User login
Transgender patients: Providing sensitive care
› Refer transgender patients to a mental health provider with experience in treating this patient population for diagnosis of gender dysphoria. B
› Confirm patients’ eligibility for hormone therapy and obtain informed consent before initiating it. B
› Treat minor adverse effects of hormone therapy whenever possible, as discontinuing the hormones may be detrimental to the well-being of patients in the process of transitioning. B
› Continue recommended screenings based on the sex the patient was assigned at birth, unless the organ or tissue in question has been surgically removed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals—although focused primarily on the “appropriate” use of restrooms—has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
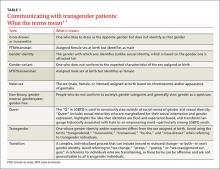
Family physicians are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (TABLE 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
Understanding and diagnosing gender dysphoria
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed. (DSM-5)8 and the International Classification of Diseases, 10th Rev. (ICD-10).9
ICD-10 criteria use the term “gender identity disorder;” DSM-5 refers to “gender dysphoria” instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient, and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet 6 or more of the following for at least 6 months:
- a repeatedly stated desire to be, or insistence that he or she is, of the other gender
- in boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- a strong rejection of toys/games typically associated with the child’s sex
- intense desire to participate in stereotypical games and pastimes of the other gender
- strong preference for playmates of the other gender
- a strong dislike of one’s sexual anatomy
- a strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet 2 or more of the following for at least 6 months:
- a noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- an intense need to do away with (or prevent) his or her primary or secondary sex features
- an intense desire to have the primary and/or secondary sex features of the other gender
- a deep desire to transform into another gender
- a profound need for society to treat them as someone of the other gender
- a powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care physician to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not a MHP confirms a diagnosis of GD, it is still up to the treating physician to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented Real-Life Experience (RLE), defined as having fully adopted the new gender role in everyday life for at least 3 months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk.. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated.11 (See “How to help transgender teens.”11,14-20)
How to help transgender teens
Gender exploration is a normal part of childhood. Changes that occur during puberty cause many adolescents to question their ideas of gender and sexuality, but those with persistent gender nonconformity or extreme body dysphoria should receive medical and mental health care as early as possible.
A mental health professional (MHP) can help families create a safe, unconditionally accepting, and supportive environment in which their child can develop into his or her authentic self. A specialist in adolescent gender dysphoria (GD) should be consulted before initiating hormone therapy. Family physicians are well positioned to provide follow-up and ongoing care.
Initiating hormone suppression for adolescents with GD early in puberty can greatly reduce the emotional trauma, as well as the need for surgery related to unwanted secondary sex characteristics. Hormone suppression also gives adolescents more time to explore their gender nonconformity and developmental needs. Treatment with a gonadotropin-releasing hormone agonist such as leuprolide or histrelin can be initiated for adolescents in Tanner Stage 2, assessed by hormone levels, as well as breast, testicle, and pubic hair development (childgrowthfoundation.org/CMS/FILES/Puberty_and_the_Tanner_Stages.pdf), to suppress ongoing puberty.11,14
The effects of puberty blockers are reversible. If preferred, however, medication management can allow teens to progress directly from hormone suppression to the puberty of their self-identified gender once initiation of adult transgender hormone therapy is started—typically around the age of 16.11
Some adolescents may seek medical care well after puberty has begun. It is possible to use hormone suppression therapy after a teen has started to develop unwanted secondary sex characteristics. However, such patients may get less benefit from puberty blockers and often derive more benefit from initiation of cross-gender hormones.
Safety—a broader perspective. Regardless of medical treatment or the age at which treatment is begun, parents and patients need to be reminded that gender transition is about more than hormones. It is crucial that either the MHP or primary care physician, or both, address safety issues and work with the transgender individual to create a comprehensive plan for his or her health, safety, and social well-being.
Psychosocial concerns for transgender youth are vast, often encompassing rejection by peers and potentially, by family and authority figures; harassment; physical, emotional, and sexual abuse; inadequate housing; legal problems; lack of financial support; and educational difficulties.15 School is a particular source of concern. A survey by the Gay, Lesbian and Straight Education Network found, for instance, that nearly 9 in 10 transgender youth had been verbally harassed at school because of their gender expression. More than half also reported being physically harassed (eg, pushed or shoved).15
Bathroom use in schools is a major issue, as well, with transgender youth often prevented from using the restroom or locker room that coincides with their gender identity. In May, 11 states filed a lawsuit against the Obama administration for directing schools to allow such students to do so or risk the loss of federal funds.16
Tools and support. In 2006, the Vancouver Coastal Health, Transcend Transgender Support & Education Society, and the Canadian Rainbow Health Coalition published guidelines for the care of transgender adolescents, including a modification of the HEEADSSS tool (used to assess teens’ psychosocial well-being) specific to transgender youth.17 (See http://www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf.) The tool can help you identify possible areas of concern and intervene, as needed.
If a patient reports feeling especially vulnerable at school, for example, encourage him or her to identify supportive friends, family members, teachers, or school counselors who can help create safe networks. Suggest that a teen who feels threatened travel in groups whenever possible. Stress to family members that their acceptance is key to nurturing positive feelings in youth with GD. And refer patients and parents to organizations that provide information and support.
Chief among them are Trans Youth Family Allies18 (www.imatyfa.org), which provides resources for parents, educators, and health care practitioners; the Human Rights Campaign, which has created a survival guide for gay and transgender youth who are forced to seek independent living19 (available at hrc-assets.s3-website-us-east-1.amazonaws.com//files/assets/resources/HRC-OnOurOwn-LGBTQYouth.pdf), and the Gay, Lesbian, Bisexual, and Transgender National Help Center, which hosts a GLBT national hotline (888-843-4564) and a weekly Trans Teen Online Talk Group.20 For more information about the online talk group, go to glbthotline.org and click on “transteens.”
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
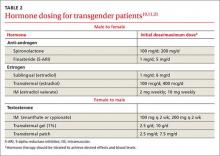
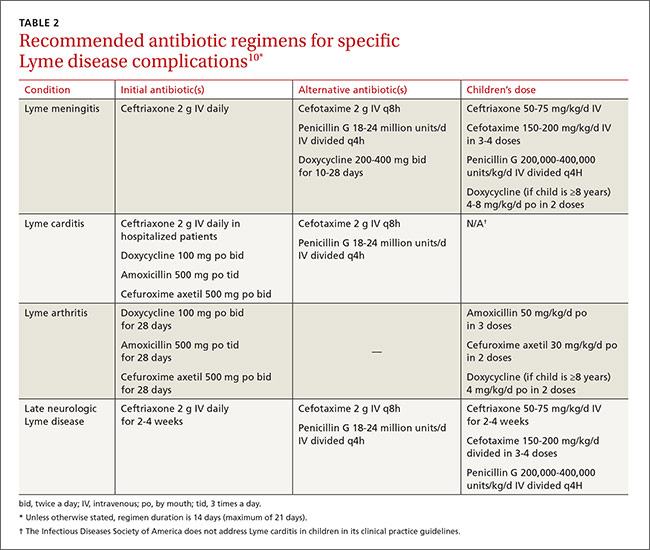
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychological more congruent. Hormone treatment (TABLE 2)10,11,21 is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body. Psychological conditions, such as depression, tend to improve as the transitioning process gets underway.22
Female-to-male transition
CASE 1 ›
Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care physician for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the last several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.11,23,24
Physical changes induced by masculinizing hormone therapy have an expected onset of one to 6 months and achieve maximum effect in approximately 2 to 5 years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk of pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk of cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks vs benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit >55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every 3 months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for >6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 ›
The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with a MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice and greater muscle mass, among others—and review the likely time frame
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of a MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
Male-to-female transition
CASE 2 ›
Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history, but reports that she has been taking spironolactone and estrogen for the past 3 years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between 3 months and 5 years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-alpha reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-alpha-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (<200 pg/mL) and the level of testosterone should be in the normal female range (<55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk of adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients on feminizing hormones (transwomen) should be seen every 2 to 3 months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every 3 months; serum electrolytes, specifically potassium, should be monitored every 2 to 3 months in the first year until stable.
CASE 2 ›
You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in 6 months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at 40 to 50 years of age (as it is for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
CORRESPONDENCE
Abbas Hyderi, MD, MPH, 1919 West Taylor Street, M/C 663, Chicago, IL 60612; [email protected].
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. Available at: http://www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed June 24, 2015.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? Available at: http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed May 25, 2016.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. Available at: http://www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed June 24, 2015.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed June 24, 2015.
6. Istar A. How queer! the development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association. 2013:451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011;13:165–232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine Treatment of transsexual persons: an Endocrine Society Clinical Practice Guideline. J Clin Endo Metabol. 2009;94:3132–3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed June 24, 2015.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S614.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. Available at: https://www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed May 29, 2016.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. Available at: https://www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed May 31, 2016.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. Available at: http://www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed May 26, 2016.
18. TransYouth Family Allies. Empowering transgender youth & families. Available at: http://www.imatyfa.org/. Accessed May 26, 2016.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. Available at: https://www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed May 26, 2016.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. Available at: www.glbthotline.org. Accessed May 31, 2016.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed December 12, 2015.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008;5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized control trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of thermablate endometrial ablation system as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed June 24, 2015.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995;82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed December 12, 2015.
› Refer transgender patients to a mental health provider with experience in treating this patient population for diagnosis of gender dysphoria. B
› Confirm patients’ eligibility for hormone therapy and obtain informed consent before initiating it. B
› Treat minor adverse effects of hormone therapy whenever possible, as discontinuing the hormones may be detrimental to the well-being of patients in the process of transitioning. B
› Continue recommended screenings based on the sex the patient was assigned at birth, unless the organ or tissue in question has been surgically removed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals—although focused primarily on the “appropriate” use of restrooms—has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Family physicians are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (TABLE 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
Understanding and diagnosing gender dysphoria
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed. (DSM-5)8 and the International Classification of Diseases, 10th Rev. (ICD-10).9
ICD-10 criteria use the term “gender identity disorder;” DSM-5 refers to “gender dysphoria” instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient, and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet 6 or more of the following for at least 6 months:
- a repeatedly stated desire to be, or insistence that he or she is, of the other gender
- in boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- a strong rejection of toys/games typically associated with the child’s sex
- intense desire to participate in stereotypical games and pastimes of the other gender
- strong preference for playmates of the other gender
- a strong dislike of one’s sexual anatomy
- a strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet 2 or more of the following for at least 6 months:
- a noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- an intense need to do away with (or prevent) his or her primary or secondary sex features
- an intense desire to have the primary and/or secondary sex features of the other gender
- a deep desire to transform into another gender
- a profound need for society to treat them as someone of the other gender
- a powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care physician to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not a MHP confirms a diagnosis of GD, it is still up to the treating physician to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented Real-Life Experience (RLE), defined as having fully adopted the new gender role in everyday life for at least 3 months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk.. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated.11 (See “How to help transgender teens.”11,14-20)
How to help transgender teens
Gender exploration is a normal part of childhood. Changes that occur during puberty cause many adolescents to question their ideas of gender and sexuality, but those with persistent gender nonconformity or extreme body dysphoria should receive medical and mental health care as early as possible.
A mental health professional (MHP) can help families create a safe, unconditionally accepting, and supportive environment in which their child can develop into his or her authentic self. A specialist in adolescent gender dysphoria (GD) should be consulted before initiating hormone therapy. Family physicians are well positioned to provide follow-up and ongoing care.
Initiating hormone suppression for adolescents with GD early in puberty can greatly reduce the emotional trauma, as well as the need for surgery related to unwanted secondary sex characteristics. Hormone suppression also gives adolescents more time to explore their gender nonconformity and developmental needs. Treatment with a gonadotropin-releasing hormone agonist such as leuprolide or histrelin can be initiated for adolescents in Tanner Stage 2, assessed by hormone levels, as well as breast, testicle, and pubic hair development (childgrowthfoundation.org/CMS/FILES/Puberty_and_the_Tanner_Stages.pdf), to suppress ongoing puberty.11,14
The effects of puberty blockers are reversible. If preferred, however, medication management can allow teens to progress directly from hormone suppression to the puberty of their self-identified gender once initiation of adult transgender hormone therapy is started—typically around the age of 16.11
Some adolescents may seek medical care well after puberty has begun. It is possible to use hormone suppression therapy after a teen has started to develop unwanted secondary sex characteristics. However, such patients may get less benefit from puberty blockers and often derive more benefit from initiation of cross-gender hormones.
Safety—a broader perspective. Regardless of medical treatment or the age at which treatment is begun, parents and patients need to be reminded that gender transition is about more than hormones. It is crucial that either the MHP or primary care physician, or both, address safety issues and work with the transgender individual to create a comprehensive plan for his or her health, safety, and social well-being.
Psychosocial concerns for transgender youth are vast, often encompassing rejection by peers and potentially, by family and authority figures; harassment; physical, emotional, and sexual abuse; inadequate housing; legal problems; lack of financial support; and educational difficulties.15 School is a particular source of concern. A survey by the Gay, Lesbian and Straight Education Network found, for instance, that nearly 9 in 10 transgender youth had been verbally harassed at school because of their gender expression. More than half also reported being physically harassed (eg, pushed or shoved).15
Bathroom use in schools is a major issue, as well, with transgender youth often prevented from using the restroom or locker room that coincides with their gender identity. In May, 11 states filed a lawsuit against the Obama administration for directing schools to allow such students to do so or risk the loss of federal funds.16
Tools and support. In 2006, the Vancouver Coastal Health, Transcend Transgender Support & Education Society, and the Canadian Rainbow Health Coalition published guidelines for the care of transgender adolescents, including a modification of the HEEADSSS tool (used to assess teens’ psychosocial well-being) specific to transgender youth.17 (See http://www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf.) The tool can help you identify possible areas of concern and intervene, as needed.
If a patient reports feeling especially vulnerable at school, for example, encourage him or her to identify supportive friends, family members, teachers, or school counselors who can help create safe networks. Suggest that a teen who feels threatened travel in groups whenever possible. Stress to family members that their acceptance is key to nurturing positive feelings in youth with GD. And refer patients and parents to organizations that provide information and support.
Chief among them are Trans Youth Family Allies18 (www.imatyfa.org), which provides resources for parents, educators, and health care practitioners; the Human Rights Campaign, which has created a survival guide for gay and transgender youth who are forced to seek independent living19 (available at hrc-assets.s3-website-us-east-1.amazonaws.com//files/assets/resources/HRC-OnOurOwn-LGBTQYouth.pdf), and the Gay, Lesbian, Bisexual, and Transgender National Help Center, which hosts a GLBT national hotline (888-843-4564) and a weekly Trans Teen Online Talk Group.20 For more information about the online talk group, go to glbthotline.org and click on “transteens.”
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychological more congruent. Hormone treatment (TABLE 2)10,11,21 is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body. Psychological conditions, such as depression, tend to improve as the transitioning process gets underway.22
Female-to-male transition
CASE 1 ›
Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care physician for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the last several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.11,23,24
Physical changes induced by masculinizing hormone therapy have an expected onset of one to 6 months and achieve maximum effect in approximately 2 to 5 years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk of pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk of cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks vs benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit >55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every 3 months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for >6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 ›
The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with a MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice and greater muscle mass, among others—and review the likely time frame
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of a MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
Male-to-female transition
CASE 2 ›
Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history, but reports that she has been taking spironolactone and estrogen for the past 3 years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between 3 months and 5 years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-alpha reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-alpha-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (<200 pg/mL) and the level of testosterone should be in the normal female range (<55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk of adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients on feminizing hormones (transwomen) should be seen every 2 to 3 months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every 3 months; serum electrolytes, specifically potassium, should be monitored every 2 to 3 months in the first year until stable.
CASE 2 ›
You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in 6 months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at 40 to 50 years of age (as it is for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
CORRESPONDENCE
Abbas Hyderi, MD, MPH, 1919 West Taylor Street, M/C 663, Chicago, IL 60612; [email protected].
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
› Refer transgender patients to a mental health provider with experience in treating this patient population for diagnosis of gender dysphoria. B
› Confirm patients’ eligibility for hormone therapy and obtain informed consent before initiating it. B
› Treat minor adverse effects of hormone therapy whenever possible, as discontinuing the hormones may be detrimental to the well-being of patients in the process of transitioning. B
› Continue recommended screenings based on the sex the patient was assigned at birth, unless the organ or tissue in question has been surgically removed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals—although focused primarily on the “appropriate” use of restrooms—has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Family physicians are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (TABLE 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
Understanding and diagnosing gender dysphoria
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed. (DSM-5)8 and the International Classification of Diseases, 10th Rev. (ICD-10).9
ICD-10 criteria use the term “gender identity disorder;” DSM-5 refers to “gender dysphoria” instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient, and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet 6 or more of the following for at least 6 months:
- a repeatedly stated desire to be, or insistence that he or she is, of the other gender
- in boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- a strong rejection of toys/games typically associated with the child’s sex
- intense desire to participate in stereotypical games and pastimes of the other gender
- strong preference for playmates of the other gender
- a strong dislike of one’s sexual anatomy
- a strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet 2 or more of the following for at least 6 months:
- a noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- an intense need to do away with (or prevent) his or her primary or secondary sex features
- an intense desire to have the primary and/or secondary sex features of the other gender
- a deep desire to transform into another gender
- a profound need for society to treat them as someone of the other gender
- a powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care physician to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not a MHP confirms a diagnosis of GD, it is still up to the treating physician to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented Real-Life Experience (RLE), defined as having fully adopted the new gender role in everyday life for at least 3 months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk.. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated.11 (See “How to help transgender teens.”11,14-20)
How to help transgender teens
Gender exploration is a normal part of childhood. Changes that occur during puberty cause many adolescents to question their ideas of gender and sexuality, but those with persistent gender nonconformity or extreme body dysphoria should receive medical and mental health care as early as possible.
A mental health professional (MHP) can help families create a safe, unconditionally accepting, and supportive environment in which their child can develop into his or her authentic self. A specialist in adolescent gender dysphoria (GD) should be consulted before initiating hormone therapy. Family physicians are well positioned to provide follow-up and ongoing care.
Initiating hormone suppression for adolescents with GD early in puberty can greatly reduce the emotional trauma, as well as the need for surgery related to unwanted secondary sex characteristics. Hormone suppression also gives adolescents more time to explore their gender nonconformity and developmental needs. Treatment with a gonadotropin-releasing hormone agonist such as leuprolide or histrelin can be initiated for adolescents in Tanner Stage 2, assessed by hormone levels, as well as breast, testicle, and pubic hair development (childgrowthfoundation.org/CMS/FILES/Puberty_and_the_Tanner_Stages.pdf), to suppress ongoing puberty.11,14
The effects of puberty blockers are reversible. If preferred, however, medication management can allow teens to progress directly from hormone suppression to the puberty of their self-identified gender once initiation of adult transgender hormone therapy is started—typically around the age of 16.11
Some adolescents may seek medical care well after puberty has begun. It is possible to use hormone suppression therapy after a teen has started to develop unwanted secondary sex characteristics. However, such patients may get less benefit from puberty blockers and often derive more benefit from initiation of cross-gender hormones.
Safety—a broader perspective. Regardless of medical treatment or the age at which treatment is begun, parents and patients need to be reminded that gender transition is about more than hormones. It is crucial that either the MHP or primary care physician, or both, address safety issues and work with the transgender individual to create a comprehensive plan for his or her health, safety, and social well-being.
Psychosocial concerns for transgender youth are vast, often encompassing rejection by peers and potentially, by family and authority figures; harassment; physical, emotional, and sexual abuse; inadequate housing; legal problems; lack of financial support; and educational difficulties.15 School is a particular source of concern. A survey by the Gay, Lesbian and Straight Education Network found, for instance, that nearly 9 in 10 transgender youth had been verbally harassed at school because of their gender expression. More than half also reported being physically harassed (eg, pushed or shoved).15
Bathroom use in schools is a major issue, as well, with transgender youth often prevented from using the restroom or locker room that coincides with their gender identity. In May, 11 states filed a lawsuit against the Obama administration for directing schools to allow such students to do so or risk the loss of federal funds.16
Tools and support. In 2006, the Vancouver Coastal Health, Transcend Transgender Support & Education Society, and the Canadian Rainbow Health Coalition published guidelines for the care of transgender adolescents, including a modification of the HEEADSSS tool (used to assess teens’ psychosocial well-being) specific to transgender youth.17 (See http://www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf.) The tool can help you identify possible areas of concern and intervene, as needed.
If a patient reports feeling especially vulnerable at school, for example, encourage him or her to identify supportive friends, family members, teachers, or school counselors who can help create safe networks. Suggest that a teen who feels threatened travel in groups whenever possible. Stress to family members that their acceptance is key to nurturing positive feelings in youth with GD. And refer patients and parents to organizations that provide information and support.
Chief among them are Trans Youth Family Allies18 (www.imatyfa.org), which provides resources for parents, educators, and health care practitioners; the Human Rights Campaign, which has created a survival guide for gay and transgender youth who are forced to seek independent living19 (available at hrc-assets.s3-website-us-east-1.amazonaws.com//files/assets/resources/HRC-OnOurOwn-LGBTQYouth.pdf), and the Gay, Lesbian, Bisexual, and Transgender National Help Center, which hosts a GLBT national hotline (888-843-4564) and a weekly Trans Teen Online Talk Group.20 For more information about the online talk group, go to glbthotline.org and click on “transteens.”
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychological more congruent. Hormone treatment (TABLE 2)10,11,21 is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body. Psychological conditions, such as depression, tend to improve as the transitioning process gets underway.22
Female-to-male transition
CASE 1 ›
Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care physician for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the last several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.11,23,24
Physical changes induced by masculinizing hormone therapy have an expected onset of one to 6 months and achieve maximum effect in approximately 2 to 5 years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk of pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk of cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks vs benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit >55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every 3 months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for >6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 ›
The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with a MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice and greater muscle mass, among others—and review the likely time frame
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of a MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
Male-to-female transition
CASE 2 ›
Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history, but reports that she has been taking spironolactone and estrogen for the past 3 years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between 3 months and 5 years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-alpha reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-alpha-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (<200 pg/mL) and the level of testosterone should be in the normal female range (<55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk of adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients on feminizing hormones (transwomen) should be seen every 2 to 3 months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every 3 months; serum electrolytes, specifically potassium, should be monitored every 2 to 3 months in the first year until stable.
CASE 2 ›
You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in 6 months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at 40 to 50 years of age (as it is for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
CORRESPONDENCE
Abbas Hyderi, MD, MPH, 1919 West Taylor Street, M/C 663, Chicago, IL 60612; [email protected].
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. Available at: http://www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed June 24, 2015.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? Available at: http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed May 25, 2016.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. Available at: http://www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed June 24, 2015.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed June 24, 2015.
6. Istar A. How queer! the development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association. 2013:451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011;13:165–232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine Treatment of transsexual persons: an Endocrine Society Clinical Practice Guideline. J Clin Endo Metabol. 2009;94:3132–3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed June 24, 2015.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S614.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. Available at: https://www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed May 29, 2016.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. Available at: https://www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed May 31, 2016.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. Available at: http://www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed May 26, 2016.
18. TransYouth Family Allies. Empowering transgender youth & families. Available at: http://www.imatyfa.org/. Accessed May 26, 2016.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. Available at: https://www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed May 26, 2016.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. Available at: www.glbthotline.org. Accessed May 31, 2016.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed December 12, 2015.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008;5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized control trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of thermablate endometrial ablation system as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed June 24, 2015.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995;82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed December 12, 2015.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. Available at: http://www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed June 24, 2015.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? Available at: http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed May 25, 2016.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. Available at: http://www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed June 24, 2015.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed June 24, 2015.
6. Istar A. How queer! the development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association. 2013:451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011;13:165–232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine Treatment of transsexual persons: an Endocrine Society Clinical Practice Guideline. J Clin Endo Metabol. 2009;94:3132–3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed June 24, 2015.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S614.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. Available at: https://www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed May 29, 2016.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. Available at: https://www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed May 31, 2016.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. Available at: http://www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed May 26, 2016.
18. TransYouth Family Allies. Empowering transgender youth & families. Available at: http://www.imatyfa.org/. Accessed May 26, 2016.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. Available at: https://www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed May 26, 2016.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. Available at: www.glbthotline.org. Accessed May 31, 2016.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed December 12, 2015.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008;5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized control trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of thermablate endometrial ablation system as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed June 24, 2015.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995;82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. Available at: http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed December 12, 2015.
Antibiotic interactions: Answers to 4 common questions
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected].
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected].
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected].
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
Hand compression neuropathy: An assessment guide
› Use provocative testing to confirm a suspected diagnosis in a patient who presents with peripheral entrapment mononeuropathy. B
› Consider electrodiagnostic testing for help in diagnosing a challenging presentation, ruling out a competing diagnosis, or clarifying an atypical clinical picture or vague subjective history. A
› Evaluate any patient who presents with non-anatomic nerve distribution of symptoms—eg, burning, numbness, and tingling of the entire hand—for a metabolic, rather than an entrapment, neuropathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neuropathic hand complaints—for which patients typically seek medical attention when the pain or paresthesia starts to interfere with their daily routine—are common and diverse. The ability to assess and accurately diagnose upper extremity compression neuropathies is critical for physicians in primary care.
Assessment starts, of course, with a thorough history of the present illness and past medical history, which helps define a broad differential diagnosis and identify comorbidities. Physical examination, including judicious use of provocative testing, allows you to objectively identify the pathologic deficit, evaluate function and coordination of multiple organ systems, and detect nerve dysfunction. The results determine whether additional tools, such as electrodiagnostic testing, are needed.
We’ve created this guide, detailed in the text, tables, and figures that follow, to help you hone your ability to accurately diagnose patients who present with compression neuropathies of the hand.
The medical history: Knowing what to ask
To clearly define a patient’s symptoms and disability, start with a thorough history of the presenting complaint.
Inquire about symptom onset and chronicity. Did the pain or paresthesia begin after an injury? Are the symptoms associated with repetitive use of the extremity? Do they occur at night?
Pinpoint the location or distribution of pain or paresthesia. It is paramount to identify the affected nerve.1,2 Ask patients to complete a hand or upper extremity profile documenting location and/or type of numbness, tingling, or decreased sensation. A diagram of the peripheral nerves responsible for sensory innervation of the hand (FIGURE 1) is an effective way to screen individuals at high risk of carpal tunnel syndrome (CTS) or ulnar tunnel syndrome (UTS).1,2
A patient report such as, “My whole hand is numb,” calls for a follow-up question to determine whether the little finger is affected,3 which would indicate that the ulnar nerve, rather than just the median nerve, is involved. And if a patient reports feeling as if he or she is wearing gloves or mittens, it is essential to consider the possibility of a systemic neuropathy rather than a single peripheral neuropathy.3
Gather basic patient information. Inquire about hand dominance, occupation, and baseline function, any or all of which may be critical in the assessment and initiation of treatment.4,5
Review systemic conditions and medications
A broad range of comorbidities, such as cervical radiculopathy, diabetes, hypothyroidism, and vitamin deficiencies (TABLE 1),6,7 may be responsible for neuropathic hand complaints, and a thorough review of systemic complaints and past medical history is critical. Include a medication history and a review of prior procedures, such as post-traumatic surgeries of the hand or upper extremity or nerve decompression surgeries, which may provide additional insight into disease etiology.
Symptoms guide physical exam, provocative testing
A physical examination, including provocative testing, follows based on reported symptoms, medical history, and suspected source of nerve compression.
Carpal tunnel syndrome
CTS is the most common peripheral neuropathy.8 Patients often report nocturnal pain or paresthesia in the distal median nerve distribution, comprising the palmar surface of the thumb, index, middle, and radial half of the ring finger.
Researchers have identified 6 standardized clinical criteria for the diagnosis of CTS. Two criteria—numbness mostly in median nerve territory and nocturnal numbness—can be ascertained during the history of present illness.The other 4, detailed below, will be found during the physical exam.9
Thenar weakness or atrophy.9 Begin your evaluation by inspecting the thenar musculature for atrophic changes. Motor exam of intrinsic musculature innervated by the recurrent motor branch of the median nerve includes assessment of thumb abduction strength (assessed by applying resistance to the metacarpophalangeal joint [MCPJ] base towards the palm in the position of maximal abduction) and opposition strength (assessed by applying force to the MCPJ from the ulnar aspect).10
Positive Phalen’s test.9 Provocative testing for CTS includes Phalen’s test (sensitivity 43%-86%, specificity 48%-67%),11 which is an attempt to reproduce the numbness or tingling in the median nerve territory within 60 seconds of full wrist flexion. Ask the patient to hold his or her forearms vertically with elbows resting on the table (allowing gravity to flex the wrists),12 and to tell you if numbness or tingling occurs.
Positive Tinel’s sign.9 Tinel’s sign (sensitivity 45%-75%, specificity 48%-67%)11 is performed by lightly tapping the median nerve from the proximal to distal end over the carpal tunnel. The test is positive if paresthesia results. Provocative testing may also include Durkan’s test, also known as the carpal compression test. Durkan’s test (sensitivity 49%-89%, specificity 54%-96%)11 involves placing your thumb directly over the carpal tunnel and holding light compression for 60 seconds, or until paresthesia is reported.
Positive 2-point discrimination test.9 To assess CTS disease severity, use 2-point discrimination to evaluate the patient’s sensation qualitatively and quantitatively. Two-point discrimination can only be tested, however, if light touch sensation is intact. It is typically performed by lightly applying 2 caliper points at fixed distances sufficient to blanch the skin, but some clinicians have used other tools, such as a modified paperclip.13 The smallest distance at which the patient can detect 2 distinct stimuli is then recorded.
Researchers have reported an average of 3 to 5 mm for 2-point discrimination at the fingertipand a normal 2-point discrimination of 6 to 9 mm in the volar surface of the hand (TABLE 2).14,15
The scratch collapse test (sensitivity 64%, specificity 99%) is a supplemental exam that uses a different outcome measure to diagnose CTS.16 It involves lightly scratching the skin over the compressed carpal tunnel while the patient performs sustained resisted bilateral shoulder external rotation in an adducted position. A momentary loss of muscle resistance to external rotation indicates a positive test.
Pronator syndrome
Pronator syndrome (PS) is a proximal median neuropathy that may present in isolation or in combination with CTS as a double crush syndrome. Clinical symptoms include features of CTS and sensory paresthesias in the palm and distal forearm in the distribution of the palmar cutaneous branch of the median nerve. PS is commonly associated with volar proximal forearm pain exacerbated by repetitive activities involving pronation and supination.
PS is not easy to assess. Palpatory examination of a large supracondylar process at the distal humerus proximal to the medial epicondyle on its anteromedial aspect can be difficult, especially if the patient is overweight. And motor weakness is not a prominent feature. What’s more, power assessment of the pronator teres, flexor carpi radialis, and flexor digitorum superficialis may exacerbate symptoms.
Because the symptoms of PS and CTS may be the same, PS provocation maneuvers should be performed on patients with CTS symptoms and paresthesia involving the palm. Start by testing for Tinel’s sign over the pronator teres muscle, although this has been found to be positive in less than 50% of PS cases.17 Palpate the antecubital fossa and the proximal aspect of the pronator teres muscle to assess for discomfort or tenderness.
Pronator compression test. The pronator compression test has been found to be the most sensitive way to assess PS.18,19 This test involves direct compression of the proximal and radial edge of the pronator teres muscle belly along the proximal volar forearm with the thumb.20 It is performed bilaterally on supinated upper extremities, with the clinician applying pressure on each forearm simultaneously (FIGURE 2). If the symptoms in the hand are reproduced in ≤30 seconds, the test is positive. In a study of 10 patients with surgically confirmed PS, the pronator compression test was positive in every case.20,21
Resistance testing. You can also evaluate the pronator teres compression site by testing the patient’s ability to resist pronation with his or her elbow extended and the forearm in neutral position. To test for compression from the bicipital aponeurosis, ask the patient to flex the elbow to approximately 120° to 130° and apply active supinated resistance.22 Likewise, resistance of the long finger proximal interphalangeal joint (IPJ) to flexion—a maneuver performed with elbow fully extended—assesses compression from the fibrous arcade of the flexor digitorum superficialis (FDS).21 A positive resistance test will reproduce the reported symptoms.
