User login
Irritable bowel syndrome: Minimize testing, let symptoms guide treatment
- For patients aged <50 years without alarm symptoms, diagnostic testing is unnecessary. Consider celiac sprue testing for patients with diarrhea (C).
- Treatment is indicated when both the patient with irritable bowel syndrome and the physician agree that quality of life has been diminished (C). The goal of therapy is to alleviate global IBS symptoms (abdominal discomfort, bloating, and altered bowel habits that are life-impacting) (C).
- Tegaserod, a 5HT4 receptor agonist, is more effective than placebo at relieving global IBS symptoms in women with constipation (A). Its effectiveness in men is unknown.
- Alosetron, a 5HT3 receptor antagonist, is more effective than placebo at relieving global IBS symptoms in women with diarrhea (A).
- Behavior therapy–relaxation therapy, hypnotherapy, or cognitive therapy–is more effective than placebo at relieving individual symptoms, but no data are available for quality-of-life improvement (B).
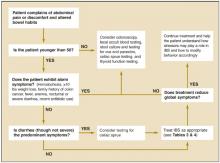
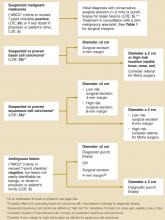
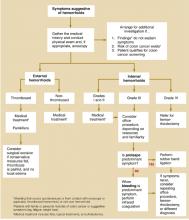
An extensive and expensive evaluation for irritable bowel syndrome (IBS) can be avoided if your patient is aged <50 years and is not experiencing alarm symptoms (hematochezia, 10 lbs weight loss, fever, anemia, nocturnal or severe diarrhea), has not recently taken antibiotics, and has no family history of colon cancer. An algorithm ( Figure ) indicates when work-up is needed and what it should entail.
Newer medications that act on 5HT receptors have proven effective in improving quality of life (global symptom reduction). Evidence supports the use of several traditional medications to reduce individual symptoms of IBS, but not for global symptom reduction.
FIGURE
Evaluating possible irritable bowel syndrome
Who gets irritable bowel syndrome?
Ten percent to 15% of the North American population has IBS, and twice as many women as men have it.1 Symptoms usually begin before the age of 35 years, and many patients can trace their symptoms back to childhood.2 Onset in the elderly is rare.3 The disorder is responsible for approximately 50% of referrals to gastroenterologists.4
The company IBS keeps
Comorbid psychiatric illness is common with IBS, but few patients seek psychiatric care.5 Depression, anxiety, and somatoform disorders are seen in 94% of patients with IBS. IBS is common in patients with chronic fatigue syndrome (51%), fibromyalgia (49%), temporomandibular joint syndrome (64%), and chronic pelvic pain (50%).6 IBS often follows stressful life events,5,7,8 such as a death in the family or divorce. It tends to be chronic, intermittent, and relapsing.3
The symptoms of IBS can overlap with those of other illnesses, including thyroid dysfunction (diarrhea or constipation), functional dyspepsia (abdominal pain), Crohn’s disease or ulcerative colitis (diarrhea, abdominal pain), celiac sprue (diarrhea), polyps and cancers (constipation or abdominal pain), and infectious diarrhea.
Elusive physiologic mechanism
Several physiologic mechanisms have been proposed for IBS symptoms: altered gut reactivity in response to luminal stimuli, hypersensitive gut with enhanced pain response, and altered brain-gut biochemical axis.9 Though the symptoms of irritable bowel syndrome appear to have a physiologic basis, there are no structural or biochemical markers for the disease.
Use symptom-based criteria for diagnosis
Consider a diagnosis of IBS when a patient complains of abdominal discomfort and altered bowel habits. In the absence of a structural or biochemical marker, IBS must be diagnosed according to symptom-based criteria–such as Manning, Rome I, or Rome II–which have been developed for research and epidemiologic purposes. Though their clinical utility remains unproven, these criteria (delineated in Table 1 ) are the crux of clinical diagnosis for IBS.4,10-14 Subtypes of IBS have been described (diarrheapredominant IBS or constipation-predominant IBS), but they are not diagnostically useful, since the treatment goal is improved quality of life.
TABLE 1
Symptom-based criteria for irritable bowel syndrome
| Symptom-based criteria | Symptoms | Sn | Sp | PV+ |
|---|---|---|---|---|
| Manning4,10,13,14 |
| 42%–90% | 70%–100% | 74% |
| Rome I4,10,13 |
| 65%–84% | 100% | 69%–100% |
| Rome II11-13 |
| 49%-65%* | 100%* | 69%-100%* |
| Supportive symptoms | ||||
| Fewer than 3 bowel movements per week | ||||
| More than 3 bowel movements per day | ||||
| Hard or lumpy stools | ||||
| *Found to have similar sensitivity and specificity to Rome I.14 | ||||
| Sn, sensitivity; Sp, specificity; PV+, positive predictive value | ||||
Dubious value of diagnostic tests
The literature regarding the value of diagnostic testing for IBS is controversial. Symptom-based criteria have varied in many studies, as have the criteria used to enroll patients and the measured outcomes of treatment (reduction in abdominal pain, in diarrhea, or in constipation, or improvement in quality of life). Because of these discrepancies, it is difficult to apply the literature clinically. Of the 6 landmark studies that considered the value of diagnostic testing for IBS patients,15-20 only 2 compared IBS patients with groups of healthy controls.17,19
Test results yield little. Most research in this area has compared the prevalence of specific illnesses in the general population with the yield of positive test results for these illnesses among persons meeting the symptom-based diagnostic criteria for IBS.
Two studies15,16 determined the incidence of abnormal test results in patients who met the Manning or Rome I criteria for IBS. In these studies, most diagnostic tests yielded positive results in 2% (range, 0%-8.2%) of patients or less, except for thyroid and lactose intolerance testing. That is equivalent to the incidence in the general population. The prevalence of thyroid disorders and lactose malabsorption was higher in IBS patients (6% and 22%-26%, respectively), but prevalence in the general population is similarly higher (5%-9% and 25%). Based on these results, testing for thyroid disease or lactose malabsorption is indicated only for patients exhibiting symptoms of these disorders (fatigue/weight change or diarrhea related to diertary intake of dairy products, respectively).
An exception. Some clinicians propose that diagnostic testing for patients with IBS symptoms should be driven by the pretest probability of organic disease (prevalence) compared with the general population. Cash21 found the pretest probability of inflammatory bowel disease, colorectal cancer, and infectious diarrhea is less than 1% among IBS patients without alarm symptoms ( Table 2 ). He demonstrated that patients with IBS had a 5% pretest probability of celiac sprue compared with healthy patients (<1% prevalence). Therefore, testing for celiac sprue (eg, complete blood count, antiendomysial antibody, and antigliadin antibody) may be considered for patients with diarrhea.6,21,22 Sigmoidoscopy,15,17 rectal biopsy,17 and abdominal ultrasound18 have low positive yield in patients meeting the diagnostic criteria for IBS.
TABLE 2
Probability of organic disease in irritable bowel syndrome patients
| Disease | Pretest probability-IBS patients (%) | Prevalence-general population (%) | Comments |
|---|---|---|---|
| Colitis/inflammatory inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 | Structural colon lesions were detected with barium enema, colonscopy, sigmoidoscopy |
| Colon cancer | 0-0.51 | 4-6 | Structural colon lesions were detected with barium enema, colonoscopy, sigmoidoscopy |
| Celiac disease | 4.67 | 0.25-0.5 | Note: celiac disease prevalence higher than in general population. |
| Gastrointestinal infection | 0-1.7 | N/A | |
| Thyroid dysfunction | 6 | 5-9 | Prevalence high in both groups |
| Lactose malabsorption | 22-26 | 25 | Prevalence high in both groups |
| Adapted from: Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002; 97:2812-2819. | |||
| Results are from multiple studies: n=125-306. | |||
How to proceed
Those under 50 years of age who have no alarm symptoms can forgo further testing. Testing for celiac sprue and lactose malabsorption might be considered for patients with diarrhea that improves or worsens with change in diet (strength of recommendation [SOR]: C).
Threshold for treatment
Treatment for IBS is indicated when both patient and physician believe global symptoms (abdominal discomfort, bloating, altered bowel habits) have diminished the quality of life (SOR: C). The goal of treatment is to alleviate all IBS symptoms (SOR: C). Treating altered bowel habits (constipation, diarrhea, and fecal urgency) without addressing other IBS symptoms (eg, abdominal pain) is inferior treatment.23,24
Treatment options for IBS
Treatments for IBS include medications, behavior therapy, and complimentary and alternative therapies. Medications traditionally prescribed include bulking agents, anticholinergics/antispasmodics, antidiarrheals, and antidepressants. A 5HT3 receptor antagonist and a 5HT4 receptor partial agonist are now available. Table 3 summarizes the traditional treatments in terms of efficacy, strength of recommendations, and outcomes measured. Alternative and complimentary therapies appear in Table 4 .
As Brandt24 has noted, the evidence for treatment effectiveness is difficult to review and summarize, because the quality of studies has been poor. Most studies did not use healthy control groups, and the numbers of participants were small. Many studies did not use blinded placebo groups. Outcomes measured varied among the studies, with most of them measuring reductions of individual bowel symptoms (eg, diarrhea or constipation). Quality-of-life tools were used in other studies to measure reduction in global IBS symptoms (eg, IBS Quality of Life 25 ). Because of these discrepancies, there is no sound evidence for traditional therapies.
TABLE 3
Treatments for irritable bowel syndrome
| Treatment | Efficacy (NNT) | SOR (studies) | Outcomes measured | Comments |
|---|---|---|---|---|
| 5HT4 receptor agonist (tegaserod)23,24,26-30 | More effective than placebo at relieving global IBS symptoms in women with constipation (3.9-17) | A (4) | Global IBS symptoms, individual IBS symptoms | 83%-100% of study participants were women with IBS and constipation. Rome I and II criteria for entry. May cause diarrhea |
| 5HT4 receptor agonist(alosetron)23,24,26- 35 | More effective than placebo at relieving global IBS symptoms in women with diarrhea (2.5-8.3) | A (4) | Global IBS symptoms, individual IBS symptoms, adverse events | 82%-93% of study participants were women. Rome I and II criteria for entry. May cause severe constipation; restricted use |
| Tricylic antidepressants (trimipramine, desipramine)23,24,36- 39 | Reduces abdominal pain. No more effective than placebo at relieving gloal IBS symptoms (3.2-5) | B (6) | GI symptoms | May cause constipation; no studies done with SSRIs |
| Loperamide23,24,36-39 | Relieves diarrhea. No more effective than placebo at relieving global IBS symptoms (3.2-5) | B (3) | Global IBS symptoms, diarrhea | Constipation or paralytic ileus can occur |
| Bulking agents (corn fiber, wheat bran, psyllium, ispaghula husks, calcium polycabophil)23,24,31,40-42 | Improves constipation. No more effective than placebo in studies considering global symptom improvement (2.2-8.6) | B (13) | GI symptoms, global IBS symptoms | May increase bloating. All studiessmall numbers of patients |
| Anti-spasmodics (hyoscyamine dicyclomine)23,24,26-30 | No evidence on improvement of global IBS symptoms (5.9) | B (3) | Individual IBS and global symptoms | Studies were short, small numbers, inconsistent effectiveness. Could worsen constipation; 15 additional studies done on drugs not available in the US |
| Behavioral therapies (hypnotherapy, relaxation therapy, psychotherapy, biofeedback)23,24,44, 52-57 | More effective than placebo at relieving individuals IBS symptoms (1.4-1.9) | B (16) | GI symptoms, psychological sypmtoms | None measures global IBS symptom improvement. Small numbers of patients |
| SSRI antidepressants (paroxtetine, fluoxetine)23,24, 50-51 | Improved quality of life, decreased abdominal pain | B (16) | Abdominal | One study severe IBS, other study only 10 participants quality of life |
| SOR, strength of recommendation; IBS, irritable bowel syndrome; GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor. For an explanation of SORs. | ||||
TABLE 4
Complementary and alternative treatments for irritable bowel syndrome
| Treatment | Efficacy | SOR | Outcomes measured | Comments |
|---|---|---|---|---|
| Neomycin 20 | Treatment for 1 week improved symptoms of abdominal pain, diarrhea, and constipation | A | Abdominal pain, diarrhea, or constipation | Studies measuring global symptom improvement lacking |
| Peppermint oil 31,4849 | Some demonstrated improvement in abdominal pain | B | Individual IBS symptoms | Studies measuring global symptom improvement lacking |
| Guar gum 44 | Improved abdominal pain and bowel alterations | B | Study compared fiber to guar gum–equal affect on abdominal pain. Gum was better toleratEd | No placebo-controlled trials |
| Probiotics48 (lactobacillus) | Improvement of abdominal pain and flatulence | C | Abdominal pain, flatulence | Two studies with small numbers |
| Elimination diets 48 | Improvement of diarrhea | C | Diarrhea | Milk, wheat, eggs eliminated; 15%-71% improvement of diarrhea |
| Lactose and fructose avoidance 48 | Conflicting evidence results | D | No controlled studies available | |
| Pancreatic enzymes 48 | No evidence | D | Evidence lacking | |
| Ginger 48 | No evidence | D | No studies |
Medications
Strength of recommendation: A. The recently approved 5HT4 receptor agonist tegaserod (Zelnorm) is more effective than placebo at relieving global symptoms in women with constipation (number needed to treat [NNT]=3.9-17).26-30 Diarrhea can be a serious side effect.
The 5HT3 receptor antagonist alosetron (Lotronex) is more effective than placebo at relieving global IBS symptoms in women with diarrhea (NNT=2.5-8.3).31-35 Severe constipation can be an adverse effect. The prescribing of alosetron is currently restricted to physicians who participate in the manufacturer’s risk management program.
In addition to these serotoninergic agents, others in this class are being developed and undergoing clinical trials. The knowledge being gained about 5HT receptors may revolutionize the care of patients with IBS.
Strength of recommendation: B. Tricyclic antidepressants are no more effective than placebo at relieving global IBS symptoms, but they do decrease abdominal pain (NNT=3.2-5).36-39
Loperamide is no more effective than placebo at relieving IBS global symptoms, but it may be used to treat diarrhea (NNT=2.3-5).31,40-42
Bulking agents (such as calcium polycarbophil or psyllium) are no more effective than placebo at relieving IBS global symptoms, but they may decrease constipation (NNT=2.2-8.6).31,36,43-47
Peppermint oil may be helpful for abdominal pain, but global symptom reduction has not been demonstrated.31,48-49 Only a few studies have looked at the use of antispasmodic agents for IBS. They are of poor quality and used small numbers with no placebo controls.23,31,36,43
Strength of recommendation: C. There are limited studies evaluating the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine. Paroxetine was shown in 1 study to improve quality of life.50 Fluoxetine reduced abdominal pain, but did not improve quality of life.51
Behavioral and complementary/alternative therapies
Relaxation therapy, hypnotherapy, and cognitive therapy are effective at relieving individual IBS symptoms, but have not been shown to reduce global IBS symptoms (SOR: B).52-57 Other alternative therapies (eg, guar gum44 [SOR: B], ginger48 [SOR: B], and pancreatic enzymes48 [SOR: C]) have been studied, but high-quality studies considering global improvement have not been published.
The position statement of the American College of Gastroenterology on the management of IBS23 and Brandt’s systematic review of this subject24 were the starting points for this review. The majority of the references from these sources were reviewed and a Medline search was completed to identify new evidence. The Oxford Centre for Evidence-Based Medicine grades of recommendations were applied to this evidence, a care algorithm was created, summary tables were developed, and numbers needed to treat were calculated.
Promote self-awareness
Quality-of-life assessment should be done routinely in the care of IBS patients. Provide support, empathy, and basic behavior modification tools. Educate patients and their families on the theoretical biochemical basis of this illness, and help them connect symptoms with stressors, to facilitate lifestyle modification.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastrenterol 2002;97:1910-1915.
2. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995;109:1736-1741.
3. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-1695.
4. Fass R, Longstreth GF, Pimental M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001;161:2081-2088.
5. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry 1997;42:835-840.
6. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140-1156.
7. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am 2000;84:1313-1327.
8. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221-227.
9. Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Internal Med 1992;116:1001-1008.
10. Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. JAMC 1999;161:154-160.
11. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002;122:1701-1714.
12. Tosetti C, Stanghellini V, Corinaldesi R. The Rome II criteria for patients with functional gastroduodenal disorders. J Clin Gastroenterol 2003;37:92-93.
13. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in US women. Am J Gastroenterol 2002;97:2803-2811.
14. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 1999;94:2912-2917.
15. Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol 1999;94:1279-1282.
16. Tolliver BA, Herrara JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol 1994;89:176-178.
17. MacIntosh DG, Thompson WG, Patel DP, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol 1992;87:1407-1409.
18. Francis CY, Duffy JN, Whorwell PJ, Martin DF. Does routine ultrasound enhance diagnostic accuracy in irritable bowel syndrome? Am J Gastroenterol 1996;91:1348-1350.
19. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult celiac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet 2001;358:1504-1508.
20. Pimental M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome; a double blind, randomized, placebo-controlled trial. Am J Gastroenterol 2003;98:412-419.
21. Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002;97:2812-2819.
22. O’Leary CO, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol 2002;97:1463-1467.
23. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastrenterol 2002;97:s1-s5.
24. Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, et al. Systematic review on the management of irritable bowel syndrome in north America. Am J Gastroenterol 2002;97:s7-s26.
25. Drossman DA, Patrick DL, Whitehead WE, NE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999-1007.
26. Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for the treatment of irritable bowel syndrome. J Clin Pharm Therapeutics 2002;27:343-352.
27. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877-1888.
28. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001;15:1655-1666.
29. Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003;52:671-676.
30. Jones RH, Holtmann G, Rodrigo L, Ehsanullah, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1419-1427.
31. Jailwala J, Imperiale TF, Kroeneke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-147.
32. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2002;15:79-86.
33. Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000;355:1035-1040.
34. Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149-1159.
35. Lembo T, Wright RA, Bagby B, et al. Lotronex Investigator Team. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predom-inant irritable bowel syndrome. Am J Gastroenterol 2001;96:2662-2670.
36. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomized control trials. Gut 2001;48:272-282.
37. Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroeneke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a metaanalysis. Am J Med 2000;108:65-72.
38. Myren J, Lovland B, Larssen SE, Larsen S. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol 1984;19:835-843.
39. Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32:257-266.
40. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
41. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl 1987;130:81-84.
42. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463-468.
43. Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376-378.
44. Parisi GC, Zilli M, Miani MP, E, et al. High-fiber diet supplementation in patients with irritable bowel syndrome. A multi-center, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum. Dig Dis Sci 2002;47:1697-1704.
45. Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (A randomized double-blind crossover study). JAPI 1982;30:353-354.
46. Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. BMJ 1983;76:253.-
47. Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: Improvement in overall well being is related to reduction in bowel dissatisfaction. J Gastroenterol Hep 1990;5:507-513.
48. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in irritable bowel syndrome. Arch Intern Med 2003;163:265-274.
49. Pittler MH, Ernst E. Peppermint Oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol 1998;93:1131-1135.
50. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303-317.
51. Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219-228.
52. Heymann-Monnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
53. Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: A critique of controlled treatment trials. Am J Gastroenterol 1996;91:277-286.
54. Svedlund J, Sjodin I, Ottoson JO, Dotevall G. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;2:589-592.
55. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consulting Clinic Psychology 1994;62:576-582.
56. Guthrie E, Creed F, Dawson D, Tomenson B. A ramdomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. B J Psychiatry 1993;163:315-321.
57. Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
- For patients aged <50 years without alarm symptoms, diagnostic testing is unnecessary. Consider celiac sprue testing for patients with diarrhea (C).
- Treatment is indicated when both the patient with irritable bowel syndrome and the physician agree that quality of life has been diminished (C). The goal of therapy is to alleviate global IBS symptoms (abdominal discomfort, bloating, and altered bowel habits that are life-impacting) (C).
- Tegaserod, a 5HT4 receptor agonist, is more effective than placebo at relieving global IBS symptoms in women with constipation (A). Its effectiveness in men is unknown.
- Alosetron, a 5HT3 receptor antagonist, is more effective than placebo at relieving global IBS symptoms in women with diarrhea (A).
- Behavior therapy–relaxation therapy, hypnotherapy, or cognitive therapy–is more effective than placebo at relieving individual symptoms, but no data are available for quality-of-life improvement (B).
An extensive and expensive evaluation for irritable bowel syndrome (IBS) can be avoided if your patient is aged <50 years and is not experiencing alarm symptoms (hematochezia, 10 lbs weight loss, fever, anemia, nocturnal or severe diarrhea), has not recently taken antibiotics, and has no family history of colon cancer. An algorithm ( Figure ) indicates when work-up is needed and what it should entail.
Newer medications that act on 5HT receptors have proven effective in improving quality of life (global symptom reduction). Evidence supports the use of several traditional medications to reduce individual symptoms of IBS, but not for global symptom reduction.
FIGURE
Evaluating possible irritable bowel syndrome
Who gets irritable bowel syndrome?
Ten percent to 15% of the North American population has IBS, and twice as many women as men have it.1 Symptoms usually begin before the age of 35 years, and many patients can trace their symptoms back to childhood.2 Onset in the elderly is rare.3 The disorder is responsible for approximately 50% of referrals to gastroenterologists.4
The company IBS keeps
Comorbid psychiatric illness is common with IBS, but few patients seek psychiatric care.5 Depression, anxiety, and somatoform disorders are seen in 94% of patients with IBS. IBS is common in patients with chronic fatigue syndrome (51%), fibromyalgia (49%), temporomandibular joint syndrome (64%), and chronic pelvic pain (50%).6 IBS often follows stressful life events,5,7,8 such as a death in the family or divorce. It tends to be chronic, intermittent, and relapsing.3
The symptoms of IBS can overlap with those of other illnesses, including thyroid dysfunction (diarrhea or constipation), functional dyspepsia (abdominal pain), Crohn’s disease or ulcerative colitis (diarrhea, abdominal pain), celiac sprue (diarrhea), polyps and cancers (constipation or abdominal pain), and infectious diarrhea.
Elusive physiologic mechanism
Several physiologic mechanisms have been proposed for IBS symptoms: altered gut reactivity in response to luminal stimuli, hypersensitive gut with enhanced pain response, and altered brain-gut biochemical axis.9 Though the symptoms of irritable bowel syndrome appear to have a physiologic basis, there are no structural or biochemical markers for the disease.
Use symptom-based criteria for diagnosis
Consider a diagnosis of IBS when a patient complains of abdominal discomfort and altered bowel habits. In the absence of a structural or biochemical marker, IBS must be diagnosed according to symptom-based criteria–such as Manning, Rome I, or Rome II–which have been developed for research and epidemiologic purposes. Though their clinical utility remains unproven, these criteria (delineated in Table 1 ) are the crux of clinical diagnosis for IBS.4,10-14 Subtypes of IBS have been described (diarrheapredominant IBS or constipation-predominant IBS), but they are not diagnostically useful, since the treatment goal is improved quality of life.
TABLE 1
Symptom-based criteria for irritable bowel syndrome
| Symptom-based criteria | Symptoms | Sn | Sp | PV+ |
|---|---|---|---|---|
| Manning4,10,13,14 |
| 42%–90% | 70%–100% | 74% |
| Rome I4,10,13 |
| 65%–84% | 100% | 69%–100% |
| Rome II11-13 |
| 49%-65%* | 100%* | 69%-100%* |
| Supportive symptoms | ||||
| Fewer than 3 bowel movements per week | ||||
| More than 3 bowel movements per day | ||||
| Hard or lumpy stools | ||||
| *Found to have similar sensitivity and specificity to Rome I.14 | ||||
| Sn, sensitivity; Sp, specificity; PV+, positive predictive value | ||||
Dubious value of diagnostic tests
The literature regarding the value of diagnostic testing for IBS is controversial. Symptom-based criteria have varied in many studies, as have the criteria used to enroll patients and the measured outcomes of treatment (reduction in abdominal pain, in diarrhea, or in constipation, or improvement in quality of life). Because of these discrepancies, it is difficult to apply the literature clinically. Of the 6 landmark studies that considered the value of diagnostic testing for IBS patients,15-20 only 2 compared IBS patients with groups of healthy controls.17,19
Test results yield little. Most research in this area has compared the prevalence of specific illnesses in the general population with the yield of positive test results for these illnesses among persons meeting the symptom-based diagnostic criteria for IBS.
Two studies15,16 determined the incidence of abnormal test results in patients who met the Manning or Rome I criteria for IBS. In these studies, most diagnostic tests yielded positive results in 2% (range, 0%-8.2%) of patients or less, except for thyroid and lactose intolerance testing. That is equivalent to the incidence in the general population. The prevalence of thyroid disorders and lactose malabsorption was higher in IBS patients (6% and 22%-26%, respectively), but prevalence in the general population is similarly higher (5%-9% and 25%). Based on these results, testing for thyroid disease or lactose malabsorption is indicated only for patients exhibiting symptoms of these disorders (fatigue/weight change or diarrhea related to diertary intake of dairy products, respectively).
An exception. Some clinicians propose that diagnostic testing for patients with IBS symptoms should be driven by the pretest probability of organic disease (prevalence) compared with the general population. Cash21 found the pretest probability of inflammatory bowel disease, colorectal cancer, and infectious diarrhea is less than 1% among IBS patients without alarm symptoms ( Table 2 ). He demonstrated that patients with IBS had a 5% pretest probability of celiac sprue compared with healthy patients (<1% prevalence). Therefore, testing for celiac sprue (eg, complete blood count, antiendomysial antibody, and antigliadin antibody) may be considered for patients with diarrhea.6,21,22 Sigmoidoscopy,15,17 rectal biopsy,17 and abdominal ultrasound18 have low positive yield in patients meeting the diagnostic criteria for IBS.
TABLE 2
Probability of organic disease in irritable bowel syndrome patients
| Disease | Pretest probability-IBS patients (%) | Prevalence-general population (%) | Comments |
|---|---|---|---|
| Colitis/inflammatory inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 | Structural colon lesions were detected with barium enema, colonscopy, sigmoidoscopy |
| Colon cancer | 0-0.51 | 4-6 | Structural colon lesions were detected with barium enema, colonoscopy, sigmoidoscopy |
| Celiac disease | 4.67 | 0.25-0.5 | Note: celiac disease prevalence higher than in general population. |
| Gastrointestinal infection | 0-1.7 | N/A | |
| Thyroid dysfunction | 6 | 5-9 | Prevalence high in both groups |
| Lactose malabsorption | 22-26 | 25 | Prevalence high in both groups |
| Adapted from: Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002; 97:2812-2819. | |||
| Results are from multiple studies: n=125-306. | |||
How to proceed
Those under 50 years of age who have no alarm symptoms can forgo further testing. Testing for celiac sprue and lactose malabsorption might be considered for patients with diarrhea that improves or worsens with change in diet (strength of recommendation [SOR]: C).
Threshold for treatment
Treatment for IBS is indicated when both patient and physician believe global symptoms (abdominal discomfort, bloating, altered bowel habits) have diminished the quality of life (SOR: C). The goal of treatment is to alleviate all IBS symptoms (SOR: C). Treating altered bowel habits (constipation, diarrhea, and fecal urgency) without addressing other IBS symptoms (eg, abdominal pain) is inferior treatment.23,24
Treatment options for IBS
Treatments for IBS include medications, behavior therapy, and complimentary and alternative therapies. Medications traditionally prescribed include bulking agents, anticholinergics/antispasmodics, antidiarrheals, and antidepressants. A 5HT3 receptor antagonist and a 5HT4 receptor partial agonist are now available. Table 3 summarizes the traditional treatments in terms of efficacy, strength of recommendations, and outcomes measured. Alternative and complimentary therapies appear in Table 4 .
As Brandt24 has noted, the evidence for treatment effectiveness is difficult to review and summarize, because the quality of studies has been poor. Most studies did not use healthy control groups, and the numbers of participants were small. Many studies did not use blinded placebo groups. Outcomes measured varied among the studies, with most of them measuring reductions of individual bowel symptoms (eg, diarrhea or constipation). Quality-of-life tools were used in other studies to measure reduction in global IBS symptoms (eg, IBS Quality of Life 25 ). Because of these discrepancies, there is no sound evidence for traditional therapies.
TABLE 3
Treatments for irritable bowel syndrome
| Treatment | Efficacy (NNT) | SOR (studies) | Outcomes measured | Comments |
|---|---|---|---|---|
| 5HT4 receptor agonist (tegaserod)23,24,26-30 | More effective than placebo at relieving global IBS symptoms in women with constipation (3.9-17) | A (4) | Global IBS symptoms, individual IBS symptoms | 83%-100% of study participants were women with IBS and constipation. Rome I and II criteria for entry. May cause diarrhea |
| 5HT4 receptor agonist(alosetron)23,24,26- 35 | More effective than placebo at relieving global IBS symptoms in women with diarrhea (2.5-8.3) | A (4) | Global IBS symptoms, individual IBS symptoms, adverse events | 82%-93% of study participants were women. Rome I and II criteria for entry. May cause severe constipation; restricted use |
| Tricylic antidepressants (trimipramine, desipramine)23,24,36- 39 | Reduces abdominal pain. No more effective than placebo at relieving gloal IBS symptoms (3.2-5) | B (6) | GI symptoms | May cause constipation; no studies done with SSRIs |
| Loperamide23,24,36-39 | Relieves diarrhea. No more effective than placebo at relieving global IBS symptoms (3.2-5) | B (3) | Global IBS symptoms, diarrhea | Constipation or paralytic ileus can occur |
| Bulking agents (corn fiber, wheat bran, psyllium, ispaghula husks, calcium polycabophil)23,24,31,40-42 | Improves constipation. No more effective than placebo in studies considering global symptom improvement (2.2-8.6) | B (13) | GI symptoms, global IBS symptoms | May increase bloating. All studiessmall numbers of patients |
| Anti-spasmodics (hyoscyamine dicyclomine)23,24,26-30 | No evidence on improvement of global IBS symptoms (5.9) | B (3) | Individual IBS and global symptoms | Studies were short, small numbers, inconsistent effectiveness. Could worsen constipation; 15 additional studies done on drugs not available in the US |
| Behavioral therapies (hypnotherapy, relaxation therapy, psychotherapy, biofeedback)23,24,44, 52-57 | More effective than placebo at relieving individuals IBS symptoms (1.4-1.9) | B (16) | GI symptoms, psychological sypmtoms | None measures global IBS symptom improvement. Small numbers of patients |
| SSRI antidepressants (paroxtetine, fluoxetine)23,24, 50-51 | Improved quality of life, decreased abdominal pain | B (16) | Abdominal | One study severe IBS, other study only 10 participants quality of life |
| SOR, strength of recommendation; IBS, irritable bowel syndrome; GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor. For an explanation of SORs. | ||||
TABLE 4
Complementary and alternative treatments for irritable bowel syndrome
| Treatment | Efficacy | SOR | Outcomes measured | Comments |
|---|---|---|---|---|
| Neomycin 20 | Treatment for 1 week improved symptoms of abdominal pain, diarrhea, and constipation | A | Abdominal pain, diarrhea, or constipation | Studies measuring global symptom improvement lacking |
| Peppermint oil 31,4849 | Some demonstrated improvement in abdominal pain | B | Individual IBS symptoms | Studies measuring global symptom improvement lacking |
| Guar gum 44 | Improved abdominal pain and bowel alterations | B | Study compared fiber to guar gum–equal affect on abdominal pain. Gum was better toleratEd | No placebo-controlled trials |
| Probiotics48 (lactobacillus) | Improvement of abdominal pain and flatulence | C | Abdominal pain, flatulence | Two studies with small numbers |
| Elimination diets 48 | Improvement of diarrhea | C | Diarrhea | Milk, wheat, eggs eliminated; 15%-71% improvement of diarrhea |
| Lactose and fructose avoidance 48 | Conflicting evidence results | D | No controlled studies available | |
| Pancreatic enzymes 48 | No evidence | D | Evidence lacking | |
| Ginger 48 | No evidence | D | No studies |
Medications
Strength of recommendation: A. The recently approved 5HT4 receptor agonist tegaserod (Zelnorm) is more effective than placebo at relieving global symptoms in women with constipation (number needed to treat [NNT]=3.9-17).26-30 Diarrhea can be a serious side effect.
The 5HT3 receptor antagonist alosetron (Lotronex) is more effective than placebo at relieving global IBS symptoms in women with diarrhea (NNT=2.5-8.3).31-35 Severe constipation can be an adverse effect. The prescribing of alosetron is currently restricted to physicians who participate in the manufacturer’s risk management program.
In addition to these serotoninergic agents, others in this class are being developed and undergoing clinical trials. The knowledge being gained about 5HT receptors may revolutionize the care of patients with IBS.
Strength of recommendation: B. Tricyclic antidepressants are no more effective than placebo at relieving global IBS symptoms, but they do decrease abdominal pain (NNT=3.2-5).36-39
Loperamide is no more effective than placebo at relieving IBS global symptoms, but it may be used to treat diarrhea (NNT=2.3-5).31,40-42
Bulking agents (such as calcium polycarbophil or psyllium) are no more effective than placebo at relieving IBS global symptoms, but they may decrease constipation (NNT=2.2-8.6).31,36,43-47
Peppermint oil may be helpful for abdominal pain, but global symptom reduction has not been demonstrated.31,48-49 Only a few studies have looked at the use of antispasmodic agents for IBS. They are of poor quality and used small numbers with no placebo controls.23,31,36,43
Strength of recommendation: C. There are limited studies evaluating the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine. Paroxetine was shown in 1 study to improve quality of life.50 Fluoxetine reduced abdominal pain, but did not improve quality of life.51
Behavioral and complementary/alternative therapies
Relaxation therapy, hypnotherapy, and cognitive therapy are effective at relieving individual IBS symptoms, but have not been shown to reduce global IBS symptoms (SOR: B).52-57 Other alternative therapies (eg, guar gum44 [SOR: B], ginger48 [SOR: B], and pancreatic enzymes48 [SOR: C]) have been studied, but high-quality studies considering global improvement have not been published.
The position statement of the American College of Gastroenterology on the management of IBS23 and Brandt’s systematic review of this subject24 were the starting points for this review. The majority of the references from these sources were reviewed and a Medline search was completed to identify new evidence. The Oxford Centre for Evidence-Based Medicine grades of recommendations were applied to this evidence, a care algorithm was created, summary tables were developed, and numbers needed to treat were calculated.
Promote self-awareness
Quality-of-life assessment should be done routinely in the care of IBS patients. Provide support, empathy, and basic behavior modification tools. Educate patients and their families on the theoretical biochemical basis of this illness, and help them connect symptoms with stressors, to facilitate lifestyle modification.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- For patients aged <50 years without alarm symptoms, diagnostic testing is unnecessary. Consider celiac sprue testing for patients with diarrhea (C).
- Treatment is indicated when both the patient with irritable bowel syndrome and the physician agree that quality of life has been diminished (C). The goal of therapy is to alleviate global IBS symptoms (abdominal discomfort, bloating, and altered bowel habits that are life-impacting) (C).
- Tegaserod, a 5HT4 receptor agonist, is more effective than placebo at relieving global IBS symptoms in women with constipation (A). Its effectiveness in men is unknown.
- Alosetron, a 5HT3 receptor antagonist, is more effective than placebo at relieving global IBS symptoms in women with diarrhea (A).
- Behavior therapy–relaxation therapy, hypnotherapy, or cognitive therapy–is more effective than placebo at relieving individual symptoms, but no data are available for quality-of-life improvement (B).
An extensive and expensive evaluation for irritable bowel syndrome (IBS) can be avoided if your patient is aged <50 years and is not experiencing alarm symptoms (hematochezia, 10 lbs weight loss, fever, anemia, nocturnal or severe diarrhea), has not recently taken antibiotics, and has no family history of colon cancer. An algorithm ( Figure ) indicates when work-up is needed and what it should entail.
Newer medications that act on 5HT receptors have proven effective in improving quality of life (global symptom reduction). Evidence supports the use of several traditional medications to reduce individual symptoms of IBS, but not for global symptom reduction.
FIGURE
Evaluating possible irritable bowel syndrome
Who gets irritable bowel syndrome?
Ten percent to 15% of the North American population has IBS, and twice as many women as men have it.1 Symptoms usually begin before the age of 35 years, and many patients can trace their symptoms back to childhood.2 Onset in the elderly is rare.3 The disorder is responsible for approximately 50% of referrals to gastroenterologists.4
The company IBS keeps
Comorbid psychiatric illness is common with IBS, but few patients seek psychiatric care.5 Depression, anxiety, and somatoform disorders are seen in 94% of patients with IBS. IBS is common in patients with chronic fatigue syndrome (51%), fibromyalgia (49%), temporomandibular joint syndrome (64%), and chronic pelvic pain (50%).6 IBS often follows stressful life events,5,7,8 such as a death in the family or divorce. It tends to be chronic, intermittent, and relapsing.3
The symptoms of IBS can overlap with those of other illnesses, including thyroid dysfunction (diarrhea or constipation), functional dyspepsia (abdominal pain), Crohn’s disease or ulcerative colitis (diarrhea, abdominal pain), celiac sprue (diarrhea), polyps and cancers (constipation or abdominal pain), and infectious diarrhea.
Elusive physiologic mechanism
Several physiologic mechanisms have been proposed for IBS symptoms: altered gut reactivity in response to luminal stimuli, hypersensitive gut with enhanced pain response, and altered brain-gut biochemical axis.9 Though the symptoms of irritable bowel syndrome appear to have a physiologic basis, there are no structural or biochemical markers for the disease.
Use symptom-based criteria for diagnosis
Consider a diagnosis of IBS when a patient complains of abdominal discomfort and altered bowel habits. In the absence of a structural or biochemical marker, IBS must be diagnosed according to symptom-based criteria–such as Manning, Rome I, or Rome II–which have been developed for research and epidemiologic purposes. Though their clinical utility remains unproven, these criteria (delineated in Table 1 ) are the crux of clinical diagnosis for IBS.4,10-14 Subtypes of IBS have been described (diarrheapredominant IBS or constipation-predominant IBS), but they are not diagnostically useful, since the treatment goal is improved quality of life.
TABLE 1
Symptom-based criteria for irritable bowel syndrome
| Symptom-based criteria | Symptoms | Sn | Sp | PV+ |
|---|---|---|---|---|
| Manning4,10,13,14 |
| 42%–90% | 70%–100% | 74% |
| Rome I4,10,13 |
| 65%–84% | 100% | 69%–100% |
| Rome II11-13 |
| 49%-65%* | 100%* | 69%-100%* |
| Supportive symptoms | ||||
| Fewer than 3 bowel movements per week | ||||
| More than 3 bowel movements per day | ||||
| Hard or lumpy stools | ||||
| *Found to have similar sensitivity and specificity to Rome I.14 | ||||
| Sn, sensitivity; Sp, specificity; PV+, positive predictive value | ||||
Dubious value of diagnostic tests
The literature regarding the value of diagnostic testing for IBS is controversial. Symptom-based criteria have varied in many studies, as have the criteria used to enroll patients and the measured outcomes of treatment (reduction in abdominal pain, in diarrhea, or in constipation, or improvement in quality of life). Because of these discrepancies, it is difficult to apply the literature clinically. Of the 6 landmark studies that considered the value of diagnostic testing for IBS patients,15-20 only 2 compared IBS patients with groups of healthy controls.17,19
Test results yield little. Most research in this area has compared the prevalence of specific illnesses in the general population with the yield of positive test results for these illnesses among persons meeting the symptom-based diagnostic criteria for IBS.
Two studies15,16 determined the incidence of abnormal test results in patients who met the Manning or Rome I criteria for IBS. In these studies, most diagnostic tests yielded positive results in 2% (range, 0%-8.2%) of patients or less, except for thyroid and lactose intolerance testing. That is equivalent to the incidence in the general population. The prevalence of thyroid disorders and lactose malabsorption was higher in IBS patients (6% and 22%-26%, respectively), but prevalence in the general population is similarly higher (5%-9% and 25%). Based on these results, testing for thyroid disease or lactose malabsorption is indicated only for patients exhibiting symptoms of these disorders (fatigue/weight change or diarrhea related to diertary intake of dairy products, respectively).
An exception. Some clinicians propose that diagnostic testing for patients with IBS symptoms should be driven by the pretest probability of organic disease (prevalence) compared with the general population. Cash21 found the pretest probability of inflammatory bowel disease, colorectal cancer, and infectious diarrhea is less than 1% among IBS patients without alarm symptoms ( Table 2 ). He demonstrated that patients with IBS had a 5% pretest probability of celiac sprue compared with healthy patients (<1% prevalence). Therefore, testing for celiac sprue (eg, complete blood count, antiendomysial antibody, and antigliadin antibody) may be considered for patients with diarrhea.6,21,22 Sigmoidoscopy,15,17 rectal biopsy,17 and abdominal ultrasound18 have low positive yield in patients meeting the diagnostic criteria for IBS.
TABLE 2
Probability of organic disease in irritable bowel syndrome patients
| Disease | Pretest probability-IBS patients (%) | Prevalence-general population (%) | Comments |
|---|---|---|---|
| Colitis/inflammatory inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 | Structural colon lesions were detected with barium enema, colonscopy, sigmoidoscopy |
| Colon cancer | 0-0.51 | 4-6 | Structural colon lesions were detected with barium enema, colonoscopy, sigmoidoscopy |
| Celiac disease | 4.67 | 0.25-0.5 | Note: celiac disease prevalence higher than in general population. |
| Gastrointestinal infection | 0-1.7 | N/A | |
| Thyroid dysfunction | 6 | 5-9 | Prevalence high in both groups |
| Lactose malabsorption | 22-26 | 25 | Prevalence high in both groups |
| Adapted from: Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002; 97:2812-2819. | |||
| Results are from multiple studies: n=125-306. | |||
How to proceed
Those under 50 years of age who have no alarm symptoms can forgo further testing. Testing for celiac sprue and lactose malabsorption might be considered for patients with diarrhea that improves or worsens with change in diet (strength of recommendation [SOR]: C).
Threshold for treatment
Treatment for IBS is indicated when both patient and physician believe global symptoms (abdominal discomfort, bloating, altered bowel habits) have diminished the quality of life (SOR: C). The goal of treatment is to alleviate all IBS symptoms (SOR: C). Treating altered bowel habits (constipation, diarrhea, and fecal urgency) without addressing other IBS symptoms (eg, abdominal pain) is inferior treatment.23,24
Treatment options for IBS
Treatments for IBS include medications, behavior therapy, and complimentary and alternative therapies. Medications traditionally prescribed include bulking agents, anticholinergics/antispasmodics, antidiarrheals, and antidepressants. A 5HT3 receptor antagonist and a 5HT4 receptor partial agonist are now available. Table 3 summarizes the traditional treatments in terms of efficacy, strength of recommendations, and outcomes measured. Alternative and complimentary therapies appear in Table 4 .
As Brandt24 has noted, the evidence for treatment effectiveness is difficult to review and summarize, because the quality of studies has been poor. Most studies did not use healthy control groups, and the numbers of participants were small. Many studies did not use blinded placebo groups. Outcomes measured varied among the studies, with most of them measuring reductions of individual bowel symptoms (eg, diarrhea or constipation). Quality-of-life tools were used in other studies to measure reduction in global IBS symptoms (eg, IBS Quality of Life 25 ). Because of these discrepancies, there is no sound evidence for traditional therapies.
TABLE 3
Treatments for irritable bowel syndrome
| Treatment | Efficacy (NNT) | SOR (studies) | Outcomes measured | Comments |
|---|---|---|---|---|
| 5HT4 receptor agonist (tegaserod)23,24,26-30 | More effective than placebo at relieving global IBS symptoms in women with constipation (3.9-17) | A (4) | Global IBS symptoms, individual IBS symptoms | 83%-100% of study participants were women with IBS and constipation. Rome I and II criteria for entry. May cause diarrhea |
| 5HT4 receptor agonist(alosetron)23,24,26- 35 | More effective than placebo at relieving global IBS symptoms in women with diarrhea (2.5-8.3) | A (4) | Global IBS symptoms, individual IBS symptoms, adverse events | 82%-93% of study participants were women. Rome I and II criteria for entry. May cause severe constipation; restricted use |
| Tricylic antidepressants (trimipramine, desipramine)23,24,36- 39 | Reduces abdominal pain. No more effective than placebo at relieving gloal IBS symptoms (3.2-5) | B (6) | GI symptoms | May cause constipation; no studies done with SSRIs |
| Loperamide23,24,36-39 | Relieves diarrhea. No more effective than placebo at relieving global IBS symptoms (3.2-5) | B (3) | Global IBS symptoms, diarrhea | Constipation or paralytic ileus can occur |
| Bulking agents (corn fiber, wheat bran, psyllium, ispaghula husks, calcium polycabophil)23,24,31,40-42 | Improves constipation. No more effective than placebo in studies considering global symptom improvement (2.2-8.6) | B (13) | GI symptoms, global IBS symptoms | May increase bloating. All studiessmall numbers of patients |
| Anti-spasmodics (hyoscyamine dicyclomine)23,24,26-30 | No evidence on improvement of global IBS symptoms (5.9) | B (3) | Individual IBS and global symptoms | Studies were short, small numbers, inconsistent effectiveness. Could worsen constipation; 15 additional studies done on drugs not available in the US |
| Behavioral therapies (hypnotherapy, relaxation therapy, psychotherapy, biofeedback)23,24,44, 52-57 | More effective than placebo at relieving individuals IBS symptoms (1.4-1.9) | B (16) | GI symptoms, psychological sypmtoms | None measures global IBS symptom improvement. Small numbers of patients |
| SSRI antidepressants (paroxtetine, fluoxetine)23,24, 50-51 | Improved quality of life, decreased abdominal pain | B (16) | Abdominal | One study severe IBS, other study only 10 participants quality of life |
| SOR, strength of recommendation; IBS, irritable bowel syndrome; GI, gastrointestinal; SSRI, selective serotonin reuptake inhibitor. For an explanation of SORs. | ||||
TABLE 4
Complementary and alternative treatments for irritable bowel syndrome
| Treatment | Efficacy | SOR | Outcomes measured | Comments |
|---|---|---|---|---|
| Neomycin 20 | Treatment for 1 week improved symptoms of abdominal pain, diarrhea, and constipation | A | Abdominal pain, diarrhea, or constipation | Studies measuring global symptom improvement lacking |
| Peppermint oil 31,4849 | Some demonstrated improvement in abdominal pain | B | Individual IBS symptoms | Studies measuring global symptom improvement lacking |
| Guar gum 44 | Improved abdominal pain and bowel alterations | B | Study compared fiber to guar gum–equal affect on abdominal pain. Gum was better toleratEd | No placebo-controlled trials |
| Probiotics48 (lactobacillus) | Improvement of abdominal pain and flatulence | C | Abdominal pain, flatulence | Two studies with small numbers |
| Elimination diets 48 | Improvement of diarrhea | C | Diarrhea | Milk, wheat, eggs eliminated; 15%-71% improvement of diarrhea |
| Lactose and fructose avoidance 48 | Conflicting evidence results | D | No controlled studies available | |
| Pancreatic enzymes 48 | No evidence | D | Evidence lacking | |
| Ginger 48 | No evidence | D | No studies |
Medications
Strength of recommendation: A. The recently approved 5HT4 receptor agonist tegaserod (Zelnorm) is more effective than placebo at relieving global symptoms in women with constipation (number needed to treat [NNT]=3.9-17).26-30 Diarrhea can be a serious side effect.
The 5HT3 receptor antagonist alosetron (Lotronex) is more effective than placebo at relieving global IBS symptoms in women with diarrhea (NNT=2.5-8.3).31-35 Severe constipation can be an adverse effect. The prescribing of alosetron is currently restricted to physicians who participate in the manufacturer’s risk management program.
In addition to these serotoninergic agents, others in this class are being developed and undergoing clinical trials. The knowledge being gained about 5HT receptors may revolutionize the care of patients with IBS.
Strength of recommendation: B. Tricyclic antidepressants are no more effective than placebo at relieving global IBS symptoms, but they do decrease abdominal pain (NNT=3.2-5).36-39
Loperamide is no more effective than placebo at relieving IBS global symptoms, but it may be used to treat diarrhea (NNT=2.3-5).31,40-42
Bulking agents (such as calcium polycarbophil or psyllium) are no more effective than placebo at relieving IBS global symptoms, but they may decrease constipation (NNT=2.2-8.6).31,36,43-47
Peppermint oil may be helpful for abdominal pain, but global symptom reduction has not been demonstrated.31,48-49 Only a few studies have looked at the use of antispasmodic agents for IBS. They are of poor quality and used small numbers with no placebo controls.23,31,36,43
Strength of recommendation: C. There are limited studies evaluating the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and paroxetine. Paroxetine was shown in 1 study to improve quality of life.50 Fluoxetine reduced abdominal pain, but did not improve quality of life.51
Behavioral and complementary/alternative therapies
Relaxation therapy, hypnotherapy, and cognitive therapy are effective at relieving individual IBS symptoms, but have not been shown to reduce global IBS symptoms (SOR: B).52-57 Other alternative therapies (eg, guar gum44 [SOR: B], ginger48 [SOR: B], and pancreatic enzymes48 [SOR: C]) have been studied, but high-quality studies considering global improvement have not been published.
The position statement of the American College of Gastroenterology on the management of IBS23 and Brandt’s systematic review of this subject24 were the starting points for this review. The majority of the references from these sources were reviewed and a Medline search was completed to identify new evidence. The Oxford Centre for Evidence-Based Medicine grades of recommendations were applied to this evidence, a care algorithm was created, summary tables were developed, and numbers needed to treat were calculated.
Promote self-awareness
Quality-of-life assessment should be done routinely in the care of IBS patients. Provide support, empathy, and basic behavior modification tools. Educate patients and their families on the theoretical biochemical basis of this illness, and help them connect symptoms with stressors, to facilitate lifestyle modification.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastrenterol 2002;97:1910-1915.
2. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995;109:1736-1741.
3. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-1695.
4. Fass R, Longstreth GF, Pimental M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001;161:2081-2088.
5. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry 1997;42:835-840.
6. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140-1156.
7. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am 2000;84:1313-1327.
8. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221-227.
9. Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Internal Med 1992;116:1001-1008.
10. Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. JAMC 1999;161:154-160.
11. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002;122:1701-1714.
12. Tosetti C, Stanghellini V, Corinaldesi R. The Rome II criteria for patients with functional gastroduodenal disorders. J Clin Gastroenterol 2003;37:92-93.
13. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in US women. Am J Gastroenterol 2002;97:2803-2811.
14. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 1999;94:2912-2917.
15. Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol 1999;94:1279-1282.
16. Tolliver BA, Herrara JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol 1994;89:176-178.
17. MacIntosh DG, Thompson WG, Patel DP, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol 1992;87:1407-1409.
18. Francis CY, Duffy JN, Whorwell PJ, Martin DF. Does routine ultrasound enhance diagnostic accuracy in irritable bowel syndrome? Am J Gastroenterol 1996;91:1348-1350.
19. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult celiac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet 2001;358:1504-1508.
20. Pimental M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome; a double blind, randomized, placebo-controlled trial. Am J Gastroenterol 2003;98:412-419.
21. Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002;97:2812-2819.
22. O’Leary CO, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol 2002;97:1463-1467.
23. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastrenterol 2002;97:s1-s5.
24. Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, et al. Systematic review on the management of irritable bowel syndrome in north America. Am J Gastroenterol 2002;97:s7-s26.
25. Drossman DA, Patrick DL, Whitehead WE, NE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999-1007.
26. Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for the treatment of irritable bowel syndrome. J Clin Pharm Therapeutics 2002;27:343-352.
27. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877-1888.
28. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001;15:1655-1666.
29. Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003;52:671-676.
30. Jones RH, Holtmann G, Rodrigo L, Ehsanullah, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1419-1427.
31. Jailwala J, Imperiale TF, Kroeneke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-147.
32. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2002;15:79-86.
33. Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000;355:1035-1040.
34. Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149-1159.
35. Lembo T, Wright RA, Bagby B, et al. Lotronex Investigator Team. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predom-inant irritable bowel syndrome. Am J Gastroenterol 2001;96:2662-2670.
36. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomized control trials. Gut 2001;48:272-282.
37. Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroeneke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a metaanalysis. Am J Med 2000;108:65-72.
38. Myren J, Lovland B, Larssen SE, Larsen S. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol 1984;19:835-843.
39. Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32:257-266.
40. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
41. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl 1987;130:81-84.
42. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463-468.
43. Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376-378.
44. Parisi GC, Zilli M, Miani MP, E, et al. High-fiber diet supplementation in patients with irritable bowel syndrome. A multi-center, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum. Dig Dis Sci 2002;47:1697-1704.
45. Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (A randomized double-blind crossover study). JAPI 1982;30:353-354.
46. Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. BMJ 1983;76:253.-
47. Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: Improvement in overall well being is related to reduction in bowel dissatisfaction. J Gastroenterol Hep 1990;5:507-513.
48. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in irritable bowel syndrome. Arch Intern Med 2003;163:265-274.
49. Pittler MH, Ernst E. Peppermint Oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol 1998;93:1131-1135.
50. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303-317.
51. Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219-228.
52. Heymann-Monnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
53. Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: A critique of controlled treatment trials. Am J Gastroenterol 1996;91:277-286.
54. Svedlund J, Sjodin I, Ottoson JO, Dotevall G. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;2:589-592.
55. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consulting Clinic Psychology 1994;62:576-582.
56. Guthrie E, Creed F, Dawson D, Tomenson B. A ramdomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. B J Psychiatry 1993;163:315-321.
57. Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
1. Saito YA, Schoenfeld P, Locke R. The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastrenterol 2002;97:1910-1915.
2. Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995;109:1736-1741.
3. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-1695.
4. Fass R, Longstreth GF, Pimental M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med 2001;161:2081-2088.
5. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry 1997;42:835-840.
6. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140-1156.
7. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am 2000;84:1313-1327.
8. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160:221-227.
9. Camilleri M, Prather CM. The irritable bowel syndrome: mechanisms and a practical approach to management. Ann Internal Med 1992;116:1001-1008.
10. Paterson WG, Thompson WG, Vanner SJ, et al. Recommendations for the management of irritable bowel syndrome in family practice. JAMC 1999;161:154-160.
11. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002;122:1701-1714.
12. Tosetti C, Stanghellini V, Corinaldesi R. The Rome II criteria for patients with functional gastroduodenal disorders. J Clin Gastroenterol 2003;37:92-93.
13. Chey WD, Olden K, Carter E, Boyle J, Drossman D, Chang L. Utility of the Rome I and Rome II criteria for irritable bowel syndrome in US women. Am J Gastroenterol 2002;97:2803-2811.
14. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 1999;94:2912-2917.
15. Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol 1999;94:1279-1282.
16. Tolliver BA, Herrara JL, DiPalma JA. Evaluation of patients who meet clinical criteria for irritable bowel syndrome. Am J Gastroenterol 1994;89:176-178.
17. MacIntosh DG, Thompson WG, Patel DP, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol 1992;87:1407-1409.
18. Francis CY, Duffy JN, Whorwell PJ, Martin DF. Does routine ultrasound enhance diagnostic accuracy in irritable bowel syndrome? Am J Gastroenterol 1996;91:1348-1350.
19. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult celiac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet 2001;358:1504-1508.
20. Pimental M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome; a double blind, randomized, placebo-controlled trial. Am J Gastroenterol 2003;98:412-419.
21. Cash BD, Schonfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol 2002;97:2812-2819.
22. O’Leary CO, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol 2002;97:1463-1467.
23. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastrenterol 2002;97:s1-s5.
24. Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, et al. Systematic review on the management of irritable bowel syndrome in north America. Am J Gastroenterol 2002;97:s7-s26.
25. Drossman DA, Patrick DL, Whitehead WE, NE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999-1007.
26. Jones BW, Moore DJ, Robinson SM, Song F. A systematic review of tegaserod for the treatment of irritable bowel syndrome. J Clin Pharm Therapeutics 2002;27:343-352.
27. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877-1888.
28. Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther 2001;15:1655-1666.
29. Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut 2003;52:671-676.
30. Jones RH, Holtmann G, Rodrigo L, Ehsanullah, Crompton PM, Jacques LA, Mills JG. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1419-1427.
31. Jailwala J, Imperiale TF, Kroeneke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-147.
32. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2002;15:79-86.
33. Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000;355:1035-1040.
34. Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149-1159.
35. Lembo T, Wright RA, Bagby B, et al. Lotronex Investigator Team. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predom-inant irritable bowel syndrome. Am J Gastroenterol 2001;96:2662-2670.
36. Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomized control trials. Gut 2001;48:272-282.
37. Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroeneke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a metaanalysis. Am J Med 2000;108:65-72.
38. Myren J, Lovland B, Larssen SE, Larsen S. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol 1984;19:835-843.
39. Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci 1987;32:257-266.
40. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984;29:239-247.
41. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl 1987;130:81-84.
42. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996;31:463-468.
43. Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376-378.
44. Parisi GC, Zilli M, Miani MP, E, et al. High-fiber diet supplementation in patients with irritable bowel syndrome. A multi-center, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum. Dig Dis Sci 2002;47:1697-1704.
45. Golechha AC, Chadda VS, Chadda S, Sharma SK, Mishra SN. Role of ispaghula husk in the management of irritable bowel syndrome (A randomized double-blind crossover study). JAPI 1982;30:353-354.
46. Arthurs Y, Fielding JF. Double blind trial of ispaghula/poloxamer in the irritable bowel syndrome. BMJ 1983;76:253.-
47. Jalihal A, Kurian G. Ispaghula therapy in irritable bowel syndrome: Improvement in overall well being is related to reduction in bowel dissatisfaction. J Gastroenterol Hep 1990;5:507-513.
48. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in irritable bowel syndrome. Arch Intern Med 2003;163:265-274.
49. Pittler MH, Ernst E. Peppermint Oil for irritable bowel syndrome: A critical review and meta-analysis. Am J Gastroenterol 1998;93:1131-1135.
50. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303-317.
51. Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219-228.
52. Heymann-Monnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
53. Talley NJ, Owen BK, Boyce P, Paterson K. Psychological treatments for irritable bowel syndrome: A critique of controlled treatment trials. Am J Gastroenterol 1996;91:277-286.
54. Svedlund J, Sjodin I, Ottoson JO, Dotevall G. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;2:589-592.
55. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consulting Clinic Psychology 1994;62:576-582.
56. Guthrie E, Creed F, Dawson D, Tomenson B. A ramdomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. B J Psychiatry 1993;163:315-321.
57. Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-994.
Clear choices in managing epidermal tinea infections
- Potassium hydroxide preparation should be used as an aid to diagnosis for all erythrosquamous lesions (B).
- Fungal culture should be used in cases in which history, physical examination, and potassium hydroxide preparation fail to clearly exclude a diagnosis of tinea (B).
- Short-duration topical therapy with terbinafine, naftifine, and butenafine is efficacious for most epidermal tinea infections (A).
- Oral antifungal agents are important in the treatment of tinea infections thatare widespread, fail to respond to topical treatment, involve the thick stratum corneum of the soles and palms, or occur in immunosuppressed patients.
- Short courses of oral itraconazole and terbinafine are safe and effective in treating tinea infections (A).
Though findings on history and physical examination are sometimes sufficient to make a diagnosis of tinea infection, a potassium hydroxide (KOH) study usually is required for confirmation. Even the KOH study can be misleading, however, if a patient recently self-administered a topical antifungal agent. This article describes the varying appearance of tinea infections according to their anatomic location, and outlines a careful work-up.
Highly effective and affordable over-the-counter medications have proliferated, and short-course therapy is available. Based on systematic reviews of randomized, controlled studies, it is possible to recommend specific first-line therapies for tinea infections.
Manifestations of tinea infection
Clinical manifestations of tinea vary with anatomic location, duration of infection, and pathogen. In general, zoophilic dermatophytes evoke a more vigorous host response than the anthropophilic species.5 Shared features of many dermatophyte infections include erythema, scaling, pruritus, ring formation, and central clearing of lesions. Table 1 reviews published data on the diagnostic value of selected clinical signs in suspected tinea infection.6
TABLE 1
Diagnostic value of selected signs and symptoms in tinea infection
| Sign/symptom | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|
| Scaling | 77% | 20% | 17% | 80% | 0.96 | 1.15 |
| Erythema | 69% | 31% | 18% | 83% | 1.00 | 1.00 |
| Pruritus | 54% | 40% | 16% | 80% | 0.90 | 1.15 |
| Central clearing | 42% | 65% | 20% | 84% | 1.20 | 0.89 |
| Concentric rings | 27% | 80% | 23% | 84% | 1.35 | 0.91 |
| Maceration | 27% | 84% | 26% | 84% | 1.69 | 0.87 |
| Note: Signs and symptoms were compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from 148 consecutive patients with erythematosquamous lesions of glabrous skin. Culture results were considered the gold standard. | ||||||
| PV+, positive predictive value; PV–, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||||
| Adapted from Lousbergh et al, Fam Pract 1999; 16:611–615.6 | ||||||
| Level of evidence=2b. For an explanation of levels of evidence, see page 865. | ||||||
Tinea pedis
Tinea of the foot may manifest as interdigital, plantar, or acute vesicular disease. Toe webs and soles of the feet are the sites most commonly affected. Tinea pedis occurs most commonly in postpubertal adolescents and adults, but may be seen in children.7
With interdigital infection, toe webs may become scaly, pruritic, and fissured. Interaction of bacteria and infecting dermatophytes may lead to a white, soggy maceration.8 Extension from the web space to the dorsal or plantar surface commonly occurs.
In chronic plantar or moccasin-type tinea pedis, the entire surface of the sole may be covered with fine, white scale and may assume a hyperkeratotic appearance. Chronic web infection may lead to acute inflammation characterized by vesicles, pustules, and bullae over the sole or the dorsum of the foot.5
Differential diagnosis. The differential diagnosis includes dry skin, pitted keratolysis, erythrasma, and contact dermatitis. Some conditions may appear very similar to tinea pedis, increasing the importance of KOH prep and fungal culture.
Tinea pedis may be distinguished from erythrasma by lack of bright coral appearance when examined with a Wood’s lamp. Tinea pedis infections also lack the well-demarcated erosions, or pits, of pitted keratolysis. Evidence of concurrent onychomycosis should increase the suspicion that tinea pedis is the correct diagnosis.
Tinea manuum
Tinea of the hand is usually analogous to moccasin-type tinea pedis. The palm appears hyperkeratotic and has very fine white scale that emphasizes the normal lines of the hand. Tinea of the dorsal surface of the hand usually occurs in the classic ringworm pattern. Tinea manuum is often seen in association with tinea pedis and onychomycosis. Many clinicians are familiar with the “one hand, two feet” syndrome, in which the palmar surfaces of both feet and one hand are infected (Figure 1).9 Onychomycosis often occurs in association with this presentation of tinea.
Differential diagnosis. The pattern of tinea manuum may be confused with those of eczema, contact dermatitis, palmar psoriasis, or even normal, rough hands. Unilateral involvement, presence of fingernail onychomycosis, lack of history indicating irritant or allergen exposure, and absence of psoriatic nail changes should increase the suspicion of palmar tinea manuum.
FIGURE 1
Tinea pedis
The common “1 hand, 2 feet” syndrome of tinea pedis. This syndrome usually requires systemic therapy.
Tinea cruris
Tinea of the groin is most common in adult males and is promoted by a warm, moist environment. Tinea cruris begins in the crural fold and spreads onto the thigh. The interior portion of the lesion is usually erythematous or slightly brown in light-skinned individuals. The leading edge often advances in a sharply demarcated semicircle with a raised, slightly scaling border. The lesion is most often bilateral, sparing the skin of the scrotum. Pruritus is common and increases as sweat macerates the irritated skin.
Differential diagnosis. Candidiasis, intertrigo, and erythrasma can cause similar lesions. It is helpful to recall that candidiasis may involve the scrotum and penis while tinea cruris does so rarely. An additional helpful feature is the characteristic bright coral appearance of erythrasma when examined with a Wood’s lamp, absent in cases of tinea cruris.
Tinea corporis and tinea faciale
Tinea infections of the face and body begin as flat, scaly, and often pruritic macules that subsequently develop a raised border and begin to spread radially. As the ring expands, the central portion of the lesion often clears. This pattern leads to the formation of irregular circles that gives tinea corporis its common name, ringworm (Figure 2).
Tinea faciale is less common, but generally has a similar appearance with central clearing of lesions. Tinea faciale may not always exhibit the sharply demarcated border of tinea corporis.
Differential diagnosis. Eczema, impetigo, early pityriasis rosea, and localized psoriasis can mimic tinea corporis. History of exposure to persons or animals (typically house pets) with known ringworm should increase suspicion of tinea corporis. Current or past evidence of psoriasis or eczema should broaden the differential to include atypical presentations of these diseases.
FIGURE 2
Tinea corporis
Widespread tinea corporis. This patient would not be a candidate for topical treatment.
Diagnosing tinea: history and exam not enough
In some cases, findings on history and physical evaluation are so characteristic of tinea that they alone may allow the clinician to make a firm diagnosis. However, studies have shown that relying upon history and physical examination may result in a many missed diagnoses.6 Accordingly, consider tinea in all instances of papulosquamous skin disease and follow up appropriately. Figure 3 presents a simple algorithm for diagnosis and treatment of suspected tinea infection.
FIGURE 3
Evaluating possible tinea infection of the skin
Koh preparation
An important adjunct in diagnosing tinea is the office-based KOH wet mount preparation ( Figure 4 ), permitting direct visualization of fungal hyphae in keratinized material of the stratum corneum. Detailed protocols for preparation and interpretation of the KOH slide for diagnosis of dermatophytosis are well described in many other sources.10
FIGURE 4
KOH preparation
Potassium hydroxide dissolves epithelial cells to reveal hyphae (A), with their characteristic branching pattern (B). To increase the likelihood of detecting hyphae on microscopic exam of skin scrapings, begin with low light and low power. If fungal elements are detected, increase magnification to confirm the diagnosis. If fungal elements are not detected, yet clinical suspicion of tinea is high, consider arranging for a fungal culture.
Performing the test
The slide is prepared by combining skin scrapings from the leading edge of a lesion with a small amount of 20% potassium hydroxide. A cover slip is applied and the slide is gently heated prior to microscopy examination under high power. Table 2 summarizes the utility of clinical diagnosis and KOH preparation in diagnosis of tinea infection. History and physical examination should be combined with KOH preparation when making the diagnosis of epidermal dermatophyte infection (strength of recommendation [SOR]=B).6 (For an explanation of evidence ratings, see page 865.)
TABLE 2
Diagnostic value of clinical diagnosis and KOH prep in tinea infection
| Test | Quality | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| a. Clinical diagnosis6 | 2b | 81% | 45% | 24 | 92% | 1.47 | 0.42 |
| b. KOH prep50 | 2b | 88% | 95% | 73% | 98% | 17.6 | 0.13 |
| c. KOH prep51 | 2b | 77% | 62% | 59% | 79% | 2.02 | 0.37 |
| a. The clinical diagnosis set was compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from consecutive patients with erythrosquamous lesions. Culture results were considered the gold standard. Study quality=2b. | |||||||
| b/c. Both studies of KOH preps were open analyses of patients with suspicious lesions. Paired fungal culture was initiated simultaneously with KOH prep and was considered the gold standard. Study quality=2b. For an explanation of levels of evidence, see page 865. | |||||||
Caveats
Though a rapid and inexpensive test, the KOH preparation has limitations. Some physicians have little experience interpreting KOH preparations, thus reducing the value of the test. Some patients with clinically significant tinea infections may be using over-the-counter topical antifungal medications, thus reducing the likelihood that fungal hyphae will be visualized at the office visit.
In cases where clinical suspicion of tinea infection is high, and the result of a KOH prep is negative, tissue should be submitted for fungal culture. Fungal culture is not typically available in the primary care office setting and usually requires submission of the sample to a reference or hospital laboratory.
Dermatophyte test medium
Dermatophyte test medium may be prepared and used in the office. It is mixed with a phenol indicator that turns red on exposure to alkaline metabolites of fungal growth. This medium is sensitive and usually exhibits color change within one week.11 Most primary care offices do not use dermatophyte test medium, however, because of its short shelf life and because the Clinical Laboratory Improvement Amendments classification requires that this test be performed by qualified laboratory personnel.
Treatment: topical and oral agents
Treatment of tinea infections may rely on antifungal medications singly or in combination. Antifungal agents are classified by their chemical structure—imidazoles, allylamines, benzylamines, and others—and act by different mechanisms to limit the availability of ergosterol, an essential component for normal function of fungal cell membranes.
Most tinea infections may be treated with topical agents alone. Oral therapy is required most often for:
- hyperkeratotic areas as on the palms or soles
- widespread or extensive infection
- immunocompromised patients
- persons intolerant of topical therapy
- failure of topical therapy
- chronic infection
Efforts to educate patients in proper hygiene and infection control may help. Specifically, remind patients that risk of tinea cruris and tinea pedis may be reduced if they wear nonocclusive footwear, use only clean and dry socks and undergarments, wear shower shoes, and apply absorbent powders. Eradication of fungal nail infections may help in controlling tinea pedis and tinea manuum. Caution in making contact with animals or people that have known tinea infections may reduce the incidence of tinea corporis.
Topical therapy
Topical antifungal agents are widely available in both prescription and over-the-counter forms. Preference of a specific agent may be difficult due to the limited number of head-to-head comparisons among these drugs. When recommending or prescribing an agent, consider efficacy, dosing regimen, cost, formulation, and availability. The most important topical antifungal agents are divided into 2 major classes—imidazoles and allylamines—although other agents are also used. Table 3 provides information on commonly used topical antifungal agents.
Imidazoles. Imidazoles are widely available as over-the-counter and prescription forms. Topical members of this class are clotrimazole, miconazole, econazole, ketoconazole, oxiconazole, and sulconazole. Miconazole and clotrimazole are available without a prescription. As a class, imidazoles are primarily fungistatic and are generally well-tolerated.12 Meta-analysis has failed to reveal significant differences in efficacy among members of this class.13 Imidazoles are efficacious with a pooled relative risk of failure to cure of 0.38 at 6 weeks after the initiation of therapy for tinea pedis (level of evidence [LOE]=1a).13
Current guidelines recommend twice-daily application for clotrimazole, miconazole, and econazole; ketoconazole, oxiconazole, and sulconazole may be applied once daily.14 When using imidazoles, usually prescribe a 2-week course for tinea cruris or tinea corporis, and a 4-week course for tinea pedis.15 In cases of inflammatory dermatophytosis, a combination agent containing clotrimazole and the corticosteroid betamethasone dipropionate may be used for a short initial period (LOE=4).16 Many experts recommend that combination steroid agents be used with caution, for no more than a few days, and with a plan for short-term follow-up.
Allylamines. A second, newer group of antifungal agents are the allylamines. Topical allylamines, including terbinafine and naftifine, are generally considered fungicidal. Terbinafine has recently been made available over-the-counter as 1% cream and 1% solution, while naftifine remains a prescription medication. Both agents are efficacious, with cure rates for dermatomycosis greater than 75%.17,18 Naftifine and terbinafine have exhibited long periods of activity in the skin and are therefore administered only once a day.19 Additionally, terbinafine has been shown effective in treating superficial mycoses in much shorter courses than typically required for imidazoles.20
Tinea infections are among the most common of all skin diseases. They are caused by dermatophytic fungi that digest keratin in the cells of the stratum corneum. Tinea infections are typically named according to the affected anatomic region: tinea corporis for the body, tinea pedis for the feet, tinea cruris for the groin, and so on.
The true prevalence of tinea is difficult to ascertain because many people self-treat or live with chronic infection. Approximately 8.6 million office visits occur each year for tinea infections. Family or general practitioners handle more than 35% of these visits. The estimated cost of office visits plus prescribed medications for cutaneous fungal infections for the 4-year period from 1990 to 1994 is just over $1 billion.1 Tinea infections occur in all age groups and in both genders; however, males have a higher incidence of tinea pedis and tinea cruris.
Dermatophytes affecting humans are from the genera Trichophyton, Microsporum, and Epidermophyton. Dermatophytes, which are ubiquitous in the environment, are categorized as geophilic, zoophilic, or anthropophilic according to their ecologic reservoir. Surveys of dermatophytes isolated from human patients in the United States from 1993 to 1995 indicate T tonsurans (44.9%), T rubrum (41.3%), T mentagrophytes (8.5%), and M canis (3.3%) are the most commonly encountered pathogens.2
The basic pathophysiology of tinea infection is inoculation of keratinized skin by dermatophytic fungi followed by release of keratinases and proteolytic enzymes. Symptoms follow as a result of host immune and epidermal response.3 Host factors, such as immunocompromised state and genetic susceptibility, play a role in infection. Warm temperature, moisture, and occlusion encourage dermatophyte growth.4
In a systematic review of available randomized controlled trials, allylamines were found to be more efficacious than imidazoles in treating tinea pedis (LOE=1a).13 Terbinafine has also been compared with imidazoles for treatment of tinea cruris and tinea corporis and found to be significantly more effective (LOE=1b).21
Previous pharmacoeconomic analyses of imidazole versus allylamine topical therapy for dermatophyte infection have presented conflicting conclusions, with some reporting that the greater cost of allylamine agents outweighs their slightly greater efficacy,22 while others concluded that the more efficacious medication initially is more cost effective.23 In the United States, terbinafine was approved for over-the-counter sale in 1999. Since then, the cost of terbinafine to consumers has declined significantly, to the point where it is now comparable to over-the-counter imidazoles (see Table 3 ). Allylamines have demonstrated some degree of intrinsic anti-inflammatory activity.24
Butenafine. Butenafine is a topical benzylamine antifungal structurally related to the allylamines. Butenafine is fungicidal and has efficacy similar to the allylamines. In a 1-week trial of therapy for tinea pedis with twice-daily butenafine, mycological cure rates in excess of 75% were observed (LOE=1b).25 In a trial of short-term therapy of tinea corporis, butenafine applied once-daily for 14 days led to high cure rates, symptom improvement, and increasing effectiveness 4 weeks after therapy was concluded (LOE=1b).26 In treatment of tinea cruris, butenafine was highly effective in achieving mycologic cure after 2 weeks of once-daily treatment (LOE=1b).27
Few trials directly compare butenafine and other topical agents, but in stand-alone or placebo-controlled trials, butenafine has demonstrated efficacy similar to the allylamines terbinafine and naftifine. Butenafine was approved for over-the-counter sale in the United States in 2000.
Ciclopirox. Ciclopirox is a substituted pyridone unrelated to the imidazole or allylamine agents. In double-blind, placebo-controlled trials, ciclopirox demonstrated significant efficacy in treating dermatophyte infections (LOE=1b).28 In head-to-head trials with the imidazole agent clotrimazole, ciclopirox demonstrated equivalent or slightly greater efficacy (LOE=1b).29,30
Ciclopirox applied twice daily is usually effective within 4 weeks of treatment. Ciclopirox is available only by prescription in the United States and is more expensive than most other topical antifungal agents. Ciclopirox has demonstrated intrinsic antiinflammatory activity equivalent to the allylamines (LOE=1b).31 Due to the availability of agents with comparable or superior efficacy at much lower costs, ciclopirox should not be considered a first- or second-line agent in treating epidermal tinea infections.
Tolnaftate. Tolnaftate is an over-the-counter antifungal agent that has been available in the US since 1965. Tolnaftate is inexpensive and has often been used in powder formulation as prophylaxis against tinea, especially tinea pedis.
In systematic reviews of available placebo-controlled trials, 1% tolnaftate was shown to be less effective against tinea pedis than the azoles and allylamines, with a number needed to treat of 3.6 for tinea pedis (LOE=1a).13 A comparative study has shown tolnaftate to be inferior to clotrimazole in the treatment of tinea pedis.32 With its demonstrated inferiority to the azoles and allylamines, tolnaftate should not be considered a first- or second-line treatment for tinea infections.
