User login
Surgical management of early pregnancy loss
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
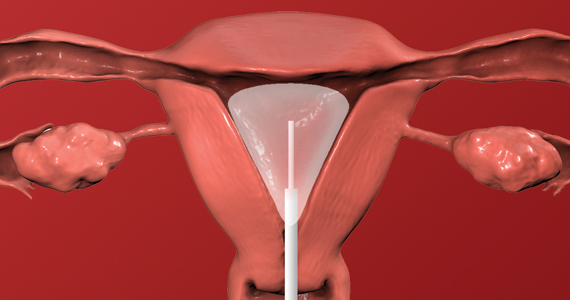
Hysteroscopic management
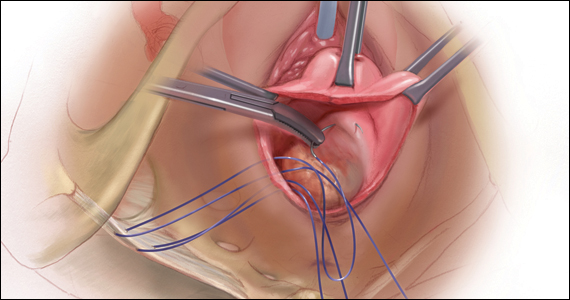
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
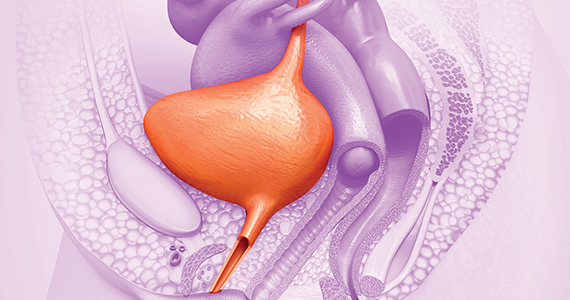
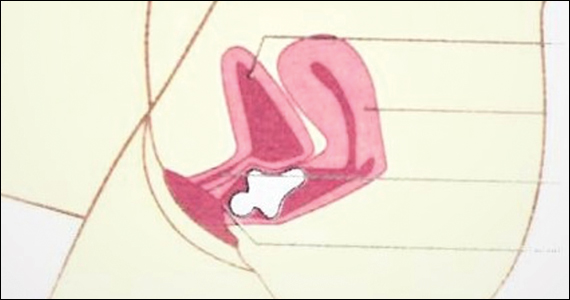
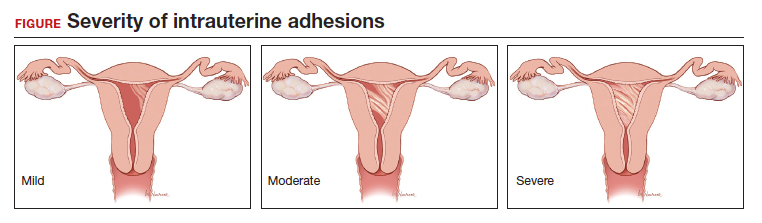
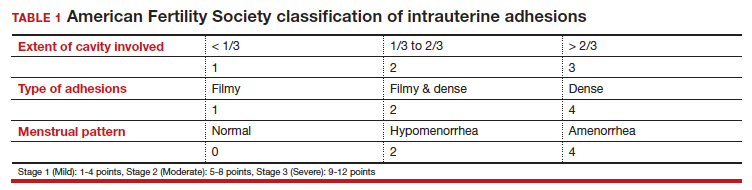
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).
Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
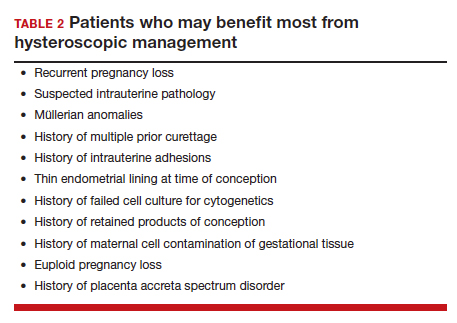
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.
CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
2022 Update on pelvic floor dysfunction
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Knowledge of the latest evidence on the management of pelvic floor disorders is essential for all practicing ObGyns. In this Update, we review long-term outcomes for a polyacrylamide hydrogel urethral bulking agent for the treatment of stress urinary incontinence (SUI) that presents a viable alternative to the gold standard, midurethral sling. We review the new recommendations from the American Urogynecologic Society (AUGS) regarding the administration of anticholinergics, highlighting a paradigm shift in the management of overactive bladder (OAB). In addition, we present data on a proposed threshold glycosylated hemoglobin A1c (HbA1c) level for patients undergoing pelvic organ prolapse (POP) surgery that may help reduce the risk of perioperative complications. Finally, we consider new evidence on the long-term efficacy and safety of transvaginal mesh for repair of POP.
Periurethral injection with polyacrylamide hydrogel is a long-term durable and safe option for women with SUI
Brosche T, Kuhn A, Lobodasch K, et al. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40:502-508. doi:10.1002/nau.24589.
Urethral bulking agents are a less invasive management option for women with SUI compared with the gold standard, midurethral sling. Treatment with a polyacrylamide hydrogel (PAHG; Bulkamid)—a nonparticulate hydrogel bulking agent—showed long-term efficacy and a favorable safety profile at 7 years’ follow-up.
Study details
Brosche and colleagues conducted a retrospective cohort study that included women with SUI or stress-predominant mixed urinary incontinence (MUI) who underwent transurethral PAHG injections for primary treatment of their incontinence symptoms. The study objective was to evaluate the long-term efficacy of PAHG based on patient satisfaction. Treatment safety was a secondary outcome.
Pad counts and validated questionnaires were used to determine treatment effectiveness. Additional data on reinjection rates, perioperative complications, and postoperative complications also were collected.
Long-term outcomes favorable
During the study time period, 1,200 patients were treated with PAHG, and 7-year data were available for 553 women. Of the 553 patients, 67% reported improvement or cure of their SUI symptoms when PAHG was performed as a primary procedure, consistent with previously published 12-month data. There were no perioperative complications. Postoperative complications were transient. Short-term subjective prolonged bladder emptying was the most common complication and occurred in 15% of patients.
PAHG injection is a durable and safe alternative for the treatment of stress urinary incontinence in women who are not candidates for or who decline treatment with alternative methods, such as a midurethral sling.
Continue to: New society guidance...
New society guidance on the use of anticholinergic medications for the treatment of OAB
AUGS Clinical Consensus Statement: Association of anticholinergic medication use and cognition in women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27:69-71. doi:10.1097/ SPV.0000000000001008.
In 2021, AUGS updated its consensus statement on the use of anticholinergic medications for the treatment of OAB. This action was in response to growing evidence that supports the association of anticholinergic medications with long-term cognitive adverse effects, including cognitive impairment, dementia, and Alzheimer disease.
Here, we summarize the most recent modifications, which differentiate the updated statement from the preceding consensus document published in 2017.
Updated AUGS recommendations
- If considering anticholinergic medications, counsel patients about the risk of cognitive adverse effects and weigh these risks against the potential benefits to their quality of life and overall health.
- Use the lowest possible dose when prescribing anticholinergics and consider alternatives such as β3 agonists (for example, mirabegron or vibegron).
- Avoid using anticholinergic medications in women older than age 70. However, if an anticholinergic must be used, consider a medication that has low potential to cross the blood-brain barrier (for example, trospium).
For patients who are unresponsive to behavioral therapies for OAB, medical management may be considered. However, the risks of anticholinergic medications may outweigh the benefits—especially for older women—and these medications should be prescribed with caution after discussing the potential cognitive adverse effects with patients. β3 agonists should be preferentially prescribed when appropriate. Consider referral to a urogynecologist for discussion of third-line therapies in patients who prefer to forego or may not be candidates for medical management of their OAB symptoms.
HbA1c levels > 8% may increase complications risk in urogyn surgery
Ringel NE, de Winter KL, Siddique M, et al. Surgical outcomes in urogynecology—assessment of perioperative and postoperative complications relative to preoperative hemoglobin A1c—a Fellows Pelvic Research Network study. Female Pelvic Med Reconstr Surg. 2022;28:7-13. doi:10.1097/ SPV.0000000000001057.
Diabetes mellitus is a known risk factor for complications following surgery. Adoption of an HbA1c level threshold for risk stratification before urogynecologic surgery may help improve patient outcomes.
Study details
Ringel and colleagues conducted a multicenter retrospective cohort study that included women with diabetes mellitus who underwent prolapse and/or SUI surgery between 2013 and 2018. The aim of the study was to identify a hemoglobin A1C threshold that would help predict increased risk for perioperative complications in women undergoing pelvic reconstructive surgery. Demographics, preoperative HbA1c levels, and surgical data were collected.
Complication risks correlated with higher HbA1c threshold
The study included 807 women with HbA1c values that ranged from 5% to 12%. The overall complication rate was 44%. Sensitivity analysis was performed to compare complication rates between patients with varying HbA1c levels and determine a threshold HbA1c value with the greatest difference in complication rates.
The authors concluded that women with an HbA1c level ≥ 8% showed the greatest increase of perioperative complications. Patients with an HbA1c ≥ 8%, compared with those who had an HbA1c < 8%, had a statistically significantly increased rate of overall (58% vs 42%, P = .002) and severe (27% vs 13%, P< .001) perioperative complications.
After multivariate logistic regression, the risk of overall complications remained elevated, with a 1.9-times higher risk of perioperative complications for women with an HbA1c ≥ 8%.
Women should be medically optimized before undergoing surgery and, while this study was restricted to urogynecologic surgery patients, it seems reasonable to assume that a similar HbA1c threshold would be beneficial for women undergoing other gynecologic procedures. Appropriately screening patients and referring them for early intervention with their primary care clinician or endocrinologist may improve surgical outcomes, especially in women with an HbA1c level > 8%.
Continue to: Success is similar for TV mesh and native tissue repair...
Success is similar for TV mesh and native tissue repair
Kahn B, Varner RE, Murphy M, et al. Transvaginal mesh compared with native tissue repair for pelvic organ prolapse. Obstet Gynecol. 2022;139:975-985. doi:10.1097/AOG.0000000000004794.
The distribution of vaginal mesh kits for the repair of POP was halted by the US Food and Drug Administration (FDA) in 2019. However, concerns have been raised about the measures used by the FDA to justify pulling these devices from the market. A cohort study compared 36-month outcomes between women who underwent prolapse repair with newer generation transvaginal mesh versus native tissue repair.
Study details
In a nonrandomized prospective multicenter cohort study, Kahn and colleagues compared outcomes in women with POP who underwent native tissue repair or transvaginal mesh repair with the Uphold LITE vaginal support system. The study’s objective was to compare the safety and efficacy of native tissue and transvaginal mesh prolapse repairs at 36 months postoperatively.
Treatment success was measured based on composite and individual measures of anatomic and subjective success, need for retreatment, and the occurrence of adverse events. Quality of life (QoL) measures also were obtained using validated questionnaires. Intention-to-treat and per-protocol analyses were performed.
Composite success rate was higher for mesh repair
A total of 710 patients were screened for eligibility (225 received transvaginal mesh and 485 received native tissue repair). Transvaginal mesh placement was found to be significantly superior to native tissue repair for composite success (84% vs 73%, P = .009) when prolapse within the hymen (that is, Ba and/or C < 0 on the Pelvic Organ Prolapse Quantification System) was used to define anatomic success.
Adverse events were similar between transvaginal mesh and native tissue repair groups, with most adverse events occurring within the first 6 months. The mesh exposure rate was 4.9%. Of the 13 incidents of mesh exposure, 4 patients required surgical intervention and 1 incident was considered a serious adverse event. QoL measures demonstrated improvement without any statistically significant differences between the treatment cohorts. ●
This study established the superiority and safety of newer generation transvaginal mesh used for the treatment of pelvic organ prolapse. Women who received newer generation transvaginal mesh can be reassured that the prolapse recurrence rates are low and that adverse events related to their mesh are rare—even when compared with those of native tissue repair. Patients also may be reassured that most adverse events would have occurred within 6 months of the initial prolapse repair surgery
Treating recurrent vulvovaginal candidiasis

