User login
An unexpected cause of shoulder pain
A 58-year-old woman who sustained right-sided traumatic rib fractures after falling down stairs 8 months earlier presented with right shoulder pain that had been present for 6 months. She received nonsteroidal anti-inflammatory drugs at another hospital, which were partially effective. Magnetic resonance imaging of the neck and right shoulder had shown no abnormalities.
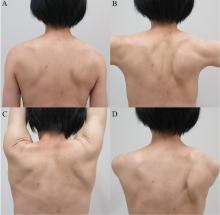
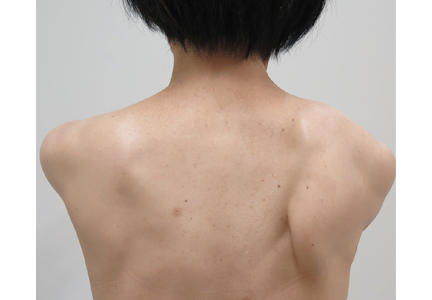
On physical examination, her right scapula was found to protrude abnormally (ie, to “wing”) during forward flexion and abduction of the right arm (Figure 1). Electromyography showed evidence of right serratus anterior paralysis and denervation of the right long thoracic nerve, leading to a diagnosis of traumatic long thoracic nerve paralysis. A course of physical therapy was initiated to improve her symptoms.
LONG THORACIC NERVE PARALYSIS
Scapular winging is caused by dysfunction of any of the 3 main muscles that attach the scapula to the posterior thoracic wall—the serratus anterior, the trapezius, and the rhomboid. The problem is most often in the serratus anterior muscle, innervated by the long thoracic nerve, a pure motor nerve that originates from the fifth, sixth, and seventh cervical nerves and descends along the lateral thoracic wall.
Long thoracic nerve paralysis can have traumatic, nontraumatic, or iatrogenic causes. Traumatic injuries result from blunt trauma to the neck, shoulder girdle, and thorax, while nontraumatic causes include viral illness, toxic exposure, apical pulmonary tumor, and C7 radiculopathy.1–3 Iatrogenic injuries may be caused by mastectomy with axillary dissection, chest tube thoracostomy, first-rib resection, or scalenotomy, or occur after general anesthesia.1,2,4
Scapular winging due to paralysis of the serratus anterior muscle is accentuated by forward elevation and—particularly—by pushing against a wall, and the entire scapula is displaced more medially and superiorly.2 The compensatory muscular activity required for shoulder stability induces secondary shoulder pain.5
The diagnosis is often delayed, as the clinical presentation may mimic the symptoms of shoulder joint or rotator cuff pathology. Although physical therapy resolves the pain and improves the function of the arm, mild endurance deficits and asymptomatic scapular winging may persist. Tendon transfer surgery is considered if adequate recovery is not achieved after a 6- to-24-month course of physical therapy.2
- Vastamäki M, Kauppila LI. Etiologic factors in isolated paralysis of the serratus anterior muscle: a report of 197 cases. J Shoulder Elbow Surg 1993; 2:240–243.
- Martin RM, Fish DE. Scapular winging: anatomical review, diagnosis, and treatments. Curr Rev Musculoskelet Med 2008; 1:1–11.
- Toshkezi G, Dejesus J, Jabre JF, Hohler A, Davies K. Long thoracic neuropathy caused by an apical pulmonary tumor. J Neurosurg 2009; 110:754–757.
- Kauppila LI, Vastamäki M. Iatrogenic serratus anterior paralysis. Long-term outcome in 26 patients. Chest 1996; 109:31–34.
- Nath RK, Lyons AB, Bietz G. Microneurolysis and decompression of long thoracic nerve injury are effective in reversing scapular winging: long-term results in 50 cases. BMC Musculoskelet Disord 2007; 8:25.
A 58-year-old woman who sustained right-sided traumatic rib fractures after falling down stairs 8 months earlier presented with right shoulder pain that had been present for 6 months. She received nonsteroidal anti-inflammatory drugs at another hospital, which were partially effective. Magnetic resonance imaging of the neck and right shoulder had shown no abnormalities.
On physical examination, her right scapula was found to protrude abnormally (ie, to “wing”) during forward flexion and abduction of the right arm (Figure 1). Electromyography showed evidence of right serratus anterior paralysis and denervation of the right long thoracic nerve, leading to a diagnosis of traumatic long thoracic nerve paralysis. A course of physical therapy was initiated to improve her symptoms.
LONG THORACIC NERVE PARALYSIS
Scapular winging is caused by dysfunction of any of the 3 main muscles that attach the scapula to the posterior thoracic wall—the serratus anterior, the trapezius, and the rhomboid. The problem is most often in the serratus anterior muscle, innervated by the long thoracic nerve, a pure motor nerve that originates from the fifth, sixth, and seventh cervical nerves and descends along the lateral thoracic wall.
Long thoracic nerve paralysis can have traumatic, nontraumatic, or iatrogenic causes. Traumatic injuries result from blunt trauma to the neck, shoulder girdle, and thorax, while nontraumatic causes include viral illness, toxic exposure, apical pulmonary tumor, and C7 radiculopathy.1–3 Iatrogenic injuries may be caused by mastectomy with axillary dissection, chest tube thoracostomy, first-rib resection, or scalenotomy, or occur after general anesthesia.1,2,4
Scapular winging due to paralysis of the serratus anterior muscle is accentuated by forward elevation and—particularly—by pushing against a wall, and the entire scapula is displaced more medially and superiorly.2 The compensatory muscular activity required for shoulder stability induces secondary shoulder pain.5
The diagnosis is often delayed, as the clinical presentation may mimic the symptoms of shoulder joint or rotator cuff pathology. Although physical therapy resolves the pain and improves the function of the arm, mild endurance deficits and asymptomatic scapular winging may persist. Tendon transfer surgery is considered if adequate recovery is not achieved after a 6- to-24-month course of physical therapy.2
A 58-year-old woman who sustained right-sided traumatic rib fractures after falling down stairs 8 months earlier presented with right shoulder pain that had been present for 6 months. She received nonsteroidal anti-inflammatory drugs at another hospital, which were partially effective. Magnetic resonance imaging of the neck and right shoulder had shown no abnormalities.
On physical examination, her right scapula was found to protrude abnormally (ie, to “wing”) during forward flexion and abduction of the right arm (Figure 1). Electromyography showed evidence of right serratus anterior paralysis and denervation of the right long thoracic nerve, leading to a diagnosis of traumatic long thoracic nerve paralysis. A course of physical therapy was initiated to improve her symptoms.
LONG THORACIC NERVE PARALYSIS
Scapular winging is caused by dysfunction of any of the 3 main muscles that attach the scapula to the posterior thoracic wall—the serratus anterior, the trapezius, and the rhomboid. The problem is most often in the serratus anterior muscle, innervated by the long thoracic nerve, a pure motor nerve that originates from the fifth, sixth, and seventh cervical nerves and descends along the lateral thoracic wall.
Long thoracic nerve paralysis can have traumatic, nontraumatic, or iatrogenic causes. Traumatic injuries result from blunt trauma to the neck, shoulder girdle, and thorax, while nontraumatic causes include viral illness, toxic exposure, apical pulmonary tumor, and C7 radiculopathy.1–3 Iatrogenic injuries may be caused by mastectomy with axillary dissection, chest tube thoracostomy, first-rib resection, or scalenotomy, or occur after general anesthesia.1,2,4
Scapular winging due to paralysis of the serratus anterior muscle is accentuated by forward elevation and—particularly—by pushing against a wall, and the entire scapula is displaced more medially and superiorly.2 The compensatory muscular activity required for shoulder stability induces secondary shoulder pain.5
The diagnosis is often delayed, as the clinical presentation may mimic the symptoms of shoulder joint or rotator cuff pathology. Although physical therapy resolves the pain and improves the function of the arm, mild endurance deficits and asymptomatic scapular winging may persist. Tendon transfer surgery is considered if adequate recovery is not achieved after a 6- to-24-month course of physical therapy.2
- Vastamäki M, Kauppila LI. Etiologic factors in isolated paralysis of the serratus anterior muscle: a report of 197 cases. J Shoulder Elbow Surg 1993; 2:240–243.
