User login
Does increasing methylphenidate dose aid symptom control in ADHD?
Most children with attention deficit/hyperactivity disorder (ADHD) who are started on methylphenidate will respond favorably to a dose increase if the initial dose does not sufficiently reduce symptoms. Once titrated to an effective maintenance dose, frequent follow-up is necessary to monitor for side effects and recurring symptoms. The dose of methylphenidate can then be increased further for better symptom control, which may be warranted in most cases.
In some children, methylphenidate may not achieve response even at high doses or may cause intolerable side effects. For these children, start a different stimulant medication (strength of recommendation: B, based on extrapolation of 1 randomized controlled trial).
Evidence summary
Most studies of ADHD medication have lasted fewer than 4 months. The National Institute of Mental Health Collaborative Multisite Multimodal Treatment Study of Children with Attention Deficit/Hyperactivity Disorder (known as the MTA study) is the longest treatment study of children to date. This study—a 28-day, double-blind placebo-controlled trial—enrolled children aged 7 to 9 years with ADHD and compared 4 treatment strategies (including medication and behavioral interventions) over 14 months.1-3
Of particular interest was the dose-titration evaluation at the beginning of the study. Daily dose-switching titration of methylphenidate was used to identify the optimal starting dose for each child assigned to receive medication. In all, 289 children were randomized to receive methylphenidate, and 256 completed the titration (17 children refused to take medication, 1 moved, 4 had side effects, 4 had missing data, and 7 stopped mid-titration because of inability to follow the titration protocol).
Of the 256 children who completed titration, 198 (77%) responded favorably to 1 of the following doses: low (15 mg/day), intermediate (25 mg/day), or high (35 mg/day for children weighing less than 25 kg; or 50 mg/day for children weighing 25 kg or more). Of the remaining 23%, 32 children responded best to placebo and 26 were methylphenidate nonresponders and were subsequently placed on dextroamphetamine.
Children who responded to methylphenidate entered the 13-month maintenance phase on the optimal dose identified in the titration trial. They were monitored by monthly re-examination and review of information from parents and teachers regarding ADHD symptoms and potential drug side effects. The dose was changed if symptoms were not well controlled or if side effects were present. Subsequently, if no effective and well-tolerated dose of methylphenidate could be identified, the drug was deemed ineffective for that child and was replaced by another medication.
Of the children who responded to methylphenidate, 88% were still taking it at the end of the maintenance trial; 29% were still taking the titration-determined dose of methylphenidate, 18% took a lower dose, and 41% took a higher dose as their “optimal” dose, at which there were no clinically significant symptoms, or “no room for improvement.” The mean dose increased from 31 mg/day at the start to 34 mg/day at the end of the study. Of the 430 total changes in dose made during the maintenance period, 62% were dose increases.
While commendable for its design and large study population, the MTA study had several limitations. The titration trial’s complex method of determining each child’s “best dose” may not be feasible in clinical practice. Furthermore, the study enrolled only children aged 7 to 9 years, while ADHD affects a much broader age range. Finally, the chronic nature of ADHD limits the generalizability of this study beyond 14 months. Additional long-term studies are needed.
Recommendations from others
The most common strategy for managing children taking methylphenidate is to start with a low dose and gradually adjust upward, as required by residual symptoms and as allowed by side effects. This escalating-dose titration reflects typical practice in the United States, as described in several clinical guides.4,5 The Physician’s Desk Reference states that the maximum total daily dose is 60 mg for methylphenidate, and experts often limit the upper range to 25 mg for a single dose.6
The American Academy of Child and Adolescent Psychiatry suggests using a consistent titration schedule with weekly increases in increments of 5–10 mg per dose to achieve symptom control. Alternatively, a fixed-dose titration trial similar to that found in the MTA study may be employed, in which a full set of different doses is switched on a weekly basis. If the top recommended dose does not help, a change in drug or psychosocial intervention may be more beneficial than an increase in methylphenidate dose.6
John Hill, DO
University of Colorado Health Sciences Center, Denver
It is disheartening to watch a bright child receive D’s in school just because he or she cannot pay attention. Treating children with ADHD is one of the most clinically rewarding behavioral issues we can address as primary care physicians.
The escalating-dose titration and effective maintenance of methylphenidate can seem intimidating. We fear the side effects and are unsure if raising the dose of methylphenidate will have any benefits.
Clearly, it is shown that raising the methylphenidate dose brings further benefits for most children, but short-acting forms (such as Ritalin) frequently have intolerable side effects. Several long-acting forms of methylphenidate (Concerta, Metadate CD, Methylin ER, and Ritalin SR) are now on the market. This allows us to raise the dose as high as 54–60 mg/day with much less drug intolerance. For children who are benefiting from methylphenidate but cannot tolerate the side effects, consider the long-acting form.
1. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-1313.
2. Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry 2001;40:180-187.
3. Vitiello B, Severe JB, Greenhill LL, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry 2001;40:188-196.
4. Dulcan M. Using psychostimulants to treat behavior disorders of children and adolescents. J Child Adolesc Psychopharmacol. 1990;1:7-20.
5. Szymanski ML, Zolotor A. Attention-deficit/hyperactivity disorder: management. Am Fam Physician 2001;64:1355-1362.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medication in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S-49S.
Most children with attention deficit/hyperactivity disorder (ADHD) who are started on methylphenidate will respond favorably to a dose increase if the initial dose does not sufficiently reduce symptoms. Once titrated to an effective maintenance dose, frequent follow-up is necessary to monitor for side effects and recurring symptoms. The dose of methylphenidate can then be increased further for better symptom control, which may be warranted in most cases.
In some children, methylphenidate may not achieve response even at high doses or may cause intolerable side effects. For these children, start a different stimulant medication (strength of recommendation: B, based on extrapolation of 1 randomized controlled trial).
Evidence summary
Most studies of ADHD medication have lasted fewer than 4 months. The National Institute of Mental Health Collaborative Multisite Multimodal Treatment Study of Children with Attention Deficit/Hyperactivity Disorder (known as the MTA study) is the longest treatment study of children to date. This study—a 28-day, double-blind placebo-controlled trial—enrolled children aged 7 to 9 years with ADHD and compared 4 treatment strategies (including medication and behavioral interventions) over 14 months.1-3
Of particular interest was the dose-titration evaluation at the beginning of the study. Daily dose-switching titration of methylphenidate was used to identify the optimal starting dose for each child assigned to receive medication. In all, 289 children were randomized to receive methylphenidate, and 256 completed the titration (17 children refused to take medication, 1 moved, 4 had side effects, 4 had missing data, and 7 stopped mid-titration because of inability to follow the titration protocol).
Of the 256 children who completed titration, 198 (77%) responded favorably to 1 of the following doses: low (15 mg/day), intermediate (25 mg/day), or high (35 mg/day for children weighing less than 25 kg; or 50 mg/day for children weighing 25 kg or more). Of the remaining 23%, 32 children responded best to placebo and 26 were methylphenidate nonresponders and were subsequently placed on dextroamphetamine.
Children who responded to methylphenidate entered the 13-month maintenance phase on the optimal dose identified in the titration trial. They were monitored by monthly re-examination and review of information from parents and teachers regarding ADHD symptoms and potential drug side effects. The dose was changed if symptoms were not well controlled or if side effects were present. Subsequently, if no effective and well-tolerated dose of methylphenidate could be identified, the drug was deemed ineffective for that child and was replaced by another medication.
Of the children who responded to methylphenidate, 88% were still taking it at the end of the maintenance trial; 29% were still taking the titration-determined dose of methylphenidate, 18% took a lower dose, and 41% took a higher dose as their “optimal” dose, at which there were no clinically significant symptoms, or “no room for improvement.” The mean dose increased from 31 mg/day at the start to 34 mg/day at the end of the study. Of the 430 total changes in dose made during the maintenance period, 62% were dose increases.
While commendable for its design and large study population, the MTA study had several limitations. The titration trial’s complex method of determining each child’s “best dose” may not be feasible in clinical practice. Furthermore, the study enrolled only children aged 7 to 9 years, while ADHD affects a much broader age range. Finally, the chronic nature of ADHD limits the generalizability of this study beyond 14 months. Additional long-term studies are needed.
Recommendations from others
The most common strategy for managing children taking methylphenidate is to start with a low dose and gradually adjust upward, as required by residual symptoms and as allowed by side effects. This escalating-dose titration reflects typical practice in the United States, as described in several clinical guides.4,5 The Physician’s Desk Reference states that the maximum total daily dose is 60 mg for methylphenidate, and experts often limit the upper range to 25 mg for a single dose.6
The American Academy of Child and Adolescent Psychiatry suggests using a consistent titration schedule with weekly increases in increments of 5–10 mg per dose to achieve symptom control. Alternatively, a fixed-dose titration trial similar to that found in the MTA study may be employed, in which a full set of different doses is switched on a weekly basis. If the top recommended dose does not help, a change in drug or psychosocial intervention may be more beneficial than an increase in methylphenidate dose.6
John Hill, DO
University of Colorado Health Sciences Center, Denver
It is disheartening to watch a bright child receive D’s in school just because he or she cannot pay attention. Treating children with ADHD is one of the most clinically rewarding behavioral issues we can address as primary care physicians.
The escalating-dose titration and effective maintenance of methylphenidate can seem intimidating. We fear the side effects and are unsure if raising the dose of methylphenidate will have any benefits.
Clearly, it is shown that raising the methylphenidate dose brings further benefits for most children, but short-acting forms (such as Ritalin) frequently have intolerable side effects. Several long-acting forms of methylphenidate (Concerta, Metadate CD, Methylin ER, and Ritalin SR) are now on the market. This allows us to raise the dose as high as 54–60 mg/day with much less drug intolerance. For children who are benefiting from methylphenidate but cannot tolerate the side effects, consider the long-acting form.
Most children with attention deficit/hyperactivity disorder (ADHD) who are started on methylphenidate will respond favorably to a dose increase if the initial dose does not sufficiently reduce symptoms. Once titrated to an effective maintenance dose, frequent follow-up is necessary to monitor for side effects and recurring symptoms. The dose of methylphenidate can then be increased further for better symptom control, which may be warranted in most cases.
In some children, methylphenidate may not achieve response even at high doses or may cause intolerable side effects. For these children, start a different stimulant medication (strength of recommendation: B, based on extrapolation of 1 randomized controlled trial).
Evidence summary
Most studies of ADHD medication have lasted fewer than 4 months. The National Institute of Mental Health Collaborative Multisite Multimodal Treatment Study of Children with Attention Deficit/Hyperactivity Disorder (known as the MTA study) is the longest treatment study of children to date. This study—a 28-day, double-blind placebo-controlled trial—enrolled children aged 7 to 9 years with ADHD and compared 4 treatment strategies (including medication and behavioral interventions) over 14 months.1-3
Of particular interest was the dose-titration evaluation at the beginning of the study. Daily dose-switching titration of methylphenidate was used to identify the optimal starting dose for each child assigned to receive medication. In all, 289 children were randomized to receive methylphenidate, and 256 completed the titration (17 children refused to take medication, 1 moved, 4 had side effects, 4 had missing data, and 7 stopped mid-titration because of inability to follow the titration protocol).
Of the 256 children who completed titration, 198 (77%) responded favorably to 1 of the following doses: low (15 mg/day), intermediate (25 mg/day), or high (35 mg/day for children weighing less than 25 kg; or 50 mg/day for children weighing 25 kg or more). Of the remaining 23%, 32 children responded best to placebo and 26 were methylphenidate nonresponders and were subsequently placed on dextroamphetamine.
Children who responded to methylphenidate entered the 13-month maintenance phase on the optimal dose identified in the titration trial. They were monitored by monthly re-examination and review of information from parents and teachers regarding ADHD symptoms and potential drug side effects. The dose was changed if symptoms were not well controlled or if side effects were present. Subsequently, if no effective and well-tolerated dose of methylphenidate could be identified, the drug was deemed ineffective for that child and was replaced by another medication.
Of the children who responded to methylphenidate, 88% were still taking it at the end of the maintenance trial; 29% were still taking the titration-determined dose of methylphenidate, 18% took a lower dose, and 41% took a higher dose as their “optimal” dose, at which there were no clinically significant symptoms, or “no room for improvement.” The mean dose increased from 31 mg/day at the start to 34 mg/day at the end of the study. Of the 430 total changes in dose made during the maintenance period, 62% were dose increases.
While commendable for its design and large study population, the MTA study had several limitations. The titration trial’s complex method of determining each child’s “best dose” may not be feasible in clinical practice. Furthermore, the study enrolled only children aged 7 to 9 years, while ADHD affects a much broader age range. Finally, the chronic nature of ADHD limits the generalizability of this study beyond 14 months. Additional long-term studies are needed.
Recommendations from others
The most common strategy for managing children taking methylphenidate is to start with a low dose and gradually adjust upward, as required by residual symptoms and as allowed by side effects. This escalating-dose titration reflects typical practice in the United States, as described in several clinical guides.4,5 The Physician’s Desk Reference states that the maximum total daily dose is 60 mg for methylphenidate, and experts often limit the upper range to 25 mg for a single dose.6
The American Academy of Child and Adolescent Psychiatry suggests using a consistent titration schedule with weekly increases in increments of 5–10 mg per dose to achieve symptom control. Alternatively, a fixed-dose titration trial similar to that found in the MTA study may be employed, in which a full set of different doses is switched on a weekly basis. If the top recommended dose does not help, a change in drug or psychosocial intervention may be more beneficial than an increase in methylphenidate dose.6
John Hill, DO
University of Colorado Health Sciences Center, Denver
It is disheartening to watch a bright child receive D’s in school just because he or she cannot pay attention. Treating children with ADHD is one of the most clinically rewarding behavioral issues we can address as primary care physicians.
The escalating-dose titration and effective maintenance of methylphenidate can seem intimidating. We fear the side effects and are unsure if raising the dose of methylphenidate will have any benefits.
Clearly, it is shown that raising the methylphenidate dose brings further benefits for most children, but short-acting forms (such as Ritalin) frequently have intolerable side effects. Several long-acting forms of methylphenidate (Concerta, Metadate CD, Methylin ER, and Ritalin SR) are now on the market. This allows us to raise the dose as high as 54–60 mg/day with much less drug intolerance. For children who are benefiting from methylphenidate but cannot tolerate the side effects, consider the long-acting form.
1. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-1313.
2. Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry 2001;40:180-187.
3. Vitiello B, Severe JB, Greenhill LL, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry 2001;40:188-196.
4. Dulcan M. Using psychostimulants to treat behavior disorders of children and adolescents. J Child Adolesc Psychopharmacol. 1990;1:7-20.
5. Szymanski ML, Zolotor A. Attention-deficit/hyperactivity disorder: management. Am Fam Physician 2001;64:1355-1362.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medication in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S-49S.
1. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-1313.
2. Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry 2001;40:180-187.
3. Vitiello B, Severe JB, Greenhill LL, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry 2001;40:188-196.
4. Dulcan M. Using psychostimulants to treat behavior disorders of children and adolescents. J Child Adolesc Psychopharmacol. 1990;1:7-20.
5. Szymanski ML, Zolotor A. Attention-deficit/hyperactivity disorder: management. Am Fam Physician 2001;64:1355-1362.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medication in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;41:26S-49S.
Evidence-based answers from the Family Physicians Inquiries Network
What is the most effective beta-blocker for heart failure?
Three beta-blockers—carvedilol, metoprolol, and bisoprolol—reduce mortality in chronic heart failure caused by left ventricular systolic dysfunction, when used in addition to diuretics and angiotensin converting enzyme (ACE) inhibitors (strength of recommendation [SOR]: A, based on large randomized placebo-controlled trials). No differences in mortality or patient tolerance have been demonstrated in studies comparing carvedilol and metoprolol (SOR: B, based on small head-to-head trials).
Evidence summary
The Table shows the 5 largest trials of beta-blockers in systolic dysfunction, including patients with both ischemic and nonischemic heart disease. In all trials, the majority of subjects were taking diuretics and either an ACE inhibitor or angiotensin receptor blocker.
The Carvedilol Prospective Randomized Cumulative Survival2 (COPERNICUS) trial, Metoprolol CR/XL Randomized Intervention Trial in Heart Failure3 (MERIT-HF), and Cardiac Insufficiency Bisoprolol Study II4 (CIBIS-II) all showed similar reductions in mortality in moderately ill patients with heart failure.
In contrast, the Beta-Blocker Evaluation of Survival Trial5 (BEST) demonstrated no effect with bucindolol. This suggests there may be differences in effectiveness among beta-blockers in reducing mortality in heart failure, and that it would be unwise to assume that protection is a class effect. We found no meta-analysis that pooled data on individual drugs for comparison purposes.
The US Carvedilol trial1 demonstrated a larger reduction in mortality than that seen in other beta-blocker trials. However, it had several methodologic problems: it was a composite of 4 smaller studies that used exercise tolerance as the primary endpoint; median duration of data collection on subjects was only 6 months; it included many minimally symptomatic patients; the actual number of deaths was small (producing a wide confidence interval); and subjects who did not survive the run-in phase were excluded from analysis.6
Three randomized controlled trials have compared carvedilol and metoprolol head-to-head. The largest7 included 150 subjects with ejection fractions below 35% who were randomized to 1 of the 2 drugs and followed for more than 3 years. Symptom scores and quality of life assessments were similar in the 2 groups. A trend toward lower mortality in the carvedilol group did not reach statistical significance. Peak oxygen uptake during exercise was greater in the metoprolol group. The carvedilol group had a statistically greater improvement in ejection fraction (+10.9 ± 11.0 vs +7.2 ± 7.7 at rest). The Carvedilol or Metoprolol European Trial (COMET), a larger head-to-head trial of carvedilol and metoprolol (N=3029), is ongoing.8
No large studies of older beta-blockers adequately assess mortality in heart failure. One study of propranolol (N=158) showed a 27% reduction in mortality in mild heart failure in the setting of ischemic heart disease.9 A study of atenolol versus placebo in subjects with ejection fraction ≤25% from various causes (N=100) was halted early when atenolol produced a 50% reduction in worsening heart failure and a 71% reduction in cardiac hospitalizations.10 A trend toward improved survival was noted but did not reach statistical significance.
TABLE
Selected trials of beta-blockers for systolic dysfunction
| Study | Drug | N | Mortality reduction (%) | 95% CI (%) | Statistically significant? | NNT | Mean duration of follow-up (months) |
|---|---|---|---|---|---|---|---|
| US Carvedilol1 | Carvedilol | 1094 | (65) | 39–80 | Yes | 22 | 6.5 |
| COPERNICUS2 | Carvedilol | 2289 | (35) | 19–48 | Yes | 14 | 10.4 |
| MERIT-HF3 | Metoprolol | 3991 | (34) | 19–46 | Yes | 26 | 12 |
| CIBIS II4 | Bisoprolol | 2647 | (34) | 19–47 | Yes | 18 | 15.6 |
| BEST5 | Bucindolol | 2708 | (9) | –0.2–22 | No | — | 24 |
| CI, confidence interval; NNT, number needed to treat | |||||||
Recommendations from others
We found no guidelines that specifically endorsed one beta-blocker over another for heart failure.