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.
Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
Mental Health Outcomes Among Transgender Veterans and Active-Duty Service Members in the United States: A Systematic Review
According to the United States Transgender Survey, 39% of respondents reported experiencing serious psychological distress (based on the Kessler 6 Psychological Distress Scale) in the past 30 days compared with 5% in the general population.1 Additionally, 40% of respondents attempted suicide in their lifetime, compared with 5% in the general population.1 Almost half of respondents reported being sexually assaulted at some time in their life, and 10% reported being sexually assaulted in the past year.1
Studies have also shown that veterans and active-duty service members experience worse mental health outcomes and are at increased risk for suicide than civilians and nonveterans.2-5 About 1 in 4 active-duty service members meet the criteria for diagnosis of a mental illness.4 Service members were found to have higher rates of probable anxiety and posttraumatic stress disorder (PTSD) compared with the general population.2,6 In 2018, veteran suicide deaths accounted for about 13% of all deaths by suicide in the US even though veterans only accounted for about 7% of the adult population in that year.5,7 Also in 2018, about 17 veterans committed suicide per day.5 According to the Health Related Behaviors Survey of active-duty service members, about 18% reported thinking about attempting suicide some time in their lives compared with 4% of the general population.2,3 Additionally, 5% of service members reported previous suicide attempts compared with 0.5% in the general population.2,3 It is clear that transgender individuals, veterans, and service members have certain mental health outcomes that are worse than that of the general population.1-7
Transgender individuals along with LGB (lesbian, gay, bisexual) individuals have long faced discrimination and unfair treatment in the military.8-11 In the 1920s, the first written policies were established that banned gay men from serving in the military.9 The US Department of Defense (DoD) continued these policies until in 1993, the “Don’t Ask Don’t Tell” policy was established, which had the façade of being more inclusive for LGB individuals but forced LGB service members to hide their sexual identity and continued the anti-LGBTQ messages that previous policies had created.8,10,11 In 2010, “Don’t Ask Don’t Tell” was repealed, which allowed LGB individuals to serve in the military without concealing their sexual orientation and without fear of discharge based on their sexual identity.11 This repeal did not allow transgender individuals to serve their country as the DoD categorized transgender identity as a medical and mental health disorder.8,11
In 2016, the ban on transgender individuals serving in the military was lifted, and service members could no longer be discharged or turned away from joining the military based on gender identity.8,12 However, in 2018, this order was reversed. The new policy stated that new service members must meet requirements and standards of their sex assigned at birth, and individuals with a history of gender dysphoria or those who have received gender-affirming medical or surgical treatment were prohibited to serve in the military.8,13 This policy did not apply to service members who joined before it took effect. Finally, in April 2021, the current policy took effect, permitting transgender individuals to openly serve in the military. The current policy states that service members cannot be discharged or denied reenlistment based on their gender identity and provides support to receive gender-affirming medical care.14 Although transgender individuals are now accepted in military service, there is still much progress needed to promote equity among transgender service members.
In 2015, according to the Health Related Behaviors Survey of active-duty service members, 0.6% of service members identified as transgender, the same percentage as US adults who identify as transgender.2,15 Previous research has shown that the prevalence of gender identity disorder among veterans is higher than that among the general US population.16 Many studies have shown that worse mental health outcomes exist among LGBTQ veterans and service members compared with heterosexual, cisgender veterans and service members.17-24 However, fewer studies have focused solely on mental health outcomes among transgender veterans and active-duty service members, and there exists no current literature review on this topic. In this article, we present data from the existing literature on mental health outcomes in transgender veterans and active-duty service members. We hypothesize, based on the current literature, that transgender veterans and service members have worse mental health outcomes than their cisgender counterparts. Key terms used in this paper are defined in the Key Definitions.25-27
Methods
We conducted a systematic review of articles presenting data on mental health outcomes in transgender veterans and active-duty service members. The National Library of Medicine PubMed database was searched using the following search terms in various combinations: mental health outcomes, transgender, veterans, military, active duty, substance use, and sexual trauma. The literature search was performed in August 2021 and included articles published through July 31, 2021. Methodology, size, demographics, measures, and main findings were extracted from each article. All studies were eligible for inclusion regardless of sample size. Studies that examined the LGBTQ population without separating transgender individuals were excluded. Studies that examined mental health outcomes including, but not limited to, PTSD, depression, suicidality, anxiety, and substance use disorders (SUDs) in addition to sexual trauma were included. Studies that only examined physical health outcomes were excluded. Qualitative studies, case reports, and papers that did not present original data were excluded (Figure).
Results
Our search resulted in 86 publications. After excluding 65 articles that did not meet the inclusion criteria, 19 studies were included in this review. The Appendix shows the summary of findings from each study, including the study size and results. All studies were conducted in the United States. Most papers used a cross-sectional study design. Most of the studies focused on transgender veterans, but some included data on transgender active-duty service members.
We separated the findings into the following categories based on the variables measured: mental health, including depression, anxiety, PTSD, and serious mental illness; suicidality and self-harm; substance use; and military sexual trauma (MST). Many studies overlapped multiple categories.
Mental Health
Most of the studies included reported that transgender veterans have statistically significant worse mental health outcomes compared with cisgender veterans.28-30 In addition, transgender active-duty service members were found to have worse mental health outcomes than cisgender active-duty service members.31 MST and discrimination were associated with worse mental health outcomes among transgender veterans.32,33 One study showed a different result than others and found that transgender older adults with prior military service had higher psychological health-related quality of life and lower depressive symptoms than those without prior military service (P = .02 and .04, respectively).34 Another study compared transgender veterans with active-duty service members and found that transgender veterans reported higher rates of depression (64.6% vs 30.9%; χ2 = 11.68; P = .001) and anxiety (41.3% vs 18.2%; χ2 = 6.54; P = .01) compared with transgender service members.35
Suicidality and Self-harm
Eleven of the 19 studies included measured suicidality and/or self-harm as an outcome. Transgender veterans and active-duty service members were found to have higher odds of suicidality than their cisgender counterparts.16,28,29,31 In addition, transgender veterans may die by suicide at a younger age than cisgender veterans.36 Stigma and gender-related discrimination were found to be associated with suicidal ideation.33,37-39 Transgender veterans were less likely than transgender nonveterans to report nonsuicidal self-injury (NSSI).40
Substance Use
Two studies focused on substance use, while 5 other studies included substance use in their measures. One of these 2 studies that focused only on substance use outcomes found that transgender veterans were more likely than cisgender veterans to have any SUD (7.2% vs 3.9%; P < .001), in addition to specifically cannabis (3.4% vs 1.5%; P < .001), amphetamine (1.1% vs 0.3%; P < .001), and cocaine use disorders (1.5% vs 1.1%; P < .001).41
Another study reported that transgender veterans had lower odds of self-reported alcohol use but had greater odds of having alcohol-related diagnoses compared with cisgender veterans.42 Of the other studies, it was found that a higher percentage of transgender veterans were diagnosed with an SUD compared with transgender active-duty service members, and transgender veterans were more likely than cisgender veterans to be diagnosed with alcohol use disorder.29,31 Additionally, rural transgender veterans had increased odds of tobacco use disorder compared with transgender veterans who lived in urban areas.43
Military Sexual Trauma
Five of the studies included examined MST, defined as sexual assault or sexual harassment that is experienced during military service.44 Studies found that 15% to 17% of transgender veterans experienced MST.32,45 Transgender veterans were more likely to report MST than cisgender veterans.28,29 MST was found to be consistently associated with depression and PTSD.32,45 A high percentage (83.9%) of transgender active-duty service members reported experiencing sexual harassment and almost one-third experienced sexual assault.46
Discussion
Outcomes examined in this review included MST, substance use, suicidality, and symptoms of depression, anxiety, and PTSD among transgender active-duty service members and veterans. To our knowledge, no other review on this topic exists. There is a review of the health and well-being of LGBTQ veterans and service members, but a majority of the included studies did not separate transgender individuals from LGB persons.17 This review of transgender individuals showed similar results to the review of LGBTQ individuals.17 This review also presented similar results to previous studies that indicated that transgender individuals in the general population have worse mental health outcomes compared with their cisgender counterparts, in addition to studies that showed that veterans and active-duty service members have worse mental health outcomes compared with civilians and nonveterans.1-5 The population of focus in this review faced a unique set of challenges, being that they belonged to both of these subsets of the population, both of which experienced worse mental health outcomes, according to the literature.
Studies included in our review found that transgender veterans and service members have worse mental health outcomes than cisgender veterans and service members.28-31 This outcome was predicted based on previous data collection among transgender individuals, veterans, and active-duty service members. One of the studies included found different results and concluded that prior military service was a protective factor against poorer mental health outcomes.34 This could be, in part, due to veterans’ access to care through the US Department of Veterans Affairs (VA) system. It has been found that transgender veterans use VA services at higher rates than the general population of veterans and that barriers to care were found more for medical treatment than for mental health treatment.47 One study found that almost 70% of transgender veterans who used VA services were satisfied with their mental health care.48 In contrast, another study included in our review found that transgender veterans had worse mental health outcomes than transgender service members, possibly showing that even with access to care, the burden of stigma and discrimination worsens mental health over time.31 Although it has been shown that transgender veterans may feel comfortable disclosing their gender identity to their health care professional, many barriers to care have been identified, such as insensitivity and lack of knowledge about transgender care among clinicians.49-51 With this information, it would be useful to ensure proper training for health care professionals on providing gender-affirming care.
Most of the studies also found that transgender veterans and service members had greater odds of suicidal thoughts and events than cisgender veterans and service members.16,28,29,35 On the contrary, transgender veterans were less likely than transgender nonveterans to report NSSI, which could be for various reasons.40 Transgender veterans may report less NSSI but experience it at similar rates, or veteran status may be a protective factor for NSSI.
Very few studies included SUDs in their measurements, but it was found that transgender veterans were more likely than cisgender veterans to have any drug and alcohol use disorder.29,41 In addition, transgender veterans were more likely than transgender service members to be diagnosed with an SUD, again showing that over time and after time of service, mental health may worsen due to the burden of stigma and discrimination.31 Studies that examined MST found that transgender veterans were more likely than cisgender veterans to report MST, which replicates previous data that found high rates of sexual assault experienced among transgender individuals.1,28,29
There is a lack of literature surrounding transgender veterans and active-duty service members, especially with regard to gender-affirming care provided to these populations. To the best of our knowledge, there exists only one original study that examines the effect of gender-affirming hormone therapy and surgery on mental health outcomes among transgender veterans.52 Further research in this area is needed, specifically longitudinal studies examining the effects of gender-affirming medical care on various outcomes, including mental health. Few longitudinal studies exist that examine the mental health effects of gender-affirming hormone therapy on transgender individuals in the general population.53-60 Most of these studies have shown a significant improvement in parameters of depression and anxiety following hormonal treatment, although long-term large follow-up studies to understand whether these improvements persist over time are missing also in the general population. However, as previously described, transgender veterans and service members are a unique subset of the transgender population and require separate data collection. Hence, further research is required to provide optimal care for this population. In addition, early screening for symptoms of mental illness, substance use, and MST is important to providing optimal care.
Limitations
This review was limited due to the lack of data collected from transgender veterans and service members. The studies included did not allow for standardized comparisons and did not use identical measures. Some papers compared transgender veterans with transgender nonveterans, some transgender veterans and/or service members with cisgender veterans and/or service members, and some transgender veterans with transgender service members. There were some consistent results found across the studies, but some studies showed contradictory results or no significant differences within a certain category. It is difficult to compare such different study designs and various participant populations. Additional research is required to verify and replicate these results.
Conclusions
Although this review was limited due to the lack of consistent study designs in the literature examining the mental health of transgender veterans and active-duty service members, overall results showed that transgender veterans and service members experience worse mental health outcomes than their cisgender counterparts. With this knowledge and exploring the history of discrimination that this population has faced, improved systems must be put into place to better serve this population and improve health outcomes. Additional research is required to examine the effects of gender-affirming care on mental health among transgender veterans and service members.
1. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality. December 2016. Accessed August 22, 2022. https://www.ustranssurvey.org
2. Meadows SO, Engel CC, Collins RL, et al. 2015 Department of Defense Health Related Behaviors Survey (HRBS). Rand Health Q. 2018;8(2):434.
3. Lipari R, Piscopo K, Kroutil LA, Miller GK. Suicidal thoughts and behavior among adults: results from the 2014 National Survey on Drug Use and Health. NSDUH Data Review. 2015:1-14. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR2-2014/NSDUH-FRR2-2014.pdf
4. Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
5. U.S. Department of Veterans Affairs Office of Mental Health and Suicide Prevention. 2020 National Veteran Suicide Prevention Annual Report. November 2020. Accessed August 22, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
6. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi:10.1001/archpsyc.62.6.593
7. Vespa J. Those who SERVED: America’s veterans from World War II to the war on terror. The United States Census Bureau. June 2, 2020. Accessed August 22, 2022. https://www.census.gov/library/publications/2020/demo/acs-43.html
8. Seibert DC, Keller N, Zapor L, Archer H. Military transgender care. J Am Assoc Nurse Pract. 2020;32(11):764-770. doi:10.1097/JXX.0000000000000519
9. Rigby WC. Military penal law: A brief survey of the 1920 revision of the Articles of War. J Crim Law Criminol. 1921;12(1):84.
10. Department of Defense Directive Number 1332.14: Enlisted Administrative Separations. December 21, 1993. Accessed August 22, 2022. https://biotech.law.lsu.edu/blaw/dodd/corres/pdf/d133214wch1_122193/d133214p.pdf
11. Aford B, Lee SJ. Toward complete inclusion: lesbian, gay, bisexual, and transgender military service members after repeal of Don’t Ask, Don’t Tell. Soc Work. 2016;61(3):257-265. doi:10.1093/sw/sww033
12. Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. June 30, 2016. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DoD-Instruction-1300.28.pdf
13. Department of Defense. Directive-type Memorandum (DTM)-19-004 - Military Service by Transgender Persons and Persons with Gender Dysphoria. March 12. 2019. Accessed August 22, 2022. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
14. US Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. April 30, 2021. Accessed August 22, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/130028p.pdf
15. Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? The Williams Institute; 2016. Accessed August 22, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
16. Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27-e32. doi:10.2105/AJPH.2013.301507
17. Mark KM, McNamara KA, Gribble R, et al. The health and well-being of LGBTQ serving and ex-serving personnel: a narrative review. Int Rev Psychiatry. 2019;31(1):75-94. doi:10.1080/09540261.2019.1575190
18. Blosnich J, Foynes MM, Shipherd JC. Health disparities among sexual minority women veterans. J Womens Health (Larchmt). 2013;22(7):631-636. doi:10.1089/jwh.2012.4214
19. Blosnich JR, Bossarte RM, Silenzio VM. Suicidal ideation among sexual minority veterans: results from the 2005-2010 Massachusetts Behavioral Risk Factor Surveillance Survey. Am J Public Health. 2012;102(suppl 1):S44-S47. doi:10.2105/AJPH.2011.300565
20. Blosnich JR, Gordon AJ, Fine MJ. Associations of sexual and gender minority status with health indicators, health risk factors, and social stressors in a national sample of young adults with military experience. Ann Epidemiol. 2015;25(9):661-667. doi:10.1016/j.annepidem.2015.06.001
21. Cochran BN, Balsam K, Flentje A, Malte CA, Simpson T. Mental health characteristics of sexual minority veterans. J Homosex. 2013;60(2-3):419-435. doi:10.1080/00918369.2013.744932
22. Lehavot K, Browne KC, Simpson TL. Examining sexual orientation disparities in alcohol misuse among women veterans. Am J Prev Med. 2014;47(5):554-562. doi:10.1016/j.amepre.2014.07.002
23. Scott RL, Lasiuk GC, Norris CM. Depression in lesbian, gay, and bisexual members of the Canadian Armed Forces. LGBT Health. 2016;3(5):366-372. doi:10.1089/lgbt.2016.0050
24. Wang J, Dey M, Soldati L, Weiss MG, Gmel G, Mohler-Kuo M. Psychiatric disorders, suicidality, and personality among young men by sexual orientation. Eur Psychiatry. 2014;29(8):514-522. doi:10.1016/j.eurpsy.2014.05.001
25. American Psychological Association. Gender. APA Style. September 2019. Updated July 2022. Accessed August 22, 2022. https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
26. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association; 2013.
27. Deutsch MB. Overview of gender-affirming treatments and procedures. UCSF Transgender Care. June 17, 2016. Accessed August 22, 2022. https://transcare.ucsf.edu/guidelines/overview
28. Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015;2(4):297-305. doi:10.1089/lgbt.2015.0052
29. Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122-131. doi:10.1089/lgbt.2015.0058
30. Downing J, Conron K, Herman JL, Blosnich JR. Transgender and cisgender US veterans have few health differences. Health Aff (Millwood). 2018;37(7):1160-1168. doi:10.1377/hlthaff.2018.0027
31. Holloway IW, Green D, Pickering C, et al. Mental health and health risk behaviors of active duty sexual minority and transgender service members in the United States military. LGBT Health. 2021;8(2):152-161. doi:10.1089/lgbt.2020.0031
32. Beckman K, Shipherd J, Simpson T, Lehavot K. Military sexual assault in transgender veterans: results from a nationwide survey. J Trauma Stress. 2018;31(2):181-190. doi:10.1002/jts.22280
33. Blosnich JR, Marsiglio MC, Gao S, Gordon AJ, Shipherd JC, Kauth M, Brown GR, Fine MJ. Mental health of transgender veterans in US states with and without discrimination and hate crime legal protection. Am J Public Health. 2016;106(3):534-540. doi:10.2105/AJPH.2015.302981
34. Hoy-Ellis CP, Shiu C, Sullivan KM, Kim HJ, Sturges AM, Fredriksen-Goldsen KI. Prior military service, identity stigma, and mental health among transgender older adults. Gerontologist. 2017;57(suppl 1):S63-S71. doi:10.1093/geront/gnw173
35. Hill BJ, Bouris A, Barnett JT, Walker D. Fit to serve? Exploring mental and physical health and well-being among transgender active-duty service members and veterans in the U.S. military. Transgend Health. 2016;1(1):4-11. Published 2016 Jan 1. doi:10.1089/trgh.2015.0002
36. Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269-276. doi:10.1089/lgbt.2014.0050
37. Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: the role of social support and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
38. Lehavot K, Simpson TL, Shipherd JC. Factors associated with suicidality among a national sample of transgender veterans. Suicide Life Threat Behav. 2016;46(5):507-524. doi:10.1111/sltb.12233
39. Tucker RP, Testa RJ, Reger MA, Simpson TL, Shipherd JC, Lehavot K. Current and military-specific gender minority stress factors and their relationship with suicide ideation in transgender veterans. Suicide Life Threat Behav. 2019;49(1):155-166. doi:10.1111/sltb.12432
40. Aboussouan A, Snow A, Cerel J, Tucker RP. Non-suicidal self-injury, suicide ideation, and past suicide attempts: Comparison between transgender and gender diverse veterans and non-veterans. J Affect Disord. 2019;259:186-194. doi:10.1016/j.jad.2019.08.046
41. Frost MC, Blosnich JR, Lehavot K, Chen JA, Rubinsky AD, Glass JE, Williams EC. Disparities in documented drug use disorders between transgender and cisgender U.S. Veterans Health Administration patients. J Addict Med. 2021;15(4):334-340. doi:10.1097/ADM.0000000000000769
42. Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the Veterans Health Administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132-141. doi:10.15288/jsad.2021.82.132
43. Bukowski LA, Blosnich J, Shipherd JC, Kauth MR, Brown GR, Gordon AJ. Exploring rural disparities in medical diagnoses among veterans with transgender-related diagnoses utilizing Veterans Health Administration care. Med Care. 2017;55(suppl 9):S97-S103. doi:10.1097/MLR.0000000000000745
44. U.S. Department of Veterans Affairs. Military Sexual Trauma. Updated August 1, 2022. Accessed August 22, 2022. https://www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
45. Lindsay JA, Keo-Meier C, Hudson S, Walder A, Martin LA, Kauth MR. Mental health of transgender veterans of the Iraq and Afghanistan conflicts who experienced military sexual trauma. J Trauma Stress. 2016;29(6):563-567. doi:10.1002/jts.22146
46. Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and Non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. doi:10.1002/jts.22506
47. Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sexual Health. 2012;24(1):78-87. doi:10.1080/19317611.2011.639440
48. Lehavot K, Katon JG, Simpson TL, Shipherd JC. Transgender veterans’ satisfaction with care and unmet health needs. Med Care. 2017;55(suppl 9):S90-S96. doi:10.1097/MLR.0000000000000723
49. Kauth MR, Barrera TL, Latini DM. Lesbian, gay, and transgender veterans’ experiences in the Veterans Health Administration: positive signs and room for improvement. Psychol Serv. 2019;16(2):346-351. doi:10.1037/ser0000232

