User login
Bell’s palsy
What is Bell’s palsy?
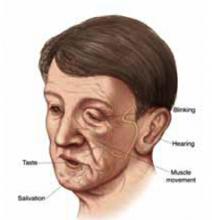
Weakness and slumping on one side of the face are common features of this condition in which the nerve controlling the face has been injured. Other symptoms are:
- Inability to move affected side of the face
- Drooping mouth, unblinking eye
- Numbness
- Twitching of facial muscles
- Taste disturbance
- Increased sensitivity to sound.
Symptoms usually start suddenly. Pain behind the ear may be felt hours to days before other symptoms appear. People between ages 30 and 60 years are most likely to be affected, but this disorder can happen at any age.
Any sudden weakness of the face also suggests the possibility of stroke—a serious emergency. You should contact your physician immediately.
Possible causes
The most common cause of Bell’s palsy is an infection of the facial nerve by the herpes simplex virus. Your doctor will also ask about possible recent trauma to the face and will check for swelling of facial tissues or a mass pressing on the facial nerve.
What to expect
In many cases, the symptoms of Bell’s palsy go away in about 3 weeks without treatment, and the face regains its normal appearance. A good sign that this will happen is if the weakness or other symptoms begin to resolve after 1 week.
In other cases, symptoms may take several months to disappear. Lasting effects are possible, though rare.
Treatments your doctor may prescribe
If a viral infection is the likely cause of your symptoms, your doctor may have you take acyclovir (Zovirax), famciclovir (Famvir), or another antiviral medication to speed your recovery. These antiviral medications are taken as pills, usually a few times a day for 10 days.
A steroid (prednisone, for example) may also help to reduce swelling that could be pressing on the facial nerve. This medication is also administered as a pill over 1 to 2 weeks.
If you cannot blink, and dryness of the eye is one of your symptoms, your doctor will ask you to use moisturizing eye drops to protect the eye from damage while you recover.
The variety of different symptoms you are experiencing is due to the fact the facial nerve controls normal functions from your forehead to your chin—including tearing, taste, muscle movement, and blinking.
What is Bell’s palsy?
Weakness and slumping on one side of the face are common features of this condition in which the nerve controlling the face has been injured. Other symptoms are:
- Inability to move affected side of the face
- Drooping mouth, unblinking eye
- Numbness
- Twitching of facial muscles
- Taste disturbance
- Increased sensitivity to sound.
Symptoms usually start suddenly. Pain behind the ear may be felt hours to days before other symptoms appear. People between ages 30 and 60 years are most likely to be affected, but this disorder can happen at any age.
Any sudden weakness of the face also suggests the possibility of stroke—a serious emergency. You should contact your physician immediately.
Possible causes
The most common cause of Bell’s palsy is an infection of the facial nerve by the herpes simplex virus. Your doctor will also ask about possible recent trauma to the face and will check for swelling of facial tissues or a mass pressing on the facial nerve.
What to expect
In many cases, the symptoms of Bell’s palsy go away in about 3 weeks without treatment, and the face regains its normal appearance. A good sign that this will happen is if the weakness or other symptoms begin to resolve after 1 week.
In other cases, symptoms may take several months to disappear. Lasting effects are possible, though rare.
Treatments your doctor may prescribe
If a viral infection is the likely cause of your symptoms, your doctor may have you take acyclovir (Zovirax), famciclovir (Famvir), or another antiviral medication to speed your recovery. These antiviral medications are taken as pills, usually a few times a day for 10 days.
A steroid (prednisone, for example) may also help to reduce swelling that could be pressing on the facial nerve. This medication is also administered as a pill over 1 to 2 weeks.
If you cannot blink, and dryness of the eye is one of your symptoms, your doctor will ask you to use moisturizing eye drops to protect the eye from damage while you recover.
The variety of different symptoms you are experiencing is due to the fact the facial nerve controls normal functions from your forehead to your chin—including tearing, taste, muscle movement, and blinking.
What is Bell’s palsy?
Weakness and slumping on one side of the face are common features of this condition in which the nerve controlling the face has been injured. Other symptoms are:
- Inability to move affected side of the face
- Drooping mouth, unblinking eye
- Numbness
- Twitching of facial muscles
- Taste disturbance
- Increased sensitivity to sound.
Symptoms usually start suddenly. Pain behind the ear may be felt hours to days before other symptoms appear. People between ages 30 and 60 years are most likely to be affected, but this disorder can happen at any age.
Any sudden weakness of the face also suggests the possibility of stroke—a serious emergency. You should contact your physician immediately.
Possible causes
The most common cause of Bell’s palsy is an infection of the facial nerve by the herpes simplex virus. Your doctor will also ask about possible recent trauma to the face and will check for swelling of facial tissues or a mass pressing on the facial nerve.
What to expect
In many cases, the symptoms of Bell’s palsy go away in about 3 weeks without treatment, and the face regains its normal appearance. A good sign that this will happen is if the weakness or other symptoms begin to resolve after 1 week.
In other cases, symptoms may take several months to disappear. Lasting effects are possible, though rare.
Treatments your doctor may prescribe
If a viral infection is the likely cause of your symptoms, your doctor may have you take acyclovir (Zovirax), famciclovir (Famvir), or another antiviral medication to speed your recovery. These antiviral medications are taken as pills, usually a few times a day for 10 days.
A steroid (prednisone, for example) may also help to reduce swelling that could be pressing on the facial nerve. This medication is also administered as a pill over 1 to 2 weeks.
If you cannot blink, and dryness of the eye is one of your symptoms, your doctor will ask you to use moisturizing eye drops to protect the eye from damage while you recover.
The variety of different symptoms you are experiencing is due to the fact the facial nerve controls normal functions from your forehead to your chin—including tearing, taste, muscle movement, and blinking.
Evidence-based answers from the Family Physicians Inquiries Network
Are drug therapies effective in treating Bell’s palsy?
Early use of corticosteroid therapy results in less autonomic synkinesis and possibly improved rates of recovery in adults (strength of recommendation: C); there is no proven benefit in children (SOR: B).
Adding acyclovir (Zovirax) to prednisone therapy may improve recovery rates compared with prednisone alone (SOR: C).
The results of 1 nonblinded study indicate that intramuscular methylcobalamin (vitamin B12) used alone or in combination with prednisone may shorten time to recovery (SOR: C).
See the Patient Information at the end of this article.
Evidence summary
Bell’s palsy is a lower motor neuron disease of the facial nerve characterized by a transient paralysis. Healing is occasionally incomplete, resulting in residual nerve dysfunction, including partial palsy and motor synkinesis (involuntary movement accompanying a voluntary one) and autonomic synkinesis (involuntary lacrimation after a voluntary muscle movement). Bell’s palsy is associated with significant edema and ischemia of the facial nerve as it passes through its bony canal.
Herpes simplex reactivation has been shown to be associated with a large proportion of cases.
Corticosteroids are the most studied form of therapy for Bell’s palsy (Table). Early work in England culminated in 1971 with a well-performed study demonstrating lower rates of incomplete recovery with prednisolone compared with corticotrophin.1 A potentially definitive randomized controlled trial in 1970 was stopped prematurely because of investigators’ subjective impression that prednisone markedly reduced postauricular pain.2 Subsequently, the highest-quality study had few patients (n=51) and reported no difference in outcomes between patients receiving 10 days of oral prednisone plus vitamins and those receiving vitamins alone.3
One open randomized controlled trial demonstrated shorter mean recovery times with intramuscular methylcobalamin (1.95 weeks) and methylcobalamin plus prednisone (2.0 weeks) compared with prednisone alone (9.6 weeks).4 Another trial of 239 patients showed improved rates of autonomic synkinesis after treatment with 16 days of prednisone compared with placebo.5
A randomized, controlled trial of children 2 to 6 years of age found no significant differences in short-term recovery after treatment with methylprednisolone compared with untreated controls.6 Eventually, all these children recovered normal facial nerve function within 12 months.
Two randomized controlled trials have assessed the efficacy of acyclovir for treatment of Bell’s palsy. One trial compared prednisone with acyclovir and found patients treated with prednisone had better complete recovery rates, 93.6% versus 77.7% (absolute risk reduction [ARR]=15.9%, 95% confidence interval [CI]=2.8%–29%], number needed to treat [NNT]=7).7
Another study demonstrated that the combination of prednisone and acyclovir had greater complete recovery rates compared with prednisone alone (92% vs. 76%, ARR=16%, 95% CI=1.7%–30.3%, NNT=7).8
Overall, the data suggest corticosteroid therapy may provide a small clinical benefit in adult patients with Bell’s palsy. In many of these studies, patients who had contraindications to steroid therapy (peptic ulcer disease, uncontrolled diabetes, hypertension, or immunosuppression) were excluded.
If no contraindications to steroids exist, it is resonable to initiate treatment with corticosteroids for an adult patient with new-onset Bell’s palsy. Most studies have started patients on steroids within 10 days of onset of symptoms.
TABLE
Therapies for Bell’s palsy
| Drug | Dosage | SOR |
|---|---|---|
| Prednisone (adults only) | Total from 410 mg over 10 days, to 760 mg over 16 days (tapering doses) | C |
| Acyclovir | 400 mg 5x/d for 10 days | C |
| Methylcobalamin | 500 (g IM 3x/wk until full recovery, or for 8 weeks | C |
| SOR, Strength of recommendation | ||
Recommendations from others
A practice parameter from the American Academy of Neurology states that steroids are safe and probably effective (SOR: B), whereas acyclovir is safe and possibly effective (SOR: C).9 Systematic reviews from the Cochrane Database report that available evidence from randomized controlled trials does not show significant benefit from treating Bell’s palsy with corticosteroids and that clinical trials on acyclovir are inconclusive and therefore cannot be used to make recommendations regarding its use.10,11
Steven H. Horowitz, MD
University of Vermont College of Medicine, Burlington
My practice of neurology began before the era of corticosteroid treatment for Bell’s palsy. Despite the lack of convincing evidenced-based data, it is my clinical impression that there are far fewer patients today with incompletely resolved Bell’s palsy than before the widespread use of steroids. Permanent facial deformities seemed more common back then. Therefore, in the absence of harmful effects, I will continue treating with steroids.
PATIENT INFORMATION
What is Bell’s palsy?
Weakness and slumping on one side of the face are common features of this condition in which the nerve controlling the face has been injured. Other symptoms are:
- Inability to move affected side of the face
- Drooping mouth, unblinking eye
- Numbness
- Twitching of facial muscles
- Taste disturbance
- Increased sensitivity to sound.
Symptoms usually start suddenly. Pain behind the ear may be felt hours to days before other symptoms appear. People between ages 30 and 60 years are most likely to be affected, but this disorder can happen at any age.
Any sudden weakness of the face also suggests the possibility of stroke—a serious emergency. You should contact your physician immediately.
Possible causes
The most common cause of Bell’s palsy is an infection of the facial nerve by the herpes simplex virus. Your doctor will also ask about possible recent trauma to the face and will check for swelling of facial tissues or a mass pressing on the facial nerve.
What to expect
In many cases, the symptoms of Bell’s palsy go away in about 3 weeks without treatment, and the face regains its normal appearance. A good sign that this will happen is if the weakness or other symptoms begin to resolve after 1 week.
In other cases, symptoms may take several months to disappear. Lasting effects are possible, though rare.
Treatments your doctor may prescribe
If a viral infection is the likely cause of your symptoms, your doctor may have you take acyclovir (Zovirax), famciclovir (Famvir), or another antiviral medication to speed your recovery. These antiviral medications are taken as pills, usually a few times a day for 10 days.
A steroid (prednisone, for example) may also help to reduce swelling that could be pressing on the facial nerve. This medication is also administered as a pill over 1 to 2 weeks.
If you cannot blink, and dryness of the eye is one of your symptoms, your doctor will ask you to use moisturizing eye drops to protect the eye from damage while you recover.
The variety of different symptoms you are experiencing is due to the fact the facial nerve controls normal functions from your forehead to your chin—including tearing, taste, muscle movement, and blinking.
1. Taverner D, Cohen SB, Hutchinson BC. Comparison of corticotrophin and prednisolone in treatment of idiopathic facial paralysis (Bell’s palsy). Br Med J 1971;4:20-2.
2. Adour KK, Wingerd J, Bell DN, Manning JJ, Hurley JP. Prednisone treatment for idiopathic facial paralysis (Bell’s palsy). N Engl J Med 1972;287:1268-72.
3. May M, Wette R, Hardin WB, Jr, Sullivan J. The use of steroids in Bell’s palsy: a prospective controlled study. Laryngoscope 1976;86:1111-22.
4. Jalaludin MA. Methylcobalamin treatment of Bell’s palsy. Methods Find Exp Clin Pharmacol 1995;17:539-44.
5. Wolf SM, Wagner JH, Davidson S, Forsythe A. Treatment of Bell palsy with prednisone: a prospective, randomized study. Neurology 1978;28:158-61.
6. Unuvar E, Oguz F, Sidal M, Kilic A. Corticosteroid treatment of childhood Bell’s palsy. Pediatr Neurol 1999;21:814-6.
7. De Diego JI, Prim MP, De Sarria MJ, Madero R, Gavilan J. Idiopathic facial paralysis: a randomized, prospective, and controlled study using single-dose prednisone versus acyclovir three times daily. Laryngoscope 1998;108:573-5.
8. Adour KK, Rubayaines JM, Von Doersten PG, Byl FM, Trent CS, Quesenberry CP, Jr, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol 1996;105:371-8.
9. Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:830-6.
10. Salinas RA, Alvarez G, Alvarez MI, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
11. Sipe J, Dunn L. Aciclovir for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
Early use of corticosteroid therapy results in less autonomic synkinesis and possibly improved rates of recovery in adults (strength of recommendation: C); there is no proven benefit in children (SOR: B).
Adding acyclovir (Zovirax) to prednisone therapy may improve recovery rates compared with prednisone alone (SOR: C).
The results of 1 nonblinded study indicate that intramuscular methylcobalamin (vitamin B12) used alone or in combination with prednisone may shorten time to recovery (SOR: C).
See the Patient Information at the end of this article.
Evidence summary
Bell’s palsy is a lower motor neuron disease of the facial nerve characterized by a transient paralysis. Healing is occasionally incomplete, resulting in residual nerve dysfunction, including partial palsy and motor synkinesis (involuntary movement accompanying a voluntary one) and autonomic synkinesis (involuntary lacrimation after a voluntary muscle movement). Bell’s palsy is associated with significant edema and ischemia of the facial nerve as it passes through its bony canal.