Ulnar tunnel syndrome
Symptoms of UTS, which is much less common than CTS, include pain in the wrist and hand that is associated with paresthesia or numbness in the small finger and ulnar half of the ring finger. Patients may report difficulty with motor tasks involving grip and pinch strength or fatigue with prolonged action of the intrinsic muscles. Many also report an exacerbation of symptoms associated with increased wrist flexion or at night.
Evaluation of UTS requires a full assessment of the upper extremity, starting with observation of hand posture and muscle bulk to identify signs of chronic nerve compression. The contralateral extremity serves as a control to the neuropathic hand. Classically, chronic ulnar nerve compression leads to intrinsic muscle atrophy, evidenced by loss of topographical soft tissue bulk in the first dorsal web space, the palmar transverse metacarpal arch, and the hypothenar area.23 Ulnar motor nerve dysfunction is limited to the intrinsic muscles of the hand. The inverted pyramid sign, signified by atrophy of the transverse head of the adductor pollicis, is another visual aberrancy,24 as is clawing of the ring finger and small finger. The clawing, which involves hyperextension of the MCPJ and flexion of the proximal and distal IPJ, is commonly known as Duchenne’s sign. FIGURE 3 demonstrates hypothenar atrophy and the loss of muscle bulk in the first dorsal web space.
When you suspect UTS, palpate the wrist and hand in an attempt to locate a mass or area of tenderness. Not all patients with a volar ganglion cyst responsible for UTS present with a palpable mass, but tenderness along the radial aspect of the pisiform or an undefined fullness in this area may be noted.25 Any patient with a palpable mass should be tested for Tinel’s sign over the mass and undergo a thorough vascular assessment. Fracture of the hook of the hamate is indicated by tenderness in the region approximated by the intersection of Kaplan’s line and the proximal extension line from the ring finger.
Perform 2-point discrimination testing at the palmar distal aspect of the small finger and ulnar half of the ring finger. This tests the superficial sensory division of the ulnar nerve that travels within Guyon’s canal. Testing the dorsal ulnar cutaneous nerve involves the skin of the dorso-ulnar hand and dorsum of the long finger proximal to the IPJ. If this area is spared and the palmar distal ulnar digits are affected, compression within Guyon’s canal is likely. If both areas are affected, suspect a more proximal compression site at the cubital tunnel, as the dorsal ulnar cutaneous nerve branches proximal to Guyon’s canal.26
Ulnar motor nerve dysfunction in UTS is limited to the intrinsic muscles of the hand. Assessment of intrinsic muscle function is described in TABLE 3.27-32 It is important to become familiar with the tests and maneuvers described, but also to be aware that a comprehensive evaluation of ulnar nerve motor function requires a combination of tests.
Cubital tunnel syndrome
Cubital tunnel syndrome—the second most common peripheral neuropathy8—involves the proximal site of ulnar nerve compression in the upper extremity. Patients typically report symptoms similar to those of UTS, with sensory paresthesia in the ulnar digits and intrinsic weakness. To learn more about the symptoms, ask if the onset of pain or paresthesia is related to a particular elbow position, such as increased elbow flexion.
Notably, pain is usually not the initial complaint, unless the disease is advanced. This may be the reason atrophic intrinsic changes are 4 times more likely to be seen in patients with cubital tunnel syndrome than in those with CTS.33 You’re more likely to hear about vague motor problems, including hand clumsiness and difficulty with fine coordination of the fingers.34 Thus, it is important to evaluate patients for concurrent UTS and/or CTS, as well as for differentiation.
Focus on the elbow. Whenever you suspect cubital tunnel syndrome, pay special attention to the elbow. Examine the carrying angle of the elbow in relation to the contralateral extremity. Deformity may provide clues to a history of trauma. Assess the ulnar nerve during active flexion and extension to identify a subluxatable nerve at the cubital tunnel. Examine the ulno-humeral joint for crepitus, and palpate the joint line for large osteophytes and/or ganglion cysts.
Motor examination of the ulnar nerve primarily focuses on the intrinsic muscles detailed in TABLE 3,27-32 although the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring finger and small finger are also innervated by the ulnar nerve. The FCU mediates the power grip, and can be tested by resisted wrist flexion and ulnar deviation. Ring finger and small finger FDP strength should be examined by resistance testing at the distal IPJ.
Provocative testing of cubital tunnel syndrome includes Tinel’s sign (performed over the cubital tunnel), the elbow flexion test (performed with elbow in maximum flexion and wrist at neutral position and held for 60 seconds), and the pressure provocation test (performed by applying pressure to the ulnar nerve just proximal to the cubital tunnel with your index and long fingers for 60 seconds while the patient’s elbow is at 20° flexion with the forearm supinated). For each test, eliciting distal paresthesia in ulnar nerve territory is a positive result. The sensitivity of these tests ranges from 70% (Tinel’s sign) to 98% (combined elbow flexion and pressure); specificity ranges from 95% to 99%.35
The scratch collapse test for cubital tunnel syndrome evaluation is similar to the version used to assess for CTS; the only difference is the location of the site that is scratched, in this case the cubital tunnel. The reported sensitivity and specificity are 69% and 99%, respectively.11
Wartenberg’s syndrome
When a patient reports paresthesia or pain along the radial aspect of the forearm that radiates into the dorsal thumb and index and middle fingers, consider Wartenberg’s syndrome—compression of the superficial radial nerve. The condition, also known as “cheiralgia paresthetica,” may exist anywhere along the course of the nerve in the forearm,36 but the compression typically occurs 9 cm proximal to the radial styloid.
Assess the skin over the forearm and hand for evidence of prior trauma, surgical scars, or external compression sources, such as a watch.37-39 Then use palpation to identify superficial or deep masses along the nerve.40 Perform Tinel’s sign by lightly tapping along the nerve course from proximal to distal in the forearm. The test is positive if paresthesias are provoked distally.41,42
Motor function of the extremity is unaffected in Wartenberg’s syndrome, unless a prior traumatic injury occurred or a comorbidity exists. Comorbidities to consider include de Quervain’s tenosynovitis, cervical radiculopathy, injury to the lateral antebrachial cutaneous nerve, and CTS.
Gross sensory testing usually is negative, but 2-point discrimination may show diminished responses. Testing distribution includes the dorsal radial wrist and hand, dorsal thumb skin proximal to the IPJ, and the dorsal surface of the index, middle, and radial half of the ring finger proximal to the IPJ.
Proceed with caution. Provocative testing with a pronated forearm and a flexed and ulnar-deviated wrist may exacerbate symptoms,12,42 and Finkelstein’s maneuver (isolated ulnar deviation at the wrist to elicit pain over the first dorsal wrist compartment) and Phalen’s test may elicit false-positive results. Upper motor neuron exams (ie, deep tendon reflexes) and Hoffman’s sign (reflexive flexion of the terminal phalanges of the thumb and index finger induced by flicking or tapping the distal phalanx of the long finger) should be symmetric to the contralateral extremity.
In patients with more than one compression injury, Spurling’s sign (neck extension and lateral rotation towards the affected extremity) may induce paresthesia when combined with axial compression, while the shoulder abduction test (shoulder 90° abduction, with external rotation) may diminish reported paresthesia. In those for whom Wartenberg’s syndrome is their only compression injury, however, these provocative maneuvers will be negative.
Anterior interosseous nerve syndrome
A rare clinical entity that affects the anterior interosseous innervated muscles in the forearm, anterior interosseous nerve syndrome (AINS)’s etiology is unclear. But it is likely related to a spontaneous neuritis rather than a compressive etiology.43 Paresis or paralysis of the flexor pollicis longus (FPL) and the flexor digitorum profundus (FDP) of the index and long finger is its hallmark, but patients may report vague forearm pain associated with weakness or absence of function.
Examination begins with a visual assessment and palpation of the forearm and hand for atrophy or a space-occupying lesion. Notably, sensory function of the hand should remain at baseline despite the motor dysfunction, as the anterior interosseous is purely a motor nerve.
Motor testing should focus on the median and anterior interosseous innervated muscles.
The FPL is tested by isolated thumb IPJ flexion and the Kiloh and Nevin sign—the inability to make an “OK” sign with the thumb and index finger.44 Assess pinch grasp with a maximally flexed thumb IPJ and distal IPJ of the index finger. Anterior interosseous nerve dysfunction results in a flattened pinch without IPJ or distal IPJ flexion, which increases the contact of the thumb and index finger pulp.45 In a series of 14 patients, researchers reported complete paralysis of FPL and FDP index finger in 5 patients, isolated paralysis of FPL in 7 patients, and isolated paralysis of FDP index and long fingers in 2 patients. None had isolated paralysis of the FDP long finger.46FIGURE 4 shows complete (A) and incomplete (B) paralysis.
Evaluation of AINS also includes assessment of FPL and FDP tendon integrity with passive tenodesis. The appearance of the hand at rest should reflect the natural digital cascade. In a completely relaxed or anesthetized hand, the forearm is placed in wrist supination and extension, allowing gravity to extend the wrist and placing the thumb MCPJ at 30° flexion, the index finger MCPJ at 40°, and the small finger MCPJ at 70°. The thumb IPJ should approximate the radial fingertip pulp of the index finger. The forearm is then pronated and flexed at the wrist, straightening the thumb MCPJ and producing a mild flexion cascade at the proximal and distal IPJ of the index, long, ring, and small fingers within 20° of full extension. This dynamic exercise is used to confirm that the patient has an intact tenodesis effect, thus excluding tendon lacerations or ruptures from the differential diagnosis. In one study, in 9 of 33 cases of partial or complete isolated index finger FPL or FDP, paralysis was initially diagnosed as tendon rupture.47
When to consider electrodiagnostic testing
Electrodiagnostic testing—a combination of electromyography and nerve conduction studies to assess the status of a peripheral nerve48—provides objective data that can help diagnose a challenging presentation, rule out a competing diagnosis, or clarify an atypical clinical picture or vague subjective history. This type of testing is also used to localize the entrapment site, identify a patient with polyneuropathy or brachial plexopathy, and assess the severity of nerve injury or presence of a double crush syndrome.49,50 Any patient with signs and symptoms of a compression neuropathy and supportive findings on physical exam should be referred for electrodiagnostic testing and/or to a surgeon specializing in treating these conditions.
CORRESPONDENCE
Kyle J. MacGillis, MD, University of Illinois at Chicago, Department of Orthopaedic Surgery, 835 South Wolcott Avenue, M/C 844, Chicago, IL 60612; [email protected].
1. Katz JN, Stirrat CR, Larson MG, et al. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495-1498.
2. Werner RA, Chiodo T, Spiegelberg T, et al. Use of hand diagrams in screening for ulnar neuropathy: comparison with electrodiagnostic studies. Muscle Nerve. 2012;46:891-894.
3. Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8:229-239.
4. Kaji H, Honma H, Usui M, et al. Hypothenar hammer syndrome in workers occupationally exposed to vibrating tools. J Hand Surg Br. 1993;18:761-766.
5. Blum J. Examination and interface with the musician. Hand Clin. 2003;19:223-230.
6. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
7. Craig, AS, Richardson JK. Acquired peripheral neuropathy. Phys Med Rehabil Clin N Am. 2003;14:365-386.
8. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
9. Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919-924.
10. Foucher G, Malizos C, Sammut D, et al. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release: a series of 73 cases. J Hand Surg Br. 1991;16:56-60.
11. Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120-127.
12. Dellon AL, Mackinnon SE. Radial sensory nerve entrapment in the forearm. J Hand Surg Am. 1986;11:199-205.
13. Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg (Am). 1985;10:906-907.
14. Louis DS, Greene TL, Jacobson KE, et al. Evaluation of normal values for stationary and moving two-point discrimination in the hand. J Hand Surg Am. 1984;9:552-555.
15. Omer GE Jr. Physical diagnosis of peripheral nerve injuries. Orthop Clin North Am. 1981;12:207-228.
16. Cheng CJ, Mackinnon-Patterson B, Beck JL, et al. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33:1518-1524.
17. Haussmann P, Patel MR. Intraepineurial constriction of nerve fascicles in pronator syndrome and anterior interosseous nerve syndrome. Orthop Clin North Am. 1996;27:339-344.
18. Lee AK, Khorsandi M, Nurbhai N, et al. Endoscopically assisted decompression for pronator syndrome. J Hand Surg Am. 2012;37:1173-1179.
19. Lee MJ, LaStayo PC. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34:601-609.
20. Gainor BJ. The pronator compression test revisited: a forgotten physical sign. Orthop Rev. 1990;19:888-892.
21. Rehak DC. Pronator syndrome. Clin Sports Med. 2001;20:531-540.
22. Eversmann WW. Compression and entrapment neuropathies of the upper extremity. J Hand Surg Am. 1983;8:759-766.
23. Masse L. Contribution a l’etude de l’achon des interosseus. J Med (Bordeaux). 1916;46:198-200.
24. Draeger RW, Stern PJ. The inverted pyramid sign and other eponymous signs of ulnar nerve palsy. J Hand Surg Am. 2014;39:2517-2520.
25. Wang B, Zhao Y, Lu A, et al. Ulnar nerve deep branch compression by a ganglion: a review of nine cases. Injury. 2014;45:1126-1130.
26. Polatsch DB, Melone CP, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283-289.
27. Buschbacher R. Side-to-side confrontational strength-testing for weakness of the intrinsic muscles of the hand. J Bone Joint Surg Am. 1997;79:401-405.
28. Wartenberg R. A sign of ulnar palsy. JAMA. 1939;112:1688.
29. Earle A, Vlastou C. Crossed fingers and other tests of ulnar nerve motor function. J Hand Surg Am. 1980;5:560-565.
30. Tsujino A, Macnicol M. Finger flexion sign for ulnar neuropathy. J Hand Surg Br. 1998;23:240-241.
31. Mannerfelt L. Studies on the hand in ulnar nerve paralysis: a clinical-experimental investigation in normal and anomalous innervations. Acta Orthop Scand. 1966;87(Suppl):S118-S129.
32. Froment J. La prehension dans les paralysies du nerf cubital et Le Signe du Pouce. Presse Med. 1915;23:409.
33. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32:855-858.
34. Huang JH, Samadani U, Zagar EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
35. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817-820.
36. Auerbach DM, Collins ED, Kunkle KL, et al. The radial sensory nerve. an anatomic study. Clin Orthop Relat Res. 1994;308:241-249.
37. Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380-383.
38. Bierman HR. Nerve compression due to a tight watchband. N Engl J Med. 1959;261:237-238.
39. Linscheid RL. Injuries to radial nerve at wrist. Arch Surg. 1965;91:942-946.
40. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9:1005-1006.
41. Poppinchalk SP, Schaffer AA. Physical examination of the upper extremity compressive neuropathies. Orthop Clin N Am. 2012;43:417-430.
42. Posner MA. Compressive neuropathies of the median and radial nerves at the elbow. Clin Sports Med. 1990;9:343-363.
43. Dang AC, Rodner CM. Unusual compression neuropathies of the forearm. Part II: median nerve. J Hand Surg Am. 2009;34A:1915-1920.
44. Kiloh LG, Nevin S. Isolated neuritis of the anterior interosseous nerve. Br Med J. 1952;1:850-851.
45. Park IJ, Roh YT, Jeong C, et al. Spontaneous anterior interosseous nerve syndrome: clinical analysis and eleven surgical cases. J Plast Surg Hand Surg. 2013;47:519-523.
46. Ulrich D, Piatkowski A, Pallua N. Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg. 2011;131:1561-1565.
47. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10:4-16.
48. Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363-370.
49. Lo JK, Finestone HM, Gilbert K, et al. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: what is the diagnosis? Arch Phys Med Rehabil. 2002;83:598-603.
50. Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012;259:1502-1508.
› Use provocative testing to confirm a suspected diagnosis in a patient who presents with peripheral entrapment mononeuropathy. B
› Consider electrodiagnostic testing for help in diagnosing a challenging presentation, ruling out a competing diagnosis, or clarifying an atypical clinical picture or vague subjective history. A
› Evaluate any patient who presents with non-anatomic nerve distribution of symptoms—eg, burning, numbness, and tingling of the entire hand—for a metabolic, rather than an entrapment, neuropathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neuropathic hand complaints—for which patients typically seek medical attention when the pain or paresthesia starts to interfere with their daily routine—are common and diverse. The ability to assess and accurately diagnose upper extremity compression neuropathies is critical for physicians in primary care.
Assessment starts, of course, with a thorough history of the present illness and past medical history, which helps define a broad differential diagnosis and identify comorbidities. Physical examination, including judicious use of provocative testing, allows you to objectively identify the pathologic deficit, evaluate function and coordination of multiple organ systems, and detect nerve dysfunction. The results determine whether additional tools, such as electrodiagnostic testing, are needed.
We’ve created this guide, detailed in the text, tables, and figures that follow, to help you hone your ability to accurately diagnose patients who present with compression neuropathies of the hand.
The medical history: Knowing what to ask
To clearly define a patient’s symptoms and disability, start with a thorough history of the presenting complaint.
Inquire about symptom onset and chronicity. Did the pain or paresthesia begin after an injury? Are the symptoms associated with repetitive use of the extremity? Do they occur at night?
Pinpoint the location or distribution of pain or paresthesia. It is paramount to identify the affected nerve.1,2 Ask patients to complete a hand or upper extremity profile documenting location and/or type of numbness, tingling, or decreased sensation. A diagram of the peripheral nerves responsible for sensory innervation of the hand (FIGURE 1) is an effective way to screen individuals at high risk of carpal tunnel syndrome (CTS) or ulnar tunnel syndrome (UTS).1,2
A patient report such as, “My whole hand is numb,” calls for a follow-up question to determine whether the little finger is affected,3 which would indicate that the ulnar nerve, rather than just the median nerve, is involved. And if a patient reports feeling as if he or she is wearing gloves or mittens, it is essential to consider the possibility of a systemic neuropathy rather than a single peripheral neuropathy.3
Gather basic patient information. Inquire about hand dominance, occupation, and baseline function, any or all of which may be critical in the assessment and initiation of treatment.4,5
Review systemic conditions and medications
A broad range of comorbidities, such as cervical radiculopathy, diabetes, hypothyroidism, and vitamin deficiencies (TABLE 1),6,7 may be responsible for neuropathic hand complaints, and a thorough review of systemic complaints and past medical history is critical. Include a medication history and a review of prior procedures, such as post-traumatic surgeries of the hand or upper extremity or nerve decompression surgeries, which may provide additional insight into disease etiology.
Symptoms guide physical exam, provocative testing
A physical examination, including provocative testing, follows based on reported symptoms, medical history, and suspected source of nerve compression.
Carpal tunnel syndrome
CTS is the most common peripheral neuropathy.8 Patients often report nocturnal pain or paresthesia in the distal median nerve distribution, comprising the palmar surface of the thumb, index, middle, and radial half of the ring finger.
Researchers have identified 6 standardized clinical criteria for the diagnosis of CTS. Two criteria—numbness mostly in median nerve territory and nocturnal numbness—can be ascertained during the history of present illness.The other 4, detailed below, will be found during the physical exam.9
Thenar weakness or atrophy.9 Begin your evaluation by inspecting the thenar musculature for atrophic changes. Motor exam of intrinsic musculature innervated by the recurrent motor branch of the median nerve includes assessment of thumb abduction strength (assessed by applying resistance to the metacarpophalangeal joint [MCPJ] base towards the palm in the position of maximal abduction) and opposition strength (assessed by applying force to the MCPJ from the ulnar aspect).10
Positive Phalen’s test.9 Provocative testing for CTS includes Phalen’s test (sensitivity 43%-86%, specificity 48%-67%),11 which is an attempt to reproduce the numbness or tingling in the median nerve territory within 60 seconds of full wrist flexion. Ask the patient to hold his or her forearms vertically with elbows resting on the table (allowing gravity to flex the wrists),12 and to tell you if numbness or tingling occurs.
Positive Tinel’s sign.9 Tinel’s sign (sensitivity 45%-75%, specificity 48%-67%)11 is performed by lightly tapping the median nerve from the proximal to distal end over the carpal tunnel. The test is positive if paresthesia results. Provocative testing may also include Durkan’s test, also known as the carpal compression test. Durkan’s test (sensitivity 49%-89%, specificity 54%-96%)11 involves placing your thumb directly over the carpal tunnel and holding light compression for 60 seconds, or until paresthesia is reported.
Positive 2-point discrimination test.9 To assess CTS disease severity, use 2-point discrimination to evaluate the patient’s sensation qualitatively and quantitatively. Two-point discrimination can only be tested, however, if light touch sensation is intact. It is typically performed by lightly applying 2 caliper points at fixed distances sufficient to blanch the skin, but some clinicians have used other tools, such as a modified paperclip.13 The smallest distance at which the patient can detect 2 distinct stimuli is then recorded.
Researchers have reported an average of 3 to 5 mm for 2-point discrimination at the fingertipand a normal 2-point discrimination of 6 to 9 mm in the volar surface of the hand (TABLE 2).14,15
The scratch collapse test (sensitivity 64%, specificity 99%) is a supplemental exam that uses a different outcome measure to diagnose CTS.16 It involves lightly scratching the skin over the compressed carpal tunnel while the patient performs sustained resisted bilateral shoulder external rotation in an adducted position. A momentary loss of muscle resistance to external rotation indicates a positive test.
Pronator syndrome
Pronator syndrome (PS) is a proximal median neuropathy that may present in isolation or in combination with CTS as a double crush syndrome. Clinical symptoms include features of CTS and sensory paresthesias in the palm and distal forearm in the distribution of the palmar cutaneous branch of the median nerve. PS is commonly associated with volar proximal forearm pain exacerbated by repetitive activities involving pronation and supination.
PS is not easy to assess. Palpatory examination of a large supracondylar process at the distal humerus proximal to the medial epicondyle on its anteromedial aspect can be difficult, especially if the patient is overweight. And motor weakness is not a prominent feature. What’s more, power assessment of the pronator teres, flexor carpi radialis, and flexor digitorum superficialis may exacerbate symptoms.
Because the symptoms of PS and CTS may be the same, PS provocation maneuvers should be performed on patients with CTS symptoms and paresthesia involving the palm. Start by testing for Tinel’s sign over the pronator teres muscle, although this has been found to be positive in less than 50% of PS cases.17 Palpate the antecubital fossa and the proximal aspect of the pronator teres muscle to assess for discomfort or tenderness.
Pronator compression test. The pronator compression test has been found to be the most sensitive way to assess PS.18,19 This test involves direct compression of the proximal and radial edge of the pronator teres muscle belly along the proximal volar forearm with the thumb.20 It is performed bilaterally on supinated upper extremities, with the clinician applying pressure on each forearm simultaneously (FIGURE 2). If the symptoms in the hand are reproduced in ≤30 seconds, the test is positive. In a study of 10 patients with surgically confirmed PS, the pronator compression test was positive in every case.20,21
Resistance testing. You can also evaluate the pronator teres compression site by testing the patient’s ability to resist pronation with his or her elbow extended and the forearm in neutral position. To test for compression from the bicipital aponeurosis, ask the patient to flex the elbow to approximately 120° to 130° and apply active supinated resistance.22 Likewise, resistance of the long finger proximal interphalangeal joint (IPJ) to flexion—a maneuver performed with elbow fully extended—assesses compression from the fibrous arcade of the flexor digitorum superficialis (FDS).21 A positive resistance test will reproduce the reported symptoms.
Ulnar tunnel syndrome
Symptoms of UTS, which is much less common than CTS, include pain in the wrist and hand that is associated with paresthesia or numbness in the small finger and ulnar half of the ring finger. Patients may report difficulty with motor tasks involving grip and pinch strength or fatigue with prolonged action of the intrinsic muscles. Many also report an exacerbation of symptoms associated with increased wrist flexion or at night.
Evaluation of UTS requires a full assessment of the upper extremity, starting with observation of hand posture and muscle bulk to identify signs of chronic nerve compression. The contralateral extremity serves as a control to the neuropathic hand. Classically, chronic ulnar nerve compression leads to intrinsic muscle atrophy, evidenced by loss of topographical soft tissue bulk in the first dorsal web space, the palmar transverse metacarpal arch, and the hypothenar area.23 Ulnar motor nerve dysfunction is limited to the intrinsic muscles of the hand. The inverted pyramid sign, signified by atrophy of the transverse head of the adductor pollicis, is another visual aberrancy,24 as is clawing of the ring finger and small finger. The clawing, which involves hyperextension of the MCPJ and flexion of the proximal and distal IPJ, is commonly known as Duchenne’s sign. FIGURE 3 demonstrates hypothenar atrophy and the loss of muscle bulk in the first dorsal web space.
When you suspect UTS, palpate the wrist and hand in an attempt to locate a mass or area of tenderness. Not all patients with a volar ganglion cyst responsible for UTS present with a palpable mass, but tenderness along the radial aspect of the pisiform or an undefined fullness in this area may be noted.25 Any patient with a palpable mass should be tested for Tinel’s sign over the mass and undergo a thorough vascular assessment. Fracture of the hook of the hamate is indicated by tenderness in the region approximated by the intersection of Kaplan’s line and the proximal extension line from the ring finger.
Perform 2-point discrimination testing at the palmar distal aspect of the small finger and ulnar half of the ring finger. This tests the superficial sensory division of the ulnar nerve that travels within Guyon’s canal. Testing the dorsal ulnar cutaneous nerve involves the skin of the dorso-ulnar hand and dorsum of the long finger proximal to the IPJ. If this area is spared and the palmar distal ulnar digits are affected, compression within Guyon’s canal is likely. If both areas are affected, suspect a more proximal compression site at the cubital tunnel, as the dorsal ulnar cutaneous nerve branches proximal to Guyon’s canal.26
Ulnar motor nerve dysfunction in UTS is limited to the intrinsic muscles of the hand. Assessment of intrinsic muscle function is described in TABLE 3.27-32 It is important to become familiar with the tests and maneuvers described, but also to be aware that a comprehensive evaluation of ulnar nerve motor function requires a combination of tests.
Cubital tunnel syndrome
Cubital tunnel syndrome—the second most common peripheral neuropathy8—involves the proximal site of ulnar nerve compression in the upper extremity. Patients typically report symptoms similar to those of UTS, with sensory paresthesia in the ulnar digits and intrinsic weakness. To learn more about the symptoms, ask if the onset of pain or paresthesia is related to a particular elbow position, such as increased elbow flexion.
Notably, pain is usually not the initial complaint, unless the disease is advanced. This may be the reason atrophic intrinsic changes are 4 times more likely to be seen in patients with cubital tunnel syndrome than in those with CTS.33 You’re more likely to hear about vague motor problems, including hand clumsiness and difficulty with fine coordination of the fingers.34 Thus, it is important to evaluate patients for concurrent UTS and/or CTS, as well as for differentiation.
Focus on the elbow. Whenever you suspect cubital tunnel syndrome, pay special attention to the elbow. Examine the carrying angle of the elbow in relation to the contralateral extremity. Deformity may provide clues to a history of trauma. Assess the ulnar nerve during active flexion and extension to identify a subluxatable nerve at the cubital tunnel. Examine the ulno-humeral joint for crepitus, and palpate the joint line for large osteophytes and/or ganglion cysts.
Motor examination of the ulnar nerve primarily focuses on the intrinsic muscles detailed in TABLE 3,27-32 although the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring finger and small finger are also innervated by the ulnar nerve. The FCU mediates the power grip, and can be tested by resisted wrist flexion and ulnar deviation. Ring finger and small finger FDP strength should be examined by resistance testing at the distal IPJ.
Provocative testing of cubital tunnel syndrome includes Tinel’s sign (performed over the cubital tunnel), the elbow flexion test (performed with elbow in maximum flexion and wrist at neutral position and held for 60 seconds), and the pressure provocation test (performed by applying pressure to the ulnar nerve just proximal to the cubital tunnel with your index and long fingers for 60 seconds while the patient’s elbow is at 20° flexion with the forearm supinated). For each test, eliciting distal paresthesia in ulnar nerve territory is a positive result. The sensitivity of these tests ranges from 70% (Tinel’s sign) to 98% (combined elbow flexion and pressure); specificity ranges from 95% to 99%.35
The scratch collapse test for cubital tunnel syndrome evaluation is similar to the version used to assess for CTS; the only difference is the location of the site that is scratched, in this case the cubital tunnel. The reported sensitivity and specificity are 69% and 99%, respectively.11
Wartenberg’s syndrome
When a patient reports paresthesia or pain along the radial aspect of the forearm that radiates into the dorsal thumb and index and middle fingers, consider Wartenberg’s syndrome—compression of the superficial radial nerve. The condition, also known as “cheiralgia paresthetica,” may exist anywhere along the course of the nerve in the forearm,36 but the compression typically occurs 9 cm proximal to the radial styloid.
Assess the skin over the forearm and hand for evidence of prior trauma, surgical scars, or external compression sources, such as a watch.37-39 Then use palpation to identify superficial or deep masses along the nerve.40 Perform Tinel’s sign by lightly tapping along the nerve course from proximal to distal in the forearm. The test is positive if paresthesias are provoked distally.41,42
Motor function of the extremity is unaffected in Wartenberg’s syndrome, unless a prior traumatic injury occurred or a comorbidity exists. Comorbidities to consider include de Quervain’s tenosynovitis, cervical radiculopathy, injury to the lateral antebrachial cutaneous nerve, and CTS.
Gross sensory testing usually is negative, but 2-point discrimination may show diminished responses. Testing distribution includes the dorsal radial wrist and hand, dorsal thumb skin proximal to the IPJ, and the dorsal surface of the index, middle, and radial half of the ring finger proximal to the IPJ.
Proceed with caution. Provocative testing with a pronated forearm and a flexed and ulnar-deviated wrist may exacerbate symptoms,12,42 and Finkelstein’s maneuver (isolated ulnar deviation at the wrist to elicit pain over the first dorsal wrist compartment) and Phalen’s test may elicit false-positive results. Upper motor neuron exams (ie, deep tendon reflexes) and Hoffman’s sign (reflexive flexion of the terminal phalanges of the thumb and index finger induced by flicking or tapping the distal phalanx of the long finger) should be symmetric to the contralateral extremity.
In patients with more than one compression injury, Spurling’s sign (neck extension and lateral rotation towards the affected extremity) may induce paresthesia when combined with axial compression, while the shoulder abduction test (shoulder 90° abduction, with external rotation) may diminish reported paresthesia. In those for whom Wartenberg’s syndrome is their only compression injury, however, these provocative maneuvers will be negative.
Anterior interosseous nerve syndrome
A rare clinical entity that affects the anterior interosseous innervated muscles in the forearm, anterior interosseous nerve syndrome (AINS)’s etiology is unclear. But it is likely related to a spontaneous neuritis rather than a compressive etiology.43 Paresis or paralysis of the flexor pollicis longus (FPL) and the flexor digitorum profundus (FDP) of the index and long finger is its hallmark, but patients may report vague forearm pain associated with weakness or absence of function.
Examination begins with a visual assessment and palpation of the forearm and hand for atrophy or a space-occupying lesion. Notably, sensory function of the hand should remain at baseline despite the motor dysfunction, as the anterior interosseous is purely a motor nerve.
Motor testing should focus on the median and anterior interosseous innervated muscles.
The FPL is tested by isolated thumb IPJ flexion and the Kiloh and Nevin sign—the inability to make an “OK” sign with the thumb and index finger.44 Assess pinch grasp with a maximally flexed thumb IPJ and distal IPJ of the index finger. Anterior interosseous nerve dysfunction results in a flattened pinch without IPJ or distal IPJ flexion, which increases the contact of the thumb and index finger pulp.45 In a series of 14 patients, researchers reported complete paralysis of FPL and FDP index finger in 5 patients, isolated paralysis of FPL in 7 patients, and isolated paralysis of FDP index and long fingers in 2 patients. None had isolated paralysis of the FDP long finger.46FIGURE 4 shows complete (A) and incomplete (B) paralysis.
