User login
Taiji Self-Care: Exploring a Pilot Self-Care Program for Extended-Care Psychiatric Nursing Staff
Evaluation of Vitamin D Supplementation in a Veteran Population
Organizing Pneumonia and Pneumothorax Associated With Daptomycin Use
Adventures in Gold Mining: Extracting a Diagnosis
Cover
UPDATE ON CONTRACEPTION
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Malpositioned IUDs: When you should intervene
(and when you should not)
Kari P. Braaten, MD, MPH; Alisa B. Goldberg, MD, MPH (August 2012)
The past 20 years have seen an explosion of new contraceptive technologies; women benefit now from a range of effective methods that can satisfy their preferences. Pharmaceutical and biotech companies jumped on board, developing and marketing new hormonal combinations, delivery systems, and inexpensive devices that offer them opportunity for great profit.
Now that many of these newer products have been available for a decade or longer, the combined motivation of women, health-care providers, and industry should have meant better success in preventing undesired pregnancies. Regrettably, we’re moving in the wrong direction: The rate of unintended pregnancy in the United States has increased.
In this Update, we address the sobering reality of the unintended pregnancy rate over 20 years. We then take the opportunity to:
- review new data and guidelines about postpartum and postprocedure insertion of an intrauterine device (IUD)
- explain the latest data and recommendations on venous thrombotic events and combined hormonal methods
- discuss the possibility of an association between depot medroxyprogesterone acetate (DMPA) and acquisition of the human immunodeficiency virus (HIV).
What are the national data on unintended pregnancy?
Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
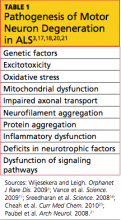
For decades, we’ve been repeating ourselves about the scope of the problem of unintended pregnancy—namely, “about half of all pregnancies in the United States are unintended,” etc. The fact that this rate has not improved in nearly 20 years is, in itself, worrisome; despite a proliferation of methods of contraception (and the hope that added options would cause the high rate of unintended pregnancy to fall), an overall benefit hasn’t been realized.
A small, but very important, decrease in the percentage of pregnancies that are unintended—from 49.2% to 48%—occurred between 1994 and 2001.1 New data assembled by Finer and Zolna show, however, that the percentage has crept back up to 49%.
The unintended pregnancy rate is another way to measure this outcome—reflecting the number of unintended pregnancies for every 1,000 women of reproductive age. The lowest rate (44.7) was seen in 1994; by 2006, the rate had increased to 52—just shy of the highest rate of 52.6 that was reported in the early 1980s.
Why haven’t new methods lowered the unintended pregnancy rate?
Both unintended pregnancy and abortion affect poorer and younger women disproportionately. In 1994, the unintended pregnancy rate among women who were below the poverty level was 2.6-fold higher than the rate among women who were 200% above the poverty level. That difference in rate increased to 5.5-fold higher by 2006 (FIGURE). The unintended pregnancy rate has increased significantly among poor women while it has continued to decrease among women who are not poor.
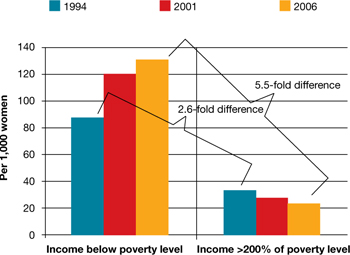
The “poverty gap” has been widening in the US rate of unintended pregnancy
Trends shown here are among women aged 15 to 44 years.
Based on data from: Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
Trends shown here are among women aged 15 to 44 years.
Based on data from: Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
Why has this happened? Perhaps newer contraceptive methods aren’t being used by, or are not available to, women who are most in need. This regrettable trend is a demonstration that unintended pregnancy is a social issue—that there are, without question, “haves” and “have-nots.”
Black women have an unintended pregnancy rate nearly double that of non-Hispanic white women, and are more likely than non-Hispanic white women to opt for an abortion when faced with an unintended pregnancy. New data also show that, from 2005 to 2008, the number of abortions and the abortion rate in the United States have remained approximately the same.2 While the rate of unintended pregnancy increases, therefore, principally among poor women, more of those pregnancies are being continued.
Contraception, recognized by the Centers for Disease Control and Prevention as one of the most important public health advances of the past century, is not having a maximal impact in the United States.3 The primary goal of contraception is to prevent unintended pregnancy; we have not continued to make strides in the last two decades against the unintended pregnancy rate so that women control when they have children and how many they have.
Advertising for contraceptives cannot take the place of education by physicians. Your care of reproductive-age women should include finding an opportunity, at every visit, to address, and educate them on, contraception.
Even more important, primary care physicians—whose ability to offer such highly effective options as IUDs, implants, and sterilization might be limited—need to be better educated to ensure that they 1) provide contraceptive counseling to women and 2) refer patients to a gynecologist or a trained primary care provider who can offer them access to the most appropriate of the full range of methods.
A final note: Continued advocacy of contraception as an important component of primary preventive medicine by the Institute of Medicine (IOM) should mean better support for seasoned providers and new trainees to give contraception and family planning the clinical attention it needs.
More evidence on postpregnancy IUD placement
Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Cremer M, Bullard KA, Mosley RM, et al. Immediate vs. delayed post-abortal copper T 380A IUD insertion in cases over 12 weeks of gestation. Contraception. 2011;83(6):522–527.
Hohmann HL, Reeves MF, Chen BA, Perriera LK, Hayes JL, Creinin MD. Immediate versus delayed insertion of the levonorgestrel-releasing intrauterine device following dilation and evacuation: a randomized controlled trial. Contraception. 2012;85(3):240–245.
Shimoni N, Davis A, Ramos ME, Rosario L, Westhoff C. Timing of copper intrauterine device insertion after medical abortion: a randomized controlled trial. Obstet Gynecol. 2011;118(3):623–628.
Betstadt SJ, Turok DK, Kapp N, Feng KT, Borgatta L. Intrauterine device insertion after medical abortion. Contraception. 2011;83(6):517–521.
Celen S, Sucak A, Yildiz Y, Danisman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception. 2011;84(3):240–243.
Intrauterine devices have received a great deal of attention in recent years. Indeed, the utilization rate has increased significantly, with 5.5% of contraceptive users—2.1 million women—now using an IUD.4 Although most women who use an IUD obtain it at an outpatient office, remote from pregnancy and where the safety profile and risk of expulsion are well documented, many women who desire effective contraception like an IUD may not be seen by a provider until they are pregnant.
A significant body of data has been published recently on the role of postpregnancy IUD placement, adding important information to the existing body of literature.
Multicenter randomized trial. A study in the United States by Bednarek and co-workers demonstrated that immediate post-aspiration placement of an IUD resulted in a higher rate (>90%) of IUD utilization at 6 months than did insertion 6 to 8 weeks postpartum (just above 75%). Furthermore, five pregnancies were documented in the group with delayed IUD insertion; none were seen in the immediate-insertion group.
Independent randomized trials. Two studies (by Cremer and colleagues and Hohmann and colleagues) showed that immediate post-dilation and evacuation placement of an IUD also yielded a significantly higher rate of continued usage at 6 months than did delayed placement. (The terms “postaspiration” and “post–dilation and evacuation” are important as they encompass elective termination procedures for miscarriage management and fetal demise among women who may have undesired fertility.) For women having such procedures who do not want another pregnancy in the near future, immediate provision of highly effective contraception can best be performed at the time of the procedure.
New data: Use of IUD after medical abortion. A randomized trial conducted by Shimoni and colleagues showed 1) no significant difference in expulsion after immediate versus delayed placement and 2) several pregnancies in the delayed group. Regrettably, the investigators did not clearly define “immediate placement.”
In another prospective cohort study, Betstadt and coworkers reported a low rate of expulsion (4.1%) when an IUD was placed within 14 days after confirmed medical abortion. The findings of that study were also limited because the researchers followed women for only 3 months after the IUD was placed.
These new studies shed important light on the safety and tolerability of immediate IUD insertion. More questions remain, however, about ideal timing of placement after medical abortion. Postpartum IUDs have also been promoted as an important method of effective contraception despite higher expulsion rates than interval insertion, which must be compared to the high rate of loss to follow-up.5
Prospective cohort study. A well-designed study recently addressed outcomes of post-placental IUD placement during cesarean delivery. Celen and colleagues followed 245 women for longer than 1 year after postplacental copper-T IUD placement and reported a 17% cumulative expulsion rate and an overall continuation rate of 62%. These rates are not significantly lower than the cumulative expulsion rate and overall continuation rate associated with postplacental insertion after vaginal delivery. The investigators also reported no increased risk of serious complications, infection, or perforation with postplacental IUD placement after cesarean delivery.
The necessity of coming to clinic in the months right after the end of a pregnancy to obtain highly effective contraception is, for women who are in this position, a well-established barrier to ensuring that they receive the protection they want. We now have important data showing that IUD placement after suction aspiration, dilation and evacuation, cesarean delivery, and vaginal delivery6 is effective and causes minimal side effects.
Better data are needed before we can make a universal recommendation about inserting an IUD shortly after medical abortion.
Overall, you should consider that the reversibility and known safety profile of an IUD continue to make this device an ideal contraceptive for many women.
VTE risk, postpartum hormonal contraception, and progestin type
Centers for Disease Control and Prevention. Update to CDC’s U.S. medical eligibility criteria for contraceptive use, 2010: Revised recommendations for the use of contraceptive methods during the postpartum period. MMWR Morb Mortal Wkly Rep. 2011;60(26):878–883.
Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423.
Combined hormonal contraception (CHC) increases a woman’s risk of venous thromboembolism (VTE), an effect that has been attributed to the thrombogenic effects of estrogen.7 The combined risk of VTE from CHC and the known independent risk of VTE postpartum has prompted the CDC to recommend against the use of any combined (i.e., estrogen-containing) method for 21 days postpartum. Although no direct evidence exists of a higher rate of VTE with CHC immediately postpartum, indirect evidence of increased risk should be considered very seriously.
Evidence from retrospective and database studies continues to suggest that one of the newer progestins, drospirenone, may play a larger role in VTE than previously understood, reigniting the debate over the risk of VTE and combined oral contraceptives (OCs).
Drospirenone was introduced in 2001 in combination with ethinyl estradiol in an OC that had the added benefits of alleviating acne and controlling premenstrual symptoms.8 A large (142,475 woman-years) prospective trial examining the role of drospirenone showed no significant difference between this hormone and other forms of progesterone in regard to adverse cardiovascular events.9 This study had minimal loss to follow-up (2.4%) and is the only cohort to confirm VTE outcomes based on medical records review (rather than insurance claims databases or national registries).10
A national cohort study in Denmark, published in 2009, found that the risk of VTE was directly related to duration of use and the dosage of estrogen.11 More significantly, those investigators found that specific progestin types, including drospirenone, desogestrel, and gestodene, were also associated with increased VTE risk.
Danish researchers conducted another retrospective study to assess the VTE risk associated with drospirenone in CHC—a review that included other progestins, the levonorgestrel-releasing IUD, and progestin-only pills. The results again suggested that contraceptives that contain drospirenone, desogestrel, or gestodene were associated with more than twice the risk of VTE, compared with OCs that contain levonorgestrel.
For gestodene and desogestrel, increasing the dosage of estrogen increased the risk of VTE; for drospirenone, however, the dosage of estrogen did not affect the rate of VTE. No association was found between the levonorgestrel-releasing IUD or progestin-only pills with VTE. Overall, the absolute number of VTE was small (4,307 VTE among 1.3 million women using hormonal contraception), which is reassuring, considering that this was a large cohort study.
No combination hormonal contraception (CHC) of any type should be prescribed for use during the 3 weeks after delivery, given indirect evidence of increased risk of VTE during this period and the known VTE risk posed by CHC.
For women who are beyond that window and who want CHC, the question becomes: How should you counsel them about progestins in different formulations?
A decade of research has yielded equivocal data on drospirenone and the risk of VTE. The only large prospective study did not show any increase in the risk of VTE; newer studies contain important retrospective data but, by their design, are inherently weaker in regard to their conclusions.
Lastly, database reviews that cannot fully control for confounding and do not include chart review for confirmation of diagnosis do not provide a rationale for avoiding certain CHC formulations, especially if one of those formulations is strongly preferred by your patient.10
Does DMPA lead to HIV?
Heffron R, Donnell D, Rees H, et al; Partners in Prevention HSV/HIV Transmission Study Team. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26.
Much controversy has arisen in recent years over the role of hormonal contraception and HIV acquisition. This led the World Health Organization (WHO) to convene an international meeting of stakeholders earlier this year to address guidelines for hormonal contraception, especially injectables, in women who are living with HIV or are at high risk of acquiring the virus12 (see “What this evidence means for practice” on page 35 for more about this meeting).
Fifteen years ago, a well-designed cohort study showed that female sex workers in Kenya who used depot medroxyprogesterone acetate (sold in the United States as Depo-Provera) for contraception were twice as likely to acquire HIV than sex workers who used a nonhormonal method.13 Since then, numerous published studies on this topic have yielded equivocal results14: for example, the largest one, of 1,536 DMPA users in Uganda and Zimbabwe, showed no increased risk of HIV acquisition with DMPA use.15
In a report of the most recent study, Heffron and coworkers analyzed data from 3,790 serodiscordant couples and found that women who used DMPA were, on average, twice as likely to acquire HIV and to transmit HIV as women who did not use DMPA. The number of seroconversions in the study was, however, low—13 women and 19 men—and investigators did not give information about the duration of DMPA use.
Furthermore, this study was a secondary analysis of a cohort study designed to assess the role of herpes simplex virus in HIV acquisition; it was not designed with the question of a DMPA-HIV link in mind. That leaves questions about contraceptive use, duration of such use, and associated sexual behavior unanswered.
In short, this study adds to an important, growing body of literature, but does not provide evidence for changing gynecologic practice regarding DMPA use and eligibility.
No study has clearly demonstrated sufficiently strong evidence of a putative link between DMPA use and an increased rate of HIV transmission in women at high risk of HIV disease for you to discourage its use in any of your patients for whom DMPA is appropriate.
Stakeholders at the WHO’s 2012 meeting on this matter concluded that 1) no change to guidelines is warranted and 2) hormonal contraception should be promoted for all women, regardless of HIV risk. That conclusion takes into account the fact that the results of more than a decade of research on the role of hormonal contraception in HIV acquisition have been equivocal.12
Given the well-known benefits of effective contraception in preventing unintended pregnancy for all women, especially those at risk of transmitting HIV, you should continue to promote DMPA and all other formulations and methods of hormonal contraception to eligible women.
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
1. Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the united states 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90-6.
2. Jones RK, Kooistra K. Abortion incidence and access to services in the United States 2008. Perspect Sex Reprod Health. 2011;43(1):41-50.
3. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States 1900-1999. MMWR. 1999;48(12):241-243.
4. Hubacher D, Finer LB, Espey E. Renewed interest in intrauterine contraception in the United States: evidence and explanation. Contraception. 2011;83(4):291-294.
5. Grimes DA, Lopez LM, Schulz KF, Van Vliet HA, Stanwood NL. Immediate post-partum insertion of intrauterine devices. Cochrane Database Syst Rev. 2010;(5):CD003036.-
6. Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116(5):1079-1087.
7. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995;346(8990):1575-1582.
8. Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH. The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential. Contraception. 1996;54(4):243-251.
9. Dinger JC, Heinemann LA, Kuhl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142475 women-years of observation. Contraception. 2007;75(5):344-354.
10. Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119(5):1039-1044.
11. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.-
12. World Health Organization. Hormonal contraception and HIV: a technical statement. 2012. http://www.who.int/reproductivehealth/topics/family_planning/Hormonal_contraception_and_HIV.pdf. Accessed June 1 2012.
13. 1Martin HL Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053-1059.
14. Heikinheimo O, Lahteenmaki P. Contraception and HIV infection in women. Hum Reprod Update. 2009;15(2):165-176.
15. Morrison CS, Richardson BA, Mmiro F, et al. Hormonal Contraception and the Risk of HIV Acquisition (HC-HIV) Study Group. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85-95.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Malpositioned IUDs: When you should intervene
(and when you should not)
Kari P. Braaten, MD, MPH; Alisa B. Goldberg, MD, MPH (August 2012)
The past 20 years have seen an explosion of new contraceptive technologies; women benefit now from a range of effective methods that can satisfy their preferences. Pharmaceutical and biotech companies jumped on board, developing and marketing new hormonal combinations, delivery systems, and inexpensive devices that offer them opportunity for great profit.
Now that many of these newer products have been available for a decade or longer, the combined motivation of women, health-care providers, and industry should have meant better success in preventing undesired pregnancies. Regrettably, we’re moving in the wrong direction: The rate of unintended pregnancy in the United States has increased.
In this Update, we address the sobering reality of the unintended pregnancy rate over 20 years. We then take the opportunity to:
- review new data and guidelines about postpartum and postprocedure insertion of an intrauterine device (IUD)
- explain the latest data and recommendations on venous thrombotic events and combined hormonal methods
- discuss the possibility of an association between depot medroxyprogesterone acetate (DMPA) and acquisition of the human immunodeficiency virus (HIV).
What are the national data on unintended pregnancy?
Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
For decades, we’ve been repeating ourselves about the scope of the problem of unintended pregnancy—namely, “about half of all pregnancies in the United States are unintended,” etc. The fact that this rate has not improved in nearly 20 years is, in itself, worrisome; despite a proliferation of methods of contraception (and the hope that added options would cause the high rate of unintended pregnancy to fall), an overall benefit hasn’t been realized.
A small, but very important, decrease in the percentage of pregnancies that are unintended—from 49.2% to 48%—occurred between 1994 and 2001.1 New data assembled by Finer and Zolna show, however, that the percentage has crept back up to 49%.
The unintended pregnancy rate is another way to measure this outcome—reflecting the number of unintended pregnancies for every 1,000 women of reproductive age. The lowest rate (44.7) was seen in 1994; by 2006, the rate had increased to 52—just shy of the highest rate of 52.6 that was reported in the early 1980s.
Why haven’t new methods lowered the unintended pregnancy rate?
Both unintended pregnancy and abortion affect poorer and younger women disproportionately. In 1994, the unintended pregnancy rate among women who were below the poverty level was 2.6-fold higher than the rate among women who were 200% above the poverty level. That difference in rate increased to 5.5-fold higher by 2006 (FIGURE). The unintended pregnancy rate has increased significantly among poor women while it has continued to decrease among women who are not poor.

The “poverty gap” has been widening in the US rate of unintended pregnancy
Trends shown here are among women aged 15 to 44 years.
Based on data from: Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
Trends shown here are among women aged 15 to 44 years.
Based on data from: Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
Why has this happened? Perhaps newer contraceptive methods aren’t being used by, or are not available to, women who are most in need. This regrettable trend is a demonstration that unintended pregnancy is a social issue—that there are, without question, “haves” and “have-nots.”
Black women have an unintended pregnancy rate nearly double that of non-Hispanic white women, and are more likely than non-Hispanic white women to opt for an abortion when faced with an unintended pregnancy. New data also show that, from 2005 to 2008, the number of abortions and the abortion rate in the United States have remained approximately the same.2 While the rate of unintended pregnancy increases, therefore, principally among poor women, more of those pregnancies are being continued.
Contraception, recognized by the Centers for Disease Control and Prevention as one of the most important public health advances of the past century, is not having a maximal impact in the United States.3 The primary goal of contraception is to prevent unintended pregnancy; we have not continued to make strides in the last two decades against the unintended pregnancy rate so that women control when they have children and how many they have.
Advertising for contraceptives cannot take the place of education by physicians. Your care of reproductive-age women should include finding an opportunity, at every visit, to address, and educate them on, contraception.
Even more important, primary care physicians—whose ability to offer such highly effective options as IUDs, implants, and sterilization might be limited—need to be better educated to ensure that they 1) provide contraceptive counseling to women and 2) refer patients to a gynecologist or a trained primary care provider who can offer them access to the most appropriate of the full range of methods.
A final note: Continued advocacy of contraception as an important component of primary preventive medicine by the Institute of Medicine (IOM) should mean better support for seasoned providers and new trainees to give contraception and family planning the clinical attention it needs.
More evidence on postpregnancy IUD placement
Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Cremer M, Bullard KA, Mosley RM, et al. Immediate vs. delayed post-abortal copper T 380A IUD insertion in cases over 12 weeks of gestation. Contraception. 2011;83(6):522–527.
Hohmann HL, Reeves MF, Chen BA, Perriera LK, Hayes JL, Creinin MD. Immediate versus delayed insertion of the levonorgestrel-releasing intrauterine device following dilation and evacuation: a randomized controlled trial. Contraception. 2012;85(3):240–245.
Shimoni N, Davis A, Ramos ME, Rosario L, Westhoff C. Timing of copper intrauterine device insertion after medical abortion: a randomized controlled trial. Obstet Gynecol. 2011;118(3):623–628.
Betstadt SJ, Turok DK, Kapp N, Feng KT, Borgatta L. Intrauterine device insertion after medical abortion. Contraception. 2011;83(6):517–521.
Celen S, Sucak A, Yildiz Y, Danisman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception. 2011;84(3):240–243.
Intrauterine devices have received a great deal of attention in recent years. Indeed, the utilization rate has increased significantly, with 5.5% of contraceptive users—2.1 million women—now using an IUD.4 Although most women who use an IUD obtain it at an outpatient office, remote from pregnancy and where the safety profile and risk of expulsion are well documented, many women who desire effective contraception like an IUD may not be seen by a provider until they are pregnant.
