User login
Should we use SSRIs to treat adolescents with depression?
Yes. Based on current evidence, fluoxetine is the most effective selective serotonin reuptake inhibitor (SSRI) for treatment of major depressive disorder in adolescents. It is the only agent approved by the US Food and Drug Administration (FDA) for use in children (strength of recommendation [SOR]: A, based on meta-analysis of RCTs).
All SSRI medications increase the risk of suicidal behavior in adolescents, but do not increase the risk of completing suicide (SOR: A, based on meta-analysis of RCTs).
First, we must spot those at risk
Beth Fox, MD
East Tennessee State University, Johnson City
Family physicians are often the primary providers of healthcare for adolescents. Most health issues affecting this unique population are social and behavioral in origin. By routinely incorporating prevention and screening techniques into the adolescent visit, we can detect at-risk individuals. We need to inquire about sleep, personal interests, eating behaviors, future plans, friends and social activities, school performance, mood, and drug and alcohol use so that we can detect early symptoms of depression.
For those family physicians who like reminders, there are mnemonics and questionnaires that evaluate the social and behavioral aspects of the adolescent visit. Family physicians should also educate parents and family members about depressive signs and symptoms and the potential warning signs of suicidality.
Evidence summary
Major depressive disorder is common among adolescents and is associated with significant morbidity, including substance abuse and eating disorders. One study of survey data from 1769 adolescents found a lifetime prevalence of 15.3% for major depression. The majority of those reporting episodes of major depression in this study had recurrent symptoms and impairment in work or school.1
Fluoxetine and therapy together have the best results
A large, multicenter, randomized controlled trial evaluated the effectiveness of fluoxetine (Prozac), cognitive behavioral therapy (CBT), or the combination of the 2. Researchers evaluated improvement with the Children’s Depression Rating Scale–Revised (CDRSR). The CDRS-R uses adolescent and parent interviews to rate 17 symptom areas: impaired schoolwork, difficulty having fun, social withdrawal, appetite disturbance, sleep disturbance, excessive fatigue, physical complaints, irritability, excessive guilt, low self-esteem, depressed feelings, morbid ideas, suicidal ideas, excessive weeping, depressed facial affect, listless speech, and hypoactivity. Combination treatment with fluoxetine and CBT was statistically superior to placebo, CBT alone, or fluoxetine alone. In addition, fluoxetine alone was superior to CBT alone.2
A meta-analysis including both published and unpublished trials of SSRI medications found that fluoxetine was more likely than placebo to cause remission of symptoms after 7 to 8 weeks of treatment (number needed to treat [NNT]=6). Fluoxetine treatment was also associated with a reduction in symptom scores as measured with the CDRS-R (NNT=5).3
Data were conflicting for the efficacy of paroxetine (Paxil), sertraline (Zoloft), and citalopram (Celexa).3,4 No data were available for escitalopram (Lexapro).
But are SSRIs safe for adolescents?
Considerable controversy surrounds the safety of SSRIs in children due to reports of increased suicidal behavior. In 2004, the FDA conducted a meta-analysis of the suicide related adverse events from the published and unpublished trials of SSRIs including fluoxetine, sertraline, paroxetine, fluvoxamine (Luvox), and citalopram. A team of experts reviewed the adverse events from each trial to evaluate for suicidality including suicidal ideation, preparatory acts, self-injurious behavior, or suicide attempts. They found a risk ratio of 1.66 (95% confidence interval, 1.02–2.68) for suicidality in the treatment arms compared with placebo. There were no completed suicides in any study.5
This review led to the FDA’s October 2004 “black box” warning regarding suicidality and antidepressant medication in adolescents. However, an ecological analysis of prescription data and US Census data found an overall decline in suicide rates as the rate of prescriptions for SSRI medications increased, suggesting a beneficial correlation of SSRI medications on suicide rates.6
Recommendations from others
The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychosocial and pharmacologic intervention for depression, with psychotherapy as the preferred initial treatment for most adolescent patients.4 This organization reviewed the current published and unpublished data including the FDA analysis to formulate its conclusions regarding safety and efficacy. AACAP concluded that fluoxetine is effective for the treatment of depression in children and adolescents.
1. Kessler R, Walters E. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depression Anxiety 1998;7:3-14.
2. March J, Silva S, Petrycki S, et al. Treatment for Adolescent Depression Study Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292:807-820.
3. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-1345.
4. Report 10 of the Council on Scientific Affairs. Safety and Efficacy of Selective Serotonin Reup-take Inhibitors (SSRIs) in Children and Adolescents. Available at: www.aacap.org/galleries/psychiatricmedication/CSA_report_10_final.pdf. Accessed August 6, 2007
5. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332-339.
6. Olfson M, Shaffer D, Marcus SC, Greenberg T. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry 2003;60:978-982.
Yes. Based on current evidence, fluoxetine is the most effective selective serotonin reuptake inhibitor (SSRI) for treatment of major depressive disorder in adolescents. It is the only agent approved by the US Food and Drug Administration (FDA) for use in children (strength of recommendation [SOR]: A, based on meta-analysis of RCTs).
All SSRI medications increase the risk of suicidal behavior in adolescents, but do not increase the risk of completing suicide (SOR: A, based on meta-analysis of RCTs).
First, we must spot those at risk
Beth Fox, MD
East Tennessee State University, Johnson City
Family physicians are often the primary providers of healthcare for adolescents. Most health issues affecting this unique population are social and behavioral in origin. By routinely incorporating prevention and screening techniques into the adolescent visit, we can detect at-risk individuals. We need to inquire about sleep, personal interests, eating behaviors, future plans, friends and social activities, school performance, mood, and drug and alcohol use so that we can detect early symptoms of depression.
For those family physicians who like reminders, there are mnemonics and questionnaires that evaluate the social and behavioral aspects of the adolescent visit. Family physicians should also educate parents and family members about depressive signs and symptoms and the potential warning signs of suicidality.
Evidence summary
Major depressive disorder is common among adolescents and is associated with significant morbidity, including substance abuse and eating disorders. One study of survey data from 1769 adolescents found a lifetime prevalence of 15.3% for major depression. The majority of those reporting episodes of major depression in this study had recurrent symptoms and impairment in work or school.1
Fluoxetine and therapy together have the best results
A large, multicenter, randomized controlled trial evaluated the effectiveness of fluoxetine (Prozac), cognitive behavioral therapy (CBT), or the combination of the 2. Researchers evaluated improvement with the Children’s Depression Rating Scale–Revised (CDRSR). The CDRS-R uses adolescent and parent interviews to rate 17 symptom areas: impaired schoolwork, difficulty having fun, social withdrawal, appetite disturbance, sleep disturbance, excessive fatigue, physical complaints, irritability, excessive guilt, low self-esteem, depressed feelings, morbid ideas, suicidal ideas, excessive weeping, depressed facial affect, listless speech, and hypoactivity. Combination treatment with fluoxetine and CBT was statistically superior to placebo, CBT alone, or fluoxetine alone. In addition, fluoxetine alone was superior to CBT alone.2
A meta-analysis including both published and unpublished trials of SSRI medications found that fluoxetine was more likely than placebo to cause remission of symptoms after 7 to 8 weeks of treatment (number needed to treat [NNT]=6). Fluoxetine treatment was also associated with a reduction in symptom scores as measured with the CDRS-R (NNT=5).3
Data were conflicting for the efficacy of paroxetine (Paxil), sertraline (Zoloft), and citalopram (Celexa).3,4 No data were available for escitalopram (Lexapro).
But are SSRIs safe for adolescents?
Considerable controversy surrounds the safety of SSRIs in children due to reports of increased suicidal behavior. In 2004, the FDA conducted a meta-analysis of the suicide related adverse events from the published and unpublished trials of SSRIs including fluoxetine, sertraline, paroxetine, fluvoxamine (Luvox), and citalopram. A team of experts reviewed the adverse events from each trial to evaluate for suicidality including suicidal ideation, preparatory acts, self-injurious behavior, or suicide attempts. They found a risk ratio of 1.66 (95% confidence interval, 1.02–2.68) for suicidality in the treatment arms compared with placebo. There were no completed suicides in any study.5
This review led to the FDA’s October 2004 “black box” warning regarding suicidality and antidepressant medication in adolescents. However, an ecological analysis of prescription data and US Census data found an overall decline in suicide rates as the rate of prescriptions for SSRI medications increased, suggesting a beneficial correlation of SSRI medications on suicide rates.6
Recommendations from others
The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychosocial and pharmacologic intervention for depression, with psychotherapy as the preferred initial treatment for most adolescent patients.4 This organization reviewed the current published and unpublished data including the FDA analysis to formulate its conclusions regarding safety and efficacy. AACAP concluded that fluoxetine is effective for the treatment of depression in children and adolescents.
Yes. Based on current evidence, fluoxetine is the most effective selective serotonin reuptake inhibitor (SSRI) for treatment of major depressive disorder in adolescents. It is the only agent approved by the US Food and Drug Administration (FDA) for use in children (strength of recommendation [SOR]: A, based on meta-analysis of RCTs).
All SSRI medications increase the risk of suicidal behavior in adolescents, but do not increase the risk of completing suicide (SOR: A, based on meta-analysis of RCTs).
First, we must spot those at risk
Beth Fox, MD
East Tennessee State University, Johnson City
Family physicians are often the primary providers of healthcare for adolescents. Most health issues affecting this unique population are social and behavioral in origin. By routinely incorporating prevention and screening techniques into the adolescent visit, we can detect at-risk individuals. We need to inquire about sleep, personal interests, eating behaviors, future plans, friends and social activities, school performance, mood, and drug and alcohol use so that we can detect early symptoms of depression.
For those family physicians who like reminders, there are mnemonics and questionnaires that evaluate the social and behavioral aspects of the adolescent visit. Family physicians should also educate parents and family members about depressive signs and symptoms and the potential warning signs of suicidality.
Evidence summary
Major depressive disorder is common among adolescents and is associated with significant morbidity, including substance abuse and eating disorders. One study of survey data from 1769 adolescents found a lifetime prevalence of 15.3% for major depression. The majority of those reporting episodes of major depression in this study had recurrent symptoms and impairment in work or school.1
Fluoxetine and therapy together have the best results
A large, multicenter, randomized controlled trial evaluated the effectiveness of fluoxetine (Prozac), cognitive behavioral therapy (CBT), or the combination of the 2. Researchers evaluated improvement with the Children’s Depression Rating Scale–Revised (CDRSR). The CDRS-R uses adolescent and parent interviews to rate 17 symptom areas: impaired schoolwork, difficulty having fun, social withdrawal, appetite disturbance, sleep disturbance, excessive fatigue, physical complaints, irritability, excessive guilt, low self-esteem, depressed feelings, morbid ideas, suicidal ideas, excessive weeping, depressed facial affect, listless speech, and hypoactivity. Combination treatment with fluoxetine and CBT was statistically superior to placebo, CBT alone, or fluoxetine alone. In addition, fluoxetine alone was superior to CBT alone.2
A meta-analysis including both published and unpublished trials of SSRI medications found that fluoxetine was more likely than placebo to cause remission of symptoms after 7 to 8 weeks of treatment (number needed to treat [NNT]=6). Fluoxetine treatment was also associated with a reduction in symptom scores as measured with the CDRS-R (NNT=5).3
Data were conflicting for the efficacy of paroxetine (Paxil), sertraline (Zoloft), and citalopram (Celexa).3,4 No data were available for escitalopram (Lexapro).
But are SSRIs safe for adolescents?
Considerable controversy surrounds the safety of SSRIs in children due to reports of increased suicidal behavior. In 2004, the FDA conducted a meta-analysis of the suicide related adverse events from the published and unpublished trials of SSRIs including fluoxetine, sertraline, paroxetine, fluvoxamine (Luvox), and citalopram. A team of experts reviewed the adverse events from each trial to evaluate for suicidality including suicidal ideation, preparatory acts, self-injurious behavior, or suicide attempts. They found a risk ratio of 1.66 (95% confidence interval, 1.02–2.68) for suicidality in the treatment arms compared with placebo. There were no completed suicides in any study.5
This review led to the FDA’s October 2004 “black box” warning regarding suicidality and antidepressant medication in adolescents. However, an ecological analysis of prescription data and US Census data found an overall decline in suicide rates as the rate of prescriptions for SSRI medications increased, suggesting a beneficial correlation of SSRI medications on suicide rates.6
Recommendations from others
The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychosocial and pharmacologic intervention for depression, with psychotherapy as the preferred initial treatment for most adolescent patients.4 This organization reviewed the current published and unpublished data including the FDA analysis to formulate its conclusions regarding safety and efficacy. AACAP concluded that fluoxetine is effective for the treatment of depression in children and adolescents.
1. Kessler R, Walters E. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depression Anxiety 1998;7:3-14.
2. March J, Silva S, Petrycki S, et al. Treatment for Adolescent Depression Study Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292:807-820.
3. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-1345.
4. Report 10 of the Council on Scientific Affairs. Safety and Efficacy of Selective Serotonin Reup-take Inhibitors (SSRIs) in Children and Adolescents. Available at: www.aacap.org/galleries/psychiatricmedication/CSA_report_10_final.pdf. Accessed August 6, 2007
5. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332-339.
6. Olfson M, Shaffer D, Marcus SC, Greenberg T. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry 2003;60:978-982.
1. Kessler R, Walters E. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depression Anxiety 1998;7:3-14.
2. March J, Silva S, Petrycki S, et al. Treatment for Adolescent Depression Study Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA 2004;292:807-820.
3. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-1345.
4. Report 10 of the Council on Scientific Affairs. Safety and Efficacy of Selective Serotonin Reup-take Inhibitors (SSRIs) in Children and Adolescents. Available at: www.aacap.org/galleries/psychiatricmedication/CSA_report_10_final.pdf. Accessed August 6, 2007
5. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332-339.
6. Olfson M, Shaffer D, Marcus SC, Greenberg T. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry 2003;60:978-982.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best treatment for gestational diabetes?
There is no single approach to glycemic control that is better than another for reducing neonatal mortality and morbidity. Glycemic control—regardless of whether it involves diet, glyburide, or insulin—leads to fewer cases of shoulder dystocia, hyperbilirubinemia requiring phototherapy, nerve palsy, bone fracture, being large for gestational age, and fetal macrosomia (strength of recommendation: A).
Customize the intervention
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Achieving solid glucose control for patients with gestational diabetes should be easy—most patients are healthy and motivated to do what is best for their babies. But a new diagnosis and blood sugar monitoring requirements can be daunting. Lifestyle changes and medications can quickly add to the sense of being overwhelmed. Fortunately, whatever brings down the blood sugar will do as therapy, so the patient can negotiate with her doctor to develop an intervention—be it diet, exercise, oral medications, insulin, or a combination—that works for her.
Evidence summary
Findings from 2 studies support the notion that the treatment of gestational diabetes decreases neonatal morbidity and mortality (TABLE).1,2 Both studies found a decrease in neonatal morbidity and mortality for those patients treated either with diet or insulin. One study found a higher rate of NICU admission in the treatment group, but the authors attributed this to physician awareness of the patient having gestational diabetes.1
TABLE
Treatment of gestational diabetes reduces neonatal morbidity and mortality
| TYPE OF STUDY | CONTROL(S) | INTERVENTION | MEONATAL MORBIDITY AND MORTALITY | ADMISSIONS TO NICU | NNT |
|---|---|---|---|---|---|
| RCT1 | GDM routine care (N=510) | GDM treated with diet or insulin (N=490) | Control: 4% Intervention: 1% | 71% diet and insulin vs 61% routine care NNH: 100 | 34 |
| Cohort2 | 1) No GDM (N=1110) | GDM treated diet or insulin (N=1110) | Control 1: 11% Control 2: 59% | Not reported | 2* |
| 2) GDM not treated (due to late entry to care) (N=555) | Intervention: 15% | ||||
| *Compared with patients presenting late. | |||||
| GDM, gestational diabetes mellitus; NNH, number needed to harm; NNT, number needed to treat. | |||||
Glyburide vs insulin
A high-quality randomized controlled trial comparing glyburide with insulin among 404 women found no difference in maternal hypoglycemia, neonatal mortality, or neonatal features and outcomes (including birthweight, NICU admissions, hyperbilirubinemia, and hypoglycemia; P ≥.25).3 Although this was a fairly large trial, it may have been underpowered since it found small differences in such rare outcomes.
Similarly, a retrospective study comparing glyburide with insulin in 584 women found little difference between the 2 approaches. Women in the glyburide group had better glycemic control, but the women in the insulin group started with higher initial blood sugars.4 The glyburide group had fewer NICU admissions than the insulin group (number needed to treat [NNT]=11), but higher rates of jaundice (number needed to harm [NNH]=25), pre-eclampsia (NNH=17), and maternal hypoglycemia (NNH=8). All other neonatal outcomes were similar between groups.
Diet alone vs diet + insulin
A meta-analysis combined 6 RCTs comparing diet alone with diet plus insulin in a total of 1281 women.5 Insulin was moderately superior to diet in preventing fetal macrosomia (NNT=11; 95% confidence interval, 6–36), but not in rates of hypoglycemia, hypocalcemia, hyperbilirubinemia, or congenital malformations.
Recommendations from others
The American Diabetes Association (ADA) recommends that women diagnosed with gestational diabetes by a 3-hour glucose tolerance test receive nutritional counseling from a registered dietician. The ADA also recommends insulin therapy if diet is unsuccessful in achieving fasting glucose <105 mg/dL, 1-hour postprandial <155 mg/dL, or 2-hour postprandial <130 mg/dL.6
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of diet or insulin to achieve 1-hour postprandial blood sugar of 130 mg/dL.7 Both ADA and ACOG indicate that further studies are needed to establish the safety of glyburide before general use can be recommended.
1. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-2486.
2. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol 2005;192:989-997.
3. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-1138.
4. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol 2005;193:118-124.
5. Giuffrida FM, Castro AA, Atallah AN, Dib SA. Diet plus insulin compared to diet alone in the treatment of gestational diabetes mellitus: A systematic review. Braz J Med Biol Res 2003;36:1297-1300.
6. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S88-S90.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
There is no single approach to glycemic control that is better than another for reducing neonatal mortality and morbidity. Glycemic control—regardless of whether it involves diet, glyburide, or insulin—leads to fewer cases of shoulder dystocia, hyperbilirubinemia requiring phototherapy, nerve palsy, bone fracture, being large for gestational age, and fetal macrosomia (strength of recommendation: A).
Customize the intervention
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Achieving solid glucose control for patients with gestational diabetes should be easy—most patients are healthy and motivated to do what is best for their babies. But a new diagnosis and blood sugar monitoring requirements can be daunting. Lifestyle changes and medications can quickly add to the sense of being overwhelmed. Fortunately, whatever brings down the blood sugar will do as therapy, so the patient can negotiate with her doctor to develop an intervention—be it diet, exercise, oral medications, insulin, or a combination—that works for her.
Evidence summary
Findings from 2 studies support the notion that the treatment of gestational diabetes decreases neonatal morbidity and mortality (TABLE).1,2 Both studies found a decrease in neonatal morbidity and mortality for those patients treated either with diet or insulin. One study found a higher rate of NICU admission in the treatment group, but the authors attributed this to physician awareness of the patient having gestational diabetes.1
TABLE
Treatment of gestational diabetes reduces neonatal morbidity and mortality
| TYPE OF STUDY | CONTROL(S) | INTERVENTION | MEONATAL MORBIDITY AND MORTALITY | ADMISSIONS TO NICU | NNT |
|---|---|---|---|---|---|
| RCT1 | GDM routine care (N=510) | GDM treated with diet or insulin (N=490) | Control: 4% Intervention: 1% | 71% diet and insulin vs 61% routine care NNH: 100 | 34 |
| Cohort2 | 1) No GDM (N=1110) | GDM treated diet or insulin (N=1110) | Control 1: 11% Control 2: 59% | Not reported | 2* |
| 2) GDM not treated (due to late entry to care) (N=555) | Intervention: 15% | ||||
| *Compared with patients presenting late. | |||||
| GDM, gestational diabetes mellitus; NNH, number needed to harm; NNT, number needed to treat. | |||||
Glyburide vs insulin
A high-quality randomized controlled trial comparing glyburide with insulin among 404 women found no difference in maternal hypoglycemia, neonatal mortality, or neonatal features and outcomes (including birthweight, NICU admissions, hyperbilirubinemia, and hypoglycemia; P ≥.25).3 Although this was a fairly large trial, it may have been underpowered since it found small differences in such rare outcomes.