Evaluation of AINS also includes assessment of FPL and FDP tendon integrity with passive tenodesis. The appearance of the hand at rest should reflect the natural digital cascade. In a completely relaxed or anesthetized hand, the forearm is placed in wrist supination and extension, allowing gravity to extend the wrist and placing the thumb MCPJ at 30° flexion, the index finger MCPJ at 40°, and the small finger MCPJ at 70°. The thumb IPJ should approximate the radial fingertip pulp of the index finger. The forearm is then pronated and flexed at the wrist, straightening the thumb MCPJ and producing a mild flexion cascade at the proximal and distal IPJ of the index, long, ring, and small fingers within 20° of full extension. This dynamic exercise is used to confirm that the patient has an intact tenodesis effect, thus excluding tendon lacerations or ruptures from the differential diagnosis. In one study, in 9 of 33 cases of partial or complete isolated index finger FPL or FDP, paralysis was initially diagnosed as tendon rupture.47
When to consider electrodiagnostic testing
Electrodiagnostic testing—a combination of electromyography and nerve conduction studies to assess the status of a peripheral nerve48—provides objective data that can help diagnose a challenging presentation, rule out a competing diagnosis, or clarify an atypical clinical picture or vague subjective history. This type of testing is also used to localize the entrapment site, identify a patient with polyneuropathy or brachial plexopathy, and assess the severity of nerve injury or presence of a double crush syndrome.49,50 Any patient with signs and symptoms of a compression neuropathy and supportive findings on physical exam should be referred for electrodiagnostic testing and/or to a surgeon specializing in treating these conditions.
CORRESPONDENCE
Kyle J. MacGillis, MD, University of Illinois at Chicago, Department of Orthopaedic Surgery, 835 South Wolcott Avenue, M/C 844, Chicago, IL 60612; [email protected].
› Use provocative testing to confirm a suspected diagnosis in a patient who presents with peripheral entrapment mononeuropathy. B
› Consider electrodiagnostic testing for help in diagnosing a challenging presentation, ruling out a competing diagnosis, or clarifying an atypical clinical picture or vague subjective history. A
› Evaluate any patient who presents with non-anatomic nerve distribution of symptoms—eg, burning, numbness, and tingling of the entire hand—for a metabolic, rather than an entrapment, neuropathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neuropathic hand complaints—for which patients typically seek medical attention when the pain or paresthesia starts to interfere with their daily routine—are common and diverse. The ability to assess and accurately diagnose upper extremity compression neuropathies is critical for physicians in primary care.
Assessment starts, of course, with a thorough history of the present illness and past medical history, which helps define a broad differential diagnosis and identify comorbidities. Physical examination, including judicious use of provocative testing, allows you to objectively identify the pathologic deficit, evaluate function and coordination of multiple organ systems, and detect nerve dysfunction. The results determine whether additional tools, such as electrodiagnostic testing, are needed.
We’ve created this guide, detailed in the text, tables, and figures that follow, to help you hone your ability to accurately diagnose patients who present with compression neuropathies of the hand.
The medical history: Knowing what to ask
To clearly define a patient’s symptoms and disability, start with a thorough history of the presenting complaint.
Inquire about symptom onset and chronicity. Did the pain or paresthesia begin after an injury? Are the symptoms associated with repetitive use of the extremity? Do they occur at night?
Pinpoint the location or distribution of pain or paresthesia. It is paramount to identify the affected nerve.1,2 Ask patients to complete a hand or upper extremity profile documenting location and/or type of numbness, tingling, or decreased sensation. A diagram of the peripheral nerves responsible for sensory innervation of the hand (FIGURE 1) is an effective way to screen individuals at high risk of carpal tunnel syndrome (CTS) or ulnar tunnel syndrome (UTS).1,2
A patient report such as, “My whole hand is numb,” calls for a follow-up question to determine whether the little finger is affected,3 which would indicate that the ulnar nerve, rather than just the median nerve, is involved. And if a patient reports feeling as if he or she is wearing gloves or mittens, it is essential to consider the possibility of a systemic neuropathy rather than a single peripheral neuropathy.3
Gather basic patient information. Inquire about hand dominance, occupation, and baseline function, any or all of which may be critical in the assessment and initiation of treatment.4,5
Review systemic conditions and medications
A broad range of comorbidities, such as cervical radiculopathy, diabetes, hypothyroidism, and vitamin deficiencies (TABLE 1),6,7 may be responsible for neuropathic hand complaints, and a thorough review of systemic complaints and past medical history is critical. Include a medication history and a review of prior procedures, such as post-traumatic surgeries of the hand or upper extremity or nerve decompression surgeries, which may provide additional insight into disease etiology.
Symptoms guide physical exam, provocative testing
A physical examination, including provocative testing, follows based on reported symptoms, medical history, and suspected source of nerve compression.
Carpal tunnel syndrome
CTS is the most common peripheral neuropathy.8 Patients often report nocturnal pain or paresthesia in the distal median nerve distribution, comprising the palmar surface of the thumb, index, middle, and radial half of the ring finger.
Researchers have identified 6 standardized clinical criteria for the diagnosis of CTS. Two criteria—numbness mostly in median nerve territory and nocturnal numbness—can be ascertained during the history of present illness.The other 4, detailed below, will be found during the physical exam.9
Thenar weakness or atrophy.9 Begin your evaluation by inspecting the thenar musculature for atrophic changes. Motor exam of intrinsic musculature innervated by the recurrent motor branch of the median nerve includes assessment of thumb abduction strength (assessed by applying resistance to the metacarpophalangeal joint [MCPJ] base towards the palm in the position of maximal abduction) and opposition strength (assessed by applying force to the MCPJ from the ulnar aspect).10
Positive Phalen’s test.9 Provocative testing for CTS includes Phalen’s test (sensitivity 43%-86%, specificity 48%-67%),11 which is an attempt to reproduce the numbness or tingling in the median nerve territory within 60 seconds of full wrist flexion. Ask the patient to hold his or her forearms vertically with elbows resting on the table (allowing gravity to flex the wrists),12 and to tell you if numbness or tingling occurs.
Positive Tinel’s sign.9 Tinel’s sign (sensitivity 45%-75%, specificity 48%-67%)11 is performed by lightly tapping the median nerve from the proximal to distal end over the carpal tunnel. The test is positive if paresthesia results. Provocative testing may also include Durkan’s test, also known as the carpal compression test. Durkan’s test (sensitivity 49%-89%, specificity 54%-96%)11 involves placing your thumb directly over the carpal tunnel and holding light compression for 60 seconds, or until paresthesia is reported.
Positive 2-point discrimination test.9 To assess CTS disease severity, use 2-point discrimination to evaluate the patient’s sensation qualitatively and quantitatively. Two-point discrimination can only be tested, however, if light touch sensation is intact. It is typically performed by lightly applying 2 caliper points at fixed distances sufficient to blanch the skin, but some clinicians have used other tools, such as a modified paperclip.13 The smallest distance at which the patient can detect 2 distinct stimuli is then recorded.
Researchers have reported an average of 3 to 5 mm for 2-point discrimination at the fingertipand a normal 2-point discrimination of 6 to 9 mm in the volar surface of the hand (TABLE 2).14,15
The scratch collapse test (sensitivity 64%, specificity 99%) is a supplemental exam that uses a different outcome measure to diagnose CTS.16 It involves lightly scratching the skin over the compressed carpal tunnel while the patient performs sustained resisted bilateral shoulder external rotation in an adducted position. A momentary loss of muscle resistance to external rotation indicates a positive test.
Pronator syndrome
Pronator syndrome (PS) is a proximal median neuropathy that may present in isolation or in combination with CTS as a double crush syndrome. Clinical symptoms include features of CTS and sensory paresthesias in the palm and distal forearm in the distribution of the palmar cutaneous branch of the median nerve. PS is commonly associated with volar proximal forearm pain exacerbated by repetitive activities involving pronation and supination.
PS is not easy to assess. Palpatory examination of a large supracondylar process at the distal humerus proximal to the medial epicondyle on its anteromedial aspect can be difficult, especially if the patient is overweight. And motor weakness is not a prominent feature. What’s more, power assessment of the pronator teres, flexor carpi radialis, and flexor digitorum superficialis may exacerbate symptoms.
Because the symptoms of PS and CTS may be the same, PS provocation maneuvers should be performed on patients with CTS symptoms and paresthesia involving the palm. Start by testing for Tinel’s sign over the pronator teres muscle, although this has been found to be positive in less than 50% of PS cases.17 Palpate the antecubital fossa and the proximal aspect of the pronator teres muscle to assess for discomfort or tenderness.
Pronator compression test. The pronator compression test has been found to be the most sensitive way to assess PS.18,19 This test involves direct compression of the proximal and radial edge of the pronator teres muscle belly along the proximal volar forearm with the thumb.20 It is performed bilaterally on supinated upper extremities, with the clinician applying pressure on each forearm simultaneously (FIGURE 2). If the symptoms in the hand are reproduced in ≤30 seconds, the test is positive. In a study of 10 patients with surgically confirmed PS, the pronator compression test was positive in every case.20,21
Resistance testing. You can also evaluate the pronator teres compression site by testing the patient’s ability to resist pronation with his or her elbow extended and the forearm in neutral position. To test for compression from the bicipital aponeurosis, ask the patient to flex the elbow to approximately 120° to 130° and apply active supinated resistance.22 Likewise, resistance of the long finger proximal interphalangeal joint (IPJ) to flexion—a maneuver performed with elbow fully extended—assesses compression from the fibrous arcade of the flexor digitorum superficialis (FDS).21 A positive resistance test will reproduce the reported symptoms.
Ulnar tunnel syndrome
Symptoms of UTS, which is much less common than CTS, include pain in the wrist and hand that is associated with paresthesia or numbness in the small finger and ulnar half of the ring finger. Patients may report difficulty with motor tasks involving grip and pinch strength or fatigue with prolonged action of the intrinsic muscles. Many also report an exacerbation of symptoms associated with increased wrist flexion or at night.
Evaluation of UTS requires a full assessment of the upper extremity, starting with observation of hand posture and muscle bulk to identify signs of chronic nerve compression. The contralateral extremity serves as a control to the neuropathic hand. Classically, chronic ulnar nerve compression leads to intrinsic muscle atrophy, evidenced by loss of topographical soft tissue bulk in the first dorsal web space, the palmar transverse metacarpal arch, and the hypothenar area.23 Ulnar motor nerve dysfunction is limited to the intrinsic muscles of the hand. The inverted pyramid sign, signified by atrophy of the transverse head of the adductor pollicis, is another visual aberrancy,24 as is clawing of the ring finger and small finger. The clawing, which involves hyperextension of the MCPJ and flexion of the proximal and distal IPJ, is commonly known as Duchenne’s sign. FIGURE 3 demonstrates hypothenar atrophy and the loss of muscle bulk in the first dorsal web space.
When you suspect UTS, palpate the wrist and hand in an attempt to locate a mass or area of tenderness. Not all patients with a volar ganglion cyst responsible for UTS present with a palpable mass, but tenderness along the radial aspect of the pisiform or an undefined fullness in this area may be noted.25 Any patient with a palpable mass should be tested for Tinel’s sign over the mass and undergo a thorough vascular assessment. Fracture of the hook of the hamate is indicated by tenderness in the region approximated by the intersection of Kaplan’s line and the proximal extension line from the ring finger.
Perform 2-point discrimination testing at the palmar distal aspect of the small finger and ulnar half of the ring finger. This tests the superficial sensory division of the ulnar nerve that travels within Guyon’s canal. Testing the dorsal ulnar cutaneous nerve involves the skin of the dorso-ulnar hand and dorsum of the long finger proximal to the IPJ. If this area is spared and the palmar distal ulnar digits are affected, compression within Guyon’s canal is likely. If both areas are affected, suspect a more proximal compression site at the cubital tunnel, as the dorsal ulnar cutaneous nerve branches proximal to Guyon’s canal.26
Ulnar motor nerve dysfunction in UTS is limited to the intrinsic muscles of the hand. Assessment of intrinsic muscle function is described in TABLE 3.27-32 It is important to become familiar with the tests and maneuvers described, but also to be aware that a comprehensive evaluation of ulnar nerve motor function requires a combination of tests.
Cubital tunnel syndrome
Cubital tunnel syndrome—the second most common peripheral neuropathy8—involves the proximal site of ulnar nerve compression in the upper extremity. Patients typically report symptoms similar to those of UTS, with sensory paresthesia in the ulnar digits and intrinsic weakness. To learn more about the symptoms, ask if the onset of pain or paresthesia is related to a particular elbow position, such as increased elbow flexion.
Notably, pain is usually not the initial complaint, unless the disease is advanced. This may be the reason atrophic intrinsic changes are 4 times more likely to be seen in patients with cubital tunnel syndrome than in those with CTS.33 You’re more likely to hear about vague motor problems, including hand clumsiness and difficulty with fine coordination of the fingers.34 Thus, it is important to evaluate patients for concurrent UTS and/or CTS, as well as for differentiation.
Focus on the elbow. Whenever you suspect cubital tunnel syndrome, pay special attention to the elbow. Examine the carrying angle of the elbow in relation to the contralateral extremity. Deformity may provide clues to a history of trauma. Assess the ulnar nerve during active flexion and extension to identify a subluxatable nerve at the cubital tunnel. Examine the ulno-humeral joint for crepitus, and palpate the joint line for large osteophytes and/or ganglion cysts.
Motor examination of the ulnar nerve primarily focuses on the intrinsic muscles detailed in TABLE 3,27-32 although the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring finger and small finger are also innervated by the ulnar nerve. The FCU mediates the power grip, and can be tested by resisted wrist flexion and ulnar deviation. Ring finger and small finger FDP strength should be examined by resistance testing at the distal IPJ.
Provocative testing of cubital tunnel syndrome includes Tinel’s sign (performed over the cubital tunnel), the elbow flexion test (performed with elbow in maximum flexion and wrist at neutral position and held for 60 seconds), and the pressure provocation test (performed by applying pressure to the ulnar nerve just proximal to the cubital tunnel with your index and long fingers for 60 seconds while the patient’s elbow is at 20° flexion with the forearm supinated). For each test, eliciting distal paresthesia in ulnar nerve territory is a positive result. The sensitivity of these tests ranges from 70% (Tinel’s sign) to 98% (combined elbow flexion and pressure); specificity ranges from 95% to 99%.35
The scratch collapse test for cubital tunnel syndrome evaluation is similar to the version used to assess for CTS; the only difference is the location of the site that is scratched, in this case the cubital tunnel. The reported sensitivity and specificity are 69% and 99%, respectively.11
Wartenberg’s syndrome
When a patient reports paresthesia or pain along the radial aspect of the forearm that radiates into the dorsal thumb and index and middle fingers, consider Wartenberg’s syndrome—compression of the superficial radial nerve. The condition, also known as “cheiralgia paresthetica,” may exist anywhere along the course of the nerve in the forearm,36 but the compression typically occurs 9 cm proximal to the radial styloid.
Assess the skin over the forearm and hand for evidence of prior trauma, surgical scars, or external compression sources, such as a watch.37-39 Then use palpation to identify superficial or deep masses along the nerve.40 Perform Tinel’s sign by lightly tapping along the nerve course from proximal to distal in the forearm. The test is positive if paresthesias are provoked distally.41,42
Motor function of the extremity is unaffected in Wartenberg’s syndrome, unless a prior traumatic injury occurred or a comorbidity exists. Comorbidities to consider include de Quervain’s tenosynovitis, cervical radiculopathy, injury to the lateral antebrachial cutaneous nerve, and CTS.
Gross sensory testing usually is negative, but 2-point discrimination may show diminished responses. Testing distribution includes the dorsal radial wrist and hand, dorsal thumb skin proximal to the IPJ, and the dorsal surface of the index, middle, and radial half of the ring finger proximal to the IPJ.
Proceed with caution. Provocative testing with a pronated forearm and a flexed and ulnar-deviated wrist may exacerbate symptoms,12,42 and Finkelstein’s maneuver (isolated ulnar deviation at the wrist to elicit pain over the first dorsal wrist compartment) and Phalen’s test may elicit false-positive results. Upper motor neuron exams (ie, deep tendon reflexes) and Hoffman’s sign (reflexive flexion of the terminal phalanges of the thumb and index finger induced by flicking or tapping the distal phalanx of the long finger) should be symmetric to the contralateral extremity.
In patients with more than one compression injury, Spurling’s sign (neck extension and lateral rotation towards the affected extremity) may induce paresthesia when combined with axial compression, while the shoulder abduction test (shoulder 90° abduction, with external rotation) may diminish reported paresthesia. In those for whom Wartenberg’s syndrome is their only compression injury, however, these provocative maneuvers will be negative.
Anterior interosseous nerve syndrome
A rare clinical entity that affects the anterior interosseous innervated muscles in the forearm, anterior interosseous nerve syndrome (AINS)’s etiology is unclear. But it is likely related to a spontaneous neuritis rather than a compressive etiology.43 Paresis or paralysis of the flexor pollicis longus (FPL) and the flexor digitorum profundus (FDP) of the index and long finger is its hallmark, but patients may report vague forearm pain associated with weakness or absence of function.
Examination begins with a visual assessment and palpation of the forearm and hand for atrophy or a space-occupying lesion. Notably, sensory function of the hand should remain at baseline despite the motor dysfunction, as the anterior interosseous is purely a motor nerve.
Motor testing should focus on the median and anterior interosseous innervated muscles.
The FPL is tested by isolated thumb IPJ flexion and the Kiloh and Nevin sign—the inability to make an “OK” sign with the thumb and index finger.44 Assess pinch grasp with a maximally flexed thumb IPJ and distal IPJ of the index finger. Anterior interosseous nerve dysfunction results in a flattened pinch without IPJ or distal IPJ flexion, which increases the contact of the thumb and index finger pulp.45 In a series of 14 patients, researchers reported complete paralysis of FPL and FDP index finger in 5 patients, isolated paralysis of FPL in 7 patients, and isolated paralysis of FDP index and long fingers in 2 patients. None had isolated paralysis of the FDP long finger.46FIGURE 4 shows complete (A) and incomplete (B) paralysis.
Evaluation of AINS also includes assessment of FPL and FDP tendon integrity with passive tenodesis. The appearance of the hand at rest should reflect the natural digital cascade. In a completely relaxed or anesthetized hand, the forearm is placed in wrist supination and extension, allowing gravity to extend the wrist and placing the thumb MCPJ at 30° flexion, the index finger MCPJ at 40°, and the small finger MCPJ at 70°. The thumb IPJ should approximate the radial fingertip pulp of the index finger. The forearm is then pronated and flexed at the wrist, straightening the thumb MCPJ and producing a mild flexion cascade at the proximal and distal IPJ of the index, long, ring, and small fingers within 20° of full extension. This dynamic exercise is used to confirm that the patient has an intact tenodesis effect, thus excluding tendon lacerations or ruptures from the differential diagnosis. In one study, in 9 of 33 cases of partial or complete isolated index finger FPL or FDP, paralysis was initially diagnosed as tendon rupture.47
When to consider electrodiagnostic testing
Electrodiagnostic testing—a combination of electromyography and nerve conduction studies to assess the status of a peripheral nerve48—provides objective data that can help diagnose a challenging presentation, rule out a competing diagnosis, or clarify an atypical clinical picture or vague subjective history. This type of testing is also used to localize the entrapment site, identify a patient with polyneuropathy or brachial plexopathy, and assess the severity of nerve injury or presence of a double crush syndrome.49,50 Any patient with signs and symptoms of a compression neuropathy and supportive findings on physical exam should be referred for electrodiagnostic testing and/or to a surgeon specializing in treating these conditions.
CORRESPONDENCE
Kyle J. MacGillis, MD, University of Illinois at Chicago, Department of Orthopaedic Surgery, 835 South Wolcott Avenue, M/C 844, Chicago, IL 60612; [email protected].
1. Katz JN, Stirrat CR, Larson MG, et al. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495-1498.
2. Werner RA, Chiodo T, Spiegelberg T, et al. Use of hand diagrams in screening for ulnar neuropathy: comparison with electrodiagnostic studies. Muscle Nerve. 2012;46:891-894.
3. Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8:229-239.
4. Kaji H, Honma H, Usui M, et al. Hypothenar hammer syndrome in workers occupationally exposed to vibrating tools. J Hand Surg Br. 1993;18:761-766.
5. Blum J. Examination and interface with the musician. Hand Clin. 2003;19:223-230.
6. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
7. Craig, AS, Richardson JK. Acquired peripheral neuropathy. Phys Med Rehabil Clin N Am. 2003;14:365-386.
8. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
9. Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919-924.
10. Foucher G, Malizos C, Sammut D, et al. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release: a series of 73 cases. J Hand Surg Br. 1991;16:56-60.
11. Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120-127.
12. Dellon AL, Mackinnon SE. Radial sensory nerve entrapment in the forearm. J Hand Surg Am. 1986;11:199-205.
13. Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg (Am). 1985;10:906-907.
14. Louis DS, Greene TL, Jacobson KE, et al. Evaluation of normal values for stationary and moving two-point discrimination in the hand. J Hand Surg Am. 1984;9:552-555.
15. Omer GE Jr. Physical diagnosis of peripheral nerve injuries. Orthop Clin North Am. 1981;12:207-228.
16. Cheng CJ, Mackinnon-Patterson B, Beck JL, et al. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33:1518-1524.
17. Haussmann P, Patel MR. Intraepineurial constriction of nerve fascicles in pronator syndrome and anterior interosseous nerve syndrome. Orthop Clin North Am. 1996;27:339-344.
18. Lee AK, Khorsandi M, Nurbhai N, et al. Endoscopically assisted decompression for pronator syndrome. J Hand Surg Am. 2012;37:1173-1179.
19. Lee MJ, LaStayo PC. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34:601-609.
20. Gainor BJ. The pronator compression test revisited: a forgotten physical sign. Orthop Rev. 1990;19:888-892.
21. Rehak DC. Pronator syndrome. Clin Sports Med. 2001;20:531-540.
22. Eversmann WW. Compression and entrapment neuropathies of the upper extremity. J Hand Surg Am. 1983;8:759-766.
23. Masse L. Contribution a l’etude de l’achon des interosseus. J Med (Bordeaux). 1916;46:198-200.
24. Draeger RW, Stern PJ. The inverted pyramid sign and other eponymous signs of ulnar nerve palsy. J Hand Surg Am. 2014;39:2517-2520.
25. Wang B, Zhao Y, Lu A, et al. Ulnar nerve deep branch compression by a ganglion: a review of nine cases. Injury. 2014;45:1126-1130.
26. Polatsch DB, Melone CP, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283-289.
27. Buschbacher R. Side-to-side confrontational strength-testing for weakness of the intrinsic muscles of the hand. J Bone Joint Surg Am. 1997;79:401-405.
28. Wartenberg R. A sign of ulnar palsy. JAMA. 1939;112:1688.
29. Earle A, Vlastou C. Crossed fingers and other tests of ulnar nerve motor function. J Hand Surg Am. 1980;5:560-565.
30. Tsujino A, Macnicol M. Finger flexion sign for ulnar neuropathy. J Hand Surg Br. 1998;23:240-241.
31. Mannerfelt L. Studies on the hand in ulnar nerve paralysis: a clinical-experimental investigation in normal and anomalous innervations. Acta Orthop Scand. 1966;87(Suppl):S118-S129.
32. Froment J. La prehension dans les paralysies du nerf cubital et Le Signe du Pouce. Presse Med. 1915;23:409.
33. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32:855-858.
34. Huang JH, Samadani U, Zagar EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
35. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817-820.
36. Auerbach DM, Collins ED, Kunkle KL, et al. The radial sensory nerve. an anatomic study. Clin Orthop Relat Res. 1994;308:241-249.
37. Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380-383.
38. Bierman HR. Nerve compression due to a tight watchband. N Engl J Med. 1959;261:237-238.
39. Linscheid RL. Injuries to radial nerve at wrist. Arch Surg. 1965;91:942-946.
40. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9:1005-1006.
41. Poppinchalk SP, Schaffer AA. Physical examination of the upper extremity compressive neuropathies. Orthop Clin N Am. 2012;43:417-430.
42. Posner MA. Compressive neuropathies of the median and radial nerves at the elbow. Clin Sports Med. 1990;9:343-363.
43. Dang AC, Rodner CM. Unusual compression neuropathies of the forearm. Part II: median nerve. J Hand Surg Am. 2009;34A:1915-1920.
44. Kiloh LG, Nevin S. Isolated neuritis of the anterior interosseous nerve. Br Med J. 1952;1:850-851.
45. Park IJ, Roh YT, Jeong C, et al. Spontaneous anterior interosseous nerve syndrome: clinical analysis and eleven surgical cases. J Plast Surg Hand Surg. 2013;47:519-523.
46. Ulrich D, Piatkowski A, Pallua N. Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg. 2011;131:1561-1565.
47. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10:4-16.
48. Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363-370.
49. Lo JK, Finestone HM, Gilbert K, et al. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: what is the diagnosis? Arch Phys Med Rehabil. 2002;83:598-603.
50. Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012;259:1502-1508.
1. Katz JN, Stirrat CR, Larson MG, et al. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol. 1990;17:1495-1498.
2. Werner RA, Chiodo T, Spiegelberg T, et al. Use of hand diagrams in screening for ulnar neuropathy: comparison with electrodiagnostic studies. Muscle Nerve. 2012;46:891-894.
3. Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8:229-239.
4. Kaji H, Honma H, Usui M, et al. Hypothenar hammer syndrome in workers occupationally exposed to vibrating tools. J Hand Surg Br. 1993;18:761-766.
5. Blum J. Examination and interface with the musician. Hand Clin. 2003;19:223-230.
6. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
7. Craig, AS, Richardson JK. Acquired peripheral neuropathy. Phys Med Rehabil Clin N Am. 2003;14:365-386.
8. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263-265.
9. Graham B, Regehr G, Naglie G, et al. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg Am. 2006;31:919-924.
10. Foucher G, Malizos C, Sammut D, et al. Primary palmaris longus transfer as an opponensplasty in carpal tunnel release: a series of 73 cases. J Hand Surg Br. 1991;16:56-60.
11. Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120-127.
12. Dellon AL, Mackinnon SE. Radial sensory nerve entrapment in the forearm. J Hand Surg Am. 1986;11:199-205.
13. Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg (Am). 1985;10:906-907.
14. Louis DS, Greene TL, Jacobson KE, et al. Evaluation of normal values for stationary and moving two-point discrimination in the hand. J Hand Surg Am. 1984;9:552-555.
15. Omer GE Jr. Physical diagnosis of peripheral nerve injuries. Orthop Clin North Am. 1981;12:207-228.
16. Cheng CJ, Mackinnon-Patterson B, Beck JL, et al. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33:1518-1524.
17. Haussmann P, Patel MR. Intraepineurial constriction of nerve fascicles in pronator syndrome and anterior interosseous nerve syndrome. Orthop Clin North Am. 1996;27:339-344.
18. Lee AK, Khorsandi M, Nurbhai N, et al. Endoscopically assisted decompression for pronator syndrome. J Hand Surg Am. 2012;37:1173-1179.
19. Lee MJ, LaStayo PC. Pronator syndrome and other nerve compressions that mimic carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34:601-609.
20. Gainor BJ. The pronator compression test revisited: a forgotten physical sign. Orthop Rev. 1990;19:888-892.
21. Rehak DC. Pronator syndrome. Clin Sports Med. 2001;20:531-540.
22. Eversmann WW. Compression and entrapment neuropathies of the upper extremity. J Hand Surg Am. 1983;8:759-766.
23. Masse L. Contribution a l’etude de l’achon des interosseus. J Med (Bordeaux). 1916;46:198-200.
24. Draeger RW, Stern PJ. The inverted pyramid sign and other eponymous signs of ulnar nerve palsy. J Hand Surg Am. 2014;39:2517-2520.
25. Wang B, Zhao Y, Lu A, et al. Ulnar nerve deep branch compression by a ganglion: a review of nine cases. Injury. 2014;45:1126-1130.
26. Polatsch DB, Melone CP, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283-289.
27. Buschbacher R. Side-to-side confrontational strength-testing for weakness of the intrinsic muscles of the hand. J Bone Joint Surg Am. 1997;79:401-405.
28. Wartenberg R. A sign of ulnar palsy. JAMA. 1939;112:1688.
29. Earle A, Vlastou C. Crossed fingers and other tests of ulnar nerve motor function. J Hand Surg Am. 1980;5:560-565.
30. Tsujino A, Macnicol M. Finger flexion sign for ulnar neuropathy. J Hand Surg Br. 1998;23:240-241.
31. Mannerfelt L. Studies on the hand in ulnar nerve paralysis: a clinical-experimental investigation in normal and anomalous innervations. Acta Orthop Scand. 1966;87(Suppl):S118-S129.
32. Froment J. La prehension dans les paralysies du nerf cubital et Le Signe du Pouce. Presse Med. 1915;23:409.
33. Mallette P, Zhao M, Zurakowski D, et al. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32:855-858.
34. Huang JH, Samadani U, Zagar EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150-1153.
35. Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817-820.
36. Auerbach DM, Collins ED, Kunkle KL, et al. The radial sensory nerve. an anatomic study. Clin Orthop Relat Res. 1994;308:241-249.
37. Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380-383.
38. Bierman HR. Nerve compression due to a tight watchband. N Engl J Med. 1959;261:237-238.
39. Linscheid RL. Injuries to radial nerve at wrist. Arch Surg. 1965;91:942-946.
40. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9:1005-1006.
41. Poppinchalk SP, Schaffer AA. Physical examination of the upper extremity compressive neuropathies. Orthop Clin N Am. 2012;43:417-430.
42. Posner MA. Compressive neuropathies of the median and radial nerves at the elbow. Clin Sports Med. 1990;9:343-363.
43. Dang AC, Rodner CM. Unusual compression neuropathies of the forearm. Part II: median nerve. J Hand Surg Am. 2009;34A:1915-1920.
44. Kiloh LG, Nevin S. Isolated neuritis of the anterior interosseous nerve. Br Med J. 1952;1:850-851.
45. Park IJ, Roh YT, Jeong C, et al. Spontaneous anterior interosseous nerve syndrome: clinical analysis and eleven surgical cases. J Plast Surg Hand Surg. 2013;47:519-523.
46. Ulrich D, Piatkowski A, Pallua N. Anterior interosseous nerve syndrome: retrospective analysis of 14 patients. Arch Orthop Trauma Surg. 2011;131:1561-1565.
47. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10:4-16.
48. Bergquist ER, Hammert WC. Timing and appropriate use of electrodiagnostic studies. Hand Clin. 2013;29:363-370.
49. Lo JK, Finestone HM, Gilbert K, et al. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: what is the diagnosis? Arch Phys Med Rehabil. 2002;83:598-603.
50. Kane NM, Oware A. Nerve conduction and electromyography studies. J Neurol. 2012;259:1502-1508.
E-cigarettes: How “safe” are they?
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
From The Journal of Family Practice | 2016;65(6):380-385.
Beyond the bull's eye: Recognizing Lyme disease
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
From The Journal of Family Practice | 2016;65(6):373-379.
Diabetes update: Your guide to the latest ADA standards
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Is platelet-rich plasma right for your patient?
› Inform patients with knee osteoarthritis that although evidence is limited, platelet-rich plasma (PRP) injections may improve pain and function in the short-term. B
› Advise patients with elbow epicondylitis that PRP injections may improve pain and function slightly more than corticosteroid injections in the short-term. B
› Counsel patients that PRP has minimal risks; however, larger studies are needed to more fully assess whether harms exist. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Ms. T is an otherwise healthy 76 year old with a history of severe osteoarthritis (OA) in her right knee. She has participated in multiple rounds of physical therapy (PT) over the last 3 years. During the past year, she received 2 intra-articular corticosteroid injections, each of which provided only 3 to 4 weeks of pain relief, and one hyaluronic acid (HA) injection, which provided no benefit whatsoever.
Today, she describes her right knee pain as an 8 out of 10 and is frustrated by her lack of symptom relief. She was planning to have a total knee replacement and is a good surgical candidate, but recently found an article regarding platelet-rich plasma (PRP) injections for knee OA. She wants your opinion as to whether she should try this approach or proceed with surgery.
CASE 2 › Mr. H is a 44-year-old, right-handed dentist who has been suffering from right lateral epicondylitis for the past year. Although he has undergone PT and has been performing exercises at home since his symptoms began, he has not noticed a significant improvement. In the last 5 months, he has been out of work a total of 8 weeks due to the pain. He received one corticosteroid injection last month, which provided no improvement in symptoms. He is not interested in surgery, as he does not want to be out of work for a prolonged period of time.
He reports that one of his friends recently received a PRP injection for lateral epicondylitis and now feels great. He is aware that PRP injections are not covered by his health insurance and says he is willing to pay out of pocket if the treatment works. He wants to know if you recommend this course of action for his elbow pain.
How would you counsel each of these patients about the use of PRP injections for pain relief from their respective orthopedic conditions?