Fred Grover, Jr, MD
University of Colorado Health Sciences Center, Denver
To provide the best care, we must go beyond the conventional ACE inhibitor and diuretic therapy for congestive heart failure patients. Adding 1 of the 3 beta-blockers (carvedilol, metoprolol, or bisoprolol), as recommended above, will further improve the survival rates and decrease hospitalization rates.
Remember these pearls when using beta-blockers in congestive heart failure:
- Do not start therapy until the patient’s fluid status has been stable for at least 1 month
- Avoid using in patients with bronchospastic disease, symptomatic bradycardia, or advanced heart blockage
- Start with low doses and titrate up slowly as tolerated every 2 weeks to the recommended target range of the beta-blocker chosen
- Decrease the dose if significant bradycardia or atrioventricular block occurs
- Let your patients know that it may take several months of beta-blocker therapy to obtain the protective benefits.
If you encounter difficulties with titration or don’t feel comfortable initiating beta-blocker therapy, consult your cardiologist for help.
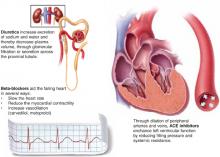
Chronic heart failure
Complementary actions of diuretics, ACE inhibitors, and beta blockers
Evidence shows that the combination of diuretics, ACE inhibitors, and 1 of 3 beta-blockers—carvedilol, metoprolol, bisoprolol—is more effective than just diuretics plus ACE inhibitors. The clinical effect of their combined actions is reduced workload on the failing heart.
1. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-1355.
2. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
3. Effect of metoprolol CR/XL in chronic heart failure: Metotprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
4. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
5. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659-1667.
6. Hart SM. Influence of beta-blockers on mortality in chronic heart failure. Ann Pharmacother 2000;34:1440-1451.
7. Metra M, Giubbini Raffaele, Nodari E, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000;102:546-551.
8. Poole-Wilson PA, Cleland JG, Di Lenarda A, et al. Rationale and design of the carvedilol or metoprolol European trail in patients with chronic heart failure: COMET. Eur J Heart Fail 2002;4:321-329.
9. Aronow WS, Ahn C, Kronzon AI. Effect of propranolol versus no propanolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-209.
10. Sturm B, Pacher R, Strametz-Juranek J, et al. Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail 2000;2:407-412.
Three beta-blockers—carvedilol, metoprolol, and bisoprolol—reduce mortality in chronic heart failure caused by left ventricular systolic dysfunction, when used in addition to diuretics and angiotensin converting enzyme (ACE) inhibitors (strength of recommendation [SOR]: A, based on large randomized placebo-controlled trials). No differences in mortality or patient tolerance have been demonstrated in studies comparing carvedilol and metoprolol (SOR: B, based on small head-to-head trials).
Evidence summary
The Table shows the 5 largest trials of beta-blockers in systolic dysfunction, including patients with both ischemic and nonischemic heart disease. In all trials, the majority of subjects were taking diuretics and either an ACE inhibitor or angiotensin receptor blocker.
The Carvedilol Prospective Randomized Cumulative Survival2 (COPERNICUS) trial, Metoprolol CR/XL Randomized Intervention Trial in Heart Failure3 (MERIT-HF), and Cardiac Insufficiency Bisoprolol Study II4 (CIBIS-II) all showed similar reductions in mortality in moderately ill patients with heart failure.
In contrast, the Beta-Blocker Evaluation of Survival Trial5 (BEST) demonstrated no effect with bucindolol. This suggests there may be differences in effectiveness among beta-blockers in reducing mortality in heart failure, and that it would be unwise to assume that protection is a class effect. We found no meta-analysis that pooled data on individual drugs for comparison purposes.
The US Carvedilol trial1 demonstrated a larger reduction in mortality than that seen in other beta-blocker trials. However, it had several methodologic problems: it was a composite of 4 smaller studies that used exercise tolerance as the primary endpoint; median duration of data collection on subjects was only 6 months; it included many minimally symptomatic patients; the actual number of deaths was small (producing a wide confidence interval); and subjects who did not survive the run-in phase were excluded from analysis.6
Three randomized controlled trials have compared carvedilol and metoprolol head-to-head. The largest7 included 150 subjects with ejection fractions below 35% who were randomized to 1 of the 2 drugs and followed for more than 3 years. Symptom scores and quality of life assessments were similar in the 2 groups. A trend toward lower mortality in the carvedilol group did not reach statistical significance. Peak oxygen uptake during exercise was greater in the metoprolol group. The carvedilol group had a statistically greater improvement in ejection fraction (+10.9 ± 11.0 vs +7.2 ± 7.7 at rest). The Carvedilol or Metoprolol European Trial (COMET), a larger head-to-head trial of carvedilol and metoprolol (N=3029), is ongoing.8
No large studies of older beta-blockers adequately assess mortality in heart failure. One study of propranolol (N=158) showed a 27% reduction in mortality in mild heart failure in the setting of ischemic heart disease.9 A study of atenolol versus placebo in subjects with ejection fraction ≤25% from various causes (N=100) was halted early when atenolol produced a 50% reduction in worsening heart failure and a 71% reduction in cardiac hospitalizations.10 A trend toward improved survival was noted but did not reach statistical significance.
TABLE
Selected trials of beta-blockers for systolic dysfunction
| Study | Drug | N | Mortality reduction (%) | 95% CI (%) | Statistically significant? | NNT | Mean duration of follow-up (months) |
|---|---|---|---|---|---|---|---|
| US Carvedilol1 | Carvedilol | 1094 | (65) | 39–80 | Yes | 22 | 6.5 |
| COPERNICUS2 | Carvedilol | 2289 | (35) | 19–48 | Yes | 14 | 10.4 |
| MERIT-HF3 | Metoprolol | 3991 | (34) | 19–46 | Yes | 26 | 12 |
| CIBIS II4 | Bisoprolol | 2647 | (34) | 19–47 | Yes | 18 | 15.6 |
| BEST5 | Bucindolol | 2708 | (9) | –0.2–22 | No | — | 24 |
| CI, confidence interval; NNT, number needed to treat | |||||||
Recommendations from others
We found no guidelines that specifically endorsed one beta-blocker over another for heart failure.
Fred Grover, Jr, MD
University of Colorado Health Sciences Center, Denver
To provide the best care, we must go beyond the conventional ACE inhibitor and diuretic therapy for congestive heart failure patients. Adding 1 of the 3 beta-blockers (carvedilol, metoprolol, or bisoprolol), as recommended above, will further improve the survival rates and decrease hospitalization rates.
Remember these pearls when using beta-blockers in congestive heart failure:
- Do not start therapy until the patient’s fluid status has been stable for at least 1 month
- Avoid using in patients with bronchospastic disease, symptomatic bradycardia, or advanced heart blockage
- Start with low doses and titrate up slowly as tolerated every 2 weeks to the recommended target range of the beta-blocker chosen
- Decrease the dose if significant bradycardia or atrioventricular block occurs
- Let your patients know that it may take several months of beta-blocker therapy to obtain the protective benefits.
If you encounter difficulties with titration or don’t feel comfortable initiating beta-blocker therapy, consult your cardiologist for help.
Chronic heart failure
Complementary actions of diuretics, ACE inhibitors, and beta blockers
Evidence shows that the combination of diuretics, ACE inhibitors, and 1 of 3 beta-blockers—carvedilol, metoprolol, bisoprolol—is more effective than just diuretics plus ACE inhibitors. The clinical effect of their combined actions is reduced workload on the failing heart.
Three beta-blockers—carvedilol, metoprolol, and bisoprolol—reduce mortality in chronic heart failure caused by left ventricular systolic dysfunction, when used in addition to diuretics and angiotensin converting enzyme (ACE) inhibitors (strength of recommendation [SOR]: A, based on large randomized placebo-controlled trials). No differences in mortality or patient tolerance have been demonstrated in studies comparing carvedilol and metoprolol (SOR: B, based on small head-to-head trials).
Evidence summary
The Table shows the 5 largest trials of beta-blockers in systolic dysfunction, including patients with both ischemic and nonischemic heart disease. In all trials, the majority of subjects were taking diuretics and either an ACE inhibitor or angiotensin receptor blocker.
The Carvedilol Prospective Randomized Cumulative Survival2 (COPERNICUS) trial, Metoprolol CR/XL Randomized Intervention Trial in Heart Failure3 (MERIT-HF), and Cardiac Insufficiency Bisoprolol Study II4 (CIBIS-II) all showed similar reductions in mortality in moderately ill patients with heart failure.
In contrast, the Beta-Blocker Evaluation of Survival Trial5 (BEST) demonstrated no effect with bucindolol. This suggests there may be differences in effectiveness among beta-blockers in reducing mortality in heart failure, and that it would be unwise to assume that protection is a class effect. We found no meta-analysis that pooled data on individual drugs for comparison purposes.
The US Carvedilol trial1 demonstrated a larger reduction in mortality than that seen in other beta-blocker trials. However, it had several methodologic problems: it was a composite of 4 smaller studies that used exercise tolerance as the primary endpoint; median duration of data collection on subjects was only 6 months; it included many minimally symptomatic patients; the actual number of deaths was small (producing a wide confidence interval); and subjects who did not survive the run-in phase were excluded from analysis.6
Three randomized controlled trials have compared carvedilol and metoprolol head-to-head. The largest7 included 150 subjects with ejection fractions below 35% who were randomized to 1 of the 2 drugs and followed for more than 3 years. Symptom scores and quality of life assessments were similar in the 2 groups. A trend toward lower mortality in the carvedilol group did not reach statistical significance. Peak oxygen uptake during exercise was greater in the metoprolol group. The carvedilol group had a statistically greater improvement in ejection fraction (+10.9 ± 11.0 vs +7.2 ± 7.7 at rest). The Carvedilol or Metoprolol European Trial (COMET), a larger head-to-head trial of carvedilol and metoprolol (N=3029), is ongoing.8
No large studies of older beta-blockers adequately assess mortality in heart failure. One study of propranolol (N=158) showed a 27% reduction in mortality in mild heart failure in the setting of ischemic heart disease.9 A study of atenolol versus placebo in subjects with ejection fraction ≤25% from various causes (N=100) was halted early when atenolol produced a 50% reduction in worsening heart failure and a 71% reduction in cardiac hospitalizations.10 A trend toward improved survival was noted but did not reach statistical significance.
TABLE
Selected trials of beta-blockers for systolic dysfunction
| Study | Drug | N | Mortality reduction (%) | 95% CI (%) | Statistically significant? | NNT | Mean duration of follow-up (months) |
|---|---|---|---|---|---|---|---|
| US Carvedilol1 | Carvedilol | 1094 | (65) | 39–80 | Yes | 22 | 6.5 |
| COPERNICUS2 | Carvedilol | 2289 | (35) | 19–48 | Yes | 14 | 10.4 |
| MERIT-HF3 | Metoprolol | 3991 | (34) | 19–46 | Yes | 26 | 12 |
| CIBIS II4 | Bisoprolol | 2647 | (34) | 19–47 | Yes | 18 | 15.6 |
| BEST5 | Bucindolol | 2708 | (9) | –0.2–22 | No | — | 24 |
| CI, confidence interval; NNT, number needed to treat | |||||||
Recommendations from others
We found no guidelines that specifically endorsed one beta-blocker over another for heart failure.
Fred Grover, Jr, MD
University of Colorado Health Sciences Center, Denver
To provide the best care, we must go beyond the conventional ACE inhibitor and diuretic therapy for congestive heart failure patients. Adding 1 of the 3 beta-blockers (carvedilol, metoprolol, or bisoprolol), as recommended above, will further improve the survival rates and decrease hospitalization rates.
Remember these pearls when using beta-blockers in congestive heart failure:
- Do not start therapy until the patient’s fluid status has been stable for at least 1 month
- Avoid using in patients with bronchospastic disease, symptomatic bradycardia, or advanced heart blockage
- Start with low doses and titrate up slowly as tolerated every 2 weeks to the recommended target range of the beta-blocker chosen
- Decrease the dose if significant bradycardia or atrioventricular block occurs
- Let your patients know that it may take several months of beta-blocker therapy to obtain the protective benefits.
If you encounter difficulties with titration or don’t feel comfortable initiating beta-blocker therapy, consult your cardiologist for help.
Chronic heart failure
Complementary actions of diuretics, ACE inhibitors, and beta blockers
Evidence shows that the combination of diuretics, ACE inhibitors, and 1 of 3 beta-blockers—carvedilol, metoprolol, bisoprolol—is more effective than just diuretics plus ACE inhibitors. The clinical effect of their combined actions is reduced workload on the failing heart.
1. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-1355.
2. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
3. Effect of metoprolol CR/XL in chronic heart failure: Metotprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
4. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
5. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659-1667.
6. Hart SM. Influence of beta-blockers on mortality in chronic heart failure. Ann Pharmacother 2000;34:1440-1451.
7. Metra M, Giubbini Raffaele, Nodari E, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000;102:546-551.
8. Poole-Wilson PA, Cleland JG, Di Lenarda A, et al. Rationale and design of the carvedilol or metoprolol European trail in patients with chronic heart failure: COMET. Eur J Heart Fail 2002;4:321-329.
9. Aronow WS, Ahn C, Kronzon AI. Effect of propranolol versus no propanolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-209.
10. Sturm B, Pacher R, Strametz-Juranek J, et al. Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail 2000;2:407-412.
1. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-1355.
2. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-1658.
3. Effect of metoprolol CR/XL in chronic heart failure: Metotprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
4. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
5. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659-1667.
6. Hart SM. Influence of beta-blockers on mortality in chronic heart failure. Ann Pharmacother 2000;34:1440-1451.
7. Metra M, Giubbini Raffaele, Nodari E, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: A prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000;102:546-551.
8. Poole-Wilson PA, Cleland JG, Di Lenarda A, et al. Rationale and design of the carvedilol or metoprolol European trail in patients with chronic heart failure: COMET. Eur J Heart Fail 2002;4:321-329.
9. Aronow WS, Ahn C, Kronzon AI. Effect of propranolol versus no propanolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol 1997;80:207-209.
10. Sturm B, Pacher R, Strametz-Juranek J, et al. Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail 2000;2:407-412.
Evidence-based answers from the Family Physicians Inquiries Network
Is osteoporosis screening in postmenopausal women effective?
No single study evaluates the effectiveness of osteoporosis screening. However, screening women over the age of 65 years—or those between 60–64 years with certain risk factors—is recommended based on available evidence.
First, osteoporosis is common, and its prevalence increases with age (strength of recommendation [SOR]: A—prospective cohort studies). Second, low bone mineral density predicts fracture risk (SOR: A—prospective cohort studies). Finally, the likelihood of osteoporotic fracture is reduced with therapy, such as alendronate 10 mg/day or risedronate 5 mg/day plus adequate daily calcium and vitamin D (SOR: A—meta-analysis of randomized clinical trials).
Women under 60 years should not be screened (SOR: B—clinical decision rule). There is no evidence to guide decisions about screening interval or at what age to stop screening. The long-term risks of newer medications used for osteoporosis are unknown.
Evidence summary
Osteoporosis results in significant morbidity and mortality. In a prospective observational study of women over 50 years of age, 39.6% had osteopenia and 7.2% had osteoporosis. Osteoporosis was associated with a fracture rate 4 times that of normal bone mineral density.1 People with vertebral or hip fractures have a reduced relative 5-year survival of 0.81. Excess mortality occurred within the first 6 months following fracture.2
One prospective cohort study identified 14 independent risk factors for hip fracture.3 The best predictors were female gender, age, low weight, and no current estrogen use. For women aged >65 years with no other risks, 12% to 28% have osteoporosis.4 Multiple risk assessment scales have been studied to identify women aged >65 years who are at increased risk; however, none of the scales had good discriminatory performance.5 As a result, it is unclear which factors for women under 65 years should trigger screening.
While multiple technologies exist to measure bone mineral density, dual-energy x-ray absorptiometry (DEXA) has been the most validated test for predicting fractures. A meta-analysis of 11 prospective cohort trials showed that all sites of bone mineral density measurements correlated with fractures (relative risk [RR], 1.5; 95% confi-dence interval [CI], 1.4–1.6.). However, DEXA of the femoral neck predicted hip fracture better than other measures (RR, 2.6; 95% CI, 2.0–3.5).6
Additionally, heel ultrasonography was compa-rable with hip DEXA for predicting hip fractures for women over 65 years (probability of fracture 0.018 vs. 0.023); no studies have compared effec-tiveness for women under 65 years.
Multiple therapeutic interventions for osteo-porosis have been demonstrated to reduce frac-tures. Adequate calcium and vitamin D appear to prevent fractures. Alendronate and rise-dronate are the only prescription medications with evidence showing they prevent hip fractures.
A meta-analysis of 11 randomized controlled trials including 11,808 women found fewer hip fractures in women taking 10 mg/day of alendronate (RR, 0.51; 95% CI, 0.38–0.69; number needed to treat [NNT]=24), and fewer vertebral fractures in women taking 5 mg/day of alendronate (RR, 0.52; 95% CI, 0.43–0.65; NNT=72).7
For these results to apply to screening, study participants must be similar to those identified by general population screening. All trials included healthy women with low bone mineral density who were not using estrogen, which is similar to women identified by general screening. However, 57% of women recruited for the second Fracture Intervention Trial (FIT-II), the largest study, were classified as ineligible. This raises concern about the study’s generalizability.8
The US Preventive Services Task Force did an outcomes estimation of screening effectiveness, combining all of the above data (Table).9 Screening 731 women aged 65 to 69 years would prevent 1 hip fracture if those with indications for treat-ment took it; screening 248 women would prevent 1 vertebral fracture. As the table demonstrates, benefits increase with age. For women under 65 years, benefits are relatively small, unless they have other risk factors for osteoporosis.
TABLE
Hip and vertebral fracture outcomes for osteoporosis screening in 10,000 postmenopausal women 9
| Age (years) | |||
|---|---|---|---|
| Screening outcomes | 55–59 | 65–69 | 75–79 |
| Identified with osteoporosis | 445 | 1200 | 2850 |
| Hip fracture prevented with medication | 2 | 14 | 70 |
| NNS to prevent 1 hip fracture | 4338 | 731 | 143 |
| NNT to prevent 1 hip fracture | 193 | 88 | 41 |
| Vertebral fractures prevented | 7 | 40 | 134 |
| NNS to prevent 1 vertebral fracture | 1338 | 248 | 75 |
| NNT to prevent 1 vertebral fracture | 60 | 30 | 21 |
| The calculations in this table assume that treatment reduces the risk of vertebral fracture by 48%, the risk of hip fracture to 36%, and that 70% of patients will adhere to therapy. Table modified from USPSTF report.9 | |||
| NNS, number needed to screen for benefit; NNT, number needed to treat for benefit | |||
Recommendations from others
Based on their outcomes model, the US Preventive Services Task Force recommends screening for women aged >65 years, and those aged 60 to 65 years who have risk factors.9 In 1998, the National Osteoporosis Foundation, in collaboration with many other professional organ-izations, recommended bone mineral density test-ing for all women aged >65 years and younger postmenopausal women who have had or are at risk for fractures.10 The 2000 Consensus Development Conference from the National Institutes of Health recommended an individual-ized approach to screening, stating evidence for universal osteoporosis screening is inconclusive.11 The American Association of Clinical Endo-crinologists revised guidelines in 2001 to include screening younger postmenopausal women with a body weight <127 lbs or a family history of nontraumatic spine or hip fracture.12
Michael L. Lefevre, MD, MSPH
Department of Family and Community Medicine, University of Missouri–Columbia
The value of screening for osteoporosis is a much bigger issue for clinicians since the pub-lication of the Women’s Health Initiative study and the consequent decline in the number of postmenopausal women using HRT. Evidence for pharmacologic prevention of fractures in women who do not meet conventional criteria for osteoporosis is lacking. Data on fracture risk with osteoporosis are short-term, and the risks and benefits of long-term treatment of women who do have osteoporosis are unknown for all of the treatment options.
