User login
Non-alcoholic fatty liver disease: What’s in our arsenal?
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; [email protected].
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; [email protected].
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; [email protected].
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
Taking an integrative approach to migraine headaches
› Ask all patients with migraines about their use of complementary and alternative medicine and what modalities, if any, they have found helpful. A
› Advise patients that while butterbur has been proven effective at reducing migraine frequency, its use requires caution, as products not processed properly may contain hepatotoxic compounds. A
› Caution women who are pregnant or attempting to conceive to avoid feverfew, which may cause uterine contractions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Americans who suffer from migraine headaches are far more likely than those who don’t to turn to complementary and alternative medicine (CAM). A 2007 National Health Interview Survey and a subsequent analysis of the results found that just under 50% of adults with migraine headaches used alternative therapies; among those without migraine, 34% did.1,2 What’s more, only about half of the migraine patients who reported the use of CAM modalities mentioned it to their health care providers.2
With migraine affecting some 36 million Americans,3 chances are you are caring for many of them. It is likely, too, that you are unaware of which of your headache patients are using alternative treatments, or what modalities they have tried. The only way to find out is to ask.
Women, who are 3 times more likely than men to suffer from migraine headache,4 are also the greatest users of CAM, particularly biologically based therapies and mind-body practices.5 Use is highly individualized and typically does not involve professional supervision.5
A number of alternative modalities look promising for migraine prevention. As a family physician, you are in an ideal position to guide patients in the use of safe and effective CAM therapies. To do so, however, you need to be familiar with the evidence for or against various options—many of which can be used in conjunction with pharmacotherapy.
An integrative approach to the treatment of migraine headaches makes use of the best available evidence for both conventional and alternative therapies and takes into account the whole person, including all aspects of his or her belief system and lifestyle. It also emphasizes a strong physician-patient relationship, which can have a powerful therapeutic effect.
We wrote this evidence-based update with such an approach in mind. In the text and table that follow, we present the latest findings. But first, a brief review of what constitutes migraine headache and an overview of conventional treatment.
A conventional approach to migraine
Migraine headache is a common and disabling neurologic disorder that frequently goes unrecognized and undertreated.6 It is generally characterized as recurrent headaches that are unilateral, pulsating, moderately severe, aggravated by physical activity, and associated with nausea, vomiting, photophobia, phonophobia, and sometimes a preceding aura. Conventional treatment typically includes abortive treatment for acute migraine, with medications such as the triptans and dihydroergotamine. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and the combination of acetaminophen/aspirin/caffeine are also effective. Opiates are efficacious, but not recommended.7
Prophylactic medications are generally offered to patients experiencing more than 4 migraines per month. The American Academy of Neurology cites strong evidence for the use of divalproex, valproate, topiramate, and beta-blockers, including metoprolol, propranolol, and timolol. Frovatriptan has strong evidence for prevention of menstrual-associated migraine. Common adverse effects include weight loss and parasthesias with topiramate and weight gain and somnolence with valproate, divalproex, and beta-blockers. There is also moderate evidence for the use of amitriptyline, venlafaxine, atenolol, and nadolol. Potential adverse effects must be considered to determine the optimal therapy for individual patients, and trial and error are often required.8
Addressing triggers
Conventional treatment also focuses on identifying and avoiding triggers to the extent possible. Physicians typically advise patients to keep a headache diary, recording details about diet and lifestyle, triggers, frequency and intensity of attacks, and possible patterns of headaches due to medication overuse.
Sleep disturbances and stress are common triggers, and instruction in sleep hygiene and stress reduction, as well as screening for anxiety or depression, can be beneficial. Other frequently reported factors believed to trigger or aggravate migraine attacks are skipping meals, particular foods, alcohol, weather changes, and exposure to light, sounds, and odors.
Despite the focus on migraine triggers, however, clinical studies of the role they play have shown conflicting results. A recent study involving 27 patients7 found that when attempting to provoke migraine with aura using participants’ self-reported triggers, only 3 individuals reported that the provocation actually led to a migraine.9 Additional studies suggest that exposure to headache triggers has the same effect as exposure to anxiety, with short-term exposure associated with an increased pain response and prolonged exposure leading to a decreased response.10,11 Thus, it may be beneficial to advise patients to learn to cope with controlled exposure to triggers rather than to aim for trigger avoidance.12
If noise is identified as a trigger, for instance, repeated exposure in a relaxed environment can help desensitize the patient. Triggers such as visual disturbances and odors are also good candidates for desensitization, while others, such as sleep deprivation and skipping meals, are better served by avoidance.12
CAM approaches: A look at the evidence
Acupuncture, butterbur, feverfew, magnesium, riboflavin, and biofeedback look promising for migraine prevention. Many of our patients are already using these and other alternative therapies. Here’s what the latest studies show (TABLE).2,9-11, 13-51
Can acupuncture help?
A 2009 Cochrane review of 22 high-quality studies with a total of 4419 participants supports the use of acupuncture for migraine prophylaxis.13 Acupuncture was found to be superior to no prophylactic treatment and acute treatment alone, and as effective as conventional preventive medications. Interestingly, though, among studies included in the Cochrane review that compared true acupuncture with sham interventions, no significant difference in results was found.
A more recent meta-analysis of 29 studies representing nearly 18,000 patients did show true acupuncture to be statistically superior to sham acupuncture, although the difference was of small clinical significance. Sham acupuncture was also shown to have a larger clinical effect than oral placebos, raising questions about the importance of exact point location.14
In a 2015 study comparing real and sham acupuncture over a 20-week period, however, the differences were more marked. Those who received real acupuncture reported significantly fewer migraine days and less severe headaches, and there were more responders in the treatment group compared with recipients of the sham procedures.15 Overall, the evidence indicates that acupuncture is at least as effective as conventional drug treatment for migraine prophylaxis, but with fewer adverse effects.13-17
Butterbur raises concerns about toxicity
Butterbur (Petasites hybridus) is one of the best-studied natural remedies for migraine. The research has primarily focused on an extract of 15% petasin and isopetasin sold under the trade name Petadolex. A study of patients using this herbal preparation for 16 weeks showed a response rate of 48% for reduction in headache frequency among those taking 75 mg twice daily and a 36% reduction in those taking 50 mg twice a day. The primary adverse effect was burping.18
Proper processing is crucial. The key concern about butterbur is that it naturally contains hepatotoxic compounds called pyrrolizidine alkaloids (PA), which may remain if the product is not properly processed.18-20 The labeling on many butterbur products states that they are “PA-free,” but because the manufacturers, rather than the US Food and Drug Administration (FDA), bear the responsibility for the safety and labeling of supplements, there is little oversight.
In fact, supplement quality is of considerable concern and subject to ongoing research. DNA bar-coding studies have confirmed that many common herbal preparations either contain ingredients not listed on the label or, conversely, fail to contain all the ingredients that are listed.52 Patients should be advised to look for evidence of quality assurance when purchasing herbal supplements, such as that offered by the US Pharmacopeia (USP) on a limited range of products. (We have not found any butterbur supplements with USP verification.)
Feverfew yields mixed results
Studies of feverfew extract for migraine have had conflicting results, probably because different extracts have been tested. A recent Cochrane review, however, cited one clinical trial (N=218) that added positive evidence to previously inconclusive findings.21
The study in question assessed a proprietary extract of feverfew (MIG-99) and found a small decrease in frequency of migraines (0.6 per month) compared with placebo. Adverse effects were gastrointestinal disturbances and mouth ulcers.22
Warn women of childbearing age that feverfew may cause uterine contractions and is contraindicated for those who are pregnant or trying to conceive.23 In addition, patients who are allergic to ragweed, chrysanthemums, or other members of the daisy family may be allergic to feverfew, as well.24
Magnesium is helpful for some
While magnesium is used for both acute relief of migraine and as prophylaxis, evidence of its efficacy is mixed. Studies have been promising in women with low magnesium levels and those who suffer from menstrual migraines, and for use in children with migraine headaches as both acute and preventive treatment.25,26
One RCT involving 160 children ages 5 to 16 years found magnesium to have a synergistic effect with acetaminophen or ibuprofen, leading to greater acute pain relief and reducing migraine frequency.27 A recent meta-analysis, however, concluded that intravenous magnesium is not likely to be effective for acute treatment.53 The main adverse effects seen with magnesium are diarrhea and soft stools.
Patients with renal disease should avoid magnesium supplementation. Food sources of magnesium include whole grains, spinach, nuts, legumes, and white potatoes.54
Riboflavin shows promise
Riboflavin (B2) plays an important role in cellular energy production and is an important antioxidant in mitochondria. Several small studies have shown promising results with high-dose (400 mg) riboflavin in migraine prevention, with evidence suggesting that it may be as effective as beta-blockers such as bisoprolol and metoprolol.28-30 Discoloration of urine, which turns bright yellow, is the primary adverse effect.
CoQ10 helps those with low levels
Like riboflavin, coenzyme Q10 (CoQ10) is involved in mitochondrial transport and plays an important role in cellular energy metabolism. Several small studies have shown efficacy in migraine prevention in doses of 150 to 300 mg/d, with response rates between 30% and 50%.31,32 Based on data in adults, the American Academy of Neurology guidelines give CoQ10 a Level C rating, indicating that it is possibly effective in preventing migraine.29
An open label study of children with migraine found that close to a third were below the reference range for CoQ10 levels. Their serum levels increased when they began taking CoQ10 supplements, resulting in a significant reduction in headache frequency and an improvement in migraine-related disability.33
Combination supplements have little efficacy
In a study published in 2015,34 a proprietary supplement containing magnesium 600 mg, riboflavin 400 mg, CoQ10 150 mg, and low-dose multivitamins, taken daily, did not show statistically significant efficacy in the reduction of migraine days. After 3 months of supplementation, however, the severity of migraine pain improved. Adverse effects included abdominal discomfort and diarrhea.
Another study compared a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg with low-dose riboflavin (25 mg) as placebo, and found that the combination did not reduce the frequency or severity of migraine any more than the placebo.35
Botox may relieve chronic migraine
Onabotulinumtoxin A (Botox) has FDA approval for the prevention of chronic migraines—ie, migraines that occur >15 days per month and at least 4 hours or more per day.55 Botox is administered by injection every 12 weeks, across 31 sites on the head and neck. The recommended dose is 155 units, with 5 units delivered into each injection site.
This protocol has been found to reduce the number of headache days by 50% in half of those being treated after one cycle, and in more than 70% of patients after 3 cycles.36 Potential adverse effects include blepharoptosis, neck muscle weakness, and the risk of botulism at sites distant from the injections.37-39
Mind-body therapies are most widely used
Of all the CAM therapies used by patients with migraine headaches, mind-body modalities are the most prevalent. Overall, 30% of headache patients use them, compared with 17% of the general population.2
Many of these modalities have been found to be effective and safe to use with the conventional migraine treatments with which patients commonly combine them.
Meditation. Both spiritual and secular forms of meditation have been studied for acute and preventive treatment of migraine and found to be effective. A recent small study suggests that spiritual meditation may be more effective,40 but secular mindfulness-based stress reduction training has also shown promise in migraine treatment.
One positive effect is that those who meditate typically use less migraine medication,41 decreasing the burden of disease. Meditation is increasingly available via a range of options, including both in-person groups and online sessions, and can easily augment conventional medical treatments.
Yoga, which typically combines physical postures, breathing techniques, and mental concentration/meditation, is increasingly widespread. While there is compelling evidence of its effect in treating chronic pain and stress-related conditions,42 studies specific to migraine are lacking. Several small studies comparing yoga to NSAIDs, educational handouts, and conventional care for headache suggest that yoga has efficacy for the treatment of migraine, but the findings are limited by methodology and sample size.42,43
Relaxation training. Various types of relaxation are described in the literature, often combining progressive muscle relaxation, diaphragmatic breathing, and relaxation-inducing imagery. Although the consensus is that these techniques are effective, differences in standards, frequency, and duration of training make it hard to draw conclusions.44
Biofeedback is similar to relaxation training, with the key difference being that it uses monitoring to train patients to alter their physiological state, thereby leading to desired changes—eg, fewer headaches and lower intensity of pain. Monitors evaluate skin temperature, electromyography, heart rate variability (blood-volume-pulse), and skin conductance, among other measures.
A robust collection of studies has shown the efficacy of skin temperature feedback, blood-volume-pulse feedback, and electromyography feedback as treatment for migraine.45 Blood-volume-pulse feedback in combination with additional home training is perhaps more effective than other modalities. Despite convincing evidence of its efficacy for migraine headaches, however, only about 1% of patients with migraine use biofeedback. That’s likely due to a lack of availability outside of urban medical centers, limited insurance coverage, and time constraints.2,45
Behavioral therapy can be of help
Cognitive behavioral therapy (CBT) focuses on adjusting maladaptive thoughts and behaviors. For migraine patients, this may include identifying and changing the patient’s response to migraine triggers such as stress, sleep deprivation, and fear of headache pain. Relaxation techniques may be incorporated into the therapy.
The effect size of CBT for prevention is comparable to prophylactic medication use, with 34% to 40% of patients achieving a clinically significant decrease in the number of attacks. Additive effects are especially promising, with more than two-thirds of patients achieving decreased frequency when CBT is combined with preventative medications.2,44,46
Acceptance and commitment therapy (ACT) is a newer variant of CBT that has recently been studied.44 Unlike CBT, in which patients are taught to control and revise their maladaptive thoughts and feelings, ACT focuses on noticing and accepting such unwanted thoughts and feelings and changing the way individuals respond to them rather than changing the thoughts themselves. Further study is needed to determine whether ACT is an effective treatment for migraine.
FDA-approved devices take aim at migraine
A transcranial magnetic stimulator (TMS) (Cerena, eNeura Inc, Sunnyvale, Calif) received FDA approval in 2013.56 The single-pulse TMS is the first device authorized for the treatment of migraine headache pain. It is geared specifically to patients suffering from migraine with aura and requires a prescription.
In a study of 201 patients, the group using the TMS device at the onset of aura had a 38% response rate, compared with a 17% response among those in the sham control group. Dizziness was reported as an adverse effect. Caution patients who express an interest in it that the device should not be used by those who are at risk for seizures or have an implanted device, such as a pacemaker or deep brain stimulator.47
Transcutaneous electrical nerve stimulation (TENS) has long been used for chronic pain, but in 2014 the FDA approved the first TENS device aimed at the prevention of migraine headaches in patients age 18 and older.57 It is also the first such device approved for use prior to the onset of pain.
The Cefaly (Cefaly US, Inc., Wilton, Conn), which requires a prescription, is worn like a headband. It is positioned on the forehead just above the eyes, using an adhesive electrode, and is worn once a day for 20 minutes. The device applies an electrical current to the skin and underlying tissues to stimulate branches of the trigeminal nerve, which can cause a tingling or massaging sensation. Several small studies have shown a decrease in migraine frequency comparable with other preventive treatments. The main adverse effect reported was sedation, but more than half of those who used it were satisfied and willing to purchase the device.48,49
Regular exercise has little downside
While physical activity can be a trigger for acute migraine, regular exercise has been shown to decrease the frequency of migraine attacks. And, although aerobic exercise is no more effective as migraine prophylaxis than conventional drug treatments, it has few adverse effects. For patients who want to stay fit and avoid taking preventive medications, exercise is a valuable adjunct to conventional treatments.50,51
CORRESPONDENCE
Laura Armstrong, MD, Memorial Hermann Family Medicine Residency Program, 14023 Southwest Freeway, Sugar Land, TX 77478; [email protected].
1. National Center for Complementary and Integrative Health. 2007 Statistics on CAM Use in the United States. National Center for Complementary and Integrative Health Web site. Available at: https://nccih.nih.gov/news/camstats/2007. Accessed January 6, 2016.
2. Wells RE, Bertisch SM, Buettner C, et al. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51:1087-1097.
3. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:S103-S122.
4. Migraine Research Foundation. Migraine in women. Migraine Research Foundation Web site. Available at: http://www.migraineresearchfoundation.org/Migraine%20in%20Women.html. Accessed January 7, 2016.
5. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015:1-16.
6. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
8. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
9. Hougaard A, Amin FM, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428-431.
10. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
11. Martin PR. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30:634-637.
12. Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483-495.
13. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218.
14. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
15. Wang Y, Xue CC, Helme R, et al. Acupuncture for frequent migraine: A randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid Based Complement Alternat Med. 2015;2015:920353.
16. Da Silva AN. Acupuncture for migraine prevention. Headache. 2015;55:470-473.
17. Meissner K, Fassler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941-1951.
18. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
19. Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6:303-310.
20. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
21. Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4:CD002286.
22. Pfaffenrath V, Diener HC, Fischer M, et al; Investigators. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523-532.
23. Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutr. 2000;3:509-514.
24. Natural Medicines. Feverfew Professional Monograph, 2016. Natural Medicines Web site. Available at: https://naturalmedicines.therapeuticresearch.com/. Accessed January 1, 2016.
25. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601-610.
26. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
27. Gallelli L, Avenoso T, Falcone D, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313-324.
28. Sándor PS, Afra J, Ambrosini A, et al. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40:30-35.
29. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
30. Boehnke C, Reuter U, Flach U, et al. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11:475-477.
31. Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64:713-715.
32. Rozen TD, Oshinsky ML, Gebeline CA, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137-141.
33. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73-80.
34. Gaul C, Diener HC, Danesch U; Migravent Study Group. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:516.
35. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.
36. Silberstein SD, Dodick DW, Aurora SK, et al. Percent of patients with chronic migraine who responded per onabotulinumtoxin A treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996-1001.
37. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
38. Aurora SK, Winner P, Freeman MC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358-1373.
39. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814.
40. Wachholtz AB, Malone CD, Pargament KI. Effect of different meditation types on migraine headache medication use. Behav Med. 2015:1-8.
41. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:13.
42. Kisan R, Sujan M, Adoor M, et al. Effect of yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126-132.
43. Büssing A, Ostermann T, Lüdtke R, et al. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13:1-9.
44. Penzien DB, Irby MB, Smitherman TA, et al. Well-established and empirically supported behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:34.
45. Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111-127.
46. Fritsche G, Kröner-Herwig B, Kropp P, et al. Psychological therapy of migraine: systematic review. Schmerz. 2013;27:263-274.
47. Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373-380.
48. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697-704.
49. Piquet M, Balestra C, Sava SL, et al. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
50. Gil-Martínez A, Kindelan-Calvo P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials. Rev Neurol. 2013;57:433-443.
51. Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
52. Newmaster SG, Grguric M, Shanmughanandhan D, et al. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
53. Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials. Eur J Emerg Med. 2014;21:2-9.
54. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4:S378-S383.
55. US Food and Drug Administration. FDA approves Botox to treat chronic migraine. US Food and Drug Administration Web site. October 15, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229782.htm. Accessed January 7, 2016.
56. US Food and Drug Administration. FDA allows marketing of first device to relieve migraine headache pain. US Food and Drug Administration Web site. December 13, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm378608.htm. Accessed January 7, 2016.
57. US Food and Drug Administration. FDA allows marketing of first medical device to prevent migraine headache. US Food and Drug Administration Web site. March 11, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm. Accessed January 7, 2016.
› Ask all patients with migraines about their use of complementary and alternative medicine and what modalities, if any, they have found helpful. A
› Advise patients that while butterbur has been proven effective at reducing migraine frequency, its use requires caution, as products not processed properly may contain hepatotoxic compounds. A
› Caution women who are pregnant or attempting to conceive to avoid feverfew, which may cause uterine contractions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Americans who suffer from migraine headaches are far more likely than those who don’t to turn to complementary and alternative medicine (CAM). A 2007 National Health Interview Survey and a subsequent analysis of the results found that just under 50% of adults with migraine headaches used alternative therapies; among those without migraine, 34% did.1,2 What’s more, only about half of the migraine patients who reported the use of CAM modalities mentioned it to their health care providers.2
With migraine affecting some 36 million Americans,3 chances are you are caring for many of them. It is likely, too, that you are unaware of which of your headache patients are using alternative treatments, or what modalities they have tried. The only way to find out is to ask.
Women, who are 3 times more likely than men to suffer from migraine headache,4 are also the greatest users of CAM, particularly biologically based therapies and mind-body practices.5 Use is highly individualized and typically does not involve professional supervision.5
A number of alternative modalities look promising for migraine prevention. As a family physician, you are in an ideal position to guide patients in the use of safe and effective CAM therapies. To do so, however, you need to be familiar with the evidence for or against various options—many of which can be used in conjunction with pharmacotherapy.
An integrative approach to the treatment of migraine headaches makes use of the best available evidence for both conventional and alternative therapies and takes into account the whole person, including all aspects of his or her belief system and lifestyle. It also emphasizes a strong physician-patient relationship, which can have a powerful therapeutic effect.
We wrote this evidence-based update with such an approach in mind. In the text and table that follow, we present the latest findings. But first, a brief review of what constitutes migraine headache and an overview of conventional treatment.
A conventional approach to migraine
Migraine headache is a common and disabling neurologic disorder that frequently goes unrecognized and undertreated.6 It is generally characterized as recurrent headaches that are unilateral, pulsating, moderately severe, aggravated by physical activity, and associated with nausea, vomiting, photophobia, phonophobia, and sometimes a preceding aura. Conventional treatment typically includes abortive treatment for acute migraine, with medications such as the triptans and dihydroergotamine. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and the combination of acetaminophen/aspirin/caffeine are also effective. Opiates are efficacious, but not recommended.7
Prophylactic medications are generally offered to patients experiencing more than 4 migraines per month. The American Academy of Neurology cites strong evidence for the use of divalproex, valproate, topiramate, and beta-blockers, including metoprolol, propranolol, and timolol. Frovatriptan has strong evidence for prevention of menstrual-associated migraine. Common adverse effects include weight loss and parasthesias with topiramate and weight gain and somnolence with valproate, divalproex, and beta-blockers. There is also moderate evidence for the use of amitriptyline, venlafaxine, atenolol, and nadolol. Potential adverse effects must be considered to determine the optimal therapy for individual patients, and trial and error are often required.8
Addressing triggers
Conventional treatment also focuses on identifying and avoiding triggers to the extent possible. Physicians typically advise patients to keep a headache diary, recording details about diet and lifestyle, triggers, frequency and intensity of attacks, and possible patterns of headaches due to medication overuse.
Sleep disturbances and stress are common triggers, and instruction in sleep hygiene and stress reduction, as well as screening for anxiety or depression, can be beneficial. Other frequently reported factors believed to trigger or aggravate migraine attacks are skipping meals, particular foods, alcohol, weather changes, and exposure to light, sounds, and odors.
Despite the focus on migraine triggers, however, clinical studies of the role they play have shown conflicting results. A recent study involving 27 patients7 found that when attempting to provoke migraine with aura using participants’ self-reported triggers, only 3 individuals reported that the provocation actually led to a migraine.9 Additional studies suggest that exposure to headache triggers has the same effect as exposure to anxiety, with short-term exposure associated with an increased pain response and prolonged exposure leading to a decreased response.10,11 Thus, it may be beneficial to advise patients to learn to cope with controlled exposure to triggers rather than to aim for trigger avoidance.12
If noise is identified as a trigger, for instance, repeated exposure in a relaxed environment can help desensitize the patient. Triggers such as visual disturbances and odors are also good candidates for desensitization, while others, such as sleep deprivation and skipping meals, are better served by avoidance.12
CAM approaches: A look at the evidence
Acupuncture, butterbur, feverfew, magnesium, riboflavin, and biofeedback look promising for migraine prevention. Many of our patients are already using these and other alternative therapies. Here’s what the latest studies show (TABLE).2,9-11, 13-51
Can acupuncture help?