- Martin RM, Fish DE. Scapular winging: anatomical review, diagnosis, and treatments. Curr Rev Musculoskelet Med 2008; 1:1–11.
- Toshkezi G, Dejesus J, Jabre JF, Hohler A, Davies K. Long thoracic neuropathy caused by an apical pulmonary tumor. J Neurosurg 2009; 110:754–757.
- Kauppila LI, Vastamäki M. Iatrogenic serratus anterior paralysis. Long-term outcome in 26 patients. Chest 1996; 109:31–34.
- Nath RK, Lyons AB, Bietz G. Microneurolysis and decompression of long thoracic nerve injury are effective in reversing scapular winging: long-term results in 50 cases. BMC Musculoskelet Disord 2007; 8:25.
- Vastamäki M, Kauppila LI. Etiologic factors in isolated paralysis of the serratus anterior muscle: a report of 197 cases. J Shoulder Elbow Surg 1993; 2:240–243.
- Martin RM, Fish DE. Scapular winging: anatomical review, diagnosis, and treatments. Curr Rev Musculoskelet Med 2008; 1:1–11.
- Toshkezi G, Dejesus J, Jabre JF, Hohler A, Davies K. Long thoracic neuropathy caused by an apical pulmonary tumor. J Neurosurg 2009; 110:754–757.
- Kauppila LI, Vastamäki M. Iatrogenic serratus anterior paralysis. Long-term outcome in 26 patients. Chest 1996; 109:31–34.
- Nath RK, Lyons AB, Bietz G. Microneurolysis and decompression of long thoracic nerve injury are effective in reversing scapular winging: long-term results in 50 cases. BMC Musculoskelet Disord 2007; 8:25.
Hypertrophic osteoarthropathy: Uncommon presentation of lung cancer
A 43-year-old woman presented to the clinic complaining of bilateral ankle joint pain for 2 months. She denied a history of fever, weight loss, addictions, cough, or trauma. On physical examinatio, she had swelling of the ankle and wrist joints and digital clubbing (Figure 1). Active movement of the ankles and wrists was restricted due to pain. The examination was otherwise unremarkable.
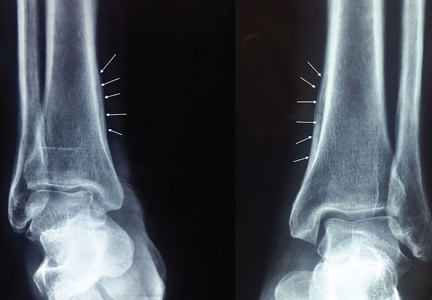
Radiography of both ankles showed a lamellar type periosteal reaction suggestive of periostitis (Figure 2). Computed tomography of the chest revealed a spiculated mass over the right lower lobe. Biopsy study of the mass was positive for squamous cell carcinoma. She was referred to the oncology center for further management.
FEATURES OF HYPERTROPHIC OSTEOARTHROPATHY
Digital clubbing is one of the oldest signs in clinical medicine. It is characterized by bulbous enlargement of the terminal segments of the fingers and toes due to proliferation of subungual connective tissue. It usually appears as a painless finger deformity and is clinically appreciated as a loss of the normal angle between the nail bed and proximal nail fold.
Hypertrophic osteoarthropathy is a symptomatic form of clubbing associated with proliferative periostosis of the distal end of long tubular bones, commonly those adjacent to the wrist and ankle joints.1 The laminated appearance of these bones on radiography is due to the excess connective tissue secondary to new osteoid material deposited under the periosteum.
There is evidence to suggest that clubbing and hypertrophic osteoarthropathy represent different stages of the same disease process.2 In most cases, finger deformity is the first manifestation; as the disease progresses, periostosis becomes evident.
Hypertrophic osteoarthropathy can be classified as primary or secondary. The primary form, also known as primary pachydermoperiostosis, is rare and constitutes only 3% of all cases.3 The exact cause is not yet known; it occurs as a hereditary disease with autosomal dominant inheritance with variable penetrance. Congenital clubbing without periostosis is of no clinical significance.4
CONDITIONS ASSOCIATED WITH CLUBBING
Primary bronchogenic carcinoma is the most common cause of clubbing and hypertrophic osteoarthropathy. In one retrospective series, 4.5% of patients with lung cancer had radiologic evidence of hypertrophic osteoarthropathy.5 Other malignancies associated with this condition are mesothelioma, hepatocellular carcinoma, and certain types of gastrointestinal adenocarcinoma.
Other conditions associated with clubbing include:
- Cardiovascular disease such as congenital cyanotic heart disease and infective endocarditis
- Gastrointestinal conditions such as cirrhosis, primary sclerosing cholangitis, Crohn disease, and ulcerative colitis
- Infections such as lung abscess and empyema.
Clubbing is generally bilaterally symmetrical. Asymmetric clubbing is rare and usually indicates impaired regional blood flow due to vascular disease. Unilateral clubbing or hypertrophic osteoarthropathy restricted to 1 upper limb can result from an anomaly of the aortic arch or from a subclavian or brachial artery aneurysm. Clubbing affecting predominantly the lower limbs has been reported in coarctation of aorta and patent ductus arteriosus.6 Rare cases of unidigital clubbing are reported in sarcoidosis.7
The importance of recognizing hypertrophic osteoarthropathy cannot be overemphasized. If any of the manifestations of the syndrome become evident in a previously healthy person, a thorough evaluation for an underlying disease should be done.
Clubbing should be differentiated from pseudoclubbing, which is seen in conditions such as hyperparathyroidism and scleroderma. The central mechanism for nail deformity in pseudoclubbing is acro-osteolysis with the resulting collapse of the subungual soft tissues. The important features differentiating it from true clubbing are preservation of the angle between the nail bed and proximal nail fold and asymmetric finger involvement.8
MANAGEMENT
The management of primary hypertrophic osteoarthropathy focuses on relieving the symptoms of periosteitis. Secondary forms require a detailed evaluation to rule out the underlying disease. In refractory cases, a bone-modifying agent (eg, zoledronic acid),9 octreotide,10 nonsteroidal anti-inflammatory drugs, or vagotomy11 may help.
- Martínez-Lavín M, Matucci-Cerinic M, Jajic I, Pineda C. Hypertrophic osteoarthropathy: consensus on its definition, classification, assessment and diagnostic criteria. J Rheumatol 1993; 20:1386–1387.
- Martínez-Lavín M. Digital clubbing and hypertrophic osteoarthropathy: a unifying hypothesis. J Rheumatol 1987; 14:6–8.
- Jajic Z, Jajic I, Nemcic T. Primary hypertrophic osteoarthropathy: clinical, radiologic, and scintigraphic characteristics. Arch Med Res 2001; 32:136–142.
- Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Izumi M, Takayama K, Yabuuchi H, Abe K, Nakanishi Y. Incidence of hypertrophic pulmonary osteoarthropathy associated with primary lung cancer. Respirology 2010; 15:809–812.
- Anoop TM, George KC. Images in clinical medicine. Differential clubbing and cyanosis. N Engl J Med 2011; 364:666.
- Singh A. Unidigital clubbing. Am J Med 2008; 121:e15.
- Santiago MB, Lima I, Feitosa AC, Braz Ade S, Miranda LG. Pseudoclubbing: is it different from clubbing? Semin Arthritis Rheum 2009; 38:452–457.
- Tachibana I, Gehi D, Rubin CD. Treatment of hypertrophic osteoarthropathy with underlying pulmonary adenocarcinoma using zoledronic acid. J Clin Rheumatol 2015; 21:333–334.
