User login
Do antibiotics improve outcomes in chronic rhinosinusitis?
For children, antibiotics do not appear to improve short-term (3-6 weeks) or long-term (3 months) outcomes of chronic rhinosinusitis (strength of recommendation [SOR]: A, randomized controlled trials). No adequate placebo-controlled trials have been performed in adults. Two consensus statements report that 10 to 21 days of antibiotics active against organisms producing beta-lactamase might be beneficial in some cases (SOR: C).
Evidence summary
The American Academy of Otolargynology-Head and Neck Surgery defines chronic rhinosinusitis as the persistence of 2 major or 1 major and 2 minor criteria lasting at least 12 weeks (Table).1 The other categories of rhinosinusitis are acute (symptoms lasting <3 weeks) and subacute (symptoms lasting 3-12 weeks).
Two placebo-controlled trials have evaluated antibiotic treatment of chronic rhinosinusitis in children. In 1 study, 141 children with chronic rhinosinusitis were randomly assigned to 1 of 4 treatment arms: saline nose drops; xylometazoline (Otrivin) drops with oral amoxicillin 3 times daily; surgical drainage; or surgical drainage, amoxicillin 3 times daily and xylometazoline drops.2 Outcomes were resolution of purulent rhinitis, no purulent drainage on exam, and no abnormalities of maxillary sinus on x-ray. The absence of all 3 findings constituted cure. At 6 weeks there was a non-statistically significant higher resolution in the fourth group, but by 26 weeks the groups were indistinguishable. At 6 weeks, 53%, 50%, 55%, and 79% of each group, respectively, were cured. These results increased to 69%, 74%, 69%, and 64% at 26 weeks.
Another study randomized 79 children with chronic sinusitis to treatment with cefaclor vs placebo following antral washout.3 Measured outcomes were similar to those in the prior study. At 6 weeks, 12.3% more patients in the antibiotic group achieved cure than the placebo group (64.8% vs 52.5%), but this difference was not statistically significant (P=.28). At 12 weeks, no differences in improvement were seen between the 2 groups (89% vs 89.5%)
No studies (since 1966) have evaluated antibiotic use compared with placebo in adults. We did not review the numerous studies comparing different antibiotics without placebo.
Recommendations from others
The American Academy of Otolaryngology-Head and Neck Surgery, in conjunction with the American Academy of Rhinology and the American
Academy of Otolaryngic Allergy, state that the use of antibiotics active against beta-lactamase producing organisms might be beneficial in some cases.3 A consensus statement from a panel convened in Belgium in 1996 stated antibiotics should be given for 5 to 7 days with repeat treatments if the child does not respond initially.5
TABLE 2
Diagnostic criteria for rhinosinusitis
| Major criteria |
| Facial pain/pressure* |
| Facial congestion/fullness |
| Nasal obstruction/blockage |
| Nasal discharge/purulence/discolored drainage |
| Hyposmia/anosmia |
| Purulence in nasal cavity on examination |
| Fever (acute only)* |
| Minor criteria |
| Headache |
| Fever (all nonacute) |
| Halitosis |
| Fatigue |
| Dental pain |
| Cough |
| Ear pain/pressure/fullness |
| *Symptom alone does not constitute a major sign in the absence of another major nasal symptom. Adapted from Lanza DC, 1997.1 |
Antibiotics provide only short-term relief, not long-term answers
William A. Hensel, MD
Moses Cone Family Residency Program, Greensboro, NC
For chronic sinusitis, I start by emphasizing nonantibiotic treatments, such as decongestants, nasal steroids, antihistamines, smoking cessation, and avoidance of passive smoke, allergens, and other irritants. With education and experience, patients realize that antibiotics provide only short-term relief, not long-term answers. Having learned this, patients can better participate in antibiotic treatment decisions. Most are able to weigh the short-term, symptomatic benefits against potential medication side effects and the cost. I believe that 2 or 3 courses of antibiotics per year are not excessive, but I try not to exceed that limit.
Finally, I don’t always choose a beta-lactamase-resistant antibiotic. Given that antibiotics do not alter the long-term prognosis, I worry less about resistance and more about minimizing cost and side-effect potential. Therefore, I occasionally treat with amoxicillin or Pen Vee K. Patients seem to appreciate my flexibility and collaborative approach to decision-making.
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S1-S7.
2. Otten FW, Grote JJ. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol 1988;15:269-278.
3. Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children? Clin Otolaryngo 1994;19:215-217.
4. Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S41-S49.
5. Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998;124:31-34.
For children, antibiotics do not appear to improve short-term (3-6 weeks) or long-term (3 months) outcomes of chronic rhinosinusitis (strength of recommendation [SOR]: A, randomized controlled trials). No adequate placebo-controlled trials have been performed in adults. Two consensus statements report that 10 to 21 days of antibiotics active against organisms producing beta-lactamase might be beneficial in some cases (SOR: C).
Evidence summary
The American Academy of Otolargynology-Head and Neck Surgery defines chronic rhinosinusitis as the persistence of 2 major or 1 major and 2 minor criteria lasting at least 12 weeks (Table).1 The other categories of rhinosinusitis are acute (symptoms lasting <3 weeks) and subacute (symptoms lasting 3-12 weeks).
Two placebo-controlled trials have evaluated antibiotic treatment of chronic rhinosinusitis in children. In 1 study, 141 children with chronic rhinosinusitis were randomly assigned to 1 of 4 treatment arms: saline nose drops; xylometazoline (Otrivin) drops with oral amoxicillin 3 times daily; surgical drainage; or surgical drainage, amoxicillin 3 times daily and xylometazoline drops.2 Outcomes were resolution of purulent rhinitis, no purulent drainage on exam, and no abnormalities of maxillary sinus on x-ray. The absence of all 3 findings constituted cure. At 6 weeks there was a non-statistically significant higher resolution in the fourth group, but by 26 weeks the groups were indistinguishable. At 6 weeks, 53%, 50%, 55%, and 79% of each group, respectively, were cured. These results increased to 69%, 74%, 69%, and 64% at 26 weeks.
Another study randomized 79 children with chronic sinusitis to treatment with cefaclor vs placebo following antral washout.3 Measured outcomes were similar to those in the prior study. At 6 weeks, 12.3% more patients in the antibiotic group achieved cure than the placebo group (64.8% vs 52.5%), but this difference was not statistically significant (P=.28). At 12 weeks, no differences in improvement were seen between the 2 groups (89% vs 89.5%)
No studies (since 1966) have evaluated antibiotic use compared with placebo in adults. We did not review the numerous studies comparing different antibiotics without placebo.
Recommendations from others
The American Academy of Otolaryngology-Head and Neck Surgery, in conjunction with the American Academy of Rhinology and the American
Academy of Otolaryngic Allergy, state that the use of antibiotics active against beta-lactamase producing organisms might be beneficial in some cases.3 A consensus statement from a panel convened in Belgium in 1996 stated antibiotics should be given for 5 to 7 days with repeat treatments if the child does not respond initially.5
TABLE 2
Diagnostic criteria for rhinosinusitis
| Major criteria |
| Facial pain/pressure* |
| Facial congestion/fullness |
| Nasal obstruction/blockage |
| Nasal discharge/purulence/discolored drainage |
| Hyposmia/anosmia |
| Purulence in nasal cavity on examination |
| Fever (acute only)* |
| Minor criteria |
| Headache |
| Fever (all nonacute) |
| Halitosis |
| Fatigue |
| Dental pain |
| Cough |
| Ear pain/pressure/fullness |
| *Symptom alone does not constitute a major sign in the absence of another major nasal symptom. Adapted from Lanza DC, 1997.1 |
Antibiotics provide only short-term relief, not long-term answers
William A. Hensel, MD
Moses Cone Family Residency Program, Greensboro, NC
For chronic sinusitis, I start by emphasizing nonantibiotic treatments, such as decongestants, nasal steroids, antihistamines, smoking cessation, and avoidance of passive smoke, allergens, and other irritants. With education and experience, patients realize that antibiotics provide only short-term relief, not long-term answers. Having learned this, patients can better participate in antibiotic treatment decisions. Most are able to weigh the short-term, symptomatic benefits against potential medication side effects and the cost. I believe that 2 or 3 courses of antibiotics per year are not excessive, but I try not to exceed that limit.
Finally, I don’t always choose a beta-lactamase-resistant antibiotic. Given that antibiotics do not alter the long-term prognosis, I worry less about resistance and more about minimizing cost and side-effect potential. Therefore, I occasionally treat with amoxicillin or Pen Vee K. Patients seem to appreciate my flexibility and collaborative approach to decision-making.
For children, antibiotics do not appear to improve short-term (3-6 weeks) or long-term (3 months) outcomes of chronic rhinosinusitis (strength of recommendation [SOR]: A, randomized controlled trials). No adequate placebo-controlled trials have been performed in adults. Two consensus statements report that 10 to 21 days of antibiotics active against organisms producing beta-lactamase might be beneficial in some cases (SOR: C).
Evidence summary
The American Academy of Otolargynology-Head and Neck Surgery defines chronic rhinosinusitis as the persistence of 2 major or 1 major and 2 minor criteria lasting at least 12 weeks (Table).1 The other categories of rhinosinusitis are acute (symptoms lasting <3 weeks) and subacute (symptoms lasting 3-12 weeks).
Two placebo-controlled trials have evaluated antibiotic treatment of chronic rhinosinusitis in children. In 1 study, 141 children with chronic rhinosinusitis were randomly assigned to 1 of 4 treatment arms: saline nose drops; xylometazoline (Otrivin) drops with oral amoxicillin 3 times daily; surgical drainage; or surgical drainage, amoxicillin 3 times daily and xylometazoline drops.2 Outcomes were resolution of purulent rhinitis, no purulent drainage on exam, and no abnormalities of maxillary sinus on x-ray. The absence of all 3 findings constituted cure. At 6 weeks there was a non-statistically significant higher resolution in the fourth group, but by 26 weeks the groups were indistinguishable. At 6 weeks, 53%, 50%, 55%, and 79% of each group, respectively, were cured. These results increased to 69%, 74%, 69%, and 64% at 26 weeks.
Another study randomized 79 children with chronic sinusitis to treatment with cefaclor vs placebo following antral washout.3 Measured outcomes were similar to those in the prior study. At 6 weeks, 12.3% more patients in the antibiotic group achieved cure than the placebo group (64.8% vs 52.5%), but this difference was not statistically significant (P=.28). At 12 weeks, no differences in improvement were seen between the 2 groups (89% vs 89.5%)
No studies (since 1966) have evaluated antibiotic use compared with placebo in adults. We did not review the numerous studies comparing different antibiotics without placebo.
Recommendations from others
The American Academy of Otolaryngology-Head and Neck Surgery, in conjunction with the American Academy of Rhinology and the American
Academy of Otolaryngic Allergy, state that the use of antibiotics active against beta-lactamase producing organisms might be beneficial in some cases.3 A consensus statement from a panel convened in Belgium in 1996 stated antibiotics should be given for 5 to 7 days with repeat treatments if the child does not respond initially.5
TABLE 2
Diagnostic criteria for rhinosinusitis
| Major criteria |
| Facial pain/pressure* |
| Facial congestion/fullness |
| Nasal obstruction/blockage |
| Nasal discharge/purulence/discolored drainage |
| Hyposmia/anosmia |
| Purulence in nasal cavity on examination |
| Fever (acute only)* |
| Minor criteria |
| Headache |
| Fever (all nonacute) |
| Halitosis |
| Fatigue |
| Dental pain |
| Cough |
| Ear pain/pressure/fullness |
| *Symptom alone does not constitute a major sign in the absence of another major nasal symptom. Adapted from Lanza DC, 1997.1 |
Antibiotics provide only short-term relief, not long-term answers
William A. Hensel, MD
Moses Cone Family Residency Program, Greensboro, NC
For chronic sinusitis, I start by emphasizing nonantibiotic treatments, such as decongestants, nasal steroids, antihistamines, smoking cessation, and avoidance of passive smoke, allergens, and other irritants. With education and experience, patients realize that antibiotics provide only short-term relief, not long-term answers. Having learned this, patients can better participate in antibiotic treatment decisions. Most are able to weigh the short-term, symptomatic benefits against potential medication side effects and the cost. I believe that 2 or 3 courses of antibiotics per year are not excessive, but I try not to exceed that limit.
Finally, I don’t always choose a beta-lactamase-resistant antibiotic. Given that antibiotics do not alter the long-term prognosis, I worry less about resistance and more about minimizing cost and side-effect potential. Therefore, I occasionally treat with amoxicillin or Pen Vee K. Patients seem to appreciate my flexibility and collaborative approach to decision-making.
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S1-S7.
2. Otten FW, Grote JJ. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol 1988;15:269-278.
3. Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children? Clin Otolaryngo 1994;19:215-217.
4. Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S41-S49.
5. Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998;124:31-34.
1. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S1-S7.
2. Otten FW, Grote JJ. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol 1988;15:269-278.
3. Otten HW, Antvelink JB, Ruyter de Wildt H, Rietema SJ, Siemelink RJ, Hordijk GJ. Is antibiotic treatment of chronic sinusitis effective in children? Clin Otolaryngo 1994;19:215-217.
4. Benninger MS, Anon J, Mabry RL. The medical management of rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):S41-S49.
5. Clement PA, Bluestone CD, Gordts F, et al. Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998;124:31-34.
Evidence-based answers from the Family Physicians Inquiries Network
Does warfarin prevent deep venous thrombosis in high-risk patients?
Warfarin (Coumadin) is effective in preventing deep venous thrombosis (DVT) among patients with a history of DVT. Conventional dosing and longer durations are the most effective, but the ideal length of therapy is unknown (strength of recommendation [SOR]: A, based on large randomized controlled trials and meta-analysis).
Warfarin is useful in preventing DVT in patients with cancer, specifically those treated with chemotherapy (SOR: B, based on small randomized controlled trials). Warfarin may be effective in pre-venting DVT in immobilized patients such as those with trauma, spinal cord injury, or stroke (SOR: B, based on an underpowered randomized controlled trial and uncontrolled studies).
Evidence summary
Warfarin, at both low and conventional doses, has been shown to be effective in preventing recurrence of DVT. A large, 4-year placebo-controlled randomized controlled trial showed that long-term low-dose warfarin (international normalized ratio [INR], 1.5-1.9) was more effective than placebo for prevention of DVT (hazard ratio=0.36; 95% confidence interval [CI], 0.19-0.67).1
A double-blind randomized controlled trial of 738 patients demonstrated that conventional-intensity warfarin therapy (INR=2.0-3.0) was more effective than low-intensity therapy (INR=1.5-1.9) in prevention of recurrent DVT. There were 1.9 vs 0.7 DVTs per 100 person-years in the low-intensity vs conventional-intensity therapy groups (hazard ratio=2.8; 95% CI, 1.1-7.0; number needed to treat [NNT]=37). No significant difference was seen in the frequency of bleeding complications between the groups.2 This and other studies suggest that low-intensity warfarin therapy reduces the relative risk of thrombosis by about 75%, and conventional-intensity therapy reduces this risk by over 90%.2
Several studies have examined the duration of warfarin therapy. A meta-analysis found treatment with warfarin for 12 to 24 weeks decreased DVT recurrence compared with 2- to 6-week regimens (relative risk [RR]=0.60; 95% CI, 0.45-0.79; NNT=21).3 A multicenter randomized controlled trial found extending warfarin treatment for 12 months vs 3 months resulted in a 95% relative risk reduction (RRR) in risk of DVT recurrence (95% CI, 63-99; NNT=5).4 A multicenter randomized trial showed similar results, but risk for recurrence was the same after treatment was stopped, regardless of the length of treatment.5
In patients with cancer, warfarin was shown to be more effective than placebo in prevention of DVT. In a trial of 311 breast cancer patients receiving chemotherapy, treatment with very-low-dose warfarin (INR=1.3-1.9) decreased thrombotic events compared with placebo, with no increase in bleeding complications (RRR=85%; P=.031; NNT=27).6 A later cost analysis showed that very-low-dose warfarin can be used in prevention of DVT in breast cancer patients on chemotherapy without an increase in health care costs.7
Although immobilized patients are at high risk for DVT, no randomized controlled trials exist for the use of warfarin in these patients. A few small studies suggest that warfarin reduces DVT rates in spinal-cord-injured patients.8 A small trial randomized stroke patients undergoing rehabilitation to placebo or fixed 1- or 2-mg doses of warfarin. This underpowered study showed a nonsignificant decrease in the risk of development of DVT (RR=0.39; 95% CI, 0.13-1.37).8
Recommendations from others
The 6th American College of Chest Physicians Consensus Conference on Antithrombotic Therapy makes these recommendations:9
Prior DVT: Oral anticoagulation therapy (INR=2.0-3.0) is indicated for at least 3 months for patients with proximal DVT or for at least 6 months in those with idiopathic proximal vein thrombosis or recurrent venous thrombosis. Indefinite anticoagulation is indicated for patients with more than 1 episode of idiopathic proximal vein thrombosis or pulmonary embolus.
Malignancy: Indefinite anticoagulation (INR= 2.0-3.0) is indicated for patients with thrombosis complicating malignancy. Prophylaxis with low-intensity warfarin in ambulatory patients with cancer to prevent initial DVT warrants further study.
Acute spinal cord injuries: Low-molecular-weight heparin or switch to full-dose oral anti-coagulation (INR=2.0-3.0) for the duration of the rehabilitation phase.
Routine prophylaxis dramatically reduces DVT cases
John P. Langlois, MD
MAHEC Family Practice Residency, Asheville, NC
I can clearly recall the dramatic reduction in the number of our patients who developed DVT when our orthopedic colleagues embraced routine prophylaxis for the high-risk surgical patients with hip surgery and knee replacements. This answer indicates that we may also be able to reduce the risk of DVT in our high-risk nonsurgical patients with previous DVT or breast cancer. Note that much of the evidence is based on the use of low-dose and very-low-dose warfarin. This may help mitigate our fear of substituting bleeding complications for the prevention of clots.
1. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-1434.
2. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity war-farin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631-639.
3. Pinede L, Duhaut P, Cucherat M, Ninet J, Pasquier J, Boissel JP. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553-562.
4. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
5. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-169.
6. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of throm-boembolism in stage IV breast cancer. Lancet 1994;343:886-889.
7. Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. J Clin Oncol 1995;13:42-46.
8. Ginsberg JS, Bates SM, Oczkowski W, et al. Low-dose warfarin in rehabilitating stroke survivors. Thromb Res 2002;107:287-290.
9. Hirsh J, Dalen J, Guyatt G. American College of Chest Physicians. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):132S-193S.
Warfarin (Coumadin) is effective in preventing deep venous thrombosis (DVT) among patients with a history of DVT. Conventional dosing and longer durations are the most effective, but the ideal length of therapy is unknown (strength of recommendation [SOR]: A, based on large randomized controlled trials and meta-analysis).
Warfarin is useful in preventing DVT in patients with cancer, specifically those treated with chemotherapy (SOR: B, based on small randomized controlled trials). Warfarin may be effective in pre-venting DVT in immobilized patients such as those with trauma, spinal cord injury, or stroke (SOR: B, based on an underpowered randomized controlled trial and uncontrolled studies).
Evidence summary
Warfarin, at both low and conventional doses, has been shown to be effective in preventing recurrence of DVT. A large, 4-year placebo-controlled randomized controlled trial showed that long-term low-dose warfarin (international normalized ratio [INR], 1.5-1.9) was more effective than placebo for prevention of DVT (hazard ratio=0.36; 95% confidence interval [CI], 0.19-0.67).1
A double-blind randomized controlled trial of 738 patients demonstrated that conventional-intensity warfarin therapy (INR=2.0-3.0) was more effective than low-intensity therapy (INR=1.5-1.9) in prevention of recurrent DVT. There were 1.9 vs 0.7 DVTs per 100 person-years in the low-intensity vs conventional-intensity therapy groups (hazard ratio=2.8; 95% CI, 1.1-7.0; number needed to treat [NNT]=37). No significant difference was seen in the frequency of bleeding complications between the groups.2 This and other studies suggest that low-intensity warfarin therapy reduces the relative risk of thrombosis by about 75%, and conventional-intensity therapy reduces this risk by over 90%.2
Several studies have examined the duration of warfarin therapy. A meta-analysis found treatment with warfarin for 12 to 24 weeks decreased DVT recurrence compared with 2- to 6-week regimens (relative risk [RR]=0.60; 95% CI, 0.45-0.79; NNT=21).3 A multicenter randomized controlled trial found extending warfarin treatment for 12 months vs 3 months resulted in a 95% relative risk reduction (RRR) in risk of DVT recurrence (95% CI, 63-99; NNT=5).4 A multicenter randomized trial showed similar results, but risk for recurrence was the same after treatment was stopped, regardless of the length of treatment.5
In patients with cancer, warfarin was shown to be more effective than placebo in prevention of DVT. In a trial of 311 breast cancer patients receiving chemotherapy, treatment with very-low-dose warfarin (INR=1.3-1.9) decreased thrombotic events compared with placebo, with no increase in bleeding complications (RRR=85%; P=.031; NNT=27).6 A later cost analysis showed that very-low-dose warfarin can be used in prevention of DVT in breast cancer patients on chemotherapy without an increase in health care costs.7
Although immobilized patients are at high risk for DVT, no randomized controlled trials exist for the use of warfarin in these patients. A few small studies suggest that warfarin reduces DVT rates in spinal-cord-injured patients.8 A small trial randomized stroke patients undergoing rehabilitation to placebo or fixed 1- or 2-mg doses of warfarin. This underpowered study showed a nonsignificant decrease in the risk of development of DVT (RR=0.39; 95% CI, 0.13-1.37).8
Recommendations from others
The 6th American College of Chest Physicians Consensus Conference on Antithrombotic Therapy makes these recommendations:9
Prior DVT: Oral anticoagulation therapy (INR=2.0-3.0) is indicated for at least 3 months for patients with proximal DVT or for at least 6 months in those with idiopathic proximal vein thrombosis or recurrent venous thrombosis. Indefinite anticoagulation is indicated for patients with more than 1 episode of idiopathic proximal vein thrombosis or pulmonary embolus.