Musculoskeletal symptoms account for 10% to 28% of patients’ complaints to primary care physicians annually.1 Treatment of both chronic tendinopathies and knee OA—2 of the most common causes of these complaints—typically follows a stepwise approach, beginning with anti-inflammatory and pain medications in addition to PT. Patients who fail to respond to these interventions are often treated with corticosteroid injections, and, in the case of knee OA, viscosupplementation (ie, HA injections) and braces. If these therapies fail, patients are often forced to choose between an invasive surgical procedure or continued pain and limited function.
A number of physicians specializing in musculoskeletal medicine have turned to prolotherapy—specifically, dextrose prolotherapy (see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768) and platelet-rich plasma (PRP) therapy—as an alternative treatment for chronic musculoskeletal conditions.
PRP has been used to enhance surgical healing and to treat muscle strains and chondropathies. It drew a great deal of attention in the media when it was used by such high-profile professional athletes as Tiger Woods and Kobe Bryant.
Although PRP therapy is not commonly reimbursed by health insurance companies because of a lack of large, definitive studies supporting its effectiveness, patients are paying anywhere from a few hundred to a few thousand dollars out of pocket for it. They’re doing so in the hope that it will treat their chronic musculoskeletal disorders or at least delay surgical procedures.
But what can these patients reasonably expect from this therapy?
The following review of the evidence for PRP in the treatment of knee OA and tendinopathies (including elbow epicondylitis, patellar tendinitis, and Achilles tendinitis) will help you counsel patients on its appropriate use.
What is PRP?
PRP is defined as a sample of autologous blood with concentrations of platelets above baseline values.2 It is made through a one- or 2-stage centrifugation process in which the liquid and solid components of whole blood are separated, and then the liquid components are further separated into portions that are platelet-rich and platelet-poor.
Significant variability in preparation methods exists, resulting in more than 40 different products.2 Some methods centrifuge only once, creating plasma that is separated from red and white blood cells, but without a huge shift in the concentration of platelets; some include white blood cells in the final preparation; and most have differing concentrations of platelets and various growth factors in the end product. Researchers have attempted to classify the various preparations by platelet concentration, inclusion or exclusion of white blood cells, and fibrin content, but no validated system yet exists. Thus, consistency in preparations is lacking.3,4
PRP is rich not only in platelets, but also in a multitude of other growth factors. It is thought to improve healing by enhancing the body’s natural regenerative processes at the tissue level. In OA, for example, a complex balance of destructive and reparative processes is at play; PRP is thought to tip the body’s response in favor of regeneration over destruction. Similarly, chronic tendinopathy involves a process of destruction, reaction, healing, and degeneration; intervening at the correct point in this pathway with a boost to healing may help the body repair an otherwise diseased tendon.3
What does the evidence show?
Overall, basic science and preclinical research support “the promise” of PRP(strength of recommendation [SOR]: A).5 However, patient-centered evidence is lacking, and tremendous variability exists between studies, not only in terms of PRP preparation, but also with regard to:
- Protocol—Was ultrasound guidance used? Did the injection include needling of the tendon? What post-injection rehabilitation was followed?
- Patient population—What treatments were tried in the past? How chronic or severe was the problem?
- Study design—What was the comparison group? How were pain and function measured? Most studies have been small in size and have included various treatment modalities in addition to the PRP injection (most often PT).
Knee OA: PRP may provide short-term benefit, especially in younger patients
Researchers have conducted a number of studies evaluating PRP for knee OA.6-12 Most have compared PRP to HA—another intra-articular injection that is plagued by mixed, limited, and poor-quality evidence. These trials have had varied results and do not consistently support PRP as superior to HA.
The most well-designed study to date demonstrated that PRP was superior to saline and as effective as HA.11 In addition, the researchers found that a series of 3 PRP injections was superior to 3 injections of HA or only one injection of PRP.
One small randomized controlled trial (RCT) compared PRP injections to saline and found that PRP improved pain and function better than placebo at 6 weeks, 3 months, and 6 months; results appeared to deteriorate after that time period.6 Also, the findings suggested that PRP delivered the strongest benefit in younger patients who had less advanced OA.
In addition, a recent systematic review found short-term improvements in functional outcomes in patients treated with PRP injections vs those treated with HA injections and those treated with placebo.12
But before experts can make any conclusive recommendations regarding the use of PRP for knee OA, standardized studies with larger numbers of participants and rigorous methodology must be designed. Notably, no evidence exists of significant harm resulting from PRP injection for knee OA. Therefore, given the mixed evidence in terms of efficacy, there may be a potential benefit to treatment with little negative consequence.
In 2013, the American Academy of Orthopaedic Surgeons (AAOS) stated that they were unable to recommend for or against PRP injection for patients with symptomatic OA of the knee because the evidence was inconclusive.13 At the same time, the AAOS was unable to recommend for or against corticosteroid injections, manual therapy, or bracing for knee OA, and recommended against HA injections.13 Recently, however, the American Medical Society for Sports Medicine (AMSSM) recommended that HA be used in appropriate patients with knee OA.14
Such disagreement indicates that evidence is lacking for many modalities employed in the management of knee OA, including the injection of corticosteroids, which is a frequent and generally accepted treatment. Compounding matters is that many of the original studies testing the efficacy of PRP injection in knee OA used HA injections as the comparison, and there is no agreement between AAOS and AMSSM as to its usefulness. Thus, the validity of using HA as a control is suspect.
Tendinopathies: PRP may have benefit, but more research is needed
A number of meta-analyses and systematic review articles have combined the results of studies involving PRP treatment for various tendinopathies.3,15-17 While most found that PRP may have a benefit (although not long-lasting) and may be of use in attempts to avoid surgery or to return to a desired activity, all reported that more rigorous studies with standardized methodologies must be conducted before PRP can be conclusively recommended for any anatomic site.
Elbow epicondylitis (tennis elbow). The majority of tendinopathy studies have examined the effect of PRP on tennis elbow, although given the small study numbers (N=20-100), high risks of bias, and very different comparison groups, the data are extremely limited. Of the 4 randomized studies,18-21 2 compared different PRP preparations to whole blood,18,20 one compared PRP to both saline and corticosteroid,19 and one compared PRP to corticosteroid alone.21
The studies comparing PRP to whole blood found similar outcomes at most time points.18,20 These studies were of extremely poor quality, and other review articles have defined whole blood as a type of PRP, so this comparison was somewhat inappropriate. One recently published meta-analysis, which included 10 studies comparing either PRP or whole blood to corticosteroid, found that PRP improved pain more than a corticosteroid.22
The one study that included a comparison of PRP to placebo (saline) suffered from a high dropout rate, and the authors were not able to analyze the primary outcome data. At 3 months, the participants remaining in each group (PRP, saline, or corticosteroid) had similar pain and disability scores.19 Although the steroid group had improved from baseline at one month, there was no difference between the steroid group and placebo group at 3 months. The PRP group did not differ from the placebo group at any time point.
The study comparing PRP to corticosteroid alone found that PRP’s effects on pain and function exceeded those of the steroid. Specifically, the steroid group initially improved and then worsened, ending the study near their baseline pain and function scores.21 The PRP group, on the other hand, showed slow improvement throughout, ending the study with less pain and disability than when they started.
Patellar tendinitis (jumper’s knee). The majority of studies examining the effect of PRP on patellar tendinitis are non-randomized, non-comparative studies. Of the 2 small RCTs that were conducted, one compared PRP to extracorporeal shockwave therapy (ESWT),23 and the other to dry needling.24
In the ESWT study, there was a slight improvement in pain and function in the PRP group relative to the ESWT group at 6 and 12 months. In the other study, although the PRP group showed an improvement in recovery at 12 weeks relative to the dry needling group, there was no difference between such outcomes as pain and activity in the 2 groups at 26 weeks.
Worth noting here is that like the studies done on OA patients, the research involving patellar tendinitis also used comparative interventions (ESWT and dry needling) that lack high-quality evidence for their use. So whether these were appropriate comparisons is debatable.
Achilles tendinitis. Only one RCT (N=54) has evaluated PRP for the treatment of Achilles tendinitis.25 This study, which compared PRP to saline, excluded patients who had previously completed a course of PT, yet both study groups participated in PT during the study. Although the trial found no difference between groups at any time point (both showed improvement), it was underpowered to detect any difference (positive or negative) between groups, given that most participants likely would have improved with PT anyway.26
PRP has few harms or adverse effects
Most individual studies involving PRP have not reported on harms or side effects; the studies that have reported on them have generally found low rates (2%-5%) of only local, short-term adverse effects.15 One review article did find that increasing the number of PRP injections increased the rate of adverse effects; however, those effects still appeared to be mild and time-limited.10
One study reported that 33% (17/51) of patients experienced systemic adverse effects including syncope, dizziness, and nausea at the time of their PRP injection.6 Overall, there is no evidence of significant harms associated with PRP treatment, but available studies have lacked the power to detect rare but serious problems.
Looking to the future: Additional considerations
In order to properly evaluate this potentially promising method of care, future studies need to include appropriately chosen controls, specifically defined formulations of PRP, standardized protocols for the injection of PRP, standardized post-injection PT regimens, and patient populations that are clearly defined in terms of severity and chronicity of disease. Furthermore, studies must be rigorously designed in terms of randomization, blinding, and analysis. (Many studies done to date did not use an intention-to-treat protocol, for example). Higher-quality studies with larger numbers of participants are the only way to determine whether PRP is worth all the “buzz.”
We should keep in mind, too, that the evidence for many of the other treatment options for both tendinopathy and knee OA are similarly problematic, and these modalities are even more widely used than PRP. Given the systemic problems associated with nonsteroidal anti-inflammatory drugs, concerns about possible tendon rupture with corticosteroid injections, and the time and compliance issues associated with PT, PRP may be a safer alternative to more traditional treatments.
An off-label use. PRP does not pass through the standard regulatory pathway of the US Food and Drug Administration (FDA). As a blood product, PRP falls under the regulatory purview of the FDA’s Center for Biologics Evaluation and Research, which has approved PRP only for use in the operative setting to enhance bone graft handling properties.27 Therefore, office-based PRP injections are an off-label use of the treatment.
CASE 1 › You explain to Ms. T that PRP injections are not covered by insurance and that there is not a significant amount of evidence to indicate that an injection would appreciably improve her pain. She decides to proceed with a knee replacement and not to pursue a PRP injection.
CASE 2 › Given the time that Mr. H has invested in traditional conservative management strategies, his time away from work, and that he is not concerned with the out-of-pocket cost associated with PRP, you explain to him that there is some limited evidence that PRP might improve his symptoms. He decides that he would rather try a PRP injection than pursue surgery.
CORRESPONDENCE
Jordan White, MD, MPH, Department of Family Medicine, 111 Brewster Street, Pawtucket, RI 02860; [email protected].
1. Washington Health Policy Fellows of the American Academy of Orthopaedic Surgeons. Musculoskeletal education in medical schools: are we making the cut? Available at: http://www.aaos.org/news/bulletin/marapr07/reimbursement2.asp. Accessed September 20, 2015.
2. Hsu WK, Mishra A, Rodeo S, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748.
3. Harmon KG, Rao AL. The use of platelet-rich plasma in the nonsurgical management of sports injuries: hype or hope? Hematology Am Soc Hematol Educ Program. 2013;2013:620-626.
4. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59.
5. Hannafin JA, Arnoczky SP, Fu FH, et al. Platelet-rich plasma: Clarifying the issues. AAOS Now. September 2010. Available at: http://www.aaos.org/AAOSNow/2010/Sep/clinical/clinical1/?ssopc=1. Accessed April 8, 2016.
6. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
7. Filardo G, Di Matteo B, Di Martino A. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1575-1582.
8. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. July 14, 2015. [Epub ahead of print]
9. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
10. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221.
11. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. August 2, 2015. [Epub ahead of print]
12. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. September 19, 2015. [Epub ahead of print]
13. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd edition. Adopted May 18, 2013. Available at: http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed March 11, 2016.
14. Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin J Sport Med. 2016;26:1-11.
15. Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071.
16. Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine. 2015;82;230-234.
17. Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99-115.
18. Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971.
19. Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635.
20. Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134.
21. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262.
22. Arirachakaran A, Sukthuayat A, Sisayanarane T, et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. September 11, 2015. [Epub ahead of print]
23. Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803.
24. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618.
25. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149.
26. Beyer R, Kongsgaard M, Hougs Kjæ B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:1704-1711.
27. Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29-34.27.
› Inform patients with knee osteoarthritis that although evidence is limited, platelet-rich plasma (PRP) injections may improve pain and function in the short-term. B
› Advise patients with elbow epicondylitis that PRP injections may improve pain and function slightly more than corticosteroid injections in the short-term. B
› Counsel patients that PRP has minimal risks; however, larger studies are needed to more fully assess whether harms exist. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Ms. T is an otherwise healthy 76 year old with a history of severe osteoarthritis (OA) in her right knee. She has participated in multiple rounds of physical therapy (PT) over the last 3 years. During the past year, she received 2 intra-articular corticosteroid injections, each of which provided only 3 to 4 weeks of pain relief, and one hyaluronic acid (HA) injection, which provided no benefit whatsoever.
Today, she describes her right knee pain as an 8 out of 10 and is frustrated by her lack of symptom relief. She was planning to have a total knee replacement and is a good surgical candidate, but recently found an article regarding platelet-rich plasma (PRP) injections for knee OA. She wants your opinion as to whether she should try this approach or proceed with surgery.
CASE 2 › Mr. H is a 44-year-old, right-handed dentist who has been suffering from right lateral epicondylitis for the past year. Although he has undergone PT and has been performing exercises at home since his symptoms began, he has not noticed a significant improvement. In the last 5 months, he has been out of work a total of 8 weeks due to the pain. He received one corticosteroid injection last month, which provided no improvement in symptoms. He is not interested in surgery, as he does not want to be out of work for a prolonged period of time.
He reports that one of his friends recently received a PRP injection for lateral epicondylitis and now feels great. He is aware that PRP injections are not covered by his health insurance and says he is willing to pay out of pocket if the treatment works. He wants to know if you recommend this course of action for his elbow pain.
How would you counsel each of these patients about the use of PRP injections for pain relief from their respective orthopedic conditions?
Musculoskeletal symptoms account for 10% to 28% of patients’ complaints to primary care physicians annually.1 Treatment of both chronic tendinopathies and knee OA—2 of the most common causes of these complaints—typically follows a stepwise approach, beginning with anti-inflammatory and pain medications in addition to PT. Patients who fail to respond to these interventions are often treated with corticosteroid injections, and, in the case of knee OA, viscosupplementation (ie, HA injections) and braces. If these therapies fail, patients are often forced to choose between an invasive surgical procedure or continued pain and limited function.
A number of physicians specializing in musculoskeletal medicine have turned to prolotherapy—specifically, dextrose prolotherapy (see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768) and platelet-rich plasma (PRP) therapy—as an alternative treatment for chronic musculoskeletal conditions.
PRP has been used to enhance surgical healing and to treat muscle strains and chondropathies. It drew a great deal of attention in the media when it was used by such high-profile professional athletes as Tiger Woods and Kobe Bryant.
Although PRP therapy is not commonly reimbursed by health insurance companies because of a lack of large, definitive studies supporting its effectiveness, patients are paying anywhere from a few hundred to a few thousand dollars out of pocket for it. They’re doing so in the hope that it will treat their chronic musculoskeletal disorders or at least delay surgical procedures.
But what can these patients reasonably expect from this therapy?
The following review of the evidence for PRP in the treatment of knee OA and tendinopathies (including elbow epicondylitis, patellar tendinitis, and Achilles tendinitis) will help you counsel patients on its appropriate use.
What is PRP?
PRP is defined as a sample of autologous blood with concentrations of platelets above baseline values.2 It is made through a one- or 2-stage centrifugation process in which the liquid and solid components of whole blood are separated, and then the liquid components are further separated into portions that are platelet-rich and platelet-poor.
Significant variability in preparation methods exists, resulting in more than 40 different products.2 Some methods centrifuge only once, creating plasma that is separated from red and white blood cells, but without a huge shift in the concentration of platelets; some include white blood cells in the final preparation; and most have differing concentrations of platelets and various growth factors in the end product. Researchers have attempted to classify the various preparations by platelet concentration, inclusion or exclusion of white blood cells, and fibrin content, but no validated system yet exists. Thus, consistency in preparations is lacking.3,4
PRP is rich not only in platelets, but also in a multitude of other growth factors. It is thought to improve healing by enhancing the body’s natural regenerative processes at the tissue level. In OA, for example, a complex balance of destructive and reparative processes is at play; PRP is thought to tip the body’s response in favor of regeneration over destruction. Similarly, chronic tendinopathy involves a process of destruction, reaction, healing, and degeneration; intervening at the correct point in this pathway with a boost to healing may help the body repair an otherwise diseased tendon.3
What does the evidence show?
Overall, basic science and preclinical research support “the promise” of PRP(strength of recommendation [SOR]: A).5 However, patient-centered evidence is lacking, and tremendous variability exists between studies, not only in terms of PRP preparation, but also with regard to:
- Protocol—Was ultrasound guidance used? Did the injection include needling of the tendon? What post-injection rehabilitation was followed?
- Patient population—What treatments were tried in the past? How chronic or severe was the problem?
- Study design—What was the comparison group? How were pain and function measured? Most studies have been small in size and have included various treatment modalities in addition to the PRP injection (most often PT).
Knee OA: PRP may provide short-term benefit, especially in younger patients
Researchers have conducted a number of studies evaluating PRP for knee OA.6-12 Most have compared PRP to HA—another intra-articular injection that is plagued by mixed, limited, and poor-quality evidence. These trials have had varied results and do not consistently support PRP as superior to HA.
The most well-designed study to date demonstrated that PRP was superior to saline and as effective as HA.11 In addition, the researchers found that a series of 3 PRP injections was superior to 3 injections of HA or only one injection of PRP.
One small randomized controlled trial (RCT) compared PRP injections to saline and found that PRP improved pain and function better than placebo at 6 weeks, 3 months, and 6 months; results appeared to deteriorate after that time period.6 Also, the findings suggested that PRP delivered the strongest benefit in younger patients who had less advanced OA.
In addition, a recent systematic review found short-term improvements in functional outcomes in patients treated with PRP injections vs those treated with HA injections and those treated with placebo.12
But before experts can make any conclusive recommendations regarding the use of PRP for knee OA, standardized studies with larger numbers of participants and rigorous methodology must be designed. Notably, no evidence exists of significant harm resulting from PRP injection for knee OA. Therefore, given the mixed evidence in terms of efficacy, there may be a potential benefit to treatment with little negative consequence.
In 2013, the American Academy of Orthopaedic Surgeons (AAOS) stated that they were unable to recommend for or against PRP injection for patients with symptomatic OA of the knee because the evidence was inconclusive.13 At the same time, the AAOS was unable to recommend for or against corticosteroid injections, manual therapy, or bracing for knee OA, and recommended against HA injections.13 Recently, however, the American Medical Society for Sports Medicine (AMSSM) recommended that HA be used in appropriate patients with knee OA.14
Such disagreement indicates that evidence is lacking for many modalities employed in the management of knee OA, including the injection of corticosteroids, which is a frequent and generally accepted treatment. Compounding matters is that many of the original studies testing the efficacy of PRP injection in knee OA used HA injections as the comparison, and there is no agreement between AAOS and AMSSM as to its usefulness. Thus, the validity of using HA as a control is suspect.
Tendinopathies: PRP may have benefit, but more research is needed
A number of meta-analyses and systematic review articles have combined the results of studies involving PRP treatment for various tendinopathies.3,15-17 While most found that PRP may have a benefit (although not long-lasting) and may be of use in attempts to avoid surgery or to return to a desired activity, all reported that more rigorous studies with standardized methodologies must be conducted before PRP can be conclusively recommended for any anatomic site.
Elbow epicondylitis (tennis elbow). The majority of tendinopathy studies have examined the effect of PRP on tennis elbow, although given the small study numbers (N=20-100), high risks of bias, and very different comparison groups, the data are extremely limited. Of the 4 randomized studies,18-21 2 compared different PRP preparations to whole blood,18,20 one compared PRP to both saline and corticosteroid,19 and one compared PRP to corticosteroid alone.21
The studies comparing PRP to whole blood found similar outcomes at most time points.18,20 These studies were of extremely poor quality, and other review articles have defined whole blood as a type of PRP, so this comparison was somewhat inappropriate. One recently published meta-analysis, which included 10 studies comparing either PRP or whole blood to corticosteroid, found that PRP improved pain more than a corticosteroid.22
The one study that included a comparison of PRP to placebo (saline) suffered from a high dropout rate, and the authors were not able to analyze the primary outcome data. At 3 months, the participants remaining in each group (PRP, saline, or corticosteroid) had similar pain and disability scores.19 Although the steroid group had improved from baseline at one month, there was no difference between the steroid group and placebo group at 3 months. The PRP group did not differ from the placebo group at any time point.
The study comparing PRP to corticosteroid alone found that PRP’s effects on pain and function exceeded those of the steroid. Specifically, the steroid group initially improved and then worsened, ending the study near their baseline pain and function scores.21 The PRP group, on the other hand, showed slow improvement throughout, ending the study with less pain and disability than when they started.
Patellar tendinitis (jumper’s knee). The majority of studies examining the effect of PRP on patellar tendinitis are non-randomized, non-comparative studies. Of the 2 small RCTs that were conducted, one compared PRP to extracorporeal shockwave therapy (ESWT),23 and the other to dry needling.24
In the ESWT study, there was a slight improvement in pain and function in the PRP group relative to the ESWT group at 6 and 12 months. In the other study, although the PRP group showed an improvement in recovery at 12 weeks relative to the dry needling group, there was no difference between such outcomes as pain and activity in the 2 groups at 26 weeks.
Worth noting here is that like the studies done on OA patients, the research involving patellar tendinitis also used comparative interventions (ESWT and dry needling) that lack high-quality evidence for their use. So whether these were appropriate comparisons is debatable.
Achilles tendinitis. Only one RCT (N=54) has evaluated PRP for the treatment of Achilles tendinitis.25 This study, which compared PRP to saline, excluded patients who had previously completed a course of PT, yet both study groups participated in PT during the study. Although the trial found no difference between groups at any time point (both showed improvement), it was underpowered to detect any difference (positive or negative) between groups, given that most participants likely would have improved with PT anyway.26
PRP has few harms or adverse effects
Most individual studies involving PRP have not reported on harms or side effects; the studies that have reported on them have generally found low rates (2%-5%) of only local, short-term adverse effects.15 One review article did find that increasing the number of PRP injections increased the rate of adverse effects; however, those effects still appeared to be mild and time-limited.10
One study reported that 33% (17/51) of patients experienced systemic adverse effects including syncope, dizziness, and nausea at the time of their PRP injection.6 Overall, there is no evidence of significant harms associated with PRP treatment, but available studies have lacked the power to detect rare but serious problems.
Looking to the future: Additional considerations
In order to properly evaluate this potentially promising method of care, future studies need to include appropriately chosen controls, specifically defined formulations of PRP, standardized protocols for the injection of PRP, standardized post-injection PT regimens, and patient populations that are clearly defined in terms of severity and chronicity of disease. Furthermore, studies must be rigorously designed in terms of randomization, blinding, and analysis. (Many studies done to date did not use an intention-to-treat protocol, for example). Higher-quality studies with larger numbers of participants are the only way to determine whether PRP is worth all the “buzz.”
We should keep in mind, too, that the evidence for many of the other treatment options for both tendinopathy and knee OA are similarly problematic, and these modalities are even more widely used than PRP. Given the systemic problems associated with nonsteroidal anti-inflammatory drugs, concerns about possible tendon rupture with corticosteroid injections, and the time and compliance issues associated with PT, PRP may be a safer alternative to more traditional treatments.
An off-label use. PRP does not pass through the standard regulatory pathway of the US Food and Drug Administration (FDA). As a blood product, PRP falls under the regulatory purview of the FDA’s Center for Biologics Evaluation and Research, which has approved PRP only for use in the operative setting to enhance bone graft handling properties.27 Therefore, office-based PRP injections are an off-label use of the treatment.
CASE 1 › You explain to Ms. T that PRP injections are not covered by insurance and that there is not a significant amount of evidence to indicate that an injection would appreciably improve her pain. She decides to proceed with a knee replacement and not to pursue a PRP injection.
CASE 2 › Given the time that Mr. H has invested in traditional conservative management strategies, his time away from work, and that he is not concerned with the out-of-pocket cost associated with PRP, you explain to him that there is some limited evidence that PRP might improve his symptoms. He decides that he would rather try a PRP injection than pursue surgery.
CORRESPONDENCE
Jordan White, MD, MPH, Department of Family Medicine, 111 Brewster Street, Pawtucket, RI 02860; [email protected].
› Inform patients with knee osteoarthritis that although evidence is limited, platelet-rich plasma (PRP) injections may improve pain and function in the short-term. B
› Advise patients with elbow epicondylitis that PRP injections may improve pain and function slightly more than corticosteroid injections in the short-term. B
› Counsel patients that PRP has minimal risks; however, larger studies are needed to more fully assess whether harms exist. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Ms. T is an otherwise healthy 76 year old with a history of severe osteoarthritis (OA) in her right knee. She has participated in multiple rounds of physical therapy (PT) over the last 3 years. During the past year, she received 2 intra-articular corticosteroid injections, each of which provided only 3 to 4 weeks of pain relief, and one hyaluronic acid (HA) injection, which provided no benefit whatsoever.
Today, she describes her right knee pain as an 8 out of 10 and is frustrated by her lack of symptom relief. She was planning to have a total knee replacement and is a good surgical candidate, but recently found an article regarding platelet-rich plasma (PRP) injections for knee OA. She wants your opinion as to whether she should try this approach or proceed with surgery.
CASE 2 › Mr. H is a 44-year-old, right-handed dentist who has been suffering from right lateral epicondylitis for the past year. Although he has undergone PT and has been performing exercises at home since his symptoms began, he has not noticed a significant improvement. In the last 5 months, he has been out of work a total of 8 weeks due to the pain. He received one corticosteroid injection last month, which provided no improvement in symptoms. He is not interested in surgery, as he does not want to be out of work for a prolonged period of time.
He reports that one of his friends recently received a PRP injection for lateral epicondylitis and now feels great. He is aware that PRP injections are not covered by his health insurance and says he is willing to pay out of pocket if the treatment works. He wants to know if you recommend this course of action for his elbow pain.
How would you counsel each of these patients about the use of PRP injections for pain relief from their respective orthopedic conditions?
Musculoskeletal symptoms account for 10% to 28% of patients’ complaints to primary care physicians annually.1 Treatment of both chronic tendinopathies and knee OA—2 of the most common causes of these complaints—typically follows a stepwise approach, beginning with anti-inflammatory and pain medications in addition to PT. Patients who fail to respond to these interventions are often treated with corticosteroid injections, and, in the case of knee OA, viscosupplementation (ie, HA injections) and braces. If these therapies fail, patients are often forced to choose between an invasive surgical procedure or continued pain and limited function.
A number of physicians specializing in musculoskeletal medicine have turned to prolotherapy—specifically, dextrose prolotherapy (see “Prolotherapy: Can it help your patient?” J Fam Pract. 2015;64:763-768) and platelet-rich plasma (PRP) therapy—as an alternative treatment for chronic musculoskeletal conditions.
PRP has been used to enhance surgical healing and to treat muscle strains and chondropathies. It drew a great deal of attention in the media when it was used by such high-profile professional athletes as Tiger Woods and Kobe Bryant.
Although PRP therapy is not commonly reimbursed by health insurance companies because of a lack of large, definitive studies supporting its effectiveness, patients are paying anywhere from a few hundred to a few thousand dollars out of pocket for it. They’re doing so in the hope that it will treat their chronic musculoskeletal disorders or at least delay surgical procedures.
But what can these patients reasonably expect from this therapy?
The following review of the evidence for PRP in the treatment of knee OA and tendinopathies (including elbow epicondylitis, patellar tendinitis, and Achilles tendinitis) will help you counsel patients on its appropriate use.
What is PRP?
PRP is defined as a sample of autologous blood with concentrations of platelets above baseline values.2 It is made through a one- or 2-stage centrifugation process in which the liquid and solid components of whole blood are separated, and then the liquid components are further separated into portions that are platelet-rich and platelet-poor.
Significant variability in preparation methods exists, resulting in more than 40 different products.2 Some methods centrifuge only once, creating plasma that is separated from red and white blood cells, but without a huge shift in the concentration of platelets; some include white blood cells in the final preparation; and most have differing concentrations of platelets and various growth factors in the end product. Researchers have attempted to classify the various preparations by platelet concentration, inclusion or exclusion of white blood cells, and fibrin content, but no validated system yet exists. Thus, consistency in preparations is lacking.3,4
PRP is rich not only in platelets, but also in a multitude of other growth factors. It is thought to improve healing by enhancing the body’s natural regenerative processes at the tissue level. In OA, for example, a complex balance of destructive and reparative processes is at play; PRP is thought to tip the body’s response in favor of regeneration over destruction. Similarly, chronic tendinopathy involves a process of destruction, reaction, healing, and degeneration; intervening at the correct point in this pathway with a boost to healing may help the body repair an otherwise diseased tendon.3
What does the evidence show?
Overall, basic science and preclinical research support “the promise” of PRP(strength of recommendation [SOR]: A).5 However, patient-centered evidence is lacking, and tremendous variability exists between studies, not only in terms of PRP preparation, but also with regard to:
- Protocol—Was ultrasound guidance used? Did the injection include needling of the tendon? What post-injection rehabilitation was followed?
- Patient population—What treatments were tried in the past? How chronic or severe was the problem?
- Study design—What was the comparison group? How were pain and function measured? Most studies have been small in size and have included various treatment modalities in addition to the PRP injection (most often PT).
Knee OA: PRP may provide short-term benefit, especially in younger patients
Researchers have conducted a number of studies evaluating PRP for knee OA.6-12 Most have compared PRP to HA—another intra-articular injection that is plagued by mixed, limited, and poor-quality evidence. These trials have had varied results and do not consistently support PRP as superior to HA.
The most well-designed study to date demonstrated that PRP was superior to saline and as effective as HA.11 In addition, the researchers found that a series of 3 PRP injections was superior to 3 injections of HA or only one injection of PRP.
One small randomized controlled trial (RCT) compared PRP injections to saline and found that PRP improved pain and function better than placebo at 6 weeks, 3 months, and 6 months; results appeared to deteriorate after that time period.6 Also, the findings suggested that PRP delivered the strongest benefit in younger patients who had less advanced OA.
In addition, a recent systematic review found short-term improvements in functional outcomes in patients treated with PRP injections vs those treated with HA injections and those treated with placebo.12
But before experts can make any conclusive recommendations regarding the use of PRP for knee OA, standardized studies with larger numbers of participants and rigorous methodology must be designed. Notably, no evidence exists of significant harm resulting from PRP injection for knee OA. Therefore, given the mixed evidence in terms of efficacy, there may be a potential benefit to treatment with little negative consequence.
In 2013, the American Academy of Orthopaedic Surgeons (AAOS) stated that they were unable to recommend for or against PRP injection for patients with symptomatic OA of the knee because the evidence was inconclusive.13 At the same time, the AAOS was unable to recommend for or against corticosteroid injections, manual therapy, or bracing for knee OA, and recommended against HA injections.13 Recently, however, the American Medical Society for Sports Medicine (AMSSM) recommended that HA be used in appropriate patients with knee OA.14
Such disagreement indicates that evidence is lacking for many modalities employed in the management of knee OA, including the injection of corticosteroids, which is a frequent and generally accepted treatment. Compounding matters is that many of the original studies testing the efficacy of PRP injection in knee OA used HA injections as the comparison, and there is no agreement between AAOS and AMSSM as to its usefulness. Thus, the validity of using HA as a control is suspect.
Tendinopathies: PRP may have benefit, but more research is needed
A number of meta-analyses and systematic review articles have combined the results of studies involving PRP treatment for various tendinopathies.3,15-17 While most found that PRP may have a benefit (although not long-lasting) and may be of use in attempts to avoid surgery or to return to a desired activity, all reported that more rigorous studies with standardized methodologies must be conducted before PRP can be conclusively recommended for any anatomic site.
