User login
Caring for Patients With Prostate Cancer Who Are BRCA Positive
There are several risk assessment tools and clinical practice guidelines used in the management of localized prostate cancer (PCa). These include the D’Amico classification, the Cancer of the Prostate Risk Assessment (CAPRA) score, the National Comprehensive Cancer Network (NCCN) risk criteria, and the American Urological Association (AUA) clinical practice guidelines.1-4 None of these tools incorporate the BRCA1 and BRCA2 genes in the risk assessment or treatment recommendations for localized PCa.5 The BRCA mutations are most strongly associated with breast and ovarian cancer risk. However, BRCA mutations also increase susceptibility and disease progression in PCa.6 This article illustrates the current knowledge gap in PCa treatment algorithms for the BRCA2-positive patient population.
Traditional risk assessment tools use clinical and pathologic features of PCa, including prostate-specific antigen (PSA) level, Gleason score, tumor stage, and disease burden to measure cancer aggressiveness.1,7,8 These criteria are the basis of the AUA and NCCN guidelines for management of clinically localized PCa, which recognize 3 categories of clinically localized disease (low, intermediate, and high risk).3,4 The NCCN guidelines (version 1.2016) include a fourth category (very low risk or pathologically insignificant PCa) among some stage T1c patients, based on additional criteria, including PSA density. Both the AUA and NCCN recommend active surveillance as a treatment option for men with low-risk PCa. The NCCN recently revised its guidelines to state that intermediate-risk patients with PCa with favorable features (Gleason grade 3 and < 50% of positive biopsy cores) may also be considered for active surveillance.3
BRCA Mutations in Prostate Cancer
Estimates of the relative risk of PCa for men with BRCA1 and BRCA2 mutations have varied, but recent data suggest that it is 3.75-fold for BRCA1 mutations and 8.6-fold for BRCA2 by age 65 years.9-11 Moreover, PCas associated with BRCA1/2 mutations, particularly those in the BRCA2 gene, are often more aggressive and characterized by poor outcomes.12,13 The presence of a BRCA2 mutation is a negative prognostic factor in PCa, independent of tumor grade, stage, and PSA levels.14 Both PCa-specific survival and metastasis-free survival rates following surgical or radiation therapy are significantly lower in the BRCA mutation carriers than in noncarriers.15 Preliminary results of the IMPACT study demonstrate that targeted PCa screening in men with BRCA1 or BRCA2 mutations may result in identification of tumors more likely to require treatment.16
As a result of these increased risks, it is recommended that men with BRCA2 mutations begin PCa screening at age 40 years; however, there are no clear guidelines for clinical management of PCa in this group of patients.5 The lack of guidelines presents a challenge for clinical management of BRCA1/2 mutation carriers with localized PCa who otherwise qualify for active surveillance. A recent editorial by Bratt and Loman specifically
calls for aggressive therapy for patients who are BRCA positive, particularly BRCA2 carriers, suggesting the need to combine early radical local treatment with adjuvant systemic therapy.17 However, data on the effectiveness of aggressive therapies in patients with PCa who carry BRCA2 mutations are sparse.5
Genomic Test for Risk
There is growing recognition of the need to include molecular testing to improve risk assessment in PCa. Using traditional risk assessment tools, about 8% of low-risk patients are found to have progressive disease postoperatively.3 Current AUA guidelines from 2007 are silent on the issue of molecular testing. The 2015 and 2016 NCCN guidelines include molecular testing for better risk stratification of patients with PCa, specifically naming Oncotype DX Prostate Cancer Assay (Genomic Health, Redwood City, CA) and Prolaris (Myriad Genetics, Salt Lake City, UT).3 However, they do not address molecular BRCA mutation testing.
There are several genomic tests aimed at improving PCa risk assessment. These include Oncotype DX PCa Assay; Prolaris; Decipher Prostate Cancer Classifier (GenomeDx Biosciences, San Diego, CA); and ProMark (Metamark Laboratories, Cambridge, MA). These assays are tissuebased and measure gene expression on the RNA or protein level to identify low- or intermediate-risk patients who may be candidates for active surveillance, as well as patients at higher risk who may benefit from closer monitoring or additional therapy after their initial treatment. By 2015, the Centers for Medicare and Medicaid Services had issued positive coverage decisions for several tests.18
The Oncotype DX test is a quantitative real-time polymerase chain reaction assay that measures the expression of 17 genes (12 cancer-related genes and 5 reference genes) representing 4 biologic pathways, including from the androgen signaling, stromal response, cellular organization, and cellular proliferation (Table). Prolaris focuses on a larger number of genes in the cell-cycle progression (CCP) pathway (31 cell-cycle-related genes and 15 reference genes). There is no overlap between the 2 gene sets. Both tests integrate genomic data with clinical and histopathologic characteristics of the tumor to arrive at prognostic information. The Oncotype DX test yields a specific Genomic Prostate Score (GPS; scaled 0-100) that is integrated with the patient’s NCCN clinical risk group to quantify the likelihood of favorable pathology, which is defined as low-grade organ-confined disease.19 The Prolaris test uses the patient’s AUA risk category and then evaluates the patient’s risk based on the cell-cycle progression
gene panel compared with that risk category. It also provides an estimate of disease-specific mortality as validated by 2 independent cohorts that were managed conservatively initially with watchful waiting.
In this article, the authors present a case report of a BRCA2-positive veteran with newly diagnosed lowrisk PCa and a history of breast cancer. In addition to evaluating clinical criteria, Oncotype DX and Prolaris gene expression tests were ordered for this patient. The authors obtained veteran and institutional review board permission. To protect the identity of the patient, minor changes were made to patient demographics.
Case Presentation
A 68-year-old white man with a history of coronary artery disease, dyslipidemia, and hypertension, was recently diagnosed with PCa. He presented to Genomic Medicine Service to discuss how his BRCA2 mutation status might impact management decisions for PCa. Priorto the PCa diagnosis, the veteran had a history of breast and skin cancer. He was diagnosed with invasive ductal carcinoma of the right breast (ER+/PR+/Her2+) at age 62 years and treated with mastectomy and tamoxifen. He had testing at that time, which revealed a BRCA2 mutation: 3773delTT. Squamous cell carcinoma was detected on his right leg and removed at age 64 years. Basal cell carcinoma was removed from his left forehead first at age 65 years, and then residual basal cell carcinoma was removed from the forehead 2 months later.
The veteran was diagnosed with PCa at age 67 years at a non-VA clinic. The urology consult note reported a sudden increase of his PSA level to 5.9. A prostate needle biopsy was performed. The Gleason score was 3 + 3 = 6 in 2 of 12, with < 1% PCa involvement and focal highgrade prostatic intraepithelial neoplasia. The patient was asymptomatic, and his cancer was identified by needle biopsy due to elevated PSA. His clinical stage was T1c. According to AUA and NCCN guidelines, the patient was categorized as low risk, defined as Gleason Score ≤ 6, PSA < 10 ng/mL, and clinical stage up to T2a.3,4 Additionally, the veteran met 3 criteria for the NCCN very low-risk category (stage T1c, < 3 positive biopsy cores and ≤ 50% cancer in any core). However, because he was initially diagnosed at a non-VA clinic, his PSA density (the remaining criterion) was not available to the VA urologist. Therefore, the low-risk category was assumed for molecular test interpretation.
The non-VA urologist recommended active surveillance. The VA urologist agreed that active surveillance was an appropriate treatment recommendation at this time. However, the veteran and his family members remained concerned that his PCa might be more aggressive due to his BRCA2 mutation, and they worried that active surveillance would result in a worse outcome. Their concern was exacerbated by the veteran’s comorbidities, which could have potential implications on the timing of surgical options. The patient expressed these concerns to his VA primary care physician, who then referred him to the VA Genomic Medicine Services.
Genetic Consult
The genetic counselor scheduled a telegenetics consult and conducted an assessment of the veteran, which included a review of his medical history, mutation status, and relevant family history. The family history was consistent with hereditary breast/ovarian cancer. However, the primary reason the veteran underwent genetic testing was the diagnosis of breast cancer in a male. The genetic counselor provided the patient with information relevant to his mutation carrier status, including that men with BRCA2 mutations are at increased risk of developing more aggressive PCa, have higher rates of lymph node involvement, and greater mortality compared with men without BRCA2 mutations. The veteran was informed that there were no published guidelines that suggest PCa in BRCA2 carriers should be treated differently from sporadic PCa.
Tumor Testing Strategy
Although the veteran was comfortable with active surveillance at the time of consultation, he was concerned that, given his comorbidities, it would be better to pursue surgery sooner. The veteran asked the genetic counselor for more information about his prognosis given his BRCA2 status. The genetic counselor discussed possible use of tumor gene expression profiling and informed him about 2 active studies within the VA that are evaluating the clinical utility of gene expression tests for PCa risk stratification (Oncotype DX at Genomic Health and Prolaris at Myriad Genetic Laboratories). The veteran expressed an interest in having his biopsy tissue tested by both assays. Tumor biopsy tissue was obtained and sent to both Genomic Health and Myriad Genetics for testing. Neither test incorporated the veteran’s other health conditions or his BRCA mutation status into risk stratification results or the patient report.
Test Results
The Oncotype DX GPS result for this NCCN low-risk patient was 31 (Figure 1). This score corresponds to a likelihood of favorable pathology at radical prostatectomy of 71% (95% confidence interval [CI]: 63%-78%). Favorable pathology is defined as freedom from highgrade (Gleason score > 4+3) and/or nonorgan-confined (pT3) disease. This GPS result was consistent with the range of risk expected for NCCN low-risk patients based on the validation cohorts for the assay. The estimate of likelihood of favorable pathology would be modified if the PSA density result were available and if it placed the patient in the NCCN very low-risk category.
The Prolaris report demonstrated a score of 0.4 (Figure 2). This puts the veteran in the 94th percentile of contemporary U.S. men who are AUA low risk. The CCP score makes his cancer more aggressive than most AUA low-risk men, and the projected 10-year disease-specific mortality is 3%. In conjunction with the patient’s BRCA2 status, he may benefit from definitive intervention. If active surveillance is chosen, careful and regular follow-up for disease progression is mandated.
Interpretation of Genomic Testing in PCa
For both tests, the results are derived from 2-tiered calculations. For Oncotype DX, the gene expression measurement yields the GPS, which is then integrated with the patient’s clinical and pathologic information to yield the likelihood of favorable pathology. Although the Oncotype DX GPS is an independent measure of disease aggressiveness, on the patient report, the GPS is combined with the NCCN clinical risk group to provide a likelihood of favorable pathology. Therefore, 2 patients with the same GPS but different levels of clinical risk will have different likelihoods of favorable pathology.
The Prolaris test provides the Prolaris CCP score as well as the percentile group of patients with a lower score within the same risk category. Also, the Prolaris test yields a numerical 10-year PCa-specific mortality risk. The Prolaris score has been shown to impact therapeutic decisions in patients with newly diagnosed PCa.20
Recently, Myriad defined a threshold for active surveillance combining the CCP and CAPRA scores.21 Myriad validated this cutoff in 2 cohorts of men initially managed conservatively. Although the model predicts up to 3.2% disease-specific mortality, there were no observed deaths during a decade of follow-up. Myriad reports that by using this cutoff in contemporary patients tested commercially with Prolaris, a health care system could increase the percentage of men who would fit current criteria for active surveillance from 36% to 60% with no increase in risk of disease-specific mortality.
The results of these 2 tests are presented in 2 different formats and provide risk estimates for different clinical endpoints, making it challenging for a clinician to directly compare them. Moreover, each genomic test is based on a different set of genes and uses different clinical risk criteria (AUA vs NCCN), which may result in different test output. Finally, and most relevant to the case described here, there is no evidence-based consensus on how to interpret these test results in the context of a BRCA2 mutation.
Based on the published literature reporting that BRCA2 mutations are associated with more aggressive disease, one prediction would be that test scores from genomic assays such as Oncotype DX and Prolaris would tend to be higher in BRCA2 carriers than those of the overall population of PCa patients. This has, in fact, been reported for the Oncotype DX Breast Cancer Assay recurrence score in women who are BRCA carriers.22 Further research is required to ascertain whether this will be true for Oncotype DX GPS and Prolaris CCP score in PCa. The mechanism of action that predisposes BRCA2 mutation carriers to develop a more aggressive variant of PCa may not be detectable by the genomic markers included in the Oncotype DX PCa and Prolaris tests. The degree to which a mutated BRCA2 gene may interact with the genes comprising these assays and the reported tumor aggressiveness is not yet understood but deserving of future study.
Treatment Recommendation and Patient’s Decision
After considering his test results, the veteran chose active surveillance. The sum of clinical, pathologic, and molecular factors, combined with the patient’s preference, determined his course of treatment. Because prostatectomy was not performed, it has not been positively determined whether or not the patient harbors aggressive disease. As the molecular test results place the patient at the high end of the low-risk group, the VA urologist recommended close monitoring and suggested a follow-up biopsy with magnetic resonance-ultrasound fusion guidance.
Conclusions
Molecular testing found that the patient’s PCa stage and grade are consistent with NCCN low risk (Oncotype DX) and that the disease-specific mortality risk is slightly higher than predicted by clinical features alone (Prolaris). Previous studies have shown that molecular testing in men with PCa provides information that influences clinical decisions. The findings reported here suggest that molecular testing may also be a vital component in the medical management of patients with complex clinical phenotypes and common chronic conditions. Additional studies are necessary to evaluate whether the finding reported here is typical of individuals diagnosed with PCa who also have a BRCA2 mutation.
For any new genomic test to be clinically useful, its results must have clinical actionability. In this case, the clinical decision point was whether to recommend immediate definitive treatment or active surveillance. For this patient, the Oncotype DX assay provided a likelihood of favorable surgical pathology of 71% (or conversely a 29% risk of unfavorable pathology); by comparison, the Prolaris CCP score provided a 3% estimate of PCaspecific
mortality at 10 years. A key question is: How do clinicians perceive the actionability of risk estimates for these different endpoints?
The current case illustrates the challenges that rapidly developing genomic medicine pose for physicians trying to optimize care and communicate results to patients in a meaningful and consistent manner. For example, some urologists find the different 2-tiered calculations confusing. When laboratories use proprietary scalesbased on internally develop algorithms, differing interpretations are to be expected. The risk-assessment tests described here use different algorithms, and their interpretations are based on clinical categories from different sets of guidelines. This underscores the need for better standardization of PCa care.23
Oncology and urology professional associations should collaborate to develop consistent guidelines for use of new technologies in the management of PCa. A positive example is the evolution of testing recommendations in lung cancer, which initially varied between professional entities. In April 2013, the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology jointly issued a unified clinical practice guideline on molecular testing in patients with lung cancer.24 In October 2014, the American Society of Clinical Oncology issued an endorsement of the CAP/IASLC/AMP guideline.25 As the number of complex tests being used in PCa increases, it will be important for professional associations such as AUA and NCCN to collaborate in evaluating utility of innovations to make consistent recommendations
Author disclosures
Myriad Genetics and Genomic Health provided funding for research on their tests within the VA. Dr. Dash and Dr. Lynch are principal investigators of the Genomic Health study. Dr. Lowrance is the principal investigator of the Myriad Genetics study.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
2. Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated November 10, 2015. Accessed December 8, 2016.
4. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
5. Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15-30.
6. Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14(3):409-414.
7. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354-1360.
8. Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11(3):117-126.
9. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11-15.
10. Leongamornlert D, Mahmud N, Tymrakiewicz M, et al; UKGPCS Collaborators. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701.
11. Kote-Jarai Z, Leongamornlert D, Saunders E, et al; UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234.
12. Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929-935.
13. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115-2121.
14. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
15. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193.
16. Bancroft EK, Page EC, Castro E, et al; IMPACT Collaborators. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-499.
17. Bratt O, Loman N. Clinical management of prostate cancer in men with BRCA mutations. Eur Urol. 2015;68(2):194-195.
18. Centers for Medicare & Medicaid Services (CMS). MCD archive site. CMS Website. http://localcoverage.cms.gov/mcd_archive/overview.aspx. Accessed January 8, 2016.
19. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550-560.
20. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2015;pii:S0022-5347(15)04811-9 [epub ahead of print].
21. Stone S, Cuzick JM, Fisher G, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. J Clin Oncol. 2015;33(suppl 15):e16040.
22. Lewin R, Rizel S, Hendler D, et al. Oncotype-DX recurrence score distribution among breast cancer patients harboring a germline mutation in the BRCA1/2 genes. J Clin Oncol. 2015;33(suppl; abstr 564).
23. Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate cancer. J Urol. 2008;180(2):451-459.
24. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
25. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
Note: Page numbers differ between the print issue and digital edition.
There are several risk assessment tools and clinical practice guidelines used in the management of localized prostate cancer (PCa). These include the D’Amico classification, the Cancer of the Prostate Risk Assessment (CAPRA) score, the National Comprehensive Cancer Network (NCCN) risk criteria, and the American Urological Association (AUA) clinical practice guidelines.1-4 None of these tools incorporate the BRCA1 and BRCA2 genes in the risk assessment or treatment recommendations for localized PCa.5 The BRCA mutations are most strongly associated with breast and ovarian cancer risk. However, BRCA mutations also increase susceptibility and disease progression in PCa.6 This article illustrates the current knowledge gap in PCa treatment algorithms for the BRCA2-positive patient population.
Traditional risk assessment tools use clinical and pathologic features of PCa, including prostate-specific antigen (PSA) level, Gleason score, tumor stage, and disease burden to measure cancer aggressiveness.1,7,8 These criteria are the basis of the AUA and NCCN guidelines for management of clinically localized PCa, which recognize 3 categories of clinically localized disease (low, intermediate, and high risk).3,4 The NCCN guidelines (version 1.2016) include a fourth category (very low risk or pathologically insignificant PCa) among some stage T1c patients, based on additional criteria, including PSA density. Both the AUA and NCCN recommend active surveillance as a treatment option for men with low-risk PCa. The NCCN recently revised its guidelines to state that intermediate-risk patients with PCa with favorable features (Gleason grade 3 and < 50% of positive biopsy cores) may also be considered for active surveillance.3
BRCA Mutations in Prostate Cancer
Estimates of the relative risk of PCa for men with BRCA1 and BRCA2 mutations have varied, but recent data suggest that it is 3.75-fold for BRCA1 mutations and 8.6-fold for BRCA2 by age 65 years.9-11 Moreover, PCas associated with BRCA1/2 mutations, particularly those in the BRCA2 gene, are often more aggressive and characterized by poor outcomes.12,13 The presence of a BRCA2 mutation is a negative prognostic factor in PCa, independent of tumor grade, stage, and PSA levels.14 Both PCa-specific survival and metastasis-free survival rates following surgical or radiation therapy are significantly lower in the BRCA mutation carriers than in noncarriers.15 Preliminary results of the IMPACT study demonstrate that targeted PCa screening in men with BRCA1 or BRCA2 mutations may result in identification of tumors more likely to require treatment.16
As a result of these increased risks, it is recommended that men with BRCA2 mutations begin PCa screening at age 40 years; however, there are no clear guidelines for clinical management of PCa in this group of patients.5 The lack of guidelines presents a challenge for clinical management of BRCA1/2 mutation carriers with localized PCa who otherwise qualify for active surveillance. A recent editorial by Bratt and Loman specifically
calls for aggressive therapy for patients who are BRCA positive, particularly BRCA2 carriers, suggesting the need to combine early radical local treatment with adjuvant systemic therapy.17 However, data on the effectiveness of aggressive therapies in patients with PCa who carry BRCA2 mutations are sparse.5
Genomic Test for Risk
There is growing recognition of the need to include molecular testing to improve risk assessment in PCa. Using traditional risk assessment tools, about 8% of low-risk patients are found to have progressive disease postoperatively.3 Current AUA guidelines from 2007 are silent on the issue of molecular testing. The 2015 and 2016 NCCN guidelines include molecular testing for better risk stratification of patients with PCa, specifically naming Oncotype DX Prostate Cancer Assay (Genomic Health, Redwood City, CA) and Prolaris (Myriad Genetics, Salt Lake City, UT).3 However, they do not address molecular BRCA mutation testing.
There are several genomic tests aimed at improving PCa risk assessment. These include Oncotype DX PCa Assay; Prolaris; Decipher Prostate Cancer Classifier (GenomeDx Biosciences, San Diego, CA); and ProMark (Metamark Laboratories, Cambridge, MA). These assays are tissuebased and measure gene expression on the RNA or protein level to identify low- or intermediate-risk patients who may be candidates for active surveillance, as well as patients at higher risk who may benefit from closer monitoring or additional therapy after their initial treatment. By 2015, the Centers for Medicare and Medicaid Services had issued positive coverage decisions for several tests.18
The Oncotype DX test is a quantitative real-time polymerase chain reaction assay that measures the expression of 17 genes (12 cancer-related genes and 5 reference genes) representing 4 biologic pathways, including from the androgen signaling, stromal response, cellular organization, and cellular proliferation (Table). Prolaris focuses on a larger number of genes in the cell-cycle progression (CCP) pathway (31 cell-cycle-related genes and 15 reference genes). There is no overlap between the 2 gene sets. Both tests integrate genomic data with clinical and histopathologic characteristics of the tumor to arrive at prognostic information. The Oncotype DX test yields a specific Genomic Prostate Score (GPS; scaled 0-100) that is integrated with the patient’s NCCN clinical risk group to quantify the likelihood of favorable pathology, which is defined as low-grade organ-confined disease.19 The Prolaris test uses the patient’s AUA risk category and then evaluates the patient’s risk based on the cell-cycle progression
gene panel compared with that risk category. It also provides an estimate of disease-specific mortality as validated by 2 independent cohorts that were managed conservatively initially with watchful waiting.
In this article, the authors present a case report of a BRCA2-positive veteran with newly diagnosed lowrisk PCa and a history of breast cancer. In addition to evaluating clinical criteria, Oncotype DX and Prolaris gene expression tests were ordered for this patient. The authors obtained veteran and institutional review board permission. To protect the identity of the patient, minor changes were made to patient demographics.
Case Presentation
A 68-year-old white man with a history of coronary artery disease, dyslipidemia, and hypertension, was recently diagnosed with PCa. He presented to Genomic Medicine Service to discuss how his BRCA2 mutation status might impact management decisions for PCa. Priorto the PCa diagnosis, the veteran had a history of breast and skin cancer. He was diagnosed with invasive ductal carcinoma of the right breast (ER+/PR+/Her2+) at age 62 years and treated with mastectomy and tamoxifen. He had testing at that time, which revealed a BRCA2 mutation: 3773delTT. Squamous cell carcinoma was detected on his right leg and removed at age 64 years. Basal cell carcinoma was removed from his left forehead first at age 65 years, and then residual basal cell carcinoma was removed from the forehead 2 months later.
The veteran was diagnosed with PCa at age 67 years at a non-VA clinic. The urology consult note reported a sudden increase of his PSA level to 5.9. A prostate needle biopsy was performed. The Gleason score was 3 + 3 = 6 in 2 of 12, with < 1% PCa involvement and focal highgrade prostatic intraepithelial neoplasia. The patient was asymptomatic, and his cancer was identified by needle biopsy due to elevated PSA. His clinical stage was T1c. According to AUA and NCCN guidelines, the patient was categorized as low risk, defined as Gleason Score ≤ 6, PSA < 10 ng/mL, and clinical stage up to T2a.3,4 Additionally, the veteran met 3 criteria for the NCCN very low-risk category (stage T1c, < 3 positive biopsy cores and ≤ 50% cancer in any core). However, because he was initially diagnosed at a non-VA clinic, his PSA density (the remaining criterion) was not available to the VA urologist. Therefore, the low-risk category was assumed for molecular test interpretation.
The non-VA urologist recommended active surveillance. The VA urologist agreed that active surveillance was an appropriate treatment recommendation at this time. However, the veteran and his family members remained concerned that his PCa might be more aggressive due to his BRCA2 mutation, and they worried that active surveillance would result in a worse outcome. Their concern was exacerbated by the veteran’s comorbidities, which could have potential implications on the timing of surgical options. The patient expressed these concerns to his VA primary care physician, who then referred him to the VA Genomic Medicine Services.
Genetic Consult
The genetic counselor scheduled a telegenetics consult and conducted an assessment of the veteran, which included a review of his medical history, mutation status, and relevant family history. The family history was consistent with hereditary breast/ovarian cancer. However, the primary reason the veteran underwent genetic testing was the diagnosis of breast cancer in a male. The genetic counselor provided the patient with information relevant to his mutation carrier status, including that men with BRCA2 mutations are at increased risk of developing more aggressive PCa, have higher rates of lymph node involvement, and greater mortality compared with men without BRCA2 mutations. The veteran was informed that there were no published guidelines that suggest PCa in BRCA2 carriers should be treated differently from sporadic PCa.
Tumor Testing Strategy
Although the veteran was comfortable with active surveillance at the time of consultation, he was concerned that, given his comorbidities, it would be better to pursue surgery sooner. The veteran asked the genetic counselor for more information about his prognosis given his BRCA2 status. The genetic counselor discussed possible use of tumor gene expression profiling and informed him about 2 active studies within the VA that are evaluating the clinical utility of gene expression tests for PCa risk stratification (Oncotype DX at Genomic Health and Prolaris at Myriad Genetic Laboratories). The veteran expressed an interest in having his biopsy tissue tested by both assays. Tumor biopsy tissue was obtained and sent to both Genomic Health and Myriad Genetics for testing. Neither test incorporated the veteran’s other health conditions or his BRCA mutation status into risk stratification results or the patient report.
Test Results
The Oncotype DX GPS result for this NCCN low-risk patient was 31 (Figure 1). This score corresponds to a likelihood of favorable pathology at radical prostatectomy of 71% (95% confidence interval [CI]: 63%-78%). Favorable pathology is defined as freedom from highgrade (Gleason score > 4+3) and/or nonorgan-confined (pT3) disease. This GPS result was consistent with the range of risk expected for NCCN low-risk patients based on the validation cohorts for the assay. The estimate of likelihood of favorable pathology would be modified if the PSA density result were available and if it placed the patient in the NCCN very low-risk category.
The Prolaris report demonstrated a score of 0.4 (Figure 2). This puts the veteran in the 94th percentile of contemporary U.S. men who are AUA low risk. The CCP score makes his cancer more aggressive than most AUA low-risk men, and the projected 10-year disease-specific mortality is 3%. In conjunction with the patient’s BRCA2 status, he may benefit from definitive intervention. If active surveillance is chosen, careful and regular follow-up for disease progression is mandated.
Interpretation of Genomic Testing in PCa
For both tests, the results are derived from 2-tiered calculations. For Oncotype DX, the gene expression measurement yields the GPS, which is then integrated with the patient’s clinical and pathologic information to yield the likelihood of favorable pathology. Although the Oncotype DX GPS is an independent measure of disease aggressiveness, on the patient report, the GPS is combined with the NCCN clinical risk group to provide a likelihood of favorable pathology. Therefore, 2 patients with the same GPS but different levels of clinical risk will have different likelihoods of favorable pathology.
The Prolaris test provides the Prolaris CCP score as well as the percentile group of patients with a lower score within the same risk category. Also, the Prolaris test yields a numerical 10-year PCa-specific mortality risk. The Prolaris score has been shown to impact therapeutic decisions in patients with newly diagnosed PCa.20
Recently, Myriad defined a threshold for active surveillance combining the CCP and CAPRA scores.21 Myriad validated this cutoff in 2 cohorts of men initially managed conservatively. Although the model predicts up to 3.2% disease-specific mortality, there were no observed deaths during a decade of follow-up. Myriad reports that by using this cutoff in contemporary patients tested commercially with Prolaris, a health care system could increase the percentage of men who would fit current criteria for active surveillance from 36% to 60% with no increase in risk of disease-specific mortality.
The results of these 2 tests are presented in 2 different formats and provide risk estimates for different clinical endpoints, making it challenging for a clinician to directly compare them. Moreover, each genomic test is based on a different set of genes and uses different clinical risk criteria (AUA vs NCCN), which may result in different test output. Finally, and most relevant to the case described here, there is no evidence-based consensus on how to interpret these test results in the context of a BRCA2 mutation.
Based on the published literature reporting that BRCA2 mutations are associated with more aggressive disease, one prediction would be that test scores from genomic assays such as Oncotype DX and Prolaris would tend to be higher in BRCA2 carriers than those of the overall population of PCa patients. This has, in fact, been reported for the Oncotype DX Breast Cancer Assay recurrence score in women who are BRCA carriers.22 Further research is required to ascertain whether this will be true for Oncotype DX GPS and Prolaris CCP score in PCa. The mechanism of action that predisposes BRCA2 mutation carriers to develop a more aggressive variant of PCa may not be detectable by the genomic markers included in the Oncotype DX PCa and Prolaris tests. The degree to which a mutated BRCA2 gene may interact with the genes comprising these assays and the reported tumor aggressiveness is not yet understood but deserving of future study.
Treatment Recommendation and Patient’s Decision
After considering his test results, the veteran chose active surveillance. The sum of clinical, pathologic, and molecular factors, combined with the patient’s preference, determined his course of treatment. Because prostatectomy was not performed, it has not been positively determined whether or not the patient harbors aggressive disease. As the molecular test results place the patient at the high end of the low-risk group, the VA urologist recommended close monitoring and suggested a follow-up biopsy with magnetic resonance-ultrasound fusion guidance.
Conclusions
Molecular testing found that the patient’s PCa stage and grade are consistent with NCCN low risk (Oncotype DX) and that the disease-specific mortality risk is slightly higher than predicted by clinical features alone (Prolaris). Previous studies have shown that molecular testing in men with PCa provides information that influences clinical decisions. The findings reported here suggest that molecular testing may also be a vital component in the medical management of patients with complex clinical phenotypes and common chronic conditions. Additional studies are necessary to evaluate whether the finding reported here is typical of individuals diagnosed with PCa who also have a BRCA2 mutation.
For any new genomic test to be clinically useful, its results must have clinical actionability. In this case, the clinical decision point was whether to recommend immediate definitive treatment or active surveillance. For this patient, the Oncotype DX assay provided a likelihood of favorable surgical pathology of 71% (or conversely a 29% risk of unfavorable pathology); by comparison, the Prolaris CCP score provided a 3% estimate of PCaspecific
mortality at 10 years. A key question is: How do clinicians perceive the actionability of risk estimates for these different endpoints?
The current case illustrates the challenges that rapidly developing genomic medicine pose for physicians trying to optimize care and communicate results to patients in a meaningful and consistent manner. For example, some urologists find the different 2-tiered calculations confusing. When laboratories use proprietary scalesbased on internally develop algorithms, differing interpretations are to be expected. The risk-assessment tests described here use different algorithms, and their interpretations are based on clinical categories from different sets of guidelines. This underscores the need for better standardization of PCa care.23
Oncology and urology professional associations should collaborate to develop consistent guidelines for use of new technologies in the management of PCa. A positive example is the evolution of testing recommendations in lung cancer, which initially varied between professional entities. In April 2013, the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology jointly issued a unified clinical practice guideline on molecular testing in patients with lung cancer.24 In October 2014, the American Society of Clinical Oncology issued an endorsement of the CAP/IASLC/AMP guideline.25 As the number of complex tests being used in PCa increases, it will be important for professional associations such as AUA and NCCN to collaborate in evaluating utility of innovations to make consistent recommendations
Author disclosures
Myriad Genetics and Genomic Health provided funding for research on their tests within the VA. Dr. Dash and Dr. Lynch are principal investigators of the Genomic Health study. Dr. Lowrance is the principal investigator of the Myriad Genetics study.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
There are several risk assessment tools and clinical practice guidelines used in the management of localized prostate cancer (PCa). These include the D’Amico classification, the Cancer of the Prostate Risk Assessment (CAPRA) score, the National Comprehensive Cancer Network (NCCN) risk criteria, and the American Urological Association (AUA) clinical practice guidelines.1-4 None of these tools incorporate the BRCA1 and BRCA2 genes in the risk assessment or treatment recommendations for localized PCa.5 The BRCA mutations are most strongly associated with breast and ovarian cancer risk. However, BRCA mutations also increase susceptibility and disease progression in PCa.6 This article illustrates the current knowledge gap in PCa treatment algorithms for the BRCA2-positive patient population.
Traditional risk assessment tools use clinical and pathologic features of PCa, including prostate-specific antigen (PSA) level, Gleason score, tumor stage, and disease burden to measure cancer aggressiveness.1,7,8 These criteria are the basis of the AUA and NCCN guidelines for management of clinically localized PCa, which recognize 3 categories of clinically localized disease (low, intermediate, and high risk).3,4 The NCCN guidelines (version 1.2016) include a fourth category (very low risk or pathologically insignificant PCa) among some stage T1c patients, based on additional criteria, including PSA density. Both the AUA and NCCN recommend active surveillance as a treatment option for men with low-risk PCa. The NCCN recently revised its guidelines to state that intermediate-risk patients with PCa with favorable features (Gleason grade 3 and < 50% of positive biopsy cores) may also be considered for active surveillance.3
BRCA Mutations in Prostate Cancer
Estimates of the relative risk of PCa for men with BRCA1 and BRCA2 mutations have varied, but recent data suggest that it is 3.75-fold for BRCA1 mutations and 8.6-fold for BRCA2 by age 65 years.9-11 Moreover, PCas associated with BRCA1/2 mutations, particularly those in the BRCA2 gene, are often more aggressive and characterized by poor outcomes.12,13 The presence of a BRCA2 mutation is a negative prognostic factor in PCa, independent of tumor grade, stage, and PSA levels.14 Both PCa-specific survival and metastasis-free survival rates following surgical or radiation therapy are significantly lower in the BRCA mutation carriers than in noncarriers.15 Preliminary results of the IMPACT study demonstrate that targeted PCa screening in men with BRCA1 or BRCA2 mutations may result in identification of tumors more likely to require treatment.16
As a result of these increased risks, it is recommended that men with BRCA2 mutations begin PCa screening at age 40 years; however, there are no clear guidelines for clinical management of PCa in this group of patients.5 The lack of guidelines presents a challenge for clinical management of BRCA1/2 mutation carriers with localized PCa who otherwise qualify for active surveillance. A recent editorial by Bratt and Loman specifically
calls for aggressive therapy for patients who are BRCA positive, particularly BRCA2 carriers, suggesting the need to combine early radical local treatment with adjuvant systemic therapy.17 However, data on the effectiveness of aggressive therapies in patients with PCa who carry BRCA2 mutations are sparse.5
Genomic Test for Risk
There is growing recognition of the need to include molecular testing to improve risk assessment in PCa. Using traditional risk assessment tools, about 8% of low-risk patients are found to have progressive disease postoperatively.3 Current AUA guidelines from 2007 are silent on the issue of molecular testing. The 2015 and 2016 NCCN guidelines include molecular testing for better risk stratification of patients with PCa, specifically naming Oncotype DX Prostate Cancer Assay (Genomic Health, Redwood City, CA) and Prolaris (Myriad Genetics, Salt Lake City, UT).3 However, they do not address molecular BRCA mutation testing.
There are several genomic tests aimed at improving PCa risk assessment. These include Oncotype DX PCa Assay; Prolaris; Decipher Prostate Cancer Classifier (GenomeDx Biosciences, San Diego, CA); and ProMark (Metamark Laboratories, Cambridge, MA). These assays are tissuebased and measure gene expression on the RNA or protein level to identify low- or intermediate-risk patients who may be candidates for active surveillance, as well as patients at higher risk who may benefit from closer monitoring or additional therapy after their initial treatment. By 2015, the Centers for Medicare and Medicaid Services had issued positive coverage decisions for several tests.18
The Oncotype DX test is a quantitative real-time polymerase chain reaction assay that measures the expression of 17 genes (12 cancer-related genes and 5 reference genes) representing 4 biologic pathways, including from the androgen signaling, stromal response, cellular organization, and cellular proliferation (Table). Prolaris focuses on a larger number of genes in the cell-cycle progression (CCP) pathway (31 cell-cycle-related genes and 15 reference genes). There is no overlap between the 2 gene sets. Both tests integrate genomic data with clinical and histopathologic characteristics of the tumor to arrive at prognostic information. The Oncotype DX test yields a specific Genomic Prostate Score (GPS; scaled 0-100) that is integrated with the patient’s NCCN clinical risk group to quantify the likelihood of favorable pathology, which is defined as low-grade organ-confined disease.19 The Prolaris test uses the patient’s AUA risk category and then evaluates the patient’s risk based on the cell-cycle progression
gene panel compared with that risk category. It also provides an estimate of disease-specific mortality as validated by 2 independent cohorts that were managed conservatively initially with watchful waiting.
In this article, the authors present a case report of a BRCA2-positive veteran with newly diagnosed lowrisk PCa and a history of breast cancer. In addition to evaluating clinical criteria, Oncotype DX and Prolaris gene expression tests were ordered for this patient. The authors obtained veteran and institutional review board permission. To protect the identity of the patient, minor changes were made to patient demographics.
Case Presentation
A 68-year-old white man with a history of coronary artery disease, dyslipidemia, and hypertension, was recently diagnosed with PCa. He presented to Genomic Medicine Service to discuss how his BRCA2 mutation status might impact management decisions for PCa. Priorto the PCa diagnosis, the veteran had a history of breast and skin cancer. He was diagnosed with invasive ductal carcinoma of the right breast (ER+/PR+/Her2+) at age 62 years and treated with mastectomy and tamoxifen. He had testing at that time, which revealed a BRCA2 mutation: 3773delTT. Squamous cell carcinoma was detected on his right leg and removed at age 64 years. Basal cell carcinoma was removed from his left forehead first at age 65 years, and then residual basal cell carcinoma was removed from the forehead 2 months later.
The veteran was diagnosed with PCa at age 67 years at a non-VA clinic. The urology consult note reported a sudden increase of his PSA level to 5.9. A prostate needle biopsy was performed. The Gleason score was 3 + 3 = 6 in 2 of 12, with < 1% PCa involvement and focal highgrade prostatic intraepithelial neoplasia. The patient was asymptomatic, and his cancer was identified by needle biopsy due to elevated PSA. His clinical stage was T1c. According to AUA and NCCN guidelines, the patient was categorized as low risk, defined as Gleason Score ≤ 6, PSA < 10 ng/mL, and clinical stage up to T2a.3,4 Additionally, the veteran met 3 criteria for the NCCN very low-risk category (stage T1c, < 3 positive biopsy cores and ≤ 50% cancer in any core). However, because he was initially diagnosed at a non-VA clinic, his PSA density (the remaining criterion) was not available to the VA urologist. Therefore, the low-risk category was assumed for molecular test interpretation.
The non-VA urologist recommended active surveillance. The VA urologist agreed that active surveillance was an appropriate treatment recommendation at this time. However, the veteran and his family members remained concerned that his PCa might be more aggressive due to his BRCA2 mutation, and they worried that active surveillance would result in a worse outcome. Their concern was exacerbated by the veteran’s comorbidities, which could have potential implications on the timing of surgical options. The patient expressed these concerns to his VA primary care physician, who then referred him to the VA Genomic Medicine Services.
Genetic Consult
The genetic counselor scheduled a telegenetics consult and conducted an assessment of the veteran, which included a review of his medical history, mutation status, and relevant family history. The family history was consistent with hereditary breast/ovarian cancer. However, the primary reason the veteran underwent genetic testing was the diagnosis of breast cancer in a male. The genetic counselor provided the patient with information relevant to his mutation carrier status, including that men with BRCA2 mutations are at increased risk of developing more aggressive PCa, have higher rates of lymph node involvement, and greater mortality compared with men without BRCA2 mutations. The veteran was informed that there were no published guidelines that suggest PCa in BRCA2 carriers should be treated differently from sporadic PCa.
Tumor Testing Strategy
Although the veteran was comfortable with active surveillance at the time of consultation, he was concerned that, given his comorbidities, it would be better to pursue surgery sooner. The veteran asked the genetic counselor for more information about his prognosis given his BRCA2 status. The genetic counselor discussed possible use of tumor gene expression profiling and informed him about 2 active studies within the VA that are evaluating the clinical utility of gene expression tests for PCa risk stratification (Oncotype DX at Genomic Health and Prolaris at Myriad Genetic Laboratories). The veteran expressed an interest in having his biopsy tissue tested by both assays. Tumor biopsy tissue was obtained and sent to both Genomic Health and Myriad Genetics for testing. Neither test incorporated the veteran’s other health conditions or his BRCA mutation status into risk stratification results or the patient report.
Test Results
The Oncotype DX GPS result for this NCCN low-risk patient was 31 (Figure 1). This score corresponds to a likelihood of favorable pathology at radical prostatectomy of 71% (95% confidence interval [CI]: 63%-78%). Favorable pathology is defined as freedom from highgrade (Gleason score > 4+3) and/or nonorgan-confined (pT3) disease. This GPS result was consistent with the range of risk expected for NCCN low-risk patients based on the validation cohorts for the assay. The estimate of likelihood of favorable pathology would be modified if the PSA density result were available and if it placed the patient in the NCCN very low-risk category.
The Prolaris report demonstrated a score of 0.4 (Figure 2). This puts the veteran in the 94th percentile of contemporary U.S. men who are AUA low risk. The CCP score makes his cancer more aggressive than most AUA low-risk men, and the projected 10-year disease-specific mortality is 3%. In conjunction with the patient’s BRCA2 status, he may benefit from definitive intervention. If active surveillance is chosen, careful and regular follow-up for disease progression is mandated.
Interpretation of Genomic Testing in PCa
For both tests, the results are derived from 2-tiered calculations. For Oncotype DX, the gene expression measurement yields the GPS, which is then integrated with the patient’s clinical and pathologic information to yield the likelihood of favorable pathology. Although the Oncotype DX GPS is an independent measure of disease aggressiveness, on the patient report, the GPS is combined with the NCCN clinical risk group to provide a likelihood of favorable pathology. Therefore, 2 patients with the same GPS but different levels of clinical risk will have different likelihoods of favorable pathology.
The Prolaris test provides the Prolaris CCP score as well as the percentile group of patients with a lower score within the same risk category. Also, the Prolaris test yields a numerical 10-year PCa-specific mortality risk. The Prolaris score has been shown to impact therapeutic decisions in patients with newly diagnosed PCa.20
Recently, Myriad defined a threshold for active surveillance combining the CCP and CAPRA scores.21 Myriad validated this cutoff in 2 cohorts of men initially managed conservatively. Although the model predicts up to 3.2% disease-specific mortality, there were no observed deaths during a decade of follow-up. Myriad reports that by using this cutoff in contemporary patients tested commercially with Prolaris, a health care system could increase the percentage of men who would fit current criteria for active surveillance from 36% to 60% with no increase in risk of disease-specific mortality.
The results of these 2 tests are presented in 2 different formats and provide risk estimates for different clinical endpoints, making it challenging for a clinician to directly compare them. Moreover, each genomic test is based on a different set of genes and uses different clinical risk criteria (AUA vs NCCN), which may result in different test output. Finally, and most relevant to the case described here, there is no evidence-based consensus on how to interpret these test results in the context of a BRCA2 mutation.
Based on the published literature reporting that BRCA2 mutations are associated with more aggressive disease, one prediction would be that test scores from genomic assays such as Oncotype DX and Prolaris would tend to be higher in BRCA2 carriers than those of the overall population of PCa patients. This has, in fact, been reported for the Oncotype DX Breast Cancer Assay recurrence score in women who are BRCA carriers.22 Further research is required to ascertain whether this will be true for Oncotype DX GPS and Prolaris CCP score in PCa. The mechanism of action that predisposes BRCA2 mutation carriers to develop a more aggressive variant of PCa may not be detectable by the genomic markers included in the Oncotype DX PCa and Prolaris tests. The degree to which a mutated BRCA2 gene may interact with the genes comprising these assays and the reported tumor aggressiveness is not yet understood but deserving of future study.
Treatment Recommendation and Patient’s Decision
After considering his test results, the veteran chose active surveillance. The sum of clinical, pathologic, and molecular factors, combined with the patient’s preference, determined his course of treatment. Because prostatectomy was not performed, it has not been positively determined whether or not the patient harbors aggressive disease. As the molecular test results place the patient at the high end of the low-risk group, the VA urologist recommended close monitoring and suggested a follow-up biopsy with magnetic resonance-ultrasound fusion guidance.
Conclusions
Molecular testing found that the patient’s PCa stage and grade are consistent with NCCN low risk (Oncotype DX) and that the disease-specific mortality risk is slightly higher than predicted by clinical features alone (Prolaris). Previous studies have shown that molecular testing in men with PCa provides information that influences clinical decisions. The findings reported here suggest that molecular testing may also be a vital component in the medical management of patients with complex clinical phenotypes and common chronic conditions. Additional studies are necessary to evaluate whether the finding reported here is typical of individuals diagnosed with PCa who also have a BRCA2 mutation.
For any new genomic test to be clinically useful, its results must have clinical actionability. In this case, the clinical decision point was whether to recommend immediate definitive treatment or active surveillance. For this patient, the Oncotype DX assay provided a likelihood of favorable surgical pathology of 71% (or conversely a 29% risk of unfavorable pathology); by comparison, the Prolaris CCP score provided a 3% estimate of PCaspecific
mortality at 10 years. A key question is: How do clinicians perceive the actionability of risk estimates for these different endpoints?
The current case illustrates the challenges that rapidly developing genomic medicine pose for physicians trying to optimize care and communicate results to patients in a meaningful and consistent manner. For example, some urologists find the different 2-tiered calculations confusing. When laboratories use proprietary scalesbased on internally develop algorithms, differing interpretations are to be expected. The risk-assessment tests described here use different algorithms, and their interpretations are based on clinical categories from different sets of guidelines. This underscores the need for better standardization of PCa care.23
Oncology and urology professional associations should collaborate to develop consistent guidelines for use of new technologies in the management of PCa. A positive example is the evolution of testing recommendations in lung cancer, which initially varied between professional entities. In April 2013, the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology jointly issued a unified clinical practice guideline on molecular testing in patients with lung cancer.24 In October 2014, the American Society of Clinical Oncology issued an endorsement of the CAP/IASLC/AMP guideline.25 As the number of complex tests being used in PCa increases, it will be important for professional associations such as AUA and NCCN to collaborate in evaluating utility of innovations to make consistent recommendations
Author disclosures
Myriad Genetics and Genomic Health provided funding for research on their tests within the VA. Dr. Dash and Dr. Lynch are principal investigators of the Genomic Health study. Dr. Lowrance is the principal investigator of the Myriad Genetics study.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
2. Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated November 10, 2015. Accessed December 8, 2016.
4. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
5. Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15-30.
6. Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14(3):409-414.
7. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354-1360.
8. Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11(3):117-126.
9. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11-15.
10. Leongamornlert D, Mahmud N, Tymrakiewicz M, et al; UKGPCS Collaborators. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701.
11. Kote-Jarai Z, Leongamornlert D, Saunders E, et al; UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234.
12. Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929-935.
13. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115-2121.
14. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
15. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193.
16. Bancroft EK, Page EC, Castro E, et al; IMPACT Collaborators. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-499.
17. Bratt O, Loman N. Clinical management of prostate cancer in men with BRCA mutations. Eur Urol. 2015;68(2):194-195.
18. Centers for Medicare & Medicaid Services (CMS). MCD archive site. CMS Website. http://localcoverage.cms.gov/mcd_archive/overview.aspx. Accessed January 8, 2016.
19. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550-560.
20. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2015;pii:S0022-5347(15)04811-9 [epub ahead of print].
21. Stone S, Cuzick JM, Fisher G, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. J Clin Oncol. 2015;33(suppl 15):e16040.
22. Lewin R, Rizel S, Hendler D, et al. Oncotype-DX recurrence score distribution among breast cancer patients harboring a germline mutation in the BRCA1/2 genes. J Clin Oncol. 2015;33(suppl; abstr 564).
23. Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate cancer. J Urol. 2008;180(2):451-459.
24. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
25. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
Note: Page numbers differ between the print issue and digital edition.
1. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-974.
2. Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173(6):1938-1942.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: prostate cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated November 10, 2015. Accessed December 8, 2016.
4. Thompson I, Thrasher JB, Aus G, et al; AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
5. Alanee SR, Glogowski EA, Schrader KA, Eastham JA, Offit K. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed). 2014;6:15-30.
6. Castro E, Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J Androl. 2012;14(3):409-414.
7. Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354-1360.
8. Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11(3):117-126.
9. Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11-15.
10. Leongamornlert D, Mahmud N, Tymrakiewicz M, et al; UKGPCS Collaborators. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701.
11. Kote-Jarai Z, Leongamornlert D, Saunders E, et al; UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234.
12. Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929-935.
13. Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16(7):2115-2121.
14. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757.
15. Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193.
16. Bancroft EK, Page EC, Castro E, et al; IMPACT Collaborators. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66(3):489-499.
17. Bratt O, Loman N. Clinical management of prostate cancer in men with BRCA mutations. Eur Urol. 2015;68(2):194-195.
18. Centers for Medicare & Medicaid Services (CMS). MCD archive site. CMS Website. http://localcoverage.cms.gov/mcd_archive/overview.aspx. Accessed January 8, 2016.
19. Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550-560.
20. Shore ND, Kella N, Moran B, et al. Impact of the cell cycle progression test on physician and patient treatment selection for localized prostate cancer. J Urol. 2015;pii:S0022-5347(15)04811-9 [epub ahead of print].