Malignancy: Indefinite anticoagulation (INR= 2.0-3.0) is indicated for patients with thrombosis complicating malignancy. Prophylaxis with low-intensity warfarin in ambulatory patients with cancer to prevent initial DVT warrants further study.
Acute spinal cord injuries: Low-molecular-weight heparin or switch to full-dose oral anti-coagulation (INR=2.0-3.0) for the duration of the rehabilitation phase.
Routine prophylaxis dramatically reduces DVT cases
John P. Langlois, MD
MAHEC Family Practice Residency, Asheville, NC
I can clearly recall the dramatic reduction in the number of our patients who developed DVT when our orthopedic colleagues embraced routine prophylaxis for the high-risk surgical patients with hip surgery and knee replacements. This answer indicates that we may also be able to reduce the risk of DVT in our high-risk nonsurgical patients with previous DVT or breast cancer. Note that much of the evidence is based on the use of low-dose and very-low-dose warfarin. This may help mitigate our fear of substituting bleeding complications for the prevention of clots.
Warfarin (Coumadin) is effective in preventing deep venous thrombosis (DVT) among patients with a history of DVT. Conventional dosing and longer durations are the most effective, but the ideal length of therapy is unknown (strength of recommendation [SOR]: A, based on large randomized controlled trials and meta-analysis).
Warfarin is useful in preventing DVT in patients with cancer, specifically those treated with chemotherapy (SOR: B, based on small randomized controlled trials). Warfarin may be effective in pre-venting DVT in immobilized patients such as those with trauma, spinal cord injury, or stroke (SOR: B, based on an underpowered randomized controlled trial and uncontrolled studies).
Evidence summary
Warfarin, at both low and conventional doses, has been shown to be effective in preventing recurrence of DVT. A large, 4-year placebo-controlled randomized controlled trial showed that long-term low-dose warfarin (international normalized ratio [INR], 1.5-1.9) was more effective than placebo for prevention of DVT (hazard ratio=0.36; 95% confidence interval [CI], 0.19-0.67).1
A double-blind randomized controlled trial of 738 patients demonstrated that conventional-intensity warfarin therapy (INR=2.0-3.0) was more effective than low-intensity therapy (INR=1.5-1.9) in prevention of recurrent DVT. There were 1.9 vs 0.7 DVTs per 100 person-years in the low-intensity vs conventional-intensity therapy groups (hazard ratio=2.8; 95% CI, 1.1-7.0; number needed to treat [NNT]=37). No significant difference was seen in the frequency of bleeding complications between the groups.2 This and other studies suggest that low-intensity warfarin therapy reduces the relative risk of thrombosis by about 75%, and conventional-intensity therapy reduces this risk by over 90%.2
Several studies have examined the duration of warfarin therapy. A meta-analysis found treatment with warfarin for 12 to 24 weeks decreased DVT recurrence compared with 2- to 6-week regimens (relative risk [RR]=0.60; 95% CI, 0.45-0.79; NNT=21).3 A multicenter randomized controlled trial found extending warfarin treatment for 12 months vs 3 months resulted in a 95% relative risk reduction (RRR) in risk of DVT recurrence (95% CI, 63-99; NNT=5).4 A multicenter randomized trial showed similar results, but risk for recurrence was the same after treatment was stopped, regardless of the length of treatment.5
In patients with cancer, warfarin was shown to be more effective than placebo in prevention of DVT. In a trial of 311 breast cancer patients receiving chemotherapy, treatment with very-low-dose warfarin (INR=1.3-1.9) decreased thrombotic events compared with placebo, with no increase in bleeding complications (RRR=85%; P=.031; NNT=27).6 A later cost analysis showed that very-low-dose warfarin can be used in prevention of DVT in breast cancer patients on chemotherapy without an increase in health care costs.7
Although immobilized patients are at high risk for DVT, no randomized controlled trials exist for the use of warfarin in these patients. A few small studies suggest that warfarin reduces DVT rates in spinal-cord-injured patients.8 A small trial randomized stroke patients undergoing rehabilitation to placebo or fixed 1- or 2-mg doses of warfarin. This underpowered study showed a nonsignificant decrease in the risk of development of DVT (RR=0.39; 95% CI, 0.13-1.37).8
Recommendations from others
The 6th American College of Chest Physicians Consensus Conference on Antithrombotic Therapy makes these recommendations:9
Prior DVT: Oral anticoagulation therapy (INR=2.0-3.0) is indicated for at least 3 months for patients with proximal DVT or for at least 6 months in those with idiopathic proximal vein thrombosis or recurrent venous thrombosis. Indefinite anticoagulation is indicated for patients with more than 1 episode of idiopathic proximal vein thrombosis or pulmonary embolus.
Malignancy: Indefinite anticoagulation (INR= 2.0-3.0) is indicated for patients with thrombosis complicating malignancy. Prophylaxis with low-intensity warfarin in ambulatory patients with cancer to prevent initial DVT warrants further study.
Acute spinal cord injuries: Low-molecular-weight heparin or switch to full-dose oral anti-coagulation (INR=2.0-3.0) for the duration of the rehabilitation phase.
Routine prophylaxis dramatically reduces DVT cases
John P. Langlois, MD
MAHEC Family Practice Residency, Asheville, NC
I can clearly recall the dramatic reduction in the number of our patients who developed DVT when our orthopedic colleagues embraced routine prophylaxis for the high-risk surgical patients with hip surgery and knee replacements. This answer indicates that we may also be able to reduce the risk of DVT in our high-risk nonsurgical patients with previous DVT or breast cancer. Note that much of the evidence is based on the use of low-dose and very-low-dose warfarin. This may help mitigate our fear of substituting bleeding complications for the prevention of clots.
1. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-1434.
2. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity war-farin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631-639.
3. Pinede L, Duhaut P, Cucherat M, Ninet J, Pasquier J, Boissel JP. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553-562.
4. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
5. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-169.
6. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of throm-boembolism in stage IV breast cancer. Lancet 1994;343:886-889.
7. Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. J Clin Oncol 1995;13:42-46.
8. Ginsberg JS, Bates SM, Oczkowski W, et al. Low-dose warfarin in rehabilitating stroke survivors. Thromb Res 2002;107:287-290.
9. Hirsh J, Dalen J, Guyatt G. American College of Chest Physicians. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):132S-193S.
1. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-1434.
2. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity war-farin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631-639.
3. Pinede L, Duhaut P, Cucherat M, Ninet J, Pasquier J, Boissel JP. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553-562.
4. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-907.
5. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-169.
6. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of throm-boembolism in stage IV breast cancer. Lancet 1994;343:886-889.
7. Rajan R, Gafni A, Levine M, Hirsh J, Gent M. Very low-dose warfarin prophylaxis to prevent thromboembolism in women with metastatic breast cancer receiving chemotherapy: an economic evaluation. J Clin Oncol 1995;13:42-46.
8. Ginsberg JS, Bates SM, Oczkowski W, et al. Low-dose warfarin in rehabilitating stroke survivors. Thromb Res 2002;107:287-290.
9. Hirsh J, Dalen J, Guyatt G. American College of Chest Physicians. The sixth (2000) ACCP guidelines for antithrombotic therapy for prevention and treatment of thrombosis. American College of Chest Physicians. Chest 2001;119(1 Suppl):132S-193S.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best macrolide for atypical pneumonia?
Erythromycin, clarithromycin, and azithromycin are equally effective in treating pneumonia caused by Mycoplasma pneumoniae or Chlamydophila (formerly Chlamydia) pneumoniae (strength of recommendation [SOR]: B, small head-to-head trials). Macrolide choice can be based on other considerations—cost, side effects, and effectiveness against other suspected pathogens (SOR: C, expert opinion).
Evidence summary
M pneumoniae and C pneumoniae account for about 30% of community-acquired pneumonia (CAP), making them the most common “atypicals.” Clinically they are indistinguishable from other causes of pneumonia; most studies use cultures to identify cases among populations with CAP.
Azithromycin and erythromycin were compared in 3 studies of children with CAP.1-3 Together, they identified 69 cases due to M pneumoniae or C pneumoniae. Only 3 patients did not respond to either antibiotic. In the largest of the 3 studies,3 side effects were noted in 10% of CAP patients on azithromycin and 20% on erythromycin (P<.05).
Another study looked at patients aged 12 to 80 years with pneumonia due to M pneumoniae (75 cases) or Chlamydophila psittaci (formerly Chlamydia psittaci, 16 cases).4 All patients responded to treatment. Clarithromycin and erythromycin were compared in children aged 3 to 12 years with CAP.5 M pneumoniae or C pneumoniae was identified in 42 cases. Two of 18 patients did not respond to erythromycin; 3 of 27 patients did not respond to clarithromycin.
Another study compared these antibiotics for patients with CAP aged 12 to 93 years.6 Subgroup analysis of those with M pneumoniae or C pneumoniae (n=27) showed similar efficacy. Pooling all 268 patients with CAP, side effects were seen in 31% of patients on clarithromycin and 59% on erythromycin (P<.001).
A comparison study of newer macrolides in 40 adults with CAP identified 13 with M pneumoniae or C pneumoniae (Table).7 One patient did not respond of the 8 treated with clarithromycin; none among the 5 treated with azithromycin. There was 1 adverse event (from clarithromycin).
TABLE
Macrolides: comparison studies
| Antibiotic | Response rates* (%) | Side-effect rates †(%) | Cost for course of therapy in adult ‡ |
|---|---|---|---|
| Erythromycin1-4 | 77-100 | 10-59 | $11 (500 mg #40) |
| Clarithromycin5 7 | 88-94 | 5-31 | $76 (250 mg #20) |
| Azithromycin1 4,7 | 87-100 | 0-14 | $57 (250 mg #6) |
| *Response rates of pneumonia due to M pneumoniae and C pneumoniae. | |||
| † In community-acquired pneumonia treated with macrolide as single agent. | |||
| ‡ Prices from www.drugstore.com. | |||
Recommendations from others
The Infectious Diseases Society of America8 recommends a macrolide for adults with pneumonia caused by M pneumoniae or C pneumoniae, and does not promote one over another. The British Thoracic Society9 recommends any of the macrolides for pneumonia caused by these pathogens in children.
Since CAP is often caused by “atypical organisms,” macrolides are sometimes recommended as empiric outpatient therapy. In this setting, the American Thoracic Society10 discourages using erythromycin, citing a higher side-effect rate and poorer effectiveness against Haemophilus influenza. However, the Canadian Infectious Disease Society11 supports the use of any of the 3 macrolides in mild CAP except for patients with chronic obstructive pulmonary disease, who are more likely to harbor H influenza.
Lower respiratory infections—a number of problematic decisions
David Mouw, MD
Mountain Area AHEC, Asheville, NC
You face several problematic decisions when treating a patient with a lower respiratory infection. First, is this pneumonia or just bronchitis? Clinical findings can be confusing, and a chest film is helpful.12 If pneumonia is likely, you consider hospitalization, and prescribe antibiotics, usually without knowing the pathogen.
Because they cover both typical and atypical pathogens, macrolides (or doxycycline) are generally recommended, with cephalosporins to be added for higher-risk patients. (Quinolones are an alternative to this combination.) Finally, if you choose a macrolide, you face yet another decision without a clear answer: which one to use? All macrolides appear to be equally effective, so the choice depends on cost balanced against convenience and side effects.
1. Wubbel L, Muniz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J 1999;18:98-104.
2. Harris JS, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia. Pediatr Infect Dis J 1998;17:865-871.
3. Manfredi R, Jannuzzi C, Mantero E, et al. Clinical comparative study of azithromycin versus erythromycin in the treatment of acute respiratory tract infections in children. J Chemother 1992;4:364-370.
4. Schonwald S, Gunjaca M, Kolacny-Babic L, Car V, Gosev M. Comparison of azithromycin and erythromycin in the treatment of atypical pneumonias. J Antimicrob Chemother 1990;25(Suppl A):123-126.
5. Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J 1995;14:471-477.
6. Chien M, Pichotta P, Siepman N, Chan CK. Treatment of community-acquired pneumonia: a multicenter, double-blind, randomized study comparing clarithromycin with erythromycin. Canada-Sweden Clarithromycin-Pneumonia Study Group. Chest 1993;103-697-701.
7. Rizzato G, Montemurro L, Fraioli P, et al. Efficacy of a three day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J 1995;8:398-402.
8. Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine M. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis 2000;31:347-382.
9. British. Thoracic Society Standards of Care Committee. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Childhood. Thorax 2002;57(Suppl 1):i1-i24.
10. American. Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-1754.
11. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis 2000;31:383-421.
12. Kelsberg G, Safranek S. How accurate is the clinical diagnosis of pneumonia? J Fam Pract 2003;52:63-64.
Erythromycin, clarithromycin, and azithromycin are equally effective in treating pneumonia caused by Mycoplasma pneumoniae or Chlamydophila (formerly Chlamydia) pneumoniae (strength of recommendation [SOR]: B, small head-to-head trials). Macrolide choice can be based on other considerations—cost, side effects, and effectiveness against other suspected pathogens (SOR: C, expert opinion).
Evidence summary
M pneumoniae and C pneumoniae account for about 30% of community-acquired pneumonia (CAP), making them the most common “atypicals.” Clinically they are indistinguishable from other causes of pneumonia; most studies use cultures to identify cases among populations with CAP.
Azithromycin and erythromycin were compared in 3 studies of children with CAP.1-3 Together, they identified 69 cases due to M pneumoniae or C pneumoniae. Only 3 patients did not respond to either antibiotic. In the largest of the 3 studies,3 side effects were noted in 10% of CAP patients on azithromycin and 20% on erythromycin (P<.05).
Another study looked at patients aged 12 to 80 years with pneumonia due to M pneumoniae (75 cases) or Chlamydophila psittaci (formerly Chlamydia psittaci, 16 cases).4 All patients responded to treatment. Clarithromycin and erythromycin were compared in children aged 3 to 12 years with CAP.5 M pneumoniae or C pneumoniae was identified in 42 cases. Two of 18 patients did not respond to erythromycin; 3 of 27 patients did not respond to clarithromycin.
Another study compared these antibiotics for patients with CAP aged 12 to 93 years.6 Subgroup analysis of those with M pneumoniae or C pneumoniae (n=27) showed similar efficacy. Pooling all 268 patients with CAP, side effects were seen in 31% of patients on clarithromycin and 59% on erythromycin (P<.001).
A comparison study of newer macrolides in 40 adults with CAP identified 13 with M pneumoniae or C pneumoniae (Table).7 One patient did not respond of the 8 treated with clarithromycin; none among the 5 treated with azithromycin. There was 1 adverse event (from clarithromycin).
TABLE
Macrolides: comparison studies
| Antibiotic | Response rates* (%) | Side-effect rates †(%) | Cost for course of therapy in adult ‡ |
|---|---|---|---|
| Erythromycin1-4 | 77-100 | 10-59 | $11 (500 mg #40) |
| Clarithromycin5 7 | 88-94 | 5-31 | $76 (250 mg #20) |
| Azithromycin1 4,7 | 87-100 | 0-14 | $57 (250 mg #6) |
| *Response rates of pneumonia due to M pneumoniae and C pneumoniae. | |||
| † In community-acquired pneumonia treated with macrolide as single agent. | |||
| ‡ Prices from www.drugstore.com. | |||
Recommendations from others
The Infectious Diseases Society of America8 recommends a macrolide for adults with pneumonia caused by M pneumoniae or C pneumoniae, and does not promote one over another. The British Thoracic Society9 recommends any of the macrolides for pneumonia caused by these pathogens in children.
Since CAP is often caused by “atypical organisms,” macrolides are sometimes recommended as empiric outpatient therapy. In this setting, the American Thoracic Society10 discourages using erythromycin, citing a higher side-effect rate and poorer effectiveness against Haemophilus influenza. However, the Canadian Infectious Disease Society11 supports the use of any of the 3 macrolides in mild CAP except for patients with chronic obstructive pulmonary disease, who are more likely to harbor H influenza.
Lower respiratory infections—a number of problematic decisions
David Mouw, MD
Mountain Area AHEC, Asheville, NC
You face several problematic decisions when treating a patient with a lower respiratory infection. First, is this pneumonia or just bronchitis? Clinical findings can be confusing, and a chest film is helpful.12 If pneumonia is likely, you consider hospitalization, and prescribe antibiotics, usually without knowing the pathogen.
Because they cover both typical and atypical pathogens, macrolides (or doxycycline) are generally recommended, with cephalosporins to be added for higher-risk patients. (Quinolones are an alternative to this combination.) Finally, if you choose a macrolide, you face yet another decision without a clear answer: which one to use? All macrolides appear to be equally effective, so the choice depends on cost balanced against convenience and side effects.
Erythromycin, clarithromycin, and azithromycin are equally effective in treating pneumonia caused by Mycoplasma pneumoniae or Chlamydophila (formerly Chlamydia) pneumoniae (strength of recommendation [SOR]: B, small head-to-head trials). Macrolide choice can be based on other considerations—cost, side effects, and effectiveness against other suspected pathogens (SOR: C, expert opinion).
Evidence summary
M pneumoniae and C pneumoniae account for about 30% of community-acquired pneumonia (CAP), making them the most common “atypicals.” Clinically they are indistinguishable from other causes of pneumonia; most studies use cultures to identify cases among populations with CAP.
Azithromycin and erythromycin were compared in 3 studies of children with CAP.1-3 Together, they identified 69 cases due to M pneumoniae or C pneumoniae. Only 3 patients did not respond to either antibiotic. In the largest of the 3 studies,3 side effects were noted in 10% of CAP patients on azithromycin and 20% on erythromycin (P<.05).
Another study looked at patients aged 12 to 80 years with pneumonia due to M pneumoniae (75 cases) or Chlamydophila psittaci (formerly Chlamydia psittaci, 16 cases).4 All patients responded to treatment. Clarithromycin and erythromycin were compared in children aged 3 to 12 years with CAP.5 M pneumoniae or C pneumoniae was identified in 42 cases. Two of 18 patients did not respond to erythromycin; 3 of 27 patients did not respond to clarithromycin.
Another study compared these antibiotics for patients with CAP aged 12 to 93 years.6 Subgroup analysis of those with M pneumoniae or C pneumoniae (n=27) showed similar efficacy. Pooling all 268 patients with CAP, side effects were seen in 31% of patients on clarithromycin and 59% on erythromycin (P<.001).
A comparison study of newer macrolides in 40 adults with CAP identified 13 with M pneumoniae or C pneumoniae (Table).7 One patient did not respond of the 8 treated with clarithromycin; none among the 5 treated with azithromycin. There was 1 adverse event (from clarithromycin).
TABLE
Macrolides: comparison studies
| Antibiotic | Response rates* (%) | Side-effect rates †(%) | Cost for course of therapy in adult ‡ |
|---|---|---|---|
| Erythromycin1-4 | 77-100 | 10-59 | $11 (500 mg #40) |
| Clarithromycin5 7 | 88-94 | 5-31 | $76 (250 mg #20) |
| Azithromycin1 4,7 | 87-100 | 0-14 | $57 (250 mg #6) |
| *Response rates of pneumonia due to M pneumoniae and C pneumoniae. | |||
| † In community-acquired pneumonia treated with macrolide as single agent. | |||
| ‡ Prices from www.drugstore.com. | |||
Recommendations from others
The Infectious Diseases Society of America8 recommends a macrolide for adults with pneumonia caused by M pneumoniae or C pneumoniae, and does not promote one over another. The British Thoracic Society9 recommends any of the macrolides for pneumonia caused by these pathogens in children.