Herpes simplex reactivation has been shown to be associated with a large proportion of cases.
Corticosteroids are the most studied form of therapy for Bell’s palsy (Table). Early work in England culminated in 1971 with a well-performed study demonstrating lower rates of incomplete recovery with prednisolone compared with corticotrophin.1 A potentially definitive randomized controlled trial in 1970 was stopped prematurely because of investigators’ subjective impression that prednisone markedly reduced postauricular pain.2 Subsequently, the highest-quality study had few patients (n=51) and reported no difference in outcomes between patients receiving 10 days of oral prednisone plus vitamins and those receiving vitamins alone.3
One open randomized controlled trial demonstrated shorter mean recovery times with intramuscular methylcobalamin (1.95 weeks) and methylcobalamin plus prednisone (2.0 weeks) compared with prednisone alone (9.6 weeks).4 Another trial of 239 patients showed improved rates of autonomic synkinesis after treatment with 16 days of prednisone compared with placebo.5
A randomized, controlled trial of children 2 to 6 years of age found no significant differences in short-term recovery after treatment with methylprednisolone compared with untreated controls.6 Eventually, all these children recovered normal facial nerve function within 12 months.
Two randomized controlled trials have assessed the efficacy of acyclovir for treatment of Bell’s palsy. One trial compared prednisone with acyclovir and found patients treated with prednisone had better complete recovery rates, 93.6% versus 77.7% (absolute risk reduction [ARR]=15.9%, 95% confidence interval [CI]=2.8%–29%], number needed to treat [NNT]=7).7
Another study demonstrated that the combination of prednisone and acyclovir had greater complete recovery rates compared with prednisone alone (92% vs. 76%, ARR=16%, 95% CI=1.7%–30.3%, NNT=7).8
Overall, the data suggest corticosteroid therapy may provide a small clinical benefit in adult patients with Bell’s palsy. In many of these studies, patients who had contraindications to steroid therapy (peptic ulcer disease, uncontrolled diabetes, hypertension, or immunosuppression) were excluded.
If no contraindications to steroids exist, it is resonable to initiate treatment with corticosteroids for an adult patient with new-onset Bell’s palsy. Most studies have started patients on steroids within 10 days of onset of symptoms.
TABLE
Therapies for Bell’s palsy
| Drug | Dosage | SOR |
|---|---|---|
| Prednisone (adults only) | Total from 410 mg over 10 days, to 760 mg over 16 days (tapering doses) | C |
| Acyclovir | 400 mg 5x/d for 10 days | C |
| Methylcobalamin | 500 (g IM 3x/wk until full recovery, or for 8 weeks | C |
| SOR, Strength of recommendation | ||
Recommendations from others
A practice parameter from the American Academy of Neurology states that steroids are safe and probably effective (SOR: B), whereas acyclovir is safe and possibly effective (SOR: C).9 Systematic reviews from the Cochrane Database report that available evidence from randomized controlled trials does not show significant benefit from treating Bell’s palsy with corticosteroids and that clinical trials on acyclovir are inconclusive and therefore cannot be used to make recommendations regarding its use.10,11
Steven H. Horowitz, MD
University of Vermont College of Medicine, Burlington
My practice of neurology began before the era of corticosteroid treatment for Bell’s palsy. Despite the lack of convincing evidenced-based data, it is my clinical impression that there are far fewer patients today with incompletely resolved Bell’s palsy than before the widespread use of steroids. Permanent facial deformities seemed more common back then. Therefore, in the absence of harmful effects, I will continue treating with steroids.
PATIENT INFORMATION
What is Bell’s palsy?
Weakness and slumping on one side of the face are common features of this condition in which the nerve controlling the face has been injured. Other symptoms are:
- Inability to move affected side of the face
- Drooping mouth, unblinking eye
- Numbness
- Twitching of facial muscles
- Taste disturbance
- Increased sensitivity to sound.
Symptoms usually start suddenly. Pain behind the ear may be felt hours to days before other symptoms appear. People between ages 30 and 60 years are most likely to be affected, but this disorder can happen at any age.
Any sudden weakness of the face also suggests the possibility of stroke—a serious emergency. You should contact your physician immediately.
Possible causes
The most common cause of Bell’s palsy is an infection of the facial nerve by the herpes simplex virus. Your doctor will also ask about possible recent trauma to the face and will check for swelling of facial tissues or a mass pressing on the facial nerve.
What to expect
In many cases, the symptoms of Bell’s palsy go away in about 3 weeks without treatment, and the face regains its normal appearance. A good sign that this will happen is if the weakness or other symptoms begin to resolve after 1 week.
In other cases, symptoms may take several months to disappear. Lasting effects are possible, though rare.
Treatments your doctor may prescribe
If a viral infection is the likely cause of your symptoms, your doctor may have you take acyclovir (Zovirax), famciclovir (Famvir), or another antiviral medication to speed your recovery. These antiviral medications are taken as pills, usually a few times a day for 10 days.
A steroid (prednisone, for example) may also help to reduce swelling that could be pressing on the facial nerve. This medication is also administered as a pill over 1 to 2 weeks.
If you cannot blink, and dryness of the eye is one of your symptoms, your doctor will ask you to use moisturizing eye drops to protect the eye from damage while you recover.
The variety of different symptoms you are experiencing is due to the fact the facial nerve controls normal functions from your forehead to your chin—including tearing, taste, muscle movement, and blinking.
Early use of corticosteroid therapy results in less autonomic synkinesis and possibly improved rates of recovery in adults (strength of recommendation: C); there is no proven benefit in children (SOR: B).
Adding acyclovir (Zovirax) to prednisone therapy may improve recovery rates compared with prednisone alone (SOR: C).
The results of 1 nonblinded study indicate that intramuscular methylcobalamin (vitamin B12) used alone or in combination with prednisone may shorten time to recovery (SOR: C).
See the Patient Information at the end of this article.
Evidence summary
Bell’s palsy is a lower motor neuron disease of the facial nerve characterized by a transient paralysis. Healing is occasionally incomplete, resulting in residual nerve dysfunction, including partial palsy and motor synkinesis (involuntary movement accompanying a voluntary one) and autonomic synkinesis (involuntary lacrimation after a voluntary muscle movement). Bell’s palsy is associated with significant edema and ischemia of the facial nerve as it passes through its bony canal.
Herpes simplex reactivation has been shown to be associated with a large proportion of cases.
Corticosteroids are the most studied form of therapy for Bell’s palsy (Table). Early work in England culminated in 1971 with a well-performed study demonstrating lower rates of incomplete recovery with prednisolone compared with corticotrophin.1 A potentially definitive randomized controlled trial in 1970 was stopped prematurely because of investigators’ subjective impression that prednisone markedly reduced postauricular pain.2 Subsequently, the highest-quality study had few patients (n=51) and reported no difference in outcomes between patients receiving 10 days of oral prednisone plus vitamins and those receiving vitamins alone.3
One open randomized controlled trial demonstrated shorter mean recovery times with intramuscular methylcobalamin (1.95 weeks) and methylcobalamin plus prednisone (2.0 weeks) compared with prednisone alone (9.6 weeks).4 Another trial of 239 patients showed improved rates of autonomic synkinesis after treatment with 16 days of prednisone compared with placebo.5
A randomized, controlled trial of children 2 to 6 years of age found no significant differences in short-term recovery after treatment with methylprednisolone compared with untreated controls.6 Eventually, all these children recovered normal facial nerve function within 12 months.
Two randomized controlled trials have assessed the efficacy of acyclovir for treatment of Bell’s palsy. One trial compared prednisone with acyclovir and found patients treated with prednisone had better complete recovery rates, 93.6% versus 77.7% (absolute risk reduction [ARR]=15.9%, 95% confidence interval [CI]=2.8%–29%], number needed to treat [NNT]=7).7
Another study demonstrated that the combination of prednisone and acyclovir had greater complete recovery rates compared with prednisone alone (92% vs. 76%, ARR=16%, 95% CI=1.7%–30.3%, NNT=7).8
Overall, the data suggest corticosteroid therapy may provide a small clinical benefit in adult patients with Bell’s palsy. In many of these studies, patients who had contraindications to steroid therapy (peptic ulcer disease, uncontrolled diabetes, hypertension, or immunosuppression) were excluded.
If no contraindications to steroids exist, it is resonable to initiate treatment with corticosteroids for an adult patient with new-onset Bell’s palsy. Most studies have started patients on steroids within 10 days of onset of symptoms.
TABLE
Therapies for Bell’s palsy
| Drug | Dosage | SOR |
|---|---|---|
| Prednisone (adults only) | Total from 410 mg over 10 days, to 760 mg over 16 days (tapering doses) | C |
| Acyclovir | 400 mg 5x/d for 10 days | C |
| Methylcobalamin | 500 (g IM 3x/wk until full recovery, or for 8 weeks | C |
| SOR, Strength of recommendation | ||
Recommendations from others
A practice parameter from the American Academy of Neurology states that steroids are safe and probably effective (SOR: B), whereas acyclovir is safe and possibly effective (SOR: C).9 Systematic reviews from the Cochrane Database report that available evidence from randomized controlled trials does not show significant benefit from treating Bell’s palsy with corticosteroids and that clinical trials on acyclovir are inconclusive and therefore cannot be used to make recommendations regarding its use.10,11
Steven H. Horowitz, MD
University of Vermont College of Medicine, Burlington
My practice of neurology began before the era of corticosteroid treatment for Bell’s palsy. Despite the lack of convincing evidenced-based data, it is my clinical impression that there are far fewer patients today with incompletely resolved Bell’s palsy than before the widespread use of steroids. Permanent facial deformities seemed more common back then. Therefore, in the absence of harmful effects, I will continue treating with steroids.
PATIENT INFORMATION
What is Bell’s palsy?
Weakness and slumping on one side of the face are common features of this condition in which the nerve controlling the face has been injured. Other symptoms are:
- Inability to move affected side of the face
- Drooping mouth, unblinking eye
- Numbness
- Twitching of facial muscles
- Taste disturbance
- Increased sensitivity to sound.
Symptoms usually start suddenly. Pain behind the ear may be felt hours to days before other symptoms appear. People between ages 30 and 60 years are most likely to be affected, but this disorder can happen at any age.
Any sudden weakness of the face also suggests the possibility of stroke—a serious emergency. You should contact your physician immediately.
Possible causes
The most common cause of Bell’s palsy is an infection of the facial nerve by the herpes simplex virus. Your doctor will also ask about possible recent trauma to the face and will check for swelling of facial tissues or a mass pressing on the facial nerve.
What to expect
In many cases, the symptoms of Bell’s palsy go away in about 3 weeks without treatment, and the face regains its normal appearance. A good sign that this will happen is if the weakness or other symptoms begin to resolve after 1 week.
In other cases, symptoms may take several months to disappear. Lasting effects are possible, though rare.
Treatments your doctor may prescribe
If a viral infection is the likely cause of your symptoms, your doctor may have you take acyclovir (Zovirax), famciclovir (Famvir), or another antiviral medication to speed your recovery. These antiviral medications are taken as pills, usually a few times a day for 10 days.
A steroid (prednisone, for example) may also help to reduce swelling that could be pressing on the facial nerve. This medication is also administered as a pill over 1 to 2 weeks.
If you cannot blink, and dryness of the eye is one of your symptoms, your doctor will ask you to use moisturizing eye drops to protect the eye from damage while you recover.
The variety of different symptoms you are experiencing is due to the fact the facial nerve controls normal functions from your forehead to your chin—including tearing, taste, muscle movement, and blinking.
1. Taverner D, Cohen SB, Hutchinson BC. Comparison of corticotrophin and prednisolone in treatment of idiopathic facial paralysis (Bell’s palsy). Br Med J 1971;4:20-2.
2. Adour KK, Wingerd J, Bell DN, Manning JJ, Hurley JP. Prednisone treatment for idiopathic facial paralysis (Bell’s palsy). N Engl J Med 1972;287:1268-72.
3. May M, Wette R, Hardin WB, Jr, Sullivan J. The use of steroids in Bell’s palsy: a prospective controlled study. Laryngoscope 1976;86:1111-22.
4. Jalaludin MA. Methylcobalamin treatment of Bell’s palsy. Methods Find Exp Clin Pharmacol 1995;17:539-44.
5. Wolf SM, Wagner JH, Davidson S, Forsythe A. Treatment of Bell palsy with prednisone: a prospective, randomized study. Neurology 1978;28:158-61.
6. Unuvar E, Oguz F, Sidal M, Kilic A. Corticosteroid treatment of childhood Bell’s palsy. Pediatr Neurol 1999;21:814-6.
7. De Diego JI, Prim MP, De Sarria MJ, Madero R, Gavilan J. Idiopathic facial paralysis: a randomized, prospective, and controlled study using single-dose prednisone versus acyclovir three times daily. Laryngoscope 1998;108:573-5.
8. Adour KK, Rubayaines JM, Von Doersten PG, Byl FM, Trent CS, Quesenberry CP, Jr, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol 1996;105:371-8.
9. Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:830-6.
10. Salinas RA, Alvarez G, Alvarez MI, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
11. Sipe J, Dunn L. Aciclovir for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
1. Taverner D, Cohen SB, Hutchinson BC. Comparison of corticotrophin and prednisolone in treatment of idiopathic facial paralysis (Bell’s palsy). Br Med J 1971;4:20-2.
2. Adour KK, Wingerd J, Bell DN, Manning JJ, Hurley JP. Prednisone treatment for idiopathic facial paralysis (Bell’s palsy). N Engl J Med 1972;287:1268-72.
3. May M, Wette R, Hardin WB, Jr, Sullivan J. The use of steroids in Bell’s palsy: a prospective controlled study. Laryngoscope 1976;86:1111-22.
4. Jalaludin MA. Methylcobalamin treatment of Bell’s palsy. Methods Find Exp Clin Pharmacol 1995;17:539-44.
5. Wolf SM, Wagner JH, Davidson S, Forsythe A. Treatment of Bell palsy with prednisone: a prospective, randomized study. Neurology 1978;28:158-61.
6. Unuvar E, Oguz F, Sidal M, Kilic A. Corticosteroid treatment of childhood Bell’s palsy. Pediatr Neurol 1999;21:814-6.
7. De Diego JI, Prim MP, De Sarria MJ, Madero R, Gavilan J. Idiopathic facial paralysis: a randomized, prospective, and controlled study using single-dose prednisone versus acyclovir three times daily. Laryngoscope 1998;108:573-5.
8. Adour KK, Rubayaines JM, Von Doersten PG, Byl FM, Trent CS, Quesenberry CP, Jr, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol 1996;105:371-8.
9. Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:830-6.
10. Salinas RA, Alvarez G, Alvarez MI, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
11. Sipe J, Dunn L. Aciclovir for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software. Updated quarterly.
Evidence-based answers from the Family Physicians Inquiries Network
Does cranberry juice prevent or treat urinary tract infection?
Cranberry juice (200 mL daily to 250 mL 3 times daily) or cranberry concentrate tablets (at least 1:30 parts concentrated juice twice daily) reduce recurrent, symptomatic urinary tract infection (UTI) in women by 12% to 20% (absolute risk reduction [ARR]) compared with placebo (number needed to treat [NNT]=58) (strength of recommendation: A). There is no conclusive evidence that cranberry juice effectively treats UTI (SOR: D).
Evidence summary
A Cochrane review found only a small number of poor-quality trials, providing insufficient support to recommend cranberry juice to prevent UTI.1 However, 2 recent randomized studies, not included in the Cochrane review, found that women taking cranberry juice have fewer symptomatic UTIs.
In women with prior Escherichia coli UTI, 50 mL of cranberry-lingonberry juice concentrate daily for 6 months reduced the recurrence of symptomatic UTI from 36% in the control group to 16% in the treated group (NNT=5).2
In a placebo-controlled randomized trial, women with prior UTI who took 1 tablet of concentrated cranberry juice (at least 1:30 parts concentrated juice) twice daily (n=50) or drank 250 mL of pure unsweetened cranberry juice 3 times a day for 12 months (n=50) reduced their incidence of symptomatic UTI.3 Women who drank the juice had an ARR of 12% (32% symptomatic UTI on placebo group, 20% in cranberry juice group, NNT=8.3) over 1 year. Use of cranberry juice tablets produced an ARR of 14% (32% symptomatic UTI in placebo group; 18% in cranberry tablet group, NNT=7.1). Self-reported compliance was 75% to 90% in the juice group and 90% in the tablet group.
No dose-response studies have been done to determine the optimal volume of juice or number of tablets needed to prevent infection. Studies have used between 200 mL once a day to 250 mL 3 times a day of the juice or 1 cranberry tablet taken twice daily. The subject dropout rate was as high as 34% in one study using juice,4 implying that cranberry juice—which is acidic and astringent at full strength—may not be acceptable to many patients as a prophylactic therapy over a long period. The 1-month compliance rate of patients taking cranberry tablets was between 88% and 100%, suggesting that this form of cranberry may improve compliance.
The cost of cranberry juice and cranberry tablets was estimated at $1400 and $624 per year, respectively.3 This must be balanced against the cost of treating symptomatic UTIs. No randomized trials have tested the more readily available and palatable cranberry juice cocktail—which is mixed with water, a sweetener, and vitamin C—to prevent recurrent UTI.
Cranberry juice does not inhibit bacterial growth and will not sterilize the urinary tract. Also, cranberry juice does not prevent or treat UTI by changing the pH of the urine. Rather, the suspected mechanism of action is that proanthocyanidins contained in cranberry juice prevent bacterial adherence to uroepithelial cells, thus reducing the development of UTI.5 Cranberry juice has been shown to reduce uroepithelial cell adherence by bacteria resistant to trimethoprim-sulfamethoxazole.6
Recommendations from others
No national practice guidelines have recommended cranberry juice as a preventive strategy for recurrent UTI but, anecdotally, patients are often advised to try cranberry juice to prevent UTI.
Brett Robbins, MD
Steven Bondi
Internal Medicine Pediatrics Residency, University of Rochester, NY.
The protective value of cranberry juice against UTI bacteria is supported by a significant body of data from in vitro studies. The published studies examining the clinical use of cranberry juice for UTI prevention suffer from a number of flaws, including small sample size, poor design, lack of randomization, lack of placebo control, heterogeneous endpoints, and a focus on the geriatric population. Even the best of these studies suffers from a major defect: failure to use commonly available cranberry juice cocktail as the experimental intervention.
Despite these flaws, the weight of the clinical evidence suggests that cranberry juice is an effective intervention for the prevention of UTIs—especially in high-risk populations. Unfortunately, cranberry juice is expensive and its taste is displeasing to some, thus limiting its usefulness. Cranberry capsules/ tablets offer a reasonable alternative, but their composition varies greatly by manufacturer, and patient compliance may be poor.
The decision to use cranberry juice should be left to the patient and her clinician.
Given the evidence, cranberry juice is best suited for secondary prevention of recurrent UTI. Patients with recurrent UTI who are being considered for antibiotic prophylaxis and are willing to drink the juice are ideal candidates. Although the studies have yet to establish an ideal dose, 3 glasses a day should be sufficient.
1. Jepson RG, Mihaljevic L, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2001:CD001321. (Updated quarterly.)
2. Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Kaskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001;322:1571-3.
3. Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol 2002;9:1558-62.
4. Foda MM, Middlebrook PF, Gatfield CT, Potvin G, Wells G, Schillinger JF. Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Can J Urol 1995;2:98-102.
5. Lowe FC, Fagelman E. Cranberry juice and urinary tract infections: what is the evidence?. Urology 2001;57:407-13.
6. Howell AB, Foxman B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 2002;287:3082-3.
Cranberry juice (200 mL daily to 250 mL 3 times daily) or cranberry concentrate tablets (at least 1:30 parts concentrated juice twice daily) reduce recurrent, symptomatic urinary tract infection (UTI) in women by 12% to 20% (absolute risk reduction [ARR]) compared with placebo (number needed to treat [NNT]=58) (strength of recommendation: A). There is no conclusive evidence that cranberry juice effectively treats UTI (SOR: D).
Evidence summary
A Cochrane review found only a small number of poor-quality trials, providing insufficient support to recommend cranberry juice to prevent UTI.1 However, 2 recent randomized studies, not included in the Cochrane review, found that women taking cranberry juice have fewer symptomatic UTIs.
In women with prior Escherichia coli UTI, 50 mL of cranberry-lingonberry juice concentrate daily for 6 months reduced the recurrence of symptomatic UTI from 36% in the control group to 16% in the treated group (NNT=5).2
In a placebo-controlled randomized trial, women with prior UTI who took 1 tablet of concentrated cranberry juice (at least 1:30 parts concentrated juice) twice daily (n=50) or drank 250 mL of pure unsweetened cranberry juice 3 times a day for 12 months (n=50) reduced their incidence of symptomatic UTI.3 Women who drank the juice had an ARR of 12% (32% symptomatic UTI on placebo group, 20% in cranberry juice group, NNT=8.3) over 1 year. Use of cranberry juice tablets produced an ARR of 14% (32% symptomatic UTI in placebo group; 18% in cranberry tablet group, NNT=7.1). Self-reported compliance was 75% to 90% in the juice group and 90% in the tablet group.
No dose-response studies have been done to determine the optimal volume of juice or number of tablets needed to prevent infection. Studies have used between 200 mL once a day to 250 mL 3 times a day of the juice or 1 cranberry tablet taken twice daily. The subject dropout rate was as high as 34% in one study using juice,4 implying that cranberry juice—which is acidic and astringent at full strength—may not be acceptable to many patients as a prophylactic therapy over a long period. The 1-month compliance rate of patients taking cranberry tablets was between 88% and 100%, suggesting that this form of cranberry may improve compliance.
The cost of cranberry juice and cranberry tablets was estimated at $1400 and $624 per year, respectively.3 This must be balanced against the cost of treating symptomatic UTIs. No randomized trials have tested the more readily available and palatable cranberry juice cocktail—which is mixed with water, a sweetener, and vitamin C—to prevent recurrent UTI.
Cranberry juice does not inhibit bacterial growth and will not sterilize the urinary tract. Also, cranberry juice does not prevent or treat UTI by changing the pH of the urine. Rather, the suspected mechanism of action is that proanthocyanidins contained in cranberry juice prevent bacterial adherence to uroepithelial cells, thus reducing the development of UTI.5 Cranberry juice has been shown to reduce uroepithelial cell adherence by bacteria resistant to trimethoprim-sulfamethoxazole.6
Recommendations from others
No national practice guidelines have recommended cranberry juice as a preventive strategy for recurrent UTI but, anecdotally, patients are often advised to try cranberry juice to prevent UTI.
Brett Robbins, MD
Steven Bondi
Internal Medicine Pediatrics Residency, University of Rochester, NY.
The protective value of cranberry juice against UTI bacteria is supported by a significant body of data from in vitro studies. The published studies examining the clinical use of cranberry juice for UTI prevention suffer from a number of flaws, including small sample size, poor design, lack of randomization, lack of placebo control, heterogeneous endpoints, and a focus on the geriatric population. Even the best of these studies suffers from a major defect: failure to use commonly available cranberry juice cocktail as the experimental intervention.
Despite these flaws, the weight of the clinical evidence suggests that cranberry juice is an effective intervention for the prevention of UTIs—especially in high-risk populations. Unfortunately, cranberry juice is expensive and its taste is displeasing to some, thus limiting its usefulness. Cranberry capsules/ tablets offer a reasonable alternative, but their composition varies greatly by manufacturer, and patient compliance may be poor.
The decision to use cranberry juice should be left to the patient and her clinician.
Given the evidence, cranberry juice is best suited for secondary prevention of recurrent UTI. Patients with recurrent UTI who are being considered for antibiotic prophylaxis and are willing to drink the juice are ideal candidates. Although the studies have yet to establish an ideal dose, 3 glasses a day should be sufficient.
Cranberry juice (200 mL daily to 250 mL 3 times daily) or cranberry concentrate tablets (at least 1:30 parts concentrated juice twice daily) reduce recurrent, symptomatic urinary tract infection (UTI) in women by 12% to 20% (absolute risk reduction [ARR]) compared with placebo (number needed to treat [NNT]=58) (strength of recommendation: A). There is no conclusive evidence that cranberry juice effectively treats UTI (SOR: D).
Evidence summary
A Cochrane review found only a small number of poor-quality trials, providing insufficient support to recommend cranberry juice to prevent UTI.1 However, 2 recent randomized studies, not included in the Cochrane review, found that women taking cranberry juice have fewer symptomatic UTIs.
In women with prior Escherichia coli UTI, 50 mL of cranberry-lingonberry juice concentrate daily for 6 months reduced the recurrence of symptomatic UTI from 36% in the control group to 16% in the treated group (NNT=5).2
In a placebo-controlled randomized trial, women with prior UTI who took 1 tablet of concentrated cranberry juice (at least 1:30 parts concentrated juice) twice daily (n=50) or drank 250 mL of pure unsweetened cranberry juice 3 times a day for 12 months (n=50) reduced their incidence of symptomatic UTI.3 Women who drank the juice had an ARR of 12% (32% symptomatic UTI on placebo group, 20% in cranberry juice group, NNT=8.3) over 1 year. Use of cranberry juice tablets produced an ARR of 14% (32% symptomatic UTI in placebo group; 18% in cranberry tablet group, NNT=7.1). Self-reported compliance was 75% to 90% in the juice group and 90% in the tablet group.
No dose-response studies have been done to determine the optimal volume of juice or number of tablets needed to prevent infection. Studies have used between 200 mL once a day to 250 mL 3 times a day of the juice or 1 cranberry tablet taken twice daily. The subject dropout rate was as high as 34% in one study using juice,4 implying that cranberry juice—which is acidic and astringent at full strength—may not be acceptable to many patients as a prophylactic therapy over a long period. The 1-month compliance rate of patients taking cranberry tablets was between 88% and 100%, suggesting that this form of cranberry may improve compliance.
The cost of cranberry juice and cranberry tablets was estimated at $1400 and $624 per year, respectively.3 This must be balanced against the cost of treating symptomatic UTIs. No randomized trials have tested the more readily available and palatable cranberry juice cocktail—which is mixed with water, a sweetener, and vitamin C—to prevent recurrent UTI.
Cranberry juice does not inhibit bacterial growth and will not sterilize the urinary tract. Also, cranberry juice does not prevent or treat UTI by changing the pH of the urine. Rather, the suspected mechanism of action is that proanthocyanidins contained in cranberry juice prevent bacterial adherence to uroepithelial cells, thus reducing the development of UTI.5 Cranberry juice has been shown to reduce uroepithelial cell adherence by bacteria resistant to trimethoprim-sulfamethoxazole.6
Recommendations from others
No national practice guidelines have recommended cranberry juice as a preventive strategy for recurrent UTI but, anecdotally, patients are often advised to try cranberry juice to prevent UTI.
Brett Robbins, MD
Steven Bondi
Internal Medicine Pediatrics Residency, University of Rochester, NY.
The protective value of cranberry juice against UTI bacteria is supported by a significant body of data from in vitro studies. The published studies examining the clinical use of cranberry juice for UTI prevention suffer from a number of flaws, including small sample size, poor design, lack of randomization, lack of placebo control, heterogeneous endpoints, and a focus on the geriatric population. Even the best of these studies suffers from a major defect: failure to use commonly available cranberry juice cocktail as the experimental intervention.
Despite these flaws, the weight of the clinical evidence suggests that cranberry juice is an effective intervention for the prevention of UTIs—especially in high-risk populations. Unfortunately, cranberry juice is expensive and its taste is displeasing to some, thus limiting its usefulness. Cranberry capsules/ tablets offer a reasonable alternative, but their composition varies greatly by manufacturer, and patient compliance may be poor.
The decision to use cranberry juice should be left to the patient and her clinician.
Given the evidence, cranberry juice is best suited for secondary prevention of recurrent UTI. Patients with recurrent UTI who are being considered for antibiotic prophylaxis and are willing to drink the juice are ideal candidates. Although the studies have yet to establish an ideal dose, 3 glasses a day should be sufficient.
1. Jepson RG, Mihaljevic L, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2001:CD001321. (Updated quarterly.)
2. Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Kaskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001;322:1571-3.
3. Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol 2002;9:1558-62.
4. Foda MM, Middlebrook PF, Gatfield CT, Potvin G, Wells G, Schillinger JF. Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Can J Urol 1995;2:98-102.
5. Lowe FC, Fagelman E. Cranberry juice and urinary tract infections: what is the evidence?. Urology 2001;57:407-13.
6. Howell AB, Foxman B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 2002;287:3082-3.
1. Jepson RG, Mihaljevic L, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2001:CD001321. (Updated quarterly.)
2. Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Kaskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001;322:1571-3.
3. Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol 2002;9:1558-62.
4. Foda MM, Middlebrook PF, Gatfield CT, Potvin G, Wells G, Schillinger JF. Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Can J Urol 1995;2:98-102.