TABLE 3
Topical antifungal medications
| Agent | Formulation | Frequency* | Duration* | NNT † | Cost ‡ |
|---|---|---|---|---|---|
| IMIDAZOLES | |||||
| Clotrimazole | 1% cream | Twice daily | 2–4 weeks | 2.9 | $ 7.99 (15 g) |
| 1% solution | $ 6.99 (10 mL) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| Econazole | 1% cream | Twice daily | 2–4 weeks | 2.6 | $16.85 (15 g) |
| Ketoconazole | 2% cream | Once daily | 2–4 weeks | No data available | $25.39 (15 g) |
| Miconazole | 2% cream | Twice daily | 2–4 weeks | 2.8 (at 8 weeks) | $ 6.99 (15 g) |
| 2% spray | $ 5.99 (3.5 oz) | ||||
| 2% powder | $ 5.99 (3.5 oz) | ||||
| Oxiconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.9 | $20.27 (15 g) |
| 1% lotion | $34.19 (30 mL) | ||||
| Sulconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.5 | $13.75 (15 g) |
| 1% solution | $26.57 (30 mL) | ||||
| ALLYLAMINES | |||||
| Naftifine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 | $27.69 (15 g) |
| 1% gel | $51.39 (40 g) | ||||
| Terbinafine | 1% cream | Once to twice daily | 1–4 weeks | 1.6 (1.7 for tinea cruris/tinea corporis at 8 weeks)18 | $ 8.99 (12 g) |
| 1% solution | $ 9.99 (30 mL) | ||||
| BENZYLAMINE | |||||
| Butenafine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 (1.4 for tinea corporis and 1.5 for tinea cruris)26,27 | $ 9.99 (12 g) |
| OTHER | |||||
| Ciclopirox | 0.77% cream | Twice daily | 2–4 weeks | 2.1 | $21.89 (15 g) |
| 0.77% lotion | $37.99 (30 mL) | ||||
| Tolnaftate | 1% powder | Twice daily | 4 weeks | 3.6 (at 8 weeks) | $ 3.99 (4 oz) |
| 1% spray | $ 5.49 (4 oz) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| *Manufacturer guidelines. | |||||
| †NNT, number needed to treat. NNT is calculated from systematic review of all randomized controlled trials for tinea pedis at 6 weeks after the initiation of treatment33 except where otherwise noted. (See “Number needed to treat,” page 866.) | |||||
| ‡Lowest cost available (including generic agents) based upon internet listings of national on-line pharmacies: www.drugstore.com, www.eckerd.com, and www.walgreens.com as of May 2003. | |||||
Oral antifungal agents
Oral antifungal agents are used for dermatophyte infections that are widespread, chronic, or markedly inflammatory, or that affect hyperkeratotic areas as in palmar or plantar tinea. They are also use for those with immuno-suppression,16 and for those in whom treatment with topical drugs has been unsatisfactory. Agents include griseofulvin, the azoles (ketoconazole, itraconazole, and fluconazole), and the allylamine terbinafine. Table 4 summarizes pertinent comparative data regarding these agents.
Griseofulvin. Griseofulvin is the oldest of the systemic antifungal agents used for tinea infections, available for more than 40 years. Griseofulvin acts on susceptible fungal cells by inhibiting microtubule function. Griseofulvin is taken by adults once daily at 500 mg, for 4 to 6 weeks, and has demonstrated efficacy in the treatment of tinea infections.33
In a systematic review of comparative trials, oral griseofulvin was found to be significantly inferior to oral terbinafine in the treatment of tinea pedis (LOE=1a).33 Likewise, terbinafine was found to be superior to griseofulvin in the treatment of tinea corporis and tinea cruris (LOE=1b).34 In comparisons with ketoconazole, griseofulvin was equivalent in the treatment of dermatophytosis (LOE=2a).35
Griseofulvin has also been compared with itraconazole in various treatment schedules for tinea corporis, tinea cruris, tinea pedis, and tinea manus; it was found to be inferior in all treatment durations to a maximum of 3 months (LOE=1b).36 In head-to-head comparisons between griseofulvin and fluconazole in the treatment of tinea corporis and tinea cruris, outcomes were not statistically significantly different, although a trend toward better clinical cures in the fluconazole arm was identified (LOE=1b).37
Azoles. Studies have made limited comparisons among the azoles (ketoconazole, itraconazole, and fluconazole) in the treatment of dermatophytosis. Small comparisons with limited power between itraconazole and fluconazole38 and between ketoconazole and fluconazole39 have demonstrated similar cure rates of about 90% for all 3 agents (LOE=1b). In a placebo-controlled comparison for treatment of tinea cruris and tinea corporis, 100 mg/d of itraconazole for 14 days was highly curative (LOE=1b).40 In placebo-controlled trials of the treatment of tinea pedis, itraconazole was found to provide statistically significant cure rates in as little as 1 week (LOE=1b).41
Systematic review of trials between itraconazole (100 mg/d for 4 weeks) and terbinafine (250 mg/d for 2 weeks) for tinea pedis showed a non-statistically significant trend toward higher cure rate in those treated with terbinafine (LOE=1a).33
There are limited placebo-controlled trials evaluating fluconazole in the treatment of superficial fungal infections of the skin. In an open, noncomparative trial employing once-weekly dosing of fluconazole 150 mg for 1 to 4 weeks for tinea corporis and tinea cruris, clinical cure rate was 92% with long-term clinical cure rate of 88% (LOE=2b).42 Another study randomized 240 adults with skin dermatophytosis or cutaneous candidiasis to either flucona-zole 150 mg/wk or fluconazole 50 mg/d for a maximum of 4 weeks (non-tinea pedis) to 6 weeks (tinea pedis) with positive clinical response of greater than 90% in both arms of treatment (LOE=2b).43 Fluconazole has not been evaluated in direct comparison with terbinafine in the treatment of dermatophytic skin infections. Comparisons between azoles and griseofulvin have been discussed above.
Terbinafine. Oral terbinafine is effective in the treatment of skin dermatophytes. In a double-blind, placebo-controlled study of terbinafine 125 mg taken twice daily for 6 weeks, 65% of patients had mycologic cure at 2 weeks post-treatment (LOE=1b).44 In an open, noncon-trolled study of terbinafine 125 mg daily for one week, 100% mycologic cure was achieved in the treatment of tinea corporis and tinea cruris (LOE=2b).45
As noted above, terbinafine has shown efficacy superior to griseofulvin and itraconazole. No well-designed, comparative study was identified that showed any oral antifungal agent to be superior to terbinafine in the treatment of dermatophytic skin infections.
TABLE 4
Oral antifungal medications in the treatment of dermatomycosis
| Comparison (with treatment costs)* | SOR | Outcomes | NNT or reduction in risk of failure to cure | Comments |
|---|---|---|---|---|
| ITRACONAZOLE ($112–$223.99) vs placebo | A 40, 41 | Itraconazole with much greater efficacy than placebo | NNT = 1.7 at 8 weeks after 1 week of treatment for tinea pedis; 1.8 at 4 weeks after 2 weeks of treatment for tinea corporis and tinea cruris | In multiple, well-designed RCT for tinea pedis, tinea corporis and tinea cruris |
| TERBINAFINE ($112.99–$329.9) vs placebo | A 33, 44, 45 | Terbinafine with much greater efficacy than with placebo | NNT = 1.5 at 8 weeks after 2 weeks of treatment for tinea pedis | Systematic review of RCT for tinea pedis and multiple, non-comparative trials for tinea corporis and tinea cruris |
| ITRACONAZOLE ($112–$223.99) vs Griseofulvin ($50.99–$71.40) | A 36 | Itraconazole with greater efficacy than Griseofulvin | Risk difference = 19% in treatment of tinea cruris/corporis and 37% in treatment of tinea pedis/manus | Systematic review of RCT for tinea corporis, tinea cruris, tinea manuus, and tinea pedis |
| TERBINAFINE ($112.99–$223.99)vs Griseofulvin ($50.99–$71.40) | A 33 | Terbinafine with greater efficacy than Griseofulvin | Risk difference = 50% at 8 weeks post-treatment | Systematic review of RCT for tinea pedis |
| KETOCONAZOLE ($30.99–$61.99) vs Griseofulvin ($50.99–71.40) | A 33 | Approximately equal efficacy between Ketoconazole and Griseofulvin | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| FLUCONAZOLE ($13.99–$50.96) vs Griseofulvin ($50.99–$71.40) | A 37 | Non-significant trend toward greater efficacy with Fluconazole | Risk difference = 12% | RCT for tinea corporis and tinea cruris |
| FLUCONAZOLE ($13.99–$50.96) vs Ketoconazole ($30.99–$61.99) | B 39 | Approximately equal efficacy between Fluconazole and Ketoconazole | Risk difference = 4% favoring fluconazole at 7 weeks post-treatment | Small RCT for all dermatomycosis |
| ITRACONAZOLE ($112–$223.99) vs Fluconazole ($13.99–$50.96) | B 38 | Approximately equal efficacy between Fluconazole and Itraconazole | Risk difference = 5% favoring itraconazole at 10 weeks post-treatment | Small RCT for tinea manuum and tinea pedis |
| TERBINAFINE ($112.99–223.99) vs Itraconazole ($112–$223.99) | A 33 | Approximately equal efficacy between Terbinafine and Itraconazole | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| SOR, strength of recommendation; NNT, number needed to treat; RCT, randomized controlled trial | ||||
| SOR: A = Multiple RCT or a systematic review of RCT; B = Trials of moderate strength, as in open trials, noncomparative trials, or RCT with small size or poor follow-up. (See page 865 for full explanation of SOR ratings.) | ||||
| * Lowest cost available (including for generic agents) based upon internet listings of national on-line pharmacies, Drugstore.com, Eckerd.com, and Walgreens.com as of May 2003. | ||||
Summary of treatment recommendations
Based upon review of the available literature, it is recommended that clinicians consider the affordable and highly effective topical agents terbinafine and butenafine as first-line therapy for patients with uncomplicated epidermal tinea infections. While also highly effective, naftifine should be considered a second-line treatment due to its prescription-only availability and increased cost. The topical azoles clotrimazole and miconazole are marginally less effective than the allylamines, but are widely available and inexpensive. The prescription drug ciclopirox is slightly superior to the azoles, but its expense and availability make it a less desirable choice for most patients. Tolnaftate is cheap and widely available, but its relative inferiority to other topical agents limits its use in treatment of tinea infections. Tolnaftate may have some utility as a prophylactic against epidermal tinea infections in susceptible individuals.46
For patients with complicated epidermal tinea infections as described above, or for those with confirmed infection and treatment failure using topical agents, oral antifungal medications should be used. Terbinafine and itraconazole are both highly effective and safe in treating tinea infections. Despite their relatively high cost, either of these agents may be considered first-line therapies. Fluconazole has been evaluated in limited trials, but data at this time are insufficient to recommend that it be considered a first-line agent in treatment of epidermal tinea infection.
Griseofulvin may be considered a second-line agent as it has been shown to be clearly less efficacious than either terbinafine or itraconazole. Oral ketoconazole has a limited role in the treatment of tinea infections. It has demonstrated efficacy equivalent to or slightly superior to griseofulvin, but its association with potentially severe liver injury makes it a very problematic choice.
Adverse reactions
As with any systemic medication, adverse effects may occur with oral antifungal agents. Oral antifungal agents are generally considered safe in the treatment of skin dermatophytoses. In systemic review of trials of oral antifungal agents in the treatment of tinea pedis, the most commonly identified adverse events were gastrointestinal complaints such as diarrhea or nausea, headaches, and skin complaints.33
Of particular note, ketoconazole has been repeatedly related to potentially serious hepatotoxicity, especially when used in longer duration of treatment.47 One large cohort study found the incident rate of acute liver injury to be 134.1 per 100,000 person-months among ketoconazole users, compared with 10.4 and 2.5 for itraconazole and terbinafine, respectively.48 Accordingly, baseline and monthly laboratory studies are advised when using ketoconazole.49
Serious complications associated with the use of topical antifungal agents are rare. As with any topical medication, local irritation and dermatitis may occur.
Correspondence
Brian Thomas, MD, NOLF-IB, Bldg 184, Box 357140, San Diego, Ca 92135. E-mail: [email protected].
1. Smith ES, Fleischer AB, Friedman SR, Willford PM. Characteristics of office-based physician visits for cutaneous fungal infection: an analysis of 1990 to 1994 National Ambulatory Medical Care Survey Data. Cutis 2002;69:191-202.
2. Weitzman I, Chin NX, Kunjukunju N, Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 1998;39:255-261.
3. Roscoe J, Farmer ER. Diseases caused by fungi. In: Farmer ER, Hood AF, eds. Pathology of the Skin. 2nd ed. New York: McGraw-Hill; 2000;611-633.
4. Odom RB, James WD, Berger TG. Diseases resulting from fungi and yeasts. >In: Diseases of the Skin: Clinical Dermatology. 9th ed. Philadelphia: WB Saunders; 2000;358-415.
5. Habif TP. Superficial fungal infections. In: Clinical Dermatology. 3rd ed. St. Louis, Mo: Mosby; 1996;362-408.
6. Lousbergh D, Buntinx F, Pierard G. Diagnosing dermatomycosis in general practice. Fam Pract 1999;16:611-615.
7. Kearse HJ, Miller OF. Tinea pedis in prepubertal children: does it occur? J Am Acad Dermatol 1988;19:619-22.
8. Kates SG, Nordstrom KM, McGinley KJ, Leyden JJ. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990;22:578-582.
9. Lookingbill DP, Marks JG. Scaling papules, plaques, and patches. In: Principles of Dermatology. 2nd ed. Philadelphia: WB Saunders; 1993;136-159.
10. Rosen T. Dermatophytosis: diagnostic pointers and therapeutic pitfalls. Consultant 1997;37:1545-1557.
11. Sinski JT, Swanson JR, Kelley LM. Dermatophyte test medium: clinical and quantitative appraisal. J Inves Dermatol 1972;58:405-411.
12. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988;32:1-8.
13. Crawford F, Hart R, Bell-Syer S, Torgerson D, Young P, Russell I. Topical treatments for fungal infections of the skin and nails of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
14. Polak A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia 1984;22:501-503.
15. Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycosis.A North American perspective. Drugs 1998;55:645-674.
16. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol 1996;34:282-286.
17. Jordon RE, Rapini RP, Rex IH, Jr, et al. Once-daily naftifine cream 1% in the treatment of tinea cruris and tinea corporis. Int J Dermatol 1990;29:441-442.
18. Budimulja U, Bramono K, Urip S, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and tinea cruris. A placebo-controlled study. Mycoses 2001;44:300-306.
19. Schuster I, Schaude M, Schatz F, et al. Preclinical characteristics of allylamines. In: Berg D, Plempel M, eds. Sterol Biosynthesis Inhibitors. Chichester, England: Ellis Horwood; 1988;449-470.
20. Evans EG, Seaman RA, James IG. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol 1994;130:83-87
21. Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol 2000;10:107-109.
22. Chren MM, Landefeld CS. A cost analysis of topical drug regimens for dermatophyte infections. JAMA 1994;272:1922-1925.
23. Davis R, Balfour JA. Terbinafine. A pharmacoeconomic evaluation of its use in superficial fungal infections. Pharmacoeconomics. 1995;8:253-269.
24. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
25. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily Butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Amer Acad Dermatol 1997;36:S15-S19.
26. Greer DL, Weiss J, Rodriguez DA, Hebert AA, Swinehart JM. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Amer Acad Dermatol 1997;37:231-235.
27. Lesher JL, Babel DE, Stewart DM, et al. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehi-cle-controlled, double-blind trial. J Amer Acad Dermatol 1997;36:S20-S24.
28. Sehgal VN. Ciclopirox: a new topical pyrodonium antimy-cotic agent. A double-blind study in superficial dermato-mycoses. Br J Dermatol 1976;95:83-88.
29. Bogaert H, Cordero C, Ollague W, Savin RC, Shalita AR, Zaias N. Multicentre double-blind clinical trials of ciclopirox olamine cream 1% in the treatment of tinea corporis and tinea cruris. J Int Med Res 1986;14:210-216.
30. Beyer M. Selected double-blind comparative studies on the efficacy and tolerance of ciclopiroxolamine solution and cream [in German]. Arzneimittelforschung 1981;31:1378-1381.
31. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
32. Woscoff A, Carageli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Curr Ther Res 1986;39:753-757.
33. Bell-Syer SEM, Hart R, Crawford F, Torgerson DJ, Tyrell W, Russell I. Oral treatments for fungal infections of the skin of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
34. Voravutinon V. Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: a randomized double-blind comparative study. J Med Assoc Thai. 1993;76:388-393.
35. Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double-blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985;112:691-696.
36. Lachapelle JM, De Doncker P, Tennstedt D, Cauwenbergh G, Janssen PA. Itraconazole compared with griseo-fulvin in the treatment of tinea corporis/cruris and tinea pedis/mannus: An interpretation of the clinical results of all completed double-blind studies with respect to the pharmacokinetic profile. Dermatology 1992;184:45-50.
37. Faergemann J, Mork NJ, Haglund A, Odegard T. A multi-centre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol 1997;136:575-577.
38. Difonzo EM, Papini M, Cilli P, et al. A double blind study comparison of itraconazole and fluconazole in tinea pedis and tinea manuum. J Eur Acad Dermatol Venereol 1995;4:148-152.
39. Fischbein A, Haneke E, Lacner K. Comparative evaluation of oral fluconazole and oral ketoconazole in the treatment of fungal infections of the skin. Intl J Dermatol 1992;31:12-16.
40. Pariser DM, Pariser RJ, Ruoff G, Ray TL. Double-blind comparison of itraconazole and placebo in the treatment of tinea corporis and tinea cruris. J Amer Acad Dermatol 1994;31:232-234.
41. Svejgaard E, Avnstorp C, Wanscher B, Nilsson J, Heremans A. Efficacy and safety of short-term itracona-zole in tinea pedis: a double-blind, randomized, placebo-controlled trial. Dermatology 1998;197:368-372.
42. Suchil P, Gei FM, Robles M, Perera-Ramirez A, Welsh O, Male O. Once-weekly oral doses of fluconazole 150 mg in the treatment of tinea corporis/cruris and cutaneous candidiasis. Clin Exp Dermatol 1992;17:397-401.
43. Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidiasis. Intl J Dermatol 1998;37:703-705.
44. Savin RC, Zaias N. Treatment of chronic moccasin-type tinea pedis with terbinafine: a double-blind, placebo-controlled trial. J Am Acad Dermatol 1990;23:804-807.
45. Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol 1994;131:684-686.
46. Smith EB, Dickson JE, Knox JM. Tolnaftate powder in prophylaxis of tinea pedis. South Med J 1974;67:776-778.
47. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy: analysis of 33 cases. Gastroenterology 1984;86:503-513.
48. Garcia Rodriguez LA, Duque A, Castellsague J, Perea-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 1999;48:847-852.
49. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419-422.
50. Haldane DJ, Robart E. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn Microbiol Infect Dis 1990;13:337-339.
51. Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol 1993;129:510-511.
- Potassium hydroxide preparation should be used as an aid to diagnosis for all erythrosquamous lesions (B).
- Fungal culture should be used in cases in which history, physical examination, and potassium hydroxide preparation fail to clearly exclude a diagnosis of tinea (B).
- Short-duration topical therapy with terbinafine, naftifine, and butenafine is efficacious for most epidermal tinea infections (A).
- Oral antifungal agents are important in the treatment of tinea infections thatare widespread, fail to respond to topical treatment, involve the thick stratum corneum of the soles and palms, or occur in immunosuppressed patients.
- Short courses of oral itraconazole and terbinafine are safe and effective in treating tinea infections (A).
Though findings on history and physical examination are sometimes sufficient to make a diagnosis of tinea infection, a potassium hydroxide (KOH) study usually is required for confirmation. Even the KOH study can be misleading, however, if a patient recently self-administered a topical antifungal agent. This article describes the varying appearance of tinea infections according to their anatomic location, and outlines a careful work-up.
Highly effective and affordable over-the-counter medications have proliferated, and short-course therapy is available. Based on systematic reviews of randomized, controlled studies, it is possible to recommend specific first-line therapies for tinea infections.
Manifestations of tinea infection
Clinical manifestations of tinea vary with anatomic location, duration of infection, and pathogen. In general, zoophilic dermatophytes evoke a more vigorous host response than the anthropophilic species.5 Shared features of many dermatophyte infections include erythema, scaling, pruritus, ring formation, and central clearing of lesions. Table 1 reviews published data on the diagnostic value of selected clinical signs in suspected tinea infection.6
TABLE 1
Diagnostic value of selected signs and symptoms in tinea infection
| Sign/symptom | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|
| Scaling | 77% | 20% | 17% | 80% | 0.96 | 1.15 |
| Erythema | 69% | 31% | 18% | 83% | 1.00 | 1.00 |
| Pruritus | 54% | 40% | 16% | 80% | 0.90 | 1.15 |
| Central clearing | 42% | 65% | 20% | 84% | 1.20 | 0.89 |
| Concentric rings | 27% | 80% | 23% | 84% | 1.35 | 0.91 |
| Maceration | 27% | 84% | 26% | 84% | 1.69 | 0.87 |
| Note: Signs and symptoms were compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from 148 consecutive patients with erythematosquamous lesions of glabrous skin. Culture results were considered the gold standard. | ||||||
| PV+, positive predictive value; PV–, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||||
| Adapted from Lousbergh et al, Fam Pract 1999; 16:611–615.6 | ||||||
| Level of evidence=2b. For an explanation of levels of evidence, see page 865. | ||||||
Tinea pedis
Tinea of the foot may manifest as interdigital, plantar, or acute vesicular disease. Toe webs and soles of the feet are the sites most commonly affected. Tinea pedis occurs most commonly in postpubertal adolescents and adults, but may be seen in children.7
With interdigital infection, toe webs may become scaly, pruritic, and fissured. Interaction of bacteria and infecting dermatophytes may lead to a white, soggy maceration.8 Extension from the web space to the dorsal or plantar surface commonly occurs.
In chronic plantar or moccasin-type tinea pedis, the entire surface of the sole may be covered with fine, white scale and may assume a hyperkeratotic appearance. Chronic web infection may lead to acute inflammation characterized by vesicles, pustules, and bullae over the sole or the dorsum of the foot.5
Differential diagnosis. The differential diagnosis includes dry skin, pitted keratolysis, erythrasma, and contact dermatitis. Some conditions may appear very similar to tinea pedis, increasing the importance of KOH prep and fungal culture.
Tinea pedis may be distinguished from erythrasma by lack of bright coral appearance when examined with a Wood’s lamp. Tinea pedis infections also lack the well-demarcated erosions, or pits, of pitted keratolysis. Evidence of concurrent onychomycosis should increase the suspicion that tinea pedis is the correct diagnosis.
Tinea manuum
Tinea of the hand is usually analogous to moccasin-type tinea pedis. The palm appears hyperkeratotic and has very fine white scale that emphasizes the normal lines of the hand. Tinea of the dorsal surface of the hand usually occurs in the classic ringworm pattern. Tinea manuum is often seen in association with tinea pedis and onychomycosis. Many clinicians are familiar with the “one hand, two feet” syndrome, in which the palmar surfaces of both feet and one hand are infected (Figure 1).9 Onychomycosis often occurs in association with this presentation of tinea.
Differential diagnosis. The pattern of tinea manuum may be confused with those of eczema, contact dermatitis, palmar psoriasis, or even normal, rough hands. Unilateral involvement, presence of fingernail onychomycosis, lack of history indicating irritant or allergen exposure, and absence of psoriatic nail changes should increase the suspicion of palmar tinea manuum.
FIGURE 1
Tinea pedis
The common “1 hand, 2 feet” syndrome of tinea pedis. This syndrome usually requires systemic therapy.
Tinea cruris
Tinea of the groin is most common in adult males and is promoted by a warm, moist environment. Tinea cruris begins in the crural fold and spreads onto the thigh. The interior portion of the lesion is usually erythematous or slightly brown in light-skinned individuals. The leading edge often advances in a sharply demarcated semicircle with a raised, slightly scaling border. The lesion is most often bilateral, sparing the skin of the scrotum. Pruritus is common and increases as sweat macerates the irritated skin.
Differential diagnosis. Candidiasis, intertrigo, and erythrasma can cause similar lesions. It is helpful to recall that candidiasis may involve the scrotum and penis while tinea cruris does so rarely. An additional helpful feature is the characteristic bright coral appearance of erythrasma when examined with a Wood’s lamp, absent in cases of tinea cruris.
Tinea corporis and tinea faciale
Tinea infections of the face and body begin as flat, scaly, and often pruritic macules that subsequently develop a raised border and begin to spread radially. As the ring expands, the central portion of the lesion often clears. This pattern leads to the formation of irregular circles that gives tinea corporis its common name, ringworm (Figure 2).
Tinea faciale is less common, but generally has a similar appearance with central clearing of lesions. Tinea faciale may not always exhibit the sharply demarcated border of tinea corporis.
Differential diagnosis. Eczema, impetigo, early pityriasis rosea, and localized psoriasis can mimic tinea corporis. History of exposure to persons or animals (typically house pets) with known ringworm should increase suspicion of tinea corporis. Current or past evidence of psoriasis or eczema should broaden the differential to include atypical presentations of these diseases.
FIGURE 2
Tinea corporis
Widespread tinea corporis. This patient would not be a candidate for topical treatment.
Diagnosing tinea: history and exam not enough
In some cases, findings on history and physical evaluation are so characteristic of tinea that they alone may allow the clinician to make a firm diagnosis. However, studies have shown that relying upon history and physical examination may result in a many missed diagnoses.6 Accordingly, consider tinea in all instances of papulosquamous skin disease and follow up appropriately. Figure 3 presents a simple algorithm for diagnosis and treatment of suspected tinea infection.
FIGURE 3
Evaluating possible tinea infection of the skin
Koh preparation
An important adjunct in diagnosing tinea is the office-based KOH wet mount preparation ( Figure 4 ), permitting direct visualization of fungal hyphae in keratinized material of the stratum corneum. Detailed protocols for preparation and interpretation of the KOH slide for diagnosis of dermatophytosis are well described in many other sources.10
FIGURE 4
KOH preparation
Potassium hydroxide dissolves epithelial cells to reveal hyphae (A), with their characteristic branching pattern (B). To increase the likelihood of detecting hyphae on microscopic exam of skin scrapings, begin with low light and low power. If fungal elements are detected, increase magnification to confirm the diagnosis. If fungal elements are not detected, yet clinical suspicion of tinea is high, consider arranging for a fungal culture.
Performing the test
The slide is prepared by combining skin scrapings from the leading edge of a lesion with a small amount of 20% potassium hydroxide. A cover slip is applied and the slide is gently heated prior to microscopy examination under high power. Table 2 summarizes the utility of clinical diagnosis and KOH preparation in diagnosis of tinea infection. History and physical examination should be combined with KOH preparation when making the diagnosis of epidermal dermatophyte infection (strength of recommendation [SOR]=B).6 (For an explanation of evidence ratings, see page 865.)
TABLE 2
Diagnostic value of clinical diagnosis and KOH prep in tinea infection
| Test | Quality | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| a. Clinical diagnosis6 | 2b | 81% | 45% | 24 | 92% | 1.47 | 0.42 |
| b. KOH prep50 | 2b | 88% | 95% | 73% | 98% | 17.6 | 0.13 |
| c. KOH prep51 | 2b | 77% | 62% | 59% | 79% | 2.02 | 0.37 |
| a. The clinical diagnosis set was compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from consecutive patients with erythrosquamous lesions. Culture results were considered the gold standard. Study quality=2b. | |||||||
| b/c. Both studies of KOH preps were open analyses of patients with suspicious lesions. Paired fungal culture was initiated simultaneously with KOH prep and was considered the gold standard. Study quality=2b. For an explanation of levels of evidence, see page 865. | |||||||
Caveats
Though a rapid and inexpensive test, the KOH preparation has limitations. Some physicians have little experience interpreting KOH preparations, thus reducing the value of the test. Some patients with clinically significant tinea infections may be using over-the-counter topical antifungal medications, thus reducing the likelihood that fungal hyphae will be visualized at the office visit.
In cases where clinical suspicion of tinea infection is high, and the result of a KOH prep is negative, tissue should be submitted for fungal culture. Fungal culture is not typically available in the primary care office setting and usually requires submission of the sample to a reference or hospital laboratory.
Dermatophyte test medium
Dermatophyte test medium may be prepared and used in the office. It is mixed with a phenol indicator that turns red on exposure to alkaline metabolites of fungal growth. This medium is sensitive and usually exhibits color change within one week.11 Most primary care offices do not use dermatophyte test medium, however, because of its short shelf life and because the Clinical Laboratory Improvement Amendments classification requires that this test be performed by qualified laboratory personnel.
Treatment: topical and oral agents
Treatment of tinea infections may rely on antifungal medications singly or in combination. Antifungal agents are classified by their chemical structure—imidazoles, allylamines, benzylamines, and others—and act by different mechanisms to limit the availability of ergosterol, an essential component for normal function of fungal cell membranes.
Most tinea infections may be treated with topical agents alone. Oral therapy is required most often for:
- hyperkeratotic areas as on the palms or soles
- widespread or extensive infection
- immunocompromised patients
- persons intolerant of topical therapy
- failure of topical therapy
- chronic infection
Efforts to educate patients in proper hygiene and infection control may help. Specifically, remind patients that risk of tinea cruris and tinea pedis may be reduced if they wear nonocclusive footwear, use only clean and dry socks and undergarments, wear shower shoes, and apply absorbent powders. Eradication of fungal nail infections may help in controlling tinea pedis and tinea manuum. Caution in making contact with animals or people that have known tinea infections may reduce the incidence of tinea corporis.
Topical therapy
Topical antifungal agents are widely available in both prescription and over-the-counter forms. Preference of a specific agent may be difficult due to the limited number of head-to-head comparisons among these drugs. When recommending or prescribing an agent, consider efficacy, dosing regimen, cost, formulation, and availability. The most important topical antifungal agents are divided into 2 major classes—imidazoles and allylamines—although other agents are also used. Table 3 provides information on commonly used topical antifungal agents.
Imidazoles. Imidazoles are widely available as over-the-counter and prescription forms. Topical members of this class are clotrimazole, miconazole, econazole, ketoconazole, oxiconazole, and sulconazole. Miconazole and clotrimazole are available without a prescription. As a class, imidazoles are primarily fungistatic and are generally well-tolerated.12 Meta-analysis has failed to reveal significant differences in efficacy among members of this class.13 Imidazoles are efficacious with a pooled relative risk of failure to cure of 0.38 at 6 weeks after the initiation of therapy for tinea pedis (level of evidence [LOE]=1a).13
Current guidelines recommend twice-daily application for clotrimazole, miconazole, and econazole; ketoconazole, oxiconazole, and sulconazole may be applied once daily.14 When using imidazoles, usually prescribe a 2-week course for tinea cruris or tinea corporis, and a 4-week course for tinea pedis.15 In cases of inflammatory dermatophytosis, a combination agent containing clotrimazole and the corticosteroid betamethasone dipropionate may be used for a short initial period (LOE=4).16 Many experts recommend that combination steroid agents be used with caution, for no more than a few days, and with a plan for short-term follow-up.
Allylamines. A second, newer group of antifungal agents are the allylamines. Topical allylamines, including terbinafine and naftifine, are generally considered fungicidal. Terbinafine has recently been made available over-the-counter as 1% cream and 1% solution, while naftifine remains a prescription medication. Both agents are efficacious, with cure rates for dermatomycosis greater than 75%.17,18 Naftifine and terbinafine have exhibited long periods of activity in the skin and are therefore administered only once a day.19 Additionally, terbinafine has been shown effective in treating superficial mycoses in much shorter courses than typically required for imidazoles.20
Tinea infections are among the most common of all skin diseases. They are caused by dermatophytic fungi that digest keratin in the cells of the stratum corneum. Tinea infections are typically named according to the affected anatomic region: tinea corporis for the body, tinea pedis for the feet, tinea cruris for the groin, and so on.
The true prevalence of tinea is difficult to ascertain because many people self-treat or live with chronic infection. Approximately 8.6 million office visits occur each year for tinea infections. Family or general practitioners handle more than 35% of these visits. The estimated cost of office visits plus prescribed medications for cutaneous fungal infections for the 4-year period from 1990 to 1994 is just over $1 billion.1 Tinea infections occur in all age groups and in both genders; however, males have a higher incidence of tinea pedis and tinea cruris.
Dermatophytes affecting humans are from the genera Trichophyton, Microsporum, and Epidermophyton. Dermatophytes, which are ubiquitous in the environment, are categorized as geophilic, zoophilic, or anthropophilic according to their ecologic reservoir. Surveys of dermatophytes isolated from human patients in the United States from 1993 to 1995 indicate T tonsurans (44.9%), T rubrum (41.3%), T mentagrophytes (8.5%), and M canis (3.3%) are the most commonly encountered pathogens.2
The basic pathophysiology of tinea infection is inoculation of keratinized skin by dermatophytic fungi followed by release of keratinases and proteolytic enzymes. Symptoms follow as a result of host immune and epidermal response.3 Host factors, such as immunocompromised state and genetic susceptibility, play a role in infection. Warm temperature, moisture, and occlusion encourage dermatophyte growth.4
In a systematic review of available randomized controlled trials, allylamines were found to be more efficacious than imidazoles in treating tinea pedis (LOE=1a).13 Terbinafine has also been compared with imidazoles for treatment of tinea cruris and tinea corporis and found to be significantly more effective (LOE=1b).21
Previous pharmacoeconomic analyses of imidazole versus allylamine topical therapy for dermatophyte infection have presented conflicting conclusions, with some reporting that the greater cost of allylamine agents outweighs their slightly greater efficacy,22 while others concluded that the more efficacious medication initially is more cost effective.23 In the United States, terbinafine was approved for over-the-counter sale in 1999. Since then, the cost of terbinafine to consumers has declined significantly, to the point where it is now comparable to over-the-counter imidazoles (see Table 3 ). Allylamines have demonstrated some degree of intrinsic anti-inflammatory activity.24
Butenafine. Butenafine is a topical benzylamine antifungal structurally related to the allylamines. Butenafine is fungicidal and has efficacy similar to the allylamines. In a 1-week trial of therapy for tinea pedis with twice-daily butenafine, mycological cure rates in excess of 75% were observed (LOE=1b).25 In a trial of short-term therapy of tinea corporis, butenafine applied once-daily for 14 days led to high cure rates, symptom improvement, and increasing effectiveness 4 weeks after therapy was concluded (LOE=1b).26 In treatment of tinea cruris, butenafine was highly effective in achieving mycologic cure after 2 weeks of once-daily treatment (LOE=1b).27
Few trials directly compare butenafine and other topical agents, but in stand-alone or placebo-controlled trials, butenafine has demonstrated efficacy similar to the allylamines terbinafine and naftifine. Butenafine was approved for over-the-counter sale in the United States in 2000.
Ciclopirox. Ciclopirox is a substituted pyridone unrelated to the imidazole or allylamine agents. In double-blind, placebo-controlled trials, ciclopirox demonstrated significant efficacy in treating dermatophyte infections (LOE=1b).28 In head-to-head trials with the imidazole agent clotrimazole, ciclopirox demonstrated equivalent or slightly greater efficacy (LOE=1b).29,30
Ciclopirox applied twice daily is usually effective within 4 weeks of treatment. Ciclopirox is available only by prescription in the United States and is more expensive than most other topical antifungal agents. Ciclopirox has demonstrated intrinsic antiinflammatory activity equivalent to the allylamines (LOE=1b).31 Due to the availability of agents with comparable or superior efficacy at much lower costs, ciclopirox should not be considered a first- or second-line agent in treating epidermal tinea infections.
Tolnaftate. Tolnaftate is an over-the-counter antifungal agent that has been available in the US since 1965. Tolnaftate is inexpensive and has often been used in powder formulation as prophylaxis against tinea, especially tinea pedis.
In systematic reviews of available placebo-controlled trials, 1% tolnaftate was shown to be less effective against tinea pedis than the azoles and allylamines, with a number needed to treat of 3.6 for tinea pedis (LOE=1a).13 A comparative study has shown tolnaftate to be inferior to clotrimazole in the treatment of tinea pedis.32 With its demonstrated inferiority to the azoles and allylamines, tolnaftate should not be considered a first- or second-line treatment for tinea infections.
TABLE 3
Topical antifungal medications
| Agent | Formulation | Frequency* | Duration* | NNT † | Cost ‡ |
|---|---|---|---|---|---|
| IMIDAZOLES | |||||
| Clotrimazole | 1% cream | Twice daily | 2–4 weeks | 2.9 | $ 7.99 (15 g) |
| 1% solution | $ 6.99 (10 mL) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| Econazole | 1% cream | Twice daily | 2–4 weeks | 2.6 | $16.85 (15 g) |
| Ketoconazole | 2% cream | Once daily | 2–4 weeks | No data available | $25.39 (15 g) |
| Miconazole | 2% cream | Twice daily | 2–4 weeks | 2.8 (at 8 weeks) | $ 6.99 (15 g) |
| 2% spray | $ 5.99 (3.5 oz) | ||||
| 2% powder | $ 5.99 (3.5 oz) | ||||
| Oxiconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.9 | $20.27 (15 g) |
| 1% lotion | $34.19 (30 mL) | ||||
| Sulconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.5 | $13.75 (15 g) |
| 1% solution | $26.57 (30 mL) | ||||
| ALLYLAMINES | |||||
| Naftifine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 | $27.69 (15 g) |
| 1% gel | $51.39 (40 g) | ||||
| Terbinafine | 1% cream | Once to twice daily | 1–4 weeks | 1.6 (1.7 for tinea cruris/tinea corporis at 8 weeks)18 | $ 8.99 (12 g) |
| 1% solution | $ 9.99 (30 mL) | ||||
| BENZYLAMINE | |||||
| Butenafine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 (1.4 for tinea corporis and 1.5 for tinea cruris)26,27 | $ 9.99 (12 g) |
| OTHER | |||||
| Ciclopirox | 0.77% cream | Twice daily | 2–4 weeks | 2.1 | $21.89 (15 g) |
| 0.77% lotion | $37.99 (30 mL) | ||||
| Tolnaftate | 1% powder | Twice daily | 4 weeks | 3.6 (at 8 weeks) | $ 3.99 (4 oz) |
| 1% spray | $ 5.49 (4 oz) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| *Manufacturer guidelines. | |||||
| †NNT, number needed to treat. NNT is calculated from systematic review of all randomized controlled trials for tinea pedis at 6 weeks after the initiation of treatment33 except where otherwise noted. (See “Number needed to treat,” page 866.) | |||||
| ‡Lowest cost available (including generic agents) based upon internet listings of national on-line pharmacies: www.drugstore.com, www.eckerd.com, and www.walgreens.com as of May 2003. | |||||
Oral antifungal agents
Oral antifungal agents are used for dermatophyte infections that are widespread, chronic, or markedly inflammatory, or that affect hyperkeratotic areas as in palmar or plantar tinea. They are also use for those with immuno-suppression,16 and for those in whom treatment with topical drugs has been unsatisfactory. Agents include griseofulvin, the azoles (ketoconazole, itraconazole, and fluconazole), and the allylamine terbinafine. Table 4 summarizes pertinent comparative data regarding these agents.
Griseofulvin. Griseofulvin is the oldest of the systemic antifungal agents used for tinea infections, available for more than 40 years. Griseofulvin acts on susceptible fungal cells by inhibiting microtubule function. Griseofulvin is taken by adults once daily at 500 mg, for 4 to 6 weeks, and has demonstrated efficacy in the treatment of tinea infections.33
In a systematic review of comparative trials, oral griseofulvin was found to be significantly inferior to oral terbinafine in the treatment of tinea pedis (LOE=1a).33 Likewise, terbinafine was found to be superior to griseofulvin in the treatment of tinea corporis and tinea cruris (LOE=1b).34 In comparisons with ketoconazole, griseofulvin was equivalent in the treatment of dermatophytosis (LOE=2a).35
Griseofulvin has also been compared with itraconazole in various treatment schedules for tinea corporis, tinea cruris, tinea pedis, and tinea manus; it was found to be inferior in all treatment durations to a maximum of 3 months (LOE=1b).36 In head-to-head comparisons between griseofulvin and fluconazole in the treatment of tinea corporis and tinea cruris, outcomes were not statistically significantly different, although a trend toward better clinical cures in the fluconazole arm was identified (LOE=1b).37
Azoles. Studies have made limited comparisons among the azoles (ketoconazole, itraconazole, and fluconazole) in the treatment of dermatophytosis. Small comparisons with limited power between itraconazole and fluconazole38 and between ketoconazole and fluconazole39 have demonstrated similar cure rates of about 90% for all 3 agents (LOE=1b). In a placebo-controlled comparison for treatment of tinea cruris and tinea corporis, 100 mg/d of itraconazole for 14 days was highly curative (LOE=1b).40 In placebo-controlled trials of the treatment of tinea pedis, itraconazole was found to provide statistically significant cure rates in as little as 1 week (LOE=1b).41
Systematic review of trials between itraconazole (100 mg/d for 4 weeks) and terbinafine (250 mg/d for 2 weeks) for tinea pedis showed a non-statistically significant trend toward higher cure rate in those treated with terbinafine (LOE=1a).33
There are limited placebo-controlled trials evaluating fluconazole in the treatment of superficial fungal infections of the skin. In an open, noncomparative trial employing once-weekly dosing of fluconazole 150 mg for 1 to 4 weeks for tinea corporis and tinea cruris, clinical cure rate was 92% with long-term clinical cure rate of 88% (LOE=2b).42 Another study randomized 240 adults with skin dermatophytosis or cutaneous candidiasis to either flucona-zole 150 mg/wk or fluconazole 50 mg/d for a maximum of 4 weeks (non-tinea pedis) to 6 weeks (tinea pedis) with positive clinical response of greater than 90% in both arms of treatment (LOE=2b).43 Fluconazole has not been evaluated in direct comparison with terbinafine in the treatment of dermatophytic skin infections. Comparisons between azoles and griseofulvin have been discussed above.
Terbinafine. Oral terbinafine is effective in the treatment of skin dermatophytes. In a double-blind, placebo-controlled study of terbinafine 125 mg taken twice daily for 6 weeks, 65% of patients had mycologic cure at 2 weeks post-treatment (LOE=1b).44 In an open, noncon-trolled study of terbinafine 125 mg daily for one week, 100% mycologic cure was achieved in the treatment of tinea corporis and tinea cruris (LOE=2b).45
As noted above, terbinafine has shown efficacy superior to griseofulvin and itraconazole. No well-designed, comparative study was identified that showed any oral antifungal agent to be superior to terbinafine in the treatment of dermatophytic skin infections.
TABLE 4
Oral antifungal medications in the treatment of dermatomycosis
| Comparison (with treatment costs)* | SOR | Outcomes | NNT or reduction in risk of failure to cure | Comments |
|---|---|---|---|---|
| ITRACONAZOLE ($112–$223.99) vs placebo | A 40, 41 | Itraconazole with much greater efficacy than placebo | NNT = 1.7 at 8 weeks after 1 week of treatment for tinea pedis; 1.8 at 4 weeks after 2 weeks of treatment for tinea corporis and tinea cruris | In multiple, well-designed RCT for tinea pedis, tinea corporis and tinea cruris |
| TERBINAFINE ($112.99–$329.9) vs placebo | A 33, 44, 45 | Terbinafine with much greater efficacy than with placebo | NNT = 1.5 at 8 weeks after 2 weeks of treatment for tinea pedis | Systematic review of RCT for tinea pedis and multiple, non-comparative trials for tinea corporis and tinea cruris |
| ITRACONAZOLE ($112–$223.99) vs Griseofulvin ($50.99–$71.40) | A 36 | Itraconazole with greater efficacy than Griseofulvin | Risk difference = 19% in treatment of tinea cruris/corporis and 37% in treatment of tinea pedis/manus | Systematic review of RCT for tinea corporis, tinea cruris, tinea manuus, and tinea pedis |
| TERBINAFINE ($112.99–$223.99)vs Griseofulvin ($50.99–$71.40) | A 33 | Terbinafine with greater efficacy than Griseofulvin | Risk difference = 50% at 8 weeks post-treatment | Systematic review of RCT for tinea pedis |
| KETOCONAZOLE ($30.99–$61.99) vs Griseofulvin ($50.99–71.40) | A 33 | Approximately equal efficacy between Ketoconazole and Griseofulvin | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| FLUCONAZOLE ($13.99–$50.96) vs Griseofulvin ($50.99–$71.40) | A 37 | Non-significant trend toward greater efficacy with Fluconazole | Risk difference = 12% | RCT for tinea corporis and tinea cruris |
| FLUCONAZOLE ($13.99–$50.96) vs Ketoconazole ($30.99–$61.99) | B 39 | Approximately equal efficacy between Fluconazole and Ketoconazole | Risk difference = 4% favoring fluconazole at 7 weeks post-treatment | Small RCT for all dermatomycosis |
| ITRACONAZOLE ($112–$223.99) vs Fluconazole ($13.99–$50.96) | B 38 | Approximately equal efficacy between Fluconazole and Itraconazole | Risk difference = 5% favoring itraconazole at 10 weeks post-treatment | Small RCT for tinea manuum and tinea pedis |
| TERBINAFINE ($112.99–223.99) vs Itraconazole ($112–$223.99) | A 33 | Approximately equal efficacy between Terbinafine and Itraconazole | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| SOR, strength of recommendation; NNT, number needed to treat; RCT, randomized controlled trial | ||||
| SOR: A = Multiple RCT or a systematic review of RCT; B = Trials of moderate strength, as in open trials, noncomparative trials, or RCT with small size or poor follow-up. (See page 865 for full explanation of SOR ratings.) | ||||
| * Lowest cost available (including for generic agents) based upon internet listings of national on-line pharmacies, Drugstore.com, Eckerd.com, and Walgreens.com as of May 2003. | ||||
Summary of treatment recommendations
Based upon review of the available literature, it is recommended that clinicians consider the affordable and highly effective topical agents terbinafine and butenafine as first-line therapy for patients with uncomplicated epidermal tinea infections. While also highly effective, naftifine should be considered a second-line treatment due to its prescription-only availability and increased cost. The topical azoles clotrimazole and miconazole are marginally less effective than the allylamines, but are widely available and inexpensive. The prescription drug ciclopirox is slightly superior to the azoles, but its expense and availability make it a less desirable choice for most patients. Tolnaftate is cheap and widely available, but its relative inferiority to other topical agents limits its use in treatment of tinea infections. Tolnaftate may have some utility as a prophylactic against epidermal tinea infections in susceptible individuals.46
For patients with complicated epidermal tinea infections as described above, or for those with confirmed infection and treatment failure using topical agents, oral antifungal medications should be used. Terbinafine and itraconazole are both highly effective and safe in treating tinea infections. Despite their relatively high cost, either of these agents may be considered first-line therapies. Fluconazole has been evaluated in limited trials, but data at this time are insufficient to recommend that it be considered a first-line agent in treatment of epidermal tinea infection.
Griseofulvin may be considered a second-line agent as it has been shown to be clearly less efficacious than either terbinafine or itraconazole. Oral ketoconazole has a limited role in the treatment of tinea infections. It has demonstrated efficacy equivalent to or slightly superior to griseofulvin, but its association with potentially severe liver injury makes it a very problematic choice.
Adverse reactions
As with any systemic medication, adverse effects may occur with oral antifungal agents. Oral antifungal agents are generally considered safe in the treatment of skin dermatophytoses. In systemic review of trials of oral antifungal agents in the treatment of tinea pedis, the most commonly identified adverse events were gastrointestinal complaints such as diarrhea or nausea, headaches, and skin complaints.33
Of particular note, ketoconazole has been repeatedly related to potentially serious hepatotoxicity, especially when used in longer duration of treatment.47 One large cohort study found the incident rate of acute liver injury to be 134.1 per 100,000 person-months among ketoconazole users, compared with 10.4 and 2.5 for itraconazole and terbinafine, respectively.48 Accordingly, baseline and monthly laboratory studies are advised when using ketoconazole.49
Serious complications associated with the use of topical antifungal agents are rare. As with any topical medication, local irritation and dermatitis may occur.
Correspondence
Brian Thomas, MD, NOLF-IB, Bldg 184, Box 357140, San Diego, Ca 92135. E-mail: [email protected].
- Potassium hydroxide preparation should be used as an aid to diagnosis for all erythrosquamous lesions (B).
- Fungal culture should be used in cases in which history, physical examination, and potassium hydroxide preparation fail to clearly exclude a diagnosis of tinea (B).
- Short-duration topical therapy with terbinafine, naftifine, and butenafine is efficacious for most epidermal tinea infections (A).
- Oral antifungal agents are important in the treatment of tinea infections thatare widespread, fail to respond to topical treatment, involve the thick stratum corneum of the soles and palms, or occur in immunosuppressed patients.
- Short courses of oral itraconazole and terbinafine are safe and effective in treating tinea infections (A).
Though findings on history and physical examination are sometimes sufficient to make a diagnosis of tinea infection, a potassium hydroxide (KOH) study usually is required for confirmation. Even the KOH study can be misleading, however, if a patient recently self-administered a topical antifungal agent. This article describes the varying appearance of tinea infections according to their anatomic location, and outlines a careful work-up.
Highly effective and affordable over-the-counter medications have proliferated, and short-course therapy is available. Based on systematic reviews of randomized, controlled studies, it is possible to recommend specific first-line therapies for tinea infections.
Manifestations of tinea infection
Clinical manifestations of tinea vary with anatomic location, duration of infection, and pathogen. In general, zoophilic dermatophytes evoke a more vigorous host response than the anthropophilic species.5 Shared features of many dermatophyte infections include erythema, scaling, pruritus, ring formation, and central clearing of lesions. Table 1 reviews published data on the diagnostic value of selected clinical signs in suspected tinea infection.6
TABLE 1
Diagnostic value of selected signs and symptoms in tinea infection
| Sign/symptom | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|
| Scaling | 77% | 20% | 17% | 80% | 0.96 | 1.15 |
| Erythema | 69% | 31% | 18% | 83% | 1.00 | 1.00 |
| Pruritus | 54% | 40% | 16% | 80% | 0.90 | 1.15 |
| Central clearing | 42% | 65% | 20% | 84% | 1.20 | 0.89 |
| Concentric rings | 27% | 80% | 23% | 84% | 1.35 | 0.91 |
| Maceration | 27% | 84% | 26% | 84% | 1.69 | 0.87 |
| Note: Signs and symptoms were compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from 148 consecutive patients with erythematosquamous lesions of glabrous skin. Culture results were considered the gold standard. | ||||||
| PV+, positive predictive value; PV–, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||||
| Adapted from Lousbergh et al, Fam Pract 1999; 16:611–615.6 | ||||||
| Level of evidence=2b. For an explanation of levels of evidence, see page 865. | ||||||
Tinea pedis
Tinea of the foot may manifest as interdigital, plantar, or acute vesicular disease. Toe webs and soles of the feet are the sites most commonly affected. Tinea pedis occurs most commonly in postpubertal adolescents and adults, but may be seen in children.7
With interdigital infection, toe webs may become scaly, pruritic, and fissured. Interaction of bacteria and infecting dermatophytes may lead to a white, soggy maceration.8 Extension from the web space to the dorsal or plantar surface commonly occurs.
In chronic plantar or moccasin-type tinea pedis, the entire surface of the sole may be covered with fine, white scale and may assume a hyperkeratotic appearance. Chronic web infection may lead to acute inflammation characterized by vesicles, pustules, and bullae over the sole or the dorsum of the foot.5
Differential diagnosis. The differential diagnosis includes dry skin, pitted keratolysis, erythrasma, and contact dermatitis. Some conditions may appear very similar to tinea pedis, increasing the importance of KOH prep and fungal culture.
Tinea pedis may be distinguished from erythrasma by lack of bright coral appearance when examined with a Wood’s lamp. Tinea pedis infections also lack the well-demarcated erosions, or pits, of pitted keratolysis. Evidence of concurrent onychomycosis should increase the suspicion that tinea pedis is the correct diagnosis.
Tinea manuum
Tinea of the hand is usually analogous to moccasin-type tinea pedis. The palm appears hyperkeratotic and has very fine white scale that emphasizes the normal lines of the hand. Tinea of the dorsal surface of the hand usually occurs in the classic ringworm pattern. Tinea manuum is often seen in association with tinea pedis and onychomycosis. Many clinicians are familiar with the “one hand, two feet” syndrome, in which the palmar surfaces of both feet and one hand are infected (Figure 1).9 Onychomycosis often occurs in association with this presentation of tinea.
Differential diagnosis. The pattern of tinea manuum may be confused with those of eczema, contact dermatitis, palmar psoriasis, or even normal, rough hands. Unilateral involvement, presence of fingernail onychomycosis, lack of history indicating irritant or allergen exposure, and absence of psoriatic nail changes should increase the suspicion of palmar tinea manuum.
FIGURE 1
Tinea pedis
The common “1 hand, 2 feet” syndrome of tinea pedis. This syndrome usually requires systemic therapy.
Tinea cruris
Tinea of the groin is most common in adult males and is promoted by a warm, moist environment. Tinea cruris begins in the crural fold and spreads onto the thigh. The interior portion of the lesion is usually erythematous or slightly brown in light-skinned individuals. The leading edge often advances in a sharply demarcated semicircle with a raised, slightly scaling border. The lesion is most often bilateral, sparing the skin of the scrotum. Pruritus is common and increases as sweat macerates the irritated skin.
Differential diagnosis. Candidiasis, intertrigo, and erythrasma can cause similar lesions. It is helpful to recall that candidiasis may involve the scrotum and penis while tinea cruris does so rarely. An additional helpful feature is the characteristic bright coral appearance of erythrasma when examined with a Wood’s lamp, absent in cases of tinea cruris.
Tinea corporis and tinea faciale
Tinea infections of the face and body begin as flat, scaly, and often pruritic macules that subsequently develop a raised border and begin to spread radially. As the ring expands, the central portion of the lesion often clears. This pattern leads to the formation of irregular circles that gives tinea corporis its common name, ringworm (Figure 2).
Tinea faciale is less common, but generally has a similar appearance with central clearing of lesions. Tinea faciale may not always exhibit the sharply demarcated border of tinea corporis.
Differential diagnosis. Eczema, impetigo, early pityriasis rosea, and localized psoriasis can mimic tinea corporis. History of exposure to persons or animals (typically house pets) with known ringworm should increase suspicion of tinea corporis. Current or past evidence of psoriasis or eczema should broaden the differential to include atypical presentations of these diseases.
FIGURE 2
Tinea corporis
Widespread tinea corporis. This patient would not be a candidate for topical treatment.
Diagnosing tinea: history and exam not enough
In some cases, findings on history and physical evaluation are so characteristic of tinea that they alone may allow the clinician to make a firm diagnosis. However, studies have shown that relying upon history and physical examination may result in a many missed diagnoses.6 Accordingly, consider tinea in all instances of papulosquamous skin disease and follow up appropriately. Figure 3 presents a simple algorithm for diagnosis and treatment of suspected tinea infection.
FIGURE 3
Evaluating possible tinea infection of the skin
Koh preparation
An important adjunct in diagnosing tinea is the office-based KOH wet mount preparation ( Figure 4 ), permitting direct visualization of fungal hyphae in keratinized material of the stratum corneum. Detailed protocols for preparation and interpretation of the KOH slide for diagnosis of dermatophytosis are well described in many other sources.10
FIGURE 4
KOH preparation
Potassium hydroxide dissolves epithelial cells to reveal hyphae (A), with their characteristic branching pattern (B). To increase the likelihood of detecting hyphae on microscopic exam of skin scrapings, begin with low light and low power. If fungal elements are detected, increase magnification to confirm the diagnosis. If fungal elements are not detected, yet clinical suspicion of tinea is high, consider arranging for a fungal culture.
Performing the test
The slide is prepared by combining skin scrapings from the leading edge of a lesion with a small amount of 20% potassium hydroxide. A cover slip is applied and the slide is gently heated prior to microscopy examination under high power. Table 2 summarizes the utility of clinical diagnosis and KOH preparation in diagnosis of tinea infection. History and physical examination should be combined with KOH preparation when making the diagnosis of epidermal dermatophyte infection (strength of recommendation [SOR]=B).6 (For an explanation of evidence ratings, see page 865.)
TABLE 2
Diagnostic value of clinical diagnosis and KOH prep in tinea infection
| Test | Quality | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| a. Clinical diagnosis6 | 2b | 81% | 45% | 24 | 92% | 1.47 | 0.42 |
| b. KOH prep50 | 2b | 88% | 95% | 73% | 98% | 17.6 | 0.13 |
| c. KOH prep51 | 2b | 77% | 62% | 59% | 79% | 2.02 | 0.37 |
| a. The clinical diagnosis set was compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from consecutive patients with erythrosquamous lesions. Culture results were considered the gold standard. Study quality=2b. | |||||||
| b/c. Both studies of KOH preps were open analyses of patients with suspicious lesions. Paired fungal culture was initiated simultaneously with KOH prep and was considered the gold standard. Study quality=2b. For an explanation of levels of evidence, see page 865. | |||||||
Caveats
Though a rapid and inexpensive test, the KOH preparation has limitations. Some physicians have little experience interpreting KOH preparations, thus reducing the value of the test. Some patients with clinically significant tinea infections may be using over-the-counter topical antifungal medications, thus reducing the likelihood that fungal hyphae will be visualized at the office visit.
In cases where clinical suspicion of tinea infection is high, and the result of a KOH prep is negative, tissue should be submitted for fungal culture. Fungal culture is not typically available in the primary care office setting and usually requires submission of the sample to a reference or hospital laboratory.
Dermatophyte test medium
Dermatophyte test medium may be prepared and used in the office. It is mixed with a phenol indicator that turns red on exposure to alkaline metabolites of fungal growth. This medium is sensitive and usually exhibits color change within one week.11 Most primary care offices do not use dermatophyte test medium, however, because of its short shelf life and because the Clinical Laboratory Improvement Amendments classification requires that this test be performed by qualified laboratory personnel.
Treatment: topical and oral agents
Treatment of tinea infections may rely on antifungal medications singly or in combination. Antifungal agents are classified by their chemical structure—imidazoles, allylamines, benzylamines, and others—and act by different mechanisms to limit the availability of ergosterol, an essential component for normal function of fungal cell membranes.
Most tinea infections may be treated with topical agents alone. Oral therapy is required most often for:
- hyperkeratotic areas as on the palms or soles
- widespread or extensive infection
- immunocompromised patients
- persons intolerant of topical therapy
- failure of topical therapy
- chronic infection
Efforts to educate patients in proper hygiene and infection control may help. Specifically, remind patients that risk of tinea cruris and tinea pedis may be reduced if they wear nonocclusive footwear, use only clean and dry socks and undergarments, wear shower shoes, and apply absorbent powders. Eradication of fungal nail infections may help in controlling tinea pedis and tinea manuum. Caution in making contact with animals or people that have known tinea infections may reduce the incidence of tinea corporis.
Topical therapy
Topical antifungal agents are widely available in both prescription and over-the-counter forms. Preference of a specific agent may be difficult due to the limited number of head-to-head comparisons among these drugs. When recommending or prescribing an agent, consider efficacy, dosing regimen, cost, formulation, and availability. The most important topical antifungal agents are divided into 2 major classes—imidazoles and allylamines—although other agents are also used. Table 3 provides information on commonly used topical antifungal agents.
Imidazoles. Imidazoles are widely available as over-the-counter and prescription forms. Topical members of this class are clotrimazole, miconazole, econazole, ketoconazole, oxiconazole, and sulconazole. Miconazole and clotrimazole are available without a prescription. As a class, imidazoles are primarily fungistatic and are generally well-tolerated.12 Meta-analysis has failed to reveal significant differences in efficacy among members of this class.13 Imidazoles are efficacious with a pooled relative risk of failure to cure of 0.38 at 6 weeks after the initiation of therapy for tinea pedis (level of evidence [LOE]=1a).13
Current guidelines recommend twice-daily application for clotrimazole, miconazole, and econazole; ketoconazole, oxiconazole, and sulconazole may be applied once daily.14 When using imidazoles, usually prescribe a 2-week course for tinea cruris or tinea corporis, and a 4-week course for tinea pedis.15 In cases of inflammatory dermatophytosis, a combination agent containing clotrimazole and the corticosteroid betamethasone dipropionate may be used for a short initial period (LOE=4).16 Many experts recommend that combination steroid agents be used with caution, for no more than a few days, and with a plan for short-term follow-up.
Allylamines. A second, newer group of antifungal agents are the allylamines. Topical allylamines, including terbinafine and naftifine, are generally considered fungicidal. Terbinafine has recently been made available over-the-counter as 1% cream and 1% solution, while naftifine remains a prescription medication. Both agents are efficacious, with cure rates for dermatomycosis greater than 75%.17,18 Naftifine and terbinafine have exhibited long periods of activity in the skin and are therefore administered only once a day.19 Additionally, terbinafine has been shown effective in treating superficial mycoses in much shorter courses than typically required for imidazoles.20
Tinea infections are among the most common of all skin diseases. They are caused by dermatophytic fungi that digest keratin in the cells of the stratum corneum. Tinea infections are typically named according to the affected anatomic region: tinea corporis for the body, tinea pedis for the feet, tinea cruris for the groin, and so on.
The true prevalence of tinea is difficult to ascertain because many people self-treat or live with chronic infection. Approximately 8.6 million office visits occur each year for tinea infections. Family or general practitioners handle more than 35% of these visits. The estimated cost of office visits plus prescribed medications for cutaneous fungal infections for the 4-year period from 1990 to 1994 is just over $1 billion.1 Tinea infections occur in all age groups and in both genders; however, males have a higher incidence of tinea pedis and tinea cruris.
Dermatophytes affecting humans are from the genera Trichophyton, Microsporum, and Epidermophyton. Dermatophytes, which are ubiquitous in the environment, are categorized as geophilic, zoophilic, or anthropophilic according to their ecologic reservoir. Surveys of dermatophytes isolated from human patients in the United States from 1993 to 1995 indicate T tonsurans (44.9%), T rubrum (41.3%), T mentagrophytes (8.5%), and M canis (3.3%) are the most commonly encountered pathogens.2
The basic pathophysiology of tinea infection is inoculation of keratinized skin by dermatophytic fungi followed by release of keratinases and proteolytic enzymes. Symptoms follow as a result of host immune and epidermal response.3 Host factors, such as immunocompromised state and genetic susceptibility, play a role in infection. Warm temperature, moisture, and occlusion encourage dermatophyte growth.4
In a systematic review of available randomized controlled trials, allylamines were found to be more efficacious than imidazoles in treating tinea pedis (LOE=1a).13 Terbinafine has also been compared with imidazoles for treatment of tinea cruris and tinea corporis and found to be significantly more effective (LOE=1b).21
Previous pharmacoeconomic analyses of imidazole versus allylamine topical therapy for dermatophyte infection have presented conflicting conclusions, with some reporting that the greater cost of allylamine agents outweighs their slightly greater efficacy,22 while others concluded that the more efficacious medication initially is more cost effective.23 In the United States, terbinafine was approved for over-the-counter sale in 1999. Since then, the cost of terbinafine to consumers has declined significantly, to the point where it is now comparable to over-the-counter imidazoles (see Table 3 ). Allylamines have demonstrated some degree of intrinsic anti-inflammatory activity.24
Butenafine. Butenafine is a topical benzylamine antifungal structurally related to the allylamines. Butenafine is fungicidal and has efficacy similar to the allylamines. In a 1-week trial of therapy for tinea pedis with twice-daily butenafine, mycological cure rates in excess of 75% were observed (LOE=1b).25 In a trial of short-term therapy of tinea corporis, butenafine applied once-daily for 14 days led to high cure rates, symptom improvement, and increasing effectiveness 4 weeks after therapy was concluded (LOE=1b).26 In treatment of tinea cruris, butenafine was highly effective in achieving mycologic cure after 2 weeks of once-daily treatment (LOE=1b).27
Few trials directly compare butenafine and other topical agents, but in stand-alone or placebo-controlled trials, butenafine has demonstrated efficacy similar to the allylamines terbinafine and naftifine. Butenafine was approved for over-the-counter sale in the United States in 2000.
Ciclopirox. Ciclopirox is a substituted pyridone unrelated to the imidazole or allylamine agents. In double-blind, placebo-controlled trials, ciclopirox demonstrated significant efficacy in treating dermatophyte infections (LOE=1b).28 In head-to-head trials with the imidazole agent clotrimazole, ciclopirox demonstrated equivalent or slightly greater efficacy (LOE=1b).29,30
Ciclopirox applied twice daily is usually effective within 4 weeks of treatment. Ciclopirox is available only by prescription in the United States and is more expensive than most other topical antifungal agents. Ciclopirox has demonstrated intrinsic antiinflammatory activity equivalent to the allylamines (LOE=1b).31 Due to the availability of agents with comparable or superior efficacy at much lower costs, ciclopirox should not be considered a first- or second-line agent in treating epidermal tinea infections.
Tolnaftate. Tolnaftate is an over-the-counter antifungal agent that has been available in the US since 1965. Tolnaftate is inexpensive and has often been used in powder formulation as prophylaxis against tinea, especially tinea pedis.
In systematic reviews of available placebo-controlled trials, 1% tolnaftate was shown to be less effective against tinea pedis than the azoles and allylamines, with a number needed to treat of 3.6 for tinea pedis (LOE=1a).13 A comparative study has shown tolnaftate to be inferior to clotrimazole in the treatment of tinea pedis.32 With its demonstrated inferiority to the azoles and allylamines, tolnaftate should not be considered a first- or second-line treatment for tinea infections.
TABLE 3
Topical antifungal medications
| Agent | Formulation | Frequency* | Duration* | NNT † | Cost ‡ |
|---|---|---|---|---|---|
| IMIDAZOLES | |||||
| Clotrimazole | 1% cream | Twice daily | 2–4 weeks | 2.9 | $ 7.99 (15 g) |
| 1% solution | $ 6.99 (10 mL) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| Econazole | 1% cream | Twice daily | 2–4 weeks | 2.6 | $16.85 (15 g) |
| Ketoconazole | 2% cream | Once daily | 2–4 weeks | No data available | $25.39 (15 g) |
| Miconazole | 2% cream | Twice daily | 2–4 weeks | 2.8 (at 8 weeks) | $ 6.99 (15 g) |
| 2% spray | $ 5.99 (3.5 oz) | ||||
| 2% powder | $ 5.99 (3.5 oz) | ||||
| Oxiconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.9 | $20.27 (15 g) |
| 1% lotion | $34.19 (30 mL) | ||||
| Sulconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.5 | $13.75 (15 g) |
| 1% solution | $26.57 (30 mL) | ||||
| ALLYLAMINES | |||||
| Naftifine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 | $27.69 (15 g) |
| 1% gel | $51.39 (40 g) | ||||
| Terbinafine | 1% cream | Once to twice daily | 1–4 weeks | 1.6 (1.7 for tinea cruris/tinea corporis at 8 weeks)18 | $ 8.99 (12 g) |
| 1% solution | $ 9.99 (30 mL) | ||||
| BENZYLAMINE | |||||
| Butenafine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 (1.4 for tinea corporis and 1.5 for tinea cruris)26,27 | $ 9.99 (12 g) |
| OTHER | |||||
| Ciclopirox | 0.77% cream | Twice daily | 2–4 weeks | 2.1 | $21.89 (15 g) |
| 0.77% lotion | $37.99 (30 mL) | ||||
| Tolnaftate | 1% powder | Twice daily | 4 weeks | 3.6 (at 8 weeks) | $ 3.99 (4 oz) |
| 1% spray | $ 5.49 (4 oz) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| *Manufacturer guidelines. | |||||
| †NNT, number needed to treat. NNT is calculated from systematic review of all randomized controlled trials for tinea pedis at 6 weeks after the initiation of treatment33 except where otherwise noted. (See “Number needed to treat,” page 866.) | |||||
| ‡Lowest cost available (including generic agents) based upon internet listings of national on-line pharmacies: www.drugstore.com, www.eckerd.com, and www.walgreens.com as of May 2003. | |||||
Oral antifungal agents
Oral antifungal agents are used for dermatophyte infections that are widespread, chronic, or markedly inflammatory, or that affect hyperkeratotic areas as in palmar or plantar tinea. They are also use for those with immuno-suppression,16 and for those in whom treatment with topical drugs has been unsatisfactory. Agents include griseofulvin, the azoles (ketoconazole, itraconazole, and fluconazole), and the allylamine terbinafine. Table 4 summarizes pertinent comparative data regarding these agents.
Griseofulvin. Griseofulvin is the oldest of the systemic antifungal agents used for tinea infections, available for more than 40 years. Griseofulvin acts on susceptible fungal cells by inhibiting microtubule function. Griseofulvin is taken by adults once daily at 500 mg, for 4 to 6 weeks, and has demonstrated efficacy in the treatment of tinea infections.33
In a systematic review of comparative trials, oral griseofulvin was found to be significantly inferior to oral terbinafine in the treatment of tinea pedis (LOE=1a).33 Likewise, terbinafine was found to be superior to griseofulvin in the treatment of tinea corporis and tinea cruris (LOE=1b).34 In comparisons with ketoconazole, griseofulvin was equivalent in the treatment of dermatophytosis (LOE=2a).35
Griseofulvin has also been compared with itraconazole in various treatment schedules for tinea corporis, tinea cruris, tinea pedis, and tinea manus; it was found to be inferior in all treatment durations to a maximum of 3 months (LOE=1b).36 In head-to-head comparisons between griseofulvin and fluconazole in the treatment of tinea corporis and tinea cruris, outcomes were not statistically significantly different, although a trend toward better clinical cures in the fluconazole arm was identified (LOE=1b).37
Azoles. Studies have made limited comparisons among the azoles (ketoconazole, itraconazole, and fluconazole) in the treatment of dermatophytosis. Small comparisons with limited power between itraconazole and fluconazole38 and between ketoconazole and fluconazole39 have demonstrated similar cure rates of about 90% for all 3 agents (LOE=1b). In a placebo-controlled comparison for treatment of tinea cruris and tinea corporis, 100 mg/d of itraconazole for 14 days was highly curative (LOE=1b).40 In placebo-controlled trials of the treatment of tinea pedis, itraconazole was found to provide statistically significant cure rates in as little as 1 week (LOE=1b).41
Systematic review of trials between itraconazole (100 mg/d for 4 weeks) and terbinafine (250 mg/d for 2 weeks) for tinea pedis showed a non-statistically significant trend toward higher cure rate in those treated with terbinafine (LOE=1a).33
There are limited placebo-controlled trials evaluating fluconazole in the treatment of superficial fungal infections of the skin. In an open, noncomparative trial employing once-weekly dosing of fluconazole 150 mg for 1 to 4 weeks for tinea corporis and tinea cruris, clinical cure rate was 92% with long-term clinical cure rate of 88% (LOE=2b).42 Another study randomized 240 adults with skin dermatophytosis or cutaneous candidiasis to either flucona-zole 150 mg/wk or fluconazole 50 mg/d for a maximum of 4 weeks (non-tinea pedis) to 6 weeks (tinea pedis) with positive clinical response of greater than 90% in both arms of treatment (LOE=2b).43 Fluconazole has not been evaluated in direct comparison with terbinafine in the treatment of dermatophytic skin infections. Comparisons between azoles and griseofulvin have been discussed above.
Terbinafine. Oral terbinafine is effective in the treatment of skin dermatophytes. In a double-blind, placebo-controlled study of terbinafine 125 mg taken twice daily for 6 weeks, 65% of patients had mycologic cure at 2 weeks post-treatment (LOE=1b).44 In an open, noncon-trolled study of terbinafine 125 mg daily for one week, 100% mycologic cure was achieved in the treatment of tinea corporis and tinea cruris (LOE=2b).45
As noted above, terbinafine has shown efficacy superior to griseofulvin and itraconazole. No well-designed, comparative study was identified that showed any oral antifungal agent to be superior to terbinafine in the treatment of dermatophytic skin infections.
TABLE 4
Oral antifungal medications in the treatment of dermatomycosis
| Comparison (with treatment costs)* | SOR | Outcomes | NNT or reduction in risk of failure to cure | Comments |
|---|---|---|---|---|
| ITRACONAZOLE ($112–$223.99) vs placebo | A 40, 41 | Itraconazole with much greater efficacy than placebo | NNT = 1.7 at 8 weeks after 1 week of treatment for tinea pedis; 1.8 at 4 weeks after 2 weeks of treatment for tinea corporis and tinea cruris | In multiple, well-designed RCT for tinea pedis, tinea corporis and tinea cruris |
| TERBINAFINE ($112.99–$329.9) vs placebo | A 33, 44, 45 | Terbinafine with much greater efficacy than with placebo | NNT = 1.5 at 8 weeks after 2 weeks of treatment for tinea pedis | Systematic review of RCT for tinea pedis and multiple, non-comparative trials for tinea corporis and tinea cruris |
| ITRACONAZOLE ($112–$223.99) vs Griseofulvin ($50.99–$71.40) | A 36 | Itraconazole with greater efficacy than Griseofulvin | Risk difference = 19% in treatment of tinea cruris/corporis and 37% in treatment of tinea pedis/manus | Systematic review of RCT for tinea corporis, tinea cruris, tinea manuus, and tinea pedis |
| TERBINAFINE ($112.99–$223.99)vs Griseofulvin ($50.99–$71.40) | A 33 | Terbinafine with greater efficacy than Griseofulvin | Risk difference = 50% at 8 weeks post-treatment | Systematic review of RCT for tinea pedis |
| KETOCONAZOLE ($30.99–$61.99) vs Griseofulvin ($50.99–71.40) | A 33 | Approximately equal efficacy between Ketoconazole and Griseofulvin | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| FLUCONAZOLE ($13.99–$50.96) vs Griseofulvin ($50.99–$71.40) | A 37 | Non-significant trend toward greater efficacy with Fluconazole | Risk difference = 12% | RCT for tinea corporis and tinea cruris |
| FLUCONAZOLE ($13.99–$50.96) vs Ketoconazole ($30.99–$61.99) | B 39 | Approximately equal efficacy between Fluconazole and Ketoconazole | Risk difference = 4% favoring fluconazole at 7 weeks post-treatment | Small RCT for all dermatomycosis |
| ITRACONAZOLE ($112–$223.99) vs Fluconazole ($13.99–$50.96) | B 38 | Approximately equal efficacy between Fluconazole and Itraconazole | Risk difference = 5% favoring itraconazole at 10 weeks post-treatment | Small RCT for tinea manuum and tinea pedis |
| TERBINAFINE ($112.99–223.99) vs Itraconazole ($112–$223.99) | A 33 | Approximately equal efficacy between Terbinafine and Itraconazole | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| SOR, strength of recommendation; NNT, number needed to treat; RCT, randomized controlled trial | ||||
| SOR: A = Multiple RCT or a systematic review of RCT; B = Trials of moderate strength, as in open trials, noncomparative trials, or RCT with small size or poor follow-up. (See page 865 for full explanation of SOR ratings.) | ||||
| * Lowest cost available (including for generic agents) based upon internet listings of national on-line pharmacies, Drugstore.com, Eckerd.com, and Walgreens.com as of May 2003. | ||||
Summary of treatment recommendations
Based upon review of the available literature, it is recommended that clinicians consider the affordable and highly effective topical agents terbinafine and butenafine as first-line therapy for patients with uncomplicated epidermal tinea infections. While also highly effective, naftifine should be considered a second-line treatment due to its prescription-only availability and increased cost. The topical azoles clotrimazole and miconazole are marginally less effective than the allylamines, but are widely available and inexpensive. The prescription drug ciclopirox is slightly superior to the azoles, but its expense and availability make it a less desirable choice for most patients. Tolnaftate is cheap and widely available, but its relative inferiority to other topical agents limits its use in treatment of tinea infections. Tolnaftate may have some utility as a prophylactic against epidermal tinea infections in susceptible individuals.46
For patients with complicated epidermal tinea infections as described above, or for those with confirmed infection and treatment failure using topical agents, oral antifungal medications should be used. Terbinafine and itraconazole are both highly effective and safe in treating tinea infections. Despite their relatively high cost, either of these agents may be considered first-line therapies. Fluconazole has been evaluated in limited trials, but data at this time are insufficient to recommend that it be considered a first-line agent in treatment of epidermal tinea infection.
Griseofulvin may be considered a second-line agent as it has been shown to be clearly less efficacious than either terbinafine or itraconazole. Oral ketoconazole has a limited role in the treatment of tinea infections. It has demonstrated efficacy equivalent to or slightly superior to griseofulvin, but its association with potentially severe liver injury makes it a very problematic choice.
Adverse reactions
As with any systemic medication, adverse effects may occur with oral antifungal agents. Oral antifungal agents are generally considered safe in the treatment of skin dermatophytoses. In systemic review of trials of oral antifungal agents in the treatment of tinea pedis, the most commonly identified adverse events were gastrointestinal complaints such as diarrhea or nausea, headaches, and skin complaints.33
Of particular note, ketoconazole has been repeatedly related to potentially serious hepatotoxicity, especially when used in longer duration of treatment.47 One large cohort study found the incident rate of acute liver injury to be 134.1 per 100,000 person-months among ketoconazole users, compared with 10.4 and 2.5 for itraconazole and terbinafine, respectively.48 Accordingly, baseline and monthly laboratory studies are advised when using ketoconazole.49
Serious complications associated with the use of topical antifungal agents are rare. As with any topical medication, local irritation and dermatitis may occur.
Correspondence
Brian Thomas, MD, NOLF-IB, Bldg 184, Box 357140, San Diego, Ca 92135. E-mail: [email protected].
1. Smith ES, Fleischer AB, Friedman SR, Willford PM. Characteristics of office-based physician visits for cutaneous fungal infection: an analysis of 1990 to 1994 National Ambulatory Medical Care Survey Data. Cutis 2002;69:191-202.
2. Weitzman I, Chin NX, Kunjukunju N, Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 1998;39:255-261.
3. Roscoe J, Farmer ER. Diseases caused by fungi. In: Farmer ER, Hood AF, eds. Pathology of the Skin. 2nd ed. New York: McGraw-Hill; 2000;611-633.
4. Odom RB, James WD, Berger TG. Diseases resulting from fungi and yeasts. >In: Diseases of the Skin: Clinical Dermatology. 9th ed. Philadelphia: WB Saunders; 2000;358-415.