Since CAP is often caused by “atypical organisms,” macrolides are sometimes recommended as empiric outpatient therapy. In this setting, the American Thoracic Society10 discourages using erythromycin, citing a higher side-effect rate and poorer effectiveness against Haemophilus influenza. However, the Canadian Infectious Disease Society11 supports the use of any of the 3 macrolides in mild CAP except for patients with chronic obstructive pulmonary disease, who are more likely to harbor H influenza.
Lower respiratory infections—a number of problematic decisions
David Mouw, MD
Mountain Area AHEC, Asheville, NC
You face several problematic decisions when treating a patient with a lower respiratory infection. First, is this pneumonia or just bronchitis? Clinical findings can be confusing, and a chest film is helpful.12 If pneumonia is likely, you consider hospitalization, and prescribe antibiotics, usually without knowing the pathogen.
Because they cover both typical and atypical pathogens, macrolides (or doxycycline) are generally recommended, with cephalosporins to be added for higher-risk patients. (Quinolones are an alternative to this combination.) Finally, if you choose a macrolide, you face yet another decision without a clear answer: which one to use? All macrolides appear to be equally effective, so the choice depends on cost balanced against convenience and side effects.
1. Wubbel L, Muniz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J 1999;18:98-104.
2. Harris JS, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia. Pediatr Infect Dis J 1998;17:865-871.
3. Manfredi R, Jannuzzi C, Mantero E, et al. Clinical comparative study of azithromycin versus erythromycin in the treatment of acute respiratory tract infections in children. J Chemother 1992;4:364-370.
4. Schonwald S, Gunjaca M, Kolacny-Babic L, Car V, Gosev M. Comparison of azithromycin and erythromycin in the treatment of atypical pneumonias. J Antimicrob Chemother 1990;25(Suppl A):123-126.
5. Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J 1995;14:471-477.
6. Chien M, Pichotta P, Siepman N, Chan CK. Treatment of community-acquired pneumonia: a multicenter, double-blind, randomized study comparing clarithromycin with erythromycin. Canada-Sweden Clarithromycin-Pneumonia Study Group. Chest 1993;103-697-701.
7. Rizzato G, Montemurro L, Fraioli P, et al. Efficacy of a three day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J 1995;8:398-402.
8. Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine M. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis 2000;31:347-382.
9. British. Thoracic Society Standards of Care Committee. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Childhood. Thorax 2002;57(Suppl 1):i1-i24.
10. American. Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-1754.
11. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis 2000;31:383-421.
12. Kelsberg G, Safranek S. How accurate is the clinical diagnosis of pneumonia? J Fam Pract 2003;52:63-64.
1. Wubbel L, Muniz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J 1999;18:98-104.
2. Harris JS, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia. Pediatr Infect Dis J 1998;17:865-871.
3. Manfredi R, Jannuzzi C, Mantero E, et al. Clinical comparative study of azithromycin versus erythromycin in the treatment of acute respiratory tract infections in children. J Chemother 1992;4:364-370.
4. Schonwald S, Gunjaca M, Kolacny-Babic L, Car V, Gosev M. Comparison of azithromycin and erythromycin in the treatment of atypical pneumonias. J Antimicrob Chemother 1990;25(Suppl A):123-126.
5. Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J 1995;14:471-477.
6. Chien M, Pichotta P, Siepman N, Chan CK. Treatment of community-acquired pneumonia: a multicenter, double-blind, randomized study comparing clarithromycin with erythromycin. Canada-Sweden Clarithromycin-Pneumonia Study Group. Chest 1993;103-697-701.
7. Rizzato G, Montemurro L, Fraioli P, et al. Efficacy of a three day course of azithromycin in moderately severe community-acquired pneumonia. Eur Respir J 1995;8:398-402.
8. Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine M. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis 2000;31:347-382.
9. British. Thoracic Society Standards of Care Committee. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Childhood. Thorax 2002;57(Suppl 1):i1-i24.
10. American. Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-1754.
11. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community-Acquired Pneumonia Working Group. Clin Infect Dis 2000;31:383-421.
12. Kelsberg G, Safranek S. How accurate is the clinical diagnosis of pneumonia? J Fam Pract 2003;52:63-64.
Evidence-based answers from the Family Physicians Inquiries Network
When should patients with mitral valve prolapse get endocarditis prophylaxis?
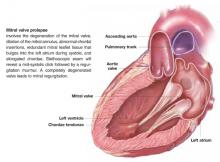
Patients with suspected mitral valve prolapse (MVP) ( Figure 1 ) should undergo echocardiography before any procedure that may place them at risk for bacteremia. Patients with MVP and documented absence of mitral regurgitation or valvular thickening likely do not need antibiotic prophylaxis against subacute bacterial endocarditis (SBE). Patients with MVP with documented mitral regurgitation, valvular thickening, or an unknown degree of valvular dysfunction may benefit from antibiotics during procedures that often lead to bacteremia (strength of recommendation: C).1
FIGURE 1 Mitral valve prolapse
Evidence summary
Only disease-oriented evidence and expert opinion address prevention for endocarditis. A randomized trial would require an estimated 6000 patients to demonstrate benefit.2
Endocarditis occurs in MVP at a rate of 0.1 cases/100 patient-years.3 However, MVP is the most common predisposing/precipitating cause of native valve endocarditis.4,5 In animal models, antibiotics prevent endocarditis following experimental bacteremia. The antibiotic can be administered either just before or up to 2 hours after the bacteremic event.2 It is worth noting that most bacteremia is not associated with medical procedures. Since endocarditis is often fatal, recommendations have been developed based on these animal models. Estimates of effectiveness of prophylaxis from case-control studies in humans (not limited to patients with MVP) estimate effectiveness from 49% to 91%.2
For patients with MVP who do not have evidence of mitral regurgitation on physical examination or echocardiography, the risk of morbidity may be greater from antibiotic therapy than the risk of endocarditis. Prophylaxis for these patients is not recommended. Patients with MVP associated with regurgitation are at moderate risk and may benefit from antibiotic prophylaxis.
Recommendations from others
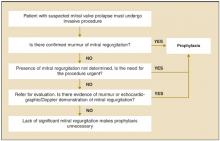
The American Heart Association has published recommendations in 1985,6 1990,7 and 1997.1 The 1997 recommendations are summarized in Figure 2 . The Swiss Working Group for Endocarditis Prophylaxis published similar recommendations in 2000.8 Recommended prophylactic regimens appear in Table 1. Table 2 shows a modified list of procedures for which prophylaxis is recommended.
FIGURE 2
Determining the need for antibiotic prophylaxis for patients with mitral valve prolapse
TABLE 1
Recommended prophylactic regimens for mitral valve prolaspe
| Situation | Medication | Dosage | |
|---|---|---|---|
| Dental, oral, respiratory, esophageal procedures | 1 hour before procedure | ||
| Standard prophylaxis | Amoxicillin | Adult:2 g | Child: 50 mg/kg |
| Allergy to penicillin | Clindamycin | Adult: 600 mg | Child: 20 mg/kg |
| Cephalexin | Adult: 2 g | Child: 50 mg/kg | |
| Azithromycin | Adult: 500 mg | Child: 15 mg/kg | |
| Genitourinary or non-esophageal gastrointestinal procedures | |||
| Moderate-risk patients | Amoxicillin | Adult: 2 g | Child: 50 mg/kg |
| 1 hour before procedure | |||
| Moderate-risk patients allergic to penicillin | Vancomycin | Adult: 1 g IV | Child: 20 mg/kg IV |
| Administer over 1-2 hrs; complete 30 minutes before procedure | |||
| High-risk patients | Add gentamicin to amoxicillin or vancomycin | 1.5 mg/kg (up to 120 mg) IV to be completed 30 minutes before procedure. If not allergic to penicillin, give penicillin give penicillin, give amoxicillin 1 g 6 hours after | |
| Modified from Dajani 1997.1 | |||
TABLE 2
Procedures for which endocarditis prophylaxis is, or is not, recommended
| Endocarditis prophylaxis recommended |
| Respiratory tract |
| Tonsillectomy or adenoidectomy |
| Surgical operations that involve respiratory mucosa |
| Bronchoscopy with a rigid bronchoscope |
| Gastrointestinal tract |
| Sclerotherapy for esophageal varices |
| Esophageal stricture dilation |
| Endoscopic retrograde cholangiography with biliary obstruction |
| Biliary tract surgery |
| Surgical operations that involve intestinal mucosa |
| Genitourinary tract |
| Prostatic surgery |
| Cystoscopy |
| Urethral dilation |
| Endocarditis prophylaxis not recommended |
| Respiratory tract |
| Endotracheal intubation |
| Flexible bronchoscopy, with or without biopsy |
| Tympanostomy tube insertion |
| Gastrointestinal tract |
| Endoscopy with or without gastrointestinal biopsy |
| Genitourinary tract |
| Circumcision |
| Vaginal hysterectomy |
| Vaginal delivery |
| Cesarean section |
| In uninfected tissue |
| Incision or biopsy of surgically scrubbed skin |
| Urethral catheterization |
| Uterine dilatation and curettage |
| Therapeutic abortion |
| Sterilization procedures |
| Insertion or removal of intrauterine devices |
| Cardiac |
| Transesophageal echocardiography |
| Cardiac catheterization, including balloon angioplasty and coronary stents |
| Implanted cardiac pacemakers, implanted defibrillators |
| Modified from Dajani et al, 1997.1 |
Guidelines assist decision-making regarding who needs SBE prophylaxis
David M. Bercaw, MD
Christiana Care Health Systems, Wilmington, Del
It is unfortunate, but not surprising, that the evidence for SBE prophylaxis for patients with MVP is disease-oriented evidence and expert opinion. Too often, the easy thing to do in a busy practice is not necessarily in the best interest of either the patient or the public. However—despite the low incidence of SBE—the high mortality of the disease and community standard of care often drive clinicians to write that prescription for antibiotics.
With the improved resolution and sensitivity of newer generations of echocardiograms, clinicians often face the dilemma of the patient with MVP and “trivial” or “mnimal” mitral regurgitation. Unfortunately, no guidelines assist us in our decision-making regarding these patients. Another consideration for the clinician is the American Heart Association’s recommendation for SBE prophylaxis for patients with MVP and thickened leaflets, regardless of whether there is associated mitral valve regurgitation.
One significant change that should lessen the frequency of unnecessary antibiotic prescribing was published recently. The echocardiographic criteria for diagnosing MVP were changed in the 2003 updated guidelines from the American College of Cardiology, American Heart Association, and American Society of Echocardiography. Valve prolapse of 2 mm or more above the mitral annulus is required for diagnosis.10 This change has effectively lowered the prevalence of MVP from 4% to 8% of the general population down to 2% to 3%.
1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-1801.
2. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44.
3. Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol 1995;75:1028-1032.
4. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol 1991;68:90-94.
5. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681-688.
6. Shulman ST, Amren DP, Bisno AL, et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am J Dis Child 1985;139:232-235.
7. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-2922.
8. Moreillon P. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Swiss Working Group for Endocarditis Prophylaxis. Schweiz Med Wochenschr 2000;130:1013-1026.
9. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Am Coll Cardiol. 2003;42:954-970.
Patients with suspected mitral valve prolapse (MVP) ( Figure 1 ) should undergo echocardiography before any procedure that may place them at risk for bacteremia. Patients with MVP and documented absence of mitral regurgitation or valvular thickening likely do not need antibiotic prophylaxis against subacute bacterial endocarditis (SBE). Patients with MVP with documented mitral regurgitation, valvular thickening, or an unknown degree of valvular dysfunction may benefit from antibiotics during procedures that often lead to bacteremia (strength of recommendation: C).1
FIGURE 1 Mitral valve prolapse
Evidence summary
Only disease-oriented evidence and expert opinion address prevention for endocarditis. A randomized trial would require an estimated 6000 patients to demonstrate benefit.2
Endocarditis occurs in MVP at a rate of 0.1 cases/100 patient-years.3 However, MVP is the most common predisposing/precipitating cause of native valve endocarditis.4,5 In animal models, antibiotics prevent endocarditis following experimental bacteremia. The antibiotic can be administered either just before or up to 2 hours after the bacteremic event.2 It is worth noting that most bacteremia is not associated with medical procedures. Since endocarditis is often fatal, recommendations have been developed based on these animal models. Estimates of effectiveness of prophylaxis from case-control studies in humans (not limited to patients with MVP) estimate effectiveness from 49% to 91%.2
For patients with MVP who do not have evidence of mitral regurgitation on physical examination or echocardiography, the risk of morbidity may be greater from antibiotic therapy than the risk of endocarditis. Prophylaxis for these patients is not recommended. Patients with MVP associated with regurgitation are at moderate risk and may benefit from antibiotic prophylaxis.
Recommendations from others
The American Heart Association has published recommendations in 1985,6 1990,7 and 1997.1 The 1997 recommendations are summarized in Figure 2 . The Swiss Working Group for Endocarditis Prophylaxis published similar recommendations in 2000.8 Recommended prophylactic regimens appear in Table 1. Table 2 shows a modified list of procedures for which prophylaxis is recommended.
FIGURE 2
Determining the need for antibiotic prophylaxis for patients with mitral valve prolapse
TABLE 1
Recommended prophylactic regimens for mitral valve prolaspe
| Situation | Medication | Dosage | |
|---|---|---|---|
| Dental, oral, respiratory, esophageal procedures | 1 hour before procedure | ||
| Standard prophylaxis | Amoxicillin | Adult:2 g | Child: 50 mg/kg |
| Allergy to penicillin | Clindamycin | Adult: 600 mg | Child: 20 mg/kg |
| Cephalexin | Adult: 2 g | Child: 50 mg/kg | |
| Azithromycin | Adult: 500 mg | Child: 15 mg/kg | |
| Genitourinary or non-esophageal gastrointestinal procedures | |||
| Moderate-risk patients | Amoxicillin | Adult: 2 g | Child: 50 mg/kg |
| 1 hour before procedure | |||
| Moderate-risk patients allergic to penicillin | Vancomycin | Adult: 1 g IV | Child: 20 mg/kg IV |
| Administer over 1-2 hrs; complete 30 minutes before procedure | |||
| High-risk patients | Add gentamicin to amoxicillin or vancomycin | 1.5 mg/kg (up to 120 mg) IV to be completed 30 minutes before procedure. If not allergic to penicillin, give penicillin give penicillin, give amoxicillin 1 g 6 hours after | |
| Modified from Dajani 1997.1 | |||
TABLE 2
Procedures for which endocarditis prophylaxis is, or is not, recommended
| Endocarditis prophylaxis recommended |
| Respiratory tract |
| Tonsillectomy or adenoidectomy |
| Surgical operations that involve respiratory mucosa |
| Bronchoscopy with a rigid bronchoscope |
| Gastrointestinal tract |
| Sclerotherapy for esophageal varices |
| Esophageal stricture dilation |
| Endoscopic retrograde cholangiography with biliary obstruction |
| Biliary tract surgery |
| Surgical operations that involve intestinal mucosa |
| Genitourinary tract |
| Prostatic surgery |
| Cystoscopy |
| Urethral dilation |
| Endocarditis prophylaxis not recommended |
| Respiratory tract |
| Endotracheal intubation |
| Flexible bronchoscopy, with or without biopsy |
| Tympanostomy tube insertion |
| Gastrointestinal tract |
| Endoscopy with or without gastrointestinal biopsy |
| Genitourinary tract |
| Circumcision |
| Vaginal hysterectomy |
| Vaginal delivery |
| Cesarean section |
| In uninfected tissue |
| Incision or biopsy of surgically scrubbed skin |
| Urethral catheterization |
| Uterine dilatation and curettage |
| Therapeutic abortion |
| Sterilization procedures |
| Insertion or removal of intrauterine devices |
| Cardiac |
| Transesophageal echocardiography |
| Cardiac catheterization, including balloon angioplasty and coronary stents |
| Implanted cardiac pacemakers, implanted defibrillators |
| Modified from Dajani et al, 1997.1 |
Guidelines assist decision-making regarding who needs SBE prophylaxis
David M. Bercaw, MD
Christiana Care Health Systems, Wilmington, Del
It is unfortunate, but not surprising, that the evidence for SBE prophylaxis for patients with MVP is disease-oriented evidence and expert opinion. Too often, the easy thing to do in a busy practice is not necessarily in the best interest of either the patient or the public. However—despite the low incidence of SBE—the high mortality of the disease and community standard of care often drive clinicians to write that prescription for antibiotics.
With the improved resolution and sensitivity of newer generations of echocardiograms, clinicians often face the dilemma of the patient with MVP and “trivial” or “mnimal” mitral regurgitation. Unfortunately, no guidelines assist us in our decision-making regarding these patients. Another consideration for the clinician is the American Heart Association’s recommendation for SBE prophylaxis for patients with MVP and thickened leaflets, regardless of whether there is associated mitral valve regurgitation.
One significant change that should lessen the frequency of unnecessary antibiotic prescribing was published recently. The echocardiographic criteria for diagnosing MVP were changed in the 2003 updated guidelines from the American College of Cardiology, American Heart Association, and American Society of Echocardiography. Valve prolapse of 2 mm or more above the mitral annulus is required for diagnosis.10 This change has effectively lowered the prevalence of MVP from 4% to 8% of the general population down to 2% to 3%.
Patients with suspected mitral valve prolapse (MVP) ( Figure 1 ) should undergo echocardiography before any procedure that may place them at risk for bacteremia. Patients with MVP and documented absence of mitral regurgitation or valvular thickening likely do not need antibiotic prophylaxis against subacute bacterial endocarditis (SBE). Patients with MVP with documented mitral regurgitation, valvular thickening, or an unknown degree of valvular dysfunction may benefit from antibiotics during procedures that often lead to bacteremia (strength of recommendation: C).1
FIGURE 1 Mitral valve prolapse
Evidence summary
Only disease-oriented evidence and expert opinion address prevention for endocarditis. A randomized trial would require an estimated 6000 patients to demonstrate benefit.2
Endocarditis occurs in MVP at a rate of 0.1 cases/100 patient-years.3 However, MVP is the most common predisposing/precipitating cause of native valve endocarditis.4,5 In animal models, antibiotics prevent endocarditis following experimental bacteremia. The antibiotic can be administered either just before or up to 2 hours after the bacteremic event.2 It is worth noting that most bacteremia is not associated with medical procedures. Since endocarditis is often fatal, recommendations have been developed based on these animal models. Estimates of effectiveness of prophylaxis from case-control studies in humans (not limited to patients with MVP) estimate effectiveness from 49% to 91%.2
For patients with MVP who do not have evidence of mitral regurgitation on physical examination or echocardiography, the risk of morbidity may be greater from antibiotic therapy than the risk of endocarditis. Prophylaxis for these patients is not recommended. Patients with MVP associated with regurgitation are at moderate risk and may benefit from antibiotic prophylaxis.
Recommendations from others
The American Heart Association has published recommendations in 1985,6 1990,7 and 1997.1 The 1997 recommendations are summarized in Figure 2 . The Swiss Working Group for Endocarditis Prophylaxis published similar recommendations in 2000.8 Recommended prophylactic regimens appear in Table 1. Table 2 shows a modified list of procedures for which prophylaxis is recommended.