21. Stone S, Cuzick JM, Fisher G, et al. Validation of an active surveillance threshold for the CCP score in conservatively managed men with localized prostate cancer. J Clin Oncol. 2015;33(suppl 15):e16040.
22. Lewin R, Rizel S, Hendler D, et al. Oncotype-DX recurrence score distribution among breast cancer patients harboring a germline mutation in the BRCA1/2 genes. J Clin Oncol. 2015;33(suppl; abstr 564).
23. Dahm P, Yeung LL, Chang SS, Cookson MS. A critical review of clinical practice guidelines for the management of clinically localized prostate cancer. J Urol. 2008;180(2):451-459.
24. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
25. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
Note: Page numbers differ between the print issue and digital edition.
A Patient Navigation Model for Veterans Traveling for Cancer Care
The VHA has a unique responsibility to provide excellent, patient-centered care to the veterans who have served the U.S. long after their active military service has ended. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely and efficient health care. The need to travel for cancer care, sometimes for long distances over long periods, adds an additional disparity and puts veterans at higher risk for delays in care. Cancer care navigation teams (CCNTs) were established at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, and throughout the Veterans Integrated Service Network, region 20 (VISN 20), which consists of a large geographical area that includes Alaska, Washington, Oregon, Idaho and one county in both Montana and California. These teams use an interdisciplinary approach to providing personalized assistance, support, and resources to veterans with cancer and their families who require travel for cancer care.
The CCNTs identify and minimize clinical and psychosocial barriers throughout the cancer care continuum. Although structured to address the unique needs and barriers of the veteran population within the VA, CCNT may also be used as a model for patients receiving cancer care within other complex and decentralized health care systems.
Patient Navigation in Cancer Care
The term navigation in the context of cancer care originated in 1990 at Harlem Hospital Center in New York City. The term described an intervention to address barriers to care experienced by a population of low income African American women with breast cancer. By applying patient navigation in addition to offering free and low-cost breast cancer screening and exams for high-risk patients, the 5-year survival rate in this disadvantaged population of women increased from 39% to 70%.1
Since then, navigation programs in cancer care have been adopted in health care settings around the world. Many different models have been described within the literature.2-5 Patient navigation is perhaps best recognized as a means to decrease health disparities by addressing barriers to health care, which may include lack of insurance, poverty, medical or psychiatric comorbidities, low health literacy, food insecurity, and homelessness. By identifying and addressing these barriers to care in high-risk populations, patient navigation programs have demonstrated positive outcomes, including improvement in cancer screening rates, timeliness of care, medication adherence, and patient satisfaction.6-10 Although there is a large amount of literature on navigation in cancer care, there is minimal literature that focuses on navigation in the veteran population and health care system.
Barriers to Cancer Care
The VA is a national health care system composed of community clinics, hospitals, and major referral centers that deliver comprehensive health care to veterans. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely, efficient health care. Research studies have documented significant differences among veterans receiving health care through the VHA compared with veterans who receive health care from other sources. Veterans enrolled at the VA are more likely to be poorer, older, African American, less well educated, unemployed or underemployed, lack social support, and in poorer physical and mental health compared with the general population or with veterans who do not use VA health care.11-13 Such health disparities have been linked to delays in timely access to health care.11
In a study comparing an age-adjusted ambulatory care population with veterans receiving care at the VA, VA patients were also found to be 3 times more likely to have ever been diagnosed with cancer.12 Exposures to carcinogens during their military service, such as Agent Orange, may contribute to this difference.14 Veterans have higher rates of posttraumatic stress disorder (PTSD) and other mental health disorders from military combat experiences or other traumas; these conditions can be exacerbated by the distress of a cancer diagnosis.15-17
Veterans requiring specialty care, such as cancer-related care, are referred within the VISN and may need to travel long distances in to access these specialty providers. Continuity of care is challenged during cancer diagnosis, staging, treatment, and surveillance when some aspects of care may be completed at geographically distant sites or by community providers if unavailable through the local VA. Appointments for care occur within each specialty service, and staff and clinic availability limit scheduling. There are no formal mechanisms for coordinating visits for efficiency or minimizing travel burden. The electronic medical record (EMR) at the VA can be helpful in accessing information from remote locations but does not easily integrate medical information from different facilities. Clinical data, such as recommendations for follow-up care, may take time and patience to access.
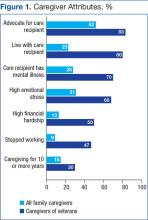
These challenges to the delivery of timely, efficient, patient-centered cancer care were documented in a cancer needs assessment performed in 2012 across VISN 20 (Figure 1). In response, a 3-year pilot program was initiated to implement a network of CCNTs in 8 VA facilities across the region.
Planning and Implementation
The VAPSHCS is a major referral center for cancer care that serves veterans living in VISN 20. On average, about 1,000 new cancers are diagnosed, and VAPSHCS sees 2,000 unique veterans for cancer care annually (Figure 2).One-quarter of these veterans are from out of state. For veterans living in Washington, nearly half traveled 50 miles or more to access cancer services at VAPSHCS. VA Puget Sound implemented its CCNT in the fall of 2014 and consists of an advanced practice registered nurse practitioner (ARNP), registered nurse (RN), social worker (SW), and program support assistant (PSA).
Veterans in identified priority cohorts thought to be at highest risk for barriers to cancer care are enrolled in navigation services. These priority groups include those veterans referred from another regional VA facility, those living more than 100 miles from the VAPSHCS, those referred for multimodality care (eg, surgery with neoadjuvant chemoradiation), and those with significant psychosocial barriers to care. Veterans are identified by the CCNT through a formal consult, notification from the CCNT at another VA facility, a cancer conference, a review of pathology results, and in some cases by veteran self-referral.
As it develops further capacity, CCNT will add other high-risk groups. Ideally, CCNT will eventually be a resource all veterans referred to VAPSHCS for cancer care, so all veterans may be assessed for potential barriers to care and be provided with much needed support and resources.
The CCNT is proactive and systematic in its navigation processes. Where possible, CCNT members are cross-trained to provide role coverage. The team reviews medical records for veterans actively enrolled in CCNT services weekly, to identify new barriers to care and address them in a timely manner. A robust data tracking system (created using a relational database) allows for storage of updated patient information and assigns tasks within the team, tracks upcoming appointments to support coordination, identifies travel and lodging needs, and assures follow-up care is completed. It also generates lists used for routine rounding on patient groups, treatment summary reports, and survivorship care plans.
The CCNT uses standardized assessment tools, including a navigation intake form, the National Comprehensive Cancer Network (NCCN) Distress Thermometer, and a functional assessment. Communication is an essential part of the navigation team, which addresses veteran’s identified needs by conducting weekly rounds within the interdisciplinary team to share information and collaborate.
The team has weekly telephone calls with its CCNTs from referring facilities to discuss veterans at all stages of the cancer continuum and facilitate transfer of information between facilities and providers, including needed diagnostic services and follow-up recommendations. The CCNT also facilitates communication with PSHCS specialty services by actively participating in multidisciplinary rounds and cancer conference.
Finally, although the CCNT follows individual veterans, the team also recognizes its role in identifying and addressing system barriers to cancer care. Collaborating with its partners within the facility and across the network, the team has improved access to services, created teaching tools that can be shared across disciplines, and implemented new procedures and policies to meet the American College of Surgeons Commission on Cancer accreditation standards and improve the cancer care system as a whole.
VAPSHCS Cancer Navigation Model
The VAPSHCS cancer navigation model is divided into 4 main processes based on the cancer care continuum. To illustrate this navigation model, this paper follows the journey of a 57-year-old male veteran referred to PSHCS with newly diagnosed head and neck cancer. He is divorced, with very little social support and lives in a remote area about 60 miles from his primary VA facility and more than 400 miles from PSHCS. His case was presented at the PSHCS facility cancer conference, where concurrent chemotherapy and radiation was recommended. This particular treatment consists of daily radiation and weekly chemotherapy over 6 to 7 weeks. The CCNT staff recognized that this veteran met criteria for navigation, entered him in the tracking database, and notified his referring facility CCNT of the plan of care.
Preconsult
Prior to veterans traveling to VAPSHCS for a new diagnosis or suspicion of cancer, the first goal is to identify any potential barriers to travel. It is a financial burden for many veterans to travel, and in the past, travel has prevented veterans from attending their specialty consult appointments. It is the role of the CCNT PSA to contact the veteran by telephone, introduce their services, provide education about available travel and lodging benefits, and schedule a visit with the CCNT RN to coincide with the veteran’s scheduled other specialty appointments.
In this case, the CCNT PSA contacted the veteran with information about the VAPSHCS, placed a lodging consult to arrange hotel accommodations for the veteran while in Seattle, and provided information regarding transportation from the hotel to the VA. The CCNT also identified that the veteran required a radiation oncology consultation and dental evaluation to proceed with a treatment plan. To decrease travel burden with additional trips to Seattle, the PSA contacted these specialty services to schedule the appointments. The PSA then assembled and mailed a packet of information to the veteran, which included details about how to pack and prepare for the trip, a facility map, and a hotel shuttle schedule.
Consult Visit and Planning
When veterans arrive at VAPSHCS, the CCNT RN meets them and completes an intake form. This standardized questionnaire identifies potential barriers to cancer care and supports the need for referrals to services, such as a dietitian, chaplain, palliative care, social work, physical and occupational therapy, travel, or lodging.
During this visit, the CCNT RN also asks the veteran to complete a NCCN Distress Thermometer. This thermometer assessment tool screens for physical, emotional, and practical needs that are specific to cancer. In this particular veteran’s situation, the distress level was 7 out of 10 (a score of 4 or greater triggersan automatic consult to social work once the results are entered in the EMR). Based on the outcomes information obtained from the intake form and NCCN Distress Thermometer, the CCNT RN made referrals to SW, chaplain services, and the oncology dietitian.
During the CCNT RN visit, nurse identified that the veteran’s financial situation had changed significantly resulting in less income and causing financial distress. The veteran was encouraged to complete an updated benefit renewal form with the SW that would likely eliminate his required copays for medical visits and prescription medications during the 6 weeks of chemotherapy and radiation. This need was communicated to the CCNT SW. The RN provided the veteran with information about VA resources to support him during cancer treatment, including meal options and support groups for both veterans and caregivers. They discussed the likely plan of care, including disease progress, information on prescribed drugs, dental evaluation and extractions as needed, placement of a feeding tube and a central line, and gave the veteran written brochures to review at his convenience. The RN also reviewed the logistics of a prolonged stay for the recommended course of chemotherapy and radiation.
During the initial CCNT intake process, the RN identified that the veteran would be without a caregiver and would be staying alone in lodging throughout his cancer treatment. The RN then completed a functional assessment of safety risks while lodging alone during this extended time. This brief questionnaire identifies any deficits in a veteran’s activity of daily living that may influence safety while lodging alone. The assessment is documented in the EMR, and if any concerns are identified, these are discussed with the veteran and a team of medical providers. If necessary, interventions are put into place before the veteran’s return for treatment. Potential safeguards may include obtaining safety equipment (eg, walker and bath chair), identifying an appropriate caregiver, or referring the veteran to a skilled nursing facility for the duration of treatment.
Following the veteran’s consultation visits, he went home with a return date 2 weeks later to start treatment. The VAPSHCS CCNT discussed the plan of care with his local CCNT, which facilitated placement of his feeding tube and addressed other symptom management concerns. The local CCNT SW completed advanced directives with the veteran and coordinated his travel back to VAPSHCS to begin treatment.
During Treatment
Veterans traveling from other VA facilities are away from their primary care providers (PCPs) for a number of weeks. Other specialty providers see a veteran during cancer treatment; however, the CCNT ARNP supports primary care needs while the veteran is away from their home VA facility. The ARNP is able to address chronic or acute medical issues before the start of treatment to prevent delays in cancer care.
Once the veteran returned to VAPSHCS to initiate therapy, the CCNT ARNP completed a history and physical examination to identify and address any active medical problems and document past medical history and current medication list in the EMR. This provides easy access to a thorough and complete baseline to both the oncology and radiation oncology providers. The ARNP examination revealed a new neck wound on the veteran, likely related to his cancer, and an urgent consult was placed to wound care. The otolaryngology, oncology, and radiation oncology departments were alerted to this development so they could assess the patient and adjust treatment plans as necessary. The veteran also required a refill of his blood pressure medication and had a number of questions regarding his upcoming treatment, which were addressed during the visit.
Within the first 2 days of the veteran’s return, he was scheduled to meet with the CCNT SW who reviewed and documented his advanced directive within the system, assessed his distress, provided therapeutic counseling, and completed the health benefit renewal form. Given the veteran’s financial status, the SW was able to help him apply for financial hardship to cover the costs of the care he had already received and assisted him with securing an appointment with the Social Security Administration (SSA) for disability benefits. The CCNT SW then helped the veteran complete a phone interview with the SSA and complete the application process. The SW also helped him complete the application for VA service-connected compensation and pension disability benefits.
Throughout his treatment course, the CCNT continued to be a resource for the veteran. Because he had PTSD and was uncomfortable attending support groups, the CCNT SW met with him weekly to provide counseling and psychosocial support. He stopped by the CCNT office on several occasions to report how he was doing, and the team provided assistance in obtaining supplies for his feeding tube and managing a complication that arose with his lodging. In preparation for his treatment completion and return home, the VAPSHCS CCNT communicated with his local CCNT to describe follow-up needs and ensure appropriate medical visits were scheduled. His travel home was arranged by the VAPSHCS PSA.
Treatment Completion
Before leaving VAPSHCS, the veteran was scheduled and seen in the clinic by the ARNP, where he received a written comprehensive treatment summary. The summary documented his cancer diagnosis, treatment, complications, and recommendations for follow-up care. He had the opportunity to ask questions about his treatment, and a clinical assessment was made for adverse effects. Appropriate interventions also were identified and addressed. A comprehensive treatment summary note was documented in the EMR and sent to his PCP and other medical specialists at his home facility to assure continuity of care.
The VAPSHCS CCNT continued to communicate weekly with the veteran’s home CCNT following his return, to ensure he received appropriate follow-up care and addressed questions and needs that arose. The veteran’s home CCNT continued to monitor the veteran for 1 year post treatment and communicate with VAPSHCS CCNT.
Conclusion
The VA is in a unique position to meet the needs of veterans by providing comprehensive care with sensitivity to military culture, access to a range of complicated benefits awarded to veterans, particularly those with servicerelated exposures or injuries, and specialists in diagnosis and treatment of physical and mental consequences of their service. Patient navigation helps ensure veterans can access these services, maintain continuity of care despite referrals across large geographic regions, and receive support while receiving cancer treatment at the VA.
Use of an interdisciplinary team, including an ARNP, RN, SW, and PSA is vital to fully address the wide range of physical, psychosocial, and practical barriers to care that a veteran may experience. Since September 2014, PSHCS has enrolled more than 500 veterans with CCNT, and nearly 200 are actively being followed and provided with navigation services at any given time (Figure 3). By proactively identifying and addressing barriers to care, the advocacy provided by CCNT has averted patient safety risks, made better use of limited veteran and VA resources, and provided patient-centered care to veterans.
Evaluation is currently underway to measure the impact of the program and develop metrics for the CCNT. Given the needs of the patient population, the team hopes to see further expansion of CCNT in order to reach more risk groups. Institutional support and funding for patient navigation should be a high priority as the VA strives to provide excellent, patient-centered care.
Acknowledgements
The authors would like first and foremost to give a special thank-you to the veterans for their service to our country. In addition, the authors would like to thank champions for the cancer care navigation team, including Dr. Daniel Wu, chief of oncology; and Dr. Peter Wu, cancer committee chair and surgical oncologist, and Sandra Solomon, nurse manager of the Cancer Care Clinic and inpatient cancer unit at VA Puget Sound Health Care System; Dr. Carol Sprague, staff physician and clinical lead VISN 20 Cancer Care Platform, Judy McConnachie, MPH, administrative director, Clinical Business Intelligence Northwest Innovation Center, VA Portland Health Care System in Portland, Oregon; and Tracy Weistreich, PhD, RN, associate director Patient Care Services at VA Roseburg Healthcare System in Roseburg, Oregon; and the VISN 20 Executive Cancer Care Platform Advisory Board.
The authors would also like to acknowledge all the VISN 20 network cancer care navigation teams at the following sites: Anchorage, Alaska; Boise, Idaho; Portland, Oregon; Roseburg, Oregon; Spokane, Washington; Walla Walla, Washington; and White City, Oregon. Team members at each site have been an integral part of the development and success of the VAPSHCS CCNT.
The authors are also grateful to all of the nurse coordinators and providers within all the specialty services at Puget Sound Health Care Systems, including oncology, radiation oncology, cancer care, otolaryngology, general surgery, palliative care, dental and primary care, for their collaboration with veteran care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Freeman HP. Patient navigation as a targeted intervention: for patients at high risk
for delays in cancer care. Cancer. 2015;121(22):3930-3932.
2. Moy B, Chabner BA. Patient navigator programs, cancer disparities, and the patient protection and affordable care act. Oncologist. 2011;16(7):926-929.
3. Meade CD, Wells KJ, Arevalo M, et al. Lay navigator model for impacting cancer health disparities. J Cancer Educ. 2014;29(3):449-457.
4. Fillion L, Cook S, Veillette AM, et al. Professional navigation: a comparative study of two Canadian models. Can Oncol Nurs J. 2012;22(4):257-277.
5. Lairson DR, Huo J, Ricks KA, Savas L, Fernández ME. The cost of implementing a 2-1-1 call center-based cancer control navigator program. Eval Program Plann. 2013:39:51-56.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly E, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ. Patient navigation
to improve follow-up of abnormal mammograms among disadvantaged women. J Womens Health (Larchmt). 2015;24(2):138-143.
8. Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority
individuals. Cancer. 2015;121(7):1088-1097.
9. Baliski C, McGahan CE, Liberto CM, et al. Influence of nurse navigation on wait times for breast cancer care in a Canadian regional cancer center. Am J Surg. 2014;207(5):686-691.
10. Hoffman JH, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;1(10):1655-1663
11. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632.
12. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans health and medical outcome studies. J Ambul Care Manage. 2004;27(3):249-262.
13. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academies Press; 1994.
15. Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23(8):921-927.
16. Mulligan EA, Wachen JS, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
17. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400.
Note: Page numbers differ between the print issue and digital edition.
The VHA has a unique responsibility to provide excellent, patient-centered care to the veterans who have served the U.S. long after their active military service has ended. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely and efficient health care. The need to travel for cancer care, sometimes for long distances over long periods, adds an additional disparity and puts veterans at higher risk for delays in care. Cancer care navigation teams (CCNTs) were established at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, and throughout the Veterans Integrated Service Network, region 20 (VISN 20), which consists of a large geographical area that includes Alaska, Washington, Oregon, Idaho and one county in both Montana and California. These teams use an interdisciplinary approach to providing personalized assistance, support, and resources to veterans with cancer and their families who require travel for cancer care.
The CCNTs identify and minimize clinical and psychosocial barriers throughout the cancer care continuum. Although structured to address the unique needs and barriers of the veteran population within the VA, CCNT may also be used as a model for patients receiving cancer care within other complex and decentralized health care systems.
Patient Navigation in Cancer Care
The term navigation in the context of cancer care originated in 1990 at Harlem Hospital Center in New York City. The term described an intervention to address barriers to care experienced by a population of low income African American women with breast cancer. By applying patient navigation in addition to offering free and low-cost breast cancer screening and exams for high-risk patients, the 5-year survival rate in this disadvantaged population of women increased from 39% to 70%.1
Since then, navigation programs in cancer care have been adopted in health care settings around the world. Many different models have been described within the literature.2-5 Patient navigation is perhaps best recognized as a means to decrease health disparities by addressing barriers to health care, which may include lack of insurance, poverty, medical or psychiatric comorbidities, low health literacy, food insecurity, and homelessness. By identifying and addressing these barriers to care in high-risk populations, patient navigation programs have demonstrated positive outcomes, including improvement in cancer screening rates, timeliness of care, medication adherence, and patient satisfaction.6-10 Although there is a large amount of literature on navigation in cancer care, there is minimal literature that focuses on navigation in the veteran population and health care system.
Barriers to Cancer Care
The VA is a national health care system composed of community clinics, hospitals, and major referral centers that deliver comprehensive health care to veterans. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely, efficient health care. Research studies have documented significant differences among veterans receiving health care through the VHA compared with veterans who receive health care from other sources. Veterans enrolled at the VA are more likely to be poorer, older, African American, less well educated, unemployed or underemployed, lack social support, and in poorer physical and mental health compared with the general population or with veterans who do not use VA health care.11-13 Such health disparities have been linked to delays in timely access to health care.11
In a study comparing an age-adjusted ambulatory care population with veterans receiving care at the VA, VA patients were also found to be 3 times more likely to have ever been diagnosed with cancer.12 Exposures to carcinogens during their military service, such as Agent Orange, may contribute to this difference.14 Veterans have higher rates of posttraumatic stress disorder (PTSD) and other mental health disorders from military combat experiences or other traumas; these conditions can be exacerbated by the distress of a cancer diagnosis.15-17
Veterans requiring specialty care, such as cancer-related care, are referred within the VISN and may need to travel long distances in to access these specialty providers. Continuity of care is challenged during cancer diagnosis, staging, treatment, and surveillance when some aspects of care may be completed at geographically distant sites or by community providers if unavailable through the local VA. Appointments for care occur within each specialty service, and staff and clinic availability limit scheduling. There are no formal mechanisms for coordinating visits for efficiency or minimizing travel burden. The electronic medical record (EMR) at the VA can be helpful in accessing information from remote locations but does not easily integrate medical information from different facilities. Clinical data, such as recommendations for follow-up care, may take time and patience to access.
These challenges to the delivery of timely, efficient, patient-centered cancer care were documented in a cancer needs assessment performed in 2012 across VISN 20 (Figure 1). In response, a 3-year pilot program was initiated to implement a network of CCNTs in 8 VA facilities across the region.
Planning and Implementation
The VAPSHCS is a major referral center for cancer care that serves veterans living in VISN 20. On average, about 1,000 new cancers are diagnosed, and VAPSHCS sees 2,000 unique veterans for cancer care annually (Figure 2).One-quarter of these veterans are from out of state. For veterans living in Washington, nearly half traveled 50 miles or more to access cancer services at VAPSHCS. VA Puget Sound implemented its CCNT in the fall of 2014 and consists of an advanced practice registered nurse practitioner (ARNP), registered nurse (RN), social worker (SW), and program support assistant (PSA).
Veterans in identified priority cohorts thought to be at highest risk for barriers to cancer care are enrolled in navigation services. These priority groups include those veterans referred from another regional VA facility, those living more than 100 miles from the VAPSHCS, those referred for multimodality care (eg, surgery with neoadjuvant chemoradiation), and those with significant psychosocial barriers to care. Veterans are identified by the CCNT through a formal consult, notification from the CCNT at another VA facility, a cancer conference, a review of pathology results, and in some cases by veteran self-referral.
As it develops further capacity, CCNT will add other high-risk groups. Ideally, CCNT will eventually be a resource all veterans referred to VAPSHCS for cancer care, so all veterans may be assessed for potential barriers to care and be provided with much needed support and resources.
The CCNT is proactive and systematic in its navigation processes. Where possible, CCNT members are cross-trained to provide role coverage. The team reviews medical records for veterans actively enrolled in CCNT services weekly, to identify new barriers to care and address them in a timely manner. A robust data tracking system (created using a relational database) allows for storage of updated patient information and assigns tasks within the team, tracks upcoming appointments to support coordination, identifies travel and lodging needs, and assures follow-up care is completed. It also generates lists used for routine rounding on patient groups, treatment summary reports, and survivorship care plans.
The CCNT uses standardized assessment tools, including a navigation intake form, the National Comprehensive Cancer Network (NCCN) Distress Thermometer, and a functional assessment. Communication is an essential part of the navigation team, which addresses veteran’s identified needs by conducting weekly rounds within the interdisciplinary team to share information and collaborate.
The team has weekly telephone calls with its CCNTs from referring facilities to discuss veterans at all stages of the cancer continuum and facilitate transfer of information between facilities and providers, including needed diagnostic services and follow-up recommendations. The CCNT also facilitates communication with PSHCS specialty services by actively participating in multidisciplinary rounds and cancer conference.
Finally, although the CCNT follows individual veterans, the team also recognizes its role in identifying and addressing system barriers to cancer care. Collaborating with its partners within the facility and across the network, the team has improved access to services, created teaching tools that can be shared across disciplines, and implemented new procedures and policies to meet the American College of Surgeons Commission on Cancer accreditation standards and improve the cancer care system as a whole.
VAPSHCS Cancer Navigation Model
The VAPSHCS cancer navigation model is divided into 4 main processes based on the cancer care continuum. To illustrate this navigation model, this paper follows the journey of a 57-year-old male veteran referred to PSHCS with newly diagnosed head and neck cancer. He is divorced, with very little social support and lives in a remote area about 60 miles from his primary VA facility and more than 400 miles from PSHCS. His case was presented at the PSHCS facility cancer conference, where concurrent chemotherapy and radiation was recommended. This particular treatment consists of daily radiation and weekly chemotherapy over 6 to 7 weeks. The CCNT staff recognized that this veteran met criteria for navigation, entered him in the tracking database, and notified his referring facility CCNT of the plan of care.
Preconsult
Prior to veterans traveling to VAPSHCS for a new diagnosis or suspicion of cancer, the first goal is to identify any potential barriers to travel. It is a financial burden for many veterans to travel, and in the past, travel has prevented veterans from attending their specialty consult appointments. It is the role of the CCNT PSA to contact the veteran by telephone, introduce their services, provide education about available travel and lodging benefits, and schedule a visit with the CCNT RN to coincide with the veteran’s scheduled other specialty appointments.
In this case, the CCNT PSA contacted the veteran with information about the VAPSHCS, placed a lodging consult to arrange hotel accommodations for the veteran while in Seattle, and provided information regarding transportation from the hotel to the VA. The CCNT also identified that the veteran required a radiation oncology consultation and dental evaluation to proceed with a treatment plan. To decrease travel burden with additional trips to Seattle, the PSA contacted these specialty services to schedule the appointments. The PSA then assembled and mailed a packet of information to the veteran, which included details about how to pack and prepare for the trip, a facility map, and a hotel shuttle schedule.
Consult Visit and Planning
When veterans arrive at VAPSHCS, the CCNT RN meets them and completes an intake form. This standardized questionnaire identifies potential barriers to cancer care and supports the need for referrals to services, such as a dietitian, chaplain, palliative care, social work, physical and occupational therapy, travel, or lodging.
During this visit, the CCNT RN also asks the veteran to complete a NCCN Distress Thermometer. This thermometer assessment tool screens for physical, emotional, and practical needs that are specific to cancer. In this particular veteran’s situation, the distress level was 7 out of 10 (a score of 4 or greater triggersan automatic consult to social work once the results are entered in the EMR). Based on the outcomes information obtained from the intake form and NCCN Distress Thermometer, the CCNT RN made referrals to SW, chaplain services, and the oncology dietitian.
During the CCNT RN visit, nurse identified that the veteran’s financial situation had changed significantly resulting in less income and causing financial distress. The veteran was encouraged to complete an updated benefit renewal form with the SW that would likely eliminate his required copays for medical visits and prescription medications during the 6 weeks of chemotherapy and radiation. This need was communicated to the CCNT SW. The RN provided the veteran with information about VA resources to support him during cancer treatment, including meal options and support groups for both veterans and caregivers. They discussed the likely plan of care, including disease progress, information on prescribed drugs, dental evaluation and extractions as needed, placement of a feeding tube and a central line, and gave the veteran written brochures to review at his convenience. The RN also reviewed the logistics of a prolonged stay for the recommended course of chemotherapy and radiation.
During the initial CCNT intake process, the RN identified that the veteran would be without a caregiver and would be staying alone in lodging throughout his cancer treatment. The RN then completed a functional assessment of safety risks while lodging alone during this extended time. This brief questionnaire identifies any deficits in a veteran’s activity of daily living that may influence safety while lodging alone. The assessment is documented in the EMR, and if any concerns are identified, these are discussed with the veteran and a team of medical providers. If necessary, interventions are put into place before the veteran’s return for treatment. Potential safeguards may include obtaining safety equipment (eg, walker and bath chair), identifying an appropriate caregiver, or referring the veteran to a skilled nursing facility for the duration of treatment.
Following the veteran’s consultation visits, he went home with a return date 2 weeks later to start treatment. The VAPSHCS CCNT discussed the plan of care with his local CCNT, which facilitated placement of his feeding tube and addressed other symptom management concerns. The local CCNT SW completed advanced directives with the veteran and coordinated his travel back to VAPSHCS to begin treatment.
During Treatment
Veterans traveling from other VA facilities are away from their primary care providers (PCPs) for a number of weeks. Other specialty providers see a veteran during cancer treatment; however, the CCNT ARNP supports primary care needs while the veteran is away from their home VA facility. The ARNP is able to address chronic or acute medical issues before the start of treatment to prevent delays in cancer care.
Once the veteran returned to VAPSHCS to initiate therapy, the CCNT ARNP completed a history and physical examination to identify and address any active medical problems and document past medical history and current medication list in the EMR. This provides easy access to a thorough and complete baseline to both the oncology and radiation oncology providers. The ARNP examination revealed a new neck wound on the veteran, likely related to his cancer, and an urgent consult was placed to wound care. The otolaryngology, oncology, and radiation oncology departments were alerted to this development so they could assess the patient and adjust treatment plans as necessary. The veteran also required a refill of his blood pressure medication and had a number of questions regarding his upcoming treatment, which were addressed during the visit.
Within the first 2 days of the veteran’s return, he was scheduled to meet with the CCNT SW who reviewed and documented his advanced directive within the system, assessed his distress, provided therapeutic counseling, and completed the health benefit renewal form. Given the veteran’s financial status, the SW was able to help him apply for financial hardship to cover the costs of the care he had already received and assisted him with securing an appointment with the Social Security Administration (SSA) for disability benefits. The CCNT SW then helped the veteran complete a phone interview with the SSA and complete the application process. The SW also helped him complete the application for VA service-connected compensation and pension disability benefits.
Throughout his treatment course, the CCNT continued to be a resource for the veteran. Because he had PTSD and was uncomfortable attending support groups, the CCNT SW met with him weekly to provide counseling and psychosocial support. He stopped by the CCNT office on several occasions to report how he was doing, and the team provided assistance in obtaining supplies for his feeding tube and managing a complication that arose with his lodging. In preparation for his treatment completion and return home, the VAPSHCS CCNT communicated with his local CCNT to describe follow-up needs and ensure appropriate medical visits were scheduled. His travel home was arranged by the VAPSHCS PSA.
Treatment Completion
Before leaving VAPSHCS, the veteran was scheduled and seen in the clinic by the ARNP, where he received a written comprehensive treatment summary. The summary documented his cancer diagnosis, treatment, complications, and recommendations for follow-up care. He had the opportunity to ask questions about his treatment, and a clinical assessment was made for adverse effects. Appropriate interventions also were identified and addressed. A comprehensive treatment summary note was documented in the EMR and sent to his PCP and other medical specialists at his home facility to assure continuity of care.
The VAPSHCS CCNT continued to communicate weekly with the veteran’s home CCNT following his return, to ensure he received appropriate follow-up care and addressed questions and needs that arose. The veteran’s home CCNT continued to monitor the veteran for 1 year post treatment and communicate with VAPSHCS CCNT.
Conclusion
The VA is in a unique position to meet the needs of veterans by providing comprehensive care with sensitivity to military culture, access to a range of complicated benefits awarded to veterans, particularly those with servicerelated exposures or injuries, and specialists in diagnosis and treatment of physical and mental consequences of their service. Patient navigation helps ensure veterans can access these services, maintain continuity of care despite referrals across large geographic regions, and receive support while receiving cancer treatment at the VA.
Use of an interdisciplinary team, including an ARNP, RN, SW, and PSA is vital to fully address the wide range of physical, psychosocial, and practical barriers to care that a veteran may experience. Since September 2014, PSHCS has enrolled more than 500 veterans with CCNT, and nearly 200 are actively being followed and provided with navigation services at any given time (Figure 3). By proactively identifying and addressing barriers to care, the advocacy provided by CCNT has averted patient safety risks, made better use of limited veteran and VA resources, and provided patient-centered care to veterans.
Evaluation is currently underway to measure the impact of the program and develop metrics for the CCNT. Given the needs of the patient population, the team hopes to see further expansion of CCNT in order to reach more risk groups. Institutional support and funding for patient navigation should be a high priority as the VA strives to provide excellent, patient-centered care.
Acknowledgements
The authors would like first and foremost to give a special thank-you to the veterans for their service to our country. In addition, the authors would like to thank champions for the cancer care navigation team, including Dr. Daniel Wu, chief of oncology; and Dr. Peter Wu, cancer committee chair and surgical oncologist, and Sandra Solomon, nurse manager of the Cancer Care Clinic and inpatient cancer unit at VA Puget Sound Health Care System; Dr. Carol Sprague, staff physician and clinical lead VISN 20 Cancer Care Platform, Judy McConnachie, MPH, administrative director, Clinical Business Intelligence Northwest Innovation Center, VA Portland Health Care System in Portland, Oregon; and Tracy Weistreich, PhD, RN, associate director Patient Care Services at VA Roseburg Healthcare System in Roseburg, Oregon; and the VISN 20 Executive Cancer Care Platform Advisory Board.
The authors would also like to acknowledge all the VISN 20 network cancer care navigation teams at the following sites: Anchorage, Alaska; Boise, Idaho; Portland, Oregon; Roseburg, Oregon; Spokane, Washington; Walla Walla, Washington; and White City, Oregon. Team members at each site have been an integral part of the development and success of the VAPSHCS CCNT.
The authors are also grateful to all of the nurse coordinators and providers within all the specialty services at Puget Sound Health Care Systems, including oncology, radiation oncology, cancer care, otolaryngology, general surgery, palliative care, dental and primary care, for their collaboration with veteran care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
The VHA has a unique responsibility to provide excellent, patient-centered care to the veterans who have served the U.S. long after their active military service has ended. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely and efficient health care. The need to travel for cancer care, sometimes for long distances over long periods, adds an additional disparity and puts veterans at higher risk for delays in care. Cancer care navigation teams (CCNTs) were established at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, and throughout the Veterans Integrated Service Network, region 20 (VISN 20), which consists of a large geographical area that includes Alaska, Washington, Oregon, Idaho and one county in both Montana and California. These teams use an interdisciplinary approach to providing personalized assistance, support, and resources to veterans with cancer and their families who require travel for cancer care.
The CCNTs identify and minimize clinical and psychosocial barriers throughout the cancer care continuum. Although structured to address the unique needs and barriers of the veteran population within the VA, CCNT may also be used as a model for patients receiving cancer care within other complex and decentralized health care systems.
Patient Navigation in Cancer Care
The term navigation in the context of cancer care originated in 1990 at Harlem Hospital Center in New York City. The term described an intervention to address barriers to care experienced by a population of low income African American women with breast cancer. By applying patient navigation in addition to offering free and low-cost breast cancer screening and exams for high-risk patients, the 5-year survival rate in this disadvantaged population of women increased from 39% to 70%.1
Since then, navigation programs in cancer care have been adopted in health care settings around the world. Many different models have been described within the literature.2-5 Patient navigation is perhaps best recognized as a means to decrease health disparities by addressing barriers to health care, which may include lack of insurance, poverty, medical or psychiatric comorbidities, low health literacy, food insecurity, and homelessness. By identifying and addressing these barriers to care in high-risk populations, patient navigation programs have demonstrated positive outcomes, including improvement in cancer screening rates, timeliness of care, medication adherence, and patient satisfaction.6-10 Although there is a large amount of literature on navigation in cancer care, there is minimal literature that focuses on navigation in the veteran population and health care system.
Barriers to Cancer Care
The VA is a national health care system composed of community clinics, hospitals, and major referral centers that deliver comprehensive health care to veterans. For veterans diagnosed with cancer, the physical, mental, and financial consequences can pose significant hardships and create barriers to obtaining timely, efficient health care. Research studies have documented significant differences among veterans receiving health care through the VHA compared with veterans who receive health care from other sources. Veterans enrolled at the VA are more likely to be poorer, older, African American, less well educated, unemployed or underemployed, lack social support, and in poorer physical and mental health compared with the general population or with veterans who do not use VA health care.11-13 Such health disparities have been linked to delays in timely access to health care.11
In a study comparing an age-adjusted ambulatory care population with veterans receiving care at the VA, VA patients were also found to be 3 times more likely to have ever been diagnosed with cancer.12 Exposures to carcinogens during their military service, such as Agent Orange, may contribute to this difference.14 Veterans have higher rates of posttraumatic stress disorder (PTSD) and other mental health disorders from military combat experiences or other traumas; these conditions can be exacerbated by the distress of a cancer diagnosis.15-17
Veterans requiring specialty care, such as cancer-related care, are referred within the VISN and may need to travel long distances in to access these specialty providers. Continuity of care is challenged during cancer diagnosis, staging, treatment, and surveillance when some aspects of care may be completed at geographically distant sites or by community providers if unavailable through the local VA. Appointments for care occur within each specialty service, and staff and clinic availability limit scheduling. There are no formal mechanisms for coordinating visits for efficiency or minimizing travel burden. The electronic medical record (EMR) at the VA can be helpful in accessing information from remote locations but does not easily integrate medical information from different facilities. Clinical data, such as recommendations for follow-up care, may take time and patience to access.
These challenges to the delivery of timely, efficient, patient-centered cancer care were documented in a cancer needs assessment performed in 2012 across VISN 20 (Figure 1). In response, a 3-year pilot program was initiated to implement a network of CCNTs in 8 VA facilities across the region.
Planning and Implementation
The VAPSHCS is a major referral center for cancer care that serves veterans living in VISN 20. On average, about 1,000 new cancers are diagnosed, and VAPSHCS sees 2,000 unique veterans for cancer care annually (Figure 2).One-quarter of these veterans are from out of state. For veterans living in Washington, nearly half traveled 50 miles or more to access cancer services at VAPSHCS. VA Puget Sound implemented its CCNT in the fall of 2014 and consists of an advanced practice registered nurse practitioner (ARNP), registered nurse (RN), social worker (SW), and program support assistant (PSA).
Veterans in identified priority cohorts thought to be at highest risk for barriers to cancer care are enrolled in navigation services. These priority groups include those veterans referred from another regional VA facility, those living more than 100 miles from the VAPSHCS, those referred for multimodality care (eg, surgery with neoadjuvant chemoradiation), and those with significant psychosocial barriers to care. Veterans are identified by the CCNT through a formal consult, notification from the CCNT at another VA facility, a cancer conference, a review of pathology results, and in some cases by veteran self-referral.
As it develops further capacity, CCNT will add other high-risk groups. Ideally, CCNT will eventually be a resource all veterans referred to VAPSHCS for cancer care, so all veterans may be assessed for potential barriers to care and be provided with much needed support and resources.
The CCNT is proactive and systematic in its navigation processes. Where possible, CCNT members are cross-trained to provide role coverage. The team reviews medical records for veterans actively enrolled in CCNT services weekly, to identify new barriers to care and address them in a timely manner. A robust data tracking system (created using a relational database) allows for storage of updated patient information and assigns tasks within the team, tracks upcoming appointments to support coordination, identifies travel and lodging needs, and assures follow-up care is completed. It also generates lists used for routine rounding on patient groups, treatment summary reports, and survivorship care plans.
The CCNT uses standardized assessment tools, including a navigation intake form, the National Comprehensive Cancer Network (NCCN) Distress Thermometer, and a functional assessment. Communication is an essential part of the navigation team, which addresses veteran’s identified needs by conducting weekly rounds within the interdisciplinary team to share information and collaborate.
The team has weekly telephone calls with its CCNTs from referring facilities to discuss veterans at all stages of the cancer continuum and facilitate transfer of information between facilities and providers, including needed diagnostic services and follow-up recommendations. The CCNT also facilitates communication with PSHCS specialty services by actively participating in multidisciplinary rounds and cancer conference.
Finally, although the CCNT follows individual veterans, the team also recognizes its role in identifying and addressing system barriers to cancer care. Collaborating with its partners within the facility and across the network, the team has improved access to services, created teaching tools that can be shared across disciplines, and implemented new procedures and policies to meet the American College of Surgeons Commission on Cancer accreditation standards and improve the cancer care system as a whole.
VAPSHCS Cancer Navigation Model
The VAPSHCS cancer navigation model is divided into 4 main processes based on the cancer care continuum. To illustrate this navigation model, this paper follows the journey of a 57-year-old male veteran referred to PSHCS with newly diagnosed head and neck cancer. He is divorced, with very little social support and lives in a remote area about 60 miles from his primary VA facility and more than 400 miles from PSHCS. His case was presented at the PSHCS facility cancer conference, where concurrent chemotherapy and radiation was recommended. This particular treatment consists of daily radiation and weekly chemotherapy over 6 to 7 weeks. The CCNT staff recognized that this veteran met criteria for navigation, entered him in the tracking database, and notified his referring facility CCNT of the plan of care.
Preconsult
Prior to veterans traveling to VAPSHCS for a new diagnosis or suspicion of cancer, the first goal is to identify any potential barriers to travel. It is a financial burden for many veterans to travel, and in the past, travel has prevented veterans from attending their specialty consult appointments. It is the role of the CCNT PSA to contact the veteran by telephone, introduce their services, provide education about available travel and lodging benefits, and schedule a visit with the CCNT RN to coincide with the veteran’s scheduled other specialty appointments.
In this case, the CCNT PSA contacted the veteran with information about the VAPSHCS, placed a lodging consult to arrange hotel accommodations for the veteran while in Seattle, and provided information regarding transportation from the hotel to the VA. The CCNT also identified that the veteran required a radiation oncology consultation and dental evaluation to proceed with a treatment plan. To decrease travel burden with additional trips to Seattle, the PSA contacted these specialty services to schedule the appointments. The PSA then assembled and mailed a packet of information to the veteran, which included details about how to pack and prepare for the trip, a facility map, and a hotel shuttle schedule.
Consult Visit and Planning
When veterans arrive at VAPSHCS, the CCNT RN meets them and completes an intake form. This standardized questionnaire identifies potential barriers to cancer care and supports the need for referrals to services, such as a dietitian, chaplain, palliative care, social work, physical and occupational therapy, travel, or lodging.
During this visit, the CCNT RN also asks the veteran to complete a NCCN Distress Thermometer. This thermometer assessment tool screens for physical, emotional, and practical needs that are specific to cancer. In this particular veteran’s situation, the distress level was 7 out of 10 (a score of 4 or greater triggersan automatic consult to social work once the results are entered in the EMR). Based on the outcomes information obtained from the intake form and NCCN Distress Thermometer, the CCNT RN made referrals to SW, chaplain services, and the oncology dietitian.
During the CCNT RN visit, nurse identified that the veteran’s financial situation had changed significantly resulting in less income and causing financial distress. The veteran was encouraged to complete an updated benefit renewal form with the SW that would likely eliminate his required copays for medical visits and prescription medications during the 6 weeks of chemotherapy and radiation. This need was communicated to the CCNT SW. The RN provided the veteran with information about VA resources to support him during cancer treatment, including meal options and support groups for both veterans and caregivers. They discussed the likely plan of care, including disease progress, information on prescribed drugs, dental evaluation and extractions as needed, placement of a feeding tube and a central line, and gave the veteran written brochures to review at his convenience. The RN also reviewed the logistics of a prolonged stay for the recommended course of chemotherapy and radiation.
During the initial CCNT intake process, the RN identified that the veteran would be without a caregiver and would be staying alone in lodging throughout his cancer treatment. The RN then completed a functional assessment of safety risks while lodging alone during this extended time. This brief questionnaire identifies any deficits in a veteran’s activity of daily living that may influence safety while lodging alone. The assessment is documented in the EMR, and if any concerns are identified, these are discussed with the veteran and a team of medical providers. If necessary, interventions are put into place before the veteran’s return for treatment. Potential safeguards may include obtaining safety equipment (eg, walker and bath chair), identifying an appropriate caregiver, or referring the veteran to a skilled nursing facility for the duration of treatment.
Following the veteran’s consultation visits, he went home with a return date 2 weeks later to start treatment. The VAPSHCS CCNT discussed the plan of care with his local CCNT, which facilitated placement of his feeding tube and addressed other symptom management concerns. The local CCNT SW completed advanced directives with the veteran and coordinated his travel back to VAPSHCS to begin treatment.
During Treatment
Veterans traveling from other VA facilities are away from their primary care providers (PCPs) for a number of weeks. Other specialty providers see a veteran during cancer treatment; however, the CCNT ARNP supports primary care needs while the veteran is away from their home VA facility. The ARNP is able to address chronic or acute medical issues before the start of treatment to prevent delays in cancer care.
Once the veteran returned to VAPSHCS to initiate therapy, the CCNT ARNP completed a history and physical examination to identify and address any active medical problems and document past medical history and current medication list in the EMR. This provides easy access to a thorough and complete baseline to both the oncology and radiation oncology providers. The ARNP examination revealed a new neck wound on the veteran, likely related to his cancer, and an urgent consult was placed to wound care. The otolaryngology, oncology, and radiation oncology departments were alerted to this development so they could assess the patient and adjust treatment plans as necessary. The veteran also required a refill of his blood pressure medication and had a number of questions regarding his upcoming treatment, which were addressed during the visit.
Within the first 2 days of the veteran’s return, he was scheduled to meet with the CCNT SW who reviewed and documented his advanced directive within the system, assessed his distress, provided therapeutic counseling, and completed the health benefit renewal form. Given the veteran’s financial status, the SW was able to help him apply for financial hardship to cover the costs of the care he had already received and assisted him with securing an appointment with the Social Security Administration (SSA) for disability benefits. The CCNT SW then helped the veteran complete a phone interview with the SSA and complete the application process. The SW also helped him complete the application for VA service-connected compensation and pension disability benefits.
Throughout his treatment course, the CCNT continued to be a resource for the veteran. Because he had PTSD and was uncomfortable attending support groups, the CCNT SW met with him weekly to provide counseling and psychosocial support. He stopped by the CCNT office on several occasions to report how he was doing, and the team provided assistance in obtaining supplies for his feeding tube and managing a complication that arose with his lodging. In preparation for his treatment completion and return home, the VAPSHCS CCNT communicated with his local CCNT to describe follow-up needs and ensure appropriate medical visits were scheduled. His travel home was arranged by the VAPSHCS PSA.
Treatment Completion
Before leaving VAPSHCS, the veteran was scheduled and seen in the clinic by the ARNP, where he received a written comprehensive treatment summary. The summary documented his cancer diagnosis, treatment, complications, and recommendations for follow-up care. He had the opportunity to ask questions about his treatment, and a clinical assessment was made for adverse effects. Appropriate interventions also were identified and addressed. A comprehensive treatment summary note was documented in the EMR and sent to his PCP and other medical specialists at his home facility to assure continuity of care.
The VAPSHCS CCNT continued to communicate weekly with the veteran’s home CCNT following his return, to ensure he received appropriate follow-up care and addressed questions and needs that arose. The veteran’s home CCNT continued to monitor the veteran for 1 year post treatment and communicate with VAPSHCS CCNT.
Conclusion
The VA is in a unique position to meet the needs of veterans by providing comprehensive care with sensitivity to military culture, access to a range of complicated benefits awarded to veterans, particularly those with servicerelated exposures or injuries, and specialists in diagnosis and treatment of physical and mental consequences of their service. Patient navigation helps ensure veterans can access these services, maintain continuity of care despite referrals across large geographic regions, and receive support while receiving cancer treatment at the VA.
Use of an interdisciplinary team, including an ARNP, RN, SW, and PSA is vital to fully address the wide range of physical, psychosocial, and practical barriers to care that a veteran may experience. Since September 2014, PSHCS has enrolled more than 500 veterans with CCNT, and nearly 200 are actively being followed and provided with navigation services at any given time (Figure 3). By proactively identifying and addressing barriers to care, the advocacy provided by CCNT has averted patient safety risks, made better use of limited veteran and VA resources, and provided patient-centered care to veterans.
Evaluation is currently underway to measure the impact of the program and develop metrics for the CCNT. Given the needs of the patient population, the team hopes to see further expansion of CCNT in order to reach more risk groups. Institutional support and funding for patient navigation should be a high priority as the VA strives to provide excellent, patient-centered care.
Acknowledgements
The authors would like first and foremost to give a special thank-you to the veterans for their service to our country. In addition, the authors would like to thank champions for the cancer care navigation team, including Dr. Daniel Wu, chief of oncology; and Dr. Peter Wu, cancer committee chair and surgical oncologist, and Sandra Solomon, nurse manager of the Cancer Care Clinic and inpatient cancer unit at VA Puget Sound Health Care System; Dr. Carol Sprague, staff physician and clinical lead VISN 20 Cancer Care Platform, Judy McConnachie, MPH, administrative director, Clinical Business Intelligence Northwest Innovation Center, VA Portland Health Care System in Portland, Oregon; and Tracy Weistreich, PhD, RN, associate director Patient Care Services at VA Roseburg Healthcare System in Roseburg, Oregon; and the VISN 20 Executive Cancer Care Platform Advisory Board.
