User login
Racial disparities in cesarean delivery rates
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
Cutaneous Manifestations in Hereditary Alpha Tryptasemia
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Practice Points
- Chronic or episodic urticaria, flushing, and pruritus are the most consistent cutaneous abnormalities associated with hereditary alpha tryptasemia (HaT), but HaT also may augment symptoms of other underlying inflammatory skin disorders, such as atopic dermatitis and psoriasis.
- Individuals with episodic dermatologic manifestations indicative of mast cell activation accompanied by symptoms affecting 1 or more organ systems should be evaluated for mast cell activation syndrome as well as HaT.
ObGyns united in a divided post-Dobbs America
While many anticipated the fall of Roe v Wade after the leaked Supreme Court of the United States (SCOTUS) decision in the Dobbs v Jackson case, few may have fully comprehended the myriad of ways this ruling would create a national health care crisis overnight. Since the ruling, abortion has been banned, or a 6-week gestational age limit has been implemented, in a total of 13 states, all within the South
The 2022 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting, held shortly after the leaked SCOTUS opinion, was unlike most others. ACOG staff appropriately recognized the vastly different ways this ruling would affect patients and providers alike, simply based on the states in which they reside. ACOG staff organized the large group of attendees according to self-identified status (ie, whether they worked in states with protected, restricted, or threatened access to abortion care). Since this is such a vast topic, attendees also were asked to identify an area upon which to focus, such as the provision of health care, advocacy, or education. As a clinician practicing in Massachusetts, Dr. Bradley found herself meeting with an ACOG leader from California as they brainstormed how to best help our own communities. In conversing with attendees from other parts of the country, it became apparent the challenges others would be facing elsewhere were far more substantive than those we would be facing in “blue states.” After the Dobbs ruling, those predictions became harsh realities.
As we begin to see and hear reports of the devastating consequences of this ruling in “red states,” those of us in protected states have been struggling to try and ascertain how to help. Many of us have worked with our own legislatures to further enshrine protections for our patients and clinicians. New York and Massachusetts exemplify these efforts.6,7 These legislative efforts have included liability protections for patients and their clinicians who care for those who travel from restricted to protected states. Others involve codifying the principles of Roe and clarifying existing law to improve access. An online fundraiser organized by physicians to assist Dr. Bernard with her legal costs as she faces politically motivated investigation by Indiana state authorities has raised more than $260,000.8 Many expressed the potential legal and medical peril for examiners and examinees if the American Board of Obstetrics and Gynecology held in-person oral examinations in Texas as previously scheduled.9 An online petition to change the format to virtual had 728 signatories, and the format was changed back to virtual.10
The implications on medical schools, residencies, and fellowships cannot be overstated. The Dobbs ruling almost immediately affected nearly half of the training programs, which is particularly problematic given the Accreditation Council for Graduate Medical Education requirement that all ObGyn residents have access to abortion training.11 Other programs already are starting to try to meet this vast training need. The University of California San Francisco started offering training to physicians from Texas who were affected by the strict restrictions that predated Dobbs in the SB8 legislation, which turned ordinary citizens into vigilantes.12
ACOG has created an online resource (https://www.acog.org/advocacy/abortion-is-essential) with a number of different sections regarding clinical care, education and training, advocacy at the state level, and how to use effective language when talking about abortion in our communities. Planned Parenthood also suggests a myriad of ways those directly and indirectly affected could get involved:
- Donate to the National Network of Abortion Funds. This fund (https://secure.actblue.com/donate/fundabortionnow) facilitates care for those without the financial means to obtain it, supporting travel, lodging, and child care.
- Share #AbortionAccess posts on social media. These stories are a powerful reminder of the incredibly harmful impact this legislation can have on our patients.
- Donate to the If When How’s Legal Repro Defense Fund (https:/www.ifwhenhow.org/), which helps cover legal costs for those facing state persecution related to reproductive health care.
- Volunteer to help protect abortion health care at the state level.
- Engage with members of Congress in their home districts. (https://www.congress.gov/members/find-your-member)
- Contact the Planned Parenthood Local Engagement Team to facilitate your group, business, or organization’s involvement.
- Partner. Facilitate your organization and other companies to partner with Planned Parenthood and sign up for Bans off our Bodies (https://docs.google.com/forms/d/e/1FAIpQLSdrmxwMcwNXJ8I NE8S2gYjDDXuT76ws_Fr7CLm3 qbtR8dcZHw/viewform).
- Record your perspective about abortion (https://www.together.plannedparenthood.org/articles/6-share-abortion-story), whether it’s having had one, supported someone who had one, or advocated for others to have access to the procedure.13
ACOG also outlines several ways those of us in protected states could help shape the landscape in other communities in addition to advocating for state medical society resolutions, writing op-eds and letters to the editor, and utilizing ACOG’s social media graphics.14 In recognition of the often sensitive, polarizing nature of these discussions, ACOG is offering a workshop entitled “Building Evidence-Based Skills for Effective Conversations about Abortion.”15
Abortion traditionally was a policy issue other medical organizations shied away from developing official policy on and speaking out in support of, but recognizing the devastating scope of the public health crisis, 75 medical professional organizations recently released a strongly worded joint statement noting, “As leading medical and health care organizations dedicated to patient care and public health, we condemn this and all interference in the patient–clinician relationship.”16 Clinicians could work to expand this list to include all aspects of organized medicine. Initiatives to get out the vote may be helpful in vulnerable states, as well.
Clinicians in protected states are not necessarily directly affected in our daily interactions with patients, but we stand in solidarity with those who are. We must remain united as a profession as different state legislatures seek to divide us. We must support those who are struggling every day. Our colleagues and fellow citizens deserve nothing less. ●
- Tracking the states where abortion is now banned. New York Times. November 23, 2022. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Accessed November 28, 2022.
- Stanton A. ‘She’s 10’: child rape victims abortion denial spreads outrage on Twitter. Newsweek. July 2, 2022. https://www.newsweek.com/shes-10-child-rape-victims-abortion-denial-sparks-outrage-twitter-1721248. Accessed November 6, 2022.
- Judge-Golden C, Flink-Bochacki R. The burden of abortion restrictions and conservative diagnostic guidelines on patient-centered care for early pregnancy loss. Obstet Gynecol 2021;138:467071.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1. doi:10.1016/j.ajog.2022.06.060.
- Winter J. The Dobbs decision has unleashed legal chaos for doctors and patients. The New Yorker. July 2, 2022. https://www.newyorker.com/news/news-desk/the-dobbs-decision-has-unleashed-legal-chaos-for-doctors-and-patients. Accessed November 6, 2022.
- Lynch B, Mallow M, Bodde K, et al. Addressing a crisis in abortion access: a case study in advocacy. Obstet Gynecol. 2022;140:110-114.
- Evans M, Bradley T, Ireland L, et al. How the fall of Roe could change abortion care in Mass. Cognoscenti. July 26, 2022. https://www.wbur.org/cognoscenti/2022/07/26/dobbs-roe-abortion-massachusetts-megan-l-evans-erin-t-bradley-luu-ireland-chloe-zera. Accessed November 6, 2022.
- Spocchia G. Over $200k raised for doctor who performed abortion on 10-year-old rape victim. Independent. July 18, 2022. https://www.independent.co.uk/news/world/americas/fundriaser-ohio-abortion-doctor-rape-b2125621.html. Accessed November 6, 2022.
- ABOG petition: convert to online examination to protect OBGYN providers. Change.org website. https://www.change.org/p/abog-petition?original_footer_petition_id=33459909&algorithm=promoted&source_location=petition_footer&grid_position=8&pt=AVBldGl0aW9uAHgWBQIAAAAAYs65vIyhbUxhZGM0MWVhZg%3D%3D. Accessed November 6, 2022.
- D’Ambrosio A. Ob/Gyn board certification exam stays virtual in light of Dobbs. MedPageToday. July 15, 2022. https://www.medpagetoday.com/special-reports/features/99758. Accessed November 6, 2022.
- Weiner S. How the repeal of Roe v. Wade will affect training in abortion and reproductive health. AAMC News. June 24, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health. Accessed November 6, 2022.
- Anderson N. The fall of Roe scrambles abortion training for university hospitals. The Washington Post. June 30, 2022. https://www.washingtonpost.com/education/2022/06/30/abortion-training-upheaval-dobbs/. Accessed November 6, 2022.
- Bans off our bodies. Planned Parenthood website. https://www.plannedparenthoodaction.org/rightfully-ours/bans-off-our-bodies. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Shape the public discourse. ACOG website. https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Building evidence-based skills for effective conversations about abortion. ACOG website. https://www.acog.org/programs/impact/activities-initiatives/building-evidence-based-skills-for-effective-conversations-about-abortion. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. More than 75 health care organizations release joint statement in opposition to legislative interference. ACOG website. Published July 7, 2022. https://www.acog.org/news/news-releases/2022/07/more-than-75-health-care-organizations-release-joint-statement-in-opposition-to-legislative-interference. Accessed November 6, 2022.
While many anticipated the fall of Roe v Wade after the leaked Supreme Court of the United States (SCOTUS) decision in the Dobbs v Jackson case, few may have fully comprehended the myriad of ways this ruling would create a national health care crisis overnight. Since the ruling, abortion has been banned, or a 6-week gestational age limit has been implemented, in a total of 13 states, all within the South
The 2022 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting, held shortly after the leaked SCOTUS opinion, was unlike most others. ACOG staff appropriately recognized the vastly different ways this ruling would affect patients and providers alike, simply based on the states in which they reside. ACOG staff organized the large group of attendees according to self-identified status (ie, whether they worked in states with protected, restricted, or threatened access to abortion care). Since this is such a vast topic, attendees also were asked to identify an area upon which to focus, such as the provision of health care, advocacy, or education. As a clinician practicing in Massachusetts, Dr. Bradley found herself meeting with an ACOG leader from California as they brainstormed how to best help our own communities. In conversing with attendees from other parts of the country, it became apparent the challenges others would be facing elsewhere were far more substantive than those we would be facing in “blue states.” After the Dobbs ruling, those predictions became harsh realities.
As we begin to see and hear reports of the devastating consequences of this ruling in “red states,” those of us in protected states have been struggling to try and ascertain how to help. Many of us have worked with our own legislatures to further enshrine protections for our patients and clinicians. New York and Massachusetts exemplify these efforts.6,7 These legislative efforts have included liability protections for patients and their clinicians who care for those who travel from restricted to protected states. Others involve codifying the principles of Roe and clarifying existing law to improve access. An online fundraiser organized by physicians to assist Dr. Bernard with her legal costs as she faces politically motivated investigation by Indiana state authorities has raised more than $260,000.8 Many expressed the potential legal and medical peril for examiners and examinees if the American Board of Obstetrics and Gynecology held in-person oral examinations in Texas as previously scheduled.9 An online petition to change the format to virtual had 728 signatories, and the format was changed back to virtual.10
The implications on medical schools, residencies, and fellowships cannot be overstated. The Dobbs ruling almost immediately affected nearly half of the training programs, which is particularly problematic given the Accreditation Council for Graduate Medical Education requirement that all ObGyn residents have access to abortion training.11 Other programs already are starting to try to meet this vast training need. The University of California San Francisco started offering training to physicians from Texas who were affected by the strict restrictions that predated Dobbs in the SB8 legislation, which turned ordinary citizens into vigilantes.12
ACOG has created an online resource (https://www.acog.org/advocacy/abortion-is-essential) with a number of different sections regarding clinical care, education and training, advocacy at the state level, and how to use effective language when talking about abortion in our communities. Planned Parenthood also suggests a myriad of ways those directly and indirectly affected could get involved:
- Donate to the National Network of Abortion Funds. This fund (https://secure.actblue.com/donate/fundabortionnow) facilitates care for those without the financial means to obtain it, supporting travel, lodging, and child care.
- Share #AbortionAccess posts on social media. These stories are a powerful reminder of the incredibly harmful impact this legislation can have on our patients.
- Donate to the If When How’s Legal Repro Defense Fund (https:/www.ifwhenhow.org/), which helps cover legal costs for those facing state persecution related to reproductive health care.
- Volunteer to help protect abortion health care at the state level.
- Engage with members of Congress in their home districts. (https://www.congress.gov/members/find-your-member)
- Contact the Planned Parenthood Local Engagement Team to facilitate your group, business, or organization’s involvement.
- Partner. Facilitate your organization and other companies to partner with Planned Parenthood and sign up for Bans off our Bodies (https://docs.google.com/forms/d/e/1FAIpQLSdrmxwMcwNXJ8I NE8S2gYjDDXuT76ws_Fr7CLm3 qbtR8dcZHw/viewform).
- Record your perspective about abortion (https://www.together.plannedparenthood.org/articles/6-share-abortion-story), whether it’s having had one, supported someone who had one, or advocated for others to have access to the procedure.13
ACOG also outlines several ways those of us in protected states could help shape the landscape in other communities in addition to advocating for state medical society resolutions, writing op-eds and letters to the editor, and utilizing ACOG’s social media graphics.14 In recognition of the often sensitive, polarizing nature of these discussions, ACOG is offering a workshop entitled “Building Evidence-Based Skills for Effective Conversations about Abortion.”15
Abortion traditionally was a policy issue other medical organizations shied away from developing official policy on and speaking out in support of, but recognizing the devastating scope of the public health crisis, 75 medical professional organizations recently released a strongly worded joint statement noting, “As leading medical and health care organizations dedicated to patient care and public health, we condemn this and all interference in the patient–clinician relationship.”16 Clinicians could work to expand this list to include all aspects of organized medicine. Initiatives to get out the vote may be helpful in vulnerable states, as well.
Clinicians in protected states are not necessarily directly affected in our daily interactions with patients, but we stand in solidarity with those who are. We must remain united as a profession as different state legislatures seek to divide us. We must support those who are struggling every day. Our colleagues and fellow citizens deserve nothing less. ●
While many anticipated the fall of Roe v Wade after the leaked Supreme Court of the United States (SCOTUS) decision in the Dobbs v Jackson case, few may have fully comprehended the myriad of ways this ruling would create a national health care crisis overnight. Since the ruling, abortion has been banned, or a 6-week gestational age limit has been implemented, in a total of 13 states, all within the South
The 2022 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting, held shortly after the leaked SCOTUS opinion, was unlike most others. ACOG staff appropriately recognized the vastly different ways this ruling would affect patients and providers alike, simply based on the states in which they reside. ACOG staff organized the large group of attendees according to self-identified status (ie, whether they worked in states with protected, restricted, or threatened access to abortion care). Since this is such a vast topic, attendees also were asked to identify an area upon which to focus, such as the provision of health care, advocacy, or education. As a clinician practicing in Massachusetts, Dr. Bradley found herself meeting with an ACOG leader from California as they brainstormed how to best help our own communities. In conversing with attendees from other parts of the country, it became apparent the challenges others would be facing elsewhere were far more substantive than those we would be facing in “blue states.” After the Dobbs ruling, those predictions became harsh realities.