FIGURE 2
Determining the need for antibiotic prophylaxis for patients with mitral valve prolapse
TABLE 1
Recommended prophylactic regimens for mitral valve prolaspe
| Situation | Medication | Dosage | |
|---|---|---|---|
| Dental, oral, respiratory, esophageal procedures | 1 hour before procedure | ||
| Standard prophylaxis | Amoxicillin | Adult:2 g | Child: 50 mg/kg |
| Allergy to penicillin | Clindamycin | Adult: 600 mg | Child: 20 mg/kg |
| Cephalexin | Adult: 2 g | Child: 50 mg/kg | |
| Azithromycin | Adult: 500 mg | Child: 15 mg/kg | |
| Genitourinary or non-esophageal gastrointestinal procedures | |||
| Moderate-risk patients | Amoxicillin | Adult: 2 g | Child: 50 mg/kg |
| 1 hour before procedure | |||
| Moderate-risk patients allergic to penicillin | Vancomycin | Adult: 1 g IV | Child: 20 mg/kg IV |
| Administer over 1-2 hrs; complete 30 minutes before procedure | |||
| High-risk patients | Add gentamicin to amoxicillin or vancomycin | 1.5 mg/kg (up to 120 mg) IV to be completed 30 minutes before procedure. If not allergic to penicillin, give penicillin give penicillin, give amoxicillin 1 g 6 hours after | |
| Modified from Dajani 1997.1 | |||
TABLE 2
Procedures for which endocarditis prophylaxis is, or is not, recommended
| Endocarditis prophylaxis recommended |
| Respiratory tract |
| Tonsillectomy or adenoidectomy |
| Surgical operations that involve respiratory mucosa |
| Bronchoscopy with a rigid bronchoscope |
| Gastrointestinal tract |
| Sclerotherapy for esophageal varices |
| Esophageal stricture dilation |
| Endoscopic retrograde cholangiography with biliary obstruction |
| Biliary tract surgery |
| Surgical operations that involve intestinal mucosa |
| Genitourinary tract |
| Prostatic surgery |
| Cystoscopy |
| Urethral dilation |
| Endocarditis prophylaxis not recommended |
| Respiratory tract |
| Endotracheal intubation |
| Flexible bronchoscopy, with or without biopsy |
| Tympanostomy tube insertion |
| Gastrointestinal tract |
| Endoscopy with or without gastrointestinal biopsy |
| Genitourinary tract |
| Circumcision |
| Vaginal hysterectomy |
| Vaginal delivery |
| Cesarean section |
| In uninfected tissue |
| Incision or biopsy of surgically scrubbed skin |
| Urethral catheterization |
| Uterine dilatation and curettage |
| Therapeutic abortion |
| Sterilization procedures |
| Insertion or removal of intrauterine devices |
| Cardiac |
| Transesophageal echocardiography |
| Cardiac catheterization, including balloon angioplasty and coronary stents |
| Implanted cardiac pacemakers, implanted defibrillators |
| Modified from Dajani et al, 1997.1 |
Guidelines assist decision-making regarding who needs SBE prophylaxis
David M. Bercaw, MD
Christiana Care Health Systems, Wilmington, Del
It is unfortunate, but not surprising, that the evidence for SBE prophylaxis for patients with MVP is disease-oriented evidence and expert opinion. Too often, the easy thing to do in a busy practice is not necessarily in the best interest of either the patient or the public. However—despite the low incidence of SBE—the high mortality of the disease and community standard of care often drive clinicians to write that prescription for antibiotics.
With the improved resolution and sensitivity of newer generations of echocardiograms, clinicians often face the dilemma of the patient with MVP and “trivial” or “mnimal” mitral regurgitation. Unfortunately, no guidelines assist us in our decision-making regarding these patients. Another consideration for the clinician is the American Heart Association’s recommendation for SBE prophylaxis for patients with MVP and thickened leaflets, regardless of whether there is associated mitral valve regurgitation.
One significant change that should lessen the frequency of unnecessary antibiotic prescribing was published recently. The echocardiographic criteria for diagnosing MVP were changed in the 2003 updated guidelines from the American College of Cardiology, American Heart Association, and American Society of Echocardiography. Valve prolapse of 2 mm or more above the mitral annulus is required for diagnosis.10 This change has effectively lowered the prevalence of MVP from 4% to 8% of the general population down to 2% to 3%.
1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-1801.
2. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44.
3. Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol 1995;75:1028-1032.
4. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol 1991;68:90-94.
5. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681-688.
6. Shulman ST, Amren DP, Bisno AL, et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am J Dis Child 1985;139:232-235.
7. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-2922.
8. Moreillon P. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Swiss Working Group for Endocarditis Prophylaxis. Schweiz Med Wochenschr 2000;130:1013-1026.
9. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Am Coll Cardiol. 2003;42:954-970.
1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-1801.
2. Durack DT. Prevention of infective endocarditis. N Engl J Med 1995;332:38-44.
3. Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol 1995;75:1028-1032.
4. Awadallah SM, Kavey RE, Byrum CJ, Smith FC, Kveselis DA, Blackman MS. The changing pattern of infective endocarditis in childhood. Am J Cardiol 1991;68:90-94.
5. McKinsey DS, Ratts TE, Bisno AL. Underlying cardiac lesions in adults with infective endocarditis. The changing spectrum. Am J Med 1987;82:681-688.
6. Shulman ST, Amren DP, Bisno AL, et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am J Dis Child 1985;139:232-235.
7. Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-2922.
8. Moreillon P. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Swiss Working Group for Endocarditis Prophylaxis. Schweiz Med Wochenschr 2000;130:1013-1026.
9. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Am Coll Cardiol. 2003;42:954-970.
Evidence-based answers from the Family Physicians Inquiries Network
Which healthy adults should take aspirin?
In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal myocardial infarction (MI). Aspirin prophylaxis does not decrease all-cause mortality, risk of fatal coronary heart disease, or risk of first stroke (strength of recommendation [SOR]: A–, based on multiple randomized controlled trials).
The benefits of aspirin use must be weighed against its potential risks, primarily gastrointestinal bleeding and cerebral hemorrhage. The benefit of aspirin increases with higher levels of cardiovascular risk, while the potential for harm remains relatively constant. Adults with a calculated 5-year coronary heart disease (CHD) event risk of 3% or greater should receive prophylaxis (SOR: A, based on multiple randomized controlled trials). The ideal dose of aspirin for prophylaxis is unknown, but it appears that low doses (75–81 mg/d) are as effective as higher doses.
Evidence summary
The leading cause of morbidity and mortality in the United States is cardiovascular disease (ischemic CHD, stroke, peripheral vascular disease).1 A meta-analysis of 5 placebo-controlled randomized controlled trials involving more than 50,000 patients free of CHD and stroke evaluated aspirin for primary prevention of cardiovascular disease. Since 3 of the trials excluded women, only 20% of the participants were female. The mean age of participants was 57 years.
The treatment groups took aspirin 75 to 500 mg/d for 3 to 7 years. The meta-analysis found that compared with placebo, aspirin significantly reduced total CHD events (odds ratio [OR]=0.72; 95% confidence interval [CI], 0.60–0.87).2 Aspirin did not reduce coronary disease mortality (OR=0.87; 95% CI, 0.70–1.09); however, results from 1 study did achieve statistical significance (OR=0.64; 95% CI, 0.42–0.99).3 No differences were found between aspirin-treated and control groups for all-cause mortality or ischemic stroke reduction.
Aspirin increased the risk of major gastrointestinal bleeding events by almost twofold (OR=1.70; 95% CI, 1.4–2.1). Three of the 5 trials showed no significant increase of intracranial hemorrhage event rates (OR=1.4; 95% CI, 0.9–2.0). Based on combined primary and secondary prevention trials, the risk of intracranial bleeding with aspirin is estimated at 0 to 2 events per 1000 patients per year.2
Although the ideal aspirin dosage is uncertain, lower dosages (75–81 mg/d) have been shown to be as beneficial as higher dosages, and may have fewer bleeding complications. Buffered and entericcoated formulations are no more protective than plain aspirin.4
In patients with no known cardiovascular disease, aspirin chemoprevention has been shown to decrease the risk of nonfatal MI and fatal CHD by 28%. At a 5-year CHD risk of 3%, the benefits of prophylaxis outweigh the harms (see Table ) by 2 to 1—assuming the events of stroke, MI, and bleeding are considered roughly equivalent in severity. (A different threshold may be appropriate for patients that perceive 1 of these events as significantly more serious than the others.) Typical patients at a 3% or greater risk for cardiovascular disease include men aged >40 years, post-menopausal women, and younger persons with risk factors for CHD. Physicians determine cardiovascular risk from the presence and severity of risk factors: gender, age, blood pressure, lipid status, diabetes, and smoking status.
Simple risk-assessment tools based on Framingham data are available for computers and palmtop devices (eg, Heart to Heart CV Risk Assessment Calculator, www.meddecisions.com; National Institutes of Health, www.nhlbi.nih.gov/health/prof/heart/). Because only 2 trials included women, it is less clear whether both sexes benefit equally from aspirin prophylaxis.1
TABLE
Net benefits and harms of aspirin prophylaxis, per 1000 patients
| Outcome | Estimated 5-year risk for CHD event | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| All-cause mortality | NS | NS | NS |
| CHD events avoided | 3 | 8 | 14 |
| Ischemic strokes avoided | NS | NS | NS |
| Hemorrhagic strokes | 1 | 1 | 1 |
| Major gastrointestinal bleeding | 3 | 3 | 3 |
| NS, not significant | |||
Recommendations from others
The US Preventive Services Task Force recommends that clinicians discuss aspirin prophylaxis with adults at increased risk for CHD (defined as a 5-year risk of 3% or more). Discussion should include the potential benefits and harms of aspirin therapy.5
The American Heart Association recommends low-dose aspirin in people at higher risk of coronary heart disease (especially those with a 10-year CHD risk of 10% or greater).6 The European Society of Cardiology says there is evidence that low-dose aspirin can reduce the risk of cardiovascular events in asymptomatic high-risk people, such as those with diabetes or well-controlled hypertension, and in men at high multifactorial risk of cardiovascular disease.7
Aspirin: effective, safe, inexpensive—and it may prevent heart attacks
Paul V. Aitken, Jr, MD, MPH
Residency in Family Medicine, University of North Carolina at Chapel Hill; New Hanover Regional Medical Center, Wilmington, NC
Acetylsalicylic acid was first compounded in Germany by chemist Felix Hoffman in 1897. According to information from the Bayer Company, aspirin’s cardioprotective effect was first recognized by Dr Lawrence Craven, a California general practitioner. He noted a decreased rate of heart attacks in patients taking this medication.
We now have evidence supporting Dr Craven’s astute clinical observation. In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal MI. For an individual at a 5-year CHD risk as low as 3%, the benefits of prophylaxis outweigh the harms. The leading cause of morbidity and mortality in the US is still cardiovascular disease. A simple, effective, safe, and inexpensive preventive measure like recommending aspirin has the potential to prevent heart attacks on a grand scale. A low-dose aspirin per day should be recommended for patients at risk for cardiovascular disease, including men aged >40 years, postmenopausal women, and younger persons with risk factors for CHD. As a 40-something male with a family history of cardiovascular disease reviewing this Clinical Inquiry, I will be taking my aspirin a day.
1. Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1-113.
2. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:161-172.
3. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-135.
4. Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events; meta-analysis and hypotheses. Arch Neurol 2000;57:326-332.
5. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: chemoprevention. January 2002. Available at www.ahrq.gov/clinic/uspstf/uspsasmi.htm. Accessed on January 6, 2004.
6. American Heart Association. Primary prevention in the adult. 2003. Available at: www.americanheart.org/presen-ter.jhtml?identifier=4704. Accessed on January 6, 2004.
7. De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal myocardial infarction (MI). Aspirin prophylaxis does not decrease all-cause mortality, risk of fatal coronary heart disease, or risk of first stroke (strength of recommendation [SOR]: A–, based on multiple randomized controlled trials).
The benefits of aspirin use must be weighed against its potential risks, primarily gastrointestinal bleeding and cerebral hemorrhage. The benefit of aspirin increases with higher levels of cardiovascular risk, while the potential for harm remains relatively constant. Adults with a calculated 5-year coronary heart disease (CHD) event risk of 3% or greater should receive prophylaxis (SOR: A, based on multiple randomized controlled trials). The ideal dose of aspirin for prophylaxis is unknown, but it appears that low doses (75–81 mg/d) are as effective as higher doses.
Evidence summary
The leading cause of morbidity and mortality in the United States is cardiovascular disease (ischemic CHD, stroke, peripheral vascular disease).1 A meta-analysis of 5 placebo-controlled randomized controlled trials involving more than 50,000 patients free of CHD and stroke evaluated aspirin for primary prevention of cardiovascular disease. Since 3 of the trials excluded women, only 20% of the participants were female. The mean age of participants was 57 years.
The treatment groups took aspirin 75 to 500 mg/d for 3 to 7 years. The meta-analysis found that compared with placebo, aspirin significantly reduced total CHD events (odds ratio [OR]=0.72; 95% confidence interval [CI], 0.60–0.87).2 Aspirin did not reduce coronary disease mortality (OR=0.87; 95% CI, 0.70–1.09); however, results from 1 study did achieve statistical significance (OR=0.64; 95% CI, 0.42–0.99).3 No differences were found between aspirin-treated and control groups for all-cause mortality or ischemic stroke reduction.
Aspirin increased the risk of major gastrointestinal bleeding events by almost twofold (OR=1.70; 95% CI, 1.4–2.1). Three of the 5 trials showed no significant increase of intracranial hemorrhage event rates (OR=1.4; 95% CI, 0.9–2.0). Based on combined primary and secondary prevention trials, the risk of intracranial bleeding with aspirin is estimated at 0 to 2 events per 1000 patients per year.2
Although the ideal aspirin dosage is uncertain, lower dosages (75–81 mg/d) have been shown to be as beneficial as higher dosages, and may have fewer bleeding complications. Buffered and entericcoated formulations are no more protective than plain aspirin.4
In patients with no known cardiovascular disease, aspirin chemoprevention has been shown to decrease the risk of nonfatal MI and fatal CHD by 28%. At a 5-year CHD risk of 3%, the benefits of prophylaxis outweigh the harms (see Table ) by 2 to 1—assuming the events of stroke, MI, and bleeding are considered roughly equivalent in severity. (A different threshold may be appropriate for patients that perceive 1 of these events as significantly more serious than the others.) Typical patients at a 3% or greater risk for cardiovascular disease include men aged >40 years, post-menopausal women, and younger persons with risk factors for CHD. Physicians determine cardiovascular risk from the presence and severity of risk factors: gender, age, blood pressure, lipid status, diabetes, and smoking status.
Simple risk-assessment tools based on Framingham data are available for computers and palmtop devices (eg, Heart to Heart CV Risk Assessment Calculator, www.meddecisions.com; National Institutes of Health, www.nhlbi.nih.gov/health/prof/heart/). Because only 2 trials included women, it is less clear whether both sexes benefit equally from aspirin prophylaxis.1
TABLE
Net benefits and harms of aspirin prophylaxis, per 1000 patients
| Outcome | Estimated 5-year risk for CHD event | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| All-cause mortality | NS | NS | NS |
| CHD events avoided | 3 | 8 | 14 |
| Ischemic strokes avoided | NS | NS | NS |
| Hemorrhagic strokes | 1 | 1 | 1 |
| Major gastrointestinal bleeding | 3 | 3 | 3 |
| NS, not significant | |||
Recommendations from others
The US Preventive Services Task Force recommends that clinicians discuss aspirin prophylaxis with adults at increased risk for CHD (defined as a 5-year risk of 3% or more). Discussion should include the potential benefits and harms of aspirin therapy.5
The American Heart Association recommends low-dose aspirin in people at higher risk of coronary heart disease (especially those with a 10-year CHD risk of 10% or greater).6 The European Society of Cardiology says there is evidence that low-dose aspirin can reduce the risk of cardiovascular events in asymptomatic high-risk people, such as those with diabetes or well-controlled hypertension, and in men at high multifactorial risk of cardiovascular disease.7
Aspirin: effective, safe, inexpensive—and it may prevent heart attacks
Paul V. Aitken, Jr, MD, MPH
Residency in Family Medicine, University of North Carolina at Chapel Hill; New Hanover Regional Medical Center, Wilmington, NC
Acetylsalicylic acid was first compounded in Germany by chemist Felix Hoffman in 1897. According to information from the Bayer Company, aspirin’s cardioprotective effect was first recognized by Dr Lawrence Craven, a California general practitioner. He noted a decreased rate of heart attacks in patients taking this medication.
We now have evidence supporting Dr Craven’s astute clinical observation. In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal MI. For an individual at a 5-year CHD risk as low as 3%, the benefits of prophylaxis outweigh the harms. The leading cause of morbidity and mortality in the US is still cardiovascular disease. A simple, effective, safe, and inexpensive preventive measure like recommending aspirin has the potential to prevent heart attacks on a grand scale. A low-dose aspirin per day should be recommended for patients at risk for cardiovascular disease, including men aged >40 years, postmenopausal women, and younger persons with risk factors for CHD. As a 40-something male with a family history of cardiovascular disease reviewing this Clinical Inquiry, I will be taking my aspirin a day.
In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal myocardial infarction (MI). Aspirin prophylaxis does not decrease all-cause mortality, risk of fatal coronary heart disease, or risk of first stroke (strength of recommendation [SOR]: A–, based on multiple randomized controlled trials).
The benefits of aspirin use must be weighed against its potential risks, primarily gastrointestinal bleeding and cerebral hemorrhage. The benefit of aspirin increases with higher levels of cardiovascular risk, while the potential for harm remains relatively constant. Adults with a calculated 5-year coronary heart disease (CHD) event risk of 3% or greater should receive prophylaxis (SOR: A, based on multiple randomized controlled trials). The ideal dose of aspirin for prophylaxis is unknown, but it appears that low doses (75–81 mg/d) are as effective as higher doses.
Evidence summary
The leading cause of morbidity and mortality in the United States is cardiovascular disease (ischemic CHD, stroke, peripheral vascular disease).1 A meta-analysis of 5 placebo-controlled randomized controlled trials involving more than 50,000 patients free of CHD and stroke evaluated aspirin for primary prevention of cardiovascular disease. Since 3 of the trials excluded women, only 20% of the participants were female. The mean age of participants was 57 years.
The treatment groups took aspirin 75 to 500 mg/d for 3 to 7 years. The meta-analysis found that compared with placebo, aspirin significantly reduced total CHD events (odds ratio [OR]=0.72; 95% confidence interval [CI], 0.60–0.87).2 Aspirin did not reduce coronary disease mortality (OR=0.87; 95% CI, 0.70–1.09); however, results from 1 study did achieve statistical significance (OR=0.64; 95% CI, 0.42–0.99).3 No differences were found between aspirin-treated and control groups for all-cause mortality or ischemic stroke reduction.
Aspirin increased the risk of major gastrointestinal bleeding events by almost twofold (OR=1.70; 95% CI, 1.4–2.1). Three of the 5 trials showed no significant increase of intracranial hemorrhage event rates (OR=1.4; 95% CI, 0.9–2.0). Based on combined primary and secondary prevention trials, the risk of intracranial bleeding with aspirin is estimated at 0 to 2 events per 1000 patients per year.2
Although the ideal aspirin dosage is uncertain, lower dosages (75–81 mg/d) have been shown to be as beneficial as higher dosages, and may have fewer bleeding complications. Buffered and entericcoated formulations are no more protective than plain aspirin.4
In patients with no known cardiovascular disease, aspirin chemoprevention has been shown to decrease the risk of nonfatal MI and fatal CHD by 28%. At a 5-year CHD risk of 3%, the benefits of prophylaxis outweigh the harms (see Table ) by 2 to 1—assuming the events of stroke, MI, and bleeding are considered roughly equivalent in severity. (A different threshold may be appropriate for patients that perceive 1 of these events as significantly more serious than the others.) Typical patients at a 3% or greater risk for cardiovascular disease include men aged >40 years, post-menopausal women, and younger persons with risk factors for CHD. Physicians determine cardiovascular risk from the presence and severity of risk factors: gender, age, blood pressure, lipid status, diabetes, and smoking status.
Simple risk-assessment tools based on Framingham data are available for computers and palmtop devices (eg, Heart to Heart CV Risk Assessment Calculator, www.meddecisions.com; National Institutes of Health, www.nhlbi.nih.gov/health/prof/heart/). Because only 2 trials included women, it is less clear whether both sexes benefit equally from aspirin prophylaxis.1
TABLE
Net benefits and harms of aspirin prophylaxis, per 1000 patients
| Outcome | Estimated 5-year risk for CHD event | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| All-cause mortality | NS | NS | NS |
| CHD events avoided | 3 | 8 | 14 |
| Ischemic strokes avoided | NS | NS | NS |
| Hemorrhagic strokes | 1 | 1 | 1 |
| Major gastrointestinal bleeding | 3 | 3 | 3 |
| NS, not significant | |||
Recommendations from others
The US Preventive Services Task Force recommends that clinicians discuss aspirin prophylaxis with adults at increased risk for CHD (defined as a 5-year risk of 3% or more). Discussion should include the potential benefits and harms of aspirin therapy.5
The American Heart Association recommends low-dose aspirin in people at higher risk of coronary heart disease (especially those with a 10-year CHD risk of 10% or greater).6 The European Society of Cardiology says there is evidence that low-dose aspirin can reduce the risk of cardiovascular events in asymptomatic high-risk people, such as those with diabetes or well-controlled hypertension, and in men at high multifactorial risk of cardiovascular disease.7
Aspirin: effective, safe, inexpensive—and it may prevent heart attacks
Paul V. Aitken, Jr, MD, MPH
Residency in Family Medicine, University of North Carolina at Chapel Hill; New Hanover Regional Medical Center, Wilmington, NC
Acetylsalicylic acid was first compounded in Germany by chemist Felix Hoffman in 1897. According to information from the Bayer Company, aspirin’s cardioprotective effect was first recognized by Dr Lawrence Craven, a California general practitioner. He noted a decreased rate of heart attacks in patients taking this medication.