50. Rosentel K, Hill BJ, Lu C, Barnett JT. Transgender veterans and the Veterans Health Administration: exploring the experiences of transgender veterans in the Veterans Affairs Healthcare System. Transgend Health. 2016;1(1):108-116. Published 2016 Jun 1. doi:10.1089/trgh.2016.0006
51. Dietert M, Dentice D, Keig Z. Addressing the needs of transgender military veterans: better access and more comprehensive care. Transgend Health. 2017;2(1):35-44. Published 2017 Mar 1. doi:10.1089/trgh.2016.0040
52. Tucker RP, Testa RJ, Simpson TL, Shipherd JC, Blosnich JR, Lehavot K. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med. 2018;48(14):2329-2336. doi:10.1017/S0033291717003853
53. Colizzi M, Costa R, Todarello O. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65-73. doi:10.1016/j.psyneuen.2013.09.029
54. Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119-126. doi:10.1111/jsm.12363
55. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. doi:10.1210/jc.2016-1276
56. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9(6):1808-1816. doi:10.1111/andr.12884
57. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321-335. doi:10.1080/0092623X.2012.736920
58. Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol. 2015;83(1):143-156. doi:10.1037/a0037599
59. Turan S‚ , Aksoy Poyraz C, Usta Sag˘lam NG, et al. Alterations in body uneasiness, eating attitudes, and psychopathology before and after cross-sex hormonal treatment in patients with female-to-male gender dysphoria. Arch Sex Behav. 2018;47(8):2349-2361. doi:10.1007/s10508-018-1189-4
60. Oda H, Kinoshita T. Efficacy of hormonal and mental treatments with MMPI in FtM individuals: cross-sectional and longitudinal studies. BMC Psychiatry. 2017;17(1):256. Published 2017 Jul 17. doi:10.1186/s12888-017-1423-y
According to the United States Transgender Survey, 39% of respondents reported experiencing serious psychological distress (based on the Kessler 6 Psychological Distress Scale) in the past 30 days compared with 5% in the general population.1 Additionally, 40% of respondents attempted suicide in their lifetime, compared with 5% in the general population.1 Almost half of respondents reported being sexually assaulted at some time in their life, and 10% reported being sexually assaulted in the past year.1
Studies have also shown that veterans and active-duty service members experience worse mental health outcomes and are at increased risk for suicide than civilians and nonveterans.2-5 About 1 in 4 active-duty service members meet the criteria for diagnosis of a mental illness.4 Service members were found to have higher rates of probable anxiety and posttraumatic stress disorder (PTSD) compared with the general population.2,6 In 2018, veteran suicide deaths accounted for about 13% of all deaths by suicide in the US even though veterans only accounted for about 7% of the adult population in that year.5,7 Also in 2018, about 17 veterans committed suicide per day.5 According to the Health Related Behaviors Survey of active-duty service members, about 18% reported thinking about attempting suicide some time in their lives compared with 4% of the general population.2,3 Additionally, 5% of service members reported previous suicide attempts compared with 0.5% in the general population.2,3 It is clear that transgender individuals, veterans, and service members have certain mental health outcomes that are worse than that of the general population.1-7
Transgender individuals along with LGB (lesbian, gay, bisexual) individuals have long faced discrimination and unfair treatment in the military.8-11 In the 1920s, the first written policies were established that banned gay men from serving in the military.9 The US Department of Defense (DoD) continued these policies until in 1993, the “Don’t Ask Don’t Tell” policy was established, which had the façade of being more inclusive for LGB individuals but forced LGB service members to hide their sexual identity and continued the anti-LGBTQ messages that previous policies had created.8,10,11 In 2010, “Don’t Ask Don’t Tell” was repealed, which allowed LGB individuals to serve in the military without concealing their sexual orientation and without fear of discharge based on their sexual identity.11 This repeal did not allow transgender individuals to serve their country as the DoD categorized transgender identity as a medical and mental health disorder.8,11
In 2016, the ban on transgender individuals serving in the military was lifted, and service members could no longer be discharged or turned away from joining the military based on gender identity.8,12 However, in 2018, this order was reversed. The new policy stated that new service members must meet requirements and standards of their sex assigned at birth, and individuals with a history of gender dysphoria or those who have received gender-affirming medical or surgical treatment were prohibited to serve in the military.8,13 This policy did not apply to service members who joined before it took effect. Finally, in April 2021, the current policy took effect, permitting transgender individuals to openly serve in the military. The current policy states that service members cannot be discharged or denied reenlistment based on their gender identity and provides support to receive gender-affirming medical care.14 Although transgender individuals are now accepted in military service, there is still much progress needed to promote equity among transgender service members.
In 2015, according to the Health Related Behaviors Survey of active-duty service members, 0.6% of service members identified as transgender, the same percentage as US adults who identify as transgender.2,15 Previous research has shown that the prevalence of gender identity disorder among veterans is higher than that among the general US population.16 Many studies have shown that worse mental health outcomes exist among LGBTQ veterans and service members compared with heterosexual, cisgender veterans and service members.17-24 However, fewer studies have focused solely on mental health outcomes among transgender veterans and active-duty service members, and there exists no current literature review on this topic. In this article, we present data from the existing literature on mental health outcomes in transgender veterans and active-duty service members. We hypothesize, based on the current literature, that transgender veterans and service members have worse mental health outcomes than their cisgender counterparts. Key terms used in this paper are defined in the Key Definitions.25-27
Methods
We conducted a systematic review of articles presenting data on mental health outcomes in transgender veterans and active-duty service members. The National Library of Medicine PubMed database was searched using the following search terms in various combinations: mental health outcomes, transgender, veterans, military, active duty, substance use, and sexual trauma. The literature search was performed in August 2021 and included articles published through July 31, 2021. Methodology, size, demographics, measures, and main findings were extracted from each article. All studies were eligible for inclusion regardless of sample size. Studies that examined the LGBTQ population without separating transgender individuals were excluded. Studies that examined mental health outcomes including, but not limited to, PTSD, depression, suicidality, anxiety, and substance use disorders (SUDs) in addition to sexual trauma were included. Studies that only examined physical health outcomes were excluded. Qualitative studies, case reports, and papers that did not present original data were excluded (Figure).
Results
Our search resulted in 86 publications. After excluding 65 articles that did not meet the inclusion criteria, 19 studies were included in this review. The Appendix shows the summary of findings from each study, including the study size and results. All studies were conducted in the United States. Most papers used a cross-sectional study design. Most of the studies focused on transgender veterans, but some included data on transgender active-duty service members.
We separated the findings into the following categories based on the variables measured: mental health, including depression, anxiety, PTSD, and serious mental illness; suicidality and self-harm; substance use; and military sexual trauma (MST). Many studies overlapped multiple categories.
Mental Health
Most of the studies included reported that transgender veterans have statistically significant worse mental health outcomes compared with cisgender veterans.28-30 In addition, transgender active-duty service members were found to have worse mental health outcomes than cisgender active-duty service members.31 MST and discrimination were associated with worse mental health outcomes among transgender veterans.32,33 One study showed a different result than others and found that transgender older adults with prior military service had higher psychological health-related quality of life and lower depressive symptoms than those without prior military service (P = .02 and .04, respectively).34 Another study compared transgender veterans with active-duty service members and found that transgender veterans reported higher rates of depression (64.6% vs 30.9%; χ2 = 11.68; P = .001) and anxiety (41.3% vs 18.2%; χ2 = 6.54; P = .01) compared with transgender service members.35
Suicidality and Self-harm
Eleven of the 19 studies included measured suicidality and/or self-harm as an outcome. Transgender veterans and active-duty service members were found to have higher odds of suicidality than their cisgender counterparts.16,28,29,31 In addition, transgender veterans may die by suicide at a younger age than cisgender veterans.36 Stigma and gender-related discrimination were found to be associated with suicidal ideation.33,37-39 Transgender veterans were less likely than transgender nonveterans to report nonsuicidal self-injury (NSSI).40
Substance Use
Two studies focused on substance use, while 5 other studies included substance use in their measures. One of these 2 studies that focused only on substance use outcomes found that transgender veterans were more likely than cisgender veterans to have any SUD (7.2% vs 3.9%; P < .001), in addition to specifically cannabis (3.4% vs 1.5%; P < .001), amphetamine (1.1% vs 0.3%; P < .001), and cocaine use disorders (1.5% vs 1.1%; P < .001).41
Another study reported that transgender veterans had lower odds of self-reported alcohol use but had greater odds of having alcohol-related diagnoses compared with cisgender veterans.42 Of the other studies, it was found that a higher percentage of transgender veterans were diagnosed with an SUD compared with transgender active-duty service members, and transgender veterans were more likely than cisgender veterans to be diagnosed with alcohol use disorder.29,31 Additionally, rural transgender veterans had increased odds of tobacco use disorder compared with transgender veterans who lived in urban areas.43
Military Sexual Trauma
Five of the studies included examined MST, defined as sexual assault or sexual harassment that is experienced during military service.44 Studies found that 15% to 17% of transgender veterans experienced MST.32,45 Transgender veterans were more likely to report MST than cisgender veterans.28,29 MST was found to be consistently associated with depression and PTSD.32,45 A high percentage (83.9%) of transgender active-duty service members reported experiencing sexual harassment and almost one-third experienced sexual assault.46
Discussion
Outcomes examined in this review included MST, substance use, suicidality, and symptoms of depression, anxiety, and PTSD among transgender active-duty service members and veterans. To our knowledge, no other review on this topic exists. There is a review of the health and well-being of LGBTQ veterans and service members, but a majority of the included studies did not separate transgender individuals from LGB persons.17 This review of transgender individuals showed similar results to the review of LGBTQ individuals.17 This review also presented similar results to previous studies that indicated that transgender individuals in the general population have worse mental health outcomes compared with their cisgender counterparts, in addition to studies that showed that veterans and active-duty service members have worse mental health outcomes compared with civilians and nonveterans.1-5 The population of focus in this review faced a unique set of challenges, being that they belonged to both of these subsets of the population, both of which experienced worse mental health outcomes, according to the literature.
Studies included in our review found that transgender veterans and service members have worse mental health outcomes than cisgender veterans and service members.28-31 This outcome was predicted based on previous data collection among transgender individuals, veterans, and active-duty service members. One of the studies included found different results and concluded that prior military service was a protective factor against poorer mental health outcomes.34 This could be, in part, due to veterans’ access to care through the US Department of Veterans Affairs (VA) system. It has been found that transgender veterans use VA services at higher rates than the general population of veterans and that barriers to care were found more for medical treatment than for mental health treatment.47 One study found that almost 70% of transgender veterans who used VA services were satisfied with their mental health care.48 In contrast, another study included in our review found that transgender veterans had worse mental health outcomes than transgender service members, possibly showing that even with access to care, the burden of stigma and discrimination worsens mental health over time.31 Although it has been shown that transgender veterans may feel comfortable disclosing their gender identity to their health care professional, many barriers to care have been identified, such as insensitivity and lack of knowledge about transgender care among clinicians.49-51 With this information, it would be useful to ensure proper training for health care professionals on providing gender-affirming care.
Most of the studies also found that transgender veterans and service members had greater odds of suicidal thoughts and events than cisgender veterans and service members.16,28,29,35 On the contrary, transgender veterans were less likely than transgender nonveterans to report NSSI, which could be for various reasons.40 Transgender veterans may report less NSSI but experience it at similar rates, or veteran status may be a protective factor for NSSI.
Very few studies included SUDs in their measurements, but it was found that transgender veterans were more likely than cisgender veterans to have any drug and alcohol use disorder.29,41 In addition, transgender veterans were more likely than transgender service members to be diagnosed with an SUD, again showing that over time and after time of service, mental health may worsen due to the burden of stigma and discrimination.31 Studies that examined MST found that transgender veterans were more likely than cisgender veterans to report MST, which replicates previous data that found high rates of sexual assault experienced among transgender individuals.1,28,29
There is a lack of literature surrounding transgender veterans and active-duty service members, especially with regard to gender-affirming care provided to these populations. To the best of our knowledge, there exists only one original study that examines the effect of gender-affirming hormone therapy and surgery on mental health outcomes among transgender veterans.52 Further research in this area is needed, specifically longitudinal studies examining the effects of gender-affirming medical care on various outcomes, including mental health. Few longitudinal studies exist that examine the mental health effects of gender-affirming hormone therapy on transgender individuals in the general population.53-60 Most of these studies have shown a significant improvement in parameters of depression and anxiety following hormonal treatment, although long-term large follow-up studies to understand whether these improvements persist over time are missing also in the general population. However, as previously described, transgender veterans and service members are a unique subset of the transgender population and require separate data collection. Hence, further research is required to provide optimal care for this population. In addition, early screening for symptoms of mental illness, substance use, and MST is important to providing optimal care.
Limitations
This review was limited due to the lack of data collected from transgender veterans and service members. The studies included did not allow for standardized comparisons and did not use identical measures. Some papers compared transgender veterans with transgender nonveterans, some transgender veterans and/or service members with cisgender veterans and/or service members, and some transgender veterans with transgender service members. There were some consistent results found across the studies, but some studies showed contradictory results or no significant differences within a certain category. It is difficult to compare such different study designs and various participant populations. Additional research is required to verify and replicate these results.
Conclusions
Although this review was limited due to the lack of consistent study designs in the literature examining the mental health of transgender veterans and active-duty service members, overall results showed that transgender veterans and service members experience worse mental health outcomes than their cisgender counterparts. With this knowledge and exploring the history of discrimination that this population has faced, improved systems must be put into place to better serve this population and improve health outcomes. Additional research is required to examine the effects of gender-affirming care on mental health among transgender veterans and service members.
According to the United States Transgender Survey, 39% of respondents reported experiencing serious psychological distress (based on the Kessler 6 Psychological Distress Scale) in the past 30 days compared with 5% in the general population.1 Additionally, 40% of respondents attempted suicide in their lifetime, compared with 5% in the general population.1 Almost half of respondents reported being sexually assaulted at some time in their life, and 10% reported being sexually assaulted in the past year.1
Studies have also shown that veterans and active-duty service members experience worse mental health outcomes and are at increased risk for suicide than civilians and nonveterans.2-5 About 1 in 4 active-duty service members meet the criteria for diagnosis of a mental illness.4 Service members were found to have higher rates of probable anxiety and posttraumatic stress disorder (PTSD) compared with the general population.2,6 In 2018, veteran suicide deaths accounted for about 13% of all deaths by suicide in the US even though veterans only accounted for about 7% of the adult population in that year.5,7 Also in 2018, about 17 veterans committed suicide per day.5 According to the Health Related Behaviors Survey of active-duty service members, about 18% reported thinking about attempting suicide some time in their lives compared with 4% of the general population.2,3 Additionally, 5% of service members reported previous suicide attempts compared with 0.5% in the general population.2,3 It is clear that transgender individuals, veterans, and service members have certain mental health outcomes that are worse than that of the general population.1-7
Transgender individuals along with LGB (lesbian, gay, bisexual) individuals have long faced discrimination and unfair treatment in the military.8-11 In the 1920s, the first written policies were established that banned gay men from serving in the military.9 The US Department of Defense (DoD) continued these policies until in 1993, the “Don’t Ask Don’t Tell” policy was established, which had the façade of being more inclusive for LGB individuals but forced LGB service members to hide their sexual identity and continued the anti-LGBTQ messages that previous policies had created.8,10,11 In 2010, “Don’t Ask Don’t Tell” was repealed, which allowed LGB individuals to serve in the military without concealing their sexual orientation and without fear of discharge based on their sexual identity.11 This repeal did not allow transgender individuals to serve their country as the DoD categorized transgender identity as a medical and mental health disorder.8,11
In 2016, the ban on transgender individuals serving in the military was lifted, and service members could no longer be discharged or turned away from joining the military based on gender identity.8,12 However, in 2018, this order was reversed. The new policy stated that new service members must meet requirements and standards of their sex assigned at birth, and individuals with a history of gender dysphoria or those who have received gender-affirming medical or surgical treatment were prohibited to serve in the military.8,13 This policy did not apply to service members who joined before it took effect. Finally, in April 2021, the current policy took effect, permitting transgender individuals to openly serve in the military. The current policy states that service members cannot be discharged or denied reenlistment based on their gender identity and provides support to receive gender-affirming medical care.14 Although transgender individuals are now accepted in military service, there is still much progress needed to promote equity among transgender service members.
In 2015, according to the Health Related Behaviors Survey of active-duty service members, 0.6% of service members identified as transgender, the same percentage as US adults who identify as transgender.2,15 Previous research has shown that the prevalence of gender identity disorder among veterans is higher than that among the general US population.16 Many studies have shown that worse mental health outcomes exist among LGBTQ veterans and service members compared with heterosexual, cisgender veterans and service members.17-24 However, fewer studies have focused solely on mental health outcomes among transgender veterans and active-duty service members, and there exists no current literature review on this topic. In this article, we present data from the existing literature on mental health outcomes in transgender veterans and active-duty service members. We hypothesize, based on the current literature, that transgender veterans and service members have worse mental health outcomes than their cisgender counterparts. Key terms used in this paper are defined in the Key Definitions.25-27
Methods
We conducted a systematic review of articles presenting data on mental health outcomes in transgender veterans and active-duty service members. The National Library of Medicine PubMed database was searched using the following search terms in various combinations: mental health outcomes, transgender, veterans, military, active duty, substance use, and sexual trauma. The literature search was performed in August 2021 and included articles published through July 31, 2021. Methodology, size, demographics, measures, and main findings were extracted from each article. All studies were eligible for inclusion regardless of sample size. Studies that examined the LGBTQ population without separating transgender individuals were excluded. Studies that examined mental health outcomes including, but not limited to, PTSD, depression, suicidality, anxiety, and substance use disorders (SUDs) in addition to sexual trauma were included. Studies that only examined physical health outcomes were excluded. Qualitative studies, case reports, and papers that did not present original data were excluded (Figure).
Results
Our search resulted in 86 publications. After excluding 65 articles that did not meet the inclusion criteria, 19 studies were included in this review. The Appendix shows the summary of findings from each study, including the study size and results. All studies were conducted in the United States. Most papers used a cross-sectional study design. Most of the studies focused on transgender veterans, but some included data on transgender active-duty service members.
We separated the findings into the following categories based on the variables measured: mental health, including depression, anxiety, PTSD, and serious mental illness; suicidality and self-harm; substance use; and military sexual trauma (MST). Many studies overlapped multiple categories.
Mental Health
Most of the studies included reported that transgender veterans have statistically significant worse mental health outcomes compared with cisgender veterans.28-30 In addition, transgender active-duty service members were found to have worse mental health outcomes than cisgender active-duty service members.31 MST and discrimination were associated with worse mental health outcomes among transgender veterans.32,33 One study showed a different result than others and found that transgender older adults with prior military service had higher psychological health-related quality of life and lower depressive symptoms than those without prior military service (P = .02 and .04, respectively).34 Another study compared transgender veterans with active-duty service members and found that transgender veterans reported higher rates of depression (64.6% vs 30.9%; χ2 = 11.68; P = .001) and anxiety (41.3% vs 18.2%; χ2 = 6.54; P = .01) compared with transgender service members.35
Suicidality and Self-harm
Eleven of the 19 studies included measured suicidality and/or self-harm as an outcome. Transgender veterans and active-duty service members were found to have higher odds of suicidality than their cisgender counterparts.16,28,29,31 In addition, transgender veterans may die by suicide at a younger age than cisgender veterans.36 Stigma and gender-related discrimination were found to be associated with suicidal ideation.33,37-39 Transgender veterans were less likely than transgender nonveterans to report nonsuicidal self-injury (NSSI).40
Substance Use
Two studies focused on substance use, while 5 other studies included substance use in their measures. One of these 2 studies that focused only on substance use outcomes found that transgender veterans were more likely than cisgender veterans to have any SUD (7.2% vs 3.9%; P < .001), in addition to specifically cannabis (3.4% vs 1.5%; P < .001), amphetamine (1.1% vs 0.3%; P < .001), and cocaine use disorders (1.5% vs 1.1%; P < .001).41
Another study reported that transgender veterans had lower odds of self-reported alcohol use but had greater odds of having alcohol-related diagnoses compared with cisgender veterans.42 Of the other studies, it was found that a higher percentage of transgender veterans were diagnosed with an SUD compared with transgender active-duty service members, and transgender veterans were more likely than cisgender veterans to be diagnosed with alcohol use disorder.29,31 Additionally, rural transgender veterans had increased odds of tobacco use disorder compared with transgender veterans who lived in urban areas.43
Military Sexual Trauma
Five of the studies included examined MST, defined as sexual assault or sexual harassment that is experienced during military service.44 Studies found that 15% to 17% of transgender veterans experienced MST.32,45 Transgender veterans were more likely to report MST than cisgender veterans.28,29 MST was found to be consistently associated with depression and PTSD.32,45 A high percentage (83.9%) of transgender active-duty service members reported experiencing sexual harassment and almost one-third experienced sexual assault.46
Discussion
Outcomes examined in this review included MST, substance use, suicidality, and symptoms of depression, anxiety, and PTSD among transgender active-duty service members and veterans. To our knowledge, no other review on this topic exists. There is a review of the health and well-being of LGBTQ veterans and service members, but a majority of the included studies did not separate transgender individuals from LGB persons.17 This review of transgender individuals showed similar results to the review of LGBTQ individuals.17 This review also presented similar results to previous studies that indicated that transgender individuals in the general population have worse mental health outcomes compared with their cisgender counterparts, in addition to studies that showed that veterans and active-duty service members have worse mental health outcomes compared with civilians and nonveterans.1-5 The population of focus in this review faced a unique set of challenges, being that they belonged to both of these subsets of the population, both of which experienced worse mental health outcomes, according to the literature.
Studies included in our review found that transgender veterans and service members have worse mental health outcomes than cisgender veterans and service members.28-31 This outcome was predicted based on previous data collection among transgender individuals, veterans, and active-duty service members. One of the studies included found different results and concluded that prior military service was a protective factor against poorer mental health outcomes.34 This could be, in part, due to veterans’ access to care through the US Department of Veterans Affairs (VA) system. It has been found that transgender veterans use VA services at higher rates than the general population of veterans and that barriers to care were found more for medical treatment than for mental health treatment.47 One study found that almost 70% of transgender veterans who used VA services were satisfied with their mental health care.48 In contrast, another study included in our review found that transgender veterans had worse mental health outcomes than transgender service members, possibly showing that even with access to care, the burden of stigma and discrimination worsens mental health over time.31 Although it has been shown that transgender veterans may feel comfortable disclosing their gender identity to their health care professional, many barriers to care have been identified, such as insensitivity and lack of knowledge about transgender care among clinicians.49-51 With this information, it would be useful to ensure proper training for health care professionals on providing gender-affirming care.
Most of the studies also found that transgender veterans and service members had greater odds of suicidal thoughts and events than cisgender veterans and service members.16,28,29,35 On the contrary, transgender veterans were less likely than transgender nonveterans to report NSSI, which could be for various reasons.40 Transgender veterans may report less NSSI but experience it at similar rates, or veteran status may be a protective factor for NSSI.
Very few studies included SUDs in their measurements, but it was found that transgender veterans were more likely than cisgender veterans to have any drug and alcohol use disorder.29,41 In addition, transgender veterans were more likely than transgender service members to be diagnosed with an SUD, again showing that over time and after time of service, mental health may worsen due to the burden of stigma and discrimination.31 Studies that examined MST found that transgender veterans were more likely than cisgender veterans to report MST, which replicates previous data that found high rates of sexual assault experienced among transgender individuals.1,28,29
There is a lack of literature surrounding transgender veterans and active-duty service members, especially with regard to gender-affirming care provided to these populations. To the best of our knowledge, there exists only one original study that examines the effect of gender-affirming hormone therapy and surgery on mental health outcomes among transgender veterans.52 Further research in this area is needed, specifically longitudinal studies examining the effects of gender-affirming medical care on various outcomes, including mental health. Few longitudinal studies exist that examine the mental health effects of gender-affirming hormone therapy on transgender individuals in the general population.53-60 Most of these studies have shown a significant improvement in parameters of depression and anxiety following hormonal treatment, although long-term large follow-up studies to understand whether these improvements persist over time are missing also in the general population. However, as previously described, transgender veterans and service members are a unique subset of the transgender population and require separate data collection. Hence, further research is required to provide optimal care for this population. In addition, early screening for symptoms of mental illness, substance use, and MST is important to providing optimal care.
Limitations
This review was limited due to the lack of data collected from transgender veterans and service members. The studies included did not allow for standardized comparisons and did not use identical measures. Some papers compared transgender veterans with transgender nonveterans, some transgender veterans and/or service members with cisgender veterans and/or service members, and some transgender veterans with transgender service members. There were some consistent results found across the studies, but some studies showed contradictory results or no significant differences within a certain category. It is difficult to compare such different study designs and various participant populations. Additional research is required to verify and replicate these results.
Conclusions
Although this review was limited due to the lack of consistent study designs in the literature examining the mental health of transgender veterans and active-duty service members, overall results showed that transgender veterans and service members experience worse mental health outcomes than their cisgender counterparts. With this knowledge and exploring the history of discrimination that this population has faced, improved systems must be put into place to better serve this population and improve health outcomes. Additional research is required to examine the effects of gender-affirming care on mental health among transgender veterans and service members.
1. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality. December 2016. Accessed August 22, 2022. https://www.ustranssurvey.org
2. Meadows SO, Engel CC, Collins RL, et al. 2015 Department of Defense Health Related Behaviors Survey (HRBS). Rand Health Q. 2018;8(2):434.
3. Lipari R, Piscopo K, Kroutil LA, Miller GK. Suicidal thoughts and behavior among adults: results from the 2014 National Survey on Drug Use and Health. NSDUH Data Review. 2015:1-14. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR2-2014/NSDUH-FRR2-2014.pdf
4. Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
5. U.S. Department of Veterans Affairs Office of Mental Health and Suicide Prevention. 2020 National Veteran Suicide Prevention Annual Report. November 2020. Accessed August 22, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
6. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi:10.1001/archpsyc.62.6.593
7. Vespa J. Those who SERVED: America’s veterans from World War II to the war on terror. The United States Census Bureau. June 2, 2020. Accessed August 22, 2022. https://www.census.gov/library/publications/2020/demo/acs-43.html
8. Seibert DC, Keller N, Zapor L, Archer H. Military transgender care. J Am Assoc Nurse Pract. 2020;32(11):764-770. doi:10.1097/JXX.0000000000000519
9. Rigby WC. Military penal law: A brief survey of the 1920 revision of the Articles of War. J Crim Law Criminol. 1921;12(1):84.
10. Department of Defense Directive Number 1332.14: Enlisted Administrative Separations. December 21, 1993. Accessed August 22, 2022. https://biotech.law.lsu.edu/blaw/dodd/corres/pdf/d133214wch1_122193/d133214p.pdf
11. Aford B, Lee SJ. Toward complete inclusion: lesbian, gay, bisexual, and transgender military service members after repeal of Don’t Ask, Don’t Tell. Soc Work. 2016;61(3):257-265. doi:10.1093/sw/sww033
12. Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. June 30, 2016. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DoD-Instruction-1300.28.pdf
13. Department of Defense. Directive-type Memorandum (DTM)-19-004 - Military Service by Transgender Persons and Persons with Gender Dysphoria. March 12. 2019. Accessed August 22, 2022. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
14. US Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. April 30, 2021. Accessed August 22, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/130028p.pdf
15. Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? The Williams Institute; 2016. Accessed August 22, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
16. Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27-e32. doi:10.2105/AJPH.2013.301507
17. Mark KM, McNamara KA, Gribble R, et al. The health and well-being of LGBTQ serving and ex-serving personnel: a narrative review. Int Rev Psychiatry. 2019;31(1):75-94. doi:10.1080/09540261.2019.1575190
18. Blosnich J, Foynes MM, Shipherd JC. Health disparities among sexual minority women veterans. J Womens Health (Larchmt). 2013;22(7):631-636. doi:10.1089/jwh.2012.4214
19. Blosnich JR, Bossarte RM, Silenzio VM. Suicidal ideation among sexual minority veterans: results from the 2005-2010 Massachusetts Behavioral Risk Factor Surveillance Survey. Am J Public Health. 2012;102(suppl 1):S44-S47. doi:10.2105/AJPH.2011.300565
20. Blosnich JR, Gordon AJ, Fine MJ. Associations of sexual and gender minority status with health indicators, health risk factors, and social stressors in a national sample of young adults with military experience. Ann Epidemiol. 2015;25(9):661-667. doi:10.1016/j.annepidem.2015.06.001
21. Cochran BN, Balsam K, Flentje A, Malte CA, Simpson T. Mental health characteristics of sexual minority veterans. J Homosex. 2013;60(2-3):419-435. doi:10.1080/00918369.2013.744932
22. Lehavot K, Browne KC, Simpson TL. Examining sexual orientation disparities in alcohol misuse among women veterans. Am J Prev Med. 2014;47(5):554-562. doi:10.1016/j.amepre.2014.07.002
23. Scott RL, Lasiuk GC, Norris CM. Depression in lesbian, gay, and bisexual members of the Canadian Armed Forces. LGBT Health. 2016;3(5):366-372. doi:10.1089/lgbt.2016.0050
24. Wang J, Dey M, Soldati L, Weiss MG, Gmel G, Mohler-Kuo M. Psychiatric disorders, suicidality, and personality among young men by sexual orientation. Eur Psychiatry. 2014;29(8):514-522. doi:10.1016/j.eurpsy.2014.05.001
25. American Psychological Association. Gender. APA Style. September 2019. Updated July 2022. Accessed August 22, 2022. https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
26. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association; 2013.
27. Deutsch MB. Overview of gender-affirming treatments and procedures. UCSF Transgender Care. June 17, 2016. Accessed August 22, 2022. https://transcare.ucsf.edu/guidelines/overview
28. Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015;2(4):297-305. doi:10.1089/lgbt.2015.0052
29. Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122-131. doi:10.1089/lgbt.2015.0058
30. Downing J, Conron K, Herman JL, Blosnich JR. Transgender and cisgender US veterans have few health differences. Health Aff (Millwood). 2018;37(7):1160-1168. doi:10.1377/hlthaff.2018.0027
31. Holloway IW, Green D, Pickering C, et al. Mental health and health risk behaviors of active duty sexual minority and transgender service members in the United States military. LGBT Health. 2021;8(2):152-161. doi:10.1089/lgbt.2020.0031
32. Beckman K, Shipherd J, Simpson T, Lehavot K. Military sexual assault in transgender veterans: results from a nationwide survey. J Trauma Stress. 2018;31(2):181-190. doi:10.1002/jts.22280
33. Blosnich JR, Marsiglio MC, Gao S, Gordon AJ, Shipherd JC, Kauth M, Brown GR, Fine MJ. Mental health of transgender veterans in US states with and without discrimination and hate crime legal protection. Am J Public Health. 2016;106(3):534-540. doi:10.2105/AJPH.2015.302981
34. Hoy-Ellis CP, Shiu C, Sullivan KM, Kim HJ, Sturges AM, Fredriksen-Goldsen KI. Prior military service, identity stigma, and mental health among transgender older adults. Gerontologist. 2017;57(suppl 1):S63-S71. doi:10.1093/geront/gnw173
35. Hill BJ, Bouris A, Barnett JT, Walker D. Fit to serve? Exploring mental and physical health and well-being among transgender active-duty service members and veterans in the U.S. military. Transgend Health. 2016;1(1):4-11. Published 2016 Jan 1. doi:10.1089/trgh.2015.0002
36. Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269-276. doi:10.1089/lgbt.2014.0050
37. Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: the role of social support and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
38. Lehavot K, Simpson TL, Shipherd JC. Factors associated with suicidality among a national sample of transgender veterans. Suicide Life Threat Behav. 2016;46(5):507-524. doi:10.1111/sltb.12233
39. Tucker RP, Testa RJ, Reger MA, Simpson TL, Shipherd JC, Lehavot K. Current and military-specific gender minority stress factors and their relationship with suicide ideation in transgender veterans. Suicide Life Threat Behav. 2019;49(1):155-166. doi:10.1111/sltb.12432
40. Aboussouan A, Snow A, Cerel J, Tucker RP. Non-suicidal self-injury, suicide ideation, and past suicide attempts: Comparison between transgender and gender diverse veterans and non-veterans. J Affect Disord. 2019;259:186-194. doi:10.1016/j.jad.2019.08.046
41. Frost MC, Blosnich JR, Lehavot K, Chen JA, Rubinsky AD, Glass JE, Williams EC. Disparities in documented drug use disorders between transgender and cisgender U.S. Veterans Health Administration patients. J Addict Med. 2021;15(4):334-340. doi:10.1097/ADM.0000000000000769
42. Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the Veterans Health Administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132-141. doi:10.15288/jsad.2021.82.132
43. Bukowski LA, Blosnich J, Shipherd JC, Kauth MR, Brown GR, Gordon AJ. Exploring rural disparities in medical diagnoses among veterans with transgender-related diagnoses utilizing Veterans Health Administration care. Med Care. 2017;55(suppl 9):S97-S103. doi:10.1097/MLR.0000000000000745
44. U.S. Department of Veterans Affairs. Military Sexual Trauma. Updated August 1, 2022. Accessed August 22, 2022. https://www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
45. Lindsay JA, Keo-Meier C, Hudson S, Walder A, Martin LA, Kauth MR. Mental health of transgender veterans of the Iraq and Afghanistan conflicts who experienced military sexual trauma. J Trauma Stress. 2016;29(6):563-567. doi:10.1002/jts.22146
46. Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and Non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. doi:10.1002/jts.22506
47. Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sexual Health. 2012;24(1):78-87. doi:10.1080/19317611.2011.639440
48. Lehavot K, Katon JG, Simpson TL, Shipherd JC. Transgender veterans’ satisfaction with care and unmet health needs. Med Care. 2017;55(suppl 9):S90-S96. doi:10.1097/MLR.0000000000000723
49. Kauth MR, Barrera TL, Latini DM. Lesbian, gay, and transgender veterans’ experiences in the Veterans Health Administration: positive signs and room for improvement. Psychol Serv. 2019;16(2):346-351. doi:10.1037/ser0000232