The authors would also like to acknowledge all the VISN 20 network cancer care navigation teams at the following sites: Anchorage, Alaska; Boise, Idaho; Portland, Oregon; Roseburg, Oregon; Spokane, Washington; Walla Walla, Washington; and White City, Oregon. Team members at each site have been an integral part of the development and success of the VAPSHCS CCNT.
The authors are also grateful to all of the nurse coordinators and providers within all the specialty services at Puget Sound Health Care Systems, including oncology, radiation oncology, cancer care, otolaryngology, general surgery, palliative care, dental and primary care, for their collaboration with veteran care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Freeman HP. Patient navigation as a targeted intervention: for patients at high risk
for delays in cancer care. Cancer. 2015;121(22):3930-3932.
2. Moy B, Chabner BA. Patient navigator programs, cancer disparities, and the patient protection and affordable care act. Oncologist. 2011;16(7):926-929.
3. Meade CD, Wells KJ, Arevalo M, et al. Lay navigator model for impacting cancer health disparities. J Cancer Educ. 2014;29(3):449-457.
4. Fillion L, Cook S, Veillette AM, et al. Professional navigation: a comparative study of two Canadian models. Can Oncol Nurs J. 2012;22(4):257-277.
5. Lairson DR, Huo J, Ricks KA, Savas L, Fernández ME. The cost of implementing a 2-1-1 call center-based cancer control navigator program. Eval Program Plann. 2013:39:51-56.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly E, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ. Patient navigation
to improve follow-up of abnormal mammograms among disadvantaged women. J Womens Health (Larchmt). 2015;24(2):138-143.
8. Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority
individuals. Cancer. 2015;121(7):1088-1097.
9. Baliski C, McGahan CE, Liberto CM, et al. Influence of nurse navigation on wait times for breast cancer care in a Canadian regional cancer center. Am J Surg. 2014;207(5):686-691.
10. Hoffman JH, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;1(10):1655-1663
11. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632.
12. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans health and medical outcome studies. J Ambul Care Manage. 2004;27(3):249-262.
13. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academies Press; 1994.
15. Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23(8):921-927.
16. Mulligan EA, Wachen JS, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
17. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400.
Note: Page numbers differ between the print issue and digital edition.
1. Freeman HP. Patient navigation as a targeted intervention: for patients at high risk
for delays in cancer care. Cancer. 2015;121(22):3930-3932.
2. Moy B, Chabner BA. Patient navigator programs, cancer disparities, and the patient protection and affordable care act. Oncologist. 2011;16(7):926-929.
3. Meade CD, Wells KJ, Arevalo M, et al. Lay navigator model for impacting cancer health disparities. J Cancer Educ. 2014;29(3):449-457.
4. Fillion L, Cook S, Veillette AM, et al. Professional navigation: a comparative study of two Canadian models. Can Oncol Nurs J. 2012;22(4):257-277.
5. Lairson DR, Huo J, Ricks KA, Savas L, Fernández ME. The cost of implementing a 2-1-1 call center-based cancer control navigator program. Eval Program Plann. 2013:39:51-56.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly E, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Percac-Lima S, Ashburner JM, McCarthy AM, Piawah S, Atlas SJ. Patient navigation
to improve follow-up of abnormal mammograms among disadvantaged women. J Womens Health (Larchmt). 2015;24(2):138-143.
8. Ladabaum U, Mannalithara A, Jandorf L, Itzkowitz SH. Cost-effectiveness of patient navigation to increase adherence with screening colonoscopy among minority
individuals. Cancer. 2015;121(7):1088-1097.
9. Baliski C, McGahan CE, Liberto CM, et al. Influence of nurse navigation on wait times for breast cancer care in a Canadian regional cancer center. Am J Surg. 2014;207(5):686-691.
10. Hoffman JH, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;1(10):1655-1663
11. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632.
12. Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans health and medical outcome studies. J Ambul Care Manage. 2004;27(3):249-262.
13. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
14. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academies Press; 1994.
15. Wachen JS, Patidar SM, Mulligan EA, Naik AD, Moye J. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psychooncology. 2014;23(8):921-927.
16. Mulligan EA, Wachen JS, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
17. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400.
Note: Page numbers differ between the print issue and digital edition.
Best Practices: Infant Formula Comparison and Recommendations
A supplement to Pediatric News. This supplement is sponsored by Perrigo Nutritionals.
Topics
- Food Insecurity and Formula Stretching
- Addressing Food Insecurity’s Effects with Store Brand Infant Formula
- Is Switching Formula Safe?
- Conclusion
Faculty/Faculty Disclosure
Jenifer R. Lightdale, MD, MPH
Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition
UMass Memorial Children’s Medical Center
Professor of Pediatrics
University of Massachusetts Medical School
Worcester, MA
Dr. Lightdale reports that she is on the medical advisory board for Perrigo Nutritionals, and is an invited speaker for Mead Johnson & Company, LLC.
A supplement to Pediatric News. This supplement is sponsored by Perrigo Nutritionals.
Topics
- Food Insecurity and Formula Stretching
- Addressing Food Insecurity’s Effects with Store Brand Infant Formula
- Is Switching Formula Safe?
- Conclusion
Faculty/Faculty Disclosure
Jenifer R. Lightdale, MD, MPH
Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition
UMass Memorial Children’s Medical Center
Professor of Pediatrics
University of Massachusetts Medical School
Worcester, MA
Dr. Lightdale reports that she is on the medical advisory board for Perrigo Nutritionals, and is an invited speaker for Mead Johnson & Company, LLC.
A supplement to Pediatric News. This supplement is sponsored by Perrigo Nutritionals.
Topics
- Food Insecurity and Formula Stretching
- Addressing Food Insecurity’s Effects with Store Brand Infant Formula
- Is Switching Formula Safe?
- Conclusion
Faculty/Faculty Disclosure
Jenifer R. Lightdale, MD, MPH
Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition
UMass Memorial Children’s Medical Center
Professor of Pediatrics
University of Massachusetts Medical School
Worcester, MA
Dr. Lightdale reports that she is on the medical advisory board for Perrigo Nutritionals, and is an invited speaker for Mead Johnson & Company, LLC.
Implementation of a Precision Oncology Program as an Exemplar of a Learning Health Care System in the VA
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
The Case for VA Leadership
A solution to these 2 central dilemmas will result in continued health care improvement and, arguably, meaningful cost reduction through elimination of inferior treatments and optimization of individual patient care strategies. Since the current research culture does not reward such accomplishments, the responsibility for moving forward is left squarely on the health care systems. Said differently, a health care research budget that is a small fraction (5%) of health care expenditures is undersized and too culturally foreign for the task.1
A critical attribute that enables the VA to promote progress to the benefit of both veterans and taxpayers is an accountable care organization incentive to use a long horizon and invest in opportunities that reduce overall cost and improve outcomes for its beneficiariesover their entire lifespan. Although this feature is common to a handful of other large health care providers (Kaiser Permanente, Intermountain Healthcare, Mayo Clinic), those systems lack the assets fundamental to solution design that are broadly represented across VA medical centers: a staff, culture, and apparatus in support of research at most medical centers; an integrated electronic health record (EHR) for data access; and a patient population receptive to participating in activities that will aid fellow veterans.
Ongoing Programs
The VA is in an excellent position to create an efficient and scalable apparatus to perform comparative effectiveness studies.The Point-of-Care clinical trials program, proposed and championed by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) and supported by the VA Cooperative Studies Program, embeds low-risk clinical trials directly into the clinical ecosystem with a resultant decreased cost and increased relevance owing to study designs driven by current patient care processes.
This methodology and program is applauded by the Institute of Medicine and the Society for Clinical Trials, and each has invited MAVERIC to present at national meetings and roundtable discussions.2 Designation as a research “transformative initiative” by the VA Office of Research and Development (ORD) provided sufficient support to culminate in the imminent launch of the first national VA Point-of-Care Clinical Trial—the Diuretic Comparison Study. The VA is proceeding with this trial at 50 VA sites for a significantly lower cost.(VA Cooperative Studies Program study #597, methods manuscript in preparation). Results will inform the optimal initial treatment for hypertension and impact the care of millions of veterans and nonveterans.
Precision Oncology Program
The VA Precision Oncology Program (POP), initiated in VISN 1 and funded through a clinical care budget, goes a step further toward creating learning opportunities. The POP sequences the DNA of tumor tissue from veterans newly diagnosed with cancer to determine the DNA mutations responsible for the tumor development and behavior. Armed with this information, HCPs can optimize therapy based on mutation status by the delivery of drugs that are targeted against particular gene products.
Systematic implementation of the POP across all VAMCs will reduce disparities in cancer care induced by variation in medical center familiarity with treatment options. Features supported by the POP include enhanced enrollment of patients into clinical trials of novel targeted therapeutics and sharing of patient outcomes data to assist in decision support for future patients. In addition, this approach could facilitate the creation of a national VA database of cancer patient characteristics, tumor mutations, and cancer-related treatments and outcomes to accelerate the pace of discovery in VA cancer care.
Million Veteran Program
The Million Veteran Program (MVP) is a VA ORD initiative that asks veterans to share their medical data, lifestyle, and genetic data with researchers to allow for the discovery of correlations between their genetic profile and their health, disease and response totreatments. Currently more than 430,000 veterans have agreed to participate and have donated data and blood samples, and researchers are performing the first projects to use this resource.
Although the knowledge gained from these studies will be indirectly relevant to veterans in general the MVP presents an opportunity to present specific findings to individual participants that will directly affect their care. While reuse of the MVP resource for precision medicine is under consideration, there are important cultural and technical barriers that must be addressed. Like POP, integration of the MVP research program with clinical care should be carried out with consideration of a community of stakeholders and not driven exclusively by a research agenda.
Challenges in Moving Forward
Central to the implementation of a learning mechanism in health care systems is the recognition by administrators of the importance of the activity and appreciation of the business argument favoring the investment. This runs counter to the current notion of separate silos for health care and medical research whereby health care systems are liberated from the cost of investigation but then suffer from a dearth of knowledge relevant to their operation.
Additionally, research enterprises are not structured for such activities. Academic investigators are incentivized to create knowledge and generate publications and they understand best the currency of grant funding. Their world is not geared to reinvent or engineer solutions for health care systems. In light of these considerations, a decentralized approach that creates institutions for local learning needs to be developed and “owned” by individual and groups of medical centers with engagement of administration, patient, scientific, and community stakeholders. The Patient-Centered Outcome Research Institute (PCORI) and the consortia it has funded, PCOR-Net, have adopted this approach.3
Importantly, a new set of ethical and regulatory standards that distinguish it from traditional research must accompany progress in the creation of a learning health care system (LHS). Sharing of patient data to benefit fellow patients must come to be expected and without the formalized sharing agreements that are required in traditional research activities. Although the digitization of medical records makes most of what this article discusses possible, execution requires access to information technology resources and a talented staff.
More than a decade ago, the decision was made to dis-integrate the Office of Information Technology from VHA. This was executed with no provision to support the small army of VA clinician-informaticists who had done much in support of patient care, including the creation of the initial iteration of the VA EHR. Although the VA includes small pockets of this clinical informatics culture throughout its organization, the community has been largely silenced and taken refuge at academic affiliates. Access to VA information systems and funding opportunities for development and implementation of tools essential for learning will draw this intellectual capital back to the VA and allow for the VA to lead in this critical arena.
The VA Precision Oncology Program
Precision medicine is a medical model that incorporates the results of genetic diagnostic testing to customize or tailor medical decision making and treatment for the individual patient. Characteristics of the VA health care system that create a favored environment for introducing precision medicine include the single-payer model, where implementation decision and authority are centralized, a standardized EHR that enables informatics requirements, and a clinician and patient culture that supports innovation. To date, the benefits of precision medicine are most robust in cancer care. Under the leadership of Michael Mayo-Smith, MD, the VA New England Healthcare System has completed a regional pilot project in precision oncology that demonstrated feasibility of incorporating a precision medicine program in the clinical care environment.
For the majority of patients with lung cancer, DNA sequencing of tumor tissue identifies driver mutations—alterations believed responsible for tumor growth and behavior. The abundance of both driver and passenger mutations (those alterations whose significance is unknown) identified within an individual cancer specimen and the diversity of alterations found across the spectrum of all patients with cancer virtually assures the unique genetic profile (hence behavior) of any given patient’s tumor. The new generation of antineoplastic agents are targeted therapies that disrupt the downstream effects of these alterations and result in improved anticancer effects and reduced toxicity compared with conventional chemotherapy. The POP approach to cancer treatment determines the mutation profile of malignancies and identifies targeted therapies with the highest likelihood of treatment success. Although many driver mutation-targeted therapy combinations have been FDA approved, many more are in development and are available only as investigational agents.
Work Accomplished
Developed over the past 2 years in VISN 1, POP is a demonstration project that standardizes the processes necessary to deliver precision oncology care for veterans with lung cancer. With approval of the cancer care specialist, targeted sequencing of cancer genes (multiple biomarker panels) is performed on formalinfixed, paraffin-embedded tissue from newly diagnosed lung cancers as part of routine POP cancer care. Samples are shipped within 48 hours of diagnosis to Personal Genome Diagnostics (CancerSelect-88 targeted genome panel: PGD, Baltimore, MD) or Personalis (ACE Extended Cancer Panel: Menlo Park, CA). Following the sequencing of the targeted gene regions for mutations, a formal report of identified genomic aberrations is collated, annotated, and transmitted for inclusion in patient medical records. Both PGD and Personalis use N-of-One (Lexington, MA) to curate the medical literature and provide mutation annotations. The VA Computerized Patient Record System shares mutation results with the treating clinician, and a consultation service, offered through Specialty Care Access Network-Extension for Community technology, is available to help clinicians incorporate the test results into a treatment plan for the patient.
The POP is highly interdisciplinary: design and implementation required buy-in and coordinated efforts from the clinical medicine, laboratory medicine, pathology, pharmacy, radiology, and research services as well as from contracting, human resources, information technology, and procurement. With more than 150 specimens processed, procedures for tissue selection, processing, shipment, and tracking have been refined, and the informatics challenges met.
A Learning Health Care System Approach
Although the standard of care in oncology is evolving to include sequencing for all solid tumors and hematologic malignancies, the lack of correlated mutation status, patient outcomes data available for analysis, and difficulties in identifying subjects eligible for clinical trials of novel therapeutics combine to slow progress. The former problem arises from the effort required to aggregate EHR data from disparate systems as well as technical and cultural barriers to data sharing. The latter problem stems from the relative rarity of patients (and the difficulty identifying them) with a given mutation that determines eligibility for a clinical trial of a particular targeted therapy.
The POP attempts to overcome these limitations by embracing the principles of a LHS with clinical trials embedded to the extent possible in the clinical care ecosystem. The creation of a precision oncology data repository derived largely from the VA Corporate Data Warehouse makes correlated data available. This repository contains patient demographics and comorbidities, tumor features and mutation status, treatments, and outcomes. Data in the repository are used to both inform individual patient care (ie, what can we learn from past patients that would inform the care of the present patient?) and to allow for generalizable discovery and validation (ie, traditional data-mining research). Given a sufficiently large POP population, clinical trial-matching algorithms will identify patients available for any number of studies open for enrollment, thus reducing the existing bottleneck in clinical trial participation.
Rationale for a National Program
Numerous organizations, including the National Comprehensive Cancer Network, the American Society of Clinical Oncology Institute for Quality, and the Society for Gynecologic Oncology, already propose tumor sequencing as the standard of care for a variety of malignancies, and there is much to suggest that additional recommendations will be forthcoming.4-6 Expanding the VISN 1 POP across the nation provides a mechanism to minimize disparities in the delivery of precision oncology across the VA. The POP will afford opportunities to create VA-centric expertise derived from the POP data repository and filtered through a national tumor board. The POP will also expand opportunities for patients to participate in clinical trials and receive state-of-the-art treatments beyond what can be offered regionally.
Both knowledge generation and the creation of a large-scale clinical trial operation require the numbers of patients that only a national POP can achieve. The economies of scale introduced by wide participation will also reduce the cost of tumor sequencing, therapeutics, and infrastructure development and will eliminate otherwise duplicate efforts that would be required to create a number of smaller regional activities. Importantly, a national POP with sufficient voice would be far more effective at moving forward the LHS agenda.
Research Activities
For the majority of POP participants, the best hope for improved quality and quantity of life lies with targeted therapeutics that are under development and available only through research protocols. The VISN 1 Clinical Trial Network (directed by Mary Brophy, MD) has developed an Oncology Consortium that includes facilities both within and outside of VISN 1. The consortium has partnered with the National Cancer Institute through a storefront mechanism with the Southwest Oncology Group to become the first national VA cancer consortium to participate in intergroup protocols. Novel therapeutics will be available to POP participants through this and other partnerships with a variety of industry sponsors.
Novel, efficient, and nationally scalable mechanisms have been proposed to facilitate clinician participation and patient enrollment in clinical trials. Additionally, MAVERIC is working with the VA Central Institutional Review Board to advance a distributed enrollment innovation, which brings the clinical trial to the patient rather than have patients travel to facilities where studies are open.
Conclusion
Unique features of the VHA enable a national rollout of the POP, which VISN 1 successfully piloted. The first of its kind effort for precision medicine within the VA holds the promise of delivering cutting-edge, life-enhancing therapy to cancer patients.
This interdisciplinary program incorporates LHS principles so that delivery of care is accompanied by analytics that can be applied to decision making for future patients. Participation in clinical trials, facilitated by the consortium model, is a cardinal feature of the POP. Opportunity exists to explore novel trial designs that meet the unique challenges presented in precision medicine, where therapeutics tailored to uncommon mutations limit patient availability.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Research America. Truth and Consequences: Health R&D Spending in the U.S. (FY11-12). Research America Website. http://www.researchamerica.org/sites/default/files/uploads/healthdollar12.pdf. Accessed January 14, 2016.
2. Institute of Medicine. Large Simple Trials and Knowledge Generation in a Learning Healthcare System. Washington, DC: National Academies Press;2013:93-114.
3. Patient-Centered Outcomes Research Institute. About us. Patient-Centered Outcomes Research Institute Website. http://www.pcori.org/about-us. Updated October 14, 2014. Accessed January 21, 2016.
4. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). nonsmall cell lung cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated January 12, 2016. Accessed January 21, 2016.
5. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
6. Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. Society of Gynecologic Oncology Website. https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Updated March 2014. Accessed January 21, 2016.
Note: Page numbers differ between the print issue and digital edition.
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
The Case for VA Leadership
A solution to these 2 central dilemmas will result in continued health care improvement and, arguably, meaningful cost reduction through elimination of inferior treatments and optimization of individual patient care strategies. Since the current research culture does not reward such accomplishments, the responsibility for moving forward is left squarely on the health care systems. Said differently, a health care research budget that is a small fraction (5%) of health care expenditures is undersized and too culturally foreign for the task.1
A critical attribute that enables the VA to promote progress to the benefit of both veterans and taxpayers is an accountable care organization incentive to use a long horizon and invest in opportunities that reduce overall cost and improve outcomes for its beneficiariesover their entire lifespan. Although this feature is common to a handful of other large health care providers (Kaiser Permanente, Intermountain Healthcare, Mayo Clinic), those systems lack the assets fundamental to solution design that are broadly represented across VA medical centers: a staff, culture, and apparatus in support of research at most medical centers; an integrated electronic health record (EHR) for data access; and a patient population receptive to participating in activities that will aid fellow veterans.
Ongoing Programs
The VA is in an excellent position to create an efficient and scalable apparatus to perform comparative effectiveness studies.The Point-of-Care clinical trials program, proposed and championed by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) and supported by the VA Cooperative Studies Program, embeds low-risk clinical trials directly into the clinical ecosystem with a resultant decreased cost and increased relevance owing to study designs driven by current patient care processes.
This methodology and program is applauded by the Institute of Medicine and the Society for Clinical Trials, and each has invited MAVERIC to present at national meetings and roundtable discussions.2 Designation as a research “transformative initiative” by the VA Office of Research and Development (ORD) provided sufficient support to culminate in the imminent launch of the first national VA Point-of-Care Clinical Trial—the Diuretic Comparison Study. The VA is proceeding with this trial at 50 VA sites for a significantly lower cost.(VA Cooperative Studies Program study #597, methods manuscript in preparation). Results will inform the optimal initial treatment for hypertension and impact the care of millions of veterans and nonveterans.
Precision Oncology Program
The VA Precision Oncology Program (POP), initiated in VISN 1 and funded through a clinical care budget, goes a step further toward creating learning opportunities. The POP sequences the DNA of tumor tissue from veterans newly diagnosed with cancer to determine the DNA mutations responsible for the tumor development and behavior. Armed with this information, HCPs can optimize therapy based on mutation status by the delivery of drugs that are targeted against particular gene products.
Systematic implementation of the POP across all VAMCs will reduce disparities in cancer care induced by variation in medical center familiarity with treatment options. Features supported by the POP include enhanced enrollment of patients into clinical trials of novel targeted therapeutics and sharing of patient outcomes data to assist in decision support for future patients. In addition, this approach could facilitate the creation of a national VA database of cancer patient characteristics, tumor mutations, and cancer-related treatments and outcomes to accelerate the pace of discovery in VA cancer care.
Million Veteran Program
The Million Veteran Program (MVP) is a VA ORD initiative that asks veterans to share their medical data, lifestyle, and genetic data with researchers to allow for the discovery of correlations between their genetic profile and their health, disease and response totreatments. Currently more than 430,000 veterans have agreed to participate and have donated data and blood samples, and researchers are performing the first projects to use this resource.
Although the knowledge gained from these studies will be indirectly relevant to veterans in general the MVP presents an opportunity to present specific findings to individual participants that will directly affect their care. While reuse of the MVP resource for precision medicine is under consideration, there are important cultural and technical barriers that must be addressed. Like POP, integration of the MVP research program with clinical care should be carried out with consideration of a community of stakeholders and not driven exclusively by a research agenda.
Challenges in Moving Forward
Central to the implementation of a learning mechanism in health care systems is the recognition by administrators of the importance of the activity and appreciation of the business argument favoring the investment. This runs counter to the current notion of separate silos for health care and medical research whereby health care systems are liberated from the cost of investigation but then suffer from a dearth of knowledge relevant to their operation.
Additionally, research enterprises are not structured for such activities. Academic investigators are incentivized to create knowledge and generate publications and they understand best the currency of grant funding. Their world is not geared to reinvent or engineer solutions for health care systems. In light of these considerations, a decentralized approach that creates institutions for local learning needs to be developed and “owned” by individual and groups of medical centers with engagement of administration, patient, scientific, and community stakeholders. The Patient-Centered Outcome Research Institute (PCORI) and the consortia it has funded, PCOR-Net, have adopted this approach.3
Importantly, a new set of ethical and regulatory standards that distinguish it from traditional research must accompany progress in the creation of a learning health care system (LHS). Sharing of patient data to benefit fellow patients must come to be expected and without the formalized sharing agreements that are required in traditional research activities. Although the digitization of medical records makes most of what this article discusses possible, execution requires access to information technology resources and a talented staff.
More than a decade ago, the decision was made to dis-integrate the Office of Information Technology from VHA. This was executed with no provision to support the small army of VA clinician-informaticists who had done much in support of patient care, including the creation of the initial iteration of the VA EHR. Although the VA includes small pockets of this clinical informatics culture throughout its organization, the community has been largely silenced and taken refuge at academic affiliates. Access to VA information systems and funding opportunities for development and implementation of tools essential for learning will draw this intellectual capital back to the VA and allow for the VA to lead in this critical arena.
The VA Precision Oncology Program
Precision medicine is a medical model that incorporates the results of genetic diagnostic testing to customize or tailor medical decision making and treatment for the individual patient. Characteristics of the VA health care system that create a favored environment for introducing precision medicine include the single-payer model, where implementation decision and authority are centralized, a standardized EHR that enables informatics requirements, and a clinician and patient culture that supports innovation. To date, the benefits of precision medicine are most robust in cancer care. Under the leadership of Michael Mayo-Smith, MD, the VA New England Healthcare System has completed a regional pilot project in precision oncology that demonstrated feasibility of incorporating a precision medicine program in the clinical care environment.
For the majority of patients with lung cancer, DNA sequencing of tumor tissue identifies driver mutations—alterations believed responsible for tumor growth and behavior. The abundance of both driver and passenger mutations (those alterations whose significance is unknown) identified within an individual cancer specimen and the diversity of alterations found across the spectrum of all patients with cancer virtually assures the unique genetic profile (hence behavior) of any given patient’s tumor. The new generation of antineoplastic agents are targeted therapies that disrupt the downstream effects of these alterations and result in improved anticancer effects and reduced toxicity compared with conventional chemotherapy. The POP approach to cancer treatment determines the mutation profile of malignancies and identifies targeted therapies with the highest likelihood of treatment success. Although many driver mutation-targeted therapy combinations have been FDA approved, many more are in development and are available only as investigational agents.
Work Accomplished
Developed over the past 2 years in VISN 1, POP is a demonstration project that standardizes the processes necessary to deliver precision oncology care for veterans with lung cancer. With approval of the cancer care specialist, targeted sequencing of cancer genes (multiple biomarker panels) is performed on formalinfixed, paraffin-embedded tissue from newly diagnosed lung cancers as part of routine POP cancer care. Samples are shipped within 48 hours of diagnosis to Personal Genome Diagnostics (CancerSelect-88 targeted genome panel: PGD, Baltimore, MD) or Personalis (ACE Extended Cancer Panel: Menlo Park, CA). Following the sequencing of the targeted gene regions for mutations, a formal report of identified genomic aberrations is collated, annotated, and transmitted for inclusion in patient medical records. Both PGD and Personalis use N-of-One (Lexington, MA) to curate the medical literature and provide mutation annotations. The VA Computerized Patient Record System shares mutation results with the treating clinician, and a consultation service, offered through Specialty Care Access Network-Extension for Community technology, is available to help clinicians incorporate the test results into a treatment plan for the patient.
The POP is highly interdisciplinary: design and implementation required buy-in and coordinated efforts from the clinical medicine, laboratory medicine, pathology, pharmacy, radiology, and research services as well as from contracting, human resources, information technology, and procurement. With more than 150 specimens processed, procedures for tissue selection, processing, shipment, and tracking have been refined, and the informatics challenges met.
A Learning Health Care System Approach
Although the standard of care in oncology is evolving to include sequencing for all solid tumors and hematologic malignancies, the lack of correlated mutation status, patient outcomes data available for analysis, and difficulties in identifying subjects eligible for clinical trials of novel therapeutics combine to slow progress. The former problem arises from the effort required to aggregate EHR data from disparate systems as well as technical and cultural barriers to data sharing. The latter problem stems from the relative rarity of patients (and the difficulty identifying them) with a given mutation that determines eligibility for a clinical trial of a particular targeted therapy.
The POP attempts to overcome these limitations by embracing the principles of a LHS with clinical trials embedded to the extent possible in the clinical care ecosystem. The creation of a precision oncology data repository derived largely from the VA Corporate Data Warehouse makes correlated data available. This repository contains patient demographics and comorbidities, tumor features and mutation status, treatments, and outcomes. Data in the repository are used to both inform individual patient care (ie, what can we learn from past patients that would inform the care of the present patient?) and to allow for generalizable discovery and validation (ie, traditional data-mining research). Given a sufficiently large POP population, clinical trial-matching algorithms will identify patients available for any number of studies open for enrollment, thus reducing the existing bottleneck in clinical trial participation.
Rationale for a National Program
Numerous organizations, including the National Comprehensive Cancer Network, the American Society of Clinical Oncology Institute for Quality, and the Society for Gynecologic Oncology, already propose tumor sequencing as the standard of care for a variety of malignancies, and there is much to suggest that additional recommendations will be forthcoming.4-6 Expanding the VISN 1 POP across the nation provides a mechanism to minimize disparities in the delivery of precision oncology across the VA. The POP will afford opportunities to create VA-centric expertise derived from the POP data repository and filtered through a national tumor board. The POP will also expand opportunities for patients to participate in clinical trials and receive state-of-the-art treatments beyond what can be offered regionally.
Both knowledge generation and the creation of a large-scale clinical trial operation require the numbers of patients that only a national POP can achieve. The economies of scale introduced by wide participation will also reduce the cost of tumor sequencing, therapeutics, and infrastructure development and will eliminate otherwise duplicate efforts that would be required to create a number of smaller regional activities. Importantly, a national POP with sufficient voice would be far more effective at moving forward the LHS agenda.
Research Activities
For the majority of POP participants, the best hope for improved quality and quantity of life lies with targeted therapeutics that are under development and available only through research protocols. The VISN 1 Clinical Trial Network (directed by Mary Brophy, MD) has developed an Oncology Consortium that includes facilities both within and outside of VISN 1. The consortium has partnered with the National Cancer Institute through a storefront mechanism with the Southwest Oncology Group to become the first national VA cancer consortium to participate in intergroup protocols. Novel therapeutics will be available to POP participants through this and other partnerships with a variety of industry sponsors.
Novel, efficient, and nationally scalable mechanisms have been proposed to facilitate clinician participation and patient enrollment in clinical trials. Additionally, MAVERIC is working with the VA Central Institutional Review Board to advance a distributed enrollment innovation, which brings the clinical trial to the patient rather than have patients travel to facilities where studies are open.
Conclusion
Unique features of the VHA enable a national rollout of the POP, which VISN 1 successfully piloted. The first of its kind effort for precision medicine within the VA holds the promise of delivering cutting-edge, life-enhancing therapy to cancer patients.
This interdisciplinary program incorporates LHS principles so that delivery of care is accompanied by analytics that can be applied to decision making for future patients. Participation in clinical trials, facilitated by the consortium model, is a cardinal feature of the POP. Opportunity exists to explore novel trial designs that meet the unique challenges presented in precision medicine, where therapeutics tailored to uncommon mutations limit patient availability.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Traditional research methods, well suited for scientific discovery and drug development, fall short of providing health care systems with pragmatic information in 2 important ways: Current funding and institutions cannot support comparative effectiveness studies in sufficient numbers to answer the plethora of important clinical questions that confront health care providers (HCPs). The resultant knowledge gap manifests in treatment variability based on clinician impression rather than on direct evidence. A second equally important deficiency is the inability to make full use of the knowledge acquired in treating past patients to determine the best treatment option for the current patient.
Digitization of medical records, creation of health care system corporate data warehouses, and state-of-the-art analytical tools already allow for this revolutionary approach to patient care. Obstructing progress, however, is a lack of understanding by health care system managers and HCPs of the capability of the approach, and unfamiliarity with the requisite informatics by traditional medical researchers. Furthermore the regulatory approach is tilted against the reuse of medical record data for learning and toward strict adherence to patient confidentiality.
The Case for VA Leadership
A solution to these 2 central dilemmas will result in continued health care improvement and, arguably, meaningful cost reduction through elimination of inferior treatments and optimization of individual patient care strategies. Since the current research culture does not reward such accomplishments, the responsibility for moving forward is left squarely on the health care systems. Said differently, a health care research budget that is a small fraction (5%) of health care expenditures is undersized and too culturally foreign for the task.1
A critical attribute that enables the VA to promote progress to the benefit of both veterans and taxpayers is an accountable care organization incentive to use a long horizon and invest in opportunities that reduce overall cost and improve outcomes for its beneficiariesover their entire lifespan. Although this feature is common to a handful of other large health care providers (Kaiser Permanente, Intermountain Healthcare, Mayo Clinic), those systems lack the assets fundamental to solution design that are broadly represented across VA medical centers: a staff, culture, and apparatus in support of research at most medical centers; an integrated electronic health record (EHR) for data access; and a patient population receptive to participating in activities that will aid fellow veterans.
Ongoing Programs
The VA is in an excellent position to create an efficient and scalable apparatus to perform comparative effectiveness studies.The Point-of-Care clinical trials program, proposed and championed by the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) and supported by the VA Cooperative Studies Program, embeds low-risk clinical trials directly into the clinical ecosystem with a resultant decreased cost and increased relevance owing to study designs driven by current patient care processes.
This methodology and program is applauded by the Institute of Medicine and the Society for Clinical Trials, and each has invited MAVERIC to present at national meetings and roundtable discussions.2 Designation as a research “transformative initiative” by the VA Office of Research and Development (ORD) provided sufficient support to culminate in the imminent launch of the first national VA Point-of-Care Clinical Trial—the Diuretic Comparison Study. The VA is proceeding with this trial at 50 VA sites for a significantly lower cost.(VA Cooperative Studies Program study #597, methods manuscript in preparation). Results will inform the optimal initial treatment for hypertension and impact the care of millions of veterans and nonveterans.
Precision Oncology Program
The VA Precision Oncology Program (POP), initiated in VISN 1 and funded through a clinical care budget, goes a step further toward creating learning opportunities. The POP sequences the DNA of tumor tissue from veterans newly diagnosed with cancer to determine the DNA mutations responsible for the tumor development and behavior. Armed with this information, HCPs can optimize therapy based on mutation status by the delivery of drugs that are targeted against particular gene products.
Systematic implementation of the POP across all VAMCs will reduce disparities in cancer care induced by variation in medical center familiarity with treatment options. Features supported by the POP include enhanced enrollment of patients into clinical trials of novel targeted therapeutics and sharing of patient outcomes data to assist in decision support for future patients. In addition, this approach could facilitate the creation of a national VA database of cancer patient characteristics, tumor mutations, and cancer-related treatments and outcomes to accelerate the pace of discovery in VA cancer care.
Million Veteran Program
The Million Veteran Program (MVP) is a VA ORD initiative that asks veterans to share their medical data, lifestyle, and genetic data with researchers to allow for the discovery of correlations between their genetic profile and their health, disease and response totreatments. Currently more than 430,000 veterans have agreed to participate and have donated data and blood samples, and researchers are performing the first projects to use this resource.
Although the knowledge gained from these studies will be indirectly relevant to veterans in general the MVP presents an opportunity to present specific findings to individual participants that will directly affect their care. While reuse of the MVP resource for precision medicine is under consideration, there are important cultural and technical barriers that must be addressed. Like POP, integration of the MVP research program with clinical care should be carried out with consideration of a community of stakeholders and not driven exclusively by a research agenda.
Challenges in Moving Forward
Central to the implementation of a learning mechanism in health care systems is the recognition by administrators of the importance of the activity and appreciation of the business argument favoring the investment. This runs counter to the current notion of separate silos for health care and medical research whereby health care systems are liberated from the cost of investigation but then suffer from a dearth of knowledge relevant to their operation.
Additionally, research enterprises are not structured for such activities. Academic investigators are incentivized to create knowledge and generate publications and they understand best the currency of grant funding. Their world is not geared to reinvent or engineer solutions for health care systems. In light of these considerations, a decentralized approach that creates institutions for local learning needs to be developed and “owned” by individual and groups of medical centers with engagement of administration, patient, scientific, and community stakeholders. The Patient-Centered Outcome Research Institute (PCORI) and the consortia it has funded, PCOR-Net, have adopted this approach.3
Importantly, a new set of ethical and regulatory standards that distinguish it from traditional research must accompany progress in the creation of a learning health care system (LHS). Sharing of patient data to benefit fellow patients must come to be expected and without the formalized sharing agreements that are required in traditional research activities. Although the digitization of medical records makes most of what this article discusses possible, execution requires access to information technology resources and a talented staff.
More than a decade ago, the decision was made to dis-integrate the Office of Information Technology from VHA. This was executed with no provision to support the small army of VA clinician-informaticists who had done much in support of patient care, including the creation of the initial iteration of the VA EHR. Although the VA includes small pockets of this clinical informatics culture throughout its organization, the community has been largely silenced and taken refuge at academic affiliates. Access to VA information systems and funding opportunities for development and implementation of tools essential for learning will draw this intellectual capital back to the VA and allow for the VA to lead in this critical arena.
The VA Precision Oncology Program
Precision medicine is a medical model that incorporates the results of genetic diagnostic testing to customize or tailor medical decision making and treatment for the individual patient. Characteristics of the VA health care system that create a favored environment for introducing precision medicine include the single-payer model, where implementation decision and authority are centralized, a standardized EHR that enables informatics requirements, and a clinician and patient culture that supports innovation. To date, the benefits of precision medicine are most robust in cancer care. Under the leadership of Michael Mayo-Smith, MD, the VA New England Healthcare System has completed a regional pilot project in precision oncology that demonstrated feasibility of incorporating a precision medicine program in the clinical care environment.
For the majority of patients with lung cancer, DNA sequencing of tumor tissue identifies driver mutations—alterations believed responsible for tumor growth and behavior. The abundance of both driver and passenger mutations (those alterations whose significance is unknown) identified within an individual cancer specimen and the diversity of alterations found across the spectrum of all patients with cancer virtually assures the unique genetic profile (hence behavior) of any given patient’s tumor. The new generation of antineoplastic agents are targeted therapies that disrupt the downstream effects of these alterations and result in improved anticancer effects and reduced toxicity compared with conventional chemotherapy. The POP approach to cancer treatment determines the mutation profile of malignancies and identifies targeted therapies with the highest likelihood of treatment success. Although many driver mutation-targeted therapy combinations have been FDA approved, many more are in development and are available only as investigational agents.
Work Accomplished
Developed over the past 2 years in VISN 1, POP is a demonstration project that standardizes the processes necessary to deliver precision oncology care for veterans with lung cancer. With approval of the cancer care specialist, targeted sequencing of cancer genes (multiple biomarker panels) is performed on formalinfixed, paraffin-embedded tissue from newly diagnosed lung cancers as part of routine POP cancer care. Samples are shipped within 48 hours of diagnosis to Personal Genome Diagnostics (CancerSelect-88 targeted genome panel: PGD, Baltimore, MD) or Personalis (ACE Extended Cancer Panel: Menlo Park, CA). Following the sequencing of the targeted gene regions for mutations, a formal report of identified genomic aberrations is collated, annotated, and transmitted for inclusion in patient medical records. Both PGD and Personalis use N-of-One (Lexington, MA) to curate the medical literature and provide mutation annotations. The VA Computerized Patient Record System shares mutation results with the treating clinician, and a consultation service, offered through Specialty Care Access Network-Extension for Community technology, is available to help clinicians incorporate the test results into a treatment plan for the patient.
The POP is highly interdisciplinary: design and implementation required buy-in and coordinated efforts from the clinical medicine, laboratory medicine, pathology, pharmacy, radiology, and research services as well as from contracting, human resources, information technology, and procurement. With more than 150 specimens processed, procedures for tissue selection, processing, shipment, and tracking have been refined, and the informatics challenges met.
A Learning Health Care System Approach
Although the standard of care in oncology is evolving to include sequencing for all solid tumors and hematologic malignancies, the lack of correlated mutation status, patient outcomes data available for analysis, and difficulties in identifying subjects eligible for clinical trials of novel therapeutics combine to slow progress. The former problem arises from the effort required to aggregate EHR data from disparate systems as well as technical and cultural barriers to data sharing. The latter problem stems from the relative rarity of patients (and the difficulty identifying them) with a given mutation that determines eligibility for a clinical trial of a particular targeted therapy.
The POP attempts to overcome these limitations by embracing the principles of a LHS with clinical trials embedded to the extent possible in the clinical care ecosystem. The creation of a precision oncology data repository derived largely from the VA Corporate Data Warehouse makes correlated data available. This repository contains patient demographics and comorbidities, tumor features and mutation status, treatments, and outcomes. Data in the repository are used to both inform individual patient care (ie, what can we learn from past patients that would inform the care of the present patient?) and to allow for generalizable discovery and validation (ie, traditional data-mining research). Given a sufficiently large POP population, clinical trial-matching algorithms will identify patients available for any number of studies open for enrollment, thus reducing the existing bottleneck in clinical trial participation.
Rationale for a National Program
Numerous organizations, including the National Comprehensive Cancer Network, the American Society of Clinical Oncology Institute for Quality, and the Society for Gynecologic Oncology, already propose tumor sequencing as the standard of care for a variety of malignancies, and there is much to suggest that additional recommendations will be forthcoming.4-6 Expanding the VISN 1 POP across the nation provides a mechanism to minimize disparities in the delivery of precision oncology across the VA. The POP will afford opportunities to create VA-centric expertise derived from the POP data repository and filtered through a national tumor board. The POP will also expand opportunities for patients to participate in clinical trials and receive state-of-the-art treatments beyond what can be offered regionally.
Both knowledge generation and the creation of a large-scale clinical trial operation require the numbers of patients that only a national POP can achieve. The economies of scale introduced by wide participation will also reduce the cost of tumor sequencing, therapeutics, and infrastructure development and will eliminate otherwise duplicate efforts that would be required to create a number of smaller regional activities. Importantly, a national POP with sufficient voice would be far more effective at moving forward the LHS agenda.
Research Activities
For the majority of POP participants, the best hope for improved quality and quantity of life lies with targeted therapeutics that are under development and available only through research protocols. The VISN 1 Clinical Trial Network (directed by Mary Brophy, MD) has developed an Oncology Consortium that includes facilities both within and outside of VISN 1. The consortium has partnered with the National Cancer Institute through a storefront mechanism with the Southwest Oncology Group to become the first national VA cancer consortium to participate in intergroup protocols. Novel therapeutics will be available to POP participants through this and other partnerships with a variety of industry sponsors.
Novel, efficient, and nationally scalable mechanisms have been proposed to facilitate clinician participation and patient enrollment in clinical trials. Additionally, MAVERIC is working with the VA Central Institutional Review Board to advance a distributed enrollment innovation, which brings the clinical trial to the patient rather than have patients travel to facilities where studies are open.
Conclusion
Unique features of the VHA enable a national rollout of the POP, which VISN 1 successfully piloted. The first of its kind effort for precision medicine within the VA holds the promise of delivering cutting-edge, life-enhancing therapy to cancer patients.
This interdisciplinary program incorporates LHS principles so that delivery of care is accompanied by analytics that can be applied to decision making for future patients. Participation in clinical trials, facilitated by the consortium model, is a cardinal feature of the POP. Opportunity exists to explore novel trial designs that meet the unique challenges presented in precision medicine, where therapeutics tailored to uncommon mutations limit patient availability.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Research America. Truth and Consequences: Health R&D Spending in the U.S. (FY11-12). Research America Website. http://www.researchamerica.org/sites/default/files/uploads/healthdollar12.pdf. Accessed January 14, 2016.
2. Institute of Medicine. Large Simple Trials and Knowledge Generation in a Learning Healthcare System. Washington, DC: National Academies Press;2013:93-114.
3. Patient-Centered Outcomes Research Institute. About us. Patient-Centered Outcomes Research Institute Website. http://www.pcori.org/about-us. Updated October 14, 2014. Accessed January 21, 2016.
4. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). nonsmall cell lung cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated January 12, 2016. Accessed January 21, 2016.
5. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
6. Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. Society of Gynecologic Oncology Website. https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Updated March 2014. Accessed January 21, 2016.
Note: Page numbers differ between the print issue and digital edition.
1. Research America. Truth and Consequences: Health R&D Spending in the U.S. (FY11-12). Research America Website. http://www.researchamerica.org/sites/default/files/uploads/healthdollar12.pdf. Accessed January 14, 2016.
2. Institute of Medicine. Large Simple Trials and Knowledge Generation in a Learning Healthcare System. Washington, DC: National Academies Press;2013:93-114.
3. Patient-Centered Outcomes Research Institute. About us. Patient-Centered Outcomes Research Institute Website. http://www.pcori.org/about-us. Updated October 14, 2014. Accessed January 21, 2016.
4. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). nonsmall cell lung cancer. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated January 12, 2016. Accessed January 21, 2016.
5. Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32(32):3673-3679.
6. Society of Gynecologic Oncology. SGO clinical practice statement: next generation cancer gene panels versus gene by gene testing. Society of Gynecologic Oncology Website. https://www.sgo.org/clinical-practice/guidelines/next-generation-cancer-gene-panels-versus-gene-by-gene-testing/. Updated March 2014. Accessed January 21, 2016.
Note: Page numbers differ between the print issue and digital edition.
A Systems Engineering and Decision-Support Tool to Enhance Care of Veterans Diagnosed With Prostate Cancer
In the U.S. in 2015, there were more than 220,800 new cases of prostate cancer and about 27,000 deaths due to prostate cancer. Across the VHA, prostate cancer is the most common nonskin cancer malignancy, and more than 25,000 patients are diagnosed yearly.1 Patients who receive treatment for prostate cancer have excellent rates of disease-specific survival: nearly 100% at 5 years, 99% at 10 years, and 94% at 15 years.
Prostate cancer is one of several cancers that can be treated successfully with radiotherapy alone, and its success or failure is defined by a discrete numerical value from the prostate specific antigen (PSA) blood test. Failure occurs when the PSA is 2.0 ng/mL greater than the lowest PSA value posttreatment.2 Multiple clinical trials have used this method to determine whether or not a certain intervention is successful.
Although high rates of survival and clear biochemical indicators exist, patients diagnosed with and treated for prostate cancer are at significant risk of PSA failure. The risk can range from 5% to 70% by 10 years, depending on the the treatment modality, risk group, and series reported.3 These patients require long-term follow-up for disease recurrence and management of adverse effects. The current guidelines recommend annual follow-up care 5 years after treatment.4
The number of veterans requiring follow-up care for prostate cancer constitutes a disproportionately large share of visits compared with those of other cancers, such as cancers of the head and neck region, chest, or gastrointestinal system, and there are many challenges to providing quality long-term care. Veterans in rural locations face barriers to accessing follow-up care for effective management.
Missed appointments can compromise long-term care, escalating the risk of nonadherence over time. Missed appointments occur commonly and may negatively impact outcomes and can restrict care for other patients.5 In a recently published article by Percac-Lima and colleagues, no-show rates among 5 cancer center clinics at the Massachusetts General Hospital were as high as 10%.6
Missed appointments have also been associated with decreased quality of care and increased resource use.7 Patients with prostate cancer who miss follow-up visits are at risk for having their cancer progress to the point it becomes symptomatic and no longer treatable with salvage therapies. These patients also risk lost efficacy of treatments that are still available.
Due to these challenges, automated PSA tracking systems can be an effective way to ensure that quality, longterm care is provided to the patient. The purpose of the PSA tracking system is to identify patients who require intervention before they present with clinical problems. A PSA tracking system helps prevent patients being inappropriately lost to follow-up or missing a needed followup PSA blood test. The tracker would serve to correctly identify, among thousands or millions of patients in the electronic medical record system (EMR), which patients were at risk of failure or active failing biochemically by triggering an alert to the cancer specialist to assess that patient’s chart and determine whether a higher level of intervention is required. It could also serve to avoid unnecessary travel or inconvenience to a patient whose prostate cancer disease status can correctly be confirmed as under control by a simple blood test and related to the patient by phone, letter, or online.
Prostate-specific antigen trackers have been used to monitor patients for postprostatectomy treatment failures on a small scale in Ireland.8 For a PSA tracker to be successful, the system must have access to all posttreatment PSA data. The VHA is uniquely positioned to leverage this information because most patients who receive treatment for prostate cancer at a VHA facility stay within the VHA system for follow-up care. All laboratory data are also collected and stored in the EMR system, which is sent daily to the VA Corporate Data Warehouse (CDW).
Project Proposal
In November 2014, the Office of Rural Health and the National Radiation Oncology Program Office issued a request for proposal for projects that would improve follow-up care for rural patients with prostate cancer following treatment with radiotherapy. A team of health care providers at the Hunter Holmes McGuire VAMC drafted a proposal to address this problem. Veterans Engineering Resource Centers (VERCs) in Pittsburgh and New England were also included in the proposal as key collaborators. Staff from these 2 centers brought expertise in analytics, implementation, and project management to help rapidly innovate and implement a PSA tracking system.
The proposal was submitted on time and required approval at multiple levels, including facility and VISN leadership. It was essential that the perceived value of the proposal be readily apparent to all stakeholders, or the necessary approvals would not have been obtainable.
The proposal was accepted, and funds were transferred in February 2015. Four core team members led rapid cycle design and prototyping of the PSA tracking system. The project lead and sponsor was a radiation oncologist and service line chief at the Hunter Holmes Mc-Guire VAMC who provided overall strategy, direction, and clinical domain knowledge. A VERC engineer provided project management and analytic expertise, and a VERC developer designed code to pull data from the VA CDW and led design of the user interface. Finally, a nurse practitioner dedicated numerous hours to review charts, contact patients, write notes, and provide user feedback on the system.
Development
The purpose of the radiation oncology-centered PSA tracking system within the VA was to identify patients who require intervention following definitive treatment with radiotherapy before they present with clinical problems from disease recurrence. The PSA tracker that the authors developed was based on a relatively simple algorithm that sorts through thousands of patient records and identifies patients who had a diagnosis of prostate cancer but did not have metastatic disease, were treated at the Hunter Homes McGuire VAMC with radiation therapy, were not seen in clinic within the past 400 days, and did not have a PSA drawn within 450 days or had a rising PSA of 0.5 or more above the lowest PSA value posttreatment. In other words, the tracker uses the power of the CDW to successfully identify the exact charts that need to be reviewed and helped ensure that patients were not lost to follow-up or did not receive appropriate care. Without the PSA tracking system, providers would not know whether or not patients were being missed.