Similarly, a retrospective study comparing glyburide with insulin in 584 women found little difference between the 2 approaches. Women in the glyburide group had better glycemic control, but the women in the insulin group started with higher initial blood sugars.4 The glyburide group had fewer NICU admissions than the insulin group (number needed to treat [NNT]=11), but higher rates of jaundice (number needed to harm [NNH]=25), pre-eclampsia (NNH=17), and maternal hypoglycemia (NNH=8). All other neonatal outcomes were similar between groups.
Diet alone vs diet + insulin
A meta-analysis combined 6 RCTs comparing diet alone with diet plus insulin in a total of 1281 women.5 Insulin was moderately superior to diet in preventing fetal macrosomia (NNT=11; 95% confidence interval, 6–36), but not in rates of hypoglycemia, hypocalcemia, hyperbilirubinemia, or congenital malformations.
Recommendations from others
The American Diabetes Association (ADA) recommends that women diagnosed with gestational diabetes by a 3-hour glucose tolerance test receive nutritional counseling from a registered dietician. The ADA also recommends insulin therapy if diet is unsuccessful in achieving fasting glucose <105 mg/dL, 1-hour postprandial <155 mg/dL, or 2-hour postprandial <130 mg/dL.6
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of diet or insulin to achieve 1-hour postprandial blood sugar of 130 mg/dL.7 Both ADA and ACOG indicate that further studies are needed to establish the safety of glyburide before general use can be recommended.
There is no single approach to glycemic control that is better than another for reducing neonatal mortality and morbidity. Glycemic control—regardless of whether it involves diet, glyburide, or insulin—leads to fewer cases of shoulder dystocia, hyperbilirubinemia requiring phototherapy, nerve palsy, bone fracture, being large for gestational age, and fetal macrosomia (strength of recommendation: A).
Customize the intervention
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Achieving solid glucose control for patients with gestational diabetes should be easy—most patients are healthy and motivated to do what is best for their babies. But a new diagnosis and blood sugar monitoring requirements can be daunting. Lifestyle changes and medications can quickly add to the sense of being overwhelmed. Fortunately, whatever brings down the blood sugar will do as therapy, so the patient can negotiate with her doctor to develop an intervention—be it diet, exercise, oral medications, insulin, or a combination—that works for her.
Evidence summary
Findings from 2 studies support the notion that the treatment of gestational diabetes decreases neonatal morbidity and mortality (TABLE).1,2 Both studies found a decrease in neonatal morbidity and mortality for those patients treated either with diet or insulin. One study found a higher rate of NICU admission in the treatment group, but the authors attributed this to physician awareness of the patient having gestational diabetes.1
TABLE
Treatment of gestational diabetes reduces neonatal morbidity and mortality
| TYPE OF STUDY | CONTROL(S) | INTERVENTION | MEONATAL MORBIDITY AND MORTALITY | ADMISSIONS TO NICU | NNT |
|---|---|---|---|---|---|
| RCT1 | GDM routine care (N=510) | GDM treated with diet or insulin (N=490) | Control: 4% Intervention: 1% | 71% diet and insulin vs 61% routine care NNH: 100 | 34 |
| Cohort2 | 1) No GDM (N=1110) | GDM treated diet or insulin (N=1110) | Control 1: 11% Control 2: 59% | Not reported | 2* |
| 2) GDM not treated (due to late entry to care) (N=555) | Intervention: 15% | ||||
| *Compared with patients presenting late. | |||||
| GDM, gestational diabetes mellitus; NNH, number needed to harm; NNT, number needed to treat. | |||||
Glyburide vs insulin
A high-quality randomized controlled trial comparing glyburide with insulin among 404 women found no difference in maternal hypoglycemia, neonatal mortality, or neonatal features and outcomes (including birthweight, NICU admissions, hyperbilirubinemia, and hypoglycemia; P ≥.25).3 Although this was a fairly large trial, it may have been underpowered since it found small differences in such rare outcomes.
Similarly, a retrospective study comparing glyburide with insulin in 584 women found little difference between the 2 approaches. Women in the glyburide group had better glycemic control, but the women in the insulin group started with higher initial blood sugars.4 The glyburide group had fewer NICU admissions than the insulin group (number needed to treat [NNT]=11), but higher rates of jaundice (number needed to harm [NNH]=25), pre-eclampsia (NNH=17), and maternal hypoglycemia (NNH=8). All other neonatal outcomes were similar between groups.
Diet alone vs diet + insulin
A meta-analysis combined 6 RCTs comparing diet alone with diet plus insulin in a total of 1281 women.5 Insulin was moderately superior to diet in preventing fetal macrosomia (NNT=11; 95% confidence interval, 6–36), but not in rates of hypoglycemia, hypocalcemia, hyperbilirubinemia, or congenital malformations.
Recommendations from others
The American Diabetes Association (ADA) recommends that women diagnosed with gestational diabetes by a 3-hour glucose tolerance test receive nutritional counseling from a registered dietician. The ADA also recommends insulin therapy if diet is unsuccessful in achieving fasting glucose <105 mg/dL, 1-hour postprandial <155 mg/dL, or 2-hour postprandial <130 mg/dL.6
The American College of Obstetricians and Gynecologists (ACOG) recommends the use of diet or insulin to achieve 1-hour postprandial blood sugar of 130 mg/dL.7 Both ADA and ACOG indicate that further studies are needed to establish the safety of glyburide before general use can be recommended.
1. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-2486.
2. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol 2005;192:989-997.
3. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-1138.
4. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol 2005;193:118-124.
5. Giuffrida FM, Castro AA, Atallah AN, Dib SA. Diet plus insulin compared to diet alone in the treatment of gestational diabetes mellitus: A systematic review. Braz J Med Biol Res 2003;36:1297-1300.
6. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S88-S90.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
1. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-2486.
2. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol 2005;192:989-997.
3. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-1138.
4. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol 2005;193:118-124.
5. Giuffrida FM, Castro AA, Atallah AN, Dib SA. Diet plus insulin compared to diet alone in the treatment of gestational diabetes mellitus: A systematic review. Braz J Med Biol Res 2003;36:1297-1300.
6. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004;27 Suppl 1:S88-S90.
7. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational diabetes. Obstet Gynecol 2001;98:525-538.
Evidence-based answers from the Family Physicians Inquiries Network
Can infants/toddlers get enough fluoride through brushing?
Yes. Brushing twice daily with topical fluoride toothpaste decreases the incidence of dental caries in infants and toddlers (strength of recommendation [SOR]: A, based on meta-analyses of randomized controlled trials [RCTs]). High-concentration fluoride toothpaste delivers superior caries protection, but causes more dental fluorosis.
Use of high-concentration fluoride toothpaste should be targeted towards children at highest risk of dental caries, such as those living in areas without fluoridated water (SOR: B).
Brushing, yes, but what about fluoride supplementation?
Laura G. Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University, Richmond
In medical school, we were taught that infants who are breastfed should start supplemental fluoride at 6 months. Pediatric dentists generally only use supplemental fluoride if the baby’s home has well water that has been tested and found deficient. The worst outcome from a lack of fluoride supplementation is caries, which usually can be managed. However, too much fluoride also has a significant downside, fluorosis, which permanently stains the teeth.
Start fluoride toothpaste in minute amounts at 1 year of age. Don’t use fluoride supplementation—even in breastfed infants—unless they are on well water proven to be low in fluoride.
Evidence summary
Toothpaste as effective as rinse or gel
A large Cochrane review evaluated topical fluoride therapy in the form of toothpaste, mouth rinse, varnish, or gel. Based on 133 randomized or quasi-randomized controlled trials (n=65,169), the meta-analysis indicated a 26% (95% confidence interval [CI], 24%–29%) reduction in decayed, missing, and filled tooth surfaces in children.1 Another Cochrane review found 17 randomized controlled trials comparing different methods of topical fluoride application in children. The limited data suggested that fluoride toothpaste is as effective as mouth rinse or gel.2 Depending on the prevalence of caries in the population, between 1.6 and 3.7 children need to use a fluoride toothpaste to prevent 1 decayed, missing, or filled tooth.3
The risk of fluorosis
Topical fluoride use has been associated with dental fluorosis, which causes staining or pitting of the enamel tooth surface. The incidence of significant dental fluorosis varies in children—from 5% to 7% with 1450 ppm fluoride toothpaste to 2% to 4% with 440 ppm fluoride toothpaste (number needed to harm [NNH]=20–100).4,5
High-fluoride concentrations
High-fluoride-concentration toothpastes (1000 ppm F) prevent 14% more caries than low-fluoride-concentration toothpastes (250 ppm F).6 Another randomized controlled trial, carried out in an area without fluoridated water, found the high-fluoride-concentration toothpaste (1450 ppm F) resulted in 16% fewer caries in children, while the low-fluoride-concentration toothpaste (440 ppm F) was no different than placebo.7
When there’s fluoridated water
A meta-analysis found that the effect of topical fluoride was independent of water fluoridation, suggesting that topical fluoride toothpaste has a beneficial effect even in communities with fluoridated water.3 No relevant studies comparing topical fluoride toothpaste with oral fluoride supplementation were found.
Recommendations from others
Both the American Academy of Pediatric Dentistry (AAPD) and the Centers for Disease Control and Prevention (CDC) recommend topical fluoride toothpaste for children as an adjunct to oral fluoride intake. The AAPD8 recommends a “pea-sized” amount of toothpaste for children under 6 years of age. The CDC9 recommends that you weigh the risks and possible other sources of fluoride in children under age 2, and using a pea-sized amount of toothpaste with supervised brushing for children 2 to 6 years of age.
The TABLE shows the AAPD’s recommended daily dose of fluoride supplementation based on the fluoride concentration in the local water.
TABLE
Oral fluoride dosing: Recommendations from the American Academy of Pediatric Dentistry8
| DRINKING WATER FLUORIDE LEVEL | |||
|---|---|---|---|
| AGE | <0.3 PPM F | 0.3–0.6 PPM F | >0.6 PPM F |
| 0–6 months | 0 | 0 | 0 |
| 6 months – 3 years | 0.25 mg daily | 0 | 0 |
| 3–6 years | 0.5 mg daily | 0.25 mg daily | 0 |
| 6–16 years | 1 mg daily | 0.5 mg daily | 0 |
| ppm F, parts per million fluoride | |||
1. Marinho VCC, Higgins JPT, Logan S, Sheiham A. Topical fluoride (toothpastes, mouth rinses, gels, or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;(4):CD002782.-
2. Marinho VCC, Higgins JPT, Sheiham A, Logan S. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2004;(1):CD002780.-
3. Marinho VCC, Higgins JPT, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;(1):CD002278.-
4. Tavener JA, Davies GM, Davies RM, Ellwood RP. The prevalence and severity of fluorosis in children who received toothpaste containing either 440 or 1450 ppm F from the age of 12 months in deprived and less deprived communities. Caries Res 2006;40:66-72.
5. Tavener JA, Davies GM, Davies RM, Ellwood RP. The prevalence and severity of fluorosis and other developmental defects of enamel in children who received free fluoride toothpaste containing either 440 or 1450 ppm F from the age of 12 months. Community Dent Health 2004;21:217-223.
6. Steiner M, Helfenstein U, Menghini G. Effect of 1000 ppm relative to 250 ppm fluoride toothpaste. A meta-analysis. Am J Dent 2004;17:85-88.
7. Davies GM, Worthington HV, Ellwood RP, et al. A randomized controlled trial of the effectiveness of providing free fluoride toothpaste from the age of 12 months on reducing caries in 5-6 year old children. Community Dent Health 2002;19:131-136.
8. American Academy of Pediatric Dentistry. Clinical guideline on fluoride therapy. Chicago, Ill: American Academy of Pediatric Dentistry; 2003. Available at: www.guideline.gov/summary/summary.aspx?doc_id=6272. Accessed August 6, 2007.
9. Recommendations for using fluoride to prevent and control dental caries in the united States. Centers for Disease Control and prevention. MMWR Recomm Rep 2001;50:1-42.
Yes. Brushing twice daily with topical fluoride toothpaste decreases the incidence of dental caries in infants and toddlers (strength of recommendation [SOR]: A, based on meta-analyses of randomized controlled trials [RCTs]). High-concentration fluoride toothpaste delivers superior caries protection, but causes more dental fluorosis.
Use of high-concentration fluoride toothpaste should be targeted towards children at highest risk of dental caries, such as those living in areas without fluoridated water (SOR: B).
Brushing, yes, but what about fluoride supplementation?
Laura G. Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University, Richmond
In medical school, we were taught that infants who are breastfed should start supplemental fluoride at 6 months. Pediatric dentists generally only use supplemental fluoride if the baby’s home has well water that has been tested and found deficient. The worst outcome from a lack of fluoride supplementation is caries, which usually can be managed. However, too much fluoride also has a significant downside, fluorosis, which permanently stains the teeth.
Start fluoride toothpaste in minute amounts at 1 year of age. Don’t use fluoride supplementation—even in breastfed infants—unless they are on well water proven to be low in fluoride.
Evidence summary
Toothpaste as effective as rinse or gel
A large Cochrane review evaluated topical fluoride therapy in the form of toothpaste, mouth rinse, varnish, or gel. Based on 133 randomized or quasi-randomized controlled trials (n=65,169), the meta-analysis indicated a 26% (95% confidence interval [CI], 24%–29%) reduction in decayed, missing, and filled tooth surfaces in children.1 Another Cochrane review found 17 randomized controlled trials comparing different methods of topical fluoride application in children. The limited data suggested that fluoride toothpaste is as effective as mouth rinse or gel.2 Depending on the prevalence of caries in the population, between 1.6 and 3.7 children need to use a fluoride toothpaste to prevent 1 decayed, missing, or filled tooth.3
The risk of fluorosis
Topical fluoride use has been associated with dental fluorosis, which causes staining or pitting of the enamel tooth surface. The incidence of significant dental fluorosis varies in children—from 5% to 7% with 1450 ppm fluoride toothpaste to 2% to 4% with 440 ppm fluoride toothpaste (number needed to harm [NNH]=20–100).4,5
High-fluoride concentrations
High-fluoride-concentration toothpastes (1000 ppm F) prevent 14% more caries than low-fluoride-concentration toothpastes (250 ppm F).6 Another randomized controlled trial, carried out in an area without fluoridated water, found the high-fluoride-concentration toothpaste (1450 ppm F) resulted in 16% fewer caries in children, while the low-fluoride-concentration toothpaste (440 ppm F) was no different than placebo.7
When there’s fluoridated water
A meta-analysis found that the effect of topical fluoride was independent of water fluoridation, suggesting that topical fluoride toothpaste has a beneficial effect even in communities with fluoridated water.3 No relevant studies comparing topical fluoride toothpaste with oral fluoride supplementation were found.
Recommendations from others
Both the American Academy of Pediatric Dentistry (AAPD) and the Centers for Disease Control and Prevention (CDC) recommend topical fluoride toothpaste for children as an adjunct to oral fluoride intake. The AAPD8 recommends a “pea-sized” amount of toothpaste for children under 6 years of age. The CDC9 recommends that you weigh the risks and possible other sources of fluoride in children under age 2, and using a pea-sized amount of toothpaste with supervised brushing for children 2 to 6 years of age.
The TABLE shows the AAPD’s recommended daily dose of fluoride supplementation based on the fluoride concentration in the local water.
TABLE
Oral fluoride dosing: Recommendations from the American Academy of Pediatric Dentistry8
| DRINKING WATER FLUORIDE LEVEL | |||
|---|---|---|---|
| AGE | <0.3 PPM F | 0.3–0.6 PPM F | >0.6 PPM F |
| 0–6 months | 0 | 0 | 0 |
| 6 months – 3 years | 0.25 mg daily | 0 | 0 |
| 3–6 years | 0.5 mg daily | 0.25 mg daily | 0 |
| 6–16 years | 1 mg daily | 0.5 mg daily | 0 |
| ppm F, parts per million fluoride | |||
Yes. Brushing twice daily with topical fluoride toothpaste decreases the incidence of dental caries in infants and toddlers (strength of recommendation [SOR]: A, based on meta-analyses of randomized controlled trials [RCTs]). High-concentration fluoride toothpaste delivers superior caries protection, but causes more dental fluorosis.
Use of high-concentration fluoride toothpaste should be targeted towards children at highest risk of dental caries, such as those living in areas without fluoridated water (SOR: B).
Brushing, yes, but what about fluoride supplementation?
Laura G. Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University, Richmond
In medical school, we were taught that infants who are breastfed should start supplemental fluoride at 6 months. Pediatric dentists generally only use supplemental fluoride if the baby’s home has well water that has been tested and found deficient. The worst outcome from a lack of fluoride supplementation is caries, which usually can be managed. However, too much fluoride also has a significant downside, fluorosis, which permanently stains the teeth.
Start fluoride toothpaste in minute amounts at 1 year of age. Don’t use fluoride supplementation—even in breastfed infants—unless they are on well water proven to be low in fluoride.
Evidence summary
Toothpaste as effective as rinse or gel
A large Cochrane review evaluated topical fluoride therapy in the form of toothpaste, mouth rinse, varnish, or gel. Based on 133 randomized or quasi-randomized controlled trials (n=65,169), the meta-analysis indicated a 26% (95% confidence interval [CI], 24%–29%) reduction in decayed, missing, and filled tooth surfaces in children.1 Another Cochrane review found 17 randomized controlled trials comparing different methods of topical fluoride application in children. The limited data suggested that fluoride toothpaste is as effective as mouth rinse or gel.2 Depending on the prevalence of caries in the population, between 1.6 and 3.7 children need to use a fluoride toothpaste to prevent 1 decayed, missing, or filled tooth.3
The risk of fluorosis
Topical fluoride use has been associated with dental fluorosis, which causes staining or pitting of the enamel tooth surface. The incidence of significant dental fluorosis varies in children—from 5% to 7% with 1450 ppm fluoride toothpaste to 2% to 4% with 440 ppm fluoride toothpaste (number needed to harm [NNH]=20–100).4,5
High-fluoride concentrations
High-fluoride-concentration toothpastes (1000 ppm F) prevent 14% more caries than low-fluoride-concentration toothpastes (250 ppm F).6 Another randomized controlled trial, carried out in an area without fluoridated water, found the high-fluoride-concentration toothpaste (1450 ppm F) resulted in 16% fewer caries in children, while the low-fluoride-concentration toothpaste (440 ppm F) was no different than placebo.7
When there’s fluoridated water
A meta-analysis found that the effect of topical fluoride was independent of water fluoridation, suggesting that topical fluoride toothpaste has a beneficial effect even in communities with fluoridated water.3 No relevant studies comparing topical fluoride toothpaste with oral fluoride supplementation were found.
Recommendations from others
Both the American Academy of Pediatric Dentistry (AAPD) and the Centers for Disease Control and Prevention (CDC) recommend topical fluoride toothpaste for children as an adjunct to oral fluoride intake. The AAPD8 recommends a “pea-sized” amount of toothpaste for children under 6 years of age. The CDC9 recommends that you weigh the risks and possible other sources of fluoride in children under age 2, and using a pea-sized amount of toothpaste with supervised brushing for children 2 to 6 years of age.
