User login
An 85-year-old woman with respiratory failure and positional hypoxemia
An 85-year-old woman was brought to our intensive care unit because of worsening hypoxemia over the past day. About 3 weeks earlier she had been diagnosed with acute bilateral pulmonary emboli in the distal branches of the left and right lower lobes and right middle lobe, for which she was receiving anticoagulation therapy.
At presentation she had generalized fatigue and dyspnea at rest that was worse with exertion, but she denied having fever, chest pain, or cough. Her medical history included hypertension, hyperlipidemia, hypothyroidism, stage 1 breast cancer in remission, thromboembolic stroke, and myasthenia gravis. Before her hospital admission, she had been taking rosuvastatin, metoprolol tartrate, pyridostigmine, prednisone, furosemide, levothyroxine, and rivaroxaban. She did not smoke, she was retired, and she had not traveled recently.
Her blood pressure was 135/66 mm Hg, pulse 73 beats per minute, respiratory rate 16, temperature 35.4ºC (95.7ºF), and oxygen saturation 88% while receiving oxygen at 6 L/min via nasal cannula. Physical examination revealed mild edema in the lower extremities and basilar decreased breath sounds. She had no finger clubbing or cyanosis and was not using accessory muscles to breathe. Of note, her oxygen saturation remained more than 93% when she was recumbent but sharply dropped to less than 85% when she was upright.
Laboratory values
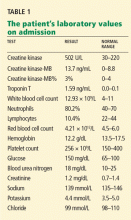
Results of initial laboratory testing were as follows:
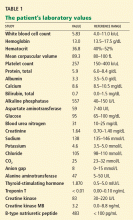
- Sodium 138 mmol/L (reference range 132–148)
- Potassium 4.2 mmol/L (3.5–5.0)
- Chloride 99 mmol/L (98–111)
- Bicarbonate 29 mmol/L (23–32)
- Creatinine 0.52 mg/dL (0.7–1.4).
- White blood cell count 11.06 × 109/L (3.7–11.0)
- Hemoglobin 12.6 g/dL (12–16)
- Platelet count 211 × 109/L (150–400).
- International normalized ratio 1.4.
Electrocardiography and imaging studies
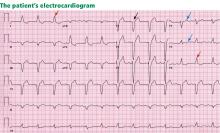
Standard 12-lead electrocardiography showed normal sinus rhythm with left axis deviation and left ventricular hypertrophy.
Chest radiography showed bilateral interstitial opacities and small pleural effusions.
Computed tomography (CT) of the chest with contrast, compared with a CT scan done 20 days earlier, showed that the pulmonary emboli had resolved.
Arterial blood gases
In view of her positional hypoxemia, blood for arterial blood gas measurements was drawn in the supine and upright positions.
Supine, with a fraction of inspired oxygen (Fio2) via high-flow nasal cannula of 45%, her values were:
- pH 7.45 (reference range 7.35–7.45)
- Pco2 34 mm Hg (36–46)
- Po2 81 mm Hg (85–95)
- Bicarbonate 23 mmol/L (22–26).
Upright, her hypoxemia was significantly worse:
- pH 7.46
- Pco2 33 mm Hg
- Po2 57 mm Hg
- Bicarbonate 23 mmol/L.
The methemoglobin level was normal on both measurements.
During her stay in the intensive care unit, she required up to 100% Fio2 because of persistent hypoxemia.
CAUSES OF HYPOXEMIA
1. So far, the patient’s laboratory tests and imaging studies point to which of the following as the most likely cause of her severe hypoxemia?
- Ventilation-perfusion (V/Q) mismatch
- Diffusion abnormality
- Hypoventilation
- Shunting
- None of the above
The arterial blood gas measurements suggested the possibility of shunting as the cause, although further imaging would be needed to confirm that.
V/Q mismatch can occur in respiratory failure due to pulmonary embolism, pulmonary edema, or shunting. If ventilation is preserved but perfusion is impaired, the V/Q ratio approaches infinity (dead-space ventilation), a situation that can be seen in pulmonary embolism. If perfusion is preserved and ventilation is impaired, the V/Q ratio approaches zero, which is consistent with a physiologic shunt.
Hypoxemia may improve in less severe forms of V/Q mismatch. In our patient, the repeat CT with contrast showed that her pulmonary embolism had resolved, so this is probably not the cause of her severe hypoxemia.
Diffusion abnormalities are due to defects in the lung parenchyma, such as in chronic obstructive pulmonary disease, interstitial lung disease, and lung fibrosis.
Hypoxemia from diffusion defects is usually aggravated by a precipitating factor that increases oxygen demand, and it usually improves with oxygen supplementation. This is unlikely in our patient, as she did not have a history of chronic interstitial lung disease and CT showed no evidence of severe lung parenchymal disease.
Hypoventilation is usually due to drugs that cause respiratory depression, to stroke, or to neuromuscular diseases such as myasthenia gravis that can cause respiratory muscle weakness. It results in elevation of Pco2 and, if not corrected, respiratory acidosis.
Our patient had a diagnosis of myasthenia gravis, though hypoventilation is unlikely in her case because she had a normal respiratory rate and low Pco2 values.
Shunting can be physiologic or anatomic and can occur in the heart or the lungs. In physiologic shunting, severe V/Q mismatch can occur when ventilation is affected, as in severe pulmonary edema and pneumonia. In anatomic shunting, a defect such as an atrial septal defect or a pulmonary arteriovenous malformation allows blood to bypass areas of ventilation from the venous to the arterial circulation, preventing it from being oxygenated. In true anatomic shunting, supplemental oxygen with 100% Fio2 has little effect, whereas in V/Q mismatch it can raise the arterial oxygen saturation.
Our patient’s radiograph did not suggest severe pneumonia or pulmonary edema, which makes these unlikely causes of her hypoxemia. At this point, because of her positional hypoxemia, further evaluation with contrast-enhanced echocardiography was needed to evaluate for anatomic shunting in the heart or lungs.
FURTHER TESTING
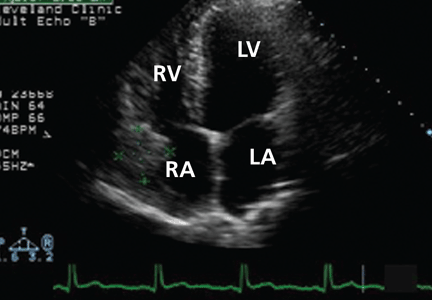
Transthoracic echocardiography (TTE) with agitated saline with a Valsalva maneuver was performed. Normally, no bubbles are seen in the left-sided chambers after intravenous injection of agitated saline contrast, whereas bubbles on the left side suggest an intracardiac or intrapulmonary shunt. In our patient, this test was negative, and her right ventricular systolic pressure was normal.
2. What further testing should be considered to evaluate our patient’s hypoxemia?
- High-resolution chest CT
- Transesophageal echocardiography (TEE)
- Pulmonary function testing
- Repeated arterial blood gas measurement
- Edrophonium testing
Repeat imaging with high-resolution CT would likely not provide additional information and would expose the patient to additional radiation without adding much clinical benefit.
TEE could help further evaluate the intracardiac anatomy and look for shunting, which may be missed on TTE because of suboptimal positioning or image quality.
Pulmonary function testing is useful in establishing the baseline function and impairment in respiratory volumes. If an acute myasthenic crisis is suspected, measuring the negative inspiratory force and the forced vital capacity can be useful in monitoring worsening respiratory muscle weakness and assessing the need for mechanical ventilation.
In our patient, it is unlikely that pulmonary function testing would help, since her acute respiratory failure was probably not caused by neuromuscular weakness.
Repeated arterial blood gas measurement would likely only confirm that the patient still has positional hypoxemia but would not help sort through the differential diagnosis.
Edrophonium testing is useful in diagnosing myasthenia gravis and differentiating it from other neuromuscular diseases, such as Lambert-Eaton syndrome. Edrophonium, a reversible acetylcholinesterase inhibitor, prevents degradation of acetylcholine and prolongs its effect at the synaptic cleft, thus improving muscle weakness.
Our patient has already been diagnosed with myasthenia gravis, so this test is not likely to uncover the cause of her hypoxemia.
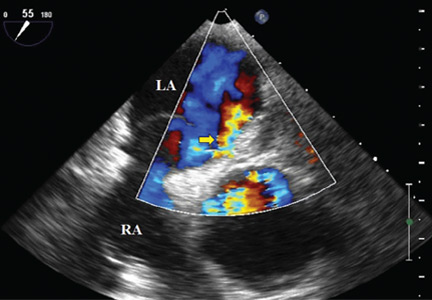
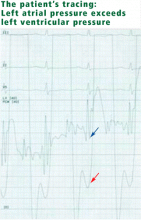
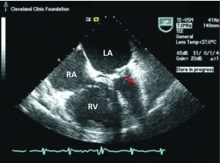
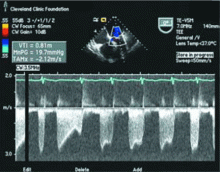
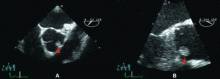
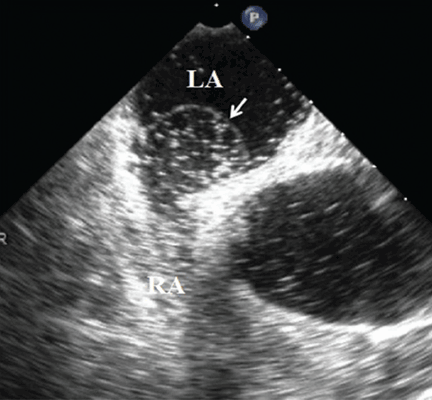
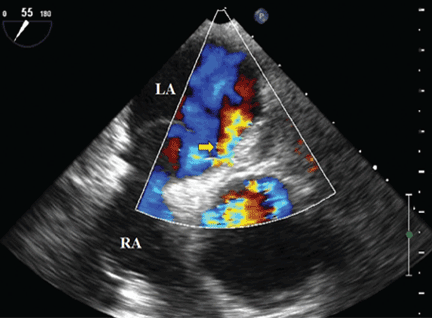
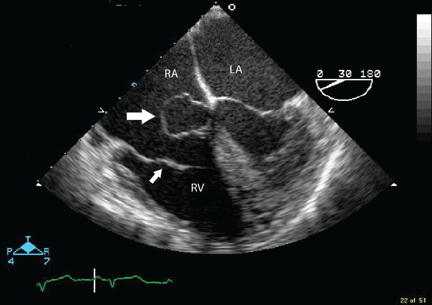
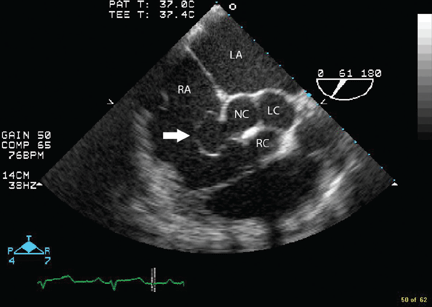
Because we still strongly suspected a shunt, TEE was performed with intravenous injection of agitated saline. TEE with the patient upright revealed intracardiac right-to-left shunting through a patent foramen ovale. The midesophageal view after saline injection showed a large interatrial septal aneurysm with total excursion of 2 cm, and right-to-left shunting within the first beat, consistent with an intracardiac shunt (Figure 1). Color Doppler imaging (Figure 2) demonstrated turbulent flow through the patent foramen ovale, consistent with right-to-left shunting, and also showed the patent foramen ovale in a closed position (Figure 3).
3. Which is now most likely the cause of our patient’s hypoxemia?
- Chronic thromboembolic pulmonary hypertension
- Myasthenic crisis
- Platypnea-orthodeoxia syndrome due to the patent foramen ovale
- Methemoglobinemia
Chronic thromboembolic pulmonary hypertension is usually a long-term result of untreated or inadequately treated thromboembolic disease (eg, pulmonary emboli), which causes vascular remodeling and pulmonary arteriopathy, which in turn leads to increased pulmonary vascular resistance and pulmonary hypertension.
This is unlikely the cause of our patient’s acute hypoxemia, as her symptoms did not suggest it. Moreover, an elevated right ventricular systolic pressure on TTE would suggest pulmonary hypertension, but TTE did not show this, and repeat chest CT indicated that her pulmonary embolism had been adequately treated and had resolved. A V/Q scan and right heart catheterization would help rule out chronic thromboembolic pulmonary hypertension, although these were not done in our patient.
Myasthenic crisis is the progressive fatiguing and paralysis of respiratory muscles ultimately requiring mechanical ventilation to sustain life. It is often brought on by infection or drug therapy.
Our patient did not require intubation and she had no signs or symptoms of myasthenic crisis such as ptosis, dysphagia, or dysarthria. She had a negative inspiratory force of −21 cm H2O, and pulmonary function testing 4 days before her hospital admission had shown a forced vital capacity of 1.84 L, making myasthenic crisis an unlikely cause of her respiratory failure.
Platypnea-orthodeoxia syndrome is a syndrome of dyspnea (platypnea) and hypoxemia (orthodeoxia) that is induced by sitting upright or standing and resolves when lying down. It is a result of right-to-left intracardiac or intrapulmonary shunting in the presence of an anatomic defect and a functional element causing redirection of shunt flow through the anatomic defect in an upright position.1 It is associated with specific cardiac, pulmonary, and hepatic diseases, such as atrial septal defect, pulmonary arteriovenous malformation, and hepatopulmonary syndrome.2 It can occur even if right-sided chamber pressures are normal, and several mechanisms of the underlying pathophysiology have been described.3
Platypnea-orthodeoxia syndrome can be triggered by an event that causes a spontaneous transient elevation of right atrial pressure and pulmonary hypertension, such as our patient’s acute pulmonary embolism. Increased right-to-left shunting occurs in an upright position, causing preferential redirection of flow from the inferior vena cava through the interatrial septum and the patent foramen ovale.4
Our patient was elderly and, like one in every four people in the world, she had had a patent foramen ovale since the day she was born. Never causing a problem, it had remained undiagnosed until complicated by platypnea-orthodeoxia syndrome after her recent pulmonary embolism.
Methemoglobinemia. Methemoglobin has a lower affinity for oxygen than normal hemoglobin. Elevations usually occur with medications such as anesthetics and nitrates and can be diagnosed through an elevated level on arterial blood gas testing.
Our patient did not have elevated methemoglobin on her blood gas measurements on admission; therefore, this is unlikely to be the diagnosis.
CASE CONCLUDED
Percutaneous closure of the patent foramen ovale with a 30-mm Amplatzer Cribriform Occluder brought significant improvement in our patient’s functional status and arterial oxygenation saturation, and 2 weeks later at follow-up she no longer needed supplemental oxygen. TEE 6 months later showed an intact closure device and no interatrial shunting.
WHEN HYPOXEMIA DOES NOT RESPOND TO OXYGEN
In the intensive care unit, time is critical, and when hypoxia is refractory to high Fio2, shunting should be considered.
In the acute-care setting, platypnea-orthodeoxia syndrome can be identified quickly by pulse oximetry and serial blood gas measurements in the upright and supine positions. A drop in arterial oxygenation in the upright position vs the supine position helps confirm the diagnosis.
Other conditions in the differential diagnosis of this syndrome include recurrent pulmonary embolism, acute respiratory distress syndrome, interstitial pulmonary fibrosis, intrapulmonary shunting due to arteriovenous malformation, and diaphragm paralysis due to neuromuscular disease.
In our patient, positional blood gas measurements demonstrated a significant drop in arterial oxygen saturation from the supine to the upright position, raising our suspicion of shunting. It helped narrow the differential diagnosis and guided our selection of additional diagnostic tests.
The initial chest radiograph in our patient was normal. TTE did not reveal shunting and showed a normal right ventricular systolic pressure. TTE with agitated saline also failed to reveal shunting. Because of suboptimal positioning and image quality, TTE may miss the shunting physiology, and that is why we proceeded to positional TEE, which can better evaluate the hemodynamic effects of positional changes on patent foramen ovale and shunting.
MORE ABOUT PATENT FORAMEN OVALE
The prevalence of patent foramen ovale is estimated at 27% in the general population, but it is usually not symptomatic. It can be associated with atrial septal aneurysm and Chiari network malformations. When associated with atrial septal aneurysm, it carries a higher risk of stroke.5
Our patient had a large atrial septal aneurysm with a septal excursion of 2 cm as well as a history of thromboembolic stroke, which was likely associated with the patent foramen ovale and the atrial septal aneurysm.
Atrial septal aneurysm is rare, with a prevalence of 1% at autopsy and 1.9% by TTE. It is defined by a septal excursion of at least 10 mm and a base diameter of at least 15 mm and is more frequently detected on TEE than on TTE.6
Studies have shown that contrast and color Doppler TEE are superior to TTE for detecting patent foramen ovale.7 Tilt-table TEE with contrast enhancement has also been used to better demonstrate the morphology of the interatrial septum and the degree of shunting due to the separation between the septum primum and septum secundum causing the patent foramen ovale.8 Contrast-enhanced transcranial Doppler has also been shown comparable to contrast TEE to detect interatrial shunting. However, TEE provides additional anatomic information.9
In our patient, atrial septal aneurysm and patent foramen ovale were exaggerated by upright positioning, which opened the aneurysm and increased the shunting through the patent foramen ovale.
The treatment of choice in symptomatic patients with platypnea-orthodeoxia syndrome is directed at the underlying cause, in this case closure of the foramen ovale. This treatment has been shown to be safe and effective in these patients,10 but caution should be used when considering foramen ovale closure in patients with pulmonary hypertension.11
In patients with irreversible or severe pulmonary hypertension, closure of the patent foramen ovale can exacerbate right heart dysfunction and lead to right heart failure. There are situations when closure of a patent foramen ovale can be considered in pulmonary hypertension; however, each decision is individualized, and caution must be used. A detailed discussion is beyond the scope of this paper.
A thorough history and physical examination are important in differentiating the various causes of hypoxemia. Appropriate diagnostic testing is needed along with prompt treatment of the underlying cause of platypnea-orthodeoxia syndrome.
- Cheng TO. Mechanisms of platypnea-orthodeoxia: what causes water to flow uphill? Circulation 2002; 105:e47.
- Natalie AA, Nichols L, Bump GM. Platypnea-orthodeoxia, an uncommon presentation of patent foramen ovale. Am J Med Sci 2010; 339:78–80.
- Acharya SS, Kartan R. A case of orthodeoxia caused by an atrial septal aneurysm. Chest 2000; 118:871–874.
- Irwin B, Ray S. Patent foramen ovale—assessment and treatment. Cardiovasc Ther 2012; 30:e128–e135.
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995; 130:1083–1088.
- Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001; 38:613–623.
- Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992; 70:668–672.
- Roxas-Timonera M, Larracas C, Gersony D, Di Tullio M, Keller A, Homma S. Patent foramen ovale presenting as platypnea-orthodeoxia: diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr 2001; 14:1039–1041.
- Sloan MA, Alexandrov AV, Tegeler CH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004; 62:1468–1481.
- Blanche C, Noble S, Roffi M, et al. Platypnea-orthodeoxia syndrome in the elderly treated by percutaneous patent foramen ovale closure: a case series and literature review. Eur J Intern Med 2013; 24:813–817.
- Tobis J, Shenoda M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol 2012; 60:1722–1732.
An 85-year-old woman was brought to our intensive care unit because of worsening hypoxemia over the past day. About 3 weeks earlier she had been diagnosed with acute bilateral pulmonary emboli in the distal branches of the left and right lower lobes and right middle lobe, for which she was receiving anticoagulation therapy.
At presentation she had generalized fatigue and dyspnea at rest that was worse with exertion, but she denied having fever, chest pain, or cough. Her medical history included hypertension, hyperlipidemia, hypothyroidism, stage 1 breast cancer in remission, thromboembolic stroke, and myasthenia gravis. Before her hospital admission, she had been taking rosuvastatin, metoprolol tartrate, pyridostigmine, prednisone, furosemide, levothyroxine, and rivaroxaban. She did not smoke, she was retired, and she had not traveled recently.
Her blood pressure was 135/66 mm Hg, pulse 73 beats per minute, respiratory rate 16, temperature 35.4ºC (95.7ºF), and oxygen saturation 88% while receiving oxygen at 6 L/min via nasal cannula. Physical examination revealed mild edema in the lower extremities and basilar decreased breath sounds. She had no finger clubbing or cyanosis and was not using accessory muscles to breathe. Of note, her oxygen saturation remained more than 93% when she was recumbent but sharply dropped to less than 85% when she was upright.
Laboratory values
Results of initial laboratory testing were as follows:
- Sodium 138 mmol/L (reference range 132–148)
- Potassium 4.2 mmol/L (3.5–5.0)
- Chloride 99 mmol/L (98–111)
- Bicarbonate 29 mmol/L (23–32)
- Creatinine 0.52 mg/dL (0.7–1.4).
- White blood cell count 11.06 × 109/L (3.7–11.0)
- Hemoglobin 12.6 g/dL (12–16)
- Platelet count 211 × 109/L (150–400).
- International normalized ratio 1.4.
Electrocardiography and imaging studies
Standard 12-lead electrocardiography showed normal sinus rhythm with left axis deviation and left ventricular hypertrophy.
Chest radiography showed bilateral interstitial opacities and small pleural effusions.
Computed tomography (CT) of the chest with contrast, compared with a CT scan done 20 days earlier, showed that the pulmonary emboli had resolved.
Arterial blood gases
In view of her positional hypoxemia, blood for arterial blood gas measurements was drawn in the supine and upright positions.
Supine, with a fraction of inspired oxygen (Fio2) via high-flow nasal cannula of 45%, her values were:
- pH 7.45 (reference range 7.35–7.45)
- Pco2 34 mm Hg (36–46)
- Po2 81 mm Hg (85–95)
- Bicarbonate 23 mmol/L (22–26).
Upright, her hypoxemia was significantly worse:
- pH 7.46
- Pco2 33 mm Hg
- Po2 57 mm Hg
- Bicarbonate 23 mmol/L.
The methemoglobin level was normal on both measurements.
During her stay in the intensive care unit, she required up to 100% Fio2 because of persistent hypoxemia.
CAUSES OF HYPOXEMIA
1. So far, the patient’s laboratory tests and imaging studies point to which of the following as the most likely cause of her severe hypoxemia?
- Ventilation-perfusion (V/Q) mismatch
- Diffusion abnormality
- Hypoventilation
- Shunting
- None of the above
The arterial blood gas measurements suggested the possibility of shunting as the cause, although further imaging would be needed to confirm that.
V/Q mismatch can occur in respiratory failure due to pulmonary embolism, pulmonary edema, or shunting. If ventilation is preserved but perfusion is impaired, the V/Q ratio approaches infinity (dead-space ventilation), a situation that can be seen in pulmonary embolism. If perfusion is preserved and ventilation is impaired, the V/Q ratio approaches zero, which is consistent with a physiologic shunt.
Hypoxemia may improve in less severe forms of V/Q mismatch. In our patient, the repeat CT with contrast showed that her pulmonary embolism had resolved, so this is probably not the cause of her severe hypoxemia.