We now have evidence supporting Dr Craven’s astute clinical observation. In adults with no history of cardiovascular disease, aspirin reduces the risk of nonfatal MI. For an individual at a 5-year CHD risk as low as 3%, the benefits of prophylaxis outweigh the harms. The leading cause of morbidity and mortality in the US is still cardiovascular disease. A simple, effective, safe, and inexpensive preventive measure like recommending aspirin has the potential to prevent heart attacks on a grand scale. A low-dose aspirin per day should be recommended for patients at risk for cardiovascular disease, including men aged >40 years, postmenopausal women, and younger persons with risk factors for CHD. As a 40-something male with a family history of cardiovascular disease reviewing this Clinical Inquiry, I will be taking my aspirin a day.
1. Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1-113.
2. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:161-172.
3. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-135.
4. Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events; meta-analysis and hypotheses. Arch Neurol 2000;57:326-332.
5. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: chemoprevention. January 2002. Available at www.ahrq.gov/clinic/uspstf/uspsasmi.htm. Accessed on January 6, 2004.
6. American Heart Association. Primary prevention in the adult. 2003. Available at: www.americanheart.org/presen-ter.jhtml?identifier=4704. Accessed on January 6, 2004.
7. De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
1. Hoyert DL, Arias E, Smith BL, Murphy SL, Kochanek KD. Deaths: final data for 1999. Natl Vital Stat Rep. 2001;49:1-113.
2. Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;136:161-172.
3. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989;321:129-135.
4. Hart RG, Halperin JL, McBride R, Benavente O, Man-Son-Hing M, Kronmal RA. Aspirin for the primary prevention of stroke and other major vascular events; meta-analysis and hypotheses. Arch Neurol 2000;57:326-332.
5. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: chemoprevention. January 2002. Available at www.ahrq.gov/clinic/uspstf/uspsasmi.htm. Accessed on January 6, 2004.
6. American Heart Association. Primary prevention in the adult. 2003. Available at: www.americanheart.org/presen-ter.jhtml?identifier=4704. Accessed on January 6, 2004.
7. De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for Osgood-Schlatter disease?
Osgood-Schlatter disease is a common cause of pain and tenderness at the tibial tuberosity in active adolescents. It is typically a self-limited condition that waxes and wanes, but which often takes months to years to resolve entirely. It is best managed with conservative measures (activity modification, ice, anti-inflammatory agents) and time (strength of recommendation [SOR]: B, several case series and retrospective studies).
In chronic cases that are refractory to conservative treatment, surgical intervention yields good results, particularly for patients with bony or cartilaginous ossicles. Excision of these ossicles produces resolution of symptoms and return to activity in several weeks (SOR: C, several case series). Corticosteroid injections are not recommended (SOR: C, case reports and expert opinion).
Evidence summary
No prospective, interventional studies evaluate the treatment of Osgood-Schlatter disease. One case series followed the natural course of the disease in 261 patients (365 symptomatic knees) for 12 to 24 months; 237 (90.8%) patients responded well to restriction of sports activity and nonsteroidal anti-inflammatory agents. The 24 patients who did not improve with conservative measures underwent surgical excision of ossicles, and all returned to normal activities (mean time, 4.5 weeks).1
In another case series of 118 patients (151 knees), 88% responded to intermittent limitation of activity (weeks to months) or cylinder casting if limiting activity was ineffective. The remaining 14 patients showed no improvement from these measures; all had surgical excision of an ossicle, sometimes combined with a tubercle-thinning procedure. Only 1 of these patients (7%) did not have complete relief and return to full activities at 6 weeks.2
Retrospective analyses also support a conservative approach. One retrospective survey of 68 young athletes with Osgood-Schlatter found they required an average of 3.2 months off all training and 7.3 months of some activity restrictions.3 In another survey, 20 of 22 (91%) adolescent athletes with Osgood-Schlatter were able to manage their symptoms with ice, aspirin, and mild activity modification. Only 2 needed to stop playing all sports for any period of time, and none required surgery.4
Another retrospective review analyzed 50 patients with Osgood-Schlatter (69 knees) for an average of 9 years. No treatments or activity restrictions were recommended. At time of follow-up, 36 (76%) had no limitations, but kneeling continued to be uncomfortable in 60%.5
No interventional studies have explicitly evaluated commonly recommended conservative treatments such as ice, analgesics, activity restriction, stretching, strengthening, or anti-inflammatory medication. Corticosteroid injections are generally not recommended, due to case reports of complications, primarily related to subcutaneous atrophy.6 One small case series demonstrated improvement in Osgood-Schlatter disease pain in 19 of 24 (79%) knees after using an infrapatellar strap for 6 to 8 weeks.7
Refractory cases have been treated with a variety of surgical interventions. In 1 case series, 67 patients (70 knees) (mean age 19.6, 77% male) with at least 18 months of symptoms despite conservative treatment underwent resection of an ossicle (62 cases) or excision of prominent tibial tubercle (8 cases). These patients were followed for 2.2 years, with 56 (90%) patients with ossicle-resection able to return to maximal sports activity without pain, tenderness, loss of motion, or atrophy.8
Another case series compared 22 patients who1 underwent drilling of the tibial tubercle (with or without the removal of the tibial tubercle) with 22 patients who had excision of loose ossicles or cartilage. Seventeen of the 22 (77%) patients with ossicle excision had complete resolution of symptoms compared with 8 of the 22 (36%) in the patients who underwent tibial tubercle drilling.9
One surgical series evaluated excision of tibial tuberosity in 35 patients (42 knees) who did not improve with conservative treatment for an average of 13.25 months. For 37 of 42 knees (88%), patients reported complete relief of pain, and all returned to activity without limitation. The average time to return to sports was 15.2 weeks.10
Recommendations from others
The American Academy of Orthopaedic Surgeons and the American Academy of Family Practice recommend activity limitation, ice, anti-inflammatories, protective padding, quadriceps/hamstring strengthening, and time in the management of Osgood-Schlatter disease.11,12
Few patients have poor results with conservative measures
James Barbee, MD
John Peter Smith Family Practice Residency Program, Ft. Worth, Tex
Osgood-Schlatter disease is a common problem that all primary care physicians must be ready to recognize and treat. While the research (primarily surgical series) indicates that 10% to 12% of patients may not improve with conservative measures, I have not had nearly that high a percentage of patients who require surgical intervention. Surgery is only offered after the tubercle attaches to the femur, or the tubercle fails to attach at all. In fact, I do not x-ray typical cases of Osgood-Schlatter disease unless evidence suggests patella tendon avulsion, or if parental concern is high. This means that, in most cases, the primary care physician has quite a while to try conservative measures before incurring the expense of radiography or an orthopedic consultation.
Drug brand names
- Candesartan • Atacand
- Felodipine • Plendil
- Spironolactone • Aldactone
- Valsartan • Diovan
1. Hussain A, Hagroo GA. Osgood-Schlatter disease. Sports Exer Injury 1996;2:202-206.
2. Mital MA, Matza RA, Cohen J. The so-called unresolved Osgood-Schlatter lesion. J Bone Joint Surg Am 1980;62:732-739.
3. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med 1985;13:236-241.
4. Beovich R, Fricker PA. Osgood-Schlatter’s disease. A review of the literature and an Australian series. Aust J Sci Med Sport 1988;20:11-13.
5. Krause BL, Williams JP, Catterall A. Natural history of Osgood-Schlatter disease. J Pediatr Orthop 1990;10:65-68.
6. Rostron PK, Calver RF. Subcutaneous atrophy following methylprednisolone injection in Osgood-Schlatter epi-physitis. J Bone Joint Surg Am 1979;61:627-628.
7. Levine J, Kashyap S. A new conservative treatment of Osgood-Schlatter disease. Clin Orthop 1981;158:126-128.
8. Orava S, Malinen L, Karpakka JJ, et al. Results of surgical treatment of unresolved Osgood-Schlatter lesion. Ann Chir Gynaecol 2000;89:298-302.
9. Glynn MK, Regan BF. Surgical treatment of Osgood-Schlatter’s disease. J Pediatr Orthop 1983;3:216-219.
10. Flowers MJ, Bhadreshwar DR. Tibial tuberosity excision for symptomatic Osgood-Schlatter disease. J Pediatr Orthop 1995;15:292-297.
11. Osgood-Schlatter disease (knee pain). American Academy of Orthopaedic Surgeons Web site. Last updated July 2000. Available at: orthoinfo.aaos.org/fact/thr_report. cfm?Thread_ID=145&topcategory=Knee. Accessed on January 14, 2004.
12. Osgood-Schlatter. Disease: A cause of knee pain in children. American Academy of Family Physicians Web site. Last updated March 2002. Available at: familydoctor.org/handouts/135.html. Accessed on January 14, 2004.
Osgood-Schlatter disease is a common cause of pain and tenderness at the tibial tuberosity in active adolescents. It is typically a self-limited condition that waxes and wanes, but which often takes months to years to resolve entirely. It is best managed with conservative measures (activity modification, ice, anti-inflammatory agents) and time (strength of recommendation [SOR]: B, several case series and retrospective studies).
In chronic cases that are refractory to conservative treatment, surgical intervention yields good results, particularly for patients with bony or cartilaginous ossicles. Excision of these ossicles produces resolution of symptoms and return to activity in several weeks (SOR: C, several case series). Corticosteroid injections are not recommended (SOR: C, case reports and expert opinion).
Evidence summary
No prospective, interventional studies evaluate the treatment of Osgood-Schlatter disease. One case series followed the natural course of the disease in 261 patients (365 symptomatic knees) for 12 to 24 months; 237 (90.8%) patients responded well to restriction of sports activity and nonsteroidal anti-inflammatory agents. The 24 patients who did not improve with conservative measures underwent surgical excision of ossicles, and all returned to normal activities (mean time, 4.5 weeks).1
In another case series of 118 patients (151 knees), 88% responded to intermittent limitation of activity (weeks to months) or cylinder casting if limiting activity was ineffective. The remaining 14 patients showed no improvement from these measures; all had surgical excision of an ossicle, sometimes combined with a tubercle-thinning procedure. Only 1 of these patients (7%) did not have complete relief and return to full activities at 6 weeks.2
Retrospective analyses also support a conservative approach. One retrospective survey of 68 young athletes with Osgood-Schlatter found they required an average of 3.2 months off all training and 7.3 months of some activity restrictions.3 In another survey, 20 of 22 (91%) adolescent athletes with Osgood-Schlatter were able to manage their symptoms with ice, aspirin, and mild activity modification. Only 2 needed to stop playing all sports for any period of time, and none required surgery.4
Another retrospective review analyzed 50 patients with Osgood-Schlatter (69 knees) for an average of 9 years. No treatments or activity restrictions were recommended. At time of follow-up, 36 (76%) had no limitations, but kneeling continued to be uncomfortable in 60%.5
No interventional studies have explicitly evaluated commonly recommended conservative treatments such as ice, analgesics, activity restriction, stretching, strengthening, or anti-inflammatory medication. Corticosteroid injections are generally not recommended, due to case reports of complications, primarily related to subcutaneous atrophy.6 One small case series demonstrated improvement in Osgood-Schlatter disease pain in 19 of 24 (79%) knees after using an infrapatellar strap for 6 to 8 weeks.7
Refractory cases have been treated with a variety of surgical interventions. In 1 case series, 67 patients (70 knees) (mean age 19.6, 77% male) with at least 18 months of symptoms despite conservative treatment underwent resection of an ossicle (62 cases) or excision of prominent tibial tubercle (8 cases). These patients were followed for 2.2 years, with 56 (90%) patients with ossicle-resection able to return to maximal sports activity without pain, tenderness, loss of motion, or atrophy.8
Another case series compared 22 patients who1 underwent drilling of the tibial tubercle (with or without the removal of the tibial tubercle) with 22 patients who had excision of loose ossicles or cartilage. Seventeen of the 22 (77%) patients with ossicle excision had complete resolution of symptoms compared with 8 of the 22 (36%) in the patients who underwent tibial tubercle drilling.9
One surgical series evaluated excision of tibial tuberosity in 35 patients (42 knees) who did not improve with conservative treatment for an average of 13.25 months. For 37 of 42 knees (88%), patients reported complete relief of pain, and all returned to activity without limitation. The average time to return to sports was 15.2 weeks.10
Recommendations from others
The American Academy of Orthopaedic Surgeons and the American Academy of Family Practice recommend activity limitation, ice, anti-inflammatories, protective padding, quadriceps/hamstring strengthening, and time in the management of Osgood-Schlatter disease.11,12
Few patients have poor results with conservative measures
James Barbee, MD
John Peter Smith Family Practice Residency Program, Ft. Worth, Tex
Osgood-Schlatter disease is a common problem that all primary care physicians must be ready to recognize and treat. While the research (primarily surgical series) indicates that 10% to 12% of patients may not improve with conservative measures, I have not had nearly that high a percentage of patients who require surgical intervention. Surgery is only offered after the tubercle attaches to the femur, or the tubercle fails to attach at all. In fact, I do not x-ray typical cases of Osgood-Schlatter disease unless evidence suggests patella tendon avulsion, or if parental concern is high. This means that, in most cases, the primary care physician has quite a while to try conservative measures before incurring the expense of radiography or an orthopedic consultation.
Drug brand names
- Candesartan • Atacand
- Felodipine • Plendil
- Spironolactone • Aldactone
- Valsartan • Diovan
Osgood-Schlatter disease is a common cause of pain and tenderness at the tibial tuberosity in active adolescents. It is typically a self-limited condition that waxes and wanes, but which often takes months to years to resolve entirely. It is best managed with conservative measures (activity modification, ice, anti-inflammatory agents) and time (strength of recommendation [SOR]: B, several case series and retrospective studies).
In chronic cases that are refractory to conservative treatment, surgical intervention yields good results, particularly for patients with bony or cartilaginous ossicles. Excision of these ossicles produces resolution of symptoms and return to activity in several weeks (SOR: C, several case series). Corticosteroid injections are not recommended (SOR: C, case reports and expert opinion).
Evidence summary
No prospective, interventional studies evaluate the treatment of Osgood-Schlatter disease. One case series followed the natural course of the disease in 261 patients (365 symptomatic knees) for 12 to 24 months; 237 (90.8%) patients responded well to restriction of sports activity and nonsteroidal anti-inflammatory agents. The 24 patients who did not improve with conservative measures underwent surgical excision of ossicles, and all returned to normal activities (mean time, 4.5 weeks).1
In another case series of 118 patients (151 knees), 88% responded to intermittent limitation of activity (weeks to months) or cylinder casting if limiting activity was ineffective. The remaining 14 patients showed no improvement from these measures; all had surgical excision of an ossicle, sometimes combined with a tubercle-thinning procedure. Only 1 of these patients (7%) did not have complete relief and return to full activities at 6 weeks.2
Retrospective analyses also support a conservative approach. One retrospective survey of 68 young athletes with Osgood-Schlatter found they required an average of 3.2 months off all training and 7.3 months of some activity restrictions.3 In another survey, 20 of 22 (91%) adolescent athletes with Osgood-Schlatter were able to manage their symptoms with ice, aspirin, and mild activity modification. Only 2 needed to stop playing all sports for any period of time, and none required surgery.4
Another retrospective review analyzed 50 patients with Osgood-Schlatter (69 knees) for an average of 9 years. No treatments or activity restrictions were recommended. At time of follow-up, 36 (76%) had no limitations, but kneeling continued to be uncomfortable in 60%.5
No interventional studies have explicitly evaluated commonly recommended conservative treatments such as ice, analgesics, activity restriction, stretching, strengthening, or anti-inflammatory medication. Corticosteroid injections are generally not recommended, due to case reports of complications, primarily related to subcutaneous atrophy.6 One small case series demonstrated improvement in Osgood-Schlatter disease pain in 19 of 24 (79%) knees after using an infrapatellar strap for 6 to 8 weeks.7
Refractory cases have been treated with a variety of surgical interventions. In 1 case series, 67 patients (70 knees) (mean age 19.6, 77% male) with at least 18 months of symptoms despite conservative treatment underwent resection of an ossicle (62 cases) or excision of prominent tibial tubercle (8 cases). These patients were followed for 2.2 years, with 56 (90%) patients with ossicle-resection able to return to maximal sports activity without pain, tenderness, loss of motion, or atrophy.8
Another case series compared 22 patients who1 underwent drilling of the tibial tubercle (with or without the removal of the tibial tubercle) with 22 patients who had excision of loose ossicles or cartilage. Seventeen of the 22 (77%) patients with ossicle excision had complete resolution of symptoms compared with 8 of the 22 (36%) in the patients who underwent tibial tubercle drilling.9
One surgical series evaluated excision of tibial tuberosity in 35 patients (42 knees) who did not improve with conservative treatment for an average of 13.25 months. For 37 of 42 knees (88%), patients reported complete relief of pain, and all returned to activity without limitation. The average time to return to sports was 15.2 weeks.10
Recommendations from others
The American Academy of Orthopaedic Surgeons and the American Academy of Family Practice recommend activity limitation, ice, anti-inflammatories, protective padding, quadriceps/hamstring strengthening, and time in the management of Osgood-Schlatter disease.11,12
Few patients have poor results with conservative measures
James Barbee, MD
John Peter Smith Family Practice Residency Program, Ft. Worth, Tex
Osgood-Schlatter disease is a common problem that all primary care physicians must be ready to recognize and treat. While the research (primarily surgical series) indicates that 10% to 12% of patients may not improve with conservative measures, I have not had nearly that high a percentage of patients who require surgical intervention. Surgery is only offered after the tubercle attaches to the femur, or the tubercle fails to attach at all. In fact, I do not x-ray typical cases of Osgood-Schlatter disease unless evidence suggests patella tendon avulsion, or if parental concern is high. This means that, in most cases, the primary care physician has quite a while to try conservative measures before incurring the expense of radiography or an orthopedic consultation.
Drug brand names
- Candesartan • Atacand
- Felodipine • Plendil
- Spironolactone • Aldactone
- Valsartan • Diovan
1. Hussain A, Hagroo GA. Osgood-Schlatter disease. Sports Exer Injury 1996;2:202-206.
2. Mital MA, Matza RA, Cohen J. The so-called unresolved Osgood-Schlatter lesion. J Bone Joint Surg Am 1980;62:732-739.
3. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med 1985;13:236-241.
4. Beovich R, Fricker PA. Osgood-Schlatter’s disease. A review of the literature and an Australian series. Aust J Sci Med Sport 1988;20:11-13.
5. Krause BL, Williams JP, Catterall A. Natural history of Osgood-Schlatter disease. J Pediatr Orthop 1990;10:65-68.
6. Rostron PK, Calver RF. Subcutaneous atrophy following methylprednisolone injection in Osgood-Schlatter epi-physitis. J Bone Joint Surg Am 1979;61:627-628.
7. Levine J, Kashyap S. A new conservative treatment of Osgood-Schlatter disease. Clin Orthop 1981;158:126-128.
8. Orava S, Malinen L, Karpakka JJ, et al. Results of surgical treatment of unresolved Osgood-Schlatter lesion. Ann Chir Gynaecol 2000;89:298-302.
9. Glynn MK, Regan BF. Surgical treatment of Osgood-Schlatter’s disease. J Pediatr Orthop 1983;3:216-219.
10. Flowers MJ, Bhadreshwar DR. Tibial tuberosity excision for symptomatic Osgood-Schlatter disease. J Pediatr Orthop 1995;15:292-297.
11. Osgood-Schlatter disease (knee pain). American Academy of Orthopaedic Surgeons Web site. Last updated July 2000. Available at: orthoinfo.aaos.org/fact/thr_report. cfm?Thread_ID=145&topcategory=Knee. Accessed on January 14, 2004.
12. Osgood-Schlatter. Disease: A cause of knee pain in children. American Academy of Family Physicians Web site. Last updated March 2002. Available at: familydoctor.org/handouts/135.html. Accessed on January 14, 2004.
1. Hussain A, Hagroo GA. Osgood-Schlatter disease. Sports Exer Injury 1996;2:202-206.
2. Mital MA, Matza RA, Cohen J. The so-called unresolved Osgood-Schlatter lesion. J Bone Joint Surg Am 1980;62:732-739.
3. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med 1985;13:236-241.
4. Beovich R, Fricker PA. Osgood-Schlatter’s disease. A review of the literature and an Australian series. Aust J Sci Med Sport 1988;20:11-13.
5. Krause BL, Williams JP, Catterall A. Natural history of Osgood-Schlatter disease. J Pediatr Orthop 1990;10:65-68.
6. Rostron PK, Calver RF. Subcutaneous atrophy following methylprednisolone injection in Osgood-Schlatter epi-physitis. J Bone Joint Surg Am 1979;61:627-628.
7. Levine J, Kashyap S. A new conservative treatment of Osgood-Schlatter disease. Clin Orthop 1981;158:126-128.
8. Orava S, Malinen L, Karpakka JJ, et al. Results of surgical treatment of unresolved Osgood-Schlatter lesion. Ann Chir Gynaecol 2000;89:298-302.