A significant body of data has been published recently on the role of postpregnancy IUD placement, adding important information to the existing body of literature.
Multicenter randomized trial. A study in the United States by Bednarek and co-workers demonstrated that immediate post-aspiration placement of an IUD resulted in a higher rate (>90%) of IUD utilization at 6 months than did insertion 6 to 8 weeks postpartum (just above 75%). Furthermore, five pregnancies were documented in the group with delayed IUD insertion; none were seen in the immediate-insertion group.
Independent randomized trials. Two studies (by Cremer and colleagues and Hohmann and colleagues) showed that immediate post-dilation and evacuation placement of an IUD also yielded a significantly higher rate of continued usage at 6 months than did delayed placement. (The terms “postaspiration” and “post–dilation and evacuation” are important as they encompass elective termination procedures for miscarriage management and fetal demise among women who may have undesired fertility.) For women having such procedures who do not want another pregnancy in the near future, immediate provision of highly effective contraception can best be performed at the time of the procedure.
New data: Use of IUD after medical abortion. A randomized trial conducted by Shimoni and colleagues showed 1) no significant difference in expulsion after immediate versus delayed placement and 2) several pregnancies in the delayed group. Regrettably, the investigators did not clearly define “immediate placement.”
In another prospective cohort study, Betstadt and coworkers reported a low rate of expulsion (4.1%) when an IUD was placed within 14 days after confirmed medical abortion. The findings of that study were also limited because the researchers followed women for only 3 months after the IUD was placed.
These new studies shed important light on the safety and tolerability of immediate IUD insertion. More questions remain, however, about ideal timing of placement after medical abortion. Postpartum IUDs have also been promoted as an important method of effective contraception despite higher expulsion rates than interval insertion, which must be compared to the high rate of loss to follow-up.5
Prospective cohort study. A well-designed study recently addressed outcomes of post-placental IUD placement during cesarean delivery. Celen and colleagues followed 245 women for longer than 1 year after postplacental copper-T IUD placement and reported a 17% cumulative expulsion rate and an overall continuation rate of 62%. These rates are not significantly lower than the cumulative expulsion rate and overall continuation rate associated with postplacental insertion after vaginal delivery. The investigators also reported no increased risk of serious complications, infection, or perforation with postplacental IUD placement after cesarean delivery.
The necessity of coming to clinic in the months right after the end of a pregnancy to obtain highly effective contraception is, for women who are in this position, a well-established barrier to ensuring that they receive the protection they want. We now have important data showing that IUD placement after suction aspiration, dilation and evacuation, cesarean delivery, and vaginal delivery6 is effective and causes minimal side effects.
Better data are needed before we can make a universal recommendation about inserting an IUD shortly after medical abortion.
Overall, you should consider that the reversibility and known safety profile of an IUD continue to make this device an ideal contraceptive for many women.
VTE risk, postpartum hormonal contraception, and progestin type
Centers for Disease Control and Prevention. Update to CDC’s U.S. medical eligibility criteria for contraceptive use, 2010: Revised recommendations for the use of contraceptive methods during the postpartum period. MMWR Morb Mortal Wkly Rep. 2011;60(26):878–883.
Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423.
Combined hormonal contraception (CHC) increases a woman’s risk of venous thromboembolism (VTE), an effect that has been attributed to the thrombogenic effects of estrogen.7 The combined risk of VTE from CHC and the known independent risk of VTE postpartum has prompted the CDC to recommend against the use of any combined (i.e., estrogen-containing) method for 21 days postpartum. Although no direct evidence exists of a higher rate of VTE with CHC immediately postpartum, indirect evidence of increased risk should be considered very seriously.
Evidence from retrospective and database studies continues to suggest that one of the newer progestins, drospirenone, may play a larger role in VTE than previously understood, reigniting the debate over the risk of VTE and combined oral contraceptives (OCs).
Drospirenone was introduced in 2001 in combination with ethinyl estradiol in an OC that had the added benefits of alleviating acne and controlling premenstrual symptoms.8 A large (142,475 woman-years) prospective trial examining the role of drospirenone showed no significant difference between this hormone and other forms of progesterone in regard to adverse cardiovascular events.9 This study had minimal loss to follow-up (2.4%) and is the only cohort to confirm VTE outcomes based on medical records review (rather than insurance claims databases or national registries).10
A national cohort study in Denmark, published in 2009, found that the risk of VTE was directly related to duration of use and the dosage of estrogen.11 More significantly, those investigators found that specific progestin types, including drospirenone, desogestrel, and gestodene, were also associated with increased VTE risk.
Danish researchers conducted another retrospective study to assess the VTE risk associated with drospirenone in CHC—a review that included other progestins, the levonorgestrel-releasing IUD, and progestin-only pills. The results again suggested that contraceptives that contain drospirenone, desogestrel, or gestodene were associated with more than twice the risk of VTE, compared with OCs that contain levonorgestrel.
For gestodene and desogestrel, increasing the dosage of estrogen increased the risk of VTE; for drospirenone, however, the dosage of estrogen did not affect the rate of VTE. No association was found between the levonorgestrel-releasing IUD or progestin-only pills with VTE. Overall, the absolute number of VTE was small (4,307 VTE among 1.3 million women using hormonal contraception), which is reassuring, considering that this was a large cohort study.
No combination hormonal contraception (CHC) of any type should be prescribed for use during the 3 weeks after delivery, given indirect evidence of increased risk of VTE during this period and the known VTE risk posed by CHC.
For women who are beyond that window and who want CHC, the question becomes: How should you counsel them about progestins in different formulations?
A decade of research has yielded equivocal data on drospirenone and the risk of VTE. The only large prospective study did not show any increase in the risk of VTE; newer studies contain important retrospective data but, by their design, are inherently weaker in regard to their conclusions.
Lastly, database reviews that cannot fully control for confounding and do not include chart review for confirmation of diagnosis do not provide a rationale for avoiding certain CHC formulations, especially if one of those formulations is strongly preferred by your patient.10
Does DMPA lead to HIV?
Heffron R, Donnell D, Rees H, et al; Partners in Prevention HSV/HIV Transmission Study Team. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26.
Much controversy has arisen in recent years over the role of hormonal contraception and HIV acquisition. This led the World Health Organization (WHO) to convene an international meeting of stakeholders earlier this year to address guidelines for hormonal contraception, especially injectables, in women who are living with HIV or are at high risk of acquiring the virus12 (see “What this evidence means for practice” on page 35 for more about this meeting).
Fifteen years ago, a well-designed cohort study showed that female sex workers in Kenya who used depot medroxyprogesterone acetate (sold in the United States as Depo-Provera) for contraception were twice as likely to acquire HIV than sex workers who used a nonhormonal method.13 Since then, numerous published studies on this topic have yielded equivocal results14: for example, the largest one, of 1,536 DMPA users in Uganda and Zimbabwe, showed no increased risk of HIV acquisition with DMPA use.15
In a report of the most recent study, Heffron and coworkers analyzed data from 3,790 serodiscordant couples and found that women who used DMPA were, on average, twice as likely to acquire HIV and to transmit HIV as women who did not use DMPA. The number of seroconversions in the study was, however, low—13 women and 19 men—and investigators did not give information about the duration of DMPA use.
Furthermore, this study was a secondary analysis of a cohort study designed to assess the role of herpes simplex virus in HIV acquisition; it was not designed with the question of a DMPA-HIV link in mind. That leaves questions about contraceptive use, duration of such use, and associated sexual behavior unanswered.
In short, this study adds to an important, growing body of literature, but does not provide evidence for changing gynecologic practice regarding DMPA use and eligibility.
No study has clearly demonstrated sufficiently strong evidence of a putative link between DMPA use and an increased rate of HIV transmission in women at high risk of HIV disease for you to discourage its use in any of your patients for whom DMPA is appropriate.
Stakeholders at the WHO’s 2012 meeting on this matter concluded that 1) no change to guidelines is warranted and 2) hormonal contraception should be promoted for all women, regardless of HIV risk. That conclusion takes into account the fact that the results of more than a decade of research on the role of hormonal contraception in HIV acquisition have been equivocal.12
Given the well-known benefits of effective contraception in preventing unintended pregnancy for all women, especially those at risk of transmitting HIV, you should continue to promote DMPA and all other formulations and methods of hormonal contraception to eligible women.
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Malpositioned IUDs: When you should intervene
(and when you should not)
Kari P. Braaten, MD, MPH; Alisa B. Goldberg, MD, MPH (August 2012)
The past 20 years have seen an explosion of new contraceptive technologies; women benefit now from a range of effective methods that can satisfy their preferences. Pharmaceutical and biotech companies jumped on board, developing and marketing new hormonal combinations, delivery systems, and inexpensive devices that offer them opportunity for great profit.
Now that many of these newer products have been available for a decade or longer, the combined motivation of women, health-care providers, and industry should have meant better success in preventing undesired pregnancies. Regrettably, we’re moving in the wrong direction: The rate of unintended pregnancy in the United States has increased.
In this Update, we address the sobering reality of the unintended pregnancy rate over 20 years. We then take the opportunity to:
- review new data and guidelines about postpartum and postprocedure insertion of an intrauterine device (IUD)
- explain the latest data and recommendations on venous thrombotic events and combined hormonal methods
- discuss the possibility of an association between depot medroxyprogesterone acetate (DMPA) and acquisition of the human immunodeficiency virus (HIV).
What are the national data on unintended pregnancy?
Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
For decades, we’ve been repeating ourselves about the scope of the problem of unintended pregnancy—namely, “about half of all pregnancies in the United States are unintended,” etc. The fact that this rate has not improved in nearly 20 years is, in itself, worrisome; despite a proliferation of methods of contraception (and the hope that added options would cause the high rate of unintended pregnancy to fall), an overall benefit hasn’t been realized.
A small, but very important, decrease in the percentage of pregnancies that are unintended—from 49.2% to 48%—occurred between 1994 and 2001.1 New data assembled by Finer and Zolna show, however, that the percentage has crept back up to 49%.
The unintended pregnancy rate is another way to measure this outcome—reflecting the number of unintended pregnancies for every 1,000 women of reproductive age. The lowest rate (44.7) was seen in 1994; by 2006, the rate had increased to 52—just shy of the highest rate of 52.6 that was reported in the early 1980s.
Why haven’t new methods lowered the unintended pregnancy rate?
Both unintended pregnancy and abortion affect poorer and younger women disproportionately. In 1994, the unintended pregnancy rate among women who were below the poverty level was 2.6-fold higher than the rate among women who were 200% above the poverty level. That difference in rate increased to 5.5-fold higher by 2006 (FIGURE). The unintended pregnancy rate has increased significantly among poor women while it has continued to decrease among women who are not poor.

The “poverty gap” has been widening in the US rate of unintended pregnancy
Trends shown here are among women aged 15 to 44 years.
Based on data from: Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
Trends shown here are among women aged 15 to 44 years.
Based on data from: Finer LB, Zolna MR. Unintended pregnancy in the United States: Incidence and disparities, 2006. Contraception. 2011;84(5):478–485.
Why has this happened? Perhaps newer contraceptive methods aren’t being used by, or are not available to, women who are most in need. This regrettable trend is a demonstration that unintended pregnancy is a social issue—that there are, without question, “haves” and “have-nots.”
Black women have an unintended pregnancy rate nearly double that of non-Hispanic white women, and are more likely than non-Hispanic white women to opt for an abortion when faced with an unintended pregnancy. New data also show that, from 2005 to 2008, the number of abortions and the abortion rate in the United States have remained approximately the same.2 While the rate of unintended pregnancy increases, therefore, principally among poor women, more of those pregnancies are being continued.
Contraception, recognized by the Centers for Disease Control and Prevention as one of the most important public health advances of the past century, is not having a maximal impact in the United States.3 The primary goal of contraception is to prevent unintended pregnancy; we have not continued to make strides in the last two decades against the unintended pregnancy rate so that women control when they have children and how many they have.
Advertising for contraceptives cannot take the place of education by physicians. Your care of reproductive-age women should include finding an opportunity, at every visit, to address, and educate them on, contraception.
Even more important, primary care physicians—whose ability to offer such highly effective options as IUDs, implants, and sterilization might be limited—need to be better educated to ensure that they 1) provide contraceptive counseling to women and 2) refer patients to a gynecologist or a trained primary care provider who can offer them access to the most appropriate of the full range of methods.
A final note: Continued advocacy of contraception as an important component of primary preventive medicine by the Institute of Medicine (IOM) should mean better support for seasoned providers and new trainees to give contraception and family planning the clinical attention it needs.
More evidence on postpregnancy IUD placement
Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT; Post-Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364(23):2208–2217.
Cremer M, Bullard KA, Mosley RM, et al. Immediate vs. delayed post-abortal copper T 380A IUD insertion in cases over 12 weeks of gestation. Contraception. 2011;83(6):522–527.
Hohmann HL, Reeves MF, Chen BA, Perriera LK, Hayes JL, Creinin MD. Immediate versus delayed insertion of the levonorgestrel-releasing intrauterine device following dilation and evacuation: a randomized controlled trial. Contraception. 2012;85(3):240–245.
Shimoni N, Davis A, Ramos ME, Rosario L, Westhoff C. Timing of copper intrauterine device insertion after medical abortion: a randomized controlled trial. Obstet Gynecol. 2011;118(3):623–628.
Betstadt SJ, Turok DK, Kapp N, Feng KT, Borgatta L. Intrauterine device insertion after medical abortion. Contraception. 2011;83(6):517–521.
Celen S, Sucak A, Yildiz Y, Danisman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception. 2011;84(3):240–243.
Intrauterine devices have received a great deal of attention in recent years. Indeed, the utilization rate has increased significantly, with 5.5% of contraceptive users—2.1 million women—now using an IUD.4 Although most women who use an IUD obtain it at an outpatient office, remote from pregnancy and where the safety profile and risk of expulsion are well documented, many women who desire effective contraception like an IUD may not be seen by a provider until they are pregnant.
A significant body of data has been published recently on the role of postpregnancy IUD placement, adding important information to the existing body of literature.
Multicenter randomized trial. A study in the United States by Bednarek and co-workers demonstrated that immediate post-aspiration placement of an IUD resulted in a higher rate (>90%) of IUD utilization at 6 months than did insertion 6 to 8 weeks postpartum (just above 75%). Furthermore, five pregnancies were documented in the group with delayed IUD insertion; none were seen in the immediate-insertion group.
Independent randomized trials. Two studies (by Cremer and colleagues and Hohmann and colleagues) showed that immediate post-dilation and evacuation placement of an IUD also yielded a significantly higher rate of continued usage at 6 months than did delayed placement. (The terms “postaspiration” and “post–dilation and evacuation” are important as they encompass elective termination procedures for miscarriage management and fetal demise among women who may have undesired fertility.) For women having such procedures who do not want another pregnancy in the near future, immediate provision of highly effective contraception can best be performed at the time of the procedure.
New data: Use of IUD after medical abortion. A randomized trial conducted by Shimoni and colleagues showed 1) no significant difference in expulsion after immediate versus delayed placement and 2) several pregnancies in the delayed group. Regrettably, the investigators did not clearly define “immediate placement.”
In another prospective cohort study, Betstadt and coworkers reported a low rate of expulsion (4.1%) when an IUD was placed within 14 days after confirmed medical abortion. The findings of that study were also limited because the researchers followed women for only 3 months after the IUD was placed.
These new studies shed important light on the safety and tolerability of immediate IUD insertion. More questions remain, however, about ideal timing of placement after medical abortion. Postpartum IUDs have also been promoted as an important method of effective contraception despite higher expulsion rates than interval insertion, which must be compared to the high rate of loss to follow-up.5
Prospective cohort study. A well-designed study recently addressed outcomes of post-placental IUD placement during cesarean delivery. Celen and colleagues followed 245 women for longer than 1 year after postplacental copper-T IUD placement and reported a 17% cumulative expulsion rate and an overall continuation rate of 62%. These rates are not significantly lower than the cumulative expulsion rate and overall continuation rate associated with postplacental insertion after vaginal delivery. The investigators also reported no increased risk of serious complications, infection, or perforation with postplacental IUD placement after cesarean delivery.
The necessity of coming to clinic in the months right after the end of a pregnancy to obtain highly effective contraception is, for women who are in this position, a well-established barrier to ensuring that they receive the protection they want. We now have important data showing that IUD placement after suction aspiration, dilation and evacuation, cesarean delivery, and vaginal delivery6 is effective and causes minimal side effects.
Better data are needed before we can make a universal recommendation about inserting an IUD shortly after medical abortion.
Overall, you should consider that the reversibility and known safety profile of an IUD continue to make this device an ideal contraceptive for many women.
VTE risk, postpartum hormonal contraception, and progestin type
Centers for Disease Control and Prevention. Update to CDC’s U.S. medical eligibility criteria for contraceptive use, 2010: Revised recommendations for the use of contraceptive methods during the postpartum period. MMWR Morb Mortal Wkly Rep. 2011;60(26):878–883.
Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;343:d6423.
Combined hormonal contraception (CHC) increases a woman’s risk of venous thromboembolism (VTE), an effect that has been attributed to the thrombogenic effects of estrogen.7 The combined risk of VTE from CHC and the known independent risk of VTE postpartum has prompted the CDC to recommend against the use of any combined (i.e., estrogen-containing) method for 21 days postpartum. Although no direct evidence exists of a higher rate of VTE with CHC immediately postpartum, indirect evidence of increased risk should be considered very seriously.
Evidence from retrospective and database studies continues to suggest that one of the newer progestins, drospirenone, may play a larger role in VTE than previously understood, reigniting the debate over the risk of VTE and combined oral contraceptives (OCs).
Drospirenone was introduced in 2001 in combination with ethinyl estradiol in an OC that had the added benefits of alleviating acne and controlling premenstrual symptoms.8 A large (142,475 woman-years) prospective trial examining the role of drospirenone showed no significant difference between this hormone and other forms of progesterone in regard to adverse cardiovascular events.9 This study had minimal loss to follow-up (2.4%) and is the only cohort to confirm VTE outcomes based on medical records review (rather than insurance claims databases or national registries).10
A national cohort study in Denmark, published in 2009, found that the risk of VTE was directly related to duration of use and the dosage of estrogen.11 More significantly, those investigators found that specific progestin types, including drospirenone, desogestrel, and gestodene, were also associated with increased VTE risk.
Danish researchers conducted another retrospective study to assess the VTE risk associated with drospirenone in CHC—a review that included other progestins, the levonorgestrel-releasing IUD, and progestin-only pills. The results again suggested that contraceptives that contain drospirenone, desogestrel, or gestodene were associated with more than twice the risk of VTE, compared with OCs that contain levonorgestrel.
For gestodene and desogestrel, increasing the dosage of estrogen increased the risk of VTE; for drospirenone, however, the dosage of estrogen did not affect the rate of VTE. No association was found between the levonorgestrel-releasing IUD or progestin-only pills with VTE. Overall, the absolute number of VTE was small (4,307 VTE among 1.3 million women using hormonal contraception), which is reassuring, considering that this was a large cohort study.
No combination hormonal contraception (CHC) of any type should be prescribed for use during the 3 weeks after delivery, given indirect evidence of increased risk of VTE during this period and the known VTE risk posed by CHC.
For women who are beyond that window and who want CHC, the question becomes: How should you counsel them about progestins in different formulations?
A decade of research has yielded equivocal data on drospirenone and the risk of VTE. The only large prospective study did not show any increase in the risk of VTE; newer studies contain important retrospective data but, by their design, are inherently weaker in regard to their conclusions.
Lastly, database reviews that cannot fully control for confounding and do not include chart review for confirmation of diagnosis do not provide a rationale for avoiding certain CHC formulations, especially if one of those formulations is strongly preferred by your patient.10
Does DMPA lead to HIV?
Heffron R, Donnell D, Rees H, et al; Partners in Prevention HSV/HIV Transmission Study Team. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26.
Much controversy has arisen in recent years over the role of hormonal contraception and HIV acquisition. This led the World Health Organization (WHO) to convene an international meeting of stakeholders earlier this year to address guidelines for hormonal contraception, especially injectables, in women who are living with HIV or are at high risk of acquiring the virus12 (see “What this evidence means for practice” on page 35 for more about this meeting).
Fifteen years ago, a well-designed cohort study showed that female sex workers in Kenya who used depot medroxyprogesterone acetate (sold in the United States as Depo-Provera) for contraception were twice as likely to acquire HIV than sex workers who used a nonhormonal method.13 Since then, numerous published studies on this topic have yielded equivocal results14: for example, the largest one, of 1,536 DMPA users in Uganda and Zimbabwe, showed no increased risk of HIV acquisition with DMPA use.15
In a report of the most recent study, Heffron and coworkers analyzed data from 3,790 serodiscordant couples and found that women who used DMPA were, on average, twice as likely to acquire HIV and to transmit HIV as women who did not use DMPA. The number of seroconversions in the study was, however, low—13 women and 19 men—and investigators did not give information about the duration of DMPA use.
Furthermore, this study was a secondary analysis of a cohort study designed to assess the role of herpes simplex virus in HIV acquisition; it was not designed with the question of a DMPA-HIV link in mind. That leaves questions about contraceptive use, duration of such use, and associated sexual behavior unanswered.
In short, this study adds to an important, growing body of literature, but does not provide evidence for changing gynecologic practice regarding DMPA use and eligibility.
No study has clearly demonstrated sufficiently strong evidence of a putative link between DMPA use and an increased rate of HIV transmission in women at high risk of HIV disease for you to discourage its use in any of your patients for whom DMPA is appropriate.