5. Lowe FC, Fagelman E. Cranberry juice and urinary tract infections: what is the evidence?. Urology 2001;57:407-13.
6. Howell AB, Foxman B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 2002;287:3082-3.
Evidence-based answers from the Family Physicians Inquiries Network
Is pneumococcal vaccine effective in nursing home patients?
Evidence from clinical trials supports the use of pneumococcal polysaccharide vaccine for prevention of pneumonia in nursing home patients (strength of recommendation: B, based on randomized, nonblinded clinical trials).
Case-control studies have consistently shown the efficacy of pneumococcal vaccine in preventing invasive pneumococcal disease and bacteremia for patients with chronic medical illnesses and the elderly, patients typically found in nursing home populations (SOR: B, based on consistent case-control studies).
Evidence summary
Two clinical trials directly addressed the prevention of pneumonia in nursing home patients. A prospective, risk-stratified, randomized study of the 14-valent pneumococcal vaccine in 1686 patients living in hospices and nursing homes in France showed an absolute risk reduction (ARR) of 2.9% in the incidence of all-cause pneumonia, corresponding to a number needed to treat (NNT) of 35.1 This study has 2 major limitations: the authors did not comment on whether the study was blinded, and 31% of patients were lost to follow-up.
A 6-year randomized clinical trial that studied the trivalent pneumococcal vaccine in preventing pneumonia in New York City Home (a nursing home) subjects showed an ARR=2.7% and NNT=37.2 While this report also did not specify whether there was blinding, any bias introduced by absence of blinding is unlikely to account for the large effect size (relative risk reduction=0.56).
Nursing home residents may be especially vulnerable to acquiring pneumococcal infection due to advanced age, chronic illnesses, and their communal setting. The Centers for Disease Control and Prevention (CDC) has reported outbreaks of invasive pneumococcal disease in nursing homes where vaccination rates are low.3 Pneumococcal bacteremia is seen in only 10%–20% of patients with pneumococcal pneumonia but confers a significant risk of death. Therefore, pneumococcal vaccination is indicated for patients ≥ 65 years or those with chronic medical conditions.
Case-control studies have consistently shown efficacy in preventing invasive pneumococcal disease. Farr and colleagues found efficacy of 70% (95% confidence interval [CI], 37%–86%) among 2 groups of patients: those ≥ 2 years of age with chronic disease or those ≥ 65 years.4 A case-control study by Sims and colleagues also found the vaccine to have efficacy of 70% (95% CI, 37%– 86%) in preventing invasive pneumococcal disease in immunocompetent patients aged ≥ 55 years.5
Recommendations from others
The CDC Advisory Committee on Immunization Practices (ACIP) recommends pneumococcal vaccination of persons aged ≥65 years and those aged 2 to 64 who have chronic cardiovascular disease, chronic pulmonary disease, or diabetes mellitus (SOR: A).6
The ACIP also recommends the pneumococcal vaccine for persons aged 2 to 64 years who have alcoholism, chronic liver disease, or cerebrospinal fluid leaks (SOR: B).
The Canadian Task Force on Preventive Health Care endorses vaccination for immunocompetent patients 55 years residing in institutions (SOR: A).7
Paul Tatum, MD, MSPH
Department of Family Medicine, University of Colorado, Boulder.
The importance of pneumococcal vaccine for the elderly is well established. However, the vaccine is underused in long-term care settings, despite being indicated for most residents.
Patient confusion about the need for both influenza and pneumococcal vaccines, poor documentation of adult immunization status, poor availability of records from previous care facilities, and frequent changes in physician all contribute to low vaccination rates.
An optimal strategy to ensure high vaccination rates is to administer the pneumococcal vaccine to patients on admission to long-term care facilities. Patients who are uncertain about their vaccination status may safely receive the vaccine, as revaccination is relatively well tolerated.8
ACKNOWLEDGMENTS
The authors wish to thank Yves LeBlanc, MD, and Khalil Nasrallah, MD, for assistance with translation.
1. Gaillet J, Zmirou D, Mallaret MR, et al. Essai clinique du vaccin antipneumococcoique chez des personnees agees vivant en institution [Clinical trial of an antipneumococcal vaccine in elderly subjects living in institutions]. Rev Epidemiol Sante Publique 1985;33:437-44.
2. Kaufman P. Pneumonia in old age. Arch Intern Med 1947;79:518-31.
3. Centers for Disease Control and Prevention. Outbreaks of pneumococcal pneumonia among unvaccinated residents of a nursing home—New Jersey, April 2001. MMWR Morb Mortal Wkly Rep 2001;50:707-10.
4. Farr BM, Johnston BL, Cobb DK, et al. Preventing pneumococcal bacteremia in patients at risk. Arch Intern Med 1995;155:2336-40.
5. Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 1988;108:653-7.
6. Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 1997;46:1-24.
7. Wang EEL. Administration of pneumococcal vaccine. Canadian Task Force on Preventive Health Care 1994;385-6.
8. Jackson LA, Benson P, Sneller VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-8.
Evidence from clinical trials supports the use of pneumococcal polysaccharide vaccine for prevention of pneumonia in nursing home patients (strength of recommendation: B, based on randomized, nonblinded clinical trials).
Case-control studies have consistently shown the efficacy of pneumococcal vaccine in preventing invasive pneumococcal disease and bacteremia for patients with chronic medical illnesses and the elderly, patients typically found in nursing home populations (SOR: B, based on consistent case-control studies).
Evidence summary
Two clinical trials directly addressed the prevention of pneumonia in nursing home patients. A prospective, risk-stratified, randomized study of the 14-valent pneumococcal vaccine in 1686 patients living in hospices and nursing homes in France showed an absolute risk reduction (ARR) of 2.9% in the incidence of all-cause pneumonia, corresponding to a number needed to treat (NNT) of 35.1 This study has 2 major limitations: the authors did not comment on whether the study was blinded, and 31% of patients were lost to follow-up.
A 6-year randomized clinical trial that studied the trivalent pneumococcal vaccine in preventing pneumonia in New York City Home (a nursing home) subjects showed an ARR=2.7% and NNT=37.2 While this report also did not specify whether there was blinding, any bias introduced by absence of blinding is unlikely to account for the large effect size (relative risk reduction=0.56).
Nursing home residents may be especially vulnerable to acquiring pneumococcal infection due to advanced age, chronic illnesses, and their communal setting. The Centers for Disease Control and Prevention (CDC) has reported outbreaks of invasive pneumococcal disease in nursing homes where vaccination rates are low.3 Pneumococcal bacteremia is seen in only 10%–20% of patients with pneumococcal pneumonia but confers a significant risk of death. Therefore, pneumococcal vaccination is indicated for patients ≥ 65 years or those with chronic medical conditions.
Case-control studies have consistently shown efficacy in preventing invasive pneumococcal disease. Farr and colleagues found efficacy of 70% (95% confidence interval [CI], 37%–86%) among 2 groups of patients: those ≥ 2 years of age with chronic disease or those ≥ 65 years.4 A case-control study by Sims and colleagues also found the vaccine to have efficacy of 70% (95% CI, 37%– 86%) in preventing invasive pneumococcal disease in immunocompetent patients aged ≥ 55 years.5
Recommendations from others
The CDC Advisory Committee on Immunization Practices (ACIP) recommends pneumococcal vaccination of persons aged ≥65 years and those aged 2 to 64 who have chronic cardiovascular disease, chronic pulmonary disease, or diabetes mellitus (SOR: A).6
The ACIP also recommends the pneumococcal vaccine for persons aged 2 to 64 years who have alcoholism, chronic liver disease, or cerebrospinal fluid leaks (SOR: B).
The Canadian Task Force on Preventive Health Care endorses vaccination for immunocompetent patients 55 years residing in institutions (SOR: A).7
Paul Tatum, MD, MSPH
Department of Family Medicine, University of Colorado, Boulder.
The importance of pneumococcal vaccine for the elderly is well established. However, the vaccine is underused in long-term care settings, despite being indicated for most residents.
Patient confusion about the need for both influenza and pneumococcal vaccines, poor documentation of adult immunization status, poor availability of records from previous care facilities, and frequent changes in physician all contribute to low vaccination rates.
An optimal strategy to ensure high vaccination rates is to administer the pneumococcal vaccine to patients on admission to long-term care facilities. Patients who are uncertain about their vaccination status may safely receive the vaccine, as revaccination is relatively well tolerated.8
ACKNOWLEDGMENTS
The authors wish to thank Yves LeBlanc, MD, and Khalil Nasrallah, MD, for assistance with translation.
Evidence from clinical trials supports the use of pneumococcal polysaccharide vaccine for prevention of pneumonia in nursing home patients (strength of recommendation: B, based on randomized, nonblinded clinical trials).
Case-control studies have consistently shown the efficacy of pneumococcal vaccine in preventing invasive pneumococcal disease and bacteremia for patients with chronic medical illnesses and the elderly, patients typically found in nursing home populations (SOR: B, based on consistent case-control studies).
Evidence summary
Two clinical trials directly addressed the prevention of pneumonia in nursing home patients. A prospective, risk-stratified, randomized study of the 14-valent pneumococcal vaccine in 1686 patients living in hospices and nursing homes in France showed an absolute risk reduction (ARR) of 2.9% in the incidence of all-cause pneumonia, corresponding to a number needed to treat (NNT) of 35.1 This study has 2 major limitations: the authors did not comment on whether the study was blinded, and 31% of patients were lost to follow-up.
A 6-year randomized clinical trial that studied the trivalent pneumococcal vaccine in preventing pneumonia in New York City Home (a nursing home) subjects showed an ARR=2.7% and NNT=37.2 While this report also did not specify whether there was blinding, any bias introduced by absence of blinding is unlikely to account for the large effect size (relative risk reduction=0.56).
Nursing home residents may be especially vulnerable to acquiring pneumococcal infection due to advanced age, chronic illnesses, and their communal setting. The Centers for Disease Control and Prevention (CDC) has reported outbreaks of invasive pneumococcal disease in nursing homes where vaccination rates are low.3 Pneumococcal bacteremia is seen in only 10%–20% of patients with pneumococcal pneumonia but confers a significant risk of death. Therefore, pneumococcal vaccination is indicated for patients ≥ 65 years or those with chronic medical conditions.
Case-control studies have consistently shown efficacy in preventing invasive pneumococcal disease. Farr and colleagues found efficacy of 70% (95% confidence interval [CI], 37%–86%) among 2 groups of patients: those ≥ 2 years of age with chronic disease or those ≥ 65 years.4 A case-control study by Sims and colleagues also found the vaccine to have efficacy of 70% (95% CI, 37%– 86%) in preventing invasive pneumococcal disease in immunocompetent patients aged ≥ 55 years.5
Recommendations from others
The CDC Advisory Committee on Immunization Practices (ACIP) recommends pneumococcal vaccination of persons aged ≥65 years and those aged 2 to 64 who have chronic cardiovascular disease, chronic pulmonary disease, or diabetes mellitus (SOR: A).6
The ACIP also recommends the pneumococcal vaccine for persons aged 2 to 64 years who have alcoholism, chronic liver disease, or cerebrospinal fluid leaks (SOR: B).
The Canadian Task Force on Preventive Health Care endorses vaccination for immunocompetent patients 55 years residing in institutions (SOR: A).7
Paul Tatum, MD, MSPH
Department of Family Medicine, University of Colorado, Boulder.
The importance of pneumococcal vaccine for the elderly is well established. However, the vaccine is underused in long-term care settings, despite being indicated for most residents.
Patient confusion about the need for both influenza and pneumococcal vaccines, poor documentation of adult immunization status, poor availability of records from previous care facilities, and frequent changes in physician all contribute to low vaccination rates.
An optimal strategy to ensure high vaccination rates is to administer the pneumococcal vaccine to patients on admission to long-term care facilities. Patients who are uncertain about their vaccination status may safely receive the vaccine, as revaccination is relatively well tolerated.8
ACKNOWLEDGMENTS
The authors wish to thank Yves LeBlanc, MD, and Khalil Nasrallah, MD, for assistance with translation.
1. Gaillet J, Zmirou D, Mallaret MR, et al. Essai clinique du vaccin antipneumococcoique chez des personnees agees vivant en institution [Clinical trial of an antipneumococcal vaccine in elderly subjects living in institutions]. Rev Epidemiol Sante Publique 1985;33:437-44.
2. Kaufman P. Pneumonia in old age. Arch Intern Med 1947;79:518-31.
3. Centers for Disease Control and Prevention. Outbreaks of pneumococcal pneumonia among unvaccinated residents of a nursing home—New Jersey, April 2001. MMWR Morb Mortal Wkly Rep 2001;50:707-10.
4. Farr BM, Johnston BL, Cobb DK, et al. Preventing pneumococcal bacteremia in patients at risk. Arch Intern Med 1995;155:2336-40.
5. Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 1988;108:653-7.
6. Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 1997;46:1-24.
7. Wang EEL. Administration of pneumococcal vaccine. Canadian Task Force on Preventive Health Care 1994;385-6.
8. Jackson LA, Benson P, Sneller VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-8.
1. Gaillet J, Zmirou D, Mallaret MR, et al. Essai clinique du vaccin antipneumococcoique chez des personnees agees vivant en institution [Clinical trial of an antipneumococcal vaccine in elderly subjects living in institutions]. Rev Epidemiol Sante Publique 1985;33:437-44.
2. Kaufman P. Pneumonia in old age. Arch Intern Med 1947;79:518-31.
3. Centers for Disease Control and Prevention. Outbreaks of pneumococcal pneumonia among unvaccinated residents of a nursing home—New Jersey, April 2001. MMWR Morb Mortal Wkly Rep 2001;50:707-10.
4. Farr BM, Johnston BL, Cobb DK, et al. Preventing pneumococcal bacteremia in patients at risk. Arch Intern Med 1995;155:2336-40.
5. Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 1988;108:653-7.
6. Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 1997;46:1-24.
7. Wang EEL. Administration of pneumococcal vaccine. Canadian Task Force on Preventive Health Care 1994;385-6.
8. Jackson LA, Benson P, Sneller VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999;281:243-8.
Evidence-based answers from the Family Physicians Inquiries Network
Which postmenopausal women should be offered combined HRT?
Recent studies have demonstrated a small but significant risk of adverse effects from combined hormone replacement therapy (HRT), including cardiovascular disease, thromboembolic disease, and breast cancer. Time-limited HRT will control intolerable menopausal symptoms and prevent risk of fractures in newly menopausal women. However, HRT achieves its maximum efficacy in 35 years, and the risk of adverse outcomes increases as time progresses. Women considering HRT, particularly those at higher risk for vascular disease and breast cancer, should be informed of the potential risks.
