User login
Youth e-cigarette use: Assessing for, and halting, the hidden habit
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23
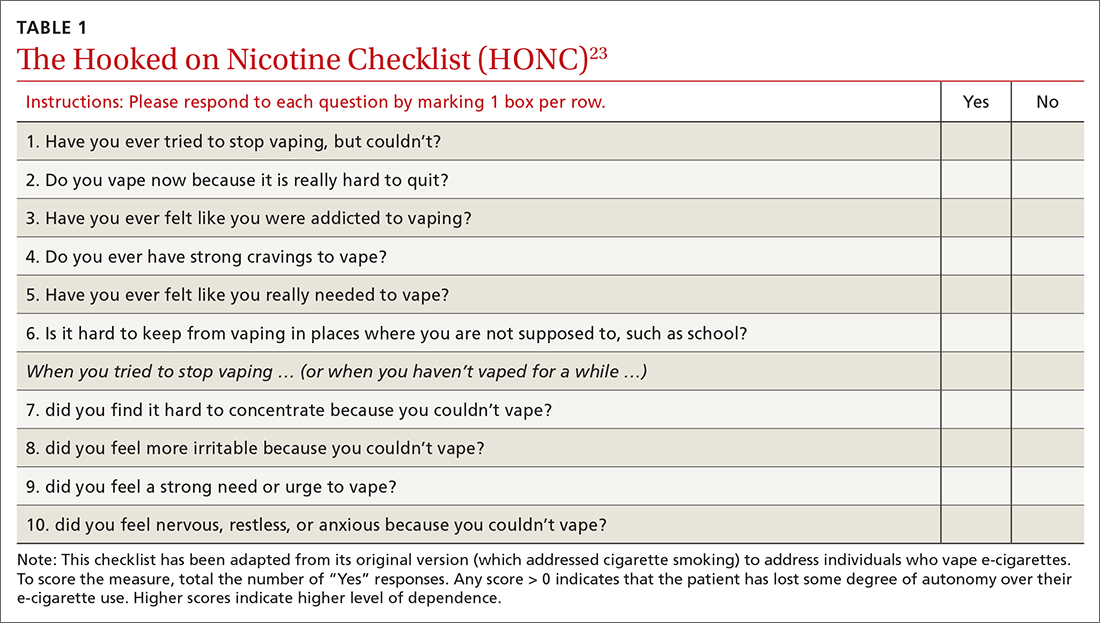
The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
Difficult patient, or something else? A review of personality disorders
Specific behaviors or expressed thoughts may signal a need for screening. Take into account an individual’s strengths and limitations when designing a Tx approach.
THE CASES
Winston S* is a 23-year-old man referred by a psychiatrist colleague for primary care. He works delivering papers in the early morning hours and spends his day alone in his apartment mainly eating frozen pizza. He has worked solitary jobs his entire life and says he prefers it that way. His answers to questions lack emotion. He doesn’t seem to have any friends or regular contact with family. He follows the medical advice he receives but can’t seem to get out of the house to exercise or socialize. His psychiatrist was treating him with a selective serotonin reuptake inhibitor for depression when he was referred.
Denise L* is a 37-year-old woman who transferred to your practice because she says the previous practice’s office manager was disrespectful and the doctor did not listen to her. She has been “very appreciative” of you and your “well-run office.” You have addressed her fibromyalgia and she has shared several personal details about her life. In the following weeks, you receive several phone calls and messages from her. At a follow-up visit, she asks questions about your family and seems agitated when you hesitate to answer. She questions whether you remember details of her history. She pushes, “Did you remember that, doctor?” She also mentions that your front desk staff seems rude to her.
Ruth B* is an 82-year-old woman whose blood pressure measured in your office is 176/94 mm Hg. When you recommend starting a medication and getting blood tests, she responds with a litany of fearful questions. She seems immobilized by worries about treatment and equally so about the risks of nontreatment. You can’t seem to get past the anxiety to decide on a satisfactory plan. She has to write everything down on a notepad and worries if she does not get every detail.
●
* This patient’s name has been changed to protect his identity. The other 2 patients are an amalgam of patients for whom the authors have provided care.
According to a survey of practicing primary care physicians, as many as 15% of patient encounters can be difficult.1 Demanding, intrusive, or angry patients who reject health care interventions are often-cited sources of these difficulties.2,3 While it is true that patient, physician, and environmental factors may contribute to challenging interactions, some patients who are “difficult” may actually have a personality disorder that requires a distinctive approach to care. Recognizing these patients can help empower physicians to provide compassionate and effective care, reduce team angst, and minimize burnout.
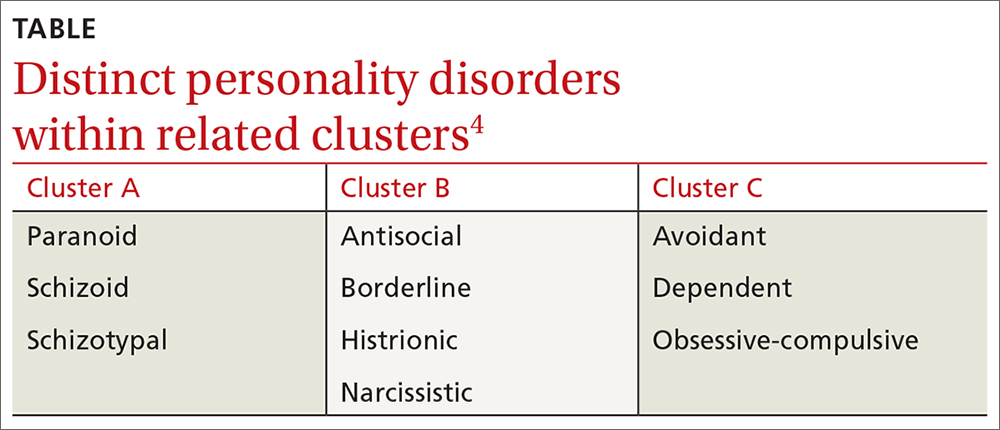
❚ What qualifies as a personality disorder? A personality disorder is an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is unchanging over time, and leads to distress or impairment in social or occupational functioning.4 The prevalence of any personality disorder seems to have increased over the past decade from 9.1%4 to 12.16%.5 The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classifies personality disorders in 3 clusters—A, B, and C (TABLE4)—with prevalence rates at 7.23%, 5.53%, and 6.7%, respectively.5 The review below will focus on the distinct personality disorders exhibited by the patients described in the opening cases.
Continue to: A closer look at the clusters...
A closer look at the clusters
Cluster A disorders
Paranoid, schizoid, and schizotypal disorders are part of this cluster. These patients exhibit odd or eccentric thinking and behavior. Individuals with schizoid personality disorder, for instance, usually lack relationships and lack the desire to acquire and maintain relationships.4 They often organize their lives to remain isolated and will choose occupations that require little social interaction. They sometimes view themselves as observers rather than participants in their own lives.6
Cluster B disorders
Dramatic, overly emotional, or unpredictable thinking and behavior are characteristic of individuals who have antisocial, borderline, histrionic, or narcissistic disorders. Patients with borderline personality disorder (BPD), for example, demonstrate a longstanding pattern of instability in affect, self-image, and relationships.4 Patients with BPD often display extreme interpersonal hypersensitivity and make frantic efforts to avoid real or imagined abandonment. Identity disturbance, feelings of emptiness, and efforts to avoid abandonment have all been associated with increased suicide risk.7
In a primary care setting, such a patient may display extremely strong reactions to minor disappointments. When the physician is unavailable for a last-minute appointment or to authorize an unscheduled medication refill or to receive an after-hours phone call, the patient may become irate. The physician, who previously was idealized by the patient as “the only person who understands me,” is now devalued as “the worst doctor I’ve ever had.”8
Cluster C disorders
With these individuals, anxious or fearful thinking and behavior predominate. Avoidant, dependent, and obsessive-compulsive disorders are included in this cluster.
Dependent personality disorder (DPD) is characterized by a pervasive and extreme need to be taken care of. Submissive and clingy behavior and fear of separation are excessive. This patient may have difficulty making everyday decisions, being assertive, or expressing disagreement with others.4
Obsessive-compulsive personality disorder falls in this cluster and is typified by a pervasive preoccupation with orderliness, perfectionism, and control, at the price of flexibility and efficiency. This individual may be reluctant to get rid of sentimental objects, have rigid moral beliefs, and have significant difficulty working with others who do not follow their rules.4
Continue to: These clues may suggest...
These clues may suggest a personality disorder
If you find that encounters with a particular patient are growing increasingly difficult, consider whether the following behaviors, attitudes, and patterns of thinking are coming into play. If they are, you may want to consider using a screening tool, which we’ll discuss in a moment.
❚ Clues to cluster A disorders
- The patient has no peer relationships outside immediate family.
- The patient almost always chooses solitary activities for work and personal enjoyment.
❚ Cluster B clues
- Hypersensitivity to treatment disagreements or cancelled appointments are common (and likely experienced as rejection).
- Mood changes occur very quickly, even during a single visit.
- There is a history of many failed relationships with providers and others.
- The patient will describe an individual as both “wonderful” and “terrible” (ie, splitting) and may do so during the course of one visit.
- The patient may also split groups (eg, medical staff) by affective extremes (eg, adoration and hatred).
- The patient may hint at suicide or acts of self-harm.7
❚ Cluster C clues
- There is an excessive dependency on family, friends, or providers.
- Significant anxiety is experienced when the patient has to make an independent decision.
- There is a fear of relationship loss and resultant vulnerability to exploitation or abuse.
- Pervasive perfectionism makes treatment planning or course changes difficult.
- Anxiety and fear are unrelieved despite support and ample information.
Consider these screening tools
Several screening tools for personality disorders can be used to follow up on your initial clinical impressions. We also highly recommend you consider concurrent screening for substance abuse, as addiction is a common comorbidity with personality disorders.
❚
❚ A sampling of screening tools. The Standardised Assessment of Personality Abbreviated Scale (SAPAS)9 is an 8-item measure that correlates well with disorders in clusters A and C.
BPD (cluster B) has many brief scale options, including the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).10 This 10-item questionnaire demonstrates sensitivity and specificity for BPD.
The International Personality Disorder Examination (IPDE) includes a 15-minute screening tool to help identify patients who may have any personality disorder, regardless of cluster.11
Improve patient encounters with these Tx pearls
In the family medicine clinic, a collaborative primary care and behavioral health team can be extremely helpful in the diagnosis and management of patients with personality disorders.12 First-line treatment of these disorders is psychotherapy, whereas medications are mainly used for symptom management. See Black and colleagues’ work for a thorough discussion on psychopharmacology considerations with personality disorders. 13
The following tips can help you to improve your interactions with patients who have personality disorders.
❚ Cluster A approaches
- Recommend treatment that respects the patient’s need for relative isolation.14
- Don’t be personally offended by your patient’s flat or disinterested affect or concrete thinking; don’t let it diminish the emotional support you provide.6
- Consult with a health psychologist (who has expertise in physical health conditions, brief treatments, and the medical system) to connect the patient with a long-term therapist. It is better to focus on fundamental changes, rather than employing brief behavioral techniques, for symptom relief. Patients with personality disorders tend to have better outcomes with long-term psychological care.15
❚ Cluster B approaches
- Set boundaries—eg, specific time limits for visits—and keep them.8
- Schedule brief, more frequent, appointments to reduce perceived feelings of abandonment.
- Coordinate plans with the entire clinic team to avoid splitting and blaming.16
- Avoid providing patients with personal information, as it may provide fodder for splitting behavior. 8
- Do not take things personally. Let patients “own” their own distress. These patients often take an emotional toll on the provider.16
- Engage the help of a health psychologist to reduce burnout and for more long-term continuity of care. A health psychologist who specializes in dialectical behavioral therapy to work on emotion regulation, distress tolerance, and interpersonal effectiveness would be ideal.17
Continue to: Cluster C approaches...
❚
❚ Cluster C approaches
- Engage the help of family and other trusted individuals in supporting treatment plans.18,19
- Try to provide just 2 treatment choices to the patient and reinforce his or her responsibility to help make the decision collaboratively. This step is important since it is difficult to enhance autonomy in these patients.20
- Engage the help of a cognitive behavioral therapist who can work on assertiveness and problem-solving skills.19
- Be empathetic with the patient and patiently build a trusting relationship, rather than “arguing” with the patient about each specific worry.20
- Make only one change at a time. Give small assignments to the patient, such as monitoring symptoms or reading up on their condition. These can help the patient feel more in control.21
- Present information in brief, clear terms. Avoid “grey areas” to reduce anxiety.21
- Engage a behavioral health provider to reduce rigid expectations and ideally increase feelings of self-esteem; this has been shown to predict better treatment outcomes.22
CASES
Mr. S displays cluster-A characteristics of schizoid personality disorder in addition to the depression he is being treated for. His physician was not put off by his flat affect and respected his limitations with social activities. Use of a stationary bike was recommended for exercise rather than walks outdoors. He also preferred phone calls to in-person encounters, so his follow-up visits were conducted by phone.
Ms. L exhibits cluster-B characteristics of BPD. You begin the tricky dance of setting limits, keeping communication clear, and not blaming yourself or others on your team for Ms. L’s feelings. You schedule regular visits with explicit time limits and discuss with your entire team how to avoid splitting. You involve a psychologist, familiar with treating BPD, who helps the patient learn positive interpersonal coping skills.
Ms. B displays cluster-C characteristics of dependent and obsessive-compulsive personality disorders. At her follow-up visit, you provide a great deal of empathy and try not to argue her out of each worry that she brings up. You make one change at a time and enlist the help of her daughter in giving her pills at home and offering reassurance. You collaborate with a cognitive behavioral therapist who works on exposing her to moderately anxiety-provoking situations/decisions.
1. Hull SK, Broquet K. How to manage difficult patient encounters. Fam Pract Manag. 2007;14:30-34.
2. Groves JE. Taking care of the hateful patient. N Engl J Med.1978;298: 883-887.
3. O’Dowd TC. Five years of heartsink patients in primary care. BMJ. 1988;297:528-530.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
5. Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. 2018;213:709-715.
6. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32:515-528.
7. Yen S, Peters JR, Nishar S, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the collaborative longitudinal study of personality disorders over 10 years of follow-up. JAMA Psychiatry. 2021;78:187-194.
8. Dubovsky AN, Kiefer MM. Borderline personality disorder in the primary care setting. Med Clin North Am. 2014;98:1049-1064.
9. Hesse M, Moran P. (2010). Screening for personality disorder with the Standardised Assessment of Personality: Abbreviated Scale (SAPAS): further evidence of concurrent validity. BMC Psychiatry. 2010;10:10.
10. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean screening instrument for borderline personality disorder (MSI-BPD). J Pers Disord. 2003;17:568-573.
11. Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215-224.12. Nelson KJ, Skodol A, Friedman M. Pharmacotherapy for personality disorders. UpToDate. Accessed April 22, 2021. www.uptodate.com/contents/pharmacotherapy-for-personality-disorders
13. Black D, Paris J, Schulz C. Evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M (ed). Cognitive Behavioral Psychopharmacology: the Clinical Practice of Evidence-Based Biopsychosocial Integration. Wiley; 2017:137-166.
14. Beck AT, Davis DD, Freeman A. Cognitive Therapy of Personality Disorders. 3rd ed. The Guilford Press; 2015.
15. Thylstrup B, Hesse M. “I am not complaining”–ambivalence construct in schizoid personality disorder. Am J Psychother. 2009;63:147-167.
16. Ricke AK, Lee MJ, Chambers JE. The difficult patient: borderline personality disorder in the obstetrical and gynecological patient. Obstet Gynecol Surv. 2012;67:495-502.
17. Seow LLY, Page AC, Hooke GR. Severity of borderline personality disorder symptoms as a moderator of the association between the use of dialectical behaviour therapy skills and treatment outcomes. Psychother Res. 2020;30:920-933.
18. Nichols WC. Integrative marital and family treatment of dependent personality disorders. In: MacFarlane MM (Ed.) Family Treatment of Personality Disorders: Advances in Clinical Practice. Haworth Clinical Practice Press; 2004:173-204.
19. Disney KL. Dependent personality disorder: a critical review. Clin Psychol Rev. 2013;33:1184-1196.
20. Bender DS. The therapeutic alliance in the treatment of personality disorders. J Psychiatr Pract. 2005;11:73-87.
21. Ward RK. Assessment and management of personality disorders. Am Fam Physician. 2004;70:1505-1512.
22. Cummings JA, Hayes AM, Cardaciotto L, et al. The dynamics of self-esteem in cognitive therapy for avoidant and obsessive-compulsive personality disorders: an adaptive role of self-esteem variability? Cognit Ther Res. 2012;36:272-281.
Specific behaviors or expressed thoughts may signal a need for screening. Take into account an individual’s strengths and limitations when designing a Tx approach.
Specific behaviors or expressed thoughts may signal a need for screening. Take into account an individual’s strengths and limitations when designing a Tx approach.
THE CASES
Winston S* is a 23-year-old man referred by a psychiatrist colleague for primary care. He works delivering papers in the early morning hours and spends his day alone in his apartment mainly eating frozen pizza. He has worked solitary jobs his entire life and says he prefers it that way. His answers to questions lack emotion. He doesn’t seem to have any friends or regular contact with family. He follows the medical advice he receives but can’t seem to get out of the house to exercise or socialize. His psychiatrist was treating him with a selective serotonin reuptake inhibitor for depression when he was referred.
Denise L* is a 37-year-old woman who transferred to your practice because she says the previous practice’s office manager was disrespectful and the doctor did not listen to her. She has been “very appreciative” of you and your “well-run office.” You have addressed her fibromyalgia and she has shared several personal details about her life. In the following weeks, you receive several phone calls and messages from her. At a follow-up visit, she asks questions about your family and seems agitated when you hesitate to answer. She questions whether you remember details of her history. She pushes, “Did you remember that, doctor?” She also mentions that your front desk staff seems rude to her.
Ruth B* is an 82-year-old woman whose blood pressure measured in your office is 176/94 mm Hg. When you recommend starting a medication and getting blood tests, she responds with a litany of fearful questions. She seems immobilized by worries about treatment and equally so about the risks of nontreatment. You can’t seem to get past the anxiety to decide on a satisfactory plan. She has to write everything down on a notepad and worries if she does not get every detail.
●
* This patient’s name has been changed to protect his identity. The other 2 patients are an amalgam of patients for whom the authors have provided care.
According to a survey of practicing primary care physicians, as many as 15% of patient encounters can be difficult.1 Demanding, intrusive, or angry patients who reject health care interventions are often-cited sources of these difficulties.2,3 While it is true that patient, physician, and environmental factors may contribute to challenging interactions, some patients who are “difficult” may actually have a personality disorder that requires a distinctive approach to care. Recognizing these patients can help empower physicians to provide compassionate and effective care, reduce team angst, and minimize burnout.
❚ What qualifies as a personality disorder? A personality disorder is an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is unchanging over time, and leads to distress or impairment in social or occupational functioning.4 The prevalence of any personality disorder seems to have increased over the past decade from 9.1%4 to 12.16%.5 The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classifies personality disorders in 3 clusters—A, B, and C (TABLE4)—with prevalence rates at 7.23%, 5.53%, and 6.7%, respectively.5 The review below will focus on the distinct personality disorders exhibited by the patients described in the opening cases.

