User login
Prolotherapy: Can it help your patient?
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
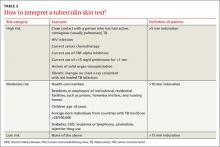
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
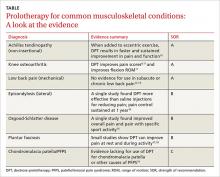
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
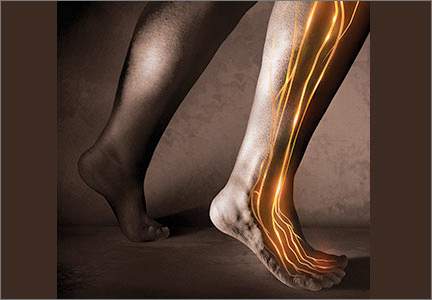
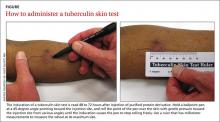
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
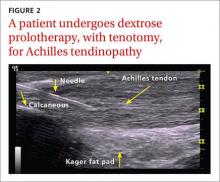
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
Causes of peripheral neuropathy: Diabetes and beyond
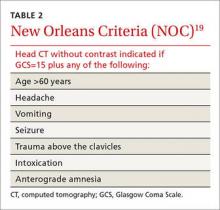
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
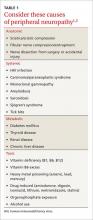
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
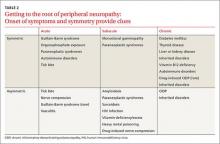
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; [email protected].
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; [email protected].
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; [email protected].
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
Home apnea monitors—when to discontinue use
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
“I feel dizzy, Doctor”
› Refer a patient who reports that his dizziness is accompanied by hearing loss to an otolaryngologist for evaluation. C
› Use the HINTS (Head Impulse, Nystagmus, and Test of Skew) procedure to differentiate central from peripheral vertigo. A
› Use the Dix-Hallpike procedure to diagnose benign paroxysmal positional vertigo. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With an estimated lifetime prevalence of 17% to 30%,1 dizziness is a relatively common clinical symptom, but the underlying cause can be difficult to diagnose. That’s because patients’ descriptions of dizziness are often imprecise, and this symptom is associated with a wide range of conditions. A careful history and physical examination are key to diagnosis, as is an understanding of the mechanisms of dizziness.
This article covers the range of diagnoses that should be considered when a patient presents with dizziness, and provides insight regarding features of the patient’s history that can better elucidate the specific etiology.
What do patients mean when they say, “I feel dizzy”?
“Dizziness” is a vague term, and patients who report dizziness should be asked to further describe the sensation. Patients may use the word dizziness in an attempt to describe many sensations, including faintness, giddiness, light-headedness, or unsteadiness.2 In 1972, Drachman and Hart proposed a classification system for dizziness that describes 4 categories—presyncope, vertigo, disequilibrium, and atypical (TABLE 1).3 These classifications are still commonly used today, and the discussion that follows describes potential causes of dizziness in each of these 4 categories. A stepwise approach for evaluating a patient who reports dizziness can be found in the ALGORITHM.3-6
Syncopal-related dizziness can have a cardiovascular cause
Presyncope is a feeling of impending loss of consciousness that’s sometimes accompanied by generalized muscle weakness and/or partial vision loss. Taking a careful history regarding the events surrounding the episode should distinguish this type of dizziness, and doing so is essential because most of the underlying pathogenesis involves the cardiovascular system and requires specific interventions.
Dysrhythmias can cause syncope and may or may not be accompanied by a feeling of palpitations. Diagnosis is made by electrocardiogram (EKG) followed by the use of a Holter monitor.
Vasovagal syncope is caused by a sudden slowing of the pulse that’s the result of stimulation of the vagal nerve. It can occur from direct stimulation of the nerve from palpation (or strangulation), or from an intense autonomic discharge, as when people are frightened or confronted with something upsetting (eg, the sight of blood.)
Orthostatic hypotension results from a change in body position in which either autonomic mechanisms cannot maintain venous tone, causing a sudden drop in blood pressure, or in which the heart cannot compensate by speeding up, as when a patient is taking a beta-adrenergic antagonist or has first-degree heart block. It can also result from hypovolemia.
Measuring the patient’s blood pressure in the recumbent, seated, and standing positions can verify the diagnosis if an episode occurred soon before the examination. This kind of dizziness can be treated by instructing the patient to rise slowly, or by making appropriate medication adjustments. If conservative measures fail, medications such as midodrine or droxidopa can be tried.7
Hypoglycemia, hypoxia, or hyperventilation can also precipitate syncopal symptoms. Taking a careful history to assess for the presence of seizure-related features such as tonic/clonic movements or loss of bowel and bladder control can be helpful in distinguishing this form of dizziness.
Vertigo can have a central or peripheral cause
Vertigo is dizziness that is characterized by the sensation of spinning. The presence of vertigo implies disease of the inner ear or central nervous system. The “wiring diagram” of the vestibulo-ocular reflex is fairly straightforward, but sorting out the symptoms that arise from lesions within the system can be a diagnostic challenge. Vertigo has classically been divided into causes that are central (originating in the central nervous system) or peripheral (originating in the peripheral nervous system).
The HINTS (Head Impulse, Nystagmus, and Test of Skew) protocol is a group of 3 tests that can be used to differentiate central from peripheral vertigo (TABLE 2).8,9 To perform the head impulse test, the examiner asks the patient to focus his gaze on a target and then rapidly turns the patient’s head to the side, watching the eyes for any corrective movements.10 When the eyes make a corrective saccade, the test is considered to be positive for a peripheral lesion.
Horizontal nystagmus is assessed by having the patient look in the direction of the fast phase of the nystagmus. If the nystagmus increases in intensity, then the test is considered positive for a peripheral lesion.
Vertigo can have many possible causes
Finally, the “test of skew” is performed by again having the patient fixate on the examiner’s nose. Each eye is tested by being covered, and then uncovered. If the uncovered eye has to move to refocus on the examiner’s nose, then the test is positive for a central lesion. A positive head impulse, positive horizontal nystagmus, and negative test of skew is 100% sensitive and 96% specific for a peripheral lesion.11
Benign paroxysmal positional vertigo (BPPV) is vertigo that is triggered by movement of the head. It occurs when otoconia that are normally embedded in gel in the utricle become dislodged and migrate into the 3 fluid-filled semicircular canals, where they interfere with the normal fluid movement these canals use to sense head motion, causing the inner ear to send false signals to the brain.12
Diagnosis is confirmed by performing the Dix-Hallpike maneuver to elicit nystagmus. The patient is moved from a seated to a supine position with her head turned 45 degrees to the right and held for 30 seconds. For a demonstration of the Dix-Hallpike maneuver, see https://youtu.be/8RYB2QlO1N4. The Dix-Hallpike maneuver is also the first step of a treatment for BBPV known as the Epley maneuver. (See “The Epley maneuver: A procedure for treating BPPV”.13,14)
Benign paroxysmal positional vertigo (BPPV) can be treated with the Epley maneuver. Like the Dix-Hallpike maneuver, the Epley maneuver isolates the posterior semicircular canal of the affected ear. However, it goes a step further to reposition otolithic debris away from the ampulla of the posterior canal, rolling it through the canal and depositing it in the utricle, where it will not stimulate nerve endings and produce symptoms.
For a demonstration of the Epley maneuver, see https://youtu.be/jBzID5nVQjk. A computer-controlled form of the Epley maneuver has been developed and can be as effective as the manual version of this procedure.13
In 38% of patients, BPPV spontaneously resolves. The Epley maneuver can improve this rate to 64% with a single treatment, and one additional maneuver improves the success rate to 83.3%.14 If this procedure doesn’t work the first time, there may be more sediment that didn’t have enough time to settle during the procedure. Therefore, the Epley maneuver can be repeated 3 times a day, and performed on subsequent days as needed.
Labyrinthitis—inflammation of the inner ear that can cause vertigo—is suggested by an acute, non-recurrent episode of dizziness that is often preceded by an upper respiratory infection. If the external canal is extremely painful and/or develops a vesicular rash, the patient might have herpes zoster of the geniculate ganglion (Ramsay Hunt syndrome type 2).
Vertigo can have many possible causes
Vestibular migraine and Meniere’s disease. When a patient who has a history of migraines experiences symptoms of vertigo, vestibular migraine should be suspected, and treatment should focus on migraine therapy rather than vestibular therapy.15
Symptoms of Meniere’s disease and vestibular migraine can overlap.16 The current definition of Meniere’s disease requires ≥2 definitive episodes of vertigo with hearing loss plus tinnitus and/or aural symptoms.17 Thirty percent of vertigo episodes in patients with Meniere's disease can be attributed to BPPV.18
Acoustic neuroma. In addition to vertigo, acoustic neuroma is often associated with gradual hearing loss, tinnitus, and facial numbness (from compression of cranial nerve V preoperatively) or facial weakness (from compression of cranial nerve VII postoperatively). Unilateral hearing loss should prompt evaluation with magnetic resonance imaging.
“Acoustic neuroma” is a misnomer. The lesion arises from the vestibular (not the acoustic) portion of the 8th cranial nerve, and isn’t a neuroma; it is a schwannoma.19 Although it actually arises peripherally within the vestibular canal, it typically expands centrally and compresses other nerves centrally, which can make the clinical diagnosis more challenging if one were using the classical schema of differentiating between peripheral and central causes of vertigo.
Age-related vestibular loss occurs when the aging process causes deterioration of most of the components of the vestibulo-ocular reflex, resulting in dizziness and vertigo. Usually, the cerebral override mechanisms can compensate for the degeneration.
Other causes of vertigo include cerebellar infarction (3% of patients with vertigo),20 sound-induced vertigo (Tullio phenomenon),21 obstructive sleep apnea,22 and systemic sclerosis.23 Diabetes can cause a reduction in vestibular sensitivity that is evidenced by an increased reliance on visual stimuli to resolve vestibulo-visual conflict.24
Disequilibrium
Disequilibrium is predominantly a loss of balance. Patients with disequilibrium have the feeling that they are about to fall, specifically without the sensation of spinning. They may appear to sway, and will reach out for something to support them. Disequilibrium can be a component of vertigo, or it may suggest a more specific diagnosis, such as ataxia, which is a lack of coordination when walking.
Atypical causes of dizziness
“Light-headedness” may have an element of euphoria or may be indistinguishable from the early part of a syncopal episode. Because other causes of light-headedness can be difficult to distinguish from presyncope, it is important to consider syncope in the differential diagnosis.
The differential of light-headedness can also include panic attack, early hyperventilation, and toxin exposure (such as diphenylarsinic acid,25 pregabalin,26 or paint thinner27).
CORRESPONDENCE
Shannon Paul Starr, MD, Louisiana State University Health Sciences Center, 200 W. Esplanade #412, Kenner, LA 70065; [email protected].
1. Murdin L, Schilder AG. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387-392.
2. Stedman TL. Stedman’s medical dictionary, illustrated. 24th ed. Baltimore, Md: William & Wilkins; 1982:419.
3. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323-334.
4. Angtuaco EJ, Wippold FJ II, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria: hearing loss and/or vertigo. 2013. American College of Radiology Web site. Available at: http://www.acr.org/~/media/914834f9cfa74e6c803e8e9c6909cd7e.pdf. Accessed September 3, 2015.
5. Dros J, Maarsingh OR, van der Windt DA, et al. Profiling dizziness in older primary care patients: an empirical study. PLoS One. 2011;6:e16481.
6. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-369.
7. Biaggioni I. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep. 2014;16:542.
8. Batuecas-Caletrío Á, Yáñez-González R, Sánchez-Blanco C, et al. [Peripheral vertigo versus central vertigo. Application of the HINTS protocol]. Rev Neurol. 2014;59:349-353.
9. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504-3510.
10. Barraclough K, Bronstein A. Vertigo. BMJ. 2009;339:b3493.
11. Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986-996.
12. Vestibular Disorders Association. Benign Paroxysmal Positional Vertigo. Vestibular Disorders Association Web site. Available at: http://vestibular.org/understanding-vestibular-disorders/types-vestibular-disorders/benign-paroxysmal-positional-vertigo. Accessed September 1, 2015.
13. Shan X, Peng X, Wang E. Efficacy of computer-controlled repositioning procedure for benign paroxysmal positional vertigo. Laryngoscope. 2015;125:715-719.
14. Lee JD, Shim DB, Park HJ, et al. A multicenter randomized double-blind study: comparison of the Epley, Semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336-341.
15. Stolte B, Holle D, Naegel S, et al. Vestibular migraine. Cephalalgia. 2015;35:262-270.
16. Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol. 2014;5:265.
17. Beasley NJ, Jones NS. Menière’s disease: evolution of a definition. J Laryngol Otol. 1996;110:1107-1113.
18. Taura A, Funabiki K, Ohgita H, et al. One-third of vertiginous episodes during the follow-up period are caused by benign paroxysmal positional vertigo in patients with Meniere’s disease. Acta Otolaryngol. 2014;134:1140-1145.
19. Pineda A, Feder BH. Acoustic neuroma: a misnomer. Is Surg. 1967;33:40-43.
20. Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32-39.
21. Harrison RV. On the biological plausibility of Wind Turbine Syndrome. Int J Environ Health Res. 2015;25:463-468.
22. Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-tosevere obstructive sleep apnea. Laryngoscope. 2015;125:1244-1248.
23. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26:337-342.
24. Razzak RA, Bagust J, Docherty S, et al. Augmented asymmetrical visual field dependence in asymptomatic diabetics: evidence of subclinical asymmetrical bilateral vestibular dysfunction. J Diabetes Complications. 2015;29:68-72.
25. Ogata T, Nakamura Y, Endo G, et al. [Subjective symptoms and miscarriage after drinking well water exposed to diphenylarsinic acid]. Nihon Koshu Eisei Zasshi. 2014;61:556-564.
26. Qu C, Xie Y, Qin F, et al. Neuropsychiatric symptoms accompanying thrombocytopenia following pregabalin treatment for neuralgia: a case report. Int J Clin Pharm. 2014;36:1138-1140.
27. Rahimi HR, Agin K, Shadnia S, et al. Clinical and biochemical analysis of acute paint thinner intoxication in adults: a retrospective descriptive study. Toxicol Mech Methods. 2015;25:42-47.
› Refer a patient who reports that his dizziness is accompanied by hearing loss to an otolaryngologist for evaluation. C
› Use the HINTS (Head Impulse, Nystagmus, and Test of Skew) procedure to differentiate central from peripheral vertigo. A
› Use the Dix-Hallpike procedure to diagnose benign paroxysmal positional vertigo. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With an estimated lifetime prevalence of 17% to 30%,1 dizziness is a relatively common clinical symptom, but the underlying cause can be difficult to diagnose. That’s because patients’ descriptions of dizziness are often imprecise, and this symptom is associated with a wide range of conditions. A careful history and physical examination are key to diagnosis, as is an understanding of the mechanisms of dizziness.
This article covers the range of diagnoses that should be considered when a patient presents with dizziness, and provides insight regarding features of the patient’s history that can better elucidate the specific etiology.
What do patients mean when they say, “I feel dizzy”?
“Dizziness” is a vague term, and patients who report dizziness should be asked to further describe the sensation. Patients may use the word dizziness in an attempt to describe many sensations, including faintness, giddiness, light-headedness, or unsteadiness.2 In 1972, Drachman and Hart proposed a classification system for dizziness that describes 4 categories—presyncope, vertigo, disequilibrium, and atypical (TABLE 1).3 These classifications are still commonly used today, and the discussion that follows describes potential causes of dizziness in each of these 4 categories. A stepwise approach for evaluating a patient who reports dizziness can be found in the ALGORITHM.3-6
Syncopal-related dizziness can have a cardiovascular cause
Presyncope is a feeling of impending loss of consciousness that’s sometimes accompanied by generalized muscle weakness and/or partial vision loss. Taking a careful history regarding the events surrounding the episode should distinguish this type of dizziness, and doing so is essential because most of the underlying pathogenesis involves the cardiovascular system and requires specific interventions.
Dysrhythmias can cause syncope and may or may not be accompanied by a feeling of palpitations. Diagnosis is made by electrocardiogram (EKG) followed by the use of a Holter monitor.
Vasovagal syncope is caused by a sudden slowing of the pulse that’s the result of stimulation of the vagal nerve. It can occur from direct stimulation of the nerve from palpation (or strangulation), or from an intense autonomic discharge, as when people are frightened or confronted with something upsetting (eg, the sight of blood.)
Orthostatic hypotension results from a change in body position in which either autonomic mechanisms cannot maintain venous tone, causing a sudden drop in blood pressure, or in which the heart cannot compensate by speeding up, as when a patient is taking a beta-adrenergic antagonist or has first-degree heart block. It can also result from hypovolemia.
Measuring the patient’s blood pressure in the recumbent, seated, and standing positions can verify the diagnosis if an episode occurred soon before the examination. This kind of dizziness can be treated by instructing the patient to rise slowly, or by making appropriate medication adjustments. If conservative measures fail, medications such as midodrine or droxidopa can be tried.7
Hypoglycemia, hypoxia, or hyperventilation can also precipitate syncopal symptoms. Taking a careful history to assess for the presence of seizure-related features such as tonic/clonic movements or loss of bowel and bladder control can be helpful in distinguishing this form of dizziness.
Vertigo can have a central or peripheral cause
Vertigo is dizziness that is characterized by the sensation of spinning. The presence of vertigo implies disease of the inner ear or central nervous system. The “wiring diagram” of the vestibulo-ocular reflex is fairly straightforward, but sorting out the symptoms that arise from lesions within the system can be a diagnostic challenge. Vertigo has classically been divided into causes that are central (originating in the central nervous system) or peripheral (originating in the peripheral nervous system).
The HINTS (Head Impulse, Nystagmus, and Test of Skew) protocol is a group of 3 tests that can be used to differentiate central from peripheral vertigo (TABLE 2).8,9 To perform the head impulse test, the examiner asks the patient to focus his gaze on a target and then rapidly turns the patient’s head to the side, watching the eyes for any corrective movements.10 When the eyes make a corrective saccade, the test is considered to be positive for a peripheral lesion.
Horizontal nystagmus is assessed by having the patient look in the direction of the fast phase of the nystagmus. If the nystagmus increases in intensity, then the test is considered positive for a peripheral lesion.
Vertigo can have many possible causes
Finally, the “test of skew” is performed by again having the patient fixate on the examiner’s nose. Each eye is tested by being covered, and then uncovered. If the uncovered eye has to move to refocus on the examiner’s nose, then the test is positive for a central lesion. A positive head impulse, positive horizontal nystagmus, and negative test of skew is 100% sensitive and 96% specific for a peripheral lesion.11
Benign paroxysmal positional vertigo (BPPV) is vertigo that is triggered by movement of the head. It occurs when otoconia that are normally embedded in gel in the utricle become dislodged and migrate into the 3 fluid-filled semicircular canals, where they interfere with the normal fluid movement these canals use to sense head motion, causing the inner ear to send false signals to the brain.12
Diagnosis is confirmed by performing the Dix-Hallpike maneuver to elicit nystagmus. The patient is moved from a seated to a supine position with her head turned 45 degrees to the right and held for 30 seconds. For a demonstration of the Dix-Hallpike maneuver, see https://youtu.be/8RYB2QlO1N4. The Dix-Hallpike maneuver is also the first step of a treatment for BBPV known as the Epley maneuver. (See “The Epley maneuver: A procedure for treating BPPV”.13,14)
Benign paroxysmal positional vertigo (BPPV) can be treated with the Epley maneuver. Like the Dix-Hallpike maneuver, the Epley maneuver isolates the posterior semicircular canal of the affected ear. However, it goes a step further to reposition otolithic debris away from the ampulla of the posterior canal, rolling it through the canal and depositing it in the utricle, where it will not stimulate nerve endings and produce symptoms.
For a demonstration of the Epley maneuver, see https://youtu.be/jBzID5nVQjk. A computer-controlled form of the Epley maneuver has been developed and can be as effective as the manual version of this procedure.13
In 38% of patients, BPPV spontaneously resolves. The Epley maneuver can improve this rate to 64% with a single treatment, and one additional maneuver improves the success rate to 83.3%.14 If this procedure doesn’t work the first time, there may be more sediment that didn’t have enough time to settle during the procedure. Therefore, the Epley maneuver can be repeated 3 times a day, and performed on subsequent days as needed.
Labyrinthitis—inflammation of the inner ear that can cause vertigo—is suggested by an acute, non-recurrent episode of dizziness that is often preceded by an upper respiratory infection. If the external canal is extremely painful and/or develops a vesicular rash, the patient might have herpes zoster of the geniculate ganglion (Ramsay Hunt syndrome type 2).