There is inadequate evidence to determine the extent of these risks in women who have had a hysterectomy and are taking unopposed estrogen (strength of recommendation: A, based on large randomized controlled trials).
Evidence summary
The Women’s Health Initiative (WHI),1 the largest randomized trial of HRT, showed that long-term use of HRT poses more risks than benefits for healthy postmenopausal women. WHI studied the use of estrogen plus progestin for prevention of coronary heart disease in 16,608 postmenopausal women age 50–79 years. After 5 years of follow-up, this arm of the study was stopped because of the adverse effects of the intervention. The researchers found that HRT increases the risk of several events:
- coronary heart disease events (number needed to harm [NNH]=1428)
- invasive breast cancer (NNH=1250)
- stroke (NNH=1250)
- venous thromboembolic events (NNH=555)
- pulmonary embolism (NNH=1250).
An ongoing arm of WHI is studying estrogen alone in postmenopausal women who have had a hysterectomy.
The Heart and Estrogen/progestin Replacement Study (HERS)2 examined the effects of HRT in postmenopausal women with coronary artery disease. HERS was a large randomized controlled trial of 2763 women with an average follow-up time of 4.1 years. It showed no statistically significant difference between the HRT (estrogen plus medroxyprog-esterone) group compared with the placebo group in either the primary outcomes (nonfatal myocardial infarction or coronary heart disease death) or in the secondary outcomes (coronary revascularization, unstable angina, congestive heart failure, resuscitated cardiac arrest, stroke or transient ischemic attack, and peripheral arterial disease). The findings of the WHI and HERS trials have been summarized in a recent meta-analysis done for the United States Preventive Services Task Force.3
Both the WHI and HERS trials demonstrated some benefits for HRT. WHI found a reduced risk of colorectal cancer (number needed to treat [NNT]=1667) and a decreased risk of any osteoporotic fracture (NNT=228). The HERS group found that HRT improved the quality of life of women with postmenopausal symptoms, particularly flushing.
Evidence indicates that women who take HRT for 3 years and then stop achieve as much protection from osteoporotic fractures as women who continue their HRT beyond 3 years.4
Continuing HRT beyond 5 years dramatically increases the risk of coronary heart disease, stroke, thromboembolic events, breast cancer, and cholecystitis.3
Recommendations from others
The American College of Obstetricians and Gynecologists has convened a multispecialty panel of experts to draft new recommendations for HRT in light of the WHI findings.
Laura Hansen, PharmD, BCPS
University of Colorado, Boulder
The WHI and HERS trials demonstrated that long-term use of HRT (>5 years) incurs significantly more risks than benefits for a postmenopausal woman who has not undergone hysterectomy. However, these trials did not evaluate postmenopausal symptoms or quality of life as primary endpoints.
Most women experience postmenopausal symptoms for more than 1 year but have resolution of symptoms within a few years after menopause. Since HRT remains the most effective therapy for hot flushes, short-term use of HRT (<5 years) may be offered to women experiencing postmenopausal symptoms.
Physicians may instruct women to attempt HRT discontinuation each year because the duration of symptoms can be variable. Discontinuation should be performed using gradual dose reductions to prevent rapid return of postmenopausal symptoms.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
3. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872-81.
4. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-72.
Recent studies have demonstrated a small but significant risk of adverse effects from combined hormone replacement therapy (HRT), including cardiovascular disease, thromboembolic disease, and breast cancer. Time-limited HRT will control intolerable menopausal symptoms and prevent risk of fractures in newly menopausal women. However, HRT achieves its maximum efficacy in 35 years, and the risk of adverse outcomes increases as time progresses. Women considering HRT, particularly those at higher risk for vascular disease and breast cancer, should be informed of the potential risks.
There is inadequate evidence to determine the extent of these risks in women who have had a hysterectomy and are taking unopposed estrogen (strength of recommendation: A, based on large randomized controlled trials).
Evidence summary
The Women’s Health Initiative (WHI),1 the largest randomized trial of HRT, showed that long-term use of HRT poses more risks than benefits for healthy postmenopausal women. WHI studied the use of estrogen plus progestin for prevention of coronary heart disease in 16,608 postmenopausal women age 50–79 years. After 5 years of follow-up, this arm of the study was stopped because of the adverse effects of the intervention. The researchers found that HRT increases the risk of several events:
- coronary heart disease events (number needed to harm [NNH]=1428)
- invasive breast cancer (NNH=1250)
- stroke (NNH=1250)
- venous thromboembolic events (NNH=555)
- pulmonary embolism (NNH=1250).
An ongoing arm of WHI is studying estrogen alone in postmenopausal women who have had a hysterectomy.
The Heart and Estrogen/progestin Replacement Study (HERS)2 examined the effects of HRT in postmenopausal women with coronary artery disease. HERS was a large randomized controlled trial of 2763 women with an average follow-up time of 4.1 years. It showed no statistically significant difference between the HRT (estrogen plus medroxyprog-esterone) group compared with the placebo group in either the primary outcomes (nonfatal myocardial infarction or coronary heart disease death) or in the secondary outcomes (coronary revascularization, unstable angina, congestive heart failure, resuscitated cardiac arrest, stroke or transient ischemic attack, and peripheral arterial disease). The findings of the WHI and HERS trials have been summarized in a recent meta-analysis done for the United States Preventive Services Task Force.3
Both the WHI and HERS trials demonstrated some benefits for HRT. WHI found a reduced risk of colorectal cancer (number needed to treat [NNT]=1667) and a decreased risk of any osteoporotic fracture (NNT=228). The HERS group found that HRT improved the quality of life of women with postmenopausal symptoms, particularly flushing.
Evidence indicates that women who take HRT for 3 years and then stop achieve as much protection from osteoporotic fractures as women who continue their HRT beyond 3 years.4
Continuing HRT beyond 5 years dramatically increases the risk of coronary heart disease, stroke, thromboembolic events, breast cancer, and cholecystitis.3
Recommendations from others
The American College of Obstetricians and Gynecologists has convened a multispecialty panel of experts to draft new recommendations for HRT in light of the WHI findings.
Laura Hansen, PharmD, BCPS
University of Colorado, Boulder
The WHI and HERS trials demonstrated that long-term use of HRT (>5 years) incurs significantly more risks than benefits for a postmenopausal woman who has not undergone hysterectomy. However, these trials did not evaluate postmenopausal symptoms or quality of life as primary endpoints.
Most women experience postmenopausal symptoms for more than 1 year but have resolution of symptoms within a few years after menopause. Since HRT remains the most effective therapy for hot flushes, short-term use of HRT (<5 years) may be offered to women experiencing postmenopausal symptoms.
Physicians may instruct women to attempt HRT discontinuation each year because the duration of symptoms can be variable. Discontinuation should be performed using gradual dose reductions to prevent rapid return of postmenopausal symptoms.
Recent studies have demonstrated a small but significant risk of adverse effects from combined hormone replacement therapy (HRT), including cardiovascular disease, thromboembolic disease, and breast cancer. Time-limited HRT will control intolerable menopausal symptoms and prevent risk of fractures in newly menopausal women. However, HRT achieves its maximum efficacy in 35 years, and the risk of adverse outcomes increases as time progresses. Women considering HRT, particularly those at higher risk for vascular disease and breast cancer, should be informed of the potential risks.
There is inadequate evidence to determine the extent of these risks in women who have had a hysterectomy and are taking unopposed estrogen (strength of recommendation: A, based on large randomized controlled trials).
Evidence summary
The Women’s Health Initiative (WHI),1 the largest randomized trial of HRT, showed that long-term use of HRT poses more risks than benefits for healthy postmenopausal women. WHI studied the use of estrogen plus progestin for prevention of coronary heart disease in 16,608 postmenopausal women age 50–79 years. After 5 years of follow-up, this arm of the study was stopped because of the adverse effects of the intervention. The researchers found that HRT increases the risk of several events:
- coronary heart disease events (number needed to harm [NNH]=1428)
- invasive breast cancer (NNH=1250)
- stroke (NNH=1250)
- venous thromboembolic events (NNH=555)
- pulmonary embolism (NNH=1250).
An ongoing arm of WHI is studying estrogen alone in postmenopausal women who have had a hysterectomy.
The Heart and Estrogen/progestin Replacement Study (HERS)2 examined the effects of HRT in postmenopausal women with coronary artery disease. HERS was a large randomized controlled trial of 2763 women with an average follow-up time of 4.1 years. It showed no statistically significant difference between the HRT (estrogen plus medroxyprog-esterone) group compared with the placebo group in either the primary outcomes (nonfatal myocardial infarction or coronary heart disease death) or in the secondary outcomes (coronary revascularization, unstable angina, congestive heart failure, resuscitated cardiac arrest, stroke or transient ischemic attack, and peripheral arterial disease). The findings of the WHI and HERS trials have been summarized in a recent meta-analysis done for the United States Preventive Services Task Force.3
Both the WHI and HERS trials demonstrated some benefits for HRT. WHI found a reduced risk of colorectal cancer (number needed to treat [NNT]=1667) and a decreased risk of any osteoporotic fracture (NNT=228). The HERS group found that HRT improved the quality of life of women with postmenopausal symptoms, particularly flushing.
Evidence indicates that women who take HRT for 3 years and then stop achieve as much protection from osteoporotic fractures as women who continue their HRT beyond 3 years.4
Continuing HRT beyond 5 years dramatically increases the risk of coronary heart disease, stroke, thromboembolic events, breast cancer, and cholecystitis.3
Recommendations from others
The American College of Obstetricians and Gynecologists has convened a multispecialty panel of experts to draft new recommendations for HRT in light of the WHI findings.
Laura Hansen, PharmD, BCPS
University of Colorado, Boulder
The WHI and HERS trials demonstrated that long-term use of HRT (>5 years) incurs significantly more risks than benefits for a postmenopausal woman who has not undergone hysterectomy. However, these trials did not evaluate postmenopausal symptoms or quality of life as primary endpoints.
Most women experience postmenopausal symptoms for more than 1 year but have resolution of symptoms within a few years after menopause. Since HRT remains the most effective therapy for hot flushes, short-term use of HRT (<5 years) may be offered to women experiencing postmenopausal symptoms.
Physicians may instruct women to attempt HRT discontinuation each year because the duration of symptoms can be variable. Discontinuation should be performed using gradual dose reductions to prevent rapid return of postmenopausal symptoms.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
3. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872-81.
4. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-72.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
3. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872-81.
4. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-72.
Evidence-based answers from the Family Physicians Inquiries Network
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
Evidence-based answers from the Family Physicians Inquiries Network
Does surgery for carpal tunnel syndrome improve outcomes?
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for bronchiolitis?
Nebulized epinephrine decreases oxygen requirements, respiratory rate, wheezing, and retractions and may lower hospitalization rates and length of stay (Grade of Recommendation: A, based on consistent randomized controlled trials [RCTs] and systematic reviews). At best, other beta-2 agonists provide modest short-term improvement in mild to moderate bronchiolitis (Grade of Recommendation: A, consistent RCTs and systematic reviews), and may be indicated in patients with preexisting asthma. Discontinue bronchodilators if patients do not respond quickly, because the bronchodilators may cause respiratory deterioration (Grade of Recommendation: D, expert opinion). Supplemental oxygen for low oxygen saturation and suctioning may improve respiratory status (Grade of Recommendation: D, expert opinion). Chest physiotherapy (Grade of Recommendation: D, expert opinion), cool mist (Grade of Recommendation: D, expert opinion), and aerosolized saline (Grade of Recommendation: A, based on RCTs) are not recommended. Steroids, routine antibiotics, ribavirin, and pooled immunoglobulins play no role in previously healthy children (Grade of Recommendation: A, systematic review, RCT and meta-analysis). See the Table for a summary of therapeutic interventions for bronchiolitis.
TABLE
Therapeutic interventions for bronchiolitis
| Intervention | Usefulness | Grade of recommendation | Notes |
|---|---|---|---|
| Nebulized epinephrine | Beneficial | A | Should be discontinued promptly in the absence of response |
| Beta-2 agonists | Not beneficial | A | May be useful in patients with preexisting asthma |
| Corticosteroids | Not beneficial | A | Not shown to impact clinical score or length of hospital stay |
| Supplemental oxygen, suctioning | Beneficial | D | Initiate at 91% and wean at 94% |
Evidence summary
Most trials of bronchiolitis treatment suffer from 2 constraints: possible inclusion of patients with asthma and inconsistent outcome measures. Five trials of nebulized epinephrine, involving 225 children, have been published in the last decade. All have shown clinical improvement in measures such as respiratory rate, wheezing, retractions, hospital admission rates, and length of stay.1
Data from other clinical trials, meta-analyses, and a comprehensive Cochrane systematic review do not support the routine use of selective beta-2 agonists. Studies with unselected patients noted some benefit, which may reflect the inclusion of asthmatic children, or the effects of suctioning in combination with inhalational therapy. Large proportions of patients admitted to hospital with bronchiolitis receive bronchodilators, and many physicians continue to advocate their use.2 The cost of routine bronchodilators for children with bronchiolitis may be as high as $37.5 million per year.2
One systematic review and 8 RCTs found conflicting evidence on the effects of corticosteroids.3 Steroid therapy, given as inhalations, intravenously, orally, or intramuscularly, does not have a consistent effect on clinical status or on length of stay.4
A 1997 systematic review showed that ribavirin had no significant effect on mortality or the risk of respiratory deterioration in children admitted to hospital with respiratory syncytial virus (RSV) bronchiolitis.3 In fact, cohort studies and randomized trials have shown that ribavirin use is associated with an increase in the number of days of mechanical ventilation, intensive care unit stay, and hospitalizations.4
Passive immunotherapy with pooled immunoglobulins remains controversial and is undergoing intense study.4 Three RCTs failed to show any effect on length of hospital stay, and subsequent studies of an RSV-specific humanized monoclonal antibody (palivizumab) have not shown improvements in outcome.
The evidence supporting the use of supplemental oxygen and suctioning of respiratory secretions is limited to expert opinion.5
Recommendations from others
Most pediatric infectious diseases specialists surveyed in Europe recommend bronchodilators. However, bronchodilators are seldom used to treat bronchiolitis in the United Kingdom.2 The present consensus from the American Academy of Pediatrics6 states that ribavirin should be considered in infants with underlying congenital heart disease, lung disease, or immunosuppression, or for infants requiring mechanical ventilation.