Continue to: A closer look at the clusters...
A closer look at the clusters
Cluster A disorders
Paranoid, schizoid, and schizotypal disorders are part of this cluster. These patients exhibit odd or eccentric thinking and behavior. Individuals with schizoid personality disorder, for instance, usually lack relationships and lack the desire to acquire and maintain relationships.4 They often organize their lives to remain isolated and will choose occupations that require little social interaction. They sometimes view themselves as observers rather than participants in their own lives.6
Cluster B disorders
Dramatic, overly emotional, or unpredictable thinking and behavior are characteristic of individuals who have antisocial, borderline, histrionic, or narcissistic disorders. Patients with borderline personality disorder (BPD), for example, demonstrate a longstanding pattern of instability in affect, self-image, and relationships.4 Patients with BPD often display extreme interpersonal hypersensitivity and make frantic efforts to avoid real or imagined abandonment. Identity disturbance, feelings of emptiness, and efforts to avoid abandonment have all been associated with increased suicide risk.7
In a primary care setting, such a patient may display extremely strong reactions to minor disappointments. When the physician is unavailable for a last-minute appointment or to authorize an unscheduled medication refill or to receive an after-hours phone call, the patient may become irate. The physician, who previously was idealized by the patient as “the only person who understands me,” is now devalued as “the worst doctor I’ve ever had.”8
Cluster C disorders
With these individuals, anxious or fearful thinking and behavior predominate. Avoidant, dependent, and obsessive-compulsive disorders are included in this cluster.
Dependent personality disorder (DPD) is characterized by a pervasive and extreme need to be taken care of. Submissive and clingy behavior and fear of separation are excessive. This patient may have difficulty making everyday decisions, being assertive, or expressing disagreement with others.4
Obsessive-compulsive personality disorder falls in this cluster and is typified by a pervasive preoccupation with orderliness, perfectionism, and control, at the price of flexibility and efficiency. This individual may be reluctant to get rid of sentimental objects, have rigid moral beliefs, and have significant difficulty working with others who do not follow their rules.4
Continue to: These clues may suggest...
These clues may suggest a personality disorder
If you find that encounters with a particular patient are growing increasingly difficult, consider whether the following behaviors, attitudes, and patterns of thinking are coming into play. If they are, you may want to consider using a screening tool, which we’ll discuss in a moment.
❚ Clues to cluster A disorders
- The patient has no peer relationships outside immediate family.
- The patient almost always chooses solitary activities for work and personal enjoyment.
❚ Cluster B clues
- Hypersensitivity to treatment disagreements or cancelled appointments are common (and likely experienced as rejection).
- Mood changes occur very quickly, even during a single visit.
- There is a history of many failed relationships with providers and others.
- The patient will describe an individual as both “wonderful” and “terrible” (ie, splitting) and may do so during the course of one visit.
- The patient may also split groups (eg, medical staff) by affective extremes (eg, adoration and hatred).
- The patient may hint at suicide or acts of self-harm.7
❚ Cluster C clues
- There is an excessive dependency on family, friends, or providers.
- Significant anxiety is experienced when the patient has to make an independent decision.
- There is a fear of relationship loss and resultant vulnerability to exploitation or abuse.
- Pervasive perfectionism makes treatment planning or course changes difficult.
- Anxiety and fear are unrelieved despite support and ample information.
Consider these screening tools
Several screening tools for personality disorders can be used to follow up on your initial clinical impressions. We also highly recommend you consider concurrent screening for substance abuse, as addiction is a common comorbidity with personality disorders.
❚
❚ A sampling of screening tools. The Standardised Assessment of Personality Abbreviated Scale (SAPAS)9 is an 8-item measure that correlates well with disorders in clusters A and C.
BPD (cluster B) has many brief scale options, including the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).10 This 10-item questionnaire demonstrates sensitivity and specificity for BPD.
The International Personality Disorder Examination (IPDE) includes a 15-minute screening tool to help identify patients who may have any personality disorder, regardless of cluster.11
Improve patient encounters with these Tx pearls
In the family medicine clinic, a collaborative primary care and behavioral health team can be extremely helpful in the diagnosis and management of patients with personality disorders.12 First-line treatment of these disorders is psychotherapy, whereas medications are mainly used for symptom management. See Black and colleagues’ work for a thorough discussion on psychopharmacology considerations with personality disorders. 13
The following tips can help you to improve your interactions with patients who have personality disorders.
❚ Cluster A approaches
- Recommend treatment that respects the patient’s need for relative isolation.14
- Don’t be personally offended by your patient’s flat or disinterested affect or concrete thinking; don’t let it diminish the emotional support you provide.6
- Consult with a health psychologist (who has expertise in physical health conditions, brief treatments, and the medical system) to connect the patient with a long-term therapist. It is better to focus on fundamental changes, rather than employing brief behavioral techniques, for symptom relief. Patients with personality disorders tend to have better outcomes with long-term psychological care.15
❚ Cluster B approaches
- Set boundaries—eg, specific time limits for visits—and keep them.8
- Schedule brief, more frequent, appointments to reduce perceived feelings of abandonment.
- Coordinate plans with the entire clinic team to avoid splitting and blaming.16
- Avoid providing patients with personal information, as it may provide fodder for splitting behavior. 8
- Do not take things personally. Let patients “own” their own distress. These patients often take an emotional toll on the provider.16
- Engage the help of a health psychologist to reduce burnout and for more long-term continuity of care. A health psychologist who specializes in dialectical behavioral therapy to work on emotion regulation, distress tolerance, and interpersonal effectiveness would be ideal.17
Continue to: Cluster C approaches...
❚
❚ Cluster C approaches
- Engage the help of family and other trusted individuals in supporting treatment plans.18,19
- Try to provide just 2 treatment choices to the patient and reinforce his or her responsibility to help make the decision collaboratively. This step is important since it is difficult to enhance autonomy in these patients.20
- Engage the help of a cognitive behavioral therapist who can work on assertiveness and problem-solving skills.19
- Be empathetic with the patient and patiently build a trusting relationship, rather than “arguing” with the patient about each specific worry.20
- Make only one change at a time. Give small assignments to the patient, such as monitoring symptoms or reading up on their condition. These can help the patient feel more in control.21
- Present information in brief, clear terms. Avoid “grey areas” to reduce anxiety.21
- Engage a behavioral health provider to reduce rigid expectations and ideally increase feelings of self-esteem; this has been shown to predict better treatment outcomes.22
CASES
Mr. S displays cluster-A characteristics of schizoid personality disorder in addition to the depression he is being treated for. His physician was not put off by his flat affect and respected his limitations with social activities. Use of a stationary bike was recommended for exercise rather than walks outdoors. He also preferred phone calls to in-person encounters, so his follow-up visits were conducted by phone.
Ms. L exhibits cluster-B characteristics of BPD. You begin the tricky dance of setting limits, keeping communication clear, and not blaming yourself or others on your team for Ms. L’s feelings. You schedule regular visits with explicit time limits and discuss with your entire team how to avoid splitting. You involve a psychologist, familiar with treating BPD, who helps the patient learn positive interpersonal coping skills.
Ms. B displays cluster-C characteristics of dependent and obsessive-compulsive personality disorders. At her follow-up visit, you provide a great deal of empathy and try not to argue her out of each worry that she brings up. You make one change at a time and enlist the help of her daughter in giving her pills at home and offering reassurance. You collaborate with a cognitive behavioral therapist who works on exposing her to moderately anxiety-provoking situations/decisions.
THE CASES
Winston S* is a 23-year-old man referred by a psychiatrist colleague for primary care. He works delivering papers in the early morning hours and spends his day alone in his apartment mainly eating frozen pizza. He has worked solitary jobs his entire life and says he prefers it that way. His answers to questions lack emotion. He doesn’t seem to have any friends or regular contact with family. He follows the medical advice he receives but can’t seem to get out of the house to exercise or socialize. His psychiatrist was treating him with a selective serotonin reuptake inhibitor for depression when he was referred.
Denise L* is a 37-year-old woman who transferred to your practice because she says the previous practice’s office manager was disrespectful and the doctor did not listen to her. She has been “very appreciative” of you and your “well-run office.” You have addressed her fibromyalgia and she has shared several personal details about her life. In the following weeks, you receive several phone calls and messages from her. At a follow-up visit, she asks questions about your family and seems agitated when you hesitate to answer. She questions whether you remember details of her history. She pushes, “Did you remember that, doctor?” She also mentions that your front desk staff seems rude to her.
Ruth B* is an 82-year-old woman whose blood pressure measured in your office is 176/94 mm Hg. When you recommend starting a medication and getting blood tests, she responds with a litany of fearful questions. She seems immobilized by worries about treatment and equally so about the risks of nontreatment. You can’t seem to get past the anxiety to decide on a satisfactory plan. She has to write everything down on a notepad and worries if she does not get every detail.
●
* This patient’s name has been changed to protect his identity. The other 2 patients are an amalgam of patients for whom the authors have provided care.
According to a survey of practicing primary care physicians, as many as 15% of patient encounters can be difficult.1 Demanding, intrusive, or angry patients who reject health care interventions are often-cited sources of these difficulties.2,3 While it is true that patient, physician, and environmental factors may contribute to challenging interactions, some patients who are “difficult” may actually have a personality disorder that requires a distinctive approach to care. Recognizing these patients can help empower physicians to provide compassionate and effective care, reduce team angst, and minimize burnout.
❚ What qualifies as a personality disorder? A personality disorder is an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is unchanging over time, and leads to distress or impairment in social or occupational functioning.4 The prevalence of any personality disorder seems to have increased over the past decade from 9.1%4 to 12.16%.5 The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) classifies personality disorders in 3 clusters—A, B, and C (TABLE4)—with prevalence rates at 7.23%, 5.53%, and 6.7%, respectively.5 The review below will focus on the distinct personality disorders exhibited by the patients described in the opening cases.