Development of the tracker required regular team meetings with well-defined, achievable goals. The team consisted of a physician as team leader, a biostatistician with structured query language experience who had access to the CDW, and a project manager with an industrial engineering background. The team met weekly. The project was broken into several components that were achieved in series and at times in parallel. The first goal was determining whether an algorithm could be written to correctly identify patients with prostate cancer treated with radiotherapy at the Hunter Holmes McGuire VAMC who did not have metastatic disease.
By using various values available within the CDW, such as ICD 9 codes, CPT codes, PSA laboratory values, dates, and other information, the authors were able to create a successful algorithm. The ability to complete the algorithm in a short time frame wasfacilitated by several factors: a very small group, weekly meetings, good communication, easy to understand concepts across all disciplines, ability to quickly determine whether the results of the algorithm were accurate or not, and high perceived value of the end product that served to motivate the team members. Each meeting ended with clear action items and a scheduled time for the next meeting. Throughout the design and implementation process, the team discussed any problems, planned solutions, and reviewed the status of project deliverables.
Results
The tracker has already been useful for reengaging patients in care and ensuring PSA testing is occurring at appropriate intervals. Of the more than 50,000 veterans currently alive who have received care at the Hunter Holmes McGuire VAMC, 1,158 were treated with radiotherapy definitively for prostate cancer. A total of 455 (39%) prostate cancer survivors had not been seen in the clinic in the past 13 months. Of these patients, 294 were being followed appropriately elsewhere within the VA system. Meanwhile, 161 neither had a PSA level nor a prostate cancer follow-up appointment recorded in the past 13 months anywhere within the entire VA system. This yielded a loss-to-follow-up rate of 14% (161/1,158).
The authors found that 21 (13%) of patients had a PSA level > 2.0 ng/mL above the posttreatment nadir.9 The authors were able to review the charts of these 21 patients to assess whether or not they required or were suitable for salvage brachytherapy. Of these, 1 has been set up for salvage high-dose rate brachytherapy treatment. Out of 50,000 patients, the PSA tracker algorithm facilitated a focus on the 21 patients who were most likely to be in need, making it possible for a nurse practitioner and physician to spend just 3 hours looking at charts instead of 3,000 hours.
Sustained use of the tracker is critically important to the Hunter Holmes McGuire VAMC project team and for the care of its veterans. Funds to support sustaining the program have been approved for fiscal year 2016. Efforts are underway to try to scale up the program and test the feasibility of disseminating the program across the enterprise. The authors estimate that an experienced advanced care provider would spend about 8 hours a week reviewing charts, contacting patients in the program, sending letters, and reviewing nuanced cases. The program would still benefit from increased automation as well as identifying a method for obtaining appropriate workload credit for this unique program.
The next phase of development will focus on improving the user interface and allowing easier transfer of information between the tracker and notes within the Computerized Patient Record System. The team will also look into automating additional parts of the process but feels that a clinician (ideally a nurse practitioner or physician assistant working with the radiation oncologist) must be part of the team, because clinical decisions must be made based on multiple variables and patient preferences.
The development of this PSA tracking system has significant future implications for improving biochemical control and extending patient survival. The tracker could be easily adapted to monitor prostatectomy patients and PSA failures requiring early intervention with salvage radiotherapy. It has been shown in several publications that early treatment with radiotherapy while PSA is relatively low results in higher rates of long-term biochemical control.10-22
Conclusions
Access to the VA CDW was essential for the success of the PSA tracking system. Furthermore, veteran patients with prostate cancer tend toward a high rate of adherence and typically stay within the system. Prostate cancer is one of the few cancers where disease recurrence is detected and determined by a quantitative laboratory value, which lends itself well to objective arithmetical tracking and detection.
Patients with prostate cancer are at risk of recurrence years after their treatment and require a long-term follow-up that includes annual PSA checks. Identifying patients who have missed follow-up appointments and not had their PSA checked is essential for combating prostate cancer recurrences. The VA CDW makes it possible to track the majority of the patients with prostate cancer who are treated in the system and identify those most in need of early treatment or early intervention before they become
symptomatic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. What are the key statistics about prostate cancer? American Cancer Society Website. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer key-statistics. Last revised March 12, 2015. Accessed January 11, 2016.
2. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
3. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22-29.
4. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guidelines endorsement. J Clin Oncol. 2015;33(9):1078-1085.
5. Husain-Gambles M, Neal RD, Dempsey O, Lawlor DA, Hodgson J. Missed appointments in primary care: questionnaire and focus group study of health professionals. Br J Gen Pract. 2004;54(499):108-113.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly DA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Hwang AS, Atlas SJ, Ashburner JM, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
8. Hennessey DB, Lynn C, Templeton H, Chambers K, Mulholland C. The PSA tracker: a computerised health care system initiative in Northern Ireland. Ulster Med J. 2013;82(3):146-149.
9. Chang M, Troeschel S, DeSotto K, et al. Development of a Post-Radiotherapy Prostate-Specific Antigen Detection and Tracking System. Poster presented at: Genito-Urinary Cancers Symposium Annual Meeting; January 2016; San Francisco, CA.
10. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific
antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol
Phys. 2000;48(2):369-375.
11. Catton C, Gospodarowicz M, Warde P, et al. Adjuvant and salvage radiation
therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother
Oncol. 2001;59(1):51-60.
12. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63(1):134-140.
13. Katz MS, Zelefsky MJ, Venkatraman ES, Hummer A, Leibal SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483-489.
14. Leventis AK, Shariat SF, Kattan MW, Butler EB, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030-1039.
15. Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for
biochemical failure of radical prostatectomy: a single-institution experience. Urology.
2003;61(6):1204-1210.
16. Maier J, Forman J, Tekyi-Mensah S, Bolton S, Patel R, Pontes JE. Salvage radiation
for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22(1):50-56.
17. Perez CA, Michalski JM, Baglan K, Andriole G, Cui Q, Lockett MA. Radiation therapy for increasing prostate-specific antigen levels after radical prostatectomy. Clin Prostate Cancer. 2003;1(4):235-241.
18. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163(3):845-850.
19. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or
persistent PSA after radical prostatectomy. Urology. 2002;60(2):281-287.
20. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325-1332.
21. Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52(6):1034-1040.
22. Vicini FA, Ziaja EL, Kestin LL, et al. Treatment outcome with adjuvant and salvage irradiation after radical prostatectomy for prostate cancer. Urology. 1999;54(1):111-117.
Note: Page numbers differ between the print issue and digital edition.
In the U.S. in 2015, there were more than 220,800 new cases of prostate cancer and about 27,000 deaths due to prostate cancer. Across the VHA, prostate cancer is the most common nonskin cancer malignancy, and more than 25,000 patients are diagnosed yearly.1 Patients who receive treatment for prostate cancer have excellent rates of disease-specific survival: nearly 100% at 5 years, 99% at 10 years, and 94% at 15 years.
Prostate cancer is one of several cancers that can be treated successfully with radiotherapy alone, and its success or failure is defined by a discrete numerical value from the prostate specific antigen (PSA) blood test. Failure occurs when the PSA is 2.0 ng/mL greater than the lowest PSA value posttreatment.2 Multiple clinical trials have used this method to determine whether or not a certain intervention is successful.
Although high rates of survival and clear biochemical indicators exist, patients diagnosed with and treated for prostate cancer are at significant risk of PSA failure. The risk can range from 5% to 70% by 10 years, depending on the the treatment modality, risk group, and series reported.3 These patients require long-term follow-up for disease recurrence and management of adverse effects. The current guidelines recommend annual follow-up care 5 years after treatment.4
The number of veterans requiring follow-up care for prostate cancer constitutes a disproportionately large share of visits compared with those of other cancers, such as cancers of the head and neck region, chest, or gastrointestinal system, and there are many challenges to providing quality long-term care. Veterans in rural locations face barriers to accessing follow-up care for effective management.
Missed appointments can compromise long-term care, escalating the risk of nonadherence over time. Missed appointments occur commonly and may negatively impact outcomes and can restrict care for other patients.5 In a recently published article by Percac-Lima and colleagues, no-show rates among 5 cancer center clinics at the Massachusetts General Hospital were as high as 10%.6
Missed appointments have also been associated with decreased quality of care and increased resource use.7 Patients with prostate cancer who miss follow-up visits are at risk for having their cancer progress to the point it becomes symptomatic and no longer treatable with salvage therapies. These patients also risk lost efficacy of treatments that are still available.
Due to these challenges, automated PSA tracking systems can be an effective way to ensure that quality, longterm care is provided to the patient. The purpose of the PSA tracking system is to identify patients who require intervention before they present with clinical problems. A PSA tracking system helps prevent patients being inappropriately lost to follow-up or missing a needed followup PSA blood test. The tracker would serve to correctly identify, among thousands or millions of patients in the electronic medical record system (EMR), which patients were at risk of failure or active failing biochemically by triggering an alert to the cancer specialist to assess that patient’s chart and determine whether a higher level of intervention is required. It could also serve to avoid unnecessary travel or inconvenience to a patient whose prostate cancer disease status can correctly be confirmed as under control by a simple blood test and related to the patient by phone, letter, or online.
Prostate-specific antigen trackers have been used to monitor patients for postprostatectomy treatment failures on a small scale in Ireland.8 For a PSA tracker to be successful, the system must have access to all posttreatment PSA data. The VHA is uniquely positioned to leverage this information because most patients who receive treatment for prostate cancer at a VHA facility stay within the VHA system for follow-up care. All laboratory data are also collected and stored in the EMR system, which is sent daily to the VA Corporate Data Warehouse (CDW).
Project Proposal
In November 2014, the Office of Rural Health and the National Radiation Oncology Program Office issued a request for proposal for projects that would improve follow-up care for rural patients with prostate cancer following treatment with radiotherapy. A team of health care providers at the Hunter Holmes McGuire VAMC drafted a proposal to address this problem. Veterans Engineering Resource Centers (VERCs) in Pittsburgh and New England were also included in the proposal as key collaborators. Staff from these 2 centers brought expertise in analytics, implementation, and project management to help rapidly innovate and implement a PSA tracking system.
The proposal was submitted on time and required approval at multiple levels, including facility and VISN leadership. It was essential that the perceived value of the proposal be readily apparent to all stakeholders, or the necessary approvals would not have been obtainable.
The proposal was accepted, and funds were transferred in February 2015. Four core team members led rapid cycle design and prototyping of the PSA tracking system. The project lead and sponsor was a radiation oncologist and service line chief at the Hunter Holmes Mc-Guire VAMC who provided overall strategy, direction, and clinical domain knowledge. A VERC engineer provided project management and analytic expertise, and a VERC developer designed code to pull data from the VA CDW and led design of the user interface. Finally, a nurse practitioner dedicated numerous hours to review charts, contact patients, write notes, and provide user feedback on the system.
Development
The purpose of the radiation oncology-centered PSA tracking system within the VA was to identify patients who require intervention following definitive treatment with radiotherapy before they present with clinical problems from disease recurrence. The PSA tracker that the authors developed was based on a relatively simple algorithm that sorts through thousands of patient records and identifies patients who had a diagnosis of prostate cancer but did not have metastatic disease, were treated at the Hunter Homes McGuire VAMC with radiation therapy, were not seen in clinic within the past 400 days, and did not have a PSA drawn within 450 days or had a rising PSA of 0.5 or more above the lowest PSA value posttreatment. In other words, the tracker uses the power of the CDW to successfully identify the exact charts that need to be reviewed and helped ensure that patients were not lost to follow-up or did not receive appropriate care. Without the PSA tracking system, providers would not know whether or not patients were being missed.
Development of the tracker required regular team meetings with well-defined, achievable goals. The team consisted of a physician as team leader, a biostatistician with structured query language experience who had access to the CDW, and a project manager with an industrial engineering background. The team met weekly. The project was broken into several components that were achieved in series and at times in parallel. The first goal was determining whether an algorithm could be written to correctly identify patients with prostate cancer treated with radiotherapy at the Hunter Holmes McGuire VAMC who did not have metastatic disease.
By using various values available within the CDW, such as ICD 9 codes, CPT codes, PSA laboratory values, dates, and other information, the authors were able to create a successful algorithm. The ability to complete the algorithm in a short time frame wasfacilitated by several factors: a very small group, weekly meetings, good communication, easy to understand concepts across all disciplines, ability to quickly determine whether the results of the algorithm were accurate or not, and high perceived value of the end product that served to motivate the team members. Each meeting ended with clear action items and a scheduled time for the next meeting. Throughout the design and implementation process, the team discussed any problems, planned solutions, and reviewed the status of project deliverables.
Results
The tracker has already been useful for reengaging patients in care and ensuring PSA testing is occurring at appropriate intervals. Of the more than 50,000 veterans currently alive who have received care at the Hunter Holmes McGuire VAMC, 1,158 were treated with radiotherapy definitively for prostate cancer. A total of 455 (39%) prostate cancer survivors had not been seen in the clinic in the past 13 months. Of these patients, 294 were being followed appropriately elsewhere within the VA system. Meanwhile, 161 neither had a PSA level nor a prostate cancer follow-up appointment recorded in the past 13 months anywhere within the entire VA system. This yielded a loss-to-follow-up rate of 14% (161/1,158).
The authors found that 21 (13%) of patients had a PSA level > 2.0 ng/mL above the posttreatment nadir.9 The authors were able to review the charts of these 21 patients to assess whether or not they required or were suitable for salvage brachytherapy. Of these, 1 has been set up for salvage high-dose rate brachytherapy treatment. Out of 50,000 patients, the PSA tracker algorithm facilitated a focus on the 21 patients who were most likely to be in need, making it possible for a nurse practitioner and physician to spend just 3 hours looking at charts instead of 3,000 hours.
Sustained use of the tracker is critically important to the Hunter Holmes McGuire VAMC project team and for the care of its veterans. Funds to support sustaining the program have been approved for fiscal year 2016. Efforts are underway to try to scale up the program and test the feasibility of disseminating the program across the enterprise. The authors estimate that an experienced advanced care provider would spend about 8 hours a week reviewing charts, contacting patients in the program, sending letters, and reviewing nuanced cases. The program would still benefit from increased automation as well as identifying a method for obtaining appropriate workload credit for this unique program.
The next phase of development will focus on improving the user interface and allowing easier transfer of information between the tracker and notes within the Computerized Patient Record System. The team will also look into automating additional parts of the process but feels that a clinician (ideally a nurse practitioner or physician assistant working with the radiation oncologist) must be part of the team, because clinical decisions must be made based on multiple variables and patient preferences.
The development of this PSA tracking system has significant future implications for improving biochemical control and extending patient survival. The tracker could be easily adapted to monitor prostatectomy patients and PSA failures requiring early intervention with salvage radiotherapy. It has been shown in several publications that early treatment with radiotherapy while PSA is relatively low results in higher rates of long-term biochemical control.10-22
Conclusions
Access to the VA CDW was essential for the success of the PSA tracking system. Furthermore, veteran patients with prostate cancer tend toward a high rate of adherence and typically stay within the system. Prostate cancer is one of the few cancers where disease recurrence is detected and determined by a quantitative laboratory value, which lends itself well to objective arithmetical tracking and detection.
Patients with prostate cancer are at risk of recurrence years after their treatment and require a long-term follow-up that includes annual PSA checks. Identifying patients who have missed follow-up appointments and not had their PSA checked is essential for combating prostate cancer recurrences. The VA CDW makes it possible to track the majority of the patients with prostate cancer who are treated in the system and identify those most in need of early treatment or early intervention before they become
symptomatic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
In the U.S. in 2015, there were more than 220,800 new cases of prostate cancer and about 27,000 deaths due to prostate cancer. Across the VHA, prostate cancer is the most common nonskin cancer malignancy, and more than 25,000 patients are diagnosed yearly.1 Patients who receive treatment for prostate cancer have excellent rates of disease-specific survival: nearly 100% at 5 years, 99% at 10 years, and 94% at 15 years.
Prostate cancer is one of several cancers that can be treated successfully with radiotherapy alone, and its success or failure is defined by a discrete numerical value from the prostate specific antigen (PSA) blood test. Failure occurs when the PSA is 2.0 ng/mL greater than the lowest PSA value posttreatment.2 Multiple clinical trials have used this method to determine whether or not a certain intervention is successful.
Although high rates of survival and clear biochemical indicators exist, patients diagnosed with and treated for prostate cancer are at significant risk of PSA failure. The risk can range from 5% to 70% by 10 years, depending on the the treatment modality, risk group, and series reported.3 These patients require long-term follow-up for disease recurrence and management of adverse effects. The current guidelines recommend annual follow-up care 5 years after treatment.4
The number of veterans requiring follow-up care for prostate cancer constitutes a disproportionately large share of visits compared with those of other cancers, such as cancers of the head and neck region, chest, or gastrointestinal system, and there are many challenges to providing quality long-term care. Veterans in rural locations face barriers to accessing follow-up care for effective management.
Missed appointments can compromise long-term care, escalating the risk of nonadherence over time. Missed appointments occur commonly and may negatively impact outcomes and can restrict care for other patients.5 In a recently published article by Percac-Lima and colleagues, no-show rates among 5 cancer center clinics at the Massachusetts General Hospital were as high as 10%.6
Missed appointments have also been associated with decreased quality of care and increased resource use.7 Patients with prostate cancer who miss follow-up visits are at risk for having their cancer progress to the point it becomes symptomatic and no longer treatable with salvage therapies. These patients also risk lost efficacy of treatments that are still available.
Due to these challenges, automated PSA tracking systems can be an effective way to ensure that quality, longterm care is provided to the patient. The purpose of the PSA tracking system is to identify patients who require intervention before they present with clinical problems. A PSA tracking system helps prevent patients being inappropriately lost to follow-up or missing a needed followup PSA blood test. The tracker would serve to correctly identify, among thousands or millions of patients in the electronic medical record system (EMR), which patients were at risk of failure or active failing biochemically by triggering an alert to the cancer specialist to assess that patient’s chart and determine whether a higher level of intervention is required. It could also serve to avoid unnecessary travel or inconvenience to a patient whose prostate cancer disease status can correctly be confirmed as under control by a simple blood test and related to the patient by phone, letter, or online.
Prostate-specific antigen trackers have been used to monitor patients for postprostatectomy treatment failures on a small scale in Ireland.8 For a PSA tracker to be successful, the system must have access to all posttreatment PSA data. The VHA is uniquely positioned to leverage this information because most patients who receive treatment for prostate cancer at a VHA facility stay within the VHA system for follow-up care. All laboratory data are also collected and stored in the EMR system, which is sent daily to the VA Corporate Data Warehouse (CDW).
Project Proposal
In November 2014, the Office of Rural Health and the National Radiation Oncology Program Office issued a request for proposal for projects that would improve follow-up care for rural patients with prostate cancer following treatment with radiotherapy. A team of health care providers at the Hunter Holmes McGuire VAMC drafted a proposal to address this problem. Veterans Engineering Resource Centers (VERCs) in Pittsburgh and New England were also included in the proposal as key collaborators. Staff from these 2 centers brought expertise in analytics, implementation, and project management to help rapidly innovate and implement a PSA tracking system.
The proposal was submitted on time and required approval at multiple levels, including facility and VISN leadership. It was essential that the perceived value of the proposal be readily apparent to all stakeholders, or the necessary approvals would not have been obtainable.
The proposal was accepted, and funds were transferred in February 2015. Four core team members led rapid cycle design and prototyping of the PSA tracking system. The project lead and sponsor was a radiation oncologist and service line chief at the Hunter Holmes Mc-Guire VAMC who provided overall strategy, direction, and clinical domain knowledge. A VERC engineer provided project management and analytic expertise, and a VERC developer designed code to pull data from the VA CDW and led design of the user interface. Finally, a nurse practitioner dedicated numerous hours to review charts, contact patients, write notes, and provide user feedback on the system.
Development
The purpose of the radiation oncology-centered PSA tracking system within the VA was to identify patients who require intervention following definitive treatment with radiotherapy before they present with clinical problems from disease recurrence. The PSA tracker that the authors developed was based on a relatively simple algorithm that sorts through thousands of patient records and identifies patients who had a diagnosis of prostate cancer but did not have metastatic disease, were treated at the Hunter Homes McGuire VAMC with radiation therapy, were not seen in clinic within the past 400 days, and did not have a PSA drawn within 450 days or had a rising PSA of 0.5 or more above the lowest PSA value posttreatment. In other words, the tracker uses the power of the CDW to successfully identify the exact charts that need to be reviewed and helped ensure that patients were not lost to follow-up or did not receive appropriate care. Without the PSA tracking system, providers would not know whether or not patients were being missed.
Development of the tracker required regular team meetings with well-defined, achievable goals. The team consisted of a physician as team leader, a biostatistician with structured query language experience who had access to the CDW, and a project manager with an industrial engineering background. The team met weekly. The project was broken into several components that were achieved in series and at times in parallel. The first goal was determining whether an algorithm could be written to correctly identify patients with prostate cancer treated with radiotherapy at the Hunter Holmes McGuire VAMC who did not have metastatic disease.
By using various values available within the CDW, such as ICD 9 codes, CPT codes, PSA laboratory values, dates, and other information, the authors were able to create a successful algorithm. The ability to complete the algorithm in a short time frame wasfacilitated by several factors: a very small group, weekly meetings, good communication, easy to understand concepts across all disciplines, ability to quickly determine whether the results of the algorithm were accurate or not, and high perceived value of the end product that served to motivate the team members. Each meeting ended with clear action items and a scheduled time for the next meeting. Throughout the design and implementation process, the team discussed any problems, planned solutions, and reviewed the status of project deliverables.
Results
The tracker has already been useful for reengaging patients in care and ensuring PSA testing is occurring at appropriate intervals. Of the more than 50,000 veterans currently alive who have received care at the Hunter Holmes McGuire VAMC, 1,158 were treated with radiotherapy definitively for prostate cancer. A total of 455 (39%) prostate cancer survivors had not been seen in the clinic in the past 13 months. Of these patients, 294 were being followed appropriately elsewhere within the VA system. Meanwhile, 161 neither had a PSA level nor a prostate cancer follow-up appointment recorded in the past 13 months anywhere within the entire VA system. This yielded a loss-to-follow-up rate of 14% (161/1,158).
The authors found that 21 (13%) of patients had a PSA level > 2.0 ng/mL above the posttreatment nadir.9 The authors were able to review the charts of these 21 patients to assess whether or not they required or were suitable for salvage brachytherapy. Of these, 1 has been set up for salvage high-dose rate brachytherapy treatment. Out of 50,000 patients, the PSA tracker algorithm facilitated a focus on the 21 patients who were most likely to be in need, making it possible for a nurse practitioner and physician to spend just 3 hours looking at charts instead of 3,000 hours.
Sustained use of the tracker is critically important to the Hunter Holmes McGuire VAMC project team and for the care of its veterans. Funds to support sustaining the program have been approved for fiscal year 2016. Efforts are underway to try to scale up the program and test the feasibility of disseminating the program across the enterprise. The authors estimate that an experienced advanced care provider would spend about 8 hours a week reviewing charts, contacting patients in the program, sending letters, and reviewing nuanced cases. The program would still benefit from increased automation as well as identifying a method for obtaining appropriate workload credit for this unique program.
The next phase of development will focus on improving the user interface and allowing easier transfer of information between the tracker and notes within the Computerized Patient Record System. The team will also look into automating additional parts of the process but feels that a clinician (ideally a nurse practitioner or physician assistant working with the radiation oncologist) must be part of the team, because clinical decisions must be made based on multiple variables and patient preferences.
The development of this PSA tracking system has significant future implications for improving biochemical control and extending patient survival. The tracker could be easily adapted to monitor prostatectomy patients and PSA failures requiring early intervention with salvage radiotherapy. It has been shown in several publications that early treatment with radiotherapy while PSA is relatively low results in higher rates of long-term biochemical control.10-22
Conclusions
Access to the VA CDW was essential for the success of the PSA tracking system. Furthermore, veteran patients with prostate cancer tend toward a high rate of adherence and typically stay within the system. Prostate cancer is one of the few cancers where disease recurrence is detected and determined by a quantitative laboratory value, which lends itself well to objective arithmetical tracking and detection.
Patients with prostate cancer are at risk of recurrence years after their treatment and require a long-term follow-up that includes annual PSA checks. Identifying patients who have missed follow-up appointments and not had their PSA checked is essential for combating prostate cancer recurrences. The VA CDW makes it possible to track the majority of the patients with prostate cancer who are treated in the system and identify those most in need of early treatment or early intervention before they become
symptomatic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. American Cancer Society. What are the key statistics about prostate cancer? American Cancer Society Website. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer key-statistics. Last revised March 12, 2015. Accessed January 11, 2016.
2. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
3. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22-29.
4. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guidelines endorsement. J Clin Oncol. 2015;33(9):1078-1085.
5. Husain-Gambles M, Neal RD, Dempsey O, Lawlor DA, Hodgson J. Missed appointments in primary care: questionnaire and focus group study of health professionals. Br J Gen Pract. 2004;54(499):108-113.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly DA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Hwang AS, Atlas SJ, Ashburner JM, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
8. Hennessey DB, Lynn C, Templeton H, Chambers K, Mulholland C. The PSA tracker: a computerised health care system initiative in Northern Ireland. Ulster Med J. 2013;82(3):146-149.
9. Chang M, Troeschel S, DeSotto K, et al. Development of a Post-Radiotherapy Prostate-Specific Antigen Detection and Tracking System. Poster presented at: Genito-Urinary Cancers Symposium Annual Meeting; January 2016; San Francisco, CA.
10. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific
antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol
Phys. 2000;48(2):369-375.
11. Catton C, Gospodarowicz M, Warde P, et al. Adjuvant and salvage radiation
therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother
Oncol. 2001;59(1):51-60.
12. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63(1):134-140.
13. Katz MS, Zelefsky MJ, Venkatraman ES, Hummer A, Leibal SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483-489.
14. Leventis AK, Shariat SF, Kattan MW, Butler EB, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030-1039.
15. Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for
biochemical failure of radical prostatectomy: a single-institution experience. Urology.
2003;61(6):1204-1210.
16. Maier J, Forman J, Tekyi-Mensah S, Bolton S, Patel R, Pontes JE. Salvage radiation
for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22(1):50-56.
17. Perez CA, Michalski JM, Baglan K, Andriole G, Cui Q, Lockett MA. Radiation therapy for increasing prostate-specific antigen levels after radical prostatectomy. Clin Prostate Cancer. 2003;1(4):235-241.
18. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163(3):845-850.
19. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or
persistent PSA after radical prostatectomy. Urology. 2002;60(2):281-287.
20. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325-1332.
21. Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52(6):1034-1040.
22. Vicini FA, Ziaja EL, Kestin LL, et al. Treatment outcome with adjuvant and salvage irradiation after radical prostatectomy for prostate cancer. Urology. 1999;54(1):111-117.
Note: Page numbers differ between the print issue and digital edition.
1. American Cancer Society. What are the key statistics about prostate cancer? American Cancer Society Website. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer key-statistics. Last revised March 12, 2015. Accessed January 11, 2016.
2. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974.
3. Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(suppl 1):22-29.
4. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guidelines endorsement. J Clin Oncol. 2015;33(9):1078-1085.
5. Husain-Gambles M, Neal RD, Dempsey O, Lawlor DA, Hodgson J. Missed appointments in primary care: questionnaire and focus group study of health professionals. Br J Gen Pract. 2004;54(499):108-113.
6. Percac-Lima S, Cronin PR, Ryan DP, Chabner BA, Daly DA, Kimball AB. Patient navigation based on predictive modeling decreases no-show rates in cancer care. Cancer. 2015;121(10):1662-1670.
7. Hwang AS, Atlas SJ, Ashburner JM, et al. Appointment “no-shows” are an independent predictor of subsequent quality of care and resource utilization outcomes. J Gen Intern Med. 2015;30(10):1426-1433.
8. Hennessey DB, Lynn C, Templeton H, Chambers K, Mulholland C. The PSA tracker: a computerised health care system initiative in Northern Ireland. Ulster Med J. 2013;82(3):146-149.
9. Chang M, Troeschel S, DeSotto K, et al. Development of a Post-Radiotherapy Prostate-Specific Antigen Detection and Tracking System. Poster presented at: Genito-Urinary Cancers Symposium Annual Meeting; January 2016; San Francisco, CA.
10. Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific
antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol
Phys. 2000;48(2):369-375.
11. Catton C, Gospodarowicz M, Warde P, et al. Adjuvant and salvage radiation
therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother
Oncol. 2001;59(1):51-60.
12. Cheung R, Kamat AM, de Crevoisier R, et al. Outcome of salvage radiotherapy for biochemical failure after radical prostatectomy with or without hormonal therapy. Int J Radiat Oncol Biol Phys. 2005;63(1):134-140.
13. Katz MS, Zelefsky MJ, Venkatraman ES, Hummer A, Leibal SA. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21(3):483-489.
14. Leventis AK, Shariat SF, Kattan MW, Butler EB, Wheeler TM, Slawin KM. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19(4):1030-1039.
15. Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for
biochemical failure of radical prostatectomy: a single-institution experience. Urology.
2003;61(6):1204-1210.
16. Maier J, Forman J, Tekyi-Mensah S, Bolton S, Patel R, Pontes JE. Salvage radiation
for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22(1):50-56.
17. Perez CA, Michalski JM, Baglan K, Andriole G, Cui Q, Lockett MA. Radiation therapy for increasing prostate-specific antigen levels after radical prostatectomy. Clin Prostate Cancer. 2003;1(4):235-241.
18. Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163(3):845-850.
19. Song DY, Thompson TL, Ramakrishnan V, et al. Salvage radiotherapy for rising or
persistent PSA after radical prostatectomy. Urology. 2002;60(2):281-287.
20. Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291(11):1325-1332.
21. Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52(6):1034-1040.
22. Vicini FA, Ziaja EL, Kestin LL, et al. Treatment outcome with adjuvant and salvage irradiation after radical prostatectomy for prostate cancer. Urology. 1999;54(1):111-117.
Note: Page numbers differ between the print issue and digital edition.
Supporting Caregivers of Veterans Online: A Partnership of the National Council on Aging and VA
Family caregivers fill a critical need in our nation’s health care system by providing essential services and support for chronically ill and disabled persons. Yet their physical, mental, and emotional well-being are often compromised due to their caregiving roles and responsibilities. Several nationally representative surveys of military caregivers have highlighted differences that are unique to caregivers of veterans.1,2 According to a RAND Corporation report, caregivers of veterans differ from other family caregivers in that they are younger with dependent children, often live with the person they are caring for, and provide care for up to a decade longer than do other caregivers.3 While most caregivers experience similar stressors, caregivers of veterans face distinct challenges, partly because veterans’ illnesses can be markedly different from the general population of disabled and/or chronically ill individuals.
Veterans and their caregivers also must navigate within large and complex health care, legal, and financial systems.3 Recently, the VA has begun to institute a number of programs and services to support veterans. One of these is the Building Better Caregivers (BBC) program. Building Better Caregivers is an online 6-week workshop aimed to equip caregivers of persons with physical and cognitive impairment with the knowledge, skills, and support to boost self-confidence in their ability to maintain and lead active and fulfilling lives. Developed at Stanford University and tested in partnership with the VA, BBC has shown significant improvements in caregivers’ health and health-related behaviors. Moreover, its online format allows for caregivers to access support and information when it is most convenient to them.4
The National Council on Aging (NCOA) has more than a decade of experience disseminating evidence-based solutions in partnership with a variety of organizations. Previously NCOA held an exclusive license to disseminate the BBC program (Canary Health now holds the license). Following a pilot study of the program, the VA partnered with NCOA to implement and sustain BBC. By integrating the program into clinical practice, NCOA and VA have positioned this program under the VA’s Caregiver Support Program (CSP). Caregiver support coordinators have referred > 5,000 caregivers to date, and > 2,654 of those caregivers expressed interest and were assigned to a workshop. Seventy percent of participants attended 4 out of 6 sessions, which is considered completing the workshop.
In the original pilot study with Stanford University, caregivers taking BBC showed significant improvements in depression, pain, stress, caregiver burden, and 63% completed at least 4 of the 6 sessions.4 Current BBC outcomes continue to show reductions in stress. In addition, participant completer rates are even greater than the original study outcomes with 75% of caregivers completing 4 out of 6 sessions. Additionally, > 50% of workshop graduates elect to participate in a BBC online community that continues to support them in their role as caregiver. Nearly half of all U.S. adults and 80% of adults aged > 55 years have more than 1 chronic condition and/or disability.3 Unlike acute care, the majority of care for chronically ill individuals is provided outside of the medical system and in homes by family caregivers. Family caregivers provide assistance with routine, daily activities, such as bathing and meal preparation, as well as more specialized tasks, such as meeting with health care providers and administering medications.
The amount of weekly care provided averages 21 hours per week for persons with physical impairment, and 22 to 47 hours per week for persons with cognitive impairment.5,6 According to the National Alliance of Caregivers, there are > 65 million individuals in the U.S. who provide care to a family member or friend who is chronically ill and/or disabled.1 As the U.S. population continues to age, so will the proportion of individuals with chronic conditions. That means a growing need for caregivers. And due to advancements in health care, more individuals are aging with disabilities, resulting in a prolonged need for caregivers.
Caregiver Challenges
Family caregivers represent a very diverse segment of the U.S. population, cutting across most demographic groups. While research indicates that living arrangements, hours of care provided, and money spent may vary by race/ethnicity, socioeconomic status, and gender, most caregivers provide similar types of care and experience similar stresses.7 However, many caregivers of veterans face a unique set of challenges and subsequently experience disproportionately poor mental and emotional health than do caregivers in the general population.3 These findings are also supported by nationally representative surveys of caregivers, one of family caregivers in general and other of caregivers of veterans.
In addition to providing assistance with daily activities and specialized tasks, caregivers of veterans usually have added roles and responsibilities that are markedly different from the general caregiver population due to the severity of veterans’ illnesses and disabilities. Many veterans experience mental illness, with > 70% of those who require caregivers having reported anxiety and/or depression, and 60% having been diagnosed with posttraumatic stress disorder (PTSD). In addition, almost half of all veterans have cognitive impairment, and nearly one-third experiencing traumatic brain injuries (TBI).8 These “invisible wounds” often require caregivers to spend a significant amount of time providing behavioral care (ie, avoiding certain triggers and providing cues), as well as emotional support, along with standard physical care.1 Behavioral care and emotional support are ongoing and more challenging than physical care, and thus more taxing on the caregiver.
Profile of Caregivers of Veterans
Caregivers of veterans also face the additional challenge of navigating large and complex systems across multiple government organizations. There are a myriad of services and benefits available to veterans, and their caregivers typically serve as care coordinators—facilitating care, services, and benefits for their loved ones. Caregivers of veterans may also handle all financial and legal matters, such as drafting wills and advance directives.3 Coordinating care and handling financial and legal matters can prove to be extremely difficult and time consuming.
It is, therefore, not surprising that caregivers of veterans experience higher levels of physical strain as well as poorer mental and emotional health. Six out of 10 caregivers of veterans report their health has declined due to their caregiving role, and the majority find themselves socially isolated and depressed (Figure 1).1,2,8
VA Support for Caregivers
The VA has long supported family caregivers of veterans through services such as home health care and programming, such as home-based primary care, teaching, and support. Following the passage of Public Law 111-163, the Caregivers and Veterans Omnibus Health Act of 2010, the VA has been able to increase its support to family caregivers to an unprecedented level. The programs and services established under this act include a national CSP and caregiver support line, as well as placement of caregiver support coordinators at each VA medical center. The VA has also developed a VA caregiver website (http://www.caregiver.va.gov), rolled out a national Peer Support Mentoring program and a number of self-care courses. Other additional supports for caregivers of veterans injured in the line of duty on or after September 11, 2001, include monthly stipends, mental health services, insurance coverage, and enhanced respite care. These caregiver programs and services utilize a variety of models to assist in the engagement of the diverse caregiver population across the military service eras.
Why Building Better Caregivers?
The VHA piloted a number of programs for caregivers prior to the implementation of the CSP. In 2009, VHA partnered with Stanford University to pilot the BBC self-management workshop for caregivers of veterans. The online pilot addressed the needs of those looking after their family members or friends with cognitive difficulties, such as dementia, TBI, PTSD, memory problems, and other care needs. The online format provided an additional option for caregivers to access support in a nontraditional format outside of their local VAMC. This format also allowed caregivers the flexibility to access support and information in the convenience of their home, based on their availability and schedule. This feature was especially important due to the challenges some caregivers experience, whether it is their rural residence, limited ability to travel to a medical center, or lack of support to leave their loved one to attend a support group.
How It Works
The BBC program, developed at Stanford University, is a 6-week workshop offered on a dedicated website. Each workshop is composed of 20 to 25 caregivers. The workshop is moderated by 2 trained facilitators—at least 1 of whom is a caregiver. Facilitators and participants together address a number of topics, including managing difficult care partner behaviors and emotions, reducing stress for the caregiver, self-care methods to improve the caregiver’s health, making decisions, finding additional help and resources, and planning for the future. Weekly activities include reading and applying new knowledge through a rich content learning center; making and posting a weekly action plan, brainstorming, problem solving, and celebrating milestones with fellow participants via 4 directed bulletin boards; and participating in any appropriate self-tests and activities.
There is no real-time attendance, so caregivers can choose the time of day and days of the week that are convenient to them to log in and participate.
VA/NCOA Partnership
An exclusive license to disseminate this program and other online programs developed and tested at Stanford is held by NCOA. In licensing the program, NCOA also offers technical assistance, training, and technologic support needed to implement and sustain the program. Following the success of the BBC pilot, VA worked with NCOA to implement the program under its CSP.
The NCOA has over a decade of experience in disseminating evidence-based programs and working with organizations at the federal, state, and local level to embed these programs into organizations so that they become standard practice and are sustainable. It also has several years of experience in disseminating programs online. The VA is a leading organization in caregiver support services and has built a national CSP that reaches tens of thousands of caregivers.
In addition to maximizing the resource potential of each organization, both organizations see the importance of clear and frequent communication in program dissemination. Each organization took the time to learn the other’s culture and have an appreciation for how each organization operates. The VA and NCOA meet weekly and work together on every aspect of the project.
Implementation
A rigorous implementation time line was developed by NCOA and VA and achieved the goal of launching the program within 90 days of the kick-off meeting. Key indicators for the success of this program are detailed in the Table.
Both NCOA and VA saw the importance of creating a program that fits well within the overarching CSP and complements its services and resources (Figure 3).
Training and Support
Adequate training and support are essential to maintaining the integrity of this program. A total of 30 facilitators and 5 mentors to support workshops were trained by NCOA, and NCOA screened all facilitator candidates and provided training with ongoing support to all certified and accredited facilitators.
In addition, an online community offers continued support for workshop participants once they have completed the workshop. Graduates can access tools and resources, as well as problem solve, brainstorm, celebrate, and set goals along with other peer graduates via moderated discussion boards.
Preliminary Findings
More than 50% of workshop participants were aged 31 to 50 years, 85% lived with the veteran they were caring for, and 78% were spouses of the care recipient. Seventy-two percent of the veterans being cared for were white, and 93% were male. Nearly 80% of the veterans had PTSD, and more than half had TBI and/or a mental health disorder.
Clinical Indicators
Caregivers in the general population and those caring for veterans consider their caregiving situation stressful. In this implementation, participants are showing a statistically significant reduction in stress when measured at week 1 and week 6 with an average change score of 1.3 on a 10-point scale. Similar reductions in stress were seen in the original Stanford University study 3 months after the workshop had ended.2
Satisfaction
Participant satisfaction was high, averaging 4.5 on a 5-point Likert scale. Caregiver participants reported that what they liked best about the workshop was the shared experience with other caregivers, timing/convenience, giving and receiving help, and goal setting.
One recent caregiver who was caring for her husband remarked “I really enjoyed the workshop. Interacting with others, heartfelt stories of celebration, and frustration. The concern for the whole woman/man; physically and emotionally. I enjoyed the helpful suggestions/encouraging words of the leaders as well. I hope more people take advantage of this program.”
“I like the informal nature and self-paced aspect. We all have crazy lives but I think this was easy to do,” said another caregiver.
“This was a place where you can put out your problem and no one will judge you,” a caregiver explained. “There was respect for each other’s situations. Learning from others’ problem and how others share the solution. I saw how important the caregivers are, taking care of our self first so we can take care of the rest.”
Recruitment
In the 36 months since implementation, > 5,000 referrals were made for BBC, resulting in 2,654 caregivers being assigned to workshops; 75% of caregivers completed a workshop. Nearly half of all workshop graduates elected to join a moderated online community.
Discussion
Internal BBC recruitment data are captured monthly and reported to the Caregiver Support Program for each of the local VAMCs. Although recruitment goals are being met, a consistent referral pattern is not occurring at local VA sites. Not all VA sites are referring participants, and the referrals to BBC at some VA sites have been low in comparison with other higher performing VA sites. The percentage of sites with no referrals is 2%; lower performing sites with less than 20 caregivers referred represent 50% of sites.
A variety of factors contribute to disparities in referrals at different sites. A primary challenge is the increased demands on caregiver support coordinators nationally as they continue to prioritize the enrollment of caregivers into a variety of other programs available within CSP. The CSP office has developed other caregiver resources and tools that may compete with referral to BBC due to the preference of the staff and caregivers. Additional factors may also include no prescribed referral metrics for local sites and variances in local marketing and promotion of BBC. As the partnership between NCOA and the VA continues, additional caregiver referral methods are being explored to facilitate local promotion and marketing to engage caregivers of all eras.
Lessons and Next Steps
This partnership and program implementation have yielded a number of lessons learned and indications for next steps. The most significant lesson also proved to be the biggest success: the program can be embedded into clinical practice. Integrating the referral process into VA staff’s daily operations and depending on them to bring forward viable participants proved highly successful, with the majority of caregiver support coordinators making ≥ 1 referral and 50% of referred caregivers electing to take the workshop.
The VA and NCOA subsequently learned through focus groups that the relationship between the CSC and caregiver is strong, but there is also a need for continual follow-up and—more often than not—these referred caregivers need reminder e-mails to complete the sign-up process. The NCOA and VA also saw a larger proportion of post 9/11 caregivers (70%) recruited to the program compared with other eras (30%). Future recruitment will focus on ways to get caregivers of older era veterans involved with BBC.
Last, the VA and NCOA learned that participants really enjoyed the program. In the future more participant testimonials and stories will be used to spread the word to other caregivers about the program.
Conclusion
Caregivers of veterans face a unique set of challenges. Throughout both the VA pilot and the current partnership with NCOA, BBC is a promising solution for improving the well-being of caregivers of veterans. The success of its integration into clinical practice and participant satisfaction speak to both the quality of the program, as well as the partnership between NCOA and VA. Both NCOA and VA are working to expand its reach and make BBC readily available to as many caregivers of veterans as possible.
1. National Alliance for Caregiving, AARP. Caregiving in the U.S. National Alliance for Caregiving Website. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed December 17, 2015.
2. National Alliance for Caregiving, United Health Foundation, Caregivers of Veterans: Serving on the home front. National Alliance for Caregiving Website. http://www.caregiving.org/data/2010_Caregivers_of_Veterans_FULLREPORT_WEB_FINAL.pdf. Published November 2010. Accessed December 17, 2015.
3. Tanielian T, Ramchand R, Fisher MP, Sims CS, Harris RS, Harrell MC. Military Caregivers: Cornerstones of Support for Our Nation’s Wounded, Ill, and Injured Veterans. Santa Monica, CA: RAND Corporation; 2013.
4. Lorig K, Thompson-Gallagher D, Traylor L, et al. Building Better Caregivers: a pilot online support workshop for family caregivers of cognitively impaired adults. J Appl Gerontol. 2012;31(3):423-437.
5. Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee.” Alzheimer’s Dement. 2006;2(1):12-32.
6. MetLife Mature Market Institute, LifePlans. The MetLife study of alzheimer’s disease: the caregiving experience. MetLife Website. https://www.metlife.com/assets/cao/mmi/publications/studies/mmi-alzheimers-disease-caregiving-experience-study.pdf. Published August 2006. Accessed December 17, 2015.
7. Pandya S. Racial and ethnic differences among older adults in long-term care service use. http://www.aarp.org/home-garden/livable-communities/info-2005/fs119_ltc.html. Published June 2005. Accessed December 17, 2015.
8. Resnik LJ, Allen SM. Using international classification of functioning, disability and health to understand challenges in community reintegration of injured veterans. J Rehab Res Dev. 2007;44(7):991-1006.
Family caregivers fill a critical need in our nation’s health care system by providing essential services and support for chronically ill and disabled persons. Yet their physical, mental, and emotional well-being are often compromised due to their caregiving roles and responsibilities. Several nationally representative surveys of military caregivers have highlighted differences that are unique to caregivers of veterans.1,2 According to a RAND Corporation report, caregivers of veterans differ from other family caregivers in that they are younger with dependent children, often live with the person they are caring for, and provide care for up to a decade longer than do other caregivers.3 While most caregivers experience similar stressors, caregivers of veterans face distinct challenges, partly because veterans’ illnesses can be markedly different from the general population of disabled and/or chronically ill individuals.
Veterans and their caregivers also must navigate within large and complex health care, legal, and financial systems.3 Recently, the VA has begun to institute a number of programs and services to support veterans. One of these is the Building Better Caregivers (BBC) program. Building Better Caregivers is an online 6-week workshop aimed to equip caregivers of persons with physical and cognitive impairment with the knowledge, skills, and support to boost self-confidence in their ability to maintain and lead active and fulfilling lives. Developed at Stanford University and tested in partnership with the VA, BBC has shown significant improvements in caregivers’ health and health-related behaviors. Moreover, its online format allows for caregivers to access support and information when it is most convenient to them.4
The National Council on Aging (NCOA) has more than a decade of experience disseminating evidence-based solutions in partnership with a variety of organizations. Previously NCOA held an exclusive license to disseminate the BBC program (Canary Health now holds the license). Following a pilot study of the program, the VA partnered with NCOA to implement and sustain BBC. By integrating the program into clinical practice, NCOA and VA have positioned this program under the VA’s Caregiver Support Program (CSP). Caregiver support coordinators have referred > 5,000 caregivers to date, and > 2,654 of those caregivers expressed interest and were assigned to a workshop. Seventy percent of participants attended 4 out of 6 sessions, which is considered completing the workshop.
In the original pilot study with Stanford University, caregivers taking BBC showed significant improvements in depression, pain, stress, caregiver burden, and 63% completed at least 4 of the 6 sessions.4 Current BBC outcomes continue to show reductions in stress. In addition, participant completer rates are even greater than the original study outcomes with 75% of caregivers completing 4 out of 6 sessions. Additionally, > 50% of workshop graduates elect to participate in a BBC online community that continues to support them in their role as caregiver. Nearly half of all U.S. adults and 80% of adults aged > 55 years have more than 1 chronic condition and/or disability.3 Unlike acute care, the majority of care for chronically ill individuals is provided outside of the medical system and in homes by family caregivers. Family caregivers provide assistance with routine, daily activities, such as bathing and meal preparation, as well as more specialized tasks, such as meeting with health care providers and administering medications.
The amount of weekly care provided averages 21 hours per week for persons with physical impairment, and 22 to 47 hours per week for persons with cognitive impairment.5,6 According to the National Alliance of Caregivers, there are > 65 million individuals in the U.S. who provide care to a family member or friend who is chronically ill and/or disabled.1 As the U.S. population continues to age, so will the proportion of individuals with chronic conditions. That means a growing need for caregivers. And due to advancements in health care, more individuals are aging with disabilities, resulting in a prolonged need for caregivers.
Caregiver Challenges
Family caregivers represent a very diverse segment of the U.S. population, cutting across most demographic groups. While research indicates that living arrangements, hours of care provided, and money spent may vary by race/ethnicity, socioeconomic status, and gender, most caregivers provide similar types of care and experience similar stresses.7 However, many caregivers of veterans face a unique set of challenges and subsequently experience disproportionately poor mental and emotional health than do caregivers in the general population.3 These findings are also supported by nationally representative surveys of caregivers, one of family caregivers in general and other of caregivers of veterans.
In addition to providing assistance with daily activities and specialized tasks, caregivers of veterans usually have added roles and responsibilities that are markedly different from the general caregiver population due to the severity of veterans’ illnesses and disabilities. Many veterans experience mental illness, with > 70% of those who require caregivers having reported anxiety and/or depression, and 60% having been diagnosed with posttraumatic stress disorder (PTSD). In addition, almost half of all veterans have cognitive impairment, and nearly one-third experiencing traumatic brain injuries (TBI).8 These “invisible wounds” often require caregivers to spend a significant amount of time providing behavioral care (ie, avoiding certain triggers and providing cues), as well as emotional support, along with standard physical care.1 Behavioral care and emotional support are ongoing and more challenging than physical care, and thus more taxing on the caregiver.
Profile of Caregivers of Veterans
Caregivers of veterans also face the additional challenge of navigating large and complex systems across multiple government organizations. There are a myriad of services and benefits available to veterans, and their caregivers typically serve as care coordinators—facilitating care, services, and benefits for their loved ones. Caregivers of veterans may also handle all financial and legal matters, such as drafting wills and advance directives.3 Coordinating care and handling financial and legal matters can prove to be extremely difficult and time consuming.