Elbow epicondylitis (tennis elbow). The majority of tendinopathy studies have examined the effect of PRP on tennis elbow, although given the small study numbers (N=20-100), high risks of bias, and very different comparison groups, the data are extremely limited. Of the 4 randomized studies,18-21 2 compared different PRP preparations to whole blood,18,20 one compared PRP to both saline and corticosteroid,19 and one compared PRP to corticosteroid alone.21
The studies comparing PRP to whole blood found similar outcomes at most time points.18,20 These studies were of extremely poor quality, and other review articles have defined whole blood as a type of PRP, so this comparison was somewhat inappropriate. One recently published meta-analysis, which included 10 studies comparing either PRP or whole blood to corticosteroid, found that PRP improved pain more than a corticosteroid.22
The one study that included a comparison of PRP to placebo (saline) suffered from a high dropout rate, and the authors were not able to analyze the primary outcome data. At 3 months, the participants remaining in each group (PRP, saline, or corticosteroid) had similar pain and disability scores.19 Although the steroid group had improved from baseline at one month, there was no difference between the steroid group and placebo group at 3 months. The PRP group did not differ from the placebo group at any time point.
The study comparing PRP to corticosteroid alone found that PRP’s effects on pain and function exceeded those of the steroid. Specifically, the steroid group initially improved and then worsened, ending the study near their baseline pain and function scores.21 The PRP group, on the other hand, showed slow improvement throughout, ending the study with less pain and disability than when they started.
Patellar tendinitis (jumper’s knee). The majority of studies examining the effect of PRP on patellar tendinitis are non-randomized, non-comparative studies. Of the 2 small RCTs that were conducted, one compared PRP to extracorporeal shockwave therapy (ESWT),23 and the other to dry needling.24
In the ESWT study, there was a slight improvement in pain and function in the PRP group relative to the ESWT group at 6 and 12 months. In the other study, although the PRP group showed an improvement in recovery at 12 weeks relative to the dry needling group, there was no difference between such outcomes as pain and activity in the 2 groups at 26 weeks.
Worth noting here is that like the studies done on OA patients, the research involving patellar tendinitis also used comparative interventions (ESWT and dry needling) that lack high-quality evidence for their use. So whether these were appropriate comparisons is debatable.
Achilles tendinitis. Only one RCT (N=54) has evaluated PRP for the treatment of Achilles tendinitis.25 This study, which compared PRP to saline, excluded patients who had previously completed a course of PT, yet both study groups participated in PT during the study. Although the trial found no difference between groups at any time point (both showed improvement), it was underpowered to detect any difference (positive or negative) between groups, given that most participants likely would have improved with PT anyway.26
PRP has few harms or adverse effects
Most individual studies involving PRP have not reported on harms or side effects; the studies that have reported on them have generally found low rates (2%-5%) of only local, short-term adverse effects.15 One review article did find that increasing the number of PRP injections increased the rate of adverse effects; however, those effects still appeared to be mild and time-limited.10
One study reported that 33% (17/51) of patients experienced systemic adverse effects including syncope, dizziness, and nausea at the time of their PRP injection.6 Overall, there is no evidence of significant harms associated with PRP treatment, but available studies have lacked the power to detect rare but serious problems.
Looking to the future: Additional considerations
In order to properly evaluate this potentially promising method of care, future studies need to include appropriately chosen controls, specifically defined formulations of PRP, standardized protocols for the injection of PRP, standardized post-injection PT regimens, and patient populations that are clearly defined in terms of severity and chronicity of disease. Furthermore, studies must be rigorously designed in terms of randomization, blinding, and analysis. (Many studies done to date did not use an intention-to-treat protocol, for example). Higher-quality studies with larger numbers of participants are the only way to determine whether PRP is worth all the “buzz.”
We should keep in mind, too, that the evidence for many of the other treatment options for both tendinopathy and knee OA are similarly problematic, and these modalities are even more widely used than PRP. Given the systemic problems associated with nonsteroidal anti-inflammatory drugs, concerns about possible tendon rupture with corticosteroid injections, and the time and compliance issues associated with PT, PRP may be a safer alternative to more traditional treatments.
An off-label use. PRP does not pass through the standard regulatory pathway of the US Food and Drug Administration (FDA). As a blood product, PRP falls under the regulatory purview of the FDA’s Center for Biologics Evaluation and Research, which has approved PRP only for use in the operative setting to enhance bone graft handling properties.27 Therefore, office-based PRP injections are an off-label use of the treatment.
CASE 1 › You explain to Ms. T that PRP injections are not covered by insurance and that there is not a significant amount of evidence to indicate that an injection would appreciably improve her pain. She decides to proceed with a knee replacement and not to pursue a PRP injection.
CASE 2 › Given the time that Mr. H has invested in traditional conservative management strategies, his time away from work, and that he is not concerned with the out-of-pocket cost associated with PRP, you explain to him that there is some limited evidence that PRP might improve his symptoms. He decides that he would rather try a PRP injection than pursue surgery.
CORRESPONDENCE
Jordan White, MD, MPH, Department of Family Medicine, 111 Brewster Street, Pawtucket, RI 02860; [email protected].
1. Washington Health Policy Fellows of the American Academy of Orthopaedic Surgeons. Musculoskeletal education in medical schools: are we making the cut? Available at: http://www.aaos.org/news/bulletin/marapr07/reimbursement2.asp. Accessed September 20, 2015.
2. Hsu WK, Mishra A, Rodeo S, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748.
3. Harmon KG, Rao AL. The use of platelet-rich plasma in the nonsurgical management of sports injuries: hype or hope? Hematology Am Soc Hematol Educ Program. 2013;2013:620-626.
4. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59.
5. Hannafin JA, Arnoczky SP, Fu FH, et al. Platelet-rich plasma: Clarifying the issues. AAOS Now. September 2010. Available at: http://www.aaos.org/AAOSNow/2010/Sep/clinical/clinical1/?ssopc=1. Accessed April 8, 2016.
6. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
7. Filardo G, Di Matteo B, Di Martino A. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1575-1582.
8. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. July 14, 2015. [Epub ahead of print]
9. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
10. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221.
11. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. August 2, 2015. [Epub ahead of print]
12. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. September 19, 2015. [Epub ahead of print]
13. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd edition. Adopted May 18, 2013. Available at: http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed March 11, 2016.
14. Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin J Sport Med. 2016;26:1-11.
15. Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071.
16. Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine. 2015;82;230-234.
17. Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99-115.
18. Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971.
19. Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635.
20. Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134.
21. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262.
22. Arirachakaran A, Sukthuayat A, Sisayanarane T, et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. September 11, 2015. [Epub ahead of print]
23. Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803.
24. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618.
25. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149.
26. Beyer R, Kongsgaard M, Hougs Kjæ B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:1704-1711.
27. Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29-34.27.
1. Washington Health Policy Fellows of the American Academy of Orthopaedic Surgeons. Musculoskeletal education in medical schools: are we making the cut? Available at: http://www.aaos.org/news/bulletin/marapr07/reimbursement2.asp. Accessed September 20, 2015.
2. Hsu WK, Mishra A, Rodeo S, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748.
3. Harmon KG, Rao AL. The use of platelet-rich plasma in the nonsurgical management of sports injuries: hype or hope? Hematology Am Soc Hematol Educ Program. 2013;2013:620-626.
4. Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. PM R. 2015;7:S53-S59.
5. Hannafin JA, Arnoczky SP, Fu FH, et al. Platelet-rich plasma: Clarifying the issues. AAOS Now. September 2010. Available at: http://www.aaos.org/AAOSNow/2010/Sep/clinical/clinical1/?ssopc=1. Accessed April 8, 2016.
6. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
7. Filardo G, Di Matteo B, Di Martino A. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 2015;43:1575-1582.
8. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. July 14, 2015. [Epub ahead of print]
9. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
10. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221.
11. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. August 2, 2015. [Epub ahead of print]
12. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. September 19, 2015. [Epub ahead of print]
13. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd edition. Adopted May 18, 2013. Available at: http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Accessed March 11, 2016.
14. Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes. Clin J Sport Med. 2016;26:1-11.
15. Moraes VY, Lenza M, Tamaoki MJ, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071.
16. Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine. 2015;82;230-234.
17. Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99-115.
18. Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971.
19. Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635.
20. Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134.
21. Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262.
22. Arirachakaran A, Sukthuayat A, Sisayanarane T, et al. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. September 11, 2015. [Epub ahead of print]
23. Vetrano M, Castorina A, Vulpiani MC, et al. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803.
24. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618.
25. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149.
26. Beyer R, Kongsgaard M, Hougs Kjæ B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:1704-1711.
27. Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29-34.27.
Improving your care of patients with spinal cord injury/disease
› Have a high index of suspicion for the leading causes of hospitalization among patients with spinal cord injury and disease (SCI/D). These include respiratory infections, urinary tract infections, and pressure ulcers. A
› Treat respiratory infections early and aggressively in patients with SCI/D; strongly consider inpatient management because of the high risk of respiratory failure. C
› Be alert to atypical signs and symptoms of urinary tract infection in patients with SCI/D, such as fever, chills, spasm, autonomic dysfunction, nausea and vomiting, abdominal discomfort, and fatigue. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 5 million Americans are living with paralysis, and for nearly one in 4 of them the cause is spinal cord injury or disease (SCI/D).1 More common than multiple sclerosis (17%) as a cause for the loss of movement, SCI/D is second only to stroke (29%).1
The percentage of people living with paralysis due to SCI/D is increasing, partly because the population is aging and partly because management of infections has improved. Prior to the 1970s, life expectancy for people with SCI/D was significantly shortened, largely because of urologic and respiratory infections. But improved bladder management, in particular, has increased life expectancy—especially for the least severely injured.2 Respiratory diseases and septicemia remain the leading causes of death, but with increased longevity, other causes, such as endocrine, metabolic and nutritional diseases, accidents, nervous system diseases, and musculoskeletal disorders, are becoming increasingly common.2,3
Primary care’s pivotal role. Given the size of the population affected by SCI/D and the increase in life expectancy, family physicians (FPs) are more likely than ever before to care for these patients, most of whom have highly specific needs. However, little information about the primary care of patients with SCI/D exists. This patient population tends to consume a relatively large share of practices’ resources because of high case complexity.4
A recent Canadian report confirms our clinical experience that FPs report knowledge gaps in the area of SCI/D care, yet the same report found that 90% of people with SCI/D identify FPs as their “regular doctors.”5 Although a large number of patients with SCI/D identify their physiatrist as their primary care physician (PCP), one study reported that fewer than half of physiatrists are willing to assume that role.6 And while more than half of all patients with SCI/D have both specialists and PCPs involved in their care,5 communication breakdowns are a concern for patients receiving medical and rehabilitative direction from multiple health care professionals.
Below we take a closer look at the distinct patient populations affected by SCI/D, summarize several clinical conditions that contribute to hospitalization, and provide clinical management recommendations (TABLE7-26).
2 patient populations, one diagnosis
Paralysis due to spinal trauma occurs predominantly in non-Hispanic white and black males because of vehicular accidents, falls, violence, and sports.2 The mean age of injury has increased from 29 years during the 1970s to 42 years since 2010.2 However, this calculated average is misleading because there is an emerging bimodal distribution of people injured during early adulthood and a new increase in older adults injured primarily because of falls.27 In addition to those injured traumatically, a broader cohort of approximately 1 million patients represents a largely undefined group of people with paralysis due to diseases such as spinal stenosis, cancer, infection, multiple sclerosis, or other non-traumatic causes.
As a result, the population with SCI/D is comprised primarily of young adult males who have relatively few chronic medical conditions at the time of their injury and age with SCI/D, and older patients who are more likely to have already developed chronic medical conditions by the time of their SCI/D. Approximately 60% of SCI/Ds result in tetraplegia (ie, 4 limbs affected), although approximately two-thirds are incomplete, meaning that patients have some residual motor or sensory function below the level of injury.2 Not surprisingly, the level and severity of SCI/D impact life expectancy inversely and lifetime financial costs directly.
High health care utilization. Morbidity data largely parallel mortality data, often resulting in high health care utilization and cost among SCI/D patients.28 In a recent prospective observational study of nearly 1000 people with new traumatic SCI, 36.2% were rehospitalized at least once and 12.5% were rehospitalized at least twice during the 12-month period after discharge following injury.29
Rehospitalization, an outcome often quoted as a proxy for inadequate primary care, remains unacceptably high (36%-50%) for people with SCI/D.29,30 The leading causes of rehospitalization—pneumonia, urinary tract infection (UTI), and pressure ulcers29—have not changed over the years and persist over the lifetime of individuals with SCI/D.30
Take steps to prevent pneumonia, other respiratory complications
Many people with SCI/D are at high risk for respiratory complications because of their weakened respiratory muscles. This is particularly true for individuals who have injuries occurring above T10; those with injuries that are high on the spinal cord have the highest complication risk.7,8 In fact, pneumonia, atelectasis, and other respiratory complications are the leading causes of mortality in patients with tetraplegia, occurring in 40% to 70% of these patients.7
The diaphragm, innervated by the phrenic nerve (C3-C5), is the primary muscle of inspiration. Accessory muscles of inspiration include the scalenes (C5-C8), sternocleidomastoid and trapezius (C1-C4), and intercostals (T1-T11); whereas forced exhalation (cough) occurs with contraction of the abdominals (T5-T12).9 Diminished inspiration in individuals with higher level lesions can lead to microatelectasis, dyspnea with exertion, and even respiratory insufficiency.
In SCI/D above T8, weakened expiration can severely decrease cough effectiveness and secretion clearance, increasing susceptibility to lower respiratory tract infections. In addition, experts have described asthma-like disorders of airway function, particularly in those with higher lesions, due to unopposed parasympathetic innervation of respiratory smooth muscle.10
Management of this neurogenic pulmonary dysfunction after SCI/D relies on extensive preventive measures, including positioning and postural changes, breathing techniques, coughing (assisted for patients with tetraplegia), postural drainage, chest compression and percussion, and suctioning to avoid atelectasis, aspiration, and pneumonia. Ensure that patients receive influenza and pneumococcal vaccinations, and encourage smoking cessation. Obtain a chest x-ray if the patient demonstrates a decrease in respiratory function, deteriorating vital signs, reduced vital capacity, an increase in subjective dyspnea, or a change in sputum quantity. Treat respiratory infections early and aggressively,7-10 and strongly consider inpatient management because of the high risk of respiratory failure.
Pneumococcus is the most common cause of respiratory infections, although up to 21% of cases of community-acquired pneumonia in patients with SCI/D are caused by Pseudomonas.11-13 Avoid the use of antibiotics in patients who do not have signs or symptoms of a respiratory infection to minimize the development of resistant organisms. Target antibiotic therapy as per general population guidelines, as guidelines validated for use in the population with SCI/D do not currently exist.7,11
Be alert for UTIs—typical signs, symptoms don’t apply
The bladder receives innervation from S2 to S4 via the hypogastric, pudendal, and pelvic nerves. As such, the vast majority—70% to 84%—of patients with SCI/D report some degree of bladder dysfunction.14 Generally, SCI/D contributes to a combination of a failure to empty the bladder and a failure to store urine. The former is more frequent and the latter occurs more often in people with bladder outlet flaccidity, which usually occurs with low injury, such as that of the lumbar spine.14
The majority of people with SCI/D who are unable to empty their bladder require the use of some type of bladder catheter, either intermittent, indwelling (urethral or suprapubic), or condom. The choice of bladder management technique depends on gender, hand function, body habitus, caregiver assistance, and medical comorbidities. People with SCI/D are at greater risk for bladder and renal stones, UTI, vesicoureteral reflux, and bladder cancer.15,16 That said, the risk of bladder and renal stones declines somewhat after the first 6 months following an injury due to an immobility-induced loss of calcium.
Patients with SCI/D are often found to have bacteruria and even pyuria, and although they are at high risk for recurrent UTIs, these can be difficult to diagnose because signs and symptoms may differ from those seen in people with neurologically intact bladders. Symptomatic UTIs may present with fever, hematuria, abdominal discomfort, and/or increased spasticity, among other symptoms. They may cause increased bouts of autonomic dysreflexia, malaise, or a change in functional status. One cannot rely on the typical symptoms of dysuria and increased urinary frequency in this patient population. Further, the Infectious Diseases Society of America (IDSA) states that cloudy or foul-smelling urine in adults with catheters is not a symptom or sign mandating treatment.17
Because there is a lack of consensus as to what constitutes UTI symptoms in patients with SCI/D, PCPs need to be aware of changes from baseline in patients; these, combined with urine dip and culture results, should guide initiation of treatment.16
Prophylactic antibiotics have no role in the prevention of UTIs in patients with SCI/D. The minimal benefits associated with prophylaxis are outweighed by the risks of increased bacterial resistance to antibiotics. Research shows no significant benefit associated with the use of non-antibiotic prophylaxis, including the use of cranberry products and mannose, but further studies are needed in this patient population.18
Focus on bowel function; it correlates with quality of life
Bowel dysfunction is nearly universal in patients with SCI/D. The enteric nervous system is modulated via the sympathetic, parasympathetic, and somatic systems, and intrinsic control occurs via the myenteric and submucosal plexi. The loss of volitional control of defecation can result in prolonged transit time, reduced colonic motility, fecal incontinence, and difficulty with evacuation.
Because bowel care and function are highly correlated with quality of life,19 recommend bowel emptying every day or every other day, as well as adequate fiber in the diet, intake of fluids, stool softeners, bulk forming agents, contact irritants (eg, bisacodyl), and prokinetic agents to achieve optimal bowel care.
Prevent and treat pressure ulcers whenever possible
Accompanying the paralysis associated with SCI/D is often some degree of sensory loss of pain, light touch, temperature, and/or proprioception. The combination of insensate skin, immobility, and sarcopenia with resultant body composition changes places individuals with SCI/D at high risk for skin breakdown.21,22 Blood flow and oxygen tension at the skin surface are also decreased in patients with SCI/D compared to those without, further contributing to the problem.21,23 Increased latency from the time of injury correlates with increased likelihood of pressure ulcer development.21,22,24
External risk factors for pressure ulcers include prolonged pressure exposure, or intense pressure over a short period, shear forces, poor nutrition, smoking, moisture, and immobility. The incidence of pressure ulcers in patients with SCI/D is 25% to 66%, compared with 0.38% in the general population.21,22 Research indicates that US hospitals spend $11 billion annually on the treatment of the condition.22
To minimize pressure ulcers in this population, perform a risk assessment, using, for example, the Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) available at https://www.scireproject.com/outcome-measures-new/spinal-cord-injury-pressure-ulcer-scale-acute-scipus. In addition, recommend that patients use pressure redistribution surfaces for beds and wheelchairs, turn while in bed, perform frequent (approximately every 15-30 minutes) pressure reliefs, exercise or move regularly, and that they or a caregiver inspect the skin daily. If pressure ulcers do occur, start treatment immediately and document the stage of the ulcer.
Ensure that screening efforts go beyond what’s standard
Preventive care for patients with SCI/D is similar in many ways to that recommended for the general population. Screening for colorectal cancer,31 cervical cancer, and breast cancer32 should follow the same evidence-based intervals and age ranges suggested by groups such as the US Preventive Services Task Force (USPSTF). The only difference is to give special consideration to patients’ physical limitations and the set-up of exam rooms when scheduling and conducting procedures, such as Pap smears, colonoscopies, and mammograms.33,34
Bladder cancer. Because of the high risk for bladder cancer (ie, squamous cell carcinoma, as opposed to the more common transitional cell carcinoma) in this population, experts recommend annual cystoscopy for bladder cancer surveillance in patients who have had indwelling catheters for more than 5 to 10 years.35
Osteoporosis. Screening for osteoporosis is another preventive health area in which recommendations differ from those addressing the general population. Paralysis contributes to a decrease in mechanical stress on bone and to accelerated bone loss, and, thus, to osteoporosis.36
In patients with SCI/D, osteoporosis affects primarily weight-bearing areas below the injured lesion, such as the distal femur and proximal tibia. Fractures in patients with SCI/D may occur during minor trauma (eg, during transfers from wheelchair to bed). Although screening and treatment guidelines for osteoporosis in patients with SCI/D are not established, most experts recommend early screening and early and aggressive treatment.36
Depression reportedly occurs more frequently in individuals with SCI/D than in the general population,37,38 affecting adjustment, quality of life, and social, behavioral, and physical functioning. In light of this, it’s advisable to use screening tools, such as The Patient Health Questionnaire (PHQ)-9, routinely.39
Sexuality and sexual function are often adversely affected in both men and women with SCI/D. Loss of sensation in the sexual organs, combined with difficulty with positioning and mobility and bowel and bladder dysfunction, contribute not only to sexual dysfunction, but to lower self-esteem and altered body image.40
It is important to remember that fertility is often unaffected in women, so routine discussions about contraception with women who have SCI/D and who are sexually active are imperative. At the same time, male fertility is usually profoundly affected by SCI/D; patients and their partners who are interested in having children will require specialized interventions. Address sexuality and fertility during primary care visits and refer patients to counseling or specialists as necessary.41-43
SCI/D requires a whole-person approach
The care of individuals with SCI/D requires a holistic approach that takes into consideration physical, psychological, environmental, and interpersonal factors44,45 and involves ongoing support from a variety of specialists. FPs, with their whole-person orientation, can be instrumental in ensuring the successful rehabilitation of patients affected by SCI/D, and in helping individuals attain, preserve, and enhance their health and well-being.
CORRESPONDENCE
Ranit Mishori, MD, MHS, FAAFP, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Pre-clinical Building GB-01D, Washington, DC 20007; [email protected].
1. Christopher and Dana Reeve Foundation. One degree of separation. Paralysis and spinal cord injury in the United States. Available at: https://www.heart.us/uploads/userfiles/files/one-degree-of-separation.pdf. Accessed April 23, 2015.
2. National Spinal Cord Injury Statistical Center. 2014 Annual Statistical Report-Complete public version. Available at: https://www.nscisc.uab.edu/reports. Accessed November 1, 2015.
3. van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27:1517-1528.
4. Smith KM, Naumann DN, McDiarmid AL, et al. Using developmental research to design innovative knowledge translation technology for spinal cord injury in primary care: Actionable Nuggets on SkillScribe. J Spinal Cord Med. 2014;37:582-588.
5. McColl MA, Aiken A, McColl A, et al. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58:1207-1216.
6. Francisco GE, Chae JC, DeLisa JA. Physiatry as a primary care specialty. Am J Phys Med Rehabil. 1995;74:186-192.
7. Consortium for Spinal Cord Medicine. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. January 2005.
8. Weaver FM, Smith B, LaVela S, et al. Interventions to increase influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10-19.
9. McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
10. Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med. 2007;30: 307-308.
11. Burns SP, Weaver FM, Parada JP, et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004;42:450-458.
12. Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129-141.
13. Waites KB, Canupp KC, Chen Y, et al. Revaccination of adults with spinal cord injury using the 23-valent pneumococcal polysaccharide vaccine. J Spinal Cord Med. 2008;31: 53-59.
14. Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012:816274.
15. Taweel W, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85-99.
16. Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am. 2011;95:111-120.
17. Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
18. Goets L, Klausner A. Strategies for prevention of urinary tract infections in neurogenic bladder dysfunction. Phys Med Rehabil Clin N Am. 2014;25:605-618.
19. Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-S102.
20. Paralyzed Veterans of America. Consortium for Spinal Cord Medicine. Neurogenic Bowel Management in Adults with Spinal Cord Injury. Available at: http://www.pva.org/site/c.ajIRK9NJbcJ2E/b.6305815/k.A19D/Publications.htm#CPG. Accessed October 30, 2015.
21. Groah SL, Schladen M, Pineda CG, et al. Prevention of Pressure Ulcers Among People With Spinal Cord Injury: A Systematic Review. PM R. 2015;7:613-636.
22. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24:S40-S101.
23. Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572-585.
24. Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: analysis of educational needs. Adv Skin Wound Care. 2008;21:322-329.
25. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. 2nd ed. Cambridge Media. 2014.
26. Ghaisas S, Pyatak EA, Blanche E, et al. Lifestyle changes and pressure ulcer prevention in adults with spinal cord injury in the pressure ulcer prevention study lifestyle intervention. Am J Occup Ther. 2015;69:6901290020p1-6901290020p10.
27. Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012;91:80-93.
28. Noonan VK, Fallah N, Park SE, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289-301.
29. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87-S97.
30. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763.
31. Hayman AV, Guihan M, Fisher MJ, et al. Colonoscopy is high yield in spinal cord injury. J Spinal Cord Med. 2013;36:436-442.
32. Guilcher SJ, Newman A, Jaglal SB. A comparison of cervical cancer screening rates among women with traumatic spinal cord injury and the general population. J Womens Health. 2010;19:57-63.
33. Lezzoni LI, Park ER, Kilbridge KL. Implications of mobility impairment on the diagnosis and treatment of breast cancer. J Womens Health. 2011;20:45-52.
34. Graham A, Savic G, Gardner B. Cervical and breast cancer screening in wheelchair dependent females. Spinal Cord. 1998;36:340-344.
35. Groah SL, Weitzenkamp DA, Lammertse DP, et al. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346-351.
36. Charmetant C, Phaner V, Condemine A, et al. Diagnosis and treatment of osteoporosis in spinal cord injury patients: a literature review. Ann Phys Rehabil Med. 2010;53:655-668.
37. Bombardier CH, Richards JS, Krause JS, et al. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749-1756.
38. Elliott TR. Studying depression following spinal cord injury: evidence, policy and practice. J Spinal Cord Med. 2015;38:584-586.
39. Kalpakjian CZ, Bombardier CH, Schomer K, et al. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32:6-24.
40. Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol. 2015;130:225-245.
41. Kreuter M, Taft C, Siösteen A, et al. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49:154-160.
42. Fritz HA, Dillaway H, Lysack CL. “Don’t think paralysis takes away your womanhood”: Sexual intimacy after spinal cord injury. Am J Occup Ther. 2015;69:6902260030p1-6902260030p10.
43. Smith AE, Molton IR, McMullen K, et al. Sexual function, satisfaction, and use of aids for sexual activity in middle-aged adults with long-term physical disability. Top Spinal Cord Inj Rehabil. 2015;21:227-232.
44. Chiodo AE, Scelza WM, Kirshblum SC, et al. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76-S83.
45. Middleton JW, Ramakrishnan K, Cameron ID. Health Maintenance for Adults with Spinal Cord Injuries. NSW Agency for Clinical Innovation. Chatswood, NSW, Australia. February 2014. Available at: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155167/Health-Maintenance.pdf. Accessed November 1, 2015.
› Have a high index of suspicion for the leading causes of hospitalization among patients with spinal cord injury and disease (SCI/D). These include respiratory infections, urinary tract infections, and pressure ulcers. A
› Treat respiratory infections early and aggressively in patients with SCI/D; strongly consider inpatient management because of the high risk of respiratory failure. C
› Be alert to atypical signs and symptoms of urinary tract infection in patients with SCI/D, such as fever, chills, spasm, autonomic dysfunction, nausea and vomiting, abdominal discomfort, and fatigue. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 5 million Americans are living with paralysis, and for nearly one in 4 of them the cause is spinal cord injury or disease (SCI/D).1 More common than multiple sclerosis (17%) as a cause for the loss of movement, SCI/D is second only to stroke (29%).1
The percentage of people living with paralysis due to SCI/D is increasing, partly because the population is aging and partly because management of infections has improved. Prior to the 1970s, life expectancy for people with SCI/D was significantly shortened, largely because of urologic and respiratory infections. But improved bladder management, in particular, has increased life expectancy—especially for the least severely injured.2 Respiratory diseases and septicemia remain the leading causes of death, but with increased longevity, other causes, such as endocrine, metabolic and nutritional diseases, accidents, nervous system diseases, and musculoskeletal disorders, are becoming increasingly common.2,3
Primary care’s pivotal role. Given the size of the population affected by SCI/D and the increase in life expectancy, family physicians (FPs) are more likely than ever before to care for these patients, most of whom have highly specific needs. However, little information about the primary care of patients with SCI/D exists. This patient population tends to consume a relatively large share of practices’ resources because of high case complexity.4
A recent Canadian report confirms our clinical experience that FPs report knowledge gaps in the area of SCI/D care, yet the same report found that 90% of people with SCI/D identify FPs as their “regular doctors.”5 Although a large number of patients with SCI/D identify their physiatrist as their primary care physician (PCP), one study reported that fewer than half of physiatrists are willing to assume that role.6 And while more than half of all patients with SCI/D have both specialists and PCPs involved in their care,5 communication breakdowns are a concern for patients receiving medical and rehabilitative direction from multiple health care professionals.
Below we take a closer look at the distinct patient populations affected by SCI/D, summarize several clinical conditions that contribute to hospitalization, and provide clinical management recommendations (TABLE7-26).
2 patient populations, one diagnosis
Paralysis due to spinal trauma occurs predominantly in non-Hispanic white and black males because of vehicular accidents, falls, violence, and sports.2 The mean age of injury has increased from 29 years during the 1970s to 42 years since 2010.2 However, this calculated average is misleading because there is an emerging bimodal distribution of people injured during early adulthood and a new increase in older adults injured primarily because of falls.27 In addition to those injured traumatically, a broader cohort of approximately 1 million patients represents a largely undefined group of people with paralysis due to diseases such as spinal stenosis, cancer, infection, multiple sclerosis, or other non-traumatic causes.
As a result, the population with SCI/D is comprised primarily of young adult males who have relatively few chronic medical conditions at the time of their injury and age with SCI/D, and older patients who are more likely to have already developed chronic medical conditions by the time of their SCI/D. Approximately 60% of SCI/Ds result in tetraplegia (ie, 4 limbs affected), although approximately two-thirds are incomplete, meaning that patients have some residual motor or sensory function below the level of injury.2 Not surprisingly, the level and severity of SCI/D impact life expectancy inversely and lifetime financial costs directly.
High health care utilization. Morbidity data largely parallel mortality data, often resulting in high health care utilization and cost among SCI/D patients.28 In a recent prospective observational study of nearly 1000 people with new traumatic SCI, 36.2% were rehospitalized at least once and 12.5% were rehospitalized at least twice during the 12-month period after discharge following injury.29
Rehospitalization, an outcome often quoted as a proxy for inadequate primary care, remains unacceptably high (36%-50%) for people with SCI/D.29,30 The leading causes of rehospitalization—pneumonia, urinary tract infection (UTI), and pressure ulcers29—have not changed over the years and persist over the lifetime of individuals with SCI/D.30
Take steps to prevent pneumonia, other respiratory complications
Many people with SCI/D are at high risk for respiratory complications because of their weakened respiratory muscles. This is particularly true for individuals who have injuries occurring above T10; those with injuries that are high on the spinal cord have the highest complication risk.7,8 In fact, pneumonia, atelectasis, and other respiratory complications are the leading causes of mortality in patients with tetraplegia, occurring in 40% to 70% of these patients.7
The diaphragm, innervated by the phrenic nerve (C3-C5), is the primary muscle of inspiration. Accessory muscles of inspiration include the scalenes (C5-C8), sternocleidomastoid and trapezius (C1-C4), and intercostals (T1-T11); whereas forced exhalation (cough) occurs with contraction of the abdominals (T5-T12).9 Diminished inspiration in individuals with higher level lesions can lead to microatelectasis, dyspnea with exertion, and even respiratory insufficiency.
In SCI/D above T8, weakened expiration can severely decrease cough effectiveness and secretion clearance, increasing susceptibility to lower respiratory tract infections. In addition, experts have described asthma-like disorders of airway function, particularly in those with higher lesions, due to unopposed parasympathetic innervation of respiratory smooth muscle.10
Management of this neurogenic pulmonary dysfunction after SCI/D relies on extensive preventive measures, including positioning and postural changes, breathing techniques, coughing (assisted for patients with tetraplegia), postural drainage, chest compression and percussion, and suctioning to avoid atelectasis, aspiration, and pneumonia. Ensure that patients receive influenza and pneumococcal vaccinations, and encourage smoking cessation. Obtain a chest x-ray if the patient demonstrates a decrease in respiratory function, deteriorating vital signs, reduced vital capacity, an increase in subjective dyspnea, or a change in sputum quantity. Treat respiratory infections early and aggressively,7-10 and strongly consider inpatient management because of the high risk of respiratory failure.