Continue to: A closer look at the clusters...
A closer look at the clusters
Cluster A disorders
Paranoid, schizoid, and schizotypal disorders are part of this cluster. These patients exhibit odd or eccentric thinking and behavior. Individuals with schizoid personality disorder, for instance, usually lack relationships and lack the desire to acquire and maintain relationships.4 They often organize their lives to remain isolated and will choose occupations that require little social interaction. They sometimes view themselves as observers rather than participants in their own lives.6
Cluster B disorders
Dramatic, overly emotional, or unpredictable thinking and behavior are characteristic of individuals who have antisocial, borderline, histrionic, or narcissistic disorders. Patients with borderline personality disorder (BPD), for example, demonstrate a longstanding pattern of instability in affect, self-image, and relationships.4 Patients with BPD often display extreme interpersonal hypersensitivity and make frantic efforts to avoid real or imagined abandonment. Identity disturbance, feelings of emptiness, and efforts to avoid abandonment have all been associated with increased suicide risk.7
In a primary care setting, such a patient may display extremely strong reactions to minor disappointments. When the physician is unavailable for a last-minute appointment or to authorize an unscheduled medication refill or to receive an after-hours phone call, the patient may become irate. The physician, who previously was idealized by the patient as “the only person who understands me,” is now devalued as “the worst doctor I’ve ever had.”8
Cluster C disorders
With these individuals, anxious or fearful thinking and behavior predominate. Avoidant, dependent, and obsessive-compulsive disorders are included in this cluster.
Dependent personality disorder (DPD) is characterized by a pervasive and extreme need to be taken care of. Submissive and clingy behavior and fear of separation are excessive. This patient may have difficulty making everyday decisions, being assertive, or expressing disagreement with others.4
Obsessive-compulsive personality disorder falls in this cluster and is typified by a pervasive preoccupation with orderliness, perfectionism, and control, at the price of flexibility and efficiency. This individual may be reluctant to get rid of sentimental objects, have rigid moral beliefs, and have significant difficulty working with others who do not follow their rules.4
Continue to: These clues may suggest...
These clues may suggest a personality disorder
If you find that encounters with a particular patient are growing increasingly difficult, consider whether the following behaviors, attitudes, and patterns of thinking are coming into play. If they are, you may want to consider using a screening tool, which we’ll discuss in a moment.
❚ Clues to cluster A disorders
- The patient has no peer relationships outside immediate family.
- The patient almost always chooses solitary activities for work and personal enjoyment.
❚ Cluster B clues
- Hypersensitivity to treatment disagreements or cancelled appointments are common (and likely experienced as rejection).
- Mood changes occur very quickly, even during a single visit.
- There is a history of many failed relationships with providers and others.
- The patient will describe an individual as both “wonderful” and “terrible” (ie, splitting) and may do so during the course of one visit.
- The patient may also split groups (eg, medical staff) by affective extremes (eg, adoration and hatred).
- The patient may hint at suicide or acts of self-harm.7
❚ Cluster C clues
- There is an excessive dependency on family, friends, or providers.
- Significant anxiety is experienced when the patient has to make an independent decision.
- There is a fear of relationship loss and resultant vulnerability to exploitation or abuse.
- Pervasive perfectionism makes treatment planning or course changes difficult.
- Anxiety and fear are unrelieved despite support and ample information.
Consider these screening tools
Several screening tools for personality disorders can be used to follow up on your initial clinical impressions. We also highly recommend you consider concurrent screening for substance abuse, as addiction is a common comorbidity with personality disorders.
❚
❚ A sampling of screening tools. The Standardised Assessment of Personality Abbreviated Scale (SAPAS)9 is an 8-item measure that correlates well with disorders in clusters A and C.
BPD (cluster B) has many brief scale options, including the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD).10 This 10-item questionnaire demonstrates sensitivity and specificity for BPD.
The International Personality Disorder Examination (IPDE) includes a 15-minute screening tool to help identify patients who may have any personality disorder, regardless of cluster.11
Improve patient encounters with these Tx pearls
In the family medicine clinic, a collaborative primary care and behavioral health team can be extremely helpful in the diagnosis and management of patients with personality disorders.12 First-line treatment of these disorders is psychotherapy, whereas medications are mainly used for symptom management. See Black and colleagues’ work for a thorough discussion on psychopharmacology considerations with personality disorders. 13
The following tips can help you to improve your interactions with patients who have personality disorders.
❚ Cluster A approaches
- Recommend treatment that respects the patient’s need for relative isolation.14
- Don’t be personally offended by your patient’s flat or disinterested affect or concrete thinking; don’t let it diminish the emotional support you provide.6
- Consult with a health psychologist (who has expertise in physical health conditions, brief treatments, and the medical system) to connect the patient with a long-term therapist. It is better to focus on fundamental changes, rather than employing brief behavioral techniques, for symptom relief. Patients with personality disorders tend to have better outcomes with long-term psychological care.15
❚ Cluster B approaches
- Set boundaries—eg, specific time limits for visits—and keep them.8
- Schedule brief, more frequent, appointments to reduce perceived feelings of abandonment.
- Coordinate plans with the entire clinic team to avoid splitting and blaming.16
- Avoid providing patients with personal information, as it may provide fodder for splitting behavior. 8
- Do not take things personally. Let patients “own” their own distress. These patients often take an emotional toll on the provider.16
- Engage the help of a health psychologist to reduce burnout and for more long-term continuity of care. A health psychologist who specializes in dialectical behavioral therapy to work on emotion regulation, distress tolerance, and interpersonal effectiveness would be ideal.17
Continue to: Cluster C approaches...
❚
❚ Cluster C approaches
- Engage the help of family and other trusted individuals in supporting treatment plans.18,19
- Try to provide just 2 treatment choices to the patient and reinforce his or her responsibility to help make the decision collaboratively. This step is important since it is difficult to enhance autonomy in these patients.20
- Engage the help of a cognitive behavioral therapist who can work on assertiveness and problem-solving skills.19
- Be empathetic with the patient and patiently build a trusting relationship, rather than “arguing” with the patient about each specific worry.20
- Make only one change at a time. Give small assignments to the patient, such as monitoring symptoms or reading up on their condition. These can help the patient feel more in control.21
- Present information in brief, clear terms. Avoid “grey areas” to reduce anxiety.21
- Engage a behavioral health provider to reduce rigid expectations and ideally increase feelings of self-esteem; this has been shown to predict better treatment outcomes.22
CASES
Mr. S displays cluster-A characteristics of schizoid personality disorder in addition to the depression he is being treated for. His physician was not put off by his flat affect and respected his limitations with social activities. Use of a stationary bike was recommended for exercise rather than walks outdoors. He also preferred phone calls to in-person encounters, so his follow-up visits were conducted by phone.
Ms. L exhibits cluster-B characteristics of BPD. You begin the tricky dance of setting limits, keeping communication clear, and not blaming yourself or others on your team for Ms. L’s feelings. You schedule regular visits with explicit time limits and discuss with your entire team how to avoid splitting. You involve a psychologist, familiar with treating BPD, who helps the patient learn positive interpersonal coping skills.
Ms. B displays cluster-C characteristics of dependent and obsessive-compulsive personality disorders. At her follow-up visit, you provide a great deal of empathy and try not to argue her out of each worry that she brings up. You make one change at a time and enlist the help of her daughter in giving her pills at home and offering reassurance. You collaborate with a cognitive behavioral therapist who works on exposing her to moderately anxiety-provoking situations/decisions.
1. Hull SK, Broquet K. How to manage difficult patient encounters. Fam Pract Manag. 2007;14:30-34.
2. Groves JE. Taking care of the hateful patient. N Engl J Med.1978;298: 883-887.
3. O’Dowd TC. Five years of heartsink patients in primary care. BMJ. 1988;297:528-530.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
5. Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. 2018;213:709-715.
6. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32:515-528.
7. Yen S, Peters JR, Nishar S, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the collaborative longitudinal study of personality disorders over 10 years of follow-up. JAMA Psychiatry. 2021;78:187-194.
8. Dubovsky AN, Kiefer MM. Borderline personality disorder in the primary care setting. Med Clin North Am. 2014;98:1049-1064.
9. Hesse M, Moran P. (2010). Screening for personality disorder with the Standardised Assessment of Personality: Abbreviated Scale (SAPAS): further evidence of concurrent validity. BMC Psychiatry. 2010;10:10.
10. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean screening instrument for borderline personality disorder (MSI-BPD). J Pers Disord. 2003;17:568-573.
11. Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215-224.12. Nelson KJ, Skodol A, Friedman M. Pharmacotherapy for personality disorders. UpToDate. Accessed April 22, 2021. www.uptodate.com/contents/pharmacotherapy-for-personality-disorders
13. Black D, Paris J, Schulz C. Evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M (ed). Cognitive Behavioral Psychopharmacology: the Clinical Practice of Evidence-Based Biopsychosocial Integration. Wiley; 2017:137-166.
14. Beck AT, Davis DD, Freeman A. Cognitive Therapy of Personality Disorders. 3rd ed. The Guilford Press; 2015.
15. Thylstrup B, Hesse M. “I am not complaining”–ambivalence construct in schizoid personality disorder. Am J Psychother. 2009;63:147-167.
16. Ricke AK, Lee MJ, Chambers JE. The difficult patient: borderline personality disorder in the obstetrical and gynecological patient. Obstet Gynecol Surv. 2012;67:495-502.
17. Seow LLY, Page AC, Hooke GR. Severity of borderline personality disorder symptoms as a moderator of the association between the use of dialectical behaviour therapy skills and treatment outcomes. Psychother Res. 2020;30:920-933.
18. Nichols WC. Integrative marital and family treatment of dependent personality disorders. In: MacFarlane MM (Ed.) Family Treatment of Personality Disorders: Advances in Clinical Practice. Haworth Clinical Practice Press; 2004:173-204.
19. Disney KL. Dependent personality disorder: a critical review. Clin Psychol Rev. 2013;33:1184-1196.
20. Bender DS. The therapeutic alliance in the treatment of personality disorders. J Psychiatr Pract. 2005;11:73-87.
21. Ward RK. Assessment and management of personality disorders. Am Fam Physician. 2004;70:1505-1512.
22. Cummings JA, Hayes AM, Cardaciotto L, et al. The dynamics of self-esteem in cognitive therapy for avoidant and obsessive-compulsive personality disorders: an adaptive role of self-esteem variability? Cognit Ther Res. 2012;36:272-281.
1. Hull SK, Broquet K. How to manage difficult patient encounters. Fam Pract Manag. 2007;14:30-34.
2. Groves JE. Taking care of the hateful patient. N Engl J Med.1978;298: 883-887.
3. O’Dowd TC. Five years of heartsink patients in primary care. BMJ. 1988;297:528-530.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
5. Volkert J, Gablonski TC, Rabung S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. 2018;213:709-715.
6. Esterberg ML, Goulding SM, Walker EF. Cluster A personality disorders: schizotypal, schizoid and paranoid personality disorders in childhood and adolescence. J Psychopathol Behav Assess. 2010;32:515-528.
7. Yen S, Peters JR, Nishar S, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the collaborative longitudinal study of personality disorders over 10 years of follow-up. JAMA Psychiatry. 2021;78:187-194.
8. Dubovsky AN, Kiefer MM. Borderline personality disorder in the primary care setting. Med Clin North Am. 2014;98:1049-1064.
9. Hesse M, Moran P. (2010). Screening for personality disorder with the Standardised Assessment of Personality: Abbreviated Scale (SAPAS): further evidence of concurrent validity. BMC Psychiatry. 2010;10:10.
10. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean screening instrument for borderline personality disorder (MSI-BPD). J Pers Disord. 2003;17:568-573.
11. Loranger AW, Sartorius N, Andreoli A, et al. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatry. 1994;51:215-224.12. Nelson KJ, Skodol A, Friedman M. Pharmacotherapy for personality disorders. UpToDate. Accessed April 22, 2021. www.uptodate.com/contents/pharmacotherapy-for-personality-disorders
13. Black D, Paris J, Schulz C. Evidence-based integrated biopsychosocial treatment of borderline personality disorder. In: Muse M (ed). Cognitive Behavioral Psychopharmacology: the Clinical Practice of Evidence-Based Biopsychosocial Integration. Wiley; 2017:137-166.
14. Beck AT, Davis DD, Freeman A. Cognitive Therapy of Personality Disorders. 3rd ed. The Guilford Press; 2015.
15. Thylstrup B, Hesse M. “I am not complaining”–ambivalence construct in schizoid personality disorder. Am J Psychother. 2009;63:147-167.
16. Ricke AK, Lee MJ, Chambers JE. The difficult patient: borderline personality disorder in the obstetrical and gynecological patient. Obstet Gynecol Surv. 2012;67:495-502.
17. Seow LLY, Page AC, Hooke GR. Severity of borderline personality disorder symptoms as a moderator of the association between the use of dialectical behaviour therapy skills and treatment outcomes. Psychother Res. 2020;30:920-933.
18. Nichols WC. Integrative marital and family treatment of dependent personality disorders. In: MacFarlane MM (Ed.) Family Treatment of Personality Disorders: Advances in Clinical Practice. Haworth Clinical Practice Press; 2004:173-204.