A 2009 Cochrane review of 22 high-quality studies with a total of 4419 participants supports the use of acupuncture for migraine prophylaxis.13 Acupuncture was found to be superior to no prophylactic treatment and acute treatment alone, and as effective as conventional preventive medications. Interestingly, though, among studies included in the Cochrane review that compared true acupuncture with sham interventions, no significant difference in results was found.
A more recent meta-analysis of 29 studies representing nearly 18,000 patients did show true acupuncture to be statistically superior to sham acupuncture, although the difference was of small clinical significance. Sham acupuncture was also shown to have a larger clinical effect than oral placebos, raising questions about the importance of exact point location.14
In a 2015 study comparing real and sham acupuncture over a 20-week period, however, the differences were more marked. Those who received real acupuncture reported significantly fewer migraine days and less severe headaches, and there were more responders in the treatment group compared with recipients of the sham procedures.15 Overall, the evidence indicates that acupuncture is at least as effective as conventional drug treatment for migraine prophylaxis, but with fewer adverse effects.13-17
Butterbur raises concerns about toxicity
Butterbur (Petasites hybridus) is one of the best-studied natural remedies for migraine. The research has primarily focused on an extract of 15% petasin and isopetasin sold under the trade name Petadolex. A study of patients using this herbal preparation for 16 weeks showed a response rate of 48% for reduction in headache frequency among those taking 75 mg twice daily and a 36% reduction in those taking 50 mg twice a day. The primary adverse effect was burping.18
Proper processing is crucial. The key concern about butterbur is that it naturally contains hepatotoxic compounds called pyrrolizidine alkaloids (PA), which may remain if the product is not properly processed.18-20 The labeling on many butterbur products states that they are “PA-free,” but because the manufacturers, rather than the US Food and Drug Administration (FDA), bear the responsibility for the safety and labeling of supplements, there is little oversight.
In fact, supplement quality is of considerable concern and subject to ongoing research. DNA bar-coding studies have confirmed that many common herbal preparations either contain ingredients not listed on the label or, conversely, fail to contain all the ingredients that are listed.52 Patients should be advised to look for evidence of quality assurance when purchasing herbal supplements, such as that offered by the US Pharmacopeia (USP) on a limited range of products. (We have not found any butterbur supplements with USP verification.)
Feverfew yields mixed results
Studies of feverfew extract for migraine have had conflicting results, probably because different extracts have been tested. A recent Cochrane review, however, cited one clinical trial (N=218) that added positive evidence to previously inconclusive findings.21
The study in question assessed a proprietary extract of feverfew (MIG-99) and found a small decrease in frequency of migraines (0.6 per month) compared with placebo. Adverse effects were gastrointestinal disturbances and mouth ulcers.22
Warn women of childbearing age that feverfew may cause uterine contractions and is contraindicated for those who are pregnant or trying to conceive.23 In addition, patients who are allergic to ragweed, chrysanthemums, or other members of the daisy family may be allergic to feverfew, as well.24
Magnesium is helpful for some
While magnesium is used for both acute relief of migraine and as prophylaxis, evidence of its efficacy is mixed. Studies have been promising in women with low magnesium levels and those who suffer from menstrual migraines, and for use in children with migraine headaches as both acute and preventive treatment.25,26
One RCT involving 160 children ages 5 to 16 years found magnesium to have a synergistic effect with acetaminophen or ibuprofen, leading to greater acute pain relief and reducing migraine frequency.27 A recent meta-analysis, however, concluded that intravenous magnesium is not likely to be effective for acute treatment.53 The main adverse effects seen with magnesium are diarrhea and soft stools.
Patients with renal disease should avoid magnesium supplementation. Food sources of magnesium include whole grains, spinach, nuts, legumes, and white potatoes.54
Riboflavin shows promise
Riboflavin (B2) plays an important role in cellular energy production and is an important antioxidant in mitochondria. Several small studies have shown promising results with high-dose (400 mg) riboflavin in migraine prevention, with evidence suggesting that it may be as effective as beta-blockers such as bisoprolol and metoprolol.28-30 Discoloration of urine, which turns bright yellow, is the primary adverse effect.
CoQ10 helps those with low levels
Like riboflavin, coenzyme Q10 (CoQ10) is involved in mitochondrial transport and plays an important role in cellular energy metabolism. Several small studies have shown efficacy in migraine prevention in doses of 150 to 300 mg/d, with response rates between 30% and 50%.31,32 Based on data in adults, the American Academy of Neurology guidelines give CoQ10 a Level C rating, indicating that it is possibly effective in preventing migraine.29
An open label study of children with migraine found that close to a third were below the reference range for CoQ10 levels. Their serum levels increased when they began taking CoQ10 supplements, resulting in a significant reduction in headache frequency and an improvement in migraine-related disability.33
Combination supplements have little efficacy
In a study published in 2015,34 a proprietary supplement containing magnesium 600 mg, riboflavin 400 mg, CoQ10 150 mg, and low-dose multivitamins, taken daily, did not show statistically significant efficacy in the reduction of migraine days. After 3 months of supplementation, however, the severity of migraine pain improved. Adverse effects included abdominal discomfort and diarrhea.
Another study compared a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg with low-dose riboflavin (25 mg) as placebo, and found that the combination did not reduce the frequency or severity of migraine any more than the placebo.35
Botox may relieve chronic migraine
Onabotulinumtoxin A (Botox) has FDA approval for the prevention of chronic migraines—ie, migraines that occur >15 days per month and at least 4 hours or more per day.55 Botox is administered by injection every 12 weeks, across 31 sites on the head and neck. The recommended dose is 155 units, with 5 units delivered into each injection site.
This protocol has been found to reduce the number of headache days by 50% in half of those being treated after one cycle, and in more than 70% of patients after 3 cycles.36 Potential adverse effects include blepharoptosis, neck muscle weakness, and the risk of botulism at sites distant from the injections.37-39
Mind-body therapies are most widely used
Of all the CAM therapies used by patients with migraine headaches, mind-body modalities are the most prevalent. Overall, 30% of headache patients use them, compared with 17% of the general population.2
Many of these modalities have been found to be effective and safe to use with the conventional migraine treatments with which patients commonly combine them.
Meditation. Both spiritual and secular forms of meditation have been studied for acute and preventive treatment of migraine and found to be effective. A recent small study suggests that spiritual meditation may be more effective,40 but secular mindfulness-based stress reduction training has also shown promise in migraine treatment.
One positive effect is that those who meditate typically use less migraine medication,41 decreasing the burden of disease. Meditation is increasingly available via a range of options, including both in-person groups and online sessions, and can easily augment conventional medical treatments.
Yoga, which typically combines physical postures, breathing techniques, and mental concentration/meditation, is increasingly widespread. While there is compelling evidence of its effect in treating chronic pain and stress-related conditions,42 studies specific to migraine are lacking. Several small studies comparing yoga to NSAIDs, educational handouts, and conventional care for headache suggest that yoga has efficacy for the treatment of migraine, but the findings are limited by methodology and sample size.42,43
Relaxation training. Various types of relaxation are described in the literature, often combining progressive muscle relaxation, diaphragmatic breathing, and relaxation-inducing imagery. Although the consensus is that these techniques are effective, differences in standards, frequency, and duration of training make it hard to draw conclusions.44
Biofeedback is similar to relaxation training, with the key difference being that it uses monitoring to train patients to alter their physiological state, thereby leading to desired changes—eg, fewer headaches and lower intensity of pain. Monitors evaluate skin temperature, electromyography, heart rate variability (blood-volume-pulse), and skin conductance, among other measures.
A robust collection of studies has shown the efficacy of skin temperature feedback, blood-volume-pulse feedback, and electromyography feedback as treatment for migraine.45 Blood-volume-pulse feedback in combination with additional home training is perhaps more effective than other modalities. Despite convincing evidence of its efficacy for migraine headaches, however, only about 1% of patients with migraine use biofeedback. That’s likely due to a lack of availability outside of urban medical centers, limited insurance coverage, and time constraints.2,45
Behavioral therapy can be of help
Cognitive behavioral therapy (CBT) focuses on adjusting maladaptive thoughts and behaviors. For migraine patients, this may include identifying and changing the patient’s response to migraine triggers such as stress, sleep deprivation, and fear of headache pain. Relaxation techniques may be incorporated into the therapy.
The effect size of CBT for prevention is comparable to prophylactic medication use, with 34% to 40% of patients achieving a clinically significant decrease in the number of attacks. Additive effects are especially promising, with more than two-thirds of patients achieving decreased frequency when CBT is combined with preventative medications.2,44,46
Acceptance and commitment therapy (ACT) is a newer variant of CBT that has recently been studied.44 Unlike CBT, in which patients are taught to control and revise their maladaptive thoughts and feelings, ACT focuses on noticing and accepting such unwanted thoughts and feelings and changing the way individuals respond to them rather than changing the thoughts themselves. Further study is needed to determine whether ACT is an effective treatment for migraine.
FDA-approved devices take aim at migraine
A transcranial magnetic stimulator (TMS) (Cerena, eNeura Inc, Sunnyvale, Calif) received FDA approval in 2013.56 The single-pulse TMS is the first device authorized for the treatment of migraine headache pain. It is geared specifically to patients suffering from migraine with aura and requires a prescription.
In a study of 201 patients, the group using the TMS device at the onset of aura had a 38% response rate, compared with a 17% response among those in the sham control group. Dizziness was reported as an adverse effect. Caution patients who express an interest in it that the device should not be used by those who are at risk for seizures or have an implanted device, such as a pacemaker or deep brain stimulator.47
Transcutaneous electrical nerve stimulation (TENS) has long been used for chronic pain, but in 2014 the FDA approved the first TENS device aimed at the prevention of migraine headaches in patients age 18 and older.57 It is also the first such device approved for use prior to the onset of pain.
The Cefaly (Cefaly US, Inc., Wilton, Conn), which requires a prescription, is worn like a headband. It is positioned on the forehead just above the eyes, using an adhesive electrode, and is worn once a day for 20 minutes. The device applies an electrical current to the skin and underlying tissues to stimulate branches of the trigeminal nerve, which can cause a tingling or massaging sensation. Several small studies have shown a decrease in migraine frequency comparable with other preventive treatments. The main adverse effect reported was sedation, but more than half of those who used it were satisfied and willing to purchase the device.48,49
Regular exercise has little downside
While physical activity can be a trigger for acute migraine, regular exercise has been shown to decrease the frequency of migraine attacks. And, although aerobic exercise is no more effective as migraine prophylaxis than conventional drug treatments, it has few adverse effects. For patients who want to stay fit and avoid taking preventive medications, exercise is a valuable adjunct to conventional treatments.50,51
CORRESPONDENCE
Laura Armstrong, MD, Memorial Hermann Family Medicine Residency Program, 14023 Southwest Freeway, Sugar Land, TX 77478; [email protected].
› Ask all patients with migraines about their use of complementary and alternative medicine and what modalities, if any, they have found helpful. A
› Advise patients that while butterbur has been proven effective at reducing migraine frequency, its use requires caution, as products not processed properly may contain hepatotoxic compounds. A
› Caution women who are pregnant or attempting to conceive to avoid feverfew, which may cause uterine contractions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Americans who suffer from migraine headaches are far more likely than those who don’t to turn to complementary and alternative medicine (CAM). A 2007 National Health Interview Survey and a subsequent analysis of the results found that just under 50% of adults with migraine headaches used alternative therapies; among those without migraine, 34% did.1,2 What’s more, only about half of the migraine patients who reported the use of CAM modalities mentioned it to their health care providers.2
With migraine affecting some 36 million Americans,3 chances are you are caring for many of them. It is likely, too, that you are unaware of which of your headache patients are using alternative treatments, or what modalities they have tried. The only way to find out is to ask.
Women, who are 3 times more likely than men to suffer from migraine headache,4 are also the greatest users of CAM, particularly biologically based therapies and mind-body practices.5 Use is highly individualized and typically does not involve professional supervision.5
A number of alternative modalities look promising for migraine prevention. As a family physician, you are in an ideal position to guide patients in the use of safe and effective CAM therapies. To do so, however, you need to be familiar with the evidence for or against various options—many of which can be used in conjunction with pharmacotherapy.
An integrative approach to the treatment of migraine headaches makes use of the best available evidence for both conventional and alternative therapies and takes into account the whole person, including all aspects of his or her belief system and lifestyle. It also emphasizes a strong physician-patient relationship, which can have a powerful therapeutic effect.
We wrote this evidence-based update with such an approach in mind. In the text and table that follow, we present the latest findings. But first, a brief review of what constitutes migraine headache and an overview of conventional treatment.
A conventional approach to migraine
Migraine headache is a common and disabling neurologic disorder that frequently goes unrecognized and undertreated.6 It is generally characterized as recurrent headaches that are unilateral, pulsating, moderately severe, aggravated by physical activity, and associated with nausea, vomiting, photophobia, phonophobia, and sometimes a preceding aura. Conventional treatment typically includes abortive treatment for acute migraine, with medications such as the triptans and dihydroergotamine. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and the combination of acetaminophen/aspirin/caffeine are also effective. Opiates are efficacious, but not recommended.7
Prophylactic medications are generally offered to patients experiencing more than 4 migraines per month. The American Academy of Neurology cites strong evidence for the use of divalproex, valproate, topiramate, and beta-blockers, including metoprolol, propranolol, and timolol. Frovatriptan has strong evidence for prevention of menstrual-associated migraine. Common adverse effects include weight loss and parasthesias with topiramate and weight gain and somnolence with valproate, divalproex, and beta-blockers. There is also moderate evidence for the use of amitriptyline, venlafaxine, atenolol, and nadolol. Potential adverse effects must be considered to determine the optimal therapy for individual patients, and trial and error are often required.8
Addressing triggers
Conventional treatment also focuses on identifying and avoiding triggers to the extent possible. Physicians typically advise patients to keep a headache diary, recording details about diet and lifestyle, triggers, frequency and intensity of attacks, and possible patterns of headaches due to medication overuse.
Sleep disturbances and stress are common triggers, and instruction in sleep hygiene and stress reduction, as well as screening for anxiety or depression, can be beneficial. Other frequently reported factors believed to trigger or aggravate migraine attacks are skipping meals, particular foods, alcohol, weather changes, and exposure to light, sounds, and odors.
Despite the focus on migraine triggers, however, clinical studies of the role they play have shown conflicting results. A recent study involving 27 patients7 found that when attempting to provoke migraine with aura using participants’ self-reported triggers, only 3 individuals reported that the provocation actually led to a migraine.9 Additional studies suggest that exposure to headache triggers has the same effect as exposure to anxiety, with short-term exposure associated with an increased pain response and prolonged exposure leading to a decreased response.10,11 Thus, it may be beneficial to advise patients to learn to cope with controlled exposure to triggers rather than to aim for trigger avoidance.12
If noise is identified as a trigger, for instance, repeated exposure in a relaxed environment can help desensitize the patient. Triggers such as visual disturbances and odors are also good candidates for desensitization, while others, such as sleep deprivation and skipping meals, are better served by avoidance.12
CAM approaches: A look at the evidence
Acupuncture, butterbur, feverfew, magnesium, riboflavin, and biofeedback look promising for migraine prevention. Many of our patients are already using these and other alternative therapies. Here’s what the latest studies show (TABLE).2,9-11, 13-51
Can acupuncture help?
A 2009 Cochrane review of 22 high-quality studies with a total of 4419 participants supports the use of acupuncture for migraine prophylaxis.13 Acupuncture was found to be superior to no prophylactic treatment and acute treatment alone, and as effective as conventional preventive medications. Interestingly, though, among studies included in the Cochrane review that compared true acupuncture with sham interventions, no significant difference in results was found.
A more recent meta-analysis of 29 studies representing nearly 18,000 patients did show true acupuncture to be statistically superior to sham acupuncture, although the difference was of small clinical significance. Sham acupuncture was also shown to have a larger clinical effect than oral placebos, raising questions about the importance of exact point location.14
In a 2015 study comparing real and sham acupuncture over a 20-week period, however, the differences were more marked. Those who received real acupuncture reported significantly fewer migraine days and less severe headaches, and there were more responders in the treatment group compared with recipients of the sham procedures.15 Overall, the evidence indicates that acupuncture is at least as effective as conventional drug treatment for migraine prophylaxis, but with fewer adverse effects.13-17
Butterbur raises concerns about toxicity
Butterbur (Petasites hybridus) is one of the best-studied natural remedies for migraine. The research has primarily focused on an extract of 15% petasin and isopetasin sold under the trade name Petadolex. A study of patients using this herbal preparation for 16 weeks showed a response rate of 48% for reduction in headache frequency among those taking 75 mg twice daily and a 36% reduction in those taking 50 mg twice a day. The primary adverse effect was burping.18
Proper processing is crucial. The key concern about butterbur is that it naturally contains hepatotoxic compounds called pyrrolizidine alkaloids (PA), which may remain if the product is not properly processed.18-20 The labeling on many butterbur products states that they are “PA-free,” but because the manufacturers, rather than the US Food and Drug Administration (FDA), bear the responsibility for the safety and labeling of supplements, there is little oversight.
In fact, supplement quality is of considerable concern and subject to ongoing research. DNA bar-coding studies have confirmed that many common herbal preparations either contain ingredients not listed on the label or, conversely, fail to contain all the ingredients that are listed.52 Patients should be advised to look for evidence of quality assurance when purchasing herbal supplements, such as that offered by the US Pharmacopeia (USP) on a limited range of products. (We have not found any butterbur supplements with USP verification.)
Feverfew yields mixed results
Studies of feverfew extract for migraine have had conflicting results, probably because different extracts have been tested. A recent Cochrane review, however, cited one clinical trial (N=218) that added positive evidence to previously inconclusive findings.21
The study in question assessed a proprietary extract of feverfew (MIG-99) and found a small decrease in frequency of migraines (0.6 per month) compared with placebo. Adverse effects were gastrointestinal disturbances and mouth ulcers.22
Warn women of childbearing age that feverfew may cause uterine contractions and is contraindicated for those who are pregnant or trying to conceive.23 In addition, patients who are allergic to ragweed, chrysanthemums, or other members of the daisy family may be allergic to feverfew, as well.24
Magnesium is helpful for some
While magnesium is used for both acute relief of migraine and as prophylaxis, evidence of its efficacy is mixed. Studies have been promising in women with low magnesium levels and those who suffer from menstrual migraines, and for use in children with migraine headaches as both acute and preventive treatment.25,26
One RCT involving 160 children ages 5 to 16 years found magnesium to have a synergistic effect with acetaminophen or ibuprofen, leading to greater acute pain relief and reducing migraine frequency.27 A recent meta-analysis, however, concluded that intravenous magnesium is not likely to be effective for acute treatment.53 The main adverse effects seen with magnesium are diarrhea and soft stools.
Patients with renal disease should avoid magnesium supplementation. Food sources of magnesium include whole grains, spinach, nuts, legumes, and white potatoes.54
Riboflavin shows promise
Riboflavin (B2) plays an important role in cellular energy production and is an important antioxidant in mitochondria. Several small studies have shown promising results with high-dose (400 mg) riboflavin in migraine prevention, with evidence suggesting that it may be as effective as beta-blockers such as bisoprolol and metoprolol.28-30 Discoloration of urine, which turns bright yellow, is the primary adverse effect.
CoQ10 helps those with low levels
Like riboflavin, coenzyme Q10 (CoQ10) is involved in mitochondrial transport and plays an important role in cellular energy metabolism. Several small studies have shown efficacy in migraine prevention in doses of 150 to 300 mg/d, with response rates between 30% and 50%.31,32 Based on data in adults, the American Academy of Neurology guidelines give CoQ10 a Level C rating, indicating that it is possibly effective in preventing migraine.29
An open label study of children with migraine found that close to a third were below the reference range for CoQ10 levels. Their serum levels increased when they began taking CoQ10 supplements, resulting in a significant reduction in headache frequency and an improvement in migraine-related disability.33
Combination supplements have little efficacy
In a study published in 2015,34 a proprietary supplement containing magnesium 600 mg, riboflavin 400 mg, CoQ10 150 mg, and low-dose multivitamins, taken daily, did not show statistically significant efficacy in the reduction of migraine days. After 3 months of supplementation, however, the severity of migraine pain improved. Adverse effects included abdominal discomfort and diarrhea.
Another study compared a combination of riboflavin 400 mg, magnesium 300 mg, and feverfew 100 mg with low-dose riboflavin (25 mg) as placebo, and found that the combination did not reduce the frequency or severity of migraine any more than the placebo.35
Botox may relieve chronic migraine
Onabotulinumtoxin A (Botox) has FDA approval for the prevention of chronic migraines—ie, migraines that occur >15 days per month and at least 4 hours or more per day.55 Botox is administered by injection every 12 weeks, across 31 sites on the head and neck. The recommended dose is 155 units, with 5 units delivered into each injection site.
This protocol has been found to reduce the number of headache days by 50% in half of those being treated after one cycle, and in more than 70% of patients after 3 cycles.36 Potential adverse effects include blepharoptosis, neck muscle weakness, and the risk of botulism at sites distant from the injections.37-39
Mind-body therapies are most widely used
Of all the CAM therapies used by patients with migraine headaches, mind-body modalities are the most prevalent. Overall, 30% of headache patients use them, compared with 17% of the general population.2
Many of these modalities have been found to be effective and safe to use with the conventional migraine treatments with which patients commonly combine them.
Meditation. Both spiritual and secular forms of meditation have been studied for acute and preventive treatment of migraine and found to be effective. A recent small study suggests that spiritual meditation may be more effective,40 but secular mindfulness-based stress reduction training has also shown promise in migraine treatment.
One positive effect is that those who meditate typically use less migraine medication,41 decreasing the burden of disease. Meditation is increasingly available via a range of options, including both in-person groups and online sessions, and can easily augment conventional medical treatments.
Yoga, which typically combines physical postures, breathing techniques, and mental concentration/meditation, is increasingly widespread. While there is compelling evidence of its effect in treating chronic pain and stress-related conditions,42 studies specific to migraine are lacking. Several small studies comparing yoga to NSAIDs, educational handouts, and conventional care for headache suggest that yoga has efficacy for the treatment of migraine, but the findings are limited by methodology and sample size.42,43
Relaxation training. Various types of relaxation are described in the literature, often combining progressive muscle relaxation, diaphragmatic breathing, and relaxation-inducing imagery. Although the consensus is that these techniques are effective, differences in standards, frequency, and duration of training make it hard to draw conclusions.44
Biofeedback is similar to relaxation training, with the key difference being that it uses monitoring to train patients to alter their physiological state, thereby leading to desired changes—eg, fewer headaches and lower intensity of pain. Monitors evaluate skin temperature, electromyography, heart rate variability (blood-volume-pulse), and skin conductance, among other measures.