Diffusion abnormalities are due to defects in the lung parenchyma, such as in chronic obstructive pulmonary disease, interstitial lung disease, and lung fibrosis.
Hypoxemia from diffusion defects is usually aggravated by a precipitating factor that increases oxygen demand, and it usually improves with oxygen supplementation. This is unlikely in our patient, as she did not have a history of chronic interstitial lung disease and CT showed no evidence of severe lung parenchymal disease.
Hypoventilation is usually due to drugs that cause respiratory depression, to stroke, or to neuromuscular diseases such as myasthenia gravis that can cause respiratory muscle weakness. It results in elevation of Pco2 and, if not corrected, respiratory acidosis.
Our patient had a diagnosis of myasthenia gravis, though hypoventilation is unlikely in her case because she had a normal respiratory rate and low Pco2 values.
Shunting can be physiologic or anatomic and can occur in the heart or the lungs. In physiologic shunting, severe V/Q mismatch can occur when ventilation is affected, as in severe pulmonary edema and pneumonia. In anatomic shunting, a defect such as an atrial septal defect or a pulmonary arteriovenous malformation allows blood to bypass areas of ventilation from the venous to the arterial circulation, preventing it from being oxygenated. In true anatomic shunting, supplemental oxygen with 100% Fio2 has little effect, whereas in V/Q mismatch it can raise the arterial oxygen saturation.
Our patient’s radiograph did not suggest severe pneumonia or pulmonary edema, which makes these unlikely causes of her hypoxemia. At this point, because of her positional hypoxemia, further evaluation with contrast-enhanced echocardiography was needed to evaluate for anatomic shunting in the heart or lungs.
FURTHER TESTING
Transthoracic echocardiography (TTE) with agitated saline with a Valsalva maneuver was performed. Normally, no bubbles are seen in the left-sided chambers after intravenous injection of agitated saline contrast, whereas bubbles on the left side suggest an intracardiac or intrapulmonary shunt. In our patient, this test was negative, and her right ventricular systolic pressure was normal.
2. What further testing should be considered to evaluate our patient’s hypoxemia?
- High-resolution chest CT
- Transesophageal echocardiography (TEE)
- Pulmonary function testing
- Repeated arterial blood gas measurement
- Edrophonium testing
Repeat imaging with high-resolution CT would likely not provide additional information and would expose the patient to additional radiation without adding much clinical benefit.
TEE could help further evaluate the intracardiac anatomy and look for shunting, which may be missed on TTE because of suboptimal positioning or image quality.
Pulmonary function testing is useful in establishing the baseline function and impairment in respiratory volumes. If an acute myasthenic crisis is suspected, measuring the negative inspiratory force and the forced vital capacity can be useful in monitoring worsening respiratory muscle weakness and assessing the need for mechanical ventilation.
In our patient, it is unlikely that pulmonary function testing would help, since her acute respiratory failure was probably not caused by neuromuscular weakness.
Repeated arterial blood gas measurement would likely only confirm that the patient still has positional hypoxemia but would not help sort through the differential diagnosis.
Edrophonium testing is useful in diagnosing myasthenia gravis and differentiating it from other neuromuscular diseases, such as Lambert-Eaton syndrome. Edrophonium, a reversible acetylcholinesterase inhibitor, prevents degradation of acetylcholine and prolongs its effect at the synaptic cleft, thus improving muscle weakness.
Our patient has already been diagnosed with myasthenia gravis, so this test is not likely to uncover the cause of her hypoxemia.
Because we still strongly suspected a shunt, TEE was performed with intravenous injection of agitated saline. TEE with the patient upright revealed intracardiac right-to-left shunting through a patent foramen ovale. The midesophageal view after saline injection showed a large interatrial septal aneurysm with total excursion of 2 cm, and right-to-left shunting within the first beat, consistent with an intracardiac shunt (Figure 1). Color Doppler imaging (Figure 2) demonstrated turbulent flow through the patent foramen ovale, consistent with right-to-left shunting, and also showed the patent foramen ovale in a closed position (Figure 3).



3. Which is now most likely the cause of our patient’s hypoxemia?
- Chronic thromboembolic pulmonary hypertension
- Myasthenic crisis
- Platypnea-orthodeoxia syndrome due to the patent foramen ovale
- Methemoglobinemia
Chronic thromboembolic pulmonary hypertension is usually a long-term result of untreated or inadequately treated thromboembolic disease (eg, pulmonary emboli), which causes vascular remodeling and pulmonary arteriopathy, which in turn leads to increased pulmonary vascular resistance and pulmonary hypertension.
This is unlikely the cause of our patient’s acute hypoxemia, as her symptoms did not suggest it. Moreover, an elevated right ventricular systolic pressure on TTE would suggest pulmonary hypertension, but TTE did not show this, and repeat chest CT indicated that her pulmonary embolism had been adequately treated and had resolved. A V/Q scan and right heart catheterization would help rule out chronic thromboembolic pulmonary hypertension, although these were not done in our patient.
Myasthenic crisis is the progressive fatiguing and paralysis of respiratory muscles ultimately requiring mechanical ventilation to sustain life. It is often brought on by infection or drug therapy.
Our patient did not require intubation and she had no signs or symptoms of myasthenic crisis such as ptosis, dysphagia, or dysarthria. She had a negative inspiratory force of −21 cm H2O, and pulmonary function testing 4 days before her hospital admission had shown a forced vital capacity of 1.84 L, making myasthenic crisis an unlikely cause of her respiratory failure.
Platypnea-orthodeoxia syndrome is a syndrome of dyspnea (platypnea) and hypoxemia (orthodeoxia) that is induced by sitting upright or standing and resolves when lying down. It is a result of right-to-left intracardiac or intrapulmonary shunting in the presence of an anatomic defect and a functional element causing redirection of shunt flow through the anatomic defect in an upright position.1 It is associated with specific cardiac, pulmonary, and hepatic diseases, such as atrial septal defect, pulmonary arteriovenous malformation, and hepatopulmonary syndrome.2 It can occur even if right-sided chamber pressures are normal, and several mechanisms of the underlying pathophysiology have been described.3
Platypnea-orthodeoxia syndrome can be triggered by an event that causes a spontaneous transient elevation of right atrial pressure and pulmonary hypertension, such as our patient’s acute pulmonary embolism. Increased right-to-left shunting occurs in an upright position, causing preferential redirection of flow from the inferior vena cava through the interatrial septum and the patent foramen ovale.4
Our patient was elderly and, like one in every four people in the world, she had had a patent foramen ovale since the day she was born. Never causing a problem, it had remained undiagnosed until complicated by platypnea-orthodeoxia syndrome after her recent pulmonary embolism.
Methemoglobinemia. Methemoglobin has a lower affinity for oxygen than normal hemoglobin. Elevations usually occur with medications such as anesthetics and nitrates and can be diagnosed through an elevated level on arterial blood gas testing.
Our patient did not have elevated methemoglobin on her blood gas measurements on admission; therefore, this is unlikely to be the diagnosis.
CASE CONCLUDED
Percutaneous closure of the patent foramen ovale with a 30-mm Amplatzer Cribriform Occluder brought significant improvement in our patient’s functional status and arterial oxygenation saturation, and 2 weeks later at follow-up she no longer needed supplemental oxygen. TEE 6 months later showed an intact closure device and no interatrial shunting.
WHEN HYPOXEMIA DOES NOT RESPOND TO OXYGEN
In the intensive care unit, time is critical, and when hypoxia is refractory to high Fio2, shunting should be considered.
In the acute-care setting, platypnea-orthodeoxia syndrome can be identified quickly by pulse oximetry and serial blood gas measurements in the upright and supine positions. A drop in arterial oxygenation in the upright position vs the supine position helps confirm the diagnosis.
Other conditions in the differential diagnosis of this syndrome include recurrent pulmonary embolism, acute respiratory distress syndrome, interstitial pulmonary fibrosis, intrapulmonary shunting due to arteriovenous malformation, and diaphragm paralysis due to neuromuscular disease.
In our patient, positional blood gas measurements demonstrated a significant drop in arterial oxygen saturation from the supine to the upright position, raising our suspicion of shunting. It helped narrow the differential diagnosis and guided our selection of additional diagnostic tests.
The initial chest radiograph in our patient was normal. TTE did not reveal shunting and showed a normal right ventricular systolic pressure. TTE with agitated saline also failed to reveal shunting. Because of suboptimal positioning and image quality, TTE may miss the shunting physiology, and that is why we proceeded to positional TEE, which can better evaluate the hemodynamic effects of positional changes on patent foramen ovale and shunting.
MORE ABOUT PATENT FORAMEN OVALE
The prevalence of patent foramen ovale is estimated at 27% in the general population, but it is usually not symptomatic. It can be associated with atrial septal aneurysm and Chiari network malformations. When associated with atrial septal aneurysm, it carries a higher risk of stroke.5
Our patient had a large atrial septal aneurysm with a septal excursion of 2 cm as well as a history of thromboembolic stroke, which was likely associated with the patent foramen ovale and the atrial septal aneurysm.
Atrial septal aneurysm is rare, with a prevalence of 1% at autopsy and 1.9% by TTE. It is defined by a septal excursion of at least 10 mm and a base diameter of at least 15 mm and is more frequently detected on TEE than on TTE.6
Studies have shown that contrast and color Doppler TEE are superior to TTE for detecting patent foramen ovale.7 Tilt-table TEE with contrast enhancement has also been used to better demonstrate the morphology of the interatrial septum and the degree of shunting due to the separation between the septum primum and septum secundum causing the patent foramen ovale.8 Contrast-enhanced transcranial Doppler has also been shown comparable to contrast TEE to detect interatrial shunting. However, TEE provides additional anatomic information.9
In our patient, atrial septal aneurysm and patent foramen ovale were exaggerated by upright positioning, which opened the aneurysm and increased the shunting through the patent foramen ovale.
The treatment of choice in symptomatic patients with platypnea-orthodeoxia syndrome is directed at the underlying cause, in this case closure of the foramen ovale. This treatment has been shown to be safe and effective in these patients,10 but caution should be used when considering foramen ovale closure in patients with pulmonary hypertension.11
In patients with irreversible or severe pulmonary hypertension, closure of the patent foramen ovale can exacerbate right heart dysfunction and lead to right heart failure. There are situations when closure of a patent foramen ovale can be considered in pulmonary hypertension; however, each decision is individualized, and caution must be used. A detailed discussion is beyond the scope of this paper.
A thorough history and physical examination are important in differentiating the various causes of hypoxemia. Appropriate diagnostic testing is needed along with prompt treatment of the underlying cause of platypnea-orthodeoxia syndrome.
An 85-year-old woman was brought to our intensive care unit because of worsening hypoxemia over the past day. About 3 weeks earlier she had been diagnosed with acute bilateral pulmonary emboli in the distal branches of the left and right lower lobes and right middle lobe, for which she was receiving anticoagulation therapy.
At presentation she had generalized fatigue and dyspnea at rest that was worse with exertion, but she denied having fever, chest pain, or cough. Her medical history included hypertension, hyperlipidemia, hypothyroidism, stage 1 breast cancer in remission, thromboembolic stroke, and myasthenia gravis. Before her hospital admission, she had been taking rosuvastatin, metoprolol tartrate, pyridostigmine, prednisone, furosemide, levothyroxine, and rivaroxaban. She did not smoke, she was retired, and she had not traveled recently.
Her blood pressure was 135/66 mm Hg, pulse 73 beats per minute, respiratory rate 16, temperature 35.4ºC (95.7ºF), and oxygen saturation 88% while receiving oxygen at 6 L/min via nasal cannula. Physical examination revealed mild edema in the lower extremities and basilar decreased breath sounds. She had no finger clubbing or cyanosis and was not using accessory muscles to breathe. Of note, her oxygen saturation remained more than 93% when she was recumbent but sharply dropped to less than 85% when she was upright.
Laboratory values
Results of initial laboratory testing were as follows:
- Sodium 138 mmol/L (reference range 132–148)
- Potassium 4.2 mmol/L (3.5–5.0)
- Chloride 99 mmol/L (98–111)
- Bicarbonate 29 mmol/L (23–32)
- Creatinine 0.52 mg/dL (0.7–1.4).
- White blood cell count 11.06 × 109/L (3.7–11.0)
- Hemoglobin 12.6 g/dL (12–16)
- Platelet count 211 × 109/L (150–400).
- International normalized ratio 1.4.
Electrocardiography and imaging studies
Standard 12-lead electrocardiography showed normal sinus rhythm with left axis deviation and left ventricular hypertrophy.
Chest radiography showed bilateral interstitial opacities and small pleural effusions.
Computed tomography (CT) of the chest with contrast, compared with a CT scan done 20 days earlier, showed that the pulmonary emboli had resolved.
Arterial blood gases
In view of her positional hypoxemia, blood for arterial blood gas measurements was drawn in the supine and upright positions.
Supine, with a fraction of inspired oxygen (Fio2) via high-flow nasal cannula of 45%, her values were:
- pH 7.45 (reference range 7.35–7.45)
- Pco2 34 mm Hg (36–46)
- Po2 81 mm Hg (85–95)
- Bicarbonate 23 mmol/L (22–26).
Upright, her hypoxemia was significantly worse:
- pH 7.46
- Pco2 33 mm Hg
- Po2 57 mm Hg
- Bicarbonate 23 mmol/L.
The methemoglobin level was normal on both measurements.
During her stay in the intensive care unit, she required up to 100% Fio2 because of persistent hypoxemia.
CAUSES OF HYPOXEMIA
1. So far, the patient’s laboratory tests and imaging studies point to which of the following as the most likely cause of her severe hypoxemia?
- Ventilation-perfusion (V/Q) mismatch
- Diffusion abnormality
- Hypoventilation
- Shunting
- None of the above
The arterial blood gas measurements suggested the possibility of shunting as the cause, although further imaging would be needed to confirm that.
V/Q mismatch can occur in respiratory failure due to pulmonary embolism, pulmonary edema, or shunting. If ventilation is preserved but perfusion is impaired, the V/Q ratio approaches infinity (dead-space ventilation), a situation that can be seen in pulmonary embolism. If perfusion is preserved and ventilation is impaired, the V/Q ratio approaches zero, which is consistent with a physiologic shunt.
Hypoxemia may improve in less severe forms of V/Q mismatch. In our patient, the repeat CT with contrast showed that her pulmonary embolism had resolved, so this is probably not the cause of her severe hypoxemia.
Diffusion abnormalities are due to defects in the lung parenchyma, such as in chronic obstructive pulmonary disease, interstitial lung disease, and lung fibrosis.
Hypoxemia from diffusion defects is usually aggravated by a precipitating factor that increases oxygen demand, and it usually improves with oxygen supplementation. This is unlikely in our patient, as she did not have a history of chronic interstitial lung disease and CT showed no evidence of severe lung parenchymal disease.
Hypoventilation is usually due to drugs that cause respiratory depression, to stroke, or to neuromuscular diseases such as myasthenia gravis that can cause respiratory muscle weakness. It results in elevation of Pco2 and, if not corrected, respiratory acidosis.
Our patient had a diagnosis of myasthenia gravis, though hypoventilation is unlikely in her case because she had a normal respiratory rate and low Pco2 values.
Shunting can be physiologic or anatomic and can occur in the heart or the lungs. In physiologic shunting, severe V/Q mismatch can occur when ventilation is affected, as in severe pulmonary edema and pneumonia. In anatomic shunting, a defect such as an atrial septal defect or a pulmonary arteriovenous malformation allows blood to bypass areas of ventilation from the venous to the arterial circulation, preventing it from being oxygenated. In true anatomic shunting, supplemental oxygen with 100% Fio2 has little effect, whereas in V/Q mismatch it can raise the arterial oxygen saturation.
Our patient’s radiograph did not suggest severe pneumonia or pulmonary edema, which makes these unlikely causes of her hypoxemia. At this point, because of her positional hypoxemia, further evaluation with contrast-enhanced echocardiography was needed to evaluate for anatomic shunting in the heart or lungs.
FURTHER TESTING
Transthoracic echocardiography (TTE) with agitated saline with a Valsalva maneuver was performed. Normally, no bubbles are seen in the left-sided chambers after intravenous injection of agitated saline contrast, whereas bubbles on the left side suggest an intracardiac or intrapulmonary shunt. In our patient, this test was negative, and her right ventricular systolic pressure was normal.
2. What further testing should be considered to evaluate our patient’s hypoxemia?
- High-resolution chest CT
- Transesophageal echocardiography (TEE)
- Pulmonary function testing
- Repeated arterial blood gas measurement
- Edrophonium testing
Repeat imaging with high-resolution CT would likely not provide additional information and would expose the patient to additional radiation without adding much clinical benefit.
TEE could help further evaluate the intracardiac anatomy and look for shunting, which may be missed on TTE because of suboptimal positioning or image quality.
Pulmonary function testing is useful in establishing the baseline function and impairment in respiratory volumes. If an acute myasthenic crisis is suspected, measuring the negative inspiratory force and the forced vital capacity can be useful in monitoring worsening respiratory muscle weakness and assessing the need for mechanical ventilation.
In our patient, it is unlikely that pulmonary function testing would help, since her acute respiratory failure was probably not caused by neuromuscular weakness.
Repeated arterial blood gas measurement would likely only confirm that the patient still has positional hypoxemia but would not help sort through the differential diagnosis.
Edrophonium testing is useful in diagnosing myasthenia gravis and differentiating it from other neuromuscular diseases, such as Lambert-Eaton syndrome. Edrophonium, a reversible acetylcholinesterase inhibitor, prevents degradation of acetylcholine and prolongs its effect at the synaptic cleft, thus improving muscle weakness.
Our patient has already been diagnosed with myasthenia gravis, so this test is not likely to uncover the cause of her hypoxemia.
Because we still strongly suspected a shunt, TEE was performed with intravenous injection of agitated saline. TEE with the patient upright revealed intracardiac right-to-left shunting through a patent foramen ovale. The midesophageal view after saline injection showed a large interatrial septal aneurysm with total excursion of 2 cm, and right-to-left shunting within the first beat, consistent with an intracardiac shunt (Figure 1). Color Doppler imaging (Figure 2) demonstrated turbulent flow through the patent foramen ovale, consistent with right-to-left shunting, and also showed the patent foramen ovale in a closed position (Figure 3).



3. Which is now most likely the cause of our patient’s hypoxemia?
- Chronic thromboembolic pulmonary hypertension
- Myasthenic crisis
- Platypnea-orthodeoxia syndrome due to the patent foramen ovale
- Methemoglobinemia
Chronic thromboembolic pulmonary hypertension is usually a long-term result of untreated or inadequately treated thromboembolic disease (eg, pulmonary emboli), which causes vascular remodeling and pulmonary arteriopathy, which in turn leads to increased pulmonary vascular resistance and pulmonary hypertension.
This is unlikely the cause of our patient’s acute hypoxemia, as her symptoms did not suggest it. Moreover, an elevated right ventricular systolic pressure on TTE would suggest pulmonary hypertension, but TTE did not show this, and repeat chest CT indicated that her pulmonary embolism had been adequately treated and had resolved. A V/Q scan and right heart catheterization would help rule out chronic thromboembolic pulmonary hypertension, although these were not done in our patient.
Myasthenic crisis is the progressive fatiguing and paralysis of respiratory muscles ultimately requiring mechanical ventilation to sustain life. It is often brought on by infection or drug therapy.
Our patient did not require intubation and she had no signs or symptoms of myasthenic crisis such as ptosis, dysphagia, or dysarthria. She had a negative inspiratory force of −21 cm H2O, and pulmonary function testing 4 days before her hospital admission had shown a forced vital capacity of 1.84 L, making myasthenic crisis an unlikely cause of her respiratory failure.
Platypnea-orthodeoxia syndrome is a syndrome of dyspnea (platypnea) and hypoxemia (orthodeoxia) that is induced by sitting upright or standing and resolves when lying down. It is a result of right-to-left intracardiac or intrapulmonary shunting in the presence of an anatomic defect and a functional element causing redirection of shunt flow through the anatomic defect in an upright position.1 It is associated with specific cardiac, pulmonary, and hepatic diseases, such as atrial septal defect, pulmonary arteriovenous malformation, and hepatopulmonary syndrome.2 It can occur even if right-sided chamber pressures are normal, and several mechanisms of the underlying pathophysiology have been described.3
Platypnea-orthodeoxia syndrome can be triggered by an event that causes a spontaneous transient elevation of right atrial pressure and pulmonary hypertension, such as our patient’s acute pulmonary embolism. Increased right-to-left shunting occurs in an upright position, causing preferential redirection of flow from the inferior vena cava through the interatrial septum and the patent foramen ovale.4
Our patient was elderly and, like one in every four people in the world, she had had a patent foramen ovale since the day she was born. Never causing a problem, it had remained undiagnosed until complicated by platypnea-orthodeoxia syndrome after her recent pulmonary embolism.
Methemoglobinemia. Methemoglobin has a lower affinity for oxygen than normal hemoglobin. Elevations usually occur with medications such as anesthetics and nitrates and can be diagnosed through an elevated level on arterial blood gas testing.
Our patient did not have elevated methemoglobin on her blood gas measurements on admission; therefore, this is unlikely to be the diagnosis.
CASE CONCLUDED
Percutaneous closure of the patent foramen ovale with a 30-mm Amplatzer Cribriform Occluder brought significant improvement in our patient’s functional status and arterial oxygenation saturation, and 2 weeks later at follow-up she no longer needed supplemental oxygen. TEE 6 months later showed an intact closure device and no interatrial shunting.
WHEN HYPOXEMIA DOES NOT RESPOND TO OXYGEN
In the intensive care unit, time is critical, and when hypoxia is refractory to high Fio2, shunting should be considered.
In the acute-care setting, platypnea-orthodeoxia syndrome can be identified quickly by pulse oximetry and serial blood gas measurements in the upright and supine positions. A drop in arterial oxygenation in the upright position vs the supine position helps confirm the diagnosis.
Other conditions in the differential diagnosis of this syndrome include recurrent pulmonary embolism, acute respiratory distress syndrome, interstitial pulmonary fibrosis, intrapulmonary shunting due to arteriovenous malformation, and diaphragm paralysis due to neuromuscular disease.
In our patient, positional blood gas measurements demonstrated a significant drop in arterial oxygen saturation from the supine to the upright position, raising our suspicion of shunting. It helped narrow the differential diagnosis and guided our selection of additional diagnostic tests.
The initial chest radiograph in our patient was normal. TTE did not reveal shunting and showed a normal right ventricular systolic pressure. TTE with agitated saline also failed to reveal shunting. Because of suboptimal positioning and image quality, TTE may miss the shunting physiology, and that is why we proceeded to positional TEE, which can better evaluate the hemodynamic effects of positional changes on patent foramen ovale and shunting.
MORE ABOUT PATENT FORAMEN OVALE
The prevalence of patent foramen ovale is estimated at 27% in the general population, but it is usually not symptomatic. It can be associated with atrial septal aneurysm and Chiari network malformations. When associated with atrial septal aneurysm, it carries a higher risk of stroke.5
Our patient had a large atrial septal aneurysm with a septal excursion of 2 cm as well as a history of thromboembolic stroke, which was likely associated with the patent foramen ovale and the atrial septal aneurysm.