It is, therefore, not surprising that caregivers of veterans experience higher levels of physical strain as well as poorer mental and emotional health. Six out of 10 caregivers of veterans report their health has declined due to their caregiving role, and the majority find themselves socially isolated and depressed (Figure 1).1,2,8
VA Support for Caregivers
The VA has long supported family caregivers of veterans through services such as home health care and programming, such as home-based primary care, teaching, and support. Following the passage of Public Law 111-163, the Caregivers and Veterans Omnibus Health Act of 2010, the VA has been able to increase its support to family caregivers to an unprecedented level. The programs and services established under this act include a national CSP and caregiver support line, as well as placement of caregiver support coordinators at each VA medical center. The VA has also developed a VA caregiver website (http://www.caregiver.va.gov), rolled out a national Peer Support Mentoring program and a number of self-care courses. Other additional supports for caregivers of veterans injured in the line of duty on or after September 11, 2001, include monthly stipends, mental health services, insurance coverage, and enhanced respite care. These caregiver programs and services utilize a variety of models to assist in the engagement of the diverse caregiver population across the military service eras.
Why Building Better Caregivers?
The VHA piloted a number of programs for caregivers prior to the implementation of the CSP. In 2009, VHA partnered with Stanford University to pilot the BBC self-management workshop for caregivers of veterans. The online pilot addressed the needs of those looking after their family members or friends with cognitive difficulties, such as dementia, TBI, PTSD, memory problems, and other care needs. The online format provided an additional option for caregivers to access support in a nontraditional format outside of their local VAMC. This format also allowed caregivers the flexibility to access support and information in the convenience of their home, based on their availability and schedule. This feature was especially important due to the challenges some caregivers experience, whether it is their rural residence, limited ability to travel to a medical center, or lack of support to leave their loved one to attend a support group.
How It Works
The BBC program, developed at Stanford University, is a 6-week workshop offered on a dedicated website. Each workshop is composed of 20 to 25 caregivers. The workshop is moderated by 2 trained facilitators—at least 1 of whom is a caregiver. Facilitators and participants together address a number of topics, including managing difficult care partner behaviors and emotions, reducing stress for the caregiver, self-care methods to improve the caregiver’s health, making decisions, finding additional help and resources, and planning for the future. Weekly activities include reading and applying new knowledge through a rich content learning center; making and posting a weekly action plan, brainstorming, problem solving, and celebrating milestones with fellow participants via 4 directed bulletin boards; and participating in any appropriate self-tests and activities.
There is no real-time attendance, so caregivers can choose the time of day and days of the week that are convenient to them to log in and participate.
VA/NCOA Partnership
An exclusive license to disseminate this program and other online programs developed and tested at Stanford is held by NCOA. In licensing the program, NCOA also offers technical assistance, training, and technologic support needed to implement and sustain the program. Following the success of the BBC pilot, VA worked with NCOA to implement the program under its CSP.
The NCOA has over a decade of experience in disseminating evidence-based programs and working with organizations at the federal, state, and local level to embed these programs into organizations so that they become standard practice and are sustainable. It also has several years of experience in disseminating programs online. The VA is a leading organization in caregiver support services and has built a national CSP that reaches tens of thousands of caregivers.
In addition to maximizing the resource potential of each organization, both organizations see the importance of clear and frequent communication in program dissemination. Each organization took the time to learn the other’s culture and have an appreciation for how each organization operates. The VA and NCOA meet weekly and work together on every aspect of the project.
Implementation
A rigorous implementation time line was developed by NCOA and VA and achieved the goal of launching the program within 90 days of the kick-off meeting. Key indicators for the success of this program are detailed in the Table.
Both NCOA and VA saw the importance of creating a program that fits well within the overarching CSP and complements its services and resources (Figure 3).
Training and Support
Adequate training and support are essential to maintaining the integrity of this program. A total of 30 facilitators and 5 mentors to support workshops were trained by NCOA, and NCOA screened all facilitator candidates and provided training with ongoing support to all certified and accredited facilitators.
In addition, an online community offers continued support for workshop participants once they have completed the workshop. Graduates can access tools and resources, as well as problem solve, brainstorm, celebrate, and set goals along with other peer graduates via moderated discussion boards.
Preliminary Findings
More than 50% of workshop participants were aged 31 to 50 years, 85% lived with the veteran they were caring for, and 78% were spouses of the care recipient. Seventy-two percent of the veterans being cared for were white, and 93% were male. Nearly 80% of the veterans had PTSD, and more than half had TBI and/or a mental health disorder.
Clinical Indicators
Caregivers in the general population and those caring for veterans consider their caregiving situation stressful. In this implementation, participants are showing a statistically significant reduction in stress when measured at week 1 and week 6 with an average change score of 1.3 on a 10-point scale. Similar reductions in stress were seen in the original Stanford University study 3 months after the workshop had ended.2
Satisfaction
Participant satisfaction was high, averaging 4.5 on a 5-point Likert scale. Caregiver participants reported that what they liked best about the workshop was the shared experience with other caregivers, timing/convenience, giving and receiving help, and goal setting.
One recent caregiver who was caring for her husband remarked “I really enjoyed the workshop. Interacting with others, heartfelt stories of celebration, and frustration. The concern for the whole woman/man; physically and emotionally. I enjoyed the helpful suggestions/encouraging words of the leaders as well. I hope more people take advantage of this program.”
“I like the informal nature and self-paced aspect. We all have crazy lives but I think this was easy to do,” said another caregiver.
“This was a place where you can put out your problem and no one will judge you,” a caregiver explained. “There was respect for each other’s situations. Learning from others’ problem and how others share the solution. I saw how important the caregivers are, taking care of our self first so we can take care of the rest.”
Recruitment
In the 36 months since implementation, > 5,000 referrals were made for BBC, resulting in 2,654 caregivers being assigned to workshops; 75% of caregivers completed a workshop. Nearly half of all workshop graduates elected to join a moderated online community.
Discussion
Internal BBC recruitment data are captured monthly and reported to the Caregiver Support Program for each of the local VAMCs. Although recruitment goals are being met, a consistent referral pattern is not occurring at local VA sites. Not all VA sites are referring participants, and the referrals to BBC at some VA sites have been low in comparison with other higher performing VA sites. The percentage of sites with no referrals is 2%; lower performing sites with less than 20 caregivers referred represent 50% of sites.
A variety of factors contribute to disparities in referrals at different sites. A primary challenge is the increased demands on caregiver support coordinators nationally as they continue to prioritize the enrollment of caregivers into a variety of other programs available within CSP. The CSP office has developed other caregiver resources and tools that may compete with referral to BBC due to the preference of the staff and caregivers. Additional factors may also include no prescribed referral metrics for local sites and variances in local marketing and promotion of BBC. As the partnership between NCOA and the VA continues, additional caregiver referral methods are being explored to facilitate local promotion and marketing to engage caregivers of all eras.
Lessons and Next Steps
This partnership and program implementation have yielded a number of lessons learned and indications for next steps. The most significant lesson also proved to be the biggest success: the program can be embedded into clinical practice. Integrating the referral process into VA staff’s daily operations and depending on them to bring forward viable participants proved highly successful, with the majority of caregiver support coordinators making ≥ 1 referral and 50% of referred caregivers electing to take the workshop.
The VA and NCOA subsequently learned through focus groups that the relationship between the CSC and caregiver is strong, but there is also a need for continual follow-up and—more often than not—these referred caregivers need reminder e-mails to complete the sign-up process. The NCOA and VA also saw a larger proportion of post 9/11 caregivers (70%) recruited to the program compared with other eras (30%). Future recruitment will focus on ways to get caregivers of older era veterans involved with BBC.
Last, the VA and NCOA learned that participants really enjoyed the program. In the future more participant testimonials and stories will be used to spread the word to other caregivers about the program.
Conclusion
Caregivers of veterans face a unique set of challenges. Throughout both the VA pilot and the current partnership with NCOA, BBC is a promising solution for improving the well-being of caregivers of veterans. The success of its integration into clinical practice and participant satisfaction speak to both the quality of the program, as well as the partnership between NCOA and VA. Both NCOA and VA are working to expand its reach and make BBC readily available to as many caregivers of veterans as possible.
Family caregivers fill a critical need in our nation’s health care system by providing essential services and support for chronically ill and disabled persons. Yet their physical, mental, and emotional well-being are often compromised due to their caregiving roles and responsibilities. Several nationally representative surveys of military caregivers have highlighted differences that are unique to caregivers of veterans.1,2 According to a RAND Corporation report, caregivers of veterans differ from other family caregivers in that they are younger with dependent children, often live with the person they are caring for, and provide care for up to a decade longer than do other caregivers.3 While most caregivers experience similar stressors, caregivers of veterans face distinct challenges, partly because veterans’ illnesses can be markedly different from the general population of disabled and/or chronically ill individuals.
Veterans and their caregivers also must navigate within large and complex health care, legal, and financial systems.3 Recently, the VA has begun to institute a number of programs and services to support veterans. One of these is the Building Better Caregivers (BBC) program. Building Better Caregivers is an online 6-week workshop aimed to equip caregivers of persons with physical and cognitive impairment with the knowledge, skills, and support to boost self-confidence in their ability to maintain and lead active and fulfilling lives. Developed at Stanford University and tested in partnership with the VA, BBC has shown significant improvements in caregivers’ health and health-related behaviors. Moreover, its online format allows for caregivers to access support and information when it is most convenient to them.4
The National Council on Aging (NCOA) has more than a decade of experience disseminating evidence-based solutions in partnership with a variety of organizations. Previously NCOA held an exclusive license to disseminate the BBC program (Canary Health now holds the license). Following a pilot study of the program, the VA partnered with NCOA to implement and sustain BBC. By integrating the program into clinical practice, NCOA and VA have positioned this program under the VA’s Caregiver Support Program (CSP). Caregiver support coordinators have referred > 5,000 caregivers to date, and > 2,654 of those caregivers expressed interest and were assigned to a workshop. Seventy percent of participants attended 4 out of 6 sessions, which is considered completing the workshop.
In the original pilot study with Stanford University, caregivers taking BBC showed significant improvements in depression, pain, stress, caregiver burden, and 63% completed at least 4 of the 6 sessions.4 Current BBC outcomes continue to show reductions in stress. In addition, participant completer rates are even greater than the original study outcomes with 75% of caregivers completing 4 out of 6 sessions. Additionally, > 50% of workshop graduates elect to participate in a BBC online community that continues to support them in their role as caregiver. Nearly half of all U.S. adults and 80% of adults aged > 55 years have more than 1 chronic condition and/or disability.3 Unlike acute care, the majority of care for chronically ill individuals is provided outside of the medical system and in homes by family caregivers. Family caregivers provide assistance with routine, daily activities, such as bathing and meal preparation, as well as more specialized tasks, such as meeting with health care providers and administering medications.
The amount of weekly care provided averages 21 hours per week for persons with physical impairment, and 22 to 47 hours per week for persons with cognitive impairment.5,6 According to the National Alliance of Caregivers, there are > 65 million individuals in the U.S. who provide care to a family member or friend who is chronically ill and/or disabled.1 As the U.S. population continues to age, so will the proportion of individuals with chronic conditions. That means a growing need for caregivers. And due to advancements in health care, more individuals are aging with disabilities, resulting in a prolonged need for caregivers.
Caregiver Challenges
Family caregivers represent a very diverse segment of the U.S. population, cutting across most demographic groups. While research indicates that living arrangements, hours of care provided, and money spent may vary by race/ethnicity, socioeconomic status, and gender, most caregivers provide similar types of care and experience similar stresses.7 However, many caregivers of veterans face a unique set of challenges and subsequently experience disproportionately poor mental and emotional health than do caregivers in the general population.3 These findings are also supported by nationally representative surveys of caregivers, one of family caregivers in general and other of caregivers of veterans.
In addition to providing assistance with daily activities and specialized tasks, caregivers of veterans usually have added roles and responsibilities that are markedly different from the general caregiver population due to the severity of veterans’ illnesses and disabilities. Many veterans experience mental illness, with > 70% of those who require caregivers having reported anxiety and/or depression, and 60% having been diagnosed with posttraumatic stress disorder (PTSD). In addition, almost half of all veterans have cognitive impairment, and nearly one-third experiencing traumatic brain injuries (TBI).8 These “invisible wounds” often require caregivers to spend a significant amount of time providing behavioral care (ie, avoiding certain triggers and providing cues), as well as emotional support, along with standard physical care.1 Behavioral care and emotional support are ongoing and more challenging than physical care, and thus more taxing on the caregiver.
Profile of Caregivers of Veterans
Caregivers of veterans also face the additional challenge of navigating large and complex systems across multiple government organizations. There are a myriad of services and benefits available to veterans, and their caregivers typically serve as care coordinators—facilitating care, services, and benefits for their loved ones. Caregivers of veterans may also handle all financial and legal matters, such as drafting wills and advance directives.3 Coordinating care and handling financial and legal matters can prove to be extremely difficult and time consuming.
It is, therefore, not surprising that caregivers of veterans experience higher levels of physical strain as well as poorer mental and emotional health. Six out of 10 caregivers of veterans report their health has declined due to their caregiving role, and the majority find themselves socially isolated and depressed (Figure 1).1,2,8
VA Support for Caregivers
The VA has long supported family caregivers of veterans through services such as home health care and programming, such as home-based primary care, teaching, and support. Following the passage of Public Law 111-163, the Caregivers and Veterans Omnibus Health Act of 2010, the VA has been able to increase its support to family caregivers to an unprecedented level. The programs and services established under this act include a national CSP and caregiver support line, as well as placement of caregiver support coordinators at each VA medical center. The VA has also developed a VA caregiver website (http://www.caregiver.va.gov), rolled out a national Peer Support Mentoring program and a number of self-care courses. Other additional supports for caregivers of veterans injured in the line of duty on or after September 11, 2001, include monthly stipends, mental health services, insurance coverage, and enhanced respite care. These caregiver programs and services utilize a variety of models to assist in the engagement of the diverse caregiver population across the military service eras.
Why Building Better Caregivers?
The VHA piloted a number of programs for caregivers prior to the implementation of the CSP. In 2009, VHA partnered with Stanford University to pilot the BBC self-management workshop for caregivers of veterans. The online pilot addressed the needs of those looking after their family members or friends with cognitive difficulties, such as dementia, TBI, PTSD, memory problems, and other care needs. The online format provided an additional option for caregivers to access support in a nontraditional format outside of their local VAMC. This format also allowed caregivers the flexibility to access support and information in the convenience of their home, based on their availability and schedule. This feature was especially important due to the challenges some caregivers experience, whether it is their rural residence, limited ability to travel to a medical center, or lack of support to leave their loved one to attend a support group.
How It Works
The BBC program, developed at Stanford University, is a 6-week workshop offered on a dedicated website. Each workshop is composed of 20 to 25 caregivers. The workshop is moderated by 2 trained facilitators—at least 1 of whom is a caregiver. Facilitators and participants together address a number of topics, including managing difficult care partner behaviors and emotions, reducing stress for the caregiver, self-care methods to improve the caregiver’s health, making decisions, finding additional help and resources, and planning for the future. Weekly activities include reading and applying new knowledge through a rich content learning center; making and posting a weekly action plan, brainstorming, problem solving, and celebrating milestones with fellow participants via 4 directed bulletin boards; and participating in any appropriate self-tests and activities.
There is no real-time attendance, so caregivers can choose the time of day and days of the week that are convenient to them to log in and participate.
VA/NCOA Partnership
An exclusive license to disseminate this program and other online programs developed and tested at Stanford is held by NCOA. In licensing the program, NCOA also offers technical assistance, training, and technologic support needed to implement and sustain the program. Following the success of the BBC pilot, VA worked with NCOA to implement the program under its CSP.
The NCOA has over a decade of experience in disseminating evidence-based programs and working with organizations at the federal, state, and local level to embed these programs into organizations so that they become standard practice and are sustainable. It also has several years of experience in disseminating programs online. The VA is a leading organization in caregiver support services and has built a national CSP that reaches tens of thousands of caregivers.
In addition to maximizing the resource potential of each organization, both organizations see the importance of clear and frequent communication in program dissemination. Each organization took the time to learn the other’s culture and have an appreciation for how each organization operates. The VA and NCOA meet weekly and work together on every aspect of the project.
Implementation
A rigorous implementation time line was developed by NCOA and VA and achieved the goal of launching the program within 90 days of the kick-off meeting. Key indicators for the success of this program are detailed in the Table.
Both NCOA and VA saw the importance of creating a program that fits well within the overarching CSP and complements its services and resources (Figure 3).
Training and Support
Adequate training and support are essential to maintaining the integrity of this program. A total of 30 facilitators and 5 mentors to support workshops were trained by NCOA, and NCOA screened all facilitator candidates and provided training with ongoing support to all certified and accredited facilitators.
In addition, an online community offers continued support for workshop participants once they have completed the workshop. Graduates can access tools and resources, as well as problem solve, brainstorm, celebrate, and set goals along with other peer graduates via moderated discussion boards.
Preliminary Findings
More than 50% of workshop participants were aged 31 to 50 years, 85% lived with the veteran they were caring for, and 78% were spouses of the care recipient. Seventy-two percent of the veterans being cared for were white, and 93% were male. Nearly 80% of the veterans had PTSD, and more than half had TBI and/or a mental health disorder.
Clinical Indicators
Caregivers in the general population and those caring for veterans consider their caregiving situation stressful. In this implementation, participants are showing a statistically significant reduction in stress when measured at week 1 and week 6 with an average change score of 1.3 on a 10-point scale. Similar reductions in stress were seen in the original Stanford University study 3 months after the workshop had ended.2
Satisfaction
Participant satisfaction was high, averaging 4.5 on a 5-point Likert scale. Caregiver participants reported that what they liked best about the workshop was the shared experience with other caregivers, timing/convenience, giving and receiving help, and goal setting.
One recent caregiver who was caring for her husband remarked “I really enjoyed the workshop. Interacting with others, heartfelt stories of celebration, and frustration. The concern for the whole woman/man; physically and emotionally. I enjoyed the helpful suggestions/encouraging words of the leaders as well. I hope more people take advantage of this program.”
“I like the informal nature and self-paced aspect. We all have crazy lives but I think this was easy to do,” said another caregiver.
“This was a place where you can put out your problem and no one will judge you,” a caregiver explained. “There was respect for each other’s situations. Learning from others’ problem and how others share the solution. I saw how important the caregivers are, taking care of our self first so we can take care of the rest.”
Recruitment
In the 36 months since implementation, > 5,000 referrals were made for BBC, resulting in 2,654 caregivers being assigned to workshops; 75% of caregivers completed a workshop. Nearly half of all workshop graduates elected to join a moderated online community.
Discussion
Internal BBC recruitment data are captured monthly and reported to the Caregiver Support Program for each of the local VAMCs. Although recruitment goals are being met, a consistent referral pattern is not occurring at local VA sites. Not all VA sites are referring participants, and the referrals to BBC at some VA sites have been low in comparison with other higher performing VA sites. The percentage of sites with no referrals is 2%; lower performing sites with less than 20 caregivers referred represent 50% of sites.
A variety of factors contribute to disparities in referrals at different sites. A primary challenge is the increased demands on caregiver support coordinators nationally as they continue to prioritize the enrollment of caregivers into a variety of other programs available within CSP. The CSP office has developed other caregiver resources and tools that may compete with referral to BBC due to the preference of the staff and caregivers. Additional factors may also include no prescribed referral metrics for local sites and variances in local marketing and promotion of BBC. As the partnership between NCOA and the VA continues, additional caregiver referral methods are being explored to facilitate local promotion and marketing to engage caregivers of all eras.
Lessons and Next Steps
This partnership and program implementation have yielded a number of lessons learned and indications for next steps. The most significant lesson also proved to be the biggest success: the program can be embedded into clinical practice. Integrating the referral process into VA staff’s daily operations and depending on them to bring forward viable participants proved highly successful, with the majority of caregiver support coordinators making ≥ 1 referral and 50% of referred caregivers electing to take the workshop.
The VA and NCOA subsequently learned through focus groups that the relationship between the CSC and caregiver is strong, but there is also a need for continual follow-up and—more often than not—these referred caregivers need reminder e-mails to complete the sign-up process. The NCOA and VA also saw a larger proportion of post 9/11 caregivers (70%) recruited to the program compared with other eras (30%). Future recruitment will focus on ways to get caregivers of older era veterans involved with BBC.
Last, the VA and NCOA learned that participants really enjoyed the program. In the future more participant testimonials and stories will be used to spread the word to other caregivers about the program.
Conclusion
Caregivers of veterans face a unique set of challenges. Throughout both the VA pilot and the current partnership with NCOA, BBC is a promising solution for improving the well-being of caregivers of veterans. The success of its integration into clinical practice and participant satisfaction speak to both the quality of the program, as well as the partnership between NCOA and VA. Both NCOA and VA are working to expand its reach and make BBC readily available to as many caregivers of veterans as possible.
1. National Alliance for Caregiving, AARP. Caregiving in the U.S. National Alliance for Caregiving Website. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed December 17, 2015.
2. National Alliance for Caregiving, United Health Foundation, Caregivers of Veterans: Serving on the home front. National Alliance for Caregiving Website. http://www.caregiving.org/data/2010_Caregivers_of_Veterans_FULLREPORT_WEB_FINAL.pdf. Published November 2010. Accessed December 17, 2015.
3. Tanielian T, Ramchand R, Fisher MP, Sims CS, Harris RS, Harrell MC. Military Caregivers: Cornerstones of Support for Our Nation’s Wounded, Ill, and Injured Veterans. Santa Monica, CA: RAND Corporation; 2013.
4. Lorig K, Thompson-Gallagher D, Traylor L, et al. Building Better Caregivers: a pilot online support workshop for family caregivers of cognitively impaired adults. J Appl Gerontol. 2012;31(3):423-437.
5. Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee.” Alzheimer’s Dement. 2006;2(1):12-32.
6. MetLife Mature Market Institute, LifePlans. The MetLife study of alzheimer’s disease: the caregiving experience. MetLife Website. https://www.metlife.com/assets/cao/mmi/publications/studies/mmi-alzheimers-disease-caregiving-experience-study.pdf. Published August 2006. Accessed December 17, 2015.
7. Pandya S. Racial and ethnic differences among older adults in long-term care service use. http://www.aarp.org/home-garden/livable-communities/info-2005/fs119_ltc.html. Published June 2005. Accessed December 17, 2015.
8. Resnik LJ, Allen SM. Using international classification of functioning, disability and health to understand challenges in community reintegration of injured veterans. J Rehab Res Dev. 2007;44(7):991-1006.
1. National Alliance for Caregiving, AARP. Caregiving in the U.S. National Alliance for Caregiving Website. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed December 17, 2015.
2. National Alliance for Caregiving, United Health Foundation, Caregivers of Veterans: Serving on the home front. National Alliance for Caregiving Website. http://www.caregiving.org/data/2010_Caregivers_of_Veterans_FULLREPORT_WEB_FINAL.pdf. Published November 2010. Accessed December 17, 2015.
3. Tanielian T, Ramchand R, Fisher MP, Sims CS, Harris RS, Harrell MC. Military Caregivers: Cornerstones of Support for Our Nation’s Wounded, Ill, and Injured Veterans. Santa Monica, CA: RAND Corporation; 2013.
4. Lorig K, Thompson-Gallagher D, Traylor L, et al. Building Better Caregivers: a pilot online support workshop for family caregivers of cognitively impaired adults. J Appl Gerontol. 2012;31(3):423-437.
5. Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee.” Alzheimer’s Dement. 2006;2(1):12-32.
6. MetLife Mature Market Institute, LifePlans. The MetLife study of alzheimer’s disease: the caregiving experience. MetLife Website. https://www.metlife.com/assets/cao/mmi/publications/studies/mmi-alzheimers-disease-caregiving-experience-study.pdf. Published August 2006. Accessed December 17, 2015.
7. Pandya S. Racial and ethnic differences among older adults in long-term care service use. http://www.aarp.org/home-garden/livable-communities/info-2005/fs119_ltc.html. Published June 2005. Accessed December 17, 2015.
8. Resnik LJ, Allen SM. Using international classification of functioning, disability and health to understand challenges in community reintegration of injured veterans. J Rehab Res Dev. 2007;44(7):991-1006.
The Social Worker’s Role in Delirium Care for Hospitalized Veterans
Delirium, or the state of mental confusion that may occur with physical or mental illness, is common, morbid, and costly; however, of the diagnosed cases, delirium is mentioned in hospital discharge summaries only 16% to 55% of the time.1-3
Social workers often coordinate care transitions for hospitalized older veterans. They serve as interdisciplinary team members who communicate with VA medical staff as well as with the patient and family. This position, in addition to their training in communication and advocacy, primes social workers for a role in delirium care and provides the needed support for veterans who experience delirium and their families.
Background
Delirium is a sudden disturbance of attention with reduced awareness of the environment. Because attention is impaired, other changes in cognition are common, including perceptual and thought disturbances. Additionally, delirium includes fluctuations in consciousness over the course of a day. The acute development of these cognitive disturbances is distinct from a preexisting chronic cognitive impairment, such as dementia. Delirium is a direct consequence of underlying medical conditions, such as infections, polypharmacy, dehydration, and surgery.4
Delirium subtypes all have inattention as a core symptom. In half of the cases, patients are hypoactive and will not awaken easily or participate in daily care plans readily.4 Hyperactive delirium occurs in a quarter of cases. In the remaining mixed delirium cases patients fluctuate between the 2 states.4
Delirium is often falsely mistaken for dementia. Although delirium and dementia can present similarly, delirium has a sudden onset, which can alert health care professionals (HCPs) to the likelihood of delirium. Another important distinction is that delirium is typically reversible. Symptom manifestations of delirium may also be confused with depression.
Related: Delirium in the Cardiac ICU
Preventing delirium is important due to its many negative health outcomes. Older adults who develop delirium are more likely to die sooner. In a Canadian study of hospitalized patients aged ≥ 65 years, 41.6% of the delirium cohort and 14.4% of the control group died within 12 months of hospital admission.5 The death rate predicted by delirium in the Canadian study was comparable to the death rate of those who experience other serious medical conditions, such as sepsis or a heart attack.6
Those who survive delirium experience other serious outcomes, such as a negative impact on function and cognition and an increase in long-term care placement.7 Even when the condition resolves quickly, lasting functional impairment may be evident without return to baseline functioning.8 Hospitalized veterans are generally older, making them susceptible to developing delirium.9
Prevalence
Delirium can result from multiple medical conditions and develops in up to 50% of patients after general surgery and up to 80% of patients in the intensive care unit.10,11 From 20% to 40% of hospitalized older adults and from 50% to 89% of patients with preexisting Alzheimer disease may develop delirium.12-15 The increasing number of aging adults who will be hospitalized may also result in an increased prevalence of delirium.1,16
Delirium is a result of various predisposing and precipitating factors.1 Predisposing vulnerabilities are intrinsic to the individual, whereas precipitating external stressors are found in the environment. External stressors may trigger delirium in an individual who is vulnerable due to predisposing risk. The primary risk factors for delirium include dementia, advanced age, sensory impairment, fracture, infection, and dehydration (Table 1).12
Predisposing factors for delirium, such as age and sex, lifestyle choices (alcohol, tobacco), and chronic conditions (atherosclerosis, depression, prior stroke/transient ischemic attack) are more prevalent in the veteran population.9,17-20 In 2011, the median age for male veterans was 64 and the median age for male nonveterans was 41. Of male veterans, 49.9% are aged ≥ 65 years in comparison with 10.5% of the nonveteran male population.21 Veterans also have higher rates of comorbidities; a significant risk factor for delirium.20 A study by Agha and colleagues found that veterans were 14 times more likely to have 5 or more medical conditions than that of the general population.9 In a study comparing veterans aged ≥ 65 years with their age matched nonveteran peers, the health status of the veterans was poorer overall.22 Veterans are more likely to have posttraumatic stress disorder, which can increase the risk of postsurgery delirium and dementia, a primary risk factor for delirium.23-26
Delirium Intervention
Up to 40% of delirium cases can be prevented.27 But delirium may remain undetected in older veterans because its symptoms are sometimes thought to be the unavoidable consequences of aging, dementia, preexisting mental health conditions, substance abuse, a disease process, or the hospital environment.28 Therefore, to avoid the negative consequences of delirium, prevention is critical.28
The goals of delirium treatment are to identify and reverse its underlying cause(s).29 Because delirium is typically multifactorial, an HCP must carefully consider the various sources that could have initiated a change in mental status. Delirium may be prevented if HCPs can reduce patient risk factors. The 2010 National Institute for Health and Clinical Excellence (NICE) Delirium Guideline recommended a set of prevention strategies to address delirium risk factors (Table 2).12
As a member of the health care team, social workers can help prevent delirium through attention to pain management, infection control, medication review, sensory improvement, adequate nutrition and hydration, hypoxia prevention, and mobilization.12No pharmacologic approach has been approved for the treatment of delirium.30 Drugs may manage symptoms associated with delirium, but they do not treat the disease and could be associated with toxicity in high-risk patients. However, there are a variety of nonpharmacologic preventative measures that have proven effective. Environmental interventions to prevent delirium include orientation, cognitive stimulation, and sensory aids. A 2013 meta-analysis of 19 delirium prevention programs found that most were effective in preventing delirium in patients at risk during hospitalization.31 This review found that the most successful programs included multidisciplinary teams providing staff education and therapeutic cognitive activities.31 Social workers can encourage and directly provide such services. The Delirium Toolbox is a delirium risk modification program that was piloted with frontline staff, including social workers, at the VA Boston Healthcare System in West Roxbury, Massachusetts, and has been associated with restraint reduction, shortened length of stay (LOS), and lower variable direct costs.32
Social Worker Role
Several studies, both national and international, have indicated that little has been done over the past 2 decades to increase the diagnosis of delirium, because only 12% to 35% of delirium cases are clinically detected within the emergency department and in acute care settings.33-37 Patients may hesitate to report their experience due to a sense of embarrassment or because of an inability to describe it.38
Social workers are skilled at helping individuals feel more at ease when disclosing distressing experiences. Delirium is relevant to HCPs and hospital social workers with care transition responsibilities, because delirium detection should impact discharge planning.1,39 Delirium education needs to be included in efforts to improve transitions from intensive care settings to lower levels of care and from lower levels of care to discharge.40 Hospital social workers are in a position to offer additional support because they see patients at a critical juncture in their care and can take steps to improve postdischarge outcomes.41
Prior to Onset
Social workers can play an important role prior to delirium onset.42 Patient education on delirium needs to be provided during the routine hospital intake assessment. Informing patients in advance that delirium is common, based on their risk factors, as well as what to expect if delirium is experienced has been found to provide comfort.38 Families who anticipated possible delirium-related confusion reported that they experienced less distress.38
Related: Baseball Reminiscence Therapy for Cognitively Impaired Veterans
During hospitalization, social workers can ascertain from families whether an alteration in mental status is a rapid change, possibly indicating delirium, or a gradual dementia onset. The social work skills of advocacy and education can be used to support delirium-risk identification to avoid adverse outcomes.43 When no family caregiver is present to provide a history of the individual’s cognitive function prior to hospitalization, the social worker may be the first to notice an acute change in cognitive status and can report this to the medical team.
During Delirium
Lack of patient responsiveness and difficulty following a conversation are possible signs of delirium. This situation should be reported to the medical team for further delirium assessment and diagnosis.4 The social worker can also attempt to determine whether a patient’s presentation is unusual by contacting the family. Social work training recognizes the important role of the family.44 Social workers often interact with families at the critical period between acute onset of delirium in the hospital and discharge.42 Studies have shown that delirium causes stress for the patient’s loved ones. Moreover, caregivers of patients who experience the syndrome are at a 12 times increased risk of meeting the criteria for generalized anxiety disorder.30 In one study, delirium was rated as more distressing for the caregivers who witnessed it than for the patients who experienced it.38 Education has been shown to reduce delirium-related distress.30
In cases where delirium is irreversible, such as during the active dying process, social workers can serve in a palliative role to ease family confusion and provide comfort.30 The presence of family and other familiar people are considered part of the nonpharmacologic management of delirium.28
Posthospitalization
Delirium complicates physical aspects of care for families, as their loved one may need direct care in areas where they were previously independent due to a loss of function. Logistic considerations such as increased supervision may be necessary due to delirium, and the patient’s condition may be upsetting and confusing for family members, triggering the need for emotional support. During the discharge process, social workers can provide support and education to family members or placement facilities.38
Social workers in the hospital setting are often responsible for discharge planning, including the reduction of extended LOS and unnecessary readmissions to the hospital.45 Increased LOS and hospital readmissions are 2 of the primary negative outcomes associated with delirium. Delirium can persist for months beyond hospitalization, making it a relevant issue at the time of discharge and beyond.46 Distress related to delirium has been documented up to 2 years after onset, due to manifestations of anxiety and depression.38
Distress impacts patients as well as caregivers who witness the delirium and provide care to the patient afterward.38 Long-term changes in mood in addition to loss of function as a result of delirium can lead to an increase in stress for both patients and their caregivers.30 The social work emphasis on counseling and family dynamics as well as the common role of coordinating post-discharge arrangements makes the profession uniquely suited for delirium care.
Barriers
Social workers can play a key role in delirium risk identification and coordination of care but face substantial barriers. Delirium assessments are complex and require training and education in the features of delirium and cognitive assessment.47 To date, social workers receive limited education about delirium and typically do not make deliberate efforts in prevention, support, and follow-up care.
Conclusion
Social workers will encounter delirium, and their training makes them particularly suited to address this health concern. An understanding of the larger ecologic system is a foundational aspect of social work and an essential component of delirium prevention and care.41 The multipathway nature of delirium as well as the importance of prevention suggests that multiple disciplines, including social work, should be involved.1 The American Delirium Society and the European Delirium Association both recognize the need for all HCPs to be engaged in delirium care.1,48
Related: Sharing Alzheimer Research, FasterSharing Alzheimer Research, Faster
Social workers in the hospital setting provide communication, advocacy, and education to other HCPs, as well as to patients and families (Figure). Because delirium directly impacts the emotional and logistic needs of patients and their families, it would be advantageous for social workers to take a more active role in delirium risk identification, prevention, and care. Fortunately, the nonpharmacologic approaches that social workers are skilled in providing (eg, education and emotional support) have been shown to benefit patients with delirium and their families.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K; American Delirium Society. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(suppl 2):S237-S240.
2. Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208.
3. van Zyl LT, Davidson PR. Delirium in hospital: an underreported event at discharge. Can J Psychiatry. 2003;48(8):555-560.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
5. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463.
6. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
7. McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575-583.
8. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(suppl 2):S301-S304.
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
10. Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4-9.
11. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598.
12. National Institute for Health and Clinical Excellence. Delirium: Diagnosis, Prevention and Management. National Institute for Health and Clinical Excellence Website. https://www.nice.org.uk/guidance/cg103/resources/delirium-174507018181. Published July 2010.
13. Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26(1):30-40.
14. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723-1732.
15. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335-1340.
16. Popejoy LL, Galambos C, Moylan K, Madsen R. Challenges to hospital discharge planning for older adults. Clin Nurs Res. 2012;21(4):431-449.
17. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139.
18. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807-813.
19. Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462-466.
20. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229-236.
21. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013 Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed November 14, 2015.
22. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276.
23. McGuire JM. The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs. 2012;27(4):236-245.
24. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747-753.
25. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
26. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204-212.
27. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
28. Conley DM. The gerontological clinical nurse specialist's role in prevention, early recognition, and management of delirium in hospitalized older adults. Urol Nurs. 2011;31(6):337-342.
29. Meagher DJ. Delirium: optimising management. BMJ. 2001;322(7279):144-149.
30. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris FD. Clarifying delirium management: practical, evidenced-based, expert recommendations for clinical practice. J Palliat Med. 2013;16(4):423-435.
31. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, pt 2):375-380.
32. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-957.e11.
33. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765.
34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248-253.
35. Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32-38.
36. Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685-689.
37. Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23-29.
38. Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804-812.
39. Simons K, Connolly RP, Bonifas R, et al. Psychosocial assessment of nursing home residents via MDS 3.0: recommendations for social service training, staffing, and roles in interdisciplinary care. J Am Med Dir Assoc. 2012;13(2):190.e9-190.e15.
40. Alici Y. Interventions to improve recognition of delirium: a sine qua non for successful transitional care programs. Arch Intern Med. 2012;172(1):80-82.
41. Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Soc Work Health Care. 2010;49(9):856-871.
42. Duffy F, Healy JP. Social work with older people in a hospital setting. Soc Work Health Care. 2011;50(2):109-123.
43. Anderson CP, Ngo LH, Marcantonio ER. Complications in post-acute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122-1127.
44. Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539-2546.
45. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
46. McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18(9):696-704.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473.
48. Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr. 2012;12:55.
Delirium, or the state of mental confusion that may occur with physical or mental illness, is common, morbid, and costly; however, of the diagnosed cases, delirium is mentioned in hospital discharge summaries only 16% to 55% of the time.1-3
Social workers often coordinate care transitions for hospitalized older veterans. They serve as interdisciplinary team members who communicate with VA medical staff as well as with the patient and family. This position, in addition to their training in communication and advocacy, primes social workers for a role in delirium care and provides the needed support for veterans who experience delirium and their families.
Background
Delirium is a sudden disturbance of attention with reduced awareness of the environment. Because attention is impaired, other changes in cognition are common, including perceptual and thought disturbances. Additionally, delirium includes fluctuations in consciousness over the course of a day. The acute development of these cognitive disturbances is distinct from a preexisting chronic cognitive impairment, such as dementia. Delirium is a direct consequence of underlying medical conditions, such as infections, polypharmacy, dehydration, and surgery.4
Delirium subtypes all have inattention as a core symptom. In half of the cases, patients are hypoactive and will not awaken easily or participate in daily care plans readily.4 Hyperactive delirium occurs in a quarter of cases. In the remaining mixed delirium cases patients fluctuate between the 2 states.4
Delirium is often falsely mistaken for dementia. Although delirium and dementia can present similarly, delirium has a sudden onset, which can alert health care professionals (HCPs) to the likelihood of delirium. Another important distinction is that delirium is typically reversible. Symptom manifestations of delirium may also be confused with depression.
Related: Delirium in the Cardiac ICU
Preventing delirium is important due to its many negative health outcomes. Older adults who develop delirium are more likely to die sooner. In a Canadian study of hospitalized patients aged ≥ 65 years, 41.6% of the delirium cohort and 14.4% of the control group died within 12 months of hospital admission.5 The death rate predicted by delirium in the Canadian study was comparable to the death rate of those who experience other serious medical conditions, such as sepsis or a heart attack.6
Those who survive delirium experience other serious outcomes, such as a negative impact on function and cognition and an increase in long-term care placement.7 Even when the condition resolves quickly, lasting functional impairment may be evident without return to baseline functioning.8 Hospitalized veterans are generally older, making them susceptible to developing delirium.9
Prevalence
Delirium can result from multiple medical conditions and develops in up to 50% of patients after general surgery and up to 80% of patients in the intensive care unit.10,11 From 20% to 40% of hospitalized older adults and from 50% to 89% of patients with preexisting Alzheimer disease may develop delirium.12-15 The increasing number of aging adults who will be hospitalized may also result in an increased prevalence of delirium.1,16
Delirium is a result of various predisposing and precipitating factors.1 Predisposing vulnerabilities are intrinsic to the individual, whereas precipitating external stressors are found in the environment. External stressors may trigger delirium in an individual who is vulnerable due to predisposing risk. The primary risk factors for delirium include dementia, advanced age, sensory impairment, fracture, infection, and dehydration (Table 1).12
Predisposing factors for delirium, such as age and sex, lifestyle choices (alcohol, tobacco), and chronic conditions (atherosclerosis, depression, prior stroke/transient ischemic attack) are more prevalent in the veteran population.9,17-20 In 2011, the median age for male veterans was 64 and the median age for male nonveterans was 41. Of male veterans, 49.9% are aged ≥ 65 years in comparison with 10.5% of the nonveteran male population.21 Veterans also have higher rates of comorbidities; a significant risk factor for delirium.20 A study by Agha and colleagues found that veterans were 14 times more likely to have 5 or more medical conditions than that of the general population.9 In a study comparing veterans aged ≥ 65 years with their age matched nonveteran peers, the health status of the veterans was poorer overall.22 Veterans are more likely to have posttraumatic stress disorder, which can increase the risk of postsurgery delirium and dementia, a primary risk factor for delirium.23-26
Delirium Intervention
Up to 40% of delirium cases can be prevented.27 But delirium may remain undetected in older veterans because its symptoms are sometimes thought to be the unavoidable consequences of aging, dementia, preexisting mental health conditions, substance abuse, a disease process, or the hospital environment.28 Therefore, to avoid the negative consequences of delirium, prevention is critical.28
The goals of delirium treatment are to identify and reverse its underlying cause(s).29 Because delirium is typically multifactorial, an HCP must carefully consider the various sources that could have initiated a change in mental status. Delirium may be prevented if HCPs can reduce patient risk factors. The 2010 National Institute for Health and Clinical Excellence (NICE) Delirium Guideline recommended a set of prevention strategies to address delirium risk factors (Table 2).12
As a member of the health care team, social workers can help prevent delirium through attention to pain management, infection control, medication review, sensory improvement, adequate nutrition and hydration, hypoxia prevention, and mobilization.12No pharmacologic approach has been approved for the treatment of delirium.30 Drugs may manage symptoms associated with delirium, but they do not treat the disease and could be associated with toxicity in high-risk patients. However, there are a variety of nonpharmacologic preventative measures that have proven effective. Environmental interventions to prevent delirium include orientation, cognitive stimulation, and sensory aids. A 2013 meta-analysis of 19 delirium prevention programs found that most were effective in preventing delirium in patients at risk during hospitalization.31 This review found that the most successful programs included multidisciplinary teams providing staff education and therapeutic cognitive activities.31 Social workers can encourage and directly provide such services. The Delirium Toolbox is a delirium risk modification program that was piloted with frontline staff, including social workers, at the VA Boston Healthcare System in West Roxbury, Massachusetts, and has been associated with restraint reduction, shortened length of stay (LOS), and lower variable direct costs.32
Social Worker Role
Several studies, both national and international, have indicated that little has been done over the past 2 decades to increase the diagnosis of delirium, because only 12% to 35% of delirium cases are clinically detected within the emergency department and in acute care settings.33-37 Patients may hesitate to report their experience due to a sense of embarrassment or because of an inability to describe it.38
Social workers are skilled at helping individuals feel more at ease when disclosing distressing experiences. Delirium is relevant to HCPs and hospital social workers with care transition responsibilities, because delirium detection should impact discharge planning.1,39 Delirium education needs to be included in efforts to improve transitions from intensive care settings to lower levels of care and from lower levels of care to discharge.40 Hospital social workers are in a position to offer additional support because they see patients at a critical juncture in their care and can take steps to improve postdischarge outcomes.41
Prior to Onset
Social workers can play an important role prior to delirium onset.42 Patient education on delirium needs to be provided during the routine hospital intake assessment. Informing patients in advance that delirium is common, based on their risk factors, as well as what to expect if delirium is experienced has been found to provide comfort.38 Families who anticipated possible delirium-related confusion reported that they experienced less distress.38
Related: Baseball Reminiscence Therapy for Cognitively Impaired Veterans
During hospitalization, social workers can ascertain from families whether an alteration in mental status is a rapid change, possibly indicating delirium, or a gradual dementia onset. The social work skills of advocacy and education can be used to support delirium-risk identification to avoid adverse outcomes.43 When no family caregiver is present to provide a history of the individual’s cognitive function prior to hospitalization, the social worker may be the first to notice an acute change in cognitive status and can report this to the medical team.
During Delirium
Lack of patient responsiveness and difficulty following a conversation are possible signs of delirium. This situation should be reported to the medical team for further delirium assessment and diagnosis.4 The social worker can also attempt to determine whether a patient’s presentation is unusual by contacting the family. Social work training recognizes the important role of the family.44 Social workers often interact with families at the critical period between acute onset of delirium in the hospital and discharge.42 Studies have shown that delirium causes stress for the patient’s loved ones. Moreover, caregivers of patients who experience the syndrome are at a 12 times increased risk of meeting the criteria for generalized anxiety disorder.30 In one study, delirium was rated as more distressing for the caregivers who witnessed it than for the patients who experienced it.38 Education has been shown to reduce delirium-related distress.30
In cases where delirium is irreversible, such as during the active dying process, social workers can serve in a palliative role to ease family confusion and provide comfort.30 The presence of family and other familiar people are considered part of the nonpharmacologic management of delirium.28
Posthospitalization
Delirium complicates physical aspects of care for families, as their loved one may need direct care in areas where they were previously independent due to a loss of function. Logistic considerations such as increased supervision may be necessary due to delirium, and the patient’s condition may be upsetting and confusing for family members, triggering the need for emotional support. During the discharge process, social workers can provide support and education to family members or placement facilities.38
Social workers in the hospital setting are often responsible for discharge planning, including the reduction of extended LOS and unnecessary readmissions to the hospital.45 Increased LOS and hospital readmissions are 2 of the primary negative outcomes associated with delirium. Delirium can persist for months beyond hospitalization, making it a relevant issue at the time of discharge and beyond.46 Distress related to delirium has been documented up to 2 years after onset, due to manifestations of anxiety and depression.38
Distress impacts patients as well as caregivers who witness the delirium and provide care to the patient afterward.38 Long-term changes in mood in addition to loss of function as a result of delirium can lead to an increase in stress for both patients and their caregivers.30 The social work emphasis on counseling and family dynamics as well as the common role of coordinating post-discharge arrangements makes the profession uniquely suited for delirium care.
Barriers
Social workers can play a key role in delirium risk identification and coordination of care but face substantial barriers. Delirium assessments are complex and require training and education in the features of delirium and cognitive assessment.47 To date, social workers receive limited education about delirium and typically do not make deliberate efforts in prevention, support, and follow-up care.
Conclusion
Social workers will encounter delirium, and their training makes them particularly suited to address this health concern. An understanding of the larger ecologic system is a foundational aspect of social work and an essential component of delirium prevention and care.41 The multipathway nature of delirium as well as the importance of prevention suggests that multiple disciplines, including social work, should be involved.1 The American Delirium Society and the European Delirium Association both recognize the need for all HCPs to be engaged in delirium care.1,48
Related: Sharing Alzheimer Research, FasterSharing Alzheimer Research, Faster
Social workers in the hospital setting provide communication, advocacy, and education to other HCPs, as well as to patients and families (Figure). Because delirium directly impacts the emotional and logistic needs of patients and their families, it would be advantageous for social workers to take a more active role in delirium risk identification, prevention, and care. Fortunately, the nonpharmacologic approaches that social workers are skilled in providing (eg, education and emotional support) have been shown to benefit patients with delirium and their families.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Delirium, or the state of mental confusion that may occur with physical or mental illness, is common, morbid, and costly; however, of the diagnosed cases, delirium is mentioned in hospital discharge summaries only 16% to 55% of the time.1-3
Social workers often coordinate care transitions for hospitalized older veterans. They serve as interdisciplinary team members who communicate with VA medical staff as well as with the patient and family. This position, in addition to their training in communication and advocacy, primes social workers for a role in delirium care and provides the needed support for veterans who experience delirium and their families.
Background
Delirium is a sudden disturbance of attention with reduced awareness of the environment. Because attention is impaired, other changes in cognition are common, including perceptual and thought disturbances. Additionally, delirium includes fluctuations in consciousness over the course of a day. The acute development of these cognitive disturbances is distinct from a preexisting chronic cognitive impairment, such as dementia. Delirium is a direct consequence of underlying medical conditions, such as infections, polypharmacy, dehydration, and surgery.4
Delirium subtypes all have inattention as a core symptom. In half of the cases, patients are hypoactive and will not awaken easily or participate in daily care plans readily.4 Hyperactive delirium occurs in a quarter of cases. In the remaining mixed delirium cases patients fluctuate between the 2 states.4
Delirium is often falsely mistaken for dementia. Although delirium and dementia can present similarly, delirium has a sudden onset, which can alert health care professionals (HCPs) to the likelihood of delirium. Another important distinction is that delirium is typically reversible. Symptom manifestations of delirium may also be confused with depression.