As we begin to see and hear reports of the devastating consequences of this ruling in “red states,” those of us in protected states have been struggling to try and ascertain how to help. Many of us have worked with our own legislatures to further enshrine protections for our patients and clinicians. New York and Massachusetts exemplify these efforts.6,7 These legislative efforts have included liability protections for patients and their clinicians who care for those who travel from restricted to protected states. Others involve codifying the principles of Roe and clarifying existing law to improve access. An online fundraiser organized by physicians to assist Dr. Bernard with her legal costs as she faces politically motivated investigation by Indiana state authorities has raised more than $260,000.8 Many expressed the potential legal and medical peril for examiners and examinees if the American Board of Obstetrics and Gynecology held in-person oral examinations in Texas as previously scheduled.9 An online petition to change the format to virtual had 728 signatories, and the format was changed back to virtual.10
The implications on medical schools, residencies, and fellowships cannot be overstated. The Dobbs ruling almost immediately affected nearly half of the training programs, which is particularly problematic given the Accreditation Council for Graduate Medical Education requirement that all ObGyn residents have access to abortion training.11 Other programs already are starting to try to meet this vast training need. The University of California San Francisco started offering training to physicians from Texas who were affected by the strict restrictions that predated Dobbs in the SB8 legislation, which turned ordinary citizens into vigilantes.12
ACOG has created an online resource (https://www.acog.org/advocacy/abortion-is-essential) with a number of different sections regarding clinical care, education and training, advocacy at the state level, and how to use effective language when talking about abortion in our communities. Planned Parenthood also suggests a myriad of ways those directly and indirectly affected could get involved:
- Donate to the National Network of Abortion Funds. This fund (https://secure.actblue.com/donate/fundabortionnow) facilitates care for those without the financial means to obtain it, supporting travel, lodging, and child care.
- Share #AbortionAccess posts on social media. These stories are a powerful reminder of the incredibly harmful impact this legislation can have on our patients.
- Donate to the If When How’s Legal Repro Defense Fund (https:/www.ifwhenhow.org/), which helps cover legal costs for those facing state persecution related to reproductive health care.
- Volunteer to help protect abortion health care at the state level.
- Engage with members of Congress in their home districts. (https://www.congress.gov/members/find-your-member)
- Contact the Planned Parenthood Local Engagement Team to facilitate your group, business, or organization’s involvement.
- Partner. Facilitate your organization and other companies to partner with Planned Parenthood and sign up for Bans off our Bodies (https://docs.google.com/forms/d/e/1FAIpQLSdrmxwMcwNXJ8I NE8S2gYjDDXuT76ws_Fr7CLm3 qbtR8dcZHw/viewform).
- Record your perspective about abortion (https://www.together.plannedparenthood.org/articles/6-share-abortion-story), whether it’s having had one, supported someone who had one, or advocated for others to have access to the procedure.13
ACOG also outlines several ways those of us in protected states could help shape the landscape in other communities in addition to advocating for state medical society resolutions, writing op-eds and letters to the editor, and utilizing ACOG’s social media graphics.14 In recognition of the often sensitive, polarizing nature of these discussions, ACOG is offering a workshop entitled “Building Evidence-Based Skills for Effective Conversations about Abortion.”15
Abortion traditionally was a policy issue other medical organizations shied away from developing official policy on and speaking out in support of, but recognizing the devastating scope of the public health crisis, 75 medical professional organizations recently released a strongly worded joint statement noting, “As leading medical and health care organizations dedicated to patient care and public health, we condemn this and all interference in the patient–clinician relationship.”16 Clinicians could work to expand this list to include all aspects of organized medicine. Initiatives to get out the vote may be helpful in vulnerable states, as well.
Clinicians in protected states are not necessarily directly affected in our daily interactions with patients, but we stand in solidarity with those who are. We must remain united as a profession as different state legislatures seek to divide us. We must support those who are struggling every day. Our colleagues and fellow citizens deserve nothing less. ●
- Tracking the states where abortion is now banned. New York Times. November 23, 2022. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Accessed November 28, 2022.
- Stanton A. ‘She’s 10’: child rape victims abortion denial spreads outrage on Twitter. Newsweek. July 2, 2022. https://www.newsweek.com/shes-10-child-rape-victims-abortion-denial-sparks-outrage-twitter-1721248. Accessed November 6, 2022.
- Judge-Golden C, Flink-Bochacki R. The burden of abortion restrictions and conservative diagnostic guidelines on patient-centered care for early pregnancy loss. Obstet Gynecol 2021;138:467071.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1. doi:10.1016/j.ajog.2022.06.060.
- Winter J. The Dobbs decision has unleashed legal chaos for doctors and patients. The New Yorker. July 2, 2022. https://www.newyorker.com/news/news-desk/the-dobbs-decision-has-unleashed-legal-chaos-for-doctors-and-patients. Accessed November 6, 2022.
- Lynch B, Mallow M, Bodde K, et al. Addressing a crisis in abortion access: a case study in advocacy. Obstet Gynecol. 2022;140:110-114.
- Evans M, Bradley T, Ireland L, et al. How the fall of Roe could change abortion care in Mass. Cognoscenti. July 26, 2022. https://www.wbur.org/cognoscenti/2022/07/26/dobbs-roe-abortion-massachusetts-megan-l-evans-erin-t-bradley-luu-ireland-chloe-zera. Accessed November 6, 2022.
- Spocchia G. Over $200k raised for doctor who performed abortion on 10-year-old rape victim. Independent. July 18, 2022. https://www.independent.co.uk/news/world/americas/fundriaser-ohio-abortion-doctor-rape-b2125621.html. Accessed November 6, 2022.
- ABOG petition: convert to online examination to protect OBGYN providers. Change.org website. https://www.change.org/p/abog-petition?original_footer_petition_id=33459909&algorithm=promoted&source_location=petition_footer&grid_position=8&pt=AVBldGl0aW9uAHgWBQIAAAAAYs65vIyhbUxhZGM0MWVhZg%3D%3D. Accessed November 6, 2022.
- D’Ambrosio A. Ob/Gyn board certification exam stays virtual in light of Dobbs. MedPageToday. July 15, 2022. https://www.medpagetoday.com/special-reports/features/99758. Accessed November 6, 2022.
- Weiner S. How the repeal of Roe v. Wade will affect training in abortion and reproductive health. AAMC News. June 24, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health. Accessed November 6, 2022.
- Anderson N. The fall of Roe scrambles abortion training for university hospitals. The Washington Post. June 30, 2022. https://www.washingtonpost.com/education/2022/06/30/abortion-training-upheaval-dobbs/. Accessed November 6, 2022.
- Bans off our bodies. Planned Parenthood website. https://www.plannedparenthoodaction.org/rightfully-ours/bans-off-our-bodies. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Shape the public discourse. ACOG website. https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Building evidence-based skills for effective conversations about abortion. ACOG website. https://www.acog.org/programs/impact/activities-initiatives/building-evidence-based-skills-for-effective-conversations-about-abortion. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. More than 75 health care organizations release joint statement in opposition to legislative interference. ACOG website. Published July 7, 2022. https://www.acog.org/news/news-releases/2022/07/more-than-75-health-care-organizations-release-joint-statement-in-opposition-to-legislative-interference. Accessed November 6, 2022.
- Tracking the states where abortion is now banned. New York Times. November 23, 2022. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Accessed November 28, 2022.
- Stanton A. ‘She’s 10’: child rape victims abortion denial spreads outrage on Twitter. Newsweek. July 2, 2022. https://www.newsweek.com/shes-10-child-rape-victims-abortion-denial-sparks-outrage-twitter-1721248. Accessed November 6, 2022.
- Judge-Golden C, Flink-Bochacki R. The burden of abortion restrictions and conservative diagnostic guidelines on patient-centered care for early pregnancy loss. Obstet Gynecol 2021;138:467071.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1. doi:10.1016/j.ajog.2022.06.060.
- Winter J. The Dobbs decision has unleashed legal chaos for doctors and patients. The New Yorker. July 2, 2022. https://www.newyorker.com/news/news-desk/the-dobbs-decision-has-unleashed-legal-chaos-for-doctors-and-patients. Accessed November 6, 2022.
- Lynch B, Mallow M, Bodde K, et al. Addressing a crisis in abortion access: a case study in advocacy. Obstet Gynecol. 2022;140:110-114.
- Evans M, Bradley T, Ireland L, et al. How the fall of Roe could change abortion care in Mass. Cognoscenti. July 26, 2022. https://www.wbur.org/cognoscenti/2022/07/26/dobbs-roe-abortion-massachusetts-megan-l-evans-erin-t-bradley-luu-ireland-chloe-zera. Accessed November 6, 2022.
- Spocchia G. Over $200k raised for doctor who performed abortion on 10-year-old rape victim. Independent. July 18, 2022. https://www.independent.co.uk/news/world/americas/fundriaser-ohio-abortion-doctor-rape-b2125621.html. Accessed November 6, 2022.
- ABOG petition: convert to online examination to protect OBGYN providers. Change.org website. https://www.change.org/p/abog-petition?original_footer_petition_id=33459909&algorithm=promoted&source_location=petition_footer&grid_position=8&pt=AVBldGl0aW9uAHgWBQIAAAAAYs65vIyhbUxhZGM0MWVhZg%3D%3D. Accessed November 6, 2022.
- D’Ambrosio A. Ob/Gyn board certification exam stays virtual in light of Dobbs. MedPageToday. July 15, 2022. https://www.medpagetoday.com/special-reports/features/99758. Accessed November 6, 2022.
- Weiner S. How the repeal of Roe v. Wade will affect training in abortion and reproductive health. AAMC News. June 24, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health. Accessed November 6, 2022.
- Anderson N. The fall of Roe scrambles abortion training for university hospitals. The Washington Post. June 30, 2022. https://www.washingtonpost.com/education/2022/06/30/abortion-training-upheaval-dobbs/. Accessed November 6, 2022.
- Bans off our bodies. Planned Parenthood website. https://www.plannedparenthoodaction.org/rightfully-ours/bans-off-our-bodies. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Shape the public discourse. ACOG website. https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Building evidence-based skills for effective conversations about abortion. ACOG website. https://www.acog.org/programs/impact/activities-initiatives/building-evidence-based-skills-for-effective-conversations-about-abortion. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. More than 75 health care organizations release joint statement in opposition to legislative interference. ACOG website. Published July 7, 2022. https://www.acog.org/news/news-releases/2022/07/more-than-75-health-care-organizations-release-joint-statement-in-opposition-to-legislative-interference. Accessed November 6, 2022.
Focus on menopause
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
Criminal liability: What are the risks for medical professionals?
Medical professionals are well aware that civil liability (malpractice) may incur when a patient is harmed because of carelessness (negligence). Recent criminal charges against physicians and a nurse, however, have called medical professionals’ attention to the fact that they also may face criminal charges for inappropriate practice.
We cite 2 cases in which criminal liability resulted from bad medical practice. In both instances, there was considerable concern among medical professionals that criminal charges for making a mistake would make it difficult to practice without fear of criminal charges. One concern is that criminal charges could drive good people out of the profession or make them too cautious.1
We look more closely at those 2 cases in which criminal liability was imposed. These cases are outliers. Relatively few criminal cases against medical professionals are based on the quality of care. (There are, however, more criminal charges related to fraudulent billing and other insurance fraud, kickbacks, Medicare and Medicaid abuse, and the like.2) At the same time, the criminal law does not stop at the front door of a clinic or hospital.3 When medical professionals engage in seriously inappropriate health care conduct that directly harms someone, criminal liability may result.4
Anatomy of a crime
Crimes generally require a specific mental state (mens rea) and an act (actus reus). The law specifies the mental state required for conviction. It can range from premeditation—once commonly called “malice aforethought”—to negligence. The mens rea requirement is an essential element of the crime—as we will see in the discussion of the prescription drug cases. A few offenses do not require even negligence, but overwhelmingly, crimes require something more than simple negligence.5
The act requirement is generally obvious, such as firing a gun, driving while intoxicated, or recklessly giving inappropriate medication to a patient. It may include “attempts,” crimes where an act was not completed. For example, attempted murder or conspiracy to commit do not require a completed offense, only intent plus overt acts toward carrying out the crime. Similarly, the wrongful act usually has to produce some harm, but again there are exceptions (attempts). To obtain a conviction, the prosecution must prove all of the elements of the crime, including the required mens rea, beyond a reasonable doubt.6
With this general background, we turn to the first case, in which the charge was a form of homicide. Please note that the following case description was derived from news descriptions of the case, because juries do not publish opinions concerning their conclusions and court documents are unavailable. The public reports therefore may contain factual gaps and errors.
CASE 1 Patient dies after nurse administers wrong drug
RaDonda Vaught, a 38-year-old experienced registered nurse employed at Vanderbilt University Medical Center (VUMC) in the intensive care unit (ICU), was providing care for a 76-year-old patient who was admitted to VUMC’s ICU in December 2017 in association with a brain injury. The brain injury involved a fall with resultant subdural hematoma. In preparation for a positron emission tomography (PET) scan to assess the patient’s injury, the physician team prescribed the sedative Versed (midazolam) because of the patient’s claustrophobia. During the course of treatment, Ms. Vaught inadvertently administered the wrong drug, a fatal dose of the muscle relaxant vecuronium, to the patient, which resulted in the patient being unable to breathe. Apparently, Ms. Vaught had been unable to find the midazolam and disengaged a safeguard, proceeding into override mode, and thus vecuronium was dispensed. By the time the error was noticed, the patient was already in cardiac arrest with resultant brain damage (partial brain death). The patient died soon thereafter.
How this medication error occurred
The medication error occurred when Ms. Vaught overrode a computer in the medical system when she could not find the “Versed” entry and typed in “VE,” which was the abbreviation for vecuronium. The prosecutors in the case stated that she failed to distinguish that vecuronium is dispensed as a powder and Versed as a liquid formula. The vecuronium has a red cap, which warns that it is a paralyzing agent. Ms. Vaught ignored these red flags, according to the prosecutors. Furthermore, the lawsuit filing documented her discussion that she was “distracted with something” at the time and admitted to overriding the medication warning.