50. Rosentel K, Hill BJ, Lu C, Barnett JT. Transgender veterans and the Veterans Health Administration: exploring the experiences of transgender veterans in the Veterans Affairs Healthcare System. Transgend Health. 2016;1(1):108-116. Published 2016 Jun 1. doi:10.1089/trgh.2016.0006
51. Dietert M, Dentice D, Keig Z. Addressing the needs of transgender military veterans: better access and more comprehensive care. Transgend Health. 2017;2(1):35-44. Published 2017 Mar 1. doi:10.1089/trgh.2016.0040
52. Tucker RP, Testa RJ, Simpson TL, Shipherd JC, Blosnich JR, Lehavot K. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med. 2018;48(14):2329-2336. doi:10.1017/S0033291717003853
53. Colizzi M, Costa R, Todarello O. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65-73. doi:10.1016/j.psyneuen.2013.09.029
54. Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119-126. doi:10.1111/jsm.12363
55. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. doi:10.1210/jc.2016-1276
56. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9(6):1808-1816. doi:10.1111/andr.12884
57. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321-335. doi:10.1080/0092623X.2012.736920
58. Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol. 2015;83(1):143-156. doi:10.1037/a0037599
59. Turan S‚ , Aksoy Poyraz C, Usta Sag˘lam NG, et al. Alterations in body uneasiness, eating attitudes, and psychopathology before and after cross-sex hormonal treatment in patients with female-to-male gender dysphoria. Arch Sex Behav. 2018;47(8):2349-2361. doi:10.1007/s10508-018-1189-4
60. Oda H, Kinoshita T. Efficacy of hormonal and mental treatments with MMPI in FtM individuals: cross-sectional and longitudinal studies. BMC Psychiatry. 2017;17(1):256. Published 2017 Jul 17. doi:10.1186/s12888-017-1423-y
1. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality. December 2016. Accessed August 22, 2022. https://www.ustranssurvey.org
2. Meadows SO, Engel CC, Collins RL, et al. 2015 Department of Defense Health Related Behaviors Survey (HRBS). Rand Health Q. 2018;8(2):434.
3. Lipari R, Piscopo K, Kroutil LA, Miller GK. Suicidal thoughts and behavior among adults: results from the 2014 National Survey on Drug Use and Health. NSDUH Data Review. 2015:1-14. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR2-2014/NSDUH-FRR2-2014.pdf
4. Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
5. U.S. Department of Veterans Affairs Office of Mental Health and Suicide Prevention. 2020 National Veteran Suicide Prevention Annual Report. November 2020. Accessed August 22, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
6. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi:10.1001/archpsyc.62.6.593
7. Vespa J. Those who SERVED: America’s veterans from World War II to the war on terror. The United States Census Bureau. June 2, 2020. Accessed August 22, 2022. https://www.census.gov/library/publications/2020/demo/acs-43.html
8. Seibert DC, Keller N, Zapor L, Archer H. Military transgender care. J Am Assoc Nurse Pract. 2020;32(11):764-770. doi:10.1097/JXX.0000000000000519
9. Rigby WC. Military penal law: A brief survey of the 1920 revision of the Articles of War. J Crim Law Criminol. 1921;12(1):84.
10. Department of Defense Directive Number 1332.14: Enlisted Administrative Separations. December 21, 1993. Accessed August 22, 2022. https://biotech.law.lsu.edu/blaw/dodd/corres/pdf/d133214wch1_122193/d133214p.pdf
11. Aford B, Lee SJ. Toward complete inclusion: lesbian, gay, bisexual, and transgender military service members after repeal of Don’t Ask, Don’t Tell. Soc Work. 2016;61(3):257-265. doi:10.1093/sw/sww033
12. Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. June 30, 2016. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DoD-Instruction-1300.28.pdf
13. Department of Defense. Directive-type Memorandum (DTM)-19-004 - Military Service by Transgender Persons and Persons with Gender Dysphoria. March 12. 2019. Accessed August 22, 2022. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
14. US Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. April 30, 2021. Accessed August 22, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/130028p.pdf
15. Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? The Williams Institute; 2016. Accessed August 22, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
16. Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27-e32. doi:10.2105/AJPH.2013.301507
17. Mark KM, McNamara KA, Gribble R, et al. The health and well-being of LGBTQ serving and ex-serving personnel: a narrative review. Int Rev Psychiatry. 2019;31(1):75-94. doi:10.1080/09540261.2019.1575190
18. Blosnich J, Foynes MM, Shipherd JC. Health disparities among sexual minority women veterans. J Womens Health (Larchmt). 2013;22(7):631-636. doi:10.1089/jwh.2012.4214
19. Blosnich JR, Bossarte RM, Silenzio VM. Suicidal ideation among sexual minority veterans: results from the 2005-2010 Massachusetts Behavioral Risk Factor Surveillance Survey. Am J Public Health. 2012;102(suppl 1):S44-S47. doi:10.2105/AJPH.2011.300565
20. Blosnich JR, Gordon AJ, Fine MJ. Associations of sexual and gender minority status with health indicators, health risk factors, and social stressors in a national sample of young adults with military experience. Ann Epidemiol. 2015;25(9):661-667. doi:10.1016/j.annepidem.2015.06.001
21. Cochran BN, Balsam K, Flentje A, Malte CA, Simpson T. Mental health characteristics of sexual minority veterans. J Homosex. 2013;60(2-3):419-435. doi:10.1080/00918369.2013.744932
22. Lehavot K, Browne KC, Simpson TL. Examining sexual orientation disparities in alcohol misuse among women veterans. Am J Prev Med. 2014;47(5):554-562. doi:10.1016/j.amepre.2014.07.002
23. Scott RL, Lasiuk GC, Norris CM. Depression in lesbian, gay, and bisexual members of the Canadian Armed Forces. LGBT Health. 2016;3(5):366-372. doi:10.1089/lgbt.2016.0050
24. Wang J, Dey M, Soldati L, Weiss MG, Gmel G, Mohler-Kuo M. Psychiatric disorders, suicidality, and personality among young men by sexual orientation. Eur Psychiatry. 2014;29(8):514-522. doi:10.1016/j.eurpsy.2014.05.001
25. American Psychological Association. Gender. APA Style. September 2019. Updated July 2022. Accessed August 22, 2022. https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
26. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association; 2013.
27. Deutsch MB. Overview of gender-affirming treatments and procedures. UCSF Transgender Care. June 17, 2016. Accessed August 22, 2022. https://transcare.ucsf.edu/guidelines/overview
28. Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015;2(4):297-305. doi:10.1089/lgbt.2015.0052
29. Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122-131. doi:10.1089/lgbt.2015.0058
30. Downing J, Conron K, Herman JL, Blosnich JR. Transgender and cisgender US veterans have few health differences. Health Aff (Millwood). 2018;37(7):1160-1168. doi:10.1377/hlthaff.2018.0027
31. Holloway IW, Green D, Pickering C, et al. Mental health and health risk behaviors of active duty sexual minority and transgender service members in the United States military. LGBT Health. 2021;8(2):152-161. doi:10.1089/lgbt.2020.0031
32. Beckman K, Shipherd J, Simpson T, Lehavot K. Military sexual assault in transgender veterans: results from a nationwide survey. J Trauma Stress. 2018;31(2):181-190. doi:10.1002/jts.22280
33. Blosnich JR, Marsiglio MC, Gao S, Gordon AJ, Shipherd JC, Kauth M, Brown GR, Fine MJ. Mental health of transgender veterans in US states with and without discrimination and hate crime legal protection. Am J Public Health. 2016;106(3):534-540. doi:10.2105/AJPH.2015.302981
34. Hoy-Ellis CP, Shiu C, Sullivan KM, Kim HJ, Sturges AM, Fredriksen-Goldsen KI. Prior military service, identity stigma, and mental health among transgender older adults. Gerontologist. 2017;57(suppl 1):S63-S71. doi:10.1093/geront/gnw173
35. Hill BJ, Bouris A, Barnett JT, Walker D. Fit to serve? Exploring mental and physical health and well-being among transgender active-duty service members and veterans in the U.S. military. Transgend Health. 2016;1(1):4-11. Published 2016 Jan 1. doi:10.1089/trgh.2015.0002
36. Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269-276. doi:10.1089/lgbt.2014.0050
37. Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: the role of social support and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
38. Lehavot K, Simpson TL, Shipherd JC. Factors associated with suicidality among a national sample of transgender veterans. Suicide Life Threat Behav. 2016;46(5):507-524. doi:10.1111/sltb.12233
39. Tucker RP, Testa RJ, Reger MA, Simpson TL, Shipherd JC, Lehavot K. Current and military-specific gender minority stress factors and their relationship with suicide ideation in transgender veterans. Suicide Life Threat Behav. 2019;49(1):155-166. doi:10.1111/sltb.12432
40. Aboussouan A, Snow A, Cerel J, Tucker RP. Non-suicidal self-injury, suicide ideation, and past suicide attempts: Comparison between transgender and gender diverse veterans and non-veterans. J Affect Disord. 2019;259:186-194. doi:10.1016/j.jad.2019.08.046
41. Frost MC, Blosnich JR, Lehavot K, Chen JA, Rubinsky AD, Glass JE, Williams EC. Disparities in documented drug use disorders between transgender and cisgender U.S. Veterans Health Administration patients. J Addict Med. 2021;15(4):334-340. doi:10.1097/ADM.0000000000000769
42. Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the Veterans Health Administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132-141. doi:10.15288/jsad.2021.82.132
43. Bukowski LA, Blosnich J, Shipherd JC, Kauth MR, Brown GR, Gordon AJ. Exploring rural disparities in medical diagnoses among veterans with transgender-related diagnoses utilizing Veterans Health Administration care. Med Care. 2017;55(suppl 9):S97-S103. doi:10.1097/MLR.0000000000000745
44. U.S. Department of Veterans Affairs. Military Sexual Trauma. Updated August 1, 2022. Accessed August 22, 2022. https://www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
45. Lindsay JA, Keo-Meier C, Hudson S, Walder A, Martin LA, Kauth MR. Mental health of transgender veterans of the Iraq and Afghanistan conflicts who experienced military sexual trauma. J Trauma Stress. 2016;29(6):563-567. doi:10.1002/jts.22146
46. Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and Non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. doi:10.1002/jts.22506
47. Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sexual Health. 2012;24(1):78-87. doi:10.1080/19317611.2011.639440
48. Lehavot K, Katon JG, Simpson TL, Shipherd JC. Transgender veterans’ satisfaction with care and unmet health needs. Med Care. 2017;55(suppl 9):S90-S96. doi:10.1097/MLR.0000000000000723
49. Kauth MR, Barrera TL, Latini DM. Lesbian, gay, and transgender veterans’ experiences in the Veterans Health Administration: positive signs and room for improvement. Psychol Serv. 2019;16(2):346-351. doi:10.1037/ser0000232