9. Glynn MK, Regan BF. Surgical treatment of Osgood-Schlatter’s disease. J Pediatr Orthop 1983;3:216-219.
10. Flowers MJ, Bhadreshwar DR. Tibial tuberosity excision for symptomatic Osgood-Schlatter disease. J Pediatr Orthop 1995;15:292-297.
11. Osgood-Schlatter disease (knee pain). American Academy of Orthopaedic Surgeons Web site. Last updated July 2000. Available at: orthoinfo.aaos.org/fact/thr_report. cfm?Thread_ID=145&topcategory=Knee. Accessed on January 14, 2004.
12. Osgood-Schlatter. Disease: A cause of knee pain in children. American Academy of Family Physicians Web site. Last updated March 2002. Available at: familydoctor.org/handouts/135.html. Accessed on January 14, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Should we screen adults for asymptomatic microhematuria?
Screening patients for asymptomatic microhematuria does not appear to improve outcomes, since screening does not identify a population with increased prevalence of urologic malignancy (strength of recommendation [SOR]: A, based on prospective cohort studies) or the presence of urologic disease of any type (SOR: B, based on 1 cohort study). Asymptomatic microhematuria is sometimes associated with urologic disease that requires intervention to prevent death or disability (SOR: B, based on cohort studies). However, no studies demonstrate improved outcomes from screening for asymptomatic microhematuria.
Evidence summary
Asymptomatic microhematuria is common in adult primary care populations, with a prevalence ranging from 2.5% to 4.3% in 3 studies.1-3 It is variably associated with urologic disease.
A retrospective cohort study of 2005 British men aged >40 years found 85 (4%) with asymptomatic microhematuria. Subsequent evaluation including intravenous pyelogram and cystoscopy found 2 men with infections—1 with bladder cancer and 1 with polycystic kidneys. Benign prostatic hypertrophy, prostatitis, anatomic abnormalities, and stones accounted for the rest.3
A prospective cohort study similarly evaluated 1034 patients with asymptomatic micro-hematuria found through annual health screening of Japanese adults; 471 (45%) had some urologic diagnosis, including 30 (2.9%) with serious disease (urologic malignancies or progressive glomerulopathy), 195 (18.9%) with moderate disease (such as stones, infection, stable glomerulopathy), and the remainder with less serious disease.4
However, it is unclear whether asymptomatic microhematuria is a useful marker for detecting urologic disease. Two retrospective cohort studies assessed the prevalence of urologic disease in patients with asymptomatic microhematuria compared with those without. Of 501 male steel-workers—an occupation believed to have a higher risk for urologic malignancy—57 men had urologic disease of any type. Six men with urolog-ic disease had asymptomatic microhematuria, while 51 men with urologic disease did not. The correlation between asymptomatic microhema-turia and the presence of urologic disease was not significant (P>.05). There were 3 cases of urolog-ic cancer in the study, all diagnosed in men without asymptomatic microhematuria.5
Among 20,751 California HMO patients who had a periodic health appraisal, screening identified 598 patients with asymptomatic microhematuria (prevalence=2.9%). The medical records for all patients were reviewed for the year prior to screening to find pre-existing urologic disease and then reviewed for new diagnoses over the next 6 years. Three cases of urologic cancer occurred in the group of patients with asymptomatic microhematuria (incidence=0.5%) and 102 cancer cases among the 20,153 patients without asymptomatic microhematuria (incidence=0.5%). Its presence was not significantly associated with either uro-logic cancers or other serious urologic disease.2
No studies demonstrate improved outcomes from screening for asymptomatic microhematuria. Earlier discovery of serious diseases would not often change patient outcome, according to expert opinion.6,7 Invasive studies, such as intravenous pyelogram and cystoscopy, used to evaluate asymptomatic microhematuria have a rate of serious complications approaching 0.3% (number needed to harm=333).7
Recommendations from others
The American Urological Association recommends that all patients with asymptomatic microhematuria be evaluated. However, they do not recommend routine screening for asympto-matic microhematuria to detect urologic malig-nancy.8 The US Preventive Services Task Force does not recommend routine screening for bladder cancer by any means, including screening for hematuria.9
This poor screening measure is not helpful
Dan DePietropaolo, MD
Director, Family Practice Residency Program; Medical Director, Heartland Hospice, Christianacare Health System, Wilmington, Del
A fairly sensitive and specific way to screen for urological malignancies would certainly be worthwhile, but, as this inquiry points out, none exists. The presence of asymptomatic microhematuria in the adult population does not aid in detecting urologic malignancies or any other serious pathology. The incidence of serious disease in the control group is just as high as in the patients with a positive screen for hematuria. A poor screening measure like this one not only is not helpful but also holds the potential to harm patients because of false positive results and the ensuing invasive workups. The USPSTF does not recommend this screening measure.
1. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Brit Med J (Clin Res Ed) 1986;292:681-683.
2. Hiatt RA, Ordenez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev 1994;3:439-443.
3. Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol 1987;138:1189-1190.
4. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol 1990;144:99-101.
5. Choi BC, Farmilo JA. Microscopic haematuria as a predictor of urological diseases among steel workers. J Soc Occup Med 1990;40:47-52.
6. Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224-229.
7. Froom P, Froom J, Ribak J. Asymptomatic microscopic hematuria—is investigation necessary? J Clin Epidemiol 1997;50:1197-1200.
8. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence and etiology. Urology 2001;57:559-603.
9. USPSTF website Screening: bladder cancer. Last updated 1996. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm. Accessed on January 6, 2004.
Screening patients for asymptomatic microhematuria does not appear to improve outcomes, since screening does not identify a population with increased prevalence of urologic malignancy (strength of recommendation [SOR]: A, based on prospective cohort studies) or the presence of urologic disease of any type (SOR: B, based on 1 cohort study). Asymptomatic microhematuria is sometimes associated with urologic disease that requires intervention to prevent death or disability (SOR: B, based on cohort studies). However, no studies demonstrate improved outcomes from screening for asymptomatic microhematuria.
Evidence summary
Asymptomatic microhematuria is common in adult primary care populations, with a prevalence ranging from 2.5% to 4.3% in 3 studies.1-3 It is variably associated with urologic disease.
A retrospective cohort study of 2005 British men aged >40 years found 85 (4%) with asymptomatic microhematuria. Subsequent evaluation including intravenous pyelogram and cystoscopy found 2 men with infections—1 with bladder cancer and 1 with polycystic kidneys. Benign prostatic hypertrophy, prostatitis, anatomic abnormalities, and stones accounted for the rest.3
A prospective cohort study similarly evaluated 1034 patients with asymptomatic micro-hematuria found through annual health screening of Japanese adults; 471 (45%) had some urologic diagnosis, including 30 (2.9%) with serious disease (urologic malignancies or progressive glomerulopathy), 195 (18.9%) with moderate disease (such as stones, infection, stable glomerulopathy), and the remainder with less serious disease.4
However, it is unclear whether asymptomatic microhematuria is a useful marker for detecting urologic disease. Two retrospective cohort studies assessed the prevalence of urologic disease in patients with asymptomatic microhematuria compared with those without. Of 501 male steel-workers—an occupation believed to have a higher risk for urologic malignancy—57 men had urologic disease of any type. Six men with urolog-ic disease had asymptomatic microhematuria, while 51 men with urologic disease did not. The correlation between asymptomatic microhema-turia and the presence of urologic disease was not significant (P>.05). There were 3 cases of urolog-ic cancer in the study, all diagnosed in men without asymptomatic microhematuria.5
Among 20,751 California HMO patients who had a periodic health appraisal, screening identified 598 patients with asymptomatic microhematuria (prevalence=2.9%). The medical records for all patients were reviewed for the year prior to screening to find pre-existing urologic disease and then reviewed for new diagnoses over the next 6 years. Three cases of urologic cancer occurred in the group of patients with asymptomatic microhematuria (incidence=0.5%) and 102 cancer cases among the 20,153 patients without asymptomatic microhematuria (incidence=0.5%). Its presence was not significantly associated with either uro-logic cancers or other serious urologic disease.2
No studies demonstrate improved outcomes from screening for asymptomatic microhematuria. Earlier discovery of serious diseases would not often change patient outcome, according to expert opinion.6,7 Invasive studies, such as intravenous pyelogram and cystoscopy, used to evaluate asymptomatic microhematuria have a rate of serious complications approaching 0.3% (number needed to harm=333).7
Recommendations from others
The American Urological Association recommends that all patients with asymptomatic microhematuria be evaluated. However, they do not recommend routine screening for asympto-matic microhematuria to detect urologic malig-nancy.8 The US Preventive Services Task Force does not recommend routine screening for bladder cancer by any means, including screening for hematuria.9
This poor screening measure is not helpful
Dan DePietropaolo, MD
Director, Family Practice Residency Program; Medical Director, Heartland Hospice, Christianacare Health System, Wilmington, Del
A fairly sensitive and specific way to screen for urological malignancies would certainly be worthwhile, but, as this inquiry points out, none exists. The presence of asymptomatic microhematuria in the adult population does not aid in detecting urologic malignancies or any other serious pathology. The incidence of serious disease in the control group is just as high as in the patients with a positive screen for hematuria. A poor screening measure like this one not only is not helpful but also holds the potential to harm patients because of false positive results and the ensuing invasive workups. The USPSTF does not recommend this screening measure.
Screening patients for asymptomatic microhematuria does not appear to improve outcomes, since screening does not identify a population with increased prevalence of urologic malignancy (strength of recommendation [SOR]: A, based on prospective cohort studies) or the presence of urologic disease of any type (SOR: B, based on 1 cohort study). Asymptomatic microhematuria is sometimes associated with urologic disease that requires intervention to prevent death or disability (SOR: B, based on cohort studies). However, no studies demonstrate improved outcomes from screening for asymptomatic microhematuria.
Evidence summary
Asymptomatic microhematuria is common in adult primary care populations, with a prevalence ranging from 2.5% to 4.3% in 3 studies.1-3 It is variably associated with urologic disease.
A retrospective cohort study of 2005 British men aged >40 years found 85 (4%) with asymptomatic microhematuria. Subsequent evaluation including intravenous pyelogram and cystoscopy found 2 men with infections—1 with bladder cancer and 1 with polycystic kidneys. Benign prostatic hypertrophy, prostatitis, anatomic abnormalities, and stones accounted for the rest.3
A prospective cohort study similarly evaluated 1034 patients with asymptomatic micro-hematuria found through annual health screening of Japanese adults; 471 (45%) had some urologic diagnosis, including 30 (2.9%) with serious disease (urologic malignancies or progressive glomerulopathy), 195 (18.9%) with moderate disease (such as stones, infection, stable glomerulopathy), and the remainder with less serious disease.4
However, it is unclear whether asymptomatic microhematuria is a useful marker for detecting urologic disease. Two retrospective cohort studies assessed the prevalence of urologic disease in patients with asymptomatic microhematuria compared with those without. Of 501 male steel-workers—an occupation believed to have a higher risk for urologic malignancy—57 men had urologic disease of any type. Six men with urolog-ic disease had asymptomatic microhematuria, while 51 men with urologic disease did not. The correlation between asymptomatic microhema-turia and the presence of urologic disease was not significant (P>.05). There were 3 cases of urolog-ic cancer in the study, all diagnosed in men without asymptomatic microhematuria.5
Among 20,751 California HMO patients who had a periodic health appraisal, screening identified 598 patients with asymptomatic microhematuria (prevalence=2.9%). The medical records for all patients were reviewed for the year prior to screening to find pre-existing urologic disease and then reviewed for new diagnoses over the next 6 years. Three cases of urologic cancer occurred in the group of patients with asymptomatic microhematuria (incidence=0.5%) and 102 cancer cases among the 20,153 patients without asymptomatic microhematuria (incidence=0.5%). Its presence was not significantly associated with either uro-logic cancers or other serious urologic disease.2
No studies demonstrate improved outcomes from screening for asymptomatic microhematuria. Earlier discovery of serious diseases would not often change patient outcome, according to expert opinion.6,7 Invasive studies, such as intravenous pyelogram and cystoscopy, used to evaluate asymptomatic microhematuria have a rate of serious complications approaching 0.3% (number needed to harm=333).7
Recommendations from others
The American Urological Association recommends that all patients with asymptomatic microhematuria be evaluated. However, they do not recommend routine screening for asympto-matic microhematuria to detect urologic malig-nancy.8 The US Preventive Services Task Force does not recommend routine screening for bladder cancer by any means, including screening for hematuria.9
This poor screening measure is not helpful
Dan DePietropaolo, MD
Director, Family Practice Residency Program; Medical Director, Heartland Hospice, Christianacare Health System, Wilmington, Del
A fairly sensitive and specific way to screen for urological malignancies would certainly be worthwhile, but, as this inquiry points out, none exists. The presence of asymptomatic microhematuria in the adult population does not aid in detecting urologic malignancies or any other serious pathology. The incidence of serious disease in the control group is just as high as in the patients with a positive screen for hematuria. A poor screening measure like this one not only is not helpful but also holds the potential to harm patients because of false positive results and the ensuing invasive workups. The USPSTF does not recommend this screening measure.
1. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Brit Med J (Clin Res Ed) 1986;292:681-683.
2. Hiatt RA, Ordenez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev 1994;3:439-443.
3. Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol 1987;138:1189-1190.
4. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol 1990;144:99-101.
5. Choi BC, Farmilo JA. Microscopic haematuria as a predictor of urological diseases among steel workers. J Soc Occup Med 1990;40:47-52.
6. Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224-229.
7. Froom P, Froom J, Ribak J. Asymptomatic microscopic hematuria—is investigation necessary? J Clin Epidemiol 1997;50:1197-1200.
8. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence and etiology. Urology 2001;57:559-603.
9. USPSTF website Screening: bladder cancer. Last updated 1996. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm. Accessed on January 6, 2004.
1. Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Brit Med J (Clin Res Ed) 1986;292:681-683.
2. Hiatt RA, Ordenez JD. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol Biomarkers Prev 1994;3:439-443.
3. Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol 1987;138:1189-1190.
4. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol 1990;144:99-101.
5. Choi BC, Farmilo JA. Microscopic haematuria as a predictor of urological diseases among steel workers. J Soc Occup Med 1990;40:47-52.
6. Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224-229.
7. Froom P, Froom J, Ribak J. Asymptomatic microscopic hematuria—is investigation necessary? J Clin Epidemiol 1997;50:1197-1200.
8. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence and etiology. Urology 2001;57:559-603.
9. USPSTF website Screening: bladder cancer. Last updated 1996. Available at www.ahrq.gov/clinic/uspstf/uspsblad.htm. Accessed on January 6, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Does lowering diastolic BP to less than 90 mm Hg decrease cardiovascular risk?
Although lowering diastolic blood pressure (DBP) is associated with reduced cardiovascular events, systolic blood pressure (SBP) is a more robust predictor of cardiovascular risk than DBP and should now be used to diagnose, stage, and treat hypertension.
Lowering diastolic blood pressure (DBP) to <90 mm Hg in hypertensive individuals of all ages decreases the risk of cardiovascular events including myocardial infarction (MI), heart failure, and sudden death (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). However, there is no consensus regarding how far to lower DBP. A “J-shaped” increase in cardiovascular risks with DBP <85 mm Hg may apply under certain conditions.
Evidence summary
The concept of a continuous graded relationship between DBP and cardiovascular risk is supported by a meta-analysis of 14 randomized clinical trials showing that lowering DBP by 6 mm Hg reduced the risk of coronary heart disease by 14% (95% confidence interval [CI], 4%–22%; P<.01; NNT=200).1 Throughout the range of DBP in study subjects, 70–115 mm Hg, a lower DBP was associated with a lower risk of coronary heart disease.
However, there is concern that lowering DBP too much may actually increase cardiovascular risk. A 10-year observational study showed that in patients with a history of ischemic heart disease, the incidence of fatal MI was lowest when DBP was between 85 to 90 mm Hg and increased with DBP <85 mm Hg, thus demonstrating a J-shaped curve.2
Farnett et al3 derived a summary curve from 13 studies that stratified cardiovascular outcomes by level of achieved blood pressure; the nadir of the curve for ischemic heart disease events occurred at 86 to 89 mm Hg DBP. The risk was independent of type of drug therapy, and more pronounced in study subjects with known cardiovascular disease.
A meta-analysis of 7 randomized controlled trials involving 40,233 hypertensive patients used statistical modeling to determine the shape of the “mortality curve” over a range of DBP categories, defined in 10-mm Hg increments from 65 to 106. The subjects received mainly beta-blockers or thiazide diuretics; controls received placebo or no treatment.4 Both groups demonstrated increased risk for cardiovascular and all-cause death at the lowest DBP levels. Among treated patients, overall death rate was lowest with a DBP in the range of 76 to 85 mm Hg; among controls the nadir was 86 to 95 mm Hg.
The Hypertension Optimal Treatment (HOT) trial5 was specifically designed to determine the optimal target blood pressure for hypertensive patients: 18,790 men and women with DBP 100 to 115 mm Hg were randomly assigned to target DBP groups of <90, <85, or <80 mm Hg. All were treated with felodipine and other agents in a stepped-care protocol; average follow-up was 3.8 years. The lowest incidence of cardiovascular events occurred at a mean DBP of 82.6 mm Hg and fewest cardiovascular deaths at 86.5 mm Hg. Further reductions in DBP neither lowered nor increased cardiovascular risk.
A French cohort study6 followed over 4700 hyper-tensive men for an average of 14 years. These men had their hypertension treated in usual fashion by their own physicians. In this group, SBP was much more accurate than DBP in classifying severity of hypertension and in predicting cardiovascular risk.
Recommendations from others
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)7 and the World Health Organization–International Society of Hyper-tension Guidelines8 state that the relationship between cardiovascular risk and blood pressure is continuous, without a lower threshold. Target blood pressure goals are <140/90 mm Hg in uncomplicated hypertension and <130/80 mm Hg for individuals with diabetes or kidney disease. The National High Blood Pressure Education Program stressed that SBP, not DBP, should become the major criterion for diagnosis and treatment of hypertension.9
Emphasize education and focus on systolic blood pressure
Randy Ward, MD
Director, Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
In light of JNC VII, there may be some confusion on the part of patients as to “normal” blood pressure and indications for treatment. In fact, on the first page of the NHLBI web site, “Your Guide to Lowering Blood Pressure,” the statement is made that “normal blood pressure is less than 120 mm Hg systolic and less than 80 mm Hg diastolic.” They later go on to describe the category of prehypertension. It is important to understand the concept and implications of prehypertension, and the “J-shaped” curve in counseling our patients on achieving optimal blood pressure control.
1. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-838.
2. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-584.
3. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489-495.
4. Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. Jshaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002;136:438-448.
5. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients treated with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-1762.
6. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-581.
7. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003;289:2560-2572.
8. 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-183.
9. Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021-1024.
Although lowering diastolic blood pressure (DBP) is associated with reduced cardiovascular events, systolic blood pressure (SBP) is a more robust predictor of cardiovascular risk than DBP and should now be used to diagnose, stage, and treat hypertension.
Lowering diastolic blood pressure (DBP) to <90 mm Hg in hypertensive individuals of all ages decreases the risk of cardiovascular events including myocardial infarction (MI), heart failure, and sudden death (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). However, there is no consensus regarding how far to lower DBP. A “J-shaped” increase in cardiovascular risks with DBP <85 mm Hg may apply under certain conditions.
Evidence summary
The concept of a continuous graded relationship between DBP and cardiovascular risk is supported by a meta-analysis of 14 randomized clinical trials showing that lowering DBP by 6 mm Hg reduced the risk of coronary heart disease by 14% (95% confidence interval [CI], 4%–22%; P<.01; NNT=200).1 Throughout the range of DBP in study subjects, 70–115 mm Hg, a lower DBP was associated with a lower risk of coronary heart disease.
However, there is concern that lowering DBP too much may actually increase cardiovascular risk. A 10-year observational study showed that in patients with a history of ischemic heart disease, the incidence of fatal MI was lowest when DBP was between 85 to 90 mm Hg and increased with DBP <85 mm Hg, thus demonstrating a J-shaped curve.2
Farnett et al3 derived a summary curve from 13 studies that stratified cardiovascular outcomes by level of achieved blood pressure; the nadir of the curve for ischemic heart disease events occurred at 86 to 89 mm Hg DBP. The risk was independent of type of drug therapy, and more pronounced in study subjects with known cardiovascular disease.
A meta-analysis of 7 randomized controlled trials involving 40,233 hypertensive patients used statistical modeling to determine the shape of the “mortality curve” over a range of DBP categories, defined in 10-mm Hg increments from 65 to 106. The subjects received mainly beta-blockers or thiazide diuretics; controls received placebo or no treatment.4 Both groups demonstrated increased risk for cardiovascular and all-cause death at the lowest DBP levels. Among treated patients, overall death rate was lowest with a DBP in the range of 76 to 85 mm Hg; among controls the nadir was 86 to 95 mm Hg.