5. Habif TP. Superficial fungal infections. In: Clinical Dermatology. 3rd ed. St. Louis, Mo: Mosby; 1996;362-408.
6. Lousbergh D, Buntinx F, Pierard G. Diagnosing dermatomycosis in general practice. Fam Pract 1999;16:611-615.
7. Kearse HJ, Miller OF. Tinea pedis in prepubertal children: does it occur? J Am Acad Dermatol 1988;19:619-22.
8. Kates SG, Nordstrom KM, McGinley KJ, Leyden JJ. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990;22:578-582.
9. Lookingbill DP, Marks JG. Scaling papules, plaques, and patches. In: Principles of Dermatology. 2nd ed. Philadelphia: WB Saunders; 1993;136-159.
10. Rosen T. Dermatophytosis: diagnostic pointers and therapeutic pitfalls. Consultant 1997;37:1545-1557.
11. Sinski JT, Swanson JR, Kelley LM. Dermatophyte test medium: clinical and quantitative appraisal. J Inves Dermatol 1972;58:405-411.
12. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988;32:1-8.
13. Crawford F, Hart R, Bell-Syer S, Torgerson D, Young P, Russell I. Topical treatments for fungal infections of the skin and nails of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
14. Polak A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia 1984;22:501-503.
15. Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycosis.A North American perspective. Drugs 1998;55:645-674.
16. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol 1996;34:282-286.
17. Jordon RE, Rapini RP, Rex IH, Jr, et al. Once-daily naftifine cream 1% in the treatment of tinea cruris and tinea corporis. Int J Dermatol 1990;29:441-442.
18. Budimulja U, Bramono K, Urip S, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and tinea cruris. A placebo-controlled study. Mycoses 2001;44:300-306.
19. Schuster I, Schaude M, Schatz F, et al. Preclinical characteristics of allylamines. In: Berg D, Plempel M, eds. Sterol Biosynthesis Inhibitors. Chichester, England: Ellis Horwood; 1988;449-470.
20. Evans EG, Seaman RA, James IG. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol 1994;130:83-87
21. Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol 2000;10:107-109.
22. Chren MM, Landefeld CS. A cost analysis of topical drug regimens for dermatophyte infections. JAMA 1994;272:1922-1925.
23. Davis R, Balfour JA. Terbinafine. A pharmacoeconomic evaluation of its use in superficial fungal infections. Pharmacoeconomics. 1995;8:253-269.
24. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
25. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily Butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Amer Acad Dermatol 1997;36:S15-S19.
26. Greer DL, Weiss J, Rodriguez DA, Hebert AA, Swinehart JM. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Amer Acad Dermatol 1997;37:231-235.
27. Lesher JL, Babel DE, Stewart DM, et al. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehi-cle-controlled, double-blind trial. J Amer Acad Dermatol 1997;36:S20-S24.
28. Sehgal VN. Ciclopirox: a new topical pyrodonium antimy-cotic agent. A double-blind study in superficial dermato-mycoses. Br J Dermatol 1976;95:83-88.
29. Bogaert H, Cordero C, Ollague W, Savin RC, Shalita AR, Zaias N. Multicentre double-blind clinical trials of ciclopirox olamine cream 1% in the treatment of tinea corporis and tinea cruris. J Int Med Res 1986;14:210-216.
30. Beyer M. Selected double-blind comparative studies on the efficacy and tolerance of ciclopiroxolamine solution and cream [in German]. Arzneimittelforschung 1981;31:1378-1381.
31. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
32. Woscoff A, Carageli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Curr Ther Res 1986;39:753-757.
33. Bell-Syer SEM, Hart R, Crawford F, Torgerson DJ, Tyrell W, Russell I. Oral treatments for fungal infections of the skin of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
34. Voravutinon V. Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: a randomized double-blind comparative study. J Med Assoc Thai. 1993;76:388-393.
35. Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double-blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985;112:691-696.
36. Lachapelle JM, De Doncker P, Tennstedt D, Cauwenbergh G, Janssen PA. Itraconazole compared with griseo-fulvin in the treatment of tinea corporis/cruris and tinea pedis/mannus: An interpretation of the clinical results of all completed double-blind studies with respect to the pharmacokinetic profile. Dermatology 1992;184:45-50.
37. Faergemann J, Mork NJ, Haglund A, Odegard T. A multi-centre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol 1997;136:575-577.
38. Difonzo EM, Papini M, Cilli P, et al. A double blind study comparison of itraconazole and fluconazole in tinea pedis and tinea manuum. J Eur Acad Dermatol Venereol 1995;4:148-152.
39. Fischbein A, Haneke E, Lacner K. Comparative evaluation of oral fluconazole and oral ketoconazole in the treatment of fungal infections of the skin. Intl J Dermatol 1992;31:12-16.
40. Pariser DM, Pariser RJ, Ruoff G, Ray TL. Double-blind comparison of itraconazole and placebo in the treatment of tinea corporis and tinea cruris. J Amer Acad Dermatol 1994;31:232-234.
41. Svejgaard E, Avnstorp C, Wanscher B, Nilsson J, Heremans A. Efficacy and safety of short-term itracona-zole in tinea pedis: a double-blind, randomized, placebo-controlled trial. Dermatology 1998;197:368-372.
42. Suchil P, Gei FM, Robles M, Perera-Ramirez A, Welsh O, Male O. Once-weekly oral doses of fluconazole 150 mg in the treatment of tinea corporis/cruris and cutaneous candidiasis. Clin Exp Dermatol 1992;17:397-401.
43. Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidiasis. Intl J Dermatol 1998;37:703-705.
44. Savin RC, Zaias N. Treatment of chronic moccasin-type tinea pedis with terbinafine: a double-blind, placebo-controlled trial. J Am Acad Dermatol 1990;23:804-807.
45. Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol 1994;131:684-686.
46. Smith EB, Dickson JE, Knox JM. Tolnaftate powder in prophylaxis of tinea pedis. South Med J 1974;67:776-778.
47. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy: analysis of 33 cases. Gastroenterology 1984;86:503-513.
48. Garcia Rodriguez LA, Duque A, Castellsague J, Perea-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 1999;48:847-852.
49. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419-422.
50. Haldane DJ, Robart E. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn Microbiol Infect Dis 1990;13:337-339.
51. Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol 1993;129:510-511.
1. Smith ES, Fleischer AB, Friedman SR, Willford PM. Characteristics of office-based physician visits for cutaneous fungal infection: an analysis of 1990 to 1994 National Ambulatory Medical Care Survey Data. Cutis 2002;69:191-202.
2. Weitzman I, Chin NX, Kunjukunju N, Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 1998;39:255-261.
3. Roscoe J, Farmer ER. Diseases caused by fungi. In: Farmer ER, Hood AF, eds. Pathology of the Skin. 2nd ed. New York: McGraw-Hill; 2000;611-633.
4. Odom RB, James WD, Berger TG. Diseases resulting from fungi and yeasts. >In: Diseases of the Skin: Clinical Dermatology. 9th ed. Philadelphia: WB Saunders; 2000;358-415.
5. Habif TP. Superficial fungal infections. In: Clinical Dermatology. 3rd ed. St. Louis, Mo: Mosby; 1996;362-408.
6. Lousbergh D, Buntinx F, Pierard G. Diagnosing dermatomycosis in general practice. Fam Pract 1999;16:611-615.
7. Kearse HJ, Miller OF. Tinea pedis in prepubertal children: does it occur? J Am Acad Dermatol 1988;19:619-22.
8. Kates SG, Nordstrom KM, McGinley KJ, Leyden JJ. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990;22:578-582.
9. Lookingbill DP, Marks JG. Scaling papules, plaques, and patches. In: Principles of Dermatology. 2nd ed. Philadelphia: WB Saunders; 1993;136-159.
10. Rosen T. Dermatophytosis: diagnostic pointers and therapeutic pitfalls. Consultant 1997;37:1545-1557.
11. Sinski JT, Swanson JR, Kelley LM. Dermatophyte test medium: clinical and quantitative appraisal. J Inves Dermatol 1972;58:405-411.
12. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988;32:1-8.
13. Crawford F, Hart R, Bell-Syer S, Torgerson D, Young P, Russell I. Topical treatments for fungal infections of the skin and nails of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
14. Polak A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia 1984;22:501-503.
15. Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycosis.A North American perspective. Drugs 1998;55:645-674.
16. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol 1996;34:282-286.
17. Jordon RE, Rapini RP, Rex IH, Jr, et al. Once-daily naftifine cream 1% in the treatment of tinea cruris and tinea corporis. Int J Dermatol 1990;29:441-442.
18. Budimulja U, Bramono K, Urip S, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and tinea cruris. A placebo-controlled study. Mycoses 2001;44:300-306.
19. Schuster I, Schaude M, Schatz F, et al. Preclinical characteristics of allylamines. In: Berg D, Plempel M, eds. Sterol Biosynthesis Inhibitors. Chichester, England: Ellis Horwood; 1988;449-470.
20. Evans EG, Seaman RA, James IG. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol 1994;130:83-87
21. Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol 2000;10:107-109.
22. Chren MM, Landefeld CS. A cost analysis of topical drug regimens for dermatophyte infections. JAMA 1994;272:1922-1925.
23. Davis R, Balfour JA. Terbinafine. A pharmacoeconomic evaluation of its use in superficial fungal infections. Pharmacoeconomics. 1995;8:253-269.
24. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
25. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily Butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Amer Acad Dermatol 1997;36:S15-S19.
26. Greer DL, Weiss J, Rodriguez DA, Hebert AA, Swinehart JM. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Amer Acad Dermatol 1997;37:231-235.
27. Lesher JL, Babel DE, Stewart DM, et al. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehi-cle-controlled, double-blind trial. J Amer Acad Dermatol 1997;36:S20-S24.
28. Sehgal VN. Ciclopirox: a new topical pyrodonium antimy-cotic agent. A double-blind study in superficial dermato-mycoses. Br J Dermatol 1976;95:83-88.
29. Bogaert H, Cordero C, Ollague W, Savin RC, Shalita AR, Zaias N. Multicentre double-blind clinical trials of ciclopirox olamine cream 1% in the treatment of tinea corporis and tinea cruris. J Int Med Res 1986;14:210-216.
30. Beyer M. Selected double-blind comparative studies on the efficacy and tolerance of ciclopiroxolamine solution and cream [in German]. Arzneimittelforschung 1981;31:1378-1381.
31. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
32. Woscoff A, Carageli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Curr Ther Res 1986;39:753-757.
33. Bell-Syer SEM, Hart R, Crawford F, Torgerson DJ, Tyrell W, Russell I. Oral treatments for fungal infections of the skin of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
34. Voravutinon V. Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: a randomized double-blind comparative study. J Med Assoc Thai. 1993;76:388-393.
35. Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double-blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985;112:691-696.
36. Lachapelle JM, De Doncker P, Tennstedt D, Cauwenbergh G, Janssen PA. Itraconazole compared with griseo-fulvin in the treatment of tinea corporis/cruris and tinea pedis/mannus: An interpretation of the clinical results of all completed double-blind studies with respect to the pharmacokinetic profile. Dermatology 1992;184:45-50.
37. Faergemann J, Mork NJ, Haglund A, Odegard T. A multi-centre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol 1997;136:575-577.
38. Difonzo EM, Papini M, Cilli P, et al. A double blind study comparison of itraconazole and fluconazole in tinea pedis and tinea manuum. J Eur Acad Dermatol Venereol 1995;4:148-152.
39. Fischbein A, Haneke E, Lacner K. Comparative evaluation of oral fluconazole and oral ketoconazole in the treatment of fungal infections of the skin. Intl J Dermatol 1992;31:12-16.
40. Pariser DM, Pariser RJ, Ruoff G, Ray TL. Double-blind comparison of itraconazole and placebo in the treatment of tinea corporis and tinea cruris. J Amer Acad Dermatol 1994;31:232-234.
41. Svejgaard E, Avnstorp C, Wanscher B, Nilsson J, Heremans A. Efficacy and safety of short-term itracona-zole in tinea pedis: a double-blind, randomized, placebo-controlled trial. Dermatology 1998;197:368-372.
42. Suchil P, Gei FM, Robles M, Perera-Ramirez A, Welsh O, Male O. Once-weekly oral doses of fluconazole 150 mg in the treatment of tinea corporis/cruris and cutaneous candidiasis. Clin Exp Dermatol 1992;17:397-401.
43. Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidiasis. Intl J Dermatol 1998;37:703-705.
44. Savin RC, Zaias N. Treatment of chronic moccasin-type tinea pedis with terbinafine: a double-blind, placebo-controlled trial. J Am Acad Dermatol 1990;23:804-807.
45. Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol 1994;131:684-686.
46. Smith EB, Dickson JE, Knox JM. Tolnaftate powder in prophylaxis of tinea pedis. South Med J 1974;67:776-778.
47. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy: analysis of 33 cases. Gastroenterology 1984;86:503-513.
48. Garcia Rodriguez LA, Duque A, Castellsague J, Perea-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 1999;48:847-852.
49. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419-422.
50. Haldane DJ, Robart E. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn Microbiol Infect Dis 1990;13:337-339.
51. Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol 1993;129:510-511.
Evaluating idiopathic venous thromboembolism: What is necessary, what is not
- In low-risk patients, the physician should conduct a thorough history and physical examination. Routine laboratory testing may be useful, but further evaluation for underlying malignancy is unnecessary (B).
- Test for disorders associated with hypercoagulability under the following circumstances: when a thrombotic event occurs in a person younger than age 50; if a patient has a family history of venous thromboembolism; or if there are recurrent episodes of unexplained venous thromboembolism (C).
- When homocysteine levels are elevated in the presence of factor V Leiden or the prothrombin gene G20210A mutation, risk of recurrent thrombosis appears to be increased beyond the r isk associated with any one defect alone (B).
In which direction, and how aggressively, should the investigation proceed when common and obvious causes of venous thromboembolism—recent surgery, trauma, immobilization, or malignancy—are absent from a patient’s history?
Two causes of hypercoagulability warrant consideration: occult malignancy and coagulation disorders resulting in thrombophilia. This review provides guidance on diagnostic testing and extent of the work-up, summarized in an algorithm (Figure).
FIGURE
Evaluating idiopathic venous thromboembolism
Malignancy and Venous Thromboembolism
Armand Trousseau first described the association between VTE and cancer nearly 150 years ago.5 For patients with known malignancy, a search for other possible causes of thrombosis is seldom needed (strength of recommendation [SOR]=B).
However, for individuals with idiopathic DVT or pulmonary embolism (PE), the clinician’s dilemma is in deciding how aggressively to look for occult malignancy. In a prospective study of 738 patients with objectively verified symptomatic deep vein thrombosis (DVT), cancer was the most common underlying cause and emerged as a major predictor of recurrent thrombotic events.6 Unfortunately, no laboratory test predicts occult cancer among patients with VTE.7
Risk of malignancy in per spective
Evidence of malignancy is usually discovered when taking a patient’s history and conducting a physical examination. Searching beyond the history and physical exam is seldom revealing.
In 1992, Prandoni and colleagues8 published a report of a study of 262 patients with symptomatic DVT, 250 of whom were followed for 2 years. One hundred seven patients had recognized nonmalignant risk factors for DVT and were not evaluated for cancer. Of the 155 patients with idiopathic venous thrombosis, 5 (3.3%) were discovered to have occult carcinoma. Malignancy was suggested in 4 of the 5 by history or physical examination.
In a similar study, Hettiarachchi et al9 evaluated 400 patients with confirmed DVT and found 70 (18%) had a diagnosis of cancer at the time of presentation. Of the remaining 326 patients (4 were lost to follow-up), 189 had recognized risk factors for DVT, 3 (1.6%) of whom were also found to have cancer; and 137 patients had unexplained DVT, 10 of whom (7.3%) were found to have occult carcinoma. As in the Prandoni study, most of the patients subsequently discovered to have cancer (10 of 13, 77%) had suggestive clinical findings in the history or physical examination.9
Venous thromboembolism and specific types of cancer
Two large, retrospective epidemiologic studies reviewed cases of thousands of patients in the Danish and Swedish National Patient Registries.10,11 Investigators for these studies found an approximately 30% increase in the diagnosis of cancer among patients with VTE compared with the general population. Because of their large size, both of these studies were able to demonstrate a significant association between thrombosis and pancreatic, liver, and ovarian cancers.
For liver and pancreatic cancers, consensus opinion suggests that early diagnosis does not change prognosis. Similarly, although some experts recommend ultrasound and Ca-125 testing to investigate possible ovarian cancer, no data support use of these tests in ovarian cancer screening.12
An evaluation of the Danish National Registry data has suggested that cancer diagnosed at the time of, or within a year of, the diagnosis of VTE is usually advanced and is associated with a poor prognosis.13 Indeed, the authors of the Danish study concluded that for patients with VTE, “[our] pragmatic recommendation [is] to use only simple methods of screening and to look for cancer in patients with signs and symptoms of cancer.”13
Recommended work-up
We conclude the literature8-13 does not support an aggressive search for hidden cancer in a patient with idiopathic VTE (SOR=B). Routine evaluation should include a careful history and physical examination. Because of their low cost, reliability, and ready availability, studies such as a complete blood count, basic chemistry panel, liver function tests, and urinalysis may be considered (SOR=B ). Examples of findings during the initial history, physical exam, and laboratory studies that should prompt further evaluation include anorexia, weight loss, cough, abdominal bloating, unexplained anemia, hyponatremia, hematuria, and abnormal liver enzymes.
It is estimated that more than 250,000 patients are hospitalized for venous thromboembolism (VTE) in the United Sates each year.1 The number of VTE cases annually in this country ranges from 600,000 to 2 million.2,3 The most common causes of VTE include surgery, trauma, hospital or nursing home confinement and malignant neoplasm.4
Unnecessary studies. Some authorities recommend a chest radiograph in the routine evaluation for occult malignancy,9 but its clinical utility for patients without pulmonary symptoms has not been clearly demonstrated. Because of their expense and low test yield, we do not advocate the use of more elaborate screens for occult malignancy, such as computerized tomography, magnetic resonance imaging, or serologic tumor markers.
Caveat. In the past, nearly all patients with pulmonary embolism or DVT were hospitalized to receive treatment with continuous intravenous heparin. Presumably these patients routinely received a careful history evaluation, physical examination, and standard blood work.
With increased use of low-molecular-weight heparins (given subcutaneously once or twice daily), many individuals with VTE are candidates for treatment partially or totally on an outpatient basis.14,15 Be sure that individuals receiving outpatient treatment for idiopathic VTE receive the same attention and routine work-up as hospitalized patients.
Coagulation disorders and Venous Thromboembolism
With advances in laboratory testing, more than half of idiopathic VTE cases can be attributed to a specific coagulation disorder. Data show that in a randomly selected group of patients with newly discovered DVT, 24% to 37% have an inherited predisposition to thrombosis.16-19
Deficiencies in protein C, protein S, and antithrombin were the first causes of inherited thrombophilia to be identified;20-22 however, these deficiencies are not particularly common. The most common causes of thrombophilic coagulation disorders are activated protein C resistance, the prothrombin gene G20210A mutation, and hyperhomocysteinemia.
When to look for prothrombotic defects
If, after ruling out occult malignancy, the cause of VTE remains uncertain, be judicious in deciding whether to run more tests for prothrombotic defects. We advocate pursuing further testing for patients likely to have an underlying hypercoagulable disorder, and for whom the identification of such a disorder would have management implications.
A decision to pursue testing with other patients should be made on a case-by-case basis. The yield in testing is low for many prothrombotic disorders, some tests are affected adversely by anticoagulation therapy, and the influence of a positive test result on patient management decisions remains unclear in many cases. Finally, serologic evaluation for thrombophilia would be costly if conducted for all patients with VTE, and the potential clinical benefit would be small.
Although large epidemiologic studies are lacking to help identify patients at increased risk of a hypercoagulable disorder, patients with clinically significant inherited thrombophilia tend to have VTE at a young age.23-25
In addition, advanced age alone is often regarded as an identifiable risk factor for DVT. A recent retrospective study demonstrated the risk for DVT rose rapidly during the 6th through 8th decades of life.26
We generally recommend testing for hypercoagulable disorders upon discovering one of the following findings:
- The first thrombotic event occurs when the patient is younger than 50 years
- A family history of VTE exists
- Episodes of unexplained VTE recur (SOR=C).
Activated protein C resistance (Factor V Leiden mutation)
Prevalence. Approximately 90% of cases of activated protein C resistance are due to a substitution of glutamine for arginine at position 506 on the factor V gene, the so-called Leiden mutation.27
Factor V Leiden mutation varies greatly by race. In North America, this mutation is found in the heterozygous form in approximately 5% of Caucasians, 2% of Hispanics, 1% of African Americans, and less than 0.5% of Asians.28 Persons heterozygous for factor V Leiden have approximately a 7-fold increase in the relative risk;29 persons homozygous for the mutation have approximately an 80-fold increase in the relative risk.30
Detection. Activated protein C resistance is typically assessed by mixing patient plasma with factor V-deficient plasma. This clotting assay is not affected by oral anticoagulation medication but is affected by heparin. A positive test result for activated protein C resistance typically warrants a polymerase chain reaction assay to distinguish the factor V Leiden mutation from other causes of activated protein C resistance.
Testing considerations. Although factor V Leiden mutation is the cause of activated protein C resistance in most cases, we recommend that activated protein C resistance testing be done first, as it is a less expensive and more widely available test than the DNA-based factor V Leiden mutation test. However, if a patient is taking heparin at the time of evaluation for thrombophilia, it may be necessary to defer activated protein C resistance testing or proceed directly to factor V Leiden DNA-based testing.
Prothrombin gene G2 0210A mutation
Prevalence. Like the factor V Leiden mutation, the prothrombin gene G20210A mutation is more common among Caucasians than among those of African or Asian descent.31 Prothrombin mutation is estimated to cause a 2.5-fold relative risk of first DVT.32
Testing considerations. If cost or availability of the polymerase chain reaction assay are issues, a reasonable course of action would be to reserve the assay for patients with factor V Leiden mutation. Data suggest that patients with both factor V and prothrombin gene G20210A mutations are at significantly higher risk for recurrent VTE; consider prolonged anticoagulation.33
Hyperhomocysteinemia
Evidence accumulating over the past decade has shown an increased risk of VTE with elevated homocysteine levels. A case-control study by den Heijer et al34 in 1996 demonstrated a relative risk for first thrombosis of 2.5 in those with a homocysteine level above the 95th percentile of the control group’s levels (which in that study corresponded to a homocysteine level of 18.5 micromoles per liter or above). This predisposition to thrombosis has also been demonstrated in subsequent meta-analyses.35,36
When homocysteine levels are elevated in the presence of factor V Leiden or the prothrombin gene G20210A mutation, risk of thrombosis appears to increase beyond that associated with any one defect alone, perhaps as high as 50-fold risk.37-39
Management Implications of Test Results
If testing for activated protein C resistance is possible and the result is positive, confirmatory testing for factor V Leiden is indicated. If the patient is homozygous for factor V Leiden (rare), or is heterozygous for factor V Leiden and has the prothrombin gene G20210A defect, consider a prolonged course of anticoagulation, perhaps even for the rest of the patient’s life.
We base these suggestions on the high rate of VTE detected among individuals who are homozygous for factor V Leiden30 and on a recent study that showed the relative risk of recurrent thrombosis to be 2.6 for those with factor V Leiden and prothrombin gene G20210A mutations, versus for those heterozygous for factor V Leiden alone.33 This recent study also showed that the relative risk of recurrent thrombosis was not increased in those heterozygous for the Factor V Leiden mutation alone compared to those without this mutation.33
If either the factor V Leiden or the prothrombin gene G20210A mutation is present, consider prolonging the planned course of anticoagulation (although the data to support this decision are less compelling). Data are limited and conflicting regarding the appropriate length and intensity of anticoagulation therapy for patients with inherited prothrombotic defects.33,40-42 When prolonging anticoagulation for these patients, try to balance the risk of a recurrent thrombotic event with the risk of a bleeding complication from chronic anticoagulation.
Prolonged anticoagulation may also be indicated if the homocysteine level is elevated and either the factor V Leiden or the prothrombin gene G20210A mutation is present. In addition, therapy with folate, pyridoxine, and vitamin B12 should be initiated in cases of elevated homocyteine levels. We recommend the use of these vitamins based on a meta-analysis which demonstrated that folate supplementation at a dose of 0.5 mg to 5 mg/day lowered homocysteine levels by approximately 25% and vitamin B12 supplementation at a dose of 0.5 mg/day led to a further reduction of approximately 7%. In this same meta-analysis, pyridoxine at a mean dose of 16.5 mg daily did not demonstrate further lowering of the homocysteine level; however, as it is safe, inexpensive and well-tolerated, we still recommend its use.43
Finally, while less common than the hypercoagulable states discussed above, the antiphospholipid antibody syndrome should be targeted as part of first-tier testing because of its clear impact on management. If the antiphospholipid antibody testing (such as the lupus anticoagulant or anticardiolipin antibody) results are positive, lifelong anticoagulation44 and perhaps a higher target international normalized ratio are considerations.
Second-tier testing: Pursuing less common defects
If the preceding evaluation is unrevealing for a patient with high-risk characteristics, consider pursuing “second-tier” testing. Such testing may include functional and immunologic assays for protein C, protein S, and anti-thrombin III deficiencies, all of which are heterozygous abnormalities caused by multiple mutations and, thus, not detectable with genetic assays.
Patients will likely be taking anticoagulation medication when these tests are administered; thus, test results must be interpreted with caution. Testing for these deficiencies is most reliable if conducted at least 2 weeks after anticoagulation has been discontinued. Again, the incidence of protein C, protein S, and antithrombin III deficiencies is low, and the yield of testing therefore is also likely to be low.
We would not recommend discontinuing anticoagulation to conduct testing for these uncommon defects once it has been initiated following an acute thrombotic event. Rather, testing should be pursued once the planned course of anticoagulation with warfarin has been completed (for example, 6 to 9 months).
Other inherited thrombophilic disorders include heparin cofactor II deficiency, plasminogen deficiency, dysfibrinogenemia, factor XII deficiency, and increased factor VIII coagulant activity. These disorders are rare and their clinical importance remains unclear. Testing for these disorders, if desired, is best conducted in consultation with a hematologist or coagulation specialist.
Corresponding author
Charles F.S. Locke, MD, Johns Hopkins Community Physicians, 2360 W. Joppa Rd., Baltimore, MD 21093. E-mail: [email protected].
1. Goldhaber SZ. Pulmonary embolism. N Engl J Med 1998;339:93-104.
2. Deitcher SR. Overview of enoxaparin in the treatment of deep vein thrombosis. Am J Manag Care 2000;6(suppl):S1026-S1033.
3. Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996;93:2212-2245.
4. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism. Arch Int Med 2000;160:809-815.
5. Trousseau A. Phlegmasia alba dolens. In: Clinique Medicale de l’Hotel-Dieu de Paris. Vol. 3. 2nd ed. Paris: JB Bailliere; 1865;654-712.
6. Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med 2000;160:769-774.
7. Naschitz JE, Yeshurun D, Lev LM. Thromboembolism in cancer. Cancer 1993;71:1384-1390.
8. Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med 1992;327:1128-1133.
9. Hettiarachchi RJ, Lok J, Prins MH, et al. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer 1998;83:180-185.
10. Baron JA, Gridley G, Weiderpass E, et al. Venous thromboembolism and cancer. Lancet 1998;351:1077-1080.
11. Sorenson HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med 1998;338:1169-1173.
12. Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ 1993;306:1030-1034.
13. Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-1850.
14. Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996;334:677-681.
15. Koopman M, Prandoni P, Piovella F, et al. for the Tasman Study Group. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. N Engl J Med 1996;334:682-687.
16. Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:444-451.
17. Ben-Tal O, Zivelin A, Seligsohn U. The relative frequency of hereditary thrombotic disorders among 107 patients with thrombophilia in Israel. Thromb Haemost 1989;61:50-54.
18. Ridker PM, Hennekens CH, Lindpaintner K, et al. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995;332:912-917.
19. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G—>A20210 gene variant. Ann Intern Med 1998;129:89-93.
20. Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh 1965;13:516.-
21. Griffith JH, Evatt B, Zimmerman TS, et al. Deficiency of protein C in congenital thrombotic disease. J Clin Invest 1981;68:1370-1373.
22. Comp PC, Nixon RR, Cooper MR, et al. Familial protein S deficiency is associated with recurrent thrombosis. J Clin Invest 1984;74:2082-2088.
23. Hillarp A, Dahlback B, Zoller B. Activated protein C resistance: from phenotype to genotype and clinical practice. Blood Rev 1995;9:201-212.
24. Zoller B, Berntsdotter A, Garcia de Frutos P, et al. Resistance to activated protein C as an additional genetic risk factor in hereditary deficiency of protein S. Blood 1995;85:3518-3523.
25. Van Boven HH, Reitsma PH, Rosendaal FR, et al. Factor V Leiden (FV R506Q) in families with inherited antithrombin deficiency. Thromb Haemost 1996;75:417-421.
26. Heit JA, Silverstein MD, Mohr DN, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000;160:809-815.
27. Bertina RM, Koeleman BPC, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994;369:64-67.
28. Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997;277:1305-1307.
29. Koster T, Rosendaal FR, de Ronde H, Briet E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden thrombophilia study. Lancet 1993;342:1503-1506.
30. Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995;85:1504-1508.
31. Rosendaal FR, Doggen CJ, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost 1998;79:706-708.
32. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3´ untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996;88:3698-3703.
33. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med 1999;341:801-806.
34. den Heijer M, Koster T, Blom HJ, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med 1996;334:759-762.
35. Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med 1998;158:2101-2106.
36. den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost 1998;80:874-877.
37. Ridker PM, Hennekens CH, Selhub J, Miletich JP, Malinow MR, Stampfer MJ. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation 1997;95:1777-1782.
38. Kluijtmans LA, den Heijer M, Reitsma PH, Heil SG, Blom HJ, Rosendaal FR. Thermolabile methylenetetrahydrofolate reductase and factor V Leiden in the risk of deep-vein thrombosis. Thromb Haemost 1998;79:254-258.
39. DeStefano V, Zappacosta B, Persichilli S, et al. Prevalence of mild hyperhomocysteinaemia and association with thrombophilic genotypes (factor V Leiden and prothrombin G20210A) in Italian patients with venous thromboembolic disease. Br J Haematol 1999;106:564-568.
40. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
41. Simioni P, Prandoni P, Lensing AWA, et al. The risk of recurrent venous thromboembolism in patients with an Arg506—>Gln mutation in the gene for factor V (factor V Leiden). N Engl J Med 1997;336:399-403.
42. Ridker PM, Miletich JP, Stampfer MJ, Goldhaber SZ, Lindpaintner K, Hennekens CH. Factor V Leiden and risks of recurrent idiopathic venous thromboembolism. Circulation 1995;92:2800-2802.
43. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998;316:894-898.
44. McCrae KR. Antiphospholipid antibody associated thrombosis: a consensus for treatment? Lupus 1996;5:560-570.
- In low-risk patients, the physician should conduct a thorough history and physical examination. Routine laboratory testing may be useful, but further evaluation for underlying malignancy is unnecessary (B).
- Test for disorders associated with hypercoagulability under the following circumstances: when a thrombotic event occurs in a person younger than age 50; if a patient has a family history of venous thromboembolism; or if there are recurrent episodes of unexplained venous thromboembolism (C).
- When homocysteine levels are elevated in the presence of factor V Leiden or the prothrombin gene G20210A mutation, risk of recurrent thrombosis appears to be increased beyond the r isk associated with any one defect alone (B).
In which direction, and how aggressively, should the investigation proceed when common and obvious causes of venous thromboembolism—recent surgery, trauma, immobilization, or malignancy—are absent from a patient’s history?
Two causes of hypercoagulability warrant consideration: occult malignancy and coagulation disorders resulting in thrombophilia. This review provides guidance on diagnostic testing and extent of the work-up, summarized in an algorithm (Figure).
FIGURE
Evaluating idiopathic venous thromboembolism
Malignancy and Venous Thromboembolism
Armand Trousseau first described the association between VTE and cancer nearly 150 years ago.5 For patients with known malignancy, a search for other possible causes of thrombosis is seldom needed (strength of recommendation [SOR]=B).
However, for individuals with idiopathic DVT or pulmonary embolism (PE), the clinician’s dilemma is in deciding how aggressively to look for occult malignancy. In a prospective study of 738 patients with objectively verified symptomatic deep vein thrombosis (DVT), cancer was the most common underlying cause and emerged as a major predictor of recurrent thrombotic events.6 Unfortunately, no laboratory test predicts occult cancer among patients with VTE.7
Risk of malignancy in per spective
Evidence of malignancy is usually discovered when taking a patient’s history and conducting a physical examination. Searching beyond the history and physical exam is seldom revealing.
In 1992, Prandoni and colleagues8 published a report of a study of 262 patients with symptomatic DVT, 250 of whom were followed for 2 years. One hundred seven patients had recognized nonmalignant risk factors for DVT and were not evaluated for cancer. Of the 155 patients with idiopathic venous thrombosis, 5 (3.3%) were discovered to have occult carcinoma. Malignancy was suggested in 4 of the 5 by history or physical examination.
In a similar study, Hettiarachchi et al9 evaluated 400 patients with confirmed DVT and found 70 (18%) had a diagnosis of cancer at the time of presentation. Of the remaining 326 patients (4 were lost to follow-up), 189 had recognized risk factors for DVT, 3 (1.6%) of whom were also found to have cancer; and 137 patients had unexplained DVT, 10 of whom (7.3%) were found to have occult carcinoma. As in the Prandoni study, most of the patients subsequently discovered to have cancer (10 of 13, 77%) had suggestive clinical findings in the history or physical examination.9
Venous thromboembolism and specific types of cancer
Two large, retrospective epidemiologic studies reviewed cases of thousands of patients in the Danish and Swedish National Patient Registries.10,11 Investigators for these studies found an approximately 30% increase in the diagnosis of cancer among patients with VTE compared with the general population. Because of their large size, both of these studies were able to demonstrate a significant association between thrombosis and pancreatic, liver, and ovarian cancers.
For liver and pancreatic cancers, consensus opinion suggests that early diagnosis does not change prognosis. Similarly, although some experts recommend ultrasound and Ca-125 testing to investigate possible ovarian cancer, no data support use of these tests in ovarian cancer screening.12
An evaluation of the Danish National Registry data has suggested that cancer diagnosed at the time of, or within a year of, the diagnosis of VTE is usually advanced and is associated with a poor prognosis.13 Indeed, the authors of the Danish study concluded that for patients with VTE, “[our] pragmatic recommendation [is] to use only simple methods of screening and to look for cancer in patients with signs and symptoms of cancer.”13
Recommended work-up
We conclude the literature8-13 does not support an aggressive search for hidden cancer in a patient with idiopathic VTE (SOR=B). Routine evaluation should include a careful history and physical examination. Because of their low cost, reliability, and ready availability, studies such as a complete blood count, basic chemistry panel, liver function tests, and urinalysis may be considered (SOR=B ). Examples of findings during the initial history, physical exam, and laboratory studies that should prompt further evaluation include anorexia, weight loss, cough, abdominal bloating, unexplained anemia, hyponatremia, hematuria, and abnormal liver enzymes.
It is estimated that more than 250,000 patients are hospitalized for venous thromboembolism (VTE) in the United Sates each year.1 The number of VTE cases annually in this country ranges from 600,000 to 2 million.2,3 The most common causes of VTE include surgery, trauma, hospital or nursing home confinement and malignant neoplasm.4
Unnecessary studies. Some authorities recommend a chest radiograph in the routine evaluation for occult malignancy,9 but its clinical utility for patients without pulmonary symptoms has not been clearly demonstrated. Because of their expense and low test yield, we do not advocate the use of more elaborate screens for occult malignancy, such as computerized tomography, magnetic resonance imaging, or serologic tumor markers.
Caveat. In the past, nearly all patients with pulmonary embolism or DVT were hospitalized to receive treatment with continuous intravenous heparin. Presumably these patients routinely received a careful history evaluation, physical examination, and standard blood work.
With increased use of low-molecular-weight heparins (given subcutaneously once or twice daily), many individuals with VTE are candidates for treatment partially or totally on an outpatient basis.14,15 Be sure that individuals receiving outpatient treatment for idiopathic VTE receive the same attention and routine work-up as hospitalized patients.
Coagulation disorders and Venous Thromboembolism
With advances in laboratory testing, more than half of idiopathic VTE cases can be attributed to a specific coagulation disorder. Data show that in a randomly selected group of patients with newly discovered DVT, 24% to 37% have an inherited predisposition to thrombosis.16-19
Deficiencies in protein C, protein S, and antithrombin were the first causes of inherited thrombophilia to be identified;20-22 however, these deficiencies are not particularly common. The most common causes of thrombophilic coagulation disorders are activated protein C resistance, the prothrombin gene G20210A mutation, and hyperhomocysteinemia.
When to look for prothrombotic defects
If, after ruling out occult malignancy, the cause of VTE remains uncertain, be judicious in deciding whether to run more tests for prothrombotic defects. We advocate pursuing further testing for patients likely to have an underlying hypercoagulable disorder, and for whom the identification of such a disorder would have management implications.
A decision to pursue testing with other patients should be made on a case-by-case basis. The yield in testing is low for many prothrombotic disorders, some tests are affected adversely by anticoagulation therapy, and the influence of a positive test result on patient management decisions remains unclear in many cases. Finally, serologic evaluation for thrombophilia would be costly if conducted for all patients with VTE, and the potential clinical benefit would be small.
Although large epidemiologic studies are lacking to help identify patients at increased risk of a hypercoagulable disorder, patients with clinically significant inherited thrombophilia tend to have VTE at a young age.23-25
In addition, advanced age alone is often regarded as an identifiable risk factor for DVT. A recent retrospective study demonstrated the risk for DVT rose rapidly during the 6th through 8th decades of life.26
We generally recommend testing for hypercoagulable disorders upon discovering one of the following findings:
- The first thrombotic event occurs when the patient is younger than 50 years
- A family history of VTE exists
- Episodes of unexplained VTE recur (SOR=C).
Activated protein C resistance (Factor V Leiden mutation)
Prevalence. Approximately 90% of cases of activated protein C resistance are due to a substitution of glutamine for arginine at position 506 on the factor V gene, the so-called Leiden mutation.27
Factor V Leiden mutation varies greatly by race. In North America, this mutation is found in the heterozygous form in approximately 5% of Caucasians, 2% of Hispanics, 1% of African Americans, and less than 0.5% of Asians.28 Persons heterozygous for factor V Leiden have approximately a 7-fold increase in the relative risk;29 persons homozygous for the mutation have approximately an 80-fold increase in the relative risk.30
Detection. Activated protein C resistance is typically assessed by mixing patient plasma with factor V-deficient plasma. This clotting assay is not affected by oral anticoagulation medication but is affected by heparin. A positive test result for activated protein C resistance typically warrants a polymerase chain reaction assay to distinguish the factor V Leiden mutation from other causes of activated protein C resistance.
Testing considerations. Although factor V Leiden mutation is the cause of activated protein C resistance in most cases, we recommend that activated protein C resistance testing be done first, as it is a less expensive and more widely available test than the DNA-based factor V Leiden mutation test. However, if a patient is taking heparin at the time of evaluation for thrombophilia, it may be necessary to defer activated protein C resistance testing or proceed directly to factor V Leiden DNA-based testing.
Prothrombin gene G2 0210A mutation
Prevalence. Like the factor V Leiden mutation, the prothrombin gene G20210A mutation is more common among Caucasians than among those of African or Asian descent.31 Prothrombin mutation is estimated to cause a 2.5-fold relative risk of first DVT.32
Testing considerations. If cost or availability of the polymerase chain reaction assay are issues, a reasonable course of action would be to reserve the assay for patients with factor V Leiden mutation. Data suggest that patients with both factor V and prothrombin gene G20210A mutations are at significantly higher risk for recurrent VTE; consider prolonged anticoagulation.33
Hyperhomocysteinemia
Evidence accumulating over the past decade has shown an increased risk of VTE with elevated homocysteine levels. A case-control study by den Heijer et al34 in 1996 demonstrated a relative risk for first thrombosis of 2.5 in those with a homocysteine level above the 95th percentile of the control group’s levels (which in that study corresponded to a homocysteine level of 18.5 micromoles per liter or above). This predisposition to thrombosis has also been demonstrated in subsequent meta-analyses.35,36
When homocysteine levels are elevated in the presence of factor V Leiden or the prothrombin gene G20210A mutation, risk of thrombosis appears to increase beyond that associated with any one defect alone, perhaps as high as 50-fold risk.37-39
Management Implications of Test Results
If testing for activated protein C resistance is possible and the result is positive, confirmatory testing for factor V Leiden is indicated. If the patient is homozygous for factor V Leiden (rare), or is heterozygous for factor V Leiden and has the prothrombin gene G20210A defect, consider a prolonged course of anticoagulation, perhaps even for the rest of the patient’s life.
We base these suggestions on the high rate of VTE detected among individuals who are homozygous for factor V Leiden30 and on a recent study that showed the relative risk of recurrent thrombosis to be 2.6 for those with factor V Leiden and prothrombin gene G20210A mutations, versus for those heterozygous for factor V Leiden alone.33 This recent study also showed that the relative risk of recurrent thrombosis was not increased in those heterozygous for the Factor V Leiden mutation alone compared to those without this mutation.33
If either the factor V Leiden or the prothrombin gene G20210A mutation is present, consider prolonging the planned course of anticoagulation (although the data to support this decision are less compelling). Data are limited and conflicting regarding the appropriate length and intensity of anticoagulation therapy for patients with inherited prothrombotic defects.33,40-42 When prolonging anticoagulation for these patients, try to balance the risk of a recurrent thrombotic event with the risk of a bleeding complication from chronic anticoagulation.
Prolonged anticoagulation may also be indicated if the homocysteine level is elevated and either the factor V Leiden or the prothrombin gene G20210A mutation is present. In addition, therapy with folate, pyridoxine, and vitamin B12 should be initiated in cases of elevated homocyteine levels. We recommend the use of these vitamins based on a meta-analysis which demonstrated that folate supplementation at a dose of 0.5 mg to 5 mg/day lowered homocysteine levels by approximately 25% and vitamin B12 supplementation at a dose of 0.5 mg/day led to a further reduction of approximately 7%. In this same meta-analysis, pyridoxine at a mean dose of 16.5 mg daily did not demonstrate further lowering of the homocysteine level; however, as it is safe, inexpensive and well-tolerated, we still recommend its use.43
Finally, while less common than the hypercoagulable states discussed above, the antiphospholipid antibody syndrome should be targeted as part of first-tier testing because of its clear impact on management. If the antiphospholipid antibody testing (such as the lupus anticoagulant or anticardiolipin antibody) results are positive, lifelong anticoagulation44 and perhaps a higher target international normalized ratio are considerations.
Second-tier testing: Pursuing less common defects
If the preceding evaluation is unrevealing for a patient with high-risk characteristics, consider pursuing “second-tier” testing. Such testing may include functional and immunologic assays for protein C, protein S, and anti-thrombin III deficiencies, all of which are heterozygous abnormalities caused by multiple mutations and, thus, not detectable with genetic assays.
Patients will likely be taking anticoagulation medication when these tests are administered; thus, test results must be interpreted with caution. Testing for these deficiencies is most reliable if conducted at least 2 weeks after anticoagulation has been discontinued. Again, the incidence of protein C, protein S, and antithrombin III deficiencies is low, and the yield of testing therefore is also likely to be low.
We would not recommend discontinuing anticoagulation to conduct testing for these uncommon defects once it has been initiated following an acute thrombotic event. Rather, testing should be pursued once the planned course of anticoagulation with warfarin has been completed (for example, 6 to 9 months).
Other inherited thrombophilic disorders include heparin cofactor II deficiency, plasminogen deficiency, dysfibrinogenemia, factor XII deficiency, and increased factor VIII coagulant activity. These disorders are rare and their clinical importance remains unclear. Testing for these disorders, if desired, is best conducted in consultation with a hematologist or coagulation specialist.
Corresponding author
Charles F.S. Locke, MD, Johns Hopkins Community Physicians, 2360 W. Joppa Rd., Baltimore, MD 21093. E-mail: [email protected].
- In low-risk patients, the physician should conduct a thorough history and physical examination. Routine laboratory testing may be useful, but further evaluation for underlying malignancy is unnecessary (B).
- Test for disorders associated with hypercoagulability under the following circumstances: when a thrombotic event occurs in a person younger than age 50; if a patient has a family history of venous thromboembolism; or if there are recurrent episodes of unexplained venous thromboembolism (C).
- When homocysteine levels are elevated in the presence of factor V Leiden or the prothrombin gene G20210A mutation, risk of recurrent thrombosis appears to be increased beyond the r isk associated with any one defect alone (B).
In which direction, and how aggressively, should the investigation proceed when common and obvious causes of venous thromboembolism—recent surgery, trauma, immobilization, or malignancy—are absent from a patient’s history?
Two causes of hypercoagulability warrant consideration: occult malignancy and coagulation disorders resulting in thrombophilia. This review provides guidance on diagnostic testing and extent of the work-up, summarized in an algorithm (Figure).
FIGURE
Evaluating idiopathic venous thromboembolism
Malignancy and Venous Thromboembolism
Armand Trousseau first described the association between VTE and cancer nearly 150 years ago.5 For patients with known malignancy, a search for other possible causes of thrombosis is seldom needed (strength of recommendation [SOR]=B).
However, for individuals with idiopathic DVT or pulmonary embolism (PE), the clinician’s dilemma is in deciding how aggressively to look for occult malignancy. In a prospective study of 738 patients with objectively verified symptomatic deep vein thrombosis (DVT), cancer was the most common underlying cause and emerged as a major predictor of recurrent thrombotic events.6 Unfortunately, no laboratory test predicts occult cancer among patients with VTE.7
Risk of malignancy in per spective
Evidence of malignancy is usually discovered when taking a patient’s history and conducting a physical examination. Searching beyond the history and physical exam is seldom revealing.
In 1992, Prandoni and colleagues8 published a report of a study of 262 patients with symptomatic DVT, 250 of whom were followed for 2 years. One hundred seven patients had recognized nonmalignant risk factors for DVT and were not evaluated for cancer. Of the 155 patients with idiopathic venous thrombosis, 5 (3.3%) were discovered to have occult carcinoma. Malignancy was suggested in 4 of the 5 by history or physical examination.
In a similar study, Hettiarachchi et al9 evaluated 400 patients with confirmed DVT and found 70 (18%) had a diagnosis of cancer at the time of presentation. Of the remaining 326 patients (4 were lost to follow-up), 189 had recognized risk factors for DVT, 3 (1.6%) of whom were also found to have cancer; and 137 patients had unexplained DVT, 10 of whom (7.3%) were found to have occult carcinoma. As in the Prandoni study, most of the patients subsequently discovered to have cancer (10 of 13, 77%) had suggestive clinical findings in the history or physical examination.9
Venous thromboembolism and specific types of cancer
Two large, retrospective epidemiologic studies reviewed cases of thousands of patients in the Danish and Swedish National Patient Registries.10,11 Investigators for these studies found an approximately 30% increase in the diagnosis of cancer among patients with VTE compared with the general population. Because of their large size, both of these studies were able to demonstrate a significant association between thrombosis and pancreatic, liver, and ovarian cancers.
For liver and pancreatic cancers, consensus opinion suggests that early diagnosis does not change prognosis. Similarly, although some experts recommend ultrasound and Ca-125 testing to investigate possible ovarian cancer, no data support use of these tests in ovarian cancer screening.12
An evaluation of the Danish National Registry data has suggested that cancer diagnosed at the time of, or within a year of, the diagnosis of VTE is usually advanced and is associated with a poor prognosis.13 Indeed, the authors of the Danish study concluded that for patients with VTE, “[our] pragmatic recommendation [is] to use only simple methods of screening and to look for cancer in patients with signs and symptoms of cancer.”13
Recommended work-up
We conclude the literature8-13 does not support an aggressive search for hidden cancer in a patient with idiopathic VTE (SOR=B). Routine evaluation should include a careful history and physical examination. Because of their low cost, reliability, and ready availability, studies such as a complete blood count, basic chemistry panel, liver function tests, and urinalysis may be considered (SOR=B ). Examples of findings during the initial history, physical exam, and laboratory studies that should prompt further evaluation include anorexia, weight loss, cough, abdominal bloating, unexplained anemia, hyponatremia, hematuria, and abnormal liver enzymes.
It is estimated that more than 250,000 patients are hospitalized for venous thromboembolism (VTE) in the United Sates each year.1 The number of VTE cases annually in this country ranges from 600,000 to 2 million.2,3 The most common causes of VTE include surgery, trauma, hospital or nursing home confinement and malignant neoplasm.4
Unnecessary studies. Some authorities recommend a chest radiograph in the routine evaluation for occult malignancy,9 but its clinical utility for patients without pulmonary symptoms has not been clearly demonstrated. Because of their expense and low test yield, we do not advocate the use of more elaborate screens for occult malignancy, such as computerized tomography, magnetic resonance imaging, or serologic tumor markers.
Caveat. In the past, nearly all patients with pulmonary embolism or DVT were hospitalized to receive treatment with continuous intravenous heparin. Presumably these patients routinely received a careful history evaluation, physical examination, and standard blood work.
With increased use of low-molecular-weight heparins (given subcutaneously once or twice daily), many individuals with VTE are candidates for treatment partially or totally on an outpatient basis.14,15 Be sure that individuals receiving outpatient treatment for idiopathic VTE receive the same attention and routine work-up as hospitalized patients.
Coagulation disorders and Venous Thromboembolism
With advances in laboratory testing, more than half of idiopathic VTE cases can be attributed to a specific coagulation disorder. Data show that in a randomly selected group of patients with newly discovered DVT, 24% to 37% have an inherited predisposition to thrombosis.16-19
Deficiencies in protein C, protein S, and antithrombin were the first causes of inherited thrombophilia to be identified;20-22 however, these deficiencies are not particularly common. The most common causes of thrombophilic coagulation disorders are activated protein C resistance, the prothrombin gene G20210A mutation, and hyperhomocysteinemia.
When to look for prothrombotic defects
If, after ruling out occult malignancy, the cause of VTE remains uncertain, be judicious in deciding whether to run more tests for prothrombotic defects. We advocate pursuing further testing for patients likely to have an underlying hypercoagulable disorder, and for whom the identification of such a disorder would have management implications.
A decision to pursue testing with other patients should be made on a case-by-case basis. The yield in testing is low for many prothrombotic disorders, some tests are affected adversely by anticoagulation therapy, and the influence of a positive test result on patient management decisions remains unclear in many cases. Finally, serologic evaluation for thrombophilia would be costly if conducted for all patients with VTE, and the potential clinical benefit would be small.
Although large epidemiologic studies are lacking to help identify patients at increased risk of a hypercoagulable disorder, patients with clinically significant inherited thrombophilia tend to have VTE at a young age.23-25
In addition, advanced age alone is often regarded as an identifiable risk factor for DVT. A recent retrospective study demonstrated the risk for DVT rose rapidly during the 6th through 8th decades of life.26
We generally recommend testing for hypercoagulable disorders upon discovering one of the following findings:
- The first thrombotic event occurs when the patient is younger than 50 years
- A family history of VTE exists
- Episodes of unexplained VTE recur (SOR=C).
Activated protein C resistance (Factor V Leiden mutation)
Prevalence. Approximately 90% of cases of activated protein C resistance are due to a substitution of glutamine for arginine at position 506 on the factor V gene, the so-called Leiden mutation.27
Factor V Leiden mutation varies greatly by race. In North America, this mutation is found in the heterozygous form in approximately 5% of Caucasians, 2% of Hispanics, 1% of African Americans, and less than 0.5% of Asians.28 Persons heterozygous for factor V Leiden have approximately a 7-fold increase in the relative risk;29 persons homozygous for the mutation have approximately an 80-fold increase in the relative risk.30
Detection. Activated protein C resistance is typically assessed by mixing patient plasma with factor V-deficient plasma. This clotting assay is not affected by oral anticoagulation medication but is affected by heparin. A positive test result for activated protein C resistance typically warrants a polymerase chain reaction assay to distinguish the factor V Leiden mutation from other causes of activated protein C resistance.
Testing considerations. Although factor V Leiden mutation is the cause of activated protein C resistance in most cases, we recommend that activated protein C resistance testing be done first, as it is a less expensive and more widely available test than the DNA-based factor V Leiden mutation test. However, if a patient is taking heparin at the time of evaluation for thrombophilia, it may be necessary to defer activated protein C resistance testing or proceed directly to factor V Leiden DNA-based testing.
Prothrombin gene G2 0210A mutation
Prevalence. Like the factor V Leiden mutation, the prothrombin gene G20210A mutation is more common among Caucasians than among those of African or Asian descent.31 Prothrombin mutation is estimated to cause a 2.5-fold relative risk of first DVT.32
Testing considerations. If cost or availability of the polymerase chain reaction assay are issues, a reasonable course of action would be to reserve the assay for patients with factor V Leiden mutation. Data suggest that patients with both factor V and prothrombin gene G20210A mutations are at significantly higher risk for recurrent VTE; consider prolonged anticoagulation.33
Hyperhomocysteinemia
Evidence accumulating over the past decade has shown an increased risk of VTE with elevated homocysteine levels. A case-control study by den Heijer et al34 in 1996 demonstrated a relative risk for first thrombosis of 2.5 in those with a homocysteine level above the 95th percentile of the control group’s levels (which in that study corresponded to a homocysteine level of 18.5 micromoles per liter or above). This predisposition to thrombosis has also been demonstrated in subsequent meta-analyses.35,36
When homocysteine levels are elevated in the presence of factor V Leiden or the prothrombin gene G20210A mutation, risk of thrombosis appears to increase beyond that associated with any one defect alone, perhaps as high as 50-fold risk.37-39
Management Implications of Test Results
If testing for activated protein C resistance is possible and the result is positive, confirmatory testing for factor V Leiden is indicated. If the patient is homozygous for factor V Leiden (rare), or is heterozygous for factor V Leiden and has the prothrombin gene G20210A defect, consider a prolonged course of anticoagulation, perhaps even for the rest of the patient’s life.
We base these suggestions on the high rate of VTE detected among individuals who are homozygous for factor V Leiden30 and on a recent study that showed the relative risk of recurrent thrombosis to be 2.6 for those with factor V Leiden and prothrombin gene G20210A mutations, versus for those heterozygous for factor V Leiden alone.33 This recent study also showed that the relative risk of recurrent thrombosis was not increased in those heterozygous for the Factor V Leiden mutation alone compared to those without this mutation.33
If either the factor V Leiden or the prothrombin gene G20210A mutation is present, consider prolonging the planned course of anticoagulation (although the data to support this decision are less compelling). Data are limited and conflicting regarding the appropriate length and intensity of anticoagulation therapy for patients with inherited prothrombotic defects.33,40-42 When prolonging anticoagulation for these patients, try to balance the risk of a recurrent thrombotic event with the risk of a bleeding complication from chronic anticoagulation.
Prolonged anticoagulation may also be indicated if the homocysteine level is elevated and either the factor V Leiden or the prothrombin gene G20210A mutation is present. In addition, therapy with folate, pyridoxine, and vitamin B12 should be initiated in cases of elevated homocyteine levels. We recommend the use of these vitamins based on a meta-analysis which demonstrated that folate supplementation at a dose of 0.5 mg to 5 mg/day lowered homocysteine levels by approximately 25% and vitamin B12 supplementation at a dose of 0.5 mg/day led to a further reduction of approximately 7%. In this same meta-analysis, pyridoxine at a mean dose of 16.5 mg daily did not demonstrate further lowering of the homocysteine level; however, as it is safe, inexpensive and well-tolerated, we still recommend its use.43
Finally, while less common than the hypercoagulable states discussed above, the antiphospholipid antibody syndrome should be targeted as part of first-tier testing because of its clear impact on management. If the antiphospholipid antibody testing (such as the lupus anticoagulant or anticardiolipin antibody) results are positive, lifelong anticoagulation44 and perhaps a higher target international normalized ratio are considerations.
Second-tier testing: Pursuing less common defects
If the preceding evaluation is unrevealing for a patient with high-risk characteristics, consider pursuing “second-tier” testing. Such testing may include functional and immunologic assays for protein C, protein S, and anti-thrombin III deficiencies, all of which are heterozygous abnormalities caused by multiple mutations and, thus, not detectable with genetic assays.
Patients will likely be taking anticoagulation medication when these tests are administered; thus, test results must be interpreted with caution. Testing for these deficiencies is most reliable if conducted at least 2 weeks after anticoagulation has been discontinued. Again, the incidence of protein C, protein S, and antithrombin III deficiencies is low, and the yield of testing therefore is also likely to be low.
We would not recommend discontinuing anticoagulation to conduct testing for these uncommon defects once it has been initiated following an acute thrombotic event. Rather, testing should be pursued once the planned course of anticoagulation with warfarin has been completed (for example, 6 to 9 months).
Other inherited thrombophilic disorders include heparin cofactor II deficiency, plasminogen deficiency, dysfibrinogenemia, factor XII deficiency, and increased factor VIII coagulant activity. These disorders are rare and their clinical importance remains unclear. Testing for these disorders, if desired, is best conducted in consultation with a hematologist or coagulation specialist.
Corresponding author
Charles F.S. Locke, MD, Johns Hopkins Community Physicians, 2360 W. Joppa Rd., Baltimore, MD 21093. E-mail: [email protected].
1. Goldhaber SZ. Pulmonary embolism. N Engl J Med 1998;339:93-104.
2. Deitcher SR. Overview of enoxaparin in the treatment of deep vein thrombosis. Am J Manag Care 2000;6(suppl):S1026-S1033.
3. Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996;93:2212-2245.
4. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism. Arch Int Med 2000;160:809-815.
5. Trousseau A. Phlegmasia alba dolens. In: Clinique Medicale de l’Hotel-Dieu de Paris. Vol. 3. 2nd ed. Paris: JB Bailliere; 1865;654-712.
6. Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med 2000;160:769-774.
7. Naschitz JE, Yeshurun D, Lev LM. Thromboembolism in cancer. Cancer 1993;71:1384-1390.
8. Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med 1992;327:1128-1133.
9. Hettiarachchi RJ, Lok J, Prins MH, et al. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer 1998;83:180-185.
10. Baron JA, Gridley G, Weiderpass E, et al. Venous thromboembolism and cancer. Lancet 1998;351:1077-1080.
11. Sorenson HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med 1998;338:1169-1173.
12. Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ 1993;306:1030-1034.
13. Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-1850.
14. Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996;334:677-681.
15. Koopman M, Prandoni P, Piovella F, et al. for the Tasman Study Group. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. N Engl J Med 1996;334:682-687.
16. Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:444-451.
17. Ben-Tal O, Zivelin A, Seligsohn U. The relative frequency of hereditary thrombotic disorders among 107 patients with thrombophilia in Israel. Thromb Haemost 1989;61:50-54.
18. Ridker PM, Hennekens CH, Lindpaintner K, et al. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995;332:912-917.
19. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G—>A20210 gene variant. Ann Intern Med 1998;129:89-93.
20. Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh 1965;13:516.-
21. Griffith JH, Evatt B, Zimmerman TS, et al. Deficiency of protein C in congenital thrombotic disease. J Clin Invest 1981;68:1370-1373.
22. Comp PC, Nixon RR, Cooper MR, et al. Familial protein S deficiency is associated with recurrent thrombosis. J Clin Invest 1984;74:2082-2088.
23. Hillarp A, Dahlback B, Zoller B. Activated protein C resistance: from phenotype to genotype and clinical practice. Blood Rev 1995;9:201-212.
24. Zoller B, Berntsdotter A, Garcia de Frutos P, et al. Resistance to activated protein C as an additional genetic risk factor in hereditary deficiency of protein S. Blood 1995;85:3518-3523.
25. Van Boven HH, Reitsma PH, Rosendaal FR, et al. Factor V Leiden (FV R506Q) in families with inherited antithrombin deficiency. Thromb Haemost 1996;75:417-421.
26. Heit JA, Silverstein MD, Mohr DN, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000;160:809-815.
27. Bertina RM, Koeleman BPC, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994;369:64-67.
28. Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997;277:1305-1307.
29. Koster T, Rosendaal FR, de Ronde H, Briet E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden thrombophilia study. Lancet 1993;342:1503-1506.
30. Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995;85:1504-1508.
31. Rosendaal FR, Doggen CJ, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost 1998;79:706-708.
32. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3´ untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996;88:3698-3703.
33. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med 1999;341:801-806.
34. den Heijer M, Koster T, Blom HJ, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med 1996;334:759-762.
35. Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med 1998;158:2101-2106.
36. den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost 1998;80:874-877.
37. Ridker PM, Hennekens CH, Selhub J, Miletich JP, Malinow MR, Stampfer MJ. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation 1997;95:1777-1782.
38. Kluijtmans LA, den Heijer M, Reitsma PH, Heil SG, Blom HJ, Rosendaal FR. Thermolabile methylenetetrahydrofolate reductase and factor V Leiden in the risk of deep-vein thrombosis. Thromb Haemost 1998;79:254-258.
39. DeStefano V, Zappacosta B, Persichilli S, et al. Prevalence of mild hyperhomocysteinaemia and association with thrombophilic genotypes (factor V Leiden and prothrombin G20210A) in Italian patients with venous thromboembolic disease. Br J Haematol 1999;106:564-568.
40. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
41. Simioni P, Prandoni P, Lensing AWA, et al. The risk of recurrent venous thromboembolism in patients with an Arg506—>Gln mutation in the gene for factor V (factor V Leiden). N Engl J Med 1997;336:399-403.
42. Ridker PM, Miletich JP, Stampfer MJ, Goldhaber SZ, Lindpaintner K, Hennekens CH. Factor V Leiden and risks of recurrent idiopathic venous thromboembolism. Circulation 1995;92:2800-2802.
43. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998;316:894-898.
44. McCrae KR. Antiphospholipid antibody associated thrombosis: a consensus for treatment? Lupus 1996;5:560-570.
1. Goldhaber SZ. Pulmonary embolism. N Engl J Med 1998;339:93-104.
2. Deitcher SR. Overview of enoxaparin in the treatment of deep vein thrombosis. Am J Manag Care 2000;6(suppl):S1026-S1033.
3. Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation. 1996;93:2212-2245.
4. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism. Arch Int Med 2000;160:809-815.
5. Trousseau A. Phlegmasia alba dolens. In: Clinique Medicale de l’Hotel-Dieu de Paris. Vol. 3. 2nd ed. Paris: JB Bailliere; 1865;654-712.
6. Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med 2000;160:769-774.
7. Naschitz JE, Yeshurun D, Lev LM. Thromboembolism in cancer. Cancer 1993;71:1384-1390.
8. Prandoni P, Lensing AW, Buller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med 1992;327:1128-1133.
9. Hettiarachchi RJ, Lok J, Prins MH, et al. Undiagnosed malignancy in patients with deep vein thrombosis: incidence, risk indicators, and diagnosis. Cancer 1998;83:180-185.
10. Baron JA, Gridley G, Weiderpass E, et al. Venous thromboembolism and cancer. Lancet 1998;351:1077-1080.
11. Sorenson HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med 1998;338:1169-1173.
12. Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ 1993;306:1030-1034.
13. Sorensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-1850.
14. Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996;334:677-681.
15. Koopman M, Prandoni P, Piovella F, et al. for the Tasman Study Group. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. N Engl J Med 1996;334:682-687.
16. Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:444-451.
17. Ben-Tal O, Zivelin A, Seligsohn U. The relative frequency of hereditary thrombotic disorders among 107 patients with thrombophilia in Israel. Thromb Haemost 1989;61:50-54.
18. Ridker PM, Hennekens CH, Lindpaintner K, et al. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995;332:912-917.
19. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G—>A20210 gene variant. Ann Intern Med 1998;129:89-93.
20. Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh 1965;13:516.-
21. Griffith JH, Evatt B, Zimmerman TS, et al. Deficiency of protein C in congenital thrombotic disease. J Clin Invest 1981;68:1370-1373.
22. Comp PC, Nixon RR, Cooper MR, et al. Familial protein S deficiency is associated with recurrent thrombosis. J Clin Invest 1984;74:2082-2088.
23. Hillarp A, Dahlback B, Zoller B. Activated protein C resistance: from phenotype to genotype and clinical practice. Blood Rev 1995;9:201-212.
24. Zoller B, Berntsdotter A, Garcia de Frutos P, et al. Resistance to activated protein C as an additional genetic risk factor in hereditary deficiency of protein S. Blood 1995;85:3518-3523.
25. Van Boven HH, Reitsma PH, Rosendaal FR, et al. Factor V Leiden (FV R506Q) in families with inherited antithrombin deficiency. Thromb Haemost 1996;75:417-421.
26. Heit JA, Silverstein MD, Mohr DN, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000;160:809-815.
27. Bertina RM, Koeleman BPC, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994;369:64-67.
28. Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997;277:1305-1307.
29. Koster T, Rosendaal FR, de Ronde H, Briet E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden thrombophilia study. Lancet 1993;342:1503-1506.
30. Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995;85:1504-1508.
31. Rosendaal FR, Doggen CJ, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost 1998;79:706-708.
32. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3´ untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996;88:3698-3703.
33. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med 1999;341:801-806.
34. den Heijer M, Koster T, Blom HJ, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med 1996;334:759-762.
35. Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med 1998;158:2101-2106.
36. den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost 1998;80:874-877.
37. Ridker PM, Hennekens CH, Selhub J, Miletich JP, Malinow MR, Stampfer MJ. Interrelation of hyperhomocyst(e)inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation 1997;95:1777-1782.
38. Kluijtmans LA, den Heijer M, Reitsma PH, Heil SG, Blom HJ, Rosendaal FR. Thermolabile methylenetetrahydrofolate reductase and factor V Leiden in the risk of deep-vein thrombosis. Thromb Haemost 1998;79:254-258.
39. DeStefano V, Zappacosta B, Persichilli S, et al. Prevalence of mild hyperhomocysteinaemia and association with thrombophilic genotypes (factor V Leiden and prothrombin G20210A) in Italian patients with venous thromboembolic disease. Br J Haematol 1999;106:564-568.
40. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
41. Simioni P, Prandoni P, Lensing AWA, et al. The risk of recurrent venous thromboembolism in patients with an Arg506—>Gln mutation in the gene for factor V (factor V Leiden). N Engl J Med 1997;336:399-403.
42. Ridker PM, Miletich JP, Stampfer MJ, Goldhaber SZ, Lindpaintner K, Hennekens CH. Factor V Leiden and risks of recurrent idiopathic venous thromboembolism. Circulation 1995;92:2800-2802.
43. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998;316:894-898.
44. McCrae KR. Antiphospholipid antibody associated thrombosis: a consensus for treatment? Lupus 1996;5:560-570.
Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: A meta-analysis
- Reliable data are scarce regarding the accuracy of physical diagnostic tests in diagnosing anterior cruciate ligament ruptures in primary care.
- The pivot shift test has a favorable positive predictive value, and the Lachman test has a good negative predictive value. The anterior drawer test is of unproven benefit in diagnosing rupture of the anterior cruciate ligament (ACL).
- Although of limited predictive value, the history and physical examination, coupled with patient preference and physical demands, should form the basis for further investigation of possible ACL rupture.
Objective: This systematic review summarizes the evidence on the accuracy of tests for assessing ACL ruptures of the knee.
Search strategy A computerized search of MEDLINE (1966–2003) and EMBASE (1980–2003) with additional reference tracking.
Selection criteria Articles included were written in English, French, German, or Dutch, and addressed the accuracy of at least 1 physical diagnostic test for ACL rupture, using arthrotomy, arthroscopy, or magnetic resonance imaging as the gold standard.
Data collection and analysis: Two reviewers independently selected studies, assessed the methodological quality, and abstracted data using a standardized protocol. We calculated sensitivity, specificity, and likelihood ratios for each test and summary estimates, when appropriate and possible.
Main results: Seventeen studies met the inclusion criteria. None assessed the index test and reference test independently (with blinding), and all but 2 displayed verification bias. Study results were heterogeneous. The pivot shift test seems to have favorable positive predictive value, and the Lachman test has good negative predictive value. The anterior drawer test is of unproven value.
Conclusions: Reliable data are rare regarding the accuracy of physical diagnostic tests for ACL ruptures, especially in a primary care setting. For the time being, history taking and physical examination, albeit of limited use, should be considered with individual patient demands to provide the basis for further evaluation.
To evaluate possible rupture of the anterior cruciate ligament (ACL), family physicians rely on the history and physical examination and primarily 3 diagnostic assessments: the anterior drawer test, the Lachman test, and the pivot shift test.1-3 Preliminary findings from these tests, coupled with patient preference and physical demands, help select those who may need further work-up with arthroscopy or magnetic resonance imaging (MRI).4
We summarize the evidence for the diagnostic accuracy of physical diagnostic tests in assessing ACL ruptures of the knee.
If a patient’s physical demands are low, one might proceed with a trial of conservative therapy (especially when Lachman’s test is negative), which has shown to be favorable for selected patients.5 However, when a patient has high demands (as is the case with athletes), more advanced diagnostic tests (eg, MRI) seem to be indicated, irrespective of the findings of physical examination.
Methods
Selection of studies
A computerized literature search of MEDLINE (from 1966 to February 14, 2003) and EMBASE (1980 to February 14, 2003) was conducted to identify articles written in English, French, German, or Dutch. Key words were the medical subject headings “knee injuries,”knee joint,” and “knee,” and the text word “knee.” This set was combined with a set consisting of the main headings “joint instability” and “anterior cruciate ligament,” and the text words “laxity,” “instability,” “cruciate,” and “effusion.”
Finally, the results of these strategies were combined with a validated search strategy for the identification of diagnostic studies using the subject headings “sensitivity and specificity” (exploded), “physical examination” and “not (animal not [human and animal])” and the text words “sensitivit$,” “specificit$,” “false positive,” “false negative,” “accuracy,” and “screening,”6 supplemented with the text words “physical examination” and “clinical examination.” Also, the cited references of included publications were examined.
Studies were selected by 2 reviewers independently. Studies were eligible for inclusion if they addressed the accuracy of at least 1 physical diagnostic test for the assessment of ACL ruptures of the knee, and used arthrotomy, arthroscopy, or MRI as the gold standard.
Assessment of methodological quality and data abstraction
The methodological quality of the selected studies was assessed and data were abstracted by 2 reviewers independently. Quality assessment was accomplished with a checklist adapted from Irwig and colleagues7 and the Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests.8 (Table W1 and Table W1 cont.).
Statistical analysis
Statistical analysis was performed9 with a strategy adapted from Midgette and colleagues.10 The method consists of estimating a summary receiver operating characteristic (SROC) curve by metaregression, and exploring heterogeneity by adding study characteristics and study validity items to the regression model (a full description of this strategy is available online as Appendix A).7-11
We performed an additional analysis according to a bivariate random effects model that accounts for heterogeneity of both sensitivity and specificity simultaneously, reflected in the width of the 95% confidence intervals.12,13
The summary estimates of sensitivity and specificity were used to calculate the predictive value of a positive (PV+) and negative test result (PV–) for circumstances with varying prevalences of ACL ruptures. When summary estimates of both sensitivity and specificity could not be calculated, the summary estimate of sensitivity and the accompanying specificity, estimated from the SROC curve, were used to calculate predictive values.
Results
Selection of studies
The literature search revealed a total of 1090 potentially eligible studies, 17 of which were selected.14-30 Two reports pertained to the same study,15,16 and 1 additional study was found by reference tracking.2 Thus, a total of 17 studies met the selection criteria.
Methodological quality and study characteristics
No study measured the index test (ie, the object of study) and reference standard independently (with blinding). Patients whose physical test results were abnormal were more likely to undergo the gold standard test—a factor that inflates sensitivity and decreases specificity. This verification bias was present in all but 2 studies.15,27 No study was performed in a primary care setting.
A detailed description of the characteristics and methodological quality of the 17 included studies is available online (Appendix B, Table W2-1, Table W2-2, Table W2-3, and Table W3).
Accuracy of ACL tests
Details of the process of selecting studies for further meta-analysis are presented online (Appendix C).
Diagnostic accuracy of the ACL tests is shown in Table 1. Significant heterogeneity of sensitivity and specificity was seen with all ACL tests, and no significant subgroups were detected for any of the tests. The power of metaregression analysis, however, was low due to the small number of available studies (4 to 6) and because some characteristics exhibited no variation.
Anterior drawer test. Correlation of sensitivity and specificity for the anterior drawer test was positive (6 studies); thus, no SROC curve was estimated. Sensitivity of the anterior drawer test was 0.18–0.92, and specificity 0.78–0.98. According to the bivariate random effects model, the pooled sensitivity was 0.62 (95% confidence interval [CI], 0.42–0.78) and the pooled specificity was 0.88 (95% CI, 0.83–0.92) ( Figure 1A ).
TABLE 1
Diagnostic accuracy of the anterior drawer sign, Lachman test, and pivot shift test
| First author | Type of ACL rupture | N | Prevalence | Sn | Sp | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Anterior drawer sign | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.18 | — | — | — |
| Tonino19*† | Partial + complete | 52 | 0.58 | 0.27 | 0.98 | 12.6 | 0.7 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.76)§ | (0.86)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.56 | 0.92 | 6.7 | 0.5 |
| Lee21* | Not specified | 79 | 0.29 | 0.77 | 0.99 | 87.9 | 0.2 |
| Richter29* | Not specified | 74 | 0.78 | 0.67 | 0.88 | 5.4 | 0.4 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.92 | 0.91 | 10.4 | 0.1 |
| Sandberg18*‡ | Not specified | 182 | 0.68 | 0.39 | 0.78 | 1.7 | 0.8 |
| Lachman test | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.74 | — | — | — |
| Tonino19* | Partial + complete | 52 | 0.58 | 0.89 | 0.98 | 40.8 | 0.1 |
| Schwarz30*‡ | Partial + complete | 58 | 0.81 | 0.91 | 0.55 | 2.0 | 0.2 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.96)§ | (1.00)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.63 | 0.90 | 6.5 | 0.4 |
| Lee21* | Not specified | 79 | 0.29 | 0.90 | 0.99 | 102.1 | 0.1 |
| Richter29*‡ | Not specified | 74 | 0.78 | 0.93 | 0.88 | 7.4 | 0.1 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.86 | 0.95 | 17.9 | 0.1 |
| Cooperman24 | Not specified | 32 | 0.41 | (0.65)║ | (0.42)║ | — | — |
| Pivot shift test | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.29 | — | — | — |
| Tonino19* | Partial + complete | 52 | 0.58 | 0.18 | 0.98 | 8.2 | 0.8 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.93)§ | (0.89)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.31 | 0.97 | 8.8 | 0.7 |
| Richter29*†‡ | Not specified | 74 | 0.78 | 0.48 | 0.97 | 16.4 | 0.5 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.22 | 0.99 | 26.9 | 0.8 |
| * Study results used for meta-analysis | |||||||
| † 0.5 added to each cell of the 2x2 table | |||||||
| ‡ 2x2 table reconstructed | |||||||
| § Mean result of 5 orthopedic surgeons | |||||||
| ║ Sum of results of 2 physiotherapists | |||||||
| ACL, anterior cruciate ligament; Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio | |||||||
FIGURE 1
Sensitivity vs 1–specificity of the 3 tests
Scatterplots of sensitivity versus 1–specificity of A) the anterior drawer test (6 studies), B) the Lachman test (6 studies), and C) the pivot shift test (4 studies). Summary receiver operating characteristic curves and summary estimates of sensitivity and specificity (including 95% confidence intervals) are shown as appropriate.
Lachman test (Figure 2). The SROC curve of the Lachman test (6 studies) is shown in Figure 1B . Sensitivity ranged from 0.63 to 0.93, and specificity from 0.55 to 0.99. According to the bivariate random effects model the pooled sensitivity was 0.86 (95% CI, 0.76–0.92) and the pooled specificity was 0.91 (95% CI, 0.79–0.96).
Pivot shift test. The SROC curve of the pivot shift test (4 studies) is shown in Figure 1C . Sensitivity ranged from 0.18 to 0.48, and specificity from 0.97 to 0.99. Bivariate random effects pooling could not be performed; in this model 5 parameters must be estimated and only 4 studies were available.
Figure 3 shows the PV+ and PV– for all tests according to varying prevalences of ACL ruptures. The pivot shift test has the highest PV+ and the Lachman test the highest PV–. If the pivot shift test is positive, there is high probability of an ACL rupture, whereas a negative Lachman test rules out a rupture.
FIGURE 2
Lachman test
To perform the Lachman test, grasp the back of the proximal tibia posteriorly and place thumb over joint line anterolaterally. Pull the proximal tibia anteriorly and posteriorly, and compare sides for endpoint laxity.
FIGURE 3
Predictive value vs prevalence of positive and negative test results
Predictive value (posttest probability of presence of ACL rupture) vs prevalence (prior probability of presence of ACL rupture) of positive and negative test results of the anterior drawer test (sensitivity=0.62, specificity=0.88), Lachman test (sensitivity=0.86, specificity=0.91), and the pivot shift test (sensitivity=0.32, specificity=0.98).