19. Disney KL. Dependent personality disorder: a critical review. Clin Psychol Rev. 2013;33:1184-1196.
20. Bender DS. The therapeutic alliance in the treatment of personality disorders. J Psychiatr Pract. 2005;11:73-87.
21. Ward RK. Assessment and management of personality disorders. Am Fam Physician. 2004;70:1505-1512.
22. Cummings JA, Hayes AM, Cardaciotto L, et al. The dynamics of self-esteem in cognitive therapy for avoidant and obsessive-compulsive personality disorders: an adaptive role of self-esteem variability? Cognit Ther Res. 2012;36:272-281.
Integrating primary care into a community mental health center
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
THE CASE
John C* is a 57-year-old man with hypertension, hyperlipidemia, and schizophrenia who followed up with a psychiatrist monthly at the community mental health center (CMHC). He had no primary care doctor. His psychiatrist referred him to our new Integrated Behavioral Health (IBH) clinic, also located in the CMHC, to see a family physician for complaints of urinary frequency, blurred vision, thirst, and weight loss. An on-site fingerstick revealed his blood glucose to be 357 mg/dL. Given the presumptive diagnosis of diabetes, we checked his bloodwork, prescribed metformin, and referred him for diabetes education. That evening, his lab results showed a hemoglobin A1C > 17%, a basic metabolic panel with an anion gap, ketones in the urine, and a low C-peptide level. We were unable to reach Mr. C by phone for further management.
● How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
Coordination of behavioral health and primary care can take many forms, from simple synchronized care via referral, to co-located services, to fully integrated care.1 Reverse integration, the subject of this article, is the provision of primary care in mental health or substance use disorder treatment settings. Published evidence to date regarding this model is minimal. This article describes our experience in developing a model of reverse integration in which family physicians and nurse practitioners are embedded in a CMHC with psychiatric providers, counselors, and social workers to jointly address physical and behavioral health care issues and address social determinants of health.
The rationale for reverse integration
Many individuals with serious mental illness (SMI), including schizophrenia and bipolar disorder, have rates of comorbid chronic physical health conditions that are higher than in the general population. These conditions include obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, HIV, viral hepatitis, and tuberculosis.2 Outcomes in the SMI group are also considerably worse than in the general population. People with SMI have a demonstrated loss of up to 32 years of potential life per patient compared with the general-population average, primarily due to poor physical health.2 Maladaptive health behaviors such as poor diet, lack of physical activity, tobacco use, and substance use contribute to this increased mortality.2,3 Social determinants of poor health are more prevalent among individuals with SMI, and a relative inability to collaborate in one’s own health care due to psychiatric symptoms further exacerbates the challenges.
Many individuals with SMI receive psychiatric care, case management, counseling, and psychosocial services in CMHCs. Their psychiatric caregiver may be their only regular health care provider. Family physicians—who receive residency training in behavioral health and social determinants of health in community settings—are distinctively capable of improving overall health care outcomes of patients with SMI.
THE ADVANTAGES OF A REVERSE-INTEGRATION PRACTICE MODEL
Delivering primary care in a CMHC with a behavioral health team can benefit patients with SMI and be a satisfying practice for family physicians. Specifically, family physicians
- find that caring for complex patients can be less stressful because they benefit from the knowledge and resources of the CMHC team. The CMHC team offers case management, counseling, employment services, and housing assistance, so the primary care provider and patient are well supported.
- see fewer patients per hour due to higher visit complexity (and coding). In our experience, team-based care and additional time with patients make complex patient care more enjoyable and less frustrating.
- benefit from a situation in which patients feel safe because the CMHC support staff knows them well.
Continue to: Other benefits
Other benefits. When primary care is delivered in a CMHC, there are “huddles” and warm handoffs that allow for bidirectional collaboration and care coordination between the primary care and behavioral health teams in real time. In addition, family medicine residents, medical students, and other learners can be successfully included in an IBH clinic for patients with SMI. The behavioral health team provides the mentorship, education, and modelling of skills needed to work with this population, including limit-setting, empathy, patience, and motivational interviewing.
For their part, learners self-report increased comfort and interest in working with underserved populations and improved awareness of the social determinants of health after these experiences.4,5 Many patients at CMHCs are comfortable working with learners if continuity is maintained with a primary care provider.
Challenges we’ve faced, tips we can offer
For primary care providers, the unique workplace culture, terminology, and patient population encountered in a CMHC can be challenging. Also challenging can be the combining of things such as electronic medical records (EMRs).
Culture. The CMHC model focuses on team-based care spearheaded by case managers, in contrast to the traditional family medicine model wherein the physician coordinates services. Case managers provide assessments of client stability and readiness to be seen. They also attend primary care visits to support patient interactions, provide important psychosocial information, and assess adherence to care.
Terminology. It’s not always easy to shift to different terminology in this culture. Thus, orientation needs to address things such as the use of the word “patient,” rather than “client,” when charting.
Continue to: The complexities of the patient population
The complexities of the patient population. Many patients treated at a CMHC have a history of trauma, anxiety, and paranoia, requiring adjustments to exam practices such as using smaller speculums, providing more physical space, and offering to leave examination room doors open while patients are waiting.
In addition, individuals with SMI often have multiple health conditions, but they may become uncomfortable with physical closeness, grow tired of conversation, or feel overwhelmed when asked to complete multiple tasks in 1 visit. As a result, visits may need to be shorter and more frequent.
It’s also worth noting that, in our experience, CMHC patients may have a higher no-show rate than typical primary care clinics, requiring flexibility in scheduling. To fill vacant primary care time slots, our front desk staff uses strategies such as waiting lists and offering walk-in visits to patients who are on site for other services.
Ideally, IBH clinics use a single, fully integrated EMR, but this is not always possible. If the primary care and CMHC EMR systems do not connect, then record review and repeat documentation is needed, while care is taken to adhere to the confidentiality standards of a particular state.
Standards of care and state policies. Written standards of care, procedures, and accreditation in CMHCs rarely include provisions for common primary care practice, such as vaccines, in-clinic medications, and implements for simple procedures. To provide these services in our clinic, we ordered/stocked the needed supplies and instituted protocols that mirrored our other outpatient family medicine clinical sites.
Continue to: Some states may have...
Some states may have policies that prevent reimbursement for mental health and primary care services billed on the same day. Seeing a family physician and a psychiatry provider on the same day is convenient for patients and allows for collaboration between providers. But reimbursement rules can vary by state, so starting an IBH clinic like this requires research into local billing regulations.
WANT TO START AN INTEGRATED BEHAVIORAL HEALTH CLINIC?
Detailed instruction on starting a primary care clinic in a CMHC is beyond the scope of this article. However, the Substance Abuse and Mental Health Services Administration provides guidance on integrating primary care services into a local CMHC.6 Start by performing a baseline needs assessment of the CMHC and its patients to help guide clinic design. Leadership buy-in is key.
Leadership must provide adequate time and financial and technological support. This includes identifying appropriate space for primary care, offering training on using the EMR, and obtaining support from Finance to develop a realistic and competent business plan with an appropriate budgetary runway for start-up. (This may include securing grants in the beginning.)
We recommend starting small and expanding slowly. Once the clinic is operational, formal pathways for good communication are necessary. This includes holding regular team meetings to develop and revise clinic workflows—eg, patient enrollment, protocols, and administrative procedures such as managing medications and vaccinations—as well as addressing space, staffing, and training issues that arise. The IBH transitional leadership structure must include clinicians from both primary care and behavioral health, support staff, and the administration. Finally, you need the right staff—people who are passionate, flexible, and interested in trying something new.
THE CASE
The next day, an outreach was made to the CMHC nurse, who had the case manager go to Mr. C’s house and bring him to the CMHC for education on insulin injection, glucometer use, and diabetes nutrition. Mr. C was prescribed long-acting insulin at bedtime; his metformin was stopped and he was monitored closely.
Continue to: Mr. C now calls...
Mr. C now calls the CMHC nurse every few weeks to report his blood sugar levels, have his insulin dose adjusted, or just say “hello.” He continues to see his psychiatrist every month and his family physician every 4 months. The team collaborates as issues arise. His diabetes has been well controlled for more than 3 years.
The IBH clinic has grown in number of patients and family medicine providers, is self-sustaining, and has expanded services to include hepatitis C treatment.
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
1. Rajesh R, Tampi R, Balachandran S. The case for behavioral health integration into primary care. J Fam Pract. 2019;68:278-284.
2. Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. 2006. Accessed March 24, 2021. www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
3. Dickerson F, Stallings, CR, Origoni AE, et al. Cigarette Smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44-50.
4. Raddock M, Antenucci C, Chrisman L. Innovative primary care training: caring for the urban underserved. Innovations in Education Poster Session, Case School of Medicine Annual Education Retreat, Cleveland, OH, March 3, 2016.
5. Berg K, Antenucci C, Raddock M, et al. Deciding to care: medical students and patients’ social circumstances. Poster: Annual meeting of the Society for Medical Decision Making. Pittsburgh, PA. October 2017.
6. Heath B, Wise Romero P, and Reynolds K. A standard framework for levels of integrated healthcare. Washington, D.C. SAMHSA-HRSA Center for Integrated Health Solutions. March 2013. Accessed March 24, 2021. www.pcpcc.org/resource/standard-framework-levels-integrated-healthcare
Recognizing and intervening in child sex trafficking
THE CASE
Emily T.* is a 15-year-old, cisgender, homeless runaway. While on the streets, she was lured to a hotel where a “pimp” informed her she was going to work for him. She repeatedly tried to leave, but he would strike her, so she eventually succumbed. She was forced to have sex with several men and rarely allowed to use condoms.
On 1 occasion, when she went to a hospital with her pimp to visit a patient, her aunt (a nurse on duty at that facility) saw Ms. T and called the police. The pimp was arrested. Ms. T was interviewed by the police and gave a statement but refused a forensic exam.
Because of her involvement with the pimp, she was incarcerated. In prison, she was seen by a physician. On evaluation, she reported difficulty sleeping, flashbacks, and feelings of shame and guilt.1
●
* The patient’s name has been changed to protect her identity.
Child and adolescent sex trafficking is defined as the sexual exploitation of minors through force, fraud, or coercion. Specifically, it includes the recruitment, harboring, transportation, or advertising of a minor, and includes the exchange of anything of value in return for sexual activity. Commercial sexual exploitation and sex trafficking against minors include crimes such as prostitution; survival sex (exchanging sex/sexual acts for money or something of value, such as shelter, food, or drugs); pornography; sex tourism, mail-order-bride trade, and early marriage; or sexual performances (peep shows or strip clubs).2
Providing optimal care for children and adolescents exploited by sex trafficking depends on knowing risk factors, having an awareness of recommended screening and assessment tools, and employing a trauma-informed approach to interviews, examination, and support.2
Continue to: Recognize clues to trafficking
Recognize clues to trafficking
The Centers for Disease Control and Prevention (CDC) offers a framework for trafficking prevention. Health care providers are encouraged to use the CDC Social-Ecological Model which describes targeted prevention strategies at the individual, relationship, community, and societal levels.3
Risk factors for entering trafficking. Younger age increases a child’s vulnerability to exploitation, due to a lack of maturity, limited cognitive development, and ease of deception. The mean age of trafficking survivors is 15 years.4 History of child abuse and other traumatic experiences can lead children to run away from home. It is estimated that, once on the streets, most teens will be recruited by a trafficker within 48 hours.5 Poor self-esteem, depression, substance abuse, history of truancy, and early sexual maturation also increase the risk of becoming involved in trafficking (TABLE 1).2,6-8
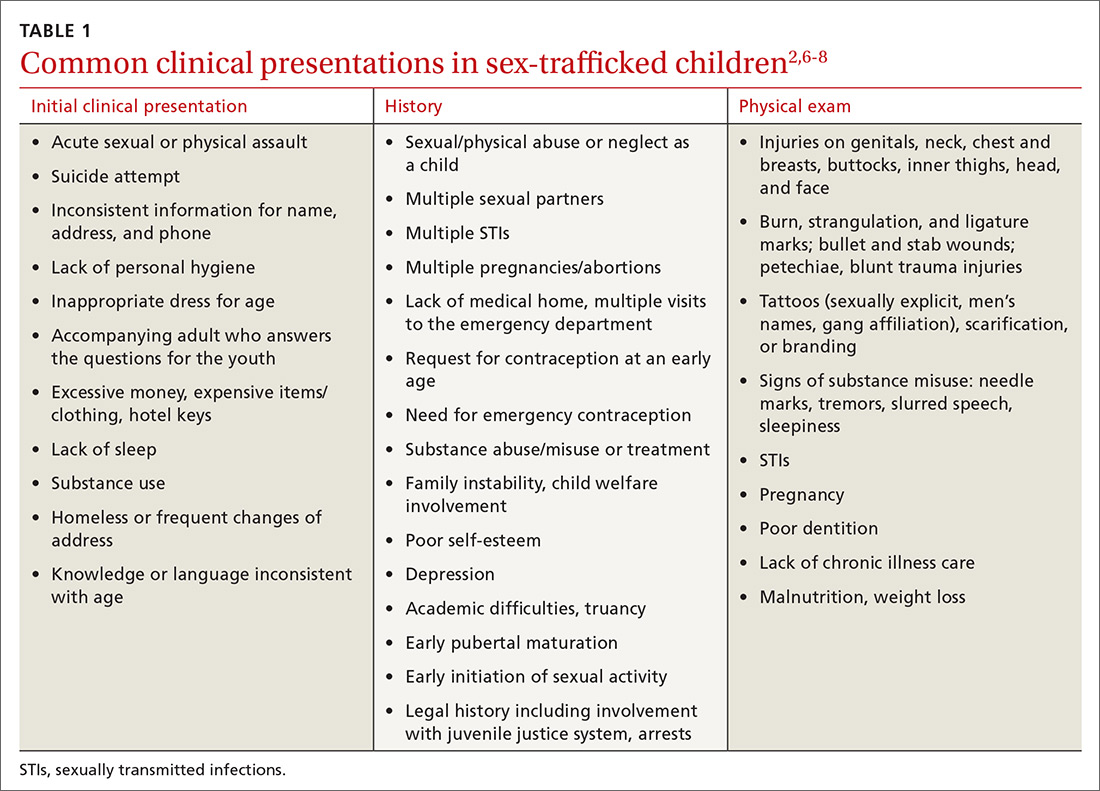
Clinical findings suggestive of trafficking. Many physicians grasp the critical role they play in confronting trafficking, but they lack specific training, experience, and assessment tools.3 Notably, in 1 retrospective study, 46% of victims had been seen by a provider within the previous 2 months.6 One of the major challenges to identifying survivors of trafficking is to recognize critical signs, of which there are many (TABLE 12,6-8). Often these include a history of sexual assault, multiple pregnancies, requests for contraception at an early age, or evidence of physical injury.