Vertigo can have many possible causes
Vestibular migraine and Meniere’s disease. When a patient who has a history of migraines experiences symptoms of vertigo, vestibular migraine should be suspected, and treatment should focus on migraine therapy rather than vestibular therapy.15
Symptoms of Meniere’s disease and vestibular migraine can overlap.16 The current definition of Meniere’s disease requires ≥2 definitive episodes of vertigo with hearing loss plus tinnitus and/or aural symptoms.17 Thirty percent of vertigo episodes in patients with Meniere's disease can be attributed to BPPV.18
Acoustic neuroma. In addition to vertigo, acoustic neuroma is often associated with gradual hearing loss, tinnitus, and facial numbness (from compression of cranial nerve V preoperatively) or facial weakness (from compression of cranial nerve VII postoperatively). Unilateral hearing loss should prompt evaluation with magnetic resonance imaging.
“Acoustic neuroma” is a misnomer. The lesion arises from the vestibular (not the acoustic) portion of the 8th cranial nerve, and isn’t a neuroma; it is a schwannoma.19 Although it actually arises peripherally within the vestibular canal, it typically expands centrally and compresses other nerves centrally, which can make the clinical diagnosis more challenging if one were using the classical schema of differentiating between peripheral and central causes of vertigo.
Age-related vestibular loss occurs when the aging process causes deterioration of most of the components of the vestibulo-ocular reflex, resulting in dizziness and vertigo. Usually, the cerebral override mechanisms can compensate for the degeneration.
Other causes of vertigo include cerebellar infarction (3% of patients with vertigo),20 sound-induced vertigo (Tullio phenomenon),21 obstructive sleep apnea,22 and systemic sclerosis.23 Diabetes can cause a reduction in vestibular sensitivity that is evidenced by an increased reliance on visual stimuli to resolve vestibulo-visual conflict.24
Disequilibrium
Disequilibrium is predominantly a loss of balance. Patients with disequilibrium have the feeling that they are about to fall, specifically without the sensation of spinning. They may appear to sway, and will reach out for something to support them. Disequilibrium can be a component of vertigo, or it may suggest a more specific diagnosis, such as ataxia, which is a lack of coordination when walking.
Atypical causes of dizziness
“Light-headedness” may have an element of euphoria or may be indistinguishable from the early part of a syncopal episode. Because other causes of light-headedness can be difficult to distinguish from presyncope, it is important to consider syncope in the differential diagnosis.
The differential of light-headedness can also include panic attack, early hyperventilation, and toxin exposure (such as diphenylarsinic acid,25 pregabalin,26 or paint thinner27).
CORRESPONDENCE
Shannon Paul Starr, MD, Louisiana State University Health Sciences Center, 200 W. Esplanade #412, Kenner, LA 70065; [email protected].
› Refer a patient who reports that his dizziness is accompanied by hearing loss to an otolaryngologist for evaluation. C
› Use the HINTS (Head Impulse, Nystagmus, and Test of Skew) procedure to differentiate central from peripheral vertigo. A
› Use the Dix-Hallpike procedure to diagnose benign paroxysmal positional vertigo. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With an estimated lifetime prevalence of 17% to 30%,1 dizziness is a relatively common clinical symptom, but the underlying cause can be difficult to diagnose. That’s because patients’ descriptions of dizziness are often imprecise, and this symptom is associated with a wide range of conditions. A careful history and physical examination are key to diagnosis, as is an understanding of the mechanisms of dizziness.
This article covers the range of diagnoses that should be considered when a patient presents with dizziness, and provides insight regarding features of the patient’s history that can better elucidate the specific etiology.
What do patients mean when they say, “I feel dizzy”?
“Dizziness” is a vague term, and patients who report dizziness should be asked to further describe the sensation. Patients may use the word dizziness in an attempt to describe many sensations, including faintness, giddiness, light-headedness, or unsteadiness.2 In 1972, Drachman and Hart proposed a classification system for dizziness that describes 4 categories—presyncope, vertigo, disequilibrium, and atypical (TABLE 1).3 These classifications are still commonly used today, and the discussion that follows describes potential causes of dizziness in each of these 4 categories. A stepwise approach for evaluating a patient who reports dizziness can be found in the ALGORITHM.3-6
Syncopal-related dizziness can have a cardiovascular cause
Presyncope is a feeling of impending loss of consciousness that’s sometimes accompanied by generalized muscle weakness and/or partial vision loss. Taking a careful history regarding the events surrounding the episode should distinguish this type of dizziness, and doing so is essential because most of the underlying pathogenesis involves the cardiovascular system and requires specific interventions.
Dysrhythmias can cause syncope and may or may not be accompanied by a feeling of palpitations. Diagnosis is made by electrocardiogram (EKG) followed by the use of a Holter monitor.
Vasovagal syncope is caused by a sudden slowing of the pulse that’s the result of stimulation of the vagal nerve. It can occur from direct stimulation of the nerve from palpation (or strangulation), or from an intense autonomic discharge, as when people are frightened or confronted with something upsetting (eg, the sight of blood.)
Orthostatic hypotension results from a change in body position in which either autonomic mechanisms cannot maintain venous tone, causing a sudden drop in blood pressure, or in which the heart cannot compensate by speeding up, as when a patient is taking a beta-adrenergic antagonist or has first-degree heart block. It can also result from hypovolemia.
Measuring the patient’s blood pressure in the recumbent, seated, and standing positions can verify the diagnosis if an episode occurred soon before the examination. This kind of dizziness can be treated by instructing the patient to rise slowly, or by making appropriate medication adjustments. If conservative measures fail, medications such as midodrine or droxidopa can be tried.7
Hypoglycemia, hypoxia, or hyperventilation can also precipitate syncopal symptoms. Taking a careful history to assess for the presence of seizure-related features such as tonic/clonic movements or loss of bowel and bladder control can be helpful in distinguishing this form of dizziness.
Vertigo can have a central or peripheral cause
Vertigo is dizziness that is characterized by the sensation of spinning. The presence of vertigo implies disease of the inner ear or central nervous system. The “wiring diagram” of the vestibulo-ocular reflex is fairly straightforward, but sorting out the symptoms that arise from lesions within the system can be a diagnostic challenge. Vertigo has classically been divided into causes that are central (originating in the central nervous system) or peripheral (originating in the peripheral nervous system).
The HINTS (Head Impulse, Nystagmus, and Test of Skew) protocol is a group of 3 tests that can be used to differentiate central from peripheral vertigo (TABLE 2).8,9 To perform the head impulse test, the examiner asks the patient to focus his gaze on a target and then rapidly turns the patient’s head to the side, watching the eyes for any corrective movements.10 When the eyes make a corrective saccade, the test is considered to be positive for a peripheral lesion.
Horizontal nystagmus is assessed by having the patient look in the direction of the fast phase of the nystagmus. If the nystagmus increases in intensity, then the test is considered positive for a peripheral lesion.
Vertigo can have many possible causes
Finally, the “test of skew” is performed by again having the patient fixate on the examiner’s nose. Each eye is tested by being covered, and then uncovered. If the uncovered eye has to move to refocus on the examiner’s nose, then the test is positive for a central lesion. A positive head impulse, positive horizontal nystagmus, and negative test of skew is 100% sensitive and 96% specific for a peripheral lesion.11
Benign paroxysmal positional vertigo (BPPV) is vertigo that is triggered by movement of the head. It occurs when otoconia that are normally embedded in gel in the utricle become dislodged and migrate into the 3 fluid-filled semicircular canals, where they interfere with the normal fluid movement these canals use to sense head motion, causing the inner ear to send false signals to the brain.12
Diagnosis is confirmed by performing the Dix-Hallpike maneuver to elicit nystagmus. The patient is moved from a seated to a supine position with her head turned 45 degrees to the right and held for 30 seconds. For a demonstration of the Dix-Hallpike maneuver, see https://youtu.be/8RYB2QlO1N4. The Dix-Hallpike maneuver is also the first step of a treatment for BBPV known as the Epley maneuver. (See “The Epley maneuver: A procedure for treating BPPV”.13,14)
Benign paroxysmal positional vertigo (BPPV) can be treated with the Epley maneuver. Like the Dix-Hallpike maneuver, the Epley maneuver isolates the posterior semicircular canal of the affected ear. However, it goes a step further to reposition otolithic debris away from the ampulla of the posterior canal, rolling it through the canal and depositing it in the utricle, where it will not stimulate nerve endings and produce symptoms.
For a demonstration of the Epley maneuver, see https://youtu.be/jBzID5nVQjk. A computer-controlled form of the Epley maneuver has been developed and can be as effective as the manual version of this procedure.13
In 38% of patients, BPPV spontaneously resolves. The Epley maneuver can improve this rate to 64% with a single treatment, and one additional maneuver improves the success rate to 83.3%.14 If this procedure doesn’t work the first time, there may be more sediment that didn’t have enough time to settle during the procedure. Therefore, the Epley maneuver can be repeated 3 times a day, and performed on subsequent days as needed.
Labyrinthitis—inflammation of the inner ear that can cause vertigo—is suggested by an acute, non-recurrent episode of dizziness that is often preceded by an upper respiratory infection. If the external canal is extremely painful and/or develops a vesicular rash, the patient might have herpes zoster of the geniculate ganglion (Ramsay Hunt syndrome type 2).
Vertigo can have many possible causes
Vestibular migraine and Meniere’s disease. When a patient who has a history of migraines experiences symptoms of vertigo, vestibular migraine should be suspected, and treatment should focus on migraine therapy rather than vestibular therapy.15
Symptoms of Meniere’s disease and vestibular migraine can overlap.16 The current definition of Meniere’s disease requires ≥2 definitive episodes of vertigo with hearing loss plus tinnitus and/or aural symptoms.17 Thirty percent of vertigo episodes in patients with Meniere's disease can be attributed to BPPV.18
Acoustic neuroma. In addition to vertigo, acoustic neuroma is often associated with gradual hearing loss, tinnitus, and facial numbness (from compression of cranial nerve V preoperatively) or facial weakness (from compression of cranial nerve VII postoperatively). Unilateral hearing loss should prompt evaluation with magnetic resonance imaging.
“Acoustic neuroma” is a misnomer. The lesion arises from the vestibular (not the acoustic) portion of the 8th cranial nerve, and isn’t a neuroma; it is a schwannoma.19 Although it actually arises peripherally within the vestibular canal, it typically expands centrally and compresses other nerves centrally, which can make the clinical diagnosis more challenging if one were using the classical schema of differentiating between peripheral and central causes of vertigo.
Age-related vestibular loss occurs when the aging process causes deterioration of most of the components of the vestibulo-ocular reflex, resulting in dizziness and vertigo. Usually, the cerebral override mechanisms can compensate for the degeneration.
Other causes of vertigo include cerebellar infarction (3% of patients with vertigo),20 sound-induced vertigo (Tullio phenomenon),21 obstructive sleep apnea,22 and systemic sclerosis.23 Diabetes can cause a reduction in vestibular sensitivity that is evidenced by an increased reliance on visual stimuli to resolve vestibulo-visual conflict.24
Disequilibrium
Disequilibrium is predominantly a loss of balance. Patients with disequilibrium have the feeling that they are about to fall, specifically without the sensation of spinning. They may appear to sway, and will reach out for something to support them. Disequilibrium can be a component of vertigo, or it may suggest a more specific diagnosis, such as ataxia, which is a lack of coordination when walking.
Atypical causes of dizziness
“Light-headedness” may have an element of euphoria or may be indistinguishable from the early part of a syncopal episode. Because other causes of light-headedness can be difficult to distinguish from presyncope, it is important to consider syncope in the differential diagnosis.
The differential of light-headedness can also include panic attack, early hyperventilation, and toxin exposure (such as diphenylarsinic acid,25 pregabalin,26 or paint thinner27).
CORRESPONDENCE
Shannon Paul Starr, MD, Louisiana State University Health Sciences Center, 200 W. Esplanade #412, Kenner, LA 70065; [email protected].
1. Murdin L, Schilder AG. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387-392.
2. Stedman TL. Stedman’s medical dictionary, illustrated. 24th ed. Baltimore, Md: William & Wilkins; 1982:419.
3. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323-334.
4. Angtuaco EJ, Wippold FJ II, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria: hearing loss and/or vertigo. 2013. American College of Radiology Web site. Available at: http://www.acr.org/~/media/914834f9cfa74e6c803e8e9c6909cd7e.pdf. Accessed September 3, 2015.
5. Dros J, Maarsingh OR, van der Windt DA, et al. Profiling dizziness in older primary care patients: an empirical study. PLoS One. 2011;6:e16481.
6. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-369.
7. Biaggioni I. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep. 2014;16:542.
8. Batuecas-Caletrío Á, Yáñez-González R, Sánchez-Blanco C, et al. [Peripheral vertigo versus central vertigo. Application of the HINTS protocol]. Rev Neurol. 2014;59:349-353.
9. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504-3510.
10. Barraclough K, Bronstein A. Vertigo. BMJ. 2009;339:b3493.
11. Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986-996.
12. Vestibular Disorders Association. Benign Paroxysmal Positional Vertigo. Vestibular Disorders Association Web site. Available at: http://vestibular.org/understanding-vestibular-disorders/types-vestibular-disorders/benign-paroxysmal-positional-vertigo. Accessed September 1, 2015.
13. Shan X, Peng X, Wang E. Efficacy of computer-controlled repositioning procedure for benign paroxysmal positional vertigo. Laryngoscope. 2015;125:715-719.
14. Lee JD, Shim DB, Park HJ, et al. A multicenter randomized double-blind study: comparison of the Epley, Semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336-341.
15. Stolte B, Holle D, Naegel S, et al. Vestibular migraine. Cephalalgia. 2015;35:262-270.
16. Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol. 2014;5:265.
17. Beasley NJ, Jones NS. Menière’s disease: evolution of a definition. J Laryngol Otol. 1996;110:1107-1113.
18. Taura A, Funabiki K, Ohgita H, et al. One-third of vertiginous episodes during the follow-up period are caused by benign paroxysmal positional vertigo in patients with Meniere’s disease. Acta Otolaryngol. 2014;134:1140-1145.
19. Pineda A, Feder BH. Acoustic neuroma: a misnomer. Is Surg. 1967;33:40-43.
20. Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32-39.
21. Harrison RV. On the biological plausibility of Wind Turbine Syndrome. Int J Environ Health Res. 2015;25:463-468.
22. Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-tosevere obstructive sleep apnea. Laryngoscope. 2015;125:1244-1248.
23. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26:337-342.
24. Razzak RA, Bagust J, Docherty S, et al. Augmented asymmetrical visual field dependence in asymptomatic diabetics: evidence of subclinical asymmetrical bilateral vestibular dysfunction. J Diabetes Complications. 2015;29:68-72.
25. Ogata T, Nakamura Y, Endo G, et al. [Subjective symptoms and miscarriage after drinking well water exposed to diphenylarsinic acid]. Nihon Koshu Eisei Zasshi. 2014;61:556-564.
26. Qu C, Xie Y, Qin F, et al. Neuropsychiatric symptoms accompanying thrombocytopenia following pregabalin treatment for neuralgia: a case report. Int J Clin Pharm. 2014;36:1138-1140.
27. Rahimi HR, Agin K, Shadnia S, et al. Clinical and biochemical analysis of acute paint thinner intoxication in adults: a retrospective descriptive study. Toxicol Mech Methods. 2015;25:42-47.
1. Murdin L, Schilder AG. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387-392.
2. Stedman TL. Stedman’s medical dictionary, illustrated. 24th ed. Baltimore, Md: William & Wilkins; 1982:419.
3. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972;22:323-334.
4. Angtuaco EJ, Wippold FJ II, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria: hearing loss and/or vertigo. 2013. American College of Radiology Web site. Available at: http://www.acr.org/~/media/914834f9cfa74e6c803e8e9c6909cd7e.pdf. Accessed September 3, 2015.
5. Dros J, Maarsingh OR, van der Windt DA, et al. Profiling dizziness in older primary care patients: an empirical study. PLoS One. 2011;6:e16481.
6. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-369.
7. Biaggioni I. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep. 2014;16:542.
8. Batuecas-Caletrío Á, Yáñez-González R, Sánchez-Blanco C, et al. [Peripheral vertigo versus central vertigo. Application of the HINTS protocol]. Rev Neurol. 2014;59:349-353.
9. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504-3510.
10. Barraclough K, Bronstein A. Vertigo. BMJ. 2009;339:b3493.
11. Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. 2013;20:986-996.
12. Vestibular Disorders Association. Benign Paroxysmal Positional Vertigo. Vestibular Disorders Association Web site. Available at: http://vestibular.org/understanding-vestibular-disorders/types-vestibular-disorders/benign-paroxysmal-positional-vertigo. Accessed September 1, 2015.
13. Shan X, Peng X, Wang E. Efficacy of computer-controlled repositioning procedure for benign paroxysmal positional vertigo. Laryngoscope. 2015;125:715-719.
14. Lee JD, Shim DB, Park HJ, et al. A multicenter randomized double-blind study: comparison of the Epley, Semont, and sham maneuvers for the treatment of posterior canal benign paroxysmal positional vertigo. Audiol Neurootol. 2014;19:336-341.
15. Stolte B, Holle D, Naegel S, et al. Vestibular migraine. Cephalalgia. 2015;35:262-270.
16. Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol. 2014;5:265.
17. Beasley NJ, Jones NS. Menière’s disease: evolution of a definition. J Laryngol Otol. 1996;110:1107-1113.
18. Taura A, Funabiki K, Ohgita H, et al. One-third of vertiginous episodes during the follow-up period are caused by benign paroxysmal positional vertigo in patients with Meniere’s disease. Acta Otolaryngol. 2014;134:1140-1145.
19. Pineda A, Feder BH. Acoustic neuroma: a misnomer. Is Surg. 1967;33:40-43.
20. Seemungal BM. Neuro-otological emergencies. Curr Opin Neurol. 2007;20:32-39.
21. Harrison RV. On the biological plausibility of Wind Turbine Syndrome. Int J Environ Health Res. 2015;25:463-468.
22. Kayabasi S, Iriz A, Cayonu M, et al. Vestibular functions were found to be impaired in patients with moderate-tosevere obstructive sleep apnea. Laryngoscope. 2015;125:1244-1248.
23. Rabelo MB, Corona AP. Auditory and vestibular dysfunctions in systemic sclerosis: literature review. Codas. 2014;26:337-342.
24. Razzak RA, Bagust J, Docherty S, et al. Augmented asymmetrical visual field dependence in asymptomatic diabetics: evidence of subclinical asymmetrical bilateral vestibular dysfunction. J Diabetes Complications. 2015;29:68-72.
25. Ogata T, Nakamura Y, Endo G, et al. [Subjective symptoms and miscarriage after drinking well water exposed to diphenylarsinic acid]. Nihon Koshu Eisei Zasshi. 2014;61:556-564.
26. Qu C, Xie Y, Qin F, et al. Neuropsychiatric symptoms accompanying thrombocytopenia following pregabalin treatment for neuralgia: a case report. Int J Clin Pharm. 2014;36:1138-1140.
27. Rahimi HR, Agin K, Shadnia S, et al. Clinical and biochemical analysis of acute paint thinner intoxication in adults: a retrospective descriptive study. Toxicol Mech Methods. 2015;25:42-47.
Test your skills: Which imaging studies would you order for these neurologic complaints?
› Use clinical decision tools and American College of Radiology Appropriateness Criteria to determine levels of evidence for ordering specific imaging studies. A
› Consider ordering an imaging study when headache is accompanied by a new neurologic symptom. Computed tomography (CT) without contrast is best for patients who are acutely ill. In the non-emergent setting, magnetic resonance imaging (MRI) with contrast is the optimal study. A
› Order structural neuroimaging as part of the initial work-up of suspected dementia. MRI is the test of choice. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When patients present with neurologic complaints in outpatient primary care practice, 2 key questions often arise: Should brain imaging be ordered, and if so, which study? Careful history-taking and physical exam findings can suggest differential diagnoses and help determine whether imaging studies could identify potential underlying causes. Further considerations in making a decision are the type of information each modality offers, the possible need for contrast media, benefits vs radiation exposure risks, potential contraindications, and cost and local availability. In this article, we present 3 common outpatient scenarios, and in each case we describe the evidence to support clinical decision-making about imaging.
The American College of Radiology (ACR) Appropriateness Criteria Web site (http://www.acr.org/Quality-Safety/Appropriateness-Criteria) provides radiation exposure information, numerical ratings of imaging studies for individual clinical scenarios, evidence tables, and reference tables for each of its recommendations.1 ACR’s recommendations were developed by expert panels of diagnostic radiologists, interventional radiologists, and radiation oncologists, and designed to help physicians order the most appropriate imaging studies based on patients’ clinical conditions.2 We used these criteria to develop an evaluation strategy for each of our clinical scenarios.
CASE 1 › Carrie D is a 45-year-old woman with a history of migraine without aura generally controlled with Excedrin (acetaminophen, aspirin, and caffeine). She arrives at your office with a 2-day history of severe headache over the top of her head and associated tingling sensation over the left side of her face, but with no vision changes, weakness, or slurred speech. She denies any prior history of numbness or tinging with past headaches. She is a business executive and reports that in the last few weeks, her company has been involved in a high-profile merger. On physical exam, her vital signs are within normal limits. She does not appear acutely ill, but on exam she reports diminished sensation to light touch over the left side of her face, left arm, and left leg compared with the right side.