The conclusion to focus our screening efforts on women aged 65 years and older, where the near-term benefits seem to clearly outweigh the risks, is certainly clinically prudent. Irrespective of our wishes, many women in their fifties are getting osteoporosis screening at health fairs or shopping malls. Although I do not encourage this age group to be screened, when faced with results showing osteoporosis, I do still treat with a bisphosphonate, based on the trials noted above.
1. Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286:2815-2822.
2. Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ, 3rd. Population-based study of survival after osteo-porotic fractures. Am J Epidemiol 1993;137:1001-1005.
3. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767-773.
4. Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV. Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ 2000;162:1289-1294.
5. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual-energy x-ray absorptiometry. JAMA 2001;286:57-63.
6. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone marrow density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-1259.
7. Cranney A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 2002;23:517-523.
8. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-2082.
9. Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:529-541.
10. Physicians Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation. Wash-ington, DC: National Osteoporosis Foundation; 1999. Available at: www.nof.org/physguide. Accessed on February 24, 2003.
11. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 2000; 17:1–45. Available at: http://odp.od.nih.gov/consensus/cons/111/111_state-ment.htm. Accessed on February 24, 2003.
12. American Association of Clinical Endocrinologists. 2001 Medical Guidelines for Clinical Practice for the Prevention and Management of Postmenopausal Osteoporosis. Available at: www.aace.com/clin/guidelines/osteoporosis2001.pdf. Accessed on February 24, 2003.
No single study evaluates the effectiveness of osteoporosis screening. However, screening women over the age of 65 years—or those between 60–64 years with certain risk factors—is recommended based on available evidence.
First, osteoporosis is common, and its prevalence increases with age (strength of recommendation [SOR]: A—prospective cohort studies). Second, low bone mineral density predicts fracture risk (SOR: A—prospective cohort studies). Finally, the likelihood of osteoporotic fracture is reduced with therapy, such as alendronate 10 mg/day or risedronate 5 mg/day plus adequate daily calcium and vitamin D (SOR: A—meta-analysis of randomized clinical trials).
Women under 60 years should not be screened (SOR: B—clinical decision rule). There is no evidence to guide decisions about screening interval or at what age to stop screening. The long-term risks of newer medications used for osteoporosis are unknown.
Evidence summary
Osteoporosis results in significant morbidity and mortality. In a prospective observational study of women over 50 years of age, 39.6% had osteopenia and 7.2% had osteoporosis. Osteoporosis was associated with a fracture rate 4 times that of normal bone mineral density.1 People with vertebral or hip fractures have a reduced relative 5-year survival of 0.81. Excess mortality occurred within the first 6 months following fracture.2
One prospective cohort study identified 14 independent risk factors for hip fracture.3 The best predictors were female gender, age, low weight, and no current estrogen use. For women aged >65 years with no other risks, 12% to 28% have osteoporosis.4 Multiple risk assessment scales have been studied to identify women aged >65 years who are at increased risk; however, none of the scales had good discriminatory performance.5 As a result, it is unclear which factors for women under 65 years should trigger screening.
While multiple technologies exist to measure bone mineral density, dual-energy x-ray absorptiometry (DEXA) has been the most validated test for predicting fractures. A meta-analysis of 11 prospective cohort trials showed that all sites of bone mineral density measurements correlated with fractures (relative risk [RR], 1.5; 95% confi-dence interval [CI], 1.4–1.6.). However, DEXA of the femoral neck predicted hip fracture better than other measures (RR, 2.6; 95% CI, 2.0–3.5).6
Additionally, heel ultrasonography was compa-rable with hip DEXA for predicting hip fractures for women over 65 years (probability of fracture 0.018 vs. 0.023); no studies have compared effec-tiveness for women under 65 years.
Multiple therapeutic interventions for osteo-porosis have been demonstrated to reduce frac-tures. Adequate calcium and vitamin D appear to prevent fractures. Alendronate and rise-dronate are the only prescription medications with evidence showing they prevent hip fractures.
A meta-analysis of 11 randomized controlled trials including 11,808 women found fewer hip fractures in women taking 10 mg/day of alendronate (RR, 0.51; 95% CI, 0.38–0.69; number needed to treat [NNT]=24), and fewer vertebral fractures in women taking 5 mg/day of alendronate (RR, 0.52; 95% CI, 0.43–0.65; NNT=72).7
For these results to apply to screening, study participants must be similar to those identified by general population screening. All trials included healthy women with low bone mineral density who were not using estrogen, which is similar to women identified by general screening. However, 57% of women recruited for the second Fracture Intervention Trial (FIT-II), the largest study, were classified as ineligible. This raises concern about the study’s generalizability.8
The US Preventive Services Task Force did an outcomes estimation of screening effectiveness, combining all of the above data (Table).9 Screening 731 women aged 65 to 69 years would prevent 1 hip fracture if those with indications for treat-ment took it; screening 248 women would prevent 1 vertebral fracture. As the table demonstrates, benefits increase with age. For women under 65 years, benefits are relatively small, unless they have other risk factors for osteoporosis.
TABLE
Hip and vertebral fracture outcomes for osteoporosis screening in 10,000 postmenopausal women 9
| Age (years) | |||
|---|---|---|---|
| Screening outcomes | 55–59 | 65–69 | 75–79 |
| Identified with osteoporosis | 445 | 1200 | 2850 |
| Hip fracture prevented with medication | 2 | 14 | 70 |
| NNS to prevent 1 hip fracture | 4338 | 731 | 143 |
| NNT to prevent 1 hip fracture | 193 | 88 | 41 |
| Vertebral fractures prevented | 7 | 40 | 134 |
| NNS to prevent 1 vertebral fracture | 1338 | 248 | 75 |
| NNT to prevent 1 vertebral fracture | 60 | 30 | 21 |
| The calculations in this table assume that treatment reduces the risk of vertebral fracture by 48%, the risk of hip fracture to 36%, and that 70% of patients will adhere to therapy. Table modified from USPSTF report.9 | |||
| NNS, number needed to screen for benefit; NNT, number needed to treat for benefit | |||
Recommendations from others
Based on their outcomes model, the US Preventive Services Task Force recommends screening for women aged >65 years, and those aged 60 to 65 years who have risk factors.9 In 1998, the National Osteoporosis Foundation, in collaboration with many other professional organ-izations, recommended bone mineral density test-ing for all women aged >65 years and younger postmenopausal women who have had or are at risk for fractures.10 The 2000 Consensus Development Conference from the National Institutes of Health recommended an individual-ized approach to screening, stating evidence for universal osteoporosis screening is inconclusive.11 The American Association of Clinical Endo-crinologists revised guidelines in 2001 to include screening younger postmenopausal women with a body weight <127 lbs or a family history of nontraumatic spine or hip fracture.12
Michael L. Lefevre, MD, MSPH
Department of Family and Community Medicine, University of Missouri–Columbia
The value of screening for osteoporosis is a much bigger issue for clinicians since the pub-lication of the Women’s Health Initiative study and the consequent decline in the number of postmenopausal women using HRT. Evidence for pharmacologic prevention of fractures in women who do not meet conventional criteria for osteoporosis is lacking. Data on fracture risk with osteoporosis are short-term, and the risks and benefits of long-term treatment of women who do have osteoporosis are unknown for all of the treatment options.
The conclusion to focus our screening efforts on women aged 65 years and older, where the near-term benefits seem to clearly outweigh the risks, is certainly clinically prudent. Irrespective of our wishes, many women in their fifties are getting osteoporosis screening at health fairs or shopping malls. Although I do not encourage this age group to be screened, when faced with results showing osteoporosis, I do still treat with a bisphosphonate, based on the trials noted above.
No single study evaluates the effectiveness of osteoporosis screening. However, screening women over the age of 65 years—or those between 60–64 years with certain risk factors—is recommended based on available evidence.
First, osteoporosis is common, and its prevalence increases with age (strength of recommendation [SOR]: A—prospective cohort studies). Second, low bone mineral density predicts fracture risk (SOR: A—prospective cohort studies). Finally, the likelihood of osteoporotic fracture is reduced with therapy, such as alendronate 10 mg/day or risedronate 5 mg/day plus adequate daily calcium and vitamin D (SOR: A—meta-analysis of randomized clinical trials).
Women under 60 years should not be screened (SOR: B—clinical decision rule). There is no evidence to guide decisions about screening interval or at what age to stop screening. The long-term risks of newer medications used for osteoporosis are unknown.
Evidence summary
Osteoporosis results in significant morbidity and mortality. In a prospective observational study of women over 50 years of age, 39.6% had osteopenia and 7.2% had osteoporosis. Osteoporosis was associated with a fracture rate 4 times that of normal bone mineral density.1 People with vertebral or hip fractures have a reduced relative 5-year survival of 0.81. Excess mortality occurred within the first 6 months following fracture.2
One prospective cohort study identified 14 independent risk factors for hip fracture.3 The best predictors were female gender, age, low weight, and no current estrogen use. For women aged >65 years with no other risks, 12% to 28% have osteoporosis.4 Multiple risk assessment scales have been studied to identify women aged >65 years who are at increased risk; however, none of the scales had good discriminatory performance.5 As a result, it is unclear which factors for women under 65 years should trigger screening.
While multiple technologies exist to measure bone mineral density, dual-energy x-ray absorptiometry (DEXA) has been the most validated test for predicting fractures. A meta-analysis of 11 prospective cohort trials showed that all sites of bone mineral density measurements correlated with fractures (relative risk [RR], 1.5; 95% confi-dence interval [CI], 1.4–1.6.). However, DEXA of the femoral neck predicted hip fracture better than other measures (RR, 2.6; 95% CI, 2.0–3.5).6
Additionally, heel ultrasonography was compa-rable with hip DEXA for predicting hip fractures for women over 65 years (probability of fracture 0.018 vs. 0.023); no studies have compared effec-tiveness for women under 65 years.
Multiple therapeutic interventions for osteo-porosis have been demonstrated to reduce frac-tures. Adequate calcium and vitamin D appear to prevent fractures. Alendronate and rise-dronate are the only prescription medications with evidence showing they prevent hip fractures.
A meta-analysis of 11 randomized controlled trials including 11,808 women found fewer hip fractures in women taking 10 mg/day of alendronate (RR, 0.51; 95% CI, 0.38–0.69; number needed to treat [NNT]=24), and fewer vertebral fractures in women taking 5 mg/day of alendronate (RR, 0.52; 95% CI, 0.43–0.65; NNT=72).7
For these results to apply to screening, study participants must be similar to those identified by general population screening. All trials included healthy women with low bone mineral density who were not using estrogen, which is similar to women identified by general screening. However, 57% of women recruited for the second Fracture Intervention Trial (FIT-II), the largest study, were classified as ineligible. This raises concern about the study’s generalizability.8
The US Preventive Services Task Force did an outcomes estimation of screening effectiveness, combining all of the above data (Table).9 Screening 731 women aged 65 to 69 years would prevent 1 hip fracture if those with indications for treat-ment took it; screening 248 women would prevent 1 vertebral fracture. As the table demonstrates, benefits increase with age. For women under 65 years, benefits are relatively small, unless they have other risk factors for osteoporosis.
TABLE
Hip and vertebral fracture outcomes for osteoporosis screening in 10,000 postmenopausal women 9
| Age (years) | |||
|---|---|---|---|
| Screening outcomes | 55–59 | 65–69 | 75–79 |
| Identified with osteoporosis | 445 | 1200 | 2850 |
| Hip fracture prevented with medication | 2 | 14 | 70 |
| NNS to prevent 1 hip fracture | 4338 | 731 | 143 |
| NNT to prevent 1 hip fracture | 193 | 88 | 41 |
| Vertebral fractures prevented | 7 | 40 | 134 |
| NNS to prevent 1 vertebral fracture | 1338 | 248 | 75 |
| NNT to prevent 1 vertebral fracture | 60 | 30 | 21 |
| The calculations in this table assume that treatment reduces the risk of vertebral fracture by 48%, the risk of hip fracture to 36%, and that 70% of patients will adhere to therapy. Table modified from USPSTF report.9 | |||
| NNS, number needed to screen for benefit; NNT, number needed to treat for benefit | |||
Recommendations from others
Based on their outcomes model, the US Preventive Services Task Force recommends screening for women aged >65 years, and those aged 60 to 65 years who have risk factors.9 In 1998, the National Osteoporosis Foundation, in collaboration with many other professional organ-izations, recommended bone mineral density test-ing for all women aged >65 years and younger postmenopausal women who have had or are at risk for fractures.10 The 2000 Consensus Development Conference from the National Institutes of Health recommended an individual-ized approach to screening, stating evidence for universal osteoporosis screening is inconclusive.11 The American Association of Clinical Endo-crinologists revised guidelines in 2001 to include screening younger postmenopausal women with a body weight <127 lbs or a family history of nontraumatic spine or hip fracture.12
Michael L. Lefevre, MD, MSPH
Department of Family and Community Medicine, University of Missouri–Columbia
The value of screening for osteoporosis is a much bigger issue for clinicians since the pub-lication of the Women’s Health Initiative study and the consequent decline in the number of postmenopausal women using HRT. Evidence for pharmacologic prevention of fractures in women who do not meet conventional criteria for osteoporosis is lacking. Data on fracture risk with osteoporosis are short-term, and the risks and benefits of long-term treatment of women who do have osteoporosis are unknown for all of the treatment options.
The conclusion to focus our screening efforts on women aged 65 years and older, where the near-term benefits seem to clearly outweigh the risks, is certainly clinically prudent. Irrespective of our wishes, many women in their fifties are getting osteoporosis screening at health fairs or shopping malls. Although I do not encourage this age group to be screened, when faced with results showing osteoporosis, I do still treat with a bisphosphonate, based on the trials noted above.
1. Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286:2815-2822.
2. Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ, 3rd. Population-based study of survival after osteo-porotic fractures. Am J Epidemiol 1993;137:1001-1005.
3. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767-773.
4. Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV. Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ 2000;162:1289-1294.
5. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual-energy x-ray absorptiometry. JAMA 2001;286:57-63.
6. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone marrow density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-1259.
7. Cranney A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 2002;23:517-523.
8. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-2082.
9. Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:529-541.
10. Physicians Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation. Wash-ington, DC: National Osteoporosis Foundation; 1999. Available at: www.nof.org/physguide. Accessed on February 24, 2003.
11. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 2000; 17:1–45. Available at: http://odp.od.nih.gov/consensus/cons/111/111_state-ment.htm. Accessed on February 24, 2003.
12. American Association of Clinical Endocrinologists. 2001 Medical Guidelines for Clinical Practice for the Prevention and Management of Postmenopausal Osteoporosis. Available at: www.aace.com/clin/guidelines/osteoporosis2001.pdf. Accessed on February 24, 2003.
1. Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286:2815-2822.
2. Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ, 3rd. Population-based study of survival after osteo-porotic fractures. Am J Epidemiol 1993;137:1001-1005.
3. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767-773.
4. Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV. Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ 2000;162:1289-1294.
5. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual-energy x-ray absorptiometry. JAMA 2001;286:57-63.
6. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone marrow density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-1259.
7. Cranney A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 2002;23:517-523.
8. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-2082.
9. Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:529-541.
10. Physicians Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation. Wash-ington, DC: National Osteoporosis Foundation; 1999. Available at: www.nof.org/physguide. Accessed on February 24, 2003.
11. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 2000; 17:1–45. Available at: http://odp.od.nih.gov/consensus/cons/111/111_state-ment.htm. Accessed on February 24, 2003.
12. American Association of Clinical Endocrinologists. 2001 Medical Guidelines for Clinical Practice for the Prevention and Management of Postmenopausal Osteoporosis. Available at: www.aace.com/clin/guidelines/osteoporosis2001.pdf. Accessed on February 24, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
What treatments are effective for varicose veins?
For larger trunk varicose veins, as in the saphenous vein, therapeutic options include conservative measures (such as leg elevation and compression stockings), injection sclerotherapy, and surgical vein ligation, with or without stripping.Long-term outcomes appear superior with surgical treatment.
For mid-sized reticular veins and spider telangiectasias, several options are available, including sclerotherapy, laser ablation, and thermal ablation. However, no randomized trials have compared the relative effectiveness of these treatments.
Venotonic medications (primarily plantderived and synthetic flavonoids, such as horse chestnut seed extract, that improve venous tone) provide symptom relief. Head-to-head comparisons are needed to identify the most efficacious therapies (strength of recommendation: C, based on case series and extrapolations from small trials.)
Evidence summary
Graduated elastic compression stockings improve lower-extremity hemodynamics (including reflux and residual volume measured by color flow duplex scanning) in patients with varicosities, and can improve symptoms such as swelling, discomfort, and leg tightness.1,2
A Cochrane review concluded that existing evidence supports the use of sclerotherapy for recurrent varicose veins after surgery and for relatively minor “thread” veins.3 Data did not show that any particular type of sclerosant or pressure dressing or duration of post-treatment compression have significant effect on outcomes, such as disappearance of varicosities and cosmetic improvement.3
A Cochrane protocol is in progress regarding comparison of the outcomes of surgery and sclerotherapy.4 Few randomized trials have compared surgery and sclerotherapy.
Belcaro reported results of a 10-year randomized trial including 121 subjects, 96 of whom completed the study.5 Surgery consisted of ligation of the saphenopopliteal junction without stripping. At 10 years, 16.1% of patients receiving surgery plus sclerotherapy had distal venous incompetence (assessed with color duplex scanning and ambulatory venous pressure measurement), compared with 36.4% of those who underwent surgery alone and 43.8% of those who received sclerotherapy alone. The authors concluded that long-term outcomes (defined as saphenofemoral junction competence) are superior with strategies that included surgery, but at greater cost.
