountered by family physicians involve fractures,2 and approximately 15% of these are foot fractures.2 Diagnosis requires radiographic evaluation, but ultrasound is proving useful, too. This article reviews the diagnosis and management of adult foot fractures, with an emphasis on when advanced imaging and referral are indicated (TABLE1,3-10).

Phalanx fractures: The most common foot fractures
Phalanx fractures typically occur by crush injury, hyperextension, or direct axial force (eg, stubbing the toe).3 Patients with phalanx fractures typically present with pain at or near the site of injury, edema, ecchymosis, and erythema. Throbbing pain is characteristic, and dependent position may worsen the pain.1 Emergently evaluate any fracture causing tenting of the skin, protrusion from the skin, or neurovascular compromise, and attempt realignment to regain neurovascular function.

Most patients with phalanx fractures have point tenderness over the site of the fracture; however, this may also occur with contusions. Placing a gentle loading force along the long axis of the bone distal to the injury may help you differentiate between a contusion and a fracture.4 Pain observed with axial loading of the bone during examination points to a fracture rather than a contusion.
Differential diagnosis
Obtain imaging, including anterior-posterior (AP), lateral, and oblique views at a minimum, for all patients in whom you suspect fractures.5 Multiple fractures of the phalanges are common; therefore, always thoroughly examine the phalanges adjacent to the injured one.
Sesamoid bone fractures are uncommon but do occur and are usually due to direct injury from jumping or landing. The most common sesamoid to be injured is the medial sesamoid of the great toe, although the lateral sesamoid can also be injured. Bipartite sesamoids can occur and may confuse the examiner due to their similar appearance on x-rays to a sesamoid fracture.1 These normal variants often appear smooth and are commonly bilateral as opposed to the jagged or abrupt edges of a fracture. Stress fractures occur as well and are typically due to overuse-type injuries.
Other causes of pain similar to that experienced with phalanx fractures include soft tissue injuries to adjacent ligaments, tendons, and muscles. To help discern the cause of pain, evaluate nail beds for subungual hematomas, indicating injury to the nail bed causing bleeding and pressure under the nail. Obvious deformities of the toes or metatarsal-phalangeal joints signal the possibility of a fracture-dislocation. First metatarsophalangeal (MTP) sprain (“turf toe,” a condition common in athletes who hyperextend the toe, such as when pushing off from hard surfaces like turf) and gout can also present with acute pain in the first phalanx.
Treatment
Due to the role of the great toe in weight bearing and balance, great toe fractures are sometimes managed differently than fractures in Toes 2 through 5. Proper alignment and healing from a fracture in the first toe are critical to prevent future pain and other sequelae. Refer for orthopedic evaluation great toe fractures with displacement, angulation, rotational deformity, neurovascular compromise, >25% involvement of the joint space, or obvious dislocation.1 If referral is not indicated, treat great toe fractures with a short leg walking cast/boot for 2 to 3 weeks followed by buddy taping and use of a hard-soled shoe for 3 to 4 weeks.1
Continue to: With regard to the lesser toes...
With regard to the lesser toes, refer patients with fracture-dislocations, displaced intra-articular fractures, and fractures that do not reduce easily. Nondisplaced fractures of the lesser toes do not require surgical referral.1 These can be treated with splinting (buddy taping) and use of rigid-sole shoes for 4 to 6 weeks. Treatment duration depends largely on patient compliance; generally, continue treatment until point tenderness resolves.1
Treatment of sesamoid fractures consists of resting the affected foot with a walking boot, hard-soled shoe, or “donut” pad under the sesamoid bone to help distribute weight on the foot when standing. Length of treatment is approximately 6 to 8 weeks for most fractures.1 Consider surgical referral if nonoperative management is unsuccessful.
Metatarsal fractures: Look for malalignment
Metatarsal fractures account for 5% of all foot fractures encountered in primary care.2 These fractures typically occur as a result of falls, direct trauma, or rotational injuries (eg, ankle and foot sprains).1 In athletes, the most common cause of these fractures is high rotational force. Patients typically present with pain over the injury site, swelling, bruising, and pain with weight bearing.
As part of your exam, look for malalignment, rotational deformities, and evidence of open fracture. Palpation at the site of the fracture may increase the pain; however, as is true with phalanx fractures, contusions may also cause significant tenderness upon palpation. Also assess range of motion—with special attention to signs of malrotation—and evaluate the adjacent metatarsals, as multiple bones, ligaments, or both are often involved.1
Fifth metatarsal fractures are the most common in adults, likely because of decreased cortical thickness as compared with the other metatarsals.3,11 In addition, multiple soft tissue attachments connect at the proximal aspect of the fifth metatarsal. Classification of these types of metatarsal fractures is based on anatomic location.3 Jones fractures are one type of fracture at the proximal aspect of the fifth metatarsal that occur at the metaphyseal-diaphyseal junction specifically (FIGURE 1). Because this area receives its blood supply from small terminal vessels, fractures here have a high risk of non-union and, thus, should be top of mind in any patient with tenderness at the base of the fifth metatarsal.

Continue to: MRI/ultrasound in addition to plain films?
MRI/ultrasound in addition to plain films?
Use the Ottawa Ankle and Foot Rules to determine the need for foot radiographs in the acute setting.12 If indicated, imaging should include AP, lateral, and oblique views of the foot.5 Consider magnetic resonance imaging (MRI) if you suspect a stress fracture, which typically presents as an overuse injury in athletes.
Ultrasound may be effective for identifying metatarsal fractures, as well.13 Ultrasound can be used to visualize fractures of the long metatarsal bones and identify displacement, angulation, and step-offs. Although its use in the United States has been limited for this purpose, studies in other countries are showing that it yields results comparable to plain films in the emergency department setting for diagnosis and initial management of these fractures.13
Differential diagnosis
Multiple other diagnoses present similarly to metatarsal fractures including: ligamentous/tendon/soft tissue injuries, interdigital neuroma, sesamoid fractures, and Lisfranc ligament injury (which we'll discuss in a bit). Stress fractures of the metatarsal, most commonly seen in the second metatarsal, are insidious in nature and are common with repetitive movement such as that made by gymnasts, dancers, and track athletes.14 Metatarsalgia, which causes pain at the ball of the foot, is a condition that can stem from a myriad of causes including a low or high arch, biomechanics, and previous injury.
Treatment
Nondisplaced, minimally displaced (<3 mm), and minimally angulated (<10° dorsal/plantar angulation) fractures of any metatarsal shaft may be managed conservatively with a hard-soled shoe or walking boot with weight bearing as tolerated for 4 to 6 weeks.1 Most stress fractures (excluding fifth metatarsal stress fractures) may be treated similarly. Surgical referral is indicated for patients with any open fracture, first metatarsal fractures with displacement, central metatarsal fractures with >10° deformity, >3 mm of translation, multiple fractures, or fifth metatarsal stress fracture.1
Base of fifth metatarsal tuberosity avulsion fractures can be managed nonoperatively with protected weight bearing in a boot or cast for 2 weeks or hard sole shoe for 4 to 8 weeks if the fracture is at the proximal tubercle (see Zone I, FIGURE 2). Jones fractures (Zone II, FIGURE 2) or fractures of the proximal diaphysis (Zone III, FIGURE 2) of the fifth metatarsal may also be managed nonoperatively in patients who are not competitive athletes. This can be done with a non-weight bearing (NWB) short leg cast for 6 to 8 weeks at a minimum.4 If the patient is an athlete who wishes to resume competition, surgical referral is indicated, as the risk of nonunion is high with these fractures.15

Continue to: Stress fractures and stress reactions...
Stress fractures and stress reactions (early evidence of bone edema seen on MRI, indicating progression to stress fracture) of the proximal fifth metatarsal should be managed with strict activity modifications and a NWB short leg cast for 6 to 8 weeks, given the high risk of nonunion.
Midfoot fractures: The cuboid, cuneiforms, and navicular bone
Although fractures are less common in the midfoot, the midfoot serves an important role in weight bearing and stabilization. In addition, along with the metatarsal bones, the midfoot is critical to proper alignment and articulation.
Cuboid and cuneiform fractures
Cuboid fractures may occur with high-velocity trauma, foot inversion with external rotation of the tibia, a direct blow, or axial load on a plantar-flexed heel. Pain with weight bearing or with walking on toes is usually present. Cuneiform fractures are less common and rarely occur in isolation.6 Mechanisms of injury for cuneiform fractures include direct impact, axial loading on a dorsiflexed or plantar-flexed foot with rotational force, and severe rotation of the midfoot section in a fixed foot. Pain is usually localized to the dorsal or dorsomedial aspect of the foot.
With these 2 midfoot injuries, the exam should include palpation of the cuboid, navicular, and cuneiform bones and careful inspection of the Lisfranc joint, as this can be injured in midfoot fractures.7 Obtain AP, lateral, and oblique views of the foot,5 and strongly consider bilateral weight-bearing films to evaluate for tarsometatarsal (TMT) joint complex injuries (typically Lisfranc joint complex injuries), given their association with midfoot fractures1 (FIGURE 3).

If the fracture is at or near the TMT joint complex, obtain a CT scan or MRI regardless of plain film findings to evaluate the Lisfranc joint complex. Because the Lisfranc joint is particularly important in midfoot stability, untreated injuries can lead to impaired gait or chronic foot pain and deformity. Early identification and surgical referral for these injuries is crucial.
Continue to: Navicular fractures
Navicular fractures
Navicular fractures are typically caused by a twisting mechanism with forced plantar flexion or forced dorsiflexion of the midfoot. They present with severe pain over the dorsal or dorsomedial foot, particularly while bearing weight. Tenderness to palpation over the navicular bone generally warrants imaging studies to rule out fracture, as undiagnosed fractures can lead to severe long-term disability. Use Ottawa Ankle and Foot Rules12 in the acute setting to determine the need for radiographs. If imaging is indicated, obtain AP, lateral, and oblique views.
Tuberosity fractures may be seen on the AP view, while dorsal avulsions, talonavicular joint disruptions, and naviculo-cuneiform joint injuries are better seen on lateral views.16 Patients, particularly cross-country and track athletes, presenting with insidious onset of pain over the navicular bone should be evaluated for stress fracture using MRI, even in the presence of normal radiographs.
Differential diagnosis. Suspect Lisfranc joint complex injuries in any patient with mid-foot pain or fracture. The transverse arch of the foot is reliant upon the articulation of the second metatarsal with all 5 neighboring bones. The Lisfranc ligament is the strongest of 3 supporting ligaments to anchor the TMT joint complex. Other causes of mid-foot pain include soft tissue injury, contusion, and tendinopathy. In addition, other conditions that may cause pain in this area include cuboid syndrome, peroneal tendinopathy, Jones fracture, stress fracture, anterior calcaneal fracture, and sinus tarsi syndrome.
Treatment. Nondisplaced fractures of the cuboid or cuneiform may be treated with a short leg walking cast/boot for 6 weeks followed by the use of a shoe with a thin, rigid, longitudinal arch support for an additional 6 weeks.1 Fractures requiring referral for surgical evaluation include fractures that are open and fractures with vascular or neurological compromise. Also refer comminuted fractures and those that present with >2 mm step-off. Lastly, midfoot fractures that involve the Lisfranc joint should be immobilized and referred for orthopedic evaluation with instructions to the patient to avoid weight bearing until orthopedic evaluation.8
Avulsion fractures of the navicular bone may be managed nonoperatively with a short leg walking cast/boot if there is <20% involvement of the talonavicular surface. Simple nondisplaced body fractures may also be managed conservatively with immobilization and protected weight bearing for 6 to 8 weeks.15 Refer for surgical evaluation avulsion fractures that are intra-articular or dorsal involving 20% or more of the talonavicular surface and tuberosity fractures, given their risk of nonunion.9 All navicular body fractures that are not longitudinal in nature should also be referred for surgical evaluation. Navicular stress fractures that do not extend into the plantar cortex may be managed conservatively with a minimum of 6 weeks of a short leg cast and strict NWB with close follow-up.17
Continue to: Calcaneal fractures
Calcaneal fractures
Calcaneal fractures typically occur from severe axial load or fall from a height. Weight bearing is usually limited and secondary to significant pain. Tenderness to palpation over the calcaneus or with squeezing of the heel will produce pain on exam. Initial x-rays should include a lateral and axial view of the calcaneus. Additional imaging, including a CT scan, may be indicated for further evaluation to determine the extent of the fracture or to determine if a fracture is present despite normal x-rays.10
Acute compartment syndrome occurs in 10% of calcaneal fractures and must be considered in patients with calcaneal fractures from severe trauma.18 Tendon injuries of the ankle, hindfoot, and midfoot may present similarly but can be ruled out with clinical exam and appropriate imaging.
Treatment
Avulsion fractures that do not involve more than 25% of the calcaneocuboid joint and nondisplaced calcaneal fractures may be managed conservatively by instructing patients to wear a NWB short leg cast/boot for 4 to 6 weeks.1 Refer for surgical evaluation patients with calcaneal fracture fragments >1 cm, displacement >3 mm, open fractures, joint involvement >25%, and those whose symptoms fail to resolve with conservative management. Stress fractures can be managed conservatively with cessation of aggravating activities and immobilization in a walking boot until symptoms resolve, which typically takes 4 to 6 weeks.1
CORRESPONDENCE
Michael Seth Smith, MD, CAQSM, PharmD, 3450 Hull Road, Gainesville, FL 32611; [email protected]
The evaluation and management of acute musculoskeletal conditions are frequently handled by primary care providers.1 It’s estimated that up to 14% of orthopedic complaints encountered by family physicians involve fractures,2 and approximately 15% of these are foot fractures.2 Diagnosis requires radiographic evaluation, but ultrasound is proving useful, too. This article reviews the diagnosis and management of adult foot fractures, with an emphasis on when advanced imaging and referral are indicated (TABLE1,3-10).

Phalanx fractures: The most common foot fractures
Phalanx fractures typically occur by crush injury, hyperextension, or direct axial force (eg, stubbing the toe).3 Patients with phalanx fractures typically present with pain at or near the site of injury, edema, ecchymosis, and erythema. Throbbing pain is characteristic, and dependent position may worsen the pain.1 Emergently evaluate any fracture causing tenting of the skin, protrusion from the skin, or neurovascular compromise, and attempt realignment to regain neurovascular function.

Most patients with phalanx fractures have point tenderness over the site of the fracture; however, this may also occur with contusions. Placing a gentle loading force along the long axis of the bone distal to the injury may help you differentiate between a contusion and a fracture.4 Pain observed with axial loading of the bone during examination points to a fracture rather than a contusion.
Differential diagnosis
Obtain imaging, including anterior-posterior (AP), lateral, and oblique views at a minimum, for all patients in whom you suspect fractures.5 Multiple fractures of the phalanges are common; therefore, always thoroughly examine the phalanges adjacent to the injured one.
Sesamoid bone fractures are uncommon but do occur and are usually due to direct injury from jumping or landing. The most common sesamoid to be injured is the medial sesamoid of the great toe, although the lateral sesamoid can also be injured. Bipartite sesamoids can occur and may confuse the examiner due to their similar appearance on x-rays to a sesamoid fracture.1 These normal variants often appear smooth and are commonly bilateral as opposed to the jagged or abrupt edges of a fracture. Stress fractures occur as well and are typically due to overuse-type injuries.
Other causes of pain similar to that experienced with phalanx fractures include soft tissue injuries to adjacent ligaments, tendons, and muscles. To help discern the cause of pain, evaluate nail beds for subungual hematomas, indicating injury to the nail bed causing bleeding and pressure under the nail. Obvious deformities of the toes or metatarsal-phalangeal joints signal the possibility of a fracture-dislocation. First metatarsophalangeal (MTP) sprain (“turf toe,” a condition common in athletes who hyperextend the toe, such as when pushing off from hard surfaces like turf) and gout can also present with acute pain in the first phalanx.
Treatment
Due to the role of the great toe in weight bearing and balance, great toe fractures are sometimes managed differently than fractures in Toes 2 through 5. Proper alignment and healing from a fracture in the first toe are critical to prevent future pain and other sequelae. Refer for orthopedic evaluation great toe fractures with displacement, angulation, rotational deformity, neurovascular compromise, >25% involvement of the joint space, or obvious dislocation.1 If referral is not indicated, treat great toe fractures with a short leg walking cast/boot for 2 to 3 weeks followed by buddy taping and use of a hard-soled shoe for 3 to 4 weeks.1
Continue to: With regard to the lesser toes...
With regard to the lesser toes, refer patients with fracture-dislocations, displaced intra-articular fractures, and fractures that do not reduce easily. Nondisplaced fractures of the lesser toes do not require surgical referral.1 These can be treated with splinting (buddy taping) and use of rigid-sole shoes for 4 to 6 weeks. Treatment duration depends largely on patient compliance; generally, continue treatment until point tenderness resolves.1
Treatment of sesamoid fractures consists of resting the affected foot with a walking boot, hard-soled shoe, or “donut” pad under the sesamoid bone to help distribute weight on the foot when standing. Length of treatment is approximately 6 to 8 weeks for most fractures.1 Consider surgical referral if nonoperative management is unsuccessful.
Metatarsal fractures: Look for malalignment
Metatarsal fractures account for 5% of all foot fractures encountered in primary care.2 These fractures typically occur as a result of falls, direct trauma, or rotational injuries (eg, ankle and foot sprains).1 In athletes, the most common cause of these fractures is high rotational force. Patients typically present with pain over the injury site, swelling, bruising, and pain with weight bearing.
As part of your exam, look for malalignment, rotational deformities, and evidence of open fracture. Palpation at the site of the fracture may increase the pain; however, as is true with phalanx fractures, contusions may also cause significant tenderness upon palpation. Also assess range of motion—with special attention to signs of malrotation—and evaluate the adjacent metatarsals, as multiple bones, ligaments, or both are often involved.1
Fifth metatarsal fractures are the most common in adults, likely because of decreased cortical thickness as compared with the other metatarsals.3,11 In addition, multiple soft tissue attachments connect at the proximal aspect of the fifth metatarsal. Classification of these types of metatarsal fractures is based on anatomic location.3 Jones fractures are one type of fracture at the proximal aspect of the fifth metatarsal that occur at the metaphyseal-diaphyseal junction specifically (FIGURE 1). Because this area receives its blood supply from small terminal vessels, fractures here have a high risk of non-union and, thus, should be top of mind in any patient with tenderness at the base of the fifth metatarsal.