›› What imaging options might you consider?
A prospective review of physicians in an ambulatory family practice setting found that neurologic imaging was typically ordered for patients with headache who were suspected of having a brain tumor or subarachnoid hemorrhage.3 For our patient, who has a history of migraines without aura and whose current severe headache is accompanied by an abnormal sensation on one side of the face, the following questions are relevant: Is this presentation part of her primary headache syndrome or could there be a different cause? If there is a different cause, is it likely to be detected with brain imaging such as computed tomography (CT) or magnetic resonance imaging (MRI)?
Patients with isolated headache and an absence of neurologic symptoms or abnormalities on neurologic exam are unlikely to have a clinically significant intracranial abnormality.4-10 Imaging of the brain is typically not indicated for these patients.2 However, given that this patient does have a positive focal neurologic finding, a CT or MRI is indicated, as findings are more likely to influence management decisions.
The decision to order CT or MRI should be based on how acutely ill the patient is. CT without contrast is an excellent tool to rule out suspected emergent intracranial abnormalities such as an intracranial hemorrhage, hydrocephalus, or a mass.11 In patients presenting with symptoms suggesting acute illness such as carotid or vertebral artery dissection, the most appropriate test would be CT angiography of the head and neck.2
However, the list of less dire causes of headache is vast. Included in this list would be trauma, other vascular disorders such as arteriovenous malformation or temporal arteritis, infection, abnormal intracranial pressure (mass, pseudotumor cerebri, intracranial hypotension), and disorders of the head/face/spine (eg, temporomandibular joint disorder).12
In the non-acute setting where a patient has stable vital signs and is not in acute distress, an MRI with contrast would be the most appropriate test to identify such causes. Avoid contrast only if there is a firm contraindication, such as pregnancy, severely impaired renal function, or known allergy to gadolinium. If history and physical exam findings suggest possible stroke, arrange for MRI and MR angiography with contrast, even if the result of a head CT scan is negative. The ALGORITHM13 offers guidance for choosing imaging studies for headache based on history, physical exam, and laboratory findings.
›› And you order...
…an MRI of the brain with contrast.
Though Ms. D does have a focal neurologic finding in addition to her headache, she does not appear to be acutely ill. Ordering an MRI with contrast is the best first step.
CASE 2 › Anne B is a 72-year-old woman with a history of hypertension, hyperlipidemia, and type 2 diabetes. Her daughter brings her in to see you because she is concerned about Ms. B’s memory. Ms. B’s daughter reports that she has become increasingly forgetful over the past 6 months, often forgetting recent events. At first the forgetfulness was occasional, but now it seems to happen daily and interfere with activities of daily living (ADLs). The week before this visit, Ms. B left a pot heating on the stove because she forgot she had started cooking. She realized what had happened only when her smoke alarm went off. Ms. B’s daughter also thinks her mother may be taking some of her medications incorrectly.
Physical exam and laboratory findings are unremarkable. On the mental status exam, Ms. B has difficulty with registration and recall.
›› What imaging options might you consider?
Ms. B has exhibited progressive memory loss over 6 months and it is now affecting her ADLs. Her symptoms could be secondary to any one of many reversible medical causes, such as adverse medication effects, depression, or vitamin B12 deficiency. If clinical and laboratory evaluations exclude these reversible causes, consider dementia.
With numerous disorders having overlapping symptoms, the diagnosis of degenerative central nervous system (CNS) disease can be extremely tricky. Complicating the issue is the fact that a single patient can have 2 or more concurrent neurodegenerative diseases. Clinical testing is essential in the diagnosis and management of degenerative CNS diseases, but testing sensitivity and specificity are highly variable depending upon the disease.14
Neuroimaging is an important supplement to clinical testing in excluding intracranial abnormalities. There are significant negative consequences of missing reversible causes of memory problems and incorrectly assigning a clinical diagnosis of dementia. Neuroimaging can be subdivided into structural and functional imaging, and structural imaging is the first step in evaluation.15
The American Academy of Neurology recommends the use of structural neuroimaging with CT or MRI in the initial evaluation of patients with dementia to detect such treatable problems as a subdural hematoma, frontal lobe mass, or hydrocephalus.12 Structural imaging may also identify anatomic changes characteristic of degenerative CNS diseases such as Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia, vascular dementia, Creutzfeldt-Jakob disease, and Huntington’s disease; however, sensitivities and specificities of testing are low.14
›› And you order...
…an MRI of the brain without contrast.
In Ms. B’s case, structural neuroimaging is indicated as part of the initial work-up of supposed dementia. An MRI without contrast is recommended over CT because it is more sensitive in detecting white matter changes in vascular dementia.16 In cases where an MRI >is unavailable or contraindicated (eg, a patient with a pacemaker), a CT is a reasonable alternative.
CASE 3 › Bob C is a 78-year-old man with a history of chronic obstructive pulmonary disease and hypertension who arrives at your walk-in clinic accompanied by his home health aide a few hours after having tripped and fallen over a rug at home. At baseline, Mr. C is ambulatory and independent in ADLs.
He takes all of his medications, including a daily baby aspirin (81 mg). Mr. C says he did not lose consciousness at the time of the fall and insists he feels fine, but you notice a bruise developing over his right temporal skull.
›› What imaging options might you consider?
With acute head trauma deemed severe enough clinically to warrant imaging, non-contrast CT is the most appropriate initial test to identify possible intracranial hemorrhage.11 The Glasgow Coma Scale (GCS) is the tool most widely used for clinical evaluation17 (TABLE 118). The score is based on an assessment of 3 features: eye response, speech, and movement. Head injury is classified as mild (13-15), moderate (9-12), or severe (≤8). It is universally agreed that patients with moderate or severe head injury should be further evaluated with a head CT.
With mild head injury, recommendations for follow-up are less straightforward. The New Orleans Criteria (NOC) and Canadian CT Head Rule (CCHR) are commonly used in triaging patients with minor head trauma in a cost effective way11 (TABLES 219 and 320). The cost-effectiveness of these assessment tools is still questionable, but both have very high sensitivity for identifying patients who will require neurosurgery intervention.21,22 Although the NOC is slightly more sensitive at identifying patients with nonsurgical clinically significant abnormalities, it has a greatly reduced specificity compared with the CCHR.23-25
›› And you order...
…a non-contrast head CT.
Mr. C presents with a GCS of 15, indicating mild head trauma. However, in elderly patients, especially ones taking anticoagulation medication, even mild trauma can result in clinically significant abnormalities such as a subdural hematoma.1 Although Mr. C’s physical and neurologic exams are not worrisome, both the NOC and CCHR recommend further evaluation with a non-contrast head CT based on his age.
CORRESPONDENCE
Urmi A. Desai, MD, MS, Columbia University Medical Center, 610 West 158th Street, New York, NY 10032; [email protected].
1. American College of Radiology. Appropriateness criteria. American College of Radiology Web site. Available at: https://acsearch.acr.org/list. Accessed January 4, 2015.
2. American College of Radiology. About the ACR appropriateness criteria. American College of Radiology Web site. Available at: http://www.acr.org/Quality-Safety/Appropriateness-Criteria/About-AC. Accessed July 20, 2015.
3. Becker LA, Green LA, Beaufait D, et al. Use of CT scans for the investigation of headache: a report from ASPN, Part 1. J Fam Pract. 1993;37:129-134.
4. Sandrini G, Friberg L, Jänig W, et al. Neurophysiological tests and neuroimaging procedures in non-acute headache: guidelines and recommendations. Eur J Neurol. 2004;11:217-224.
5. Sempere AP, Porta-Etessam J, Medrano V, et al. Neuroimaging in the evaluation of patients with non-acute headache. Cephalalgia. 2005;25:30-35.
6. Gilbert JW, Johnson KM, Larkin GL, et al. Atraumatic headache in US emergency departments: recent trends in CT/MRI utilisation and factors associated with severe intracranial pathology. Emerg Med J. 2012;29:576-581.
7. Tsushima Y, Endo K. MR imaging in the evaluation of chronic or recurrent headache. Radiology. 2005;235:575-579.
8. Jordan JE, Ramirez GF, Bradley WG, et al. Economic and outcomes assessment of magnetic resonance imaging in the evaluation of headache. J Natl Med Assoc. 2000;92:573-578.
9. Mitchell CS, Osborn RE, Grosskreutz SR. Computed tomography in the headache patient: is routine evaluation really necessary? Headache. 1993;33:82-86.
10. Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology. 1994;44:1191-1197.
11. Osborn AG. Osborn’s Brain: Imaging, Pathology, and Anatomy. Salt Lake City, Utah: Amirsys Publishing; 2013.
12. Silberstein SD. Chronic daily headache. J Am Osteopath Assoc. 2005;105:23S-29S.
13. Douglas AC, Wippold FJ 2nd, Broderick DF, et al. ACR Appropriateness Criteria Headache. J Am Coll Radiol. 2014;11:657-667.
14. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143-1153.
15. Wippold FJ 2nd, Brown DC, Broderick DF, et al. ACR Appropriateness Criteria Dementia and Movement Disorders. J Am Coll Radiol. 2015;12:19-28.
16. Kantarci K, Jack CR Jr. Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197-209.
17. Teasdale G, Maas A, Lecky F, et al. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13:844-854.
18. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84.
19. Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100-105.
20. Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391-1396.
21. Davis PC, Drayer BP, Anderson RE, et al. Head trauma. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215 Suppl:507-524.
22. Stiell IG, Clement CM, Grimshaw JM, et al. A prospective clusterrandomized trial to implement the Canadian CT Head Rule in emergency departments. CMAJ. 2010;182:1527-1532.
23. Smits M, Dippel DW, Nederkoorn PJ, et al. Minor head injury: CT-based strategies for management—a cost-effectiveness analysis. Radiology. 2010;254:532-540.
24. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294:1511-1518.
25. Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519-1525.
› Use clinical decision tools and American College of Radiology Appropriateness Criteria to determine levels of evidence for ordering specific imaging studies. A
› Consider ordering an imaging study when headache is accompanied by a new neurologic symptom. Computed tomography (CT) without contrast is best for patients who are acutely ill. In the non-emergent setting, magnetic resonance imaging (MRI) with contrast is the optimal study. A
› Order structural neuroimaging as part of the initial work-up of suspected dementia. MRI is the test of choice. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When patients present with neurologic complaints in outpatient primary care practice, 2 key questions often arise: Should brain imaging be ordered, and if so, which study? Careful history-taking and physical exam findings can suggest differential diagnoses and help determine whether imaging studies could identify potential underlying causes. Further considerations in making a decision are the type of information each modality offers, the possible need for contrast media, benefits vs radiation exposure risks, potential contraindications, and cost and local availability. In this article, we present 3 common outpatient scenarios, and in each case we describe the evidence to support clinical decision-making about imaging.
The American College of Radiology (ACR) Appropriateness Criteria Web site (http://www.acr.org/Quality-Safety/Appropriateness-Criteria) provides radiation exposure information, numerical ratings of imaging studies for individual clinical scenarios, evidence tables, and reference tables for each of its recommendations.1 ACR’s recommendations were developed by expert panels of diagnostic radiologists, interventional radiologists, and radiation oncologists, and designed to help physicians order the most appropriate imaging studies based on patients’ clinical conditions.2 We used these criteria to develop an evaluation strategy for each of our clinical scenarios.
CASE 1 › Carrie D is a 45-year-old woman with a history of migraine without aura generally controlled with Excedrin (acetaminophen, aspirin, and caffeine). She arrives at your office with a 2-day history of severe headache over the top of her head and associated tingling sensation over the left side of her face, but with no vision changes, weakness, or slurred speech. She denies any prior history of numbness or tinging with past headaches. She is a business executive and reports that in the last few weeks, her company has been involved in a high-profile merger. On physical exam, her vital signs are within normal limits. She does not appear acutely ill, but on exam she reports diminished sensation to light touch over the left side of her face, left arm, and left leg compared with the right side.
›› What imaging options might you consider?
A prospective review of physicians in an ambulatory family practice setting found that neurologic imaging was typically ordered for patients with headache who were suspected of having a brain tumor or subarachnoid hemorrhage.3 For our patient, who has a history of migraines without aura and whose current severe headache is accompanied by an abnormal sensation on one side of the face, the following questions are relevant: Is this presentation part of her primary headache syndrome or could there be a different cause? If there is a different cause, is it likely to be detected with brain imaging such as computed tomography (CT) or magnetic resonance imaging (MRI)?
Patients with isolated headache and an absence of neurologic symptoms or abnormalities on neurologic exam are unlikely to have a clinically significant intracranial abnormality.4-10 Imaging of the brain is typically not indicated for these patients.2 However, given that this patient does have a positive focal neurologic finding, a CT or MRI is indicated, as findings are more likely to influence management decisions.
The decision to order CT or MRI should be based on how acutely ill the patient is. CT without contrast is an excellent tool to rule out suspected emergent intracranial abnormalities such as an intracranial hemorrhage, hydrocephalus, or a mass.11 In patients presenting with symptoms suggesting acute illness such as carotid or vertebral artery dissection, the most appropriate test would be CT angiography of the head and neck.2
However, the list of less dire causes of headache is vast. Included in this list would be trauma, other vascular disorders such as arteriovenous malformation or temporal arteritis, infection, abnormal intracranial pressure (mass, pseudotumor cerebri, intracranial hypotension), and disorders of the head/face/spine (eg, temporomandibular joint disorder).12
In the non-acute setting where a patient has stable vital signs and is not in acute distress, an MRI with contrast would be the most appropriate test to identify such causes. Avoid contrast only if there is a firm contraindication, such as pregnancy, severely impaired renal function, or known allergy to gadolinium. If history and physical exam findings suggest possible stroke, arrange for MRI and MR angiography with contrast, even if the result of a head CT scan is negative. The ALGORITHM13 offers guidance for choosing imaging studies for headache based on history, physical exam, and laboratory findings.
›› And you order...
…an MRI of the brain with contrast.
Though Ms. D does have a focal neurologic finding in addition to her headache, she does not appear to be acutely ill. Ordering an MRI with contrast is the best first step.
CASE 2 › Anne B is a 72-year-old woman with a history of hypertension, hyperlipidemia, and type 2 diabetes. Her daughter brings her in to see you because she is concerned about Ms. B’s memory. Ms. B’s daughter reports that she has become increasingly forgetful over the past 6 months, often forgetting recent events. At first the forgetfulness was occasional, but now it seems to happen daily and interfere with activities of daily living (ADLs). The week before this visit, Ms. B left a pot heating on the stove because she forgot she had started cooking. She realized what had happened only when her smoke alarm went off. Ms. B’s daughter also thinks her mother may be taking some of her medications incorrectly.
Physical exam and laboratory findings are unremarkable. On the mental status exam, Ms. B has difficulty with registration and recall.
›› What imaging options might you consider?
Ms. B has exhibited progressive memory loss over 6 months and it is now affecting her ADLs. Her symptoms could be secondary to any one of many reversible medical causes, such as adverse medication effects, depression, or vitamin B12 deficiency. If clinical and laboratory evaluations exclude these reversible causes, consider dementia.
With numerous disorders having overlapping symptoms, the diagnosis of degenerative central nervous system (CNS) disease can be extremely tricky. Complicating the issue is the fact that a single patient can have 2 or more concurrent neurodegenerative diseases. Clinical testing is essential in the diagnosis and management of degenerative CNS diseases, but testing sensitivity and specificity are highly variable depending upon the disease.14
Neuroimaging is an important supplement to clinical testing in excluding intracranial abnormalities. There are significant negative consequences of missing reversible causes of memory problems and incorrectly assigning a clinical diagnosis of dementia. Neuroimaging can be subdivided into structural and functional imaging, and structural imaging is the first step in evaluation.15
The American Academy of Neurology recommends the use of structural neuroimaging with CT or MRI in the initial evaluation of patients with dementia to detect such treatable problems as a subdural hematoma, frontal lobe mass, or hydrocephalus.12 Structural imaging may also identify anatomic changes characteristic of degenerative CNS diseases such as Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia, vascular dementia, Creutzfeldt-Jakob disease, and Huntington’s disease; however, sensitivities and specificities of testing are low.14
›› And you order...
…an MRI of the brain without contrast.
In Ms. B’s case, structural neuroimaging is indicated as part of the initial work-up of supposed dementia. An MRI without contrast is recommended over CT because it is more sensitive in detecting white matter changes in vascular dementia.16 In cases where an MRI >is unavailable or contraindicated (eg, a patient with a pacemaker), a CT is a reasonable alternative.
CASE 3 › Bob C is a 78-year-old man with a history of chronic obstructive pulmonary disease and hypertension who arrives at your walk-in clinic accompanied by his home health aide a few hours after having tripped and fallen over a rug at home. At baseline, Mr. C is ambulatory and independent in ADLs.
He takes all of his medications, including a daily baby aspirin (81 mg). Mr. C says he did not lose consciousness at the time of the fall and insists he feels fine, but you notice a bruise developing over his right temporal skull.
›› What imaging options might you consider?
With acute head trauma deemed severe enough clinically to warrant imaging, non-contrast CT is the most appropriate initial test to identify possible intracranial hemorrhage.11 The Glasgow Coma Scale (GCS) is the tool most widely used for clinical evaluation17 (TABLE 118). The score is based on an assessment of 3 features: eye response, speech, and movement. Head injury is classified as mild (13-15), moderate (9-12), or severe (≤8). It is universally agreed that patients with moderate or severe head injury should be further evaluated with a head CT.
With mild head injury, recommendations for follow-up are less straightforward. The New Orleans Criteria (NOC) and Canadian CT Head Rule (CCHR) are commonly used in triaging patients with minor head trauma in a cost effective way11 (TABLES 219 and 320). The cost-effectiveness of these assessment tools is still questionable, but both have very high sensitivity for identifying patients who will require neurosurgery intervention.21,22 Although the NOC is slightly more sensitive at identifying patients with nonsurgical clinically significant abnormalities, it has a greatly reduced specificity compared with the CCHR.23-25
›› And you order...
…a non-contrast head CT.
Mr. C presents with a GCS of 15, indicating mild head trauma. However, in elderly patients, especially ones taking anticoagulation medication, even mild trauma can result in clinically significant abnormalities such as a subdural hematoma.1 Although Mr. C’s physical and neurologic exams are not worrisome, both the NOC and CCHR recommend further evaluation with a non-contrast head CT based on his age.
CORRESPONDENCE
Urmi A. Desai, MD, MS, Columbia University Medical Center, 610 West 158th Street, New York, NY 10032; [email protected].
› Use clinical decision tools and American College of Radiology Appropriateness Criteria to determine levels of evidence for ordering specific imaging studies. A
› Consider ordering an imaging study when headache is accompanied by a new neurologic symptom. Computed tomography (CT) without contrast is best for patients who are acutely ill. In the non-emergent setting, magnetic resonance imaging (MRI) with contrast is the optimal study. A
› Order structural neuroimaging as part of the initial work-up of suspected dementia. MRI is the test of choice. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When patients present with neurologic complaints in outpatient primary care practice, 2 key questions often arise: Should brain imaging be ordered, and if so, which study? Careful history-taking and physical exam findings can suggest differential diagnoses and help determine whether imaging studies could identify potential underlying causes. Further considerations in making a decision are the type of information each modality offers, the possible need for contrast media, benefits vs radiation exposure risks, potential contraindications, and cost and local availability. In this article, we present 3 common outpatient scenarios, and in each case we describe the evidence to support clinical decision-making about imaging.
The American College of Radiology (ACR) Appropriateness Criteria Web site (http://www.acr.org/Quality-Safety/Appropriateness-Criteria) provides radiation exposure information, numerical ratings of imaging studies for individual clinical scenarios, evidence tables, and reference tables for each of its recommendations.1 ACR’s recommendations were developed by expert panels of diagnostic radiologists, interventional radiologists, and radiation oncologists, and designed to help physicians order the most appropriate imaging studies based on patients’ clinical conditions.2 We used these criteria to develop an evaluation strategy for each of our clinical scenarios.
CASE 1 › Carrie D is a 45-year-old woman with a history of migraine without aura generally controlled with Excedrin (acetaminophen, aspirin, and caffeine). She arrives at your office with a 2-day history of severe headache over the top of her head and associated tingling sensation over the left side of her face, but with no vision changes, weakness, or slurred speech. She denies any prior history of numbness or tinging with past headaches. She is a business executive and reports that in the last few weeks, her company has been involved in a high-profile merger. On physical exam, her vital signs are within normal limits. She does not appear acutely ill, but on exam she reports diminished sensation to light touch over the left side of her face, left arm, and left leg compared with the right side.
›› What imaging options might you consider?
A prospective review of physicians in an ambulatory family practice setting found that neurologic imaging was typically ordered for patients with headache who were suspected of having a brain tumor or subarachnoid hemorrhage.3 For our patient, who has a history of migraines without aura and whose current severe headache is accompanied by an abnormal sensation on one side of the face, the following questions are relevant: Is this presentation part of her primary headache syndrome or could there be a different cause? If there is a different cause, is it likely to be detected with brain imaging such as computed tomography (CT) or magnetic resonance imaging (MRI)?