A robust collection of studies has shown the efficacy of skin temperature feedback, blood-volume-pulse feedback, and electromyography feedback as treatment for migraine.45 Blood-volume-pulse feedback in combination with additional home training is perhaps more effective than other modalities. Despite convincing evidence of its efficacy for migraine headaches, however, only about 1% of patients with migraine use biofeedback. That’s likely due to a lack of availability outside of urban medical centers, limited insurance coverage, and time constraints.2,45
Behavioral therapy can be of help
Cognitive behavioral therapy (CBT) focuses on adjusting maladaptive thoughts and behaviors. For migraine patients, this may include identifying and changing the patient’s response to migraine triggers such as stress, sleep deprivation, and fear of headache pain. Relaxation techniques may be incorporated into the therapy.
The effect size of CBT for prevention is comparable to prophylactic medication use, with 34% to 40% of patients achieving a clinically significant decrease in the number of attacks. Additive effects are especially promising, with more than two-thirds of patients achieving decreased frequency when CBT is combined with preventative medications.2,44,46
Acceptance and commitment therapy (ACT) is a newer variant of CBT that has recently been studied.44 Unlike CBT, in which patients are taught to control and revise their maladaptive thoughts and feelings, ACT focuses on noticing and accepting such unwanted thoughts and feelings and changing the way individuals respond to them rather than changing the thoughts themselves. Further study is needed to determine whether ACT is an effective treatment for migraine.
FDA-approved devices take aim at migraine
A transcranial magnetic stimulator (TMS) (Cerena, eNeura Inc, Sunnyvale, Calif) received FDA approval in 2013.56 The single-pulse TMS is the first device authorized for the treatment of migraine headache pain. It is geared specifically to patients suffering from migraine with aura and requires a prescription.
In a study of 201 patients, the group using the TMS device at the onset of aura had a 38% response rate, compared with a 17% response among those in the sham control group. Dizziness was reported as an adverse effect. Caution patients who express an interest in it that the device should not be used by those who are at risk for seizures or have an implanted device, such as a pacemaker or deep brain stimulator.47
Transcutaneous electrical nerve stimulation (TENS) has long been used for chronic pain, but in 2014 the FDA approved the first TENS device aimed at the prevention of migraine headaches in patients age 18 and older.57 It is also the first such device approved for use prior to the onset of pain.
The Cefaly (Cefaly US, Inc., Wilton, Conn), which requires a prescription, is worn like a headband. It is positioned on the forehead just above the eyes, using an adhesive electrode, and is worn once a day for 20 minutes. The device applies an electrical current to the skin and underlying tissues to stimulate branches of the trigeminal nerve, which can cause a tingling or massaging sensation. Several small studies have shown a decrease in migraine frequency comparable with other preventive treatments. The main adverse effect reported was sedation, but more than half of those who used it were satisfied and willing to purchase the device.48,49
Regular exercise has little downside
While physical activity can be a trigger for acute migraine, regular exercise has been shown to decrease the frequency of migraine attacks. And, although aerobic exercise is no more effective as migraine prophylaxis than conventional drug treatments, it has few adverse effects. For patients who want to stay fit and avoid taking preventive medications, exercise is a valuable adjunct to conventional treatments.50,51
CORRESPONDENCE
Laura Armstrong, MD, Memorial Hermann Family Medicine Residency Program, 14023 Southwest Freeway, Sugar Land, TX 77478; [email protected].
1. National Center for Complementary and Integrative Health. 2007 Statistics on CAM Use in the United States. National Center for Complementary and Integrative Health Web site. Available at: https://nccih.nih.gov/news/camstats/2007. Accessed January 6, 2016.
2. Wells RE, Bertisch SM, Buettner C, et al. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51:1087-1097.
3. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:S103-S122.
4. Migraine Research Foundation. Migraine in women. Migraine Research Foundation Web site. Available at: http://www.migraineresearchfoundation.org/Migraine%20in%20Women.html. Accessed January 7, 2016.
5. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015:1-16.
6. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
8. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
9. Hougaard A, Amin FM, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428-431.
10. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
11. Martin PR. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30:634-637.
12. Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483-495.
13. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218.
14. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
15. Wang Y, Xue CC, Helme R, et al. Acupuncture for frequent migraine: A randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid Based Complement Alternat Med. 2015;2015:920353.
16. Da Silva AN. Acupuncture for migraine prevention. Headache. 2015;55:470-473.
17. Meissner K, Fassler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941-1951.
18. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
19. Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6:303-310.
20. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
21. Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4:CD002286.
22. Pfaffenrath V, Diener HC, Fischer M, et al; Investigators. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523-532.
23. Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutr. 2000;3:509-514.
24. Natural Medicines. Feverfew Professional Monograph, 2016. Natural Medicines Web site. Available at: https://naturalmedicines.therapeuticresearch.com/. Accessed January 1, 2016.
25. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601-610.
26. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
27. Gallelli L, Avenoso T, Falcone D, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313-324.
28. Sándor PS, Afra J, Ambrosini A, et al. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40:30-35.
29. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
30. Boehnke C, Reuter U, Flach U, et al. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11:475-477.
31. Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64:713-715.
32. Rozen TD, Oshinsky ML, Gebeline CA, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137-141.
33. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73-80.
34. Gaul C, Diener HC, Danesch U; Migravent Study Group. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:516.
35. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.
36. Silberstein SD, Dodick DW, Aurora SK, et al. Percent of patients with chronic migraine who responded per onabotulinumtoxin A treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996-1001.
37. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
38. Aurora SK, Winner P, Freeman MC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358-1373.
39. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814.
40. Wachholtz AB, Malone CD, Pargament KI. Effect of different meditation types on migraine headache medication use. Behav Med. 2015:1-8.
41. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:13.
42. Kisan R, Sujan M, Adoor M, et al. Effect of yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126-132.
43. Büssing A, Ostermann T, Lüdtke R, et al. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13:1-9.
44. Penzien DB, Irby MB, Smitherman TA, et al. Well-established and empirically supported behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:34.
45. Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111-127.
46. Fritsche G, Kröner-Herwig B, Kropp P, et al. Psychological therapy of migraine: systematic review. Schmerz. 2013;27:263-274.
47. Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373-380.
48. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697-704.
49. Piquet M, Balestra C, Sava SL, et al. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
50. Gil-Martínez A, Kindelan-Calvo P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials. Rev Neurol. 2013;57:433-443.
51. Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
52. Newmaster SG, Grguric M, Shanmughanandhan D, et al. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
53. Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials. Eur J Emerg Med. 2014;21:2-9.
54. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4:S378-S383.
55. US Food and Drug Administration. FDA approves Botox to treat chronic migraine. US Food and Drug Administration Web site. October 15, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229782.htm. Accessed January 7, 2016.
56. US Food and Drug Administration. FDA allows marketing of first device to relieve migraine headache pain. US Food and Drug Administration Web site. December 13, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm378608.htm. Accessed January 7, 2016.
57. US Food and Drug Administration. FDA allows marketing of first medical device to prevent migraine headache. US Food and Drug Administration Web site. March 11, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm. Accessed January 7, 2016.
1. National Center for Complementary and Integrative Health. 2007 Statistics on CAM Use in the United States. National Center for Complementary and Integrative Health Web site. Available at: https://nccih.nih.gov/news/camstats/2007. Accessed January 6, 2016.
2. Wells RE, Bertisch SM, Buettner C, et al. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51:1087-1097.
3. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:S103-S122.
4. Migraine Research Foundation. Migraine in women. Migraine Research Foundation Web site. Available at: http://www.migraineresearchfoundation.org/Migraine%20in%20Women.html. Accessed January 7, 2016.
5. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015:1-16.
6. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd ed. Cephalalgia. 2013;33:629-808.
7. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
8. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
9. Hougaard A, Amin FM, Hauge AW, et al. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428-431.
10. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
11. Martin PR. Managing headache triggers: Think ‘coping’ not ‘avoidance’. Cephalalgia. 2010;30:634-637.
12. Martin PR, MacLeod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev. 2009;29:483-495.
13. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009:CD001218.
14. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
15. Wang Y, Xue CC, Helme R, et al. Acupuncture for frequent migraine: A randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid Based Complement Alternat Med. 2015;2015:920353.
16. Da Silva AN. Acupuncture for migraine prevention. Headache. 2015;55:470-473.
17. Meissner K, Fassler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941-1951.
18. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
19. Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6:303-310.
20. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
21. Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;4:CD002286.
22. Pfaffenrath V, Diener HC, Fischer M, et al; Investigators. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523-532.
23. Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): an update of a systematic review. Public Health Nutr. 2000;3:509-514.
24. Natural Medicines. Feverfew Professional Monograph, 2016. Natural Medicines Web site. Available at: https://naturalmedicines.therapeuticresearch.com/. Accessed January 1, 2016.
25. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43:601-610.
26. Facchinetti F, Sances G, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
27. Gallelli L, Avenoso T, Falcone D, et al. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache. 2014;54:313-324.
28. Sándor PS, Afra J, Ambrosini A, et al. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40:30-35.
29. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
30. Boehnke C, Reuter U, Flach U, et al. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11:475-477.
31. Sándor PS, Di Clemente L, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64:713-715.
32. Rozen TD, Oshinsky ML, Gebeline CA, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia. 2002;22:137-141.
33. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache. 2007;47:73-80.
34. Gaul C, Diener HC, Danesch U; Migravent Study Group. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J Headache Pain. 2015;16:516.
35. Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885-890.
36. Silberstein SD, Dodick DW, Aurora SK, et al. Percent of patients with chronic migraine who responded per onabotulinumtoxin A treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996-1001.
37. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
38. Aurora SK, Winner P, Freeman MC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358-1373.
39. Diener HC, Dodick DW, Aurora SK, et al; PREEMPT 2 Chronic Migraine Study Group. Onabotulinumtoxin A for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814.
40. Wachholtz AB, Malone CD, Pargament KI. Effect of different meditation types on migraine headache medication use. Behav Med. 2015:1-8.
41. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:13.
42. Kisan R, Sujan M, Adoor M, et al. Effect of yoga on migraine: A comprehensive study using clinical profile and cardiac autonomic functions. Int J Yoga. 2014;7:126-132.
43. Büssing A, Ostermann T, Lüdtke R, et al. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13:1-9.
44. Penzien DB, Irby MB, Smitherman TA, et al. Well-established and empirically supported behavioral treatments for migraine. Curr Pain Headache Rep. 2015;19:34.
45. Nestoriuc Y, Martin A. Efficacy of biofeedback for migraine: a meta-analysis. Pain. 2007;128:111-127.
46. Fritsche G, Kröner-Herwig B, Kropp P, et al. Psychological therapy of migraine: systematic review. Schmerz. 2013;27:263-274.
47. Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373-380.
48. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697-704.
49. Piquet M, Balestra C, Sava SL, et al. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135.
50. Gil-Martínez A, Kindelan-Calvo P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trials. Rev Neurol. 2013;57:433-443.
51. Varkey E, Cider A, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
52. Newmaster SG, Grguric M, Shanmughanandhan D, et al. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
53. Choi H, Parmar N. The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials. Eur J Emerg Med. 2014;21:2-9.
54. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4:S378-S383.
55. US Food and Drug Administration. FDA approves Botox to treat chronic migraine. US Food and Drug Administration Web site. October 15, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229782.htm. Accessed January 7, 2016.
56. US Food and Drug Administration. FDA allows marketing of first device to relieve migraine headache pain. US Food and Drug Administration Web site. December 13, 2013. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm378608.htm. Accessed January 7, 2016.
57. US Food and Drug Administration. FDA allows marketing of first medical device to prevent migraine headache. US Food and Drug Administration Web site. March 11, 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm388765.htm. Accessed January 7, 2016.
Medical marijuana: A treatment worth trying?
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
Helping patients with cystic fibrosis live longer
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
Personality disorders: A measured response
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
What next when metformin isn't enough for type 2 diabetes?
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5
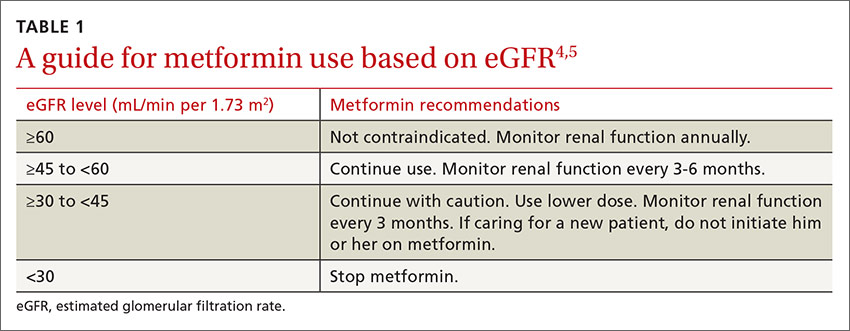
What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1
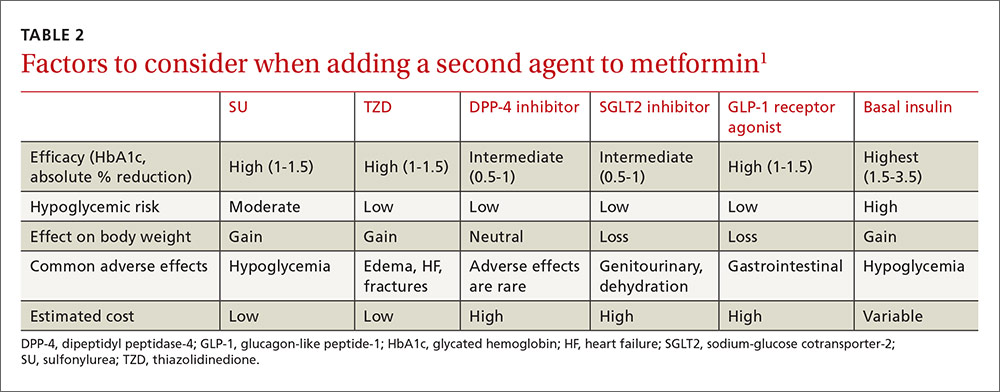
Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1
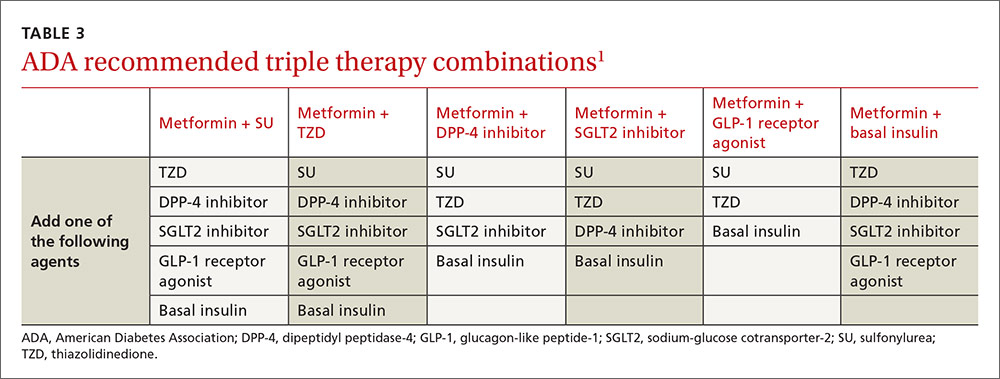
In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral medsFor type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12
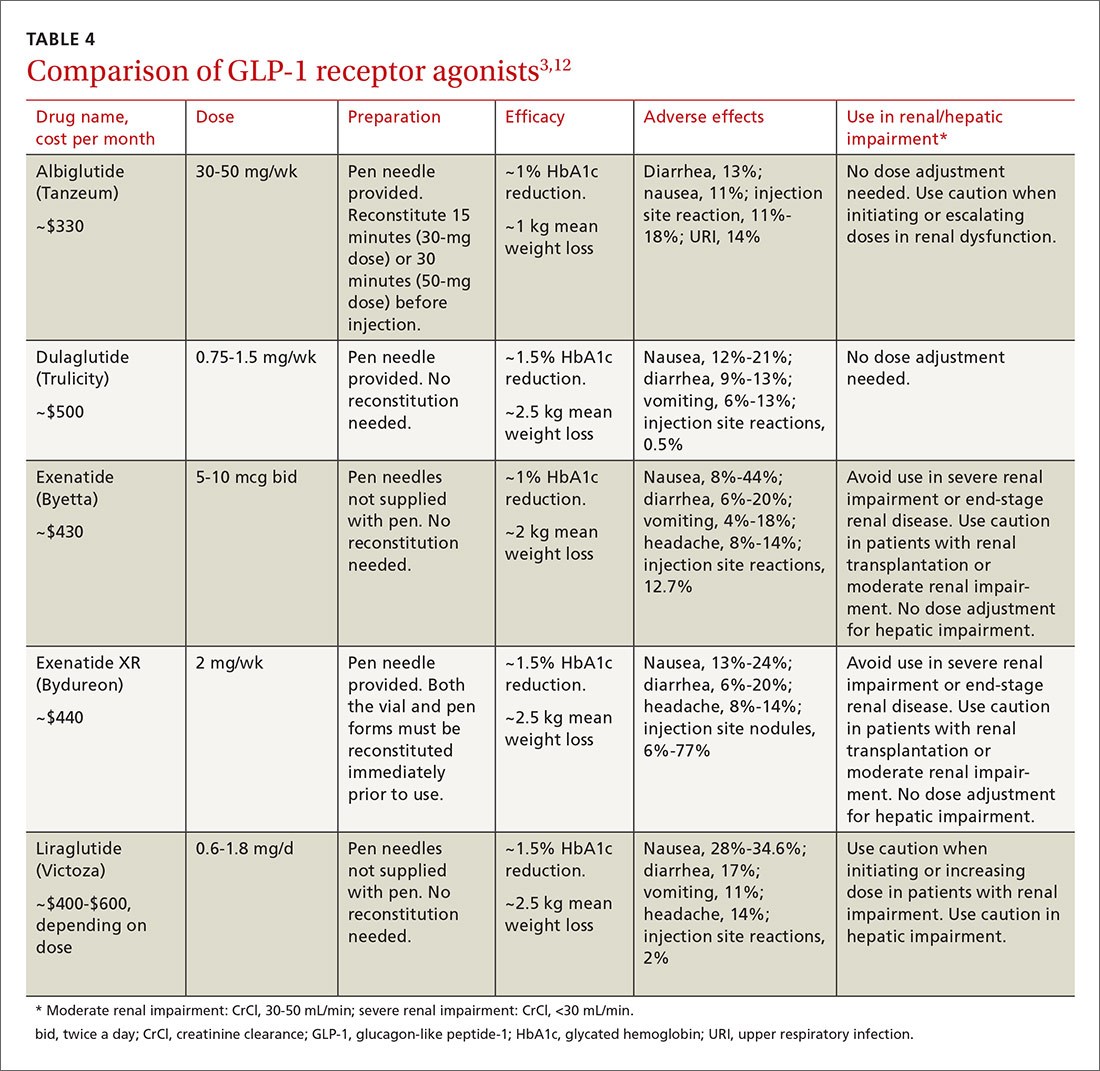
All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merckmanuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5

What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1

Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1

In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral medsFor type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12

All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5

What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1

Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1

In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral medsFor type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12

All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merckmanuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merckmanuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
Pulmonary nodule on x-ray: An algorithmic approach
› Order a computed tomography chest scan, preferably with thin sections through the nodule, to help characterize an indeterminate pulmonary nodule identified on x-ray. B
› Estimate the pretest probability of malignancy for a patient with a pulmonary nodule using your clinical judgment and/or by using a validated model. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › George D is a 67-year-old patient who has never smoked and who has no history of malignancy. An x-ray of his ribs performed after a fall shows a 13-mm solitary nodule in his right upper lung.
CASE 2 › Cathy B is a healthy 80-year-old with no history of smoking. During a trip to the emergency department for chest pain, she had a computed tomography (CT) scan of her chest. While the chest pain was subsequently attributed to gastroesophageal reflux, the CT revealed a 9-mm part solid nodule that was 75% solid.
How should the physicians caring for each of these patients proceed with their care?
The widespread use of sensitive imaging techniques often leads to the incidental discovery of unrelated—but possibly significant—pulmonary findings. Pulmonary nodules are incidentally discovered on an estimated 0.09% to 0.2% of all chest x-rays, 13% of all chest CT angiograms,1 31% of all cardiac CTs performed for coronary calcium scoring,2 and up to 50% of thin-section chest CT scans.1
The widespread implementation of the US Preventive Services Task Force recommendations on lung cancer screening has further expanded the number of patients in whom asymptomatic pulmonary nodules will be detected. As a result, family physicians (FPs) will frequently encounter this challenging clinical dilemma and will need to:
- assess the patient’s risk profile
- address the patient’s concerns about malignancy while eliciting his or her preference for management
- minimize the risks of surveillance testing
- minimize patient distress while ensuring compliance with a follow-up that may extend up to 4 years
- determine when it’s appropriate to refer the patient to a pulmonologist and/or pulmonary nodule clinic or registry.
Taking these steps, however, can be challenging. In interviews, 15 primary care clinicians who care for patients with pulmonary nodules expressed concerns about limitations in time, knowledge, and resources, as well as a fear about such patients “falling through the cracks.”3 Familiarity with current evidence-based guidelines such as those from the American College of Chest Physicians (ACCP) and knowledge of emerging data on the management of various types of nodules are imperative.
To that end, this review will fill in the information gaps and provide guidance on how best to communicate what is known about a particular type of nodule with the patient who has one. (See “What to say to improve joint decision-making.”4-7) But first, a word about terminology.
What to say to improve joint decision-making4-7
Diagnosis and follow-up of a pulmonary nodule takes an emotional toll on patients, who often have a poor sense of what the presence of a nodule signifies. When caring for a patient with a pulmonary nodule, it’s essential to have an effective communication strategy to ensure that he or she is a well-informed partner in decision-making.
Specifically, you'll need to describe the type of nodule that the patient has, how fast it might grow and its malignancy potential, steps that will need to be taken, and the importance of smoking cessation (if the patient smokes).
Ask the patient about any concerns/fears he or she may have, and provide resources to reduce them. Emphasize shared decision-making and discuss the rationale for various management plans and the limitations of diagnostic tests. Do not minimize the issue; emphasize the need for, and importance of, prolonged follow-up—even for a patient who has a small, low-risk nodule.
Solid vs subsolid pulmonary nodules
Solid pulmonary nodules. Traditionally, the term “solitary pulmonary nodule” has been used to describe a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is completely surrounded by aerated lung.1,8 The term “solitary” is now less useful because increasingly sensitive imaging techniques often reveal more than one nodule. In the absence of evidence of features that strongly suggest a benign etiology, these are now commonly referred to as indeterminate solid nodules.
Subsolid nodules are pulmonary nodules that have unique characteristics and require separate guidelines for management. Subsolid nodules include pure ground glass nodules (GGNs) and part solid nodules. GGNs are focal nodular areas of increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa can be visualized.1,8 Part solid nodules have a solid component. They are usually, but not necessarily, >50% ground glass in appearance.
Lung masses. Focal pulmonary lesions >3 cm in diameter are called lung masses and are presumed to be malignant (bronchogenic carcinoma) unless proven otherwise.1,8
The specific approach to evaluating and monitoring a pulmonary nodule varies depending on whether the nodule is solid or subsolid and other factors, including the nodule’s size.