Stakeholders at the WHO’s 2012 meeting on this matter concluded that 1) no change to guidelines is warranted and 2) hormonal contraception should be promoted for all women, regardless of HIV risk. That conclusion takes into account the fact that the results of more than a decade of research on the role of hormonal contraception in HIV acquisition have been equivocal.12
Given the well-known benefits of effective contraception in preventing unintended pregnancy for all women, especially those at risk of transmitting HIV, you should continue to promote DMPA and all other formulations and methods of hormonal contraception to eligible women.
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
1. Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the united states 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90-6.
2. Jones RK, Kooistra K. Abortion incidence and access to services in the United States 2008. Perspect Sex Reprod Health. 2011;43(1):41-50.
3. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States 1900-1999. MMWR. 1999;48(12):241-243.
4. Hubacher D, Finer LB, Espey E. Renewed interest in intrauterine contraception in the United States: evidence and explanation. Contraception. 2011;83(4):291-294.
5. Grimes DA, Lopez LM, Schulz KF, Van Vliet HA, Stanwood NL. Immediate post-partum insertion of intrauterine devices. Cochrane Database Syst Rev. 2010;(5):CD003036.-
6. Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116(5):1079-1087.
7. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995;346(8990):1575-1582.
8. Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH. The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential. Contraception. 1996;54(4):243-251.
9. Dinger JC, Heinemann LA, Kuhl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142475 women-years of observation. Contraception. 2007;75(5):344-354.
10. Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119(5):1039-1044.
11. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.-
12. World Health Organization. Hormonal contraception and HIV: a technical statement. 2012. http://www.who.int/reproductivehealth/topics/family_planning/Hormonal_contraception_and_HIV.pdf. Accessed June 1 2012.
13. 1Martin HL Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053-1059.
14. Heikinheimo O, Lahteenmaki P. Contraception and HIV infection in women. Hum Reprod Update. 2009;15(2):165-176.
15. Morrison CS, Richardson BA, Mmiro F, et al. Hormonal Contraception and the Risk of HIV Acquisition (HC-HIV) Study Group. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85-95.
1. Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the united states 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90-6.
2. Jones RK, Kooistra K. Abortion incidence and access to services in the United States 2008. Perspect Sex Reprod Health. 2011;43(1):41-50.
3. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States 1900-1999. MMWR. 1999;48(12):241-243.
4. Hubacher D, Finer LB, Espey E. Renewed interest in intrauterine contraception in the United States: evidence and explanation. Contraception. 2011;83(4):291-294.
5. Grimes DA, Lopez LM, Schulz KF, Van Vliet HA, Stanwood NL. Immediate post-partum insertion of intrauterine devices. Cochrane Database Syst Rev. 2010;(5):CD003036.-
6. Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116(5):1079-1087.
7. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet. 1995;346(8990):1575-1582.
8. Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH. The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential. Contraception. 1996;54(4):243-251.
9. Dinger JC, Heinemann LA, Kuhl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142475 women-years of observation. Contraception. 2007;75(5):344-354.
10. Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119(5):1039-1044.
11. Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.-
12. World Health Organization. Hormonal contraception and HIV: a technical statement. 2012. http://www.who.int/reproductivehealth/topics/family_planning/Hormonal_contraception_and_HIV.pdf. Accessed June 1 2012.
13. 1Martin HL Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053-1059.
14. Heikinheimo O, Lahteenmaki P. Contraception and HIV infection in women. Hum Reprod Update. 2009;15(2):165-176.
15. Morrison CS, Richardson BA, Mmiro F, et al. Hormonal Contraception and the Risk of HIV Acquisition (HC-HIV) Study Group. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85-95.
Malpositioned IUDs: When you should intervene (and when you should not)
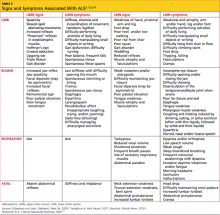
CASE Embedded IUD, currently asymptomatic patient
A 32-year-old G4, P3 presents 1 day after pelvic ultrasonography (US) is performed to evaluate a previous report of intermittent left lower quadrant pain. She is using a levonorgestrel-intrauterine system (LNG-IUS) for contraception, which was placed 1 year ago when she was 6-weeks postpartum. She previously had heavy menses but now has minimal bleeding and is happy with her intrauterine device (IUD). US showed that the IUD is in the lower uterine segment, with the left arm embedded in the myometrium (FIGURE 1). The patient’s pain resolved spontaneously 2 weeks ago, and she is now asymptomatic.
What were this patient’s risk factors for IUD malpositioning? How would you manage her at this time?
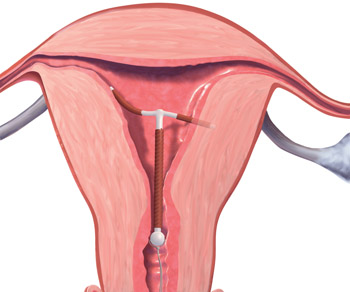
FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.IUDs are an increasingly common form of birth control, now used by 5.5% of contracepting women in the United States.1 With frequent use of pelvic US to evaluate gynecologic complaints, the discovery of malpositioned IUDs also has become an increasingly common occurrence. Clinicians often find themselves faced with dilemmas regarding how to manage a malpositioned IUD, especially in the setting of an asymptomatic patient.
In this article, we review: 1) what constitutes a malpositioned IUD, 2) the consequences of malpositioning, 3) how or if malpositioning can be avoided, and 4) how to manage a malpositioned IUD.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Update on Contraception
Tami Rowen, MD, MS; Mitchell D. Creinin, MD (August 2012)
What constitutes malpositioning?
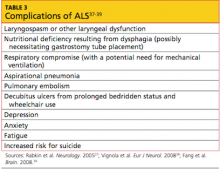
A correctly positioned IUD should be located at the fundus of the uterus, with the arms fully expanded and extending toward the uterine cornua. The vertical portion of the “T” should extend straight down in the uterine corpus. When noted on US, malpositioned IUDs may be described as:
- located in the lower uterine segment or cervix
- rotated (FIGURE 2)
- embedded in the myometrium (one or both arms) (FIGURE 3)
- partially expelled (if an IUD is low enough in the cervix that the hub extends through the external os), or
- protruding through the uterine serosa or completely outside the uterus and within the abdominal cavity. (This is how a perforated or partially perforated IUD may be described.)
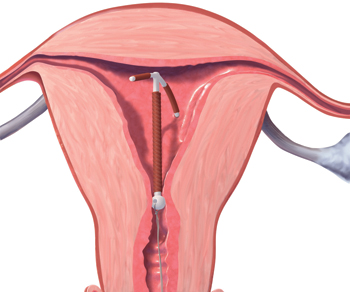
FIGURE 2 Rotated IUD
Copper intrauterine device rotated horizontally.
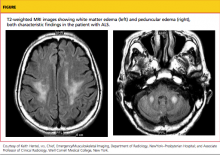
FIGURE 3 Embedded IUD
Three-dimensional sonogram of a 26-year-old patient, showing an intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.If US does not show an IUD that has been placed, x-ray should performed to explore for perforation (see algorithm.)
What damage can malpositioning cause?
For many women, a malpositioned IUD may have minimal or no adverse consequences. The most common negative sequelae of women with a malpositioned IUD, however, include an increase in bleeding or pain, compared with women with fundally positioned IUDs.
Bernacerraf and colleagues retrospectively reviewed the medical records of 167 consecutive women who had ultrasound examination with an IUD in place and found that 28 (16.8%) of them had malpositioned devices.2 Of these 28 women, 75% presented with either bleeding or pain, compared with 34.5% of women with normally positioned devices (P=.0001). Twenty of the 21 patients with a malpositioned device and symptoms reported improvement in their symptoms after IUD removal. In this study, the type of IUD was not specified.
Similar to Bernacerraf and colleagues, authors of a case-controlled study, in which women with malpositioned IUDs were compared with women with normally positioned IUDs, found a higher proportion of symptoms, including bleeding and pain, among women with malpositioned IUDs.3 This study included both copper and levonorgestrel IUDs.
The bowel can suffer, though on rare occasion. The rarest, though most serious form of IUD malpositioning, is the IUD that has perforated the uterine corpus and is intraperitoneally located (FIGURE 4). Studies suggest that approximately 15% of these perforated IUDs cause injury or damage to surrounding organs, primarily the bowel. Management of intraperitoneal IUDs generally involves laparoscopy or laparotomy for removal and exploration of the surrounding structures.4
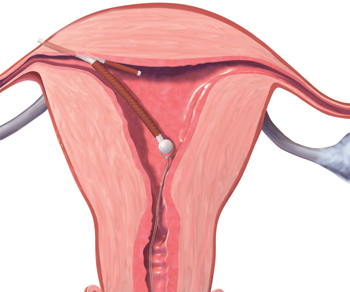
FIGURE 4 Perforation
A copper intrauterine device perforating the serosa.
What about risk of pregnancy?
For the asymptomatic patient, your biggest concern often is whether a malpositioned IUD poses an increased risk of pregnancy. Though data are limited, the available literature suggests that malpositioned, specifically cervically located, copper IUDs may pose an increased risk of pregnancy.
In a prospective study, the authors compared 97 women who had a Cu375 Multiload IUD inserted with 25 women in whom pregnancy was discovered with an IUD in place.5 They found a greater occurrence of intracervical IUDs among the pregnant women, with an odds ratio of 13.93 for pregnancy among women with cervically versus correctly positioned IUDs. Similarly, findings from a case-control study, in which 318 women with pregnancies with CuT380A IUDs in place were compared with 300 controls also using the CuT380A IUD, revealed a 64% rate of IUD malpositioning among the pregnant cases, compared with an 11% rate among the nonpregnant controls (P<.05).6
Does pregnancy or malpositioned IUD come first? None of these studies are able to clarify if it is low placement of the IUD that leads to increased risk of pregnancy or if the pregnancy itself causes malpositioning of the IUD. It is also not known if other types of malpositioning, such as arms extending into the myometrium, are associated with any greater risk of pregnancy. Finally, because there are no prospective studies that have followed a cohort of women with IUDs in situ and assessed pregnancy status according to IUD position, we do not have any data on the absolute risk of pregnancy with a malpositioned IUD in place, though it is likely very small.
IUD type makes a difference. The LNG-IUS does not appear to pose the same risk of pregnancy as copper IUDs if malpositioned. The LNG-IUS prevents pregnancy primarily through hormonal effects on the cervical mucus and endometrium. It seems that the local effects of levonorgestrel are likely adequate for contraception even if the device is not at the fundus, as long as it remains within the uterine cavity. This hypothesis is supported by a randomized clinical trial in which researchers compared the efficacy of an intracervical device that releases the same dose of levonorgestrel as the LNG-IUS, with the efficacy of an LNG-IUS placed at the fundus.7 This study demonstrated no difference in pregnancy rates between the intracervically and the fundally positioned devices.
You also may worry that a downwardly displaced IUD represents risk for expulsion. Although two small studies have suggested that IUDs positioned more than 3 mm from the fundus might have a higher risk of expulsion, most downwardly displaced IUDs are not expelled.8,9 Removal and replacement of downwardly displaced IUDs for the purpose of preventing expulsion would result in a large number of unnecessary removals. Also, studies have shown that not all downwardly displaced IUDs remain so. In fact, the vast majority of IUDs that are downwardly displaced shortly after insertion move to a fundal position within 3 months.10,11
Can malpositioning be avoided?
It is not clear to what extent prevention is possible. Risk factors for IUD malpositioning were examined in a recent case-controlled study. Its authors found that suspected adenomyosis increased the risk of IUD malpositioning and that prior vaginal delivery was protective. No effect of delayed postpartum insertion was seen. The authors also found that public or no insurance was associated with an increased risk of malpositioning; they suggest that this may be related to higher rates of insertion by trainees. Indeed, other studies have found that IUD complications, such as failed insertion and early removals due to pain or bleeding, are associated with insertion by less experienced providers12,13; more skilled providers experience lower rates of IUD malpositioning. Enhancing IUD insertion training may decrease the risk of malpositioning; however, a learning curve may remain.
Despite the fact that some women may be at higher risk for IUD malpositioning, it does not mean they are not IUD candidates. It may be prudent to consider US guidance for IUD insertion in cases of:
- a previous difficult insertion
- obesity precluding the accurate assessment of uterine position, or
- suspected abnormal or distorted uterine cavity.
Integrating evidence and experience
The greatest risk for pregnancy may be unnecessary removal of an IUD. In a recent case-controlled study, Braaten and colleagues compared 182 women with malpositioned IUDs noted on US with 182 women found to have normally positioned IUDs on US. An important finding of this study was that women initially found to have a malpositioned device had a higher rate of pregnancy in the subsequent 2 years. There were no pregnancies among women with malpositioned IUDs left in place; rather, the higher pregnancy rate was due to higher rates of IUD removals (approximately two-thirds of malpositioned IUDs were removed), without replacement with another highly effective method of contraception.
While findings from earlier studies suggest there may be a small increased risk of pregnancy with a malpositioned copper IUD left in situ, as compared with a fundally placed device, this study demonstrates that the real-life risk of pregnancy with removal of an IUD and use of less effective methods of contraception is significantly higher.
Clinicians should be cognizant of this risk prior to removal of a malpositioned IUD and try to ensure that, if a malpositioned IUD is removed, it is quickly replaced with another highly effective form of contraception, such as another IUD, subdermal implant, or sterilization.
Coding for insertion of intrauterine devices (IUDs) can be a hassle if you aren’t familiar with the right code combinations. Here is some advice you can use right now to ensure reimbursement for the usual and unusual situations.
If the purpose of the visit is insertion of an IUD, you only code for that insertion plus the supply. (Even if patient history is repeated at the visit, a separate significant E/M service is not warranted.) Coding is 58300 and J7300 for a copper IUD or J7302 for a levonorgestrel-containing IUD. Note, however, that Blue Cross/Blue Shield payers may require the HCPCS code S4989 (Contraceptive IUD [eg, Progestacert], including implants and supplies), rather than the CPT code.
If you require ultrasound guidance in placing the IUD, the code 76998 can be reported as well.
In some cases, the patient may have a stenotic cervix; if cervical dilation is performed that too can be billed using either 57800, Dilation of cervical canal, instrumental (separate procedure) or 59200, Insertion of cervical dilator (eg, laminaria, prostaglandin) (separate procedure). Because both of these codes are CPT “separate procedures,” a modifier -59 should be added to indicate that a distinct procedure was performed.
In cases in which the IUD is placed immediately following birth, 58300 can be billed but will require a modifier -51. When the IUD is placed 24 hours or more after birth, 58300 requires the addition of the modifier -79 (Unrelated procedure or service by the same physician during the postoperative period).
Sometimes the insertion does not go as planned. If insertion:
- fails due to cervical stenosis, report 58300 with a modifier -52 (Reduced services) since, after considerable work is performed, the decision is made to not insert the device.
- must be stopped because of an unexpected physical reaction by the patient (fainting or a sudden increase or drop in blood pressure), a modifier -53 (Discontinued procedure) is more appropriate.
- is successful but the IUD is expelled from the uterus, repeat insertion may be performed by adding a modifier -76 (Repeat procedure) to 58300.
- is successful but the IUD perforates the uterus to lodge in the abdominal cavity and laparoscopic surgery is required to remove it, the correct code is 49329 (Unlisted laparoscopy procedure, abdomen, peritoneum and omentum). Be sure to compare the work to code 49402, (Removal of peritoneal foreign body from peritoneal cavity) to ensure fair reimbursement.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Our recommendations
The management of malpositioned IUDs can be clinically challenging. Given the available evidence, we suggest the following:
- If patients present with symptoms that may be attributable to the malpositioned device, such as bleeding or pain, the device should be removed and the patient should be offered immediate replacement or immediate initiation of another form of highly effective contraception. Many women will show improvement in their symptoms if the malpositioned device is replaced with one that is correctly placed.
- The asymptomatic patient with a malpositioned LNG-IUS that is still in the uterine cavity can be expectantly managed. She may be offered replacement if she desires.
- An asymptomatic patient with a malpositioned copper IUD should be counseled that she is potentially at higher risk for pregnancy than she would be if her IUD were correctly positioned. This risk cannot be quantified easily, but it is likely lower than the risk of pregnancy associated with most forms of short-acting contraception. She should be counseled for IUD replacement or removal and immediate initiation of another form of highly effective contraception—but you and she also may opt for expectant management if initiating another highly effective form of contraception is not feasible.
CASE Conclusion
The patient is asymptomatic with an LNG-IUS in the lower uterine segment and embedded in the myometrium. She has no obvious risk factors for IUD malpositioning, but given that her menses were heavy prior to placement, she may have adenomyosis.
Given that she is currently asymptomatic with a hormone-containing IUD, she may be managed expectantly. Were she to become symptomatic, she should be offered IUD replacement (versus another form of highly effective contraception such as an implant or sterilization).
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
1. Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):1-44.
2. Bernacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110-115.
3. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014-1020.
4. Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81(1):112-114.
5. Inal MM, Ertopçu K, Özelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005;10(4):266-271.
6. Pakarinen P, Luukkainen T. Five years’ experience with a small intracervical/intrauterine levonorgestrel-releasing device. Contraception. 2005;72(5):342-325.
7. Tangtonpet O, Choktanasiri W, Patrachai S, Israngura Na, Ayudhya N. Intrauterine location and expulsion of intrauterine device. Thai J Obstet Gynecol. 2003;15:45-50.
8. Petta CA, Faúndes D, Pimentel E, Diaz J, Bahamondes L. The use of vaginal ultrasound to identify copper T IUDs at high risk of expulsion. Contraception. 1996;54(5):287-289.
9. Morales-Roselló J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430-431.
10. Faúndes D, Perdigão A, Faúndes A, Bahamondes L, Petta CA. T-shaped IUDs accommodate in their position during the first 3 months after insertion. Contraception. 2000;62(4):165-168.
11. Arslan A, Kanat-Pektas M, Yesilyurt H, Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstet. 2009;279(3):395-397.
12. Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003;29(4):227-231.
13. Zhang J. Factors associated with copper T IUD removal for bleeding/pain: a mulitvariate analysis. Contraception. 1993;48(1):13-21.
CASE Embedded IUD, currently asymptomatic patient
A 32-year-old G4, P3 presents 1 day after pelvic ultrasonography (US) is performed to evaluate a previous report of intermittent left lower quadrant pain. She is using a levonorgestrel-intrauterine system (LNG-IUS) for contraception, which was placed 1 year ago when she was 6-weeks postpartum. She previously had heavy menses but now has minimal bleeding and is happy with her intrauterine device (IUD). US showed that the IUD is in the lower uterine segment, with the left arm embedded in the myometrium (FIGURE 1). The patient’s pain resolved spontaneously 2 weeks ago, and she is now asymptomatic.
What were this patient’s risk factors for IUD malpositioning? How would you manage her at this time?

FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.IUDs are an increasingly common form of birth control, now used by 5.5% of contracepting women in the United States.1 With frequent use of pelvic US to evaluate gynecologic complaints, the discovery of malpositioned IUDs also has become an increasingly common occurrence. Clinicians often find themselves faced with dilemmas regarding how to manage a malpositioned IUD, especially in the setting of an asymptomatic patient.
In this article, we review: 1) what constitutes a malpositioned IUD, 2) the consequences of malpositioning, 3) how or if malpositioning can be avoided, and 4) how to manage a malpositioned IUD.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Update on Contraception
Tami Rowen, MD, MS; Mitchell D. Creinin, MD (August 2012)
What constitutes malpositioning?
A correctly positioned IUD should be located at the fundus of the uterus, with the arms fully expanded and extending toward the uterine cornua. The vertical portion of the “T” should extend straight down in the uterine corpus. When noted on US, malpositioned IUDs may be described as:
- located in the lower uterine segment or cervix
- rotated (FIGURE 2)
- embedded in the myometrium (one or both arms) (FIGURE 3)
- partially expelled (if an IUD is low enough in the cervix that the hub extends through the external os), or
- protruding through the uterine serosa or completely outside the uterus and within the abdominal cavity. (This is how a perforated or partially perforated IUD may be described.)

FIGURE 2 Rotated IUD
Copper intrauterine device rotated horizontally.
FIGURE 3 Embedded IUD
Three-dimensional sonogram of a 26-year-old patient, showing an intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.If US does not show an IUD that has been placed, x-ray should performed to explore for perforation (see algorithm.)

What damage can malpositioning cause?
For many women, a malpositioned IUD may have minimal or no adverse consequences. The most common negative sequelae of women with a malpositioned IUD, however, include an increase in bleeding or pain, compared with women with fundally positioned IUDs.
Bernacerraf and colleagues retrospectively reviewed the medical records of 167 consecutive women who had ultrasound examination with an IUD in place and found that 28 (16.8%) of them had malpositioned devices.2 Of these 28 women, 75% presented with either bleeding or pain, compared with 34.5% of women with normally positioned devices (P=.0001). Twenty of the 21 patients with a malpositioned device and symptoms reported improvement in their symptoms after IUD removal. In this study, the type of IUD was not specified.
Similar to Bernacerraf and colleagues, authors of a case-controlled study, in which women with malpositioned IUDs were compared with women with normally positioned IUDs, found a higher proportion of symptoms, including bleeding and pain, among women with malpositioned IUDs.3 This study included both copper and levonorgestrel IUDs.