Patients with isolated headache and an absence of neurologic symptoms or abnormalities on neurologic exam are unlikely to have a clinically significant intracranial abnormality.4-10 Imaging of the brain is typically not indicated for these patients.2 However, given that this patient does have a positive focal neurologic finding, a CT or MRI is indicated, as findings are more likely to influence management decisions.
The decision to order CT or MRI should be based on how acutely ill the patient is. CT without contrast is an excellent tool to rule out suspected emergent intracranial abnormalities such as an intracranial hemorrhage, hydrocephalus, or a mass.11 In patients presenting with symptoms suggesting acute illness such as carotid or vertebral artery dissection, the most appropriate test would be CT angiography of the head and neck.2
However, the list of less dire causes of headache is vast. Included in this list would be trauma, other vascular disorders such as arteriovenous malformation or temporal arteritis, infection, abnormal intracranial pressure (mass, pseudotumor cerebri, intracranial hypotension), and disorders of the head/face/spine (eg, temporomandibular joint disorder).12
In the non-acute setting where a patient has stable vital signs and is not in acute distress, an MRI with contrast would be the most appropriate test to identify such causes. Avoid contrast only if there is a firm contraindication, such as pregnancy, severely impaired renal function, or known allergy to gadolinium. If history and physical exam findings suggest possible stroke, arrange for MRI and MR angiography with contrast, even if the result of a head CT scan is negative. The ALGORITHM13 offers guidance for choosing imaging studies for headache based on history, physical exam, and laboratory findings.
›› And you order...
…an MRI of the brain with contrast.
Though Ms. D does have a focal neurologic finding in addition to her headache, she does not appear to be acutely ill. Ordering an MRI with contrast is the best first step.
CASE 2 › Anne B is a 72-year-old woman with a history of hypertension, hyperlipidemia, and type 2 diabetes. Her daughter brings her in to see you because she is concerned about Ms. B’s memory. Ms. B’s daughter reports that she has become increasingly forgetful over the past 6 months, often forgetting recent events. At first the forgetfulness was occasional, but now it seems to happen daily and interfere with activities of daily living (ADLs). The week before this visit, Ms. B left a pot heating on the stove because she forgot she had started cooking. She realized what had happened only when her smoke alarm went off. Ms. B’s daughter also thinks her mother may be taking some of her medications incorrectly.
Physical exam and laboratory findings are unremarkable. On the mental status exam, Ms. B has difficulty with registration and recall.
›› What imaging options might you consider?
Ms. B has exhibited progressive memory loss over 6 months and it is now affecting her ADLs. Her symptoms could be secondary to any one of many reversible medical causes, such as adverse medication effects, depression, or vitamin B12 deficiency. If clinical and laboratory evaluations exclude these reversible causes, consider dementia.
With numerous disorders having overlapping symptoms, the diagnosis of degenerative central nervous system (CNS) disease can be extremely tricky. Complicating the issue is the fact that a single patient can have 2 or more concurrent neurodegenerative diseases. Clinical testing is essential in the diagnosis and management of degenerative CNS diseases, but testing sensitivity and specificity are highly variable depending upon the disease.14
Neuroimaging is an important supplement to clinical testing in excluding intracranial abnormalities. There are significant negative consequences of missing reversible causes of memory problems and incorrectly assigning a clinical diagnosis of dementia. Neuroimaging can be subdivided into structural and functional imaging, and structural imaging is the first step in evaluation.15
The American Academy of Neurology recommends the use of structural neuroimaging with CT or MRI in the initial evaluation of patients with dementia to detect such treatable problems as a subdural hematoma, frontal lobe mass, or hydrocephalus.12 Structural imaging may also identify anatomic changes characteristic of degenerative CNS diseases such as Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia, vascular dementia, Creutzfeldt-Jakob disease, and Huntington’s disease; however, sensitivities and specificities of testing are low.14
›› And you order...
…an MRI of the brain without contrast.
In Ms. B’s case, structural neuroimaging is indicated as part of the initial work-up of supposed dementia. An MRI without contrast is recommended over CT because it is more sensitive in detecting white matter changes in vascular dementia.16 In cases where an MRI >is unavailable or contraindicated (eg, a patient with a pacemaker), a CT is a reasonable alternative.
CASE 3 › Bob C is a 78-year-old man with a history of chronic obstructive pulmonary disease and hypertension who arrives at your walk-in clinic accompanied by his home health aide a few hours after having tripped and fallen over a rug at home. At baseline, Mr. C is ambulatory and independent in ADLs.
He takes all of his medications, including a daily baby aspirin (81 mg). Mr. C says he did not lose consciousness at the time of the fall and insists he feels fine, but you notice a bruise developing over his right temporal skull.
›› What imaging options might you consider?
With acute head trauma deemed severe enough clinically to warrant imaging, non-contrast CT is the most appropriate initial test to identify possible intracranial hemorrhage.11 The Glasgow Coma Scale (GCS) is the tool most widely used for clinical evaluation17 (TABLE 118). The score is based on an assessment of 3 features: eye response, speech, and movement. Head injury is classified as mild (13-15), moderate (9-12), or severe (≤8). It is universally agreed that patients with moderate or severe head injury should be further evaluated with a head CT.
With mild head injury, recommendations for follow-up are less straightforward. The New Orleans Criteria (NOC) and Canadian CT Head Rule (CCHR) are commonly used in triaging patients with minor head trauma in a cost effective way11 (TABLES 219 and 320). The cost-effectiveness of these assessment tools is still questionable, but both have very high sensitivity for identifying patients who will require neurosurgery intervention.21,22 Although the NOC is slightly more sensitive at identifying patients with nonsurgical clinically significant abnormalities, it has a greatly reduced specificity compared with the CCHR.23-25
›› And you order...
…a non-contrast head CT.
Mr. C presents with a GCS of 15, indicating mild head trauma. However, in elderly patients, especially ones taking anticoagulation medication, even mild trauma can result in clinically significant abnormalities such as a subdural hematoma.1 Although Mr. C’s physical and neurologic exams are not worrisome, both the NOC and CCHR recommend further evaluation with a non-contrast head CT based on his age.
CORRESPONDENCE
Urmi A. Desai, MD, MS, Columbia University Medical Center, 610 West 158th Street, New York, NY 10032; [email protected].
1. American College of Radiology. Appropriateness criteria. American College of Radiology Web site. Available at: https://acsearch.acr.org/list. Accessed January 4, 2015.
2. American College of Radiology. About the ACR appropriateness criteria. American College of Radiology Web site. Available at: http://www.acr.org/Quality-Safety/Appropriateness-Criteria/About-AC. Accessed July 20, 2015.
3. Becker LA, Green LA, Beaufait D, et al. Use of CT scans for the investigation of headache: a report from ASPN, Part 1. J Fam Pract. 1993;37:129-134.
4. Sandrini G, Friberg L, Jänig W, et al. Neurophysiological tests and neuroimaging procedures in non-acute headache: guidelines and recommendations. Eur J Neurol. 2004;11:217-224.
5. Sempere AP, Porta-Etessam J, Medrano V, et al. Neuroimaging in the evaluation of patients with non-acute headache. Cephalalgia. 2005;25:30-35.
6. Gilbert JW, Johnson KM, Larkin GL, et al. Atraumatic headache in US emergency departments: recent trends in CT/MRI utilisation and factors associated with severe intracranial pathology. Emerg Med J. 2012;29:576-581.
7. Tsushima Y, Endo K. MR imaging in the evaluation of chronic or recurrent headache. Radiology. 2005;235:575-579.
8. Jordan JE, Ramirez GF, Bradley WG, et al. Economic and outcomes assessment of magnetic resonance imaging in the evaluation of headache. J Natl Med Assoc. 2000;92:573-578.
9. Mitchell CS, Osborn RE, Grosskreutz SR. Computed tomography in the headache patient: is routine evaluation really necessary? Headache. 1993;33:82-86.
10. Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology. 1994;44:1191-1197.
11. Osborn AG. Osborn’s Brain: Imaging, Pathology, and Anatomy. Salt Lake City, Utah: Amirsys Publishing; 2013.
12. Silberstein SD. Chronic daily headache. J Am Osteopath Assoc. 2005;105:23S-29S.
13. Douglas AC, Wippold FJ 2nd, Broderick DF, et al. ACR Appropriateness Criteria Headache. J Am Coll Radiol. 2014;11:657-667.
14. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143-1153.
15. Wippold FJ 2nd, Brown DC, Broderick DF, et al. ACR Appropriateness Criteria Dementia and Movement Disorders. J Am Coll Radiol. 2015;12:19-28.
16. Kantarci K, Jack CR Jr. Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197-209.
17. Teasdale G, Maas A, Lecky F, et al. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13:844-854.
18. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84.
19. Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100-105.
20. Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391-1396.
21. Davis PC, Drayer BP, Anderson RE, et al. Head trauma. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215 Suppl:507-524.
22. Stiell IG, Clement CM, Grimshaw JM, et al. A prospective clusterrandomized trial to implement the Canadian CT Head Rule in emergency departments. CMAJ. 2010;182:1527-1532.
23. Smits M, Dippel DW, Nederkoorn PJ, et al. Minor head injury: CT-based strategies for management—a cost-effectiveness analysis. Radiology. 2010;254:532-540.
24. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294:1511-1518.
25. Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519-1525.
1. American College of Radiology. Appropriateness criteria. American College of Radiology Web site. Available at: https://acsearch.acr.org/list. Accessed January 4, 2015.
2. American College of Radiology. About the ACR appropriateness criteria. American College of Radiology Web site. Available at: http://www.acr.org/Quality-Safety/Appropriateness-Criteria/About-AC. Accessed July 20, 2015.
3. Becker LA, Green LA, Beaufait D, et al. Use of CT scans for the investigation of headache: a report from ASPN, Part 1. J Fam Pract. 1993;37:129-134.
4. Sandrini G, Friberg L, Jänig W, et al. Neurophysiological tests and neuroimaging procedures in non-acute headache: guidelines and recommendations. Eur J Neurol. 2004;11:217-224.
5. Sempere AP, Porta-Etessam J, Medrano V, et al. Neuroimaging in the evaluation of patients with non-acute headache. Cephalalgia. 2005;25:30-35.
6. Gilbert JW, Johnson KM, Larkin GL, et al. Atraumatic headache in US emergency departments: recent trends in CT/MRI utilisation and factors associated with severe intracranial pathology. Emerg Med J. 2012;29:576-581.
7. Tsushima Y, Endo K. MR imaging in the evaluation of chronic or recurrent headache. Radiology. 2005;235:575-579.
8. Jordan JE, Ramirez GF, Bradley WG, et al. Economic and outcomes assessment of magnetic resonance imaging in the evaluation of headache. J Natl Med Assoc. 2000;92:573-578.
9. Mitchell CS, Osborn RE, Grosskreutz SR. Computed tomography in the headache patient: is routine evaluation really necessary? Headache. 1993;33:82-86.
10. Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology. 1994;44:1191-1197.
11. Osborn AG. Osborn’s Brain: Imaging, Pathology, and Anatomy. Salt Lake City, Utah: Amirsys Publishing; 2013.
12. Silberstein SD. Chronic daily headache. J Am Osteopath Assoc. 2005;105:23S-29S.
13. Douglas AC, Wippold FJ 2nd, Broderick DF, et al. ACR Appropriateness Criteria Headache. J Am Coll Radiol. 2014;11:657-667.
14. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143-1153.
15. Wippold FJ 2nd, Brown DC, Broderick DF, et al. ACR Appropriateness Criteria Dementia and Movement Disorders. J Am Coll Radiol. 2015;12:19-28.
16. Kantarci K, Jack CR Jr. Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197-209.
17. Teasdale G, Maas A, Lecky F, et al. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13:844-854.
18. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84.
19. Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100-105.
20. Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391-1396.
21. Davis PC, Drayer BP, Anderson RE, et al. Head trauma. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215 Suppl:507-524.
22. Stiell IG, Clement CM, Grimshaw JM, et al. A prospective clusterrandomized trial to implement the Canadian CT Head Rule in emergency departments. CMAJ. 2010;182:1527-1532.
23. Smits M, Dippel DW, Nederkoorn PJ, et al. Minor head injury: CT-based strategies for management—a cost-effectiveness analysis. Radiology. 2010;254:532-540.
24. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294:1511-1518.
25. Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519-1525.
Contraception for the perimenopausal woman: What’s best?
› Consider long-acting reversible contraception, such as an intrauterine device or an implant, as a first-line option for women who have mild or no symptoms of perimenopause. A
› Unless contraindicated, prescribe combination hormonal contraceptives for women in their 40s who desire them, as they are generally safe and effective in treating perimenopausal symptoms. A
› Use the Centers for Disease Control and Prevention’s evidence-based recommendations to guide your choice of contraceptive for perimenopausal patients based on individual medical history. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It is no secret that about half of all pregnancies in the United States are unintended, and that teens have the highest rate of unplanned pregnancy. What’s not so well known is that women in their 40s have the second highest rate.1
Optimal use of contraception throughout perimenopause is crucial, but finding the right method of birth control for this patient population can be a bit of a balancing act. Long-acting reversible contraceptives (LARCs), such as an intrauterine device or progestin-only implant, are preferred first-line contraceptive options when preventing pregnancy is the primary goal, given their increased efficacy and limited number of contraindications.2,3 However, women experiencing perimenopausal symptoms often need a combination hormonal contraceptive (CHC)—typically an estrogen-containing pill, a patch, or a vaginal ring—for relief of vasomotor symptoms and cycle control.
Women in their 40s should have access to a full array of options to help improve adherence. However, physicians may be reluctant to prescribe estrogen-containing products for patients who often have a more complex medical history than their younger counterparts, including increased risks for breast cancer, cardiovascular disease, and venous thromboembolism (VTE).
With this in mind, the Centers for Disease Control and Prevention (CDC) has identified medical conditions that may affect the use of the various types of contraceptives by perimenopausal women and issued evidence-based recommendations on the appropriateness of each method using a one-to-4 rating system (TABLE 1).2 To help you address the contraceptive needs of such patients, we review the key risk factors, CDC guidelines, and optimal choices in the 4 case studies that follow.
CASE 1 › Sara G: VTE risk
Sara G, a healthy 45-year-old, recently started dating again following her divorce. She wants to avoid pregnancy. She has no personal or family history of clotting disorders and does not smoke. However, she is obese (body mass index [BMI]=32 kg/m2), and her job as a visiting nurse requires her to spend most of the day in her car. Ms. G also has acne and wants an estrogen-containing contraceptive to help treat it.
If Ms. G were your patient, what would you offer her?
The risk for VTE increases substantially for women older than 40 years. In a recent cohort study, those ages 45 to 49 faced approximately twice the risk of women ages 25 to 29. However, the absolute risk for the older women was still low (4.7-5.3 per 10,000 woman-years).4 What’s more, the risk of VTE from the use of a CHC is substantially less than the risk associated with pregnancy and the postpartum period (TABLE 2).5
Obesity increases the risk. Women like Ms. G who are obese (BMI >30) have an increased risk for VTE associated with CHCs, but the CDC rates them as a Category 2 risk, even for obese women in their 40s—a determination that the advantages outweigh the risks.2
Progestin choice and estrogen dose matter. Combination oral contraceptives (COCs) that contain certain third-generation progestins (gestodene and desogestrel) may be more thrombophilic than those containing first- or second-generation progestins (TABLE 3).6 The relative risk (RR) for VTE with third-generation vs second-generation progestins is 1.3 (95% confidence interval [CI], 1.0-1.8).7 Formulations containing higher doses of estrogen are also more likely to be associated with VTE.7
Drospirenone is a newer progestin. Found in several COCs, drospirenone has antimineralocorticoid properties that help to minimize bloating and fluid retention but may also lead to a hypercoagulable state.5 Numerous studies have investigated the association between drospirenone and VTE risk, with conflicting results.8 Most recently, a large international prospective observational study involving more than 85,000 women showed no increased risk for VTE among women taking COCs with drospirenone compared with pills that do not contain this progestin.9
Non-oral CHCs, including the vaginal ring and the patch, offer the convenience of weekly or monthly use while providing similar benefits to COCs. Some fear that the continuous exposure to hormones associated with these methods may increase the risk for VTE, but evidence is mixed.
A large (N=1.6 million) Danish registry study published in 2012 demonstrated a 2-fold increased risk of VTE among vaginal ring users vs women taking COCs.4 But a multinational prospective cohort study of more than 33,000 women found no increased VTE risk in ring users,10 and a recent US database study involving more than 800,000 women reported nonsignificant VTE risk estimates for both the ring (RR=1.09; 95% CI, 0.55-2.16) and the patch (RR=1.35; 95% CI, 0.90-2.02) compared with COCs.11
THE BOTTOM LINE For Ms. G, the benefits of contraception likely outweigh any small increase in her absolute risk for VTE. To minimize her risk, however, select a pill that contains a low dose (20-35 mcg) of ethinyl estradiol (EE) combined with a progestin that has not been associated with an increased VTE risk. Because of their mechanism of action, most COCs will improve acne, regardless of the progestin in the formulation.12-14
CASE 2 › Stephanie T: CV risk
Stephanie T, 47, is in need of contraception and treatment for severe hot flashes. She has no significant past medical history, but she is obese (BMI =36), her blood pressure (BP) is 130/80 mm Hg, and her most recent labs reveal a fasting glucose of 115 and a hemoglobin A1c of 6.1%. Ms. T is concerned about arterial thromboembolic disease because of her family history: Her father had a myocardial infarction (MI) at age 56 and a maternal aunt had a stroke when she was 65.
What evidence should you consider?
Baseline arterial thromboembolic events are considerably more rare in premenopausal women than VTEs (13.2 MIs vs 24.2 thrombotic strokes per 100,000 woman-years).15 Thus, a small increased RR from a CHC is unlikely to have a significant clinical impact.
A systemic review and meta-analysis of studies between 1995 and 2012 showed that the odds ratio (OR) of ischemic stroke in users of COCs vs nonusers was 1.9 (95% CI, 1.24–2.91).16 This study included very few estrogen formulations with <35 mcg EE, however; even so, no increased risk of MI was found (OR=1.34; 95% CI, 0.87–2.08).16 A 15-year retrospective cohort study of 1.6 million Danish women showed that lowering the dose of EE to 20 mcg (from 30-40 mcg) significantly reduced the risk of arterial events.15 It is unclear whether the vaginal ring is associated with an increased RR of stroke compared with COCs because studies have had mixed results.10,15 There is no compelling evidence to suggest a difference in the risk of arterial events based on the type of progestin used in the COC.15
Hypertension is a key consideration. It is important to remember that perimenopausal women may have comorbid conditions that increase their risk of arterial thromboembolic events. CHCs should be used with caution in women with hypertension, even if BP is adequately controlled—a Category 3 recommendation from the CDC. In such patients, LARC or a progestin-only pill is preferred unless there is a compelling reason to use a CHC, such as acne, vasomotor symptoms, or hirsutism.2
CHCs are contraindicated for women with a BP ≥160/100 mm Hg and/or any manifestation of vascular disease (Category 4).2 Although progestin-only methods are often preferred for women with established vascular disease, depot medroxyprogesterone acetate (DMPA) is an exception (Category 3).2 DMPA is not a first-line choice for such patients because of its potential to cause weight gain and worsening lipids, glucose, and insulin metabolism. Women with hypertriglyceridemia should have follow-up testing of lipid levels after initiation of hormonal contraception, especially if it contains estrogen.
Diabetes is not an absolute contraindication. Many women with diabetes can safely use CHCs (Category 2). The exceptions: those who have vascular disease, nephropathy, retinopathy, or neuropathy (Category 4) or have had diabetes for >20 years and therefore have the potential for undiagnosed vascular disease.2 Generally, the use of insulin should not affect decisions regarding CHCs, and patients can be reassured that the hormones will not worsen their diabetes control.
When caring for women who have multiple risk factors for cardiovascular disease, it is important to exercise clinical judgment regarding the appropriateness of CHCs (Categories 3 and 4). Progestin-only methods have a more favorable risk profile for women at the highest risk and may provide ample relief of perimenopausal symptoms.2
THE BOTTOM LINE Ms. T may benefit from a CHC due to her severe hot flashes. She should be encouraged to adopt healthy lifestyle changes, including diet and exercise, to decrease her risk of arterial thromboembolism and VTE, but she has no contraindications to the use of a CHC at this time.
CASE 3 › Leslie C: Bone health
Leslie C, age 45, is happy with the contraceptive he has used for the past 3 years—DMPA injections every 3 months. She has no perimenopausal symptoms. However, her mother had an osteoporotic hip fracture at age 70 and Ms. C is concerned about the long-term use of DMPA.
Should Ms. C be worried?