Atrial septal aneurysm is rare, with a prevalence of 1% at autopsy and 1.9% by TTE. It is defined by a septal excursion of at least 10 mm and a base diameter of at least 15 mm and is more frequently detected on TEE than on TTE.6
Studies have shown that contrast and color Doppler TEE are superior to TTE for detecting patent foramen ovale.7 Tilt-table TEE with contrast enhancement has also been used to better demonstrate the morphology of the interatrial septum and the degree of shunting due to the separation between the septum primum and septum secundum causing the patent foramen ovale.8 Contrast-enhanced transcranial Doppler has also been shown comparable to contrast TEE to detect interatrial shunting. However, TEE provides additional anatomic information.9
In our patient, atrial septal aneurysm and patent foramen ovale were exaggerated by upright positioning, which opened the aneurysm and increased the shunting through the patent foramen ovale.
The treatment of choice in symptomatic patients with platypnea-orthodeoxia syndrome is directed at the underlying cause, in this case closure of the foramen ovale. This treatment has been shown to be safe and effective in these patients,10 but caution should be used when considering foramen ovale closure in patients with pulmonary hypertension.11
In patients with irreversible or severe pulmonary hypertension, closure of the patent foramen ovale can exacerbate right heart dysfunction and lead to right heart failure. There are situations when closure of a patent foramen ovale can be considered in pulmonary hypertension; however, each decision is individualized, and caution must be used. A detailed discussion is beyond the scope of this paper.
A thorough history and physical examination are important in differentiating the various causes of hypoxemia. Appropriate diagnostic testing is needed along with prompt treatment of the underlying cause of platypnea-orthodeoxia syndrome.
- Cheng TO. Mechanisms of platypnea-orthodeoxia: what causes water to flow uphill? Circulation 2002; 105:e47.
- Natalie AA, Nichols L, Bump GM. Platypnea-orthodeoxia, an uncommon presentation of patent foramen ovale. Am J Med Sci 2010; 339:78–80.
- Acharya SS, Kartan R. A case of orthodeoxia caused by an atrial septal aneurysm. Chest 2000; 118:871–874.
- Irwin B, Ray S. Patent foramen ovale—assessment and treatment. Cardiovasc Ther 2012; 30:e128–e135.
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995; 130:1083–1088.
- Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001; 38:613–623.
- Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992; 70:668–672.
- Roxas-Timonera M, Larracas C, Gersony D, Di Tullio M, Keller A, Homma S. Patent foramen ovale presenting as platypnea-orthodeoxia: diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr 2001; 14:1039–1041.
- Sloan MA, Alexandrov AV, Tegeler CH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004; 62:1468–1481.
- Blanche C, Noble S, Roffi M, et al. Platypnea-orthodeoxia syndrome in the elderly treated by percutaneous patent foramen ovale closure: a case series and literature review. Eur J Intern Med 2013; 24:813–817.
- Tobis J, Shenoda M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol 2012; 60:1722–1732.
- Cheng TO. Mechanisms of platypnea-orthodeoxia: what causes water to flow uphill? Circulation 2002; 105:e47.
- Natalie AA, Nichols L, Bump GM. Platypnea-orthodeoxia, an uncommon presentation of patent foramen ovale. Am J Med Sci 2010; 339:78–80.
- Acharya SS, Kartan R. A case of orthodeoxia caused by an atrial septal aneurysm. Chest 2000; 118:871–874.
- Irwin B, Ray S. Patent foramen ovale—assessment and treatment. Cardiovasc Ther 2012; 30:e128–e135.
- Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J 1995; 130:1083–1088.
- Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001; 38:613–623.
- Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992; 70:668–672.
- Roxas-Timonera M, Larracas C, Gersony D, Di Tullio M, Keller A, Homma S. Patent foramen ovale presenting as platypnea-orthodeoxia: diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr 2001; 14:1039–1041.
- Sloan MA, Alexandrov AV, Tegeler CH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004; 62:1468–1481.
- Blanche C, Noble S, Roffi M, et al. Platypnea-orthodeoxia syndrome in the elderly treated by percutaneous patent foramen ovale closure: a case series and literature review. Eur J Intern Med 2013; 24:813–817.
- Tobis J, Shenoda M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol 2012; 60:1722–1732.
A continuous cardiac murmur
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
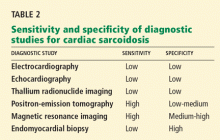
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.
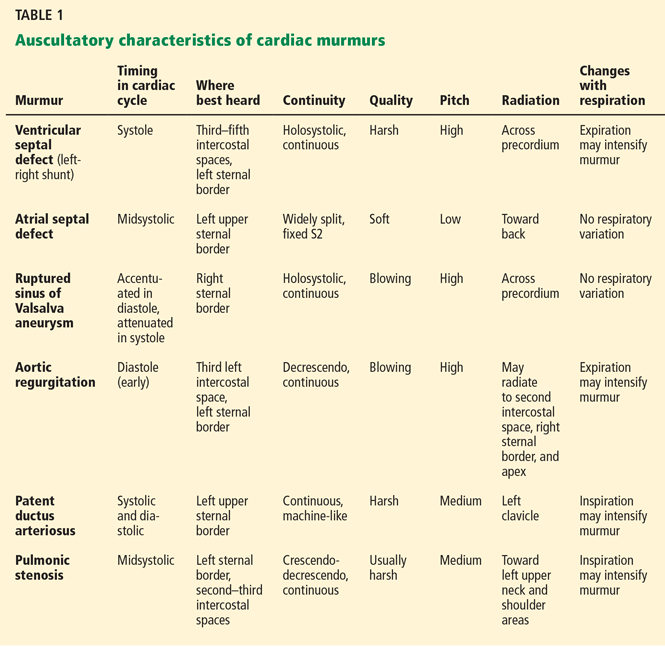
Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
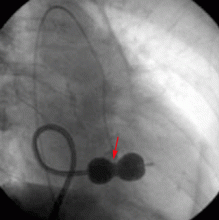
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
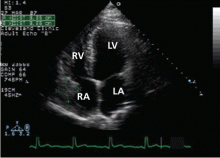
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
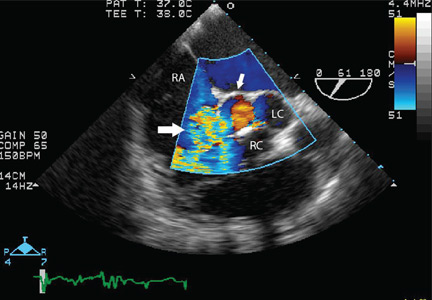
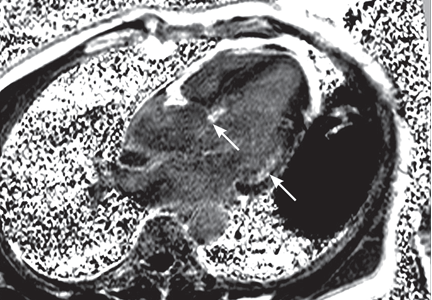
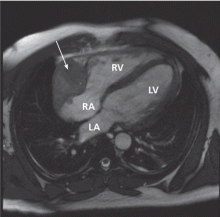
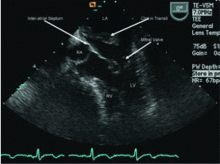
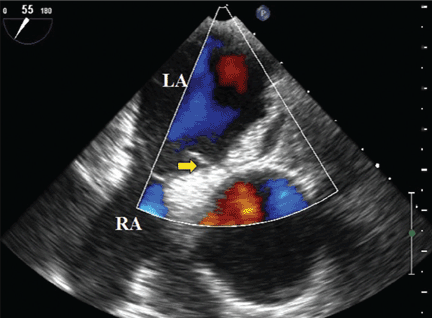
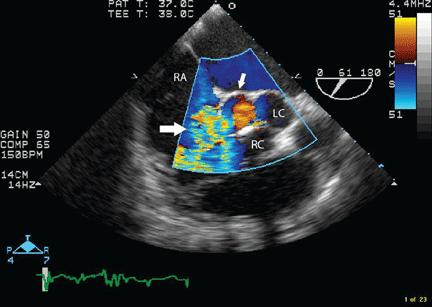
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.
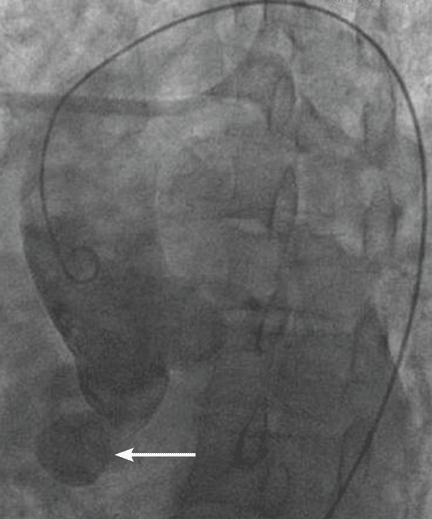
The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.
This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.

Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.



The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.

This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.

Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.



The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.

This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
A middle-aged man with progressive fatigue
A 61-year-old white man presents with progressive fatigue, which began several months ago and has accelerated in severity over the past week. He says he has had no shortness of breath, chest pain, or symptoms of heart failure, but he has noticed a decrease in exertional capacity and now has trouble completing his daily 5-mile walk.
He saw his primary physician, who obtained an electrocardiogram that showed a new left bundle branch block. Transthoracic echocardiography indicated that his left ventricular ejection fraction, which was 60% a year earlier, was now 35%.
He has hypertension, dyslipidemia, type 2 diabetes, and chronic kidney disease. Although he was previously morbidly obese, he has lost more than 100 pounds with diet and exercise over the past 10 years. He also used to smoke; in fact, he has a 30-pack-year history, but he quit in 1987. He has a family history of premature coronary artery disease.
Physical examination. His heart rate is 75 beats per minute, blood pressure 142/85 mm Hg, and blood oxygen saturation 96% while breathing room air. He weighs 169 pounds (76.6 kg) and he is 6 feet tall (182.9 cm), so his body mass index is 22.9 kg/m2.
Electrocardiography reveals sinus rhythm with a left bundle branch block and left axis deviation (Figure 1), which were not present 1 year ago.
Chest roentgenography is normal.
A WORRISOME PICTURE
1. Which of the following is associated with left bundle branch block?
- Myocardial injury
- Hypertension
- Aortic stenosis
- Intrinsic conduction system disease
- All of the above
All of the above are true. For left bundle branch block to be diagnosed, the rhythm must be supraventricular and the QRS duration must be 120 ms or more. There should be a QS or RS complex in V1 and a monophasic R wave in I and V6. Also, the T wave should be deflected opposite the terminal deflection of the QRS complex. This is known as appropriate T-wave discordance with bundle branch block. A concordant T wave is nonspecific but suggests ischemia or myocardial infarction.
Potential causes of a new left bundle branch block include hypertension, acute myocardial infarction, aortic stenosis, and conduction system disease. A new left bundle branch block with a concomitant decrease in ejection fraction, especially in a patient with cardiac risk factors, is very worrisome, raising the possibility of ischemic heart disease.
MORE CARDIAC TESTING
The patient undergoes more cardiac testing.
Transthoracic echocardiography is done again. The left ventricle is normal in size, but the ejection fraction is 35%. In addition, stage 1 diastolic dysfunction (abnormal relaxation) and evidence of mechanical dyssynchrony (disruption in the normal sequence of activation and contraction of segments of the left ventricular wall) are seen. The right ventricle is normal in size and function. There is trivial mitral regurgitation and mild tricuspid regurgitation with normal right-sided pressures.
A gated rubidium-82 dipyridamole stress test yields no evidence of a fixed or reversible perfusion defect.
Left heart catheterization reveals angiographically normal coronary arteries.
Magnetic resonance imaging (MRI) shows a moderately hypertrophied left ventricle with moderately to severely depressed systolic function (left ventricular ejection fraction 27%). The left ventricle appears dyssynchronous. Delayed-enhancement imaging reveals patchy delayed enhancement in the basal septum and the basal inferolateral walls. These findings suggest cardiac sarcoidosis, with a sensitivity of nearly 100% and a specificity of approximately 78%.1
SARCOIDOSIS IS A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Almost any organ can be affected, but it most commonly involves the respiratory and lymphatic systems.2 Although infectious, environmental, and genetic factors have been implicated, the cause remains unknown. The prevalence is approximately 20 per 100,000, being higher in black3 and Japanese 4 populations.
CARDIAC SARCOIDOSIS
2. What percentage of patients with sarcoidosis have cardiac involvement?
- 10%–20%
- 20%–30%
- 50%
- 80%
Cardiac involvement is seen in 20% to 30% of patients with sarcoidosis.5–7 However, most cases are subclinical, and symptomatic cardiac involvement is present in only about 5% of patients with systemic sarcoidosis.8 Isolated cardiac sarcoidosis has been described in case reports but is rare.9
The clinical manifestations of cardiac sarcoidosis depend on the location and extent of granulomatous inflammation. In a necropsy study of 113 patients with cardiac sarcoidosis, the left ventricular free wall was the most common location, followed by the interventricular septum.10
3. How does cardiac sarcoidosis most commonly present?
- Conduction abnormalities
- Ventricular tachycardia
- Cardiomyopathy
- Sudden death
- None of the above
Common presentations of cardiac sarcoidosis include conduction system disease and arrhythmias (which can sometimes result in sudden death), and heart failure.
Conduction abnormalities due to granulomas (in the active phase of sarcoidosis) and fibrosis (in the fibrotic phase) in the atrioventricular node or bundle of His are the most common presentation of cardiac sarcoidosis.9 These lesions may result in relatively benign first-degree heart block or may be as potentially devastating as complete heart block.
In almost all patients with conduction abnormalities, the basal interventricular septum is involved.11 Patients who develop complete heart block from sarcoidosis tend to be younger than those with idiopathic heart block. Therefore, complete heart block in a young patient should raise the possibility of this diagnosis. 12
Ventricular tachycardia (sustained or nonsustained) and ventricular premature beats are the second most common presentation. Up to 22% of patients with sarcoidosis have electrocardiographic evidence of ventricular arrythmias. 13 The cause is believed to be myocardial scar tissue resulting from the sarcoid granulomas, leading to electrical reentry.14 Sudden death due to ventricular tachyarrhythmias or conduction blocks accounts for 25% to 65% of deaths from cardiac sarcoidosis.9,15,16
Heart failure may result from sarcoidosis when there is extensive granulomatous disease in the myocardium. Depending on the location of the granulomas, both systolic and diastolic dysfunction can occur. In severe cases, extensive granulomas can cause left ventricular aneurysms.
The diagnosis of cardiac sarcoidosis as the cause of heart failure can be difficult to establish, especially in patients without evidence of sarcoidosis elsewhere. Such patients are often given a diagnosis of idiopathic dilated cardiomyopathy. However, compared with patients with idiopathic dilated cardiomyopathy, those with cardiac sarcoidosis have a greater incidence of advanced atrioventricular block, abnormal wall thickness, focal wall motion abnormalities, and perfusion defects of the anteroseptal and apical regions.17
Progressive heart failure is the second most frequent cause of death (after sudden death) and accounts for 25% to 75% of sarcoid-related cardiac deaths.9,18,19
DIAGNOSING CARDIAC SARCOIDOSIS
4. How is cardiac sarcoidosis diagnosed?
- Electrocardiography
- Echocardiography
- Computed tomography
- Endomyocardial biopsy
- There are no guidelines for diagnosis
Given the variable extent and location of granulomas in sarcoidosis, the diagnosis of cardiac sarcoidosis is often challenging.
To make the diagnosis of sarcoidosis in general, the American Thoracic Society2 says that the clinical picture should be compatible with this diagnosis, noncaseating granulomas should be histologically confirmed, and other diseases capable of producing a similar clinical or histologic picture should be excluded.
A newer diagnostic tool, the Sarcoidosis Three-Dimensional Assessment Instrument,20 incorporates two earlier tools.20,21 It assesses three axes: organ involvement, sarcoidosis severity, and sarcoidosis activity and categorizes the diagnosis of sarcoidosis as “definite,” “probable,” or “possible.”20
In Japan, where sarcoidosis is more common, the Ministry of Health and Welfare22 says that cardiac sarcoidosis can be diagnosed histologically if operative or endomyocardial biopsy specimens contain noncaseating granuloma. In addition, the diagnosis can be suspected in patients who have a histologic diagnosis of extracardiac sarcoidosis if the first item in the list below and one or more of the rest are present:
- Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contractions (> grade 2 of the Lown classification), or Q or ST-T wave abnormalities
- Abnormal wall motion, regional wall thinning, or dilation of the left ventricle on echocardiography
- Perfusion defects on thallium 201 myocardial scintigraphy or abnormal accumulation of gallium citrate Ga 67 or technetium 99m on myocardial scintigraphy
- Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed left ventricular ejection fraction on cardiac catheterization
- Nonspecific interstitial fibrosis or cellular infiltration on myocardial biopsy.
The current diagnostic guidelines from the American Thoracic Society2 and the Japanese Ministry of Health and Welfare22 and the Sarcoidosis Three-Dimensional Assessment Instrument,20 however, do not incorporate newer imaging studies as part of their criteria.
A DEFINITIVE DIAGNOSIS
5. Endomyocardial biopsy often provides the definitive diagnosis of cardiac sarcoidosis.
- True
- False
False. Endomyocardial biopsy often fails to reveal noncaseating granulomas, which have a patchy distribution.13 Table 2 summarizes the accuracy of tests for cardiac sarcoidosis.
Electrocardiography is abnormal in up to 50% of patients with sarcoidosis,23 reflecting the conduction disease or arrhythmias commonly seen in cardiac involvement.
Chest radiography classically shows hilar lymphadenopathy and interstitial disease, and may show cardiomegaly, pericardial effusion, or left ventricular aneurysm.
Echocardiography is nonspecific for sarcoid disease, but granulomatous involvement and scar tissue of cardiac tissue may appear hyperechogenic, particularly in the ventricular septum or left ventricular free wall.24
Angiography. Primary sarcoidosis rarely involves the coronary arteries,25 so angiography is most useful in excluding the diagnosis of atherosclerotic coronary artery disease.
Radionuclide imaging with thallium 201 in patients with suspected cardiac sarcoidosis may be useful to suggest myocardial involvement and to exclude cardiac dysfunction secondary to coronary artery disease. Segmental areas with defective thallium 201 uptake correspond to fibrogranulomatous tissue. In resting images, the pattern may be similar to that seen in coronary artery disease. However, during exercise, perfusion defects increase in patients who have ischemia but actually decrease in those with cardiac sarcoidosis.26
Nevertheless, some conclude that thallium scanning is too nonspecific for it to be used as a diagnostic or screening test.27,28 The combined use of thallium 201 and gallium 67 may better detect cardiac sarcoidosis, as gallium is taken up in areas of active inflammation.
Positron-emission tomography (PET) with fluorodeoxyglucose F 18 (FDG), with the patient fasting, appears to be useful in detecting the early inflammation of cardiac sarcoidosis29–34 and monitoring disease activity.30,31 FDG is a glucose analogue that is taken up by granulomatous tissue in the myocardium.34 The uptake in cardiac sarcoidosis is in a focal distribution.30,31,34 The abnormal FDG uptake resolves with steroid treatment.31,32
MRI has promise for diagnosing cardiac sarcoidosis. With gadolinium contrast, MRI has superior image resolution and can detect cardiac involvement early in its course.27,29,35–44
Inflammation of the myocardium due to sarcoid involvement appears as focal zones of increased signal intensity on both T2-weighted and early gadolinium T1-weighted images. Late myocardial enhancement after gadolinium infusion is the most typical finding of cardiac sarcoidosis on MRI, and likely represents fibrogranulomatous tissue.27 Delayed gadolinium enhancement is also seen in myocardial infarction but differs in its distribution.1,35,45 Cardiac sarcoidosis most commonly affects the basal and lateral segments. In one study, the finding of delayed enhancement had a sensitivity of 100% and a specificity of 78%,1,27 though it may not sufficiently differentiate active inflammation from scar.30
Like FDG-PET, MRI has also been shown to be useful for monitoring treatment.33,46 However, PET is more useful for follow-up in patients who receive a pacemaker or implantable cardioverter-defibrillator, in whom MRI is contraindicated. One case report29 described using both delayed-enhancement MRI and FDG-PET to diagnose cardiac sarcoidosis.
TREATMENT
6. How is cardiac sarcoidosis currently treated?
- Implantable cardioverter-defibrillator
- Corticosteroids
- Heart transplantation
- All of the above
- None of the above
Corticosteroids
Corticosteroids are the mainstay of treatment of cardiac sarcoidosis, as they attenuate the characteristic inflammation and fibrosis of sarcoid granulomas. The goal is to prevent compromise of cardiac structure or function.47 Although most of the supporting data are anecdotal, steroids have been shown to improve ventricular contractility,48 arrhythmias,49 and conduction abnormalities.50 MRI and FDG-PET studies have shown cardiac lesions resolving after steroids were started.31,45,46
The optimal dosage remains unknown. Initial doses of 30 to 60 mg daily, gradually tapered over 6 to 12 months to maintenance doses of 5 to 10 mg daily, have been effective.45,51
Relapses are common and require vigilant monitoring.
Alternative agents such as cyclophosphamide (Cytoxan),52 methotrexate (Rheumatrex), 53 and cyclosporine (Sandimmune)54 can be given to patients whose disease does not respond to corticosteroids or who cannot tolerate their side effects.
Implantable cardioverter-defibrillator
Sudden death due to ventricular tachyarrhythmias or conduction block accounts for 30% to 65% of deaths in patients with cardiac sarcoidosis.10 The rates of recurrent ventricular tachycardia and sudden death are high, even with antiarrhythmic drug therapy.55
Although experience with implantable cardiac defibrillators is limited in patients with cardiac sarcoidosis,55–58 some have argued that they be strongly considered to prevent sudden cardiac death in this high-risk group.57,58
Heart transplantation
The largest body of data on transplantation comes from the United Network for Organ Sharing database. In the 65 patients with cardiac sarcoidosis who underwent cardiac transplantation in the 18 years from October 1987 to September 2005, the 1-year post-transplant survival rate was 88%, which was better than in patients with all other diagnoses (85%). The 5-year survival rate was 80%.59,60
Recurrence of sarcoidosis within the cardiac allograft and transmission of sarcoidosis from donor to recipient have both been documented after heart transplantation.61,62
CAUSES OF DEATH
7. What is the most common cause of death in patients with cardiac sarcoidosis?
- Respiratory failure
- Conduction disturbances
- Progressive heart failure
- Ventricular tachyarrhythmias
- None of the above
The prognosis of symptomatic cardiac sarcoidosis is not well defined, owing to the variable extent and severity of the disease. The mortality rate in sarcoidosis without cardiac involvement is about 1% to 5% per year.63,64 Cardiac involvement portends a worse prognosis, with a 55% survival rate at 5 years and 44% at 10 years.17,65 Most patients in the reported series ultimately died of cardiac complications of sarcoidosis, including ventricular tachyarrhythmias, conduction disturbances, and progressive cardiomyopathy.10,17
Since corticosteroids, pacemakers, and implantable cardioverter-defibrillators have begun to be used, the cause of death has shifted from sudden death to progressive heart failure.66
CASE CONTINUED
Electrophysiologic testing revealed inducible monomorphic sustained ventricular tachycardia. The patient subsequently had a biventricular cardioverter-defibrillator implanted. He was started on an angiotensin-converting enzyme inhibitor and a beta-blocker for his heart failure. Further imaging of his chest and abdomen revealed lesions in his thyroid and liver. As of this writing, he is undergoing further workup. Because of active infection with Clostridium difficile, steroid therapy was deferred.
- Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–241.
- Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278:455–469.
- Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004; 83:315–334.
- Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11:26–31.
- Thomsen TK, Eriksson T. Myocardial sarcoidosis in forensic medicine. Am J Forensic Med Pathol 1999; 20:52–56.
- Silverman KJ, Hutchins GM, Buckley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204–1211.
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63:86–108.
- Bargout R, Kelly R. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97:173–182.
- Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J 1979; 97:701–707.
- Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond 1981; 15:245–253.
- Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980; 44:249–263.
- Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 2004; 27:217–222.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997; 89:145–153.
- Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82:537–540.
- Fleming H. Cardiac sarcoidosis. In:James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Dekker 1994; 73:323–334.
- Padilla M. Cardiac sarcoidosis. In:Baughman R, editor. Lung Biology in Health and Disease (Sarcoidosis), vol 210. New York, NY: Taylor & Francis Group; 2006:515–552.
- Judson MA. A proposed solution to the clinical assessment of sarcoidosis: the sarcoidosis three-dimensional assessment instrument (STAI). Med Hypotheses 2007; 68:1080–1087.
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:75–86.
- Hiraga H, Yuwai K, Hiroe M, et al. Guideline for diagnosis of cardiac sarcoidosis. Study report of diffuse pulmonary diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare, 1993:23–24 (in Japanese).
- Stein E, Jackler I, Stimmel B, Stein W, Siltzbach LE. Asymptomatic electrocardiographic alterations in sarcoidosis. Am Heart J 1973; 86:474–477.
- Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109:62–66.
- Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: a case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15:222–227.
- Haywood LJ, Sharma OP, Siegel ME, et al. Detection of myocardial sarcoidosis by thallium-201 imaging. J Natl Med Assoc 1982; 74:959–964.
- Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185:110–115.
- Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990; 41:573–576.
- Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J 2007; 29:418–422.
- Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35:933–941.
- Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44:1030–1036.
- Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002; 105:1144–1145.
- Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26:1538–1543.
- Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45:1989–1998.
- Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92:399–400.
- Kiuchi S, Teraoka K, Koizumi K, Takazawa K, Yamashina A. Usefulness of late gadolinium enhancement combined with MRI and 67-Ga scintigraphy in the diagnosis of cardiac sarcoidosis and disease activity evaluation. Int J Cardiovasc Imaging 2007; 23:237–241.
- Matsuki M, Matsuo M. MR findings of myocardial sarcoidosis. Clin Radiol 2000; 55:323–325.
- Inoue S, Shimada T, Murakami Y. Clinical significance of gadolinium-DTPA-enhanced MRI for detection of myocardial lesions in a patient with sarcoidosis. Clin Radiol 1999; 54:70–72.
- Vignaux O, Dhote R, Dudoc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrastenhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26:762–767.
- Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol 2005; 184:249–254.
- Smedema JP, Snoep G, Van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Doherty MJ, Kumar SK, Nicholson AA, McGivern DV. Cardiac sarcoidosis: the value of magnetic resonance imagine in diagnosis and assessment of response to treatment. Respir Med 1998; 92:697–699.
- Smedema JP, Truter R, de Klerk PA, Zaaiman L, White L, Doubell AF. Cardiac sarcoidosis evaluated with gadolinium-enhanced magnetic resonance and contrast-enhanced 64-slice computed tomography. Int J Cardiol 2006; 112:261–263.
- Kanao S, Tadamura E, Yamamuro M, et al. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2005; 29:745–748.
- Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002; 122:1895–1901.
- Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110:520–527.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Ishikawa T, Kondoh H, Nakagawa S, Koiwaya Y, Tanaka K. Steroid therapy in cardiac sarcoidosis. Increased left ventricular contractility concomitant with electrocardiographic improvement after prednisolone. Chest 1984; 85:445–447.
- Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978; 40:931–933.
- Lash R, Coker J, Wong BY. Treatment of heart block due to sarcoid heart disease. J Electrocardiol 1979; 12:325–329.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and implications. Ann N Y Acad Sci 1986; 465:702–712.
- Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94:202–203.
- Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155:846–851.
- York EL, Kovithavongs T, Man SF, Rebuck AS, Sproule BJ. Cyclosporine and chronic sarcoidosis. Chest 1990; 98:1026–1029.
- Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18:937–943.
- Bajaj AK, Kopelman HA, Echt DS. Cardiac sarcoidosis with sudden death: treatment with automatic implantable cardioverter defibrillator. Am Heart J 1988; 116:557–560.
- Paz HL, McCormick DJ, Kutalek SP, Patchefsky A. The automated implantable cardiac defibrillator. Prophylaxis in cardiac sarcoidosis. Chest 1994; 106:1603–1607.
- Becker D, Berger E, Chmielewski C. Cardiac sarcoidosis: a report of four cases with ventricular tachycardia. J Cardiovasc Electrophysiol 1990; 1:214–219.
- Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26:714–717.
- Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant 1987; 6:244–250.
- Oni AA, Hershberger RE, Norman DJ, et al. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 1992; 11:367–369.
- Burke WM, Keogh A, Maloney PJ, Delprado W, Bryant DH, Spratt P. Transmission of sarcoidosis via cardiac transplantation. Lancet 1990; 336:1579.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 1986; 465:702–712.
- Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996; 100:423–427.
- Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986; 465:543–550.
- Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan. Clinical evaluation of corticosteroid therapy. Sarcoidosis 1994; 11:113–117.
A 61-year-old white man presents with progressive fatigue, which began several months ago and has accelerated in severity over the past week. He says he has had no shortness of breath, chest pain, or symptoms of heart failure, but he has noticed a decrease in exertional capacity and now has trouble completing his daily 5-mile walk.
He saw his primary physician, who obtained an electrocardiogram that showed a new left bundle branch block. Transthoracic echocardiography indicated that his left ventricular ejection fraction, which was 60% a year earlier, was now 35%.
He has hypertension, dyslipidemia, type 2 diabetes, and chronic kidney disease. Although he was previously morbidly obese, he has lost more than 100 pounds with diet and exercise over the past 10 years. He also used to smoke; in fact, he has a 30-pack-year history, but he quit in 1987. He has a family history of premature coronary artery disease.
Physical examination. His heart rate is 75 beats per minute, blood pressure 142/85 mm Hg, and blood oxygen saturation 96% while breathing room air. He weighs 169 pounds (76.6 kg) and he is 6 feet tall (182.9 cm), so his body mass index is 22.9 kg/m2.
Electrocardiography reveals sinus rhythm with a left bundle branch block and left axis deviation (Figure 1), which were not present 1 year ago.
Chest roentgenography is normal.
A WORRISOME PICTURE
1. Which of the following is associated with left bundle branch block?
- Myocardial injury
- Hypertension
- Aortic stenosis
- Intrinsic conduction system disease
- All of the above
All of the above are true. For left bundle branch block to be diagnosed, the rhythm must be supraventricular and the QRS duration must be 120 ms or more. There should be a QS or RS complex in V1 and a monophasic R wave in I and V6. Also, the T wave should be deflected opposite the terminal deflection of the QRS complex. This is known as appropriate T-wave discordance with bundle branch block. A concordant T wave is nonspecific but suggests ischemia or myocardial infarction.
Potential causes of a new left bundle branch block include hypertension, acute myocardial infarction, aortic stenosis, and conduction system disease. A new left bundle branch block with a concomitant decrease in ejection fraction, especially in a patient with cardiac risk factors, is very worrisome, raising the possibility of ischemic heart disease.
MORE CARDIAC TESTING
The patient undergoes more cardiac testing.
Transthoracic echocardiography is done again. The left ventricle is normal in size, but the ejection fraction is 35%. In addition, stage 1 diastolic dysfunction (abnormal relaxation) and evidence of mechanical dyssynchrony (disruption in the normal sequence of activation and contraction of segments of the left ventricular wall) are seen. The right ventricle is normal in size and function. There is trivial mitral regurgitation and mild tricuspid regurgitation with normal right-sided pressures.
A gated rubidium-82 dipyridamole stress test yields no evidence of a fixed or reversible perfusion defect.
Left heart catheterization reveals angiographically normal coronary arteries.
Magnetic resonance imaging (MRI) shows a moderately hypertrophied left ventricle with moderately to severely depressed systolic function (left ventricular ejection fraction 27%). The left ventricle appears dyssynchronous. Delayed-enhancement imaging reveals patchy delayed enhancement in the basal septum and the basal inferolateral walls. These findings suggest cardiac sarcoidosis, with a sensitivity of nearly 100% and a specificity of approximately 78%.1
SARCOIDOSIS IS A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Almost any organ can be affected, but it most commonly involves the respiratory and lymphatic systems.2 Although infectious, environmental, and genetic factors have been implicated, the cause remains unknown. The prevalence is approximately 20 per 100,000, being higher in black3 and Japanese 4 populations.
CARDIAC SARCOIDOSIS
2. What percentage of patients with sarcoidosis have cardiac involvement?
- 10%–20%
- 20%–30%
- 50%
- 80%
Cardiac involvement is seen in 20% to 30% of patients with sarcoidosis.5–7 However, most cases are subclinical, and symptomatic cardiac involvement is present in only about 5% of patients with systemic sarcoidosis.8 Isolated cardiac sarcoidosis has been described in case reports but is rare.9
The clinical manifestations of cardiac sarcoidosis depend on the location and extent of granulomatous inflammation. In a necropsy study of 113 patients with cardiac sarcoidosis, the left ventricular free wall was the most common location, followed by the interventricular septum.10
3. How does cardiac sarcoidosis most commonly present?
- Conduction abnormalities
- Ventricular tachycardia
- Cardiomyopathy
- Sudden death
- None of the above
Common presentations of cardiac sarcoidosis include conduction system disease and arrhythmias (which can sometimes result in sudden death), and heart failure.
Conduction abnormalities due to granulomas (in the active phase of sarcoidosis) and fibrosis (in the fibrotic phase) in the atrioventricular node or bundle of His are the most common presentation of cardiac sarcoidosis.9 These lesions may result in relatively benign first-degree heart block or may be as potentially devastating as complete heart block.
In almost all patients with conduction abnormalities, the basal interventricular septum is involved.11 Patients who develop complete heart block from sarcoidosis tend to be younger than those with idiopathic heart block. Therefore, complete heart block in a young patient should raise the possibility of this diagnosis. 12
Ventricular tachycardia (sustained or nonsustained) and ventricular premature beats are the second most common presentation. Up to 22% of patients with sarcoidosis have electrocardiographic evidence of ventricular arrythmias. 13 The cause is believed to be myocardial scar tissue resulting from the sarcoid granulomas, leading to electrical reentry.14 Sudden death due to ventricular tachyarrhythmias or conduction blocks accounts for 25% to 65% of deaths from cardiac sarcoidosis.9,15,16
Heart failure may result from sarcoidosis when there is extensive granulomatous disease in the myocardium. Depending on the location of the granulomas, both systolic and diastolic dysfunction can occur. In severe cases, extensive granulomas can cause left ventricular aneurysms.
The diagnosis of cardiac sarcoidosis as the cause of heart failure can be difficult to establish, especially in patients without evidence of sarcoidosis elsewhere. Such patients are often given a diagnosis of idiopathic dilated cardiomyopathy. However, compared with patients with idiopathic dilated cardiomyopathy, those with cardiac sarcoidosis have a greater incidence of advanced atrioventricular block, abnormal wall thickness, focal wall motion abnormalities, and perfusion defects of the anteroseptal and apical regions.17
Progressive heart failure is the second most frequent cause of death (after sudden death) and accounts for 25% to 75% of sarcoid-related cardiac deaths.9,18,19
DIAGNOSING CARDIAC SARCOIDOSIS
4. How is cardiac sarcoidosis diagnosed?
- Electrocardiography
- Echocardiography
- Computed tomography
- Endomyocardial biopsy
- There are no guidelines for diagnosis
Given the variable extent and location of granulomas in sarcoidosis, the diagnosis of cardiac sarcoidosis is often challenging.
To make the diagnosis of sarcoidosis in general, the American Thoracic Society2 says that the clinical picture should be compatible with this diagnosis, noncaseating granulomas should be histologically confirmed, and other diseases capable of producing a similar clinical or histologic picture should be excluded.
A newer diagnostic tool, the Sarcoidosis Three-Dimensional Assessment Instrument,20 incorporates two earlier tools.20,21 It assesses three axes: organ involvement, sarcoidosis severity, and sarcoidosis activity and categorizes the diagnosis of sarcoidosis as “definite,” “probable,” or “possible.”20
In Japan, where sarcoidosis is more common, the Ministry of Health and Welfare22 says that cardiac sarcoidosis can be diagnosed histologically if operative or endomyocardial biopsy specimens contain noncaseating granuloma. In addition, the diagnosis can be suspected in patients who have a histologic diagnosis of extracardiac sarcoidosis if the first item in the list below and one or more of the rest are present:
- Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contractions (> grade 2 of the Lown classification), or Q or ST-T wave abnormalities
- Abnormal wall motion, regional wall thinning, or dilation of the left ventricle on echocardiography
- Perfusion defects on thallium 201 myocardial scintigraphy or abnormal accumulation of gallium citrate Ga 67 or technetium 99m on myocardial scintigraphy
- Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed left ventricular ejection fraction on cardiac catheterization
- Nonspecific interstitial fibrosis or cellular infiltration on myocardial biopsy.
The current diagnostic guidelines from the American Thoracic Society2 and the Japanese Ministry of Health and Welfare22 and the Sarcoidosis Three-Dimensional Assessment Instrument,20 however, do not incorporate newer imaging studies as part of their criteria.
A DEFINITIVE DIAGNOSIS
5. Endomyocardial biopsy often provides the definitive diagnosis of cardiac sarcoidosis.
- True
- False
False. Endomyocardial biopsy often fails to reveal noncaseating granulomas, which have a patchy distribution.13 Table 2 summarizes the accuracy of tests for cardiac sarcoidosis.
Electrocardiography is abnormal in up to 50% of patients with sarcoidosis,23 reflecting the conduction disease or arrhythmias commonly seen in cardiac involvement.
Chest radiography classically shows hilar lymphadenopathy and interstitial disease, and may show cardiomegaly, pericardial effusion, or left ventricular aneurysm.
Echocardiography is nonspecific for sarcoid disease, but granulomatous involvement and scar tissue of cardiac tissue may appear hyperechogenic, particularly in the ventricular septum or left ventricular free wall.24
Angiography. Primary sarcoidosis rarely involves the coronary arteries,25 so angiography is most useful in excluding the diagnosis of atherosclerotic coronary artery disease.
Radionuclide imaging with thallium 201 in patients with suspected cardiac sarcoidosis may be useful to suggest myocardial involvement and to exclude cardiac dysfunction secondary to coronary artery disease. Segmental areas with defective thallium 201 uptake correspond to fibrogranulomatous tissue. In resting images, the pattern may be similar to that seen in coronary artery disease. However, during exercise, perfusion defects increase in patients who have ischemia but actually decrease in those with cardiac sarcoidosis.26
Nevertheless, some conclude that thallium scanning is too nonspecific for it to be used as a diagnostic or screening test.27,28 The combined use of thallium 201 and gallium 67 may better detect cardiac sarcoidosis, as gallium is taken up in areas of active inflammation.
Positron-emission tomography (PET) with fluorodeoxyglucose F 18 (FDG), with the patient fasting, appears to be useful in detecting the early inflammation of cardiac sarcoidosis29–34 and monitoring disease activity.30,31 FDG is a glucose analogue that is taken up by granulomatous tissue in the myocardium.34 The uptake in cardiac sarcoidosis is in a focal distribution.30,31,34 The abnormal FDG uptake resolves with steroid treatment.31,32
MRI has promise for diagnosing cardiac sarcoidosis. With gadolinium contrast, MRI has superior image resolution and can detect cardiac involvement early in its course.27,29,35–44
Inflammation of the myocardium due to sarcoid involvement appears as focal zones of increased signal intensity on both T2-weighted and early gadolinium T1-weighted images. Late myocardial enhancement after gadolinium infusion is the most typical finding of cardiac sarcoidosis on MRI, and likely represents fibrogranulomatous tissue.27 Delayed gadolinium enhancement is also seen in myocardial infarction but differs in its distribution.1,35,45 Cardiac sarcoidosis most commonly affects the basal and lateral segments. In one study, the finding of delayed enhancement had a sensitivity of 100% and a specificity of 78%,1,27 though it may not sufficiently differentiate active inflammation from scar.30
Like FDG-PET, MRI has also been shown to be useful for monitoring treatment.33,46 However, PET is more useful for follow-up in patients who receive a pacemaker or implantable cardioverter-defibrillator, in whom MRI is contraindicated. One case report29 described using both delayed-enhancement MRI and FDG-PET to diagnose cardiac sarcoidosis.
TREATMENT
6. How is cardiac sarcoidosis currently treated?
- Implantable cardioverter-defibrillator
- Corticosteroids
- Heart transplantation
- All of the above
- None of the above
Corticosteroids
Corticosteroids are the mainstay of treatment of cardiac sarcoidosis, as they attenuate the characteristic inflammation and fibrosis of sarcoid granulomas. The goal is to prevent compromise of cardiac structure or function.47 Although most of the supporting data are anecdotal, steroids have been shown to improve ventricular contractility,48 arrhythmias,49 and conduction abnormalities.50 MRI and FDG-PET studies have shown cardiac lesions resolving after steroids were started.31,45,46
The optimal dosage remains unknown. Initial doses of 30 to 60 mg daily, gradually tapered over 6 to 12 months to maintenance doses of 5 to 10 mg daily, have been effective.45,51
Relapses are common and require vigilant monitoring.
Alternative agents such as cyclophosphamide (Cytoxan),52 methotrexate (Rheumatrex), 53 and cyclosporine (Sandimmune)54 can be given to patients whose disease does not respond to corticosteroids or who cannot tolerate their side effects.
Implantable cardioverter-defibrillator
Sudden death due to ventricular tachyarrhythmias or conduction block accounts for 30% to 65% of deaths in patients with cardiac sarcoidosis.10 The rates of recurrent ventricular tachycardia and sudden death are high, even with antiarrhythmic drug therapy.55
Although experience with implantable cardiac defibrillators is limited in patients with cardiac sarcoidosis,55–58 some have argued that they be strongly considered to prevent sudden cardiac death in this high-risk group.57,58
Heart transplantation
The largest body of data on transplantation comes from the United Network for Organ Sharing database. In the 65 patients with cardiac sarcoidosis who underwent cardiac transplantation in the 18 years from October 1987 to September 2005, the 1-year post-transplant survival rate was 88%, which was better than in patients with all other diagnoses (85%). The 5-year survival rate was 80%.59,60
Recurrence of sarcoidosis within the cardiac allograft and transmission of sarcoidosis from donor to recipient have both been documented after heart transplantation.61,62
CAUSES OF DEATH
7. What is the most common cause of death in patients with cardiac sarcoidosis?
- Respiratory failure
- Conduction disturbances
- Progressive heart failure
- Ventricular tachyarrhythmias
- None of the above
The prognosis of symptomatic cardiac sarcoidosis is not well defined, owing to the variable extent and severity of the disease. The mortality rate in sarcoidosis without cardiac involvement is about 1% to 5% per year.63,64 Cardiac involvement portends a worse prognosis, with a 55% survival rate at 5 years and 44% at 10 years.17,65 Most patients in the reported series ultimately died of cardiac complications of sarcoidosis, including ventricular tachyarrhythmias, conduction disturbances, and progressive cardiomyopathy.10,17
Since corticosteroids, pacemakers, and implantable cardioverter-defibrillators have begun to be used, the cause of death has shifted from sudden death to progressive heart failure.66
CASE CONTINUED
Electrophysiologic testing revealed inducible monomorphic sustained ventricular tachycardia. The patient subsequently had a biventricular cardioverter-defibrillator implanted. He was started on an angiotensin-converting enzyme inhibitor and a beta-blocker for his heart failure. Further imaging of his chest and abdomen revealed lesions in his thyroid and liver. As of this writing, he is undergoing further workup. Because of active infection with Clostridium difficile, steroid therapy was deferred.
A 61-year-old white man presents with progressive fatigue, which began several months ago and has accelerated in severity over the past week. He says he has had no shortness of breath, chest pain, or symptoms of heart failure, but he has noticed a decrease in exertional capacity and now has trouble completing his daily 5-mile walk.
He saw his primary physician, who obtained an electrocardiogram that showed a new left bundle branch block. Transthoracic echocardiography indicated that his left ventricular ejection fraction, which was 60% a year earlier, was now 35%.
He has hypertension, dyslipidemia, type 2 diabetes, and chronic kidney disease. Although he was previously morbidly obese, he has lost more than 100 pounds with diet and exercise over the past 10 years. He also used to smoke; in fact, he has a 30-pack-year history, but he quit in 1987. He has a family history of premature coronary artery disease.
Physical examination. His heart rate is 75 beats per minute, blood pressure 142/85 mm Hg, and blood oxygen saturation 96% while breathing room air. He weighs 169 pounds (76.6 kg) and he is 6 feet tall (182.9 cm), so his body mass index is 22.9 kg/m2.
Electrocardiography reveals sinus rhythm with a left bundle branch block and left axis deviation (Figure 1), which were not present 1 year ago.
Chest roentgenography is normal.
A WORRISOME PICTURE
1. Which of the following is associated with left bundle branch block?
- Myocardial injury
- Hypertension
- Aortic stenosis
- Intrinsic conduction system disease
- All of the above
All of the above are true. For left bundle branch block to be diagnosed, the rhythm must be supraventricular and the QRS duration must be 120 ms or more. There should be a QS or RS complex in V1 and a monophasic R wave in I and V6. Also, the T wave should be deflected opposite the terminal deflection of the QRS complex. This is known as appropriate T-wave discordance with bundle branch block. A concordant T wave is nonspecific but suggests ischemia or myocardial infarction.
Potential causes of a new left bundle branch block include hypertension, acute myocardial infarction, aortic stenosis, and conduction system disease. A new left bundle branch block with a concomitant decrease in ejection fraction, especially in a patient with cardiac risk factors, is very worrisome, raising the possibility of ischemic heart disease.
MORE CARDIAC TESTING
The patient undergoes more cardiac testing.