- Birch E, Jenkins D, Noble S. Treatment of painful hypertrophic osteoarthropathy associated with non-small cell lung cancer with octreotide: a case report and review of the literature. BMJ Support Palliat Care 2011; 1:189–192.
- Nguyen S, Hojjati M. Review of current therapies for secondary hypertrophic pulmonary osteoarthropathy. Clin Rheumatol 2011; 30:7–13.
A 43-year-old woman presented to the clinic complaining of bilateral ankle joint pain for 2 months. She denied a history of fever, weight loss, addictions, cough, or trauma. On physical examinatio, she had swelling of the ankle and wrist joints and digital clubbing (Figure 1). Active movement of the ankles and wrists was restricted due to pain. The examination was otherwise unremarkable.
Radiography of both ankles showed a lamellar type periosteal reaction suggestive of periostitis (Figure 2). Computed tomography of the chest revealed a spiculated mass over the right lower lobe. Biopsy study of the mass was positive for squamous cell carcinoma. She was referred to the oncology center for further management.
FEATURES OF HYPERTROPHIC OSTEOARTHROPATHY
Digital clubbing is one of the oldest signs in clinical medicine. It is characterized by bulbous enlargement of the terminal segments of the fingers and toes due to proliferation of subungual connective tissue. It usually appears as a painless finger deformity and is clinically appreciated as a loss of the normal angle between the nail bed and proximal nail fold.
Hypertrophic osteoarthropathy is a symptomatic form of clubbing associated with proliferative periostosis of the distal end of long tubular bones, commonly those adjacent to the wrist and ankle joints.1 The laminated appearance of these bones on radiography is due to the excess connective tissue secondary to new osteoid material deposited under the periosteum.
There is evidence to suggest that clubbing and hypertrophic osteoarthropathy represent different stages of the same disease process.2 In most cases, finger deformity is the first manifestation; as the disease progresses, periostosis becomes evident.
Hypertrophic osteoarthropathy can be classified as primary or secondary. The primary form, also known as primary pachydermoperiostosis, is rare and constitutes only 3% of all cases.3 The exact cause is not yet known; it occurs as a hereditary disease with autosomal dominant inheritance with variable penetrance. Congenital clubbing without periostosis is of no clinical significance.4
CONDITIONS ASSOCIATED WITH CLUBBING
Primary bronchogenic carcinoma is the most common cause of clubbing and hypertrophic osteoarthropathy. In one retrospective series, 4.5% of patients with lung cancer had radiologic evidence of hypertrophic osteoarthropathy.5 Other malignancies associated with this condition are mesothelioma, hepatocellular carcinoma, and certain types of gastrointestinal adenocarcinoma.
Other conditions associated with clubbing include:
- Cardiovascular disease such as congenital cyanotic heart disease and infective endocarditis
- Gastrointestinal conditions such as cirrhosis, primary sclerosing cholangitis, Crohn disease, and ulcerative colitis
- Infections such as lung abscess and empyema.
Clubbing is generally bilaterally symmetrical. Asymmetric clubbing is rare and usually indicates impaired regional blood flow due to vascular disease. Unilateral clubbing or hypertrophic osteoarthropathy restricted to 1 upper limb can result from an anomaly of the aortic arch or from a subclavian or brachial artery aneurysm. Clubbing affecting predominantly the lower limbs has been reported in coarctation of aorta and patent ductus arteriosus.6 Rare cases of unidigital clubbing are reported in sarcoidosis.7
The importance of recognizing hypertrophic osteoarthropathy cannot be overemphasized. If any of the manifestations of the syndrome become evident in a previously healthy person, a thorough evaluation for an underlying disease should be done.
Clubbing should be differentiated from pseudoclubbing, which is seen in conditions such as hyperparathyroidism and scleroderma. The central mechanism for nail deformity in pseudoclubbing is acro-osteolysis with the resulting collapse of the subungual soft tissues. The important features differentiating it from true clubbing are preservation of the angle between the nail bed and proximal nail fold and asymmetric finger involvement.8
MANAGEMENT
The management of primary hypertrophic osteoarthropathy focuses on relieving the symptoms of periosteitis. Secondary forms require a detailed evaluation to rule out the underlying disease. In refractory cases, a bone-modifying agent (eg, zoledronic acid),9 octreotide,10 nonsteroidal anti-inflammatory drugs, or vagotomy11 may help.
A 43-year-old woman presented to the clinic complaining of bilateral ankle joint pain for 2 months. She denied a history of fever, weight loss, addictions, cough, or trauma. On physical examinatio, she had swelling of the ankle and wrist joints and digital clubbing (Figure 1). Active movement of the ankles and wrists was restricted due to pain. The examination was otherwise unremarkable.
Radiography of both ankles showed a lamellar type periosteal reaction suggestive of periostitis (Figure 2). Computed tomography of the chest revealed a spiculated mass over the right lower lobe. Biopsy study of the mass was positive for squamous cell carcinoma. She was referred to the oncology center for further management.
FEATURES OF HYPERTROPHIC OSTEOARTHROPATHY
Digital clubbing is one of the oldest signs in clinical medicine. It is characterized by bulbous enlargement of the terminal segments of the fingers and toes due to proliferation of subungual connective tissue. It usually appears as a painless finger deformity and is clinically appreciated as a loss of the normal angle between the nail bed and proximal nail fold.
Hypertrophic osteoarthropathy is a symptomatic form of clubbing associated with proliferative periostosis of the distal end of long tubular bones, commonly those adjacent to the wrist and ankle joints.1 The laminated appearance of these bones on radiography is due to the excess connective tissue secondary to new osteoid material deposited under the periosteum.
There is evidence to suggest that clubbing and hypertrophic osteoarthropathy represent different stages of the same disease process.2 In most cases, finger deformity is the first manifestation; as the disease progresses, periostosis becomes evident.
Hypertrophic osteoarthropathy can be classified as primary or secondary. The primary form, also known as primary pachydermoperiostosis, is rare and constitutes only 3% of all cases.3 The exact cause is not yet known; it occurs as a hereditary disease with autosomal dominant inheritance with variable penetrance. Congenital clubbing without periostosis is of no clinical significance.4
CONDITIONS ASSOCIATED WITH CLUBBING
Primary bronchogenic carcinoma is the most common cause of clubbing and hypertrophic osteoarthropathy. In one retrospective series, 4.5% of patients with lung cancer had radiologic evidence of hypertrophic osteoarthropathy.5 Other malignancies associated with this condition are mesothelioma, hepatocellular carcinoma, and certain types of gastrointestinal adenocarcinoma.
Other conditions associated with clubbing include:
- Cardiovascular disease such as congenital cyanotic heart disease and infective endocarditis
- Gastrointestinal conditions such as cirrhosis, primary sclerosing cholangitis, Crohn disease, and ulcerative colitis
- Infections such as lung abscess and empyema.
Clubbing is generally bilaterally symmetrical. Asymmetric clubbing is rare and usually indicates impaired regional blood flow due to vascular disease. Unilateral clubbing or hypertrophic osteoarthropathy restricted to 1 upper limb can result from an anomaly of the aortic arch or from a subclavian or brachial artery aneurysm. Clubbing affecting predominantly the lower limbs has been reported in coarctation of aorta and patent ductus arteriosus.6 Rare cases of unidigital clubbing are reported in sarcoidosis.7
The importance of recognizing hypertrophic osteoarthropathy cannot be overemphasized. If any of the manifestations of the syndrome become evident in a previously healthy person, a thorough evaluation for an underlying disease should be done.