Pneumococcus is the most common cause of respiratory infections, although up to 21% of cases of community-acquired pneumonia in patients with SCI/D are caused by Pseudomonas.11-13 Avoid the use of antibiotics in patients who do not have signs or symptoms of a respiratory infection to minimize the development of resistant organisms. Target antibiotic therapy as per general population guidelines, as guidelines validated for use in the population with SCI/D do not currently exist.7,11
Be alert for UTIs—typical signs, symptoms don’t apply
The bladder receives innervation from S2 to S4 via the hypogastric, pudendal, and pelvic nerves. As such, the vast majority—70% to 84%—of patients with SCI/D report some degree of bladder dysfunction.14 Generally, SCI/D contributes to a combination of a failure to empty the bladder and a failure to store urine. The former is more frequent and the latter occurs more often in people with bladder outlet flaccidity, which usually occurs with low injury, such as that of the lumbar spine.14
The majority of people with SCI/D who are unable to empty their bladder require the use of some type of bladder catheter, either intermittent, indwelling (urethral or suprapubic), or condom. The choice of bladder management technique depends on gender, hand function, body habitus, caregiver assistance, and medical comorbidities. People with SCI/D are at greater risk for bladder and renal stones, UTI, vesicoureteral reflux, and bladder cancer.15,16 That said, the risk of bladder and renal stones declines somewhat after the first 6 months following an injury due to an immobility-induced loss of calcium.
Patients with SCI/D are often found to have bacteruria and even pyuria, and although they are at high risk for recurrent UTIs, these can be difficult to diagnose because signs and symptoms may differ from those seen in people with neurologically intact bladders. Symptomatic UTIs may present with fever, hematuria, abdominal discomfort, and/or increased spasticity, among other symptoms. They may cause increased bouts of autonomic dysreflexia, malaise, or a change in functional status. One cannot rely on the typical symptoms of dysuria and increased urinary frequency in this patient population. Further, the Infectious Diseases Society of America (IDSA) states that cloudy or foul-smelling urine in adults with catheters is not a symptom or sign mandating treatment.17
Because there is a lack of consensus as to what constitutes UTI symptoms in patients with SCI/D, PCPs need to be aware of changes from baseline in patients; these, combined with urine dip and culture results, should guide initiation of treatment.16
Prophylactic antibiotics have no role in the prevention of UTIs in patients with SCI/D. The minimal benefits associated with prophylaxis are outweighed by the risks of increased bacterial resistance to antibiotics. Research shows no significant benefit associated with the use of non-antibiotic prophylaxis, including the use of cranberry products and mannose, but further studies are needed in this patient population.18
Focus on bowel function; it correlates with quality of life
Bowel dysfunction is nearly universal in patients with SCI/D. The enteric nervous system is modulated via the sympathetic, parasympathetic, and somatic systems, and intrinsic control occurs via the myenteric and submucosal plexi. The loss of volitional control of defecation can result in prolonged transit time, reduced colonic motility, fecal incontinence, and difficulty with evacuation.
Because bowel care and function are highly correlated with quality of life,19 recommend bowel emptying every day or every other day, as well as adequate fiber in the diet, intake of fluids, stool softeners, bulk forming agents, contact irritants (eg, bisacodyl), and prokinetic agents to achieve optimal bowel care.
Prevent and treat pressure ulcers whenever possible
Accompanying the paralysis associated with SCI/D is often some degree of sensory loss of pain, light touch, temperature, and/or proprioception. The combination of insensate skin, immobility, and sarcopenia with resultant body composition changes places individuals with SCI/D at high risk for skin breakdown.21,22 Blood flow and oxygen tension at the skin surface are also decreased in patients with SCI/D compared to those without, further contributing to the problem.21,23 Increased latency from the time of injury correlates with increased likelihood of pressure ulcer development.21,22,24
External risk factors for pressure ulcers include prolonged pressure exposure, or intense pressure over a short period, shear forces, poor nutrition, smoking, moisture, and immobility. The incidence of pressure ulcers in patients with SCI/D is 25% to 66%, compared with 0.38% in the general population.21,22 Research indicates that US hospitals spend $11 billion annually on the treatment of the condition.22
To minimize pressure ulcers in this population, perform a risk assessment, using, for example, the Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) available at https://www.scireproject.com/outcome-measures-new/spinal-cord-injury-pressure-ulcer-scale-acute-scipus. In addition, recommend that patients use pressure redistribution surfaces for beds and wheelchairs, turn while in bed, perform frequent (approximately every 15-30 minutes) pressure reliefs, exercise or move regularly, and that they or a caregiver inspect the skin daily. If pressure ulcers do occur, start treatment immediately and document the stage of the ulcer.
Ensure that screening efforts go beyond what’s standard
Preventive care for patients with SCI/D is similar in many ways to that recommended for the general population. Screening for colorectal cancer,31 cervical cancer, and breast cancer32 should follow the same evidence-based intervals and age ranges suggested by groups such as the US Preventive Services Task Force (USPSTF). The only difference is to give special consideration to patients’ physical limitations and the set-up of exam rooms when scheduling and conducting procedures, such as Pap smears, colonoscopies, and mammograms.33,34
Bladder cancer. Because of the high risk for bladder cancer (ie, squamous cell carcinoma, as opposed to the more common transitional cell carcinoma) in this population, experts recommend annual cystoscopy for bladder cancer surveillance in patients who have had indwelling catheters for more than 5 to 10 years.35
Osteoporosis. Screening for osteoporosis is another preventive health area in which recommendations differ from those addressing the general population. Paralysis contributes to a decrease in mechanical stress on bone and to accelerated bone loss, and, thus, to osteoporosis.36
In patients with SCI/D, osteoporosis affects primarily weight-bearing areas below the injured lesion, such as the distal femur and proximal tibia. Fractures in patients with SCI/D may occur during minor trauma (eg, during transfers from wheelchair to bed). Although screening and treatment guidelines for osteoporosis in patients with SCI/D are not established, most experts recommend early screening and early and aggressive treatment.36
Depression reportedly occurs more frequently in individuals with SCI/D than in the general population,37,38 affecting adjustment, quality of life, and social, behavioral, and physical functioning. In light of this, it’s advisable to use screening tools, such as The Patient Health Questionnaire (PHQ)-9, routinely.39
Sexuality and sexual function are often adversely affected in both men and women with SCI/D. Loss of sensation in the sexual organs, combined with difficulty with positioning and mobility and bowel and bladder dysfunction, contribute not only to sexual dysfunction, but to lower self-esteem and altered body image.40
It is important to remember that fertility is often unaffected in women, so routine discussions about contraception with women who have SCI/D and who are sexually active are imperative. At the same time, male fertility is usually profoundly affected by SCI/D; patients and their partners who are interested in having children will require specialized interventions. Address sexuality and fertility during primary care visits and refer patients to counseling or specialists as necessary.41-43
SCI/D requires a whole-person approach
The care of individuals with SCI/D requires a holistic approach that takes into consideration physical, psychological, environmental, and interpersonal factors44,45 and involves ongoing support from a variety of specialists. FPs, with their whole-person orientation, can be instrumental in ensuring the successful rehabilitation of patients affected by SCI/D, and in helping individuals attain, preserve, and enhance their health and well-being.
CORRESPONDENCE
Ranit Mishori, MD, MHS, FAAFP, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Pre-clinical Building GB-01D, Washington, DC 20007; [email protected].
› Have a high index of suspicion for the leading causes of hospitalization among patients with spinal cord injury and disease (SCI/D). These include respiratory infections, urinary tract infections, and pressure ulcers. A
› Treat respiratory infections early and aggressively in patients with SCI/D; strongly consider inpatient management because of the high risk of respiratory failure. C
› Be alert to atypical signs and symptoms of urinary tract infection in patients with SCI/D, such as fever, chills, spasm, autonomic dysfunction, nausea and vomiting, abdominal discomfort, and fatigue. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
More than 5 million Americans are living with paralysis, and for nearly one in 4 of them the cause is spinal cord injury or disease (SCI/D).1 More common than multiple sclerosis (17%) as a cause for the loss of movement, SCI/D is second only to stroke (29%).1
The percentage of people living with paralysis due to SCI/D is increasing, partly because the population is aging and partly because management of infections has improved. Prior to the 1970s, life expectancy for people with SCI/D was significantly shortened, largely because of urologic and respiratory infections. But improved bladder management, in particular, has increased life expectancy—especially for the least severely injured.2 Respiratory diseases and septicemia remain the leading causes of death, but with increased longevity, other causes, such as endocrine, metabolic and nutritional diseases, accidents, nervous system diseases, and musculoskeletal disorders, are becoming increasingly common.2,3
Primary care’s pivotal role. Given the size of the population affected by SCI/D and the increase in life expectancy, family physicians (FPs) are more likely than ever before to care for these patients, most of whom have highly specific needs. However, little information about the primary care of patients with SCI/D exists. This patient population tends to consume a relatively large share of practices’ resources because of high case complexity.4
A recent Canadian report confirms our clinical experience that FPs report knowledge gaps in the area of SCI/D care, yet the same report found that 90% of people with SCI/D identify FPs as their “regular doctors.”5 Although a large number of patients with SCI/D identify their physiatrist as their primary care physician (PCP), one study reported that fewer than half of physiatrists are willing to assume that role.6 And while more than half of all patients with SCI/D have both specialists and PCPs involved in their care,5 communication breakdowns are a concern for patients receiving medical and rehabilitative direction from multiple health care professionals.
Below we take a closer look at the distinct patient populations affected by SCI/D, summarize several clinical conditions that contribute to hospitalization, and provide clinical management recommendations (TABLE7-26).
2 patient populations, one diagnosis
Paralysis due to spinal trauma occurs predominantly in non-Hispanic white and black males because of vehicular accidents, falls, violence, and sports.2 The mean age of injury has increased from 29 years during the 1970s to 42 years since 2010.2 However, this calculated average is misleading because there is an emerging bimodal distribution of people injured during early adulthood and a new increase in older adults injured primarily because of falls.27 In addition to those injured traumatically, a broader cohort of approximately 1 million patients represents a largely undefined group of people with paralysis due to diseases such as spinal stenosis, cancer, infection, multiple sclerosis, or other non-traumatic causes.
As a result, the population with SCI/D is comprised primarily of young adult males who have relatively few chronic medical conditions at the time of their injury and age with SCI/D, and older patients who are more likely to have already developed chronic medical conditions by the time of their SCI/D. Approximately 60% of SCI/Ds result in tetraplegia (ie, 4 limbs affected), although approximately two-thirds are incomplete, meaning that patients have some residual motor or sensory function below the level of injury.2 Not surprisingly, the level and severity of SCI/D impact life expectancy inversely and lifetime financial costs directly.
High health care utilization. Morbidity data largely parallel mortality data, often resulting in high health care utilization and cost among SCI/D patients.28 In a recent prospective observational study of nearly 1000 people with new traumatic SCI, 36.2% were rehospitalized at least once and 12.5% were rehospitalized at least twice during the 12-month period after discharge following injury.29
Rehospitalization, an outcome often quoted as a proxy for inadequate primary care, remains unacceptably high (36%-50%) for people with SCI/D.29,30 The leading causes of rehospitalization—pneumonia, urinary tract infection (UTI), and pressure ulcers29—have not changed over the years and persist over the lifetime of individuals with SCI/D.30
Take steps to prevent pneumonia, other respiratory complications
Many people with SCI/D are at high risk for respiratory complications because of their weakened respiratory muscles. This is particularly true for individuals who have injuries occurring above T10; those with injuries that are high on the spinal cord have the highest complication risk.7,8 In fact, pneumonia, atelectasis, and other respiratory complications are the leading causes of mortality in patients with tetraplegia, occurring in 40% to 70% of these patients.7
The diaphragm, innervated by the phrenic nerve (C3-C5), is the primary muscle of inspiration. Accessory muscles of inspiration include the scalenes (C5-C8), sternocleidomastoid and trapezius (C1-C4), and intercostals (T1-T11); whereas forced exhalation (cough) occurs with contraction of the abdominals (T5-T12).9 Diminished inspiration in individuals with higher level lesions can lead to microatelectasis, dyspnea with exertion, and even respiratory insufficiency.
In SCI/D above T8, weakened expiration can severely decrease cough effectiveness and secretion clearance, increasing susceptibility to lower respiratory tract infections. In addition, experts have described asthma-like disorders of airway function, particularly in those with higher lesions, due to unopposed parasympathetic innervation of respiratory smooth muscle.10
Management of this neurogenic pulmonary dysfunction after SCI/D relies on extensive preventive measures, including positioning and postural changes, breathing techniques, coughing (assisted for patients with tetraplegia), postural drainage, chest compression and percussion, and suctioning to avoid atelectasis, aspiration, and pneumonia. Ensure that patients receive influenza and pneumococcal vaccinations, and encourage smoking cessation. Obtain a chest x-ray if the patient demonstrates a decrease in respiratory function, deteriorating vital signs, reduced vital capacity, an increase in subjective dyspnea, or a change in sputum quantity. Treat respiratory infections early and aggressively,7-10 and strongly consider inpatient management because of the high risk of respiratory failure.
Pneumococcus is the most common cause of respiratory infections, although up to 21% of cases of community-acquired pneumonia in patients with SCI/D are caused by Pseudomonas.11-13 Avoid the use of antibiotics in patients who do not have signs or symptoms of a respiratory infection to minimize the development of resistant organisms. Target antibiotic therapy as per general population guidelines, as guidelines validated for use in the population with SCI/D do not currently exist.7,11
Be alert for UTIs—typical signs, symptoms don’t apply
The bladder receives innervation from S2 to S4 via the hypogastric, pudendal, and pelvic nerves. As such, the vast majority—70% to 84%—of patients with SCI/D report some degree of bladder dysfunction.14 Generally, SCI/D contributes to a combination of a failure to empty the bladder and a failure to store urine. The former is more frequent and the latter occurs more often in people with bladder outlet flaccidity, which usually occurs with low injury, such as that of the lumbar spine.14
The majority of people with SCI/D who are unable to empty their bladder require the use of some type of bladder catheter, either intermittent, indwelling (urethral or suprapubic), or condom. The choice of bladder management technique depends on gender, hand function, body habitus, caregiver assistance, and medical comorbidities. People with SCI/D are at greater risk for bladder and renal stones, UTI, vesicoureteral reflux, and bladder cancer.15,16 That said, the risk of bladder and renal stones declines somewhat after the first 6 months following an injury due to an immobility-induced loss of calcium.
Patients with SCI/D are often found to have bacteruria and even pyuria, and although they are at high risk for recurrent UTIs, these can be difficult to diagnose because signs and symptoms may differ from those seen in people with neurologically intact bladders. Symptomatic UTIs may present with fever, hematuria, abdominal discomfort, and/or increased spasticity, among other symptoms. They may cause increased bouts of autonomic dysreflexia, malaise, or a change in functional status. One cannot rely on the typical symptoms of dysuria and increased urinary frequency in this patient population. Further, the Infectious Diseases Society of America (IDSA) states that cloudy or foul-smelling urine in adults with catheters is not a symptom or sign mandating treatment.17
Because there is a lack of consensus as to what constitutes UTI symptoms in patients with SCI/D, PCPs need to be aware of changes from baseline in patients; these, combined with urine dip and culture results, should guide initiation of treatment.16
Prophylactic antibiotics have no role in the prevention of UTIs in patients with SCI/D. The minimal benefits associated with prophylaxis are outweighed by the risks of increased bacterial resistance to antibiotics. Research shows no significant benefit associated with the use of non-antibiotic prophylaxis, including the use of cranberry products and mannose, but further studies are needed in this patient population.18
Focus on bowel function; it correlates with quality of life
Bowel dysfunction is nearly universal in patients with SCI/D. The enteric nervous system is modulated via the sympathetic, parasympathetic, and somatic systems, and intrinsic control occurs via the myenteric and submucosal plexi. The loss of volitional control of defecation can result in prolonged transit time, reduced colonic motility, fecal incontinence, and difficulty with evacuation.
Because bowel care and function are highly correlated with quality of life,19 recommend bowel emptying every day or every other day, as well as adequate fiber in the diet, intake of fluids, stool softeners, bulk forming agents, contact irritants (eg, bisacodyl), and prokinetic agents to achieve optimal bowel care.
Prevent and treat pressure ulcers whenever possible
Accompanying the paralysis associated with SCI/D is often some degree of sensory loss of pain, light touch, temperature, and/or proprioception. The combination of insensate skin, immobility, and sarcopenia with resultant body composition changes places individuals with SCI/D at high risk for skin breakdown.21,22 Blood flow and oxygen tension at the skin surface are also decreased in patients with SCI/D compared to those without, further contributing to the problem.21,23 Increased latency from the time of injury correlates with increased likelihood of pressure ulcer development.21,22,24
External risk factors for pressure ulcers include prolonged pressure exposure, or intense pressure over a short period, shear forces, poor nutrition, smoking, moisture, and immobility. The incidence of pressure ulcers in patients with SCI/D is 25% to 66%, compared with 0.38% in the general population.21,22 Research indicates that US hospitals spend $11 billion annually on the treatment of the condition.22
To minimize pressure ulcers in this population, perform a risk assessment, using, for example, the Spinal Cord Injury Pressure Ulcer Scale-Acute (SCIPUS-A) available at https://www.scireproject.com/outcome-measures-new/spinal-cord-injury-pressure-ulcer-scale-acute-scipus. In addition, recommend that patients use pressure redistribution surfaces for beds and wheelchairs, turn while in bed, perform frequent (approximately every 15-30 minutes) pressure reliefs, exercise or move regularly, and that they or a caregiver inspect the skin daily. If pressure ulcers do occur, start treatment immediately and document the stage of the ulcer.
Ensure that screening efforts go beyond what’s standard
Preventive care for patients with SCI/D is similar in many ways to that recommended for the general population. Screening for colorectal cancer,31 cervical cancer, and breast cancer32 should follow the same evidence-based intervals and age ranges suggested by groups such as the US Preventive Services Task Force (USPSTF). The only difference is to give special consideration to patients’ physical limitations and the set-up of exam rooms when scheduling and conducting procedures, such as Pap smears, colonoscopies, and mammograms.33,34
Bladder cancer. Because of the high risk for bladder cancer (ie, squamous cell carcinoma, as opposed to the more common transitional cell carcinoma) in this population, experts recommend annual cystoscopy for bladder cancer surveillance in patients who have had indwelling catheters for more than 5 to 10 years.35
Osteoporosis. Screening for osteoporosis is another preventive health area in which recommendations differ from those addressing the general population. Paralysis contributes to a decrease in mechanical stress on bone and to accelerated bone loss, and, thus, to osteoporosis.36
In patients with SCI/D, osteoporosis affects primarily weight-bearing areas below the injured lesion, such as the distal femur and proximal tibia. Fractures in patients with SCI/D may occur during minor trauma (eg, during transfers from wheelchair to bed). Although screening and treatment guidelines for osteoporosis in patients with SCI/D are not established, most experts recommend early screening and early and aggressive treatment.36
Depression reportedly occurs more frequently in individuals with SCI/D than in the general population,37,38 affecting adjustment, quality of life, and social, behavioral, and physical functioning. In light of this, it’s advisable to use screening tools, such as The Patient Health Questionnaire (PHQ)-9, routinely.39
Sexuality and sexual function are often adversely affected in both men and women with SCI/D. Loss of sensation in the sexual organs, combined with difficulty with positioning and mobility and bowel and bladder dysfunction, contribute not only to sexual dysfunction, but to lower self-esteem and altered body image.40
It is important to remember that fertility is often unaffected in women, so routine discussions about contraception with women who have SCI/D and who are sexually active are imperative. At the same time, male fertility is usually profoundly affected by SCI/D; patients and their partners who are interested in having children will require specialized interventions. Address sexuality and fertility during primary care visits and refer patients to counseling or specialists as necessary.41-43
SCI/D requires a whole-person approach
The care of individuals with SCI/D requires a holistic approach that takes into consideration physical, psychological, environmental, and interpersonal factors44,45 and involves ongoing support from a variety of specialists. FPs, with their whole-person orientation, can be instrumental in ensuring the successful rehabilitation of patients affected by SCI/D, and in helping individuals attain, preserve, and enhance their health and well-being.
CORRESPONDENCE
Ranit Mishori, MD, MHS, FAAFP, Georgetown University School of Medicine, 3900 Reservoir Road, NW, Pre-clinical Building GB-01D, Washington, DC 20007; [email protected].
1. Christopher and Dana Reeve Foundation. One degree of separation. Paralysis and spinal cord injury in the United States. Available at: https://www.heart.us/uploads/userfiles/files/one-degree-of-separation.pdf. Accessed April 23, 2015.
2. National Spinal Cord Injury Statistical Center. 2014 Annual Statistical Report-Complete public version. Available at: https://www.nscisc.uab.edu/reports. Accessed November 1, 2015.
3. van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27:1517-1528.
4. Smith KM, Naumann DN, McDiarmid AL, et al. Using developmental research to design innovative knowledge translation technology for spinal cord injury in primary care: Actionable Nuggets on SkillScribe. J Spinal Cord Med. 2014;37:582-588.
5. McColl MA, Aiken A, McColl A, et al. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58:1207-1216.
6. Francisco GE, Chae JC, DeLisa JA. Physiatry as a primary care specialty. Am J Phys Med Rehabil. 1995;74:186-192.
7. Consortium for Spinal Cord Medicine. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. January 2005.
8. Weaver FM, Smith B, LaVela S, et al. Interventions to increase influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10-19.
9. McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
10. Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med. 2007;30: 307-308.
11. Burns SP, Weaver FM, Parada JP, et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004;42:450-458.
12. Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129-141.
13. Waites KB, Canupp KC, Chen Y, et al. Revaccination of adults with spinal cord injury using the 23-valent pneumococcal polysaccharide vaccine. J Spinal Cord Med. 2008;31: 53-59.
14. Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012:816274.
15. Taweel W, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85-99.
16. Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am. 2011;95:111-120.
17. Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
18. Goets L, Klausner A. Strategies for prevention of urinary tract infections in neurogenic bladder dysfunction. Phys Med Rehabil Clin N Am. 2014;25:605-618.
19. Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-S102.
20. Paralyzed Veterans of America. Consortium for Spinal Cord Medicine. Neurogenic Bowel Management in Adults with Spinal Cord Injury. Available at: http://www.pva.org/site/c.ajIRK9NJbcJ2E/b.6305815/k.A19D/Publications.htm#CPG. Accessed October 30, 2015.
21. Groah SL, Schladen M, Pineda CG, et al. Prevention of Pressure Ulcers Among People With Spinal Cord Injury: A Systematic Review. PM R. 2015;7:613-636.
22. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24:S40-S101.
23. Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572-585.
24. Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: analysis of educational needs. Adv Skin Wound Care. 2008;21:322-329.
25. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. 2nd ed. Cambridge Media. 2014.
26. Ghaisas S, Pyatak EA, Blanche E, et al. Lifestyle changes and pressure ulcer prevention in adults with spinal cord injury in the pressure ulcer prevention study lifestyle intervention. Am J Occup Ther. 2015;69:6901290020p1-6901290020p10.
27. Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012;91:80-93.
28. Noonan VK, Fallah N, Park SE, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289-301.
29. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87-S97.
30. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763.
31. Hayman AV, Guihan M, Fisher MJ, et al. Colonoscopy is high yield in spinal cord injury. J Spinal Cord Med. 2013;36:436-442.
32. Guilcher SJ, Newman A, Jaglal SB. A comparison of cervical cancer screening rates among women with traumatic spinal cord injury and the general population. J Womens Health. 2010;19:57-63.
33. Lezzoni LI, Park ER, Kilbridge KL. Implications of mobility impairment on the diagnosis and treatment of breast cancer. J Womens Health. 2011;20:45-52.
34. Graham A, Savic G, Gardner B. Cervical and breast cancer screening in wheelchair dependent females. Spinal Cord. 1998;36:340-344.
35. Groah SL, Weitzenkamp DA, Lammertse DP, et al. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346-351.
36. Charmetant C, Phaner V, Condemine A, et al. Diagnosis and treatment of osteoporosis in spinal cord injury patients: a literature review. Ann Phys Rehabil Med. 2010;53:655-668.
37. Bombardier CH, Richards JS, Krause JS, et al. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749-1756.
38. Elliott TR. Studying depression following spinal cord injury: evidence, policy and practice. J Spinal Cord Med. 2015;38:584-586.
39. Kalpakjian CZ, Bombardier CH, Schomer K, et al. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32:6-24.
40. Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol. 2015;130:225-245.
41. Kreuter M, Taft C, Siösteen A, et al. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49:154-160.
42. Fritz HA, Dillaway H, Lysack CL. “Don’t think paralysis takes away your womanhood”: Sexual intimacy after spinal cord injury. Am J Occup Ther. 2015;69:6902260030p1-6902260030p10.
43. Smith AE, Molton IR, McMullen K, et al. Sexual function, satisfaction, and use of aids for sexual activity in middle-aged adults with long-term physical disability. Top Spinal Cord Inj Rehabil. 2015;21:227-232.
44. Chiodo AE, Scelza WM, Kirshblum SC, et al. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76-S83.
45. Middleton JW, Ramakrishnan K, Cameron ID. Health Maintenance for Adults with Spinal Cord Injuries. NSW Agency for Clinical Innovation. Chatswood, NSW, Australia. February 2014. Available at: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155167/Health-Maintenance.pdf. Accessed November 1, 2015.
1. Christopher and Dana Reeve Foundation. One degree of separation. Paralysis and spinal cord injury in the United States. Available at: https://www.heart.us/uploads/userfiles/files/one-degree-of-separation.pdf. Accessed April 23, 2015.
2. National Spinal Cord Injury Statistical Center. 2014 Annual Statistical Report-Complete public version. Available at: https://www.nscisc.uab.edu/reports. Accessed November 1, 2015.
3. van den Berg ME, Castellote JM, de Pedro-Cuesta J, et al. Survival after spinal cord injury: a systematic review. J Neurotrauma. 2010;27:1517-1528.
4. Smith KM, Naumann DN, McDiarmid AL, et al. Using developmental research to design innovative knowledge translation technology for spinal cord injury in primary care: Actionable Nuggets on SkillScribe. J Spinal Cord Med. 2014;37:582-588.
5. McColl MA, Aiken A, McColl A, et al. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58:1207-1216.
6. Francisco GE, Chae JC, DeLisa JA. Physiatry as a primary care specialty. Am J Phys Med Rehabil. 1995;74:186-192.
7. Consortium for Spinal Cord Medicine. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. January 2005.
8. Weaver FM, Smith B, LaVela S, et al. Interventions to increase influenza vaccination rates in veterans with spinal cord injuries and disorders. J Spinal Cord Med. 2007;30:10-19.
9. McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402-1410.
10. Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med. 2007;30: 307-308.
11. Burns SP, Weaver FM, Parada JP, et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004;42:450-458.
12. Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129-141.
13. Waites KB, Canupp KC, Chen Y, et al. Revaccination of adults with spinal cord injury using the 23-valent pneumococcal polysaccharide vaccine. J Spinal Cord Med. 2008;31: 53-59.
14. Dorsher PT, McIntosh PM. Neurogenic bladder. Adv Urol. 2012:816274.
15. Taweel W, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85-99.
16. Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am. 2011;95:111-120.
17. Hooten TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625-663.
18. Goets L, Klausner A. Strategies for prevention of urinary tract infections in neurogenic bladder dysfunction. Phys Med Rehabil Clin N Am. 2014;25:605-618.
19. Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-S102.
20. Paralyzed Veterans of America. Consortium for Spinal Cord Medicine. Neurogenic Bowel Management in Adults with Spinal Cord Injury. Available at: http://www.pva.org/site/c.ajIRK9NJbcJ2E/b.6305815/k.A19D/Publications.htm#CPG. Accessed October 30, 2015.
21. Groah SL, Schladen M, Pineda CG, et al. Prevention of Pressure Ulcers Among People With Spinal Cord Injury: A Systematic Review. PM R. 2015;7:613-636.
22. Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2001;24:S40-S101.
23. Kruger EA, Pires M, Ngann Y, et al. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572-585.
24. Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: analysis of educational needs. Adv Skin Wound Care. 2008;21:322-329.
25. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: quick reference guide. 2nd ed. Cambridge Media. 2014.
26. Ghaisas S, Pyatak EA, Blanche E, et al. Lifestyle changes and pressure ulcer prevention in adults with spinal cord injury in the pressure ulcer prevention study lifestyle intervention. Am J Occup Ther. 2015;69:6901290020p1-6901290020p10.
27. Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012;91:80-93.
28. Noonan VK, Fallah N, Park SE, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20:289-301.
29. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94:S87-S97.
30. Cardenas DD, Hoffman JM, Kirshblum S, et al. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757-1763.
31. Hayman AV, Guihan M, Fisher MJ, et al. Colonoscopy is high yield in spinal cord injury. J Spinal Cord Med. 2013;36:436-442.
32. Guilcher SJ, Newman A, Jaglal SB. A comparison of cervical cancer screening rates among women with traumatic spinal cord injury and the general population. J Womens Health. 2010;19:57-63.
33. Lezzoni LI, Park ER, Kilbridge KL. Implications of mobility impairment on the diagnosis and treatment of breast cancer. J Womens Health. 2011;20:45-52.
34. Graham A, Savic G, Gardner B. Cervical and breast cancer screening in wheelchair dependent females. Spinal Cord. 1998;36:340-344.
35. Groah SL, Weitzenkamp DA, Lammertse DP, et al. Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehabil. 2002;83:346-351.
36. Charmetant C, Phaner V, Condemine A, et al. Diagnosis and treatment of osteoporosis in spinal cord injury patients: a literature review. Ann Phys Rehabil Med. 2010;53:655-668.
37. Bombardier CH, Richards JS, Krause JS, et al. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749-1756.
38. Elliott TR. Studying depression following spinal cord injury: evidence, policy and practice. J Spinal Cord Med. 2015;38:584-586.
39. Kalpakjian CZ, Bombardier CH, Schomer K, et al. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009;32:6-24.
40. Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol. 2015;130:225-245.
41. Kreuter M, Taft C, Siösteen A, et al. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49:154-160.
42. Fritz HA, Dillaway H, Lysack CL. “Don’t think paralysis takes away your womanhood”: Sexual intimacy after spinal cord injury. Am J Occup Ther. 2015;69:6902260030p1-6902260030p10.
43. Smith AE, Molton IR, McMullen K, et al. Sexual function, satisfaction, and use of aids for sexual activity in middle-aged adults with long-term physical disability. Top Spinal Cord Inj Rehabil. 2015;21:227-232.
44. Chiodo AE, Scelza WM, Kirshblum SC, et al. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil. 2007;88:S76-S83.
45. Middleton JW, Ramakrishnan K, Cameron ID. Health Maintenance for Adults with Spinal Cord Injuries. NSW Agency for Clinical Innovation. Chatswood, NSW, Australia. February 2014. Available at: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0007/155167/Health-Maintenance.pdf. Accessed November 1, 2015.
From The Journal of Family Practice | 2016;65(5):302-306,308-309.
Anxiety and depression: Easing the burden in COPD patients
› Initiate both pharmacologic and psychological therapies for anxiety or depression coexisting with COPD to improve patient outcomes. B
› Consider buspirone as an alternative to benzodiazepines for anxiety coexistent with COPD. B
› Consider motivational interviewing as a behavioral approach to help patients who are ambivalent about or resistant to change. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 66-year-old man you have seen many times for issues related to his chronic obstructive pulmonary disease (COPD) comes in to your clinic for a routine visit. He has been taking budesonide/formoterol twice a day for the last 3 years; however, he has not always been compliant with his medications and has been hospitalized within the last 6 months for disease exacerbations. Today, he says he has difficulty falling asleep and often becomes short of breath, even when physically inactive. His wife, who is accompanying him today, tells you he has become increasingly distant over the past few months and is not as engaged at family outings, which he attributes to labored breathing. They’re both concerned about this change and ask for advice.
Despite the increased awareness that generalized anxiety disorder (GAD) and major depressive disorder (MDD) are common comorbidities of COPD, they remain underdiagnosed and undertreated in patients with COPD. The results are increased rates of symptom exacerbation and rehospitalization.1 Family physicians, who are the primary caregivers for most patients with the disease,2 can maximize patients’ quality of life by recognizing comorbid mental illness, motivating and engaging patients in their disease management, and initiating appropriate treatment.
