User login
2023 Update on bone health
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Janus Kinase Inhibitors: A Promising Therapeutic Option for Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29
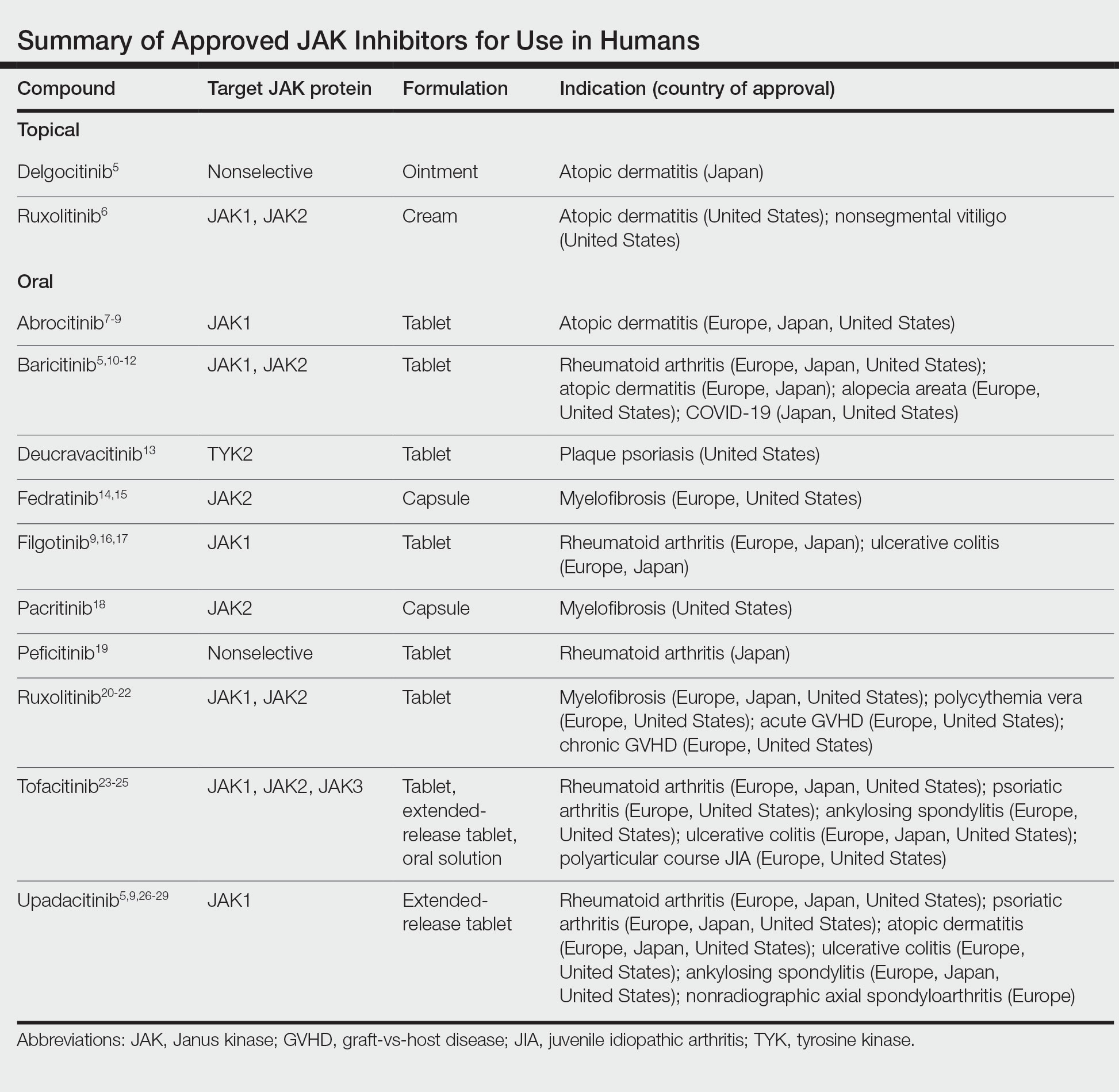
Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
PRACTICE POINTS
- Janus kinase (JAK) inhibitors are a novel class of small molecule inhibitors that modulate the JAK/signal transducer and activator of transcription signaling pathway.
- Select JAK inhibitors have been approved by the US Food and Drug Administration for the management of atopic dermatitis. Their use in allergic contact dermatitis is under active investigation.
- Regular follow-up and laboratory monitoring for patients on oral JAK inhibitors is recommended, given the potential for treatment-related adverse effects.
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
Diagnostic Errors in Hospitalized Patients
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21
The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.
A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).
The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73;application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73;application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73;application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
How the Dobbs decision shapes the ObGyn workforce and training landscape

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
2023 Update on obstetrics
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
Liability in robotic gyn surgery
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
Racial disparities in cesarean delivery rates
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.