Related: Delirium in the Cardiac ICU
Preventing delirium is important due to its many negative health outcomes. Older adults who develop delirium are more likely to die sooner. In a Canadian study of hospitalized patients aged ≥ 65 years, 41.6% of the delirium cohort and 14.4% of the control group died within 12 months of hospital admission.5 The death rate predicted by delirium in the Canadian study was comparable to the death rate of those who experience other serious medical conditions, such as sepsis or a heart attack.6
Those who survive delirium experience other serious outcomes, such as a negative impact on function and cognition and an increase in long-term care placement.7 Even when the condition resolves quickly, lasting functional impairment may be evident without return to baseline functioning.8 Hospitalized veterans are generally older, making them susceptible to developing delirium.9
Prevalence
Delirium can result from multiple medical conditions and develops in up to 50% of patients after general surgery and up to 80% of patients in the intensive care unit.10,11 From 20% to 40% of hospitalized older adults and from 50% to 89% of patients with preexisting Alzheimer disease may develop delirium.12-15 The increasing number of aging adults who will be hospitalized may also result in an increased prevalence of delirium.1,16
Delirium is a result of various predisposing and precipitating factors.1 Predisposing vulnerabilities are intrinsic to the individual, whereas precipitating external stressors are found in the environment. External stressors may trigger delirium in an individual who is vulnerable due to predisposing risk. The primary risk factors for delirium include dementia, advanced age, sensory impairment, fracture, infection, and dehydration (Table 1).12
Predisposing factors for delirium, such as age and sex, lifestyle choices (alcohol, tobacco), and chronic conditions (atherosclerosis, depression, prior stroke/transient ischemic attack) are more prevalent in the veteran population.9,17-20 In 2011, the median age for male veterans was 64 and the median age for male nonveterans was 41. Of male veterans, 49.9% are aged ≥ 65 years in comparison with 10.5% of the nonveteran male population.21 Veterans also have higher rates of comorbidities; a significant risk factor for delirium.20 A study by Agha and colleagues found that veterans were 14 times more likely to have 5 or more medical conditions than that of the general population.9 In a study comparing veterans aged ≥ 65 years with their age matched nonveteran peers, the health status of the veterans was poorer overall.22 Veterans are more likely to have posttraumatic stress disorder, which can increase the risk of postsurgery delirium and dementia, a primary risk factor for delirium.23-26
Delirium Intervention
Up to 40% of delirium cases can be prevented.27 But delirium may remain undetected in older veterans because its symptoms are sometimes thought to be the unavoidable consequences of aging, dementia, preexisting mental health conditions, substance abuse, a disease process, or the hospital environment.28 Therefore, to avoid the negative consequences of delirium, prevention is critical.28
The goals of delirium treatment are to identify and reverse its underlying cause(s).29 Because delirium is typically multifactorial, an HCP must carefully consider the various sources that could have initiated a change in mental status. Delirium may be prevented if HCPs can reduce patient risk factors. The 2010 National Institute for Health and Clinical Excellence (NICE) Delirium Guideline recommended a set of prevention strategies to address delirium risk factors (Table 2).12
As a member of the health care team, social workers can help prevent delirium through attention to pain management, infection control, medication review, sensory improvement, adequate nutrition and hydration, hypoxia prevention, and mobilization.12No pharmacologic approach has been approved for the treatment of delirium.30 Drugs may manage symptoms associated with delirium, but they do not treat the disease and could be associated with toxicity in high-risk patients. However, there are a variety of nonpharmacologic preventative measures that have proven effective. Environmental interventions to prevent delirium include orientation, cognitive stimulation, and sensory aids. A 2013 meta-analysis of 19 delirium prevention programs found that most were effective in preventing delirium in patients at risk during hospitalization.31 This review found that the most successful programs included multidisciplinary teams providing staff education and therapeutic cognitive activities.31 Social workers can encourage and directly provide such services. The Delirium Toolbox is a delirium risk modification program that was piloted with frontline staff, including social workers, at the VA Boston Healthcare System in West Roxbury, Massachusetts, and has been associated with restraint reduction, shortened length of stay (LOS), and lower variable direct costs.32
Social Worker Role
Several studies, both national and international, have indicated that little has been done over the past 2 decades to increase the diagnosis of delirium, because only 12% to 35% of delirium cases are clinically detected within the emergency department and in acute care settings.33-37 Patients may hesitate to report their experience due to a sense of embarrassment or because of an inability to describe it.38
Social workers are skilled at helping individuals feel more at ease when disclosing distressing experiences. Delirium is relevant to HCPs and hospital social workers with care transition responsibilities, because delirium detection should impact discharge planning.1,39 Delirium education needs to be included in efforts to improve transitions from intensive care settings to lower levels of care and from lower levels of care to discharge.40 Hospital social workers are in a position to offer additional support because they see patients at a critical juncture in their care and can take steps to improve postdischarge outcomes.41
Prior to Onset
Social workers can play an important role prior to delirium onset.42 Patient education on delirium needs to be provided during the routine hospital intake assessment. Informing patients in advance that delirium is common, based on their risk factors, as well as what to expect if delirium is experienced has been found to provide comfort.38 Families who anticipated possible delirium-related confusion reported that they experienced less distress.38
Related: Baseball Reminiscence Therapy for Cognitively Impaired Veterans
During hospitalization, social workers can ascertain from families whether an alteration in mental status is a rapid change, possibly indicating delirium, or a gradual dementia onset. The social work skills of advocacy and education can be used to support delirium-risk identification to avoid adverse outcomes.43 When no family caregiver is present to provide a history of the individual’s cognitive function prior to hospitalization, the social worker may be the first to notice an acute change in cognitive status and can report this to the medical team.
During Delirium
Lack of patient responsiveness and difficulty following a conversation are possible signs of delirium. This situation should be reported to the medical team for further delirium assessment and diagnosis.4 The social worker can also attempt to determine whether a patient’s presentation is unusual by contacting the family. Social work training recognizes the important role of the family.44 Social workers often interact with families at the critical period between acute onset of delirium in the hospital and discharge.42 Studies have shown that delirium causes stress for the patient’s loved ones. Moreover, caregivers of patients who experience the syndrome are at a 12 times increased risk of meeting the criteria for generalized anxiety disorder.30 In one study, delirium was rated as more distressing for the caregivers who witnessed it than for the patients who experienced it.38 Education has been shown to reduce delirium-related distress.30
In cases where delirium is irreversible, such as during the active dying process, social workers can serve in a palliative role to ease family confusion and provide comfort.30 The presence of family and other familiar people are considered part of the nonpharmacologic management of delirium.28
Posthospitalization
Delirium complicates physical aspects of care for families, as their loved one may need direct care in areas where they were previously independent due to a loss of function. Logistic considerations such as increased supervision may be necessary due to delirium, and the patient’s condition may be upsetting and confusing for family members, triggering the need for emotional support. During the discharge process, social workers can provide support and education to family members or placement facilities.38
Social workers in the hospital setting are often responsible for discharge planning, including the reduction of extended LOS and unnecessary readmissions to the hospital.45 Increased LOS and hospital readmissions are 2 of the primary negative outcomes associated with delirium. Delirium can persist for months beyond hospitalization, making it a relevant issue at the time of discharge and beyond.46 Distress related to delirium has been documented up to 2 years after onset, due to manifestations of anxiety and depression.38
Distress impacts patients as well as caregivers who witness the delirium and provide care to the patient afterward.38 Long-term changes in mood in addition to loss of function as a result of delirium can lead to an increase in stress for both patients and their caregivers.30 The social work emphasis on counseling and family dynamics as well as the common role of coordinating post-discharge arrangements makes the profession uniquely suited for delirium care.
Barriers
Social workers can play a key role in delirium risk identification and coordination of care but face substantial barriers. Delirium assessments are complex and require training and education in the features of delirium and cognitive assessment.47 To date, social workers receive limited education about delirium and typically do not make deliberate efforts in prevention, support, and follow-up care.
Conclusion
Social workers will encounter delirium, and their training makes them particularly suited to address this health concern. An understanding of the larger ecologic system is a foundational aspect of social work and an essential component of delirium prevention and care.41 The multipathway nature of delirium as well as the importance of prevention suggests that multiple disciplines, including social work, should be involved.1 The American Delirium Society and the European Delirium Association both recognize the need for all HCPs to be engaged in delirium care.1,48
Related: Sharing Alzheimer Research, FasterSharing Alzheimer Research, Faster
Social workers in the hospital setting provide communication, advocacy, and education to other HCPs, as well as to patients and families (Figure). Because delirium directly impacts the emotional and logistic needs of patients and their families, it would be advantageous for social workers to take a more active role in delirium risk identification, prevention, and care. Fortunately, the nonpharmacologic approaches that social workers are skilled in providing (eg, education and emotional support) have been shown to benefit patients with delirium and their families.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K; American Delirium Society. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(suppl 2):S237-S240.
2. Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208.
3. van Zyl LT, Davidson PR. Delirium in hospital: an underreported event at discharge. Can J Psychiatry. 2003;48(8):555-560.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
5. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463.
6. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
7. McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575-583.
8. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(suppl 2):S301-S304.
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
10. Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4-9.
11. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598.
12. National Institute for Health and Clinical Excellence. Delirium: Diagnosis, Prevention and Management. National Institute for Health and Clinical Excellence Website. https://www.nice.org.uk/guidance/cg103/resources/delirium-174507018181. Published July 2010.
13. Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26(1):30-40.
14. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723-1732.
15. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335-1340.
16. Popejoy LL, Galambos C, Moylan K, Madsen R. Challenges to hospital discharge planning for older adults. Clin Nurs Res. 2012;21(4):431-449.
17. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139.
18. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807-813.
19. Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462-466.
20. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229-236.
21. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013 Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed November 14, 2015.
22. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276.
23. McGuire JM. The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs. 2012;27(4):236-245.
24. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747-753.
25. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
26. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204-212.
27. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
28. Conley DM. The gerontological clinical nurse specialist's role in prevention, early recognition, and management of delirium in hospitalized older adults. Urol Nurs. 2011;31(6):337-342.
29. Meagher DJ. Delirium: optimising management. BMJ. 2001;322(7279):144-149.
30. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris FD. Clarifying delirium management: practical, evidenced-based, expert recommendations for clinical practice. J Palliat Med. 2013;16(4):423-435.
31. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, pt 2):375-380.
32. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-957.e11.
33. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765.
34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248-253.
35. Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32-38.
36. Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685-689.
37. Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23-29.
38. Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804-812.
39. Simons K, Connolly RP, Bonifas R, et al. Psychosocial assessment of nursing home residents via MDS 3.0: recommendations for social service training, staffing, and roles in interdisciplinary care. J Am Med Dir Assoc. 2012;13(2):190.e9-190.e15.
40. Alici Y. Interventions to improve recognition of delirium: a sine qua non for successful transitional care programs. Arch Intern Med. 2012;172(1):80-82.
41. Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Soc Work Health Care. 2010;49(9):856-871.
42. Duffy F, Healy JP. Social work with older people in a hospital setting. Soc Work Health Care. 2011;50(2):109-123.
43. Anderson CP, Ngo LH, Marcantonio ER. Complications in post-acute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122-1127.
44. Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539-2546.
45. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
46. McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18(9):696-704.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473.
48. Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr. 2012;12:55.
1. Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K; American Delirium Society. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(suppl 2):S237-S240.
2. Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208.
3. van Zyl LT, Davidson PR. Delirium in hospital: an underreported event at discharge. Can J Psychiatry. 2003;48(8):555-560.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
5. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463.
6. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
7. McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575-583.
8. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(suppl 2):S301-S304.
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
10. Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4-9.
11. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598.
12. National Institute for Health and Clinical Excellence. Delirium: Diagnosis, Prevention and Management. National Institute for Health and Clinical Excellence Website. https://www.nice.org.uk/guidance/cg103/resources/delirium-174507018181. Published July 2010.
13. Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26(1):30-40.
14. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723-1732.
15. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335-1340.
16. Popejoy LL, Galambos C, Moylan K, Madsen R. Challenges to hospital discharge planning for older adults. Clin Nurs Res. 2012;21(4):431-449.
17. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139.
18. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807-813.
19. Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462-466.
20. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229-236.
21. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013 Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed November 14, 2015.
22. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276.
23. McGuire JM. The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs. 2012;27(4):236-245.
24. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747-753.
25. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
26. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204-212.
27. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
28. Conley DM. The gerontological clinical nurse specialist's role in prevention, early recognition, and management of delirium in hospitalized older adults. Urol Nurs. 2011;31(6):337-342.
29. Meagher DJ. Delirium: optimising management. BMJ. 2001;322(7279):144-149.
30. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris FD. Clarifying delirium management: practical, evidenced-based, expert recommendations for clinical practice. J Palliat Med. 2013;16(4):423-435.
31. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, pt 2):375-380.
32. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-957.e11.
33. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765.
34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248-253.
35. Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32-38.
36. Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685-689.
37. Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23-29.
38. Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804-812.
39. Simons K, Connolly RP, Bonifas R, et al. Psychosocial assessment of nursing home residents via MDS 3.0: recommendations for social service training, staffing, and roles in interdisciplinary care. J Am Med Dir Assoc. 2012;13(2):190.e9-190.e15.
40. Alici Y. Interventions to improve recognition of delirium: a sine qua non for successful transitional care programs. Arch Intern Med. 2012;172(1):80-82.
41. Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Soc Work Health Care. 2010;49(9):856-871.
42. Duffy F, Healy JP. Social work with older people in a hospital setting. Soc Work Health Care. 2011;50(2):109-123.
43. Anderson CP, Ngo LH, Marcantonio ER. Complications in post-acute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122-1127.
44. Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539-2546.
45. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
46. McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18(9):696-704.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473.
48. Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr. 2012;12:55.
Yoga-Based Classes for Veterans With Severe Mental Illness: Development, Dissemination, and Assessment
There is growing interest in developing a holistic and integrative approach for the treatment of severe mental illnesses (SMI), such as schizophrenia, major depression, posttraumatic stress disorder (PTSD), and anxiety disorders. Western medicine has traditionally focused on the direct treatment of symptoms and separated the management of physical and mental health, but increasing attention is being given to complementary and alternative medicine (CAM) for patients with SMI.
Recognizing the connectedness of the mind and body, these complementary or alternative approaches incorporate nontraditional therapeutic techniques with mainstream treatment methods, including psychopharmacology and psychotherapy.1 Patients with SMI may particularly benefit from a mind-body therapeutic approach, because they often experience psychological symptoms such as stress, anxiety, depression and psychosis, as well as a preponderance of medical comorbidities, including obesity, diabetes mellitus, and cardiovascular disease, some of which are compounded by adverse effects (AEs) of essential pharmacologic treatments.2-4 Mind-body interventions might also be particularly advantageous for veterans, who often experience a range of interconnected physical and psychological difficulties due to trauma exposure and challenges transitioning from military to civilian life.5
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
In 2002, the White House Commission on Complementary and Alternative Medicine Policy issued a report supporting CAM research and integration into existing medical systems.6 The DoD later established Total Force Fitness, a holistic health care program for active-duty military personnel.7 The VA has also incorporated mind-body and holistic strategies into veteran care.8 One such mind-body intervention, yoga, is becoming increasingly popular within the health care field.
Recent research has documented the effectiveness of yoga, underscoring its utility as a mind-body therapeutic approach. Yoga is associated with improvement in balance and flexibility,9 fatigue,10 blood pressure,11 sleep,12 strength,13 and pain14 in both healthy individuals and patients with medical and psychiatric disorders.15 The literature also illustrates that yoga has led to significant improvements in stress and psychiatric symptoms in individuals with PTSD, schizophrenia, depression, and anxiety.16-22 A previous meta-analysis conducted by the authors, which considered studies of the effectiveness of yoga as an adjunctive treatment for patients with mental illness, found that 212 studies with null results would need to be located and incorporated to negate the positive effects of yoga found in the literature.17
Because yoga emphasizes the practice of mindfulness and timing movement with breath awareness, it is a calming practice that may decrease stress and relieve psychiatric symptoms not treated through psychopharmacology and psychotherapy.17,21 Recent research has postulated that the physiological mechanisms by which this occurs may include (a) reduction in sympathetic and increase in parasympathetic activity23,24; (b) increases in heart-rate variability and respiratory sinus arrhythmia, low levels of which are associated with anxiety, panic disorder, and depression23,24; (c) increases in melatonin and serotonin 25-27; and (d) decrease in cortisol.28,29
Related: Enhancing Patient Satisfaction Through the Use of Complementary Therapies
As yoga may calm the autonomic nervous system and reduce stress, it may benefit patients with SMI, whose symptoms are often aggravated by stress.30 In addition, veterans experience both acute stressors and high levels of chronic stress.5 Therefore, because they experience mind-body comorbid illnesses as well as high levels of stress, the authors believe that veterans with SMI could benefit greatly from a tailored yoga-based program as part of a holistic approach that includes necessary medication and evidence-based therapies.
In order to evaluate the effects of a yoga program on veterans receiving mental health treatment across the VA Greater Los Angeles Healthcare System (VAGLAHS), the authors developed a set of yoga-based wellness classes called Breathing, Stretching, Relaxation (BSR) classes. This article describes the process of developing these classes and outlines the procedures and results of a study to assess their effects.
BSR Classes
The development of BSR classes took place at the West Los Angeles VA Medical Center (WLAVAMC), within the Psychosocial Rehabilitation and Recovery Center (PRRC) program. The PRRC is a psychoeducational program that focuses on the biological, psychological, social, and spiritual aspects of life in order to help veterans with SMI rehabilitate and reintegrate into the community. The program allows veterans to create their own recovery curriculum by selecting from diverse classes led by program staff members, including physicians, psychologists, nurses, social workers, nutritionists, and recreational therapists.
Development of BSR Protocols
The primary goal of this project was to develop a yoga-based program tailored to the specific needs of veterans with SMI. To the authors’ knowledge, BSR is the first yoga-based program customized for SMI. The BSR classes were developed within interdisciplinary focus groups that included professional yoga teachers, the director of the PRRC, psychiatrists, psychologists, nurses, occupational therapists, and physical therapists. Drawing on their experience with SMI and yoga, members of the focus groups identified 3 aspects of yoga that would be most beneficial to veterans with SMI, and the program was designed to optimize these effects. Because SMI can both create and be exacerbated by stress, BSR classes were designed to reduce stress and provide veterans with the tools to monitor and manage their stress.
Breathing and meditative techniques were adapted from yoga in order to facilitate stress reduction. In addition, aerobic elements of yoga have the potential to help veterans manage their incidence of medical diseases, such as cardiovascular disease, obesity, and diabetes. Patients with SMI are at a greater risk for developing these diseases, so classes were designed to incorporate physical stretching elements to promote overall health.4,31-33 Finally BSR was designed to improve veteran self- efficacy and self-esteem, and to place veterans at the center of their care by equipping them with skills to practice BSR independently.
Related: Mindfulness to Reduce Stress
The focus groups also identified the logistic requirements when implementing a yoga-based program for veterans with SMI, including (a) obtaining participant or conservator consent; (b) obtaining medical clearance from care providers, given the high prevalence of medical comorbidities; (c) removing the traditional yoga terms, taking a secular approach, and naming the class “Breathing, Stretching, Relaxation” without directly referencing yoga; (d) asking veterans’ permission before incorporating physical contact into demonstrations, because veterans with SMI, especially those with PTSD, might be uncomfortable with touching from instructors; (e) creating protocols of varying duration and intensity so that BSR was approachable for veterans with diverse levels of physical ability; and (f) ensuring that a clinician who regularly works with SMI patients be present to supervise classes for the safety of patients and instructors.
Yoga instructors and clinicians collaborated to create adaptable 30- and 50-minute protocols that reflected best practices for an SMI population. The 30-minute seated BSR class protocol is included in eAppendix A. Once protocols were finalized, a Train the Trainer program was established to facilitate dissemination of BSR to clinicians working with veterans with SMI throughout the VAGLAHS.
Interested clinicians were given protocols and trained to lead BSR classes on their own. Subsequently, clinician-led BSR classes of various lengths (depending on clinician preference and program scheduling) were established at PRRCs and other mental health programs, such as Mental Health Recovery and Intensive Treatment and Dual Diagnosis Treatment Program, throughout the VAGLAHS. These programs were selected, because they are centered on recovery and improvements in symptoms of SMI. The adoption of a Train the Trainer model, through which VA clinicians were trained by professional yoga instructors, allowed for seamless integration of BSR into VA usual care for veterans with SMI.
Assessment of Classes
The authors conducted a study to assess the quality and effectiveness of BSR classes. This survey research was approved by the VAGLAHS institutional review board for human subjects. The authors hypothesized that there would be significant improvements in veterans’ stress, pain, well-being, and perception of the benefits of BSR over 8 weeks of participation in classes. Also hypothesized was that there would be greater benefits in veterans who participated in longer classes and who attended classes more frequently.
Methods
A total of 120 veterans completed surveys after participating in clinician- and yoga instructor-led BSR classes at the 3 sites within the VAGLAHS: WLAVAMC, Los Angeles Ambulatory Care Center (LAACC), and Sepulveda Ambulatory Care Center (SACC). At the WLAVAMC, surveys were collected at 10-, 30-, 60-, and 90-minute classes. At LAACC, surveys were collected at 30- and 60-minute classes. At SACC, surveys were collected at 20- and 45-minute classes. A researcher noted the duration of the class and was available to assist with comprehension. Veterans completed identical surveys after classes at a designated week 0 (baseline), week 4, and week 8. Of the 120 patients with an initial survey, 82 completed at least 1 follow-up survey and 49 completed both follow-up surveys.
Survey packets included (a) demographic questions, including age, gender, and ethnicity; (b) class participation questions, including frequency of class attendance, patients’ favorite aspect of class, and dura tion of class attendance (in months of prior participation); (c) a pain rating from 0 (no pain) to 10 (the worst pain imaginable); (d) the BioPsychoSocial-Spiritual (BPSS) Scale (eAppendix B), developed at the WLAVAMC, which provides wellness scores from 0 (low) to 10 (high) in 4 areas as well as a holistic wellness score from 0 (low) to 40 (high); (e) the Perceived Stress Scale (PSS), developed by Cohen and colleagues, which generates a stress score for the past month from 0 (low) to 40 (high)34; and (f) the Perceived Benefits of Yoga Questionnaire (PBYQ) (eAppendix C), which rates participants’ opinions about the benefits of yoga from 12 (low) to 60 (high) and is based on the Perceived Benefits of Dance Questionnaire.35
Statistical Analysis
Pearson’s r correlation coefficients were calculated between PBYQ scores and quantitative survey items at each time point (weeks 0, 4, and 8). Linear mixed-effects models were used to test for effects of multiple predictor variables on individual outcomes. Each model had a random intercept by participant, and regressors included main effects for the following: survey week (0, 4, or 8), class duration (in minutes), age, sex, ethnicity, frequency of attendance (in days per week), and duration of attendance (in months). For all statistical analyses, a 2-tailed significance criterion of α = .05 was used.
Results
Veterans who completed surveys were predominantly male (90.8%) and averaged 61.4 years of age. Table 1 shows demographic information. Table 2 displays the number of participants who were involved in short (< 30 min), medium (30-59 min), and long (> 60 min) classes. Veteran participants also had a wide range of prior BSR experience (Table 3).
At all time points, PBYQ scores were significantly positively correlated with class duration and biological, psychological, social, spiritual, and total well-being as measured by the BPSS. The PBYQ scores at all time points were also significantly negatively correlated with age, pain ratings, and PSS scores. Table 4 includes specific Pearson’s r values.
Survey week was not significantly associated with any individual outcome measures. There were no significant regressors for total PSS score or total BPSS score within the linear models. However, participants’ PBYQ scores were significantly associated with age (t(98) = -2.13, P = .036), frequency of attendance (t(103) = 2.10, P = .038), and class duration (t(98) = 4.35, P < .001). Additionally, class duration was significantly associated with pain (t(98) = -3.01, P = .003), with longer duration associated with less pain. Ethnicity was also associated with pain, with African American veterans reporting less pain than did white (t(98) = -2.41, P = .017) and Hispanic (t(98) = -2.31, P = .023) veterans. Because ethnicity was significantly associated with class duration (F(5,339) = 3.81, P = .002), the authors used an analysis of covariance to test for a mediating effect of ethnicity on the relationship between class duration and pain. Although there was a partial mediation (F(5,203) = 2.57, P = .028), the main effect of class duration remained significant.
Discussion and Limitations
The goals of this project were to develop a yoga-based program tailored for veterans with SMI and assess the program in a sample of veterans with SMI on subjective reports of stress, pain, well-being, and benefits of yoga. The authors hypothesized that significant improvements in these measures in veterans with SMI would be observed over 8 weeks of participation in BSR classes and that there would be greater benefits in veterans who participated in longer classes and who attended classes more frequently.
The authors succeeded in developing an adaptable yoga-based wellness program for veterans with SMI that can be both practiced in structured classes and incorporated into veterans’ everyday routines. The BSR classes were well tolerated by veterans with SMI, caused no discernible AEs, and are readily available for dissemination across other mental health programs. Veterans described integrating the tools they learned within BSR classes into their daily lives, helping them to manage pain; feel more flexible; reduce stress, anxiety, depression, and PTSD symptoms; and increase relaxation and feelings of self-control and confidence. Table 5 shows qualitative feedback collected from veterans. In addition, the Train the Trainer model optimized clinical applicability and flexibility, demonstrating that clinicians can seamlessly integrate BSR classes into a health care plan for veterans with SMI.
In assessing quantitative measures of stress, pain, well-being, and perceived benefits of a yoga-based program, veterans who reported BSR classes as beneficial experienced lower levels of pain and stress and higher levels of biological, psychological, social, spiritual, and overall well-being. Those veterans who perceived BSR as more beneficial tended to be younger and attend longer classes with greater frequency. Veterans who attended longer classes also reported experiencing less pain. This may be because the more rigorous stretching and posing involved in longer BSR classes made them more effective at reducing pain; however, it is also possible that veterans who were experiencing more pain avoided these longer classes due to their rigor and length.
Results suggest that longer classes attended with greater regularity may be more beneficial to veterans than short and infrequent classes, particularly in regards to their pain. Despite the relationships between class and outcome variables, the authors did not find significant improvements in measures of wellness, pain, stress, or perceived benefits of BSR over time, as was hypothesized. This may be because the data collection for this study began after classes had been established for some time. In fact, only 35 of 120 veterans included in this study reported having < 1 month of BSR experience at week 0, suggesting that results collected from week 0 did not represent a true baseline measurement. Although no relationship was found between prior duration of attendance and any outcome measures, the fact that most veterans in the sample had attended classes for several months prior to completing surveys may have biased the results by favoring the responses of veterans who were more invested in the classes. Improvements may have been better captured in a BSR-naïve sample.
The finding that the PBYQ score was significantly correlated with all other outcome measures (pain, BPSS score, and PSS score) raises some questions about the ways in which these classes were beneficial to veterans. It may be that veterans who experienced more positive outcomes from classes saw BSR as more beneficial, but it is also possible that veterans who entered classes with greater expectations experienced better outcomes due to a placebo effect—that is, outcomes may have been influenced more by the expectation than by the content of the classes. In the case of well-being (BPSS scores) and stress (PSS score), this could explain why these outcomes were significantly correlated with perceived benefits of BSR but were not significantly related to any class-related variables such as duration and frequency of attendance. Pain ratings, however, were related to class variables and perceived benefits of BSR.
In a post hoc analysis, the main effect of the PBYQ score as a regressor was added to the linear model for pain, resulting in the PBYQ score having a significant main effect (t(100) = -2.98, P = .004). The main effect of class duration remained significant (t(97) = -1.99, P = .050) but was less substantial when the PBYQ score was added, suggesting some correlation between class duration and pain, independent of BSR’s perceived benefits. Future research should consider the possible mediating effect of perceptions of the effectiveness of a yoga-based program a priori or control for placebo effects in order to address the degree to which outcomes are influenced by participant expectations.
This study had a few other notable limitations. Because it was an observational study administered within a clinical mental health program, a control group was not included. Measurement began after BSR classes were established, so veterans had varying levels of prior experience. Specific SMI and medical diagnosis information was not collected from individual veterans. Data were collected from classes of varying length and intensity. The BSR classes often took place within larger programs at the VA, which offered comprehensive care, so some effects of the BSR classes might have been confounded with concurrent evidenced-based treatments or other holistic care programs. Due to these limitations, particularly the absence of a control group, the relationships between BSR participation and health outcomes cannot be assumed to be causal, because a multitude of other variables, such as patient contact and expectancy effects, may have influenced outcomes.
Future Directions
Future research should aim to utilize a control group and collect data from classes of the same intensity and length to better examine whether BSR can be causally linked with improvements in measures of stress, pain, and well-being and to attempt to control for expectancy and contact effects. In addition, future research should aim to recruit a BSR-naïve sample to account for prior experience. Future studies should also aim to parse out whether BSR is differentially effective for each SMI or medical diagnosis, whether there is a relationship between class time and outcomes (as these results suggest that longer class times might be more beneficial for pain), and whether any pain-management benefits from BSR influence other measures of functionality and well-being. Finally, future research can further divide BSR into its active components, such as meditative breathing and aerobic stretching, in order to examine which aspect leads to the greatest effect on each measure; the current results imply that the more rigorous components of BSR classes have the greatest effects on pain.
Conclusion
A yoga-based class affects the mind and body, making it particularly useful for veterans with SMI who experience a range of physical and psychological symptoms and comorbidities.4 Other studies have demonstrated that when practiced alone, yoga leads to improvements in both physical and psychological symptoms.15,17 Looking forward, yoga-based classes may be implemented as part of a larger biopsychosocial-spiritual care plan that is being embraced both within and outside of the VA.7,8,36,37 This integrative health care model suggests that psychosocial and CAM modalities are additive and should be practiced concurrently.37
Although little research has assessed the effects of comprehensive psychosocial and CAM treatment programs, initial research indicates that these programs are associated with reductions in symptoms of medical and mental illness.37-40 Participants in BSR classes may derive further benefits if classes are incorporated into a larger holistic health care plan that includes both traditional psychiatric treatment modalities and CAM therapies that integrate biopsychosocial-spiritual components.
Acknowledgements
The authors would like to thank Nancy Mohler, Anne Platt, and Matthew Crowder, all professional yoga instructors, for their integral role in developing classes, providing classes to veterans, and assisting VA staff with training. The authors also acknowledge Rosie Dominguez, LCSW, for expertly leading BSR classes at LAACC. The authors also thank Irina Arnold, MS, MD, and Vanessa Streiff, MA, for their vital assistance with data collection at SACC.
Funding for the development of BSR protocols, quality improvement research, and expenses associated with classes was provided by the Disabled American Veterans Charitable Service Trust. Support for dissemination of classes to multiple VA programs was provided by a grant from the VA Center of Innovation for Patient Centered Care, headed by Sandra Robertson, RN, MSN, PH-CNS.
The authors are very grateful to the veterans and staff of the Mental Health Intensive Case Management, Psychosocial Rehabilitation and Recovery Centers, Mental Health Clinics, and Domiciliary throughout Greater Los Angeles for their support of the BSR classes.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what's in a name? U.S. Department of Health and Human Services, National Institutes of Health Website. http://nccam.nih.gov/health/whatiscam. Updated March 2015. Accessed August 27, 2015.
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69(4):514-519.
4. Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63(10):856-865.
5. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
6. White House Commission on Complementary and Alternative Medicine Policy. Final Report. Washington, DC: White House Commission on Complementary and Alternative Medicine Policy; 2002.
7. Land BC. Current Department of Defense guidance for total force fitness. Mil Med. 2010;175(suppl 8):3-5.
8. U.S. Department of Veterans Affairs, Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: U.S. Department of Veterans Affairs, Veterans Health Administration; 2009.
9. Oken BS, Zajdel D, Kishiyama S, et al. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006;12(1):40-47.
10. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766-3775.
11. Cade WT, Reeds DN, Mondy KE, et al. Yoga lifestyle intervention reduces blood pressure in HIV-infected adults with cardiovascular disease risk factors. HIV Med. 2010;11(6):379-388.
12. Innes KE, Selfe TK. The effects of a gentle yoga program on sleep, mood, and blood pressure in older women with restless leg syndrome (RLS): a preliminary randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:294058.
13. Van Puymbroeck M, Payne LL, Hsieh PC. A phase I feasibility study of yoga on the physical health and coping of informal caregivers. Evid Based Complement Alternat Med. 2007;4(4):519-529.
14. Rani K, Tiwari SC, Singh U, Agrawal GG, Srivastava N. Six-month trial of Yoga Nidra in menstrual disorder patients: effects on somatoform symptoms. Ind Psychiatry J. 2011;20(2):97-102.
15. Ross A, Thomas S. The health benefits of yoga and exercise: a review of comparison studies. J Altern Complement Med. 2010;16(1):3-12.
16. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6(3):242-250.
17. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4):doi:10.4088/PCC.10r01068.
18. Katzman MA, Vermani M, Gerbarg PL, et al. A multicomponent yoga-based, breath intervention program as an adjunctive treatment in patients suffering from generalized anxiety disorder with or without comorbidities. Int J Yoga. 2012;5(1):57-65.
19. Köhn M, Persson Lundholm U, Bryngelsson IL, Anderzén-Carlsson A, Westerdahl E. Medical yoga for patients with stress-related symptoms and diagnoses in primary health care: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:215348.
20. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs. 2007;33(2):17-23.
21. Meyer HB, Katsman A, Sones AC, Auerbach DE, Ames D, Rubin RT. Yoga as an ancillary treatment for neurological and psychiatric disorders: a review. J Neuropsychiatry Clin Neurosci. 2012;24(2):152-164.
22. Visceglia E, Lewis S. Yoga therapy as an adjunctive treatment for schizophrenia: a randomized, controlled pilot study. J Altern Complement Med. 2011;17(7):601-607.
23. Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression, part I-neurophysiologic model. J Altern Complement Med. 2005;11(1):189-201.
24. Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Ann N Y Acad Sci. 2009;1172:54-62.
25. Harinath K, Malhotra AS, Pal K, et al. Effects of hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J Altern Complement Med. 2004;10(2):261-268.
26. Tooley GA, Armstrong SM, Norman TR, Sali A. Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol. 2000;53(1):69-78.
27. Walton KG, Pugh ND, Gelderloos P, Macrae P. Stress reduction and preventing hypertension: preliminary support of a psychoneuroendocrine mechanism. J Altern Complement Med. 1995;1(3):263-283.
28. Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23(3):135-142.
29. Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8(1):37-46.
30. Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23(3):199-208.
31. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1-100.
32. Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: a review. Obes Rev. 2004;5(2):115-121.
33. Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC. Novel antipsychotics and new onset diabetes. Biol Psychiatry. 1998;44(8):778-783.
34. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
35. Quiroga Murcia C, Kreutz G, Clift S, Bongard S. Shall we dance? An exploration of the perceived benefits of dancing on well-being. Arts Health. 2010;2(2):149-163.
36. The Duke Center for Integrative Medicine; Liebowitz R, Smith L. The Duke Encyclopedia of New Medicine: Conventional and Alternative Medicine for All Ages. London, UK: Rodale Books International; 2006.
37. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
38. Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319-1323.
39. Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12.
40. Sidhu KS, Vandana P, Balon R. Exercise prescription: a practical, effective therapy for depression. Curr Psychiatr. 2009;8(6):38-51.
There is growing interest in developing a holistic and integrative approach for the treatment of severe mental illnesses (SMI), such as schizophrenia, major depression, posttraumatic stress disorder (PTSD), and anxiety disorders. Western medicine has traditionally focused on the direct treatment of symptoms and separated the management of physical and mental health, but increasing attention is being given to complementary and alternative medicine (CAM) for patients with SMI.
Recognizing the connectedness of the mind and body, these complementary or alternative approaches incorporate nontraditional therapeutic techniques with mainstream treatment methods, including psychopharmacology and psychotherapy.1 Patients with SMI may particularly benefit from a mind-body therapeutic approach, because they often experience psychological symptoms such as stress, anxiety, depression and psychosis, as well as a preponderance of medical comorbidities, including obesity, diabetes mellitus, and cardiovascular disease, some of which are compounded by adverse effects (AEs) of essential pharmacologic treatments.2-4 Mind-body interventions might also be particularly advantageous for veterans, who often experience a range of interconnected physical and psychological difficulties due to trauma exposure and challenges transitioning from military to civilian life.5
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
In 2002, the White House Commission on Complementary and Alternative Medicine Policy issued a report supporting CAM research and integration into existing medical systems.6 The DoD later established Total Force Fitness, a holistic health care program for active-duty military personnel.7 The VA has also incorporated mind-body and holistic strategies into veteran care.8 One such mind-body intervention, yoga, is becoming increasingly popular within the health care field.
Recent research has documented the effectiveness of yoga, underscoring its utility as a mind-body therapeutic approach. Yoga is associated with improvement in balance and flexibility,9 fatigue,10 blood pressure,11 sleep,12 strength,13 and pain14 in both healthy individuals and patients with medical and psychiatric disorders.15 The literature also illustrates that yoga has led to significant improvements in stress and psychiatric symptoms in individuals with PTSD, schizophrenia, depression, and anxiety.16-22 A previous meta-analysis conducted by the authors, which considered studies of the effectiveness of yoga as an adjunctive treatment for patients with mental illness, found that 212 studies with null results would need to be located and incorporated to negate the positive effects of yoga found in the literature.17
Because yoga emphasizes the practice of mindfulness and timing movement with breath awareness, it is a calming practice that may decrease stress and relieve psychiatric symptoms not treated through psychopharmacology and psychotherapy.17,21 Recent research has postulated that the physiological mechanisms by which this occurs may include (a) reduction in sympathetic and increase in parasympathetic activity23,24; (b) increases in heart-rate variability and respiratory sinus arrhythmia, low levels of which are associated with anxiety, panic disorder, and depression23,24; (c) increases in melatonin and serotonin 25-27; and (d) decrease in cortisol.28,29
Related: Enhancing Patient Satisfaction Through the Use of Complementary Therapies
As yoga may calm the autonomic nervous system and reduce stress, it may benefit patients with SMI, whose symptoms are often aggravated by stress.30 In addition, veterans experience both acute stressors and high levels of chronic stress.5 Therefore, because they experience mind-body comorbid illnesses as well as high levels of stress, the authors believe that veterans with SMI could benefit greatly from a tailored yoga-based program as part of a holistic approach that includes necessary medication and evidence-based therapies.
In order to evaluate the effects of a yoga program on veterans receiving mental health treatment across the VA Greater Los Angeles Healthcare System (VAGLAHS), the authors developed a set of yoga-based wellness classes called Breathing, Stretching, Relaxation (BSR) classes. This article describes the process of developing these classes and outlines the procedures and results of a study to assess their effects.
BSR Classes
The development of BSR classes took place at the West Los Angeles VA Medical Center (WLAVAMC), within the Psychosocial Rehabilitation and Recovery Center (PRRC) program. The PRRC is a psychoeducational program that focuses on the biological, psychological, social, and spiritual aspects of life in order to help veterans with SMI rehabilitate and reintegrate into the community. The program allows veterans to create their own recovery curriculum by selecting from diverse classes led by program staff members, including physicians, psychologists, nurses, social workers, nutritionists, and recreational therapists.
Development of BSR Protocols
The primary goal of this project was to develop a yoga-based program tailored to the specific needs of veterans with SMI. To the authors’ knowledge, BSR is the first yoga-based program customized for SMI. The BSR classes were developed within interdisciplinary focus groups that included professional yoga teachers, the director of the PRRC, psychiatrists, psychologists, nurses, occupational therapists, and physical therapists. Drawing on their experience with SMI and yoga, members of the focus groups identified 3 aspects of yoga that would be most beneficial to veterans with SMI, and the program was designed to optimize these effects. Because SMI can both create and be exacerbated by stress, BSR classes were designed to reduce stress and provide veterans with the tools to monitor and manage their stress.
Breathing and meditative techniques were adapted from yoga in order to facilitate stress reduction. In addition, aerobic elements of yoga have the potential to help veterans manage their incidence of medical diseases, such as cardiovascular disease, obesity, and diabetes. Patients with SMI are at a greater risk for developing these diseases, so classes were designed to incorporate physical stretching elements to promote overall health.4,31-33 Finally BSR was designed to improve veteran self- efficacy and self-esteem, and to place veterans at the center of their care by equipping them with skills to practice BSR independently.
Related: Mindfulness to Reduce Stress
The focus groups also identified the logistic requirements when implementing a yoga-based program for veterans with SMI, including (a) obtaining participant or conservator consent; (b) obtaining medical clearance from care providers, given the high prevalence of medical comorbidities; (c) removing the traditional yoga terms, taking a secular approach, and naming the class “Breathing, Stretching, Relaxation” without directly referencing yoga; (d) asking veterans’ permission before incorporating physical contact into demonstrations, because veterans with SMI, especially those with PTSD, might be uncomfortable with touching from instructors; (e) creating protocols of varying duration and intensity so that BSR was approachable for veterans with diverse levels of physical ability; and (f) ensuring that a clinician who regularly works with SMI patients be present to supervise classes for the safety of patients and instructors.
Yoga instructors and clinicians collaborated to create adaptable 30- and 50-minute protocols that reflected best practices for an SMI population. The 30-minute seated BSR class protocol is included in eAppendix A. Once protocols were finalized, a Train the Trainer program was established to facilitate dissemination of BSR to clinicians working with veterans with SMI throughout the VAGLAHS.
Interested clinicians were given protocols and trained to lead BSR classes on their own. Subsequently, clinician-led BSR classes of various lengths (depending on clinician preference and program scheduling) were established at PRRCs and other mental health programs, such as Mental Health Recovery and Intensive Treatment and Dual Diagnosis Treatment Program, throughout the VAGLAHS. These programs were selected, because they are centered on recovery and improvements in symptoms of SMI. The adoption of a Train the Trainer model, through which VA clinicians were trained by professional yoga instructors, allowed for seamless integration of BSR into VA usual care for veterans with SMI.
Assessment of Classes
The authors conducted a study to assess the quality and effectiveness of BSR classes. This survey research was approved by the VAGLAHS institutional review board for human subjects. The authors hypothesized that there would be significant improvements in veterans’ stress, pain, well-being, and perception of the benefits of BSR over 8 weeks of participation in classes. Also hypothesized was that there would be greater benefits in veterans who participated in longer classes and who attended classes more frequently.
Methods
A total of 120 veterans completed surveys after participating in clinician- and yoga instructor-led BSR classes at the 3 sites within the VAGLAHS: WLAVAMC, Los Angeles Ambulatory Care Center (LAACC), and Sepulveda Ambulatory Care Center (SACC). At the WLAVAMC, surveys were collected at 10-, 30-, 60-, and 90-minute classes. At LAACC, surveys were collected at 30- and 60-minute classes. At SACC, surveys were collected at 20- and 45-minute classes. A researcher noted the duration of the class and was available to assist with comprehension. Veterans completed identical surveys after classes at a designated week 0 (baseline), week 4, and week 8. Of the 120 patients with an initial survey, 82 completed at least 1 follow-up survey and 49 completed both follow-up surveys.
Survey packets included (a) demographic questions, including age, gender, and ethnicity; (b) class participation questions, including frequency of class attendance, patients’ favorite aspect of class, and dura tion of class attendance (in months of prior participation); (c) a pain rating from 0 (no pain) to 10 (the worst pain imaginable); (d) the BioPsychoSocial-Spiritual (BPSS) Scale (eAppendix B), developed at the WLAVAMC, which provides wellness scores from 0 (low) to 10 (high) in 4 areas as well as a holistic wellness score from 0 (low) to 40 (high); (e) the Perceived Stress Scale (PSS), developed by Cohen and colleagues, which generates a stress score for the past month from 0 (low) to 40 (high)34; and (f) the Perceived Benefits of Yoga Questionnaire (PBYQ) (eAppendix C), which rates participants’ opinions about the benefits of yoga from 12 (low) to 60 (high) and is based on the Perceived Benefits of Dance Questionnaire.35
Statistical Analysis
Pearson’s r correlation coefficients were calculated between PBYQ scores and quantitative survey items at each time point (weeks 0, 4, and 8). Linear mixed-effects models were used to test for effects of multiple predictor variables on individual outcomes. Each model had a random intercept by participant, and regressors included main effects for the following: survey week (0, 4, or 8), class duration (in minutes), age, sex, ethnicity, frequency of attendance (in days per week), and duration of attendance (in months). For all statistical analyses, a 2-tailed significance criterion of α = .05 was used.
Results
Veterans who completed surveys were predominantly male (90.8%) and averaged 61.4 years of age. Table 1 shows demographic information. Table 2 displays the number of participants who were involved in short (< 30 min), medium (30-59 min), and long (> 60 min) classes. Veteran participants also had a wide range of prior BSR experience (Table 3).
At all time points, PBYQ scores were significantly positively correlated with class duration and biological, psychological, social, spiritual, and total well-being as measured by the BPSS. The PBYQ scores at all time points were also significantly negatively correlated with age, pain ratings, and PSS scores. Table 4 includes specific Pearson’s r values.
Survey week was not significantly associated with any individual outcome measures. There were no significant regressors for total PSS score or total BPSS score within the linear models. However, participants’ PBYQ scores were significantly associated with age (t(98) = -2.13, P = .036), frequency of attendance (t(103) = 2.10, P = .038), and class duration (t(98) = 4.35, P < .001). Additionally, class duration was significantly associated with pain (t(98) = -3.01, P = .003), with longer duration associated with less pain. Ethnicity was also associated with pain, with African American veterans reporting less pain than did white (t(98) = -2.41, P = .017) and Hispanic (t(98) = -2.31, P = .023) veterans. Because ethnicity was significantly associated with class duration (F(5,339) = 3.81, P = .002), the authors used an analysis of covariance to test for a mediating effect of ethnicity on the relationship between class duration and pain. Although there was a partial mediation (F(5,203) = 2.57, P = .028), the main effect of class duration remained significant.
Discussion and Limitations
The goals of this project were to develop a yoga-based program tailored for veterans with SMI and assess the program in a sample of veterans with SMI on subjective reports of stress, pain, well-being, and benefits of yoga. The authors hypothesized that significant improvements in these measures in veterans with SMI would be observed over 8 weeks of participation in BSR classes and that there would be greater benefits in veterans who participated in longer classes and who attended classes more frequently.
The authors succeeded in developing an adaptable yoga-based wellness program for veterans with SMI that can be both practiced in structured classes and incorporated into veterans’ everyday routines. The BSR classes were well tolerated by veterans with SMI, caused no discernible AEs, and are readily available for dissemination across other mental health programs. Veterans described integrating the tools they learned within BSR classes into their daily lives, helping them to manage pain; feel more flexible; reduce stress, anxiety, depression, and PTSD symptoms; and increase relaxation and feelings of self-control and confidence. Table 5 shows qualitative feedback collected from veterans. In addition, the Train the Trainer model optimized clinical applicability and flexibility, demonstrating that clinicians can seamlessly integrate BSR classes into a health care plan for veterans with SMI.
In assessing quantitative measures of stress, pain, well-being, and perceived benefits of a yoga-based program, veterans who reported BSR classes as beneficial experienced lower levels of pain and stress and higher levels of biological, psychological, social, spiritual, and overall well-being. Those veterans who perceived BSR as more beneficial tended to be younger and attend longer classes with greater frequency. Veterans who attended longer classes also reported experiencing less pain. This may be because the more rigorous stretching and posing involved in longer BSR classes made them more effective at reducing pain; however, it is also possible that veterans who were experiencing more pain avoided these longer classes due to their rigor and length.
Results suggest that longer classes attended with greater regularity may be more beneficial to veterans than short and infrequent classes, particularly in regards to their pain. Despite the relationships between class and outcome variables, the authors did not find significant improvements in measures of wellness, pain, stress, or perceived benefits of BSR over time, as was hypothesized. This may be because the data collection for this study began after classes had been established for some time. In fact, only 35 of 120 veterans included in this study reported having < 1 month of BSR experience at week 0, suggesting that results collected from week 0 did not represent a true baseline measurement. Although no relationship was found between prior duration of attendance and any outcome measures, the fact that most veterans in the sample had attended classes for several months prior to completing surveys may have biased the results by favoring the responses of veterans who were more invested in the classes. Improvements may have been better captured in a BSR-naïve sample.
The finding that the PBYQ score was significantly correlated with all other outcome measures (pain, BPSS score, and PSS score) raises some questions about the ways in which these classes were beneficial to veterans. It may be that veterans who experienced more positive outcomes from classes saw BSR as more beneficial, but it is also possible that veterans who entered classes with greater expectations experienced better outcomes due to a placebo effect—that is, outcomes may have been influenced more by the expectation than by the content of the classes. In the case of well-being (BPSS scores) and stress (PSS score), this could explain why these outcomes were significantly correlated with perceived benefits of BSR but were not significantly related to any class-related variables such as duration and frequency of attendance. Pain ratings, however, were related to class variables and perceived benefits of BSR.
In a post hoc analysis, the main effect of the PBYQ score as a regressor was added to the linear model for pain, resulting in the PBYQ score having a significant main effect (t(100) = -2.98, P = .004). The main effect of class duration remained significant (t(97) = -1.99, P = .050) but was less substantial when the PBYQ score was added, suggesting some correlation between class duration and pain, independent of BSR’s perceived benefits. Future research should consider the possible mediating effect of perceptions of the effectiveness of a yoga-based program a priori or control for placebo effects in order to address the degree to which outcomes are influenced by participant expectations.
This study had a few other notable limitations. Because it was an observational study administered within a clinical mental health program, a control group was not included. Measurement began after BSR classes were established, so veterans had varying levels of prior experience. Specific SMI and medical diagnosis information was not collected from individual veterans. Data were collected from classes of varying length and intensity. The BSR classes often took place within larger programs at the VA, which offered comprehensive care, so some effects of the BSR classes might have been confounded with concurrent evidenced-based treatments or other holistic care programs. Due to these limitations, particularly the absence of a control group, the relationships between BSR participation and health outcomes cannot be assumed to be causal, because a multitude of other variables, such as patient contact and expectancy effects, may have influenced outcomes.