DISCUSSION
We reviewed 17 studies that examined the accuracy of physical diagnostic tests for assessing ACL ruptures of the knee. Of those tests, the pivot shift test seems to have favorable positive predictive value, and the Lachman test good negative predictive value. The anterior drawer test is of unproven diagnostic value in this setting. In view of the potential biases in the original studies, however, the accuracy of the various ACL tests might be overestimated and the poor quality of the studies impede sound conclusions about the usefulness of the tests for daily practice. In addition, no study has been performed in primary care.
Because test characteristics may be influenced substantially by referral filters leading to spectrum bias,31 and because primary care physicians will be less experienced in performing these tests, the tests will presumably be less accurate in a primary care setting. Furthermore, the pivot shift test is very difficult to perform, making it less attractive for the average primary care physician.
Future research
Useful answers would be derived from sound research on the diagnostic accuracy of the various tests (determined for each test separately and for all tests jointly) combined with patient characteristics (eg, age, physical fitness, and functional demands) and elements of the medical history (eg, type of trauma and nature of the complaints). The emergence of MRI will facilitate this research. Relevance to clinical practice would be enhanced by an assessment of the effect of a correct diagnosis on the functional outcome of patients.
Acknowledgments
The authors thank Afina Glas, MD, and Professor Koos Zwinderman, PhD, for their statistical advice. We are much obliged to 1 of the referees for his/her useful suggestions.
Corresponding author
Rob J.P.M. Scholten, MD, PhD, Dutch Cochrane Centre, Department of Clinical Epidemiology and Biostatistics, J2-273, Academic Medical Center, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands. E-mail: [email protected].
1. Slocum DB, Larson RL. Rotatory instability of the knee. Its pathogenesis and a clinical test to demonstrate its presence. J Bone Joint Surg Am 1968;50:211-225.
2. Torg JS, Conrad W, Kalen V. Clinical diagnosis of anterior cruciate ligament instability in the athlete. Am J Sports Med 1976;4:84-93.
3. Galway HR, MacIntosh DL. The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop 1980;147:45-50.
4. Boeve BF, Davidson RA, Staab EV. Magnetic resonance imaging in the evaluation of knee injuries. South Med J 1991;84:1123-1127.
5. Buss DD, Min R, Skyhar M, Galinat B, Warren RF, Wickiewicz TL. Nonoperative treatment of acute anterior cruciate ligament injuries in a selected group of patients. Am J Sports Med 1995;23:160-165.
6. Devillè WLJM, Bezemer PD, Bouter LM. Publications on diagnostic test evaluation in family medicine journals: an optimal search strategy. J Clin Epidemiol 2000;53:65-69.
7. Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 1995;48:119-130.
8. Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests: Recommended, Methods, updated 6 June 1996. Available at http://www.cochrane.de/cochrane/sadtdoc1.htm.
9. Scholten RJPM, Devillè WLJM, Opstelten W, Bijl D, van der Plas CG, Bouter LM. The accuracy of physical diagnostic tests for assessing meniscal lesions of the knee: a meta-analysis. J Fam Pract 2001;50:938-944.
10. Midgette AS, Stukel TA, Littenberg B. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med Decis Making 1993;13:253-257.
11. Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-1316.
12. van Houwelingen JC, Zwinderman K, Stijnen T. A bivariate approach to meta-analysis. Stat Med. 1993;12:2272-2284.
13. van Houwelingen JC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589-624.
14. Warren RF, Marshall JL. Injuries of the anterior cruciate and medial collateral ligaments of the knee. A retrospective analysis of clinical records—part I. Clin Orthop 1978;136:191-197.
15. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg 1980;62A:687-695.
16. Noyes FR, Paulos L, Mooar LA, Signer B. Knee sprains and acute knee hemarthrosis: misdiagnosis of anterior cruciate ligament tears. Phys Ther 1980;60:1596-1601.
17. Braunstein EM. Anterior cruciate ligament injuries: a comparison of arthrographic and physical diagnosis. AJR Am J Roentgenol 1982;138:423-425.
18. Sandberg R, Balkfors B, Henricson A, Westlin N. Stability tests in knee ligament injuries. Arch Orthop Trauma Surg 1986;106:5-7.
19. Tonino AJ, Huy J, Schaafsma J. The diagnostic accuracy of knee testing in the acutely injured knee. Initial examination versus examination under anaesthesia with arthroscopy. Acta Orthop Belg 1986;52:479-487.
20. Harilainen A. Evaluation of knee instability in acute liga-mentous injuries. Ann Chir Gynaecol 1987;76:269-273.
21. Lee JK, Yao L, Phelps CT, Wirth CR, Czajka J, Lozman J. Anterior cruciate ligament tears: MR imaging compared with arthroscopy and clinical tests. Radiology 1988;166:861-864.
22. SteinbrÜck K, Wiehmann JC. Examination of the knee joint. The value of clinical findings in arthroscopic control [in German]. Z Orthop Ihre Grenzgeb 1988;126:289-295.
23. Anderson AF, Lipscomb AB. Preoperative instrumented testing of anterior and posterior knee laxity. Am J Sports Med 1989;17:387-392.
24. Cooperman JM, Riddle DL, Rothstein JM. Reliability and validity of judgments of the integrity of the anterior cruci-ate ligament of the knee using the Lachman’s test. Phys Ther 1990;70:225-233.
25. Hardaker WT Jr, Garrett WE Jr, Bassett FH 3d. Evaluation of acute traumatic hemarthrosis of the knee joint. South Med J 1990;83:640-644.
26. Boeree NR, Ackroyd CE. Assessment of the menisci and cruciate ligaments: an audit of clinical practice. Injury 1991;22:291-294.
27. al-Duri Z. Relation of the fibular head sign to other signs of anterior cruciate ligament insufficiency. A follow-up letter to the editor. Clin Orthop 1992;275:220-225.
28. Rubinstein RA Jr, Shelbourne KD, McCarroll JR, VanMeter CD, Rettig AC. The accuracy of the clinical examination in the setting of posterior cruciate ligament injuries. Am J Sports Med 1994;22:550-557.
29. Richter J, David A, Pape HG, Ostermann PA, Muhr G. Diagnosis of acute rupture of the anterior cruciate liga-ment. Value of ultrasonic in addition to clinical examination [in German]. Unfallchirurg 1996;99:124-129.
30. Schwarz W, Hagelstein J, Minholz R, Schierlinger M, Danz B, Gerngross H. Manual ultrasound of the knee joint. A general practice method for diagnosis of fresh rupture of the anterior cruciate ligament [in German]. Unfallchirurg 1997;100:280-285.
31. Knottnerus JA, Leffers P. The influence of referral patterns on the characteristics of diagnostic tests. J Clin Epidemiol 1992;45:1143-1154.
- Reliable data are scarce regarding the accuracy of physical diagnostic tests in diagnosing anterior cruciate ligament ruptures in primary care.
- The pivot shift test has a favorable positive predictive value, and the Lachman test has a good negative predictive value. The anterior drawer test is of unproven benefit in diagnosing rupture of the anterior cruciate ligament (ACL).
- Although of limited predictive value, the history and physical examination, coupled with patient preference and physical demands, should form the basis for further investigation of possible ACL rupture.
Objective: This systematic review summarizes the evidence on the accuracy of tests for assessing ACL ruptures of the knee.
Search strategy A computerized search of MEDLINE (1966–2003) and EMBASE (1980–2003) with additional reference tracking.
Selection criteria Articles included were written in English, French, German, or Dutch, and addressed the accuracy of at least 1 physical diagnostic test for ACL rupture, using arthrotomy, arthroscopy, or magnetic resonance imaging as the gold standard.
Data collection and analysis: Two reviewers independently selected studies, assessed the methodological quality, and abstracted data using a standardized protocol. We calculated sensitivity, specificity, and likelihood ratios for each test and summary estimates, when appropriate and possible.
Main results: Seventeen studies met the inclusion criteria. None assessed the index test and reference test independently (with blinding), and all but 2 displayed verification bias. Study results were heterogeneous. The pivot shift test seems to have favorable positive predictive value, and the Lachman test has good negative predictive value. The anterior drawer test is of unproven value.
Conclusions: Reliable data are rare regarding the accuracy of physical diagnostic tests for ACL ruptures, especially in a primary care setting. For the time being, history taking and physical examination, albeit of limited use, should be considered with individual patient demands to provide the basis for further evaluation.
To evaluate possible rupture of the anterior cruciate ligament (ACL), family physicians rely on the history and physical examination and primarily 3 diagnostic assessments: the anterior drawer test, the Lachman test, and the pivot shift test.1-3 Preliminary findings from these tests, coupled with patient preference and physical demands, help select those who may need further work-up with arthroscopy or magnetic resonance imaging (MRI).4
We summarize the evidence for the diagnostic accuracy of physical diagnostic tests in assessing ACL ruptures of the knee.
If a patient’s physical demands are low, one might proceed with a trial of conservative therapy (especially when Lachman’s test is negative), which has shown to be favorable for selected patients.5 However, when a patient has high demands (as is the case with athletes), more advanced diagnostic tests (eg, MRI) seem to be indicated, irrespective of the findings of physical examination.
Methods
Selection of studies
A computerized literature search of MEDLINE (from 1966 to February 14, 2003) and EMBASE (1980 to February 14, 2003) was conducted to identify articles written in English, French, German, or Dutch. Key words were the medical subject headings “knee injuries,”knee joint,” and “knee,” and the text word “knee.” This set was combined with a set consisting of the main headings “joint instability” and “anterior cruciate ligament,” and the text words “laxity,” “instability,” “cruciate,” and “effusion.”
Finally, the results of these strategies were combined with a validated search strategy for the identification of diagnostic studies using the subject headings “sensitivity and specificity” (exploded), “physical examination” and “not (animal not [human and animal])” and the text words “sensitivit$,” “specificit$,” “false positive,” “false negative,” “accuracy,” and “screening,”6 supplemented with the text words “physical examination” and “clinical examination.” Also, the cited references of included publications were examined.
Studies were selected by 2 reviewers independently. Studies were eligible for inclusion if they addressed the accuracy of at least 1 physical diagnostic test for the assessment of ACL ruptures of the knee, and used arthrotomy, arthroscopy, or MRI as the gold standard.
Assessment of methodological quality and data abstraction
The methodological quality of the selected studies was assessed and data were abstracted by 2 reviewers independently. Quality assessment was accomplished with a checklist adapted from Irwig and colleagues7 and the Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests.8 (Table W1 and Table W1 cont.).
Statistical analysis
Statistical analysis was performed9 with a strategy adapted from Midgette and colleagues.10 The method consists of estimating a summary receiver operating characteristic (SROC) curve by metaregression, and exploring heterogeneity by adding study characteristics and study validity items to the regression model (a full description of this strategy is available online as Appendix A).7-11
We performed an additional analysis according to a bivariate random effects model that accounts for heterogeneity of both sensitivity and specificity simultaneously, reflected in the width of the 95% confidence intervals.12,13
The summary estimates of sensitivity and specificity were used to calculate the predictive value of a positive (PV+) and negative test result (PV–) for circumstances with varying prevalences of ACL ruptures. When summary estimates of both sensitivity and specificity could not be calculated, the summary estimate of sensitivity and the accompanying specificity, estimated from the SROC curve, were used to calculate predictive values.
Results
Selection of studies
The literature search revealed a total of 1090 potentially eligible studies, 17 of which were selected.14-30 Two reports pertained to the same study,15,16 and 1 additional study was found by reference tracking.2 Thus, a total of 17 studies met the selection criteria.
Methodological quality and study characteristics
No study measured the index test (ie, the object of study) and reference standard independently (with blinding). Patients whose physical test results were abnormal were more likely to undergo the gold standard test—a factor that inflates sensitivity and decreases specificity. This verification bias was present in all but 2 studies.15,27 No study was performed in a primary care setting.
A detailed description of the characteristics and methodological quality of the 17 included studies is available online (Appendix B, Table W2-1, Table W2-2, Table W2-3, and Table W3).
Accuracy of ACL tests
Details of the process of selecting studies for further meta-analysis are presented online (Appendix C).
Diagnostic accuracy of the ACL tests is shown in Table 1. Significant heterogeneity of sensitivity and specificity was seen with all ACL tests, and no significant subgroups were detected for any of the tests. The power of metaregression analysis, however, was low due to the small number of available studies (4 to 6) and because some characteristics exhibited no variation.
Anterior drawer test. Correlation of sensitivity and specificity for the anterior drawer test was positive (6 studies); thus, no SROC curve was estimated. Sensitivity of the anterior drawer test was 0.18–0.92, and specificity 0.78–0.98. According to the bivariate random effects model, the pooled sensitivity was 0.62 (95% confidence interval [CI], 0.42–0.78) and the pooled specificity was 0.88 (95% CI, 0.83–0.92) ( Figure 1A ).
TABLE 1
Diagnostic accuracy of the anterior drawer sign, Lachman test, and pivot shift test
| First author | Type of ACL rupture | N | Prevalence | Sn | Sp | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Anterior drawer sign | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.18 | — | — | — |
| Tonino19*† | Partial + complete | 52 | 0.58 | 0.27 | 0.98 | 12.6 | 0.7 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.76)§ | (0.86)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.56 | 0.92 | 6.7 | 0.5 |
| Lee21* | Not specified | 79 | 0.29 | 0.77 | 0.99 | 87.9 | 0.2 |
| Richter29* | Not specified | 74 | 0.78 | 0.67 | 0.88 | 5.4 | 0.4 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.92 | 0.91 | 10.4 | 0.1 |
| Sandberg18*‡ | Not specified | 182 | 0.68 | 0.39 | 0.78 | 1.7 | 0.8 |
| Lachman test | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.74 | — | — | — |
| Tonino19* | Partial + complete | 52 | 0.58 | 0.89 | 0.98 | 40.8 | 0.1 |
| Schwarz30*‡ | Partial + complete | 58 | 0.81 | 0.91 | 0.55 | 2.0 | 0.2 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.96)§ | (1.00)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.63 | 0.90 | 6.5 | 0.4 |
| Lee21* | Not specified | 79 | 0.29 | 0.90 | 0.99 | 102.1 | 0.1 |
| Richter29*‡ | Not specified | 74 | 0.78 | 0.93 | 0.88 | 7.4 | 0.1 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.86 | 0.95 | 17.9 | 0.1 |
| Cooperman24 | Not specified | 32 | 0.41 | (0.65)║ | (0.42)║ | — | — |
| Pivot shift test | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.29 | — | — | — |
| Tonino19* | Partial + complete | 52 | 0.58 | 0.18 | 0.98 | 8.2 | 0.8 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.93)§ | (0.89)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.31 | 0.97 | 8.8 | 0.7 |
| Richter29*†‡ | Not specified | 74 | 0.78 | 0.48 | 0.97 | 16.4 | 0.5 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.22 | 0.99 | 26.9 | 0.8 |
| * Study results used for meta-analysis | |||||||
| † 0.5 added to each cell of the 2x2 table | |||||||
| ‡ 2x2 table reconstructed | |||||||
| § Mean result of 5 orthopedic surgeons | |||||||
| ║ Sum of results of 2 physiotherapists | |||||||
| ACL, anterior cruciate ligament; Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio | |||||||
FIGURE 1
Sensitivity vs 1–specificity of the 3 tests
Scatterplots of sensitivity versus 1–specificity of A) the anterior drawer test (6 studies), B) the Lachman test (6 studies), and C) the pivot shift test (4 studies). Summary receiver operating characteristic curves and summary estimates of sensitivity and specificity (including 95% confidence intervals) are shown as appropriate.
Lachman test (Figure 2). The SROC curve of the Lachman test (6 studies) is shown in Figure 1B . Sensitivity ranged from 0.63 to 0.93, and specificity from 0.55 to 0.99. According to the bivariate random effects model the pooled sensitivity was 0.86 (95% CI, 0.76–0.92) and the pooled specificity was 0.91 (95% CI, 0.79–0.96).
Pivot shift test. The SROC curve of the pivot shift test (4 studies) is shown in Figure 1C . Sensitivity ranged from 0.18 to 0.48, and specificity from 0.97 to 0.99. Bivariate random effects pooling could not be performed; in this model 5 parameters must be estimated and only 4 studies were available.
Figure 3 shows the PV+ and PV– for all tests according to varying prevalences of ACL ruptures. The pivot shift test has the highest PV+ and the Lachman test the highest PV–. If the pivot shift test is positive, there is high probability of an ACL rupture, whereas a negative Lachman test rules out a rupture.
FIGURE 2
Lachman test
To perform the Lachman test, grasp the back of the proximal tibia posteriorly and place thumb over joint line anterolaterally. Pull the proximal tibia anteriorly and posteriorly, and compare sides for endpoint laxity.
FIGURE 3
Predictive value vs prevalence of positive and negative test results
Predictive value (posttest probability of presence of ACL rupture) vs prevalence (prior probability of presence of ACL rupture) of positive and negative test results of the anterior drawer test (sensitivity=0.62, specificity=0.88), Lachman test (sensitivity=0.86, specificity=0.91), and the pivot shift test (sensitivity=0.32, specificity=0.98).
DISCUSSION
We reviewed 17 studies that examined the accuracy of physical diagnostic tests for assessing ACL ruptures of the knee. Of those tests, the pivot shift test seems to have favorable positive predictive value, and the Lachman test good negative predictive value. The anterior drawer test is of unproven diagnostic value in this setting. In view of the potential biases in the original studies, however, the accuracy of the various ACL tests might be overestimated and the poor quality of the studies impede sound conclusions about the usefulness of the tests for daily practice. In addition, no study has been performed in primary care.
Because test characteristics may be influenced substantially by referral filters leading to spectrum bias,31 and because primary care physicians will be less experienced in performing these tests, the tests will presumably be less accurate in a primary care setting. Furthermore, the pivot shift test is very difficult to perform, making it less attractive for the average primary care physician.
Future research
Useful answers would be derived from sound research on the diagnostic accuracy of the various tests (determined for each test separately and for all tests jointly) combined with patient characteristics (eg, age, physical fitness, and functional demands) and elements of the medical history (eg, type of trauma and nature of the complaints). The emergence of MRI will facilitate this research. Relevance to clinical practice would be enhanced by an assessment of the effect of a correct diagnosis on the functional outcome of patients.
Acknowledgments
The authors thank Afina Glas, MD, and Professor Koos Zwinderman, PhD, for their statistical advice. We are much obliged to 1 of the referees for his/her useful suggestions.
Corresponding author
Rob J.P.M. Scholten, MD, PhD, Dutch Cochrane Centre, Department of Clinical Epidemiology and Biostatistics, J2-273, Academic Medical Center, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands. E-mail: [email protected].
- Reliable data are scarce regarding the accuracy of physical diagnostic tests in diagnosing anterior cruciate ligament ruptures in primary care.
- The pivot shift test has a favorable positive predictive value, and the Lachman test has a good negative predictive value. The anterior drawer test is of unproven benefit in diagnosing rupture of the anterior cruciate ligament (ACL).
- Although of limited predictive value, the history and physical examination, coupled with patient preference and physical demands, should form the basis for further investigation of possible ACL rupture.
Objective: This systematic review summarizes the evidence on the accuracy of tests for assessing ACL ruptures of the knee.
Search strategy A computerized search of MEDLINE (1966–2003) and EMBASE (1980–2003) with additional reference tracking.
Selection criteria Articles included were written in English, French, German, or Dutch, and addressed the accuracy of at least 1 physical diagnostic test for ACL rupture, using arthrotomy, arthroscopy, or magnetic resonance imaging as the gold standard.
Data collection and analysis: Two reviewers independently selected studies, assessed the methodological quality, and abstracted data using a standardized protocol. We calculated sensitivity, specificity, and likelihood ratios for each test and summary estimates, when appropriate and possible.
Main results: Seventeen studies met the inclusion criteria. None assessed the index test and reference test independently (with blinding), and all but 2 displayed verification bias. Study results were heterogeneous. The pivot shift test seems to have favorable positive predictive value, and the Lachman test has good negative predictive value. The anterior drawer test is of unproven value.
Conclusions: Reliable data are rare regarding the accuracy of physical diagnostic tests for ACL ruptures, especially in a primary care setting. For the time being, history taking and physical examination, albeit of limited use, should be considered with individual patient demands to provide the basis for further evaluation.
To evaluate possible rupture of the anterior cruciate ligament (ACL), family physicians rely on the history and physical examination and primarily 3 diagnostic assessments: the anterior drawer test, the Lachman test, and the pivot shift test.1-3 Preliminary findings from these tests, coupled with patient preference and physical demands, help select those who may need further work-up with arthroscopy or magnetic resonance imaging (MRI).4
We summarize the evidence for the diagnostic accuracy of physical diagnostic tests in assessing ACL ruptures of the knee.
If a patient’s physical demands are low, one might proceed with a trial of conservative therapy (especially when Lachman’s test is negative), which has shown to be favorable for selected patients.5 However, when a patient has high demands (as is the case with athletes), more advanced diagnostic tests (eg, MRI) seem to be indicated, irrespective of the findings of physical examination.
Methods
Selection of studies
A computerized literature search of MEDLINE (from 1966 to February 14, 2003) and EMBASE (1980 to February 14, 2003) was conducted to identify articles written in English, French, German, or Dutch. Key words were the medical subject headings “knee injuries,”knee joint,” and “knee,” and the text word “knee.” This set was combined with a set consisting of the main headings “joint instability” and “anterior cruciate ligament,” and the text words “laxity,” “instability,” “cruciate,” and “effusion.”
Finally, the results of these strategies were combined with a validated search strategy for the identification of diagnostic studies using the subject headings “sensitivity and specificity” (exploded), “physical examination” and “not (animal not [human and animal])” and the text words “sensitivit$,” “specificit$,” “false positive,” “false negative,” “accuracy,” and “screening,”6 supplemented with the text words “physical examination” and “clinical examination.” Also, the cited references of included publications were examined.
Studies were selected by 2 reviewers independently. Studies were eligible for inclusion if they addressed the accuracy of at least 1 physical diagnostic test for the assessment of ACL ruptures of the knee, and used arthrotomy, arthroscopy, or MRI as the gold standard.
Assessment of methodological quality and data abstraction
The methodological quality of the selected studies was assessed and data were abstracted by 2 reviewers independently. Quality assessment was accomplished with a checklist adapted from Irwig and colleagues7 and the Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests.8 (Table W1 and Table W1 cont.).
Statistical analysis
Statistical analysis was performed9 with a strategy adapted from Midgette and colleagues.10 The method consists of estimating a summary receiver operating characteristic (SROC) curve by metaregression, and exploring heterogeneity by adding study characteristics and study validity items to the regression model (a full description of this strategy is available online as Appendix A).7-11
We performed an additional analysis according to a bivariate random effects model that accounts for heterogeneity of both sensitivity and specificity simultaneously, reflected in the width of the 95% confidence intervals.12,13
The summary estimates of sensitivity and specificity were used to calculate the predictive value of a positive (PV+) and negative test result (PV–) for circumstances with varying prevalences of ACL ruptures. When summary estimates of both sensitivity and specificity could not be calculated, the summary estimate of sensitivity and the accompanying specificity, estimated from the SROC curve, were used to calculate predictive values.
Results
Selection of studies
The literature search revealed a total of 1090 potentially eligible studies, 17 of which were selected.14-30 Two reports pertained to the same study,15,16 and 1 additional study was found by reference tracking.2 Thus, a total of 17 studies met the selection criteria.
Methodological quality and study characteristics
No study measured the index test (ie, the object of study) and reference standard independently (with blinding). Patients whose physical test results were abnormal were more likely to undergo the gold standard test—a factor that inflates sensitivity and decreases specificity. This verification bias was present in all but 2 studies.15,27 No study was performed in a primary care setting.
A detailed description of the characteristics and methodological quality of the 17 included studies is available online (Appendix B, Table W2-1, Table W2-2, Table W2-3, and Table W3).
Accuracy of ACL tests
Details of the process of selecting studies for further meta-analysis are presented online (Appendix C).
Diagnostic accuracy of the ACL tests is shown in Table 1. Significant heterogeneity of sensitivity and specificity was seen with all ACL tests, and no significant subgroups were detected for any of the tests. The power of metaregression analysis, however, was low due to the small number of available studies (4 to 6) and because some characteristics exhibited no variation.
Anterior drawer test. Correlation of sensitivity and specificity for the anterior drawer test was positive (6 studies); thus, no SROC curve was estimated. Sensitivity of the anterior drawer test was 0.18–0.92, and specificity 0.78–0.98. According to the bivariate random effects model, the pooled sensitivity was 0.62 (95% confidence interval [CI], 0.42–0.78) and the pooled specificity was 0.88 (95% CI, 0.83–0.92) ( Figure 1A ).
TABLE 1
Diagnostic accuracy of the anterior drawer sign, Lachman test, and pivot shift test
| First author | Type of ACL rupture | N | Prevalence | Sn | Sp | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Anterior drawer sign | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.18 | — | — | — |
| Tonino19*† | Partial + complete | 52 | 0.58 | 0.27 | 0.98 | 12.6 | 0.7 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.76)§ | (0.86)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.56 | 0.92 | 6.7 | 0.5 |
| Lee21* | Not specified | 79 | 0.29 | 0.77 | 0.99 | 87.9 | 0.2 |
| Richter29* | Not specified | 74 | 0.78 | 0.67 | 0.88 | 5.4 | 0.4 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.92 | 0.91 | 10.4 | 0.1 |
| Sandberg18*‡ | Not specified | 182 | 0.68 | 0.39 | 0.78 | 1.7 | 0.8 |
| Lachman test | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.74 | — | — | — |
| Tonino19* | Partial + complete | 52 | 0.58 | 0.89 | 0.98 | 40.8 | 0.1 |
| Schwarz30*‡ | Partial + complete | 58 | 0.81 | 0.91 | 0.55 | 2.0 | 0.2 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.96)§ | (1.00)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.63 | 0.90 | 6.5 | 0.4 |
| Lee21* | Not specified | 79 | 0.29 | 0.90 | 0.99 | 102.1 | 0.1 |
| Richter29*‡ | Not specified | 74 | 0.78 | 0.93 | 0.88 | 7.4 | 0.1 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.86 | 0.95 | 17.9 | 0.1 |
| Cooperman24 | Not specified | 32 | 0.41 | (0.65)║ | (0.42)║ | — | — |
| Pivot shift test | |||||||
| Hardaker25 | Partial + complete | 132 | 0.77 | 0.29 | — | — | — |
| Tonino19* | Partial + complete | 52 | 0.58 | 0.18 | 0.98 | 8.2 | 0.8 |
| Rubinstein28 | “ACL-deficient” | 39 | 0.23 | (0.93)§ | (0.89)§ | — | — |
| Boeree26* | Not specified | 203 | 0.29 | 0.31 | 0.97 | 8.8 | 0.7 |
| Richter29*†‡ | Not specified | 74 | 0.78 | 0.48 | 0.97 | 16.4 | 0.5 |
| SteinbrÜck22* | Not specified | 300 | 0.17 | 0.22 | 0.99 | 26.9 | 0.8 |
| * Study results used for meta-analysis | |||||||
| † 0.5 added to each cell of the 2x2 table | |||||||
| ‡ 2x2 table reconstructed | |||||||
| § Mean result of 5 orthopedic surgeons | |||||||
| ║ Sum of results of 2 physiotherapists | |||||||
| ACL, anterior cruciate ligament; Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio | |||||||
FIGURE 1
Sensitivity vs 1–specificity of the 3 tests
Scatterplots of sensitivity versus 1–specificity of A) the anterior drawer test (6 studies), B) the Lachman test (6 studies), and C) the pivot shift test (4 studies). Summary receiver operating characteristic curves and summary estimates of sensitivity and specificity (including 95% confidence intervals) are shown as appropriate.
Lachman test (Figure 2). The SROC curve of the Lachman test (6 studies) is shown in Figure 1B . Sensitivity ranged from 0.63 to 0.93, and specificity from 0.55 to 0.99. According to the bivariate random effects model the pooled sensitivity was 0.86 (95% CI, 0.76–0.92) and the pooled specificity was 0.91 (95% CI, 0.79–0.96).
Pivot shift test. The SROC curve of the pivot shift test (4 studies) is shown in Figure 1C . Sensitivity ranged from 0.18 to 0.48, and specificity from 0.97 to 0.99. Bivariate random effects pooling could not be performed; in this model 5 parameters must be estimated and only 4 studies were available.
Figure 3 shows the PV+ and PV– for all tests according to varying prevalences of ACL ruptures. The pivot shift test has the highest PV+ and the Lachman test the highest PV–. If the pivot shift test is positive, there is high probability of an ACL rupture, whereas a negative Lachman test rules out a rupture.
FIGURE 2
Lachman test
To perform the Lachman test, grasp the back of the proximal tibia posteriorly and place thumb over joint line anterolaterally. Pull the proximal tibia anteriorly and posteriorly, and compare sides for endpoint laxity.
FIGURE 3
Predictive value vs prevalence of positive and negative test results
Predictive value (posttest probability of presence of ACL rupture) vs prevalence (prior probability of presence of ACL rupture) of positive and negative test results of the anterior drawer test (sensitivity=0.62, specificity=0.88), Lachman test (sensitivity=0.86, specificity=0.91), and the pivot shift test (sensitivity=0.32, specificity=0.98).
DISCUSSION
We reviewed 17 studies that examined the accuracy of physical diagnostic tests for assessing ACL ruptures of the knee. Of those tests, the pivot shift test seems to have favorable positive predictive value, and the Lachman test good negative predictive value. The anterior drawer test is of unproven diagnostic value in this setting. In view of the potential biases in the original studies, however, the accuracy of the various ACL tests might be overestimated and the poor quality of the studies impede sound conclusions about the usefulness of the tests for daily practice. In addition, no study has been performed in primary care.
Because test characteristics may be influenced substantially by referral filters leading to spectrum bias,31 and because primary care physicians will be less experienced in performing these tests, the tests will presumably be less accurate in a primary care setting. Furthermore, the pivot shift test is very difficult to perform, making it less attractive for the average primary care physician.
Future research
Useful answers would be derived from sound research on the diagnostic accuracy of the various tests (determined for each test separately and for all tests jointly) combined with patient characteristics (eg, age, physical fitness, and functional demands) and elements of the medical history (eg, type of trauma and nature of the complaints). The emergence of MRI will facilitate this research. Relevance to clinical practice would be enhanced by an assessment of the effect of a correct diagnosis on the functional outcome of patients.
Acknowledgments
The authors thank Afina Glas, MD, and Professor Koos Zwinderman, PhD, for their statistical advice. We are much obliged to 1 of the referees for his/her useful suggestions.
Corresponding author
Rob J.P.M. Scholten, MD, PhD, Dutch Cochrane Centre, Department of Clinical Epidemiology and Biostatistics, J2-273, Academic Medical Center, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands. E-mail: [email protected].
1. Slocum DB, Larson RL. Rotatory instability of the knee. Its pathogenesis and a clinical test to demonstrate its presence. J Bone Joint Surg Am 1968;50:211-225.
2. Torg JS, Conrad W, Kalen V. Clinical diagnosis of anterior cruciate ligament instability in the athlete. Am J Sports Med 1976;4:84-93.
3. Galway HR, MacIntosh DL. The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop 1980;147:45-50.
4. Boeve BF, Davidson RA, Staab EV. Magnetic resonance imaging in the evaluation of knee injuries. South Med J 1991;84:1123-1127.
5. Buss DD, Min R, Skyhar M, Galinat B, Warren RF, Wickiewicz TL. Nonoperative treatment of acute anterior cruciate ligament injuries in a selected group of patients. Am J Sports Med 1995;23:160-165.
6. Devillè WLJM, Bezemer PD, Bouter LM. Publications on diagnostic test evaluation in family medicine journals: an optimal search strategy. J Clin Epidemiol 2000;53:65-69.
7. Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 1995;48:119-130.
8. Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests: Recommended, Methods, updated 6 June 1996. Available at http://www.cochrane.de/cochrane/sadtdoc1.htm.
9. Scholten RJPM, Devillè WLJM, Opstelten W, Bijl D, van der Plas CG, Bouter LM. The accuracy of physical diagnostic tests for assessing meniscal lesions of the knee: a meta-analysis. J Fam Pract 2001;50:938-944.
10. Midgette AS, Stukel TA, Littenberg B. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med Decis Making 1993;13:253-257.
11. Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-1316.
12. van Houwelingen JC, Zwinderman K, Stijnen T. A bivariate approach to meta-analysis. Stat Med. 1993;12:2272-2284.
13. van Houwelingen JC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589-624.
14. Warren RF, Marshall JL. Injuries of the anterior cruciate and medial collateral ligaments of the knee. A retrospective analysis of clinical records—part I. Clin Orthop 1978;136:191-197.
15. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg 1980;62A:687-695.
16. Noyes FR, Paulos L, Mooar LA, Signer B. Knee sprains and acute knee hemarthrosis: misdiagnosis of anterior cruciate ligament tears. Phys Ther 1980;60:1596-1601.
17. Braunstein EM. Anterior cruciate ligament injuries: a comparison of arthrographic and physical diagnosis. AJR Am J Roentgenol 1982;138:423-425.
18. Sandberg R, Balkfors B, Henricson A, Westlin N. Stability tests in knee ligament injuries. Arch Orthop Trauma Surg 1986;106:5-7.
19. Tonino AJ, Huy J, Schaafsma J. The diagnostic accuracy of knee testing in the acutely injured knee. Initial examination versus examination under anaesthesia with arthroscopy. Acta Orthop Belg 1986;52:479-487.
20. Harilainen A. Evaluation of knee instability in acute liga-mentous injuries. Ann Chir Gynaecol 1987;76:269-273.
21. Lee JK, Yao L, Phelps CT, Wirth CR, Czajka J, Lozman J. Anterior cruciate ligament tears: MR imaging compared with arthroscopy and clinical tests. Radiology 1988;166:861-864.
22. SteinbrÜck K, Wiehmann JC. Examination of the knee joint. The value of clinical findings in arthroscopic control [in German]. Z Orthop Ihre Grenzgeb 1988;126:289-295.
23. Anderson AF, Lipscomb AB. Preoperative instrumented testing of anterior and posterior knee laxity. Am J Sports Med 1989;17:387-392.
24. Cooperman JM, Riddle DL, Rothstein JM. Reliability and validity of judgments of the integrity of the anterior cruci-ate ligament of the knee using the Lachman’s test. Phys Ther 1990;70:225-233.
25. Hardaker WT Jr, Garrett WE Jr, Bassett FH 3d. Evaluation of acute traumatic hemarthrosis of the knee joint. South Med J 1990;83:640-644.
26. Boeree NR, Ackroyd CE. Assessment of the menisci and cruciate ligaments: an audit of clinical practice. Injury 1991;22:291-294.
27. al-Duri Z. Relation of the fibular head sign to other signs of anterior cruciate ligament insufficiency. A follow-up letter to the editor. Clin Orthop 1992;275:220-225.
28. Rubinstein RA Jr, Shelbourne KD, McCarroll JR, VanMeter CD, Rettig AC. The accuracy of the clinical examination in the setting of posterior cruciate ligament injuries. Am J Sports Med 1994;22:550-557.
29. Richter J, David A, Pape HG, Ostermann PA, Muhr G. Diagnosis of acute rupture of the anterior cruciate liga-ment. Value of ultrasonic in addition to clinical examination [in German]. Unfallchirurg 1996;99:124-129.
30. Schwarz W, Hagelstein J, Minholz R, Schierlinger M, Danz B, Gerngross H. Manual ultrasound of the knee joint. A general practice method for diagnosis of fresh rupture of the anterior cruciate ligament [in German]. Unfallchirurg 1997;100:280-285.
31. Knottnerus JA, Leffers P. The influence of referral patterns on the characteristics of diagnostic tests. J Clin Epidemiol 1992;45:1143-1154.
1. Slocum DB, Larson RL. Rotatory instability of the knee. Its pathogenesis and a clinical test to demonstrate its presence. J Bone Joint Surg Am 1968;50:211-225.
2. Torg JS, Conrad W, Kalen V. Clinical diagnosis of anterior cruciate ligament instability in the athlete. Am J Sports Med 1976;4:84-93.
3. Galway HR, MacIntosh DL. The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop 1980;147:45-50.
4. Boeve BF, Davidson RA, Staab EV. Magnetic resonance imaging in the evaluation of knee injuries. South Med J 1991;84:1123-1127.
5. Buss DD, Min R, Skyhar M, Galinat B, Warren RF, Wickiewicz TL. Nonoperative treatment of acute anterior cruciate ligament injuries in a selected group of patients. Am J Sports Med 1995;23:160-165.
6. Devillè WLJM, Bezemer PD, Bouter LM. Publications on diagnostic test evaluation in family medicine journals: an optimal search strategy. J Clin Epidemiol 2000;53:65-69.
7. Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 1995;48:119-130.
8. Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests: Recommended, Methods, updated 6 June 1996. Available at http://www.cochrane.de/cochrane/sadtdoc1.htm.
9. Scholten RJPM, Devillè WLJM, Opstelten W, Bijl D, van der Plas CG, Bouter LM. The accuracy of physical diagnostic tests for assessing meniscal lesions of the knee: a meta-analysis. J Fam Pract 2001;50:938-944.
10. Midgette AS, Stukel TA, Littenberg B. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med Decis Making 1993;13:253-257.
11. Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-1316.
12. van Houwelingen JC, Zwinderman K, Stijnen T. A bivariate approach to meta-analysis. Stat Med. 1993;12:2272-2284.
13. van Houwelingen JC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589-624.
14. Warren RF, Marshall JL. Injuries of the anterior cruciate and medial collateral ligaments of the knee. A retrospective analysis of clinical records—part I. Clin Orthop 1978;136:191-197.
15. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg 1980;62A:687-695.
16. Noyes FR, Paulos L, Mooar LA, Signer B. Knee sprains and acute knee hemarthrosis: misdiagnosis of anterior cruciate ligament tears. Phys Ther 1980;60:1596-1601.
17. Braunstein EM. Anterior cruciate ligament injuries: a comparison of arthrographic and physical diagnosis. AJR Am J Roentgenol 1982;138:423-425.
18. Sandberg R, Balkfors B, Henricson A, Westlin N. Stability tests in knee ligament injuries. Arch Orthop Trauma Surg 1986;106:5-7.
19. Tonino AJ, Huy J, Schaafsma J. The diagnostic accuracy of knee testing in the acutely injured knee. Initial examination versus examination under anaesthesia with arthroscopy. Acta Orthop Belg 1986;52:479-487.
20. Harilainen A. Evaluation of knee instability in acute liga-mentous injuries. Ann Chir Gynaecol 1987;76:269-273.
21. Lee JK, Yao L, Phelps CT, Wirth CR, Czajka J, Lozman J. Anterior cruciate ligament tears: MR imaging compared with arthroscopy and clinical tests. Radiology 1988;166:861-864.
22. SteinbrÜck K, Wiehmann JC. Examination of the knee joint. The value of clinical findings in arthroscopic control [in German]. Z Orthop Ihre Grenzgeb 1988;126:289-295.
23. Anderson AF, Lipscomb AB. Preoperative instrumented testing of anterior and posterior knee laxity. Am J Sports Med 1989;17:387-392.
24. Cooperman JM, Riddle DL, Rothstein JM. Reliability and validity of judgments of the integrity of the anterior cruci-ate ligament of the knee using the Lachman’s test. Phys Ther 1990;70:225-233.
25. Hardaker WT Jr, Garrett WE Jr, Bassett FH 3d. Evaluation of acute traumatic hemarthrosis of the knee joint. South Med J 1990;83:640-644.
26. Boeree NR, Ackroyd CE. Assessment of the menisci and cruciate ligaments: an audit of clinical practice. Injury 1991;22:291-294.
27. al-Duri Z. Relation of the fibular head sign to other signs of anterior cruciate ligament insufficiency. A follow-up letter to the editor. Clin Orthop 1992;275:220-225.
28. Rubinstein RA Jr, Shelbourne KD, McCarroll JR, VanMeter CD, Rettig AC. The accuracy of the clinical examination in the setting of posterior cruciate ligament injuries. Am J Sports Med 1994;22:550-557.
29. Richter J, David A, Pape HG, Ostermann PA, Muhr G. Diagnosis of acute rupture of the anterior cruciate liga-ment. Value of ultrasonic in addition to clinical examination [in German]. Unfallchirurg 1996;99:124-129.
30. Schwarz W, Hagelstein J, Minholz R, Schierlinger M, Danz B, Gerngross H. Manual ultrasound of the knee joint. A general practice method for diagnosis of fresh rupture of the anterior cruciate ligament [in German]. Unfallchirurg 1997;100:280-285.
31. Knottnerus JA, Leffers P. The influence of referral patterns on the characteristics of diagnostic tests. J Clin Epidemiol 1992;45:1143-1154.
Complementary medicine: Where is the evidence?
- Herbal medicines have been submitted to systematic reviews more frequently than any other complementary therapy, and it is here where the most positive evidence can be found.
- There is not much research into potential serious risks of complementary medicine. Possible risks range from the toxicity of herbs to vertebral artery dissection or nerve damage after chiropractic manipulation.
- Currently the Cochrane Library contains 34 systematic reviews of complementary medicine: 20 of herbal medicines, 7 of acupuncture, 3 of homeopathy, 2 of manual therapies, and 2 of other forms.
Complementary or alternative medicine has moved from the fringe of health care toward its center; recent figures show that in Germany, for instance, no less than three quarters of the general population use at least 1 complementary therapy.1 In the United States, the equivalent figures have increased from 33% in 1990 to 42% in 1997.2
Virtually all survey data agree that those most fascinated with complementary medicine are predominantly female, affluent, middleaged, and well-educated. Seventy-eight percent of all Medicaid programs provide coverage of at least 1 form of complementary medicine.3
At the same time, critics of complementary medicine often insist there is no good evidence to support these therapies,4,5 and that it is a waste of resources and a misuse of science to try establishing an evidence base for therapies that are essentially nonscientific, irrational, and implausible fads.6 But is this really true?
Research in complementary medicine
Over the last 30 years, the level of original research activity in complementary medicine has increased considerably.7 The best quality of evidence for or against the effectiveness of any therapy is usually provided by Cochrane reviews.8,9
Cochrane reviews
Currently the Cochrane Library contains 34 systematic reviews and 35 protocols of complementary medicine10 (depending on what one considers complementary/alternative and what mainstream, this figure might vary marginally). Twenty of the reviews are of herbal medicines, 7 of acupuncture, 3 of homeopathy, 2 of manual therapies, and 2 of other forms of comple-mentary medicine. Twelve reviews include a meta-analytic approach.
The 34 reviews comprise a total of 286 clinical, mostly randomized and often placebo-controlled, double-blind studies. In 1999, the Cochrane Library listed more than 4000 controlled trials of complementary medicine and a further 4000 awaited assessment.11
The single largest Cochrane review of complementary medicine is a meta-analysis of randomized clinical trials of St John’s wort for depression, based on 27 primary studies with a total of 2291 patients.12 Seven of the 34 Cochrane reviews are “negative”ie, do not suggest a positive clinical effect of the intervention under evaluation. Eleven are entirely inconclusive and 16 draw at least tentatively positive conclusions (Table). Given the dire funding situation for research in complementary medicine,13 this evidence base is remarkable.
TABLE
Cochrane reviews in complementary medicine with (tentatively) positive results
| First author (primary studies)* | Therapy | Indication | Reservations* |
|---|---|---|---|
| Furlan (8) | Massage | Low back pain | More studies required, some trials of poor quality |
| Green (4) | Acupuncture | Lateral elbow pain | More studies required, most trials of poor quality |
| Linde (7) | Acupuncture | Asthma | Evidence only positive for peak expiratory flow rate, effect size small |
| Linde (27) | St John’s wort (Hypericum perforatum)† | Depression | Some trials of poor quality, few equivalence studies |
| Little (11) | Various herbal medicines | Rheumatoid arthritis | More studies required, some trials of poor quality |
| Little (5) | Various herbal medicines | Osteoarthritis | More studies required, some trials of poor quality |
| Melchart (26) | Acupuncture | Headache | Evidence positive only for migraine headaches, effect size small |
| Pittler (7) | Kava (Piper methysticum)† | Anxiety | Concern over safety |
| Pittler (13) | Horse chestnut (Aesculus hippocastanum)† | Chronic venous insufficiency | Scarcity of long-term studies |
| Pittler (4) | Feverfew (Tanacetum parthenium)† | Migraine prevention | More studies required, effect size small |
| Pittler (2) | Globe artichoke (Cynara scolymus)† | Hypercholesterolemia | More studies required effect size small |
| Vickers (7) | Oscillococcinum | Influenza | More studies required, effect size small |
| Wilt (21) | Saw palmetto (Serenoa repens)† | Benign prostate hypertrophy | Effect size moderate, scarcity of long-term studies |
| Wilt (18) | African prune (Pygeum africanum)† | Benign prostate hypertrophy | Scarcity of long-term studies |
| Wilt (4) | Cernilton† | Benign prostate hypertrophy | More studies required, scarcity of long-term trials |
| Wilt (4) | Beta-sitosterols† | Benign prostate hypertrophy | More studies required, scarcity of long-term trials |
| All data extracted from The Cochrane Library, 2003. “More studies required” means that volume of data was small; “trials of poor quality” means that the average quality of the evidence was lowered by flawed studies; “effect size” describes the difference in clinical response to active and control treatment. | |||
| *As expressed by authors of respective review. | |||
| †Plant-based treatments. | |||
Other reviews
The Cochrane database may be the best but certainly is not the only source of systematic reviews of complementary medicine. My unit has published about 100 systematic reviews (a full list is available from the author), and most were not in the Cochrane format.
Linde and Willich have analyzed selected systematic reviews of acupuncture, homeopathy, and herbal medicine, and have shown that their methodological approach differed considerably.14
Methods of reviewing complementary medicine
The conclusions of these methodologically diverse articles were still surprisingly consistent.15 Some maintain that complementary medicine cannot be evidence-based in the conventional sense of the word16 ; that “softer” types of evidence need to be taken into consideration as well17 ; that placebo effects must not be dismissed as nonbeneficial18 ; that the healing encounter includes significant factors that may never be quantifiable19 ; that “the scientific method cannot measure hope, divine intervention, or the power of belief.”20 And, obviously, research in complementary medicine “must consider social, cultural, political, and economic contexts.”21
The debate about what constitutes the best research methods for complementary medicine has been going on for decades. There are no simple answers except that, like in any type of scientific inquiry, there are no intrinsically good or bad methods, only good and bad matches between the research question posed and the methodology employed.22
Herbal medicines have been submitted to systematic reviews more frequently than any other complementary therapy, and it is in this area where most of the positive evidence can be found (also outside Cochrane reviews).23 The medical conditions treated with complementary medicine are often chronic benign diseases for which existing conventional treatments fail to offer a cure or a risk-free reduction of symptoms (Table). Given the popularity of complementary medicine and the economic importance of these conditions, it seems ill-conceived to argue against further research in this area. 6
Firm conclusions of the Cochrane (or other) reviews of complementary medicine are often hampered by the paucity of primary studies; 10 of the 16 reviews in Table are based on fewer than 10 primary studies. The average methodological quality of the primary data is in some but by no means all disappointing.24-26
Problems in testing complementary medicine
There is little doubt that rigorous trials of complementary medicine can pose formidable problems.22 What, for instance, is an adequate placebo for a study of massage therapy, and how should one blind patients in such a trial? The biggest obstacle to good research is perhaps the notorious lack of research funding in this area, which is all the more acute because costs can be particularly high for trials of time-intensive forms of complementary medicine.13
The average size of the overall therapeutic effect associated with complementary medicine is usually modest and the numbers needed to treat are often high. In other words, the difference between benefit from complementary medicine and no therapy or placebo may be statistically significant but critics might argue that it is of debatable clinical relevance.5 Even minor adverse effects would therefore critically disturb the delicate balance of risk and benefit.23
Assessing the risks
It follows that the potential risks of complementary medicine require careful attention and more systematic study. Our fragmentary knowledge indicates that the issues are complex.27 They range from toxicity of herbs to vertebral artery dissection or nerve damage after chiropractic manipulation.
They also include more subtle indirect hazards. Some practitioners of complementary medicine, for instance, tend to advise their clients against employing important medical interventions.28 At present there are no reliable incident figures regarding serious adverse effects of complementary medicine,5,23,27 rendering this area perhaps the most urgent topic for further research.
Moving forward
At a time when healthcare systems universally are strapped for money, the decision whether to integrate complementary medicine into routine medicine will undoubtedly be influenced by economic considerations. Virtually all research on this issue is inconclusive or flawed or both.29 We therefore cannot be sure whether such an integration would save public funds or cost extra money.
In the US, about 41 million people have no health insurance and are thus not covered for even the most basic health care. About the same number of Americans are underinsured. In this situation, it seems difficult to argue without convincing data that the integration of complementary medicine would present a solution to the economic problems in healthcare.
In conclusion, during recent years the evidence in support of complementary medicine has been considerably strengthened, primarily through numerous Cochrane reviews10 and other documents.7,23,30 A large range of promising interventions could be at our fingertips. At a time when whole populations are voting with their feet in favor of complementary medicine,1,2 it would be in everyone’s interest to invest in rigorous research of this area.
Correspondence
Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, 25 Victoria Park Road, Exeter EX2 4NT, United Kingdom. E-mail: [email protected].
1. Institut fÜr Demoskopie Allensbach. Naturheilmittel: Wichtigste Erkenntnisse aus Allensbacher Trendstudien. [Natural cures: The most important conclusions from Allensbach trend studies.] Available at: www.demoskopie.de.Accessed on May 27, 2003.
2. Eisenberg DM, David RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998;280:1569-1575.
3. Steyer TE, Freed GL, Lantz PM. Medicaid reimbursement for alternative therapies. Alt Ther Health Med 2002;8:84-88.
4. Marcus DM. Alternative medicine and the Arthritis Foundation. Arthritis Rheum 2002;47:5-7.
5. Fitzcharles M. Is it time for rheumatologists to rethink the use of manual therapies? J Rheumatol 2002;29:1117-1120.
6. Charlton BG. Randomized trials in alternative/complementary medicine. Q J Med 2002;95:643-645.
7. Barnes J, Abbot NC, Harkness E, Ernst E. Articles on complementary medicine in the mainstream medical literature: an investigation of MEDLINE, 1966 through 1996. Arch Intern Med 1999;159:1721-1725.
8. Olsen O, Middleton P, Ezzo J, et al. Quality of Cochrane reviews: assessment of sample from 1998. BMJ 2001;323:829-832.
9. Jadad AR, Moher M, Browman GP, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
10. The Cochrane Library [database online] Oxford, UK: Update Software; 2002.Available at: www.cochranelibrary.com. Accessed on May 23, 2003.
11. Vickers A. Evidence-based medicine and complementary medicine. ACP J Club 1999;130:A13-14.
12. Linde K. St John’s wort for depression. The Cochrane Library 2000;4:1-17.
13. Ernst E. Funding research into complementary medicine: the situation in Britain. Complement Ther Med 1999;7:250-253.
14. Linde K, Willich SN. How objective are systematic reviews? Differences between reviews on complementary medicine. J R Soc Med 2003;96:17-22.
15. Ernst E. How objective are systematic reviews? J R Soc Med 2003;96:56-57.
16. Tonelli MR, Callahan TC. Why alternative medicine cannot be evidence-based. Acad Med 2001;76:1213-1220.
17. Jonas WB. The evidence house: how to build an inclusive base for complementary medicine. West J Med 2001;175:79-80.
18. Walach H. The efficacy paradox in randomized controlled trials of CAM and elsewhere: beware of the placebo trap. J Altern Complement Med 2001;7:213-218.
19. Redwood D. Methodological changes in the evaluation of complementary and alternative medicine: issued raised by Sherman et al and Hawk et al. J Altern Complement Med 2002;8:5-6.
20. Puchalski CM. Reconnecting the science and art of medicine. Acad Med 2001;76:1224-1225.
21. Bodeker G, Kronenberg F. A public health agenda for traditional, complementary, and alternative medicine. Am J Public Health 2002;92:1582-1591.
22. Vickers A, Cassileth B, Ernst E, et al. How should we research unconventional therapies? A panel report from the conference on Complementary and Alternative Medicine Research Methodology, National Institute of Health. Int J Technol Assess Health Care 1997;13:111-121.
23. Ernst E, Pittler MH, Stevinson C, White AR. The desktop guide to complementary and alternative medicine. London: Mosby; 2001.
24. Bloom BS, Retbi A, Dahan S, Jonsson E. Evaluation of randomized controlled trials on complementary and alternative medicine. Int J Technol Assess Health Care 2000;16:13-21.
25. Linde K, Jonas WB, Melchart D, Willich S. The methodological quality of randomised controlled trials of homeopathy, herbal medicines and acupuncture. Int J Epidemiol 2001;30:526-531.
26. Moher D, Sampson M, Campbell K, et al. Assessing the quality of reports of randomised trials in pediatric complementary and alternative medicine. BMC Pediatr 2002;2:1-6.
27. Ernst E. Risks associated with complementary therapies. In MNG Dukes and JR Aronson, eds, Meyler’s Side Effects of Drugs. 14th ed. Oxford and New York: Elsevier Science; 2000:1649-1681.
28. Schmidt K, Ernst E. Aspects of MMR. Survey shows that some homeopaths and chiropractors advise against MMR. BMJ 2002;325-597.
29. White AR, Ernst E. Economic analysis of complementary medicine a systematic review. Complement Ther Med 2000;8:111-118.
30. Yuan C-S, Bieber EJ, eds. Textbook of complementary and alternative medicine. Boca Raton, Fla: Parthenon; 2003.
- Herbal medicines have been submitted to systematic reviews more frequently than any other complementary therapy, and it is here where the most positive evidence can be found.
- There is not much research into potential serious risks of complementary medicine. Possible risks range from the toxicity of herbs to vertebral artery dissection or nerve damage after chiropractic manipulation.
- Currently the Cochrane Library contains 34 systematic reviews of complementary medicine: 20 of herbal medicines, 7 of acupuncture, 3 of homeopathy, 2 of manual therapies, and 2 of other forms.
Complementary or alternative medicine has moved from the fringe of health care toward its center; recent figures show that in Germany, for instance, no less than three quarters of the general population use at least 1 complementary therapy.1 In the United States, the equivalent figures have increased from 33% in 1990 to 42% in 1997.2
Virtually all survey data agree that those most fascinated with complementary medicine are predominantly female, affluent, middleaged, and well-educated. Seventy-eight percent of all Medicaid programs provide coverage of at least 1 form of complementary medicine.3
At the same time, critics of complementary medicine often insist there is no good evidence to support these therapies,4,5 and that it is a waste of resources and a misuse of science to try establishing an evidence base for therapies that are essentially nonscientific, irrational, and implausible fads.6 But is this really true?
Research in complementary medicine
Over the last 30 years, the level of original research activity in complementary medicine has increased considerably.7 The best quality of evidence for or against the effectiveness of any therapy is usually provided by Cochrane reviews.8,9
Cochrane reviews
Currently the Cochrane Library contains 34 systematic reviews and 35 protocols of complementary medicine10 (depending on what one considers complementary/alternative and what mainstream, this figure might vary marginally). Twenty of the reviews are of herbal medicines, 7 of acupuncture, 3 of homeopathy, 2 of manual therapies, and 2 of other forms of comple-mentary medicine. Twelve reviews include a meta-analytic approach.
The 34 reviews comprise a total of 286 clinical, mostly randomized and often placebo-controlled, double-blind studies. In 1999, the Cochrane Library listed more than 4000 controlled trials of complementary medicine and a further 4000 awaited assessment.11
The single largest Cochrane review of complementary medicine is a meta-analysis of randomized clinical trials of St John’s wort for depression, based on 27 primary studies with a total of 2291 patients.12 Seven of the 34 Cochrane reviews are “negative”ie, do not suggest a positive clinical effect of the intervention under evaluation. Eleven are entirely inconclusive and 16 draw at least tentatively positive conclusions (Table). Given the dire funding situation for research in complementary medicine,13 this evidence base is remarkable.
TABLE
Cochrane reviews in complementary medicine with (tentatively) positive results
| First author (primary studies)* | Therapy | Indication | Reservations* |
|---|---|---|---|
| Furlan (8) | Massage | Low back pain | More studies required, some trials of poor quality |
| Green (4) | Acupuncture | Lateral elbow pain | More studies required, most trials of poor quality |
| Linde (7) | Acupuncture | Asthma | Evidence only positive for peak expiratory flow rate, effect size small |
| Linde (27) | St John’s wort (Hypericum perforatum)† | Depression | Some trials of poor quality, few equivalence studies |
| Little (11) | Various herbal medicines | Rheumatoid arthritis | More studies required, some trials of poor quality |
| Little (5) | Various herbal medicines | Osteoarthritis | More studies required, some trials of poor quality |
| Melchart (26) | Acupuncture | Headache | Evidence positive only for migraine headaches, effect size small |
| Pittler (7) | Kava (Piper methysticum)† | Anxiety | Concern over safety |
| Pittler (13) | Horse chestnut (Aesculus hippocastanum)† | Chronic venous insufficiency | Scarcity of long-term studies |
| Pittler (4) | Feverfew (Tanacetum parthenium)† | Migraine prevention | More studies required, effect size small |
| Pittler (2) | Globe artichoke (Cynara scolymus)† | Hypercholesterolemia | More studies required effect size small |
| Vickers (7) | Oscillococcinum | Influenza | More studies required, effect size small |
| Wilt (21) | Saw palmetto (Serenoa repens)† | Benign prostate hypertrophy | Effect size moderate, scarcity of long-term studies |
| Wilt (18) | African prune (Pygeum africanum)† | Benign prostate hypertrophy | Scarcity of long-term studies |
| Wilt (4) | Cernilton† | Benign prostate hypertrophy | More studies required, scarcity of long-term trials |
| Wilt (4) | Beta-sitosterols† | Benign prostate hypertrophy | More studies required, scarcity of long-term trials |
| All data extracted from The Cochrane Library, 2003. “More studies required” means that volume of data was small; “trials of poor quality” means that the average quality of the evidence was lowered by flawed studies; “effect size” describes the difference in clinical response to active and control treatment. | |||
| *As expressed by authors of respective review. | |||
| †Plant-based treatments. | |||
Other reviews
The Cochrane database may be the best but certainly is not the only source of systematic reviews of complementary medicine. My unit has published about 100 systematic reviews (a full list is available from the author), and most were not in the Cochrane format.
Linde and Willich have analyzed selected systematic reviews of acupuncture, homeopathy, and herbal medicine, and have shown that their methodological approach differed considerably.14
Methods of reviewing complementary medicine
The conclusions of these methodologically diverse articles were still surprisingly consistent.15 Some maintain that complementary medicine cannot be evidence-based in the conventional sense of the word16 ; that “softer” types of evidence need to be taken into consideration as well17 ; that placebo effects must not be dismissed as nonbeneficial18 ; that the healing encounter includes significant factors that may never be quantifiable19 ; that “the scientific method cannot measure hope, divine intervention, or the power of belief.”20 And, obviously, research in complementary medicine “must consider social, cultural, political, and economic contexts.”21
The debate about what constitutes the best research methods for complementary medicine has been going on for decades. There are no simple answers except that, like in any type of scientific inquiry, there are no intrinsically good or bad methods, only good and bad matches between the research question posed and the methodology employed.22
Herbal medicines have been submitted to systematic reviews more frequently than any other complementary therapy, and it is in this area where most of the positive evidence can be found (also outside Cochrane reviews).23 The medical conditions treated with complementary medicine are often chronic benign diseases for which existing conventional treatments fail to offer a cure or a risk-free reduction of symptoms (Table). Given the popularity of complementary medicine and the economic importance of these conditions, it seems ill-conceived to argue against further research in this area. 6
Firm conclusions of the Cochrane (or other) reviews of complementary medicine are often hampered by the paucity of primary studies; 10 of the 16 reviews in Table are based on fewer than 10 primary studies. The average methodological quality of the primary data is in some but by no means all disappointing.24-26
Problems in testing complementary medicine
There is little doubt that rigorous trials of complementary medicine can pose formidable problems.22 What, for instance, is an adequate placebo for a study of massage therapy, and how should one blind patients in such a trial? The biggest obstacle to good research is perhaps the notorious lack of research funding in this area, which is all the more acute because costs can be particularly high for trials of time-intensive forms of complementary medicine.13
The average size of the overall therapeutic effect associated with complementary medicine is usually modest and the numbers needed to treat are often high. In other words, the difference between benefit from complementary medicine and no therapy or placebo may be statistically significant but critics might argue that it is of debatable clinical relevance.5 Even minor adverse effects would therefore critically disturb the delicate balance of risk and benefit.23
Assessing the risks
It follows that the potential risks of complementary medicine require careful attention and more systematic study. Our fragmentary knowledge indicates that the issues are complex.27 They range from toxicity of herbs to vertebral artery dissection or nerve damage after chiropractic manipulation.
They also include more subtle indirect hazards. Some practitioners of complementary medicine, for instance, tend to advise their clients against employing important medical interventions.28 At present there are no reliable incident figures regarding serious adverse effects of complementary medicine,5,23,27 rendering this area perhaps the most urgent topic for further research.
Moving forward
At a time when healthcare systems universally are strapped for money, the decision whether to integrate complementary medicine into routine medicine will undoubtedly be influenced by economic considerations. Virtually all research on this issue is inconclusive or flawed or both.29 We therefore cannot be sure whether such an integration would save public funds or cost extra money.
In the US, about 41 million people have no health insurance and are thus not covered for even the most basic health care. About the same number of Americans are underinsured. In this situation, it seems difficult to argue without convincing data that the integration of complementary medicine would present a solution to the economic problems in healthcare.
In conclusion, during recent years the evidence in support of complementary medicine has been considerably strengthened, primarily through numerous Cochrane reviews10 and other documents.7,23,30 A large range of promising interventions could be at our fingertips. At a time when whole populations are voting with their feet in favor of complementary medicine,1,2 it would be in everyone’s interest to invest in rigorous research of this area.
Correspondence
Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, 25 Victoria Park Road, Exeter EX2 4NT, United Kingdom. E-mail: [email protected].
- Herbal medicines have been submitted to systematic reviews more frequently than any other complementary therapy, and it is here where the most positive evidence can be found.
- There is not much research into potential serious risks of complementary medicine. Possible risks range from the toxicity of herbs to vertebral artery dissection or nerve damage after chiropractic manipulation.
- Currently the Cochrane Library contains 34 systematic reviews of complementary medicine: 20 of herbal medicines, 7 of acupuncture, 3 of homeopathy, 2 of manual therapies, and 2 of other forms.
Complementary or alternative medicine has moved from the fringe of health care toward its center; recent figures show that in Germany, for instance, no less than three quarters of the general population use at least 1 complementary therapy.1 In the United States, the equivalent figures have increased from 33% in 1990 to 42% in 1997.2
Virtually all survey data agree that those most fascinated with complementary medicine are predominantly female, affluent, middleaged, and well-educated. Seventy-eight percent of all Medicaid programs provide coverage of at least 1 form of complementary medicine.3
At the same time, critics of complementary medicine often insist there is no good evidence to support these therapies,4,5 and that it is a waste of resources and a misuse of science to try establishing an evidence base for therapies that are essentially nonscientific, irrational, and implausible fads.6 But is this really true?
Research in complementary medicine
Over the last 30 years, the level of original research activity in complementary medicine has increased considerably.7 The best quality of evidence for or against the effectiveness of any therapy is usually provided by Cochrane reviews.8,9
Cochrane reviews
Currently the Cochrane Library contains 34 systematic reviews and 35 protocols of complementary medicine10 (depending on what one considers complementary/alternative and what mainstream, this figure might vary marginally). Twenty of the reviews are of herbal medicines, 7 of acupuncture, 3 of homeopathy, 2 of manual therapies, and 2 of other forms of comple-mentary medicine. Twelve reviews include a meta-analytic approach.
The 34 reviews comprise a total of 286 clinical, mostly randomized and often placebo-controlled, double-blind studies. In 1999, the Cochrane Library listed more than 4000 controlled trials of complementary medicine and a further 4000 awaited assessment.11
The single largest Cochrane review of complementary medicine is a meta-analysis of randomized clinical trials of St John’s wort for depression, based on 27 primary studies with a total of 2291 patients.12 Seven of the 34 Cochrane reviews are “negative”ie, do not suggest a positive clinical effect of the intervention under evaluation. Eleven are entirely inconclusive and 16 draw at least tentatively positive conclusions (Table). Given the dire funding situation for research in complementary medicine,13 this evidence base is remarkable.
TABLE
Cochrane reviews in complementary medicine with (tentatively) positive results
| First author (primary studies)* | Therapy | Indication | Reservations* |
|---|---|---|---|
| Furlan (8) | Massage | Low back pain | More studies required, some trials of poor quality |
| Green (4) | Acupuncture | Lateral elbow pain | More studies required, most trials of poor quality |
| Linde (7) | Acupuncture | Asthma | Evidence only positive for peak expiratory flow rate, effect size small |
| Linde (27) | St John’s wort (Hypericum perforatum)† | Depression | Some trials of poor quality, few equivalence studies |
| Little (11) | Various herbal medicines | Rheumatoid arthritis | More studies required, some trials of poor quality |
| Little (5) | Various herbal medicines | Osteoarthritis | More studies required, some trials of poor quality |
| Melchart (26) | Acupuncture | Headache | Evidence positive only for migraine headaches, effect size small |
| Pittler (7) | Kava (Piper methysticum)† | Anxiety | Concern over safety |
| Pittler (13) | Horse chestnut (Aesculus hippocastanum)† | Chronic venous insufficiency | Scarcity of long-term studies |
| Pittler (4) | Feverfew (Tanacetum parthenium)† | Migraine prevention | More studies required, effect size small |
| Pittler (2) | Globe artichoke (Cynara scolymus)† | Hypercholesterolemia | More studies required effect size small |
| Vickers (7) | Oscillococcinum | Influenza | More studies required, effect size small |
| Wilt (21) | Saw palmetto (Serenoa repens)† | Benign prostate hypertrophy | Effect size moderate, scarcity of long-term studies |
| Wilt (18) | African prune (Pygeum africanum)† | Benign prostate hypertrophy | Scarcity of long-term studies |
| Wilt (4) | Cernilton† | Benign prostate hypertrophy | More studies required, scarcity of long-term trials |
| Wilt (4) | Beta-sitosterols† | Benign prostate hypertrophy | More studies required, scarcity of long-term trials |
| All data extracted from The Cochrane Library, 2003. “More studies required” means that volume of data was small; “trials of poor quality” means that the average quality of the evidence was lowered by flawed studies; “effect size” describes the difference in clinical response to active and control treatment. | |||
| *As expressed by authors of respective review. | |||
| †Plant-based treatments. | |||
Other reviews
The Cochrane database may be the best but certainly is not the only source of systematic reviews of complementary medicine. My unit has published about 100 systematic reviews (a full list is available from the author), and most were not in the Cochrane format.
Linde and Willich have analyzed selected systematic reviews of acupuncture, homeopathy, and herbal medicine, and have shown that their methodological approach differed considerably.14
Methods of reviewing complementary medicine
The conclusions of these methodologically diverse articles were still surprisingly consistent.15 Some maintain that complementary medicine cannot be evidence-based in the conventional sense of the word16 ; that “softer” types of evidence need to be taken into consideration as well17 ; that placebo effects must not be dismissed as nonbeneficial18 ; that the healing encounter includes significant factors that may never be quantifiable19 ; that “the scientific method cannot measure hope, divine intervention, or the power of belief.”20 And, obviously, research in complementary medicine “must consider social, cultural, political, and economic contexts.”21
The debate about what constitutes the best research methods for complementary medicine has been going on for decades. There are no simple answers except that, like in any type of scientific inquiry, there are no intrinsically good or bad methods, only good and bad matches between the research question posed and the methodology employed.22
Herbal medicines have been submitted to systematic reviews more frequently than any other complementary therapy, and it is in this area where most of the positive evidence can be found (also outside Cochrane reviews).23 The medical conditions treated with complementary medicine are often chronic benign diseases for which existing conventional treatments fail to offer a cure or a risk-free reduction of symptoms (Table). Given the popularity of complementary medicine and the economic importance of these conditions, it seems ill-conceived to argue against further research in this area. 6
Firm conclusions of the Cochrane (or other) reviews of complementary medicine are often hampered by the paucity of primary studies; 10 of the 16 reviews in Table are based on fewer than 10 primary studies. The average methodological quality of the primary data is in some but by no means all disappointing.24-26
Problems in testing complementary medicine
There is little doubt that rigorous trials of complementary medicine can pose formidable problems.22 What, for instance, is an adequate placebo for a study of massage therapy, and how should one blind patients in such a trial? The biggest obstacle to good research is perhaps the notorious lack of research funding in this area, which is all the more acute because costs can be particularly high for trials of time-intensive forms of complementary medicine.13
The average size of the overall therapeutic effect associated with complementary medicine is usually modest and the numbers needed to treat are often high. In other words, the difference between benefit from complementary medicine and no therapy or placebo may be statistically significant but critics might argue that it is of debatable clinical relevance.5 Even minor adverse effects would therefore critically disturb the delicate balance of risk and benefit.23
Assessing the risks
It follows that the potential risks of complementary medicine require careful attention and more systematic study. Our fragmentary knowledge indicates that the issues are complex.27 They range from toxicity of herbs to vertebral artery dissection or nerve damage after chiropractic manipulation.
They also include more subtle indirect hazards. Some practitioners of complementary medicine, for instance, tend to advise their clients against employing important medical interventions.28 At present there are no reliable incident figures regarding serious adverse effects of complementary medicine,5,23,27 rendering this area perhaps the most urgent topic for further research.
Moving forward
At a time when healthcare systems universally are strapped for money, the decision whether to integrate complementary medicine into routine medicine will undoubtedly be influenced by economic considerations. Virtually all research on this issue is inconclusive or flawed or both.29 We therefore cannot be sure whether such an integration would save public funds or cost extra money.
In the US, about 41 million people have no health insurance and are thus not covered for even the most basic health care. About the same number of Americans are underinsured. In this situation, it seems difficult to argue without convincing data that the integration of complementary medicine would present a solution to the economic problems in healthcare.
In conclusion, during recent years the evidence in support of complementary medicine has been considerably strengthened, primarily through numerous Cochrane reviews10 and other documents.7,23,30 A large range of promising interventions could be at our fingertips. At a time when whole populations are voting with their feet in favor of complementary medicine,1,2 it would be in everyone’s interest to invest in rigorous research of this area.
Correspondence
Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, 25 Victoria Park Road, Exeter EX2 4NT, United Kingdom. E-mail: [email protected].
1. Institut fÜr Demoskopie Allensbach. Naturheilmittel: Wichtigste Erkenntnisse aus Allensbacher Trendstudien. [Natural cures: The most important conclusions from Allensbach trend studies.] Available at: www.demoskopie.de.Accessed on May 27, 2003.
2. Eisenberg DM, David RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998;280:1569-1575.
3. Steyer TE, Freed GL, Lantz PM. Medicaid reimbursement for alternative therapies. Alt Ther Health Med 2002;8:84-88.
4. Marcus DM. Alternative medicine and the Arthritis Foundation. Arthritis Rheum 2002;47:5-7.
5. Fitzcharles M. Is it time for rheumatologists to rethink the use of manual therapies? J Rheumatol 2002;29:1117-1120.
6. Charlton BG. Randomized trials in alternative/complementary medicine. Q J Med 2002;95:643-645.
7. Barnes J, Abbot NC, Harkness E, Ernst E. Articles on complementary medicine in the mainstream medical literature: an investigation of MEDLINE, 1966 through 1996. Arch Intern Med 1999;159:1721-1725.
8. Olsen O, Middleton P, Ezzo J, et al. Quality of Cochrane reviews: assessment of sample from 1998. BMJ 2001;323:829-832.
9. Jadad AR, Moher M, Browman GP, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
10. The Cochrane Library [database online] Oxford, UK: Update Software; 2002.Available at: www.cochranelibrary.com. Accessed on May 23, 2003.
11. Vickers A. Evidence-based medicine and complementary medicine. ACP J Club 1999;130:A13-14.
12. Linde K. St John’s wort for depression. The Cochrane Library 2000;4:1-17.
13. Ernst E. Funding research into complementary medicine: the situation in Britain. Complement Ther Med 1999;7:250-253.
14. Linde K, Willich SN. How objective are systematic reviews? Differences between reviews on complementary medicine. J R Soc Med 2003;96:17-22.
15. Ernst E. How objective are systematic reviews? J R Soc Med 2003;96:56-57.
16. Tonelli MR, Callahan TC. Why alternative medicine cannot be evidence-based. Acad Med 2001;76:1213-1220.
17. Jonas WB. The evidence house: how to build an inclusive base for complementary medicine. West J Med 2001;175:79-80.
18. Walach H. The efficacy paradox in randomized controlled trials of CAM and elsewhere: beware of the placebo trap. J Altern Complement Med 2001;7:213-218.
19. Redwood D. Methodological changes in the evaluation of complementary and alternative medicine: issued raised by Sherman et al and Hawk et al. J Altern Complement Med 2002;8:5-6.
20. Puchalski CM. Reconnecting the science and art of medicine. Acad Med 2001;76:1224-1225.
21. Bodeker G, Kronenberg F. A public health agenda for traditional, complementary, and alternative medicine. Am J Public Health 2002;92:1582-1591.
22. Vickers A, Cassileth B, Ernst E, et al. How should we research unconventional therapies? A panel report from the conference on Complementary and Alternative Medicine Research Methodology, National Institute of Health. Int J Technol Assess Health Care 1997;13:111-121.
23. Ernst E, Pittler MH, Stevinson C, White AR. The desktop guide to complementary and alternative medicine. London: Mosby; 2001.
24. Bloom BS, Retbi A, Dahan S, Jonsson E. Evaluation of randomized controlled trials on complementary and alternative medicine. Int J Technol Assess Health Care 2000;16:13-21.
25. Linde K, Jonas WB, Melchart D, Willich S. The methodological quality of randomised controlled trials of homeopathy, herbal medicines and acupuncture. Int J Epidemiol 2001;30:526-531.
26. Moher D, Sampson M, Campbell K, et al. Assessing the quality of reports of randomised trials in pediatric complementary and alternative medicine. BMC Pediatr 2002;2:1-6.
27. Ernst E. Risks associated with complementary therapies. In MNG Dukes and JR Aronson, eds, Meyler’s Side Effects of Drugs. 14th ed. Oxford and New York: Elsevier Science; 2000:1649-1681.
28. Schmidt K, Ernst E. Aspects of MMR. Survey shows that some homeopaths and chiropractors advise against MMR. BMJ 2002;325-597.
29. White AR, Ernst E. Economic analysis of complementary medicine a systematic review. Complement Ther Med 2000;8:111-118.
30. Yuan C-S, Bieber EJ, eds. Textbook of complementary and alternative medicine. Boca Raton, Fla: Parthenon; 2003.
1. Institut fÜr Demoskopie Allensbach. Naturheilmittel: Wichtigste Erkenntnisse aus Allensbacher Trendstudien. [Natural cures: The most important conclusions from Allensbach trend studies.] Available at: www.demoskopie.de.Accessed on May 27, 2003.
2. Eisenberg DM, David RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998;280:1569-1575.
3. Steyer TE, Freed GL, Lantz PM. Medicaid reimbursement for alternative therapies. Alt Ther Health Med 2002;8:84-88.
4. Marcus DM. Alternative medicine and the Arthritis Foundation. Arthritis Rheum 2002;47:5-7.
5. Fitzcharles M. Is it time for rheumatologists to rethink the use of manual therapies? J Rheumatol 2002;29:1117-1120.
6. Charlton BG. Randomized trials in alternative/complementary medicine. Q J Med 2002;95:643-645.
7. Barnes J, Abbot NC, Harkness E, Ernst E. Articles on complementary medicine in the mainstream medical literature: an investigation of MEDLINE, 1966 through 1996. Arch Intern Med 1999;159:1721-1725.
8. Olsen O, Middleton P, Ezzo J, et al. Quality of Cochrane reviews: assessment of sample from 1998. BMJ 2001;323:829-832.
9. Jadad AR, Moher M, Browman GP, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
10. The Cochrane Library [database online] Oxford, UK: Update Software; 2002.Available at: www.cochranelibrary.com. Accessed on May 23, 2003.
11. Vickers A. Evidence-based medicine and complementary medicine. ACP J Club 1999;130:A13-14.
12. Linde K. St John’s wort for depression. The Cochrane Library 2000;4:1-17.
13. Ernst E. Funding research into complementary medicine: the situation in Britain. Complement Ther Med 1999;7:250-253.
14. Linde K, Willich SN. How objective are systematic reviews? Differences between reviews on complementary medicine. J R Soc Med 2003;96:17-22.
15. Ernst E. How objective are systematic reviews? J R Soc Med 2003;96:56-57.
16. Tonelli MR, Callahan TC. Why alternative medicine cannot be evidence-based. Acad Med 2001;76:1213-1220.
17. Jonas WB. The evidence house: how to build an inclusive base for complementary medicine. West J Med 2001;175:79-80.
18. Walach H. The efficacy paradox in randomized controlled trials of CAM and elsewhere: beware of the placebo trap. J Altern Complement Med 2001;7:213-218.
19. Redwood D. Methodological changes in the evaluation of complementary and alternative medicine: issued raised by Sherman et al and Hawk et al. J Altern Complement Med 2002;8:5-6.