Monitoring solid nodules
Monitoring of an indeterminate solid nodule is largely determined by the patient’s risk profile and the characteristics of the identified nodule.1 Independent patient predictors of malignancy include older age, smoking status, and history of prior malignancy (>5 years ago). Less established predictors are the presence of moderate or severe obstructive lung disease and exposure to particulate or sulfur oxide-related pollution.9
Patients who have an indeterminate nodule identified on chest x-ray should undergo a chest CT scan, preferably with thin sections through the nodule to help characterize it.1 Nodule characteristics that can help predict a patient’s risk of malignancy include the size (>8 mm confers higher risk), malignant rate of growth, edge characteristics (spiculation or irregular edges), thickness of the wall of a cavitary pulmonary nodule (≥16 mm has a likelihood ratio [LR] 37.97 of malignancy), and the location of the nodule (upper or middle lobe [LR=1.2 to 1.6]). 10 A lack of growth over 2 years and a benign pattern of calcification eliminate the need for further evaluation.
Validated tools to help guide decision-making. Although many physicians estimate pretest probability of malignancy intuitively, validated tools are readily available and can help in clinical decision-making.11 One such tool is the Mayo model, which is available at http://reference.medscape.com/calculator/solitary-pulmonary-nodule-risk. This model takes into account the patient’s age, smoking status, history of cancer, and characteristics of the nodule.
Solid nodules >8 mm to 3 cm
For a patient with a solid nodule >8 mm to 3 cm, ACCP guidelines suggest that physicians estimate the pretest probability of malignancy qualitatively using their clinical judgment and/or quantitatively by using a validated model, such as the Mayo model described above.
Based on the patient’s probability of malignancy, management options include continued CT surveillance, positron emission tomography (PET) imaging, CT-guided needle lung biopsy, bronchoscopy with biopsy, or surgical wedge resection (ALGORITHM 1).1
CT surveillance is recommended for individuals:
- with very low (<5%) probability of malignancy
- with low to moderate (5% to 30%) or moderate to high (31% to 65%) probability of malignancy with negative functional imaging (PET)
- with high probability of malignancy (>65%) when needle biopsy is nondiagnostic and the lesion is not hypermetabolic on PET scan.
Surveillance is also recommended when a fully informed patient prefers nonaggressive management. The intervals for serial CT in this population are at 3 to 6 months, 9 to 12 months, and 18 to 24 months.
In an individual with a solid indeterminate nodule with a high probability of malignancy (>65%), functional imaging should not be performed to characterize the nodule. It may, however, be performed for staging.
Time for biopsy or resection? If a nodule shows evidence of malignant growth on serial imaging, nonsurgical biopsy (CT scan-guided transthoracic needle biopsy, bronchoscopy guided by fluoroscopy, endobronchial ultrasound, electromagnetic navigation bronchoscopy, or virtual bronchoscopy navigation) or surgical resection is recommended.
Nonsurgical biopsy is also recommended when the patient’s pretest probability and imaging test results are discordant, when a benign diagnosis requires specific medical treatment, or if a fully informed patient desires proof of diagnosis prior to surgery.
Thoracoscopy with wedge resection is the gold standard for diagnosis of a malignant nodule. It is recommended:
- when the clinical probability of malignancy is high (>65%)
- when the nodule is intensely hypermetabolic by PET scan or positive by other functional imaging tests
- when nonsurgical biopsy is suggestive of malignancy
- when a fully informed patient prefers a definitive diagnostic procedure.
CASE 1 › The FP contacts Mr. D and advises that he get a chest CT to better characterize his pulmonary nodule. A thin-slice CT of the lung reveals that the 13-mm solid nodule in the right upper lobe has spiculated margins. According to the Mayo risk calculator, Mr. D is at moderate risk of malignancy (32.5%). Mr. D and his physician discuss the findings and possible management options, and Mr. D opts to have a PET scan. The FP gives Mr. D literature on pulmonary nodules and contact information for the provider team. A PET scan shows negative uptake. Mr. D and his physician discuss CT surveillance and nonsurgical biopsy. He opts for CT surveillance. The next CT is scheduled for 3 months.
Solid nodules ≤8 mm
Management of these lesions generally follows the consensus-based guidelines that were first published by the Fleischner Society and subsequently endorsed by the ACCP.1 The 2 main determinants that guide management of nodules ≤8 mm are the patient’s risk factors for cancer and nodule size (ALGORITHM 2).1 The Fleischner guidelines pertain only to patients older than age 35 with no current extra pulmonary malignancy or unexplained fevers. The ACCP guidelines, although similar, do not include these limitations. Patient risk factors include history of smoking, older age, and a history of malignancy.1
Patients with no risk factors for malignancy. The frequency of surveillance CT is determined by the size of the nodule. Nodules ≤4 mm do not need to be followed. For nodules >4 mm to 6 mm, a repeat CT in 12 months is recommended with no follow-up if stable. For nodules >6 to <8 mm, repeat CT is recommended at 6 to 12 months, and again between 18 and 24 months if unchanged.1Patients with one or more risk factors for malignancy. Nodules ≤4 mm should be reevaluated at 12 months in patients with one or more risk factors; no additional follow-up is needed if unchanged. For nodules >4 mm to 6 mm, CT should be repeated between 6 and 12 months and again between 18 and 24 months. Nodules >6 mm to <8 mm should be followed initially between 3 to 6 months, then between 9 and 12 months and again at 24 months if unchanged.1
Subsolid nodules require a different approach
Subsolid nodules have a high prevalence of premalignant and malignant disease (adenocarcinoma in situ, minimally invasive adenocarcinoma, and adenocarcinoma). Studies have reported subsolid nodule malignancy rates ranging from 20% to 75%.11-15 This wide range may be a function of different nodule sizes or rates of biopsy. The prevalence increases even further in nodules with a part solid component.
These factors, plus challenges in measuring serial growth on CT and the uncertain prognosis of untreated premalignant disease, make it necessary to have separate guidelines for managing subsolid nodules. The Fleischner Society, National Comprehensive Cancer Network, and the American College of Radiology (LungRads) each have differing recommendations on the frequency of follow-up for different-sized subsolid nodules. Newer studies favor a more conservative approach.16 Here we describe the current ACCP guidelines for managing subsolid nodules (ALGORITHM 3).1
GGNs. In an individual with a pure GGN ≤5 mm in diameter, no further evaluation is recommended. In an individual with a pure GGN >5 mm in diameter, annual surveillance with chest CT for at least 3 years is recommended.1
Part solid nodules. In an individual with a part solid nodule ≤8 mm, conduct CT surveillance at 3, 12, and 24 months and then annually for an additional one to 3 years. In a patient with a part solid nodule >8 mm to 15 mm, repeat chest CT at 3 months followed by a PET scan, nonsurgical biopsy, and/or surgical resection if the nodule persists. A patient with a part solid nodule >15 mm should undergo a PET scan, nonsurgical biopsy, and/or surgical resection.
CASE 2 › Ms. G is seen in the office by her FP, and they discuss management options. A repeat CT is done in 3 months and shows a persistent, unchanged nodule. Ms. G opts for a transthoracic biopsy, which reveals adenocarcinoma. Following a PET scan, which shows no evidence of metastasis, curative surgical wedge resection is done.
Multiple subsolid nodules. In a patient who has a dominant nodule and one or more additional nodules, each nodule should be evaluated individually, according to recommendations from the Fleischner Society (the ACCP currently does not have guidelines for managing multiple subsolid nodules). An individual with multiple GGNs that all measure ≤5 mm should receive CT exams at 2 and 4 years.13 A patient with multiple GGNs that include at least one nodule >5 mm but no dominant nodule should undergo follow-up CT at 3 months and annual CT surveillance for at least 3 years.13
CORRESPONDENCE
Samina Yunus, MD, MPH, Cleveland Clinic, Family Medicine, 551 East Washington Street, Chagrin Falls, OH 44022; [email protected].
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S.
2. Burt JR, Iribarren C, Fair JM, et al; Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology (ADVANCE) Study. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168:756-761.
3. Golden SE, Wiener RS, Sullivan D, et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422-1429.
4. Sullivan DR, Golden SE, Ganzini L, et al. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028.
5. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154-161.
6. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672-677.
7. Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355-365.
8. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
9. Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132-1141.
10. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
11. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388.
12. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365-373.
13. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317.
14. Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67-73.
15. Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391-408.
16. Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis. 2015;7:1103-1106.
› Order a computed tomography chest scan, preferably with thin sections through the nodule, to help characterize an indeterminate pulmonary nodule identified on x-ray. B
› Estimate the pretest probability of malignancy for a patient with a pulmonary nodule using your clinical judgment and/or by using a validated model. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › George D is a 67-year-old patient who has never smoked and who has no history of malignancy. An x-ray of his ribs performed after a fall shows a 13-mm solitary nodule in his right upper lung.
CASE 2 › Cathy B is a healthy 80-year-old with no history of smoking. During a trip to the emergency department for chest pain, she had a computed tomography (CT) scan of her chest. While the chest pain was subsequently attributed to gastroesophageal reflux, the CT revealed a 9-mm part solid nodule that was 75% solid.
How should the physicians caring for each of these patients proceed with their care?
The widespread use of sensitive imaging techniques often leads to the incidental discovery of unrelated—but possibly significant—pulmonary findings. Pulmonary nodules are incidentally discovered on an estimated 0.09% to 0.2% of all chest x-rays, 13% of all chest CT angiograms,1 31% of all cardiac CTs performed for coronary calcium scoring,2 and up to 50% of thin-section chest CT scans.1
The widespread implementation of the US Preventive Services Task Force recommendations on lung cancer screening has further expanded the number of patients in whom asymptomatic pulmonary nodules will be detected. As a result, family physicians (FPs) will frequently encounter this challenging clinical dilemma and will need to:
- assess the patient’s risk profile
- address the patient’s concerns about malignancy while eliciting his or her preference for management
- minimize the risks of surveillance testing
- minimize patient distress while ensuring compliance with a follow-up that may extend up to 4 years
- determine when it’s appropriate to refer the patient to a pulmonologist and/or pulmonary nodule clinic or registry.
Taking these steps, however, can be challenging. In interviews, 15 primary care clinicians who care for patients with pulmonary nodules expressed concerns about limitations in time, knowledge, and resources, as well as a fear about such patients “falling through the cracks.”3 Familiarity with current evidence-based guidelines such as those from the American College of Chest Physicians (ACCP) and knowledge of emerging data on the management of various types of nodules are imperative.
To that end, this review will fill in the information gaps and provide guidance on how best to communicate what is known about a particular type of nodule with the patient who has one. (See “What to say to improve joint decision-making.”4-7) But first, a word about terminology.
What to say to improve joint decision-making4-7
Diagnosis and follow-up of a pulmonary nodule takes an emotional toll on patients, who often have a poor sense of what the presence of a nodule signifies. When caring for a patient with a pulmonary nodule, it’s essential to have an effective communication strategy to ensure that he or she is a well-informed partner in decision-making.
Specifically, you'll need to describe the type of nodule that the patient has, how fast it might grow and its malignancy potential, steps that will need to be taken, and the importance of smoking cessation (if the patient smokes).
Ask the patient about any concerns/fears he or she may have, and provide resources to reduce them. Emphasize shared decision-making and discuss the rationale for various management plans and the limitations of diagnostic tests. Do not minimize the issue; emphasize the need for, and importance of, prolonged follow-up—even for a patient who has a small, low-risk nodule.
Solid vs subsolid pulmonary nodules
Solid pulmonary nodules. Traditionally, the term “solitary pulmonary nodule” has been used to describe a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is completely surrounded by aerated lung.1,8 The term “solitary” is now less useful because increasingly sensitive imaging techniques often reveal more than one nodule. In the absence of evidence of features that strongly suggest a benign etiology, these are now commonly referred to as indeterminate solid nodules.
Subsolid nodules are pulmonary nodules that have unique characteristics and require separate guidelines for management. Subsolid nodules include pure ground glass nodules (GGNs) and part solid nodules. GGNs are focal nodular areas of increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa can be visualized.1,8 Part solid nodules have a solid component. They are usually, but not necessarily, >50% ground glass in appearance.
Lung masses. Focal pulmonary lesions >3 cm in diameter are called lung masses and are presumed to be malignant (bronchogenic carcinoma) unless proven otherwise.1,8
The specific approach to evaluating and monitoring a pulmonary nodule varies depending on whether the nodule is solid or subsolid and other factors, including the nodule’s size.
Monitoring solid nodules
Monitoring of an indeterminate solid nodule is largely determined by the patient’s risk profile and the characteristics of the identified nodule.1 Independent patient predictors of malignancy include older age, smoking status, and history of prior malignancy (>5 years ago). Less established predictors are the presence of moderate or severe obstructive lung disease and exposure to particulate or sulfur oxide-related pollution.9
Patients who have an indeterminate nodule identified on chest x-ray should undergo a chest CT scan, preferably with thin sections through the nodule to help characterize it.1 Nodule characteristics that can help predict a patient’s risk of malignancy include the size (>8 mm confers higher risk), malignant rate of growth, edge characteristics (spiculation or irregular edges), thickness of the wall of a cavitary pulmonary nodule (≥16 mm has a likelihood ratio [LR] 37.97 of malignancy), and the location of the nodule (upper or middle lobe [LR=1.2 to 1.6]). 10 A lack of growth over 2 years and a benign pattern of calcification eliminate the need for further evaluation.
Validated tools to help guide decision-making. Although many physicians estimate pretest probability of malignancy intuitively, validated tools are readily available and can help in clinical decision-making.11 One such tool is the Mayo model, which is available at http://reference.medscape.com/calculator/solitary-pulmonary-nodule-risk. This model takes into account the patient’s age, smoking status, history of cancer, and characteristics of the nodule.
Solid nodules >8 mm to 3 cm
For a patient with a solid nodule >8 mm to 3 cm, ACCP guidelines suggest that physicians estimate the pretest probability of malignancy qualitatively using their clinical judgment and/or quantitatively by using a validated model, such as the Mayo model described above.
Based on the patient’s probability of malignancy, management options include continued CT surveillance, positron emission tomography (PET) imaging, CT-guided needle lung biopsy, bronchoscopy with biopsy, or surgical wedge resection (ALGORITHM 1).1
CT surveillance is recommended for individuals:
- with very low (<5%) probability of malignancy
- with low to moderate (5% to 30%) or moderate to high (31% to 65%) probability of malignancy with negative functional imaging (PET)
- with high probability of malignancy (>65%) when needle biopsy is nondiagnostic and the lesion is not hypermetabolic on PET scan.
Surveillance is also recommended when a fully informed patient prefers nonaggressive management. The intervals for serial CT in this population are at 3 to 6 months, 9 to 12 months, and 18 to 24 months.
In an individual with a solid indeterminate nodule with a high probability of malignancy (>65%), functional imaging should not be performed to characterize the nodule. It may, however, be performed for staging.
Time for biopsy or resection? If a nodule shows evidence of malignant growth on serial imaging, nonsurgical biopsy (CT scan-guided transthoracic needle biopsy, bronchoscopy guided by fluoroscopy, endobronchial ultrasound, electromagnetic navigation bronchoscopy, or virtual bronchoscopy navigation) or surgical resection is recommended.
Nonsurgical biopsy is also recommended when the patient’s pretest probability and imaging test results are discordant, when a benign diagnosis requires specific medical treatment, or if a fully informed patient desires proof of diagnosis prior to surgery.
Thoracoscopy with wedge resection is the gold standard for diagnosis of a malignant nodule. It is recommended:
- when the clinical probability of malignancy is high (>65%)
- when the nodule is intensely hypermetabolic by PET scan or positive by other functional imaging tests
- when nonsurgical biopsy is suggestive of malignancy
- when a fully informed patient prefers a definitive diagnostic procedure.
CASE 1 › The FP contacts Mr. D and advises that he get a chest CT to better characterize his pulmonary nodule. A thin-slice CT of the lung reveals that the 13-mm solid nodule in the right upper lobe has spiculated margins. According to the Mayo risk calculator, Mr. D is at moderate risk of malignancy (32.5%). Mr. D and his physician discuss the findings and possible management options, and Mr. D opts to have a PET scan. The FP gives Mr. D literature on pulmonary nodules and contact information for the provider team. A PET scan shows negative uptake. Mr. D and his physician discuss CT surveillance and nonsurgical biopsy. He opts for CT surveillance. The next CT is scheduled for 3 months.
Solid nodules ≤8 mm
Management of these lesions generally follows the consensus-based guidelines that were first published by the Fleischner Society and subsequently endorsed by the ACCP.1 The 2 main determinants that guide management of nodules ≤8 mm are the patient’s risk factors for cancer and nodule size (ALGORITHM 2).1 The Fleischner guidelines pertain only to patients older than age 35 with no current extra pulmonary malignancy or unexplained fevers. The ACCP guidelines, although similar, do not include these limitations. Patient risk factors include history of smoking, older age, and a history of malignancy.1
Patients with no risk factors for malignancy. The frequency of surveillance CT is determined by the size of the nodule. Nodules ≤4 mm do not need to be followed. For nodules >4 mm to 6 mm, a repeat CT in 12 months is recommended with no follow-up if stable. For nodules >6 to <8 mm, repeat CT is recommended at 6 to 12 months, and again between 18 and 24 months if unchanged.1Patients with one or more risk factors for malignancy. Nodules ≤4 mm should be reevaluated at 12 months in patients with one or more risk factors; no additional follow-up is needed if unchanged. For nodules >4 mm to 6 mm, CT should be repeated between 6 and 12 months and again between 18 and 24 months. Nodules >6 mm to <8 mm should be followed initially between 3 to 6 months, then between 9 and 12 months and again at 24 months if unchanged.1
Subsolid nodules require a different approach
Subsolid nodules have a high prevalence of premalignant and malignant disease (adenocarcinoma in situ, minimally invasive adenocarcinoma, and adenocarcinoma). Studies have reported subsolid nodule malignancy rates ranging from 20% to 75%.11-15 This wide range may be a function of different nodule sizes or rates of biopsy. The prevalence increases even further in nodules with a part solid component.
These factors, plus challenges in measuring serial growth on CT and the uncertain prognosis of untreated premalignant disease, make it necessary to have separate guidelines for managing subsolid nodules. The Fleischner Society, National Comprehensive Cancer Network, and the American College of Radiology (LungRads) each have differing recommendations on the frequency of follow-up for different-sized subsolid nodules. Newer studies favor a more conservative approach.16 Here we describe the current ACCP guidelines for managing subsolid nodules (ALGORITHM 3).1
GGNs. In an individual with a pure GGN ≤5 mm in diameter, no further evaluation is recommended. In an individual with a pure GGN >5 mm in diameter, annual surveillance with chest CT for at least 3 years is recommended.1
Part solid nodules. In an individual with a part solid nodule ≤8 mm, conduct CT surveillance at 3, 12, and 24 months and then annually for an additional one to 3 years. In a patient with a part solid nodule >8 mm to 15 mm, repeat chest CT at 3 months followed by a PET scan, nonsurgical biopsy, and/or surgical resection if the nodule persists. A patient with a part solid nodule >15 mm should undergo a PET scan, nonsurgical biopsy, and/or surgical resection.
CASE 2 › Ms. G is seen in the office by her FP, and they discuss management options. A repeat CT is done in 3 months and shows a persistent, unchanged nodule. Ms. G opts for a transthoracic biopsy, which reveals adenocarcinoma. Following a PET scan, which shows no evidence of metastasis, curative surgical wedge resection is done.
Multiple subsolid nodules. In a patient who has a dominant nodule and one or more additional nodules, each nodule should be evaluated individually, according to recommendations from the Fleischner Society (the ACCP currently does not have guidelines for managing multiple subsolid nodules). An individual with multiple GGNs that all measure ≤5 mm should receive CT exams at 2 and 4 years.13 A patient with multiple GGNs that include at least one nodule >5 mm but no dominant nodule should undergo follow-up CT at 3 months and annual CT surveillance for at least 3 years.13
CORRESPONDENCE
Samina Yunus, MD, MPH, Cleveland Clinic, Family Medicine, 551 East Washington Street, Chagrin Falls, OH 44022; [email protected].
› Order a computed tomography chest scan, preferably with thin sections through the nodule, to help characterize an indeterminate pulmonary nodule identified on x-ray. B
› Estimate the pretest probability of malignancy for a patient with a pulmonary nodule using your clinical judgment and/or by using a validated model. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › George D is a 67-year-old patient who has never smoked and who has no history of malignancy. An x-ray of his ribs performed after a fall shows a 13-mm solitary nodule in his right upper lung.
CASE 2 › Cathy B is a healthy 80-year-old with no history of smoking. During a trip to the emergency department for chest pain, she had a computed tomography (CT) scan of her chest. While the chest pain was subsequently attributed to gastroesophageal reflux, the CT revealed a 9-mm part solid nodule that was 75% solid.
How should the physicians caring for each of these patients proceed with their care?
The widespread use of sensitive imaging techniques often leads to the incidental discovery of unrelated—but possibly significant—pulmonary findings. Pulmonary nodules are incidentally discovered on an estimated 0.09% to 0.2% of all chest x-rays, 13% of all chest CT angiograms,1 31% of all cardiac CTs performed for coronary calcium scoring,2 and up to 50% of thin-section chest CT scans.1
The widespread implementation of the US Preventive Services Task Force recommendations on lung cancer screening has further expanded the number of patients in whom asymptomatic pulmonary nodules will be detected. As a result, family physicians (FPs) will frequently encounter this challenging clinical dilemma and will need to:
- assess the patient’s risk profile
- address the patient’s concerns about malignancy while eliciting his or her preference for management
- minimize the risks of surveillance testing
- minimize patient distress while ensuring compliance with a follow-up that may extend up to 4 years
- determine when it’s appropriate to refer the patient to a pulmonologist and/or pulmonary nodule clinic or registry.
Taking these steps, however, can be challenging. In interviews, 15 primary care clinicians who care for patients with pulmonary nodules expressed concerns about limitations in time, knowledge, and resources, as well as a fear about such patients “falling through the cracks.”3 Familiarity with current evidence-based guidelines such as those from the American College of Chest Physicians (ACCP) and knowledge of emerging data on the management of various types of nodules are imperative.
To that end, this review will fill in the information gaps and provide guidance on how best to communicate what is known about a particular type of nodule with the patient who has one. (See “What to say to improve joint decision-making.”4-7) But first, a word about terminology.
What to say to improve joint decision-making4-7
Diagnosis and follow-up of a pulmonary nodule takes an emotional toll on patients, who often have a poor sense of what the presence of a nodule signifies. When caring for a patient with a pulmonary nodule, it’s essential to have an effective communication strategy to ensure that he or she is a well-informed partner in decision-making.
Specifically, you'll need to describe the type of nodule that the patient has, how fast it might grow and its malignancy potential, steps that will need to be taken, and the importance of smoking cessation (if the patient smokes).
Ask the patient about any concerns/fears he or she may have, and provide resources to reduce them. Emphasize shared decision-making and discuss the rationale for various management plans and the limitations of diagnostic tests. Do not minimize the issue; emphasize the need for, and importance of, prolonged follow-up—even for a patient who has a small, low-risk nodule.
Solid vs subsolid pulmonary nodules
Solid pulmonary nodules. Traditionally, the term “solitary pulmonary nodule” has been used to describe a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is completely surrounded by aerated lung.1,8 The term “solitary” is now less useful because increasingly sensitive imaging techniques often reveal more than one nodule. In the absence of evidence of features that strongly suggest a benign etiology, these are now commonly referred to as indeterminate solid nodules.