Transthoracic echocardiography is done again. The left ventricle is normal in size, but the ejection fraction is 35%. In addition, stage 1 diastolic dysfunction (abnormal relaxation) and evidence of mechanical dyssynchrony (disruption in the normal sequence of activation and contraction of segments of the left ventricular wall) are seen. The right ventricle is normal in size and function. There is trivial mitral regurgitation and mild tricuspid regurgitation with normal right-sided pressures.
A gated rubidium-82 dipyridamole stress test yields no evidence of a fixed or reversible perfusion defect.
Left heart catheterization reveals angiographically normal coronary arteries.
Magnetic resonance imaging (MRI) shows a moderately hypertrophied left ventricle with moderately to severely depressed systolic function (left ventricular ejection fraction 27%). The left ventricle appears dyssynchronous. Delayed-enhancement imaging reveals patchy delayed enhancement in the basal septum and the basal inferolateral walls. These findings suggest cardiac sarcoidosis, with a sensitivity of nearly 100% and a specificity of approximately 78%.1
SARCOIDOSIS IS A MULTISYSTEM DISEASE
Sarcoidosis is a multisystem disease characterized by noncaseating granulomas. Almost any organ can be affected, but it most commonly involves the respiratory and lymphatic systems.2 Although infectious, environmental, and genetic factors have been implicated, the cause remains unknown. The prevalence is approximately 20 per 100,000, being higher in black3 and Japanese 4 populations.
CARDIAC SARCOIDOSIS
2. What percentage of patients with sarcoidosis have cardiac involvement?
- 10%–20%
- 20%–30%
- 50%
- 80%
Cardiac involvement is seen in 20% to 30% of patients with sarcoidosis.5–7 However, most cases are subclinical, and symptomatic cardiac involvement is present in only about 5% of patients with systemic sarcoidosis.8 Isolated cardiac sarcoidosis has been described in case reports but is rare.9
The clinical manifestations of cardiac sarcoidosis depend on the location and extent of granulomatous inflammation. In a necropsy study of 113 patients with cardiac sarcoidosis, the left ventricular free wall was the most common location, followed by the interventricular septum.10
3. How does cardiac sarcoidosis most commonly present?
- Conduction abnormalities
- Ventricular tachycardia
- Cardiomyopathy
- Sudden death
- None of the above
Common presentations of cardiac sarcoidosis include conduction system disease and arrhythmias (which can sometimes result in sudden death), and heart failure.
Conduction abnormalities due to granulomas (in the active phase of sarcoidosis) and fibrosis (in the fibrotic phase) in the atrioventricular node or bundle of His are the most common presentation of cardiac sarcoidosis.9 These lesions may result in relatively benign first-degree heart block or may be as potentially devastating as complete heart block.
In almost all patients with conduction abnormalities, the basal interventricular septum is involved.11 Patients who develop complete heart block from sarcoidosis tend to be younger than those with idiopathic heart block. Therefore, complete heart block in a young patient should raise the possibility of this diagnosis. 12
Ventricular tachycardia (sustained or nonsustained) and ventricular premature beats are the second most common presentation. Up to 22% of patients with sarcoidosis have electrocardiographic evidence of ventricular arrythmias. 13 The cause is believed to be myocardial scar tissue resulting from the sarcoid granulomas, leading to electrical reentry.14 Sudden death due to ventricular tachyarrhythmias or conduction blocks accounts for 25% to 65% of deaths from cardiac sarcoidosis.9,15,16
Heart failure may result from sarcoidosis when there is extensive granulomatous disease in the myocardium. Depending on the location of the granulomas, both systolic and diastolic dysfunction can occur. In severe cases, extensive granulomas can cause left ventricular aneurysms.
The diagnosis of cardiac sarcoidosis as the cause of heart failure can be difficult to establish, especially in patients without evidence of sarcoidosis elsewhere. Such patients are often given a diagnosis of idiopathic dilated cardiomyopathy. However, compared with patients with idiopathic dilated cardiomyopathy, those with cardiac sarcoidosis have a greater incidence of advanced atrioventricular block, abnormal wall thickness, focal wall motion abnormalities, and perfusion defects of the anteroseptal and apical regions.17
Progressive heart failure is the second most frequent cause of death (after sudden death) and accounts for 25% to 75% of sarcoid-related cardiac deaths.9,18,19
DIAGNOSING CARDIAC SARCOIDOSIS
4. How is cardiac sarcoidosis diagnosed?
- Electrocardiography
- Echocardiography
- Computed tomography
- Endomyocardial biopsy
- There are no guidelines for diagnosis
Given the variable extent and location of granulomas in sarcoidosis, the diagnosis of cardiac sarcoidosis is often challenging.
To make the diagnosis of sarcoidosis in general, the American Thoracic Society2 says that the clinical picture should be compatible with this diagnosis, noncaseating granulomas should be histologically confirmed, and other diseases capable of producing a similar clinical or histologic picture should be excluded.
A newer diagnostic tool, the Sarcoidosis Three-Dimensional Assessment Instrument,20 incorporates two earlier tools.20,21 It assesses three axes: organ involvement, sarcoidosis severity, and sarcoidosis activity and categorizes the diagnosis of sarcoidosis as “definite,” “probable,” or “possible.”20
In Japan, where sarcoidosis is more common, the Ministry of Health and Welfare22 says that cardiac sarcoidosis can be diagnosed histologically if operative or endomyocardial biopsy specimens contain noncaseating granuloma. In addition, the diagnosis can be suspected in patients who have a histologic diagnosis of extracardiac sarcoidosis if the first item in the list below and one or more of the rest are present:
- Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contractions (> grade 2 of the Lown classification), or Q or ST-T wave abnormalities
- Abnormal wall motion, regional wall thinning, or dilation of the left ventricle on echocardiography
- Perfusion defects on thallium 201 myocardial scintigraphy or abnormal accumulation of gallium citrate Ga 67 or technetium 99m on myocardial scintigraphy
- Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed left ventricular ejection fraction on cardiac catheterization
- Nonspecific interstitial fibrosis or cellular infiltration on myocardial biopsy.
The current diagnostic guidelines from the American Thoracic Society2 and the Japanese Ministry of Health and Welfare22 and the Sarcoidosis Three-Dimensional Assessment Instrument,20 however, do not incorporate newer imaging studies as part of their criteria.
A DEFINITIVE DIAGNOSIS
5. Endomyocardial biopsy often provides the definitive diagnosis of cardiac sarcoidosis.
- True
- False
False. Endomyocardial biopsy often fails to reveal noncaseating granulomas, which have a patchy distribution.13 Table 2 summarizes the accuracy of tests for cardiac sarcoidosis.
Electrocardiography is abnormal in up to 50% of patients with sarcoidosis,23 reflecting the conduction disease or arrhythmias commonly seen in cardiac involvement.
Chest radiography classically shows hilar lymphadenopathy and interstitial disease, and may show cardiomegaly, pericardial effusion, or left ventricular aneurysm.
Echocardiography is nonspecific for sarcoid disease, but granulomatous involvement and scar tissue of cardiac tissue may appear hyperechogenic, particularly in the ventricular septum or left ventricular free wall.24
Angiography. Primary sarcoidosis rarely involves the coronary arteries,25 so angiography is most useful in excluding the diagnosis of atherosclerotic coronary artery disease.
Radionuclide imaging with thallium 201 in patients with suspected cardiac sarcoidosis may be useful to suggest myocardial involvement and to exclude cardiac dysfunction secondary to coronary artery disease. Segmental areas with defective thallium 201 uptake correspond to fibrogranulomatous tissue. In resting images, the pattern may be similar to that seen in coronary artery disease. However, during exercise, perfusion defects increase in patients who have ischemia but actually decrease in those with cardiac sarcoidosis.26
Nevertheless, some conclude that thallium scanning is too nonspecific for it to be used as a diagnostic or screening test.27,28 The combined use of thallium 201 and gallium 67 may better detect cardiac sarcoidosis, as gallium is taken up in areas of active inflammation.
Positron-emission tomography (PET) with fluorodeoxyglucose F 18 (FDG), with the patient fasting, appears to be useful in detecting the early inflammation of cardiac sarcoidosis29–34 and monitoring disease activity.30,31 FDG is a glucose analogue that is taken up by granulomatous tissue in the myocardium.34 The uptake in cardiac sarcoidosis is in a focal distribution.30,31,34 The abnormal FDG uptake resolves with steroid treatment.31,32
MRI has promise for diagnosing cardiac sarcoidosis. With gadolinium contrast, MRI has superior image resolution and can detect cardiac involvement early in its course.27,29,35–44
Inflammation of the myocardium due to sarcoid involvement appears as focal zones of increased signal intensity on both T2-weighted and early gadolinium T1-weighted images. Late myocardial enhancement after gadolinium infusion is the most typical finding of cardiac sarcoidosis on MRI, and likely represents fibrogranulomatous tissue.27 Delayed gadolinium enhancement is also seen in myocardial infarction but differs in its distribution.1,35,45 Cardiac sarcoidosis most commonly affects the basal and lateral segments. In one study, the finding of delayed enhancement had a sensitivity of 100% and a specificity of 78%,1,27 though it may not sufficiently differentiate active inflammation from scar.30
Like FDG-PET, MRI has also been shown to be useful for monitoring treatment.33,46 However, PET is more useful for follow-up in patients who receive a pacemaker or implantable cardioverter-defibrillator, in whom MRI is contraindicated. One case report29 described using both delayed-enhancement MRI and FDG-PET to diagnose cardiac sarcoidosis.
TREATMENT
6. How is cardiac sarcoidosis currently treated?
- Implantable cardioverter-defibrillator
- Corticosteroids
- Heart transplantation
- All of the above
- None of the above
Corticosteroids
Corticosteroids are the mainstay of treatment of cardiac sarcoidosis, as they attenuate the characteristic inflammation and fibrosis of sarcoid granulomas. The goal is to prevent compromise of cardiac structure or function.47 Although most of the supporting data are anecdotal, steroids have been shown to improve ventricular contractility,48 arrhythmias,49 and conduction abnormalities.50 MRI and FDG-PET studies have shown cardiac lesions resolving after steroids were started.31,45,46
The optimal dosage remains unknown. Initial doses of 30 to 60 mg daily, gradually tapered over 6 to 12 months to maintenance doses of 5 to 10 mg daily, have been effective.45,51
Relapses are common and require vigilant monitoring.
Alternative agents such as cyclophosphamide (Cytoxan),52 methotrexate (Rheumatrex), 53 and cyclosporine (Sandimmune)54 can be given to patients whose disease does not respond to corticosteroids or who cannot tolerate their side effects.
Implantable cardioverter-defibrillator
Sudden death due to ventricular tachyarrhythmias or conduction block accounts for 30% to 65% of deaths in patients with cardiac sarcoidosis.10 The rates of recurrent ventricular tachycardia and sudden death are high, even with antiarrhythmic drug therapy.55
Although experience with implantable cardiac defibrillators is limited in patients with cardiac sarcoidosis,55–58 some have argued that they be strongly considered to prevent sudden cardiac death in this high-risk group.57,58
Heart transplantation
The largest body of data on transplantation comes from the United Network for Organ Sharing database. In the 65 patients with cardiac sarcoidosis who underwent cardiac transplantation in the 18 years from October 1987 to September 2005, the 1-year post-transplant survival rate was 88%, which was better than in patients with all other diagnoses (85%). The 5-year survival rate was 80%.59,60
Recurrence of sarcoidosis within the cardiac allograft and transmission of sarcoidosis from donor to recipient have both been documented after heart transplantation.61,62
CAUSES OF DEATH
7. What is the most common cause of death in patients with cardiac sarcoidosis?
- Respiratory failure
- Conduction disturbances
- Progressive heart failure
- Ventricular tachyarrhythmias
- None of the above
The prognosis of symptomatic cardiac sarcoidosis is not well defined, owing to the variable extent and severity of the disease. The mortality rate in sarcoidosis without cardiac involvement is about 1% to 5% per year.63,64 Cardiac involvement portends a worse prognosis, with a 55% survival rate at 5 years and 44% at 10 years.17,65 Most patients in the reported series ultimately died of cardiac complications of sarcoidosis, including ventricular tachyarrhythmias, conduction disturbances, and progressive cardiomyopathy.10,17
Since corticosteroids, pacemakers, and implantable cardioverter-defibrillators have begun to be used, the cause of death has shifted from sudden death to progressive heart failure.66
CASE CONTINUED
Electrophysiologic testing revealed inducible monomorphic sustained ventricular tachycardia. The patient subsequently had a biventricular cardioverter-defibrillator implanted. He was started on an angiotensin-converting enzyme inhibitor and a beta-blocker for his heart failure. Further imaging of his chest and abdomen revealed lesions in his thyroid and liver. As of this writing, he is undergoing further workup. Because of active infection with Clostridium difficile, steroid therapy was deferred.
- Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–241.
- Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278:455–469.
- Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004; 83:315–334.
- Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11:26–31.
- Thomsen TK, Eriksson T. Myocardial sarcoidosis in forensic medicine. Am J Forensic Med Pathol 1999; 20:52–56.
- Silverman KJ, Hutchins GM, Buckley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204–1211.
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63:86–108.
- Bargout R, Kelly R. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97:173–182.
- Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J 1979; 97:701–707.
- Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond 1981; 15:245–253.
- Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980; 44:249–263.
- Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 2004; 27:217–222.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997; 89:145–153.
- Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82:537–540.
- Fleming H. Cardiac sarcoidosis. In:James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Dekker 1994; 73:323–334.
- Padilla M. Cardiac sarcoidosis. In:Baughman R, editor. Lung Biology in Health and Disease (Sarcoidosis), vol 210. New York, NY: Taylor & Francis Group; 2006:515–552.
- Judson MA. A proposed solution to the clinical assessment of sarcoidosis: the sarcoidosis three-dimensional assessment instrument (STAI). Med Hypotheses 2007; 68:1080–1087.
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:75–86.
- Hiraga H, Yuwai K, Hiroe M, et al. Guideline for diagnosis of cardiac sarcoidosis. Study report of diffuse pulmonary diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare, 1993:23–24 (in Japanese).
- Stein E, Jackler I, Stimmel B, Stein W, Siltzbach LE. Asymptomatic electrocardiographic alterations in sarcoidosis. Am Heart J 1973; 86:474–477.
- Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109:62–66.
- Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: a case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15:222–227.
- Haywood LJ, Sharma OP, Siegel ME, et al. Detection of myocardial sarcoidosis by thallium-201 imaging. J Natl Med Assoc 1982; 74:959–964.
- Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185:110–115.
- Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990; 41:573–576.
- Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J 2007; 29:418–422.
- Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35:933–941.
- Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44:1030–1036.
- Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002; 105:1144–1145.
- Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26:1538–1543.
- Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45:1989–1998.
- Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92:399–400.
- Kiuchi S, Teraoka K, Koizumi K, Takazawa K, Yamashina A. Usefulness of late gadolinium enhancement combined with MRI and 67-Ga scintigraphy in the diagnosis of cardiac sarcoidosis and disease activity evaluation. Int J Cardiovasc Imaging 2007; 23:237–241.
- Matsuki M, Matsuo M. MR findings of myocardial sarcoidosis. Clin Radiol 2000; 55:323–325.
- Inoue S, Shimada T, Murakami Y. Clinical significance of gadolinium-DTPA-enhanced MRI for detection of myocardial lesions in a patient with sarcoidosis. Clin Radiol 1999; 54:70–72.
- Vignaux O, Dhote R, Dudoc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrastenhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26:762–767.
- Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol 2005; 184:249–254.
- Smedema JP, Snoep G, Van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Doherty MJ, Kumar SK, Nicholson AA, McGivern DV. Cardiac sarcoidosis: the value of magnetic resonance imagine in diagnosis and assessment of response to treatment. Respir Med 1998; 92:697–699.
- Smedema JP, Truter R, de Klerk PA, Zaaiman L, White L, Doubell AF. Cardiac sarcoidosis evaluated with gadolinium-enhanced magnetic resonance and contrast-enhanced 64-slice computed tomography. Int J Cardiol 2006; 112:261–263.
- Kanao S, Tadamura E, Yamamuro M, et al. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2005; 29:745–748.
- Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002; 122:1895–1901.
- Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110:520–527.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Ishikawa T, Kondoh H, Nakagawa S, Koiwaya Y, Tanaka K. Steroid therapy in cardiac sarcoidosis. Increased left ventricular contractility concomitant with electrocardiographic improvement after prednisolone. Chest 1984; 85:445–447.
- Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978; 40:931–933.
- Lash R, Coker J, Wong BY. Treatment of heart block due to sarcoid heart disease. J Electrocardiol 1979; 12:325–329.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and implications. Ann N Y Acad Sci 1986; 465:702–712.
- Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94:202–203.
- Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155:846–851.
- York EL, Kovithavongs T, Man SF, Rebuck AS, Sproule BJ. Cyclosporine and chronic sarcoidosis. Chest 1990; 98:1026–1029.
- Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18:937–943.
- Bajaj AK, Kopelman HA, Echt DS. Cardiac sarcoidosis with sudden death: treatment with automatic implantable cardioverter defibrillator. Am Heart J 1988; 116:557–560.
- Paz HL, McCormick DJ, Kutalek SP, Patchefsky A. The automated implantable cardiac defibrillator. Prophylaxis in cardiac sarcoidosis. Chest 1994; 106:1603–1607.
- Becker D, Berger E, Chmielewski C. Cardiac sarcoidosis: a report of four cases with ventricular tachycardia. J Cardiovasc Electrophysiol 1990; 1:214–219.
- Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26:714–717.
- Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant 1987; 6:244–250.
- Oni AA, Hershberger RE, Norman DJ, et al. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 1992; 11:367–369.
- Burke WM, Keogh A, Maloney PJ, Delprado W, Bryant DH, Spratt P. Transmission of sarcoidosis via cardiac transplantation. Lancet 1990; 336:1579.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 1986; 465:702–712.
- Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996; 100:423–427.
- Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986; 465:543–550.
- Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan. Clinical evaluation of corticosteroid therapy. Sarcoidosis 1994; 11:113–117.
- Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160:736–755.
- Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–241.
- Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278:455–469.
- Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004; 83:315–334.
- Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11:26–31.
- Thomsen TK, Eriksson T. Myocardial sarcoidosis in forensic medicine. Am J Forensic Med Pathol 1999; 20:52–56.
- Silverman KJ, Hutchins GM, Buckley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58:1204–1211.
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63:86–108.
- Bargout R, Kelly R. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97:173–182.
- Abeler V. Sarcoidosis of the cardiac conducting system. Am Heart J 1979; 97:701–707.
- Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond 1981; 15:245–253.
- Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J 1980; 44:249–263.
- Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 2004; 27:217–222.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997; 89:145–153.
- Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol 1998; 82:537–540.
- Fleming H. Cardiac sarcoidosis. In:James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Dekker 1994; 73:323–334.
- Padilla M. Cardiac sarcoidosis. In:Baughman R, editor. Lung Biology in Health and Disease (Sarcoidosis), vol 210. New York, NY: Taylor & Francis Group; 2006:515–552.
- Judson MA. A proposed solution to the clinical assessment of sarcoidosis: the sarcoidosis three-dimensional assessment instrument (STAI). Med Hypotheses 2007; 68:1080–1087.
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16:75–86.
- Hiraga H, Yuwai K, Hiroe M, et al. Guideline for diagnosis of cardiac sarcoidosis. Study report of diffuse pulmonary diseases. Tokyo, Japan: The Japanese Ministry of Health and Welfare, 1993:23–24 (in Japanese).
- Stein E, Jackler I, Stimmel B, Stein W, Siltzbach LE. Asymptomatic electrocardiographic alterations in sarcoidosis. Am Heart J 1973; 86:474–477.
- Fahy GJ, Marwick T, McCreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109:62–66.
- Butany J, Bahl NE, Morales K, et al. The intricacies of cardiac sarcoidosis: a case report involving the coronary arteries and a review of the literature. Cardiovasc Pathol 2006; 15:222–227.
- Haywood LJ, Sharma OP, Siegel ME, et al. Detection of myocardial sarcoidosis by thallium-201 imaging. J Natl Med Assoc 1982; 74:959–964.
- Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol 2005; 185:110–115.
- Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990; 41:573–576.
- Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J 2007; 29:418–422.
- Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35:933–941.
- Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with 13N-NH3/18F-FDG PET. J Nucl Med 2003; 44:1030–1036.
- Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation 2002; 105:1144–1145.
- Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26:1538–1543.
- Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004; 45:1989–1998.
- Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006; 92:399–400.
- Kiuchi S, Teraoka K, Koizumi K, Takazawa K, Yamashina A. Usefulness of late gadolinium enhancement combined with MRI and 67-Ga scintigraphy in the diagnosis of cardiac sarcoidosis and disease activity evaluation. Int J Cardiovasc Imaging 2007; 23:237–241.
- Matsuki M, Matsuo M. MR findings of myocardial sarcoidosis. Clin Radiol 2000; 55:323–325.
- Inoue S, Shimada T, Murakami Y. Clinical significance of gadolinium-DTPA-enhanced MRI for detection of myocardial lesions in a patient with sarcoidosis. Clin Radiol 1999; 54:70–72.
- Vignaux O, Dhote R, Dudoc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrastenhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26:762–767.
- Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol 2005; 184:249–254.
- Smedema JP, Snoep G, Van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005; 45:1683–1690.
- Doherty MJ, Kumar SK, Nicholson AA, McGivern DV. Cardiac sarcoidosis: the value of magnetic resonance imagine in diagnosis and assessment of response to treatment. Respir Med 1998; 92:697–699.
- Smedema JP, Truter R, de Klerk PA, Zaaiman L, White L, Doubell AF. Cardiac sarcoidosis evaluated with gadolinium-enhanced magnetic resonance and contrast-enhanced 64-slice computed tomography. Int J Cardiol 2006; 112:261–263.
- Kanao S, Tadamura E, Yamamuro M, et al. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2005; 29:745–748.
- Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002; 122:1895–1901.
- Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001; 110:520–527.
- Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of longterm survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001; 88:1006–1010.
- Ishikawa T, Kondoh H, Nakagawa S, Koiwaya Y, Tanaka K. Steroid therapy in cardiac sarcoidosis. Increased left ventricular contractility concomitant with electrocardiographic improvement after prednisolone. Chest 1984; 85:445–447.
- Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978; 40:931–933.
- Lash R, Coker J, Wong BY. Treatment of heart block due to sarcoid heart disease. J Electrocardiol 1979; 12:325–329.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and implications. Ann N Y Acad Sci 1986; 465:702–712.
- Demeter SL. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988; 94:202–203.
- Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155:846–851.
- York EL, Kovithavongs T, Man SF, Rebuck AS, Sproule BJ. Cyclosporine and chronic sarcoidosis. Chest 1990; 98:1026–1029.
- Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991; 18:937–943.
- Bajaj AK, Kopelman HA, Echt DS. Cardiac sarcoidosis with sudden death: treatment with automatic implantable cardioverter defibrillator. Am Heart J 1988; 116:557–560.
- Paz HL, McCormick DJ, Kutalek SP, Patchefsky A. The automated implantable cardiac defibrillator. Prophylaxis in cardiac sarcoidosis. Chest 1994; 106:1603–1607.