The TABLE shows the AAPD’s recommended daily dose of fluoride supplementation based on the fluoride concentration in the local water.
TABLE
Oral fluoride dosing: Recommendations from the American Academy of Pediatric Dentistry8
| DRINKING WATER FLUORIDE LEVEL | |||
|---|---|---|---|
| AGE | <0.3 PPM F | 0.3–0.6 PPM F | >0.6 PPM F |
| 0–6 months | 0 | 0 | 0 |
| 6 months – 3 years | 0.25 mg daily | 0 | 0 |
| 3–6 years | 0.5 mg daily | 0.25 mg daily | 0 |
| 6–16 years | 1 mg daily | 0.5 mg daily | 0 |
| ppm F, parts per million fluoride | |||
1. Marinho VCC, Higgins JPT, Logan S, Sheiham A. Topical fluoride (toothpastes, mouth rinses, gels, or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;(4):CD002782.-
2. Marinho VCC, Higgins JPT, Sheiham A, Logan S. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2004;(1):CD002780.-
3. Marinho VCC, Higgins JPT, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;(1):CD002278.-
4. Tavener JA, Davies GM, Davies RM, Ellwood RP. The prevalence and severity of fluorosis in children who received toothpaste containing either 440 or 1450 ppm F from the age of 12 months in deprived and less deprived communities. Caries Res 2006;40:66-72.
5. Tavener JA, Davies GM, Davies RM, Ellwood RP. The prevalence and severity of fluorosis and other developmental defects of enamel in children who received free fluoride toothpaste containing either 440 or 1450 ppm F from the age of 12 months. Community Dent Health 2004;21:217-223.
6. Steiner M, Helfenstein U, Menghini G. Effect of 1000 ppm relative to 250 ppm fluoride toothpaste. A meta-analysis. Am J Dent 2004;17:85-88.
7. Davies GM, Worthington HV, Ellwood RP, et al. A randomized controlled trial of the effectiveness of providing free fluoride toothpaste from the age of 12 months on reducing caries in 5-6 year old children. Community Dent Health 2002;19:131-136.
8. American Academy of Pediatric Dentistry. Clinical guideline on fluoride therapy. Chicago, Ill: American Academy of Pediatric Dentistry; 2003. Available at: www.guideline.gov/summary/summary.aspx?doc_id=6272. Accessed August 6, 2007.
9. Recommendations for using fluoride to prevent and control dental caries in the united States. Centers for Disease Control and prevention. MMWR Recomm Rep 2001;50:1-42.
1. Marinho VCC, Higgins JPT, Logan S, Sheiham A. Topical fluoride (toothpastes, mouth rinses, gels, or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;(4):CD002782.-
2. Marinho VCC, Higgins JPT, Sheiham A, Logan S. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2004;(1):CD002780.-
3. Marinho VCC, Higgins JPT, Sheiham A, Logan S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;(1):CD002278.-
4. Tavener JA, Davies GM, Davies RM, Ellwood RP. The prevalence and severity of fluorosis in children who received toothpaste containing either 440 or 1450 ppm F from the age of 12 months in deprived and less deprived communities. Caries Res 2006;40:66-72.
5. Tavener JA, Davies GM, Davies RM, Ellwood RP. The prevalence and severity of fluorosis and other developmental defects of enamel in children who received free fluoride toothpaste containing either 440 or 1450 ppm F from the age of 12 months. Community Dent Health 2004;21:217-223.
6. Steiner M, Helfenstein U, Menghini G. Effect of 1000 ppm relative to 250 ppm fluoride toothpaste. A meta-analysis. Am J Dent 2004;17:85-88.
7. Davies GM, Worthington HV, Ellwood RP, et al. A randomized controlled trial of the effectiveness of providing free fluoride toothpaste from the age of 12 months on reducing caries in 5-6 year old children. Community Dent Health 2002;19:131-136.
8. American Academy of Pediatric Dentistry. Clinical guideline on fluoride therapy. Chicago, Ill: American Academy of Pediatric Dentistry; 2003. Available at: www.guideline.gov/summary/summary.aspx?doc_id=6272. Accessed August 6, 2007.
9. Recommendations for using fluoride to prevent and control dental caries in the united States. Centers for Disease Control and prevention. MMWR Recomm Rep 2001;50:1-42.
Evidence-based answers from the Family Physicians Inquiries Network
Does the age you introduce food to an infant affect allergies later?
Yes. In children at high risk for atopy (those with a family history of allergy, asthma, or eczema in at least 1 first-degree relative), breastfeeding or giving hydrolyzed protein formula during the first 4 to 6 months reduces the risk of atopy in the first year of life, when compared with introducing cow’s milk or soy formula (strength of recommendation [SOR]: B, based on a systematic review that included only 2 double-blinded randomized controlled trials [RCTs]).
There is inconsistent evidence to show that early introduction of solid food increases the incidence of atopic disease (SOR: B, systematic review of inconsistent studies).
Begin talking about infant feeding during the first postnatal visit
Mary M. Stephens, MD, MPH
East Tennessee State University, Johnson City
Having found a surprising number of children on cereal or other solids at the 2-month visit, I make it a practice to begin talking about infant feeding during the first postnatal visit. I encourage parents to wait until at least 4 months (but not more than 6 months) to start cereal, and to wait 3 to 4 days between introducing any new foods to make sure the child does not have an adverse reaction.
I generally tell all parents to keep their child on breast milk or formula and avoid whole milk until age 1, although small amounts of cheese and yogurt are fine. Additionally, I recommend avoiding citrus, honey, and eggs until age 1 and peanut butter until age 2 or 3.
For the child at high risk of atopy, the American Academy of Pediatrics recommendation to wait to introduce solid foods until 6 months of age and to wait to introduce peanuts and fish until 3 years of age seems reasonable, although I’d let parents know that they could try these foods earlier if they wanted since there is no definite evidence that those changes will make a difference.
Although I’ve never recommended hydrolyzed protein formula for children at high risk of atopy, it is an option for discussion. practical considerations to bring up in the discussion are the higher cost of these formulas and the palatability. Children receiving their formula through Women, Infant and Children (WIC) programs will need a prescription that includes the indication for the formula.
Evidence summary
Systematic reviews analyzing the modification of early feeding practices to prevent atopic disease have all been limited by a paucity of double-blinded RCTs of sufficient duration.1-3
Breastfeeding, hydrolyzed formulas confer lowest atopy risk
Breastfeeding and the use of hydrolyzed protein formulas confer the lowest risk of atopy in high-risk children when compared with cow’s milk or soy-based formulas.1,3 The relative risk for wheeze or asthma in the first year of life was 0.4 (95% confidence interval [CI] 0.19–0.85) for children fed hydrolyzed protein formulas when compared with cow’s milk.1 Studies have not found a significant difference among these forms of milk for infants without a strong family history of atopic disease.3
Delaying solid food may reduce allergies
There is speculation that introducing certain solid foods early increases the risk of allergies to these foods, as well as causing generalized atopic symptoms. Few studies have examined this, and no systematic reviews focus on atopic disease.4
A cohort study (n=1265) comparing children who had been given 4 or more types of solid food before 4 months of age with those whose caregivers delayed solid foods showed an increased incidence of eczema by 10 years of age (relative risk=2.35; P<.05) in the early feeding group.5
However, a prospective interventional cohort study using a retrospective cohort as a control (n=375) compared children who had strictly avoided fish and citrus products until 1 year of age with those who had an unrestricted diet. There was no difference in the frequency of allergy to these foods as quantified by history and positive challenge test, although the authors did not include a statistical analysis of their results.6
Another study randomized 165 high-risk children into groups with standard feeding practices or an allergy prophylaxis regimen, which included avoidance of milk protein until age 1 year, eggs until 2 years, and fish and peanuts until 3 years. Although prophylaxis decreased the prevalence of atopic disorders at 1 year, there was no difference in any atopic disease between the 2 groups at age 7.7
Recommendations from others
The American Academy of Pediatrics recommends that for high-risk infants, solid foods should not be introduced into the diet until 6 months of age, with dairy products delayed until 1 year, eggs until 2 years, and peanuts, tree nuts, and fish until 3 years of age.8
1. Ram FS, Ducharme FM, Scarlett J. Cow’s milk protein avoidance and development of childhood wheeze in children with a family history of atopy. Cochrane Database Syst Rev 2002;(3):CD003795.-
2. Smethurst D, Macfarlane S. Atopic eczema (web archive): prolonged breast feeding in predisposed infants. Clinical Evidence 2002;(8):1664-1682.
3. Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2006;(4):CD003664.-
4. Lanigan JA, Bishop J, Kimber AC, Morgan J. Systematic review concerning the age of introduction of complementary foods to the healthy full-term infant. Eur J Clin Nutr 2001;55:309-320.
5. Fergusson DM, Horwood LJ, Shannon FT. Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics 1990;86:541-546.
6. Saarinen UM, Kajosaari M. Does dietary elimination in infancy prevent or only postpone a food allergy? A study of fish and citrus allergy in 375 children. Lancet 1980;1:166-167.
7. Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol 1995;95:1179-1190.
8. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346-349.
Yes. In children at high risk for atopy (those with a family history of allergy, asthma, or eczema in at least 1 first-degree relative), breastfeeding or giving hydrolyzed protein formula during the first 4 to 6 months reduces the risk of atopy in the first year of life, when compared with introducing cow’s milk or soy formula (strength of recommendation [SOR]: B, based on a systematic review that included only 2 double-blinded randomized controlled trials [RCTs]).
There is inconsistent evidence to show that early introduction of solid food increases the incidence of atopic disease (SOR: B, systematic review of inconsistent studies).
Begin talking about infant feeding during the first postnatal visit
Mary M. Stephens, MD, MPH
East Tennessee State University, Johnson City
Having found a surprising number of children on cereal or other solids at the 2-month visit, I make it a practice to begin talking about infant feeding during the first postnatal visit. I encourage parents to wait until at least 4 months (but not more than 6 months) to start cereal, and to wait 3 to 4 days between introducing any new foods to make sure the child does not have an adverse reaction.
I generally tell all parents to keep their child on breast milk or formula and avoid whole milk until age 1, although small amounts of cheese and yogurt are fine. Additionally, I recommend avoiding citrus, honey, and eggs until age 1 and peanut butter until age 2 or 3.
For the child at high risk of atopy, the American Academy of Pediatrics recommendation to wait to introduce solid foods until 6 months of age and to wait to introduce peanuts and fish until 3 years of age seems reasonable, although I’d let parents know that they could try these foods earlier if they wanted since there is no definite evidence that those changes will make a difference.
Although I’ve never recommended hydrolyzed protein formula for children at high risk of atopy, it is an option for discussion. practical considerations to bring up in the discussion are the higher cost of these formulas and the palatability. Children receiving their formula through Women, Infant and Children (WIC) programs will need a prescription that includes the indication for the formula.
Evidence summary
Systematic reviews analyzing the modification of early feeding practices to prevent atopic disease have all been limited by a paucity of double-blinded RCTs of sufficient duration.1-3
Breastfeeding, hydrolyzed formulas confer lowest atopy risk
Breastfeeding and the use of hydrolyzed protein formulas confer the lowest risk of atopy in high-risk children when compared with cow’s milk or soy-based formulas.1,3 The relative risk for wheeze or asthma in the first year of life was 0.4 (95% confidence interval [CI] 0.19–0.85) for children fed hydrolyzed protein formulas when compared with cow’s milk.1 Studies have not found a significant difference among these forms of milk for infants without a strong family history of atopic disease.3
Delaying solid food may reduce allergies
There is speculation that introducing certain solid foods early increases the risk of allergies to these foods, as well as causing generalized atopic symptoms. Few studies have examined this, and no systematic reviews focus on atopic disease.4
A cohort study (n=1265) comparing children who had been given 4 or more types of solid food before 4 months of age with those whose caregivers delayed solid foods showed an increased incidence of eczema by 10 years of age (relative risk=2.35; P<.05) in the early feeding group.5
However, a prospective interventional cohort study using a retrospective cohort as a control (n=375) compared children who had strictly avoided fish and citrus products until 1 year of age with those who had an unrestricted diet. There was no difference in the frequency of allergy to these foods as quantified by history and positive challenge test, although the authors did not include a statistical analysis of their results.6
Another study randomized 165 high-risk children into groups with standard feeding practices or an allergy prophylaxis regimen, which included avoidance of milk protein until age 1 year, eggs until 2 years, and fish and peanuts until 3 years. Although prophylaxis decreased the prevalence of atopic disorders at 1 year, there was no difference in any atopic disease between the 2 groups at age 7.7
Recommendations from others
The American Academy of Pediatrics recommends that for high-risk infants, solid foods should not be introduced into the diet until 6 months of age, with dairy products delayed until 1 year, eggs until 2 years, and peanuts, tree nuts, and fish until 3 years of age.8
Yes. In children at high risk for atopy (those with a family history of allergy, asthma, or eczema in at least 1 first-degree relative), breastfeeding or giving hydrolyzed protein formula during the first 4 to 6 months reduces the risk of atopy in the first year of life, when compared with introducing cow’s milk or soy formula (strength of recommendation [SOR]: B, based on a systematic review that included only 2 double-blinded randomized controlled trials [RCTs]).
There is inconsistent evidence to show that early introduction of solid food increases the incidence of atopic disease (SOR: B, systematic review of inconsistent studies).
Begin talking about infant feeding during the first postnatal visit
Mary M. Stephens, MD, MPH
East Tennessee State University, Johnson City
Having found a surprising number of children on cereal or other solids at the 2-month visit, I make it a practice to begin talking about infant feeding during the first postnatal visit. I encourage parents to wait until at least 4 months (but not more than 6 months) to start cereal, and to wait 3 to 4 days between introducing any new foods to make sure the child does not have an adverse reaction.
I generally tell all parents to keep their child on breast milk or formula and avoid whole milk until age 1, although small amounts of cheese and yogurt are fine. Additionally, I recommend avoiding citrus, honey, and eggs until age 1 and peanut butter until age 2 or 3.
For the child at high risk of atopy, the American Academy of Pediatrics recommendation to wait to introduce solid foods until 6 months of age and to wait to introduce peanuts and fish until 3 years of age seems reasonable, although I’d let parents know that they could try these foods earlier if they wanted since there is no definite evidence that those changes will make a difference.
Although I’ve never recommended hydrolyzed protein formula for children at high risk of atopy, it is an option for discussion. practical considerations to bring up in the discussion are the higher cost of these formulas and the palatability. Children receiving their formula through Women, Infant and Children (WIC) programs will need a prescription that includes the indication for the formula.
Evidence summary
Systematic reviews analyzing the modification of early feeding practices to prevent atopic disease have all been limited by a paucity of double-blinded RCTs of sufficient duration.1-3
Breastfeeding, hydrolyzed formulas confer lowest atopy risk
Breastfeeding and the use of hydrolyzed protein formulas confer the lowest risk of atopy in high-risk children when compared with cow’s milk or soy-based formulas.1,3 The relative risk for wheeze or asthma in the first year of life was 0.4 (95% confidence interval [CI] 0.19–0.85) for children fed hydrolyzed protein formulas when compared with cow’s milk.1 Studies have not found a significant difference among these forms of milk for infants without a strong family history of atopic disease.3
Delaying solid food may reduce allergies
There is speculation that introducing certain solid foods early increases the risk of allergies to these foods, as well as causing generalized atopic symptoms. Few studies have examined this, and no systematic reviews focus on atopic disease.4
A cohort study (n=1265) comparing children who had been given 4 or more types of solid food before 4 months of age with those whose caregivers delayed solid foods showed an increased incidence of eczema by 10 years of age (relative risk=2.35; P<.05) in the early feeding group.5
However, a prospective interventional cohort study using a retrospective cohort as a control (n=375) compared children who had strictly avoided fish and citrus products until 1 year of age with those who had an unrestricted diet. There was no difference in the frequency of allergy to these foods as quantified by history and positive challenge test, although the authors did not include a statistical analysis of their results.6
Another study randomized 165 high-risk children into groups with standard feeding practices or an allergy prophylaxis regimen, which included avoidance of milk protein until age 1 year, eggs until 2 years, and fish and peanuts until 3 years. Although prophylaxis decreased the prevalence of atopic disorders at 1 year, there was no difference in any atopic disease between the 2 groups at age 7.7
Recommendations from others
The American Academy of Pediatrics recommends that for high-risk infants, solid foods should not be introduced into the diet until 6 months of age, with dairy products delayed until 1 year, eggs until 2 years, and peanuts, tree nuts, and fish until 3 years of age.8
1. Ram FS, Ducharme FM, Scarlett J. Cow’s milk protein avoidance and development of childhood wheeze in children with a family history of atopy. Cochrane Database Syst Rev 2002;(3):CD003795.-
2. Smethurst D, Macfarlane S. Atopic eczema (web archive): prolonged breast feeding in predisposed infants. Clinical Evidence 2002;(8):1664-1682.
3. Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2006;(4):CD003664.-
4. Lanigan JA, Bishop J, Kimber AC, Morgan J. Systematic review concerning the age of introduction of complementary foods to the healthy full-term infant. Eur J Clin Nutr 2001;55:309-320.
5. Fergusson DM, Horwood LJ, Shannon FT. Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics 1990;86:541-546.
6. Saarinen UM, Kajosaari M. Does dietary elimination in infancy prevent or only postpone a food allergy? A study of fish and citrus allergy in 375 children. Lancet 1980;1:166-167.
7. Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol 1995;95:1179-1190.
8. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346-349.
1. Ram FS, Ducharme FM, Scarlett J. Cow’s milk protein avoidance and development of childhood wheeze in children with a family history of atopy. Cochrane Database Syst Rev 2002;(3):CD003795.-
2. Smethurst D, Macfarlane S. Atopic eczema (web archive): prolonged breast feeding in predisposed infants. Clinical Evidence 2002;(8):1664-1682.
3. Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2006;(4):CD003664.-
4. Lanigan JA, Bishop J, Kimber AC, Morgan J. Systematic review concerning the age of introduction of complementary foods to the healthy full-term infant. Eur J Clin Nutr 2001;55:309-320.
5. Fergusson DM, Horwood LJ, Shannon FT. Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics 1990;86:541-546.
6. Saarinen UM, Kajosaari M. Does dietary elimination in infancy prevent or only postpone a food allergy? A study of fish and citrus allergy in 375 children. Lancet 1980;1:166-167.
7. Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol 1995;95:1179-1190.
8. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106:346-349.
Evidence-based answers from the Family Physicians Inquiries Network
Which technique for removing nevi is least scarring?
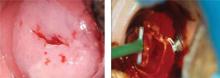
A shave biopsy with a razor blade or #15 scalpel is the best approach for a facial nevus, assuming malignancy is not suspected. the resulting scar is usually flat, smaller than the lesion, has no suture lines, and—if shaved in mid or upper dermis—has a low risk of producing a hypertrophic or hypotrophic scar (strength of recommendation: C, expert opinion, committee guidelines).
Shave biopsies are quick and well-tolerated
Parul Harsora, MD
University of Texas Southwestern, Dallas
If you suspect malignancy in a nevus, obtain an excisional or incisional biopsy. Shave biopsies are best suited for raised, flesh-colored nevi and are generally quick, well-tolerated, and cost-effective. tissue from a shave biopsy can be submitted for histological evaluation.
Shave biopsies are preferred by patients because there are no sutures and scarring is minimized. the site may be pink and may take several months to develop a normal appearance. the final result may be unnoticeable, or leave an indentation or be hypo- or hyperpigmented.
Hairy, pigmented, and compound nevi are likely to do better with a punch biopsy. to prevent recurrence, seek histologic confirmation that the entire nevus has been removed.
Evidence summary
Numerous reports and guidelines indicate that if a nevus is even slightly suspicious for malignancy, it should be removed by excisional biopsy or sampled for diagnosis by punch or incisional biopsy. There are no randomized controlled trials or cohort studies comparing techniques for removing raised nevi from the face.