Clubbing should be differentiated from pseudoclubbing, which is seen in conditions such as hyperparathyroidism and scleroderma. The central mechanism for nail deformity in pseudoclubbing is acro-osteolysis with the resulting collapse of the subungual soft tissues. The important features differentiating it from true clubbing are preservation of the angle between the nail bed and proximal nail fold and asymmetric finger involvement.8
MANAGEMENT
The management of primary hypertrophic osteoarthropathy focuses on relieving the symptoms of periosteitis. Secondary forms require a detailed evaluation to rule out the underlying disease. In refractory cases, a bone-modifying agent (eg, zoledronic acid),9 octreotide,10 nonsteroidal anti-inflammatory drugs, or vagotomy11 may help.
- Martínez-Lavín M, Matucci-Cerinic M, Jajic I, Pineda C. Hypertrophic osteoarthropathy: consensus on its definition, classification, assessment and diagnostic criteria. J Rheumatol 1993; 20:1386–1387.
- Martínez-Lavín M. Digital clubbing and hypertrophic osteoarthropathy: a unifying hypothesis. J Rheumatol 1987; 14:6–8.
- Jajic Z, Jajic I, Nemcic T. Primary hypertrophic osteoarthropathy: clinical, radiologic, and scintigraphic characteristics. Arch Med Res 2001; 32:136–142.
- Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Izumi M, Takayama K, Yabuuchi H, Abe K, Nakanishi Y. Incidence of hypertrophic pulmonary osteoarthropathy associated with primary lung cancer. Respirology 2010; 15:809–812.
- Anoop TM, George KC. Images in clinical medicine. Differential clubbing and cyanosis. N Engl J Med 2011; 364:666.
- Singh A. Unidigital clubbing. Am J Med 2008; 121:e15.
- Santiago MB, Lima I, Feitosa AC, Braz Ade S, Miranda LG. Pseudoclubbing: is it different from clubbing? Semin Arthritis Rheum 2009; 38:452–457.
- Tachibana I, Gehi D, Rubin CD. Treatment of hypertrophic osteoarthropathy with underlying pulmonary adenocarcinoma using zoledronic acid. J Clin Rheumatol 2015; 21:333–334.
- Birch E, Jenkins D, Noble S. Treatment of painful hypertrophic osteoarthropathy associated with non-small cell lung cancer with octreotide: a case report and review of the literature. BMJ Support Palliat Care 2011; 1:189–192.
- Nguyen S, Hojjati M. Review of current therapies for secondary hypertrophic pulmonary osteoarthropathy. Clin Rheumatol 2011; 30:7–13.
- Martínez-Lavín M, Matucci-Cerinic M, Jajic I, Pineda C. Hypertrophic osteoarthropathy: consensus on its definition, classification, assessment and diagnostic criteria. J Rheumatol 1993; 20:1386–1387.
- Martínez-Lavín M. Digital clubbing and hypertrophic osteoarthropathy: a unifying hypothesis. J Rheumatol 1987; 14:6–8.
- Jajic Z, Jajic I, Nemcic T. Primary hypertrophic osteoarthropathy: clinical, radiologic, and scintigraphic characteristics. Arch Med Res 2001; 32:136–142.
- Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Izumi M, Takayama K, Yabuuchi H, Abe K, Nakanishi Y. Incidence of hypertrophic pulmonary osteoarthropathy associated with primary lung cancer. Respirology 2010; 15:809–812.
- Anoop TM, George KC. Images in clinical medicine. Differential clubbing and cyanosis. N Engl J Med 2011; 364:666.
- Singh A. Unidigital clubbing. Am J Med 2008; 121:e15.
- Santiago MB, Lima I, Feitosa AC, Braz Ade S, Miranda LG. Pseudoclubbing: is it different from clubbing? Semin Arthritis Rheum 2009; 38:452–457.
- Tachibana I, Gehi D, Rubin CD. Treatment of hypertrophic osteoarthropathy with underlying pulmonary adenocarcinoma using zoledronic acid. J Clin Rheumatol 2015; 21:333–334.
- Birch E, Jenkins D, Noble S. Treatment of painful hypertrophic osteoarthropathy associated with non-small cell lung cancer with octreotide: a case report and review of the literature. BMJ Support Palliat Care 2011; 1:189–192.
- Nguyen S, Hojjati M. Review of current therapies for secondary hypertrophic pulmonary osteoarthropathy. Clin Rheumatol 2011; 30:7–13.
Porcelain heart in a uremic patient
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
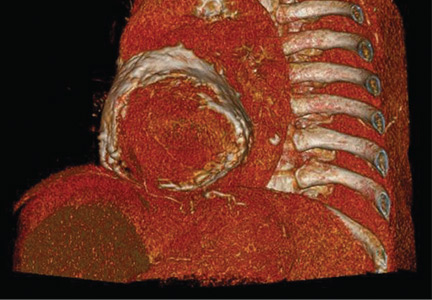
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
A 58-year-old man with end-stage renal disease due to diabetic nephropathy was admitted with aggravated exertional dyspnea and intermittent chest pain for 1 week. He had been on hemodialysis for 15 years.
His blood pressure was 124/69 mm Hg, pulse 96 beats per minute, and temperature 35.8°C. On physical examination, he had bilateral diffuse crackles, elevated jugular venous pressure (9.5 cm H2O) with positive hepatojugular reflux, and apparent dependent pedal edema. The Kussmaul sign was not observed.
Cardiac enzymes were in the normal range (creatine kinase 73 U/L, troponin I 0.032 ng/mL), but the brain-natriuretic peptide level was elevated at 340 pg/mL. Other laboratory findings included calcium 9 mg/dL (reference range 8.4–10.2 mg/dL), inorganic phosphate 5 mg/dL (2.5–4.5 mg/dL), and intact parathyroid hormone 1,457 pg/mL (10–69 pg/mL).
Electrocardiography showed sinus tachycardia with low voltage in diffuse leads and generalized flattening of the T wave. Chest radiography showed a bilateral reticulonodular pattern, mild costophrenic angle obliteration, and notable calcifications along the cardiac contour. Thoracic computed tomography showed a porcelain-like encasement of the heart (Figure 1). Transthoracic echocardiography showed thickened pericardium, pericardial calcification, and mild interventricular septal bounce in diastole, with no dyskinesia of ventricular wall motion. We decided not to perform an invasive hemodynamic assessment.
CAUSES OF PERICARDIAL CALCIFICATION
Pericardial calcification, abnormal calcium deposits in response to inflammation,1 has become more widely reported as the use of chest computed tomography has become more widespread. The common identifiable causes of pericardial calcification include recurrent or chronic pericarditis, radiation therapy for Hodgkin lymphoma or breast cancer, tuberculosis, and end-stage kidney disease.2,3 Other possible causes are retention of uremic metabolites, metastatic calcification induced by secondary hyperparathyroidism, and calcium-phosphate deposition induced by hyperphosphatemia.4
In chronic kidney disease, the amount of pericardial fluid and fibrinous pericardial deposition is thought to contribute to increased pericardial thickness and constriction. In some patients, pericardial calcification and thickening would lead to constrictive pericarditis, which could be confirmed by echocardiography and cardiac catheterization. About 25% to 50% of cases of pericardial calcification are complicated by constrictive pericarditis.5,6 Constrictive pericarditis occurs in up to 4% of patients with end-stage renal disease, even with successful dialysis.7
Partial clinical improvement may be obtained with intensive hemodialysis, strict volume control, and decreased catabolism in patients with multiple comorbidities.8 However, the definite treatment is total pericardiectomy, which reduces symptoms substantially and offers a favorable long-term outcome.7
SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common complication in patients with end-stage renal disease and is characterized by derangements in the homeostasis of calcium, phosphorus, and vitamin D.9
Because renal function is decreased, phosphate is retained and calcitriol synthesis is reduced, resulting in hypocalcemia, which induces parathyroid gland hyperplasia and parathyroid hormone secretion.10 Moreover, some patents with long-standing secondary hyperparathyroidism may develop tertiary hyperparathyroidism associated with autonomous parathyroid hormone secretion, hypercalcemia, and hyperphosphatemia.11
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommends screening for and managing secondary hyperparathyroidism in all patients with stage 3 chronic kidney disease (estimated glomerular filtration rate < 60 mL/min). In patients with stage 5 chronic kidney disease or on dialysis, the serum calcium and phosphorus levels should be monitored every 1 to 3 months and the parathyroid hormone levels every 3 to 6 months.12
According to KDIGO guidelines, the target level of calcium is less than 10.2 mg/dL, and the target phosphorus level is less than 4.6 mg/dL. The level of parathyroid hormone should be maintained at 2 to 9 times the upper limit of normal for the assay.