- Becker D, Berger E, Chmielewski C. Cardiac sarcoidosis: a report of four cases with ventricular tachycardia. J Cardiovasc Electrophysiol 1990; 1:214–219.
- Zaidi AR, Zaidi A, Vaitkus PT. Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 2007; 26:714–717.
- Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant 1987; 6:244–250.
- Oni AA, Hershberger RE, Norman DJ, et al. Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 1992; 11:367–369.
- Burke WM, Keogh A, Maloney PJ, Delprado W, Bryant DH, Spratt P. Transmission of sarcoidosis via cardiac transplantation. Lancet 1990; 336:1579.
- Johns CJ, Schonfeld SA, Scott PP, Zachary JB, MacGregor MI. Longitudinal study of chronic sarcoidosis with low-dose maintenance corticosteroid therapy. Outcome and complications. Ann N Y Acad Sci 1986; 465:702–712.
- Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996; 100:423–427.
- Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986; 465:543–550.
- Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan. Clinical evaluation of corticosteroid therapy. Sarcoidosis 1994; 11:113–117.
A 37-year-old man with chest pain, ECG changes, and elevated cardiac enzymes
A 37-year-old African American man presents to the emergency department with chest pain and dyspnea, which began suddenly 30 minutes ago. The pain is severe, pressure-like, nonradiating, and pleuritic.
His heart rate is 88 beats per minute, blood pressure 135/72 mm Hg, respiratory rate 12 per minute, and oral temperature 38.5°C (101.3°F). His oxygen saturation by pulse oximetry is 99% while breathing room air. He is given sublingual nitroglycerin, but this does not alleviate his pain.
While blood samples are being drawn, we learn more about his history. He has hypertension, for which he takes amlodipine (Norvasc), and gastroesophageal reflux under control with esomeprazole (Nexium). He says he does not have hyperlipidemia, diabetes, or coronary artery disease and his surgical history is unremarkable. He says he does not smoke, rarely drinks, and does not use any drugs. No one in his family has had premature coronary artery disease.
He says he has had similar symptoms in the past few months, which resulted in two emergency room visits. Electrocardiograms at those times were unremarkable, and a stress test was negative for ischemia.
A computed tomographic (CT) scan of the chest was also obtained during one of those visits. The scan was negative for a pulmonary embolus but incidentally showed liver hemangiomas.
The patient’s initial laboratory results are shown in Table 1.
WHAT IS THE CAUSE OF HIS CHEST PAIN?
1. Which is the most likely cause of this patient’s chest pain?
- Acute myocardial infarction
- Acute pericarditis
- Myocarditis
- Pulmonary embolism
- Aortic dissection
- Pneumonia
Acute myocardial infarction. This is a young man with chest pain, ST-segment elevation, and elevated cardiac enzymes. Acute myocardial infarction should always be included in the differential diagnosis of such a patient, as recognizing it early and making an effort to rapidly restore blood flow to the myocardium can greatly improve the clinical outcome. However, particular features in his electrocardiogram and the duration and nature of his chest pain suggest another diagnosis.
Acute pericarditis causes pleuritic chest pain with diffuse ST-segment elevation, and its electrocardiographic changes may be difficult to distinguish from those of ischemia. The features in our patient’s electrocardiogram that point to pericarditis are1:
- ST-segment elevation that is concave upward, occurring in all leads except aVR
- T waves concordant with ST-segment deviation
- PR-segment depression, sparing V1 and aVR
- PR-segment elevation and ST depression in aVR.
Pleuritic chest pain is the most common symptom in acute pericarditis. A prodrome of fever, myalgia, and malaise is also common, especially in younger patients.2 On physical examination, a pericardial friction rub is pathognomonic.
Our patient has most if not all of the classic features of acute pericarditis. Elevated cardiac enzymes, which this patient has, are not a classic feature of pericarditis and are generally considered a marker of cardiac ischemia. However, because the myocardium is adjacent to the pericardium, the acute inflammatory process of acute pericarditis may also result in myocardial injury, resulting in release of creatine kinase-MB.3
An increase in cardiac troponin is also frequently observed in acute pericarditis, reflecting biochemical evidence of inflammatory myocardial cell damage.4 Furthermore, cardiac troponin can be elevated in several other medical conditions,5 such as ischemic heart disease, congestive heart failure, myocarditis, pulmonary embolism, severe pulmonary hypertension, significant left ventricular hypertrophy, renal failure, sepsis, critical illness, and subarachnoid hemorrhage. Therefore, cardiac enzymes are not good markers to distinguish between acute myocardial infarction and acute pericarditis. However, echocardiography is an effective way to help differentiate pericarditis from myocardial ischemia in the setting of elevated troponins and electrocardiographic changes, by determining if wall-motion abnormalities are present or absent.
Hence, the diagnosis of acute pericarditis should take into account the combination of the clinical picture, electrocardiographic findings, and laboratory values. Overreliance on any of these in isolation can lead to misdiagnosis.
Pulmonary embolism is another common cause of acute-onset pleuritic chest pain and dyspnea. Electrocardiographic changes can include ST-segment elevation, and cardiac enzymes can be elevated, although this is uncommon.
Myocarditis is commonly due to infections, collagen vascular diseases, or medications. Hallmarks of this disease are elevated cardiac enzymes and myocardial damage that results in reduction in heart function.
Aortic dissection typically causes a sharp, tearing chest pain that radiates to the back. This diagnosis is unlikely in this patient.
Pneumonia. Although our patient did not have a cough and no crackles were heard on lung examination to suggest pneumonia, his fever, pleuritic chest pain, and leukocytosis with a left shift warrant a workup for it. A parapneumonic effusion could manifest with fevers and pleuritic chest pain. However, the acuity of the symptoms and the characteristic electrocardiographic changes and elevated cardiac enzymes are better explained by the other diagnoses, notably acute pericarditis.
ACUTE PERICARDITIS: WHAT IS THE CAUSE?
2. Which is the most common cause of acute pericarditis?
- Idiopathic
- Neoplasm
- Autoimmune
- Tuberculosis
Most (approximately 80%) of cases of acute pericarditis are idiopathic.6,7 In a study in 100 patients with acute pericarditis,6 a specific cause was identified in only 22. The most common identified cause was neoplasm, which was present in seven patients: four with lung cancer and one each with breast carcinoma, cystic duct adenocarcinoma, and cardiac angiosarcoma.
CASE CONTINUES: PERICARDIAL EFFUSION
The patient is admitted to the hospital for additional workup. His fever, myalgia, and chest pain persist, though the pain is less intense than before.
A chest roentgenogram and transthoracic echocardiogram are ordered and blood cultures are drawn.
The roentgenogram shows marked cardiomegaly, bilateral small pleural effusions, and minimal atelectatic changes in the lungs.
Echocardiography reveals a normal ejection fraction (60%) and a moderate-sized pericardial effusion without evidence of tamponade.
3. Which is the most common cause of pericardial effusion?
- Idiopathic
- Infection
- Malignancy
- Collagen vascular disease
Pericardial effusion is relatively common after acute pericarditis but also has many other possible causes. In a study of 204 patients with pericardial effusion,8 48% of cases were labeled as idiopathic. Of the remaining 52%, the most common specific diagnoses were infection (16%) and cancer (15%). Collagen vascular disease accounted for 8% of the cases and included systemic lupus erythematosus, rheumatoid arthritis, and scleroderma.
Although small pericardial effusions are common in pericarditis, larger pericardial effusions or failure to respond to therapy necessitates additional workup.2
In our patient, an extensive workup is initiated to look for bacterial, viral, fungal, and autoimmune causes of pericardial effusion, but the results of the workup are negative.
TREATING ACUTE PERICARDITIS
4. Which is the most appropriate treatment for acute pericarditis?
- Steroids
- A nonsteroidal anti-inflammatory drug (NSAID) or aspirin
- Opioids
- Colchicine
- Colchicine plus an NSAID or aspirin
An NSAID or aspirin is the basis of treatment for acute pericarditis and is very effective in relieving symptoms. Aspirin 2–4 g daily, indomethacin (Indocin) 75–225 mg daily, or ibuprofen (Motrin) 1,600–3,200 mg daily are prescribed most often; ibuprofen is preferred because it has a lower incidence of adverse effects than the others.9
Colchicine is recommended in addition to aspirin or NSAIDs for the treatment of acute pericarditis. Although in the past colchicine was reserved for recurrent pericarditis, the Colchicine for Acute Pericarditis (COPE) trial10 found it to be beneficial for first episodes of pericarditis as well.10 In this study, patients were randomized to receive conventional treatment with aspirin 800 mg every 6 or 8 hours or aspirin at the same dose combined with colchicine 0.5 to 1.0 mg daily. Colchicine showed significant benefit over conventional therapy, resulting in reduced rates of recurrence.
CASE CONTINUES: HEPATIC LESIONS ON MRI
Although aspirin and colchicine were started at the time of admission, our patient’s symptoms fail to improve. A suspicion remains that a neoplastic disorder could be the underlying cause of the presentation and could explain his chronic malaise, pericardial disease, and fever. In view of the liver hemangiomas reported previously on CT, we decide to evaluate the liver further with magnetic resonance imaging (MRI).
Since our patient’s symptoms have improved significantly during the past few days and his fever has resolved, biopsy is scheduled on an outpatient basis. Biopsy with ultrasonographic guidance is performed a week later and yields a pathologic diagnosis of hemangioma. The improvement, however, is short-lived, and his pain and dyspnea recur after 2 months. A follow-up echocardiogram is ordered.
A remarkable echocardiographic finding
The original echocardiogram that was performed a little over 2-1/2 months ago is re-reviewed. It very subtly suggests a complexity to the pericardial effusion in the area of the current mass, apparent only when the two studies are directly compared. Clearly, there has been interval development of a mass easily detectable by echocardiography. Although a small mass may have been obscured by the pericardial effusion in the original echocardiogram, the development of a mass of this size in such a short time suggests a rapidly growing tumor.
CARDIAC TUMORS
5. Which is the most common primary cardiac tumor?
- Myxoma
- Papillary fibroelastoma
- Sarcoma
- Lymphoma
Primary cardiac tumors are rare, with an incidence on autopsy series ranging between 0.0017% and 0.33%,11,12 making them far less common than metastases to the heart.
Myxomas are benign cardiac tumors and are the most common primary cardiac neoplasm. Approximately 80% of myxomas originate in the left atrium, typically presenting with one or more of the triad of intracardiac obstruction, systemic embolization, and constitutional symptoms.14
Cardiac papillary fibroelastomas, the second most common cardiac tumors, are benign and predominantly affect the cardiac valves.15
Only one-fourth of all cardiac tumors are malignant. Nearly all of these malignant tumors are sarcomas, with angiosarcoma being the most common morphologic type, accounting for 30% of primary cardiac sarcomas.13
Primary cardiac lymphomas are extremely rare and account for only 1.3% of all primary cardiac tumors.16
A DIAGNOSIS IS MADE
CARDIAC ANGIOSARCOMA
Cardiac angiosarcoma, the most common malignant primary cardiac tumor, has a predilection for the right atrium.13 These tumors tend to occur between the third and fifth decade of life and are three times more common in men than in women. Cardiac sarcomas proliferate rapidly and commonly extend into the pericardial space, causing pericardial effusion in up to one-fourth of patients.
Surgical resection is the treatment of choice, but due to the location and extent of involvement, complete resection is often difficult. Also, distant metastases are present at the time of diagnosis in 80% of cases, precluding a surgical cure.17 Adjuvant chemotherapy, radiotherapy, and even heart transplantation do not substantially improve the survival of these patients.18–20 Because no effective treatment is available, the prognosis is dismal, with a median survival of 6 to 12 months.
Our patient is discharged home to follow up with an oncologist and initiate chemotherapy.
Acknowledgment: We thank Lisa M. Yerian, MD, for interpreting the biopsy specimens described in this article.
- Ariyarajah V, Spodick DH. Acute pericarditis: diagnostic cues and common electrocardiographic manifestations. Cardiol Rev 2007; 15:24–30.
- LeWinter MM, Kabbani S. Pericardial disease. In: Zipes DP, et al, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier/WB Saunders; 2005:1757–1781.
- Karjalainen J, Heikkila J. “Acute pericarditis”: myocardial enzyme release as evidence for myocarditis”. Am Heart J 1986; 111:546–552.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol 1995; 75:378–382.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007; 115:2739–2744.
- Levy PY, Corey R, Berger P, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore) 2003; 82:385–391.
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med 2004; 351:2195–2202.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: Results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112:2012–2016.
- Heath D. Pathology of cardiac tumors. Am J Cardiol 1968; 21:315–327.
- Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol 1980; 101:219–240.
- Sabatine MS, Colucci WS, Schoen FS. Primary tumors of the heart. In: Zipes DP, et al, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier/WB Saunders; 2005:1741–1757.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Glancy DL, Morales JB, Roberts WC. Angiosarcoma of the heart. Am J Cardiol 1968; 21:413–419.
- Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer 1997; 80:1497–1506.
- Bear PA, Moodie DS. Malignant primary cardiac tumors. The Cleveland Clinic experience, 1956 to 1986. Chest 1987; 92:860–862.
- Llombart–Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer 1998; 78:1624–1628.
- Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg 1991; 51:906–910.
- Conklin LD, Reardon MJ. Autotransplantation of the heart for primary cardiac malignancy: development and surgical technique. Tex Heart Inst J 2002; 29:105–108.
A 37-year-old African American man presents to the emergency department with chest pain and dyspnea, which began suddenly 30 minutes ago. The pain is severe, pressure-like, nonradiating, and pleuritic.
His heart rate is 88 beats per minute, blood pressure 135/72 mm Hg, respiratory rate 12 per minute, and oral temperature 38.5°C (101.3°F). His oxygen saturation by pulse oximetry is 99% while breathing room air. He is given sublingual nitroglycerin, but this does not alleviate his pain.
While blood samples are being drawn, we learn more about his history. He has hypertension, for which he takes amlodipine (Norvasc), and gastroesophageal reflux under control with esomeprazole (Nexium). He says he does not have hyperlipidemia, diabetes, or coronary artery disease and his surgical history is unremarkable. He says he does not smoke, rarely drinks, and does not use any drugs. No one in his family has had premature coronary artery disease.
He says he has had similar symptoms in the past few months, which resulted in two emergency room visits. Electrocardiograms at those times were unremarkable, and a stress test was negative for ischemia.
A computed tomographic (CT) scan of the chest was also obtained during one of those visits. The scan was negative for a pulmonary embolus but incidentally showed liver hemangiomas.
The patient’s initial laboratory results are shown in Table 1.
WHAT IS THE CAUSE OF HIS CHEST PAIN?
1. Which is the most likely cause of this patient’s chest pain?
- Acute myocardial infarction
- Acute pericarditis
- Myocarditis
- Pulmonary embolism
- Aortic dissection
- Pneumonia
Acute myocardial infarction. This is a young man with chest pain, ST-segment elevation, and elevated cardiac enzymes. Acute myocardial infarction should always be included in the differential diagnosis of such a patient, as recognizing it early and making an effort to rapidly restore blood flow to the myocardium can greatly improve the clinical outcome. However, particular features in his electrocardiogram and the duration and nature of his chest pain suggest another diagnosis.
Acute pericarditis causes pleuritic chest pain with diffuse ST-segment elevation, and its electrocardiographic changes may be difficult to distinguish from those of ischemia. The features in our patient’s electrocardiogram that point to pericarditis are1:
- ST-segment elevation that is concave upward, occurring in all leads except aVR
- T waves concordant with ST-segment deviation
- PR-segment depression, sparing V1 and aVR
- PR-segment elevation and ST depression in aVR.
Pleuritic chest pain is the most common symptom in acute pericarditis. A prodrome of fever, myalgia, and malaise is also common, especially in younger patients.2 On physical examination, a pericardial friction rub is pathognomonic.
Our patient has most if not all of the classic features of acute pericarditis. Elevated cardiac enzymes, which this patient has, are not a classic feature of pericarditis and are generally considered a marker of cardiac ischemia. However, because the myocardium is adjacent to the pericardium, the acute inflammatory process of acute pericarditis may also result in myocardial injury, resulting in release of creatine kinase-MB.3
An increase in cardiac troponin is also frequently observed in acute pericarditis, reflecting biochemical evidence of inflammatory myocardial cell damage.4 Furthermore, cardiac troponin can be elevated in several other medical conditions,5 such as ischemic heart disease, congestive heart failure, myocarditis, pulmonary embolism, severe pulmonary hypertension, significant left ventricular hypertrophy, renal failure, sepsis, critical illness, and subarachnoid hemorrhage. Therefore, cardiac enzymes are not good markers to distinguish between acute myocardial infarction and acute pericarditis. However, echocardiography is an effective way to help differentiate pericarditis from myocardial ischemia in the setting of elevated troponins and electrocardiographic changes, by determining if wall-motion abnormalities are present or absent.
Hence, the diagnosis of acute pericarditis should take into account the combination of the clinical picture, electrocardiographic findings, and laboratory values. Overreliance on any of these in isolation can lead to misdiagnosis.
Pulmonary embolism is another common cause of acute-onset pleuritic chest pain and dyspnea. Electrocardiographic changes can include ST-segment elevation, and cardiac enzymes can be elevated, although this is uncommon.
Myocarditis is commonly due to infections, collagen vascular diseases, or medications. Hallmarks of this disease are elevated cardiac enzymes and myocardial damage that results in reduction in heart function.
Aortic dissection typically causes a sharp, tearing chest pain that radiates to the back. This diagnosis is unlikely in this patient.
Pneumonia. Although our patient did not have a cough and no crackles were heard on lung examination to suggest pneumonia, his fever, pleuritic chest pain, and leukocytosis with a left shift warrant a workup for it. A parapneumonic effusion could manifest with fevers and pleuritic chest pain. However, the acuity of the symptoms and the characteristic electrocardiographic changes and elevated cardiac enzymes are better explained by the other diagnoses, notably acute pericarditis.
ACUTE PERICARDITIS: WHAT IS THE CAUSE?
2. Which is the most common cause of acute pericarditis?
- Idiopathic
- Neoplasm
- Autoimmune
- Tuberculosis
Most (approximately 80%) of cases of acute pericarditis are idiopathic.6,7 In a study in 100 patients with acute pericarditis,6 a specific cause was identified in only 22. The most common identified cause was neoplasm, which was present in seven patients: four with lung cancer and one each with breast carcinoma, cystic duct adenocarcinoma, and cardiac angiosarcoma.
CASE CONTINUES: PERICARDIAL EFFUSION
The patient is admitted to the hospital for additional workup. His fever, myalgia, and chest pain persist, though the pain is less intense than before.
A chest roentgenogram and transthoracic echocardiogram are ordered and blood cultures are drawn.
The roentgenogram shows marked cardiomegaly, bilateral small pleural effusions, and minimal atelectatic changes in the lungs.
Echocardiography reveals a normal ejection fraction (60%) and a moderate-sized pericardial effusion without evidence of tamponade.
3. Which is the most common cause of pericardial effusion?
- Idiopathic
- Infection
- Malignancy
- Collagen vascular disease
Pericardial effusion is relatively common after acute pericarditis but also has many other possible causes. In a study of 204 patients with pericardial effusion,8 48% of cases were labeled as idiopathic. Of the remaining 52%, the most common specific diagnoses were infection (16%) and cancer (15%). Collagen vascular disease accounted for 8% of the cases and included systemic lupus erythematosus, rheumatoid arthritis, and scleroderma.
Although small pericardial effusions are common in pericarditis, larger pericardial effusions or failure to respond to therapy necessitates additional workup.2
In our patient, an extensive workup is initiated to look for bacterial, viral, fungal, and autoimmune causes of pericardial effusion, but the results of the workup are negative.
TREATING ACUTE PERICARDITIS
4. Which is the most appropriate treatment for acute pericarditis?
- Steroids
- A nonsteroidal anti-inflammatory drug (NSAID) or aspirin
- Opioids
- Colchicine
- Colchicine plus an NSAID or aspirin
An NSAID or aspirin is the basis of treatment for acute pericarditis and is very effective in relieving symptoms. Aspirin 2–4 g daily, indomethacin (Indocin) 75–225 mg daily, or ibuprofen (Motrin) 1,600–3,200 mg daily are prescribed most often; ibuprofen is preferred because it has a lower incidence of adverse effects than the others.9
Colchicine is recommended in addition to aspirin or NSAIDs for the treatment of acute pericarditis. Although in the past colchicine was reserved for recurrent pericarditis, the Colchicine for Acute Pericarditis (COPE) trial10 found it to be beneficial for first episodes of pericarditis as well.10 In this study, patients were randomized to receive conventional treatment with aspirin 800 mg every 6 or 8 hours or aspirin at the same dose combined with colchicine 0.5 to 1.0 mg daily. Colchicine showed significant benefit over conventional therapy, resulting in reduced rates of recurrence.
CASE CONTINUES: HEPATIC LESIONS ON MRI
Although aspirin and colchicine were started at the time of admission, our patient’s symptoms fail to improve. A suspicion remains that a neoplastic disorder could be the underlying cause of the presentation and could explain his chronic malaise, pericardial disease, and fever. In view of the liver hemangiomas reported previously on CT, we decide to evaluate the liver further with magnetic resonance imaging (MRI).
Since our patient’s symptoms have improved significantly during the past few days and his fever has resolved, biopsy is scheduled on an outpatient basis. Biopsy with ultrasonographic guidance is performed a week later and yields a pathologic diagnosis of hemangioma. The improvement, however, is short-lived, and his pain and dyspnea recur after 2 months. A follow-up echocardiogram is ordered.
A remarkable echocardiographic finding
The original echocardiogram that was performed a little over 2-1/2 months ago is re-reviewed. It very subtly suggests a complexity to the pericardial effusion in the area of the current mass, apparent only when the two studies are directly compared. Clearly, there has been interval development of a mass easily detectable by echocardiography. Although a small mass may have been obscured by the pericardial effusion in the original echocardiogram, the development of a mass of this size in such a short time suggests a rapidly growing tumor.
CARDIAC TUMORS
5. Which is the most common primary cardiac tumor?
- Myxoma
- Papillary fibroelastoma
- Sarcoma
- Lymphoma
Primary cardiac tumors are rare, with an incidence on autopsy series ranging between 0.0017% and 0.33%,11,12 making them far less common than metastases to the heart.
Myxomas are benign cardiac tumors and are the most common primary cardiac neoplasm. Approximately 80% of myxomas originate in the left atrium, typically presenting with one or more of the triad of intracardiac obstruction, systemic embolization, and constitutional symptoms.14
Cardiac papillary fibroelastomas, the second most common cardiac tumors, are benign and predominantly affect the cardiac valves.15
Only one-fourth of all cardiac tumors are malignant. Nearly all of these malignant tumors are sarcomas, with angiosarcoma being the most common morphologic type, accounting for 30% of primary cardiac sarcomas.13
Primary cardiac lymphomas are extremely rare and account for only 1.3% of all primary cardiac tumors.16
A DIAGNOSIS IS MADE
CARDIAC ANGIOSARCOMA
Cardiac angiosarcoma, the most common malignant primary cardiac tumor, has a predilection for the right atrium.13 These tumors tend to occur between the third and fifth decade of life and are three times more common in men than in women. Cardiac sarcomas proliferate rapidly and commonly extend into the pericardial space, causing pericardial effusion in up to one-fourth of patients.