Beresford and colleagues also concluded that surgery lessened the likelihood of additional treatment.6 Another randomized trial showed that saphenous vein stripping reduced by two thirds the need for reoperation due to recurrent saphenofemoral incompetence, compared with saphenofemoral junction ligation alone.7
A meta-analysis studied the effectiveness of venotonic medications (such as rutoside, flunarizine, and dihydroergotamine) in chronic venous insufficiency.8 These agents significantly reduced pain, leg heaviness, cramps, and paresthesias. However, a Cochrane Collaboration reviewer questioned the validity of pooling results from this heterogeneous group of studies into a single meta-analysis.9
A Cochrane Review did find that horse chestnut seed extract significantly improves leg pain, edema, pruritus, and lower leg volume and circumference, but suggests that larger randomized trials are needed to establish conclusively this agent’s efficacy.10
Recommendations from others
A recent clinical review indicated that patients whose main symptoms are swelling or aching can be treated with compression stockings alone; trunk varicosities should be treated with saphenofemoral or saphenopopliteal ligation, plus stripping of the long saphenous vein for long saphenous varicosities.11 They suggest that sclerotherapy should be reserved for varicosities that persist after surgery.
The Venous Insufficiency Epidemiologic and Economic Studies (VEINES) program recommends sclerotherapy for telangiectasias and reticular veins, and surgery for saphenous varicosities.12 However, they noted the need for randomized trials to compare therapies.
Alan Adelman, MD, MS
Penn State University, State College, Pa
Choosing the best treatment for varicose veins can be complicated. Symptoms and the type of varicose veins (truncal varices, reticular varices, or telangiectasia) can guide the clinician in selecting therapy. Asymptomatic varicosities can usually be observed without treatment. Patients with symptomatic varicosities may be treated conservatively before referring for invasive treatment.
Surgery is probably the best treatment for truncal varices, whereas sclerotherapy is better for reticular veins or telangiectasia. The long-term risks and benefits of newer modalities such as laser and thermal ablation need further evaluation. Regardless of the treatment chosen, patients with varicose veins should first undergo a thorough investigation.
1. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999;25:701-704.
2. Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-123.
3. Tisi PV, Beverley CA. Injection sclerotherapy for varicose veins (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
4. Michaels JA, Kendall RJ. Surgery for varicose veins (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Belcaro G, Nicolaides AN, Ricci A, et al. Endovascular sclerotherapy, surgery, and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow-up trial—final results. Angiology 2000;51:529-534.
6. Beresford SAA, Chant ADB, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Five-year follow-up. Lancet. 1978;1:921-924.
7. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg 1999;29:589-592.
8. Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis. Clin Drug Invest 1999;18:413-432.
9. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
10. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
11. London NJM, Nash R. ABC of arterial and venous disease. Varicose veins. BMJ 2000;320:1391-1394.
12. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83-102.
For larger trunk varicose veins, as in the saphenous vein, therapeutic options include conservative measures (such as leg elevation and compression stockings), injection sclerotherapy, and surgical vein ligation, with or without stripping.Long-term outcomes appear superior with surgical treatment.
For mid-sized reticular veins and spider telangiectasias, several options are available, including sclerotherapy, laser ablation, and thermal ablation. However, no randomized trials have compared the relative effectiveness of these treatments.
Venotonic medications (primarily plantderived and synthetic flavonoids, such as horse chestnut seed extract, that improve venous tone) provide symptom relief. Head-to-head comparisons are needed to identify the most efficacious therapies (strength of recommendation: C, based on case series and extrapolations from small trials.)
Evidence summary
Graduated elastic compression stockings improve lower-extremity hemodynamics (including reflux and residual volume measured by color flow duplex scanning) in patients with varicosities, and can improve symptoms such as swelling, discomfort, and leg tightness.1,2
A Cochrane review concluded that existing evidence supports the use of sclerotherapy for recurrent varicose veins after surgery and for relatively minor “thread” veins.3 Data did not show that any particular type of sclerosant or pressure dressing or duration of post-treatment compression have significant effect on outcomes, such as disappearance of varicosities and cosmetic improvement.3
A Cochrane protocol is in progress regarding comparison of the outcomes of surgery and sclerotherapy.4 Few randomized trials have compared surgery and sclerotherapy.
Belcaro reported results of a 10-year randomized trial including 121 subjects, 96 of whom completed the study.5 Surgery consisted of ligation of the saphenopopliteal junction without stripping. At 10 years, 16.1% of patients receiving surgery plus sclerotherapy had distal venous incompetence (assessed with color duplex scanning and ambulatory venous pressure measurement), compared with 36.4% of those who underwent surgery alone and 43.8% of those who received sclerotherapy alone. The authors concluded that long-term outcomes (defined as saphenofemoral junction competence) are superior with strategies that included surgery, but at greater cost.
Beresford and colleagues also concluded that surgery lessened the likelihood of additional treatment.6 Another randomized trial showed that saphenous vein stripping reduced by two thirds the need for reoperation due to recurrent saphenofemoral incompetence, compared with saphenofemoral junction ligation alone.7
A meta-analysis studied the effectiveness of venotonic medications (such as rutoside, flunarizine, and dihydroergotamine) in chronic venous insufficiency.8 These agents significantly reduced pain, leg heaviness, cramps, and paresthesias. However, a Cochrane Collaboration reviewer questioned the validity of pooling results from this heterogeneous group of studies into a single meta-analysis.9
A Cochrane Review did find that horse chestnut seed extract significantly improves leg pain, edema, pruritus, and lower leg volume and circumference, but suggests that larger randomized trials are needed to establish conclusively this agent’s efficacy.10
Recommendations from others
A recent clinical review indicated that patients whose main symptoms are swelling or aching can be treated with compression stockings alone; trunk varicosities should be treated with saphenofemoral or saphenopopliteal ligation, plus stripping of the long saphenous vein for long saphenous varicosities.11 They suggest that sclerotherapy should be reserved for varicosities that persist after surgery.
The Venous Insufficiency Epidemiologic and Economic Studies (VEINES) program recommends sclerotherapy for telangiectasias and reticular veins, and surgery for saphenous varicosities.12 However, they noted the need for randomized trials to compare therapies.
Alan Adelman, MD, MS
Penn State University, State College, Pa
Choosing the best treatment for varicose veins can be complicated. Symptoms and the type of varicose veins (truncal varices, reticular varices, or telangiectasia) can guide the clinician in selecting therapy. Asymptomatic varicosities can usually be observed without treatment. Patients with symptomatic varicosities may be treated conservatively before referring for invasive treatment.
Surgery is probably the best treatment for truncal varices, whereas sclerotherapy is better for reticular veins or telangiectasia. The long-term risks and benefits of newer modalities such as laser and thermal ablation need further evaluation. Regardless of the treatment chosen, patients with varicose veins should first undergo a thorough investigation.
For larger trunk varicose veins, as in the saphenous vein, therapeutic options include conservative measures (such as leg elevation and compression stockings), injection sclerotherapy, and surgical vein ligation, with or without stripping.Long-term outcomes appear superior with surgical treatment.
For mid-sized reticular veins and spider telangiectasias, several options are available, including sclerotherapy, laser ablation, and thermal ablation. However, no randomized trials have compared the relative effectiveness of these treatments.
Venotonic medications (primarily plantderived and synthetic flavonoids, such as horse chestnut seed extract, that improve venous tone) provide symptom relief. Head-to-head comparisons are needed to identify the most efficacious therapies (strength of recommendation: C, based on case series and extrapolations from small trials.)
Evidence summary
Graduated elastic compression stockings improve lower-extremity hemodynamics (including reflux and residual volume measured by color flow duplex scanning) in patients with varicosities, and can improve symptoms such as swelling, discomfort, and leg tightness.1,2
A Cochrane review concluded that existing evidence supports the use of sclerotherapy for recurrent varicose veins after surgery and for relatively minor “thread” veins.3 Data did not show that any particular type of sclerosant or pressure dressing or duration of post-treatment compression have significant effect on outcomes, such as disappearance of varicosities and cosmetic improvement.3
A Cochrane protocol is in progress regarding comparison of the outcomes of surgery and sclerotherapy.4 Few randomized trials have compared surgery and sclerotherapy.
Belcaro reported results of a 10-year randomized trial including 121 subjects, 96 of whom completed the study.5 Surgery consisted of ligation of the saphenopopliteal junction without stripping. At 10 years, 16.1% of patients receiving surgery plus sclerotherapy had distal venous incompetence (assessed with color duplex scanning and ambulatory venous pressure measurement), compared with 36.4% of those who underwent surgery alone and 43.8% of those who received sclerotherapy alone. The authors concluded that long-term outcomes (defined as saphenofemoral junction competence) are superior with strategies that included surgery, but at greater cost.
Beresford and colleagues also concluded that surgery lessened the likelihood of additional treatment.6 Another randomized trial showed that saphenous vein stripping reduced by two thirds the need for reoperation due to recurrent saphenofemoral incompetence, compared with saphenofemoral junction ligation alone.7
A meta-analysis studied the effectiveness of venotonic medications (such as rutoside, flunarizine, and dihydroergotamine) in chronic venous insufficiency.8 These agents significantly reduced pain, leg heaviness, cramps, and paresthesias. However, a Cochrane Collaboration reviewer questioned the validity of pooling results from this heterogeneous group of studies into a single meta-analysis.9
A Cochrane Review did find that horse chestnut seed extract significantly improves leg pain, edema, pruritus, and lower leg volume and circumference, but suggests that larger randomized trials are needed to establish conclusively this agent’s efficacy.10
Recommendations from others
A recent clinical review indicated that patients whose main symptoms are swelling or aching can be treated with compression stockings alone; trunk varicosities should be treated with saphenofemoral or saphenopopliteal ligation, plus stripping of the long saphenous vein for long saphenous varicosities.11 They suggest that sclerotherapy should be reserved for varicosities that persist after surgery.
The Venous Insufficiency Epidemiologic and Economic Studies (VEINES) program recommends sclerotherapy for telangiectasias and reticular veins, and surgery for saphenous varicosities.12 However, they noted the need for randomized trials to compare therapies.
Alan Adelman, MD, MS
Penn State University, State College, Pa
Choosing the best treatment for varicose veins can be complicated. Symptoms and the type of varicose veins (truncal varices, reticular varices, or telangiectasia) can guide the clinician in selecting therapy. Asymptomatic varicosities can usually be observed without treatment. Patients with symptomatic varicosities may be treated conservatively before referring for invasive treatment.
Surgery is probably the best treatment for truncal varices, whereas sclerotherapy is better for reticular veins or telangiectasia. The long-term risks and benefits of newer modalities such as laser and thermal ablation need further evaluation. Regardless of the treatment chosen, patients with varicose veins should first undergo a thorough investigation.
1. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999;25:701-704.
2. Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-123.
3. Tisi PV, Beverley CA. Injection sclerotherapy for varicose veins (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
4. Michaels JA, Kendall RJ. Surgery for varicose veins (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Belcaro G, Nicolaides AN, Ricci A, et al. Endovascular sclerotherapy, surgery, and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow-up trial—final results. Angiology 2000;51:529-534.
6. Beresford SAA, Chant ADB, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Five-year follow-up. Lancet. 1978;1:921-924.
7. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg 1999;29:589-592.
8. Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis. Clin Drug Invest 1999;18:413-432.
9. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
10. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
11. London NJM, Nash R. ABC of arterial and venous disease. Varicose veins. BMJ 2000;320:1391-1394.
12. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83-102.
1. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999;25:701-704.
2. Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-123.
3. Tisi PV, Beverley CA. Injection sclerotherapy for varicose veins (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
4. Michaels JA, Kendall RJ. Surgery for varicose veins (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Belcaro G, Nicolaides AN, Ricci A, et al. Endovascular sclerotherapy, surgery, and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow-up trial—final results. Angiology 2000;51:529-534.
6. Beresford SAA, Chant ADB, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Five-year follow-up. Lancet. 1978;1:921-924.
7. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg 1999;29:589-592.
8. Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis. Clin Drug Invest 1999;18:413-432.
9. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
10. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
11. London NJM, Nash R. ABC of arterial and venous disease. Varicose veins. BMJ 2000;320:1391-1394.
12. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83-102.
Evidence-based answers from the Family Physicians Inquiries Network
What nonhormonal therapies are effective for postmenopausal vasomotor symptoms?
Regular exercise may reduce vasomotor symptoms of menopause (strength of recommendation [SOR]: C—single observational study).1
Soy products/isoflavones, either through diet or supplementation, may reduce the incidence of hot flushes (SOR: D—inconsistent results of randomized trials).2
Clonidine, as an oral or transdermal preparation, reduces hot flushes (SOR: A—randomized clinical trials),3 as does gabapentin (SOR: A— single randomized clinical trial).4
In cancer patients who have had surgical menopause, selective serotonin reuptake inhibitors5 and megestrol6 (Megase) have been effective in reducing hot flushes (SOR: A; B for extrapolation to the general population).
Other therapies—including Bellergal (a combination of belladonna, ergotamine, and phenobarbital), methyldopa, evening primrose oil, mai quan, flaxseed, ginseng, and topical wild yam extract—have not been effective.7 Black cohosh may be effective, but the evidence for this is of poor quality (SOR: C). (See Table.)
TABLE
Nonhormonal therapies for postmenopausal vasomotor symptoms
| Agent | Effective | SOR † | Comments |
|---|---|---|---|
| Soy/isoflavones | Maybe | D | Multiple RCTs with conflicting results, no formal meta-analysis. Does have a positive effect on lipid profile |
| Clonidine (Catapres) | Yes | A | Multiple small RCTs |
| Venlafaxine* (Effexor) | Yes | B | Single RCT |
| Fluoxetine* (Prozac) | Yes | B | Single RCT |
| Gabapentin (Neurontin) | Yes | A | Single RCT |
| Megestrol* (Megace) | Yes | B | Single RCT |
| Exercise | Maybe | C | Single observational study |
| Black cohosh | Maybe | C | German E commission recommenda tion positive in 1989, but only 1 of 7 trials cited had placebo control. Recent RCT showed no benefit |
| Other: Bellergal, methyldopa, evening of effect | No | C | All have been advocated but no positive trials for any evidence primrose oil, ginseng, wild yam extract, mai quan, flaxseed |
| *Trials conducted only with patients with breast cancer and interventional menopause, most of whom were on anti-estrogen therapy. | |||
| †See page 290 for a description of strength of recommendation. | |||
| SOR, strength of recommendation; RCT, randomized controlled trial | |||
Evidence summary
Hormone replacement therapy (HRT) is the standard treatment for vasomotor symptoms of menopause, and it is effective for this indication. With recent studies showing no benefit from long-term HRT for menopausal women and increased adverse effects with its use (especially for women at risk for coronary heart disease), there has been increased interest in nonhormonal treatments for these symptoms.
A small number of randomized clinical trials have studied treatments other than HRT for the control of vasomotor symptoms of menopause. As a group, these trials have been short-term and have involved small numbers of patients. A disproportionate number of trials have been completed in breast cancer survivors, since these patients tend to have more severe vasomotor symptoms as a result of their anti-estrogenic therapies. Whether these results can be generalized to all postmenopausal women with vasomotor symptoms cannot be determined from the evidence.
Eleven randomized trials of soy protein/isoflavone used placebo controls. Results were mixed, with 7 trials showing no effect and 4 showing a reduction in hot flushes in comparison with placebo. Studies reporting a positive effect showed approximately a 15% reduction in episodes in comparison with placebo. In one 6-month trial, there was a correlation between hot flushes and urinary isoflavone excretion regardless of treatment group, suggesting a confounding effect of dietary intake of isoflavone.
Five of six randomized controlled trials of cloni-dine have shown a reduction in frequency of hot flushes ranging from 14%–50% compared with placebo. One trial, which used oral clonidine 0.1 mg daily, also reported an improved quality of life for the treatment group. A single randomized trial has shown that gabapentin, at a dose of 900 mg/day, is effective in reducing both frequency and severity of hot flashes.4
Trials of specific selective serotonin reuptake inhibitors have been completed in patients with vasomotor symptoms secondary to breast cancer therapies. Individual randomized controlled trials of venlafaxine and fluoxetine have proven these agents effective, and a preliminary open-labeled trial of paroxetine has also suggested benefit.
Several reviews suggest black cohosh may be effective for short-term treatment, and it is used in Germany for this indication. The trials we found were not placebo-controlled, however, and the safety of this agent is controversial. A single English-language placebo-controlled trial did not show any benefit for black cohosh.
Recommendations from others
The American College of Obstetrics and Gynecology clinical management guideline, “The use of botanicals for management of menopausal symptoms,” gives a level C recommendation (consensus and expert opinion) that “Soy and isoflavone may be helpful in short-term (2 years) treatment of vasomotor symptoms” and “black cohosh may be helpful in the short-term (6 months) treatment of women with vasomotor symptoms.” They note that “given the possibility that these compounds may interact with estrogen, these agents should not be considered free of potential harm in women with estrogen-dependent cancers.”8
The North American Menopause Society notes that behavior changes, such as moderate exercise and avoidance of hot-flush triggers, may prevent some hot flushes, although there is only anecdotal evidence for this. The efficacy of paced respiration—deep, slow abdominal breathing—to lessen hot flushes has been shown in a small trial. The society states that other alternative therapies have not been shown to be efficacious, except for moderate quantities of soy products.9 The Medical Letter says the evidence that phytoestrogens are helpful for menopausal women comes mostly from epidemiological studies. The long-term adverse effects of phytoestrogen consumption are not known.10
Laura B. Hansen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Behavioral modifications may be the first approach to reduce the incidence of vasomotor symptoms in menopausal women. Recommendations include wearing several layers of breathable clothing; keeping a glass of cold water, ice pack, or small fan by the bedside and nearby at work; performing deep breathing relaxation techniques; and exercising routinely.
Effective nonhormonal treatments include phytoestrogens (2 years), black cohosh (6 months), clonidine, selective serotonin reuptake inhibitors, and venlafaxine. Overall, there are few well-designed clinical trials regarding the safety and effectiveness of botanical agents used for vasomotor symptoms. Since the Food and Drug Administration does not regulate the marketing and standardization of these products, patients should be advised to purchase products from reputable companies with internal standardization processes.
Additionally, patients should talk with their health care provider prior to initiating any alternative medication to avoid drug-disease and drug-drug interactions.
1. Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Maturitas 1998;29:139-146.
2. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236-242.
3. Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med 2000;132:788-793.
4. Guttuso T, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337-345.
5. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med 1994;331:347-352.
6. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578-1583.
7. Taylor M. Alternative medicine and the perimenopause: an evidence-based review. Obstet Gynecol Clin North Am 2002;29:555-573.
8. Use of botanicals for management of menopausal symptoms. ACOG Pract Bull No. 28. Washington, DC: American College of Obstetricians and Gynecologists, 2001.
9. Clinical challenges of perimenopause: consensus opinions of the North American Menopause Society. Menopause 2000;7:5-13.
10. Phytoestrogens. Med Lett Drugs Ther 2000;1072:17-18.
Regular exercise may reduce vasomotor symptoms of menopause (strength of recommendation [SOR]: C—single observational study).1
Soy products/isoflavones, either through diet or supplementation, may reduce the incidence of hot flushes (SOR: D—inconsistent results of randomized trials).2
Clonidine, as an oral or transdermal preparation, reduces hot flushes (SOR: A—randomized clinical trials),3 as does gabapentin (SOR: A— single randomized clinical trial).4
In cancer patients who have had surgical menopause, selective serotonin reuptake inhibitors5 and megestrol6 (Megase) have been effective in reducing hot flushes (SOR: A; B for extrapolation to the general population).