The management of secondary hyperparathyroidism includes a low-phosphorus diet, calcium-containing or calcium-free phosphate binders, a calcitriol supplement, and calcimimetics. If medical treatment fails and manifestations are significant, parathyroidectomy may be indicated.13
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
- Alpert MA, Ravenscraft MD. Pericardial involvement in end-stage renal disease. Am J Med Sci 2003; 325:228–236.
- Gowda RM, Boxt LM. Calcifications of the heart. Radiol Clin North Am 2004; 42:603–617.
- Kleynberg RL, Kleynberg VM, Kleynberg LM, Farahmandian D. Chronic constrictive pericarditis in association with end-stage renal disease. Int J Nephrol 2011; 2011:469602.
- Rao N, Crail S. Metastatic calcification and long-term hemodialysis. N Engl J Med 2013; 368:2415.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100:1380–1386.
- Bergman M, Vitrai J, Salman H. Constrictive pericarditis: a reminder of a not so rare disease. Eur J Intern Med 2006; 17:457–464.
- Szabó G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothorac Surg 2013; 44:1023–1028.
- Feldman V, Dovrish Z, Weisenberg N, Neuman Y, Amital H. Uremic pericarditis. Isr Med Assoc J 2011; 13:256–257.
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38.
- Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 2007; 18:875–885.
- Kerby J, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Operative treatment of tertiary hyperparathyroidism: a single-center experience. Ann Surg 1998; 227:878–886.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease—mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; 76:S1–130.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 suppl 3):S1–201.
Disseminated molluscum contagiosum lesions in an HIV patient
A 37-year-old woman with a 3-month history of disorientation and depression was admitted to the infectious disease unit. In addition, she had had multiple painless exophytic lesions on the face, abdomen, and genital area for the past 3 years.
Physical examination revealed multiple waxy lesions, which were skin-colored dome-shaped papules with an umbilicated top, with diameters of 2 to 10 mm (Figure 1).
Skin biopsy study (Figure 2) showed lobulated endophytic hyperplasia with an intradermal pseudotumor (Henderson-Paterson bodies). Dimorphic fungal infection with Cryptococcus species was excluded. A final diagnosis of molluscum contagiosum was made based on the clinical appearance of the lesions and the histologic findings.
The patient was known to be positive for human immunodeficiency virus (HIV) and to have discontinued medications and follow-up visits in 2011. She was severely immunodepressed, at stage C3 (the worst stage) in the US Centers for Disease Control and Prevention classification. Her CD4 cell count was 26 × 106/L (reference range 533–1,674) and 11% (34%–61%); her viral load was 252,085 copies/mm3.
Subsequently, she was diagnosed with HIV-related encephalopathy and disseminated Mycobacterium tuberculosis infection. Highly active antiretoroviral therapy (HAART) and tuberculosis treatment were started.
LINKED TO IMMUNOCOMPROMISE
Molluscum contagiosum virus is an important human skin pathogen. Transmitted through direct skin-to-skin contact, it can cause disfigurement and suffering in affected patients. It often affects children, but abundant or atypical lesions in an adult usually indicate underlying immunodeficiency1 and are usually related to impaired cell-mediated immunity.2
In the mid-1980s, atypical molluscum contagiosum was recognized as a feature of HIV infection,3 but with widespread use of HAART, lesions are now less frequently observed in Western countries. Cases of molluscum contagiosum have also been reported in patients receiving immunosuppressive drugs such as methrotrexate and tumor necrosis factor alpha inhibitors.4 A high burden of lesions such as our patient had is uncommon.
Optimal treatment in HIV patients is restoration of immunologic competence with HAART. Adjunctive treatment with surgical excision, curettage, cryotherapy, and various chemical removal methods can also be applied.4,5 Severe infection secondary to iatrogenic immunosuppression may be resistant to standard therapy, and when the condition does not respond to combination treatment, withdrawal of immunosuppressive therapies may be necessary.4
The bottom line. Molluscum contagiosum is less frequently seen in the HAART era; however, when present it usually indicates a high level of immunosuppression. Clinicians need to keep the relation in mind.
Acknowledgment: We thank Dr. Isabel Faro Viana for the histologic image.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–888.
- Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 1998; 13:131–136.
- Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J 2016; 22:pii:13030/qt8vz669cj.
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS 2008; 19:503–506.
- Filo-Rogulska M, Pindycka-Piaszczynska M, Januszewski K, Jarzab J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol 2013; 30:56–58.
A 37-year-old woman with a 3-month history of disorientation and depression was admitted to the infectious disease unit. In addition, she had had multiple painless exophytic lesions on the face, abdomen, and genital area for the past 3 years.
Physical examination revealed multiple waxy lesions, which were skin-colored dome-shaped papules with an umbilicated top, with diameters of 2 to 10 mm (Figure 1).
Skin biopsy study (Figure 2) showed lobulated endophytic hyperplasia with an intradermal pseudotumor (Henderson-Paterson bodies). Dimorphic fungal infection with Cryptococcus species was excluded. A final diagnosis of molluscum contagiosum was made based on the clinical appearance of the lesions and the histologic findings.
The patient was known to be positive for human immunodeficiency virus (HIV) and to have discontinued medications and follow-up visits in 2011. She was severely immunodepressed, at stage C3 (the worst stage) in the US Centers for Disease Control and Prevention classification. Her CD4 cell count was 26 × 106/L (reference range 533–1,674) and 11% (34%–61%); her viral load was 252,085 copies/mm3.
Subsequently, she was diagnosed with HIV-related encephalopathy and disseminated Mycobacterium tuberculosis infection. Highly active antiretoroviral therapy (HAART) and tuberculosis treatment were started.
LINKED TO IMMUNOCOMPROMISE
Molluscum contagiosum virus is an important human skin pathogen. Transmitted through direct skin-to-skin contact, it can cause disfigurement and suffering in affected patients. It often affects children, but abundant or atypical lesions in an adult usually indicate underlying immunodeficiency1 and are usually related to impaired cell-mediated immunity.2
In the mid-1980s, atypical molluscum contagiosum was recognized as a feature of HIV infection,3 but with widespread use of HAART, lesions are now less frequently observed in Western countries. Cases of molluscum contagiosum have also been reported in patients receiving immunosuppressive drugs such as methrotrexate and tumor necrosis factor alpha inhibitors.4 A high burden of lesions such as our patient had is uncommon.
Optimal treatment in HIV patients is restoration of immunologic competence with HAART. Adjunctive treatment with surgical excision, curettage, cryotherapy, and various chemical removal methods can also be applied.4,5 Severe infection secondary to iatrogenic immunosuppression may be resistant to standard therapy, and when the condition does not respond to combination treatment, withdrawal of immunosuppressive therapies may be necessary.4
The bottom line. Molluscum contagiosum is less frequently seen in the HAART era; however, when present it usually indicates a high level of immunosuppression. Clinicians need to keep the relation in mind.
Acknowledgment: We thank Dr. Isabel Faro Viana for the histologic image.
A 37-year-old woman with a 3-month history of disorientation and depression was admitted to the infectious disease unit. In addition, she had had multiple painless exophytic lesions on the face, abdomen, and genital area for the past 3 years.