Shave biopsy has good outcomes
Expert opinion and individual prospective case series show acceptable outcomes for shave biopsy. One prospective study followed 55 patients after removal of nevi from the head and neck. These nevi were removed using a shave procedure with a#15 scalpel and hot cautery for bleeding. Of the 55 sites, 4 retained pigment and 30 had a visible scar with a mean diameter of 5 mm at 6- to 8-month follow-up.1 The mean diameter of the original lesions was 6 mm. There was no difference between the size of those lesions that scarred and those that didn’t.
Researchers conducting a second retrospective study, done at least 1 year after the procedure, used a questionnaire to ask 76 patients (with a total of 83 nevi removed from the face by shave excision) about their perceptions of the scar.2 Patients described their lesions as: no scar (33%), white and flat (25%), depressed (19%), raised (15%), and pigmented (7%). Eighty-six percent thought their scars looked better than the nevus and 79% were “happy with the way the scar looks now.” Two additional studies, based on both patient and provider perceptions, with similar conclusions, are presented in the (TABLE).3,4
TABLE
Favorable cosmetic results following shave biopsy of facial nevi
| STUDY | NO. PTS/NEVI | % WITH RET AINED PIGMENT OR RECURRENCE | % WITH VISIBLE SCARRING | FOLLOW-UP INTERVAL | EVALUATION/DONE BY |
|---|---|---|---|---|---|
| Hudson-Peacock1 | 55/55 | 13 | 55 | 6-8 mo | Cosmetically acceptable/patients |
| Bong2 | 76/83 | 28 | 67 | ≥1 yr | 86%: better than nevus/patients |
| Zanardini3 | 206/ 215 | 4 | 9 | 3 mo | 90%: excellent* 9%: good/surgeons |
| Ferrandiz4 | Not known/59 | 20† | 67 | 3 mo | 98%: better than nevus/pts; 92%: excellent or acceptable‡/surgeons |
| Excellent=no noticeable scar, good=slightly noticeable scar with normochromia or hypochromia, poor=depressed scar or intense dyschromia. | |||||
| † Some lesions not papular. | |||||
| ‡ Excellent cosmetic result=imperceptible scar without erythema, hyper- or hypo-pigmentation, hypertrophy or atrophy. Acceptable=scar better than original mole. poor=left scar worse than original mole. | |||||
Atypical lesions should be excised
Atypical lesions require excisional biopsy. The depth and architecture of the lesion, if melanoma, cannot be determined by shave biopsy, and both treatment and prognosis depend on those characteristics.
These guidelines derive from well-designed, nonexperimental descriptive studies.5 However, a recent retrospective study compared the Breslow depth determination of 4 different biopsy techniques, performed by experienced dermatologists, with the subsequent depth on definitive surgery for melanoma. This study found that superficial shave, deep shave, and punch biopsy predicted the Breslow depth 88% (95/108) of the time.6 As expected, excisional biopsy predicted the depth 100% (30/30) of the time. The location of the biopsy sites were not reported. The choice of biopsy was influenced by the suspicion of melanoma; thin (< 1 mm) melanomas were more likely to be superficially shaved than deep-shaved or punched.
Recommendations from others
Guidelines on nevocellular nevi from the American Academy of Dermatology recommend a simple excisional or incisional biopsy; they do not discuss the method of removal for benign appearing facial lesions.7
The UK Guidelines for the Management of Cutaneous Melanoma recommend that suspicious lesions be excised completely (excisional biopsy) and sent for confirmatory histopathological examination.5 A biopsy that transects the depth of the lesion (for example, superficial shave biopsy) should be avoided because histological depth of invasion is the basic criterion for staging and shave biopsy makes the staging impossible in some cases.
1. Hudson-Peacock MJ, Bishop J, Lawrence CM. Shave excision of benign papular naevocytic nevi. Br J Plast Surg 1995;48:318-322.
2. Bong JL, Perkins W. Shave excision of benign facial melanocytic naevi: a patient’s satisfaction survey. Dermatol Surg 2003;29:227-229.
3. Zanardini Pereira CA, Alchorne AOA. angential excision of nevocellular nevus on the face. Int J Dermatol 2004;43:533-537.
4. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg 2005;3(Pt 1)1:1112-1115.
5. Roberts DLL, Anstey AV, Barlow RJ, et al. U.K.Guidelines for the management of cutaneous melanoma. Br J Dermatol 2002;146:7-17.
6. Ng PC, Barzilai DA, Ismail SA, Averitte RL Jr, Gilliam AC. valuating invasive cutaneous melanoma: Is the initial biopsy representative of the final depth? J Am Acad Dermatol 2005;48:420-424.
7. Drake L A, Ceilley R I, Cornelison RL , et al Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for nevi I (nevocellular nevi and seborrheic keratoses). J Am Acad Dermatol 1992;26:629-631.
A shave biopsy with a razor blade or #15 scalpel is the best approach for a facial nevus, assuming malignancy is not suspected. the resulting scar is usually flat, smaller than the lesion, has no suture lines, and—if shaved in mid or upper dermis—has a low risk of producing a hypertrophic or hypotrophic scar (strength of recommendation: C, expert opinion, committee guidelines).
Shave biopsies are quick and well-tolerated
Parul Harsora, MD
University of Texas Southwestern, Dallas
If you suspect malignancy in a nevus, obtain an excisional or incisional biopsy. Shave biopsies are best suited for raised, flesh-colored nevi and are generally quick, well-tolerated, and cost-effective. tissue from a shave biopsy can be submitted for histological evaluation.
Shave biopsies are preferred by patients because there are no sutures and scarring is minimized. the site may be pink and may take several months to develop a normal appearance. the final result may be unnoticeable, or leave an indentation or be hypo- or hyperpigmented.
Hairy, pigmented, and compound nevi are likely to do better with a punch biopsy. to prevent recurrence, seek histologic confirmation that the entire nevus has been removed.
Evidence summary
Numerous reports and guidelines indicate that if a nevus is even slightly suspicious for malignancy, it should be removed by excisional biopsy or sampled for diagnosis by punch or incisional biopsy. There are no randomized controlled trials or cohort studies comparing techniques for removing raised nevi from the face.
Shave biopsy has good outcomes
Expert opinion and individual prospective case series show acceptable outcomes for shave biopsy. One prospective study followed 55 patients after removal of nevi from the head and neck. These nevi were removed using a shave procedure with a#15 scalpel and hot cautery for bleeding. Of the 55 sites, 4 retained pigment and 30 had a visible scar with a mean diameter of 5 mm at 6- to 8-month follow-up.1 The mean diameter of the original lesions was 6 mm. There was no difference between the size of those lesions that scarred and those that didn’t.
Researchers conducting a second retrospective study, done at least 1 year after the procedure, used a questionnaire to ask 76 patients (with a total of 83 nevi removed from the face by shave excision) about their perceptions of the scar.2 Patients described their lesions as: no scar (33%), white and flat (25%), depressed (19%), raised (15%), and pigmented (7%). Eighty-six percent thought their scars looked better than the nevus and 79% were “happy with the way the scar looks now.” Two additional studies, based on both patient and provider perceptions, with similar conclusions, are presented in the (TABLE).3,4
TABLE
Favorable cosmetic results following shave biopsy of facial nevi
| STUDY | NO. PTS/NEVI | % WITH RET AINED PIGMENT OR RECURRENCE | % WITH VISIBLE SCARRING | FOLLOW-UP INTERVAL | EVALUATION/DONE BY |
|---|---|---|---|---|---|
| Hudson-Peacock1 | 55/55 | 13 | 55 | 6-8 mo | Cosmetically acceptable/patients |
| Bong2 | 76/83 | 28 | 67 | ≥1 yr | 86%: better than nevus/patients |
| Zanardini3 | 206/ 215 | 4 | 9 | 3 mo | 90%: excellent* 9%: good/surgeons |
| Ferrandiz4 | Not known/59 | 20† | 67 | 3 mo | 98%: better than nevus/pts; 92%: excellent or acceptable‡/surgeons |
| Excellent=no noticeable scar, good=slightly noticeable scar with normochromia or hypochromia, poor=depressed scar or intense dyschromia. | |||||
| † Some lesions not papular. | |||||
| ‡ Excellent cosmetic result=imperceptible scar without erythema, hyper- or hypo-pigmentation, hypertrophy or atrophy. Acceptable=scar better than original mole. poor=left scar worse than original mole. | |||||
Atypical lesions should be excised
Atypical lesions require excisional biopsy. The depth and architecture of the lesion, if melanoma, cannot be determined by shave biopsy, and both treatment and prognosis depend on those characteristics.
These guidelines derive from well-designed, nonexperimental descriptive studies.5 However, a recent retrospective study compared the Breslow depth determination of 4 different biopsy techniques, performed by experienced dermatologists, with the subsequent depth on definitive surgery for melanoma. This study found that superficial shave, deep shave, and punch biopsy predicted the Breslow depth 88% (95/108) of the time.6 As expected, excisional biopsy predicted the depth 100% (30/30) of the time. The location of the biopsy sites were not reported. The choice of biopsy was influenced by the suspicion of melanoma; thin (< 1 mm) melanomas were more likely to be superficially shaved than deep-shaved or punched.
Recommendations from others
Guidelines on nevocellular nevi from the American Academy of Dermatology recommend a simple excisional or incisional biopsy; they do not discuss the method of removal for benign appearing facial lesions.7
The UK Guidelines for the Management of Cutaneous Melanoma recommend that suspicious lesions be excised completely (excisional biopsy) and sent for confirmatory histopathological examination.5 A biopsy that transects the depth of the lesion (for example, superficial shave biopsy) should be avoided because histological depth of invasion is the basic criterion for staging and shave biopsy makes the staging impossible in some cases.
A shave biopsy with a razor blade or #15 scalpel is the best approach for a facial nevus, assuming malignancy is not suspected. the resulting scar is usually flat, smaller than the lesion, has no suture lines, and—if shaved in mid or upper dermis—has a low risk of producing a hypertrophic or hypotrophic scar (strength of recommendation: C, expert opinion, committee guidelines).
Shave biopsies are quick and well-tolerated
Parul Harsora, MD
University of Texas Southwestern, Dallas
If you suspect malignancy in a nevus, obtain an excisional or incisional biopsy. Shave biopsies are best suited for raised, flesh-colored nevi and are generally quick, well-tolerated, and cost-effective. tissue from a shave biopsy can be submitted for histological evaluation.
Shave biopsies are preferred by patients because there are no sutures and scarring is minimized. the site may be pink and may take several months to develop a normal appearance. the final result may be unnoticeable, or leave an indentation or be hypo- or hyperpigmented.
Hairy, pigmented, and compound nevi are likely to do better with a punch biopsy. to prevent recurrence, seek histologic confirmation that the entire nevus has been removed.
Evidence summary
Numerous reports and guidelines indicate that if a nevus is even slightly suspicious for malignancy, it should be removed by excisional biopsy or sampled for diagnosis by punch or incisional biopsy. There are no randomized controlled trials or cohort studies comparing techniques for removing raised nevi from the face.
Shave biopsy has good outcomes
Expert opinion and individual prospective case series show acceptable outcomes for shave biopsy. One prospective study followed 55 patients after removal of nevi from the head and neck. These nevi were removed using a shave procedure with a#15 scalpel and hot cautery for bleeding. Of the 55 sites, 4 retained pigment and 30 had a visible scar with a mean diameter of 5 mm at 6- to 8-month follow-up.1 The mean diameter of the original lesions was 6 mm. There was no difference between the size of those lesions that scarred and those that didn’t.
Researchers conducting a second retrospective study, done at least 1 year after the procedure, used a questionnaire to ask 76 patients (with a total of 83 nevi removed from the face by shave excision) about their perceptions of the scar.2 Patients described their lesions as: no scar (33%), white and flat (25%), depressed (19%), raised (15%), and pigmented (7%). Eighty-six percent thought their scars looked better than the nevus and 79% were “happy with the way the scar looks now.” Two additional studies, based on both patient and provider perceptions, with similar conclusions, are presented in the (TABLE).3,4
TABLE
Favorable cosmetic results following shave biopsy of facial nevi
| STUDY | NO. PTS/NEVI | % WITH RET AINED PIGMENT OR RECURRENCE | % WITH VISIBLE SCARRING | FOLLOW-UP INTERVAL | EVALUATION/DONE BY |
|---|---|---|---|---|---|
| Hudson-Peacock1 | 55/55 | 13 | 55 | 6-8 mo | Cosmetically acceptable/patients |
| Bong2 | 76/83 | 28 | 67 | ≥1 yr | 86%: better than nevus/patients |
| Zanardini3 | 206/ 215 | 4 | 9 | 3 mo | 90%: excellent* 9%: good/surgeons |
| Ferrandiz4 | Not known/59 | 20† | 67 | 3 mo | 98%: better than nevus/pts; 92%: excellent or acceptable‡/surgeons |
| Excellent=no noticeable scar, good=slightly noticeable scar with normochromia or hypochromia, poor=depressed scar or intense dyschromia. | |||||
| † Some lesions not papular. | |||||
| ‡ Excellent cosmetic result=imperceptible scar without erythema, hyper- or hypo-pigmentation, hypertrophy or atrophy. Acceptable=scar better than original mole. poor=left scar worse than original mole. | |||||
Atypical lesions should be excised
Atypical lesions require excisional biopsy. The depth and architecture of the lesion, if melanoma, cannot be determined by shave biopsy, and both treatment and prognosis depend on those characteristics.
These guidelines derive from well-designed, nonexperimental descriptive studies.5 However, a recent retrospective study compared the Breslow depth determination of 4 different biopsy techniques, performed by experienced dermatologists, with the subsequent depth on definitive surgery for melanoma. This study found that superficial shave, deep shave, and punch biopsy predicted the Breslow depth 88% (95/108) of the time.6 As expected, excisional biopsy predicted the depth 100% (30/30) of the time. The location of the biopsy sites were not reported. The choice of biopsy was influenced by the suspicion of melanoma; thin (< 1 mm) melanomas were more likely to be superficially shaved than deep-shaved or punched.
Recommendations from others
Guidelines on nevocellular nevi from the American Academy of Dermatology recommend a simple excisional or incisional biopsy; they do not discuss the method of removal for benign appearing facial lesions.7
The UK Guidelines for the Management of Cutaneous Melanoma recommend that suspicious lesions be excised completely (excisional biopsy) and sent for confirmatory histopathological examination.5 A biopsy that transects the depth of the lesion (for example, superficial shave biopsy) should be avoided because histological depth of invasion is the basic criterion for staging and shave biopsy makes the staging impossible in some cases.
1. Hudson-Peacock MJ, Bishop J, Lawrence CM. Shave excision of benign papular naevocytic nevi. Br J Plast Surg 1995;48:318-322.
2. Bong JL, Perkins W. Shave excision of benign facial melanocytic naevi: a patient’s satisfaction survey. Dermatol Surg 2003;29:227-229.
3. Zanardini Pereira CA, Alchorne AOA. angential excision of nevocellular nevus on the face. Int J Dermatol 2004;43:533-537.
4. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg 2005;3(Pt 1)1:1112-1115.
5. Roberts DLL, Anstey AV, Barlow RJ, et al. U.K.Guidelines for the management of cutaneous melanoma. Br J Dermatol 2002;146:7-17.
6. Ng PC, Barzilai DA, Ismail SA, Averitte RL Jr, Gilliam AC. valuating invasive cutaneous melanoma: Is the initial biopsy representative of the final depth? J Am Acad Dermatol 2005;48:420-424.
7. Drake L A, Ceilley R I, Cornelison RL , et al Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for nevi I (nevocellular nevi and seborrheic keratoses). J Am Acad Dermatol 1992;26:629-631.
1. Hudson-Peacock MJ, Bishop J, Lawrence CM. Shave excision of benign papular naevocytic nevi. Br J Plast Surg 1995;48:318-322.
2. Bong JL, Perkins W. Shave excision of benign facial melanocytic naevi: a patient’s satisfaction survey. Dermatol Surg 2003;29:227-229.
3. Zanardini Pereira CA, Alchorne AOA. angential excision of nevocellular nevus on the face. Int J Dermatol 2004;43:533-537.
4. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg 2005;3(Pt 1)1:1112-1115.
5. Roberts DLL, Anstey AV, Barlow RJ, et al. U.K.Guidelines for the management of cutaneous melanoma. Br J Dermatol 2002;146:7-17.
6. Ng PC, Barzilai DA, Ismail SA, Averitte RL Jr, Gilliam AC. valuating invasive cutaneous melanoma: Is the initial biopsy representative of the final depth? J Am Acad Dermatol 2005;48:420-424.
7. Drake L A, Ceilley R I, Cornelison RL , et al Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for nevi I (nevocellular nevi and seborrheic keratoses). J Am Acad Dermatol 1992;26:629-631.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best treatment for CIN 2 or 3?
Excision or ablation of the transformation zone are equally effective for treating an initial diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3 in women with a satisfactory colposcopy and no suggestion of microinvasive or invasive disease (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]).
Laser or loop electrosurgical excision procedure (LEEP) are the preferred treatment methods for recurrent CIN 2 and CIN 3 (SOR: B, based on clinical trials without randomization).
For women with an unsatisfactory colposcopy or suspicion of invasive disease, a diagnostic excisional procedure is recommended (SOR: C, based on consensus guidelines).
Observation or deferred treatment may be acceptable for CIN 2 in adolescents with satisfactory colposcopy and negative endocervical sampling (SOR: C, based on consensus guidelines).
Limit diagnostic excisional procedures in pregnancy to cases where suspicion of invasive cancer is high (SOR: C, based on consensus guidelines).
LEEP provides tissue for examination and a short recovery time
Timothy Huber, MD
Oroville, Calif
Close follow-up of observable disease and aggressive intervention continue to drive down the number of cervical cancer deaths each year.
It remains to be seen what the true effect of the HPV vaccine will be, although the presumed result will be a dramatic decline in high-grade lesions (CIN 2 and 3), carcinoma in situ, and invasive disease.
When intervention is necessary, my preferred method is LEEP because it provides tissue for examination and the recovery time is short.
Cryotherapy is an acceptable alternative, but the 4 to 8 weeks of leukorrhea and the lack of a tissue diagnosis often make it a less desirable option for patient and physician.
Evidence summary
Morbidity profile makes LEEP appear best
Similar efficacy. All 7 available surgical techniques were found to have similar efficacy, in a 2005 Cochrane review of 28 randomized trials.1 Resolution of CIN 2 or 3 lesions was 77% to 98%, using knife cone biopsy, laser conization, loop excision, laser ablation, cryotherapy, or 2 techniques not used commonly in the US, cold coagulation and radical diathermy. Surgical techniques were tested in various combinations, but no trial compared all of the techniques with one another. Most studies were underpowered, limiting the results.
Pap test: Good, but underused We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality. Yet, even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. The Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab. 9
Dr. J. Thomas cox, university of California. Member, American Cancer society Cervical guidelines Committee, the 2002 Bethesda Workshop; ACS HPV vaccine Advisory Committee; author, ASCCP guidelines Committee
Each year in the united states approximately 500,000 women are diagnosed with high-grade cervical cancer precursor lesions, CIN 2 and CIN III.4 If left untreated, 22% of CIN 2 lesions progress to carcinoma in situ or invasive cervical cancer, 43% regress, and 35% persist at the same level. Fourteen percent of untreated CIN 3 lesions progress, 32% regress, and 56% persist at the same level.
HPV vaccine: Won’t replace prevention or protection Although an effective vaccine is a major advance in the prevention of genital HPV and cervical cancer, it will not replace other prevention strategies, such as cervical cancer screening for women or protective sexual behaviors. Women should continue to get Pap tests as a safeguard against cervical cancer.