Because of DMPA’s association with bone loss, the US Food and Drug Administration issued a black box warning in 2004 recommending that this method be used for more than 2 years only by women for whom other birth control methods are deemed inappropriate.17
The bone loss may be reversed. Evidence suggests that the bone loss is reversible, however, and the American College of Obstetricians and Gynecologists has stated that a potential fracture risk need not limit a woman’s use of DMPA to 2 years.18 A retrospective cohort review of 312,295 women in the United Kingdom did not find evidence of an increased risk of fracture with long-term use of DMPA.19 It is important to note, however, that because of declining estrogen levels, perimenopausal women have fewer years than their younger counterparts to recover bone density upon discontinuation of DMPA.20,21
THE BOTTOM LINE Because Ms. C has no perimenopausal symptoms, she may do well with LARC, which—like DMPA —would free her of the need to remember to take, apply, or insert a contraceptive regularly. It may help to point out that LARCs provide superior contraceptive efficacy compared with DMPA injections (99% vs 94%).3 Nonetheless, she and other women in their 40s who need ongoing contraception should not be discouraged from using DMPA if that is their preference.
CASE 4 › Alissa B: Breast cancer risk
Alissa B, 49, has polycystic ovaries and wonders if it is safe for her to continue her COC. She has been happy with the treatment for years because it gives her relief from hot flashes and regulates her cycles. Her 46-year-old sister was recently diagnosed with invasive breast cancer, however, and Ms. B is afraid that the hormones she takes put her at increased risk.
Should you recommend another method?
Breast cancer is an important concern for many women as they age. Although Ms. B’s family history increases her risk for developing breast cancer, a systematic review indicates that COCs do not add to this risk.22
Weak association between family history and OC use. The review included 10 observational studies and one meta-analysis that investigated the association between COC use and breast cancer in women with a family history of the disease. Only 2 fair-quality studies showed an association, one of which included women who had begun taking the pill before 1975, when formulations typically contained higher doses of estrogen than present-day preparations.22
Data from a recently published meta-analysis also indicate that there is no increased risk for breast cancer from COCs among women with BRCA 1 or BRCA 2 mutations. The summary RR for breast cancer in such patients was 1.13 (95% CI, 0.88-1.45), but OC users had a lower risk for ovarian cancer (summary RR=0.50; 95% CI, 0.33-0.75).23 Additionally, investigators found no association between specific currently used COC formulations and breast cancer.24
THE BOTTOM LINE Based on an independent review of the evidence, the CDC has given a family history of breast cancer a Category 1 rating. Thus, Ms. B can be reassured that she may safely continue taking her COC, which is unlikely to increase her breast cancer risk.
CORRESPONDENCE
Pelin Batur, MD, NCMP, CCD, Cleveland Clinic Independence Family Health Center, 5001 Rockside Road, IN30, Cleveland, OH 44131; [email protected].
1. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478-485.
2. Centers for Disease Control and Prevention (CDC). U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
3. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60.
4. Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
5. Committee on gynecologic practice. ACOG committee opinion number 540: Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
6. McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med. 2015;162:ITC1-15.
7. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
8. Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
9. Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the international active surveillance study of women taking oral contraceptives. Contraception. 2014;89:253-263.
10. Dinger J, Möhner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol. 2013;122:800-808.
11. Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93-100.
12. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;6:CD004425.
13. Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
14. Thorneycroft IH. Update on androgenicity. Am J Obstet Gynecol. 1999;180:288-294.
15. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
16. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389.
17. U.S. Food and Drug Administration. Safety: Depo-Provera (medroxyprogesterone acetate injectable suspension). U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm. Accessed April 20, 2015.
18. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123:1398-1402.
19. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121:593-600.
20. Ettinger B, Pressman A, Sklarin P, et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239-2243.
21. Reginster JY, Sarlet N, Deroisy R, et al. Minimal levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
22. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
23. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
24. Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342-350.
› Consider long-acting reversible contraception, such as an intrauterine device or an implant, as a first-line option for women who have mild or no symptoms of perimenopause. A
› Unless contraindicated, prescribe combination hormonal contraceptives for women in their 40s who desire them, as they are generally safe and effective in treating perimenopausal symptoms. A
› Use the Centers for Disease Control and Prevention’s evidence-based recommendations to guide your choice of contraceptive for perimenopausal patients based on individual medical history. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It is no secret that about half of all pregnancies in the United States are unintended, and that teens have the highest rate of unplanned pregnancy. What’s not so well known is that women in their 40s have the second highest rate.1
Optimal use of contraception throughout perimenopause is crucial, but finding the right method of birth control for this patient population can be a bit of a balancing act. Long-acting reversible contraceptives (LARCs), such as an intrauterine device or progestin-only implant, are preferred first-line contraceptive options when preventing pregnancy is the primary goal, given their increased efficacy and limited number of contraindications.2,3 However, women experiencing perimenopausal symptoms often need a combination hormonal contraceptive (CHC)—typically an estrogen-containing pill, a patch, or a vaginal ring—for relief of vasomotor symptoms and cycle control.
Women in their 40s should have access to a full array of options to help improve adherence. However, physicians may be reluctant to prescribe estrogen-containing products for patients who often have a more complex medical history than their younger counterparts, including increased risks for breast cancer, cardiovascular disease, and venous thromboembolism (VTE).
With this in mind, the Centers for Disease Control and Prevention (CDC) has identified medical conditions that may affect the use of the various types of contraceptives by perimenopausal women and issued evidence-based recommendations on the appropriateness of each method using a one-to-4 rating system (TABLE 1).2 To help you address the contraceptive needs of such patients, we review the key risk factors, CDC guidelines, and optimal choices in the 4 case studies that follow.
CASE 1 › Sara G: VTE risk
Sara G, a healthy 45-year-old, recently started dating again following her divorce. She wants to avoid pregnancy. She has no personal or family history of clotting disorders and does not smoke. However, she is obese (body mass index [BMI]=32 kg/m2), and her job as a visiting nurse requires her to spend most of the day in her car. Ms. G also has acne and wants an estrogen-containing contraceptive to help treat it.
If Ms. G were your patient, what would you offer her?
The risk for VTE increases substantially for women older than 40 years. In a recent cohort study, those ages 45 to 49 faced approximately twice the risk of women ages 25 to 29. However, the absolute risk for the older women was still low (4.7-5.3 per 10,000 woman-years).4 What’s more, the risk of VTE from the use of a CHC is substantially less than the risk associated with pregnancy and the postpartum period (TABLE 2).5
Obesity increases the risk. Women like Ms. G who are obese (BMI >30) have an increased risk for VTE associated with CHCs, but the CDC rates them as a Category 2 risk, even for obese women in their 40s—a determination that the advantages outweigh the risks.2
Progestin choice and estrogen dose matter. Combination oral contraceptives (COCs) that contain certain third-generation progestins (gestodene and desogestrel) may be more thrombophilic than those containing first- or second-generation progestins (TABLE 3).6 The relative risk (RR) for VTE with third-generation vs second-generation progestins is 1.3 (95% confidence interval [CI], 1.0-1.8).7 Formulations containing higher doses of estrogen are also more likely to be associated with VTE.7
Drospirenone is a newer progestin. Found in several COCs, drospirenone has antimineralocorticoid properties that help to minimize bloating and fluid retention but may also lead to a hypercoagulable state.5 Numerous studies have investigated the association between drospirenone and VTE risk, with conflicting results.8 Most recently, a large international prospective observational study involving more than 85,000 women showed no increased risk for VTE among women taking COCs with drospirenone compared with pills that do not contain this progestin.9
Non-oral CHCs, including the vaginal ring and the patch, offer the convenience of weekly or monthly use while providing similar benefits to COCs. Some fear that the continuous exposure to hormones associated with these methods may increase the risk for VTE, but evidence is mixed.
A large (N=1.6 million) Danish registry study published in 2012 demonstrated a 2-fold increased risk of VTE among vaginal ring users vs women taking COCs.4 But a multinational prospective cohort study of more than 33,000 women found no increased VTE risk in ring users,10 and a recent US database study involving more than 800,000 women reported nonsignificant VTE risk estimates for both the ring (RR=1.09; 95% CI, 0.55-2.16) and the patch (RR=1.35; 95% CI, 0.90-2.02) compared with COCs.11
THE BOTTOM LINE For Ms. G, the benefits of contraception likely outweigh any small increase in her absolute risk for VTE. To minimize her risk, however, select a pill that contains a low dose (20-35 mcg) of ethinyl estradiol (EE) combined with a progestin that has not been associated with an increased VTE risk. Because of their mechanism of action, most COCs will improve acne, regardless of the progestin in the formulation.12-14
CASE 2 › Stephanie T: CV risk
Stephanie T, 47, is in need of contraception and treatment for severe hot flashes. She has no significant past medical history, but she is obese (BMI =36), her blood pressure (BP) is 130/80 mm Hg, and her most recent labs reveal a fasting glucose of 115 and a hemoglobin A1c of 6.1%. Ms. T is concerned about arterial thromboembolic disease because of her family history: Her father had a myocardial infarction (MI) at age 56 and a maternal aunt had a stroke when she was 65.
What evidence should you consider?
Baseline arterial thromboembolic events are considerably more rare in premenopausal women than VTEs (13.2 MIs vs 24.2 thrombotic strokes per 100,000 woman-years).15 Thus, a small increased RR from a CHC is unlikely to have a significant clinical impact.
A systemic review and meta-analysis of studies between 1995 and 2012 showed that the odds ratio (OR) of ischemic stroke in users of COCs vs nonusers was 1.9 (95% CI, 1.24–2.91).16 This study included very few estrogen formulations with <35 mcg EE, however; even so, no increased risk of MI was found (OR=1.34; 95% CI, 0.87–2.08).16 A 15-year retrospective cohort study of 1.6 million Danish women showed that lowering the dose of EE to 20 mcg (from 30-40 mcg) significantly reduced the risk of arterial events.15 It is unclear whether the vaginal ring is associated with an increased RR of stroke compared with COCs because studies have had mixed results.10,15 There is no compelling evidence to suggest a difference in the risk of arterial events based on the type of progestin used in the COC.15
Hypertension is a key consideration. It is important to remember that perimenopausal women may have comorbid conditions that increase their risk of arterial thromboembolic events. CHCs should be used with caution in women with hypertension, even if BP is adequately controlled—a Category 3 recommendation from the CDC. In such patients, LARC or a progestin-only pill is preferred unless there is a compelling reason to use a CHC, such as acne, vasomotor symptoms, or hirsutism.2
CHCs are contraindicated for women with a BP ≥160/100 mm Hg and/or any manifestation of vascular disease (Category 4).2 Although progestin-only methods are often preferred for women with established vascular disease, depot medroxyprogesterone acetate (DMPA) is an exception (Category 3).2 DMPA is not a first-line choice for such patients because of its potential to cause weight gain and worsening lipids, glucose, and insulin metabolism. Women with hypertriglyceridemia should have follow-up testing of lipid levels after initiation of hormonal contraception, especially if it contains estrogen.
Diabetes is not an absolute contraindication. Many women with diabetes can safely use CHCs (Category 2). The exceptions: those who have vascular disease, nephropathy, retinopathy, or neuropathy (Category 4) or have had diabetes for >20 years and therefore have the potential for undiagnosed vascular disease.2 Generally, the use of insulin should not affect decisions regarding CHCs, and patients can be reassured that the hormones will not worsen their diabetes control.
When caring for women who have multiple risk factors for cardiovascular disease, it is important to exercise clinical judgment regarding the appropriateness of CHCs (Categories 3 and 4). Progestin-only methods have a more favorable risk profile for women at the highest risk and may provide ample relief of perimenopausal symptoms.2
THE BOTTOM LINE Ms. T may benefit from a CHC due to her severe hot flashes. She should be encouraged to adopt healthy lifestyle changes, including diet and exercise, to decrease her risk of arterial thromboembolism and VTE, but she has no contraindications to the use of a CHC at this time.
CASE 3 › Leslie C: Bone health
Leslie C, age 45, is happy with the contraceptive he has used for the past 3 years—DMPA injections every 3 months. She has no perimenopausal symptoms. However, her mother had an osteoporotic hip fracture at age 70 and Ms. C is concerned about the long-term use of DMPA.
Should Ms. C be worried?
Because of DMPA’s association with bone loss, the US Food and Drug Administration issued a black box warning in 2004 recommending that this method be used for more than 2 years only by women for whom other birth control methods are deemed inappropriate.17
The bone loss may be reversed. Evidence suggests that the bone loss is reversible, however, and the American College of Obstetricians and Gynecologists has stated that a potential fracture risk need not limit a woman’s use of DMPA to 2 years.18 A retrospective cohort review of 312,295 women in the United Kingdom did not find evidence of an increased risk of fracture with long-term use of DMPA.19 It is important to note, however, that because of declining estrogen levels, perimenopausal women have fewer years than their younger counterparts to recover bone density upon discontinuation of DMPA.20,21
THE BOTTOM LINE Because Ms. C has no perimenopausal symptoms, she may do well with LARC, which—like DMPA —would free her of the need to remember to take, apply, or insert a contraceptive regularly. It may help to point out that LARCs provide superior contraceptive efficacy compared with DMPA injections (99% vs 94%).3 Nonetheless, she and other women in their 40s who need ongoing contraception should not be discouraged from using DMPA if that is their preference.
CASE 4 › Alissa B: Breast cancer risk
Alissa B, 49, has polycystic ovaries and wonders if it is safe for her to continue her COC. She has been happy with the treatment for years because it gives her relief from hot flashes and regulates her cycles. Her 46-year-old sister was recently diagnosed with invasive breast cancer, however, and Ms. B is afraid that the hormones she takes put her at increased risk.
Should you recommend another method?
Breast cancer is an important concern for many women as they age. Although Ms. B’s family history increases her risk for developing breast cancer, a systematic review indicates that COCs do not add to this risk.22
Weak association between family history and OC use. The review included 10 observational studies and one meta-analysis that investigated the association between COC use and breast cancer in women with a family history of the disease. Only 2 fair-quality studies showed an association, one of which included women who had begun taking the pill before 1975, when formulations typically contained higher doses of estrogen than present-day preparations.22
Data from a recently published meta-analysis also indicate that there is no increased risk for breast cancer from COCs among women with BRCA 1 or BRCA 2 mutations. The summary RR for breast cancer in such patients was 1.13 (95% CI, 0.88-1.45), but OC users had a lower risk for ovarian cancer (summary RR=0.50; 95% CI, 0.33-0.75).23 Additionally, investigators found no association between specific currently used COC formulations and breast cancer.24
THE BOTTOM LINE Based on an independent review of the evidence, the CDC has given a family history of breast cancer a Category 1 rating. Thus, Ms. B can be reassured that she may safely continue taking her COC, which is unlikely to increase her breast cancer risk.
CORRESPONDENCE
Pelin Batur, MD, NCMP, CCD, Cleveland Clinic Independence Family Health Center, 5001 Rockside Road, IN30, Cleveland, OH 44131; [email protected].
› Consider long-acting reversible contraception, such as an intrauterine device or an implant, as a first-line option for women who have mild or no symptoms of perimenopause. A
› Unless contraindicated, prescribe combination hormonal contraceptives for women in their 40s who desire them, as they are generally safe and effective in treating perimenopausal symptoms. A
› Use the Centers for Disease Control and Prevention’s evidence-based recommendations to guide your choice of contraceptive for perimenopausal patients based on individual medical history. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It is no secret that about half of all pregnancies in the United States are unintended, and that teens have the highest rate of unplanned pregnancy. What’s not so well known is that women in their 40s have the second highest rate.1
Optimal use of contraception throughout perimenopause is crucial, but finding the right method of birth control for this patient population can be a bit of a balancing act. Long-acting reversible contraceptives (LARCs), such as an intrauterine device or progestin-only implant, are preferred first-line contraceptive options when preventing pregnancy is the primary goal, given their increased efficacy and limited number of contraindications.2,3 However, women experiencing perimenopausal symptoms often need a combination hormonal contraceptive (CHC)—typically an estrogen-containing pill, a patch, or a vaginal ring—for relief of vasomotor symptoms and cycle control.
Women in their 40s should have access to a full array of options to help improve adherence. However, physicians may be reluctant to prescribe estrogen-containing products for patients who often have a more complex medical history than their younger counterparts, including increased risks for breast cancer, cardiovascular disease, and venous thromboembolism (VTE).
With this in mind, the Centers for Disease Control and Prevention (CDC) has identified medical conditions that may affect the use of the various types of contraceptives by perimenopausal women and issued evidence-based recommendations on the appropriateness of each method using a one-to-4 rating system (TABLE 1).2 To help you address the contraceptive needs of such patients, we review the key risk factors, CDC guidelines, and optimal choices in the 4 case studies that follow.
CASE 1 › Sara G: VTE risk
Sara G, a healthy 45-year-old, recently started dating again following her divorce. She wants to avoid pregnancy. She has no personal or family history of clotting disorders and does not smoke. However, she is obese (body mass index [BMI]=32 kg/m2), and her job as a visiting nurse requires her to spend most of the day in her car. Ms. G also has acne and wants an estrogen-containing contraceptive to help treat it.
If Ms. G were your patient, what would you offer her?
The risk for VTE increases substantially for women older than 40 years. In a recent cohort study, those ages 45 to 49 faced approximately twice the risk of women ages 25 to 29. However, the absolute risk for the older women was still low (4.7-5.3 per 10,000 woman-years).4 What’s more, the risk of VTE from the use of a CHC is substantially less than the risk associated with pregnancy and the postpartum period (TABLE 2).5
Obesity increases the risk. Women like Ms. G who are obese (BMI >30) have an increased risk for VTE associated with CHCs, but the CDC rates them as a Category 2 risk, even for obese women in their 40s—a determination that the advantages outweigh the risks.2
Progestin choice and estrogen dose matter. Combination oral contraceptives (COCs) that contain certain third-generation progestins (gestodene and desogestrel) may be more thrombophilic than those containing first- or second-generation progestins (TABLE 3).6 The relative risk (RR) for VTE with third-generation vs second-generation progestins is 1.3 (95% confidence interval [CI], 1.0-1.8).7 Formulations containing higher doses of estrogen are also more likely to be associated with VTE.7
Drospirenone is a newer progestin. Found in several COCs, drospirenone has antimineralocorticoid properties that help to minimize bloating and fluid retention but may also lead to a hypercoagulable state.5 Numerous studies have investigated the association between drospirenone and VTE risk, with conflicting results.8 Most recently, a large international prospective observational study involving more than 85,000 women showed no increased risk for VTE among women taking COCs with drospirenone compared with pills that do not contain this progestin.9
Non-oral CHCs, including the vaginal ring and the patch, offer the convenience of weekly or monthly use while providing similar benefits to COCs. Some fear that the continuous exposure to hormones associated with these methods may increase the risk for VTE, but evidence is mixed.
A large (N=1.6 million) Danish registry study published in 2012 demonstrated a 2-fold increased risk of VTE among vaginal ring users vs women taking COCs.4 But a multinational prospective cohort study of more than 33,000 women found no increased VTE risk in ring users,10 and a recent US database study involving more than 800,000 women reported nonsignificant VTE risk estimates for both the ring (RR=1.09; 95% CI, 0.55-2.16) and the patch (RR=1.35; 95% CI, 0.90-2.02) compared with COCs.11
THE BOTTOM LINE For Ms. G, the benefits of contraception likely outweigh any small increase in her absolute risk for VTE. To minimize her risk, however, select a pill that contains a low dose (20-35 mcg) of ethinyl estradiol (EE) combined with a progestin that has not been associated with an increased VTE risk. Because of their mechanism of action, most COCs will improve acne, regardless of the progestin in the formulation.12-14
CASE 2 › Stephanie T: CV risk
Stephanie T, 47, is in need of contraception and treatment for severe hot flashes. She has no significant past medical history, but she is obese (BMI =36), her blood pressure (BP) is 130/80 mm Hg, and her most recent labs reveal a fasting glucose of 115 and a hemoglobin A1c of 6.1%. Ms. T is concerned about arterial thromboembolic disease because of her family history: Her father had a myocardial infarction (MI) at age 56 and a maternal aunt had a stroke when she was 65.
What evidence should you consider?
Baseline arterial thromboembolic events are considerably more rare in premenopausal women than VTEs (13.2 MIs vs 24.2 thrombotic strokes per 100,000 woman-years).15 Thus, a small increased RR from a CHC is unlikely to have a significant clinical impact.
A systemic review and meta-analysis of studies between 1995 and 2012 showed that the odds ratio (OR) of ischemic stroke in users of COCs vs nonusers was 1.9 (95% CI, 1.24–2.91).16 This study included very few estrogen formulations with <35 mcg EE, however; even so, no increased risk of MI was found (OR=1.34; 95% CI, 0.87–2.08).16 A 15-year retrospective cohort study of 1.6 million Danish women showed that lowering the dose of EE to 20 mcg (from 30-40 mcg) significantly reduced the risk of arterial events.15 It is unclear whether the vaginal ring is associated with an increased RR of stroke compared with COCs because studies have had mixed results.10,15 There is no compelling evidence to suggest a difference in the risk of arterial events based on the type of progestin used in the COC.15
Hypertension is a key consideration. It is important to remember that perimenopausal women may have comorbid conditions that increase their risk of arterial thromboembolic events. CHCs should be used with caution in women with hypertension, even if BP is adequately controlled—a Category 3 recommendation from the CDC. In such patients, LARC or a progestin-only pill is preferred unless there is a compelling reason to use a CHC, such as acne, vasomotor symptoms, or hirsutism.2
CHCs are contraindicated for women with a BP ≥160/100 mm Hg and/or any manifestation of vascular disease (Category 4).2 Although progestin-only methods are often preferred for women with established vascular disease, depot medroxyprogesterone acetate (DMPA) is an exception (Category 3).2 DMPA is not a first-line choice for such patients because of its potential to cause weight gain and worsening lipids, glucose, and insulin metabolism. Women with hypertriglyceridemia should have follow-up testing of lipid levels after initiation of hormonal contraception, especially if it contains estrogen.