Physical examination revealed multiple waxy lesions, which were skin-colored dome-shaped papules with an umbilicated top, with diameters of 2 to 10 mm (Figure 1).
Skin biopsy study (Figure 2) showed lobulated endophytic hyperplasia with an intradermal pseudotumor (Henderson-Paterson bodies). Dimorphic fungal infection with Cryptococcus species was excluded. A final diagnosis of molluscum contagiosum was made based on the clinical appearance of the lesions and the histologic findings.
The patient was known to be positive for human immunodeficiency virus (HIV) and to have discontinued medications and follow-up visits in 2011. She was severely immunodepressed, at stage C3 (the worst stage) in the US Centers for Disease Control and Prevention classification. Her CD4 cell count was 26 × 106/L (reference range 533–1,674) and 11% (34%–61%); her viral load was 252,085 copies/mm3.
Subsequently, she was diagnosed with HIV-related encephalopathy and disseminated Mycobacterium tuberculosis infection. Highly active antiretoroviral therapy (HAART) and tuberculosis treatment were started.
LINKED TO IMMUNOCOMPROMISE
Molluscum contagiosum virus is an important human skin pathogen. Transmitted through direct skin-to-skin contact, it can cause disfigurement and suffering in affected patients. It often affects children, but abundant or atypical lesions in an adult usually indicate underlying immunodeficiency1 and are usually related to impaired cell-mediated immunity.2
In the mid-1980s, atypical molluscum contagiosum was recognized as a feature of HIV infection,3 but with widespread use of HAART, lesions are now less frequently observed in Western countries. Cases of molluscum contagiosum have also been reported in patients receiving immunosuppressive drugs such as methrotrexate and tumor necrosis factor alpha inhibitors.4 A high burden of lesions such as our patient had is uncommon.
Optimal treatment in HIV patients is restoration of immunologic competence with HAART. Adjunctive treatment with surgical excision, curettage, cryotherapy, and various chemical removal methods can also be applied.4,5 Severe infection secondary to iatrogenic immunosuppression may be resistant to standard therapy, and when the condition does not respond to combination treatment, withdrawal of immunosuppressive therapies may be necessary.4
The bottom line. Molluscum contagiosum is less frequently seen in the HAART era; however, when present it usually indicates a high level of immunosuppression. Clinicians need to keep the relation in mind.
Acknowledgment: We thank Dr. Isabel Faro Viana for the histologic image.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–888.
- Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 1998; 13:131–136.
- Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J 2016; 22:pii:13030/qt8vz669cj.
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS 2008; 19:503–506.
- Filo-Rogulska M, Pindycka-Piaszczynska M, Januszewski K, Jarzab J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol 2013; 30:56–58.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis 2013; 13:877–888.
- Jung AC, Paauw DS. Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 1998; 13:131–136.
- Beutler BD, Cohen PR. Molluscum contagiosum of the eyelid: case report in a man receiving methotrexate and literature review of molluscum contagiosum in patients who are immunosuppressed secondary to methotrexate or HIV infection. Dermatol Online J 2016; 22:pii:13030/qt8vz669cj.
- Gur I. The epidemiology of Molluscum contagiosum in HIV-seropositive patients: a unique entity or insignificant finding? Int J STD AIDS 2008; 19:503–506.
- Filo-Rogulska M, Pindycka-Piaszczynska M, Januszewski K, Jarzab J. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol 2013; 30:56–58.
Worsening migraine due to neurocysticercosis
A 35-year-old woman with a history of migraine presented with a headache that had worsened over the past 2 weeks. The headache was occipital and was associated with blurred vision, photophobia, tingling of the hands, episodes of flashing lights and images, and difficulty concentrating. The headache was similar to her typical migraines, but with the addition of flashing lights and images.
Her medical history included a cystic mass in the right occipital lobe that had been found incidentally on magnetic resonance imaging (MRI) during a workup for pituitary adenoma. The mass was thought to be a congenital lesion or arachnoid cyst, and intermittent screening had been recommended.
The patient had grown up in Honduras and had lived in the jungle until age 12, when she moved to the United States.
EVALUATION AND MANAGEMENT
Physical examination was remarkable for partial visual field loss in the periphery of the left temporal quadrant in both eyes (partial homonymous hemianopia). Repeat MRI showed a cystic lesion with scolex (the anterior end of a tapeworm) in the right occipital lobe, with surrounding edema (Figure 1).
Cystic brain lesions are associated with arachnoid cyst, glioma, and malignancy, but the presence of the scolex placed neurocysticercosis as the leading diagnosis. Testing for cysticercus antibody was negative. This test was done in the hope of confirming our high suspicion; while a negative test result does not exclude this diagnosis, a positive test would have been helpful to corroborate what we suspected. However, her imaging and clinical features were sufficient to warrant treating her for neurocysticercosis
She was treated with albendazole 400 mg twice a day for 10 days, and prednisone 1 mg/kg/day for 10 days followed by a taper. Because of the frequency with which neurocysticercosis causes seizures, an antiepileptic drug is also recommended, at least until active lesions have subsided.1 In this patient, levetiracetam 1,000 mg twice a day was prescribed for 6 months for seizure prophylaxis.
Repeat MRI 2 months later showed improvement (Figure 2). Her acute neurologic signs and symptoms had resolved, but she continued to be followed for chronic migraines (Figure 3). She has had no seizures despite weaning from levetiracetam.
TAPEWORM AND MIGRAINE
Neurocysticercosis is caused by the cestode Taenia solium, acquired by eating undercooked pork contaminated with the cysts or eggs.1 The oncospheres released by the eggs migrate through the host body and encyst in end organs.
Neuroimaging can show 4 stages of the cysts—vesicular with living larva, colloidal with larva degeneration, granulonodular with thickening of the cyst, and calcification.1
For patients who have lived in or visited high-risk areas of the world such as Central America, South America, sub-Saharan Africa, India, and Asia, it is important to include neurocysticercosis in the differential diagnosis of migraine with focal deficits or migraine with an evolving quality. Encysted larvae can remain asymptomatic for years but can cause brain edema, often leading to seizures.
Serum testing for cysticercus antibody can indicate acute infection, chronic infection, and possibly the immune response to treatment; however, serum testing has limited sensitivity in patients who have single or calcified lesions.2 A negative test result does not exclude infection and is more likely to be a false negative in patients with a single or calcified lesion.
Current treatment guidelines recommend albendazole 400 mg twice daily along with dexamethasone or prednisolone to decrease the number of cysts and the development of lesional epilepsy.1 Albendazole in combination with praziquantel 50 mg/kg/day kills more cysts than albendazole alone and should be considered in patients with more than 2 cysts.3
- Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–1429.
- Garcia HH, Wittner M, Coyle CM, Tanowitz HB, White AC Jr. Cysticercosis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:1289–1303.
- Garcia HH, Gonzales I, Lescano AG, et al; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687–695.
A 35-year-old woman with a history of migraine presented with a headache that had worsened over the past 2 weeks. The headache was occipital and was associated with blurred vision, photophobia, tingling of the hands, episodes of flashing lights and images, and difficulty concentrating. The headache was similar to her typical migraines, but with the addition of flashing lights and images.
Her medical history included a cystic mass in the right occipital lobe that had been found incidentally on magnetic resonance imaging (MRI) during a workup for pituitary adenoma. The mass was thought to be a congenital lesion or arachnoid cyst, and intermittent screening had been recommended.
The patient had grown up in Honduras and had lived in the jungle until age 12, when she moved to the United States.
EVALUATION AND MANAGEMENT
Physical examination was remarkable for partial visual field loss in the periphery of the left temporal quadrant in both eyes (partial homonymous hemianopia). Repeat MRI showed a cystic lesion with scolex (the anterior end of a tapeworm) in the right occipital lobe, with surrounding edema (Figure 1).