Continue to: The charges in this case...
The charges in this case
The charges revolved around “criminally negligent homicide and gross neglect of an impaired adult,” the most notable charge being criminally negligent homicide. Potential consequences were up to an 8 years’ prison sentence.7
Furthermore, the Tennessee Board of Nursing revoked Ms. Vaught’s license in July 2021.8 The Board also reportedly fined her $3,000.9
The criminal proceedings were filed in Davidson County Criminal Court, with Judge Jennifer Smith presiding. Ms. Vaught repeatedly manifested remorse for the event. The patient’s family, including her son Michael and her daughters-in-law, provided tearful testimonies at the hearing. Ms. Vaught repeatedly cried during the testimonies. The nurse did not provide an apology, according to one daughter-in-law. The news media reported that the family did not want jail time for Ms. Vaught.7 Nurses across the country were “jolted,” as expressed by the news media.10
Why the controversy?
The entire issue of medical errors continues to be discussed among both the medical and the legal professions. To have a nursing personnel held to the level of criminal liability is unusual.
It was clear that Ms. Vaught took responsibility for her actions, and neither the prosecutors nor defendant attorneys sensed any evidence of malice on her part. On the other hand, there was enough evidence and concern for District Attorney Glenn Funk to proceed with prosecution-related action. Ms. Vaught was facing years in prison if convicted.
WHAT’S THE VERDICT?
In March 2022, the jury convicted Ms. Vaught of criminally negligent homicide—but not of reckless homicide, a more serious offense.
Judge Smith granted a judicial diversion, that is, the conviction would be expunged from the record if Ms. Vaught completed a 3-year probation. Judge Smith noted the “credible remorse expressed by Nurse Vaught” and went on to state, “this is a terrible, terrible, mistake and there have been consequences to the defendant.” In the courtroom, Ms. Vaught apologized to the patient’s family and conveyed that she will “forever be haunted by her role in the (patient’s) passing.”
Overall, this served as an opportunity for health care workers to address oftentimes poor working conditions, which have been exacerbated by the COVID-19 pandemic.
The Davidson County District Attorney’s office conveyed that this was one case of a careless nurse and not a reflection of the nursing profession. The prosecutors were in accord with a probation verdict. The family felt that their mother, the patient, would not want to see the nurse serve a jail sentence: “Mom was a very forgiving person.”
The patient’s cause of death was listed as “intracerebral hemorrhage and cardiac arrest.” One year later, a new death certificate was issued and noted vecuronium intoxication as the cause of death.
Continue to: The health care institution’s involvement...
The health care institution’s involvement
Approximately 1 year after an apparent anonymous tip was made to health care officials, an unscheduled state and federal investigation, with the threat of possible sanctions, occurred at the VUMC. This was predicated on the criminal indictment related to Ms. Vaught. In the end, her nursing license was revoked, as noted earlier. The family earlier reached an out-of-court settlement with the hospital and there were a number of problems identified at the university medical center.11
Legal principles in the case
Most criminal cases are state cases. Crimes are defined in state statutes, and the trial takes place in state courts. Thus, crimes are defined a little differently from state to state. Ms. Vaught, for example, was tried in Tennessee under the laws of that state.
Homicide involves the killing of a human being. It may not be a crime. For example, there is “justifiable homicide,” such as self-defense. At the other extreme is first-degree murder, an intentional and planned killing. In this case, Ms. Vaught was charged with criminally negligent homicide, which is usually the least serious of criminal homicides but is still a felony. (Some states have misdemeanor manslaughter, which was not an issue in this case.) In some states, criminally negligent homicide is sometimes referred to as involuntary manslaughter. The mens rea for involuntary manslaughter is generally recklessness or “criminal negligence.” This crime goes by various names depending on the state, but involuntary manslaughter and criminally negligent homicide are common names.
Ordinary negligence versus criminal negligence. Criminal negligence is usually considered a more serious mistake than ordinary negligence. This is where there is a difference between civil malpractice negligence and criminal negligence. Criminal negligence is somewhat more careless than ordinary negligence. To use a driving example, if Dr. A was driving home from the hospital, missed seeing a red light, and killed Joe Pedestrian, it could be ordinary negligence. If, however, Dr. B was texting or drinking while driving, causing Dr. B to be distracted and miss seeing the red light, killing a pedestrian, it could be criminal negligence and result in the conviction for the homicide. Of course, in either case there could be civil liability for causing the death.
Applying these legal principles to the reported facts in Ms. Vaught’s case, it appears there was more than simple negligence. That is, the nurse was more than careless. Using “VE” for the wrong drug might have been negligent. In addition, however, she disengaged a safeguard meant to prevent wrongful use of the drug, failed to notice that the drug was a powder instead of a liquid, and ignored the red cap warning that the drug was a paralyzing agent. It becomes apparent why the jury could have found aggravated or criminal negligence.
It is worth emphasizing that in this case, the criminal charges were unusual. For years, studies have suggested that many deaths result from medical errors. The Institute of Medicine famously said that the number of deaths from medical errors was equivalent to that of a 747 airplane crashing every day.12,13 These events result in a relatively small number of malpractice actions but an infinitesimally small number of homicide charges. Among other things, prosecutors are reluctant to pursue such cases regarding acts carried out as part of clinical duties unless there is strong evidence, and grand juries may be reluctant to indict medical professionals.14
Nonetheless, medical professionals ultimately can be criminally responsible for deaths resulting from intentional, or criminally negligent, careless practice. Such liability should not dissuade nurses or others from medical practice any more than the much more common homicide charges that can occur from driving an automobile carelessly that results in someone’s death. A fundamental purpose of the criminal law is to disincentivize unnecessarily harmful (deadly) conduct, whether it is distracted driving or distracted nursing.
Continue to: The drug-prescribing crimes...
The drug-prescribing crimes
The US Supreme Court considered a much different kind of criminal medical practice in 2 (consolidated) cases in its 2021–2022 Term. Physicians in 2 states were each tried and convicted of federal charges of illegally dispensing or distributing (prescribing) controlled substances.15 A federal statute makes it a felony for a physician, or others, “except as authorized” to “knowingly or intentionally distribute, or dispense a controlled substance.”16 Federal regulations clarify the statute. The regulation provides that a prescription is authorized only if a doctor issues it “for a legitimate medical purpose . . . acting in the usual course of professional practice.”17
CASE 2 Physicians charged with overprescribing controlled substances
In these 2 drug-prescribing cases, the physicians had grossly overprescribed the opioids. One reportedly wrote prescriptions in 2 states in exchange for payments in cash or, infrequently, firearms, approximating the cost of the prescriptions to street drugs. The other had a clinic that, over about 4 years, issued 300,000 prescriptions for controlled substances and was a significant source for some kinds of fentanyl.18
WHAT’S THE VERDICT?
In each trial, the juries found the defendant guilty of improper distribution of controlled substances. Although the charges were not homicides, the sentencing judges were much more severe than the court had been in the nursing case discussed above. One physician received a prison term of 20 years, the other, a 25-year term. These undoubtedly reflect both the outrageous conduct and the likely great harm the defendants did.
The Supreme Court heard the cases
The Supreme Court reversed these physicians’ convictions. The Court held that the lower courts had not correctly described for the juries the mens rea required for a conviction under these charges. The Supreme Court held that to be convicted of these offenses, the government had to prove “beyond a reasonable doubt that the defendant [physician] knew that he or she was acting in an unauthorized manner.”19 Both can be retried and probably will be unless they reach a plea agreement with the federal government. Nonetheless, the Court established a very high standard. Carelessness is not enough, but rather “knowingly” acting in an unauthorized way is required. Although these physicians were prosecuted under federal law, other physicians have been prosecuted under state laws limiting the distribution of controlled substances.20
Some physicians have expressed concern that the Supreme Court, in these cases, made the practice of medicine more dangerous for physicians (the threat of criminal sanctions) and patients (making it more difficult to obtain pain control, for example).21,22 That view may be overly pessimistic for 2 reasons. First, the Court actually made it more difficult to convict physicians of writing excessive prescriptions. It did so by setting a higher mens rea standard than lower courts were using, that is, the physician had to “knowingly” act in an unauthorized way. Because “knowingly” can be implied by the circumstances, taking guns or cash would be evidence that the physician knowingly misprescribed.
More fundamentally, the actions of these physicians appear to be well outside even a generous legitimate level of controlled substance prescription. These convictions should not be misunderstood as a way of federal courts to crack down on pain medications. However, the original convictions are a warning to the small handful who grossly overprescribe controlled substances.
Lessons about criminal law and the practice of medicine
Medical professionals’ strong reaction to criminal charges is understandable. Criminal charges can result in jail time (the physicians involved in the controlled substance case were sentenced to 20 years or more) and hefty fines; bring social and professional disapprobation; may lead to license discipline; and are terribly disruptive even for those found not guilty. To make matters worse, malpractice insurance ordinarily does not cover criminal charges, so any fines and the cost of defense are likely out of pocket for those charged—and that can be very expensive. Therefore, the strong reaction to the cases we have described is understandable.
At the same time, the probability of criminal charges against medical personnel for their medical treatment is very low compared with, for example, fraudulent billing, their driving habits, or tax avoidance. Criminal charges are much more likely to arise from insurance fraud, Medicare or Medicaid dishonesty, kickbacks, false statements, and similar corruption crimes rather than inadequate practice. In the cases we examined here, there is an enhanced or aggravated negligence in one case and grossly inappropriate prescribing in the others (which the Supreme Court held must be “knowingly” wrong).
Finally, there is an irony. Medical professionals worried about practice-related criminal charges should be thankful for the malpractice system. Civil malpractice is, as a practical matter, an alternative for patients who believe they were mistreated or harmed by physicians or other providers. They have the option of finding a private attorney to file a civil complaint. In the absence of that system, they would be much more likely to take their grievance and complaint to the prosecutor to seek answers and retribution. Criminal law and civil liability are each a way of allowing someone harmed by another to seek redress. Both are intended to deter harmful conduct and provide some individual and social retribution for such behavior. The civil system, of course, also provides the potential for compensation to those injured. An injured patient without the possibility of a civil suit sometimes would turn to the criminal system for satisfaction. This way, the malpractice system is a better alternative to criminal charges. ●
- Kelman B. As a nurse faces prison for a deadly error, her colleagues worry: could I be next? NPR. March 22, 2022. Accessed November 7, 2022. https://www.npr.org/sections/health-shots/2022/03/22/1087903348/as-a-nurse-faces-prison-for-a-deadly-error-her-colleagues-worry-could-i-be-next
- US Department of Justice. National health care fraud enforcement action results in charges involving over $1.4 billion in alleged losses. September 17, 2021. Accessed November 7, 2022. https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-charges-involving-over-14-billion
- Steinman G. Stuff of nightmares: criminal prosecution for malpractice. OBG Manag. 2008;20(8):35-45.
- Maher V, Cwiek M. Criminal liability for nursing and medical harm. Hosp Top. 2022 July 13;1-8.
- Singer RG. The resurgence of mens rea: III—the rise and fall of strict criminal liability. Boston Coll Law Rev. 1989;30:337-408. Accessed November 7, 2022. https://lawdigitalcommons.bc.edu/cgi/viewcontent.cgi?article=2431&context=bclr
- Sarch AF. Knowledge, recklessness and the connection requirement between actus reus and mens rea. Penn State Law Rev. 2015;120:1-51. Accessed November 7, 2022. https://ideas.dickinsonlaw.psu.edu/cgi/viewcontent.cgi?article=4120&context=dlra
- Timms M, Gluck F, Wegner R, et al. RaDonda Vaught sentenced to three years probation on a diverted sentence, could see record wiped. Tennessean. May 13, 2022. Accessed November 7, 2022. http://www.tennessean.com/story/news/crime/2022/05/13/radonda-vaught-sentened-vanderbilt-nurse/9717529002/
- Tennessee Board of Nursing. Disciplinary hearing: RaDonda Vaught, RN #205702, minutes. July 22-23, 2021. Accessed November 7, 2022. https://www.tn.gov/content/dam/tn/health/healthprofboards/nursing/meeting-minutes/Nursing%20Meeting%20Minutes%20July%2022-23,%202021.pdf
- Institute for Safe Medication Practices. TN Board of Nursing’s unjust decision to revoke nurse’s license: travesty on top of tragedy! August 12, 2021. Accessed November 7, 2022. https://www.ismp.org/resources/tn-board-nursings-unjust-decision-revoke-nurses-license-travesty-top-tragedy
- Medina E. Ex-nurse convicted in fatal medication error gets probation. New York Times. May 15, 2022. Accessed November 7, 2022. https://www.nytimes.com/2022/05/15/us/tennessee-nurse-sentencing.html
- Kelman B. In nurse’s trial, investigator says hospital bears ‘heavy’ responsibility for patient death. KHN. March 24, 2022. Accessed November 15, 2022. https://khn.org/news/article/radonda-vaught-fatal-drug-error-vanderbilt-hospital-responsibility/
- Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, ed. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
- Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Affairs. 2018;37:1736-1743.
- Eisenberg RL, Berlin L. When does malpractice become manslaughter? Am J Roentgenol. 2002;179:331-335.
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1410_1an2.pdf
- 84 Stat. 1260, 21 U. S. C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Liptak A. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022.
- Xiulu Ruan v United States, at 2 (slip opinion).
- Pedemonte S. State v. Christensen: criminalizing medical malpractice. Montana Law Rev. 2022;83(1):183-193. Accessed November 7, 2022. https://scholarworks.umt.edu/cgi/view content.cgi?article=2497&context=mlr
- Szalavitz M. A recent Supreme Court ruling will help people in pain. Scientific American. September 19, 2022. Accessed November 15, 2022. https://www.scientificamerican.com/ article/a-recent-supreme-court-ruling-will-help-people-in -pain/
- Lopez I. Opioid pill peddling case threatens future of pain treatment. Bloomberg Law. March 29, 2022. Accessed November 15, 2022. https://news.bloomberglaw.com/health -law-and-business/opioid-pill-peddling-case-threatens -future-of-pain-treatment
Medical professionals are well aware that civil liability (malpractice) may incur when a patient is harmed because of carelessness (negligence). Recent criminal charges against physicians and a nurse, however, have called medical professionals’ attention to the fact that they also may face criminal charges for inappropriate practice.