50. Rosentel K, Hill BJ, Lu C, Barnett JT. Transgender veterans and the Veterans Health Administration: exploring the experiences of transgender veterans in the Veterans Affairs Healthcare System. Transgend Health. 2016;1(1):108-116. Published 2016 Jun 1. doi:10.1089/trgh.2016.0006
51. Dietert M, Dentice D, Keig Z. Addressing the needs of transgender military veterans: better access and more comprehensive care. Transgend Health. 2017;2(1):35-44. Published 2017 Mar 1. doi:10.1089/trgh.2016.0040
52. Tucker RP, Testa RJ, Simpson TL, Shipherd JC, Blosnich JR, Lehavot K. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med. 2018;48(14):2329-2336. doi:10.1017/S0033291717003853
53. Colizzi M, Costa R, Todarello O. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65-73. doi:10.1016/j.psyneuen.2013.09.029
54. Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119-126. doi:10.1111/jsm.12363
55. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. doi:10.1210/jc.2016-1276
56. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9(6):1808-1816. doi:10.1111/andr.12884
57. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321-335. doi:10.1080/0092623X.2012.736920
58. Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol. 2015;83(1):143-156. doi:10.1037/a0037599
59. Turan S‚ , Aksoy Poyraz C, Usta Sag˘lam NG, et al. Alterations in body uneasiness, eating attitudes, and psychopathology before and after cross-sex hormonal treatment in patients with female-to-male gender dysphoria. Arch Sex Behav. 2018;47(8):2349-2361. doi:10.1007/s10508-018-1189-4
60. Oda H, Kinoshita T. Efficacy of hormonal and mental treatments with MMPI in FtM individuals: cross-sectional and longitudinal studies. BMC Psychiatry. 2017;17(1):256. Published 2017 Jul 17. doi:10.1186/s12888-017-1423-y
COMMENT & CONTROVERSY
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Options and outcomes for uterine preservation at the time of prolapse surgery
CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
2022 Update on contraception
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.
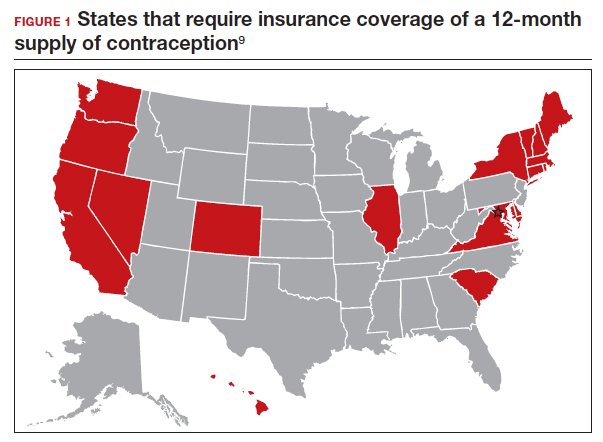
Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14
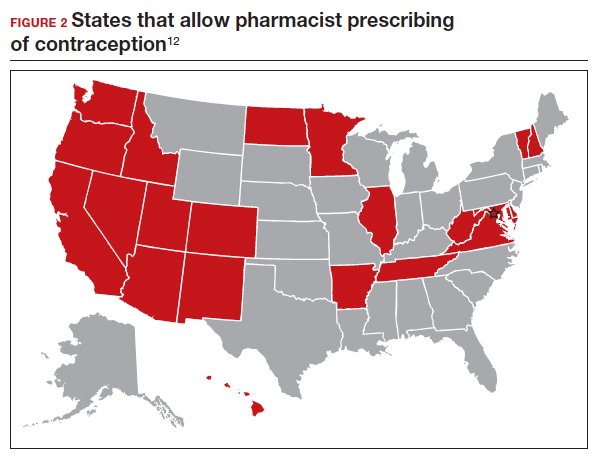
Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).
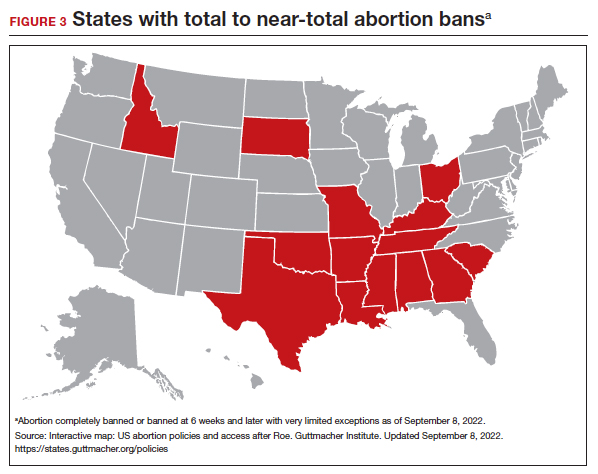
Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●
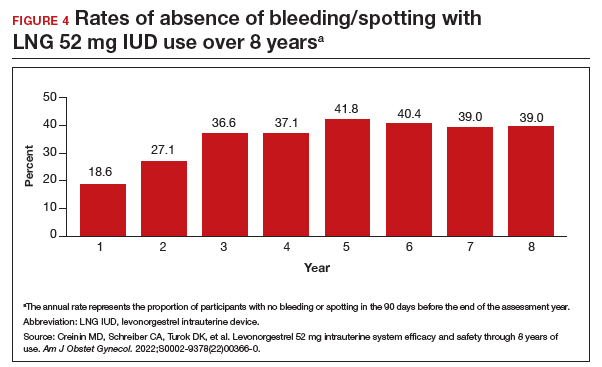
As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
Nonsurgical treatments for patients with urinary incontinence
CASE Patient has urine leakage that worsens with exercise
At her annual preventative health visit, a 39-year-old woman reports that she has leakage of urine. She states that she drinks “a gallon of water daily” to help her lose the 20 lb she gained during the COVID-19 pandemic. She wants to resume Zumba fitness classes, but exercise makes her urine leakage worse. She started wearing protective pads because she finds herself often leaking urine on the way to the bathroom.
What nonsurgical treatment options are available for this patient?
Nearly half of all women experience urinary incontinence (UI), the involuntary loss of urine, and the condition increases with age.1 This common condition negatively impacts physical and psychological health and has been associated with social isolation, sexual dysfunction, and reduced independence.2,3 Symptoms of UI are underreported, and therefore universal screening is recommended for women of all ages.4 The diversity of available treatments (TABLE 1) provides patients and clinicians an opportunity to develop a plan that aligns with their symptom severity, goals, preferences, and resources.
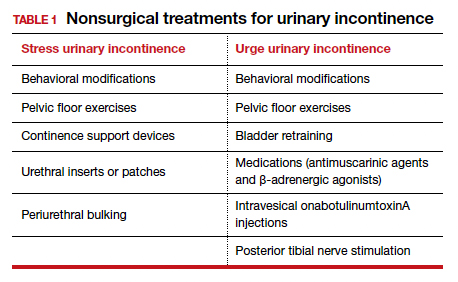
Types of UI
The most common types of UI are stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). Mixed urinary incontinence (MUI) occurs when symptoms of both SUI and UUI are present. Although the mechanisms that lead to urine leakage vary by the type of incontinence, many primary interventions improve both types of leakage, so a clinical diagnosis is sufficient to initiate treatment.
Stress urinary incontinence results from an impaired or weakened sphincter, which leads to involuntary, yet predictable, urine loss during increased abdominal pressure, such as coughing, laughing, sneezing, lifting, or physical activity.5 In UUI, involuntary loss of urine often accompanies the sudden urge to void. UUI is associated with overactive bladder (OAB), defined as urinary urgency, with or without urinary incontinence, usually accompanied by urinary frequency and/or nocturia (urination that interrupts sleep).6
In OAB, the detrusor muscle contracts randomly, leading to a sudden urge to void. When bladder pressure exceeds urethral sphincter closure pressure, urine leakage occurs. Women describe the urgency episodes as unpredictable, the urine leakage as prolonged with large volumes, and often occurring as they seek the toilet. Risk factors include age, obesity, parity, history of vaginal delivery, family history, ethnicity/race, medical comorbidities, menopausal status, and tobacco use.5
Making a diagnosis
A basic office evaluation is the most key step for diagnostic accuracy that leads to treatment success. This includes a detailed history, assessment of symptom severity, physical exam, pelvic exam, urinalysis, postvoid residual (to rule out urinary retention), and a cough stress test (to demonstrate SUI). The goal is to assess symptom severity, determine the type of UI, and identify contributing and potentially reversible factors, such as a urinary tract infection, medications, pelvic organ prolapse, incomplete bladder emptying, or impaired neurologic status. In the absence of the latter, advanced diagnostic tests, such as urodynamics, contribute little toward discerning the type of incontinence or changing first-line treatment plans.7
During the COVID-19 pandemic, abbreviated, virtual assessments for urinary symptoms were associated with high degrees of satisfaction (91% for fulfillment of personal needs, 94% overall satisfaction).8 This highlights the value of validated symptom questionnaires that help establish a working diagnosis and treatment plan in the absence of a physical exam. Questionnaire-based diagnoses have acceptable accuracy for classifying UUI and SUI among women with uncomplicated medical and surgical histories and for initiating low-risk therapies for defined intervals.
The 3 incontinence questions (3IQ) screen is an example of a useful, quick diagnostic tool designed for the primary care setting (FIGURE 1).9 It has been used in pharmaceutical treatment trials for UUI, with low frequency of misdiagnosis (1%–4%), resulting in no harm by the drug treatment prescribed or by the delay in appropriate care.10 Due to the limitations of an abbreviated remote evaluation, however, clinicians should assess patient response to primary interventions in a timely window. Patients who fail to experience satisfactory symptom reduction within 6 to 12 weeks should complete their evaluation in person or through a referral to a urogynecology program.

Continue to: Primary therapies for UI...
Primary therapies for UI
Primary therapies for UUI and SUI target strength training of the pelvic floor muscles, moderation of fluid intake, and adjustment in voiding behaviors and medications. Any functional barriers to continence also should be identified and addressed. Simple interventions, including a daily bowel regimen to address constipation, a bedside commode, and scheduled voiding, may reduce incontinence episodes without incurring significant cost or risk. For women suspected of having MUI, the treatment plan should prioritize their most bothersome symptoms.
Lifestyle and behavioral modifications
Everyday habits, medical comorbidities, and medications may exacerbate the severity of both SUI and UUI. Behavioral therapy alone or in combination with other interventions effectively reduces both SUI and UUI symptoms and has been shown to improve the efficacy of continence surgery.11 Information gained from a 3-day bladder diary (FIGURE 2)12 can guide clinicians on personalized patient recommendations, such as reducing excessive consumption of fluids and bladder irritants, limiting late evening drinking in the setting of bothersome nocturia, and scheduling voids (every 2–3 hours) to preempt incontinence episodes.