Harold A. Williamson, Jr, MD, MSPH
Department of Family and Community Medicine, University of Missouri– Columbia
Wheezing children are usually hospitalized when they have hypoxemia, lethargy, and fatigue associated with tachypnea and decreased oral intake. Because of the difficulty in differentiating between “bronchiolitis” and a first episode of “asthma,” many wheezing children will continue to receive bronchodilators. Discontinuing bronchodilators seems prudent if oxygenation and respiratory rate do not improve after 6 hours. Supportive care with fluids, oxygen, and suctioning of secretions is usually all that is required in even moderately sick patients. As in other situations involving sick children, the temptation to intervene is overwhelming, hence the many ineffective treatments available. RSV is by far the most common viral pathogen causing bronchiolitis; effective immunization for RSV would probably markedly decrease hospitalizations from bronchiolitis.
1. Schindler M. Do bronchodilators have an effect on bronchiolitis? Crit Care 2002;6:111-2.
2. Kellner JD, Ohlsson A, Gadomski AM, et al. Bronchodilators for bronchiolitis (Cochrane Review). In:The Cochrane Library, Issue 2, 2002. Oxford, England: Update Software.
3. Wang E. What are the effects of treatment for children with bronchiolitis? Clin Evid 2001 December.;
4. Barr F, Graham B. Respiratory syncytial virus. Up To Date 2001 June.;
5. National Guideline Clearinghouse.Evidence based clinical practice guidelines for the infant with bronchiolitis. Cincinnati, OH: Cincinnati Children’s Hospital Medical Center; 2001 Nov 28.
6. Committee on Infect Diseases, American Academy of Pediatrics. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996;97:137.-
Nebulized epinephrine decreases oxygen requirements, respiratory rate, wheezing, and retractions and may lower hospitalization rates and length of stay (Grade of Recommendation: A, based on consistent randomized controlled trials [RCTs] and systematic reviews). At best, other beta-2 agonists provide modest short-term improvement in mild to moderate bronchiolitis (Grade of Recommendation: A, consistent RCTs and systematic reviews), and may be indicated in patients with preexisting asthma. Discontinue bronchodilators if patients do not respond quickly, because the bronchodilators may cause respiratory deterioration (Grade of Recommendation: D, expert opinion). Supplemental oxygen for low oxygen saturation and suctioning may improve respiratory status (Grade of Recommendation: D, expert opinion). Chest physiotherapy (Grade of Recommendation: D, expert opinion), cool mist (Grade of Recommendation: D, expert opinion), and aerosolized saline (Grade of Recommendation: A, based on RCTs) are not recommended. Steroids, routine antibiotics, ribavirin, and pooled immunoglobulins play no role in previously healthy children (Grade of Recommendation: A, systematic review, RCT and meta-analysis). See the Table for a summary of therapeutic interventions for bronchiolitis.
TABLE
Therapeutic interventions for bronchiolitis
| Intervention | Usefulness | Grade of recommendation | Notes |
|---|---|---|---|
| Nebulized epinephrine | Beneficial | A | Should be discontinued promptly in the absence of response |
| Beta-2 agonists | Not beneficial | A | May be useful in patients with preexisting asthma |
| Corticosteroids | Not beneficial | A | Not shown to impact clinical score or length of hospital stay |
| Supplemental oxygen, suctioning | Beneficial | D | Initiate at 91% and wean at 94% |
Evidence summary
Most trials of bronchiolitis treatment suffer from 2 constraints: possible inclusion of patients with asthma and inconsistent outcome measures. Five trials of nebulized epinephrine, involving 225 children, have been published in the last decade. All have shown clinical improvement in measures such as respiratory rate, wheezing, retractions, hospital admission rates, and length of stay.1
Data from other clinical trials, meta-analyses, and a comprehensive Cochrane systematic review do not support the routine use of selective beta-2 agonists. Studies with unselected patients noted some benefit, which may reflect the inclusion of asthmatic children, or the effects of suctioning in combination with inhalational therapy. Large proportions of patients admitted to hospital with bronchiolitis receive bronchodilators, and many physicians continue to advocate their use.2 The cost of routine bronchodilators for children with bronchiolitis may be as high as $37.5 million per year.2
One systematic review and 8 RCTs found conflicting evidence on the effects of corticosteroids.3 Steroid therapy, given as inhalations, intravenously, orally, or intramuscularly, does not have a consistent effect on clinical status or on length of stay.4
A 1997 systematic review showed that ribavirin had no significant effect on mortality or the risk of respiratory deterioration in children admitted to hospital with respiratory syncytial virus (RSV) bronchiolitis.3 In fact, cohort studies and randomized trials have shown that ribavirin use is associated with an increase in the number of days of mechanical ventilation, intensive care unit stay, and hospitalizations.4
Passive immunotherapy with pooled immunoglobulins remains controversial and is undergoing intense study.4 Three RCTs failed to show any effect on length of hospital stay, and subsequent studies of an RSV-specific humanized monoclonal antibody (palivizumab) have not shown improvements in outcome.
The evidence supporting the use of supplemental oxygen and suctioning of respiratory secretions is limited to expert opinion.5
Recommendations from others
Most pediatric infectious diseases specialists surveyed in Europe recommend bronchodilators. However, bronchodilators are seldom used to treat bronchiolitis in the United Kingdom.2 The present consensus from the American Academy of Pediatrics6 states that ribavirin should be considered in infants with underlying congenital heart disease, lung disease, or immunosuppression, or for infants requiring mechanical ventilation.
Harold A. Williamson, Jr, MD, MSPH
Department of Family and Community Medicine, University of Missouri– Columbia
Wheezing children are usually hospitalized when they have hypoxemia, lethargy, and fatigue associated with tachypnea and decreased oral intake. Because of the difficulty in differentiating between “bronchiolitis” and a first episode of “asthma,” many wheezing children will continue to receive bronchodilators. Discontinuing bronchodilators seems prudent if oxygenation and respiratory rate do not improve after 6 hours. Supportive care with fluids, oxygen, and suctioning of secretions is usually all that is required in even moderately sick patients. As in other situations involving sick children, the temptation to intervene is overwhelming, hence the many ineffective treatments available. RSV is by far the most common viral pathogen causing bronchiolitis; effective immunization for RSV would probably markedly decrease hospitalizations from bronchiolitis.
Nebulized epinephrine decreases oxygen requirements, respiratory rate, wheezing, and retractions and may lower hospitalization rates and length of stay (Grade of Recommendation: A, based on consistent randomized controlled trials [RCTs] and systematic reviews). At best, other beta-2 agonists provide modest short-term improvement in mild to moderate bronchiolitis (Grade of Recommendation: A, consistent RCTs and systematic reviews), and may be indicated in patients with preexisting asthma. Discontinue bronchodilators if patients do not respond quickly, because the bronchodilators may cause respiratory deterioration (Grade of Recommendation: D, expert opinion). Supplemental oxygen for low oxygen saturation and suctioning may improve respiratory status (Grade of Recommendation: D, expert opinion). Chest physiotherapy (Grade of Recommendation: D, expert opinion), cool mist (Grade of Recommendation: D, expert opinion), and aerosolized saline (Grade of Recommendation: A, based on RCTs) are not recommended. Steroids, routine antibiotics, ribavirin, and pooled immunoglobulins play no role in previously healthy children (Grade of Recommendation: A, systematic review, RCT and meta-analysis). See the Table for a summary of therapeutic interventions for bronchiolitis.
TABLE
Therapeutic interventions for bronchiolitis
| Intervention | Usefulness | Grade of recommendation | Notes |
|---|---|---|---|
| Nebulized epinephrine | Beneficial | A | Should be discontinued promptly in the absence of response |
| Beta-2 agonists | Not beneficial | A | May be useful in patients with preexisting asthma |
| Corticosteroids | Not beneficial | A | Not shown to impact clinical score or length of hospital stay |
| Supplemental oxygen, suctioning | Beneficial | D | Initiate at 91% and wean at 94% |
Evidence summary
Most trials of bronchiolitis treatment suffer from 2 constraints: possible inclusion of patients with asthma and inconsistent outcome measures. Five trials of nebulized epinephrine, involving 225 children, have been published in the last decade. All have shown clinical improvement in measures such as respiratory rate, wheezing, retractions, hospital admission rates, and length of stay.1
Data from other clinical trials, meta-analyses, and a comprehensive Cochrane systematic review do not support the routine use of selective beta-2 agonists. Studies with unselected patients noted some benefit, which may reflect the inclusion of asthmatic children, or the effects of suctioning in combination with inhalational therapy. Large proportions of patients admitted to hospital with bronchiolitis receive bronchodilators, and many physicians continue to advocate their use.2 The cost of routine bronchodilators for children with bronchiolitis may be as high as $37.5 million per year.2
One systematic review and 8 RCTs found conflicting evidence on the effects of corticosteroids.3 Steroid therapy, given as inhalations, intravenously, orally, or intramuscularly, does not have a consistent effect on clinical status or on length of stay.4
A 1997 systematic review showed that ribavirin had no significant effect on mortality or the risk of respiratory deterioration in children admitted to hospital with respiratory syncytial virus (RSV) bronchiolitis.3 In fact, cohort studies and randomized trials have shown that ribavirin use is associated with an increase in the number of days of mechanical ventilation, intensive care unit stay, and hospitalizations.4
Passive immunotherapy with pooled immunoglobulins remains controversial and is undergoing intense study.4 Three RCTs failed to show any effect on length of hospital stay, and subsequent studies of an RSV-specific humanized monoclonal antibody (palivizumab) have not shown improvements in outcome.
The evidence supporting the use of supplemental oxygen and suctioning of respiratory secretions is limited to expert opinion.5
Recommendations from others
Most pediatric infectious diseases specialists surveyed in Europe recommend bronchodilators. However, bronchodilators are seldom used to treat bronchiolitis in the United Kingdom.2 The present consensus from the American Academy of Pediatrics6 states that ribavirin should be considered in infants with underlying congenital heart disease, lung disease, or immunosuppression, or for infants requiring mechanical ventilation.
Harold A. Williamson, Jr, MD, MSPH
Department of Family and Community Medicine, University of Missouri– Columbia
Wheezing children are usually hospitalized when they have hypoxemia, lethargy, and fatigue associated with tachypnea and decreased oral intake. Because of the difficulty in differentiating between “bronchiolitis” and a first episode of “asthma,” many wheezing children will continue to receive bronchodilators. Discontinuing bronchodilators seems prudent if oxygenation and respiratory rate do not improve after 6 hours. Supportive care with fluids, oxygen, and suctioning of secretions is usually all that is required in even moderately sick patients. As in other situations involving sick children, the temptation to intervene is overwhelming, hence the many ineffective treatments available. RSV is by far the most common viral pathogen causing bronchiolitis; effective immunization for RSV would probably markedly decrease hospitalizations from bronchiolitis.
1. Schindler M. Do bronchodilators have an effect on bronchiolitis? Crit Care 2002;6:111-2.
2. Kellner JD, Ohlsson A, Gadomski AM, et al. Bronchodilators for bronchiolitis (Cochrane Review). In:The Cochrane Library, Issue 2, 2002. Oxford, England: Update Software.
3. Wang E. What are the effects of treatment for children with bronchiolitis? Clin Evid 2001 December.;
4. Barr F, Graham B. Respiratory syncytial virus. Up To Date 2001 June.;
5. National Guideline Clearinghouse.Evidence based clinical practice guidelines for the infant with bronchiolitis. Cincinnati, OH: Cincinnati Children’s Hospital Medical Center; 2001 Nov 28.
6. Committee on Infect Diseases, American Academy of Pediatrics. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996;97:137.-
1. Schindler M. Do bronchodilators have an effect on bronchiolitis? Crit Care 2002;6:111-2.
2. Kellner JD, Ohlsson A, Gadomski AM, et al. Bronchodilators for bronchiolitis (Cochrane Review). In:The Cochrane Library, Issue 2, 2002. Oxford, England: Update Software.
3. Wang E. What are the effects of treatment for children with bronchiolitis? Clin Evid 2001 December.;
4. Barr F, Graham B. Respiratory syncytial virus. Up To Date 2001 June.;
5. National Guideline Clearinghouse.Evidence based clinical practice guidelines for the infant with bronchiolitis. Cincinnati, OH: Cincinnati Children’s Hospital Medical Center; 2001 Nov 28.
6. Committee on Infect Diseases, American Academy of Pediatrics. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996;97:137.-
Evidence-based answers from the Family Physicians Inquiries Network
Who should have colposcopy?
Colposcopy is the preferred test in the work-up of patients with abnormal cervical cytology:
- Low-grade squamous intraepithelial lesion (LSIL): mild dysplasia
- High-grade squamous intraepithelial lesion (HSIL): moderate to severe dysplasia.
- Atypical squamous cells of undetermined significance (ASC-US) with high-risk human papillo-mavirus (HPV) DNA
- Atypical squamous cells, cannot rule out HSIL (ASC-H)
- Atypical glandular cells (AGC)
- Adenocarcinoma in situ (AIS)
Colposcopy is also recommended for patients with symptoms suggestive of cervical cancer (abnormal appearance of the cervix, persistent and undiagnosed vaginal discharge or bleeding) regardless of cytology results, and in the follow-up of patients previously treated for cervical dysplasia (Grade of Recommendation: B). Colposcopy is not recommended for routine cervical cancer screening.
Evidence summary
The primary role of colposcopy is to identify cervical lesions, allowing directed biopsies to identify invasive cancer or its precursors. Although colposcopy has been studied as a primary screening technique, issues of cost, accessibility, invasiveness, and low specificity severely limit its usefulness in this role.1 Using histology as the gold standard, the sensitivity of colposcopy for cervical abnormalities is high (96%; 95% confidence interval [CI], 95%–97%), but the specificity is much lower (48%; 95% CI, 47%–49%).2 This low specificity means that more than half of women with no cervical pathology will have an abnormal colposcopy result. The corresponding positive and negative likelihood ratios are 2 and 0.1, respectively. Consequently, a normal colposcopy result can effectively rule out cervical pathology, thus supporting its role as a diagnostic rather than a screening tool.
While most lesions are found by abnormal cytology, the sensitivity of the Papanicolaou smear ranges from 30% to 89%.3 Therefore, colposcopy is also indicated for patients with symptoms suggestive of cervical dysplasia or cancer (abnormal appearance of the cervix, or persistent and undiagnosed vaginal discharge or bleeding), even in the setting of normal cytology.4
Colposcopy is also indicated for follow-up after treatment of cervical dysplasia. One study5 identified 3 risk factors for recurrence of dysplasia after a loop electrocautery excision procedure (LEEP): residual disease at either the endocervical or ectocervical margins, and involvement of endocervical glands. The presence of these risk factors predicted a recurrence rate of almost 70%.5 Because 8% of the recurrences were missed on cytology, the authors recommended colposcopy 6 months after LEEP for patients with these risk factors.