We cite 2 cases in which criminal liability resulted from bad medical practice. In both instances, there was considerable concern among medical professionals that criminal charges for making a mistake would make it difficult to practice without fear of criminal charges. One concern is that criminal charges could drive good people out of the profession or make them too cautious.1
We look more closely at those 2 cases in which criminal liability was imposed. These cases are outliers. Relatively few criminal cases against medical professionals are based on the quality of care. (There are, however, more criminal charges related to fraudulent billing and other insurance fraud, kickbacks, Medicare and Medicaid abuse, and the like.2) At the same time, the criminal law does not stop at the front door of a clinic or hospital.3 When medical professionals engage in seriously inappropriate health care conduct that directly harms someone, criminal liability may result.4
Anatomy of a crime
Crimes generally require a specific mental state (mens rea) and an act (actus reus). The law specifies the mental state required for conviction. It can range from premeditation—once commonly called “malice aforethought”—to negligence. The mens rea requirement is an essential element of the crime—as we will see in the discussion of the prescription drug cases. A few offenses do not require even negligence, but overwhelmingly, crimes require something more than simple negligence.5
The act requirement is generally obvious, such as firing a gun, driving while intoxicated, or recklessly giving inappropriate medication to a patient. It may include “attempts,” crimes where an act was not completed. For example, attempted murder or conspiracy to commit do not require a completed offense, only intent plus overt acts toward carrying out the crime. Similarly, the wrongful act usually has to produce some harm, but again there are exceptions (attempts). To obtain a conviction, the prosecution must prove all of the elements of the crime, including the required mens rea, beyond a reasonable doubt.6
With this general background, we turn to the first case, in which the charge was a form of homicide. Please note that the following case description was derived from news descriptions of the case, because juries do not publish opinions concerning their conclusions and court documents are unavailable. The public reports therefore may contain factual gaps and errors.
CASE 1 Patient dies after nurse administers wrong drug
RaDonda Vaught, a 38-year-old experienced registered nurse employed at Vanderbilt University Medical Center (VUMC) in the intensive care unit (ICU), was providing care for a 76-year-old patient who was admitted to VUMC’s ICU in December 2017 in association with a brain injury. The brain injury involved a fall with resultant subdural hematoma. In preparation for a positron emission tomography (PET) scan to assess the patient’s injury, the physician team prescribed the sedative Versed (midazolam) because of the patient’s claustrophobia. During the course of treatment, Ms. Vaught inadvertently administered the wrong drug, a fatal dose of the muscle relaxant vecuronium, to the patient, which resulted in the patient being unable to breathe. Apparently, Ms. Vaught had been unable to find the midazolam and disengaged a safeguard, proceeding into override mode, and thus vecuronium was dispensed. By the time the error was noticed, the patient was already in cardiac arrest with resultant brain damage (partial brain death). The patient died soon thereafter.
How this medication error occurred
The medication error occurred when Ms. Vaught overrode a computer in the medical system when she could not find the “Versed” entry and typed in “VE,” which was the abbreviation for vecuronium. The prosecutors in the case stated that she failed to distinguish that vecuronium is dispensed as a powder and Versed as a liquid formula. The vecuronium has a red cap, which warns that it is a paralyzing agent. Ms. Vaught ignored these red flags, according to the prosecutors. Furthermore, the lawsuit filing documented her discussion that she was “distracted with something” at the time and admitted to overriding the medication warning.
Continue to: The charges in this case...
The charges in this case
The charges revolved around “criminally negligent homicide and gross neglect of an impaired adult,” the most notable charge being criminally negligent homicide. Potential consequences were up to an 8 years’ prison sentence.7
Furthermore, the Tennessee Board of Nursing revoked Ms. Vaught’s license in July 2021.8 The Board also reportedly fined her $3,000.9
The criminal proceedings were filed in Davidson County Criminal Court, with Judge Jennifer Smith presiding. Ms. Vaught repeatedly manifested remorse for the event. The patient’s family, including her son Michael and her daughters-in-law, provided tearful testimonies at the hearing. Ms. Vaught repeatedly cried during the testimonies. The nurse did not provide an apology, according to one daughter-in-law. The news media reported that the family did not want jail time for Ms. Vaught.7 Nurses across the country were “jolted,” as expressed by the news media.10
Why the controversy?
The entire issue of medical errors continues to be discussed among both the medical and the legal professions. To have a nursing personnel held to the level of criminal liability is unusual.
It was clear that Ms. Vaught took responsibility for her actions, and neither the prosecutors nor defendant attorneys sensed any evidence of malice on her part. On the other hand, there was enough evidence and concern for District Attorney Glenn Funk to proceed with prosecution-related action. Ms. Vaught was facing years in prison if convicted.
WHAT’S THE VERDICT?
In March 2022, the jury convicted Ms. Vaught of criminally negligent homicide—but not of reckless homicide, a more serious offense.
Judge Smith granted a judicial diversion, that is, the conviction would be expunged from the record if Ms. Vaught completed a 3-year probation. Judge Smith noted the “credible remorse expressed by Nurse Vaught” and went on to state, “this is a terrible, terrible, mistake and there have been consequences to the defendant.” In the courtroom, Ms. Vaught apologized to the patient’s family and conveyed that she will “forever be haunted by her role in the (patient’s) passing.”
Overall, this served as an opportunity for health care workers to address oftentimes poor working conditions, which have been exacerbated by the COVID-19 pandemic.
The Davidson County District Attorney’s office conveyed that this was one case of a careless nurse and not a reflection of the nursing profession. The prosecutors were in accord with a probation verdict. The family felt that their mother, the patient, would not want to see the nurse serve a jail sentence: “Mom was a very forgiving person.”
The patient’s cause of death was listed as “intracerebral hemorrhage and cardiac arrest.” One year later, a new death certificate was issued and noted vecuronium intoxication as the cause of death.
Continue to: The health care institution’s involvement...
The health care institution’s involvement
Approximately 1 year after an apparent anonymous tip was made to health care officials, an unscheduled state and federal investigation, with the threat of possible sanctions, occurred at the VUMC. This was predicated on the criminal indictment related to Ms. Vaught. In the end, her nursing license was revoked, as noted earlier. The family earlier reached an out-of-court settlement with the hospital and there were a number of problems identified at the university medical center.11
Legal principles in the case
Most criminal cases are state cases. Crimes are defined in state statutes, and the trial takes place in state courts. Thus, crimes are defined a little differently from state to state. Ms. Vaught, for example, was tried in Tennessee under the laws of that state.
Homicide involves the killing of a human being. It may not be a crime. For example, there is “justifiable homicide,” such as self-defense. At the other extreme is first-degree murder, an intentional and planned killing. In this case, Ms. Vaught was charged with criminally negligent homicide, which is usually the least serious of criminal homicides but is still a felony. (Some states have misdemeanor manslaughter, which was not an issue in this case.) In some states, criminally negligent homicide is sometimes referred to as involuntary manslaughter. The mens rea for involuntary manslaughter is generally recklessness or “criminal negligence.” This crime goes by various names depending on the state, but involuntary manslaughter and criminally negligent homicide are common names.
Ordinary negligence versus criminal negligence. Criminal negligence is usually considered a more serious mistake than ordinary negligence. This is where there is a difference between civil malpractice negligence and criminal negligence. Criminal negligence is somewhat more careless than ordinary negligence. To use a driving example, if Dr. A was driving home from the hospital, missed seeing a red light, and killed Joe Pedestrian, it could be ordinary negligence. If, however, Dr. B was texting or drinking while driving, causing Dr. B to be distracted and miss seeing the red light, killing a pedestrian, it could be criminal negligence and result in the conviction for the homicide. Of course, in either case there could be civil liability for causing the death.
Applying these legal principles to the reported facts in Ms. Vaught’s case, it appears there was more than simple negligence. That is, the nurse was more than careless. Using “VE” for the wrong drug might have been negligent. In addition, however, she disengaged a safeguard meant to prevent wrongful use of the drug, failed to notice that the drug was a powder instead of a liquid, and ignored the red cap warning that the drug was a paralyzing agent. It becomes apparent why the jury could have found aggravated or criminal negligence.
It is worth emphasizing that in this case, the criminal charges were unusual. For years, studies have suggested that many deaths result from medical errors. The Institute of Medicine famously said that the number of deaths from medical errors was equivalent to that of a 747 airplane crashing every day.12,13 These events result in a relatively small number of malpractice actions but an infinitesimally small number of homicide charges. Among other things, prosecutors are reluctant to pursue such cases regarding acts carried out as part of clinical duties unless there is strong evidence, and grand juries may be reluctant to indict medical professionals.14
Nonetheless, medical professionals ultimately can be criminally responsible for deaths resulting from intentional, or criminally negligent, careless practice. Such liability should not dissuade nurses or others from medical practice any more than the much more common homicide charges that can occur from driving an automobile carelessly that results in someone’s death. A fundamental purpose of the criminal law is to disincentivize unnecessarily harmful (deadly) conduct, whether it is distracted driving or distracted nursing.
Continue to: The drug-prescribing crimes...
The drug-prescribing crimes
The US Supreme Court considered a much different kind of criminal medical practice in 2 (consolidated) cases in its 2021–2022 Term. Physicians in 2 states were each tried and convicted of federal charges of illegally dispensing or distributing (prescribing) controlled substances.15 A federal statute makes it a felony for a physician, or others, “except as authorized” to “knowingly or intentionally distribute, or dispense a controlled substance.”16 Federal regulations clarify the statute. The regulation provides that a prescription is authorized only if a doctor issues it “for a legitimate medical purpose . . . acting in the usual course of professional practice.”17
CASE 2 Physicians charged with overprescribing controlled substances
In these 2 drug-prescribing cases, the physicians had grossly overprescribed the opioids. One reportedly wrote prescriptions in 2 states in exchange for payments in cash or, infrequently, firearms, approximating the cost of the prescriptions to street drugs. The other had a clinic that, over about 4 years, issued 300,000 prescriptions for controlled substances and was a significant source for some kinds of fentanyl.18
WHAT’S THE VERDICT?
In each trial, the juries found the defendant guilty of improper distribution of controlled substances. Although the charges were not homicides, the sentencing judges were much more severe than the court had been in the nursing case discussed above. One physician received a prison term of 20 years, the other, a 25-year term. These undoubtedly reflect both the outrageous conduct and the likely great harm the defendants did.
The Supreme Court heard the cases
The Supreme Court reversed these physicians’ convictions. The Court held that the lower courts had not correctly described for the juries the mens rea required for a conviction under these charges. The Supreme Court held that to be convicted of these offenses, the government had to prove “beyond a reasonable doubt that the defendant [physician] knew that he or she was acting in an unauthorized manner.”19 Both can be retried and probably will be unless they reach a plea agreement with the federal government. Nonetheless, the Court established a very high standard. Carelessness is not enough, but rather “knowingly” acting in an unauthorized way is required. Although these physicians were prosecuted under federal law, other physicians have been prosecuted under state laws limiting the distribution of controlled substances.20
Some physicians have expressed concern that the Supreme Court, in these cases, made the practice of medicine more dangerous for physicians (the threat of criminal sanctions) and patients (making it more difficult to obtain pain control, for example).21,22 That view may be overly pessimistic for 2 reasons. First, the Court actually made it more difficult to convict physicians of writing excessive prescriptions. It did so by setting a higher mens rea standard than lower courts were using, that is, the physician had to “knowingly” act in an unauthorized way. Because “knowingly” can be implied by the circumstances, taking guns or cash would be evidence that the physician knowingly misprescribed.
More fundamentally, the actions of these physicians appear to be well outside even a generous legitimate level of controlled substance prescription. These convictions should not be misunderstood as a way of federal courts to crack down on pain medications. However, the original convictions are a warning to the small handful who grossly overprescribe controlled substances.
Lessons about criminal law and the practice of medicine
Medical professionals’ strong reaction to criminal charges is understandable. Criminal charges can result in jail time (the physicians involved in the controlled substance case were sentenced to 20 years or more) and hefty fines; bring social and professional disapprobation; may lead to license discipline; and are terribly disruptive even for those found not guilty. To make matters worse, malpractice insurance ordinarily does not cover criminal charges, so any fines and the cost of defense are likely out of pocket for those charged—and that can be very expensive. Therefore, the strong reaction to the cases we have described is understandable.
At the same time, the probability of criminal charges against medical personnel for their medical treatment is very low compared with, for example, fraudulent billing, their driving habits, or tax avoidance. Criminal charges are much more likely to arise from insurance fraud, Medicare or Medicaid dishonesty, kickbacks, false statements, and similar corruption crimes rather than inadequate practice. In the cases we examined here, there is an enhanced or aggravated negligence in one case and grossly inappropriate prescribing in the others (which the Supreme Court held must be “knowingly” wrong).
Finally, there is an irony. Medical professionals worried about practice-related criminal charges should be thankful for the malpractice system. Civil malpractice is, as a practical matter, an alternative for patients who believe they were mistreated or harmed by physicians or other providers. They have the option of finding a private attorney to file a civil complaint. In the absence of that system, they would be much more likely to take their grievance and complaint to the prosecutor to seek answers and retribution. Criminal law and civil liability are each a way of allowing someone harmed by another to seek redress. Both are intended to deter harmful conduct and provide some individual and social retribution for such behavior. The civil system, of course, also provides the potential for compensation to those injured. An injured patient without the possibility of a civil suit sometimes would turn to the criminal system for satisfaction. This way, the malpractice system is a better alternative to criminal charges. ●
Medical professionals are well aware that civil liability (malpractice) may incur when a patient is harmed because of carelessness (negligence). Recent criminal charges against physicians and a nurse, however, have called medical professionals’ attention to the fact that they also may face criminal charges for inappropriate practice.
We cite 2 cases in which criminal liability resulted from bad medical practice. In both instances, there was considerable concern among medical professionals that criminal charges for making a mistake would make it difficult to practice without fear of criminal charges. One concern is that criminal charges could drive good people out of the profession or make them too cautious.1
We look more closely at those 2 cases in which criminal liability was imposed. These cases are outliers. Relatively few criminal cases against medical professionals are based on the quality of care. (There are, however, more criminal charges related to fraudulent billing and other insurance fraud, kickbacks, Medicare and Medicaid abuse, and the like.2) At the same time, the criminal law does not stop at the front door of a clinic or hospital.3 When medical professionals engage in seriously inappropriate health care conduct that directly harms someone, criminal liability may result.4
Anatomy of a crime
Crimes generally require a specific mental state (mens rea) and an act (actus reus). The law specifies the mental state required for conviction. It can range from premeditation—once commonly called “malice aforethought”—to negligence. The mens rea requirement is an essential element of the crime—as we will see in the discussion of the prescription drug cases. A few offenses do not require even negligence, but overwhelmingly, crimes require something more than simple negligence.5
The act requirement is generally obvious, such as firing a gun, driving while intoxicated, or recklessly giving inappropriate medication to a patient. It may include “attempts,” crimes where an act was not completed. For example, attempted murder or conspiracy to commit do not require a completed offense, only intent plus overt acts toward carrying out the crime. Similarly, the wrongful act usually has to produce some harm, but again there are exceptions (attempts). To obtain a conviction, the prosecution must prove all of the elements of the crime, including the required mens rea, beyond a reasonable doubt.6
With this general background, we turn to the first case, in which the charge was a form of homicide. Please note that the following case description was derived from news descriptions of the case, because juries do not publish opinions concerning their conclusions and court documents are unavailable. The public reports therefore may contain factual gaps and errors.