Continue to: MRI/ultrasound in addition to plain films?
MRI/ultrasound in addition to plain films?
Use the Ottawa Ankle and Foot Rules to determine the need for foot radiographs in the acute setting.12 If indicated, imaging should include AP, lateral, and oblique views of the foot.5 Consider magnetic resonance imaging (MRI) if you suspect a stress fracture, which typically presents as an overuse injury in athletes.
Ultrasound may be effective for identifying metatarsal fractures, as well.13 Ultrasound can be used to visualize fractures of the long metatarsal bones and identify displacement, angulation, and step-offs. Although its use in the United States has been limited for this purpose, studies in other countries are showing that it yields results comparable to plain films in the emergency department setting for diagnosis and initial management of these fractures.13
Differential diagnosis
Multiple other diagnoses present similarly to metatarsal fractures including: ligamentous/tendon/soft tissue injuries, interdigital neuroma, sesamoid fractures, and Lisfranc ligament injury (which we'll discuss in a bit). Stress fractures of the metatarsal, most commonly seen in the second metatarsal, are insidious in nature and are common with repetitive movement such as that made by gymnasts, dancers, and track athletes.14 Metatarsalgia, which causes pain at the ball of the foot, is a condition that can stem from a myriad of causes including a low or high arch, biomechanics, and previous injury.
Treatment
Nondisplaced, minimally displaced (<3 mm), and minimally angulated (<10° dorsal/plantar angulation) fractures of any metatarsal shaft may be managed conservatively with a hard-soled shoe or walking boot with weight bearing as tolerated for 4 to 6 weeks.1 Most stress fractures (excluding fifth metatarsal stress fractures) may be treated similarly. Surgical referral is indicated for patients with any open fracture, first metatarsal fractures with displacement, central metatarsal fractures with >10° deformity, >3 mm of translation, multiple fractures, or fifth metatarsal stress fracture.1
Base of fifth metatarsal tuberosity avulsion fractures can be managed nonoperatively with protected weight bearing in a boot or cast for 2 weeks or hard sole shoe for 4 to 8 weeks if the fracture is at the proximal tubercle (see Zone I, FIGURE 2). Jones fractures (Zone II, FIGURE 2) or fractures of the proximal diaphysis (Zone III, FIGURE 2) of the fifth metatarsal may also be managed nonoperatively in patients who are not competitive athletes. This can be done with a non-weight bearing (NWB) short leg cast for 6 to 8 weeks at a minimum.4 If the patient is an athlete who wishes to resume competition, surgical referral is indicated, as the risk of nonunion is high with these fractures.15

Continue to: Stress fractures and stress reactions...
Stress fractures and stress reactions (early evidence of bone edema seen on MRI, indicating progression to stress fracture) of the proximal fifth metatarsal should be managed with strict activity modifications and a NWB short leg cast for 6 to 8 weeks, given the high risk of nonunion.
Midfoot fractures: The cuboid, cuneiforms, and navicular bone
Although fractures are less common in the midfoot, the midfoot serves an important role in weight bearing and stabilization. In addition, along with the metatarsal bones, the midfoot is critical to proper alignment and articulation.
Cuboid and cuneiform fractures
Cuboid fractures may occur with high-velocity trauma, foot inversion with external rotation of the tibia, a direct blow, or axial load on a plantar-flexed heel. Pain with weight bearing or with walking on toes is usually present. Cuneiform fractures are less common and rarely occur in isolation.6 Mechanisms of injury for cuneiform fractures include direct impact, axial loading on a dorsiflexed or plantar-flexed foot with rotational force, and severe rotation of the midfoot section in a fixed foot. Pain is usually localized to the dorsal or dorsomedial aspect of the foot.
With these 2 midfoot injuries, the exam should include palpation of the cuboid, navicular, and cuneiform bones and careful inspection of the Lisfranc joint, as this can be injured in midfoot fractures.7 Obtain AP, lateral, and oblique views of the foot,5 and strongly consider bilateral weight-bearing films to evaluate for tarsometatarsal (TMT) joint complex injuries (typically Lisfranc joint complex injuries), given their association with midfoot fractures1 (FIGURE 3).

If the fracture is at or near the TMT joint complex, obtain a CT scan or MRI regardless of plain film findings to evaluate the Lisfranc joint complex. Because the Lisfranc joint is particularly important in midfoot stability, untreated injuries can lead to impaired gait or chronic foot pain and deformity. Early identification and surgical referral for these injuries is crucial.
Continue to: Navicular fractures
Navicular fractures
Navicular fractures are typically caused by a twisting mechanism with forced plantar flexion or forced dorsiflexion of the midfoot. They present with severe pain over the dorsal or dorsomedial foot, particularly while bearing weight. Tenderness to palpation over the navicular bone generally warrants imaging studies to rule out fracture, as undiagnosed fractures can lead to severe long-term disability. Use Ottawa Ankle and Foot Rules12 in the acute setting to determine the need for radiographs. If imaging is indicated, obtain AP, lateral, and oblique views.
Tuberosity fractures may be seen on the AP view, while dorsal avulsions, talonavicular joint disruptions, and naviculo-cuneiform joint injuries are better seen on lateral views.16 Patients, particularly cross-country and track athletes, presenting with insidious onset of pain over the navicular bone should be evaluated for stress fracture using MRI, even in the presence of normal radiographs.
Differential diagnosis. Suspect Lisfranc joint complex injuries in any patient with mid-foot pain or fracture. The transverse arch of the foot is reliant upon the articulation of the second metatarsal with all 5 neighboring bones. The Lisfranc ligament is the strongest of 3 supporting ligaments to anchor the TMT joint complex. Other causes of mid-foot pain include soft tissue injury, contusion, and tendinopathy. In addition, other conditions that may cause pain in this area include cuboid syndrome, peroneal tendinopathy, Jones fracture, stress fracture, anterior calcaneal fracture, and sinus tarsi syndrome.
Treatment. Nondisplaced fractures of the cuboid or cuneiform may be treated with a short leg walking cast/boot for 6 weeks followed by the use of a shoe with a thin, rigid, longitudinal arch support for an additional 6 weeks.1 Fractures requiring referral for surgical evaluation include fractures that are open and fractures with vascular or neurological compromise. Also refer comminuted fractures and those that present with >2 mm step-off. Lastly, midfoot fractures that involve the Lisfranc joint should be immobilized and referred for orthopedic evaluation with instructions to the patient to avoid weight bearing until orthopedic evaluation.8
Avulsion fractures of the navicular bone may be managed nonoperatively with a short leg walking cast/boot if there is <20% involvement of the talonavicular surface. Simple nondisplaced body fractures may also be managed conservatively with immobilization and protected weight bearing for 6 to 8 weeks.15 Refer for surgical evaluation avulsion fractures that are intra-articular or dorsal involving 20% or more of the talonavicular surface and tuberosity fractures, given their risk of nonunion.9 All navicular body fractures that are not longitudinal in nature should also be referred for surgical evaluation. Navicular stress fractures that do not extend into the plantar cortex may be managed conservatively with a minimum of 6 weeks of a short leg cast and strict NWB with close follow-up.17
Continue to: Calcaneal fractures
Calcaneal fractures
Calcaneal fractures typically occur from severe axial load or fall from a height. Weight bearing is usually limited and secondary to significant pain. Tenderness to palpation over the calcaneus or with squeezing of the heel will produce pain on exam. Initial x-rays should include a lateral and axial view of the calcaneus. Additional imaging, including a CT scan, may be indicated for further evaluation to determine the extent of the fracture or to determine if a fracture is present despite normal x-rays.10
Acute compartment syndrome occurs in 10% of calcaneal fractures and must be considered in patients with calcaneal fractures from severe trauma.18 Tendon injuries of the ankle, hindfoot, and midfoot may present similarly but can be ruled out with clinical exam and appropriate imaging.
Treatment
Avulsion fractures that do not involve more than 25% of the calcaneocuboid joint and nondisplaced calcaneal fractures may be managed conservatively by instructing patients to wear a NWB short leg cast/boot for 4 to 6 weeks.1 Refer for surgical evaluation patients with calcaneal fracture fragments >1 cm, displacement >3 mm, open fractures, joint involvement >25%, and those whose symptoms fail to resolve with conservative management. Stress fractures can be managed conservatively with cessation of aggravating activities and immobilization in a walking boot until symptoms resolve, which typically takes 4 to 6 weeks.1
CORRESPONDENCE
Michael Seth Smith, MD, CAQSM, PharmD, 3450 Hull Road, Gainesville, FL 32611; [email protected]
1. Eiff MP, Hatch RL, et al. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
2. Hatch RL, Rosenbaum Cl. Fracture care by family physicians. A review of 295 cases. J Fam Pract. 1994;38:238-244.
3. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
4. Hatch RL, Hacking S. Evaluation and management of toe fractures. Am Fam Physician. 2003;68:2413-2418.
5. Snider RK. Essentials of Musculoskeletal Care. 2nd ed. Rosemont, IL: American Orthopedic Surgeons; 2001.
6. Guler F, Baz AB, Turan A, et al. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Spec. 2011;4:306-309.
7. Borrelli J, De S, Van Pelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20:472.
8. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001:32:21-33.
9. Rosenbaum AJ, Uhl R, DiPreta JA. Acute fractures of the tarsal navicular. Orthopedics. 2014;37:541-546.
10. Sanders RW, Clare MP. Calcaneous fractures. In: Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:2064.
11. Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772-776.
12. Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384-390.
13. Ekinci S, Polat O, Günalp M, et al. The accuracy of ultrasound evaluation in foot and ankle trauma. Am J Emerg Med. 2013;31:1551-1555.
14. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
15. Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817-826.
16. Schildhauer TA, Coulibaly MO, Hoffman MF. Fractures and dislocations of the midfoot and forefoot. In: Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:2690.
17. Mayer SW, Joyner PW, Almekinders LC, et al. Stress fractures of the foot and ankle in athletes. Sports Health. 2014;6:481-491.
18. Kalsi R, Dempsey A, Bunney EB. Compartment syndrome of the foot after calcaneal fracture. J Emerg Med. 2012;43:e101-106.
1. Eiff MP, Hatch RL, et al. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
2. Hatch RL, Rosenbaum Cl. Fracture care by family physicians. A review of 295 cases. J Fam Pract. 1994;38:238-244.
3. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
4. Hatch RL, Hacking S. Evaluation and management of toe fractures. Am Fam Physician. 2003;68:2413-2418.
5. Snider RK. Essentials of Musculoskeletal Care. 2nd ed. Rosemont, IL: American Orthopedic Surgeons; 2001.
6. Guler F, Baz AB, Turan A, et al. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Spec. 2011;4:306-309.
7. Borrelli J, De S, Van Pelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20:472.
8. Pinney SJ, Sangeorzan BJ. Fractures of the tarsal bones. Orthop Clin North Am. 2001:32:21-33.
9. Rosenbaum AJ, Uhl R, DiPreta JA. Acute fractures of the tarsal navicular. Orthopedics. 2014;37:541-546.
10. Sanders RW, Clare MP. Calcaneous fractures. In: Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:2064.
11. Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772-776.
12. Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21:384-390.
13. Ekinci S, Polat O, Günalp M, et al. The accuracy of ultrasound evaluation in foot and ankle trauma. Am J Emerg Med. 2013;31:1551-1555.
14. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
15. Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817-826.
16. Schildhauer TA, Coulibaly MO, Hoffman MF. Fractures and dislocations of the midfoot and forefoot. In: Rockwood and Green’s Fractures in Adults. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:2690.
17. Mayer SW, Joyner PW, Almekinders LC, et al. Stress fractures of the foot and ankle in athletes. Sports Health. 2014;6:481-491.
18. Kalsi R, Dempsey A, Bunney EB. Compartment syndrome of the foot after calcaneal fracture. J Emerg Med. 2012;43:e101-106.
PRACTICE RECOMMENDATIONS
› Manage most fractures of the proximal fifth metatarsal metaphyseal-diaphyseal junction (Jones fracture) conservatively with appropriate treatment and close follow-up; refer for early surgical evaluation only those patients who are highly active and who are interested in a faster return to activity. B
› Use the Ottawa Ankle and Foot Rules to determine whether x-rays are needed in a patient with foot pain and a suspected fracture. A
› Start with weight-bearing x-rays and then consider computed tomography or magnetic resonance imaging for complete evaluation of suspected injury to the Lisfranc joint. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
MSK injury? Make splinting choices based on the evidence
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).
Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.
Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.
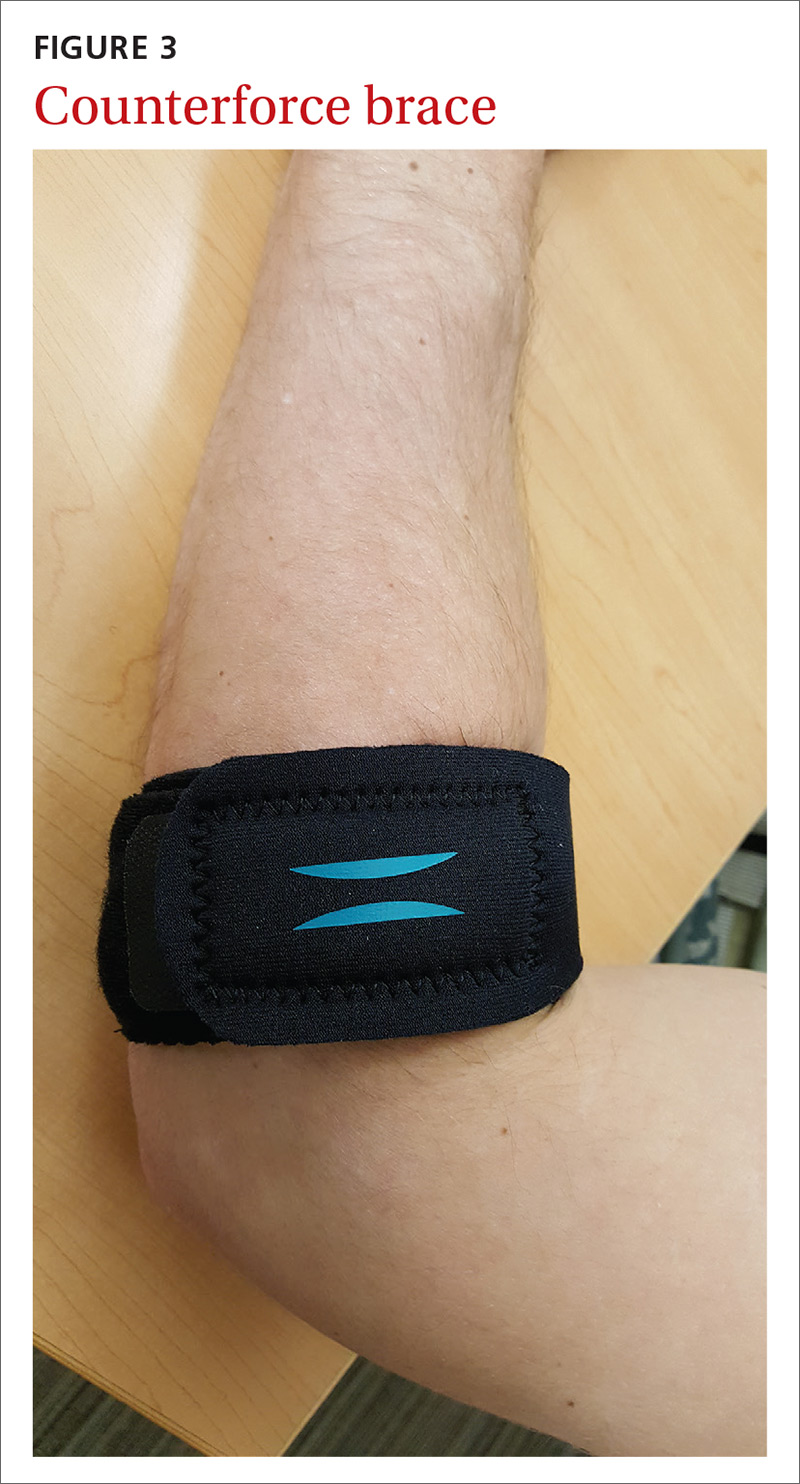
Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.
Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).
Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
About 25% of all outpatient visits to family physicians include musculoskeletal (MSK) complaints.1 Splinting, bracing, or wrapping are used in 25% of these visits.2 The goals of splinting/bracing are multifold: accommodate a correct movement pattern, restrict poor movement patterns, and decrease the use of an injured area to allow for healing.
Splints and braces are generally noncircumferential and are easily put on and taken off. (The terms splints and braces can be used interchangeably.) The devices can be adjusted for swelling and are more comfortable than casts, but have the potential for poor patient adherence, may require frequent adjustment, and can allow for excessive motion.
Making the most of these devices requires an understanding of when the evidence supports (and doesn’t support) their use for particular injuries. In this article, we review the evidence for the use of splints/braces for common upper and lower extremity MSK conditions seen in family practice. We have confined our discussion to readily obtainable, off-the-shelf products. These products come in a variety of sizes and are easily kept on hand, or ordered through a durable medical equipment provider.
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve at the level of the wrist. It is caused by several different conditions.
Goal of splinting: Minimize wrist movement to decrease any concomitant swelling in the carpal tunnel contributing to the compression. The two different types of orthoses commonly used are a neutral wrist splint (FIGURE 1) and a cock-up wrist splint (20° wrist extension).