Weight loss
Obesity is a strong, independent, modifiable risk factor for both SUI and UUI. Each 5 kg/m2 increase in body mass index (BMI) has been associated with a 20% to 70% increased risk of UI, while weight loss of 5% or greater in overweight or obese women can lead to at least a 50% decrease in UI frequency.13
Reducing fluid intake and bladder irritants
Overactive bladder symptoms often respond to moderation of excessive fluid intake and reduction of bladder irritants (caffeine, carbonated beverages, diet beverages, and alcohol). While there is no established definition of excess caffeine intake, one study categorized high caffeine intake as greater than 400 mg/day (approximately four 8-oz cups of coffee).14
Information provided in a bladder diary can guide individualized recommendations for reducing fluid intake, particularly when 24-hour urine production exceeds the normative range (> 50–60 oz or 1.5-1.8 L/day).15 Hydration needs vary by activity, environment, and food; some general guidelines suggest 48 to 64 oz/day.5,16
Continue to: Pelvic floor muscle training...
Pelvic floor muscle training
An effective treatment for both UUI and SUI symptoms, pelvic floor muscle training (PFMT) leads to high degrees of patient satisfaction and improvement in quality of life.17 The presumed mechanisms of action of PFMT include improved urethral closure pressure and inhibition of detrusor muscle contractions.
Common exercise protocols recommend 3 sets of 10 contractions, held for 6 to 10 seconds per day, in varying positions of sitting, standing, and lying. While many women may be familiar with Kegel exercises, poor technique with straining and recruitment of gluteal and abdominal muscles can undermine the effect of PFMT. Clinicians can confirm successful pelvic muscle contractions by placing a finger in the vagina to appreciate contraction around and elevation of the finger toward the pubic symphysis in the absence of pushing.
Referral to supervised physical therapy and use of such teaching aid tools as booklets, mobile applications, and biofeedback can improve exercise adherence and outcomes.18,19 Systematic reviews report initial cure or improvement of incontinence symptoms as high as 74%, although little information is available about the long-term duration of effect.17
Vaginal pessaries
Vaginal continence support pessaries and devices work by stabilizing urethral mobility and compression of the bladder neck. Continence devices are particularly effective for situational SUI (such as during exercise).
The reusable medical grade silicone pessaries are available in numerous shapes and sizes and are fitted by a health care clinician (FIGURE 3). Uresta is a self-fitted intravaginal device that women can purchase online with a prescription. The Poise Impressa bladder support is a disposable intravaginal device marketed for incontinence and available over-the-counter, without a prescription (FIGURE 4). Anecdotally, many women find that menstrual tampons provide a similar effect, but outcome data are lacking.
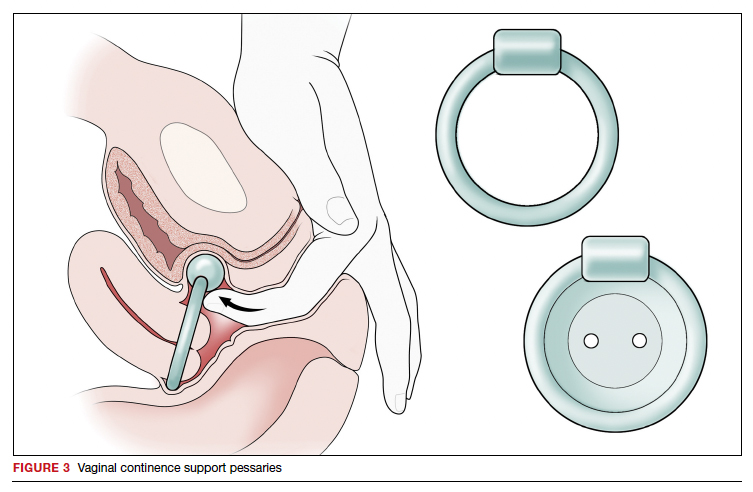
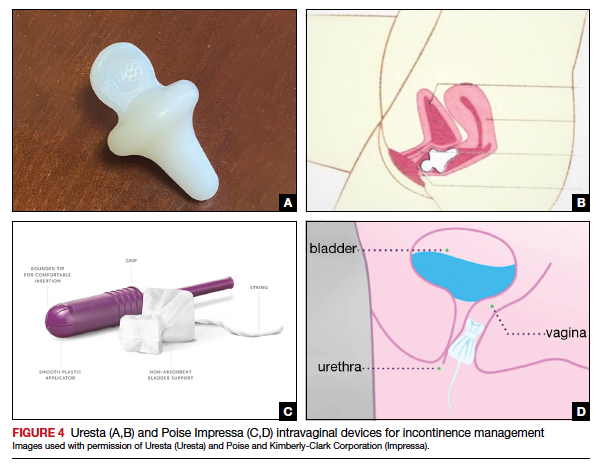
In a comparative effectiveness trial of a continence pessary and behavior therapy, behavioral therapy was more likely to result in no bothersome incontinence symptoms (49% vs 33%, P = .006) and greater treatment satisfaction at 3 months.20 However, these short-term group differences did not persist at 12 months, presumably due to waning adherence.
UUI-specific nonsurgical treatments
Drug therapy
All medications approved by the US Food and Drug Administration (FDA) for UI are for the indications of OAB or UUI. These second-line treatments are most effective as adjuncts to behavioral modifications and PFMT.
A multicenter randomized trial that evaluated the efficacy of drug therapy alone compared with drug therapy in combination with behavioral modification, PFMT, urge suppression strategies, timed voiding, and fluid management for UUI found that combined therapy was more successful in achieving greater than 70% reduction in incontinence episodes (58% for drug therapy vs 69% for combined therapy).21
Of the 8 medications currently marketed in the United States for OAB or UUI, 6 are anticholinergic agents that block muscarinic receptors in the smooth muscle of the bladder, leading to inhibition of detrusor contractions, and 2 are β-adrenergic receptor agonists that promote bladder storage capacity by relaxing the detrusor muscle (TABLE 2). Similar efficacies lead most clinicians to initiate drug therapy based on formulary coverage and tolerance for adverse effects. Patients can expect a 53% to 80% reduction in UUI episodes and a 12% to 32% reduction in urinary frequency.22
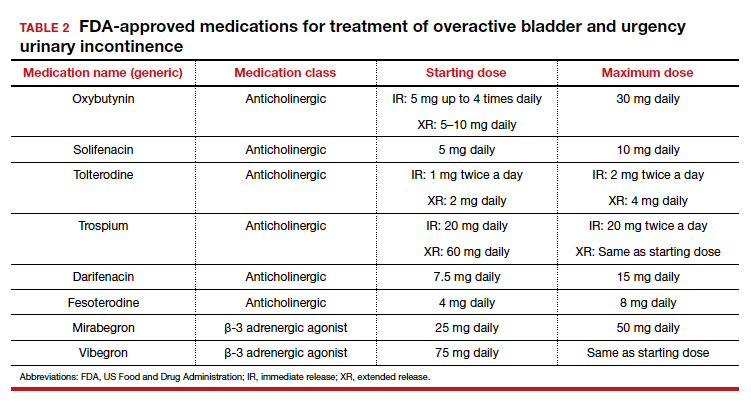
Extended-release formulations are associated with reduced anticholinergic side effects (dry mouth, constipation, somnolence, dry eyes), leading to improved adherence. Notably, the anticholinergic medications are contraindicated in patients with untreated narrow-angle glaucoma, gastric retention, and supraventricular tachycardia. Mirabegron should be used with caution in patients with poorly controlled hypertension. 5 Due to concerns regarding the association between cumulative anticholinergic burden and the development of dementia, clinicians may consider avoiding the anticholinergic medications in older and at-risk patients.23
Continue to: UUI office-based procedure treatments...
UUI office-based procedure treatments
If behavioral therapies and medications are ineffective, contraindicated, or not the patient’s preference, additional FDA-approved therapies for UUI are available, typically through referral to a urogynecologist, urologist, or continence center.
Posterior tibial nerve stimulation (PTNS) is a nondrug treatment that delivers electrical stimulation using an acupuncture needle for 12 weekly 30-minute sessions followed by monthly maintenance for responders. The time commitment for this treatment plan can be a barrier for some patients. However, patients who adhere to the recommended protocol can expect a 60% improvement in symptoms, with minimal adverse events. Treatment efficacy is comparable to that of anticholinergic medication.24
OnabotulinumtoxinA injections into the bladder muscle are performed cystoscopically under local anesthetic. The toxin blocks the presynaptic release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. This treatment is associated with high satisfaction. Efficacy varies by study population and outcome measure.
In one US comparative effectiveness trial, 67% of study participants with UUI symptoms refractory to oral medication reported a greater than 50% reduction in OAB symptoms at 6 months, 20% reported complete resolution of UUI, and 72% requested a second injection within 24 months.25 The interval between the first and second injection was nearly 1 year (350 days).Risks include urinary tract infection (12% within 1 month of the procedure and 35% through 6 months); urinary retention requiring catheterization has decreased to 6% with recognition that most moderate retention is tolerated by patients.
Some insurers limit onabotulinumtoxinA treatment coverage to patients who have failed to achieve symptom control with first- and second-line treatments.
SUI-specific nonsurgical treatments
Cystoscopic injection of urethral bulking agents into the urethral submucosa is designed to improve urethral coaptation. It is a minor procedure that can be performed in an ambulatory setting under local anesthetic with or without sedation.
Various bulking agents have been approved for use in the United States, some of which have been withdrawn due to complications of migration, erosion, and pseudoabscess formation. Cure or improvement after bulking agent injection was found to be superior to a home pelvic floor exercise program but inferior to a midurethral sling procedure for cure (9% vs 89%).26
The durability of currently available urethral bulking agents beyond 1 year is unknown. Complications are typically minor and transient and include pain at the injection site, urinary retention, de novo urgency, and implant leakage. The advantages include no postprocedure activity restrictions.
CASE Symptom presentation guides treatment plan
Our patient described symptoms of stress-predominant MUI. She was counseled to moderate her fluid intake to 2 L per day and to strategically time voids (before exercise, and at least every 4 hours). The patient was fitted with an incontinence pessary, and she elected to pursue a course of supervised physical therapy for pelvic floor muscle strengthening. Her follow-up visit is scheduled in 3 months to determine if other interventions are warranted. ●
1. Lee UJ, Feinstein L, Ward JB, et al. Prevalence of urinary incontinence among a nationally representative sample of women, 2005–2016: findings from the Urologic Diseases in America Project. J Urol. 2021;205:1718-1724. doi:10.1097 /JU.0000000000001634
2. Sims J, Browning C, Lundgren-Lindquist B, et al. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389-1398. doi:10.3109/09638288.2010.532284
3. Sarikaya S, Yildiz FG, Senocak C, et al. Urinary incontinence as a cause of depression and sexual dysfunction: questionnaire-based study. Rev Int Androl. 2020:18:50-54. doi:10.1016 /j.androl.2018.08.003
4. O’Reilly N, Nelson HD, Conry JM, et al; Women’s Preventive Services Initiative. Screening for urinary incontinence in women: a recommendation from the Women’s Preventive Services Initiative. Ann Intern Med. 2018;169(5):320-328. doi:10.7326/M18-0595
5. Barber MD, Walters MD, Karram MM, et al. Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier Saunders; 2021.
6. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21: 5-26. doi:10.1007/s00192-009-0976-9
7. ACOG practice bulletin no. 155. Urinary incontinence in women. Obstet Gynecol. 2015;126:e66-e81. doi:10.1097 /AOG.0000000000001148
8. Sansone S, Lu J, Drangsholt S, et al. No pelvic exam, no problem: patient satisfaction following the integration of comprehensive urogynecology telemedicine. Int Urogynecol J. 2022;1:3. doi:10.1007/s00192-022-05104-w
9. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715723. doi:10.7326/0003-4819-144-10-200605160-00005
10. Hess R, Huang AJ, Richter HE, et al. Long-term efficacy and safety of questionnaire-based initiation of urgency urinary incontinence treatment. Am J Obstet Gynecol. 2013;209:244. e1-9. doi:10.1016/j.ajog.2013.05.008
11. Sung VW, Borello-France D, Newman DK, et al; NICHD Pelvic Floor Disorders Network. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence. JAMA. 2019;322:1066-1076. doi:10.1001 /jama.2019.12467
12. American Urogynecologic Society. Voices for PFD: intake and voiding diary. Accessed August 11, 2022. https://www .voicesforpfd.org/assets/2/6/Voiding_Diary.pdf
13. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 suppl):S2-7. doi:10.1016/j.juro.2009.08.071
14. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89. doi:10.1016/s0029-7844(00)00808-5
15. Wyman JF, Zhou J, LaCoursiere DY, et al. Normative noninvasive bladder function measurements in healthy women: a systematic review and meta-analysis. Neurourol Urodyn. 2020;39:507-522. doi:10.1002/nau.24265
16. Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10: 428-433. doi:10.1007/s11934-009-0068-x
17. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
18. Araujo CC, de A Marques A, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26:697-703. doi:10.1097/SPV.0000000000000670
19. Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: a randomized controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112:362-372. doi:10.1111/j.1464 -410X.2012.11713.x
20. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609617. doi:10.1097/AOG.0b013e3181d055d4
21. Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3): 161-169. doi:10.7326/0003-4819-149-3-200808050 -00005
22. Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary incontinence in women: a review. JAMA. 2017;318:1592-1604. doi:10.1001/jama.2017.12137
23. Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686-700. doi:10.1038/s41585-021-00504-x
24. Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206-1216. doi:10.1002/nau.22251
25. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: A randomized clinical trial. JAMA. 2016;316:1366-1374. doi:10.1001/jama.2016.14617
26. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi:10.1002/14651858.CD003881.pub4
CASE Patient has urine leakage that worsens with exercise
At her annual preventative health visit, a 39-year-old woman reports that she has leakage of urine. She states that she drinks “a gallon of water daily” to help her lose the 20 lb she gained during the COVID-19 pandemic. She wants to resume Zumba fitness classes, but exercise makes her urine leakage worse. She started wearing protective pads because she finds herself often leaking urine on the way to the bathroom.
What nonsurgical treatment options are available for this patient?
Nearly half of all women experience urinary incontinence (UI), the involuntary loss of urine, and the condition increases with age.1 This common condition negatively impacts physical and psychological health and has been associated with social isolation, sexual dysfunction, and reduced independence.2,3 Symptoms of UI are underreported, and therefore universal screening is recommended for women of all ages.4 The diversity of available treatments (TABLE 1) provides patients and clinicians an opportunity to develop a plan that aligns with their symptom severity, goals, preferences, and resources.

Types of UI
The most common types of UI are stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). Mixed urinary incontinence (MUI) occurs when symptoms of both SUI and UUI are present. Although the mechanisms that lead to urine leakage vary by the type of incontinence, many primary interventions improve both types of leakage, so a clinical diagnosis is sufficient to initiate treatment.
Stress urinary incontinence results from an impaired or weakened sphincter, which leads to involuntary, yet predictable, urine loss during increased abdominal pressure, such as coughing, laughing, sneezing, lifting, or physical activity.5 In UUI, involuntary loss of urine often accompanies the sudden urge to void. UUI is associated with overactive bladder (OAB), defined as urinary urgency, with or without urinary incontinence, usually accompanied by urinary frequency and/or nocturia (urination that interrupts sleep).6
In OAB, the detrusor muscle contracts randomly, leading to a sudden urge to void. When bladder pressure exceeds urethral sphincter closure pressure, urine leakage occurs. Women describe the urgency episodes as unpredictable, the urine leakage as prolonged with large volumes, and often occurring as they seek the toilet. Risk factors include age, obesity, parity, history of vaginal delivery, family history, ethnicity/race, medical comorbidities, menopausal status, and tobacco use.5
Making a diagnosis
A basic office evaluation is the most key step for diagnostic accuracy that leads to treatment success. This includes a detailed history, assessment of symptom severity, physical exam, pelvic exam, urinalysis, postvoid residual (to rule out urinary retention), and a cough stress test (to demonstrate SUI). The goal is to assess symptom severity, determine the type of UI, and identify contributing and potentially reversible factors, such as a urinary tract infection, medications, pelvic organ prolapse, incomplete bladder emptying, or impaired neurologic status. In the absence of the latter, advanced diagnostic tests, such as urodynamics, contribute little toward discerning the type of incontinence or changing first-line treatment plans.7
During the COVID-19 pandemic, abbreviated, virtual assessments for urinary symptoms were associated with high degrees of satisfaction (91% for fulfillment of personal needs, 94% overall satisfaction).8 This highlights the value of validated symptom questionnaires that help establish a working diagnosis and treatment plan in the absence of a physical exam. Questionnaire-based diagnoses have acceptable accuracy for classifying UUI and SUI among women with uncomplicated medical and surgical histories and for initiating low-risk therapies for defined intervals.
The 3 incontinence questions (3IQ) screen is an example of a useful, quick diagnostic tool designed for the primary care setting (FIGURE 1).9 It has been used in pharmaceutical treatment trials for UUI, with low frequency of misdiagnosis (1%–4%), resulting in no harm by the drug treatment prescribed or by the delay in appropriate care.10 Due to the limitations of an abbreviated remote evaluation, however, clinicians should assess patient response to primary interventions in a timely window. Patients who fail to experience satisfactory symptom reduction within 6 to 12 weeks should complete their evaluation in person or through a referral to a urogynecology program.

Continue to: Primary therapies for UI...
Primary therapies for UI
Primary therapies for UUI and SUI target strength training of the pelvic floor muscles, moderation of fluid intake, and adjustment in voiding behaviors and medications. Any functional barriers to continence also should be identified and addressed. Simple interventions, including a daily bowel regimen to address constipation, a bedside commode, and scheduled voiding, may reduce incontinence episodes without incurring significant cost or risk. For women suspected of having MUI, the treatment plan should prioritize their most bothersome symptoms.
Lifestyle and behavioral modifications
Everyday habits, medical comorbidities, and medications may exacerbate the severity of both SUI and UUI. Behavioral therapy alone or in combination with other interventions effectively reduces both SUI and UUI symptoms and has been shown to improve the efficacy of continence surgery.11 Information gained from a 3-day bladder diary (FIGURE 2)12 can guide clinicians on personalized patient recommendations, such as reducing excessive consumption of fluids and bladder irritants, limiting late evening drinking in the setting of bothersome nocturia, and scheduling voids (every 2–3 hours) to preempt incontinence episodes.