CASE 1 Patient dies after nurse administers wrong drug
RaDonda Vaught, a 38-year-old experienced registered nurse employed at Vanderbilt University Medical Center (VUMC) in the intensive care unit (ICU), was providing care for a 76-year-old patient who was admitted to VUMC’s ICU in December 2017 in association with a brain injury. The brain injury involved a fall with resultant subdural hematoma. In preparation for a positron emission tomography (PET) scan to assess the patient’s injury, the physician team prescribed the sedative Versed (midazolam) because of the patient’s claustrophobia. During the course of treatment, Ms. Vaught inadvertently administered the wrong drug, a fatal dose of the muscle relaxant vecuronium, to the patient, which resulted in the patient being unable to breathe. Apparently, Ms. Vaught had been unable to find the midazolam and disengaged a safeguard, proceeding into override mode, and thus vecuronium was dispensed. By the time the error was noticed, the patient was already in cardiac arrest with resultant brain damage (partial brain death). The patient died soon thereafter.
How this medication error occurred
The medication error occurred when Ms. Vaught overrode a computer in the medical system when she could not find the “Versed” entry and typed in “VE,” which was the abbreviation for vecuronium. The prosecutors in the case stated that she failed to distinguish that vecuronium is dispensed as a powder and Versed as a liquid formula. The vecuronium has a red cap, which warns that it is a paralyzing agent. Ms. Vaught ignored these red flags, according to the prosecutors. Furthermore, the lawsuit filing documented her discussion that she was “distracted with something” at the time and admitted to overriding the medication warning.
Continue to: The charges in this case...
The charges in this case
The charges revolved around “criminally negligent homicide and gross neglect of an impaired adult,” the most notable charge being criminally negligent homicide. Potential consequences were up to an 8 years’ prison sentence.7
Furthermore, the Tennessee Board of Nursing revoked Ms. Vaught’s license in July 2021.8 The Board also reportedly fined her $3,000.9
The criminal proceedings were filed in Davidson County Criminal Court, with Judge Jennifer Smith presiding. Ms. Vaught repeatedly manifested remorse for the event. The patient’s family, including her son Michael and her daughters-in-law, provided tearful testimonies at the hearing. Ms. Vaught repeatedly cried during the testimonies. The nurse did not provide an apology, according to one daughter-in-law. The news media reported that the family did not want jail time for Ms. Vaught.7 Nurses across the country were “jolted,” as expressed by the news media.10
Why the controversy?
The entire issue of medical errors continues to be discussed among both the medical and the legal professions. To have a nursing personnel held to the level of criminal liability is unusual.
It was clear that Ms. Vaught took responsibility for her actions, and neither the prosecutors nor defendant attorneys sensed any evidence of malice on her part. On the other hand, there was enough evidence and concern for District Attorney Glenn Funk to proceed with prosecution-related action. Ms. Vaught was facing years in prison if convicted.
WHAT’S THE VERDICT?
In March 2022, the jury convicted Ms. Vaught of criminally negligent homicide—but not of reckless homicide, a more serious offense.
Judge Smith granted a judicial diversion, that is, the conviction would be expunged from the record if Ms. Vaught completed a 3-year probation. Judge Smith noted the “credible remorse expressed by Nurse Vaught” and went on to state, “this is a terrible, terrible, mistake and there have been consequences to the defendant.” In the courtroom, Ms. Vaught apologized to the patient’s family and conveyed that she will “forever be haunted by her role in the (patient’s) passing.”
Overall, this served as an opportunity for health care workers to address oftentimes poor working conditions, which have been exacerbated by the COVID-19 pandemic.
The Davidson County District Attorney’s office conveyed that this was one case of a careless nurse and not a reflection of the nursing profession. The prosecutors were in accord with a probation verdict. The family felt that their mother, the patient, would not want to see the nurse serve a jail sentence: “Mom was a very forgiving person.”
The patient’s cause of death was listed as “intracerebral hemorrhage and cardiac arrest.” One year later, a new death certificate was issued and noted vecuronium intoxication as the cause of death.
Continue to: The health care institution’s involvement...
The health care institution’s involvement
Approximately 1 year after an apparent anonymous tip was made to health care officials, an unscheduled state and federal investigation, with the threat of possible sanctions, occurred at the VUMC. This was predicated on the criminal indictment related to Ms. Vaught. In the end, her nursing license was revoked, as noted earlier. The family earlier reached an out-of-court settlement with the hospital and there were a number of problems identified at the university medical center.11
Legal principles in the case
Most criminal cases are state cases. Crimes are defined in state statutes, and the trial takes place in state courts. Thus, crimes are defined a little differently from state to state. Ms. Vaught, for example, was tried in Tennessee under the laws of that state.
Homicide involves the killing of a human being. It may not be a crime. For example, there is “justifiable homicide,” such as self-defense. At the other extreme is first-degree murder, an intentional and planned killing. In this case, Ms. Vaught was charged with criminally negligent homicide, which is usually the least serious of criminal homicides but is still a felony. (Some states have misdemeanor manslaughter, which was not an issue in this case.) In some states, criminally negligent homicide is sometimes referred to as involuntary manslaughter. The mens rea for involuntary manslaughter is generally recklessness or “criminal negligence.” This crime goes by various names depending on the state, but involuntary manslaughter and criminally negligent homicide are common names.
Ordinary negligence versus criminal negligence. Criminal negligence is usually considered a more serious mistake than ordinary negligence. This is where there is a difference between civil malpractice negligence and criminal negligence. Criminal negligence is somewhat more careless than ordinary negligence. To use a driving example, if Dr. A was driving home from the hospital, missed seeing a red light, and killed Joe Pedestrian, it could be ordinary negligence. If, however, Dr. B was texting or drinking while driving, causing Dr. B to be distracted and miss seeing the red light, killing a pedestrian, it could be criminal negligence and result in the conviction for the homicide. Of course, in either case there could be civil liability for causing the death.
Applying these legal principles to the reported facts in Ms. Vaught’s case, it appears there was more than simple negligence. That is, the nurse was more than careless. Using “VE” for the wrong drug might have been negligent. In addition, however, she disengaged a safeguard meant to prevent wrongful use of the drug, failed to notice that the drug was a powder instead of a liquid, and ignored the red cap warning that the drug was a paralyzing agent. It becomes apparent why the jury could have found aggravated or criminal negligence.
It is worth emphasizing that in this case, the criminal charges were unusual. For years, studies have suggested that many deaths result from medical errors. The Institute of Medicine famously said that the number of deaths from medical errors was equivalent to that of a 747 airplane crashing every day.12,13 These events result in a relatively small number of malpractice actions but an infinitesimally small number of homicide charges. Among other things, prosecutors are reluctant to pursue such cases regarding acts carried out as part of clinical duties unless there is strong evidence, and grand juries may be reluctant to indict medical professionals.14
Nonetheless, medical professionals ultimately can be criminally responsible for deaths resulting from intentional, or criminally negligent, careless practice. Such liability should not dissuade nurses or others from medical practice any more than the much more common homicide charges that can occur from driving an automobile carelessly that results in someone’s death. A fundamental purpose of the criminal law is to disincentivize unnecessarily harmful (deadly) conduct, whether it is distracted driving or distracted nursing.
Continue to: The drug-prescribing crimes...
The drug-prescribing crimes
The US Supreme Court considered a much different kind of criminal medical practice in 2 (consolidated) cases in its 2021–2022 Term. Physicians in 2 states were each tried and convicted of federal charges of illegally dispensing or distributing (prescribing) controlled substances.15 A federal statute makes it a felony for a physician, or others, “except as authorized” to “knowingly or intentionally distribute, or dispense a controlled substance.”16 Federal regulations clarify the statute. The regulation provides that a prescription is authorized only if a doctor issues it “for a legitimate medical purpose . . . acting in the usual course of professional practice.”17
CASE 2 Physicians charged with overprescribing controlled substances
In these 2 drug-prescribing cases, the physicians had grossly overprescribed the opioids. One reportedly wrote prescriptions in 2 states in exchange for payments in cash or, infrequently, firearms, approximating the cost of the prescriptions to street drugs. The other had a clinic that, over about 4 years, issued 300,000 prescriptions for controlled substances and was a significant source for some kinds of fentanyl.18
WHAT’S THE VERDICT?
In each trial, the juries found the defendant guilty of improper distribution of controlled substances. Although the charges were not homicides, the sentencing judges were much more severe than the court had been in the nursing case discussed above. One physician received a prison term of 20 years, the other, a 25-year term. These undoubtedly reflect both the outrageous conduct and the likely great harm the defendants did.
The Supreme Court heard the cases
The Supreme Court reversed these physicians’ convictions. The Court held that the lower courts had not correctly described for the juries the mens rea required for a conviction under these charges. The Supreme Court held that to be convicted of these offenses, the government had to prove “beyond a reasonable doubt that the defendant [physician] knew that he or she was acting in an unauthorized manner.”19 Both can be retried and probably will be unless they reach a plea agreement with the federal government. Nonetheless, the Court established a very high standard. Carelessness is not enough, but rather “knowingly” acting in an unauthorized way is required. Although these physicians were prosecuted under federal law, other physicians have been prosecuted under state laws limiting the distribution of controlled substances.20
Some physicians have expressed concern that the Supreme Court, in these cases, made the practice of medicine more dangerous for physicians (the threat of criminal sanctions) and patients (making it more difficult to obtain pain control, for example).21,22 That view may be overly pessimistic for 2 reasons. First, the Court actually made it more difficult to convict physicians of writing excessive prescriptions. It did so by setting a higher mens rea standard than lower courts were using, that is, the physician had to “knowingly” act in an unauthorized way. Because “knowingly” can be implied by the circumstances, taking guns or cash would be evidence that the physician knowingly misprescribed.
More fundamentally, the actions of these physicians appear to be well outside even a generous legitimate level of controlled substance prescription. These convictions should not be misunderstood as a way of federal courts to crack down on pain medications. However, the original convictions are a warning to the small handful who grossly overprescribe controlled substances.
Lessons about criminal law and the practice of medicine
Medical professionals’ strong reaction to criminal charges is understandable. Criminal charges can result in jail time (the physicians involved in the controlled substance case were sentenced to 20 years or more) and hefty fines; bring social and professional disapprobation; may lead to license discipline; and are terribly disruptive even for those found not guilty. To make matters worse, malpractice insurance ordinarily does not cover criminal charges, so any fines and the cost of defense are likely out of pocket for those charged—and that can be very expensive. Therefore, the strong reaction to the cases we have described is understandable.
At the same time, the probability of criminal charges against medical personnel for their medical treatment is very low compared with, for example, fraudulent billing, their driving habits, or tax avoidance. Criminal charges are much more likely to arise from insurance fraud, Medicare or Medicaid dishonesty, kickbacks, false statements, and similar corruption crimes rather than inadequate practice. In the cases we examined here, there is an enhanced or aggravated negligence in one case and grossly inappropriate prescribing in the others (which the Supreme Court held must be “knowingly” wrong).
Finally, there is an irony. Medical professionals worried about practice-related criminal charges should be thankful for the malpractice system. Civil malpractice is, as a practical matter, an alternative for patients who believe they were mistreated or harmed by physicians or other providers. They have the option of finding a private attorney to file a civil complaint. In the absence of that system, they would be much more likely to take their grievance and complaint to the prosecutor to seek answers and retribution. Criminal law and civil liability are each a way of allowing someone harmed by another to seek redress. Both are intended to deter harmful conduct and provide some individual and social retribution for such behavior. The civil system, of course, also provides the potential for compensation to those injured. An injured patient without the possibility of a civil suit sometimes would turn to the criminal system for satisfaction. This way, the malpractice system is a better alternative to criminal charges. ●
- Kelman B. As a nurse faces prison for a deadly error, her colleagues worry: could I be next? NPR. March 22, 2022. Accessed November 7, 2022. https://www.npr.org/sections/health-shots/2022/03/22/1087903348/as-a-nurse-faces-prison-for-a-deadly-error-her-colleagues-worry-could-i-be-next
- US Department of Justice. National health care fraud enforcement action results in charges involving over $1.4 billion in alleged losses. September 17, 2021. Accessed November 7, 2022. https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-charges-involving-over-14-billion
- Steinman G. Stuff of nightmares: criminal prosecution for malpractice. OBG Manag. 2008;20(8):35-45.
- Maher V, Cwiek M. Criminal liability for nursing and medical harm. Hosp Top. 2022 July 13;1-8.
- Singer RG. The resurgence of mens rea: III—the rise and fall of strict criminal liability. Boston Coll Law Rev. 1989;30:337-408. Accessed November 7, 2022. https://lawdigitalcommons.bc.edu/cgi/viewcontent.cgi?article=2431&context=bclr
- Sarch AF. Knowledge, recklessness and the connection requirement between actus reus and mens rea. Penn State Law Rev. 2015;120:1-51. Accessed November 7, 2022. https://ideas.dickinsonlaw.psu.edu/cgi/viewcontent.cgi?article=4120&context=dlra
- Timms M, Gluck F, Wegner R, et al. RaDonda Vaught sentenced to three years probation on a diverted sentence, could see record wiped. Tennessean. May 13, 2022. Accessed November 7, 2022. http://www.tennessean.com/story/news/crime/2022/05/13/radonda-vaught-sentened-vanderbilt-nurse/9717529002/
- Tennessee Board of Nursing. Disciplinary hearing: RaDonda Vaught, RN #205702, minutes. July 22-23, 2021. Accessed November 7, 2022. https://www.tn.gov/content/dam/tn/health/healthprofboards/nursing/meeting-minutes/Nursing%20Meeting%20Minutes%20July%2022-23,%202021.pdf
- Institute for Safe Medication Practices. TN Board of Nursing’s unjust decision to revoke nurse’s license: travesty on top of tragedy! August 12, 2021. Accessed November 7, 2022. https://www.ismp.org/resources/tn-board-nursings-unjust-decision-revoke-nurses-license-travesty-top-tragedy
- Medina E. Ex-nurse convicted in fatal medication error gets probation. New York Times. May 15, 2022. Accessed November 7, 2022. https://www.nytimes.com/2022/05/15/us/tennessee-nurse-sentencing.html
- Kelman B. In nurse’s trial, investigator says hospital bears ‘heavy’ responsibility for patient death. KHN. March 24, 2022. Accessed November 15, 2022. https://khn.org/news/article/radonda-vaught-fatal-drug-error-vanderbilt-hospital-responsibility/
- Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, ed. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
- Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Affairs. 2018;37:1736-1743.