Evidence: A 2003 Cochrane review concluded that short-term symptom relief was achievable with bracing; however, better outcomes were seen with combination therapies (eg, medications, occupational therapy) vs splinting alone.3 A more recent Cochrane review in 2012 found poor or limited evidence that splint use at night was better than no treatment or any other nonsurgical treatment.4 There was also insufficient evidence to recommend one type of splint over another, although several poor-quality studies found neutral splinting to be more beneficial.5
A 2016 clinical practice guideline (CPG) from the American Academy of Orthopaedic Surgeons (AAOS) reported strong evidence supporting the use of immobilization.6 (Strong evidence is defined by the AAOS as 2 or more “high” strength studies with consistent findings for the intervention.6) Interestingly, of the 2 studies that AAOS used to make its conclusions,7,8 only the study by Manente et al8 was available at the time of the Cochrane 2012 review, and the Cochrane authors came to a different conclusion. The AAOS CPG does not comment on a specific type of brace.
Continue to: Harms
Harms: Both the 2012 Cochrane review and the AAOS statement indicate that there are no long-term harms other than some subjective discomfort in a minority of patients while wearing the splint.
Bottom line: A wrist splint should be considered in the treatment of CTS—especially if the condition is likely the result of repetitive wrist motion. If the patient can tolerate continuous use for 2 to 4 weeks, this should be employed. But at a minimum, nocturnal use for this duration would constitute a therapeutic trial. Combination therapy (ie, medication, occupational therapy, and splinting) is better than splinting alone.
de Quervain tendinopathy
This form of tendinopathy involves pain at the tendon sheaths of the abductor pollicis longus and the extensor pollicis brevis. Onset of symptoms has been attributed to overuse or repetitive movements of the wrist and thumb.
Goal of splinting: Immobilize the affected tendons to reduce irritation and/or inflammation. A thumb spica splint (FIGURE 2) is used to achieve this restriction.

Evidence: Three randomized controlled trials (RCTs) suggested that the natural course is not affected by splint use for patients with prolonged symptoms (>3 months), and eventual resolution was noted in about 12 months—regardless of intervention with bracing. Symptoms improved more rapidly with the combination of medications and splint wear for those with a shorter duration of symptoms.9-11 Symptom severity driven wear compared with full-time use yielded equivalent outcomes.9 Those patients with longer duration and increased severity of symptoms fared worse regardless of treatment.10
Continue to: Harms
Harms: No documented harmful adverse effects (AEs) have been reported with splinting for this condition.
Bottom line: A thumb spica splint remains an option for de Quervain tendinopathy. It may provide symptomatic relief, especially if used early in the disease, but does not alter the natural disease course.
Lateral/medial epicondyle pain
Also known as tennis/golfer’s elbow, lateral/medial epicondyle pain is thought to result from overuse of the common wrist extensor/flexor muscle origins at the site of the myotendinous junctions.
Goal of splinting: To dampen or disperse the forces at the painful area via a counterforce brace (FIGURE 3). In addition, braces are used to decrease wrist use, specifically extension or flexion.

Evidence: A 2002 Cochrane Review found insufficient data to support the use of counterforce braces for relief of acute or chronic pain symptoms associated with epicondyle pain.Several studies supporting their use within this review were of varying quality with weak evidence.12
Continue to: Volar wrist braces have also been...
Volar wrist braces have also been studied for conservative management of epicondyle pain. Equivalent outcomes were noted comparing volar wrist bracing with a counterforce brace. Higher rates of recovery were seen in patients who participated in combination therapies (ie, bracing, physical therapy, and medication use).12
Harms: Use of counterforce braces for ≥30 days resulted in higher rates of braces restriction, more medical visits per patients, and higher medical costs. Derebery et al13 concluded that this was due to deconditioning on returning to normal activity. Use of a volar wrist brace should be discouraged as it reduces the active range of wrist motion, further contributing to deconditioning with long-term application.14
Bottom line: A trial of counterforce bracing should be used if pain precludes active rehabilitation or vocational pursuits, but should not be used as the sole therapy.
Knee osteoarthritis
Knee osteoarthritis (OA) can result from multiple (often commingled) etiologies, which ultimately result in loss of cartilage, ensuing bony abnormalities, and affected joint/soft tissue structures. Patients can present with severe symptoms with little loss of structural architecture or major structural changes with a paucity of symptoms.

Goal of splinting: Depending on the orthoses used, the goals of splinting vary. A simple knee sleeve (FIGURE 4) provides warmth and proprioception, and a valgus unloader brace (FIGURE 5) provides valgus stress to open and unload the medial compartment.

Continue to: Evidence
Evidence: A single study evaluating a neutral knee sleeve vs control exhibited improved pain scores following several months of treatment. Mixed results were demonstrated with patient perceived quality of life improvement though.15 Currently, there is inconclusive evidence to support the use of valgus offloader braces per AAOS guidelines.16 This decision is based on 3 separate studies of moderate to high strength evidence. Improvements in the domains of pain, stiffness, self-reported functional capacity, and physical performance were unclear and no conclusions were able to be drawn.17,18
Harms: To date, no harmful AEs have been demonstrated with the use of knee sleeves. Valgus knee bracing can be uncomfortable, leading to poor adherence, but there are no long-term negative consequences.
Bottom line: Use of knee sleeves is worthwhile in patients with mild-to-moderate OA to improve functional scores. Inconclusive support for valgus knee bracing, along with the high cost of equipment, should reserve this option for patients with advanced OA who do not respond to typical conservative management and who are unwilling or ill-advised to undergo knee arthroplasty.16-18
Medial collateral ligament injury
An injury of the medial collateral ligament (MCL)—the medial stabilizer of the knee—can result from either a direct blow or a noncontact twisting injury. Grade 1 injuries have no actual ligament tear, grade 2 injuries have partial disruption, and grade 3 injuries denote a complete tear.
Goal of splinting: A hinged knee brace (FIGURE 6) allows for full extension but limited valgus and varus stresses.

Continue to: Evidence
Evidence: A conservative management strategy for an isolated injury is generally adequate to allow for sufficient healing, and “return to play” without prolonged disability. With conservative management, the affected joint is protected with a hinged knee brace for about 3 to 6 weeks.19,20 Data gathered on patients 9 years postinjury support the use of bracing of grades 1 to 2 injuries, but it is unclear what the optimal strategy is for grade 3 injuries.19
Harms: Generally well tolerated, and no harms have been reported.
Bottom line: Isolated grades 1 to 2 MCL injuries can be treated conservatively, and a hinged knee brace should be used as part of the rehabilitative process. It is unclear how to optimally manage grade 3 injuries.
Lateral ankle sprain
Lateral ankle sprains involve inversion injury to 1 or more of the 3 lateral ankle ligaments. Injuries are graded using the same grade schema as MCL injuries.
Goal of splinting: There are a variety of braces designed to provide lateral stability to patients with lateral ankle sprains. These stirrup braces differ in degree of support and additional fixation points—rigid (pneumatic) vs semirigid (Velcro, lace-up, etc) (FIGURE 7).

Continue to: Evidence
Evidence: A 2017 meta-analysis of systematic reviews found improved (self-reported) function when patients used external support devices such as tape, compression bandages, semirigid braces or boots, or walking casts.21 Secondary prevention utilizing brace wear during at-risk activities has been found to be the most important intervention to reduce recurrence.21,22
Harms: No direct injury from brace use has been reported, but consistent evidence exists that lack of early mobilization and rehabilitation can substantially affect the recovery from these injuries.
Bottom line: Consensus opinion recommends stirrup bracing for the treatment of grades 1 and 2 injuries.23,24 Controversy remains regarding brace use or complete immobilization for grade 3 injuries. Regardless of injury grade, early mobilization should be integrated into the treatment plan, coupled with active rehabilitation, including restoration of strength and proprioception. Prevention of second injuries is best accomplished with full rehabilitation and bracing during at-risk activities (eg, sports practices and competitions).21,22,25
A useful tool, but one not always covered by insurance
Bracing is a useful tool in the armamentarium of treating the common MSK complaints seen in everyday practice. Bracing must always be accompanied by a functional, active rehabilitation program.
Keep in mind, though, that many insurance plans may not cover the cost of bracing. Therefore, knowledge of its efficacy for a particular injury (or lack thereof) should guide treatment recommendations, along with shared decision making.
CORRESPONDENCE
Jeffrey C. Leggit, MD, CAQSM, 9706 Ethan Ridge Avenue, Frederick, MD 21704; [email protected].
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
1. MacKay C, Canizares M, Davis AM, et al. Health care utilization for musculoskeletal disorders. Arthritis Care Res (Hoboken). 2010;62:161-169.
2. CDC, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 outpatient department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_outpatient/2010_opd_web_tables.pdf. Accessed August 16, 2018.
3. O’Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003:CD003219.
4. Page MJ, Massy‐Westropp N, O’Connor D, et al. Splinting for carpal tunnel syndrome. Cochrane Database of Syst Rev. 2012:CD010003.
5. Burke DT, Burke MM, Stewart GW, et al. Splinting for carpal tunnel syndrome: in search of the optimal angle. Arch Phys Med Rehabil. 1994;75:1241-1244.
6. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome Evidence-Based Clinical Practice Guideline. http://www.aaos.org/uploadedFiles/PreProduction/Quality/Guidelines_and_Reviews/guidelines/CTS%20CPG_2.29.16.pdf. Published February 29, 2016. Accessed August 16, 2018.
7. Hall B, Lee HC, Fitzgerald H, et al. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. Am J Occup Ther. 2013;7:448-459.
8. Manente G, Torrieri F, di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;8:1020-1025.
9. Menendez ME, Thornton E, Kent S, et al. A prospective randomized clinical trial of prescription of full-time versus as-directed splint wear for de Quervain tendinopathy. Int Orthop. 2015;39:1563-1569.
10. Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258-260.
11. Ring D, Schnellen A. Patient-centered care of de Quervain’s disease. J Hand Microsurg. 2009;1:68-71.
12. Struijs PA, Smidt N, Arola H, et al. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002:CD001821.
13. Derebery VJ, Devenport JN, Giang GM, et al. The effects of splinting on outcomes of epicondylitis. Arch Phys Med Rehabil. 2005;86:1081-1088.
14. van de Streek MD, van der Schans CP, de Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment of lateral epicondylitis. Prosthet Orthot Int. 2004;28:183-189.
15. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
16. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee, 2nd ed. Summary of recommendations. https://www.aaos.org/research/guidelines/OAKSummaryofRecommendations.pdf. Accessed August 16, 2018.
17. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539-548.
18. van Raaij TM, Reijman M, Brouwer RW, et al. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin Orthop Relat Res. 2010;468:1926-1932.
19. Kannus P. Long-term use of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103-112.
20. Stannard J. Medial and posteromedial instability of the knee: evaluation, treatment, and results. Sports Med Arthrosc Rev. 2010;18:263-268.
21. Doherty C, Bleakley C, Delahund E, et al. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51:113-125.
22. Janssen KW, Hendriks MR, van Mechelen W, et al. The cost-effectiveness of measures to prevent recurrent ankle sprains: results of a 3-arm randomized controlled trial. Am J Sports Med. 2014;42:1534-1541.
23. Beynnon B, Renström P, Haugh L, et al. A prospective, randomized clinical investigation of the treatment of first-time ankle sprains. Am J Sports Med. 2006;34:1401-1412.
24. Seah R, Mani-Badu S. Managing ankle sprains in primary care: what is best practice? A systemic review of the last 10 years of evidence. Br Med Bull. 2011;97:105-135.
25. Kaminski TW, Hertel J, Amendola N, et al; National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
From The Journal of Family Practice | 2018;67(11):678-683.
PRACTICE RECOMMENDATIONS
› Consider a wrist splint for carpal tunnel syndrome secondary to repetitive motion. B
› Recommend a simple knee sleeve to help patients with osteoarthritis reduce their pain and improve daily function. B
› Use ankle bracing for secondary prevention of a recurrent ankle sprain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood adversity & lifelong health: From research to action
The rising prevalence of obesity, widespread community violence, and the opioid epidemic are urgent health crises that we have, so far, failed to solve. Physicians must therefore ask: Are we employing the right framework to effectively understand and address these complex problems?
Careful review of the literature reveals that these problems and many others begin with, and are profoundly affected by, childhood adversity. Compounding this, studies over the past 20 years that have focused on abuse and neglect without including community, structural, and historical adversity demonstrate that our definitions of adversity and trauma have been too narrow. The prevalence and diversity of factors affecting development and health is much greater than our medical model anticipates.1,2
CASE
Eileen W, a 55-year-old married, self-employed woman with a 20-year history of autoimmune thyroiditis, longstanding insomnia, and anxiety presents with intense episodes of terror related to public speaking, which are compromising her work performance. Her history is significant for tobacco and alcohol use beginning in early adolescence and continuing into young adulthood, as well as 2 unplanned pregnancies in her 20s. Additional adversities included the murder of her maternal aunt while Ms. W was in utero, resulting in her parents having fostered 2 young cousins; bullying; and the premature death of a special-needs sibling.
What treatment strategies might have been undertaken to manage consequences of the adversities of Ms. W’s childhood—both on her own initiative and as interventions by her health care providers?