Screen to identify trafficking
Universal, validated screening tools to accurately identify trafficked youth is an area of growing research. Some tools have been validated, but only for specific populations such as homeless or incarcerated youth or in emergency department (ED) patients. Tools for sex trafficking identification that may be useful in primary care include the Child Sex Trafficking (CST) screen validated in ED settings (TABLE 2),9 the Commercial Sexual Exploitation-Identification Tool (CSE-IT) in multiple settings (TABLE 3),10,11 and the Quick Youth Indicators for Trafficking (QYIT) in homeless youth (TABLE 4).11 The QYIT is the first validated labor and sex trafficking screening tool in homeless young adults. Children who screen positive for sex trafficking should be further assessed using a comprehensive tool.

Barriers to effective recognition of trafficked individuals. Financial factors limit access to health care. Also, survivors have cited multiple barriers for health professionals that prevent identification of survivors’ trafficked status.12,13 Once in the medical setting, disclosure is impacted by time constraints, fear of judgment by clinicians, and the risk of re-traumatization. Survivors have also cited lack of privacy, control strategies by their traffickers, lack of provider empathy, and fear of police as barriers to disclosure.14
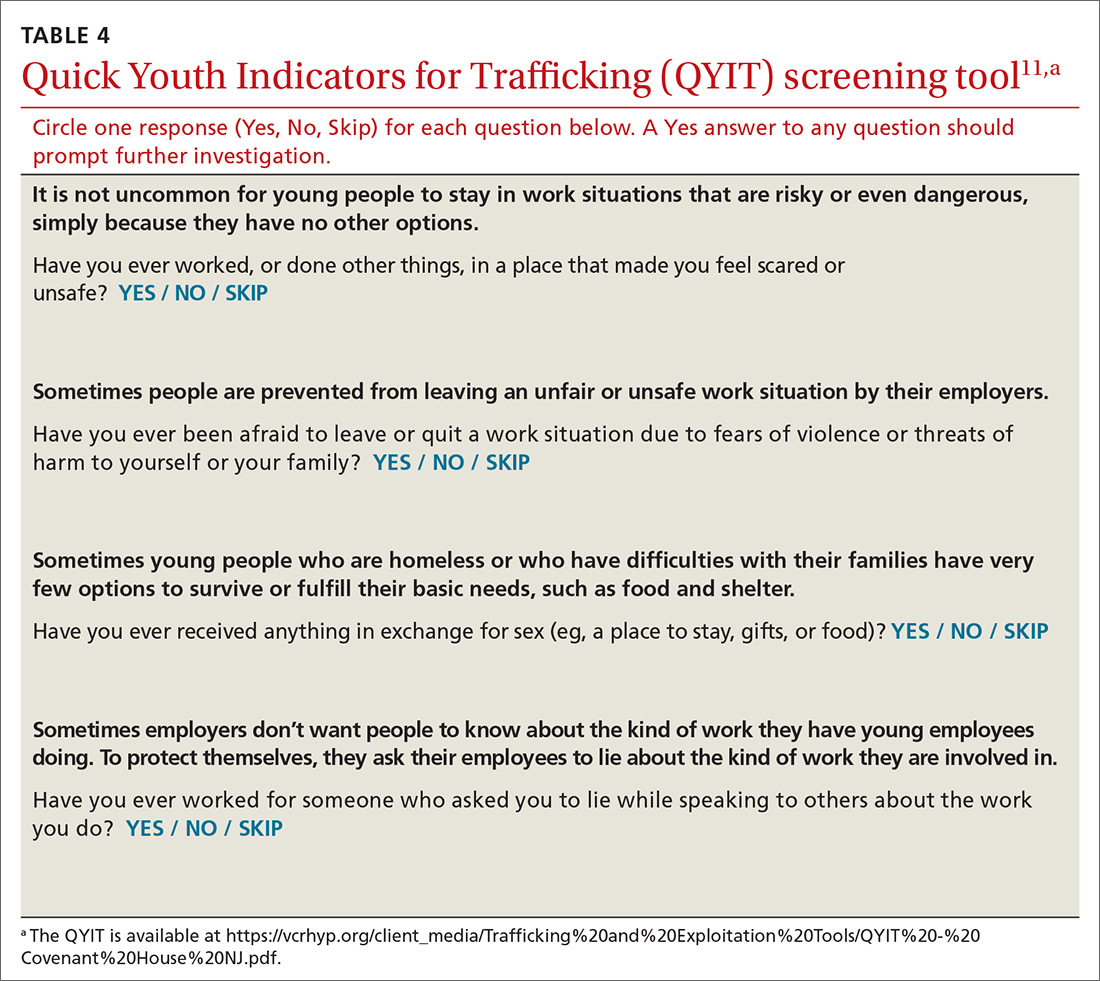
Continue to: The medical impact of trafficking
The medical impact of trafficking
Sexual exploitation is a traumatic experience that is known to cause harm across multiple domains including serious physical injuries related to violence, as well as reproductive and mental health consequences.15,16
Acute and chronic illnesses. In an initial evaluation, assess the survivor’s acute medical conditions (TABLE 5).15,16 Common acute issues include physical injuries due to assault, infections, and reproductive complications.17 Health concerns can also result from stressors such as deprivation of food and sleep, hazardous living conditions, and limited access to care.17