Weight loss
Obesity is a strong, independent, modifiable risk factor for both SUI and UUI. Each 5 kg/m2 increase in body mass index (BMI) has been associated with a 20% to 70% increased risk of UI, while weight loss of 5% or greater in overweight or obese women can lead to at least a 50% decrease in UI frequency.13
Reducing fluid intake and bladder irritants
Overactive bladder symptoms often respond to moderation of excessive fluid intake and reduction of bladder irritants (caffeine, carbonated beverages, diet beverages, and alcohol). While there is no established definition of excess caffeine intake, one study categorized high caffeine intake as greater than 400 mg/day (approximately four 8-oz cups of coffee).14
Information provided in a bladder diary can guide individualized recommendations for reducing fluid intake, particularly when 24-hour urine production exceeds the normative range (> 50–60 oz or 1.5-1.8 L/day).15 Hydration needs vary by activity, environment, and food; some general guidelines suggest 48 to 64 oz/day.5,16
Continue to: Pelvic floor muscle training...
Pelvic floor muscle training
An effective treatment for both UUI and SUI symptoms, pelvic floor muscle training (PFMT) leads to high degrees of patient satisfaction and improvement in quality of life.17 The presumed mechanisms of action of PFMT include improved urethral closure pressure and inhibition of detrusor muscle contractions.
Common exercise protocols recommend 3 sets of 10 contractions, held for 6 to 10 seconds per day, in varying positions of sitting, standing, and lying. While many women may be familiar with Kegel exercises, poor technique with straining and recruitment of gluteal and abdominal muscles can undermine the effect of PFMT. Clinicians can confirm successful pelvic muscle contractions by placing a finger in the vagina to appreciate contraction around and elevation of the finger toward the pubic symphysis in the absence of pushing.
Referral to supervised physical therapy and use of such teaching aid tools as booklets, mobile applications, and biofeedback can improve exercise adherence and outcomes.18,19 Systematic reviews report initial cure or improvement of incontinence symptoms as high as 74%, although little information is available about the long-term duration of effect.17
Vaginal pessaries
Vaginal continence support pessaries and devices work by stabilizing urethral mobility and compression of the bladder neck. Continence devices are particularly effective for situational SUI (such as during exercise).
The reusable medical grade silicone pessaries are available in numerous shapes and sizes and are fitted by a health care clinician (FIGURE 3). Uresta is a self-fitted intravaginal device that women can purchase online with a prescription. The Poise Impressa bladder support is a disposable intravaginal device marketed for incontinence and available over-the-counter, without a prescription (FIGURE 4). Anecdotally, many women find that menstrual tampons provide a similar effect, but outcome data are lacking.


In a comparative effectiveness trial of a continence pessary and behavior therapy, behavioral therapy was more likely to result in no bothersome incontinence symptoms (49% vs 33%, P = .006) and greater treatment satisfaction at 3 months.20 However, these short-term group differences did not persist at 12 months, presumably due to waning adherence.
UUI-specific nonsurgical treatments
Drug therapy
All medications approved by the US Food and Drug Administration (FDA) for UI are for the indications of OAB or UUI. These second-line treatments are most effective as adjuncts to behavioral modifications and PFMT.
A multicenter randomized trial that evaluated the efficacy of drug therapy alone compared with drug therapy in combination with behavioral modification, PFMT, urge suppression strategies, timed voiding, and fluid management for UUI found that combined therapy was more successful in achieving greater than 70% reduction in incontinence episodes (58% for drug therapy vs 69% for combined therapy).21
Of the 8 medications currently marketed in the United States for OAB or UUI, 6 are anticholinergic agents that block muscarinic receptors in the smooth muscle of the bladder, leading to inhibition of detrusor contractions, and 2 are β-adrenergic receptor agonists that promote bladder storage capacity by relaxing the detrusor muscle (TABLE 2). Similar efficacies lead most clinicians to initiate drug therapy based on formulary coverage and tolerance for adverse effects. Patients can expect a 53% to 80% reduction in UUI episodes and a 12% to 32% reduction in urinary frequency.22

Extended-release formulations are associated with reduced anticholinergic side effects (dry mouth, constipation, somnolence, dry eyes), leading to improved adherence. Notably, the anticholinergic medications are contraindicated in patients with untreated narrow-angle glaucoma, gastric retention, and supraventricular tachycardia. Mirabegron should be used with caution in patients with poorly controlled hypertension. 5 Due to concerns regarding the association between cumulative anticholinergic burden and the development of dementia, clinicians may consider avoiding the anticholinergic medications in older and at-risk patients.23
Continue to: UUI office-based procedure treatments...
UUI office-based procedure treatments
If behavioral therapies and medications are ineffective, contraindicated, or not the patient’s preference, additional FDA-approved therapies for UUI are available, typically through referral to a urogynecologist, urologist, or continence center.
Posterior tibial nerve stimulation (PTNS) is a nondrug treatment that delivers electrical stimulation using an acupuncture needle for 12 weekly 30-minute sessions followed by monthly maintenance for responders. The time commitment for this treatment plan can be a barrier for some patients. However, patients who adhere to the recommended protocol can expect a 60% improvement in symptoms, with minimal adverse events. Treatment efficacy is comparable to that of anticholinergic medication.24
OnabotulinumtoxinA injections into the bladder muscle are performed cystoscopically under local anesthetic. The toxin blocks the presynaptic release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. This treatment is associated with high satisfaction. Efficacy varies by study population and outcome measure.
In one US comparative effectiveness trial, 67% of study participants with UUI symptoms refractory to oral medication reported a greater than 50% reduction in OAB symptoms at 6 months, 20% reported complete resolution of UUI, and 72% requested a second injection within 24 months.25 The interval between the first and second injection was nearly 1 year (350 days).Risks include urinary tract infection (12% within 1 month of the procedure and 35% through 6 months); urinary retention requiring catheterization has decreased to 6% with recognition that most moderate retention is tolerated by patients.
Some insurers limit onabotulinumtoxinA treatment coverage to patients who have failed to achieve symptom control with first- and second-line treatments.
SUI-specific nonsurgical treatments
Cystoscopic injection of urethral bulking agents into the urethral submucosa is designed to improve urethral coaptation. It is a minor procedure that can be performed in an ambulatory setting under local anesthetic with or without sedation.
Various bulking agents have been approved for use in the United States, some of which have been withdrawn due to complications of migration, erosion, and pseudoabscess formation. Cure or improvement after bulking agent injection was found to be superior to a home pelvic floor exercise program but inferior to a midurethral sling procedure for cure (9% vs 89%).26
The durability of currently available urethral bulking agents beyond 1 year is unknown. Complications are typically minor and transient and include pain at the injection site, urinary retention, de novo urgency, and implant leakage. The advantages include no postprocedure activity restrictions.
CASE Symptom presentation guides treatment plan
Our patient described symptoms of stress-predominant MUI. She was counseled to moderate her fluid intake to 2 L per day and to strategically time voids (before exercise, and at least every 4 hours). The patient was fitted with an incontinence pessary, and she elected to pursue a course of supervised physical therapy for pelvic floor muscle strengthening. Her follow-up visit is scheduled in 3 months to determine if other interventions are warranted. ●
CASE Patient has urine leakage that worsens with exercise
At her annual preventative health visit, a 39-year-old woman reports that she has leakage of urine. She states that she drinks “a gallon of water daily” to help her lose the 20 lb she gained during the COVID-19 pandemic. She wants to resume Zumba fitness classes, but exercise makes her urine leakage worse. She started wearing protective pads because she finds herself often leaking urine on the way to the bathroom.
What nonsurgical treatment options are available for this patient?
Nearly half of all women experience urinary incontinence (UI), the involuntary loss of urine, and the condition increases with age.1 This common condition negatively impacts physical and psychological health and has been associated with social isolation, sexual dysfunction, and reduced independence.2,3 Symptoms of UI are underreported, and therefore universal screening is recommended for women of all ages.4 The diversity of available treatments (TABLE 1) provides patients and clinicians an opportunity to develop a plan that aligns with their symptom severity, goals, preferences, and resources.

Types of UI
The most common types of UI are stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). Mixed urinary incontinence (MUI) occurs when symptoms of both SUI and UUI are present. Although the mechanisms that lead to urine leakage vary by the type of incontinence, many primary interventions improve both types of leakage, so a clinical diagnosis is sufficient to initiate treatment.
Stress urinary incontinence results from an impaired or weakened sphincter, which leads to involuntary, yet predictable, urine loss during increased abdominal pressure, such as coughing, laughing, sneezing, lifting, or physical activity.5 In UUI, involuntary loss of urine often accompanies the sudden urge to void. UUI is associated with overactive bladder (OAB), defined as urinary urgency, with or without urinary incontinence, usually accompanied by urinary frequency and/or nocturia (urination that interrupts sleep).6
In OAB, the detrusor muscle contracts randomly, leading to a sudden urge to void. When bladder pressure exceeds urethral sphincter closure pressure, urine leakage occurs. Women describe the urgency episodes as unpredictable, the urine leakage as prolonged with large volumes, and often occurring as they seek the toilet. Risk factors include age, obesity, parity, history of vaginal delivery, family history, ethnicity/race, medical comorbidities, menopausal status, and tobacco use.5
Making a diagnosis
A basic office evaluation is the most key step for diagnostic accuracy that leads to treatment success. This includes a detailed history, assessment of symptom severity, physical exam, pelvic exam, urinalysis, postvoid residual (to rule out urinary retention), and a cough stress test (to demonstrate SUI). The goal is to assess symptom severity, determine the type of UI, and identify contributing and potentially reversible factors, such as a urinary tract infection, medications, pelvic organ prolapse, incomplete bladder emptying, or impaired neurologic status. In the absence of the latter, advanced diagnostic tests, such as urodynamics, contribute little toward discerning the type of incontinence or changing first-line treatment plans.7
During the COVID-19 pandemic, abbreviated, virtual assessments for urinary symptoms were associated with high degrees of satisfaction (91% for fulfillment of personal needs, 94% overall satisfaction).8 This highlights the value of validated symptom questionnaires that help establish a working diagnosis and treatment plan in the absence of a physical exam. Questionnaire-based diagnoses have acceptable accuracy for classifying UUI and SUI among women with uncomplicated medical and surgical histories and for initiating low-risk therapies for defined intervals.
The 3 incontinence questions (3IQ) screen is an example of a useful, quick diagnostic tool designed for the primary care setting (FIGURE 1).9 It has been used in pharmaceutical treatment trials for UUI, with low frequency of misdiagnosis (1%–4%), resulting in no harm by the drug treatment prescribed or by the delay in appropriate care.10 Due to the limitations of an abbreviated remote evaluation, however, clinicians should assess patient response to primary interventions in a timely window. Patients who fail to experience satisfactory symptom reduction within 6 to 12 weeks should complete their evaluation in person or through a referral to a urogynecology program.

Continue to: Primary therapies for UI...
Primary therapies for UI
Primary therapies for UUI and SUI target strength training of the pelvic floor muscles, moderation of fluid intake, and adjustment in voiding behaviors and medications. Any functional barriers to continence also should be identified and addressed. Simple interventions, including a daily bowel regimen to address constipation, a bedside commode, and scheduled voiding, may reduce incontinence episodes without incurring significant cost or risk. For women suspected of having MUI, the treatment plan should prioritize their most bothersome symptoms.
Lifestyle and behavioral modifications
Everyday habits, medical comorbidities, and medications may exacerbate the severity of both SUI and UUI. Behavioral therapy alone or in combination with other interventions effectively reduces both SUI and UUI symptoms and has been shown to improve the efficacy of continence surgery.11 Information gained from a 3-day bladder diary (FIGURE 2)12 can guide clinicians on personalized patient recommendations, such as reducing excessive consumption of fluids and bladder irritants, limiting late evening drinking in the setting of bothersome nocturia, and scheduling voids (every 2–3 hours) to preempt incontinence episodes.

Weight loss
Obesity is a strong, independent, modifiable risk factor for both SUI and UUI. Each 5 kg/m2 increase in body mass index (BMI) has been associated with a 20% to 70% increased risk of UI, while weight loss of 5% or greater in overweight or obese women can lead to at least a 50% decrease in UI frequency.13
Reducing fluid intake and bladder irritants
Overactive bladder symptoms often respond to moderation of excessive fluid intake and reduction of bladder irritants (caffeine, carbonated beverages, diet beverages, and alcohol). While there is no established definition of excess caffeine intake, one study categorized high caffeine intake as greater than 400 mg/day (approximately four 8-oz cups of coffee).14
Information provided in a bladder diary can guide individualized recommendations for reducing fluid intake, particularly when 24-hour urine production exceeds the normative range (> 50–60 oz or 1.5-1.8 L/day).15 Hydration needs vary by activity, environment, and food; some general guidelines suggest 48 to 64 oz/day.5,16
Continue to: Pelvic floor muscle training...
Pelvic floor muscle training
An effective treatment for both UUI and SUI symptoms, pelvic floor muscle training (PFMT) leads to high degrees of patient satisfaction and improvement in quality of life.17 The presumed mechanisms of action of PFMT include improved urethral closure pressure and inhibition of detrusor muscle contractions.
Common exercise protocols recommend 3 sets of 10 contractions, held for 6 to 10 seconds per day, in varying positions of sitting, standing, and lying. While many women may be familiar with Kegel exercises, poor technique with straining and recruitment of gluteal and abdominal muscles can undermine the effect of PFMT. Clinicians can confirm successful pelvic muscle contractions by placing a finger in the vagina to appreciate contraction around and elevation of the finger toward the pubic symphysis in the absence of pushing.
Referral to supervised physical therapy and use of such teaching aid tools as booklets, mobile applications, and biofeedback can improve exercise adherence and outcomes.18,19 Systematic reviews report initial cure or improvement of incontinence symptoms as high as 74%, although little information is available about the long-term duration of effect.17
Vaginal pessaries
Vaginal continence support pessaries and devices work by stabilizing urethral mobility and compression of the bladder neck. Continence devices are particularly effective for situational SUI (such as during exercise).
The reusable medical grade silicone pessaries are available in numerous shapes and sizes and are fitted by a health care clinician (FIGURE 3). Uresta is a self-fitted intravaginal device that women can purchase online with a prescription. The Poise Impressa bladder support is a disposable intravaginal device marketed for incontinence and available over-the-counter, without a prescription (FIGURE 4). Anecdotally, many women find that menstrual tampons provide a similar effect, but outcome data are lacking.


In a comparative effectiveness trial of a continence pessary and behavior therapy, behavioral therapy was more likely to result in no bothersome incontinence symptoms (49% vs 33%, P = .006) and greater treatment satisfaction at 3 months.20 However, these short-term group differences did not persist at 12 months, presumably due to waning adherence.
UUI-specific nonsurgical treatments
Drug therapy
All medications approved by the US Food and Drug Administration (FDA) for UI are for the indications of OAB or UUI. These second-line treatments are most effective as adjuncts to behavioral modifications and PFMT.
A multicenter randomized trial that evaluated the efficacy of drug therapy alone compared with drug therapy in combination with behavioral modification, PFMT, urge suppression strategies, timed voiding, and fluid management for UUI found that combined therapy was more successful in achieving greater than 70% reduction in incontinence episodes (58% for drug therapy vs 69% for combined therapy).21
Of the 8 medications currently marketed in the United States for OAB or UUI, 6 are anticholinergic agents that block muscarinic receptors in the smooth muscle of the bladder, leading to inhibition of detrusor contractions, and 2 are β-adrenergic receptor agonists that promote bladder storage capacity by relaxing the detrusor muscle (TABLE 2). Similar efficacies lead most clinicians to initiate drug therapy based on formulary coverage and tolerance for adverse effects. Patients can expect a 53% to 80% reduction in UUI episodes and a 12% to 32% reduction in urinary frequency.22