Subsolid nodules are pulmonary nodules that have unique characteristics and require separate guidelines for management. Subsolid nodules include pure ground glass nodules (GGNs) and part solid nodules. GGNs are focal nodular areas of increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa can be visualized.1,8 Part solid nodules have a solid component. They are usually, but not necessarily, >50% ground glass in appearance.
Lung masses. Focal pulmonary lesions >3 cm in diameter are called lung masses and are presumed to be malignant (bronchogenic carcinoma) unless proven otherwise.1,8
The specific approach to evaluating and monitoring a pulmonary nodule varies depending on whether the nodule is solid or subsolid and other factors, including the nodule’s size.
Monitoring solid nodules
Monitoring of an indeterminate solid nodule is largely determined by the patient’s risk profile and the characteristics of the identified nodule.1 Independent patient predictors of malignancy include older age, smoking status, and history of prior malignancy (>5 years ago). Less established predictors are the presence of moderate or severe obstructive lung disease and exposure to particulate or sulfur oxide-related pollution.9
Patients who have an indeterminate nodule identified on chest x-ray should undergo a chest CT scan, preferably with thin sections through the nodule to help characterize it.1 Nodule characteristics that can help predict a patient’s risk of malignancy include the size (>8 mm confers higher risk), malignant rate of growth, edge characteristics (spiculation or irregular edges), thickness of the wall of a cavitary pulmonary nodule (≥16 mm has a likelihood ratio [LR] 37.97 of malignancy), and the location of the nodule (upper or middle lobe [LR=1.2 to 1.6]). 10 A lack of growth over 2 years and a benign pattern of calcification eliminate the need for further evaluation.
Validated tools to help guide decision-making. Although many physicians estimate pretest probability of malignancy intuitively, validated tools are readily available and can help in clinical decision-making.11 One such tool is the Mayo model, which is available at http://reference.medscape.com/calculator/solitary-pulmonary-nodule-risk. This model takes into account the patient’s age, smoking status, history of cancer, and characteristics of the nodule.
Solid nodules >8 mm to 3 cm
For a patient with a solid nodule >8 mm to 3 cm, ACCP guidelines suggest that physicians estimate the pretest probability of malignancy qualitatively using their clinical judgment and/or quantitatively by using a validated model, such as the Mayo model described above.
Based on the patient’s probability of malignancy, management options include continued CT surveillance, positron emission tomography (PET) imaging, CT-guided needle lung biopsy, bronchoscopy with biopsy, or surgical wedge resection (ALGORITHM 1).1
CT surveillance is recommended for individuals:
- with very low (<5%) probability of malignancy
- with low to moderate (5% to 30%) or moderate to high (31% to 65%) probability of malignancy with negative functional imaging (PET)
- with high probability of malignancy (>65%) when needle biopsy is nondiagnostic and the lesion is not hypermetabolic on PET scan.
Surveillance is also recommended when a fully informed patient prefers nonaggressive management. The intervals for serial CT in this population are at 3 to 6 months, 9 to 12 months, and 18 to 24 months.
In an individual with a solid indeterminate nodule with a high probability of malignancy (>65%), functional imaging should not be performed to characterize the nodule. It may, however, be performed for staging.
Time for biopsy or resection? If a nodule shows evidence of malignant growth on serial imaging, nonsurgical biopsy (CT scan-guided transthoracic needle biopsy, bronchoscopy guided by fluoroscopy, endobronchial ultrasound, electromagnetic navigation bronchoscopy, or virtual bronchoscopy navigation) or surgical resection is recommended.
Nonsurgical biopsy is also recommended when the patient’s pretest probability and imaging test results are discordant, when a benign diagnosis requires specific medical treatment, or if a fully informed patient desires proof of diagnosis prior to surgery.
Thoracoscopy with wedge resection is the gold standard for diagnosis of a malignant nodule. It is recommended:
- when the clinical probability of malignancy is high (>65%)
- when the nodule is intensely hypermetabolic by PET scan or positive by other functional imaging tests
- when nonsurgical biopsy is suggestive of malignancy
- when a fully informed patient prefers a definitive diagnostic procedure.
CASE 1 › The FP contacts Mr. D and advises that he get a chest CT to better characterize his pulmonary nodule. A thin-slice CT of the lung reveals that the 13-mm solid nodule in the right upper lobe has spiculated margins. According to the Mayo risk calculator, Mr. D is at moderate risk of malignancy (32.5%). Mr. D and his physician discuss the findings and possible management options, and Mr. D opts to have a PET scan. The FP gives Mr. D literature on pulmonary nodules and contact information for the provider team. A PET scan shows negative uptake. Mr. D and his physician discuss CT surveillance and nonsurgical biopsy. He opts for CT surveillance. The next CT is scheduled for 3 months.
Solid nodules ≤8 mm
Management of these lesions generally follows the consensus-based guidelines that were first published by the Fleischner Society and subsequently endorsed by the ACCP.1 The 2 main determinants that guide management of nodules ≤8 mm are the patient’s risk factors for cancer and nodule size (ALGORITHM 2).1 The Fleischner guidelines pertain only to patients older than age 35 with no current extra pulmonary malignancy or unexplained fevers. The ACCP guidelines, although similar, do not include these limitations. Patient risk factors include history of smoking, older age, and a history of malignancy.1
Patients with no risk factors for malignancy. The frequency of surveillance CT is determined by the size of the nodule. Nodules ≤4 mm do not need to be followed. For nodules >4 mm to 6 mm, a repeat CT in 12 months is recommended with no follow-up if stable. For nodules >6 to <8 mm, repeat CT is recommended at 6 to 12 months, and again between 18 and 24 months if unchanged.1Patients with one or more risk factors for malignancy. Nodules ≤4 mm should be reevaluated at 12 months in patients with one or more risk factors; no additional follow-up is needed if unchanged. For nodules >4 mm to 6 mm, CT should be repeated between 6 and 12 months and again between 18 and 24 months. Nodules >6 mm to <8 mm should be followed initially between 3 to 6 months, then between 9 and 12 months and again at 24 months if unchanged.1
Subsolid nodules require a different approach
Subsolid nodules have a high prevalence of premalignant and malignant disease (adenocarcinoma in situ, minimally invasive adenocarcinoma, and adenocarcinoma). Studies have reported subsolid nodule malignancy rates ranging from 20% to 75%.11-15 This wide range may be a function of different nodule sizes or rates of biopsy. The prevalence increases even further in nodules with a part solid component.
These factors, plus challenges in measuring serial growth on CT and the uncertain prognosis of untreated premalignant disease, make it necessary to have separate guidelines for managing subsolid nodules. The Fleischner Society, National Comprehensive Cancer Network, and the American College of Radiology (LungRads) each have differing recommendations on the frequency of follow-up for different-sized subsolid nodules. Newer studies favor a more conservative approach.16 Here we describe the current ACCP guidelines for managing subsolid nodules (ALGORITHM 3).1
GGNs. In an individual with a pure GGN ≤5 mm in diameter, no further evaluation is recommended. In an individual with a pure GGN >5 mm in diameter, annual surveillance with chest CT for at least 3 years is recommended.1
Part solid nodules. In an individual with a part solid nodule ≤8 mm, conduct CT surveillance at 3, 12, and 24 months and then annually for an additional one to 3 years. In a patient with a part solid nodule >8 mm to 15 mm, repeat chest CT at 3 months followed by a PET scan, nonsurgical biopsy, and/or surgical resection if the nodule persists. A patient with a part solid nodule >15 mm should undergo a PET scan, nonsurgical biopsy, and/or surgical resection.
CASE 2 › Ms. G is seen in the office by her FP, and they discuss management options. A repeat CT is done in 3 months and shows a persistent, unchanged nodule. Ms. G opts for a transthoracic biopsy, which reveals adenocarcinoma. Following a PET scan, which shows no evidence of metastasis, curative surgical wedge resection is done.
Multiple subsolid nodules. In a patient who has a dominant nodule and one or more additional nodules, each nodule should be evaluated individually, according to recommendations from the Fleischner Society (the ACCP currently does not have guidelines for managing multiple subsolid nodules). An individual with multiple GGNs that all measure ≤5 mm should receive CT exams at 2 and 4 years.13 A patient with multiple GGNs that include at least one nodule >5 mm but no dominant nodule should undergo follow-up CT at 3 months and annual CT surveillance for at least 3 years.13
CORRESPONDENCE
Samina Yunus, MD, MPH, Cleveland Clinic, Family Medicine, 551 East Washington Street, Chagrin Falls, OH 44022; [email protected].
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S.
2. Burt JR, Iribarren C, Fair JM, et al; Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology (ADVANCE) Study. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168:756-761.
3. Golden SE, Wiener RS, Sullivan D, et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422-1429.
4. Sullivan DR, Golden SE, Ganzini L, et al. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028.
5. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154-161.
6. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672-677.
7. Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355-365.
8. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
9. Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132-1141.
10. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
11. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388.
12. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365-373.
13. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317.
14. Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67-73.
15. Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391-408.
16. Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis. 2015;7:1103-1106.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S.
2. Burt JR, Iribarren C, Fair JM, et al; Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology (ADVANCE) Study. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168:756-761.
3. Golden SE, Wiener RS, Sullivan D, et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422-1429.
4. Sullivan DR, Golden SE, Ganzini L, et al. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028.
5. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154-161.
6. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672-677.
7. Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355-365.
8. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
9. Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132-1141.
10. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
11. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388.
12. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365-373.
13. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317.
14. Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67-73.
15. Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391-408.
16. Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis. 2015;7:1103-1106.
Targeting gut flora to treat and prevent disease
› Encourage patients to eat a healthy diet that includes an adequate amount of soluble fiber to maintain a healthy, diverse microbiome. B
› Recommend combination probiotics to treat symptoms of irritable bowel syndrome. A
› Encourage patients to take probiotics containing Lactobacillus species to prevent antibiotic-associated diarrhea and Saccharomyces to prevent Clostridium difficile infection. A
› Recommend probiotics containing Lactobacillus species and/or Saccharomyces to treat acute infectious diarrhea. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sheila S, age 27, has irritable bowel syndrome (IBS) and comes to your office for a follow-up visit. Over the past 6 months she has started taking a fiber supplement, drinking more water, and looking for links between stress and her symptoms. She has read about probiotics and wonders if you would consider recommending them in her situation.
CASE 2 › Mark M, age 45, has type 2 diabetes and is overweight. He is motivated to change his diet and has started to exercise more. He is taking metformin 2000 mg/d but his hemoglobin A1c remains slightly elevated at 7.2%. He heard on television that probiotics might help to keep him from needing to add another medication.
Most of the living organisms that comprise the human microbiome—all of the microbes that live on or in humans—are found in the gastrointestinal (GI) tract. The gut flora contribute 99% of the genetic material in the human body. The composition of the gut flora is remarkably diverse across the population; each individual has a unique microbial footprint. Within this microbial diversity, there appears to be a stable number of genes that are responsible for the major functions of the gut flora.1 These microbes:
- supply essential nutrients by breaking down complex carbohydrates;
- generate secondary bile acids that assist in digesting fats;2
- synthesize vitamins such as K, B12, folate, and biotin;3
- contribute to the defensive barrier in the colon by keeping pathogenic bacteria from crossing the colonic mucosa; and
- interact with our systemic immune system in a way that maintains a level of homeostasis, allowing for appropriate activation in the face of pathogens without developing autoimmunity.4
The gut flora also play a role in the communication between the central nervous system and the enteric nervous system by modulating the hormonal and neural pathways that have been labeled the “gut-brain axis.” The gut-brain axis has been associated with numerous disease states, including irritable bowel syndrome and certain psychiatric disorders.5
Researchers are investigating interventions that target the microbiome to increase microbial diversity and the presence of certain species to prevent or treat various diseases. The use of probiotics and dietary changes to increase intake of soluble fiber have been the most studied of these interventions. The thought is that these interventions can correct an imbalance, or dysbiosis, of the gut flora.6 Studies have shown that decreased microbial diversity is associated with elevations of certain disease markers (eg, adiposity, insulin, triglycerides, C–reactive protein)7 and that increases in soluble fiber lead to the greatest long-term improvement in microbial diversity.8 Fecal transplant—the transfer of a processed mixture of stool that contains “healthy” bacteria from a donor into the intestines of a patient—is being explored as a method of replacing colonic gut flora, but evidence is limited.
The following review takes a closer look at these options and identifies those that are most likely to benefit patients in the treatment—and prevention—of several diseases (TABLE 1).9-16
Evidence is best for using probiotics for digestive diseases
Dietary interventions for digestive diseases have long been studied, but are getting renewed attention for their potential impact on the microbiome.17 Beyond dietary modification, other similar treatment options include probiotics (live microorganisms thought to confer a beneficial effect on the host), prebiotics (non-digestible food ingredients, including oligosaccharides and inulin, thought to promote the growth of “helpful” gut flora), and synbiotics (combinations of the 2).18
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized by altered intestinal transit, low-grade colonic inflammation, and/or alterations in the gutbrain axis. Research has increasingly focused on recently discovered increases in intestinal immune activation, intestinal permeability, and alterations in the colonic microbiome (decreased diversity and increased pathogenic bacteria) associated with IBS.19
A meta-analysis of 43 randomized control trials (RCTs) found probiotics ranging from Lactobacillus to Saccharomyces can significantly decrease global IBS symptoms, abdominal pain, bloating, and flatulence.9 For a patient such as Ms. S, the evidence suggests a probiotic that contains a mixture of Lactobacillus and Bifidobacterium might help relieve her symptoms.9 In terms of dietary modifications, soluble fiber, which is already known to help treat IBS,20 has profound effects on improving microbiota diversity and in shifting the composition toward less pathogenic strains.21 The Institute of Medicine's daily recommended intake of soluble fiber is about 15 g/d.22
Inflammatory bowel disease (IBD) is caused by inflammation of the GI lining due to an overactive immune response. Evidence shows that patients with IBD have an altered microbial composition—specifically, an increase in bacteria that produce pro-inflammatory molecules and a decrease in bacteria that have a dampening effect on immune activation.23
Most studies evaluating probiotics as a treatment for IBD have been small and have used a wide variety of bacterial mixtures, which makes comparisons difficult. Recent meta-analyses found combination probiotics can both induce and maintain remission in patients with ulcerative colitis, but have no beneficial effects in Crohn’s disease.10 In a review of 9 case series of patients with IBD, fecal transplant reduced IBD symptoms, and patients were able to decrease medication use.24
Diarrheal illness. The human intestine is protected from diarrheal illness by healthy bacteria that block the actions of pathogenic bacteria. This mechanism is called colonization resistance. Moderate levels of evidence support the use of probiotics to prevent or treat several types of diarrheal illness.14
Antibiotic-associated diarrhea (AAD) is caused when antibiotic use alters the microbial balance. Recent meta-analyses have shown probiotics can prevent AAD and Clostridium difficile-associated diarrhea.11,12 Several case series and one RCT have found that fecal transplants are safe and efficacious for treating recurrent Clostridium difficile infection.25 Using probiotics to treat symptoms of AAD has been less studied.
Acute infectious diarrhea and traveler’s diarrhea (TD). A Cochrane review found that probiotics decreased the duration of diarrheal episodes by 25 hours, decreased the risk of an episode lasting more than 4 days by 59%, and led to one less diarrheal stool per day by the second day of the intervention.13 In a separate meta-analysis of 12 studies, probiotics significantly prevented 85% of cases of TD.14
Encouraging early evidence for several other illnesses
Metabolic disorders. Both animal and human studies support the theory that gut flora contribute to energy homeostasis, and in some genetically predisposed people dysbiosis may lead to obesity and diabetes. The traditional western diet4 and possibly decreased physical activity26 are major contributors to gut flora dysbiosis. Healthy bacteria in the gut break down soluble fiber into short chain fatty acids (SCFAs). SCFAs are associated with increased satiety, decreased food intake, lower levels of inflammation, and improvement in insulin signaling in adipose tissue. In addition to decreased SFCA production, dysbiosis also leads to increased lipid deposition through higher levels of lipoprotein lipase.27
Obesity. The bacteria in our gut affect energy metabolism. In patients with obesity, increased amounts of bacteria in the taxa Firmicutes and a corresponding decrease in Bacteroidetes is associated with an increased energy harvest and decreased SCFA production, which leads to a pro-inflammatory state.28 Probiotics that contain Bifidobacterium and Lactobacillus are thought to help correct this dysbiosis by increasing production of SCFAs.28
A recent meta-analysis of 4 RCTs found no significant difference between supplementation with probiotics and placebo on weight reduction.29 However, lower-quality studies with more subjects and longer duration have shown a statistically significant improvement in weight reduction with probiotic use compared to placebo.29
Diabetes. Although dietary interventions to improve glycemic control have long been an important cornerstone of treatment, probiotic supplementation to further alter gut flora composition is also being evaluated. Studies have found probiotics have largely beneficial effects on glycemic control, especially in animals. The largest systematic review to date looked at 33 studies, including 5 human trials. The human studies each found a significant reduction in at least one of 6 parameters of glycemic control (levels of fasting plasma glucose, postprandial blood glucose, glycated hemoglobin, insulin, insulin resistance, and onset of diabetes).16 It is unclear which probiotic strains confer benefit, and if those benefits are sustainable without dietary modification and increased physical activity.
Psychiatric illnesses. The gut-brain axis is thought to impact mental health by several mechanisms, including modulating the hypothalamic-pituitary-adrenal axis, activating the immune system, producing active metabolites, and affecting the vagus nerve. It is unclear which of these pathways may be clinically relevant.5,30 The few human studies that have looked for a potential link between gut flora and psychiatric illness have focused on depression and autism spectrum disorders (ASD).
Depression. Small studies comparing the microbiome composition of depressed patients vs healthy controls have found differences in patterns of both over- and underrepresented microbiota species in depressed patients, although the patterns across studies have been inconsistent.31,32 One small functional magnetic resonance imaging study of healthy women showed that a fermented milk product that contained probiotics affected activity in areas of the brain that control emotion and sensation.33 A few small studies have shown that patients who used probiotics had improved depression scores.34 Further studies are needed.
ASD. Children with ASD have GI disturbances—most commonly diarrhea, constipation, and/or bloating—more often than healthy controls.35,36 This association has led to speculation of a connection between the gut and brain. The microbial composition and diversity appears to be different in individuals with ASD; several studies have found an increase in Clostridia species.37
Research on probiotics for treating ASD has been primarily in preclinical models. Human studies of probiotics for ASD are lacking.38 Small studies on dietary modifications such as gluten-free and casein-free diets have had varying results; to what extent these dietary changes exert their influence via the intestinal microbiome is unknown.38
Eczema. Several studies have looked at the role of prebiotics and probiotics in reducing the risk for allergic disease. A 2013 Cochrane review found strong evidence that certain prebiotics can prevent eczema in children under age 2.15 There is limited evidence that probiotics may also play a role in preventing eczema.39,40 However, probiotics do not appear to be effective for treating eczema.41
Rheumatoid arthritis (RA). Patients with RA have a change in the balance of function of different T helper cells subsets, and several studies have shown that changes in the gut microbiome can affect this balance.42 A recent small study of patients with RA found that 75% of those with new onset RA had Prevotella copri bacteria as the predominant species, and patients with chronic RA had a decrease in Bacteroides species compared to healthy counterparts.42-44 The exact influence of gut flora dysbiosis on RA is unknown.45 Small studies suggest dietary changes may improve RA symptoms, while data on the use of probiotics to alleviate symptoms is mixed.46
What to tell patients about gut flora and health
There is increasing evidence that the gut microbiome and the genes contained therein have an impact on an individual’s health. (See TABLE 2 for additional resources.) The best preventive advice for patients and their families is to eat a diet rich in fruits and vegetables. This measure has well proven benefits beyond its potential effects on gut flora.
Correcting dysbiosis with diet or probiotics may play a role in treating chronic conditions; however, in many cases, further research is required to elucidate specific recommendations. In the meantime, given the safety profile of probiotics and dietary fiber, it is reasonable to consider using these interventions, particularly probiotics for treating IBS, ulcerative colitis, and acute infectious diarrhea; probiotics for preventing antibiotic-associated diarrhea and traveler’s diarrhea; and prebiotics for preventing eczema in high-risk infants.
CORRESPONDENCE
Jill Schneiderhan, MD, Family Medicine at Domino’s Farms, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; [email protected].
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214.
2. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17-44.
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267.
4. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-7519.
5. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404-410.
6. Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050.
7. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
8. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
9. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546,1562.
10. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1-13.
11. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
12. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801.
13. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010(11):CD003048.
14. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
15. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. The Cochrane Library. 2013. Cochrane Database Syst Rev. 2013;3:CD006474.
16. Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015. [Epub ahead of print].
17. Aguirre M, Eck A, Savelkoul PH, et al. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res Microbiol. 2015. [Epub ahead of print].
18. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958.
20. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
21. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158-179.
22. Otten JJ, Hellwig JP, Meyers LD; Institute of Medicine of the National Academies. Dietary Reference Intakes: The essential guide to nutrient requirements. 2006. US Department of Agriculture Web site. Available at: http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf. Accessed December 8, 2015.
23. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105-120.
24. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516.
25. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702.
26. Bermon S, Petriz B, Kajeniene A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70-79.
27. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198-203.
28. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628.
29. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566-575.
30. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiotabrain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984-995.
31. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194.
32. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-1162.
33. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401.
34. Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4.
35. Krajmalnik-Brown R, Lozupone C, Kang DW, et al. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914.
36. Buie T. Potential etiologic factors of microbiome disruption in autism. Clin Ther. 2015;37:976-983.
37. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 2013;25:342-353.
38. Frye RE, Slattery J, MacFabe DF, et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878.
39. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475.
40. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626-634.
41. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
42. Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696.
43. Perez-Santiago Ja, Gianella Sa, Massanella Ma, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
44. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
45. Scofield RH. Rheumatic diseases and the microbiome. Int J Rheum Dis. 2014;17:489-492.
46. Sandhya P, Danda D, Sharma D, et al. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis. 2015. [Epub ahead of print].
› Encourage patients to eat a healthy diet that includes an adequate amount of soluble fiber to maintain a healthy, diverse microbiome. B
› Recommend combination probiotics to treat symptoms of irritable bowel syndrome. A
› Encourage patients to take probiotics containing Lactobacillus species to prevent antibiotic-associated diarrhea and Saccharomyces to prevent Clostridium difficile infection. A
› Recommend probiotics containing Lactobacillus species and/or Saccharomyces to treat acute infectious diarrhea. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sheila S, age 27, has irritable bowel syndrome (IBS) and comes to your office for a follow-up visit. Over the past 6 months she has started taking a fiber supplement, drinking more water, and looking for links between stress and her symptoms. She has read about probiotics and wonders if you would consider recommending them in her situation.
CASE 2 › Mark M, age 45, has type 2 diabetes and is overweight. He is motivated to change his diet and has started to exercise more. He is taking metformin 2000 mg/d but his hemoglobin A1c remains slightly elevated at 7.2%. He heard on television that probiotics might help to keep him from needing to add another medication.