- Eisenberg RL, Berlin L. When does malpractice become manslaughter? Am J Roentgenol. 2002;179:331-335.
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1410_1an2.pdf
- 84 Stat. 1260, 21 U. S. C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Liptak A. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022.
- Xiulu Ruan v United States, at 2 (slip opinion).
- Pedemonte S. State v. Christensen: criminalizing medical malpractice. Montana Law Rev. 2022;83(1):183-193. Accessed November 7, 2022. https://scholarworks.umt.edu/cgi/view content.cgi?article=2497&context=mlr
- Szalavitz M. A recent Supreme Court ruling will help people in pain. Scientific American. September 19, 2022. Accessed November 15, 2022. https://www.scientificamerican.com/ article/a-recent-supreme-court-ruling-will-help-people-in -pain/
- Lopez I. Opioid pill peddling case threatens future of pain treatment. Bloomberg Law. March 29, 2022. Accessed November 15, 2022. https://news.bloomberglaw.com/health -law-and-business/opioid-pill-peddling-case-threatens -future-of-pain-treatment
- Kelman B. As a nurse faces prison for a deadly error, her colleagues worry: could I be next? NPR. March 22, 2022. Accessed November 7, 2022. https://www.npr.org/sections/health-shots/2022/03/22/1087903348/as-a-nurse-faces-prison-for-a-deadly-error-her-colleagues-worry-could-i-be-next
- US Department of Justice. National health care fraud enforcement action results in charges involving over $1.4 billion in alleged losses. September 17, 2021. Accessed November 7, 2022. https://www.justice.gov/opa/pr/national-health-care-fraud-enforcement-action-results-charges-involving-over-14-billion
- Steinman G. Stuff of nightmares: criminal prosecution for malpractice. OBG Manag. 2008;20(8):35-45.
- Maher V, Cwiek M. Criminal liability for nursing and medical harm. Hosp Top. 2022 July 13;1-8.
- Singer RG. The resurgence of mens rea: III—the rise and fall of strict criminal liability. Boston Coll Law Rev. 1989;30:337-408. Accessed November 7, 2022. https://lawdigitalcommons.bc.edu/cgi/viewcontent.cgi?article=2431&context=bclr
- Sarch AF. Knowledge, recklessness and the connection requirement between actus reus and mens rea. Penn State Law Rev. 2015;120:1-51. Accessed November 7, 2022. https://ideas.dickinsonlaw.psu.edu/cgi/viewcontent.cgi?article=4120&context=dlra
- Timms M, Gluck F, Wegner R, et al. RaDonda Vaught sentenced to three years probation on a diverted sentence, could see record wiped. Tennessean. May 13, 2022. Accessed November 7, 2022. http://www.tennessean.com/story/news/crime/2022/05/13/radonda-vaught-sentened-vanderbilt-nurse/9717529002/
- Tennessee Board of Nursing. Disciplinary hearing: RaDonda Vaught, RN #205702, minutes. July 22-23, 2021. Accessed November 7, 2022. https://www.tn.gov/content/dam/tn/health/healthprofboards/nursing/meeting-minutes/Nursing%20Meeting%20Minutes%20July%2022-23,%202021.pdf
- Institute for Safe Medication Practices. TN Board of Nursing’s unjust decision to revoke nurse’s license: travesty on top of tragedy! August 12, 2021. Accessed November 7, 2022. https://www.ismp.org/resources/tn-board-nursings-unjust-decision-revoke-nurses-license-travesty-top-tragedy
- Medina E. Ex-nurse convicted in fatal medication error gets probation. New York Times. May 15, 2022. Accessed November 7, 2022. https://www.nytimes.com/2022/05/15/us/tennessee-nurse-sentencing.html
- Kelman B. In nurse’s trial, investigator says hospital bears ‘heavy’ responsibility for patient death. KHN. March 24, 2022. Accessed November 15, 2022. https://khn.org/news/article/radonda-vaught-fatal-drug-error-vanderbilt-hospital-responsibility/
- Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, ed. To Err Is Human: Building a Safer Health System. National Academies Press; 2000.
- Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Affairs. 2018;37:1736-1743.
- Eisenberg RL, Berlin L. When does malpractice become manslaughter? Am J Roentgenol. 2002;179:331-335.
- Xiulu Ruan v United States, 20-1410, decided June 27, 2022. https://www.supremecourt.gov/opinions/21pdf/20 -1410_1an2.pdf
- 84 Stat. 1260, 21 U. S. C. §841(a).
- 21 CFR §1306.04(a) (2021).
- Liptak A. Supreme Court sides with doctors accused of running pill mills. The New York Times. June 27, 2022.
- Xiulu Ruan v United States, at 2 (slip opinion).
- Pedemonte S. State v. Christensen: criminalizing medical malpractice. Montana Law Rev. 2022;83(1):183-193. Accessed November 7, 2022. https://scholarworks.umt.edu/cgi/view content.cgi?article=2497&context=mlr
- Szalavitz M. A recent Supreme Court ruling will help people in pain. Scientific American. September 19, 2022. Accessed November 15, 2022. https://www.scientificamerican.com/ article/a-recent-supreme-court-ruling-will-help-people-in -pain/
- Lopez I. Opioid pill peddling case threatens future of pain treatment. Bloomberg Law. March 29, 2022. Accessed November 15, 2022. https://news.bloomberglaw.com/health -law-and-business/opioid-pill-peddling-case-threatens -future-of-pain-treatment
Overturning Roe: Exacerbating inequities in abortion care and ObGyn training
On a recent overnight shift, our ObGyn on-call team was urgently paged to the emergency room for a patient who was brought in hemorrhaging after having passed out mid-flight from Texas to Boston. She was 12-weeks pregnant. We rushed her to the operating room for surgical removal of the pregnancy by dilation and curettage to stop her bleeding. Landing in Massachusetts had saved her life.
The significance of this patient’s case was not lost on the multidisciplinary teams caring for her, as the—at the time—impending Roe v Wade decision weighed heavily on our minds. One of many, her story foreshadows the harrowing experiences that we anticipate in the coming months and highlights the danger that the Supreme Court has inflicted on pregnant people nationally.
The Supreme Court decision on Dobbs v Jackson condemns us as a nation in which abortion rights are no longer federally protected under Roe v Wade.1 Twenty-six states have been poised to ban abortion, and in at least 12 states, abortion is now illegal.2,3 Political decision making will soon deny pregnant people the right to bodily autonomy, and the United States will lag behind other nations in abortion access.4 As ObGyn resident physicians who practice in tertiary referral hospitals in Massachusetts, where the ROE Act protects abortion beyond 24 weeks’ gestational age, we affirm abortion as essential health care that saves lives.5
Collectively as physician residents, we have provided an abortion for the patient at 22 weeks with a desired pregnancy who would have otherwise died from high blood pressures, the patient who ended her pregnancy to expedite breast cancer treatment, and the 16-year-old who feared for her life after suffering an assault by her partner for disclosing her pregnancy. With the overturn of Roe v Wade, patients like these will suffer dramatically divergent fates as race, class, and, now more than ever, geography will impact who is able to access abortion care.
Ramifications of the overturn of Roe
History foreshadows the grim impact of repealing Roe. Ohio’s 2011 law that requires the use of the restrictive protocol approved by the US Food and Drug Administration for mifepristone administration deepened existing inequities in abortion access.6 Patients with private insurance, higher income, higher level of education, and those who were White were more likely to obtain abortion care.7 In Texas, after the implementation of SB8 and other restrictive laws, Hispanic women whose travel distance increased more than 100 miles had the greatest reduction in abortion rates.8,9 A recent study regarding banning abortion in the United States estimated a 7% increase in pregnancy-related deaths in 1 year, with a 21% increase in subsequent years.10
Inequities in abortion access subsequently will disparately increase deaths of pregnant individuals in certain populations.11,12 Communities with the highest rates of unintended pregnancy, medical comorbidities, and lack of access to abortion, as well as historically marginalized populations—including non-Hispanic Black people, LGBTQIA people, those with limited English proficiency, and undocumented persons—will experience the greatest increase in pregnancy-related deaths due to a total abortion ban.13-15
The US maternal mortality rate is already the highest among developed nations, and it will only climb if ObGyns are not appropriately trained to operate within our full scope of practice and, thus, are unable to provide the highest quality of care.16,17
Continue: Abortion is a medical treatment that requires resident training...
Abortion is a medical treatment that requires resident training
Abortion care must be protected. Uterine evacuation by medical management, suction curettage, or dilation and evacuation is indicated for undesired pregnancy, regardless of reasoning or life circumstance. Pregnancy carries inherent risks that can at times be deadly.18 Abortion serves as first-line treatment for certain life-threatening pregnancy risks, including septic miscarriage, maternal hemorrhage, early-onset severe preeclampsia, and certain health conditions.19 Surgical skills and medical management of abortion are therefore fundamental components of ObGyn care and residency training.20
In choosing to become ObGyns,and particularly in selecting our training program, the ability to provide safe abortion care was a calculated priority. A recent study on the implications of overturning Roe predicted that nearly half of ObGyn residents will likely or certainly lose access to in-state abortion training.21 As demonstrated already in states with restrictive abortion laws, we will lose an entire generation of medical professionals skilled in performing this lifesaving procedure.9,22 While privileged patients may travel across state borders to access care, ObGyn and other medical trainees who are contract bound to residency programs do not have such flexibility to seek out abortion training. Although we hope the reversal of Roe will be fleeting, the consequences of this lost generation are irreparable.23,24 For physicians like ourselves, who fortunately are trained in surgical abortions and safe management of medical terminations, the discrepancy between evidence-based guidelines and impending political restrictions is distressing. We are forced to imagine refusing patients necessary health care—or face incarceration to save their lives.
The idea of watching a patient die, whether by hemorrhage, sepsis, or suicide, while armed with the tools of safe abortion technique is horrific. As authors with roots in Texas, Michigan, and Georgia, where abortion has or will almost certainly become illegal now that Roe v Wade is overturned, this scene is personal. It affects our future patients, our families, our colleagues, and our ability to return to our home states to live and practice.
Political organizing is critical to protect and restore abortion rights and defend against conservative coercive politics.25 Nearly half of pregnancies in the United States are unintended, and more than half of these end in abortion.26,27 Threats to abortion access require action from every one of the 59% of Americans who believe abortion should remain legal.28 This is especially important from a social and racial justice perspective as abortion bans will disproportionately affect marginalized groups and further exacerbate inequities in maternal mortality.13
Call to action
Now is the time for community action for reproductive justice and human rights. We urge everyone to donate to abortion funds, vote for leaders who support reproductive justice, and petition your state legislators to codify Roe into law. Now is the time to expand legislation to protect abortion providers and our patients. To ObGyns, family medicine physicians, internists, and other reproductive health clinicians, now is the time to maximize your abortion training. Now is the time to act; otherwise, pregnant individuals will die and future generations of physicians will not have the training to save their lives. ●
- de Vogue A, Sneed T, Duster C, et al. Supreme Court overturns Roe v Wade. CNN Politics. June 24, 2022. Accessed July 19, 2022. https://www.cnn.com/2022/06/24/politics/dobbs-missis sippi-supreme-court-abortion-roe-wade/index.html
- Nash E, Cross L. 26 States are certain or likely to ban abortion without Roe: here’s which ones and why. Guttmacher Institute. October 28, 2021. Updated April 19, 2022. Accessed July 19, 2022. https://www.guttmacher.org/article/2021/10/26-states-are-certain-or-likely-ban-abortion-without-roe-heres-which-ones-and-why
- Messerly M. Abortion laws by state: where abortions are illegal after Roe v Wade overturned. Politico. June 24, 2022. Accessed July 19, 2022. https://www.politico.com/news/2022/06/24/abortion-laws-by-state-roe-v-wade-00037695
- Archie A. US would lag behind global abortion access if Roe v Wade is undone, advocates say. NPR. May 5, 2022. Accessed July 19, 2022. https://www.npr.org/2022/05/05/1096805490/abortion-access-supreme-court-roe-v-wade-united-nations
- Romo V. Massachusetts senate overrides veto, passes law expanding abortion access. NPR. December 29, 2020. Accessed July 19, 2022. https://www.npr.org/2020/12/29/951259506/massachusetts-senate-overrides-veto-passes-law-expanding-abortion-access
- Upadhyay UD, Johns NE, Combellick SL, et al. Comparison of outcomes before and after Ohio’s law mandating use of the FDA-approved protocol for medication abortion: a retrospective cohort study. PLoS Med. 2016;13:e1002110.
- Upadhyay UD, Johns NE, Cartwright AF, et al. Sociodemographic characteristics of women able to obtain medication abortion before and after Ohio’s law requiring use of the Food and Drug Administration protocol. Health Equity. 2018;2:122-130.
- Goyal V, Brooks IHM, Powers DA. Differences in abortion rates by race-ethnicity after implementation of a restrictive Texas law. Contraception. 2020;102:109-114.
- Noyes E Holder BH, Evans ML. Texas SB8 and the future of abortion care. OBG Manag. 2021;33. doi:12788/obgm.0151.
- Vilda D, Wallace ME, Daniel C, et al. State abortion policies and maternal death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704.
- The Lancet. Why Roe v Wade must be defended. Lancet. 2022;399:1845.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in two Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Stevenson AJ. The pregnancy-related mortality impact of a total abortion ban in the United States: a research note on increased deaths due to remaining pregnant. Demography. 2021;58:20192028.
- Medley S. Gutting abortion rights would be devastating for LGBTQ+ people. Them. September 17, 2021. Accessed July 20, 2022. https://www.them.us/story/gutting-abortion-rights-devastating-lgbtq-people
- Holter L. Detained immigrant women are facing a grueling abortion struggle. National Latina Institute for Reproductive Justice. May 10, 2017. Accessed July 20, 2022. https://www.latinainsti tute.org/es/node/4620
- Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. 2009;2:122-126.