The bowel can suffer, though on rare occasion. The rarest, though most serious form of IUD malpositioning, is the IUD that has perforated the uterine corpus and is intraperitoneally located (FIGURE 4). Studies suggest that approximately 15% of these perforated IUDs cause injury or damage to surrounding organs, primarily the bowel. Management of intraperitoneal IUDs generally involves laparoscopy or laparotomy for removal and exploration of the surrounding structures.4

FIGURE 4 Perforation
A copper intrauterine device perforating the serosa.
What about risk of pregnancy?
For the asymptomatic patient, your biggest concern often is whether a malpositioned IUD poses an increased risk of pregnancy. Though data are limited, the available literature suggests that malpositioned, specifically cervically located, copper IUDs may pose an increased risk of pregnancy.
In a prospective study, the authors compared 97 women who had a Cu375 Multiload IUD inserted with 25 women in whom pregnancy was discovered with an IUD in place.5 They found a greater occurrence of intracervical IUDs among the pregnant women, with an odds ratio of 13.93 for pregnancy among women with cervically versus correctly positioned IUDs. Similarly, findings from a case-control study, in which 318 women with pregnancies with CuT380A IUDs in place were compared with 300 controls also using the CuT380A IUD, revealed a 64% rate of IUD malpositioning among the pregnant cases, compared with an 11% rate among the nonpregnant controls (P<.05).6
Does pregnancy or malpositioned IUD come first? None of these studies are able to clarify if it is low placement of the IUD that leads to increased risk of pregnancy or if the pregnancy itself causes malpositioning of the IUD. It is also not known if other types of malpositioning, such as arms extending into the myometrium, are associated with any greater risk of pregnancy. Finally, because there are no prospective studies that have followed a cohort of women with IUDs in situ and assessed pregnancy status according to IUD position, we do not have any data on the absolute risk of pregnancy with a malpositioned IUD in place, though it is likely very small.
IUD type makes a difference. The LNG-IUS does not appear to pose the same risk of pregnancy as copper IUDs if malpositioned. The LNG-IUS prevents pregnancy primarily through hormonal effects on the cervical mucus and endometrium. It seems that the local effects of levonorgestrel are likely adequate for contraception even if the device is not at the fundus, as long as it remains within the uterine cavity. This hypothesis is supported by a randomized clinical trial in which researchers compared the efficacy of an intracervical device that releases the same dose of levonorgestrel as the LNG-IUS, with the efficacy of an LNG-IUS placed at the fundus.7 This study demonstrated no difference in pregnancy rates between the intracervically and the fundally positioned devices.
You also may worry that a downwardly displaced IUD represents risk for expulsion. Although two small studies have suggested that IUDs positioned more than 3 mm from the fundus might have a higher risk of expulsion, most downwardly displaced IUDs are not expelled.8,9 Removal and replacement of downwardly displaced IUDs for the purpose of preventing expulsion would result in a large number of unnecessary removals. Also, studies have shown that not all downwardly displaced IUDs remain so. In fact, the vast majority of IUDs that are downwardly displaced shortly after insertion move to a fundal position within 3 months.10,11
Can malpositioning be avoided?
It is not clear to what extent prevention is possible. Risk factors for IUD malpositioning were examined in a recent case-controlled study. Its authors found that suspected adenomyosis increased the risk of IUD malpositioning and that prior vaginal delivery was protective. No effect of delayed postpartum insertion was seen. The authors also found that public or no insurance was associated with an increased risk of malpositioning; they suggest that this may be related to higher rates of insertion by trainees. Indeed, other studies have found that IUD complications, such as failed insertion and early removals due to pain or bleeding, are associated with insertion by less experienced providers12,13; more skilled providers experience lower rates of IUD malpositioning. Enhancing IUD insertion training may decrease the risk of malpositioning; however, a learning curve may remain.
Despite the fact that some women may be at higher risk for IUD malpositioning, it does not mean they are not IUD candidates. It may be prudent to consider US guidance for IUD insertion in cases of:
- a previous difficult insertion
- obesity precluding the accurate assessment of uterine position, or
- suspected abnormal or distorted uterine cavity.
Integrating evidence and experience
The greatest risk for pregnancy may be unnecessary removal of an IUD. In a recent case-controlled study, Braaten and colleagues compared 182 women with malpositioned IUDs noted on US with 182 women found to have normally positioned IUDs on US. An important finding of this study was that women initially found to have a malpositioned device had a higher rate of pregnancy in the subsequent 2 years. There were no pregnancies among women with malpositioned IUDs left in place; rather, the higher pregnancy rate was due to higher rates of IUD removals (approximately two-thirds of malpositioned IUDs were removed), without replacement with another highly effective method of contraception.
While findings from earlier studies suggest there may be a small increased risk of pregnancy with a malpositioned copper IUD left in situ, as compared with a fundally placed device, this study demonstrates that the real-life risk of pregnancy with removal of an IUD and use of less effective methods of contraception is significantly higher.
Clinicians should be cognizant of this risk prior to removal of a malpositioned IUD and try to ensure that, if a malpositioned IUD is removed, it is quickly replaced with another highly effective form of contraception, such as another IUD, subdermal implant, or sterilization.
Coding for insertion of intrauterine devices (IUDs) can be a hassle if you aren’t familiar with the right code combinations. Here is some advice you can use right now to ensure reimbursement for the usual and unusual situations.
If the purpose of the visit is insertion of an IUD, you only code for that insertion plus the supply. (Even if patient history is repeated at the visit, a separate significant E/M service is not warranted.) Coding is 58300 and J7300 for a copper IUD or J7302 for a levonorgestrel-containing IUD. Note, however, that Blue Cross/Blue Shield payers may require the HCPCS code S4989 (Contraceptive IUD [eg, Progestacert], including implants and supplies), rather than the CPT code.
If you require ultrasound guidance in placing the IUD, the code 76998 can be reported as well.
In some cases, the patient may have a stenotic cervix; if cervical dilation is performed that too can be billed using either 57800, Dilation of cervical canal, instrumental (separate procedure) or 59200, Insertion of cervical dilator (eg, laminaria, prostaglandin) (separate procedure). Because both of these codes are CPT “separate procedures,” a modifier -59 should be added to indicate that a distinct procedure was performed.
In cases in which the IUD is placed immediately following birth, 58300 can be billed but will require a modifier -51. When the IUD is placed 24 hours or more after birth, 58300 requires the addition of the modifier -79 (Unrelated procedure or service by the same physician during the postoperative period).
Sometimes the insertion does not go as planned. If insertion:
- fails due to cervical stenosis, report 58300 with a modifier -52 (Reduced services) since, after considerable work is performed, the decision is made to not insert the device.
- must be stopped because of an unexpected physical reaction by the patient (fainting or a sudden increase or drop in blood pressure), a modifier -53 (Discontinued procedure) is more appropriate.
- is successful but the IUD is expelled from the uterus, repeat insertion may be performed by adding a modifier -76 (Repeat procedure) to 58300.
- is successful but the IUD perforates the uterus to lodge in the abdominal cavity and laparoscopic surgery is required to remove it, the correct code is 49329 (Unlisted laparoscopy procedure, abdomen, peritoneum and omentum). Be sure to compare the work to code 49402, (Removal of peritoneal foreign body from peritoneal cavity) to ensure fair reimbursement.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Our recommendations
The management of malpositioned IUDs can be clinically challenging. Given the available evidence, we suggest the following:
- If patients present with symptoms that may be attributable to the malpositioned device, such as bleeding or pain, the device should be removed and the patient should be offered immediate replacement or immediate initiation of another form of highly effective contraception. Many women will show improvement in their symptoms if the malpositioned device is replaced with one that is correctly placed.
- The asymptomatic patient with a malpositioned LNG-IUS that is still in the uterine cavity can be expectantly managed. She may be offered replacement if she desires.
- An asymptomatic patient with a malpositioned copper IUD should be counseled that she is potentially at higher risk for pregnancy than she would be if her IUD were correctly positioned. This risk cannot be quantified easily, but it is likely lower than the risk of pregnancy associated with most forms of short-acting contraception. She should be counseled for IUD replacement or removal and immediate initiation of another form of highly effective contraception—but you and she also may opt for expectant management if initiating another highly effective form of contraception is not feasible.
CASE Conclusion
The patient is asymptomatic with an LNG-IUS in the lower uterine segment and embedded in the myometrium. She has no obvious risk factors for IUD malpositioning, but given that her menses were heavy prior to placement, she may have adenomyosis.
Given that she is currently asymptomatic with a hormone-containing IUD, she may be managed expectantly. Were she to become symptomatic, she should be offered IUD replacement (versus another form of highly effective contraception such as an implant or sterilization).
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
CASE Embedded IUD, currently asymptomatic patient
A 32-year-old G4, P3 presents 1 day after pelvic ultrasonography (US) is performed to evaluate a previous report of intermittent left lower quadrant pain. She is using a levonorgestrel-intrauterine system (LNG-IUS) for contraception, which was placed 1 year ago when she was 6-weeks postpartum. She previously had heavy menses but now has minimal bleeding and is happy with her intrauterine device (IUD). US showed that the IUD is in the lower uterine segment, with the left arm embedded in the myometrium (FIGURE 1). The patient’s pain resolved spontaneously 2 weeks ago, and she is now asymptomatic.
What were this patient’s risk factors for IUD malpositioning? How would you manage her at this time?

FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.IUDs are an increasingly common form of birth control, now used by 5.5% of contracepting women in the United States.1 With frequent use of pelvic US to evaluate gynecologic complaints, the discovery of malpositioned IUDs also has become an increasingly common occurrence. Clinicians often find themselves faced with dilemmas regarding how to manage a malpositioned IUD, especially in the setting of an asymptomatic patient.
In this article, we review: 1) what constitutes a malpositioned IUD, 2) the consequences of malpositioning, 3) how or if malpositioning can be avoided, and 4) how to manage a malpositioned IUD.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Update on Contraception
Tami Rowen, MD, MS; Mitchell D. Creinin, MD (August 2012)
What constitutes malpositioning?
A correctly positioned IUD should be located at the fundus of the uterus, with the arms fully expanded and extending toward the uterine cornua. The vertical portion of the “T” should extend straight down in the uterine corpus. When noted on US, malpositioned IUDs may be described as:
- located in the lower uterine segment or cervix
- rotated (FIGURE 2)
- embedded in the myometrium (one or both arms) (FIGURE 3)
- partially expelled (if an IUD is low enough in the cervix that the hub extends through the external os), or
- protruding through the uterine serosa or completely outside the uterus and within the abdominal cavity. (This is how a perforated or partially perforated IUD may be described.)

FIGURE 2 Rotated IUD
Copper intrauterine device rotated horizontally.
FIGURE 3 Embedded IUD
Three-dimensional sonogram of a 26-year-old patient, showing an intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.If US does not show an IUD that has been placed, x-ray should performed to explore for perforation (see algorithm.)

What damage can malpositioning cause?
For many women, a malpositioned IUD may have minimal or no adverse consequences. The most common negative sequelae of women with a malpositioned IUD, however, include an increase in bleeding or pain, compared with women with fundally positioned IUDs.
Bernacerraf and colleagues retrospectively reviewed the medical records of 167 consecutive women who had ultrasound examination with an IUD in place and found that 28 (16.8%) of them had malpositioned devices.2 Of these 28 women, 75% presented with either bleeding or pain, compared with 34.5% of women with normally positioned devices (P=.0001). Twenty of the 21 patients with a malpositioned device and symptoms reported improvement in their symptoms after IUD removal. In this study, the type of IUD was not specified.
Similar to Bernacerraf and colleagues, authors of a case-controlled study, in which women with malpositioned IUDs were compared with women with normally positioned IUDs, found a higher proportion of symptoms, including bleeding and pain, among women with malpositioned IUDs.3 This study included both copper and levonorgestrel IUDs.
The bowel can suffer, though on rare occasion. The rarest, though most serious form of IUD malpositioning, is the IUD that has perforated the uterine corpus and is intraperitoneally located (FIGURE 4). Studies suggest that approximately 15% of these perforated IUDs cause injury or damage to surrounding organs, primarily the bowel. Management of intraperitoneal IUDs generally involves laparoscopy or laparotomy for removal and exploration of the surrounding structures.4

FIGURE 4 Perforation
A copper intrauterine device perforating the serosa.
What about risk of pregnancy?
For the asymptomatic patient, your biggest concern often is whether a malpositioned IUD poses an increased risk of pregnancy. Though data are limited, the available literature suggests that malpositioned, specifically cervically located, copper IUDs may pose an increased risk of pregnancy.
In a prospective study, the authors compared 97 women who had a Cu375 Multiload IUD inserted with 25 women in whom pregnancy was discovered with an IUD in place.5 They found a greater occurrence of intracervical IUDs among the pregnant women, with an odds ratio of 13.93 for pregnancy among women with cervically versus correctly positioned IUDs. Similarly, findings from a case-control study, in which 318 women with pregnancies with CuT380A IUDs in place were compared with 300 controls also using the CuT380A IUD, revealed a 64% rate of IUD malpositioning among the pregnant cases, compared with an 11% rate among the nonpregnant controls (P<.05).6
Does pregnancy or malpositioned IUD come first? None of these studies are able to clarify if it is low placement of the IUD that leads to increased risk of pregnancy or if the pregnancy itself causes malpositioning of the IUD. It is also not known if other types of malpositioning, such as arms extending into the myometrium, are associated with any greater risk of pregnancy. Finally, because there are no prospective studies that have followed a cohort of women with IUDs in situ and assessed pregnancy status according to IUD position, we do not have any data on the absolute risk of pregnancy with a malpositioned IUD in place, though it is likely very small.
IUD type makes a difference. The LNG-IUS does not appear to pose the same risk of pregnancy as copper IUDs if malpositioned. The LNG-IUS prevents pregnancy primarily through hormonal effects on the cervical mucus and endometrium. It seems that the local effects of levonorgestrel are likely adequate for contraception even if the device is not at the fundus, as long as it remains within the uterine cavity. This hypothesis is supported by a randomized clinical trial in which researchers compared the efficacy of an intracervical device that releases the same dose of levonorgestrel as the LNG-IUS, with the efficacy of an LNG-IUS placed at the fundus.7 This study demonstrated no difference in pregnancy rates between the intracervically and the fundally positioned devices.
You also may worry that a downwardly displaced IUD represents risk for expulsion. Although two small studies have suggested that IUDs positioned more than 3 mm from the fundus might have a higher risk of expulsion, most downwardly displaced IUDs are not expelled.8,9 Removal and replacement of downwardly displaced IUDs for the purpose of preventing expulsion would result in a large number of unnecessary removals. Also, studies have shown that not all downwardly displaced IUDs remain so. In fact, the vast majority of IUDs that are downwardly displaced shortly after insertion move to a fundal position within 3 months.10,11
Can malpositioning be avoided?
It is not clear to what extent prevention is possible. Risk factors for IUD malpositioning were examined in a recent case-controlled study. Its authors found that suspected adenomyosis increased the risk of IUD malpositioning and that prior vaginal delivery was protective. No effect of delayed postpartum insertion was seen. The authors also found that public or no insurance was associated with an increased risk of malpositioning; they suggest that this may be related to higher rates of insertion by trainees. Indeed, other studies have found that IUD complications, such as failed insertion and early removals due to pain or bleeding, are associated with insertion by less experienced providers12,13; more skilled providers experience lower rates of IUD malpositioning. Enhancing IUD insertion training may decrease the risk of malpositioning; however, a learning curve may remain.
Despite the fact that some women may be at higher risk for IUD malpositioning, it does not mean they are not IUD candidates. It may be prudent to consider US guidance for IUD insertion in cases of:
- a previous difficult insertion
- obesity precluding the accurate assessment of uterine position, or
- suspected abnormal or distorted uterine cavity.
Integrating evidence and experience
The greatest risk for pregnancy may be unnecessary removal of an IUD. In a recent case-controlled study, Braaten and colleagues compared 182 women with malpositioned IUDs noted on US with 182 women found to have normally positioned IUDs on US. An important finding of this study was that women initially found to have a malpositioned device had a higher rate of pregnancy in the subsequent 2 years. There were no pregnancies among women with malpositioned IUDs left in place; rather, the higher pregnancy rate was due to higher rates of IUD removals (approximately two-thirds of malpositioned IUDs were removed), without replacement with another highly effective method of contraception.
While findings from earlier studies suggest there may be a small increased risk of pregnancy with a malpositioned copper IUD left in situ, as compared with a fundally placed device, this study demonstrates that the real-life risk of pregnancy with removal of an IUD and use of less effective methods of contraception is significantly higher.
Clinicians should be cognizant of this risk prior to removal of a malpositioned IUD and try to ensure that, if a malpositioned IUD is removed, it is quickly replaced with another highly effective form of contraception, such as another IUD, subdermal implant, or sterilization.
Coding for insertion of intrauterine devices (IUDs) can be a hassle if you aren’t familiar with the right code combinations. Here is some advice you can use right now to ensure reimbursement for the usual and unusual situations.
If the purpose of the visit is insertion of an IUD, you only code for that insertion plus the supply. (Even if patient history is repeated at the visit, a separate significant E/M service is not warranted.) Coding is 58300 and J7300 for a copper IUD or J7302 for a levonorgestrel-containing IUD. Note, however, that Blue Cross/Blue Shield payers may require the HCPCS code S4989 (Contraceptive IUD [eg, Progestacert], including implants and supplies), rather than the CPT code.
If you require ultrasound guidance in placing the IUD, the code 76998 can be reported as well.
In some cases, the patient may have a stenotic cervix; if cervical dilation is performed that too can be billed using either 57800, Dilation of cervical canal, instrumental (separate procedure) or 59200, Insertion of cervical dilator (eg, laminaria, prostaglandin) (separate procedure). Because both of these codes are CPT “separate procedures,” a modifier -59 should be added to indicate that a distinct procedure was performed.
In cases in which the IUD is placed immediately following birth, 58300 can be billed but will require a modifier -51. When the IUD is placed 24 hours or more after birth, 58300 requires the addition of the modifier -79 (Unrelated procedure or service by the same physician during the postoperative period).
Sometimes the insertion does not go as planned. If insertion:
- fails due to cervical stenosis, report 58300 with a modifier -52 (Reduced services) since, after considerable work is performed, the decision is made to not insert the device.
- must be stopped because of an unexpected physical reaction by the patient (fainting or a sudden increase or drop in blood pressure), a modifier -53 (Discontinued procedure) is more appropriate.
- is successful but the IUD is expelled from the uterus, repeat insertion may be performed by adding a modifier -76 (Repeat procedure) to 58300.
- is successful but the IUD perforates the uterus to lodge in the abdominal cavity and laparoscopic surgery is required to remove it, the correct code is 49329 (Unlisted laparoscopy procedure, abdomen, peritoneum and omentum). Be sure to compare the work to code 49402, (Removal of peritoneal foreign body from peritoneal cavity) to ensure fair reimbursement.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Our recommendations
The management of malpositioned IUDs can be clinically challenging. Given the available evidence, we suggest the following:
- If patients present with symptoms that may be attributable to the malpositioned device, such as bleeding or pain, the device should be removed and the patient should be offered immediate replacement or immediate initiation of another form of highly effective contraception. Many women will show improvement in their symptoms if the malpositioned device is replaced with one that is correctly placed.
- The asymptomatic patient with a malpositioned LNG-IUS that is still in the uterine cavity can be expectantly managed. She may be offered replacement if she desires.
- An asymptomatic patient with a malpositioned copper IUD should be counseled that she is potentially at higher risk for pregnancy than she would be if her IUD were correctly positioned. This risk cannot be quantified easily, but it is likely lower than the risk of pregnancy associated with most forms of short-acting contraception. She should be counseled for IUD replacement or removal and immediate initiation of another form of highly effective contraception—but you and she also may opt for expectant management if initiating another highly effective form of contraception is not feasible.
CASE Conclusion
The patient is asymptomatic with an LNG-IUS in the lower uterine segment and embedded in the myometrium. She has no obvious risk factors for IUD malpositioning, but given that her menses were heavy prior to placement, she may have adenomyosis.
Given that she is currently asymptomatic with a hormone-containing IUD, she may be managed expectantly. Were she to become symptomatic, she should be offered IUD replacement (versus another form of highly effective contraception such as an implant or sterilization).
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
1. Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):1-44.
2. Bernacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110-115.
3. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014-1020.
4. Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81(1):112-114.
5. Inal MM, Ertopçu K, Özelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005;10(4):266-271.
6. Pakarinen P, Luukkainen T. Five years’ experience with a small intracervical/intrauterine levonorgestrel-releasing device. Contraception. 2005;72(5):342-325.
7. Tangtonpet O, Choktanasiri W, Patrachai S, Israngura Na, Ayudhya N. Intrauterine location and expulsion of intrauterine device. Thai J Obstet Gynecol. 2003;15:45-50.
8. Petta CA, Faúndes D, Pimentel E, Diaz J, Bahamondes L. The use of vaginal ultrasound to identify copper T IUDs at high risk of expulsion. Contraception. 1996;54(5):287-289.
9. Morales-Roselló J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430-431.
10. Faúndes D, Perdigão A, Faúndes A, Bahamondes L, Petta CA. T-shaped IUDs accommodate in their position during the first 3 months after insertion. Contraception. 2000;62(4):165-168.
11. Arslan A, Kanat-Pektas M, Yesilyurt H, Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstet. 2009;279(3):395-397.
12. Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003;29(4):227-231.
13. Zhang J. Factors associated with copper T IUD removal for bleeding/pain: a mulitvariate analysis. Contraception. 1993;48(1):13-21.
1. Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):1-44.
2. Bernacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110-115.
3. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014-1020.
4. Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81(1):112-114.
5. Inal MM, Ertopçu K, Özelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005;10(4):266-271.
6. Pakarinen P, Luukkainen T. Five years’ experience with a small intracervical/intrauterine levonorgestrel-releasing device. Contraception. 2005;72(5):342-325.
7. Tangtonpet O, Choktanasiri W, Patrachai S, Israngura Na, Ayudhya N. Intrauterine location and expulsion of intrauterine device. Thai J Obstet Gynecol. 2003;15:45-50.
8. Petta CA, Faúndes D, Pimentel E, Diaz J, Bahamondes L. The use of vaginal ultrasound to identify copper T IUDs at high risk of expulsion. Contraception. 1996;54(5):287-289.
9. Morales-Roselló J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430-431.
10. Faúndes D, Perdigão A, Faúndes A, Bahamondes L, Petta CA. T-shaped IUDs accommodate in their position during the first 3 months after insertion. Contraception. 2000;62(4):165-168.
11. Arslan A, Kanat-Pektas M, Yesilyurt H, Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstet. 2009;279(3):395-397.
12. Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003;29(4):227-231.
13. Zhang J. Factors associated with copper T IUD removal for bleeding/pain: a mulitvariate analysis. Contraception. 1993;48(1):13-21.
Amyotrophic Lateral Sclerosis: Diagnosis and Appropriate Management
Amyotrophic lateral sclerosis (ALS) is probably best known to the majority of the US population as Lou Gehrig’s disease, named for the New York Yankee who died of ALS after a stellar 17-year baseball career in the 1920s and ’30s. Gradually, the general public is gaining familiarity with the characteristics of ALS, a disease with no single identifiable cause and no known cure.
About 5,600 people receive a diagnosis of ALS in the US each year (about 15 per day), making it the most common form of motor neuron disease.1 Most patients present with progressive muscle weakness in an extremity, which ultimately leads to respiratory failure and death.
Generally, patients with ALS have no cognitive disability and are aware of their physical decline. These patients are faced with important decisions as their condition worsens: Will they want nutritional support through a gastrostomy? For respiratory assistance, will their preference be noninvasive ventilation or mechanical ventilation via tracheostomy?
Not only do ALS patients undergo deterioration of motor function, but many experience muscle cramping, generalized pain, and depression. Only one medication is currently approved to treat ALS patients, with the benefit of prolonging life by a few months. In addition to symptom management, supportive measures, including physical, occupational, and speech therapy, exercise, nutrition, support groups, and counseling, are important tools to enhance quality of life for the patient with ALS.2
Primary care providers need to be aware of ALS, recognize its symptoms in their patients, and manage those affected on a case-by-case basis. Because of the challenges in diagnostic evaluation, the rapidly evolving nature of the disease, and the dire prognosis of ALS, any patient suspected of having ALS should be referred immediately to a neurologist. Providers need to educate patients and their caregivers regarding the disease process and ensure that patients receive appropriate care to meet their needs and preferences.
ALS DEFINED
ALS is a progressive neurodegenerative disease that affects both the upper and lower motor neurons. The disease is considered terminal. Although life may be prolonged by the one currently available pharmacologic agent, no treatment option is yet capable of stopping or reversing progression of the disease.3 While ALS was once believed to be a purely motor disorder, the accompanying degeneration of nonmotor brain regions, such as frontal and temporal cortical neurons, is considered by some to be part of the clinicopathologic spectrum of ALS.4
There are two forms of ALS: sporadic and familial. Sporadic ALS is by far the more common, accounting for 90% to 95% of cases. The remaining 5% to 10% of ALS cases are of the familial form, which can be autosomal-dominant or autosomal-recessive.5
The Degenerative Motor Neuron Diseases
Weakness and muscle wasting characterize several degenerative motor neuron diseases. In addition to ALS, these include primary lateral sclerosis, progressive muscular atrophy, progressive bulbar palsy, and pseudobulbar palsy.6
Primary lateral sclerosis involves upper motor neuron (UMN) dysfunction in the limbs.7Progressive muscular atrophy results from degeneration of the anterior horn cells in the spinal cord and is associated with lower motor neuron (LMN) deficits in the limbs.
Progressive bulbar palsy is a progressive UMN and LMN disorder of the cranial muscles. This condition may occasionally stay isolated in the bulbar segment, but more commonly, UMN and LMN signs and symptoms spread to involve other segments. This is then referred to as bulbar-onset ALS. There have been no reports of specific pathology in progressive bulbar palsy.
Pseudobulbar palsy results from an UMN lesion in the corticobulbar pathway in the pyramidal tract. It is characterized by difficulty chewing and swallowing, and slurred speech (manifestations that may also represent the initial presentation of ALS). Patients with pseudobulbar palsy may exhibit inappropriate, excessive yawning and emotional outbursts; these manifestations are referred to as emotional incontinence.8
EPIDEMIOLOGY
In North America, it is estimated that ALS affects 1.5 to 2.7 people per 100,000 between ages 20 and 80; most frequently, the disease presents between ages 55 and 65. ALS develops infrequently before age 30.3,9
While no gender difference is apparent in patients with familial ALS, sporadic ALS predominately affects males more than females (although the accepted ratio of 1.5:1 appears to be in decline9). After age 65, men and women are equally impacted.10
RISK FACTORS
Established risk factors for ALS include age and family history. Accumulating evidence suggests that military service and smoking may also contribute to the development of ALS.11-15 Children and siblings of ALS patients are at increased risk for ALS, while military personnel have 1.5 times the risk.11
Investigation of the precise link between military service and ALS is ongoing, but factors may include intense exertion, traumatic injuries, viral infections, and exposure to certain chemicals or metals.11 Research suggests the risk is independent of time period, years of service, or branch of service. Geographic location appears to be an independent factor, although there does seem to be a strong association between deployment during the 1991 Gulf War and the risk for ALS.12-14
Recently, smoking has been implicated as a potential risk factor in the disease.3,15 Certain environmental exposures have also been recognized as a possible risk factor. In Guam, an ALS-like syndrome has been identified among members of the Chamorro tribe. This syndrome has been linked to a neurotoxin in the seed of the cycad nut, a tropical plant endemic to the area, which was used in the 1950s and 1960s in the human food supply.3,16
ETIOLOGY
Familial ALS is a genetically transmitted degenerative disease. Twenty percent of cases involve the long arm of chromosome 21, which is responsible for coding of superoxide dismutase (SOD1). Mutations in SOD1, an RNA-processing protein called FUS, and the DNA-binding protein TDP-43, have all been identified in cases of familial ALS.17,18
The exact etiology of sporadic ALS remains unknown, but gene mutations have also been implicated, including the ANG gene21 and TDP-43.18 Other theories include increased levels of glutamate (which have been detected in cerebrospinal fluid and serum of patients with ALS), mitochondrial dysfunction, free radical injury, programmed cell death, neurofilament defects, viral infections, and autoimmune dysfunction19,20 (see Table 13,17,18,20,21). It is possible that a combination of factors is involved in the development of sporadic ALS.
PATHOPHYSIOLOGY
The pathophysiology of ALS involves degeneration of the UMN and LMN axons, which leads to glial scarring and possible impairment of the glial cells’ ability to store excess glutamate.22 ALS affects the central nervous system, specifically the anterior horn cells in the spinal cord and the cranial nerve nuclei (X, XI, XII) of the LMNs, and the corticospinal tract and corticobulbar pathway of the UMNs. Bulbar and limb muscles innervated by LMNs are subject to atrophy, whereas cognition, coordination, sensation, the oculomotor system, and sphincters are typically spared.3
CLINICAL FEATURES
In most patients, ALS symptoms characterize either limb onset or bulbar onset (see Table 23,7,23,24), with limb onset being the more common (about 75% vs about 25% of cases, respectively).1 Typically, patients with limb-onset ALS complain of rapidly progressive, asymmetric weakness in an extremity, followed by focal muscle atrophy with cramping and fasciculations, and eventually, spasticity. Weakness generally begins in one hand, arm, foot, or leg. Patients may notice increased episodes of tripping, clumsiness when they run or walk, a “dropped foot” gait, and/or a decline in manual dexterity.25
The weakness often develops insidiously; patients may notice that symptoms are exacerbated by cold weather.3 Eventually, the bulbar muscles are affected, resulting in dysphagia, dysarthria, and dysphasia. Occasionally, patients encounter bladder dysfunction (urgent micturition), sensory symptoms, and cognitive symptoms (eg, dementia, parkinsonism).26 Multisystem involvement is possible. Ultimately, respiratory compromise or other pulmonary complications ensue, representing a primary cause of mortality in ALS patients.3,24
In bulbar-onset ALS, patients first notice symptoms of dysphasia and dysphagia. They may complain of slurred speech, nasal or low-volume speech, and/or inhibited tongue mobility. The risk for aspiration is increased. The majority of patients with bulbar-onset ALS experience sialorrhea (excessive drooling) because they have difficulty swallowing their saliva. In most patients, mild UMN-type bilateral facial weakness affects the lower half of the face.3 As is the case with limb-onset ALS, bulbar-onset ALS progresses to respiratory compromise.27
In less than 3% of patients with ALS, presentation begins with respiratory weakness and no significant limb or bulbar symptoms.24,28 Patients with respiratory-onset ALS experience symptoms associated with nocturnal hypoventilation, including daytime hypersomnolence, morning headaches, impaired concentration, irritability, anorexia, mood changes, dyspnea, orthopnea, and disturbed sleep; or they may experience type 2 respiratory failure.28,29
Patients with axial symptoms of ALS present with neck weakness and may complain of posterior neck pain or strain with a gradually worsening tendency of the head to tip forward. These patients often support the chin with one hand. Those with axial truncal weakness often complain of difficulty maintaining erect posture when standing and of stooping as they walk. Some patients support the trunk by placing their hands in their front pants pockets or on their upper thighs. They may report some relief when pushing a grocery cart.7,23,24
Symptoms of ALS can be present for weeks or months before a patient consults with a health care provider. The average time span from onset of initial symptoms to diagnosis of ALS is about one year.1 Due to the unpredictable pattern of progression and variability of symptoms among patients, it is difficult to approximate a time frame for symptom progression; for some patients, the disease progresses slowly, while others deteriorate rapidly.30
PHYSICAL EXAMINATION FINDINGS
At onset, the typical presentation of ALS includes muscle weakness in one limb as well as visible fasciculations. As the disease progresses, focal wasting of muscle groups occurs in all four extremities. Particularly involved are the muscles of the hands, forearms, or shoulders in the upper limbs; and of the proximal thigh or distal foot muscles in the lower limbs.31 Deep tendon reflexes are symmetrically brisk. Spasticity, evident in the upper limbs, may present as increased tone.32
In patients with bulbar dysfunction, dysarthria may arise from either LMN pathology or pseudobulbar palsy caused by a UMN disorder, leading to slow, slurred speech or speech with a nasal quality. Tongue fasciculations will be present, as will atrophy and diminished mobility of the tongue.33 The gag reflex remains intact, even brisk, but weakness may occur in the muscles of the soft palate.3 Facial weakness is sometimes seen late in the disease, as evidenced by difficulty sealing the lips or puffing out the cheeks. The jaw jerk will be brisk, indicating that cranial nerve V is intact.34
A pseudobulbar affect, which is best described as emotional lability, may be present. The patient may have a history of exaggerated expression of emotion, such as uncontrollable crying, laughing, or both.35 Cognition, coordination, sensation, the oculomotor system, and sphincters are generally spared. However, cases of frontotemporal dementias coexisting with ALS have been reported; affected patients exhibit cognitive impairment, compulsive behaviors, and personality changes, and they may experience shorter survival.4,24,36
Evidence of known complications of ALS may also be noted during the physical examination; see Table 3.37-39
DIAGNOSIS OF ALS
In current research, use of structural MRI, magnetic resonance spectroscopy, and diffusion tensor imaging is being examined to detect thinning in the primary motor cortex, fractional anisotropy in the corpus callosum, patterns of gray and white matter atrophy, and other proposed diagnostic markers for ALS.40-43 However, no single specific diagnostic test has yet been proven to identify ALS; rather, it remains a disease of exclusion (see Table 43,44). For a confirmed diagnosis of ALS to be made, the patient must display:
- Evidence of LMN degeneration as found through clinical, neuropathologic, or electrophysiological examination
- UMN degeneration detected by clinical examination, and
- Progressive spread of signs or symptoms within a region or to other regions.3,45-47
Other disease processes that might explain the signs of UMN and/or LMN degeneration must be excluded, such as cervical spinal disease, myasthenia gravis, multifocal motor neuropathy, lead intoxication, and Lyme disease.45
Imaging studies are not required in ALS cases that have clinically definite disease with bulbar or pseudobulbar onset.47 Otherwise, the essential role of neuroimaging is to exclude a treatable structural lesion that may mimic ALS by producing UMN and LMN signs in varying degrees.48
Typically, MRI of the head and spine is ordered in patients with suspected ALS (see figure); MRI can reveal lesions in the corticospinal tracts that occur in ALS. The most characteristic finding on T2-weighted MRI is hyperintensity of the corticospinal tracts, which is visualized best in the brain and brainstem, and to a lesser extent in the spinal cord.49 Decreased signal intensity in the motor cortex has been reported on MRI in cases of ALS.50
Additional diagnostic procedures that are useful in excluding other disease processes include blood and cerebrospinal fluid (CSF) samples,3,44 four-limb electromyography (EMG),44 nerve conduction studies, motor unit number estimations, and muscle biopsies.47
- Autopsy results of patients with ALS demonstrate:
- Neuron loss, especially in lumbar and cervical enlargements
- Nonexistent or atrophic neurons in the motor nuclei of the pons and medulla, and in the anterior horn cells of the spinal cord
- Degeneration of the lateral columns of the spinal cord, and
- Atrophy of the ventral roots.31,51
PROGNOSIS
The overall five-year survival rate for patients with ALS has been reported between 7% and 14%,52,53 and the mortality rate rises in patients older than 75 and in those with bulbar signs.30 The rate of disease progression varies greatly among ALS patients and may be hastened with advancing age, female gender, presence of bulbar features, and absence of a significant other.37,53,54
The average life span for a patient with limb-onset ALS is two to five years from diagnosis, whereas patients affected by the bulbar form usually succumb within six to 18 months.30,53 In patients who present with respiratory symptoms, Shoesmith et al24 have reported a mean time of 14.9 months from initial symptoms to need for full-time ventilation, and of 27.0 months from symptom onset to death.
Advance directives, end-of-life care, and respiratory and nutritional management become essential issues during late stages of ALS; thus, they should be discussed with patients and their relatives at the time of diagnosis or shortly thereafter.
TREATMENT
Pharmacologic Options
There are currently no treatments to halt the progression of ALS or to reverse the disease process. Riluzole, currently the only FDA-approved drug for treatment of ALS, has been shown to slow the loss of muscle strength and to prolong life by an average of two to three months.55 Riluzole targets and blocks glutamate transporters on the presynaptic neuron, decreasing glutamate release and reducing excitotoxicity20 (ie, overstimulation of the postsynaptic receptors). These effects support the theory that ALS may result from excess glutamate and help to explain the increased levels of glutamate found in the serum and CSF of ALS patients.19
Riluzole is typically dosed at 50 mg by mouth twice a day, although 200-mg/d doses have also been examined in clinical trials.20,55 Generally, the drug is well tolerated, with common adverse effects including asthenia, nausea, gastrointestinal upset, and abnormal liver test results. Liver function should be monitored regularly during riluzole therapy, with elevations in serum alanine transferase of particular concern.55
Additional agents have been used to reduce muscle spasticity, muscle cramping and fasciculations, and the associated pain some patients experience, attributable in part to lack of activity and/or inflammation.56 For spasticity, tizanidine or baclofen (orally, a maximum of 20 mg in divided doses; or lower doses administered intrathecally, for patients who experience sedation and fatigue with high oral doses56,57) are often used. Carbamazepine and phenytoin are most commonly used to relieve muscle cramps.56 For some patients, NSAIDs may be adequate to control moderate to severe pain, but others may require opioids.2,56,58
Depressive disorders must be identified accurately, using appropriate clinical tools, before SSRIs (eg, citalopram) or other medications (eg, amitriptyline) are prescribed3; these agents should not be used presumptively,37 as estimates of prevalence of depression among ALS patients range from 2% to 75%.2,37,38,54 Estimates of anxiety prevalence in these patients range from 0% to 30%, reflecting the importance of accurate diagnosis before lorazepam or other agents are prescribed.3,38
Promising results have been reported in the use of modafinil to manage fatigue in patients with ALS.59 For patients with pseudobulbar affect, dextromethorphan 20 mg/quinidine sulfate 10 mg is an FDA-approved treatment.35
Vitamins and other supplements, including creatine, vitamin E, coenzyme Q10, and acetylcysteine, have not been shown to improve survival in this patient population.3
Nonpharmacologic Interventions
Supportive measures for ALS include physical and occupational therapy. In speech and language therapy, breathing and relaxation patterns can be used to correct ineffective compensatory behaviors and help patients “economize” their speaking efforts.60
Nutritional support is also important in patients who have difficulty swallowing, although this can be alleviated somewhat by changes in posture (eg, lowering the chin before attempting to swallow) and by use of thickened fluids; those with immobility of the tongue may find swallowing easier with the head tilted back.60 Once oral alimentation is no longer possible, enteral tube feeding is an option that may prolong survival.60,61
As ALS progresses, dysphagia may be aggravated as the ability to cough, reflexively or voluntarily, is reduced.60 As breathing becomes increasingly difficult, patients may require respiratory support. Noninvasive ventilation, using either continuous or bilevel positive airway pressure, may be implemented early in patients with respiratory-onset ALS, and later in the disease process for other patients, to prevent apnea and hypoventilation.28,62 Mechanical ventilation via tracheostomy is the most invasive method to address respiratory dysfunction in patients with ALS; however, like noninvasive ventilation, nocturnal mechanical ventilation has been shown to extend survival in these patients.28,63,64
Diaphragm pacing stimulation (DPS) has emerged as a possible alternative to mechanical ventilation for ALS patients. The pacing system consists of a battery-operated external pulse generator with electrodes placed after laparoscopic mapping on the diaphragm.65 Natural respiration is mimicked as stimulation from the external pulse generator prompts the diaphragm to contract. Researchers have shown that the minimally invasive surgery (including use of general anesthesia) required to install the DPS system can be safely performed on patients with ALS, and its use can delay the need for a ventilator by 24 months.65,66 Use of the DPS device was granted FDA approval in 2011, under the Humanitarian Device Exemption program.67
Addressing Quality of Life
For the patient with ALS, quality of life is an important consideration throughout disease management. According to numerous research teams, quality of life for these patients depends less on physical function and strength and more on social relationships, existential issues, and spirituality.2,37,54 A high level of quality of life can be sustained in patients with ALS, despite the decline they experience in physical function.
Addressing depression and anxiety by nonpharmacologic means may be needed. Loss of physical strength and mobility and difficulties with speech, swallowing, and breathing can challenge even the strongest patient’s coping skills; even more difficult can be the increased dependence on caregivers, the loss of income, and the financial burdens incurred in health care–related expenses. In some patients, the severity of disease, the lack of effective treatments, and the loss of independence may trigger thoughts of suicide or the wish to “hasten death.”37,39 The importance of counseling, support groups, spirituality or religion, and palliative care, from early in the disease process, cannot be overstated.3,37
Some of these considerations may also be of benefit to spouses and other nonpaid caregivers of the patient with ALS, at least half of whom report feeling physically or psychologically unwell.37,68 Even when professional nursing services or hospice support are available, caregivers often devote 12 hours or more per day to nonprofessional patient care.69 Strategies that support the caregiver can reduce the patient’s perception of burden in that individual.37
CONCLUSION
The exact cause or causes of ALS remain unknown, making it difficult to predict who will present with a disease that appears impossible to prevent. Health care providers in any practice should be aware of the signs of ALS and familiar with its symptoms in order to provide optimal management for potentially affected patients.
Any cause for suspicion of ALS (ie, recent-onset limb weakness or atrophy; difficulty swallowing or speaking) warrants immediate patient referral to a neurologist. Evaluation should include a comprehensive history and physical examination, with emphasis on the musculoskeletal and neurologic exams; MRI of the spine and head, analysis of blood and CSF samples, EMG, and nerve conduction studies should be used to rule out treatable causes of limb weakness.
Potential complications of ALS, including nutritional deficiency, respiratory compromise, and depression, should be discussed early with patients and their caregivers. Management of the patient with ALS necessitates a multidisciplinary approach involving providers from several specialties to ensure that the many issues associated with this disease are being addressed. The priority of the health care provider should be to extend the patient’s survival while maintaining quality of life.
1. ALS Association. Facts you should know. www.alsa.org/about-als/facts-you-should-know.html. Accessed May 30, 2012.
2. Simmons Z. Management strategies for patients with amyotrophic lateral sclerosis from diagnosis through death. Neurologist. 2005;11(5):257-270.
3. Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3.
4. Yoshida M. Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology. 2004;24(1):87-102.
5. Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(6):623-627.
6. National Institute of Neurological Disorders and Stroke, NIH. Motor neuron diseases fact sheet. www.ninds.nih.gov/disorders/motor_neuron_diseases/detail_motor_neuron_diseases.htm. Accessed May 30, 2012.