Most of the living organisms that comprise the human microbiome—all of the microbes that live on or in humans—are found in the gastrointestinal (GI) tract. The gut flora contribute 99% of the genetic material in the human body. The composition of the gut flora is remarkably diverse across the population; each individual has a unique microbial footprint. Within this microbial diversity, there appears to be a stable number of genes that are responsible for the major functions of the gut flora.1 These microbes:
- supply essential nutrients by breaking down complex carbohydrates;
- generate secondary bile acids that assist in digesting fats;2
- synthesize vitamins such as K, B12, folate, and biotin;3
- contribute to the defensive barrier in the colon by keeping pathogenic bacteria from crossing the colonic mucosa; and
- interact with our systemic immune system in a way that maintains a level of homeostasis, allowing for appropriate activation in the face of pathogens without developing autoimmunity.4
The gut flora also play a role in the communication between the central nervous system and the enteric nervous system by modulating the hormonal and neural pathways that have been labeled the “gut-brain axis.” The gut-brain axis has been associated with numerous disease states, including irritable bowel syndrome and certain psychiatric disorders.5
Researchers are investigating interventions that target the microbiome to increase microbial diversity and the presence of certain species to prevent or treat various diseases. The use of probiotics and dietary changes to increase intake of soluble fiber have been the most studied of these interventions. The thought is that these interventions can correct an imbalance, or dysbiosis, of the gut flora.6 Studies have shown that decreased microbial diversity is associated with elevations of certain disease markers (eg, adiposity, insulin, triglycerides, C–reactive protein)7 and that increases in soluble fiber lead to the greatest long-term improvement in microbial diversity.8 Fecal transplant—the transfer of a processed mixture of stool that contains “healthy” bacteria from a donor into the intestines of a patient—is being explored as a method of replacing colonic gut flora, but evidence is limited.
The following review takes a closer look at these options and identifies those that are most likely to benefit patients in the treatment—and prevention—of several diseases (TABLE 1).9-16
Evidence is best for using probiotics for digestive diseases
Dietary interventions for digestive diseases have long been studied, but are getting renewed attention for their potential impact on the microbiome.17 Beyond dietary modification, other similar treatment options include probiotics (live microorganisms thought to confer a beneficial effect on the host), prebiotics (non-digestible food ingredients, including oligosaccharides and inulin, thought to promote the growth of “helpful” gut flora), and synbiotics (combinations of the 2).18
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized by altered intestinal transit, low-grade colonic inflammation, and/or alterations in the gutbrain axis. Research has increasingly focused on recently discovered increases in intestinal immune activation, intestinal permeability, and alterations in the colonic microbiome (decreased diversity and increased pathogenic bacteria) associated with IBS.19
A meta-analysis of 43 randomized control trials (RCTs) found probiotics ranging from Lactobacillus to Saccharomyces can significantly decrease global IBS symptoms, abdominal pain, bloating, and flatulence.9 For a patient such as Ms. S, the evidence suggests a probiotic that contains a mixture of Lactobacillus and Bifidobacterium might help relieve her symptoms.9 In terms of dietary modifications, soluble fiber, which is already known to help treat IBS,20 has profound effects on improving microbiota diversity and in shifting the composition toward less pathogenic strains.21 The Institute of Medicine's daily recommended intake of soluble fiber is about 15 g/d.22
Inflammatory bowel disease (IBD) is caused by inflammation of the GI lining due to an overactive immune response. Evidence shows that patients with IBD have an altered microbial composition—specifically, an increase in bacteria that produce pro-inflammatory molecules and a decrease in bacteria that have a dampening effect on immune activation.23
Most studies evaluating probiotics as a treatment for IBD have been small and have used a wide variety of bacterial mixtures, which makes comparisons difficult. Recent meta-analyses found combination probiotics can both induce and maintain remission in patients with ulcerative colitis, but have no beneficial effects in Crohn’s disease.10 In a review of 9 case series of patients with IBD, fecal transplant reduced IBD symptoms, and patients were able to decrease medication use.24
Diarrheal illness. The human intestine is protected from diarrheal illness by healthy bacteria that block the actions of pathogenic bacteria. This mechanism is called colonization resistance. Moderate levels of evidence support the use of probiotics to prevent or treat several types of diarrheal illness.14
Antibiotic-associated diarrhea (AAD) is caused when antibiotic use alters the microbial balance. Recent meta-analyses have shown probiotics can prevent AAD and Clostridium difficile-associated diarrhea.11,12 Several case series and one RCT have found that fecal transplants are safe and efficacious for treating recurrent Clostridium difficile infection.25 Using probiotics to treat symptoms of AAD has been less studied.
Acute infectious diarrhea and traveler’s diarrhea (TD). A Cochrane review found that probiotics decreased the duration of diarrheal episodes by 25 hours, decreased the risk of an episode lasting more than 4 days by 59%, and led to one less diarrheal stool per day by the second day of the intervention.13 In a separate meta-analysis of 12 studies, probiotics significantly prevented 85% of cases of TD.14
Encouraging early evidence for several other illnesses
Metabolic disorders. Both animal and human studies support the theory that gut flora contribute to energy homeostasis, and in some genetically predisposed people dysbiosis may lead to obesity and diabetes. The traditional western diet4 and possibly decreased physical activity26 are major contributors to gut flora dysbiosis. Healthy bacteria in the gut break down soluble fiber into short chain fatty acids (SCFAs). SCFAs are associated with increased satiety, decreased food intake, lower levels of inflammation, and improvement in insulin signaling in adipose tissue. In addition to decreased SFCA production, dysbiosis also leads to increased lipid deposition through higher levels of lipoprotein lipase.27
Obesity. The bacteria in our gut affect energy metabolism. In patients with obesity, increased amounts of bacteria in the taxa Firmicutes and a corresponding decrease in Bacteroidetes is associated with an increased energy harvest and decreased SCFA production, which leads to a pro-inflammatory state.28 Probiotics that contain Bifidobacterium and Lactobacillus are thought to help correct this dysbiosis by increasing production of SCFAs.28
A recent meta-analysis of 4 RCTs found no significant difference between supplementation with probiotics and placebo on weight reduction.29 However, lower-quality studies with more subjects and longer duration have shown a statistically significant improvement in weight reduction with probiotic use compared to placebo.29
Diabetes. Although dietary interventions to improve glycemic control have long been an important cornerstone of treatment, probiotic supplementation to further alter gut flora composition is also being evaluated. Studies have found probiotics have largely beneficial effects on glycemic control, especially in animals. The largest systematic review to date looked at 33 studies, including 5 human trials. The human studies each found a significant reduction in at least one of 6 parameters of glycemic control (levels of fasting plasma glucose, postprandial blood glucose, glycated hemoglobin, insulin, insulin resistance, and onset of diabetes).16 It is unclear which probiotic strains confer benefit, and if those benefits are sustainable without dietary modification and increased physical activity.
Psychiatric illnesses. The gut-brain axis is thought to impact mental health by several mechanisms, including modulating the hypothalamic-pituitary-adrenal axis, activating the immune system, producing active metabolites, and affecting the vagus nerve. It is unclear which of these pathways may be clinically relevant.5,30 The few human studies that have looked for a potential link between gut flora and psychiatric illness have focused on depression and autism spectrum disorders (ASD).
Depression. Small studies comparing the microbiome composition of depressed patients vs healthy controls have found differences in patterns of both over- and underrepresented microbiota species in depressed patients, although the patterns across studies have been inconsistent.31,32 One small functional magnetic resonance imaging study of healthy women showed that a fermented milk product that contained probiotics affected activity in areas of the brain that control emotion and sensation.33 A few small studies have shown that patients who used probiotics had improved depression scores.34 Further studies are needed.
ASD. Children with ASD have GI disturbances—most commonly diarrhea, constipation, and/or bloating—more often than healthy controls.35,36 This association has led to speculation of a connection between the gut and brain. The microbial composition and diversity appears to be different in individuals with ASD; several studies have found an increase in Clostridia species.37
Research on probiotics for treating ASD has been primarily in preclinical models. Human studies of probiotics for ASD are lacking.38 Small studies on dietary modifications such as gluten-free and casein-free diets have had varying results; to what extent these dietary changes exert their influence via the intestinal microbiome is unknown.38
Eczema. Several studies have looked at the role of prebiotics and probiotics in reducing the risk for allergic disease. A 2013 Cochrane review found strong evidence that certain prebiotics can prevent eczema in children under age 2.15 There is limited evidence that probiotics may also play a role in preventing eczema.39,40 However, probiotics do not appear to be effective for treating eczema.41
Rheumatoid arthritis (RA). Patients with RA have a change in the balance of function of different T helper cells subsets, and several studies have shown that changes in the gut microbiome can affect this balance.42 A recent small study of patients with RA found that 75% of those with new onset RA had Prevotella copri bacteria as the predominant species, and patients with chronic RA had a decrease in Bacteroides species compared to healthy counterparts.42-44 The exact influence of gut flora dysbiosis on RA is unknown.45 Small studies suggest dietary changes may improve RA symptoms, while data on the use of probiotics to alleviate symptoms is mixed.46
What to tell patients about gut flora and health
There is increasing evidence that the gut microbiome and the genes contained therein have an impact on an individual’s health. (See TABLE 2 for additional resources.) The best preventive advice for patients and their families is to eat a diet rich in fruits and vegetables. This measure has well proven benefits beyond its potential effects on gut flora.
Correcting dysbiosis with diet or probiotics may play a role in treating chronic conditions; however, in many cases, further research is required to elucidate specific recommendations. In the meantime, given the safety profile of probiotics and dietary fiber, it is reasonable to consider using these interventions, particularly probiotics for treating IBS, ulcerative colitis, and acute infectious diarrhea; probiotics for preventing antibiotic-associated diarrhea and traveler’s diarrhea; and prebiotics for preventing eczema in high-risk infants.
CORRESPONDENCE
Jill Schneiderhan, MD, Family Medicine at Domino’s Farms, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; [email protected].
› Encourage patients to eat a healthy diet that includes an adequate amount of soluble fiber to maintain a healthy, diverse microbiome. B
› Recommend combination probiotics to treat symptoms of irritable bowel syndrome. A
› Encourage patients to take probiotics containing Lactobacillus species to prevent antibiotic-associated diarrhea and Saccharomyces to prevent Clostridium difficile infection. A
› Recommend probiotics containing Lactobacillus species and/or Saccharomyces to treat acute infectious diarrhea. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sheila S, age 27, has irritable bowel syndrome (IBS) and comes to your office for a follow-up visit. Over the past 6 months she has started taking a fiber supplement, drinking more water, and looking for links between stress and her symptoms. She has read about probiotics and wonders if you would consider recommending them in her situation.
CASE 2 › Mark M, age 45, has type 2 diabetes and is overweight. He is motivated to change his diet and has started to exercise more. He is taking metformin 2000 mg/d but his hemoglobin A1c remains slightly elevated at 7.2%. He heard on television that probiotics might help to keep him from needing to add another medication.
Most of the living organisms that comprise the human microbiome—all of the microbes that live on or in humans—are found in the gastrointestinal (GI) tract. The gut flora contribute 99% of the genetic material in the human body. The composition of the gut flora is remarkably diverse across the population; each individual has a unique microbial footprint. Within this microbial diversity, there appears to be a stable number of genes that are responsible for the major functions of the gut flora.1 These microbes:
- supply essential nutrients by breaking down complex carbohydrates;
- generate secondary bile acids that assist in digesting fats;2
- synthesize vitamins such as K, B12, folate, and biotin;3
- contribute to the defensive barrier in the colon by keeping pathogenic bacteria from crossing the colonic mucosa; and
- interact with our systemic immune system in a way that maintains a level of homeostasis, allowing for appropriate activation in the face of pathogens without developing autoimmunity.4
The gut flora also play a role in the communication between the central nervous system and the enteric nervous system by modulating the hormonal and neural pathways that have been labeled the “gut-brain axis.” The gut-brain axis has been associated with numerous disease states, including irritable bowel syndrome and certain psychiatric disorders.5
Researchers are investigating interventions that target the microbiome to increase microbial diversity and the presence of certain species to prevent or treat various diseases. The use of probiotics and dietary changes to increase intake of soluble fiber have been the most studied of these interventions. The thought is that these interventions can correct an imbalance, or dysbiosis, of the gut flora.6 Studies have shown that decreased microbial diversity is associated with elevations of certain disease markers (eg, adiposity, insulin, triglycerides, C–reactive protein)7 and that increases in soluble fiber lead to the greatest long-term improvement in microbial diversity.8 Fecal transplant—the transfer of a processed mixture of stool that contains “healthy” bacteria from a donor into the intestines of a patient—is being explored as a method of replacing colonic gut flora, but evidence is limited.
The following review takes a closer look at these options and identifies those that are most likely to benefit patients in the treatment—and prevention—of several diseases (TABLE 1).9-16
Evidence is best for using probiotics for digestive diseases
Dietary interventions for digestive diseases have long been studied, but are getting renewed attention for their potential impact on the microbiome.17 Beyond dietary modification, other similar treatment options include probiotics (live microorganisms thought to confer a beneficial effect on the host), prebiotics (non-digestible food ingredients, including oligosaccharides and inulin, thought to promote the growth of “helpful” gut flora), and synbiotics (combinations of the 2).18
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized by altered intestinal transit, low-grade colonic inflammation, and/or alterations in the gutbrain axis. Research has increasingly focused on recently discovered increases in intestinal immune activation, intestinal permeability, and alterations in the colonic microbiome (decreased diversity and increased pathogenic bacteria) associated with IBS.19
A meta-analysis of 43 randomized control trials (RCTs) found probiotics ranging from Lactobacillus to Saccharomyces can significantly decrease global IBS symptoms, abdominal pain, bloating, and flatulence.9 For a patient such as Ms. S, the evidence suggests a probiotic that contains a mixture of Lactobacillus and Bifidobacterium might help relieve her symptoms.9 In terms of dietary modifications, soluble fiber, which is already known to help treat IBS,20 has profound effects on improving microbiota diversity and in shifting the composition toward less pathogenic strains.21 The Institute of Medicine's daily recommended intake of soluble fiber is about 15 g/d.22
Inflammatory bowel disease (IBD) is caused by inflammation of the GI lining due to an overactive immune response. Evidence shows that patients with IBD have an altered microbial composition—specifically, an increase in bacteria that produce pro-inflammatory molecules and a decrease in bacteria that have a dampening effect on immune activation.23
Most studies evaluating probiotics as a treatment for IBD have been small and have used a wide variety of bacterial mixtures, which makes comparisons difficult. Recent meta-analyses found combination probiotics can both induce and maintain remission in patients with ulcerative colitis, but have no beneficial effects in Crohn’s disease.10 In a review of 9 case series of patients with IBD, fecal transplant reduced IBD symptoms, and patients were able to decrease medication use.24
Diarrheal illness. The human intestine is protected from diarrheal illness by healthy bacteria that block the actions of pathogenic bacteria. This mechanism is called colonization resistance. Moderate levels of evidence support the use of probiotics to prevent or treat several types of diarrheal illness.14
Antibiotic-associated diarrhea (AAD) is caused when antibiotic use alters the microbial balance. Recent meta-analyses have shown probiotics can prevent AAD and Clostridium difficile-associated diarrhea.11,12 Several case series and one RCT have found that fecal transplants are safe and efficacious for treating recurrent Clostridium difficile infection.25 Using probiotics to treat symptoms of AAD has been less studied.
Acute infectious diarrhea and traveler’s diarrhea (TD). A Cochrane review found that probiotics decreased the duration of diarrheal episodes by 25 hours, decreased the risk of an episode lasting more than 4 days by 59%, and led to one less diarrheal stool per day by the second day of the intervention.13 In a separate meta-analysis of 12 studies, probiotics significantly prevented 85% of cases of TD.14
Encouraging early evidence for several other illnesses
Metabolic disorders. Both animal and human studies support the theory that gut flora contribute to energy homeostasis, and in some genetically predisposed people dysbiosis may lead to obesity and diabetes. The traditional western diet4 and possibly decreased physical activity26 are major contributors to gut flora dysbiosis. Healthy bacteria in the gut break down soluble fiber into short chain fatty acids (SCFAs). SCFAs are associated with increased satiety, decreased food intake, lower levels of inflammation, and improvement in insulin signaling in adipose tissue. In addition to decreased SFCA production, dysbiosis also leads to increased lipid deposition through higher levels of lipoprotein lipase.27
Obesity. The bacteria in our gut affect energy metabolism. In patients with obesity, increased amounts of bacteria in the taxa Firmicutes and a corresponding decrease in Bacteroidetes is associated with an increased energy harvest and decreased SCFA production, which leads to a pro-inflammatory state.28 Probiotics that contain Bifidobacterium and Lactobacillus are thought to help correct this dysbiosis by increasing production of SCFAs.28
A recent meta-analysis of 4 RCTs found no significant difference between supplementation with probiotics and placebo on weight reduction.29 However, lower-quality studies with more subjects and longer duration have shown a statistically significant improvement in weight reduction with probiotic use compared to placebo.29
Diabetes. Although dietary interventions to improve glycemic control have long been an important cornerstone of treatment, probiotic supplementation to further alter gut flora composition is also being evaluated. Studies have found probiotics have largely beneficial effects on glycemic control, especially in animals. The largest systematic review to date looked at 33 studies, including 5 human trials. The human studies each found a significant reduction in at least one of 6 parameters of glycemic control (levels of fasting plasma glucose, postprandial blood glucose, glycated hemoglobin, insulin, insulin resistance, and onset of diabetes).16 It is unclear which probiotic strains confer benefit, and if those benefits are sustainable without dietary modification and increased physical activity.
Psychiatric illnesses. The gut-brain axis is thought to impact mental health by several mechanisms, including modulating the hypothalamic-pituitary-adrenal axis, activating the immune system, producing active metabolites, and affecting the vagus nerve. It is unclear which of these pathways may be clinically relevant.5,30 The few human studies that have looked for a potential link between gut flora and psychiatric illness have focused on depression and autism spectrum disorders (ASD).
Depression. Small studies comparing the microbiome composition of depressed patients vs healthy controls have found differences in patterns of both over- and underrepresented microbiota species in depressed patients, although the patterns across studies have been inconsistent.31,32 One small functional magnetic resonance imaging study of healthy women showed that a fermented milk product that contained probiotics affected activity in areas of the brain that control emotion and sensation.33 A few small studies have shown that patients who used probiotics had improved depression scores.34 Further studies are needed.
ASD. Children with ASD have GI disturbances—most commonly diarrhea, constipation, and/or bloating—more often than healthy controls.35,36 This association has led to speculation of a connection between the gut and brain. The microbial composition and diversity appears to be different in individuals with ASD; several studies have found an increase in Clostridia species.37
Research on probiotics for treating ASD has been primarily in preclinical models. Human studies of probiotics for ASD are lacking.38 Small studies on dietary modifications such as gluten-free and casein-free diets have had varying results; to what extent these dietary changes exert their influence via the intestinal microbiome is unknown.38
Eczema. Several studies have looked at the role of prebiotics and probiotics in reducing the risk for allergic disease. A 2013 Cochrane review found strong evidence that certain prebiotics can prevent eczema in children under age 2.15 There is limited evidence that probiotics may also play a role in preventing eczema.39,40 However, probiotics do not appear to be effective for treating eczema.41
Rheumatoid arthritis (RA). Patients with RA have a change in the balance of function of different T helper cells subsets, and several studies have shown that changes in the gut microbiome can affect this balance.42 A recent small study of patients with RA found that 75% of those with new onset RA had Prevotella copri bacteria as the predominant species, and patients with chronic RA had a decrease in Bacteroides species compared to healthy counterparts.42-44 The exact influence of gut flora dysbiosis on RA is unknown.45 Small studies suggest dietary changes may improve RA symptoms, while data on the use of probiotics to alleviate symptoms is mixed.46
What to tell patients about gut flora and health
There is increasing evidence that the gut microbiome and the genes contained therein have an impact on an individual’s health. (See TABLE 2 for additional resources.) The best preventive advice for patients and their families is to eat a diet rich in fruits and vegetables. This measure has well proven benefits beyond its potential effects on gut flora.
Correcting dysbiosis with diet or probiotics may play a role in treating chronic conditions; however, in many cases, further research is required to elucidate specific recommendations. In the meantime, given the safety profile of probiotics and dietary fiber, it is reasonable to consider using these interventions, particularly probiotics for treating IBS, ulcerative colitis, and acute infectious diarrhea; probiotics for preventing antibiotic-associated diarrhea and traveler’s diarrhea; and prebiotics for preventing eczema in high-risk infants.
CORRESPONDENCE
Jill Schneiderhan, MD, Family Medicine at Domino’s Farms, 24 Frank Lloyd Wright Dr., Lobby H, Suite 2300, Ann Arbor, MI 48105; [email protected].
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214.
2. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17-44.
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267.
4. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-7519.
5. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404-410.
6. Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050.
7. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
8. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
9. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546,1562.
10. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1-13.
11. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
12. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801.
13. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010(11):CD003048.
14. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
15. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. The Cochrane Library. 2013. Cochrane Database Syst Rev. 2013;3:CD006474.
16. Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015. [Epub ahead of print].
17. Aguirre M, Eck A, Savelkoul PH, et al. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res Microbiol. 2015. [Epub ahead of print].
18. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958.
20. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
21. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158-179.
22. Otten JJ, Hellwig JP, Meyers LD; Institute of Medicine of the National Academies. Dietary Reference Intakes: The essential guide to nutrient requirements. 2006. US Department of Agriculture Web site. Available at: http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf. Accessed December 8, 2015.
23. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105-120.
24. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516.
25. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702.
26. Bermon S, Petriz B, Kajeniene A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70-79.
27. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198-203.
28. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628.
29. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566-575.
30. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiotabrain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984-995.
31. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194.
32. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-1162.
33. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401.
34. Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4.
35. Krajmalnik-Brown R, Lozupone C, Kang DW, et al. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914.
36. Buie T. Potential etiologic factors of microbiome disruption in autism. Clin Ther. 2015;37:976-983.
37. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 2013;25:342-353.
38. Frye RE, Slattery J, MacFabe DF, et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878.
39. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475.
40. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626-634.
41. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
42. Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696.
43. Perez-Santiago Ja, Gianella Sa, Massanella Ma, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
44. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
45. Scofield RH. Rheumatic diseases and the microbiome. Int J Rheum Dis. 2014;17:489-492.
46. Sandhya P, Danda D, Sharma D, et al. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis. 2015. [Epub ahead of print].
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207-214.
2. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17-44.
3. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267.
4. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-7519.
5. Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes. 2014;5:404-410.
6. Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050.
7. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
8. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
9. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561; quiz 1546,1562.
10. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1-13.
11. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
12. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801.
13. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010(11):CD003048.
14. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
15. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. The Cochrane Library. 2013. Cochrane Database Syst Rev. 2013;3:CD006474.
16. Razmpoosh E, Javadi M, Ejtahed HS, et al. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev. 2015. [Epub ahead of print].
17. Aguirre M, Eck A, Savelkoul PH, et al. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Res Microbiol. 2015. [Epub ahead of print].
18. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
19. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958.
20. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367-1374.
21. Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158-179.
22. Otten JJ, Hellwig JP, Meyers LD; Institute of Medicine of the National Academies. Dietary Reference Intakes: The essential guide to nutrient requirements. 2006. US Department of Agriculture Web site. Available at: http://www.nal.usda.gov/fnic/DRI/Essential_Guide/DRIEssentialGuideNutReq.pdf. Accessed December 8, 2015.