Future Directions
Future research should aim to utilize a control group and collect data from classes of the same intensity and length to better examine whether BSR can be causally linked with improvements in measures of stress, pain, and well-being and to attempt to control for expectancy and contact effects. In addition, future research should aim to recruit a BSR-naïve sample to account for prior experience. Future studies should also aim to parse out whether BSR is differentially effective for each SMI or medical diagnosis, whether there is a relationship between class time and outcomes (as these results suggest that longer class times might be more beneficial for pain), and whether any pain-management benefits from BSR influence other measures of functionality and well-being. Finally, future research can further divide BSR into its active components, such as meditative breathing and aerobic stretching, in order to examine which aspect leads to the greatest effect on each measure; the current results imply that the more rigorous components of BSR classes have the greatest effects on pain.
Conclusion
A yoga-based class affects the mind and body, making it particularly useful for veterans with SMI who experience a range of physical and psychological symptoms and comorbidities.4 Other studies have demonstrated that when practiced alone, yoga leads to improvements in both physical and psychological symptoms.15,17 Looking forward, yoga-based classes may be implemented as part of a larger biopsychosocial-spiritual care plan that is being embraced both within and outside of the VA.7,8,36,37 This integrative health care model suggests that psychosocial and CAM modalities are additive and should be practiced concurrently.37
Although little research has assessed the effects of comprehensive psychosocial and CAM treatment programs, initial research indicates that these programs are associated with reductions in symptoms of medical and mental illness.37-40 Participants in BSR classes may derive further benefits if classes are incorporated into a larger holistic health care plan that includes both traditional psychiatric treatment modalities and CAM therapies that integrate biopsychosocial-spiritual components.
Acknowledgements
The authors would like to thank Nancy Mohler, Anne Platt, and Matthew Crowder, all professional yoga instructors, for their integral role in developing classes, providing classes to veterans, and assisting VA staff with training. The authors also acknowledge Rosie Dominguez, LCSW, for expertly leading BSR classes at LAACC. The authors also thank Irina Arnold, MS, MD, and Vanessa Streiff, MA, for their vital assistance with data collection at SACC.
Funding for the development of BSR protocols, quality improvement research, and expenses associated with classes was provided by the Disabled American Veterans Charitable Service Trust. Support for dissemination of classes to multiple VA programs was provided by a grant from the VA Center of Innovation for Patient Centered Care, headed by Sandra Robertson, RN, MSN, PH-CNS.
The authors are very grateful to the veterans and staff of the Mental Health Intensive Case Management, Psychosocial Rehabilitation and Recovery Centers, Mental Health Clinics, and Domiciliary throughout Greater Los Angeles for their support of the BSR classes.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
There is growing interest in developing a holistic and integrative approach for the treatment of severe mental illnesses (SMI), such as schizophrenia, major depression, posttraumatic stress disorder (PTSD), and anxiety disorders. Western medicine has traditionally focused on the direct treatment of symptoms and separated the management of physical and mental health, but increasing attention is being given to complementary and alternative medicine (CAM) for patients with SMI.
Recognizing the connectedness of the mind and body, these complementary or alternative approaches incorporate nontraditional therapeutic techniques with mainstream treatment methods, including psychopharmacology and psychotherapy.1 Patients with SMI may particularly benefit from a mind-body therapeutic approach, because they often experience psychological symptoms such as stress, anxiety, depression and psychosis, as well as a preponderance of medical comorbidities, including obesity, diabetes mellitus, and cardiovascular disease, some of which are compounded by adverse effects (AEs) of essential pharmacologic treatments.2-4 Mind-body interventions might also be particularly advantageous for veterans, who often experience a range of interconnected physical and psychological difficulties due to trauma exposure and challenges transitioning from military to civilian life.5
Related: Complementary and Alternative Medicine for Chronic Musculoskeletal Pain
In 2002, the White House Commission on Complementary and Alternative Medicine Policy issued a report supporting CAM research and integration into existing medical systems.6 The DoD later established Total Force Fitness, a holistic health care program for active-duty military personnel.7 The VA has also incorporated mind-body and holistic strategies into veteran care.8 One such mind-body intervention, yoga, is becoming increasingly popular within the health care field.
Recent research has documented the effectiveness of yoga, underscoring its utility as a mind-body therapeutic approach. Yoga is associated with improvement in balance and flexibility,9 fatigue,10 blood pressure,11 sleep,12 strength,13 and pain14 in both healthy individuals and patients with medical and psychiatric disorders.15 The literature also illustrates that yoga has led to significant improvements in stress and psychiatric symptoms in individuals with PTSD, schizophrenia, depression, and anxiety.16-22 A previous meta-analysis conducted by the authors, which considered studies of the effectiveness of yoga as an adjunctive treatment for patients with mental illness, found that 212 studies with null results would need to be located and incorporated to negate the positive effects of yoga found in the literature.17
Because yoga emphasizes the practice of mindfulness and timing movement with breath awareness, it is a calming practice that may decrease stress and relieve psychiatric symptoms not treated through psychopharmacology and psychotherapy.17,21 Recent research has postulated that the physiological mechanisms by which this occurs may include (a) reduction in sympathetic and increase in parasympathetic activity23,24; (b) increases in heart-rate variability and respiratory sinus arrhythmia, low levels of which are associated with anxiety, panic disorder, and depression23,24; (c) increases in melatonin and serotonin 25-27; and (d) decrease in cortisol.28,29
Related: Enhancing Patient Satisfaction Through the Use of Complementary Therapies
As yoga may calm the autonomic nervous system and reduce stress, it may benefit patients with SMI, whose symptoms are often aggravated by stress.30 In addition, veterans experience both acute stressors and high levels of chronic stress.5 Therefore, because they experience mind-body comorbid illnesses as well as high levels of stress, the authors believe that veterans with SMI could benefit greatly from a tailored yoga-based program as part of a holistic approach that includes necessary medication and evidence-based therapies.
In order to evaluate the effects of a yoga program on veterans receiving mental health treatment across the VA Greater Los Angeles Healthcare System (VAGLAHS), the authors developed a set of yoga-based wellness classes called Breathing, Stretching, Relaxation (BSR) classes. This article describes the process of developing these classes and outlines the procedures and results of a study to assess their effects.
BSR Classes
The development of BSR classes took place at the West Los Angeles VA Medical Center (WLAVAMC), within the Psychosocial Rehabilitation and Recovery Center (PRRC) program. The PRRC is a psychoeducational program that focuses on the biological, psychological, social, and spiritual aspects of life in order to help veterans with SMI rehabilitate and reintegrate into the community. The program allows veterans to create their own recovery curriculum by selecting from diverse classes led by program staff members, including physicians, psychologists, nurses, social workers, nutritionists, and recreational therapists.
Development of BSR Protocols
The primary goal of this project was to develop a yoga-based program tailored to the specific needs of veterans with SMI. To the authors’ knowledge, BSR is the first yoga-based program customized for SMI. The BSR classes were developed within interdisciplinary focus groups that included professional yoga teachers, the director of the PRRC, psychiatrists, psychologists, nurses, occupational therapists, and physical therapists. Drawing on their experience with SMI and yoga, members of the focus groups identified 3 aspects of yoga that would be most beneficial to veterans with SMI, and the program was designed to optimize these effects. Because SMI can both create and be exacerbated by stress, BSR classes were designed to reduce stress and provide veterans with the tools to monitor and manage their stress.
Breathing and meditative techniques were adapted from yoga in order to facilitate stress reduction. In addition, aerobic elements of yoga have the potential to help veterans manage their incidence of medical diseases, such as cardiovascular disease, obesity, and diabetes. Patients with SMI are at a greater risk for developing these diseases, so classes were designed to incorporate physical stretching elements to promote overall health.4,31-33 Finally BSR was designed to improve veteran self- efficacy and self-esteem, and to place veterans at the center of their care by equipping them with skills to practice BSR independently.
Related: Mindfulness to Reduce Stress
The focus groups also identified the logistic requirements when implementing a yoga-based program for veterans with SMI, including (a) obtaining participant or conservator consent; (b) obtaining medical clearance from care providers, given the high prevalence of medical comorbidities; (c) removing the traditional yoga terms, taking a secular approach, and naming the class “Breathing, Stretching, Relaxation” without directly referencing yoga; (d) asking veterans’ permission before incorporating physical contact into demonstrations, because veterans with SMI, especially those with PTSD, might be uncomfortable with touching from instructors; (e) creating protocols of varying duration and intensity so that BSR was approachable for veterans with diverse levels of physical ability; and (f) ensuring that a clinician who regularly works with SMI patients be present to supervise classes for the safety of patients and instructors.
Yoga instructors and clinicians collaborated to create adaptable 30- and 50-minute protocols that reflected best practices for an SMI population. The 30-minute seated BSR class protocol is included in eAppendix A. Once protocols were finalized, a Train the Trainer program was established to facilitate dissemination of BSR to clinicians working with veterans with SMI throughout the VAGLAHS.
Interested clinicians were given protocols and trained to lead BSR classes on their own. Subsequently, clinician-led BSR classes of various lengths (depending on clinician preference and program scheduling) were established at PRRCs and other mental health programs, such as Mental Health Recovery and Intensive Treatment and Dual Diagnosis Treatment Program, throughout the VAGLAHS. These programs were selected, because they are centered on recovery and improvements in symptoms of SMI. The adoption of a Train the Trainer model, through which VA clinicians were trained by professional yoga instructors, allowed for seamless integration of BSR into VA usual care for veterans with SMI.
Assessment of Classes
The authors conducted a study to assess the quality and effectiveness of BSR classes. This survey research was approved by the VAGLAHS institutional review board for human subjects. The authors hypothesized that there would be significant improvements in veterans’ stress, pain, well-being, and perception of the benefits of BSR over 8 weeks of participation in classes. Also hypothesized was that there would be greater benefits in veterans who participated in longer classes and who attended classes more frequently.
Methods
A total of 120 veterans completed surveys after participating in clinician- and yoga instructor-led BSR classes at the 3 sites within the VAGLAHS: WLAVAMC, Los Angeles Ambulatory Care Center (LAACC), and Sepulveda Ambulatory Care Center (SACC). At the WLAVAMC, surveys were collected at 10-, 30-, 60-, and 90-minute classes. At LAACC, surveys were collected at 30- and 60-minute classes. At SACC, surveys were collected at 20- and 45-minute classes. A researcher noted the duration of the class and was available to assist with comprehension. Veterans completed identical surveys after classes at a designated week 0 (baseline), week 4, and week 8. Of the 120 patients with an initial survey, 82 completed at least 1 follow-up survey and 49 completed both follow-up surveys.
Survey packets included (a) demographic questions, including age, gender, and ethnicity; (b) class participation questions, including frequency of class attendance, patients’ favorite aspect of class, and dura tion of class attendance (in months of prior participation); (c) a pain rating from 0 (no pain) to 10 (the worst pain imaginable); (d) the BioPsychoSocial-Spiritual (BPSS) Scale (eAppendix B), developed at the WLAVAMC, which provides wellness scores from 0 (low) to 10 (high) in 4 areas as well as a holistic wellness score from 0 (low) to 40 (high); (e) the Perceived Stress Scale (PSS), developed by Cohen and colleagues, which generates a stress score for the past month from 0 (low) to 40 (high)34; and (f) the Perceived Benefits of Yoga Questionnaire (PBYQ) (eAppendix C), which rates participants’ opinions about the benefits of yoga from 12 (low) to 60 (high) and is based on the Perceived Benefits of Dance Questionnaire.35
Statistical Analysis
Pearson’s r correlation coefficients were calculated between PBYQ scores and quantitative survey items at each time point (weeks 0, 4, and 8). Linear mixed-effects models were used to test for effects of multiple predictor variables on individual outcomes. Each model had a random intercept by participant, and regressors included main effects for the following: survey week (0, 4, or 8), class duration (in minutes), age, sex, ethnicity, frequency of attendance (in days per week), and duration of attendance (in months). For all statistical analyses, a 2-tailed significance criterion of α = .05 was used.
Results
Veterans who completed surveys were predominantly male (90.8%) and averaged 61.4 years of age. Table 1 shows demographic information. Table 2 displays the number of participants who were involved in short (< 30 min), medium (30-59 min), and long (> 60 min) classes. Veteran participants also had a wide range of prior BSR experience (Table 3).
At all time points, PBYQ scores were significantly positively correlated with class duration and biological, psychological, social, spiritual, and total well-being as measured by the BPSS. The PBYQ scores at all time points were also significantly negatively correlated with age, pain ratings, and PSS scores. Table 4 includes specific Pearson’s r values.
Survey week was not significantly associated with any individual outcome measures. There were no significant regressors for total PSS score or total BPSS score within the linear models. However, participants’ PBYQ scores were significantly associated with age (t(98) = -2.13, P = .036), frequency of attendance (t(103) = 2.10, P = .038), and class duration (t(98) = 4.35, P < .001). Additionally, class duration was significantly associated with pain (t(98) = -3.01, P = .003), with longer duration associated with less pain. Ethnicity was also associated with pain, with African American veterans reporting less pain than did white (t(98) = -2.41, P = .017) and Hispanic (t(98) = -2.31, P = .023) veterans. Because ethnicity was significantly associated with class duration (F(5,339) = 3.81, P = .002), the authors used an analysis of covariance to test for a mediating effect of ethnicity on the relationship between class duration and pain. Although there was a partial mediation (F(5,203) = 2.57, P = .028), the main effect of class duration remained significant.
Discussion and Limitations
The goals of this project were to develop a yoga-based program tailored for veterans with SMI and assess the program in a sample of veterans with SMI on subjective reports of stress, pain, well-being, and benefits of yoga. The authors hypothesized that significant improvements in these measures in veterans with SMI would be observed over 8 weeks of participation in BSR classes and that there would be greater benefits in veterans who participated in longer classes and who attended classes more frequently.
The authors succeeded in developing an adaptable yoga-based wellness program for veterans with SMI that can be both practiced in structured classes and incorporated into veterans’ everyday routines. The BSR classes were well tolerated by veterans with SMI, caused no discernible AEs, and are readily available for dissemination across other mental health programs. Veterans described integrating the tools they learned within BSR classes into their daily lives, helping them to manage pain; feel more flexible; reduce stress, anxiety, depression, and PTSD symptoms; and increase relaxation and feelings of self-control and confidence. Table 5 shows qualitative feedback collected from veterans. In addition, the Train the Trainer model optimized clinical applicability and flexibility, demonstrating that clinicians can seamlessly integrate BSR classes into a health care plan for veterans with SMI.
In assessing quantitative measures of stress, pain, well-being, and perceived benefits of a yoga-based program, veterans who reported BSR classes as beneficial experienced lower levels of pain and stress and higher levels of biological, psychological, social, spiritual, and overall well-being. Those veterans who perceived BSR as more beneficial tended to be younger and attend longer classes with greater frequency. Veterans who attended longer classes also reported experiencing less pain. This may be because the more rigorous stretching and posing involved in longer BSR classes made them more effective at reducing pain; however, it is also possible that veterans who were experiencing more pain avoided these longer classes due to their rigor and length.
Results suggest that longer classes attended with greater regularity may be more beneficial to veterans than short and infrequent classes, particularly in regards to their pain. Despite the relationships between class and outcome variables, the authors did not find significant improvements in measures of wellness, pain, stress, or perceived benefits of BSR over time, as was hypothesized. This may be because the data collection for this study began after classes had been established for some time. In fact, only 35 of 120 veterans included in this study reported having < 1 month of BSR experience at week 0, suggesting that results collected from week 0 did not represent a true baseline measurement. Although no relationship was found between prior duration of attendance and any outcome measures, the fact that most veterans in the sample had attended classes for several months prior to completing surveys may have biased the results by favoring the responses of veterans who were more invested in the classes. Improvements may have been better captured in a BSR-naïve sample.
The finding that the PBYQ score was significantly correlated with all other outcome measures (pain, BPSS score, and PSS score) raises some questions about the ways in which these classes were beneficial to veterans. It may be that veterans who experienced more positive outcomes from classes saw BSR as more beneficial, but it is also possible that veterans who entered classes with greater expectations experienced better outcomes due to a placebo effect—that is, outcomes may have been influenced more by the expectation than by the content of the classes. In the case of well-being (BPSS scores) and stress (PSS score), this could explain why these outcomes were significantly correlated with perceived benefits of BSR but were not significantly related to any class-related variables such as duration and frequency of attendance. Pain ratings, however, were related to class variables and perceived benefits of BSR.
In a post hoc analysis, the main effect of the PBYQ score as a regressor was added to the linear model for pain, resulting in the PBYQ score having a significant main effect (t(100) = -2.98, P = .004). The main effect of class duration remained significant (t(97) = -1.99, P = .050) but was less substantial when the PBYQ score was added, suggesting some correlation between class duration and pain, independent of BSR’s perceived benefits. Future research should consider the possible mediating effect of perceptions of the effectiveness of a yoga-based program a priori or control for placebo effects in order to address the degree to which outcomes are influenced by participant expectations.
This study had a few other notable limitations. Because it was an observational study administered within a clinical mental health program, a control group was not included. Measurement began after BSR classes were established, so veterans had varying levels of prior experience. Specific SMI and medical diagnosis information was not collected from individual veterans. Data were collected from classes of varying length and intensity. The BSR classes often took place within larger programs at the VA, which offered comprehensive care, so some effects of the BSR classes might have been confounded with concurrent evidenced-based treatments or other holistic care programs. Due to these limitations, particularly the absence of a control group, the relationships between BSR participation and health outcomes cannot be assumed to be causal, because a multitude of other variables, such as patient contact and expectancy effects, may have influenced outcomes.
Future Directions
Future research should aim to utilize a control group and collect data from classes of the same intensity and length to better examine whether BSR can be causally linked with improvements in measures of stress, pain, and well-being and to attempt to control for expectancy and contact effects. In addition, future research should aim to recruit a BSR-naïve sample to account for prior experience. Future studies should also aim to parse out whether BSR is differentially effective for each SMI or medical diagnosis, whether there is a relationship between class time and outcomes (as these results suggest that longer class times might be more beneficial for pain), and whether any pain-management benefits from BSR influence other measures of functionality and well-being. Finally, future research can further divide BSR into its active components, such as meditative breathing and aerobic stretching, in order to examine which aspect leads to the greatest effect on each measure; the current results imply that the more rigorous components of BSR classes have the greatest effects on pain.
Conclusion
A yoga-based class affects the mind and body, making it particularly useful for veterans with SMI who experience a range of physical and psychological symptoms and comorbidities.4 Other studies have demonstrated that when practiced alone, yoga leads to improvements in both physical and psychological symptoms.15,17 Looking forward, yoga-based classes may be implemented as part of a larger biopsychosocial-spiritual care plan that is being embraced both within and outside of the VA.7,8,36,37 This integrative health care model suggests that psychosocial and CAM modalities are additive and should be practiced concurrently.37
Although little research has assessed the effects of comprehensive psychosocial and CAM treatment programs, initial research indicates that these programs are associated with reductions in symptoms of medical and mental illness.37-40 Participants in BSR classes may derive further benefits if classes are incorporated into a larger holistic health care plan that includes both traditional psychiatric treatment modalities and CAM therapies that integrate biopsychosocial-spiritual components.
Acknowledgements
The authors would like to thank Nancy Mohler, Anne Platt, and Matthew Crowder, all professional yoga instructors, for their integral role in developing classes, providing classes to veterans, and assisting VA staff with training. The authors also acknowledge Rosie Dominguez, LCSW, for expertly leading BSR classes at LAACC. The authors also thank Irina Arnold, MS, MD, and Vanessa Streiff, MA, for their vital assistance with data collection at SACC.
Funding for the development of BSR protocols, quality improvement research, and expenses associated with classes was provided by the Disabled American Veterans Charitable Service Trust. Support for dissemination of classes to multiple VA programs was provided by a grant from the VA Center of Innovation for Patient Centered Care, headed by Sandra Robertson, RN, MSN, PH-CNS.
The authors are very grateful to the veterans and staff of the Mental Health Intensive Case Management, Psychosocial Rehabilitation and Recovery Centers, Mental Health Clinics, and Domiciliary throughout Greater Los Angeles for their support of the BSR classes.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what's in a name? U.S. Department of Health and Human Services, National Institutes of Health Website. http://nccam.nih.gov/health/whatiscam. Updated March 2015. Accessed August 27, 2015.
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69(4):514-519.
4. Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63(10):856-865.
5. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
6. White House Commission on Complementary and Alternative Medicine Policy. Final Report. Washington, DC: White House Commission on Complementary and Alternative Medicine Policy; 2002.
7. Land BC. Current Department of Defense guidance for total force fitness. Mil Med. 2010;175(suppl 8):3-5.
8. U.S. Department of Veterans Affairs, Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: U.S. Department of Veterans Affairs, Veterans Health Administration; 2009.
9. Oken BS, Zajdel D, Kishiyama S, et al. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006;12(1):40-47.
10. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766-3775.
11. Cade WT, Reeds DN, Mondy KE, et al. Yoga lifestyle intervention reduces blood pressure in HIV-infected adults with cardiovascular disease risk factors. HIV Med. 2010;11(6):379-388.
12. Innes KE, Selfe TK. The effects of a gentle yoga program on sleep, mood, and blood pressure in older women with restless leg syndrome (RLS): a preliminary randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:294058.
13. Van Puymbroeck M, Payne LL, Hsieh PC. A phase I feasibility study of yoga on the physical health and coping of informal caregivers. Evid Based Complement Alternat Med. 2007;4(4):519-529.
14. Rani K, Tiwari SC, Singh U, Agrawal GG, Srivastava N. Six-month trial of Yoga Nidra in menstrual disorder patients: effects on somatoform symptoms. Ind Psychiatry J. 2011;20(2):97-102.
15. Ross A, Thomas S. The health benefits of yoga and exercise: a review of comparison studies. J Altern Complement Med. 2010;16(1):3-12.
16. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6(3):242-250.
17. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4):doi:10.4088/PCC.10r01068.
18. Katzman MA, Vermani M, Gerbarg PL, et al. A multicomponent yoga-based, breath intervention program as an adjunctive treatment in patients suffering from generalized anxiety disorder with or without comorbidities. Int J Yoga. 2012;5(1):57-65.
19. Köhn M, Persson Lundholm U, Bryngelsson IL, Anderzén-Carlsson A, Westerdahl E. Medical yoga for patients with stress-related symptoms and diagnoses in primary health care: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:215348.
20. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs. 2007;33(2):17-23.
21. Meyer HB, Katsman A, Sones AC, Auerbach DE, Ames D, Rubin RT. Yoga as an ancillary treatment for neurological and psychiatric disorders: a review. J Neuropsychiatry Clin Neurosci. 2012;24(2):152-164.
22. Visceglia E, Lewis S. Yoga therapy as an adjunctive treatment for schizophrenia: a randomized, controlled pilot study. J Altern Complement Med. 2011;17(7):601-607.
23. Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression, part I-neurophysiologic model. J Altern Complement Med. 2005;11(1):189-201.
24. Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Ann N Y Acad Sci. 2009;1172:54-62.
25. Harinath K, Malhotra AS, Pal K, et al. Effects of hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J Altern Complement Med. 2004;10(2):261-268.
26. Tooley GA, Armstrong SM, Norman TR, Sali A. Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol. 2000;53(1):69-78.
27. Walton KG, Pugh ND, Gelderloos P, Macrae P. Stress reduction and preventing hypertension: preliminary support of a psychoneuroendocrine mechanism. J Altern Complement Med. 1995;1(3):263-283.
28. Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23(3):135-142.
29. Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8(1):37-46.
30. Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23(3):199-208.
31. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1-100.
32. Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: a review. Obes Rev. 2004;5(2):115-121.
33. Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC. Novel antipsychotics and new onset diabetes. Biol Psychiatry. 1998;44(8):778-783.
34. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
35. Quiroga Murcia C, Kreutz G, Clift S, Bongard S. Shall we dance? An exploration of the perceived benefits of dancing on well-being. Arts Health. 2010;2(2):149-163.
36. The Duke Center for Integrative Medicine; Liebowitz R, Smith L. The Duke Encyclopedia of New Medicine: Conventional and Alternative Medicine for All Ages. London, UK: Rodale Books International; 2006.
37. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
38. Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319-1323.
39. Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12.
40. Sidhu KS, Vandana P, Balon R. Exercise prescription: a practical, effective therapy for depression. Curr Psychiatr. 2009;8(6):38-51.
1. National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what's in a name? U.S. Department of Health and Human Services, National Institutes of Health Website. http://nccam.nih.gov/health/whatiscam. Updated March 2015. Accessed August 27, 2015.
2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69(4):514-519.
4. Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63(10):856-865.
5. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
6. White House Commission on Complementary and Alternative Medicine Policy. Final Report. Washington, DC: White House Commission on Complementary and Alternative Medicine Policy; 2002.
7. Land BC. Current Department of Defense guidance for total force fitness. Mil Med. 2010;175(suppl 8):3-5.
8. U.S. Department of Veterans Affairs, Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: U.S. Department of Veterans Affairs, Veterans Health Administration; 2009.
9. Oken BS, Zajdel D, Kishiyama S, et al. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006;12(1):40-47.
10. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766-3775.
11. Cade WT, Reeds DN, Mondy KE, et al. Yoga lifestyle intervention reduces blood pressure in HIV-infected adults with cardiovascular disease risk factors. HIV Med. 2010;11(6):379-388.
12. Innes KE, Selfe TK. The effects of a gentle yoga program on sleep, mood, and blood pressure in older women with restless leg syndrome (RLS): a preliminary randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:294058.
13. Van Puymbroeck M, Payne LL, Hsieh PC. A phase I feasibility study of yoga on the physical health and coping of informal caregivers. Evid Based Complement Alternat Med. 2007;4(4):519-529.
14. Rani K, Tiwari SC, Singh U, Agrawal GG, Srivastava N. Six-month trial of Yoga Nidra in menstrual disorder patients: effects on somatoform symptoms. Ind Psychiatry J. 2011;20(2):97-102.
15. Ross A, Thomas S. The health benefits of yoga and exercise: a review of comparison studies. J Altern Complement Med. 2010;16(1):3-12.
16. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6(3):242-250.
17. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4):doi:10.4088/PCC.10r01068.
18. Katzman MA, Vermani M, Gerbarg PL, et al. A multicomponent yoga-based, breath intervention program as an adjunctive treatment in patients suffering from generalized anxiety disorder with or without comorbidities. Int J Yoga. 2012;5(1):57-65.
19. Köhn M, Persson Lundholm U, Bryngelsson IL, Anderzén-Carlsson A, Westerdahl E. Medical yoga for patients with stress-related symptoms and diagnoses in primary health care: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:215348.
20. Krishnamurthy MN, Telles S. Assessing depression following two ancient Indian interventions: effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs. 2007;33(2):17-23.
21. Meyer HB, Katsman A, Sones AC, Auerbach DE, Ames D, Rubin RT. Yoga as an ancillary treatment for neurological and psychiatric disorders: a review. J Neuropsychiatry Clin Neurosci. 2012;24(2):152-164.
22. Visceglia E, Lewis S. Yoga therapy as an adjunctive treatment for schizophrenia: a randomized, controlled pilot study. J Altern Complement Med. 2011;17(7):601-607.
23. Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression, part I-neurophysiologic model. J Altern Complement Med. 2005;11(1):189-201.
24. Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Ann N Y Acad Sci. 2009;1172:54-62.
25. Harinath K, Malhotra AS, Pal K, et al. Effects of hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J Altern Complement Med. 2004;10(2):261-268.
26. Tooley GA, Armstrong SM, Norman TR, Sali A. Acute increases in night-time plasma melatonin levels following a period of meditation. Biol Psychol. 2000;53(1):69-78.
27. Walton KG, Pugh ND, Gelderloos P, Macrae P. Stress reduction and preventing hypertension: preliminary support of a psychoneuroendocrine mechanism. J Altern Complement Med. 1995;1(3):263-283.
28. Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23(3):135-142.
29. Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8(1):37-46.
30. Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23(3):199-208.
31. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1-100.
32. Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: a review. Obes Rev. 2004;5(2):115-121.
33. Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC. Novel antipsychotics and new onset diabetes. Biol Psychiatry. 1998;44(8):778-783.
34. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
35. Quiroga Murcia C, Kreutz G, Clift S, Bongard S. Shall we dance? An exploration of the perceived benefits of dancing on well-being. Arts Health. 2010;2(2):149-163.
36. The Duke Center for Integrative Medicine; Liebowitz R, Smith L. The Duke Encyclopedia of New Medicine: Conventional and Alternative Medicine for All Ages. London, UK: Rodale Books International; 2006.
37. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592.
38. Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319-1323.
39. Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12.
40. Sidhu KS, Vandana P, Balon R. Exercise prescription: a practical, effective therapy for depression. Curr Psychiatr. 2009;8(6):38-51.
A Treatment Protocol for Patients With Diabetic Peripheral Neuropathy
The progressive symptoms of diabetic peripheral neuropathy (DPN) are some of the most frequent presentations of patients seeking care at the VHA. Patients with DPN often experience unmanageable pain in the lower extremities, loss of sensation in the feet, loss of balance, and an inability to perform daily functional activities.1 In addition, these patients are at significant risk for lower extremity ulceration and amputation.2 The symptoms and consequences of DPN are strongly linked to chronic use of pain medications as well as increased fall risk and injury.3 The high health care usage of veterans with these complex issues makes DPN a significant burden for the patient, the VHA, and society as a whole.
At the William Jennings Bryan Dorn VA Medical Center (WJBDVAMC) in Columbia, South Carolina, 10,763 veterans were identified to be at risk for limb loss in 2014 due to loss of protective sensation and 5,667 veterans diagnosed with DPN were treated in 2014.4 Although WJBDVAMC offers multiple clinics and programs to address the complex issues of diabetes and DPN, veterans oftentimes continue to experience uncontrolled pain, loss of protective sensation, and a decline in function even after diagnosis.
One area of improvement the authors identified in the WJBDVAMC Physical Medicine and Rehabilitation Services Department was the need for an effective, nonpharmacologic treatment for patients who experience DPN. As a result, the authors designed a pilot research study to determine whether or not a combined physical therapy intervention of monochromatic near-infrared energy (MIRE) treatments and a standardized balance exercise program would help improve the protective sensation, reduce fall risk, and decrease the adverse impact of pain on daily function. The study was approved by the institutional review board (IRB) and had no outside source of funding.
Background
Current treatments for DPN are primarily pharmacologic and are viewed as only moderately effective, limited by significant adverse effects (AEs) and drug interactions.5 Patients in the VHA at risk for amputation in low-, moderate-, and high-risk groups total 541,475 and 363,468 have a history of neuropathy. They are considered at risk due to multiple, documented factors, including weakness, callus, foot deformity, loss of protective sensation, and/or history of amputation.4 Neuropathy can affect tissues throughout the body, including organs, sensory neurons, cardiovascular status, the autonomic system, and the gastrointestinal tract as it progresses.
Individuals who develop DPN often experience severe, uncontrolled pain in the lower extremities, insensate feet, and decreased proprioceptive skills. The functional status of individuals with DPN often declines insidiously while mortality rate increases.6 Increased levels of neuropathic pain often lead to decreased activity levels, which, in turn, contribute to decreased endurance, poorly managed glycemic indexes, decreased strength, and decreased independence.
Additional DPN complications, such as decreased sensation and muscle atrophy in the lower extremities, often lead to foot deformity and increased areas of pressure during weight bearing postures. These areas of increased pressure may develop unknowingly into ulceration. If a patient’s wound becomes chronic and nonhealing, it can also lead to amputation. In such cases, early mortality may result.6,7 The cascading effects of neuropathic pain and decreased sensation place a patient with diabetes at risk for falls. Injuries from falls are widely known to be a leading cause of hospitalization and mortality in the elderly.8
Physical therapy may be prescribed for DPN and its resulting sequelae. Several studies present conflicting results regarding the benefits of therapeutic exercise in the treatment of DPN. Akbari and colleagues showed that balance exercises can increase stability in patients with DPN; whereas, a study by Kruse and colleagues noted a training program consisting of lower-extremity exercises, balance training, and walking resulted in minimal improvement of participants’ balance and leg strength over a 12-month period.9,10 Recent studies have shown that weight bearing does not increase ulceration in patients with diabetes and DPN. This is contrary to previous assumptions that patients with diabetes and DPN need to avoid weight-bearing activities.11,12
Transcutaneous electrical nerve stimulation (TENS), a modality often used in physical therapy, has been studied in the treatment of DPN with conflicting results. Gossrau and colleagues found that pain reduction with micro-TENS applied peripherally is not superior to a placebo.13 However, a case study by Somers and Somers indicated that TENS applied to the lumbar area seemed to reduce pain and insomnia associated with diabetic neuropathy.14
Several recent research studies suggest that MIRE, another available modality, may be effective in treating symptoms of DPN. Monochromatic infrared energy therapy is a noninvasive, drug-free, FDA-approved medical device that emits monochromatic near-infrared light to improve local circulation and decrease pain. A large study of 2,239 patients with DPN reported an increase in foot sensation and decreased neuropathic pain levels when treated with MIRE.15
Leonard and colleagues found that the MIRE treatments resulted in a significant increase in sensation in individuals with baseline sensation of 6.65 Semmes-Weinstein Monofilament (SWM) after 6 and 12 active treatments as well as a decrease in neuropathic symptoms as measured by the Michigan Neuropathy Screening Instrument.16 Prendergast and colleagues noted improved electrophysical changes in both large and small myelinated nerve fibers of patients with DPN following 10 MIRE treatments.17 When studying 49 patients with DPN, Kochman and colleagues found 100% of participants had improved sensation after 12 MIRE treatments when tested with monofilaments.18
An additional benefit of MIRE treatment is that it can be safely performed at home once the patient is educated on proper use and application. Home DPN treatment has the potential to decrease the burden this population places on health care systems by reducing provider visits, medication, hospitalization secondary to pain, ulceration, fall injuries, and amputations.
Methods
This was a prospective, case series pilot study designed to measure changes in patient pain levels using the visual analog scale (VAS) and Pain Outcomes Questionnaire-VA (POQ-VA), degree of protective sensation loss as measured by SWM, and fall risk as denoted by Tinetti scores from entry to 6 months. Informed consent was obtained prior to treatment, and 33 patients referred by primary care providers and specialty clinics met the criteria and enrolled in the study. Twenty-one patients completed the entire 6-month study. The nonparametric Friedman test with a Dunn’s multiple comparison (DMC) post hoc test was used to analyze the data from the initial, 4-week, 3-month, and 6-month follow-up visits.
Setting and Participants
The study was performed in the Outpatient Physical Therapy Department at WJBDVAMC. Veterans with DPN who met the inclusion/exclusion criteria were enrolled. Inclusion criteria specified that the participant must be referred by a qualified health care provider for the treatment of DPN, be able to read and write in English, have consistent transportation to and from the study location, and be able to apply MIRE therapy as directed at home.
Exclusion criteria were subjects for whom MIRE or exercise were contraindicated. Subjects were excluded if they had medical conditions that suggested a possible decline in health status in the next 6 months. Such conditions included a current regimen of chemotherapy, radiation therapy, or dialysis; recent lower extremity amputation without prosthesis; documented active alcohol and/or drug misuse; advanced chronic obstructive pulmonary disease as defined as dyspnea at rest at least once per day; unstable angina; hemiplegia or other lower extremity paralysis; and a history of central nervous system or peripheral nervous system demyelinating disorders. Additional exclusion criteria included hospitalization in the past 60 days, use of any apparatus for continuous or patient-controlled analgesia; history of chronic low back pain with documented radiculopathy; and any change in pertinent medications in the past 60 days, including pain medications, insulin, metformin, and anti-inflammatories.
Interventions
Subjects participated in a combined physical therapy approach using MIRE and a standardized balance program. Patients received treatment in the outpatient clinic 3 times each week for 4 weeks. The treatment then continued at the same frequency at home until the scheduled 6-month follow-up visit. Clinic and home treatments included application of MIRE to bilateral lower extremities and feet for 30 minutes each as well as performance of a therapeutic exercise program for balance.
In the clinic, 2 pads from the MIRE device (Anodyne Therapy, LLC, Tampa, FL) were placed along the medial and lateral aspect of each lower leg, and an additional 2 pads were placed in a T formation on the plantar surface of each foot, per the manufacturer’s recommendations. The T formation consisted of the first pad placed horizontally across the metatarsal heads and the second placed vertically down the length of the foot. Each pad was protected by plastic wrap to ensure proper hygiene and secured. The intensity of clinic treatments was set at 7 bars, which minimized the risk of burns. Home treatments were similar to those in the clinic, except that each leg had to be treated individually instead of simultaneously and home MIRE units are preset and only function at an intensity that is equivalent to around 7 bars on the clinical unit.
The standardized balance program consisted of a progression of the following exercises: ankle alphabet/ankle range of motion, standing lateral weight shifts, bilateral heel raises, bilateral toe raises, unilateral heel raises, unilateral toe raises, partial wall squats, and single leg stance. Each participant performed these exercises 3 times per week in the clinic and then 3 times per week at home following the 12th visit.
Outcomes and Follow-up
The POQ-VA, a subjective quality of life (QOL) measure for veterans, as well as VAS, SWM testing, and the Tinetti Gait and Balance Assessment scores were used to measure outcomes. Data were collected for each of these measures during the initial and 12th clinic visits and at the 3-month and 6-month follow-up visits. The POQ-VA and VAS scores were self-reported and filled out by each participant at the initial, 12th, 3-month, and 6-month visits. The POQ-VA score has proven to be reliable and valid for the assessment of noncancer, chronic pain in veterans.19 The VAS scores were measured using a scale of 0 to 10 cm.
The SWM was standardized, and 7 sites were tested on each foot during the initial, 12th, 3-month, and 6-month visits: plantar surface of the distal great toe, the distal 3rd toe, the distal 5th toe, the 1st metatarsal head, the 3rd metatarsal head, the 5th metatarsal head, and the mid-plantar arch. At each site, the SWM was applied with just enough force to initiate a bending force and held for 1.5 seconds. Each site was tested 3 times. Participants had to detect the monofilament at least twice for the monofilament value to be recorded. Monofilament testing began with 6.65 SWM and decreased to 5.07, 4.56, 4.32, and lower until the patient was no longer able to detect sensation.
The Tinetti Gait and Balance Assessments was performed on each participant at the initial, 12th, 3-month, and 6-month visits. Tinetti balance, gait, and total scores were recorded at each interval.
Results
Thirty-three patients, referred by primary care providers and specialty clinics, met the inclusion criteria and enrolled in the study. Twenty-one patients (20 men and 1 woman) completed the entire 6-month study. Causes for withdrawal included travel difficulties (5), did not show up for follow-up visits (4), lumbar radiculopathy (1), perceived minimal/no benefit (1), and unrelated death (1). No AEs were reported.
The Friedman test with DMC post hoc test was performed on the POQ-VA total score and subscale scores. The POQ-VA subscale scores were divided into the following domains: pain, activities of daily living (ADL), fear, negative affect, mobility, and vitality. The POQ-VA domains were analyzed to compare data from the initial, 12th, 3-month, and 6-month visits. The POQ-VA total score significantly decreased from the initial to the 12th visit (P < .01), from the initial to the 3-month (P < .01), and from the initial to the 6-month visit (P < .05). However, there was no significant change from the 12th visit to the 3-month follow-up, 12th visit to the 6-month follow-up, or the 3-month to 6-month follow-up.
The POQ-VA pain score decreased significantly from the initial to the 12th visit (P < .05) and from the initial to the 6-month visit (P < .05). However, there was no significant interval change from the initial to the 3-month, the 12th to 3-month, 12th to 6-month, or 3-month to 6-month visit (Figure 1). The POQ-VA vitality scores and POQ-VA fear scores did not yield significant changes. The POQ-VA negative affect scores showed significant improvement only between the initial and the 3-month visit (P < .05) (Figure 2). The POQ-VA ADL scores showed significant improvement in the initial vs 3-month score (P < .05). The POQ-VA mobility scores were significantly improved for the initial vs 12th visit (P < .01), initial vs 3-month visit (P < .01), and the initial vs 6-month visit (P < .001) (Figure 1).
Analysis of VAS scores revealed a significant decrease at the 6-month time frame compared with the initial score for the left foot (P < .05). Further VAS analysis revealed no significant difference between the initial and 6-month right foot VAS score. When both feet were compared together, there was no significant difference in VAS ratings between any 2 points in time.
Analysis of Tinetti Total Score, Tinetti Balance Score, and Tinetti Gait Score revealed a significant difference between the initial vs 3-month visit for all 3 scores (P < .001, P < .001, and P < .05, respectively). In addition, Tinetti Total (P < .001) and Tinetti Balance (P < .01) scores were significantly improved from initial to the final 6-month visit. There were no significant findings between interim scores of the initial and 12th visits, the 12th and 3-month visits, or the 3-month and 6-month scores (Figure 2).
Analysis of SWM testing indicated a significant decrease in the total number of insensate sites (> 5.07) when both feet were grouped together between the initial and 3-month visits (P < .05) as well as the initial and 6-month (P < .01) visits. When the left and right feet were compared independently of each other, there was a significant decrease in the number of insensate sites between the initial and 6-month visits (P < .01 for both) (Figure 3).
Discussion
This study investigated whether or not a multimodal physical therapy approach would reduce several of the debilitating symptoms of DPN experienced by many veterans at WJBDVAMC. The results support the idea that a combined treatment protocol of MIRE and a standardized exercise program can lead to decreased POQ-VA pain levels, improved balance, and improved protective sensation in veterans with DPN. Alleviation of these DPN complications may ultimately decrease an individual’s risk of injury and improve overall QOL.
Because the POQ-VA is a reliable, valid self-reported measure for veterans, it was chosen to quantify the impact of pain. Overall, veterans who participated in this study perceived decreased pain interference in multiple areas of their lives. The most significant findings were in overall QOL, household and community mobility, and pain ratings. This suggests that the combined treatment protocol will help veterans maintain an active lifestyle despite poorly controlled diabetes and neuropathic pain.
Along with decreased pain interference with QOL, participants demonstrated a decrease in fall risk as quantified by the Tinetti Gait and Balance Assessment. The SWM testing showed improved protective sensation as early as 3 months and continued through the 6-month visit. As protective sensation improves and fall risk decreases, the risk of injury is lessened, fear of falling is decreased, and individuals are less likely to self-impose limitations on daily activity levels, which improves QOL. In addition, decreased fall risk and improved protective sensation can reduce the financial burden on both the patient and the health care system. Many individuals are hospitalized secondary to fall injury, nonhealing wounds, resulting infections, and/or secondary complications from prolonged immobility. This treatment protocol demonstrates how a standardized physical therapy protocol, including MIRE and balance exercises, can be used preventively to reduce both the personal and financial impact of DPN.
It is interesting to note that some POQ-VA and Tinetti subscores were significantly improved at 3 months but not at 6 months. The significance achieved at 3 months may be due to the time required (ie, > 12 visits) to make significant physiological changes. The lack of significance at 6 months may be due to the natural tendency of participants to less consistently perform the home exercise program and MIRE protocol when unsupervised in the home. Differences in the VAS and POQ-VA pain score ratings were noted in the data. The POQ-VA pain rating scale indicated significant improvement in pain levels over the course of the study. However, when asked about pain using the 10-cm VAS, patients reported no significant improvements. This may be because veterans are more familiar with the numerical pain rating scale and are rarely asked to use the 10-cm VAS. It may also be because the POQ-VA pain rating asks for an average pain level over the previous week, whereas the 10-cm VAS asks for pain level at a discrete point in time.
Historically, physical therapy has had little to offer individuals with DPN. As a result of this study, however, a standardized treatment program for DPN has been implemented at the WJBDVAMC Physical Therapy Clinic. Referred patients are seen in the clinic on a trial basis. If positive results are documented during the clinic treatments, a home MIRE unit and exercise program are provided. The patients are expected to continue performing home treatments of MIRE and exercise 3 times a week after discharge.
Strengths and Limitations
Strengths of the study include a stringent IRB review, control of medication changes during the study through alerts to all VA providers, and a standardized MIRE and exercise protocol. An additional strength is the long duration of the study, which included supervised and unsupervised interventions that simulate real-life scenarios.
Limitations of the study include a small sample size, case-controlled design rather than a randomized, double-blinded study, which can contribute to selection bias, inability to differentiate between the benefits of physical therapy alone vs physical therapy and MIRE treatments, and retention of participants due to travel difficulties across a wide catchment area.
This pilot study should be expanded to a multicenter, randomized, double-blinded study to clarify the most beneficial treatments for individuals with diabetic neuropathy. Examining the number of documented falls pre- and postintervention may also be helpful to determine actual effects on an individual’s fall risk.
Conclusion
The use of a multimodal physical therapy approach seems to be effective in reducing the impact of neuropathic pain, the risk of amputation, and the risk of falls in individuals who have pursued all standard medical options but still experience the long-term effects of DPN. By adhering to a standardized treatment protocol of MIRE and therapeutic exercise, it seems that the benefits of this intervention can be maintained over time. This offers new, nonconventional treatment options in the field of physical therapy for veterans whose QOL is negatively impacted by the devastating effects of diabetic neuropathy.
Acknowledgements
Clinical support was provided by David Metzelfeld, DPT, and Cam Lendrim, PTA of William Jennings Bryan Dorn VA Medical Center. Paul Bartels, PhD, of Warren Wilson College provided data analysis support. Anodyne Therapy, LLC, provided the MIRE unit used in the clinic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. National Institute of Neurological Disorders and Stroke Website. http://www.ninds.nih.gov/disorders/peripheralneuropath/detail_peripheralneuropathy.htm#183583208. Updated April 17, 2015. Accesssed August 8, 2015.
2. Armstrong DG, Lavery LA, and Wunderlich RP. Risk factors for diabetic foot ulceration: a logical approach to treatment. J Wound Ostomy Continence Nurs. 1998;25(3):123-128.
3. Pesa J, Meyer R, Quock T, Rattana SK, Mody SH. MBA Opioid utilization patterns among medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits. 2013;6(4):188-196.
4. VHA Support Service Center. The amputation risk by facility in the ProClarity amputation risk (PAVE) cube. Department of Veterans Affairs Nonpublic Intranet. http://vssc.med.va.gov.
5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892-900
6. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598-1604.
7. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
8. Centers for Disease Control and Prevention. Older adults falls: get the facts. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html. Updated July 1, 2015. Accessed August 8, 2015.
9. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333-338.
10. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568-1579.
11. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398.
12. Tuttle LG, Hastings MK, and Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92(1):133-141.
13. Gossrau G, Wähner M, Kuschke M, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011;12(6):953-960.
14. Somers DL, Somers MF. Treatment of neuropathic pain in a patient with diabetic neuropathy using transcutaneous electrical nerve stimulation applied to the skin of the lumbar region. Phys Ther. 1999;79(8):767-775.
15. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy—MIRE. J Diabetes Complications. 2006;20(2):81-87.
16. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168-172.
17. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract. 2004;10(1):24-30.
18. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92(3):125-130.
19. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395.
The progressive symptoms of diabetic peripheral neuropathy (DPN) are some of the most frequent presentations of patients seeking care at the VHA. Patients with DPN often experience unmanageable pain in the lower extremities, loss of sensation in the feet, loss of balance, and an inability to perform daily functional activities.1 In addition, these patients are at significant risk for lower extremity ulceration and amputation.2 The symptoms and consequences of DPN are strongly linked to chronic use of pain medications as well as increased fall risk and injury.3 The high health care usage of veterans with these complex issues makes DPN a significant burden for the patient, the VHA, and society as a whole.
At the William Jennings Bryan Dorn VA Medical Center (WJBDVAMC) in Columbia, South Carolina, 10,763 veterans were identified to be at risk for limb loss in 2014 due to loss of protective sensation and 5,667 veterans diagnosed with DPN were treated in 2014.4 Although WJBDVAMC offers multiple clinics and programs to address the complex issues of diabetes and DPN, veterans oftentimes continue to experience uncontrolled pain, loss of protective sensation, and a decline in function even after diagnosis.
One area of improvement the authors identified in the WJBDVAMC Physical Medicine and Rehabilitation Services Department was the need for an effective, nonpharmacologic treatment for patients who experience DPN. As a result, the authors designed a pilot research study to determine whether or not a combined physical therapy intervention of monochromatic near-infrared energy (MIRE) treatments and a standardized balance exercise program would help improve the protective sensation, reduce fall risk, and decrease the adverse impact of pain on daily function. The study was approved by the institutional review board (IRB) and had no outside source of funding.
Background
Current treatments for DPN are primarily pharmacologic and are viewed as only moderately effective, limited by significant adverse effects (AEs) and drug interactions.5 Patients in the VHA at risk for amputation in low-, moderate-, and high-risk groups total 541,475 and 363,468 have a history of neuropathy. They are considered at risk due to multiple, documented factors, including weakness, callus, foot deformity, loss of protective sensation, and/or history of amputation.4 Neuropathy can affect tissues throughout the body, including organs, sensory neurons, cardiovascular status, the autonomic system, and the gastrointestinal tract as it progresses.
Individuals who develop DPN often experience severe, uncontrolled pain in the lower extremities, insensate feet, and decreased proprioceptive skills. The functional status of individuals with DPN often declines insidiously while mortality rate increases.6 Increased levels of neuropathic pain often lead to decreased activity levels, which, in turn, contribute to decreased endurance, poorly managed glycemic indexes, decreased strength, and decreased independence.