Recommendations from others
The place of colposcopy in the work-up of patients with abnormal cytology is well supported. With the recent revision of the Bethesda System by the National Cancer Institute,6 the American Society for Colposcopy and Cervical Pathology (ASCCP) held a consensus conference to review the literature and provide evidence-based guidelines for management of abnormal cervical cytology.7 Its recommendations on colposcopy are summarized in the Table.
The U.S. Preventive Services Task Force’s 1996 recommendations found insufficient evidence to recommend either for or against the use of colposcopy as a screening tool for cervical cancer. Based on high cost and low specificity, it recommends against screening colposcopy.8
TABLE
Recommendations for colposcopy, American Society for Colposcopy and Cervical Pathology7
| Cytology result | Recommendation for colposcopy | Strength of recommendation |
|---|---|---|
| ASC-US | Preferred for positive high-risk HPV DNA | A |
| Acceptable for any patient with ASC-US | A | |
| Also acceptable: intensive cytology follow-up alone | A | |
| Preferred for any immunosuppressed patient | B | |
| ASC-H | Preferred for all patients | A |
| AGC or AIS | Preferred for all patients (include endocervical curettage) | A |
| Preferred for those older than 35 years, or having atypical endometrial cells, or unexplained vaginal bleeding (include endometrial biopsy) | ||
| LSIL | Preferred for all patients | A |
| HSIL | Preferred for all patients (include endocervical curettage) | A |
Jacqueline M. Ruplinger, MD
Department of Family and Community Medicine, University of Missouri–Columbia
The evaluation of abnormal Pap smear results is a common problem for providers of women’s health care. Questions as to which women should be referred for colposcopy occur most commonly when the Pap smear shows ASC-US, AGC, or abnormal clinical findings. The recent evidence-based guidelines from the ASCCP provide clearer guidance as to who needs colposcopy, especially when Pap smear results are minimally abnormal.
Evaluation of LSIL confirmed by colposcopy and biopsy is another area causing confusion. It is reasonable for these patients to be followed with regular Pap smears for up to 2 years, as the smears of many women will return to normal without any treatment. I usually do not recommend they return for colposcopy unless the Pap smear result worsens or does not normalize after 2 years.
Patients, as well as providers, have many questions regarding HPV testing. Recently, the ALTS trial has shown that HPV testing in patients with ASC-US can be useful in determining which patients need colposcopy.9
1. Pete I, Toth V, Bosze P. The value of colposcopy in screening cervical carcinoma. Eur J Gynaecol Oncol 1998;19:120-2.
2. Mitchell MF, Schottenfeld D, Tortolero-Luna G, et al. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31.
3. Nanda K, Douglas C, Myers E, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000;132:810-9.
4. Newkirk GR. The colposcopic examination. In: Pfenninger J, Fowler GC, eds. Procedures for Primary Care Physicians. St. Louis, MO: Mosby; 1994;616-39.
5. Dietrich CS, 3rd, Yancey MK, Miyazawa K, et al. Risk factors for early cytologic abnormalities after loop electrosurgical excision procedure. Obstet Gynecol 2002;99:188-92.
6. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-9.
7. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9.
8. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
9. Solomon D, Schiffman M, Tarone R, for the ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-9.
Colposcopy is the preferred test in the work-up of patients with abnormal cervical cytology:
- Low-grade squamous intraepithelial lesion (LSIL): mild dysplasia
- High-grade squamous intraepithelial lesion (HSIL): moderate to severe dysplasia.
- Atypical squamous cells of undetermined significance (ASC-US) with high-risk human papillo-mavirus (HPV) DNA
- Atypical squamous cells, cannot rule out HSIL (ASC-H)
- Atypical glandular cells (AGC)
- Adenocarcinoma in situ (AIS)
Colposcopy is also recommended for patients with symptoms suggestive of cervical cancer (abnormal appearance of the cervix, persistent and undiagnosed vaginal discharge or bleeding) regardless of cytology results, and in the follow-up of patients previously treated for cervical dysplasia (Grade of Recommendation: B). Colposcopy is not recommended for routine cervical cancer screening.
Evidence summary
The primary role of colposcopy is to identify cervical lesions, allowing directed biopsies to identify invasive cancer or its precursors. Although colposcopy has been studied as a primary screening technique, issues of cost, accessibility, invasiveness, and low specificity severely limit its usefulness in this role.1 Using histology as the gold standard, the sensitivity of colposcopy for cervical abnormalities is high (96%; 95% confidence interval [CI], 95%–97%), but the specificity is much lower (48%; 95% CI, 47%–49%).2 This low specificity means that more than half of women with no cervical pathology will have an abnormal colposcopy result. The corresponding positive and negative likelihood ratios are 2 and 0.1, respectively. Consequently, a normal colposcopy result can effectively rule out cervical pathology, thus supporting its role as a diagnostic rather than a screening tool.
While most lesions are found by abnormal cytology, the sensitivity of the Papanicolaou smear ranges from 30% to 89%.3 Therefore, colposcopy is also indicated for patients with symptoms suggestive of cervical dysplasia or cancer (abnormal appearance of the cervix, or persistent and undiagnosed vaginal discharge or bleeding), even in the setting of normal cytology.4
Colposcopy is also indicated for follow-up after treatment of cervical dysplasia. One study5 identified 3 risk factors for recurrence of dysplasia after a loop electrocautery excision procedure (LEEP): residual disease at either the endocervical or ectocervical margins, and involvement of endocervical glands. The presence of these risk factors predicted a recurrence rate of almost 70%.5 Because 8% of the recurrences were missed on cytology, the authors recommended colposcopy 6 months after LEEP for patients with these risk factors.
Recommendations from others
The place of colposcopy in the work-up of patients with abnormal cytology is well supported. With the recent revision of the Bethesda System by the National Cancer Institute,6 the American Society for Colposcopy and Cervical Pathology (ASCCP) held a consensus conference to review the literature and provide evidence-based guidelines for management of abnormal cervical cytology.7 Its recommendations on colposcopy are summarized in the Table.
The U.S. Preventive Services Task Force’s 1996 recommendations found insufficient evidence to recommend either for or against the use of colposcopy as a screening tool for cervical cancer. Based on high cost and low specificity, it recommends against screening colposcopy.8
TABLE
Recommendations for colposcopy, American Society for Colposcopy and Cervical Pathology7
| Cytology result | Recommendation for colposcopy | Strength of recommendation |
|---|---|---|
| ASC-US | Preferred for positive high-risk HPV DNA | A |
| Acceptable for any patient with ASC-US | A | |
| Also acceptable: intensive cytology follow-up alone | A | |
| Preferred for any immunosuppressed patient | B | |
| ASC-H | Preferred for all patients | A |
| AGC or AIS | Preferred for all patients (include endocervical curettage) | A |
| Preferred for those older than 35 years, or having atypical endometrial cells, or unexplained vaginal bleeding (include endometrial biopsy) | ||
| LSIL | Preferred for all patients | A |
| HSIL | Preferred for all patients (include endocervical curettage) | A |
Jacqueline M. Ruplinger, MD
Department of Family and Community Medicine, University of Missouri–Columbia
The evaluation of abnormal Pap smear results is a common problem for providers of women’s health care. Questions as to which women should be referred for colposcopy occur most commonly when the Pap smear shows ASC-US, AGC, or abnormal clinical findings. The recent evidence-based guidelines from the ASCCP provide clearer guidance as to who needs colposcopy, especially when Pap smear results are minimally abnormal.
Evaluation of LSIL confirmed by colposcopy and biopsy is another area causing confusion. It is reasonable for these patients to be followed with regular Pap smears for up to 2 years, as the smears of many women will return to normal without any treatment. I usually do not recommend they return for colposcopy unless the Pap smear result worsens or does not normalize after 2 years.
Patients, as well as providers, have many questions regarding HPV testing. Recently, the ALTS trial has shown that HPV testing in patients with ASC-US can be useful in determining which patients need colposcopy.9
Colposcopy is the preferred test in the work-up of patients with abnormal cervical cytology:
- Low-grade squamous intraepithelial lesion (LSIL): mild dysplasia
- High-grade squamous intraepithelial lesion (HSIL): moderate to severe dysplasia.
- Atypical squamous cells of undetermined significance (ASC-US) with high-risk human papillo-mavirus (HPV) DNA
- Atypical squamous cells, cannot rule out HSIL (ASC-H)
- Atypical glandular cells (AGC)
- Adenocarcinoma in situ (AIS)
Colposcopy is also recommended for patients with symptoms suggestive of cervical cancer (abnormal appearance of the cervix, persistent and undiagnosed vaginal discharge or bleeding) regardless of cytology results, and in the follow-up of patients previously treated for cervical dysplasia (Grade of Recommendation: B). Colposcopy is not recommended for routine cervical cancer screening.
Evidence summary
The primary role of colposcopy is to identify cervical lesions, allowing directed biopsies to identify invasive cancer or its precursors. Although colposcopy has been studied as a primary screening technique, issues of cost, accessibility, invasiveness, and low specificity severely limit its usefulness in this role.1 Using histology as the gold standard, the sensitivity of colposcopy for cervical abnormalities is high (96%; 95% confidence interval [CI], 95%–97%), but the specificity is much lower (48%; 95% CI, 47%–49%).2 This low specificity means that more than half of women with no cervical pathology will have an abnormal colposcopy result. The corresponding positive and negative likelihood ratios are 2 and 0.1, respectively. Consequently, a normal colposcopy result can effectively rule out cervical pathology, thus supporting its role as a diagnostic rather than a screening tool.
While most lesions are found by abnormal cytology, the sensitivity of the Papanicolaou smear ranges from 30% to 89%.3 Therefore, colposcopy is also indicated for patients with symptoms suggestive of cervical dysplasia or cancer (abnormal appearance of the cervix, or persistent and undiagnosed vaginal discharge or bleeding), even in the setting of normal cytology.4
Colposcopy is also indicated for follow-up after treatment of cervical dysplasia. One study5 identified 3 risk factors for recurrence of dysplasia after a loop electrocautery excision procedure (LEEP): residual disease at either the endocervical or ectocervical margins, and involvement of endocervical glands. The presence of these risk factors predicted a recurrence rate of almost 70%.5 Because 8% of the recurrences were missed on cytology, the authors recommended colposcopy 6 months after LEEP for patients with these risk factors.
Recommendations from others
The place of colposcopy in the work-up of patients with abnormal cytology is well supported. With the recent revision of the Bethesda System by the National Cancer Institute,6 the American Society for Colposcopy and Cervical Pathology (ASCCP) held a consensus conference to review the literature and provide evidence-based guidelines for management of abnormal cervical cytology.7 Its recommendations on colposcopy are summarized in the Table.
The U.S. Preventive Services Task Force’s 1996 recommendations found insufficient evidence to recommend either for or against the use of colposcopy as a screening tool for cervical cancer. Based on high cost and low specificity, it recommends against screening colposcopy.8
TABLE
Recommendations for colposcopy, American Society for Colposcopy and Cervical Pathology7
| Cytology result | Recommendation for colposcopy | Strength of recommendation |
|---|---|---|
| ASC-US | Preferred for positive high-risk HPV DNA | A |
| Acceptable for any patient with ASC-US | A | |
| Also acceptable: intensive cytology follow-up alone | A | |
| Preferred for any immunosuppressed patient | B | |
| ASC-H | Preferred for all patients | A |
| AGC or AIS | Preferred for all patients (include endocervical curettage) | A |
| Preferred for those older than 35 years, or having atypical endometrial cells, or unexplained vaginal bleeding (include endometrial biopsy) | ||
| LSIL | Preferred for all patients | A |
| HSIL | Preferred for all patients (include endocervical curettage) | A |
Jacqueline M. Ruplinger, MD
Department of Family and Community Medicine, University of Missouri–Columbia
The evaluation of abnormal Pap smear results is a common problem for providers of women’s health care. Questions as to which women should be referred for colposcopy occur most commonly when the Pap smear shows ASC-US, AGC, or abnormal clinical findings. The recent evidence-based guidelines from the ASCCP provide clearer guidance as to who needs colposcopy, especially when Pap smear results are minimally abnormal.
Evaluation of LSIL confirmed by colposcopy and biopsy is another area causing confusion. It is reasonable for these patients to be followed with regular Pap smears for up to 2 years, as the smears of many women will return to normal without any treatment. I usually do not recommend they return for colposcopy unless the Pap smear result worsens or does not normalize after 2 years.
Patients, as well as providers, have many questions regarding HPV testing. Recently, the ALTS trial has shown that HPV testing in patients with ASC-US can be useful in determining which patients need colposcopy.9
1. Pete I, Toth V, Bosze P. The value of colposcopy in screening cervical carcinoma. Eur J Gynaecol Oncol 1998;19:120-2.
2. Mitchell MF, Schottenfeld D, Tortolero-Luna G, et al. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31.
3. Nanda K, Douglas C, Myers E, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000;132:810-9.
4. Newkirk GR. The colposcopic examination. In: Pfenninger J, Fowler GC, eds. Procedures for Primary Care Physicians. St. Louis, MO: Mosby; 1994;616-39.
5. Dietrich CS, 3rd, Yancey MK, Miyazawa K, et al. Risk factors for early cytologic abnormalities after loop electrosurgical excision procedure. Obstet Gynecol 2002;99:188-92.
6. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-9.
7. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9.
8. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
9. Solomon D, Schiffman M, Tarone R, for the ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-9.
1. Pete I, Toth V, Bosze P. The value of colposcopy in screening cervical carcinoma. Eur J Gynaecol Oncol 1998;19:120-2.
2. Mitchell MF, Schottenfeld D, Tortolero-Luna G, et al. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998;91:626-31.
3. Nanda K, Douglas C, Myers E, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000;132:810-9.
4. Newkirk GR. The colposcopic examination. In: Pfenninger J, Fowler GC, eds. Procedures for Primary Care Physicians. St. Louis, MO: Mosby; 1994;616-39.
5. Dietrich CS, 3rd, Yancey MK, Miyazawa K, et al. Risk factors for early cytologic abnormalities after loop electrosurgical excision procedure. Obstet Gynecol 2002;99:188-92.
6. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114-9.
7. Wright TC, Jr, Cox JT, Massad LS, et al. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9.
8. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
9. Solomon D, Schiffman M, Tarone R, for the ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-9.
Evidence-based answers from the Family Physicians Inquiries Network
How accurate is the clinical diagnosis of pneumonia?