Diabetes is not an absolute contraindication. Many women with diabetes can safely use CHCs (Category 2). The exceptions: those who have vascular disease, nephropathy, retinopathy, or neuropathy (Category 4) or have had diabetes for >20 years and therefore have the potential for undiagnosed vascular disease.2 Generally, the use of insulin should not affect decisions regarding CHCs, and patients can be reassured that the hormones will not worsen their diabetes control.
When caring for women who have multiple risk factors for cardiovascular disease, it is important to exercise clinical judgment regarding the appropriateness of CHCs (Categories 3 and 4). Progestin-only methods have a more favorable risk profile for women at the highest risk and may provide ample relief of perimenopausal symptoms.2
THE BOTTOM LINE Ms. T may benefit from a CHC due to her severe hot flashes. She should be encouraged to adopt healthy lifestyle changes, including diet and exercise, to decrease her risk of arterial thromboembolism and VTE, but she has no contraindications to the use of a CHC at this time.
CASE 3 › Leslie C: Bone health
Leslie C, age 45, is happy with the contraceptive he has used for the past 3 years—DMPA injections every 3 months. She has no perimenopausal symptoms. However, her mother had an osteoporotic hip fracture at age 70 and Ms. C is concerned about the long-term use of DMPA.
Should Ms. C be worried?
Because of DMPA’s association with bone loss, the US Food and Drug Administration issued a black box warning in 2004 recommending that this method be used for more than 2 years only by women for whom other birth control methods are deemed inappropriate.17
The bone loss may be reversed. Evidence suggests that the bone loss is reversible, however, and the American College of Obstetricians and Gynecologists has stated that a potential fracture risk need not limit a woman’s use of DMPA to 2 years.18 A retrospective cohort review of 312,295 women in the United Kingdom did not find evidence of an increased risk of fracture with long-term use of DMPA.19 It is important to note, however, that because of declining estrogen levels, perimenopausal women have fewer years than their younger counterparts to recover bone density upon discontinuation of DMPA.20,21
THE BOTTOM LINE Because Ms. C has no perimenopausal symptoms, she may do well with LARC, which—like DMPA —would free her of the need to remember to take, apply, or insert a contraceptive regularly. It may help to point out that LARCs provide superior contraceptive efficacy compared with DMPA injections (99% vs 94%).3 Nonetheless, she and other women in their 40s who need ongoing contraception should not be discouraged from using DMPA if that is their preference.
CASE 4 › Alissa B: Breast cancer risk
Alissa B, 49, has polycystic ovaries and wonders if it is safe for her to continue her COC. She has been happy with the treatment for years because it gives her relief from hot flashes and regulates her cycles. Her 46-year-old sister was recently diagnosed with invasive breast cancer, however, and Ms. B is afraid that the hormones she takes put her at increased risk.
Should you recommend another method?
Breast cancer is an important concern for many women as they age. Although Ms. B’s family history increases her risk for developing breast cancer, a systematic review indicates that COCs do not add to this risk.22
Weak association between family history and OC use. The review included 10 observational studies and one meta-analysis that investigated the association between COC use and breast cancer in women with a family history of the disease. Only 2 fair-quality studies showed an association, one of which included women who had begun taking the pill before 1975, when formulations typically contained higher doses of estrogen than present-day preparations.22
Data from a recently published meta-analysis also indicate that there is no increased risk for breast cancer from COCs among women with BRCA 1 or BRCA 2 mutations. The summary RR for breast cancer in such patients was 1.13 (95% CI, 0.88-1.45), but OC users had a lower risk for ovarian cancer (summary RR=0.50; 95% CI, 0.33-0.75).23 Additionally, investigators found no association between specific currently used COC formulations and breast cancer.24
THE BOTTOM LINE Based on an independent review of the evidence, the CDC has given a family history of breast cancer a Category 1 rating. Thus, Ms. B can be reassured that she may safely continue taking her COC, which is unlikely to increase her breast cancer risk.
CORRESPONDENCE
Pelin Batur, MD, NCMP, CCD, Cleveland Clinic Independence Family Health Center, 5001 Rockside Road, IN30, Cleveland, OH 44131; [email protected].
1. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478-485.
2. Centers for Disease Control and Prevention (CDC). U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
3. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60.
4. Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
5. Committee on gynecologic practice. ACOG committee opinion number 540: Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
6. McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med. 2015;162:ITC1-15.
7. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
8. Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
9. Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the international active surveillance study of women taking oral contraceptives. Contraception. 2014;89:253-263.
10. Dinger J, Möhner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol. 2013;122:800-808.
11. Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93-100.
12. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;6:CD004425.
13. Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
14. Thorneycroft IH. Update on androgenicity. Am J Obstet Gynecol. 1999;180:288-294.
15. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
16. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389.
17. U.S. Food and Drug Administration. Safety: Depo-Provera (medroxyprogesterone acetate injectable suspension). U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm. Accessed April 20, 2015.
18. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123:1398-1402.
19. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121:593-600.
20. Ettinger B, Pressman A, Sklarin P, et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239-2243.
21. Reginster JY, Sarlet N, Deroisy R, et al. Minimal levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
22. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
23. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
24. Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342-350.
1. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478-485.
2. Centers for Disease Control and Prevention (CDC). U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
3. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep. 2013;62:1-60.
4. Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
5. Committee on gynecologic practice. ACOG committee opinion number 540: Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
6. McNamara M, Batur P, DeSapri KT. In the clinic. Perimenopause. Ann Intern Med. 2015;162:ITC1-15.
7. de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
8. Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
9. Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the international active surveillance study of women taking oral contraceptives. Contraception. 2014;89:253-263.
10. Dinger J, Möhner S, Heinemann K. Cardiovascular risk associated with the use of an etonogestrel-containing vaginal ring. Obstet Gynecol. 2013;122:800-808.
11. Sidney S, Cheetham TC, Connell FA, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception. 2013;87:93-100.
12. Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;6:CD004425.
13. Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
14. Thorneycroft IH. Update on androgenicity. Am J Obstet Gynecol. 1999;180:288-294.
15. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
16. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol. 2013;122:380-389.
17. U.S. Food and Drug Administration. Safety: Depo-Provera (medroxyprogesterone acetate injectable suspension). U.S. Food and Drug Administration Web site. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154784.htm. Accessed April 20, 2015.
18. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123:1398-1402.
19. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121:593-600.
20. Ettinger B, Pressman A, Sklarin P, et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239-2243.
21. Reginster JY, Sarlet N, Deroisy R, et al. Minimal levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
22. Gaffield ME, Culwell KR, Ravi A. Oral contraceptives and family history of breast cancer. Contraception. 2009;80:372-380.
23. Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46:2275-2284.
24. Marchbanks PA, Curtis KM, Mandel MG, et al. Oral contraceptive formulation and risk of breast cancer. Contraception. 2012;85:342-350.
COPD: Optimizing treatment
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
What you can do to improve adult immunization rates
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
› Recommend immunization to patients routinely. Most adults believe vaccines are important and are likely to get them if recommended by their health care professionals. C
› Consider implementing standing orders that authorize nurses, pharmacists, or other trained health care personnel to assess a patient’s immunization status and administer vaccinations according to a protocol. C
› Explore the use of Web-based patient portals or other new-media communication formats to engage patients. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaccines have been proven effective in preventing disease and are one of the most cost-effective and successful public health initiatives of the 20th century. Nevertheless, adult vaccination rates in the United States for vaccine-preventable diseases are low for most routinely recommended vaccines.1 In 2013 alone, there were an estimated 3700 deaths in the United States (95% of which were adults) from pneumococcal infections—a vaccine-preventable disorder.2
Consider the threat posed by the flu. Annually, most people who die of influenza and its complications are adults, with estimates ranging from a low of 3000 to a high of 49,000 based on Centers for Disease Control and Prevention (CDC) data from the 1976-1977 flu season to the 2006-2007 season.3 Vaccination during the 2013-2014 season resulted in an estimated 7.2 million fewer cases of influenza, 90,000 fewer hospitalizations, and 3.1 million fewer medically attended cases than would have been expected without vaccination.4 If vaccination levels had reached the Healthy People 2020 target of 70%, an additional 5.9 million illnesses, 2.3 million medically attended illnesses, and 42,000 hospitalizations might have been averted.4
How are we doing with other vaccines? Based on the 2013 National Health Interview Survey, the CDC assessed vaccination coverage among adults ages ≥19 years for selected vaccines: pneumococcal vaccine, tetanus toxoid-containing vaccines (tetanus and diphtheria vaccine [Td] or tetanus and diphtheria with acellular pertussis vaccine [Tdap]), and vaccines for hepatitis A, hepatitis B, herpes zoster, and human papillomavirus (HPV). (With the exception of influenza vaccination, which is recommended annually for all adults, other vaccinations are directed at specific populations based on age, health conditions, behavioral risk factors, occupation, or travel conditions.)
Overall, coverage rates for hepatitis A and B, pneumococcal, Td, and human papillomavirus (HPV) for all adults did not improve from 2012 to 2013; rates increased only modestly for Tdap among adults ≥19 years, for herpes zoster among adults ≥60 years, and for HPV among men ages 19 to 26. Furthermore, racial and ethnic gaps in coverage are seen in all vaccines, and these gaps widened since 2012 for Tdap, herpes zoster, and HPV vaccination.1
Commonly cited barriers to improved vaccine uptake in adults include lack of regular assessment of vaccine status; lack of physician and other health care provider knowledge on current vaccine recommendations; cost; insufficient stocking of some vaccines; financial disincentives for vaccination in the primary care setting; limited use of electronic records, tools, and immunization registries; missed opportunities; and patient hesitancy and vaccine refusal.5
Removing barriers to immunization. Several recommendations on ways to improve adult vaccination rates are made by many federal organizations as well as by The Community Preventive Services Task Force (Task Force), an independent, nonfederal, unpaid panel of public health and prevention experts. The Task Force—which makes recommendations based on systematic reviews of the evidence of effectiveness, the applicability of the evidence, economic evaluations, and barriers to implementation of interventions6—advocates a 3-pronged approach to improve adult vaccination rates: 1) enhance access to vaccination services; 2) increase community demand for vaccinations; and 3) incorporate physician- or system-based interventions into practice.7
The CDC and other groups such as the National Vaccine Advisory Committee (NVAC) recommend that every routine adult office visit include a vaccination needs assessment, recommendation, and offer of vaccination.8 Additionally, the Task Force recommends 3 means of enhancing adult access to vaccination services: make home visits, reduce patient costs, and offer vaccination programs in the community.7
This article describes a number of simple steps physicians can take to increase the likelihood that adults will get their vaccines and reviews the literature on using new media such as smartphones and other Internet-based tools to improve immunization coverage.9
Increasing community demands for vaccinations
Physicians and other healthcare providers can increase community demand for vaccinations by improving their own knowledge on the subject, recommending vaccination to patients, and increasing their community and political involvement to strengthen or change laws to better support immunization uptake.
To increase awareness and education, keep abreast of the Advisory Committee on Immunization Practices (ACIP) recommendations and guidelines, which are updated annually and reported on in this journal’s Practice Alert column. Consider taking advantage of free immunization apps that are available from the CDC (“CDC Vaccine Schedules” http://www.cdc.gov/vaccines/schedules/hcp/schedule-app.html), the Society of Teachers of Family Medicine (STFM; “Shots Immunizations” http://www.immunizationed.org/Shots-Mobile-App), and the American College of Physicians (“ACP Immunization Advisor” http://immunization.acponline.org/app/).
Take steps to put guidelines into practice. Despite wide promulgation, clinical practice guidelines alone have had limited effect on changing physician behavior and improving patient outcomes. Interactive techniques are more effective than guidelines and didactic presentations alone at changing physician care and patient outcomes. Such techniques include audit/feedback (the reporting of an individual clinician’s vaccination rates compared with desired or target rates, for example), academic detailing/outreach, and reminders by way of electronic or other alerts.10,11
Promote immunization to patients. Physicians are highly influential in determining a patient’s decision to vaccinate, and it is well documented that a strong recommendation about the importance of immunizations makes a difference to patients.12,13
What you say and how you say it matters. A halfhearted recommendation for vaccination may result in the patient remaining unvaccinated.14 For example, “If you want, you can get your pneumonia shot today” is much less persuasive than, “I recommend you get your pneumonia vaccine today to prevent a potentially serious disease that affects thousands of adults each year.” Most adults believe that vaccines are important and are likely to get them if recommended by their health care professionals.15
The CDC recommends that physicians encourage patients to make an informed decision about vaccination by sharing critical information highlighting the importance of vaccinations and reminding patients what vaccines protect against while addressing their concerns (www.cdc.gov/vaccines/adultstandards). Free educational materials for patients can be found at www.cdc.gov/vaccines/AdultPatientEd.
Draw on community resources. Laws and policies that require vaccinations as a prerequisite for attending childcare, school, or college increase coverage. Community and faith-based organizations are likely to play an important role in reducing racial and ethnic disparities in adult immunizations because they can deliver education that is culturally sensitive and tailored to specific subpopulations.16,17 Physicians and other health care providers can get involved with community and faith-based groups and local and federal legislative efforts to improve immunization rates.
Consider implementing these system-based interventions
The following 6 system-based interventions can help improve adult immunization rates:
1. Develop a practice team. The practice team, based on the Patient-Centered Medical Home (PCMH), includes physicians, midlevel providers, nurses, medical assistants, pharmacists, social workers, and other staff. The PCMH team model can facilitate a shift of responsibilities among individuals to better orient the practice toward patients’ health and preventive services.18,19 While physicians have traditionally held all of the responsibility for patient care, including screening for disease and prevention, shifting the responsibility of vaccine screening to nurses or medical assistants can free up time for longer physician/patient interactions.18
The creation of a practice champion within the PCMH team—a physician, midlevel provider, or nurse—to oversee quality improvement for vaccine rates and work to generate support and cooperation from coworkers has also been shown to improve vaccination rates.20 The vaccine champion should keep abreast of new vaccine recommendations and relay that information to the practice through regular staff meetings, announcements, and office postings. The champion can also supervise pre-visit planning for immunizations.19
2. Use electronic immunization information systems (IIS). All states except New Hampshire have an IIS.21 Accurate tracking of adult immunizations in a registry provides a complete record and is essential to improving adult immunization rates,22 as does the use of chart notes, computerized alerts, checklists, and other tools that remind health care providers when patients are due for vaccinations.18 NVAC recommends that all physicians use their state IIS and create a process in their practice to include its use.
3. Incorporate physician feedback. Many health care systems and payers are using benchmarking and incentives to provide physician feedback on vaccination performance.23 Using achievable benchmarks enhances the effectiveness of physician performance feedback.24 The Task Force conducted a systematic review of the evidence on the effectiveness of health care provider assessment and feedback for increasing coverage rates and found that this strategy remains an effective means to increase vaccination rates.25
4. Use reminders/alerts. Even though you may intend to routinely recommend immunizations, remembering to do so at the time of each visit can be difficult when there are so many other issues to address. Reminders at the time of the visit can help. Some electronic records have reminder prompts, or “best practice alerts” (BPAs), programmed into their systems.26 These BPAs will prompt for needed immunizations whether the patient is being seen for a well, acute, or routine follow-up visit. These reminder/recall activities can be greatly simplified by participation in a population-based IIS.
Practices that don’t have an electronic health record can still improve vaccination rates by conveying the reminder with a brightly colored paper form attached to the front of a patient’s chart during the check-in process. One recent study showed that this approach increased rates of influenza vaccination in an urban practice by 12 percentage points.27
Furthermore, simply reminding patients to vaccinate increases the vaccination rate.28 Patient reminder/recall systems using telephone calls or mailings (phone calls are more effective than mailings) improve both childhood and adult vaccinations in all medical settings. More intensive systems using multiple reminders appear to be more effective than single reminders, and while costly, the benefits of increasing preventive visits/services and vaccine uptake help offset this cost.28
5. Implement standing orders. Standing orders—which allow nurses and other appropriately trained health care personnel to assess immunization status and administer vaccinations according to protocol—help improve immunization rates.29 ACIP advises that standing order programs be used in long-term care facilities under the supervision of a medical director to ensure the administration of recommended vaccinations for adults, and in inpatient and outpatient facilities. Because of the societal burden of influenza and pneumococcal disease, implementation of standing orders programs to improve adult vaccination coverage for these diseases is considered a national public health priority.30
6. Develop an encouraging communication style. Studies show that how one communicates with patients is just as important as what one communicates. Certain communication styles and techniques may be more or less effective when discussing vaccination needs with some patients, especially those with vaccine hesitancy or low confidence in vaccine safety or effectiveness. For example, styles that are “directing” are usually unhelpful in addressing concerns about vaccination. These styles typically use information and persuasion to achieve change and may be perceived as confrontational. This approach can lead to cues being missed, jargon being used, and vaccine safety being overstated.
Styles shown to be helpful are those that elicit patient concerns, ask permission to discuss, acknowledge/listen/empathize, determine readiness to change, inform about benefits and risks, and give appropriate resources. These helpful forms of communication are more of a “May I help you?” style vs a “This is what you should do” style of communication.31
Assure patients that recommendations are based on the best interest of their health and on the best available science. Listen to a patient’s concerns and acknowledge them in a nonconfrontational manner, allowing patients to express their concerns and thereby increase their willingness to listen.32 Saying that there is “absolutely no need to worry—vaccines are safe and you are silly not to get yours” is not as effective as saying, “What are your concerns regarding vaccines? Let’s talk about them.”
For the vaccine-hesitant group, building trust is essential through a respectful, nonjudgmental approach that aims to elicit and address specific concerns. For those who refuse vaccines, keep the consultation brief, keep the door open for further discussion, and provide appropriate resources if the patient wants them.33
Increase use of new media
Mass communication through smartphones and other Internet-based tools such as Facebook and Twitter brings a new dimension to health care, allowing patients and health professionals to communicate about health issues and possibly improve health outcomes.34 The number of people using social media increased by almost 570% worldwide between 2000 and 2012 and surpassed 2.75 billion in 2013.35
Sixty-one percent of adults in the United States look online for health information.36 In a survey conducted in September 2014, the Pew Research Center found that Facebook is the most popular social media site in the United States. Seventy-one percent of online-knowledgeable adults use Facebook, and multiplatform use is on the rise: 52% of adult Internet users now use 2 or more social media sites, a significant increase from 2013, when it stood at 42%. (Other platforms such as Twitter, Instagram, Pinterest, and LinkedIn saw significant increases over the past year in the proportion of online adults who use them).37
Health information provided by social media can answer medical questions and concerns and enhance health promotion and education.35 A recent review of 98 research studies provided evidence that social media can create a space to share, comment, and discuss health information.34 Compared with traditional communication methods, the widespread availability of social media makes health information more accessible, broadening access to various population groups, regardless of age, education, race, ethnicity, and locale.
New media platforms are proving effective. The first systematic assessment of available evidence on the use of new media to increase vaccine uptake and immunization coverage (a review of 7 randomized controlled trials [RCTs], 5 non-RCTs, 3 cross-sectional studies, one case-control study and 3 operational research studies published between 2000-2013) found that text messaging, accessing immunization campaign Web sites, using patient-held Web-based portals, computerized reminders, and standing orders increased immunization coverage rates.35 However, evidence was insufficient in this regard on the value of social networks, email communication, and smartphone applications.
One RCT showed that having access to a personalized Web-based portal where patients could manage health records as well as interact with both health care providers and other members of the community through social forums and messaging tools increased influenza vaccination rates.35
CORRESPONDENCE
Pamela G. Rockwell, DO, Department of Family Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, P.O. Box 431, Ann Arbor, MI 48106-0795; [email protected].
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
1. Williams WW, Lu PJ, O’Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95-102.
2. Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report, emerging infections program network, Streptococcus pneumoniae, 2013. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Accessed August 20, 2015.
3. Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057-1062.
4. Reed C, Kim IK, Singleton JA, et al; Centers for Disease Control and Prevention (CDC). Estimated influenza illnesses and hospitalizations averted by vaccination--United States, 2013-14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:1151-1154.
5. Kimmel SR, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
6. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based Guide to Community Preventive Services—methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
7. The Guide to Community Preventive Services. Increasing appropriate vaccination. The Community Guide Web site. Available at: http://www.thecommunityguide.org/vaccines/index.html. Accessed August 20, 2015.