Other therapies—including Bellergal (a combination of belladonna, ergotamine, and phenobarbital), methyldopa, evening primrose oil, mai quan, flaxseed, ginseng, and topical wild yam extract—have not been effective.7 Black cohosh may be effective, but the evidence for this is of poor quality (SOR: C). (See Table.)
TABLE
Nonhormonal therapies for postmenopausal vasomotor symptoms
| Agent | Effective | SOR † | Comments |
|---|---|---|---|
| Soy/isoflavones | Maybe | D | Multiple RCTs with conflicting results, no formal meta-analysis. Does have a positive effect on lipid profile |
| Clonidine (Catapres) | Yes | A | Multiple small RCTs |
| Venlafaxine* (Effexor) | Yes | B | Single RCT |
| Fluoxetine* (Prozac) | Yes | B | Single RCT |
| Gabapentin (Neurontin) | Yes | A | Single RCT |
| Megestrol* (Megace) | Yes | B | Single RCT |
| Exercise | Maybe | C | Single observational study |
| Black cohosh | Maybe | C | German E commission recommenda tion positive in 1989, but only 1 of 7 trials cited had placebo control. Recent RCT showed no benefit |
| Other: Bellergal, methyldopa, evening of effect | No | C | All have been advocated but no positive trials for any evidence primrose oil, ginseng, wild yam extract, mai quan, flaxseed |
| *Trials conducted only with patients with breast cancer and interventional menopause, most of whom were on anti-estrogen therapy. | |||
| †See page 290 for a description of strength of recommendation. | |||
| SOR, strength of recommendation; RCT, randomized controlled trial | |||
Evidence summary
Hormone replacement therapy (HRT) is the standard treatment for vasomotor symptoms of menopause, and it is effective for this indication. With recent studies showing no benefit from long-term HRT for menopausal women and increased adverse effects with its use (especially for women at risk for coronary heart disease), there has been increased interest in nonhormonal treatments for these symptoms.
A small number of randomized clinical trials have studied treatments other than HRT for the control of vasomotor symptoms of menopause. As a group, these trials have been short-term and have involved small numbers of patients. A disproportionate number of trials have been completed in breast cancer survivors, since these patients tend to have more severe vasomotor symptoms as a result of their anti-estrogenic therapies. Whether these results can be generalized to all postmenopausal women with vasomotor symptoms cannot be determined from the evidence.
Eleven randomized trials of soy protein/isoflavone used placebo controls. Results were mixed, with 7 trials showing no effect and 4 showing a reduction in hot flushes in comparison with placebo. Studies reporting a positive effect showed approximately a 15% reduction in episodes in comparison with placebo. In one 6-month trial, there was a correlation between hot flushes and urinary isoflavone excretion regardless of treatment group, suggesting a confounding effect of dietary intake of isoflavone.
Five of six randomized controlled trials of cloni-dine have shown a reduction in frequency of hot flushes ranging from 14%–50% compared with placebo. One trial, which used oral clonidine 0.1 mg daily, also reported an improved quality of life for the treatment group. A single randomized trial has shown that gabapentin, at a dose of 900 mg/day, is effective in reducing both frequency and severity of hot flashes.4
Trials of specific selective serotonin reuptake inhibitors have been completed in patients with vasomotor symptoms secondary to breast cancer therapies. Individual randomized controlled trials of venlafaxine and fluoxetine have proven these agents effective, and a preliminary open-labeled trial of paroxetine has also suggested benefit.
Several reviews suggest black cohosh may be effective for short-term treatment, and it is used in Germany for this indication. The trials we found were not placebo-controlled, however, and the safety of this agent is controversial. A single English-language placebo-controlled trial did not show any benefit for black cohosh.
Recommendations from others
The American College of Obstetrics and Gynecology clinical management guideline, “The use of botanicals for management of menopausal symptoms,” gives a level C recommendation (consensus and expert opinion) that “Soy and isoflavone may be helpful in short-term (2 years) treatment of vasomotor symptoms” and “black cohosh may be helpful in the short-term (6 months) treatment of women with vasomotor symptoms.” They note that “given the possibility that these compounds may interact with estrogen, these agents should not be considered free of potential harm in women with estrogen-dependent cancers.”8
The North American Menopause Society notes that behavior changes, such as moderate exercise and avoidance of hot-flush triggers, may prevent some hot flushes, although there is only anecdotal evidence for this. The efficacy of paced respiration—deep, slow abdominal breathing—to lessen hot flushes has been shown in a small trial. The society states that other alternative therapies have not been shown to be efficacious, except for moderate quantities of soy products.9 The Medical Letter says the evidence that phytoestrogens are helpful for menopausal women comes mostly from epidemiological studies. The long-term adverse effects of phytoestrogen consumption are not known.10
Laura B. Hansen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Behavioral modifications may be the first approach to reduce the incidence of vasomotor symptoms in menopausal women. Recommendations include wearing several layers of breathable clothing; keeping a glass of cold water, ice pack, or small fan by the bedside and nearby at work; performing deep breathing relaxation techniques; and exercising routinely.
Effective nonhormonal treatments include phytoestrogens (2 years), black cohosh (6 months), clonidine, selective serotonin reuptake inhibitors, and venlafaxine. Overall, there are few well-designed clinical trials regarding the safety and effectiveness of botanical agents used for vasomotor symptoms. Since the Food and Drug Administration does not regulate the marketing and standardization of these products, patients should be advised to purchase products from reputable companies with internal standardization processes.
Additionally, patients should talk with their health care provider prior to initiating any alternative medication to avoid drug-disease and drug-drug interactions.
Regular exercise may reduce vasomotor symptoms of menopause (strength of recommendation [SOR]: C—single observational study).1
Soy products/isoflavones, either through diet or supplementation, may reduce the incidence of hot flushes (SOR: D—inconsistent results of randomized trials).2
Clonidine, as an oral or transdermal preparation, reduces hot flushes (SOR: A—randomized clinical trials),3 as does gabapentin (SOR: A— single randomized clinical trial).4
In cancer patients who have had surgical menopause, selective serotonin reuptake inhibitors5 and megestrol6 (Megase) have been effective in reducing hot flushes (SOR: A; B for extrapolation to the general population).
Other therapies—including Bellergal (a combination of belladonna, ergotamine, and phenobarbital), methyldopa, evening primrose oil, mai quan, flaxseed, ginseng, and topical wild yam extract—have not been effective.7 Black cohosh may be effective, but the evidence for this is of poor quality (SOR: C). (See Table.)
TABLE
Nonhormonal therapies for postmenopausal vasomotor symptoms
| Agent | Effective | SOR † | Comments |
|---|---|---|---|
| Soy/isoflavones | Maybe | D | Multiple RCTs with conflicting results, no formal meta-analysis. Does have a positive effect on lipid profile |
| Clonidine (Catapres) | Yes | A | Multiple small RCTs |
| Venlafaxine* (Effexor) | Yes | B | Single RCT |
| Fluoxetine* (Prozac) | Yes | B | Single RCT |
| Gabapentin (Neurontin) | Yes | A | Single RCT |
| Megestrol* (Megace) | Yes | B | Single RCT |
| Exercise | Maybe | C | Single observational study |
| Black cohosh | Maybe | C | German E commission recommenda tion positive in 1989, but only 1 of 7 trials cited had placebo control. Recent RCT showed no benefit |
| Other: Bellergal, methyldopa, evening of effect | No | C | All have been advocated but no positive trials for any evidence primrose oil, ginseng, wild yam extract, mai quan, flaxseed |
| *Trials conducted only with patients with breast cancer and interventional menopause, most of whom were on anti-estrogen therapy. | |||
| †See page 290 for a description of strength of recommendation. | |||
| SOR, strength of recommendation; RCT, randomized controlled trial | |||
Evidence summary
Hormone replacement therapy (HRT) is the standard treatment for vasomotor symptoms of menopause, and it is effective for this indication. With recent studies showing no benefit from long-term HRT for menopausal women and increased adverse effects with its use (especially for women at risk for coronary heart disease), there has been increased interest in nonhormonal treatments for these symptoms.
A small number of randomized clinical trials have studied treatments other than HRT for the control of vasomotor symptoms of menopause. As a group, these trials have been short-term and have involved small numbers of patients. A disproportionate number of trials have been completed in breast cancer survivors, since these patients tend to have more severe vasomotor symptoms as a result of their anti-estrogenic therapies. Whether these results can be generalized to all postmenopausal women with vasomotor symptoms cannot be determined from the evidence.
Eleven randomized trials of soy protein/isoflavone used placebo controls. Results were mixed, with 7 trials showing no effect and 4 showing a reduction in hot flushes in comparison with placebo. Studies reporting a positive effect showed approximately a 15% reduction in episodes in comparison with placebo. In one 6-month trial, there was a correlation between hot flushes and urinary isoflavone excretion regardless of treatment group, suggesting a confounding effect of dietary intake of isoflavone.
Five of six randomized controlled trials of cloni-dine have shown a reduction in frequency of hot flushes ranging from 14%–50% compared with placebo. One trial, which used oral clonidine 0.1 mg daily, also reported an improved quality of life for the treatment group. A single randomized trial has shown that gabapentin, at a dose of 900 mg/day, is effective in reducing both frequency and severity of hot flashes.4
Trials of specific selective serotonin reuptake inhibitors have been completed in patients with vasomotor symptoms secondary to breast cancer therapies. Individual randomized controlled trials of venlafaxine and fluoxetine have proven these agents effective, and a preliminary open-labeled trial of paroxetine has also suggested benefit.
Several reviews suggest black cohosh may be effective for short-term treatment, and it is used in Germany for this indication. The trials we found were not placebo-controlled, however, and the safety of this agent is controversial. A single English-language placebo-controlled trial did not show any benefit for black cohosh.
Recommendations from others
The American College of Obstetrics and Gynecology clinical management guideline, “The use of botanicals for management of menopausal symptoms,” gives a level C recommendation (consensus and expert opinion) that “Soy and isoflavone may be helpful in short-term (2 years) treatment of vasomotor symptoms” and “black cohosh may be helpful in the short-term (6 months) treatment of women with vasomotor symptoms.” They note that “given the possibility that these compounds may interact with estrogen, these agents should not be considered free of potential harm in women with estrogen-dependent cancers.”8
The North American Menopause Society notes that behavior changes, such as moderate exercise and avoidance of hot-flush triggers, may prevent some hot flushes, although there is only anecdotal evidence for this. The efficacy of paced respiration—deep, slow abdominal breathing—to lessen hot flushes has been shown in a small trial. The society states that other alternative therapies have not been shown to be efficacious, except for moderate quantities of soy products.9 The Medical Letter says the evidence that phytoestrogens are helpful for menopausal women comes mostly from epidemiological studies. The long-term adverse effects of phytoestrogen consumption are not known.10
Laura B. Hansen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Behavioral modifications may be the first approach to reduce the incidence of vasomotor symptoms in menopausal women. Recommendations include wearing several layers of breathable clothing; keeping a glass of cold water, ice pack, or small fan by the bedside and nearby at work; performing deep breathing relaxation techniques; and exercising routinely.
Effective nonhormonal treatments include phytoestrogens (2 years), black cohosh (6 months), clonidine, selective serotonin reuptake inhibitors, and venlafaxine. Overall, there are few well-designed clinical trials regarding the safety and effectiveness of botanical agents used for vasomotor symptoms. Since the Food and Drug Administration does not regulate the marketing and standardization of these products, patients should be advised to purchase products from reputable companies with internal standardization processes.
Additionally, patients should talk with their health care provider prior to initiating any alternative medication to avoid drug-disease and drug-drug interactions.
1. Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Maturitas 1998;29:139-146.
2. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236-242.
3. Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med 2000;132:788-793.
4. Guttuso T, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337-345.
5. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med 1994;331:347-352.
6. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578-1583.
7. Taylor M. Alternative medicine and the perimenopause: an evidence-based review. Obstet Gynecol Clin North Am 2002;29:555-573.
8. Use of botanicals for management of menopausal symptoms. ACOG Pract Bull No. 28. Washington, DC: American College of Obstetricians and Gynecologists, 2001.
9. Clinical challenges of perimenopause: consensus opinions of the North American Menopause Society. Menopause 2000;7:5-13.
10. Phytoestrogens. Med Lett Drugs Ther 2000;1072:17-18.
1. Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Maturitas 1998;29:139-146.
2. Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause 2000;7:236-242.
3. Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med 2000;132:788-793.
4. Guttuso T, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337-345.
5. Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med 1994;331:347-352.
6. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578-1583.
7. Taylor M. Alternative medicine and the perimenopause: an evidence-based review. Obstet Gynecol Clin North Am 2002;29:555-573.
8. Use of botanicals for management of menopausal symptoms. ACOG Pract Bull No. 28. Washington, DC: American College of Obstetricians and Gynecologists, 2001.
9. Clinical challenges of perimenopause: consensus opinions of the North American Menopause Society. Menopause 2000;7:5-13.
10. Phytoestrogens. Med Lett Drugs Ther 2000;1072:17-18.
Evidence-based answers from the Family Physicians Inquiries Network
Are antibiotics effective for otitis media with effusion?
Antibiotics provide little or no long-term benefit for children with otitis media with effusion (OME), defined as fluid in the middle ear without signs or symptoms of infection.
Most meta-analyses show a modest, short-term reduction in effusion rates. However, the most rigorous meta-analysis shows no benefit (strength of recommendation [SOR]: D, based on conflicting meta-analyses). No significant effect was noted on longer-term (>1 month) outcomes after treatment (SOR: A, based on a meta-analysis of 8 trials). In addition, there is no reliable evidence regarding patient-oriented outcomes (hearing loss, speech delay).
Evidence summary
Longitudinal studies show spontaneous resolution in more than half of children within 3 months of the development of the effusion. After 3 months, the rate of spontaneous resolution remains constant, so that only a small percentage of children have OME a year or longer. There is a theoretical basis for the use of antibiotics for OME, since between 27%–50% of middle-ear aspirates of patients with OME contain bacteria.1
In the last 10 years, 4 meta-analyses reported mild short-term improvement in OME with antibiotic treatment (effusion clearance rates of 23%,2 16%,3 14%,1 and 4%,4 respectively—see Table). The last study was the only meta-analysis that restricted inclusion to only randomized, blinded, placebo-controlled trials. The small difference reported (4%) was not significant. None of the studies that assessed outcomes beyond a month showed a significant difference in the persistence of OME.
The meta-analyses vary significantly in methodology, inclusion/exclusion criteria, and interpretation, making a definitive conclusion on treatment results difficult. The included trials varied in antibiotics chosen, use of placebo, duration of therapy, time to measurement of OME resolution, and method of diagnosis (tympanography, otoscopy, audiometry).
The reviews commented on potential harms of antibiotic therapy, including medication cost and the development of antibiotic resistance. Nausea, vomiting, and diarrhea were reported in 2%–30% of children on antibiotic therapy.1 The reviews did not address the treatment of OME in the nonpediatric population or such long-term patient-oriented outcomes as hearing loss or speech delay.
Recommendations from others
The American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology– Head and Neck Surgery participated in the meta-analysis by Stool et al,1 under contract with the Agency for Health Care Policy and Research. The resulting clinical practice guideline has been adopted by the AAP, AAFP, and the Centers for Disease Control and Prevention. The guideline stresses that observation or antibiotics are treatment options for children with OME present less than 4 to 6 months. Antibiotic therapy is never considered a required treatment for OME of any duration. All published guidelines are applicable to the pediatric population only.
Conflicting evidence indicates short-term or no benefit for antibiotics, and complications such as nausea, vomiting, diarrhea, and rash have been reported in 2%–32% of children. Long-term antibiotics lead to poor adherence, more office visits, and antibiotic resistance.5
Lynda Montgomery, MD
Case Western University School of Medicine Cleveland, Ohio
Conflicting meta-analyses and a guideline that hedges leaves the clinician who practices evidence-based medicine in the uncomfortable position of saying “maybe” when asked whether antibiotics are helpful. In the majority of cases of OME, I would seek to avoid the possible complications of antibiotics, given that there is no clear benefit. I await more data on speech and hearing outcomes in OME, as these studies will provide the most helpful evidence to primary care physicians.
TABLE
Meta-analyses of otitis media with effusion
| Meta-analysis | # of trials | Number of subjects | Description | Rate difference (95% CI) |
|---|---|---|---|---|
| Cantekin et al4 | 8 | 775 children | Includes only non-placebo-controlled RCTs. Variable timing of outcome measure | 32 (25.8–38.8) |
| Rosenfeld et al2 | 10 | 1325 children | Includes some nonblinded and nonplacebo-controlled RCTs. Variable timing | 22.8 (10.5–35.1) |
| Williams et al3 | 12 | 1697 children | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on bilateral resolution of OME within 1 month of starting therapy | 16 (3–29) |
| Williams et al3 | 8 | 2052 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on unilateral resolution of OME within 1 month of starting therapy | 25 (10–40) |
| Williams et al3 | 8 | 1313 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Long-term outcomes measured more than 1 month after treatment was completed | 6 (-3–14) |
| Stool et al1 | 10 | 1041 children | All blinded RCTs. Not all placebocontrolled. Variable timing | 14.0 (3.6–24.2) |
| Cantekin et al4 | 8 | 1292 children | Includes only blinded, placebo-controlled RCTs. Variable timing | 4.3 (-0.1–8.6) |
| RCT, randomized clinical trial; CI, confidence interval; OME, otitis media with effusion | ||||
Otitis media with effusion
1. Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical Practice Guideline No. 12. AHCPR Publication 94-0622. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, July 1994.
2. Rosenfeld RM, Post JC. Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 1992;106:378-386.
3. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve to brouhaha. JAMA 1993;270:1344-351.
4. Cantekin EI, McGuire TW. Antibiotics are not effective for otitis media with effusion: reanalysis of meta-analyses. Otorhinolaryngol Nova 1998;8:214-222.
5. Williamson I. Otitis media with effusion. Clinical Evidence [online]. Available at: http://www.clinicalevidence.com. Accessed on February 24, 2003.
Antibiotics provide little or no long-term benefit for children with otitis media with effusion (OME), defined as fluid in the middle ear without signs or symptoms of infection.
Most meta-analyses show a modest, short-term reduction in effusion rates. However, the most rigorous meta-analysis shows no benefit (strength of recommendation [SOR]: D, based on conflicting meta-analyses). No significant effect was noted on longer-term (>1 month) outcomes after treatment (SOR: A, based on a meta-analysis of 8 trials). In addition, there is no reliable evidence regarding patient-oriented outcomes (hearing loss, speech delay).