The Hypertension Optimal Treatment (HOT) trial5 was specifically designed to determine the optimal target blood pressure for hypertensive patients: 18,790 men and women with DBP 100 to 115 mm Hg were randomly assigned to target DBP groups of <90, <85, or <80 mm Hg. All were treated with felodipine and other agents in a stepped-care protocol; average follow-up was 3.8 years. The lowest incidence of cardiovascular events occurred at a mean DBP of 82.6 mm Hg and fewest cardiovascular deaths at 86.5 mm Hg. Further reductions in DBP neither lowered nor increased cardiovascular risk.
A French cohort study6 followed over 4700 hyper-tensive men for an average of 14 years. These men had their hypertension treated in usual fashion by their own physicians. In this group, SBP was much more accurate than DBP in classifying severity of hypertension and in predicting cardiovascular risk.
Recommendations from others
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)7 and the World Health Organization–International Society of Hyper-tension Guidelines8 state that the relationship between cardiovascular risk and blood pressure is continuous, without a lower threshold. Target blood pressure goals are <140/90 mm Hg in uncomplicated hypertension and <130/80 mm Hg for individuals with diabetes or kidney disease. The National High Blood Pressure Education Program stressed that SBP, not DBP, should become the major criterion for diagnosis and treatment of hypertension.9
Emphasize education and focus on systolic blood pressure
Randy Ward, MD
Director, Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
In light of JNC VII, there may be some confusion on the part of patients as to “normal” blood pressure and indications for treatment. In fact, on the first page of the NHLBI web site, “Your Guide to Lowering Blood Pressure,” the statement is made that “normal blood pressure is less than 120 mm Hg systolic and less than 80 mm Hg diastolic.” They later go on to describe the category of prehypertension. It is important to understand the concept and implications of prehypertension, and the “J-shaped” curve in counseling our patients on achieving optimal blood pressure control.
Although lowering diastolic blood pressure (DBP) is associated with reduced cardiovascular events, systolic blood pressure (SBP) is a more robust predictor of cardiovascular risk than DBP and should now be used to diagnose, stage, and treat hypertension.
Lowering diastolic blood pressure (DBP) to <90 mm Hg in hypertensive individuals of all ages decreases the risk of cardiovascular events including myocardial infarction (MI), heart failure, and sudden death (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials). However, there is no consensus regarding how far to lower DBP. A “J-shaped” increase in cardiovascular risks with DBP <85 mm Hg may apply under certain conditions.
Evidence summary
The concept of a continuous graded relationship between DBP and cardiovascular risk is supported by a meta-analysis of 14 randomized clinical trials showing that lowering DBP by 6 mm Hg reduced the risk of coronary heart disease by 14% (95% confidence interval [CI], 4%–22%; P<.01; NNT=200).1 Throughout the range of DBP in study subjects, 70–115 mm Hg, a lower DBP was associated with a lower risk of coronary heart disease.
However, there is concern that lowering DBP too much may actually increase cardiovascular risk. A 10-year observational study showed that in patients with a history of ischemic heart disease, the incidence of fatal MI was lowest when DBP was between 85 to 90 mm Hg and increased with DBP <85 mm Hg, thus demonstrating a J-shaped curve.2
Farnett et al3 derived a summary curve from 13 studies that stratified cardiovascular outcomes by level of achieved blood pressure; the nadir of the curve for ischemic heart disease events occurred at 86 to 89 mm Hg DBP. The risk was independent of type of drug therapy, and more pronounced in study subjects with known cardiovascular disease.
A meta-analysis of 7 randomized controlled trials involving 40,233 hypertensive patients used statistical modeling to determine the shape of the “mortality curve” over a range of DBP categories, defined in 10-mm Hg increments from 65 to 106. The subjects received mainly beta-blockers or thiazide diuretics; controls received placebo or no treatment.4 Both groups demonstrated increased risk for cardiovascular and all-cause death at the lowest DBP levels. Among treated patients, overall death rate was lowest with a DBP in the range of 76 to 85 mm Hg; among controls the nadir was 86 to 95 mm Hg.
The Hypertension Optimal Treatment (HOT) trial5 was specifically designed to determine the optimal target blood pressure for hypertensive patients: 18,790 men and women with DBP 100 to 115 mm Hg were randomly assigned to target DBP groups of <90, <85, or <80 mm Hg. All were treated with felodipine and other agents in a stepped-care protocol; average follow-up was 3.8 years. The lowest incidence of cardiovascular events occurred at a mean DBP of 82.6 mm Hg and fewest cardiovascular deaths at 86.5 mm Hg. Further reductions in DBP neither lowered nor increased cardiovascular risk.
A French cohort study6 followed over 4700 hyper-tensive men for an average of 14 years. These men had their hypertension treated in usual fashion by their own physicians. In this group, SBP was much more accurate than DBP in classifying severity of hypertension and in predicting cardiovascular risk.
Recommendations from others
The Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC VII)7 and the World Health Organization–International Society of Hyper-tension Guidelines8 state that the relationship between cardiovascular risk and blood pressure is continuous, without a lower threshold. Target blood pressure goals are <140/90 mm Hg in uncomplicated hypertension and <130/80 mm Hg for individuals with diabetes or kidney disease. The National High Blood Pressure Education Program stressed that SBP, not DBP, should become the major criterion for diagnosis and treatment of hypertension.9
Emphasize education and focus on systolic blood pressure
Randy Ward, MD
Director, Family Medicine/Psychiatry Residency, Medical College of Wisconsin, Milwaukee
In light of JNC VII, there may be some confusion on the part of patients as to “normal” blood pressure and indications for treatment. In fact, on the first page of the NHLBI web site, “Your Guide to Lowering Blood Pressure,” the statement is made that “normal blood pressure is less than 120 mm Hg systolic and less than 80 mm Hg diastolic.” They later go on to describe the category of prehypertension. It is important to understand the concept and implications of prehypertension, and the “J-shaped” curve in counseling our patients on achieving optimal blood pressure control.
1. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-838.
2. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-584.
3. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489-495.
4. Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. Jshaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002;136:438-448.
5. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients treated with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-1762.
6. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-581.
7. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003;289:2560-2572.
8. 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-183.
9. Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021-1024.
1. Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-838.
2. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-584.
3. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA 1991;265:489-495.
4. Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP. Jshaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002;136:438-448.
5. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients treated with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-1762.
6. Benetos A, Thomas F, Bean K, Gautier S, Smulyan H, Guize L. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-581.
7. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003;289:2560-2572.
8. 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-183.
9. Izzo JL, Jr, Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension 2000;35:1021-1024.
Evidence-based answers from the Family Physicians Inquiries Network
Is combining ACE inhibitors and ARBs helpful or harmful?
The combination of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) has been studied for treatment of heart failure, hypertension, and proteinuric renal disease. Combination therapy with an ACE inhibitor and an ARB decreases symptoms in heart failure patients, but does not appear to have an impact on overall mortality (strength of recommendation [SOR]: A).
Preliminary data from small trials indicate that combination therapy may be more effective than monotherapy with an ACE inhibitor or an ARB for lowering blood pressure (SOR: B), although morbidity and mortality data for the combination are not currently available. Additionally, in trials involving diabetic and nondiabetic proteinuric renal disease, the combination of ACE inhibitors and ARBs delays progression of renal disease to a greater extent than monotherapy; however, mortality data are also unavailable (SOR: A).
Evidence summary
ACE inhibitors have been used most commonly for the treatment of congestive heart failure and hypertension and to slow the progression of proteinuria. Their primary mechanism of action is the suppression of angiotensin II by blocking its formation via renin and angiotensin I, thereby reducing the main deleterious effects of angiotensin II, which are mediated through vaso-constriction. Other pathways of angiotensin II formation exist and may escape inhibition of the converting enzyme.1 ARBs block the action of angiotensin II at the AT1 receptor and may, in theory, provide additive benefit.
The data describing the use of the combination of an ACE inhibitor and an ARB in heart failure are from the Valsartan Heart Failure Trial (ValHeFT),2 the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity Trial (CHARM),3 and in the Valsartan in Acute Myocardial Infarction Trial (VALIANT).4
In ValHeFT, 5010 patients with systolic dysfunction were randomized to the ARB valsartan or placebo in addition to background therapy, which included an ACE inhibitor in 93% of subjects. The primary endpoints were mortality and combined mortality and morbidity. An increase in mortality was found among patients on the triple therapy combination of valsartan, an ACE inhibitor, and a beta-blocker (relative risk [RR]=1.4; 95% confidence interval [CI], 1.1–1.9). Among those not on beta-blockers, adding valsartan to baseline therapy of an ACE inhibitor resulted in a modest improvement in the combined endpoint (RR=0.8; 95% CI, 0.7–0.9), but no change in mortality alone was found.2
In CHARM, candesartan was added to baseline therapy among patients with heart failure. Baseline therapy included diuretics (90%), beta blockers (55%), spironolactone (17%), and other cardiovascular medications as necessary. In this study, those in the treatment arm had a decrease in the combined endpoint of cardiovascular death plus congestive heart failure admission (RR=0.85; 95% CI, 0.75–0.96), but no difference was seen in overall mortality. Of note, no adverse interaction was demonstrated for those on the triple combination of ACE inhibitors, ARBs, and beta-blockers.3
Similarly, VALIANT demonstrated the safety but the lack of incremental efficacy in adding valsartan to ACE inhibitors for patients with left ventricular dysfunction after a myocardial infarction.4
Limited evidence is available from randomized controlled trials on the safety or efficacy of combination therapy exclusively for hyperten-sive patients. The available published trials were short-term and assessed blood pressure rather than more clinically significant endpoints such as risk of cardiovascular events and mortality. One trial of 177 patients found no significant difference in 24-hour ambulatory mean diastolic blood pressure with combination therapy vs ACE inhibitor or ARB monotherapy, but did show a decrease in clinic diastolic blood pressure.5 Another small trial of 20 patients demonstrated improved ambulatory blood pressure control with combination therapy vs ACE inhibitor monotherapy.6
Several trials have investigated the effect of combination therapy on diabetic and nondiabet-ic proteinuria. Conclusions from these trials are limited by their small sample size and by measurement of intermediate outcomes without mortality data. The largest trial, COOPERATE, was conducted in Japan and included 336 patients with nondiabetic renal disease.7 The investigators found that significantly fewer patients receiving combination therapy reached the combined primary endpoint of time to doubling of serum creatinine or end-stage renal disease compared with patients receiving monotherapy. The CALM study included 199 patients with hypertension, micro-albuminuria, and type 2 diabetes mellitus, and demonstrated significantly greater attenuation of urinary albumin/creatinine ratio and significantly improved blood pressure control with combination therapy compared with either therapy alone.8
Another trial, ONTARGET, is being conducted to assess the impact of ACE inhibitor or ARB monotherapy and combination therapy on reducing cardiovascular risk; it includes a combined primary endpoint of morbidity and mortality. The study involves 23,400 high-risk patients and will have a follow-up period of 5.5 years. This trial enrolls patients who have coronary disease, cere-brovascular disease, peripheral vascular disease, or diabetes with end-organ damage (inclusion and exclusion criteria are based upon those used in the HOPE study).
Recommendations from others
We were unable to find to find any recommendations regarding the addition of ARB drugs to ACE inhibitors.
Adding ARBs to ACE inhibitors: Good in theory, but clinical evidence is still weak
David Kilgore, MD
Tacoma Family Medicine, Tacoma, Wash
There is good evidence of the benefits of angiotensin inhibition in multiple diseases, so it is logical to ask if adding receptor blockers adds further benefit. For now, it appears that the addition of an ARB to an ACE inhibitor is an idea that sounds good in theory, but needs more data to prove its clinical benefit and safety.
The clinical evidence for the combo in heart failure and hypertension is weak, since mortality data are lacking and there is the troubling association with increased mortality in the presence of beta blockers. Using the combination is not currently recommended by the major national guidelines for those areas (eg, American Heart Association, Joint National Committee VII). Although the benefit for patients with proteinuria appears promising, we still await evidence for decreasing mortality. Given cost and the combination’s uncertain benefit, it would be prudent to wait until the completion of studies currently in progress before we embrace it.
1. Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study Group. Circulation 1999;99:2658-2664.
2. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-1675.
3. McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-771.
4. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-1906.
5. Azizi M, Linhart A, Alexander J, et al. Pilot study of combined blockade of the renin-angiotensin system in essential hypertensive patients. J Hypertens 2000;18:1139-1147.
6. Stergiou GS, Skeva II, Baibas NM, et al. Additive hypotensive effect of angiotensin-converting enzyme inhibition and angiotensin-receptor antagonism in essential hypertension. J Cardiovasc Pharmacol 2000;35:937-941.
7. Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 2003;361:117-124.
8. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of the renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000;321:1440-1444.
The combination of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) has been studied for treatment of heart failure, hypertension, and proteinuric renal disease. Combination therapy with an ACE inhibitor and an ARB decreases symptoms in heart failure patients, but does not appear to have an impact on overall mortality (strength of recommendation [SOR]: A).
Preliminary data from small trials indicate that combination therapy may be more effective than monotherapy with an ACE inhibitor or an ARB for lowering blood pressure (SOR: B), although morbidity and mortality data for the combination are not currently available. Additionally, in trials involving diabetic and nondiabetic proteinuric renal disease, the combination of ACE inhibitors and ARBs delays progression of renal disease to a greater extent than monotherapy; however, mortality data are also unavailable (SOR: A).
Evidence summary
ACE inhibitors have been used most commonly for the treatment of congestive heart failure and hypertension and to slow the progression of proteinuria. Their primary mechanism of action is the suppression of angiotensin II by blocking its formation via renin and angiotensin I, thereby reducing the main deleterious effects of angiotensin II, which are mediated through vaso-constriction. Other pathways of angiotensin II formation exist and may escape inhibition of the converting enzyme.1 ARBs block the action of angiotensin II at the AT1 receptor and may, in theory, provide additive benefit.
The data describing the use of the combination of an ACE inhibitor and an ARB in heart failure are from the Valsartan Heart Failure Trial (ValHeFT),2 the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity Trial (CHARM),3 and in the Valsartan in Acute Myocardial Infarction Trial (VALIANT).4
In ValHeFT, 5010 patients with systolic dysfunction were randomized to the ARB valsartan or placebo in addition to background therapy, which included an ACE inhibitor in 93% of subjects. The primary endpoints were mortality and combined mortality and morbidity. An increase in mortality was found among patients on the triple therapy combination of valsartan, an ACE inhibitor, and a beta-blocker (relative risk [RR]=1.4; 95% confidence interval [CI], 1.1–1.9). Among those not on beta-blockers, adding valsartan to baseline therapy of an ACE inhibitor resulted in a modest improvement in the combined endpoint (RR=0.8; 95% CI, 0.7–0.9), but no change in mortality alone was found.2
In CHARM, candesartan was added to baseline therapy among patients with heart failure. Baseline therapy included diuretics (90%), beta blockers (55%), spironolactone (17%), and other cardiovascular medications as necessary. In this study, those in the treatment arm had a decrease in the combined endpoint of cardiovascular death plus congestive heart failure admission (RR=0.85; 95% CI, 0.75–0.96), but no difference was seen in overall mortality. Of note, no adverse interaction was demonstrated for those on the triple combination of ACE inhibitors, ARBs, and beta-blockers.3
Similarly, VALIANT demonstrated the safety but the lack of incremental efficacy in adding valsartan to ACE inhibitors for patients with left ventricular dysfunction after a myocardial infarction.4
Limited evidence is available from randomized controlled trials on the safety or efficacy of combination therapy exclusively for hyperten-sive patients. The available published trials were short-term and assessed blood pressure rather than more clinically significant endpoints such as risk of cardiovascular events and mortality. One trial of 177 patients found no significant difference in 24-hour ambulatory mean diastolic blood pressure with combination therapy vs ACE inhibitor or ARB monotherapy, but did show a decrease in clinic diastolic blood pressure.5 Another small trial of 20 patients demonstrated improved ambulatory blood pressure control with combination therapy vs ACE inhibitor monotherapy.6
Several trials have investigated the effect of combination therapy on diabetic and nondiabet-ic proteinuria. Conclusions from these trials are limited by their small sample size and by measurement of intermediate outcomes without mortality data. The largest trial, COOPERATE, was conducted in Japan and included 336 patients with nondiabetic renal disease.7 The investigators found that significantly fewer patients receiving combination therapy reached the combined primary endpoint of time to doubling of serum creatinine or end-stage renal disease compared with patients receiving monotherapy. The CALM study included 199 patients with hypertension, micro-albuminuria, and type 2 diabetes mellitus, and demonstrated significantly greater attenuation of urinary albumin/creatinine ratio and significantly improved blood pressure control with combination therapy compared with either therapy alone.8
Another trial, ONTARGET, is being conducted to assess the impact of ACE inhibitor or ARB monotherapy and combination therapy on reducing cardiovascular risk; it includes a combined primary endpoint of morbidity and mortality. The study involves 23,400 high-risk patients and will have a follow-up period of 5.5 years. This trial enrolls patients who have coronary disease, cere-brovascular disease, peripheral vascular disease, or diabetes with end-organ damage (inclusion and exclusion criteria are based upon those used in the HOPE study).
Recommendations from others
We were unable to find to find any recommendations regarding the addition of ARB drugs to ACE inhibitors.
Adding ARBs to ACE inhibitors: Good in theory, but clinical evidence is still weak
David Kilgore, MD
Tacoma Family Medicine, Tacoma, Wash
There is good evidence of the benefits of angiotensin inhibition in multiple diseases, so it is logical to ask if adding receptor blockers adds further benefit. For now, it appears that the addition of an ARB to an ACE inhibitor is an idea that sounds good in theory, but needs more data to prove its clinical benefit and safety.
The clinical evidence for the combo in heart failure and hypertension is weak, since mortality data are lacking and there is the troubling association with increased mortality in the presence of beta blockers. Using the combination is not currently recommended by the major national guidelines for those areas (eg, American Heart Association, Joint National Committee VII). Although the benefit for patients with proteinuria appears promising, we still await evidence for decreasing mortality. Given cost and the combination’s uncertain benefit, it would be prudent to wait until the completion of studies currently in progress before we embrace it.
The combination of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) has been studied for treatment of heart failure, hypertension, and proteinuric renal disease. Combination therapy with an ACE inhibitor and an ARB decreases symptoms in heart failure patients, but does not appear to have an impact on overall mortality (strength of recommendation [SOR]: A).
Preliminary data from small trials indicate that combination therapy may be more effective than monotherapy with an ACE inhibitor or an ARB for lowering blood pressure (SOR: B), although morbidity and mortality data for the combination are not currently available. Additionally, in trials involving diabetic and nondiabetic proteinuric renal disease, the combination of ACE inhibitors and ARBs delays progression of renal disease to a greater extent than monotherapy; however, mortality data are also unavailable (SOR: A).
Evidence summary
ACE inhibitors have been used most commonly for the treatment of congestive heart failure and hypertension and to slow the progression of proteinuria. Their primary mechanism of action is the suppression of angiotensin II by blocking its formation via renin and angiotensin I, thereby reducing the main deleterious effects of angiotensin II, which are mediated through vaso-constriction. Other pathways of angiotensin II formation exist and may escape inhibition of the converting enzyme.1 ARBs block the action of angiotensin II at the AT1 receptor and may, in theory, provide additive benefit.