Surgical resection is the treatment of choice, but due to the location and extent of involvement, complete resection is often difficult. Also, distant metastases are present at the time of diagnosis in 80% of cases, precluding a surgical cure.17 Adjuvant chemotherapy, radiotherapy, and even heart transplantation do not substantially improve the survival of these patients.18–20 Because no effective treatment is available, the prognosis is dismal, with a median survival of 6 to 12 months.
Our patient is discharged home to follow up with an oncologist and initiate chemotherapy.
Acknowledgment: We thank Lisa M. Yerian, MD, for interpreting the biopsy specimens described in this article.
A 37-year-old African American man presents to the emergency department with chest pain and dyspnea, which began suddenly 30 minutes ago. The pain is severe, pressure-like, nonradiating, and pleuritic.
His heart rate is 88 beats per minute, blood pressure 135/72 mm Hg, respiratory rate 12 per minute, and oral temperature 38.5°C (101.3°F). His oxygen saturation by pulse oximetry is 99% while breathing room air. He is given sublingual nitroglycerin, but this does not alleviate his pain.
While blood samples are being drawn, we learn more about his history. He has hypertension, for which he takes amlodipine (Norvasc), and gastroesophageal reflux under control with esomeprazole (Nexium). He says he does not have hyperlipidemia, diabetes, or coronary artery disease and his surgical history is unremarkable. He says he does not smoke, rarely drinks, and does not use any drugs. No one in his family has had premature coronary artery disease.
He says he has had similar symptoms in the past few months, which resulted in two emergency room visits. Electrocardiograms at those times were unremarkable, and a stress test was negative for ischemia.
A computed tomographic (CT) scan of the chest was also obtained during one of those visits. The scan was negative for a pulmonary embolus but incidentally showed liver hemangiomas.
The patient’s initial laboratory results are shown in Table 1.
WHAT IS THE CAUSE OF HIS CHEST PAIN?
1. Which is the most likely cause of this patient’s chest pain?
- Acute myocardial infarction
- Acute pericarditis
- Myocarditis
- Pulmonary embolism
- Aortic dissection
- Pneumonia
Acute myocardial infarction. This is a young man with chest pain, ST-segment elevation, and elevated cardiac enzymes. Acute myocardial infarction should always be included in the differential diagnosis of such a patient, as recognizing it early and making an effort to rapidly restore blood flow to the myocardium can greatly improve the clinical outcome. However, particular features in his electrocardiogram and the duration and nature of his chest pain suggest another diagnosis.
Acute pericarditis causes pleuritic chest pain with diffuse ST-segment elevation, and its electrocardiographic changes may be difficult to distinguish from those of ischemia. The features in our patient’s electrocardiogram that point to pericarditis are1:
- ST-segment elevation that is concave upward, occurring in all leads except aVR
- T waves concordant with ST-segment deviation
- PR-segment depression, sparing V1 and aVR
- PR-segment elevation and ST depression in aVR.
Pleuritic chest pain is the most common symptom in acute pericarditis. A prodrome of fever, myalgia, and malaise is also common, especially in younger patients.2 On physical examination, a pericardial friction rub is pathognomonic.
Our patient has most if not all of the classic features of acute pericarditis. Elevated cardiac enzymes, which this patient has, are not a classic feature of pericarditis and are generally considered a marker of cardiac ischemia. However, because the myocardium is adjacent to the pericardium, the acute inflammatory process of acute pericarditis may also result in myocardial injury, resulting in release of creatine kinase-MB.3
An increase in cardiac troponin is also frequently observed in acute pericarditis, reflecting biochemical evidence of inflammatory myocardial cell damage.4 Furthermore, cardiac troponin can be elevated in several other medical conditions,5 such as ischemic heart disease, congestive heart failure, myocarditis, pulmonary embolism, severe pulmonary hypertension, significant left ventricular hypertrophy, renal failure, sepsis, critical illness, and subarachnoid hemorrhage. Therefore, cardiac enzymes are not good markers to distinguish between acute myocardial infarction and acute pericarditis. However, echocardiography is an effective way to help differentiate pericarditis from myocardial ischemia in the setting of elevated troponins and electrocardiographic changes, by determining if wall-motion abnormalities are present or absent.
Hence, the diagnosis of acute pericarditis should take into account the combination of the clinical picture, electrocardiographic findings, and laboratory values. Overreliance on any of these in isolation can lead to misdiagnosis.
Pulmonary embolism is another common cause of acute-onset pleuritic chest pain and dyspnea. Electrocardiographic changes can include ST-segment elevation, and cardiac enzymes can be elevated, although this is uncommon.
Myocarditis is commonly due to infections, collagen vascular diseases, or medications. Hallmarks of this disease are elevated cardiac enzymes and myocardial damage that results in reduction in heart function.
Aortic dissection typically causes a sharp, tearing chest pain that radiates to the back. This diagnosis is unlikely in this patient.
Pneumonia. Although our patient did not have a cough and no crackles were heard on lung examination to suggest pneumonia, his fever, pleuritic chest pain, and leukocytosis with a left shift warrant a workup for it. A parapneumonic effusion could manifest with fevers and pleuritic chest pain. However, the acuity of the symptoms and the characteristic electrocardiographic changes and elevated cardiac enzymes are better explained by the other diagnoses, notably acute pericarditis.
ACUTE PERICARDITIS: WHAT IS THE CAUSE?
2. Which is the most common cause of acute pericarditis?
- Idiopathic
- Neoplasm
- Autoimmune
- Tuberculosis
Most (approximately 80%) of cases of acute pericarditis are idiopathic.6,7 In a study in 100 patients with acute pericarditis,6 a specific cause was identified in only 22. The most common identified cause was neoplasm, which was present in seven patients: four with lung cancer and one each with breast carcinoma, cystic duct adenocarcinoma, and cardiac angiosarcoma.
CASE CONTINUES: PERICARDIAL EFFUSION
The patient is admitted to the hospital for additional workup. His fever, myalgia, and chest pain persist, though the pain is less intense than before.
A chest roentgenogram and transthoracic echocardiogram are ordered and blood cultures are drawn.
The roentgenogram shows marked cardiomegaly, bilateral small pleural effusions, and minimal atelectatic changes in the lungs.
Echocardiography reveals a normal ejection fraction (60%) and a moderate-sized pericardial effusion without evidence of tamponade.
3. Which is the most common cause of pericardial effusion?
- Idiopathic
- Infection
- Malignancy
- Collagen vascular disease
Pericardial effusion is relatively common after acute pericarditis but also has many other possible causes. In a study of 204 patients with pericardial effusion,8 48% of cases were labeled as idiopathic. Of the remaining 52%, the most common specific diagnoses were infection (16%) and cancer (15%). Collagen vascular disease accounted for 8% of the cases and included systemic lupus erythematosus, rheumatoid arthritis, and scleroderma.
Although small pericardial effusions are common in pericarditis, larger pericardial effusions or failure to respond to therapy necessitates additional workup.2
In our patient, an extensive workup is initiated to look for bacterial, viral, fungal, and autoimmune causes of pericardial effusion, but the results of the workup are negative.
TREATING ACUTE PERICARDITIS
4. Which is the most appropriate treatment for acute pericarditis?
- Steroids
- A nonsteroidal anti-inflammatory drug (NSAID) or aspirin
- Opioids
- Colchicine
- Colchicine plus an NSAID or aspirin
An NSAID or aspirin is the basis of treatment for acute pericarditis and is very effective in relieving symptoms. Aspirin 2–4 g daily, indomethacin (Indocin) 75–225 mg daily, or ibuprofen (Motrin) 1,600–3,200 mg daily are prescribed most often; ibuprofen is preferred because it has a lower incidence of adverse effects than the others.9
Colchicine is recommended in addition to aspirin or NSAIDs for the treatment of acute pericarditis. Although in the past colchicine was reserved for recurrent pericarditis, the Colchicine for Acute Pericarditis (COPE) trial10 found it to be beneficial for first episodes of pericarditis as well.10 In this study, patients were randomized to receive conventional treatment with aspirin 800 mg every 6 or 8 hours or aspirin at the same dose combined with colchicine 0.5 to 1.0 mg daily. Colchicine showed significant benefit over conventional therapy, resulting in reduced rates of recurrence.
CASE CONTINUES: HEPATIC LESIONS ON MRI
Although aspirin and colchicine were started at the time of admission, our patient’s symptoms fail to improve. A suspicion remains that a neoplastic disorder could be the underlying cause of the presentation and could explain his chronic malaise, pericardial disease, and fever. In view of the liver hemangiomas reported previously on CT, we decide to evaluate the liver further with magnetic resonance imaging (MRI).
Since our patient’s symptoms have improved significantly during the past few days and his fever has resolved, biopsy is scheduled on an outpatient basis. Biopsy with ultrasonographic guidance is performed a week later and yields a pathologic diagnosis of hemangioma. The improvement, however, is short-lived, and his pain and dyspnea recur after 2 months. A follow-up echocardiogram is ordered.
A remarkable echocardiographic finding
The original echocardiogram that was performed a little over 2-1/2 months ago is re-reviewed. It very subtly suggests a complexity to the pericardial effusion in the area of the current mass, apparent only when the two studies are directly compared. Clearly, there has been interval development of a mass easily detectable by echocardiography. Although a small mass may have been obscured by the pericardial effusion in the original echocardiogram, the development of a mass of this size in such a short time suggests a rapidly growing tumor.
CARDIAC TUMORS
5. Which is the most common primary cardiac tumor?
- Myxoma
- Papillary fibroelastoma
- Sarcoma
- Lymphoma
Primary cardiac tumors are rare, with an incidence on autopsy series ranging between 0.0017% and 0.33%,11,12 making them far less common than metastases to the heart.
Myxomas are benign cardiac tumors and are the most common primary cardiac neoplasm. Approximately 80% of myxomas originate in the left atrium, typically presenting with one or more of the triad of intracardiac obstruction, systemic embolization, and constitutional symptoms.14
Cardiac papillary fibroelastomas, the second most common cardiac tumors, are benign and predominantly affect the cardiac valves.15
Only one-fourth of all cardiac tumors are malignant. Nearly all of these malignant tumors are sarcomas, with angiosarcoma being the most common morphologic type, accounting for 30% of primary cardiac sarcomas.13
Primary cardiac lymphomas are extremely rare and account for only 1.3% of all primary cardiac tumors.16
A DIAGNOSIS IS MADE
CARDIAC ANGIOSARCOMA
Cardiac angiosarcoma, the most common malignant primary cardiac tumor, has a predilection for the right atrium.13 These tumors tend to occur between the third and fifth decade of life and are three times more common in men than in women. Cardiac sarcomas proliferate rapidly and commonly extend into the pericardial space, causing pericardial effusion in up to one-fourth of patients.
Surgical resection is the treatment of choice, but due to the location and extent of involvement, complete resection is often difficult. Also, distant metastases are present at the time of diagnosis in 80% of cases, precluding a surgical cure.17 Adjuvant chemotherapy, radiotherapy, and even heart transplantation do not substantially improve the survival of these patients.18–20 Because no effective treatment is available, the prognosis is dismal, with a median survival of 6 to 12 months.
Our patient is discharged home to follow up with an oncologist and initiate chemotherapy.
Acknowledgment: We thank Lisa M. Yerian, MD, for interpreting the biopsy specimens described in this article.
- Ariyarajah V, Spodick DH. Acute pericarditis: diagnostic cues and common electrocardiographic manifestations. Cardiol Rev 2007; 15:24–30.
- LeWinter MM, Kabbani S. Pericardial disease. In: Zipes DP, et al, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier/WB Saunders; 2005:1757–1781.
- Karjalainen J, Heikkila J. “Acute pericarditis”: myocardial enzyme release as evidence for myocarditis”. Am Heart J 1986; 111:546–552.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol 1995; 75:378–382.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007; 115:2739–2744.
- Levy PY, Corey R, Berger P, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore) 2003; 82:385–391.
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med 2004; 351:2195–2202.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: Results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112:2012–2016.
- Heath D. Pathology of cardiac tumors. Am J Cardiol 1968; 21:315–327.
- Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol 1980; 101:219–240.
- Sabatine MS, Colucci WS, Schoen FS. Primary tumors of the heart. In: Zipes DP, et al, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier/WB Saunders; 2005:1741–1757.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Glancy DL, Morales JB, Roberts WC. Angiosarcoma of the heart. Am J Cardiol 1968; 21:413–419.
- Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer 1997; 80:1497–1506.
- Bear PA, Moodie DS. Malignant primary cardiac tumors. The Cleveland Clinic experience, 1956 to 1986. Chest 1987; 92:860–862.
- Llombart–Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer 1998; 78:1624–1628.
- Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg 1991; 51:906–910.
- Conklin LD, Reardon MJ. Autotransplantation of the heart for primary cardiac malignancy: development and surgical technique. Tex Heart Inst J 2002; 29:105–108.
- Ariyarajah V, Spodick DH. Acute pericarditis: diagnostic cues and common electrocardiographic manifestations. Cardiol Rev 2007; 15:24–30.
- LeWinter MM, Kabbani S. Pericardial disease. In: Zipes DP, et al, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier/WB Saunders; 2005:1757–1781.
- Karjalainen J, Heikkila J. “Acute pericarditis”: myocardial enzyme release as evidence for myocarditis”. Am Heart J 1986; 111:546–552.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol 1995; 75:378–382.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007; 115:2739–2744.
- Levy PY, Corey R, Berger P, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore) 2003; 82:385–391.
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med 2004; 351:2195–2202.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: Results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112:2012–2016.
- Heath D. Pathology of cardiac tumors. Am J Cardiol 1968; 21:315–327.
- Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol 1980; 101:219–240.
- Sabatine MS, Colucci WS, Schoen FS. Primary tumors of the heart. In: Zipes DP, et al, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: Elsevier/WB Saunders; 2005:1741–1757.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Glancy DL, Morales JB, Roberts WC. Angiosarcoma of the heart. Am J Cardiol 1968; 21:413–419.
- Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer 1997; 80:1497–1506.
- Bear PA, Moodie DS. Malignant primary cardiac tumors. The Cleveland Clinic experience, 1956 to 1986. Chest 1987; 92:860–862.
- Llombart–Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer 1998; 78:1624–1628.
- Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg 1991; 51:906–910.
- Conklin LD, Reardon MJ. Autotransplantation of the heart for primary cardiac malignancy: development and surgical technique. Tex Heart Inst J 2002; 29:105–108.
An elderly woman with shortness of breath
A frail 75-year-old woman with diabetes, hyperlipidemia, and a history of rheumatic fever in childhood presents to the emergency department of a community hospital with complaints of chest pressure, shortness of breath on exertion, and easy fatigability. Her shortness of breath started 6 months ago but has become much worse over the past few days.
On examination, her pulse is 110 and irregular, and she has markedly distended neck veins and evidence of pulmonary edema. She has a systolic murmur, but it is difficult to characterize due to tachycardia. Electrocardiography shows atrial fibrillation with rapid ventricular response and right axis deviation. Chest radiography shows bilateral pleural effusions.
The patient is given a diuretic, anticoagulation is started to prevent thromboembolism, and she undergoes cardioversion for the atrial fibrillation.
Transthoracic echocardiography is performed and reveals biatrial enlargement, anterior mitral valve leaflet thickening, mitral valve calcification, and moderate mitral regurgitation. Her ejection fraction is normal, but she has mild right ventricular systolic dysfunction with moderate tricuspid regurgitation and an estimated right ventricular systolic pressure of 90 mm Hg (normal range 15–30 mm Hg).
After an uneventful hospital course, she is discharged on a stable dose of a diuretic and oral anticoagulation. Despite adequate diuresis and maintenance of normal sinus rhythm, however, she continues to experience severe dyspnea, which limits her ability to perform simple tasks, such as dusting the furniture in her home. She is referred to Cleveland Clinic for further evaluation.
WHAT IS THE CAUSE OF HER SYMPTOMS?
1. What was the most likely cause of this patient’s initial acute presentation to the emergency department?
- An acute decrease in mitral valve area
- Rheumatic mitral valve stenosis
- Acute coronary syndrome
- Atrial fibrillation
Although an acute decrease in mitral valve area caused by an atrial myxoma, thrombus, or vegetation is a possibility, this patient’s symptoms gradually increased over a period of months. She also did not have signs of infection, nor did she have any signs of embolic phenomena to suggest myxoma, thrombus, or vegetation, all of which frequently present with emboli.
Given her history of rheumatic fever and her echocardiographic findings, rheumatic mitral valve stenosis is high on the list of differential diagnoses. Rheumatic mitral valve stenosis is a chronic process in which the valve area decreases at a rate of about 0.1 cm2/year.1,2
Acute coronary syndrome is a possibility in this elderly woman with multiple risk factors for coronary artery disease. An evaluation for coronary insufficiency should be considered, but the most striking finding on her initial electrocardiogram is atrial fibrillation with a rapid ventricular response.
The onset of atrial fibrillation in the setting of valvular heart disease is the most likely cause of her acute decompensation with signs and symptoms of congestive heart failure. In severe mitral stenosis, because the mitral valve orifice is narrowed, a higher left atrial pressure and longer ventricular filling time are required to maintain forward flow. Now add atrial fibrillation to this situation: the left atrium no longer contracts properly, so less blood is forced through the narrowed valve, and with the rapid heart rate the left ventricle has less time to fill. These two conditions result in elevated left atrial and pulmonary venous pressures, which in turn result in pulmonary edema and congestive heart failure. Thus, patients with mitral stenosis tolerate atrial fibrillation poorly.
WHAT IS THE NEXT STEP?
2. What would be the most appropriate next step for our patient?
- Transesophageal echocardiography
- Right heart catheterization
- An exercise treadmill stress test0
Transesophageal echocardiography is a reasonable option, as mitral stenosis is strongly suspected but transthoracic echocardiography did not reveal severe mitral valve disease. The use of transesophageal echocardiography for the diagnosis of mitral stenosis in this situation is a class IC recommendation (ie, the procedure is recommended, although very few trials have been done) in the American College of Cardiology and American Heart Association Valvular Disease guidelines.3
Right heart catheterization is the diagnostic test of choice in this situation, as the patient has evidence of biventricular heart failure and her right ventricular systolic pressure of 90 mm Hg is consistent with severe pulmonary hypertension. Right heart catheterization would help differentiate primary pulmonary hypertension causing dyspnea from pulmonary hypertension secondary to elevated left-sided pressures. It would also provide a direct hemodynamic estimate of her cardiac function.
Exercise treadmill testing. Evaluation for myocardial ischemia is a reasonable option, as our patient is elderly and has hypertension and diabetes, both of which are risk factors for coronary artery disease. Moreover, in a patient with diabetes, myocardial ischemia can present as dyspnea without typical anginal chest pain. Because of her age and severely limiting dyspnea, however, she would be unlikely to achieve an adequate heart rate during exercise treadmill testing, so this may not be the optimal type of stress test.
Although asymptomatic patients with moderate or severe mitral stenosis should undergo evaluation of exercise capacity and change in pulmonary artery pressures with exercise to determine the need for percutaneous balloon mitral valvuloplasty (see below),3 our patient is symptomatic and already has evidence of severe pulmonary hypertension on transthoracic echocardiography.
Other options for evaluating for myocardial ischemia include pharmacologic stress testing with imaging (eg, dobutamine echocardiography or adenosine nuclear imaging) or proceeding directly to coronary angiography.
CASE CONTINUED: RIGHT HEART CATHETERIZATION, CORONARY ANGIOGRAPHY
- Mean right atrial pressure 6 mm Hg (normal 2–7 mm Hg)
- Right ventricular pressure 102/6 mm Hg (consistent with severe pulmonary hypertension) (normal 15–30/1–7 mm Hg)
- Pulmonary artery pressure 102/40 mm Hg (normal 15–30/4–12 mm Hg)
- Mean pulmonary capillary wedge pressure 25 mm Hg (normal 4–12 mm Hg)
- Cardiac output 3.16 L/min (normal 4–8 L/min)
- Cardiac index 2.10 L/min/m2 (normal 2.5–4.2 L/min/m2)
- v waves are not prominent.
Because her symptoms raise concern for ischemia, coronary angiography is also performed and shows minimal, nonobstructive coronary artery disease. Her left ventricular end-diastolic pressure is 8 mm Hg (normal 5–12 mm Hg).
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis?
- Tricuspid stenosis
- Pulmonic stenosis
- Mitral stenosis
- Mitral regurgitation
Tricuspid stenosis would result in a higher pressure in the right atrium than in the right ventricle. In our patient, the right atrial pressure and the right ventricular diastolic pressure are both 6 mm Hg, eliminating this possibility.
Similarly, pulmonic stenosis would result in a higher pressure in the right ventricle than in the pulmonary artery. In our patient both the right ventricular systolic pressure and the pulmonary artery systolic pressures are 102 mm Hg.
Acute mitral regurgitation may result in increased wedge pressure and tall v waves (reflecting left atrial filling during ventricular systole). In chronic mitral regurgitation, however, the wedge pressure may be normal and the patient may have relatively normal-appearing v waves.4
Mitral stenosis results in a marked gradient between the pulmonary capillary wedge pressure and the left ventricular diastolic pressure in the absence of pulmonary veno-occlusive disease. This gradient can be measured by simultaneous catheterization of the right heart (to measure the wedge pressure, which is an indirect measure of left atrial pressure) and the left heart (to measure the left ventricular diastolic pressure). If the patient does not have significant mitral stenosis, the wedge pressure should be approximately equal to the left ventricular diastolic pressure. In our patient, the wedge pressure (and therefore the left atrial pressure) is 25 mm Hg, and the left ventricular end-diastolic pressure is 8 mm Hg—a difference of 17 mm Hg, consistent with significant mitral stenosis.
CASE CONTINUED: TRANSESOPHAGEAL ECHOCARDIOGRAPHY
WHAT IS THE TREATMENT?
4. Which of the following is the preferred technique for correcting mitral stenosis in this patient?
- Percutaneous balloon mitral valvuloplasty
- Mitral valve surgery
- Percutaneous mitral valve replacement
Although there are several options for mechanical treatment of mitral stenosis, percutaneous balloon mitral valvuloplasty by experienced operators is the procedure of choice for patients who have symptomatic moderate-to-severe mitral stenosis with favorable valve morphology but do not have significant mitral regurgitation or left atrial thrombus.3 The hemodynamic and symptomatic improvement that can be expected after this procedure can be predicted using several echocardiographic criteria, including valve mobility, subvalvular thickening, valve leaflet thickening, and valve leaflet calcification,5 as well as the degree of commissural calcification or commissural fusion.6 Success rates are better if the valve is relatively more mobile and has lesser degrees of valvular and subvalvular thickening, calcification, and commissural fusion.