Evidence summary
Longitudinal studies show spontaneous resolution in more than half of children within 3 months of the development of the effusion. After 3 months, the rate of spontaneous resolution remains constant, so that only a small percentage of children have OME a year or longer. There is a theoretical basis for the use of antibiotics for OME, since between 27%–50% of middle-ear aspirates of patients with OME contain bacteria.1
In the last 10 years, 4 meta-analyses reported mild short-term improvement in OME with antibiotic treatment (effusion clearance rates of 23%,2 16%,3 14%,1 and 4%,4 respectively—see Table). The last study was the only meta-analysis that restricted inclusion to only randomized, blinded, placebo-controlled trials. The small difference reported (4%) was not significant. None of the studies that assessed outcomes beyond a month showed a significant difference in the persistence of OME.
The meta-analyses vary significantly in methodology, inclusion/exclusion criteria, and interpretation, making a definitive conclusion on treatment results difficult. The included trials varied in antibiotics chosen, use of placebo, duration of therapy, time to measurement of OME resolution, and method of diagnosis (tympanography, otoscopy, audiometry).
The reviews commented on potential harms of antibiotic therapy, including medication cost and the development of antibiotic resistance. Nausea, vomiting, and diarrhea were reported in 2%–30% of children on antibiotic therapy.1 The reviews did not address the treatment of OME in the nonpediatric population or such long-term patient-oriented outcomes as hearing loss or speech delay.
Recommendations from others
The American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology– Head and Neck Surgery participated in the meta-analysis by Stool et al,1 under contract with the Agency for Health Care Policy and Research. The resulting clinical practice guideline has been adopted by the AAP, AAFP, and the Centers for Disease Control and Prevention. The guideline stresses that observation or antibiotics are treatment options for children with OME present less than 4 to 6 months. Antibiotic therapy is never considered a required treatment for OME of any duration. All published guidelines are applicable to the pediatric population only.
Conflicting evidence indicates short-term or no benefit for antibiotics, and complications such as nausea, vomiting, diarrhea, and rash have been reported in 2%–32% of children. Long-term antibiotics lead to poor adherence, more office visits, and antibiotic resistance.5
Lynda Montgomery, MD
Case Western University School of Medicine Cleveland, Ohio
Conflicting meta-analyses and a guideline that hedges leaves the clinician who practices evidence-based medicine in the uncomfortable position of saying “maybe” when asked whether antibiotics are helpful. In the majority of cases of OME, I would seek to avoid the possible complications of antibiotics, given that there is no clear benefit. I await more data on speech and hearing outcomes in OME, as these studies will provide the most helpful evidence to primary care physicians.
TABLE
Meta-analyses of otitis media with effusion
| Meta-analysis | # of trials | Number of subjects | Description | Rate difference (95% CI) |
|---|---|---|---|---|
| Cantekin et al4 | 8 | 775 children | Includes only non-placebo-controlled RCTs. Variable timing of outcome measure | 32 (25.8–38.8) |
| Rosenfeld et al2 | 10 | 1325 children | Includes some nonblinded and nonplacebo-controlled RCTs. Variable timing | 22.8 (10.5–35.1) |
| Williams et al3 | 12 | 1697 children | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on bilateral resolution of OME within 1 month of starting therapy | 16 (3–29) |
| Williams et al3 | 8 | 2052 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on unilateral resolution of OME within 1 month of starting therapy | 25 (10–40) |
| Williams et al3 | 8 | 1313 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Long-term outcomes measured more than 1 month after treatment was completed | 6 (-3–14) |
| Stool et al1 | 10 | 1041 children | All blinded RCTs. Not all placebocontrolled. Variable timing | 14.0 (3.6–24.2) |
| Cantekin et al4 | 8 | 1292 children | Includes only blinded, placebo-controlled RCTs. Variable timing | 4.3 (-0.1–8.6) |
| RCT, randomized clinical trial; CI, confidence interval; OME, otitis media with effusion | ||||
Otitis media with effusion
Antibiotics provide little or no long-term benefit for children with otitis media with effusion (OME), defined as fluid in the middle ear without signs or symptoms of infection.
Most meta-analyses show a modest, short-term reduction in effusion rates. However, the most rigorous meta-analysis shows no benefit (strength of recommendation [SOR]: D, based on conflicting meta-analyses). No significant effect was noted on longer-term (>1 month) outcomes after treatment (SOR: A, based on a meta-analysis of 8 trials). In addition, there is no reliable evidence regarding patient-oriented outcomes (hearing loss, speech delay).
Evidence summary
Longitudinal studies show spontaneous resolution in more than half of children within 3 months of the development of the effusion. After 3 months, the rate of spontaneous resolution remains constant, so that only a small percentage of children have OME a year or longer. There is a theoretical basis for the use of antibiotics for OME, since between 27%–50% of middle-ear aspirates of patients with OME contain bacteria.1
In the last 10 years, 4 meta-analyses reported mild short-term improvement in OME with antibiotic treatment (effusion clearance rates of 23%,2 16%,3 14%,1 and 4%,4 respectively—see Table). The last study was the only meta-analysis that restricted inclusion to only randomized, blinded, placebo-controlled trials. The small difference reported (4%) was not significant. None of the studies that assessed outcomes beyond a month showed a significant difference in the persistence of OME.
The meta-analyses vary significantly in methodology, inclusion/exclusion criteria, and interpretation, making a definitive conclusion on treatment results difficult. The included trials varied in antibiotics chosen, use of placebo, duration of therapy, time to measurement of OME resolution, and method of diagnosis (tympanography, otoscopy, audiometry).
The reviews commented on potential harms of antibiotic therapy, including medication cost and the development of antibiotic resistance. Nausea, vomiting, and diarrhea were reported in 2%–30% of children on antibiotic therapy.1 The reviews did not address the treatment of OME in the nonpediatric population or such long-term patient-oriented outcomes as hearing loss or speech delay.
Recommendations from others
The American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology– Head and Neck Surgery participated in the meta-analysis by Stool et al,1 under contract with the Agency for Health Care Policy and Research. The resulting clinical practice guideline has been adopted by the AAP, AAFP, and the Centers for Disease Control and Prevention. The guideline stresses that observation or antibiotics are treatment options for children with OME present less than 4 to 6 months. Antibiotic therapy is never considered a required treatment for OME of any duration. All published guidelines are applicable to the pediatric population only.
Conflicting evidence indicates short-term or no benefit for antibiotics, and complications such as nausea, vomiting, diarrhea, and rash have been reported in 2%–32% of children. Long-term antibiotics lead to poor adherence, more office visits, and antibiotic resistance.5
Lynda Montgomery, MD
Case Western University School of Medicine Cleveland, Ohio
Conflicting meta-analyses and a guideline that hedges leaves the clinician who practices evidence-based medicine in the uncomfortable position of saying “maybe” when asked whether antibiotics are helpful. In the majority of cases of OME, I would seek to avoid the possible complications of antibiotics, given that there is no clear benefit. I await more data on speech and hearing outcomes in OME, as these studies will provide the most helpful evidence to primary care physicians.
TABLE
Meta-analyses of otitis media with effusion
| Meta-analysis | # of trials | Number of subjects | Description | Rate difference (95% CI) |
|---|---|---|---|---|
| Cantekin et al4 | 8 | 775 children | Includes only non-placebo-controlled RCTs. Variable timing of outcome measure | 32 (25.8–38.8) |
| Rosenfeld et al2 | 10 | 1325 children | Includes some nonblinded and nonplacebo-controlled RCTs. Variable timing | 22.8 (10.5–35.1) |
| Williams et al3 | 12 | 1697 children | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on bilateral resolution of OME within 1 month of starting therapy | 16 (3–29) |
| Williams et al3 | 8 | 2052 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Short-term outcomes focused on unilateral resolution of OME within 1 month of starting therapy | 25 (10–40) |
| Williams et al3 | 8 | 1313 ears | Includes some nonblinded and nonplacebo-controlled RCTs. Long-term outcomes measured more than 1 month after treatment was completed | 6 (-3–14) |
| Stool et al1 | 10 | 1041 children | All blinded RCTs. Not all placebocontrolled. Variable timing | 14.0 (3.6–24.2) |
| Cantekin et al4 | 8 | 1292 children | Includes only blinded, placebo-controlled RCTs. Variable timing | 4.3 (-0.1–8.6) |
| RCT, randomized clinical trial; CI, confidence interval; OME, otitis media with effusion | ||||
Otitis media with effusion
1. Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical Practice Guideline No. 12. AHCPR Publication 94-0622. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, July 1994.
2. Rosenfeld RM, Post JC. Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 1992;106:378-386.
3. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve to brouhaha. JAMA 1993;270:1344-351.
4. Cantekin EI, McGuire TW. Antibiotics are not effective for otitis media with effusion: reanalysis of meta-analyses. Otorhinolaryngol Nova 1998;8:214-222.
5. Williamson I. Otitis media with effusion. Clinical Evidence [online]. Available at: http://www.clinicalevidence.com. Accessed on February 24, 2003.
1. Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical Practice Guideline No. 12. AHCPR Publication 94-0622. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, July 1994.
2. Rosenfeld RM, Post JC. Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 1992;106:378-386.
3. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve to brouhaha. JAMA 1993;270:1344-351.
4. Cantekin EI, McGuire TW. Antibiotics are not effective for otitis media with effusion: reanalysis of meta-analyses. Otorhinolaryngol Nova 1998;8:214-222.
5. Williamson I. Otitis media with effusion. Clinical Evidence [online]. Available at: http://www.clinicalevidence.com. Accessed on February 24, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
Do calcium supplements prevent postmenopausal osteoporotic fractures?
Calcium supplementation (1000–1200 mg daily) decreases menopause-related bone loss and reduces the rate of vertebral and non-vertebral fractures. Calcium is more efficacious in conjunction with vitamin D (700–800 IU daily), particularly in elderly patients, who have a high rate of vitamin D deficiency (strength of recommendation: A, based on randomized controlled trials).
Evidence summary
Calcium supplementation lessens bone loss in postmenopausal women. One double-blind, randomized controlled trial included healthy women who were 6 or more years postmenopausal and had a dietary intake of less than 400 mg of calcium per day.1 Women who received daily calcium citrate (500 mg) for 2 years had significantly less bone loss at the spine, hip, and radius than women taking placebo. In addition, calcium carbonate supplementation maintained bone density at the hip and radius but not the spine when compared with placebo. This dose of calcium was not associated with better outcomes in women within the first 5 years after menopause, but the dose was less than most generally recommended ranges.
Another randomized controlled trial of healthy women, postmenopausal for at least 3 years, showed that calcium supplementation at 1000 mg per day for 2 years decreased bone density loss in the hip and eliminated loss in the spine.2 The effect may be greatest in the first year of supplementation and less in subsequent years.3
Several studies have shown calcium supplementation has a beneficial effect on reducing fractures in postmenopausal women. A randomized controlled trial of healthy, community-dwelling people 65 years of age and older (55% women) showed daily supplementation with 500 mg calcium and 700 IU vitamin D for 3 years decreased nonvertebral fractures vs. placebo (response rate [RR]=0.54; 95% confidence interval [CI], 0.12–0.77; number needed to treat [NNT]=15).4
Another randomized controlled trial of elderly ambulatory women showed that supplementation with 1200 mg calcium and 800 IU vitamin D per day for 18 months decreased hip fractures (RR=0.26; 95% CI, 0.03–0.44; NNT=48) and other nonvertebral fractures (RR=0.25; 95% CI, 0.09–0.38; NNT=26).5
A third randomized controlled trial in post-menopausal women with low calcium intake and previous vertebral fractures showed that 1200 mg of calcium supplementation reduced the incidence of additional fractures (RR=0.23).6
Not all studies agree. The Study of Osteoporotic Fractures showed no beneficial effect of calcium supplements on fracture risk. This cohort study found that calcium supplements were actually associated with an increased risk of hip fracture (RR=1.5; 95% CI, 1.1–2.0) and vertebral fracture (RR=1.4; 95% CI, 1.1–1.9).7
Observational studies like this are subject to bias; reviews of the more rigorous randomized trials support calcium supplementation in order to decrease the risk of vertebral fracture by approximately 35% and nonvertebral fractures by approximately 25%.8,9 Daily supplementation of calcium (500–1200 mg) along with vitamin D (700–800 IU) is the regimen best supported by the evidence. Since the absorption of calcium decreases with single doses above 500 mg, the studies that used 1000–1200 mg of calcium split the daily doses.10
Recommendations from others
Guidelines have been published by the National Osteoporosis Foundation (1999),11 the National Institutes of Health Consensus Development Panel on Osteoporosis (2000),12 and others, all recommending 1200–1500 mg of elemental calcium and 400–800 IU of vitamin D be taken daily through a combination of diet and supplementation. The United States Preventive Services Task Force13 recommends that 1000–1500 mg of calcium be used daily; they make no specific recommendation regarding vitamin D supplementation.
Karen Gunning, PharmD, BCPS
University of Utah, Salt Lake City
Calcium and vitamin D are the foundation of osteoporosis treatment and prevention. Nearly every trial evaluating the use of antiresorptive and anabolic agents for the treatment and prevention of osteoporosis have evaluated these therapies in combination with calcium and vitamin D. As evaluated in this clinical inquiry, studies have also demonstrated benefit of these agents together in the absence of other medications.
Clinicians should ensure adequate dosing of calcium and vitamin D in all patients they are evaluating for osteoporosis treatment and prevention.
Clinicians should remember some people do get a significant portion of their daily nutritional requirements through diet, and incorporation of calcium and vitamin D as a part of a healthy diet should be the first recommendation. Checking the vitamin D content of a patient’s multivitamins is also important to avoid added expense.
Use of calcium citrate should be recommended for the elderly and those with achlorhydria, as acid is necessary to absorb the less expensive calcium carbonate. There is no evidence at this time suggesting a need to recommend other calcium salts.
1. Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in post-menopausal women. N Engl J Med 1990;323:878-883.
2. Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Effect of calcium supplementation on bone loss in post-menopausal women. N Engl J Med 1993;328:460-464.
3. Mackerras D, Lumley T. First- and second-year effects in trials of calcium supplementation on the loss of bone density in postmenopausal women. Bone 1997;21:527-533.
4. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-676.
5. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637-1642.
6. Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996;11:1961-1966.
7. Cumming RG, Cummings SR, Nevitt MC, et al. Calcium intake and fracture risk: results from the study of osteoporotic fractures. Am J Epidemiol 1997;145:926-934.
8. Kanis JA. The use of calcium in the management of osteoporosis. Bone 1999;24:279-290.
9. Shea B, Wells G, Cranney A, et al. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 2002;23:552-559.
10. Heaney RP. Calcium supplements: practical considerations. Osteoporos Int 1991;1:65-71.
11. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Belle Mead, NJ: Excerpta Medica; 1999.
12. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online. 2000;17:1-36.Available at: http://odp.od.nih.gov/consensus/cons/111/osteo_abstract. pdf. Accessed on January 17, 2003.
13. U.S. Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med 2002;137:526-528.
Calcium supplementation (1000–1200 mg daily) decreases menopause-related bone loss and reduces the rate of vertebral and non-vertebral fractures. Calcium is more efficacious in conjunction with vitamin D (700–800 IU daily), particularly in elderly patients, who have a high rate of vitamin D deficiency (strength of recommendation: A, based on randomized controlled trials).
Evidence summary
Calcium supplementation lessens bone loss in postmenopausal women. One double-blind, randomized controlled trial included healthy women who were 6 or more years postmenopausal and had a dietary intake of less than 400 mg of calcium per day.1 Women who received daily calcium citrate (500 mg) for 2 years had significantly less bone loss at the spine, hip, and radius than women taking placebo. In addition, calcium carbonate supplementation maintained bone density at the hip and radius but not the spine when compared with placebo. This dose of calcium was not associated with better outcomes in women within the first 5 years after menopause, but the dose was less than most generally recommended ranges.
Another randomized controlled trial of healthy women, postmenopausal for at least 3 years, showed that calcium supplementation at 1000 mg per day for 2 years decreased bone density loss in the hip and eliminated loss in the spine.2 The effect may be greatest in the first year of supplementation and less in subsequent years.3
Several studies have shown calcium supplementation has a beneficial effect on reducing fractures in postmenopausal women. A randomized controlled trial of healthy, community-dwelling people 65 years of age and older (55% women) showed daily supplementation with 500 mg calcium and 700 IU vitamin D for 3 years decreased nonvertebral fractures vs. placebo (response rate [RR]=0.54; 95% confidence interval [CI], 0.12–0.77; number needed to treat [NNT]=15).4
Another randomized controlled trial of elderly ambulatory women showed that supplementation with 1200 mg calcium and 800 IU vitamin D per day for 18 months decreased hip fractures (RR=0.26; 95% CI, 0.03–0.44; NNT=48) and other nonvertebral fractures (RR=0.25; 95% CI, 0.09–0.38; NNT=26).5
A third randomized controlled trial in post-menopausal women with low calcium intake and previous vertebral fractures showed that 1200 mg of calcium supplementation reduced the incidence of additional fractures (RR=0.23).6
Not all studies agree. The Study of Osteoporotic Fractures showed no beneficial effect of calcium supplements on fracture risk. This cohort study found that calcium supplements were actually associated with an increased risk of hip fracture (RR=1.5; 95% CI, 1.1–2.0) and vertebral fracture (RR=1.4; 95% CI, 1.1–1.9).7
Observational studies like this are subject to bias; reviews of the more rigorous randomized trials support calcium supplementation in order to decrease the risk of vertebral fracture by approximately 35% and nonvertebral fractures by approximately 25%.8,9 Daily supplementation of calcium (500–1200 mg) along with vitamin D (700–800 IU) is the regimen best supported by the evidence. Since the absorption of calcium decreases with single doses above 500 mg, the studies that used 1000–1200 mg of calcium split the daily doses.10
Recommendations from others
Guidelines have been published by the National Osteoporosis Foundation (1999),11 the National Institutes of Health Consensus Development Panel on Osteoporosis (2000),12 and others, all recommending 1200–1500 mg of elemental calcium and 400–800 IU of vitamin D be taken daily through a combination of diet and supplementation. The United States Preventive Services Task Force13 recommends that 1000–1500 mg of calcium be used daily; they make no specific recommendation regarding vitamin D supplementation.
Karen Gunning, PharmD, BCPS
University of Utah, Salt Lake City
Calcium and vitamin D are the foundation of osteoporosis treatment and prevention. Nearly every trial evaluating the use of antiresorptive and anabolic agents for the treatment and prevention of osteoporosis have evaluated these therapies in combination with calcium and vitamin D. As evaluated in this clinical inquiry, studies have also demonstrated benefit of these agents together in the absence of other medications.
Clinicians should ensure adequate dosing of calcium and vitamin D in all patients they are evaluating for osteoporosis treatment and prevention.
Clinicians should remember some people do get a significant portion of their daily nutritional requirements through diet, and incorporation of calcium and vitamin D as a part of a healthy diet should be the first recommendation. Checking the vitamin D content of a patient’s multivitamins is also important to avoid added expense.
Use of calcium citrate should be recommended for the elderly and those with achlorhydria, as acid is necessary to absorb the less expensive calcium carbonate. There is no evidence at this time suggesting a need to recommend other calcium salts.