7. Tartaglia MC, Rowe A, Findlater K. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007;64(2):232-236.
8. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
9. Beghi E, Logroscino G, Chiò A, et al; EURALS Consortium. The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta. 2006;1762(11-12):1150-1157.
10. McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7(6):557-570.
11. Weisskopf MG, Morozova N, O’Reilly EJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):558-561.
12. ALS Association. ALS in the military: unexpected consequences of military service (2011). www.alsa.org/assets/pdfs/advocacy/als_military_paper.pdf. Accessed May 22, 2012.
13. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749.
14. Horner RD, Grambow SC, Coffman DJ, et al. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: evidence for a time-limited outbreak. Neuroepidemiology. 2008;31(1):28-32.
15. Wang H, O’Reilly EJ, Weisskopf MG, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68(2):207-213.
16. Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70(21):1984-1990.
17. Vance C, Rogelj B, Hortobágyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208-1211.
18. Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668-1672.
19. Pioro EP, Majors AAW, Mitsumoto H, et al. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology. 1999;53(1):71-79.
20. Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17(18):1942-1959.
21. Paubel A, Violette J, Amy M, et al. Mutations of the ANG gene in French patients with sporadic amyotrophic lateral sclerosis. Arch Neurol. 2008;65(10):1333-1336.
22. Neusch C, Bähr M, Schneider-Gold C. Glia cells in amyotrophic lateral sclerosis: new clues to understanding an old disease? Muscle Nerve. 2007;35(6):712-724.
23. Rowland LP. Progressive muscular atrophy and other lower motor neuron syndromes of adults. Muscle Nerve. 2010;41(12):161-165.
24. Shoesmith CL, Findlater K, Rowe A, Strong MJ. Progression of amyotrophic lateral sclerosis with respiratory onset. J Neurol Neurosurg Psychiatry. 2007;78(6):629-631.
25. Traynor BJ, Codd MB, Corr B, et al. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population-based study. Arch Neurol. 2000;57(8):1171-1176.
26. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994-1003.
27. Chen R, Grand’Maison F, Strong MJ, et al. Motor neuron disease presenting as acute respiratory failure: a clinical and pathological study. J Neurol Neurosurg Psychiatry. 1996;60(4):455-458.
28. Gautier G, Verscheuren A, Monnier A, et al. ALS with respiratory onset: clinical features and effects of non-invasive ventilation on the prognosis. Amyotroph Lateral Scler. 2010;11(4):379-382.
29. Hasan A, Saxena AB, Ahmed SM, Swamy TLN. Amyotrophic lateral sclerosis presenting with orthopnea in a patient with COPD and obstructive sleep apnea. J Med All Sci. 2011;1(1):46-49.
30. Zoccolella S, Beghi E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2008;79(1):33-37.
31. Brown RH Jr. Amyotrophic lateral sclerosis and other motor neuron diseases. In: Fauci AS, Braunwald E, Kasper DL, ed al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2572-2576.
32. Ince PG, Lowe J, Shaw PJ. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol. 1998;24(2):104-117.
33. Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;73(1):25-31.
34. Shimizu T, Komori T, Kato S, et al. Masseter inhibitory reflex in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2(4):189-195.
35. Rosen H. Dextromethorphan/quinidine sulfate (Zenvia) for pseudobulbar affect. Drugs Today (Barc). 2008;44(9):661-668.
36. Rippon GA, Scarmeas N, Gordon PH, et al. An observational study of cognitive impairment in amyotrophic lateral sclerosis. Arch Neurol. 2006;63(3):345-352.
37. Rabkin JG, Albert SM, Del Bene ML, et al. Prevalence of depressive disorders and change over time in late-stage ALS. Neurology. 2005;65(1):62-67.
38. Vignola A, Guzzo A, Calvo A, et al. Anxiety undermines quality of life in ALS patients and caregivers. Eur J Neurol. 2008;15(11):1231-1236.
39. Fang F, Valdimarsdóttir U, Fürst CJ, et al. Suicide among patients with amyotrophic lateral sclerosis. Brain. 2008;131(pt 10):2729-2733.
40. Verstraete E, Veldink JH, Hendrikse J, et al. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(4):383-388.
41. Turner MR, Modo M. Advances in the application of MRI to amyotrophic lateral sclerosis. Expert Opin Med Diagn. 2010;4(6):483-496.
42. Cirillo M, Esposito F, Tedeschi G, et al. Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol. 2012 Feb 2. [Epub ahead of print]
43. Canu E, Agosta F, Riva N, et al. The topography of brain microstructural damage in amyotrophic lateral sclerosis assessed using diffusion tensor MR imaging. AJNR Am J Neuroradiol. 2011;32(7):1307-1314.
44. Baek WS, Desai NP. ALS: pitfalls in the diagnosis. Pract Neurol. 2007;7(2):74-81.
45. ALS Association. Criteria for the diagnosis of ALS. www.alsa.org/als-care/resources/publica tions-videos/factsheets/criteria-for-diagnosis.html. Accessed May 31, 2012.
46. de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497-503.
47. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299.
48. Waragai M. MRI and clinical features of amyotrophic lateral sclerosis. Neuroradiology. 1997;39(12):847-851.
49. Thorpe JW, Moseley IF, Hawkes CH, et al. Brain and spinal cord MRI in motor neuron disease. J Neurol Neurosurg Psychiatry. 1996; 61(3):314-317.
50. Oba H, Araki T, Ohtomo K, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology. 1993;189 (3):843-846.
51. Piao YS, Wakabayashi K, Kakita A, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;13(1):10-22.
52. Mateen FJ, Carone M, Sorenson EJ. Patients who survive 5 years or more with ALS in Olmsted County, 1925-2004. J Neurol Neurosurg Psychiatry. 2010;81(10):1144-1146.
53. del Aguila MA, Longstreth WT Jr, McGuire V, et al. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813-819.
54. Matuz T, Birbaumer N, Hautzinger M, Kübler A. Coping with amyotrophic lateral sclerosis: an integrative view. J Neurol Neurosurg Psychiatry. 2010;81(8):893-898.
55. Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012 Mar 14;3:CD001447.
56. Handy CR, Krudy C, Boulis N, Federici T. Pain in amyotrophic lateral sclerosis: a neglected aspect of disease. Neurol Res Int. 2011; 2011:403808. Epub 2011 May 3.
57. McClelland S 3rd, Bethoux FA, Boulis NM, et al. Intrathecal baclofen for spasticity-related pain in amyotrophic lateral sclerosis: efficacy and factors associated with pain relief. Muscle Nerve. 2008;37(3):396-398.
58. Andersen PM, Borasio GD, Dengler R, et al; EALSC Working Group. Good practice in the management of amyotrophic lateral sclerosis: clinical guidelines. Amyotroph Lateral Scler. 2007;8(4):195-213.
59. Rabkin JG, Gordon PH, McElhiney M, et al. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle Nerve. 2009;39(3):297-303.
60. Kühnlein P, Gdynia HJ, Sperfeld AD, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366-374.
61. Katzberg HD, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD004030.
62. Lechtzin N, Scott Y, Busse AM, et al. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler. 2007;8(3):185-188.
63. Annane D, Orlikowski D, Chevret S, et al. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD001941.
64. Radunovic A, Annane D, Jewitt K, Mustfa N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD004427.
65. Onders RP, Elmo MJ, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23(7):1433-1440.
66. Onders RP, Carlin AM, Elmo M, et al. Amyotrophic lateral sclerosis: the Midwestern surgical experience with the diaphragm pacing stimulation system shows that general anesthesia can be safely performed. Am J Surg. 2009;197(3):386-390.
67. US Food and Drug Administration. Medical devices: NeuRx Diaphragm Pacing System™—H100006. www.fda.gov/MedicalDevices/Prod uctsandMedicalProcedures/DeviceApprovalsand Clearances/Recently-ApprovedDevices/ucm278684.htm. Accessed May 31, 2012.
68. Rabkin JG, Wagner GJ, Del Bene M. Resilience and distress among amyotrophic lateral sclerosis patients and caregivers. Psychosom Med. 2000; 62(2):271-279.
69. Chiò A, Gauthier A, Vignola A, et al. Caregiver time use in ALS. Neurology. 2006;67(5):902-904.
Amyotrophic lateral sclerosis (ALS) is probably best known to the majority of the US population as Lou Gehrig’s disease, named for the New York Yankee who died of ALS after a stellar 17-year baseball career in the 1920s and ’30s. Gradually, the general public is gaining familiarity with the characteristics of ALS, a disease with no single identifiable cause and no known cure.
About 5,600 people receive a diagnosis of ALS in the US each year (about 15 per day), making it the most common form of motor neuron disease.1 Most patients present with progressive muscle weakness in an extremity, which ultimately leads to respiratory failure and death.
Generally, patients with ALS have no cognitive disability and are aware of their physical decline. These patients are faced with important decisions as their condition worsens: Will they want nutritional support through a gastrostomy? For respiratory assistance, will their preference be noninvasive ventilation or mechanical ventilation via tracheostomy?
Not only do ALS patients undergo deterioration of motor function, but many experience muscle cramping, generalized pain, and depression. Only one medication is currently approved to treat ALS patients, with the benefit of prolonging life by a few months. In addition to symptom management, supportive measures, including physical, occupational, and speech therapy, exercise, nutrition, support groups, and counseling, are important tools to enhance quality of life for the patient with ALS.2
Primary care providers need to be aware of ALS, recognize its symptoms in their patients, and manage those affected on a case-by-case basis. Because of the challenges in diagnostic evaluation, the rapidly evolving nature of the disease, and the dire prognosis of ALS, any patient suspected of having ALS should be referred immediately to a neurologist. Providers need to educate patients and their caregivers regarding the disease process and ensure that patients receive appropriate care to meet their needs and preferences.
ALS DEFINED
ALS is a progressive neurodegenerative disease that affects both the upper and lower motor neurons. The disease is considered terminal. Although life may be prolonged by the one currently available pharmacologic agent, no treatment option is yet capable of stopping or reversing progression of the disease.3 While ALS was once believed to be a purely motor disorder, the accompanying degeneration of nonmotor brain regions, such as frontal and temporal cortical neurons, is considered by some to be part of the clinicopathologic spectrum of ALS.4
There are two forms of ALS: sporadic and familial. Sporadic ALS is by far the more common, accounting for 90% to 95% of cases. The remaining 5% to 10% of ALS cases are of the familial form, which can be autosomal-dominant or autosomal-recessive.5
The Degenerative Motor Neuron Diseases
Weakness and muscle wasting characterize several degenerative motor neuron diseases. In addition to ALS, these include primary lateral sclerosis, progressive muscular atrophy, progressive bulbar palsy, and pseudobulbar palsy.6
Primary lateral sclerosis involves upper motor neuron (UMN) dysfunction in the limbs.7Progressive muscular atrophy results from degeneration of the anterior horn cells in the spinal cord and is associated with lower motor neuron (LMN) deficits in the limbs.
Progressive bulbar palsy is a progressive UMN and LMN disorder of the cranial muscles. This condition may occasionally stay isolated in the bulbar segment, but more commonly, UMN and LMN signs and symptoms spread to involve other segments. This is then referred to as bulbar-onset ALS. There have been no reports of specific pathology in progressive bulbar palsy.
Pseudobulbar palsy results from an UMN lesion in the corticobulbar pathway in the pyramidal tract. It is characterized by difficulty chewing and swallowing, and slurred speech (manifestations that may also represent the initial presentation of ALS). Patients with pseudobulbar palsy may exhibit inappropriate, excessive yawning and emotional outbursts; these manifestations are referred to as emotional incontinence.8
EPIDEMIOLOGY
In North America, it is estimated that ALS affects 1.5 to 2.7 people per 100,000 between ages 20 and 80; most frequently, the disease presents between ages 55 and 65. ALS develops infrequently before age 30.3,9
While no gender difference is apparent in patients with familial ALS, sporadic ALS predominately affects males more than females (although the accepted ratio of 1.5:1 appears to be in decline9). After age 65, men and women are equally impacted.10
RISK FACTORS
Established risk factors for ALS include age and family history. Accumulating evidence suggests that military service and smoking may also contribute to the development of ALS.11-15 Children and siblings of ALS patients are at increased risk for ALS, while military personnel have 1.5 times the risk.11
Investigation of the precise link between military service and ALS is ongoing, but factors may include intense exertion, traumatic injuries, viral infections, and exposure to certain chemicals or metals.11 Research suggests the risk is independent of time period, years of service, or branch of service. Geographic location appears to be an independent factor, although there does seem to be a strong association between deployment during the 1991 Gulf War and the risk for ALS.12-14
Recently, smoking has been implicated as a potential risk factor in the disease.3,15 Certain environmental exposures have also been recognized as a possible risk factor. In Guam, an ALS-like syndrome has been identified among members of the Chamorro tribe. This syndrome has been linked to a neurotoxin in the seed of the cycad nut, a tropical plant endemic to the area, which was used in the 1950s and 1960s in the human food supply.3,16
ETIOLOGY
Familial ALS is a genetically transmitted degenerative disease. Twenty percent of cases involve the long arm of chromosome 21, which is responsible for coding of superoxide dismutase (SOD1). Mutations in SOD1, an RNA-processing protein called FUS, and the DNA-binding protein TDP-43, have all been identified in cases of familial ALS.17,18
The exact etiology of sporadic ALS remains unknown, but gene mutations have also been implicated, including the ANG gene21 and TDP-43.18 Other theories include increased levels of glutamate (which have been detected in cerebrospinal fluid and serum of patients with ALS), mitochondrial dysfunction, free radical injury, programmed cell death, neurofilament defects, viral infections, and autoimmune dysfunction19,20 (see Table 13,17,18,20,21). It is possible that a combination of factors is involved in the development of sporadic ALS.
PATHOPHYSIOLOGY
The pathophysiology of ALS involves degeneration of the UMN and LMN axons, which leads to glial scarring and possible impairment of the glial cells’ ability to store excess glutamate.22 ALS affects the central nervous system, specifically the anterior horn cells in the spinal cord and the cranial nerve nuclei (X, XI, XII) of the LMNs, and the corticospinal tract and corticobulbar pathway of the UMNs. Bulbar and limb muscles innervated by LMNs are subject to atrophy, whereas cognition, coordination, sensation, the oculomotor system, and sphincters are typically spared.3
CLINICAL FEATURES
In most patients, ALS symptoms characterize either limb onset or bulbar onset (see Table 23,7,23,24), with limb onset being the more common (about 75% vs about 25% of cases, respectively).1 Typically, patients with limb-onset ALS complain of rapidly progressive, asymmetric weakness in an extremity, followed by focal muscle atrophy with cramping and fasciculations, and eventually, spasticity. Weakness generally begins in one hand, arm, foot, or leg. Patients may notice increased episodes of tripping, clumsiness when they run or walk, a “dropped foot” gait, and/or a decline in manual dexterity.25
The weakness often develops insidiously; patients may notice that symptoms are exacerbated by cold weather.3 Eventually, the bulbar muscles are affected, resulting in dysphagia, dysarthria, and dysphasia. Occasionally, patients encounter bladder dysfunction (urgent micturition), sensory symptoms, and cognitive symptoms (eg, dementia, parkinsonism).26 Multisystem involvement is possible. Ultimately, respiratory compromise or other pulmonary complications ensue, representing a primary cause of mortality in ALS patients.3,24
In bulbar-onset ALS, patients first notice symptoms of dysphasia and dysphagia. They may complain of slurred speech, nasal or low-volume speech, and/or inhibited tongue mobility. The risk for aspiration is increased. The majority of patients with bulbar-onset ALS experience sialorrhea (excessive drooling) because they have difficulty swallowing their saliva. In most patients, mild UMN-type bilateral facial weakness affects the lower half of the face.3 As is the case with limb-onset ALS, bulbar-onset ALS progresses to respiratory compromise.27
In less than 3% of patients with ALS, presentation begins with respiratory weakness and no significant limb or bulbar symptoms.24,28 Patients with respiratory-onset ALS experience symptoms associated with nocturnal hypoventilation, including daytime hypersomnolence, morning headaches, impaired concentration, irritability, anorexia, mood changes, dyspnea, orthopnea, and disturbed sleep; or they may experience type 2 respiratory failure.28,29
Patients with axial symptoms of ALS present with neck weakness and may complain of posterior neck pain or strain with a gradually worsening tendency of the head to tip forward. These patients often support the chin with one hand. Those with axial truncal weakness often complain of difficulty maintaining erect posture when standing and of stooping as they walk. Some patients support the trunk by placing their hands in their front pants pockets or on their upper thighs. They may report some relief when pushing a grocery cart.7,23,24
Symptoms of ALS can be present for weeks or months before a patient consults with a health care provider. The average time span from onset of initial symptoms to diagnosis of ALS is about one year.1 Due to the unpredictable pattern of progression and variability of symptoms among patients, it is difficult to approximate a time frame for symptom progression; for some patients, the disease progresses slowly, while others deteriorate rapidly.30
PHYSICAL EXAMINATION FINDINGS
At onset, the typical presentation of ALS includes muscle weakness in one limb as well as visible fasciculations. As the disease progresses, focal wasting of muscle groups occurs in all four extremities. Particularly involved are the muscles of the hands, forearms, or shoulders in the upper limbs; and of the proximal thigh or distal foot muscles in the lower limbs.31 Deep tendon reflexes are symmetrically brisk. Spasticity, evident in the upper limbs, may present as increased tone.32
In patients with bulbar dysfunction, dysarthria may arise from either LMN pathology or pseudobulbar palsy caused by a UMN disorder, leading to slow, slurred speech or speech with a nasal quality. Tongue fasciculations will be present, as will atrophy and diminished mobility of the tongue.33 The gag reflex remains intact, even brisk, but weakness may occur in the muscles of the soft palate.3 Facial weakness is sometimes seen late in the disease, as evidenced by difficulty sealing the lips or puffing out the cheeks. The jaw jerk will be brisk, indicating that cranial nerve V is intact.34
A pseudobulbar affect, which is best described as emotional lability, may be present. The patient may have a history of exaggerated expression of emotion, such as uncontrollable crying, laughing, or both.35 Cognition, coordination, sensation, the oculomotor system, and sphincters are generally spared. However, cases of frontotemporal dementias coexisting with ALS have been reported; affected patients exhibit cognitive impairment, compulsive behaviors, and personality changes, and they may experience shorter survival.4,24,36
Evidence of known complications of ALS may also be noted during the physical examination; see Table 3.37-39
DIAGNOSIS OF ALS
In current research, use of structural MRI, magnetic resonance spectroscopy, and diffusion tensor imaging is being examined to detect thinning in the primary motor cortex, fractional anisotropy in the corpus callosum, patterns of gray and white matter atrophy, and other proposed diagnostic markers for ALS.40-43 However, no single specific diagnostic test has yet been proven to identify ALS; rather, it remains a disease of exclusion (see Table 43,44). For a confirmed diagnosis of ALS to be made, the patient must display:
- Evidence of LMN degeneration as found through clinical, neuropathologic, or electrophysiological examination
- UMN degeneration detected by clinical examination, and
- Progressive spread of signs or symptoms within a region or to other regions.3,45-47
Other disease processes that might explain the signs of UMN and/or LMN degeneration must be excluded, such as cervical spinal disease, myasthenia gravis, multifocal motor neuropathy, lead intoxication, and Lyme disease.45
Imaging studies are not required in ALS cases that have clinically definite disease with bulbar or pseudobulbar onset.47 Otherwise, the essential role of neuroimaging is to exclude a treatable structural lesion that may mimic ALS by producing UMN and LMN signs in varying degrees.48
Typically, MRI of the head and spine is ordered in patients with suspected ALS (see figure); MRI can reveal lesions in the corticospinal tracts that occur in ALS. The most characteristic finding on T2-weighted MRI is hyperintensity of the corticospinal tracts, which is visualized best in the brain and brainstem, and to a lesser extent in the spinal cord.49 Decreased signal intensity in the motor cortex has been reported on MRI in cases of ALS.50
Additional diagnostic procedures that are useful in excluding other disease processes include blood and cerebrospinal fluid (CSF) samples,3,44 four-limb electromyography (EMG),44 nerve conduction studies, motor unit number estimations, and muscle biopsies.47
- Autopsy results of patients with ALS demonstrate:
- Neuron loss, especially in lumbar and cervical enlargements
- Nonexistent or atrophic neurons in the motor nuclei of the pons and medulla, and in the anterior horn cells of the spinal cord
- Degeneration of the lateral columns of the spinal cord, and
- Atrophy of the ventral roots.31,51
PROGNOSIS
The overall five-year survival rate for patients with ALS has been reported between 7% and 14%,52,53 and the mortality rate rises in patients older than 75 and in those with bulbar signs.30 The rate of disease progression varies greatly among ALS patients and may be hastened with advancing age, female gender, presence of bulbar features, and absence of a significant other.37,53,54
The average life span for a patient with limb-onset ALS is two to five years from diagnosis, whereas patients affected by the bulbar form usually succumb within six to 18 months.30,53 In patients who present with respiratory symptoms, Shoesmith et al24 have reported a mean time of 14.9 months from initial symptoms to need for full-time ventilation, and of 27.0 months from symptom onset to death.
Advance directives, end-of-life care, and respiratory and nutritional management become essential issues during late stages of ALS; thus, they should be discussed with patients and their relatives at the time of diagnosis or shortly thereafter.