Cystic brain lesions are associated with arachnoid cyst, glioma, and malignancy, but the presence of the scolex placed neurocysticercosis as the leading diagnosis. Testing for cysticercus antibody was negative. This test was done in the hope of confirming our high suspicion; while a negative test result does not exclude this diagnosis, a positive test would have been helpful to corroborate what we suspected. However, her imaging and clinical features were sufficient to warrant treating her for neurocysticercosis
She was treated with albendazole 400 mg twice a day for 10 days, and prednisone 1 mg/kg/day for 10 days followed by a taper. Because of the frequency with which neurocysticercosis causes seizures, an antiepileptic drug is also recommended, at least until active lesions have subsided.1 In this patient, levetiracetam 1,000 mg twice a day was prescribed for 6 months for seizure prophylaxis.
Repeat MRI 2 months later showed improvement (Figure 2). Her acute neurologic signs and symptoms had resolved, but she continued to be followed for chronic migraines (Figure 3). She has had no seizures despite weaning from levetiracetam.


TAPEWORM AND MIGRAINE
Neurocysticercosis is caused by the cestode Taenia solium, acquired by eating undercooked pork contaminated with the cysts or eggs.1 The oncospheres released by the eggs migrate through the host body and encyst in end organs.
Neuroimaging can show 4 stages of the cysts—vesicular with living larva, colloidal with larva degeneration, granulonodular with thickening of the cyst, and calcification.1
For patients who have lived in or visited high-risk areas of the world such as Central America, South America, sub-Saharan Africa, India, and Asia, it is important to include neurocysticercosis in the differential diagnosis of migraine with focal deficits or migraine with an evolving quality. Encysted larvae can remain asymptomatic for years but can cause brain edema, often leading to seizures.
Serum testing for cysticercus antibody can indicate acute infection, chronic infection, and possibly the immune response to treatment; however, serum testing has limited sensitivity in patients who have single or calcified lesions.2 A negative test result does not exclude infection and is more likely to be a false negative in patients with a single or calcified lesion.
Current treatment guidelines recommend albendazole 400 mg twice daily along with dexamethasone or prednisolone to decrease the number of cysts and the development of lesional epilepsy.1 Albendazole in combination with praziquantel 50 mg/kg/day kills more cysts than albendazole alone and should be considered in patients with more than 2 cysts.3
A 35-year-old woman with a history of migraine presented with a headache that had worsened over the past 2 weeks. The headache was occipital and was associated with blurred vision, photophobia, tingling of the hands, episodes of flashing lights and images, and difficulty concentrating. The headache was similar to her typical migraines, but with the addition of flashing lights and images.
Her medical history included a cystic mass in the right occipital lobe that had been found incidentally on magnetic resonance imaging (MRI) during a workup for pituitary adenoma. The mass was thought to be a congenital lesion or arachnoid cyst, and intermittent screening had been recommended.
The patient had grown up in Honduras and had lived in the jungle until age 12, when she moved to the United States.
EVALUATION AND MANAGEMENT
Physical examination was remarkable for partial visual field loss in the periphery of the left temporal quadrant in both eyes (partial homonymous hemianopia). Repeat MRI showed a cystic lesion with scolex (the anterior end of a tapeworm) in the right occipital lobe, with surrounding edema (Figure 1).

Cystic brain lesions are associated with arachnoid cyst, glioma, and malignancy, but the presence of the scolex placed neurocysticercosis as the leading diagnosis. Testing for cysticercus antibody was negative. This test was done in the hope of confirming our high suspicion; while a negative test result does not exclude this diagnosis, a positive test would have been helpful to corroborate what we suspected. However, her imaging and clinical features were sufficient to warrant treating her for neurocysticercosis
She was treated with albendazole 400 mg twice a day for 10 days, and prednisone 1 mg/kg/day for 10 days followed by a taper. Because of the frequency with which neurocysticercosis causes seizures, an antiepileptic drug is also recommended, at least until active lesions have subsided.1 In this patient, levetiracetam 1,000 mg twice a day was prescribed for 6 months for seizure prophylaxis.
Repeat MRI 2 months later showed improvement (Figure 2). Her acute neurologic signs and symptoms had resolved, but she continued to be followed for chronic migraines (Figure 3). She has had no seizures despite weaning from levetiracetam.


TAPEWORM AND MIGRAINE
Neurocysticercosis is caused by the cestode Taenia solium, acquired by eating undercooked pork contaminated with the cysts or eggs.1 The oncospheres released by the eggs migrate through the host body and encyst in end organs.
Neuroimaging can show 4 stages of the cysts—vesicular with living larva, colloidal with larva degeneration, granulonodular with thickening of the cyst, and calcification.1
For patients who have lived in or visited high-risk areas of the world such as Central America, South America, sub-Saharan Africa, India, and Asia, it is important to include neurocysticercosis in the differential diagnosis of migraine with focal deficits or migraine with an evolving quality. Encysted larvae can remain asymptomatic for years but can cause brain edema, often leading to seizures.
Serum testing for cysticercus antibody can indicate acute infection, chronic infection, and possibly the immune response to treatment; however, serum testing has limited sensitivity in patients who have single or calcified lesions.2 A negative test result does not exclude infection and is more likely to be a false negative in patients with a single or calcified lesion.
Current treatment guidelines recommend albendazole 400 mg twice daily along with dexamethasone or prednisolone to decrease the number of cysts and the development of lesional epilepsy.1 Albendazole in combination with praziquantel 50 mg/kg/day kills more cysts than albendazole alone and should be considered in patients with more than 2 cysts.3
- Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–1429.
- Garcia HH, Wittner M, Coyle CM, Tanowitz HB, White AC Jr. Cysticercosis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:1289–1303.
- Garcia HH, Gonzales I, Lescano AG, et al; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687–695.
- Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013; 80:1424–1429.
- Garcia HH, Wittner M, Coyle CM, Tanowitz HB, White AC Jr. Cysticercosis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Philadelphia, PA: Elsevier Churchill Livingstone; 2006:1289–1303.
- Garcia HH, Gonzales I, Lescano AG, et al; Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double blind, randomized controlled trial. Lancet Infect Dis 2014; 14:687–695.