Calcium supplementation (1000–1200 mg daily) decreases menopause-related bone loss and reduces the rate of vertebral and non-vertebral fractures. Calcium is more efficacious in conjunction with vitamin D (700–800 IU daily), particularly in elderly patients, who have a high rate of vitamin D deficiency (strength of recommendation: A, based on randomized controlled trials).
Evidence summary
Calcium supplementation lessens bone loss in postmenopausal women. One double-blind, randomized controlled trial included healthy women who were 6 or more years postmenopausal and had a dietary intake of less than 400 mg of calcium per day.1 Women who received daily calcium citrate (500 mg) for 2 years had significantly less bone loss at the spine, hip, and radius than women taking placebo. In addition, calcium carbonate supplementation maintained bone density at the hip and radius but not the spine when compared with placebo. This dose of calcium was not associated with better outcomes in women within the first 5 years after menopause, but the dose was less than most generally recommended ranges.
Another randomized controlled trial of healthy women, postmenopausal for at least 3 years, showed that calcium supplementation at 1000 mg per day for 2 years decreased bone density loss in the hip and eliminated loss in the spine.2 The effect may be greatest in the first year of supplementation and less in subsequent years.3
Several studies have shown calcium supplementation has a beneficial effect on reducing fractures in postmenopausal women. A randomized controlled trial of healthy, community-dwelling people 65 years of age and older (55% women) showed daily supplementation with 500 mg calcium and 700 IU vitamin D for 3 years decreased nonvertebral fractures vs. placebo (response rate [RR]=0.54; 95% confidence interval [CI], 0.12–0.77; number needed to treat [NNT]=15).4
Another randomized controlled trial of elderly ambulatory women showed that supplementation with 1200 mg calcium and 800 IU vitamin D per day for 18 months decreased hip fractures (RR=0.26; 95% CI, 0.03–0.44; NNT=48) and other nonvertebral fractures (RR=0.25; 95% CI, 0.09–0.38; NNT=26).5
A third randomized controlled trial in post-menopausal women with low calcium intake and previous vertebral fractures showed that 1200 mg of calcium supplementation reduced the incidence of additional fractures (RR=0.23).6
Not all studies agree. The Study of Osteoporotic Fractures showed no beneficial effect of calcium supplements on fracture risk. This cohort study found that calcium supplements were actually associated with an increased risk of hip fracture (RR=1.5; 95% CI, 1.1–2.0) and vertebral fracture (RR=1.4; 95% CI, 1.1–1.9).7
Observational studies like this are subject to bias; reviews of the more rigorous randomized trials support calcium supplementation in order to decrease the risk of vertebral fracture by approximately 35% and nonvertebral fractures by approximately 25%.8,9 Daily supplementation of calcium (500–1200 mg) along with vitamin D (700–800 IU) is the regimen best supported by the evidence. Since the absorption of calcium decreases with single doses above 500 mg, the studies that used 1000–1200 mg of calcium split the daily doses.10
Recommendations from others
Guidelines have been published by the National Osteoporosis Foundation (1999),11 the National Institutes of Health Consensus Development Panel on Osteoporosis (2000),12 and others, all recommending 1200–1500 mg of elemental calcium and 400–800 IU of vitamin D be taken daily through a combination of diet and supplementation. The United States Preventive Services Task Force13 recommends that 1000–1500 mg of calcium be used daily; they make no specific recommendation regarding vitamin D supplementation.
Karen Gunning, PharmD, BCPS
University of Utah, Salt Lake City
Calcium and vitamin D are the foundation of osteoporosis treatment and prevention. Nearly every trial evaluating the use of antiresorptive and anabolic agents for the treatment and prevention of osteoporosis have evaluated these therapies in combination with calcium and vitamin D. As evaluated in this clinical inquiry, studies have also demonstrated benefit of these agents together in the absence of other medications.
Clinicians should ensure adequate dosing of calcium and vitamin D in all patients they are evaluating for osteoporosis treatment and prevention.
Clinicians should remember some people do get a significant portion of their daily nutritional requirements through diet, and incorporation of calcium and vitamin D as a part of a healthy diet should be the first recommendation. Checking the vitamin D content of a patient’s multivitamins is also important to avoid added expense.
Use of calcium citrate should be recommended for the elderly and those with achlorhydria, as acid is necessary to absorb the less expensive calcium carbonate. There is no evidence at this time suggesting a need to recommend other calcium salts.
1. Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in post-menopausal women. N Engl J Med 1990;323:878-883.
2. Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Effect of calcium supplementation on bone loss in post-menopausal women. N Engl J Med 1993;328:460-464.
3. Mackerras D, Lumley T. First- and second-year effects in trials of calcium supplementation on the loss of bone density in postmenopausal women. Bone 1997;21:527-533.
4. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-676.
5. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637-1642.
6. Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996;11:1961-1966.
7. Cumming RG, Cummings SR, Nevitt MC, et al. Calcium intake and fracture risk: results from the study of osteoporotic fractures. Am J Epidemiol 1997;145:926-934.
8. Kanis JA. The use of calcium in the management of osteoporosis. Bone 1999;24:279-290.
9. Shea B, Wells G, Cranney A, et al. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 2002;23:552-559.
10. Heaney RP. Calcium supplements: practical considerations. Osteoporos Int 1991;1:65-71.
11. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Belle Mead, NJ: Excerpta Medica; 1999.
12. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online. 2000;17:1-36.Available at: http://odp.od.nih.gov/consensus/cons/111/osteo_abstract. pdf. Accessed on January 17, 2003.
13. U.S. Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med 2002;137:526-528.
1. Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in post-menopausal women. N Engl J Med 1990;323:878-883.
2. Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Effect of calcium supplementation on bone loss in post-menopausal women. N Engl J Med 1993;328:460-464.
3. Mackerras D, Lumley T. First- and second-year effects in trials of calcium supplementation on the loss of bone density in postmenopausal women. Bone 1997;21:527-533.
4. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997;337:670-676.
5. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 1992;327:1637-1642.
6. Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996;11:1961-1966.
7. Cumming RG, Cummings SR, Nevitt MC, et al. Calcium intake and fracture risk: results from the study of osteoporotic fractures. Am J Epidemiol 1997;145:926-934.
8. Kanis JA. The use of calcium in the management of osteoporosis. Bone 1999;24:279-290.
9. Shea B, Wells G, Cranney A, et al. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 2002;23:552-559.
10. Heaney RP. Calcium supplements: practical considerations. Osteoporos Int 1991;1:65-71.
11. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Belle Mead, NJ: Excerpta Medica; 1999.
12. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online. 2000;17:1-36.Available at: http://odp.od.nih.gov/consensus/cons/111/osteo_abstract. pdf. Accessed on January 17, 2003.
13. U.S. Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med 2002;137:526-528.
Evidence-based answers from the Family Physicians Inquiries Network
Is MRI useful for evaluation of acute low back pain?
Magnetic resonance imaging (MRI) is rarely helpful in the evaluation of acute low back pain. Limited evidence suggests that MRI may be useful in further assessing “red flags” in the history or physical exam.
MRI has a high sensitivity and specificity in the detection of cancer or infection, but it is not particularly specific when evaluating lumbar radiculopathy. Poor specificity can lead to finding clinically irrelevant abnormalities.1 The overall evidence for the appropriate use of MRI in low back pain is limited and weak2,3 (strength of recommendation: C, based on limited randomized controlled trials).
Evidence summary
Radiologic imaging of any kind is seldom needed in the evaluation of acute low back pain unless there are “red flags” suggestive of cancer, infection, or fracture (Table). Conduct a thorough history and review of systems to risk-stratify patients that may benefit from imaging.
One study of patients with low back pain identified risk factors for cancer, including age >50 years, prior cancer, unexplained weight loss, pain lasting >1 month, and no relief with bed rest.4 An elevated erythrocyte sedimentation rate of >50 mm/hr in the setting of these risk factors should prompt the clinician to order an MRI or bone scan.5
An analysis of systematic reviews and original articles by Jarvik and Deyo reported sensitivities for MRI (83% to 93%) and for radionucleotide scanning (74% to 98%) in detecting cancer.6 MRI exhibits the best sensitivity (96%) and specificity (92%) for infection. MRI may be helpful for further evaluation of an acute neurologic deficit, suspected cauda equina syndrome, suspected active sacroiliitis, and worsening low back pain not responding to 4 or more weeks of conservative therapy.7,8
Consider contrast enhancement with gadolinium when evaluating inflammatory conditions, or for patients who have had spine surgery.9 The lower specificity of MRI for radiculopathy means that MRI can detect disk herniations that do not cause the patient’s signs or symptoms. In one study, MRI demonstrated herniated disks in 25% of asymptomatic persons.1
Unfortunately, there are too few studies to guide clinicians in the appropriate use of MRI in the evaluation of low back pain.2,4 Higher quality evidence is needed before firm guidelines can be made for the use of MRI in the evaluation of low back pain.
TABLE
Red flags for underlying causes of low back pain
| Condition | Red flags |
|---|---|
| Cancer | Age >50 |
| History of cancer | |
| Unexplained weight loss | |
| Failure to improve after 4 to 6 weeks of conservative low back pain therapy | |
| Spinal infection | Fever >38°C |
| History of intravenous drug abuse | |
| Urinary tract infection | |
| Neurologic emergencies or urgencies | Cauda equina symptoms |
| Progressive neurologic deficit | |
| Suspicion of ankylosing spondylitis | |
| Unrelenting night pain or pain at rest | |
| Pain with distal numbness or leg weakness | |
| Fracture | History of osteoporosis |
| Chronic oral steroid use | |
| Serious accident or injury | |
| Adapted from Institute for Clinical Systems Improvement10 | |
Recommendations from others
Institute for Clinical Systems Improvement guidelines recommend considering plain films for patients with risk factors for cancer or infection.
Additional indications are listed in the Table. Plain films, however, do not rule out cancer. With patients who warrant a high level of suspicion of cancer, consider using MRI, computed tomography, or bone scan. Consider MRI or computed tomography also for patients with cauda equina syndrome or a rapidly progressing neurologic deficit, while concurrently consulting neurosurgery or surgery.10
Susan L. Pereira, MD
Department of Family and Community Medicine, University of Missouri– Columbia
When a patient has acute low back pain, with or without known trauma, I rarely find it useful to order an MRI. I have found conservative therapy with anti-inflammatory agents and exercise (when a patient is able to do so) provides relief. Further intervention is rarely necessary.
For more difficult cases—when pain has been present for months and is getting worse despite conservative therapy, or for patients who demonstrate symptoms of cauda equina syndrome—I find MRI useful to help tailor therapy and make decisions regarding appropriate referral. I agree with the author that, even for patients with radicular pain, an MRI rarely changes the treatment plan. Paying attention to the risk factors identified above and performing an MRI when they are present seems to be the best recommendation.
1. Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994;331:69-73.
2. More research is needed to evaluate the clinical efficacy of MRI. ACP J Club 1994;121:49.-
3. Goh RH, Somers S, Jurriaans E, Yu J. Magnetic resonance imaging. Application to family practice. Can Fam Physician 1999;45:2118-282131-2132.
4. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med 1988;3:230-238.
5. Joines JD, McNutt Ra, Carey TS, Deyo RA, Rouhani R. Finding cancer in primary care outpatients with low back pain: a comparison of diagnostic strategies. J Gen Intern Med 2001;16:14-23.
6. Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 2002;137:586-597.
7. McNally EG, Wilson DJ, Ostlere SJ. Limited magnetic resonance imaging in low back pain instead of plain radiographs: experience with first 1000 cases. Clin Radiol 2001;56:922-925.
8. Battafarano DF, West SG, Rak KM, Fortenbery EJ, Chantelois AE. Comparison of bone scan, computer tomography, and magnetic resonance imaging in the diagnosis of active sacroiliitis. Semin Arthritis Rheum 1993;23:161-176.
9. Bradley WG. Use of contrast in MR imaging of the lumbar spine. Magn Reson Imaging Clin N Am 1999;7:439-57, vii.
10. Thorson DC. Health Care Guideline: Adult low back pain. Bloomington, MN: Institute for Clinical Systems Improvement; 1–61. Available at:www.icsi.org/knowledge. Accessed on February 5, 2003.
Magnetic resonance imaging (MRI) is rarely helpful in the evaluation of acute low back pain. Limited evidence suggests that MRI may be useful in further assessing “red flags” in the history or physical exam.
MRI has a high sensitivity and specificity in the detection of cancer or infection, but it is not particularly specific when evaluating lumbar radiculopathy. Poor specificity can lead to finding clinically irrelevant abnormalities.1 The overall evidence for the appropriate use of MRI in low back pain is limited and weak2,3 (strength of recommendation: C, based on limited randomized controlled trials).
Evidence summary
Radiologic imaging of any kind is seldom needed in the evaluation of acute low back pain unless there are “red flags” suggestive of cancer, infection, or fracture (Table). Conduct a thorough history and review of systems to risk-stratify patients that may benefit from imaging.
One study of patients with low back pain identified risk factors for cancer, including age >50 years, prior cancer, unexplained weight loss, pain lasting >1 month, and no relief with bed rest.4 An elevated erythrocyte sedimentation rate of >50 mm/hr in the setting of these risk factors should prompt the clinician to order an MRI or bone scan.5
An analysis of systematic reviews and original articles by Jarvik and Deyo reported sensitivities for MRI (83% to 93%) and for radionucleotide scanning (74% to 98%) in detecting cancer.6 MRI exhibits the best sensitivity (96%) and specificity (92%) for infection. MRI may be helpful for further evaluation of an acute neurologic deficit, suspected cauda equina syndrome, suspected active sacroiliitis, and worsening low back pain not responding to 4 or more weeks of conservative therapy.7,8
Consider contrast enhancement with gadolinium when evaluating inflammatory conditions, or for patients who have had spine surgery.9 The lower specificity of MRI for radiculopathy means that MRI can detect disk herniations that do not cause the patient’s signs or symptoms. In one study, MRI demonstrated herniated disks in 25% of asymptomatic persons.1
Unfortunately, there are too few studies to guide clinicians in the appropriate use of MRI in the evaluation of low back pain.2,4 Higher quality evidence is needed before firm guidelines can be made for the use of MRI in the evaluation of low back pain.
TABLE
Red flags for underlying causes of low back pain
| Condition | Red flags |
|---|---|
| Cancer | Age >50 |
| History of cancer | |
| Unexplained weight loss | |
| Failure to improve after 4 to 6 weeks of conservative low back pain therapy | |
| Spinal infection | Fever >38°C |
| History of intravenous drug abuse | |
| Urinary tract infection | |
| Neurologic emergencies or urgencies | Cauda equina symptoms |
| Progressive neurologic deficit | |
| Suspicion of ankylosing spondylitis | |
| Unrelenting night pain or pain at rest | |
| Pain with distal numbness or leg weakness | |
| Fracture | History of osteoporosis |
| Chronic oral steroid use | |
| Serious accident or injury | |
| Adapted from Institute for Clinical Systems Improvement10 | |
Recommendations from others
Institute for Clinical Systems Improvement guidelines recommend considering plain films for patients with risk factors for cancer or infection.
Additional indications are listed in the Table. Plain films, however, do not rule out cancer. With patients who warrant a high level of suspicion of cancer, consider using MRI, computed tomography, or bone scan. Consider MRI or computed tomography also for patients with cauda equina syndrome or a rapidly progressing neurologic deficit, while concurrently consulting neurosurgery or surgery.10
Susan L. Pereira, MD
Department of Family and Community Medicine, University of Missouri– Columbia
When a patient has acute low back pain, with or without known trauma, I rarely find it useful to order an MRI. I have found conservative therapy with anti-inflammatory agents and exercise (when a patient is able to do so) provides relief. Further intervention is rarely necessary.
For more difficult cases—when pain has been present for months and is getting worse despite conservative therapy, or for patients who demonstrate symptoms of cauda equina syndrome—I find MRI useful to help tailor therapy and make decisions regarding appropriate referral. I agree with the author that, even for patients with radicular pain, an MRI rarely changes the treatment plan. Paying attention to the risk factors identified above and performing an MRI when they are present seems to be the best recommendation.
Magnetic resonance imaging (MRI) is rarely helpful in the evaluation of acute low back pain. Limited evidence suggests that MRI may be useful in further assessing “red flags” in the history or physical exam.
MRI has a high sensitivity and specificity in the detection of cancer or infection, but it is not particularly specific when evaluating lumbar radiculopathy. Poor specificity can lead to finding clinically irrelevant abnormalities.1 The overall evidence for the appropriate use of MRI in low back pain is limited and weak2,3 (strength of recommendation: C, based on limited randomized controlled trials).
Evidence summary
Radiologic imaging of any kind is seldom needed in the evaluation of acute low back pain unless there are “red flags” suggestive of cancer, infection, or fracture (Table). Conduct a thorough history and review of systems to risk-stratify patients that may benefit from imaging.
One study of patients with low back pain identified risk factors for cancer, including age >50 years, prior cancer, unexplained weight loss, pain lasting >1 month, and no relief with bed rest.4 An elevated erythrocyte sedimentation rate of >50 mm/hr in the setting of these risk factors should prompt the clinician to order an MRI or bone scan.5
An analysis of systematic reviews and original articles by Jarvik and Deyo reported sensitivities for MRI (83% to 93%) and for radionucleotide scanning (74% to 98%) in detecting cancer.6 MRI exhibits the best sensitivity (96%) and specificity (92%) for infection. MRI may be helpful for further evaluation of an acute neurologic deficit, suspected cauda equina syndrome, suspected active sacroiliitis, and worsening low back pain not responding to 4 or more weeks of conservative therapy.7,8
Consider contrast enhancement with gadolinium when evaluating inflammatory conditions, or for patients who have had spine surgery.9 The lower specificity of MRI for radiculopathy means that MRI can detect disk herniations that do not cause the patient’s signs or symptoms. In one study, MRI demonstrated herniated disks in 25% of asymptomatic persons.1
Unfortunately, there are too few studies to guide clinicians in the appropriate use of MRI in the evaluation of low back pain.2,4 Higher quality evidence is needed before firm guidelines can be made for the use of MRI in the evaluation of low back pain.
TABLE
Red flags for underlying causes of low back pain
| Condition | Red flags |
|---|---|
| Cancer | Age >50 |
| History of cancer | |
| Unexplained weight loss | |
| Failure to improve after 4 to 6 weeks of conservative low back pain therapy | |
| Spinal infection | Fever >38°C |
| History of intravenous drug abuse | |
| Urinary tract infection | |
| Neurologic emergencies or urgencies | Cauda equina symptoms |
| Progressive neurologic deficit | |
| Suspicion of ankylosing spondylitis | |
| Unrelenting night pain or pain at rest | |
| Pain with distal numbness or leg weakness | |
| Fracture | History of osteoporosis |
| Chronic oral steroid use | |
| Serious accident or injury | |
| Adapted from Institute for Clinical Systems Improvement10 | |
Recommendations from others
Institute for Clinical Systems Improvement guidelines recommend considering plain films for patients with risk factors for cancer or infection.