As part of caring for sex-trafficking survivors, assess for, and treat, chronic health issues such as pain, gastrointestinal complaints, poor dental care, malnutrition, and fatigue.16,18 Substance use, as well as chronic mental health concerns (eg, anxiety, depression, posttraumatic stress disorder [PTSD]) may also influence the clinical presentation.
Physical injuries. A cross-sectional study of female survivors of sex trafficking in the United States found that 89% sustained physical injury resulting from violence, including fractures, open wounds, head injury, dental problems, burns, and anogenital trauma.16,17 In many cases, acute injury may not be present in the clinical setting since care is often delayed, but a full examination can reveal signs of prior trauma.16
Reproductive health concerns. Significant, long-term impact on reproductive health can result due to forced penetration by multiple perpetrators, sodomy, and sex without protection or lubricants. Survivors are therefore at high risk for unplanned and unwanted pregnancies, sexually transmitted infections (STIs), pelvic pain, and infertility.16
Continue to: Psychological effects and trauma exposure
Psychological effects and trauma exposure. Survivors often have experienced abuse and neglect prior to commercial exploitation, and they may exhibit long-term sequelae. Survivors may present with major depression, anxiety, panic attacks, suicidality, addiction, PTSD, or aggression.16 Long-term sequelae for patients can include dysfunctional relationships due to an inability to trust, self-destructive behaviors, and significant shame.2,18
Care and treatment of trafficked youth
Initial presentation may occur in a variety of health care settings. Use a trauma-informed approach emphasizing physical and emotional safety and positive relationships, to reduce risk to the survivor, staff, and providers.19 Establishing trust and rapport may provide better short-term safety, as well as help build stronger long-term relationships that can lead to better health outcomes.
Clinical examination. Provide traumatized patients with a sense of safety, control, and autonomy in the health care setting. During the physical exam, be aware of the impact of re-traumatization as patients are asked to undress, endure sensitive examinations, and undergo invasive procedures. Explain the examination, ask for permission at each step, and use slow movements. Allow the patient to guide certain sensitive exams.15 Adopt an approach that recognizes the impact of trauma, avoids revictimization, and acknowledges the resilience of survivors.20
Treatment plan. After the initial exam, treat acute physical injuries and determine if any further testing is needed. Offer emergency contraception, STI prophylaxis, pre-exposure prophylaxis, and vaccines.15 After assessing patient readiness, offer local resources, identify safe methods for communication, identify individuals who could intervene in a crisis, and consider a safety plan (TABLE 6).
Coordination of care. Consider referrals to behavioral health services, substance recovery centers, food programs, housing resources, and a primary care clinic.15 ED clinicians may be asked to complete a sexual or physical assault forensic examination. After obtaining informed consent, one needs to19
- document skin signs such as scars, bites, strangulation marks, and tattoos. Note the size, shape, color, location, and other characteristics of each lesion.
- perform an oral, genital, and rectal examination and use a sexual assault evidence kit as indicated.
- use body diagrams and take photographs of all injuries/physical findings.
- order diagnostic testing as appropriate (eg, imaging to assess fractures).
Continue to: THE CASE
THE CASE
During Ms. T’s incarceration, she was tested for STIs and treated for gonorrhea, trichomonas, and bacterial vaginosis. She was educated about sexual health, was counseled on contraception, and accepted condoms. She was referred to a therapist and given information on additional community resources she could contact upon her release.
A year after her release, she was incarcerated again. She also had an unplanned pregnancy. With the support she received from community programs, social workers, and her primary care provider, she moved in with her family, where she is currently living. She denies any ongoing trafficking activity.
CORRESPONDENCE
Piali Basu, DO, MPH, UCSF Primary Care, 185 Berry Street, Lobby 1, Suite 1000, San Francisco, CA 94107; [email protected]
1. Samko Tracey (Department of Pediatrics, LA County + University of Southern California, Los Angeles, CA). Conversation with: Vidhi Doshi (Department of Internal Medicine, University of California San Francisco, San Francisco, CA). July 8, 2019.
2. Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
3. Greenbaum VJ, Titchen K, Walker-Descartes I, et al. Multi-level prevention of human trafficking: the role of health care professionals. Prev Med. 2018;114:164-167.
4. Smith LA, Vardman SH, Snow MA. The national report on domestic minor sex trafficking: America’s prostituted children. 2009. Accessed January 11, 2021. http://sharedhope.org/wp-content/uploads/2012/09/SHI_National_Report_on_DMST_2009.pdf
5. Know the facts: commercial sexual exploitation of children. In: Connections. Summer 2011. Washington Coalition of Sexual Assault Programs. Accessed January 11, 2021. www.wcsap.org/sites/default/files/uploads/resources_publications/connections/Commercial_Sexual_Exploitation_of_Youth_2011.pdf
6. Varma S, Gillespie S, McCracken C, et al. Characteristics of child commercial sexual exploitation and sex trafficking victims presenting for medical care in the United States. Child Abuse Negl. 2015;44:98-105.
7. Walker K, California Child Welfare Council. Ending the commercial sexual exploitation of children: a call for multi-system collaboration in California. 2013. Accessed January 11, 2021. https://youthlaw.org/wp-content/uploads/2015/01/Ending-CSEC-A-Call-for-Multi-System_Collaboration-in-CA.pdf
8. Landers M, McGrath K, Johnson MH, et al. Baseline characteristics of dependent youth who have been commercially sexually exploited: findings from a specialized treatment program. J Child Sex Abus. 2017;26:692-709.
9. Greenbaum VJ, Dodd M, McCracken C. A short screening tool to identify victims of child sex trafficking in the health care setting. Pediatr Emerg Care. 2018;34:33-37.
10. Sy E, Quach T, Lee J, et al. Responding to commercially sexually exploited children (CSEC): a community health center‘s journey towards creating a primary care clinical CSEC screening tool in the United States. Inter J Soc Sci Stud. 2016;4:45-51.
11. Chisolm-Straker M, Sze J, Einbond J, et al. Screening for human trafficking among homeless young adults. Childr Youth Serv Rev. 2019;98:72-79.
12. Richie-Zavaleta AC, Villanueva A, Martinez-Donate A, et al. Sex trafficking victims at their junction with the healthcare setting—a mixed-methods inquiry. J Hum Traffick. 2020;6:1-29.
13. Chisolm-Straker M, Miller CL, Duke G, et al. A framework for the development of healthcare provider education programs on human trafficking part two: survivors. J Hum Traffick. 2019;5:410-424.
14. Heilemann T, Santhiveeran J. How do female adolescents cope and survive the hardships of prostitution? A content analysis of existing literature. J Ethn Cult Divers Soc Work. 2011;20:57-76.
15. Greenbaum J, Crawford-Jakubiak JE, Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015;135:566-574.
16. Barnert E, Iqbal Z, Bruce J, et al. Commercial sexual exploitation and sex trafficking of children and adolescents: a narrative review. Acad Pediatr. 2017;17:825-829.
17. Dovydaitis T. Human trafficking: the role of the health care provider. J Midwifery Womens Health. 2010;55:462-467.
18. English A, Kivlahan C. Human rights and human trafficking of adolescents: legal and clinical perspectives. In: Titchen K, Miller E, Eds. Medical Perspectives on Human Trafficking in Adolescents. Springer Nature; 2020:21-41.
19. Price K, Nelson BD, Macias-Konstantopoulos WL. Understanding health care access disparities among human trafficking survivors: profiles of health care experiences, access, and engagement. J Interpers Violence. 2019; doi: 10.1177/0886260519889934.
20. Chambers R, Ravi A, Paulus S. Human trafficking: how family physicians can recognize and assist victims. Am Fam Physician. 2019;100:202-204.
THE CASE
Emily T.* is a 15-year-old, cisgender, homeless runaway. While on the streets, she was lured to a hotel where a “pimp” informed her she was going to work for him. She repeatedly tried to leave, but he would strike her, so she eventually succumbed. She was forced to have sex with several men and rarely allowed to use condoms.
On 1 occasion, when she went to a hospital with her pimp to visit a patient, her aunt (a nurse on duty at that facility) saw Ms. T and called the police. The pimp was arrested. Ms. T was interviewed by the police and gave a statement but refused a forensic exam.
Because of her involvement with the pimp, she was incarcerated. In prison, she was seen by a physician. On evaluation, she reported difficulty sleeping, flashbacks, and feelings of shame and guilt.1
●
* The patient’s name has been changed to protect her identity.
Child and adolescent sex trafficking is defined as the sexual exploitation of minors through force, fraud, or coercion. Specifically, it includes the recruitment, harboring, transportation, or advertising of a minor, and includes the exchange of anything of value in return for sexual activity. Commercial sexual exploitation and sex trafficking against minors include crimes such as prostitution; survival sex (exchanging sex/sexual acts for money or something of value, such as shelter, food, or drugs); pornography; sex tourism, mail-order-bride trade, and early marriage; or sexual performances (peep shows or strip clubs).2
Providing optimal care for children and adolescents exploited by sex trafficking depends on knowing risk factors, having an awareness of recommended screening and assessment tools, and employing a trauma-informed approach to interviews, examination, and support.2
Continue to: Recognize clues to trafficking
Recognize clues to trafficking
The Centers for Disease Control and Prevention (CDC) offers a framework for trafficking prevention. Health care providers are encouraged to use the CDC Social-Ecological Model which describes targeted prevention strategies at the individual, relationship, community, and societal levels.3
Risk factors for entering trafficking. Younger age increases a child’s vulnerability to exploitation, due to a lack of maturity, limited cognitive development, and ease of deception. The mean age of trafficking survivors is 15 years.4 History of child abuse and other traumatic experiences can lead children to run away from home. It is estimated that, once on the streets, most teens will be recruited by a trafficker within 48 hours.5 Poor self-esteem, depression, substance abuse, history of truancy, and early sexual maturation also increase the risk of becoming involved in trafficking (TABLE 1).2,6-8

Clinical findings suggestive of trafficking. Many physicians grasp the critical role they play in confronting trafficking, but they lack specific training, experience, and assessment tools.3 Notably, in 1 retrospective study, 46% of victims had been seen by a provider within the previous 2 months.6 One of the major challenges to identifying survivors of trafficking is to recognize critical signs, of which there are many (TABLE 12,6-8). Often these include a history of sexual assault, multiple pregnancies, requests for contraception at an early age, or evidence of physical injury.

Screen to identify trafficking
Universal, validated screening tools to accurately identify trafficked youth is an area of growing research. Some tools have been validated, but only for specific populations such as homeless or incarcerated youth or in emergency department (ED) patients. Tools for sex trafficking identification that may be useful in primary care include the Child Sex Trafficking (CST) screen validated in ED settings (TABLE 2),9 the Commercial Sexual Exploitation-Identification Tool (CSE-IT) in multiple settings (TABLE 3),10,11 and the Quick Youth Indicators for Trafficking (QYIT) in homeless youth (TABLE 4).11 The QYIT is the first validated labor and sex trafficking screening tool in homeless young adults. Children who screen positive for sex trafficking should be further assessed using a comprehensive tool.

Barriers to effective recognition of trafficked individuals. Financial factors limit access to health care. Also, survivors have cited multiple barriers for health professionals that prevent identification of survivors’ trafficked status.12,13 Once in the medical setting, disclosure is impacted by time constraints, fear of judgment by clinicians, and the risk of re-traumatization. Survivors have also cited lack of privacy, control strategies by their traffickers, lack of provider empathy, and fear of police as barriers to disclosure.14

Continue to: The medical impact of trafficking
The medical impact of trafficking
Sexual exploitation is a traumatic experience that is known to cause harm across multiple domains including serious physical injuries related to violence, as well as reproductive and mental health consequences.15,16
Acute and chronic illnesses. In an initial evaluation, assess the survivor’s acute medical conditions (TABLE 5).15,16 Common acute issues include physical injuries due to assault, infections, and reproductive complications.17 Health concerns can also result from stressors such as deprivation of food and sleep, hazardous living conditions, and limited access to care.17

As part of caring for sex-trafficking survivors, assess for, and treat, chronic health issues such as pain, gastrointestinal complaints, poor dental care, malnutrition, and fatigue.16,18 Substance use, as well as chronic mental health concerns (eg, anxiety, depression, posttraumatic stress disorder [PTSD]) may also influence the clinical presentation.
Physical injuries. A cross-sectional study of female survivors of sex trafficking in the United States found that 89% sustained physical injury resulting from violence, including fractures, open wounds, head injury, dental problems, burns, and anogenital trauma.16,17 In many cases, acute injury may not be present in the clinical setting since care is often delayed, but a full examination can reveal signs of prior trauma.16
Reproductive health concerns. Significant, long-term impact on reproductive health can result due to forced penetration by multiple perpetrators, sodomy, and sex without protection or lubricants. Survivors are therefore at high risk for unplanned and unwanted pregnancies, sexually transmitted infections (STIs), pelvic pain, and infertility.16
Continue to: Psychological effects and trauma exposure
Psychological effects and trauma exposure. Survivors often have experienced abuse and neglect prior to commercial exploitation, and they may exhibit long-term sequelae. Survivors may present with major depression, anxiety, panic attacks, suicidality, addiction, PTSD, or aggression.16 Long-term sequelae for patients can include dysfunctional relationships due to an inability to trust, self-destructive behaviors, and significant shame.2,18
Care and treatment of trafficked youth
Initial presentation may occur in a variety of health care settings. Use a trauma-informed approach emphasizing physical and emotional safety and positive relationships, to reduce risk to the survivor, staff, and providers.19 Establishing trust and rapport may provide better short-term safety, as well as help build stronger long-term relationships that can lead to better health outcomes.
Clinical examination. Provide traumatized patients with a sense of safety, control, and autonomy in the health care setting. During the physical exam, be aware of the impact of re-traumatization as patients are asked to undress, endure sensitive examinations, and undergo invasive procedures. Explain the examination, ask for permission at each step, and use slow movements. Allow the patient to guide certain sensitive exams.15 Adopt an approach that recognizes the impact of trauma, avoids revictimization, and acknowledges the resilience of survivors.20
Treatment plan. After the initial exam, treat acute physical injuries and determine if any further testing is needed. Offer emergency contraception, STI prophylaxis, pre-exposure prophylaxis, and vaccines.15 After assessing patient readiness, offer local resources, identify safe methods for communication, identify individuals who could intervene in a crisis, and consider a safety plan (TABLE 6).