20. Puchalski CM. Reconnecting the science and art of medicine. Acad Med 2001;76:1224-1225.
21. Bodeker G, Kronenberg F. A public health agenda for traditional, complementary, and alternative medicine. Am J Public Health 2002;92:1582-1591.
22. Vickers A, Cassileth B, Ernst E, et al. How should we research unconventional therapies? A panel report from the conference on Complementary and Alternative Medicine Research Methodology, National Institute of Health. Int J Technol Assess Health Care 1997;13:111-121.
23. Ernst E, Pittler MH, Stevinson C, White AR. The desktop guide to complementary and alternative medicine. London: Mosby; 2001.
24. Bloom BS, Retbi A, Dahan S, Jonsson E. Evaluation of randomized controlled trials on complementary and alternative medicine. Int J Technol Assess Health Care 2000;16:13-21.
25. Linde K, Jonas WB, Melchart D, Willich S. The methodological quality of randomised controlled trials of homeopathy, herbal medicines and acupuncture. Int J Epidemiol 2001;30:526-531.
26. Moher D, Sampson M, Campbell K, et al. Assessing the quality of reports of randomised trials in pediatric complementary and alternative medicine. BMC Pediatr 2002;2:1-6.
27. Ernst E. Risks associated with complementary therapies. In MNG Dukes and JR Aronson, eds, Meyler’s Side Effects of Drugs. 14th ed. Oxford and New York: Elsevier Science; 2000:1649-1681.
28. Schmidt K, Ernst E. Aspects of MMR. Survey shows that some homeopaths and chiropractors advise against MMR. BMJ 2002;325-597.
29. White AR, Ernst E. Economic analysis of complementary medicine a systematic review. Complement Ther Med 2000;8:111-118.
30. Yuan C-S, Bieber EJ, eds. Textbook of complementary and alternative medicine. Boca Raton, Fla: Parthenon; 2003.
Evaluation and management of hip pain: An algorithmic approach
- Start by determining whether pain is located in the anterior, lateral, or posterior hip. As the site varies, so does the etiology.
- Besides location, consider sudden vs insidious onset, motions and positions that reproduce pain, predisposing activities, and effect of ambulation or weight bearing.
- Physical examination tests that elucidate range of motion, muscle strength, and pain replication will narrow the diagnostic search.
- Magnetic resonance imaging is usually diagnostic if plain x-rays and conservative therapy are ineffective.
- Conservative measures and selective use of injection therapy are usually effective.
Given the number of disorders capable of causing hip pain, and the fact that hip pathology can refer pain to other areas, and pathology elsewhere (particularly the lumbar spine) can refer pain to the hip,* a useful starting point in the evaluation is one that begins to narrow the search immediately.
*Medial groin pain is often included in the discussion of hip pain, but this topic is beyond the scope of this review.
In the work-up of hip pain, the first fact to establish is whether pain is felt in the anterior, lateral, or posterior part of the hip. Each location suggests a distinctive set of possible underlying causes. We provide diagnostic algorithms for all 3 scenarios, to aid in determining the best course for the work-up.
Anterior hip pain
Anterior hip pain (Figure 1), which is the most common, usually indicates pathology of the hip joint (ie, degenerative arthritis), hip flexor muscle strains or tendonitis, and iliopsoas bursitis. In a study by Lamberts and colleagues,5 by far the most common diagnosis of patients with hip complaints seen by their general practitioner was osteoarthritis. In a study of subjects older than 40 years who experienced a new episode of hip pain, 44% had evidence of osteoarthritis (level of evidence [LOE]=1b).6
Iliopsoas bursitis, a less common cause of anterior hip pain, involves inflammation of the bursa between the iliopsoas muscle and the iliopectineal eminence or “pelvic brim (Figure 2).
Stress fractures typically occur in athletes as the structural demands from training exceed bone remodeling (fatigue fractures), and may also occur in the setting of osteoporosis under normal physiologic loads (insufficiency fractures).
Labral tears have recently been recognized in younger athletic patients with unexplained hip joint pain and normal radiographic findings.7
FIGURE 1
Evaluating anterior hip pain
FIGURE 2
Hip joint
Anatomy of the hip joint and surrounding musculature.
Lateral hip pain
Lateral hip pain (Figure 3) is usually associated with greater trochanteric pain syndrome, iliotibial band syndrome, or meralgia paresthetica.
Greater trochanteric pain syndrome is a relatively new term that includes greater trochanteric bursitis and gluteus medius pathology.8,9 Trochanteric bursitis is a common cause of lateral hip pain, especially in older patients. However, a magnetic resonance imaging (MRI) study of 24 women with greater trochanteric pain syndrome (described as chronic pain and tenderness over the lateral aspect of the hip) found that 45.8% had a gluteus medius tear and 62.5% had gluteus medius tendonitis, calling into question how many of these patients actually have bursitis (LOE=4).9
Iliotibial band syndrome is particularly common in athletes. It is caused by repetitive movement of the iliotibial band over the greater trochanter.
Meralgia paresthetica, an entrapment syndrome of the lateral femoral cutaneous nerve, is another cause of lateral hip pain that occurs more frequently in middle age. Meralgia paresthetica is characterized by hyperesthesia in the anterolateral thigh, although 23% of patients with this disorder also complain of lateral hip pain.10
FIGURE 3
Evaluating lateral hip pain
Posterior hip pain
Posterior hip pain (Figure 4) is the least common pain pattern, and it usually suggests a source outside the hip joint. Posterior pain is typically referred from such disorders of the lumbar spine as degenerative disc disease, facet arthropathy, and spinal stenosis. Posterior hip pain is also caused by disorders of the sacroiliac joint, hip extensor and external rotator muscles, or, rarely, aortoiliac vascular occlusive disease.
The family physician in a typical practice can expect to see a patient with hip pain every 1 to 2 weeks, given that this complaint accounts for 0.61% of all visits to family practitioners, or about 1 in every 164 encounters.1 However, few studies shed light on the prevalence of hip disorders, and no clear consensus exists on this matter or even on terminology. Most information about causes of hip pain is drawn from expert opinion in a range of disciplines, including orthopedics, sports medicine, rheumatology, and family medicine.
Runners report an average yearly hip or pelvic injury rate of 2% to 11%.2 In the third National Health and Nutrition Examination Survey (NHANES III), 14.3% of patients aged 60 years and older reported significant hip pain on most days over the previous 6 weeks.3 Older women were more likely to report hip pain than older men. NHANES III also reported that 18.4% of those who had not participated in leisure time physical activity during the previous month reported severe hip pain as opposed to 12.6% of those who did engage in physical activity.
In younger patients, sports injuries about the hip and pelvis are most common in ballet dancers, soccer players, and runners (incidence of 44%, 13%, and 11% respectively).4
FIGURE 4
Evaluating posterior hip pain
Integrating history and physical examination
Little research has been performed to clarify the sensitivity and specificity of most history and physical examination maneuvers used in the diagnosis of hip pain. Therefore, much of the evaluation of hip pain is based on level 5 evidence: expert opinion.
The American Academy of Orthopaedic Surgeons created a clinical guideline on the evaluation of hip pain.11 Although a useful resource, this guideline focuses primarily on 3 diagnoses—osteoarthritis, inflammatory arthritis, and avascular necrosis—and does not expand upon the many other causes of hip pain that present to a primary care physician. Based on the available literature as well as our experience, we recommend the following approach to a patient with hip pain.
Medical history
After identifying whether the pain is anterior, lateral, or posterior (Figure 1, (Figure 3), and (Figure 4), focus on other characteristics of the pain—sudden vs insidious onset, movements and positions that reproduce the pain, predisposing activities, and the effect of ambulation or weight-bearing activity on the pain (Table 1).
In general, osteoarthritis and trochanteric bursitis are more common in older, less active patients, whereas stress fractures, iliopsoas strain or bursitis, and iliotibial band syndrome are more common in athletes. Complaints of a “snapping sensation may indicate iliopsoas bursitis if the snapping is anterior, or iliotibial band syndrome if the snapping is lateral.
Warning signs for other conditions. With any adult who has acute hip pain, be alert for “red flags that may indicate a more serious medical condition as the source of pain. Fever, malaise, night sweats, weight loss, night pain, intravenous drug abuse, a history of cancer, or known immunocompromised state should prompt you to consider such conditions as tumor, infection (ie, septic arthritis or osteomyelitis), or an inflammatory arthritis. Consider appropriate laboratory studies such as a complete blood count, erythrocyte sedimentation rate or C-reactive protein; and expedited imaging, diagnostic arthrocentesis, or referral. Fractures must also be excluded if there is a history of significant trauma, fall, or motor vehicle accident.
TABLE 1
Integrating the history and physical examination to diagnose hip pain
| Disorder | Presentation and exam findings | |
|---|---|---|
| Anterior pain | Osteoarthritis | Gradual onset anterior thigh/groin pain worsening with weight-bearing |
| Limited range of motion with pain, especially internal rotation (LOE=1b)12 | ||
| Abnormal FABER test | ||
| Hip flexor muscle strain/tendonitis | History of overuse or sports injury | |
| Pain with resisted muscle testing | ||
| Tenderness over specific muscle or tendon | ||
| Iliopsoas bursitis | Anterior pain and associated snapping sensation | |
| Tenderness with deep palpation over femoral triangle | ||
| Positive snapping hip maneuver | ||
| Etiology from overuse, acute trauma, or rheumatoid arthritis | ||
| Hip fracture (proximal femur) | Fall or trauma followed by inability to walk | |
| Limb externally rotated, abducted, and shortened | ||
| Pain with any movement | ||
| Stress fracture | History of overuse or osteoporosis | |
| Pain with weight-bearing activity; antalgic gait | ||
| Limited range of motion, sensitivity 87% (LOE=4)13 | ||
| Inflammatory arthritis | Morning stiffness or associated systemic symptoms | |
| Previous history of inflammatory arthritis | ||
| Limited range of motion and pain with passive motion | ||
| Acetabular labral tear | Activity-related sharp groin/anterior thigh pain, esp. upon hip extension | |
| Deep clicking felt, sensitivity 89% (LOE=4)14 | ||
| Positive Thomas flexion-extension test | ||
| Avascular necrosis of femoral head | Dull ache in groin, thigh, and buttock usually with risk factors (corticosteroid exposure, alcohol abuse) | |
| Limited range of movement with pain | ||
| Lateral pain | Greater trochanteric bursitis | Female:male 4:1, fourth to sixth decade |
| Spontaneous, insidious onset lateral hip pain | ||
| Point tenderness over greater trochanter | ||
| Gluteus medius muscle dysfunction | Pain with resisted hip abduction | |
| Tender over gluteus medius (cephalad to greater trochanter) | ||
| Trendelenburg test: sensitivity 72.7%, specificity 76.9% for detecting gluteus medius muscle tear (LOE=2b)9 | ||
| Iliotibial band syndrome | Lateral hip pain or snapping associated with walking, jogging, or cycling | |
| Positive Ober's test | ||
| Meralgia paresthetica | Numbness, tingling, and burning pain over anterolateral thigh | |
| Aggravated by extension of hip and with walking | ||
| Pressure over nerve may reproduce dysesthesia in distribution of lateral femoral cutaneous nerve (LOE=5)15 | ||
| Posterior pain | Referred pain from lumbar spine | History of low back pain |
| Pain reproduced with isolated lumbar flexion or extension | ||
| Radicular symptoms or history consistent with spinal stenosis | ||
| Sacroiliac joint dysfunction | Controversial diagnosis | |
| Posterior hip or buttocks pain usually in runners | ||
| Pelvic asymmetry found on exam | ||
| Hip extensor or rotator muscle strain | History of overuse or acute injury | |
| Pain with resisted muscle testing | ||
| Tender over gluteal muscles | ||
| LOE, level of evidence. For an explanation of levels of evidence. | ||
Physical examination
Begin your examination by observing the patient's gait and general ability to move around the examining room.
Range of motion. Carefully assess range of motion of the hip, comparing the affected side with the normal side to detect subtle limitations or painful movements. Range of motion testing includes passive hip flexion, internal and external rotation, and the flexion, abduction, and external rotation (FABER) test (Figure 5).
In the FABER test, the patient lies supine; the affected leg is flexed, abducted, and externally rotated. Lower the leg toward the table. A positive test elicits anterior or posterior pain and indicates hip or sacroiliac joint involvement.
The most predictive finding for osteoarthritis is decreased range of motion with restriction in internal rotation (LOE=1b).12 For those patients with one plane of restricted movement, the sensitivity for osteoarthritis is 100% and specificity is 42%; in 3 planes of restricted movement, sensitivity is 54% and specificity is 88% with a likelihood ratio of 4.4.12 A positive FABER test has been shown to be 88% sensitive for intra-articular pathology in an athletic population.16
Muscle testing. Test muscle strength to assess whether particular muscle groups are the source of pain. Maneuvers include resisted hip flexion, adduction, abduction, external rotation, and extension.
Other tests. With lateral hip pain, findings of weakness or pain while testing hip abduction may point to gluteus medius muscle dysfunction associated with greater trochanteric pain syndrome. The Trendelenburg test may also help. The patient stands on the affected leg. A negative test result occurs when the pelvis rises on the opposite side. A positive test result occurs when the pelvis on the opposite side drops and indicates a weak or painful gluteus medius muscle.
With Ober's test, the patient lies on his or her side with hips and knees flexed. The upper leg is passively extended then lowered to the table. Lateral hip pain or considerable tightness may indicate iliotibial band syndrome.
With the Thomas test, the contralateral hip is flexed, and the symptomatic hip is moved from full flexion to full extension. A deep click palpated may be indicative of a labral tear.
The snapping hip maneuver (Figure 6) may also be helpful in diagnosing the cause of pain. Loss of sensation to the anterolateral thigh is consistent with meralgia paresthetica.
Palpation. Finally, palpate over specific structures, such as the hip flexor muscles, greater trochanter, iliotibial band, and gluteus medius muscle, to further localize the source of pain. For instance, tenderness may be present over the anterior soft tissues in a hip flexor muscle strain or iliopsoas bursitis, and over the greater trochanter in trochanteric bursitis.
FIGURE 5
FABER test
FIGURE 6
Snapping hip maneuver
When diagnostic imaging is beneficial
In most cases, a thorough history and physical examination are adequate to establish a diagnosis. In the Lamberts study,5 only 16% of hip complaints required imaging for further elucidation. (Table 2) summarizes use of imaging studies with different disorders.
X-ray studies
Patients with a history of traumatic injury, osteoporosis, cancer, high-dose corticosteroid exposure, or alcohol abuse are at higher risk of such bony hip pathology as fracture, osteoarthritis, or avascular necrosis. These patients should undergo x-ray studies during their initial evaluation. An anteroposterior pelvic radiograph and a lateral radiograph of the hip are appropriate.
Although no specific patient age has been identified as a threshold for ordering x-ray studies, we recommend that all patients older than 65 years with new-onset hip pain undergo such studies.
We also recommend x-ray films for a patient of any age who has chronic severe hip pain.
TABLE 2
Indications for diagnostic imaging studies
| Disorder | Test | Level of evidence |
|---|---|---|
| Osteoarthritis | AP and lateral hip x-ray studies—weight-bearing17 | 2 |
| Muscle strain/tendonitis | None needed initially; consider MRI if not resolving | 5 |
| Greater trochanteric pain syndrome | None needed initially; consider MRI if not resolving9 | 4 |
| Hip fracture (proximal femur) | AP pelvis and cross table lateral x-ray studies | * |
| Stress fracture | MRI—sensitivity 100%13 | 4 |
| Iliopsoas bursitis | None needed initially; consider MRI if not resolving Can also use iliopsoas bursa imaging18-20 | 4 |
| Iliotibial band syndrome | None needed initially; consider MRI if not resolving | 5 |
| Meralgia paresthetica | Usually diagnosed by history. Can use sensory nerve conduction study21 | 4 |
| Inflammatory arthritis | Complete blood count, erythrocyte sedimentation rate or C-reactive protein, arthrocentesis, x-ray study | * |
| Referred pain from lumbar spine | MRI of lumbar spine | * |
| Avascular necrosis of femoral head | AP and lateral hip x-rays MRI for staging22 | 4 |
| Acetabular labral tear | MR arthrography—sensitivity 91%, specificity 71%23 25 | 4 |
| *Level of evidence not reported as specific references could not be found. | ||
| AP, anteroposterior; MRI, magnetic resonance imaging | ||
Magnetic resonance imaging
Advanced imaging may be required when initial conservative therapy is not effective or x-ray findings are unrevealing. Although computed tomography (CT) scan and bone scan have roles in the evaluation of some hip disorders, MRI has emerged as the study of choice in diagnosing hip pathology, especially in athletes.13
MRI offers valuable information regarding occult bony and cartilage injury such as stress fractures, avascular necrosis, and osteoarthritis, as well as soft tissue abnormalities such as muscle tears and bursitis. In a retrospective study of patients with suspected hip fracture but negative plain film results, MRI showed occult femoral fractures in 37% of patients, occult pelvic fractures in 23%, and associated soft-tissue abnormalities such as muscle edema and hematoma or joint effusion in 74%.26
Other imaging tests
In cases of suspected labral or intra-articular pathology, MR arthrography, anesthetic intraarticular injection and examination under local anesthesia, or diagnostic arthroscopy may be needed.16 These are relatively new techniques that help diagnose disorders not previously recognized.
Treatment
Depending on the presumed cause of pain, treatment options include activity modification, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, corticosteroid injections, physical therapy, and, if necessary, walking support.
Osteoarthritis. When symptoms persist despite conservative treatment for osteoarthritis, fluoroscopically guided intra-articular injection of a corticosteroid—or, more recently, viscosupplementation with hyaluronic acid preparations—may be useful in decreasing pain, and delaying or possibly avoiding hip arthroplasty (LOE=4).27-29
Greater trochanteric bursitis. Corticosteroid injection is also helpful and easily performed by a family physician for treatment of greater trochanteric bursitis, with 77% of patients improving in 1 week, and 61% with sustained improvement at 26 weeks (LOE=4).30
Iliopsoas bursitis. This disorder has been shown to respond to a physical therapy program emphasizing hip rotation strengthening (LOE=4).31 However, recalcitrant cases may require intrabursal injection or surgical lengthening of the iliopsoas muscle (LOE=4).32,33
Meralgia paresthetica. This condition may respond to an injection of corticosteroid adjacent to the anterior superior iliac spine near the emergence of the lateral femoral cutaneous nerve.10 In cases of suspected sacroiliac joint dysfunction, manipulative therapy was shown to provide short-term improvement.34
When To Refer
When hip pain is refractory to conventional treatment, consider referral to a specialist, such as a sports medicine specialist, physiatrist, rheumatologist, or orthopedic surgeon.
1. National Ambulatory Medical Care Survey. Hyattsville, Md: National Center for Health Statistics; 1995. CHS CD-ROM series 13, no. 11. Issued July 1997.
2. van Mechelen W. Running injuries. A review of the epidemiological literature. Sports Med 1992;14:320-335.
3. Christmas C, Crespo CJ, Franckowiak SC, Bathon JM, Bartlett SJ, Andersen RE. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract 2002;51:345-348.
4. Scopp JM, Moorman CT. The assessment of athletic hip injury. Clin Sports Med 2001;20:647-659.
5. Lamberts H, Brouwer HJ, Marinus AFM, Hofmans-Okkes IM. The use of ICPC in the Transition project.Episode-oriented epidemiology in general practice. In: Lamberts H, Wood M, Hofmans-Okkes IM, eds. International Classification of Primary Care in the European Community. Oxford: Oxford University Press; 1993;45-93.
6. Birrell F, Croft P, Cooper C, Hosie G, Macfarlane GJ, Silman A. Radiographic change is common in new presenters in primary care with hip pain. Rheumatology (Oxford) 2000;39:772-775.
7. Hickman JM, Peters CL. Hip pain in the young adult: diagnosis and treatment of disorders of the acetabular labrum and acetabular dysplasia. Am J Orthop 2001;30:459-467.
8. Shbeeb MI, Matteson EL. Trochanteric bursitis (greater trochanter pain syndrome). Mayo Clin Proc 1996;71:565-569.
9. Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum 2001;44:2138-2145.
10. Jones RK. Meralgia paresthetica as a cause of leg discomfort. Can Med Assoc J 1974;111:541-542.
11. Individual Clinical Guidelines: Hip Pain (non-traumatic) Phase 1. Version 1.0. Rosemont, Ill: Department of Research and Scientific Affairs, American Academy of Orthopaedic Surgeons; 1996.
12. Birrell F, Croft P, Cooper C, Hosie G, Macfarlane G, Silman A; PCR Hip Study Group. Predicting radiographic hip osteoarthritis from range of movement. Rheumatology (Oxford) 2001;40:506-512.
13. Shin AY, Morin WD, Gorman JD, Jones SB, Lapinsky AS. The superiority of magnetic resonance imaging in differentiating the cause of hip pain in endurance athletes. Am J Sports Med 1996;24:168-176.
14. McCarthy JC, Busconi B. The role of hip arthroscopy in the diagnosis and treatment of hip disease. Orthopedics 1995;18:753-756.
15. Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg 2001;9:336-344.
16. Mitchell B, McCrory P, Brukner P, O'Donnell J, Colson E, Howells R. Hip joint pathology: clinical presentation and correlation between magnetic resonance arthrography, ultrasound, and arthroscopic findings in 25 consecutive cases. Clin J Sport Med 2003;13:152-156.
17. Croft P, Cooper C, Coggon D. Case definition of hip osteoarthritis in epidemiologic studies. J Rheumatol 1994;21:591-592.
18. Harper MC, Schaberg JE, Allen WC. Primary iliopsoas bursography in the diagnosis of disorders of the hip. Clin Orthop 1987;221:238-241.
19. Vaccaro JP, Sauser DD, Beals RK. Iliopsoas bursa imaging: efficacy in depicting abnormal iliopsoas tendon motion in patients with internal snapping hip syndrome. Radiology 1995;197:853-856.
20. Janzen DL, Partridge E, Logan PM, Connell DG, Duncan CP. The snapping hip: clinical and imaging findings in transient subluxation of the iliopsoas tendon. Can Assoc Radiol J 1996;47:202-208.
21. Seror P. Lateral femoral cutaneous nerve conduction v MRI may be required when conservative therapy is not effective or x-rays are unrevealing somatosensory evoked potentials for electrodiagnosis of meralgia paresthetica. Am J Phys Med Rehabil 1999;78:313-316.
22. Mitchell DG, Rao VM, Dalinka MK, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology 1987;162:709-715.
23. Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology 1996;200:225-230.
24. Czerny C, Hofmann S, Urban M, et al. MR arthrography of the adult acetabular capsular-labral complex: correlation with surgery and anatomy. AJR Am J Roentengol 1999;173:345-349.
25. Petersilge DA, Haque MA, Petersilge WJ, Lewin JS, Lieberman JM, Buly R. Acetabular labral tears: evaluation with MR arthrography. Radiology 1996;200:231-235.
26. Bogost GA, Lizerbram EK, Crues JV. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology 1995;197:263-267.
27. Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol 1999;11:417-421.
28. Migliore A, Martin LS, Alimonti A, Valente C, Tormenta S. Efficacy and safety of viscosupplementation by ultrasound-guided intra-articular injection in osteoarthrits of the hip. Osteoarthritis Cartilage 2003;11:305-306.
29. Brocq O, Tran G, Breuil V, Grisot C, Flory P, Euller-Ziegler L. Hip osteoarthritis: short-term efficacy and safety of viscosupplementation by hylan G-F 20. An open-label study in 22 patients. Joint Bone Spine 2002;69:388-391.
30. Shbeeb MI, O'Duffy JD, Michet CJ, O'Fallon WM, Matteson EL. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol 1996;23:2104-2106.
31. Johnston CA, Lindsay DM, Wiley JP. Treatment of iliopsoas syndrome with a hip rotation strengthening program: a retrospective case series. J Orthop Sports Phys Ther 1999;29:218-224.
32. Johnston CA, Wiley JP, Lindsay DM, Wiseman DA. Iliopsoas bursitis and tendonitis. A review. Sports Med 1998;25:271-283.
33. Gruen GS, Scioscia TN, Lowenstein JE. The surgical treatment of internal snapping hip. Am J Sports Med 2002;30:607-613.
34. Cibulka MT, Delitto A. A comparison of two different methods to treat hip pain in runners. J Orthop Sports Phys Ther 1993;17:172-176.
- Start by determining whether pain is located in the anterior, lateral, or posterior hip. As the site varies, so does the etiology.
- Besides location, consider sudden vs insidious onset, motions and positions that reproduce pain, predisposing activities, and effect of ambulation or weight bearing.
- Physical examination tests that elucidate range of motion, muscle strength, and pain replication will narrow the diagnostic search.
- Magnetic resonance imaging is usually diagnostic if plain x-rays and conservative therapy are ineffective.
- Conservative measures and selective use of injection therapy are usually effective.
Given the number of disorders capable of causing hip pain, and the fact that hip pathology can refer pain to other areas, and pathology elsewhere (particularly the lumbar spine) can refer pain to the hip,* a useful starting point in the evaluation is one that begins to narrow the search immediately.
*Medial groin pain is often included in the discussion of hip pain, but this topic is beyond the scope of this review.
In the work-up of hip pain, the first fact to establish is whether pain is felt in the anterior, lateral, or posterior part of the hip. Each location suggests a distinctive set of possible underlying causes. We provide diagnostic algorithms for all 3 scenarios, to aid in determining the best course for the work-up.
Anterior hip pain
Anterior hip pain (Figure 1), which is the most common, usually indicates pathology of the hip joint (ie, degenerative arthritis), hip flexor muscle strains or tendonitis, and iliopsoas bursitis. In a study by Lamberts and colleagues,5 by far the most common diagnosis of patients with hip complaints seen by their general practitioner was osteoarthritis. In a study of subjects older than 40 years who experienced a new episode of hip pain, 44% had evidence of osteoarthritis (level of evidence [LOE]=1b).6
Iliopsoas bursitis, a less common cause of anterior hip pain, involves inflammation of the bursa between the iliopsoas muscle and the iliopectineal eminence or “pelvic brim (Figure 2).
Stress fractures typically occur in athletes as the structural demands from training exceed bone remodeling (fatigue fractures), and may also occur in the setting of osteoporosis under normal physiologic loads (insufficiency fractures).
Labral tears have recently been recognized in younger athletic patients with unexplained hip joint pain and normal radiographic findings.7
FIGURE 1
Evaluating anterior hip pain
FIGURE 2
Hip joint
Anatomy of the hip joint and surrounding musculature.
Lateral hip pain
Lateral hip pain (Figure 3) is usually associated with greater trochanteric pain syndrome, iliotibial band syndrome, or meralgia paresthetica.
Greater trochanteric pain syndrome is a relatively new term that includes greater trochanteric bursitis and gluteus medius pathology.8,9 Trochanteric bursitis is a common cause of lateral hip pain, especially in older patients. However, a magnetic resonance imaging (MRI) study of 24 women with greater trochanteric pain syndrome (described as chronic pain and tenderness over the lateral aspect of the hip) found that 45.8% had a gluteus medius tear and 62.5% had gluteus medius tendonitis, calling into question how many of these patients actually have bursitis (LOE=4).9
Iliotibial band syndrome is particularly common in athletes. It is caused by repetitive movement of the iliotibial band over the greater trochanter.
Meralgia paresthetica, an entrapment syndrome of the lateral femoral cutaneous nerve, is another cause of lateral hip pain that occurs more frequently in middle age. Meralgia paresthetica is characterized by hyperesthesia in the anterolateral thigh, although 23% of patients with this disorder also complain of lateral hip pain.10
FIGURE 3
Evaluating lateral hip pain
Posterior hip pain
Posterior hip pain (Figure 4) is the least common pain pattern, and it usually suggests a source outside the hip joint. Posterior pain is typically referred from such disorders of the lumbar spine as degenerative disc disease, facet arthropathy, and spinal stenosis. Posterior hip pain is also caused by disorders of the sacroiliac joint, hip extensor and external rotator muscles, or, rarely, aortoiliac vascular occlusive disease.
The family physician in a typical practice can expect to see a patient with hip pain every 1 to 2 weeks, given that this complaint accounts for 0.61% of all visits to family practitioners, or about 1 in every 164 encounters.1 However, few studies shed light on the prevalence of hip disorders, and no clear consensus exists on this matter or even on terminology. Most information about causes of hip pain is drawn from expert opinion in a range of disciplines, including orthopedics, sports medicine, rheumatology, and family medicine.
Runners report an average yearly hip or pelvic injury rate of 2% to 11%.2 In the third National Health and Nutrition Examination Survey (NHANES III), 14.3% of patients aged 60 years and older reported significant hip pain on most days over the previous 6 weeks.3 Older women were more likely to report hip pain than older men. NHANES III also reported that 18.4% of those who had not participated in leisure time physical activity during the previous month reported severe hip pain as opposed to 12.6% of those who did engage in physical activity.
In younger patients, sports injuries about the hip and pelvis are most common in ballet dancers, soccer players, and runners (incidence of 44%, 13%, and 11% respectively).4
FIGURE 4
Evaluating posterior hip pain
Integrating history and physical examination
Little research has been performed to clarify the sensitivity and specificity of most history and physical examination maneuvers used in the diagnosis of hip pain. Therefore, much of the evaluation of hip pain is based on level 5 evidence: expert opinion.
The American Academy of Orthopaedic Surgeons created a clinical guideline on the evaluation of hip pain.11 Although a useful resource, this guideline focuses primarily on 3 diagnoses—osteoarthritis, inflammatory arthritis, and avascular necrosis—and does not expand upon the many other causes of hip pain that present to a primary care physician. Based on the available literature as well as our experience, we recommend the following approach to a patient with hip pain.
Medical history
After identifying whether the pain is anterior, lateral, or posterior (Figure 1, (Figure 3), and (Figure 4), focus on other characteristics of the pain—sudden vs insidious onset, movements and positions that reproduce the pain, predisposing activities, and the effect of ambulation or weight-bearing activity on the pain (Table 1).
In general, osteoarthritis and trochanteric bursitis are more common in older, less active patients, whereas stress fractures, iliopsoas strain or bursitis, and iliotibial band syndrome are more common in athletes. Complaints of a “snapping sensation may indicate iliopsoas bursitis if the snapping is anterior, or iliotibial band syndrome if the snapping is lateral.
Warning signs for other conditions. With any adult who has acute hip pain, be alert for “red flags that may indicate a more serious medical condition as the source of pain. Fever, malaise, night sweats, weight loss, night pain, intravenous drug abuse, a history of cancer, or known immunocompromised state should prompt you to consider such conditions as tumor, infection (ie, septic arthritis or osteomyelitis), or an inflammatory arthritis. Consider appropriate laboratory studies such as a complete blood count, erythrocyte sedimentation rate or C-reactive protein; and expedited imaging, diagnostic arthrocentesis, or referral. Fractures must also be excluded if there is a history of significant trauma, fall, or motor vehicle accident.
TABLE 1
Integrating the history and physical examination to diagnose hip pain
| Disorder | Presentation and exam findings | |
|---|---|---|
| Anterior pain | Osteoarthritis | Gradual onset anterior thigh/groin pain worsening with weight-bearing |
| Limited range of motion with pain, especially internal rotation (LOE=1b)12 | ||
| Abnormal FABER test | ||
| Hip flexor muscle strain/tendonitis | History of overuse or sports injury | |
| Pain with resisted muscle testing | ||
| Tenderness over specific muscle or tendon | ||
| Iliopsoas bursitis | Anterior pain and associated snapping sensation | |
| Tenderness with deep palpation over femoral triangle | ||
| Positive snapping hip maneuver | ||
| Etiology from overuse, acute trauma, or rheumatoid arthritis | ||
| Hip fracture (proximal femur) | Fall or trauma followed by inability to walk | |
| Limb externally rotated, abducted, and shortened | ||
| Pain with any movement | ||
| Stress fracture | History of overuse or osteoporosis | |
| Pain with weight-bearing activity; antalgic gait | ||
| Limited range of motion, sensitivity 87% (LOE=4)13 | ||
| Inflammatory arthritis | Morning stiffness or associated systemic symptoms | |
| Previous history of inflammatory arthritis | ||
| Limited range of motion and pain with passive motion | ||
| Acetabular labral tear | Activity-related sharp groin/anterior thigh pain, esp. upon hip extension | |
| Deep clicking felt, sensitivity 89% (LOE=4)14 | ||
| Positive Thomas flexion-extension test | ||
| Avascular necrosis of femoral head | Dull ache in groin, thigh, and buttock usually with risk factors (corticosteroid exposure, alcohol abuse) | |
| Limited range of movement with pain | ||
| Lateral pain | Greater trochanteric bursitis | Female:male 4:1, fourth to sixth decade |
| Spontaneous, insidious onset lateral hip pain | ||
| Point tenderness over greater trochanter | ||
| Gluteus medius muscle dysfunction | Pain with resisted hip abduction | |
| Tender over gluteus medius (cephalad to greater trochanter) | ||
| Trendelenburg test: sensitivity 72.7%, specificity 76.9% for detecting gluteus medius muscle tear (LOE=2b)9 | ||
| Iliotibial band syndrome | Lateral hip pain or snapping associated with walking, jogging, or cycling | |
| Positive Ober's test | ||
| Meralgia paresthetica | Numbness, tingling, and burning pain over anterolateral thigh | |
| Aggravated by extension of hip and with walking | ||
| Pressure over nerve may reproduce dysesthesia in distribution of lateral femoral cutaneous nerve (LOE=5)15 | ||
| Posterior pain | Referred pain from lumbar spine | History of low back pain |
| Pain reproduced with isolated lumbar flexion or extension | ||
| Radicular symptoms or history consistent with spinal stenosis | ||
| Sacroiliac joint dysfunction | Controversial diagnosis | |
| Posterior hip or buttocks pain usually in runners | ||
| Pelvic asymmetry found on exam | ||
| Hip extensor or rotator muscle strain | History of overuse or acute injury | |
| Pain with resisted muscle testing | ||
| Tender over gluteal muscles | ||
| LOE, level of evidence. For an explanation of levels of evidence. | ||
Physical examination
Begin your examination by observing the patient's gait and general ability to move around the examining room.
Range of motion. Carefully assess range of motion of the hip, comparing the affected side with the normal side to detect subtle limitations or painful movements. Range of motion testing includes passive hip flexion, internal and external rotation, and the flexion, abduction, and external rotation (FABER) test (Figure 5).
In the FABER test, the patient lies supine; the affected leg is flexed, abducted, and externally rotated. Lower the leg toward the table. A positive test elicits anterior or posterior pain and indicates hip or sacroiliac joint involvement.
The most predictive finding for osteoarthritis is decreased range of motion with restriction in internal rotation (LOE=1b).12 For those patients with one plane of restricted movement, the sensitivity for osteoarthritis is 100% and specificity is 42%; in 3 planes of restricted movement, sensitivity is 54% and specificity is 88% with a likelihood ratio of 4.4.12 A positive FABER test has been shown to be 88% sensitive for intra-articular pathology in an athletic population.16
Muscle testing. Test muscle strength to assess whether particular muscle groups are the source of pain. Maneuvers include resisted hip flexion, adduction, abduction, external rotation, and extension.
Other tests. With lateral hip pain, findings of weakness or pain while testing hip abduction may point to gluteus medius muscle dysfunction associated with greater trochanteric pain syndrome. The Trendelenburg test may also help. The patient stands on the affected leg. A negative test result occurs when the pelvis rises on the opposite side. A positive test result occurs when the pelvis on the opposite side drops and indicates a weak or painful gluteus medius muscle.
With Ober's test, the patient lies on his or her side with hips and knees flexed. The upper leg is passively extended then lowered to the table. Lateral hip pain or considerable tightness may indicate iliotibial band syndrome.
With the Thomas test, the contralateral hip is flexed, and the symptomatic hip is moved from full flexion to full extension. A deep click palpated may be indicative of a labral tear.
The snapping hip maneuver (Figure 6) may also be helpful in diagnosing the cause of pain. Loss of sensation to the anterolateral thigh is consistent with meralgia paresthetica.
Palpation. Finally, palpate over specific structures, such as the hip flexor muscles, greater trochanter, iliotibial band, and gluteus medius muscle, to further localize the source of pain. For instance, tenderness may be present over the anterior soft tissues in a hip flexor muscle strain or iliopsoas bursitis, and over the greater trochanter in trochanteric bursitis.
FIGURE 5
FABER test
FIGURE 6
Snapping hip maneuver
When diagnostic imaging is beneficial
In most cases, a thorough history and physical examination are adequate to establish a diagnosis. In the Lamberts study,5 only 16% of hip complaints required imaging for further elucidation. (Table 2) summarizes use of imaging studies with different disorders.
X-ray studies
Patients with a history of traumatic injury, osteoporosis, cancer, high-dose corticosteroid exposure, or alcohol abuse are at higher risk of such bony hip pathology as fracture, osteoarthritis, or avascular necrosis. These patients should undergo x-ray studies during their initial evaluation. An anteroposterior pelvic radiograph and a lateral radiograph of the hip are appropriate.
Although no specific patient age has been identified as a threshold for ordering x-ray studies, we recommend that all patients older than 65 years with new-onset hip pain undergo such studies.
We also recommend x-ray films for a patient of any age who has chronic severe hip pain.
TABLE 2
Indications for diagnostic imaging studies
| Disorder | Test | Level of evidence |
|---|---|---|
| Osteoarthritis | AP and lateral hip x-ray studies—weight-bearing17 | 2 |
| Muscle strain/tendonitis | None needed initially; consider MRI if not resolving | 5 |
| Greater trochanteric pain syndrome | None needed initially; consider MRI if not resolving9 | 4 |
| Hip fracture (proximal femur) | AP pelvis and cross table lateral x-ray studies | * |
| Stress fracture | MRI—sensitivity 100%13 | 4 |
| Iliopsoas bursitis | None needed initially; consider MRI if not resolving Can also use iliopsoas bursa imaging18-20 | 4 |
| Iliotibial band syndrome | None needed initially; consider MRI if not resolving | 5 |
| Meralgia paresthetica | Usually diagnosed by history. Can use sensory nerve conduction study21 | 4 |
| Inflammatory arthritis | Complete blood count, erythrocyte sedimentation rate or C-reactive protein, arthrocentesis, x-ray study | * |
| Referred pain from lumbar spine | MRI of lumbar spine | * |
| Avascular necrosis of femoral head | AP and lateral hip x-rays MRI for staging22 | 4 |
| Acetabular labral tear | MR arthrography—sensitivity 91%, specificity 71%23 25 | 4 |
| *Level of evidence not reported as specific references could not be found. | ||
| AP, anteroposterior; MRI, magnetic resonance imaging | ||
Magnetic resonance imaging
Advanced imaging may be required when initial conservative therapy is not effective or x-ray findings are unrevealing. Although computed tomography (CT) scan and bone scan have roles in the evaluation of some hip disorders, MRI has emerged as the study of choice in diagnosing hip pathology, especially in athletes.13
MRI offers valuable information regarding occult bony and cartilage injury such as stress fractures, avascular necrosis, and osteoarthritis, as well as soft tissue abnormalities such as muscle tears and bursitis. In a retrospective study of patients with suspected hip fracture but negative plain film results, MRI showed occult femoral fractures in 37% of patients, occult pelvic fractures in 23%, and associated soft-tissue abnormalities such as muscle edema and hematoma or joint effusion in 74%.26
Other imaging tests
In cases of suspected labral or intra-articular pathology, MR arthrography, anesthetic intraarticular injection and examination under local anesthesia, or diagnostic arthroscopy may be needed.16 These are relatively new techniques that help diagnose disorders not previously recognized.
Treatment
Depending on the presumed cause of pain, treatment options include activity modification, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, corticosteroid injections, physical therapy, and, if necessary, walking support.
Osteoarthritis. When symptoms persist despite conservative treatment for osteoarthritis, fluoroscopically guided intra-articular injection of a corticosteroid—or, more recently, viscosupplementation with hyaluronic acid preparations—may be useful in decreasing pain, and delaying or possibly avoiding hip arthroplasty (LOE=4).27-29
Greater trochanteric bursitis. Corticosteroid injection is also helpful and easily performed by a family physician for treatment of greater trochanteric bursitis, with 77% of patients improving in 1 week, and 61% with sustained improvement at 26 weeks (LOE=4).30
Iliopsoas bursitis. This disorder has been shown to respond to a physical therapy program emphasizing hip rotation strengthening (LOE=4).31 However, recalcitrant cases may require intrabursal injection or surgical lengthening of the iliopsoas muscle (LOE=4).32,33
Meralgia paresthetica. This condition may respond to an injection of corticosteroid adjacent to the anterior superior iliac spine near the emergence of the lateral femoral cutaneous nerve.10 In cases of suspected sacroiliac joint dysfunction, manipulative therapy was shown to provide short-term improvement.34
When To Refer
When hip pain is refractory to conventional treatment, consider referral to a specialist, such as a sports medicine specialist, physiatrist, rheumatologist, or orthopedic surgeon.
- Start by determining whether pain is located in the anterior, lateral, or posterior hip. As the site varies, so does the etiology.
- Besides location, consider sudden vs insidious onset, motions and positions that reproduce pain, predisposing activities, and effect of ambulation or weight bearing.
- Physical examination tests that elucidate range of motion, muscle strength, and pain replication will narrow the diagnostic search.
- Magnetic resonance imaging is usually diagnostic if plain x-rays and conservative therapy are ineffective.
- Conservative measures and selective use of injection therapy are usually effective.
Given the number of disorders capable of causing hip pain, and the fact that hip pathology can refer pain to other areas, and pathology elsewhere (particularly the lumbar spine) can refer pain to the hip,* a useful starting point in the evaluation is one that begins to narrow the search immediately.
*Medial groin pain is often included in the discussion of hip pain, but this topic is beyond the scope of this review.
In the work-up of hip pain, the first fact to establish is whether pain is felt in the anterior, lateral, or posterior part of the hip. Each location suggests a distinctive set of possible underlying causes. We provide diagnostic algorithms for all 3 scenarios, to aid in determining the best course for the work-up.
Anterior hip pain
Anterior hip pain (Figure 1), which is the most common, usually indicates pathology of the hip joint (ie, degenerative arthritis), hip flexor muscle strains or tendonitis, and iliopsoas bursitis. In a study by Lamberts and colleagues,5 by far the most common diagnosis of patients with hip complaints seen by their general practitioner was osteoarthritis. In a study of subjects older than 40 years who experienced a new episode of hip pain, 44% had evidence of osteoarthritis (level of evidence [LOE]=1b).6
Iliopsoas bursitis, a less common cause of anterior hip pain, involves inflammation of the bursa between the iliopsoas muscle and the iliopectineal eminence or “pelvic brim (Figure 2).
Stress fractures typically occur in athletes as the structural demands from training exceed bone remodeling (fatigue fractures), and may also occur in the setting of osteoporosis under normal physiologic loads (insufficiency fractures).
Labral tears have recently been recognized in younger athletic patients with unexplained hip joint pain and normal radiographic findings.7
FIGURE 1
Evaluating anterior hip pain
FIGURE 2
Hip joint
Anatomy of the hip joint and surrounding musculature.
Lateral hip pain
Lateral hip pain (Figure 3) is usually associated with greater trochanteric pain syndrome, iliotibial band syndrome, or meralgia paresthetica.
Greater trochanteric pain syndrome is a relatively new term that includes greater trochanteric bursitis and gluteus medius pathology.8,9 Trochanteric bursitis is a common cause of lateral hip pain, especially in older patients. However, a magnetic resonance imaging (MRI) study of 24 women with greater trochanteric pain syndrome (described as chronic pain and tenderness over the lateral aspect of the hip) found that 45.8% had a gluteus medius tear and 62.5% had gluteus medius tendonitis, calling into question how many of these patients actually have bursitis (LOE=4).9
Iliotibial band syndrome is particularly common in athletes. It is caused by repetitive movement of the iliotibial band over the greater trochanter.
Meralgia paresthetica, an entrapment syndrome of the lateral femoral cutaneous nerve, is another cause of lateral hip pain that occurs more frequently in middle age. Meralgia paresthetica is characterized by hyperesthesia in the anterolateral thigh, although 23% of patients with this disorder also complain of lateral hip pain.10
FIGURE 3
Evaluating lateral hip pain
Posterior hip pain
Posterior hip pain (Figure 4) is the least common pain pattern, and it usually suggests a source outside the hip joint. Posterior pain is typically referred from such disorders of the lumbar spine as degenerative disc disease, facet arthropathy, and spinal stenosis. Posterior hip pain is also caused by disorders of the sacroiliac joint, hip extensor and external rotator muscles, or, rarely, aortoiliac vascular occlusive disease.
The family physician in a typical practice can expect to see a patient with hip pain every 1 to 2 weeks, given that this complaint accounts for 0.61% of all visits to family practitioners, or about 1 in every 164 encounters.1 However, few studies shed light on the prevalence of hip disorders, and no clear consensus exists on this matter or even on terminology. Most information about causes of hip pain is drawn from expert opinion in a range of disciplines, including orthopedics, sports medicine, rheumatology, and family medicine.
Runners report an average yearly hip or pelvic injury rate of 2% to 11%.2 In the third National Health and Nutrition Examination Survey (NHANES III), 14.3% of patients aged 60 years and older reported significant hip pain on most days over the previous 6 weeks.3 Older women were more likely to report hip pain than older men. NHANES III also reported that 18.4% of those who had not participated in leisure time physical activity during the previous month reported severe hip pain as opposed to 12.6% of those who did engage in physical activity.
In younger patients, sports injuries about the hip and pelvis are most common in ballet dancers, soccer players, and runners (incidence of 44%, 13%, and 11% respectively).4
FIGURE 4
Evaluating posterior hip pain
Integrating history and physical examination
Little research has been performed to clarify the sensitivity and specificity of most history and physical examination maneuvers used in the diagnosis of hip pain. Therefore, much of the evaluation of hip pain is based on level 5 evidence: expert opinion.
The American Academy of Orthopaedic Surgeons created a clinical guideline on the evaluation of hip pain.11 Although a useful resource, this guideline focuses primarily on 3 diagnoses—osteoarthritis, inflammatory arthritis, and avascular necrosis—and does not expand upon the many other causes of hip pain that present to a primary care physician. Based on the available literature as well as our experience, we recommend the following approach to a patient with hip pain.
Medical history
After identifying whether the pain is anterior, lateral, or posterior (Figure 1, (Figure 3), and (Figure 4), focus on other characteristics of the pain—sudden vs insidious onset, movements and positions that reproduce the pain, predisposing activities, and the effect of ambulation or weight-bearing activity on the pain (Table 1).
In general, osteoarthritis and trochanteric bursitis are more common in older, less active patients, whereas stress fractures, iliopsoas strain or bursitis, and iliotibial band syndrome are more common in athletes. Complaints of a “snapping sensation may indicate iliopsoas bursitis if the snapping is anterior, or iliotibial band syndrome if the snapping is lateral.
Warning signs for other conditions. With any adult who has acute hip pain, be alert for “red flags that may indicate a more serious medical condition as the source of pain. Fever, malaise, night sweats, weight loss, night pain, intravenous drug abuse, a history of cancer, or known immunocompromised state should prompt you to consider such conditions as tumor, infection (ie, septic arthritis or osteomyelitis), or an inflammatory arthritis. Consider appropriate laboratory studies such as a complete blood count, erythrocyte sedimentation rate or C-reactive protein; and expedited imaging, diagnostic arthrocentesis, or referral. Fractures must also be excluded if there is a history of significant trauma, fall, or motor vehicle accident.
TABLE 1
Integrating the history and physical examination to diagnose hip pain
| Disorder | Presentation and exam findings | |
|---|---|---|
| Anterior pain | Osteoarthritis | Gradual onset anterior thigh/groin pain worsening with weight-bearing |
| Limited range of motion with pain, especially internal rotation (LOE=1b)12 | ||
| Abnormal FABER test | ||
| Hip flexor muscle strain/tendonitis | History of overuse or sports injury | |
| Pain with resisted muscle testing | ||
| Tenderness over specific muscle or tendon | ||
| Iliopsoas bursitis | Anterior pain and associated snapping sensation | |
| Tenderness with deep palpation over femoral triangle | ||
| Positive snapping hip maneuver | ||
| Etiology from overuse, acute trauma, or rheumatoid arthritis | ||
| Hip fracture (proximal femur) | Fall or trauma followed by inability to walk | |
| Limb externally rotated, abducted, and shortened | ||
| Pain with any movement | ||
| Stress fracture | History of overuse or osteoporosis | |
| Pain with weight-bearing activity; antalgic gait | ||
| Limited range of motion, sensitivity 87% (LOE=4)13 | ||
| Inflammatory arthritis | Morning stiffness or associated systemic symptoms | |
| Previous history of inflammatory arthritis | ||
| Limited range of motion and pain with passive motion | ||
| Acetabular labral tear | Activity-related sharp groin/anterior thigh pain, esp. upon hip extension | |
| Deep clicking felt, sensitivity 89% (LOE=4)14 | ||
| Positive Thomas flexion-extension test | ||
| Avascular necrosis of femoral head | Dull ache in groin, thigh, and buttock usually with risk factors (corticosteroid exposure, alcohol abuse) | |
| Limited range of movement with pain | ||
| Lateral pain | Greater trochanteric bursitis | Female:male 4:1, fourth to sixth decade |
| Spontaneous, insidious onset lateral hip pain | ||
| Point tenderness over greater trochanter | ||
| Gluteus medius muscle dysfunction | Pain with resisted hip abduction | |
| Tender over gluteus medius (cephalad to greater trochanter) | ||
| Trendelenburg test: sensitivity 72.7%, specificity 76.9% for detecting gluteus medius muscle tear (LOE=2b)9 | ||
| Iliotibial band syndrome | Lateral hip pain or snapping associated with walking, jogging, or cycling | |
| Positive Ober's test | ||
| Meralgia paresthetica | Numbness, tingling, and burning pain over anterolateral thigh | |
| Aggravated by extension of hip and with walking | ||
| Pressure over nerve may reproduce dysesthesia in distribution of lateral femoral cutaneous nerve (LOE=5)15 | ||
| Posterior pain | Referred pain from lumbar spine | History of low back pain |
| Pain reproduced with isolated lumbar flexion or extension | ||
| Radicular symptoms or history consistent with spinal stenosis | ||
| Sacroiliac joint dysfunction | Controversial diagnosis | |
| Posterior hip or buttocks pain usually in runners | ||
| Pelvic asymmetry found on exam | ||
| Hip extensor or rotator muscle strain | History of overuse or acute injury | |
| Pain with resisted muscle testing | ||
| Tender over gluteal muscles | ||
| LOE, level of evidence. For an explanation of levels of evidence. | ||
Physical examination
Begin your examination by observing the patient's gait and general ability to move around the examining room.
Range of motion. Carefully assess range of motion of the hip, comparing the affected side with the normal side to detect subtle limitations or painful movements. Range of motion testing includes passive hip flexion, internal and external rotation, and the flexion, abduction, and external rotation (FABER) test (Figure 5).
In the FABER test, the patient lies supine; the affected leg is flexed, abducted, and externally rotated. Lower the leg toward the table. A positive test elicits anterior or posterior pain and indicates hip or sacroiliac joint involvement.
The most predictive finding for osteoarthritis is decreased range of motion with restriction in internal rotation (LOE=1b).12 For those patients with one plane of restricted movement, the sensitivity for osteoarthritis is 100% and specificity is 42%; in 3 planes of restricted movement, sensitivity is 54% and specificity is 88% with a likelihood ratio of 4.4.12 A positive FABER test has been shown to be 88% sensitive for intra-articular pathology in an athletic population.16
Muscle testing. Test muscle strength to assess whether particular muscle groups are the source of pain. Maneuvers include resisted hip flexion, adduction, abduction, external rotation, and extension.
Other tests. With lateral hip pain, findings of weakness or pain while testing hip abduction may point to gluteus medius muscle dysfunction associated with greater trochanteric pain syndrome. The Trendelenburg test may also help. The patient stands on the affected leg. A negative test result occurs when the pelvis rises on the opposite side. A positive test result occurs when the pelvis on the opposite side drops and indicates a weak or painful gluteus medius muscle.
With Ober's test, the patient lies on his or her side with hips and knees flexed. The upper leg is passively extended then lowered to the table. Lateral hip pain or considerable tightness may indicate iliotibial band syndrome.
With the Thomas test, the contralateral hip is flexed, and the symptomatic hip is moved from full flexion to full extension. A deep click palpated may be indicative of a labral tear.
The snapping hip maneuver (Figure 6) may also be helpful in diagnosing the cause of pain. Loss of sensation to the anterolateral thigh is consistent with meralgia paresthetica.
Palpation. Finally, palpate over specific structures, such as the hip flexor muscles, greater trochanter, iliotibial band, and gluteus medius muscle, to further localize the source of pain. For instance, tenderness may be present over the anterior soft tissues in a hip flexor muscle strain or iliopsoas bursitis, and over the greater trochanter in trochanteric bursitis.
FIGURE 5
FABER test
FIGURE 6
Snapping hip maneuver
When diagnostic imaging is beneficial
In most cases, a thorough history and physical examination are adequate to establish a diagnosis. In the Lamberts study,5 only 16% of hip complaints required imaging for further elucidation. (Table 2) summarizes use of imaging studies with different disorders.
X-ray studies
Patients with a history of traumatic injury, osteoporosis, cancer, high-dose corticosteroid exposure, or alcohol abuse are at higher risk of such bony hip pathology as fracture, osteoarthritis, or avascular necrosis. These patients should undergo x-ray studies during their initial evaluation. An anteroposterior pelvic radiograph and a lateral radiograph of the hip are appropriate.
Although no specific patient age has been identified as a threshold for ordering x-ray studies, we recommend that all patients older than 65 years with new-onset hip pain undergo such studies.
We also recommend x-ray films for a patient of any age who has chronic severe hip pain.
TABLE 2
Indications for diagnostic imaging studies
| Disorder | Test | Level of evidence |
|---|---|---|
| Osteoarthritis | AP and lateral hip x-ray studies—weight-bearing17 | 2 |
| Muscle strain/tendonitis | None needed initially; consider MRI if not resolving | 5 |
| Greater trochanteric pain syndrome | None needed initially; consider MRI if not resolving9 | 4 |
| Hip fracture (proximal femur) | AP pelvis and cross table lateral x-ray studies | * |
| Stress fracture | MRI—sensitivity 100%13 | 4 |
| Iliopsoas bursitis | None needed initially; consider MRI if not resolving Can also use iliopsoas bursa imaging18-20 | 4 |
| Iliotibial band syndrome | None needed initially; consider MRI if not resolving | 5 |
| Meralgia paresthetica | Usually diagnosed by history. Can use sensory nerve conduction study21 | 4 |
| Inflammatory arthritis | Complete blood count, erythrocyte sedimentation rate or C-reactive protein, arthrocentesis, x-ray study | * |
| Referred pain from lumbar spine | MRI of lumbar spine | * |
| Avascular necrosis of femoral head | AP and lateral hip x-rays MRI for staging22 | 4 |
| Acetabular labral tear | MR arthrography—sensitivity 91%, specificity 71%23 25 | 4 |
| *Level of evidence not reported as specific references could not be found. | ||
| AP, anteroposterior; MRI, magnetic resonance imaging | ||
Magnetic resonance imaging
Advanced imaging may be required when initial conservative therapy is not effective or x-ray findings are unrevealing. Although computed tomography (CT) scan and bone scan have roles in the evaluation of some hip disorders, MRI has emerged as the study of choice in diagnosing hip pathology, especially in athletes.13
MRI offers valuable information regarding occult bony and cartilage injury such as stress fractures, avascular necrosis, and osteoarthritis, as well as soft tissue abnormalities such as muscle tears and bursitis. In a retrospective study of patients with suspected hip fracture but negative plain film results, MRI showed occult femoral fractures in 37% of patients, occult pelvic fractures in 23%, and associated soft-tissue abnormalities such as muscle edema and hematoma or joint effusion in 74%.26
Other imaging tests
In cases of suspected labral or intra-articular pathology, MR arthrography, anesthetic intraarticular injection and examination under local anesthesia, or diagnostic arthroscopy may be needed.16 These are relatively new techniques that help diagnose disorders not previously recognized.
Treatment
Depending on the presumed cause of pain, treatment options include activity modification, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, corticosteroid injections, physical therapy, and, if necessary, walking support.
Osteoarthritis. When symptoms persist despite conservative treatment for osteoarthritis, fluoroscopically guided intra-articular injection of a corticosteroid—or, more recently, viscosupplementation with hyaluronic acid preparations—may be useful in decreasing pain, and delaying or possibly avoiding hip arthroplasty (LOE=4).27-29
Greater trochanteric bursitis. Corticosteroid injection is also helpful and easily performed by a family physician for treatment of greater trochanteric bursitis, with 77% of patients improving in 1 week, and 61% with sustained improvement at 26 weeks (LOE=4).30
Iliopsoas bursitis. This disorder has been shown to respond to a physical therapy program emphasizing hip rotation strengthening (LOE=4).31 However, recalcitrant cases may require intrabursal injection or surgical lengthening of the iliopsoas muscle (LOE=4).32,33
Meralgia paresthetica. This condition may respond to an injection of corticosteroid adjacent to the anterior superior iliac spine near the emergence of the lateral femoral cutaneous nerve.10 In cases of suspected sacroiliac joint dysfunction, manipulative therapy was shown to provide short-term improvement.34
When To Refer
When hip pain is refractory to conventional treatment, consider referral to a specialist, such as a sports medicine specialist, physiatrist, rheumatologist, or orthopedic surgeon.
1. National Ambulatory Medical Care Survey. Hyattsville, Md: National Center for Health Statistics; 1995. CHS CD-ROM series 13, no. 11. Issued July 1997.
2. van Mechelen W. Running injuries. A review of the epidemiological literature. Sports Med 1992;14:320-335.
3. Christmas C, Crespo CJ, Franckowiak SC, Bathon JM, Bartlett SJ, Andersen RE. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract 2002;51:345-348.
4. Scopp JM, Moorman CT. The assessment of athletic hip injury. Clin Sports Med 2001;20:647-659.
5. Lamberts H, Brouwer HJ, Marinus AFM, Hofmans-Okkes IM. The use of ICPC in the Transition project.Episode-oriented epidemiology in general practice. In: Lamberts H, Wood M, Hofmans-Okkes IM, eds. International Classification of Primary Care in the European Community. Oxford: Oxford University Press; 1993;45-93.
6. Birrell F, Croft P, Cooper C, Hosie G, Macfarlane GJ, Silman A. Radiographic change is common in new presenters in primary care with hip pain. Rheumatology (Oxford) 2000;39:772-775.
7. Hickman JM, Peters CL. Hip pain in the young adult: diagnosis and treatment of disorders of the acetabular labrum and acetabular dysplasia. Am J Orthop 2001;30:459-467.
8. Shbeeb MI, Matteson EL. Trochanteric bursitis (greater trochanter pain syndrome). Mayo Clin Proc 1996;71:565-569.
9. Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum 2001;44:2138-2145.
10. Jones RK. Meralgia paresthetica as a cause of leg discomfort. Can Med Assoc J 1974;111:541-542.
11. Individual Clinical Guidelines: Hip Pain (non-traumatic) Phase 1. Version 1.0. Rosemont, Ill: Department of Research and Scientific Affairs, American Academy of Orthopaedic Surgeons; 1996.
12. Birrell F, Croft P, Cooper C, Hosie G, Macfarlane G, Silman A; PCR Hip Study Group. Predicting radiographic hip osteoarthritis from range of movement. Rheumatology (Oxford) 2001;40:506-512.
13. Shin AY, Morin WD, Gorman JD, Jones SB, Lapinsky AS. The superiority of magnetic resonance imaging in differentiating the cause of hip pain in endurance athletes. Am J Sports Med 1996;24:168-176.
14. McCarthy JC, Busconi B. The role of hip arthroscopy in the diagnosis and treatment of hip disease. Orthopedics 1995;18:753-756.
15. Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg 2001;9:336-344.
16. Mitchell B, McCrory P, Brukner P, O'Donnell J, Colson E, Howells R. Hip joint pathology: clinical presentation and correlation between magnetic resonance arthrography, ultrasound, and arthroscopic findings in 25 consecutive cases. Clin J Sport Med 2003;13:152-156.
17. Croft P, Cooper C, Coggon D. Case definition of hip osteoarthritis in epidemiologic studies. J Rheumatol 1994;21:591-592.
18. Harper MC, Schaberg JE, Allen WC. Primary iliopsoas bursography in the diagnosis of disorders of the hip. Clin Orthop 1987;221:238-241.
19. Vaccaro JP, Sauser DD, Beals RK. Iliopsoas bursa imaging: efficacy in depicting abnormal iliopsoas tendon motion in patients with internal snapping hip syndrome. Radiology 1995;197:853-856.
20. Janzen DL, Partridge E, Logan PM, Connell DG, Duncan CP. The snapping hip: clinical and imaging findings in transient subluxation of the iliopsoas tendon. Can Assoc Radiol J 1996;47:202-208.
21. Seror P. Lateral femoral cutaneous nerve conduction v MRI may be required when conservative therapy is not effective or x-rays are unrevealing somatosensory evoked potentials for electrodiagnosis of meralgia paresthetica. Am J Phys Med Rehabil 1999;78:313-316.
22. Mitchell DG, Rao VM, Dalinka MK, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology 1987;162:709-715.
23. Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology 1996;200:225-230.
24. Czerny C, Hofmann S, Urban M, et al. MR arthrography of the adult acetabular capsular-labral complex: correlation with surgery and anatomy. AJR Am J Roentengol 1999;173:345-349.
25. Petersilge DA, Haque MA, Petersilge WJ, Lewin JS, Lieberman JM, Buly R. Acetabular labral tears: evaluation with MR arthrography. Radiology 1996;200:231-235.
26. Bogost GA, Lizerbram EK, Crues JV. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology 1995;197:263-267.
27. Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol 1999;11:417-421.
28. Migliore A, Martin LS, Alimonti A, Valente C, Tormenta S. Efficacy and safety of viscosupplementation by ultrasound-guided intra-articular injection in osteoarthrits of the hip. Osteoarthritis Cartilage 2003;11:305-306.
29. Brocq O, Tran G, Breuil V, Grisot C, Flory P, Euller-Ziegler L. Hip osteoarthritis: short-term efficacy and safety of viscosupplementation by hylan G-F 20. An open-label study in 22 patients. Joint Bone Spine 2002;69:388-391.
30. Shbeeb MI, O'Duffy JD, Michet CJ, O'Fallon WM, Matteson EL. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol 1996;23:2104-2106.
31. Johnston CA, Lindsay DM, Wiley JP. Treatment of iliopsoas syndrome with a hip rotation strengthening program: a retrospective case series. J Orthop Sports Phys Ther 1999;29:218-224.
32. Johnston CA, Wiley JP, Lindsay DM, Wiseman DA. Iliopsoas bursitis and tendonitis. A review. Sports Med 1998;25:271-283.
33. Gruen GS, Scioscia TN, Lowenstein JE. The surgical treatment of internal snapping hip. Am J Sports Med 2002;30:607-613.
34. Cibulka MT, Delitto A. A comparison of two different methods to treat hip pain in runners. J Orthop Sports Phys Ther 1993;17:172-176.
1. National Ambulatory Medical Care Survey. Hyattsville, Md: National Center for Health Statistics; 1995. CHS CD-ROM series 13, no. 11. Issued July 1997.
2. van Mechelen W. Running injuries. A review of the epidemiological literature. Sports Med 1992;14:320-335.
3. Christmas C, Crespo CJ, Franckowiak SC, Bathon JM, Bartlett SJ, Andersen RE. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract 2002;51:345-348.
4. Scopp JM, Moorman CT. The assessment of athletic hip injury. Clin Sports Med 2001;20:647-659.
5. Lamberts H, Brouwer HJ, Marinus AFM, Hofmans-Okkes IM. The use of ICPC in the Transition project.Episode-oriented epidemiology in general practice. In: Lamberts H, Wood M, Hofmans-Okkes IM, eds. International Classification of Primary Care in the European Community. Oxford: Oxford University Press; 1993;45-93.
6. Birrell F, Croft P, Cooper C, Hosie G, Macfarlane GJ, Silman A. Radiographic change is common in new presenters in primary care with hip pain. Rheumatology (Oxford) 2000;39:772-775.
7. Hickman JM, Peters CL. Hip pain in the young adult: diagnosis and treatment of disorders of the acetabular labrum and acetabular dysplasia. Am J Orthop 2001;30:459-467.
8. Shbeeb MI, Matteson EL. Trochanteric bursitis (greater trochanter pain syndrome). Mayo Clin Proc 1996;71:565-569.
9. Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum 2001;44:2138-2145.
10. Jones RK. Meralgia paresthetica as a cause of leg discomfort. Can Med Assoc J 1974;111:541-542.
11. Individual Clinical Guidelines: Hip Pain (non-traumatic) Phase 1. Version 1.0. Rosemont, Ill: Department of Research and Scientific Affairs, American Academy of Orthopaedic Surgeons; 1996.
12. Birrell F, Croft P, Cooper C, Hosie G, Macfarlane G, Silman A; PCR Hip Study Group. Predicting radiographic hip osteoarthritis from range of movement. Rheumatology (Oxford) 2001;40:506-512.
13. Shin AY, Morin WD, Gorman JD, Jones SB, Lapinsky AS. The superiority of magnetic resonance imaging in differentiating the cause of hip pain in endurance athletes. Am J Sports Med 1996;24:168-176.
14. McCarthy JC, Busconi B. The role of hip arthroscopy in the diagnosis and treatment of hip disease. Orthopedics 1995;18:753-756.
15. Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg 2001;9:336-344.
16. Mitchell B, McCrory P, Brukner P, O'Donnell J, Colson E, Howells R. Hip joint pathology: clinical presentation and correlation between magnetic resonance arthrography, ultrasound, and arthroscopic findings in 25 consecutive cases. Clin J Sport Med 2003;13:152-156.
17. Croft P, Cooper C, Coggon D. Case definition of hip osteoarthritis in epidemiologic studies. J Rheumatol 1994;21:591-592.
18. Harper MC, Schaberg JE, Allen WC. Primary iliopsoas bursography in the diagnosis of disorders of the hip. Clin Orthop 1987;221:238-241.
19. Vaccaro JP, Sauser DD, Beals RK. Iliopsoas bursa imaging: efficacy in depicting abnormal iliopsoas tendon motion in patients with internal snapping hip syndrome. Radiology 1995;197:853-856.
20. Janzen DL, Partridge E, Logan PM, Connell DG, Duncan CP. The snapping hip: clinical and imaging findings in transient subluxation of the iliopsoas tendon. Can Assoc Radiol J 1996;47:202-208.
21. Seror P. Lateral femoral cutaneous nerve conduction v MRI may be required when conservative therapy is not effective or x-rays are unrevealing somatosensory evoked potentials for electrodiagnosis of meralgia paresthetica. Am J Phys Med Rehabil 1999;78:313-316.
22. Mitchell DG, Rao VM, Dalinka MK, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology 1987;162:709-715.
23. Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology 1996;200:225-230.
24. Czerny C, Hofmann S, Urban M, et al. MR arthrography of the adult acetabular capsular-labral complex: correlation with surgery and anatomy. AJR Am J Roentengol 1999;173:345-349.
25. Petersilge DA, Haque MA, Petersilge WJ, Lewin JS, Lieberman JM, Buly R. Acetabular labral tears: evaluation with MR arthrography. Radiology 1996;200:231-235.
26. Bogost GA, Lizerbram EK, Crues JV. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology 1995;197:263-267.
27. Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol 1999;11:417-421.
28. Migliore A, Martin LS, Alimonti A, Valente C, Tormenta S. Efficacy and safety of viscosupplementation by ultrasound-guided intra-articular injection in osteoarthrits of the hip. Osteoarthritis Cartilage 2003;11:305-306.
29. Brocq O, Tran G, Breuil V, Grisot C, Flory P, Euller-Ziegler L. Hip osteoarthritis: short-term efficacy and safety of viscosupplementation by hylan G-F 20. An open-label study in 22 patients. Joint Bone Spine 2002;69:388-391.
30. Shbeeb MI, O'Duffy JD, Michet CJ, O'Fallon WM, Matteson EL. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol 1996;23:2104-2106.
31. Johnston CA, Lindsay DM, Wiley JP. Treatment of iliopsoas syndrome with a hip rotation strengthening program: a retrospective case series. J Orthop Sports Phys Ther 1999;29:218-224.
32. Johnston CA, Wiley JP, Lindsay DM, Wiseman DA. Iliopsoas bursitis and tendonitis. A review. Sports Med 1998;25:271-283.
33. Gruen GS, Scioscia TN, Lowenstein JE. The surgical treatment of internal snapping hip. Am J Sports Med 2002;30:607-613.
34. Cibulka MT, Delitto A. A comparison of two different methods to treat hip pain in runners. J Orthop Sports Phys Ther 1993;17:172-176.
Domestic violence: Screening made practical
- Physicians should routinely screen women for domestic violence (C). Although the US Domestic Task Force considers the evidence for or against specific instruments insufficient, the recommendation to include questions about physical abuse may be made on other grounds, such as the high prevalence of undetected abuse among women patients, the potential value of this information in helping such patients, and the low cost and low risk of screening.
- Offer abused patients information about community resources and advocates (B). Advocacy and connections with community agencies have proven helpful (in a randomized controlled trial) in improving quality of life and preventing violence-related injuries.
Screening is effective in detecting domestic violence, and increases the rate of referrals to community resources, resulting in improved quality of life and fewer violence-related injuries.
Screening can be accomplished with a questionnaire filled out by the patient or a directed interview conducted by you or a staff member.
Newer screening tools are briefer and easier to use than before. A self-administered questionnaire can even become part of the routine intake at annual health examinations.
These advances may be a remedy for a finding of one study—only 10% of primary care physicians routinely screen for domestic violence.1 Although 92% of women surveyed who were physically abused by their partners did not discuss these incidents with their physicians,2 studies show they would like their health care providers to ask about abuse.3-5
How screening makes a difference
Domestic violence is a chronic life-threatening condition that is treatable. If abuse is left untreated, the severity and frequency of abuse can worsen, leading to serious adverse effects to health and potentially life-threatening consequences.6,7 However, if we identify victims by screening and offer information including safety plans and referrals to advocacy services, the prognosis is improved in terms of reported quality of life and fewer violence-related injuries (LOE: 1b).8,9
Although the effectiveness of screening on every aspect of the recovery process has not been validated by randomized controlled trials, the current literature certainly suggests likely benefit in certain stages. Qualitative evidence from abuse victims supports the assumption that screening for abuse enables patients to recognize a problem, even if they are not ready for help at that point.10
Prevalence of domestic violence
A study by the Centers for Disease Control and Prevention of 1,691,600 women found that 30% had experienced domestic violence during their lifetimes.11 The prevalence of domestic violence is difficult to measure due to different definitions of abuse and factors that preclude accurate reporting by victims, such as safety and social stigma.
One anonymous survey in a family practice setting found that 23% of women had been physically assaulted by their partners in the past year,12 and another anonymous survey of 1952 female patients attending 4 different community-based primary care practices found that 1 of every 5 had experienced violence in their adult lives.13
Domestic violence is also a financial burden to victims and to society: domestic violence victims have 2.5 times greater outpatient costs than do nonvictims.14
Why screen all women?
Particular history and physical findings are associated with increased likelihood of domestic violence (Table 1).15-20 Neither victims nor batterers fit a distinct personality or profile, however, and abuse affects women of all ages, ethnicities, and socioeconomic classes. Predicting which women will be affected is difficult,21,22 which suggests that universal screening is more appropriate than targeting specific groups (LOE: 5).
The US Preventive Services Task Force (USPTF) gave a strength of recommendation of C for domestic violence screening because evidence to recommend for or against use of specific screening instruments is insufficient.23 Two recent systematic reviews concluded that evidence is lacking for the effectiveness of interventions for women experiencing abuse, and the potential harms of identifying and treating abused women are not well evaluated.24,25 However, the USPTF noted that asking questions about physical abuse is justifiable on other grounds, such as the high prevalence of undetected abuse among women patients, the potential value of this information in helping such patients, and the low cost and low risk from screening.
The American Academy of Family Physicians,26 the American College of Physicians,27 the American Medical Association,28 and the American College of Obstetricians and Gynecologists29,30 all recommend screening for domestic violence. Screening does increase the detection of domestic violence.25 The screening can be a questionnaire filled out by the patient or a directed interview conducted by a staff member or physician. Two recent studies found that questionnaires are better than interviews at detecting domestic violence (LOE: 2b).31,32
The Joint Commission on Accreditation of Healthcare Organizations now mandates that all hospitals screen patients for domestic violence.33 Educating health care providers about domestic violence and screening improves their self-reported ability to identify and manage abuse victims.34,35 In addition, screening for domestic violence increases the rate of referrals to community resources.34,35 Administrative changes, guidelines, protocols, and changes to standardized medical record forms to assist screening for domestic violence increase identification of victims35-37 and help maintain sustained change in screening behavior over more than 12 months.1
TABLE 1
History and physical findings suggestive of abuse
2 Useful screening instruments
New screening tools are briefer and more efficient than earlier devices.
The HITS Scale38 (Hurt, Insult, Threaten, Scream; Table 2) is a practical 4-item scale. It has been validated in the family practice setting in a study that compared 160 family practice patients whose abuse status was unknown with 99 selfidentified victims of abuse.
The Woman Abuse Screening Tool (WAST; Appendix A, available online at http://www.jfponline.com) was developed for the family practice setting. It was validated by a study comparing the responses between 24 self-identified abused women from shelters and 24 nonabused women recruited from the principal investigator’s professional contacts.39
The first 2 questions of the WAST screen make up the WAST-short questions:
- In general, how would you describe your relationship? (A lot of tension; some tension; no tension)
- Do you and your partner work out arguments with…? (great difficulty; some difficulty; no difficulty)
These questions assess the degree of relationship tension and the amount of difficulty the patient and her partner have in working out arguments. If a patient answers affirmatively to these 2 questions, then the physician can use the remaining WAST questions to elicit more information about the patient’s experience of abuse. A Spanish version of the WAST has been shown to be successful as well.40
The WAST and HITS scales need to be further evaluated prospectively in larger populations with a high prevalence of abuse. In addition, nonbiased samples need to be recruited and the tests need to be validated against a criterion standard.
The HITS scale has been tested in English-speaking populations only. The ability to screen different ethnic groups and ask sensitive questions across cultural barriers is important and should be studied further.
The Women’s Experience with Battering Scale41 (Table 3) is a series of 10 questions tested in a large cross-sectional survey of women (n=1152) attending 1 of 2 family practice clinics. It has been validated in a study using the Index of Spouse Abuse as a reference standard (18% of the women surveyed had experienced violence in a current or most recent intimate relationship with a male partner). For every 100 female patients seen, a physician will correctly identify 16 of 18 abuse victims and will incorrectly label 7 nonabused women as victims. For this reason, a positive screen using any instrument must be followed-up by a careful interview before further intervention.
Unlike other tests, the Women’s Experience with Battering Scale was conducted in a relatively larger, unbiased, sample population, had good accuracy, and is recommended. The only drawback is the length, but it can be self-administered as part of a routine intake for an annual health maintenance examination.
TABLE 2
The HITS screen
| Hurt | How often does your partner physically hurt you? |
| Insult | How often does your partner insult or talk down to you? |
| Threaten | How often does your partner threaten you with physical harm? |
| Scream | How often does your partner scream or curse at you? |
| Each question is answered on a 5-point scale: 1 = never, 2 = rarely, 3 = sometimes, 4 = fairly often, 5 = frequently. | |
| The score ranges from 4 to a maximum of 20. A score of 10 is considered diagnostic of abuse. | |
TABLE 3
Women’s Experience with Battering Scale
| Description of how your partner makes you feel | Agree strongly | Agree somewhat | Agree a little | Disagree a little | Disagree somewhat | Disagree strongly | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. He makes me feel unsafe even in my own home | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 2. I feel ashamed of the things he does to me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 3. I try not to rock the boat because I am afraid of what he might do | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 4. I feel like I am programmed to react in a certain way to him | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 5. I feel like he keeps me prisoner | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 6. He makes me feel like I have no control over my life, no power, no protection | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 7. I hide the truth from others because I am afraid not to | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 8. I feel owned and controlled by him | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 9. He can scare me without laying a hand on me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 10. He has a look that goes straight through me and terrifies me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| To score this scale, add the responses for items 1 through 10. The score range is 10 to 60. A score of 20 or higher is a positive screening test for battering. | |||||||||||
Older, less useful tools
The Conflicts Tactics Scale was one of the first instruments to identify partner violence by measuring interpersonal aggression. The original screen consisted of 19 questions.42 The Index of Spouse Abuse is a 30-item self-report scale designed to measure the severity or magnitude of physical and nonphysical abuse inflicted on a woman by her male partner.43 Detailed independent evaluations by experienced therapists to determine whether an individual is a victim of partner abuse, considered to be the gold standard, have been used to validate the Index of Spouse Abuse. However, the Index of Spouse Abuse and Conflicts Tactics Scale are impractical for routine use in the office due to their length and complexity. Table 4 compares these screening tests.