Additional indications are listed in the Table. Plain films, however, do not rule out cancer. With patients who warrant a high level of suspicion of cancer, consider using MRI, computed tomography, or bone scan. Consider MRI or computed tomography also for patients with cauda equina syndrome or a rapidly progressing neurologic deficit, while concurrently consulting neurosurgery or surgery.10
Susan L. Pereira, MD
Department of Family and Community Medicine, University of Missouri– Columbia
When a patient has acute low back pain, with or without known trauma, I rarely find it useful to order an MRI. I have found conservative therapy with anti-inflammatory agents and exercise (when a patient is able to do so) provides relief. Further intervention is rarely necessary.
For more difficult cases—when pain has been present for months and is getting worse despite conservative therapy, or for patients who demonstrate symptoms of cauda equina syndrome—I find MRI useful to help tailor therapy and make decisions regarding appropriate referral. I agree with the author that, even for patients with radicular pain, an MRI rarely changes the treatment plan. Paying attention to the risk factors identified above and performing an MRI when they are present seems to be the best recommendation.
1. Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994;331:69-73.
2. More research is needed to evaluate the clinical efficacy of MRI. ACP J Club 1994;121:49.-
3. Goh RH, Somers S, Jurriaans E, Yu J. Magnetic resonance imaging. Application to family practice. Can Fam Physician 1999;45:2118-282131-2132.
4. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med 1988;3:230-238.
5. Joines JD, McNutt Ra, Carey TS, Deyo RA, Rouhani R. Finding cancer in primary care outpatients with low back pain: a comparison of diagnostic strategies. J Gen Intern Med 2001;16:14-23.
6. Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 2002;137:586-597.
7. McNally EG, Wilson DJ, Ostlere SJ. Limited magnetic resonance imaging in low back pain instead of plain radiographs: experience with first 1000 cases. Clin Radiol 2001;56:922-925.
8. Battafarano DF, West SG, Rak KM, Fortenbery EJ, Chantelois AE. Comparison of bone scan, computer tomography, and magnetic resonance imaging in the diagnosis of active sacroiliitis. Semin Arthritis Rheum 1993;23:161-176.
9. Bradley WG. Use of contrast in MR imaging of the lumbar spine. Magn Reson Imaging Clin N Am 1999;7:439-57, vii.
10. Thorson DC. Health Care Guideline: Adult low back pain. Bloomington, MN: Institute for Clinical Systems Improvement; 1–61. Available at:www.icsi.org/knowledge. Accessed on February 5, 2003.
1. Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994;331:69-73.
2. More research is needed to evaluate the clinical efficacy of MRI. ACP J Club 1994;121:49.-
3. Goh RH, Somers S, Jurriaans E, Yu J. Magnetic resonance imaging. Application to family practice. Can Fam Physician 1999;45:2118-282131-2132.
4. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med 1988;3:230-238.
5. Joines JD, McNutt Ra, Carey TS, Deyo RA, Rouhani R. Finding cancer in primary care outpatients with low back pain: a comparison of diagnostic strategies. J Gen Intern Med 2001;16:14-23.
6. Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 2002;137:586-597.
7. McNally EG, Wilson DJ, Ostlere SJ. Limited magnetic resonance imaging in low back pain instead of plain radiographs: experience with first 1000 cases. Clin Radiol 2001;56:922-925.
8. Battafarano DF, West SG, Rak KM, Fortenbery EJ, Chantelois AE. Comparison of bone scan, computer tomography, and magnetic resonance imaging in the diagnosis of active sacroiliitis. Semin Arthritis Rheum 1993;23:161-176.
9. Bradley WG. Use of contrast in MR imaging of the lumbar spine. Magn Reson Imaging Clin N Am 1999;7:439-57, vii.
10. Thorson DC. Health Care Guideline: Adult low back pain. Bloomington, MN: Institute for Clinical Systems Improvement; 1–61. Available at:www.icsi.org/knowledge. Accessed on February 5, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
Does microalbuminuria screening in diabetes prevent complications?
Screening diabetic patients for microalbuminuria identifies those who may benefit from treatments that delay progression to renal failure (strength of recommendation: B, based on extrapolation from Level 1 treatment studies of patients with microalbuminuria).
No research has determined the best method for screening for microalbuminuria, or whether screening in primary care populations will produce better long-term outcomes. No studies have examined the role of microalbuminuria screening after angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) have been instituted for other indications.
Evidence summary
Patients with diabetes mellitus have a 20% to 40% lifetime risk for development of nephropathy, and microalbuminuria is the earliest easily detectable marker of renal damage.1 Improved control of blood sugar2,3 and blood pressure4 decreases but does not completely prevent development of microalbuminuria and progression to overt kidney failure. ACE inhibitors and ARBs have been shown to diminish this progression even in the absence of hypertension (the latter in type 2 diabetes only) (Table).
No prospective randomized trials of screening have been reported. There is uncertainty about what method of screening is most effective and practical in primary care settings.10 Expert opinion recommends diagnosing microalbuminuria after 2 positive test results,1 but whether repeated tests improve diagnostic accuracy is still controversial.10
A large randomized controlled trial showing better long-term renal and vascular disease outcomes would be needed to give screening for microalbuminuria a strength of recommendation of A. Recruiting patients for such a study, and interpreting its results, would be difficult: many subjects would have other indications, such as hypertension or congestive heart failure, warranting use of potentially renoprotective medications.
TABLE
Reno- and cardioprotective efficacy of treatments for diabetic patients with microalbuminuria
| DM type | Medication | NNT | Time (years) | To prevent endpoint |
|---|---|---|---|---|
| 1 | ACE inhibitor (Captopril) | 7.9* | 2 | Clinical proteinuria5 |
| 2 | ACE inhibitor (Enalapril) | 6.3* | 5 | Macroalbuminuria6 |
| 2 | ACE inhibitor (Enalapril) | 2.4* | 7 | Significant proteinuria7 |
| 2 | ARB (Losartan) | 3.6 | 3.4 | End-stage renal disease8 |
| 2 | ACE inhibitor (Ramipril) | 4 | 4.5 | Cardiovascular disease9 † |
| *Normotensive subjects | ||||
| †Myocardial infarction, revascularization procedure, stroke, cardiovascular death, congestive heart failure requiring hospitalization, overt nephropathy, renal dialysis, or laser treatment for retinopathy | ||||
| DM, diabetes mellitus; NNT, number needed to treat; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker | ||||
Recommendations from others
The American Diabetes Association recommends annual screening for microalbuminuria—after 5 years of established type 1 disease, and at time of diagnosis for type 2 diabetes without macroalbuminuria. Initial screening can use 1 of 3 methods: measurement of the albumin-to-creatinine ratio in a random, spot collection; 24-hour collection with creatinine, allowing the simultaneous measurement of creatinine clearance; timed (eg, 4-hour or overnight) collection. At least 2 of 3 tests measured within a 6-month period should show elevated levels before a patient is said to have microalbuminuria.1
Stephen A. Wilson, MD
University of Pittsburgh Medical Center, St. Margaret Family Practice Residency, Pittsburgh, Pa
Blood pressure control and ACE inhibition improve mortality and morbidity for patients with diabetes mellitus type 2. Therefore, maximize ACE inhibitor or ARB doses, as tolerated, and aim for a blood pressure of 110–120/70–80 mm Hg (130/85 mm Hg is the maximum).
Using this plan, I do not routinely screen for microalbuminuria—which is, at best, a surrogate marker for nephropathy and poor blood pressure control—unless I believe it will work as an educational and motivational tool for patients who are less committed to self-care.
If serum creatinine becomes elevated, a 24-hour urine collection to examine volume, creatinine clearance, and protein can be used to help develop a negotiated care plan with the patient, which may or may not include referral. Until there is different evidence about screening and treatment options for microalbuminuria, I see no need to screen when the above plan is in effect.
1. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2002;25:S33-S49.
2. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial (DCCT) Research Group. Kidney Int 1995;47:1703-1720.
3. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study Group. Lancet 1998;352:837-853.
4. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 UK Prospective Diabetes Study Group. BMJ 1998;317:703-713.
5. Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med 1995;99:497-504.
6. Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care 1997;20:1576-1581.
7. Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med 1996;156:286-289.
8. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-869.
9. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE study. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Lancet 2000;355:253-259.
10. Scheid DC, McCarthy LH, Lawler FH, Hamm RM, Reilly KEH. Screening for microalbuminuria to prevent nephropathy in patients with diabetes. A systematic review of the evidence. J Fam Pract 2001;50:661-s668.
Screening diabetic patients for microalbuminuria identifies those who may benefit from treatments that delay progression to renal failure (strength of recommendation: B, based on extrapolation from Level 1 treatment studies of patients with microalbuminuria).
No research has determined the best method for screening for microalbuminuria, or whether screening in primary care populations will produce better long-term outcomes. No studies have examined the role of microalbuminuria screening after angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) have been instituted for other indications.
Evidence summary
Patients with diabetes mellitus have a 20% to 40% lifetime risk for development of nephropathy, and microalbuminuria is the earliest easily detectable marker of renal damage.1 Improved control of blood sugar2,3 and blood pressure4 decreases but does not completely prevent development of microalbuminuria and progression to overt kidney failure. ACE inhibitors and ARBs have been shown to diminish this progression even in the absence of hypertension (the latter in type 2 diabetes only) (Table).
No prospective randomized trials of screening have been reported. There is uncertainty about what method of screening is most effective and practical in primary care settings.10 Expert opinion recommends diagnosing microalbuminuria after 2 positive test results,1 but whether repeated tests improve diagnostic accuracy is still controversial.10
A large randomized controlled trial showing better long-term renal and vascular disease outcomes would be needed to give screening for microalbuminuria a strength of recommendation of A. Recruiting patients for such a study, and interpreting its results, would be difficult: many subjects would have other indications, such as hypertension or congestive heart failure, warranting use of potentially renoprotective medications.
TABLE
Reno- and cardioprotective efficacy of treatments for diabetic patients with microalbuminuria
| DM type | Medication | NNT | Time (years) | To prevent endpoint |
|---|---|---|---|---|
| 1 | ACE inhibitor (Captopril) | 7.9* | 2 | Clinical proteinuria5 |
| 2 | ACE inhibitor (Enalapril) | 6.3* | 5 | Macroalbuminuria6 |
| 2 | ACE inhibitor (Enalapril) | 2.4* | 7 | Significant proteinuria7 |
| 2 | ARB (Losartan) | 3.6 | 3.4 | End-stage renal disease8 |
| 2 | ACE inhibitor (Ramipril) | 4 | 4.5 | Cardiovascular disease9 † |
| *Normotensive subjects | ||||
| †Myocardial infarction, revascularization procedure, stroke, cardiovascular death, congestive heart failure requiring hospitalization, overt nephropathy, renal dialysis, or laser treatment for retinopathy | ||||
| DM, diabetes mellitus; NNT, number needed to treat; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker | ||||
Recommendations from others
The American Diabetes Association recommends annual screening for microalbuminuria—after 5 years of established type 1 disease, and at time of diagnosis for type 2 diabetes without macroalbuminuria. Initial screening can use 1 of 3 methods: measurement of the albumin-to-creatinine ratio in a random, spot collection; 24-hour collection with creatinine, allowing the simultaneous measurement of creatinine clearance; timed (eg, 4-hour or overnight) collection. At least 2 of 3 tests measured within a 6-month period should show elevated levels before a patient is said to have microalbuminuria.1
Stephen A. Wilson, MD
University of Pittsburgh Medical Center, St. Margaret Family Practice Residency, Pittsburgh, Pa
Blood pressure control and ACE inhibition improve mortality and morbidity for patients with diabetes mellitus type 2. Therefore, maximize ACE inhibitor or ARB doses, as tolerated, and aim for a blood pressure of 110–120/70–80 mm Hg (130/85 mm Hg is the maximum).
Using this plan, I do not routinely screen for microalbuminuria—which is, at best, a surrogate marker for nephropathy and poor blood pressure control—unless I believe it will work as an educational and motivational tool for patients who are less committed to self-care.
If serum creatinine becomes elevated, a 24-hour urine collection to examine volume, creatinine clearance, and protein can be used to help develop a negotiated care plan with the patient, which may or may not include referral. Until there is different evidence about screening and treatment options for microalbuminuria, I see no need to screen when the above plan is in effect.
Screening diabetic patients for microalbuminuria identifies those who may benefit from treatments that delay progression to renal failure (strength of recommendation: B, based on extrapolation from Level 1 treatment studies of patients with microalbuminuria).
No research has determined the best method for screening for microalbuminuria, or whether screening in primary care populations will produce better long-term outcomes. No studies have examined the role of microalbuminuria screening after angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) have been instituted for other indications.
Evidence summary
Patients with diabetes mellitus have a 20% to 40% lifetime risk for development of nephropathy, and microalbuminuria is the earliest easily detectable marker of renal damage.1 Improved control of blood sugar2,3 and blood pressure4 decreases but does not completely prevent development of microalbuminuria and progression to overt kidney failure. ACE inhibitors and ARBs have been shown to diminish this progression even in the absence of hypertension (the latter in type 2 diabetes only) (Table).
No prospective randomized trials of screening have been reported. There is uncertainty about what method of screening is most effective and practical in primary care settings.10 Expert opinion recommends diagnosing microalbuminuria after 2 positive test results,1 but whether repeated tests improve diagnostic accuracy is still controversial.10
A large randomized controlled trial showing better long-term renal and vascular disease outcomes would be needed to give screening for microalbuminuria a strength of recommendation of A. Recruiting patients for such a study, and interpreting its results, would be difficult: many subjects would have other indications, such as hypertension or congestive heart failure, warranting use of potentially renoprotective medications.
TABLE
Reno- and cardioprotective efficacy of treatments for diabetic patients with microalbuminuria
| DM type | Medication | NNT | Time (years) | To prevent endpoint |
|---|---|---|---|---|
| 1 | ACE inhibitor (Captopril) | 7.9* | 2 | Clinical proteinuria5 |
| 2 | ACE inhibitor (Enalapril) | 6.3* | 5 | Macroalbuminuria6 |
| 2 | ACE inhibitor (Enalapril) | 2.4* | 7 | Significant proteinuria7 |
| 2 | ARB (Losartan) | 3.6 | 3.4 | End-stage renal disease8 |
| 2 | ACE inhibitor (Ramipril) | 4 | 4.5 | Cardiovascular disease9 † |
| *Normotensive subjects | ||||
| †Myocardial infarction, revascularization procedure, stroke, cardiovascular death, congestive heart failure requiring hospitalization, overt nephropathy, renal dialysis, or laser treatment for retinopathy | ||||
| DM, diabetes mellitus; NNT, number needed to treat; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker | ||||
Recommendations from others
The American Diabetes Association recommends annual screening for microalbuminuria—after 5 years of established type 1 disease, and at time of diagnosis for type 2 diabetes without macroalbuminuria. Initial screening can use 1 of 3 methods: measurement of the albumin-to-creatinine ratio in a random, spot collection; 24-hour collection with creatinine, allowing the simultaneous measurement of creatinine clearance; timed (eg, 4-hour or overnight) collection. At least 2 of 3 tests measured within a 6-month period should show elevated levels before a patient is said to have microalbuminuria.1
Stephen A. Wilson, MD
University of Pittsburgh Medical Center, St. Margaret Family Practice Residency, Pittsburgh, Pa
Blood pressure control and ACE inhibition improve mortality and morbidity for patients with diabetes mellitus type 2. Therefore, maximize ACE inhibitor or ARB doses, as tolerated, and aim for a blood pressure of 110–120/70–80 mm Hg (130/85 mm Hg is the maximum).
Using this plan, I do not routinely screen for microalbuminuria—which is, at best, a surrogate marker for nephropathy and poor blood pressure control—unless I believe it will work as an educational and motivational tool for patients who are less committed to self-care.
If serum creatinine becomes elevated, a 24-hour urine collection to examine volume, creatinine clearance, and protein can be used to help develop a negotiated care plan with the patient, which may or may not include referral. Until there is different evidence about screening and treatment options for microalbuminuria, I see no need to screen when the above plan is in effect.
1. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2002;25:S33-S49.
2. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial (DCCT) Research Group. Kidney Int 1995;47:1703-1720.
3. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study Group. Lancet 1998;352:837-853.
4. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 UK Prospective Diabetes Study Group. BMJ 1998;317:703-713.
5. Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med 1995;99:497-504.
6. Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care 1997;20:1576-1581.
7. Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med 1996;156:286-289.
8. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-869.
9. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE study. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Lancet 2000;355:253-259.
10. Scheid DC, McCarthy LH, Lawler FH, Hamm RM, Reilly KEH. Screening for microalbuminuria to prevent nephropathy in patients with diabetes. A systematic review of the evidence. J Fam Pract 2001;50:661-s668.
1. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2002;25:S33-S49.
2. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial (DCCT) Research Group. Kidney Int 1995;47:1703-1720.
3. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study Group. Lancet 1998;352:837-853.
4. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38 UK Prospective Diabetes Study Group. BMJ 1998;317:703-713.
5. Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med 1995;99:497-504.
6. Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care 1997;20:1576-1581.
7. Ravid M, Lang R, Rachmani R, Lishner M. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. A 7-year follow-up study. Arch Intern Med 1996;156:286-289.
8. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-869.
9. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE study. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Lancet 2000;355:253-259.
10. Scheid DC, McCarthy LH, Lawler FH, Hamm RM, Reilly KEH. Screening for microalbuminuria to prevent nephropathy in patients with diabetes. A systematic review of the evidence. J Fam Pract 2001;50:661-s668.
Evidence-based answers from the Family Physicians Inquiries Network
Do glucosamine or chondroitin cause regeneration of cartilage in osteoarthritis?
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9
Fred Tudiver, MD
East Tennessee State University, Johnson City
Most family physicians see many patients with osteoarthritis, which can be difficult to treat. My patients typically want improvement in their symptoms, function, and disease progression. Although there is good evidence that the use of glucosamine sulphate (but not chondroitin sulphate) can improve the common symptoms and functional problems of osteoarthritis, this review states it is unclear whether these substances can alter disease progression through regeneration of cartilage.
I tell my patients with osteoarthritis that glucosamine sulfate can help problems like joint pain and function, but that we do not have a safe and reliable treatment for reversing the disease or the joint damage resulting from it.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
1. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;CD002946.-
2. Hauselmann HJ. Nutripharmaceuticals for osteoarthritis. Best Pract Res Clin Rheumatol 2001;15:595-607.
3. de los Reyes GC, Koda RT, Lien EJ. Glucosamine and chondroitin sulfates in the treatment of osteoarthritis: a survey. Prog Drug Res 2000;155:81-103.
4. Jubb RW. Oral and intra-articular remedies: review of papers published from March 2001 to February 2002. Curr Opin Rheumatol 2002;14:597-602.
5. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001;357:251-256.
6. Mazzuca SA, Brandt KD, Lane KA, Katz BP. Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002;46:1223-1227.
7. Verbruggen G, Goemaere S, Veys EM. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002;21:231-243.
8. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
9. Problems with dietary supplements. Med Lett Drugs Ther 2002;44:84-86.
Evidence-based answers from the Family Physicians Inquiries Network