Coordination of care. Consider referrals to behavioral health services, substance recovery centers, food programs, housing resources, and a primary care clinic.15 ED clinicians may be asked to complete a sexual or physical assault forensic examination. After obtaining informed consent, one needs to19
- document skin signs such as scars, bites, strangulation marks, and tattoos. Note the size, shape, color, location, and other characteristics of each lesion.
- perform an oral, genital, and rectal examination and use a sexual assault evidence kit as indicated.
- use body diagrams and take photographs of all injuries/physical findings.
- order diagnostic testing as appropriate (eg, imaging to assess fractures).
Continue to: THE CASE
THE CASE
During Ms. T’s incarceration, she was tested for STIs and treated for gonorrhea, trichomonas, and bacterial vaginosis. She was educated about sexual health, was counseled on contraception, and accepted condoms. She was referred to a therapist and given information on additional community resources she could contact upon her release.
A year after her release, she was incarcerated again. She also had an unplanned pregnancy. With the support she received from community programs, social workers, and her primary care provider, she moved in with her family, where she is currently living. She denies any ongoing trafficking activity.
CORRESPONDENCE
Piali Basu, DO, MPH, UCSF Primary Care, 185 Berry Street, Lobby 1, Suite 1000, San Francisco, CA 94107; [email protected]
THE CASE
Emily T.* is a 15-year-old, cisgender, homeless runaway. While on the streets, she was lured to a hotel where a “pimp” informed her she was going to work for him. She repeatedly tried to leave, but he would strike her, so she eventually succumbed. She was forced to have sex with several men and rarely allowed to use condoms.
On 1 occasion, when she went to a hospital with her pimp to visit a patient, her aunt (a nurse on duty at that facility) saw Ms. T and called the police. The pimp was arrested. Ms. T was interviewed by the police and gave a statement but refused a forensic exam.
Because of her involvement with the pimp, she was incarcerated. In prison, she was seen by a physician. On evaluation, she reported difficulty sleeping, flashbacks, and feelings of shame and guilt.1
●
* The patient’s name has been changed to protect her identity.
Child and adolescent sex trafficking is defined as the sexual exploitation of minors through force, fraud, or coercion. Specifically, it includes the recruitment, harboring, transportation, or advertising of a minor, and includes the exchange of anything of value in return for sexual activity. Commercial sexual exploitation and sex trafficking against minors include crimes such as prostitution; survival sex (exchanging sex/sexual acts for money or something of value, such as shelter, food, or drugs); pornography; sex tourism, mail-order-bride trade, and early marriage; or sexual performances (peep shows or strip clubs).2
Providing optimal care for children and adolescents exploited by sex trafficking depends on knowing risk factors, having an awareness of recommended screening and assessment tools, and employing a trauma-informed approach to interviews, examination, and support.2
Continue to: Recognize clues to trafficking
Recognize clues to trafficking
The Centers for Disease Control and Prevention (CDC) offers a framework for trafficking prevention. Health care providers are encouraged to use the CDC Social-Ecological Model which describes targeted prevention strategies at the individual, relationship, community, and societal levels.3
Risk factors for entering trafficking. Younger age increases a child’s vulnerability to exploitation, due to a lack of maturity, limited cognitive development, and ease of deception. The mean age of trafficking survivors is 15 years.4 History of child abuse and other traumatic experiences can lead children to run away from home. It is estimated that, once on the streets, most teens will be recruited by a trafficker within 48 hours.5 Poor self-esteem, depression, substance abuse, history of truancy, and early sexual maturation also increase the risk of becoming involved in trafficking (TABLE 1).2,6-8

Clinical findings suggestive of trafficking. Many physicians grasp the critical role they play in confronting trafficking, but they lack specific training, experience, and assessment tools.3 Notably, in 1 retrospective study, 46% of victims had been seen by a provider within the previous 2 months.6 One of the major challenges to identifying survivors of trafficking is to recognize critical signs, of which there are many (TABLE 12,6-8). Often these include a history of sexual assault, multiple pregnancies, requests for contraception at an early age, or evidence of physical injury.

Screen to identify trafficking
Universal, validated screening tools to accurately identify trafficked youth is an area of growing research. Some tools have been validated, but only for specific populations such as homeless or incarcerated youth or in emergency department (ED) patients. Tools for sex trafficking identification that may be useful in primary care include the Child Sex Trafficking (CST) screen validated in ED settings (TABLE 2),9 the Commercial Sexual Exploitation-Identification Tool (CSE-IT) in multiple settings (TABLE 3),10,11 and the Quick Youth Indicators for Trafficking (QYIT) in homeless youth (TABLE 4).11 The QYIT is the first validated labor and sex trafficking screening tool in homeless young adults. Children who screen positive for sex trafficking should be further assessed using a comprehensive tool.

Barriers to effective recognition of trafficked individuals. Financial factors limit access to health care. Also, survivors have cited multiple barriers for health professionals that prevent identification of survivors’ trafficked status.12,13 Once in the medical setting, disclosure is impacted by time constraints, fear of judgment by clinicians, and the risk of re-traumatization. Survivors have also cited lack of privacy, control strategies by their traffickers, lack of provider empathy, and fear of police as barriers to disclosure.14

Continue to: The medical impact of trafficking
The medical impact of trafficking
Sexual exploitation is a traumatic experience that is known to cause harm across multiple domains including serious physical injuries related to violence, as well as reproductive and mental health consequences.15,16
Acute and chronic illnesses. In an initial evaluation, assess the survivor’s acute medical conditions (TABLE 5).15,16 Common acute issues include physical injuries due to assault, infections, and reproductive complications.17 Health concerns can also result from stressors such as deprivation of food and sleep, hazardous living conditions, and limited access to care.17

As part of caring for sex-trafficking survivors, assess for, and treat, chronic health issues such as pain, gastrointestinal complaints, poor dental care, malnutrition, and fatigue.16,18 Substance use, as well as chronic mental health concerns (eg, anxiety, depression, posttraumatic stress disorder [PTSD]) may also influence the clinical presentation.
Physical injuries. A cross-sectional study of female survivors of sex trafficking in the United States found that 89% sustained physical injury resulting from violence, including fractures, open wounds, head injury, dental problems, burns, and anogenital trauma.16,17 In many cases, acute injury may not be present in the clinical setting since care is often delayed, but a full examination can reveal signs of prior trauma.16
Reproductive health concerns. Significant, long-term impact on reproductive health can result due to forced penetration by multiple perpetrators, sodomy, and sex without protection or lubricants. Survivors are therefore at high risk for unplanned and unwanted pregnancies, sexually transmitted infections (STIs), pelvic pain, and infertility.16
Continue to: Psychological effects and trauma exposure
Psychological effects and trauma exposure. Survivors often have experienced abuse and neglect prior to commercial exploitation, and they may exhibit long-term sequelae. Survivors may present with major depression, anxiety, panic attacks, suicidality, addiction, PTSD, or aggression.16 Long-term sequelae for patients can include dysfunctional relationships due to an inability to trust, self-destructive behaviors, and significant shame.2,18
Care and treatment of trafficked youth
Initial presentation may occur in a variety of health care settings. Use a trauma-informed approach emphasizing physical and emotional safety and positive relationships, to reduce risk to the survivor, staff, and providers.19 Establishing trust and rapport may provide better short-term safety, as well as help build stronger long-term relationships that can lead to better health outcomes.
Clinical examination. Provide traumatized patients with a sense of safety, control, and autonomy in the health care setting. During the physical exam, be aware of the impact of re-traumatization as patients are asked to undress, endure sensitive examinations, and undergo invasive procedures. Explain the examination, ask for permission at each step, and use slow movements. Allow the patient to guide certain sensitive exams.15 Adopt an approach that recognizes the impact of trauma, avoids revictimization, and acknowledges the resilience of survivors.20
Treatment plan. After the initial exam, treat acute physical injuries and determine if any further testing is needed. Offer emergency contraception, STI prophylaxis, pre-exposure prophylaxis, and vaccines.15 After assessing patient readiness, offer local resources, identify safe methods for communication, identify individuals who could intervene in a crisis, and consider a safety plan (TABLE 6).