TABLE 4
Performance characteristics of domestic violence screening instruments
| Test | LOE | Sn, %* | Sp, %* | LR+, % | LR–, % | PV+, % † | PV–, % † |
|---|---|---|---|---|---|---|---|
| ISA-P 28 ∂1 | 1b | 90.7 | 92.2 | 11.4 | 0.1 | 72 | 2.15 |
| ISA-NP 28 ∂1 | 1b | 90.7 | 90.6 | 10.1 | 0.1 | 69 | 2.15 |
| WEB 32 ∂2 | 1b | 86 | 91 | 9.56 | 0.15 | 67.8 | 3.2 |
| HITS 29 ∂3 | 3b | 96 | 91 | 10.7 | 0.04 | 70.2 | 0.87 |
| WAST 30 ∂3 | 3b | 83 | 75 | 3.32 | 0.23 | 42.2 | 4.82 |
| *Sensitivity and specificity summarized as reported in individual studies. | |||||||
| † Posttest probability was calculated assuming a pretest probability of 18%. | |||||||
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PV+, probability of disease given a positive test; PV–, probability of disease given a negative test; ∂ , reference standard; ∂1, detailed interview; ∂2, ∂3, Index of Spouse Abuse self-identified abuse victims; ISA-P, Index of Spouse Abuse scale measuring the severity or magnitude of physical abuse inflicted on a woman by her male partner; ISA-NP, Index of Spouse Abuse scale measuring the severity or magnitude of nonphysical abuse inflicted on a woman by her male partner; WEB, Women’s Experience with Battery Scale; HITS, hurt, insult, threaten, scream; WAST, Woman Abuse Screening Tool | |||||||
How physicians can help ensure safety
Table 5 shows the strength of recommendation supporting different aspects of treatment. The care of the abused woman requires a multidisciplinary team approach involving institutional and community services.28 The literature suggests that once a victim of abuse is identified in an office setting, a primary care physician can improve outcome by caring for acute injuries,28 offering support, and making appropriate referrals.
A physician can help ensure safety by:
- Assessing immediate risk. Has the violence increased in frequency or severity over the past year? Has your partner threatened to kill you or your children? Are there weapons in the house? Does your partner know that you are planning to leave? (LOE: 5)44
- Discussing safety behaviors. This includes advice on self-protection (ie, removal of weapons from the home) and planning for leaving safely in a threatening situation. One study of abused pregnant mothers found that receiving a safety intervention protocol significantly increased the safety behaviors adopted during and after pregnancy (from 47.6% at visit 1 to 78.1% at visit 6; P.≤001), preventing further abuse and increasing the safety and well-being of mother and baby (LOE: 2c)8
- Helping the patient obtain a civil protection order. This can be obtained with the assistance of the police or community advocacy services. Women with permanent protection orders are less likely than those without orders to be physically abused (risk ratio in 12 months, 0.2; 95% confidence interval, 0.1–0.8; LOE: 2b).46
- A trusting relationship with the patient can help her break the cycle of abuse and enable her to change her circumstances (LOE: 4).47 A qualitative study showed that battered women have rated the following behaviors highly desirable in their physicians (LOE: 4).10
- Initially validates their experiences with compassionate messages and emphasizes their worth as human beings
- Clearly labels the abuse as wrong and criminal
- Listens in a careful, nonjudgmental manner.
Having someone to confide in and having told someone about the abuse were factors associated with diminished abuse at 3 months in one study (P=.001 and .023, respectively) (LOE: 2c).48
TABLE 5
Evidence supporting interventions for domestic violence
| SOR | Treatment |
|---|---|
| A | Community-based advocacy intervention programs40 |
| B | Safety intervention protocols35 |
| B | Civil protection order36 |
| B | Telling or confiding in someone39 |
| B | Contact with community resources on domestic violence39 |
| B | On-site advocacy programs41 |
| C | Validating the patient’s experience38 |
| C | Assessing immediate safety and emphasizing potential for lethal outcome33,34 |
| SOR, strength of recommendation. For an explanation of the recommendations. | |
Referral to community resources
A randomized controlled trial with 2-year follow-up investigated community-based advocacy for abused women who were leaving a shelter program. This study found that advocacy services led to significantly greater effectiveness in obtaining resources, a decrease in physical violence, a decrease in depression, and an improved quality of life and social support at 10 weeks post-shelter. At 2 years, advocacy services led to reduced physical violence (11% vs 24%, P..05, number needed to treat=7.7), increased likelihood of leaving the abusive relationship (96% vs 87%, number needed to treat=11, P.<.03), and improved quality of life (P..01) (LOE: 1b).9
Straus and colleagues48 associated contact with community domestic violence resources with a decreased sense of community isolation (LOE: 2c). The National Domestic Violence Hotline (800-799-SAFE) can provide physicians in every state with information on local resources.
Muelleman and Feighny49 found that advocacy programs that are available on-site can improve the use of shelters and shelter-based counseling (LOE: 2c). However, there are no studies of suitable quality comparing outcomes for women using shelters with women not using shelters.24 Biasfree samples would be difficult to recruit. One study that evaluated experiences before and after shelter found that women experienced less violence after the shelter stay (LOE: 2c).50
Acknowledgments
I thank the faculty and residents of Shadyside Family Practice for their feedback and help with the preparation of this article.
Correspondence
Mallika Punukollu, MD, PO Box B135, Huddersfield, West Yorkshire, HD1 1YG, UK. E-mail:[email protected].
1. Elliot L, Nerney M, Jones T, Friedman PD. Barriers to screening for domestic violence. J Gen Intern Med 2002;17:112-116.
2. The Commonwealth Fund National Survey. First comprehensive national survey of American women finds them at significant risk [news release]. New York, NY: Commonwealth Fund; 1993.
3. Fogarty CT, Burge S, McCord EC. Communicating with patients about intimate partner violence: screening and interviewing approaches. Fam Med 2002;34:369-375.
4. Rodriguez M, Quiroga SS, Bauer H. Breaking the silence: battered women’s perspectives on medical care. Arch Fam Med 1996;5:153-158.
5. Rodriguez MA, Sheldon WR, Bauer HM, et al. The factors associated with disclosure of intimate partner abuse to clinicians. J Fam Pract 2001;50:338-344.
6. US Bureau of Justice Statistics. Highlights from 20 years of surveying crime victims: the National Crime Victimization survey, 1973–1992. Washington, DC: US Department of Justice; 1993.
7. Berrios DC, Grady D. Domestic violence: risk factors and outcome. West J Med 1991;155:133-135.
8. McFarlane J, Parker B, Soeken K, et al. Safety behaviors of abused women after an intervention during pregnancy. J Obstet Gynecol Neonatal Nurs 1998;27:64-69.
9. Sullivan CM, Bybee DI. Reducing violence using community-based advocacy for women with abusive partners. J Consult Clin Psychol 1999;67:43-53.
10. Nicolaidis C. The voices of survivors’ documentary. Using patient narrative to educate physicians about domestic violence. J Gen Intern Med 2002;17:117-124.
11. Centers for Disease Control and Prevention. Lifetime and annual incidence of intimate partner violence and resulting injuries—Georgia, 1995. MMWR Morb Mortal Wkly Rep 47; 1998;47:849-853.
12. Hamberger KL, Saunders DG, Hovey M. Prevalence of domestic violence in community practice and rate of physician inquiry. Fam Med 1992;24:283-287.
13. McCauley J, Kern DE, Kolodner K, et al. The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices. Ann Intern Med 1995;123:737-746.
14. Koss MP, Koss PG, Woodruff WJ. Relation of criminal victimization to health perceptions among women medical patients. Arch Intern Med 1991;151:342-347.
15. Fanslow JL, Norton RN, Spinola CG. Indicators of assaultrelated injuries among women presenting to the Emergency Department. Ann Emerg Med 1998;32:341-348.
16. Drossman DA, Lesserman J, Nachman G, et al. Sexual and Physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med 1990;113:828-833.
17. Danielson KK, Moffitt TE, Caspi A, Silva PA. Comorbidity between abuse of an adult and DSM-III-R mental disorders: Evidence from an epidemiological study. Am J Psychiatry 1998;155:131-133.
18. Bergman B, Brismar B. A 5-year follow up study of 117 battered women. Am J Public Health 1991;81:1486-1489.
19. Muelleman RL, Lenaghan PA, Pakieser RA. Battered women: injury locations and types. Ann Emerg Med 1996;28:486-492.
20. Murphy CC, Schei B, Myhr TL, DuMont J. Abuse: A risk factor for low birth weight? A systematic review and metaanalysis. CMAJ 2001;164:1578-1579.
21. Saunders DG, Hamberger K, Hovey M. Indicators of woman abuse based on a chart review at a family practice center. Arch Fam Med 1993;2:537-543.
22. Wasson JH, Jette AM, Anderson J, et al. Routine, single-item screening to identify abusive relationships in women. J Fam Pract 2000;49:1017-1022.
23. Thompson CF, Atkins D. Screening for Family Violence Washington, DC: US Preventive Services Task Force; 1996.
24. Wathen CN, MacMillan HL. Interventions for violence against women: scientific review. JAMA 2003;289:589-600-e581-e510.
25. Ramsay J, Richardson J, Carter Y, Davidson L, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002;325:314-318.
26. Age Charts for Periodic Health Examination Reprint 510. Kansas City, KS: American Academy of Family Physicians; 1994.
27. Holbrook JH, Ende J, eds. Primary care internal medicine. In: Medical Knowledge Self-Assessment Program 11. Philadelphia: American College of Physicians; 1998:21-22.
28. Flitcraft A, Hadley S, Hendricks-Matthews MK, McLeer SV, Warshaw C. Diagnostic and Treatment Guidelines on Domestic Violence. Chicago, Ill: American Medical Association; 1992.
29. Dunn L, Brown C, Dickerson V, et al. The Obstetrician.-Gynecologist and Primary-Preventive Health Care. Washington, DC: American College of Obstetricians and Gynecologists; 1993.
30. Psychosocial Risk Factors: Perinatal Screening and Intervention. Educational Bulletin 255. Washington, DC: American College of Obstetricians and Gynecologists; 1999.
31. McFarlane J, Christoffel K, Bateman L, et al. Assessing for abuse: self-report vs. nurse interview. Public Health Nurs 1991;4:245-250.
32. Canterino JC, Vanham LG, Harrigan JT, et al. Domestic abuse in pregnancy: a comparison of self completed domestic abuse questionnaire with directed interview. Am J Obstet Gynecol 1999;181:1049-1051.
33. Joint Commission on Accreditation of Healthcare Organizations. Hospital Manual. Standard PE1.9. Chicago, Ill: Joint Commission Resources, Inc; 2002.
34. Glass N, Dearwater S, Campbell J. Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments. J Emerg Nurs 2001;27:141-149.
35. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence, a randomized trial. Am J Prev Med 2000;19:253-263.
36. Harwell TS, Casten RJ, Armstrong KA, et al. Results of a domestic violence training program offered to staff of urban community health centers. Am J Prev Med 1998;15:235-242.
37. Larkin GL, Rolniak S, Hyman KB, et al. Effects of an administrative intervention on rates of screening for domestic violence in an urban emergency department. Am J Public Health 2000;90:1444-1448.
38. Sherin KM, Sinacore JM, Li X, et al. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med 1998;30:508-512.
39. Brown JB, Lent B, Brett PJ, et al. Development of the woman abuse screening tool for use in family practice. Fam Med 1996;28:422-428.
40. Fogarty CT, Brown JB. Screening for abuse in Spanish-speaking women. J Am Board Fam Pract 2002;15:101-111.
41. Coker AL, Pope BO, Smith PH, et al. Assessment of clinical partner violence screening tools. J Am Med Womens Assoc 2001;56:19-23.
42. Straus MA. Measuring intrafamily conflict and violence: the conflicts tactics scale. J Marriage Fam 1979;4:75-88.
43. Hudson WW, McIntosh SR. The assessment of spouse abuse: two quantifiable dimensions. J Marriage Fam 1981;43:873-888.
44. Campbell JC. Nursing assessment for risk of homicide with battered women. ANS Adv Nurs Sci 1986;8:36.-
45. Elliot BA, Johnson MMP. Domestic violence in primary care setting: patterns and prevalence. Arch Fam Med 1995;4:113-119.
46. Holt VL, Kernic MA, Lumley T, Wolf ME, Rivara FP. Civil protection orders and risk of subsequent police reported violence. JAMA 2002;288:589-594.
47. Gerbert B, Caspers N, Milliken N, et al. Interventions that help victims of domestic violence: a qualitative analysis of physicians’ experiences. J Fam Pract 2000;49:889-895.
48. Straus HE, Rydman RJ, Roberts RR, et al. A three months prospective outcomes study of recently abused women. Acad Emerg Med 2001;8(5):461.-
49. Muelleman RL, Feighny KM. Injury prevention: effects of an emergency department based advocacy program for battered women on community resource utilization. Ann Emerg Med 1999;33:62-66.
50. Sullivan CM, Campbell R, Angelique H, et al. An advocacy intervention program for women with abusive partners: six month follow up. Am J Comm Psychol 1994;22:101-122.
- Physicians should routinely screen women for domestic violence (C). Although the US Domestic Task Force considers the evidence for or against specific instruments insufficient, the recommendation to include questions about physical abuse may be made on other grounds, such as the high prevalence of undetected abuse among women patients, the potential value of this information in helping such patients, and the low cost and low risk of screening.
- Offer abused patients information about community resources and advocates (B). Advocacy and connections with community agencies have proven helpful (in a randomized controlled trial) in improving quality of life and preventing violence-related injuries.
Screening is effective in detecting domestic violence, and increases the rate of referrals to community resources, resulting in improved quality of life and fewer violence-related injuries.
Screening can be accomplished with a questionnaire filled out by the patient or a directed interview conducted by you or a staff member.
Newer screening tools are briefer and easier to use than before. A self-administered questionnaire can even become part of the routine intake at annual health examinations.
These advances may be a remedy for a finding of one study—only 10% of primary care physicians routinely screen for domestic violence.1 Although 92% of women surveyed who were physically abused by their partners did not discuss these incidents with their physicians,2 studies show they would like their health care providers to ask about abuse.3-5
How screening makes a difference
Domestic violence is a chronic life-threatening condition that is treatable. If abuse is left untreated, the severity and frequency of abuse can worsen, leading to serious adverse effects to health and potentially life-threatening consequences.6,7 However, if we identify victims by screening and offer information including safety plans and referrals to advocacy services, the prognosis is improved in terms of reported quality of life and fewer violence-related injuries (LOE: 1b).8,9
Although the effectiveness of screening on every aspect of the recovery process has not been validated by randomized controlled trials, the current literature certainly suggests likely benefit in certain stages. Qualitative evidence from abuse victims supports the assumption that screening for abuse enables patients to recognize a problem, even if they are not ready for help at that point.10
Prevalence of domestic violence
A study by the Centers for Disease Control and Prevention of 1,691,600 women found that 30% had experienced domestic violence during their lifetimes.11 The prevalence of domestic violence is difficult to measure due to different definitions of abuse and factors that preclude accurate reporting by victims, such as safety and social stigma.
One anonymous survey in a family practice setting found that 23% of women had been physically assaulted by their partners in the past year,12 and another anonymous survey of 1952 female patients attending 4 different community-based primary care practices found that 1 of every 5 had experienced violence in their adult lives.13
Domestic violence is also a financial burden to victims and to society: domestic violence victims have 2.5 times greater outpatient costs than do nonvictims.14
Why screen all women?
Particular history and physical findings are associated with increased likelihood of domestic violence (Table 1).15-20 Neither victims nor batterers fit a distinct personality or profile, however, and abuse affects women of all ages, ethnicities, and socioeconomic classes. Predicting which women will be affected is difficult,21,22 which suggests that universal screening is more appropriate than targeting specific groups (LOE: 5).
The US Preventive Services Task Force (USPTF) gave a strength of recommendation of C for domestic violence screening because evidence to recommend for or against use of specific screening instruments is insufficient.23 Two recent systematic reviews concluded that evidence is lacking for the effectiveness of interventions for women experiencing abuse, and the potential harms of identifying and treating abused women are not well evaluated.24,25 However, the USPTF noted that asking questions about physical abuse is justifiable on other grounds, such as the high prevalence of undetected abuse among women patients, the potential value of this information in helping such patients, and the low cost and low risk from screening.
The American Academy of Family Physicians,26 the American College of Physicians,27 the American Medical Association,28 and the American College of Obstetricians and Gynecologists29,30 all recommend screening for domestic violence. Screening does increase the detection of domestic violence.25 The screening can be a questionnaire filled out by the patient or a directed interview conducted by a staff member or physician. Two recent studies found that questionnaires are better than interviews at detecting domestic violence (LOE: 2b).31,32
The Joint Commission on Accreditation of Healthcare Organizations now mandates that all hospitals screen patients for domestic violence.33 Educating health care providers about domestic violence and screening improves their self-reported ability to identify and manage abuse victims.34,35 In addition, screening for domestic violence increases the rate of referrals to community resources.34,35 Administrative changes, guidelines, protocols, and changes to standardized medical record forms to assist screening for domestic violence increase identification of victims35-37 and help maintain sustained change in screening behavior over more than 12 months.1
TABLE 1
History and physical findings suggestive of abuse
2 Useful screening instruments
New screening tools are briefer and more efficient than earlier devices.
The HITS Scale38 (Hurt, Insult, Threaten, Scream; Table 2) is a practical 4-item scale. It has been validated in the family practice setting in a study that compared 160 family practice patients whose abuse status was unknown with 99 selfidentified victims of abuse.
The Woman Abuse Screening Tool (WAST; Appendix A, available online at http://www.jfponline.com) was developed for the family practice setting. It was validated by a study comparing the responses between 24 self-identified abused women from shelters and 24 nonabused women recruited from the principal investigator’s professional contacts.39
The first 2 questions of the WAST screen make up the WAST-short questions:
- In general, how would you describe your relationship? (A lot of tension; some tension; no tension)
- Do you and your partner work out arguments with…? (great difficulty; some difficulty; no difficulty)
These questions assess the degree of relationship tension and the amount of difficulty the patient and her partner have in working out arguments. If a patient answers affirmatively to these 2 questions, then the physician can use the remaining WAST questions to elicit more information about the patient’s experience of abuse. A Spanish version of the WAST has been shown to be successful as well.40
The WAST and HITS scales need to be further evaluated prospectively in larger populations with a high prevalence of abuse. In addition, nonbiased samples need to be recruited and the tests need to be validated against a criterion standard.
The HITS scale has been tested in English-speaking populations only. The ability to screen different ethnic groups and ask sensitive questions across cultural barriers is important and should be studied further.
The Women’s Experience with Battering Scale41 (Table 3) is a series of 10 questions tested in a large cross-sectional survey of women (n=1152) attending 1 of 2 family practice clinics. It has been validated in a study using the Index of Spouse Abuse as a reference standard (18% of the women surveyed had experienced violence in a current or most recent intimate relationship with a male partner). For every 100 female patients seen, a physician will correctly identify 16 of 18 abuse victims and will incorrectly label 7 nonabused women as victims. For this reason, a positive screen using any instrument must be followed-up by a careful interview before further intervention.
Unlike other tests, the Women’s Experience with Battering Scale was conducted in a relatively larger, unbiased, sample population, had good accuracy, and is recommended. The only drawback is the length, but it can be self-administered as part of a routine intake for an annual health maintenance examination.
TABLE 2
The HITS screen
| Hurt | How often does your partner physically hurt you? |
| Insult | How often does your partner insult or talk down to you? |
| Threaten | How often does your partner threaten you with physical harm? |
| Scream | How often does your partner scream or curse at you? |
| Each question is answered on a 5-point scale: 1 = never, 2 = rarely, 3 = sometimes, 4 = fairly often, 5 = frequently. | |
| The score ranges from 4 to a maximum of 20. A score of 10 is considered diagnostic of abuse. | |
TABLE 3
Women’s Experience with Battering Scale
| Description of how your partner makes you feel | Agree strongly | Agree somewhat | Agree a little | Disagree a little | Disagree somewhat | Disagree strongly | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. He makes me feel unsafe even in my own home | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 2. I feel ashamed of the things he does to me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 3. I try not to rock the boat because I am afraid of what he might do | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 4. I feel like I am programmed to react in a certain way to him | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 5. I feel like he keeps me prisoner | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 6. He makes me feel like I have no control over my life, no power, no protection | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 7. I hide the truth from others because I am afraid not to | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 8. I feel owned and controlled by him | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 9. He can scare me without laying a hand on me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 10. He has a look that goes straight through me and terrifies me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| To score this scale, add the responses for items 1 through 10. The score range is 10 to 60. A score of 20 or higher is a positive screening test for battering. | |||||||||||
Older, less useful tools
The Conflicts Tactics Scale was one of the first instruments to identify partner violence by measuring interpersonal aggression. The original screen consisted of 19 questions.42 The Index of Spouse Abuse is a 30-item self-report scale designed to measure the severity or magnitude of physical and nonphysical abuse inflicted on a woman by her male partner.43 Detailed independent evaluations by experienced therapists to determine whether an individual is a victim of partner abuse, considered to be the gold standard, have been used to validate the Index of Spouse Abuse. However, the Index of Spouse Abuse and Conflicts Tactics Scale are impractical for routine use in the office due to their length and complexity. Table 4 compares these screening tests.
TABLE 4
Performance characteristics of domestic violence screening instruments
| Test | LOE | Sn, %* | Sp, %* | LR+, % | LR–, % | PV+, % † | PV–, % † |
|---|---|---|---|---|---|---|---|
| ISA-P 28 ∂1 | 1b | 90.7 | 92.2 | 11.4 | 0.1 | 72 | 2.15 |
| ISA-NP 28 ∂1 | 1b | 90.7 | 90.6 | 10.1 | 0.1 | 69 | 2.15 |
| WEB 32 ∂2 | 1b | 86 | 91 | 9.56 | 0.15 | 67.8 | 3.2 |
| HITS 29 ∂3 | 3b | 96 | 91 | 10.7 | 0.04 | 70.2 | 0.87 |
| WAST 30 ∂3 | 3b | 83 | 75 | 3.32 | 0.23 | 42.2 | 4.82 |
| *Sensitivity and specificity summarized as reported in individual studies. | |||||||
| † Posttest probability was calculated assuming a pretest probability of 18%. | |||||||
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PV+, probability of disease given a positive test; PV–, probability of disease given a negative test; ∂ , reference standard; ∂1, detailed interview; ∂2, ∂3, Index of Spouse Abuse self-identified abuse victims; ISA-P, Index of Spouse Abuse scale measuring the severity or magnitude of physical abuse inflicted on a woman by her male partner; ISA-NP, Index of Spouse Abuse scale measuring the severity or magnitude of nonphysical abuse inflicted on a woman by her male partner; WEB, Women’s Experience with Battery Scale; HITS, hurt, insult, threaten, scream; WAST, Woman Abuse Screening Tool | |||||||
How physicians can help ensure safety
Table 5 shows the strength of recommendation supporting different aspects of treatment. The care of the abused woman requires a multidisciplinary team approach involving institutional and community services.28 The literature suggests that once a victim of abuse is identified in an office setting, a primary care physician can improve outcome by caring for acute injuries,28 offering support, and making appropriate referrals.
A physician can help ensure safety by:
- Assessing immediate risk. Has the violence increased in frequency or severity over the past year? Has your partner threatened to kill you or your children? Are there weapons in the house? Does your partner know that you are planning to leave? (LOE: 5)44
- Discussing safety behaviors. This includes advice on self-protection (ie, removal of weapons from the home) and planning for leaving safely in a threatening situation. One study of abused pregnant mothers found that receiving a safety intervention protocol significantly increased the safety behaviors adopted during and after pregnancy (from 47.6% at visit 1 to 78.1% at visit 6; P.≤001), preventing further abuse and increasing the safety and well-being of mother and baby (LOE: 2c)8
- Helping the patient obtain a civil protection order. This can be obtained with the assistance of the police or community advocacy services. Women with permanent protection orders are less likely than those without orders to be physically abused (risk ratio in 12 months, 0.2; 95% confidence interval, 0.1–0.8; LOE: 2b).46
- A trusting relationship with the patient can help her break the cycle of abuse and enable her to change her circumstances (LOE: 4).47 A qualitative study showed that battered women have rated the following behaviors highly desirable in their physicians (LOE: 4).10
- Initially validates their experiences with compassionate messages and emphasizes their worth as human beings
- Clearly labels the abuse as wrong and criminal
- Listens in a careful, nonjudgmental manner.
Having someone to confide in and having told someone about the abuse were factors associated with diminished abuse at 3 months in one study (P=.001 and .023, respectively) (LOE: 2c).48
TABLE 5
Evidence supporting interventions for domestic violence
| SOR | Treatment |
|---|---|
| A | Community-based advocacy intervention programs40 |
| B | Safety intervention protocols35 |
| B | Civil protection order36 |
| B | Telling or confiding in someone39 |
| B | Contact with community resources on domestic violence39 |
| B | On-site advocacy programs41 |
| C | Validating the patient’s experience38 |
| C | Assessing immediate safety and emphasizing potential for lethal outcome33,34 |
| SOR, strength of recommendation. For an explanation of the recommendations. | |
Referral to community resources
A randomized controlled trial with 2-year follow-up investigated community-based advocacy for abused women who were leaving a shelter program. This study found that advocacy services led to significantly greater effectiveness in obtaining resources, a decrease in physical violence, a decrease in depression, and an improved quality of life and social support at 10 weeks post-shelter. At 2 years, advocacy services led to reduced physical violence (11% vs 24%, P..05, number needed to treat=7.7), increased likelihood of leaving the abusive relationship (96% vs 87%, number needed to treat=11, P.<.03), and improved quality of life (P..01) (LOE: 1b).9
Straus and colleagues48 associated contact with community domestic violence resources with a decreased sense of community isolation (LOE: 2c). The National Domestic Violence Hotline (800-799-SAFE) can provide physicians in every state with information on local resources.
Muelleman and Feighny49 found that advocacy programs that are available on-site can improve the use of shelters and shelter-based counseling (LOE: 2c). However, there are no studies of suitable quality comparing outcomes for women using shelters with women not using shelters.24 Biasfree samples would be difficult to recruit. One study that evaluated experiences before and after shelter found that women experienced less violence after the shelter stay (LOE: 2c).50
Acknowledgments
I thank the faculty and residents of Shadyside Family Practice for their feedback and help with the preparation of this article.
Correspondence
Mallika Punukollu, MD, PO Box B135, Huddersfield, West Yorkshire, HD1 1YG, UK. E-mail:[email protected].
- Physicians should routinely screen women for domestic violence (C). Although the US Domestic Task Force considers the evidence for or against specific instruments insufficient, the recommendation to include questions about physical abuse may be made on other grounds, such as the high prevalence of undetected abuse among women patients, the potential value of this information in helping such patients, and the low cost and low risk of screening.
- Offer abused patients information about community resources and advocates (B). Advocacy and connections with community agencies have proven helpful (in a randomized controlled trial) in improving quality of life and preventing violence-related injuries.
Screening is effective in detecting domestic violence, and increases the rate of referrals to community resources, resulting in improved quality of life and fewer violence-related injuries.
Screening can be accomplished with a questionnaire filled out by the patient or a directed interview conducted by you or a staff member.
Newer screening tools are briefer and easier to use than before. A self-administered questionnaire can even become part of the routine intake at annual health examinations.
These advances may be a remedy for a finding of one study—only 10% of primary care physicians routinely screen for domestic violence.1 Although 92% of women surveyed who were physically abused by their partners did not discuss these incidents with their physicians,2 studies show they would like their health care providers to ask about abuse.3-5
How screening makes a difference
Domestic violence is a chronic life-threatening condition that is treatable. If abuse is left untreated, the severity and frequency of abuse can worsen, leading to serious adverse effects to health and potentially life-threatening consequences.6,7 However, if we identify victims by screening and offer information including safety plans and referrals to advocacy services, the prognosis is improved in terms of reported quality of life and fewer violence-related injuries (LOE: 1b).8,9
Although the effectiveness of screening on every aspect of the recovery process has not been validated by randomized controlled trials, the current literature certainly suggests likely benefit in certain stages. Qualitative evidence from abuse victims supports the assumption that screening for abuse enables patients to recognize a problem, even if they are not ready for help at that point.10
Prevalence of domestic violence
A study by the Centers for Disease Control and Prevention of 1,691,600 women found that 30% had experienced domestic violence during their lifetimes.11 The prevalence of domestic violence is difficult to measure due to different definitions of abuse and factors that preclude accurate reporting by victims, such as safety and social stigma.
One anonymous survey in a family practice setting found that 23% of women had been physically assaulted by their partners in the past year,12 and another anonymous survey of 1952 female patients attending 4 different community-based primary care practices found that 1 of every 5 had experienced violence in their adult lives.13
Domestic violence is also a financial burden to victims and to society: domestic violence victims have 2.5 times greater outpatient costs than do nonvictims.14
Why screen all women?
Particular history and physical findings are associated with increased likelihood of domestic violence (Table 1).15-20 Neither victims nor batterers fit a distinct personality or profile, however, and abuse affects women of all ages, ethnicities, and socioeconomic classes. Predicting which women will be affected is difficult,21,22 which suggests that universal screening is more appropriate than targeting specific groups (LOE: 5).
The US Preventive Services Task Force (USPTF) gave a strength of recommendation of C for domestic violence screening because evidence to recommend for or against use of specific screening instruments is insufficient.23 Two recent systematic reviews concluded that evidence is lacking for the effectiveness of interventions for women experiencing abuse, and the potential harms of identifying and treating abused women are not well evaluated.24,25 However, the USPTF noted that asking questions about physical abuse is justifiable on other grounds, such as the high prevalence of undetected abuse among women patients, the potential value of this information in helping such patients, and the low cost and low risk from screening.
The American Academy of Family Physicians,26 the American College of Physicians,27 the American Medical Association,28 and the American College of Obstetricians and Gynecologists29,30 all recommend screening for domestic violence. Screening does increase the detection of domestic violence.25 The screening can be a questionnaire filled out by the patient or a directed interview conducted by a staff member or physician. Two recent studies found that questionnaires are better than interviews at detecting domestic violence (LOE: 2b).31,32
The Joint Commission on Accreditation of Healthcare Organizations now mandates that all hospitals screen patients for domestic violence.33 Educating health care providers about domestic violence and screening improves their self-reported ability to identify and manage abuse victims.34,35 In addition, screening for domestic violence increases the rate of referrals to community resources.34,35 Administrative changes, guidelines, protocols, and changes to standardized medical record forms to assist screening for domestic violence increase identification of victims35-37 and help maintain sustained change in screening behavior over more than 12 months.1
TABLE 1
History and physical findings suggestive of abuse
2 Useful screening instruments
New screening tools are briefer and more efficient than earlier devices.
The HITS Scale38 (Hurt, Insult, Threaten, Scream; Table 2) is a practical 4-item scale. It has been validated in the family practice setting in a study that compared 160 family practice patients whose abuse status was unknown with 99 selfidentified victims of abuse.
The Woman Abuse Screening Tool (WAST; Appendix A, available online at http://www.jfponline.com) was developed for the family practice setting. It was validated by a study comparing the responses between 24 self-identified abused women from shelters and 24 nonabused women recruited from the principal investigator’s professional contacts.39
The first 2 questions of the WAST screen make up the WAST-short questions:
- In general, how would you describe your relationship? (A lot of tension; some tension; no tension)
- Do you and your partner work out arguments with…? (great difficulty; some difficulty; no difficulty)
These questions assess the degree of relationship tension and the amount of difficulty the patient and her partner have in working out arguments. If a patient answers affirmatively to these 2 questions, then the physician can use the remaining WAST questions to elicit more information about the patient’s experience of abuse. A Spanish version of the WAST has been shown to be successful as well.40
The WAST and HITS scales need to be further evaluated prospectively in larger populations with a high prevalence of abuse. In addition, nonbiased samples need to be recruited and the tests need to be validated against a criterion standard.
The HITS scale has been tested in English-speaking populations only. The ability to screen different ethnic groups and ask sensitive questions across cultural barriers is important and should be studied further.
The Women’s Experience with Battering Scale41 (Table 3) is a series of 10 questions tested in a large cross-sectional survey of women (n=1152) attending 1 of 2 family practice clinics. It has been validated in a study using the Index of Spouse Abuse as a reference standard (18% of the women surveyed had experienced violence in a current or most recent intimate relationship with a male partner). For every 100 female patients seen, a physician will correctly identify 16 of 18 abuse victims and will incorrectly label 7 nonabused women as victims. For this reason, a positive screen using any instrument must be followed-up by a careful interview before further intervention.
Unlike other tests, the Women’s Experience with Battering Scale was conducted in a relatively larger, unbiased, sample population, had good accuracy, and is recommended. The only drawback is the length, but it can be self-administered as part of a routine intake for an annual health maintenance examination.
TABLE 2
The HITS screen
| Hurt | How often does your partner physically hurt you? |
| Insult | How often does your partner insult or talk down to you? |
| Threaten | How often does your partner threaten you with physical harm? |
| Scream | How often does your partner scream or curse at you? |
| Each question is answered on a 5-point scale: 1 = never, 2 = rarely, 3 = sometimes, 4 = fairly often, 5 = frequently. | |
| The score ranges from 4 to a maximum of 20. A score of 10 is considered diagnostic of abuse. | |
TABLE 3
Women’s Experience with Battering Scale
| Description of how your partner makes you feel | Agree strongly | Agree somewhat | Agree a little | Disagree a little | Disagree somewhat | Disagree strongly | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. He makes me feel unsafe even in my own home | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 2. I feel ashamed of the things he does to me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 3. I try not to rock the boat because I am afraid of what he might do | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 4. I feel like I am programmed to react in a certain way to him | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 5. I feel like he keeps me prisoner | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 6. He makes me feel like I have no control over my life, no power, no protection | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 7. I hide the truth from others because I am afraid not to | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 8. I feel owned and controlled by him | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 9. He can scare me without laying a hand on me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| 10. He has a look that goes straight through me and terrifies me | 6 | 5 | 4 | 3 | 2 | 1 | |||||
| To score this scale, add the responses for items 1 through 10. The score range is 10 to 60. A score of 20 or higher is a positive screening test for battering. | |||||||||||
Older, less useful tools
The Conflicts Tactics Scale was one of the first instruments to identify partner violence by measuring interpersonal aggression. The original screen consisted of 19 questions.42 The Index of Spouse Abuse is a 30-item self-report scale designed to measure the severity or magnitude of physical and nonphysical abuse inflicted on a woman by her male partner.43 Detailed independent evaluations by experienced therapists to determine whether an individual is a victim of partner abuse, considered to be the gold standard, have been used to validate the Index of Spouse Abuse. However, the Index of Spouse Abuse and Conflicts Tactics Scale are impractical for routine use in the office due to their length and complexity. Table 4 compares these screening tests.
TABLE 4
Performance characteristics of domestic violence screening instruments
| Test | LOE | Sn, %* | Sp, %* | LR+, % | LR–, % | PV+, % † | PV–, % † |
|---|---|---|---|---|---|---|---|
| ISA-P 28 ∂1 | 1b | 90.7 | 92.2 | 11.4 | 0.1 | 72 | 2.15 |
| ISA-NP 28 ∂1 | 1b | 90.7 | 90.6 | 10.1 | 0.1 | 69 | 2.15 |
| WEB 32 ∂2 | 1b | 86 | 91 | 9.56 | 0.15 | 67.8 | 3.2 |
| HITS 29 ∂3 | 3b | 96 | 91 | 10.7 | 0.04 | 70.2 | 0.87 |
| WAST 30 ∂3 | 3b | 83 | 75 | 3.32 | 0.23 | 42.2 | 4.82 |
| *Sensitivity and specificity summarized as reported in individual studies. | |||||||
| † Posttest probability was calculated assuming a pretest probability of 18%. | |||||||
| Sn, sensitivity; Sp, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; PV+, probability of disease given a positive test; PV–, probability of disease given a negative test; ∂ , reference standard; ∂1, detailed interview; ∂2, ∂3, Index of Spouse Abuse self-identified abuse victims; ISA-P, Index of Spouse Abuse scale measuring the severity or magnitude of physical abuse inflicted on a woman by her male partner; ISA-NP, Index of Spouse Abuse scale measuring the severity or magnitude of nonphysical abuse inflicted on a woman by her male partner; WEB, Women’s Experience with Battery Scale; HITS, hurt, insult, threaten, scream; WAST, Woman Abuse Screening Tool | |||||||
How physicians can help ensure safety
Table 5 shows the strength of recommendation supporting different aspects of treatment. The care of the abused woman requires a multidisciplinary team approach involving institutional and community services.28 The literature suggests that once a victim of abuse is identified in an office setting, a primary care physician can improve outcome by caring for acute injuries,28 offering support, and making appropriate referrals.
A physician can help ensure safety by:
- Assessing immediate risk. Has the violence increased in frequency or severity over the past year? Has your partner threatened to kill you or your children? Are there weapons in the house? Does your partner know that you are planning to leave? (LOE: 5)44
- Discussing safety behaviors. This includes advice on self-protection (ie, removal of weapons from the home) and planning for leaving safely in a threatening situation. One study of abused pregnant mothers found that receiving a safety intervention protocol significantly increased the safety behaviors adopted during and after pregnancy (from 47.6% at visit 1 to 78.1% at visit 6; P.≤001), preventing further abuse and increasing the safety and well-being of mother and baby (LOE: 2c)8
- Helping the patient obtain a civil protection order. This can be obtained with the assistance of the police or community advocacy services. Women with permanent protection orders are less likely than those without orders to be physically abused (risk ratio in 12 months, 0.2; 95% confidence interval, 0.1–0.8; LOE: 2b).46
- A trusting relationship with the patient can help her break the cycle of abuse and enable her to change her circumstances (LOE: 4).47 A qualitative study showed that battered women have rated the following behaviors highly desirable in their physicians (LOE: 4).10
- Initially validates their experiences with compassionate messages and emphasizes their worth as human beings
- Clearly labels the abuse as wrong and criminal
- Listens in a careful, nonjudgmental manner.
Having someone to confide in and having told someone about the abuse were factors associated with diminished abuse at 3 months in one study (P=.001 and .023, respectively) (LOE: 2c).48
TABLE 5
Evidence supporting interventions for domestic violence
| SOR | Treatment |
|---|---|
| A | Community-based advocacy intervention programs40 |
| B | Safety intervention protocols35 |
| B | Civil protection order36 |
| B | Telling or confiding in someone39 |
| B | Contact with community resources on domestic violence39 |
| B | On-site advocacy programs41 |
| C | Validating the patient’s experience38 |
| C | Assessing immediate safety and emphasizing potential for lethal outcome33,34 |
| SOR, strength of recommendation. For an explanation of the recommendations. | |
Referral to community resources
A randomized controlled trial with 2-year follow-up investigated community-based advocacy for abused women who were leaving a shelter program. This study found that advocacy services led to significantly greater effectiveness in obtaining resources, a decrease in physical violence, a decrease in depression, and an improved quality of life and social support at 10 weeks post-shelter. At 2 years, advocacy services led to reduced physical violence (11% vs 24%, P..05, number needed to treat=7.7), increased likelihood of leaving the abusive relationship (96% vs 87%, number needed to treat=11, P.<.03), and improved quality of life (P..01) (LOE: 1b).9
Straus and colleagues48 associated contact with community domestic violence resources with a decreased sense of community isolation (LOE: 2c). The National Domestic Violence Hotline (800-799-SAFE) can provide physicians in every state with information on local resources.
Muelleman and Feighny49 found that advocacy programs that are available on-site can improve the use of shelters and shelter-based counseling (LOE: 2c). However, there are no studies of suitable quality comparing outcomes for women using shelters with women not using shelters.24 Biasfree samples would be difficult to recruit. One study that evaluated experiences before and after shelter found that women experienced less violence after the shelter stay (LOE: 2c).50
Acknowledgments
I thank the faculty and residents of Shadyside Family Practice for their feedback and help with the preparation of this article.
Correspondence
Mallika Punukollu, MD, PO Box B135, Huddersfield, West Yorkshire, HD1 1YG, UK. E-mail:[email protected].
1. Elliot L, Nerney M, Jones T, Friedman PD. Barriers to screening for domestic violence. J Gen Intern Med 2002;17:112-116.
2. The Commonwealth Fund National Survey. First comprehensive national survey of American women finds them at significant risk [news release]. New York, NY: Commonwealth Fund; 1993.
3. Fogarty CT, Burge S, McCord EC. Communicating with patients about intimate partner violence: screening and interviewing approaches. Fam Med 2002;34:369-375.
4. Rodriguez M, Quiroga SS, Bauer H. Breaking the silence: battered women’s perspectives on medical care. Arch Fam Med 1996;5:153-158.
5. Rodriguez MA, Sheldon WR, Bauer HM, et al. The factors associated with disclosure of intimate partner abuse to clinicians. J Fam Pract 2001;50:338-344.
6. US Bureau of Justice Statistics. Highlights from 20 years of surveying crime victims: the National Crime Victimization survey, 1973–1992. Washington, DC: US Department of Justice; 1993.
7. Berrios DC, Grady D. Domestic violence: risk factors and outcome. West J Med 1991;155:133-135.
8. McFarlane J, Parker B, Soeken K, et al. Safety behaviors of abused women after an intervention during pregnancy. J Obstet Gynecol Neonatal Nurs 1998;27:64-69.
9. Sullivan CM, Bybee DI. Reducing violence using community-based advocacy for women with abusive partners. J Consult Clin Psychol 1999;67:43-53.
10. Nicolaidis C. The voices of survivors’ documentary. Using patient narrative to educate physicians about domestic violence. J Gen Intern Med 2002;17:117-124.
11. Centers for Disease Control and Prevention. Lifetime and annual incidence of intimate partner violence and resulting injuries—Georgia, 1995. MMWR Morb Mortal Wkly Rep 47; 1998;47:849-853.
12. Hamberger KL, Saunders DG, Hovey M. Prevalence of domestic violence in community practice and rate of physician inquiry. Fam Med 1992;24:283-287.
13. McCauley J, Kern DE, Kolodner K, et al. The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices. Ann Intern Med 1995;123:737-746.
14. Koss MP, Koss PG, Woodruff WJ. Relation of criminal victimization to health perceptions among women medical patients. Arch Intern Med 1991;151:342-347.
15. Fanslow JL, Norton RN, Spinola CG. Indicators of assaultrelated injuries among women presenting to the Emergency Department. Ann Emerg Med 1998;32:341-348.
16. Drossman DA, Lesserman J, Nachman G, et al. Sexual and Physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med 1990;113:828-833.
17. Danielson KK, Moffitt TE, Caspi A, Silva PA. Comorbidity between abuse of an adult and DSM-III-R mental disorders: Evidence from an epidemiological study. Am J Psychiatry 1998;155:131-133.
18. Bergman B, Brismar B. A 5-year follow up study of 117 battered women. Am J Public Health 1991;81:1486-1489.
19. Muelleman RL, Lenaghan PA, Pakieser RA. Battered women: injury locations and types. Ann Emerg Med 1996;28:486-492.
20. Murphy CC, Schei B, Myhr TL, DuMont J. Abuse: A risk factor for low birth weight? A systematic review and metaanalysis. CMAJ 2001;164:1578-1579.
21. Saunders DG, Hamberger K, Hovey M. Indicators of woman abuse based on a chart review at a family practice center. Arch Fam Med 1993;2:537-543.
22. Wasson JH, Jette AM, Anderson J, et al. Routine, single-item screening to identify abusive relationships in women. J Fam Pract 2000;49:1017-1022.
23. Thompson CF, Atkins D. Screening for Family Violence Washington, DC: US Preventive Services Task Force; 1996.
24. Wathen CN, MacMillan HL. Interventions for violence against women: scientific review. JAMA 2003;289:589-600-e581-e510.
25. Ramsay J, Richardson J, Carter Y, Davidson L, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002;325:314-318.
26. Age Charts for Periodic Health Examination Reprint 510. Kansas City, KS: American Academy of Family Physicians; 1994.
27. Holbrook JH, Ende J, eds. Primary care internal medicine. In: Medical Knowledge Self-Assessment Program 11. Philadelphia: American College of Physicians; 1998:21-22.
28. Flitcraft A, Hadley S, Hendricks-Matthews MK, McLeer SV, Warshaw C. Diagnostic and Treatment Guidelines on Domestic Violence. Chicago, Ill: American Medical Association; 1992.
29. Dunn L, Brown C, Dickerson V, et al. The Obstetrician.-Gynecologist and Primary-Preventive Health Care. Washington, DC: American College of Obstetricians and Gynecologists; 1993.
30. Psychosocial Risk Factors: Perinatal Screening and Intervention. Educational Bulletin 255. Washington, DC: American College of Obstetricians and Gynecologists; 1999.
31. McFarlane J, Christoffel K, Bateman L, et al. Assessing for abuse: self-report vs. nurse interview. Public Health Nurs 1991;4:245-250.
32. Canterino JC, Vanham LG, Harrigan JT, et al. Domestic abuse in pregnancy: a comparison of self completed domestic abuse questionnaire with directed interview. Am J Obstet Gynecol 1999;181:1049-1051.
33. Joint Commission on Accreditation of Healthcare Organizations. Hospital Manual. Standard PE1.9. Chicago, Ill: Joint Commission Resources, Inc; 2002.
34. Glass N, Dearwater S, Campbell J. Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments. J Emerg Nurs 2001;27:141-149.
35. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence, a randomized trial. Am J Prev Med 2000;19:253-263.
36. Harwell TS, Casten RJ, Armstrong KA, et al. Results of a domestic violence training program offered to staff of urban community health centers. Am J Prev Med 1998;15:235-242.
37. Larkin GL, Rolniak S, Hyman KB, et al. Effects of an administrative intervention on rates of screening for domestic violence in an urban emergency department. Am J Public Health 2000;90:1444-1448.
38. Sherin KM, Sinacore JM, Li X, et al. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med 1998;30:508-512.
39. Brown JB, Lent B, Brett PJ, et al. Development of the woman abuse screening tool for use in family practice. Fam Med 1996;28:422-428.
40. Fogarty CT, Brown JB. Screening for abuse in Spanish-speaking women. J Am Board Fam Pract 2002;15:101-111.
41. Coker AL, Pope BO, Smith PH, et al. Assessment of clinical partner violence screening tools. J Am Med Womens Assoc 2001;56:19-23.
42. Straus MA. Measuring intrafamily conflict and violence: the conflicts tactics scale. J Marriage Fam 1979;4:75-88.
43. Hudson WW, McIntosh SR. The assessment of spouse abuse: two quantifiable dimensions. J Marriage Fam 1981;43:873-888.
44. Campbell JC. Nursing assessment for risk of homicide with battered women. ANS Adv Nurs Sci 1986;8:36.-
45. Elliot BA, Johnson MMP. Domestic violence in primary care setting: patterns and prevalence. Arch Fam Med 1995;4:113-119.
46. Holt VL, Kernic MA, Lumley T, Wolf ME, Rivara FP. Civil protection orders and risk of subsequent police reported violence. JAMA 2002;288:589-594.
47. Gerbert B, Caspers N, Milliken N, et al. Interventions that help victims of domestic violence: a qualitative analysis of physicians’ experiences. J Fam Pract 2000;49:889-895.
48. Straus HE, Rydman RJ, Roberts RR, et al. A three months prospective outcomes study of recently abused women. Acad Emerg Med 2001;8(5):461.-
49. Muelleman RL, Feighny KM. Injury prevention: effects of an emergency department based advocacy program for battered women on community resource utilization. Ann Emerg Med 1999;33:62-66.
50. Sullivan CM, Campbell R, Angelique H, et al. An advocacy intervention program for women with abusive partners: six month follow up. Am J Comm Psychol 1994;22:101-122.
1. Elliot L, Nerney M, Jones T, Friedman PD. Barriers to screening for domestic violence. J Gen Intern Med 2002;17:112-116.
2. The Commonwealth Fund National Survey. First comprehensive national survey of American women finds them at significant risk [news release]. New York, NY: Commonwealth Fund; 1993.
3. Fogarty CT, Burge S, McCord EC. Communicating with patients about intimate partner violence: screening and interviewing approaches. Fam Med 2002;34:369-375.
4. Rodriguez M, Quiroga SS, Bauer H. Breaking the silence: battered women’s perspectives on medical care. Arch Fam Med 1996;5:153-158.
5. Rodriguez MA, Sheldon WR, Bauer HM, et al. The factors associated with disclosure of intimate partner abuse to clinicians. J Fam Pract 2001;50:338-344.
6. US Bureau of Justice Statistics. Highlights from 20 years of surveying crime victims: the National Crime Victimization survey, 1973–1992. Washington, DC: US Department of Justice; 1993.
7. Berrios DC, Grady D. Domestic violence: risk factors and outcome. West J Med 1991;155:133-135.
8. McFarlane J, Parker B, Soeken K, et al. Safety behaviors of abused women after an intervention during pregnancy. J Obstet Gynecol Neonatal Nurs 1998;27:64-69.
9. Sullivan CM, Bybee DI. Reducing violence using community-based advocacy for women with abusive partners. J Consult Clin Psychol 1999;67:43-53.
10. Nicolaidis C. The voices of survivors’ documentary. Using patient narrative to educate physicians about domestic violence. J Gen Intern Med 2002;17:117-124.
11. Centers for Disease Control and Prevention. Lifetime and annual incidence of intimate partner violence and resulting injuries—Georgia, 1995. MMWR Morb Mortal Wkly Rep 47; 1998;47:849-853.
12. Hamberger KL, Saunders DG, Hovey M. Prevalence of domestic violence in community practice and rate of physician inquiry. Fam Med 1992;24:283-287.
13. McCauley J, Kern DE, Kolodner K, et al. The “battering syndrome”: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices. Ann Intern Med 1995;123:737-746.
14. Koss MP, Koss PG, Woodruff WJ. Relation of criminal victimization to health perceptions among women medical patients. Arch Intern Med 1991;151:342-347.
15. Fanslow JL, Norton RN, Spinola CG. Indicators of assaultrelated injuries among women presenting to the Emergency Department. Ann Emerg Med 1998;32:341-348.
16. Drossman DA, Lesserman J, Nachman G, et al. Sexual and Physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med 1990;113:828-833.
17. Danielson KK, Moffitt TE, Caspi A, Silva PA. Comorbidity between abuse of an adult and DSM-III-R mental disorders: Evidence from an epidemiological study. Am J Psychiatry 1998;155:131-133.
18. Bergman B, Brismar B. A 5-year follow up study of 117 battered women. Am J Public Health 1991;81:1486-1489.
19. Muelleman RL, Lenaghan PA, Pakieser RA. Battered women: injury locations and types. Ann Emerg Med 1996;28:486-492.
20. Murphy CC, Schei B, Myhr TL, DuMont J. Abuse: A risk factor for low birth weight? A systematic review and metaanalysis. CMAJ 2001;164:1578-1579.
21. Saunders DG, Hamberger K, Hovey M. Indicators of woman abuse based on a chart review at a family practice center. Arch Fam Med 1993;2:537-543.
22. Wasson JH, Jette AM, Anderson J, et al. Routine, single-item screening to identify abusive relationships in women. J Fam Pract 2000;49:1017-1022.
23. Thompson CF, Atkins D. Screening for Family Violence Washington, DC: US Preventive Services Task Force; 1996.
24. Wathen CN, MacMillan HL. Interventions for violence against women: scientific review. JAMA 2003;289:589-600-e581-e510.
25. Ramsay J, Richardson J, Carter Y, Davidson L, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002;325:314-318.
26. Age Charts for Periodic Health Examination Reprint 510. Kansas City, KS: American Academy of Family Physicians; 1994.
27. Holbrook JH, Ende J, eds. Primary care internal medicine. In: Medical Knowledge Self-Assessment Program 11. Philadelphia: American College of Physicians; 1998:21-22.
28. Flitcraft A, Hadley S, Hendricks-Matthews MK, McLeer SV, Warshaw C. Diagnostic and Treatment Guidelines on Domestic Violence. Chicago, Ill: American Medical Association; 1992.
29. Dunn L, Brown C, Dickerson V, et al. The Obstetrician.-Gynecologist and Primary-Preventive Health Care. Washington, DC: American College of Obstetricians and Gynecologists; 1993.
30. Psychosocial Risk Factors: Perinatal Screening and Intervention. Educational Bulletin 255. Washington, DC: American College of Obstetricians and Gynecologists; 1999.
31. McFarlane J, Christoffel K, Bateman L, et al. Assessing for abuse: self-report vs. nurse interview. Public Health Nurs 1991;4:245-250.
32. Canterino JC, Vanham LG, Harrigan JT, et al. Domestic abuse in pregnancy: a comparison of self completed domestic abuse questionnaire with directed interview. Am J Obstet Gynecol 1999;181:1049-1051.
33. Joint Commission on Accreditation of Healthcare Organizations. Hospital Manual. Standard PE1.9. Chicago, Ill: Joint Commission Resources, Inc; 2002.
34. Glass N, Dearwater S, Campbell J. Intimate partner violence screening and intervention: data from eleven Pennsylvania and California community hospital emergency departments. J Emerg Nurs 2001;27:141-149.
35. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence, a randomized trial. Am J Prev Med 2000;19:253-263.
36. Harwell TS, Casten RJ, Armstrong KA, et al. Results of a domestic violence training program offered to staff of urban community health centers. Am J Prev Med 1998;15:235-242.
37. Larkin GL, Rolniak S, Hyman KB, et al. Effects of an administrative intervention on rates of screening for domestic violence in an urban emergency department. Am J Public Health 2000;90:1444-1448.
38. Sherin KM, Sinacore JM, Li X, et al. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med 1998;30:508-512.
39. Brown JB, Lent B, Brett PJ, et al. Development of the woman abuse screening tool for use in family practice. Fam Med 1996;28:422-428.
40. Fogarty CT, Brown JB. Screening for abuse in Spanish-speaking women. J Am Board Fam Pract 2002;15:101-111.
41. Coker AL, Pope BO, Smith PH, et al. Assessment of clinical partner violence screening tools. J Am Med Womens Assoc 2001;56:19-23.
42. Straus MA. Measuring intrafamily conflict and violence: the conflicts tactics scale. J Marriage Fam 1979;4:75-88.
43. Hudson WW, McIntosh SR. The assessment of spouse abuse: two quantifiable dimensions. J Marriage Fam 1981;43:873-888.
44. Campbell JC. Nursing assessment for risk of homicide with battered women. ANS Adv Nurs Sci 1986;8:36.-
45. Elliot BA, Johnson MMP. Domestic violence in primary care setting: patterns and prevalence. Arch Fam Med 1995;4:113-119.
46. Holt VL, Kernic MA, Lumley T, Wolf ME, Rivara FP. Civil protection orders and risk of subsequent police reported violence. JAMA 2002;288:589-594.
47. Gerbert B, Caspers N, Milliken N, et al. Interventions that help victims of domestic violence: a qualitative analysis of physicians’ experiences. J Fam Pract 2000;49:889-895.
48. Straus HE, Rydman RJ, Roberts RR, et al. A three months prospective outcomes study of recently abused women. Acad Emerg Med 2001;8(5):461.-
49. Muelleman RL, Feighny KM. Injury prevention: effects of an emergency department based advocacy program for battered women on community resource utilization. Ann Emerg Med 1999;33:62-66.
50. Sullivan CM, Campbell R, Angelique H, et al. An advocacy intervention program for women with abusive partners: six month follow up. Am J Comm Psychol 1994;22:101-122.
Treatment of skin malignancies
- Malignant melanomas in situ can usually be treated in consultation with a specialist (C). Larger lesions may require referral.
- Based on best available evidence, surgical excision is the first-line treatment for most nonmelanoma skin cancers, with cure rates as high as 98% with proper margins (C).
- Consider Mohs surgery for larger lesions, sclerosing lesions with morpheaform histology, or for cosmetically sensitive areas (A).
- With properly selected lesions, curettage/electrodesiccation and cryosurgery have cure rates comparable to that of surgical excision (B).
Reliable criteria exist to guide primary care physicians in the evaluation of malignant melanoma.1 Once a diagnosis of malignant melanoma is made, physicians should promptly consult with a skin malignancy specialist (Figure 1).
Several well-accepted treatment options exist for primary nonmelanoma skin cancer, but direct comparisons are lacking. The best evidence recommends surgical excision as first-line treatment for most nonmelanoma skin cancer. Cure rates up to 98% can be expected with appropriate margins.
Curettage/electrodesiccation and cryosurgery are probably equally effective with properly selected lesions. Physicians should be aware of those cases when nonmelanoma skin cancers are at higher risk for recurrence or metastasis and tailor their treatment plan accordingly.
Mohs micrographic surgery is the preferred treatment for high-risk and recurrent primary nonmelanoma skin cancer.
In this Applied Evidence review, we examine the guidance that can be gleaned from currently available evidence, and review each option in detail.
FIGURE 1
Surgical excision of suspected skin malignancies
Confirming the diagnosis
Our previous article in THE JOURNAL OF FAMILY PRACTICE, “Diagnosing skin malignancy: Assessment of predictive clinical criteria and risk factors,”1 presented a comprehensive review for the initial evaluation of skin malignancies. (This article is available online at www.jfponline.com.)
Nonmelanoma skin cancer includes basal cell carcinomas and squamous cell carcinomas. Nonmelanoma skin cancer, which accounts for most cases of skin cancer, has a high 5-year survival rate, more than 95%.2 Malignant melanoma represents only 1% of skin malignancies but leads to more than 75% of skin cancer deaths.3 Up to 83% of family physicians treat skin malignancies in their offices.4
When the diagnosis is uncertain on clinical grounds alone, an initial diagnostic biopsy may be used. Techniques include excisional, punch, and shave biopsies.
Excisional biopsy (Figure 2) is the preferred method for primary care evaluation of suspected skin malignancy (level of evidence [LOE]: 5).5,6 Excision wounds are easily cared for by patients and provide good cosmesis. Surgical excision may also be used for definitive treatment, as discussed below.
Punch biopsy or incisional biopsy (Figure 3) provides a full-thickness tissue sample with minimal scarring. A 3-mm punch is sufficient for most lesions (LOE: 5).5 Punch biopsy is a reasonable alternative when excision is impractical due to the size or location of a lesion.
Punch biopsy is not recommended if malignant melanoma is suspected by history or physical exam—an excisional biopsy should be done (LOE: 5).6 Even when performed mistakenly on a malignant melanoma, however, punch biopsy has not been shown to alter prognosis or cause dissemination of a tumor (LOE: 1b).7
Shave biopsies (Figure 4) are useful for benign-appearing lesions or elevated, nodular lesions suggestive of basal cell carcinoma or squamous cell carcinoma. Shave biopsy sites typically heal more slowly than excisional biopsies but do so with good cosmesis. In general, shave biopsy of pigmented lesions is contraindicated, but may be performed by an experienced physician confident of a benign clinical diagnosis. According to a National Institutes of Health consensus panel, suspected malignant melanoma should not be shaved, as the maximal depth of the tumor cannot be assessed.8
FIGURE 2
Excisional biopsy
FIGURE 3
Punch/Incisional biopsy
FIGURE 4
Shave biopsy
Malignant melanoma treatment
Overview
If a skin lesion is suspected clinically to be malignant melanoma, use the ABCD criteria and the revised 7-point checklist to guide biopsy decisions.1 If the physician or patient has any doubt, perform a biopsy.
If malignant melanoma is suspected, the initial biopsy should be conservative, with 1–2 mm margins (LOE: 5),9,10 and excisional, in order to determine the thickness of the tumor (Breslow’s measurement) and the histologic level of invasion (Clark’s level). Tumor depth at the time of diagnosis is the main factor guiding treatment and is the principal indicator predicting death due to malignant melanoma.8 Long-term survival of patients (10 years) with malignant melanoma <0.76 mm in thickness is greater than 90%; mortality rises linearly with increasing tumor thickness (LOE: 2b).11
Although knowledge of biopsy type is important for proper pathologic evaluation, it does not affect survival (LOE: 1b).7 Reviewing initial pathology results, a skin malignancy specialist (dermatologist, surgical oncologist, or plastic surgeon) can help determine the surgical margin necessary for curative excision. While most primary care physicians can treat lesions in situ, larger lesions require referral. Current recommendations for excision margins are shown in Table 1.
Options for lymph node management include observation, sentinel lymph node biopsy, and elective lymph node dissection. The choice of treatment depends on clinical staging, tumor thickness, ulceration, location, and patient age, and should be managed by a melanoma specialist.
TABLE 1
Excision margins for malignant melanoma
| Tumor thickness | Surgical margin | Level of evidence |
|---|---|---|
| In situ | 5 mm | 58 |
| <1 mm | 1 cm | 1b12 |
| 1–4 mm | 2 cm | 1b13 |
| >4 mm | 3 cm | 1b12,13 |
Systemic therapy
Dacarbazine is the accepted treatment of choice for metastatic malignant melanoma. A systematic review found no randomized controlled trials comparing systemic therapy with supportive care or placebo. Several trials used dacarbazine as the control agent and found response rates varying from 9.1% to 29% (no test agent was found superior). Combination chemotherapy should be reserved for clinical studies and trials (LOE: 1a).14
Interferon alfa-2b is used as adjuvant therapy after surgical resection. It has an overall 5-year survival advantage over placebo (46% vs. 37%; number needed to treat [NNT]= 11, P=.0237) (LOE: 1b).15 Additionally, an economic analysis of this treatment found it to be cost-effective for high-risk melanomas (LOE: 2b).16
Vaccine therapy has shown preliminary benefit in several case-control studies but needs to be investigated further before it can be recommended (LOE: 3b).17
Nonmelanoma skin cancer treatment
Overview
Treatments for nonmelanoma skin cancer include surgical excision, Mohs micrographic surgery, curettage and electrodesiccation, cryosurgery, fractionated radiotherapy, topical chemotherapy, carbon dioxide laser, photo-dynamic therapy, intralesional interferon, and retinoids.
Some of these therapies are untested or not widely available. Immunotherapy and photodynamic therapy remain experimental.18 Laser treatment offers theoretical advantages for certain patients, such as those taking anticoagulants. However, safety hazards and inconvenience limit their use even by dermatologists (LOE: 5).19
Two meta-analysis reviews have attempted to compare cure rates for treatment of basal cell carcinoma by conventional methods.20,21 In these studies, standard excision was recommended for nodular-ulcerative and superficial basal cell carcinoma <2 cm in diameter and away from the face. Consideration of Mohs surgery was recommended for larger lesions, sclerosing lesions with morpheaform histology, and for cosmetically sensitive areas where large tissue loss or recurrence would be disfiguring (eyelid, ear, nose, lips) (LOE: 1a).20 Results are summarized in Table 2.
It should be noted that lack of uniformity in the method of reporting prohibited direct comparison of recurrence rates for different treatments. No similar meta-analysis reviews of treatment for squamous cell carcinoma were found.
TABLE 2
Effectiveness of treatment modalities for basal cell carcinoma 20
| Treatment modality | Raw recurrence rate %* | Mean rawrecurrence % | Cumulative 5-year recurrence rate % † |
|---|---|---|---|
| Surgical excision | 1.4–2.9 | N/A | 5.3 |
| Mohs micrographic surgery | 0.5–1.3 | 0.8 (21/2660) | N/A |
| Curettage and electrodesiccation | 3.8–18.1 | N/A | 13.2 |
| Cryosurgery | 0–11.4 | 3.0 (24/798) | N/A |
| N/A, not available | |||
| *Absolute number of patients with recurrence divided by number of patients with primary basal cell carcinoma at start of study (unknown number lost to follow-up). | |||
| †Life-table cumulative 5-year recurrence rate. | |||
Recurrence and metastasis
The average rate of metastasis is 3.6% for squamous cell carcinoma. However, certain nonmelanoma skin cancer lesions are at higher risk for recurrence or metastasis and merit special consideration: larger lesions, those involving a mucous membrane, and lesions located on the scalp, ears, eyelids, nose, lips, or genitals. The rate of metastasis with lesions at high-risk locations may approach 30%.5
The majority of squamous cell carcinoma is actinically induced on sun-exposed areas. Squamous cell carcinoma occurring at a chronic scar or ulcer, such as that caused by a thermal or radiation burn, is also more likely to metastasize (LOE: 5).22 Metastatic spread most commonly involves regional lymph nodes, lungs, and liver. Basal cell carcinomas have an extremely low rate of metastasis (<0.1%).23
The overall rate of recurrence is also low—less than 1% for lesions removed from the neck, trunk, and extremities.24 Risk factors for recurrence include size of lesion and morpheaform histology found with sclerosing basal cell carcinoma (with associated subclinical infiltration). Lesions of the face are at higher risk for recurrence with rates up to 43% on the lateral canthus, 33% on the superior orbital rim and brow, 24% on the ear, and 19% on the nose.18 Primary care physicians should consider referral for such high-risk lesions, depending on their level of training and experience.
Surgical excision
Surgical excision is generally considered the gold standard for evaluation and treatment of suspected skin malignancy (LOE: 5).5,6 Advantages include rapid healing, excellent cosmesis, and an option to obtain a pathological evaluation of the excised tissue.
Conversely, excision is a more time-consuming prodedure than curettage and electrodesiccation or cryosurgery, and it may sacrifice more normal tissue than Mohs surgery. A surgical margin of normal-appearing tissue is routinely excised to eliminate microscopic tumor extension.
Two studies provide objective data regarding the margins necessary to ensure tumor clearance for basal cell carcinoma and squamous cell carcinoma. Both were prospective studies using Mohs surgery as the gold standard. Histological, subclinical tumor extension was then compared with ink markings placed preoperatively on the normal-appearing skin.
The first study enrolled 111 consecutive patients, aged 51 to 95 years, presenting with invasive squamous cell carcinoma. The results are summarized in Table 3.25 Importantly, 30% of the tumors extended into the subcutaneous fat. The researchers recommend that excision of all squamous cell carcinomas should include subcutaneous fat (LOE: 2b).
The second study enrolled 117 patients with previously untreated, well-demarcated basal cell carcinoma. The investigators concluded that a 4-mm lateral margin will totally eradicate 98% of tumors <2 cm in diameter (3.79 mm; 95% confidence interval [CI], 3.21–4.86) (LOE: 2b).26 Vertical extension of tumor was not addressed. No recommendations were made for tumors 2 cm in diameter.
Interestingly, no tumors in this study required a 4-mm margin in all directions. Rather, the basal cell carcinomas were found to send out extensions in an irregular pattern.
TABLE 3
Surgical margins for treatment of squamous cell carcinomas 25
| Tumor diameter and risk level | Margin needed to completely clear 95% or more of tumors |
|---|---|
| 0–19 mm | 4 mm |
| ≥20 mm | 6 mm |
| “High-risk,”* 0–19 mm | 6 mm |
| “High-risk,”* 20 mm | Indeterminate† |
| *High risk of metastasis if located on scalp, ears, eyelids, nose, or lips (47 of 141 tumors in study located here). | |
| †Only 90% of tumors completely cleared even with 6 mm margin; author recommends Mohs surgery in this case. | |
Mohs micrographic surgery
Mohs surgery is generally considered the preferred treatment for high-risk primary non-melanoma skin cancer and recurrent non-melanoma skin cancer (LOE: 5).18 It is clearly superior for treatment of locally recurrent squamous cell carcinoma, with a 5-year cure rate of up to 90%, compared with 76.7% obtained with standard excision (LOE: 2a).27
The benefit of Mohs surgery for small (<1 cm), low-risk skin cancers is probably slight. Cure rates for primary nonmelanoma skin cancer are as high as 99.8% for basal cell carcinoma and 98.8% for squamous cell carcinoma, but standard excision also has high cure rates, up to 98% with proper surgical margins.19
Disadvantages include length of the procedure, need for special equipment, and dependence on highly trained personnel (a fellowshiptrained dermatologist and histotechnologist). Not surprisingly, availability of this procedure is limited. The high initial cost may be balanced by the lower cost of treating recurrent tumors, but no cost-effectiveness studies are available.28
Curettage and electrodesiccation
Curettage and electrodesiccation is performed using a sharp curette to remove soft material from the tumor until the underlying dermis is reached. The base of the tumor is then destroyed using hyfrecation. The procedure may be repeated once or twice to achieve a base of healthy tissue (LOE: 5).19,29
Curettage and electrodesiccation is the most common way dermatologists treat small (<1 cm) primary basal cell carcinoma.18 It is safe, well tolerated, and has 5-year cure rates >90%.5 Other advantages include speed and suitability for patients with multiple lesions.19 Curettage and electrodesiccation is also appropriate for small (<1 cm) primary squamous cell carcinoma below the neck (LOE: 5).22 This technique should not be used for malignant melanoma and is not suitable for tumors with high risk of metastasis or recurrence (LOE: 5).22
Cryosurgery
Cryosurgery with liquid nitrogen or nitrous oxide is used for small (<1 cm) superficial lesions and those locations where surgery is technically difficult, such as the lips, nose, ears, eyelids, and hands. It is also ideal for patients on chronic anticoagulation or with significant comorbidities.
For properly selected lesions, 5-year cure rates are excellent at 97% (LOE: 1a).20 Cure rates for larger lesions are reportedly high, but study follow-up was inconsistent (LOE: 2b).30 Small lesions heal completely in 4 to 6 weeks, while larger lesions may take up to 14 weeks to resolve (LOE: 5).31 Despite the prolonged healing time, cosmesis is often excellent.
Guidelines for the treatment of non-melanoma skin cancer with cryosurgery are presented in Figure 5. Importantly, published cure rates for cryosurgery are based on several case series, the largest of which employed a temperature-monitoring thermocouple needle to guarantee adequate depth of freezing. Cryosurgery without a thermocouple device may therefore result in higher recurrence rates than expected.31
FIGURE 5
Guidelines for cryosurgery
Topical agents
Topical chemotherapeutic agents offer great promise in the treatment of properly selected tumors, but quality evidence is lacking. The most commonly used topical agent is the DNA synthesis inhibitor 5-fluorouracil (5-FU). 5-FU is a proven treatment for actinic keratoses and has been used to treat squamous cell carcinoma in situ and superficial basal cell carcinoma. However, 5-FU is contraindicated in invasive squamous cell carcinoma and basal cell carcinoma, as it may bury tumor tissue and lead to continued, subclinical spread (LOE: 5).19
Imiquimod 5% cream also shows great promise for the treatment of superficial basal cell carcinoma. This cytokine and interferon inducer is primarily used for treatment of external genital and anal warts. Twice daily, once daily, or 3 times weekly application over 10 to 16 weeks produces histological clearing of low-risk, small superficial basal cell carcinoma (LOE: 2b).32 Adverse events are limited to local skin reactions with severity increasing with more frequent dosing. Research examining rates of recurrence is ongoing. As with 5-FU, effectiveness for large tumors and those at high-risk locations has not been established.
Radiotherapy
Currently, radiotherapy is used in special situations, such as nonmelanoma skin cancer near the eye, nose, and ear (LOE: 5).22 In specialty centers, radiation is used in conjunction with other modalities for recurrent or highly aggressive lesions.
While cure rates in low-risk areas are over 90%, long-term cosmesis, particularly for young patients with basal cell carcinoma, is less favorable than with other modalities.20 Other disadvantages include long-term radiation risks, high cost, and need for multiple visits over several weeks.22
Follow-up
Periodic population-based screening for non-melanoma skin cancer has not been proven to extend life. However, in patients who have already had nonmelanoma skin cancer, periodic surveillance is probably important.
Of patients with squamous cell carcinoma, 30% will develop an additional squamous cell carcinoma after 5 years and over 50% will develop an additional nonmelanoma skin cancer.33 More than one third of patients with basal cell carcinoma will develop an additional basal cell carcinoma after 5 years.33
One accepted approach is to have patients with newly diagnosed nonmelanoma skin cancer follow-up every 3 months for the first year and then at 6-month intervals thereafter (LOE: 5).5 New primary lesions should be treated accordingly, while recurrent lesions may be referred to a dermatologist.
Acknowledgments
The authors wish to thank Barbara Zuckerman and Michael Campese, PhD for their assistance in preparation of this manuscript.
Corresponding author
Scott M. Strayer, MD, MPH, University of Virginia Health System, Department of Family Medicine, P.O. Box 800729, Charlottesville, VA 22908-0729. E-mail: [email protected].
1. Strayer SM, Reynolds PL. Diagnosing skin malignancy: assessment of predictive clinical criteria and risk factors. J Fam Pract 2003;52:210-218.
2. Gloster HM, Jr, Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996;22:217-226.
3. Skin tumors. In: Sauer GC, Hall JC, eds. A manual of skin diseases. Philadelphia, Pa: Lippincott-Raven;1996:342.
4. American Academy of Family Physicians. Practice Profile II Survey, May 2000. Shawnee Mission, Kansas: American Academy of Family Physicians; 2000.
5. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27:447-458.
6. Bruce AJ, Brodland DG. Overview of skin cancer detection and prevention for the primary care physician. Mayo Clin Proc 2000;75:491-500.
7. Lederman JS, Sober AJ. Does biopsy type influence survival in clinical stage I cutaneous melanoma? J Am Acad Dermatol 1985;13:983-987.
8. NIH Consensus conference Diagnosis and treatment of early melanoma. JAMA 1992;268:1314-1319.
9. Martinez JC, Otley CC. The management of melanoma and nonmelanoma skin cancer: a review for the primary care physician. Mayo Clin Proc 2001;76:1253-1265.
10. Goldstein BG, Goldstein AO. Diagnosis and management of malignant melanoma. Am Fam Physician 2001;63:1359-1368.
11. Melanoma in the Southern United States. In: Balch CM, Milton GW, eds. Cutaneous melanoma: clinical management and treatment results worldwide. Philadelphia: Lippincott; 1985:397-406.
12. Veronesi U, Cascinelli N. Narrow excision (1-cm margin): a safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-441.
13. Balch CM, Urist M, Karakousis C. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1-4 mm): results of a multi-institutional randomized surgical trial. Ann Surg 1993;218:262-267.
14. Crosby T, Fish R, Coles B, Mason MD. Systemic treatments for metastaic cutaneous melanoma. The Cochrane Library 2002;(2).:
15. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17.
16. Hillner B, Kirkwood J, Atkins M, Johnson E, Smith T. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol 1997;15:2351-2358.
17. Ollila DW, Kelley MC, Gammon G, Morton DL. Overview of melanoma vaccines: active specific immunotherapy for melanoma patients. Semin Surg Oncol 1998;14:328-336.
18. McGovern TW, Leffell DJ. Mohs surgery: the informed view. Arch Dermatol 1999;135:1255-1259.
19. Hochman M, Lang P. Skin cancer of the head and neck. Med Clin North Am 1999;83:261-282.
20. Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol 1999;135:1255-1259.
21. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol 1995;13:617-620.
22. Alam M, Ratner D. Primary care: cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-983.
23. Von Domarus H, Stern PJ. Metastatic basal cell carcinoma report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 1984;10:1043-1060.
24. Silverman MK, Kopf AW, Bart RS, Grin CM, Levenstein MS. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol 1993;18:471-476.
25. Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol 1992;27:241-248.
26. Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol 1987;123:340-344.
27. Rowe DE, Carroll RJ, Day CL, Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol 1992;26:976-990.
28. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol 1998;39:698-703.
29. Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for basal cell carcinoma. The American Academy of Dermatology Committee on Guidelines of Care. J Am Acad Dermatol 1992;26:117-120.
30. Graham GC, Clark LC. Statistical analysis in cryosurgery of skin cancer. Clin Dermatol 1990;8:101-107.
31. Kuflik EG. Cryosurgery for cutaneous malignancy. Dermatol Surg 1997;23:1081-1087.
32. Beutner KR, Geisse JK, Helman D, Fox TL, Ginkel A, Owens ML. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol 1999;41:1002-1007.
33. Rhodes AR. Public education and cancer of the skin. Cancer 1995;75:613-636.
- Malignant melanomas in situ can usually be treated in consultation with a specialist (C). Larger lesions may require referral.
- Based on best available evidence, surgical excision is the first-line treatment for most nonmelanoma skin cancers, with cure rates as high as 98% with proper margins (C).
- Consider Mohs surgery for larger lesions, sclerosing lesions with morpheaform histology, or for cosmetically sensitive areas (A).
- With properly selected lesions, curettage/electrodesiccation and cryosurgery have cure rates comparable to that of surgical excision (B).
Reliable criteria exist to guide primary care physicians in the evaluation of malignant melanoma.1 Once a diagnosis of malignant melanoma is made, physicians should promptly consult with a skin malignancy specialist (Figure 1).
Several well-accepted treatment options exist for primary nonmelanoma skin cancer, but direct comparisons are lacking. The best evidence recommends surgical excision as first-line treatment for most nonmelanoma skin cancer. Cure rates up to 98% can be expected with appropriate margins.
Curettage/electrodesiccation and cryosurgery are probably equally effective with properly selected lesions. Physicians should be aware of those cases when nonmelanoma skin cancers are at higher risk for recurrence or metastasis and tailor their treatment plan accordingly.
Mohs micrographic surgery is the preferred treatment for high-risk and recurrent primary nonmelanoma skin cancer.
In this Applied Evidence review, we examine the guidance that can be gleaned from currently available evidence, and review each option in detail.