23. Hansen JJ, Sartor RB. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr Treat Options Gastroenterol. 2015;13:105-120.
24. Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516.
25. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702.
26. Bermon S, Petriz B, Kajeniene A, et al. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70-79.
27. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198-203.
28. Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59:617-628.
29. Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35:566-575.
30. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiotabrain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984-995.
31. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194.
32. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155-1162.
33. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401.
34. Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III - convergence toward clinical trials. Gut Pathog. 2013;5:4.
35. Krajmalnik-Brown R, Lozupone C, Kang DW, et al. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914.
36. Buie T. Potential etiologic factors of microbiome disruption in autism. Clin Ther. 2015;37:976-983.
37. Cao X, Lin P, Jiang P, et al. Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry. 2013;25:342-353.
38. Frye RE, Slattery J, MacFabe DF, et al. Approaches to studying and manipulating the enteric microbiome to improve autism symptoms. Microb Ecol Health Dis. 2015;26:26878.
39. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;(4):CD006475.
40. Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626-634.
41. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;(4):CD006135.
42. Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696.
43. Perez-Santiago Ja, Gianella Sa, Massanella Ma, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
44. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
45. Scofield RH. Rheumatic diseases and the microbiome. Int J Rheum Dis. 2014;17:489-492.
46. Sandhya P, Danda D, Sharma D, et al. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis. 2015. [Epub ahead of print].
HIV prevention: A 3-pronged approach
› Screen all pregnant women and individuals ages 15 to 65 for human immunodeficiency virus (HIV) infection. A
› Prescribe tenofovir disoproxil fumarate/emtricitabine (Truvada) for pre-exposure prophylaxis for patients at high risk of acquiring HIV. A
› Offer needle and syringe exchange programs and, when appropriate, opioid substitution therapy to individuals who inject drugs. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite advances in human immunodeficiency virus (HIV) screening and treatment over the last 30 years, HIV remains a public health concern. In the United States, after an initial decline, total HIV incidence has failed to significantly decrease in the last 25 years. More than 1.2 million people are living with HIV in the United States, and 12.8% of them (156,300) are unaware they are affected.1 Of those diagnosed with HIV, only 30% are receiving treatment and are virally suppressed.2 Due to structural inequalities and psychosocial factors, African American and Latino patients remain disproportionately affected.3 The incidence of HIV infection among men who have sex with men has increased, and the incidence of HIV infection among people who inject drugs has plateaued after years of progressive decline.4
HIV prevention strategies are highly effective, but in general are underutilized. This article reviews 3 prevention strategies that can be administered by family physicians: HIV screening, pre-exposure prophylaxis (PrEP), and harm reduction.
Who and how to screen for HIV
Early identification of HIV infection generally leads to reduced transmission because diagnosis is associated with decreases in risky behavior.5,6 In addition, antiretroviral therapy (ART) is more effective when initiated early, before the development of advanced immunosuppression.7-9
The “window period” of acute HIV infection (AHI) is the time from when the virus is transmitted to when markers of infection can be detected. Because this window period is associated with high viral transmission rates, family physicians must be familiar with symptoms of AHI (TABLE 1)10,11 and associated risk factors (eg, recent condomless sex or sharing of drug injection equipment with someone who is HIV-positive or of unknown HIV status).
Screening for HIV solely based on the presence of risk factors or clinical symptoms is not enough, however. The United States Preventive Services Task Force (USPSTF) recommends screening all pregnant women and individuals ages 15 to 65 for HIV.12 Screening based solely on risk factors or clinical symptoms frequently leads to missed diagnoses and identification of HIV infection at more advanced stages.13,14 Both the USPSTF and the Centers for Disease Control and Prevention (CDC) recommend universal opt-out screening (patients are informed that HIV screening will be performed and that they may decline testing) because such screening identifies HIV earlier and is associated with higher testing rates than opt-in screening, which requires explicit written consent and extensive pre-test counseling.
Which test to use. HIV screening with a fourth-generation antigen/antibody combination immunoassay—which detects both HIV p24 antigen and HIV antibodies—provides greater diagnostic accuracy than older tests.15 These newer tests detect HIV approximately 15 days after initial infection, reducing the window period of AHI.15,16 If you suspect a patient has AHI, consider early repeat HIV testing with a fourth-generation assay, or initial co-testing with a fourth-generation assay and a nucleic acid amplification test for HIV RNA, which makes it possible to detect infection approximately 5 days earlier than fourth-generation assays.15
Offer pre-exposure prophylaxis to high-risk patients
PrEP is the use of ART prior to HIV exposure to prevent transmission of the virus. It should be used with conventional risk reduction strategies, such as providing condoms, counseling patients about reducing risky behaviors, supporting medication adherence, and screening for and treating other sexually transmitted infections.
The US Food and Drug Administration (FDA) has approved only one medication, Truvada (tenofovir disoproxil fumarate/emtricitabine; TDF/FTC), for use as PrEP. Oral tenofovir-based regimens can effectively prevent HIV transmission,17-20 and strong adherence is associated with a risk reduction of 90% to 100%.17-23 The protective effect of oral PrEP is particularly strong in high-risk populations (eg, men who have sex with men, people who inject drugs), where the number needed to treat to prevent one HIV infection ranges from 12 to 100, depending on the patients’ risk profile.24-26 The CDC and Department of Health and Human Services have issued guidelines for using PrEP in high-risk patients.27
Barriers to implementing PrEP. Despite being highly effective, PrEP is not routinely prescribed to high-risk patients; modeling suggests that current use of PrEP is insufficient to significantly impact the incidence of HIV.28 Demand for PrEP is high among target groups,21,29,30 but patients have expressed concerns about adverse effects31 and stigma related to ART, HIV, and being at risk for HIV.32,33 Young age, lack of social support, low perception of risk, and failure to show up for appointments are also barriers to PrEP use.28,30,34
Some physicians have expressed concern that prescribing PrEP may promote high-risk sexual behavior.35 However, because PrEP is most beneficial in individuals who already engage in high-risk sexual behavior, strategic delivery of PrEP remains an effective risk-reducing strategy.17,18,21,26,36,37 Even in instances where PrEP has been associated with higher-risk sexual behavior and higher rates of sexually transmitted infections, it still prevents as much as 100% of new HIV infections.38
Fear of drug resistance also contributes to slow implementation of PrEP. Drug resistance has been observed in clinical trials of PrEP, but it has been exceedingly rare and predominantly limited to patients who had unrecognized AHI when they started PrEP.39 Furthermore, the few cases of drug resistance attributable to PrEP pale in comparison to the large number of estimated HIV infections averted—infections that would require lifelong ART with its own associated risks of drug resistance. By decreasing HIV transmission, PrEP is expected to decrease total drug resistance.40
Cost is another obstacle. Truvada costs approximately $1,540 per month.41 However, analysis has demonstrated that PrEP is cost-effective when targeted to high-risk patients.42 Most insurance plans cover PrEP, but often require high deductibles and copays; fortunately, this financial burden for patients can be mitigated or eliminated by co-pay assistance programs. The manufacturer of Truvada offers assistance programs for both insured and uninsured patients who are candidates for PrEP; details are available at http://www.truvada.com/truvada-patient-assistance.
Stigma has historically burdened individuals who seek to protect their sexual health, including HIV-negative individuals who are candidates for PrEP. Stigma surrounding HIV may decrease ART-based HIV prevention in men who have sex with men,43 while increasing high-risk behaviors44 and all-cause mortality.45
The resources listed in TABLE 2 can help physicians overcome some of the barriers to implementing PrEP.
How to deliver PrEP
Whether HIV specialists or primary care clinicians are best suited to provide PrEP is a subject of debate. HIV specialists are most familiar with ART and routine monitoring of adherence; however, they have less access to HIV-negative patients, who are the target group for PrEP.35 Family physicians tend to work in closer proximity and maintain longitudinal relationships with PrEP target groups, but in general have less experience with ART and evaluating AHI. Some may argue that competing demands may make it impractical to take a detailed sexual history during a primary care visit.46 In truth, both HIV specialists and family physicians can be appropriately equipped to provide PrEP.
TABLE 3 outlines the steps necessary to provide a patient with PrEP.47 Assessing risk is the initial step; PrEP is beneficial for patients who have one or more risk factors for HIV infection. To be eligible for TDF/FTC, a patient must be HIV-negative, and should be tested for hepatitis B virus (HBV) infection and kidney disease. Because TDF/FTC treats HBV infection, candidates for PrEP who test positive for HBV should be evaluated for treatment of HBV before initiating PrEP. Candidates for PrEP who test negative for HBV infection and immunity should be vaccinated.
Candidates for PrEP should also be screened and monitored for kidney disease. TDF can cause increased serum creatinine due to tubular toxicity. A patient who has an estimated creatinine clearance <60 mL/min should not receive TDF/FTC for PrEP. If a patient’s estimated creatinine clearance falls below 60 mL/min or serum creatinine increases by 0.3 mg/dL above baseline after PrEP is started, TDF/FTC should be discontinued, and the patient should be evaluated for the underlying cause of the kidney disease.27
Before starting PrEP, candidates should be screened for HIV infection and symptoms of AHI. Strongly consider testing for sexually transmitted infections that may increase the risk of HIV transmission, such as syphilis, gonorrhea, or chlamydia.
Candidates who are eligible for PrEP must be counseled on medication adverse effects, adherence strategies, and symptoms of sexually transmitted infections. To initiate PrEP, candidates may be given a one-month supply of TDF/FTC; adherence, adverse effects, and other risk-reduction strategies are assessed at an office visit 3 to 4 weeks later. Subsequent prescriptions are then dispensed as a 3-month supply, with office visits to monitor PrEP scheduled for at least once every 3 months. During these monitoring visits, evaluate the patient’s HIV status, pregnancy status, adherence, adverse effects, risk-reduction behaviors, and indications for continued PrEP. Every 6 months, renal function and sexually transmitted infection status should be reassessed.
Reducing risk of harm among patients who inject drugs
Nonsexual transmission of HIV is a route of high infectivity.48 It includes transfusion of infected blood, sharing of equipment during injection drug use, and percutaneous needle sticks. Sharing of equipment during injection drug use is estimated to account for 8% of new infections in the United States.4
Harm reduction is a collection of strategies meant to reduce complications of illicit drug use, including HIV transmission. These strategies include needle and syringe programs that provide injection drug users with sterile equipment, and opioid substitution therapy.
Needle and syringe programs decrease HIV transmission49 and risky behaviors related to injection drug use,50 but federal funding of such programs is prohibited. Opioid substitution therapy reduces the incidence of HIV,50,51 injection drug use, sharing of drug preparation and injection equipment, and drug-related behaviors associated with a high risk of HIV transmission.50,52 However, in the United States, the quality of these programs varies; a study of opioid substitution therapy delivery found that 22.8% of programs provided doses that were too low to be effective.53
FDA-approved medications for opioid substitution therapy include sublingual buprenorphine, sublingual buprenorphine/naloxone tablets or strips (Suboxone), and oral methadone. Buprenorphine-based regimens can be provided by appropriately trained primary care clinicians; methadone requires a referral to a narcotic treatment program. TABLE 4 provides training and support resources for physicians who want to integrate opioid substitution therapy into their clinical practice.
CORRESPONDENCE
Richard Moore II, MD, 250 Smith Church Road, Roanoke Rapids, NC 27870; [email protected].
1. Hall HI, An Q, Tang T, et al; Centers for Disease Control and Prevention (CDC). Prevalence of diagnosed and undiagnosed HIV infection--United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2015;64:657-662.
2. Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113-1117.
3. Maulsby C, Millet G, Lindsey K, et al. HIV among black men who have sex with men (MSM) in the United States: a review of the literature. AIDS Behav. 2014;18:10-25.
4. Centers for Disease Control and Prevention. Estimated HIV incidence among adults and adolescents in the United States, 2007-2010, HIV Surveillance Supplemental Report. 2012. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed October 8, 2015.
5. Cleary PD, Van Devanter N, Rogers TF, et al. Behavior changes after notification of HIV infection. Am J Public Health. 1991;81:1586-1590.
6. Higgins DL, Galavotti C, O’Reilly KR, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266:2419-2429.
7. Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17-26.
8. Palella FJ Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4 cell strata. Ann Intern Med. 2003;138:620-626.
9. INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
10. Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:10-15.
11. Tindall B, Barker S, Donovan B, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148:945-949.
12. Moyer V, US Preventative Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
13. Jenkins T, Gardner E, Thrun M, et al. Risk-based HIV testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329-333.
14. Klein D, Hurley LB, Merrill D, et al. Review of medical encounters in the 5 years before a diagnosis of HIV-1 infection: implications for early detection. J Acquir Immune Defic Syndr. 2003;32:143-152.
15. Pandori M, Hackett J Jr, Louie B, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol. 2009;47:2639-2642.
16. Branson BM. The future of HIV testing. J Acquir Imm Defic Syndr. 2010;55 Suppl 2:S102-S105.
17. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
18. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
19. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
20. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, doubleblind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083-2090.
21. Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820-829.
22. Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125.
23. Henderson FL, Taylor AW, Chirwa LI, et al. Characteristics and oral PrEP adherence in the TDF2 open-label extension in Botswana. Paper presented at International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 1922, 2015; Vancouver, Canada.
24. Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27:2155-2160.
25. Heffron R, Mugo N, Were E, et al. Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS. 2014;28:2771-2776.
26. Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14:468-475.
27. Center for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed June 18, 2015.
28. Grant RM. Scale-up of preexposure prophylaxis in San Francisco to impact HIV incidence. Abstract 25. Paper presented at Conference on Retroviruses and Opportunistic Infections; February 23-26, 2015; Seattle, WA.
29. Cohen SE, Vittinghoff E, Bacon O, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68:439-448.
30. Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511.
31. Gilmore H, Koester K, Liu A, et al. To PrEP or not to PrEP: Perspectives from US iPrEx open label extension (OLE) participants. Abstract 440. Paper presented at 9th International Conference on HIV Treatment and Prevention Adherence; June 9, 2014; Miami Beach, FL.
32. Jain S, Gregor C, Krakower D, et al. Attitudes and interest toward HIV pre-exposure prophylaxis (PrEP) among participants using HIV non-occupational post-exposure prophylaxis (NPEP). Poster Abstract 1523. Poster presented at Infectious Disease Society of America Conference; October 8-12, 2014; Philadelphia, PA.
33. van der Straten A, Stadler J, Luecke E, et al; VOICE-C Study Team, Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17:19146.
34. Corneli AL, McKenna K, Headley J, et al; FEM-PrEP Study Group. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc. 2014;17:19152.
35. Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014;18:1712-1721.
36. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015. [Epub ahead of print].
37. Mugwanya KK, Donnell D, Celum C, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013;13:1021-1028.
38. Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61:1601-1603.
39. Lehman DA, Baeten JM, McCoy CO, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
40. Supervie V, Garcia-Lerma JG, Heneine W, et al. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci U S A. 2010;107:12381-12386.
41. AIDSinfo. Cost considerations and antiretroviral therapy. AIDSinfo Web site. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/459/cost-considerations-and-antiretroviral-therapy. Accessed December 14, 2015.
42. Gomez GB, Borquez A, Case KK, et al. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10:e1001401.
43. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
44. Hatzenbuehler ML, O’Cleirigh C, Mayer KH, et al. Prospective associations between HIV-related stigma, transmission risk behaviors, and adverse mental health outcomes in men who have sex with men. Ann Behav Med. 2011;42:227-234.
45. Hatzenbuehler ML, Bellatorre A, Lee Y, et al. Structural stigma and all-cause mortality in sexual minority populations. Soc Sci Med. 2014;103:33-41.
46. Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One. 2012;7:e40603.
47. North Carolina AIDS Training and Education Center. For PrEP Providers. North Carolina AIDS Training and Education Center Web site. Available at: http://www.med.unc.edu/ncaidstraining/prep/for-providers/for-prep-prescribers. Accessed July 7, 2015.
48. Patel P, Borkowf CB, Brook JT, et al. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509-1519.
49. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and metaanalysis. Int J Epidemiol. 2014;43:235-248.
50. MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34-52.
51. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945.
52. Gowing L, Farrell MF, Bornemann R, et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;(8):CD004145.
53. D’Aunno T, Pollack HA, Frimpong JA, et al. Evidence-based treatment for opioid disorders: a 23-year national study of methadone dose levels. J Subst Abuse Treat. 2014;47:245-250.
› Screen all pregnant women and individuals ages 15 to 65 for human immunodeficiency virus (HIV) infection. A
› Prescribe tenofovir disoproxil fumarate/emtricitabine (Truvada) for pre-exposure prophylaxis for patients at high risk of acquiring HIV. A
› Offer needle and syringe exchange programs and, when appropriate, opioid substitution therapy to individuals who inject drugs. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite advances in human immunodeficiency virus (HIV) screening and treatment over the last 30 years, HIV remains a public health concern. In the United States, after an initial decline, total HIV incidence has failed to significantly decrease in the last 25 years. More than 1.2 million people are living with HIV in the United States, and 12.8% of them (156,300) are unaware they are affected.1 Of those diagnosed with HIV, only 30% are receiving treatment and are virally suppressed.2 Due to structural inequalities and psychosocial factors, African American and Latino patients remain disproportionately affected.3 The incidence of HIV infection among men who have sex with men has increased, and the incidence of HIV infection among people who inject drugs has plateaued after years of progressive decline.4
HIV prevention strategies are highly effective, but in general are underutilized. This article reviews 3 prevention strategies that can be administered by family physicians: HIV screening, pre-exposure prophylaxis (PrEP), and harm reduction.
Who and how to screen for HIV
Early identification of HIV infection generally leads to reduced transmission because diagnosis is associated with decreases in risky behavior.5,6 In addition, antiretroviral therapy (ART) is more effective when initiated early, before the development of advanced immunosuppression.7-9
The “window period” of acute HIV infection (AHI) is the time from when the virus is transmitted to when markers of infection can be detected. Because this window period is associated with high viral transmission rates, family physicians must be familiar with symptoms of AHI (TABLE 1)10,11 and associated risk factors (eg, recent condomless sex or sharing of drug injection equipment with someone who is HIV-positive or of unknown HIV status).
Screening for HIV solely based on the presence of risk factors or clinical symptoms is not enough, however. The United States Preventive Services Task Force (USPSTF) recommends screening all pregnant women and individuals ages 15 to 65 for HIV.12 Screening based solely on risk factors or clinical symptoms frequently leads to missed diagnoses and identification of HIV infection at more advanced stages.13,14 Both the USPSTF and the Centers for Disease Control and Prevention (CDC) recommend universal opt-out screening (patients are informed that HIV screening will be performed and that they may decline testing) because such screening identifies HIV earlier and is associated with higher testing rates than opt-in screening, which requires explicit written consent and extensive pre-test counseling.
Which test to use. HIV screening with a fourth-generation antigen/antibody combination immunoassay—which detects both HIV p24 antigen and HIV antibodies—provides greater diagnostic accuracy than older tests.15 These newer tests detect HIV approximately 15 days after initial infection, reducing the window period of AHI.15,16 If you suspect a patient has AHI, consider early repeat HIV testing with a fourth-generation assay, or initial co-testing with a fourth-generation assay and a nucleic acid amplification test for HIV RNA, which makes it possible to detect infection approximately 5 days earlier than fourth-generation assays.15
Offer pre-exposure prophylaxis to high-risk patients
PrEP is the use of ART prior to HIV exposure to prevent transmission of the virus. It should be used with conventional risk reduction strategies, such as providing condoms, counseling patients about reducing risky behaviors, supporting medication adherence, and screening for and treating other sexually transmitted infections.
The US Food and Drug Administration (FDA) has approved only one medication, Truvada (tenofovir disoproxil fumarate/emtricitabine; TDF/FTC), for use as PrEP. Oral tenofovir-based regimens can effectively prevent HIV transmission,17-20 and strong adherence is associated with a risk reduction of 90% to 100%.17-23 The protective effect of oral PrEP is particularly strong in high-risk populations (eg, men who have sex with men, people who inject drugs), where the number needed to treat to prevent one HIV infection ranges from 12 to 100, depending on the patients’ risk profile.24-26 The CDC and Department of Health and Human Services have issued guidelines for using PrEP in high-risk patients.27
Barriers to implementing PrEP. Despite being highly effective, PrEP is not routinely prescribed to high-risk patients; modeling suggests that current use of PrEP is insufficient to significantly impact the incidence of HIV.28 Demand for PrEP is high among target groups,21,29,30 but patients have expressed concerns about adverse effects31 and stigma related to ART, HIV, and being at risk for HIV.32,33 Young age, lack of social support, low perception of risk, and failure to show up for appointments are also barriers to PrEP use.28,30,34
Some physicians have expressed concern that prescribing PrEP may promote high-risk sexual behavior.35 However, because PrEP is most beneficial in individuals who already engage in high-risk sexual behavior, strategic delivery of PrEP remains an effective risk-reducing strategy.17,18,21,26,36,37 Even in instances where PrEP has been associated with higher-risk sexual behavior and higher rates of sexually transmitted infections, it still prevents as much as 100% of new HIV infections.38
Fear of drug resistance also contributes to slow implementation of PrEP. Drug resistance has been observed in clinical trials of PrEP, but it has been exceedingly rare and predominantly limited to patients who had unrecognized AHI when they started PrEP.39 Furthermore, the few cases of drug resistance attributable to PrEP pale in comparison to the large number of estimated HIV infections averted—infections that would require lifelong ART with its own associated risks of drug resistance. By decreasing HIV transmission, PrEP is expected to decrease total drug resistance.40
Cost is another obstacle. Truvada costs approximately $1,540 per month.41 However, analysis has demonstrated that PrEP is cost-effective when targeted to high-risk patients.42 Most insurance plans cover PrEP, but often require high deductibles and copays; fortunately, this financial burden for patients can be mitigated or eliminated by co-pay assistance programs. The manufacturer of Truvada offers assistance programs for both insured and uninsured patients who are candidates for PrEP; details are available at http://www.truvada.com/truvada-patient-assistance.
Stigma has historically burdened individuals who seek to protect their sexual health, including HIV-negative individuals who are candidates for PrEP. Stigma surrounding HIV may decrease ART-based HIV prevention in men who have sex with men,43 while increasing high-risk behaviors44 and all-cause mortality.45
The resources listed in TABLE 2 can help physicians overcome some of the barriers to implementing PrEP.
How to deliver PrEP
Whether HIV specialists or primary care clinicians are best suited to provide PrEP is a subject of debate. HIV specialists are most familiar with ART and routine monitoring of adherence; however, they have less access to HIV-negative patients, who are the target group for PrEP.35 Family physicians tend to work in closer proximity and maintain longitudinal relationships with PrEP target groups, but in general have less experience with ART and evaluating AHI. Some may argue that competing demands may make it impractical to take a detailed sexual history during a primary care visit.46 In truth, both HIV specialists and family physicians can be appropriately equipped to provide PrEP.