TREATMENT
Pharmacologic Options
There are currently no treatments to halt the progression of ALS or to reverse the disease process. Riluzole, currently the only FDA-approved drug for treatment of ALS, has been shown to slow the loss of muscle strength and to prolong life by an average of two to three months.55 Riluzole targets and blocks glutamate transporters on the presynaptic neuron, decreasing glutamate release and reducing excitotoxicity20 (ie, overstimulation of the postsynaptic receptors). These effects support the theory that ALS may result from excess glutamate and help to explain the increased levels of glutamate found in the serum and CSF of ALS patients.19
Riluzole is typically dosed at 50 mg by mouth twice a day, although 200-mg/d doses have also been examined in clinical trials.20,55 Generally, the drug is well tolerated, with common adverse effects including asthenia, nausea, gastrointestinal upset, and abnormal liver test results. Liver function should be monitored regularly during riluzole therapy, with elevations in serum alanine transferase of particular concern.55
Additional agents have been used to reduce muscle spasticity, muscle cramping and fasciculations, and the associated pain some patients experience, attributable in part to lack of activity and/or inflammation.56 For spasticity, tizanidine or baclofen (orally, a maximum of 20 mg in divided doses; or lower doses administered intrathecally, for patients who experience sedation and fatigue with high oral doses56,57) are often used. Carbamazepine and phenytoin are most commonly used to relieve muscle cramps.56 For some patients, NSAIDs may be adequate to control moderate to severe pain, but others may require opioids.2,56,58
Depressive disorders must be identified accurately, using appropriate clinical tools, before SSRIs (eg, citalopram) or other medications (eg, amitriptyline) are prescribed3; these agents should not be used presumptively,37 as estimates of prevalence of depression among ALS patients range from 2% to 75%.2,37,38,54 Estimates of anxiety prevalence in these patients range from 0% to 30%, reflecting the importance of accurate diagnosis before lorazepam or other agents are prescribed.3,38
Promising results have been reported in the use of modafinil to manage fatigue in patients with ALS.59 For patients with pseudobulbar affect, dextromethorphan 20 mg/quinidine sulfate 10 mg is an FDA-approved treatment.35
Vitamins and other supplements, including creatine, vitamin E, coenzyme Q10, and acetylcysteine, have not been shown to improve survival in this patient population.3
Nonpharmacologic Interventions
Supportive measures for ALS include physical and occupational therapy. In speech and language therapy, breathing and relaxation patterns can be used to correct ineffective compensatory behaviors and help patients “economize” their speaking efforts.60
Nutritional support is also important in patients who have difficulty swallowing, although this can be alleviated somewhat by changes in posture (eg, lowering the chin before attempting to swallow) and by use of thickened fluids; those with immobility of the tongue may find swallowing easier with the head tilted back.60 Once oral alimentation is no longer possible, enteral tube feeding is an option that may prolong survival.60,61
As ALS progresses, dysphagia may be aggravated as the ability to cough, reflexively or voluntarily, is reduced.60 As breathing becomes increasingly difficult, patients may require respiratory support. Noninvasive ventilation, using either continuous or bilevel positive airway pressure, may be implemented early in patients with respiratory-onset ALS, and later in the disease process for other patients, to prevent apnea and hypoventilation.28,62 Mechanical ventilation via tracheostomy is the most invasive method to address respiratory dysfunction in patients with ALS; however, like noninvasive ventilation, nocturnal mechanical ventilation has been shown to extend survival in these patients.28,63,64
Diaphragm pacing stimulation (DPS) has emerged as a possible alternative to mechanical ventilation for ALS patients. The pacing system consists of a battery-operated external pulse generator with electrodes placed after laparoscopic mapping on the diaphragm.65 Natural respiration is mimicked as stimulation from the external pulse generator prompts the diaphragm to contract. Researchers have shown that the minimally invasive surgery (including use of general anesthesia) required to install the DPS system can be safely performed on patients with ALS, and its use can delay the need for a ventilator by 24 months.65,66 Use of the DPS device was granted FDA approval in 2011, under the Humanitarian Device Exemption program.67
Addressing Quality of Life
For the patient with ALS, quality of life is an important consideration throughout disease management. According to numerous research teams, quality of life for these patients depends less on physical function and strength and more on social relationships, existential issues, and spirituality.2,37,54 A high level of quality of life can be sustained in patients with ALS, despite the decline they experience in physical function.
Addressing depression and anxiety by nonpharmacologic means may be needed. Loss of physical strength and mobility and difficulties with speech, swallowing, and breathing can challenge even the strongest patient’s coping skills; even more difficult can be the increased dependence on caregivers, the loss of income, and the financial burdens incurred in health care–related expenses. In some patients, the severity of disease, the lack of effective treatments, and the loss of independence may trigger thoughts of suicide or the wish to “hasten death.”37,39 The importance of counseling, support groups, spirituality or religion, and palliative care, from early in the disease process, cannot be overstated.3,37
Some of these considerations may also be of benefit to spouses and other nonpaid caregivers of the patient with ALS, at least half of whom report feeling physically or psychologically unwell.37,68 Even when professional nursing services or hospice support are available, caregivers often devote 12 hours or more per day to nonprofessional patient care.69 Strategies that support the caregiver can reduce the patient’s perception of burden in that individual.37
CONCLUSION
The exact cause or causes of ALS remain unknown, making it difficult to predict who will present with a disease that appears impossible to prevent. Health care providers in any practice should be aware of the signs of ALS and familiar with its symptoms in order to provide optimal management for potentially affected patients.
Any cause for suspicion of ALS (ie, recent-onset limb weakness or atrophy; difficulty swallowing or speaking) warrants immediate patient referral to a neurologist. Evaluation should include a comprehensive history and physical examination, with emphasis on the musculoskeletal and neurologic exams; MRI of the spine and head, analysis of blood and CSF samples, EMG, and nerve conduction studies should be used to rule out treatable causes of limb weakness.
Potential complications of ALS, including nutritional deficiency, respiratory compromise, and depression, should be discussed early with patients and their caregivers. Management of the patient with ALS necessitates a multidisciplinary approach involving providers from several specialties to ensure that the many issues associated with this disease are being addressed. The priority of the health care provider should be to extend the patient’s survival while maintaining quality of life.
Amyotrophic lateral sclerosis (ALS) is probably best known to the majority of the US population as Lou Gehrig’s disease, named for the New York Yankee who died of ALS after a stellar 17-year baseball career in the 1920s and ’30s. Gradually, the general public is gaining familiarity with the characteristics of ALS, a disease with no single identifiable cause and no known cure.
About 5,600 people receive a diagnosis of ALS in the US each year (about 15 per day), making it the most common form of motor neuron disease.1 Most patients present with progressive muscle weakness in an extremity, which ultimately leads to respiratory failure and death.
Generally, patients with ALS have no cognitive disability and are aware of their physical decline. These patients are faced with important decisions as their condition worsens: Will they want nutritional support through a gastrostomy? For respiratory assistance, will their preference be noninvasive ventilation or mechanical ventilation via tracheostomy?
Not only do ALS patients undergo deterioration of motor function, but many experience muscle cramping, generalized pain, and depression. Only one medication is currently approved to treat ALS patients, with the benefit of prolonging life by a few months. In addition to symptom management, supportive measures, including physical, occupational, and speech therapy, exercise, nutrition, support groups, and counseling, are important tools to enhance quality of life for the patient with ALS.2
Primary care providers need to be aware of ALS, recognize its symptoms in their patients, and manage those affected on a case-by-case basis. Because of the challenges in diagnostic evaluation, the rapidly evolving nature of the disease, and the dire prognosis of ALS, any patient suspected of having ALS should be referred immediately to a neurologist. Providers need to educate patients and their caregivers regarding the disease process and ensure that patients receive appropriate care to meet their needs and preferences.
ALS DEFINED
ALS is a progressive neurodegenerative disease that affects both the upper and lower motor neurons. The disease is considered terminal. Although life may be prolonged by the one currently available pharmacologic agent, no treatment option is yet capable of stopping or reversing progression of the disease.3 While ALS was once believed to be a purely motor disorder, the accompanying degeneration of nonmotor brain regions, such as frontal and temporal cortical neurons, is considered by some to be part of the clinicopathologic spectrum of ALS.4
There are two forms of ALS: sporadic and familial. Sporadic ALS is by far the more common, accounting for 90% to 95% of cases. The remaining 5% to 10% of ALS cases are of the familial form, which can be autosomal-dominant or autosomal-recessive.5
The Degenerative Motor Neuron Diseases
Weakness and muscle wasting characterize several degenerative motor neuron diseases. In addition to ALS, these include primary lateral sclerosis, progressive muscular atrophy, progressive bulbar palsy, and pseudobulbar palsy.6
Primary lateral sclerosis involves upper motor neuron (UMN) dysfunction in the limbs.7Progressive muscular atrophy results from degeneration of the anterior horn cells in the spinal cord and is associated with lower motor neuron (LMN) deficits in the limbs.
Progressive bulbar palsy is a progressive UMN and LMN disorder of the cranial muscles. This condition may occasionally stay isolated in the bulbar segment, but more commonly, UMN and LMN signs and symptoms spread to involve other segments. This is then referred to as bulbar-onset ALS. There have been no reports of specific pathology in progressive bulbar palsy.
Pseudobulbar palsy results from an UMN lesion in the corticobulbar pathway in the pyramidal tract. It is characterized by difficulty chewing and swallowing, and slurred speech (manifestations that may also represent the initial presentation of ALS). Patients with pseudobulbar palsy may exhibit inappropriate, excessive yawning and emotional outbursts; these manifestations are referred to as emotional incontinence.8
EPIDEMIOLOGY
In North America, it is estimated that ALS affects 1.5 to 2.7 people per 100,000 between ages 20 and 80; most frequently, the disease presents between ages 55 and 65. ALS develops infrequently before age 30.3,9
While no gender difference is apparent in patients with familial ALS, sporadic ALS predominately affects males more than females (although the accepted ratio of 1.5:1 appears to be in decline9). After age 65, men and women are equally impacted.10
RISK FACTORS
Established risk factors for ALS include age and family history. Accumulating evidence suggests that military service and smoking may also contribute to the development of ALS.11-15 Children and siblings of ALS patients are at increased risk for ALS, while military personnel have 1.5 times the risk.11
Investigation of the precise link between military service and ALS is ongoing, but factors may include intense exertion, traumatic injuries, viral infections, and exposure to certain chemicals or metals.11 Research suggests the risk is independent of time period, years of service, or branch of service. Geographic location appears to be an independent factor, although there does seem to be a strong association between deployment during the 1991 Gulf War and the risk for ALS.12-14
Recently, smoking has been implicated as a potential risk factor in the disease.3,15 Certain environmental exposures have also been recognized as a possible risk factor. In Guam, an ALS-like syndrome has been identified among members of the Chamorro tribe. This syndrome has been linked to a neurotoxin in the seed of the cycad nut, a tropical plant endemic to the area, which was used in the 1950s and 1960s in the human food supply.3,16
ETIOLOGY
Familial ALS is a genetically transmitted degenerative disease. Twenty percent of cases involve the long arm of chromosome 21, which is responsible for coding of superoxide dismutase (SOD1). Mutations in SOD1, an RNA-processing protein called FUS, and the DNA-binding protein TDP-43, have all been identified in cases of familial ALS.17,18
The exact etiology of sporadic ALS remains unknown, but gene mutations have also been implicated, including the ANG gene21 and TDP-43.18 Other theories include increased levels of glutamate (which have been detected in cerebrospinal fluid and serum of patients with ALS), mitochondrial dysfunction, free radical injury, programmed cell death, neurofilament defects, viral infections, and autoimmune dysfunction19,20 (see Table 13,17,18,20,21). It is possible that a combination of factors is involved in the development of sporadic ALS.
PATHOPHYSIOLOGY
The pathophysiology of ALS involves degeneration of the UMN and LMN axons, which leads to glial scarring and possible impairment of the glial cells’ ability to store excess glutamate.22 ALS affects the central nervous system, specifically the anterior horn cells in the spinal cord and the cranial nerve nuclei (X, XI, XII) of the LMNs, and the corticospinal tract and corticobulbar pathway of the UMNs. Bulbar and limb muscles innervated by LMNs are subject to atrophy, whereas cognition, coordination, sensation, the oculomotor system, and sphincters are typically spared.3
CLINICAL FEATURES
In most patients, ALS symptoms characterize either limb onset or bulbar onset (see Table 23,7,23,24), with limb onset being the more common (about 75% vs about 25% of cases, respectively).1 Typically, patients with limb-onset ALS complain of rapidly progressive, asymmetric weakness in an extremity, followed by focal muscle atrophy with cramping and fasciculations, and eventually, spasticity. Weakness generally begins in one hand, arm, foot, or leg. Patients may notice increased episodes of tripping, clumsiness when they run or walk, a “dropped foot” gait, and/or a decline in manual dexterity.25
The weakness often develops insidiously; patients may notice that symptoms are exacerbated by cold weather.3 Eventually, the bulbar muscles are affected, resulting in dysphagia, dysarthria, and dysphasia. Occasionally, patients encounter bladder dysfunction (urgent micturition), sensory symptoms, and cognitive symptoms (eg, dementia, parkinsonism).26 Multisystem involvement is possible. Ultimately, respiratory compromise or other pulmonary complications ensue, representing a primary cause of mortality in ALS patients.3,24
In bulbar-onset ALS, patients first notice symptoms of dysphasia and dysphagia. They may complain of slurred speech, nasal or low-volume speech, and/or inhibited tongue mobility. The risk for aspiration is increased. The majority of patients with bulbar-onset ALS experience sialorrhea (excessive drooling) because they have difficulty swallowing their saliva. In most patients, mild UMN-type bilateral facial weakness affects the lower half of the face.3 As is the case with limb-onset ALS, bulbar-onset ALS progresses to respiratory compromise.27
In less than 3% of patients with ALS, presentation begins with respiratory weakness and no significant limb or bulbar symptoms.24,28 Patients with respiratory-onset ALS experience symptoms associated with nocturnal hypoventilation, including daytime hypersomnolence, morning headaches, impaired concentration, irritability, anorexia, mood changes, dyspnea, orthopnea, and disturbed sleep; or they may experience type 2 respiratory failure.28,29
Patients with axial symptoms of ALS present with neck weakness and may complain of posterior neck pain or strain with a gradually worsening tendency of the head to tip forward. These patients often support the chin with one hand. Those with axial truncal weakness often complain of difficulty maintaining erect posture when standing and of stooping as they walk. Some patients support the trunk by placing their hands in their front pants pockets or on their upper thighs. They may report some relief when pushing a grocery cart.7,23,24
Symptoms of ALS can be present for weeks or months before a patient consults with a health care provider. The average time span from onset of initial symptoms to diagnosis of ALS is about one year.1 Due to the unpredictable pattern of progression and variability of symptoms among patients, it is difficult to approximate a time frame for symptom progression; for some patients, the disease progresses slowly, while others deteriorate rapidly.30
PHYSICAL EXAMINATION FINDINGS
At onset, the typical presentation of ALS includes muscle weakness in one limb as well as visible fasciculations. As the disease progresses, focal wasting of muscle groups occurs in all four extremities. Particularly involved are the muscles of the hands, forearms, or shoulders in the upper limbs; and of the proximal thigh or distal foot muscles in the lower limbs.31 Deep tendon reflexes are symmetrically brisk. Spasticity, evident in the upper limbs, may present as increased tone.32
In patients with bulbar dysfunction, dysarthria may arise from either LMN pathology or pseudobulbar palsy caused by a UMN disorder, leading to slow, slurred speech or speech with a nasal quality. Tongue fasciculations will be present, as will atrophy and diminished mobility of the tongue.33 The gag reflex remains intact, even brisk, but weakness may occur in the muscles of the soft palate.3 Facial weakness is sometimes seen late in the disease, as evidenced by difficulty sealing the lips or puffing out the cheeks. The jaw jerk will be brisk, indicating that cranial nerve V is intact.34
A pseudobulbar affect, which is best described as emotional lability, may be present. The patient may have a history of exaggerated expression of emotion, such as uncontrollable crying, laughing, or both.35 Cognition, coordination, sensation, the oculomotor system, and sphincters are generally spared. However, cases of frontotemporal dementias coexisting with ALS have been reported; affected patients exhibit cognitive impairment, compulsive behaviors, and personality changes, and they may experience shorter survival.4,24,36
Evidence of known complications of ALS may also be noted during the physical examination; see Table 3.37-39
DIAGNOSIS OF ALS
In current research, use of structural MRI, magnetic resonance spectroscopy, and diffusion tensor imaging is being examined to detect thinning in the primary motor cortex, fractional anisotropy in the corpus callosum, patterns of gray and white matter atrophy, and other proposed diagnostic markers for ALS.40-43 However, no single specific diagnostic test has yet been proven to identify ALS; rather, it remains a disease of exclusion (see Table 43,44). For a confirmed diagnosis of ALS to be made, the patient must display:
- Evidence of LMN degeneration as found through clinical, neuropathologic, or electrophysiological examination
- UMN degeneration detected by clinical examination, and
- Progressive spread of signs or symptoms within a region or to other regions.3,45-47
Other disease processes that might explain the signs of UMN and/or LMN degeneration must be excluded, such as cervical spinal disease, myasthenia gravis, multifocal motor neuropathy, lead intoxication, and Lyme disease.45
Imaging studies are not required in ALS cases that have clinically definite disease with bulbar or pseudobulbar onset.47 Otherwise, the essential role of neuroimaging is to exclude a treatable structural lesion that may mimic ALS by producing UMN and LMN signs in varying degrees.48
Typically, MRI of the head and spine is ordered in patients with suspected ALS (see figure); MRI can reveal lesions in the corticospinal tracts that occur in ALS. The most characteristic finding on T2-weighted MRI is hyperintensity of the corticospinal tracts, which is visualized best in the brain and brainstem, and to a lesser extent in the spinal cord.49 Decreased signal intensity in the motor cortex has been reported on MRI in cases of ALS.50
Additional diagnostic procedures that are useful in excluding other disease processes include blood and cerebrospinal fluid (CSF) samples,3,44 four-limb electromyography (EMG),44 nerve conduction studies, motor unit number estimations, and muscle biopsies.47
- Autopsy results of patients with ALS demonstrate:
- Neuron loss, especially in lumbar and cervical enlargements
- Nonexistent or atrophic neurons in the motor nuclei of the pons and medulla, and in the anterior horn cells of the spinal cord
- Degeneration of the lateral columns of the spinal cord, and
- Atrophy of the ventral roots.31,51
PROGNOSIS
The overall five-year survival rate for patients with ALS has been reported between 7% and 14%,52,53 and the mortality rate rises in patients older than 75 and in those with bulbar signs.30 The rate of disease progression varies greatly among ALS patients and may be hastened with advancing age, female gender, presence of bulbar features, and absence of a significant other.37,53,54
The average life span for a patient with limb-onset ALS is two to five years from diagnosis, whereas patients affected by the bulbar form usually succumb within six to 18 months.30,53 In patients who present with respiratory symptoms, Shoesmith et al24 have reported a mean time of 14.9 months from initial symptoms to need for full-time ventilation, and of 27.0 months from symptom onset to death.
Advance directives, end-of-life care, and respiratory and nutritional management become essential issues during late stages of ALS; thus, they should be discussed with patients and their relatives at the time of diagnosis or shortly thereafter.
TREATMENT
Pharmacologic Options
There are currently no treatments to halt the progression of ALS or to reverse the disease process. Riluzole, currently the only FDA-approved drug for treatment of ALS, has been shown to slow the loss of muscle strength and to prolong life by an average of two to three months.55 Riluzole targets and blocks glutamate transporters on the presynaptic neuron, decreasing glutamate release and reducing excitotoxicity20 (ie, overstimulation of the postsynaptic receptors). These effects support the theory that ALS may result from excess glutamate and help to explain the increased levels of glutamate found in the serum and CSF of ALS patients.19
Riluzole is typically dosed at 50 mg by mouth twice a day, although 200-mg/d doses have also been examined in clinical trials.20,55 Generally, the drug is well tolerated, with common adverse effects including asthenia, nausea, gastrointestinal upset, and abnormal liver test results. Liver function should be monitored regularly during riluzole therapy, with elevations in serum alanine transferase of particular concern.55
Additional agents have been used to reduce muscle spasticity, muscle cramping and fasciculations, and the associated pain some patients experience, attributable in part to lack of activity and/or inflammation.56 For spasticity, tizanidine or baclofen (orally, a maximum of 20 mg in divided doses; or lower doses administered intrathecally, for patients who experience sedation and fatigue with high oral doses56,57) are often used. Carbamazepine and phenytoin are most commonly used to relieve muscle cramps.56 For some patients, NSAIDs may be adequate to control moderate to severe pain, but others may require opioids.2,56,58
Depressive disorders must be identified accurately, using appropriate clinical tools, before SSRIs (eg, citalopram) or other medications (eg, amitriptyline) are prescribed3; these agents should not be used presumptively,37 as estimates of prevalence of depression among ALS patients range from 2% to 75%.2,37,38,54 Estimates of anxiety prevalence in these patients range from 0% to 30%, reflecting the importance of accurate diagnosis before lorazepam or other agents are prescribed.3,38
Promising results have been reported in the use of modafinil to manage fatigue in patients with ALS.59 For patients with pseudobulbar affect, dextromethorphan 20 mg/quinidine sulfate 10 mg is an FDA-approved treatment.35
Vitamins and other supplements, including creatine, vitamin E, coenzyme Q10, and acetylcysteine, have not been shown to improve survival in this patient population.3
Nonpharmacologic Interventions
Supportive measures for ALS include physical and occupational therapy. In speech and language therapy, breathing and relaxation patterns can be used to correct ineffective compensatory behaviors and help patients “economize” their speaking efforts.60
Nutritional support is also important in patients who have difficulty swallowing, although this can be alleviated somewhat by changes in posture (eg, lowering the chin before attempting to swallow) and by use of thickened fluids; those with immobility of the tongue may find swallowing easier with the head tilted back.60 Once oral alimentation is no longer possible, enteral tube feeding is an option that may prolong survival.60,61
As ALS progresses, dysphagia may be aggravated as the ability to cough, reflexively or voluntarily, is reduced.60 As breathing becomes increasingly difficult, patients may require respiratory support. Noninvasive ventilation, using either continuous or bilevel positive airway pressure, may be implemented early in patients with respiratory-onset ALS, and later in the disease process for other patients, to prevent apnea and hypoventilation.28,62 Mechanical ventilation via tracheostomy is the most invasive method to address respiratory dysfunction in patients with ALS; however, like noninvasive ventilation, nocturnal mechanical ventilation has been shown to extend survival in these patients.28,63,64
Diaphragm pacing stimulation (DPS) has emerged as a possible alternative to mechanical ventilation for ALS patients. The pacing system consists of a battery-operated external pulse generator with electrodes placed after laparoscopic mapping on the diaphragm.65 Natural respiration is mimicked as stimulation from the external pulse generator prompts the diaphragm to contract. Researchers have shown that the minimally invasive surgery (including use of general anesthesia) required to install the DPS system can be safely performed on patients with ALS, and its use can delay the need for a ventilator by 24 months.65,66 Use of the DPS device was granted FDA approval in 2011, under the Humanitarian Device Exemption program.67
Addressing Quality of Life
For the patient with ALS, quality of life is an important consideration throughout disease management. According to numerous research teams, quality of life for these patients depends less on physical function and strength and more on social relationships, existential issues, and spirituality.2,37,54 A high level of quality of life can be sustained in patients with ALS, despite the decline they experience in physical function.