Mitral valve surgery (repair if possible) is indicated in patients with acceptable operative risk who have symptomatic (New York Heart Association class III or IV) moderate-to-severe mitral stenosis if percutaneous balloon mitral valvuloplasty is unavailable, in cases in which an atrial thrombus or moderate-to-severe mitral regurgitation precludes balloon valvuloplasty, or when the valve morphology is not favorable for balloon valvulo-plasty.3
Although she has moderate mitral regurgitation and poor valve morphology, our patient is a poor surgical candidate because of her advanced age, severe pulmonary hypertension, and poor functional status. Patients with moderate-to-severe mitral stenosis and class III or IV symptoms who have nonpliable, calcified valves but are not candidates for open heart surgery have a class IIb indication for percutaneous balloon mitral valvuloplasty—ie, the procedure may be considered.3
In addition, the procedure also carries a class IIb recommendation in patients with moderate-to-severe mitral stenosis and new-onset atrial fibrillation (provided that they do not have a thrombus in the left atrium or moderate-to-severe mitral regurgitation), even without symptoms.3
Percutaneous mitral valve replacement is not available in clinical practice, although this is an active area of clinical research and may be available in the future.
CASE CONTINUED: THE PATIENT UNDERGOES BALLOON VALVULOPLASTY
Transthoracic echocardiography performed 4 months later shows moderate mitral stenosis with a mean gradient of 9.0 mm Hg, a mitral valve area of 1.7 cm2, and moderate mitral regurgitation. Her right ventricular systolic pressure is estimated to be 74 mm Hg. The patient reports less dyspnea during her housework and now has New York Heart Association class II symptoms. Her treatment regimen includes warfarin (Coumadin) for atrial fibrillation, a beta-blocker to control her heart rate in atrial fibrillation and increase her left ventricular filling time, and a low-dose diuretic.
- Gordon SP, Douglas PS, Come PC, et al. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Coll Cardiol 1992; 19:968–973.
- Sagie A, Freitas N, Padial LR, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol 1996; 28:472–479.
- Bonow ROC, Blase A, Chatterjee K, et al. ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:598–675.
- Braunwald E. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation 1963; 27:29–35.
- Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308.
- Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol 1997; 29:175–180.
A frail 75-year-old woman with diabetes, hyperlipidemia, and a history of rheumatic fever in childhood presents to the emergency department of a community hospital with complaints of chest pressure, shortness of breath on exertion, and easy fatigability. Her shortness of breath started 6 months ago but has become much worse over the past few days.
On examination, her pulse is 110 and irregular, and she has markedly distended neck veins and evidence of pulmonary edema. She has a systolic murmur, but it is difficult to characterize due to tachycardia. Electrocardiography shows atrial fibrillation with rapid ventricular response and right axis deviation. Chest radiography shows bilateral pleural effusions.
The patient is given a diuretic, anticoagulation is started to prevent thromboembolism, and she undergoes cardioversion for the atrial fibrillation.
Transthoracic echocardiography is performed and reveals biatrial enlargement, anterior mitral valve leaflet thickening, mitral valve calcification, and moderate mitral regurgitation. Her ejection fraction is normal, but she has mild right ventricular systolic dysfunction with moderate tricuspid regurgitation and an estimated right ventricular systolic pressure of 90 mm Hg (normal range 15–30 mm Hg).
After an uneventful hospital course, she is discharged on a stable dose of a diuretic and oral anticoagulation. Despite adequate diuresis and maintenance of normal sinus rhythm, however, she continues to experience severe dyspnea, which limits her ability to perform simple tasks, such as dusting the furniture in her home. She is referred to Cleveland Clinic for further evaluation.
WHAT IS THE CAUSE OF HER SYMPTOMS?
1. What was the most likely cause of this patient’s initial acute presentation to the emergency department?
- An acute decrease in mitral valve area
- Rheumatic mitral valve stenosis
- Acute coronary syndrome
- Atrial fibrillation
Although an acute decrease in mitral valve area caused by an atrial myxoma, thrombus, or vegetation is a possibility, this patient’s symptoms gradually increased over a period of months. She also did not have signs of infection, nor did she have any signs of embolic phenomena to suggest myxoma, thrombus, or vegetation, all of which frequently present with emboli.
Given her history of rheumatic fever and her echocardiographic findings, rheumatic mitral valve stenosis is high on the list of differential diagnoses. Rheumatic mitral valve stenosis is a chronic process in which the valve area decreases at a rate of about 0.1 cm2/year.1,2
Acute coronary syndrome is a possibility in this elderly woman with multiple risk factors for coronary artery disease. An evaluation for coronary insufficiency should be considered, but the most striking finding on her initial electrocardiogram is atrial fibrillation with a rapid ventricular response.
The onset of atrial fibrillation in the setting of valvular heart disease is the most likely cause of her acute decompensation with signs and symptoms of congestive heart failure. In severe mitral stenosis, because the mitral valve orifice is narrowed, a higher left atrial pressure and longer ventricular filling time are required to maintain forward flow. Now add atrial fibrillation to this situation: the left atrium no longer contracts properly, so less blood is forced through the narrowed valve, and with the rapid heart rate the left ventricle has less time to fill. These two conditions result in elevated left atrial and pulmonary venous pressures, which in turn result in pulmonary edema and congestive heart failure. Thus, patients with mitral stenosis tolerate atrial fibrillation poorly.
WHAT IS THE NEXT STEP?
2. What would be the most appropriate next step for our patient?
- Transesophageal echocardiography
- Right heart catheterization
- An exercise treadmill stress test0
Transesophageal echocardiography is a reasonable option, as mitral stenosis is strongly suspected but transthoracic echocardiography did not reveal severe mitral valve disease. The use of transesophageal echocardiography for the diagnosis of mitral stenosis in this situation is a class IC recommendation (ie, the procedure is recommended, although very few trials have been done) in the American College of Cardiology and American Heart Association Valvular Disease guidelines.3
Right heart catheterization is the diagnostic test of choice in this situation, as the patient has evidence of biventricular heart failure and her right ventricular systolic pressure of 90 mm Hg is consistent with severe pulmonary hypertension. Right heart catheterization would help differentiate primary pulmonary hypertension causing dyspnea from pulmonary hypertension secondary to elevated left-sided pressures. It would also provide a direct hemodynamic estimate of her cardiac function.
Exercise treadmill testing. Evaluation for myocardial ischemia is a reasonable option, as our patient is elderly and has hypertension and diabetes, both of which are risk factors for coronary artery disease. Moreover, in a patient with diabetes, myocardial ischemia can present as dyspnea without typical anginal chest pain. Because of her age and severely limiting dyspnea, however, she would be unlikely to achieve an adequate heart rate during exercise treadmill testing, so this may not be the optimal type of stress test.
Although asymptomatic patients with moderate or severe mitral stenosis should undergo evaluation of exercise capacity and change in pulmonary artery pressures with exercise to determine the need for percutaneous balloon mitral valvuloplasty (see below),3 our patient is symptomatic and already has evidence of severe pulmonary hypertension on transthoracic echocardiography.
Other options for evaluating for myocardial ischemia include pharmacologic stress testing with imaging (eg, dobutamine echocardiography or adenosine nuclear imaging) or proceeding directly to coronary angiography.
CASE CONTINUED: RIGHT HEART CATHETERIZATION, CORONARY ANGIOGRAPHY
- Mean right atrial pressure 6 mm Hg (normal 2–7 mm Hg)
- Right ventricular pressure 102/6 mm Hg (consistent with severe pulmonary hypertension) (normal 15–30/1–7 mm Hg)
- Pulmonary artery pressure 102/40 mm Hg (normal 15–30/4–12 mm Hg)
- Mean pulmonary capillary wedge pressure 25 mm Hg (normal 4–12 mm Hg)
- Cardiac output 3.16 L/min (normal 4–8 L/min)
- Cardiac index 2.10 L/min/m2 (normal 2.5–4.2 L/min/m2)
- v waves are not prominent.
Because her symptoms raise concern for ischemia, coronary angiography is also performed and shows minimal, nonobstructive coronary artery disease. Her left ventricular end-diastolic pressure is 8 mm Hg (normal 5–12 mm Hg).
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis?
- Tricuspid stenosis
- Pulmonic stenosis
- Mitral stenosis
- Mitral regurgitation
Tricuspid stenosis would result in a higher pressure in the right atrium than in the right ventricle. In our patient, the right atrial pressure and the right ventricular diastolic pressure are both 6 mm Hg, eliminating this possibility.
Similarly, pulmonic stenosis would result in a higher pressure in the right ventricle than in the pulmonary artery. In our patient both the right ventricular systolic pressure and the pulmonary artery systolic pressures are 102 mm Hg.
Acute mitral regurgitation may result in increased wedge pressure and tall v waves (reflecting left atrial filling during ventricular systole). In chronic mitral regurgitation, however, the wedge pressure may be normal and the patient may have relatively normal-appearing v waves.4
Mitral stenosis results in a marked gradient between the pulmonary capillary wedge pressure and the left ventricular diastolic pressure in the absence of pulmonary veno-occlusive disease. This gradient can be measured by simultaneous catheterization of the right heart (to measure the wedge pressure, which is an indirect measure of left atrial pressure) and the left heart (to measure the left ventricular diastolic pressure). If the patient does not have significant mitral stenosis, the wedge pressure should be approximately equal to the left ventricular diastolic pressure. In our patient, the wedge pressure (and therefore the left atrial pressure) is 25 mm Hg, and the left ventricular end-diastolic pressure is 8 mm Hg—a difference of 17 mm Hg, consistent with significant mitral stenosis.
CASE CONTINUED: TRANSESOPHAGEAL ECHOCARDIOGRAPHY
WHAT IS THE TREATMENT?
4. Which of the following is the preferred technique for correcting mitral stenosis in this patient?
- Percutaneous balloon mitral valvuloplasty
- Mitral valve surgery
- Percutaneous mitral valve replacement
Although there are several options for mechanical treatment of mitral stenosis, percutaneous balloon mitral valvuloplasty by experienced operators is the procedure of choice for patients who have symptomatic moderate-to-severe mitral stenosis with favorable valve morphology but do not have significant mitral regurgitation or left atrial thrombus.3 The hemodynamic and symptomatic improvement that can be expected after this procedure can be predicted using several echocardiographic criteria, including valve mobility, subvalvular thickening, valve leaflet thickening, and valve leaflet calcification,5 as well as the degree of commissural calcification or commissural fusion.6 Success rates are better if the valve is relatively more mobile and has lesser degrees of valvular and subvalvular thickening, calcification, and commissural fusion.
Mitral valve surgery (repair if possible) is indicated in patients with acceptable operative risk who have symptomatic (New York Heart Association class III or IV) moderate-to-severe mitral stenosis if percutaneous balloon mitral valvuloplasty is unavailable, in cases in which an atrial thrombus or moderate-to-severe mitral regurgitation precludes balloon valvuloplasty, or when the valve morphology is not favorable for balloon valvulo-plasty.3
Although she has moderate mitral regurgitation and poor valve morphology, our patient is a poor surgical candidate because of her advanced age, severe pulmonary hypertension, and poor functional status. Patients with moderate-to-severe mitral stenosis and class III or IV symptoms who have nonpliable, calcified valves but are not candidates for open heart surgery have a class IIb indication for percutaneous balloon mitral valvuloplasty—ie, the procedure may be considered.3
In addition, the procedure also carries a class IIb recommendation in patients with moderate-to-severe mitral stenosis and new-onset atrial fibrillation (provided that they do not have a thrombus in the left atrium or moderate-to-severe mitral regurgitation), even without symptoms.3
Percutaneous mitral valve replacement is not available in clinical practice, although this is an active area of clinical research and may be available in the future.
CASE CONTINUED: THE PATIENT UNDERGOES BALLOON VALVULOPLASTY
Transthoracic echocardiography performed 4 months later shows moderate mitral stenosis with a mean gradient of 9.0 mm Hg, a mitral valve area of 1.7 cm2, and moderate mitral regurgitation. Her right ventricular systolic pressure is estimated to be 74 mm Hg. The patient reports less dyspnea during her housework and now has New York Heart Association class II symptoms. Her treatment regimen includes warfarin (Coumadin) for atrial fibrillation, a beta-blocker to control her heart rate in atrial fibrillation and increase her left ventricular filling time, and a low-dose diuretic.
A frail 75-year-old woman with diabetes, hyperlipidemia, and a history of rheumatic fever in childhood presents to the emergency department of a community hospital with complaints of chest pressure, shortness of breath on exertion, and easy fatigability. Her shortness of breath started 6 months ago but has become much worse over the past few days.
On examination, her pulse is 110 and irregular, and she has markedly distended neck veins and evidence of pulmonary edema. She has a systolic murmur, but it is difficult to characterize due to tachycardia. Electrocardiography shows atrial fibrillation with rapid ventricular response and right axis deviation. Chest radiography shows bilateral pleural effusions.
The patient is given a diuretic, anticoagulation is started to prevent thromboembolism, and she undergoes cardioversion for the atrial fibrillation.
Transthoracic echocardiography is performed and reveals biatrial enlargement, anterior mitral valve leaflet thickening, mitral valve calcification, and moderate mitral regurgitation. Her ejection fraction is normal, but she has mild right ventricular systolic dysfunction with moderate tricuspid regurgitation and an estimated right ventricular systolic pressure of 90 mm Hg (normal range 15–30 mm Hg).
After an uneventful hospital course, she is discharged on a stable dose of a diuretic and oral anticoagulation. Despite adequate diuresis and maintenance of normal sinus rhythm, however, she continues to experience severe dyspnea, which limits her ability to perform simple tasks, such as dusting the furniture in her home. She is referred to Cleveland Clinic for further evaluation.
WHAT IS THE CAUSE OF HER SYMPTOMS?
1. What was the most likely cause of this patient’s initial acute presentation to the emergency department?
- An acute decrease in mitral valve area
- Rheumatic mitral valve stenosis
- Acute coronary syndrome
- Atrial fibrillation
Although an acute decrease in mitral valve area caused by an atrial myxoma, thrombus, or vegetation is a possibility, this patient’s symptoms gradually increased over a period of months. She also did not have signs of infection, nor did she have any signs of embolic phenomena to suggest myxoma, thrombus, or vegetation, all of which frequently present with emboli.
Given her history of rheumatic fever and her echocardiographic findings, rheumatic mitral valve stenosis is high on the list of differential diagnoses. Rheumatic mitral valve stenosis is a chronic process in which the valve area decreases at a rate of about 0.1 cm2/year.1,2
Acute coronary syndrome is a possibility in this elderly woman with multiple risk factors for coronary artery disease. An evaluation for coronary insufficiency should be considered, but the most striking finding on her initial electrocardiogram is atrial fibrillation with a rapid ventricular response.
The onset of atrial fibrillation in the setting of valvular heart disease is the most likely cause of her acute decompensation with signs and symptoms of congestive heart failure. In severe mitral stenosis, because the mitral valve orifice is narrowed, a higher left atrial pressure and longer ventricular filling time are required to maintain forward flow. Now add atrial fibrillation to this situation: the left atrium no longer contracts properly, so less blood is forced through the narrowed valve, and with the rapid heart rate the left ventricle has less time to fill. These two conditions result in elevated left atrial and pulmonary venous pressures, which in turn result in pulmonary edema and congestive heart failure. Thus, patients with mitral stenosis tolerate atrial fibrillation poorly.
WHAT IS THE NEXT STEP?
2. What would be the most appropriate next step for our patient?
- Transesophageal echocardiography
- Right heart catheterization
- An exercise treadmill stress test0
Transesophageal echocardiography is a reasonable option, as mitral stenosis is strongly suspected but transthoracic echocardiography did not reveal severe mitral valve disease. The use of transesophageal echocardiography for the diagnosis of mitral stenosis in this situation is a class IC recommendation (ie, the procedure is recommended, although very few trials have been done) in the American College of Cardiology and American Heart Association Valvular Disease guidelines.3
Right heart catheterization is the diagnostic test of choice in this situation, as the patient has evidence of biventricular heart failure and her right ventricular systolic pressure of 90 mm Hg is consistent with severe pulmonary hypertension. Right heart catheterization would help differentiate primary pulmonary hypertension causing dyspnea from pulmonary hypertension secondary to elevated left-sided pressures. It would also provide a direct hemodynamic estimate of her cardiac function.
Exercise treadmill testing. Evaluation for myocardial ischemia is a reasonable option, as our patient is elderly and has hypertension and diabetes, both of which are risk factors for coronary artery disease. Moreover, in a patient with diabetes, myocardial ischemia can present as dyspnea without typical anginal chest pain. Because of her age and severely limiting dyspnea, however, she would be unlikely to achieve an adequate heart rate during exercise treadmill testing, so this may not be the optimal type of stress test.
Although asymptomatic patients with moderate or severe mitral stenosis should undergo evaluation of exercise capacity and change in pulmonary artery pressures with exercise to determine the need for percutaneous balloon mitral valvuloplasty (see below),3 our patient is symptomatic and already has evidence of severe pulmonary hypertension on transthoracic echocardiography.
Other options for evaluating for myocardial ischemia include pharmacologic stress testing with imaging (eg, dobutamine echocardiography or adenosine nuclear imaging) or proceeding directly to coronary angiography.
CASE CONTINUED: RIGHT HEART CATHETERIZATION, CORONARY ANGIOGRAPHY
- Mean right atrial pressure 6 mm Hg (normal 2–7 mm Hg)
- Right ventricular pressure 102/6 mm Hg (consistent with severe pulmonary hypertension) (normal 15–30/1–7 mm Hg)
- Pulmonary artery pressure 102/40 mm Hg (normal 15–30/4–12 mm Hg)
- Mean pulmonary capillary wedge pressure 25 mm Hg (normal 4–12 mm Hg)
- Cardiac output 3.16 L/min (normal 4–8 L/min)
- Cardiac index 2.10 L/min/m2 (normal 2.5–4.2 L/min/m2)
- v waves are not prominent.
Because her symptoms raise concern for ischemia, coronary angiography is also performed and shows minimal, nonobstructive coronary artery disease. Her left ventricular end-diastolic pressure is 8 mm Hg (normal 5–12 mm Hg).
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis?
- Tricuspid stenosis
- Pulmonic stenosis
- Mitral stenosis
- Mitral regurgitation
Tricuspid stenosis would result in a higher pressure in the right atrium than in the right ventricle. In our patient, the right atrial pressure and the right ventricular diastolic pressure are both 6 mm Hg, eliminating this possibility.
Similarly, pulmonic stenosis would result in a higher pressure in the right ventricle than in the pulmonary artery. In our patient both the right ventricular systolic pressure and the pulmonary artery systolic pressures are 102 mm Hg.
Acute mitral regurgitation may result in increased wedge pressure and tall v waves (reflecting left atrial filling during ventricular systole). In chronic mitral regurgitation, however, the wedge pressure may be normal and the patient may have relatively normal-appearing v waves.4
Mitral stenosis results in a marked gradient between the pulmonary capillary wedge pressure and the left ventricular diastolic pressure in the absence of pulmonary veno-occlusive disease. This gradient can be measured by simultaneous catheterization of the right heart (to measure the wedge pressure, which is an indirect measure of left atrial pressure) and the left heart (to measure the left ventricular diastolic pressure). If the patient does not have significant mitral stenosis, the wedge pressure should be approximately equal to the left ventricular diastolic pressure. In our patient, the wedge pressure (and therefore the left atrial pressure) is 25 mm Hg, and the left ventricular end-diastolic pressure is 8 mm Hg—a difference of 17 mm Hg, consistent with significant mitral stenosis.
CASE CONTINUED: TRANSESOPHAGEAL ECHOCARDIOGRAPHY
WHAT IS THE TREATMENT?
4. Which of the following is the preferred technique for correcting mitral stenosis in this patient?
- Percutaneous balloon mitral valvuloplasty
- Mitral valve surgery
- Percutaneous mitral valve replacement
Although there are several options for mechanical treatment of mitral stenosis, percutaneous balloon mitral valvuloplasty by experienced operators is the procedure of choice for patients who have symptomatic moderate-to-severe mitral stenosis with favorable valve morphology but do not have significant mitral regurgitation or left atrial thrombus.3 The hemodynamic and symptomatic improvement that can be expected after this procedure can be predicted using several echocardiographic criteria, including valve mobility, subvalvular thickening, valve leaflet thickening, and valve leaflet calcification,5 as well as the degree of commissural calcification or commissural fusion.6 Success rates are better if the valve is relatively more mobile and has lesser degrees of valvular and subvalvular thickening, calcification, and commissural fusion.
Mitral valve surgery (repair if possible) is indicated in patients with acceptable operative risk who have symptomatic (New York Heart Association class III or IV) moderate-to-severe mitral stenosis if percutaneous balloon mitral valvuloplasty is unavailable, in cases in which an atrial thrombus or moderate-to-severe mitral regurgitation precludes balloon valvuloplasty, or when the valve morphology is not favorable for balloon valvulo-plasty.3
Although she has moderate mitral regurgitation and poor valve morphology, our patient is a poor surgical candidate because of her advanced age, severe pulmonary hypertension, and poor functional status. Patients with moderate-to-severe mitral stenosis and class III or IV symptoms who have nonpliable, calcified valves but are not candidates for open heart surgery have a class IIb indication for percutaneous balloon mitral valvuloplasty—ie, the procedure may be considered.3
In addition, the procedure also carries a class IIb recommendation in patients with moderate-to-severe mitral stenosis and new-onset atrial fibrillation (provided that they do not have a thrombus in the left atrium or moderate-to-severe mitral regurgitation), even without symptoms.3
Percutaneous mitral valve replacement is not available in clinical practice, although this is an active area of clinical research and may be available in the future.
CASE CONTINUED: THE PATIENT UNDERGOES BALLOON VALVULOPLASTY
Transthoracic echocardiography performed 4 months later shows moderate mitral stenosis with a mean gradient of 9.0 mm Hg, a mitral valve area of 1.7 cm2, and moderate mitral regurgitation. Her right ventricular systolic pressure is estimated to be 74 mm Hg. The patient reports less dyspnea during her housework and now has New York Heart Association class II symptoms. Her treatment regimen includes warfarin (Coumadin) for atrial fibrillation, a beta-blocker to control her heart rate in atrial fibrillation and increase her left ventricular filling time, and a low-dose diuretic.
- Gordon SP, Douglas PS, Come PC, et al. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Coll Cardiol 1992; 19:968–973.
- Sagie A, Freitas N, Padial LR, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol 1996; 28:472–479.
- Bonow ROC, Blase A, Chatterjee K, et al. ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:598–675.
- Braunwald E. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation 1963; 27:29–35.
- Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308.
- Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol 1997; 29:175–180.
- Gordon SP, Douglas PS, Come PC, et al. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Coll Cardiol 1992; 19:968–973.
- Sagie A, Freitas N, Padial LR, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol 1996; 28:472–479.
- Bonow ROC, Blase A, Chatterjee K, et al. ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:598–675.
- Braunwald E. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation 1963; 27:29–35.
- Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308.
- Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol 1997; 29:175–180.
A young man with acute weakness of his right arm
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.