Coordination of care. Consider referrals to behavioral health services, substance recovery centers, food programs, housing resources, and a primary care clinic.15 ED clinicians may be asked to complete a sexual or physical assault forensic examination. After obtaining informed consent, one needs to19
- document skin signs such as scars, bites, strangulation marks, and tattoos. Note the size, shape, color, location, and other characteristics of each lesion.
- perform an oral, genital, and rectal examination and use a sexual assault evidence kit as indicated.
- use body diagrams and take photographs of all injuries/physical findings.
- order diagnostic testing as appropriate (eg, imaging to assess fractures).
Continue to: THE CASE
THE CASE
During Ms. T’s incarceration, she was tested for STIs and treated for gonorrhea, trichomonas, and bacterial vaginosis. She was educated about sexual health, was counseled on contraception, and accepted condoms. She was referred to a therapist and given information on additional community resources she could contact upon her release.
A year after her release, she was incarcerated again. She also had an unplanned pregnancy. With the support she received from community programs, social workers, and her primary care provider, she moved in with her family, where she is currently living. She denies any ongoing trafficking activity.
CORRESPONDENCE
Piali Basu, DO, MPH, UCSF Primary Care, 185 Berry Street, Lobby 1, Suite 1000, San Francisco, CA 94107; [email protected]
1. Samko Tracey (Department of Pediatrics, LA County + University of Southern California, Los Angeles, CA). Conversation with: Vidhi Doshi (Department of Internal Medicine, University of California San Francisco, San Francisco, CA). July 8, 2019.
2. Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
3. Greenbaum VJ, Titchen K, Walker-Descartes I, et al. Multi-level prevention of human trafficking: the role of health care professionals. Prev Med. 2018;114:164-167.
4. Smith LA, Vardman SH, Snow MA. The national report on domestic minor sex trafficking: America’s prostituted children. 2009. Accessed January 11, 2021. http://sharedhope.org/wp-content/uploads/2012/09/SHI_National_Report_on_DMST_2009.pdf
5. Know the facts: commercial sexual exploitation of children. In: Connections. Summer 2011. Washington Coalition of Sexual Assault Programs. Accessed January 11, 2021. www.wcsap.org/sites/default/files/uploads/resources_publications/connections/Commercial_Sexual_Exploitation_of_Youth_2011.pdf
6. Varma S, Gillespie S, McCracken C, et al. Characteristics of child commercial sexual exploitation and sex trafficking victims presenting for medical care in the United States. Child Abuse Negl. 2015;44:98-105.
7. Walker K, California Child Welfare Council. Ending the commercial sexual exploitation of children: a call for multi-system collaboration in California. 2013. Accessed January 11, 2021. https://youthlaw.org/wp-content/uploads/2015/01/Ending-CSEC-A-Call-for-Multi-System_Collaboration-in-CA.pdf
8. Landers M, McGrath K, Johnson MH, et al. Baseline characteristics of dependent youth who have been commercially sexually exploited: findings from a specialized treatment program. J Child Sex Abus. 2017;26:692-709.
9. Greenbaum VJ, Dodd M, McCracken C. A short screening tool to identify victims of child sex trafficking in the health care setting. Pediatr Emerg Care. 2018;34:33-37.
10. Sy E, Quach T, Lee J, et al. Responding to commercially sexually exploited children (CSEC): a community health center‘s journey towards creating a primary care clinical CSEC screening tool in the United States. Inter J Soc Sci Stud. 2016;4:45-51.
11. Chisolm-Straker M, Sze J, Einbond J, et al. Screening for human trafficking among homeless young adults. Childr Youth Serv Rev. 2019;98:72-79.
12. Richie-Zavaleta AC, Villanueva A, Martinez-Donate A, et al. Sex trafficking victims at their junction with the healthcare setting—a mixed-methods inquiry. J Hum Traffick. 2020;6:1-29.
13. Chisolm-Straker M, Miller CL, Duke G, et al. A framework for the development of healthcare provider education programs on human trafficking part two: survivors. J Hum Traffick. 2019;5:410-424.
14. Heilemann T, Santhiveeran J. How do female adolescents cope and survive the hardships of prostitution? A content analysis of existing literature. J Ethn Cult Divers Soc Work. 2011;20:57-76.
15. Greenbaum J, Crawford-Jakubiak JE, Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015;135:566-574.
16. Barnert E, Iqbal Z, Bruce J, et al. Commercial sexual exploitation and sex trafficking of children and adolescents: a narrative review. Acad Pediatr. 2017;17:825-829.
17. Dovydaitis T. Human trafficking: the role of the health care provider. J Midwifery Womens Health. 2010;55:462-467.
18. English A, Kivlahan C. Human rights and human trafficking of adolescents: legal and clinical perspectives. In: Titchen K, Miller E, Eds. Medical Perspectives on Human Trafficking in Adolescents. Springer Nature; 2020:21-41.
19. Price K, Nelson BD, Macias-Konstantopoulos WL. Understanding health care access disparities among human trafficking survivors: profiles of health care experiences, access, and engagement. J Interpers Violence. 2019; doi: 10.1177/0886260519889934.
20. Chambers R, Ravi A, Paulus S. Human trafficking: how family physicians can recognize and assist victims. Am Fam Physician. 2019;100:202-204.
1. Samko Tracey (Department of Pediatrics, LA County + University of Southern California, Los Angeles, CA). Conversation with: Vidhi Doshi (Department of Internal Medicine, University of California San Francisco, San Francisco, CA). July 8, 2019.
2. Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
3. Greenbaum VJ, Titchen K, Walker-Descartes I, et al. Multi-level prevention of human trafficking: the role of health care professionals. Prev Med. 2018;114:164-167.
4. Smith LA, Vardman SH, Snow MA. The national report on domestic minor sex trafficking: America’s prostituted children. 2009. Accessed January 11, 2021. http://sharedhope.org/wp-content/uploads/2012/09/SHI_National_Report_on_DMST_2009.pdf
5. Know the facts: commercial sexual exploitation of children. In: Connections. Summer 2011. Washington Coalition of Sexual Assault Programs. Accessed January 11, 2021. www.wcsap.org/sites/default/files/uploads/resources_publications/connections/Commercial_Sexual_Exploitation_of_Youth_2011.pdf
6. Varma S, Gillespie S, McCracken C, et al. Characteristics of child commercial sexual exploitation and sex trafficking victims presenting for medical care in the United States. Child Abuse Negl. 2015;44:98-105.
7. Walker K, California Child Welfare Council. Ending the commercial sexual exploitation of children: a call for multi-system collaboration in California. 2013. Accessed January 11, 2021. https://youthlaw.org/wp-content/uploads/2015/01/Ending-CSEC-A-Call-for-Multi-System_Collaboration-in-CA.pdf
8. Landers M, McGrath K, Johnson MH, et al. Baseline characteristics of dependent youth who have been commercially sexually exploited: findings from a specialized treatment program. J Child Sex Abus. 2017;26:692-709.
9. Greenbaum VJ, Dodd M, McCracken C. A short screening tool to identify victims of child sex trafficking in the health care setting. Pediatr Emerg Care. 2018;34:33-37.
10. Sy E, Quach T, Lee J, et al. Responding to commercially sexually exploited children (CSEC): a community health center‘s journey towards creating a primary care clinical CSEC screening tool in the United States. Inter J Soc Sci Stud. 2016;4:45-51.
11. Chisolm-Straker M, Sze J, Einbond J, et al. Screening for human trafficking among homeless young adults. Childr Youth Serv Rev. 2019;98:72-79.
12. Richie-Zavaleta AC, Villanueva A, Martinez-Donate A, et al. Sex trafficking victims at their junction with the healthcare setting—a mixed-methods inquiry. J Hum Traffick. 2020;6:1-29.
13. Chisolm-Straker M, Miller CL, Duke G, et al. A framework for the development of healthcare provider education programs on human trafficking part two: survivors. J Hum Traffick. 2019;5:410-424.
14. Heilemann T, Santhiveeran J. How do female adolescents cope and survive the hardships of prostitution? A content analysis of existing literature. J Ethn Cult Divers Soc Work. 2011;20:57-76.
15. Greenbaum J, Crawford-Jakubiak JE, Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015;135:566-574.
16. Barnert E, Iqbal Z, Bruce J, et al. Commercial sexual exploitation and sex trafficking of children and adolescents: a narrative review. Acad Pediatr. 2017;17:825-829.
17. Dovydaitis T. Human trafficking: the role of the health care provider. J Midwifery Womens Health. 2010;55:462-467.
18. English A, Kivlahan C. Human rights and human trafficking of adolescents: legal and clinical perspectives. In: Titchen K, Miller E, Eds. Medical Perspectives on Human Trafficking in Adolescents. Springer Nature; 2020:21-41.
19. Price K, Nelson BD, Macias-Konstantopoulos WL. Understanding health care access disparities among human trafficking survivors: profiles of health care experiences, access, and engagement. J Interpers Violence. 2019; doi: 10.1177/0886260519889934.
20. Chambers R, Ravi A, Paulus S. Human trafficking: how family physicians can recognize and assist victims. Am Fam Physician. 2019;100:202-204.
Which behavioral health screening tool should you use—and when?
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3
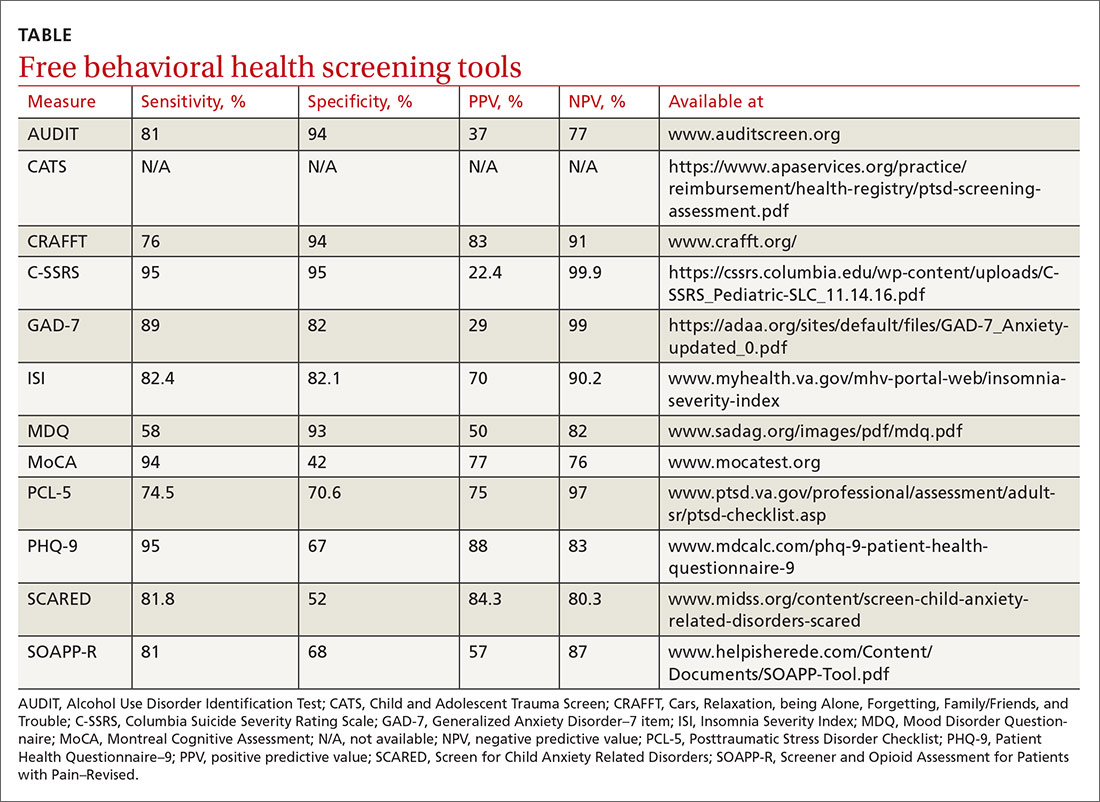
Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
Continue to: The C-SSRS covers...
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
Continue to: The Insomnia Severity Index...
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Continue to: Opioid medications...
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Posttraumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher,
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
Continue to: The MoCA is a simple...
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues.
The FIGURE can be used to aid in the clinical decision-making process.
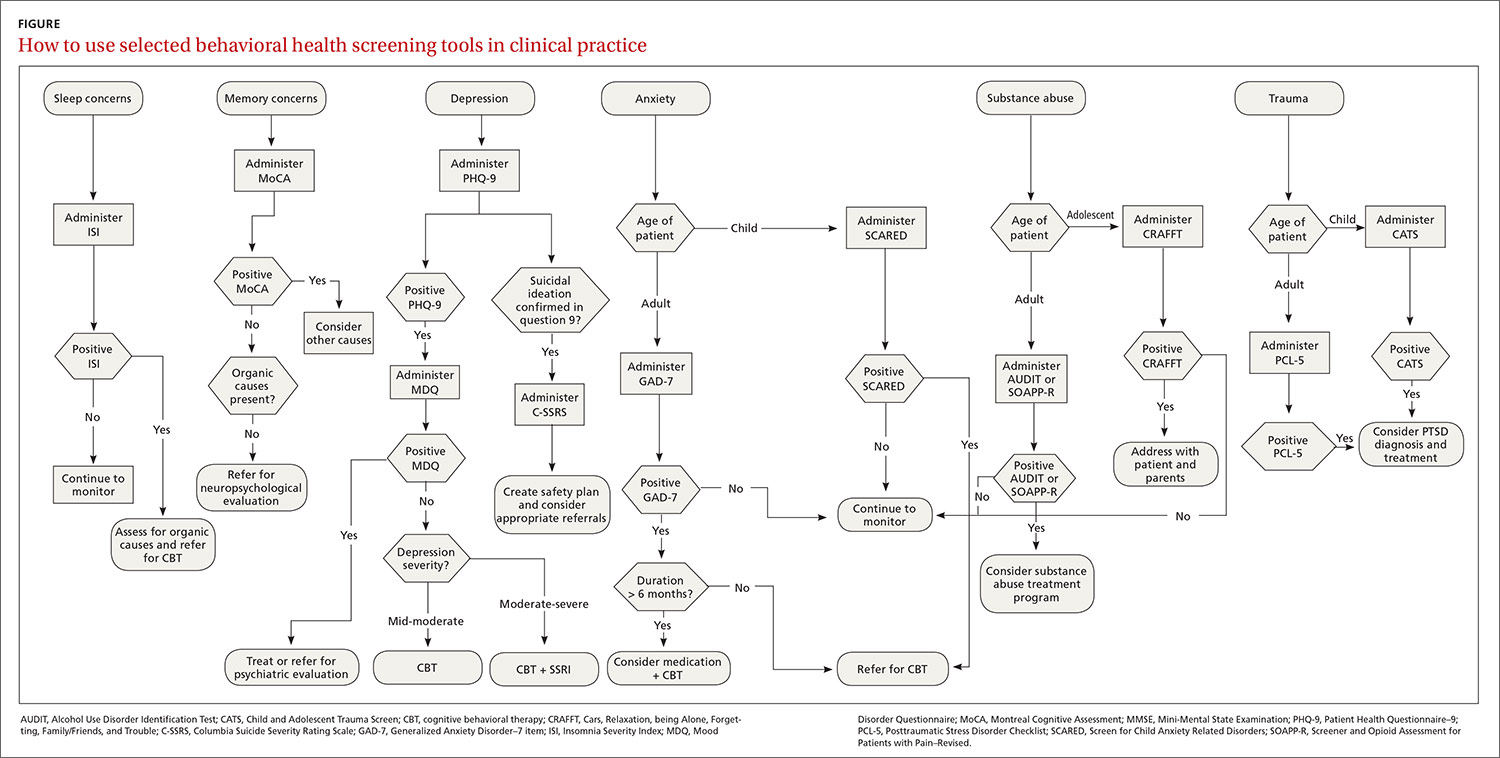
CORRESPONDENCE
Rebecca Sewell, PsyD, Bon Secours Mercy Health, 2213 Cherry Street, Toledo, OH 4360; [email protected].
1. Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
2. Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
3. Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
4. Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
5. Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016,2:127-138.
6. Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
7. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
9. Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
10. CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
11. Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
12. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
13. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
14. DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
15. Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
16. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
17. Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
18. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
19. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
21. Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
22. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
24. Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
26. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
27. Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
28. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360-372.
29. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
30. DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23, 2020.
31. Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
32. Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
34. Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
35. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
Continue to: The C-SSRS covers...
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
Continue to: The Insomnia Severity Index...
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Continue to: Opioid medications...
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Posttraumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher,
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
Continue to: The MoCA is a simple...
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues.
The FIGURE can be used to aid in the clinical decision-making process.

CORRESPONDENCE
Rebecca Sewell, PsyD, Bon Secours Mercy Health, 2213 Cherry Street, Toledo, OH 4360; [email protected].
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
Continue to: The C-SSRS covers...
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
Continue to: The Insomnia Severity Index...
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Continue to: Opioid medications...
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Posttraumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher,
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
Continue to: The MoCA is a simple...
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues.
The FIGURE can be used to aid in the clinical decision-making process.

CORRESPONDENCE
Rebecca Sewell, PsyD, Bon Secours Mercy Health, 2213 Cherry Street, Toledo, OH 4360; [email protected].
1. Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
2. Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
3. Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
4. Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
5. Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016,2:127-138.
6. Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
7. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
9. Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
10. CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
11. Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
12. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
13. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
14. DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
15. Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
16. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
17. Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
18. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
19. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
21. Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
22. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
24. Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
26. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
27. Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
28. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360-372.
29. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
30. DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23, 2020.
31. Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
32. Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
34. Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
35. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
1. Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
2. Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
3. Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
4. Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
5. Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016,2:127-138.
6. Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
7. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
9. Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
10. CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
11. Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
12. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
13. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
14. DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
15. Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
16. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
17. Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
18. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
19. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
21. Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
22. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
24. Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
26. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
27. Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
28. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360-372.
29. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
30. DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23, 2020.
31. Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
32. Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
34. Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
35. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.