Dr. Anne Schuchat, Director, CDC National Center for Immunization and respiratory Diseases, June 29, 2006 press release (www.cdc.gov/od/oc/media/pressrel/r060629.htm)
The HPV vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18, was approved by the us Food and Drug Administration in June 2006 for use in females 9 to 26 years oof age. Shortly after, the Advisory Committee on Immunization Practices issued guidelines, stating that vaccination is recommended for all women <26 years of age. (www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm).
HPV testing: Adjunct to cytology The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are underway. 10
Dr. Thomas C. Wright, Columbia university. Author, 2001 Consensus guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim guidance for use of HPV DNA testing for Primary screening, and the 2001 Bethesda system
Least morbidity with LEEP. Morbidity was compared with one-on-one trials of different techniques ( TABLE 1 ). The review noted that LEEP has the least morbidity (such as hemorrhage, infection, cervical stenosis, and midtrimester pregnancy loss) while providing the most reliable histology by excising tissue without causing thermal artifact ( FIGURE ).
Higher rate of hemorrhage with cone biopsy. Another systematic review of 21 controlled trials comparing treatments for CIN 2 or 3 found a similar efficacy of all the modalities, including cone biopsy, cryotherapy, laser ablation, and LEEP. However, it also found a trend toward a higher rate of significant hemorrhage among women who received cone biopsies compared with women who received either laser ablation or LEEP.2
FIGURE
CIN 2 and 3 and its treatment by LEEP
TABLE 1
CIN 2 and 3 treatment options: An outcomes comparison
| COMPARISON OF TREATMENTS | OUTCOME/MORBIDITY | ODDS RATIO (95% CI) |
|---|---|---|
| Laser ablation vs cryotherapy | Laser ablation had more perioperative severe bleeding | 7.45 (1.68–33) |
| Laser ablation had higher rates of future adequate colposcopy | 4.64 (2.98–7.27) | |
| Laser conization vs knife conization | Laser conization had higher rates of future adequate colposcopy | 2.73 (1.47–5.08) |
| Laser conization had less cervical stenosis | 0.39 (0.25–0.61) | |
| Laser conization vs LEEP | Laser conization had more severe pain during procedure | 5.36 (1.02–17.2) |
| LEEP had fewer inadequate future colposcopies | 0.27 (0.08–0.89) | |
| Source: Martin-Hirsch et al, Cochrane Database Syst Rev 2000.1 | ||
Surgical treatment raises obstetric risks
There is a concern regarding future obstetric outcomes for women who have undergone surgical treatment of a high-grade cervical lesion. A recent meta-analysis of 27 controlled cohort studies found that cold knife conization and LEEP were associated with increased obstetrical risks, such as delivery at less than 37 weeks’ gestation and a birth weight <2500 g ( TABLE 2 ). Any resection that was more than 10 mm deep increased the risk of prematurity with future pregnancies (pooled relative risk=2.6; 95%] CI, 1.3–5.3).3
TABLE 2
Obstetrical outcomes for CIN 2 and 3 treatment options
| TREATMENT TYPE | OBSTETRICAL OUTCOME | RELATIVE RISK (95% CI) |
|---|---|---|
| Cold knife conization | Preterm delivery | 2.59 (1.80–3.72) |
| Low birth weight | 2.53 (1.19–5.36) | |
| Cesarean delivery | 3.17 (1.07–9.40) | |
| Laser conization | Preterm delivery | 1.71 (0.93–3.14) |
| LEEP | Preterm delivery | 1.70 (1.24–2.35) |
| Low birth weight | 1.82 (1.09–3.06) | |
| Preterm premature rupture of membranes | 2.69 (1.62–4.46) | |
| Source: Kyrgiou et al, Lancet 2006.3 | ||
Recommendations from others
Consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and a practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) both recommend immediate removal of the entire transformation zone, with either ablative or excisional treatment as initial treatment of CIN 2 and 3 for patients who are not pregnant.4,5
Value of excisional treatment. The ASCCP guidelines note that there is a benefit to excisional treatment, as it allows pathologic assessment of the excised tissue. Some of the ASCCP guideline authors recommend excisional procedures for the management of large CIN 2 and 3 lesions, which are at increased risk of having microinvasive disease.4
For women with unsatisfactory colposcopy and biopsy-proven CIN 2 or 3, there is up to a 7% risk for an occult invasive cervical carcinoma.1,4 Performing a diagnostic excisional procedure is recommended on these patients.4,6
ASCCP and ACOG make special recommendations for both adolescents and pregnant women.
For adolescent patients with biopsy-proven CIN 2, a recent ACOG Committee Opinion recommends close follow-up—with Pap smears or colposcopies every 4 to 6 months—due to the high rates of resolution of CIN 2 in adolescents.7
For pregnant patients, diagnostic excisional procedures are associated with complications such as bleeding and preterm delivery, while there is minimal risk of CIN 2 or 3 progressing to invasive cervical cancer.4,8 In pregnancy, follow CIN 2 and 3 with colposcopy each trimester, and reevaluate at 6 to 12 weeks postpartum. Limit any diagnostic excisional procedures to cases where you cannot rule out invasive cancer.4,5
1. Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;(2)CD001318.-
2. Nuovo J, Melnikow J, Willan AR, Chan BKS. Treatment outcomes for squamous intraepithelial lesions. Int J gynaecol obstet 2000;68:25-33.
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-498.
4. Wright TC, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
5. American College of obstetricians and gynecologists. ACOG Practice Bulletin number 66, september 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol 2005;106:645-664.
6. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol 1999;180:276-282.
7. American College of obstetricians and gynecologists. ACOG Committee opinion. Evaluation and management of abnormal cervical cytology and histology in the adolescent. Number 330, April 2006. Obstet Gynecol 2006;107:963-968.
8. Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 1993;81:915-918.
9. Cox JT. We’re on the way to ending cervical cancer. OBG Management 2006;18(3):62-72.
10. Wright TC. Cervical disease update. OBG Management 2007;19(3):52-60.
Excision or ablation of the transformation zone are equally effective for treating an initial diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3 in women with a satisfactory colposcopy and no suggestion of microinvasive or invasive disease (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]).
Laser or loop electrosurgical excision procedure (LEEP) are the preferred treatment methods for recurrent CIN 2 and CIN 3 (SOR: B, based on clinical trials without randomization).
For women with an unsatisfactory colposcopy or suspicion of invasive disease, a diagnostic excisional procedure is recommended (SOR: C, based on consensus guidelines).
Observation or deferred treatment may be acceptable for CIN 2 in adolescents with satisfactory colposcopy and negative endocervical sampling (SOR: C, based on consensus guidelines).
Limit diagnostic excisional procedures in pregnancy to cases where suspicion of invasive cancer is high (SOR: C, based on consensus guidelines).
LEEP provides tissue for examination and a short recovery time
Timothy Huber, MD
Oroville, Calif
Close follow-up of observable disease and aggressive intervention continue to drive down the number of cervical cancer deaths each year.
It remains to be seen what the true effect of the HPV vaccine will be, although the presumed result will be a dramatic decline in high-grade lesions (CIN 2 and 3), carcinoma in situ, and invasive disease.
When intervention is necessary, my preferred method is LEEP because it provides tissue for examination and the recovery time is short.
Cryotherapy is an acceptable alternative, but the 4 to 8 weeks of leukorrhea and the lack of a tissue diagnosis often make it a less desirable option for patient and physician.
Evidence summary
Morbidity profile makes LEEP appear best
Similar efficacy. All 7 available surgical techniques were found to have similar efficacy, in a 2005 Cochrane review of 28 randomized trials.1 Resolution of CIN 2 or 3 lesions was 77% to 98%, using knife cone biopsy, laser conization, loop excision, laser ablation, cryotherapy, or 2 techniques not used commonly in the US, cold coagulation and radical diathermy. Surgical techniques were tested in various combinations, but no trial compared all of the techniques with one another. Most studies were underpowered, limiting the results.
Pap test: Good, but underused We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality. Yet, even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. The Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab. 9
Dr. J. Thomas cox, university of California. Member, American Cancer society Cervical guidelines Committee, the 2002 Bethesda Workshop; ACS HPV vaccine Advisory Committee; author, ASCCP guidelines Committee
Each year in the united states approximately 500,000 women are diagnosed with high-grade cervical cancer precursor lesions, CIN 2 and CIN III.4 If left untreated, 22% of CIN 2 lesions progress to carcinoma in situ or invasive cervical cancer, 43% regress, and 35% persist at the same level. Fourteen percent of untreated CIN 3 lesions progress, 32% regress, and 56% persist at the same level.
HPV vaccine: Won’t replace prevention or protection Although an effective vaccine is a major advance in the prevention of genital HPV and cervical cancer, it will not replace other prevention strategies, such as cervical cancer screening for women or protective sexual behaviors. Women should continue to get Pap tests as a safeguard against cervical cancer.
Dr. Anne Schuchat, Director, CDC National Center for Immunization and respiratory Diseases, June 29, 2006 press release (www.cdc.gov/od/oc/media/pressrel/r060629.htm)
The HPV vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18, was approved by the us Food and Drug Administration in June 2006 for use in females 9 to 26 years oof age. Shortly after, the Advisory Committee on Immunization Practices issued guidelines, stating that vaccination is recommended for all women <26 years of age. (www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm).
HPV testing: Adjunct to cytology The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are underway. 10
Dr. Thomas C. Wright, Columbia university. Author, 2001 Consensus guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim guidance for use of HPV DNA testing for Primary screening, and the 2001 Bethesda system
Least morbidity with LEEP. Morbidity was compared with one-on-one trials of different techniques ( TABLE 1 ). The review noted that LEEP has the least morbidity (such as hemorrhage, infection, cervical stenosis, and midtrimester pregnancy loss) while providing the most reliable histology by excising tissue without causing thermal artifact ( FIGURE ).
Higher rate of hemorrhage with cone biopsy. Another systematic review of 21 controlled trials comparing treatments for CIN 2 or 3 found a similar efficacy of all the modalities, including cone biopsy, cryotherapy, laser ablation, and LEEP. However, it also found a trend toward a higher rate of significant hemorrhage among women who received cone biopsies compared with women who received either laser ablation or LEEP.2
FIGURE
CIN 2 and 3 and its treatment by LEEP
TABLE 1
CIN 2 and 3 treatment options: An outcomes comparison
| COMPARISON OF TREATMENTS | OUTCOME/MORBIDITY | ODDS RATIO (95% CI) |
|---|---|---|
| Laser ablation vs cryotherapy | Laser ablation had more perioperative severe bleeding | 7.45 (1.68–33) |
| Laser ablation had higher rates of future adequate colposcopy | 4.64 (2.98–7.27) | |
| Laser conization vs knife conization | Laser conization had higher rates of future adequate colposcopy | 2.73 (1.47–5.08) |
| Laser conization had less cervical stenosis | 0.39 (0.25–0.61) | |
| Laser conization vs LEEP | Laser conization had more severe pain during procedure | 5.36 (1.02–17.2) |
| LEEP had fewer inadequate future colposcopies | 0.27 (0.08–0.89) | |
| Source: Martin-Hirsch et al, Cochrane Database Syst Rev 2000.1 | ||
Surgical treatment raises obstetric risks
There is a concern regarding future obstetric outcomes for women who have undergone surgical treatment of a high-grade cervical lesion. A recent meta-analysis of 27 controlled cohort studies found that cold knife conization and LEEP were associated with increased obstetrical risks, such as delivery at less than 37 weeks’ gestation and a birth weight <2500 g ( TABLE 2 ). Any resection that was more than 10 mm deep increased the risk of prematurity with future pregnancies (pooled relative risk=2.6; 95%] CI, 1.3–5.3).3
TABLE 2
Obstetrical outcomes for CIN 2 and 3 treatment options
| TREATMENT TYPE | OBSTETRICAL OUTCOME | RELATIVE RISK (95% CI) |
|---|---|---|
| Cold knife conization | Preterm delivery | 2.59 (1.80–3.72) |
| Low birth weight | 2.53 (1.19–5.36) | |
| Cesarean delivery | 3.17 (1.07–9.40) | |
| Laser conization | Preterm delivery | 1.71 (0.93–3.14) |
| LEEP | Preterm delivery | 1.70 (1.24–2.35) |
| Low birth weight | 1.82 (1.09–3.06) | |
| Preterm premature rupture of membranes | 2.69 (1.62–4.46) | |
| Source: Kyrgiou et al, Lancet 2006.3 | ||
Recommendations from others
Consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and a practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) both recommend immediate removal of the entire transformation zone, with either ablative or excisional treatment as initial treatment of CIN 2 and 3 for patients who are not pregnant.4,5
Value of excisional treatment. The ASCCP guidelines note that there is a benefit to excisional treatment, as it allows pathologic assessment of the excised tissue. Some of the ASCCP guideline authors recommend excisional procedures for the management of large CIN 2 and 3 lesions, which are at increased risk of having microinvasive disease.4
For women with unsatisfactory colposcopy and biopsy-proven CIN 2 or 3, there is up to a 7% risk for an occult invasive cervical carcinoma.1,4 Performing a diagnostic excisional procedure is recommended on these patients.4,6
ASCCP and ACOG make special recommendations for both adolescents and pregnant women.
For adolescent patients with biopsy-proven CIN 2, a recent ACOG Committee Opinion recommends close follow-up—with Pap smears or colposcopies every 4 to 6 months—due to the high rates of resolution of CIN 2 in adolescents.7
For pregnant patients, diagnostic excisional procedures are associated with complications such as bleeding and preterm delivery, while there is minimal risk of CIN 2 or 3 progressing to invasive cervical cancer.4,8 In pregnancy, follow CIN 2 and 3 with colposcopy each trimester, and reevaluate at 6 to 12 weeks postpartum. Limit any diagnostic excisional procedures to cases where you cannot rule out invasive cancer.4,5
Excision or ablation of the transformation zone are equally effective for treating an initial diagnosis of cervical intraepithelial neoplasia (CIN) grades 2 or 3 in women with a satisfactory colposcopy and no suggestion of microinvasive or invasive disease (strength of recommendation [SOR]: A, based on randomized controlled trials [RCTs]).
Laser or loop electrosurgical excision procedure (LEEP) are the preferred treatment methods for recurrent CIN 2 and CIN 3 (SOR: B, based on clinical trials without randomization).
For women with an unsatisfactory colposcopy or suspicion of invasive disease, a diagnostic excisional procedure is recommended (SOR: C, based on consensus guidelines).
Observation or deferred treatment may be acceptable for CIN 2 in adolescents with satisfactory colposcopy and negative endocervical sampling (SOR: C, based on consensus guidelines).
Limit diagnostic excisional procedures in pregnancy to cases where suspicion of invasive cancer is high (SOR: C, based on consensus guidelines).
LEEP provides tissue for examination and a short recovery time
Timothy Huber, MD
Oroville, Calif
Close follow-up of observable disease and aggressive intervention continue to drive down the number of cervical cancer deaths each year.
It remains to be seen what the true effect of the HPV vaccine will be, although the presumed result will be a dramatic decline in high-grade lesions (CIN 2 and 3), carcinoma in situ, and invasive disease.
When intervention is necessary, my preferred method is LEEP because it provides tissue for examination and the recovery time is short.
Cryotherapy is an acceptable alternative, but the 4 to 8 weeks of leukorrhea and the lack of a tissue diagnosis often make it a less desirable option for patient and physician.
Evidence summary
Morbidity profile makes LEEP appear best
Similar efficacy. All 7 available surgical techniques were found to have similar efficacy, in a 2005 Cochrane review of 28 randomized trials.1 Resolution of CIN 2 or 3 lesions was 77% to 98%, using knife cone biopsy, laser conization, loop excision, laser ablation, cryotherapy, or 2 techniques not used commonly in the US, cold coagulation and radical diathermy. Surgical techniques were tested in various combinations, but no trial compared all of the techniques with one another. Most studies were underpowered, limiting the results.
Pap test: Good, but underused We’ve made great progress. What was the 2nd leading cancer in US women in incidence and mortality is now 11th in incidence and 13th in mortality. Yet, even with perfect attendance at annual screenings, women still get cervical cancer. And many still do not have screenings—they account for about half of all cervical cancers. The Pap, as good as it is, has flaws. The test is subjective, and sensitivity varies from lab to lab. 9
Dr. J. Thomas cox, university of California. Member, American Cancer society Cervical guidelines Committee, the 2002 Bethesda Workshop; ACS HPV vaccine Advisory Committee; author, ASCCP guidelines Committee
Each year in the united states approximately 500,000 women are diagnosed with high-grade cervical cancer precursor lesions, CIN 2 and CIN III.4 If left untreated, 22% of CIN 2 lesions progress to carcinoma in situ or invasive cervical cancer, 43% regress, and 35% persist at the same level. Fourteen percent of untreated CIN 3 lesions progress, 32% regress, and 56% persist at the same level.
HPV vaccine: Won’t replace prevention or protection Although an effective vaccine is a major advance in the prevention of genital HPV and cervical cancer, it will not replace other prevention strategies, such as cervical cancer screening for women or protective sexual behaviors. Women should continue to get Pap tests as a safeguard against cervical cancer.
Dr. Anne Schuchat, Director, CDC National Center for Immunization and respiratory Diseases, June 29, 2006 press release (www.cdc.gov/od/oc/media/pressrel/r060629.htm)
The HPV vaccine (Gardasil) that prevents the development of lesions caused by HPV types 6, 11, 16, and 18, was approved by the us Food and Drug Administration in June 2006 for use in females 9 to 26 years oof age. Shortly after, the Advisory Committee on Immunization Practices issued guidelines, stating that vaccination is recommended for all women <26 years of age. (www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm).
HPV testing: Adjunct to cytology The United States is falling behind other countries in assessing how best to utilize HPV testing for screening. Ongoing trials in The Netherlands, Italy, United Kingdom, Canada, and Finland are evaluating whether cytology can be replaced by HPV DNA testing for screening. Currently, HPV testing is only approved as an adjunct to cytology for cervical cancer screening in the United States, and no similar trials are underway. 10
Dr. Thomas C. Wright, Columbia university. Author, 2001 Consensus guidelines on Managing Women with Cytological and Histological Abnormalities, the 2004 Interim guidance for use of HPV DNA testing for Primary screening, and the 2001 Bethesda system
Least morbidity with LEEP. Morbidity was compared with one-on-one trials of different techniques ( TABLE 1 ). The review noted that LEEP has the least morbidity (such as hemorrhage, infection, cervical stenosis, and midtrimester pregnancy loss) while providing the most reliable histology by excising tissue without causing thermal artifact ( FIGURE ).