FIGURE 1
Surgical excision of suspected skin malignancies
Confirming the diagnosis
Our previous article in THE JOURNAL OF FAMILY PRACTICE, “Diagnosing skin malignancy: Assessment of predictive clinical criteria and risk factors,”1 presented a comprehensive review for the initial evaluation of skin malignancies. (This article is available online at www.jfponline.com.)
Nonmelanoma skin cancer includes basal cell carcinomas and squamous cell carcinomas. Nonmelanoma skin cancer, which accounts for most cases of skin cancer, has a high 5-year survival rate, more than 95%.2 Malignant melanoma represents only 1% of skin malignancies but leads to more than 75% of skin cancer deaths.3 Up to 83% of family physicians treat skin malignancies in their offices.4
When the diagnosis is uncertain on clinical grounds alone, an initial diagnostic biopsy may be used. Techniques include excisional, punch, and shave biopsies.
Excisional biopsy (Figure 2) is the preferred method for primary care evaluation of suspected skin malignancy (level of evidence [LOE]: 5).5,6 Excision wounds are easily cared for by patients and provide good cosmesis. Surgical excision may also be used for definitive treatment, as discussed below.
Punch biopsy or incisional biopsy (Figure 3) provides a full-thickness tissue sample with minimal scarring. A 3-mm punch is sufficient for most lesions (LOE: 5).5 Punch biopsy is a reasonable alternative when excision is impractical due to the size or location of a lesion.
Punch biopsy is not recommended if malignant melanoma is suspected by history or physical exam—an excisional biopsy should be done (LOE: 5).6 Even when performed mistakenly on a malignant melanoma, however, punch biopsy has not been shown to alter prognosis or cause dissemination of a tumor (LOE: 1b).7
Shave biopsies (Figure 4) are useful for benign-appearing lesions or elevated, nodular lesions suggestive of basal cell carcinoma or squamous cell carcinoma. Shave biopsy sites typically heal more slowly than excisional biopsies but do so with good cosmesis. In general, shave biopsy of pigmented lesions is contraindicated, but may be performed by an experienced physician confident of a benign clinical diagnosis. According to a National Institutes of Health consensus panel, suspected malignant melanoma should not be shaved, as the maximal depth of the tumor cannot be assessed.8
FIGURE 2
Excisional biopsy
FIGURE 3
Punch/Incisional biopsy
FIGURE 4
Shave biopsy
Malignant melanoma treatment
Overview
If a skin lesion is suspected clinically to be malignant melanoma, use the ABCD criteria and the revised 7-point checklist to guide biopsy decisions.1 If the physician or patient has any doubt, perform a biopsy.
If malignant melanoma is suspected, the initial biopsy should be conservative, with 1–2 mm margins (LOE: 5),9,10 and excisional, in order to determine the thickness of the tumor (Breslow’s measurement) and the histologic level of invasion (Clark’s level). Tumor depth at the time of diagnosis is the main factor guiding treatment and is the principal indicator predicting death due to malignant melanoma.8 Long-term survival of patients (10 years) with malignant melanoma <0.76 mm in thickness is greater than 90%; mortality rises linearly with increasing tumor thickness (LOE: 2b).11
Although knowledge of biopsy type is important for proper pathologic evaluation, it does not affect survival (LOE: 1b).7 Reviewing initial pathology results, a skin malignancy specialist (dermatologist, surgical oncologist, or plastic surgeon) can help determine the surgical margin necessary for curative excision. While most primary care physicians can treat lesions in situ, larger lesions require referral. Current recommendations for excision margins are shown in Table 1.
Options for lymph node management include observation, sentinel lymph node biopsy, and elective lymph node dissection. The choice of treatment depends on clinical staging, tumor thickness, ulceration, location, and patient age, and should be managed by a melanoma specialist.
TABLE 1
Excision margins for malignant melanoma
| Tumor thickness | Surgical margin | Level of evidence |
|---|---|---|
| In situ | 5 mm | 58 |
| <1 mm | 1 cm | 1b12 |
| 1–4 mm | 2 cm | 1b13 |
| >4 mm | 3 cm | 1b12,13 |
Systemic therapy
Dacarbazine is the accepted treatment of choice for metastatic malignant melanoma. A systematic review found no randomized controlled trials comparing systemic therapy with supportive care or placebo. Several trials used dacarbazine as the control agent and found response rates varying from 9.1% to 29% (no test agent was found superior). Combination chemotherapy should be reserved for clinical studies and trials (LOE: 1a).14
Interferon alfa-2b is used as adjuvant therapy after surgical resection. It has an overall 5-year survival advantage over placebo (46% vs. 37%; number needed to treat [NNT]= 11, P=.0237) (LOE: 1b).15 Additionally, an economic analysis of this treatment found it to be cost-effective for high-risk melanomas (LOE: 2b).16
Vaccine therapy has shown preliminary benefit in several case-control studies but needs to be investigated further before it can be recommended (LOE: 3b).17
Nonmelanoma skin cancer treatment
Overview
Treatments for nonmelanoma skin cancer include surgical excision, Mohs micrographic surgery, curettage and electrodesiccation, cryosurgery, fractionated radiotherapy, topical chemotherapy, carbon dioxide laser, photo-dynamic therapy, intralesional interferon, and retinoids.
Some of these therapies are untested or not widely available. Immunotherapy and photodynamic therapy remain experimental.18 Laser treatment offers theoretical advantages for certain patients, such as those taking anticoagulants. However, safety hazards and inconvenience limit their use even by dermatologists (LOE: 5).19
Two meta-analysis reviews have attempted to compare cure rates for treatment of basal cell carcinoma by conventional methods.20,21 In these studies, standard excision was recommended for nodular-ulcerative and superficial basal cell carcinoma <2 cm in diameter and away from the face. Consideration of Mohs surgery was recommended for larger lesions, sclerosing lesions with morpheaform histology, and for cosmetically sensitive areas where large tissue loss or recurrence would be disfiguring (eyelid, ear, nose, lips) (LOE: 1a).20 Results are summarized in Table 2.
It should be noted that lack of uniformity in the method of reporting prohibited direct comparison of recurrence rates for different treatments. No similar meta-analysis reviews of treatment for squamous cell carcinoma were found.
TABLE 2
Effectiveness of treatment modalities for basal cell carcinoma 20
| Treatment modality | Raw recurrence rate %* | Mean rawrecurrence % | Cumulative 5-year recurrence rate % † |
|---|---|---|---|
| Surgical excision | 1.4–2.9 | N/A | 5.3 |
| Mohs micrographic surgery | 0.5–1.3 | 0.8 (21/2660) | N/A |
| Curettage and electrodesiccation | 3.8–18.1 | N/A | 13.2 |
| Cryosurgery | 0–11.4 | 3.0 (24/798) | N/A |
| N/A, not available | |||
| *Absolute number of patients with recurrence divided by number of patients with primary basal cell carcinoma at start of study (unknown number lost to follow-up). | |||
| †Life-table cumulative 5-year recurrence rate. | |||
Recurrence and metastasis
The average rate of metastasis is 3.6% for squamous cell carcinoma. However, certain nonmelanoma skin cancer lesions are at higher risk for recurrence or metastasis and merit special consideration: larger lesions, those involving a mucous membrane, and lesions located on the scalp, ears, eyelids, nose, lips, or genitals. The rate of metastasis with lesions at high-risk locations may approach 30%.5
The majority of squamous cell carcinoma is actinically induced on sun-exposed areas. Squamous cell carcinoma occurring at a chronic scar or ulcer, such as that caused by a thermal or radiation burn, is also more likely to metastasize (LOE: 5).22 Metastatic spread most commonly involves regional lymph nodes, lungs, and liver. Basal cell carcinomas have an extremely low rate of metastasis (<0.1%).23
The overall rate of recurrence is also low—less than 1% for lesions removed from the neck, trunk, and extremities.24 Risk factors for recurrence include size of lesion and morpheaform histology found with sclerosing basal cell carcinoma (with associated subclinical infiltration). Lesions of the face are at higher risk for recurrence with rates up to 43% on the lateral canthus, 33% on the superior orbital rim and brow, 24% on the ear, and 19% on the nose.18 Primary care physicians should consider referral for such high-risk lesions, depending on their level of training and experience.
Surgical excision
Surgical excision is generally considered the gold standard for evaluation and treatment of suspected skin malignancy (LOE: 5).5,6 Advantages include rapid healing, excellent cosmesis, and an option to obtain a pathological evaluation of the excised tissue.
Conversely, excision is a more time-consuming prodedure than curettage and electrodesiccation or cryosurgery, and it may sacrifice more normal tissue than Mohs surgery. A surgical margin of normal-appearing tissue is routinely excised to eliminate microscopic tumor extension.
Two studies provide objective data regarding the margins necessary to ensure tumor clearance for basal cell carcinoma and squamous cell carcinoma. Both were prospective studies using Mohs surgery as the gold standard. Histological, subclinical tumor extension was then compared with ink markings placed preoperatively on the normal-appearing skin.
The first study enrolled 111 consecutive patients, aged 51 to 95 years, presenting with invasive squamous cell carcinoma. The results are summarized in Table 3.25 Importantly, 30% of the tumors extended into the subcutaneous fat. The researchers recommend that excision of all squamous cell carcinomas should include subcutaneous fat (LOE: 2b).
The second study enrolled 117 patients with previously untreated, well-demarcated basal cell carcinoma. The investigators concluded that a 4-mm lateral margin will totally eradicate 98% of tumors <2 cm in diameter (3.79 mm; 95% confidence interval [CI], 3.21–4.86) (LOE: 2b).26 Vertical extension of tumor was not addressed. No recommendations were made for tumors 2 cm in diameter.
Interestingly, no tumors in this study required a 4-mm margin in all directions. Rather, the basal cell carcinomas were found to send out extensions in an irregular pattern.
TABLE 3
Surgical margins for treatment of squamous cell carcinomas 25
| Tumor diameter and risk level | Margin needed to completely clear 95% or more of tumors |
|---|---|
| 0–19 mm | 4 mm |
| ≥20 mm | 6 mm |
| “High-risk,”* 0–19 mm | 6 mm |
| “High-risk,”* 20 mm | Indeterminate† |
| *High risk of metastasis if located on scalp, ears, eyelids, nose, or lips (47 of 141 tumors in study located here). | |
| †Only 90% of tumors completely cleared even with 6 mm margin; author recommends Mohs surgery in this case. | |
Mohs micrographic surgery
Mohs surgery is generally considered the preferred treatment for high-risk primary non-melanoma skin cancer and recurrent non-melanoma skin cancer (LOE: 5).18 It is clearly superior for treatment of locally recurrent squamous cell carcinoma, with a 5-year cure rate of up to 90%, compared with 76.7% obtained with standard excision (LOE: 2a).27
The benefit of Mohs surgery for small (<1 cm), low-risk skin cancers is probably slight. Cure rates for primary nonmelanoma skin cancer are as high as 99.8% for basal cell carcinoma and 98.8% for squamous cell carcinoma, but standard excision also has high cure rates, up to 98% with proper surgical margins.19
Disadvantages include length of the procedure, need for special equipment, and dependence on highly trained personnel (a fellowshiptrained dermatologist and histotechnologist). Not surprisingly, availability of this procedure is limited. The high initial cost may be balanced by the lower cost of treating recurrent tumors, but no cost-effectiveness studies are available.28
Curettage and electrodesiccation
Curettage and electrodesiccation is performed using a sharp curette to remove soft material from the tumor until the underlying dermis is reached. The base of the tumor is then destroyed using hyfrecation. The procedure may be repeated once or twice to achieve a base of healthy tissue (LOE: 5).19,29
Curettage and electrodesiccation is the most common way dermatologists treat small (<1 cm) primary basal cell carcinoma.18 It is safe, well tolerated, and has 5-year cure rates >90%.5 Other advantages include speed and suitability for patients with multiple lesions.19 Curettage and electrodesiccation is also appropriate for small (<1 cm) primary squamous cell carcinoma below the neck (LOE: 5).22 This technique should not be used for malignant melanoma and is not suitable for tumors with high risk of metastasis or recurrence (LOE: 5).22
Cryosurgery
Cryosurgery with liquid nitrogen or nitrous oxide is used for small (<1 cm) superficial lesions and those locations where surgery is technically difficult, such as the lips, nose, ears, eyelids, and hands. It is also ideal for patients on chronic anticoagulation or with significant comorbidities.
For properly selected lesions, 5-year cure rates are excellent at 97% (LOE: 1a).20 Cure rates for larger lesions are reportedly high, but study follow-up was inconsistent (LOE: 2b).30 Small lesions heal completely in 4 to 6 weeks, while larger lesions may take up to 14 weeks to resolve (LOE: 5).31 Despite the prolonged healing time, cosmesis is often excellent.
Guidelines for the treatment of non-melanoma skin cancer with cryosurgery are presented in Figure 5. Importantly, published cure rates for cryosurgery are based on several case series, the largest of which employed a temperature-monitoring thermocouple needle to guarantee adequate depth of freezing. Cryosurgery without a thermocouple device may therefore result in higher recurrence rates than expected.31
FIGURE 5
Guidelines for cryosurgery
Topical agents
Topical chemotherapeutic agents offer great promise in the treatment of properly selected tumors, but quality evidence is lacking. The most commonly used topical agent is the DNA synthesis inhibitor 5-fluorouracil (5-FU). 5-FU is a proven treatment for actinic keratoses and has been used to treat squamous cell carcinoma in situ and superficial basal cell carcinoma. However, 5-FU is contraindicated in invasive squamous cell carcinoma and basal cell carcinoma, as it may bury tumor tissue and lead to continued, subclinical spread (LOE: 5).19
Imiquimod 5% cream also shows great promise for the treatment of superficial basal cell carcinoma. This cytokine and interferon inducer is primarily used for treatment of external genital and anal warts. Twice daily, once daily, or 3 times weekly application over 10 to 16 weeks produces histological clearing of low-risk, small superficial basal cell carcinoma (LOE: 2b).32 Adverse events are limited to local skin reactions with severity increasing with more frequent dosing. Research examining rates of recurrence is ongoing. As with 5-FU, effectiveness for large tumors and those at high-risk locations has not been established.
Radiotherapy
Currently, radiotherapy is used in special situations, such as nonmelanoma skin cancer near the eye, nose, and ear (LOE: 5).22 In specialty centers, radiation is used in conjunction with other modalities for recurrent or highly aggressive lesions.
While cure rates in low-risk areas are over 90%, long-term cosmesis, particularly for young patients with basal cell carcinoma, is less favorable than with other modalities.20 Other disadvantages include long-term radiation risks, high cost, and need for multiple visits over several weeks.22
Follow-up
Periodic population-based screening for non-melanoma skin cancer has not been proven to extend life. However, in patients who have already had nonmelanoma skin cancer, periodic surveillance is probably important.
Of patients with squamous cell carcinoma, 30% will develop an additional squamous cell carcinoma after 5 years and over 50% will develop an additional nonmelanoma skin cancer.33 More than one third of patients with basal cell carcinoma will develop an additional basal cell carcinoma after 5 years.33
One accepted approach is to have patients with newly diagnosed nonmelanoma skin cancer follow-up every 3 months for the first year and then at 6-month intervals thereafter (LOE: 5).5 New primary lesions should be treated accordingly, while recurrent lesions may be referred to a dermatologist.
Acknowledgments
The authors wish to thank Barbara Zuckerman and Michael Campese, PhD for their assistance in preparation of this manuscript.
Corresponding author
Scott M. Strayer, MD, MPH, University of Virginia Health System, Department of Family Medicine, P.O. Box 800729, Charlottesville, VA 22908-0729. E-mail: [email protected].
- Malignant melanomas in situ can usually be treated in consultation with a specialist (C). Larger lesions may require referral.
- Based on best available evidence, surgical excision is the first-line treatment for most nonmelanoma skin cancers, with cure rates as high as 98% with proper margins (C).
- Consider Mohs surgery for larger lesions, sclerosing lesions with morpheaform histology, or for cosmetically sensitive areas (A).
- With properly selected lesions, curettage/electrodesiccation and cryosurgery have cure rates comparable to that of surgical excision (B).
Reliable criteria exist to guide primary care physicians in the evaluation of malignant melanoma.1 Once a diagnosis of malignant melanoma is made, physicians should promptly consult with a skin malignancy specialist (Figure 1).
Several well-accepted treatment options exist for primary nonmelanoma skin cancer, but direct comparisons are lacking. The best evidence recommends surgical excision as first-line treatment for most nonmelanoma skin cancer. Cure rates up to 98% can be expected with appropriate margins.
Curettage/electrodesiccation and cryosurgery are probably equally effective with properly selected lesions. Physicians should be aware of those cases when nonmelanoma skin cancers are at higher risk for recurrence or metastasis and tailor their treatment plan accordingly.
Mohs micrographic surgery is the preferred treatment for high-risk and recurrent primary nonmelanoma skin cancer.
In this Applied Evidence review, we examine the guidance that can be gleaned from currently available evidence, and review each option in detail.
FIGURE 1
Surgical excision of suspected skin malignancies
Confirming the diagnosis
Our previous article in THE JOURNAL OF FAMILY PRACTICE, “Diagnosing skin malignancy: Assessment of predictive clinical criteria and risk factors,”1 presented a comprehensive review for the initial evaluation of skin malignancies. (This article is available online at www.jfponline.com.)
Nonmelanoma skin cancer includes basal cell carcinomas and squamous cell carcinomas. Nonmelanoma skin cancer, which accounts for most cases of skin cancer, has a high 5-year survival rate, more than 95%.2 Malignant melanoma represents only 1% of skin malignancies but leads to more than 75% of skin cancer deaths.3 Up to 83% of family physicians treat skin malignancies in their offices.4
When the diagnosis is uncertain on clinical grounds alone, an initial diagnostic biopsy may be used. Techniques include excisional, punch, and shave biopsies.
Excisional biopsy (Figure 2) is the preferred method for primary care evaluation of suspected skin malignancy (level of evidence [LOE]: 5).5,6 Excision wounds are easily cared for by patients and provide good cosmesis. Surgical excision may also be used for definitive treatment, as discussed below.
Punch biopsy or incisional biopsy (Figure 3) provides a full-thickness tissue sample with minimal scarring. A 3-mm punch is sufficient for most lesions (LOE: 5).5 Punch biopsy is a reasonable alternative when excision is impractical due to the size or location of a lesion.
Punch biopsy is not recommended if malignant melanoma is suspected by history or physical exam—an excisional biopsy should be done (LOE: 5).6 Even when performed mistakenly on a malignant melanoma, however, punch biopsy has not been shown to alter prognosis or cause dissemination of a tumor (LOE: 1b).7
Shave biopsies (Figure 4) are useful for benign-appearing lesions or elevated, nodular lesions suggestive of basal cell carcinoma or squamous cell carcinoma. Shave biopsy sites typically heal more slowly than excisional biopsies but do so with good cosmesis. In general, shave biopsy of pigmented lesions is contraindicated, but may be performed by an experienced physician confident of a benign clinical diagnosis. According to a National Institutes of Health consensus panel, suspected malignant melanoma should not be shaved, as the maximal depth of the tumor cannot be assessed.8
FIGURE 2
Excisional biopsy
FIGURE 3
Punch/Incisional biopsy
FIGURE 4
Shave biopsy
Malignant melanoma treatment
Overview
If a skin lesion is suspected clinically to be malignant melanoma, use the ABCD criteria and the revised 7-point checklist to guide biopsy decisions.1 If the physician or patient has any doubt, perform a biopsy.
If malignant melanoma is suspected, the initial biopsy should be conservative, with 1–2 mm margins (LOE: 5),9,10 and excisional, in order to determine the thickness of the tumor (Breslow’s measurement) and the histologic level of invasion (Clark’s level). Tumor depth at the time of diagnosis is the main factor guiding treatment and is the principal indicator predicting death due to malignant melanoma.8 Long-term survival of patients (10 years) with malignant melanoma <0.76 mm in thickness is greater than 90%; mortality rises linearly with increasing tumor thickness (LOE: 2b).11
Although knowledge of biopsy type is important for proper pathologic evaluation, it does not affect survival (LOE: 1b).7 Reviewing initial pathology results, a skin malignancy specialist (dermatologist, surgical oncologist, or plastic surgeon) can help determine the surgical margin necessary for curative excision. While most primary care physicians can treat lesions in situ, larger lesions require referral. Current recommendations for excision margins are shown in Table 1.
Options for lymph node management include observation, sentinel lymph node biopsy, and elective lymph node dissection. The choice of treatment depends on clinical staging, tumor thickness, ulceration, location, and patient age, and should be managed by a melanoma specialist.
TABLE 1
Excision margins for malignant melanoma
| Tumor thickness | Surgical margin | Level of evidence |
|---|---|---|
| In situ | 5 mm | 58 |
| <1 mm | 1 cm | 1b12 |
| 1–4 mm | 2 cm | 1b13 |
| >4 mm | 3 cm | 1b12,13 |
Systemic therapy
Dacarbazine is the accepted treatment of choice for metastatic malignant melanoma. A systematic review found no randomized controlled trials comparing systemic therapy with supportive care or placebo. Several trials used dacarbazine as the control agent and found response rates varying from 9.1% to 29% (no test agent was found superior). Combination chemotherapy should be reserved for clinical studies and trials (LOE: 1a).14
Interferon alfa-2b is used as adjuvant therapy after surgical resection. It has an overall 5-year survival advantage over placebo (46% vs. 37%; number needed to treat [NNT]= 11, P=.0237) (LOE: 1b).15 Additionally, an economic analysis of this treatment found it to be cost-effective for high-risk melanomas (LOE: 2b).16
Vaccine therapy has shown preliminary benefit in several case-control studies but needs to be investigated further before it can be recommended (LOE: 3b).17
Nonmelanoma skin cancer treatment
Overview
Treatments for nonmelanoma skin cancer include surgical excision, Mohs micrographic surgery, curettage and electrodesiccation, cryosurgery, fractionated radiotherapy, topical chemotherapy, carbon dioxide laser, photo-dynamic therapy, intralesional interferon, and retinoids.
Some of these therapies are untested or not widely available. Immunotherapy and photodynamic therapy remain experimental.18 Laser treatment offers theoretical advantages for certain patients, such as those taking anticoagulants. However, safety hazards and inconvenience limit their use even by dermatologists (LOE: 5).19
Two meta-analysis reviews have attempted to compare cure rates for treatment of basal cell carcinoma by conventional methods.20,21 In these studies, standard excision was recommended for nodular-ulcerative and superficial basal cell carcinoma <2 cm in diameter and away from the face. Consideration of Mohs surgery was recommended for larger lesions, sclerosing lesions with morpheaform histology, and for cosmetically sensitive areas where large tissue loss or recurrence would be disfiguring (eyelid, ear, nose, lips) (LOE: 1a).20 Results are summarized in Table 2.
It should be noted that lack of uniformity in the method of reporting prohibited direct comparison of recurrence rates for different treatments. No similar meta-analysis reviews of treatment for squamous cell carcinoma were found.
TABLE 2
Effectiveness of treatment modalities for basal cell carcinoma 20
| Treatment modality | Raw recurrence rate %* | Mean rawrecurrence % | Cumulative 5-year recurrence rate % † |
|---|---|---|---|
| Surgical excision | 1.4–2.9 | N/A | 5.3 |
| Mohs micrographic surgery | 0.5–1.3 | 0.8 (21/2660) | N/A |
| Curettage and electrodesiccation | 3.8–18.1 | N/A | 13.2 |
| Cryosurgery | 0–11.4 | 3.0 (24/798) | N/A |
| N/A, not available | |||
| *Absolute number of patients with recurrence divided by number of patients with primary basal cell carcinoma at start of study (unknown number lost to follow-up). | |||
| †Life-table cumulative 5-year recurrence rate. | |||
Recurrence and metastasis
The average rate of metastasis is 3.6% for squamous cell carcinoma. However, certain nonmelanoma skin cancer lesions are at higher risk for recurrence or metastasis and merit special consideration: larger lesions, those involving a mucous membrane, and lesions located on the scalp, ears, eyelids, nose, lips, or genitals. The rate of metastasis with lesions at high-risk locations may approach 30%.5
The majority of squamous cell carcinoma is actinically induced on sun-exposed areas. Squamous cell carcinoma occurring at a chronic scar or ulcer, such as that caused by a thermal or radiation burn, is also more likely to metastasize (LOE: 5).22 Metastatic spread most commonly involves regional lymph nodes, lungs, and liver. Basal cell carcinomas have an extremely low rate of metastasis (<0.1%).23
The overall rate of recurrence is also low—less than 1% for lesions removed from the neck, trunk, and extremities.24 Risk factors for recurrence include size of lesion and morpheaform histology found with sclerosing basal cell carcinoma (with associated subclinical infiltration). Lesions of the face are at higher risk for recurrence with rates up to 43% on the lateral canthus, 33% on the superior orbital rim and brow, 24% on the ear, and 19% on the nose.18 Primary care physicians should consider referral for such high-risk lesions, depending on their level of training and experience.
Surgical excision
Surgical excision is generally considered the gold standard for evaluation and treatment of suspected skin malignancy (LOE: 5).5,6 Advantages include rapid healing, excellent cosmesis, and an option to obtain a pathological evaluation of the excised tissue.
Conversely, excision is a more time-consuming prodedure than curettage and electrodesiccation or cryosurgery, and it may sacrifice more normal tissue than Mohs surgery. A surgical margin of normal-appearing tissue is routinely excised to eliminate microscopic tumor extension.
Two studies provide objective data regarding the margins necessary to ensure tumor clearance for basal cell carcinoma and squamous cell carcinoma. Both were prospective studies using Mohs surgery as the gold standard. Histological, subclinical tumor extension was then compared with ink markings placed preoperatively on the normal-appearing skin.
The first study enrolled 111 consecutive patients, aged 51 to 95 years, presenting with invasive squamous cell carcinoma. The results are summarized in Table 3.25 Importantly, 30% of the tumors extended into the subcutaneous fat. The researchers recommend that excision of all squamous cell carcinomas should include subcutaneous fat (LOE: 2b).
The second study enrolled 117 patients with previously untreated, well-demarcated basal cell carcinoma. The investigators concluded that a 4-mm lateral margin will totally eradicate 98% of tumors <2 cm in diameter (3.79 mm; 95% confidence interval [CI], 3.21–4.86) (LOE: 2b).26 Vertical extension of tumor was not addressed. No recommendations were made for tumors 2 cm in diameter.
Interestingly, no tumors in this study required a 4-mm margin in all directions. Rather, the basal cell carcinomas were found to send out extensions in an irregular pattern.
TABLE 3
Surgical margins for treatment of squamous cell carcinomas 25
| Tumor diameter and risk level | Margin needed to completely clear 95% or more of tumors |
|---|---|
| 0–19 mm | 4 mm |
| ≥20 mm | 6 mm |
| “High-risk,”* 0–19 mm | 6 mm |
| “High-risk,”* 20 mm | Indeterminate† |
| *High risk of metastasis if located on scalp, ears, eyelids, nose, or lips (47 of 141 tumors in study located here). | |
| †Only 90% of tumors completely cleared even with 6 mm margin; author recommends Mohs surgery in this case. | |
Mohs micrographic surgery
Mohs surgery is generally considered the preferred treatment for high-risk primary non-melanoma skin cancer and recurrent non-melanoma skin cancer (LOE: 5).18 It is clearly superior for treatment of locally recurrent squamous cell carcinoma, with a 5-year cure rate of up to 90%, compared with 76.7% obtained with standard excision (LOE: 2a).27
The benefit of Mohs surgery for small (<1 cm), low-risk skin cancers is probably slight. Cure rates for primary nonmelanoma skin cancer are as high as 99.8% for basal cell carcinoma and 98.8% for squamous cell carcinoma, but standard excision also has high cure rates, up to 98% with proper surgical margins.19
Disadvantages include length of the procedure, need for special equipment, and dependence on highly trained personnel (a fellowshiptrained dermatologist and histotechnologist). Not surprisingly, availability of this procedure is limited. The high initial cost may be balanced by the lower cost of treating recurrent tumors, but no cost-effectiveness studies are available.28
Curettage and electrodesiccation
Curettage and electrodesiccation is performed using a sharp curette to remove soft material from the tumor until the underlying dermis is reached. The base of the tumor is then destroyed using hyfrecation. The procedure may be repeated once or twice to achieve a base of healthy tissue (LOE: 5).19,29
Curettage and electrodesiccation is the most common way dermatologists treat small (<1 cm) primary basal cell carcinoma.18 It is safe, well tolerated, and has 5-year cure rates >90%.5 Other advantages include speed and suitability for patients with multiple lesions.19 Curettage and electrodesiccation is also appropriate for small (<1 cm) primary squamous cell carcinoma below the neck (LOE: 5).22 This technique should not be used for malignant melanoma and is not suitable for tumors with high risk of metastasis or recurrence (LOE: 5).22
Cryosurgery
Cryosurgery with liquid nitrogen or nitrous oxide is used for small (<1 cm) superficial lesions and those locations where surgery is technically difficult, such as the lips, nose, ears, eyelids, and hands. It is also ideal for patients on chronic anticoagulation or with significant comorbidities.
For properly selected lesions, 5-year cure rates are excellent at 97% (LOE: 1a).20 Cure rates for larger lesions are reportedly high, but study follow-up was inconsistent (LOE: 2b).30 Small lesions heal completely in 4 to 6 weeks, while larger lesions may take up to 14 weeks to resolve (LOE: 5).31 Despite the prolonged healing time, cosmesis is often excellent.
Guidelines for the treatment of non-melanoma skin cancer with cryosurgery are presented in Figure 5. Importantly, published cure rates for cryosurgery are based on several case series, the largest of which employed a temperature-monitoring thermocouple needle to guarantee adequate depth of freezing. Cryosurgery without a thermocouple device may therefore result in higher recurrence rates than expected.31
FIGURE 5
Guidelines for cryosurgery
Topical agents
Topical chemotherapeutic agents offer great promise in the treatment of properly selected tumors, but quality evidence is lacking. The most commonly used topical agent is the DNA synthesis inhibitor 5-fluorouracil (5-FU). 5-FU is a proven treatment for actinic keratoses and has been used to treat squamous cell carcinoma in situ and superficial basal cell carcinoma. However, 5-FU is contraindicated in invasive squamous cell carcinoma and basal cell carcinoma, as it may bury tumor tissue and lead to continued, subclinical spread (LOE: 5).19
Imiquimod 5% cream also shows great promise for the treatment of superficial basal cell carcinoma. This cytokine and interferon inducer is primarily used for treatment of external genital and anal warts. Twice daily, once daily, or 3 times weekly application over 10 to 16 weeks produces histological clearing of low-risk, small superficial basal cell carcinoma (LOE: 2b).32 Adverse events are limited to local skin reactions with severity increasing with more frequent dosing. Research examining rates of recurrence is ongoing. As with 5-FU, effectiveness for large tumors and those at high-risk locations has not been established.
Radiotherapy
Currently, radiotherapy is used in special situations, such as nonmelanoma skin cancer near the eye, nose, and ear (LOE: 5).22 In specialty centers, radiation is used in conjunction with other modalities for recurrent or highly aggressive lesions.
While cure rates in low-risk areas are over 90%, long-term cosmesis, particularly for young patients with basal cell carcinoma, is less favorable than with other modalities.20 Other disadvantages include long-term radiation risks, high cost, and need for multiple visits over several weeks.22
Follow-up
Periodic population-based screening for non-melanoma skin cancer has not been proven to extend life. However, in patients who have already had nonmelanoma skin cancer, periodic surveillance is probably important.
Of patients with squamous cell carcinoma, 30% will develop an additional squamous cell carcinoma after 5 years and over 50% will develop an additional nonmelanoma skin cancer.33 More than one third of patients with basal cell carcinoma will develop an additional basal cell carcinoma after 5 years.33
One accepted approach is to have patients with newly diagnosed nonmelanoma skin cancer follow-up every 3 months for the first year and then at 6-month intervals thereafter (LOE: 5).5 New primary lesions should be treated accordingly, while recurrent lesions may be referred to a dermatologist.
Acknowledgments
The authors wish to thank Barbara Zuckerman and Michael Campese, PhD for their assistance in preparation of this manuscript.
Corresponding author
Scott M. Strayer, MD, MPH, University of Virginia Health System, Department of Family Medicine, P.O. Box 800729, Charlottesville, VA 22908-0729. E-mail: [email protected].
1. Strayer SM, Reynolds PL. Diagnosing skin malignancy: assessment of predictive clinical criteria and risk factors. J Fam Pract 2003;52:210-218.
2. Gloster HM, Jr, Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996;22:217-226.
3. Skin tumors. In: Sauer GC, Hall JC, eds. A manual of skin diseases. Philadelphia, Pa: Lippincott-Raven;1996:342.
4. American Academy of Family Physicians. Practice Profile II Survey, May 2000. Shawnee Mission, Kansas: American Academy of Family Physicians; 2000.
5. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27:447-458.
6. Bruce AJ, Brodland DG. Overview of skin cancer detection and prevention for the primary care physician. Mayo Clin Proc 2000;75:491-500.
7. Lederman JS, Sober AJ. Does biopsy type influence survival in clinical stage I cutaneous melanoma? J Am Acad Dermatol 1985;13:983-987.
8. NIH Consensus conference Diagnosis and treatment of early melanoma. JAMA 1992;268:1314-1319.
9. Martinez JC, Otley CC. The management of melanoma and nonmelanoma skin cancer: a review for the primary care physician. Mayo Clin Proc 2001;76:1253-1265.
10. Goldstein BG, Goldstein AO. Diagnosis and management of malignant melanoma. Am Fam Physician 2001;63:1359-1368.
11. Melanoma in the Southern United States. In: Balch CM, Milton GW, eds. Cutaneous melanoma: clinical management and treatment results worldwide. Philadelphia: Lippincott; 1985:397-406.
12. Veronesi U, Cascinelli N. Narrow excision (1-cm margin): a safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-441.
13. Balch CM, Urist M, Karakousis C. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1-4 mm): results of a multi-institutional randomized surgical trial. Ann Surg 1993;218:262-267.
14. Crosby T, Fish R, Coles B, Mason MD. Systemic treatments for metastaic cutaneous melanoma. The Cochrane Library 2002;(2).:
15. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17.
16. Hillner B, Kirkwood J, Atkins M, Johnson E, Smith T. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol 1997;15:2351-2358.
17. Ollila DW, Kelley MC, Gammon G, Morton DL. Overview of melanoma vaccines: active specific immunotherapy for melanoma patients. Semin Surg Oncol 1998;14:328-336.
18. McGovern TW, Leffell DJ. Mohs surgery: the informed view. Arch Dermatol 1999;135:1255-1259.
19. Hochman M, Lang P. Skin cancer of the head and neck. Med Clin North Am 1999;83:261-282.
20. Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol 1999;135:1255-1259.
21. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol 1995;13:617-620.
22. Alam M, Ratner D. Primary care: cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-983.
23. Von Domarus H, Stern PJ. Metastatic basal cell carcinoma report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 1984;10:1043-1060.
24. Silverman MK, Kopf AW, Bart RS, Grin CM, Levenstein MS. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol 1993;18:471-476.
25. Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol 1992;27:241-248.
26. Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol 1987;123:340-344.
27. Rowe DE, Carroll RJ, Day CL, Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol 1992;26:976-990.
28. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol 1998;39:698-703.
29. Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for basal cell carcinoma. The American Academy of Dermatology Committee on Guidelines of Care. J Am Acad Dermatol 1992;26:117-120.
30. Graham GC, Clark LC. Statistical analysis in cryosurgery of skin cancer. Clin Dermatol 1990;8:101-107.
31. Kuflik EG. Cryosurgery for cutaneous malignancy. Dermatol Surg 1997;23:1081-1087.
32. Beutner KR, Geisse JK, Helman D, Fox TL, Ginkel A, Owens ML. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol 1999;41:1002-1007.
33. Rhodes AR. Public education and cancer of the skin. Cancer 1995;75:613-636.
1. Strayer SM, Reynolds PL. Diagnosing skin malignancy: assessment of predictive clinical criteria and risk factors. J Fam Pract 2003;52:210-218.
2. Gloster HM, Jr, Brodland DG. The epidemiology of skin cancer. Dermatol Surg 1996;22:217-226.
3. Skin tumors. In: Sauer GC, Hall JC, eds. A manual of skin diseases. Philadelphia, Pa: Lippincott-Raven;1996:342.
4. American Academy of Family Physicians. Practice Profile II Survey, May 2000. Shawnee Mission, Kansas: American Academy of Family Physicians; 2000.
5. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27:447-458.
6. Bruce AJ, Brodland DG. Overview of skin cancer detection and prevention for the primary care physician. Mayo Clin Proc 2000;75:491-500.
7. Lederman JS, Sober AJ. Does biopsy type influence survival in clinical stage I cutaneous melanoma? J Am Acad Dermatol 1985;13:983-987.
8. NIH Consensus conference Diagnosis and treatment of early melanoma. JAMA 1992;268:1314-1319.
9. Martinez JC, Otley CC. The management of melanoma and nonmelanoma skin cancer: a review for the primary care physician. Mayo Clin Proc 2001;76:1253-1265.
10. Goldstein BG, Goldstein AO. Diagnosis and management of malignant melanoma. Am Fam Physician 2001;63:1359-1368.
11. Melanoma in the Southern United States. In: Balch CM, Milton GW, eds. Cutaneous melanoma: clinical management and treatment results worldwide. Philadelphia: Lippincott; 1985:397-406.
12. Veronesi U, Cascinelli N. Narrow excision (1-cm margin): a safe procedure for thin cutaneous melanoma. Arch Surg 1991;126:438-441.
13. Balch CM, Urist M, Karakousis C. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1-4 mm): results of a multi-institutional randomized surgical trial. Ann Surg 1993;218:262-267.
14. Crosby T, Fish R, Coles B, Mason MD. Systemic treatments for metastaic cutaneous melanoma. The Cochrane Library 2002;(2).:
15. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17.
16. Hillner B, Kirkwood J, Atkins M, Johnson E, Smith T. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol 1997;15:2351-2358.
17. Ollila DW, Kelley MC, Gammon G, Morton DL. Overview of melanoma vaccines: active specific immunotherapy for melanoma patients. Semin Surg Oncol 1998;14:328-336.
18. McGovern TW, Leffell DJ. Mohs surgery: the informed view. Arch Dermatol 1999;135:1255-1259.
19. Hochman M, Lang P. Skin cancer of the head and neck. Med Clin North Am 1999;83:261-282.
20. Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol 1999;135:1255-1259.
21. Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol 1995;13:617-620.
22. Alam M, Ratner D. Primary care: cutaneous squamous-cell carcinoma. N Engl J Med 2001;344:975-983.
23. Von Domarus H, Stern PJ. Metastatic basal cell carcinoma report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 1984;10:1043-1060.
24. Silverman MK, Kopf AW, Bart RS, Grin CM, Levenstein MS. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol 1993;18:471-476.
25. Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol 1992;27:241-248.
26. Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol 1987;123:340-344.
27. Rowe DE, Carroll RJ, Day CL, Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol 1992;26:976-990.
28. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol 1998;39:698-703.
29. Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for basal cell carcinoma. The American Academy of Dermatology Committee on Guidelines of Care. J Am Acad Dermatol 1992;26:117-120.
30. Graham GC, Clark LC. Statistical analysis in cryosurgery of skin cancer. Clin Dermatol 1990;8:101-107.
31. Kuflik EG. Cryosurgery for cutaneous malignancy. Dermatol Surg 1997;23:1081-1087.
32. Beutner KR, Geisse JK, Helman D, Fox TL, Ginkel A, Owens ML. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol 1999;41:1002-1007.
33. Rhodes AR. Public education and cancer of the skin. Cancer 1995;75:613-636.
Office evaluation and treatment of hemorrhoids
- Only symptomatic hemorrhoids require treatment. Most patients can be treated with conservative therapy alone or an office procedure.
- Anoscopy detects more lesions in the anorectal region than does flexible sigmoidoscopy. Visualization is best achieved with the slotted anoscope.
- Rubber band ligation is the preferred office procedure for treatment of grade I/II hemorrhoids that do not respond to medical therapy, and treatment of all grade III hemorrhoids.
Most cases of hemorrhoids can be managed in the primary care setting with simple measures and office procedures, including anoscopy. This paper reviews the advantages, disadvantages, and levels of evidence regarding specific treatments for different grades of hemorrhoids.
Prevalence
Prevalence varies from 4.4% in the general population to 36.4% in general practice.1 The annual rate of office visits for hemorrhoids is 12 for every 1000 patients in the United States2 ; its prevalence is similar between the sexes and increases with age until the seventh decade.3,4 Only a third of patients with symptomatic hemorrhoids seek medical help.4
Characteristics
Hemorrhoidal padding, which is critical to maintaining continence, accounts for approximately 15% to 20% of the anal resting pressure and supplies important sensory information that enables the differentiation between liquid, solid, and gas. When an individual coughs or performs a Valsalva maneuver, this vascular padding increases in area and volume, thereby enabling the anal canal to remain closed and avoid the loss of stools.
Hemorrhoids are associated with chronic straining secondary to constipation, diarrhea, tenesmus, or long periods trying to defecate, and are common during pregnancy and child-birth.5 The pathophysiology is not clearly understood, but current theories suggest that structural or vascular changes may be involved.
The mucocutaneous junction of the ano-rectum, or dentate line, divides hemorrhoids anatomically into internal (above the junction) and external (below). This anatomic “border” is of special clinical interest because external pain fibers end at this point, and most people have no sensation above this line.
Hemorrhoids originating above the junction, even if prolapsed, are still classified as internal hemorrhoids, and are divided into 4 categories depending on the grade of prolapse:
- Grade I—Protrudes into the anal canal but does not prolapse
- Grade II—Prolapses but reduces spontaneously
- Grade III—Prolapses and requires manual reduction
- Grade IV—Irreducible prolapse.
Hemorrhoids, especially if external, sometimes thrombose (Figure 1). Distention of overlying perianal skin and inflammation associated with the process of thrombosis can cause severe pain and discomfort.
FIGURE 1
Thrombosed hemorrhoids Anoscopy
Thrombosis can be particularly painful due to distention of perianal skin and inflammation. Used with permission, National Procedures Institute, Midland, Mich.
Diagnosis
Symptoms
The most common symptoms of hemorrhoids are bleeding and prolapse. Less frequently, symptoms also include discomfort, pain, soiling, or itching.
Internal hemorrhoids are usually painless; bleeding or prolapse generally prompt a visit to the physician. Bleeding is described as bright red spotting on toilet tissue or as dripping in the toilet bowl and normally occurs at the end of defecation and separately from the stool.
External hemorrhoids may be asymptomatic, associated with discomfort, or a cause of acute pain in the event of a thrombosis. They generally do not bleed except in the case of a spontaneously resolved local thrombosis. Some individuals may have both types of hemorrhoids simultaneously (mixed).
The term hemorrhoids is commonly misused by patients to refer to any anal symptoms. Other diagnoses such as anal fissures, pruritus ani, abscess, fistula, and condyloma should be ruled out by examining the anus, the perianal region, and the anal canal. However, we have found no studies reporting on the accuracy of the medical history or the physical examination.
How to conduct a digital examination
The patient should be positioned in the left lateral decubitus position for the anorectal exam-ination.6 This position is more comfortable and less intimidating for the patient than the traditional head-down position, and it permits optimal visualization.
Digital palpation allows the entire circumference of the canal to be examined and rectal masses or tender points to be ruled out. Internal hem-orrhoids cannot be detected this way, however.6 The procedure, which must be done gently and with prior reassurance to the patient, is generally quite simple. Intense pain may prevent further examination and suggests the possibility of anal fissure or thrombosed external hemorrhoid.
Anoscopy: safe and essential office procedure
The most accurate method for examining the anal canal and distal rectum is anoscopy. Although several types of anoscopes are available, visualization is best achieved with the slotted anoscope (Figure 2).6 Once it has been inserted, the anoscope is gradually withdrawn while rotating right and left to allow inspection of the mucous membrane. Hemorrhoids appear as pink swellings of the mucosa. Ask the patient to strain during the examination to improve visualization.
Two prospective studies found that anoscopy detects a higher percentage of lesions in the anorectal region than does flexible sigmoidoscopy (99% vs 78%).7,8 It is the procedure of choice for evaluating rectal diseases.
In skilled hands, anoscopy is safe; complications are unusual, and it can be performed in a physician’s office on short notice and without bowel preparation. After appropriate training, primary care physicians can perform routine examinations safely and accurately.
FIGURE 2
Anoscopy
The slotted anoscope provides the best visualization. Used with permission, National Procedures Institute, Midland, Mich.
Office-Based Treatment
Primary care physicians can safely use simple treatment measures to manage most cases of symptomatic hemorrhoids (Figure 3). Only the more symptomatic patients require surgical intervention.
Patients should be made aware of the nature of the condition and the advantages and disadvantages of the different treatment options. The evidence regarding these treatments is summarized in the Table.
FIGURE 3
Management of adult patients with hemorrhoids
TABLE
Medical and surgical treatments for hemorrhoids
| Treatment | SOR* | Indication | Comments |
|---|---|---|---|
| Analgesics and anti-inflammatories2 | D | Grades I–IV and thrombosed external hemorrhoids | No trials comparing analgesics with anti-inflammatories |
| Topical treatments with corticosteroids or anesthetics3 | D | Grades I–III | No controlled trials with placebo are available, but patients report empiric benefit with their use; use only for brief periods |
| High-fiber diet or fiber supplements10-13 | B | Grades I–II | NNT=2.8 for reduction of rectal bleeding and 3.6 for pain relief23 |
| Office procedures23-25 | A | Grades I and II that do not respond to medical therapy, and grade III | Rubber band ligation was more effective and required fewer additional treatments for symptomatic recurrence than did infrared coagulation (NNT=9) and sclerotherapy (NNT=6.9); but rubber band ligation produced more complications than did infrared coagulation (pain: NNH=6) |
| Phlebotonics14-21 | B | Grades I–II | Moderate reduction of duration of bleeding during acute episodes of internal hemorrhoids; conflicting results for other outcomes (pain and prolapse) |
| Hemorrhoidectomy24 | A | Grades II–IV | Hemorrhoidectomy is more effective than office procedures, but it is more painful and presents more complications; office procedures are cheaper and require no time off from work |
| Stapling technique31-36 | A | Grades III–IV | Stapling technique is as effective as hemorrhoidectomy, is less painful, and requires less time off from work; more long-term data are needed37 |
| *For an explanation of strength of recommendation, see page 379. | |||
| SOR, strength of recommendation; NNT, number needed to treat; NNH, number needed to harm | |||
Benefits of lifestyle changes
The main purpose of lifestyle changes is to minimize prolonged straining during bowel movements, which is thought to contribute to the development of hemorrhoids. Such changes include increasing the amount of fiber in the diet, which is especially helpful for grade I and II internal hemorrhoids. Preventing constipation also helps alleviate more severe hemorrhoids and can help to prevent future episodes.10
Rectal bleeding can mask the diagnosis of cancer. The probability of colorectal cancer increases in the elderly, those with a family or personal history of colorectal cancer, and those with any other symptoms of colorectal cancer (fatigue, weight loss, palpable tumor, anemia). Patients at risk need a more thorough evaluation, including endoscopy, to rule out malignancy.9
It is important to document that observed hemorrhoids account for the bleeding episode (bleeding at the contact site with the anoscope or an applicator, thrombosed hemorrhoids, or a clot over the hemorrhoid).
Several clinical trials have reported the benefits of a high-fiber diet or fiber supplements compared with placebo in relieving pain, bleeding, and prolapse (strength of recommendation [SOR]: B).10-13 We found no studies comparing the different types of bulk laxatives.
Although the role of certain foods in the pathogenesis of hemorrhoids or their acute exacerbation is accepted empirically, this has not been proved. Also, no firm evidence to date shows that increasing physical exercise, limiting time on the commode, or improving local hygiene are beneficial. However, these measures are usually recommended because they have other health benefits or are thought to do no harm (SOR: D).
Medical therapy
No rigorous evidence exists to support the use of topical therapies, physical or pharmacologic (sitz baths, anesthetics, phlebotonics, corticoids, or ice). Most studies have employed poor methods, lacked placebo control, and addressed heterogeneous preparations with multiple associated components. It is therefore not possible to formulate firm recommendations.
Soothing agents. Popular topical soothing agents are often combined with corticosteroids or anesthetics and are available over the counter as creams or enemas. Many patients report some empirical benefit with their use, especially corticosteroids and anesthetics (SOR: D).3 Advise patients against prolonged use due to possible local allergic effects and sensitization of skin (SOR: D).
Phlebotonics. Several phlebotonics have been evaluated in the literature; diosmin is the best-studied agent, but it is not commercially available in the United States at this time. In studies of patients with acute attacks of internal hemorrhoids (grades I to II), the main perceived effect was reduced bleeding duration;14,15 the results were conflicting for other outcomes (mainly pain and prolapse)14-18 (SOR: B).
Other phlebotonics (the botanicals ginkgo biloba, troxerutin, and calcium dobesilate), when compared with placebo19,20 or diosmin,21 have shown similar effects (SOR: B). No studies thus far have evaluated the role of phlebotonics in thrombosis of external hemorrhoids.
Anti-inflammatories and analgesics. Most episodes of acute thrombosed external hemorrhoids improve spontaneously and therefore can be treated with symptomatic measures, including anti-inflammatory agents, analgesics, and stool softeners. Anti-inflammatories and analgesics can be effective during episodes of external and internal thromboses3 (SOR: D). Several clinical trials have reported benefits of fiber in relieving pain, bleeding, and prolapse
In a small randomized clinical trial, the addition of topical nifedipine (0.3%) to a lidocaine ointment (1.5%) was more effective than lidocaine alone in reducing pain and shortening resolution time.22
Surgical treatment
All office (nonoperative) and surgical procedures fix the sliding hemorrhoidal tissue back onto the muscle wall. The fixation takes place by directly promoting tissue fibrosis (eg, sclerotherapy or infrared coagulation) or by tissue destruction with subsequent fibrosis (eg, hemorrhoidectomy).
Office procedures. The most commonly used methods are rubber band ligation and infrared coagulation. Other methods include bipolar electrocoagulation, low-voltage direct current, sclerotherapy, laser therapy, and cryosurgery.
Two meta-analyses compared these nonoperative methods and concluded that rubber band ligation and infrared coagulation are the most effective. The first meta-analysis reported that ligation was more effective because it required fewer additional treatments for symptomatic recurrence than did coagulation (number needed to treat [NNT]=9) and sclerotherapy (NNT=6.9).23 However, ligation produced more complications than did coagulation (pain: number needed to harm=6) (SOR: A).
The second, more recent meta-analysis found ligation to have similar beneficial effect and a similar complication rate,24 although it was more painful. It appeared to be the therapy of choice for grades I to III (SOR: A). No difference was found between sclerotherapy and infrared coagulation for any outcome measure, but the authors of these meta-analyses commented that the overall quality of the studies was not high and their conclusions were therefore limited. One subsequent randomized clinical trial confirmed the advantages of rubber band ligation.25
In the event of a thrombosed hemorrhoid, whether to remove the clot promptly or wait for spontaneous resolution is controversial. We found no studies comparing these approaches. Excision should be performed when local measures fail, the thrombosis is painful, and there is no local edema (SOR: D).3
In the treatment of perianal thrombosis, one clinical trial found excision more effective than topically applied 0.2% glyceryl trinitrate or incision in reducing pain and the number of recurrences at 1 year (SOR: A).26 Residual hemorrhoidal tissue following an episode of acute thrombosis of external hemorrhoids also may cause symptoms, especially pruritus. These external anal tags can make it difficult to clean the anus and can be excised if symptoms warrant.
Surgery. Surgical treatment, though more invasive and expensive, is the most effective and definitive course for symptomatic hemorrhoids. The aim is to decrease blood flow to the anorectal ring and excise redundant hemorrhoidal tissue.
There are several techniques. In the United States, the Ferguson (closed) hemorrhoidectomy is preferred. The one used most commonly in Europe is the Milligan-Morgan technique (open). Both techniques have been shown to be similarly effective, although there is debate over healing time.27-30 Only a competently performed technique will produce satisfying results.
In their meta-analysis, MacRae and McLeod found that hemorrhoidectomy is more effective than all other treatment modalities, though complications such as pain and costs were greater (SOR: A).24
A new surgical procedure, the stapled hemorrhoidectomy, has been introduced as an alternative to the standard hemorrhoidectomy. Several randomized clinical trials have shown the procedure to be as effective, cause less pain, and require less time off from work compared with standard techniques (SOR: A).31-36 However, it is more expensive and requires advanced surgical skills. More long-term data are also needed.37
Anal stretch, or manual anal dilatation, has been reported to be effective in the treatment of hemorrhoids, but a high rate of incontinence after the procedure has led to abandonment of this technique.38,39 Antibiotic prophylaxis in colorectal surgery is highly recommended and has been shown to reduce infection and mortality (SOR: A).40
Surgery vs office procedures
Several clinical practice guidelines3,38 and meta-analyses23,24 have recommended office procedures for hemorrhoids of grades I through III. Although there is some discrepancy about the procedure of choice, rubber band ligation appears to be the most effective technique.
An evidence-based clinical practice guideline3 has recommended coagulation techniques for bleeding nonprolapsed hemorrhoids or those with a low grade of prolapse (grades I and II), and reserving rubber band ligation for hemorrhoids more severely prolapsed (grade III). The basis for this recommendation is that flat bleeding hemorrhoids may not provide enough tissue to grasp.
Surgical hemorrhoidectomy should be reserved for grade IV hemorrhoids and for grade III lesions that do not respond to other procedures.
This is an approximate rather than a rigid approach, and the final decision will depend on the physician’s technical training, the patient’s preferences, clinical circumstances, and local resources.
Prognosis: 90% require no surgery
Hemorrhoids are generally a chronic problem and tend to worsen with time. According to a retrospective cohort study, most patients will have several episodes during their lives; however, it can be considered a benign disorder, and approximately 90% of patients will not require surgery to alleviate their symptoms (SOR: B).41
It is worth noting that 26% of patients who require a hemorrhoidectomy may have a recurrence, and 11% will need further treatment.39 Similarly, approximately half of those who undergo office procedures may require further treatment or surgery in 5 to 10 years.42,43
ACKNOWLEDGMENTS
We thank E. Thompson for help in editing this manuscript and J. A. Ferrus and A. Hervas for their clinical comments. Dr Alonso-Coello holds a postgraduate research fellowship at the Instituto Carlos III, Spanish Ministry of Health, Spain.
Corresponding author
Pablo Alonso-Coello, MD, Ibero-american Cochrane Centre, Hospital de la Santa Creu i Sant Pau, Sant Antoni Mª Claret 171, 08041 Barcelona, Spain. E-mail: [email protected].
1. Hulme-Moir M, Bartolo DC. Hemorrhoids. Gastroenterol Clin North Am 2001;30:183-197.
2. Johanson JF, Sonnenberg A. Temporal changes in the occurrence of hemorrhoids in the United States and England. Dis Colon Rectum 1991;34:585-591.
3. Abramowitz L, Godeberge P, Staumont G, Soudan D. Clinical practice guidelines for the treatment of hemorrhoid disease [in French]. Gastroenterol Clin Biol 2001;25:674-702.
4. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 1990;98:380-386.
5. Beck DE. Hemorrhoidal disease. In: Beck DE, Wexner SD, eds. Fundamentals of Anorectal Surgery. 2nd ed. London: WB Saunders 1998;237-253.
6. Pfenninger JL, Zainea GG. Common anorectal conditions: part I. Symptoms and complaints. Am Fam Physician 2001;63:2391-2398.
7. Kelly SM, Sanowski RA, Foutch PG, Bellapravalu S, Haynes WC. A prospective comparison of anoscopy and fiber endoscopy in detecting anal lesions. J Clin Gastroenterol 1986;8:658-660.
8. Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci 1995;40:1520-1523.
9. Wauters H, Van Casteren V, Buntinx F. Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ 2000;321:998-999.
10. Moesgaard F, Nielsen ML, Hansen JB, Knudsen JT. High-fiber diet reduces bleeding and pain in patients with hemorrhoids: a double-blind trial of Vi-Siblin. Dis Colon Rectum 1982;25:454-456.
11. Broader JH, Gunn IF, Alexander-Williams J. Evaluation of a bulk-forming evacuant in the management of haemorrhoids. Br J Surg 1974;61:142-144.
12. Webster DJ, Gough DC, Craven JL. The use of bulk evacuant in patients with haemorrhoids. Br J Surg 1978;65:291-292.
13. Perez-Miranda M, Gomez-Cedenilla A, Leon-Colombo T, Pajares J, Mate-Jimenez J. Effect of fiber supplements on internal bleeding hemorrhoids. Hepatogastroenterology 1996;43:1504-1507.
14. Godeberge P. Daflon 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology 1994;45:574-578.
15. Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum 2000;43:66-69.
16. Thanapongsathorn W, Vajrabukka T. Clinical trial of oral diosmin (Daflon) in the treatment of hemorrhoids. Dis Colon Rectum 1992;35:1085-1088.
17. Cospite M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology 1994;45:566-573.
18. Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg 2000;87:868-872.
19. Annoni F, Boccasanta P, Chiurazzi D, Mozzi E, Oberhauser V. Treatment of acute symptoms of hemorrhoid disease with high-dose oral O-(beta-hydroxyethyl)-rutosides [in Italian]. Minerva Med 1986;77:1663-1668
20. Mentes BB, Gorgul A, Tatlicioglu E, Ayoglu F, Unal S. Efficacy of calcium dobesilate in treating acute attacks of hemorrhoidal disease. Dis Colon Rectum 2001;44:1489-1495.
21. Debien P, Denis J. Traitements des signes fonctionnels de la maladie hèmorroïaigüe: essai multicentrique, randomisè, diosmine d’hèmisynthèse versus association extrait de Ginko biloba-heptaminol-troxèrutine. Med Chir Dig 1996;25:259-264.
22. Perrotti P, Antropoli C, Molino D, De Stefano G, Antropoli M. Conservative treatment of acute thrombosed external hemorrhoids with topical nifedipine. Dis Colon Rectum 2001;44:405-409.
23. Johanson JF, Rimm A. Optimal nonsurgical treatment of hemorrhoids: a comparative analysis of infrared coagulation, rubber band ligation, and injection sclerotherapy. Am J Gastroenterol 1992;87:1600-1606.
24. MacRae HM, McLeod RS. Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum. 1995;38:687-694.
25. Poen AC, Felt-Bersma RJ, Cuesta MA, Deville W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infra-red coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol 2000;12:535-539.
26. Cavcic J, Turcic J, Martinac P, Mestrovic T, Mladina R, Pezerovic-Panijan R. Comparison of topically applied 0.2% glyceryl trinitrate ointment, incision and excision in the treatment of perianal thrombosis. Dig Liver Dis 2001;33:335-340.
27. Ho YH, Seow-Choen F, Tan M, Leong AF. Randomized controlled trial of open and closed haemorrhoidectomy. Br J Surg 1997;84:1729-1730.
28. Carapeti EA, Kamm MA, McDonald PJ, Chadwick SJ, Phillips RK. Randomized trial of open versus closed day-case haemorrhoidectomy. Br J Surg 1999;86:612-613.
29. Arbman G, Krook H, Haapaniemi S. Closed vs. open hemorrhoidectomy—is there any difference? Dis Colon Rectum 2000;43:31-34.
30. Gencosmanoglu R, Sad O, Koc D, Inceoglu R. Hemorrhoidectomy: open or closed technique? A prospective, randomized clinical trial. Dis Colon Rectum 2002;45:70-75.
31. Mehigan BJ, Monson JR, Hartley JE. Stapling procedure for haemorrhoids versus Milligan-Morgan haemorrhoidectomy: randomised controlled trial. Lancet 2000;355:782-785.
32. Rowsell M, Bello M, Hemingway DM. Circumferential mucosectomy (stapled haemorrhoidectomy) versus conventional haemorrhoidectomy: randomised controlled trial. Lancet. 2000;355:779-781.
33. Khalil KH, O’Bichere A, Sellu D. Randomized clinical trial of sutured versus stapled closed haemorrhoidectomy. Br J Surg 2000;87:1352-1355.
34. Boccasanta P, Capretti PG, Venturi M, et al. Randomised controlled trial between stapled circumferential mucosectomy and conventional circular hemorrhoidectomy in advanced hemorrhoids with external mucosal prolapse. Am J Surg 2001;182:64-68.
35. Ganio E, Altomare DF, Gabrielli F, Milito G, Canuti S. Prospective randomized multicentre trial comparing stapled with open haemorrhoidectomy. Br J Surg 2001;88:669-674.
36. Shalaby R, Desoky A. Randomized clinical trial of stapled versus Milligan-Morgan haemorrhoidectomy. Br J Surg 2001;88:1049-1053.
37. Hetzer FH, Demartines N, Handschin AE, Clavien PA. Stapled vs excision hemorrhoidectomy: long-term results of a prospective randomized trial. Arch Surg 2002;137:337-340.
38. Standards Task Force of American Society of Colon and Rectal Surgeons Practice parameters for the treatment of hemorrhoids. Dis Colon Rectum 1993;36:1118-1120.
39. Konsten J, Baeten CG. Hemorrhoidectomy vs. Lord’s method: 17-year follow-up of a prospective, randomized trial. Dis Colon Rectum 2000;43:503-506.
40. Baum ML, Anish DS, Chalmers TC, Sacks HS, Smith H, Fagerstrom RM. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med 1981;305:795-799.
41. Bleday R, Pena JP, Rothenberger DA, Goldberg SM, Buls JG. Symptomatic hemorrhoids: current incidence and complications of operative therapy. Dis Colon Rectum 1992;35:477-481.
42. Wrobleski DE, Corman ML, Veidenheimer MC, Coller JA. Long-term evaluation of rubber ring ligation in hemorrhoidal disease. Dis Colon Rectum 1980;23:478-482.
43. Savioz D, Roche B, Glauser T, Dobrinov A, Ludwig C, Marti MC. Rubber band ligation of hemorrhoids: relapse as a function of time. Int J Colorectal Dis 1998;13:154-156.
- Only symptomatic hemorrhoids require treatment. Most patients can be treated with conservative therapy alone or an office procedure.
- Anoscopy detects more lesions in the anorectal region than does flexible sigmoidoscopy. Visualization is best achieved with the slotted anoscope.
- Rubber band ligation is the preferred office procedure for treatment of grade I/II hemorrhoids that do not respond to medical therapy, and treatment of all grade III hemorrhoids.
Most cases of hemorrhoids can be managed in the primary care setting with simple measures and office procedures, including anoscopy. This paper reviews the advantages, disadvantages, and levels of evidence regarding specific treatments for different grades of hemorrhoids.
Prevalence
Prevalence varies from 4.4% in the general population to 36.4% in general practice.1 The annual rate of office visits for hemorrhoids is 12 for every 1000 patients in the United States2 ; its prevalence is similar between the sexes and increases with age until the seventh decade.3,4 Only a third of patients with symptomatic hemorrhoids seek medical help.4
Characteristics
Hemorrhoidal padding, which is critical to maintaining continence, accounts for approximately 15% to 20% of the anal resting pressure and supplies important sensory information that enables the differentiation between liquid, solid, and gas. When an individual coughs or performs a Valsalva maneuver, this vascular padding increases in area and volume, thereby enabling the anal canal to remain closed and avoid the loss of stools.
Hemorrhoids are associated with chronic straining secondary to constipation, diarrhea, tenesmus, or long periods trying to defecate, and are common during pregnancy and child-birth.5 The pathophysiology is not clearly understood, but current theories suggest that structural or vascular changes may be involved.
The mucocutaneous junction of the ano-rectum, or dentate line, divides hemorrhoids anatomically into internal (above the junction) and external (below). This anatomic “border” is of special clinical interest because external pain fibers end at this point, and most people have no sensation above this line.
Hemorrhoids originating above the junction, even if prolapsed, are still classified as internal hemorrhoids, and are divided into 4 categories depending on the grade of prolapse:
- Grade I—Protrudes into the anal canal but does not prolapse
- Grade II—Prolapses but reduces spontaneously
- Grade III—Prolapses and requires manual reduction
- Grade IV—Irreducible prolapse.
Hemorrhoids, especially if external, sometimes thrombose (Figure 1). Distention of overlying perianal skin and inflammation associated with the process of thrombosis can cause severe pain and discomfort.
FIGURE 1
Thrombosed hemorrhoids Anoscopy
Thrombosis can be particularly painful due to distention of perianal skin and inflammation. Used with permission, National Procedures Institute, Midland, Mich.
Diagnosis
Symptoms
The most common symptoms of hemorrhoids are bleeding and prolapse. Less frequently, symptoms also include discomfort, pain, soiling, or itching.
Internal hemorrhoids are usually painless; bleeding or prolapse generally prompt a visit to the physician. Bleeding is described as bright red spotting on toilet tissue or as dripping in the toilet bowl and normally occurs at the end of defecation and separately from the stool.
External hemorrhoids may be asymptomatic, associated with discomfort, or a cause of acute pain in the event of a thrombosis. They generally do not bleed except in the case of a spontaneously resolved local thrombosis. Some individuals may have both types of hemorrhoids simultaneously (mixed).
The term hemorrhoids is commonly misused by patients to refer to any anal symptoms. Other diagnoses such as anal fissures, pruritus ani, abscess, fistula, and condyloma should be ruled out by examining the anus, the perianal region, and the anal canal. However, we have found no studies reporting on the accuracy of the medical history or the physical examination.
How to conduct a digital examination
The patient should be positioned in the left lateral decubitus position for the anorectal exam-ination.6 This position is more comfortable and less intimidating for the patient than the traditional head-down position, and it permits optimal visualization.
Digital palpation allows the entire circumference of the canal to be examined and rectal masses or tender points to be ruled out. Internal hem-orrhoids cannot be detected this way, however.6 The procedure, which must be done gently and with prior reassurance to the patient, is generally quite simple. Intense pain may prevent further examination and suggests the possibility of anal fissure or thrombosed external hemorrhoid.
Anoscopy: safe and essential office procedure
The most accurate method for examining the anal canal and distal rectum is anoscopy. Although several types of anoscopes are available, visualization is best achieved with the slotted anoscope (Figure 2).6 Once it has been inserted, the anoscope is gradually withdrawn while rotating right and left to allow inspection of the mucous membrane. Hemorrhoids appear as pink swellings of the mucosa. Ask the patient to strain during the examination to improve visualization.
Two prospective studies found that anoscopy detects a higher percentage of lesions in the anorectal region than does flexible sigmoidoscopy (99% vs 78%).7,8 It is the procedure of choice for evaluating rectal diseases.
In skilled hands, anoscopy is safe; complications are unusual, and it can be performed in a physician’s office on short notice and without bowel preparation. After appropriate training, primary care physicians can perform routine examinations safely and accurately.
FIGURE 2
Anoscopy
The slotted anoscope provides the best visualization. Used with permission, National Procedures Institute, Midland, Mich.
Office-Based Treatment
Primary care physicians can safely use simple treatment measures to manage most cases of symptomatic hemorrhoids (Figure 3). Only the more symptomatic patients require surgical intervention.
Patients should be made aware of the nature of the condition and the advantages and disadvantages of the different treatment options. The evidence regarding these treatments is summarized in the Table.
FIGURE 3
Management of adult patients with hemorrhoids
TABLE
Medical and surgical treatments for hemorrhoids
| Treatment | SOR* | Indication | Comments |
|---|---|---|---|
| Analgesics and anti-inflammatories2 | D | Grades I–IV and thrombosed external hemorrhoids | No trials comparing analgesics with anti-inflammatories |
| Topical treatments with corticosteroids or anesthetics3 | D | Grades I–III | No controlled trials with placebo are available, but patients report empiric benefit with their use; use only for brief periods |
| High-fiber diet or fiber supplements10-13 | B | Grades I–II | NNT=2.8 for reduction of rectal bleeding and 3.6 for pain relief23 |
| Office procedures23-25 | A | Grades I and II that do not respond to medical therapy, and grade III | Rubber band ligation was more effective and required fewer additional treatments for symptomatic recurrence than did infrared coagulation (NNT=9) and sclerotherapy (NNT=6.9); but rubber band ligation produced more complications than did infrared coagulation (pain: NNH=6) |
| Phlebotonics14-21 | B | Grades I–II | Moderate reduction of duration of bleeding during acute episodes of internal hemorrhoids; conflicting results for other outcomes (pain and prolapse) |
| Hemorrhoidectomy24 | A | Grades II–IV | Hemorrhoidectomy is more effective than office procedures, but it is more painful and presents more complications; office procedures are cheaper and require no time off from work |
| Stapling technique31-36 | A | Grades III–IV | Stapling technique is as effective as hemorrhoidectomy, is less painful, and requires less time off from work; more long-term data are needed37 |
| *For an explanation of strength of recommendation, see page 379. | |||
| SOR, strength of recommendation; NNT, number needed to treat; NNH, number needed to harm | |||
Benefits of lifestyle changes
The main purpose of lifestyle changes is to minimize prolonged straining during bowel movements, which is thought to contribute to the development of hemorrhoids. Such changes include increasing the amount of fiber in the diet, which is especially helpful for grade I and II internal hemorrhoids. Preventing constipation also helps alleviate more severe hemorrhoids and can help to prevent future episodes.10
Rectal bleeding can mask the diagnosis of cancer. The probability of colorectal cancer increases in the elderly, those with a family or personal history of colorectal cancer, and those with any other symptoms of colorectal cancer (fatigue, weight loss, palpable tumor, anemia). Patients at risk need a more thorough evaluation, including endoscopy, to rule out malignancy.9
It is important to document that observed hemorrhoids account for the bleeding episode (bleeding at the contact site with the anoscope or an applicator, thrombosed hemorrhoids, or a clot over the hemorrhoid).
Several clinical trials have reported the benefits of a high-fiber diet or fiber supplements compared with placebo in relieving pain, bleeding, and prolapse (strength of recommendation [SOR]: B).10-13 We found no studies comparing the different types of bulk laxatives.
Although the role of certain foods in the pathogenesis of hemorrhoids or their acute exacerbation is accepted empirically, this has not been proved. Also, no firm evidence to date shows that increasing physical exercise, limiting time on the commode, or improving local hygiene are beneficial. However, these measures are usually recommended because they have other health benefits or are thought to do no harm (SOR: D).
Medical therapy
No rigorous evidence exists to support the use of topical therapies, physical or pharmacologic (sitz baths, anesthetics, phlebotonics, corticoids, or ice). Most studies have employed poor methods, lacked placebo control, and addressed heterogeneous preparations with multiple associated components. It is therefore not possible to formulate firm recommendations.
Soothing agents. Popular topical soothing agents are often combined with corticosteroids or anesthetics and are available over the counter as creams or enemas. Many patients report some empirical benefit with their use, especially corticosteroids and anesthetics (SOR: D).3 Advise patients against prolonged use due to possible local allergic effects and sensitization of skin (SOR: D).
Phlebotonics. Several phlebotonics have been evaluated in the literature; diosmin is the best-studied agent, but it is not commercially available in the United States at this time. In studies of patients with acute attacks of internal hemorrhoids (grades I to II), the main perceived effect was reduced bleeding duration;14,15 the results were conflicting for other outcomes (mainly pain and prolapse)14-18 (SOR: B).
Other phlebotonics (the botanicals ginkgo biloba, troxerutin, and calcium dobesilate), when compared with placebo19,20 or diosmin,21 have shown similar effects (SOR: B). No studies thus far have evaluated the role of phlebotonics in thrombosis of external hemorrhoids.
Anti-inflammatories and analgesics. Most episodes of acute thrombosed external hemorrhoids improve spontaneously and therefore can be treated with symptomatic measures, including anti-inflammatory agents, analgesics, and stool softeners. Anti-inflammatories and analgesics can be effective during episodes of external and internal thromboses3 (SOR: D). Several clinical trials have reported benefits of fiber in relieving pain, bleeding, and prolapse
In a small randomized clinical trial, the addition of topical nifedipine (0.3%) to a lidocaine ointment (1.5%) was more effective than lidocaine alone in reducing pain and shortening resolution time.22
Surgical treatment
All office (nonoperative) and surgical procedures fix the sliding hemorrhoidal tissue back onto the muscle wall. The fixation takes place by directly promoting tissue fibrosis (eg, sclerotherapy or infrared coagulation) or by tissue destruction with subsequent fibrosis (eg, hemorrhoidectomy).
Office procedures. The most commonly used methods are rubber band ligation and infrared coagulation. Other methods include bipolar electrocoagulation, low-voltage direct current, sclerotherapy, laser therapy, and cryosurgery.
Two meta-analyses compared these nonoperative methods and concluded that rubber band ligation and infrared coagulation are the most effective. The first meta-analysis reported that ligation was more effective because it required fewer additional treatments for symptomatic recurrence than did coagulation (number needed to treat [NNT]=9) and sclerotherapy (NNT=6.9).23 However, ligation produced more complications than did coagulation (pain: number needed to harm=6) (SOR: A).
The second, more recent meta-analysis found ligation to have similar beneficial effect and a similar complication rate,24 although it was more painful. It appeared to be the therapy of choice for grades I to III (SOR: A). No difference was found between sclerotherapy and infrared coagulation for any outcome measure, but the authors of these meta-analyses commented that the overall quality of the studies was not high and their conclusions were therefore limited. One subsequent randomized clinical trial confirmed the advantages of rubber band ligation.25
In the event of a thrombosed hemorrhoid, whether to remove the clot promptly or wait for spontaneous resolution is controversial. We found no studies comparing these approaches. Excision should be performed when local measures fail, the thrombosis is painful, and there is no local edema (SOR: D).3
In the treatment of perianal thrombosis, one clinical trial found excision more effective than topically applied 0.2% glyceryl trinitrate or incision in reducing pain and the number of recurrences at 1 year (SOR: A).26 Residual hemorrhoidal tissue following an episode of acute thrombosis of external hemorrhoids also may cause symptoms, especially pruritus. These external anal tags can make it difficult to clean the anus and can be excised if symptoms warrant.
Surgery. Surgical treatment, though more invasive and expensive, is the most effective and definitive course for symptomatic hemorrhoids. The aim is to decrease blood flow to the anorectal ring and excise redundant hemorrhoidal tissue.
There are several techniques. In the United States, the Ferguson (closed) hemorrhoidectomy is preferred. The one used most commonly in Europe is the Milligan-Morgan technique (open). Both techniques have been shown to be similarly effective, although there is debate over healing time.27-30 Only a competently performed technique will produce satisfying results.
In their meta-analysis, MacRae and McLeod found that hemorrhoidectomy is more effective than all other treatment modalities, though complications such as pain and costs were greater (SOR: A).24
A new surgical procedure, the stapled hemorrhoidectomy, has been introduced as an alternative to the standard hemorrhoidectomy. Several randomized clinical trials have shown the procedure to be as effective, cause less pain, and require less time off from work compared with standard techniques (SOR: A).31-36 However, it is more expensive and requires advanced surgical skills. More long-term data are also needed.37
Anal stretch, or manual anal dilatation, has been reported to be effective in the treatment of hemorrhoids, but a high rate of incontinence after the procedure has led to abandonment of this technique.38,39 Antibiotic prophylaxis in colorectal surgery is highly recommended and has been shown to reduce infection and mortality (SOR: A).40
Surgery vs office procedures
Several clinical practice guidelines3,38 and meta-analyses23,24 have recommended office procedures for hemorrhoids of grades I through III. Although there is some discrepancy about the procedure of choice, rubber band ligation appears to be the most effective technique.
An evidence-based clinical practice guideline3 has recommended coagulation techniques for bleeding nonprolapsed hemorrhoids or those with a low grade of prolapse (grades I and II), and reserving rubber band ligation for hemorrhoids more severely prolapsed (grade III). The basis for this recommendation is that flat bleeding hemorrhoids may not provide enough tissue to grasp.
Surgical hemorrhoidectomy should be reserved for grade IV hemorrhoids and for grade III lesions that do not respond to other procedures.
This is an approximate rather than a rigid approach, and the final decision will depend on the physician’s technical training, the patient’s preferences, clinical circumstances, and local resources.
Prognosis: 90% require no surgery
Hemorrhoids are generally a chronic problem and tend to worsen with time. According to a retrospective cohort study, most patients will have several episodes during their lives; however, it can be considered a benign disorder, and approximately 90% of patients will not require surgery to alleviate their symptoms (SOR: B).41
It is worth noting that 26% of patients who require a hemorrhoidectomy may have a recurrence, and 11% will need further treatment.39 Similarly, approximately half of those who undergo office procedures may require further treatment or surgery in 5 to 10 years.42,43
ACKNOWLEDGMENTS
We thank E. Thompson for help in editing this manuscript and J. A. Ferrus and A. Hervas for their clinical comments. Dr Alonso-Coello holds a postgraduate research fellowship at the Instituto Carlos III, Spanish Ministry of Health, Spain.
Corresponding author
Pablo Alonso-Coello, MD, Ibero-american Cochrane Centre, Hospital de la Santa Creu i Sant Pau, Sant Antoni Mª Claret 171, 08041 Barcelona, Spain. E-mail: [email protected].
- Only symptomatic hemorrhoids require treatment. Most patients can be treated with conservative therapy alone or an office procedure.