TABLE 3 outlines the steps necessary to provide a patient with PrEP.47 Assessing risk is the initial step; PrEP is beneficial for patients who have one or more risk factors for HIV infection. To be eligible for TDF/FTC, a patient must be HIV-negative, and should be tested for hepatitis B virus (HBV) infection and kidney disease. Because TDF/FTC treats HBV infection, candidates for PrEP who test positive for HBV should be evaluated for treatment of HBV before initiating PrEP. Candidates for PrEP who test negative for HBV infection and immunity should be vaccinated.
Candidates for PrEP should also be screened and monitored for kidney disease. TDF can cause increased serum creatinine due to tubular toxicity. A patient who has an estimated creatinine clearance <60 mL/min should not receive TDF/FTC for PrEP. If a patient’s estimated creatinine clearance falls below 60 mL/min or serum creatinine increases by 0.3 mg/dL above baseline after PrEP is started, TDF/FTC should be discontinued, and the patient should be evaluated for the underlying cause of the kidney disease.27
Before starting PrEP, candidates should be screened for HIV infection and symptoms of AHI. Strongly consider testing for sexually transmitted infections that may increase the risk of HIV transmission, such as syphilis, gonorrhea, or chlamydia.
Candidates who are eligible for PrEP must be counseled on medication adverse effects, adherence strategies, and symptoms of sexually transmitted infections. To initiate PrEP, candidates may be given a one-month supply of TDF/FTC; adherence, adverse effects, and other risk-reduction strategies are assessed at an office visit 3 to 4 weeks later. Subsequent prescriptions are then dispensed as a 3-month supply, with office visits to monitor PrEP scheduled for at least once every 3 months. During these monitoring visits, evaluate the patient’s HIV status, pregnancy status, adherence, adverse effects, risk-reduction behaviors, and indications for continued PrEP. Every 6 months, renal function and sexually transmitted infection status should be reassessed.
Reducing risk of harm among patients who inject drugs
Nonsexual transmission of HIV is a route of high infectivity.48 It includes transfusion of infected blood, sharing of equipment during injection drug use, and percutaneous needle sticks. Sharing of equipment during injection drug use is estimated to account for 8% of new infections in the United States.4
Harm reduction is a collection of strategies meant to reduce complications of illicit drug use, including HIV transmission. These strategies include needle and syringe programs that provide injection drug users with sterile equipment, and opioid substitution therapy.
Needle and syringe programs decrease HIV transmission49 and risky behaviors related to injection drug use,50 but federal funding of such programs is prohibited. Opioid substitution therapy reduces the incidence of HIV,50,51 injection drug use, sharing of drug preparation and injection equipment, and drug-related behaviors associated with a high risk of HIV transmission.50,52 However, in the United States, the quality of these programs varies; a study of opioid substitution therapy delivery found that 22.8% of programs provided doses that were too low to be effective.53
FDA-approved medications for opioid substitution therapy include sublingual buprenorphine, sublingual buprenorphine/naloxone tablets or strips (Suboxone), and oral methadone. Buprenorphine-based regimens can be provided by appropriately trained primary care clinicians; methadone requires a referral to a narcotic treatment program. TABLE 4 provides training and support resources for physicians who want to integrate opioid substitution therapy into their clinical practice.
CORRESPONDENCE
Richard Moore II, MD, 250 Smith Church Road, Roanoke Rapids, NC 27870; [email protected].
› Screen all pregnant women and individuals ages 15 to 65 for human immunodeficiency virus (HIV) infection. A
› Prescribe tenofovir disoproxil fumarate/emtricitabine (Truvada) for pre-exposure prophylaxis for patients at high risk of acquiring HIV. A
› Offer needle and syringe exchange programs and, when appropriate, opioid substitution therapy to individuals who inject drugs. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite advances in human immunodeficiency virus (HIV) screening and treatment over the last 30 years, HIV remains a public health concern. In the United States, after an initial decline, total HIV incidence has failed to significantly decrease in the last 25 years. More than 1.2 million people are living with HIV in the United States, and 12.8% of them (156,300) are unaware they are affected.1 Of those diagnosed with HIV, only 30% are receiving treatment and are virally suppressed.2 Due to structural inequalities and psychosocial factors, African American and Latino patients remain disproportionately affected.3 The incidence of HIV infection among men who have sex with men has increased, and the incidence of HIV infection among people who inject drugs has plateaued after years of progressive decline.4
HIV prevention strategies are highly effective, but in general are underutilized. This article reviews 3 prevention strategies that can be administered by family physicians: HIV screening, pre-exposure prophylaxis (PrEP), and harm reduction.
Who and how to screen for HIV
Early identification of HIV infection generally leads to reduced transmission because diagnosis is associated with decreases in risky behavior.5,6 In addition, antiretroviral therapy (ART) is more effective when initiated early, before the development of advanced immunosuppression.7-9
The “window period” of acute HIV infection (AHI) is the time from when the virus is transmitted to when markers of infection can be detected. Because this window period is associated with high viral transmission rates, family physicians must be familiar with symptoms of AHI (TABLE 1)10,11 and associated risk factors (eg, recent condomless sex or sharing of drug injection equipment with someone who is HIV-positive or of unknown HIV status).
Screening for HIV solely based on the presence of risk factors or clinical symptoms is not enough, however. The United States Preventive Services Task Force (USPSTF) recommends screening all pregnant women and individuals ages 15 to 65 for HIV.12 Screening based solely on risk factors or clinical symptoms frequently leads to missed diagnoses and identification of HIV infection at more advanced stages.13,14 Both the USPSTF and the Centers for Disease Control and Prevention (CDC) recommend universal opt-out screening (patients are informed that HIV screening will be performed and that they may decline testing) because such screening identifies HIV earlier and is associated with higher testing rates than opt-in screening, which requires explicit written consent and extensive pre-test counseling.
Which test to use. HIV screening with a fourth-generation antigen/antibody combination immunoassay—which detects both HIV p24 antigen and HIV antibodies—provides greater diagnostic accuracy than older tests.15 These newer tests detect HIV approximately 15 days after initial infection, reducing the window period of AHI.15,16 If you suspect a patient has AHI, consider early repeat HIV testing with a fourth-generation assay, or initial co-testing with a fourth-generation assay and a nucleic acid amplification test for HIV RNA, which makes it possible to detect infection approximately 5 days earlier than fourth-generation assays.15
Offer pre-exposure prophylaxis to high-risk patients
PrEP is the use of ART prior to HIV exposure to prevent transmission of the virus. It should be used with conventional risk reduction strategies, such as providing condoms, counseling patients about reducing risky behaviors, supporting medication adherence, and screening for and treating other sexually transmitted infections.
The US Food and Drug Administration (FDA) has approved only one medication, Truvada (tenofovir disoproxil fumarate/emtricitabine; TDF/FTC), for use as PrEP. Oral tenofovir-based regimens can effectively prevent HIV transmission,17-20 and strong adherence is associated with a risk reduction of 90% to 100%.17-23 The protective effect of oral PrEP is particularly strong in high-risk populations (eg, men who have sex with men, people who inject drugs), where the number needed to treat to prevent one HIV infection ranges from 12 to 100, depending on the patients’ risk profile.24-26 The CDC and Department of Health and Human Services have issued guidelines for using PrEP in high-risk patients.27
Barriers to implementing PrEP. Despite being highly effective, PrEP is not routinely prescribed to high-risk patients; modeling suggests that current use of PrEP is insufficient to significantly impact the incidence of HIV.28 Demand for PrEP is high among target groups,21,29,30 but patients have expressed concerns about adverse effects31 and stigma related to ART, HIV, and being at risk for HIV.32,33 Young age, lack of social support, low perception of risk, and failure to show up for appointments are also barriers to PrEP use.28,30,34
Some physicians have expressed concern that prescribing PrEP may promote high-risk sexual behavior.35 However, because PrEP is most beneficial in individuals who already engage in high-risk sexual behavior, strategic delivery of PrEP remains an effective risk-reducing strategy.17,18,21,26,36,37 Even in instances where PrEP has been associated with higher-risk sexual behavior and higher rates of sexually transmitted infections, it still prevents as much as 100% of new HIV infections.38
Fear of drug resistance also contributes to slow implementation of PrEP. Drug resistance has been observed in clinical trials of PrEP, but it has been exceedingly rare and predominantly limited to patients who had unrecognized AHI when they started PrEP.39 Furthermore, the few cases of drug resistance attributable to PrEP pale in comparison to the large number of estimated HIV infections averted—infections that would require lifelong ART with its own associated risks of drug resistance. By decreasing HIV transmission, PrEP is expected to decrease total drug resistance.40
Cost is another obstacle. Truvada costs approximately $1,540 per month.41 However, analysis has demonstrated that PrEP is cost-effective when targeted to high-risk patients.42 Most insurance plans cover PrEP, but often require high deductibles and copays; fortunately, this financial burden for patients can be mitigated or eliminated by co-pay assistance programs. The manufacturer of Truvada offers assistance programs for both insured and uninsured patients who are candidates for PrEP; details are available at http://www.truvada.com/truvada-patient-assistance.
Stigma has historically burdened individuals who seek to protect their sexual health, including HIV-negative individuals who are candidates for PrEP. Stigma surrounding HIV may decrease ART-based HIV prevention in men who have sex with men,43 while increasing high-risk behaviors44 and all-cause mortality.45
The resources listed in TABLE 2 can help physicians overcome some of the barriers to implementing PrEP.
How to deliver PrEP
Whether HIV specialists or primary care clinicians are best suited to provide PrEP is a subject of debate. HIV specialists are most familiar with ART and routine monitoring of adherence; however, they have less access to HIV-negative patients, who are the target group for PrEP.35 Family physicians tend to work in closer proximity and maintain longitudinal relationships with PrEP target groups, but in general have less experience with ART and evaluating AHI. Some may argue that competing demands may make it impractical to take a detailed sexual history during a primary care visit.46 In truth, both HIV specialists and family physicians can be appropriately equipped to provide PrEP.
TABLE 3 outlines the steps necessary to provide a patient with PrEP.47 Assessing risk is the initial step; PrEP is beneficial for patients who have one or more risk factors for HIV infection. To be eligible for TDF/FTC, a patient must be HIV-negative, and should be tested for hepatitis B virus (HBV) infection and kidney disease. Because TDF/FTC treats HBV infection, candidates for PrEP who test positive for HBV should be evaluated for treatment of HBV before initiating PrEP. Candidates for PrEP who test negative for HBV infection and immunity should be vaccinated.
Candidates for PrEP should also be screened and monitored for kidney disease. TDF can cause increased serum creatinine due to tubular toxicity. A patient who has an estimated creatinine clearance <60 mL/min should not receive TDF/FTC for PrEP. If a patient’s estimated creatinine clearance falls below 60 mL/min or serum creatinine increases by 0.3 mg/dL above baseline after PrEP is started, TDF/FTC should be discontinued, and the patient should be evaluated for the underlying cause of the kidney disease.27
Before starting PrEP, candidates should be screened for HIV infection and symptoms of AHI. Strongly consider testing for sexually transmitted infections that may increase the risk of HIV transmission, such as syphilis, gonorrhea, or chlamydia.
Candidates who are eligible for PrEP must be counseled on medication adverse effects, adherence strategies, and symptoms of sexually transmitted infections. To initiate PrEP, candidates may be given a one-month supply of TDF/FTC; adherence, adverse effects, and other risk-reduction strategies are assessed at an office visit 3 to 4 weeks later. Subsequent prescriptions are then dispensed as a 3-month supply, with office visits to monitor PrEP scheduled for at least once every 3 months. During these monitoring visits, evaluate the patient’s HIV status, pregnancy status, adherence, adverse effects, risk-reduction behaviors, and indications for continued PrEP. Every 6 months, renal function and sexually transmitted infection status should be reassessed.
Reducing risk of harm among patients who inject drugs
Nonsexual transmission of HIV is a route of high infectivity.48 It includes transfusion of infected blood, sharing of equipment during injection drug use, and percutaneous needle sticks. Sharing of equipment during injection drug use is estimated to account for 8% of new infections in the United States.4
Harm reduction is a collection of strategies meant to reduce complications of illicit drug use, including HIV transmission. These strategies include needle and syringe programs that provide injection drug users with sterile equipment, and opioid substitution therapy.
Needle and syringe programs decrease HIV transmission49 and risky behaviors related to injection drug use,50 but federal funding of such programs is prohibited. Opioid substitution therapy reduces the incidence of HIV,50,51 injection drug use, sharing of drug preparation and injection equipment, and drug-related behaviors associated with a high risk of HIV transmission.50,52 However, in the United States, the quality of these programs varies; a study of opioid substitution therapy delivery found that 22.8% of programs provided doses that were too low to be effective.53
FDA-approved medications for opioid substitution therapy include sublingual buprenorphine, sublingual buprenorphine/naloxone tablets or strips (Suboxone), and oral methadone. Buprenorphine-based regimens can be provided by appropriately trained primary care clinicians; methadone requires a referral to a narcotic treatment program. TABLE 4 provides training and support resources for physicians who want to integrate opioid substitution therapy into their clinical practice.
CORRESPONDENCE
Richard Moore II, MD, 250 Smith Church Road, Roanoke Rapids, NC 27870; [email protected].
1. Hall HI, An Q, Tang T, et al; Centers for Disease Control and Prevention (CDC). Prevalence of diagnosed and undiagnosed HIV infection--United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2015;64:657-662.
2. Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113-1117.
3. Maulsby C, Millet G, Lindsey K, et al. HIV among black men who have sex with men (MSM) in the United States: a review of the literature. AIDS Behav. 2014;18:10-25.
4. Centers for Disease Control and Prevention. Estimated HIV incidence among adults and adolescents in the United States, 2007-2010, HIV Surveillance Supplemental Report. 2012. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed October 8, 2015.
5. Cleary PD, Van Devanter N, Rogers TF, et al. Behavior changes after notification of HIV infection. Am J Public Health. 1991;81:1586-1590.
6. Higgins DL, Galavotti C, O’Reilly KR, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266:2419-2429.
7. Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17-26.
8. Palella FJ Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4 cell strata. Ann Intern Med. 2003;138:620-626.
9. INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
10. Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:10-15.
11. Tindall B, Barker S, Donovan B, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148:945-949.
12. Moyer V, US Preventative Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
13. Jenkins T, Gardner E, Thrun M, et al. Risk-based HIV testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329-333.
14. Klein D, Hurley LB, Merrill D, et al. Review of medical encounters in the 5 years before a diagnosis of HIV-1 infection: implications for early detection. J Acquir Immune Defic Syndr. 2003;32:143-152.
15. Pandori M, Hackett J Jr, Louie B, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol. 2009;47:2639-2642.
16. Branson BM. The future of HIV testing. J Acquir Imm Defic Syndr. 2010;55 Suppl 2:S102-S105.
17. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
18. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
19. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
20. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, doubleblind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083-2090.
21. Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820-829.
22. Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125.
23. Henderson FL, Taylor AW, Chirwa LI, et al. Characteristics and oral PrEP adherence in the TDF2 open-label extension in Botswana. Paper presented at International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 1922, 2015; Vancouver, Canada.
24. Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27:2155-2160.
25. Heffron R, Mugo N, Were E, et al. Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS. 2014;28:2771-2776.
26. Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14:468-475.
27. Center for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed June 18, 2015.
28. Grant RM. Scale-up of preexposure prophylaxis in San Francisco to impact HIV incidence. Abstract 25. Paper presented at Conference on Retroviruses and Opportunistic Infections; February 23-26, 2015; Seattle, WA.
29. Cohen SE, Vittinghoff E, Bacon O, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68:439-448.
30. Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511.
31. Gilmore H, Koester K, Liu A, et al. To PrEP or not to PrEP: Perspectives from US iPrEx open label extension (OLE) participants. Abstract 440. Paper presented at 9th International Conference on HIV Treatment and Prevention Adherence; June 9, 2014; Miami Beach, FL.
32. Jain S, Gregor C, Krakower D, et al. Attitudes and interest toward HIV pre-exposure prophylaxis (PrEP) among participants using HIV non-occupational post-exposure prophylaxis (NPEP). Poster Abstract 1523. Poster presented at Infectious Disease Society of America Conference; October 8-12, 2014; Philadelphia, PA.
33. van der Straten A, Stadler J, Luecke E, et al; VOICE-C Study Team, Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17:19146.
34. Corneli AL, McKenna K, Headley J, et al; FEM-PrEP Study Group. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc. 2014;17:19152.
35. Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014;18:1712-1721.
36. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015. [Epub ahead of print].
37. Mugwanya KK, Donnell D, Celum C, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013;13:1021-1028.
38. Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61:1601-1603.
39. Lehman DA, Baeten JM, McCoy CO, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
40. Supervie V, Garcia-Lerma JG, Heneine W, et al. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci U S A. 2010;107:12381-12386.
41. AIDSinfo. Cost considerations and antiretroviral therapy. AIDSinfo Web site. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/459/cost-considerations-and-antiretroviral-therapy. Accessed December 14, 2015.
42. Gomez GB, Borquez A, Case KK, et al. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10:e1001401.
43. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
44. Hatzenbuehler ML, O’Cleirigh C, Mayer KH, et al. Prospective associations between HIV-related stigma, transmission risk behaviors, and adverse mental health outcomes in men who have sex with men. Ann Behav Med. 2011;42:227-234.
45. Hatzenbuehler ML, Bellatorre A, Lee Y, et al. Structural stigma and all-cause mortality in sexual minority populations. Soc Sci Med. 2014;103:33-41.
46. Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One. 2012;7:e40603.
47. North Carolina AIDS Training and Education Center. For PrEP Providers. North Carolina AIDS Training and Education Center Web site. Available at: http://www.med.unc.edu/ncaidstraining/prep/for-providers/for-prep-prescribers. Accessed July 7, 2015.
48. Patel P, Borkowf CB, Brook JT, et al. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509-1519.
49. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and metaanalysis. Int J Epidemiol. 2014;43:235-248.
50. MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34-52.
51. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945.
52. Gowing L, Farrell MF, Bornemann R, et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;(8):CD004145.
53. D’Aunno T, Pollack HA, Frimpong JA, et al. Evidence-based treatment for opioid disorders: a 23-year national study of methadone dose levels. J Subst Abuse Treat. 2014;47:245-250.
1. Hall HI, An Q, Tang T, et al; Centers for Disease Control and Prevention (CDC). Prevalence of diagnosed and undiagnosed HIV infection--United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2015;64:657-662.
2. Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113-1117.
3. Maulsby C, Millet G, Lindsey K, et al. HIV among black men who have sex with men (MSM) in the United States: a review of the literature. AIDS Behav. 2014;18:10-25.
4. Centers for Disease Control and Prevention. Estimated HIV incidence among adults and adolescents in the United States, 2007-2010, HIV Surveillance Supplemental Report. 2012. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed October 8, 2015.
5. Cleary PD, Van Devanter N, Rogers TF, et al. Behavior changes after notification of HIV infection. Am J Public Health. 1991;81:1586-1590.
6. Higgins DL, Galavotti C, O’Reilly KR, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266:2419-2429.
7. Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17-26.
8. Palella FJ Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4 cell strata. Ann Intern Med. 2003;138:620-626.
9. INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795-807.
10. Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:10-15.
11. Tindall B, Barker S, Donovan B, et al. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Arch Intern Med. 1988;148:945-949.
12. Moyer V, US Preventative Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
13. Jenkins T, Gardner E, Thrun M, et al. Risk-based HIV testing fails to detect the majority of HIV-infected persons in medical care settings. Sex Transm Dis. 2006;33:329-333.
14. Klein D, Hurley LB, Merrill D, et al. Review of medical encounters in the 5 years before a diagnosis of HIV-1 infection: implications for early detection. J Acquir Immune Defic Syndr. 2003;32:143-152.
15. Pandori M, Hackett J Jr, Louie B, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol. 2009;47:2639-2642.
16. Branson BM. The future of HIV testing. J Acquir Imm Defic Syndr. 2010;55 Suppl 2:S102-S105.
17. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
18. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
19. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
20. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, doubleblind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083-2090.
21. Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820-829.
22. Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125.
23. Henderson FL, Taylor AW, Chirwa LI, et al. Characteristics and oral PrEP adherence in the TDF2 open-label extension in Botswana. Paper presented at International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 1922, 2015; Vancouver, Canada.
24. Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27:2155-2160.
25. Heffron R, Mugo N, Were E, et al. Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS. 2014;28:2771-2776.
26. Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14:468-475.
27. Center for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. Accessed June 18, 2015.
28. Grant RM. Scale-up of preexposure prophylaxis in San Francisco to impact HIV incidence. Abstract 25. Paper presented at Conference on Retroviruses and Opportunistic Infections; February 23-26, 2015; Seattle, WA.
29. Cohen SE, Vittinghoff E, Bacon O, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68:439-448.
30. Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511.
31. Gilmore H, Koester K, Liu A, et al. To PrEP or not to PrEP: Perspectives from US iPrEx open label extension (OLE) participants. Abstract 440. Paper presented at 9th International Conference on HIV Treatment and Prevention Adherence; June 9, 2014; Miami Beach, FL.
32. Jain S, Gregor C, Krakower D, et al. Attitudes and interest toward HIV pre-exposure prophylaxis (PrEP) among participants using HIV non-occupational post-exposure prophylaxis (NPEP). Poster Abstract 1523. Poster presented at Infectious Disease Society of America Conference; October 8-12, 2014; Philadelphia, PA.
33. van der Straten A, Stadler J, Luecke E, et al; VOICE-C Study Team, Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17:19146.
34. Corneli AL, McKenna K, Headley J, et al; FEM-PrEP Study Group. A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc. 2014;17:19152.
35. Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014;18:1712-1721.
36. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015. [Epub ahead of print].
37. Mugwanya KK, Donnell D, Celum C, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013;13:1021-1028.
38. Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61:1601-1603.
39. Lehman DA, Baeten JM, McCoy CO, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
40. Supervie V, Garcia-Lerma JG, Heneine W, et al. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci U S A. 2010;107:12381-12386.
41. AIDSinfo. Cost considerations and antiretroviral therapy. AIDSinfo Web site. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/459/cost-considerations-and-antiretroviral-therapy. Accessed December 14, 2015.
42. Gomez GB, Borquez A, Case KK, et al. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med. 2013;10:e1001401.
43. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
44. Hatzenbuehler ML, O’Cleirigh C, Mayer KH, et al. Prospective associations between HIV-related stigma, transmission risk behaviors, and adverse mental health outcomes in men who have sex with men. Ann Behav Med. 2011;42:227-234.
45. Hatzenbuehler ML, Bellatorre A, Lee Y, et al. Structural stigma and all-cause mortality in sexual minority populations. Soc Sci Med. 2014;103:33-41.
46. Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One. 2012;7:e40603.
47. North Carolina AIDS Training and Education Center. For PrEP Providers. North Carolina AIDS Training and Education Center Web site. Available at: http://www.med.unc.edu/ncaidstraining/prep/for-providers/for-prep-prescribers. Accessed July 7, 2015.
48. Patel P, Borkowf CB, Brook JT, et al. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509-1519.
49. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and metaanalysis. Int J Epidemiol. 2014;43:235-248.
50. MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34-52.
51. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945.
52. Gowing L, Farrell MF, Bornemann R, et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;(8):CD004145.
53. D’Aunno T, Pollack HA, Frimpong JA, et al. Evidence-based treatment for opioid disorders: a 23-year national study of methadone dose levels. J Subst Abuse Treat. 2014;47:245-250.
Smoking cessation: What should you recommend?
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.