Our medical model must be updated to be effective
Because at least 60% of Americans have had 1 or more experiences of childhood adversity, family physicians care for affected patients every day—a reality incompletely addressed by our conventional theories and practices.1,3 Consequently, updating our medical model to incorporate research that confirms the critical and widespread impact of childhood experience on health and illness is an essential task for family medicine.
Core values of family medicine integrate biological, clinical, and behavioral sciences. They include comprehensive and compassionate care that is provided within the context of family and community across the lifespan.4,5 Family medicine is therefore the ideal specialty to lead a movement that will translate scientific evidence of the effects of childhood adversity on health into training, delivery of care, and research—transforming clinical practice and patient health across the lifespan.
This article describes the dramatic impact of childhood adversity on health and well-being and calls on family physicians to play a crucial role in preventing, mitigating, and treating the consequences of childhood adversity, an important root cause of disease.
Continue to: Childhood adversity makes us sick
Childhood adversity makes us sick
The first paper about the landmark Adverse Childhood Experiences (ACE) Study, published 20 years ago, is 1 of more than 90 on this topic.3 This study explored the relationship of physical, emotional, and social health in adulthood and self-reported childhood adversity, and comprised 10 categories of abuse, neglect, and household distress between birth and 18 years of age. One of the largest epidemiological studies of its kind, the ACE Study surveyed more than 17,000 mostly white, middle-aged, educated, and insured participants. Study researchers developed an “ACE Score”—the total number of ACEs faced by a person before her (his) 18th birthday—and found that 64% of respondents endorsed 1 or more ACEs; 27% reported 3 or more ACEs; and 5% experienced 6 or more.
The ACE Study revealed a dose–response relationship between ACEs and more than 40 health-compromising behaviors, negative health conditions, and poor social outcomes. Examples include cardiac, autoimmune disease, obesity, intravenous drug abuse, depression and anxiety, adolescent pregnancy, and worker absenteeism. Tragically, an ACE score of ≥6 conferred a significant risk for premature death.1
ACE data have been collected in diverse populations in 32 states and many countries through the Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention3; the Child & Adolescent Health Measurement Initiative’s National Survey of Children’s Health6; and The World Health Organization’s ACE International Questionnaire7—underscoring the pervasiveness of childhood adversity. Evaluation of ACEs in special populations, such as people experiencing homelessness,8 incarcerated youth,9 people struggling with addiction,10 and even health care workers,11 uncovers notably higher rates of ACEs in these populations than in the general population.
Is childhood adversity a true cause of bad outcomes?
Or is the relationship between the 2 entities merely an association? To help answer this question, researchers evaluated the ACE Study using Bradford Hill criteria—9 epidemiological principles employed to infer causation. Their findings strongly support the hypothesis that not only are ACEs associated with myriad negative outcomes, they are their root cause12 and therefore a powerful determinant of our most pressing and expensive health and social problems.Nevertheless, strategies to prevent and address childhood adversity, which are critical to meeting national health goals of successful prevention and treatment of myriad conditions, are absent from the paradigm and practice of most physicians.
The body of research about the health impact of additional adverse experiences is growing to include community violence, poverty, longstanding discrimination,2 and other experiences that we describe as social determinants of health. Furthermore, social determinants of health, or adverse community experiences, appear to maintain a dose–response relationship with health and social outcomes.2 ,13 Along with adverse collective historical experiences (historical trauma),14 these community experiences are forcing further re-examination of existing paradigms of health.
Continue to: The biological pathway from experience to illness
The biological pathway from experience to illness
Neuroscience supports the epidemiology of ACEs.12 The brain develops from the bottom up, in a use-dependent fashion, contingent on genetic potential and, most importantly, on our experiences, which also influence genetic expression. Although present across the lifespan, the brain’s capacity to change—neuroplasticity—is most robust from the prenatal period until about 3 years of age.15 The autonomic nervous system receives information from the body about our internal world and from sensory organs about our external environment and sends it to the brain for processing and interpretation, resulting in micro- and macro-adaptations in structure and function, both within the brain and in the rest of the body.16
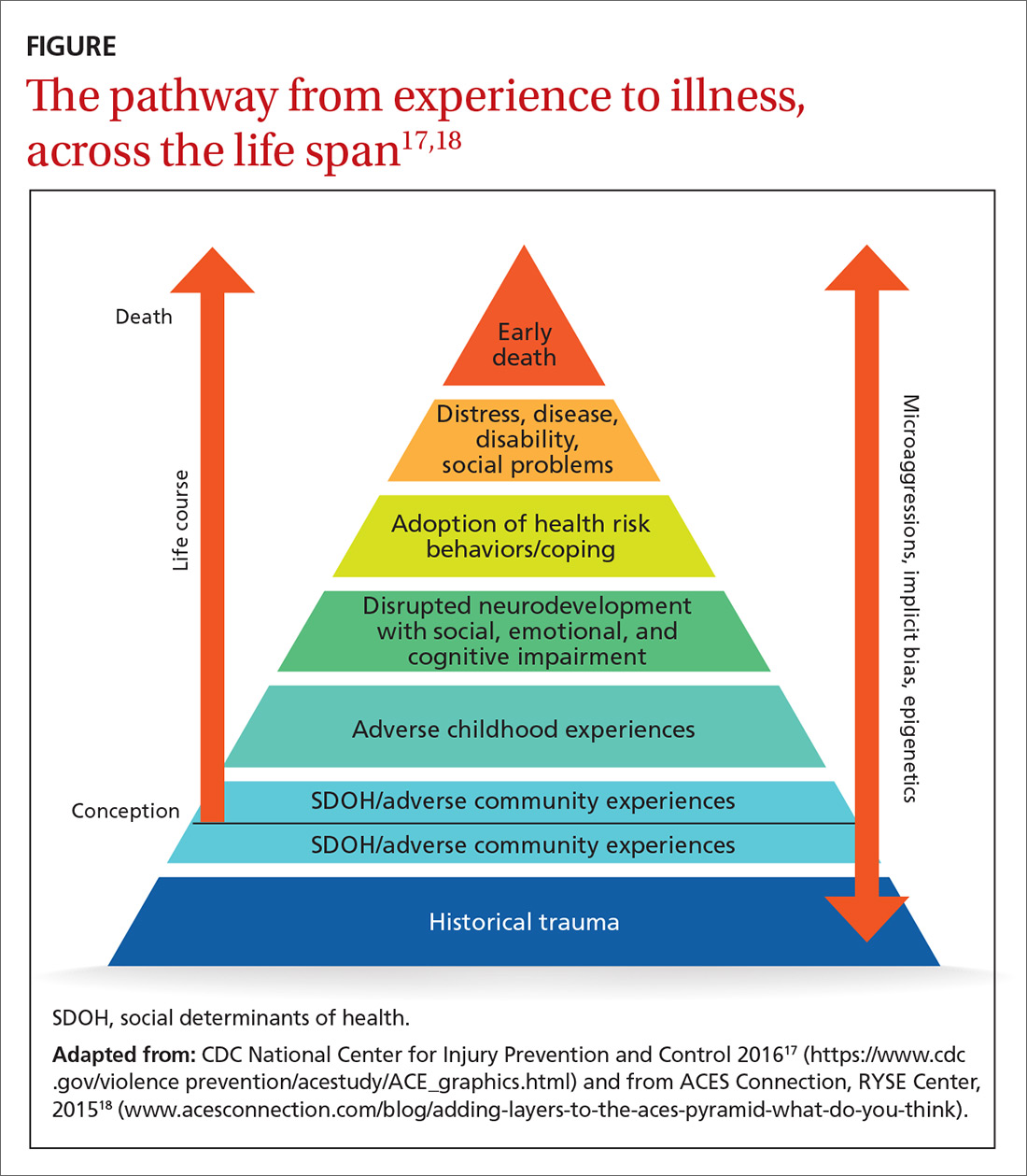
Neuroscience demonstrates that adverse experiences, in the context of insufficient protective factors and depending on their timing, severity, and frequency, cause overactivation or prolonged activation, or both, of the stress response system, thus derailing optimal growth and development of the brain and disrupting healthy signaling in all body systems. The dysregulated stress response drives inflammation and subsequent chronic disease (FIGURE17,18), and may influence genetic expression in this, and future, generations.12,14,19 Using neuroimaging and assessment of biomarkers, researchers can see the harm caused by inadequately buffered adversity on overall anatomy and physiology. Protective factors such as a safe environment and positive relationships provide hope that normal biological responses to adverse circumstances can be prevented or reversed, leading to clinical, cognitive, and functional improvement11 (TABLE 120-22).
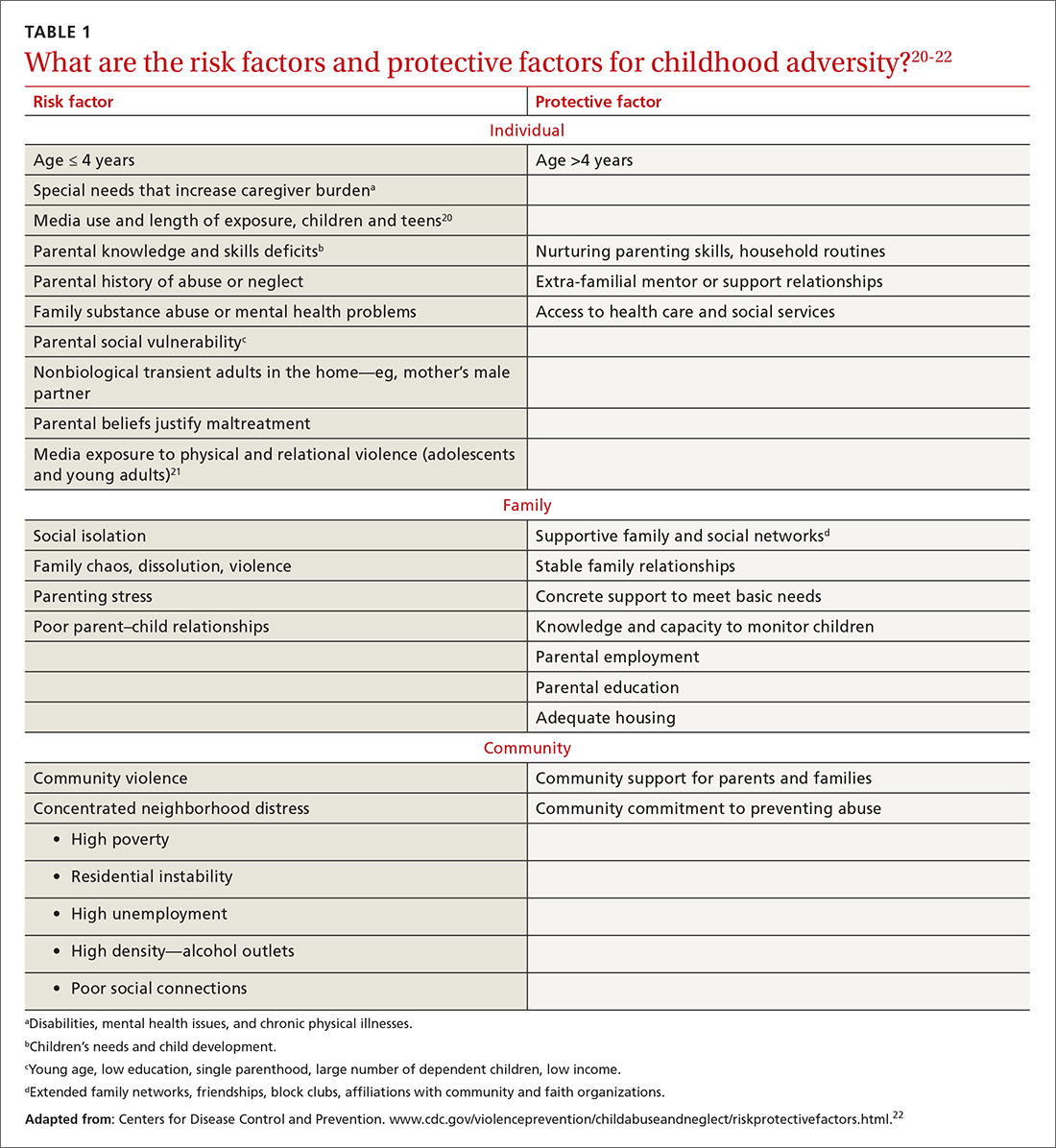
Evidence-based primary prevention of childhood adversity succeeds
Primary prevention of childhood adversity offers significant benefits across the lifespan and, likely, into the next generation. It ensures that every infant has at least 1 nurturing, attuned caregiver with whom to develop a secure attachment relationship that is essential for optimal growth and development of brain and body.
Primary prevention is most effective when it focuses on supporting caregivers during the perinatal and early childhood periods of their families, before children’s brains are fully organized. Primary prevention involves evidence-based program implementation; collaboration among multiple sectors, including early childhood education, child welfare, criminal justice, business, faith, and health care; and, ultimately, policy change. It incorporates individual, family, and community-based strategies to meet basic needs, ensure safety, fortify a sense of love and belonging in families, and support parents in developing optimal parenting skills. This allows caregivers to devote attention to their children, thus strengthening attunement and attachment, reducing toxic stress, and building protective factors and resilience. Evidence-based and -informed prevention programs include the Nurse–Family Partnership (NFP), Positive Parenting Program (Triple P), and the Family-Centered Medical Home.
NFP. Randomized controlled trials of the NFP, a perinatal home visiting program for low-income, first-time pregnant women and their offspring, showed a reduction in the incidence of domestic violence, child maltreatment, and maternal smoking, with improvement in maternal financial stability, cognitive and socioemotional outcomes, and rates of substance abuse and incarceration in children and/or youth.23
Continue to: Triple P
Triple P. A randomized controlled trial of Triple P, an evidence-based, multilevel, population-based preventive intervention system that was designed to support parents and enhance parenting practices for families with at least 1 child (birth to 12 years old), demonstrated a statistically significant reduction in substantiated child maltreatment cases, out-of-home placements, and emergency room visits and hospitalizations for childhood injuries that were the result of child maltreatment.24
The Family-Centered Medical Home, a primary care strategy to reduce premature and low-birth-weight deliveries, used Medicaid dollars for services not traditionally considered “medical” to address all physical and emotional needs of mothers and families as part of the medical relationship. This program eliminated premature delivery and low birth weight,25 both considered evidence of in utero toxic stress.26
Screening can be brief: In some cases, a single question
The prevalence and impact of childhood adversity, along with the opportunity for significant health improvements and savings, inspires providers to explore screening. Existing screening programs have consistent goals27,28:
- identify unique experiences shaping our patients’ health
- reframe “What’s wrong with you?” as “What happened to you?” “What’s right with you?” and “What matters to you?”
- facilitate health education and neuro-education, particularly meaning-making and self-regulation
- prevent and mitigate the sequelae of exposure to ACEs
- promote health in this and subsequent generations.
The ACE Study screened patients in the context of a comprehensive periodic health assessment. Study participants completed an at-home questionnaire and reviewed it with their physician.1 The Urban ACE Survey added important community stressors such as neighborhood violence, bullying, and food insecurity to the original ACE questionnaire.2
Primary care tool. Wade developed a short, 2-question ACE pre-screener for primary care29 and is exploring screening for childhood adversity in pediatric practice, as are primary care clinicians around the country.
Continue to: Single-question screener
Single-question screener. A Chicago internist interviewed more than 500 patients using a single-question screener that asked whether growing up was “mostly okay or pretty difficult.” This tool accurately confirmed childhood adversity in patients with complex chronic illness, prevented re-traumatization by allowing patients control over disclosure, and opened the door to collaborative healing work over time.30
The Hague Protocol, now mandated in the Netherlands for health and justice professionals, focuses its efforts upstream by offering early detection of children at risk for adverse experiences. The protocol requires asking adults who present with intimate partner violence, suicidality, psychiatric disturbance, or severe substance abuse whether they care for children in any capacity. Those who are so identified are referred to a center at which support services are offered.31
Uncertainty about the utility of existing tools. Many screening tools appear to be promising in terms of identification of the risk for, or actual, childhood adversity, patient and provider satisfaction, and their “fit” in the clinical workflow. Even so, no best practice guidelines exist in primary care to steer screening efforts. Questions remain about27-29:
- broad implementation of a specific tool
- how, when, and where screening should take place
- whether to screen adults, parents, or children—or all 3
- how best to use the content and pacing of screening questions to promote self-regulation and prevent re-traumatization
- best strategies for training and supporting health care workers around screening activities
- how to optimally manage a positive screen.
How best to approach treatment
Treatment includes trauma-informed care, an organizational transformation process (described in TABLE 232; in “The lexicon of childhood adversity: Concepts and tools for care”33-45; and in the subsection, “Lessons from neuroscience”), and individual treatment strategies. The Substance Abuse and Mental Health Services Administration (SAMHSA) of the US Department of Health and Human Services is advocating for implementation of trauma-informed approaches in health systems.