Additional DPN complications, such as decreased sensation and muscle atrophy in the lower extremities, often lead to foot deformity and increased areas of pressure during weight bearing postures. These areas of increased pressure may develop unknowingly into ulceration. If a patient’s wound becomes chronic and nonhealing, it can also lead to amputation. In such cases, early mortality may result.6,7 The cascading effects of neuropathic pain and decreased sensation place a patient with diabetes at risk for falls. Injuries from falls are widely known to be a leading cause of hospitalization and mortality in the elderly.8
Physical therapy may be prescribed for DPN and its resulting sequelae. Several studies present conflicting results regarding the benefits of therapeutic exercise in the treatment of DPN. Akbari and colleagues showed that balance exercises can increase stability in patients with DPN; whereas, a study by Kruse and colleagues noted a training program consisting of lower-extremity exercises, balance training, and walking resulted in minimal improvement of participants’ balance and leg strength over a 12-month period.9,10 Recent studies have shown that weight bearing does not increase ulceration in patients with diabetes and DPN. This is contrary to previous assumptions that patients with diabetes and DPN need to avoid weight-bearing activities.11,12
Transcutaneous electrical nerve stimulation (TENS), a modality often used in physical therapy, has been studied in the treatment of DPN with conflicting results. Gossrau and colleagues found that pain reduction with micro-TENS applied peripherally is not superior to a placebo.13 However, a case study by Somers and Somers indicated that TENS applied to the lumbar area seemed to reduce pain and insomnia associated with diabetic neuropathy.14
Several recent research studies suggest that MIRE, another available modality, may be effective in treating symptoms of DPN. Monochromatic infrared energy therapy is a noninvasive, drug-free, FDA-approved medical device that emits monochromatic near-infrared light to improve local circulation and decrease pain. A large study of 2,239 patients with DPN reported an increase in foot sensation and decreased neuropathic pain levels when treated with MIRE.15
Leonard and colleagues found that the MIRE treatments resulted in a significant increase in sensation in individuals with baseline sensation of 6.65 Semmes-Weinstein Monofilament (SWM) after 6 and 12 active treatments as well as a decrease in neuropathic symptoms as measured by the Michigan Neuropathy Screening Instrument.16 Prendergast and colleagues noted improved electrophysical changes in both large and small myelinated nerve fibers of patients with DPN following 10 MIRE treatments.17 When studying 49 patients with DPN, Kochman and colleagues found 100% of participants had improved sensation after 12 MIRE treatments when tested with monofilaments.18
An additional benefit of MIRE treatment is that it can be safely performed at home once the patient is educated on proper use and application. Home DPN treatment has the potential to decrease the burden this population places on health care systems by reducing provider visits, medication, hospitalization secondary to pain, ulceration, fall injuries, and amputations.
Methods
This was a prospective, case series pilot study designed to measure changes in patient pain levels using the visual analog scale (VAS) and Pain Outcomes Questionnaire-VA (POQ-VA), degree of protective sensation loss as measured by SWM, and fall risk as denoted by Tinetti scores from entry to 6 months. Informed consent was obtained prior to treatment, and 33 patients referred by primary care providers and specialty clinics met the criteria and enrolled in the study. Twenty-one patients completed the entire 6-month study. The nonparametric Friedman test with a Dunn’s multiple comparison (DMC) post hoc test was used to analyze the data from the initial, 4-week, 3-month, and 6-month follow-up visits.
Setting and Participants
The study was performed in the Outpatient Physical Therapy Department at WJBDVAMC. Veterans with DPN who met the inclusion/exclusion criteria were enrolled. Inclusion criteria specified that the participant must be referred by a qualified health care provider for the treatment of DPN, be able to read and write in English, have consistent transportation to and from the study location, and be able to apply MIRE therapy as directed at home.
Exclusion criteria were subjects for whom MIRE or exercise were contraindicated. Subjects were excluded if they had medical conditions that suggested a possible decline in health status in the next 6 months. Such conditions included a current regimen of chemotherapy, radiation therapy, or dialysis; recent lower extremity amputation without prosthesis; documented active alcohol and/or drug misuse; advanced chronic obstructive pulmonary disease as defined as dyspnea at rest at least once per day; unstable angina; hemiplegia or other lower extremity paralysis; and a history of central nervous system or peripheral nervous system demyelinating disorders. Additional exclusion criteria included hospitalization in the past 60 days, use of any apparatus for continuous or patient-controlled analgesia; history of chronic low back pain with documented radiculopathy; and any change in pertinent medications in the past 60 days, including pain medications, insulin, metformin, and anti-inflammatories.
Interventions
Subjects participated in a combined physical therapy approach using MIRE and a standardized balance program. Patients received treatment in the outpatient clinic 3 times each week for 4 weeks. The treatment then continued at the same frequency at home until the scheduled 6-month follow-up visit. Clinic and home treatments included application of MIRE to bilateral lower extremities and feet for 30 minutes each as well as performance of a therapeutic exercise program for balance.
In the clinic, 2 pads from the MIRE device (Anodyne Therapy, LLC, Tampa, FL) were placed along the medial and lateral aspect of each lower leg, and an additional 2 pads were placed in a T formation on the plantar surface of each foot, per the manufacturer’s recommendations. The T formation consisted of the first pad placed horizontally across the metatarsal heads and the second placed vertically down the length of the foot. Each pad was protected by plastic wrap to ensure proper hygiene and secured. The intensity of clinic treatments was set at 7 bars, which minimized the risk of burns. Home treatments were similar to those in the clinic, except that each leg had to be treated individually instead of simultaneously and home MIRE units are preset and only function at an intensity that is equivalent to around 7 bars on the clinical unit.
The standardized balance program consisted of a progression of the following exercises: ankle alphabet/ankle range of motion, standing lateral weight shifts, bilateral heel raises, bilateral toe raises, unilateral heel raises, unilateral toe raises, partial wall squats, and single leg stance. Each participant performed these exercises 3 times per week in the clinic and then 3 times per week at home following the 12th visit.
Outcomes and Follow-up
The POQ-VA, a subjective quality of life (QOL) measure for veterans, as well as VAS, SWM testing, and the Tinetti Gait and Balance Assessment scores were used to measure outcomes. Data were collected for each of these measures during the initial and 12th clinic visits and at the 3-month and 6-month follow-up visits. The POQ-VA and VAS scores were self-reported and filled out by each participant at the initial, 12th, 3-month, and 6-month visits. The POQ-VA score has proven to be reliable and valid for the assessment of noncancer, chronic pain in veterans.19 The VAS scores were measured using a scale of 0 to 10 cm.
The SWM was standardized, and 7 sites were tested on each foot during the initial, 12th, 3-month, and 6-month visits: plantar surface of the distal great toe, the distal 3rd toe, the distal 5th toe, the 1st metatarsal head, the 3rd metatarsal head, the 5th metatarsal head, and the mid-plantar arch. At each site, the SWM was applied with just enough force to initiate a bending force and held for 1.5 seconds. Each site was tested 3 times. Participants had to detect the monofilament at least twice for the monofilament value to be recorded. Monofilament testing began with 6.65 SWM and decreased to 5.07, 4.56, 4.32, and lower until the patient was no longer able to detect sensation.
The Tinetti Gait and Balance Assessments was performed on each participant at the initial, 12th, 3-month, and 6-month visits. Tinetti balance, gait, and total scores were recorded at each interval.
Results
Thirty-three patients, referred by primary care providers and specialty clinics, met the inclusion criteria and enrolled in the study. Twenty-one patients (20 men and 1 woman) completed the entire 6-month study. Causes for withdrawal included travel difficulties (5), did not show up for follow-up visits (4), lumbar radiculopathy (1), perceived minimal/no benefit (1), and unrelated death (1). No AEs were reported.
The Friedman test with DMC post hoc test was performed on the POQ-VA total score and subscale scores. The POQ-VA subscale scores were divided into the following domains: pain, activities of daily living (ADL), fear, negative affect, mobility, and vitality. The POQ-VA domains were analyzed to compare data from the initial, 12th, 3-month, and 6-month visits. The POQ-VA total score significantly decreased from the initial to the 12th visit (P < .01), from the initial to the 3-month (P < .01), and from the initial to the 6-month visit (P < .05). However, there was no significant change from the 12th visit to the 3-month follow-up, 12th visit to the 6-month follow-up, or the 3-month to 6-month follow-up.
The POQ-VA pain score decreased significantly from the initial to the 12th visit (P < .05) and from the initial to the 6-month visit (P < .05). However, there was no significant interval change from the initial to the 3-month, the 12th to 3-month, 12th to 6-month, or 3-month to 6-month visit (Figure 1). The POQ-VA vitality scores and POQ-VA fear scores did not yield significant changes. The POQ-VA negative affect scores showed significant improvement only between the initial and the 3-month visit (P < .05) (Figure 2). The POQ-VA ADL scores showed significant improvement in the initial vs 3-month score (P < .05). The POQ-VA mobility scores were significantly improved for the initial vs 12th visit (P < .01), initial vs 3-month visit (P < .01), and the initial vs 6-month visit (P < .001) (Figure 1).
Analysis of VAS scores revealed a significant decrease at the 6-month time frame compared with the initial score for the left foot (P < .05). Further VAS analysis revealed no significant difference between the initial and 6-month right foot VAS score. When both feet were compared together, there was no significant difference in VAS ratings between any 2 points in time.
Analysis of Tinetti Total Score, Tinetti Balance Score, and Tinetti Gait Score revealed a significant difference between the initial vs 3-month visit for all 3 scores (P < .001, P < .001, and P < .05, respectively). In addition, Tinetti Total (P < .001) and Tinetti Balance (P < .01) scores were significantly improved from initial to the final 6-month visit. There were no significant findings between interim scores of the initial and 12th visits, the 12th and 3-month visits, or the 3-month and 6-month scores (Figure 2).
Analysis of SWM testing indicated a significant decrease in the total number of insensate sites (> 5.07) when both feet were grouped together between the initial and 3-month visits (P < .05) as well as the initial and 6-month (P < .01) visits. When the left and right feet were compared independently of each other, there was a significant decrease in the number of insensate sites between the initial and 6-month visits (P < .01 for both) (Figure 3).
Discussion
This study investigated whether or not a multimodal physical therapy approach would reduce several of the debilitating symptoms of DPN experienced by many veterans at WJBDVAMC. The results support the idea that a combined treatment protocol of MIRE and a standardized exercise program can lead to decreased POQ-VA pain levels, improved balance, and improved protective sensation in veterans with DPN. Alleviation of these DPN complications may ultimately decrease an individual’s risk of injury and improve overall QOL.
Because the POQ-VA is a reliable, valid self-reported measure for veterans, it was chosen to quantify the impact of pain. Overall, veterans who participated in this study perceived decreased pain interference in multiple areas of their lives. The most significant findings were in overall QOL, household and community mobility, and pain ratings. This suggests that the combined treatment protocol will help veterans maintain an active lifestyle despite poorly controlled diabetes and neuropathic pain.
Along with decreased pain interference with QOL, participants demonstrated a decrease in fall risk as quantified by the Tinetti Gait and Balance Assessment. The SWM testing showed improved protective sensation as early as 3 months and continued through the 6-month visit. As protective sensation improves and fall risk decreases, the risk of injury is lessened, fear of falling is decreased, and individuals are less likely to self-impose limitations on daily activity levels, which improves QOL. In addition, decreased fall risk and improved protective sensation can reduce the financial burden on both the patient and the health care system. Many individuals are hospitalized secondary to fall injury, nonhealing wounds, resulting infections, and/or secondary complications from prolonged immobility. This treatment protocol demonstrates how a standardized physical therapy protocol, including MIRE and balance exercises, can be used preventively to reduce both the personal and financial impact of DPN.
It is interesting to note that some POQ-VA and Tinetti subscores were significantly improved at 3 months but not at 6 months. The significance achieved at 3 months may be due to the time required (ie, > 12 visits) to make significant physiological changes. The lack of significance at 6 months may be due to the natural tendency of participants to less consistently perform the home exercise program and MIRE protocol when unsupervised in the home. Differences in the VAS and POQ-VA pain score ratings were noted in the data. The POQ-VA pain rating scale indicated significant improvement in pain levels over the course of the study. However, when asked about pain using the 10-cm VAS, patients reported no significant improvements. This may be because veterans are more familiar with the numerical pain rating scale and are rarely asked to use the 10-cm VAS. It may also be because the POQ-VA pain rating asks for an average pain level over the previous week, whereas the 10-cm VAS asks for pain level at a discrete point in time.
Historically, physical therapy has had little to offer individuals with DPN. As a result of this study, however, a standardized treatment program for DPN has been implemented at the WJBDVAMC Physical Therapy Clinic. Referred patients are seen in the clinic on a trial basis. If positive results are documented during the clinic treatments, a home MIRE unit and exercise program are provided. The patients are expected to continue performing home treatments of MIRE and exercise 3 times a week after discharge.
Strengths and Limitations
Strengths of the study include a stringent IRB review, control of medication changes during the study through alerts to all VA providers, and a standardized MIRE and exercise protocol. An additional strength is the long duration of the study, which included supervised and unsupervised interventions that simulate real-life scenarios.
Limitations of the study include a small sample size, case-controlled design rather than a randomized, double-blinded study, which can contribute to selection bias, inability to differentiate between the benefits of physical therapy alone vs physical therapy and MIRE treatments, and retention of participants due to travel difficulties across a wide catchment area.
This pilot study should be expanded to a multicenter, randomized, double-blinded study to clarify the most beneficial treatments for individuals with diabetic neuropathy. Examining the number of documented falls pre- and postintervention may also be helpful to determine actual effects on an individual’s fall risk.
Conclusion
The use of a multimodal physical therapy approach seems to be effective in reducing the impact of neuropathic pain, the risk of amputation, and the risk of falls in individuals who have pursued all standard medical options but still experience the long-term effects of DPN. By adhering to a standardized treatment protocol of MIRE and therapeutic exercise, it seems that the benefits of this intervention can be maintained over time. This offers new, nonconventional treatment options in the field of physical therapy for veterans whose QOL is negatively impacted by the devastating effects of diabetic neuropathy.
Acknowledgements
Clinical support was provided by David Metzelfeld, DPT, and Cam Lendrim, PTA of William Jennings Bryan Dorn VA Medical Center. Paul Bartels, PhD, of Warren Wilson College provided data analysis support. Anodyne Therapy, LLC, provided the MIRE unit used in the clinic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The progressive symptoms of diabetic peripheral neuropathy (DPN) are some of the most frequent presentations of patients seeking care at the VHA. Patients with DPN often experience unmanageable pain in the lower extremities, loss of sensation in the feet, loss of balance, and an inability to perform daily functional activities.1 In addition, these patients are at significant risk for lower extremity ulceration and amputation.2 The symptoms and consequences of DPN are strongly linked to chronic use of pain medications as well as increased fall risk and injury.3 The high health care usage of veterans with these complex issues makes DPN a significant burden for the patient, the VHA, and society as a whole.
At the William Jennings Bryan Dorn VA Medical Center (WJBDVAMC) in Columbia, South Carolina, 10,763 veterans were identified to be at risk for limb loss in 2014 due to loss of protective sensation and 5,667 veterans diagnosed with DPN were treated in 2014.4 Although WJBDVAMC offers multiple clinics and programs to address the complex issues of diabetes and DPN, veterans oftentimes continue to experience uncontrolled pain, loss of protective sensation, and a decline in function even after diagnosis.
One area of improvement the authors identified in the WJBDVAMC Physical Medicine and Rehabilitation Services Department was the need for an effective, nonpharmacologic treatment for patients who experience DPN. As a result, the authors designed a pilot research study to determine whether or not a combined physical therapy intervention of monochromatic near-infrared energy (MIRE) treatments and a standardized balance exercise program would help improve the protective sensation, reduce fall risk, and decrease the adverse impact of pain on daily function. The study was approved by the institutional review board (IRB) and had no outside source of funding.
Background
Current treatments for DPN are primarily pharmacologic and are viewed as only moderately effective, limited by significant adverse effects (AEs) and drug interactions.5 Patients in the VHA at risk for amputation in low-, moderate-, and high-risk groups total 541,475 and 363,468 have a history of neuropathy. They are considered at risk due to multiple, documented factors, including weakness, callus, foot deformity, loss of protective sensation, and/or history of amputation.4 Neuropathy can affect tissues throughout the body, including organs, sensory neurons, cardiovascular status, the autonomic system, and the gastrointestinal tract as it progresses.
Individuals who develop DPN often experience severe, uncontrolled pain in the lower extremities, insensate feet, and decreased proprioceptive skills. The functional status of individuals with DPN often declines insidiously while mortality rate increases.6 Increased levels of neuropathic pain often lead to decreased activity levels, which, in turn, contribute to decreased endurance, poorly managed glycemic indexes, decreased strength, and decreased independence.
Additional DPN complications, such as decreased sensation and muscle atrophy in the lower extremities, often lead to foot deformity and increased areas of pressure during weight bearing postures. These areas of increased pressure may develop unknowingly into ulceration. If a patient’s wound becomes chronic and nonhealing, it can also lead to amputation. In such cases, early mortality may result.6,7 The cascading effects of neuropathic pain and decreased sensation place a patient with diabetes at risk for falls. Injuries from falls are widely known to be a leading cause of hospitalization and mortality in the elderly.8
Physical therapy may be prescribed for DPN and its resulting sequelae. Several studies present conflicting results regarding the benefits of therapeutic exercise in the treatment of DPN. Akbari and colleagues showed that balance exercises can increase stability in patients with DPN; whereas, a study by Kruse and colleagues noted a training program consisting of lower-extremity exercises, balance training, and walking resulted in minimal improvement of participants’ balance and leg strength over a 12-month period.9,10 Recent studies have shown that weight bearing does not increase ulceration in patients with diabetes and DPN. This is contrary to previous assumptions that patients with diabetes and DPN need to avoid weight-bearing activities.11,12
Transcutaneous electrical nerve stimulation (TENS), a modality often used in physical therapy, has been studied in the treatment of DPN with conflicting results. Gossrau and colleagues found that pain reduction with micro-TENS applied peripherally is not superior to a placebo.13 However, a case study by Somers and Somers indicated that TENS applied to the lumbar area seemed to reduce pain and insomnia associated with diabetic neuropathy.14
Several recent research studies suggest that MIRE, another available modality, may be effective in treating symptoms of DPN. Monochromatic infrared energy therapy is a noninvasive, drug-free, FDA-approved medical device that emits monochromatic near-infrared light to improve local circulation and decrease pain. A large study of 2,239 patients with DPN reported an increase in foot sensation and decreased neuropathic pain levels when treated with MIRE.15
Leonard and colleagues found that the MIRE treatments resulted in a significant increase in sensation in individuals with baseline sensation of 6.65 Semmes-Weinstein Monofilament (SWM) after 6 and 12 active treatments as well as a decrease in neuropathic symptoms as measured by the Michigan Neuropathy Screening Instrument.16 Prendergast and colleagues noted improved electrophysical changes in both large and small myelinated nerve fibers of patients with DPN following 10 MIRE treatments.17 When studying 49 patients with DPN, Kochman and colleagues found 100% of participants had improved sensation after 12 MIRE treatments when tested with monofilaments.18
An additional benefit of MIRE treatment is that it can be safely performed at home once the patient is educated on proper use and application. Home DPN treatment has the potential to decrease the burden this population places on health care systems by reducing provider visits, medication, hospitalization secondary to pain, ulceration, fall injuries, and amputations.
Methods
This was a prospective, case series pilot study designed to measure changes in patient pain levels using the visual analog scale (VAS) and Pain Outcomes Questionnaire-VA (POQ-VA), degree of protective sensation loss as measured by SWM, and fall risk as denoted by Tinetti scores from entry to 6 months. Informed consent was obtained prior to treatment, and 33 patients referred by primary care providers and specialty clinics met the criteria and enrolled in the study. Twenty-one patients completed the entire 6-month study. The nonparametric Friedman test with a Dunn’s multiple comparison (DMC) post hoc test was used to analyze the data from the initial, 4-week, 3-month, and 6-month follow-up visits.
Setting and Participants
The study was performed in the Outpatient Physical Therapy Department at WJBDVAMC. Veterans with DPN who met the inclusion/exclusion criteria were enrolled. Inclusion criteria specified that the participant must be referred by a qualified health care provider for the treatment of DPN, be able to read and write in English, have consistent transportation to and from the study location, and be able to apply MIRE therapy as directed at home.
Exclusion criteria were subjects for whom MIRE or exercise were contraindicated. Subjects were excluded if they had medical conditions that suggested a possible decline in health status in the next 6 months. Such conditions included a current regimen of chemotherapy, radiation therapy, or dialysis; recent lower extremity amputation without prosthesis; documented active alcohol and/or drug misuse; advanced chronic obstructive pulmonary disease as defined as dyspnea at rest at least once per day; unstable angina; hemiplegia or other lower extremity paralysis; and a history of central nervous system or peripheral nervous system demyelinating disorders. Additional exclusion criteria included hospitalization in the past 60 days, use of any apparatus for continuous or patient-controlled analgesia; history of chronic low back pain with documented radiculopathy; and any change in pertinent medications in the past 60 days, including pain medications, insulin, metformin, and anti-inflammatories.
Interventions
Subjects participated in a combined physical therapy approach using MIRE and a standardized balance program. Patients received treatment in the outpatient clinic 3 times each week for 4 weeks. The treatment then continued at the same frequency at home until the scheduled 6-month follow-up visit. Clinic and home treatments included application of MIRE to bilateral lower extremities and feet for 30 minutes each as well as performance of a therapeutic exercise program for balance.
In the clinic, 2 pads from the MIRE device (Anodyne Therapy, LLC, Tampa, FL) were placed along the medial and lateral aspect of each lower leg, and an additional 2 pads were placed in a T formation on the plantar surface of each foot, per the manufacturer’s recommendations. The T formation consisted of the first pad placed horizontally across the metatarsal heads and the second placed vertically down the length of the foot. Each pad was protected by plastic wrap to ensure proper hygiene and secured. The intensity of clinic treatments was set at 7 bars, which minimized the risk of burns. Home treatments were similar to those in the clinic, except that each leg had to be treated individually instead of simultaneously and home MIRE units are preset and only function at an intensity that is equivalent to around 7 bars on the clinical unit.
The standardized balance program consisted of a progression of the following exercises: ankle alphabet/ankle range of motion, standing lateral weight shifts, bilateral heel raises, bilateral toe raises, unilateral heel raises, unilateral toe raises, partial wall squats, and single leg stance. Each participant performed these exercises 3 times per week in the clinic and then 3 times per week at home following the 12th visit.
Outcomes and Follow-up
The POQ-VA, a subjective quality of life (QOL) measure for veterans, as well as VAS, SWM testing, and the Tinetti Gait and Balance Assessment scores were used to measure outcomes. Data were collected for each of these measures during the initial and 12th clinic visits and at the 3-month and 6-month follow-up visits. The POQ-VA and VAS scores were self-reported and filled out by each participant at the initial, 12th, 3-month, and 6-month visits. The POQ-VA score has proven to be reliable and valid for the assessment of noncancer, chronic pain in veterans.19 The VAS scores were measured using a scale of 0 to 10 cm.
The SWM was standardized, and 7 sites were tested on each foot during the initial, 12th, 3-month, and 6-month visits: plantar surface of the distal great toe, the distal 3rd toe, the distal 5th toe, the 1st metatarsal head, the 3rd metatarsal head, the 5th metatarsal head, and the mid-plantar arch. At each site, the SWM was applied with just enough force to initiate a bending force and held for 1.5 seconds. Each site was tested 3 times. Participants had to detect the monofilament at least twice for the monofilament value to be recorded. Monofilament testing began with 6.65 SWM and decreased to 5.07, 4.56, 4.32, and lower until the patient was no longer able to detect sensation.
The Tinetti Gait and Balance Assessments was performed on each participant at the initial, 12th, 3-month, and 6-month visits. Tinetti balance, gait, and total scores were recorded at each interval.
Results
Thirty-three patients, referred by primary care providers and specialty clinics, met the inclusion criteria and enrolled in the study. Twenty-one patients (20 men and 1 woman) completed the entire 6-month study. Causes for withdrawal included travel difficulties (5), did not show up for follow-up visits (4), lumbar radiculopathy (1), perceived minimal/no benefit (1), and unrelated death (1). No AEs were reported.
The Friedman test with DMC post hoc test was performed on the POQ-VA total score and subscale scores. The POQ-VA subscale scores were divided into the following domains: pain, activities of daily living (ADL), fear, negative affect, mobility, and vitality. The POQ-VA domains were analyzed to compare data from the initial, 12th, 3-month, and 6-month visits. The POQ-VA total score significantly decreased from the initial to the 12th visit (P < .01), from the initial to the 3-month (P < .01), and from the initial to the 6-month visit (P < .05). However, there was no significant change from the 12th visit to the 3-month follow-up, 12th visit to the 6-month follow-up, or the 3-month to 6-month follow-up.
The POQ-VA pain score decreased significantly from the initial to the 12th visit (P < .05) and from the initial to the 6-month visit (P < .05). However, there was no significant interval change from the initial to the 3-month, the 12th to 3-month, 12th to 6-month, or 3-month to 6-month visit (Figure 1). The POQ-VA vitality scores and POQ-VA fear scores did not yield significant changes. The POQ-VA negative affect scores showed significant improvement only between the initial and the 3-month visit (P < .05) (Figure 2). The POQ-VA ADL scores showed significant improvement in the initial vs 3-month score (P < .05). The POQ-VA mobility scores were significantly improved for the initial vs 12th visit (P < .01), initial vs 3-month visit (P < .01), and the initial vs 6-month visit (P < .001) (Figure 1).
Analysis of VAS scores revealed a significant decrease at the 6-month time frame compared with the initial score for the left foot (P < .05). Further VAS analysis revealed no significant difference between the initial and 6-month right foot VAS score. When both feet were compared together, there was no significant difference in VAS ratings between any 2 points in time.
Analysis of Tinetti Total Score, Tinetti Balance Score, and Tinetti Gait Score revealed a significant difference between the initial vs 3-month visit for all 3 scores (P < .001, P < .001, and P < .05, respectively). In addition, Tinetti Total (P < .001) and Tinetti Balance (P < .01) scores were significantly improved from initial to the final 6-month visit. There were no significant findings between interim scores of the initial and 12th visits, the 12th and 3-month visits, or the 3-month and 6-month scores (Figure 2).
Analysis of SWM testing indicated a significant decrease in the total number of insensate sites (> 5.07) when both feet were grouped together between the initial and 3-month visits (P < .05) as well as the initial and 6-month (P < .01) visits. When the left and right feet were compared independently of each other, there was a significant decrease in the number of insensate sites between the initial and 6-month visits (P < .01 for both) (Figure 3).
Discussion
This study investigated whether or not a multimodal physical therapy approach would reduce several of the debilitating symptoms of DPN experienced by many veterans at WJBDVAMC. The results support the idea that a combined treatment protocol of MIRE and a standardized exercise program can lead to decreased POQ-VA pain levels, improved balance, and improved protective sensation in veterans with DPN. Alleviation of these DPN complications may ultimately decrease an individual’s risk of injury and improve overall QOL.
Because the POQ-VA is a reliable, valid self-reported measure for veterans, it was chosen to quantify the impact of pain. Overall, veterans who participated in this study perceived decreased pain interference in multiple areas of their lives. The most significant findings were in overall QOL, household and community mobility, and pain ratings. This suggests that the combined treatment protocol will help veterans maintain an active lifestyle despite poorly controlled diabetes and neuropathic pain.
Along with decreased pain interference with QOL, participants demonstrated a decrease in fall risk as quantified by the Tinetti Gait and Balance Assessment. The SWM testing showed improved protective sensation as early as 3 months and continued through the 6-month visit. As protective sensation improves and fall risk decreases, the risk of injury is lessened, fear of falling is decreased, and individuals are less likely to self-impose limitations on daily activity levels, which improves QOL. In addition, decreased fall risk and improved protective sensation can reduce the financial burden on both the patient and the health care system. Many individuals are hospitalized secondary to fall injury, nonhealing wounds, resulting infections, and/or secondary complications from prolonged immobility. This treatment protocol demonstrates how a standardized physical therapy protocol, including MIRE and balance exercises, can be used preventively to reduce both the personal and financial impact of DPN.
It is interesting to note that some POQ-VA and Tinetti subscores were significantly improved at 3 months but not at 6 months. The significance achieved at 3 months may be due to the time required (ie, > 12 visits) to make significant physiological changes. The lack of significance at 6 months may be due to the natural tendency of participants to less consistently perform the home exercise program and MIRE protocol when unsupervised in the home. Differences in the VAS and POQ-VA pain score ratings were noted in the data. The POQ-VA pain rating scale indicated significant improvement in pain levels over the course of the study. However, when asked about pain using the 10-cm VAS, patients reported no significant improvements. This may be because veterans are more familiar with the numerical pain rating scale and are rarely asked to use the 10-cm VAS. It may also be because the POQ-VA pain rating asks for an average pain level over the previous week, whereas the 10-cm VAS asks for pain level at a discrete point in time.
Historically, physical therapy has had little to offer individuals with DPN. As a result of this study, however, a standardized treatment program for DPN has been implemented at the WJBDVAMC Physical Therapy Clinic. Referred patients are seen in the clinic on a trial basis. If positive results are documented during the clinic treatments, a home MIRE unit and exercise program are provided. The patients are expected to continue performing home treatments of MIRE and exercise 3 times a week after discharge.
Strengths and Limitations
Strengths of the study include a stringent IRB review, control of medication changes during the study through alerts to all VA providers, and a standardized MIRE and exercise protocol. An additional strength is the long duration of the study, which included supervised and unsupervised interventions that simulate real-life scenarios.
Limitations of the study include a small sample size, case-controlled design rather than a randomized, double-blinded study, which can contribute to selection bias, inability to differentiate between the benefits of physical therapy alone vs physical therapy and MIRE treatments, and retention of participants due to travel difficulties across a wide catchment area.
This pilot study should be expanded to a multicenter, randomized, double-blinded study to clarify the most beneficial treatments for individuals with diabetic neuropathy. Examining the number of documented falls pre- and postintervention may also be helpful to determine actual effects on an individual’s fall risk.
Conclusion
The use of a multimodal physical therapy approach seems to be effective in reducing the impact of neuropathic pain, the risk of amputation, and the risk of falls in individuals who have pursued all standard medical options but still experience the long-term effects of DPN. By adhering to a standardized treatment protocol of MIRE and therapeutic exercise, it seems that the benefits of this intervention can be maintained over time. This offers new, nonconventional treatment options in the field of physical therapy for veterans whose QOL is negatively impacted by the devastating effects of diabetic neuropathy.
Acknowledgements
Clinical support was provided by David Metzelfeld, DPT, and Cam Lendrim, PTA of William Jennings Bryan Dorn VA Medical Center. Paul Bartels, PhD, of Warren Wilson College provided data analysis support. Anodyne Therapy, LLC, provided the MIRE unit used in the clinic.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. National Institute of Neurological Disorders and Stroke Website. http://www.ninds.nih.gov/disorders/peripheralneuropath/detail_peripheralneuropathy.htm#183583208. Updated April 17, 2015. Accesssed August 8, 2015.
2. Armstrong DG, Lavery LA, and Wunderlich RP. Risk factors for diabetic foot ulceration: a logical approach to treatment. J Wound Ostomy Continence Nurs. 1998;25(3):123-128.
3. Pesa J, Meyer R, Quock T, Rattana SK, Mody SH. MBA Opioid utilization patterns among medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits. 2013;6(4):188-196.
4. VHA Support Service Center. The amputation risk by facility in the ProClarity amputation risk (PAVE) cube. Department of Veterans Affairs Nonpublic Intranet. http://vssc.med.va.gov.
5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892-900
6. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598-1604.
7. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
8. Centers for Disease Control and Prevention. Older adults falls: get the facts. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html. Updated July 1, 2015. Accessed August 8, 2015.
9. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333-338.
10. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568-1579.
11. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398.
12. Tuttle LG, Hastings MK, and Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92(1):133-141.
13. Gossrau G, Wähner M, Kuschke M, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011;12(6):953-960.
14. Somers DL, Somers MF. Treatment of neuropathic pain in a patient with diabetic neuropathy using transcutaneous electrical nerve stimulation applied to the skin of the lumbar region. Phys Ther. 1999;79(8):767-775.
15. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy—MIRE. J Diabetes Complications. 2006;20(2):81-87.
16. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168-172.
17. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract. 2004;10(1):24-30.
18. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92(3):125-130.
19. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395.
1. National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. National Institute of Neurological Disorders and Stroke Website. http://www.ninds.nih.gov/disorders/peripheralneuropath/detail_peripheralneuropathy.htm#183583208. Updated April 17, 2015. Accesssed August 8, 2015.
2. Armstrong DG, Lavery LA, and Wunderlich RP. Risk factors for diabetic foot ulceration: a logical approach to treatment. J Wound Ostomy Continence Nurs. 1998;25(3):123-128.
3. Pesa J, Meyer R, Quock T, Rattana SK, Mody SH. MBA Opioid utilization patterns among medicare patients with diabetic peripheral neuropathy. Am Health Drug Benefits. 2013;6(4):188-196.
4. VHA Support Service Center. The amputation risk by facility in the ProClarity amputation risk (PAVE) cube. Department of Veterans Affairs Nonpublic Intranet. http://vssc.med.va.gov.
5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892-900
6. Tentolouris N, Al-Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5-year follow-up study. Diabetes Care. 2004;27(7):1598-1604.
7. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-1042.
8. Centers for Disease Control and Prevention. Older adults falls: get the facts. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html. Updated July 1, 2015. Accessed August 8, 2015.
9. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49(2):333-338.
10. Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568-1579.
11. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385-1398.
12. Tuttle LG, Hastings MK, and Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92(1):133-141.
13. Gossrau G, Wähner M, Kuschke M, et al. Microcurrent transcutaneous electric nerve stimulation in painful diabetic neuropathy: a randomized placebo-controlled study. Pain Med. 2011;12(6):953-960.
14. Somers DL, Somers MF. Treatment of neuropathic pain in a patient with diabetic neuropathy using transcutaneous electrical nerve stimulation applied to the skin of the lumbar region. Phys Ther. 1999;79(8):767-775.
15. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy—MIRE. J Diabetes Complications. 2006;20(2):81-87.
16. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatment. Diabetes Care. 2004;27(1):168-172.
17. Prendergast JJ, Miranda G, Sanchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract. 2004;10(1):24-30.
18. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92(3):125-130.
19. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395.
The VA/DoD Chronic Effects of Neurotrauma Consortium: An Overview at Year 1
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
The Chronic Effects of Neuro-trauma Consortium (CENC) is a federally funded research project devised to address the long-term effects of mild traumatic brain injury (mTBI) in military service members (SMs) and veterans. Announced by President Barack Obama on August 20, 2013, the CENC is one of 2 major initiatives developed in response to injuries incurred by U.S. service personnel during Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) as part of the National Research Action Plan. The CENC is jointly funded by the DoD and the VA, with a budget of $62.175 million over 5 years.
The consortium funds basic science, clinical, and translational research efforts with a closely integrated supportive infrastructure, including administrative services, regulatory guidance, study design, biostatistical consultation, data management, common data element application, and interdisciplinary communication. In addition, the consortium facilitates and integrates the activities of a diverse group of skilled specialty research teams, allowing them to fully focus their efforts on understanding and clarifying the relationship between combat-related mTBI and chronic neurotrauma effects, including neurodegeneration.
Background
Nearly 20% of the more than 2.6 million U.S. SMs deployed since 2003 to OEF and OIF have sustained at least 1 TBI, predominantly mTBI. Almost 8% of all OEF/OIF veterans demonstrate persistent post-TBI symptoms more than 6 months postinjury. Acute mTBI effects are typically transient, with headache, cognitive, behavioral, balance, and sleep symptoms most often seen, but symptoms may persist and even lead to lifelong disability. In these individuals, additional chronic effects, such as neuroendocrinologic abnormalities, seizures and seizurelike disorders, fatigue, vision and hearing abnormalities, and numerous other somatic symptoms are more common over time. The long-term effects from single or repeated mTBIs on the persistence of these symptoms, on combat and trauma-related comorbidities, and on long-term brain functioning are unknown.
Increasing evidence supports the link between both concussions and combat-related trauma with chronic traumatic encephalopathy (CTE), which results in progressive cognitive and behavioral decline in subpopulations 5 to 50 years out from repeated or cumulative mTBI exposures. The possibility of a link between mTBI, persistent symptoms, and early dementia has widespread implications for SMs and veterans; however, these chronic and late-life effects of mTBI are poorly understood.
Traumatic brain injuries of mixed severity have been linked to a higher incidence of Alzheimer disease (AD) and other dementias and an earlier onset of AD, although negative findings have also been reported. Chronic traumatic encephalopathy has been reported to occur in retired boxers at higher rates and at younger ages compared with dementia in the general population. More recently, brain autopsies of athletes from a variety of sports with confirmed CTE have demonstrated elevated tau proteins, tau-immunoreactive neurofibrillary tangles, and neuropil threads, suggesting that pathologic processes similar to those occurring in AD may be involved. Longitudinal research bridging SMs, veterans, and athletes with neurotrauma has been fragmented and incompletely focused on the strategic needs (eg, troop readiness) and vision of the DoD and VA.
Critical gaps exist in the literature with few prospective, well-controlled, longitudinal studies on late-life outcomes and neurodegeneration after mTBI, as well as in related basic science research. These research gaps are particularly prominent in the potentially unique injuries and difficulties seen in combat-exposed populations. The existing research, although suggestive, is not rigorous or robust enough to allow for a clear understanding of the relationships, risks, and potential effective interventions for mTBI, chronic symptoms, and neurodegeneration.
The CENC was developed to create a road map of existing knowledge gaps, to recruit the top relevant subject matter experts in the country, to develop and establish a cohesive set of rigorously designed studies to address these knowledge voids, and to leverage core consortium resources both efficiently and effectively.
Related: The Right Care at the Right Time and in the Right Place: The Role of Technology in the VHA
Given these gaps in scientific research and knowledge, the DoD and VA jointly issued a request for proposals to fund a project to address these concerns. After a competitive application process, an integrated proposal, led by researchers at Virginia Commonwealth University (VCU) was announced as the recipient of the Presidential award.
Consortium Structure
The CENC, serving as the comprehensive research network for DoD and VA, focuses on (1) identifying and characterizing the anatomic, molecular, and physiologic mechanisms of chronic injury from mTBI and potential neurodegeneration; (2) investigating the relationship of comorbidities (psychological, neurologic, sensory, motor, pain, cognitive, and neuroendocrine) of trauma and combat exposure to TBI with neurodegeneration; and (3) assessing the efficacy of existing and novel treatment and rehabilitation strategies for chronic effects and neurodegeneration following TBI.
The consortium is a collaboration among more than 30 universities, nonprofit research organizations, VAMCs, and military medical centers made up of a leadership core, 5 research infrastructure cores, 8 active studies, a data safety monitoring committee, a consumer advisory board, a scientific advisory board, and an independent granting mechanism to foster additional research in chronic effects after mTBI.
Leadership Core
The principal investigator for CENC is David X. Cifu, MD, chairman and professor of the VCU Department of Physical Medicine and Rehabilitation in Richmond, Virginia. The consortium co-principal investigators are Ramon Diaz-Arrastia, MD, PhD, professor of neurology, Uniformed Services University of the Health Sciences (USUHS) and director of the clinical research at the Center for Neuroscience and Regenerative Medicine in Bethesda, Maryland, and Rick L. Williams, PhD, co-principal investigator for CENC and senior statistician at RTI International in Raleigh, North Carolina.
Research Cores
The CENC operates 5 research infrastructure cores. The Biorepository Core, led by Dr. Diaz-Arrastia at USUHS, manages the storage and processing of biologic (blood and saliva) samples collected through all CENC protocols. The Biostatistics Core, led by Dr. Williams; Nancy Temkin, PhD; and Heather Belanger, PhD at RTI, provides study design guidance and biostatistical analysis to facilitate knowledge translation and dissemination.
The Data and Study Management Core is led by Dr. Williams at RTI. It centrally and securely maintains all collected data; oversees the clinical monitoring of research sites; provides a consortium research manager for each study who interacts with the study leadership, study site leaders, and staff; expedites and guides clinical protocols through regulatory approval processes; coordinates patient accrual and study activities across sites; develops and monitors data acquisition compliance; and facilitates exportation of all data collection to the Federal Interagency Traumatic Brain Injury Research informatics system.
The Neuroimaging Core is led by Elisabeth Wilde, PhD, at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, Texas. This core facilitates sequence development and pulse programming; provides training and supervision of technologists and support personnel; ensures acquisition, transfer, and storage of imaging data; oversees quality assurance; performs conventional and advanced imaging analysis; and interprets neuroimaging data.
The Neuropathology Core is led by Dr. Dan Perl and colocated at USUHS and Edith Norse Rogers Memorial Veterans Hospital/VA Boston Healthcare System. Dr. Perl manages the collection of brain specimens from the participants, using an existing national network of dieners and neuropathologists, catalogs and stores tissues, and administers requests for use of these tissues.
Active Research Studies
The Longitudinal Cohort Study addresses a critical research gap by identifying and characterizing the late effects of mTBI and assessing the influence and interaction of the many potential risk factors for early dementia. The study uses a wide array of self-report, laboratory, biophysical, neuropsychologic, and imaging assessment tools to evaluate a cohort (n = 880) of U.S. OEF/OIF combatants who have had at least 1 mTBI and a control group of participants (n = 220) who have experienced combat but have not had a mTBI, and then re-assesses them annually (in person or via telephone), with the goal of following the cohort for as long as resources are available.
Collaborating sites for this study include Hunter Holmes McGuire VAMC in Richmond, Virginia; James A. Haley Veterans’ Hospital in Tampa, Florida; Michael E. DeBakey VAMC in Houston, Texas; Audie L. Murphy Memorial Veterans Hospital in San Antonio, Texas; VA Boston Healthcare System; Minneapolis VA Health Care System in Minnesota; and Fort Belvoir in Virginia. Dr. Cifu and Dr. William Walker lead this study.
Epidemiology of mTBI and Neurosensory Outcomes
This project integrates and analyzes several VA, DoD, and Centers for Medicare and Medicaid Services health care system data sets to study the chronic effects of mTBI on neurodegenerative disease and other comorbidities. The primary aims of the project include evaluating the association between mTBI and short-term clinical outcomes, including factors associated with resilience and effects of treatment; investigating long-term clinical outcomes, including neurosensory disorders and mortality; and identifying factors associated with low- and high-distress trajectories of comorbid burden after mTBI. Dr. Kristine Yaffe, Dr. Mary Jo Pugh, and Dr. Michael McCrea, are the leads of this study.
Tau Modification and Aggregation in TBI
This study aims to develop an animal model of repetitive-mTBI, which will allow the tracking of progressive intraneuronal tau alterations that can be correlated with behavioral dysfunction, neuronal protein, and gene expression signatures that can be used to assess the effects of interventions. The observations made in the animal model will be compared with findings generated from tissue obtained at autopsy from deceased SMs and veterans who sustained repetitive-mTBI. Dr. Fiona Crawford and Dr. Elliott Mufson lead this study.
Otolith Dysfunction
This study is examining the effect of inner ear dysfunction on balance, gait, and quality of life (QOL). Recent evidence suggests that otolith organ dysfunction can occur in patients with mTBI or blast exposure. If the dizziness and imbalance symptoms that occur following head injury or blast exposure are related to injury to the otolith organs rather than to the horizontal semicircular canal, then new treatment approaches may be necessary to focus on otolith organ pathway recovery. Performance on balance tasks while standing and walking and questionnaires on the impact on QOL will be compared in 4 groups of individuals (n = 120) with and without head injury/blast exposure (otolith organ dysfunction, horizontal canal dysfunction, both otolith and horizontal canal dysfunction, and healthy individuals). Dr. Faith Akin leads this study.
ADAPT
The ADAPT study (Assessment and Long-term Outcome and Disability in Active Duty Military Prospectively Examined following Concussive TBI) is investigating the association of early clinical and imaging measures with late (5 year) clinical outcome after blast-related mTBI from combat. The study (n = 100) will use 5-year follow-up advanced magnetic resonance imaging (MRI) and clinical outcome measures of combat mTBI, as a continuation of previous longitudinal research efforts (n = 575). Two groups of subjects will be studied: subjects who sustained a mTBI from blast during deployment and subjects without history of blast exposure and no diagnosis of deployment mTBI. Dr. Christine MacDonald leads this study.
Diffusion Tensor Imaging Phantom Study
This study involves the development and testing of a novel phantom that would be used to enhance accuracy, consistency, and reliability in both isotropic and anisotropic measurements derived from diffusion imaging, as well as other MRI-based measurements, using universal fluid disk chambers in a single phantom. Currently, the acquisition of diffusion data in large studies and clinical trials lacks standardization, and important differences exist in how data are acquired on scanners of different manufacturers, using different hardware or software, or when different acquisition parameters are used. As a result, development of large pools of data and the creation of normative data are hampered by inhomogeneity in the data set, which is difficult to analyze. The study team will perform detailed testing of the phantom materials and phantoms themselves, as well as examine diffusion imaging on 1 to 2 human volunteers at each of the 4 sites. Intra- and interscanner differences will be measured, and based on these findings, a more standardized imaging protocol that will provide optimal uniformity of diffusion imaging will be designed. Dr. Elisabeth Wilde leads this study.
Novel White Matter Imaging to Improve mTBI Diagnosis
This study will use myelin-sensitive novel imaging techniques (McDespot [multi-component driven equilibrium single pulse observation of T1/T2]) to improve correspondence with diagnostic groups after trauma exposure and correlation with cognitive deficits in mTBI. The study will recruit individuals (n = 82) from 4 groups, comorbid mTBI and posttraumatic stress disorder (PTSD), only mTBI, only PTSD, and controls who will be prospectively comprehensively assessed clinically (clinical interview, physical exam, neuropsychological assessment) and with advanced imaging (including McDespot, diffusion tensor imaging, and other forms of imaging). Dr. Amy Jak leads this study.
Peer Review Program
The CENC has an integrated grant program to identify scientifically valid and strategically important research projects. To date, 2 rounds of proposal requests and project support have been completed. Scientific review is conducted under the CENC Peer Review Program. Scientifically meritorious studies are identified by independent peer review and then undergo a Programmatic Review by CENC leadership before being recommended for funding to the Government Steering Committee (GSC). Studies that are recommended must address road map gaps, develop innovative approaches, or provide an avenue for new researchers and novel research approaches to contribute to the consortium mission to advance the science of brain injury treatment and prevention. The CENC grant program is administered by Dr. Steven L. West.
Consumer Advisory Board
The Consumer Advisory Board (CAB) advises and makes nonbinding recommendations to CENC. The responsibilities of the committee members include (1) providing information that helps CENC leadership better appreciate and understand the issues and needs of TBI survivors and their support networks so appropriate research can be designed and implemented; (2) evaluating existing research and making recommendations for additions and/or modifications to project procedures; (3) providing input for the road map for future research based on members’ personal experiences and knowledge; and (4) providing linkages to targeted communities for direct feedback and to assist in forming collaborative partnerships.
The CAB is composed of survivors of TBI, family members of survivors of TBI, providers of TBI services, service organizations with specific ties to SMs and veterans, and clinical and corporate representatives of transportation services for the disabled, the independent living movement, and assistive technology. Persons who are heavily engaged in political activity or who actively endorse a specific device or product are not eligible for membership on the CAB. Membership is composed of persons nominated by CENC leadership and approved by the GSC. The CAB is co-chaired by Charles Gatlin, MS, and General (Ret.) Peter Chiarelli.
Scientific Advisory Board
The members of the Scientific Advisory Board (SAB) advise and make nonbinding recommendations to CENC. Responsibilities of the committee members include (1) providing information that may help the consortium leadership better understand the issues related to TBI; (2) evaluating existing research; (3) recommending additions and/or modifications to project procedures; and (4) assisting CENC by helping leverage relationships with other researchers. The SAB is composed of members of the research community on TBI who are not part of CENC. Persons who may be considered to have positions of authority, such as active or retired flag officers or chief executive officers, may be eligible for general SAB membership but are not be eligible for chair positions. Membership is composed of persons nominated by CENC leadership and approved by the GSC. Col. Jamie Grimes, MD, and Henry Lew, MD, PhD, co-chair the SAB.
Federal Oversight
The GSC oversees CENC. Members of the GSC are DoD and VA appointed and represent both government agencies and nongovernment subject matter experts. The GSC approves all studies to be conducted, recommends new studies, and identifies existing and new requirements. The GSC is the overall main governing and management committee for the project and the committee through which the DoD and VA interact and collaborate with the CENC. The GSC determines all major scientific decisions, and clinical studies proposed by the CENC committee proceed to the implementation stage only with the approval of the GSC.
Acknowledgements
This research is supported by grants 1-I01-RX-001135-01-A2 (PI: F. Aiken), 1-I01-RX-001774-01 (PI: F. Crawford), 1-I01-RX-001880-01 (PI: E. Wilde), 1-I01-CX-001135-01 (PI: S. Cifu), and 1-I01-CX-001246-01 (PI: K. Yaffe) from the U.S. Department of Veterans Affairs and by grant W81XWH-13-2-0095 (PI: D. Cifu) from the U.S. Department of Defense, Congressionally Directed Medical Research Programs. The ideas and opinions expressed in this paper do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or the U.S. Government.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.