- Tikkanen R, Gunja MZ, FitzGerald M, et al. Maternal mortality and maternity care in the United States compared to 10 other developed countries. The Commonwealth Fund. November 18, 2020. Accessed November 17, 2022. https://www .commonwealthfund.org/publications/issue -briefs/2020/nov/maternal-mortality-maternity -care-us-compared-10-countries
- Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews. 2019;20:e561-e574.
- ACOG practice bulletin no. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394-1406.
- Committee on Health Care for Underserved Women. ACOG Committee opinion no. 612: Abortion training and education. Obstet Gynecol. 2014;124:1055-1059.
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- Horvath S, Turk J, Steinauer J, et al. Increase in obstetrics and gynecology resident self-assessed competence in early pregnancy loss management with routine abortion care training. Obstet Gynecol. 2022;139:116-119.
- Anderson N. The fall of Roe scrambles abortion training in university hospitals. The Washington Post. June 30, 2022. Accessed July 20, 2022. https://www.washingtonpost.com/educa tion/2022/06/30/abortion-training-upheaval-dobbs/
- Weiner S. How the repeal of Roe v Wade will affect training in abortion and reproductive health. AAMC. June 24, 2022. Accessed July 20, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health
- Dreweke J. Coercion is at the heart of social conservatives’ reproductive health agenda. Guttmacher Institute. February 7, 2018. Accessed July 20, 2022. https://www.guttmacher.org/gpr/2018/02/coercion-heart-social-conservatives-reproduc tive-health-agenda
- Unintended pregnancy and abortion worldwide. Guttmacher Institute. March 2022. Accessed July 20, 2022. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Hartig H. About six-in-ten Americans say abortion should be legal in all or most cases. Pew Research Center. June 13, 2022. Accessed July 20, 2022. https://www.pewresearch.org/fact-tank/2022/06/13/about-six-in-ten-americans-say-abortion-should-be-legal-in-all-or-most-cases-2/
On a recent overnight shift, our ObGyn on-call team was urgently paged to the emergency room for a patient who was brought in hemorrhaging after having passed out mid-flight from Texas to Boston. She was 12-weeks pregnant. We rushed her to the operating room for surgical removal of the pregnancy by dilation and curettage to stop her bleeding. Landing in Massachusetts had saved her life.
The significance of this patient’s case was not lost on the multidisciplinary teams caring for her, as the—at the time—impending Roe v Wade decision weighed heavily on our minds. One of many, her story foreshadows the harrowing experiences that we anticipate in the coming months and highlights the danger that the Supreme Court has inflicted on pregnant people nationally.
The Supreme Court decision on Dobbs v Jackson condemns us as a nation in which abortion rights are no longer federally protected under Roe v Wade.1 Twenty-six states have been poised to ban abortion, and in at least 12 states, abortion is now illegal.2,3 Political decision making will soon deny pregnant people the right to bodily autonomy, and the United States will lag behind other nations in abortion access.4 As ObGyn resident physicians who practice in tertiary referral hospitals in Massachusetts, where the ROE Act protects abortion beyond 24 weeks’ gestational age, we affirm abortion as essential health care that saves lives.5
Collectively as physician residents, we have provided an abortion for the patient at 22 weeks with a desired pregnancy who would have otherwise died from high blood pressures, the patient who ended her pregnancy to expedite breast cancer treatment, and the 16-year-old who feared for her life after suffering an assault by her partner for disclosing her pregnancy. With the overturn of Roe v Wade, patients like these will suffer dramatically divergent fates as race, class, and, now more than ever, geography will impact who is able to access abortion care.
Ramifications of the overturn of Roe
History foreshadows the grim impact of repealing Roe. Ohio’s 2011 law that requires the use of the restrictive protocol approved by the US Food and Drug Administration for mifepristone administration deepened existing inequities in abortion access.6 Patients with private insurance, higher income, higher level of education, and those who were White were more likely to obtain abortion care.7 In Texas, after the implementation of SB8 and other restrictive laws, Hispanic women whose travel distance increased more than 100 miles had the greatest reduction in abortion rates.8,9 A recent study regarding banning abortion in the United States estimated a 7% increase in pregnancy-related deaths in 1 year, with a 21% increase in subsequent years.10
Inequities in abortion access subsequently will disparately increase deaths of pregnant individuals in certain populations.11,12 Communities with the highest rates of unintended pregnancy, medical comorbidities, and lack of access to abortion, as well as historically marginalized populations—including non-Hispanic Black people, LGBTQIA people, those with limited English proficiency, and undocumented persons—will experience the greatest increase in pregnancy-related deaths due to a total abortion ban.13-15
The US maternal mortality rate is already the highest among developed nations, and it will only climb if ObGyns are not appropriately trained to operate within our full scope of practice and, thus, are unable to provide the highest quality of care.16,17
Continue: Abortion is a medical treatment that requires resident training...
Abortion is a medical treatment that requires resident training
Abortion care must be protected. Uterine evacuation by medical management, suction curettage, or dilation and evacuation is indicated for undesired pregnancy, regardless of reasoning or life circumstance. Pregnancy carries inherent risks that can at times be deadly.18 Abortion serves as first-line treatment for certain life-threatening pregnancy risks, including septic miscarriage, maternal hemorrhage, early-onset severe preeclampsia, and certain health conditions.19 Surgical skills and medical management of abortion are therefore fundamental components of ObGyn care and residency training.20
In choosing to become ObGyns,and particularly in selecting our training program, the ability to provide safe abortion care was a calculated priority. A recent study on the implications of overturning Roe predicted that nearly half of ObGyn residents will likely or certainly lose access to in-state abortion training.21 As demonstrated already in states with restrictive abortion laws, we will lose an entire generation of medical professionals skilled in performing this lifesaving procedure.9,22 While privileged patients may travel across state borders to access care, ObGyn and other medical trainees who are contract bound to residency programs do not have such flexibility to seek out abortion training. Although we hope the reversal of Roe will be fleeting, the consequences of this lost generation are irreparable.23,24 For physicians like ourselves, who fortunately are trained in surgical abortions and safe management of medical terminations, the discrepancy between evidence-based guidelines and impending political restrictions is distressing. We are forced to imagine refusing patients necessary health care—or face incarceration to save their lives.
The idea of watching a patient die, whether by hemorrhage, sepsis, or suicide, while armed with the tools of safe abortion technique is horrific. As authors with roots in Texas, Michigan, and Georgia, where abortion has or will almost certainly become illegal now that Roe v Wade is overturned, this scene is personal. It affects our future patients, our families, our colleagues, and our ability to return to our home states to live and practice.
Political organizing is critical to protect and restore abortion rights and defend against conservative coercive politics.25 Nearly half of pregnancies in the United States are unintended, and more than half of these end in abortion.26,27 Threats to abortion access require action from every one of the 59% of Americans who believe abortion should remain legal.28 This is especially important from a social and racial justice perspective as abortion bans will disproportionately affect marginalized groups and further exacerbate inequities in maternal mortality.13
Call to action
Now is the time for community action for reproductive justice and human rights. We urge everyone to donate to abortion funds, vote for leaders who support reproductive justice, and petition your state legislators to codify Roe into law. Now is the time to expand legislation to protect abortion providers and our patients. To ObGyns, family medicine physicians, internists, and other reproductive health clinicians, now is the time to maximize your abortion training. Now is the time to act; otherwise, pregnant individuals will die and future generations of physicians will not have the training to save their lives. ●
On a recent overnight shift, our ObGyn on-call team was urgently paged to the emergency room for a patient who was brought in hemorrhaging after having passed out mid-flight from Texas to Boston. She was 12-weeks pregnant. We rushed her to the operating room for surgical removal of the pregnancy by dilation and curettage to stop her bleeding. Landing in Massachusetts had saved her life.
The significance of this patient’s case was not lost on the multidisciplinary teams caring for her, as the—at the time—impending Roe v Wade decision weighed heavily on our minds. One of many, her story foreshadows the harrowing experiences that we anticipate in the coming months and highlights the danger that the Supreme Court has inflicted on pregnant people nationally.
The Supreme Court decision on Dobbs v Jackson condemns us as a nation in which abortion rights are no longer federally protected under Roe v Wade.1 Twenty-six states have been poised to ban abortion, and in at least 12 states, abortion is now illegal.2,3 Political decision making will soon deny pregnant people the right to bodily autonomy, and the United States will lag behind other nations in abortion access.4 As ObGyn resident physicians who practice in tertiary referral hospitals in Massachusetts, where the ROE Act protects abortion beyond 24 weeks’ gestational age, we affirm abortion as essential health care that saves lives.5
Collectively as physician residents, we have provided an abortion for the patient at 22 weeks with a desired pregnancy who would have otherwise died from high blood pressures, the patient who ended her pregnancy to expedite breast cancer treatment, and the 16-year-old who feared for her life after suffering an assault by her partner for disclosing her pregnancy. With the overturn of Roe v Wade, patients like these will suffer dramatically divergent fates as race, class, and, now more than ever, geography will impact who is able to access abortion care.
Ramifications of the overturn of Roe
History foreshadows the grim impact of repealing Roe. Ohio’s 2011 law that requires the use of the restrictive protocol approved by the US Food and Drug Administration for mifepristone administration deepened existing inequities in abortion access.6 Patients with private insurance, higher income, higher level of education, and those who were White were more likely to obtain abortion care.7 In Texas, after the implementation of SB8 and other restrictive laws, Hispanic women whose travel distance increased more than 100 miles had the greatest reduction in abortion rates.8,9 A recent study regarding banning abortion in the United States estimated a 7% increase in pregnancy-related deaths in 1 year, with a 21% increase in subsequent years.10
Inequities in abortion access subsequently will disparately increase deaths of pregnant individuals in certain populations.11,12 Communities with the highest rates of unintended pregnancy, medical comorbidities, and lack of access to abortion, as well as historically marginalized populations—including non-Hispanic Black people, LGBTQIA people, those with limited English proficiency, and undocumented persons—will experience the greatest increase in pregnancy-related deaths due to a total abortion ban.13-15
The US maternal mortality rate is already the highest among developed nations, and it will only climb if ObGyns are not appropriately trained to operate within our full scope of practice and, thus, are unable to provide the highest quality of care.16,17
Continue: Abortion is a medical treatment that requires resident training...
Abortion is a medical treatment that requires resident training
Abortion care must be protected. Uterine evacuation by medical management, suction curettage, or dilation and evacuation is indicated for undesired pregnancy, regardless of reasoning or life circumstance. Pregnancy carries inherent risks that can at times be deadly.18 Abortion serves as first-line treatment for certain life-threatening pregnancy risks, including septic miscarriage, maternal hemorrhage, early-onset severe preeclampsia, and certain health conditions.19 Surgical skills and medical management of abortion are therefore fundamental components of ObGyn care and residency training.20
In choosing to become ObGyns,and particularly in selecting our training program, the ability to provide safe abortion care was a calculated priority. A recent study on the implications of overturning Roe predicted that nearly half of ObGyn residents will likely or certainly lose access to in-state abortion training.21 As demonstrated already in states with restrictive abortion laws, we will lose an entire generation of medical professionals skilled in performing this lifesaving procedure.9,22 While privileged patients may travel across state borders to access care, ObGyn and other medical trainees who are contract bound to residency programs do not have such flexibility to seek out abortion training. Although we hope the reversal of Roe will be fleeting, the consequences of this lost generation are irreparable.23,24 For physicians like ourselves, who fortunately are trained in surgical abortions and safe management of medical terminations, the discrepancy between evidence-based guidelines and impending political restrictions is distressing. We are forced to imagine refusing patients necessary health care—or face incarceration to save their lives.
The idea of watching a patient die, whether by hemorrhage, sepsis, or suicide, while armed with the tools of safe abortion technique is horrific. As authors with roots in Texas, Michigan, and Georgia, where abortion has or will almost certainly become illegal now that Roe v Wade is overturned, this scene is personal. It affects our future patients, our families, our colleagues, and our ability to return to our home states to live and practice.
Political organizing is critical to protect and restore abortion rights and defend against conservative coercive politics.25 Nearly half of pregnancies in the United States are unintended, and more than half of these end in abortion.26,27 Threats to abortion access require action from every one of the 59% of Americans who believe abortion should remain legal.28 This is especially important from a social and racial justice perspective as abortion bans will disproportionately affect marginalized groups and further exacerbate inequities in maternal mortality.13
Call to action
Now is the time for community action for reproductive justice and human rights. We urge everyone to donate to abortion funds, vote for leaders who support reproductive justice, and petition your state legislators to codify Roe into law. Now is the time to expand legislation to protect abortion providers and our patients. To ObGyns, family medicine physicians, internists, and other reproductive health clinicians, now is the time to maximize your abortion training. Now is the time to act; otherwise, pregnant individuals will die and future generations of physicians will not have the training to save their lives. ●
- de Vogue A, Sneed T, Duster C, et al. Supreme Court overturns Roe v Wade. CNN Politics. June 24, 2022. Accessed July 19, 2022. https://www.cnn.com/2022/06/24/politics/dobbs-missis sippi-supreme-court-abortion-roe-wade/index.html
- Nash E, Cross L. 26 States are certain or likely to ban abortion without Roe: here’s which ones and why. Guttmacher Institute. October 28, 2021. Updated April 19, 2022. Accessed July 19, 2022. https://www.guttmacher.org/article/2021/10/26-states-are-certain-or-likely-ban-abortion-without-roe-heres-which-ones-and-why
- Messerly M. Abortion laws by state: where abortions are illegal after Roe v Wade overturned. Politico. June 24, 2022. Accessed July 19, 2022. https://www.politico.com/news/2022/06/24/abortion-laws-by-state-roe-v-wade-00037695
- Archie A. US would lag behind global abortion access if Roe v Wade is undone, advocates say. NPR. May 5, 2022. Accessed July 19, 2022. https://www.npr.org/2022/05/05/1096805490/abortion-access-supreme-court-roe-v-wade-united-nations
- Romo V. Massachusetts senate overrides veto, passes law expanding abortion access. NPR. December 29, 2020. Accessed July 19, 2022. https://www.npr.org/2020/12/29/951259506/massachusetts-senate-overrides-veto-passes-law-expanding-abortion-access
- Upadhyay UD, Johns NE, Combellick SL, et al. Comparison of outcomes before and after Ohio’s law mandating use of the FDA-approved protocol for medication abortion: a retrospective cohort study. PLoS Med. 2016;13:e1002110.