Higher rate of hemorrhage with cone biopsy. Another systematic review of 21 controlled trials comparing treatments for CIN 2 or 3 found a similar efficacy of all the modalities, including cone biopsy, cryotherapy, laser ablation, and LEEP. However, it also found a trend toward a higher rate of significant hemorrhage among women who received cone biopsies compared with women who received either laser ablation or LEEP.2
FIGURE
CIN 2 and 3 and its treatment by LEEP
TABLE 1
CIN 2 and 3 treatment options: An outcomes comparison
| COMPARISON OF TREATMENTS | OUTCOME/MORBIDITY | ODDS RATIO (95% CI) |
|---|---|---|
| Laser ablation vs cryotherapy | Laser ablation had more perioperative severe bleeding | 7.45 (1.68–33) |
| Laser ablation had higher rates of future adequate colposcopy | 4.64 (2.98–7.27) | |
| Laser conization vs knife conization | Laser conization had higher rates of future adequate colposcopy | 2.73 (1.47–5.08) |
| Laser conization had less cervical stenosis | 0.39 (0.25–0.61) | |
| Laser conization vs LEEP | Laser conization had more severe pain during procedure | 5.36 (1.02–17.2) |
| LEEP had fewer inadequate future colposcopies | 0.27 (0.08–0.89) | |
| Source: Martin-Hirsch et al, Cochrane Database Syst Rev 2000.1 | ||
Surgical treatment raises obstetric risks
There is a concern regarding future obstetric outcomes for women who have undergone surgical treatment of a high-grade cervical lesion. A recent meta-analysis of 27 controlled cohort studies found that cold knife conization and LEEP were associated with increased obstetrical risks, such as delivery at less than 37 weeks’ gestation and a birth weight <2500 g ( TABLE 2 ). Any resection that was more than 10 mm deep increased the risk of prematurity with future pregnancies (pooled relative risk=2.6; 95%] CI, 1.3–5.3).3
TABLE 2
Obstetrical outcomes for CIN 2 and 3 treatment options
| TREATMENT TYPE | OBSTETRICAL OUTCOME | RELATIVE RISK (95% CI) |
|---|---|---|
| Cold knife conization | Preterm delivery | 2.59 (1.80–3.72) |
| Low birth weight | 2.53 (1.19–5.36) | |
| Cesarean delivery | 3.17 (1.07–9.40) | |
| Laser conization | Preterm delivery | 1.71 (0.93–3.14) |
| LEEP | Preterm delivery | 1.70 (1.24–2.35) |
| Low birth weight | 1.82 (1.09–3.06) | |
| Preterm premature rupture of membranes | 2.69 (1.62–4.46) | |
| Source: Kyrgiou et al, Lancet 2006.3 | ||
Recommendations from others
Consensus guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) and a practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) both recommend immediate removal of the entire transformation zone, with either ablative or excisional treatment as initial treatment of CIN 2 and 3 for patients who are not pregnant.4,5
Value of excisional treatment. The ASCCP guidelines note that there is a benefit to excisional treatment, as it allows pathologic assessment of the excised tissue. Some of the ASCCP guideline authors recommend excisional procedures for the management of large CIN 2 and 3 lesions, which are at increased risk of having microinvasive disease.4
For women with unsatisfactory colposcopy and biopsy-proven CIN 2 or 3, there is up to a 7% risk for an occult invasive cervical carcinoma.1,4 Performing a diagnostic excisional procedure is recommended on these patients.4,6
ASCCP and ACOG make special recommendations for both adolescents and pregnant women.
For adolescent patients with biopsy-proven CIN 2, a recent ACOG Committee Opinion recommends close follow-up—with Pap smears or colposcopies every 4 to 6 months—due to the high rates of resolution of CIN 2 in adolescents.7
For pregnant patients, diagnostic excisional procedures are associated with complications such as bleeding and preterm delivery, while there is minimal risk of CIN 2 or 3 progressing to invasive cervical cancer.4,8 In pregnancy, follow CIN 2 and 3 with colposcopy each trimester, and reevaluate at 6 to 12 weeks postpartum. Limit any diagnostic excisional procedures to cases where you cannot rule out invasive cancer.4,5
1. Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;(2)CD001318.-
2. Nuovo J, Melnikow J, Willan AR, Chan BKS. Treatment outcomes for squamous intraepithelial lesions. Int J gynaecol obstet 2000;68:25-33.
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-498.
4. Wright TC, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
5. American College of obstetricians and gynecologists. ACOG Practice Bulletin number 66, september 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol 2005;106:645-664.
6. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol 1999;180:276-282.
7. American College of obstetricians and gynecologists. ACOG Committee opinion. Evaluation and management of abnormal cervical cytology and histology in the adolescent. Number 330, April 2006. Obstet Gynecol 2006;107:963-968.
8. Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 1993;81:915-918.
9. Cox JT. We’re on the way to ending cervical cancer. OBG Management 2006;18(3):62-72.
10. Wright TC. Cervical disease update. OBG Management 2007;19(3):52-60.
1. Martin-Hirsch PL, Paraskevaidis E, Kitchener H. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2000;(2)CD001318.-
2. Nuovo J, Melnikow J, Willan AR, Chan BKS. Treatment outcomes for squamous intraepithelial lesions. Int J gynaecol obstet 2000;68:25-33.
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489-498.
4. Wright TC, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 ASCCP-sponsored Consensus Workshop. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol 2003;189:295-304.
5. American College of obstetricians and gynecologists. ACOG Practice Bulletin number 66, september 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol 2005;106:645-664.
6. Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol 1999;180:276-282.
7. American College of obstetricians and gynecologists. ACOG Committee opinion. Evaluation and management of abnormal cervical cytology and histology in the adolescent. Number 330, April 2006. Obstet Gynecol 2006;107:963-968.
8. Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 1993;81:915-918.
9. Cox JT. We’re on the way to ending cervical cancer. OBG Management 2006;18(3):62-72.
10. Wright TC. Cervical disease update. OBG Management 2007;19(3):52-60.
Evidence-based answers from the Family Physicians Inquiries Network
Alcoholic liver disease: Is acetaminophen safe?
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
Yes—acetaminophen is a safe and effective analgesic that can be appropriately used for adult patients with stable chronic alcoholic liver disease for at least a short period of time (studies have been limited to a maximum of 48–72 hours), up to the maximum recommended dosage of 4 g daily (strength of recommendation: A, based on 2 RCTs and other studies). There are little data to guide longer-term use of acetaminophen in this situation.
“Lesser of all evils”
Joseph J. Saseen, PharmD, FCCP, BCPS
University of Colorado at Denver and Health Sciences Center, Denver
Selecting an appropriate analgesic for patients with chronic alcoholic liver disease is complicated. Narcotics are potentially addictive, and nonsteroidal anti-inflammatory drugs (NSAIDs) can cause gastrointestinal bleeding and other adverse events. Alcoholic liver disease predisposes patients to these potential drug-related complications, so these options are not ideal.
Acetaminophen is the “lesser of all evils” in this population, based on some data suggesting it is safe when used within approved dosing parameters. However, these parameters vary significantly.
Although a maximum daily dose of 4 g is widely accepted as normal, the American Geriatric Society recommends no more than 2 to 3 g daily for older patients with hepatic insufficiency or a history of alcohol abuse.
Moreover, the American Liver Foundation issued a warning to not exceed 3 g daily for any prolonged period of time in response to a 2006 clinical trial that demonstrated aminotransferase increases in healthy volunteers treated with 4 g of acetaminophen daily for 14 days.1
Regardless of the exact maximum dose, none are greater than 4 g daily.
Always judiciously monitor dosing of acetaminophen because patients continue to experience unintentional overdose and hepatic failure caused by inadvertent use of multiple acetaminophen-containing products.2
Evidence summary
Acetaminophen, while widely used, is hepatotoxic in supra-therapeutic doses.3 Many studies purporting to show evidence of hepatic damage from therapeutic doses of acetaminophen have also been reported. Particularly in the 1970s and 1980s, there were a number of case reports and small literature reviews indicating that hepatic injury among regular users of alcohol (particularly chronic alcoholics) who take acetaminophen with therapeutic intent could be a “therapeutic misadventure.”4
Recent studies suggest short-term safety
- A systematic review (published in 2000) identified reports of acetaminophen toxicity, poisoning, or adverse events for alcohol patients.5
- In a randomized, double-blinded, placebo-controlled study, 102 alcoholic patients were given 4 g of acetaminophen daily for 2 days.6
Recommendations from others
American College of Gastroenterology. The American College of Gastroenterology states that it’s generally safe to take acetaminophen in the amount specified in the package labeling. Furthermore, they recommend that patients diagnosed with liver conditions consult their physician for advice on dosing for acetaminophen or any other pain reliever.7
In Liver and Biliary Disease, the author concludes that chronic alcoholics are at increased risk for hepatotoxicity secondary to acetaminophen even at therapeutic doses; therefore, advise them to take no more than 2 g daily.8
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
1. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. A randomized controlled trial. JAMA 2006;296:87-93.
2. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.
3. Barker JD, Jr, de Carle DJ, Anuras S. Chronic excessive acetaminophen use and liver damage. Ann Intern Med 1977;87:299-301.
4. Zimmerman HJ, Maddery WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-773.
5. Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Therapeutics 2000;7:123-134.
6. Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Inter Med 2001;161:2247-2252.
7. Herrera JL, O’Brien BL. Important information for patients with chronic liver disease and/or cirrhosis. In McNally PR, DeVault KR, Surawicz CM, eds. Common GI Problems. vol 3. Available at: www.acg.gi.org/patients/cgp/cgpvol3.asp#liver. Accessed on June 26, 2007.
8. Kaplowitz N. Liver and Biliary Diseases. Baltimore, Md: Williams and Wilkins; 1996.
Evidence-based answers from the Family Physicians Inquiries Network
How should you treat Candida vaginitis in patients on antibiotics?
Oral and intravaginal antifungals for the treatment of uncomplicated vulvovaginal candidiasis (VVC) have similar effectiveness (strength of recommendation [SOR]: A, systematic review). However, no randomized controlled trials (RCTs) have addressed treatment options for patients taking antibiotics. Oral antifungals are contraindicated in pregnancy. While shorter courses of intravaginal therapy can be used by nonpregnant women, 7-day treatment may be necessary during pregnancy (SOR: A, systematic review). Products containing Lactobacillus species do not prevent postantibiotic vulvovaginitis (SOR: A, systematic review and RCT).
Most women would rather prevent than treat
Laura Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University
Many women complain about getting yeast infections after receiving antibiotics. Usually the patient will inform me of this while I’m writing the prescription for the antibiotic, asking for Diflucan “just in case.” Women prefer the convenience of the oral medicine over the hassle with topical applications. Some state that 1 dose of Diflucan does not cut it, and that they usually need 2. As a result, I find myself writing a prescription for Diflucan to be started along with the antibiotic, and then to be repeated as a second dose in 3 days. I have not heard any complaints from these patients about postantibiotic yeast infections.
Evidence summary
VVC is a common cause of vaginitis; Candida albicans accounts for 85% to 90% of cases. Risk factors include pregnancy, diabetes mellitus, and systemic antibiotics.1 Incidence increases with onset of sexual activity, but there is no direct evidence it is sexually transmitted.1 About 75% of women experience 1 VVC episode during their lifetime, 40% to 45% have 2 or more, and 5% to 8% have recurrent VVC (defined as 4 or more annually).1,2
The candidiasis/antibiotics link
A 2003 systematic review found the evidence supporting the association between antibiotics and VVC limited and contradictory.3 Most were case-control or cohort studies with small sample sizes. No RCTs compare the incidence of culture-confirmed VVC among women receiving antibiotics or placebo.
Nineteen reports of 18 original studies had sufficient data to calculate a relative risk or odds ratio for antibiotic-associated VVC. Thirteen of the 19 reports showed an increase (around twofold; range, 0.43–5) in vaginal Candida prevalence; however, 3 of the 13 reports had no mycological culture data. Six studies did not show significant association between antibiotics and vaginal yeast.3
Antibiotics are thought to increase risk of VVC by killing endogenous vaginal flora (particularly Lactobacillus), allowing microorganisms resistant to the antibiotics, like Candida, to flourish.1 Yet there is evidence that numbers of genital Lactobacillus are similar for women with and without symptomatic VVC.4 Further, decreasing Lactobacillus does not increase the risk of VVC.5
Topical and oral antifungals—both do the job
For the treatment of uncomplicated VVC, both topical and oral antifungals are clinically and mycologically effective, with comparable clinical cure rates >80%.6 No difference in persistent symptoms between single and multiple doses, or different durations of multiple dose regimens have been found, but samples may have been too small to detect clinically significant effects. An RCT found less nausea, headache, and abdominal pain with intravaginal imidazoles, but more vulvar irritation and vaginal discharge than oral fluconazole.6
For treatment of recurrent VVC, RCTs have shown the effectiveness of oral fluconazole and itraconazole maintenance therapy taken for 6 months after an initial regimen.7,8 Treating male sexual partners did not significantly improve resolution of the woman’s symptoms or reduce the rate of symptomatic relapse.9
Yogurt may not live up to its rep
Two poor-quality crossover RCTs provided insufficient evidence regarding effectiveness of a diet containing oral Lactobacillus yogurt to prevent recurrent VVC.9 A recent RCT of 278 women on short courses of antibiotics were randomized to oral lactobacilli or placebo and vaginal lactobacilli or placebo.10 The study was stopped early because there was no effect seen. Overall, 23% developed symptomatic vulvovaginitis.
Recommendations from others
The Infectious Diseases Society of America11 recommends treating uncomplicated VVC with short-course of oral or topical antifungals; treating complicated VVC with antimycotic therapy for 7 days, either daily as topical therapy or as two 150-mg doses of fluconazole 72 hours apart; treating non-albicans species of Candida with topical boric acid (600 mg/day for 14 days) or topical flucytosine; and treating recurrent VVC with induction therapy with 2 weeks of a topical or oral azole followed by a maintenance regimen for 6 months (fluconazole once a week or itraconazole twice a week).11
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
2. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
3. Xu J, Sobel JD. Antibiotic-associated vulvovaginal candidiasis. Curr Infect Dis Rep 2003;5:481-487.
4. Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol 1996;34:2497-2499.
5. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
6. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole antifungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2001;(1):CD002845.-
7. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004;351:876-883.
8. Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med 1997;42:83-87.
9. Spence D. Candidiasis (vulvovaginal). Clin Evid 2006;15:1-8.
10. Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing postantibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 2004;329:548.-
11. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
Oral and intravaginal antifungals for the treatment of uncomplicated vulvovaginal candidiasis (VVC) have similar effectiveness (strength of recommendation [SOR]: A, systematic review). However, no randomized controlled trials (RCTs) have addressed treatment options for patients taking antibiotics. Oral antifungals are contraindicated in pregnancy. While shorter courses of intravaginal therapy can be used by nonpregnant women, 7-day treatment may be necessary during pregnancy (SOR: A, systematic review). Products containing Lactobacillus species do not prevent postantibiotic vulvovaginitis (SOR: A, systematic review and RCT).
Most women would rather prevent than treat
Laura Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University
Many women complain about getting yeast infections after receiving antibiotics. Usually the patient will inform me of this while I’m writing the prescription for the antibiotic, asking for Diflucan “just in case.” Women prefer the convenience of the oral medicine over the hassle with topical applications. Some state that 1 dose of Diflucan does not cut it, and that they usually need 2. As a result, I find myself writing a prescription for Diflucan to be started along with the antibiotic, and then to be repeated as a second dose in 3 days. I have not heard any complaints from these patients about postantibiotic yeast infections.
Evidence summary
VVC is a common cause of vaginitis; Candida albicans accounts for 85% to 90% of cases. Risk factors include pregnancy, diabetes mellitus, and systemic antibiotics.1 Incidence increases with onset of sexual activity, but there is no direct evidence it is sexually transmitted.1 About 75% of women experience 1 VVC episode during their lifetime, 40% to 45% have 2 or more, and 5% to 8% have recurrent VVC (defined as 4 or more annually).1,2
The candidiasis/antibiotics link
A 2003 systematic review found the evidence supporting the association between antibiotics and VVC limited and contradictory.3 Most were case-control or cohort studies with small sample sizes. No RCTs compare the incidence of culture-confirmed VVC among women receiving antibiotics or placebo.
Nineteen reports of 18 original studies had sufficient data to calculate a relative risk or odds ratio for antibiotic-associated VVC. Thirteen of the 19 reports showed an increase (around twofold; range, 0.43–5) in vaginal Candida prevalence; however, 3 of the 13 reports had no mycological culture data. Six studies did not show significant association between antibiotics and vaginal yeast.3
Antibiotics are thought to increase risk of VVC by killing endogenous vaginal flora (particularly Lactobacillus), allowing microorganisms resistant to the antibiotics, like Candida, to flourish.1 Yet there is evidence that numbers of genital Lactobacillus are similar for women with and without symptomatic VVC.4 Further, decreasing Lactobacillus does not increase the risk of VVC.5
Topical and oral antifungals—both do the job
For the treatment of uncomplicated VVC, both topical and oral antifungals are clinically and mycologically effective, with comparable clinical cure rates >80%.6 No difference in persistent symptoms between single and multiple doses, or different durations of multiple dose regimens have been found, but samples may have been too small to detect clinically significant effects. An RCT found less nausea, headache, and abdominal pain with intravaginal imidazoles, but more vulvar irritation and vaginal discharge than oral fluconazole.6
For treatment of recurrent VVC, RCTs have shown the effectiveness of oral fluconazole and itraconazole maintenance therapy taken for 6 months after an initial regimen.7,8 Treating male sexual partners did not significantly improve resolution of the woman’s symptoms or reduce the rate of symptomatic relapse.9
Yogurt may not live up to its rep
Two poor-quality crossover RCTs provided insufficient evidence regarding effectiveness of a diet containing oral Lactobacillus yogurt to prevent recurrent VVC.9 A recent RCT of 278 women on short courses of antibiotics were randomized to oral lactobacilli or placebo and vaginal lactobacilli or placebo.10 The study was stopped early because there was no effect seen. Overall, 23% developed symptomatic vulvovaginitis.
Recommendations from others
The Infectious Diseases Society of America11 recommends treating uncomplicated VVC with short-course of oral or topical antifungals; treating complicated VVC with antimycotic therapy for 7 days, either daily as topical therapy or as two 150-mg doses of fluconazole 72 hours apart; treating non-albicans species of Candida with topical boric acid (600 mg/day for 14 days) or topical flucytosine; and treating recurrent VVC with induction therapy with 2 weeks of a topical or oral azole followed by a maintenance regimen for 6 months (fluconazole once a week or itraconazole twice a week).11
Oral and intravaginal antifungals for the treatment of uncomplicated vulvovaginal candidiasis (VVC) have similar effectiveness (strength of recommendation [SOR]: A, systematic review). However, no randomized controlled trials (RCTs) have addressed treatment options for patients taking antibiotics. Oral antifungals are contraindicated in pregnancy. While shorter courses of intravaginal therapy can be used by nonpregnant women, 7-day treatment may be necessary during pregnancy (SOR: A, systematic review). Products containing Lactobacillus species do not prevent postantibiotic vulvovaginitis (SOR: A, systematic review and RCT).
Most women would rather prevent than treat
Laura Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University
Many women complain about getting yeast infections after receiving antibiotics. Usually the patient will inform me of this while I’m writing the prescription for the antibiotic, asking for Diflucan “just in case.” Women prefer the convenience of the oral medicine over the hassle with topical applications. Some state that 1 dose of Diflucan does not cut it, and that they usually need 2. As a result, I find myself writing a prescription for Diflucan to be started along with the antibiotic, and then to be repeated as a second dose in 3 days. I have not heard any complaints from these patients about postantibiotic yeast infections.
Evidence summary
VVC is a common cause of vaginitis; Candida albicans accounts for 85% to 90% of cases. Risk factors include pregnancy, diabetes mellitus, and systemic antibiotics.1 Incidence increases with onset of sexual activity, but there is no direct evidence it is sexually transmitted.1 About 75% of women experience 1 VVC episode during their lifetime, 40% to 45% have 2 or more, and 5% to 8% have recurrent VVC (defined as 4 or more annually).1,2
The candidiasis/antibiotics link
A 2003 systematic review found the evidence supporting the association between antibiotics and VVC limited and contradictory.3 Most were case-control or cohort studies with small sample sizes. No RCTs compare the incidence of culture-confirmed VVC among women receiving antibiotics or placebo.