Addressing depression and anxiety by nonpharmacologic means may be needed. Loss of physical strength and mobility and difficulties with speech, swallowing, and breathing can challenge even the strongest patient’s coping skills; even more difficult can be the increased dependence on caregivers, the loss of income, and the financial burdens incurred in health care–related expenses. In some patients, the severity of disease, the lack of effective treatments, and the loss of independence may trigger thoughts of suicide or the wish to “hasten death.”37,39 The importance of counseling, support groups, spirituality or religion, and palliative care, from early in the disease process, cannot be overstated.3,37
Some of these considerations may also be of benefit to spouses and other nonpaid caregivers of the patient with ALS, at least half of whom report feeling physically or psychologically unwell.37,68 Even when professional nursing services or hospice support are available, caregivers often devote 12 hours or more per day to nonprofessional patient care.69 Strategies that support the caregiver can reduce the patient’s perception of burden in that individual.37
CONCLUSION
The exact cause or causes of ALS remain unknown, making it difficult to predict who will present with a disease that appears impossible to prevent. Health care providers in any practice should be aware of the signs of ALS and familiar with its symptoms in order to provide optimal management for potentially affected patients.
Any cause for suspicion of ALS (ie, recent-onset limb weakness or atrophy; difficulty swallowing or speaking) warrants immediate patient referral to a neurologist. Evaluation should include a comprehensive history and physical examination, with emphasis on the musculoskeletal and neurologic exams; MRI of the spine and head, analysis of blood and CSF samples, EMG, and nerve conduction studies should be used to rule out treatable causes of limb weakness.
Potential complications of ALS, including nutritional deficiency, respiratory compromise, and depression, should be discussed early with patients and their caregivers. Management of the patient with ALS necessitates a multidisciplinary approach involving providers from several specialties to ensure that the many issues associated with this disease are being addressed. The priority of the health care provider should be to extend the patient’s survival while maintaining quality of life.
1. ALS Association. Facts you should know. www.alsa.org/about-als/facts-you-should-know.html. Accessed May 30, 2012.
2. Simmons Z. Management strategies for patients with amyotrophic lateral sclerosis from diagnosis through death. Neurologist. 2005;11(5):257-270.
3. Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3.
4. Yoshida M. Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology. 2004;24(1):87-102.
5. Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(6):623-627.
6. National Institute of Neurological Disorders and Stroke, NIH. Motor neuron diseases fact sheet. www.ninds.nih.gov/disorders/motor_neuron_diseases/detail_motor_neuron_diseases.htm. Accessed May 30, 2012.
7. Tartaglia MC, Rowe A, Findlater K. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007;64(2):232-236.
8. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
9. Beghi E, Logroscino G, Chiò A, et al; EURALS Consortium. The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta. 2006;1762(11-12):1150-1157.
10. McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7(6):557-570.
11. Weisskopf MG, Morozova N, O’Reilly EJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):558-561.
12. ALS Association. ALS in the military: unexpected consequences of military service (2011). www.alsa.org/assets/pdfs/advocacy/als_military_paper.pdf. Accessed May 22, 2012.
13. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749.
14. Horner RD, Grambow SC, Coffman DJ, et al. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: evidence for a time-limited outbreak. Neuroepidemiology. 2008;31(1):28-32.
15. Wang H, O’Reilly EJ, Weisskopf MG, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68(2):207-213.
16. Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70(21):1984-1990.
17. Vance C, Rogelj B, Hortobágyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208-1211.
18. Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668-1672.
19. Pioro EP, Majors AAW, Mitsumoto H, et al. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology. 1999;53(1):71-79.
20. Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17(18):1942-1959.
21. Paubel A, Violette J, Amy M, et al. Mutations of the ANG gene in French patients with sporadic amyotrophic lateral sclerosis. Arch Neurol. 2008;65(10):1333-1336.
22. Neusch C, Bähr M, Schneider-Gold C. Glia cells in amyotrophic lateral sclerosis: new clues to understanding an old disease? Muscle Nerve. 2007;35(6):712-724.
23. Rowland LP. Progressive muscular atrophy and other lower motor neuron syndromes of adults. Muscle Nerve. 2010;41(12):161-165.
24. Shoesmith CL, Findlater K, Rowe A, Strong MJ. Progression of amyotrophic lateral sclerosis with respiratory onset. J Neurol Neurosurg Psychiatry. 2007;78(6):629-631.
25. Traynor BJ, Codd MB, Corr B, et al. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population-based study. Arch Neurol. 2000;57(8):1171-1176.
26. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994-1003.
27. Chen R, Grand’Maison F, Strong MJ, et al. Motor neuron disease presenting as acute respiratory failure: a clinical and pathological study. J Neurol Neurosurg Psychiatry. 1996;60(4):455-458.
28. Gautier G, Verscheuren A, Monnier A, et al. ALS with respiratory onset: clinical features and effects of non-invasive ventilation on the prognosis. Amyotroph Lateral Scler. 2010;11(4):379-382.
29. Hasan A, Saxena AB, Ahmed SM, Swamy TLN. Amyotrophic lateral sclerosis presenting with orthopnea in a patient with COPD and obstructive sleep apnea. J Med All Sci. 2011;1(1):46-49.
30. Zoccolella S, Beghi E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2008;79(1):33-37.
31. Brown RH Jr. Amyotrophic lateral sclerosis and other motor neuron diseases. In: Fauci AS, Braunwald E, Kasper DL, ed al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2572-2576.
32. Ince PG, Lowe J, Shaw PJ. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol. 1998;24(2):104-117.
33. Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;73(1):25-31.
34. Shimizu T, Komori T, Kato S, et al. Masseter inhibitory reflex in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2(4):189-195.
35. Rosen H. Dextromethorphan/quinidine sulfate (Zenvia) for pseudobulbar affect. Drugs Today (Barc). 2008;44(9):661-668.
36. Rippon GA, Scarmeas N, Gordon PH, et al. An observational study of cognitive impairment in amyotrophic lateral sclerosis. Arch Neurol. 2006;63(3):345-352.
37. Rabkin JG, Albert SM, Del Bene ML, et al. Prevalence of depressive disorders and change over time in late-stage ALS. Neurology. 2005;65(1):62-67.
38. Vignola A, Guzzo A, Calvo A, et al. Anxiety undermines quality of life in ALS patients and caregivers. Eur J Neurol. 2008;15(11):1231-1236.
39. Fang F, Valdimarsdóttir U, Fürst CJ, et al. Suicide among patients with amyotrophic lateral sclerosis. Brain. 2008;131(pt 10):2729-2733.
40. Verstraete E, Veldink JH, Hendrikse J, et al. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(4):383-388.
41. Turner MR, Modo M. Advances in the application of MRI to amyotrophic lateral sclerosis. Expert Opin Med Diagn. 2010;4(6):483-496.
42. Cirillo M, Esposito F, Tedeschi G, et al. Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol. 2012 Feb 2. [Epub ahead of print]
43. Canu E, Agosta F, Riva N, et al. The topography of brain microstructural damage in amyotrophic lateral sclerosis assessed using diffusion tensor MR imaging. AJNR Am J Neuroradiol. 2011;32(7):1307-1314.
44. Baek WS, Desai NP. ALS: pitfalls in the diagnosis. Pract Neurol. 2007;7(2):74-81.
45. ALS Association. Criteria for the diagnosis of ALS. www.alsa.org/als-care/resources/publica tions-videos/factsheets/criteria-for-diagnosis.html. Accessed May 31, 2012.
46. de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497-503.
47. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299.
48. Waragai M. MRI and clinical features of amyotrophic lateral sclerosis. Neuroradiology. 1997;39(12):847-851.
49. Thorpe JW, Moseley IF, Hawkes CH, et al. Brain and spinal cord MRI in motor neuron disease. J Neurol Neurosurg Psychiatry. 1996; 61(3):314-317.
50. Oba H, Araki T, Ohtomo K, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology. 1993;189 (3):843-846.
51. Piao YS, Wakabayashi K, Kakita A, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;13(1):10-22.
52. Mateen FJ, Carone M, Sorenson EJ. Patients who survive 5 years or more with ALS in Olmsted County, 1925-2004. J Neurol Neurosurg Psychiatry. 2010;81(10):1144-1146.
53. del Aguila MA, Longstreth WT Jr, McGuire V, et al. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813-819.
54. Matuz T, Birbaumer N, Hautzinger M, Kübler A. Coping with amyotrophic lateral sclerosis: an integrative view. J Neurol Neurosurg Psychiatry. 2010;81(8):893-898.
55. Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012 Mar 14;3:CD001447.
56. Handy CR, Krudy C, Boulis N, Federici T. Pain in amyotrophic lateral sclerosis: a neglected aspect of disease. Neurol Res Int. 2011; 2011:403808. Epub 2011 May 3.
57. McClelland S 3rd, Bethoux FA, Boulis NM, et al. Intrathecal baclofen for spasticity-related pain in amyotrophic lateral sclerosis: efficacy and factors associated with pain relief. Muscle Nerve. 2008;37(3):396-398.
58. Andersen PM, Borasio GD, Dengler R, et al; EALSC Working Group. Good practice in the management of amyotrophic lateral sclerosis: clinical guidelines. Amyotroph Lateral Scler. 2007;8(4):195-213.
59. Rabkin JG, Gordon PH, McElhiney M, et al. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle Nerve. 2009;39(3):297-303.
60. Kühnlein P, Gdynia HJ, Sperfeld AD, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366-374.
61. Katzberg HD, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD004030.
62. Lechtzin N, Scott Y, Busse AM, et al. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler. 2007;8(3):185-188.
63. Annane D, Orlikowski D, Chevret S, et al. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD001941.
64. Radunovic A, Annane D, Jewitt K, Mustfa N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD004427.
65. Onders RP, Elmo MJ, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23(7):1433-1440.
66. Onders RP, Carlin AM, Elmo M, et al. Amyotrophic lateral sclerosis: the Midwestern surgical experience with the diaphragm pacing stimulation system shows that general anesthesia can be safely performed. Am J Surg. 2009;197(3):386-390.
67. US Food and Drug Administration. Medical devices: NeuRx Diaphragm Pacing System™—H100006. www.fda.gov/MedicalDevices/Prod uctsandMedicalProcedures/DeviceApprovalsand Clearances/Recently-ApprovedDevices/ucm278684.htm. Accessed May 31, 2012.
68. Rabkin JG, Wagner GJ, Del Bene M. Resilience and distress among amyotrophic lateral sclerosis patients and caregivers. Psychosom Med. 2000; 62(2):271-279.
69. Chiò A, Gauthier A, Vignola A, et al. Caregiver time use in ALS. Neurology. 2006;67(5):902-904.
1. ALS Association. Facts you should know. www.alsa.org/about-als/facts-you-should-know.html. Accessed May 30, 2012.
2. Simmons Z. Management strategies for patients with amyotrophic lateral sclerosis from diagnosis through death. Neurologist. 2005;11(5):257-270.
3. Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3.
4. Yoshida M. Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology. 2004;24(1):87-102.
5. Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(6):623-627.
6. National Institute of Neurological Disorders and Stroke, NIH. Motor neuron diseases fact sheet. www.ninds.nih.gov/disorders/motor_neuron_diseases/detail_motor_neuron_diseases.htm. Accessed May 30, 2012.
7. Tartaglia MC, Rowe A, Findlater K. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007;64(2):232-236.
8. Strowd RE, Cartwright MS, Okun MS, et al. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J Neurol. 2010;257(8):1382-1387.
9. Beghi E, Logroscino G, Chiò A, et al; EURALS Consortium. The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta. 2006;1762(11-12):1150-1157.
10. McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7(6):557-570.
11. Weisskopf MG, Morozova N, O’Reilly EJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):558-561.
12. ALS Association. ALS in the military: unexpected consequences of military service (2011). www.alsa.org/assets/pdfs/advocacy/als_military_paper.pdf. Accessed May 22, 2012.
13. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749.
14. Horner RD, Grambow SC, Coffman DJ, et al. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: evidence for a time-limited outbreak. Neuroepidemiology. 2008;31(1):28-32.
15. Wang H, O’Reilly EJ, Weisskopf MG, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68(2):207-213.
16. Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70(21):1984-1990.
17. Vance C, Rogelj B, Hortobágyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208-1211.
18. Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668-1672.
19. Pioro EP, Majors AAW, Mitsumoto H, et al. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology. 1999;53(1):71-79.
20. Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17(18):1942-1959.
21. Paubel A, Violette J, Amy M, et al. Mutations of the ANG gene in French patients with sporadic amyotrophic lateral sclerosis. Arch Neurol. 2008;65(10):1333-1336.
22. Neusch C, Bähr M, Schneider-Gold C. Glia cells in amyotrophic lateral sclerosis: new clues to understanding an old disease? Muscle Nerve. 2007;35(6):712-724.
23. Rowland LP. Progressive muscular atrophy and other lower motor neuron syndromes of adults. Muscle Nerve. 2010;41(12):161-165.
24. Shoesmith CL, Findlater K, Rowe A, Strong MJ. Progression of amyotrophic lateral sclerosis with respiratory onset. J Neurol Neurosurg Psychiatry. 2007;78(6):629-631.
25. Traynor BJ, Codd MB, Corr B, et al. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population-based study. Arch Neurol. 2000;57(8):1171-1176.
26. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994-1003.
27. Chen R, Grand’Maison F, Strong MJ, et al. Motor neuron disease presenting as acute respiratory failure: a clinical and pathological study. J Neurol Neurosurg Psychiatry. 1996;60(4):455-458.
28. Gautier G, Verscheuren A, Monnier A, et al. ALS with respiratory onset: clinical features and effects of non-invasive ventilation on the prognosis. Amyotroph Lateral Scler. 2010;11(4):379-382.
29. Hasan A, Saxena AB, Ahmed SM, Swamy TLN. Amyotrophic lateral sclerosis presenting with orthopnea in a patient with COPD and obstructive sleep apnea. J Med All Sci. 2011;1(1):46-49.
30. Zoccolella S, Beghi E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2008;79(1):33-37.
31. Brown RH Jr. Amyotrophic lateral sclerosis and other motor neuron diseases. In: Fauci AS, Braunwald E, Kasper DL, ed al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2572-2576.
32. Ince PG, Lowe J, Shaw PJ. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol. 1998;24(2):104-117.
33. Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;73(1):25-31.
34. Shimizu T, Komori T, Kato S, et al. Masseter inhibitory reflex in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2(4):189-195.
35. Rosen H. Dextromethorphan/quinidine sulfate (Zenvia) for pseudobulbar affect. Drugs Today (Barc). 2008;44(9):661-668.
36. Rippon GA, Scarmeas N, Gordon PH, et al. An observational study of cognitive impairment in amyotrophic lateral sclerosis. Arch Neurol. 2006;63(3):345-352.
37. Rabkin JG, Albert SM, Del Bene ML, et al. Prevalence of depressive disorders and change over time in late-stage ALS. Neurology. 2005;65(1):62-67.
38. Vignola A, Guzzo A, Calvo A, et al. Anxiety undermines quality of life in ALS patients and caregivers. Eur J Neurol. 2008;15(11):1231-1236.
39. Fang F, Valdimarsdóttir U, Fürst CJ, et al. Suicide among patients with amyotrophic lateral sclerosis. Brain. 2008;131(pt 10):2729-2733.
40. Verstraete E, Veldink JH, Hendrikse J, et al. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(4):383-388.
41. Turner MR, Modo M. Advances in the application of MRI to amyotrophic lateral sclerosis. Expert Opin Med Diagn. 2010;4(6):483-496.
42. Cirillo M, Esposito F, Tedeschi G, et al. Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol. 2012 Feb 2. [Epub ahead of print]
43. Canu E, Agosta F, Riva N, et al. The topography of brain microstructural damage in amyotrophic lateral sclerosis assessed using diffusion tensor MR imaging. AJNR Am J Neuroradiol. 2011;32(7):1307-1314.
44. Baek WS, Desai NP. ALS: pitfalls in the diagnosis. Pract Neurol. 2007;7(2):74-81.
45. ALS Association. Criteria for the diagnosis of ALS. www.alsa.org/als-care/resources/publica tions-videos/factsheets/criteria-for-diagnosis.html. Accessed May 31, 2012.
46. de Carvalho M, Dengler R, Eisen A, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119(3):497-503.
47. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299.
48. Waragai M. MRI and clinical features of amyotrophic lateral sclerosis. Neuroradiology. 1997;39(12):847-851.
49. Thorpe JW, Moseley IF, Hawkes CH, et al. Brain and spinal cord MRI in motor neuron disease. J Neurol Neurosurg Psychiatry. 1996; 61(3):314-317.
50. Oba H, Araki T, Ohtomo K, et al. Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology. 1993;189 (3):843-846.
51. Piao YS, Wakabayashi K, Kakita A, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;13(1):10-22.
52. Mateen FJ, Carone M, Sorenson EJ. Patients who survive 5 years or more with ALS in Olmsted County, 1925-2004. J Neurol Neurosurg Psychiatry. 2010;81(10):1144-1146.
53. del Aguila MA, Longstreth WT Jr, McGuire V, et al. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813-819.
54. Matuz T, Birbaumer N, Hautzinger M, Kübler A. Coping with amyotrophic lateral sclerosis: an integrative view. J Neurol Neurosurg Psychiatry. 2010;81(8):893-898.
55. Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012 Mar 14;3:CD001447.
56. Handy CR, Krudy C, Boulis N, Federici T. Pain in amyotrophic lateral sclerosis: a neglected aspect of disease. Neurol Res Int. 2011; 2011:403808. Epub 2011 May 3.
57. McClelland S 3rd, Bethoux FA, Boulis NM, et al. Intrathecal baclofen for spasticity-related pain in amyotrophic lateral sclerosis: efficacy and factors associated with pain relief. Muscle Nerve. 2008;37(3):396-398.
58. Andersen PM, Borasio GD, Dengler R, et al; EALSC Working Group. Good practice in the management of amyotrophic lateral sclerosis: clinical guidelines. Amyotroph Lateral Scler. 2007;8(4):195-213.
59. Rabkin JG, Gordon PH, McElhiney M, et al. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle Nerve. 2009;39(3):297-303.
60. Kühnlein P, Gdynia HJ, Sperfeld AD, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366-374.
61. Katzberg HD, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD004030.
62. Lechtzin N, Scott Y, Busse AM, et al. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler. 2007;8(3):185-188.
63. Annane D, Orlikowski D, Chevret S, et al. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD001941.
64. Radunovic A, Annane D, Jewitt K, Mustfa N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD004427.
65. Onders RP, Elmo MJ, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23(7):1433-1440.
66. Onders RP, Carlin AM, Elmo M, et al. Amyotrophic lateral sclerosis: the Midwestern surgical experience with the diaphragm pacing stimulation system shows that general anesthesia can be safely performed. Am J Surg. 2009;197(3):386-390.
67. US Food and Drug Administration. Medical devices: NeuRx Diaphragm Pacing System™—H100006. www.fda.gov/MedicalDevices/Prod uctsandMedicalProcedures/DeviceApprovalsand Clearances/Recently-ApprovedDevices/ucm278684.htm. Accessed May 31, 2012.
68. Rabkin JG, Wagner GJ, Del Bene M. Resilience and distress among amyotrophic lateral sclerosis patients and caregivers. Psychosom Med. 2000; 62(2):271-279.
69. Chiò A, Gauthier A, Vignola A, et al. Caregiver time use in ALS. Neurology. 2006;67(5):902-904.