Continue to: The lexicon of childhood adversity...
SIDEBAR
The lexicon of childhood adversity: Concepts and tools for care33-45
Adversity A state or instance of serious or continued difficulty or misfortune. 33
Attachment A special, enduring form of emotional relationship with a specific person involving soothing, pleasure, and comfort.34
Attunement The ability to read and respond to the cues of another.35
Eye-movement desensitization and reprocessing (EMDR) An evidence-based psychotherapy for posttraumatic stress disorder and other psychiatric disorders, mental health problems, and somatic symptoms. EMDR facilitates resumption of normal information processing and integration; the patient attends to emotionally disturbing material in brief sequential doses while simultaneously focusing on an external stimulus. EMDR targets past experience, current triggers, and future potential challenges, and results in alleviation of presenting symptoms; a decrease or elimination of distress from the disturbing memory; improved view of the self; relief from bodily disturbance; and resolution of present and future anticipated triggers.36
Historical trauma Cumulative emotional and psychological wounding, resulting from group traumatic experiences, transmitted across generations within a community.37
Neurofeedback Electroencephalographic biofeedback is a method for retraining brainwave patterns through operant conditioning; it is used to treat posttraumatic stress disorder, various mental health conditions, addiction, chronic pain, epilepsy, and other disorders.38
Neuromodulatory Having the capacity to alter nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body to help restore function or relieve symptoms.39
Social determinants of health/adverse community experiences Conditions in which people are born, grow, live, work, and age and that are shaped by distribution of money, power, and resources at all levels.40,41
Trauma An event or circumstance experienced or observed by a person as physically or emotionally harmful or threatening and having lasting adverse effects on that person's functioning and well-being.42
Trauma-focused cognitive behavioral therapy An evidence-based trauma treatment for children 3 to 18 years and their parents comprising the elements of the acronym PRACTICE: Psychoeducation and parenting; Relaxation methods; Affective expression and regulation skills; Cognitive coping skills and processing; Trauma narrative and processing; In vivo exposure; Conjoint parent-child therapy sessions; and Enhancing personal safety and growth.43
Trauma-informed approach This "4-R" approach can be implemented in any type of service setting, organization, or program that: Realizes the widespread impact of trauma and understands potential paths for recovery; recognizes signs and symptoms of trauma in clients, families, staff, and others involved with the system; responds by fully integrating knowledge about trauma into policies, procedures, and practices; and seeks to actively resist re-traumatization.44
Use-dependent The organization and function of neurons, the neural system, and the brain depends on repetitive, patterned stimulation.45
Continue to: Trauma-informed care is a model...
Trauma-informed care is a model intended to promote healing and reduce the risk for re-traumatization of patients by staff—significant concerns in clinical settings, where the dynamics of loss of power, control, and safety that are inherent in traumatic experience can be replicated.46 To operationalize trauma-informed care more formally, the Center for Health Care Strategies, Inc., and the National Council for Behavioral Health are developing recommendations for 1) standardized screening and assessment tools, evidence-based clinical interventions, implementation processes, and relevant and replicable outcome measures, and 2) policy changes to improve patient and staff engagement, enhance health outcomes, and reduce avoidable care and excess costs.47,48
Lessons from neuroscience guide effective treatment.16 Treatment begins with bottom-up strategies that are focused on decreasing suboptimal excitatory input from the survival brainstem to create safety, connect patients to resources to meet basic needs, teach self-regulation skills, and improve relational health in and outside of the office. Later-stage top-down methods, such as education and other cognitive activities, focus on strengthening the regulatory capacity of the thinking cortex.16 In many ways, treatment mirrors prevention: It emphasizes first helping patients feel safe and loved.
In a follow-up to the ACE Study, 100,000 patients had a primary care visit in which their practitioner reviewed the ACE questionnaire with them; said “I see that you have________. Tell me how that has affected you later in your life” for every “Yes” response; and listened to the answers without passing judgment. This simple intervention profoundly decreased health resource utilization by these patients during the following year: a reduction of 35% in office visits, 11% in emergency room visits, and 3% in hospitalizations.1
The neurosequential model of therapeutics assesses neurodevelopment in the context of childhood adversity and relational health to evaluate consequences of childhood adversity and direct treatment. Adopted domestically and internationally, this model has had statistically significant success facilitating improvement in patients’ physical, emotional, and social health status.16,49
Trauma-specific treatment modalities such as trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing (EMDR),50 a trauma-specific treatment effective in resolving painful childhood memories, are evidence-based treatments that reduce trauma-related symptoms; evidence is also emerging about the efficacy of yoga51 and neurofeedback.52 These therapies have been best studied as treatment for posttraumatic stress disorder and other mental health disorders and also hold promise for addressing physical and social consequences of adversity. They present a low risk for harm, appear to be cost-effective, and improve outcomes.
Continue to: Best regimens involve a multifaceted approach that combines...
Best regimens involve a multifaceted approach that combines health-system resources with referral to other community practitioners and agencies. An excellent example is a current collaboration between health systems and affordable housing programs to reduce and, ultimately, eliminate chronic homelessness. Positive outcomes of this collaboration include both improved health and life satisfaction for participants and cost savings to the health system.53
CASE
Beginning in adulthood, Ms. W began long-term psychotherapy and had a therapeutic trial of antidepressants, without significant improvement. None of her medical or mental-health providers educated her about the connection between childhood adversity and illness to help her make sense of her health history and autoimmune disease, or to guide treatment. She learned from a friend about the relationship between childhood adversity and poor health and self-administered the ACE questionnaire, scoring 5 points out of a possible 10.
Ms. W enjoyed loving relationships with her mother, sisters, and friends. She had long-standing personal practices of individual and group physical activity, journaling, and spending time in nature.
About 10 years ago, Ms. W committed to regular yoga practice and later saw a functional medicine provider, who focused on nutrition and restorative sleep. She noticed improvement in all signs and symptoms; however, the terror of public speaking remained. Through friends, she found a practitioner who offered EMDR. Over the past 2 years, her terror has resolved and general anxiety and insomnia have continued to improve; she is now able to speak with fluency and comfort in any arena.
Addressing childhood adversity: Our “natural domain”
Experiences, positive and negative, shape our psychology and biology; they are powerful determinants of health—or illness. Prevention of, and response to, childhood adversity demand a systems approach to the whole person in context—the natural domain of family medicine.
Continue to: Although clinical translation is still unfolding...
Although clinical translation is still unfolding, the risks of implementing promising prevention and treatment strategies are low, the stakes are high, and the potential benefits are vast. Therefore, we as family physicians can—must—learn and incorporate the science of childhood adversity, neurobiology, and life course into our training, research, and clinical paradigm and practice; we can do that by embedding this framework throughout our training and continuing education in formal didactics, case discussions, hands-on skill-building, scientific investigation, and patient care.
We must make our offices and hospitals trauma-informed; connect patients with resources to meet basic needs and with home-visiting and parent education programs; educate patients about the impact of protective and adverse factors on health; provide and practice self-regulation training in our offices or by referral; and advocate for equity.
Using these strategies, family physicians will play a crucial role in the prevention, mitigation, and treatment of the root cause of disease and society’s deepest individual and collective suffering.
CORRESPONDENCE
Audrey Stillerman, MD, ABFM, ABIHM, ABOIM, Office of Community Engagement and Neighborhood Health Partnerships, 808 South Wolcott Street, Room 809, Chicago, IL 60612; [email protected].
ACKNOWLEDGMENT
Patricia Rush, MD, MBA, and Adrienne Williams, PhD, reviewed the manuscript of this article.
1. Felitti V, Anda R. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
2. Wade R Jr, Shea JA, Rubin D, et al. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134:e13-e20.
3. Centers for Disease Control and Prevention. Child abuse and neglect prevention. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/index.html. Accessed September 20, 2018.
4. American Academy of Family Physicians. Definition of family medicine. www.aafp.org/about/policies/all/family-medicine-definition.html. Accessed March 5, 2018.
5. Martin JC, Avant RF, Bowman MA, et al; The Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2 Suppl 1:S3-S32.
6. Child & Adolescent Health Measurement Initiative (CAHMI). A national and across-state profile on Adverse Childhood Experiences among U.S. children and possibilities to heal and thrive. Issue Brief. October 2017. www.cahmi.org/wp-content/uploads/2018/05/aces_brief_final.pdf. Accessed September 20, 2018.
7. World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ). www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/. Accessed September 20, 2018.
8. Roos LE, Mota N, Afifi TO, et al. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am J Public Health. 2013;103(Suppl 2):S275-S281.
9. Baglivio MT. Wolff KT. Piquero AR, et al. The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J Crim Justice. 2015;43:229-241.
10. Dube SR. Felitti VF. Dong M, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564-572.
11. Maunder RG, Peladeau N, Savage D, et al. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34:114-123.
12. Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174-186.
13. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186-S196.
14. Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41:232-244.
15. Perry BD. Memories of fears: How the brain stores and retrieves traumatic experiences. In: Goodwin J, Attias R, eds. Splintered Reflections: Images of the Body in Trauma. New York, NY: Basic Books; 1999:9-38.
16. Perry BD. Examining child maltreatment through a neurodevelopmental lens: clinical application of the Neurosequential Model of Therapeutics. J Loss Trauma. 2009;14:240-255.
17.
18. Adding layers to the ACEs pyramid—What do you think? Trauma and social location. ACES Connection, RYSE Center. 2015. www.acesconnection.com/blog/adding-layers-to-the-aces-pyramid-what-do-you-think. Accessed October 10, 2018.
19. Berens AE, Jensen SKG, Nelson CA 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135.
20. Rostad WL, Basile KC, Clayton HB. Association among television and computer/video game use, victimization, and suicide risk among U.S. high school students. J Interpers Violence. 2018 Mar 1:886260518760020.
21. Coyne SM, Nelson DA, Graham-Kevan N, et al. Media depictions of physical and relational aggression: connections with aggression in young adults’ romantic relationships. Aggress Behav. 2011;37:56-62.
22. Centers for Disease Control and Prevention. Violence prevention: Child abuse and neglect: risk and protective factors. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/riskprotectivefactors.html. Accessed October 10, 2018.
23. Miller TR. Projected outcomes of nurse-family partnership home visitation during 1996-2013, United States. Prev Sci. 2015;16:765-777.
24. Prinz RJ, Sanders MR, Shapiro CJ, et al. Population-based prevention of child maltreatment: the U.S. Triple P system population trial. Prev Sci. 2009;10:1-12.
25. Kraft C. Building capacity & support for two generation primary care. 2015 Midwest Regional Summit on Adverse Childhood Experiences. March 13, 2015. www.hmprg.org/assets/root/PDFs/2015/Summit%20Notes%20for%20Day%20Two.pdf. Accessed September 20, 2018.
26. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20:790-798.
27. Leitch L. Action steps using ACEs and trauma-informed care: a resilience model. Health & Justice. 2017;5:1-10.
28. Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51-S69.
29. Wade R Jr, Becker BD, Bevans KB, et al. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52:163-172.
30. Rush P. How learning about emotional trauma led me to a new understanding of chronic illness and health disparity. Becoming trauma-informed: Perspectives from public health, faith communities, education and medicine. Presented at 2016 Advocate Symposium, “Becoming a Trauma-Informed Children’s Hospital and Community: Building Foundations of Care, Collaboration and Practice.” Oaklawn, IL: Advocate Children’s Hospital; November 16, 2016.
31. Diderich HM, Fekkes M, Verkerk PH, et al. A new protocol for screening adults presenting with their own medical problems at the Emergency Department to identify children at high risk for maltreatment. Child Abuse Negl. 2013;37:1122-1131.
32. Fact Sheet: Key ingredients for trauma-informed care. Center for Health Care Strategies, Inc. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 22, 2018.
33. Adversity. In: Merriam-Webster Online Dictionary. Springfield, MA: Merriam-Webster, Inc. www.merriam-webster.com/dictionary/adversity. Accessed September 21, 2018.
34. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:12. Child Trauma Academy (http://childtrauma.org/).
35. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:19. Child Trauma Academy (http://childtrauma.org/).
36. EMDRIA’s definition of EMDR (eye movement desensitization and reprocessing). Austin, TX: EMDRIA: EMDR International Association. http://c.ymcdn.com/sites/www.emdria.org/resource/resmgr/imported/EMDRIA%20Definition%20of%20EMDR.pdf. Revised February 25 2012. Accessed September 21, 2018.
37. Types of trauma and violence: Historical trauma. Washington, DC: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/trauma-violence/types. Accessed September 21, 2018.
38. Hammond DC. What is neurofeedback? An update. J Neurotherapy. 2011;15:305-336.
39. International Neuromodulation Society. Neuromodulation, or neuromodulatory effect. www.neuromodulation.com/neuromodulation-defined. November 9, 2017. Accessed September 21, 2018.
40. World Health Organization. Social determinants of health. www.who.int/social_determinants/sdh_definition/en/. Accessed September 21, 2018.
41. Davis R, Pinderhughes H, Williams M. Adverse community experiences and resilience: a framework for addressing and preventing community trauma. Oakland, CA: Prevention Institute; 2015:4-5. www.preventioninstitute.org/publications/adverse-community-experiences-and-resilience-framework-addressing-and-preventing. Accessed September 30, 2018.
42. SAMHSA-HRSA Center for Integrated Health Solutions. Trauma. Rockville, MD: Substance Abuse and Mental Health Services Administration and Health Resources and Services Administration, US Department of Health and Human Services. www.integration.samhsa.gov/clinical-practice/trauma. Accessed September 21, 2018.
43. Cohen JA, Mandarino AP. Trauma-focused cognitive behavioural therapy for children and parents. Child Adolesc Ment Health. 2008;13:158-162.
44. Trauma-informed approach and trauma-specific interventions: Trauma-informed approach. Washington, DC: National Center for Trauma Informed Care and Alternatives to Seclusion and Restraints; Substance Abuse and Mental Health Services Administration. www.samhsa.gov/nctic/trauma-interventions. Accessed September 21, 2018.
45. Perry BD. How the brain develops: the importance of early childhood. Child Trauma Academy Video Training Series, Video 1;2004:21. Child Trauma Academy (http://childtrauma.org/).
46. Huang LN, Sharp CS, Gunther T. It’s just good medicine: trauma-informed primary care. (SAMHSA-HRSA Center for Integrated Health Solutions webinar); August 6, 2013. www.integration.samhsa.gov/about-us/CIHS_TIC_Webinar_PDF.pdf. Accessed September 20, 2018.
47. CHCS: Center for Health Care Strategies, Inc. Fact sheet: Key ingredients for trauma-informed care. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 20, 2018.
48. National Council for Behavioral Health. Trauma-informed primary care: fostering resilience and recovery. www.thenationalcouncil.org/consulting-areas-of-expertise/trauma-informed-primary-care/. Accessed September 20, 2018.
49. Child Trauma Academy. The Neurosequential Model of Therapeutics as evidence-based practice. https://childtrauma.org/wp-content/uploads/2015/05/NMT_EvidenceBasedPract_5_2_15.pdf. Accessed September 30, 2018.
50. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
51. Metcalf O, Varker T, Forbes D, et al. Efficacy of fifteen emerging interventions for the treatment of posttraumatic stress disorder: a systematic review. J Trauma Stress. 2016;29:88-92.
52. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. 2016; PLoS One. 2016;11:e0166752.
53. Bryan M. A hospital offers frequent ER patients an out—free housing. “All Things Considered.” National Public Radio. June 29, 2016. www.npr.org/sections/health-shots/2016/06/29/482994000/a-hospital-offers-frequent-er-patients-an-out-free-housing. Acces-sed September 20, 2018.
The rising prevalence of obesity, widespread community violence, and the opioid epidemic are urgent health crises that we have, so far, failed to solve. Physicians must therefore ask: Are we employing the right framework to effectively understand and address these complex problems?
Careful review of the literature reveals that these problems and many others begin with, and are profoundly affected by, childhood adversity. Compounding this, studies over the past 20 years that have focused on abuse and neglect without including community, structural, and historical adversity demonstrate that our definitions of adversity and trauma have been too narrow. The prevalence and diversity of factors affecting development and health is much greater than our medical model anticipates.1,2
CASE
Eileen W, a 55-year-old married, self-employed woman with a 20-year history of autoimmune thyroiditis, longstanding insomnia, and anxiety presents with intense episodes of terror related to public speaking, which are compromising her work performance. Her history is significant for tobacco and alcohol use beginning in early adolescence and continuing into young adulthood, as well as 2 unplanned pregnancies in her 20s. Additional adversities included the murder of her maternal aunt while Ms. W was in utero, resulting in her parents having fostered 2 young cousins; bullying; and the premature death of a special-needs sibling.
What treatment strategies might have been undertaken to manage consequences of the adversities of Ms. W’s childhood—both on her own initiative and as interventions by her health care providers?