Nineteen reports of 18 original studies had sufficient data to calculate a relative risk or odds ratio for antibiotic-associated VVC. Thirteen of the 19 reports showed an increase (around twofold; range, 0.43–5) in vaginal Candida prevalence; however, 3 of the 13 reports had no mycological culture data. Six studies did not show significant association between antibiotics and vaginal yeast.3
Antibiotics are thought to increase risk of VVC by killing endogenous vaginal flora (particularly Lactobacillus), allowing microorganisms resistant to the antibiotics, like Candida, to flourish.1 Yet there is evidence that numbers of genital Lactobacillus are similar for women with and without symptomatic VVC.4 Further, decreasing Lactobacillus does not increase the risk of VVC.5
Topical and oral antifungals—both do the job
For the treatment of uncomplicated VVC, both topical and oral antifungals are clinically and mycologically effective, with comparable clinical cure rates >80%.6 No difference in persistent symptoms between single and multiple doses, or different durations of multiple dose regimens have been found, but samples may have been too small to detect clinically significant effects. An RCT found less nausea, headache, and abdominal pain with intravaginal imidazoles, but more vulvar irritation and vaginal discharge than oral fluconazole.6
For treatment of recurrent VVC, RCTs have shown the effectiveness of oral fluconazole and itraconazole maintenance therapy taken for 6 months after an initial regimen.7,8 Treating male sexual partners did not significantly improve resolution of the woman’s symptoms or reduce the rate of symptomatic relapse.9
Yogurt may not live up to its rep
Two poor-quality crossover RCTs provided insufficient evidence regarding effectiveness of a diet containing oral Lactobacillus yogurt to prevent recurrent VVC.9 A recent RCT of 278 women on short courses of antibiotics were randomized to oral lactobacilli or placebo and vaginal lactobacilli or placebo.10 The study was stopped early because there was no effect seen. Overall, 23% developed symptomatic vulvovaginitis.
Recommendations from others
The Infectious Diseases Society of America11 recommends treating uncomplicated VVC with short-course of oral or topical antifungals; treating complicated VVC with antimycotic therapy for 7 days, either daily as topical therapy or as two 150-mg doses of fluconazole 72 hours apart; treating non-albicans species of Candida with topical boric acid (600 mg/day for 14 days) or topical flucytosine; and treating recurrent VVC with induction therapy with 2 weeks of a topical or oral azole followed by a maintenance regimen for 6 months (fluconazole once a week or itraconazole twice a week).11
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
2. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
3. Xu J, Sobel JD. Antibiotic-associated vulvovaginal candidiasis. Curr Infect Dis Rep 2003;5:481-487.
4. Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol 1996;34:2497-2499.
5. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
6. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole antifungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2001;(1):CD002845.-
7. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004;351:876-883.
8. Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med 1997;42:83-87.
9. Spence D. Candidiasis (vulvovaginal). Clin Evid 2006;15:1-8.
10. Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing postantibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 2004;329:548.-
11. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
2. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
3. Xu J, Sobel JD. Antibiotic-associated vulvovaginal candidiasis. Curr Infect Dis Rep 2003;5:481-487.
4. Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol 1996;34:2497-2499.
5. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
6. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole antifungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2001;(1):CD002845.-
7. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004;351:876-883.
8. Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med 1997;42:83-87.
9. Spence D. Candidiasis (vulvovaginal). Clin Evid 2006;15:1-8.
10. Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing postantibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 2004;329:548.-
11. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
Evidence-based answers from the Family Physicians Inquiries Network
Is guaifenesin safe during pregnancy?
It’s not clear; little evidence supports or refutes the safety of guaifenesin, a common expectorant, in pregnancy. A small number of observational and case-control studies suggest a weak association between guaifenesin use and inguinal hernias and neural tube defects in newborns. However, substantial methodological flaws, the absence of statistical significance, and low rates of prevalence cast a shadow of a doubt over the data (strength of recommendation [SOR]: B, based on observational and case-control studies).
Always take a conservative approach to obstetrics
Christopher P. Paulson, MD, FAAFP
Eglin Family Medicine residency, Eglin Air Force Base, Fla
In my practice as a family physician, a conservative approach to obstetrical care has been the rule. It seems prudent to avoid guaifenesin during the first trimester. Although the evidence is inconclusive on potential harm to the fetus, the marginal benefit gained seems insufficient to justify treatment early in pregnancy when the fetus is most vulnerable to teratogenic effects in general.
As with most other Category C medications (except those with clear clinical benefit and no safer alternative), I typically avoid guaifenesin throughout pregnancy. However, it may be reasonable to prescribe it in the latter half of pregnancy when the potential risk is lower; particularly in light of the lack of any clear evidence demonstrating harmful effects.
Evidence summary
Guaifenesin, available in numerous preparations (eg, Mucinex, Robitussin), is one of the most commonly used over-the-counter medications in pregnancy. The National Birth Defects Prevention Study surveyed 2970 pregnant women from 1997 to 2001; 6.2% reported taking guaifenesin during pregnancy.1 A second survey evaluated 7563 mothers from 1998 to 2004; 9.2% of the mothers reported that they took it during pregnancy.1
A weak link to inguinal hernias
Guaifenesin use in pregnancy has been associated with inguinal hernias in newborns. From 1958 to 1965, the Collaborative Perinatal Project recruited 132,500 women to participate in a multicenter study; however, selection and exclusion criteria were not consistent. From this initial group, only 50,282 mother-child pairs were studied. Trained examiners interviewed the women at the child’s 4, 8, 12, and 24-month visits, and then annually thereafter to 8 years of age.
The examiners identified 7 children with inguinal hernias among 197 mothers who had used guaifenesin during their first trimester (standardized relative risk [RR] of 2.6; no CI or P value reported). Twenty children had inguinal hernias among the 1337 mothers who had used guaifenesin during any trimester of their pregnancy (RR=1.1; no confidence interval [CI] or P value reported).2 The authors acknowledged that reporting bias among the participating centers prevented them from drawing any conclusions from the data.
A possible trend toward neural tube defects?
Guaifenesin use in pregnancy may also be associated with neural tube defects. In a case-control study, researchers identified 538 fetuses and live-born infants with neural tube defects between 1989 and 1991.3 Twelve patients with neural tube defects were exposed to guaifenesin during gestation; 6 in the control group reported exposure.
The authors reported a trend towards increased risk of neural tube defects in offspring of guaifenesin-exposed mothers (odds ratio=2.04; 95% CI, 0.79–5.28).3 However, since the results were not statistically significant, the authors concluded that guaifenesin had not contributed to the occurrence of neural tube defects.
In a study evaluating 6509 women whose pregnancies resulted in live births, 241 women reported first-trimester exposure to guaifenesin.4 Five of the guaifenesin-exposed infants (2.1%) had 1 of the birth defects studied (types of disorders not reported). The calculated RR of birth defect after in utero guaifenesin exposure was 1.3 (no CIs or P values reported); the authors concluded that there was no strong association between guaifenesin and the malformations studied.4
Interpret results with caution
Because of the significant potential for recall bias, interpret the findings of these case-control studies with caution.5 The mother of a child with a birth defect may search her memory more aggressively for potential causes than a mother of a healthy infant, leading to different rates of recalled, rather than actual, exposures. In the absence of confirmatory prospective data, such as medication diaries or pharmacy databases, recall bias accounts for many spurious positive findings in case control studies.
Recommendations from others
Food and Drug Administration. The FDA assigned guaifenesin to category C—that is, its risk cannot be ruled out. Human studies are lacking, and animal studies are either positive for fetal risk or lacking. Guaifenesin is recommended if the benefit to the pregnant woman warrants the risk to the fetus.6
ACOG. The American College of Obstetricians and Gynecologists (ACOG) makes no recommendation.7 The textbook Drugs in Pregnancy and Lactation reports that guaifenesin use during pregnancy exhibits very low risk to the fetus.6 The National Collaborating Centre for Women’s and Children’s Health suggests that guaifenesin be used sparingly during pregnancy.8
1. Werler MM, Mitchel AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005;193:771-777.
2. Heinonen OP, Sione D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton, Mass: Publishing Sciences Group, 1977.
3. Shaw GM, Todoroff K, Velie EM, Lammer EJ. Maternal Illness, including fever, and medication use as risk factors for neural tube defects. Teratology 1998;57:1-7.
4. Aselton P, Jick H, Milunsky A, Hunter JR, Stergachis A. First-trimester drug use and congenital disorders. Obstet Gynecol 1985;65:451-455.
5. Chouinard E, Walter S. Title recall bias in case-control studies: an empirical analysis and theoretical framework. J Clin Epidemiology 1995;48:245-254.
6. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation 7th ed. Baltimore, Md: Lippincott Williams & Wilkins;2005.
7. The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
8. National Collaborating Centre for Women’s and Children’s Health. Antenatal Care: Routine Care for the Healthy Pregnant Woman London: RCOG Press; 2003.
It’s not clear; little evidence supports or refutes the safety of guaifenesin, a common expectorant, in pregnancy. A small number of observational and case-control studies suggest a weak association between guaifenesin use and inguinal hernias and neural tube defects in newborns. However, substantial methodological flaws, the absence of statistical significance, and low rates of prevalence cast a shadow of a doubt over the data (strength of recommendation [SOR]: B, based on observational and case-control studies).
Always take a conservative approach to obstetrics
Christopher P. Paulson, MD, FAAFP
Eglin Family Medicine residency, Eglin Air Force Base, Fla
In my practice as a family physician, a conservative approach to obstetrical care has been the rule. It seems prudent to avoid guaifenesin during the first trimester. Although the evidence is inconclusive on potential harm to the fetus, the marginal benefit gained seems insufficient to justify treatment early in pregnancy when the fetus is most vulnerable to teratogenic effects in general.
As with most other Category C medications (except those with clear clinical benefit and no safer alternative), I typically avoid guaifenesin throughout pregnancy. However, it may be reasonable to prescribe it in the latter half of pregnancy when the potential risk is lower; particularly in light of the lack of any clear evidence demonstrating harmful effects.
Evidence summary
Guaifenesin, available in numerous preparations (eg, Mucinex, Robitussin), is one of the most commonly used over-the-counter medications in pregnancy. The National Birth Defects Prevention Study surveyed 2970 pregnant women from 1997 to 2001; 6.2% reported taking guaifenesin during pregnancy.1 A second survey evaluated 7563 mothers from 1998 to 2004; 9.2% of the mothers reported that they took it during pregnancy.1
A weak link to inguinal hernias
Guaifenesin use in pregnancy has been associated with inguinal hernias in newborns. From 1958 to 1965, the Collaborative Perinatal Project recruited 132,500 women to participate in a multicenter study; however, selection and exclusion criteria were not consistent. From this initial group, only 50,282 mother-child pairs were studied. Trained examiners interviewed the women at the child’s 4, 8, 12, and 24-month visits, and then annually thereafter to 8 years of age.
The examiners identified 7 children with inguinal hernias among 197 mothers who had used guaifenesin during their first trimester (standardized relative risk [RR] of 2.6; no CI or P value reported). Twenty children had inguinal hernias among the 1337 mothers who had used guaifenesin during any trimester of their pregnancy (RR=1.1; no confidence interval [CI] or P value reported).2 The authors acknowledged that reporting bias among the participating centers prevented them from drawing any conclusions from the data.
A possible trend toward neural tube defects?
Guaifenesin use in pregnancy may also be associated with neural tube defects. In a case-control study, researchers identified 538 fetuses and live-born infants with neural tube defects between 1989 and 1991.3 Twelve patients with neural tube defects were exposed to guaifenesin during gestation; 6 in the control group reported exposure.
The authors reported a trend towards increased risk of neural tube defects in offspring of guaifenesin-exposed mothers (odds ratio=2.04; 95% CI, 0.79–5.28).3 However, since the results were not statistically significant, the authors concluded that guaifenesin had not contributed to the occurrence of neural tube defects.
In a study evaluating 6509 women whose pregnancies resulted in live births, 241 women reported first-trimester exposure to guaifenesin.4 Five of the guaifenesin-exposed infants (2.1%) had 1 of the birth defects studied (types of disorders not reported). The calculated RR of birth defect after in utero guaifenesin exposure was 1.3 (no CIs or P values reported); the authors concluded that there was no strong association between guaifenesin and the malformations studied.4
Interpret results with caution
Because of the significant potential for recall bias, interpret the findings of these case-control studies with caution.5 The mother of a child with a birth defect may search her memory more aggressively for potential causes than a mother of a healthy infant, leading to different rates of recalled, rather than actual, exposures. In the absence of confirmatory prospective data, such as medication diaries or pharmacy databases, recall bias accounts for many spurious positive findings in case control studies.
Recommendations from others
Food and Drug Administration. The FDA assigned guaifenesin to category C—that is, its risk cannot be ruled out. Human studies are lacking, and animal studies are either positive for fetal risk or lacking. Guaifenesin is recommended if the benefit to the pregnant woman warrants the risk to the fetus.6
ACOG. The American College of Obstetricians and Gynecologists (ACOG) makes no recommendation.7 The textbook Drugs in Pregnancy and Lactation reports that guaifenesin use during pregnancy exhibits very low risk to the fetus.6 The National Collaborating Centre for Women’s and Children’s Health suggests that guaifenesin be used sparingly during pregnancy.8
It’s not clear; little evidence supports or refutes the safety of guaifenesin, a common expectorant, in pregnancy. A small number of observational and case-control studies suggest a weak association between guaifenesin use and inguinal hernias and neural tube defects in newborns. However, substantial methodological flaws, the absence of statistical significance, and low rates of prevalence cast a shadow of a doubt over the data (strength of recommendation [SOR]: B, based on observational and case-control studies).
Always take a conservative approach to obstetrics
Christopher P. Paulson, MD, FAAFP
Eglin Family Medicine residency, Eglin Air Force Base, Fla
In my practice as a family physician, a conservative approach to obstetrical care has been the rule. It seems prudent to avoid guaifenesin during the first trimester. Although the evidence is inconclusive on potential harm to the fetus, the marginal benefit gained seems insufficient to justify treatment early in pregnancy when the fetus is most vulnerable to teratogenic effects in general.
As with most other Category C medications (except those with clear clinical benefit and no safer alternative), I typically avoid guaifenesin throughout pregnancy. However, it may be reasonable to prescribe it in the latter half of pregnancy when the potential risk is lower; particularly in light of the lack of any clear evidence demonstrating harmful effects.
Evidence summary
Guaifenesin, available in numerous preparations (eg, Mucinex, Robitussin), is one of the most commonly used over-the-counter medications in pregnancy. The National Birth Defects Prevention Study surveyed 2970 pregnant women from 1997 to 2001; 6.2% reported taking guaifenesin during pregnancy.1 A second survey evaluated 7563 mothers from 1998 to 2004; 9.2% of the mothers reported that they took it during pregnancy.1
A weak link to inguinal hernias
Guaifenesin use in pregnancy has been associated with inguinal hernias in newborns. From 1958 to 1965, the Collaborative Perinatal Project recruited 132,500 women to participate in a multicenter study; however, selection and exclusion criteria were not consistent. From this initial group, only 50,282 mother-child pairs were studied. Trained examiners interviewed the women at the child’s 4, 8, 12, and 24-month visits, and then annually thereafter to 8 years of age.
The examiners identified 7 children with inguinal hernias among 197 mothers who had used guaifenesin during their first trimester (standardized relative risk [RR] of 2.6; no CI or P value reported). Twenty children had inguinal hernias among the 1337 mothers who had used guaifenesin during any trimester of their pregnancy (RR=1.1; no confidence interval [CI] or P value reported).2 The authors acknowledged that reporting bias among the participating centers prevented them from drawing any conclusions from the data.
A possible trend toward neural tube defects?
Guaifenesin use in pregnancy may also be associated with neural tube defects. In a case-control study, researchers identified 538 fetuses and live-born infants with neural tube defects between 1989 and 1991.3 Twelve patients with neural tube defects were exposed to guaifenesin during gestation; 6 in the control group reported exposure.
The authors reported a trend towards increased risk of neural tube defects in offspring of guaifenesin-exposed mothers (odds ratio=2.04; 95% CI, 0.79–5.28).3 However, since the results were not statistically significant, the authors concluded that guaifenesin had not contributed to the occurrence of neural tube defects.
In a study evaluating 6509 women whose pregnancies resulted in live births, 241 women reported first-trimester exposure to guaifenesin.4 Five of the guaifenesin-exposed infants (2.1%) had 1 of the birth defects studied (types of disorders not reported). The calculated RR of birth defect after in utero guaifenesin exposure was 1.3 (no CIs or P values reported); the authors concluded that there was no strong association between guaifenesin and the malformations studied.4
Interpret results with caution
Because of the significant potential for recall bias, interpret the findings of these case-control studies with caution.5 The mother of a child with a birth defect may search her memory more aggressively for potential causes than a mother of a healthy infant, leading to different rates of recalled, rather than actual, exposures. In the absence of confirmatory prospective data, such as medication diaries or pharmacy databases, recall bias accounts for many spurious positive findings in case control studies.
Recommendations from others
Food and Drug Administration. The FDA assigned guaifenesin to category C—that is, its risk cannot be ruled out. Human studies are lacking, and animal studies are either positive for fetal risk or lacking. Guaifenesin is recommended if the benefit to the pregnant woman warrants the risk to the fetus.6
ACOG. The American College of Obstetricians and Gynecologists (ACOG) makes no recommendation.7 The textbook Drugs in Pregnancy and Lactation reports that guaifenesin use during pregnancy exhibits very low risk to the fetus.6 The National Collaborating Centre for Women’s and Children’s Health suggests that guaifenesin be used sparingly during pregnancy.8
1. Werler MM, Mitchel AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005;193:771-777.
2. Heinonen OP, Sione D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton, Mass: Publishing Sciences Group, 1977.
3. Shaw GM, Todoroff K, Velie EM, Lammer EJ. Maternal Illness, including fever, and medication use as risk factors for neural tube defects. Teratology 1998;57:1-7.
4. Aselton P, Jick H, Milunsky A, Hunter JR, Stergachis A. First-trimester drug use and congenital disorders. Obstet Gynecol 1985;65:451-455.
5. Chouinard E, Walter S. Title recall bias in case-control studies: an empirical analysis and theoretical framework. J Clin Epidemiology 1995;48:245-254.
6. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation 7th ed. Baltimore, Md: Lippincott Williams & Wilkins;2005.
7. The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
8. National Collaborating Centre for Women’s and Children’s Health. Antenatal Care: Routine Care for the Healthy Pregnant Woman London: RCOG Press; 2003.
1. Werler MM, Mitchel AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005;193:771-777.
2. Heinonen OP, Sione D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton, Mass: Publishing Sciences Group, 1977.
3. Shaw GM, Todoroff K, Velie EM, Lammer EJ. Maternal Illness, including fever, and medication use as risk factors for neural tube defects. Teratology 1998;57:1-7.
4. Aselton P, Jick H, Milunsky A, Hunter JR, Stergachis A. First-trimester drug use and congenital disorders. Obstet Gynecol 1985;65:451-455.
5. Chouinard E, Walter S. Title recall bias in case-control studies: an empirical analysis and theoretical framework. J Clin Epidemiology 1995;48:245-254.
6. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation 7th ed. Baltimore, Md: Lippincott Williams & Wilkins;2005.
7. The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
8. National Collaborating Centre for Women’s and Children’s Health. Antenatal Care: Routine Care for the Healthy Pregnant Woman London: RCOG Press; 2003.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best initial treatment for orbital cellulitis in children?
Although antibiotics are the best initial treatment, surgical intervention is warranted when a child has:
- visual impairment, complete ophthalmoplegia, or well-defined abscess on presentation, or
- no clearly apparent clinical improvement by 24 hours (strength of recommendation [SOR]: C, based on patient-oriented case-series studies).
Target antimicrobial therapy toward the common pathogens associated with predisposing factors for orbital cellulitis, such as sinusitis—and pay attention to local resistance patterns (SOR: C, based on patient-oriented case series).
Rare but serious risk factors
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
The incidence of Haemophilus influenzae–related periorbital cellulitis appears to have plummeted with the advent of Hib vaccine. And while no national data have been published, case series support my clinical observation that the overall incidence of periorbital cellulitis has dropped as well.
The arrival of heptavalent pneumococcal vaccine may further contribute to its welcome scarcity. take this changing bacteriology—in conjunction with local resistance patterns—into account when considering antibiotic coverage.