Anxiety and depression in COPD: A 2-way street
Several studies have assessed the prevalence of psychological disorders in patients with COPD. Affective disorders, mainly GAD and MDD, are the ones most commonly associated with poor COPD prognoses.3,4 GAD is at least 3 times more prevalent in patients with COPD than in the general US population,5 reaching upwards of 55%.1,6 Prevalence of MDD is also high, affecting approximately 40% of patients with the disease.1
GAD and MDD are more prevalent as comorbidities of COPD than they are with other chronic diseases such as orthopedic conditions, pulmonary tuberculosis, hypertension and heart disease, stroke, diabetes, and cancer.5,7-9 Patients with COPD, more so than patients with other serious chronic diseases, report heightened edginess, anxiousness, tiredness, distractibility, and irritability,5 perhaps owing in part to breathlessness and “air hunger.”10
The connection between COPD and GAD or MDD is not unidirectional, with progression of lung disease exacerbating its psychological comorbidities. The interaction is reciprocal, as clarified by Atlantis, et al, in a 2013 systematic review and meta-analysis that assessed key variables in the development of COPD and GAD or MDD.11
COPD increases the risk of MDD, which is associated with increased tobacco consumption, poor adherence with COPD medications, and decreased physical activity.11 Compounding the problem of inactivity is the fact that COPD—particularly longstanding disease—can lead to volume reductions in the anterior cingulate cortex of patients, which correlates with a persistent fear of performing physical activity.12 MDD in the setting of COPD also complicates the already complex interplay between nicotine dependence and attempts at smoking cessation.11
GAD/MDD worsens COPD outcomes
Comorbid GAD and MDD increase demands on our health care system and decrease the quality of life for patients with COPD. Anxious or depressed patients have higher 30-day readmission rates and less frequent outpatient follow-up than COPD patients without these mental comorbidities.6 Patients with comorbidities tend to have a higher prevalence of systemic symptoms independent of COPD severity,7 exhibit poorer physical and social functioning,13 and experience greater impairment of quality of life than patients with lung dysfunction alone.1,14 Patients with GAD or MDD have a 43% increased risk of any adverse COPD outcome, which can include exacerbations, COPD-related diagnoses (eg, emphysema), new anxiety or depression events, and death.11 Specifically, the risk of a COPD exacerbation rises by 31% in patients with comorbid GAD or MDD, and risk of death in those with comorbid MDD increases by 83%.11
GAD or MDD with COPD increases health care utilization and costs per patient when compared with patients who have COPD alone.9 Annual physician visits, emergency-room visits, and hospitalizations for any cause are higher in anxious or depressed COPD patients, and they have a 77% increased chance annually of a COPD-related hospitalization.9 Annual COPD-related health care costs for patients with GAD or MDD are significantly higher than the average COPD-related costs for patients without depression or anxiety, leading to significantly increased all-cause health care costs: $28,961 vs $22,512.9 Addressing and managing comorbid GAD or MDD in COPD patients could substantially reduce health care costs.
Be vigilant for anxiety, depression—even when COPD is mild
One reason comorbid GAD or MDD may be overlooked and underdiagnosed is that the symptoms can overlap those of COPD. In cases where suspicion of GAD or MDD is warranted, providers must keep separate the diagnostic inquiries for COPD and these comorbidities.6
Somatic symptoms of anxiety, such as hyperventilation, shortness of breath, and sweating, may easily be attributed to pulmonary disease instead of a psychological disorder. Differentiating the 2 processes becomes more difficult with patients younger than 60 years, as they are more likely to experience symptoms of GAD or MDD than older patients, regardless of COPD severity.15 Therefore, when assessing COPD patients, physicians need to be more vigilant for anxiety and depression, even in the mildest cases.14
Several methods exist for assessing anxiety and depression, including the Generalized Anxiety Disorder Screener 7 (GAD-7) and the Patient Health Questionnaire (PHQ) 2 or 9.16 All PHQ and GAD-7 screeners and translations are downloadable from www.phqscreeners.com/select-screener and permission is not required to reproduce, translate, display, or distribute them (FIGURE).16 Other anxiety and depression screening instruments are also available.
No one method has been shown to be most effective for rapid screening, and the physician’s comfort level or familiarity with a particular assessment tool may guide selection. One advantage of short screening instruments is that they can be incorporated into electronic health records for easy use across continuity visits. Although routine screening for these mental comorbidities takes slightly more time—especially in high-volume family practice clinics—it needs to become standard practice to protect patients’ quality of life.
Managing psychiatric conditions in COPD
Treatment for GAD and MDD in COPD is often suboptimal and may diminish a patient’s quality of life. In one study, COPD patients with a mental illness were 46% less likely than those with COPD alone to receive medications such as short- or long-acting bronchodilators and inhaled corticosteroids.17 Therapy for both the physiologic abnormalities and mental disturbances should be initiated promptly to maintain an acceptable state of health.
Pharmacotherapy. Reluctance to give traditional psychiatric medications to COPD patients contributes to the under-treatment of mental comorbidities. While benzodiazepines are generally not recommended—especially in severe COPD cases due to their sedative effect on respiratory drive—alternatives such as buspirone, tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and selective norepinephrine reuptake inhibitors (SNRIs) have been shown to effectively reduce GAD, MDD, and dyspnea in these patients5,14(TABLE18,19).
Non-pharmacotherapy approaches. Having patients apply behavioral-modification principles to their own behavior20 has been proposed as a standard of care in the treatment of COPD.21 A recent systematic review found that self-management (behavior change) interventions in patients with COPD improved health-related quality of life, reduced hospital admissions, and helped alleviate dyspnea.22 While that review could not make clear recommendations regarding the most effective form and content of self-management in COPD,22 patient engagement and motivation in creating treatment goals are considered critical ingredients for effective self-management.21
Motivational Interviewing (MI) is an evidence-based behavioral approach designed for patients who are ambivalent about or resistant to change.23 MI works by supporting a patient’s autonomy and by activating his/her own internal motivation for change or adherence to treatment. In MI, the physician’s involvement with the patient relies on collaboration, evocation, and autonomy, rather than confrontation, education, and authority. MI involves exploration more than exhortation, and support rather than persuasion or argument. The overall goal of MI is to increase intrinsic motivation so that change arises from within and serves the patient’s goals and values.23
Benzo, et al, provide a very detailed description of a self-management process that includes MI.21 Their protocol proved to be feasible in severe COPD and helped increase patient engagement and commitment to self-management.21 This finding and similar evidence of MI’s effectiveness in a variety of other health conditions suggest that pharmacotherapy and cognitive-behavior therapy can be delivered in combination with an MI approach.
Self-management depends on a patient’s readiness to implement behavioral changes. Patients engaged in unhealthy behavior may be reluctant to change at a particular time, so the physician may focus efforts on such behaviors as self-monitoring or examining values that may lead to future behavior change.
For example, a patient may not want to stop smoking, but the physician’s willingness to ask about smoking in subsequent visits may catch the patient at a time when motivation has changed—eg, perhaps there is a new child in the home, prompting a recognition that smoking is now inconsistent with one’s values and can be resolved with smoking cessation. Awareness of an individual’s baseline behavior and readiness to change assists physicians and other health professionals in tailoring interventions for the most favorable outcome.
Several other non-pharmacologic methods to reduce symptoms of GAD and MDD in patients with COPD have been studied and supported by the literature.
- Progressive muscle relaxation, stress management, biofeedback, and guided imagery have been shown to decrease symptoms of anxiety, dyspnea, and airway obstruction.5,14
- Pulmonary rehabilitation programs including psychotherapy sessions have also relieved symptoms of GAD and MDD for patients with COPD.
- Programs that include physiotherapy, physical exercise (arm and leg exercise, aerobic conditioning, flexibility training), patient education, and psychotherapy sessions have significantly lowered GAD and MDD scores when compared with similar rehabilitation programs not offering psychotherapy.24
- Cognitive-behavioral therapy has been variably effective in treating comorbid GAD and MDD, with studies citing either superiority5 or equivalence25 to COPD education alone.
Increasingly, psychologists have been integrated into primary care with implementation of the Patient-Centered Medical Home.26 However, if primary care physicians do not have behavioral specialists available, they can contact the American Psychological Association, their state psychological association, or professional organizations, such as the Society of Behavioral Medicine, for referral to professionals trained in behavioral self-management skills.
Initiation of treatment, whether pharmacological or non-pharmacological, and emphasis on self-management of the disease can greatly improve patients' perceptions of their condition and overall quality of life.
CASE › The patient screens positive for GAD and you give him a prescription for venlafaxine to begin immediately. Using an MI approach, you help the patient clarify that being more engaged with his family is important to him. Acknowledging that your recommendations are consistent with his values, the patient agrees to pursue pulmonary rehabilitation and, with the aid of a behavioral health specialist, learn self-management techniques for medication adherence and social reengagement.
CORRESPONDENCE
Ms. Sydney Marsh, 3009 S 35th Ave., Omaha, NE 68105; [email protected].
1. Yohannes AM, Willgoss TG, Baldwin RC, et al. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25:1209-1221.
2. Punturieri A, Croxton TL, Weinmann G, et al. The changing face of COPD. Am Fam Physician. 2007;1:315-316.
3. Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58:858-866.
4. Porthirat C, Chaiwong W, Phetsuk N, et al. Major affective disorders in chronic obstructive pulmonary disease compared with other chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2015;10:1583-1590.
5. Brenes GA. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med. 2003; 65:963-970.
6. Singh G, Zhang W, Kuo YF, et al. Association of psychological disorders with 30-day readmission rates in patients with Chronic Obstructive Pulmonary Disease. Chest. 2015;Jul 23:[Epub ahead of print].
7. Vögele C, von Leupoldt A. Mental disorders in chronic obstructive pulmonary disease. Respir Med. 2008;102:764-773.
8. Aydin IO, Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. Gen Hosp Psychiatry. 2001;23:77-83.
9. Dalal AA, Shah M, Lunacsek O, et al. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8:293-299.
10. Janssen DJA, Wouters EFM, Spruit MA. Psychosocial consequences of living with breathlessness due to advanced disease. Curr Opin Support Palliat Care. 2015;9:232-237.
11. Atlantis E, Fahey P, Cochrane B, et al. Bidirectional associations between clinically relevant depression or anxiety and COPD. Chest. 2013;144:766-777.
12. Esser RW, Stoeckel MC, Kirsten A, et al. Structural brain changes in patients with chronic obstructive pulmonary disease. Chest. 2015;Jul 23:[Epub ahead of print].
13. Ng TP, Niti M, Tan WC, et al. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60-67.
14. Kim HF, Kunik ME, Molinari VA, et al. Functional impairment in COPD patients. Psychosomatics. 2000;41:465-471.
15. Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. 2007;24:217-223.
16. Kroenke K, Spitzer RL, Williams JB et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359.
17. Ajmera M, Sambamoorthi U, Metzger A, et al. Multimorbidity and COPD medication receipt among Medicaid beneficiaries with newly diagnosed COPD. Respir Care. 2015;60:1592-1602.
18. Medscape. Psychiatrics. Available at: http://reference.medscape.com/drugs/psychiatrics. Accessed March 1, 2016.
19. Physicians’ Desk Reference. Available at: http://www.pdr.net. Accessed March 1, 2016.
20. Kazdin AE. Behavior Modification in Applied Settings. Belmont, CA: Wadsworth/Thomson Learning; 2001.
21. Benzo R, Vickers K, Ernst D, et al. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. J Cardiopulm Rehabil. 2013;33:113-123.
22. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database System Rev. 2014;(3):CD002990.
23. Miller WR, Rollnick S. Motivational Interviewing. 3rd ed. New York, NY: Guilford Press; 2013.
24. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84:1154-1157.
25. Kunik ME, Veazey C, Cully JA, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med. 2008;38:385-396.
26. McDaniel SH, Fogarty CT. What primary care psychology has to offer the patient-centered medical home. Prof Psych Res Pract. 2009;40:483-492.
› Initiate both pharmacologic and psychological therapies for anxiety or depression coexisting with COPD to improve patient outcomes. B
› Consider buspirone as an alternative to benzodiazepines for anxiety coexistent with COPD. B
› Consider motivational interviewing as a behavioral approach to help patients who are ambivalent about or resistant to change. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 66-year-old man you have seen many times for issues related to his chronic obstructive pulmonary disease (COPD) comes in to your clinic for a routine visit. He has been taking budesonide/formoterol twice a day for the last 3 years; however, he has not always been compliant with his medications and has been hospitalized within the last 6 months for disease exacerbations. Today, he says he has difficulty falling asleep and often becomes short of breath, even when physically inactive. His wife, who is accompanying him today, tells you he has become increasingly distant over the past few months and is not as engaged at family outings, which he attributes to labored breathing. They’re both concerned about this change and ask for advice.
Despite the increased awareness that generalized anxiety disorder (GAD) and major depressive disorder (MDD) are common comorbidities of COPD, they remain underdiagnosed and undertreated in patients with COPD. The results are increased rates of symptom exacerbation and rehospitalization.1 Family physicians, who are the primary caregivers for most patients with the disease,2 can maximize patients’ quality of life by recognizing comorbid mental illness, motivating and engaging patients in their disease management, and initiating appropriate treatment.
Anxiety and depression in COPD: A 2-way street
Several studies have assessed the prevalence of psychological disorders in patients with COPD. Affective disorders, mainly GAD and MDD, are the ones most commonly associated with poor COPD prognoses.3,4 GAD is at least 3 times more prevalent in patients with COPD than in the general US population,5 reaching upwards of 55%.1,6 Prevalence of MDD is also high, affecting approximately 40% of patients with the disease.1
GAD and MDD are more prevalent as comorbidities of COPD than they are with other chronic diseases such as orthopedic conditions, pulmonary tuberculosis, hypertension and heart disease, stroke, diabetes, and cancer.5,7-9 Patients with COPD, more so than patients with other serious chronic diseases, report heightened edginess, anxiousness, tiredness, distractibility, and irritability,5 perhaps owing in part to breathlessness and “air hunger.”10
The connection between COPD and GAD or MDD is not unidirectional, with progression of lung disease exacerbating its psychological comorbidities. The interaction is reciprocal, as clarified by Atlantis, et al, in a 2013 systematic review and meta-analysis that assessed key variables in the development of COPD and GAD or MDD.11
COPD increases the risk of MDD, which is associated with increased tobacco consumption, poor adherence with COPD medications, and decreased physical activity.11 Compounding the problem of inactivity is the fact that COPD—particularly longstanding disease—can lead to volume reductions in the anterior cingulate cortex of patients, which correlates with a persistent fear of performing physical activity.12 MDD in the setting of COPD also complicates the already complex interplay between nicotine dependence and attempts at smoking cessation.11
GAD/MDD worsens COPD outcomes
Comorbid GAD and MDD increase demands on our health care system and decrease the quality of life for patients with COPD. Anxious or depressed patients have higher 30-day readmission rates and less frequent outpatient follow-up than COPD patients without these mental comorbidities.6 Patients with comorbidities tend to have a higher prevalence of systemic symptoms independent of COPD severity,7 exhibit poorer physical and social functioning,13 and experience greater impairment of quality of life than patients with lung dysfunction alone.1,14 Patients with GAD or MDD have a 43% increased risk of any adverse COPD outcome, which can include exacerbations, COPD-related diagnoses (eg, emphysema), new anxiety or depression events, and death.11 Specifically, the risk of a COPD exacerbation rises by 31% in patients with comorbid GAD or MDD, and risk of death in those with comorbid MDD increases by 83%.11
GAD or MDD with COPD increases health care utilization and costs per patient when compared with patients who have COPD alone.9 Annual physician visits, emergency-room visits, and hospitalizations for any cause are higher in anxious or depressed COPD patients, and they have a 77% increased chance annually of a COPD-related hospitalization.9 Annual COPD-related health care costs for patients with GAD or MDD are significantly higher than the average COPD-related costs for patients without depression or anxiety, leading to significantly increased all-cause health care costs: $28,961 vs $22,512.9 Addressing and managing comorbid GAD or MDD in COPD patients could substantially reduce health care costs.
Be vigilant for anxiety, depression—even when COPD is mild
One reason comorbid GAD or MDD may be overlooked and underdiagnosed is that the symptoms can overlap those of COPD. In cases where suspicion of GAD or MDD is warranted, providers must keep separate the diagnostic inquiries for COPD and these comorbidities.6
Somatic symptoms of anxiety, such as hyperventilation, shortness of breath, and sweating, may easily be attributed to pulmonary disease instead of a psychological disorder. Differentiating the 2 processes becomes more difficult with patients younger than 60 years, as they are more likely to experience symptoms of GAD or MDD than older patients, regardless of COPD severity.15 Therefore, when assessing COPD patients, physicians need to be more vigilant for anxiety and depression, even in the mildest cases.14
Several methods exist for assessing anxiety and depression, including the Generalized Anxiety Disorder Screener 7 (GAD-7) and the Patient Health Questionnaire (PHQ) 2 or 9.16 All PHQ and GAD-7 screeners and translations are downloadable from www.phqscreeners.com/select-screener and permission is not required to reproduce, translate, display, or distribute them (FIGURE).16 Other anxiety and depression screening instruments are also available.
No one method has been shown to be most effective for rapid screening, and the physician’s comfort level or familiarity with a particular assessment tool may guide selection. One advantage of short screening instruments is that they can be incorporated into electronic health records for easy use across continuity visits. Although routine screening for these mental comorbidities takes slightly more time—especially in high-volume family practice clinics—it needs to become standard practice to protect patients’ quality of life.
Managing psychiatric conditions in COPD
Treatment for GAD and MDD in COPD is often suboptimal and may diminish a patient’s quality of life. In one study, COPD patients with a mental illness were 46% less likely than those with COPD alone to receive medications such as short- or long-acting bronchodilators and inhaled corticosteroids.17 Therapy for both the physiologic abnormalities and mental disturbances should be initiated promptly to maintain an acceptable state of health.
Pharmacotherapy. Reluctance to give traditional psychiatric medications to COPD patients contributes to the under-treatment of mental comorbidities. While benzodiazepines are generally not recommended—especially in severe COPD cases due to their sedative effect on respiratory drive—alternatives such as buspirone, tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and selective norepinephrine reuptake inhibitors (SNRIs) have been shown to effectively reduce GAD, MDD, and dyspnea in these patients5,14(TABLE18,19).
Non-pharmacotherapy approaches. Having patients apply behavioral-modification principles to their own behavior20 has been proposed as a standard of care in the treatment of COPD.21 A recent systematic review found that self-management (behavior change) interventions in patients with COPD improved health-related quality of life, reduced hospital admissions, and helped alleviate dyspnea.22 While that review could not make clear recommendations regarding the most effective form and content of self-management in COPD,22 patient engagement and motivation in creating treatment goals are considered critical ingredients for effective self-management.21
Motivational Interviewing (MI) is an evidence-based behavioral approach designed for patients who are ambivalent about or resistant to change.23 MI works by supporting a patient’s autonomy and by activating his/her own internal motivation for change or adherence to treatment. In MI, the physician’s involvement with the patient relies on collaboration, evocation, and autonomy, rather than confrontation, education, and authority. MI involves exploration more than exhortation, and support rather than persuasion or argument. The overall goal of MI is to increase intrinsic motivation so that change arises from within and serves the patient’s goals and values.23
Benzo, et al, provide a very detailed description of a self-management process that includes MI.21 Their protocol proved to be feasible in severe COPD and helped increase patient engagement and commitment to self-management.21 This finding and similar evidence of MI’s effectiveness in a variety of other health conditions suggest that pharmacotherapy and cognitive-behavior therapy can be delivered in combination with an MI approach.
Self-management depends on a patient’s readiness to implement behavioral changes. Patients engaged in unhealthy behavior may be reluctant to change at a particular time, so the physician may focus efforts on such behaviors as self-monitoring or examining values that may lead to future behavior change.
For example, a patient may not want to stop smoking, but the physician’s willingness to ask about smoking in subsequent visits may catch the patient at a time when motivation has changed—eg, perhaps there is a new child in the home, prompting a recognition that smoking is now inconsistent with one’s values and can be resolved with smoking cessation. Awareness of an individual’s baseline behavior and readiness to change assists physicians and other health professionals in tailoring interventions for the most favorable outcome.
Several other non-pharmacologic methods to reduce symptoms of GAD and MDD in patients with COPD have been studied and supported by the literature.
- Progressive muscle relaxation, stress management, biofeedback, and guided imagery have been shown to decrease symptoms of anxiety, dyspnea, and airway obstruction.5,14
- Pulmonary rehabilitation programs including psychotherapy sessions have also relieved symptoms of GAD and MDD for patients with COPD.
- Programs that include physiotherapy, physical exercise (arm and leg exercise, aerobic conditioning, flexibility training), patient education, and psychotherapy sessions have significantly lowered GAD and MDD scores when compared with similar rehabilitation programs not offering psychotherapy.24
- Cognitive-behavioral therapy has been variably effective in treating comorbid GAD and MDD, with studies citing either superiority5 or equivalence25 to COPD education alone.
Increasingly, psychologists have been integrated into primary care with implementation of the Patient-Centered Medical Home.26 However, if primary care physicians do not have behavioral specialists available, they can contact the American Psychological Association, their state psychological association, or professional organizations, such as the Society of Behavioral Medicine, for referral to professionals trained in behavioral self-management skills.
Initiation of treatment, whether pharmacological or non-pharmacological, and emphasis on self-management of the disease can greatly improve patients' perceptions of their condition and overall quality of life.
CASE › The patient screens positive for GAD and you give him a prescription for venlafaxine to begin immediately. Using an MI approach, you help the patient clarify that being more engaged with his family is important to him. Acknowledging that your recommendations are consistent with his values, the patient agrees to pursue pulmonary rehabilitation and, with the aid of a behavioral health specialist, learn self-management techniques for medication adherence and social reengagement.
CORRESPONDENCE
Ms. Sydney Marsh, 3009 S 35th Ave., Omaha, NE 68105; [email protected].
› Initiate both pharmacologic and psychological therapies for anxiety or depression coexisting with COPD to improve patient outcomes. B
› Consider buspirone as an alternative to benzodiazepines for anxiety coexistent with COPD. B
› Consider motivational interviewing as a behavioral approach to help patients who are ambivalent about or resistant to change. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 66-year-old man you have seen many times for issues related to his chronic obstructive pulmonary disease (COPD) comes in to your clinic for a routine visit. He has been taking budesonide/formoterol twice a day for the last 3 years; however, he has not always been compliant with his medications and has been hospitalized within the last 6 months for disease exacerbations. Today, he says he has difficulty falling asleep and often becomes short of breath, even when physically inactive. His wife, who is accompanying him today, tells you he has become increasingly distant over the past few months and is not as engaged at family outings, which he attributes to labored breathing. They’re both concerned about this change and ask for advice.
Despite the increased awareness that generalized anxiety disorder (GAD) and major depressive disorder (MDD) are common comorbidities of COPD, they remain underdiagnosed and undertreated in patients with COPD. The results are increased rates of symptom exacerbation and rehospitalization.1 Family physicians, who are the primary caregivers for most patients with the disease,2 can maximize patients’ quality of life by recognizing comorbid mental illness, motivating and engaging patients in their disease management, and initiating appropriate treatment.
Anxiety and depression in COPD: A 2-way street
Several studies have assessed the prevalence of psychological disorders in patients with COPD. Affective disorders, mainly GAD and MDD, are the ones most commonly associated with poor COPD prognoses.3,4 GAD is at least 3 times more prevalent in patients with COPD than in the general US population,5 reaching upwards of 55%.1,6 Prevalence of MDD is also high, affecting approximately 40% of patients with the disease.1
GAD and MDD are more prevalent as comorbidities of COPD than they are with other chronic diseases such as orthopedic conditions, pulmonary tuberculosis, hypertension and heart disease, stroke, diabetes, and cancer.5,7-9 Patients with COPD, more so than patients with other serious chronic diseases, report heightened edginess, anxiousness, tiredness, distractibility, and irritability,5 perhaps owing in part to breathlessness and “air hunger.”10
The connection between COPD and GAD or MDD is not unidirectional, with progression of lung disease exacerbating its psychological comorbidities. The interaction is reciprocal, as clarified by Atlantis, et al, in a 2013 systematic review and meta-analysis that assessed key variables in the development of COPD and GAD or MDD.11
COPD increases the risk of MDD, which is associated with increased tobacco consumption, poor adherence with COPD medications, and decreased physical activity.11 Compounding the problem of inactivity is the fact that COPD—particularly longstanding disease—can lead to volume reductions in the anterior cingulate cortex of patients, which correlates with a persistent fear of performing physical activity.12 MDD in the setting of COPD also complicates the already complex interplay between nicotine dependence and attempts at smoking cessation.11
GAD/MDD worsens COPD outcomes
Comorbid GAD and MDD increase demands on our health care system and decrease the quality of life for patients with COPD. Anxious or depressed patients have higher 30-day readmission rates and less frequent outpatient follow-up than COPD patients without these mental comorbidities.6 Patients with comorbidities tend to have a higher prevalence of systemic symptoms independent of COPD severity,7 exhibit poorer physical and social functioning,13 and experience greater impairment of quality of life than patients with lung dysfunction alone.1,14 Patients with GAD or MDD have a 43% increased risk of any adverse COPD outcome, which can include exacerbations, COPD-related diagnoses (eg, emphysema), new anxiety or depression events, and death.11 Specifically, the risk of a COPD exacerbation rises by 31% in patients with comorbid GAD or MDD, and risk of death in those with comorbid MDD increases by 83%.11
GAD or MDD with COPD increases health care utilization and costs per patient when compared with patients who have COPD alone.9 Annual physician visits, emergency-room visits, and hospitalizations for any cause are higher in anxious or depressed COPD patients, and they have a 77% increased chance annually of a COPD-related hospitalization.9 Annual COPD-related health care costs for patients with GAD or MDD are significantly higher than the average COPD-related costs for patients without depression or anxiety, leading to significantly increased all-cause health care costs: $28,961 vs $22,512.9 Addressing and managing comorbid GAD or MDD in COPD patients could substantially reduce health care costs.
Be vigilant for anxiety, depression—even when COPD is mild
One reason comorbid GAD or MDD may be overlooked and underdiagnosed is that the symptoms can overlap those of COPD. In cases where suspicion of GAD or MDD is warranted, providers must keep separate the diagnostic inquiries for COPD and these comorbidities.6
Somatic symptoms of anxiety, such as hyperventilation, shortness of breath, and sweating, may easily be attributed to pulmonary disease instead of a psychological disorder. Differentiating the 2 processes becomes more difficult with patients younger than 60 years, as they are more likely to experience symptoms of GAD or MDD than older patients, regardless of COPD severity.15 Therefore, when assessing COPD patients, physicians need to be more vigilant for anxiety and depression, even in the mildest cases.14
Several methods exist for assessing anxiety and depression, including the Generalized Anxiety Disorder Screener 7 (GAD-7) and the Patient Health Questionnaire (PHQ) 2 or 9.16 All PHQ and GAD-7 screeners and translations are downloadable from www.phqscreeners.com/select-screener and permission is not required to reproduce, translate, display, or distribute them (FIGURE).16 Other anxiety and depression screening instruments are also available.
No one method has been shown to be most effective for rapid screening, and the physician’s comfort level or familiarity with a particular assessment tool may guide selection. One advantage of short screening instruments is that they can be incorporated into electronic health records for easy use across continuity visits. Although routine screening for these mental comorbidities takes slightly more time—especially in high-volume family practice clinics—it needs to become standard practice to protect patients’ quality of life.
Managing psychiatric conditions in COPD
Treatment for GAD and MDD in COPD is often suboptimal and may diminish a patient’s quality of life. In one study, COPD patients with a mental illness were 46% less likely than those with COPD alone to receive medications such as short- or long-acting bronchodilators and inhaled corticosteroids.17 Therapy for both the physiologic abnormalities and mental disturbances should be initiated promptly to maintain an acceptable state of health.
Pharmacotherapy. Reluctance to give traditional psychiatric medications to COPD patients contributes to the under-treatment of mental comorbidities. While benzodiazepines are generally not recommended—especially in severe COPD cases due to their sedative effect on respiratory drive—alternatives such as buspirone, tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and selective norepinephrine reuptake inhibitors (SNRIs) have been shown to effectively reduce GAD, MDD, and dyspnea in these patients5,14(TABLE18,19).
Non-pharmacotherapy approaches. Having patients apply behavioral-modification principles to their own behavior20 has been proposed as a standard of care in the treatment of COPD.21 A recent systematic review found that self-management (behavior change) interventions in patients with COPD improved health-related quality of life, reduced hospital admissions, and helped alleviate dyspnea.22 While that review could not make clear recommendations regarding the most effective form and content of self-management in COPD,22 patient engagement and motivation in creating treatment goals are considered critical ingredients for effective self-management.21
Motivational Interviewing (MI) is an evidence-based behavioral approach designed for patients who are ambivalent about or resistant to change.23 MI works by supporting a patient’s autonomy and by activating his/her own internal motivation for change or adherence to treatment. In MI, the physician’s involvement with the patient relies on collaboration, evocation, and autonomy, rather than confrontation, education, and authority. MI involves exploration more than exhortation, and support rather than persuasion or argument. The overall goal of MI is to increase intrinsic motivation so that change arises from within and serves the patient’s goals and values.23
Benzo, et al, provide a very detailed description of a self-management process that includes MI.21 Their protocol proved to be feasible in severe COPD and helped increase patient engagement and commitment to self-management.21 This finding and similar evidence of MI’s effectiveness in a variety of other health conditions suggest that pharmacotherapy and cognitive-behavior therapy can be delivered in combination with an MI approach.
Self-management depends on a patient’s readiness to implement behavioral changes. Patients engaged in unhealthy behavior may be reluctant to change at a particular time, so the physician may focus efforts on such behaviors as self-monitoring or examining values that may lead to future behavior change.
For example, a patient may not want to stop smoking, but the physician’s willingness to ask about smoking in subsequent visits may catch the patient at a time when motivation has changed—eg, perhaps there is a new child in the home, prompting a recognition that smoking is now inconsistent with one’s values and can be resolved with smoking cessation. Awareness of an individual’s baseline behavior and readiness to change assists physicians and other health professionals in tailoring interventions for the most favorable outcome.
Several other non-pharmacologic methods to reduce symptoms of GAD and MDD in patients with COPD have been studied and supported by the literature.
- Progressive muscle relaxation, stress management, biofeedback, and guided imagery have been shown to decrease symptoms of anxiety, dyspnea, and airway obstruction.5,14
- Pulmonary rehabilitation programs including psychotherapy sessions have also relieved symptoms of GAD and MDD for patients with COPD.
- Programs that include physiotherapy, physical exercise (arm and leg exercise, aerobic conditioning, flexibility training), patient education, and psychotherapy sessions have significantly lowered GAD and MDD scores when compared with similar rehabilitation programs not offering psychotherapy.24
- Cognitive-behavioral therapy has been variably effective in treating comorbid GAD and MDD, with studies citing either superiority5 or equivalence25 to COPD education alone.
Increasingly, psychologists have been integrated into primary care with implementation of the Patient-Centered Medical Home.26 However, if primary care physicians do not have behavioral specialists available, they can contact the American Psychological Association, their state psychological association, or professional organizations, such as the Society of Behavioral Medicine, for referral to professionals trained in behavioral self-management skills.
Initiation of treatment, whether pharmacological or non-pharmacological, and emphasis on self-management of the disease can greatly improve patients' perceptions of their condition and overall quality of life.
CASE › The patient screens positive for GAD and you give him a prescription for venlafaxine to begin immediately. Using an MI approach, you help the patient clarify that being more engaged with his family is important to him. Acknowledging that your recommendations are consistent with his values, the patient agrees to pursue pulmonary rehabilitation and, with the aid of a behavioral health specialist, learn self-management techniques for medication adherence and social reengagement.
CORRESPONDENCE
Ms. Sydney Marsh, 3009 S 35th Ave., Omaha, NE 68105; [email protected].
1. Yohannes AM, Willgoss TG, Baldwin RC, et al. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25:1209-1221.
2. Punturieri A, Croxton TL, Weinmann G, et al. The changing face of COPD. Am Fam Physician. 2007;1:315-316.
3. Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58:858-866.
4. Porthirat C, Chaiwong W, Phetsuk N, et al. Major affective disorders in chronic obstructive pulmonary disease compared with other chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2015;10:1583-1590.
5. Brenes GA. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med. 2003; 65:963-970.
6. Singh G, Zhang W, Kuo YF, et al. Association of psychological disorders with 30-day readmission rates in patients with Chronic Obstructive Pulmonary Disease. Chest. 2015;Jul 23:[Epub ahead of print].
7. Vögele C, von Leupoldt A. Mental disorders in chronic obstructive pulmonary disease. Respir Med. 2008;102:764-773.
8. Aydin IO, Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. Gen Hosp Psychiatry. 2001;23:77-83.
9. Dalal AA, Shah M, Lunacsek O, et al. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8:293-299.
10. Janssen DJA, Wouters EFM, Spruit MA. Psychosocial consequences of living with breathlessness due to advanced disease. Curr Opin Support Palliat Care. 2015;9:232-237.