Our medical model must be updated to be effective
Because at least 60% of Americans have had 1 or more experiences of childhood adversity, family physicians care for affected patients every day—a reality incompletely addressed by our conventional theories and practices.1,3 Consequently, updating our medical model to incorporate research that confirms the critical and widespread impact of childhood experience on health and illness is an essential task for family medicine.
Core values of family medicine integrate biological, clinical, and behavioral sciences. They include comprehensive and compassionate care that is provided within the context of family and community across the lifespan.4,5 Family medicine is therefore the ideal specialty to lead a movement that will translate scientific evidence of the effects of childhood adversity on health into training, delivery of care, and research—transforming clinical practice and patient health across the lifespan.
This article describes the dramatic impact of childhood adversity on health and well-being and calls on family physicians to play a crucial role in preventing, mitigating, and treating the consequences of childhood adversity, an important root cause of disease.
Continue to: Childhood adversity makes us sick
Childhood adversity makes us sick
The first paper about the landmark Adverse Childhood Experiences (ACE) Study, published 20 years ago, is 1 of more than 90 on this topic.3 This study explored the relationship of physical, emotional, and social health in adulthood and self-reported childhood adversity, and comprised 10 categories of abuse, neglect, and household distress between birth and 18 years of age. One of the largest epidemiological studies of its kind, the ACE Study surveyed more than 17,000 mostly white, middle-aged, educated, and insured participants. Study researchers developed an “ACE Score”—the total number of ACEs faced by a person before her (his) 18th birthday—and found that 64% of respondents endorsed 1 or more ACEs; 27% reported 3 or more ACEs; and 5% experienced 6 or more.
The ACE Study revealed a dose–response relationship between ACEs and more than 40 health-compromising behaviors, negative health conditions, and poor social outcomes. Examples include cardiac, autoimmune disease, obesity, intravenous drug abuse, depression and anxiety, adolescent pregnancy, and worker absenteeism. Tragically, an ACE score of ≥6 conferred a significant risk for premature death.1
ACE data have been collected in diverse populations in 32 states and many countries through the Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention3; the Child & Adolescent Health Measurement Initiative’s National Survey of Children’s Health6; and The World Health Organization’s ACE International Questionnaire7—underscoring the pervasiveness of childhood adversity. Evaluation of ACEs in special populations, such as people experiencing homelessness,8 incarcerated youth,9 people struggling with addiction,10 and even health care workers,11 uncovers notably higher rates of ACEs in these populations than in the general population.
Is childhood adversity a true cause of bad outcomes?
Or is the relationship between the 2 entities merely an association? To help answer this question, researchers evaluated the ACE Study using Bradford Hill criteria—9 epidemiological principles employed to infer causation. Their findings strongly support the hypothesis that not only are ACEs associated with myriad negative outcomes, they are their root cause12 and therefore a powerful determinant of our most pressing and expensive health and social problems.Nevertheless, strategies to prevent and address childhood adversity, which are critical to meeting national health goals of successful prevention and treatment of myriad conditions, are absent from the paradigm and practice of most physicians.
The body of research about the health impact of additional adverse experiences is growing to include community violence, poverty, longstanding discrimination,2 and other experiences that we describe as social determinants of health. Furthermore, social determinants of health, or adverse community experiences, appear to maintain a dose–response relationship with health and social outcomes.2 ,13 Along with adverse collective historical experiences (historical trauma),14 these community experiences are forcing further re-examination of existing paradigms of health.
Continue to: The biological pathway from experience to illness
The biological pathway from experience to illness
Neuroscience supports the epidemiology of ACEs.12 The brain develops from the bottom up, in a use-dependent fashion, contingent on genetic potential and, most importantly, on our experiences, which also influence genetic expression. Although present across the lifespan, the brain’s capacity to change—neuroplasticity—is most robust from the prenatal period until about 3 years of age.15 The autonomic nervous system receives information from the body about our internal world and from sensory organs about our external environment and sends it to the brain for processing and interpretation, resulting in micro- and macro-adaptations in structure and function, both within the brain and in the rest of the body.16

Neuroscience demonstrates that adverse experiences, in the context of insufficient protective factors and depending on their timing, severity, and frequency, cause overactivation or prolonged activation, or both, of the stress response system, thus derailing optimal growth and development of the brain and disrupting healthy signaling in all body systems. The dysregulated stress response drives inflammation and subsequent chronic disease (FIGURE17,18), and may influence genetic expression in this, and future, generations.12,14,19 Using neuroimaging and assessment of biomarkers, researchers can see the harm caused by inadequately buffered adversity on overall anatomy and physiology. Protective factors such as a safe environment and positive relationships provide hope that normal biological responses to adverse circumstances can be prevented or reversed, leading to clinical, cognitive, and functional improvement11 (TABLE 120-22).

Evidence-based primary prevention of childhood adversity succeeds
Primary prevention of childhood adversity offers significant benefits across the lifespan and, likely, into the next generation. It ensures that every infant has at least 1 nurturing, attuned caregiver with whom to develop a secure attachment relationship that is essential for optimal growth and development of brain and body.
Primary prevention is most effective when it focuses on supporting caregivers during the perinatal and early childhood periods of their families, before children’s brains are fully organized. Primary prevention involves evidence-based program implementation; collaboration among multiple sectors, including early childhood education, child welfare, criminal justice, business, faith, and health care; and, ultimately, policy change. It incorporates individual, family, and community-based strategies to meet basic needs, ensure safety, fortify a sense of love and belonging in families, and support parents in developing optimal parenting skills. This allows caregivers to devote attention to their children, thus strengthening attunement and attachment, reducing toxic stress, and building protective factors and resilience. Evidence-based and -informed prevention programs include the Nurse–Family Partnership (NFP), Positive Parenting Program (Triple P), and the Family-Centered Medical Home.
NFP. Randomized controlled trials of the NFP, a perinatal home visiting program for low-income, first-time pregnant women and their offspring, showed a reduction in the incidence of domestic violence, child maltreatment, and maternal smoking, with improvement in maternal financial stability, cognitive and socioemotional outcomes, and rates of substance abuse and incarceration in children and/or youth.23
Continue to: Triple P
Triple P. A randomized controlled trial of Triple P, an evidence-based, multilevel, population-based preventive intervention system that was designed to support parents and enhance parenting practices for families with at least 1 child (birth to 12 years old), demonstrated a statistically significant reduction in substantiated child maltreatment cases, out-of-home placements, and emergency room visits and hospitalizations for childhood injuries that were the result of child maltreatment.24
The Family-Centered Medical Home, a primary care strategy to reduce premature and low-birth-weight deliveries, used Medicaid dollars for services not traditionally considered “medical” to address all physical and emotional needs of mothers and families as part of the medical relationship. This program eliminated premature delivery and low birth weight,25 both considered evidence of in utero toxic stress.26
Screening can be brief: In some cases, a single question
The prevalence and impact of childhood adversity, along with the opportunity for significant health improvements and savings, inspires providers to explore screening. Existing screening programs have consistent goals27,28:
- identify unique experiences shaping our patients’ health
- reframe “What’s wrong with you?” as “What happened to you?” “What’s right with you?” and “What matters to you?”
- facilitate health education and neuro-education, particularly meaning-making and self-regulation
- prevent and mitigate the sequelae of exposure to ACEs
- promote health in this and subsequent generations.
The ACE Study screened patients in the context of a comprehensive periodic health assessment. Study participants completed an at-home questionnaire and reviewed it with their physician.1 The Urban ACE Survey added important community stressors such as neighborhood violence, bullying, and food insecurity to the original ACE questionnaire.2
Primary care tool. Wade developed a short, 2-question ACE pre-screener for primary care29 and is exploring screening for childhood adversity in pediatric practice, as are primary care clinicians around the country.
Continue to: Single-question screener
Single-question screener. A Chicago internist interviewed more than 500 patients using a single-question screener that asked whether growing up was “mostly okay or pretty difficult.” This tool accurately confirmed childhood adversity in patients with complex chronic illness, prevented re-traumatization by allowing patients control over disclosure, and opened the door to collaborative healing work over time.30
The Hague Protocol, now mandated in the Netherlands for health and justice professionals, focuses its efforts upstream by offering early detection of children at risk for adverse experiences. The protocol requires asking adults who present with intimate partner violence, suicidality, psychiatric disturbance, or severe substance abuse whether they care for children in any capacity. Those who are so identified are referred to a center at which support services are offered.31
Uncertainty about the utility of existing tools. Many screening tools appear to be promising in terms of identification of the risk for, or actual, childhood adversity, patient and provider satisfaction, and their “fit” in the clinical workflow. Even so, no best practice guidelines exist in primary care to steer screening efforts. Questions remain about27-29:
- broad implementation of a specific tool
- how, when, and where screening should take place
- whether to screen adults, parents, or children—or all 3
- how best to use the content and pacing of screening questions to promote self-regulation and prevent re-traumatization
- best strategies for training and supporting health care workers around screening activities
- how to optimally manage a positive screen.
How best to approach treatment
Treatment includes trauma-informed care, an organizational transformation process (described in TABLE 232; in “The lexicon of childhood adversity: Concepts and tools for care”33-45; and in the subsection, “Lessons from neuroscience”), and individual treatment strategies. The Substance Abuse and Mental Health Services Administration (SAMHSA) of the US Department of Health and Human Services is advocating for implementation of trauma-informed approaches in health systems.

Continue to: The lexicon of childhood adversity...
SIDEBAR
The lexicon of childhood adversity: Concepts and tools for care33-45
Adversity A state or instance of serious or continued difficulty or misfortune. 33
Attachment A special, enduring form of emotional relationship with a specific person involving soothing, pleasure, and comfort.34
Attunement The ability to read and respond to the cues of another.35
Eye-movement desensitization and reprocessing (EMDR) An evidence-based psychotherapy for posttraumatic stress disorder and other psychiatric disorders, mental health problems, and somatic symptoms. EMDR facilitates resumption of normal information processing and integration; the patient attends to emotionally disturbing material in brief sequential doses while simultaneously focusing on an external stimulus. EMDR targets past experience, current triggers, and future potential challenges, and results in alleviation of presenting symptoms; a decrease or elimination of distress from the disturbing memory; improved view of the self; relief from bodily disturbance; and resolution of present and future anticipated triggers.36
Historical trauma Cumulative emotional and psychological wounding, resulting from group traumatic experiences, transmitted across generations within a community.37
Neurofeedback Electroencephalographic biofeedback is a method for retraining brainwave patterns through operant conditioning; it is used to treat posttraumatic stress disorder, various mental health conditions, addiction, chronic pain, epilepsy, and other disorders.38
Neuromodulatory Having the capacity to alter nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body to help restore function or relieve symptoms.39
Social determinants of health/adverse community experiences Conditions in which people are born, grow, live, work, and age and that are shaped by distribution of money, power, and resources at all levels.40,41
Trauma An event or circumstance experienced or observed by a person as physically or emotionally harmful or threatening and having lasting adverse effects on that person's functioning and well-being.42
Trauma-focused cognitive behavioral therapy An evidence-based trauma treatment for children 3 to 18 years and their parents comprising the elements of the acronym PRACTICE: Psychoeducation and parenting; Relaxation methods; Affective expression and regulation skills; Cognitive coping skills and processing; Trauma narrative and processing; In vivo exposure; Conjoint parent-child therapy sessions; and Enhancing personal safety and growth.43
Trauma-informed approach This "4-R" approach can be implemented in any type of service setting, organization, or program that: Realizes the widespread impact of trauma and understands potential paths for recovery; recognizes signs and symptoms of trauma in clients, families, staff, and others involved with the system; responds by fully integrating knowledge about trauma into policies, procedures, and practices; and seeks to actively resist re-traumatization.44
Use-dependent The organization and function of neurons, the neural system, and the brain depends on repetitive, patterned stimulation.45
Continue to: Trauma-informed care is a model...
Trauma-informed care is a model intended to promote healing and reduce the risk for re-traumatization of patients by staff—significant concerns in clinical settings, where the dynamics of loss of power, control, and safety that are inherent in traumatic experience can be replicated.46 To operationalize trauma-informed care more formally, the Center for Health Care Strategies, Inc., and the National Council for Behavioral Health are developing recommendations for 1) standardized screening and assessment tools, evidence-based clinical interventions, implementation processes, and relevant and replicable outcome measures, and 2) policy changes to improve patient and staff engagement, enhance health outcomes, and reduce avoidable care and excess costs.47,48
Lessons from neuroscience guide effective treatment.16 Treatment begins with bottom-up strategies that are focused on decreasing suboptimal excitatory input from the survival brainstem to create safety, connect patients to resources to meet basic needs, teach self-regulation skills, and improve relational health in and outside of the office. Later-stage top-down methods, such as education and other cognitive activities, focus on strengthening the regulatory capacity of the thinking cortex.16 In many ways, treatment mirrors prevention: It emphasizes first helping patients feel safe and loved.
In a follow-up to the ACE Study, 100,000 patients had a primary care visit in which their practitioner reviewed the ACE questionnaire with them; said “I see that you have________. Tell me how that has affected you later in your life” for every “Yes” response; and listened to the answers without passing judgment. This simple intervention profoundly decreased health resource utilization by these patients during the following year: a reduction of 35% in office visits, 11% in emergency room visits, and 3% in hospitalizations.1
The neurosequential model of therapeutics assesses neurodevelopment in the context of childhood adversity and relational health to evaluate consequences of childhood adversity and direct treatment. Adopted domestically and internationally, this model has had statistically significant success facilitating improvement in patients’ physical, emotional, and social health status.16,49
Trauma-specific treatment modalities such as trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing (EMDR),50 a trauma-specific treatment effective in resolving painful childhood memories, are evidence-based treatments that reduce trauma-related symptoms; evidence is also emerging about the efficacy of yoga51 and neurofeedback.52 These therapies have been best studied as treatment for posttraumatic stress disorder and other mental health disorders and also hold promise for addressing physical and social consequences of adversity. They present a low risk for harm, appear to be cost-effective, and improve outcomes.
Continue to: Best regimens involve a multifaceted approach that combines...
Best regimens involve a multifaceted approach that combines health-system resources with referral to other community practitioners and agencies. An excellent example is a current collaboration between health systems and affordable housing programs to reduce and, ultimately, eliminate chronic homelessness. Positive outcomes of this collaboration include both improved health and life satisfaction for participants and cost savings to the health system.53
CASE
Beginning in adulthood, Ms. W began long-term psychotherapy and had a therapeutic trial of antidepressants, without significant improvement. None of her medical or mental-health providers educated her about the connection between childhood adversity and illness to help her make sense of her health history and autoimmune disease, or to guide treatment. She learned from a friend about the relationship between childhood adversity and poor health and self-administered the ACE questionnaire, scoring 5 points out of a possible 10.
Ms. W enjoyed loving relationships with her mother, sisters, and friends. She had long-standing personal practices of individual and group physical activity, journaling, and spending time in nature.
About 10 years ago, Ms. W committed to regular yoga practice and later saw a functional medicine provider, who focused on nutrition and restorative sleep. She noticed improvement in all signs and symptoms; however, the terror of public speaking remained. Through friends, she found a practitioner who offered EMDR. Over the past 2 years, her terror has resolved and general anxiety and insomnia have continued to improve; she is now able to speak with fluency and comfort in any arena.
Addressing childhood adversity: Our “natural domain”
Experiences, positive and negative, shape our psychology and biology; they are powerful determinants of health—or illness. Prevention of, and response to, childhood adversity demand a systems approach to the whole person in context—the natural domain of family medicine.
Continue to: Although clinical translation is still unfolding...
Although clinical translation is still unfolding, the risks of implementing promising prevention and treatment strategies are low, the stakes are high, and the potential benefits are vast. Therefore, we as family physicians can—must—learn and incorporate the science of childhood adversity, neurobiology, and life course into our training, research, and clinical paradigm and practice; we can do that by embedding this framework throughout our training and continuing education in formal didactics, case discussions, hands-on skill-building, scientific investigation, and patient care.
We must make our offices and hospitals trauma-informed; connect patients with resources to meet basic needs and with home-visiting and parent education programs; educate patients about the impact of protective and adverse factors on health; provide and practice self-regulation training in our offices or by referral; and advocate for equity.
Using these strategies, family physicians will play a crucial role in the prevention, mitigation, and treatment of the root cause of disease and society’s deepest individual and collective suffering.
CORRESPONDENCE
Audrey Stillerman, MD, ABFM, ABIHM, ABOIM, Office of Community Engagement and Neighborhood Health Partnerships, 808 South Wolcott Street, Room 809, Chicago, IL 60612; [email protected].
ACKNOWLEDGMENT
Patricia Rush, MD, MBA, and Adrienne Williams, PhD, reviewed the manuscript of this article.
The rising prevalence of obesity, widespread community violence, and the opioid epidemic are urgent health crises that we have, so far, failed to solve. Physicians must therefore ask: Are we employing the right framework to effectively understand and address these complex problems?
Careful review of the literature reveals that these problems and many others begin with, and are profoundly affected by, childhood adversity. Compounding this, studies over the past 20 years that have focused on abuse and neglect without including community, structural, and historical adversity demonstrate that our definitions of adversity and trauma have been too narrow. The prevalence and diversity of factors affecting development and health is much greater than our medical model anticipates.1,2
CASE
Eileen W, a 55-year-old married, self-employed woman with a 20-year history of autoimmune thyroiditis, longstanding insomnia, and anxiety presents with intense episodes of terror related to public speaking, which are compromising her work performance. Her history is significant for tobacco and alcohol use beginning in early adolescence and continuing into young adulthood, as well as 2 unplanned pregnancies in her 20s. Additional adversities included the murder of her maternal aunt while Ms. W was in utero, resulting in her parents having fostered 2 young cousins; bullying; and the premature death of a special-needs sibling.
What treatment strategies might have been undertaken to manage consequences of the adversities of Ms. W’s childhood—both on her own initiative and as interventions by her health care providers?