When confronted by the rare case of periorbital cellulitis, I always consider risk factors that may change my management, such as immunization status and asplenia. Also, meningitis is a rare but serious complication, so I also keep meningitis risk factors in mind, such as immunosuppression, coincident trauma, or a poor response to initial medical therapy.
Finally, any question of orbital involvement should prompt an emergent consultation.
Evidence summary
Orbital cellulitis is a serious soft-tissue infection of childhood with very different etiologies.
- Periorbital (or preseptal) cellulitis is synonymous with stage I orbital cellulitis, in which there is induration, erythema, warmth, and tenderness of the periorbital soft tissues, usually secondary to external inoculation, but the inflammation does not extend into the bony orbit.
- Stages II, III, and IV orbital cellulitis are progressively more invasive infections that generally arise from the sinuses; they may involve the retro-orbital area. These stages of orbital cellulitis can cause proptosis, decrease visual acuity, or appear as abscesses on computed tomography scan.1,2
Staged treatment
Many retrospective studies of stage II–IV orbital cellulitis with relatively few subjects and small prospective case series have been published with common themes for management recommendations:
- early intravenous antibiotics (likely for an inpatient), and
- involvement of otolaryngology and ophthalmology specialists.
No head-to-head trials have been completed to evaluate efficacy of specific antimicrobial regimens.
Oral antibiotics. First, treat stage I orbital cellulitis with oral antibiotics.
IV antibiotics. Modify treatment to intravenous antibiotics when there is no improvement within 24 hours or if you discover any characteristic of more severe orbital cellulitis.
Medical management of stage II–IV orbital cellulitis with intravenous antibiotics is the current standard of care until it is clear that one of the following is present:
- no improvement by 24 to 48 hours
- visual impairment
- complete ophthalmoplegia, or
- well-defined periosteal abscess.1,2
Surgery. For refractory cases, surgical decompression will likely be required.
The evidence. A small case series (n=9) found 21 children admitted to hospital for preseptal cellulitis, of whom 4 later were diagnosed with orbital cellulitis. There was a total of 9 cases of orbital cellulitis; however, only 1 required operative management of orbital cellulitis.3 In a prospective study to evaluate medical management (n=23), 87% of patients responded to intravenous antibiotics.4 No statistically significant long-term difference in subperiosteal abscesses (as a complication of orbital cellulitis) was found in another retrospective study comparing medical to surgical management.5
Target the likely pathogens
Direct antimicrobial therapy toward common pathogens for likely sources of infection, paying attention to local resistance patterns and the pathogens usually associated with sinusitis (TABLE).1,2,6-8
A retrospective case series of 94 patients of all ages in China implicated Staphylococcus aureus and streptococcal species based on cultures taken from eye purulence and abscesses.6 Another retrospective case series from Vanderbilt (n=80) found streptococci as the most common cause, based on blood and wound cultures in the Hib vaccination era; however, only 12 wounds returned positive cultures.7
TABLE
Choose antibiotic based on cause and likely pathogen1,2,6-8
| ANTECEDENT EVENT | LIKELY PATHOGENS | BEST DRUGS |
|---|---|---|
| Acute sinusitis | Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis | Penicillinase-resistant penicillins |
| Trauma | Staphylococcus aureus Group A β-hernolytic streptococci Increasing concern for methicillin-resistant S aureus | Penicillinase-resistant penicillins First-generation cephalosporins Consider drugs appropriate for methicillin-resistant S aureus |
| Chronic sinusitis | Anaerobes | Metronidazole Clindamycin |
Steroids have no proven benefit
Systemic steroids have no proven benefit in the treatment of pediatric orbital cellulitis with subperiosteal abscess.
A small retrospective cohort study of the benefit of intravenous steroids in addition to antibiotics showed no decrease in hospital stay or need for surgical decompression (n=23, P=.26 and .20, respectively).9 Without prospective data and a power analysis, lack of benefit of steroids cannot be definitively shown.
Recommendations from others
Infectious Disease Society of America. The guidelines for the management of skin and soft-tissue infections implicate β-hemolytic streptococci as the most common cellulitis pathogen, but also recommend empiric coverage against S aureus.
Periorbital and orbital cellulitis are not specifically addressed in these guidelines, but oral dicloxacillin, cephalexin, clindamycin, or erythromycin are recommended for superficial cellulitis, provided there is no known resistance to these antibiotics.
Intravenous penicillinase-resistant penicillins (nafcillin) or a first-generation cephalosporin (cefazolin) may be used for more severe infections.
For penicillin-allergic patients, the IDSA recommends clindamycin or vancomycin.10
Sanford Guide to Antimicrobial Therapy. Nafcillin plus ceftriaxone and metronidazole is the recommended treatment for orbital cellulitis.
For patients allergic to penicillin, vancomycin plus levofloxacin and metronidazole are recommended.8
Neither the American Academy of Ophthalmology nor the International Council of Ophthalmology offers clinical statements on orbital cellulitis.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical service or the US Air Force at large.
1. Nageswaran S, Woods CR, Benjamin DK, Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J 2006;25:695-699.
2. Vayalumkal JV, Jadavji T. Children hospitalized with skin and soft tissue infections: a guide to antibacterial selection and treatment. Paediatr Drugs 2006;8:99-111.
3. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J 2001;20:1002-1005.
4. Noel LP, Clarke WN, MacDonald N. Clinical management of orbital cellulitis in children. Can J Ophthalmol 1990;25:11-16.
5. Greenberg MF, Pollard ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS 1998;2:351-355.
6. Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: a 10-year review of hospitalized patients. J Chin Med Assoc 2006;69:415-422.
7. Donohue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:1902;1905.
8. Gilbert DM, Eliopoulos GM, Moellering RC, Sande MA. The Sanford Guide to Antimicrobial Therapy 2006 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006;12.-
9. Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthal Plast Reconstr Surg 2005;21:363-366.
10. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-1406.
Although antibiotics are the best initial treatment, surgical intervention is warranted when a child has:
- visual impairment, complete ophthalmoplegia, or well-defined abscess on presentation, or
- no clearly apparent clinical improvement by 24 hours (strength of recommendation [SOR]: C, based on patient-oriented case-series studies).
Target antimicrobial therapy toward the common pathogens associated with predisposing factors for orbital cellulitis, such as sinusitis—and pay attention to local resistance patterns (SOR: C, based on patient-oriented case series).
Rare but serious risk factors
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
The incidence of Haemophilus influenzae–related periorbital cellulitis appears to have plummeted with the advent of Hib vaccine. And while no national data have been published, case series support my clinical observation that the overall incidence of periorbital cellulitis has dropped as well.
The arrival of heptavalent pneumococcal vaccine may further contribute to its welcome scarcity. take this changing bacteriology—in conjunction with local resistance patterns—into account when considering antibiotic coverage.
When confronted by the rare case of periorbital cellulitis, I always consider risk factors that may change my management, such as immunization status and asplenia. Also, meningitis is a rare but serious complication, so I also keep meningitis risk factors in mind, such as immunosuppression, coincident trauma, or a poor response to initial medical therapy.
Finally, any question of orbital involvement should prompt an emergent consultation.
Evidence summary
Orbital cellulitis is a serious soft-tissue infection of childhood with very different etiologies.
- Periorbital (or preseptal) cellulitis is synonymous with stage I orbital cellulitis, in which there is induration, erythema, warmth, and tenderness of the periorbital soft tissues, usually secondary to external inoculation, but the inflammation does not extend into the bony orbit.
- Stages II, III, and IV orbital cellulitis are progressively more invasive infections that generally arise from the sinuses; they may involve the retro-orbital area. These stages of orbital cellulitis can cause proptosis, decrease visual acuity, or appear as abscesses on computed tomography scan.1,2
Staged treatment
Many retrospective studies of stage II–IV orbital cellulitis with relatively few subjects and small prospective case series have been published with common themes for management recommendations:
- early intravenous antibiotics (likely for an inpatient), and
- involvement of otolaryngology and ophthalmology specialists.
No head-to-head trials have been completed to evaluate efficacy of specific antimicrobial regimens.
Oral antibiotics. First, treat stage I orbital cellulitis with oral antibiotics.
IV antibiotics. Modify treatment to intravenous antibiotics when there is no improvement within 24 hours or if you discover any characteristic of more severe orbital cellulitis.
Medical management of stage II–IV orbital cellulitis with intravenous antibiotics is the current standard of care until it is clear that one of the following is present:
- no improvement by 24 to 48 hours
- visual impairment
- complete ophthalmoplegia, or
- well-defined periosteal abscess.1,2
Surgery. For refractory cases, surgical decompression will likely be required.
The evidence. A small case series (n=9) found 21 children admitted to hospital for preseptal cellulitis, of whom 4 later were diagnosed with orbital cellulitis. There was a total of 9 cases of orbital cellulitis; however, only 1 required operative management of orbital cellulitis.3 In a prospective study to evaluate medical management (n=23), 87% of patients responded to intravenous antibiotics.4 No statistically significant long-term difference in subperiosteal abscesses (as a complication of orbital cellulitis) was found in another retrospective study comparing medical to surgical management.5
Target the likely pathogens
Direct antimicrobial therapy toward common pathogens for likely sources of infection, paying attention to local resistance patterns and the pathogens usually associated with sinusitis (TABLE).1,2,6-8
A retrospective case series of 94 patients of all ages in China implicated Staphylococcus aureus and streptococcal species based on cultures taken from eye purulence and abscesses.6 Another retrospective case series from Vanderbilt (n=80) found streptococci as the most common cause, based on blood and wound cultures in the Hib vaccination era; however, only 12 wounds returned positive cultures.7
TABLE
Choose antibiotic based on cause and likely pathogen1,2,6-8
| ANTECEDENT EVENT | LIKELY PATHOGENS | BEST DRUGS |
|---|---|---|
| Acute sinusitis | Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis | Penicillinase-resistant penicillins |
| Trauma | Staphylococcus aureus Group A β-hernolytic streptococci Increasing concern for methicillin-resistant S aureus | Penicillinase-resistant penicillins First-generation cephalosporins Consider drugs appropriate for methicillin-resistant S aureus |
| Chronic sinusitis | Anaerobes | Metronidazole Clindamycin |
Steroids have no proven benefit
Systemic steroids have no proven benefit in the treatment of pediatric orbital cellulitis with subperiosteal abscess.
A small retrospective cohort study of the benefit of intravenous steroids in addition to antibiotics showed no decrease in hospital stay or need for surgical decompression (n=23, P=.26 and .20, respectively).9 Without prospective data and a power analysis, lack of benefit of steroids cannot be definitively shown.
Recommendations from others
Infectious Disease Society of America. The guidelines for the management of skin and soft-tissue infections implicate β-hemolytic streptococci as the most common cellulitis pathogen, but also recommend empiric coverage against S aureus.
Periorbital and orbital cellulitis are not specifically addressed in these guidelines, but oral dicloxacillin, cephalexin, clindamycin, or erythromycin are recommended for superficial cellulitis, provided there is no known resistance to these antibiotics.
Intravenous penicillinase-resistant penicillins (nafcillin) or a first-generation cephalosporin (cefazolin) may be used for more severe infections.
For penicillin-allergic patients, the IDSA recommends clindamycin or vancomycin.10
Sanford Guide to Antimicrobial Therapy. Nafcillin plus ceftriaxone and metronidazole is the recommended treatment for orbital cellulitis.
For patients allergic to penicillin, vancomycin plus levofloxacin and metronidazole are recommended.8
Neither the American Academy of Ophthalmology nor the International Council of Ophthalmology offers clinical statements on orbital cellulitis.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical service or the US Air Force at large.
Although antibiotics are the best initial treatment, surgical intervention is warranted when a child has:
- visual impairment, complete ophthalmoplegia, or well-defined abscess on presentation, or
- no clearly apparent clinical improvement by 24 hours (strength of recommendation [SOR]: C, based on patient-oriented case-series studies).
Target antimicrobial therapy toward the common pathogens associated with predisposing factors for orbital cellulitis, such as sinusitis—and pay attention to local resistance patterns (SOR: C, based on patient-oriented case series).
Rare but serious risk factors
Peter C. Smith, MD
Rose Family Medicine Residency, University of Colorado Health Sciences Center, Denver
The incidence of Haemophilus influenzae–related periorbital cellulitis appears to have plummeted with the advent of Hib vaccine. And while no national data have been published, case series support my clinical observation that the overall incidence of periorbital cellulitis has dropped as well.
The arrival of heptavalent pneumococcal vaccine may further contribute to its welcome scarcity. take this changing bacteriology—in conjunction with local resistance patterns—into account when considering antibiotic coverage.
When confronted by the rare case of periorbital cellulitis, I always consider risk factors that may change my management, such as immunization status and asplenia. Also, meningitis is a rare but serious complication, so I also keep meningitis risk factors in mind, such as immunosuppression, coincident trauma, or a poor response to initial medical therapy.
Finally, any question of orbital involvement should prompt an emergent consultation.
Evidence summary
Orbital cellulitis is a serious soft-tissue infection of childhood with very different etiologies.
- Periorbital (or preseptal) cellulitis is synonymous with stage I orbital cellulitis, in which there is induration, erythema, warmth, and tenderness of the periorbital soft tissues, usually secondary to external inoculation, but the inflammation does not extend into the bony orbit.
- Stages II, III, and IV orbital cellulitis are progressively more invasive infections that generally arise from the sinuses; they may involve the retro-orbital area. These stages of orbital cellulitis can cause proptosis, decrease visual acuity, or appear as abscesses on computed tomography scan.1,2
Staged treatment
Many retrospective studies of stage II–IV orbital cellulitis with relatively few subjects and small prospective case series have been published with common themes for management recommendations:
- early intravenous antibiotics (likely for an inpatient), and
- involvement of otolaryngology and ophthalmology specialists.
No head-to-head trials have been completed to evaluate efficacy of specific antimicrobial regimens.
Oral antibiotics. First, treat stage I orbital cellulitis with oral antibiotics.
IV antibiotics. Modify treatment to intravenous antibiotics when there is no improvement within 24 hours or if you discover any characteristic of more severe orbital cellulitis.
Medical management of stage II–IV orbital cellulitis with intravenous antibiotics is the current standard of care until it is clear that one of the following is present:
- no improvement by 24 to 48 hours
- visual impairment
- complete ophthalmoplegia, or
- well-defined periosteal abscess.1,2
Surgery. For refractory cases, surgical decompression will likely be required.
The evidence. A small case series (n=9) found 21 children admitted to hospital for preseptal cellulitis, of whom 4 later were diagnosed with orbital cellulitis. There was a total of 9 cases of orbital cellulitis; however, only 1 required operative management of orbital cellulitis.3 In a prospective study to evaluate medical management (n=23), 87% of patients responded to intravenous antibiotics.4 No statistically significant long-term difference in subperiosteal abscesses (as a complication of orbital cellulitis) was found in another retrospective study comparing medical to surgical management.5
Target the likely pathogens
Direct antimicrobial therapy toward common pathogens for likely sources of infection, paying attention to local resistance patterns and the pathogens usually associated with sinusitis (TABLE).1,2,6-8
A retrospective case series of 94 patients of all ages in China implicated Staphylococcus aureus and streptococcal species based on cultures taken from eye purulence and abscesses.6 Another retrospective case series from Vanderbilt (n=80) found streptococci as the most common cause, based on blood and wound cultures in the Hib vaccination era; however, only 12 wounds returned positive cultures.7
TABLE
Choose antibiotic based on cause and likely pathogen1,2,6-8
| ANTECEDENT EVENT | LIKELY PATHOGENS | BEST DRUGS |
|---|---|---|
| Acute sinusitis | Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis | Penicillinase-resistant penicillins |
| Trauma | Staphylococcus aureus Group A β-hernolytic streptococci Increasing concern for methicillin-resistant S aureus | Penicillinase-resistant penicillins First-generation cephalosporins Consider drugs appropriate for methicillin-resistant S aureus |
| Chronic sinusitis | Anaerobes | Metronidazole Clindamycin |
Steroids have no proven benefit
Systemic steroids have no proven benefit in the treatment of pediatric orbital cellulitis with subperiosteal abscess.
A small retrospective cohort study of the benefit of intravenous steroids in addition to antibiotics showed no decrease in hospital stay or need for surgical decompression (n=23, P=.26 and .20, respectively).9 Without prospective data and a power analysis, lack of benefit of steroids cannot be definitively shown.
Recommendations from others
Infectious Disease Society of America. The guidelines for the management of skin and soft-tissue infections implicate β-hemolytic streptococci as the most common cellulitis pathogen, but also recommend empiric coverage against S aureus.
Periorbital and orbital cellulitis are not specifically addressed in these guidelines, but oral dicloxacillin, cephalexin, clindamycin, or erythromycin are recommended for superficial cellulitis, provided there is no known resistance to these antibiotics.
Intravenous penicillinase-resistant penicillins (nafcillin) or a first-generation cephalosporin (cefazolin) may be used for more severe infections.
For penicillin-allergic patients, the IDSA recommends clindamycin or vancomycin.10
Sanford Guide to Antimicrobial Therapy. Nafcillin plus ceftriaxone and metronidazole is the recommended treatment for orbital cellulitis.
For patients allergic to penicillin, vancomycin plus levofloxacin and metronidazole are recommended.8
Neither the American Academy of Ophthalmology nor the International Council of Ophthalmology offers clinical statements on orbital cellulitis.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and not to be construed as official, or as reflecting the views of the US Air Force Medical service or the US Air Force at large.
1. Nageswaran S, Woods CR, Benjamin DK, Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J 2006;25:695-699.
2. Vayalumkal JV, Jadavji T. Children hospitalized with skin and soft tissue infections: a guide to antibacterial selection and treatment. Paediatr Drugs 2006;8:99-111.
3. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J 2001;20:1002-1005.
4. Noel LP, Clarke WN, MacDonald N. Clinical management of orbital cellulitis in children. Can J Ophthalmol 1990;25:11-16.
5. Greenberg MF, Pollard ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS 1998;2:351-355.
6. Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: a 10-year review of hospitalized patients. J Chin Med Assoc 2006;69:415-422.
7. Donohue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:1902;1905.
8. Gilbert DM, Eliopoulos GM, Moellering RC, Sande MA. The Sanford Guide to Antimicrobial Therapy 2006 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006;12.-
9. Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthal Plast Reconstr Surg 2005;21:363-366.
10. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-1406.
1. Nageswaran S, Woods CR, Benjamin DK, Jr, Givner LB, Shetty AK. Orbital cellulitis in children. Pediatr Infect Dis J 2006;25:695-699.
2. Vayalumkal JV, Jadavji T. Children hospitalized with skin and soft tissue infections: a guide to antibacterial selection and treatment. Paediatr Drugs 2006;8:99-111.
3. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J 2001;20:1002-1005.
4. Noel LP, Clarke WN, MacDonald N. Clinical management of orbital cellulitis in children. Can J Ophthalmol 1990;25:11-16.
5. Greenberg MF, Pollard ZF. Medical treatment of pediatric subperiosteal orbital abscess secondary to sinusitis. J AAPOS 1998;2:351-355.
6. Liu IT, Kao SC, Wang AG, Tsai CC, Liang CK, Hsu WM. Preseptal and orbital cellulitis: a 10-year review of hospitalized patients. J Chin Med Assoc 2006;69:415-422.
7. Donohue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:1902;1905.
8. Gilbert DM, Eliopoulos GM, Moellering RC, Sande MA. The Sanford Guide to Antimicrobial Therapy 2006 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006;12.-
9. Yen MT, Yen KG. Effect of corticosteroids in the acute management of pediatric orbital cellulitis with subperiosteal abscess. Ophthal Plast Reconstr Surg 2005;21:363-366.
10. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-1406.
Evidence-based answers from the Family Physicians Inquiries Network