8. National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129:115-123.
9. Househ M. The use of social media in healthcare: organizational, clinical, and patient perspectives. Stud Health Technol Inform. 2013;183:244-248.
10. Bloom BS. Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380-385.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
12. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
13. Zimmerman RK, Santibanez TA, Janosky JE, et al. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am J Med. 2003;114:31-38.
14. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed August 20, 2015.
15. National Foundation for Infectious Diseases. Survey: adults do not recognize infectious disease risks. National Foundation for Infectious Diseases Web site. Available at: http://www.adultvaccination.org/newsroom/events/2009-vaccination-news-conference/NFID-Survey-Fact-Sheet.pdf. Accessed July 7, 2015.
16. Wang E, Clymer J, Davis-Hayes C, et al. Nonmedical exemptions from school immunization requirements: a systematic review. Am J Public Health. 2014;104:e62-e84.
17. National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 14, 2011. Public Health Rep. 2012;127:1-42.
18. Gannon M, Qaseem A, Snooks Q, et al. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102:e46-e52.
19. Bottino CJ, Cox JE, Kahlon PS, et al. Improving immunization rates in a hospital-based primary care practice. Pediatrics. 2014;133:e1047-e1054.
20. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
21. Centers for Disease Control and Prevention (CDC). Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005-1008.
22. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
23. Kerr EA, McGlynn EA, Adams J, et al. Profiling the quality of care in twelve communities: results from the CQI study. Health Aff (Millwood). 2004;23:247-256.
24. Kiefe CI, Allison JJ, Williams OD, et al. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285:2871-2879.
25. National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64.
26. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
27. Pierson RC, Malone AM, Haas DM. Increasing influenza vaccination rates in a busy urban clinic. J Nat Sci. 2015;1.
28. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;CD003941.
29. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:92-96.
30. McKibben LJ, Stange PV, Sneller VP, et al; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49:15-16.
31. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154.
32. Kimmel SR, Wolfe RM. Communicating the benefits and risks of vaccines. J Fam Pract. 2005;54:S51-S57.
33. Danchin M, Nolan T. A positive approach to parents with concerns about vaccination for the family physician. Aust Fam Physician. 2014;43:690-694.
34. Moorhead SA, Hazlett DE, Harrison L, et al. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15:e85.
35. Odone A, Ferrari A, Spagnoli F, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11:72-82.
36. Pew Research Center. Fox S. The Social Life of Health Information, 2011. Pew Research Center Web site. Available at: http://www.pewinternet.org/2011/05/12/the-social-life-of-health-information-2011/. Accessed August 20, 2015.
37. Pew Research Center. Duggan M, Ellison NB, Lampe C, et al. Social Media Update 2014. Pew Research Center Web site. Available at: http://www.pewinternet.org/2015/01/09/social-media-update-2014/. Accessed August 20, 2015.
Drug-induced liver injury: Diagnosing (and treating) it early
› If you suspect your patient may have drug-induced liver injury (DILI), take a careful medication history, assess for risk factors, and investigate other possible causes. B
› Immediately stop any drugs you suspect are causing DILI, especially when the patient’s liver enzymes are rapidly increasing or there is evidence of acute liver failure. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James A, age 68, presents to his family physician (FP) with anorexia, nausea, and vague upper abdominal pain that he’s had for 2 weeks. Mr. A has diabetes and hypertension, both of which are well controlled by medications. He also consumes more than 4 alcoholic beverages daily.
On examination, the FP notes icterus and tenderness in the right hypochondrium. Liver function testing reveals elevated liver enzyme levels: aspartate aminotransferase (AST), 864 IU/L (normal range: 10-40 IU/L); alanine aminotransferase (ALT), 1012 IU/L (normal range: 7-56 IU/L); serum bilirubin, 4.8 mg/dL (normal range: 0.3-1.9 mg/dL); and alkaline phosphatase (ALP), 200 IU/L (normal range: 44-147 IU/L). Mr. A’s coagulation profile is slightly abnormal. He is provisionally diagnosed with acute hepatitis. The FP sends blood samples to the lab to assess for viral markers, and starts symptomatic management.
If Mr. A were your patient, how would you proceed?
In the United States, drug-induced liver injury (DILI) is the most common cause of acute liver failure.1,2 It can occur due to ingestion of any therapeutic drug, herbal product, or xenobiotic. Further complicating matters is the fact that it has an unpredictable and heterogeneous course, ranging from an asymptomatic rise in liver enzymes to acute liver failure. This article describes the risk factors, common causative agents, tools for early diagnosis, and effective management of DILI.
Two types of risk factors for DILI
Risk factors for DILI can be classified as drug-related (eg, dose, concomitant medications, polypharmacy) or host-related (eg, age, gender, alcohol intake, concomitant infections).3-5
Drug-related factors. Hundreds of agents can lead to liver injury. In fact, the US National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases have created LiverTox (http://www.livertox.nih.gov/), an online database that provides detailed information on more than 600 such agents.6 Antibiotics are the most common cause of DILI, followed by neuropsychiatric drugs, immunomodulatory agents, antihypertensives, analgesics, antineoplastic drugs, and lipid-lowering agents.2
Among antibiotics, the specific medication most often responsible for DILI varies by geographical region. Amoxicillin/clavulanic acid is the most common causative antibiotic in the United States, whereas anti-tuberculosis agents such as isoniazid, rifampin, and pyrazinamide are the most common causative drugs in developing countries such as India, where the prevalence of tuberculosis is still high.7,8 Herbal and dietary supplements are emerging as an important cause of DILI.5,9,10
The use of multiple drugs further increases the risk of developing DILI.10 Drugs with a recommended daily dose of <50 mg are rarely associated with DILI.11
Host-related factors. Vulnerability to DILI is influenced by a patient’s age and sex.3,12 Very young and very old patients have an increased risk of developing DILI, and a patient’s age may make him or her particularly susceptible to the effects of certain medications.3,12,13 For example, children are more susceptible to DILI as a result of taking valproate or aspirin, whereas older patients are more likely to experience DILI brought on by amoxicillin/clavulanic acid.13 The pattern of liver injury also varies by age. Younger patients present most commonly with a hepatocellular pattern of injury, whereas older patients mostly present with a cholestatic pattern of liver injury.3
Some studies have found that women have a greater risk of developing DILI than men.13 The presence of chronic liver diseases, alcoholism, and nonalcoholic fatty liver disease (NAFLD) increase the risk of developing DILI.14 Diabetes is an independent risk factor for DILI.3
Clinical presentation of DILI varies widely
Some degree of liver injury may occur in any patient who ingests a drug that is metabolized in the liver. The clinical presentation of a patient with DILI can vary from an asymptomatic rise in liver enzymes to acute liver failure. Unexplained transaminitis should raise the possibility of DILI, especially when the patient has started a new drug in the preceding 3 months. However, in most patients, an asymptomatic rise in liver enzymes is due to hepatic adaptation or tolerance. In such cases, liver enzyme levels tend to normalize even if the patient continues to take the drug in the same dose.15
Apart from nonspecific symptoms such as anorexia, nausea, and vomiting, a patient with DILI may exhibit right upper quadrant pain, skin rash, or itching. A patient with severe DILI might exhibit jaundice, ascites, or encephalopathy.15
A stepwise approach to evaluation
DILI is a diagnosis of exclusion.16 Guidelines from the American College of Gastroenterology (ACG) recommend a stepwise approach to evaluating a patient you suspect may have DILI (TABLE).16 First, take a detailed history regarding the onset of symptoms, time latency, and use of hepatotoxic and other drugs (dosage and duration of use). Also ask the patient about his or her use of herbal products, dietary supplements, and alcohol. Check the patient’s history for the presence of other liver diseases such as NAFLD.
Next, make sure initial laboratory testing includes liver function tests and an eosinophil count. In order to classify the pattern of liver injury as hepatocellular, cholestatic, or mixed, you’ll need to calculate the patient’s R value (ALGORITHM16-18). This value is calculated by dividing the patient’s ALT level by the ALP, using the upper limit of the normal range (ULN) as follows: R = (ALT value ÷ ALT ULN) ÷ (ALP value ÷ ALP ULN).17 A hepatocellular pattern of liver injury is indicated by an R value >5, a cholestatic pattern is an R value <2, and a mixed pattern is suggested by an R value between 2 and 5.17
Quite often, the pattern of liver damage is characteristic of a particular drug or drug class. For example, DILI induced by amoxicillin/clavulanic acid typically will exhibit a cholestatic injury pattern, whereas DILI resulting from a nonsteroidal anti-inflammatory drug typically is associated with a hepatocellular injury pattern.16
Rule out other causes. Further investigations should be directed at ruling out other possible causes of liver injury. If a patient has a hepatocellular pattern of liver injury, order serological tests to rule out acute viral hepatitis (hepatitis A, B, C, and E). Such patients should also be evaluated for autoimmune hepatitis, Budd-Chiari syndrome, Wilson’s disease, and ischemic hepatitis.16 In patients with a predominant cholestatic pattern, imaging studies and other serological tests should be ordered to rule out pancreato-biliary diseases.
Once DILI is confirmed, identify offending agent, grade severity
Which medication is responsible for DILI is determined by the physician based on his or her clinical experience and judgment. Guiding points are improvement of liver function tests after stopping the suspected drug (more on that in a bit), exclusion of other possible causes of liver injury, and the results of liver biopsy. (See “Time for a biopsy?”16)
DILI severity can be graded as mild (1+) to fatal (5+).19 Mild forms of DILI are associated with increased levels of liver enzymes (AST, ALT, or ALP) without raised serum bilirubin or clinical jaundice, whereas moderately severe DILI is associated with clinical jaundice or hyperbilirubinemia (bilirubin >2 mg/dL).19 Severe forms of DILI are associated with features of hepatic failure, such as ascites, encephalopathy, and an elevated international normalized ratio (>1.5), in addition to hyperbilirubinemia or jaundice.
American College of Gastroenterology guidelines recommend liver biopsy if autoimmune hepatitis is suspected, liver enzymes remain elevated for more than 6 months, or liver enzymes continue to rise even after stopping the suspected offending drug.16
Biopsy should also be considered if a patient’s alanine aminotransferase level fails to fall by at least half 60 days after stopping the suspected medication (in a patient with a hepatocellular pattern) or if a patient’s peak alkaline phosphatase level doesn’t fall by at least half at 180 days after stopping the suspected medication (in a patient with a cholestatic pattern).
CASE › Mr. A’s lab results are negative for viral markers. On further questioning, he reveals that he had recovered from a sore throat 3 weeks earlier, for which he had been prescribed an unknown dose of amoxicillin/clavulanic acid. A review of Mr. A’s drug history finds that he is taking metformin, pioglitazone, telmisartan, and atorvastatin for his chronic conditions. The FP suspects DILI , and refers Mr. A to a liver specialist for further investigation.
For many patients, stopping the offending drug will be sufficient
The first step in managing DILI is to stop the medication suspected of causing the liver injury.20 Discontinuing the suspected medication may not always be necessary in patients who have only slightly elevated liver enzymes, but should be strongly considered for a patient who has a considerable increase in liver enzymes levels (ie, an AST, ALT, or serum bilirubin level more than 3 times the ULN or an ALP more than 1.5 times the ULN at any time after initiating a new drug).18 Certain drugs, such as those used to treat tuberculosis, are associated with hepatic adaptation, in which there is spontaneous resolution of the increased liver enzymes level even while the drug is continued in the same dose.
In patients with mild to moderate DILI, stopping the offending drug typically results in normalization of liver enzyme levels.20 Management of patients with moderate to severe DILI is mainly supportive; however, a patient with acute liver failure will require intensive care support.21,22 Consider hospital admission for patients who exhibit severe symptoms, such as intractable vomiting or severe dehydration, those who experience bleeding due to coagulation failure, and those who develop hepatic encephalopathy.21
When more aggressive steps are needed
N-acetylcysteine (NAC) should be considered for all patients with DILI who present with acute liver failure.12,23-25 NAC can be administered either orally or intravenously. The following 3 regimens have been well studied for patients with acetaminophen-induced liver injury:26
• Oral 72-hour regimen: Loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours up to 72 hours
• Intravenous 72-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 418.75 mg/kg over 67 hours
• Intravenous 21-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
Of these regimens, the 72-hour IV regimen has been found to be more effective than the 21-hour regimen for patients with acetaminophen-induced liver toxicity.26 A study of NAC administered as continuous infusion for 72 hours in patients with acute liver failure found that the transplant-free survival rate was 40% for NAC in comparison with 27% for placebo.26
L-carnitine can be used to treat valproate-induced hepatotoxicity. In a case-control study of 92 patients with severe, symptomatic, valproate-induced hepatotoxicity, nearly half of 42 patients treated with L-carnitine survived, but only 10% of 50 patients treated solely with aggressive supportive care survived.27 Greater benefit has been found for IV vs oral L-carnitine.27,28
Ursodeoxycholic acid (UDCA), 13 to 15 mg/kg, may be helpful for DILI patients with a cholestatic pattern of liver injury.
Other therapies. Steroids have no defined role in management of DILI except in autoimmune-type DILI. Other drugs, such as silymarin and antioxidants, have been used to treat other forms of hepatic toxicities and might be beneficial for patients with DILI.29,30
Liver transplantation may be necessary to prevent death due to acute liver failure in patients with severe DILI. Various criteria, including Kings College criteria,31 can be used to select which patients may best benefit from liver transplantation.
For most patients, hospitalization will not be necessary
Generally, patients with DILI have a good prognosis.20,30 About 70% of patients with DILI do not require hospitalization, and approximately 90% recover without reaching the threshold of acute liver failure. However, patients with acute liver failure have a poor prognosis; 40% will require liver transplantation.16,20
Traditionally, patients with a cholestatic pattern of liver injury have been considered to have a better prognosis than those with a hepatocellular pattern of liver injury. Patients whose DILI is the result of a hypersensitivity reaction to a drug also have a good prognosis. This may be because features such as skin rash prompt early diagnosis and discontinuation of the offending drugs.7
CASE › A liver specialist evaluates Mr. A and concludes that his liver injury was caused by his long-term heavy alcohol consumption and exacerbated by the amoxicillin/clavulanic acid he had recently been prescribed. After 2 days, Mr. A develops drowsiness and is admitted to the hospital for further management. He is managed in the intensive care unit under supervision of a gastroenterologist. A NAC infusion is started at a loading dose of 150 mg/kg to manage acute liver failure. Unfortunately, however, Mr. A succumbs to his illness.
CORRESPONDENCE
Piyush Ranjan, MD, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029; [email protected].
1. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73.
2. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076.
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
4. Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115-122.
5. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380-1387.
6. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox. United States National Library of Medicine Web site. Available at: http://livertox.nih.gov/. Accessed April 5, 2015.
7. Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404.
8. Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327-334.
9. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257.
10. Herbals and dietary supplements. United States National Library of Medicine Web site. Available from: http://livertox.nih.gov/Herbals_and_Dietary_Supplements.htm. Accessed April 4, 2015.
11. Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009.
12. Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425.
13. Lucena MI, Andrade RJ, Kaplowitz N, et al; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001-2009.
14. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415.
15. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134-144.
16. Chalasani NP, Hayashi PH, Bonkovsky HL, et al; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966.
17. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. United States National Library of Medicine Web site. Available at: http://www.livertox.nih.gov/rucam.html. Accessed September 8, 2015.
18. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785.
19. Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742.
20. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106.
21. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27-33.
22. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
23. Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015.
24. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89:193-199.
25. Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251-258.
26. Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther. 2012;342:529-540.
27. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409.
28. Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19:206-210.
29. Ghabril M, Chalasani N, Björnsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-226.
30. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-259.
31. Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford). 2006;8:29-34.
› If you suspect your patient may have drug-induced liver injury (DILI), take a careful medication history, assess for risk factors, and investigate other possible causes. B
› Immediately stop any drugs you suspect are causing DILI, especially when the patient’s liver enzymes are rapidly increasing or there is evidence of acute liver failure. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James A, age 68, presents to his family physician (FP) with anorexia, nausea, and vague upper abdominal pain that he’s had for 2 weeks. Mr. A has diabetes and hypertension, both of which are well controlled by medications. He also consumes more than 4 alcoholic beverages daily.
On examination, the FP notes icterus and tenderness in the right hypochondrium. Liver function testing reveals elevated liver enzyme levels: aspartate aminotransferase (AST), 864 IU/L (normal range: 10-40 IU/L); alanine aminotransferase (ALT), 1012 IU/L (normal range: 7-56 IU/L); serum bilirubin, 4.8 mg/dL (normal range: 0.3-1.9 mg/dL); and alkaline phosphatase (ALP), 200 IU/L (normal range: 44-147 IU/L). Mr. A’s coagulation profile is slightly abnormal. He is provisionally diagnosed with acute hepatitis. The FP sends blood samples to the lab to assess for viral markers, and starts symptomatic management.
If Mr. A were your patient, how would you proceed?
In the United States, drug-induced liver injury (DILI) is the most common cause of acute liver failure.1,2 It can occur due to ingestion of any therapeutic drug, herbal product, or xenobiotic. Further complicating matters is the fact that it has an unpredictable and heterogeneous course, ranging from an asymptomatic rise in liver enzymes to acute liver failure. This article describes the risk factors, common causative agents, tools for early diagnosis, and effective management of DILI.
Two types of risk factors for DILI
Risk factors for DILI can be classified as drug-related (eg, dose, concomitant medications, polypharmacy) or host-related (eg, age, gender, alcohol intake, concomitant infections).3-5
Drug-related factors. Hundreds of agents can lead to liver injury. In fact, the US National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases have created LiverTox (http://www.livertox.nih.gov/), an online database that provides detailed information on more than 600 such agents.6 Antibiotics are the most common cause of DILI, followed by neuropsychiatric drugs, immunomodulatory agents, antihypertensives, analgesics, antineoplastic drugs, and lipid-lowering agents.2
Among antibiotics, the specific medication most often responsible for DILI varies by geographical region. Amoxicillin/clavulanic acid is the most common causative antibiotic in the United States, whereas anti-tuberculosis agents such as isoniazid, rifampin, and pyrazinamide are the most common causative drugs in developing countries such as India, where the prevalence of tuberculosis is still high.7,8 Herbal and dietary supplements are emerging as an important cause of DILI.5,9,10
The use of multiple drugs further increases the risk of developing DILI.10 Drugs with a recommended daily dose of <50 mg are rarely associated with DILI.11
Host-related factors. Vulnerability to DILI is influenced by a patient’s age and sex.3,12 Very young and very old patients have an increased risk of developing DILI, and a patient’s age may make him or her particularly susceptible to the effects of certain medications.3,12,13 For example, children are more susceptible to DILI as a result of taking valproate or aspirin, whereas older patients are more likely to experience DILI brought on by amoxicillin/clavulanic acid.13 The pattern of liver injury also varies by age. Younger patients present most commonly with a hepatocellular pattern of injury, whereas older patients mostly present with a cholestatic pattern of liver injury.3
Some studies have found that women have a greater risk of developing DILI than men.13 The presence of chronic liver diseases, alcoholism, and nonalcoholic fatty liver disease (NAFLD) increase the risk of developing DILI.14 Diabetes is an independent risk factor for DILI.3
Clinical presentation of DILI varies widely
Some degree of liver injury may occur in any patient who ingests a drug that is metabolized in the liver. The clinical presentation of a patient with DILI can vary from an asymptomatic rise in liver enzymes to acute liver failure. Unexplained transaminitis should raise the possibility of DILI, especially when the patient has started a new drug in the preceding 3 months. However, in most patients, an asymptomatic rise in liver enzymes is due to hepatic adaptation or tolerance. In such cases, liver enzyme levels tend to normalize even if the patient continues to take the drug in the same dose.15
Apart from nonspecific symptoms such as anorexia, nausea, and vomiting, a patient with DILI may exhibit right upper quadrant pain, skin rash, or itching. A patient with severe DILI might exhibit jaundice, ascites, or encephalopathy.15
A stepwise approach to evaluation
DILI is a diagnosis of exclusion.16 Guidelines from the American College of Gastroenterology (ACG) recommend a stepwise approach to evaluating a patient you suspect may have DILI (TABLE).16 First, take a detailed history regarding the onset of symptoms, time latency, and use of hepatotoxic and other drugs (dosage and duration of use). Also ask the patient about his or her use of herbal products, dietary supplements, and alcohol. Check the patient’s history for the presence of other liver diseases such as NAFLD.