Our medical model must be updated to be effective
Because at least 60% of Americans have had 1 or more experiences of childhood adversity, family physicians care for affected patients every day—a reality incompletely addressed by our conventional theories and practices.1,3 Consequently, updating our medical model to incorporate research that confirms the critical and widespread impact of childhood experience on health and illness is an essential task for family medicine.
Core values of family medicine integrate biological, clinical, and behavioral sciences. They include comprehensive and compassionate care that is provided within the context of family and community across the lifespan.4,5 Family medicine is therefore the ideal specialty to lead a movement that will translate scientific evidence of the effects of childhood adversity on health into training, delivery of care, and research—transforming clinical practice and patient health across the lifespan.
This article describes the dramatic impact of childhood adversity on health and well-being and calls on family physicians to play a crucial role in preventing, mitigating, and treating the consequences of childhood adversity, an important root cause of disease.
Continue to: Childhood adversity makes us sick
Childhood adversity makes us sick
The first paper about the landmark Adverse Childhood Experiences (ACE) Study, published 20 years ago, is 1 of more than 90 on this topic.3 This study explored the relationship of physical, emotional, and social health in adulthood and self-reported childhood adversity, and comprised 10 categories of abuse, neglect, and household distress between birth and 18 years of age. One of the largest epidemiological studies of its kind, the ACE Study surveyed more than 17,000 mostly white, middle-aged, educated, and insured participants. Study researchers developed an “ACE Score”—the total number of ACEs faced by a person before her (his) 18th birthday—and found that 64% of respondents endorsed 1 or more ACEs; 27% reported 3 or more ACEs; and 5% experienced 6 or more.
The ACE Study revealed a dose–response relationship between ACEs and more than 40 health-compromising behaviors, negative health conditions, and poor social outcomes. Examples include cardiac, autoimmune disease, obesity, intravenous drug abuse, depression and anxiety, adolescent pregnancy, and worker absenteeism. Tragically, an ACE score of ≥6 conferred a significant risk for premature death.1
ACE data have been collected in diverse populations in 32 states and many countries through the Behavioral Risk Factor Surveillance Survey conducted by the Centers for Disease Control and Prevention3; the Child & Adolescent Health Measurement Initiative’s National Survey of Children’s Health6; and The World Health Organization’s ACE International Questionnaire7—underscoring the pervasiveness of childhood adversity. Evaluation of ACEs in special populations, such as people experiencing homelessness,8 incarcerated youth,9 people struggling with addiction,10 and even health care workers,11 uncovers notably higher rates of ACEs in these populations than in the general population.
Is childhood adversity a true cause of bad outcomes?
Or is the relationship between the 2 entities merely an association? To help answer this question, researchers evaluated the ACE Study using Bradford Hill criteria—9 epidemiological principles employed to infer causation. Their findings strongly support the hypothesis that not only are ACEs associated with myriad negative outcomes, they are their root cause12 and therefore a powerful determinant of our most pressing and expensive health and social problems.Nevertheless, strategies to prevent and address childhood adversity, which are critical to meeting national health goals of successful prevention and treatment of myriad conditions, are absent from the paradigm and practice of most physicians.
The body of research about the health impact of additional adverse experiences is growing to include community violence, poverty, longstanding discrimination,2 and other experiences that we describe as social determinants of health. Furthermore, social determinants of health, or adverse community experiences, appear to maintain a dose–response relationship with health and social outcomes.2 ,13 Along with adverse collective historical experiences (historical trauma),14 these community experiences are forcing further re-examination of existing paradigms of health.
Continue to: The biological pathway from experience to illness
The biological pathway from experience to illness
Neuroscience supports the epidemiology of ACEs.12 The brain develops from the bottom up, in a use-dependent fashion, contingent on genetic potential and, most importantly, on our experiences, which also influence genetic expression. Although present across the lifespan, the brain’s capacity to change—neuroplasticity—is most robust from the prenatal period until about 3 years of age.15 The autonomic nervous system receives information from the body about our internal world and from sensory organs about our external environment and sends it to the brain for processing and interpretation, resulting in micro- and macro-adaptations in structure and function, both within the brain and in the rest of the body.16

Neuroscience demonstrates that adverse experiences, in the context of insufficient protective factors and depending on their timing, severity, and frequency, cause overactivation or prolonged activation, or both, of the stress response system, thus derailing optimal growth and development of the brain and disrupting healthy signaling in all body systems. The dysregulated stress response drives inflammation and subsequent chronic disease (FIGURE17,18), and may influence genetic expression in this, and future, generations.12,14,19 Using neuroimaging and assessment of biomarkers, researchers can see the harm caused by inadequately buffered adversity on overall anatomy and physiology. Protective factors such as a safe environment and positive relationships provide hope that normal biological responses to adverse circumstances can be prevented or reversed, leading to clinical, cognitive, and functional improvement11 (TABLE 120-22).

Evidence-based primary prevention of childhood adversity succeeds
Primary prevention of childhood adversity offers significant benefits across the lifespan and, likely, into the next generation. It ensures that every infant has at least 1 nurturing, attuned caregiver with whom to develop a secure attachment relationship that is essential for optimal growth and development of brain and body.
Primary prevention is most effective when it focuses on supporting caregivers during the perinatal and early childhood periods of their families, before children’s brains are fully organized. Primary prevention involves evidence-based program implementation; collaboration among multiple sectors, including early childhood education, child welfare, criminal justice, business, faith, and health care; and, ultimately, policy change. It incorporates individual, family, and community-based strategies to meet basic needs, ensure safety, fortify a sense of love and belonging in families, and support parents in developing optimal parenting skills. This allows caregivers to devote attention to their children, thus strengthening attunement and attachment, reducing toxic stress, and building protective factors and resilience. Evidence-based and -informed prevention programs include the Nurse–Family Partnership (NFP), Positive Parenting Program (Triple P), and the Family-Centered Medical Home.
NFP. Randomized controlled trials of the NFP, a perinatal home visiting program for low-income, first-time pregnant women and their offspring, showed a reduction in the incidence of domestic violence, child maltreatment, and maternal smoking, with improvement in maternal financial stability, cognitive and socioemotional outcomes, and rates of substance abuse and incarceration in children and/or youth.23
Continue to: Triple P
Triple P. A randomized controlled trial of Triple P, an evidence-based, multilevel, population-based preventive intervention system that was designed to support parents and enhance parenting practices for families with at least 1 child (birth to 12 years old), demonstrated a statistically significant reduction in substantiated child maltreatment cases, out-of-home placements, and emergency room visits and hospitalizations for childhood injuries that were the result of child maltreatment.24
The Family-Centered Medical Home, a primary care strategy to reduce premature and low-birth-weight deliveries, used Medicaid dollars for services not traditionally considered “medical” to address all physical and emotional needs of mothers and families as part of the medical relationship. This program eliminated premature delivery and low birth weight,25 both considered evidence of in utero toxic stress.26
Screening can be brief: In some cases, a single question
The prevalence and impact of childhood adversity, along with the opportunity for significant health improvements and savings, inspires providers to explore screening. Existing screening programs have consistent goals27,28:
- identify unique experiences shaping our patients’ health
- reframe “What’s wrong with you?” as “What happened to you?” “What’s right with you?” and “What matters to you?”
- facilitate health education and neuro-education, particularly meaning-making and self-regulation
- prevent and mitigate the sequelae of exposure to ACEs
- promote health in this and subsequent generations.
The ACE Study screened patients in the context of a comprehensive periodic health assessment. Study participants completed an at-home questionnaire and reviewed it with their physician.1 The Urban ACE Survey added important community stressors such as neighborhood violence, bullying, and food insecurity to the original ACE questionnaire.2
Primary care tool. Wade developed a short, 2-question ACE pre-screener for primary care29 and is exploring screening for childhood adversity in pediatric practice, as are primary care clinicians around the country.
Continue to: Single-question screener
Single-question screener. A Chicago internist interviewed more than 500 patients using a single-question screener that asked whether growing up was “mostly okay or pretty difficult.” This tool accurately confirmed childhood adversity in patients with complex chronic illness, prevented re-traumatization by allowing patients control over disclosure, and opened the door to collaborative healing work over time.30
The Hague Protocol, now mandated in the Netherlands for health and justice professionals, focuses its efforts upstream by offering early detection of children at risk for adverse experiences. The protocol requires asking adults who present with intimate partner violence, suicidality, psychiatric disturbance, or severe substance abuse whether they care for children in any capacity. Those who are so identified are referred to a center at which support services are offered.31
Uncertainty about the utility of existing tools. Many screening tools appear to be promising in terms of identification of the risk for, or actual, childhood adversity, patient and provider satisfaction, and their “fit” in the clinical workflow. Even so, no best practice guidelines exist in primary care to steer screening efforts. Questions remain about27-29:
- broad implementation of a specific tool
- how, when, and where screening should take place
- whether to screen adults, parents, or children—or all 3
- how best to use the content and pacing of screening questions to promote self-regulation and prevent re-traumatization
- best strategies for training and supporting health care workers around screening activities
- how to optimally manage a positive screen.
How best to approach treatment
Treatment includes trauma-informed care, an organizational transformation process (described in TABLE 232; in “The lexicon of childhood adversity: Concepts and tools for care”33-45; and in the subsection, “Lessons from neuroscience”), and individual treatment strategies. The Substance Abuse and Mental Health Services Administration (SAMHSA) of the US Department of Health and Human Services is advocating for implementation of trauma-informed approaches in health systems.