- Upadhyay UD, Johns NE, Cartwright AF, et al. Sociodemographic characteristics of women able to obtain medication abortion before and after Ohio’s law requiring use of the Food and Drug Administration protocol. Health Equity. 2018;2:122-130.
- Goyal V, Brooks IHM, Powers DA. Differences in abortion rates by race-ethnicity after implementation of a restrictive Texas law. Contraception. 2020;102:109-114.
- Noyes E Holder BH, Evans ML. Texas SB8 and the future of abortion care. OBG Manag. 2021;33. doi:12788/obgm.0151.
- Vilda D, Wallace ME, Daniel C, et al. State abortion policies and maternal death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704.
- The Lancet. Why Roe v Wade must be defended. Lancet. 2022;399:1845.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in two Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Stevenson AJ. The pregnancy-related mortality impact of a total abortion ban in the United States: a research note on increased deaths due to remaining pregnant. Demography. 2021;58:20192028.
- Medley S. Gutting abortion rights would be devastating for LGBTQ+ people. Them. September 17, 2021. Accessed July 20, 2022. https://www.them.us/story/gutting-abortion-rights-devastating-lgbtq-people
- Holter L. Detained immigrant women are facing a grueling abortion struggle. National Latina Institute for Reproductive Justice. May 10, 2017. Accessed July 20, 2022. https://www.latinainsti tute.org/es/node/4620
- Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. 2009;2:122-126.
- Tikkanen R, Gunja MZ, FitzGerald M, et al. Maternal mortality and maternity care in the United States compared to 10 other developed countries. The Commonwealth Fund. November 18, 2020. Accessed November 17, 2022. https://www .commonwealthfund.org/publications/issue -briefs/2020/nov/maternal-mortality-maternity -care-us-compared-10-countries
- Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews. 2019;20:e561-e574.
- ACOG practice bulletin no. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394-1406.
- Committee on Health Care for Underserved Women. ACOG Committee opinion no. 612: Abortion training and education. Obstet Gynecol. 2014;124:1055-1059.
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- Horvath S, Turk J, Steinauer J, et al. Increase in obstetrics and gynecology resident self-assessed competence in early pregnancy loss management with routine abortion care training. Obstet Gynecol. 2022;139:116-119.
- Anderson N. The fall of Roe scrambles abortion training in university hospitals. The Washington Post. June 30, 2022. Accessed July 20, 2022. https://www.washingtonpost.com/educa tion/2022/06/30/abortion-training-upheaval-dobbs/
- Weiner S. How the repeal of Roe v Wade will affect training in abortion and reproductive health. AAMC. June 24, 2022. Accessed July 20, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health
- Dreweke J. Coercion is at the heart of social conservatives’ reproductive health agenda. Guttmacher Institute. February 7, 2018. Accessed July 20, 2022. https://www.guttmacher.org/gpr/2018/02/coercion-heart-social-conservatives-reproduc tive-health-agenda
- Unintended pregnancy and abortion worldwide. Guttmacher Institute. March 2022. Accessed July 20, 2022. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Hartig H. About six-in-ten Americans say abortion should be legal in all or most cases. Pew Research Center. June 13, 2022. Accessed July 20, 2022. https://www.pewresearch.org/fact-tank/2022/06/13/about-six-in-ten-americans-say-abortion-should-be-legal-in-all-or-most-cases-2/
- de Vogue A, Sneed T, Duster C, et al. Supreme Court overturns Roe v Wade. CNN Politics. June 24, 2022. Accessed July 19, 2022. https://www.cnn.com/2022/06/24/politics/dobbs-missis sippi-supreme-court-abortion-roe-wade/index.html
- Nash E, Cross L. 26 States are certain or likely to ban abortion without Roe: here’s which ones and why. Guttmacher Institute. October 28, 2021. Updated April 19, 2022. Accessed July 19, 2022. https://www.guttmacher.org/article/2021/10/26-states-are-certain-or-likely-ban-abortion-without-roe-heres-which-ones-and-why
- Messerly M. Abortion laws by state: where abortions are illegal after Roe v Wade overturned. Politico. June 24, 2022. Accessed July 19, 2022. https://www.politico.com/news/2022/06/24/abortion-laws-by-state-roe-v-wade-00037695
- Archie A. US would lag behind global abortion access if Roe v Wade is undone, advocates say. NPR. May 5, 2022. Accessed July 19, 2022. https://www.npr.org/2022/05/05/1096805490/abortion-access-supreme-court-roe-v-wade-united-nations
- Romo V. Massachusetts senate overrides veto, passes law expanding abortion access. NPR. December 29, 2020. Accessed July 19, 2022. https://www.npr.org/2020/12/29/951259506/massachusetts-senate-overrides-veto-passes-law-expanding-abortion-access
- Upadhyay UD, Johns NE, Combellick SL, et al. Comparison of outcomes before and after Ohio’s law mandating use of the FDA-approved protocol for medication abortion: a retrospective cohort study. PLoS Med. 2016;13:e1002110.
- Upadhyay UD, Johns NE, Cartwright AF, et al. Sociodemographic characteristics of women able to obtain medication abortion before and after Ohio’s law requiring use of the Food and Drug Administration protocol. Health Equity. 2018;2:122-130.
- Goyal V, Brooks IHM, Powers DA. Differences in abortion rates by race-ethnicity after implementation of a restrictive Texas law. Contraception. 2020;102:109-114.
- Noyes E Holder BH, Evans ML. Texas SB8 and the future of abortion care. OBG Manag. 2021;33. doi:12788/obgm.0151.
- Vilda D, Wallace ME, Daniel C, et al. State abortion policies and maternal death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704.
- The Lancet. Why Roe v Wade must be defended. Lancet. 2022;399:1845.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in two Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Stevenson AJ. The pregnancy-related mortality impact of a total abortion ban in the United States: a research note on increased deaths due to remaining pregnant. Demography. 2021;58:20192028.
- Medley S. Gutting abortion rights would be devastating for LGBTQ+ people. Them. September 17, 2021. Accessed July 20, 2022. https://www.them.us/story/gutting-abortion-rights-devastating-lgbtq-people
- Holter L. Detained immigrant women are facing a grueling abortion struggle. National Latina Institute for Reproductive Justice. May 10, 2017. Accessed July 20, 2022. https://www.latinainsti tute.org/es/node/4620
- Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. 2009;2:122-126.
- Tikkanen R, Gunja MZ, FitzGerald M, et al. Maternal mortality and maternity care in the United States compared to 10 other developed countries. The Commonwealth Fund. November 18, 2020. Accessed November 17, 2022. https://www .commonwealthfund.org/publications/issue -briefs/2020/nov/maternal-mortality-maternity -care-us-compared-10-countries
- Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews. 2019;20:e561-e574.
- ACOG practice bulletin no. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394-1406.
- Committee on Health Care for Underserved Women. ACOG Committee opinion no. 612: Abortion training and education. Obstet Gynecol. 2014;124:1055-1059.
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- Horvath S, Turk J, Steinauer J, et al. Increase in obstetrics and gynecology resident self-assessed competence in early pregnancy loss management with routine abortion care training. Obstet Gynecol. 2022;139:116-119.
- Anderson N. The fall of Roe scrambles abortion training in university hospitals. The Washington Post. June 30, 2022. Accessed July 20, 2022. https://www.washingtonpost.com/educa tion/2022/06/30/abortion-training-upheaval-dobbs/
- Weiner S. How the repeal of Roe v Wade will affect training in abortion and reproductive health. AAMC. June 24, 2022. Accessed July 20, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health
- Dreweke J. Coercion is at the heart of social conservatives’ reproductive health agenda. Guttmacher Institute. February 7, 2018. Accessed July 20, 2022. https://www.guttmacher.org/gpr/2018/02/coercion-heart-social-conservatives-reproduc tive-health-agenda
- Unintended pregnancy and abortion worldwide. Guttmacher Institute. March 2022. Accessed July 20, 2022. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Hartig H. About six-in-ten Americans say abortion should be legal in all or most cases. Pew Research Center. June 13, 2022. Accessed July 20, 2022. https://www.pewresearch.org/fact-tank/2022/06/13/about-six-in-ten-americans-say-abortion-should-be-legal-in-all-or-most-cases-2/
How to advocate in a post-Roe world, no matter your zip code
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
For many, the recent Supreme Court decision in the Dobbs v Jackson case that removed the constitutional right to an abortion has introduced outrage, fear, and confusion throughout the country. While the American College of Obstetricians and Gynecologists (ACOG) clearly has established that abortion is essential health care and has published resources regarding the issue (www.acog.org/advocacy/abortion-is-essential), and many providers know what to do medically, they do not know what they can do legally. In a country where 45% of pregnancies are unplanned and 25% of women will access abortion services in their lifetime, this decision will completely change the landscape of providing and receiving abortion care. This decision will affect every provider and their patients and will affect them differently in each state. The country likely will be divided into 24 destination states that will protect the right to abortion and another 26 states that have or will soon ban abortion or severely restrict access to it.
Regardless of the state you practice in, it is clear that our voices, actions, and advocacy are essential during these challenging times. It can feel difficult to find ways to advocate, especially if you are in a state or have an employer that supports anti-abortion legislation or has been silent after the Dobbs decision was released. We have created a guide to help and encourage all ObGyn providers to find ways to advocate, no matter their zip code.
1. Donate
Many of our patients will need to travel out of state to seek abortion care. The cost of abortion care can be expensive, and travel, child care, and time off of work add to the costs of the procedure itself, making access to abortion care financially out of reach for some. There are many well-established abortion funds throughout the country; consider donating to one of them or organizing a fundraiser in your community. Go to abortionfunds.org/funds to find an abortion fund that will support patients in your community, or donate generally to support them all.
2. Save your stories
We already are hearing the devastating impact abortion bans have on patient care around the country. If you had to deny or delay care because of the new legal landscape surrounding abortion, write down or record the experience. Your stories can be critical in discussing the impact of legislation. If you choose to share on social media, ask the involved patients if they are comfortable with their story being shared online (as long as their identity is protected).
3. Talk about it
Talking about abortion is a critical step in destigmatizing it and supporting our patients as well as our field. These conversations can be challenging, but ACOG has provided an important guide that includes key phrases and statements to help shape the conversation and avoid polarizing language (https://www.acog.org/advocacy/abortion-is-essential/come-prepared). This guide also can be helpful to keep in mind when talking to members of the media.
Continue to: 4. Write about it...
4. Write about it
There are many opportunities to write about the impact of the Dobbs decision, especially locally. As a clinician and trusted member of the community, you can uniquely share your and your patients’ experiences. Your article does not have to appear in a major publication; you can still have an important impact in your local paper. See resources on how to write an op-ed and letter to the editor (https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community/legislative-rx-op-eds-and-letters-to-the-editor).
5. Teach about it
These legislative changes uniquely impact our ObGyn residents; 44% of residents likely will be in a training program in a state that will ban or severely restrict abortion access. Abortion is health care, and a vast majority of our residents could graduate without important skills to save lives. As we strategize to ensure all ObGyn residents are able to receive this important training, work on incorporating an advocacy curriculum into your residents’ educational experience. Teaching about how to advocate is an important skill for supporting our patients and ensuring critical health policy. ACOG has published guides focused on education and training (www.acog.org/advocacy/abortion-is-essential/education-and-training). We also have included our own medical center’s advocacy curriculum (https://docs.google.com/document/d/1STxLzE0j55mlDEbF0_wZbo9O QryAcs6RpfZ47Mwfs4I/edit).
6. Get involved and seek out allies
It’s important that ObGyns be at the table for all discussions surrounding abortion care and reproductive health. Join hospital committees and help influence policy within your own institution. Refer back to those abortion talking points—this will help in some of these challenging conversations.
7. Get on social media
Using social media can be a powerful tool for advocacy. You can help elevate issues and encourage others to get active as well. Using a common hashtag, such as #AbortionisHealthcare, on different platforms can help connect you to other advocates. Share simple and important graphics provided by ACOG on important topics in our field (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state/social-media) and review ACOG’s recommendation for professionalism in social media (https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/10/professional-use-of-digital-and-social-media).
8. Get active locally
We have seen the introduction of hundreds of bills in states around the country not only on abortion but also on other legislation that directly impacts the care we provide. It is critical that we get involved in advocating for important reproductive health legislation and against bills that cause harm and interfere with the doctor-patient relationship. Stay up to date on legislative issues with your local ACOG and medical chapters (https://www.acog.org/advocacy/abortion-is-essential/advocate-in-your-state). Consider testifying at your State house, providing written or oral testimony. Connect with ACOG or your state medical chapter to help with talking points!
9. Read up
There have been many new policies at the federal level that could impact the care you provide. Take some time to read up on these new changes. Patients also may ask you about self-managed abortion. There are guides and resources (https://www.acog.org/advocacy/abortion-is-essential/practice-management) for patients that may seek medication online, and we want to ensure that patients have the resources to make informed decisions.
10. Hit the Capitol
Consider making time to come to the annual Congressional Leadership Conference in Washington, DC (https://www.acog.org/education-and-events/meetings/acog-congressional-leadership-conference), or other advocacy events offered through the American Medical Association or other subspecialty organizations. When we all come together as an organization, a field, and a community, it sends a powerful message that we are standing up together for our patients and our colleagues.
Make a difference
There is no advocacy too big or too small. It is critical that we continue to use our voices and our platforms to stand up for health care and access to critical services, including abortion care. ●
Incorporating medication abortion into your ObGyn practice: Why and how
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.
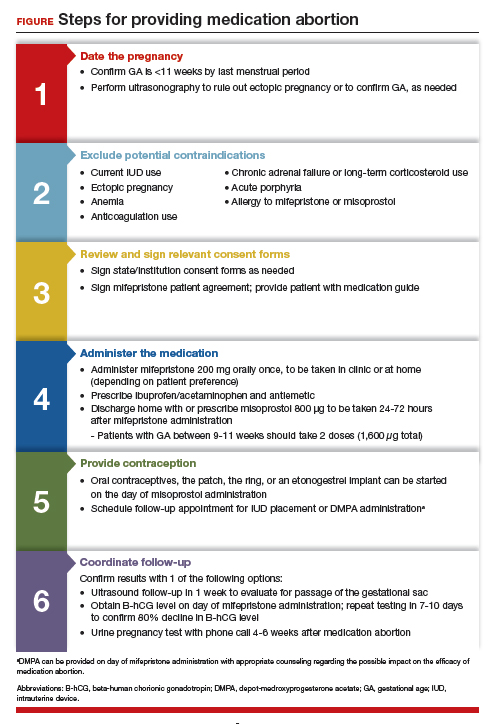
Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.