Next, make sure initial laboratory testing includes liver function tests and an eosinophil count. In order to classify the pattern of liver injury as hepatocellular, cholestatic, or mixed, you’ll need to calculate the patient’s R value (ALGORITHM16-18). This value is calculated by dividing the patient’s ALT level by the ALP, using the upper limit of the normal range (ULN) as follows: R = (ALT value ÷ ALT ULN) ÷ (ALP value ÷ ALP ULN).17 A hepatocellular pattern of liver injury is indicated by an R value >5, a cholestatic pattern is an R value <2, and a mixed pattern is suggested by an R value between 2 and 5.17
Quite often, the pattern of liver damage is characteristic of a particular drug or drug class. For example, DILI induced by amoxicillin/clavulanic acid typically will exhibit a cholestatic injury pattern, whereas DILI resulting from a nonsteroidal anti-inflammatory drug typically is associated with a hepatocellular injury pattern.16
Rule out other causes. Further investigations should be directed at ruling out other possible causes of liver injury. If a patient has a hepatocellular pattern of liver injury, order serological tests to rule out acute viral hepatitis (hepatitis A, B, C, and E). Such patients should also be evaluated for autoimmune hepatitis, Budd-Chiari syndrome, Wilson’s disease, and ischemic hepatitis.16 In patients with a predominant cholestatic pattern, imaging studies and other serological tests should be ordered to rule out pancreato-biliary diseases.
Once DILI is confirmed, identify offending agent, grade severity
Which medication is responsible for DILI is determined by the physician based on his or her clinical experience and judgment. Guiding points are improvement of liver function tests after stopping the suspected drug (more on that in a bit), exclusion of other possible causes of liver injury, and the results of liver biopsy. (See “Time for a biopsy?”16)
DILI severity can be graded as mild (1+) to fatal (5+).19 Mild forms of DILI are associated with increased levels of liver enzymes (AST, ALT, or ALP) without raised serum bilirubin or clinical jaundice, whereas moderately severe DILI is associated with clinical jaundice or hyperbilirubinemia (bilirubin >2 mg/dL).19 Severe forms of DILI are associated with features of hepatic failure, such as ascites, encephalopathy, and an elevated international normalized ratio (>1.5), in addition to hyperbilirubinemia or jaundice.
American College of Gastroenterology guidelines recommend liver biopsy if autoimmune hepatitis is suspected, liver enzymes remain elevated for more than 6 months, or liver enzymes continue to rise even after stopping the suspected offending drug.16
Biopsy should also be considered if a patient’s alanine aminotransferase level fails to fall by at least half 60 days after stopping the suspected medication (in a patient with a hepatocellular pattern) or if a patient’s peak alkaline phosphatase level doesn’t fall by at least half at 180 days after stopping the suspected medication (in a patient with a cholestatic pattern).
CASE › Mr. A’s lab results are negative for viral markers. On further questioning, he reveals that he had recovered from a sore throat 3 weeks earlier, for which he had been prescribed an unknown dose of amoxicillin/clavulanic acid. A review of Mr. A’s drug history finds that he is taking metformin, pioglitazone, telmisartan, and atorvastatin for his chronic conditions. The FP suspects DILI , and refers Mr. A to a liver specialist for further investigation.
For many patients, stopping the offending drug will be sufficient
The first step in managing DILI is to stop the medication suspected of causing the liver injury.20 Discontinuing the suspected medication may not always be necessary in patients who have only slightly elevated liver enzymes, but should be strongly considered for a patient who has a considerable increase in liver enzymes levels (ie, an AST, ALT, or serum bilirubin level more than 3 times the ULN or an ALP more than 1.5 times the ULN at any time after initiating a new drug).18 Certain drugs, such as those used to treat tuberculosis, are associated with hepatic adaptation, in which there is spontaneous resolution of the increased liver enzymes level even while the drug is continued in the same dose.
In patients with mild to moderate DILI, stopping the offending drug typically results in normalization of liver enzyme levels.20 Management of patients with moderate to severe DILI is mainly supportive; however, a patient with acute liver failure will require intensive care support.21,22 Consider hospital admission for patients who exhibit severe symptoms, such as intractable vomiting or severe dehydration, those who experience bleeding due to coagulation failure, and those who develop hepatic encephalopathy.21
When more aggressive steps are needed
N-acetylcysteine (NAC) should be considered for all patients with DILI who present with acute liver failure.12,23-25 NAC can be administered either orally or intravenously. The following 3 regimens have been well studied for patients with acetaminophen-induced liver injury:26
• Oral 72-hour regimen: Loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours up to 72 hours
• Intravenous 72-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 418.75 mg/kg over 67 hours
• Intravenous 21-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
Of these regimens, the 72-hour IV regimen has been found to be more effective than the 21-hour regimen for patients with acetaminophen-induced liver toxicity.26 A study of NAC administered as continuous infusion for 72 hours in patients with acute liver failure found that the transplant-free survival rate was 40% for NAC in comparison with 27% for placebo.26
L-carnitine can be used to treat valproate-induced hepatotoxicity. In a case-control study of 92 patients with severe, symptomatic, valproate-induced hepatotoxicity, nearly half of 42 patients treated with L-carnitine survived, but only 10% of 50 patients treated solely with aggressive supportive care survived.27 Greater benefit has been found for IV vs oral L-carnitine.27,28
Ursodeoxycholic acid (UDCA), 13 to 15 mg/kg, may be helpful for DILI patients with a cholestatic pattern of liver injury.
Other therapies. Steroids have no defined role in management of DILI except in autoimmune-type DILI. Other drugs, such as silymarin and antioxidants, have been used to treat other forms of hepatic toxicities and might be beneficial for patients with DILI.29,30
Liver transplantation may be necessary to prevent death due to acute liver failure in patients with severe DILI. Various criteria, including Kings College criteria,31 can be used to select which patients may best benefit from liver transplantation.
For most patients, hospitalization will not be necessary
Generally, patients with DILI have a good prognosis.20,30 About 70% of patients with DILI do not require hospitalization, and approximately 90% recover without reaching the threshold of acute liver failure. However, patients with acute liver failure have a poor prognosis; 40% will require liver transplantation.16,20
Traditionally, patients with a cholestatic pattern of liver injury have been considered to have a better prognosis than those with a hepatocellular pattern of liver injury. Patients whose DILI is the result of a hypersensitivity reaction to a drug also have a good prognosis. This may be because features such as skin rash prompt early diagnosis and discontinuation of the offending drugs.7
CASE › A liver specialist evaluates Mr. A and concludes that his liver injury was caused by his long-term heavy alcohol consumption and exacerbated by the amoxicillin/clavulanic acid he had recently been prescribed. After 2 days, Mr. A develops drowsiness and is admitted to the hospital for further management. He is managed in the intensive care unit under supervision of a gastroenterologist. A NAC infusion is started at a loading dose of 150 mg/kg to manage acute liver failure. Unfortunately, however, Mr. A succumbs to his illness.
CORRESPONDENCE
Piyush Ranjan, MD, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029; [email protected].
› If you suspect your patient may have drug-induced liver injury (DILI), take a careful medication history, assess for risk factors, and investigate other possible causes. B
› Immediately stop any drugs you suspect are causing DILI, especially when the patient’s liver enzymes are rapidly increasing or there is evidence of acute liver failure. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › James A, age 68, presents to his family physician (FP) with anorexia, nausea, and vague upper abdominal pain that he’s had for 2 weeks. Mr. A has diabetes and hypertension, both of which are well controlled by medications. He also consumes more than 4 alcoholic beverages daily.
On examination, the FP notes icterus and tenderness in the right hypochondrium. Liver function testing reveals elevated liver enzyme levels: aspartate aminotransferase (AST), 864 IU/L (normal range: 10-40 IU/L); alanine aminotransferase (ALT), 1012 IU/L (normal range: 7-56 IU/L); serum bilirubin, 4.8 mg/dL (normal range: 0.3-1.9 mg/dL); and alkaline phosphatase (ALP), 200 IU/L (normal range: 44-147 IU/L). Mr. A’s coagulation profile is slightly abnormal. He is provisionally diagnosed with acute hepatitis. The FP sends blood samples to the lab to assess for viral markers, and starts symptomatic management.
If Mr. A were your patient, how would you proceed?
In the United States, drug-induced liver injury (DILI) is the most common cause of acute liver failure.1,2 It can occur due to ingestion of any therapeutic drug, herbal product, or xenobiotic. Further complicating matters is the fact that it has an unpredictable and heterogeneous course, ranging from an asymptomatic rise in liver enzymes to acute liver failure. This article describes the risk factors, common causative agents, tools for early diagnosis, and effective management of DILI.
Two types of risk factors for DILI
Risk factors for DILI can be classified as drug-related (eg, dose, concomitant medications, polypharmacy) or host-related (eg, age, gender, alcohol intake, concomitant infections).3-5
Drug-related factors. Hundreds of agents can lead to liver injury. In fact, the US National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases have created LiverTox (http://www.livertox.nih.gov/), an online database that provides detailed information on more than 600 such agents.6 Antibiotics are the most common cause of DILI, followed by neuropsychiatric drugs, immunomodulatory agents, antihypertensives, analgesics, antineoplastic drugs, and lipid-lowering agents.2
Among antibiotics, the specific medication most often responsible for DILI varies by geographical region. Amoxicillin/clavulanic acid is the most common causative antibiotic in the United States, whereas anti-tuberculosis agents such as isoniazid, rifampin, and pyrazinamide are the most common causative drugs in developing countries such as India, where the prevalence of tuberculosis is still high.7,8 Herbal and dietary supplements are emerging as an important cause of DILI.5,9,10
The use of multiple drugs further increases the risk of developing DILI.10 Drugs with a recommended daily dose of <50 mg are rarely associated with DILI.11
Host-related factors. Vulnerability to DILI is influenced by a patient’s age and sex.3,12 Very young and very old patients have an increased risk of developing DILI, and a patient’s age may make him or her particularly susceptible to the effects of certain medications.3,12,13 For example, children are more susceptible to DILI as a result of taking valproate or aspirin, whereas older patients are more likely to experience DILI brought on by amoxicillin/clavulanic acid.13 The pattern of liver injury also varies by age. Younger patients present most commonly with a hepatocellular pattern of injury, whereas older patients mostly present with a cholestatic pattern of liver injury.3
Some studies have found that women have a greater risk of developing DILI than men.13 The presence of chronic liver diseases, alcoholism, and nonalcoholic fatty liver disease (NAFLD) increase the risk of developing DILI.14 Diabetes is an independent risk factor for DILI.3
Clinical presentation of DILI varies widely
Some degree of liver injury may occur in any patient who ingests a drug that is metabolized in the liver. The clinical presentation of a patient with DILI can vary from an asymptomatic rise in liver enzymes to acute liver failure. Unexplained transaminitis should raise the possibility of DILI, especially when the patient has started a new drug in the preceding 3 months. However, in most patients, an asymptomatic rise in liver enzymes is due to hepatic adaptation or tolerance. In such cases, liver enzyme levels tend to normalize even if the patient continues to take the drug in the same dose.15
Apart from nonspecific symptoms such as anorexia, nausea, and vomiting, a patient with DILI may exhibit right upper quadrant pain, skin rash, or itching. A patient with severe DILI might exhibit jaundice, ascites, or encephalopathy.15
A stepwise approach to evaluation
DILI is a diagnosis of exclusion.16 Guidelines from the American College of Gastroenterology (ACG) recommend a stepwise approach to evaluating a patient you suspect may have DILI (TABLE).16 First, take a detailed history regarding the onset of symptoms, time latency, and use of hepatotoxic and other drugs (dosage and duration of use). Also ask the patient about his or her use of herbal products, dietary supplements, and alcohol. Check the patient’s history for the presence of other liver diseases such as NAFLD.
Next, make sure initial laboratory testing includes liver function tests and an eosinophil count. In order to classify the pattern of liver injury as hepatocellular, cholestatic, or mixed, you’ll need to calculate the patient’s R value (ALGORITHM16-18). This value is calculated by dividing the patient’s ALT level by the ALP, using the upper limit of the normal range (ULN) as follows: R = (ALT value ÷ ALT ULN) ÷ (ALP value ÷ ALP ULN).17 A hepatocellular pattern of liver injury is indicated by an R value >5, a cholestatic pattern is an R value <2, and a mixed pattern is suggested by an R value between 2 and 5.17
Quite often, the pattern of liver damage is characteristic of a particular drug or drug class. For example, DILI induced by amoxicillin/clavulanic acid typically will exhibit a cholestatic injury pattern, whereas DILI resulting from a nonsteroidal anti-inflammatory drug typically is associated with a hepatocellular injury pattern.16
Rule out other causes. Further investigations should be directed at ruling out other possible causes of liver injury. If a patient has a hepatocellular pattern of liver injury, order serological tests to rule out acute viral hepatitis (hepatitis A, B, C, and E). Such patients should also be evaluated for autoimmune hepatitis, Budd-Chiari syndrome, Wilson’s disease, and ischemic hepatitis.16 In patients with a predominant cholestatic pattern, imaging studies and other serological tests should be ordered to rule out pancreato-biliary diseases.
Once DILI is confirmed, identify offending agent, grade severity
Which medication is responsible for DILI is determined by the physician based on his or her clinical experience and judgment. Guiding points are improvement of liver function tests after stopping the suspected drug (more on that in a bit), exclusion of other possible causes of liver injury, and the results of liver biopsy. (See “Time for a biopsy?”16)
DILI severity can be graded as mild (1+) to fatal (5+).19 Mild forms of DILI are associated with increased levels of liver enzymes (AST, ALT, or ALP) without raised serum bilirubin or clinical jaundice, whereas moderately severe DILI is associated with clinical jaundice or hyperbilirubinemia (bilirubin >2 mg/dL).19 Severe forms of DILI are associated with features of hepatic failure, such as ascites, encephalopathy, and an elevated international normalized ratio (>1.5), in addition to hyperbilirubinemia or jaundice.
American College of Gastroenterology guidelines recommend liver biopsy if autoimmune hepatitis is suspected, liver enzymes remain elevated for more than 6 months, or liver enzymes continue to rise even after stopping the suspected offending drug.16
Biopsy should also be considered if a patient’s alanine aminotransferase level fails to fall by at least half 60 days after stopping the suspected medication (in a patient with a hepatocellular pattern) or if a patient’s peak alkaline phosphatase level doesn’t fall by at least half at 180 days after stopping the suspected medication (in a patient with a cholestatic pattern).
CASE › Mr. A’s lab results are negative for viral markers. On further questioning, he reveals that he had recovered from a sore throat 3 weeks earlier, for which he had been prescribed an unknown dose of amoxicillin/clavulanic acid. A review of Mr. A’s drug history finds that he is taking metformin, pioglitazone, telmisartan, and atorvastatin for his chronic conditions. The FP suspects DILI , and refers Mr. A to a liver specialist for further investigation.
For many patients, stopping the offending drug will be sufficient
The first step in managing DILI is to stop the medication suspected of causing the liver injury.20 Discontinuing the suspected medication may not always be necessary in patients who have only slightly elevated liver enzymes, but should be strongly considered for a patient who has a considerable increase in liver enzymes levels (ie, an AST, ALT, or serum bilirubin level more than 3 times the ULN or an ALP more than 1.5 times the ULN at any time after initiating a new drug).18 Certain drugs, such as those used to treat tuberculosis, are associated with hepatic adaptation, in which there is spontaneous resolution of the increased liver enzymes level even while the drug is continued in the same dose.
In patients with mild to moderate DILI, stopping the offending drug typically results in normalization of liver enzyme levels.20 Management of patients with moderate to severe DILI is mainly supportive; however, a patient with acute liver failure will require intensive care support.21,22 Consider hospital admission for patients who exhibit severe symptoms, such as intractable vomiting or severe dehydration, those who experience bleeding due to coagulation failure, and those who develop hepatic encephalopathy.21
When more aggressive steps are needed
N-acetylcysteine (NAC) should be considered for all patients with DILI who present with acute liver failure.12,23-25 NAC can be administered either orally or intravenously. The following 3 regimens have been well studied for patients with acetaminophen-induced liver injury:26
• Oral 72-hour regimen: Loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours up to 72 hours
• Intravenous 72-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 418.75 mg/kg over 67 hours
• Intravenous 21-hour regimen: Loading infusion of 150 mg/kg over one hour, followed by 50 mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
Of these regimens, the 72-hour IV regimen has been found to be more effective than the 21-hour regimen for patients with acetaminophen-induced liver toxicity.26 A study of NAC administered as continuous infusion for 72 hours in patients with acute liver failure found that the transplant-free survival rate was 40% for NAC in comparison with 27% for placebo.26
L-carnitine can be used to treat valproate-induced hepatotoxicity. In a case-control study of 92 patients with severe, symptomatic, valproate-induced hepatotoxicity, nearly half of 42 patients treated with L-carnitine survived, but only 10% of 50 patients treated solely with aggressive supportive care survived.27 Greater benefit has been found for IV vs oral L-carnitine.27,28
Ursodeoxycholic acid (UDCA), 13 to 15 mg/kg, may be helpful for DILI patients with a cholestatic pattern of liver injury.
Other therapies. Steroids have no defined role in management of DILI except in autoimmune-type DILI. Other drugs, such as silymarin and antioxidants, have been used to treat other forms of hepatic toxicities and might be beneficial for patients with DILI.29,30
Liver transplantation may be necessary to prevent death due to acute liver failure in patients with severe DILI. Various criteria, including Kings College criteria,31 can be used to select which patients may best benefit from liver transplantation.
For most patients, hospitalization will not be necessary
Generally, patients with DILI have a good prognosis.20,30 About 70% of patients with DILI do not require hospitalization, and approximately 90% recover without reaching the threshold of acute liver failure. However, patients with acute liver failure have a poor prognosis; 40% will require liver transplantation.16,20
Traditionally, patients with a cholestatic pattern of liver injury have been considered to have a better prognosis than those with a hepatocellular pattern of liver injury. Patients whose DILI is the result of a hypersensitivity reaction to a drug also have a good prognosis. This may be because features such as skin rash prompt early diagnosis and discontinuation of the offending drugs.7
CASE › A liver specialist evaluates Mr. A and concludes that his liver injury was caused by his long-term heavy alcohol consumption and exacerbated by the amoxicillin/clavulanic acid he had recently been prescribed. After 2 days, Mr. A develops drowsiness and is admitted to the hospital for further management. He is managed in the intensive care unit under supervision of a gastroenterologist. A NAC infusion is started at a loading dose of 150 mg/kg to manage acute liver failure. Unfortunately, however, Mr. A succumbs to his illness.
CORRESPONDENCE
Piyush Ranjan, MD, All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India 110029; [email protected].
1. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73.
2. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076.
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
4. Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115-122.
5. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380-1387.
6. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox. United States National Library of Medicine Web site. Available at: http://livertox.nih.gov/. Accessed April 5, 2015.
7. Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404.
8. Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327-334.
9. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257.
10. Herbals and dietary supplements. United States National Library of Medicine Web site. Available from: http://livertox.nih.gov/Herbals_and_Dietary_Supplements.htm. Accessed April 4, 2015.
11. Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009.
12. Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425.
13. Lucena MI, Andrade RJ, Kaplowitz N, et al; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001-2009.
14. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415.
15. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134-144.
16. Chalasani NP, Hayashi PH, Bonkovsky HL, et al; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966.
17. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. United States National Library of Medicine Web site. Available at: http://www.livertox.nih.gov/rucam.html. Accessed September 8, 2015.
18. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785.
19. Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742.
20. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106.
21. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27-33.
22. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
23. Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015.
24. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89:193-199.
25. Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251-258.
26. Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther. 2012;342:529-540.
27. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409.
28. Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19:206-210.
29. Ghabril M, Chalasani N, Björnsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-226.
30. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-259.
31. Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford). 2006;8:29-34.
1. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73.
2. Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065-2076.
3. Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924-1934.
4. Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115-122.
5. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380-1387.
6. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox. United States National Library of Medicine Web site. Available at: http://livertox.nih.gov/. Accessed April 5, 2015.
7. Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396-2404.
8. Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327-334.
9. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18:249-257.
10. Herbals and dietary supplements. United States National Library of Medicine Web site. Available from: http://livertox.nih.gov/Herbals_and_Dietary_Supplements.htm. Accessed April 4, 2015.
11. Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009.
12. Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425.
13. Lucena MI, Andrade RJ, Kaplowitz N, et al; Spanish Group for the Study of Drug-Induced Liver Disease. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001-2009.
14. Tarantino G, Conca P, Basile V, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415.
15. Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34:134-144.
16. Chalasani NP, Hayashi PH, Bonkovsky HL, et al; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-966.
17. United States National Library of Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. United States National Library of Medicine Web site. Available at: http://www.livertox.nih.gov/rucam.html. Accessed September 8, 2015.
18. Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774-6785.
19. Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742.
20. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106.
21. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19:27-33.
22. Lee WM, Hynan LS, Rossaro L, et al; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864.
23. Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol. 2015.
24. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89:193-199.
25. Carter BA, Karpen SJ. Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis. 2007;27:251-258.
26. Woodhead JL, Howell BA, Yang Y, et al. An analysis of N-acetylcysteine treatment for acetaminophen overdose using a systems model of drug-induced liver injury. J Pharmacol Exp Ther. 2012;342:529-540.
27. Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409.
28. Russell S. Carnitine as an antidote for acute valproate toxicity in children. Curr Opin Pediatr. 2007;19:206-210.
29. Ghabril M, Chalasani N, Björnsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-226.
30. Devarbhavi H. An update on drug-induced liver injury. J Clin Exp Hepatol. 2012;2:247-259.
31. Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford). 2006;8:29-34.
Tuberculosis testing: Which patients, which test?
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.