Continue to: The lexicon of childhood adversity...
SIDEBAR
The lexicon of childhood adversity: Concepts and tools for care33-45
Adversity A state or instance of serious or continued difficulty or misfortune. 33
Attachment A special, enduring form of emotional relationship with a specific person involving soothing, pleasure, and comfort.34
Attunement The ability to read and respond to the cues of another.35
Eye-movement desensitization and reprocessing (EMDR) An evidence-based psychotherapy for posttraumatic stress disorder and other psychiatric disorders, mental health problems, and somatic symptoms. EMDR facilitates resumption of normal information processing and integration; the patient attends to emotionally disturbing material in brief sequential doses while simultaneously focusing on an external stimulus. EMDR targets past experience, current triggers, and future potential challenges, and results in alleviation of presenting symptoms; a decrease or elimination of distress from the disturbing memory; improved view of the self; relief from bodily disturbance; and resolution of present and future anticipated triggers.36
Historical trauma Cumulative emotional and psychological wounding, resulting from group traumatic experiences, transmitted across generations within a community.37
Neurofeedback Electroencephalographic biofeedback is a method for retraining brainwave patterns through operant conditioning; it is used to treat posttraumatic stress disorder, various mental health conditions, addiction, chronic pain, epilepsy, and other disorders.38
Neuromodulatory Having the capacity to alter nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body to help restore function or relieve symptoms.39
Social determinants of health/adverse community experiences Conditions in which people are born, grow, live, work, and age and that are shaped by distribution of money, power, and resources at all levels.40,41
Trauma An event or circumstance experienced or observed by a person as physically or emotionally harmful or threatening and having lasting adverse effects on that person's functioning and well-being.42
Trauma-focused cognitive behavioral therapy An evidence-based trauma treatment for children 3 to 18 years and their parents comprising the elements of the acronym PRACTICE: Psychoeducation and parenting; Relaxation methods; Affective expression and regulation skills; Cognitive coping skills and processing; Trauma narrative and processing; In vivo exposure; Conjoint parent-child therapy sessions; and Enhancing personal safety and growth.43
Trauma-informed approach This "4-R" approach can be implemented in any type of service setting, organization, or program that: Realizes the widespread impact of trauma and understands potential paths for recovery; recognizes signs and symptoms of trauma in clients, families, staff, and others involved with the system; responds by fully integrating knowledge about trauma into policies, procedures, and practices; and seeks to actively resist re-traumatization.44
Use-dependent The organization and function of neurons, the neural system, and the brain depends on repetitive, patterned stimulation.45
Continue to: Trauma-informed care is a model...
Trauma-informed care is a model intended to promote healing and reduce the risk for re-traumatization of patients by staff—significant concerns in clinical settings, where the dynamics of loss of power, control, and safety that are inherent in traumatic experience can be replicated.46 To operationalize trauma-informed care more formally, the Center for Health Care Strategies, Inc., and the National Council for Behavioral Health are developing recommendations for 1) standardized screening and assessment tools, evidence-based clinical interventions, implementation processes, and relevant and replicable outcome measures, and 2) policy changes to improve patient and staff engagement, enhance health outcomes, and reduce avoidable care and excess costs.47,48
Lessons from neuroscience guide effective treatment.16 Treatment begins with bottom-up strategies that are focused on decreasing suboptimal excitatory input from the survival brainstem to create safety, connect patients to resources to meet basic needs, teach self-regulation skills, and improve relational health in and outside of the office. Later-stage top-down methods, such as education and other cognitive activities, focus on strengthening the regulatory capacity of the thinking cortex.16 In many ways, treatment mirrors prevention: It emphasizes first helping patients feel safe and loved.
In a follow-up to the ACE Study, 100,000 patients had a primary care visit in which their practitioner reviewed the ACE questionnaire with them; said “I see that you have________. Tell me how that has affected you later in your life” for every “Yes” response; and listened to the answers without passing judgment. This simple intervention profoundly decreased health resource utilization by these patients during the following year: a reduction of 35% in office visits, 11% in emergency room visits, and 3% in hospitalizations.1
The neurosequential model of therapeutics assesses neurodevelopment in the context of childhood adversity and relational health to evaluate consequences of childhood adversity and direct treatment. Adopted domestically and internationally, this model has had statistically significant success facilitating improvement in patients’ physical, emotional, and social health status.16,49
Trauma-specific treatment modalities such as trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing (EMDR),50 a trauma-specific treatment effective in resolving painful childhood memories, are evidence-based treatments that reduce trauma-related symptoms; evidence is also emerging about the efficacy of yoga51 and neurofeedback.52 These therapies have been best studied as treatment for posttraumatic stress disorder and other mental health disorders and also hold promise for addressing physical and social consequences of adversity. They present a low risk for harm, appear to be cost-effective, and improve outcomes.
Continue to: Best regimens involve a multifaceted approach that combines...
Best regimens involve a multifaceted approach that combines health-system resources with referral to other community practitioners and agencies. An excellent example is a current collaboration between health systems and affordable housing programs to reduce and, ultimately, eliminate chronic homelessness. Positive outcomes of this collaboration include both improved health and life satisfaction for participants and cost savings to the health system.53
CASE
Beginning in adulthood, Ms. W began long-term psychotherapy and had a therapeutic trial of antidepressants, without significant improvement. None of her medical or mental-health providers educated her about the connection between childhood adversity and illness to help her make sense of her health history and autoimmune disease, or to guide treatment. She learned from a friend about the relationship between childhood adversity and poor health and self-administered the ACE questionnaire, scoring 5 points out of a possible 10.
Ms. W enjoyed loving relationships with her mother, sisters, and friends. She had long-standing personal practices of individual and group physical activity, journaling, and spending time in nature.
About 10 years ago, Ms. W committed to regular yoga practice and later saw a functional medicine provider, who focused on nutrition and restorative sleep. She noticed improvement in all signs and symptoms; however, the terror of public speaking remained. Through friends, she found a practitioner who offered EMDR. Over the past 2 years, her terror has resolved and general anxiety and insomnia have continued to improve; she is now able to speak with fluency and comfort in any arena.
Addressing childhood adversity: Our “natural domain”
Experiences, positive and negative, shape our psychology and biology; they are powerful determinants of health—or illness. Prevention of, and response to, childhood adversity demand a systems approach to the whole person in context—the natural domain of family medicine.
Continue to: Although clinical translation is still unfolding...
Although clinical translation is still unfolding, the risks of implementing promising prevention and treatment strategies are low, the stakes are high, and the potential benefits are vast. Therefore, we as family physicians can—must—learn and incorporate the science of childhood adversity, neurobiology, and life course into our training, research, and clinical paradigm and practice; we can do that by embedding this framework throughout our training and continuing education in formal didactics, case discussions, hands-on skill-building, scientific investigation, and patient care.
We must make our offices and hospitals trauma-informed; connect patients with resources to meet basic needs and with home-visiting and parent education programs; educate patients about the impact of protective and adverse factors on health; provide and practice self-regulation training in our offices or by referral; and advocate for equity.
Using these strategies, family physicians will play a crucial role in the prevention, mitigation, and treatment of the root cause of disease and society’s deepest individual and collective suffering.
CORRESPONDENCE
Audrey Stillerman, MD, ABFM, ABIHM, ABOIM, Office of Community Engagement and Neighborhood Health Partnerships, 808 South Wolcott Street, Room 809, Chicago, IL 60612; [email protected].
ACKNOWLEDGMENT
Patricia Rush, MD, MBA, and Adrienne Williams, PhD, reviewed the manuscript of this article.
1. Felitti V, Anda R. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
2. Wade R Jr, Shea JA, Rubin D, et al. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134:e13-e20.
3. Centers for Disease Control and Prevention. Child abuse and neglect prevention. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/index.html. Accessed September 20, 2018.
4. American Academy of Family Physicians. Definition of family medicine. www.aafp.org/about/policies/all/family-medicine-definition.html. Accessed March 5, 2018.
5. Martin JC, Avant RF, Bowman MA, et al; The Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2 Suppl 1:S3-S32.
6. Child & Adolescent Health Measurement Initiative (CAHMI). A national and across-state profile on Adverse Childhood Experiences among U.S. children and possibilities to heal and thrive. Issue Brief. October 2017. www.cahmi.org/wp-content/uploads/2018/05/aces_brief_final.pdf. Accessed September 20, 2018.
7. World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ). www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/. Accessed September 20, 2018.
8. Roos LE, Mota N, Afifi TO, et al. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am J Public Health. 2013;103(Suppl 2):S275-S281.
9. Baglivio MT. Wolff KT. Piquero AR, et al. The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J Crim Justice. 2015;43:229-241.
10. Dube SR. Felitti VF. Dong M, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564-572.
11. Maunder RG, Peladeau N, Savage D, et al. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34:114-123.
12. Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174-186.
13. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186-S196.
14. Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41:232-244.
15. Perry BD. Memories of fears: How the brain stores and retrieves traumatic experiences. In: Goodwin J, Attias R, eds. Splintered Reflections: Images of the Body in Trauma. New York, NY: Basic Books; 1999:9-38.
16. Perry BD. Examining child maltreatment through a neurodevelopmental lens: clinical application of the Neurosequential Model of Therapeutics. J Loss Trauma. 2009;14:240-255.
17.
18. Adding layers to the ACEs pyramid—What do you think? Trauma and social location. ACES Connection, RYSE Center. 2015. www.acesconnection.com/blog/adding-layers-to-the-aces-pyramid-what-do-you-think. Accessed October 10, 2018.
19. Berens AE, Jensen SKG, Nelson CA 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135.
20. Rostad WL, Basile KC, Clayton HB. Association among television and computer/video game use, victimization, and suicide risk among U.S. high school students. J Interpers Violence. 2018 Mar 1:886260518760020.
21. Coyne SM, Nelson DA, Graham-Kevan N, et al. Media depictions of physical and relational aggression: connections with aggression in young adults’ romantic relationships. Aggress Behav. 2011;37:56-62.
22. Centers for Disease Control and Prevention. Violence prevention: Child abuse and neglect: risk and protective factors. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/riskprotectivefactors.html. Accessed October 10, 2018.
23. Miller TR. Projected outcomes of nurse-family partnership home visitation during 1996-2013, United States. Prev Sci. 2015;16:765-777.
24. Prinz RJ, Sanders MR, Shapiro CJ, et al. Population-based prevention of child maltreatment: the U.S. Triple P system population trial. Prev Sci. 2009;10:1-12.
25. Kraft C. Building capacity & support for two generation primary care. 2015 Midwest Regional Summit on Adverse Childhood Experiences. March 13, 2015. www.hmprg.org/assets/root/PDFs/2015/Summit%20Notes%20for%20Day%20Two.pdf. Accessed September 20, 2018.
26. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20:790-798.
27. Leitch L. Action steps using ACEs and trauma-informed care: a resilience model. Health & Justice. 2017;5:1-10.
28. Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51-S69.
29. Wade R Jr, Becker BD, Bevans KB, et al. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52:163-172.
30. Rush P. How learning about emotional trauma led me to a new understanding of chronic illness and health disparity. Becoming trauma-informed: Perspectives from public health, faith communities, education and medicine. Presented at 2016 Advocate Symposium, “Becoming a Trauma-Informed Children’s Hospital and Community: Building Foundations of Care, Collaboration and Practice.” Oaklawn, IL: Advocate Children’s Hospital; November 16, 2016.
31. Diderich HM, Fekkes M, Verkerk PH, et al. A new protocol for screening adults presenting with their own medical problems at the Emergency Department to identify children at high risk for maltreatment. Child Abuse Negl. 2013;37:1122-1131.
32. Fact Sheet: Key ingredients for trauma-informed care. Center for Health Care Strategies, Inc. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 22, 2018.
33. Adversity. In: Merriam-Webster Online Dictionary. Springfield, MA: Merriam-Webster, Inc. www.merriam-webster.com/dictionary/adversity. Accessed September 21, 2018.
34. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:12. Child Trauma Academy (http://childtrauma.org/).
35. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:19. Child Trauma Academy (http://childtrauma.org/).
36. EMDRIA’s definition of EMDR (eye movement desensitization and reprocessing). Austin, TX: EMDRIA: EMDR International Association. http://c.ymcdn.com/sites/www.emdria.org/resource/resmgr/imported/EMDRIA%20Definition%20of%20EMDR.pdf. Revised February 25 2012. Accessed September 21, 2018.
37. Types of trauma and violence: Historical trauma. Washington, DC: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/trauma-violence/types. Accessed September 21, 2018.
38. Hammond DC. What is neurofeedback? An update. J Neurotherapy. 2011;15:305-336.
39. International Neuromodulation Society. Neuromodulation, or neuromodulatory effect. www.neuromodulation.com/neuromodulation-defined. November 9, 2017. Accessed September 21, 2018.
40. World Health Organization. Social determinants of health. www.who.int/social_determinants/sdh_definition/en/. Accessed September 21, 2018.
41. Davis R, Pinderhughes H, Williams M. Adverse community experiences and resilience: a framework for addressing and preventing community trauma. Oakland, CA: Prevention Institute; 2015:4-5. www.preventioninstitute.org/publications/adverse-community-experiences-and-resilience-framework-addressing-and-preventing. Accessed September 30, 2018.
42. SAMHSA-HRSA Center for Integrated Health Solutions. Trauma. Rockville, MD: Substance Abuse and Mental Health Services Administration and Health Resources and Services Administration, US Department of Health and Human Services. www.integration.samhsa.gov/clinical-practice/trauma. Accessed September 21, 2018.
43. Cohen JA, Mandarino AP. Trauma-focused cognitive behavioural therapy for children and parents. Child Adolesc Ment Health. 2008;13:158-162.
44. Trauma-informed approach and trauma-specific interventions: Trauma-informed approach. Washington, DC: National Center for Trauma Informed Care and Alternatives to Seclusion and Restraints; Substance Abuse and Mental Health Services Administration. www.samhsa.gov/nctic/trauma-interventions. Accessed September 21, 2018.
45. Perry BD. How the brain develops: the importance of early childhood. Child Trauma Academy Video Training Series, Video 1;2004:21. Child Trauma Academy (http://childtrauma.org/).
46. Huang LN, Sharp CS, Gunther T. It’s just good medicine: trauma-informed primary care. (SAMHSA-HRSA Center for Integrated Health Solutions webinar); August 6, 2013. www.integration.samhsa.gov/about-us/CIHS_TIC_Webinar_PDF.pdf. Accessed September 20, 2018.
47. CHCS: Center for Health Care Strategies, Inc. Fact sheet: Key ingredients for trauma-informed care. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 20, 2018.
48. National Council for Behavioral Health. Trauma-informed primary care: fostering resilience and recovery. www.thenationalcouncil.org/consulting-areas-of-expertise/trauma-informed-primary-care/. Accessed September 20, 2018.
49. Child Trauma Academy. The Neurosequential Model of Therapeutics as evidence-based practice. https://childtrauma.org/wp-content/uploads/2015/05/NMT_EvidenceBasedPract_5_2_15.pdf. Accessed September 30, 2018.
50. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
51. Metcalf O, Varker T, Forbes D, et al. Efficacy of fifteen emerging interventions for the treatment of posttraumatic stress disorder: a systematic review. J Trauma Stress. 2016;29:88-92.
52. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. 2016; PLoS One. 2016;11:e0166752.
53. Bryan M. A hospital offers frequent ER patients an out—free housing. “All Things Considered.” National Public Radio. June 29, 2016. www.npr.org/sections/health-shots/2016/06/29/482994000/a-hospital-offers-frequent-er-patients-an-out-free-housing. Acces-sed September 20, 2018.
1. Felitti V, Anda R. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders and sexual behavior: implications for healthcare. In: Lanius RA, Vermetten E, Pain C, eds. The Impact of Early Life Trauma on Health and Disease: The Hidden Epidemic. Cambridge, UK: Cambridge University Press; 2011:77-87.
2. Wade R Jr, Shea JA, Rubin D, et al. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134:e13-e20.
3. Centers for Disease Control and Prevention. Child abuse and neglect prevention. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/index.html. Accessed September 20, 2018.
4. American Academy of Family Physicians. Definition of family medicine. www.aafp.org/about/policies/all/family-medicine-definition.html. Accessed March 5, 2018.
5. Martin JC, Avant RF, Bowman MA, et al; The Future of Family Medicine Project Leadership Committee. The Future of Family Medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2 Suppl 1:S3-S32.
6. Child & Adolescent Health Measurement Initiative (CAHMI). A national and across-state profile on Adverse Childhood Experiences among U.S. children and possibilities to heal and thrive. Issue Brief. October 2017. www.cahmi.org/wp-content/uploads/2018/05/aces_brief_final.pdf. Accessed September 20, 2018.
7. World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ). www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/. Accessed September 20, 2018.
8. Roos LE, Mota N, Afifi TO, et al. Relationship between adverse childhood experiences and homelessness and the impact of axis I and II disorders. Am J Public Health. 2013;103(Suppl 2):S275-S281.
9. Baglivio MT. Wolff KT. Piquero AR, et al. The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J Crim Justice. 2015;43:229-241.
10. Dube SR. Felitti VF. Dong M, et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564-572.
11. Maunder RG, Peladeau N, Savage D, et al. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010;34:114-123.
12. Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174-186.
13. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186-S196.
14. Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41:232-244.
15. Perry BD. Memories of fears: How the brain stores and retrieves traumatic experiences. In: Goodwin J, Attias R, eds. Splintered Reflections: Images of the Body in Trauma. New York, NY: Basic Books; 1999:9-38.
16. Perry BD. Examining child maltreatment through a neurodevelopmental lens: clinical application of the Neurosequential Model of Therapeutics. J Loss Trauma. 2009;14:240-255.
17.
18. Adding layers to the ACEs pyramid—What do you think? Trauma and social location. ACES Connection, RYSE Center. 2015. www.acesconnection.com/blog/adding-layers-to-the-aces-pyramid-what-do-you-think. Accessed October 10, 2018.
19. Berens AE, Jensen SKG, Nelson CA 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135.
20. Rostad WL, Basile KC, Clayton HB. Association among television and computer/video game use, victimization, and suicide risk among U.S. high school students. J Interpers Violence. 2018 Mar 1:886260518760020.
21. Coyne SM, Nelson DA, Graham-Kevan N, et al. Media depictions of physical and relational aggression: connections with aggression in young adults’ romantic relationships. Aggress Behav. 2011;37:56-62.
22. Centers for Disease Control and Prevention. Violence prevention: Child abuse and neglect: risk and protective factors. April 10, 2018. www.cdc.gov/violenceprevention/childabuseandneglect/riskprotectivefactors.html. Accessed October 10, 2018.
23. Miller TR. Projected outcomes of nurse-family partnership home visitation during 1996-2013, United States. Prev Sci. 2015;16:765-777.
24. Prinz RJ, Sanders MR, Shapiro CJ, et al. Population-based prevention of child maltreatment: the U.S. Triple P system population trial. Prev Sci. 2009;10:1-12.
25. Kraft C. Building capacity & support for two generation primary care. 2015 Midwest Regional Summit on Adverse Childhood Experiences. March 13, 2015. www.hmprg.org/assets/root/PDFs/2015/Summit%20Notes%20for%20Day%20Two.pdf. Accessed September 20, 2018.
26. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20:790-798.
27. Leitch L. Action steps using ACEs and trauma-informed care: a resilience model. Health & Justice. 2017;5:1-10.
28. Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51-S69.
29. Wade R Jr, Becker BD, Bevans KB, et al. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52:163-172.
30. Rush P. How learning about emotional trauma led me to a new understanding of chronic illness and health disparity. Becoming trauma-informed: Perspectives from public health, faith communities, education and medicine. Presented at 2016 Advocate Symposium, “Becoming a Trauma-Informed Children’s Hospital and Community: Building Foundations of Care, Collaboration and Practice.” Oaklawn, IL: Advocate Children’s Hospital; November 16, 2016.
31. Diderich HM, Fekkes M, Verkerk PH, et al. A new protocol for screening adults presenting with their own medical problems at the Emergency Department to identify children at high risk for maltreatment. Child Abuse Negl. 2013;37:1122-1131.
32. Fact Sheet: Key ingredients for trauma-informed care. Center for Health Care Strategies, Inc. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 22, 2018.
33. Adversity. In: Merriam-Webster Online Dictionary. Springfield, MA: Merriam-Webster, Inc. www.merriam-webster.com/dictionary/adversity. Accessed September 21, 2018.
34. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:12. Child Trauma Academy (http://childtrauma.org/).
35. Perry BD. Understanding traumatized and maltreated children: the core concepts. Child Trauma Academy Video Training Series, Video 4;2004:19. Child Trauma Academy (http://childtrauma.org/).
36. EMDRIA’s definition of EMDR (eye movement desensitization and reprocessing). Austin, TX: EMDRIA: EMDR International Association. http://c.ymcdn.com/sites/www.emdria.org/resource/resmgr/imported/EMDRIA%20Definition%20of%20EMDR.pdf. Revised February 25 2012. Accessed September 21, 2018.
37. Types of trauma and violence: Historical trauma. Washington, DC: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/trauma-violence/types. Accessed September 21, 2018.
38. Hammond DC. What is neurofeedback? An update. J Neurotherapy. 2011;15:305-336.
39. International Neuromodulation Society. Neuromodulation, or neuromodulatory effect. www.neuromodulation.com/neuromodulation-defined. November 9, 2017. Accessed September 21, 2018.
40. World Health Organization. Social determinants of health. www.who.int/social_determinants/sdh_definition/en/. Accessed September 21, 2018.
41. Davis R, Pinderhughes H, Williams M. Adverse community experiences and resilience: a framework for addressing and preventing community trauma. Oakland, CA: Prevention Institute; 2015:4-5. www.preventioninstitute.org/publications/adverse-community-experiences-and-resilience-framework-addressing-and-preventing. Accessed September 30, 2018.
42. SAMHSA-HRSA Center for Integrated Health Solutions. Trauma. Rockville, MD: Substance Abuse and Mental Health Services Administration and Health Resources and Services Administration, US Department of Health and Human Services. www.integration.samhsa.gov/clinical-practice/trauma. Accessed September 21, 2018.
43. Cohen JA, Mandarino AP. Trauma-focused cognitive behavioural therapy for children and parents. Child Adolesc Ment Health. 2008;13:158-162.
44. Trauma-informed approach and trauma-specific interventions: Trauma-informed approach. Washington, DC: National Center for Trauma Informed Care and Alternatives to Seclusion and Restraints; Substance Abuse and Mental Health Services Administration. www.samhsa.gov/nctic/trauma-interventions. Accessed September 21, 2018.
45. Perry BD. How the brain develops: the importance of early childhood. Child Trauma Academy Video Training Series, Video 1;2004:21. Child Trauma Academy (http://childtrauma.org/).
46. Huang LN, Sharp CS, Gunther T. It’s just good medicine: trauma-informed primary care. (SAMHSA-HRSA Center for Integrated Health Solutions webinar); August 6, 2013. www.integration.samhsa.gov/about-us/CIHS_TIC_Webinar_PDF.pdf. Accessed September 20, 2018.
47. CHCS: Center for Health Care Strategies, Inc. Fact sheet: Key ingredients for trauma-informed care. August 2017. www.chcs.org/media/ATC-Key-Ingredients-Fact-Sheet_081417.pdf. Accessed September 20, 2018.
48. National Council for Behavioral Health. Trauma-informed primary care: fostering resilience and recovery. www.thenationalcouncil.org/consulting-areas-of-expertise/trauma-informed-primary-care/. Accessed September 20, 2018.
49. Child Trauma Academy. The Neurosequential Model of Therapeutics as evidence-based practice. https://childtrauma.org/wp-content/uploads/2015/05/NMT_EvidenceBasedPract_5_2_15.pdf. Accessed September 30, 2018.
50. Bisson JI, Ehlers A, Matthews R, et al. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry. 2007;190:97-104.
51. Metcalf O, Varker T, Forbes D, et al. Efficacy of fifteen emerging interventions for the treatment of posttraumatic stress disorder: a systematic review. J Trauma Stress. 2016;29:88-92.
52. van der Kolk BA, Hodgdon H, Gapen M, et al. A randomized controlled study of neurofeedback for chronic PTSD. 2016; PLoS One. 2016;11:e0166752.
53. Bryan M. A hospital offers frequent ER patients an out—free housing. “All Things Considered.” National Public Radio. June 29, 2016. www.npr.org/sections/health-shots/2016/06/29/482994000/a-hospital-offers-frequent-er-patients-an-out-free-housing. Acces-sed September 20, 2018.
PRACTICE RECOMMENDATIONS
› Refer eligible patients to an evidence-based perinatal home-visiting program and all parents to an evidence-based parenting program to prevent childhood adversity. A
› Consider screening adult patients and parents for their own history (and their children’s history) of childhood adversity. B
› Recommend trauma-focused cognitive behavioral therapy and eye-movement desensitization and reprocessing as first-line treatments for adversity and trauma. A
› Consider prescribing yoga, neurofeedback, and other neuromodulatory modalities to treat the consequences of childhood adversity and trauma. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series