Extended-release formulations are associated with reduced anticholinergic side effects (dry mouth, constipation, somnolence, dry eyes), leading to improved adherence. Notably, the anticholinergic medications are contraindicated in patients with untreated narrow-angle glaucoma, gastric retention, and supraventricular tachycardia. Mirabegron should be used with caution in patients with poorly controlled hypertension. 5 Due to concerns regarding the association between cumulative anticholinergic burden and the development of dementia, clinicians may consider avoiding the anticholinergic medications in older and at-risk patients.23
Continue to: UUI office-based procedure treatments...
UUI office-based procedure treatments
If behavioral therapies and medications are ineffective, contraindicated, or not the patient’s preference, additional FDA-approved therapies for UUI are available, typically through referral to a urogynecologist, urologist, or continence center.
Posterior tibial nerve stimulation (PTNS) is a nondrug treatment that delivers electrical stimulation using an acupuncture needle for 12 weekly 30-minute sessions followed by monthly maintenance for responders. The time commitment for this treatment plan can be a barrier for some patients. However, patients who adhere to the recommended protocol can expect a 60% improvement in symptoms, with minimal adverse events. Treatment efficacy is comparable to that of anticholinergic medication.24
OnabotulinumtoxinA injections into the bladder muscle are performed cystoscopically under local anesthetic. The toxin blocks the presynaptic release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. This treatment is associated with high satisfaction. Efficacy varies by study population and outcome measure.
In one US comparative effectiveness trial, 67% of study participants with UUI symptoms refractory to oral medication reported a greater than 50% reduction in OAB symptoms at 6 months, 20% reported complete resolution of UUI, and 72% requested a second injection within 24 months.25 The interval between the first and second injection was nearly 1 year (350 days).Risks include urinary tract infection (12% within 1 month of the procedure and 35% through 6 months); urinary retention requiring catheterization has decreased to 6% with recognition that most moderate retention is tolerated by patients.
Some insurers limit onabotulinumtoxinA treatment coverage to patients who have failed to achieve symptom control with first- and second-line treatments.
SUI-specific nonsurgical treatments
Cystoscopic injection of urethral bulking agents into the urethral submucosa is designed to improve urethral coaptation. It is a minor procedure that can be performed in an ambulatory setting under local anesthetic with or without sedation.
Various bulking agents have been approved for use in the United States, some of which have been withdrawn due to complications of migration, erosion, and pseudoabscess formation. Cure or improvement after bulking agent injection was found to be superior to a home pelvic floor exercise program but inferior to a midurethral sling procedure for cure (9% vs 89%).26
The durability of currently available urethral bulking agents beyond 1 year is unknown. Complications are typically minor and transient and include pain at the injection site, urinary retention, de novo urgency, and implant leakage. The advantages include no postprocedure activity restrictions.
CASE Symptom presentation guides treatment plan
Our patient described symptoms of stress-predominant MUI. She was counseled to moderate her fluid intake to 2 L per day and to strategically time voids (before exercise, and at least every 4 hours). The patient was fitted with an incontinence pessary, and she elected to pursue a course of supervised physical therapy for pelvic floor muscle strengthening. Her follow-up visit is scheduled in 3 months to determine if other interventions are warranted. ●
1. Lee UJ, Feinstein L, Ward JB, et al. Prevalence of urinary incontinence among a nationally representative sample of women, 2005–2016: findings from the Urologic Diseases in America Project. J Urol. 2021;205:1718-1724. doi:10.1097 /JU.0000000000001634
2. Sims J, Browning C, Lundgren-Lindquist B, et al. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389-1398. doi:10.3109/09638288.2010.532284
3. Sarikaya S, Yildiz FG, Senocak C, et al. Urinary incontinence as a cause of depression and sexual dysfunction: questionnaire-based study. Rev Int Androl. 2020:18:50-54. doi:10.1016 /j.androl.2018.08.003
4. O’Reilly N, Nelson HD, Conry JM, et al; Women’s Preventive Services Initiative. Screening for urinary incontinence in women: a recommendation from the Women’s Preventive Services Initiative. Ann Intern Med. 2018;169(5):320-328. doi:10.7326/M18-0595
5. Barber MD, Walters MD, Karram MM, et al. Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier Saunders; 2021.
6. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21: 5-26. doi:10.1007/s00192-009-0976-9
7. ACOG practice bulletin no. 155. Urinary incontinence in women. Obstet Gynecol. 2015;126:e66-e81. doi:10.1097 /AOG.0000000000001148
8. Sansone S, Lu J, Drangsholt S, et al. No pelvic exam, no problem: patient satisfaction following the integration of comprehensive urogynecology telemedicine. Int Urogynecol J. 2022;1:3. doi:10.1007/s00192-022-05104-w
9. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715723. doi:10.7326/0003-4819-144-10-200605160-00005
10. Hess R, Huang AJ, Richter HE, et al. Long-term efficacy and safety of questionnaire-based initiation of urgency urinary incontinence treatment. Am J Obstet Gynecol. 2013;209:244. e1-9. doi:10.1016/j.ajog.2013.05.008
11. Sung VW, Borello-France D, Newman DK, et al; NICHD Pelvic Floor Disorders Network. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence. JAMA. 2019;322:1066-1076. doi:10.1001 /jama.2019.12467
12. American Urogynecologic Society. Voices for PFD: intake and voiding diary. Accessed August 11, 2022. https://www .voicesforpfd.org/assets/2/6/Voiding_Diary.pdf
13. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 suppl):S2-7. doi:10.1016/j.juro.2009.08.071
14. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89. doi:10.1016/s0029-7844(00)00808-5
15. Wyman JF, Zhou J, LaCoursiere DY, et al. Normative noninvasive bladder function measurements in healthy women: a systematic review and meta-analysis. Neurourol Urodyn. 2020;39:507-522. doi:10.1002/nau.24265
16. Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10: 428-433. doi:10.1007/s11934-009-0068-x
17. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
18. Araujo CC, de A Marques A, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26:697-703. doi:10.1097/SPV.0000000000000670
19. Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: a randomized controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112:362-372. doi:10.1111/j.1464 -410X.2012.11713.x
20. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609617. doi:10.1097/AOG.0b013e3181d055d4
21. Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3): 161-169. doi:10.7326/0003-4819-149-3-200808050 -00005
22. Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary incontinence in women: a review. JAMA. 2017;318:1592-1604. doi:10.1001/jama.2017.12137
23. Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686-700. doi:10.1038/s41585-021-00504-x
24. Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206-1216. doi:10.1002/nau.22251
25. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: A randomized clinical trial. JAMA. 2016;316:1366-1374. doi:10.1001/jama.2016.14617
26. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi:10.1002/14651858.CD003881.pub4
1. Lee UJ, Feinstein L, Ward JB, et al. Prevalence of urinary incontinence among a nationally representative sample of women, 2005–2016: findings from the Urologic Diseases in America Project. J Urol. 2021;205:1718-1724. doi:10.1097 /JU.0000000000001634
2. Sims J, Browning C, Lundgren-Lindquist B, et al. Urinary incontinence in a community sample of older adults: prevalence and impact on quality of life. Disabil Rehabil. 2011;33:1389-1398. doi:10.3109/09638288.2010.532284
3. Sarikaya S, Yildiz FG, Senocak C, et al. Urinary incontinence as a cause of depression and sexual dysfunction: questionnaire-based study. Rev Int Androl. 2020:18:50-54. doi:10.1016 /j.androl.2018.08.003
4. O’Reilly N, Nelson HD, Conry JM, et al; Women’s Preventive Services Initiative. Screening for urinary incontinence in women: a recommendation from the Women’s Preventive Services Initiative. Ann Intern Med. 2018;169(5):320-328. doi:10.7326/M18-0595
5. Barber MD, Walters MD, Karram MM, et al. Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier Saunders; 2021.
6. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21: 5-26. doi:10.1007/s00192-009-0976-9
7. ACOG practice bulletin no. 155. Urinary incontinence in women. Obstet Gynecol. 2015;126:e66-e81. doi:10.1097 /AOG.0000000000001148
8. Sansone S, Lu J, Drangsholt S, et al. No pelvic exam, no problem: patient satisfaction following the integration of comprehensive urogynecology telemedicine. Int Urogynecol J. 2022;1:3. doi:10.1007/s00192-022-05104-w
9. Brown JS, Bradley CS, Subak LL, et al; Diagnostic Aspects of Incontinence Study (DAISy) Research Group. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715723. doi:10.7326/0003-4819-144-10-200605160-00005
10. Hess R, Huang AJ, Richter HE, et al. Long-term efficacy and safety of questionnaire-based initiation of urgency urinary incontinence treatment. Am J Obstet Gynecol. 2013;209:244. e1-9. doi:10.1016/j.ajog.2013.05.008
11. Sung VW, Borello-France D, Newman DK, et al; NICHD Pelvic Floor Disorders Network. Effect of behavioral and pelvic floor muscle therapy combined with surgery vs surgery alone on incontinence symptoms among women with mixed urinary incontinence. JAMA. 2019;322:1066-1076. doi:10.1001 /jama.2019.12467
12. American Urogynecologic Society. Voices for PFD: intake and voiding diary. Accessed August 11, 2022. https://www .voicesforpfd.org/assets/2/6/Voiding_Diary.pdf
13. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 suppl):S2-7. doi:10.1016/j.juro.2009.08.071
14. Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstet Gynecol. 2000;96:85-89. doi:10.1016/s0029-7844(00)00808-5
15. Wyman JF, Zhou J, LaCoursiere DY, et al. Normative noninvasive bladder function measurements in healthy women: a systematic review and meta-analysis. Neurourol Urodyn. 2020;39:507-522. doi:10.1002/nau.24265
16. Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10: 428-433. doi:10.1007/s11934-009-0068-x
17. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10:CD005654. doi:10.1002/14651858.CD005654.pub4
18. Araujo CC, de A Marques A, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26:697-703. doi:10.1097/SPV.0000000000000670
19. Sjöström M, Umefjord G, Stenlund H, et al. Internet-based treatment of stress urinary incontinence: a randomized controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112:362-372. doi:10.1111/j.1464 -410X.2012.11713.x
20. Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609617. doi:10.1097/AOG.0b013e3181d055d4
21. Burgio KL, Kraus SR, Menefee S, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3): 161-169. doi:10.7326/0003-4819-149-3-200808050 -00005
22. Lukacz ES, Santiago-Lastra Y, Albo ME, et al. Urinary incontinence in women: a review. JAMA. 2017;318:1592-1604. doi:10.1001/jama.2017.12137
23. Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686-700. doi:10.1038/s41585-021-00504-x
24. Burton C, Sajja A, Latthe PM. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31:1206-1216. doi:10.1002/nau.22251
25. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: A randomized clinical trial. JAMA. 2016;316:1366-1374. doi:10.1001/jama.2016.14617
26. Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881. doi:10.1002/14651858.CD003881.pub4
2022 Update on abnormal uterine bleeding
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
In this Update, we focus on therapies for abnormal uterine bleeding (AUB) that include a new formulation of a progesterone-only pill (POP), drospirenone 4 mg in a 24/4 regimen (24 days of drospirenone/4 days of inert tablets), which recently showed benefit over the use of desogestrel in a European randomized clinical trial (RCT). Two other commonly used treatments for AUB— the levonorgestrel-releasing intrauterine system (LNG IUS) and endometrial ablation—were studied in terms of cost-effectiveness as well as whether they should be used in combination for added efficacy. In addition, although at times either COVID-19 disease or the COVID-19 vaccine has been blamed for societal and medical problems, one study showed that it is unlikely that significant changes in the menstrual cycle are a result of the COVID-19 vaccine.
COVID-19 vaccination had minimal effects on menstrual cycle length
Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
Does receiving the COVID-19 vaccination result in abnormal menstrual cycles? Patients often ask this question, and it has been a topic of social media discussion (including NPR) and concerns about the possibility of vaccine hesitancy,1,2 as the menstrual cycle is often considered a sign of health and fertility.
To better understand this possible association, Edelman and colleagues conducted a study that prospectively tracked menstrual cycle data using the digital app Natural Cycles in US residents aged 18 to 45 years for 3 consecutive cycles in both a vaccinated and an unvaccinated cohort.3 Almost 4,000 individuals were studied; 2,403 were vaccinated and 1,556 were unvaccinated. The study vaccine types included the BioNTech (Pfizer), Moderna, Johnson & Johnson/Janssen, and unspecified vaccines.
The primary outcome was the within-individual change in cycle length in days, comparing a 3-cycle postvaccine average to a 3-cycle prevaccination average in the 2 groups. (For the unvaccinated group, cycles 1, 2, and 3 were considered the equivalent of prevaccination cycles; cycle 4 was designated as the artificial first vaccine dose-cycle and cycle 5 as the artificial second-dose cycle.)
Increase in cycle length clinically negligible
The investigators found that the vaccinated cohort had less than a 1-day unadjusted increase in the length of their menstrual cycle, which was essentially a 0.71-day increase (98.75% confidence interval [CI], 0.47–0.94). Although this is considered statistically significant, it is likely clinically insignificant in that the overlaid histograms comparing the distribution of change showed a cycle length distribution in vaccinated individuals that is essentially equivalent to that in unvaccinated individuals. After adjusting for confounders, the difference in cycle length was reduced to a 0.64 day (98.75% CI, 0.27–1.01).
An interesting finding was that a subset of individuals who received both vaccine doses in a single cycle had, on average, an adjusted 2-day increase in their menstrual cycle compared with unvaccinated individuals. To explain this slightly longer cycle length, the authors postulated that mRNA vaccines create an immune response, or stressor, which could temporarily affect the hypothalamic-pituitary-ovarian axis if timed correctly. It is certainly possible for an individual to receive 2 doses in a single cycle, which could have both been administered in the early follicular phase. Such cycle length variability can be caused by events, including stressors, that affect the recruitment and maturation of the dominant follicle.
Counseling takeaway
This study provides reassurance to most individuals who receive a COVID-19 vaccine that it likely will not affect their menstrual cycle in a clinically significant manner.
This robust study by Edelman and colleagues on COVID-19 vaccination effects on menstrual cycle length had more than 99% power to detect an unadjusted 1-day difference in cycle length. However, given that most of the study participants were White and had access to the Natural Cycles app, the results may not be generalizable to all individuals who receive the vaccine.
Continue to: Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel...
Drospirenone improved bleeding profiles, lowered discontinuation rates compared with desogestrel
Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
A new POP, marketed under the name Slynd, recently came to market. It contains the progestin drospirenone (DRSP) 4 mg in a 24/4 regimen. This formulation has the advantage of being an antiandrogenic progestin, with a long enough half-life to allow for managing a missed pill in the same fashion as combined oral contraceptives (COCs).
Investigators in Europe conducted a double-blind, randomized trial to assess discontinuation rates due to adverse events (mainly bleeding disorders) in participants taking DRSP 4 mg in a 24/4 regimen compared with those taking the POP desogestrel (DSG) 0.075 mg, which is commonly used in Europe.4 Regidor and colleagues compared 858 women with 6,691 DRSP treatment cycles with 332 women with 2,487 DSG treatment cycles.
Top reasons for stopping a POP
The discontinuation rate for abnormal bleeding was 3.7% in the DRSP group versus 7.3% in the DSG group (55.7% lower). The most common reasons for stopping either POP formulation were vaginal bleeding and acne. Both of these adverse events were less common in the DRSP group. Pill discontinuation due to vaginal bleeding was 2.6% in the DRSP group versus 5.4% in the DSG group, while discontinuation due to acne occurred in 1% in the DRSP group versus 2.7% in the DSG group.
New oral contraception option
This study shows improved acceptability and bleeding profiles in women using this new DRSP contraception pill regimen.
Adherence to a contraceptive method is influenced by patient satisfaction, and this is particularly important in patients who cannot take COCs. It also should be noted that the discontinuation rate for DRSP as a POP used in this 24/4 regimen was similar to discontinuation rates for COCs containing 20 µg and 30 µg of ethinyl estradiol. Cost, however, may be an issue with DRSP, depending on a patient’s insurance coverage.
Continue to: Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy...
Placing an LNG IUS after endometrial ablation for heavy menstrual bleeding reduced risk of hysterectomy
Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. The combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
Over the years, a smattering of articles have suggested that a reduction in uterine bleeding was associated with placement of an LNG IUS at the conclusion of endometrial ablation. We now have a systematic review of this surgical modification.
Oderkerk and colleagues sifted through 747 articles to find 7 publications that could provide meaningful data on the impact of combined use of endometrial ablation and LNG IUS insertion for women with heavy menstrual bleeding.5 These included 4 retrospective cohort studies with control groups, 2 retrospective studies without control groups, and 1 case series. The primary outcome was the hysterectomy rate after therapy.
Promising results for combined therapy
Although no statistically significant intergroup differences were seen in the combined treatment group versus the endometrial ablation alone group for the first 6 months of treatment, significant differences existed at the 12- and 24-month mark. Hysterectomy rates after combined treatment varied from 0% to 11% versus 9.4% to 24% after endometrial ablation alone. Complication rates for combined treatment did not appear higher than those for endometrial ablation alone.
The authors postulated that the failure of endometrial ablation is generally caused by either remaining or regenerating endometrial tissue and that the addition of an LNG IUS allows for suppression of endometrial tissue. Also encouraging was that, in general, the removal of the LNG IUS was relatively simple. A single difficult removal was described due to uterine synechiae, but hysteroscopic resection was not necessary. The authors acknowledged that the data from these 7 retrospective studies are limited and that high-quality research from prospective studies is needed.
Bottom line
The data available from this systematic review suggest that placement of an LNG IUS at the completion of an endometrial ablation may result in lower hysterectomy rates, without apparent risk, and without significantly difficult LNG IUS removal when needed.
The data provided by Oderkerk and colleagues’ systematic review are promising and, although not studied in the reviewed publications, the potential may exist to reduce the risk of endometrial hyperplasia and endometrial cancer by adding an LNG IUS.
Continue to: LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows...
LNG IUS is less expensive, and less effective, than endometrial ablation for heavy menstrual bleeding, cost analysis shows
van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
To assess the cost-effectiveness of the LNG IUS versus endometrial ablation in the treatment of heavy menstrual bleeding, van den Brink and colleagues conducted a randomized, noninferiority trial.6
Part of the rationale for this study was to better understand the cost differences between the LNG IUS and second-generation endometrial ablation. Some data have suggested that the LNG IUS is cost-effective when compared with first-generation endometrial ablation; however, definitive evidence about its cost compared with second-generation endometrial ablation is lacking, as these procedures should be less expensive than first-generation endometrial ablation since they frequently are performed in the office rather than in an operating room.
Cost-effectiveness and noninferiority assessed
A total of 270 women were randomly assigned to 1 of 2 treatment strategies. Eventually, 132 women were treated first with the 52-mg LNG IUS, and 138 were treated first with endometrial ablation by radiofrequency ablation. Menstrual blood loss after 24 months was the primary outcome.
At 24 months, the mean pictorial blood loss assessment chart (PBAC) scores were 64.8 in the LNG IUS group compared with 14.2 in the endometrial ablation group. Given that the noninferiority margin was defined as 25 points, noninferiority could not be demonstrated. However, when looking at PBAC scores less than 75 points, the LNG IUS group met this secondary end point in 87% of women versus 94% in the endometrial ablation group. When satisfaction was assessed, 74% of women in the LNG IUS group were satisfied compared with 84% in the endometrial ablation group.
Overall, the total costs per patient were €2,285 in the LNG IUS strategy and €3,465 in the endometrial ablation strategy (costs convert to $2,285 and $3,465 as of this writing).
Key takeaway
Treatment of heavy menstrual bleeding starting with the LNG IUS is cheaper, but it is slightly less effective than endometrial ablation. ●
It is interesting that there are minimal differences between satisfaction rates and PBAC scores less than 75, yet the mean PBAC scores were significantly more favorable for endometrial ablation. This study’s results support the use of a sequential therapy of a less invasive therapy, such as the LNG IUS, prior to performing endometrial ablation.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.
- Blumfiel G. Why reports of menstrual changes after COVID vaccine are tough to study. NPR. August 9, 2021. Accessed August 30, 2022. https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research
- Lee KMN, Junkins EJ, Fatima UA, et al. Characterizing menstrual bleeding changes occurring after SARSCoV-2 vaccinations. MedRxiv. February 11, 2022. doi:10.1101/2021.10.11.21264863
- Edelman A, Boniface ER, Benhar W, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481-489.
- Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. 2021;37:1121-1127.
- Oderkerk TJ, van de Kar MMA, van der Zanden CHM, et al. T he combined use of endometrial ablation or resection and levonorgestrel-releasing intrauterine system in women with heavy menstrual bleeding: a systematic review. Acta Obstet Gynecol Scand. 2021;100:1779-1787.
- van den Brink MJ, Beelen P, Herman MC, et al. The levonorgestrel intrauterine system versus endometrial ablation for heavy menstrual bleeding: a cost-effectiveness analysis. BJOG. 2021;128:2003-2011.