No element or combination of elements from the clinical history and physical examination are sufficiently sensitive or specific to confirm or exclude acute community-acquired pneumonia (CAP). A chest x-ray is recommended to make the diagnosis (Grade of Recommendation: A, based on well-designed cohort studies). No studies specifically demonstrate improved patient outcomes through use of chest x-ray in adults; however, accurate diagnosis is expected to reduce the number of unnecessary antibiotic prescriptions (Grade of Recommendation: D, based on expert opinion).
Evidence summary
Metlay and colleagues1 found only 4 high-quality, prospective cohort trials evaluating the sensitivity and specificity of the clinical history and physical examination in pneumonia. In each of the 4 studies, the reference standard for the diagnosis of pneumonia was a new infiltrate on chest radiograph. Subjects were community-dwelling adults with acute cough who were seen in ambulatory settings, and who had an average pneumonia prevalence of 7% (range, 3%–38%).1 Although no study specifically addressed the interobserver reliability of the history and physical examination findings in pneumonia, other studies of chest findings typically found variable reproducibility. In a study by Spiteri and associates,2 24 physicians examined 24 patients with a variety of respiratory conditions: only 4 had pneumonia on chest x-ray. The most reliable findings (dullness to percussion and wheezing) had only fair agreement among examiners (kappa approximately 0.5).
Nine symptoms (cough, dyspnea, sputum production, subjective fever, chills, night sweats, myalgias, sore throat, and rhinorrhea) and 3 items in the past medical history (asthma, immunosuppression, and dementia) were associated with pneumonia. For most elements of history, both the positive and negative likelihood ratios (LR+, LR−) were in the indeterminate range of 0.5 to 2.0. No single feature was sufficient to either rule in or rule out the diagnosis.1
Regarding the physical examination, tachypnea, tachycardia, and fever had LR+s between 1.5 and 2.4 in an ambulatory setting. In one study, the absence of any vital sign abnormalities reduced the likelihood of pneumonia substantially (LR− = 0.18), but did not rule out the diagnosis completely.1 Egophony had an LR+ of 5.3. Other physical findings (rhonchi, crackles, decreased breath sounds, dullness to percussion, and bronchial breath sounds) yielded LR+s from 1.5 to 3.5, respectively. Most individual findings were insufficient to diagnose pneumonia. For example, if the baseline prevalence of pneumonia was 5%, the presence of crackles raised the probability to 10% and their absence decreased the probability to 3%.
The sensitivity and specificity of clinical diagnosis varied with the prevalence of pneumonia. In a general practice setting, 20 of 402 patients with cough were diagnosed with pneumonia by chest x-ray.3 Physicians correctly diagnosed 7 patients clinically, and incorrectly diagnosed pneumonia in 22 additional patients.3 At a Veterans Administration hospital, a prospective cohort of 52 men with acute cough and change in sputum production underwent sequential blinded examination by 3 physicians. Rales and bronchial breath sounds were common, and chest x-ray confirmed pneumonia in 28 patients. Sensitivity of clinical diagnosis ranged from 47% to 69%, and specificity from 58% to 75%.4
Several researchers improved diagnostic accuracy by combining multiple elements from the history and physical examination. For example, according to Metlay and colleagues,1 Heckerling et al calculated the probability of pneumonia if up to 5 predictors were present. However, if the prevalence of pneumonia in a primary care population is 5%, the presence of all 5 predictors raises the probability of pneumonia only to 53%.1 The absence of 4 of the 5 findings (fever >37.8°C, heart rate >100 beats per minute, decreased breath sounds, crackles) reduces the risk of pneumonia to 1%, thus eliminating the need for radiography or antibiotics in most situations. If the patient also has asthma, the risk drops even further.
Recommendations from others
The Infectious Diseases Society of North America states that a chest x-ray is necessary for accurate diagnosis. In otherwise healthy adults with acute cough illness, antibiotic therapy is indicated only for pneumonia. A normal chest x-ray obviates the need for antibiotics.5,6
John W. Ely, MD, MSPH
University of Iowa Iowa City
[email protected].
The immediate question for clinicians is “Can you treat pneumonia based on clinical findings alone?” Apparently, the answer is “no” unless the radiograph would be unacceptably difficult to obtain (eg, certain nursing home or homebound patients). Can the patient have pneumonia even if the chest radiograph is negative? Subtle early pneumonias sometimes blossom on chest film after a day or two. The diagnosis of pneumonia can be just as much a subjective “call” for the radiologist as “a few crackles” can be for the clinician, so the bottom line is: If you suspect pneumonia, order a chest film.
1. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-5.
2. Spiteri MA, Cook DG, Clarke SW. Reliability of eliciting physical signs in examination of the chest. Lancet 1988;1:873-5.
3. Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992;10:226-33.
4. Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: relevant or relic? Arch Intern Med 1999;159:1082-7.
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521-9.
6. Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults.Infectious Diseases Society of America. Clin Infect Dis 2000;31:347-82.
No element or combination of elements from the clinical history and physical examination are sufficiently sensitive or specific to confirm or exclude acute community-acquired pneumonia (CAP). A chest x-ray is recommended to make the diagnosis (Grade of Recommendation: A, based on well-designed cohort studies). No studies specifically demonstrate improved patient outcomes through use of chest x-ray in adults; however, accurate diagnosis is expected to reduce the number of unnecessary antibiotic prescriptions (Grade of Recommendation: D, based on expert opinion).
Evidence summary
Metlay and colleagues1 found only 4 high-quality, prospective cohort trials evaluating the sensitivity and specificity of the clinical history and physical examination in pneumonia. In each of the 4 studies, the reference standard for the diagnosis of pneumonia was a new infiltrate on chest radiograph. Subjects were community-dwelling adults with acute cough who were seen in ambulatory settings, and who had an average pneumonia prevalence of 7% (range, 3%–38%).1 Although no study specifically addressed the interobserver reliability of the history and physical examination findings in pneumonia, other studies of chest findings typically found variable reproducibility. In a study by Spiteri and associates,2 24 physicians examined 24 patients with a variety of respiratory conditions: only 4 had pneumonia on chest x-ray. The most reliable findings (dullness to percussion and wheezing) had only fair agreement among examiners (kappa approximately 0.5).
Nine symptoms (cough, dyspnea, sputum production, subjective fever, chills, night sweats, myalgias, sore throat, and rhinorrhea) and 3 items in the past medical history (asthma, immunosuppression, and dementia) were associated with pneumonia. For most elements of history, both the positive and negative likelihood ratios (LR+, LR−) were in the indeterminate range of 0.5 to 2.0. No single feature was sufficient to either rule in or rule out the diagnosis.1
Regarding the physical examination, tachypnea, tachycardia, and fever had LR+s between 1.5 and 2.4 in an ambulatory setting. In one study, the absence of any vital sign abnormalities reduced the likelihood of pneumonia substantially (LR− = 0.18), but did not rule out the diagnosis completely.1 Egophony had an LR+ of 5.3. Other physical findings (rhonchi, crackles, decreased breath sounds, dullness to percussion, and bronchial breath sounds) yielded LR+s from 1.5 to 3.5, respectively. Most individual findings were insufficient to diagnose pneumonia. For example, if the baseline prevalence of pneumonia was 5%, the presence of crackles raised the probability to 10% and their absence decreased the probability to 3%.
The sensitivity and specificity of clinical diagnosis varied with the prevalence of pneumonia. In a general practice setting, 20 of 402 patients with cough were diagnosed with pneumonia by chest x-ray.3 Physicians correctly diagnosed 7 patients clinically, and incorrectly diagnosed pneumonia in 22 additional patients.3 At a Veterans Administration hospital, a prospective cohort of 52 men with acute cough and change in sputum production underwent sequential blinded examination by 3 physicians. Rales and bronchial breath sounds were common, and chest x-ray confirmed pneumonia in 28 patients. Sensitivity of clinical diagnosis ranged from 47% to 69%, and specificity from 58% to 75%.4
Several researchers improved diagnostic accuracy by combining multiple elements from the history and physical examination. For example, according to Metlay and colleagues,1 Heckerling et al calculated the probability of pneumonia if up to 5 predictors were present. However, if the prevalence of pneumonia in a primary care population is 5%, the presence of all 5 predictors raises the probability of pneumonia only to 53%.1 The absence of 4 of the 5 findings (fever >37.8°C, heart rate >100 beats per minute, decreased breath sounds, crackles) reduces the risk of pneumonia to 1%, thus eliminating the need for radiography or antibiotics in most situations. If the patient also has asthma, the risk drops even further.
Recommendations from others
The Infectious Diseases Society of North America states that a chest x-ray is necessary for accurate diagnosis. In otherwise healthy adults with acute cough illness, antibiotic therapy is indicated only for pneumonia. A normal chest x-ray obviates the need for antibiotics.5,6
John W. Ely, MD, MSPH
University of Iowa Iowa City
[email protected].
The immediate question for clinicians is “Can you treat pneumonia based on clinical findings alone?” Apparently, the answer is “no” unless the radiograph would be unacceptably difficult to obtain (eg, certain nursing home or homebound patients). Can the patient have pneumonia even if the chest radiograph is negative? Subtle early pneumonias sometimes blossom on chest film after a day or two. The diagnosis of pneumonia can be just as much a subjective “call” for the radiologist as “a few crackles” can be for the clinician, so the bottom line is: If you suspect pneumonia, order a chest film.
No element or combination of elements from the clinical history and physical examination are sufficiently sensitive or specific to confirm or exclude acute community-acquired pneumonia (CAP). A chest x-ray is recommended to make the diagnosis (Grade of Recommendation: A, based on well-designed cohort studies). No studies specifically demonstrate improved patient outcomes through use of chest x-ray in adults; however, accurate diagnosis is expected to reduce the number of unnecessary antibiotic prescriptions (Grade of Recommendation: D, based on expert opinion).
Evidence summary
Metlay and colleagues1 found only 4 high-quality, prospective cohort trials evaluating the sensitivity and specificity of the clinical history and physical examination in pneumonia. In each of the 4 studies, the reference standard for the diagnosis of pneumonia was a new infiltrate on chest radiograph. Subjects were community-dwelling adults with acute cough who were seen in ambulatory settings, and who had an average pneumonia prevalence of 7% (range, 3%–38%).1 Although no study specifically addressed the interobserver reliability of the history and physical examination findings in pneumonia, other studies of chest findings typically found variable reproducibility. In a study by Spiteri and associates,2 24 physicians examined 24 patients with a variety of respiratory conditions: only 4 had pneumonia on chest x-ray. The most reliable findings (dullness to percussion and wheezing) had only fair agreement among examiners (kappa approximately 0.5).
Nine symptoms (cough, dyspnea, sputum production, subjective fever, chills, night sweats, myalgias, sore throat, and rhinorrhea) and 3 items in the past medical history (asthma, immunosuppression, and dementia) were associated with pneumonia. For most elements of history, both the positive and negative likelihood ratios (LR+, LR−) were in the indeterminate range of 0.5 to 2.0. No single feature was sufficient to either rule in or rule out the diagnosis.1
Regarding the physical examination, tachypnea, tachycardia, and fever had LR+s between 1.5 and 2.4 in an ambulatory setting. In one study, the absence of any vital sign abnormalities reduced the likelihood of pneumonia substantially (LR− = 0.18), but did not rule out the diagnosis completely.1 Egophony had an LR+ of 5.3. Other physical findings (rhonchi, crackles, decreased breath sounds, dullness to percussion, and bronchial breath sounds) yielded LR+s from 1.5 to 3.5, respectively. Most individual findings were insufficient to diagnose pneumonia. For example, if the baseline prevalence of pneumonia was 5%, the presence of crackles raised the probability to 10% and their absence decreased the probability to 3%.
The sensitivity and specificity of clinical diagnosis varied with the prevalence of pneumonia. In a general practice setting, 20 of 402 patients with cough were diagnosed with pneumonia by chest x-ray.3 Physicians correctly diagnosed 7 patients clinically, and incorrectly diagnosed pneumonia in 22 additional patients.3 At a Veterans Administration hospital, a prospective cohort of 52 men with acute cough and change in sputum production underwent sequential blinded examination by 3 physicians. Rales and bronchial breath sounds were common, and chest x-ray confirmed pneumonia in 28 patients. Sensitivity of clinical diagnosis ranged from 47% to 69%, and specificity from 58% to 75%.4
Several researchers improved diagnostic accuracy by combining multiple elements from the history and physical examination. For example, according to Metlay and colleagues,1 Heckerling et al calculated the probability of pneumonia if up to 5 predictors were present. However, if the prevalence of pneumonia in a primary care population is 5%, the presence of all 5 predictors raises the probability of pneumonia only to 53%.1 The absence of 4 of the 5 findings (fever >37.8°C, heart rate >100 beats per minute, decreased breath sounds, crackles) reduces the risk of pneumonia to 1%, thus eliminating the need for radiography or antibiotics in most situations. If the patient also has asthma, the risk drops even further.
Recommendations from others
The Infectious Diseases Society of North America states that a chest x-ray is necessary for accurate diagnosis. In otherwise healthy adults with acute cough illness, antibiotic therapy is indicated only for pneumonia. A normal chest x-ray obviates the need for antibiotics.5,6
John W. Ely, MD, MSPH
University of Iowa Iowa City
[email protected].
The immediate question for clinicians is “Can you treat pneumonia based on clinical findings alone?” Apparently, the answer is “no” unless the radiograph would be unacceptably difficult to obtain (eg, certain nursing home or homebound patients). Can the patient have pneumonia even if the chest radiograph is negative? Subtle early pneumonias sometimes blossom on chest film after a day or two. The diagnosis of pneumonia can be just as much a subjective “call” for the radiologist as “a few crackles” can be for the clinician, so the bottom line is: If you suspect pneumonia, order a chest film.
1. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-5.
2. Spiteri MA, Cook DG, Clarke SW. Reliability of eliciting physical signs in examination of the chest. Lancet 1988;1:873-5.
3. Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992;10:226-33.
4. Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: relevant or relic? Arch Intern Med 1999;159:1082-7.
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521-9.
6. Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults.Infectious Diseases Society of America. Clin Infect Dis 2000;31:347-82.
1. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-5.
2. Spiteri MA, Cook DG, Clarke SW. Reliability of eliciting physical signs in examination of the chest. Lancet 1988;1:873-5.
3. Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992;10:226-33.
4. Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: relevant or relic? Arch Intern Med 1999;159:1082-7.
5. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521-9.
6. Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults.Infectious Diseases Society of America. Clin Infect Dis 2000;31:347-82.
Evidence-based answers from the Family Physicians Inquiries Network