- Anoscopy detects more lesions in the anorectal region than does flexible sigmoidoscopy. Visualization is best achieved with the slotted anoscope.
- Rubber band ligation is the preferred office procedure for treatment of grade I/II hemorrhoids that do not respond to medical therapy, and treatment of all grade III hemorrhoids.
Most cases of hemorrhoids can be managed in the primary care setting with simple measures and office procedures, including anoscopy. This paper reviews the advantages, disadvantages, and levels of evidence regarding specific treatments for different grades of hemorrhoids.
Prevalence
Prevalence varies from 4.4% in the general population to 36.4% in general practice.1 The annual rate of office visits for hemorrhoids is 12 for every 1000 patients in the United States2 ; its prevalence is similar between the sexes and increases with age until the seventh decade.3,4 Only a third of patients with symptomatic hemorrhoids seek medical help.4
Characteristics
Hemorrhoidal padding, which is critical to maintaining continence, accounts for approximately 15% to 20% of the anal resting pressure and supplies important sensory information that enables the differentiation between liquid, solid, and gas. When an individual coughs or performs a Valsalva maneuver, this vascular padding increases in area and volume, thereby enabling the anal canal to remain closed and avoid the loss of stools.
Hemorrhoids are associated with chronic straining secondary to constipation, diarrhea, tenesmus, or long periods trying to defecate, and are common during pregnancy and child-birth.5 The pathophysiology is not clearly understood, but current theories suggest that structural or vascular changes may be involved.
The mucocutaneous junction of the ano-rectum, or dentate line, divides hemorrhoids anatomically into internal (above the junction) and external (below). This anatomic “border” is of special clinical interest because external pain fibers end at this point, and most people have no sensation above this line.
Hemorrhoids originating above the junction, even if prolapsed, are still classified as internal hemorrhoids, and are divided into 4 categories depending on the grade of prolapse:
- Grade I—Protrudes into the anal canal but does not prolapse
- Grade II—Prolapses but reduces spontaneously
- Grade III—Prolapses and requires manual reduction
- Grade IV—Irreducible prolapse.
Hemorrhoids, especially if external, sometimes thrombose (Figure 1). Distention of overlying perianal skin and inflammation associated with the process of thrombosis can cause severe pain and discomfort.
FIGURE 1
Thrombosed hemorrhoids Anoscopy
Thrombosis can be particularly painful due to distention of perianal skin and inflammation. Used with permission, National Procedures Institute, Midland, Mich.
Diagnosis
Symptoms
The most common symptoms of hemorrhoids are bleeding and prolapse. Less frequently, symptoms also include discomfort, pain, soiling, or itching.
Internal hemorrhoids are usually painless; bleeding or prolapse generally prompt a visit to the physician. Bleeding is described as bright red spotting on toilet tissue or as dripping in the toilet bowl and normally occurs at the end of defecation and separately from the stool.
External hemorrhoids may be asymptomatic, associated with discomfort, or a cause of acute pain in the event of a thrombosis. They generally do not bleed except in the case of a spontaneously resolved local thrombosis. Some individuals may have both types of hemorrhoids simultaneously (mixed).
The term hemorrhoids is commonly misused by patients to refer to any anal symptoms. Other diagnoses such as anal fissures, pruritus ani, abscess, fistula, and condyloma should be ruled out by examining the anus, the perianal region, and the anal canal. However, we have found no studies reporting on the accuracy of the medical history or the physical examination.
How to conduct a digital examination
The patient should be positioned in the left lateral decubitus position for the anorectal exam-ination.6 This position is more comfortable and less intimidating for the patient than the traditional head-down position, and it permits optimal visualization.
Digital palpation allows the entire circumference of the canal to be examined and rectal masses or tender points to be ruled out. Internal hem-orrhoids cannot be detected this way, however.6 The procedure, which must be done gently and with prior reassurance to the patient, is generally quite simple. Intense pain may prevent further examination and suggests the possibility of anal fissure or thrombosed external hemorrhoid.
Anoscopy: safe and essential office procedure
The most accurate method for examining the anal canal and distal rectum is anoscopy. Although several types of anoscopes are available, visualization is best achieved with the slotted anoscope (Figure 2).6 Once it has been inserted, the anoscope is gradually withdrawn while rotating right and left to allow inspection of the mucous membrane. Hemorrhoids appear as pink swellings of the mucosa. Ask the patient to strain during the examination to improve visualization.
Two prospective studies found that anoscopy detects a higher percentage of lesions in the anorectal region than does flexible sigmoidoscopy (99% vs 78%).7,8 It is the procedure of choice for evaluating rectal diseases.
In skilled hands, anoscopy is safe; complications are unusual, and it can be performed in a physician’s office on short notice and without bowel preparation. After appropriate training, primary care physicians can perform routine examinations safely and accurately.
FIGURE 2
Anoscopy
The slotted anoscope provides the best visualization. Used with permission, National Procedures Institute, Midland, Mich.
Office-Based Treatment
Primary care physicians can safely use simple treatment measures to manage most cases of symptomatic hemorrhoids (Figure 3). Only the more symptomatic patients require surgical intervention.
Patients should be made aware of the nature of the condition and the advantages and disadvantages of the different treatment options. The evidence regarding these treatments is summarized in the Table.
FIGURE 3
Management of adult patients with hemorrhoids
TABLE
Medical and surgical treatments for hemorrhoids
| Treatment | SOR* | Indication | Comments |
|---|---|---|---|
| Analgesics and anti-inflammatories2 | D | Grades I–IV and thrombosed external hemorrhoids | No trials comparing analgesics with anti-inflammatories |
| Topical treatments with corticosteroids or anesthetics3 | D | Grades I–III | No controlled trials with placebo are available, but patients report empiric benefit with their use; use only for brief periods |
| High-fiber diet or fiber supplements10-13 | B | Grades I–II | NNT=2.8 for reduction of rectal bleeding and 3.6 for pain relief23 |
| Office procedures23-25 | A | Grades I and II that do not respond to medical therapy, and grade III | Rubber band ligation was more effective and required fewer additional treatments for symptomatic recurrence than did infrared coagulation (NNT=9) and sclerotherapy (NNT=6.9); but rubber band ligation produced more complications than did infrared coagulation (pain: NNH=6) |
| Phlebotonics14-21 | B | Grades I–II | Moderate reduction of duration of bleeding during acute episodes of internal hemorrhoids; conflicting results for other outcomes (pain and prolapse) |
| Hemorrhoidectomy24 | A | Grades II–IV | Hemorrhoidectomy is more effective than office procedures, but it is more painful and presents more complications; office procedures are cheaper and require no time off from work |
| Stapling technique31-36 | A | Grades III–IV | Stapling technique is as effective as hemorrhoidectomy, is less painful, and requires less time off from work; more long-term data are needed37 |
| *For an explanation of strength of recommendation, see page 379. | |||
| SOR, strength of recommendation; NNT, number needed to treat; NNH, number needed to harm | |||
Benefits of lifestyle changes
The main purpose of lifestyle changes is to minimize prolonged straining during bowel movements, which is thought to contribute to the development of hemorrhoids. Such changes include increasing the amount of fiber in the diet, which is especially helpful for grade I and II internal hemorrhoids. Preventing constipation also helps alleviate more severe hemorrhoids and can help to prevent future episodes.10
Rectal bleeding can mask the diagnosis of cancer. The probability of colorectal cancer increases in the elderly, those with a family or personal history of colorectal cancer, and those with any other symptoms of colorectal cancer (fatigue, weight loss, palpable tumor, anemia). Patients at risk need a more thorough evaluation, including endoscopy, to rule out malignancy.9
It is important to document that observed hemorrhoids account for the bleeding episode (bleeding at the contact site with the anoscope or an applicator, thrombosed hemorrhoids, or a clot over the hemorrhoid).
Several clinical trials have reported the benefits of a high-fiber diet or fiber supplements compared with placebo in relieving pain, bleeding, and prolapse (strength of recommendation [SOR]: B).10-13 We found no studies comparing the different types of bulk laxatives.
Although the role of certain foods in the pathogenesis of hemorrhoids or their acute exacerbation is accepted empirically, this has not been proved. Also, no firm evidence to date shows that increasing physical exercise, limiting time on the commode, or improving local hygiene are beneficial. However, these measures are usually recommended because they have other health benefits or are thought to do no harm (SOR: D).
Medical therapy
No rigorous evidence exists to support the use of topical therapies, physical or pharmacologic (sitz baths, anesthetics, phlebotonics, corticoids, or ice). Most studies have employed poor methods, lacked placebo control, and addressed heterogeneous preparations with multiple associated components. It is therefore not possible to formulate firm recommendations.
Soothing agents. Popular topical soothing agents are often combined with corticosteroids or anesthetics and are available over the counter as creams or enemas. Many patients report some empirical benefit with their use, especially corticosteroids and anesthetics (SOR: D).3 Advise patients against prolonged use due to possible local allergic effects and sensitization of skin (SOR: D).
Phlebotonics. Several phlebotonics have been evaluated in the literature; diosmin is the best-studied agent, but it is not commercially available in the United States at this time. In studies of patients with acute attacks of internal hemorrhoids (grades I to II), the main perceived effect was reduced bleeding duration;14,15 the results were conflicting for other outcomes (mainly pain and prolapse)14-18 (SOR: B).
Other phlebotonics (the botanicals ginkgo biloba, troxerutin, and calcium dobesilate), when compared with placebo19,20 or diosmin,21 have shown similar effects (SOR: B). No studies thus far have evaluated the role of phlebotonics in thrombosis of external hemorrhoids.
Anti-inflammatories and analgesics. Most episodes of acute thrombosed external hemorrhoids improve spontaneously and therefore can be treated with symptomatic measures, including anti-inflammatory agents, analgesics, and stool softeners. Anti-inflammatories and analgesics can be effective during episodes of external and internal thromboses3 (SOR: D). Several clinical trials have reported benefits of fiber in relieving pain, bleeding, and prolapse
In a small randomized clinical trial, the addition of topical nifedipine (0.3%) to a lidocaine ointment (1.5%) was more effective than lidocaine alone in reducing pain and shortening resolution time.22
Surgical treatment
All office (nonoperative) and surgical procedures fix the sliding hemorrhoidal tissue back onto the muscle wall. The fixation takes place by directly promoting tissue fibrosis (eg, sclerotherapy or infrared coagulation) or by tissue destruction with subsequent fibrosis (eg, hemorrhoidectomy).
Office procedures. The most commonly used methods are rubber band ligation and infrared coagulation. Other methods include bipolar electrocoagulation, low-voltage direct current, sclerotherapy, laser therapy, and cryosurgery.
Two meta-analyses compared these nonoperative methods and concluded that rubber band ligation and infrared coagulation are the most effective. The first meta-analysis reported that ligation was more effective because it required fewer additional treatments for symptomatic recurrence than did coagulation (number needed to treat [NNT]=9) and sclerotherapy (NNT=6.9).23 However, ligation produced more complications than did coagulation (pain: number needed to harm=6) (SOR: A).
The second, more recent meta-analysis found ligation to have similar beneficial effect and a similar complication rate,24 although it was more painful. It appeared to be the therapy of choice for grades I to III (SOR: A). No difference was found between sclerotherapy and infrared coagulation for any outcome measure, but the authors of these meta-analyses commented that the overall quality of the studies was not high and their conclusions were therefore limited. One subsequent randomized clinical trial confirmed the advantages of rubber band ligation.25
In the event of a thrombosed hemorrhoid, whether to remove the clot promptly or wait for spontaneous resolution is controversial. We found no studies comparing these approaches. Excision should be performed when local measures fail, the thrombosis is painful, and there is no local edema (SOR: D).3
In the treatment of perianal thrombosis, one clinical trial found excision more effective than topically applied 0.2% glyceryl trinitrate or incision in reducing pain and the number of recurrences at 1 year (SOR: A).26 Residual hemorrhoidal tissue following an episode of acute thrombosis of external hemorrhoids also may cause symptoms, especially pruritus. These external anal tags can make it difficult to clean the anus and can be excised if symptoms warrant.
Surgery. Surgical treatment, though more invasive and expensive, is the most effective and definitive course for symptomatic hemorrhoids. The aim is to decrease blood flow to the anorectal ring and excise redundant hemorrhoidal tissue.
There are several techniques. In the United States, the Ferguson (closed) hemorrhoidectomy is preferred. The one used most commonly in Europe is the Milligan-Morgan technique (open). Both techniques have been shown to be similarly effective, although there is debate over healing time.27-30 Only a competently performed technique will produce satisfying results.
In their meta-analysis, MacRae and McLeod found that hemorrhoidectomy is more effective than all other treatment modalities, though complications such as pain and costs were greater (SOR: A).24
A new surgical procedure, the stapled hemorrhoidectomy, has been introduced as an alternative to the standard hemorrhoidectomy. Several randomized clinical trials have shown the procedure to be as effective, cause less pain, and require less time off from work compared with standard techniques (SOR: A).31-36 However, it is more expensive and requires advanced surgical skills. More long-term data are also needed.37
Anal stretch, or manual anal dilatation, has been reported to be effective in the treatment of hemorrhoids, but a high rate of incontinence after the procedure has led to abandonment of this technique.38,39 Antibiotic prophylaxis in colorectal surgery is highly recommended and has been shown to reduce infection and mortality (SOR: A).40
Surgery vs office procedures
Several clinical practice guidelines3,38 and meta-analyses23,24 have recommended office procedures for hemorrhoids of grades I through III. Although there is some discrepancy about the procedure of choice, rubber band ligation appears to be the most effective technique.
An evidence-based clinical practice guideline3 has recommended coagulation techniques for bleeding nonprolapsed hemorrhoids or those with a low grade of prolapse (grades I and II), and reserving rubber band ligation for hemorrhoids more severely prolapsed (grade III). The basis for this recommendation is that flat bleeding hemorrhoids may not provide enough tissue to grasp.
Surgical hemorrhoidectomy should be reserved for grade IV hemorrhoids and for grade III lesions that do not respond to other procedures.
This is an approximate rather than a rigid approach, and the final decision will depend on the physician’s technical training, the patient’s preferences, clinical circumstances, and local resources.
Prognosis: 90% require no surgery
Hemorrhoids are generally a chronic problem and tend to worsen with time. According to a retrospective cohort study, most patients will have several episodes during their lives; however, it can be considered a benign disorder, and approximately 90% of patients will not require surgery to alleviate their symptoms (SOR: B).41
It is worth noting that 26% of patients who require a hemorrhoidectomy may have a recurrence, and 11% will need further treatment.39 Similarly, approximately half of those who undergo office procedures may require further treatment or surgery in 5 to 10 years.42,43
ACKNOWLEDGMENTS
We thank E. Thompson for help in editing this manuscript and J. A. Ferrus and A. Hervas for their clinical comments. Dr Alonso-Coello holds a postgraduate research fellowship at the Instituto Carlos III, Spanish Ministry of Health, Spain.
Corresponding author
Pablo Alonso-Coello, MD, Ibero-american Cochrane Centre, Hospital de la Santa Creu i Sant Pau, Sant Antoni Mª Claret 171, 08041 Barcelona, Spain. E-mail: [email protected].
1. Hulme-Moir M, Bartolo DC. Hemorrhoids. Gastroenterol Clin North Am 2001;30:183-197.
2. Johanson JF, Sonnenberg A. Temporal changes in the occurrence of hemorrhoids in the United States and England. Dis Colon Rectum 1991;34:585-591.
3. Abramowitz L, Godeberge P, Staumont G, Soudan D. Clinical practice guidelines for the treatment of hemorrhoid disease [in French]. Gastroenterol Clin Biol 2001;25:674-702.
4. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 1990;98:380-386.
5. Beck DE. Hemorrhoidal disease. In: Beck DE, Wexner SD, eds. Fundamentals of Anorectal Surgery. 2nd ed. London: WB Saunders 1998;237-253.
6. Pfenninger JL, Zainea GG. Common anorectal conditions: part I. Symptoms and complaints. Am Fam Physician 2001;63:2391-2398.
7. Kelly SM, Sanowski RA, Foutch PG, Bellapravalu S, Haynes WC. A prospective comparison of anoscopy and fiber endoscopy in detecting anal lesions. J Clin Gastroenterol 1986;8:658-660.
8. Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci 1995;40:1520-1523.
9. Wauters H, Van Casteren V, Buntinx F. Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ 2000;321:998-999.
10. Moesgaard F, Nielsen ML, Hansen JB, Knudsen JT. High-fiber diet reduces bleeding and pain in patients with hemorrhoids: a double-blind trial of Vi-Siblin. Dis Colon Rectum 1982;25:454-456.
11. Broader JH, Gunn IF, Alexander-Williams J. Evaluation of a bulk-forming evacuant in the management of haemorrhoids. Br J Surg 1974;61:142-144.
12. Webster DJ, Gough DC, Craven JL. The use of bulk evacuant in patients with haemorrhoids. Br J Surg 1978;65:291-292.
13. Perez-Miranda M, Gomez-Cedenilla A, Leon-Colombo T, Pajares J, Mate-Jimenez J. Effect of fiber supplements on internal bleeding hemorrhoids. Hepatogastroenterology 1996;43:1504-1507.
14. Godeberge P. Daflon 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology 1994;45:574-578.
15. Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum 2000;43:66-69.
16. Thanapongsathorn W, Vajrabukka T. Clinical trial of oral diosmin (Daflon) in the treatment of hemorrhoids. Dis Colon Rectum 1992;35:1085-1088.
17. Cospite M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology 1994;45:566-573.
18. Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg 2000;87:868-872.
19. Annoni F, Boccasanta P, Chiurazzi D, Mozzi E, Oberhauser V. Treatment of acute symptoms of hemorrhoid disease with high-dose oral O-(beta-hydroxyethyl)-rutosides [in Italian]. Minerva Med 1986;77:1663-1668
20. Mentes BB, Gorgul A, Tatlicioglu E, Ayoglu F, Unal S. Efficacy of calcium dobesilate in treating acute attacks of hemorrhoidal disease. Dis Colon Rectum 2001;44:1489-1495.
21. Debien P, Denis J. Traitements des signes fonctionnels de la maladie hèmorroïaigüe: essai multicentrique, randomisè, diosmine d’hèmisynthèse versus association extrait de Ginko biloba-heptaminol-troxèrutine. Med Chir Dig 1996;25:259-264.
22. Perrotti P, Antropoli C, Molino D, De Stefano G, Antropoli M. Conservative treatment of acute thrombosed external hemorrhoids with topical nifedipine. Dis Colon Rectum 2001;44:405-409.
23. Johanson JF, Rimm A. Optimal nonsurgical treatment of hemorrhoids: a comparative analysis of infrared coagulation, rubber band ligation, and injection sclerotherapy. Am J Gastroenterol 1992;87:1600-1606.
24. MacRae HM, McLeod RS. Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum. 1995;38:687-694.
25. Poen AC, Felt-Bersma RJ, Cuesta MA, Deville W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infra-red coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol 2000;12:535-539.
26. Cavcic J, Turcic J, Martinac P, Mestrovic T, Mladina R, Pezerovic-Panijan R. Comparison of topically applied 0.2% glyceryl trinitrate ointment, incision and excision in the treatment of perianal thrombosis. Dig Liver Dis 2001;33:335-340.
27. Ho YH, Seow-Choen F, Tan M, Leong AF. Randomized controlled trial of open and closed haemorrhoidectomy. Br J Surg 1997;84:1729-1730.
28. Carapeti EA, Kamm MA, McDonald PJ, Chadwick SJ, Phillips RK. Randomized trial of open versus closed day-case haemorrhoidectomy. Br J Surg 1999;86:612-613.
29. Arbman G, Krook H, Haapaniemi S. Closed vs. open hemorrhoidectomy—is there any difference? Dis Colon Rectum 2000;43:31-34.
30. Gencosmanoglu R, Sad O, Koc D, Inceoglu R. Hemorrhoidectomy: open or closed technique? A prospective, randomized clinical trial. Dis Colon Rectum 2002;45:70-75.
31. Mehigan BJ, Monson JR, Hartley JE. Stapling procedure for haemorrhoids versus Milligan-Morgan haemorrhoidectomy: randomised controlled trial. Lancet 2000;355:782-785.
32. Rowsell M, Bello M, Hemingway DM. Circumferential mucosectomy (stapled haemorrhoidectomy) versus conventional haemorrhoidectomy: randomised controlled trial. Lancet. 2000;355:779-781.
33. Khalil KH, O’Bichere A, Sellu D. Randomized clinical trial of sutured versus stapled closed haemorrhoidectomy. Br J Surg 2000;87:1352-1355.
34. Boccasanta P, Capretti PG, Venturi M, et al. Randomised controlled trial between stapled circumferential mucosectomy and conventional circular hemorrhoidectomy in advanced hemorrhoids with external mucosal prolapse. Am J Surg 2001;182:64-68.
35. Ganio E, Altomare DF, Gabrielli F, Milito G, Canuti S. Prospective randomized multicentre trial comparing stapled with open haemorrhoidectomy. Br J Surg 2001;88:669-674.
36. Shalaby R, Desoky A. Randomized clinical trial of stapled versus Milligan-Morgan haemorrhoidectomy. Br J Surg 2001;88:1049-1053.
37. Hetzer FH, Demartines N, Handschin AE, Clavien PA. Stapled vs excision hemorrhoidectomy: long-term results of a prospective randomized trial. Arch Surg 2002;137:337-340.
38. Standards Task Force of American Society of Colon and Rectal Surgeons Practice parameters for the treatment of hemorrhoids. Dis Colon Rectum 1993;36:1118-1120.
39. Konsten J, Baeten CG. Hemorrhoidectomy vs. Lord’s method: 17-year follow-up of a prospective, randomized trial. Dis Colon Rectum 2000;43:503-506.
40. Baum ML, Anish DS, Chalmers TC, Sacks HS, Smith H, Fagerstrom RM. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med 1981;305:795-799.
41. Bleday R, Pena JP, Rothenberger DA, Goldberg SM, Buls JG. Symptomatic hemorrhoids: current incidence and complications of operative therapy. Dis Colon Rectum 1992;35:477-481.
42. Wrobleski DE, Corman ML, Veidenheimer MC, Coller JA. Long-term evaluation of rubber ring ligation in hemorrhoidal disease. Dis Colon Rectum 1980;23:478-482.
43. Savioz D, Roche B, Glauser T, Dobrinov A, Ludwig C, Marti MC. Rubber band ligation of hemorrhoids: relapse as a function of time. Int J Colorectal Dis 1998;13:154-156.
1. Hulme-Moir M, Bartolo DC. Hemorrhoids. Gastroenterol Clin North Am 2001;30:183-197.
2. Johanson JF, Sonnenberg A. Temporal changes in the occurrence of hemorrhoids in the United States and England. Dis Colon Rectum 1991;34:585-591.
3. Abramowitz L, Godeberge P, Staumont G, Soudan D. Clinical practice guidelines for the treatment of hemorrhoid disease [in French]. Gastroenterol Clin Biol 2001;25:674-702.
4. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 1990;98:380-386.
5. Beck DE. Hemorrhoidal disease. In: Beck DE, Wexner SD, eds. Fundamentals of Anorectal Surgery. 2nd ed. London: WB Saunders 1998;237-253.
6. Pfenninger JL, Zainea GG. Common anorectal conditions: part I. Symptoms and complaints. Am Fam Physician 2001;63:2391-2398.
7. Kelly SM, Sanowski RA, Foutch PG, Bellapravalu S, Haynes WC. A prospective comparison of anoscopy and fiber endoscopy in detecting anal lesions. J Clin Gastroenterol 1986;8:658-660.
8. Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci 1995;40:1520-1523.
9. Wauters H, Van Casteren V, Buntinx F. Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ 2000;321:998-999.
10. Moesgaard F, Nielsen ML, Hansen JB, Knudsen JT. High-fiber diet reduces bleeding and pain in patients with hemorrhoids: a double-blind trial of Vi-Siblin. Dis Colon Rectum 1982;25:454-456.
11. Broader JH, Gunn IF, Alexander-Williams J. Evaluation of a bulk-forming evacuant in the management of haemorrhoids. Br J Surg 1974;61:142-144.
12. Webster DJ, Gough DC, Craven JL. The use of bulk evacuant in patients with haemorrhoids. Br J Surg 1978;65:291-292.
13. Perez-Miranda M, Gomez-Cedenilla A, Leon-Colombo T, Pajares J, Mate-Jimenez J. Effect of fiber supplements on internal bleeding hemorrhoids. Hepatogastroenterology 1996;43:1504-1507.
14. Godeberge P. Daflon 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology 1994;45:574-578.
15. Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum 2000;43:66-69.
16. Thanapongsathorn W, Vajrabukka T. Clinical trial of oral diosmin (Daflon) in the treatment of hemorrhoids. Dis Colon Rectum 1992;35:1085-1088.
17. Cospite M. Double-blind, placebo-controlled evaluation of clinical activity and safety of Daflon 500 mg in the treatment of acute hemorrhoids. Angiology 1994;45:566-573.
18. Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg 2000;87:868-872.
19. Annoni F, Boccasanta P, Chiurazzi D, Mozzi E, Oberhauser V. Treatment of acute symptoms of hemorrhoid disease with high-dose oral O-(beta-hydroxyethyl)-rutosides [in Italian]. Minerva Med 1986;77:1663-1668
20. Mentes BB, Gorgul A, Tatlicioglu E, Ayoglu F, Unal S. Efficacy of calcium dobesilate in treating acute attacks of hemorrhoidal disease. Dis Colon Rectum 2001;44:1489-1495.
21. Debien P, Denis J. Traitements des signes fonctionnels de la maladie hèmorroïaigüe: essai multicentrique, randomisè, diosmine d’hèmisynthèse versus association extrait de Ginko biloba-heptaminol-troxèrutine. Med Chir Dig 1996;25:259-264.
22. Perrotti P, Antropoli C, Molino D, De Stefano G, Antropoli M. Conservative treatment of acute thrombosed external hemorrhoids with topical nifedipine. Dis Colon Rectum 2001;44:405-409.
23. Johanson JF, Rimm A. Optimal nonsurgical treatment of hemorrhoids: a comparative analysis of infrared coagulation, rubber band ligation, and injection sclerotherapy. Am J Gastroenterol 1992;87:1600-1606.
24. MacRae HM, McLeod RS. Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum. 1995;38:687-694.
25. Poen AC, Felt-Bersma RJ, Cuesta MA, Deville W, Meuwissen SG. A randomized controlled trial of rubber band ligation versus infra-red coagulation in the treatment of internal haemorrhoids. Eur J Gastroenterol Hepatol 2000;12:535-539.
26. Cavcic J, Turcic J, Martinac P, Mestrovic T, Mladina R, Pezerovic-Panijan R. Comparison of topically applied 0.2% glyceryl trinitrate ointment, incision and excision in the treatment of perianal thrombosis. Dig Liver Dis 2001;33:335-340.
27. Ho YH, Seow-Choen F, Tan M, Leong AF. Randomized controlled trial of open and closed haemorrhoidectomy. Br J Surg 1997;84:1729-1730.
28. Carapeti EA, Kamm MA, McDonald PJ, Chadwick SJ, Phillips RK. Randomized trial of open versus closed day-case haemorrhoidectomy. Br J Surg 1999;86:612-613.
29. Arbman G, Krook H, Haapaniemi S. Closed vs. open hemorrhoidectomy—is there any difference? Dis Colon Rectum 2000;43:31-34.
30. Gencosmanoglu R, Sad O, Koc D, Inceoglu R. Hemorrhoidectomy: open or closed technique? A prospective, randomized clinical trial. Dis Colon Rectum 2002;45:70-75.
31. Mehigan BJ, Monson JR, Hartley JE. Stapling procedure for haemorrhoids versus Milligan-Morgan haemorrhoidectomy: randomised controlled trial. Lancet 2000;355:782-785.
32. Rowsell M, Bello M, Hemingway DM. Circumferential mucosectomy (stapled haemorrhoidectomy) versus conventional haemorrhoidectomy: randomised controlled trial. Lancet. 2000;355:779-781.
33. Khalil KH, O’Bichere A, Sellu D. Randomized clinical trial of sutured versus stapled closed haemorrhoidectomy. Br J Surg 2000;87:1352-1355.
34. Boccasanta P, Capretti PG, Venturi M, et al. Randomised controlled trial between stapled circumferential mucosectomy and conventional circular hemorrhoidectomy in advanced hemorrhoids with external mucosal prolapse. Am J Surg 2001;182:64-68.
35. Ganio E, Altomare DF, Gabrielli F, Milito G, Canuti S. Prospective randomized multicentre trial comparing stapled with open haemorrhoidectomy. Br J Surg 2001;88:669-674.
36. Shalaby R, Desoky A. Randomized clinical trial of stapled versus Milligan-Morgan haemorrhoidectomy. Br J Surg 2001;88:1049-1053.
37. Hetzer FH, Demartines N, Handschin AE, Clavien PA. Stapled vs excision hemorrhoidectomy: long-term results of a prospective randomized trial. Arch Surg 2002;137:337-340.
38. Standards Task Force of American Society of Colon and Rectal Surgeons Practice parameters for the treatment of hemorrhoids. Dis Colon Rectum 1993;36:1118-1120.
39. Konsten J, Baeten CG. Hemorrhoidectomy vs. Lord’s method: 17-year follow-up of a prospective, randomized trial. Dis Colon Rectum 2000;43:503-506.
40. Baum ML, Anish DS, Chalmers TC, Sacks HS, Smith H, Fagerstrom RM. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med 1981;305:795-799.
41. Bleday R, Pena JP, Rothenberger DA, Goldberg SM, Buls JG. Symptomatic hemorrhoids: current incidence and complications of operative therapy. Dis Colon Rectum 1992;35:477-481.
42. Wrobleski DE, Corman ML, Veidenheimer MC, Coller JA. Long-term evaluation of rubber ring ligation in hemorrhoidal disease. Dis Colon Rectum 1980;23:478-482.
43. Savioz D, Roche B, Glauser T, Dobrinov A, Ludwig C, Marti MC. Rubber band ligation of hemorrhoids: relapse as a function of time. Int J Colorectal Dis 1998;13:154-156.
Treating hot flushes without hormone replacement therapy
- Ask your patients about complementary and alternative therapies; 21% of women say they use complementary or alternative therapies only, and another 25% say they use both conventional and alternative methods.
- Women who take 50 mg of soy isoflavones daily report a 10% to 20% absolute risk reduction (number needed to treat, 5–10) in the frequency of hot flashes. The duration of this effect is unknown.
- Black cohosh yields up to an 80% improvement in hot flashes.
- Patients should use an alternative therapy for at least 1 month and keep a symptom diary to adequately assess its effect.
Physicians may recommend alternative treatments for hot flashes with the same confidence they have in prescription drugs if they understand the expected results, risks and benefits, and interactions with other medications.
For treatment of hot flashes, an increasing number of menopausal women are choosing plantbased alternatives to hormone replacement therapy (HRT). Despite HRT’s proven efficacy in treating this plaguing symptom, many patients are fearful that HRT might lead to an increased risk of breast or uterine cancer, increase the risk of vascular disease including heart attack, or cause unpleasant side effects such as mood swings, depression, or continued menstrual periods.1
Dr. Seibel, an expert in non-hormone replacement therapy and a proponent of soy, provides his view of options in this important area. He argues that treatment with the more promising soy and black cohosh preparations is worth considering as part of a careful “N of 1” trial. Read this issue’s Clinical Inquiry, “What nonhormonal therapies are effective for postmenopausal vasomotor symptoms?” (pages 324–329), and you be the judge. —Jeffrey L. Susman, MD
One study of 2500 postmenopausal women found that 20% to 30% never fill their initial HRT prescriptions, 10% of those who use estrogen do so only intermittently, an additional 20% discontinue their therapy within 8 months, and only 15% to 20% of women will take HRT for more than a year.2
HRT is contraindicated in about 10% of postmenopausal women3 ; the Womens’ Health Initiative4 and the Heart and Estrogen/progestin Replacement Study5 (HERS) trials have suggested caution in using HRT even for those without contraindications.
With such an enormous number of women either unwilling or unable to take HRT, it is important to consider the alternatives you can offer.6,7 (See “How pervasive are alternative therapies?”) Whichever alternative treatment you and a patient select, give it at least 1 month (and preferably 3 months) to assess its effectiveness. Keeping a symptom diary will allow patients to objectively track their progress.
So-called alternative approaches to menopause are used so widely it might be more accurate to consider hormone replacement therapy as the true alternative medicine. Statistics presented at the National Institutes of Health on October 27, 2000, indicate that nearly half of all menopausal women are using complementary therapies—including vitamins, herbs, and soy products—to help treat their symptoms. Twenty-one percent of the women surveyed used complementary or alternative therapies alone, and 25% said they used both conventional and alternative methods.
Taken together, that is more than twice the 19% who said they used conventional hormone replacement therapy only. Given this enormous usage, it should come as no surprise that, in 2001, the dietary supplement industry likely exceeded $12 billion in sales in the United States alone.4 For many women, the decision to use an alternative is not so much dissatisfaction with conventional treatment, but that they regard the complementary agents as more congruent with their own values, beliefs, and philosophical orientations toward health and life.5
Soy
Much of the excitement about the health benefits of soy—a staple of the Asian diet for 5000 years—stems from epidemiological studies. The Asian diet, which is rich in isoflavones, is associated with a reduced risk of breast cancer, heart disease, and osteoporosis. Asian women also report fewer hot flashes than do their Western counterparts.8 One study showed that women in Western countries have an 80% incidence of hot flashes, while Asian women living in China have an incidence of only 20%.9
Clearly, factors other than soy also must be considered before we can make a direct cause-and-effect correlation. To that end, many studies have been conducted on the health benefits of soybeans, a rich source of the isoflavones genistein and daidzein.
Physiologic activity
Isoflavones are phytoestrogens with a hetero-cyclic phenol structure that is similar to estrogen. Their potency is between 1 x 104 and 1 x 103 the activity of 17Β-estradiol.10 Although their potency is low, their serum concentrations can reach levels several orders of magnitude higher than those of physiologic estrogens. It is generally believed that isoflavones act as a selective estrogen receptor modulator, exerting antiestrogenic effects in the high-estrogen environment of premenopause and estrogenic effects in the low-estrogen environment of post-menopause.
Clinical efficacy
Hot flushes. An increasing number of studies suggest that soy and soy isoflavones—in the form of soy flour, soy protein, and dietary supplements—may play an important role in the treatment of hot flushes. In general, the average amount of isoflavones consumed in a typical Asian diet is approximately 50 mg/day. (One gram of soy protein contains approximately 1 mg of isoflavones.)
Unfortunately, clinical trial data are confounded by varying preparations of soy, length of therapy, outcomes measured, and small sample sizes. The absolute risk reduction of soy preparations versus placebo or comparators in positive trials ranges from 10% to 20% (number needed to treat [NNT]=6–10) when frequency of hot flushes is the outcome measured (Table 1).11-15
Placebo effect. All soy studies also confirm the existence of a placebo effect on the treatment of hot flushes. The large placebo effect and varied efficacy has left many skeptics questioning the clinical efficacy of soy products.16
I believe that thinking is wrong. Women who have hot flushes tend to have lower sleep efficiencies and longer REM latencies than women who do not experience this vasomotor symptom.17 By lowering the frequency of hot flushes, soy may produce greater sleep efficiencies and improve quality of life.
TABLE 1
Positive trials* of soy in the treatment of hot flushes
| Absolute risk reduction of hot flushes (%) | ||||||
|---|---|---|---|---|---|---|
| Study | N | Design | Soy | Control | NNT | LOE† |
| Murkies 199511 | 58 | 45 g soy flour | 42 | 25 | 6 | 1b |
| Brzezinski 199712 | 73/72 | Mostly soy, 1/4 diet, some flaxseed | 54 | 35 | ||
| Albertazzi 199813 | 51/53 | 60 g soy protein | 43.6 | 31.3 | 8.1 | 2b |
| Scambia 200014 | 39 | 50 mg/d isoflavones | 45 | 25 | 1b | |
| Upmalis 200015 | 177 | 50 mg/d isoflavones | 30 | 20 | 1b | |
| From Seibel MM, The soy solution for menopause: an alternative to estrogen. New York, NY: Fireside Press, 2003. | ||||||
| *None of the above trials were of high quality—all either had a large number of dropouts, substantial divergence of outcomes, a small “n” (number of subjects) or other substantive methodological concerns. | ||||||
| NNT, number needed to treat; LOE, level of evidence | ||||||
| †See page 290 for a description of strength of recommendation. | ||||||
Safety
Soy has been safely consumed by hundreds of millions of people without complications, with t he possible exception of soy allergies (Table 2). The literature suggests it is safe and effective in dosages of either 40 g of soy protein or 50 mg of soy isoflavones per day. Soy does not stimulate the uterine lining and may be protective of the endometrium if taken with estrogen.
The incidence of breast cancer is one fourth as high in Asia as in the US; many studies have shown soy to be protective of breast tissue. However, soy may stimulate breast tissue in women with a history of breast cancer. The evidence in these areas is conflicting and controversial.
In contrast to herbal alternatives to HRT, soy has been thought to lower cholesterol, make blood vessels more elastic (increase vascular elasticity), and slow osteoclast activity in bones. It is also an excellent source of protein, and is lactose- and cholesterol-free.18
TABLE 2
Remedies for hot flushes and their adverse effects, interactions, and contraindications
| Adverse effects | Important drug interactions | Contraindications | |
|---|---|---|---|
| Soy | Bloating, flatulence | Thyroid hormone, if taken simultaneously | Use with caution in patients being treated for hypothyroidism (soy may bind thyroid medication, thus lowering absorption; patients on thyroid hormone should use soy only in supplement form or take at a time distant from thyroid medication) |
| Black cohosh | Abdominal pain, nausea, headaches, dizziness, trembling limbs | Iron therapy medications | Patients with iron deficiencies; use cautiously in patients with breast cancer or high risk of breast cancer |
| Dong quai | Photodermatitis, rash | Warfarin (effects potentiated) | Patients with coagulopathies or very heavy menstruation and acute viral infections such as colds or influenza; pregnancy |
| Evening primrose oil | Nausea, softening of stools, headaches, seizures | Anticonvulsant and tricyclic antidepressants | Patients taking anticonvulsants and tricyclic antidepressants (lowers efficacy) |
| Red clover | Blood thinning | Anticoagulants such as coumadin, heparin, clopidrogel, pentoxifylline, or aspirin | Patients with coagulopathies |
Black cohosh
A member of the buttercup family, black cohosh (Actaea [formerly Cimicifuga] racemosa) has a long history in folk medicine, especially among Native Americans, who boiled the root in water and drank the resulting beverage to treat dysmenorrhea, labor pain, upset stomach, and arthritis. In Germany, extracts of black cohosh have been used since the 1940s.
Many substances have been identified in the rhizome, but it is uncertain what the majority of them do or, in fact, which are active ingredients.19 The effectiveness of black cohosh is based on the total amount of triterpenoid glycosides, typically standardized to 2.5%.20
Several studies, most of them in the German literature, have shown that black cohosh yields a significant improvement in hot flushes, with reductions of up to 80% reported (level of evidence [LOE]: 2b).21-23 The usual dosage is 40 drops of the extract twice daily for 6 to 8 weeks, or one to two 20-mg tablets twice daily with liquid (not to be chewed or sucked).
Side effects are uncommon, but occasional stomach pains and intestinal discomfort, dizziness, nausea, severe headaches, stiffness, and trembling limbs have been noted. Germany’s Commission E, which is similar to the US Food and Drug Administration, recommends that black cohosh not be used for more than 6 months, since no studies have been conducted for longer periods.
Black cohosh has weak estrogenic effect on the breast and should be used cautiously in patients with breast cancer or a high risk of breast cancer. A 2-month double-blind, randomized controlled trial of black cohosh in breast cancer survivors demonstrated no short-term side effects; however, therapy was only significantly beneficial in relieving sweating and ineffective in reducing flushes.24 The long-term effects are unknown. A recent systematic review suggests that the side effects of black cohosh are transient, and severe adverse event reports are unproven (Table 2).24
Dong quai
Dong quai, a common Chinese herb extracted from the Angelica sinensis root, has become popular in the US. In contrast to China, where it is sold as part of a mixture that includes several other herbs, dong quai typically is sold in the US as a single herb.
Although some women report improvements in their vasomotor symptoms, there have been very few studies on the effects of dong quai on menopause. In one 24-week study of 71 postmenopausal women, researchers could not demonstrate a significant difference between dong quai and placebo in alleviating vasomotor symptoms (LOE: 2b).25 The investigators suggested that studying the effects of dong quai alone, rather than in combination with other herbs, may have been a factor in their findings.
Two caveats: Dong quai increases photosensitivity, so women taking the herb should be cautioned that too much exposure to sunlight may result in a rash. Also it has been reported to potentiate the effects of warfarin (Table 2). It should not be used during pregnancy.26
Evening primrose
Native Americans consumed the leaves, roots, and seedpods of evening primrose (Oenothera biennis) for food, and made extracts from it to treat a variety of conditions. Today, the flowers and seeds are pressed to make oil that is high in the omega-6 fatty acid gamma-linolenic acid (known as GLA) and essential polyunsaturated fatty acids, which convert into prostaglandins. Evening primrose oil also is a good source of linoleic acid.
Although there are a number of good studies in which evening primrose oil has been used to successfully treat eczema and several other conditions with few side effects, it appears to have no benefit over placebo for hot flushes (LOE: 2b).27
Patients should be warned that mild upset stomach, indigestion, nausea, softening of stools, and mild headaches may occasionally occur. Also, evening primrose is contraindicated in women taking seizure medications or antipsychotics because it lowers the seizure threshold in patients on phenothiazines (Table 2).
Red clover
Red clover (Trifolium pratense) is a plant that contains the phytoestrogens formononetin, biochanin A, daidzein, and genistein. It was originally used by Native Americans to treat whooping cough, gout, and cancer.
Two clinical trials conducted in Australia failed to demonstrate that red clover extract was more effective than placebo in reducing vasomotor symptoms (LOE: 2b).28,29 However, one recent presentation found that women who took 40 mg of red clover per day—the recommended dosage—experienced a significant reduction in hot flushes.30
There is still little information on whether red clover will have any effect on the uterine lining or breast tissue. Because red clover contains coumarin-like substances, high dosages may cause the blood to thin (Table 2).31
1. Salamone LM, Pressman AR, Seeley DG, Cauley JA. Estrogen replacement therapy. A survey of older women’s attitudes. Arch Int Med 1996;156:1293-1297.
2. Ravnikar VA. Compliance with hormone therapy. Am J Obstet Gynecol 1987;156:1332-1334.
3. Kessel B. Alternatives to estrogen for menopausal women. Proc Soc Esp Biol Med 1998;217:38-44.
4. Roussouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-333.
5. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone replacement therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002;288:49-57.
6. Seibel MM. The role of nutrition and nutritional supplements in women’s health. Fertil Steril 1999;72:579-591.
7. Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998;279:1548-1553.
8. Haines CJ, Chung TKH, Leung DHY. A prospective study of the frequency of acute menopausal symptoms in Hog Dong Chinese women. Maturitas 1994;18:175-181.
9. Tang GWK. The climacteric of Chinese factory workers. Maturitas 1994;19:177-182.
10. Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol 1993;45:399-405.
11. Murkies AL, Lombard C, Strauss BJD, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases postmenopausal hot flushes: effect of soy and wheat. Maturitas 1995;21:189-195.
12. Brzezinski A, Adlercreutz H, Shaoul R, Rosler A, Shmueli A, Tanos V, Schenker JG. Short-term effects of phytoestrogen-rich diet on postmenopausal women. Menopause 1997;4:89-94.
13. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
14. Scambia G, Mango D, Signorile PG, et al. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause 2000;7:105-111.
15. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236-242.
16. Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med 2002;137:805-811.
17. Shaver JLF, Giblin E, Paulsen V. Sleep quality subtypes in midlife women. Sleep 1991;14:18-23.
18. From Seibel MM. The soy solution for menopause: an alternative to estrogen. New York, NY: Fireside Press, 2003.
19. Struck D, Tegtmeier M, Harnishfeger G. Flavones in extracts of Cimicifuga racemosa. Planta Med 1997;63:289-290.
20. Beuscher N. Cimicifuga racemosa L.Black Cohosh. HerbalGram 1996;19-27.
21. Vorberg G. Therapy of climacteric complaints. Zeitschrift fur Allgemeinmedizin 1984;60:626-629.
22. Warnecke G. Influence of a phytopharmaceutical on climacteric complaints. Die Meizinische Welt 1985;36:871-874.
23. Stoll W. Phytopharmacon influences atrophic vaginal epithelium: double-blind study—Cimicifuga vs. estrogenic substances. Therapeuticum 1987;1:23-31.
24. Jacobson JS, Troxel AB, Evans J, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-2745.
25. Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause 2003;10:58-64.
26. Hirata JD, Swiers LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind placebo-controlled trial. Fertil Steril 1997;68:981-986.
27. Page RL 2nd, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy 1999;19:870-876.
28. Chenoy R, Hussain S, Tayob Y, O’Brien PMS, Moss MY, Morse PF. Effect of oral gamalenic acid from evening primrose oil on menopausal flushing. BMJ 1994;308:501-503.
29. Barber RJ, Templeman C, Morton T, Delley GE, Weat L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999;2:85-92.
30. Knight DC, Howes JB, Eden JA. The effect of Promensil an isoflavone extract, on menopausal symptoms. Climacteric 1999;2:79-84.
31. Nachtigall LB, LaGrega L, Lee WW, et al. The effects of isoflavones derived from red clover on vasomotor symptoms and endometrial thickness. 9th International Menopause Society World Congress on Menopause, October 17-21, 1999.
32. Fugh-Berman A, Kronenberg F. Red clover (Trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-337.
- Ask your patients about complementary and alternative therapies; 21% of women say they use complementary or alternative therapies only, and another 25% say they use both conventional and alternative methods.
- Women who take 50 mg of soy isoflavones daily report a 10% to 20% absolute risk reduction (number needed to treat, 5–10) in the frequency of hot flashes. The duration of this effect is unknown.
- Black cohosh yields up to an 80% improvement in hot flashes.
- Patients should use an alternative therapy for at least 1 month and keep a symptom diary to adequately assess its effect.
Physicians may recommend alternative treatments for hot flashes with the same confidence they have in prescription drugs if they understand the expected results, risks and benefits, and interactions with other medications.
For treatment of hot flashes, an increasing number of menopausal women are choosing plantbased alternatives to hormone replacement therapy (HRT). Despite HRT’s proven efficacy in treating this plaguing symptom, many patients are fearful that HRT might lead to an increased risk of breast or uterine cancer, increase the risk of vascular disease including heart attack, or cause unpleasant side effects such as mood swings, depression, or continued menstrual periods.1
Dr. Seibel, an expert in non-hormone replacement therapy and a proponent of soy, provides his view of options in this important area. He argues that treatment with the more promising soy and black cohosh preparations is worth considering as part of a careful “N of 1” trial. Read this issue’s Clinical Inquiry, “What nonhormonal therapies are effective for postmenopausal vasomotor symptoms?” (pages 324–329), and you be the judge. —Jeffrey L. Susman, MD
One study of 2500 postmenopausal women found that 20% to 30% never fill their initial HRT prescriptions, 10% of those who use estrogen do so only intermittently, an additional 20% discontinue their therapy within 8 months, and only 15% to 20% of women will take HRT for more than a year.2
HRT is contraindicated in about 10% of postmenopausal women3 ; the Womens’ Health Initiative4 and the Heart and Estrogen/progestin Replacement Study5 (HERS) trials have suggested caution in using HRT even for those without contraindications.
With such an enormous number of women either unwilling or unable to take HRT, it is important to consider the alternatives you can offer.6,7 (See “How pervasive are alternative therapies?”) Whichever alternative treatment you and a patient select, give it at least 1 month (and preferably 3 months) to assess its effectiveness. Keeping a symptom diary will allow patients to objectively track their progress.
So-called alternative approaches to menopause are used so widely it might be more accurate to consider hormone replacement therapy as the true alternative medicine. Statistics presented at the National Institutes of Health on October 27, 2000, indicate that nearly half of all menopausal women are using complementary therapies—including vitamins, herbs, and soy products—to help treat their symptoms. Twenty-one percent of the women surveyed used complementary or alternative therapies alone, and 25% said they used both conventional and alternative methods.
Taken together, that is more than twice the 19% who said they used conventional hormone replacement therapy only. Given this enormous usage, it should come as no surprise that, in 2001, the dietary supplement industry likely exceeded $12 billion in sales in the United States alone.4 For many women, the decision to use an alternative is not so much dissatisfaction with conventional treatment, but that they regard the complementary agents as more congruent with their own values, beliefs, and philosophical orientations toward health and life.5
Soy
Much of the excitement about the health benefits of soy—a staple of the Asian diet for 5000 years—stems from epidemiological studies. The Asian diet, which is rich in isoflavones, is associated with a reduced risk of breast cancer, heart disease, and osteoporosis. Asian women also report fewer hot flashes than do their Western counterparts.8 One study showed that women in Western countries have an 80% incidence of hot flashes, while Asian women living in China have an incidence of only 20%.9
Clearly, factors other than soy also must be considered before we can make a direct cause-and-effect correlation. To that end, many studies have been conducted on the health benefits of soybeans, a rich source of the isoflavones genistein and daidzein.
Physiologic activity
Isoflavones are phytoestrogens with a hetero-cyclic phenol structure that is similar to estrogen. Their potency is between 1 x 104 and 1 x 103 the activity of 17Β-estradiol.10 Although their potency is low, their serum concentrations can reach levels several orders of magnitude higher than those of physiologic estrogens. It is generally believed that isoflavones act as a selective estrogen receptor modulator, exerting antiestrogenic effects in the high-estrogen environment of premenopause and estrogenic effects in the low-estrogen environment of post-menopause.
Clinical efficacy
Hot flushes. An increasing number of studies suggest that soy and soy isoflavones—in the form of soy flour, soy protein, and dietary supplements—may play an important role in the treatment of hot flushes. In general, the average amount of isoflavones consumed in a typical Asian diet is approximately 50 mg/day. (One gram of soy protein contains approximately 1 mg of isoflavones.)
Unfortunately, clinical trial data are confounded by varying preparations of soy, length of therapy, outcomes measured, and small sample sizes. The absolute risk reduction of soy preparations versus placebo or comparators in positive trials ranges from 10% to 20% (number needed to treat [NNT]=6–10) when frequency of hot flushes is the outcome measured (Table 1).11-15
Placebo effect. All soy studies also confirm the existence of a placebo effect on the treatment of hot flushes. The large placebo effect and varied efficacy has left many skeptics questioning the clinical efficacy of soy products.16
I believe that thinking is wrong. Women who have hot flushes tend to have lower sleep efficiencies and longer REM latencies than women who do not experience this vasomotor symptom.17 By lowering the frequency of hot flushes, soy may produce greater sleep efficiencies and improve quality of life.
TABLE 1
Positive trials* of soy in the treatment of hot flushes
| Absolute risk reduction of hot flushes (%) | ||||||
|---|---|---|---|---|---|---|
| Study | N | Design | Soy | Control | NNT | LOE† |
| Murkies 199511 | 58 | 45 g soy flour | 42 | 25 | 6 | 1b |
| Brzezinski 199712 | 73/72 | Mostly soy, 1/4 diet, some flaxseed | 54 | 35 | ||
| Albertazzi 199813 | 51/53 | 60 g soy protein | 43.6 | 31.3 | 8.1 | 2b |
| Scambia 200014 | 39 | 50 mg/d isoflavones | 45 | 25 | 1b | |
| Upmalis 200015 | 177 | 50 mg/d isoflavones | 30 | 20 | 1b | |
| From Seibel MM, The soy solution for menopause: an alternative to estrogen. New York, NY: Fireside Press, 2003. | ||||||
| *None of the above trials were of high quality—all either had a large number of dropouts, substantial divergence of outcomes, a small “n” (number of subjects) or other substantive methodological concerns. | ||||||
| NNT, number needed to treat; LOE, level of evidence | ||||||
| †See page 290 for a description of strength of recommendation. | ||||||
Safety
Soy has been safely consumed by hundreds of millions of people without complications, with t he possible exception of soy allergies (Table 2). The literature suggests it is safe and effective in dosages of either 40 g of soy protein or 50 mg of soy isoflavones per day. Soy does not stimulate the uterine lining and may be protective of the endometrium if taken with estrogen.
The incidence of breast cancer is one fourth as high in Asia as in the US; many studies have shown soy to be protective of breast tissue. However, soy may stimulate breast tissue in women with a history of breast cancer. The evidence in these areas is conflicting and controversial.
In contrast to herbal alternatives to HRT, soy has been thought to lower cholesterol, make blood vessels more elastic (increase vascular elasticity), and slow osteoclast activity in bones. It is also an excellent source of protein, and is lactose- and cholesterol-free.18
TABLE 2
Remedies for hot flushes and their adverse effects, interactions, and contraindications
| Adverse effects | Important drug interactions | Contraindications | |
|---|---|---|---|
| Soy | Bloating, flatulence | Thyroid hormone, if taken simultaneously | Use with caution in patients being treated for hypothyroidism (soy may bind thyroid medication, thus lowering absorption; patients on thyroid hormone should use soy only in supplement form or take at a time distant from thyroid medication) |
| Black cohosh | Abdominal pain, nausea, headaches, dizziness, trembling limbs | Iron therapy medications | Patients with iron deficiencies; use cautiously in patients with breast cancer or high risk of breast cancer |
| Dong quai | Photodermatitis, rash | Warfarin (effects potentiated) | Patients with coagulopathies or very heavy menstruation and acute viral infections such as colds or influenza; pregnancy |
| Evening primrose oil | Nausea, softening of stools, headaches, seizures | Anticonvulsant and tricyclic antidepressants | Patients taking anticonvulsants and tricyclic antidepressants (lowers efficacy) |
| Red clover | Blood thinning | Anticoagulants such as coumadin, heparin, clopidrogel, pentoxifylline, or aspirin | Patients with coagulopathies |
Black cohosh
A member of the buttercup family, black cohosh (Actaea [formerly Cimicifuga] racemosa) has a long history in folk medicine, especially among Native Americans, who boiled the root in water and drank the resulting beverage to treat dysmenorrhea, labor pain, upset stomach, and arthritis. In Germany, extracts of black cohosh have been used since the 1940s.
Many substances have been identified in the rhizome, but it is uncertain what the majority of them do or, in fact, which are active ingredients.19 The effectiveness of black cohosh is based on the total amount of triterpenoid glycosides, typically standardized to 2.5%.20
Several studies, most of them in the German literature, have shown that black cohosh yields a significant improvement in hot flushes, with reductions of up to 80% reported (level of evidence [LOE]: 2b).21-23 The usual dosage is 40 drops of the extract twice daily for 6 to 8 weeks, or one to two 20-mg tablets twice daily with liquid (not to be chewed or sucked).
Side effects are uncommon, but occasional stomach pains and intestinal discomfort, dizziness, nausea, severe headaches, stiffness, and trembling limbs have been noted. Germany’s Commission E, which is similar to the US Food and Drug Administration, recommends that black cohosh not be used for more than 6 months, since no studies have been conducted for longer periods.
Black cohosh has weak estrogenic effect on the breast and should be used cautiously in patients with breast cancer or a high risk of breast cancer. A 2-month double-blind, randomized controlled trial of black cohosh in breast cancer survivors demonstrated no short-term side effects; however, therapy was only significantly beneficial in relieving sweating and ineffective in reducing flushes.24 The long-term effects are unknown. A recent systematic review suggests that the side effects of black cohosh are transient, and severe adverse event reports are unproven (Table 2).24
Dong quai
Dong quai, a common Chinese herb extracted from the Angelica sinensis root, has become popular in the US. In contrast to China, where it is sold as part of a mixture that includes several other herbs, dong quai typically is sold in the US as a single herb.
Although some women report improvements in their vasomotor symptoms, there have been very few studies on the effects of dong quai on menopause. In one 24-week study of 71 postmenopausal women, researchers could not demonstrate a significant difference between dong quai and placebo in alleviating vasomotor symptoms (LOE: 2b).25 The investigators suggested that studying the effects of dong quai alone, rather than in combination with other herbs, may have been a factor in their findings.
Two caveats: Dong quai increases photosensitivity, so women taking the herb should be cautioned that too much exposure to sunlight may result in a rash. Also it has been reported to potentiate the effects of warfarin (Table 2). It should not be used during pregnancy.26
Evening primrose
Native Americans consumed the leaves, roots, and seedpods of evening primrose (Oenothera biennis) for food, and made extracts from it to treat a variety of conditions. Today, the flowers and seeds are pressed to make oil that is high in the omega-6 fatty acid gamma-linolenic acid (known as GLA) and essential polyunsaturated fatty acids, which convert into prostaglandins. Evening primrose oil also is a good source of linoleic acid.
Although there are a number of good studies in which evening primrose oil has been used to successfully treat eczema and several other conditions with few side effects, it appears to have no benefit over placebo for hot flushes (LOE: 2b).27
Patients should be warned that mild upset stomach, indigestion, nausea, softening of stools, and mild headaches may occasionally occur. Also, evening primrose is contraindicated in women taking seizure medications or antipsychotics because it lowers the seizure threshold in patients on phenothiazines (Table 2).
Red clover
Red clover (Trifolium pratense) is a plant that contains the phytoestrogens formononetin, biochanin A, daidzein, and genistein. It was originally used by Native Americans to treat whooping cough, gout, and cancer.
Two clinical trials conducted in Australia failed to demonstrate that red clover extract was more effective than placebo in reducing vasomotor symptoms (LOE: 2b).28,29 However, one recent presentation found that women who took 40 mg of red clover per day—the recommended dosage—experienced a significant reduction in hot flushes.30
There is still little information on whether red clover will have any effect on the uterine lining or breast tissue. Because red clover contains coumarin-like substances, high dosages may cause the blood to thin (Table 2).31
- Ask your patients about complementary and alternative therapies; 21% of women say they use complementary or alternative therapies only, and another 25% say they use both conventional and alternative methods.
- Women who take 50 mg of soy isoflavones daily report a 10% to 20% absolute risk reduction (number needed to treat, 5–10) in the frequency of hot flashes. The duration of this effect is unknown.
- Black cohosh yields up to an 80% improvement in hot flashes.
- Patients should use an alternative therapy for at least 1 month and keep a symptom diary to adequately assess its effect.
Physicians may recommend alternative treatments for hot flashes with the same confidence they have in prescription drugs if they understand the expected results, risks and benefits, and interactions with other medications.
For treatment of hot flashes, an increasing number of menopausal women are choosing plantbased alternatives to hormone replacement therapy (HRT). Despite HRT’s proven efficacy in treating this plaguing symptom, many patients are fearful that HRT might lead to an increased risk of breast or uterine cancer, increase the risk of vascular disease including heart attack, or cause unpleasant side effects such as mood swings, depression, or continued menstrual periods.1
Dr. Seibel, an expert in non-hormone replacement therapy and a proponent of soy, provides his view of options in this important area. He argues that treatment with the more promising soy and black cohosh preparations is worth considering as part of a careful “N of 1” trial. Read this issue’s Clinical Inquiry, “What nonhormonal therapies are effective for postmenopausal vasomotor symptoms?” (pages 324–329), and you be the judge. —Jeffrey L. Susman, MD
One study of 2500 postmenopausal women found that 20% to 30% never fill their initial HRT prescriptions, 10% of those who use estrogen do so only intermittently, an additional 20% discontinue their therapy within 8 months, and only 15% to 20% of women will take HRT for more than a year.2
HRT is contraindicated in about 10% of postmenopausal women3 ; the Womens’ Health Initiative4 and the Heart and Estrogen/progestin Replacement Study5 (HERS) trials have suggested caution in using HRT even for those without contraindications.
With such an enormous number of women either unwilling or unable to take HRT, it is important to consider the alternatives you can offer.6,7 (See “How pervasive are alternative therapies?”) Whichever alternative treatment you and a patient select, give it at least 1 month (and preferably 3 months) to assess its effectiveness. Keeping a symptom diary will allow patients to objectively track their progress.
So-called alternative approaches to menopause are used so widely it might be more accurate to consider hormone replacement therapy as the true alternative medicine. Statistics presented at the National Institutes of Health on October 27, 2000, indicate that nearly half of all menopausal women are using complementary therapies—including vitamins, herbs, and soy products—to help treat their symptoms. Twenty-one percent of the women surveyed used complementary or alternative therapies alone, and 25% said they used both conventional and alternative methods.
Taken together, that is more than twice the 19% who said they used conventional hormone replacement therapy only. Given this enormous usage, it should come as no surprise that, in 2001, the dietary supplement industry likely exceeded $12 billion in sales in the United States alone.4 For many women, the decision to use an alternative is not so much dissatisfaction with conventional treatment, but that they regard the complementary agents as more congruent with their own values, beliefs, and philosophical orientations toward health and life.5
Soy
Much of the excitement about the health benefits of soy—a staple of the Asian diet for 5000 years—stems from epidemiological studies. The Asian diet, which is rich in isoflavones, is associated with a reduced risk of breast cancer, heart disease, and osteoporosis. Asian women also report fewer hot flashes than do their Western counterparts.8 One study showed that women in Western countries have an 80% incidence of hot flashes, while Asian women living in China have an incidence of only 20%.9
Clearly, factors other than soy also must be considered before we can make a direct cause-and-effect correlation. To that end, many studies have been conducted on the health benefits of soybeans, a rich source of the isoflavones genistein and daidzein.
Physiologic activity
Isoflavones are phytoestrogens with a hetero-cyclic phenol structure that is similar to estrogen. Their potency is between 1 x 104 and 1 x 103 the activity of 17Β-estradiol.10 Although their potency is low, their serum concentrations can reach levels several orders of magnitude higher than those of physiologic estrogens. It is generally believed that isoflavones act as a selective estrogen receptor modulator, exerting antiestrogenic effects in the high-estrogen environment of premenopause and estrogenic effects in the low-estrogen environment of post-menopause.
Clinical efficacy
Hot flushes. An increasing number of studies suggest that soy and soy isoflavones—in the form of soy flour, soy protein, and dietary supplements—may play an important role in the treatment of hot flushes. In general, the average amount of isoflavones consumed in a typical Asian diet is approximately 50 mg/day. (One gram of soy protein contains approximately 1 mg of isoflavones.)
Unfortunately, clinical trial data are confounded by varying preparations of soy, length of therapy, outcomes measured, and small sample sizes. The absolute risk reduction of soy preparations versus placebo or comparators in positive trials ranges from 10% to 20% (number needed to treat [NNT]=6–10) when frequency of hot flushes is the outcome measured (Table 1).11-15
Placebo effect. All soy studies also confirm the existence of a placebo effect on the treatment of hot flushes. The large placebo effect and varied efficacy has left many skeptics questioning the clinical efficacy of soy products.16
I believe that thinking is wrong. Women who have hot flushes tend to have lower sleep efficiencies and longer REM latencies than women who do not experience this vasomotor symptom.17 By lowering the frequency of hot flushes, soy may produce greater sleep efficiencies and improve quality of life.
TABLE 1
Positive trials* of soy in the treatment of hot flushes
| Absolute risk reduction of hot flushes (%) | ||||||
|---|---|---|---|---|---|---|
| Study | N | Design | Soy | Control | NNT | LOE† |
| Murkies 199511 | 58 | 45 g soy flour | 42 | 25 | 6 | 1b |
| Brzezinski 199712 | 73/72 | Mostly soy, 1/4 diet, some flaxseed | 54 | 35 | ||
| Albertazzi 199813 | 51/53 | 60 g soy protein | 43.6 | 31.3 | 8.1 | 2b |
| Scambia 200014 | 39 | 50 mg/d isoflavones | 45 | 25 | 1b | |
| Upmalis 200015 | 177 | 50 mg/d isoflavones | 30 | 20 | 1b | |
| From Seibel MM, The soy solution for menopause: an alternative to estrogen. New York, NY: Fireside Press, 2003. | ||||||
| *None of the above trials were of high quality—all either had a large number of dropouts, substantial divergence of outcomes, a small “n” (number of subjects) or other substantive methodological concerns. | ||||||
| NNT, number needed to treat; LOE, level of evidence | ||||||
| †See page 290 for a description of strength of recommendation. | ||||||
Safety
Soy has been safely consumed by hundreds of millions of people without complications, with t he possible exception of soy allergies (Table 2). The literature suggests it is safe and effective in dosages of either 40 g of soy protein or 50 mg of soy isoflavones per day. Soy does not stimulate the uterine lining and may be protective of the endometrium if taken with estrogen.
The incidence of breast cancer is one fourth as high in Asia as in the US; many studies have shown soy to be protective of breast tissue. However, soy may stimulate breast tissue in women with a history of breast cancer. The evidence in these areas is conflicting and controversial.
In contrast to herbal alternatives to HRT, soy has been thought to lower cholesterol, make blood vessels more elastic (increase vascular elasticity), and slow osteoclast activity in bones. It is also an excellent source of protein, and is lactose- and cholesterol-free.18
TABLE 2
Remedies for hot flushes and their adverse effects, interactions, and contraindications
| Adverse effects | Important drug interactions | Contraindications | |
|---|---|---|---|
| Soy | Bloating, flatulence | Thyroid hormone, if taken simultaneously | Use with caution in patients being treated for hypothyroidism (soy may bind thyroid medication, thus lowering absorption; patients on thyroid hormone should use soy only in supplement form or take at a time distant from thyroid medication) |
| Black cohosh | Abdominal pain, nausea, headaches, dizziness, trembling limbs | Iron therapy medications | Patients with iron deficiencies; use cautiously in patients with breast cancer or high risk of breast cancer |
| Dong quai | Photodermatitis, rash | Warfarin (effects potentiated) | Patients with coagulopathies or very heavy menstruation and acute viral infections such as colds or influenza; pregnancy |
| Evening primrose oil | Nausea, softening of stools, headaches, seizures | Anticonvulsant and tricyclic antidepressants | Patients taking anticonvulsants and tricyclic antidepressants (lowers efficacy) |
| Red clover | Blood thinning | Anticoagulants such as coumadin, heparin, clopidrogel, pentoxifylline, or aspirin | Patients with coagulopathies |
Black cohosh
A member of the buttercup family, black cohosh (Actaea [formerly Cimicifuga] racemosa) has a long history in folk medicine, especially among Native Americans, who boiled the root in water and drank the resulting beverage to treat dysmenorrhea, labor pain, upset stomach, and arthritis. In Germany, extracts of black cohosh have been used since the 1940s.
Many substances have been identified in the rhizome, but it is uncertain what the majority of them do or, in fact, which are active ingredients.19 The effectiveness of black cohosh is based on the total amount of triterpenoid glycosides, typically standardized to 2.5%.20
Several studies, most of them in the German literature, have shown that black cohosh yields a significant improvement in hot flushes, with reductions of up to 80% reported (level of evidence [LOE]: 2b).21-23 The usual dosage is 40 drops of the extract twice daily for 6 to 8 weeks, or one to two 20-mg tablets twice daily with liquid (not to be chewed or sucked).
Side effects are uncommon, but occasional stomach pains and intestinal discomfort, dizziness, nausea, severe headaches, stiffness, and trembling limbs have been noted. Germany’s Commission E, which is similar to the US Food and Drug Administration, recommends that black cohosh not be used for more than 6 months, since no studies have been conducted for longer periods.
Black cohosh has weak estrogenic effect on the breast and should be used cautiously in patients with breast cancer or a high risk of breast cancer. A 2-month double-blind, randomized controlled trial of black cohosh in breast cancer survivors demonstrated no short-term side effects; however, therapy was only significantly beneficial in relieving sweating and ineffective in reducing flushes.24 The long-term effects are unknown. A recent systematic review suggests that the side effects of black cohosh are transient, and severe adverse event reports are unproven (Table 2).24
Dong quai
Dong quai, a common Chinese herb extracted from the Angelica sinensis root, has become popular in the US. In contrast to China, where it is sold as part of a mixture that includes several other herbs, dong quai typically is sold in the US as a single herb.
Although some women report improvements in their vasomotor symptoms, there have been very few studies on the effects of dong quai on menopause. In one 24-week study of 71 postmenopausal women, researchers could not demonstrate a significant difference between dong quai and placebo in alleviating vasomotor symptoms (LOE: 2b).25 The investigators suggested that studying the effects of dong quai alone, rather than in combination with other herbs, may have been a factor in their findings.
Two caveats: Dong quai increases photosensitivity, so women taking the herb should be cautioned that too much exposure to sunlight may result in a rash. Also it has been reported to potentiate the effects of warfarin (Table 2). It should not be used during pregnancy.26
Evening primrose
Native Americans consumed the leaves, roots, and seedpods of evening primrose (Oenothera biennis) for food, and made extracts from it to treat a variety of conditions. Today, the flowers and seeds are pressed to make oil that is high in the omega-6 fatty acid gamma-linolenic acid (known as GLA) and essential polyunsaturated fatty acids, which convert into prostaglandins. Evening primrose oil also is a good source of linoleic acid.
Although there are a number of good studies in which evening primrose oil has been used to successfully treat eczema and several other conditions with few side effects, it appears to have no benefit over placebo for hot flushes (LOE: 2b).27
Patients should be warned that mild upset stomach, indigestion, nausea, softening of stools, and mild headaches may occasionally occur. Also, evening primrose is contraindicated in women taking seizure medications or antipsychotics because it lowers the seizure threshold in patients on phenothiazines (Table 2).
Red clover
Red clover (Trifolium pratense) is a plant that contains the phytoestrogens formononetin, biochanin A, daidzein, and genistein. It was originally used by Native Americans to treat whooping cough, gout, and cancer.
Two clinical trials conducted in Australia failed to demonstrate that red clover extract was more effective than placebo in reducing vasomotor symptoms (LOE: 2b).28,29 However, one recent presentation found that women who took 40 mg of red clover per day—the recommended dosage—experienced a significant reduction in hot flushes.30
There is still little information on whether red clover will have any effect on the uterine lining or breast tissue. Because red clover contains coumarin-like substances, high dosages may cause the blood to thin (Table 2).31
1. Salamone LM, Pressman AR, Seeley DG, Cauley JA. Estrogen replacement therapy. A survey of older women’s attitudes. Arch Int Med 1996;156:1293-1297.
2. Ravnikar VA. Compliance with hormone therapy. Am J Obstet Gynecol 1987;156:1332-1334.
3. Kessel B. Alternatives to estrogen for menopausal women. Proc Soc Esp Biol Med 1998;217:38-44.
4. Roussouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-333.
5. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone replacement therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002;288:49-57.
6. Seibel MM. The role of nutrition and nutritional supplements in women’s health. Fertil Steril 1999;72:579-591.
7. Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998;279:1548-1553.
8. Haines CJ, Chung TKH, Leung DHY. A prospective study of the frequency of acute menopausal symptoms in Hog Dong Chinese women. Maturitas 1994;18:175-181.
9. Tang GWK. The climacteric of Chinese factory workers. Maturitas 1994;19:177-182.
10. Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol 1993;45:399-405.
11. Murkies AL, Lombard C, Strauss BJD, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases postmenopausal hot flushes: effect of soy and wheat. Maturitas 1995;21:189-195.
12. Brzezinski A, Adlercreutz H, Shaoul R, Rosler A, Shmueli A, Tanos V, Schenker JG. Short-term effects of phytoestrogen-rich diet on postmenopausal women. Menopause 1997;4:89-94.
13. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
14. Scambia G, Mango D, Signorile PG, et al. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause 2000;7:105-111.
15. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236-242.
16. Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med 2002;137:805-811.
17. Shaver JLF, Giblin E, Paulsen V. Sleep quality subtypes in midlife women. Sleep 1991;14:18-23.
18. From Seibel MM. The soy solution for menopause: an alternative to estrogen. New York, NY: Fireside Press, 2003.
19. Struck D, Tegtmeier M, Harnishfeger G. Flavones in extracts of Cimicifuga racemosa. Planta Med 1997;63:289-290.
20. Beuscher N. Cimicifuga racemosa L.Black Cohosh. HerbalGram 1996;19-27.
21. Vorberg G. Therapy of climacteric complaints. Zeitschrift fur Allgemeinmedizin 1984;60:626-629.
22. Warnecke G. Influence of a phytopharmaceutical on climacteric complaints. Die Meizinische Welt 1985;36:871-874.
23. Stoll W. Phytopharmacon influences atrophic vaginal epithelium: double-blind study—Cimicifuga vs. estrogenic substances. Therapeuticum 1987;1:23-31.
24. Jacobson JS, Troxel AB, Evans J, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-2745.
25. Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause 2003;10:58-64.
26. Hirata JD, Swiers LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind placebo-controlled trial. Fertil Steril 1997;68:981-986.
27. Page RL 2nd, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy 1999;19:870-876.
28. Chenoy R, Hussain S, Tayob Y, O’Brien PMS, Moss MY, Morse PF. Effect of oral gamalenic acid from evening primrose oil on menopausal flushing. BMJ 1994;308:501-503.
29. Barber RJ, Templeman C, Morton T, Delley GE, Weat L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999;2:85-92.
30. Knight DC, Howes JB, Eden JA. The effect of Promensil an isoflavone extract, on menopausal symptoms. Climacteric 1999;2:79-84.
31. Nachtigall LB, LaGrega L, Lee WW, et al. The effects of isoflavones derived from red clover on vasomotor symptoms and endometrial thickness. 9th International Menopause Society World Congress on Menopause, October 17-21, 1999.
32. Fugh-Berman A, Kronenberg F. Red clover (Trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-337.
1. Salamone LM, Pressman AR, Seeley DG, Cauley JA. Estrogen replacement therapy. A survey of older women’s attitudes. Arch Int Med 1996;156:1293-1297.
2. Ravnikar VA. Compliance with hormone therapy. Am J Obstet Gynecol 1987;156:1332-1334.
3. Kessel B. Alternatives to estrogen for menopausal women. Proc Soc Esp Biol Med 1998;217:38-44.
4. Roussouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-333.
5. Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone replacement therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002;288:49-57.
6. Seibel MM. The role of nutrition and nutritional supplements in women’s health. Fertil Steril 1999;72:579-591.
7. Astin JA. Why patients use alternative medicine: results of a national study. JAMA 1998;279:1548-1553.
8. Haines CJ, Chung TKH, Leung DHY. A prospective study of the frequency of acute menopausal symptoms in Hog Dong Chinese women. Maturitas 1994;18:175-181.
9. Tang GWK. The climacteric of Chinese factory workers. Maturitas 1994;19:177-182.
10. Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol 1993;45:399-405.
11. Murkies AL, Lombard C, Strauss BJD, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases postmenopausal hot flushes: effect of soy and wheat. Maturitas 1995;21:189-195.
12. Brzezinski A, Adlercreutz H, Shaoul R, Rosler A, Shmueli A, Tanos V, Schenker JG. Short-term effects of phytoestrogen-rich diet on postmenopausal women. Menopause 1997;4:89-94.
13. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
14. Scambia G, Mango D, Signorile PG, et al. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause 2000;7:105-111.
15. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236-242.
16. Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med 2002;137:805-811.
17. Shaver JLF, Giblin E, Paulsen V. Sleep quality subtypes in midlife women. Sleep 1991;14:18-23.
18. From Seibel MM. The soy solution for menopause: an alternative to estrogen. New York, NY: Fireside Press, 2003.
19. Struck D, Tegtmeier M, Harnishfeger G. Flavones in extracts of Cimicifuga racemosa. Planta Med 1997;63:289-290.
20. Beuscher N. Cimicifuga racemosa L.Black Cohosh. HerbalGram 1996;19-27.
21. Vorberg G. Therapy of climacteric complaints. Zeitschrift fur Allgemeinmedizin 1984;60:626-629.
22. Warnecke G. Influence of a phytopharmaceutical on climacteric complaints. Die Meizinische Welt 1985;36:871-874.
23. Stoll W. Phytopharmacon influences atrophic vaginal epithelium: double-blind study—Cimicifuga vs. estrogenic substances. Therapeuticum 1987;1:23-31.
24. Jacobson JS, Troxel AB, Evans J, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-2745.
25. Huntley A, Ernst E. A systematic review of the safety of black cohosh. Menopause 2003;10:58-64.
26. Hirata JD, Swiers LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind placebo-controlled trial. Fertil Steril 1997;68:981-986.
27. Page RL 2nd, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy 1999;19:870-876.
28. Chenoy R, Hussain S, Tayob Y, O’Brien PMS, Moss MY, Morse PF. Effect of oral gamalenic acid from evening primrose oil on menopausal flushing. BMJ 1994;308:501-503.
29. Barber RJ, Templeman C, Morton T, Delley GE, Weat L. Randomized placebo-controlled trial of an isoflavone supplement and menopausal symptoms in women. Climacteric 1999;2:85-92.
30. Knight DC, Howes JB, Eden JA. The effect of Promensil an isoflavone extract, on menopausal symptoms. Climacteric 1999;2:79-84.
31. Nachtigall LB, LaGrega L, Lee WW, et al. The effects of isoflavones derived from red clover on vasomotor symptoms and endometrial thickness. 9th International Menopause Society World Congress on Menopause, October 17-21, 1999.
32. Fugh-Berman A, Kronenberg F. Red clover (Trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-337.