Ring-enhancing cerebral lesions
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
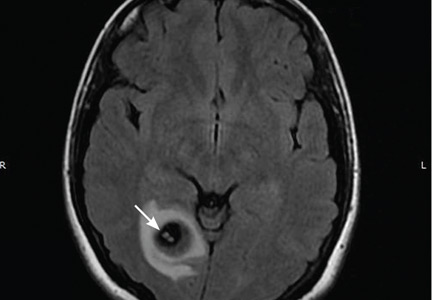
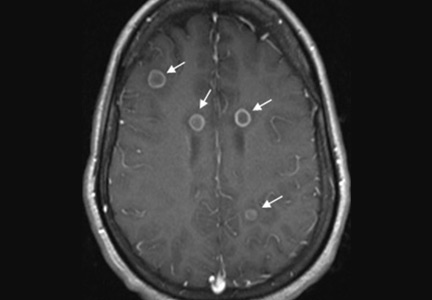
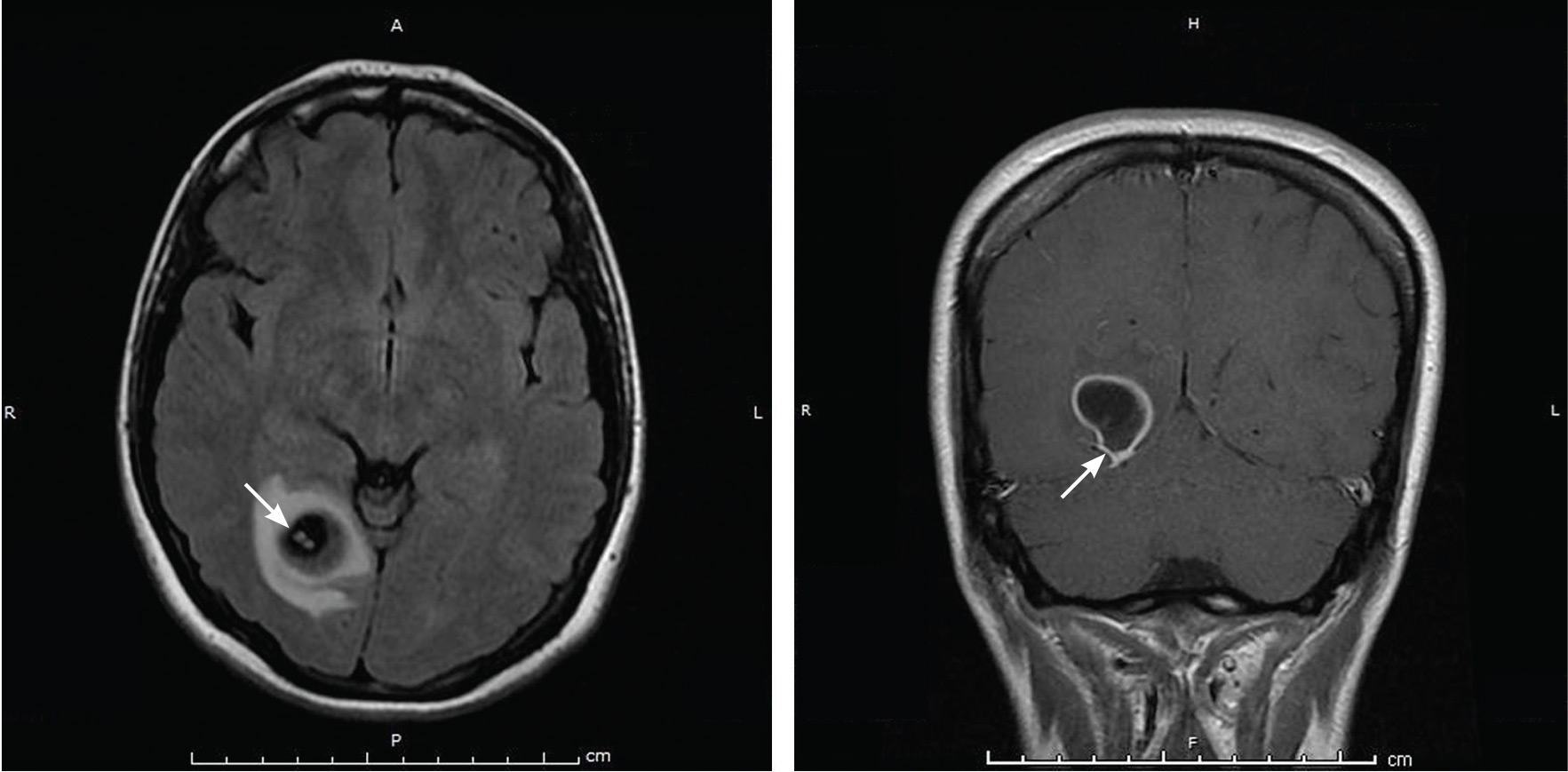
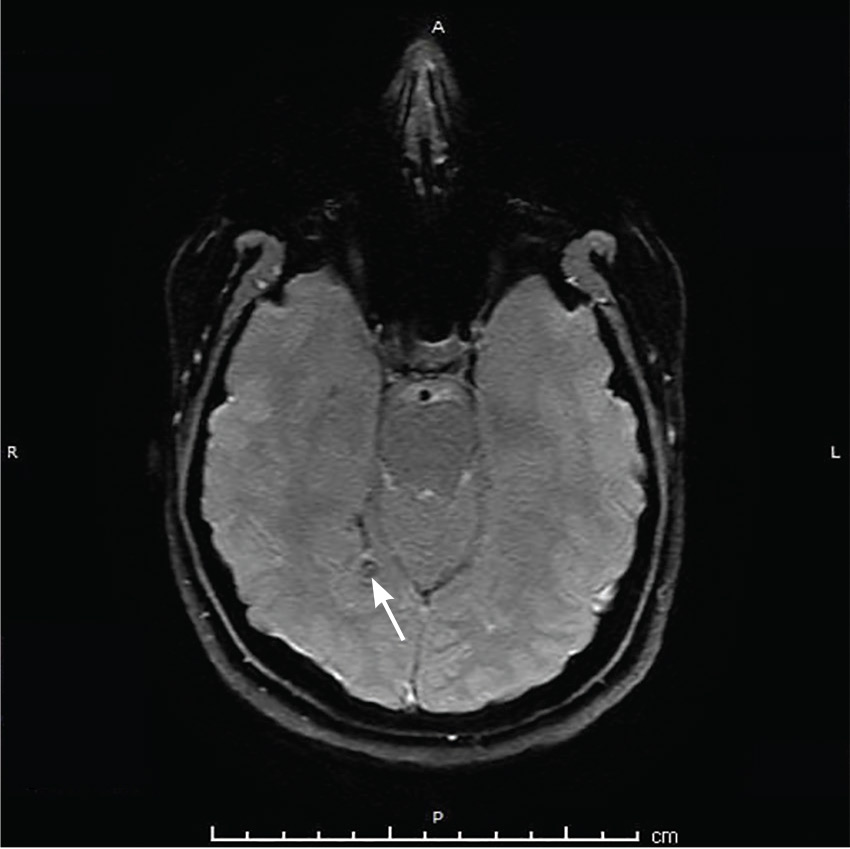
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
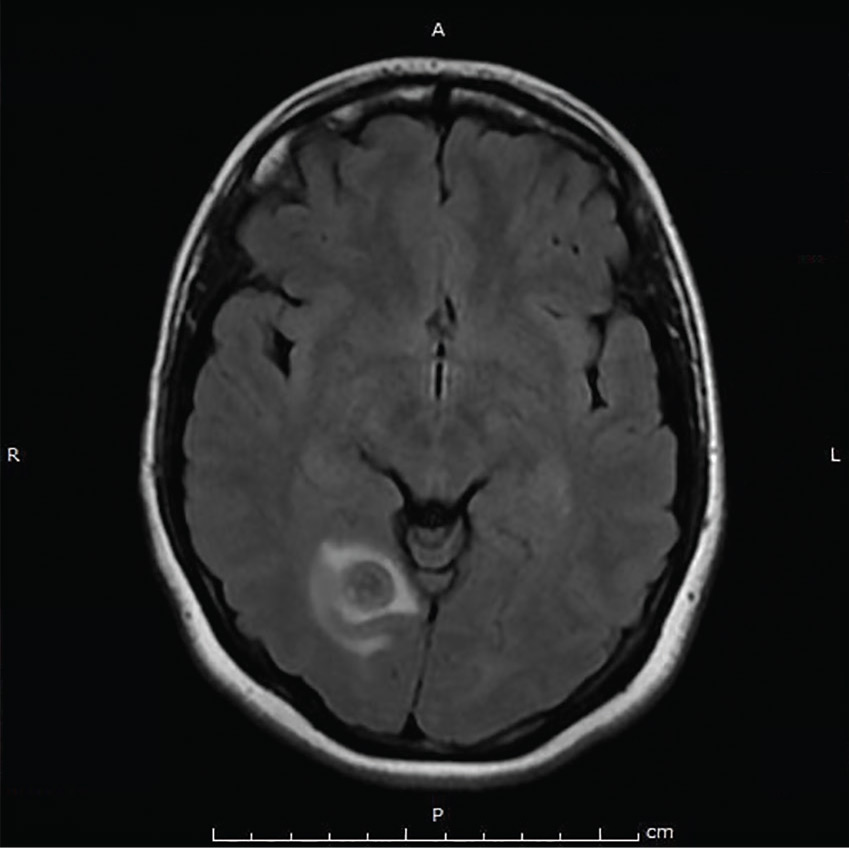
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.