11. Atlantis E, Fahey P, Cochrane B, et al. Bidirectional associations between clinically relevant depression or anxiety and COPD. Chest. 2013;144:766-777.
12. Esser RW, Stoeckel MC, Kirsten A, et al. Structural brain changes in patients with chronic obstructive pulmonary disease. Chest. 2015;Jul 23:[Epub ahead of print].
13. Ng TP, Niti M, Tan WC, et al. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60-67.
14. Kim HF, Kunik ME, Molinari VA, et al. Functional impairment in COPD patients. Psychosomatics. 2000;41:465-471.
15. Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. 2007;24:217-223.
16. Kroenke K, Spitzer RL, Williams JB et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359.
17. Ajmera M, Sambamoorthi U, Metzger A, et al. Multimorbidity and COPD medication receipt among Medicaid beneficiaries with newly diagnosed COPD. Respir Care. 2015;60:1592-1602.
18. Medscape. Psychiatrics. Available at: http://reference.medscape.com/drugs/psychiatrics. Accessed March 1, 2016.
19. Physicians’ Desk Reference. Available at: http://www.pdr.net. Accessed March 1, 2016.
20. Kazdin AE. Behavior Modification in Applied Settings. Belmont, CA: Wadsworth/Thomson Learning; 2001.
21. Benzo R, Vickers K, Ernst D, et al. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. J Cardiopulm Rehabil. 2013;33:113-123.
22. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database System Rev. 2014;(3):CD002990.
23. Miller WR, Rollnick S. Motivational Interviewing. 3rd ed. New York, NY: Guilford Press; 2013.
24. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84:1154-1157.
25. Kunik ME, Veazey C, Cully JA, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med. 2008;38:385-396.
26. McDaniel SH, Fogarty CT. What primary care psychology has to offer the patient-centered medical home. Prof Psych Res Pract. 2009;40:483-492.
1. Yohannes AM, Willgoss TG, Baldwin RC, et al. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25:1209-1221.
2. Punturieri A, Croxton TL, Weinmann G, et al. The changing face of COPD. Am Fam Physician. 2007;1:315-316.
3. Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58:858-866.
4. Porthirat C, Chaiwong W, Phetsuk N, et al. Major affective disorders in chronic obstructive pulmonary disease compared with other chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2015;10:1583-1590.
5. Brenes GA. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med. 2003; 65:963-970.
6. Singh G, Zhang W, Kuo YF, et al. Association of psychological disorders with 30-day readmission rates in patients with Chronic Obstructive Pulmonary Disease. Chest. 2015;Jul 23:[Epub ahead of print].
7. Vögele C, von Leupoldt A. Mental disorders in chronic obstructive pulmonary disease. Respir Med. 2008;102:764-773.
8. Aydin IO, Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. Gen Hosp Psychiatry. 2001;23:77-83.
9. Dalal AA, Shah M, Lunacsek O, et al. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8:293-299.
10. Janssen DJA, Wouters EFM, Spruit MA. Psychosocial consequences of living with breathlessness due to advanced disease. Curr Opin Support Palliat Care. 2015;9:232-237.
11. Atlantis E, Fahey P, Cochrane B, et al. Bidirectional associations between clinically relevant depression or anxiety and COPD. Chest. 2013;144:766-777.
12. Esser RW, Stoeckel MC, Kirsten A, et al. Structural brain changes in patients with chronic obstructive pulmonary disease. Chest. 2015;Jul 23:[Epub ahead of print].
13. Ng TP, Niti M, Tan WC, et al. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60-67.
14. Kim HF, Kunik ME, Molinari VA, et al. Functional impairment in COPD patients. Psychosomatics. 2000;41:465-471.
15. Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. 2007;24:217-223.
16. Kroenke K, Spitzer RL, Williams JB et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359.
17. Ajmera M, Sambamoorthi U, Metzger A, et al. Multimorbidity and COPD medication receipt among Medicaid beneficiaries with newly diagnosed COPD. Respir Care. 2015;60:1592-1602.
18. Medscape. Psychiatrics. Available at: http://reference.medscape.com/drugs/psychiatrics. Accessed March 1, 2016.
19. Physicians’ Desk Reference. Available at: http://www.pdr.net. Accessed March 1, 2016.
20. Kazdin AE. Behavior Modification in Applied Settings. Belmont, CA: Wadsworth/Thomson Learning; 2001.
21. Benzo R, Vickers K, Ernst D, et al. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. J Cardiopulm Rehabil. 2013;33:113-123.
22. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database System Rev. 2014;(3):CD002990.
23. Miller WR, Rollnick S. Motivational Interviewing. 3rd ed. New York, NY: Guilford Press; 2013.
24. de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84:1154-1157.
25. Kunik ME, Veazey C, Cully JA, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med. 2008;38:385-396.
26. McDaniel SH, Fogarty CT. What primary care psychology has to offer the patient-centered medical home. Prof Psych Res Pract. 2009;40:483-492.
Tips and algorithms to get your patient's BP to goal
The Systolic Blood Pressure Intervention Trial (SPRINT),1 a study of more than 9000 patients published late last year, was stopped prematurely when it became clear that those receiving intensive treatment (systolic target 120 mm Hg) had significantly lower rates of myocardial infarction (MI), stroke, cardiovascular death, and other severe heart disease than those getting standard treatment (systolic target 140 mm Hg). Participants had an elevated cardiovascular risk at baseline (age ≥75 years, history of cardiovascular disease [CVD], chronic kidney disease [CKD], or elevated 10-year Framingham CVD risk score ≥15%); those with diabetes, history of stroke, or polycystic kidney disease were excluded.
Although serious adverse events were not significantly different between the intensive and standard treatment groups, syncope, acute renal failure, electrolyte abnormalities, and hyponatremia were all statistically more common in the aggressively treated group. (To learn more, see “Is lower BP worth it in higher-risk patients with diabetes or coronary disease?” Clinical Inquiries, J Fam Pract. 2016;65:129-131.)
Taking an aggressive approach. This trial shined a light on an important topic in medicine—the aggressive treatment of hypertension. And while this article will not discuss the finer points of the SPRINT trial or the limitations of generalizing aggressive treatment to the broad population of patients with hypertension, it will outline important considerations for physicians who wish to aggressively treat hypertension. I offer recommendations based on my 34 years of clinical practice and experience as a co-investigator on a number of hypertension studies to help you better balance each patient’s risks (eg, age, frailty, fall risk) and potential benefits (prevention of stroke, MI, and congestive heart failure).
Taking an aggressive approach, however, starts with ensuring that the diagnosis and treatment are based on accurate measures.
How accurate are your BP readings?
Measuring BP in clinical practice is markedly different from measurements taken in a research setting.2 This can result in large, clinically significant differences in readings and adversely affect treatment decisions.
Quiet time, multiple readings
SPRINT used techniques similar to those followed by other hypertension outcomes studies I’ve been involved in—methods that are rare in medical practice. Each study participant sat quietly in a chair for 5 minutes prior to the first BP reading. In addition, the researchers used an automatic oscillatory BP device (Omron Healthcare, Lake Forest, Ill), recording the average of 3 readings.
Practices that compromise accuracy. In clinical practice, BP is rarely measured after the patient has had 5 minutes of rest in a quiet room. Nor are readings done in triplicate. Instead, BP is typically measured while patient and clinician are engaged in conversation, often using a BP cuff that is too small (in my experience, most Americans require a large cuff).
BP is usually taken shortly after the patient has walked, frequently with some difficulty, from the waiting area to the exam room. Often, too, patients are weighed before their BP is measured, a common source of concern that can lead to a short-term rise in pressure. (Conversely, rapid deflation during the auscultatory measurement [>2 mm Hg/sec] can have the opposite effect, resulting in under-reading the true value.)
Compounding matters is the failure to consider the approximately 20% of patients who develop White Coat Syndrome. Such individuals, who typically have elevated office measurements but normal out-of-office readings, may develop further hypotensive symptoms if their treatment is based solely on in-office findings. Overtreatment of frail patients who often have marked orthostatic hypotension is an additional concern.
How to get more accurate readings
It’s clear that taking the treatments that led to optimal outcomes in clinical trials and applying them to clinic patients based on their office measurements is likely to result in overtreatment, leading to hypotension and endangering patients. The following steps, however, can ensure more accurate readings and thus, a proper starting place for treatment.
Use an oscillatory device. I suggest that clinical practices switch to oscillatory digital devices like those used in virtually all clinical research studies I’ve been involved in for the past 20 years. There are oscillatory digital devices designed for medical offices that automatically record BP readings. However, these are much more expensive.2 The home oscillatory devices I’m referring to can be purchased for each exam room, with various sized cuffs.
Go slow, repeat as needed. Have the rooming staff or medical assistant measure BP only after the patient interview is complete. The patient should sit down, with both feet on the floor, legs uncrossed.
If the reading is elevated, the staffer should show the patient how to repeat the measurement, then prepare to leave the room, advising him or her to sit quietly for 3 to 5 minutes before doing so. This method is both practical and time efficient. Occasionally, oscillometric measures result in an extremely elevated diastolic reading; in such a case, I recommend that a clinician manually remeasure BP.
Incorporate home monitoring. Out-of-office readings are important, not only for the initial diagnosis of hypertension, but for clinical management of established hypertension, as well.3 Guidelines from both the US Preventive Services Task Force (USPSTF) and the National Institute for Health and Care Excellence (NICE) call for 24-hour ambulatory monitoring to establish a hypertension diagnosis.3,4 Accuracy is imperative, as this is commonly a lifelong diagnosis that should not be established based on a few, often inaccurately measured office readings.
Home monitoring improves BP control and correlates more closely with ambulatory monitoring than with office readings.5,6 I use an Excel spreadsheet (Microsoft, Bellevue, Wash.) to have patients send me their home BP readings, but commercially available software programs, if available, and smart phone apps may be used instead.
My preference is to have patients measure and record their BP at breakfast and dinnertime (always after a 3-to-5-minute rest) for a month after any change in the medication regimen (FIGURE), and then send the chart to the office. (There are other protocols for how often and how long to monitor home BP, but this is the format I use.) Adjustments in medications can be continued based on the home readings until the goal is reached.
I advise all patients I treat for hypertension to check their BP on the first day of each month and record the measurements for review at their next office visit.
What to consider for optimal treatment
Screening patients for concurrent disease and hypertensive end-organ damage, of course, should be routine for primary care physicians. Baseline tests should include a complete blood count, electrolytes with creatinine clearance, and an electrocardiogram. A review of a recent echocardiogram and spot urine for microalbuminuria will also be useful, if clinically indicated.
Cost, compliance, and concurrent disease. Generic drugs with a long half-life to ensure 24-hour coverage are the optimal choice due to both cost and compliance. Some agents may be chosen because they also treat concurrent disease—a beta-blocker for a patient with heart failure with reduced ejection fraction or migraines, an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) for diabetes, a diuretic for fluid overload, or spironolactone for systolic congestive heart failure.
Single agent or combination?
Most single drugs lower BP by approximately 10 mm Hg systolic and 5 mm Hg diastolic, with 2-drug combinations lowering pressure by 20 mm Hg and 10 mm Hg, respectively.7 Amlodipine, chlorthalidone, and azilsartan medoxomil, all of which have long half-lives, are approximately 50% more potent than other antihypertensive agents.
When the target BP is a reduction ≥20/10 mm Hg, starting with dual drug therapy is often useful. In such cases, it is prudent not only to be sure that BP has been accurately measured, but to begin with half-tablet doses for several days to allow the patient to acclimate to the change in pressure. Beta-blockers, central sympatholytic drugs, direct vasodilators, and alpha antagonists are not considered first-, second-, or third-line agents.
Spironolactone, an aldosterone receptor antagonist, is very useful in resistant hypertension,8 defined as inadequate BP control despite a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic. (For more information, see "Resistant hypertension? Time to consider this fourth-line drug.") Patients on spironolactone require electrolyte monitoring due to the risks of hyponatremia and hyperkalemia, especially in combination with an ACE inhibitor or ARB.
I advocate monitoring such patients after one month, although every 2 weeks for at least the first 6 weeks of treatment is prudent for patients with CKD. Mild hyperkalemia (<5.5 mEq/L) or hyponatremia (>130 mEq/L) is well tolerated, but conditions associated with sudden dehydration, such as diarrhea or vomiting, can rapidly worsen these imbalances and be clinically significant.
Treatment algorithms can help
SPRINT and other hypertension trials have used algorithm-based drug additions to reach the desired goals. In SPRINT, one or more antihypertensive drug classes with the strongest evidence to prevent cardiovascular disease outcomes were initiated and adjusted at the discretion of the investigators. The initial drug classes were thiazide-type diuretics (chlorthalidone was preferred unless advanced CKD was present, and then loop diuretics), calcium channel blockers (amlodipine preferred), ACE inhibitors (lisinopril was preferred), and ARBs (losartan or azilsartan medoxomil preferred).
The algorithms in this article may be considered for the treatment of hypertension. They are based on my experience, as well as on guidance from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.9
ALGORITHM 1 is suitable for patients who initially need only 10/5 mm Hg lowering.10ALGORITHM 2A may be used for patients for whom you wish to lower BP by ≥20/10 mm Hg. I also recommend 2A for patients of Asian descent; that’s because ARBs are preferable to ACE inhibitors, which are associated with a high incidence of cough in this patient population. Either ALGORITHM 2A or 2B may be used for African-American patients with hypertension, as ACE inhibitors and ARBs alone are less effective for this group.
CORRESPONDENCE
Steven Yarows, MD, FACP, FASH, IHA Chelsea Family and Internal Medicine, 128 Van Buren St, Chelsea, MI 48118; [email protected].
1. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Myers MG, Goodwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195-200.
3. Siu A. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. McCormack T, Krause T. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163-164.
5. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725–731.
6. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17:1017-1022.
7. Law MR, Wald NJ, Morris JK, et al. Value of low-dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326:1427.
8. Bloch MJ, Basile JN. Ambulatory blood pressure monitoring to diagnose hypertension—an idea whose time has come. J Am Soc Hypertens. 2016;10:89-91.
9. National Institutes of Health. JNC 7 Express. The Seventh Report of the Joint Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed February 26, 2016.
10. Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041-1046.
The Systolic Blood Pressure Intervention Trial (SPRINT),1 a study of more than 9000 patients published late last year, was stopped prematurely when it became clear that those receiving intensive treatment (systolic target 120 mm Hg) had significantly lower rates of myocardial infarction (MI), stroke, cardiovascular death, and other severe heart disease than those getting standard treatment (systolic target 140 mm Hg). Participants had an elevated cardiovascular risk at baseline (age ≥75 years, history of cardiovascular disease [CVD], chronic kidney disease [CKD], or elevated 10-year Framingham CVD risk score ≥15%); those with diabetes, history of stroke, or polycystic kidney disease were excluded.
Although serious adverse events were not significantly different between the intensive and standard treatment groups, syncope, acute renal failure, electrolyte abnormalities, and hyponatremia were all statistically more common in the aggressively treated group. (To learn more, see “Is lower BP worth it in higher-risk patients with diabetes or coronary disease?” Clinical Inquiries, J Fam Pract. 2016;65:129-131.)
Taking an aggressive approach. This trial shined a light on an important topic in medicine—the aggressive treatment of hypertension. And while this article will not discuss the finer points of the SPRINT trial or the limitations of generalizing aggressive treatment to the broad population of patients with hypertension, it will outline important considerations for physicians who wish to aggressively treat hypertension. I offer recommendations based on my 34 years of clinical practice and experience as a co-investigator on a number of hypertension studies to help you better balance each patient’s risks (eg, age, frailty, fall risk) and potential benefits (prevention of stroke, MI, and congestive heart failure).
Taking an aggressive approach, however, starts with ensuring that the diagnosis and treatment are based on accurate measures.
How accurate are your BP readings?
Measuring BP in clinical practice is markedly different from measurements taken in a research setting.2 This can result in large, clinically significant differences in readings and adversely affect treatment decisions.
Quiet time, multiple readings
SPRINT used techniques similar to those followed by other hypertension outcomes studies I’ve been involved in—methods that are rare in medical practice. Each study participant sat quietly in a chair for 5 minutes prior to the first BP reading. In addition, the researchers used an automatic oscillatory BP device (Omron Healthcare, Lake Forest, Ill), recording the average of 3 readings.
Practices that compromise accuracy. In clinical practice, BP is rarely measured after the patient has had 5 minutes of rest in a quiet room. Nor are readings done in triplicate. Instead, BP is typically measured while patient and clinician are engaged in conversation, often using a BP cuff that is too small (in my experience, most Americans require a large cuff).
BP is usually taken shortly after the patient has walked, frequently with some difficulty, from the waiting area to the exam room. Often, too, patients are weighed before their BP is measured, a common source of concern that can lead to a short-term rise in pressure. (Conversely, rapid deflation during the auscultatory measurement [>2 mm Hg/sec] can have the opposite effect, resulting in under-reading the true value.)
Compounding matters is the failure to consider the approximately 20% of patients who develop White Coat Syndrome. Such individuals, who typically have elevated office measurements but normal out-of-office readings, may develop further hypotensive symptoms if their treatment is based solely on in-office findings. Overtreatment of frail patients who often have marked orthostatic hypotension is an additional concern.
How to get more accurate readings
It’s clear that taking the treatments that led to optimal outcomes in clinical trials and applying them to clinic patients based on their office measurements is likely to result in overtreatment, leading to hypotension and endangering patients. The following steps, however, can ensure more accurate readings and thus, a proper starting place for treatment.
Use an oscillatory device. I suggest that clinical practices switch to oscillatory digital devices like those used in virtually all clinical research studies I’ve been involved in for the past 20 years. There are oscillatory digital devices designed for medical offices that automatically record BP readings. However, these are much more expensive.2 The home oscillatory devices I’m referring to can be purchased for each exam room, with various sized cuffs.
Go slow, repeat as needed. Have the rooming staff or medical assistant measure BP only after the patient interview is complete. The patient should sit down, with both feet on the floor, legs uncrossed.
If the reading is elevated, the staffer should show the patient how to repeat the measurement, then prepare to leave the room, advising him or her to sit quietly for 3 to 5 minutes before doing so. This method is both practical and time efficient. Occasionally, oscillometric measures result in an extremely elevated diastolic reading; in such a case, I recommend that a clinician manually remeasure BP.
Incorporate home monitoring. Out-of-office readings are important, not only for the initial diagnosis of hypertension, but for clinical management of established hypertension, as well.3 Guidelines from both the US Preventive Services Task Force (USPSTF) and the National Institute for Health and Care Excellence (NICE) call for 24-hour ambulatory monitoring to establish a hypertension diagnosis.3,4 Accuracy is imperative, as this is commonly a lifelong diagnosis that should not be established based on a few, often inaccurately measured office readings.
Home monitoring improves BP control and correlates more closely with ambulatory monitoring than with office readings.5,6 I use an Excel spreadsheet (Microsoft, Bellevue, Wash.) to have patients send me their home BP readings, but commercially available software programs, if available, and smart phone apps may be used instead.
My preference is to have patients measure and record their BP at breakfast and dinnertime (always after a 3-to-5-minute rest) for a month after any change in the medication regimen (FIGURE), and then send the chart to the office. (There are other protocols for how often and how long to monitor home BP, but this is the format I use.) Adjustments in medications can be continued based on the home readings until the goal is reached.
I advise all patients I treat for hypertension to check their BP on the first day of each month and record the measurements for review at their next office visit.
What to consider for optimal treatment
Screening patients for concurrent disease and hypertensive end-organ damage, of course, should be routine for primary care physicians. Baseline tests should include a complete blood count, electrolytes with creatinine clearance, and an electrocardiogram. A review of a recent echocardiogram and spot urine for microalbuminuria will also be useful, if clinically indicated.
Cost, compliance, and concurrent disease. Generic drugs with a long half-life to ensure 24-hour coverage are the optimal choice due to both cost and compliance. Some agents may be chosen because they also treat concurrent disease—a beta-blocker for a patient with heart failure with reduced ejection fraction or migraines, an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) for diabetes, a diuretic for fluid overload, or spironolactone for systolic congestive heart failure.
Single agent or combination?
Most single drugs lower BP by approximately 10 mm Hg systolic and 5 mm Hg diastolic, with 2-drug combinations lowering pressure by 20 mm Hg and 10 mm Hg, respectively.7 Amlodipine, chlorthalidone, and azilsartan medoxomil, all of which have long half-lives, are approximately 50% more potent than other antihypertensive agents.
When the target BP is a reduction ≥20/10 mm Hg, starting with dual drug therapy is often useful. In such cases, it is prudent not only to be sure that BP has been accurately measured, but to begin with half-tablet doses for several days to allow the patient to acclimate to the change in pressure. Beta-blockers, central sympatholytic drugs, direct vasodilators, and alpha antagonists are not considered first-, second-, or third-line agents.
Spironolactone, an aldosterone receptor antagonist, is very useful in resistant hypertension,8 defined as inadequate BP control despite a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic. (For more information, see "Resistant hypertension? Time to consider this fourth-line drug.") Patients on spironolactone require electrolyte monitoring due to the risks of hyponatremia and hyperkalemia, especially in combination with an ACE inhibitor or ARB.
I advocate monitoring such patients after one month, although every 2 weeks for at least the first 6 weeks of treatment is prudent for patients with CKD. Mild hyperkalemia (<5.5 mEq/L) or hyponatremia (>130 mEq/L) is well tolerated, but conditions associated with sudden dehydration, such as diarrhea or vomiting, can rapidly worsen these imbalances and be clinically significant.
Treatment algorithms can help
SPRINT and other hypertension trials have used algorithm-based drug additions to reach the desired goals. In SPRINT, one or more antihypertensive drug classes with the strongest evidence to prevent cardiovascular disease outcomes were initiated and adjusted at the discretion of the investigators. The initial drug classes were thiazide-type diuretics (chlorthalidone was preferred unless advanced CKD was present, and then loop diuretics), calcium channel blockers (amlodipine preferred), ACE inhibitors (lisinopril was preferred), and ARBs (losartan or azilsartan medoxomil preferred).
The algorithms in this article may be considered for the treatment of hypertension. They are based on my experience, as well as on guidance from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.9
ALGORITHM 1 is suitable for patients who initially need only 10/5 mm Hg lowering.10ALGORITHM 2A may be used for patients for whom you wish to lower BP by ≥20/10 mm Hg. I also recommend 2A for patients of Asian descent; that’s because ARBs are preferable to ACE inhibitors, which are associated with a high incidence of cough in this patient population. Either ALGORITHM 2A or 2B may be used for African-American patients with hypertension, as ACE inhibitors and ARBs alone are less effective for this group.
CORRESPONDENCE
Steven Yarows, MD, FACP, FASH, IHA Chelsea Family and Internal Medicine, 128 Van Buren St, Chelsea, MI 48118; [email protected].
The Systolic Blood Pressure Intervention Trial (SPRINT),1 a study of more than 9000 patients published late last year, was stopped prematurely when it became clear that those receiving intensive treatment (systolic target 120 mm Hg) had significantly lower rates of myocardial infarction (MI), stroke, cardiovascular death, and other severe heart disease than those getting standard treatment (systolic target 140 mm Hg). Participants had an elevated cardiovascular risk at baseline (age ≥75 years, history of cardiovascular disease [CVD], chronic kidney disease [CKD], or elevated 10-year Framingham CVD risk score ≥15%); those with diabetes, history of stroke, or polycystic kidney disease were excluded.
Although serious adverse events were not significantly different between the intensive and standard treatment groups, syncope, acute renal failure, electrolyte abnormalities, and hyponatremia were all statistically more common in the aggressively treated group. (To learn more, see “Is lower BP worth it in higher-risk patients with diabetes or coronary disease?” Clinical Inquiries, J Fam Pract. 2016;65:129-131.)
Taking an aggressive approach. This trial shined a light on an important topic in medicine—the aggressive treatment of hypertension. And while this article will not discuss the finer points of the SPRINT trial or the limitations of generalizing aggressive treatment to the broad population of patients with hypertension, it will outline important considerations for physicians who wish to aggressively treat hypertension. I offer recommendations based on my 34 years of clinical practice and experience as a co-investigator on a number of hypertension studies to help you better balance each patient’s risks (eg, age, frailty, fall risk) and potential benefits (prevention of stroke, MI, and congestive heart failure).
Taking an aggressive approach, however, starts with ensuring that the diagnosis and treatment are based on accurate measures.
How accurate are your BP readings?
Measuring BP in clinical practice is markedly different from measurements taken in a research setting.2 This can result in large, clinically significant differences in readings and adversely affect treatment decisions.
Quiet time, multiple readings
SPRINT used techniques similar to those followed by other hypertension outcomes studies I’ve been involved in—methods that are rare in medical practice. Each study participant sat quietly in a chair for 5 minutes prior to the first BP reading. In addition, the researchers used an automatic oscillatory BP device (Omron Healthcare, Lake Forest, Ill), recording the average of 3 readings.
Practices that compromise accuracy. In clinical practice, BP is rarely measured after the patient has had 5 minutes of rest in a quiet room. Nor are readings done in triplicate. Instead, BP is typically measured while patient and clinician are engaged in conversation, often using a BP cuff that is too small (in my experience, most Americans require a large cuff).
BP is usually taken shortly after the patient has walked, frequently with some difficulty, from the waiting area to the exam room. Often, too, patients are weighed before their BP is measured, a common source of concern that can lead to a short-term rise in pressure. (Conversely, rapid deflation during the auscultatory measurement [>2 mm Hg/sec] can have the opposite effect, resulting in under-reading the true value.)
Compounding matters is the failure to consider the approximately 20% of patients who develop White Coat Syndrome. Such individuals, who typically have elevated office measurements but normal out-of-office readings, may develop further hypotensive symptoms if their treatment is based solely on in-office findings. Overtreatment of frail patients who often have marked orthostatic hypotension is an additional concern.
How to get more accurate readings
It’s clear that taking the treatments that led to optimal outcomes in clinical trials and applying them to clinic patients based on their office measurements is likely to result in overtreatment, leading to hypotension and endangering patients. The following steps, however, can ensure more accurate readings and thus, a proper starting place for treatment.
Use an oscillatory device. I suggest that clinical practices switch to oscillatory digital devices like those used in virtually all clinical research studies I’ve been involved in for the past 20 years. There are oscillatory digital devices designed for medical offices that automatically record BP readings. However, these are much more expensive.2 The home oscillatory devices I’m referring to can be purchased for each exam room, with various sized cuffs.
Go slow, repeat as needed. Have the rooming staff or medical assistant measure BP only after the patient interview is complete. The patient should sit down, with both feet on the floor, legs uncrossed.
If the reading is elevated, the staffer should show the patient how to repeat the measurement, then prepare to leave the room, advising him or her to sit quietly for 3 to 5 minutes before doing so. This method is both practical and time efficient. Occasionally, oscillometric measures result in an extremely elevated diastolic reading; in such a case, I recommend that a clinician manually remeasure BP.
Incorporate home monitoring. Out-of-office readings are important, not only for the initial diagnosis of hypertension, but for clinical management of established hypertension, as well.3 Guidelines from both the US Preventive Services Task Force (USPSTF) and the National Institute for Health and Care Excellence (NICE) call for 24-hour ambulatory monitoring to establish a hypertension diagnosis.3,4 Accuracy is imperative, as this is commonly a lifelong diagnosis that should not be established based on a few, often inaccurately measured office readings.
Home monitoring improves BP control and correlates more closely with ambulatory monitoring than with office readings.5,6 I use an Excel spreadsheet (Microsoft, Bellevue, Wash.) to have patients send me their home BP readings, but commercially available software programs, if available, and smart phone apps may be used instead.
My preference is to have patients measure and record their BP at breakfast and dinnertime (always after a 3-to-5-minute rest) for a month after any change in the medication regimen (FIGURE), and then send the chart to the office. (There are other protocols for how often and how long to monitor home BP, but this is the format I use.) Adjustments in medications can be continued based on the home readings until the goal is reached.
I advise all patients I treat for hypertension to check their BP on the first day of each month and record the measurements for review at their next office visit.
What to consider for optimal treatment
Screening patients for concurrent disease and hypertensive end-organ damage, of course, should be routine for primary care physicians. Baseline tests should include a complete blood count, electrolytes with creatinine clearance, and an electrocardiogram. A review of a recent echocardiogram and spot urine for microalbuminuria will also be useful, if clinically indicated.
Cost, compliance, and concurrent disease. Generic drugs with a long half-life to ensure 24-hour coverage are the optimal choice due to both cost and compliance. Some agents may be chosen because they also treat concurrent disease—a beta-blocker for a patient with heart failure with reduced ejection fraction or migraines, an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) for diabetes, a diuretic for fluid overload, or spironolactone for systolic congestive heart failure.
Single agent or combination?
Most single drugs lower BP by approximately 10 mm Hg systolic and 5 mm Hg diastolic, with 2-drug combinations lowering pressure by 20 mm Hg and 10 mm Hg, respectively.7 Amlodipine, chlorthalidone, and azilsartan medoxomil, all of which have long half-lives, are approximately 50% more potent than other antihypertensive agents.
When the target BP is a reduction ≥20/10 mm Hg, starting with dual drug therapy is often useful. In such cases, it is prudent not only to be sure that BP has been accurately measured, but to begin with half-tablet doses for several days to allow the patient to acclimate to the change in pressure. Beta-blockers, central sympatholytic drugs, direct vasodilators, and alpha antagonists are not considered first-, second-, or third-line agents.
Spironolactone, an aldosterone receptor antagonist, is very useful in resistant hypertension,8 defined as inadequate BP control despite a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic. (For more information, see "Resistant hypertension? Time to consider this fourth-line drug.") Patients on spironolactone require electrolyte monitoring due to the risks of hyponatremia and hyperkalemia, especially in combination with an ACE inhibitor or ARB.
I advocate monitoring such patients after one month, although every 2 weeks for at least the first 6 weeks of treatment is prudent for patients with CKD. Mild hyperkalemia (<5.5 mEq/L) or hyponatremia (>130 mEq/L) is well tolerated, but conditions associated with sudden dehydration, such as diarrhea or vomiting, can rapidly worsen these imbalances and be clinically significant.
Treatment algorithms can help
SPRINT and other hypertension trials have used algorithm-based drug additions to reach the desired goals. In SPRINT, one or more antihypertensive drug classes with the strongest evidence to prevent cardiovascular disease outcomes were initiated and adjusted at the discretion of the investigators. The initial drug classes were thiazide-type diuretics (chlorthalidone was preferred unless advanced CKD was present, and then loop diuretics), calcium channel blockers (amlodipine preferred), ACE inhibitors (lisinopril was preferred), and ARBs (losartan or azilsartan medoxomil preferred).
The algorithms in this article may be considered for the treatment of hypertension. They are based on my experience, as well as on guidance from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.9
ALGORITHM 1 is suitable for patients who initially need only 10/5 mm Hg lowering.10ALGORITHM 2A may be used for patients for whom you wish to lower BP by ≥20/10 mm Hg. I also recommend 2A for patients of Asian descent; that’s because ARBs are preferable to ACE inhibitors, which are associated with a high incidence of cough in this patient population. Either ALGORITHM 2A or 2B may be used for African-American patients with hypertension, as ACE inhibitors and ARBs alone are less effective for this group.
CORRESPONDENCE
Steven Yarows, MD, FACP, FASH, IHA Chelsea Family and Internal Medicine, 128 Van Buren St, Chelsea, MI 48118; [email protected].
1. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Myers MG, Goodwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195-200.
3. Siu A. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. McCormack T, Krause T. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163-164.
5. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725–731.
6. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17:1017-1022.
7. Law MR, Wald NJ, Morris JK, et al. Value of low-dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326:1427.
8. Bloch MJ, Basile JN. Ambulatory blood pressure monitoring to diagnose hypertension—an idea whose time has come. J Am Soc Hypertens. 2016;10:89-91.
9. National Institutes of Health. JNC 7 Express. The Seventh Report of the Joint Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed February 26, 2016.
10. Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041-1046.
1. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Myers MG, Goodwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195-200.
3. Siu A. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. McCormack T, Krause T. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163-164.
5. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725–731.
6. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17:1017-1022.
7. Law MR, Wald NJ, Morris JK, et al. Value of low-dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326:1427.
8. Bloch MJ, Basile JN. Ambulatory blood pressure monitoring to diagnose hypertension—an idea whose time has come. J Am Soc Hypertens. 2016;10:89-91.
9. National Institutes of Health. JNC 7 Express. The Seventh Report of the Joint Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed February 26, 2016.
10. Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041-1046.