The data describing the use of the combination of an ACE inhibitor and an ARB in heart failure are from the Valsartan Heart Failure Trial (ValHeFT),2 the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity Trial (CHARM),3 and in the Valsartan in Acute Myocardial Infarction Trial (VALIANT).4
In ValHeFT, 5010 patients with systolic dysfunction were randomized to the ARB valsartan or placebo in addition to background therapy, which included an ACE inhibitor in 93% of subjects. The primary endpoints were mortality and combined mortality and morbidity. An increase in mortality was found among patients on the triple therapy combination of valsartan, an ACE inhibitor, and a beta-blocker (relative risk [RR]=1.4; 95% confidence interval [CI], 1.1–1.9). Among those not on beta-blockers, adding valsartan to baseline therapy of an ACE inhibitor resulted in a modest improvement in the combined endpoint (RR=0.8; 95% CI, 0.7–0.9), but no change in mortality alone was found.2
In CHARM, candesartan was added to baseline therapy among patients with heart failure. Baseline therapy included diuretics (90%), beta blockers (55%), spironolactone (17%), and other cardiovascular medications as necessary. In this study, those in the treatment arm had a decrease in the combined endpoint of cardiovascular death plus congestive heart failure admission (RR=0.85; 95% CI, 0.75–0.96), but no difference was seen in overall mortality. Of note, no adverse interaction was demonstrated for those on the triple combination of ACE inhibitors, ARBs, and beta-blockers.3
Similarly, VALIANT demonstrated the safety but the lack of incremental efficacy in adding valsartan to ACE inhibitors for patients with left ventricular dysfunction after a myocardial infarction.4
Limited evidence is available from randomized controlled trials on the safety or efficacy of combination therapy exclusively for hyperten-sive patients. The available published trials were short-term and assessed blood pressure rather than more clinically significant endpoints such as risk of cardiovascular events and mortality. One trial of 177 patients found no significant difference in 24-hour ambulatory mean diastolic blood pressure with combination therapy vs ACE inhibitor or ARB monotherapy, but did show a decrease in clinic diastolic blood pressure.5 Another small trial of 20 patients demonstrated improved ambulatory blood pressure control with combination therapy vs ACE inhibitor monotherapy.6
Several trials have investigated the effect of combination therapy on diabetic and nondiabet-ic proteinuria. Conclusions from these trials are limited by their small sample size and by measurement of intermediate outcomes without mortality data. The largest trial, COOPERATE, was conducted in Japan and included 336 patients with nondiabetic renal disease.7 The investigators found that significantly fewer patients receiving combination therapy reached the combined primary endpoint of time to doubling of serum creatinine or end-stage renal disease compared with patients receiving monotherapy. The CALM study included 199 patients with hypertension, micro-albuminuria, and type 2 diabetes mellitus, and demonstrated significantly greater attenuation of urinary albumin/creatinine ratio and significantly improved blood pressure control with combination therapy compared with either therapy alone.8
Another trial, ONTARGET, is being conducted to assess the impact of ACE inhibitor or ARB monotherapy and combination therapy on reducing cardiovascular risk; it includes a combined primary endpoint of morbidity and mortality. The study involves 23,400 high-risk patients and will have a follow-up period of 5.5 years. This trial enrolls patients who have coronary disease, cere-brovascular disease, peripheral vascular disease, or diabetes with end-organ damage (inclusion and exclusion criteria are based upon those used in the HOPE study).
Recommendations from others
We were unable to find to find any recommendations regarding the addition of ARB drugs to ACE inhibitors.
Adding ARBs to ACE inhibitors: Good in theory, but clinical evidence is still weak
David Kilgore, MD
Tacoma Family Medicine, Tacoma, Wash
There is good evidence of the benefits of angiotensin inhibition in multiple diseases, so it is logical to ask if adding receptor blockers adds further benefit. For now, it appears that the addition of an ARB to an ACE inhibitor is an idea that sounds good in theory, but needs more data to prove its clinical benefit and safety.
The clinical evidence for the combo in heart failure and hypertension is weak, since mortality data are lacking and there is the troubling association with increased mortality in the presence of beta blockers. Using the combination is not currently recommended by the major national guidelines for those areas (eg, American Heart Association, Joint National Committee VII). Although the benefit for patients with proteinuria appears promising, we still await evidence for decreasing mortality. Given cost and the combination’s uncertain benefit, it would be prudent to wait until the completion of studies currently in progress before we embrace it.
1. Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study Group. Circulation 1999;99:2658-2664.
2. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-1675.
3. McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-771.
4. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-1906.
5. Azizi M, Linhart A, Alexander J, et al. Pilot study of combined blockade of the renin-angiotensin system in essential hypertensive patients. J Hypertens 2000;18:1139-1147.
6. Stergiou GS, Skeva II, Baibas NM, et al. Additive hypotensive effect of angiotensin-converting enzyme inhibition and angiotensin-receptor antagonism in essential hypertension. J Cardiovasc Pharmacol 2000;35:937-941.
7. Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 2003;361:117-124.
8. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of the renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000;321:1440-1444.
1. Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study Group. Circulation 1999;99:2658-2664.
2. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-1675.
3. McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-771.
4. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-1906.
5. Azizi M, Linhart A, Alexander J, et al. Pilot study of combined blockade of the renin-angiotensin system in essential hypertensive patients. J Hypertens 2000;18:1139-1147.
6. Stergiou GS, Skeva II, Baibas NM, et al. Additive hypotensive effect of angiotensin-converting enzyme inhibition and angiotensin-receptor antagonism in essential hypertension. J Cardiovasc Pharmacol 2000;35:937-941.
7. Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 2003;361:117-124.
8. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of the renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000;321:1440-1444.
Evidence-based answers from the Family Physicians Inquiries Network
When should we treat isolated high triglycerides?
No evidence exists that treating isolated high triglyceride levels in the absence of other risk factors prevents coronary events. Although elevated triglycerides in some studies correlates with coronary events, the association weakens when controlled for factors such as diabetes, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, body mass index, and other cardiac risk factors.
Coincident lowering of triglycerides, while treating other dyslipidemias (such as high LDL and low HDL), can contribute to decreasing coronary events (strength of recommendation [SOR]: A, based randomized controlled trials). Treating triglyceride levels over 500 to 1000 mg/dL may reduce the risk of pancreatitis (SOR: C, expert opinion).
Evidence summary
Truly isolated hypertriglyceridemia is rare. To date, no good trials directly address the effect of reducing truly isolated hypertriglyceridemia on cardiovascular morbidity or mortality. High triglycerides are usually accompanied by other features of the “metabolic syndrome” (low HDL, high LDL, insulin resistance, diabetes, hypertension, and obesity), making it almost impossible to look at these in isolation or attribute risk to a specific component.1
Whether high triglyceride levels pose risk in the true absence of these other metabolic factors is controversial. One meta-analysis of 17 population-based prospective studies of triglycerides and cardiovascular disease (including 57,000 patients) showed high triglyceride levels to be predictive of cardiac events, even when adjusted for HDL and other risk factors (age, total and LDL cholesterol, smoking, body mass index, and blood pressure).2 After adjusting for these other risk factors, the authors found an increased risk for all cardiac endpoints (myocardial infarction, death, etc) of 14% for men and 32% for women (Men: relative risk [RR]=1.14; 95% confidence interval [CI], 1.05–1.28; Women: RR=1.37; 95% CI, 1.13–1.66).
Another meta-analysis of 3 prospective intervention trials with 15,880 enrolled subjects found that triglyceride levels did not provide any clinically meaningful information about risk beyond that provided by other cholesterol subfractions.3
In treatment trials, the most impressive risk reductions come from the groups who fit the lipid triad of low HDL, high LDL, and high triglycerides. Low levels of HDL appear to interact with hypertriglyceridemia to increase coronary risk, and all studies showing improved outcomes have simultaneously increased HDL while lowering triglycerides.4-6 In 3 large-scale prospective, placebo-controlled trials (the Helsinki Heart Study, a primary prevention study, and the VA-HIT and Bezafibrate Infarction Prevention trials, both secondary prevention studies), lowering triglycerides and raising HDL concurrently improved outcomes.5 Successful dietary and medical interventions, especially with statins and fibrates, improved overall lipid profiles—not just triglyceride levels.
Accordingly, elevated triglycerides should prompt providers to rigorously identify these other risk factors for cardiovascular morbidity and mortality, which may not be immediately obvious. In the absence of such other factors, no evidence exists to guide therapy. Expert opinion7,8 supported by epidemiologic evidence9 suggests that patients with triglyceride levels of 500 to 1000 mg/dL may have an increased risk of pancreatitis. Accordingly, providers should consider therapy to lower triglycerides to less than 500 in these patients, regardless of accompanying risk factors.
Recommendations from others
The American College of Physicians, the European Society of Cardiology, and the US Preventive Services Task Force do not recommend screening for hypertriglyceridemia. Clinical guidelines of the National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III), American Heart Association/American College of Cardiology, and the American Diabetes Association all support LDL lowering as the primary target of therapy based on the patients risk profile.10 NCEP/ATP III has identified triglyceride levels of <150 as normal, 150–199 as borderline high, 200–499 as high, and 500 as very high.7
A patient with high triglycerides should prompt a search for components of the “meta-bolic syndrome” and secondary causes, including high dietary fat, high alcohol intake, drugs (steroids, beta-blockers, high-estrogen oral contraceptives), medical conditions (hypothyroidism, nephrosis, renal failure, liver disease, Cushing disease, and lupus), and rare familial dyslipidemias.7,10,11
Elevated triglyceride level? First look at the big picture
Donald C. Spencer, MD, MBA
University of North Carolina at Chapel Hill
Observing the pendulum swings of medical knowledge over time is one of the hallmarks of the experienced family physician. As a student, I was warned of the evils of high triglycerides, only to enter a period in the 1970s and 1980s of therapeutic nihilism when triglycerides were not thought to be an independent coronary risk factor.
As outlined here, the pendulum is moving toward a more complex consideration of the effect of triglycerides on heart disease—and what we should do about it. Our patients are better served when we focus on total coronary risk rather than a specific level of triglycerides. An elevated triglyceride level leads me first to look at the glucose. I have found several poorly controlled or even new diabetic patients this way. By then following the adage to “major on the majors and minor on the minors,” I have focused on glucose and LDL control to the benefit of my patients.
1. Forrester JS. Triglycerides: risk factor or fellow traveler? Curr Opin Cardiol 2001;16:261-264.
2. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998;81(4A):7B-12B.
3. Avins AL, Neuhaus JM. Do triglycerides provide meaningful information about heart disease risk? Arch Intern Med 2000;160:1937-1944.
4. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410-418.
5. Secondary prevention by raising HDL cholesterol and reducing triglyceride in patients with coronary disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-27.
6. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study a randomised study. Lancet 2001;357:905-910.
7. Expert Panel on Detection. Evaluation and. Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
8. Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Intern Med 1992;37:249-273.
9. Athyros VG, Giouleme OI, Nikolaidis NL, et al. Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis. J Clin Gastroenterol 2002;34:472-475.
10. Breuer HW. Hypertriglyceridemia: a review of clinical relevance and treatment options: focus on cerivastatin. Curr Med Res Opin 2001;17:60-73.
11. Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med 2001;47:111-136.
No evidence exists that treating isolated high triglyceride levels in the absence of other risk factors prevents coronary events. Although elevated triglycerides in some studies correlates with coronary events, the association weakens when controlled for factors such as diabetes, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, body mass index, and other cardiac risk factors.
Coincident lowering of triglycerides, while treating other dyslipidemias (such as high LDL and low HDL), can contribute to decreasing coronary events (strength of recommendation [SOR]: A, based randomized controlled trials). Treating triglyceride levels over 500 to 1000 mg/dL may reduce the risk of pancreatitis (SOR: C, expert opinion).
Evidence summary
Truly isolated hypertriglyceridemia is rare. To date, no good trials directly address the effect of reducing truly isolated hypertriglyceridemia on cardiovascular morbidity or mortality. High triglycerides are usually accompanied by other features of the “metabolic syndrome” (low HDL, high LDL, insulin resistance, diabetes, hypertension, and obesity), making it almost impossible to look at these in isolation or attribute risk to a specific component.1
Whether high triglyceride levels pose risk in the true absence of these other metabolic factors is controversial. One meta-analysis of 17 population-based prospective studies of triglycerides and cardiovascular disease (including 57,000 patients) showed high triglyceride levels to be predictive of cardiac events, even when adjusted for HDL and other risk factors (age, total and LDL cholesterol, smoking, body mass index, and blood pressure).2 After adjusting for these other risk factors, the authors found an increased risk for all cardiac endpoints (myocardial infarction, death, etc) of 14% for men and 32% for women (Men: relative risk [RR]=1.14; 95% confidence interval [CI], 1.05–1.28; Women: RR=1.37; 95% CI, 1.13–1.66).
Another meta-analysis of 3 prospective intervention trials with 15,880 enrolled subjects found that triglyceride levels did not provide any clinically meaningful information about risk beyond that provided by other cholesterol subfractions.3
In treatment trials, the most impressive risk reductions come from the groups who fit the lipid triad of low HDL, high LDL, and high triglycerides. Low levels of HDL appear to interact with hypertriglyceridemia to increase coronary risk, and all studies showing improved outcomes have simultaneously increased HDL while lowering triglycerides.4-6 In 3 large-scale prospective, placebo-controlled trials (the Helsinki Heart Study, a primary prevention study, and the VA-HIT and Bezafibrate Infarction Prevention trials, both secondary prevention studies), lowering triglycerides and raising HDL concurrently improved outcomes.5 Successful dietary and medical interventions, especially with statins and fibrates, improved overall lipid profiles—not just triglyceride levels.
Accordingly, elevated triglycerides should prompt providers to rigorously identify these other risk factors for cardiovascular morbidity and mortality, which may not be immediately obvious. In the absence of such other factors, no evidence exists to guide therapy. Expert opinion7,8 supported by epidemiologic evidence9 suggests that patients with triglyceride levels of 500 to 1000 mg/dL may have an increased risk of pancreatitis. Accordingly, providers should consider therapy to lower triglycerides to less than 500 in these patients, regardless of accompanying risk factors.
Recommendations from others
The American College of Physicians, the European Society of Cardiology, and the US Preventive Services Task Force do not recommend screening for hypertriglyceridemia. Clinical guidelines of the National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III), American Heart Association/American College of Cardiology, and the American Diabetes Association all support LDL lowering as the primary target of therapy based on the patients risk profile.10 NCEP/ATP III has identified triglyceride levels of <150 as normal, 150–199 as borderline high, 200–499 as high, and 500 as very high.7
A patient with high triglycerides should prompt a search for components of the “meta-bolic syndrome” and secondary causes, including high dietary fat, high alcohol intake, drugs (steroids, beta-blockers, high-estrogen oral contraceptives), medical conditions (hypothyroidism, nephrosis, renal failure, liver disease, Cushing disease, and lupus), and rare familial dyslipidemias.7,10,11
Elevated triglyceride level? First look at the big picture
Donald C. Spencer, MD, MBA
University of North Carolina at Chapel Hill
Observing the pendulum swings of medical knowledge over time is one of the hallmarks of the experienced family physician. As a student, I was warned of the evils of high triglycerides, only to enter a period in the 1970s and 1980s of therapeutic nihilism when triglycerides were not thought to be an independent coronary risk factor.
As outlined here, the pendulum is moving toward a more complex consideration of the effect of triglycerides on heart disease—and what we should do about it. Our patients are better served when we focus on total coronary risk rather than a specific level of triglycerides. An elevated triglyceride level leads me first to look at the glucose. I have found several poorly controlled or even new diabetic patients this way. By then following the adage to “major on the majors and minor on the minors,” I have focused on glucose and LDL control to the benefit of my patients.
No evidence exists that treating isolated high triglyceride levels in the absence of other risk factors prevents coronary events. Although elevated triglycerides in some studies correlates with coronary events, the association weakens when controlled for factors such as diabetes, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, body mass index, and other cardiac risk factors.
Coincident lowering of triglycerides, while treating other dyslipidemias (such as high LDL and low HDL), can contribute to decreasing coronary events (strength of recommendation [SOR]: A, based randomized controlled trials). Treating triglyceride levels over 500 to 1000 mg/dL may reduce the risk of pancreatitis (SOR: C, expert opinion).
Evidence summary
Truly isolated hypertriglyceridemia is rare. To date, no good trials directly address the effect of reducing truly isolated hypertriglyceridemia on cardiovascular morbidity or mortality. High triglycerides are usually accompanied by other features of the “metabolic syndrome” (low HDL, high LDL, insulin resistance, diabetes, hypertension, and obesity), making it almost impossible to look at these in isolation or attribute risk to a specific component.1
Whether high triglyceride levels pose risk in the true absence of these other metabolic factors is controversial. One meta-analysis of 17 population-based prospective studies of triglycerides and cardiovascular disease (including 57,000 patients) showed high triglyceride levels to be predictive of cardiac events, even when adjusted for HDL and other risk factors (age, total and LDL cholesterol, smoking, body mass index, and blood pressure).2 After adjusting for these other risk factors, the authors found an increased risk for all cardiac endpoints (myocardial infarction, death, etc) of 14% for men and 32% for women (Men: relative risk [RR]=1.14; 95% confidence interval [CI], 1.05–1.28; Women: RR=1.37; 95% CI, 1.13–1.66).
Another meta-analysis of 3 prospective intervention trials with 15,880 enrolled subjects found that triglyceride levels did not provide any clinically meaningful information about risk beyond that provided by other cholesterol subfractions.3
In treatment trials, the most impressive risk reductions come from the groups who fit the lipid triad of low HDL, high LDL, and high triglycerides. Low levels of HDL appear to interact with hypertriglyceridemia to increase coronary risk, and all studies showing improved outcomes have simultaneously increased HDL while lowering triglycerides.4-6 In 3 large-scale prospective, placebo-controlled trials (the Helsinki Heart Study, a primary prevention study, and the VA-HIT and Bezafibrate Infarction Prevention trials, both secondary prevention studies), lowering triglycerides and raising HDL concurrently improved outcomes.5 Successful dietary and medical interventions, especially with statins and fibrates, improved overall lipid profiles—not just triglyceride levels.
Accordingly, elevated triglycerides should prompt providers to rigorously identify these other risk factors for cardiovascular morbidity and mortality, which may not be immediately obvious. In the absence of such other factors, no evidence exists to guide therapy. Expert opinion7,8 supported by epidemiologic evidence9 suggests that patients with triglyceride levels of 500 to 1000 mg/dL may have an increased risk of pancreatitis. Accordingly, providers should consider therapy to lower triglycerides to less than 500 in these patients, regardless of accompanying risk factors.
Recommendations from others
The American College of Physicians, the European Society of Cardiology, and the US Preventive Services Task Force do not recommend screening for hypertriglyceridemia. Clinical guidelines of the National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III), American Heart Association/American College of Cardiology, and the American Diabetes Association all support LDL lowering as the primary target of therapy based on the patients risk profile.10 NCEP/ATP III has identified triglyceride levels of <150 as normal, 150–199 as borderline high, 200–499 as high, and 500 as very high.7
A patient with high triglycerides should prompt a search for components of the “meta-bolic syndrome” and secondary causes, including high dietary fat, high alcohol intake, drugs (steroids, beta-blockers, high-estrogen oral contraceptives), medical conditions (hypothyroidism, nephrosis, renal failure, liver disease, Cushing disease, and lupus), and rare familial dyslipidemias.7,10,11
Elevated triglyceride level? First look at the big picture
Donald C. Spencer, MD, MBA
University of North Carolina at Chapel Hill
Observing the pendulum swings of medical knowledge over time is one of the hallmarks of the experienced family physician. As a student, I was warned of the evils of high triglycerides, only to enter a period in the 1970s and 1980s of therapeutic nihilism when triglycerides were not thought to be an independent coronary risk factor.
As outlined here, the pendulum is moving toward a more complex consideration of the effect of triglycerides on heart disease—and what we should do about it. Our patients are better served when we focus on total coronary risk rather than a specific level of triglycerides. An elevated triglyceride level leads me first to look at the glucose. I have found several poorly controlled or even new diabetic patients this way. By then following the adage to “major on the majors and minor on the minors,” I have focused on glucose and LDL control to the benefit of my patients.
1. Forrester JS. Triglycerides: risk factor or fellow traveler? Curr Opin Cardiol 2001;16:261-264.
2. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998;81(4A):7B-12B.
3. Avins AL, Neuhaus JM. Do triglycerides provide meaningful information about heart disease risk? Arch Intern Med 2000;160:1937-1944.
4. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410-418.
5. Secondary prevention by raising HDL cholesterol and reducing triglyceride in patients with coronary disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-27.
6. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study a randomised study. Lancet 2001;357:905-910.
7. Expert Panel on Detection. Evaluation and. Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
8. Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Intern Med 1992;37:249-273.
9. Athyros VG, Giouleme OI, Nikolaidis NL, et al. Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis. J Clin Gastroenterol 2002;34:472-475.
10. Breuer HW. Hypertriglyceridemia: a review of clinical relevance and treatment options: focus on cerivastatin. Curr Med Res Opin 2001;17:60-73.
11. Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med 2001;47:111-136.
1. Forrester JS. Triglycerides: risk factor or fellow traveler? Curr Opin Cardiol 2001;16:261-264.
2. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998;81(4A):7B-12B.
3. Avins AL, Neuhaus JM. Do triglycerides provide meaningful information about heart disease risk? Arch Intern Med 2000;160:1937-1944.
4. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410-418.
5. Secondary prevention by raising HDL cholesterol and reducing triglyceride in patients with coronary disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-27.
6. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study a randomised study. Lancet 2001;357:905-910.
7. Expert Panel on Detection. Evaluation and. Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-2497.
8. Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Intern Med 1992;37:249-273.
9. Athyros VG, Giouleme OI, Nikolaidis NL, et al. Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis. J Clin Gastroenterol 2002;34:472-475.
10. Breuer HW. Hypertriglyceridemia: a review of clinical relevance and treatment options: focus on cerivastatin. Curr Med Res Opin 2001;17:60-73.
11. Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med 2001;47:111-136.
Evidence-based answers from the Family Physicians Inquiries Network